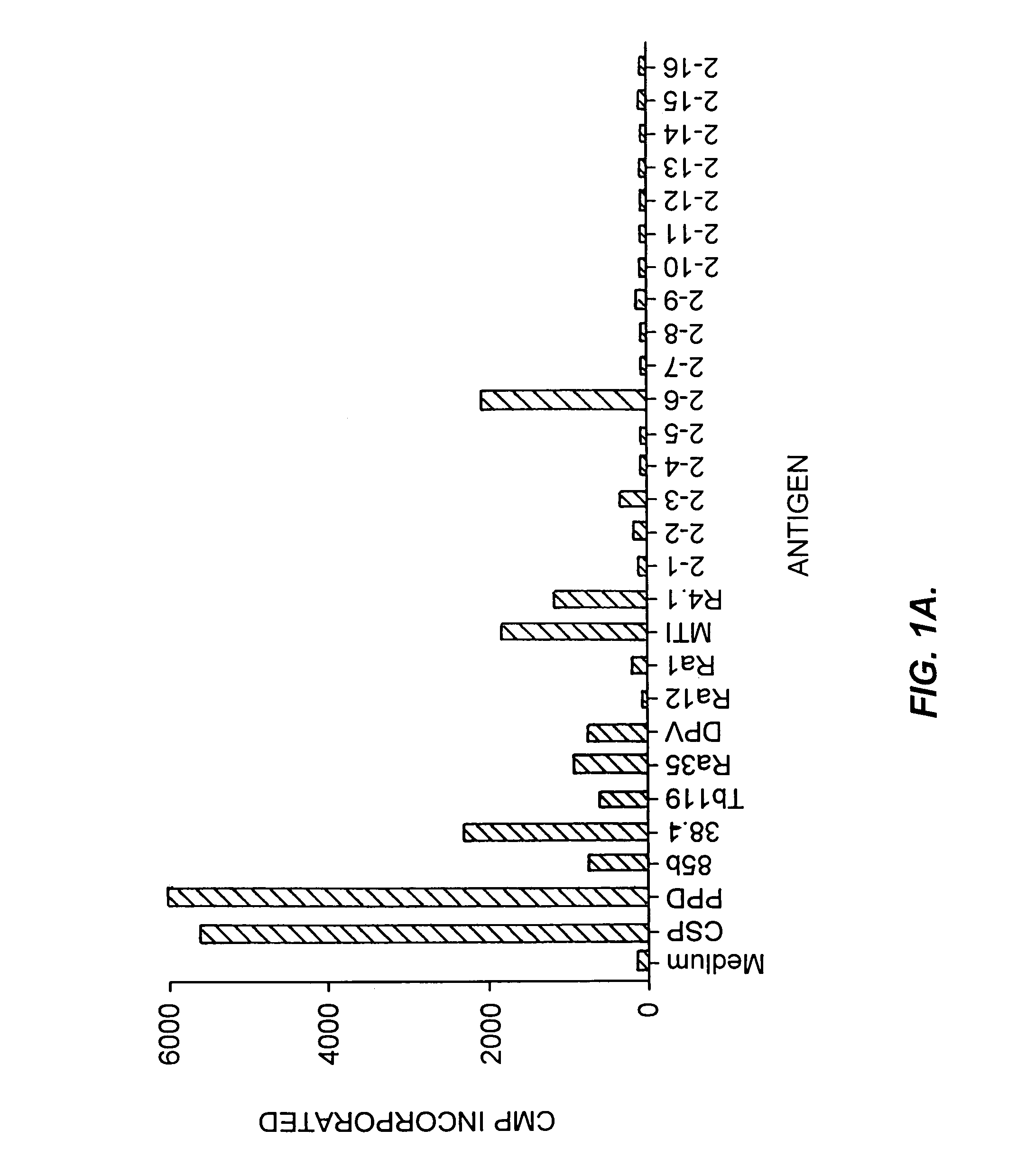
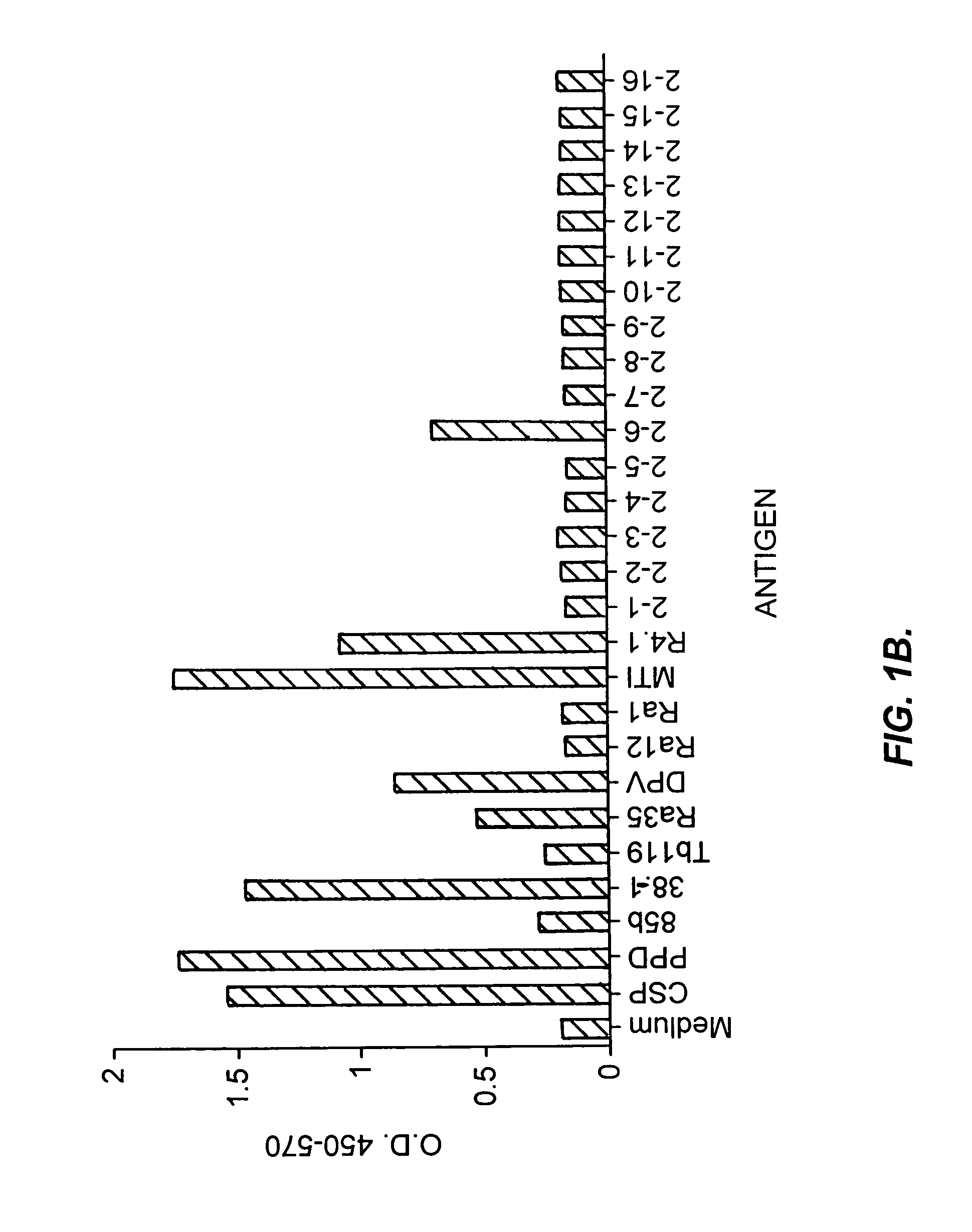
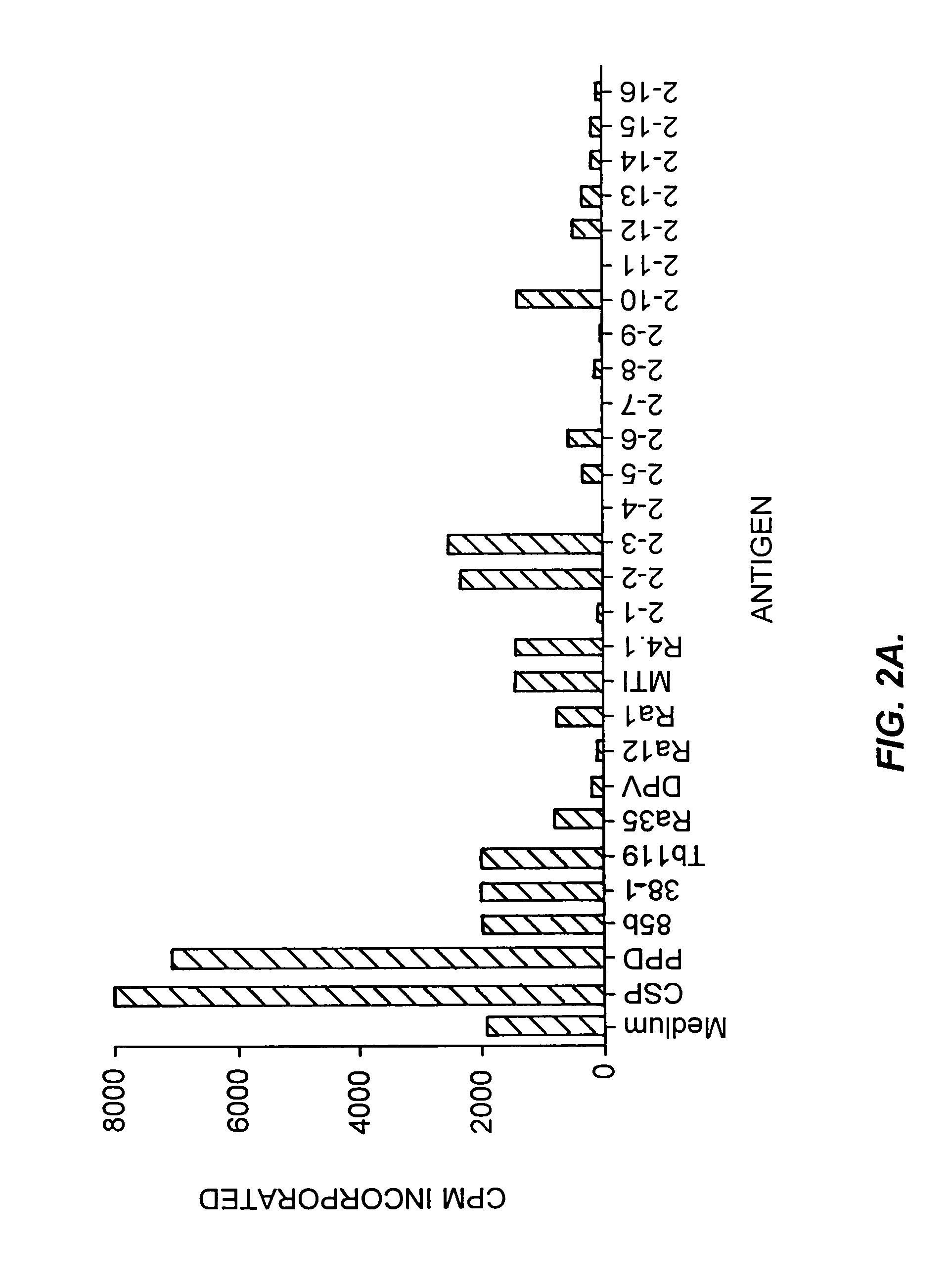
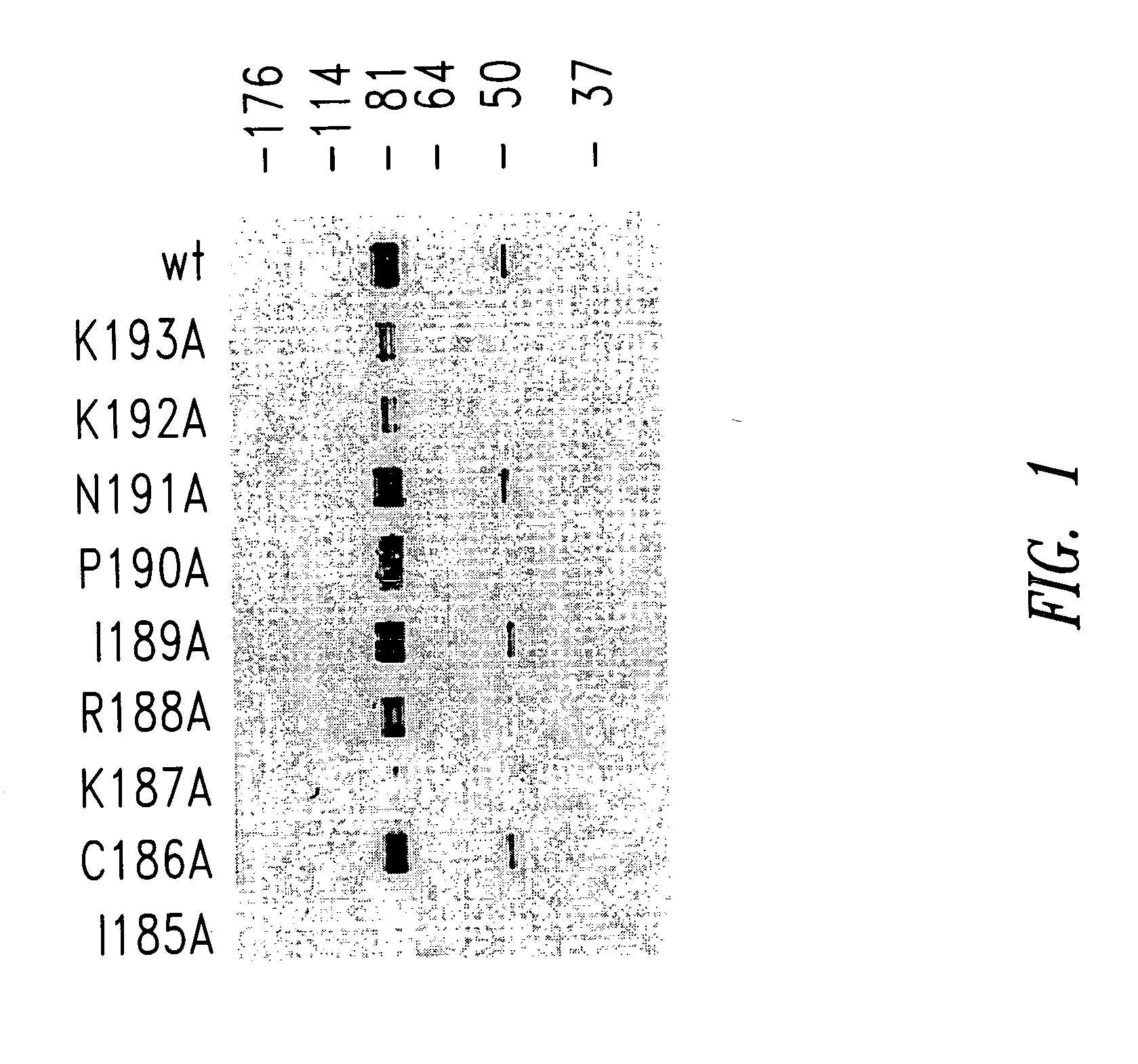
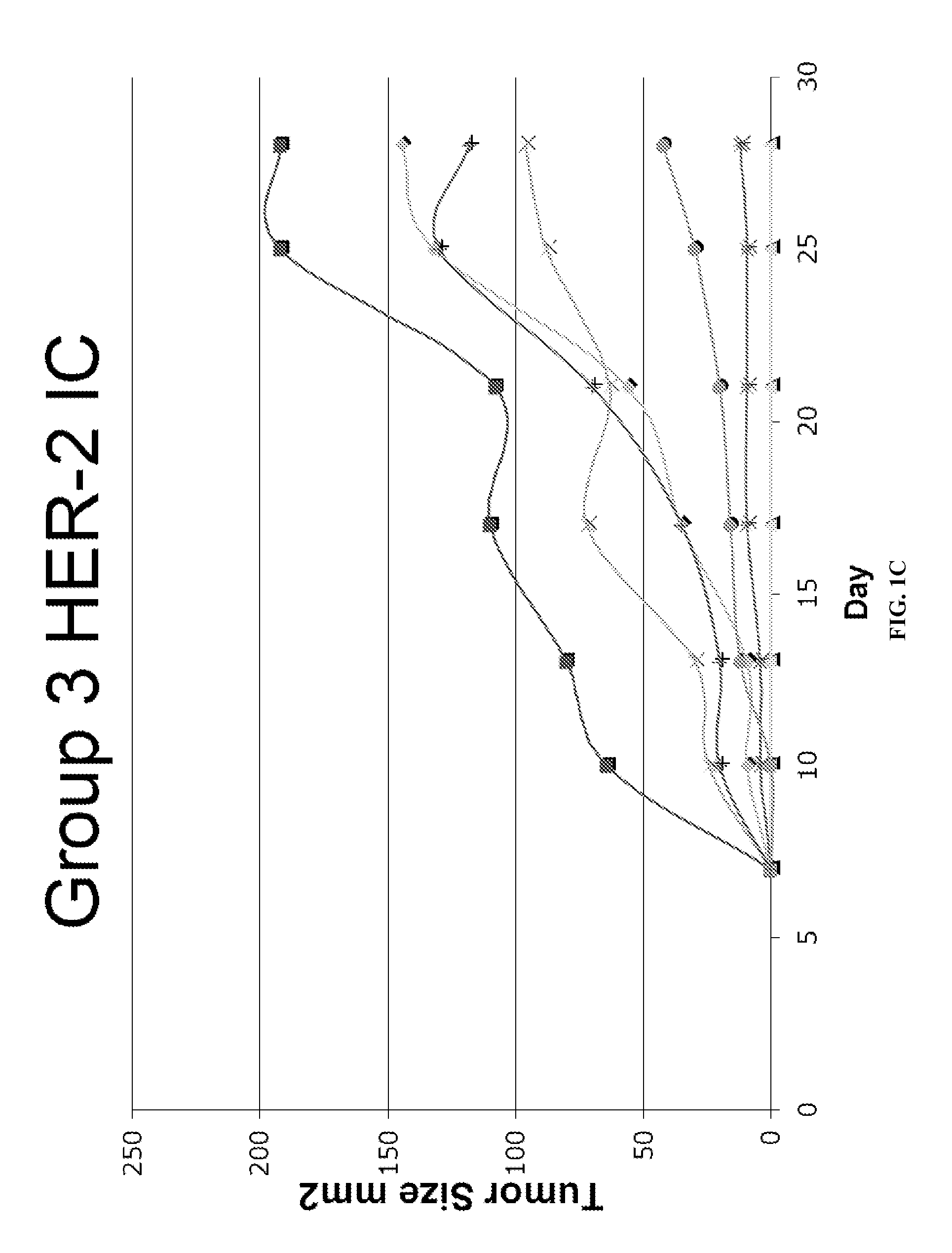
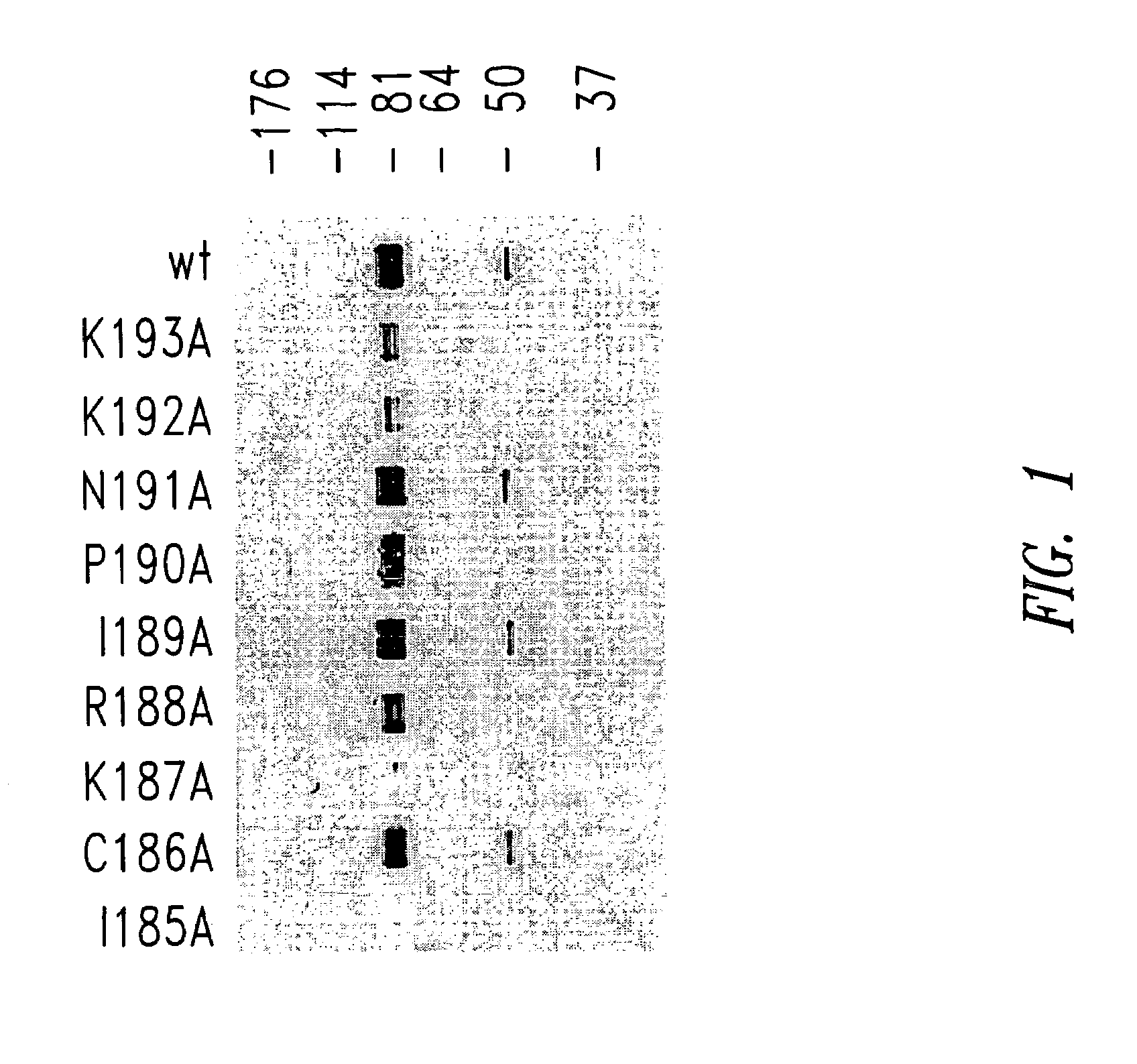
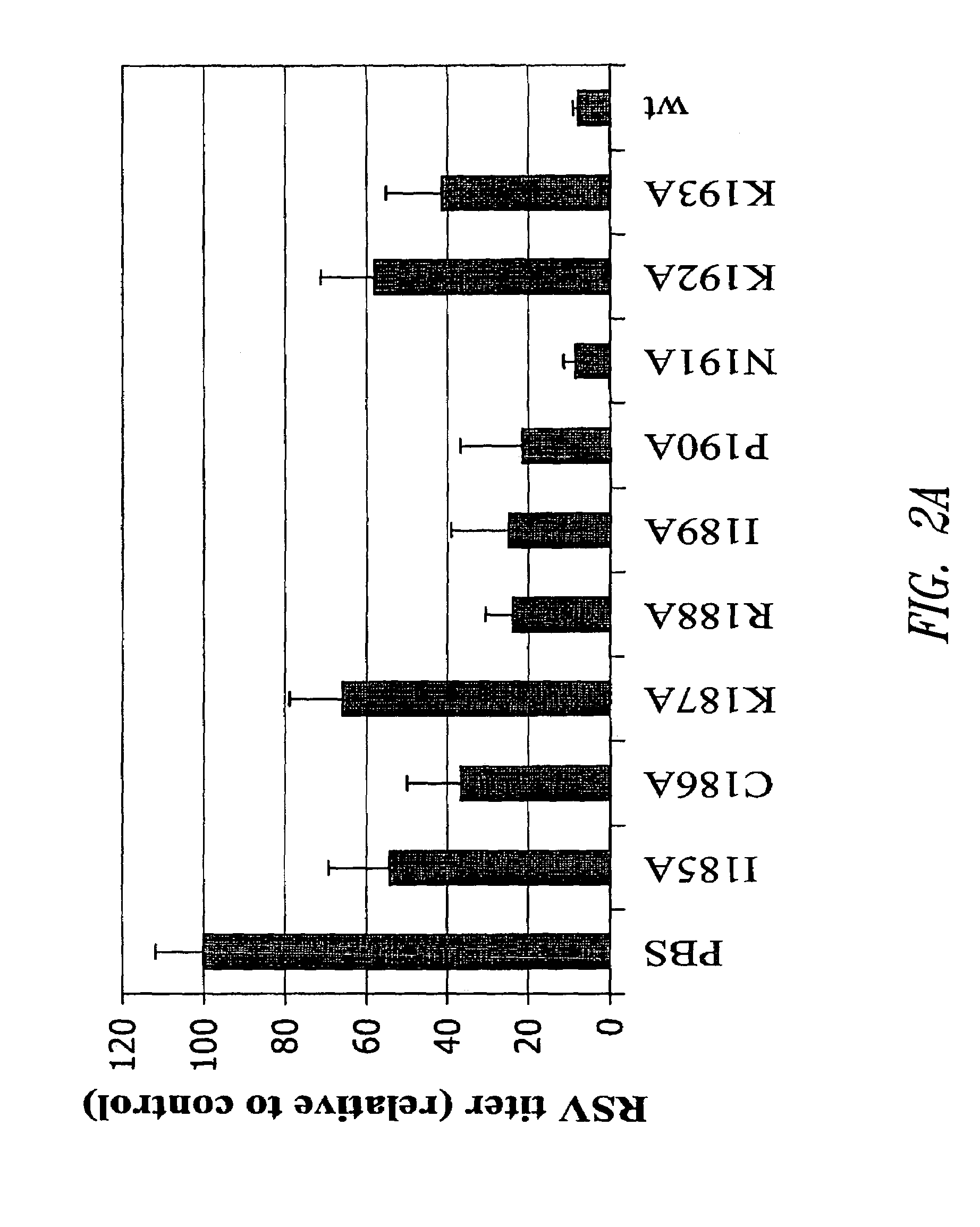
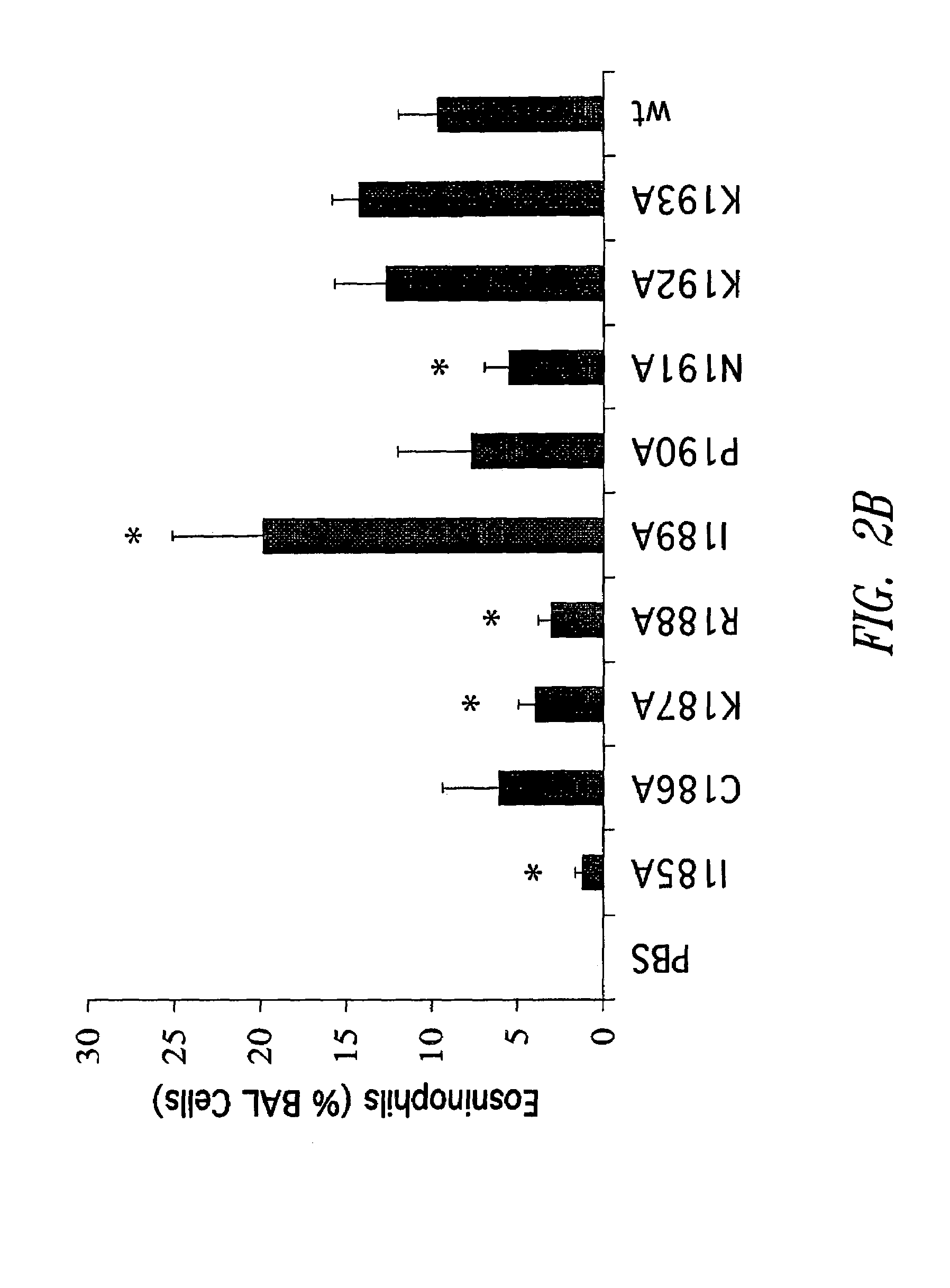
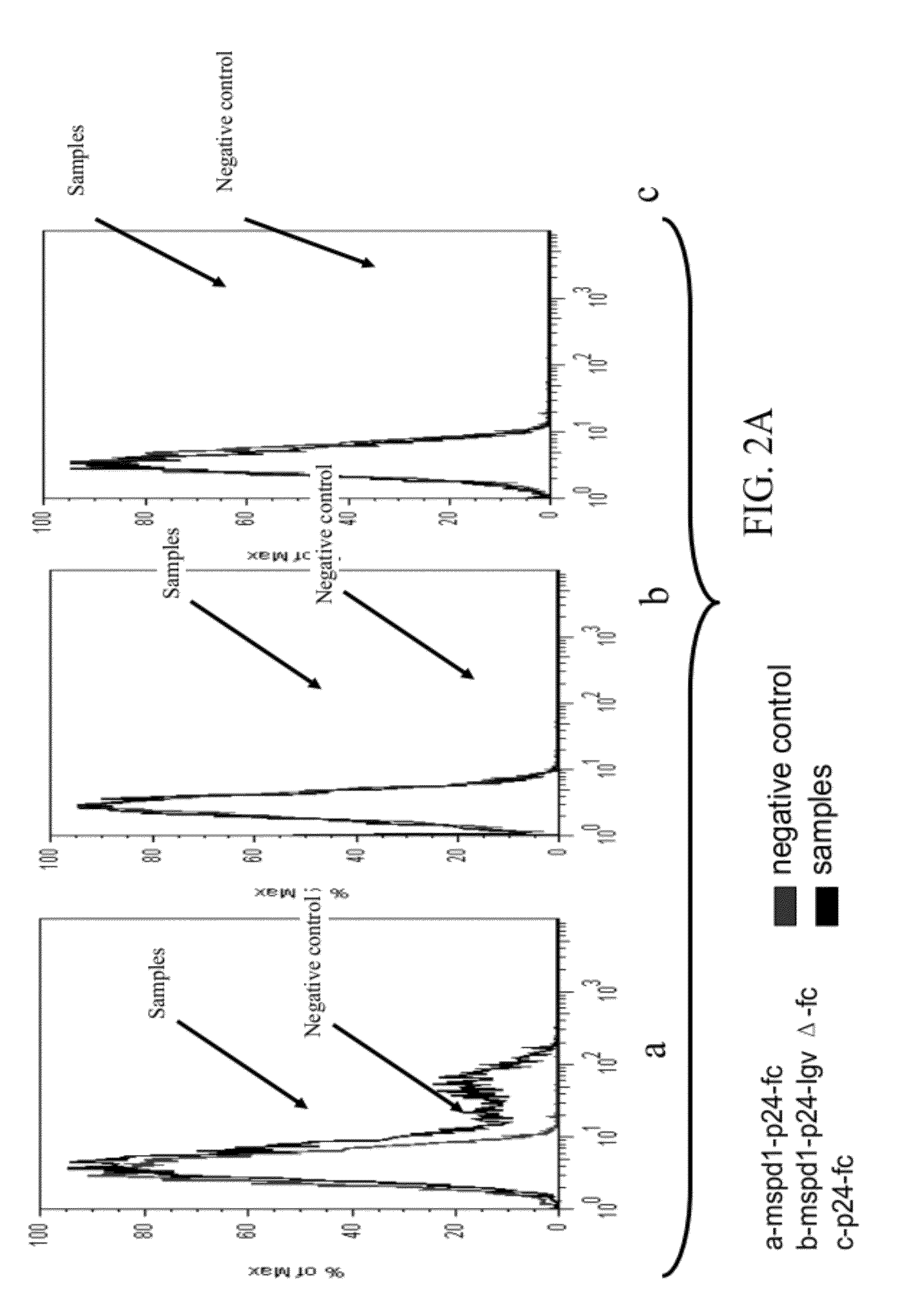
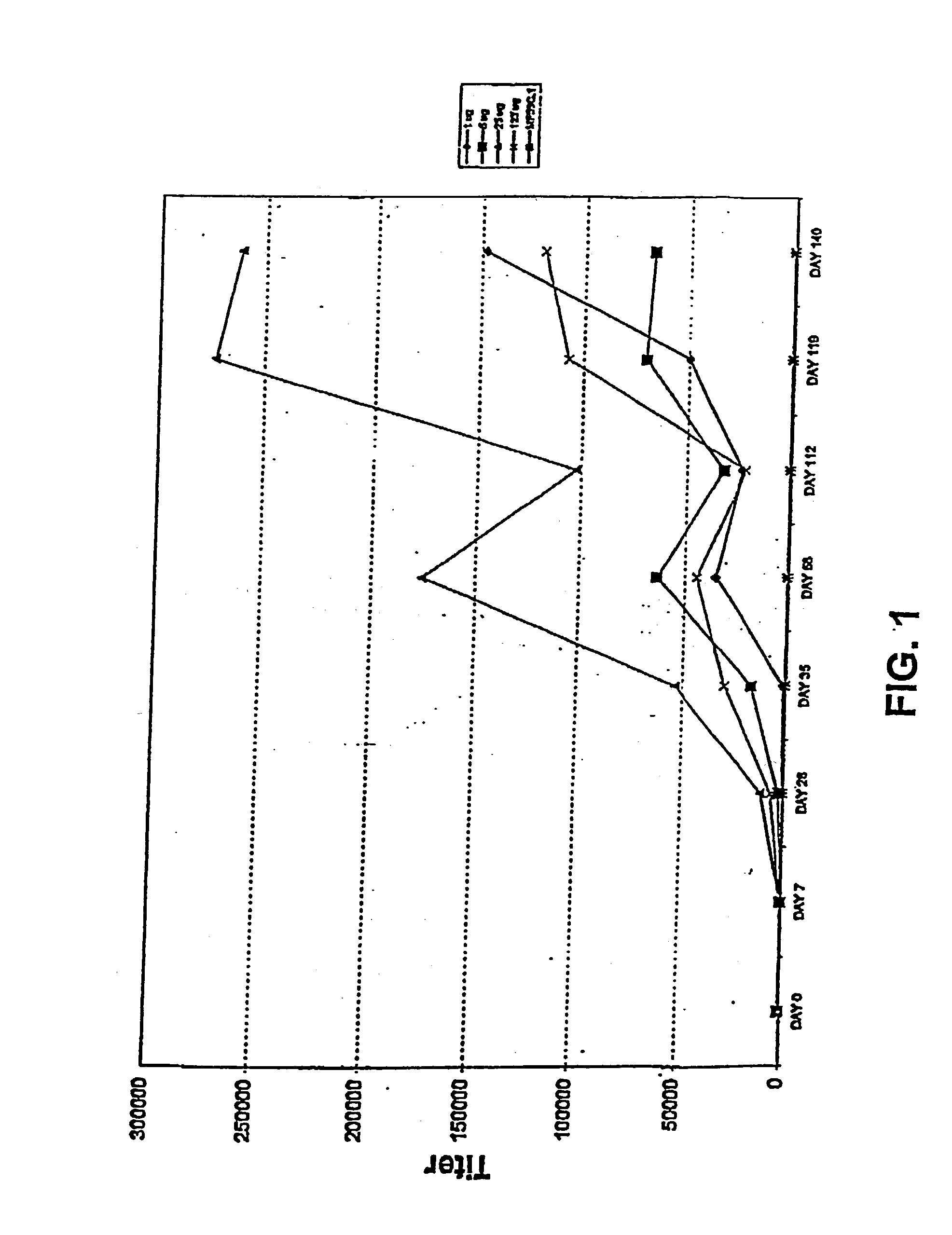
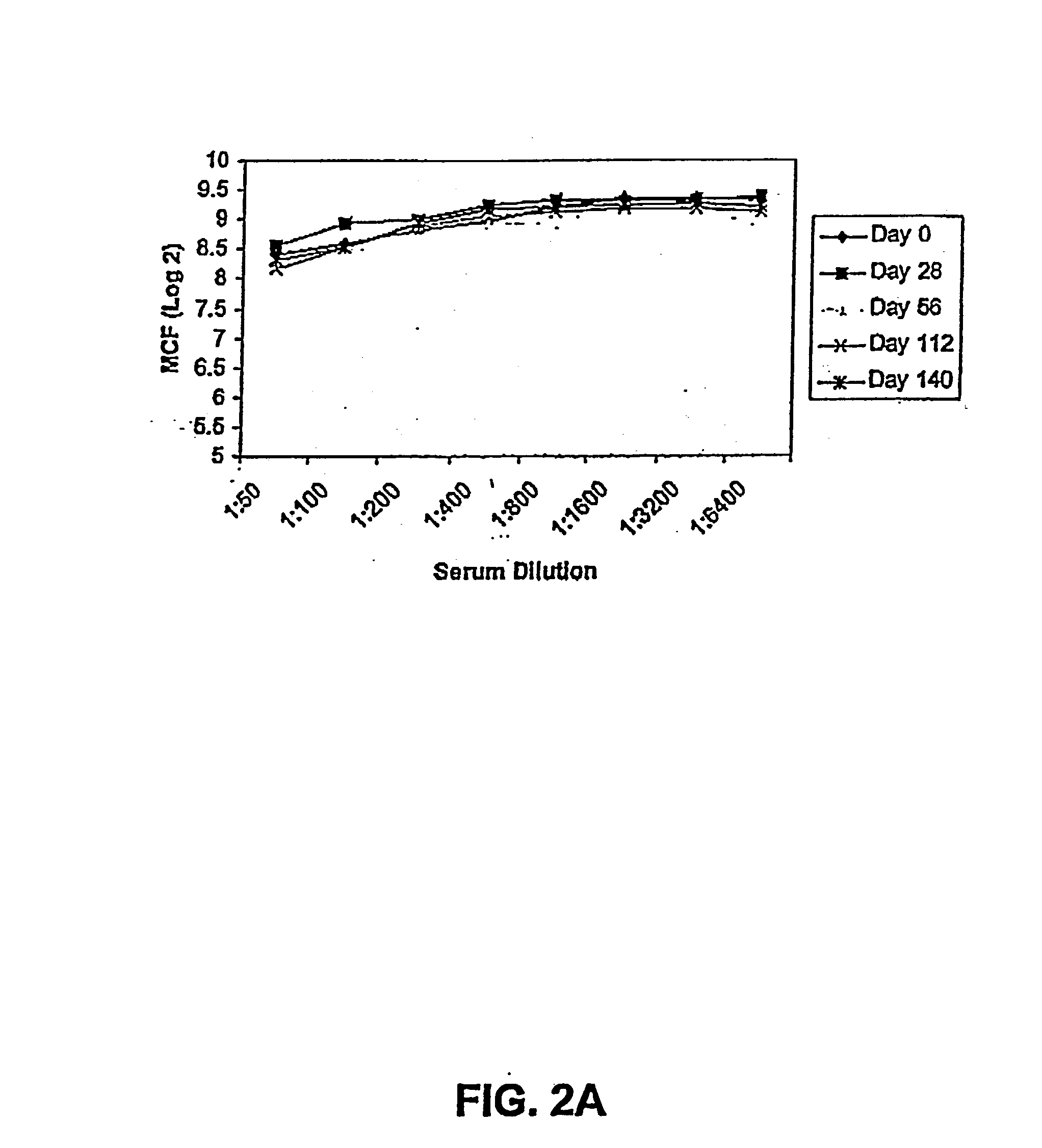
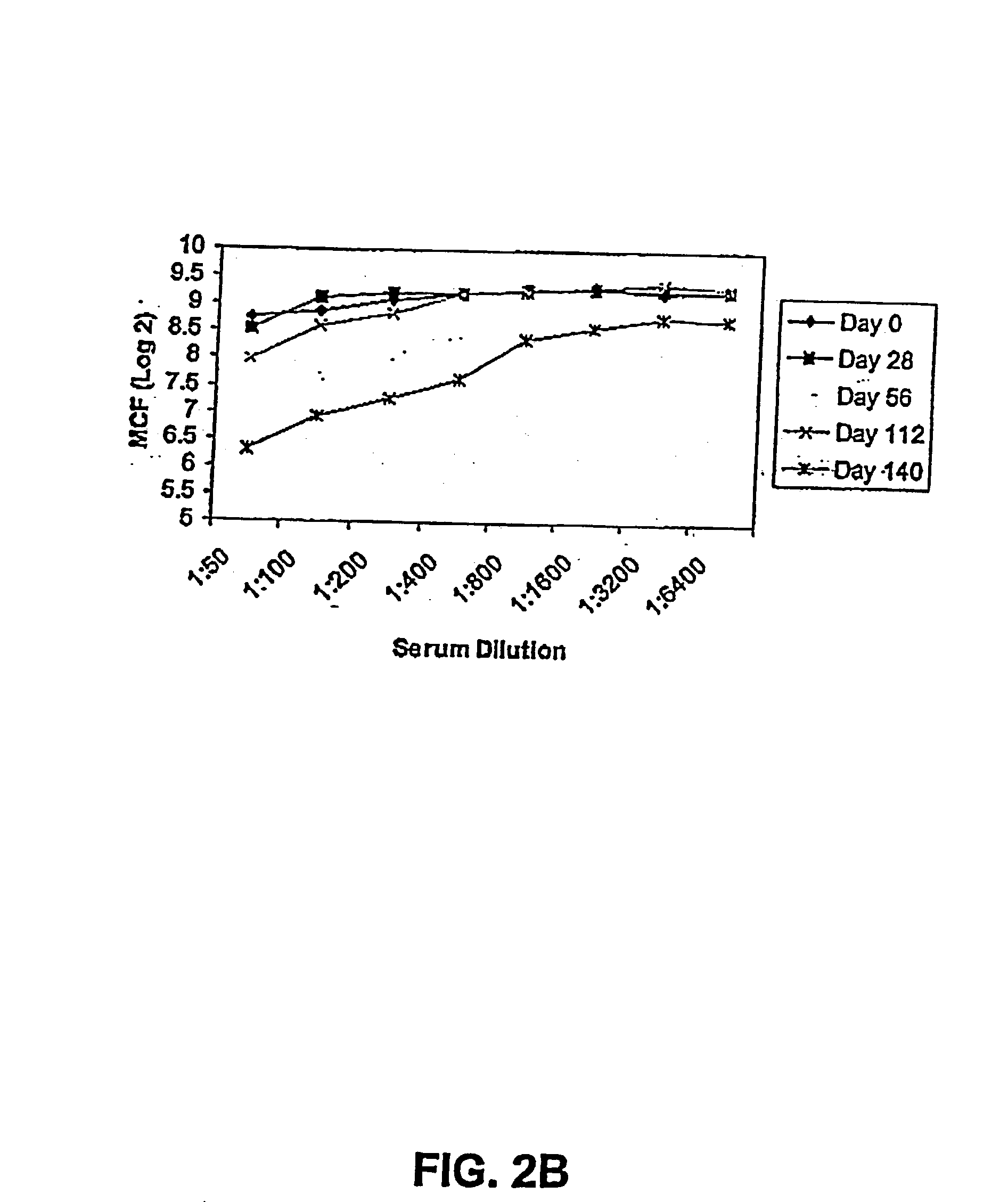
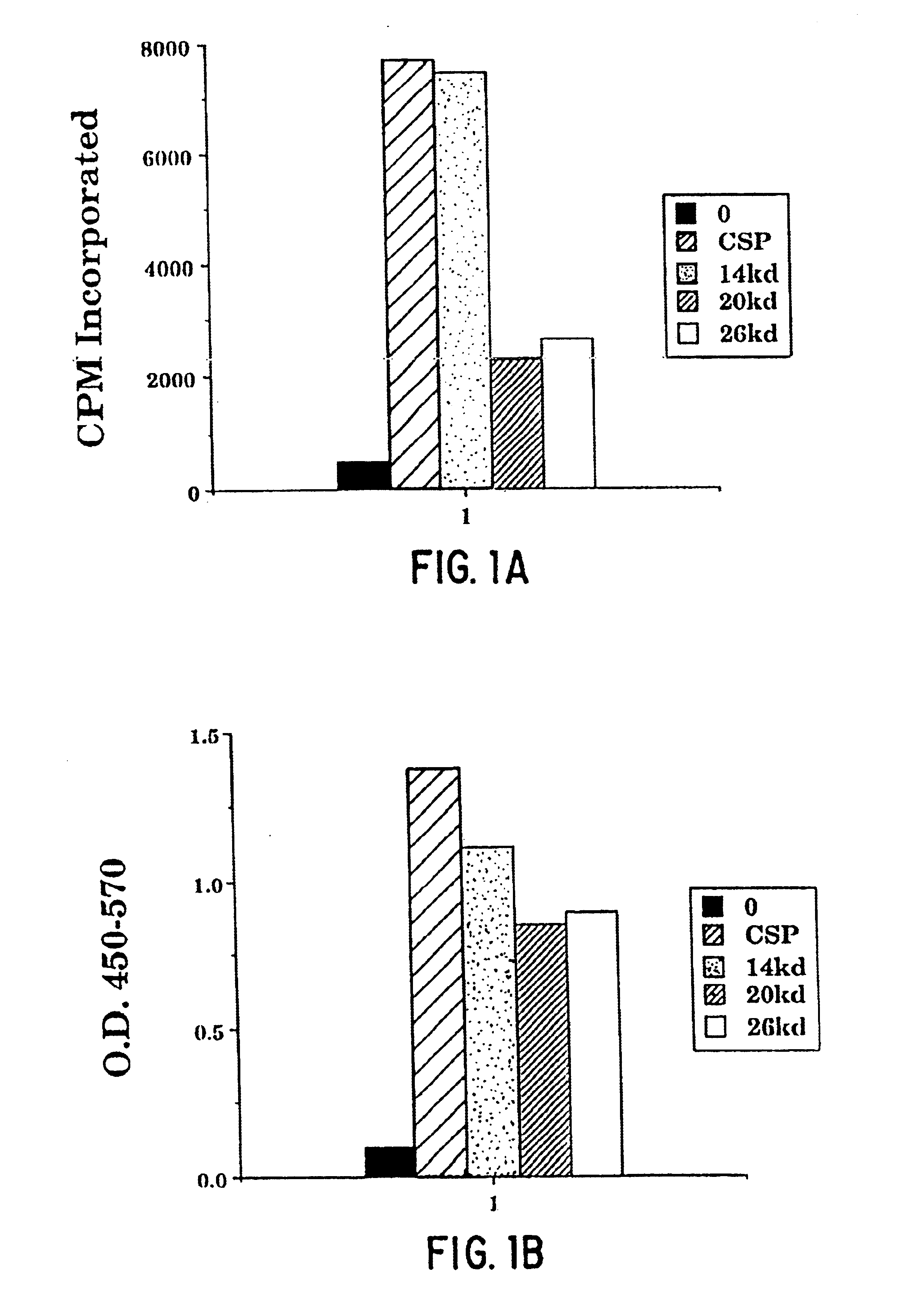
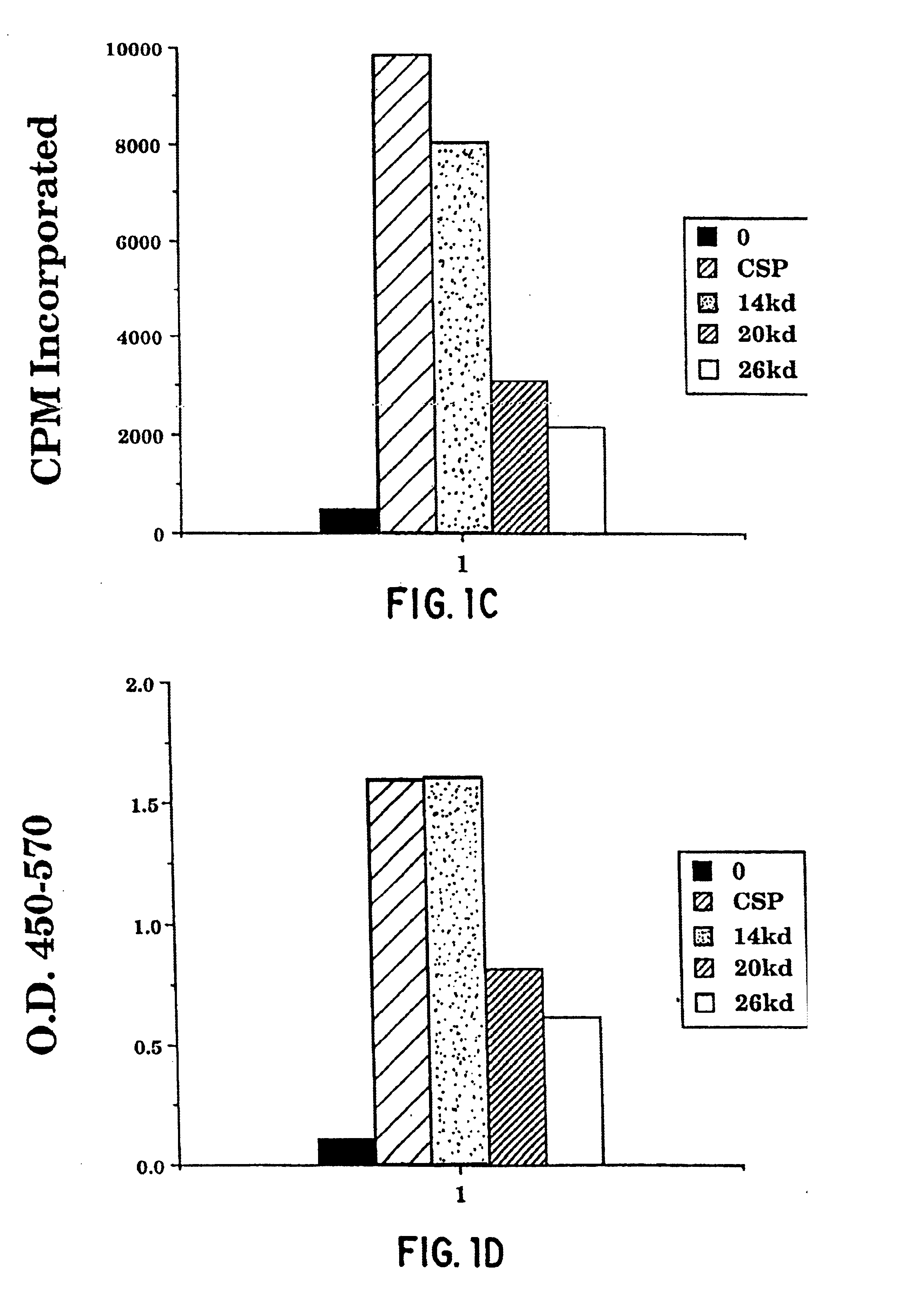
Patents
Literature
271 results about "Protective immunity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
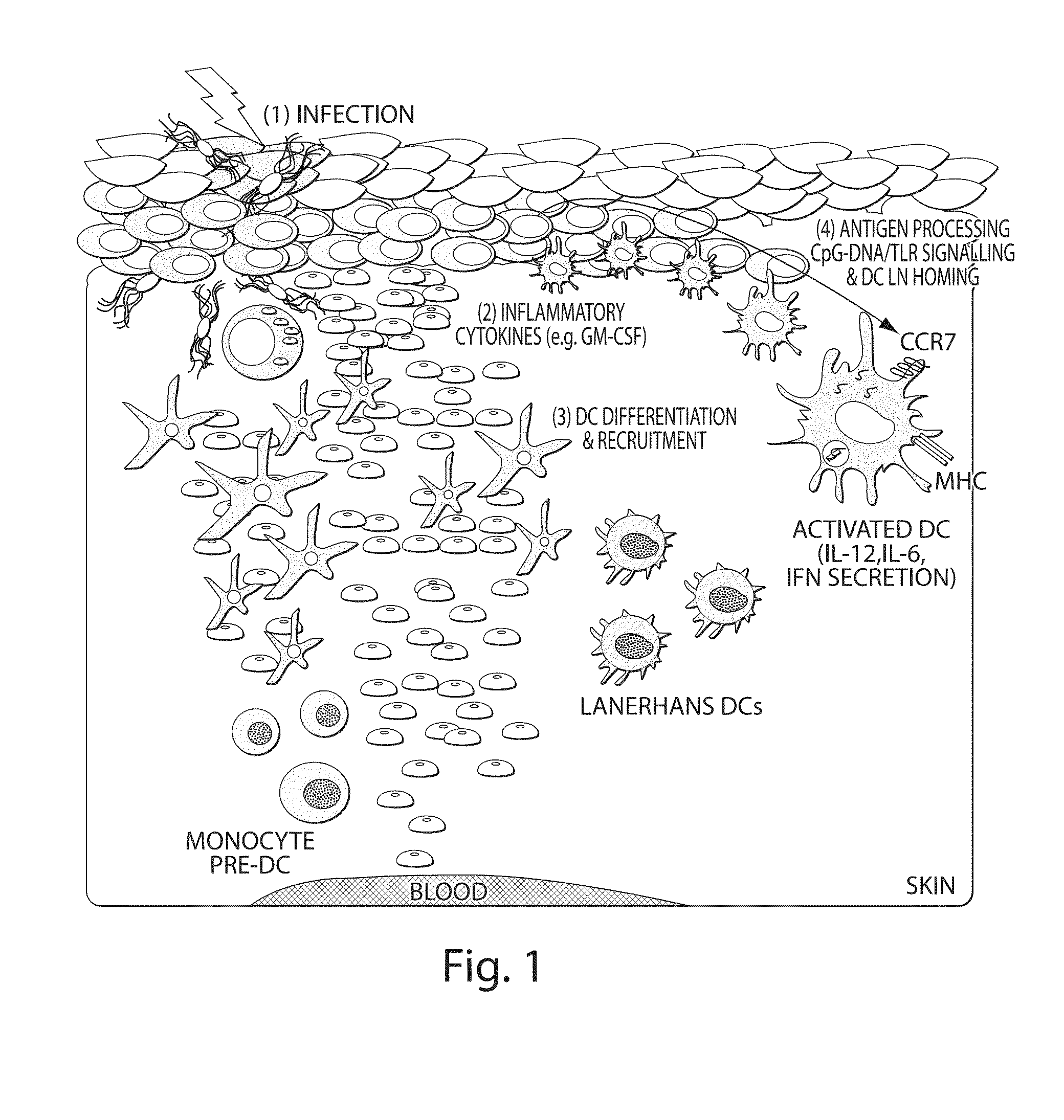
Protective immunity is the body’s ability to resist a certain disease. The body’s source of protective immunity against microbes comes from the antibodies created by B cells, as well as the memories stored in the body’s specialized lymphocytes (B and T cells).
Compounds and methods for diagnosis and immunotherapy of tuberculosis
InactiveUS7087713B2Preventing and diagnosing tuberculosisAntibacterial agentsBacteriaProtective immunityImmunotherapy
Compounds and methods for diagnosing tuberculosis or for inducing protective immunity against tuberculosis are disclosed. The compounds provided include polypeptides that contain at least one immunogenic portion of one or more Mycobacterium proteins and DNA molecules encoding such polypeptides. Diagnostic kits containing such polypeptides or DNA sequences and a suitable detection reagent may be used for the detection of Mycobacterium infection in patients and biological samples. Antibodies directed against such polypeptides are also provided. In addition, such compounds may be formulated into vaccines and / or pharmaceutical compositions for immunization against Mycobacterium infection.
Owner:CORIXA CORP
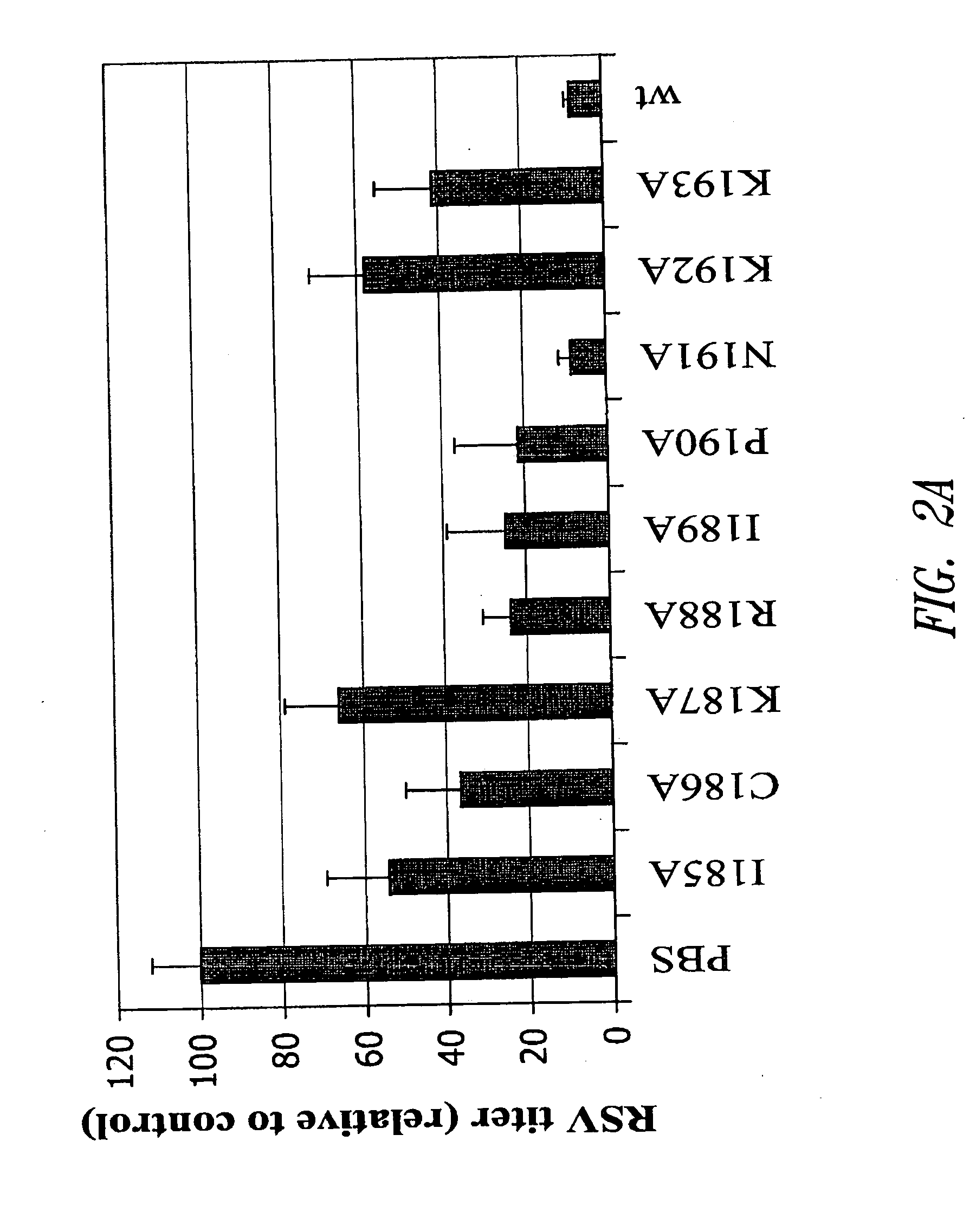
Subunit vaccine against respiratory syncytial virus infection
InactiveUS20050042230A1Reduced responseSsRNA viruses negative-senseVirus peptidesPrevention infectionProtection sex
The present invention relates generally to methods of treating or preventing RSV infections, and more specifically, to compositions, and the use thereof, comprising one or more RSV G protein immunogen or fragment thereof capable of eliciting protective immunity without eliciting an immunopathological response or eliciting a reduced immunopathological response.
Owner:ID BIOMEDICAL CORP LAVAL
Continuous Cell Programming Devices
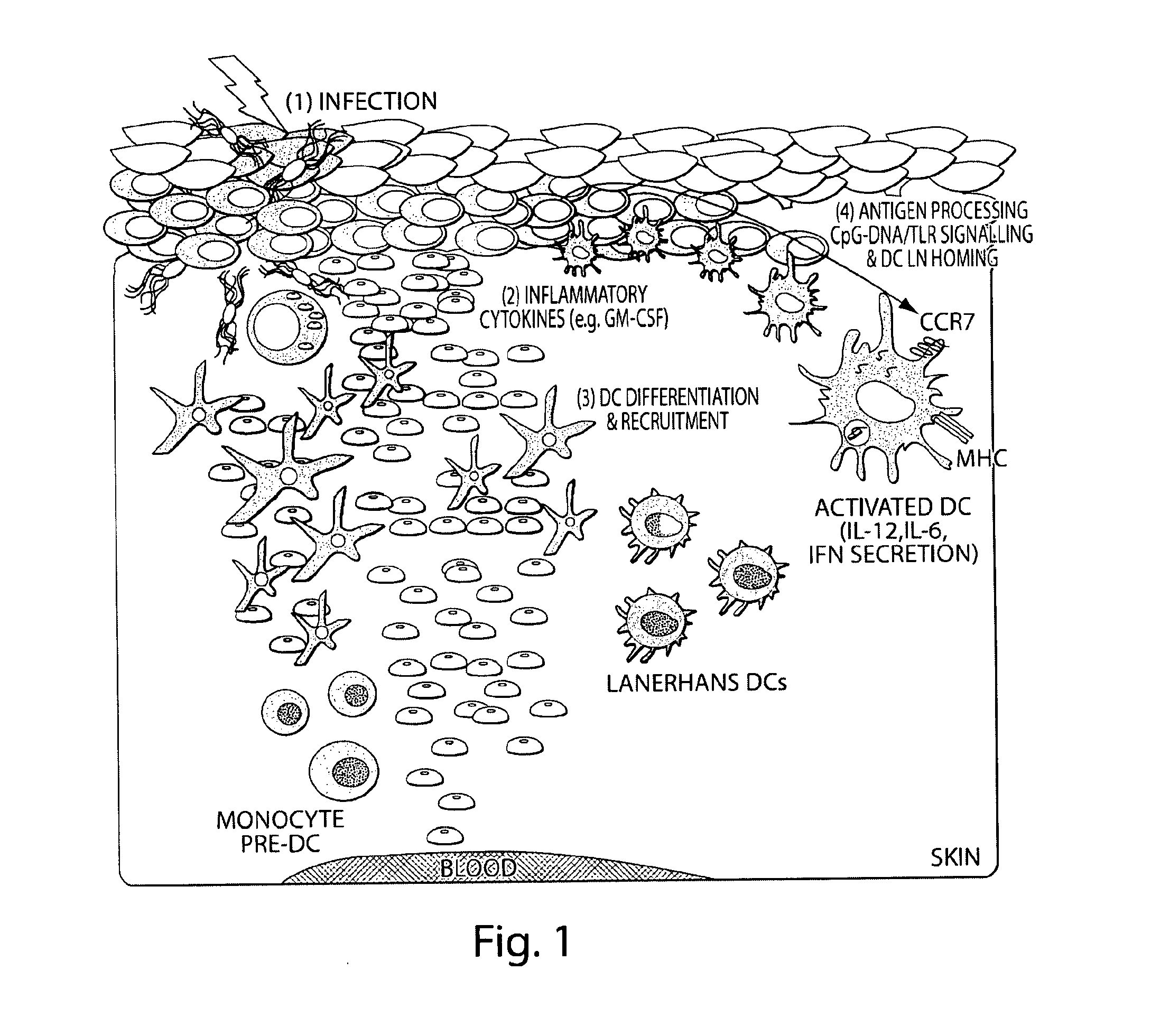
ActiveUS20120100182A1Easy to controlClinical effectiveness of has been limitedOrganic active ingredientsPeptide/protein ingredientsDendritic cellProtective immunity
The present invention comprises compositions, methods and devices for creating an infection-mimicking environment within a polymer scaffold to stimulate antigen-specific dendritic cell activation. Devices of the present invention are used to provide protective immunity to subjects against infection and cancer.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE +1
SPAS-1 cancer antigen
InactiveUS20020150588A1Tumor rejection antigen precursorsPeptide/protein ingredientsCancer antigenProtective immunity
Compounds and methods for inducing protective immunity against cancer are disclosed. The compounds provided include polypeptides that contain at least one immunogenic portion of one or more SPAS-1 proteins and DNA molecules encoding such polypeptides. Such compounds may be formulated into vaccines and pharmaceutical compositions for immunization against cancer, or can be used for the diagnosis of cancer and the monitoring of cancer progression
Owner:RGT UNIV OF CALIFORNIA
Methods for converting or inducing protective immunity
InactiveUS20090214533A1Reduced activityReduce functionAntibody ingredientsImmunoglobulinsRegulatory T cellImmune complex deposition
The invention is based in part on the finding that suppressing regulatory T cell function is needed in order to convert passive immunity into active antigen-specific immunity. Generally, the methods of the invention comprise at least the combination of: (1) increasing the amount of immune complexes in the subject, wherein the immune complex comprises a target antigen and a immunoglobulin molecule comprising (i) a variable region specific to the target antigen and (ii) a Fc receptor binding region; and (2) inhibiting regulatory T cell function or decreasing / depleting the regulatory T cell population in the subject.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
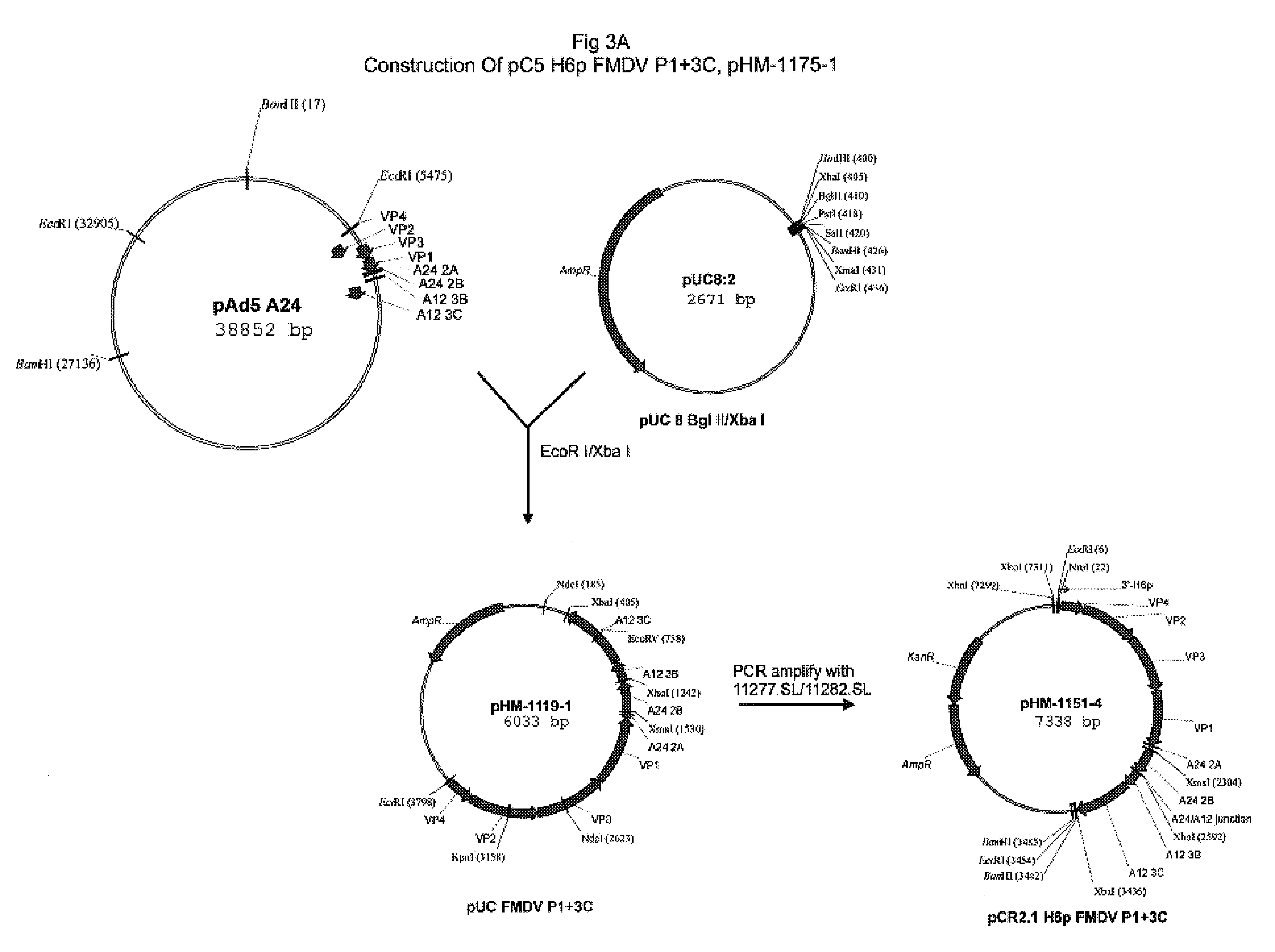
Avipox recombinants expressing foot and mouth disease virus genes
ActiveUS20050287672A1SsRNA viruses positive-senseViral antigen ingredientsGene productProtective immunity
The present invention relates to modified poxviral vectors and to methods of making and using the same. In particular, the invention relates to recombinant avipox that expresses gene products of foot and mouth disease virus (FMDV), and to compositions or vaccines that elicit immune responses directed to FMDV gene products and which can confer protective immunity against infection by FMDV.
Owner:MERIAL INC
Subunit vaccine against respiratory syncytial virus infection
The present invention relates generally to methods of treating or preventing RSV infections, and more specifically, to compositions, and the use thereof, comprising one or more RSV G protein immunogen or fragment thereof capable of eliciting protective immunity without eliciting an immunopathological response or eliciting a reduced immunopathological response.
Owner:ID BIOMEDICAL CORP LAVAL
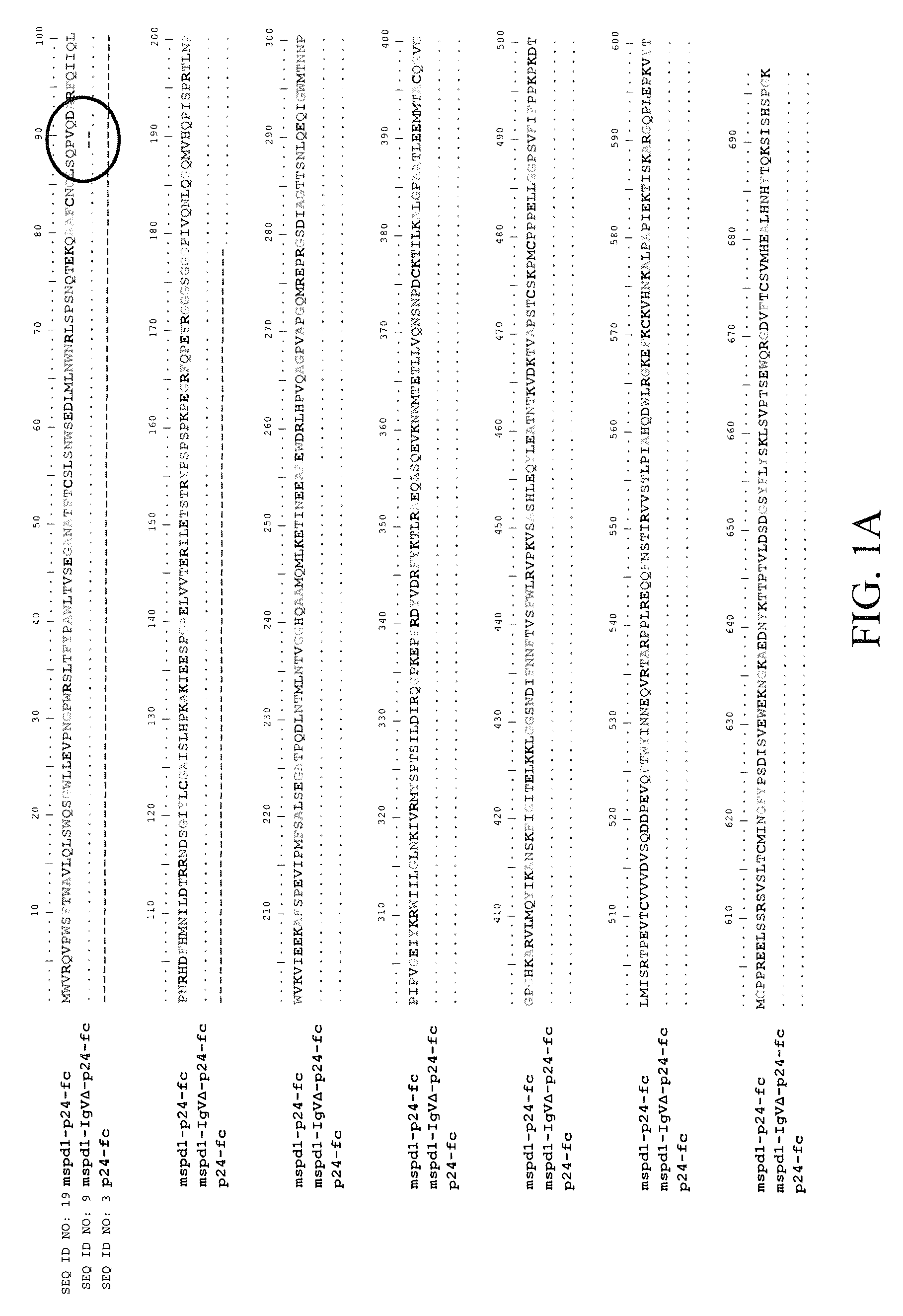
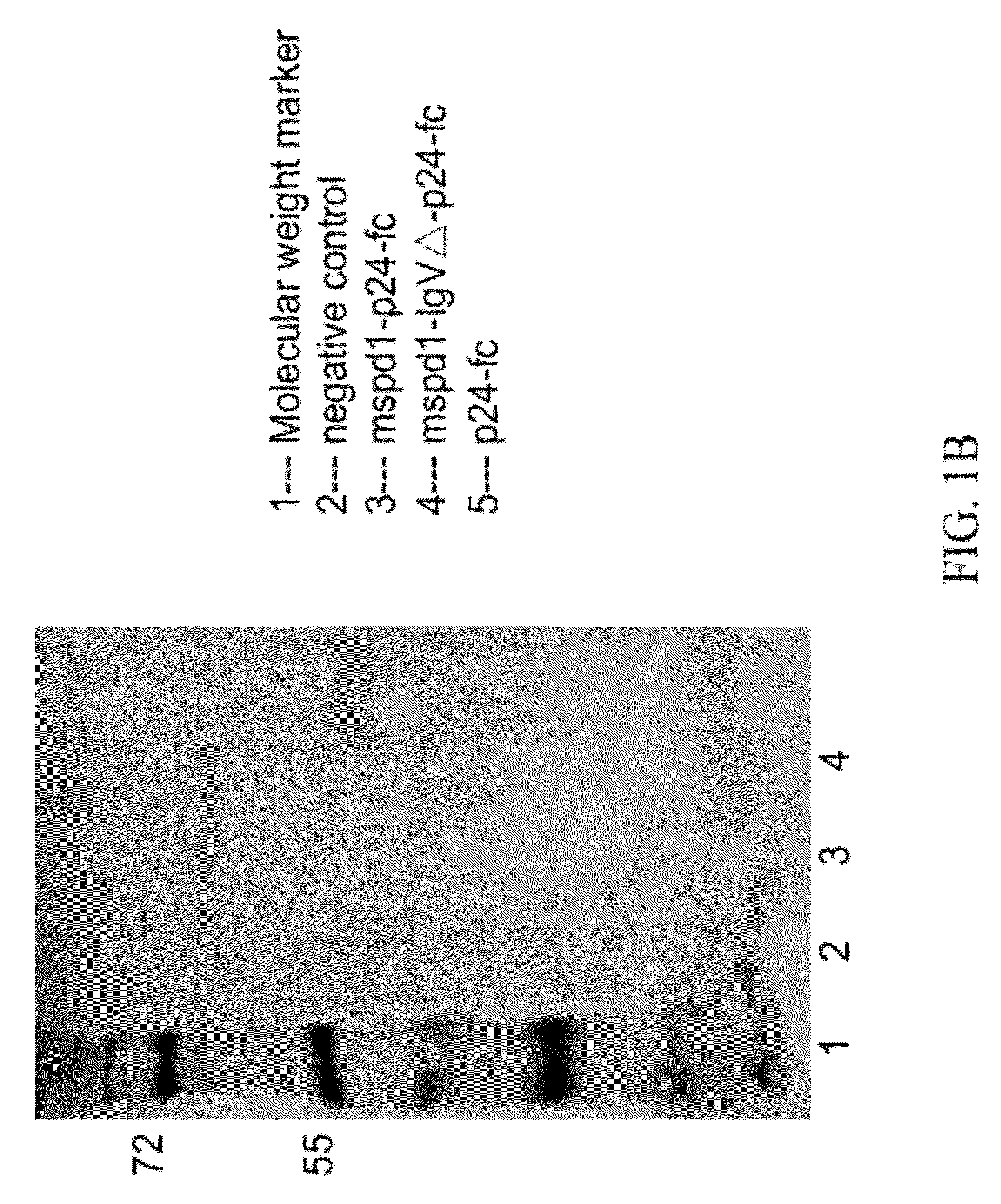
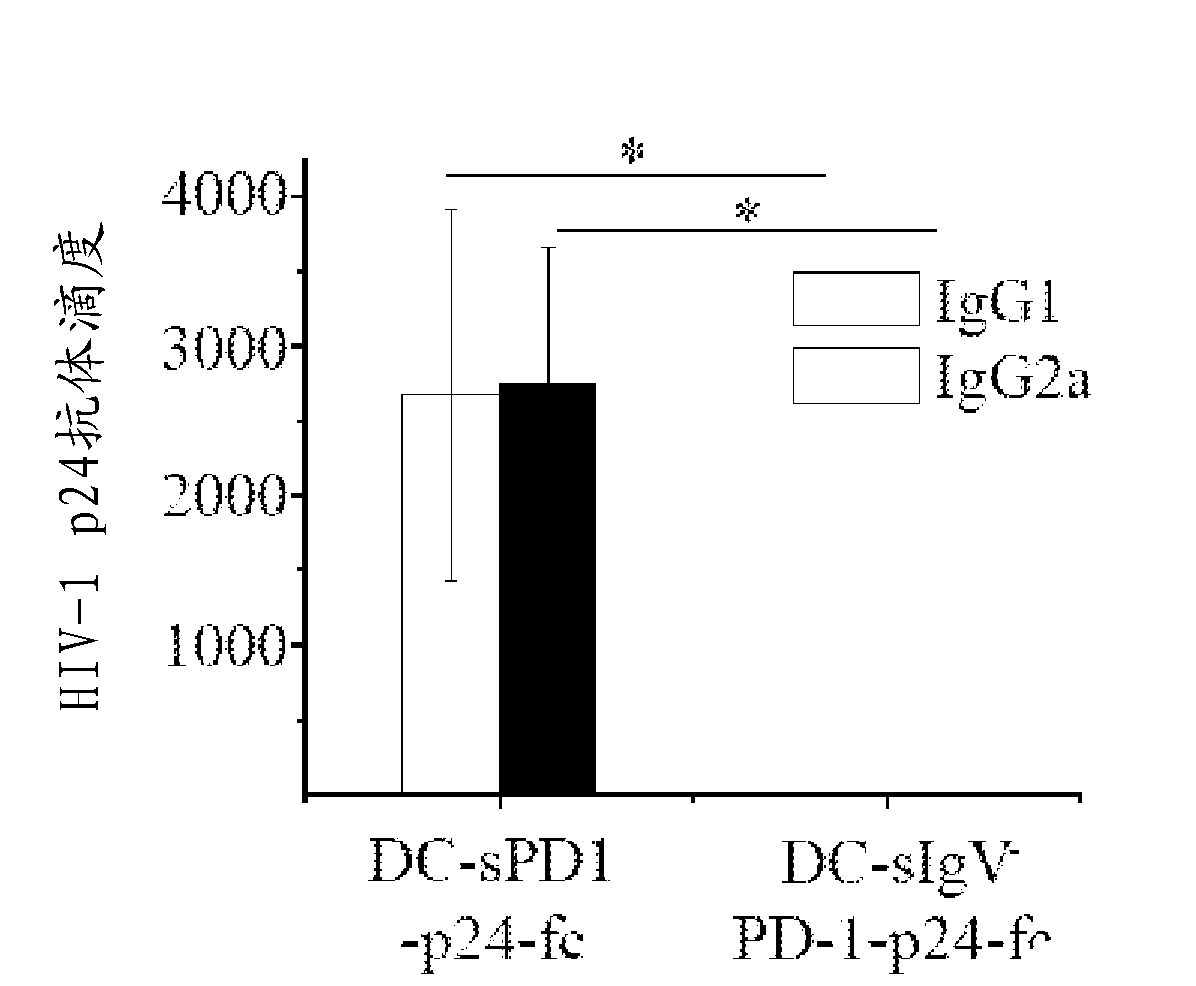
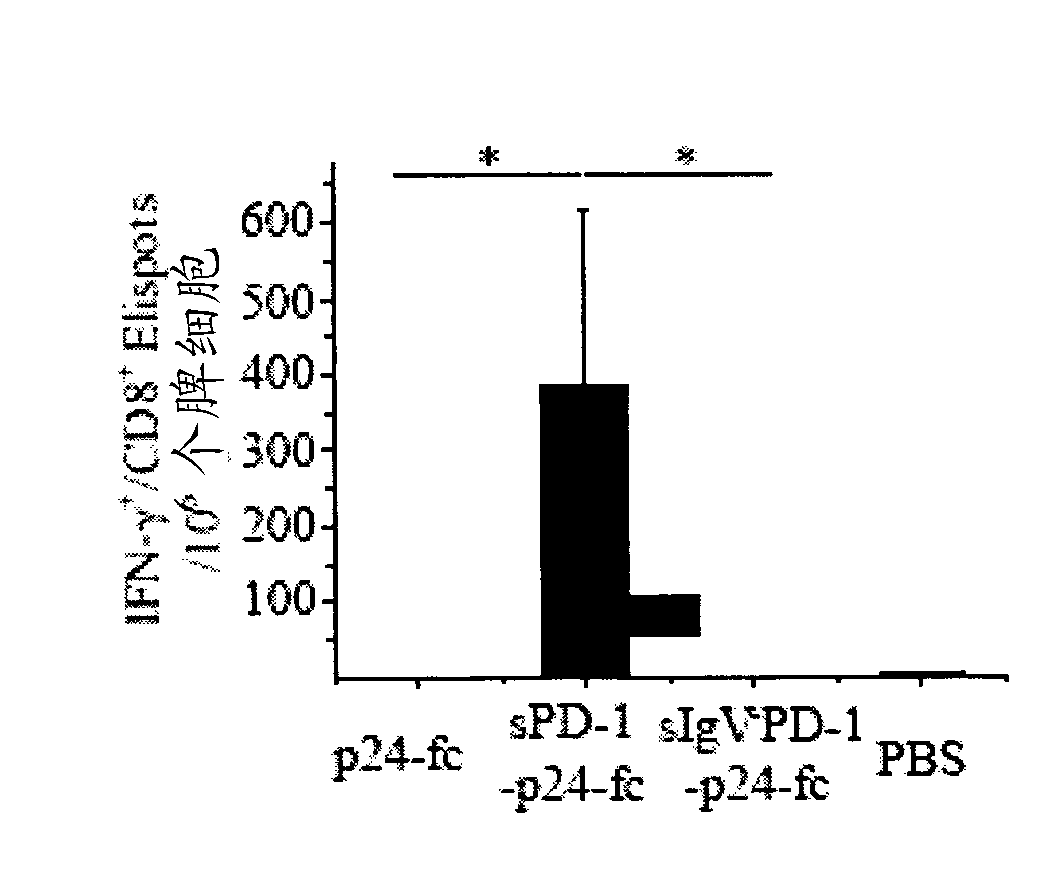
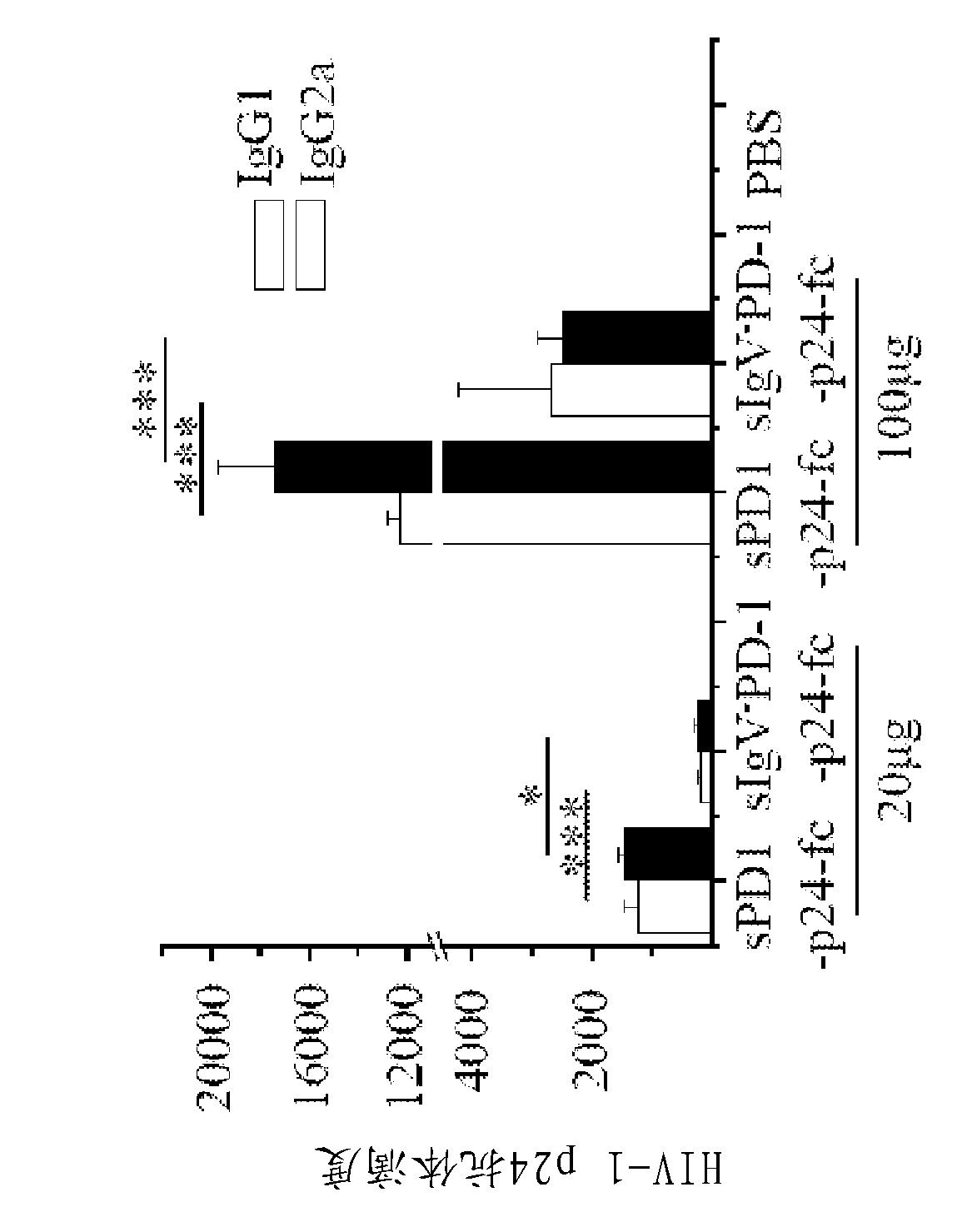
Soluble pd-1 variants, fusion constructs, and uses thereof
ActiveUS20120121634A1Enhance host humoralEnhance cell-mediated immunityVirusesPeptide/protein ingredientsProtective immunityCell mediated immunity
The subject invention provides novel soluble PD-1 (sPD-1) proteins, nucleic acids, and fusion constructs thereof, for enhancing humoral and cell-mediated immunity of a subject. Also provided are therapeutic compositions comprising the sPD-1 proteins, nucleic acids, and fusion constructs of the subject invention. In a preferred embodiment, the therapeutic composition is formulated as a vaccine composition. Advantageously, the sPD-1 proteins, nucleic acids, and therapeutic compositions provide protective immunity against pathogenic infection including HIV infection. In additon, the subject invention can be used in the prevention and / or treatment of tumor or cancer.
Owner:VERSITECH LTD
Method of administering FimH protein as a vaccine for urinary tract infections
InactiveUS20030138449A1Reduce morbidityInhibit bindingAntibacterial agentsBacterial antigen ingredientsDiseaseBacteroides
The present invention relates to methods of stimulating an immune response in a primate utilizing compositions comprising bacterial adhesin proteins and / or immunogenic fragments thereof. The compositions are useful for the prevention and treatment of bacterial induced diseases involving bacterial adherence to a target cell, such as diseases of the urinary tract. More specifically, the invention relates to the vaccination of primates, preferably humans, with protein complexes, such as a purified FimH polypeptides, a purified FimC-FimH (FimCH) polypeptide complex, or immunogenic fragments thereof, to stimulate protective immunity in the recipient against infection by pathogenic bacteria, including all types of Enterobacteriaceae, preferably E. coli to produce specific immunoglobin molecules in the serum and urine or mucosal secretions of the subject.
Owner:MEDIMMUNE LLC
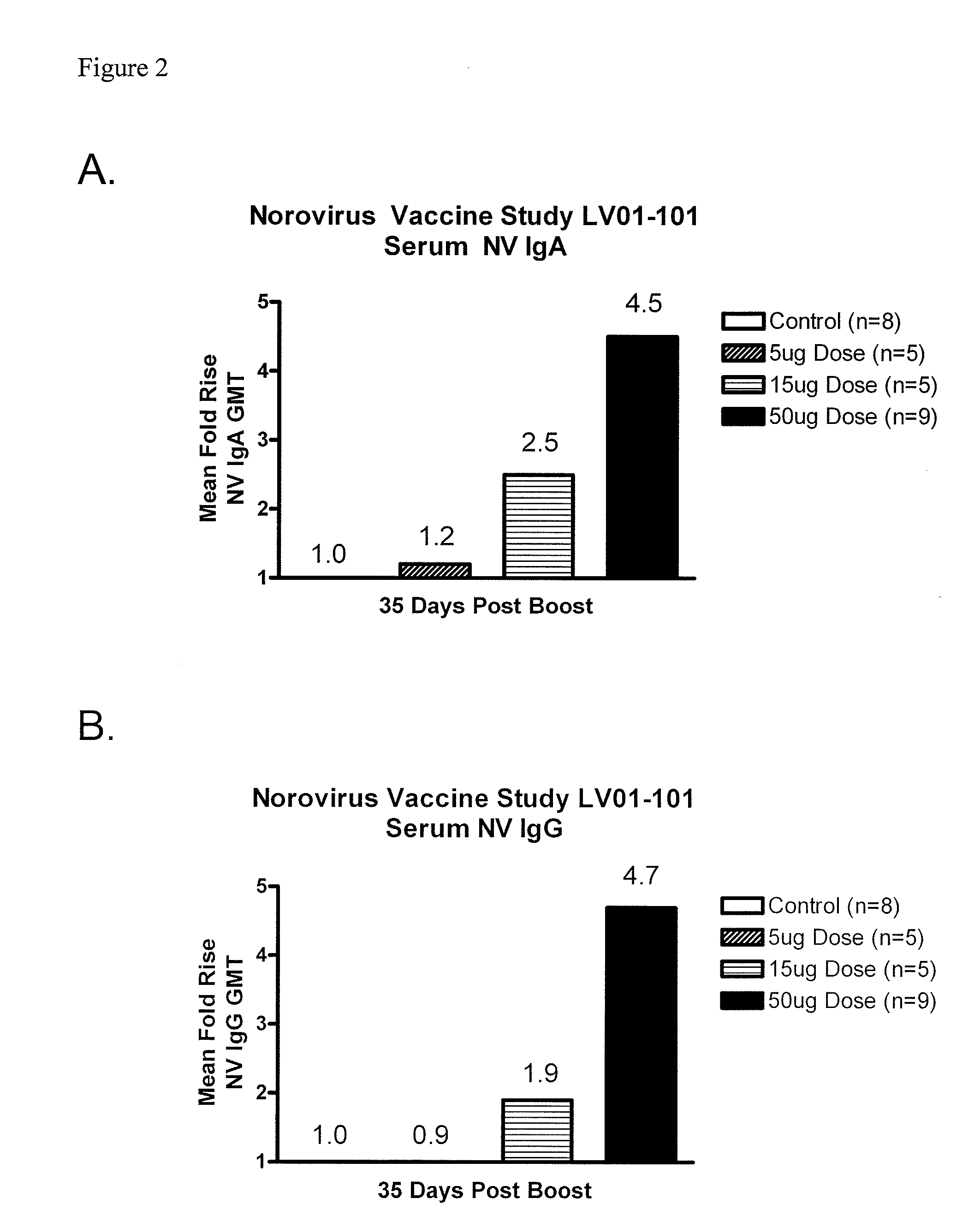
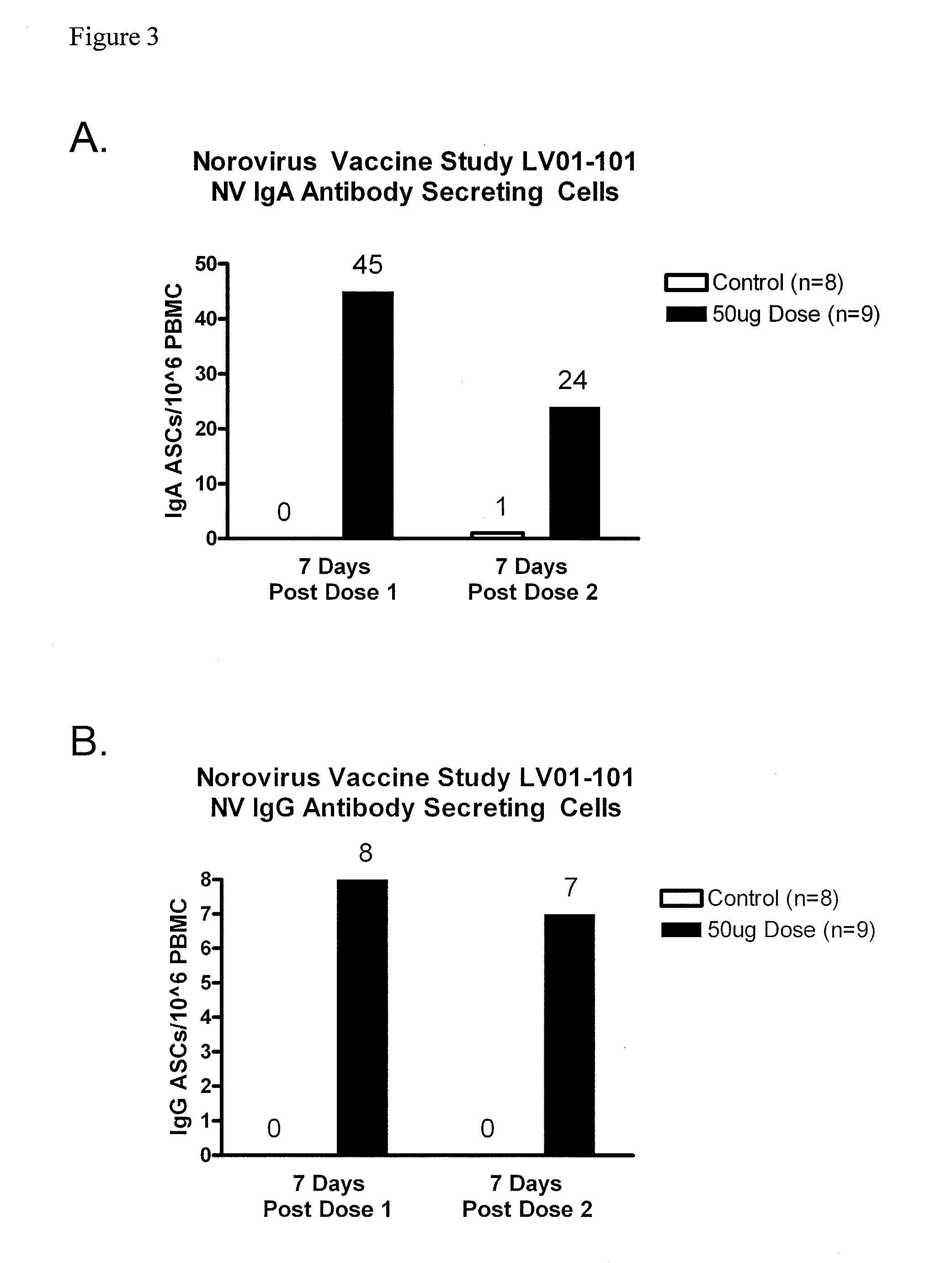
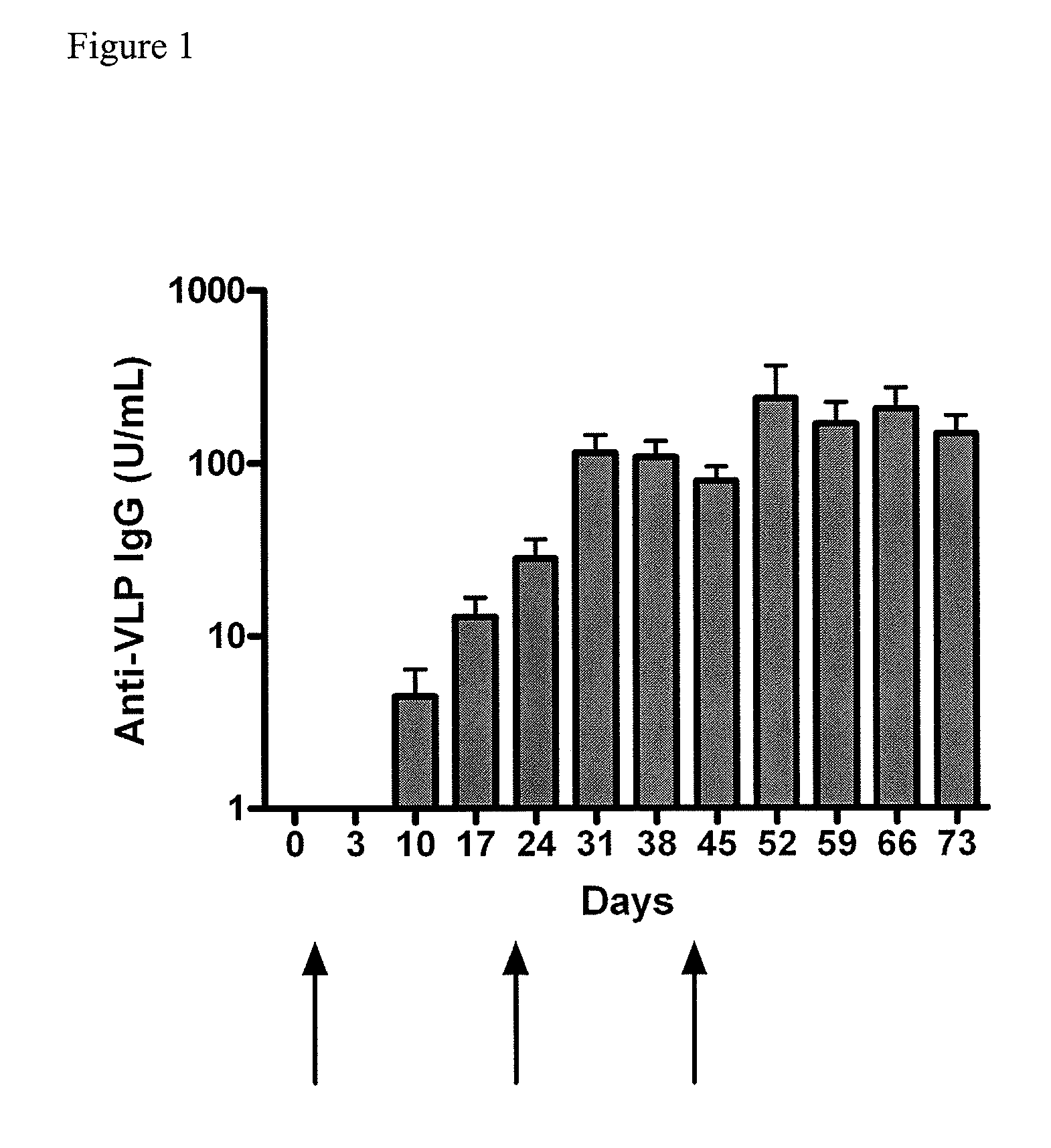
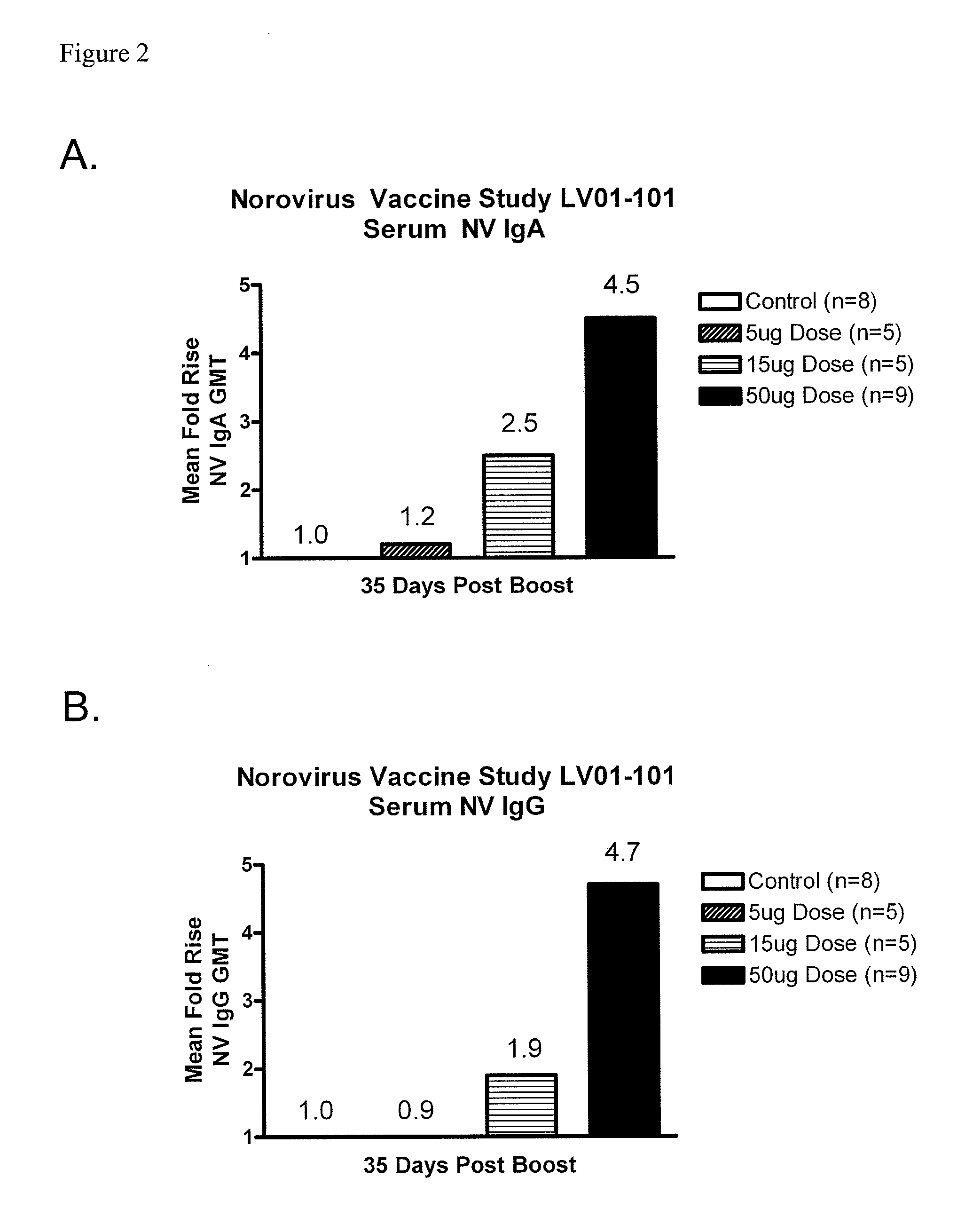
Method of conferring a protective immune response to norovirus
InactiveUS20100266636A1Increase antigen retention timeEnhance immune responseSsRNA viruses positive-senseViral antigen ingredientsAntigenAdjuvant
The present invention relates to vaccine compositions comprising Norovirus antigens and adjuvants, in particular, mixtures of monovalent VLPs and mixtures of multivalent VLPs, and to methods of conferring protective immunity to Norovirus infections in a human subject.
Owner:TAKEDA VACCINES MONTANA
Soluble PD-1 variants, fusion constructs, and uses thereof
The subject invention provides novel soluble PD-1 (sPD-1) proteins, nucleic acids, and fusion constructs thereof, for enhancing humoral and cell-mediated immunity of a subject. Also provided are therapeutic compositions comprising the sPD-1 proteins, nucleic acids, and fusion constructs of the subject invention. In a preferred embodiment, the therapeutic composition is formulated as a vaccine composition. Advantageously, the sPD-1 proteins, nucleic acids, and therapeutic compositions provide protective immunity against pathogenic infection including HIV infection. In addition, the subject invention can be used in the prevention and / or treatment of tumor or cancer.
Owner:VERSITECH LTD
Avian influenza virus live attenuated vaccine and uses thereof
ActiveUS20110150912A1Poor replicationLess virulentSsRNA viruses negative-senseBacteriaHeterologousDonor strain
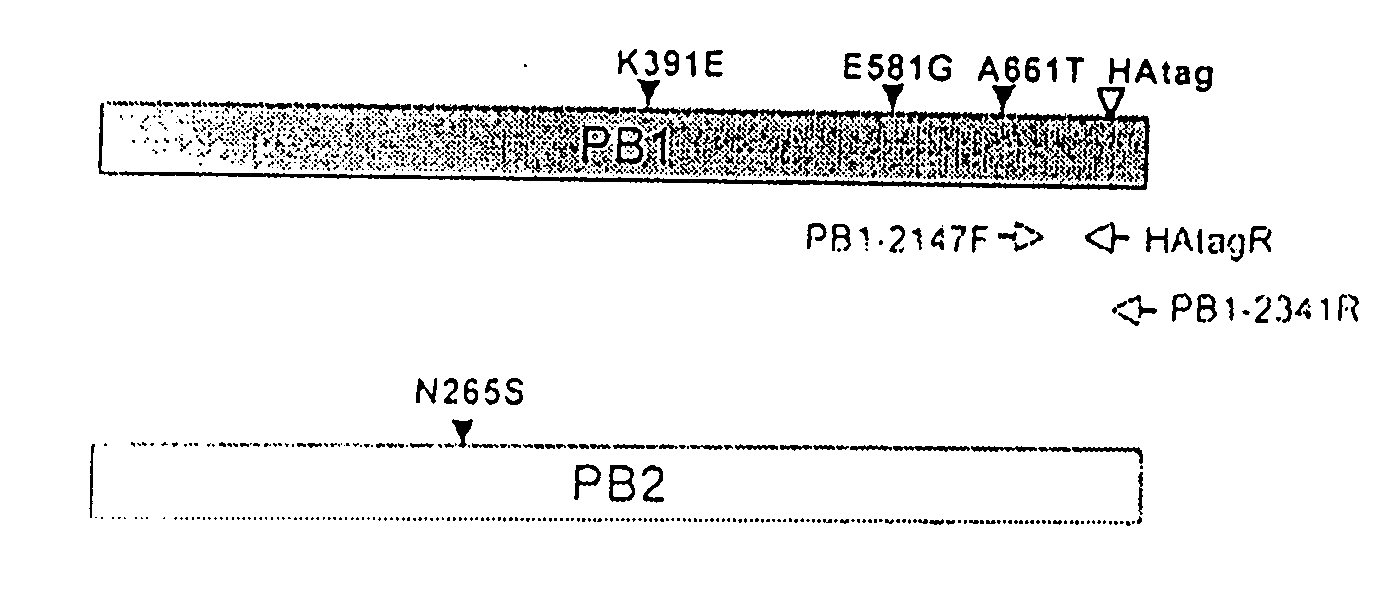
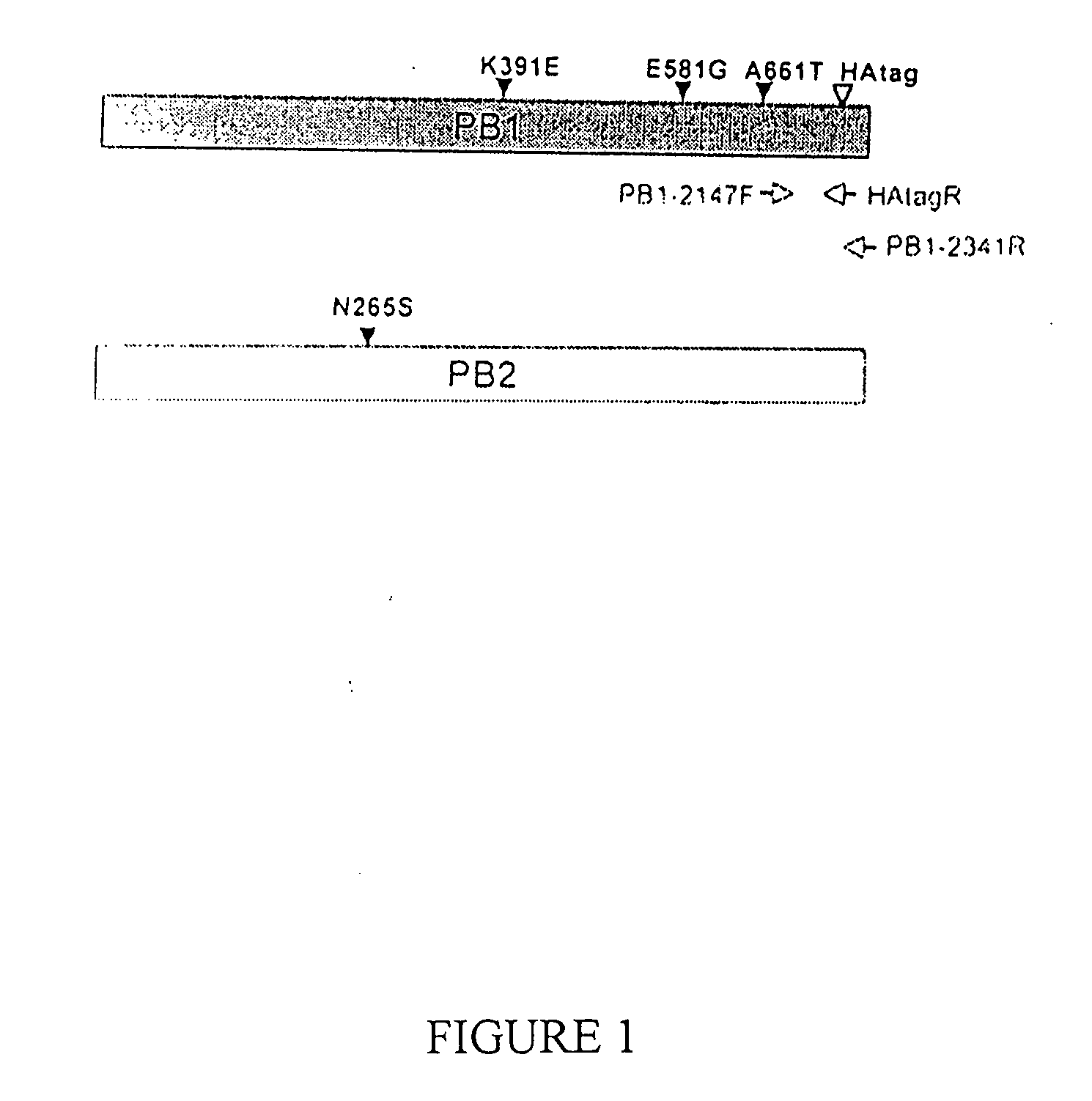
Described in this application are attenuated strains of avian influenza virus containing temperature sensitive mutations in addition to a genetic tag in the PB1 gene. The attenuated viruses are useful as avian and mammalian vaccine for protective immunity against homologous and heterologous lethal challenges with influenza virus. A genetically modified avian influenza virus backbone is described which can be used as a master donor strain for the generation of live attenuated vaccines for epidemic and pandemic influenza.
Owner:UNIV OF MARYLAND
Compositions comprising pathogen-associated molecular patterns and antigens and methods of use thereof
InactiveUS20080063657A1Stimulate immune responseImprove immunitySsRNA viruses positive-sensePeptide/protein ingredientsAntigenYellow fever
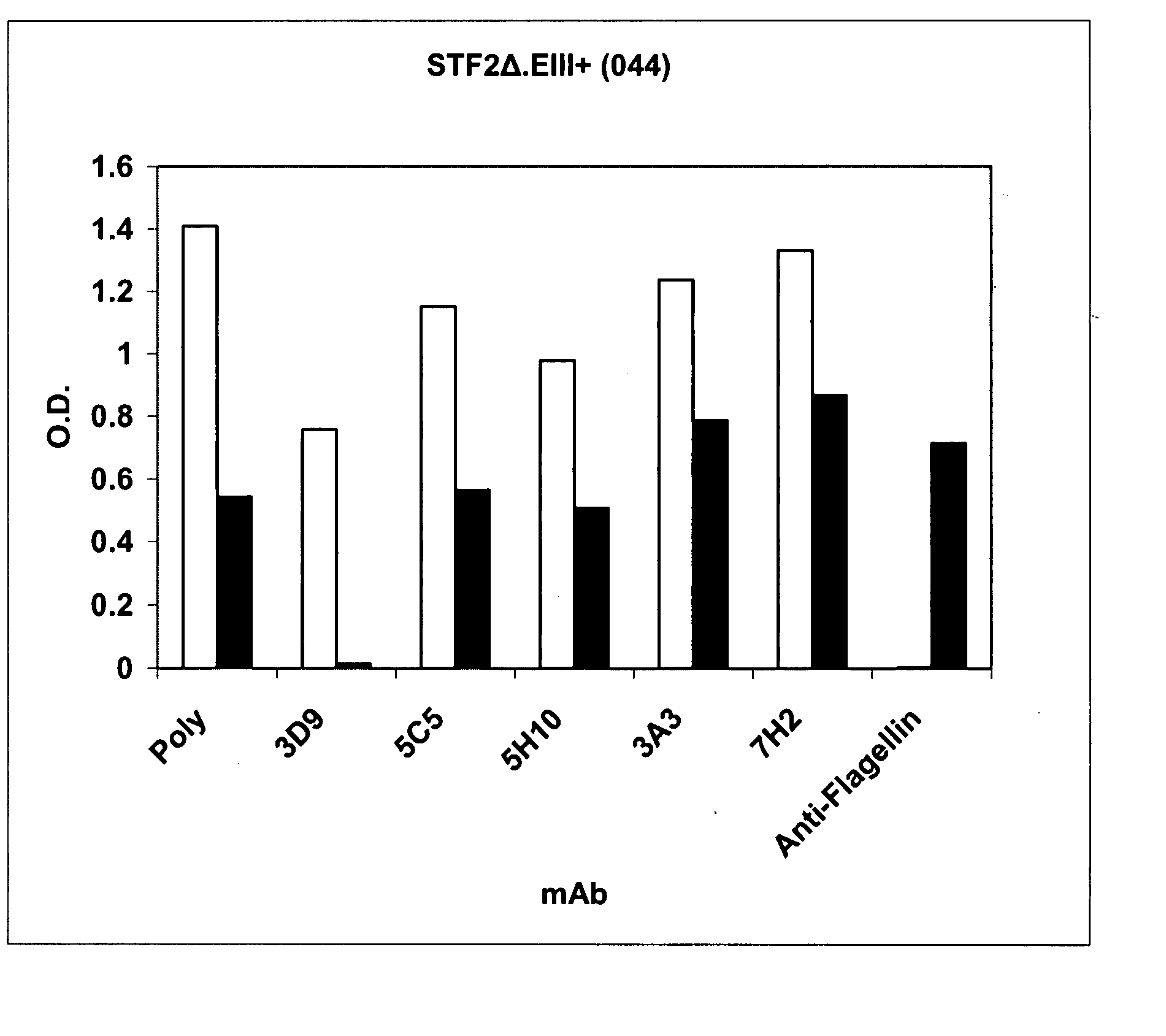
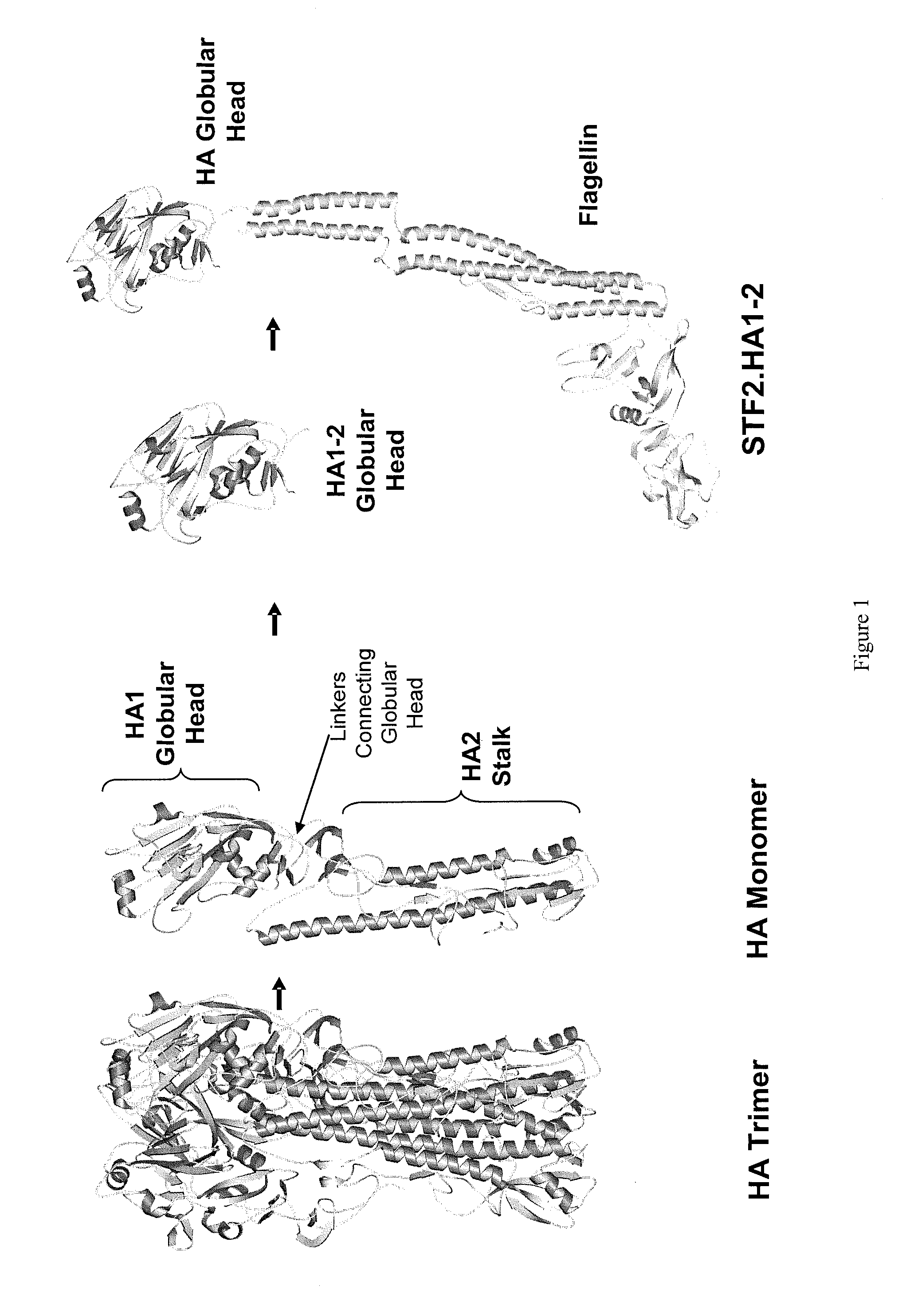
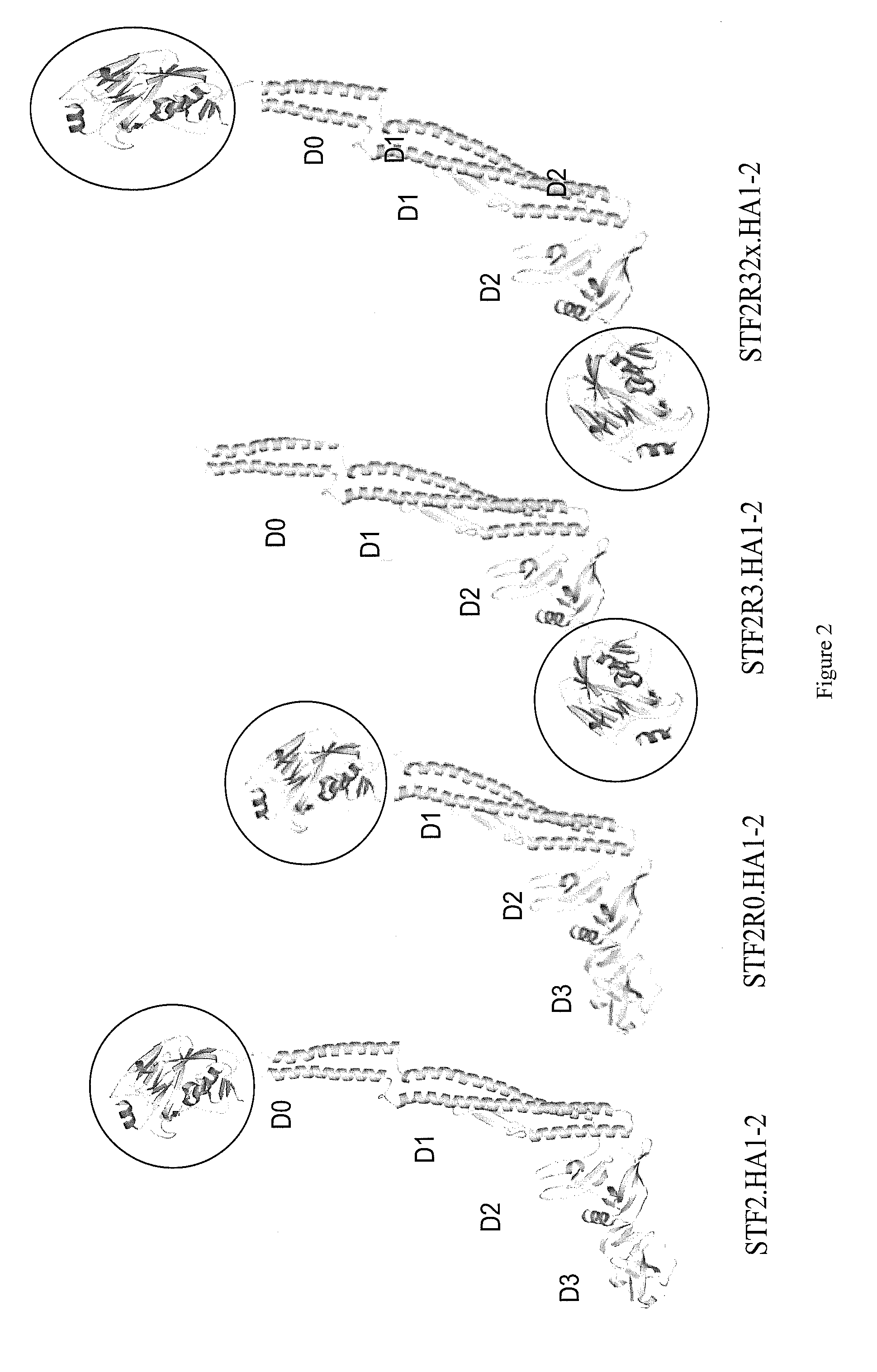
Compositions comprise at least a portion of an antigen and at least a portion of a flagellin that lacks a hinge region. Compositions, fusion proteins and polypeptides comprise at least a portion of at least one pathogen-associated molecular pattern and at least a portion of at least one viral protein. The viral protein of the compositions, fusion proteins and polypeptides of the invention are flaviviral proteins, including a West Nile flaviviral protein, a Dengue flaviviral protein, a Langat flaviviral protein, a Kunjin flaviviral protein, a Murray Valley encephalitis flaviviral protein, a Japanese encephalitis flaviviral protein, a Tick-borne encephalitis flaviviral protein, a Yellow fever flaviviral protein and a hepatitis C flaviviral protein. The compositions, fusion proteins and polypeptides are used to stimulate an immune response and protective immunity in a subject.
Owner:VAXINNATE
DNA vaccine expressing HA1 of equine-2 influenza virus
InactiveUS7244435B2Reduce riskReduce dosageSsRNA viruses negative-senseViral antigen ingredientsHemagglutininA-DNA
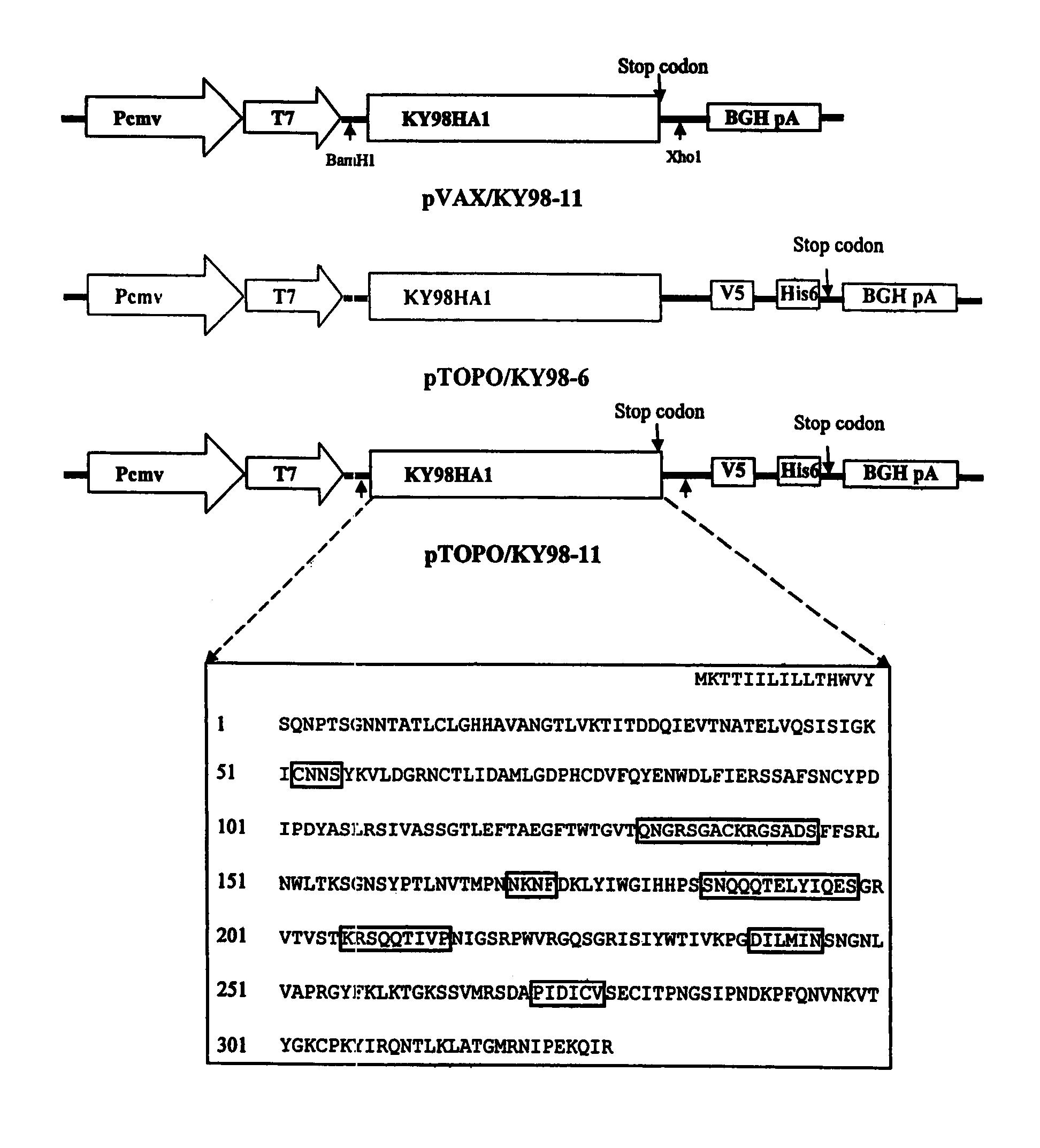
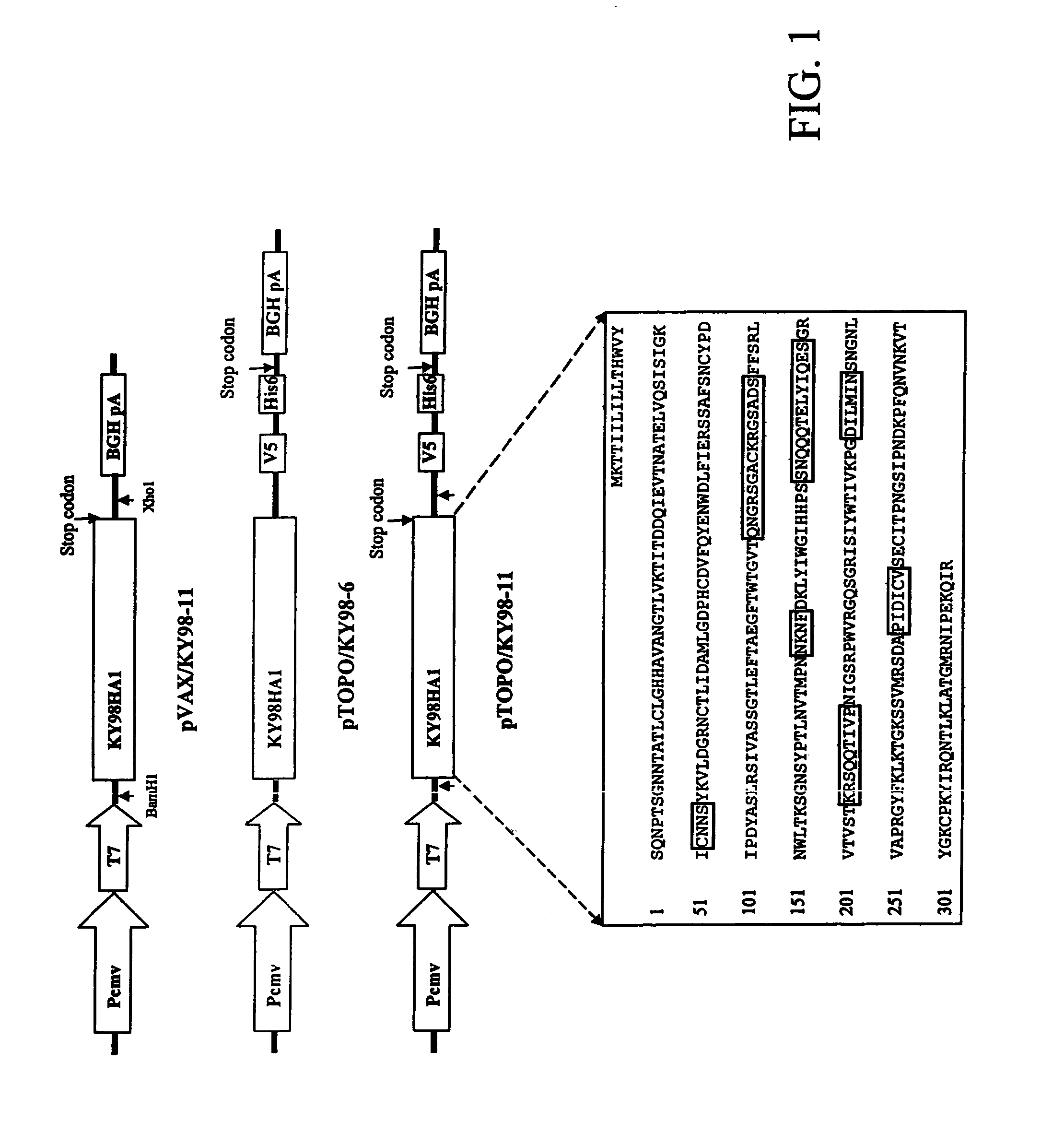
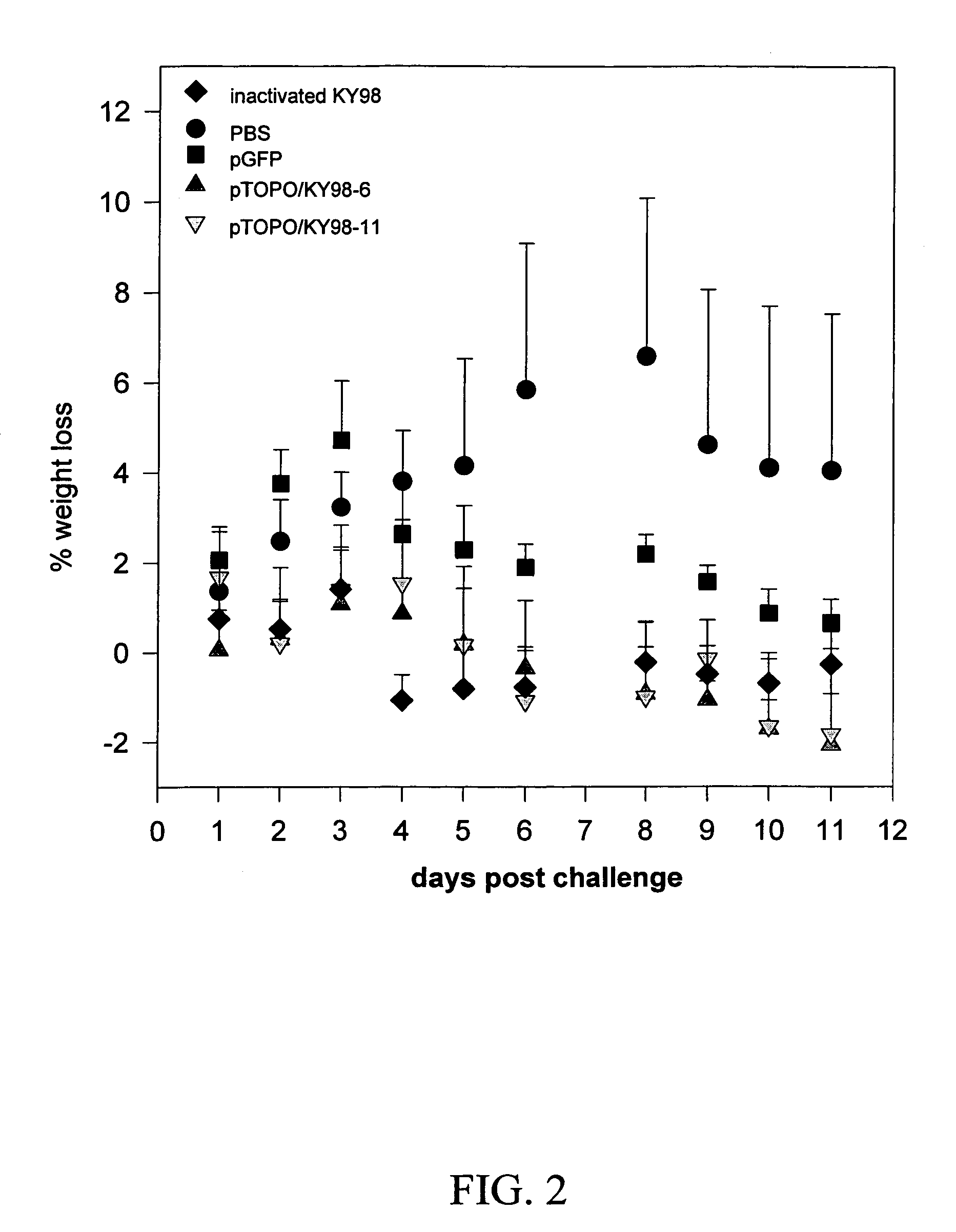
The invention is for a DNA vaccine expressing the hemagglutinin (HA1) gene of equine-2 influenza virus. By engineering a stop codon within HA1, expression of HA1 is ensured. By encapsulation of the DNA vaccine in liposome and by intranasal inoculation, it is sufficient to elicit protective immunity at a significantly lower dosage compared to a DNA vaccine expressing the full length HA gene. Lower dosage reduces the risk of induction of anti-DNA antibodies. Intranasal inoculation directly to the respiratory epithelial cells reduces the risk of DNA integration. The inventive vaccine is advantageous over current inactivated or live attenuated vaccines, as updating of the vaccine requires only the replacement of the encoding sequence with the new virus.
Owner:BOARD OF REGENTS FOR OKLAHOMA STATE UNIVERSITY
PRRSV subunit vaccines
ActiveUS7722878B2SsRNA viruses positive-senseViral antigen ingredientsProtective immunityProtection sex
Vaccines effective against PRRSV include at least one portion of PRRSV ORF1. Such vaccines, upon administration, provoke an immune response in PRRSV-susceptible animals. Moreover, compositions in accordance with the present invention provide immune response up to and including protective immunity against PRRSV as well as reduce the severity of PRRSV and / or incidence of PRRSV. Selected portions of ORF1 can be used singularly, in combination with one another, in combination with other PRRSV ORFs, and in combination with other PRRSV vaccines.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
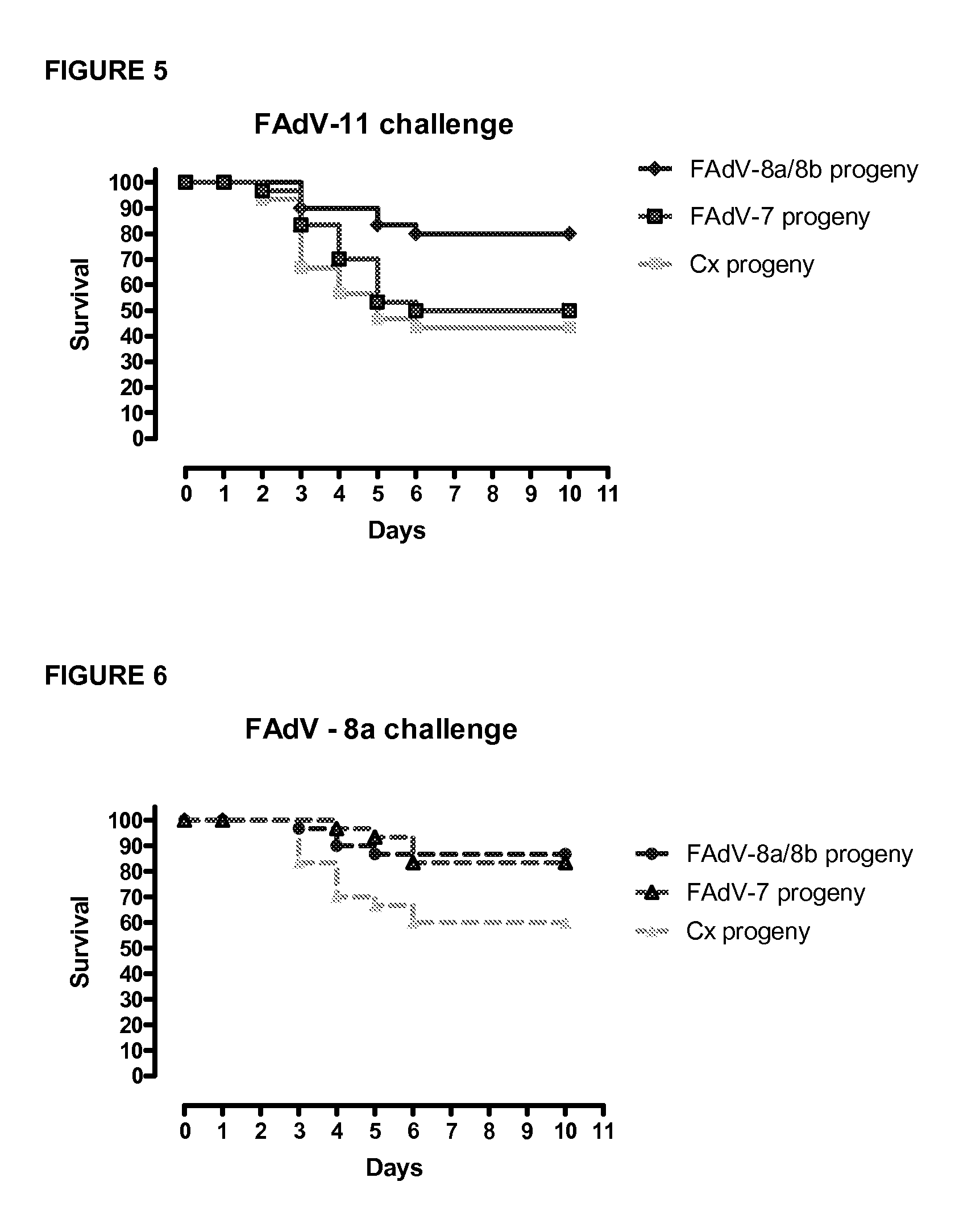
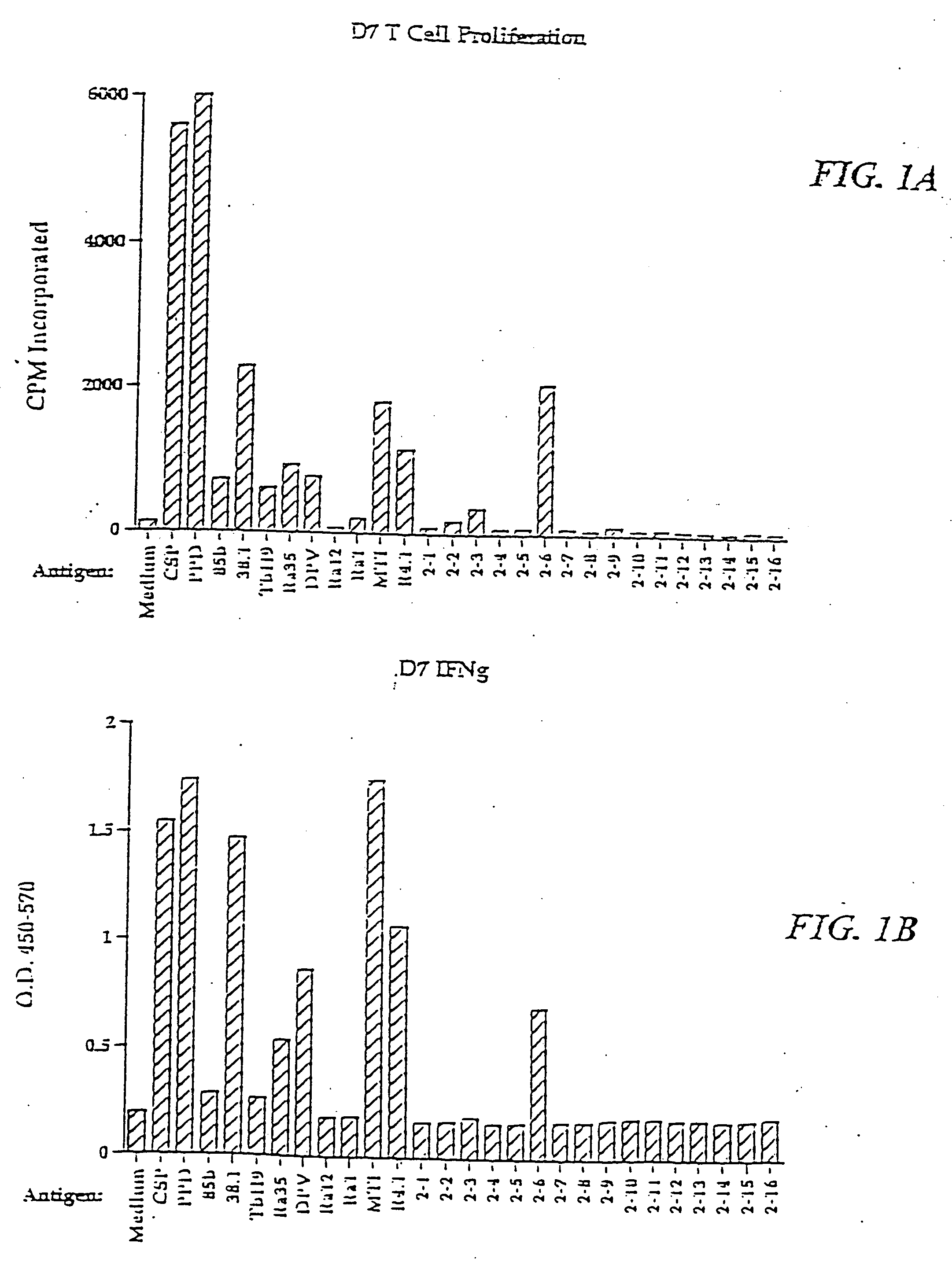
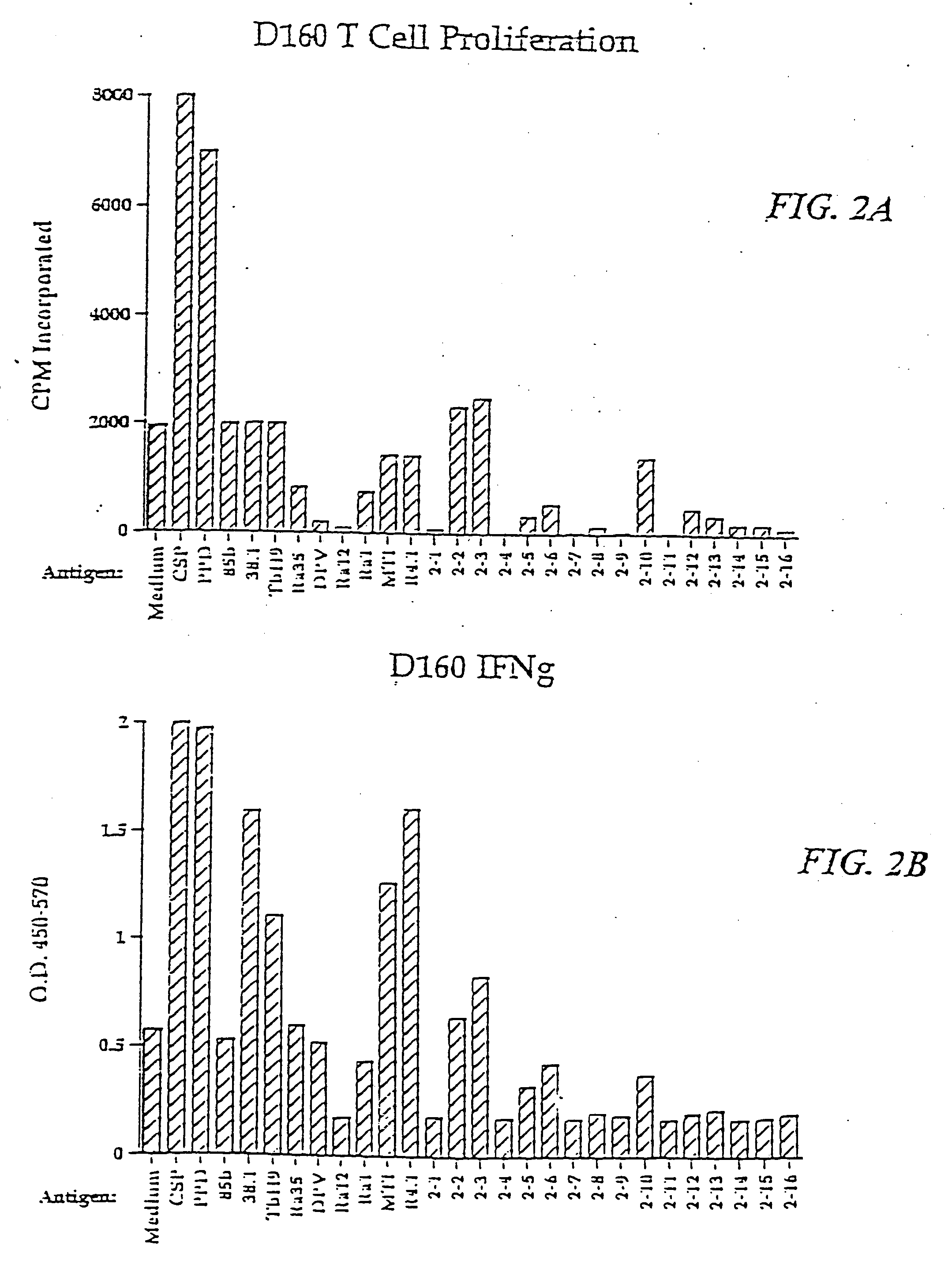
Vaccines for inclusion body hepatitis
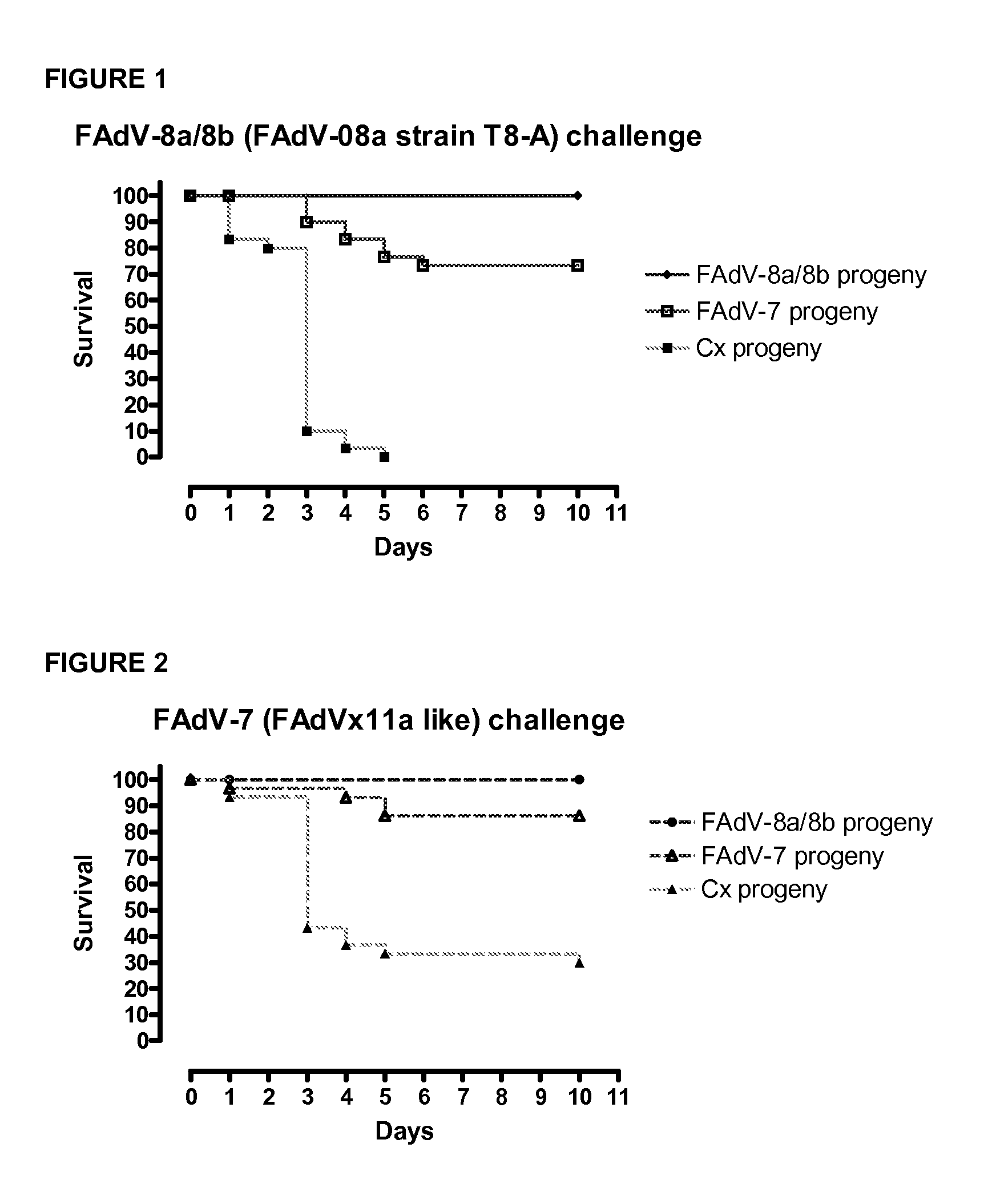
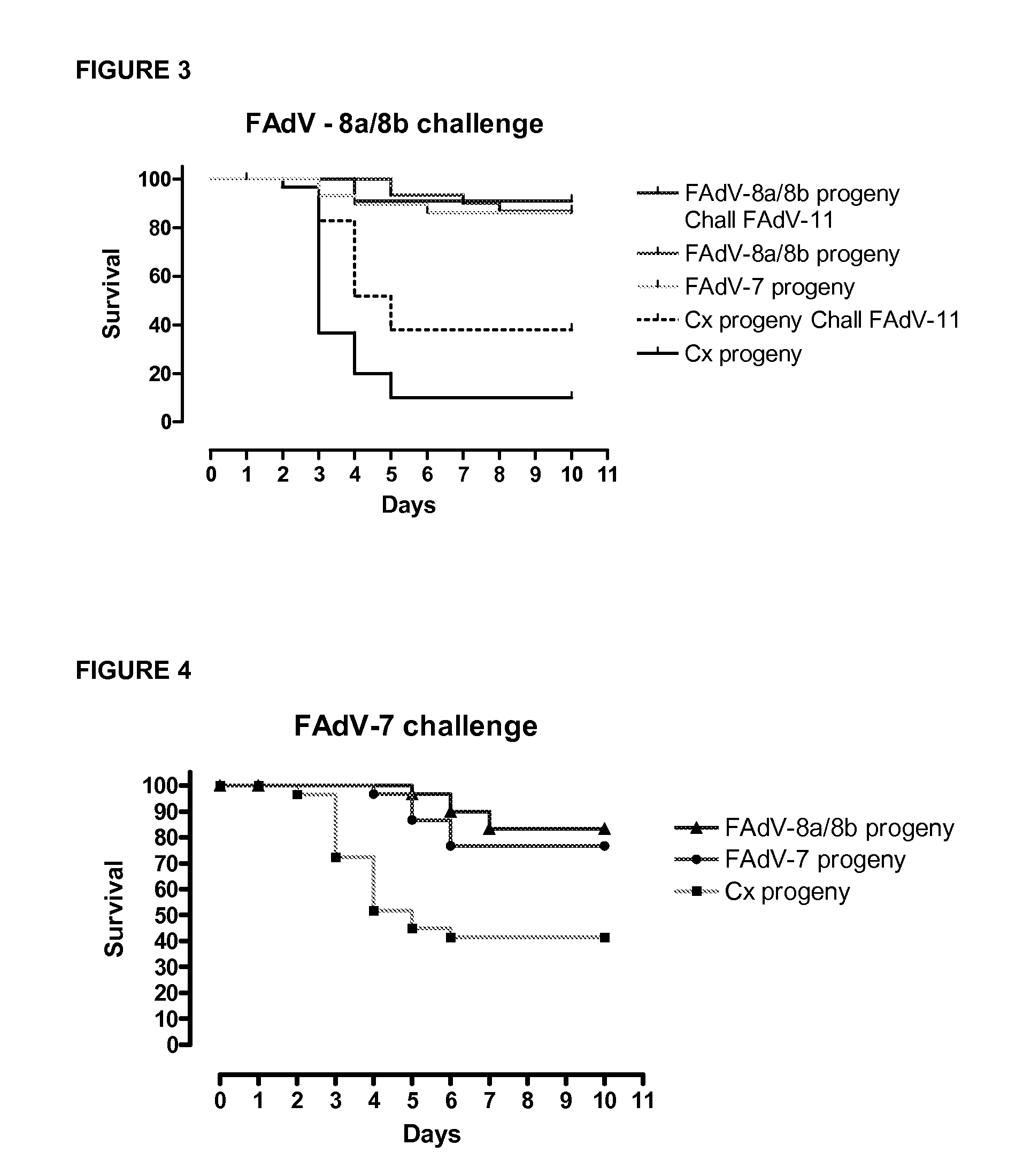
A composition comprising an isolated fowl adenovirus (FAdV), wherein the FAdV is a strain selected from FAdV-2, FAdV-7, FAdv-8a, FAdV-8b, FAdV-8a / 8b or FAdV-11 serotype strains; and a suitable carrier and methods for inducing protective immunity in a subject and / or its progeny.
Owner:UNIVERSITY OF GUELPH +1
Compounds and methods for diagnosis and immunotherapy of tuberculosis
Compounds and methods for diagnosing tuberculosis or for inducing protective immunity against tuberculosis are disclosed. The compounds provided include polypeptides that contain at least one immunogenic portion of one or more Mycobacterium proteins and DNA molecules encoding such polypeptides. Diagnostic kits containing such polypeptides or DNA sequences and a suitable detection reagent may be used for the detection of Mycobacterium infection in patients and biological samples. Antibodies directed against such polypeptides are also provided. In addition, such compounds may be formulated into vaccines and / or pharmaceutical compositions for immunization against Mycobacterium infection.
Owner:CORIXA CORP
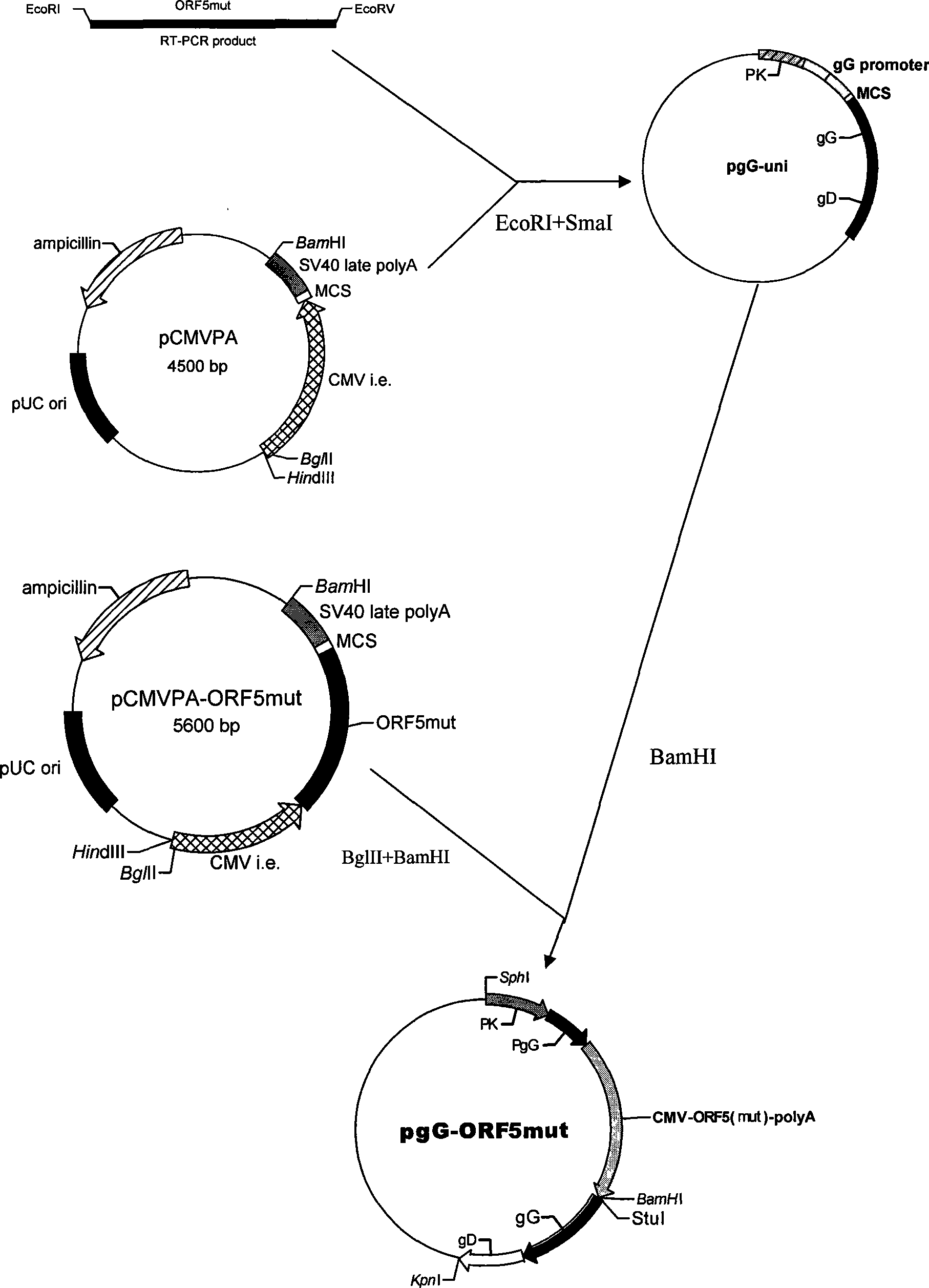
Recombinant porcine pseudorabies virus-porcine propagate and breath complex virus-porcine circovirus genetic engineering strain and application
InactiveCN101457215AAvoid infectionPrevention of swine pseudorabiesViral antigen ingredientsMicroorganism based processesAnimal virusAntigen
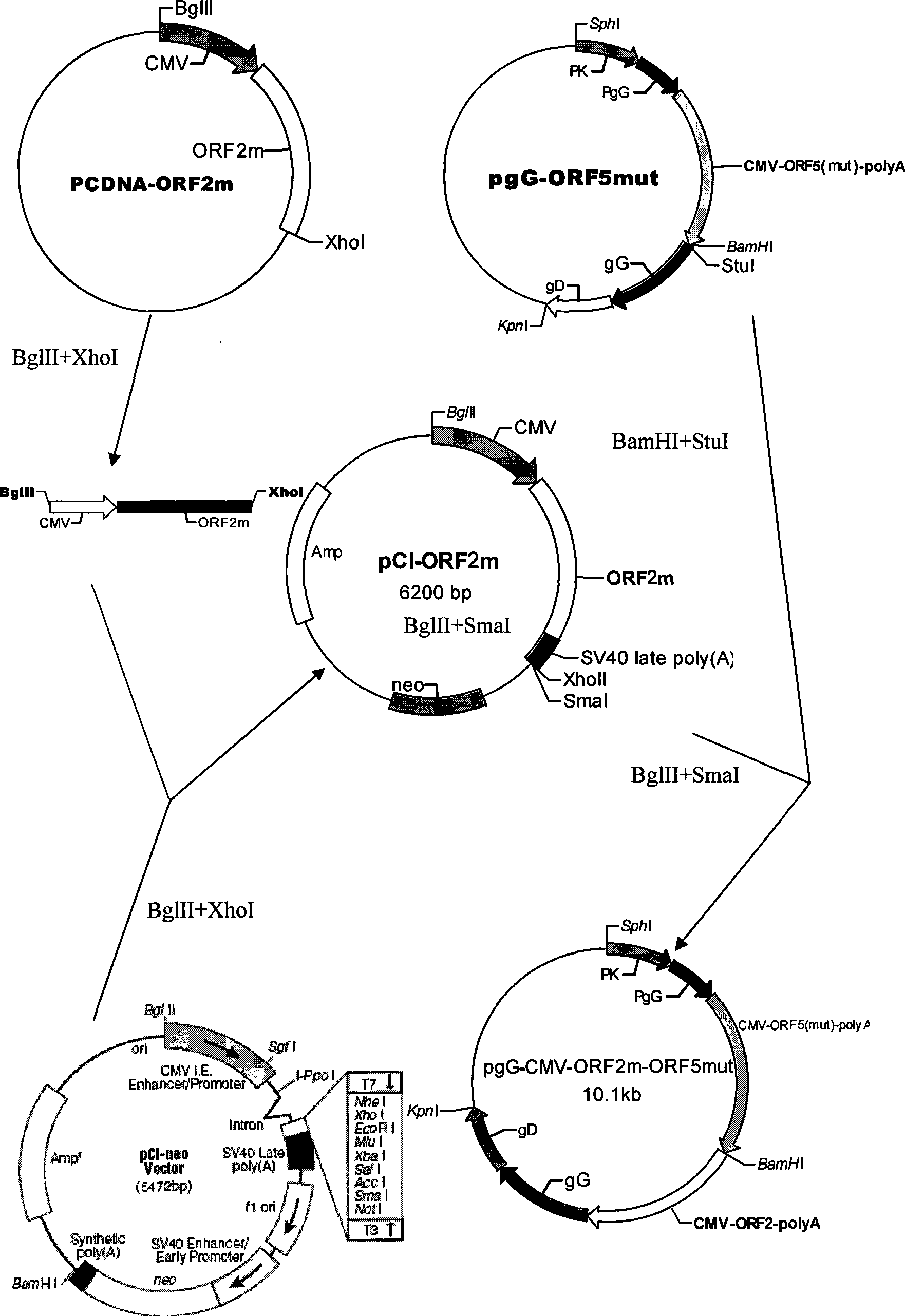
The invention belongs to the animal virus genetic engineering technique field, especially to a recombinant porcine pseudorabies virus-porcine propagate and breath complex virus-porcine circovirus gene engineering strain construction, a vaccine preparation and applications. The recombinant porcine pseudorabies virus-porcine propagate and breath complex virus-porcine circovirus gene engineering strain E001 lacks the pseudorabies virus main virulence gene TK, glycoprotein genes gG, gE and gI, does not express the functional glycoprotein gG and gE / gI of the pseudorabies virus; and simultaneously expresses a GP5m / M protein antigen of the porcine propagate and breath complex virus classical strain and the porcine propagate and breath complex virus internal variant GP5 protein and porcine circovirus ORF2. The strain can stimulate the porcine to produce protective immunity reaction for resisting pseudorabies virus-porcine propagate and breath complex virus-porcine circovirus, effectively prevent the infection of the pseudorabies virus, the porcine propagate and breath complex virus and porcine circovirus. The invention also discloses a preparation and application of a tervalence genetic engineering vaccine.
Owner:HUAZHONG AGRI UNIV
Coccidial vaccine and methods of making and using same
ActiveUS6908620B2Reduce cross infectionBird performanceProtozoa antigen ingredientsAllergen ingredientsCoccidiosisEscherichia coli
The present invention relates to a vaccine for coccidiosis in chickens prepared from four attenuated Eimeria species: E. acervulina, E. maxima, E. mitis and E. tenella. The vaccine was similar to or superior to other anticoccidial drugs in stimulating protective immunity against coccidiosis.
Owner:UNIV OF GEORGIA RES FOUND INC
Method of conferring a protective immune response to norovirus
ActiveUS20110182975A1Increase antigen retention timeEnhance immune responseAntibacterial agentsSsRNA viruses positive-senseAdjuvantProtective immunity
The present invention relates to vaccine compositions comprising Norovirus antigens and adjuvants, in particular, mixtures of monovalent VLPs and mixtures of multivalent VLPs, and to methods of conferring protective immunity to Norovirus infections in a human subject.
Owner:TAKEDA VACCINES INC
Avipox recombinants expressing foot and mouth disease virus genes
ActiveUS7527960B2SsRNA viruses positive-senseViral antigen ingredientsGene productProtective immunity
The present invention relates to modified poxviral vectors and to methods of making and using the same. In particular, the invention relates to recombinant avipox that expresses gene products of foot and mouth disease virus (FMDV), and to compositions or vaccines that elicit immune responses directed to FMDV gene products and which can confer protective immunity against infection by FMDV.
Owner:MERIAL INC
Deletion mutants of flagellin and methods of use
InactiveUS20110135680A1Cost effectiveMaximize immunogenic responseAntibacterial agentsPowder deliveryToll-like receptorProtective immunity
Compositions that include Toll-like Receptor 5 agonists and at least a portion of at least one viral antigen can be employed in methods that stimulate an immune response in a subject, in particular, a protective immune response in a subject. Compositions can be associated with particles and employed in the methods in relatively low doses to provide protective immunity to viral infection.
Owner:VAXINNATE
Continuous Cell Programming Devices
InactiveUS20160220668A1Easy to controlClinical effectiveness of has been limitedOrganic active ingredientsPeptide/protein ingredientsDendritic cellProtective immunity
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE +1
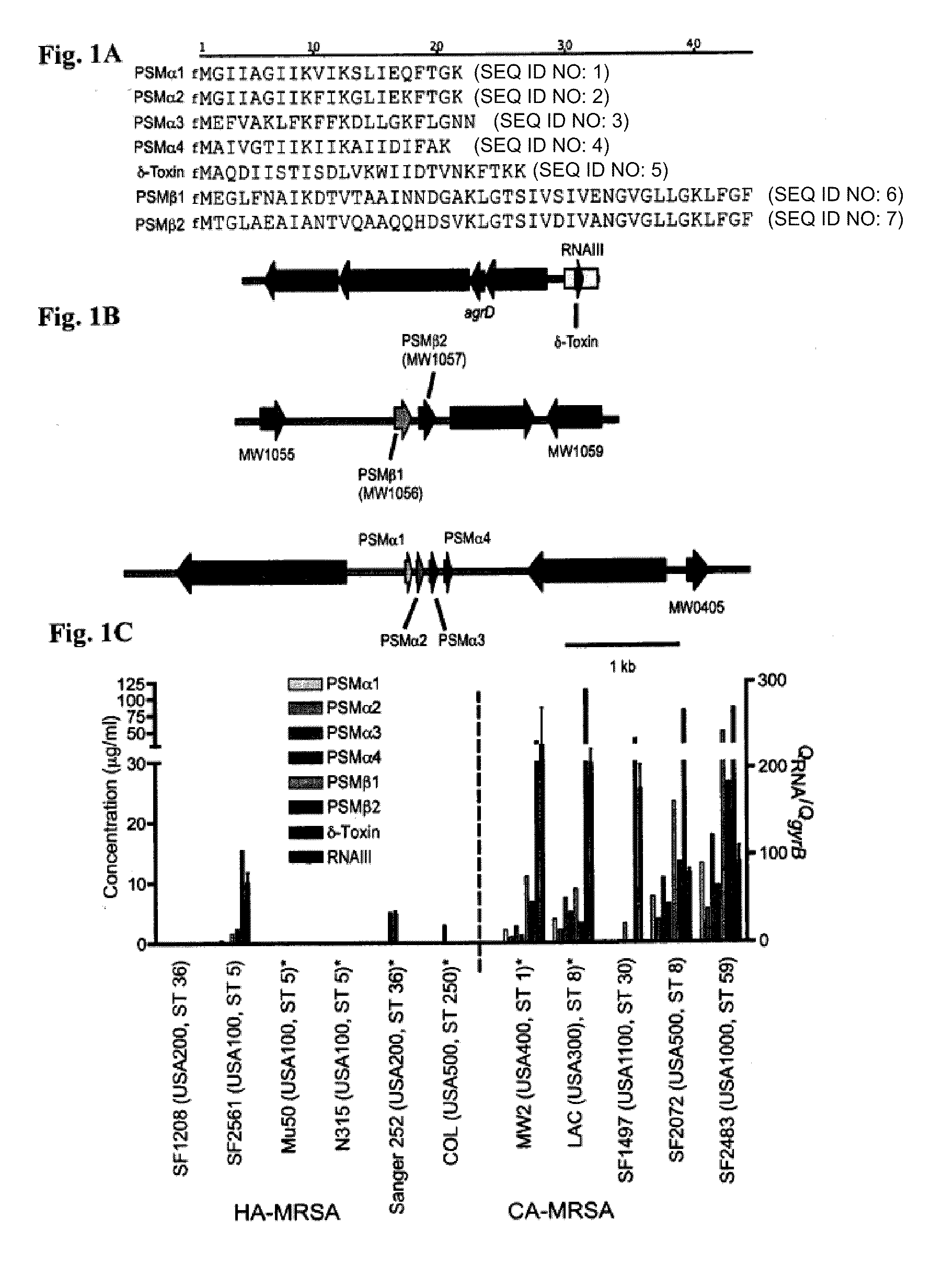
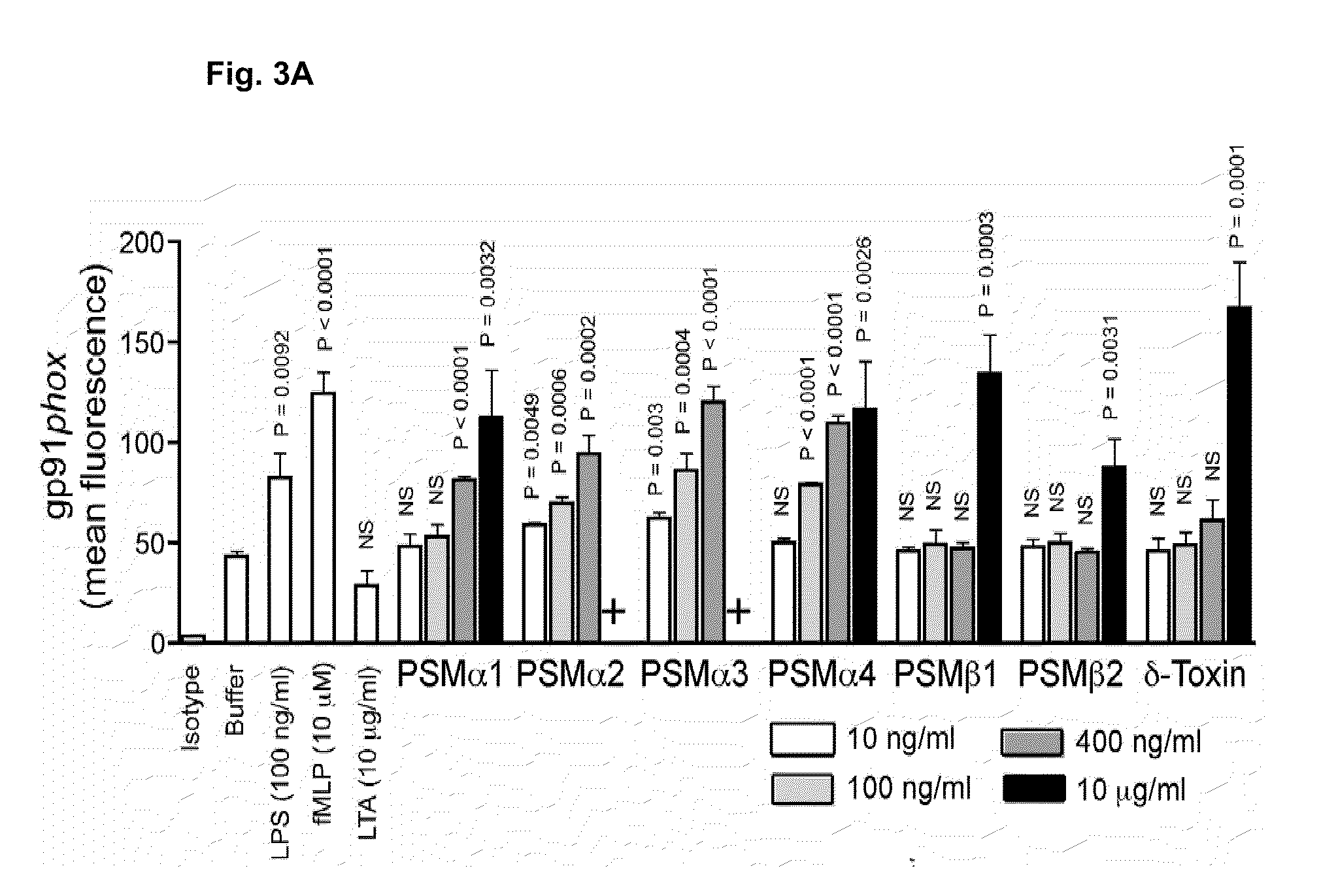
Psm peptides as vaccine targets against methicillin-resistant staphylococcus
ActiveUS20100119477A1Enable productionAntibacterial agentsPeptide/protein ingredientsProtective immunityImmunogenic peptide
This disclosure concerns compositions and methods for the treatment and inhibition of infectious disease, particularly methicillin-resistant Staphylococcus. In certain embodiments, the disclosure concerns immunogenic peptides, for instance PSM peptides, which can be used to induce protective immunity against methicillin-resistant Staphylococcus. Also disclosed are methods of detecting methicillin-resistant staphylococcus in a sample, and methods of diagnosing methicillin-resistant staphylococcus in a subject.
Owner:UNITED STATES OF AMERICA
Soluble pd-1 variants, fusion constructs, and uses thereof
Provided are soluble PD-1 (sPD-1) proteins, fusion proteins comprising said sPD-1 proteins, nucleic acids encoding said sPD-1 proteins and fusion proteins. The sPD-1 proteins have the amino acid sequences as shown in SEQ ID NO: 11, SEQ ID NO: 15 and SEQ ID NO: 25. The fusion proteins have the amino acid sequences as shown in SEQ ID NO: 13, SEQ ID NO: 17, SEQ ID NO: 19, SEQ ID NO: 23 and SEQ ID NO: 27. Also provided is pharmaceutical composition, especially a vaccine composition, comprising the sPD-1 proteins, fusion proteins or nucleic acids. The sPD-1 proteins, fusion proteins, nucleic acids and therapeutic composition of the present invention can provide protective immunity against pathogenic infection including HIV infection, and can be used in the prevention and / or treatment of tumor or cancer.
Owner:VERSITECH LTD
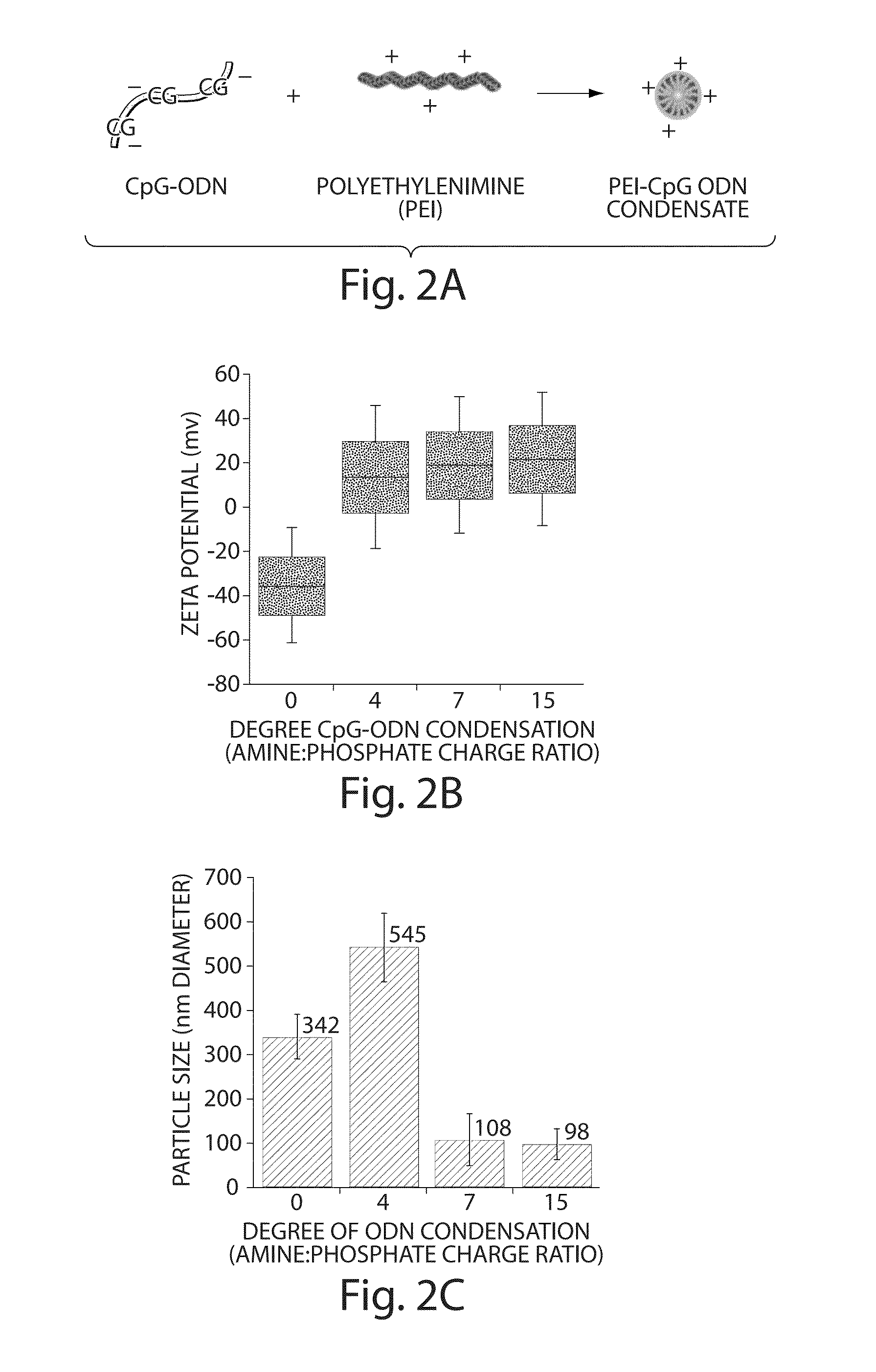
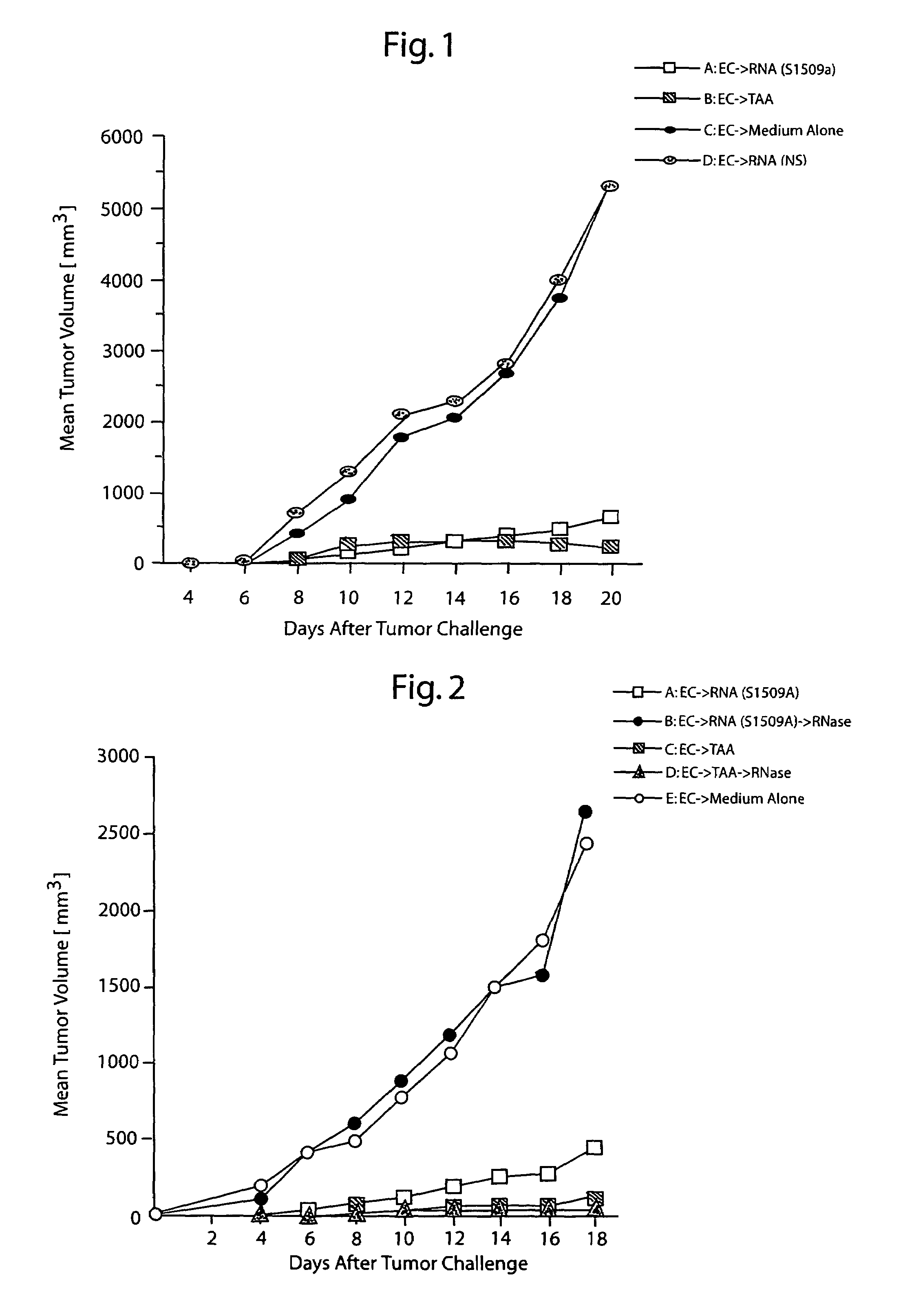
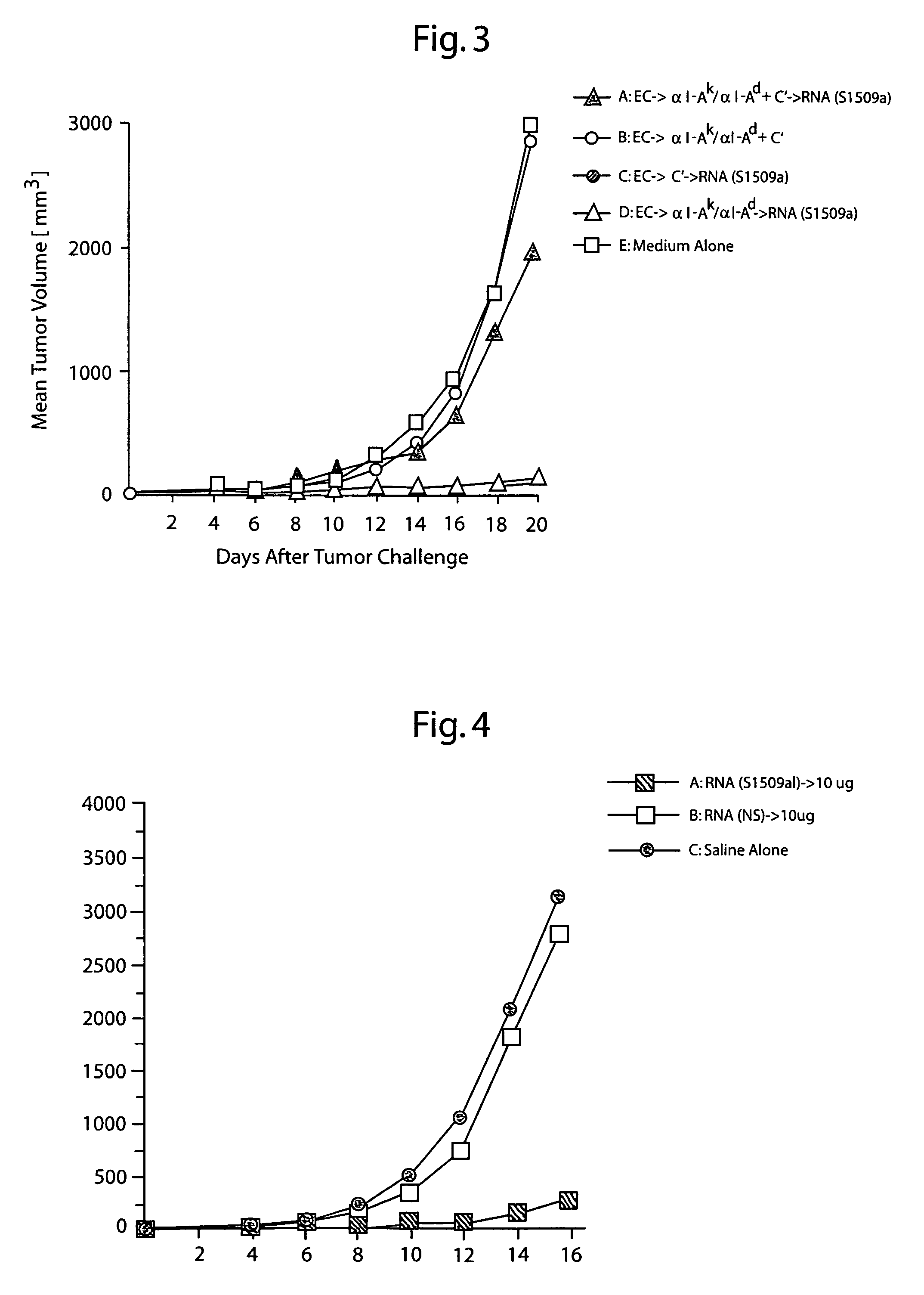
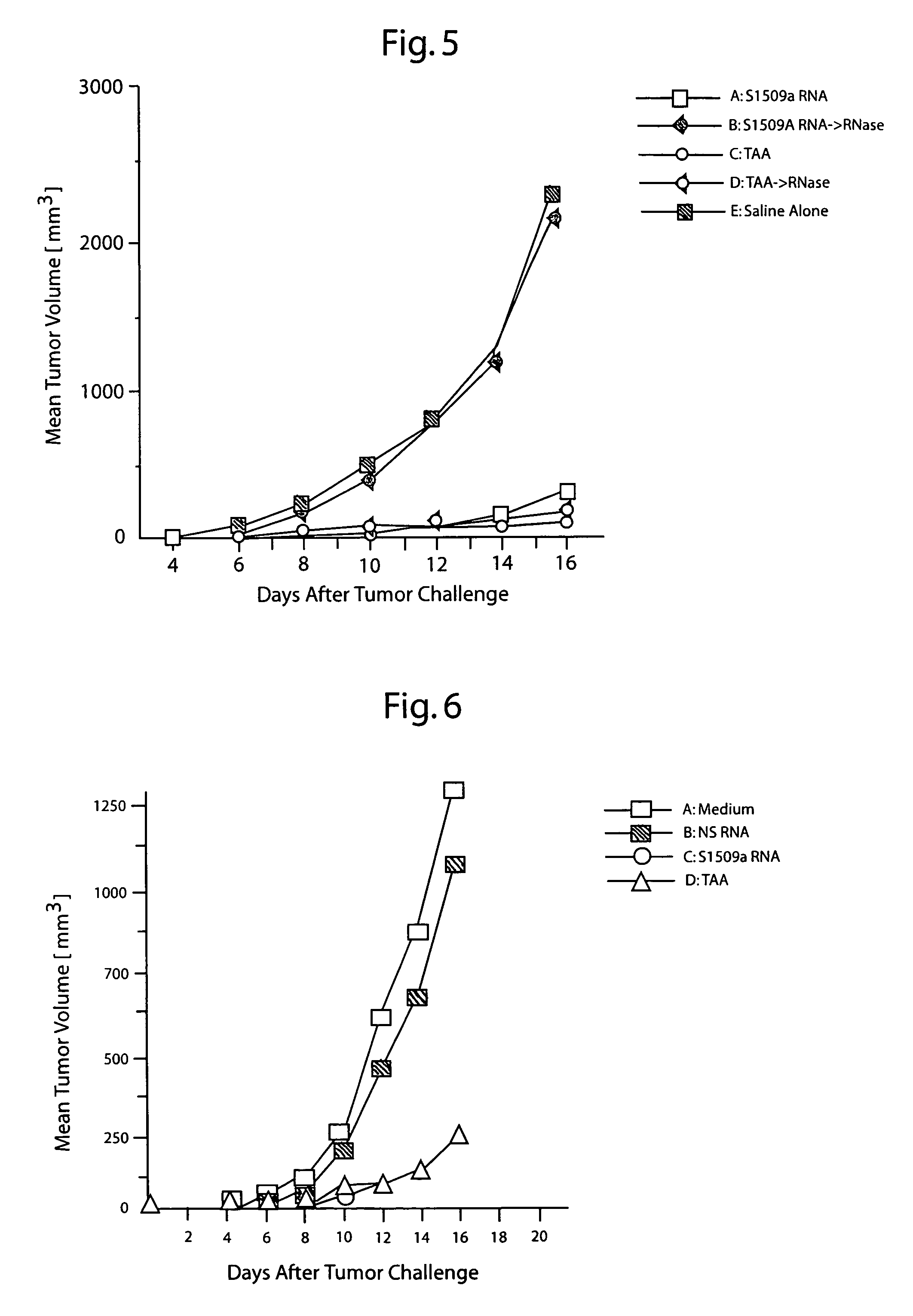
Protective immunity or immunological tolerance induced with RNA particularly total cellular RNA
InactiveUS7015204B1Reducing and inhibiting growth of tumorPathogen infection can be reduced and inhibitedBiocideSugar derivativesAbnormal tissue growthCuticle
Effective anti-tumor immunity is induced in mice utilizing RNA-pulsed epidermal cells (EC) for in vivo immunization or by injecting RNA intradermally into naïve mice. A vaccine comprising total cell RNA and a pharmaceutically acceptable carrier for inducing an immune response to reduce or prevent the occurrence of a tumor.
Owner:CORNELL RES FOUNDATION INC
Immunogenic compositions comprising nanoemulsion and hepatitis b virus immunogen and methods of using the same
ActiveUS20100028433A1Increased cellularityReduce the risk of infectionAntibacterial agentsPowder deliveryVaccinationPreventative Medicine
The present invention provides methods and compositions for the stimulation of immune responses. Specifically, the present invention provides immunogenic compositions and methods of using the same to induce immune responses (e.g., immunity (e.g., protective immunity)) against Hepatitis B virus (HBV)). Compositions and methods of the present invention find use in, among other things, clinical (e.g. therapeutic and preventative medicine (e.g., vaccination)) and research applications.
Owner:RGT UNIV OF MICHIGAN
Genetically engineered rabies recombinant vaccine for immunization of stray dogs and wildlife
InactiveUS7074413B2Reduce absorptionEnhance immune responseSsRNA viruses negative-senseVectorsWildlifeRecombinant vaccines
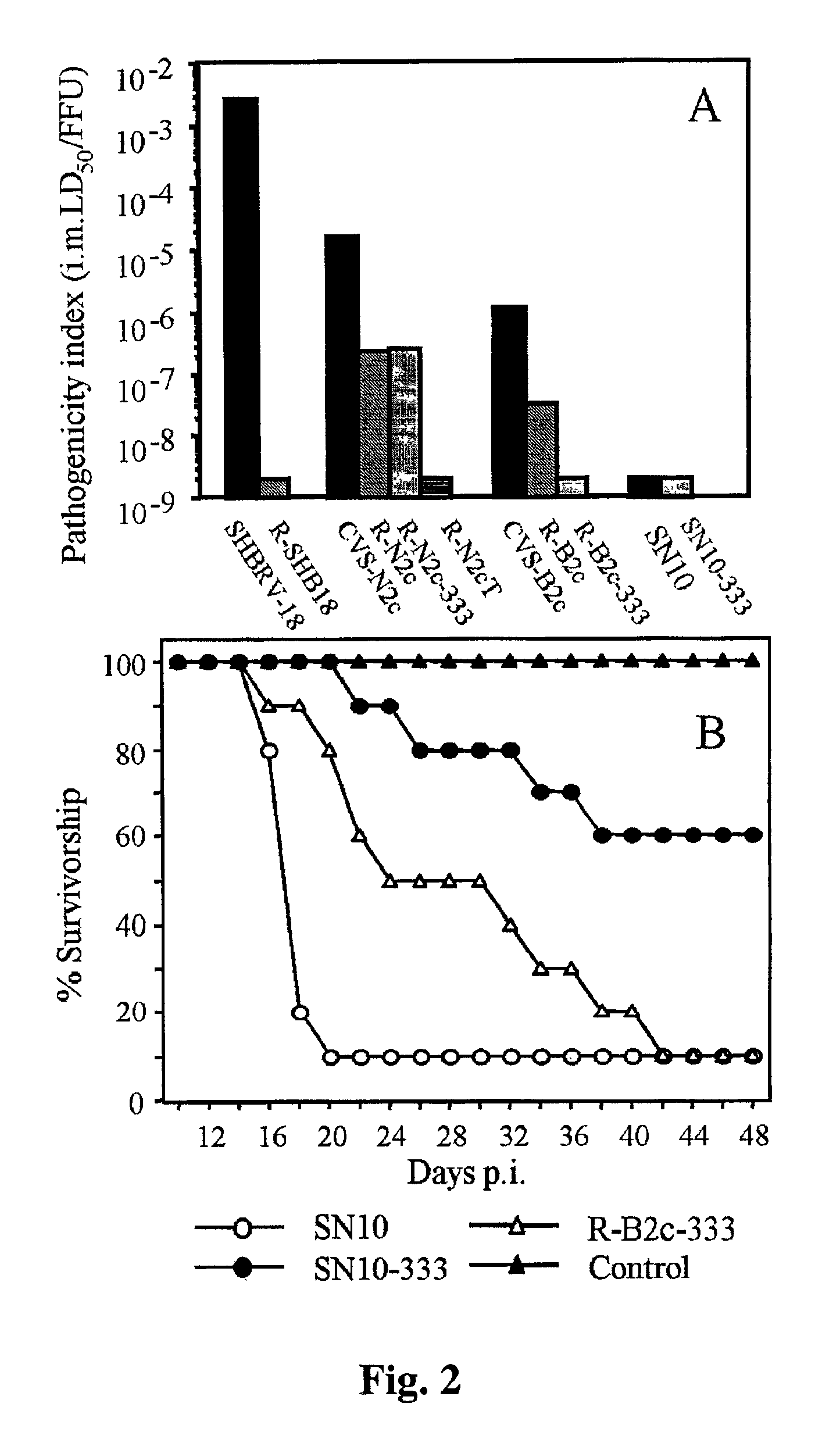
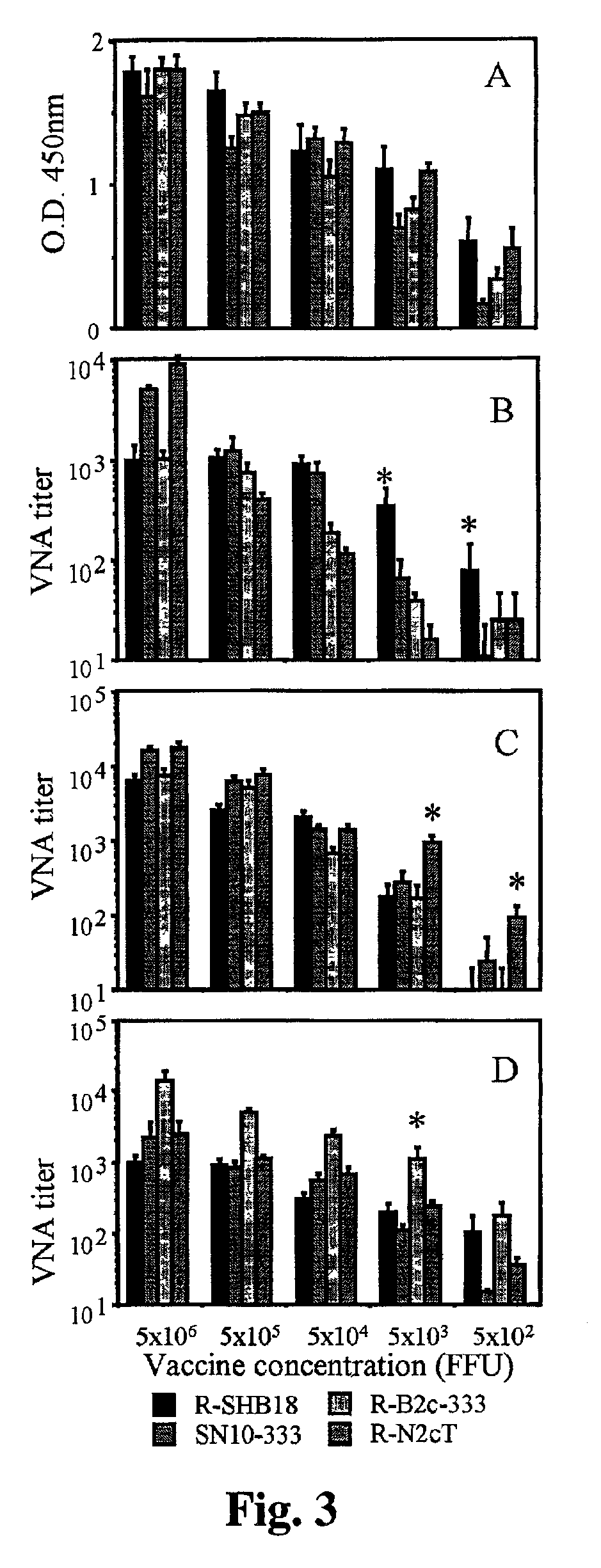
Live, attenuated recombinant rabies virus vaccines are generated using reverse genetics to combine the antigenic determinants that render the rabies virus non-pathogenic with the determinants that are responsible for the elicitation of an effective anti-rabies immune response. These vaccines do not affect the antigenic, and therefore the immunogenic, properties of the virus. The present invention further relates to recombinant rabies virus vaccines that express a pro-apoptotic protein, such as cytochrome c, to increase the capacity to induce apoptosis, thereby enhancing the protective immunity against rabies. This new generation of live rabies virus vaccines represents a safe and effective approach to the eradication of rabies in wildlife, and subsequently humans and livestock.
Owner:UNITED STATES OF AMERICA +1
Continuous cell programming devices
ActiveUS20160220667A1Easy to controlClinical effectiveness of has been limitedOrganic active ingredientsPeptide/protein ingredientsDendritic cellProtective immunity
The present invention comprises compositions, methods, and devices for creating an infection-mimicking environment within a polymer scaffold to stimulate antigen-specific dendritic cell activation. Devices of the present invention are used to provide protective immunity to subjects against infection and cancer.
Owner:DANA FARBER CANCER INST INC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com