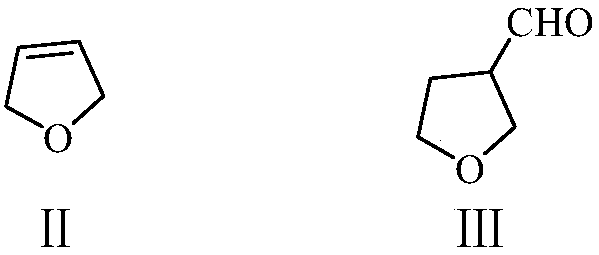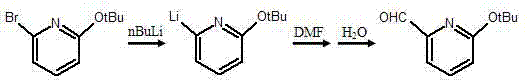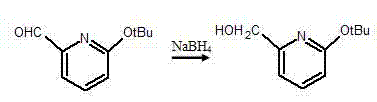Patents
Literature
47results about How to "Specific response" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
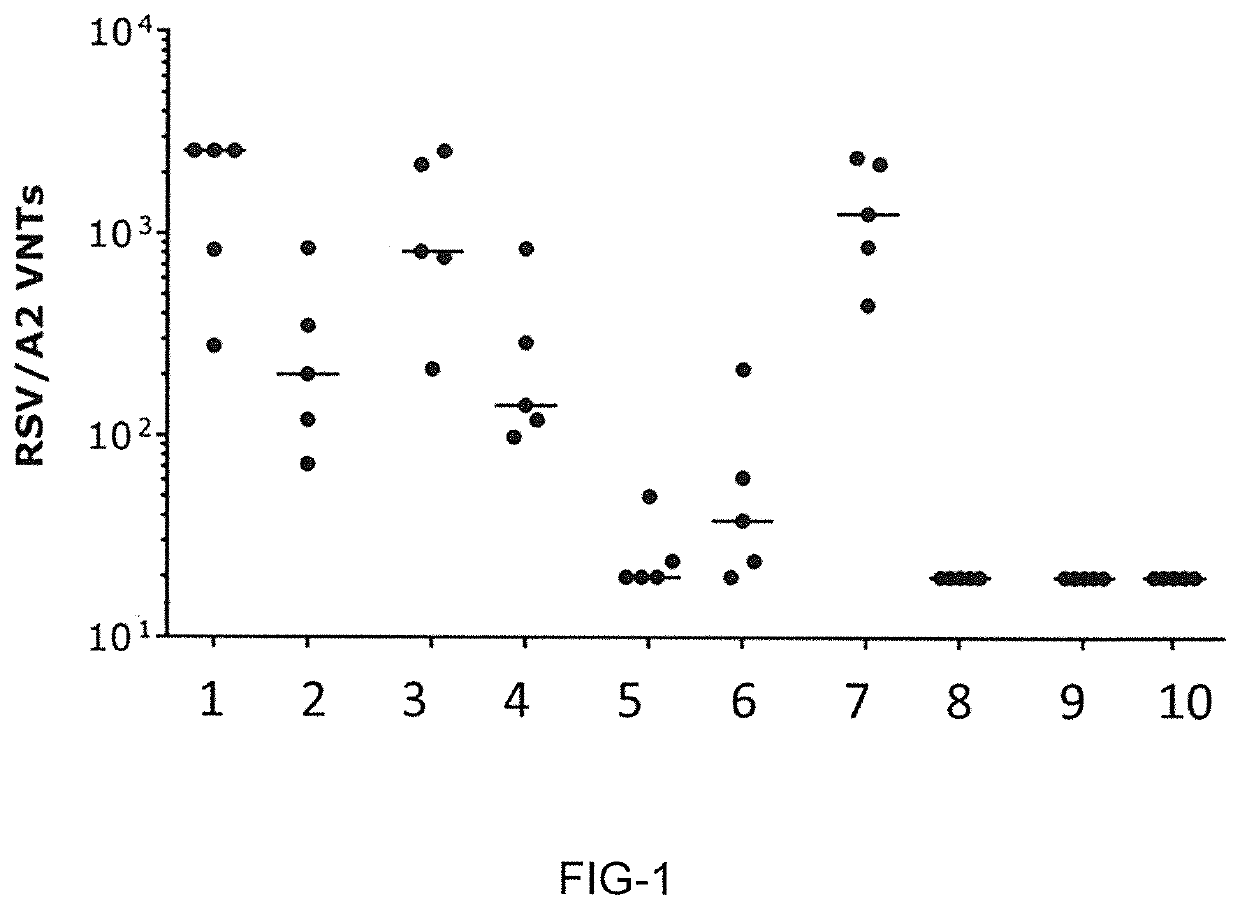
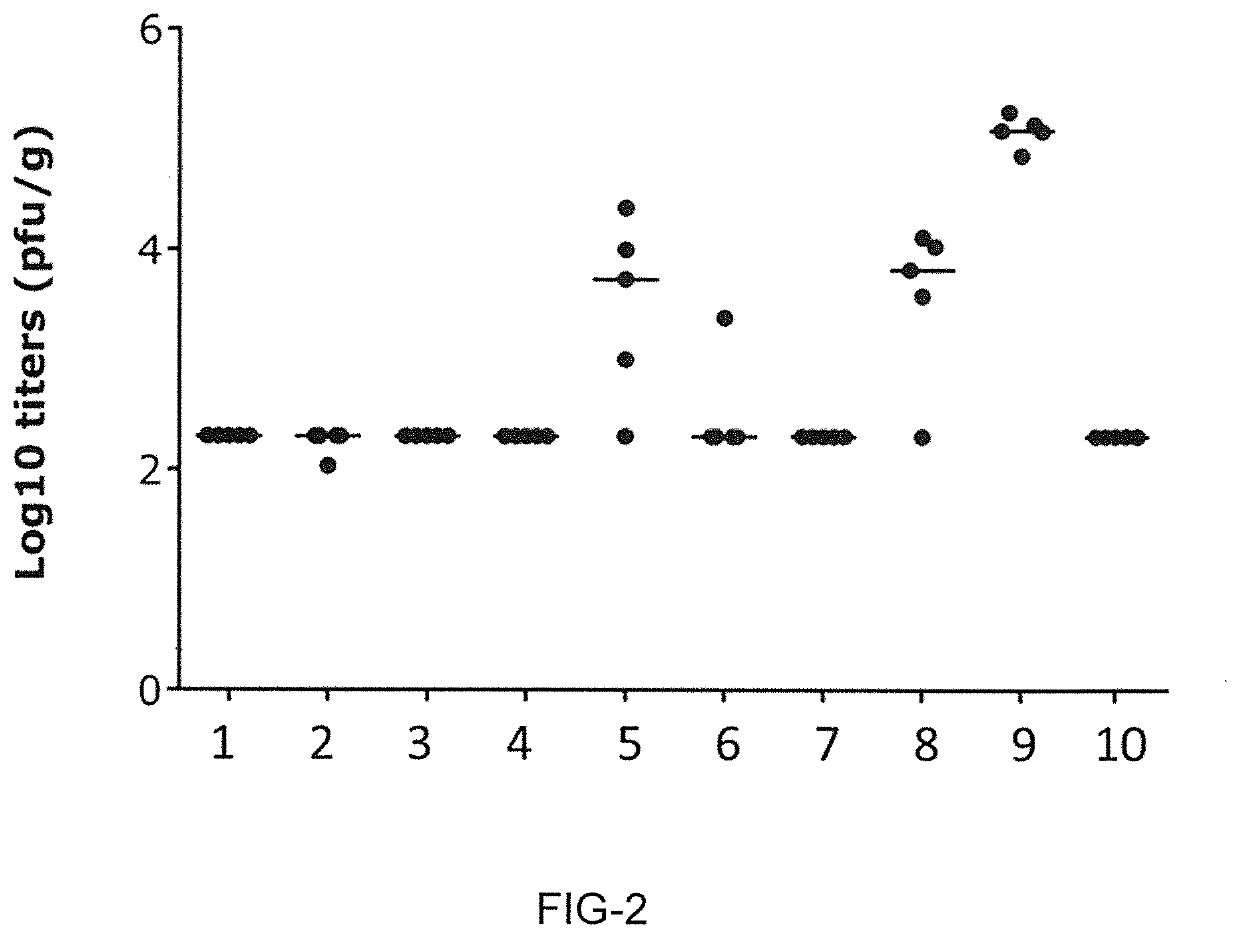
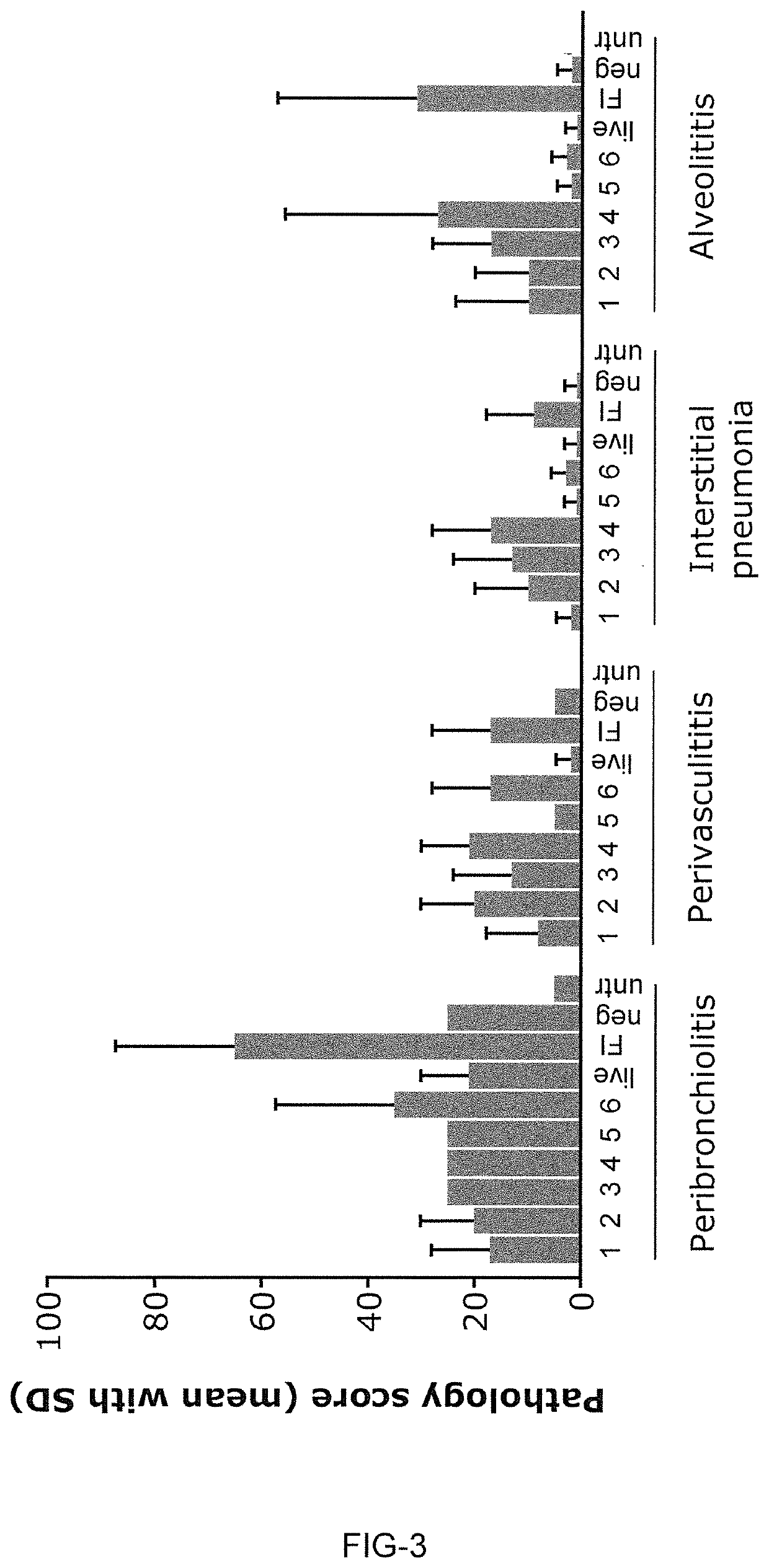
Novel rsv RNA molecules and compositions for vaccination
PendingUS20210170017A1Efficiently induces antigen-specific immune responseSpecific functionalSsRNA viruses negative-senseViral antigen ingredientsDiseaseVaccination
The present invention is directed to an artificial nucleic acid, particularly to an artificial RNA suitable for use in treatment and / or prophylaxis of an infection with Respiratory syncytial virus (RSV) or a disorder related to such an infection. The invention further concerns a method of treating or preventing a disorder or a disease, first and second medical uses of the artificial RNA, compositions, and vaccines. Further, the invention is directed to a kit, particularly to a kit of parts, comprising the artificial RNA, compositions and vaccines.
Owner:CUREVAC SE
Biological macromolecule detection method based on nano homogeneous time-resolved fluoroimmunoassay and droplet-based micro-fluidic technology
InactiveCN106324236AReduce volumeLow concentration requirementBiological testingFluorescenceEngineering
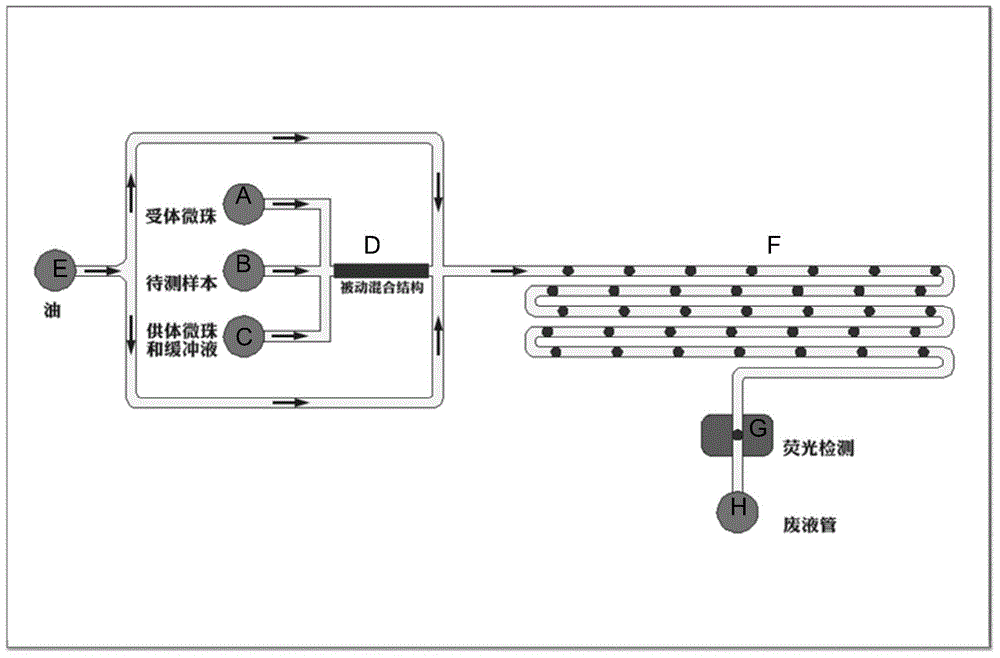
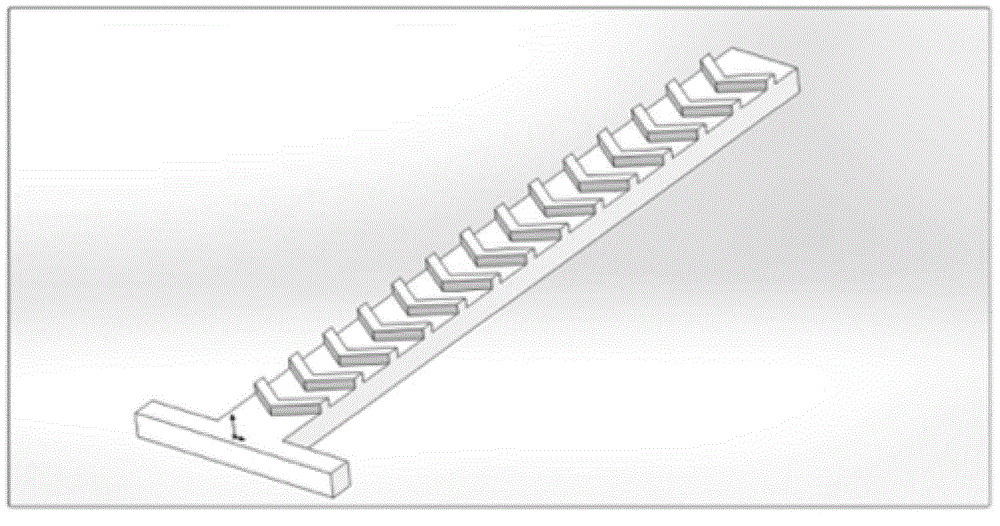
The invention belongs to the field of micro-fluidic chips, and relates to a biological macromolecule detection method. An alpha-LISA technology and a droplet-based microfluidics technology are combined and optimized to prepare a micro-fluidic chip liquid. A novel biological macromolecule detection method with a high sensitivity is provided. A three-phase mixing technology is adopted. A passive mixing structure is used to fully and evenly mix the reaction liquid. A cross focusing technology is used to form droplets. The generated droplets have the advantage of smaller volume, and thus the reactions become faster. Moreover, the requirements on the samples are lower, the reactions are specific, sensitive, fast, and full; the washing does not need to be carried out after reactions; complicated complexes such as complete proteins, enzyme complex, bacteriophage, and the like, can be detected; the technical bottleneck of conventional commercial liquid chips at present can be broken through; the provided method can be applied to fast clinical biological macromolecule detection; the detection sensitivity and specificity are both improved, and the required sample amount is reduced.
Owner:SHANGHAI FIRST PEOPLES HOSPITAL
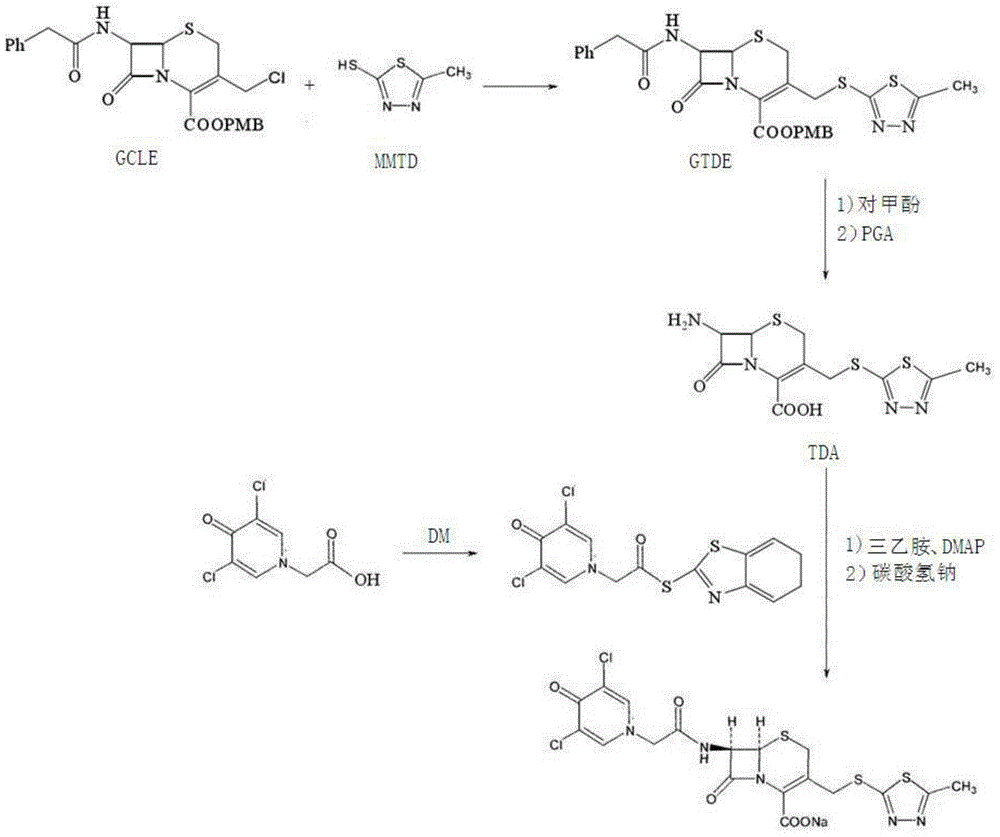
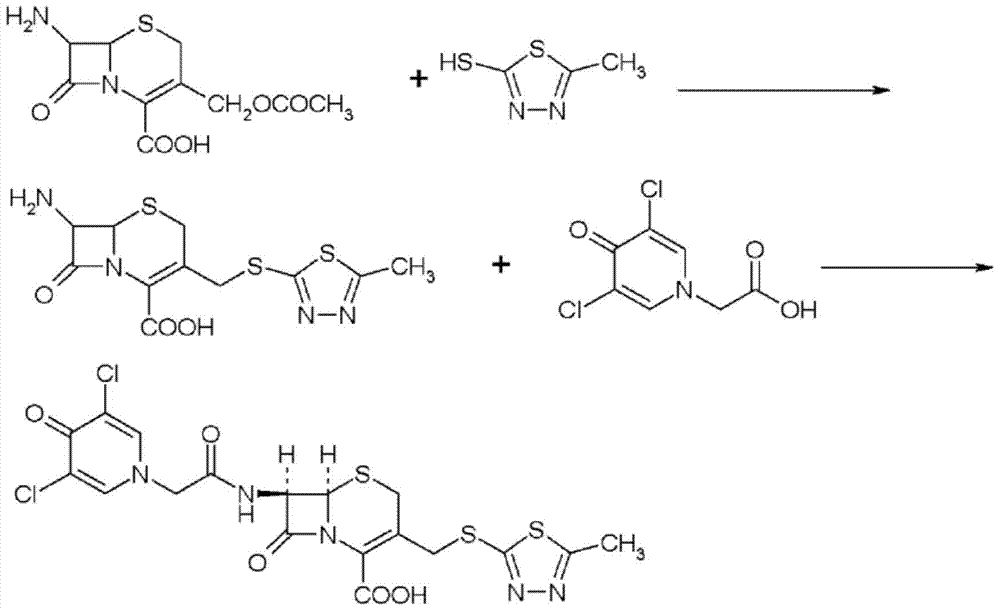
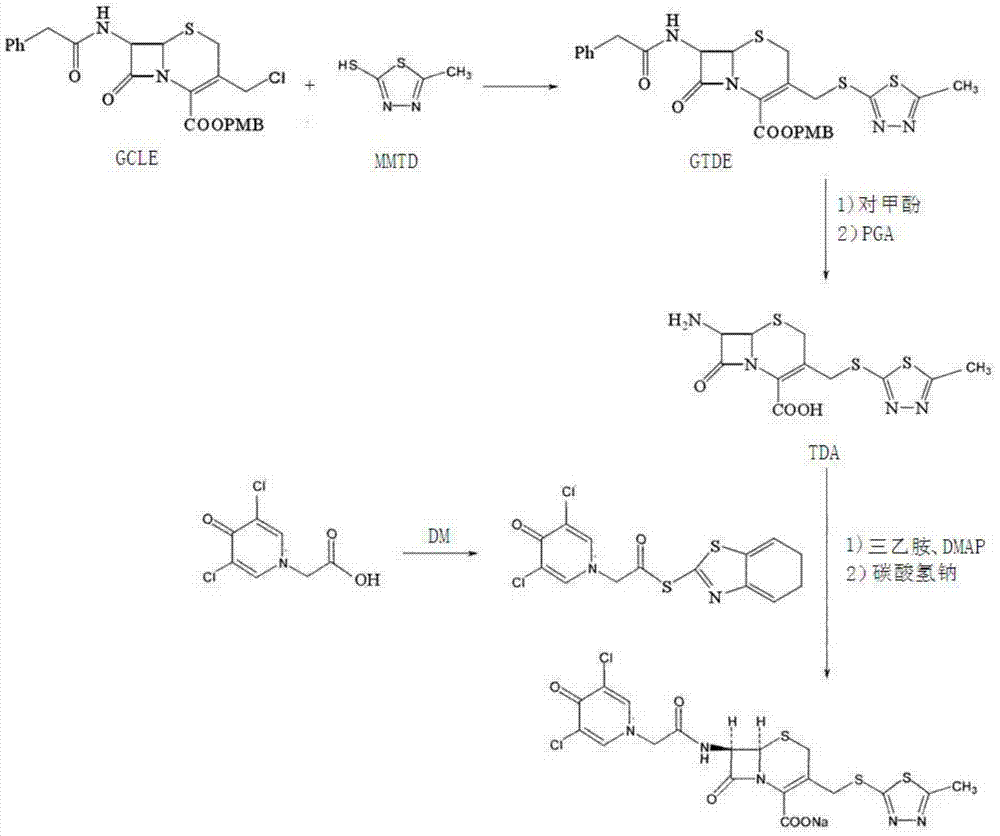
Preparation method for cephalosporin anti-infective drug
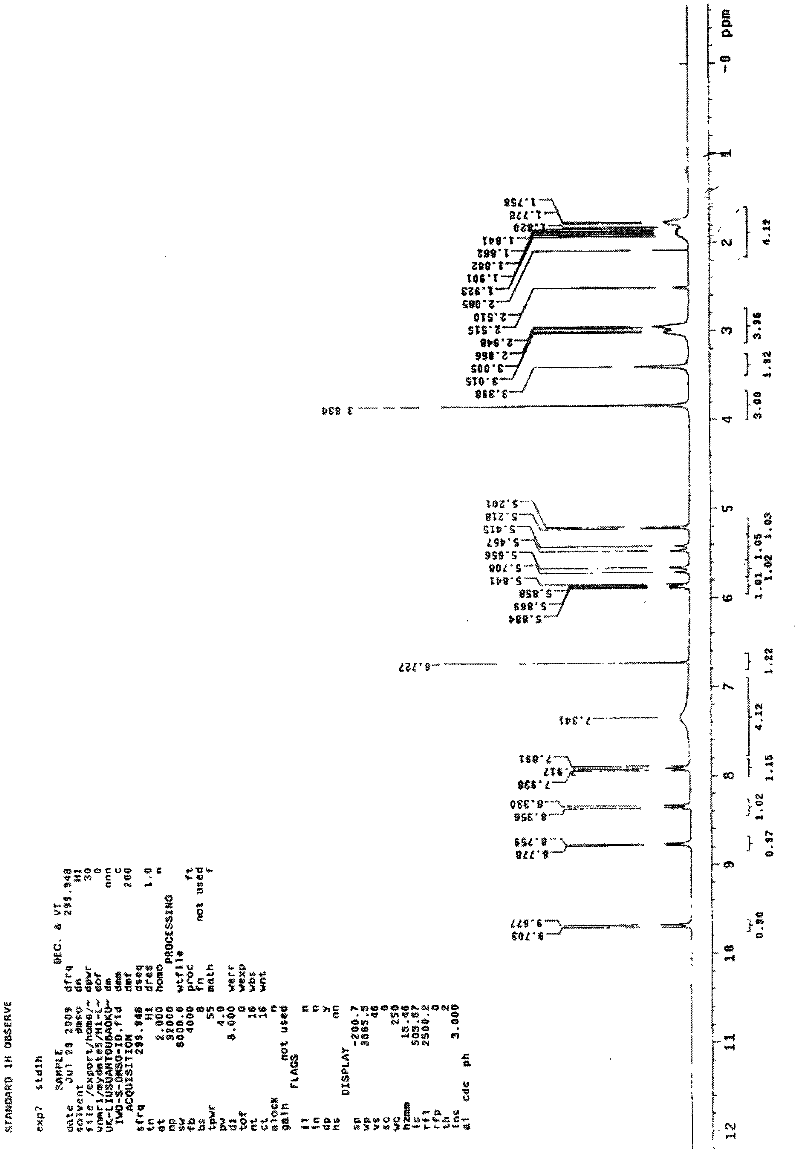
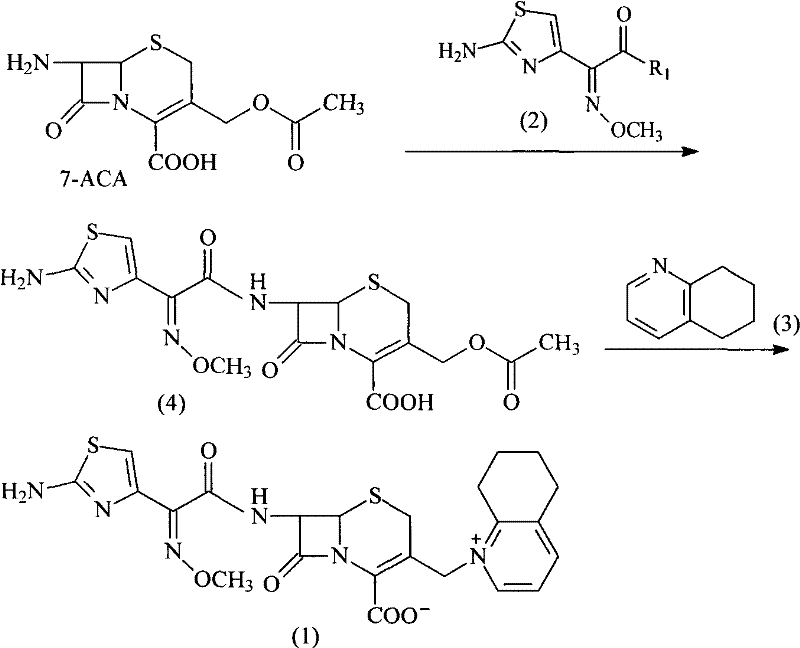
ActiveCN105017286ASimple preparation processReduce generationOrganic chemistry7-ACACefazedone sodium
The invention relates to a preparation method for a cephalosporin anti-infective drug-cefazedone sodium, belonging to the field of pharmaceutical synthesis. According to the invention, the method uses GCLE as a raw material to substitute 7-ACA and overcomes the defects of low yield, high pollution and the like in prior art; the preparation method with mild reaction conditions, little side reaction and simple process is provided; meanwhile, the method has the advantages of cheap and easily-available raw materials, low cost, high product yield, high product purity and applicability to industrial production.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
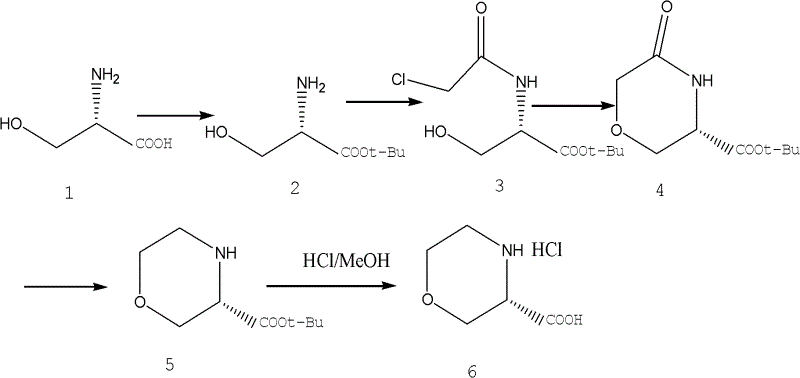
Novel synthetic method of (S)-3-morpholinyl carboxylic acid
ActiveCN102617503AMild reaction conditionsSpecific responseOrganic chemistryCarboxylic acidTert butyl
The invention discloses a synthetic method of (S)-3-morpholinyl carboxylic acid, which comprises the following steps: (1) taking L-serine as a raw material to synthesize L-serine tert-butyl ester; (2) dissolving L-serine tert-butyl ester in dichloromethane, adding a dichloromethane solution of chloroacetyl chloride drop by drop to obtain N-chloroacetyl-L-serine tert-butyl ester; (3) dissolving N-chloroacetyl-L-serine tert-butyl ester in a toluene solution, adding the toluene solution of sodium ethoxide drop by drop to obtain (S)-5-oxo 3-morpholinyl carboxylic acid tert-butyl ester; (4) dissolving (S)-5-oxo 3-morpholinyl carboxylic acid tert-butyl ester in methanol, successively adding aluminum trichloride and sodium borohydride to carry out a reaction to obtain the (S)-3-morpholinyl carboxylic acid tert-butyl ester; (5) dissolving (S)-3-morpholinyl carboxylic acid tert-butyl ester in methanol, adding a methanol solution of hydrogen chloride for reacting to obtain the (S)-3-morpholinyl carboxylic acid. The method of the invention has the advantages of mild reaction condition, easily available raw material and less three waste, and is suitable for industrial production.
Owner:上海常丰生物医药科技有限公司
Total bilirubin determination reagent kit
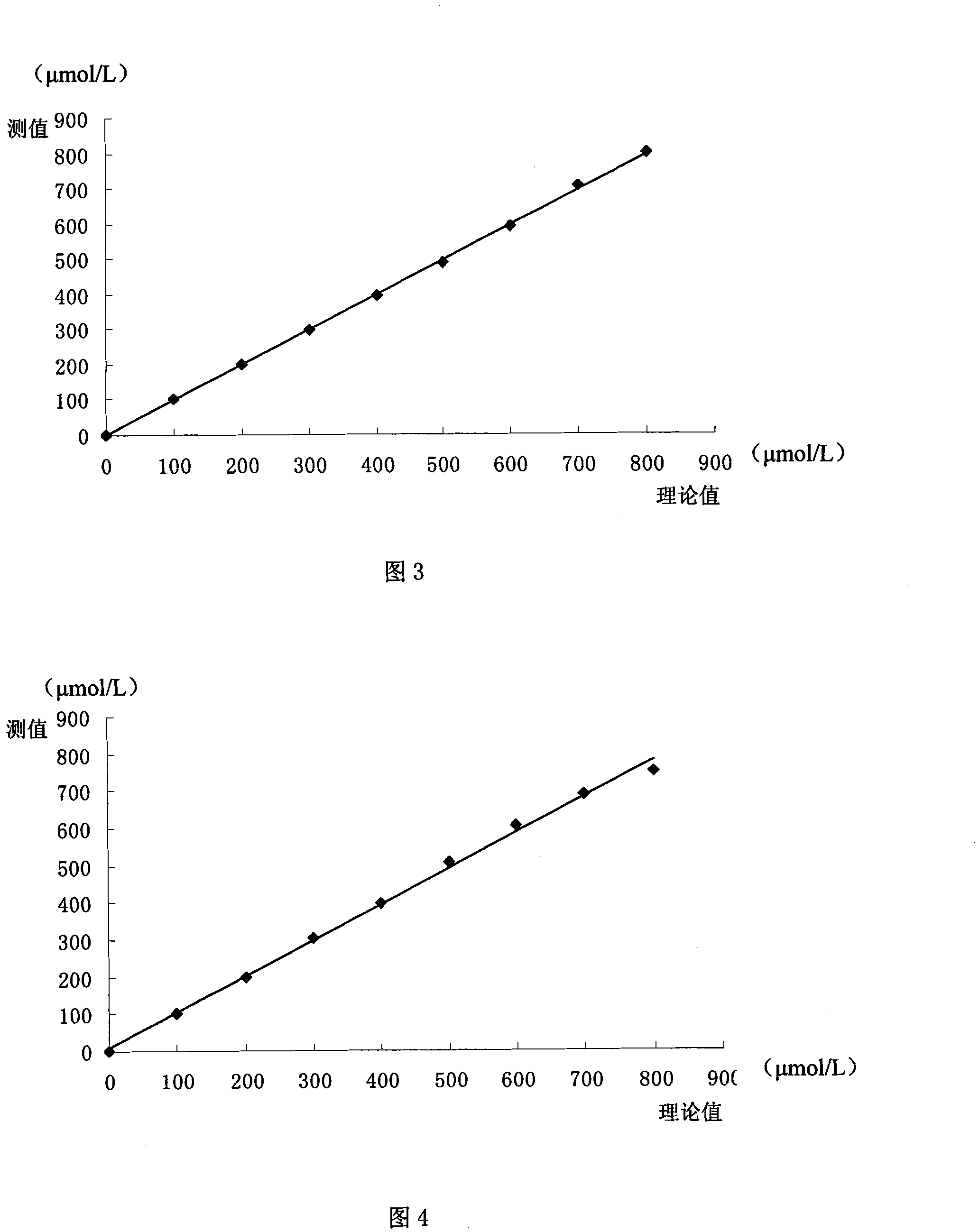
ActiveCN101144824AStrong interference abilityLong storage timeBiological testingAcetic acidAnti jamming
The present invention discloses a total bilirubin determining reagent box, which is liquid double reagent formed by reagent 1 and reagent 2. The constituents and the consistence range of the reagent 1 are: disodium of 13.5-81g / L, sodium dihydrogenphosphate of 0.6-3.6g / L, sodium chloride of 9.0g / L, ethylenediaminetetraacetic acid disodium salt of 0.2-1g / L, tween of -80 0.5-2ml / L, and sodium dodecyl sulfate of 1-10g / L. The constituents and the consistence range of the reagent 2 are: disodium of 1g / L, sodium chloride of 9g / L, sodium persulfate of 11.9-23.8g / L, and sulphuric acid of 0.1-2ml / L. The total bilirubin determining reagent box is stable and environment-friendly, and has proper linearity range, high test accuracy, high precision, high sensitivity and strong anti-jamming performance.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
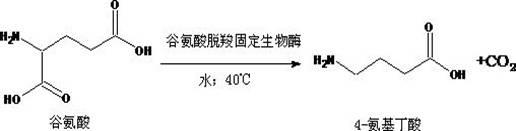
Method for catalytically synthesizing gamma-aminobutyric acid from glutamate biological solid-phase enzyme
InactiveCN102690846ASpecific responseHigh yieldOn/in organic carrierFermentationActivated carbonEnzyme catalysis
The invention provides a method for catalytically synthesizing gamma-aminobutyric acid from a glutamate biological solid-phase enzyme, The method comprises the following steps: (1) catalytically synthesizing substrate glutamic acid solution with solid-phase enzyme to generate gamma-aminobutyric acid solution, and then filtering or centrifugally separating the biological solid-phase enzyme from reaction solution; (2) after the activated carbon fading of the gamma-aminobutyric acid solution, concentrating the obtained product in vacuum by a thin film to obtain concentrated gamma-aminobutyric acid solution; and (3) adding 95% alcohol in the gamma-aminobutyric acid solution, crystallizing, centrifugally separating and drying the precipitated gamma-aminobutyric acid white precipitate in vacuum to obtain white gamma-aminobutyric acid powder. The process has the advantages of special reaction, high yield and purity, short period and low energy consumption and is suitable for industrial production.
Owner:广东乐尔康生物科技股份有限公司
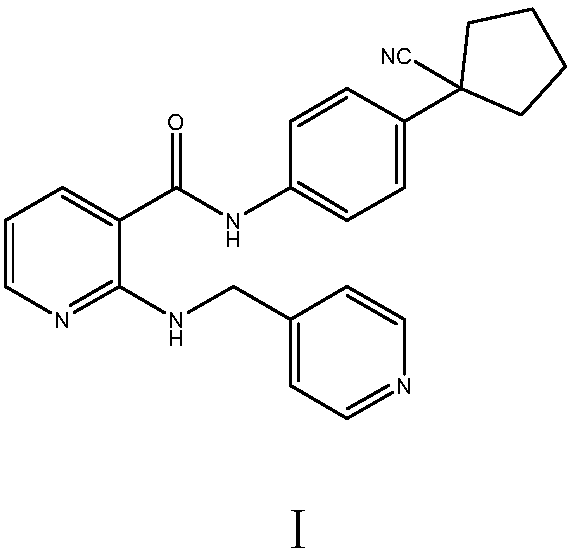
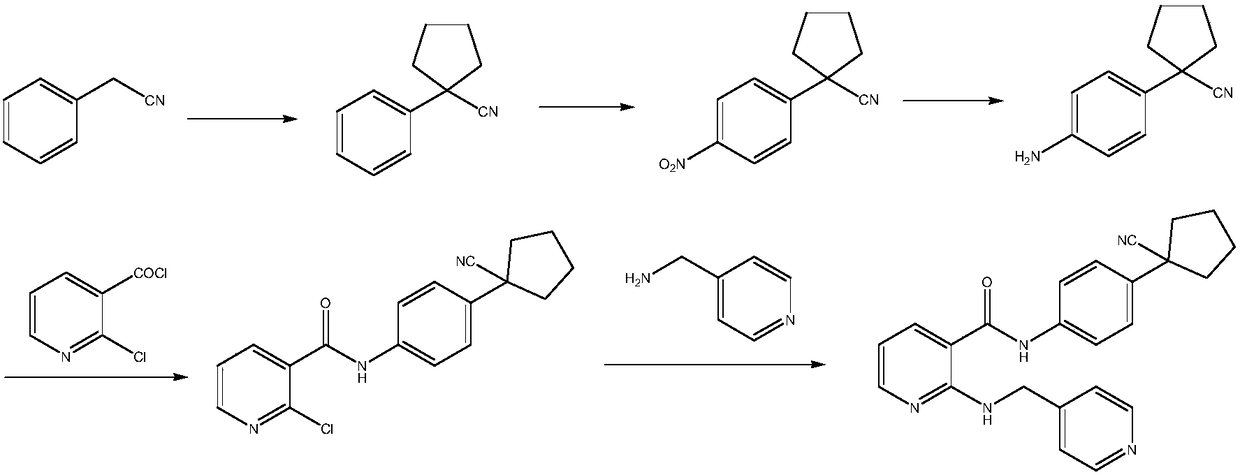
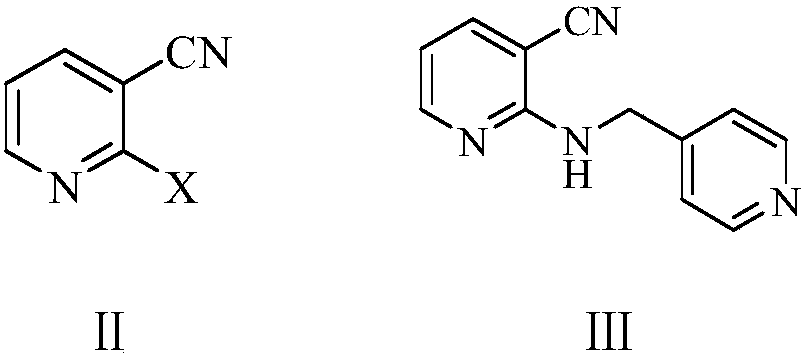
Preparation method of apatinib
The invention provides a preparation method of apatinib. The preparation method comprises that 2-halogenated-3-cyanopyridine as a raw material and 4-aminomethylpyridine undergo a substitution reactionto produce N-(pyridin-4-yl-methyl)-2-amino-3-cyanopyridine, the N-(pyridin-4-yl-methyl)-2-amino-3-cyanopyridine undergoes an esterification reaction to produce N-(pyridin-4-yl-methyl)-2-amino-3-picolinate, and the N-(pyridin-4-yl-methyl)-2-amino-3-picolinate and 1-(4-aminophenyl)cyclopentylformonitrile undergo an amidation reaction to produce apatinib (I). The preparation method has the advantages of low cost and easy availability of raw materials, simple processes, low cost, less waste water generation, safety and environmental protection, easy realization of reaction conditions, high reactivity and selectivity, few side reactions, few apatinib impurities, high purity and high yield.
Owner:XINFA PHARMA
Method for producing gamma-aminobutyric acid by using banana biological enzyme method
The invention discloses a method for producing gamma-aminobutyric acid by using a banana biological enzyme method, which comprises the following steps of: cutting up and grinding bananas, adding 1-3L of leaching liquor into every 1 kg of bananas, and after the obtained mixture is uniformly stirred, carrying out standing extraction on the obtained mixture for 3-5 h at a temperature of 4-25 DEG C; carrying out centrifugation on the obtained product for 5-10 min at a speed of 5000-7000 rpm so as to obtain glutamic acid decarboxylase crude-enzyme; adding 30-50 g of glutamic acid and sodium glutamate with pH of 4.5-6.0 into every 1L of enzyme, so that the obtained product can be converted completely in 12-24 h under the condition of 37 DEG C; and adding 0.1-3.0% of active carbon into the obtained product, stirring for 30-60 min, and carrying out filtering, rotary steaming and crystallization on the obtained product so as to obtain 20-40 g of gamma-aminobutyric acid. The method is simple in production process, specific in reaction, low in cost, high in yield, good in purity, short in cycle, less in waste, and environmental-friendly, and leaching liquor can be repeatedly used, therefore, the method is suitable for industrial production.
Owner:CATCH BIO SCI & TECH
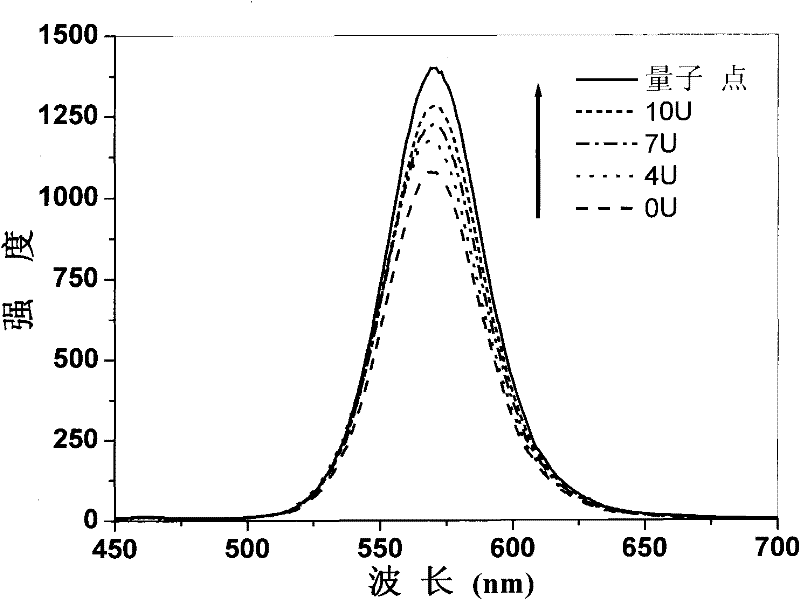
Enzyme chip based on quantum dot fluorescence detection, preparation method and application
InactiveCN102384902AGood biocompatibilityHigh affinityAnalysis by subjecting material to chemical reactionFluorescence/phosphorescenceSolubilityOligomer
The invention relates to an enzyme chip based on quantum dot fluorescence detection, a preparation method and application, which manufactures substrates of the enzyme chip through a mask photoetching. A plurality of mini-chambers are obtained on the substrates. A reaction system formed by a water-solubility quantum dot, enzyme oligomer and coenzyme or formed by the water-solubility quantum dot, enzyme and the coenzyme is loaded in each mini-chamber which is used for detection and arranged on the substrates provided with the plurality of mini-chambers. Or one reaction system formed by the water-solubility quantum dot, the enzyme oligomer and the coenzyme or formed by the water-solubility quantum dot, the enzyme and the coenzyme is respectively loaded in different mini-chambers which are arranged on the substrate and used for detection. By means of the reaction of the enzyme to be detected and the enzyme oligomer or the reaction of the enzyme and the enzyme oligomer to be detected, fluorescence strength of the water-solubility quantum dot changes. High-selectivity quantitative detection of activity of the enzyme to be detected and / or density of the enzyme oligomer to be detected canbe achieved through detection of the quantity of the change of the fluorescence strength of the water-solubility quantum dot.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
Preparation method of 2-methyl-4-acetoxy-2-butenal with high yield
ActiveCN110734374AReduce productionImprove stabilityOrganic compound preparationCarboxylic acid esters preparationPtru catalystIsomerization
The invention provides a preparation method of 2-methyl-4-acetoxy-2-butenal (I) with high yield. The preparation method comprises the steps: with 2,5-dihydrofuran (II) and synthesis gas as raw materials, carrying out hydroformylation reaction under the action of a catalyst to prepare 3-formyl tetrahydrofuran (III); then carrying out a reaction with an acetylation reagent under the action of a catalyst to prepare 2-formyl-4-acetoxy-1-butene (IV); and carrying out double bond isomerization under the action of a catalyst to obtain 2-methyl-4-acetoxy-2-butenal (I). The method has the advantages ofcheap and accessible raw materials and low cost; the process flow is short, the reaction is easy to realize, the operation is safe, simple and convenient, the wastewater yield is low, and the green and environment-friendly effects are achieved; the method has the advantages of stable reaction intermediate product, proper reaction activity, high reaction selectivity, fewer side reactions and highyield, and is suitable for industrial production.
Owner:XINFA PHARMA
Preparation method of p-chlorophenylglycine
InactiveCN111470994AAvoid poisoningAvoid pollutionOrganic compound preparationCatalystsChlorobenzenePtru catalyst
The invention provides a preparation method of p-chlorophenylglycine, which comprises the following steps: adding a mixed solution of a chloroform solution, a catalyst and p-chlorobenzaldehyde into asodium hydroxide solution, mixing, and dropwisely adding liquid ammonia while stirring for 2-6 hours; after finishing dropwise adding the liquid ammonia, adding an ammonium bicarbonate solution, and reacting for 5-10 hours at room temperature; after the reaction is finished, distilling and concentrating a reaction solution, decolorizing and filtering by using activated carbon, adjusting the pH value of a filtrate to 6.0 by using an inorganic acid, cooling and filtering to obtain a crude product and mother liquor, washing the crude product by using water, ethanol and diethyl ether, and drying to obtain a finished product p-chlorophenylglycine; wherein the catalyst is trioctyl methyl ammonium chloride; wherein the molar ratio of the p-chlorobenzaldehyde to the trioctyl methyl ammonium chloride to the chloroform to the sodium hydroxide to the ammonium bicarbonate is 1.0 : (0.02-0.38) : (1.5-2.5) : (6.0-10.0) : (0.03-0.07). The synthesis process is simple, raw materials are easy to obtain,the reaction is milder, and toxicity and pollution of cyanide are avoided.
Owner:上海开荣化工科技有限公司
Method for catalytically synthesizing gamma-aminobutyric acid by using sodium glutamate and immobilized bio-enzyme
InactiveCN102676598ASpecific responseHigh yieldOn/in organic carrierFermentationActivated carbonCombinatorial chemistry
The invention provides a method for catalytically synthesizing gamma-aminobutyric acid by using sodium glutamate and an immobilized bio-enzyme. The main process comprises the following steps: (1) performing catalytic synthesis on a substrate, namely a sodium glutamate solution and an immobilized enzyme to obtain gamma-aminobutyric acid, and performing centrifugal separation on the immobilized bio-enzyme from a reaction liquid; (2) allowing the reaction liquid to pass through a cation exchange resin to remove sodium chloride from the reaction liquid so as to obtain purified gamma-aminobutyric acid; (3) decoloring the aqueous solution of gamma-aminobutyric acid by using active carbon, and concentrating by using a vacuum membrane to obtain a concentrated solution of gamma-aminobutyric acid; and (4) adding 95 percent alcohol into the concentrated solution of gamma-aminobutyric acid, separating out white gamma-aminobutyric acid precipitate crystals, performing centrifugal separation and vacuum drying to obtain white gamma-aminobutyric acid powder. The process has the advantages of reaction specificity, high yield, high purity, short period and low energy consumption, and is suitable for industrial production.
Owner:广东乐尔康生物科技股份有限公司
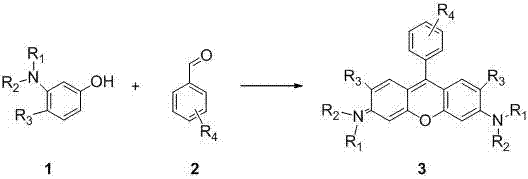
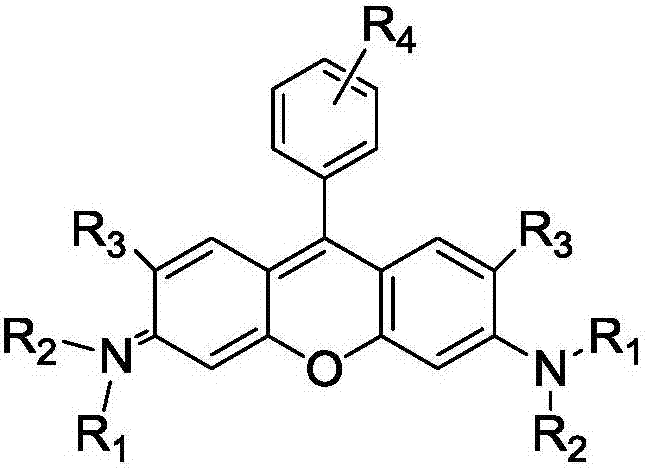
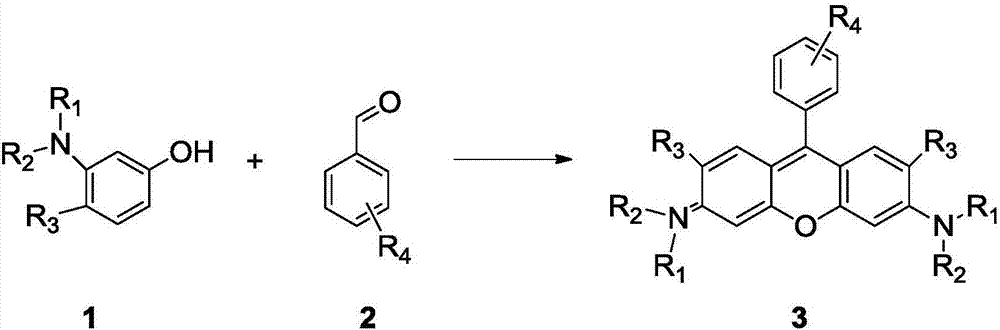
Method of rapidly preparing rhodamine dye with a plurality of active functional groups under mild condition
InactiveCN106946838AExcellent photophysical propertiesSpecific responseOrganic chemistryPyronine/xanthon/thioxanthon/selenoxanthan/telluroxanthan dyesFluorescenceDerivatization
The invention relates to a method of rapidly preparing a rhodamine dye with a plurality of active functional groups under the mild condition and belongs to the technical field of fine chemicals. The method is used for synthesizing rhodamine with specific functional groups by carrying out simple chemical modification on raw materials and has an important guiding significance to derivatization of the rhodamine dye. Compared with the commercially available rhodamine dye, the rhodamine dye has multiple active functional groups and is beneficial to derivatization of the rhodamine dye. By adopting the method, the rhodamine dye with the specific functional groups can be synthesized by 6-7h only without protection of inert gas, so that the synthesis time of the rhodamine dye is greatly saved, the purpose of the rhodamine dye is expanded, the synthesis yield of the rhodamine dye is greatly increased, and the separation difficulty of the rhodamine dye is prevented. Through the advantages, the purpose of the rhodamine dye is expanded, so that the rhodamine dye can be fully developed in the fields of biolabeling, cell imaging, fluorescent probes and the like.
Owner:苏州高德瑞仪器有限公司
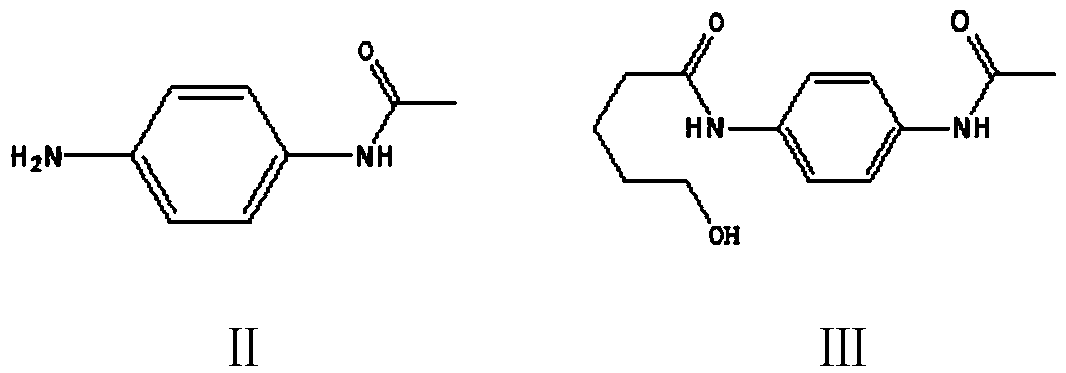
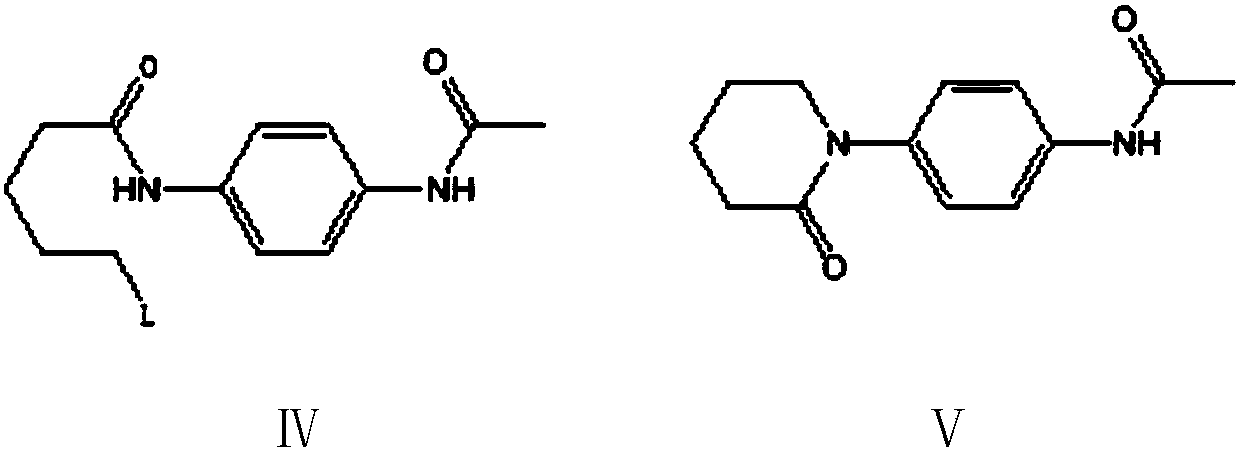
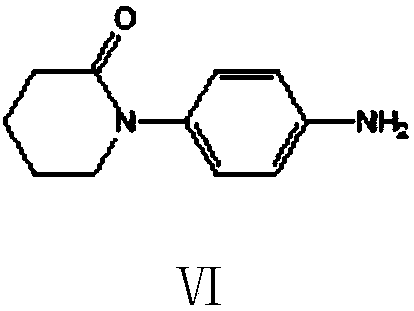
Preparation method of 5,6-dihydropyridine-2 (1H)-one derivative
The invention provides a preparation method of a 5,6-dihydropyridine-2 (1H)-one derivative, and particularly relates to a 1-(piperidine-2-keto-1-yl)-4-(5,6-dihydro-3-R substituent pyridine-2 (1H)-keto-1-yl) benzene preparation method, wherein the R substituent is chlorine or morpholine-4-yl. According to the invention, p-acetamido aniline is used as a raw material, and amidation with delta-valerolactone, halogenation with a halogenation reagent or sulfonylation with sulfonyl chloride, condensation, deacetylation, amidation with 2,2-dichloro-delta-valerolactone, halogenation with a halogenationreagent or sulfonylation with sulfonyl chloride, condensation elimination or condensation elimination substitution in the presence of morpholine are performed to obtain the target product. Accordingto the invention, the raw materials used in the preparation method are cheap, easy to obtain and low in cost; the process operation is simple, reaction conditions are easy to realize, the wastewater yield is low, and safety and greenness are realized; and the reaction selectivity of each step is high, the product yield and purity are high, and industrial production is facilitated.
Owner:XINFA PHARMA
Synthetic method of cefquinome sulfate
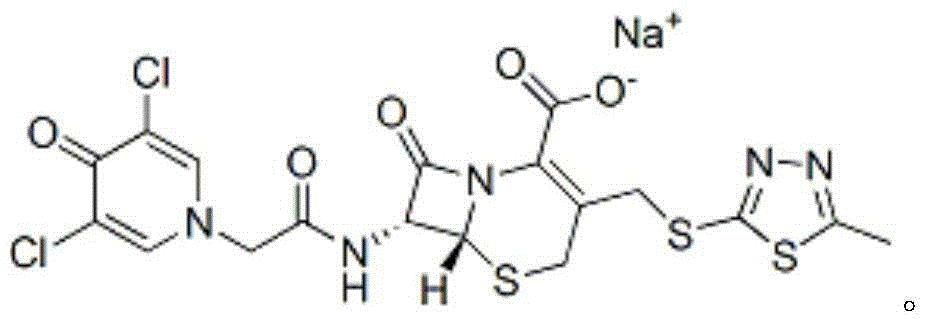
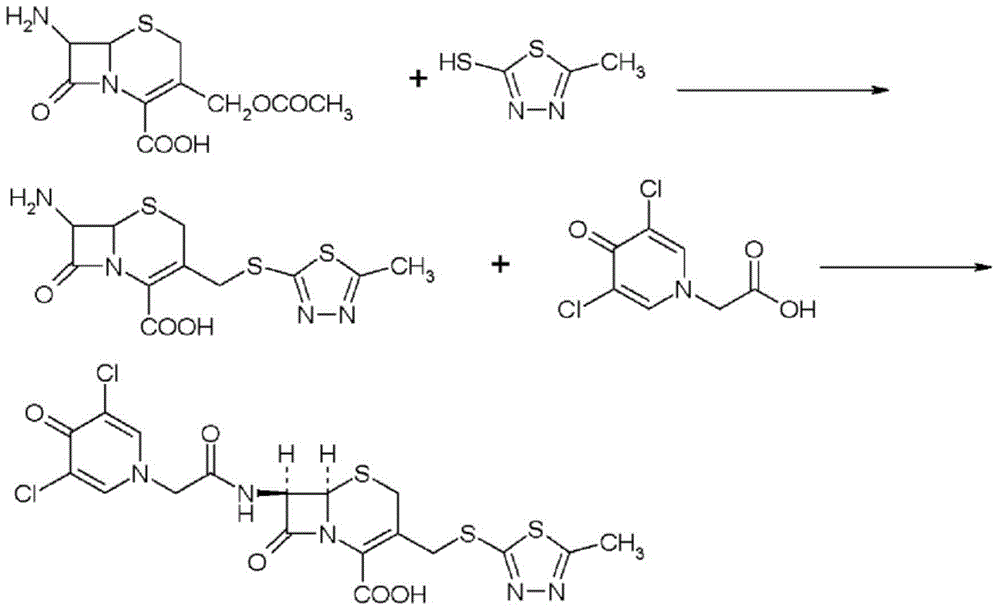
ActiveCN102002058BSpecific responseLess side effectsOrganic chemistryChemical synthesisCEFQUINOME SULFATE
Owner:AMICOGEN CHINA BIOPHARM CO LTD
Preparation method of 2-chloro-5-nitropyridine
The invention provides a preparation method of 2-chloro-5-nitropyridine. The preparation method comprises the following steps: preparing 2-hydroxy-5-nitropyridine by taking 2-nitroacetaldehyde diethylacetal as an initial raw material through two methods; and then carrying out a chlorination reaction on the 2-hydroxy-5-nitropyridine and a chlorination reagent to prepare the 2-chloro-5-nitropyridine. The method has the advantages of cheap and accessible raw materials and low cost, does not use a diazotization hydrolysis reaction, is safe, simple and convenient to operate, does not use mixed acid, is less in wastewater yield and environmentally-friendly, does not use a nitration reaction, is high in reaction selectivity, few in side reactions, simple in post-treatment and high in product yield and product, and is suitable for industrial production.
Owner:XINFA PHARMA
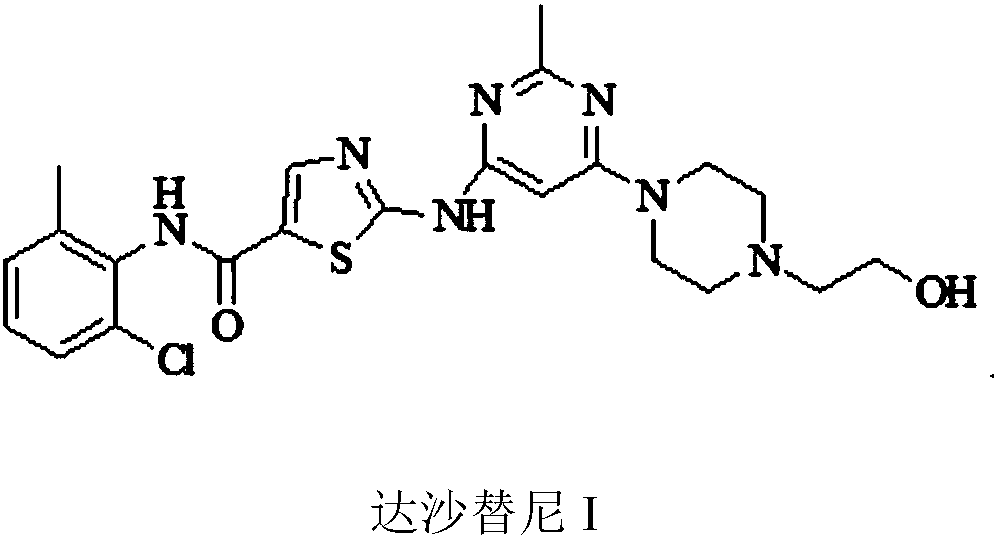
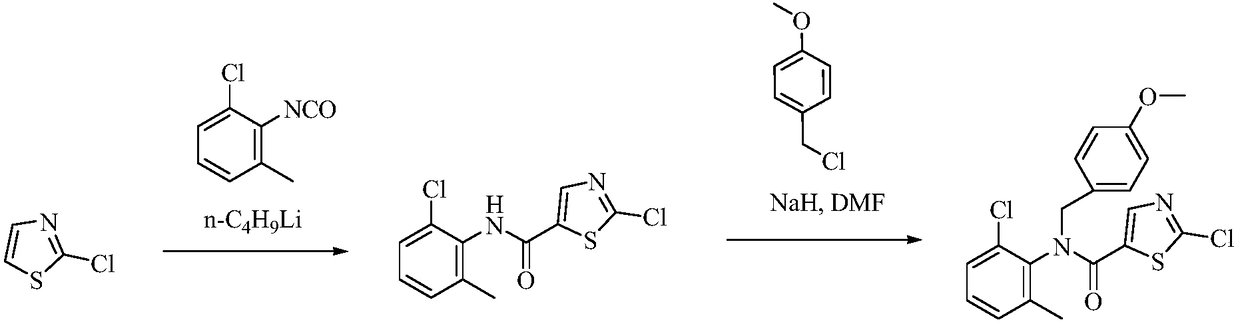
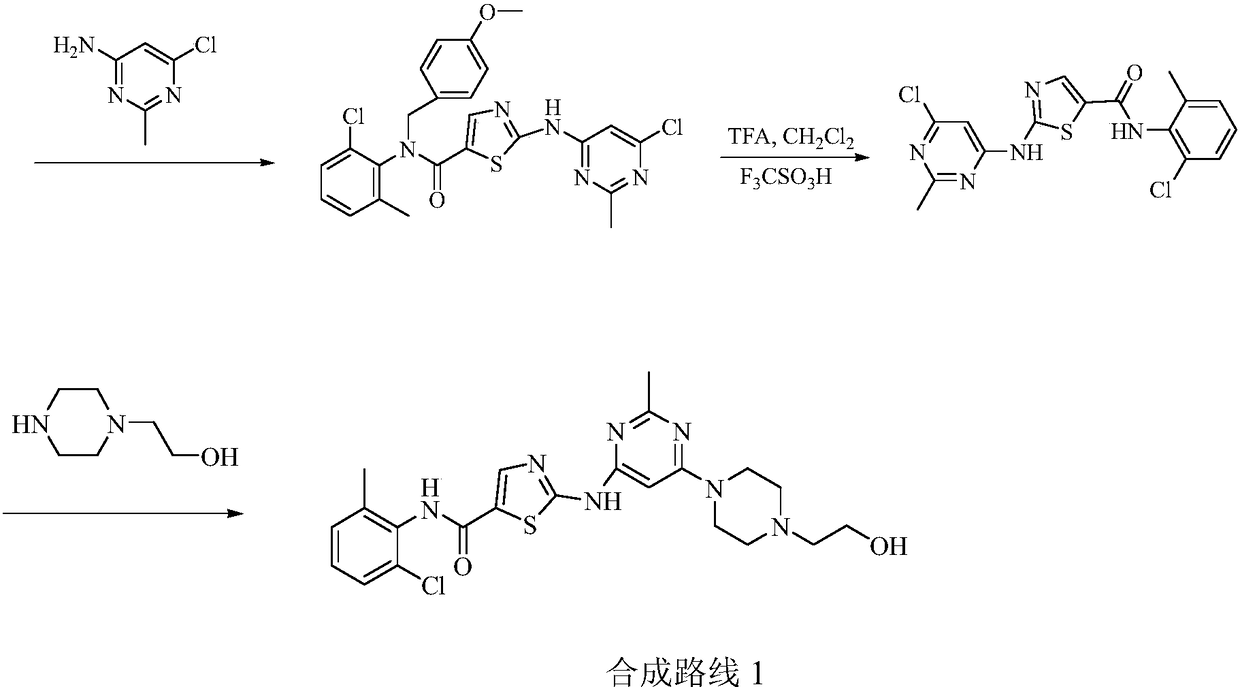
Preparation method of Dasatinib
The invention provides a preparation method of Dasatinib. 2-Bromothiazole-5-formic acid and 2-methyl-4-amino-6-cloro pyridine are adopted as raw materials, a first-time substitution reaction is performed, the raw materials and 4-(2-acetyl oxyl) Ethylpiperazine are subjected to a second-time substitution reaction to prepare 2-[[6-[4-(2-acetyloxy ethyl)-1-piperazinyl]-2-methl-4-pyrimidyl]amino]-5-Febuxostat; then, the product and an acylating chlorination reagent are subjected to an acylating chlorination reaction and subjected to an amidation reaction with 2-cholo-6-methylaniline, and finally ahydrolysis reaction is performed to remove acetyl to prepare dasatinib. The raw materials are cheap and easy to obtain and low in cost; the technological process is simple, operation is safe and easy, technological wastewater generation amount is small, and the method is environmentally friendly; raw materials and intermediate products stability is suitable, the reaction activity and selectivityare high, reaction conditions are easy to obtain, side reactions are few, the manufactured dasatinib contains few impurities, the purity and yield are high, and industrial production of dasatinib is facilitated.
Owner:XINFA PHARMA
Method for producing fermentative cordycep fungal powder and cordyceps polysaccharide powder through fermentation technology
InactiveCN104620844AHigh technical contentStrong competitivenessFungiCultivating equipmentsEnzyme methodThallus
The invention relates to a method for producing fermentative cordycep fungal powder and cordyceps polysaccharide powder through the fermentation technology. The method comprises the steps that inoculation is carried out on cordyceps sinensis pure bacterial strain culture on solid synthetic medium or liquid synthetic medium liquid fermentation equipment, fermental cultivation is carried out on the cordyceps sinensis pure bacterial strain culture and the media, drying and cell smashing are carried out on thalluses after the media are separated from the growing thalluses, and the fermentative cordycep fungal powder is made; then the obtained fermentative cordycep fungal powder is used as a raw material, and active immunization cordyceps polysaccharide freeze-dried powder is made through the organic solvent precipitation method, the compound enzyme method, the adsorption method and other working procedures. According to the technical scheme, the method is of significance in releasing contradiction between supply and demand of traditional Chinese medicinal materials, preserving the ecological environment, achieving valuable and rare wild medicinal material manual cultivation, producing high value-added Chinese herbal medicine products and the like.
Owner:孙悦迎
Synthesis method of diphenylcarbinol decanoate
InactiveCN102108050AStable structureWide range of usesOrganic compound preparationCarboxylic acid esters preparationDiphenylmethanolAlcohol
The invention discloses a synthesis method of diphenylcarbinol decanoate. The synthesis method is characterized in that esterification is carried out on capric acid and diphenylcarbinol which are utilized as raw materials at the atmosphere of nitrogen in the presence of an organic or inorganic tin catalyst, then dealcoholization is carried out after the esterification is completed, and the diphenylcarbinol decanoate is obtained after filtration, wherein the weight of the organic or inorganic tin catalyst is 0.05%-0.8% of that of the added diphenylcarbinol. The synthesis method has the characteristics of one-step esterification and simple process and equipment; and the recovered alcohol can be used as a solvent.
Owner:XUZHOU B&C CHEM CO LTD +1
Preparation method of kresoxim-methyl
ActiveCN112409206AAvoid hydrolysisImprove stabilityOrganic compound preparationCarboxylic acid esters preparationGrignard reagentGrignard reaction
The invention provides a preparation method of kresoxim-methyl, which comprises the following steps: carrying out an etherification reaction on 2-halogenated methyl halogenated benzene and 2-methylphenol to prepare 2 -(2-methylphenoxy methyl) halogenated benzene, and carrying out a Grignard reaction on the 2-(2-methylphenoxy methyl) halogenated benzene and magnesium metal to prepare reaction liquid containing a Grignard reagent; dropwise adding the obtained reaction liquid containing the Grignard reagent into dimethyl oxalate to prepare 2-(2-methyl phenoxy methyl) phenyl methyl oxalate, and finally performing condensation reaction with methoxylamine salt to prepare kresoxim-methyl. The method has the advantages of cheap and accessible raw materials and low cost; the method has the advantages of short process flow, easy realization of reaction conditions, safe, simple and convenient operation, less process wastewater generation amount, greenness and environmental protection, and can prepare kresoxim-methyl only through three steps of reactions; raw materials and intermediate products are high in stability, high in reaction activity and selectivity and less in side reaction; and theobtained kresoxim-methyl has few impurities and high purity and yield, and is beneficial to industrial production of kresoxim-methyl.
Owner:XINFA PHARMA
A kind of preparation method of cephalosporin anti-infection medicine
ActiveCN105017286BSimple preparation processReduce generationOrganic chemistry7-ACACefazedone sodium
The invention relates to a preparation method for a cephalosporin anti-infective drug-cefazedone sodium, belonging to the field of pharmaceutical synthesis. According to the invention, the method uses GCLE as a raw material to substitute 7-ACA and overcomes the defects of low yield, high pollution and the like in prior art; the preparation method with mild reaction conditions, little side reaction and simple process is provided; meanwhile, the method has the advantages of cheap and easily-available raw materials, low cost, high product yield, high product purity and applicability to industrial production.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD
Fasudil impurity I preparation method
The invention discloses a fasudil impurity I preparation method. A fasudil impurity I (shown in a formula I) is an oxidization product of fasudil, is one of important impurities for fasudil medicine or preparations of the fasudil medicine, can be applied to toxicological and pharmacological researches for absorption, metabolism and the like of the fasudil in the body and also can be applied to researches for aspects of stability, quality control and the like of the fasudil preparations. The invention discloses a method which can be used for preparing the fasudil impurity I in high efficiency.According to the method, the fasudil or salt of the fasudil reacts with acyl peroxide under the alkali action, and generated intermediates are hydrolyzed to prepare the fasudil impurity I. According to the fasudil impurity I preparation method disclosed by the invention, cheap reactants and a dedicated reaction method are utilized, and a total yield of products can be improved to 90% or more.The formula I is shown in the description.
Owner:深圳市祥根生物科技有限公司
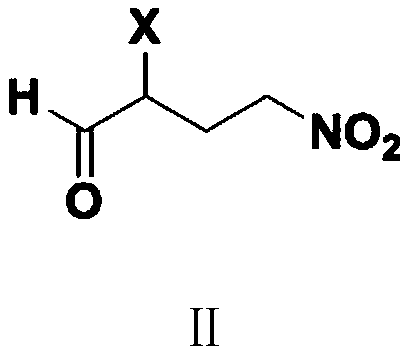
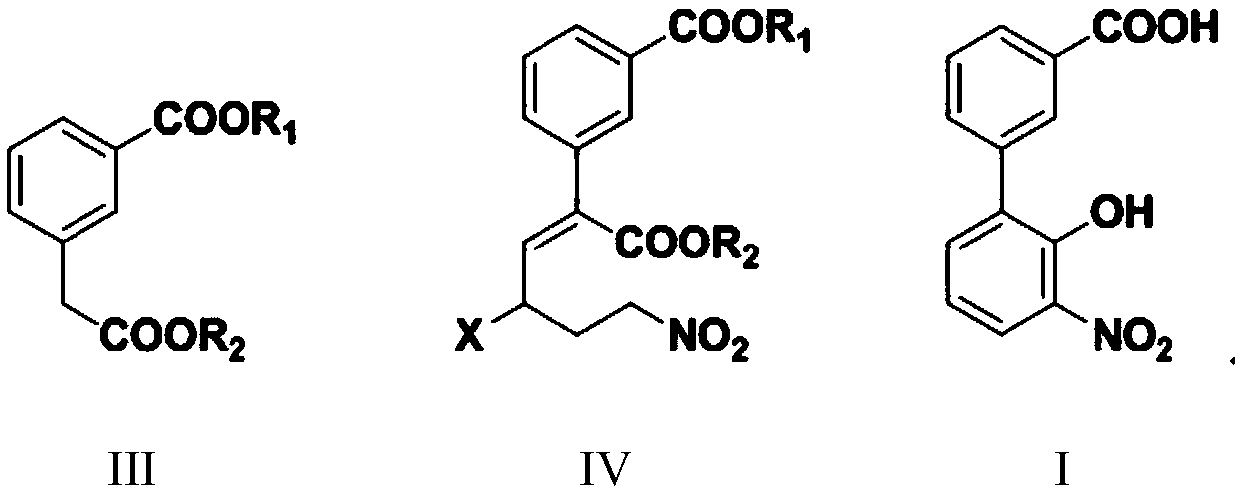
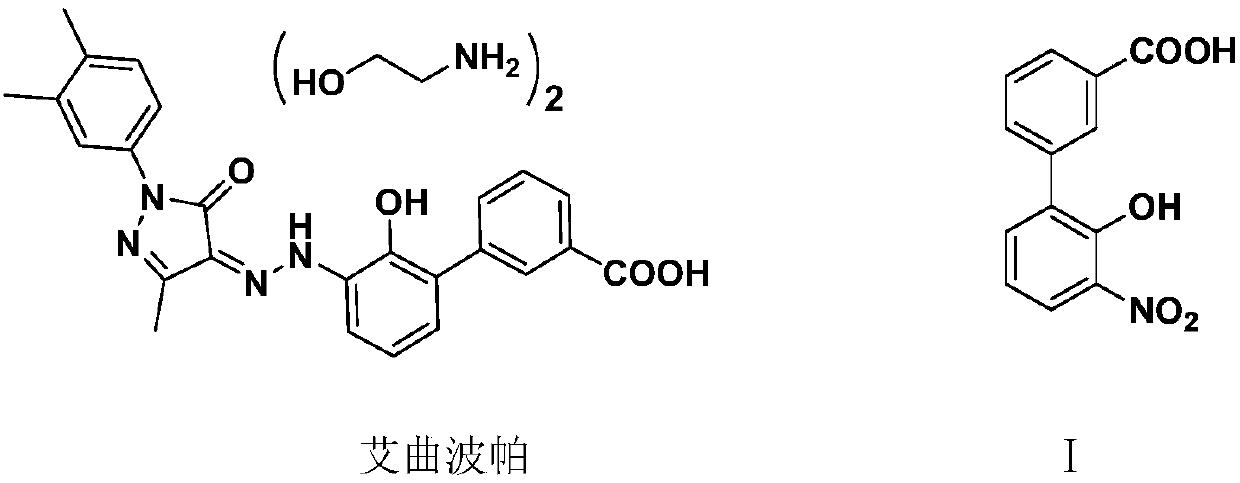
Preparation method of 3'-nitro-2'-hydroxybiphenyl-3-formic acid
ActiveCN110467531AReduce productionLow costOrganic chemistryOrganic compound preparationAcroleinWastewater
The invention provides a preparation method of 3'-nitro-2'-hydroxybiphenyl-3-formic acid. The method uses 2-halogenated acrolein and nitromethane as starting raw materials, obtains 2-halo-4-nitro-n-butyraldehyde through an addition reaction, obtains 2-(3-alkoxycarbonyl)phenyl-4-halo-6-nitro-n-hexa-2-enoate through dehydration and condensation with 3-alkoxycarbonyl phenylacetate, obtains 3'-nitro-2'-hydroxybiphenyl-3-formate through a cyclisation reaction and a hydrolysis reaction, and obtains the 3'-nitro-2'-hydroxybiphenyl-3-formic acid through acidification. The method of the invention has the advantages of cheap and easily available raw materials, simple and short process flow, low cost, less waste acid and wastewater production amount, environment protection, good reaction selectivity,less side reactions and high product yield and purity, and is suitable for large-scale production.
Owner:XINFA PHARMA
Method for preparing cholesterol by microbial conversion method
The invention discloses a method for preparing cholesterol by a microbial conversion method. The method is characterized in that cholesterol is prepared from raw material lanolin completely by microbial conversion through the steps of: 1) crushing raw material and adding a carbon source and a nitrogen source to prepare a seed medium and a fermentation medium; 2) screening strains; 3) inoculating the screened strain and then stirring and fermenting; and 4) separating, purifying and crystallizing. The method has the characteristics of mild conditions, specific reaction, small pollution and the like in preparing cholesterol. The cholesterol prepared by the method has high purity and yield, and conforms to the concept of environment protection.
Owner:HENAN LIWEI BIOLOGICAL PHARMA
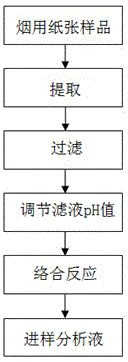
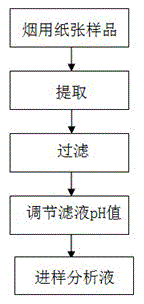
A pretreatment method for HPLC-ICP/MS analysis of chromium content in cigarette paper
ActiveCN103728397BAvoid cumbersome steps such as reactionsSpecific responseComponent separationChromatographic separationPretreatment method
The invention belongs to the technical field of tobacco analysis and detection and discloses a pretreatment method for analyzing the content of chrome in cigarette paper by HPLC-ICP / MS. The cigarette paper sample is subjected to shaking extraction by using a NaOH solution, the extraction solution is filtered by an aqueous filter membrane to obtain an initial filter solution, the pH value of the initial filter solution is adjusted by using an HCl solution, and finally the obtained filter solution is used as the analysis solution of the hexavalent chrome; or after the pH value of the initial filter solution is adjusted, the adjusted filter solution is allowed to complex with EDTA solution, and finally the complex reaction solution is used as the analysis solution of the trivalent chrome and the hexavalent chrome in the cigarette paper by HPLC-ICP / MS. The invention provides the chromatographic separation pretreatment method for the separation and measurement process of the trivalent chrome and the hexavalent chrome in the cigarette paper by using the high performance lquid chromatography (HPLC)-inductively coupled plasma mass spectrometry(ICP / MS). The pretreatment method has the advantages of convenience in operation, specific reaction and easiness in control, and provides powerful technical basis for measurement of the trivalent chrome and the hexavalent chrome in bulk samples.
Owner:CHINA TOBACCO GUANGDONG IND
Synthesis method of pyridine derivative 2-tert-butoxy-6-methylene chloropyridine
InactiveCN104496891AFew reaction stepsShort reaction timeOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsOrganometallic catalysisSynthesis methods
The invention discloses a synthesis method of a pyridine derivative 2-tert-butoxy-6-methylene chloropyridine. The synthesis method of the pyridine derivative 2-tert-butoxy-6-methylene chloropyridine comprises the following steps: carrying out reaction on low-cost 2-bromo-6-tert-butoxy pyridine taken as a raw material as well as n-butyl lithium and DMF (dimethyl formamide) sequentially in presence of N2 and at low temperature, thus preparing a pyridine aldehyde compound; reducing aldehyde to obtain alcohol in a corresponding structure by utilizing sodium borohydride or lithium aluminium hydride as a reducing agent; and replacing hydroxyl in alcohol by utilizing a chlorine atom, thus obtaining a pyridine derivative containing methylene chloride and tert-butoxy groups with relatively high activity. The synthesis method of the pyridine derivative 2-tert-butoxy-6-methylene chloropyridine has the advantages that overall reaction steps are less, the reaction time is short, and the yield is high; and the prepared 2-tert-butoxy-6-methylene chloropyridine is easy to coordinate with a metal atom or other atoms and can be taken as a pyridine derivative ligand to be coordinated with metal, so as to prepare an organic metal catalyst or be applied to synthesis of a pyridine derivative drug.
Owner:HARBIN INST OF TECH
A kind of preparation method of 5,6-dihydropyridin-2(1h)-one derivative
The invention provides a preparation method of 5,6-dihydropyridine-2(1H)-one derivatives, specifically 1-(piperidin-2-one-1-yl)-4-(5,6-di The preparation method of hydrogen-3-R substituent pyridine-2(1H)-one-1-yl)benzene, the R substituent is chlorine or morpholin-4-yl. The present invention uses p-acetamidoaniline as a raw material, undergoes amidation with δ-valerolactone, halogenates with a halogenating agent or sulfonylates with sulfonyl chloride, condenses, deacetylates, and then reacts with 2,2-dichloro-δ - Amidation of valerolactone, halogenation with halogenating reagents or sulfonylation with sulfonyl chloride, followed by condensation elimination or condensation elimination substitution in the presence of morpholine to prepare the target product. The raw materials used in the preparation method of the invention are cheap and easy to obtain, and the cost is low; the process is simple and easy to operate, the reaction conditions are easy to realize, the amount of waste water generated is small, and it is safe and green; the reaction selectivity of each step is high, the product yield and purity are high, and it is beneficial to industrial production.
Owner:XINFA PHARMA
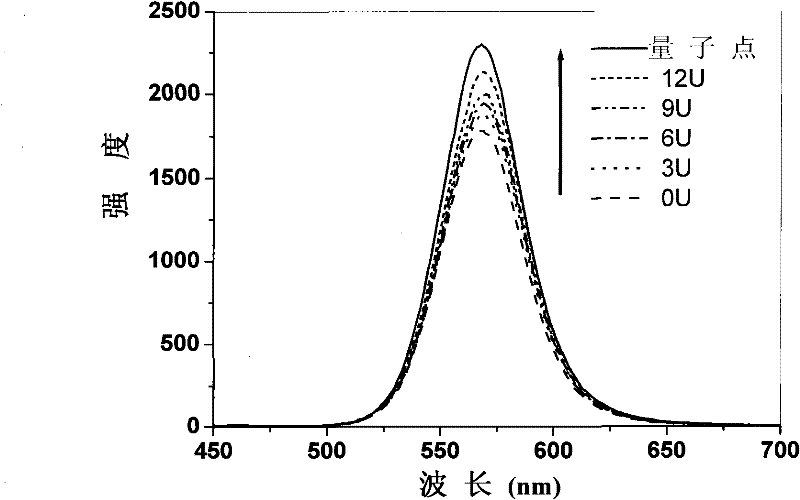
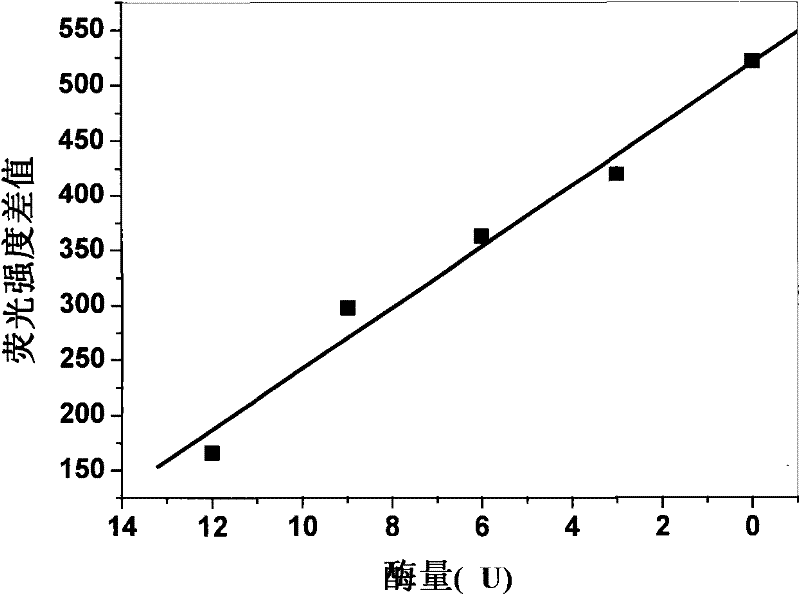
Method for detecting enzymatic activity by using quantum dot fluorescence
InactiveCN102081040BGood biocompatibilityHigh affinityFluorescence/phosphorescenceQuantum dotWater soluble
The invention belongs to the field of biological detection, in particular relates to a method for detecting enzymatic activity by using quantum dot fluorescence. The method provided by the invention is used for detecting the enzymatic activity through measuring the influence of a reaction system to water-soluble quantum dot fluorescence property in the enzyme reaction process. The method concretely comprises the following steps of: adding a certain quantity of enzymes to be detected in a reaction system containing water-soluble quantum dots or further in a reaction system containing coenzymes, enabling the fluorescence intensity of the water-soluble quantum dots to be varied due to the reaction of the enzymes to be detected, and realizing the high selective quantitative detection on the activity of the enzymes to be detected through the variable quantity of the fluorescence intensity of the water-soluble quantum dots. The method provided by the invention is simple in operation, rapid to detect and low in cost and can be used for detecting the activity of various physiological enzymes relevant to disease diagnosis.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
Preparation method of Dapagliflozin isomer impurities I
ActiveCN108314613ASpecific responseEasy to operateOrganic compound preparationCarbonyl compound preparation by condensationSolventCompound c
The invention discloses a preparation method of Dapagliflozin isomer impurities I. The preparation method comprises the following steps that a, 2-chlorine-5-bromobenzoic acid is dissolved in a first solvent; an acylation reagent is added; reaction is performed for 1 to 8h at 20 to 60 DEG C to generate a compound A; b, methoxyl methylamine hydrochloride is dissolved in a second solvent; alkali is added at -10 to 10 DEG C; then, the compound A capable of being dissolved in the second solvent is dropwise added; the temperature is maintained; stirring reaction is performed for 1 to 4h to obtain acompound B; c, a compound C is dissolved in a third solvent; a hexane solution of n-butyllithium is added at -78 DEG C; then, the compound B capable of being dissolved in the third solvent is dropwiseadded; after reaction, treatment is performed to obtain the Dapagliflozin isomer impurities I. The carbonyl substitution reaction is used, so that the reaction is exclusive; by-products are few; theoperation is simple; the process is stable; the repeatability is high; the total yield of the product is improved by 90 percent or higher; the cost is reduced; the commercialized production and the batch supply are facilitated.
Owner:SHENZHEN SUNGENING BIO-MEDICAL CO LTD
Total bilirubin determination reagent kit
ActiveCN101144824BStrong interference abilityLong storage timeBiological testingAcetic acidAnti jamming
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com