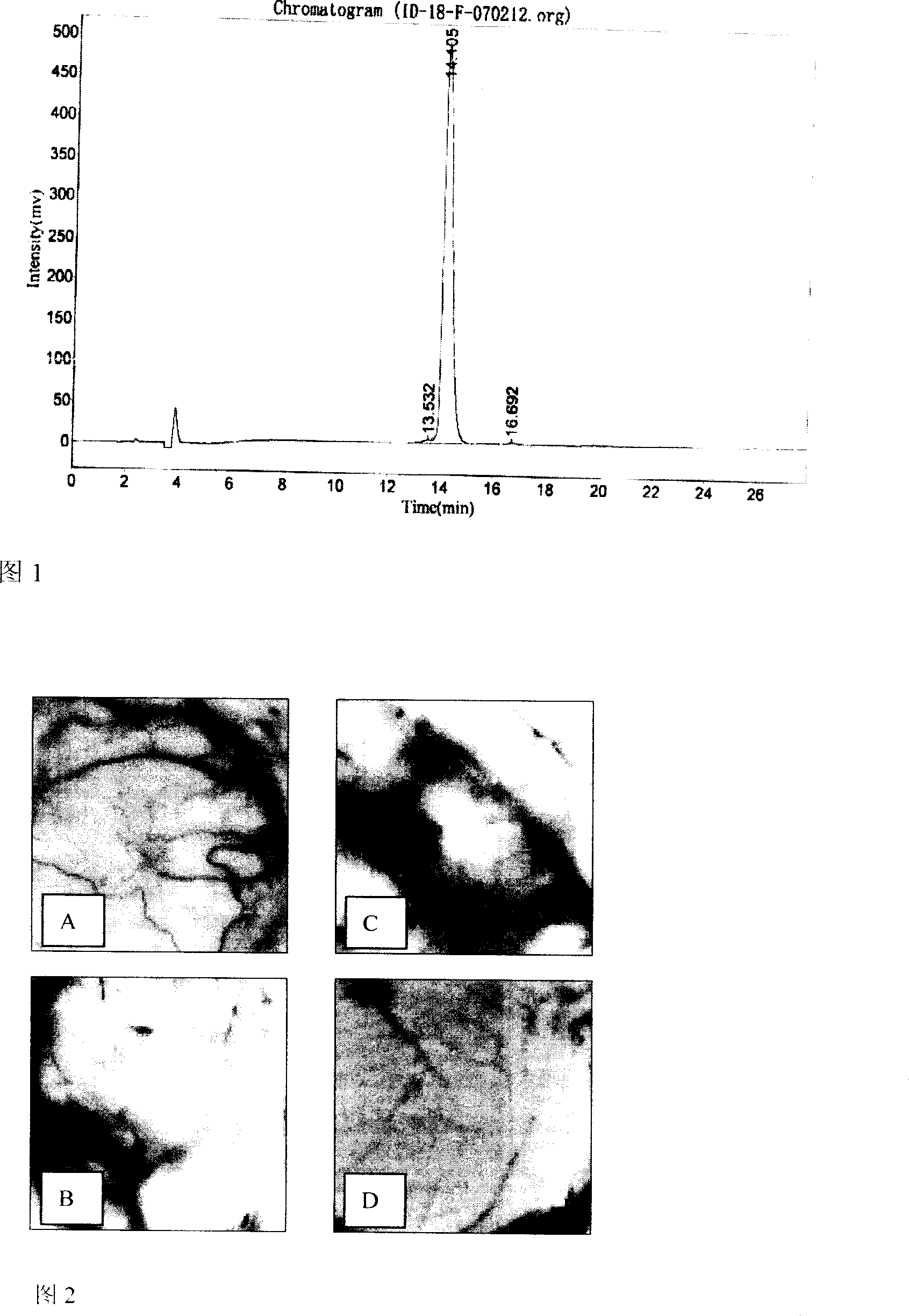
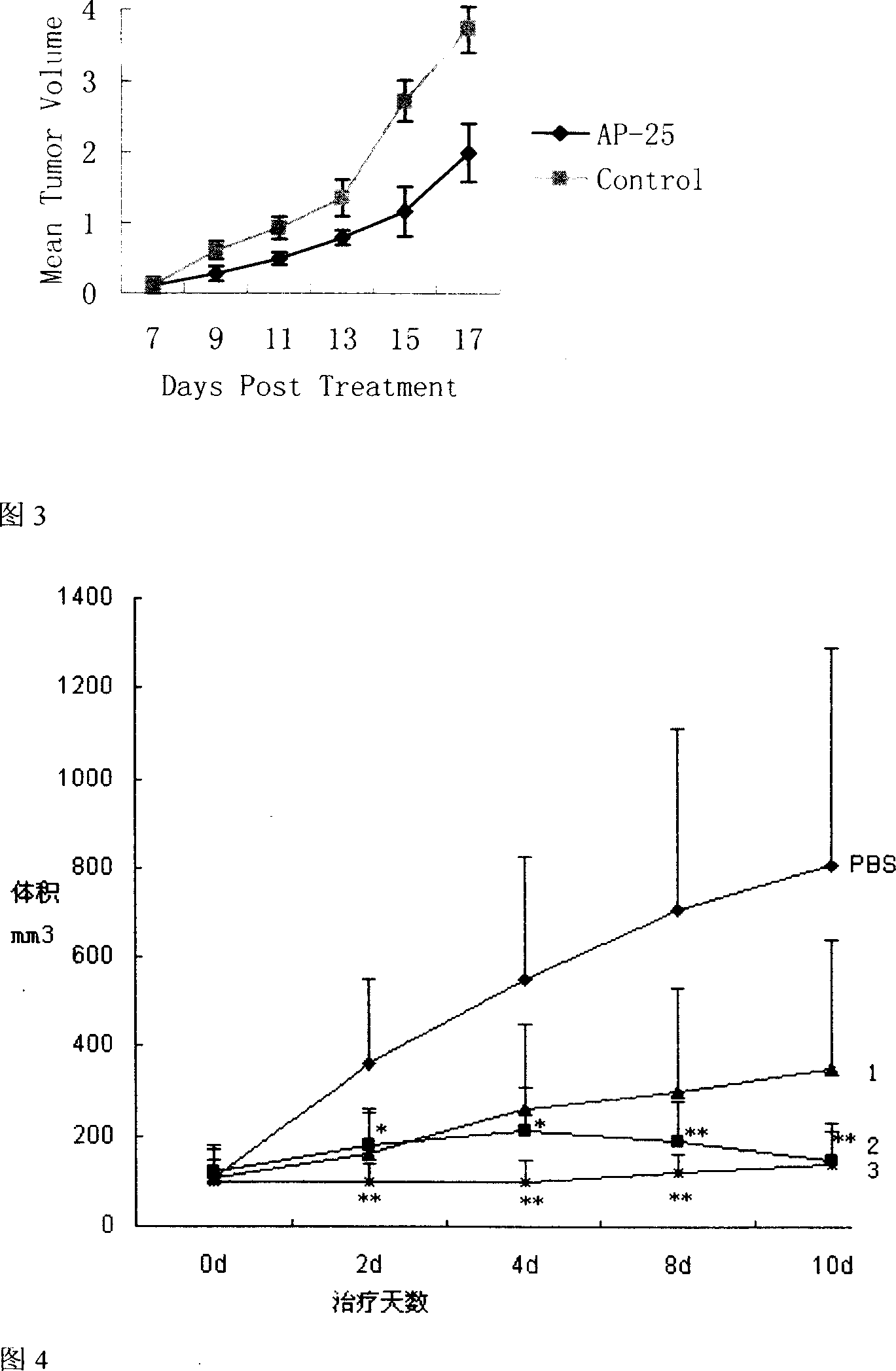
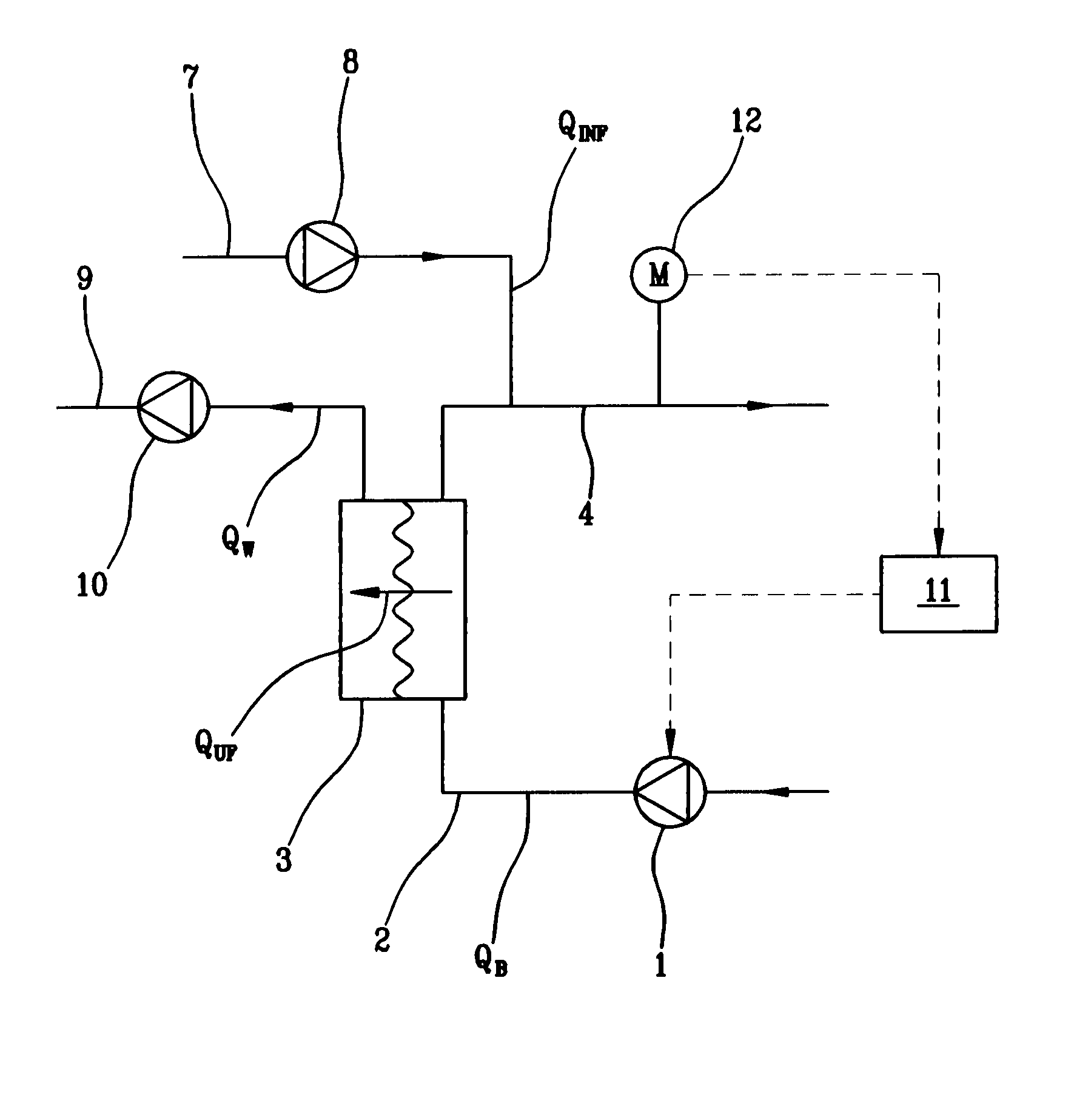
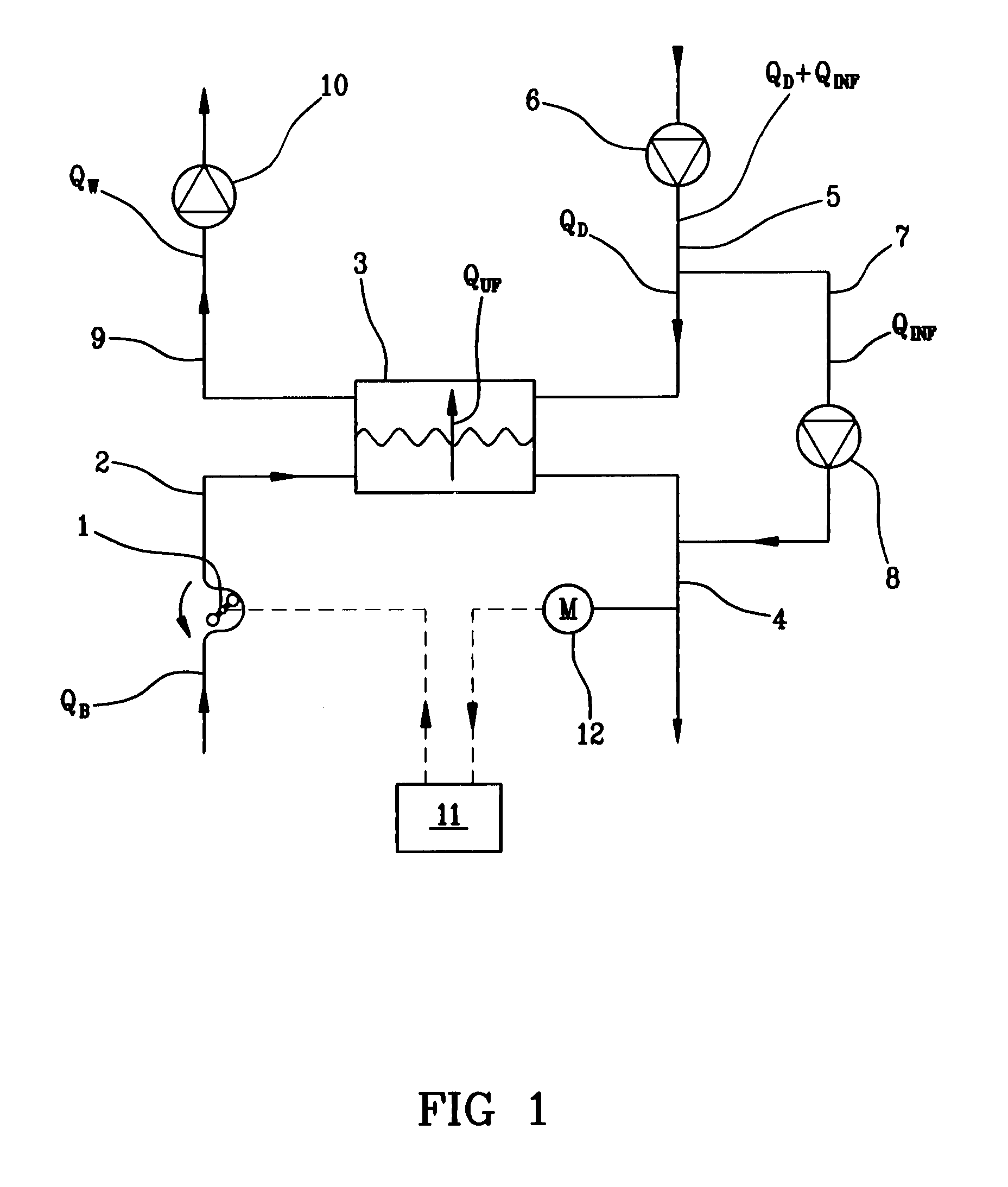
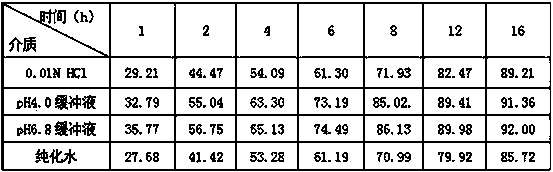
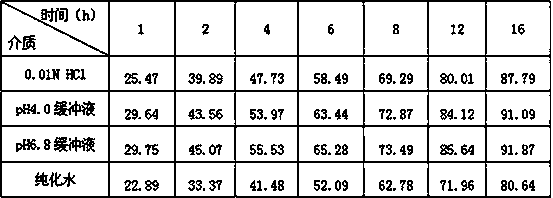
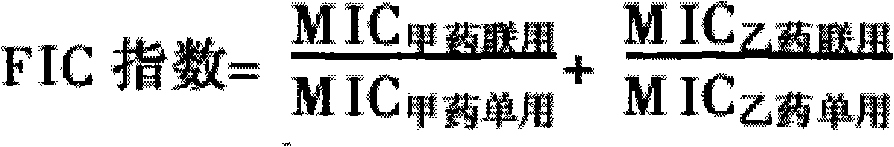Patents
Literature
433results about How to "Increase blood concentration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
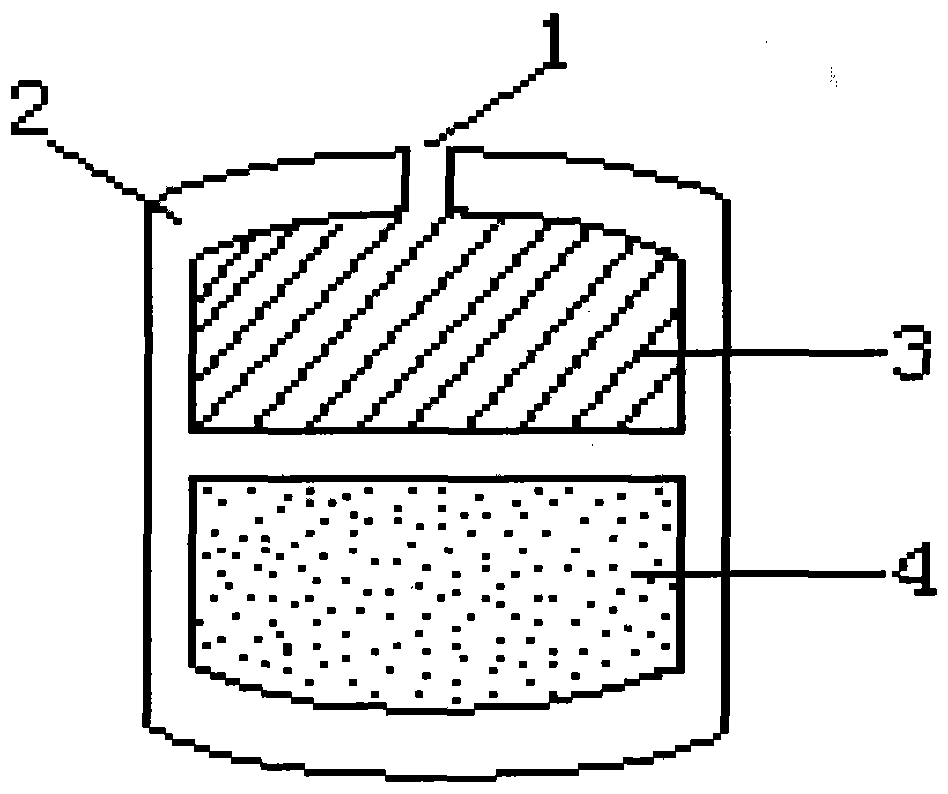
Controlled-release multilayer drug-loaded artificial bone and preparation method thereof
InactiveCN101862230AIncrease concentrationProlong the action timeBone implantMedical devicesControlled releaseIndividualized treatment
The invention discloses a controlled-release multilayer drug-loaded artificial bone and a preparation method thereof. The controlled-release multilayer drug-loaded artificial bone is a multilayer structure which is coated layer by layer and is composed of artificial bone carrier materials loaded with drugs or a multilayer structure which is coated layer by layer and is formed by alternately arranging the artificial bone carrier materials loaded with the drugs and artificial bone carrier materials loaded with no drugs; the outline of the controlled-release multilayer drug-loaded artificial bone can be cylindrical, cuboid, square or irregular; and the artificial bone carrier materials loaded with the drugs at different layers comprise the same or different drugs. The controlled-release multilayer drug-loaded artificial bone is prepared by adopting a three-dimensional stereoscopic printing rapid prototyping technology, and the drugs distributed at the different layers are released from outside to inside layer by layer, thereby being capable of realizing multidrug combined action, regulating the releasing sequence and time of the drugs and selecting appropriate drugs to load so as to achieve the individualized treatment goal. The invention can be applied to carrying out local chemotherapy and filling and repairing bone coloboma after eradication focuses of various infections, concretions, tumours and the like of the orthopedics department.
Owner:XIEHE HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI & TECH UNIV
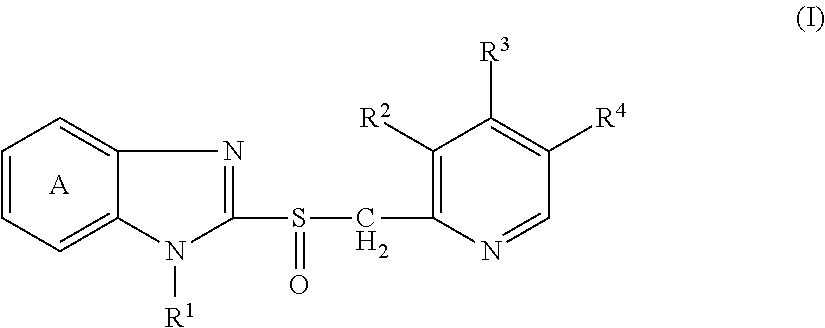
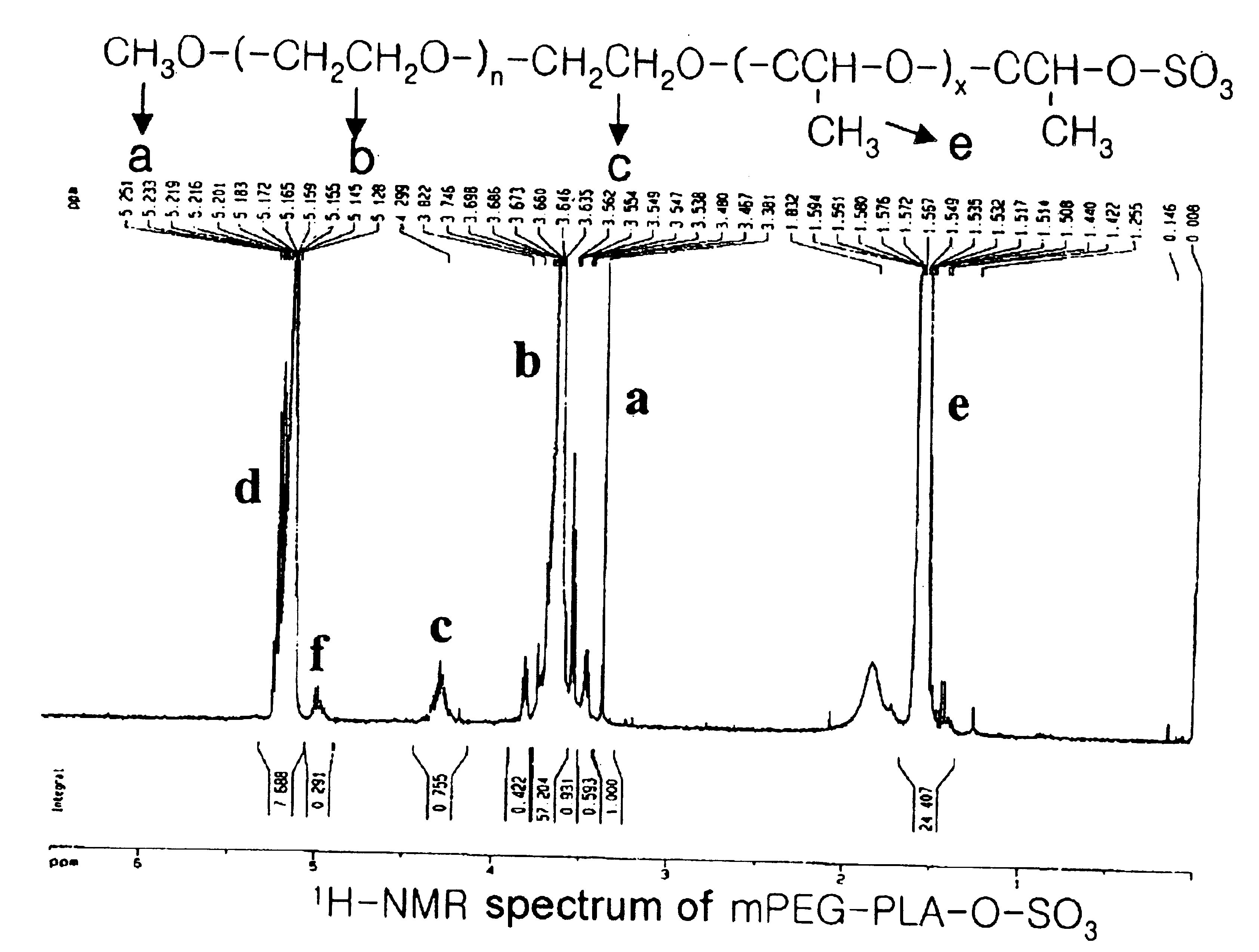
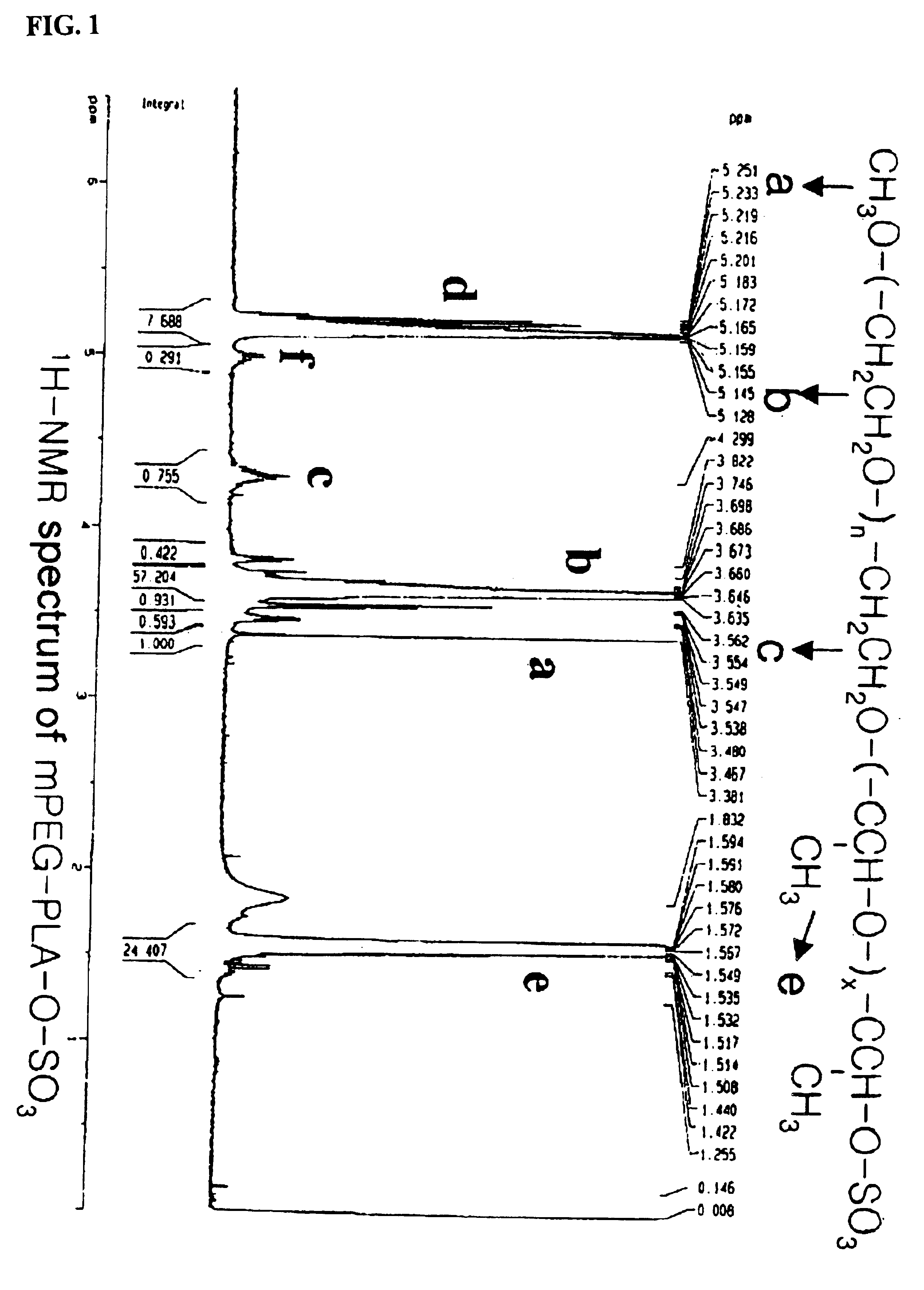
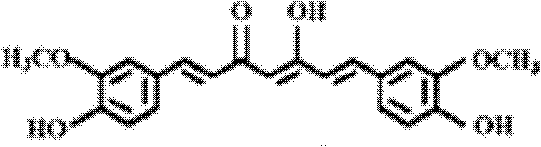
[(indole-3-yl)pyrimidine-2-yl]aminophenylpropyl-2-eneamide derivative and its salt, preparation method of derivative, and application of derivative and salt
ActiveCN105153122AExtended half-lifeIncrease blood concentrationOrganic active ingredientsOrganic chemistryHigh concentrationSide effect
The invention provides a [(indole-3-yl)pyrimidine-2-yl]aminophenylpropyl-2-eneamide derivative and its salt, a preparation method of the derivative, and application of the derivative and the salt. The [(indole-3-yl)pyrimidine-2-yl]aminophenylpropyl-2-eneamide derivative has a structure shown in the formula I below. Deuterium-carbon bonds in the derivative enable the derivative to decompose slowly in a human body, a medicament of the derivative has a longer half-life period and a higher concentration in blood, the dosage of the medicament is finally reduced, and toxic and side effects of the medicament are decreased. Experiments show that compared with AZD 9291 mesylates, AZD 929-D9 mesylate of the deuterium-substituted derivative has Cmax which is 1.32 times as high as that of AZD 9291, exposed dose 1.41 times as high as that of AZD 9291, and elimination half-life 1.31 times as long as that of AZD 9291.
Owner:河南英诺唯医药科技有限公司
Effervescent tablet containing cefixime and its preparing method
InactiveCN100417383CEasy to storeEasy to carryAntibacterial agentsOrganic active ingredientsEffervescent tabletPharmaceutical formulation
The present invention relates to an effervescent tablet containing cefixime and its preparation method. It contains 25-400 mg of cefixime and pharmaceutically-acceptable acid-dbase pair. Besides, the pharmaceutically-acceptable filling agent, adhesive, disintegrating agent, lubricating agent, sweetener and corrective also can be added according to the requirements.
Owner:CHINA PHARM UNIV
Highly effective polypeptide for inhibiting angiogenesis, physical chemistry modifying method and application thereof
InactiveCN101143894APrevent proliferationLittle side effectsPeptide/protein ingredientsPeptidesIon exchangeGenetic engineering
The invention relates to a high-performance angiogenesis inhibitor and a production method, which belongs to the field of the biological engineering pharmaceutical technology or protein polypeptide drugs. The invention designs a high-performance angiogenesis inhibitor RGD-ED with integrin compatibility, the inhibitor contains angiogenesis inhibition polypeptide isoleucine-valine-arginine-arginine-alanine-aspartate-arginine-alanine-alanine-valine-proline, and one or two ends of the inhibitor are respectively connected with polypeptides containing arginine-glycin-aspartate sequence. The RGD-ED of the invention can be synthesized. By the method of genetic engineering, the invention also expresses one of RGD-Eds in escherichia coli or other eukaryotic cells, and the RGD-ED is obtained by carrying out the separation, dissolution and renaturation of inclusion body protein and separation and purification by ion exchange chromatography. All the polypeptide sequences of the invention are modified by Polyethylene Glycol (PEG), heparin, dextran, polyvinylpyrrolidone (PVP), polyglycol-poly (amino acid) copolymer, palmitic acid, colominic acid and liposome, which includes liposome (REV), drying liposomal (DRV) and multivesicular liposome (Mvl). In vivo and in vitro experiments, the synthetic polypeptide sequence, product of genetic engineering and modified product of the invention can notably increase the effects of inhibiting the growth of endothelial cells, inhibiting angiogenesis and resisting tumor of the present angiogenesis inhibitors, and moreover, the high-performance angiogenesis inhibitor can be used as a drug curing solid tumors and rheumatoid arthritis.
Owner:CHINA PHARM UNIV
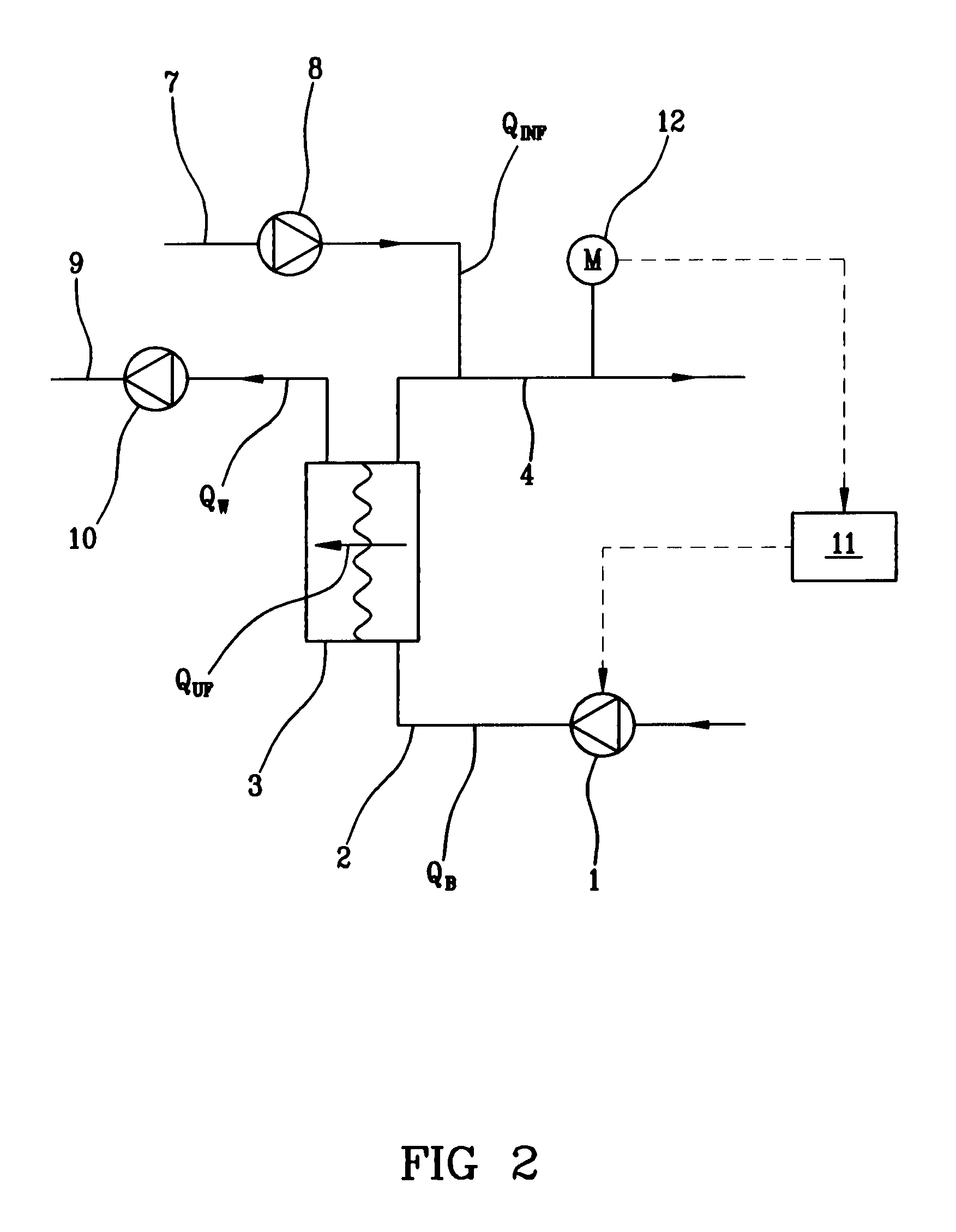
Hemodialysis or hemo(dia)filtration apparatus and a method for controlling a hemodialysis or hemo(dia)filtration apparatus
ActiveUS8647290B2Reduce riskIncrease blood concentrationHaemofiltrationUltrafiltrationHemodialysisHaemodialysis machine
Owner:GAMBRO LUNDIA AB
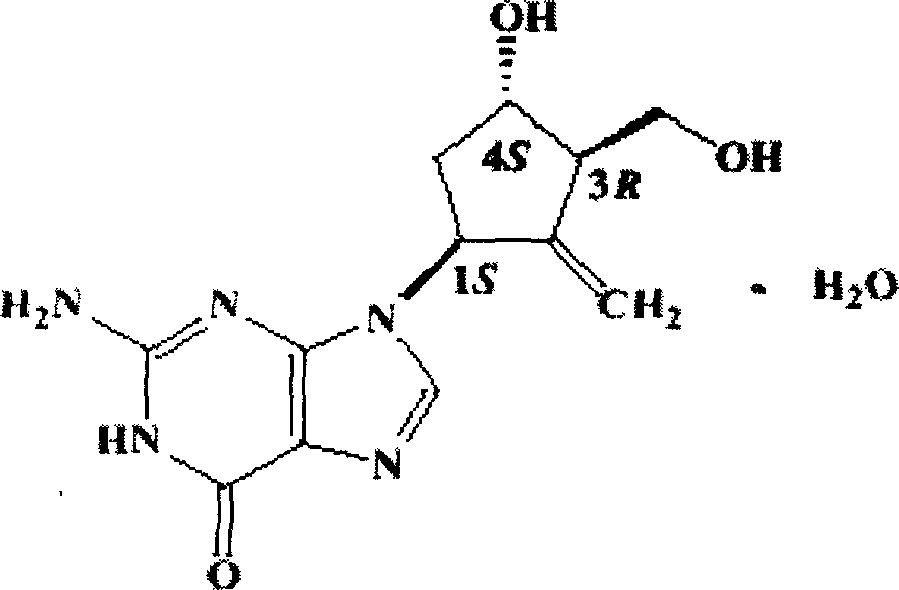
Entecavir dispersible tablet and its preparation process
ActiveCN1732944ASlow disintegrationImprove bioavailabilityDigestive systemAntiviralsPharmaceutical industryEntecavir
The invention relates to an Entecavir dispersible tablet for treating hepatitis B and process for its preparation by using Entecavir as raw material, charging auxiliary materials of specific species and proportions, and preparing tablet through the conventional techniques in the pharmaceutical industry.
Owner:HAINAN ZHONGHE PHARM CO LTD
Methods and formulations for the treatment of medical conditions related to elevated dihydrotestosterone
InactiveUS20050153948A1Enhances small intestinal absorptionIncrease blood concentrationBiocideDispersion deliveryHydrogenAdditive ingredient
The present invention describes a composition that contains a plant sterol or plant stanol or their fatty acid esters and an emulsifier for treating conditions that are related to elevated dihydrotestosterone. The compositions can be prepared in a dry form for use as a food ingredient, tablet or capsule. Alternatively, the compositions can be dissolved in oil.
Owner:ZOMANEX
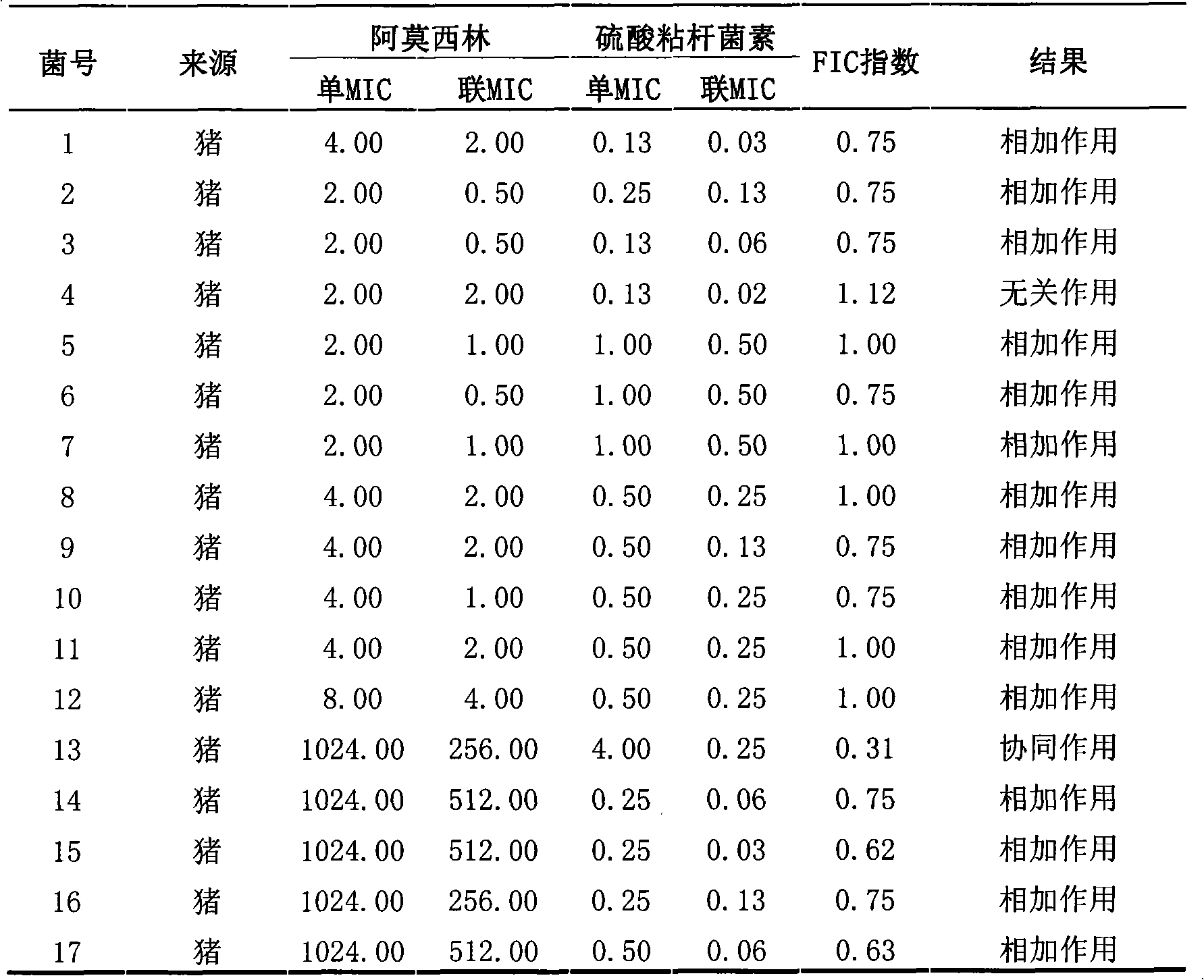
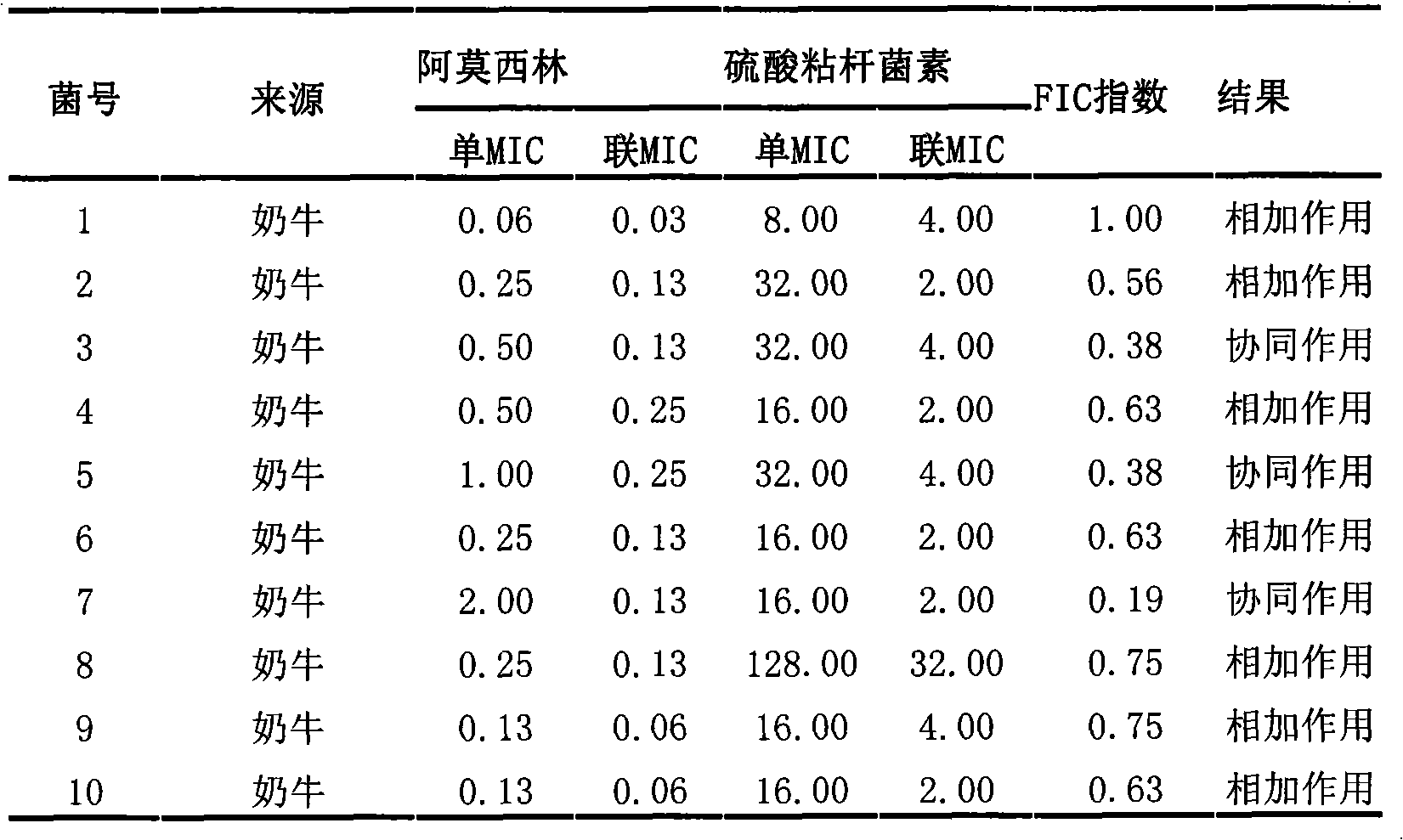
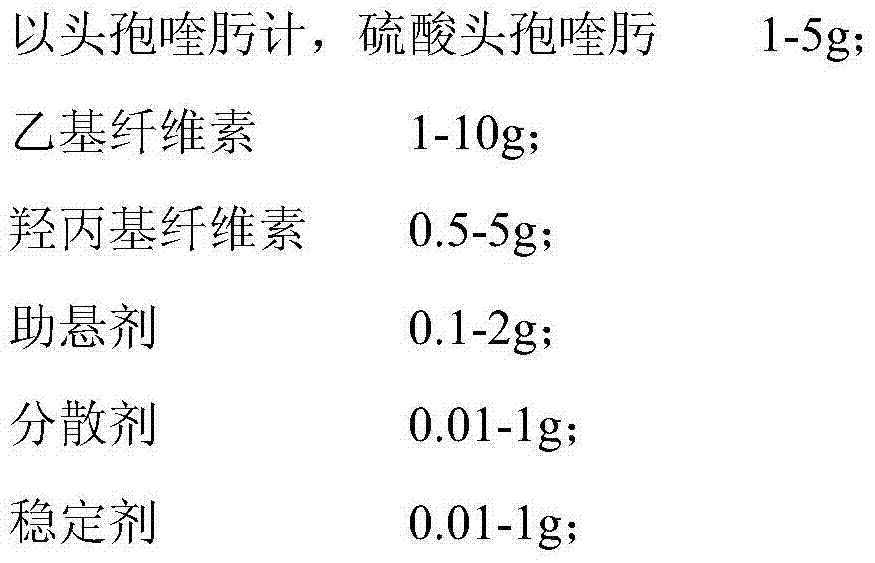
Veterinary suspension containing amoxicillin, colistin sulfate and prednisolone and preparation method thereof
InactiveCN101953784AExpanded antimicrobial spectrumHigh antibacterial efficacyAntibacterial agentsSolution deliveryPrednisoloneColistin Sulfate
The invention discloses a veterinary suspension containing amoxicillin, colistin sulfate and prednisolone and a preparation method thereof. The veterinary suspension adopts the amoxicillin, the colistin sulfate and the prednisolone as active ingredients. The preparation method of the veterinary suspension containing the amoxicillin, the colistin sulfate and the prednisolone comprises the following steps of: (1) dissolving or dispersing a suspending agent, an antioxidant and a preservative in a hot dispersion medium to obtain a solution (A); (2) adding 40-80 percent of dispersion medium in a formula ratio in a colloid mill, starting the colloid mill, then slowly adding the solution (A) and adding a wetting agent while stirring after the solution (A) is fully added; (3) sequentially adding the amoxicillin, the colistin sulfate and the prednisolone after fully adding all the accessories, and grinding by adopting two alternate modes, i.e. an endless grinding mode and a non-endless grinding mode; and (4) detecting the grain fineness, stopping grinding when the grain fineness accords with the requirement, adding the dispersion medium to the formula ratio, mixing, canning, sealing and sterilizing to obtain the veterinary suspension containing the amoxicillin, the colistin sulfate and the prednisolone.
Owner:CHINA AGRI UNIV +1
Orally-disintergrating solid preparation
ActiveUS20110091563A1Reduce breakageSufficient variationBiocideDigestive systemControlled releaseTableting
The present invention provides an orally-disintegrating solid preparation such as a tablet produced by tabletting fine granules showing controlled release of a pharmaceutically active ingredient and an additive, and the like, and the orally-disintegrating solid preparation containing fine granules coated with a coating layer containing a polymer affording a casting film having an elongation at break of about 100-about 700%. With the preparation, breakage of fine granules during tabletting can be suppressed in the production of an orally-disintegrating solid preparation containing fine granules showing controlled release of a pharmaceutically active ingredient.
Owner:TAKEDA PHARMA CO LTD
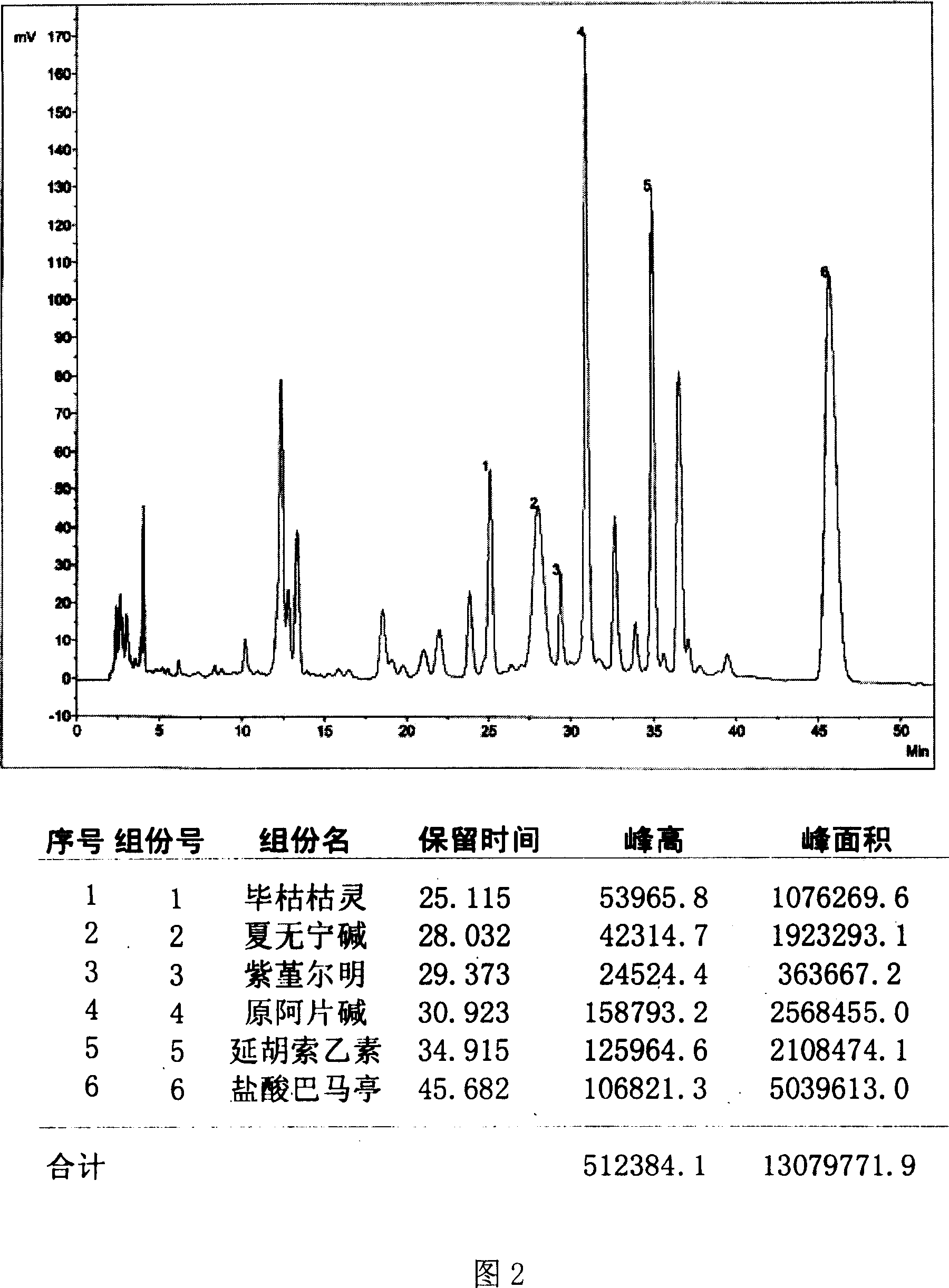
Rhizoma corydalis decumbentis extract, preparation method thereof, medicament composition containing the rhizoma corydalis decumbentis and application thereof
InactiveCN101058576AConsistent with multi-pathway mode of actionVarious ingredientsPowder deliveryAlkaloids chemistryFreeze-dryingPalmatine
The invention discloses a total alkaloid extract and making method of eye drip, which comprises the following parts: opium alkaline, tetrahydropalmatine, cumic acid, alcaine palmatine, corydaline, summer alkaline and other biological alkalines. The preparing method comprises the following steps: adopting summer non as raw material; optimizing hypercritical CO2 flow to extract; collecting extract; drying; obtaining the total alkalilne extract; fitting for tablet, pill, suppository, percutaneous absorption agent, oral rapid-breaking agent, release-control agent and freeze dried.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
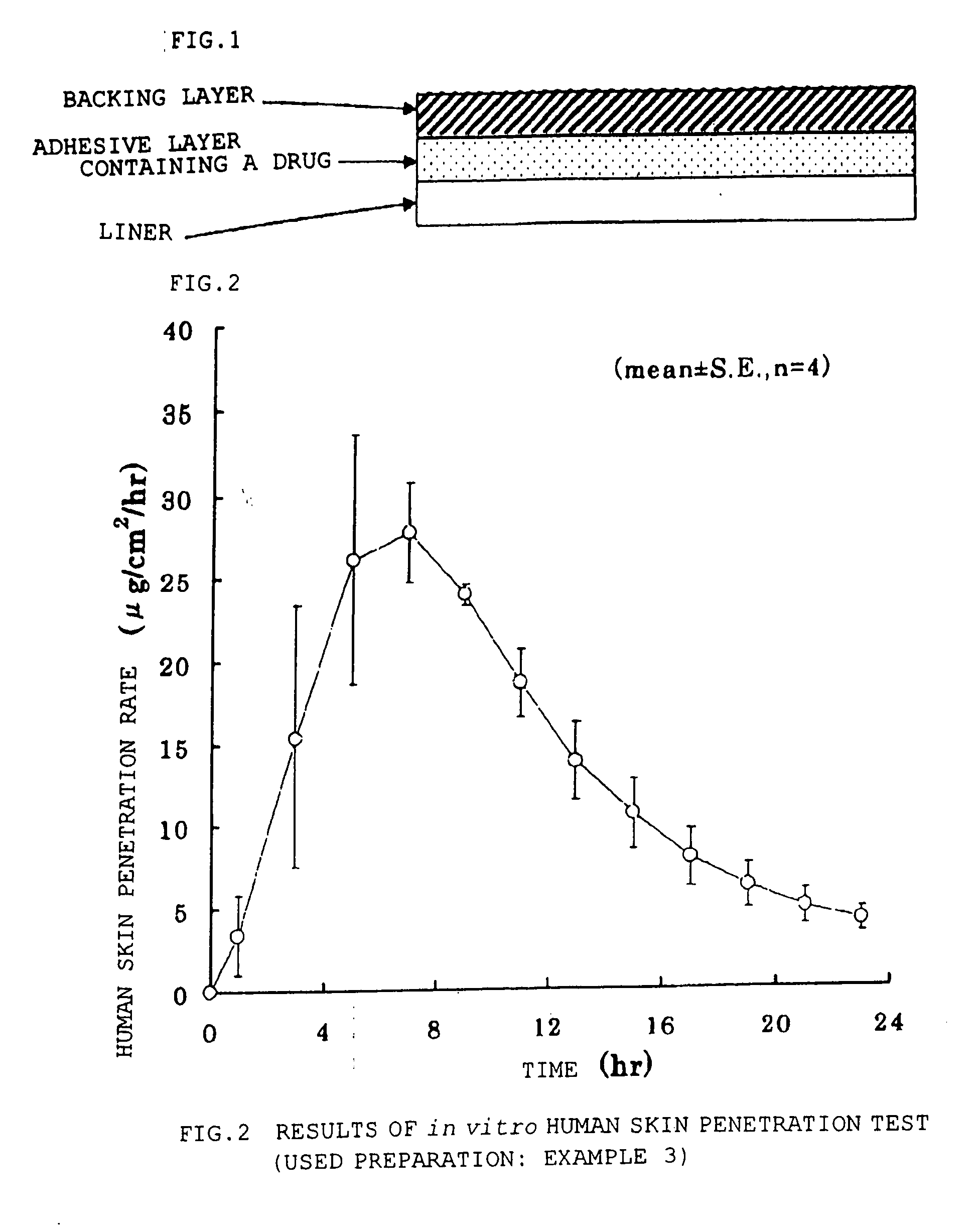
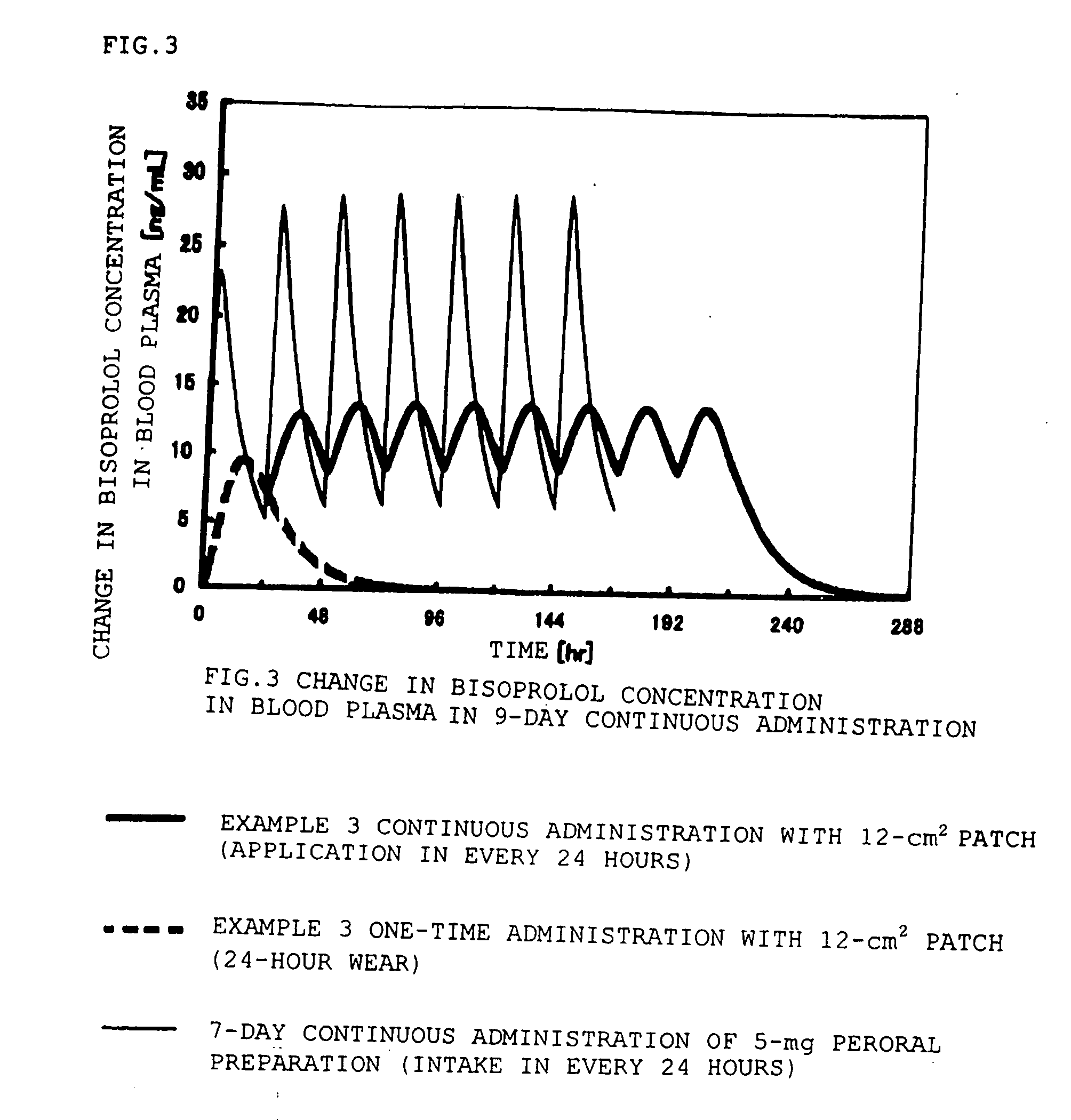
Adhesive patch
InactiveUS20060240086A1Improve permeabilityEffective absorptionOrganic active ingredientsPharmaceutical non-active ingredientsMethacrylateAcrylic polymer
An adhesive patch which includes a pressure-sensitive adhesive layer comprising a composition which comprises: bisoprolol and / or a pharmaceutically acceptable salt thereof; and an acrylic polymer obtained by copolymerizing a (meth)acrylic ester with a carboxylated (meth)acrylic acid. The adhesive patch is stable and excellent in penetration through the skin.
Owner:HISAMITSU PHARM CO INC
Negatively charged amphiphilic block copolymer as drug carrier
InactiveUS6890560B2Increase blood concentrationImprove drug stabilityPowder deliveryMaterial nanotechnologyBlood concentrationDrug carrier
The present invention provides an anionic group-containing amphiphilic block copolymer that is biocompatible and biodegradable and, when used as a drug carrier for a cationic drug, provides several advantages such as increased blood concentration and improved stability of the drug.
Owner:SAMYANG BIOPHARMLS CORP
Bicyclo-ethanol submicron emulsion and preparation method thereof
ActiveCN101524329AImprove solubilityGood chemical stabilityDigestive systemEmulsion deliveryOrganosolvOil phase
The invention discloses a bicyclo-ethanol submicron emulsion and a preparation method thereof. the preparation method comprises the steps of dissolving bicyclo-ethanol and emulsifying agent in an oil phase, adding an assistant for emulsifying agent, a stabilizing agent, other additive and a water phase, adopting a cutting dispersing and high-pressure homogeneous emulsification process to prepare an oil-in-water(O / W) submicron emulsion with the average grain diameter below 500 nm and drug loading dosage between 0.01mg / ml and 5mg / ml. The bicyclo-ethanol submicron emulsion is injected through vein and used for treating medium and serious hepatitis. The prepared submicron emulsion does not contain solubilizer such as Tween-80 or organic solvent, can be mixed with glucose injection, physiological saline or distilled water according to random proportion, and can not easily generate insoluble particulates when stored, used or matched with other components, thereby having high security and good stability. The invention also relates to various preparations of the bicyclo-ethanol submicron emulsion.
Owner:INST OF MATERIA MEDICA CHINESE ACAD OF MEDICAL SCI +1
Immunomodulator slow-release preparation and preparation method thereof
ActiveCN103610658AProlong the action timeUniform and constant action timeOrganic active ingredientsPill deliveryBlood concentrationProlonged-release tablet
The invention discloses an immunomodulator slow-release preparation and a preparation method thereof. A lenalidomide slow-release tablet is composed of a slow-release layer and an optional quick-release layer, wherein the slow-release layer contains active ingredients of lenalidomide and a slow-release framework material simultaneously; the quick-release layer does not contain the slow-release framework material. The lenalidomide slow-release tablet disclosed by the invention is capable of slowly and uniformly releasing medicines by virtue of the slow-release framework material, so as to reduce the release speed, delay the time to peak, prolong the action time of lenalidomide, and provide a uniform and constant blood concentration. Moreover, The lenalidomide slow-release tablet disclosed by the invention is simple in prescription and excellent in quality stability; the preparation process is simple to operate, free from special treatment and production equipment, low in production cost, and beneficial to batch-enlarged industrial production for the product; the preparation method is high in yield, the granulation and crushing procedures are simple and practicable to operate, the intermediate material is good in stability, flowability, compressibility and content uniformity, and completely meets the requirements of tabletting, and the surface of the prepared tablet is smooth and beautiful.
Owner:AC PHARMA CO LTD
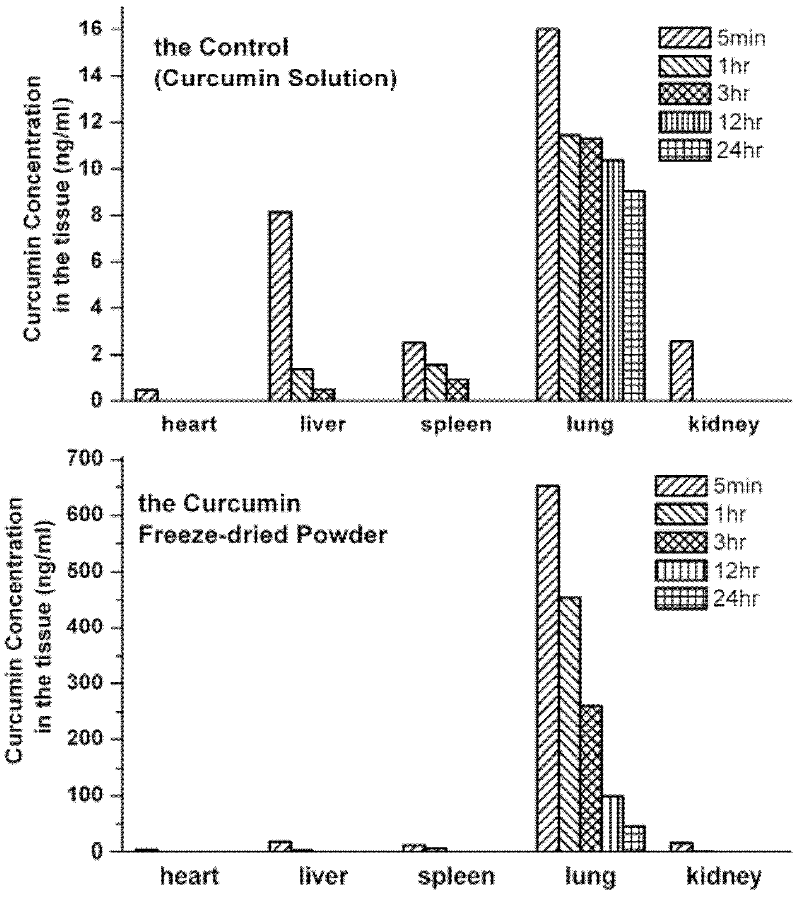
Lung targeting preparation of curcumin class compound as well as preparation method and application thereof
InactiveCN102512404AIncrease loading capacityGood storage stabilityKetone active ingredientsRespiratory disorderDrugSelf-assembly
The invention relates to a lung targeting preparation of curcumin and / or an analog thereof as well as a preparation method and application thereof. The medicine preparation contains a surface active substance and curcumin and / or an analog thereof; the curcumin and / or the analog are(is) loaded in a micell-shaped self-assembly structure formed by the surface active substance; and the lung targeting preparation of the curcumin and / or the analog thereof has the advantages of high medicine carrying quantity and favorable safety and stability, and can be used for intravenous injection. After the intravenous injection of the lung targeting preparation of the curcumin and / or the analog thereof is carried out, the curcumin can be concentrated at the tissue of the lung, so that the blood concentration of the lung is increased, the curative effect of medicine is enhanced, and the dosage of the medicine is obviously lowered.
Owner:SHANGHAI JIAO TONG UNIV
Arenobufagin nanoliposome and preparation method thereof
InactiveCN1951400AHigh encapsulation efficiencyReduce leak rateAmphibian material medical ingredientsAntiviralsToad VenomArenobufagin
The invention discloses a secretio bufonis nano liposome which comprises the following constituents (by weight portions): 0.01-1 part of toad venoms extract, 0.1-20 of phospholipids, 0.05-10 parts of cholesterin, and 0.1-10 parts of emulsifying agent. The invention also discloses the process for preparation through film membrane dispersion method. The secretio bufonis nano liposome has a grain size between 10-100nm and the advantages of high medicinal encapsulation efficiency, low medicinal percolation ratio and oxidation index, and better constancy.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
Chinese herbal piece ultrasonic wall breaking method and device thereof
InactiveCN106423490AIncrease blood concentrationEasy to pass throughGrain treatmentsMedicineFine powder
The invention discloses a Chinese herbal piece ultrasonic wall breaking method. The Chinese herbal piece ultrasonic wall breaking method comprises the following steps: 1) pretreatment: Chinese herbal medicines are put in a drying chamber from a feed port to obtain dried Chinese herbal medicines; 2) the dried Chinese herbal medicines obtained in the step 1) are roughly crushed by a 80-mesh rough crushing device to obtain rough powder of 80-100 meshes; 3) ultrasonic wall breaking: the rough powder obtained in the step 2) is controlled through a flow valve to add in a first wall breaking reaction cavity for ultrasonic crushing to reach a particle size of 10-100 microns so as to obtain wall breaking fine powder; 4) secondary ultrasonic wall breaking: the wall breaking fine powder obtained in the step 3) is added in a second wall breaking reaction cavity for secondary wall breaking by 30-90 min to obtain secondary wall breaking powder; and 5) ternary ultrasonic wall breaking: the secondary wall breaking powder is put in a third wall breaking reaction cavity for ultrasonic wall breaking by at least 20 min to obtain wall breaking ultrafine powder; and the wall breaking ultrafine powder flows out from a discharge port. The Chinese herbal piece ultrasonic wall breaking method can be applied to manufacture ultrafine wall breaking Chinese herbal pieces, and is better in wall breaking effect of the Chinese herbal pieces through three times of continuous ultrasonic wall breaking.
Owner:SUZHOU YINGHUANG PHARMA
Effervescent tablet containing cefixime and its preparing method
InactiveCN1850087AEasy to storeEasy to carryAntibacterial agentsOrganic active ingredientsEffervescent tabletPharmaceutical formulation
The present invention relates to an effervescent tablet containing cefixime and its preparation method. It contains 25-400 mg of cefixime and pharmaceutically-acceptable acid-dbase pair. Besides, the pharmaceutically-acceptable filling agent, adhesive, disintegrating agent, lubricating agent, sweetener and corrective also can be added according to the requirements.
Owner:CHINA PHARM UNIV
Tablet of isosorbide mononitrate
ActiveCN101732276AEasy to pumpStable release ratePill deliveryHeterocyclic compound active ingredientsMedicineSemipermeable membrane
The invention relates to a tablet of isosorbide mononitrate, in particular to a double-layer osmotic pump controlled release tablet of isosorbide mononitrate, which belongs to the field of medicine preparation. A single-chamber double-layer osmotic pump tablet of the isosorbide mononitrate is characterized in that a semi-transparent coating film cover a double-layer core body consisting of a medicine-containing layer and a boosting layer; and the coating film is provided with a medicine releasing pore on the surface of the medicine-containing layer. The gastrointestinal tract water enters a double-layer tablet chip through the semi-transparent film; the medicines forms a mixed suspension liquid or solution when contacting water in the medicine-containing layer; a penetration enhancer enables the solution of the medicine-containing layer to be hypertonic so that an osmotic pressure difference exists between the inner side and the outer side of the film, which is beneficial to pumping the medicines out; and the pressure is generated in the boosting layer through water absorption, dissolution and expansion of a penetrating agent so as to further boost a medicine liquid to eject the pore.
Owner:LUNAN BETTER PHARMA
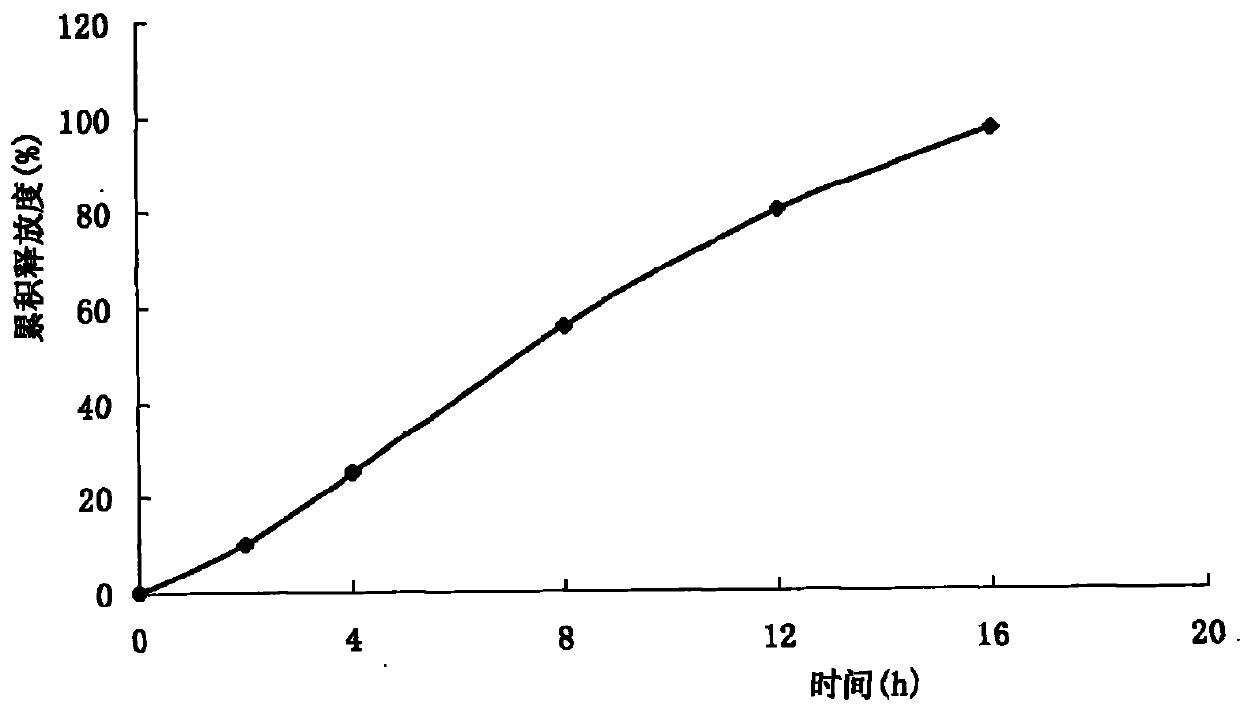
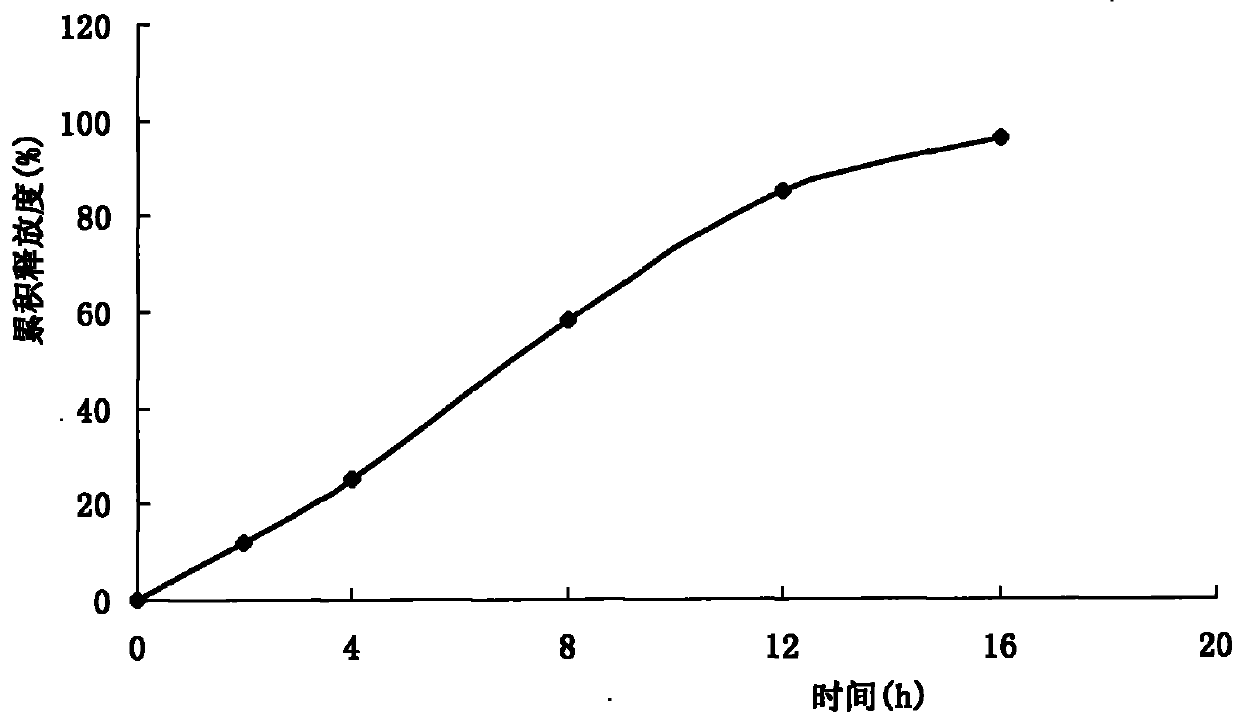
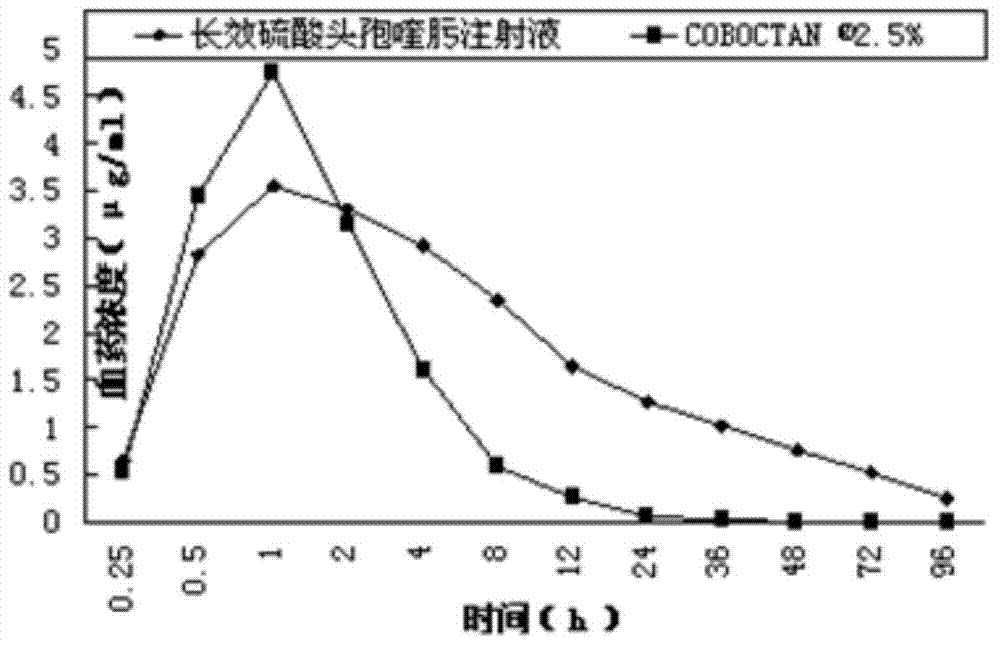
Long-acting cefquinome sulfate injection and preparation method thereof
ActiveCN103751103AProlong the duration of action of the drugImprove redispersibilityAntibacterial agentsOrganic active ingredientsSolventAdministration time
The invention relates to a long-acting cefquinome sulfate injection. The injection is prepared by a method in the following steps: adding cefquinome sulfate into an ethanol solution containing ethyecellulose and hydroxy propyl cellulose; filtering, sterilizing and performing spray-drying to obtain a cefquinome sulfate carrier; adding a suspending agent, a dispersing agent and a stabilizing agent into partial injection solvent, dissolving at 100 DEG C, and adding an injection solvent to the whole amount, and performing dry heat sterilization at 150 DEG C for 1 hour; cooling the dispersion liquid to room temperature, adding the cefquinome sulfate carrier into the dispersion liquid, and uniformly stirring; passing through a high-speed cutting dispersion machine to prepare the long-acting cefquinome sulfate injection. The prepared suspension injection is uniform in granularity, good in fluidity, slow in settlement, good in redispersion property, wall adhesion prevention of liquid medicine, good needle cleaning performance, no discoloration after long-term storage and stable chemical property. After the preparation is injected in a pig in an intramuscular manner, the preparation is slowly released, long in elimination half life and smooth in plasma concentration, can be used for prolonging the effective action duration of the medicine in the body and reducing the number of administration times, and is convenient to use.
Owner:AMICOGEN CHINA BIOPHARM CO LTD
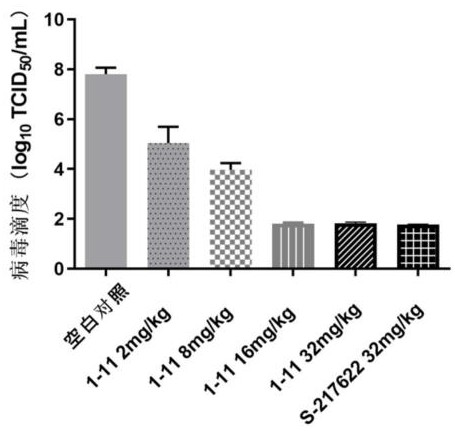
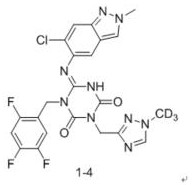
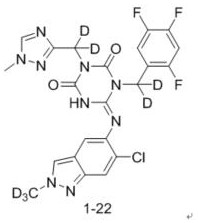
Triazine compound and application thereof in preparation of antiviral drugs
ActiveCN114507221AStrong inhibitory activityIncrease blood concentrationOrganic chemistryAntiviralsViral infectious diseaseSevere acute respiratory syndrome-related coronavirus
The invention relates to a triazine compound with a structure represented by a formula A, or a stereoisomer, a prodrug, an active metabolite or a pharmaceutically acceptable salt, a solvate or a crystal form thereof, a pharmaceutical composition of the triazine compound, the stereoisomer, the prodrug, the active metabolite or the pharmaceutically acceptable salt, the solvate or the crystal form, and a use method of the triazine compound. In addition, the invention also relates to a method for preparing a 3C-like cysteine protease inhibitor or a medicine for treating and / or preventing virus infectious diseases. In particular, the present invention relates to the use for treating viral infectious diseases such as Middle East Syndrome Related Coronavirus (MERS-CoV), Severe Acute Respiratory Syndrome Related Coronavirus (SARS-CoV), Influenza A virus, Influenza B virus, Novel Coronavirus Pneumonia (COVID-19) and the like.
Owner:YAOKANG ZHONGTUO (JIANGSU) PHARMA TECH CO LTD
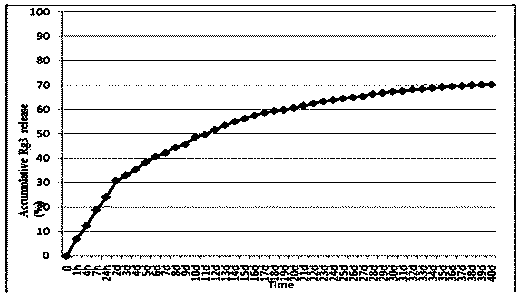
Ginsenoside Rg3 poly(lactic-co-glycolic acid) nano microsphere and preparation method thereof
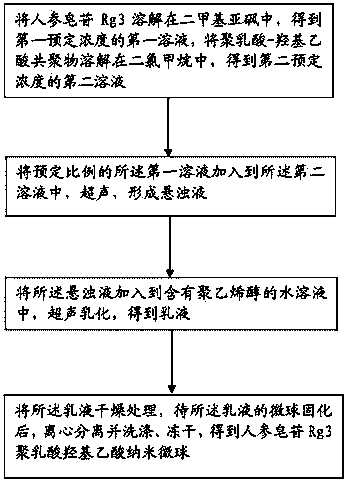
InactiveCN104288111ASolve the shortcomings of poor water solubility and difficult absorption after oral administrationLittle side effectsPowder deliveryOrganic active ingredientsFreeze-dryingMicrosphere
The invention discloses a ginsenoside Rg3 poly(lactic-co-glycolic acid) (PLGA) nano microsphere and a preparation method thereof. The preparation method of the ginsenoside Rg3 poly(lactic-co-glycolic acid) nano microsphere comprises the following steps: S1, dissolving ginsenoside Rg3 in dimethyl sulfoxide to obtain a first liquid with a first predetermined concentration, and dissolving poly(lactic-co-glycolic acid) copolymer into dichloromethane to obtain a second liquid with a second predetermined concentration; S2, adding the first liquid in a predetermined proportion into the second liquid to perform ultrasonic treatment to form a turbid liquid; S3, adding the turbid liquid into an aqueous liquid containing polyvinyl alcohol to perform ultrasonic emulsion to obtain emulsion; and S4, drying the emulsion, centrifugally separating and washing, and freeze-drying the emulsion after curing the microspheres of the microsphere of the emulsion to obtain the ginsenoside Rg3 poly(lactic-co-glycolic acid) nano microsphere. According to the preparation method of the ginsenoside Rg3 poly(lactic-co-glycolic acid) nano microsphere of the embodiment of the invention, the prepared ginsenoside PLGA microsphere is good in pesticide effect.
Owner:BEIJING JIAOTONG UNIV
Percutaneous absorption preparation containing donepezil, and method for preparing same
InactiveUS9622986B2Minimize contactInhibition releaseNervous disorderSheet deliveryAdhesiveCombinatorial chemistry
Provided is a transdermal delivery system (for example, in the form of a patch) having a double-layer structure including a drug-containing matrix layer and an adhesive layer, wherein the drug-containing matrix layer is obtained by completely dissolving donepezil using a certain polymer and then formulating along with an adhesive; and a process for preparing the same. The transdermal delivery system according to the present invention does not show any crystallization of donepezil in the formulation, can release donepezil at a uniform rate for a long time, and can inhibit the release dumping phenomenon that occurs in a transdermal delivery system having a single-layer structure.
Owner:SK CHEM CO LTD
Ground substance and plaster of Chinese traditional medicine as well as its preparing method
InactiveCN1480129AOvercoming irritationLow allergy rateAerosol deliveryOintment deliveryMedicineIrritation
A matrix for Chinese-medicinal plaster is prepared from polyisobutylene, viscosity increaser, softening agent, filler, percutaneous absorption promoter, and solvent. Its advantages are low hypersensitivity and irritation to skin, and controllable viscosity. Its Chinese-medicinal plaster is prepared through proportionally mixing said matrix with the Chinese-medicinal extract.
Owner:桂林华润天和药业有限公司
Implants using ultrasonic communication for modulating splenic nerve activity
PendingUS20190321640A1Desired effectReduce concentrationSpinal electrodesCircuit arrangementsTreatment hypertensionMedical treatment
Described herein are methods for monitoring or modulating an immune system in a subject; treating, reducing or monitoring inflammation; monitoring blood pressure; treating hypertension; or administering or adjusting a therapy for inflammation or hypertension in a patient by electrically stimulating the splenic nerve or detecting splenic nerve activity using an implanted medical device. Also described herein are implantable medical devices for performing such methods. The implanted medical device includes an ultrasonic transducer configured to receive ultrasonic waves and convert energy from the ultrasonic waves into an electrical energy that powers the device, two or more electrodes in electrical communication with the ultrasonic transducer that are configured to electrically stimulate a splenic nerve or detect a splenic nerve activity, and optionally a splenic nerve attachment member.
Owner:IOTA BIOSCI INC
FAK inhibitor and combined medicine thereof
ActiveCN111377871AIncrease exposureExtended half-lifeOrganic chemistry methodsAntibody medical ingredientsPharmaceutical medicineBlood drug concentration
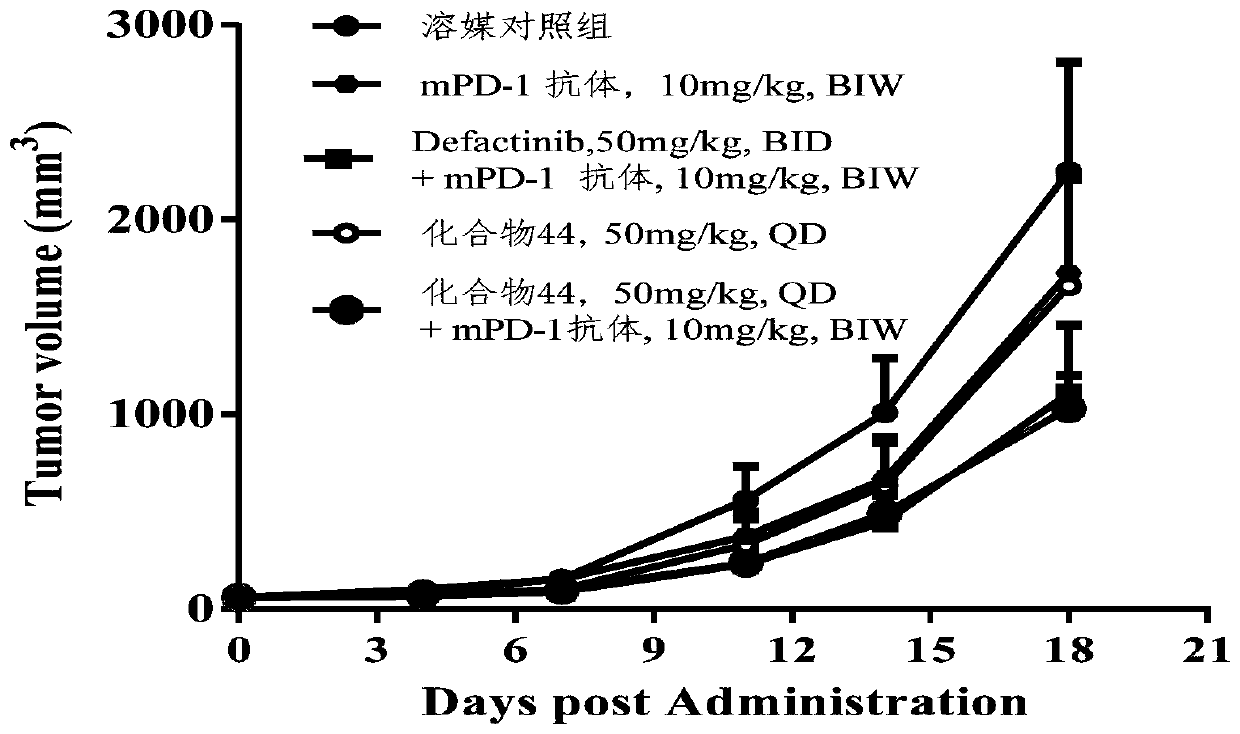
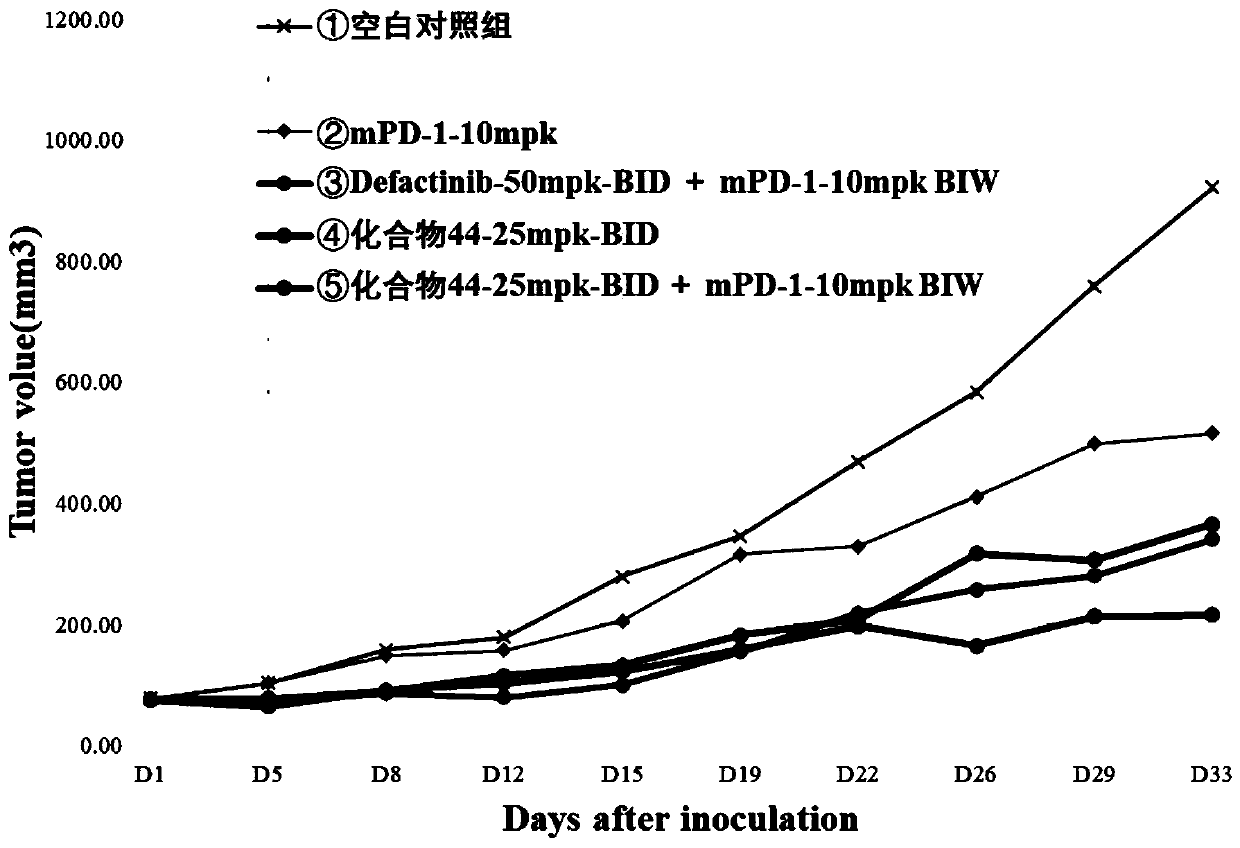
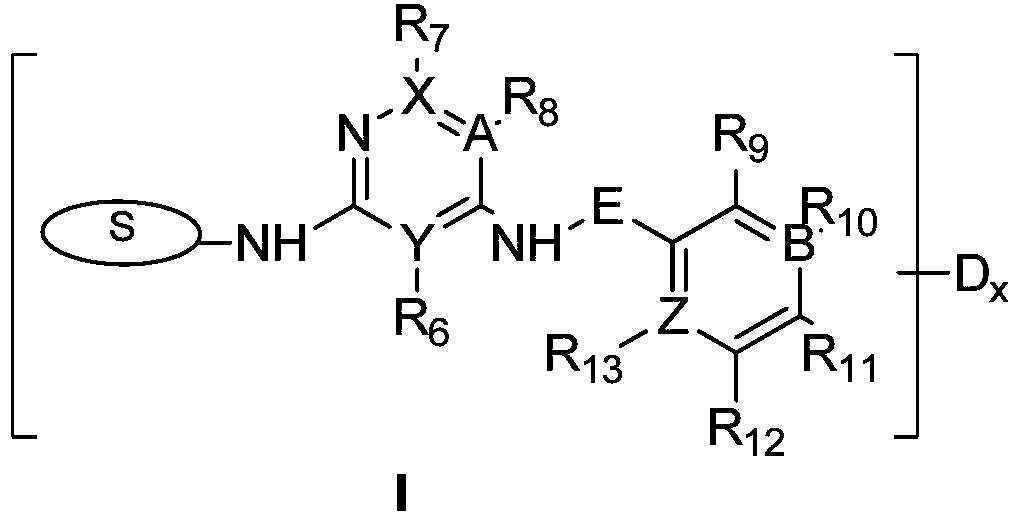
The invention provides a deuterated compound shown as a formula (I) or an optical isomer, a tautomer, a pharmaceutically acceptable salt, a prodrug, a hydrate or a solvate thereof. Compared with a compound before deuteration, the deuterated compound disclosed by the invention shows better pharmacokinetics, higher highest blood concentration, higher exposure and longer half-life period, and has more excellent metabolic performance. Moreover, the deuterated compound provided by the invention can effectively inhibit the activity of FAK, and has a very good application prospect in preparation of an FAK inhibitor and / or a drug for treating cancers. Meanwhile, the deuterated compound and an anti-cancer drug (such as a PD-1 inhibitor) are combined for use, so that the synergistic effect can be achieved, the tumor inhibition effect is remarkably improved, and a better choice is provided for clinical treatment of cancers.
Owner:HINOVA PHARM INC
Application of high-solubility berberine in preparing medicine
InactiveCN1771944ASelf-regulatingSafe to takePowder deliveryOrganic active ingredientsSolubilityThrombus
The present invention relates to the new use of high solubility berberine in preparing medicine. The high solubility berberine may be dissolved in 500 times of water and is easy to be absorbed by human body to make best use and raise curative effect. It may be used in treating and preventing hypertension, hyperlipemia, arrhythmia, heart disease, hepatitis, etc.
Owner:吴开敏
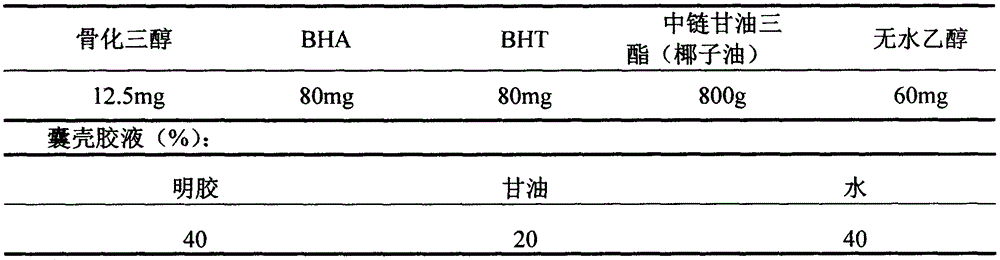
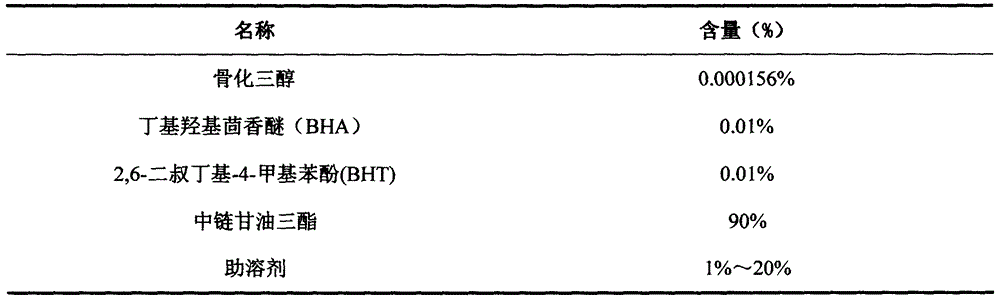
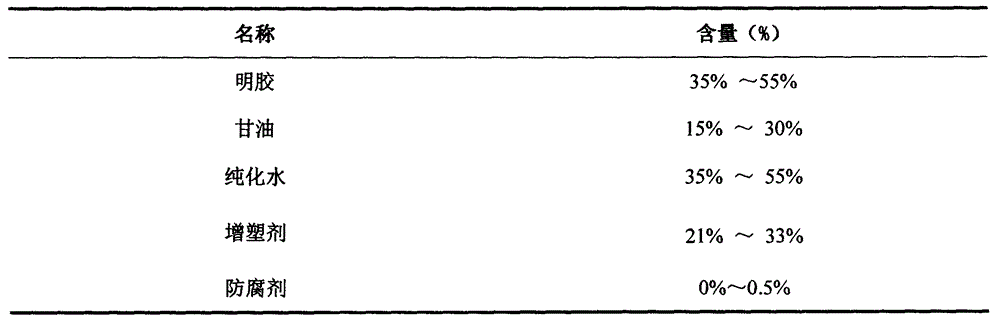
Calcitriol soft capsule preparation production method
InactiveCN106265586AShort half-lifeReduce adverse reactionsOrganic active ingredientsMetabolism disorderGlycerolSide effect
The present invention relates to a calcitriol soft capsule and a preparation method thereof. According to the preparation method, a content is prepared from calcitriol, medium chain triglyceride, butylhydroxy anisole (BHA), 2,6-di-tert-butyl-4-methylphenol (BHT) and dehydrated alcohol, a capsule skin material is prepared from gelatin, glycerol, sorbitol, purified water, methylparaben, propylparaben, sunset yellow and titanium dioxide, and the soft capsule is formed through a pressing method. According to the present invention, the calcitriol soft capsule has advantages of reasonable types and reasonable dose ratio of the auxiliary material and the main drug in the soft capsule dosage form, convenient medication, rapid effect, high bioavailability and low side effect, and the preparation method has characteristics of simple, easy control and stable product quality, and is suitable for large-scale production application.
Owner:ZHENGZHOU TAIFENG PHARMA CO LTD
Peptide pharmaceuticals for nasal delivery
InactiveUS20100256060A1Improve bioavailabilityIncrease blood concentrationPeptide/protein ingredientsAerosol delivery(R)-CarnitineActive agent
Pharmaceutical products for nasal administration contain peptide active agents and are formulated with compounds that enhance bioavailability of the peptide active agents. In particular, citrates, fatty acids, sugar esters of fatty acids or acyl carnitines are used. In some embodiments, a sugar ester of a fatty acid is used in combination with either a fatty acid, or alternatively, an acyl carnitine.
Owner:ENTERIS BIOPHARMA
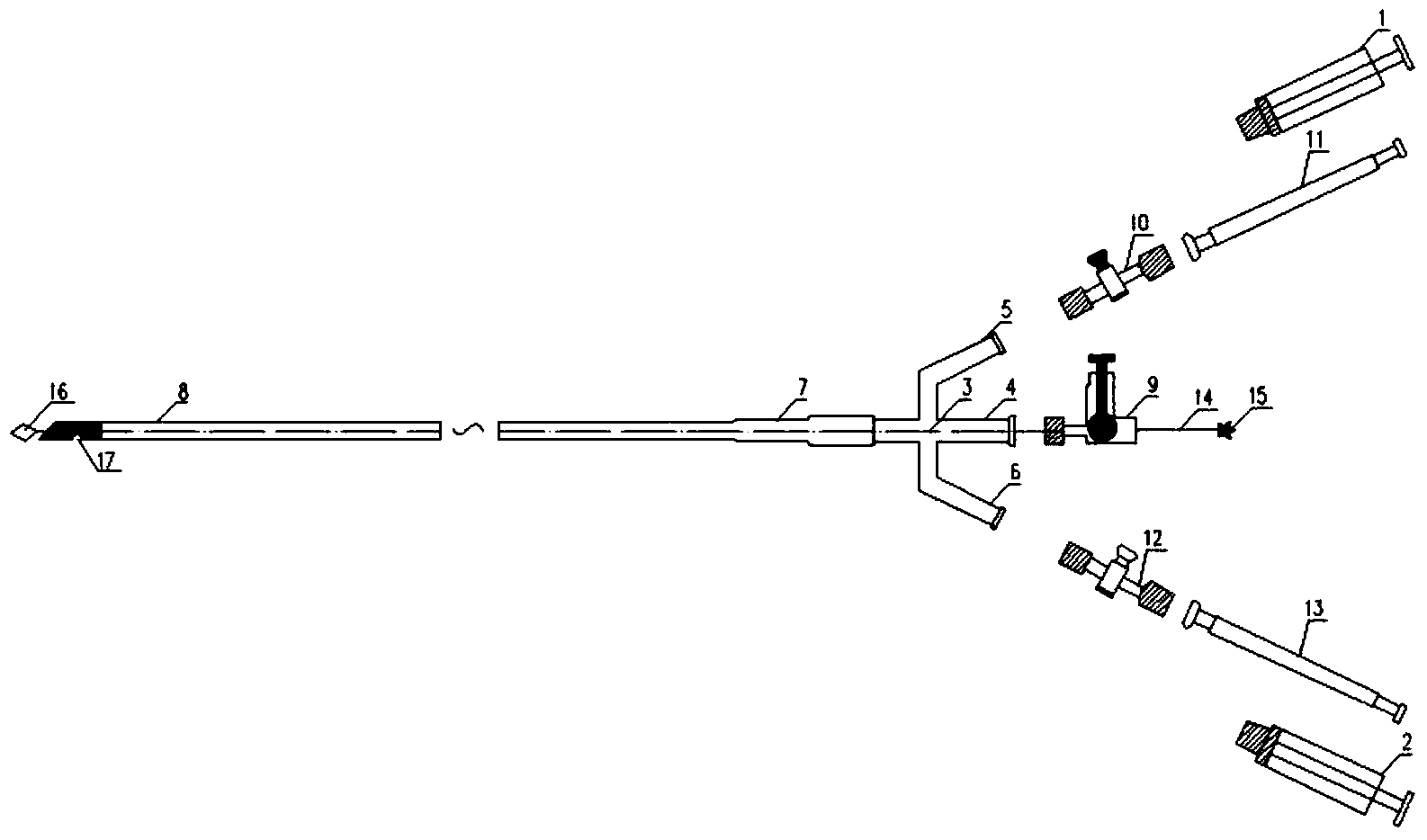
Thrombus aspiration catheter system
The invention relates to a thrombus aspiration catheter system. The thrombus aspiration catheter system comprises an aspirator, a medicine injection device and a four-way tube; the four-way tube consists of a main tube, and an upper branch tube and a lower branch tube which are symmetrically distributed on the main tube; one end of the main tube is connected with a front-section catheter through a front end connecting tube, and the other end of the main tube is connected with a shut-off valve I; the upper branch tube is connected with one end of a back-end catheter I through a shut-off valve II; the other end of the back-end catheter I is connected with the aspirator; the lower branch tube is connected with one end of a back-end catheter II through a shut-off valve III; the other end of the back-end catheter II is connected with the medicine injection device; a center shaft penetrating through the front-section catheter, the front-end connecting tube and the main tube is provided with a metal stirring wire; the near end of the metal stirring wire is provided with a handle I; the far end of the metal stirring wire is provided with a diamond ring. The thrombus aspiration catheter system can implement treatment on thrombus and plaques before aspiration, can also implement aspiration, can also timely inject thrombolytic agents, and is simple in structure and convenient to operate.
Owner:HENAN YADU IND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![[(indole-3-yl)pyrimidine-2-yl]aminophenylpropyl-2-eneamide derivative and its salt, preparation method of derivative, and application of derivative and salt [(indole-3-yl)pyrimidine-2-yl]aminophenylpropyl-2-eneamide derivative and its salt, preparation method of derivative, and application of derivative and salt](https://images-eureka.patsnap.com/patent_img/eb66cca8-32dc-4f07-9f38-12677107a7ba/HDA0000790432580000011.PNG)
![[(indole-3-yl)pyrimidine-2-yl]aminophenylpropyl-2-eneamide derivative and its salt, preparation method of derivative, and application of derivative and salt [(indole-3-yl)pyrimidine-2-yl]aminophenylpropyl-2-eneamide derivative and its salt, preparation method of derivative, and application of derivative and salt](https://images-eureka.patsnap.com/patent_img/eb66cca8-32dc-4f07-9f38-12677107a7ba/HDA0000790432580000012.PNG)
![[(indole-3-yl)pyrimidine-2-yl]aminophenylpropyl-2-eneamide derivative and its salt, preparation method of derivative, and application of derivative and salt [(indole-3-yl)pyrimidine-2-yl]aminophenylpropyl-2-eneamide derivative and its salt, preparation method of derivative, and application of derivative and salt](https://images-eureka.patsnap.com/patent_img/eb66cca8-32dc-4f07-9f38-12677107a7ba/HDA0000790432580000021.PNG)