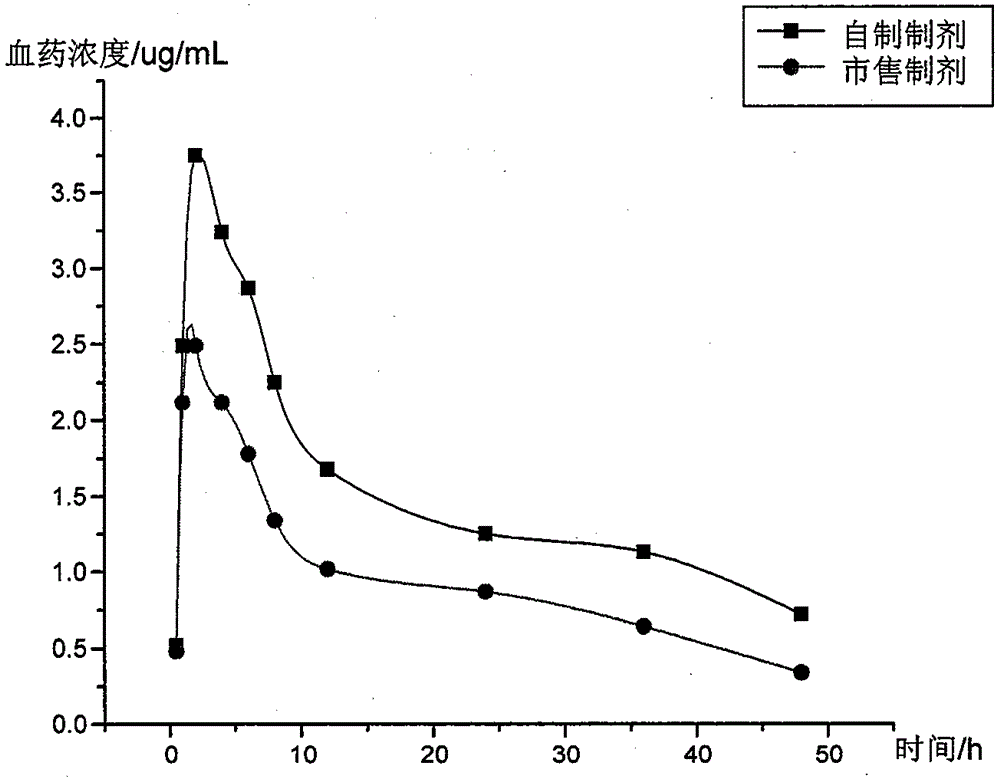
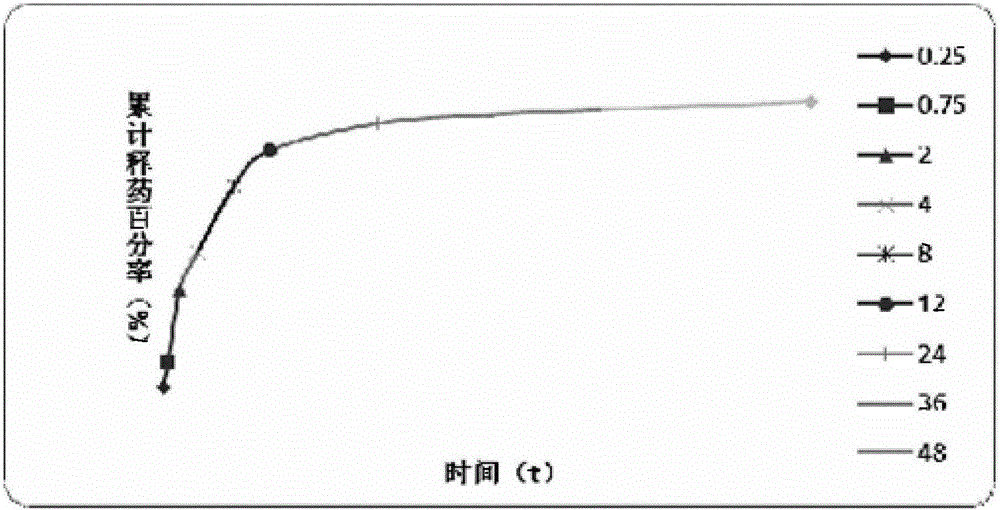
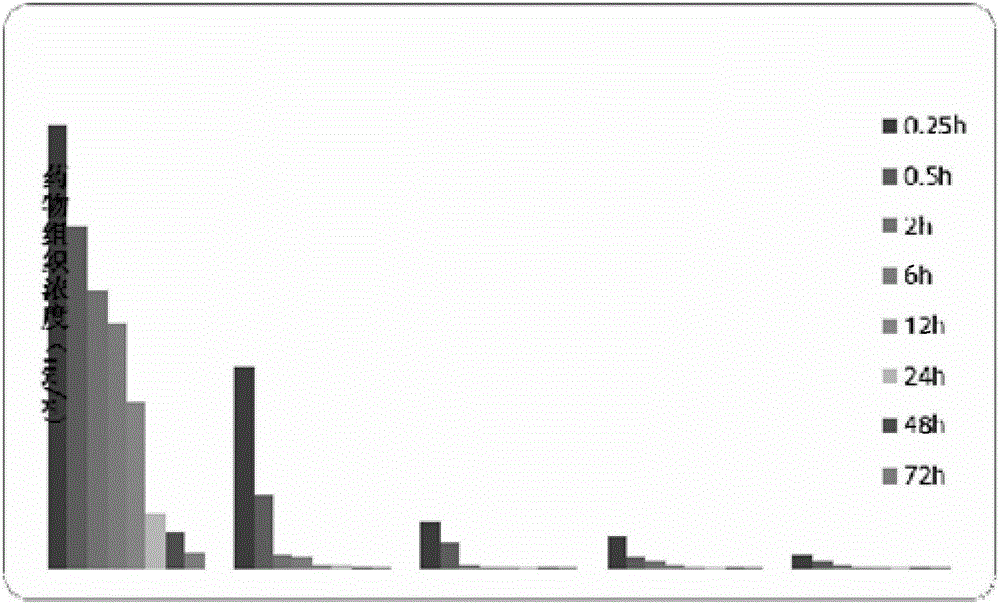
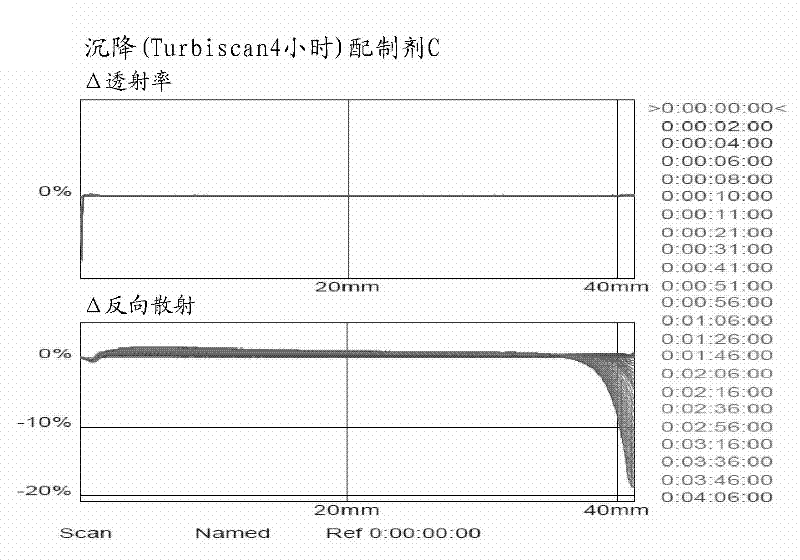
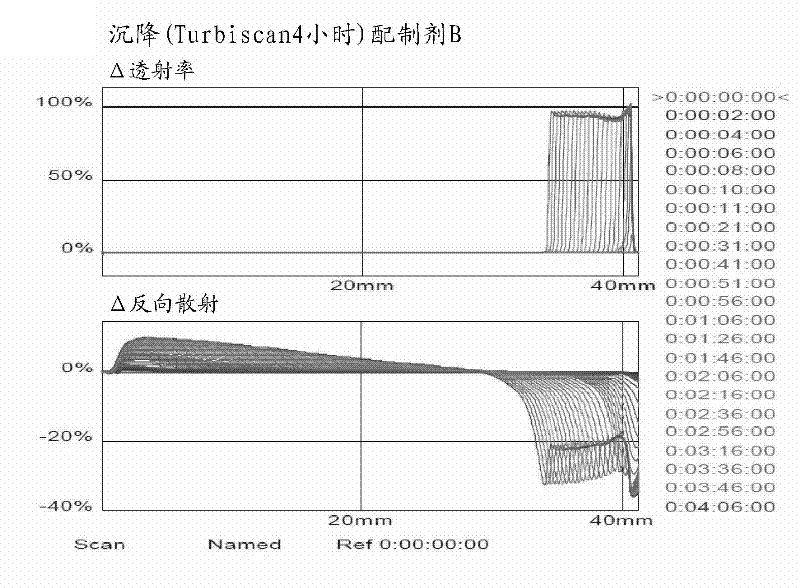
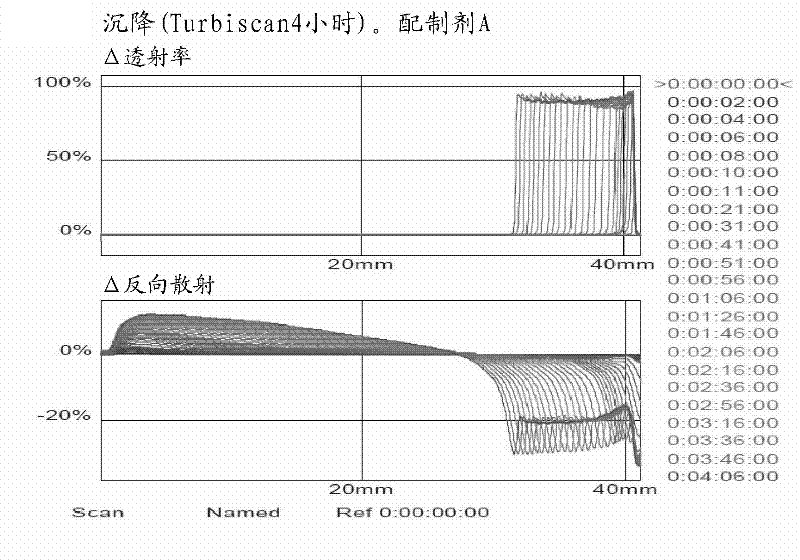
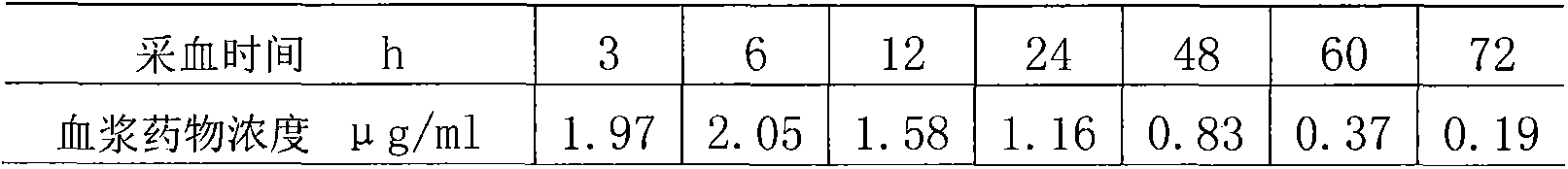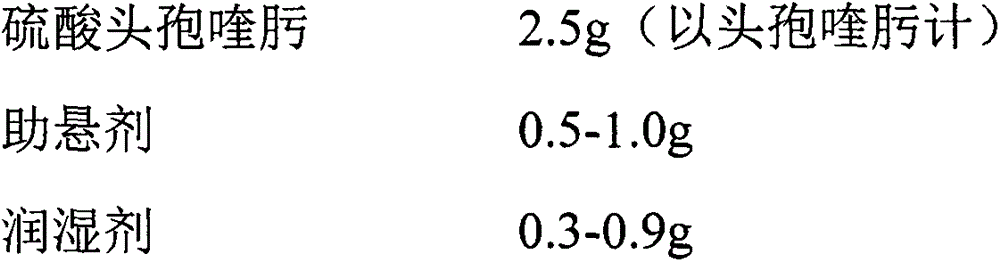Patents
Literature
75 results about "CEFQUINOME SULFATE" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
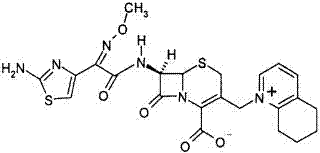
Cefquinome Sulfate is the sulfate form of cefquinome, a semisynthetic, broad-spectrum, fourth-generation aminothiazolyl cephalosporin with antibacterial activity. Cefquinome binds to and inactivates penicillin -binding proteins (PBPs) located on the inner membrane of the bacterial cell wall.
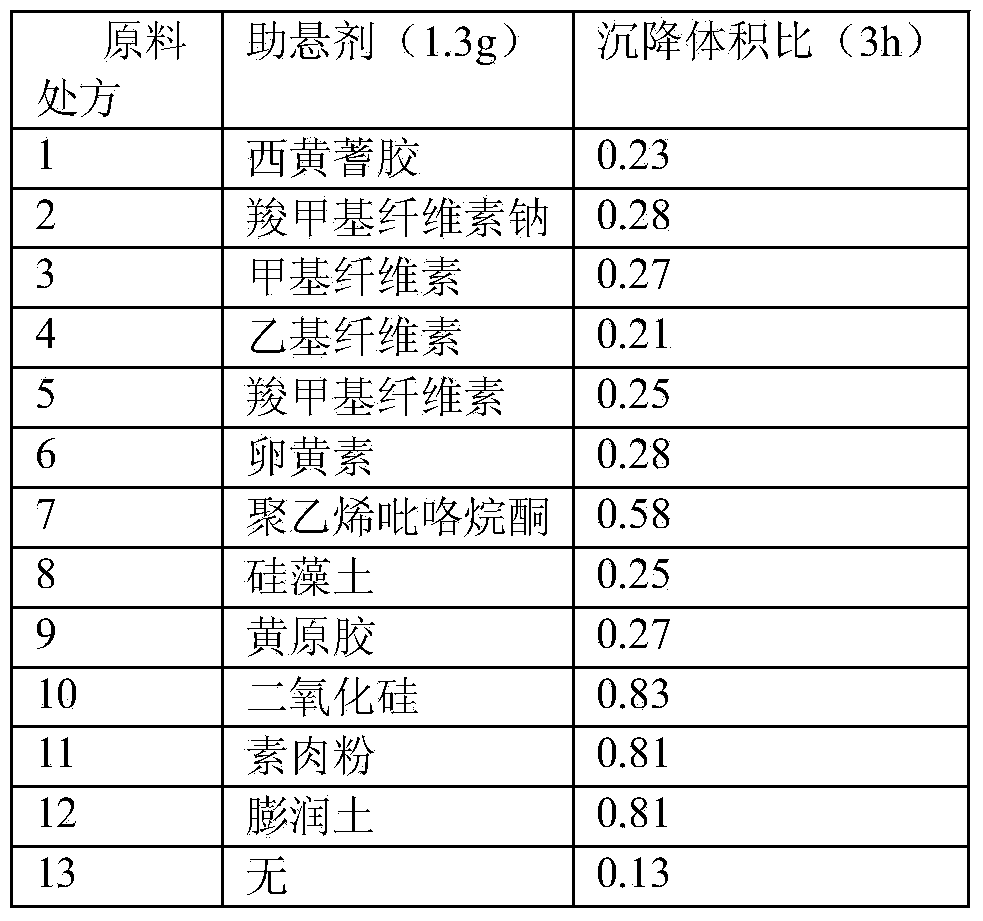
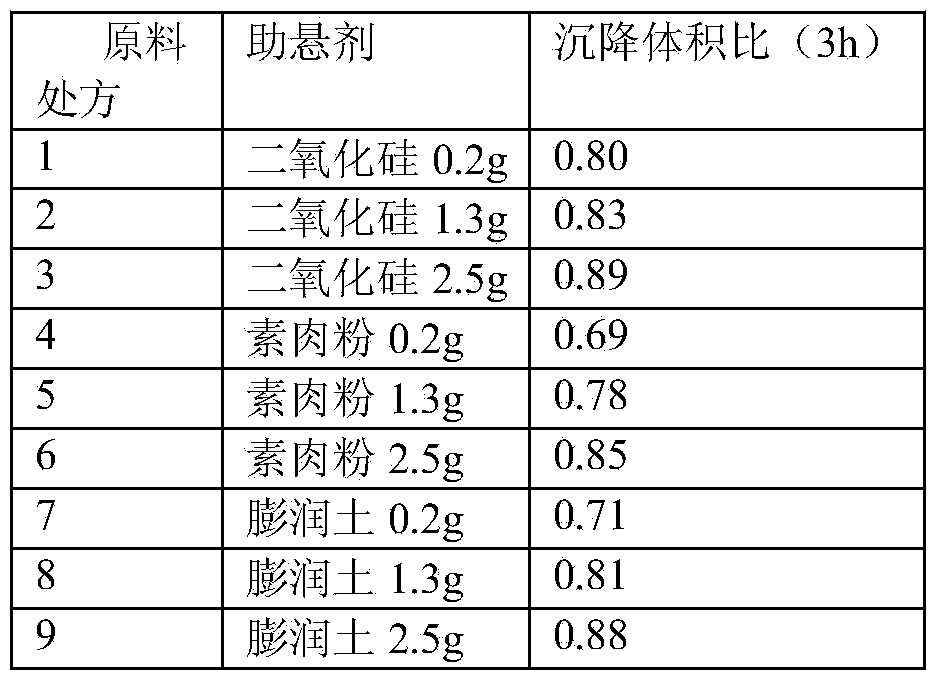
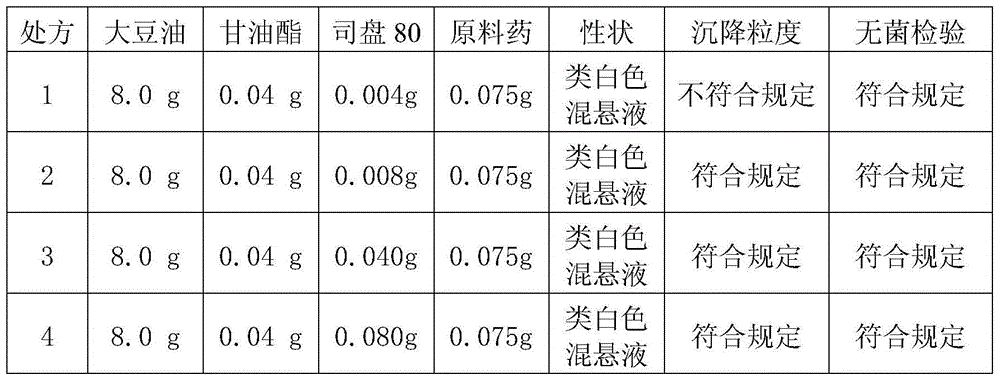
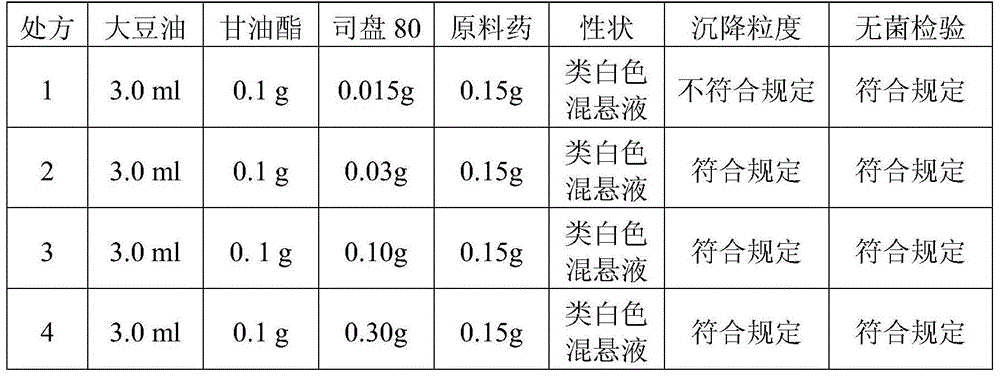
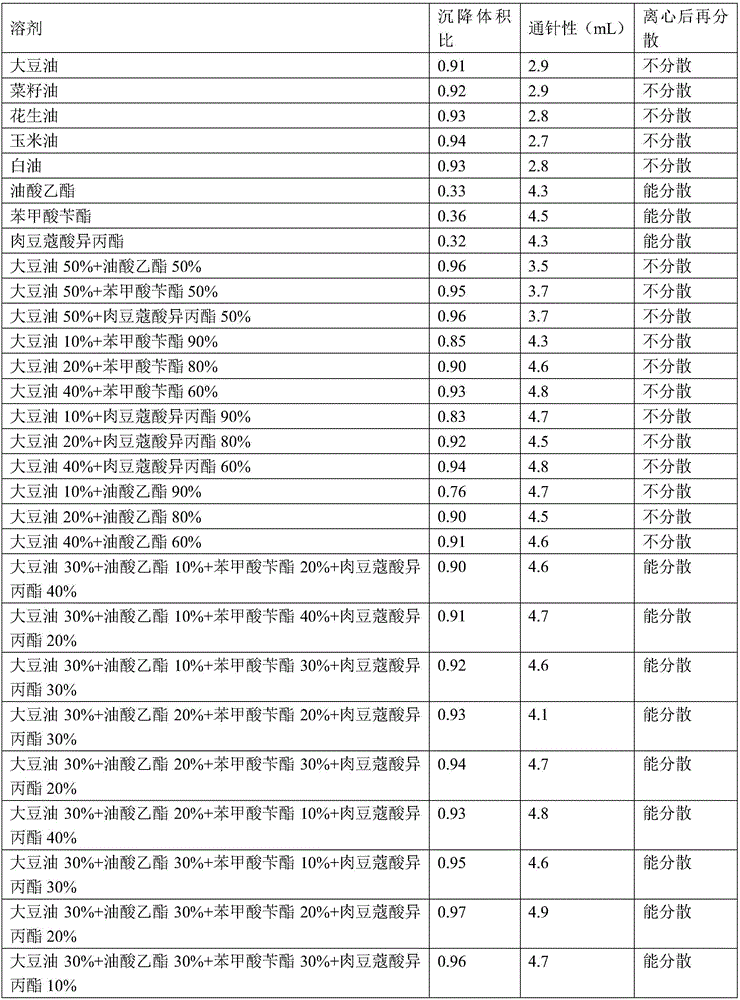
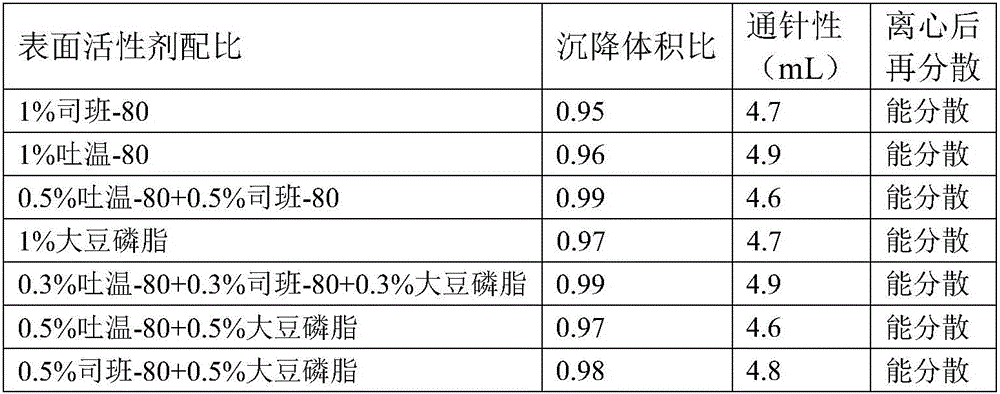
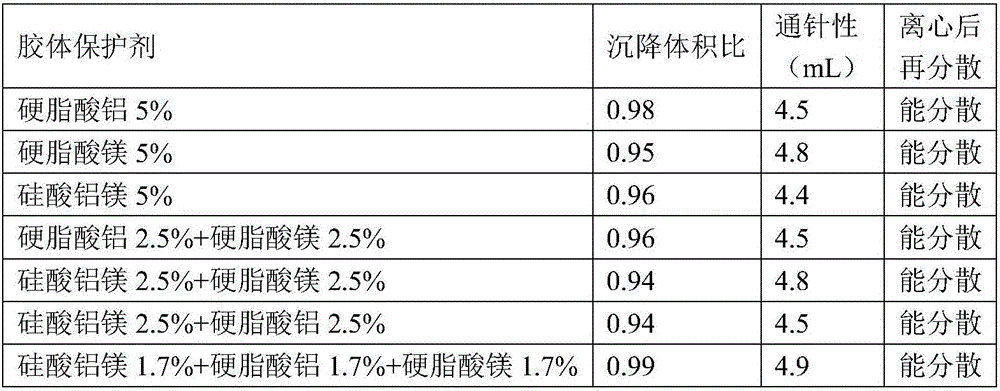
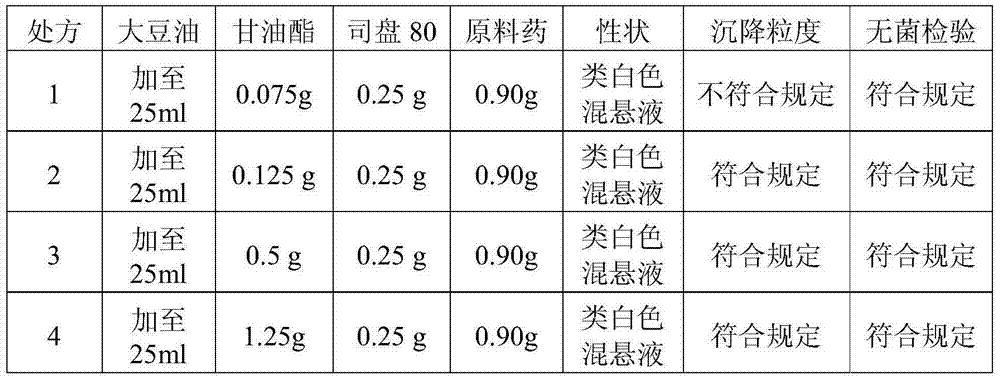
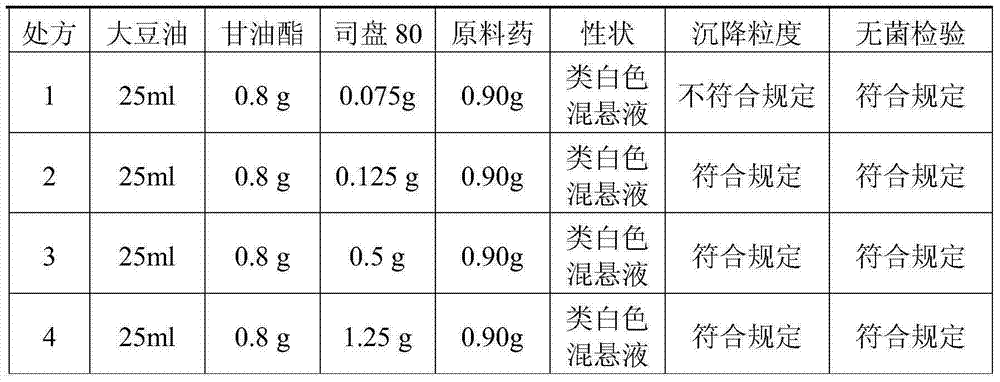
A cefquinome sulfate oily suspension injection and a preparing method thereof
ActiveCN104758245AGood suspensionNot prone to stratificationAntibacterial agentsOrganic active ingredientsBiotechnologyOil emulsion
The invention belongs to the technical field of medicines, and particularly relates to a cefquinome sulfate oily suspension injection and a preparing method thereof. 100 mL of the injection comprises following components: 0.1-10.0 g of cefquinome sulfate, 0.05-0.5 g of an antioxidant, 1.0-3.0 g of a bacteriostatic agent, 0.5-7.0 g of an emulsifying agent, 0.1-5.0 g of a suspending agent and 0.1-1.0 g of a thixotropic agent, with the balance being oil for injection. The preparing method includes following steps: dispersing the suspending agent into a part of the oil for injection, adding the antioxidant and the bacteriostatic agent in sequence, stirring, adding the emulsifying agent, and stirring to obtain a solution system; adding the cefquinome sulfate into a part of the oil for injection and grinding to obtain a solution system; mixing the two solution systems; adding the oil for injection until the total volume is 100 mL; and sterilizing. The injection has good compatibility with oil emulsion vaccines commonly used for livestock and poultry, and can be injected together, thus reducing working load. The injection is good in syringeability and obvious in slow release effects.
Owner:HENAN SOAR VETERINARY PHARMA
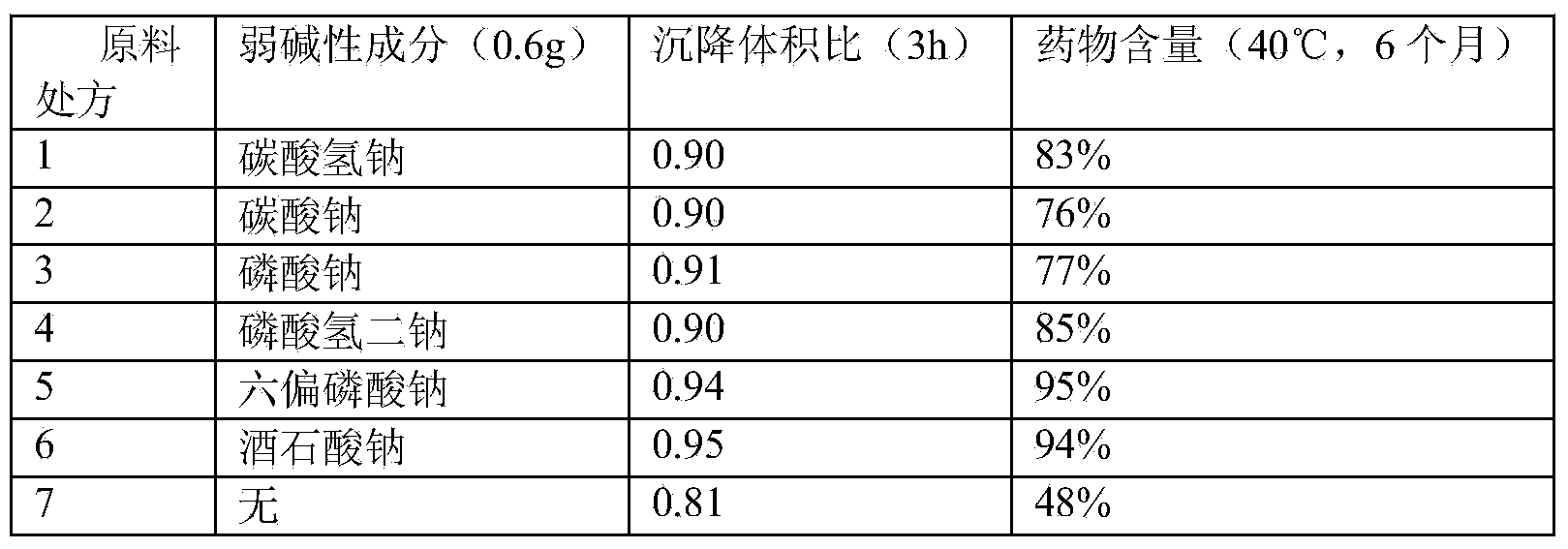
Stable cefquinome sulfate oil suspension injection
InactiveCN104127419AImprove the suspension effectImprove physical stabilityAntibacterial agentsOrganic active ingredientsAdditive ingredientAntioxidant
The invention provides a stable cefquinome sulfate oil suspension injection. The injection per 100mL comprises the following components: 1-5g of cefquinome sulfate, 0.2-2.5g of suspending agent, 0.2-1.0g of weakly alkaline ingredient, 1-6g of wetting agent, 0.05-0.2g of antioxidant, 0.01-1g of bacteriostatic agent, and oil or fat for injection added to 100mL. The oil suspension injection has the advantages of low viscosity, easily injectable property, good medicine suspension property and redispersibility, good chemical stability in long-term storage of the medicine, and the like.
Owner:CHINA PHARM UNIV
Perfusion medicament for treating milk cow mastitis and preparation method thereof
ActiveCN102488694ATreat both symptoms and root causesSolve the problem of easily inducing endotoxemia in dairy cowsAntibacterial agentsSexual disorderFLUNIXIN MEGLUMINEThird generation
The invention discloses a perfusion medicament for treating milk cow mastitis and a preparation method thereof. The perfusion medicament is prepared from the following raw materials by weight: 0.9-4.3g of cefquinome sulphate, 0.15-0.25g of prednisolone, 0.5-1.3g of flunixin meglumine, 1-1.5g of glycerin monostearate, 3-8g of Vaseline, 0.05g of ethylparaben and 65-70g of whiteruss. The preparationmethod comprises the following specific steps of: (1) putting one tenth of the liquid paraffin into a burdening tank in a certain weight ratio; (2) adding one third of the liquid paraffin into a heating tank; and (3) putting the rest of the liquid paraffin left after the steps (1) and (2) and auxiliary materials in the step (2) into an emulsifying tank, adding 0.9-4.3g of cefquinome sulfate in a certain weight ratio, and stirring till uniform distribution to obtain the perfusion medicament for treating milk cow mastitis. The perfusion medicament has the advantages of no induction of endotoxemia in a milk cow mastitis treating process, remarkable curative effect on mastitis and freeness from medicament tolerance.
Owner:QILU ANIMAL HEALTH PROD
Cefquinome sulfate injection and process for producing the same
InactiveCN101347407ABroad spectrum antibacterialHigh antibacterial activityAntibacterial agentsOrganic active ingredientsAntioxidantCefamandole
The invention relates to a preparation method of a medicine, in particular to a cefquinome sulfate injection and a preparation process thereof. The cefquinome sulfate injection consists of cefquinome sulfate micro-powder which contains not less than 50-300g of cefquinome, 0.4-40g of antioxidant and a fat or ester organic solvent which is added to be 10,000ml. The preparation process is as follows: the antioxidant is dissolved into the fat or ester organic solvent, the mixture is filtrated into a mixing tank, added with the cefquinome sulfate micro-powder, stirred evenly and milled through a colloid mill, thus even suspension is made, and then the suspension is stirred, separately filled and sterilized. The cefquinome sulfate injection and the preparation process solve the problem of no domestic fourth generation cefamandole antibiotic which is special for animals existing in the prior art. The product prepared has wider antimicrobial spectrum, stronger antibacterial activity and more stable Beta-lactamase for bacteria compared with the third generation cephalosporin, fills up the gap of no domestic fourth generation cefamandole antibiotic preparation which is special for animals, and the whole preparation process is simple and practicable.
Owner:河北远征药业有限公司
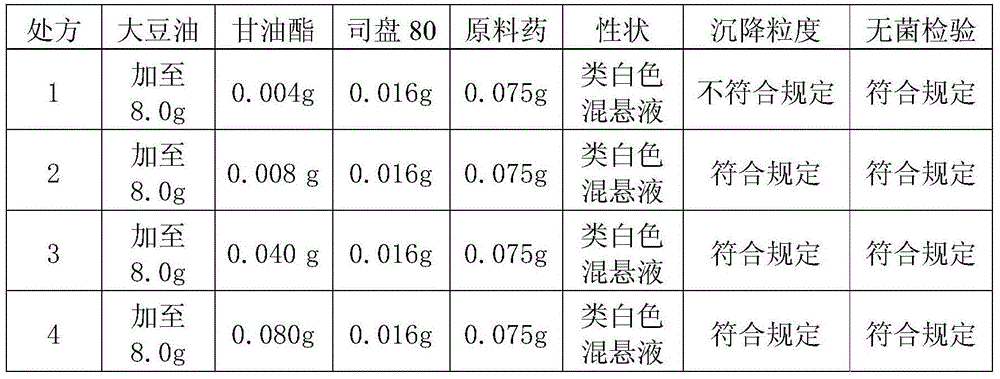
Cefquinome sulfate breast injection for dairy cow in lactation period and preparation method thereof
ActiveCN104546703AEasy to administerSave manpower and material resourcesAntibacterial agentsOrganic active ingredientsVegetable oilIrritation
The invention discloses a cefquinome sulfate breast injection for dairy cow in the lactation period and a preparation method thereof. The cefquinome sulfate breast injection is prepared from the following raw and auxiliary materials: 0.05-0.15g of cefquinome sulfate (based on cefquinome), 0.008-0.08g of glyceryl monostearate, 0.008-0.08g of a suspending agent and the balance of vegetable oil which is added until the total weight is 8.0g. The preparation method comprises the following steps: adding glyceryl monostearate into a proper amount of pre-treated vegetable oil, heating and melting; then, adding the suspending agent and cefquinome sulfate, and adding the vegetable oil to the total amount; and uniformly grinding. The breast injection has the advantages of low drug release speed, high bioavailability and small irritation to local tissues; each lactation region of each dairy cow is independently administrated, so that cross infection is avoided; the injection is injected through breast and is convenient to use, so that animal stress reaction caused by parenteral administration can be avoided, and convenience is brought to clinical administration; the injection has a remarkable curative effect and extremely high popularization value.
Owner:FEED RESEARCH INSTITUTE CHINESE ACADEMY OF AGRICULTURAL SCIENCES
Method for preparing Cefquinome sulfate of antibiotics of animal
InactiveCN101050220AGood treatment effectDrug resistance has no effectOrganic chemistryThiazoleOctene
This invention discloses a method for preparing cefquinome sulfate as an animal antibiotic. The method comprises: synthesizing 1-[[(6R,7R)-7-[[(2Z)-(2-amino-4-thiazole)(methoxyimino)acetyl]amino]-2-carboxyl-8-O-5-S-1-diazabicyclo[4.2.0]-2-octene-3-yl]methyl]-5,6,7,8-tetrahydroquinoline hydroiodate cefquinome dihydroiodate, and then preparing cefquinome sulfate.
Owner:蒋春茂
Breast injectant prescription of cefquinome sulfate in dry milk period and preparation method thereof
InactiveCN103893108AGood prevention effectInvulnerableAntibacterial agentsOrganic active ingredientsCEFQUINOME SULFATEBiochemistry
The invention relates to a breast injectant prescription of cefquinome sulfate in a dry milk period and a preparation method thereof. The prescription comprises a suspending aid and a solid dispersing carrier, and is prepared into an almost white and light brown ointment by virtue of a special process. The prescription disclosed by the invention has such advantages as simple preparation process, constant quality, easiness for injection and soft matrix. The prescription, which is in the dosage form of an ointment, has multiple advantages such as high bioavailability, slow release and long action time compared with an injection; the ointment, after being filled into a milk chamber through a milk vessel, can be sealed on a nipple hole, so as to prevent bacteria from moving reversely and invading the milk chamber through the nipple hole and other phenomenons.
Owner:RINGPU TIANJIN BIOLOGICAL PHARMA
Cefquinome sulfate suspension injection and preparation method thereof
InactiveCN104473868AImprove stabilityHigh viscosityAntibacterial agentsOrganic active ingredientsMedicineAntioxidant
The invention relates to a cefquinome sulfate suspension injection. The cefquinome sulfate suspension injection is characterized by comprising the following components in mass-volume ratio: 2.5% of cefquinome sulfate, 1%-3% of a suspending agent, 0.1%-0.3% of an antioxidant, and balance of a solvent for injection, totaling 100%. The invention also provides a preparation method of the cefquinome sulfate suspension injection. Since a surfactant is used as the suspending agent, the stability of the suspension can be improved; the stability of the medicine is increased; the irritation of the medicine is reduced; and the cefquinome sulfate suspension injection is simple in preparation process, is prepared from cheap and easily available raw materials, and is suitable for industrial production, so that the cefquinome sulfate suspension injection has a wide application prospect.
Owner:SHANGHAI TONGREN PHARM CO LTD
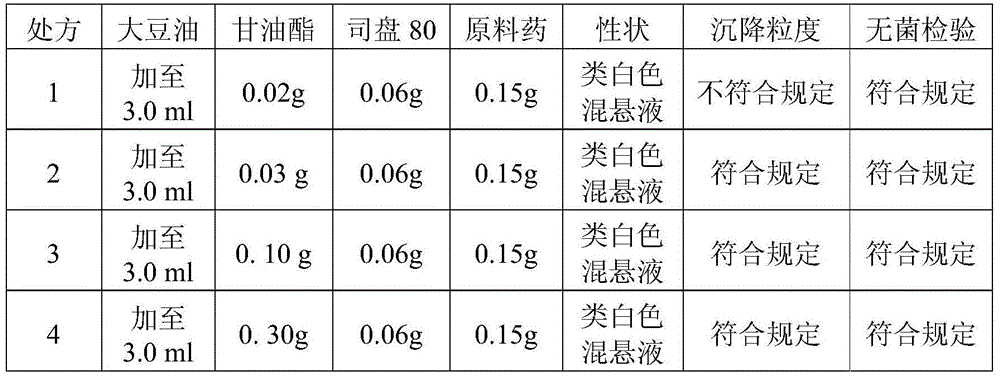
Cefquinome-sulfate breast injection agent for dry period of dairy cows and preparation method thereof
ActiveCN104873462AFast and convenient administrationSave manpower and material resourcesOrganic active ingredientsSolution deliveryVegetable oilMedicine
The invention discloses a cefquinome-sulfate breast injection agent for the dry period of dairy cows and a preparation method thereof. The cefquinome-sulfate breast injection agent is prepared from the following raw auxiliary materials by weight: 0.05 to 0.3g of cefquinome sulfate (calculated by cefquinome), 0.03 to 0.3g of glyceryl monostearate, 0.03 to 0.3g of suspending agent and vegetable oil added to 3.0ml. The preparation process comprises the following steps : taking proper amount of vegetable oil pretreated, adding glyceryl monostearate, heating for melting, then adding the suspending agent and the cefquinome sulfate, then adding the vegetable oil to the total amount, grinding to be uniform and obtaining the cefquinome-sulfate breast injection agent. The cefquinome-sulfate breast injection agent disclosed by the invention has the following advantages that the drug release is slow, the bioavailability is high and the stimulation to local tissues is small; single medication is adopted for each breast area for each cow, so that cross infection is avoided; by breast injection, the use is convenient, the animal stress response caused by injection administration is avoided, and convenience is provided for clinical medication; and the curative effect is obvious and the promotion value is extremely high.
Owner:FEED RESEARCH INSTITUTE CHINESE ACADEMY OF AGRICULTURAL SCIENCES
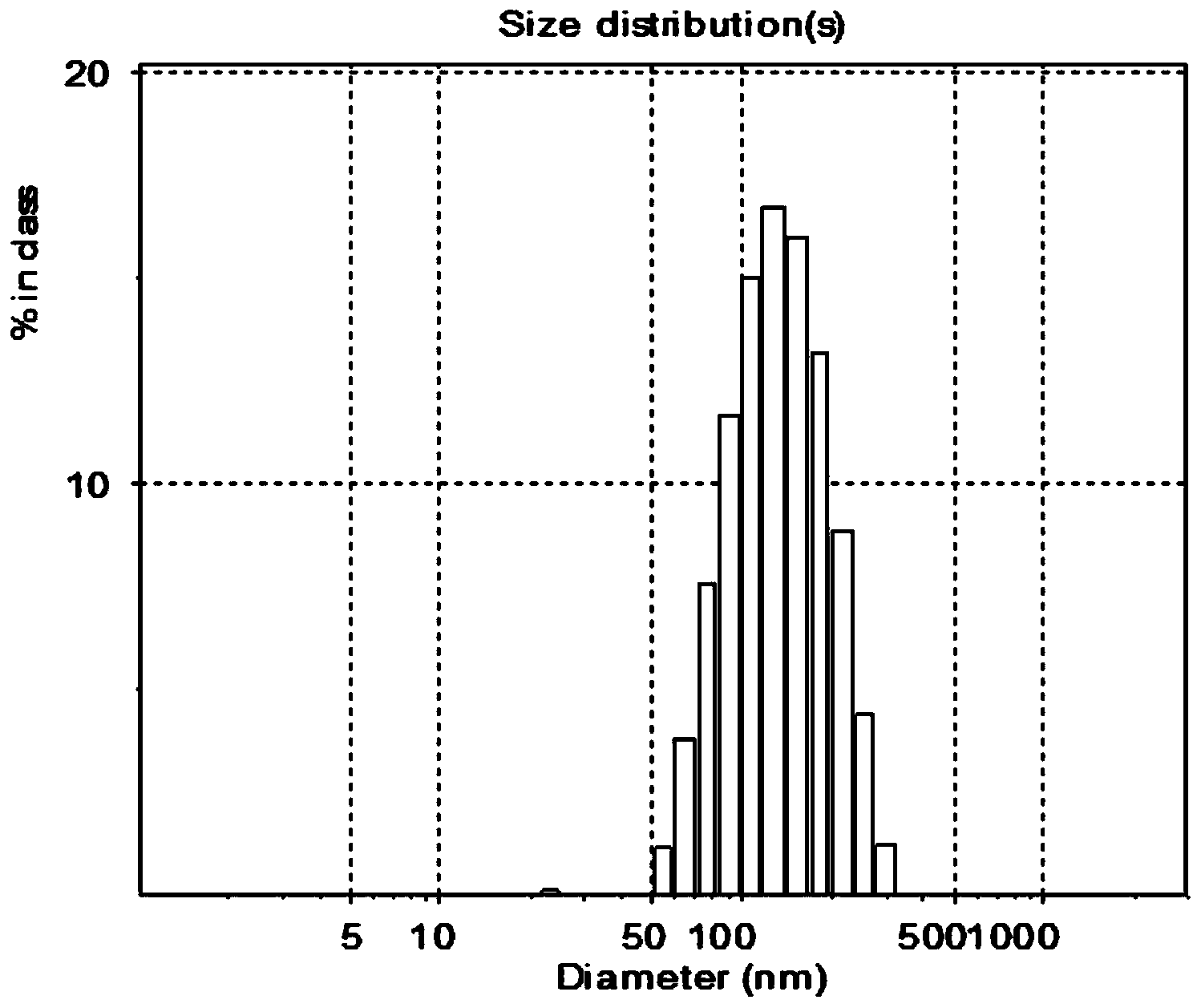
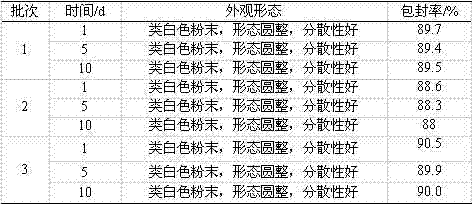
Monodisperse nano cefquinome sulfate liposome preparation and preparation method thereof
InactiveCN103637993AUniform sizeHigh encapsulation efficiencyAntibacterial agentsOrganic active ingredientsMembrane emulsificationCholesterol
The invention relates to a monodisperse nano cefquinome sulfate liposome preparation and a preparation method thereof. The monodisperse nano cefquinome sulfate liposome preparation is prepared by adopting a membrane emulsification technique. The preparation method comprises the following steps: directly suspending sterile cefquinome sulfate into injection water, adding sterile L-lysine to adjust the PH value, and completely dissolving the sterile cefquinome sulfate to obtain the aqueous solution of cefquinome sulfate / L-lysine, wherein the the aqueous solution of cefquinome sulfate / L-lysine is a continuous phase; dissolving an anti-oxidant-containing injection phospholipid and cholesterol in ethyl alcohol, wherein the obtained solution is a dispersed phase; dispersing the dispersed phase in the continuous phase through a porous membrane under the action of a pressure; removing the ethyl alcohol under reduced pressure; sterilizing, packaging, and freeze-drying to finally obtain the monodisperse nano cefquinome sulfate liposome preparation for injection. The preparation method provided by the invention is simple; the prepared particles are uniform in size, high in encapsulation rate and high in stability; in an emulsification process, the energy consumption is low, the conditions are mild, and the reproducibility is high; the stability of a cefquinome sulfate medicament is stable, toxic or side effects are reduced, and the medicament utilization degree is improved; moreover, the preparation process is simple and is suitable for industrial production.
Owner:湖北领盛制药有限公司
Injection for treating cow escherichia coli mastitis and pig bacterial respiratory tract infection and preparation thereof
InactiveCN101214222AResidue reductionLow toxicityAntibacterial agentsOrganic active ingredientsEscherichia coliMastitis
The present invention provides an injection for remedying dairy cattle coli mastitis and porcine bacterial respiratory tract infection, which contains 10g to 70g of cefquinome sulfate which is mixed with 500g to 1000g of soybean oil for injection. The present invention also provides a preparation method of the injection for remedying the dairy cattle coli mastitis and the porcine bacterial respiratory tract infection, which comprises the following steps that firstly, 500g to 1000g of the soybean oil for injection is filtered and then is led into filtered nitrogen, and finally the soybean oil for injection is added into a germfree mixing bottle which is equipped with a homogenizer and is filled with the nitrogen; secondly, 10g to 70g of the cefquinome sulfate is weighed up to be added into the germfree mixing bottle to be mixed with the soybean oil for injection and then is stirred into uniform suspension, and the injection is obtained; thirdly, the suspension is poured into an ampoule which is led with the nitrogen for sealing.
Owner:陆广富 +1
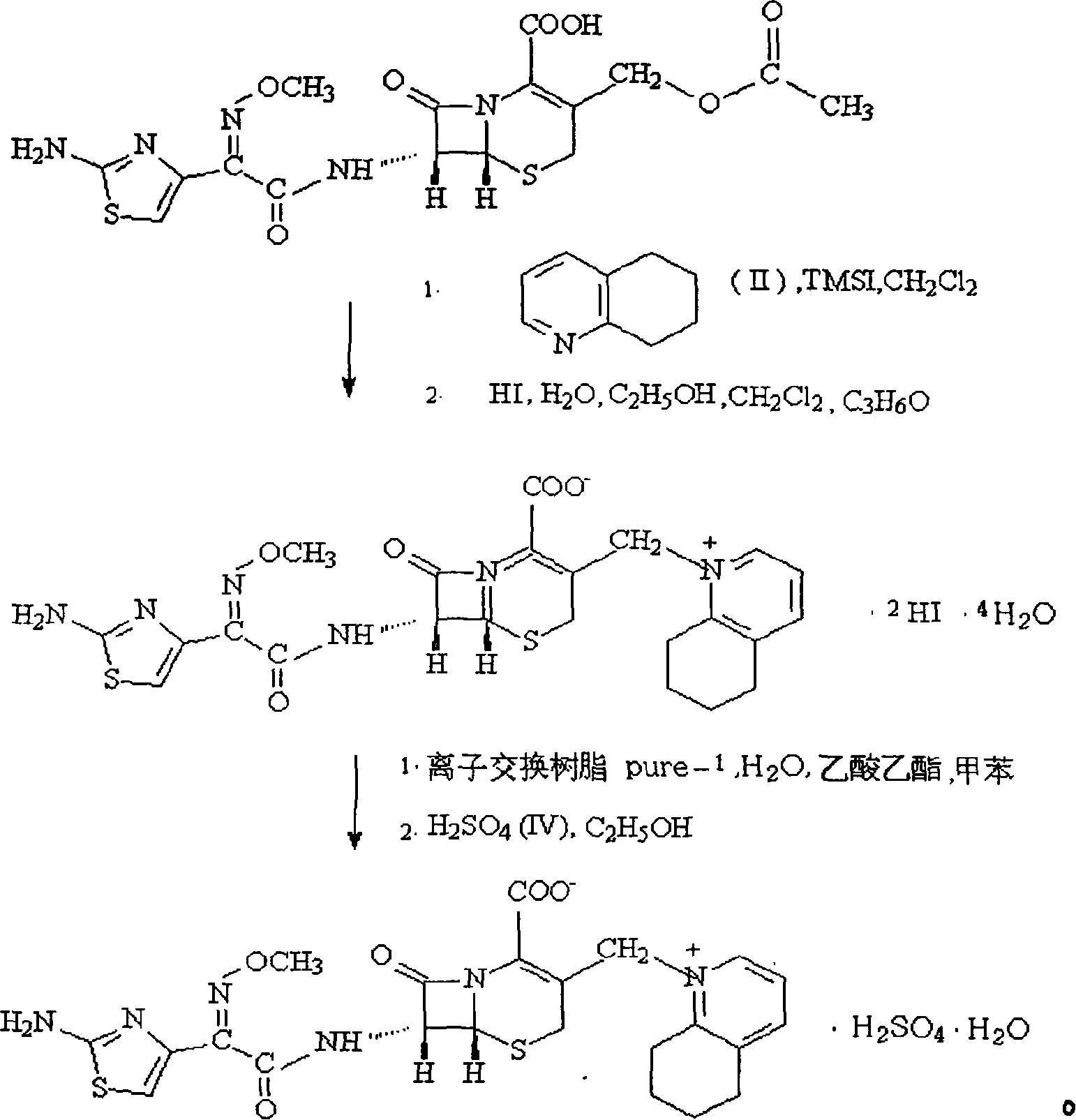
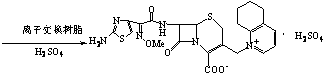
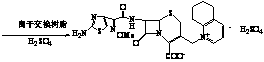
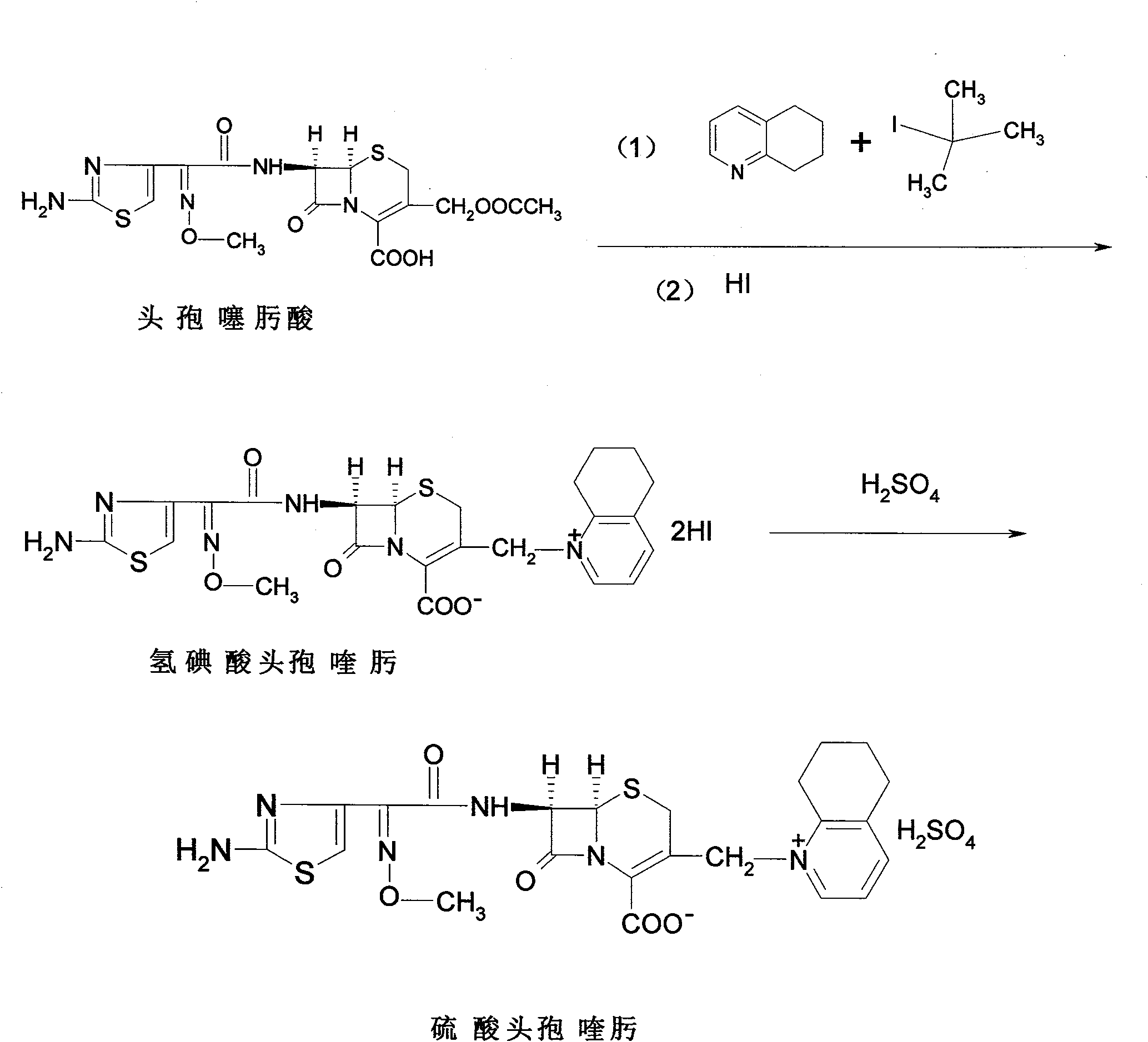
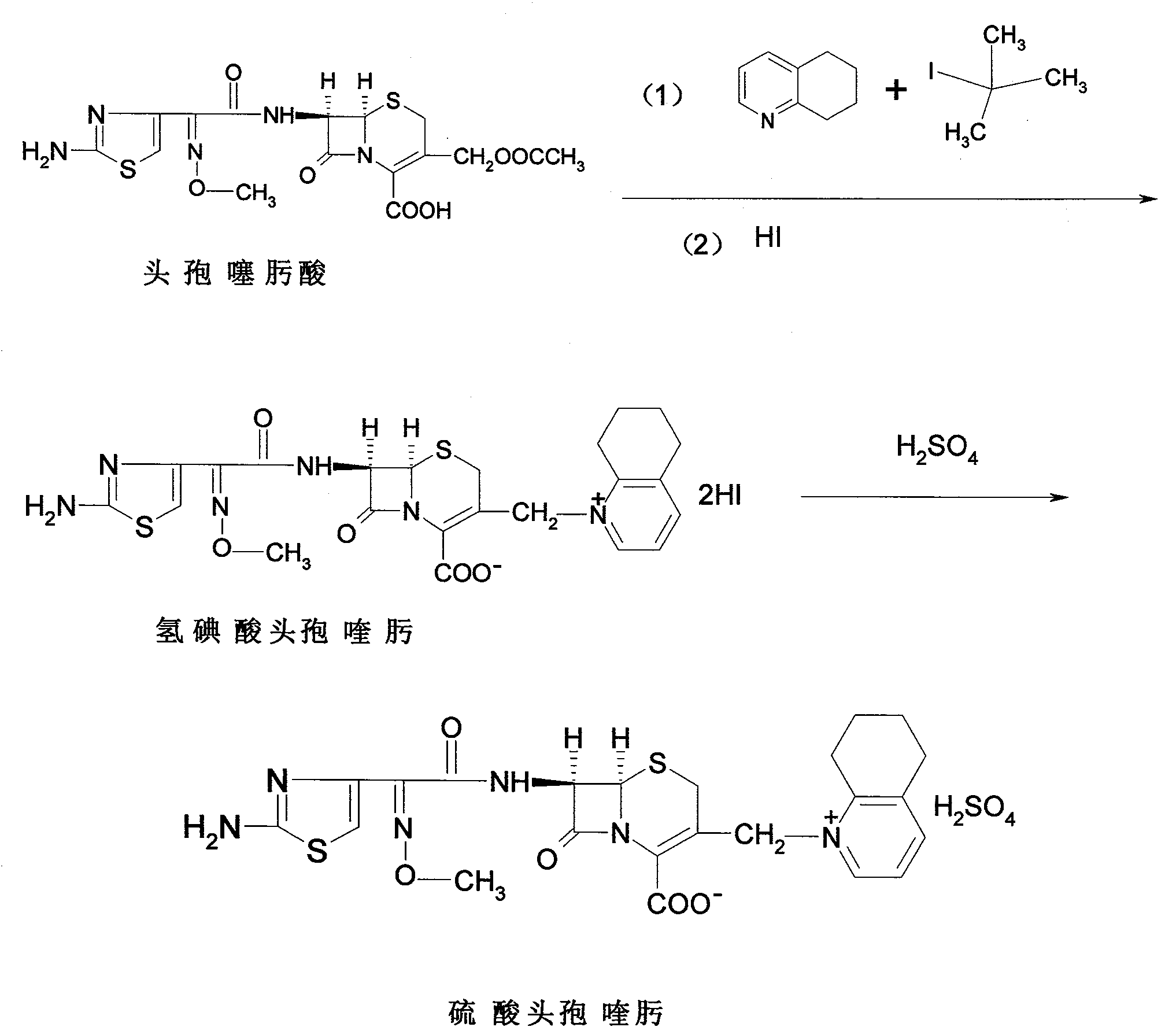
Process for preparing cefquinome sulfate
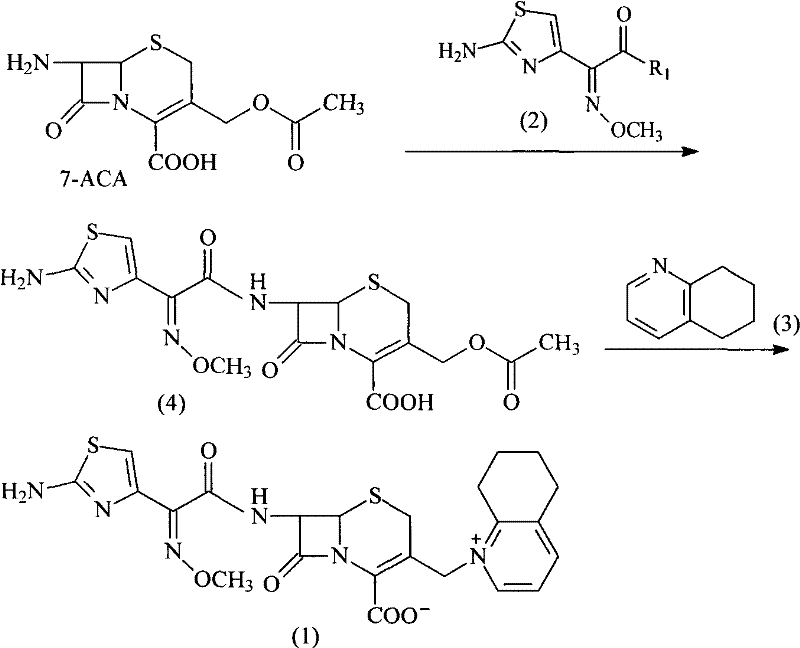
InactiveCN101307064AGood treatment effectLow toxicityAntibacterial agentsOrganic chemistryCefotaxime7-ACA
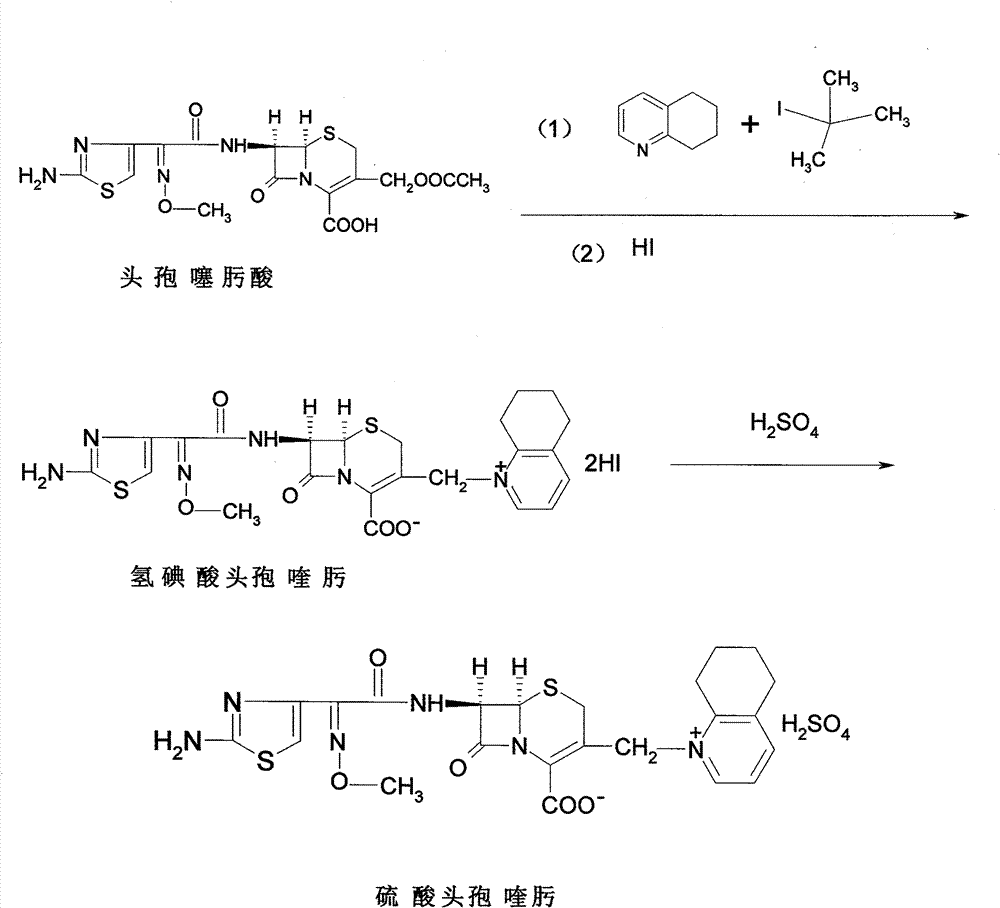
The invention discloses a method for preparing cefquinome sulfate, belonging to the cephalosporin antibiotics special for animals. The method comprises the following: A. a step of preparing 7-amino-cefquinome, during which, iodotrimethylsilane reacts with 5, 6, 7, 8-tetrahydroquinoline, and a reaction product reacts with 7-amino-cephalosporanic acid, thereby obtaining the 7-amino-cefquinome; B. a step of preparing the cefquinome sulfate, during which, the 7-amino-cefquinome reacts with AE active ester after the 7-amino-cefquinome is dissolved and is decolored by sulphuric acid, and the cefquinome sulfate is obtained after the reaction product is subject to extraction, suction filtering, leaching and drying. The method uses 7-ACA which is widely used in industry, is low in cost and is easily obtained as a raw material, without using cefotaxime acid with unstable service quality; meanwhile, the method has a mild reaction condition and simple operation, reduces the dosage of the 5, 6, 7, 8-tetrahydroquinoline which is an important intermediate, and greatly improves the product quality with the yield of 64.1 percent and the purity of 99.7 percent, thereby the method is suitable for industrial production.
Owner:崔增学
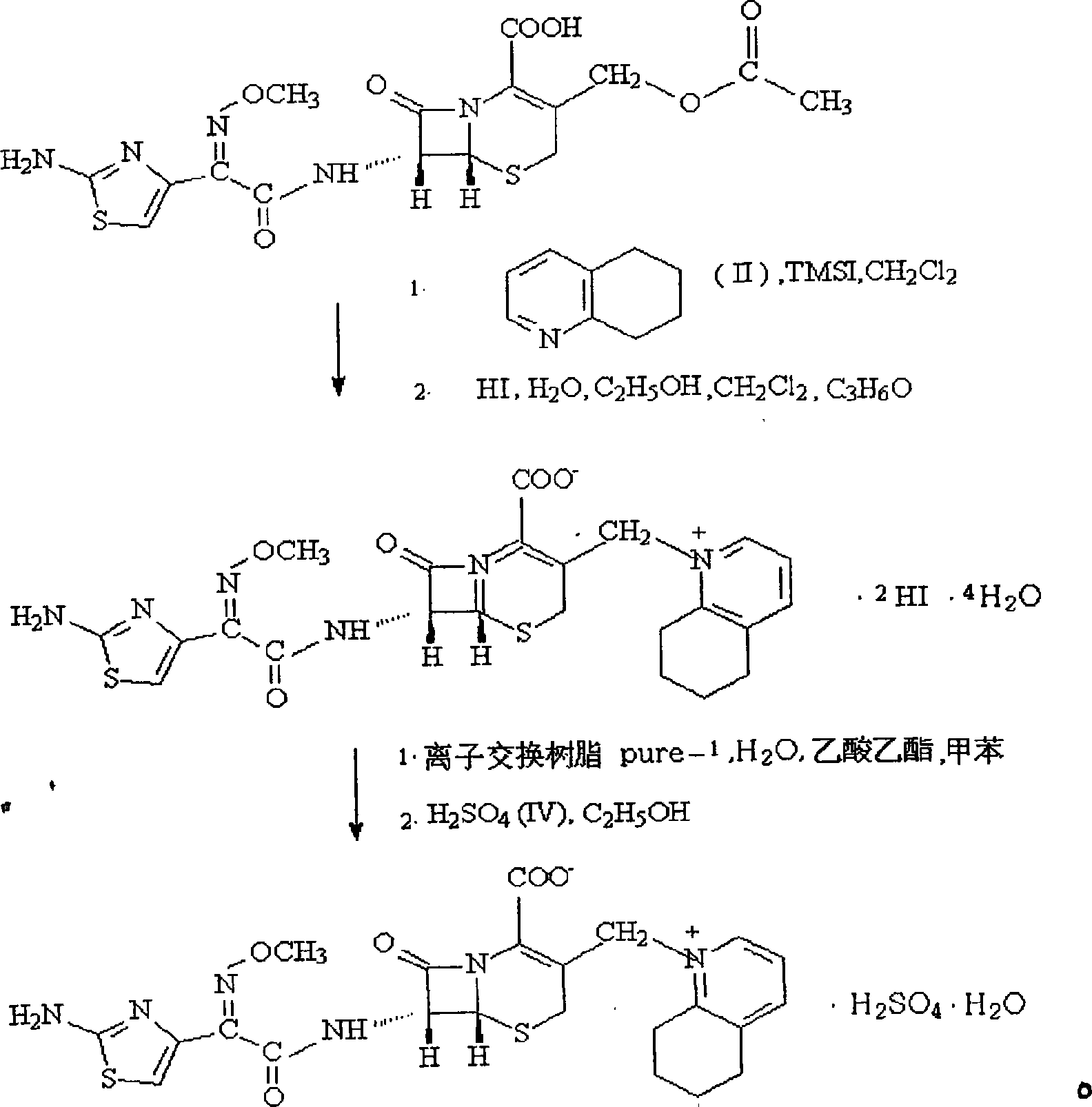
Method for preparing cefquinome sulfate
InactiveCN103275103AReduce manufacturing costMild reaction conditionsOrganic chemistryCefotaximePhosphoric acid
The invention discloses a method for preparing cefquinome sulfate. The method comprises the steps as follows: 7-aminocephalosporanic acid is taken as a raw material; a C-3 bite ester group is hydrolyzed under the action of alkali and reacts with 2-(2-Amino-4-thiazolyl)-(z)-methoxyiminoacetic, thiobenzothiazole ester, so that an intermediate A is obtained; an iodo substance 3-iodine methyl cefotaxime is obtained through the intermediate and potassium iodide under the action of phosphoric acid; the 3-iodine methyl cefotaxime reacts with 5, 6, 7, 8-tetrahydroquinoline, so that an intermediate B of cefquinome hydriodate is obtained; and finally, the intermediate B of cefquinome hydriodate reacts with sulfuric acid under the action of an alkaline anion exchange resin, so that the cefquinome sulfate is obtained. According to the method for preparing the cefquinome sulfate, the 7-aminocephalosporanic acid which is cheap and easy to obtain is taken as the raw material, so that the use of iodotrimethylsilane which is expensive and prone to decompose when contacted with light and water is avoided, and the production cost is reduced; and the reaction condition is mild, the operation is simple, the yield is high, and the cefquinome sulfate is suitable for industrial production.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Cefquinome sulfate injection and low-temperature high-shear preparation method thereof
ActiveCN107334730AEasy preparation stepsEasy to operateAntibacterial agentsOrganic active ingredientsALUMINUM STEARATESLiquid temperature
The invention discloses a cefquinome sulfate injection and a low-temperature high-shear preparation method thereof, wherein the cefquinome sulfate injection comprises, by mass, 2.25-2.75% of cefquinome sulphate, 0.9-1.1% of lecithin, 1.8-2.2% of aluminum stearate, and the balance of ethyl oleate. The preparation method comprises: carrying out low-temperature high shear on cefquinome sulfate to obtain an ultra-fine suspension, and homogenizing by using a colloid mill to finally prepare the cefquinome sulfate injection. Compared to the preparation method in the prior art, the preparation method of the present invention has the following advantages that the drug liquid temperature is controlled at 2-8 DEG C during the shear, the finally obtained product is detected according to the quality standard, various detection indexes meet the regulations, the sum of various impurity peak area in the related substance detection is 3 times (3.0%) less than the main peak area of the control solution, the yield of the product is high, and the preparation steps are simple and are easy to operate.
Owner:GUANGDONG WENS DAHUANONG BIOTECH
Preparation method of cefquinome sulfate
The invention relates to a method for synthesizing cefquinome sulfate and relates to a preparation method of a medicament for animals. The preparation method comprises the following steps: taking cefotaxime, 2,3-cyclohexyl pyridine and trimethyliodosilane as initial raw materials; preparing cefotaxime hydriodate from the cefotaxime; carrying out decoloring and silica column chromatography on the cefotaxime; and reacting the treated cefotaxime with sulphuric acid to prepare a cefquinome sulfate product. Compared with the prior art, the invention has the advantages of high yield of products, stable production quality and suitability for industrial production.
Owner:PU LIKE BIO ENG
Cefquinome sulfate injection and preparation process
InactiveCN106491532ANot easy to layerNot easy to settleAntibacterial agentsOrganic active ingredientsALUMINUM STEARATESAntioxidant
The invention belongs to the field of the animal medicine, and particularly relates to cefquinome sulfate injection and a preparation process. The formula comprises the following components by weight: 2.5 parts of cefquinome sulfate, 1.8-2.2 parts of aluminum stearate, 0.8-1.2 parts of lecithin, 1.8-2.2 parts of span E, 0.08-0.12 parts of phenol, 0.08-0.12 parts of vitamin E, and 100 parts of ethyl oleate. After the cefquinome sulfate is superfine-grinded, the prepared suspension preparation is not easy to layering and settling, the sedimentation rate is reduced above 50%. The preparation is easy to shake uniformly in use, the needle cleaning property is better, the injection is easy, and the stress after the injection is smaller. A specific pure natural antioxidant is added, so the stability is better, and the content descent rate is reduced by 18%.
Owner:SICHUAN MEIJIALONG BIOTECH
Cefquinome sulfate oil suspension and preparation method thereof
ActiveCN106344509AImprove qualityReduce volumeAntibacterial agentsOrganic active ingredientsDispersityDrug content
The invention discloses a cefquinome sulfate oil suspension. Every 100mL of the suspension contains the following raw and auxiliary materials: 2-15g of cefquinome sulfate, 0.9-1.0g of surfactant, 5.0-5.1g of colloid protective agent, 2.0-8.0g of suspending agent, 0-0.1g of antioxidant and the balance of oil phase. The high-quality cefquinome sulfate oil suspension with the main drug content of 2-15% is obtained by screening the formula. The used auxiliary materials lower the drug sedimentation volume and sedimentation rate. The product has the advantages of low flowability, low sedimentation rate, high sedimentation volume, high needle penetration property and favorable dispersity after centrifugation. In addition, the inspection on the stability under different temperature conditions further proves the high quality of the suspension.
Owner:CHENGDU QIANKUN VETERINARY PHARMA
Oil injection containing antibacterial agents/polyethylene glycol drug-loading particles
InactiveCN103721263APromote absorptionRetain water swellingAntibacterial agentsPharmaceutical delivery mechanismCelluloseDoxycycline hydrochloride
The invention discloses an oil injection containing antibacterial agents / solid polyethylene glycol drug-loading particles. The oil injection is prepared by consisting of the drug-loading particles through antibacterial agents and solid polyethylene glycol and suspending the drug-loading particles in an oil medium. The antibacterial agents comprise enrofloxacin, danofloxacin mesylate, Marbofloxacin, mequindox, tilmicosin, tylosin, oxytetracycline hydrochloride, ceftiofur hydrochloride, cefquinome sulfate, lincomycin hydrochloride, florfenicol, erythrocin and doxycycline hydrochloride; preferentially the polyethylene glycol with the molecular weight of more than 6000 is used for preparing the preparation; more preferentially, any one of isopropyl myristate, soybean oil for injection, corn oil and tea-seed oil is used for preparing the preparation. Hydroxypropyl methyl cellulose or high-substituted hydroxy propyl cellulose can be added into the preparation.
Owner:王玉万
Cefquinome sulfate muscle injection and preparation method thereof
InactiveCN105640881AStable physical propertiesFine granularityAntibacterial agentsOrganic active ingredientsIntramuscular injectionAntioxidant
The invention discloses a cefquinome sulfate muscle injection and a preparation method of the cefquinome sulfate muscle injection. The cefquinome sulfate muscle injection is prepared from cefquinome sulfate, a suspending agent, a wetting agent, an antioxidant and an oily solvent. The preparation method is characterized by preparing a suspension of which the color is similar to white and is sandy beige through grinding of a high-speed shear dispersion instrument, and sterilizing the cefquinome sulfate muscle injection by adopting an irradiation sterilization technology. The cefquinome sulfate muscle injection prepared by the invention has the advantages of good physical and chemical stability, fine granularity, slow sedimentation, good redispersibility, good needling performance, stable content, simplicity and feasibility in preparation technology and capability of being beneficial for industrial large-scale production; after the cefquinome sulfate muscle injection is injected intramuscularly, the release is slow, the elimination half life is long, the effect is durable, and the bioavailability is high.
Owner:CHINA PHARM UNIV
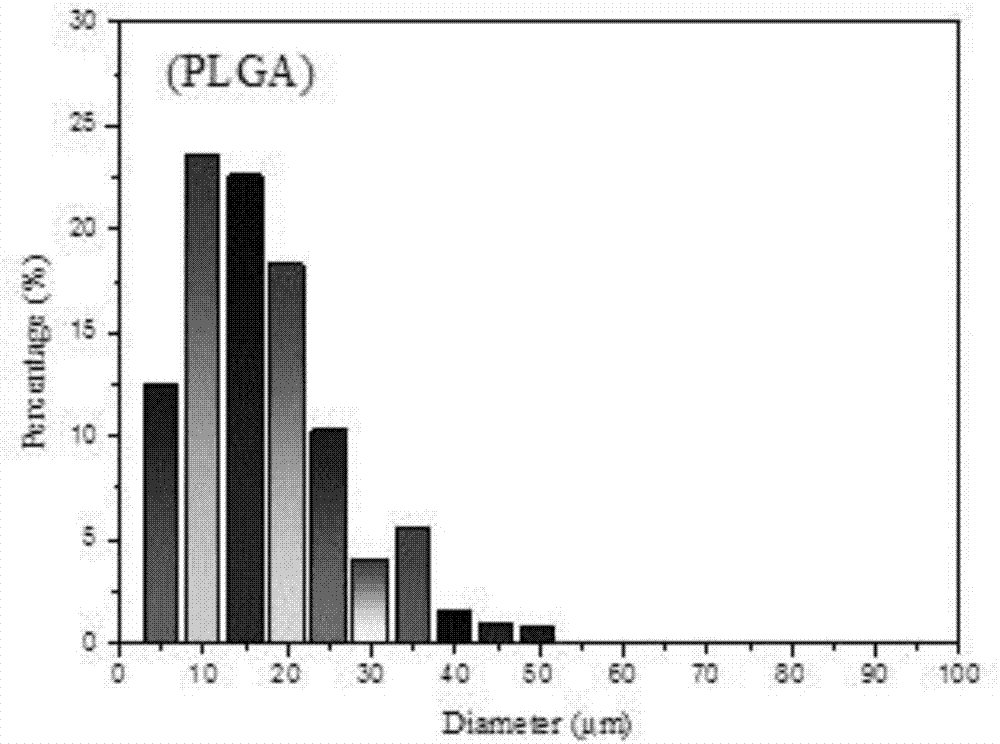
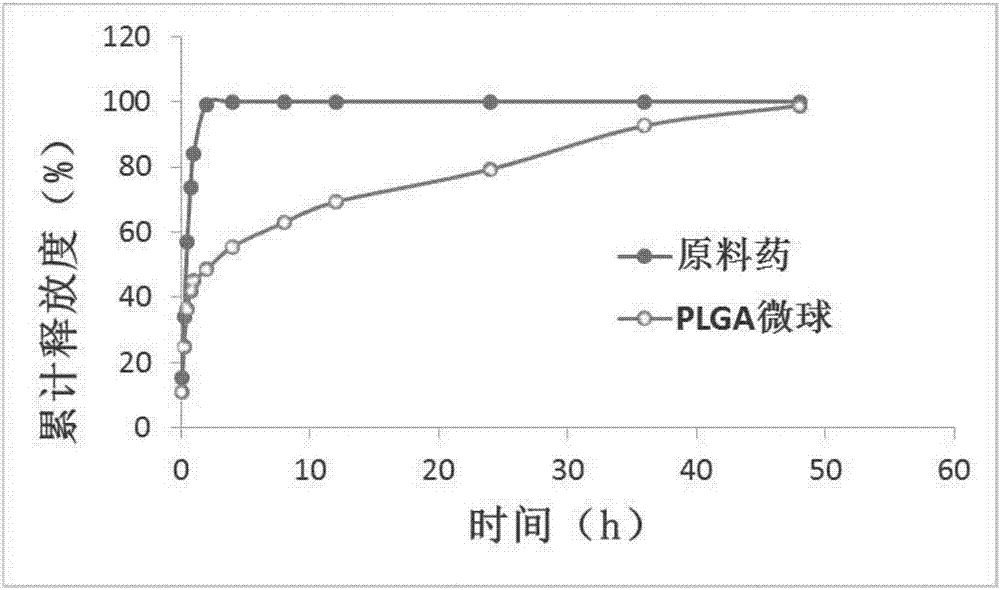
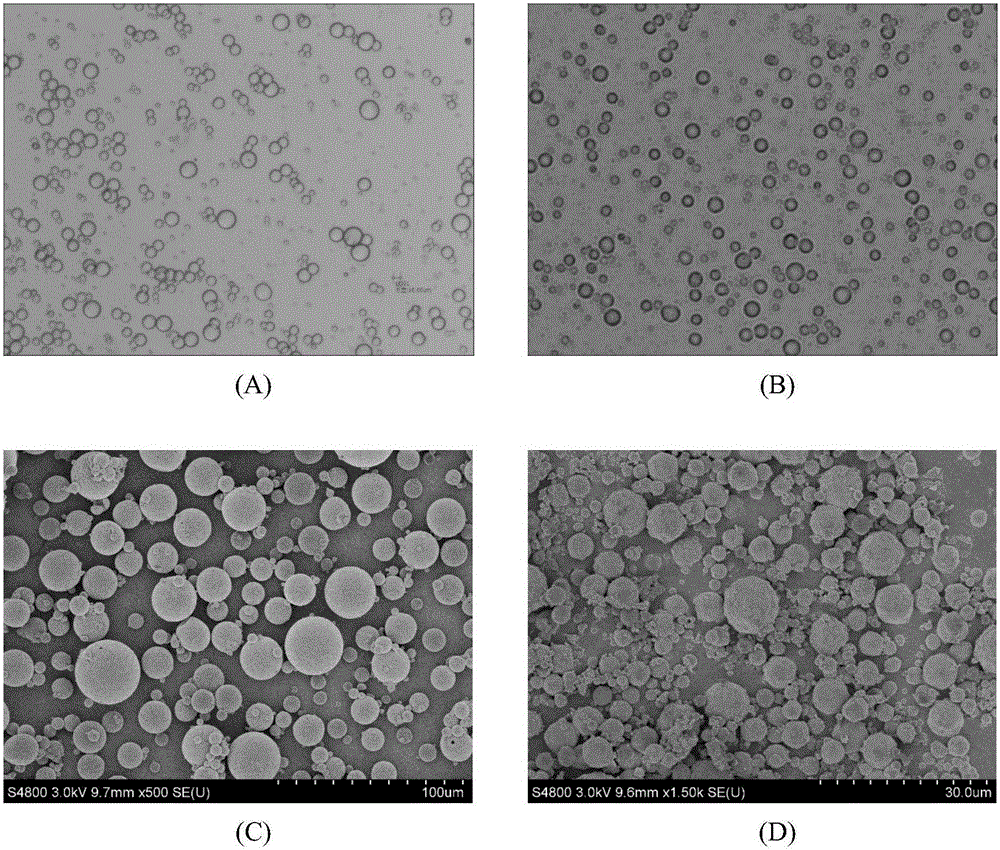
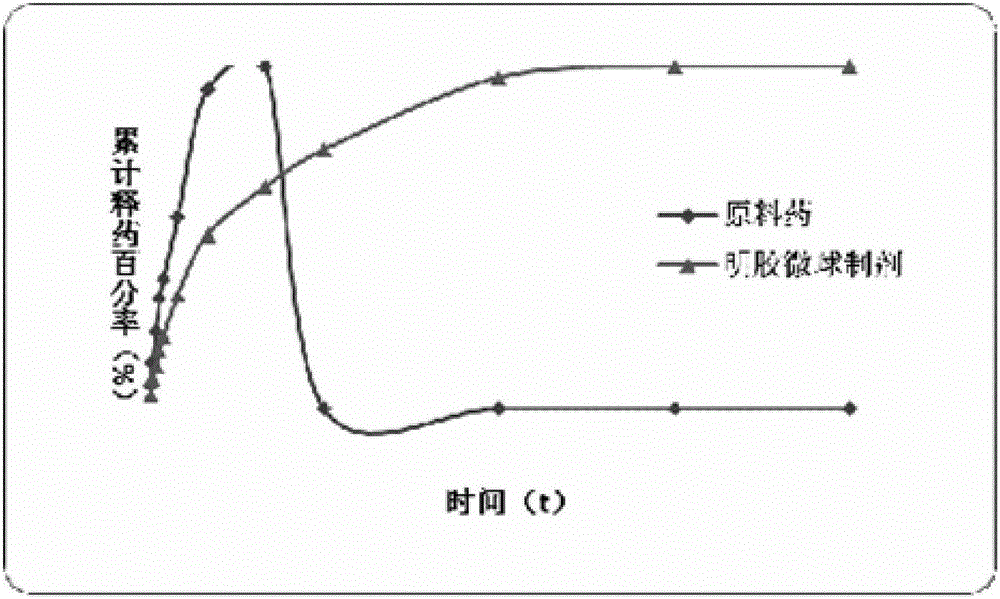
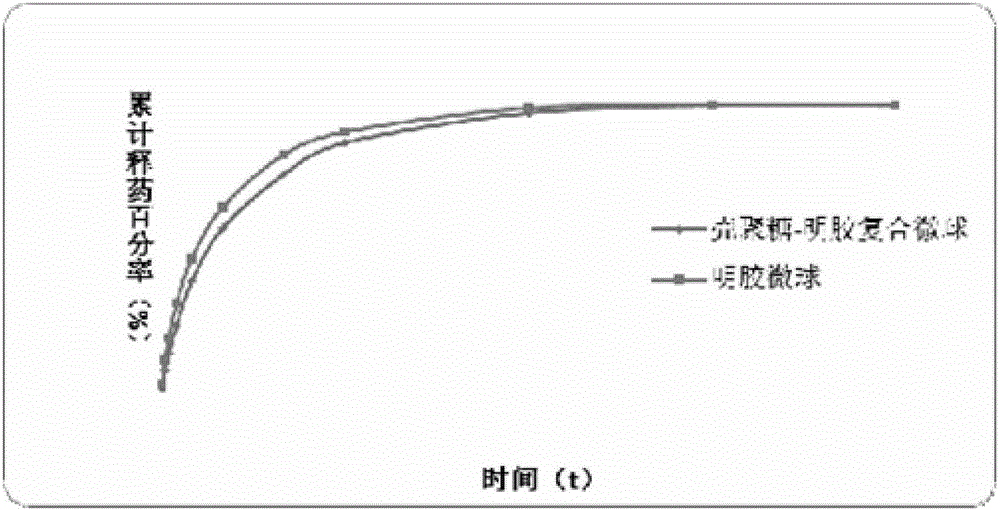
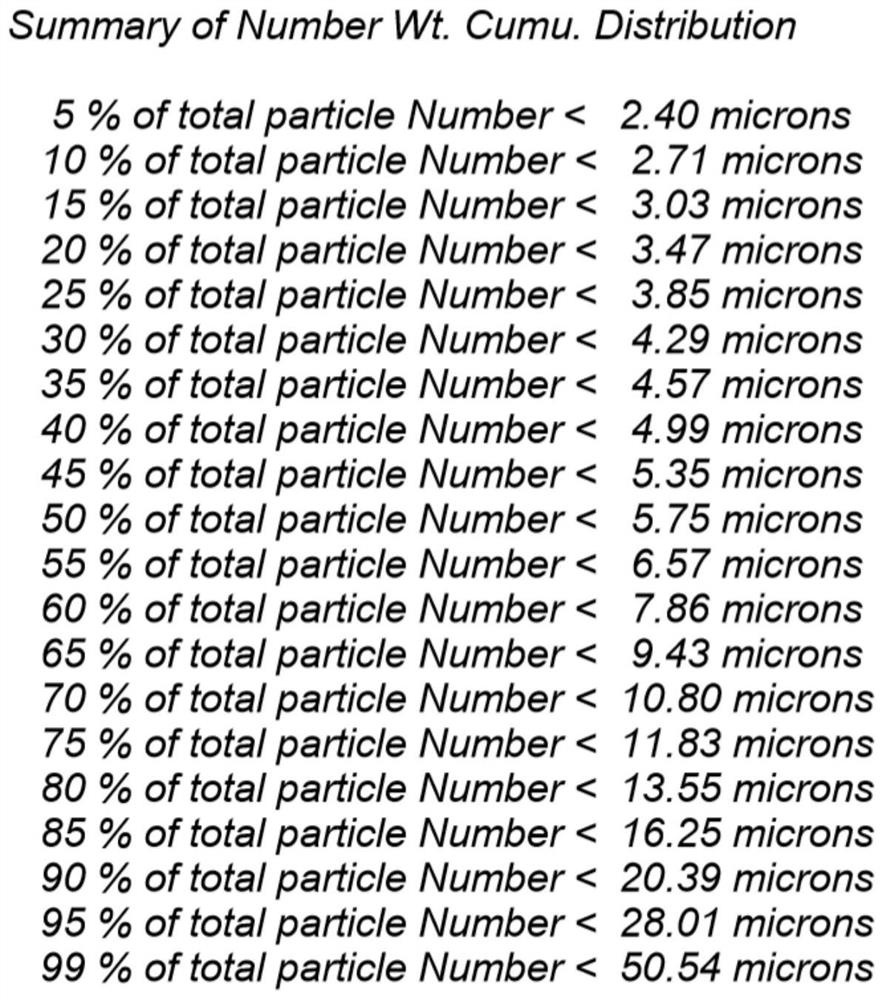
Lung-targeted PLGA (polylactic-co-glycolic-acid) microsphere preparation of cefquinome sulfate and preparation method of lung-targeted PLGA microsphere preparation
InactiveCN105030692AGood curative effectSmall toxicityAntibacterial agentsOrganic active ingredientsSide effectAdditive ingredient
The invention discloses a lung-targeted PLGA (polylactic-co-glycolic-acid) microsphere preparation of cefquinome sulfate. The lung-targeted PLGA microsphere preparation is prepared by cefquinome sulfate serving as an active pharmaceutical ingredient and PLGA serving as a carrier according to a weight ratio of 1:5-20. The invention further provides a preparation method of the lung-targeted PLGA microsphere preparation. The lung-targeted PLGA microsphere preparation and the preparation method have the advantages that encapsulation efficiency of prepared microspheres is higher than 60%, and grain diameter of more than 85% of the microspheres ranges from 7um to 35um, so that the microsphere preparation can be gathered in a lung in a targeted manner, curative effect of the microsphere preparation is improved effectively, and toxic and side effect of the microsphere preparation is lowered; detention time of the microsphere combination in the lung can be increased, and blood-drug concentration can be maintained stable to realize long-acting effect.
Owner:QINGDAO AGRI UNIV
Composition and application and preparation thereof
ActiveCN105079000AGood dispersionImprove distributionAntibacterial agentsOrganic active ingredientsTreatment effectIrritation
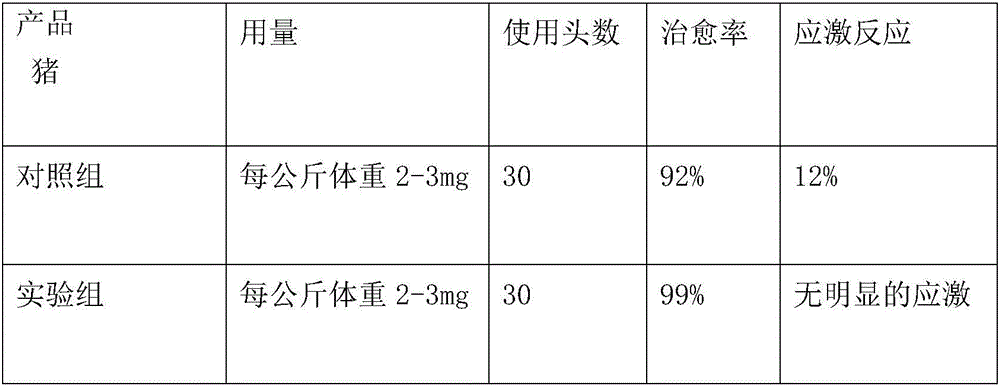
The invention relates to the field of animal cultivation, in particular to a composition and an application and preparation thereof. The composition comprises cefquinome or a cefquinome salt and an auxiliary material, and the auxiliary material comprises a combination of glidant and one or a mixture (including two or more) of a stabilizer, a suspending agent, synergist, antioxidant and solvent. Safety test results show that milk cows have no adverse reaction to the provided preparation. Local irritation and anaphylaxis test results show that cefquinome sulfate breast injectant (the lactation period) is free of irritant reaction. Curative effect test results show that the tested drug cefquinome sulfate breast injectant has the clearing effect on treating pathogenic bacteria of a milk area, relieves mastitis clinical symptoms of the milk cows, reduces the somatic number of milk, improves the milk quality and has a good treating effect on the clinic mastitis of the milk cows, and the cure rate reaches 80-85%.
Owner:FOSHAN NANHAI EASTERN ALONG PHARMA CO LTD
Preparation method of cefquinome sulfate
The invention relates to a method for synthesizing cefquinome sulfate and relates to a preparation method of a medicament for animals. The preparation method comprises the following steps: taking cefotaxime, 2,3-cyclohexyl pyridine and trimethyliodosilane as initial raw materials; preparing cefotaxime hydriodate from the cefotaxime; carrying out decoloring and silica column chromatography on the cefotaxime; and reacting the treated cefotaxime with sulphuric acid to prepare a cefquinome sulfate product. Compared with the prior art, the invention has the advantages of high yield of products, stable production quality and suitability for industrial production.
Owner:PU LIKE BIO ENG
Method of making cefquinome particles
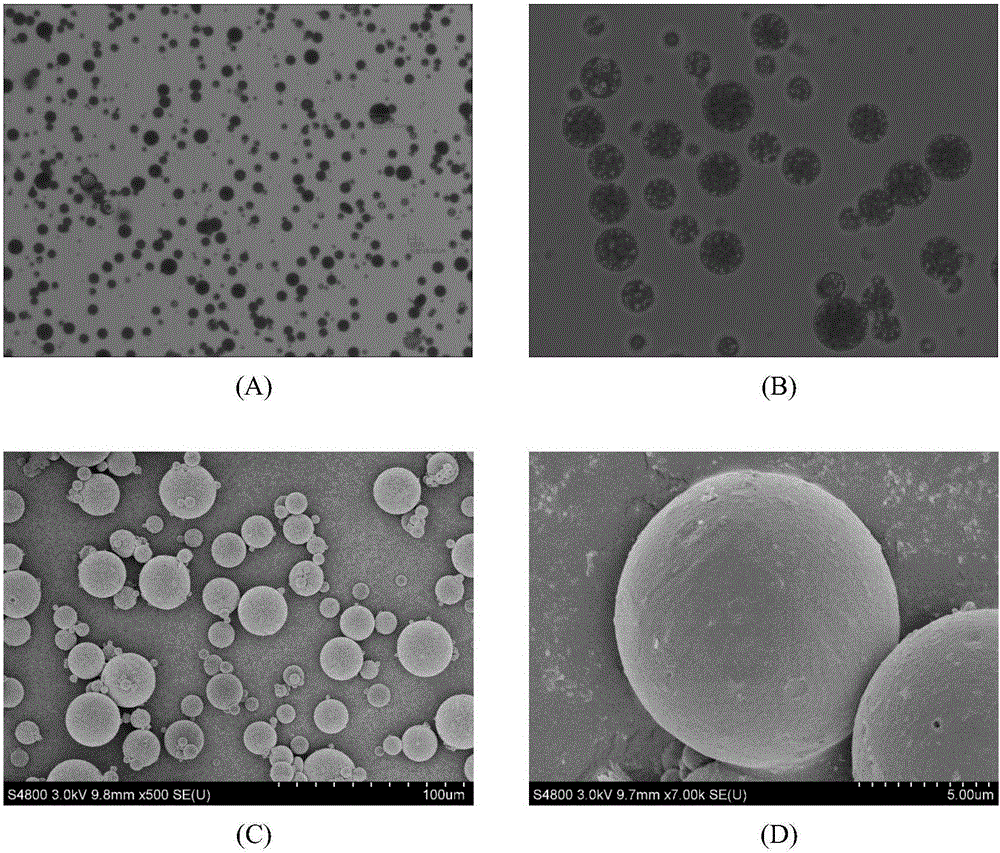
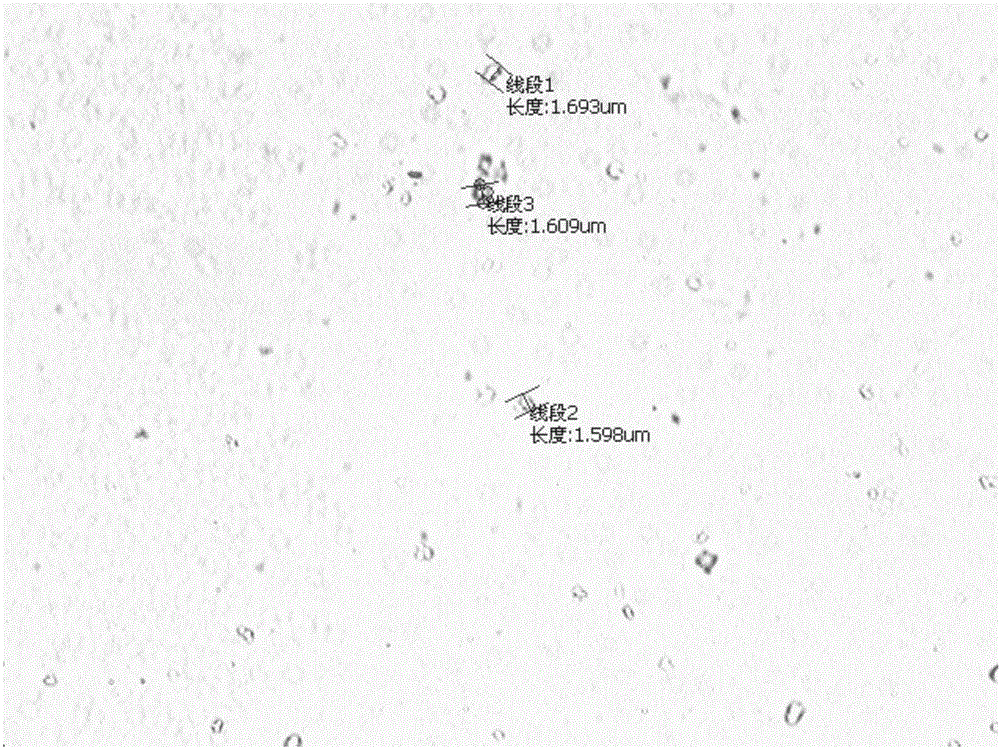
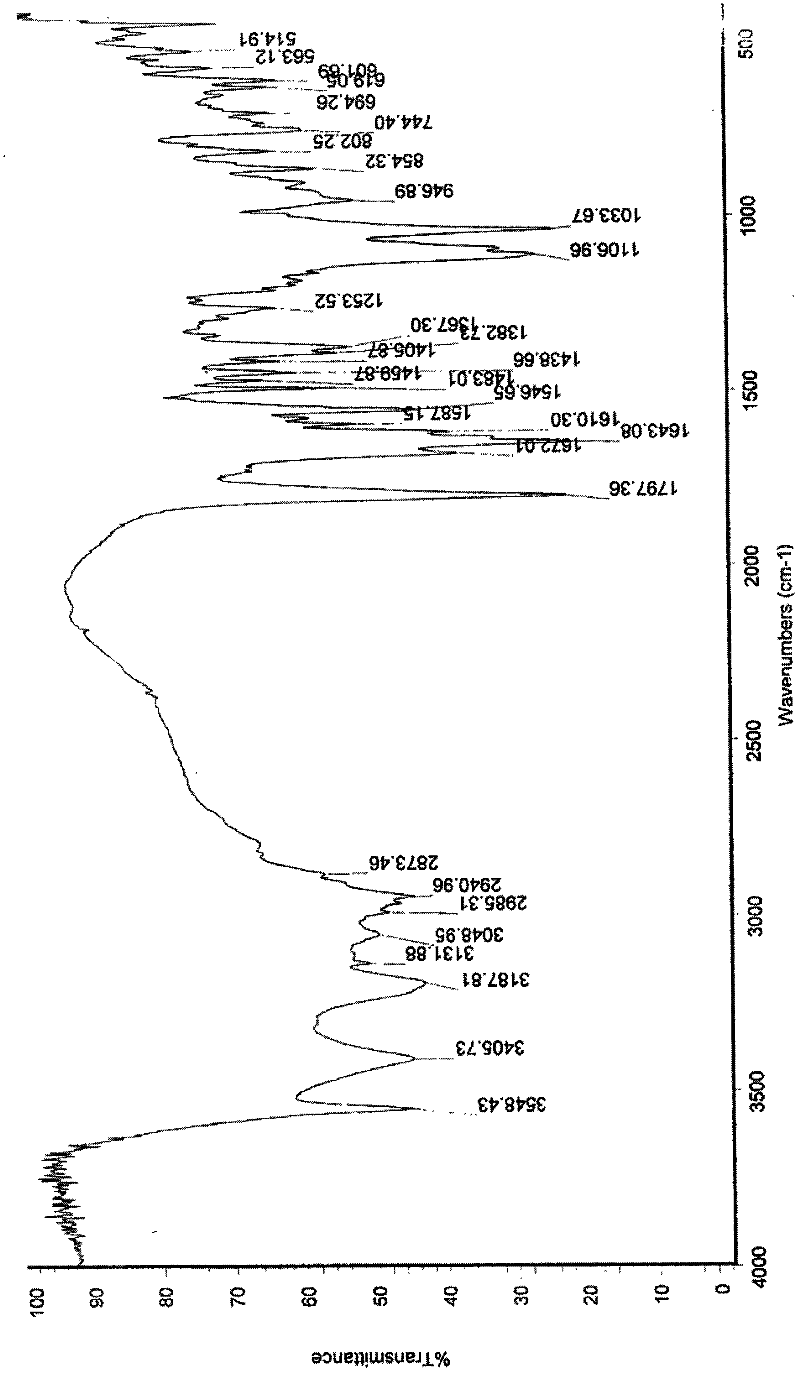
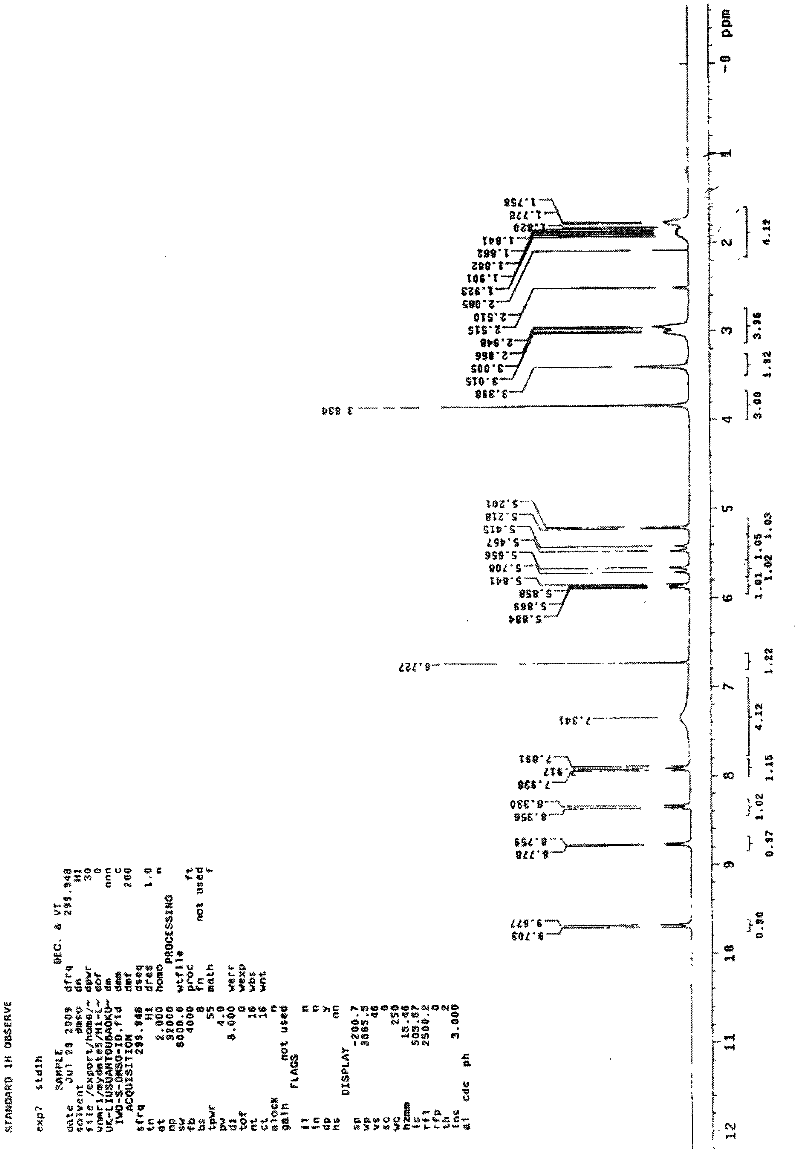
Disclosed is a process for the production of particles of a cefquinome acid addition salt, preferably cefquinome sulfate particles, by precipitation of cefquinome acid addition salt, preferably the sulfate, from a cefquinome betaine solution, wherein acid, preferably sulfuric acid, is added to the betaine solution. According to the invention the acid, preferably sulfuric acid, is added quickly in a single shot, in a molar excess of 40% to less than 100%. As a result, particles are formed that comprise agglomerates of microscale primary crystalline particles. This enables providing particles of cefquinome acid addition salt, preferably cefquinome sulfate, in particle sizes commensurate with micronized material, but with improved stability.
Owner:INTERVET INT BV
Lung targeted cefquinome sulfate PLGA microspheres and preparation method thereof
ActiveCN107308118AGood dispersionUniform appearanceAntibacterial agentsOrganic active ingredientsDispersityMicrosphere
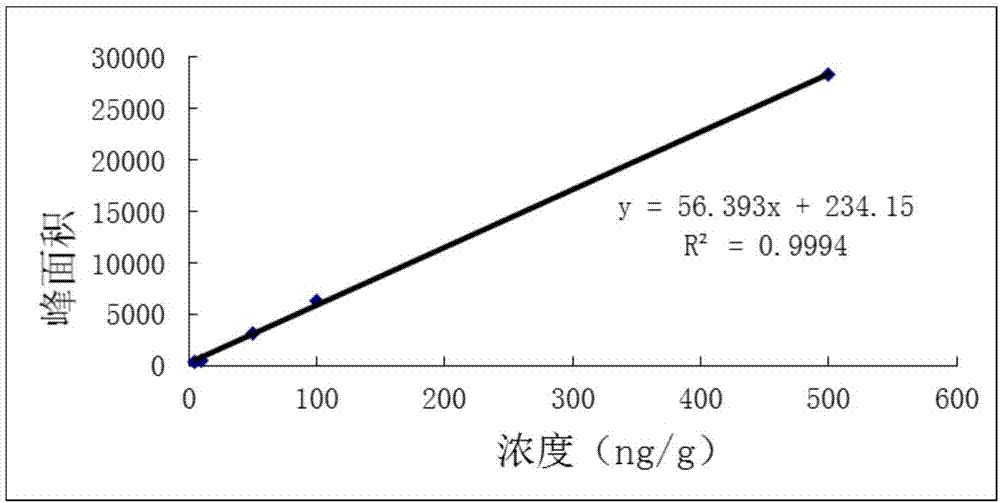
The invention provides lung targeted cefquinome sulfate PLGA microspheres and a preparation method thereof and belongs to the technical field of antibiotics targeted preparations for animals. The preparation method comprises the following steps: uniformly mixing a bulk drug with a dispersant, performing ball-milling, and completely evaporating liquid to obtain treated bulk drug cefquinome; weighing PLGA and PLA as carriers, dissolving the carriers in a mixed solvent, adding the bulk drug after complete dissolution, then adding a flow aid, performing spray-drying through ultrasonic mixing, meanwhile, performing magnetic stirring, and then adopting a vortex separator to obtain the microspheres. The microsphere encapsulation ratio is 95% or above, the drug loading capacity is 20% or above, and 85% or more microspheres have the grain sizes of 10-25 [mu]m; the operation method is simple and convenient, the microsphere preparation efficiency is high, and the obtained microspheres are good in dispersity and have a favorable slow-release effect and lung targeting characteristic. An established tissue Chinese herbal medicine detection method is good in specificity, high in sensitiveness and recovery rate and excellent in degree of precision.
Owner:QINGDAO AGRI UNIV
Lung-targeting gelatin microsphere agent containing cefquinome sulfate and preparation method of lung-targeting gelatin microsphere agents
InactiveCN105030693AGood curative effectSmall toxicityAntibacterial agentsOrganic active ingredientsGelatin microspheresSide effect
The invention discloses a lung-targeting gelatin microsphere agent containing cefquinome sulfate and a preparation method of the lung-targeting gelatin microsphere agent. According to the lung-targeting gelatin microsphere agent, cefquinome sulfate is taken as the active ingredient, gelatin or gelatin-chitosan compound is taken as a carrier, and the weight ratio of the active ingredient to the carrier is 1 to 2-6. The invention further provides the preparation method of the lung-targeting gelatin microsphere agent. The encapsulation efficiency of the prepared microspheres is 65% or above, the particle sizes of 80% or more of the microspheres are distributed within 7-35 um, so that the microsphere agent can gather to the lung in a targeted manner, the therapeutic effect of the medicine is effectively improved, the toxic and side effects of the medicine are reduced, meanwhile, the retaining time of the medicine in the lug is prolonged, the concentration of the blood concentration is kept stable, and the long-term effect is achieved.
Owner:QINGDAO AGRI UNIV
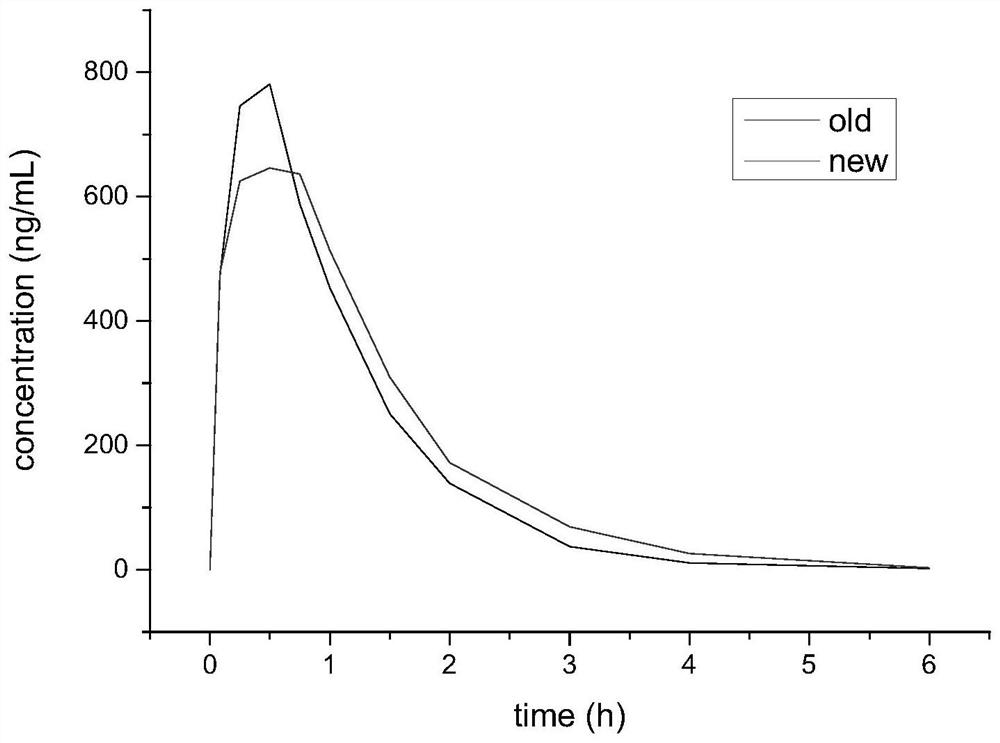
Preparation method and application of cefquinome sulfate sustained-release suspension injection
PendingCN112569186AQuality improvementGood curative effectAntibacterial agentsOrganic active ingredientsFormularyButylated hydroxytoluene
The invention belongs to the technical field of chemical pharmaceutical preparations, and particularly relates to a preparation method and application of a cefquinome sulfate sustained-release suspension injection, the optimal prescription ratio of the cefquinome sulfate injection is screened by an orthogonal design method, ethyl oleate for injection is used as a dispersing medium, hydrogenated castor oil is used as a suspending aid, span 85 is used as a wetting agent, the butylated hydroxytoluene is used as an antioxidant, the long-acting cefquinome sulfate injection which is more stable in quality and more reliable in curative effect is prepared by taking cefquinome sulfate as a raw material and butylated hydroxytoluene as an antioxidant, the preparation process is optimized through colloid mill grinding, and the colloid mill grinding time is 15-20 minutes. An optimal formula is selected through a preferable formula and an orthogonal test, a colloid mill is used for grinding, and compared with cefquinome sulfate injection of an old process, the cefquinome sulfate injection of the new process has the advantages that within 0-6 hours, the new process shows the effects of slow absorption and slow release compared with a preparation of the old process, and the slow release effect is prolonged; the quality is more stable and the curative effect is more reliable.
Owner:杭州爱力迈动物药业有限公司
Cefquinome sulfate uterine injectant for milk cows and preparation method of cefquinome sulfate uterine injectant
ActiveCN104840420AEasy to administerSave manpower and material resourcesOrganic active ingredientsSolution deliveryVegetable oilIrritation
The invention discloses a cefquinome sulfate uterine injectant for milk cows and a preparation method of the cefquinome sulfate uterine injectant. The cefquinome sulfate uterine injectant for milk cows is prepared from the following raw materials and auxiliary materials: 0.5-2.5g of cefquinome sulfate (measured by cefquinome), 0.125-1.25g of glycerin monostearate, 0.125-1.25g of a suspending agent and the balance of vegetable oil added to be up to 25ml. The preparation method comprises the following steps of weighing a proper amount of pretreated vegetable oil, adding glycerin monostearate, and melting by heating; then, adding the suspending agent and cefquinome sulfate; next, adding the vegetable oil to be up to the total quantity; and uniformly grinding to obtain the cefquinome sulfate uterine injectant. The uterine injectant has the following advantages that a drug is slowly released, the bioavailability is high, and the irritation to local tissues is little; due to the adoption of the local drug delivery of the uterus, effects are rapidly taken, and the animal stress response caused by drug delivery by injection is avoided; and the drug is rapidly eliminated, the milk withdrawal period is short, the economic loss is effectively avoided, and the clinical popularization value is very high.
Owner:FEED RESEARCH INSTITUTE CHINESE ACADEMY OF AGRICULTURAL SCIENCES
Cefquinome sulfate lipidosome and preparation method thereof
ActiveCN103784404ASustained releaseObvious functionAntibacterial agentsOrganic active ingredientsOrganic solventCholesterol
The invention relates to a cefquinome sulfate lipidosome and a preparation method thereof. The cefquinome sulfate lipidosome consists of cefquinome sulfate, phospholipid, cholesterol, vitamin E and a freeze-drying supporting agent. The preparation method comprises the following steps: adding phospholipid, cholesterol and vitamin E into an organic solvent to dissolve; dissolving cefquinome sulfate in a buffer liquid; and mixing, stirring and filtrating the products by a membrane obtained by the two steps to obtain the cefquinome sulfate lipidosome. The cefquinome sulfate lipidosome provided by the invention has good stability and encapsulation efficiency, and has a remarkable slow release effect after being delivered to animals.
Owner:江西派尼生物药业有限公司
Method for improving dissolution rate of cefquinome sulfate and method for detecting dissolution rate of cefquinome sulfate
InactiveCN111714501ASolve the problem of low dissolution rateImprove drug dissolutionAntibacterial agentsOrganic active ingredientsCEFQUINOME SULFATEDissolution
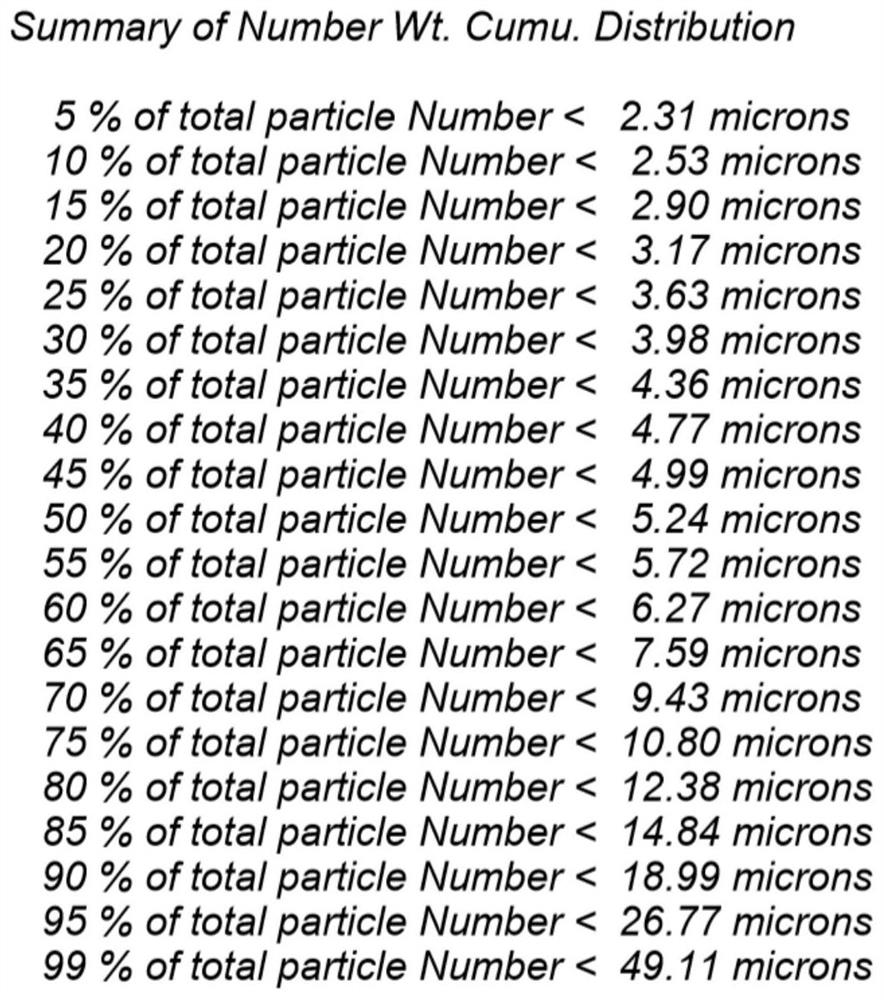
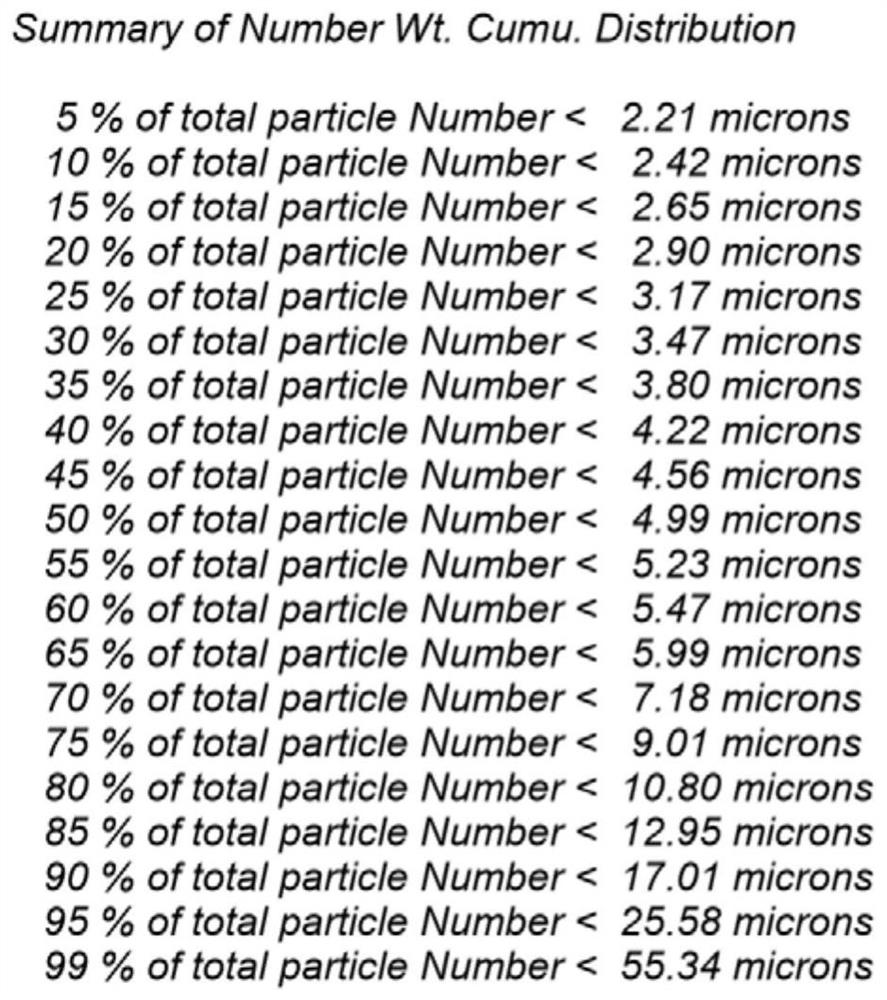
The invention discloses a method for improving the dissolution rate of cefquinome sulfate and a method for detecting the dissolution rate of the cefquinome sulfate. The method comprises the followingsteps of 1) mixing soybean oil for injection and ethyl oleate according to a ratio of 5: 5, heating mixed liquid to 120 DEG C under the protection of N2, keeping the temperature for 2 hours, and cooling the liquid to the room temperature; 2) heating the mixed liquid to 60 DEG C, adding BHT, performing stirring and melting, adding hydrogenated castor oil and span 60 when the liquid is cooled to 40DEG C, performing stirring for 30 minutes, and performing cooling to the room temperature; and 3) adding a prescription amount of the cefquinome sulfate after all medicinal auxiliary materials are added, pouring the cefquinome sulfate into a colloid mill, and performing grinding for 5-20 minutes in a manner of alternating circulating grinding and non-circulating grinding. The particle size of thecefquinome sulfate is changed through the colloid mill, so that the drug dissolution rate of the cefquinome sulfate is increased; and through particle size detection and dissolution rate analysis, after grinding is conducted for 15-20 min, the cefquinome sulfate with the particle size of 10 microns or below accounts for 80%, and the dissolution rate is the best.
Owner:杭州爱力迈动物药业有限公司
Synthetic method of cefquinome sulfate
ActiveCN102002058BSpecific responseLess side effectsOrganic chemistryChemical synthesisCEFQUINOME SULFATE
Owner:AMICOGEN CHINA BIOPHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com