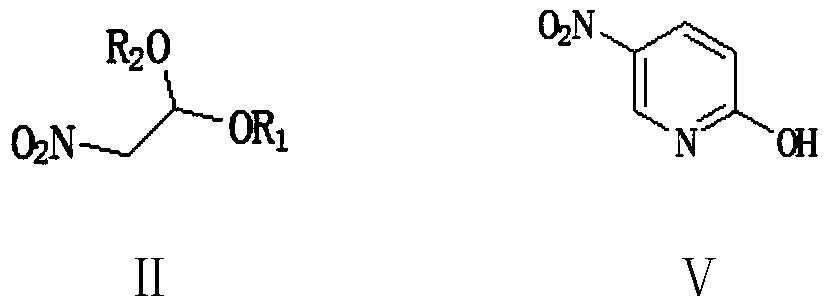Preparation method of 2-chloro-5-nitropyridine
A nitropyridine and alkyl technology, which is applied in the field of preparation of 2-chloro-5-nitropyridine, can solve problems such as large waste water, unfavorable production scale-up and safe operation, and not meeting green production requirements.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Embodiment 1: Preparation of 2-hydroxyl-5-nitropyridine (Ⅴ) (approach 1 of step (1))
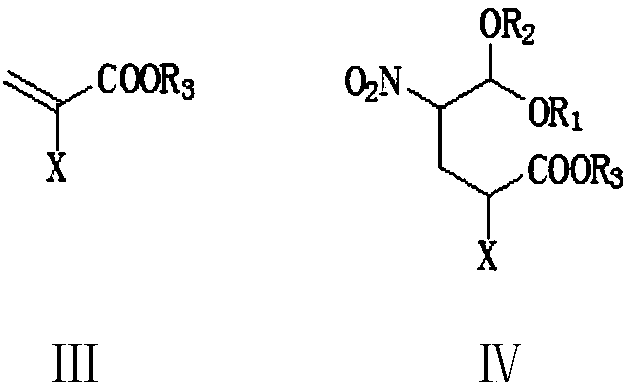
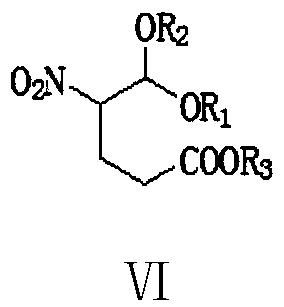
[0074] To a 500 ml four-necked flask connected with stirring, a thermometer, and a reflux condenser, add 200 g of dichloromethane, 27.0 g (0.2 moles) of 2-nitroacetaldehyde dimethyl acetal (II1), 0.5 g of piperidine, 25.3 gram (0.21 moles) of methyl 2-chloroacrylate (Ⅲ1), stirred and reacted at 40-45°C for 3 hours, lowered the reaction solution to 25°C, added 5.0 grams of ammonium chloride, 100 grams of 17% ammonia water, 35-40°C The reaction was stirred for 5 hours. Add 100 grams of water, recover dichloromethane under reduced pressure, cool to 20-25°C, filter, wash once with 20 grams of water, and dry to obtain 25.8 grams of 2-hydroxy-5-nitropyridine with a liquid phase purity of 99.6% , yield 92.1%.
Embodiment 2
[0075] Embodiment 2: the preparation of 2-hydroxyl-5-nitropyridine (Ⅴ) (way 1 of step (1))
[0076] In a 500 ml four-necked flask connected with stirring, a thermometer and a reflux condenser, add 200 grams of tetrahydrofuran, 26.6 grams (0.2 moles) of 2-nitroacetaldehyde ethylene acetal (II2), 0.5 grams of DBU, 34.7 grams ( 0.21 moles) methyl 2-bromoacrylate (Ⅲ2), stirred and reacted at 40-45°C for 3 hours, lowered the reaction solution to 25°C, added 3.5 grams of ammonium chloride, 100 grams of 17% ammonia in methanol solution, 50-55 The reaction was stirred at °C for 3 hours. Add 150 grams of water, recover tetrahydrofuran and methanol under reduced pressure, cool to 20-25°C, filter, wash once with 20 grams of water, and dry to obtain 26.2 grams of 2-hydroxy-5-nitropyridine, with a liquid phase purity of 99.5% , yield 93.6%.
Embodiment 3
[0077] Embodiment 3: Preparation of 2-hydroxyl-5-nitropyridine (Ⅴ) (way 1 of step (1))
[0078] To a 500 ml four-necked flask connected with stirring, a thermometer, and a reflux condenser, add 200 g of 1,2-dichloroethane, 27.0 g (0.2 moles) of 2-nitroacetaldehyde dimethyl acetal (II1), 0.5 Kepiperidine, 28.2 grams (0.21 moles) of ethyl 2-chloroacrylate (Ⅲ3), stirred and reacted at 55-60°C for 2 hours, lowered the reaction solution to 25°C, added 3.5 grams of ammonium chloride, 100 grams of 17% ammonia water , Stir the reaction at 50-55°C for 3 hours. Add 100 grams of water, recover 1,2-dichloroethane under reduced pressure, cool to 20-25°C, filter, wash once with 20 grams of water, and dry to obtain 25.3 grams of 2-hydroxy-5-nitropyridine, liquid The phase purity was 99.2%, and the yield was 90.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com