Novel rsv RNA molecules and compositions for vaccination
a technology of rsv and rna, which is applied in the field of new rsv rna molecules and compositions for vaccination, can solve the problems of unlicensed human rsv vaccine, too expensive and impractical for universal use, and the use of dna as a vaccine may be dangerous
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
on of DNA and mRNA Constructs and Compositions for In Vitro and In Vivo Experiments
[0818]The present Example provides methods of obtaining the artificial RNA of the invention as well as methods of generating a composition or a vaccine of the invention.
[0819]1.1. Preparation of DNA and mRNA constructs:
[0820]For the present examples, DNA sequences encoding different RSV F proteins (e.g. F0, F-del, F-del_DSCav1, F-del_DSCav1_mut5, etc.) were prepared and used for subsequent RNA in vitro transcription reactions. Said DNA sequences were prepared by modifying the wild type encoding DNA sequences by introducing a G / C optimized coding sequence (e.g., “cds opt1”) for stabilization. Sequences were introduced into a pUC19 derived vector to comprise stabilizing 3′-UTR sequences derived from a 3′-UTR of a gene selected from PSMB3, ALB7, alpha-globin, CASP1, COX6B1, GNAS, NDUFA1 and RPS9 and 5′-UTR sequences derived from a 5′-UTR of a gene selected from HSD17B4, RPL32, ASAH1, ATP5A1, MP68, NDUFA4...
example 2
on of Cotton Rats with LNP-Formulated mRNA Encoding RSV-F and RSV Cotton Rat Challenge Study
[0833]The present Example shows that LNP-formulated mRNA encoding RSV-F induces strong and functional immune responses in cotton rats.
[0834]For the development of RSV vaccines the cotton rat is an accepted animal model, especially for the challenge infection. Cotton rats that have received formalin-inactivated RSV virus vaccine preparations respond to RSV infection with enhanced lung pathology. This allows the evaluation of the safety of a vaccination in terms of enhanced disease phenomenon.
[0835]To optimize the RSV-specific immune response, mRNA vaccines encoding the pre-fusion stabilized RSV F protein F-del_DSCav were prepared according to Example 1, formulated either with LNPs (see Example 1.4.) or with protamine (see Example 1.5.) and were applied on days 0 and 28 intramuscularly (i.m.) or intradermally (i.d) with different doses of RNA as shown in Table 10. Control animals received a sin...
example 3
on of Cotton Rats with LNP-Formulated mRNA Encoding RSV-F and RSV Cotton Rat Challenge Study
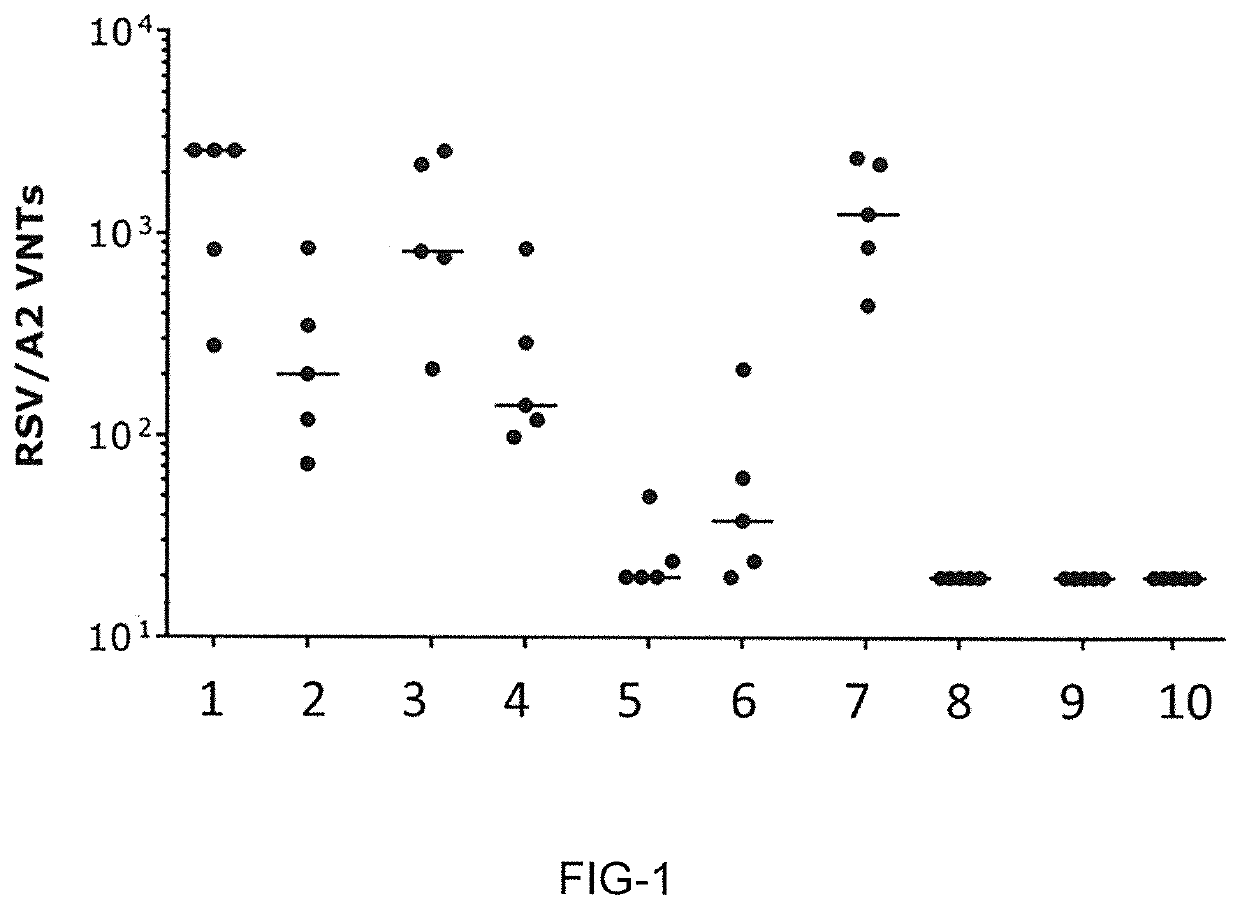
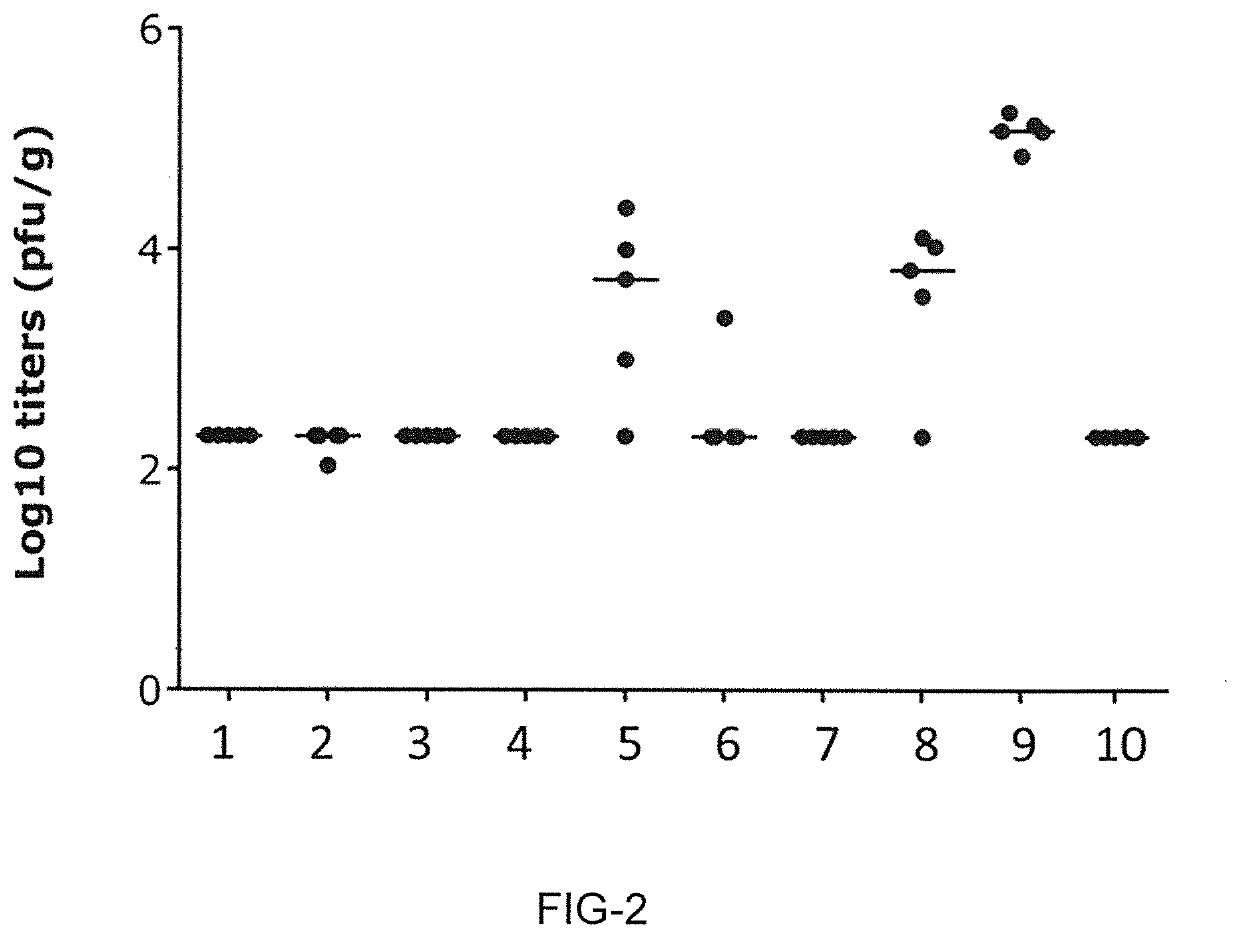
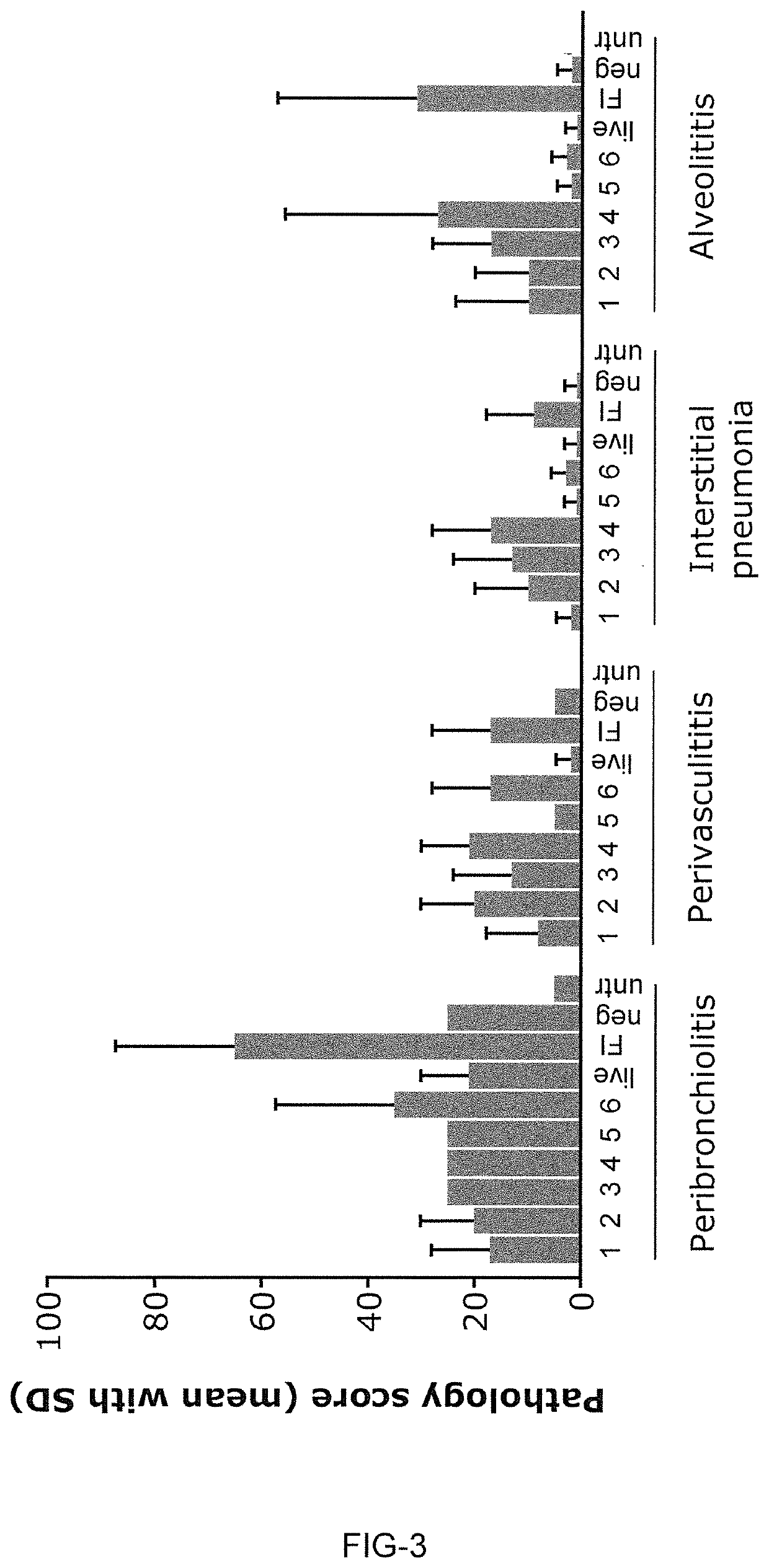
[0848]The present Example shows that LNP-formulated mRNA encoding different RSV-F antigens induce strong and functional immune responses in cotton rats for mRNA constructs having UTR combination RPL32 / ALB7 (i-2).
[0849]This experiment compared mRNA-LNPs encoding three different RSV-F variants: full-length RSV F0, truncated RSV F-del, and pre-fusion stabilized truncated F-del_DSCav1. The mRNA-LNP vaccines were prepared according to Example 1. Cotton rats received two intramuscular vaccinations with mRNA-LNP vaccines on days 0 and 28. Each dose comprised 10 μg or 100 μg mRNA-LNPs. Control animals received a single vaccination at day 0 with 105 pfu live RSV / A2 virus intranasally or two vaccinations at days 0 and 28 with formalin-inactivated RSV virus (FI RSV) intramuscularly. Additional control animals received buffer only (see Table 11).
TABLE 11Animal groups and vaccination schedule of Example 3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| nucleic acid | aaaaa | aaaaa |
| composition | aaaaa | aaaaa |
| structures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com