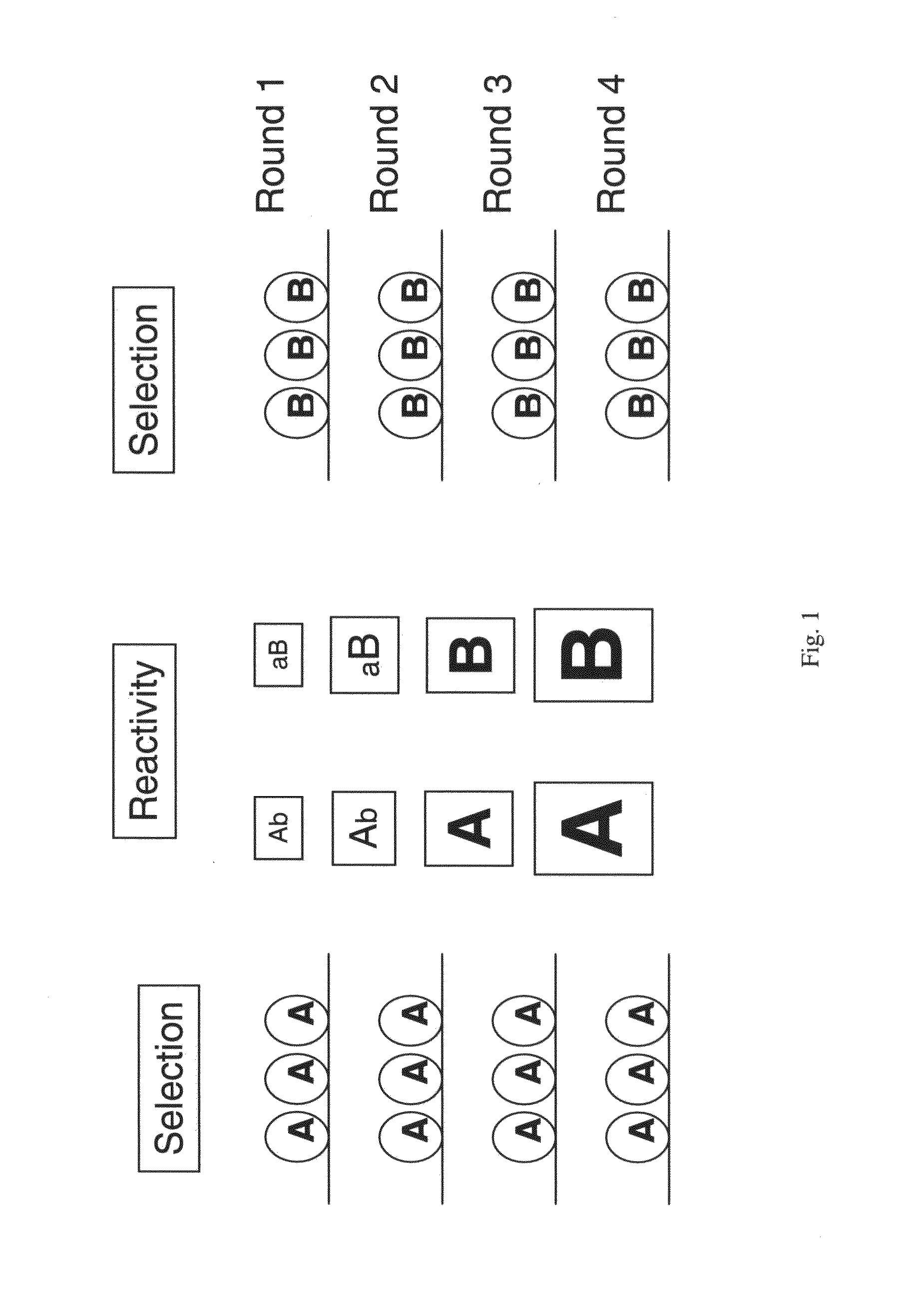
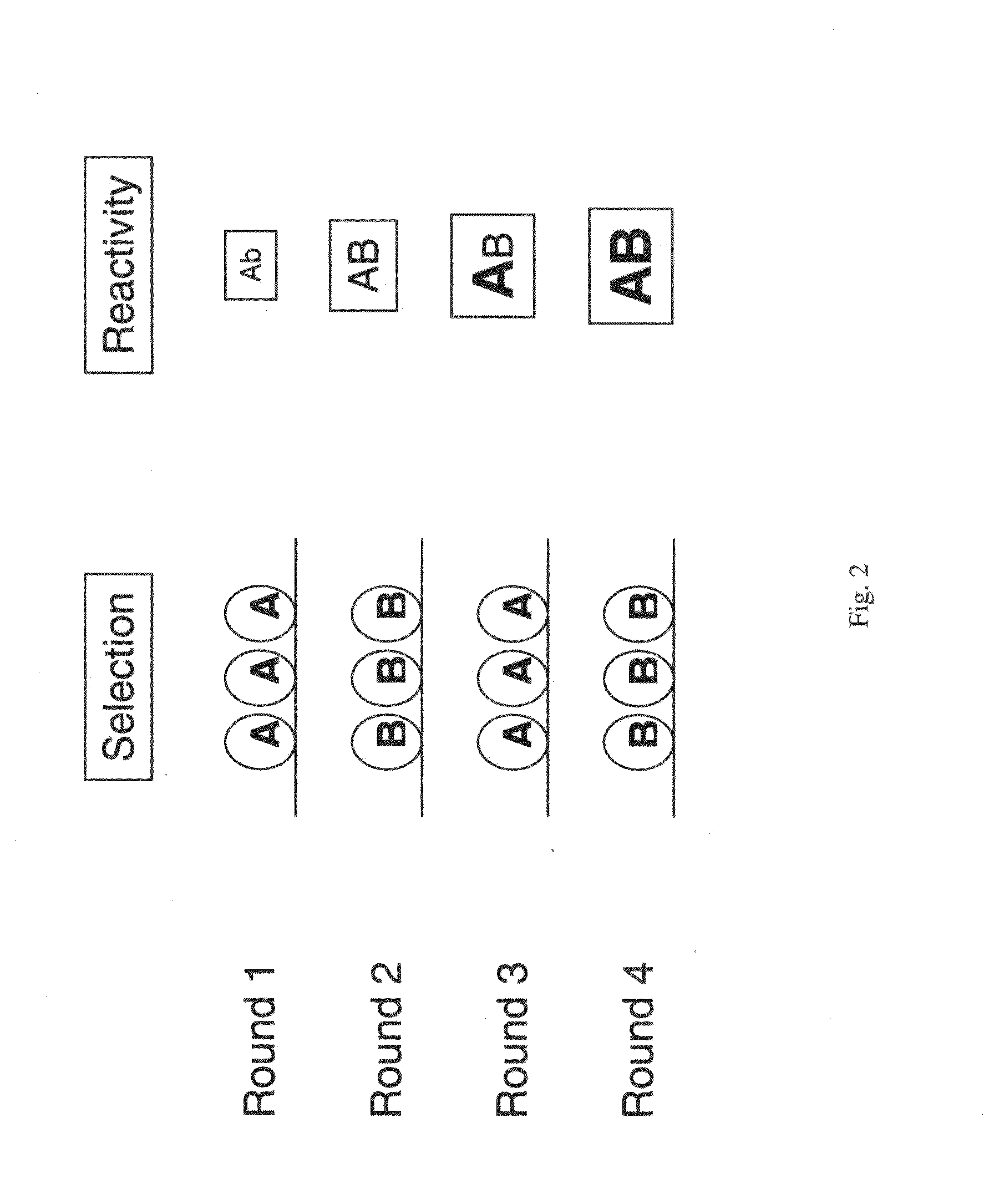
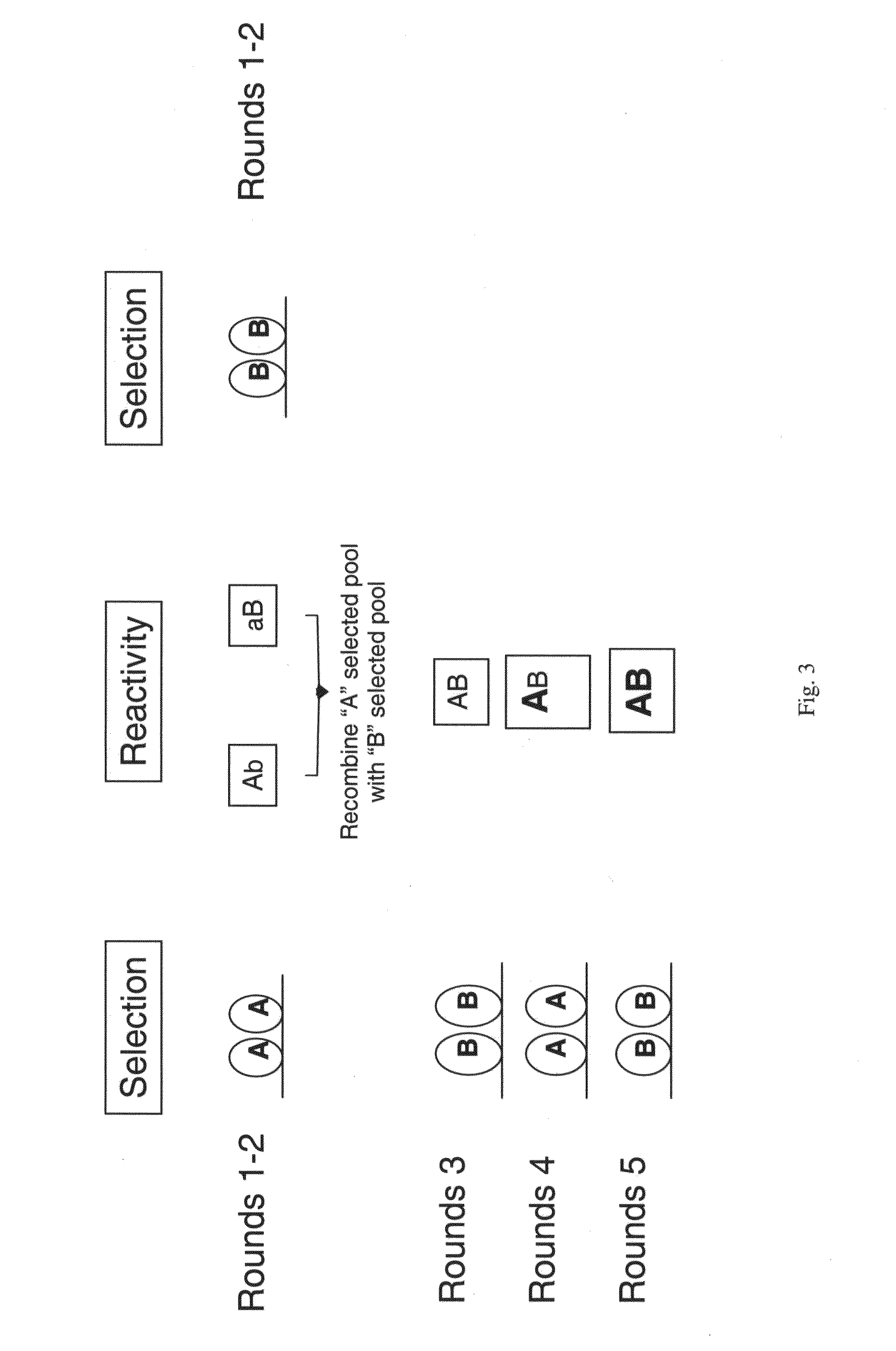
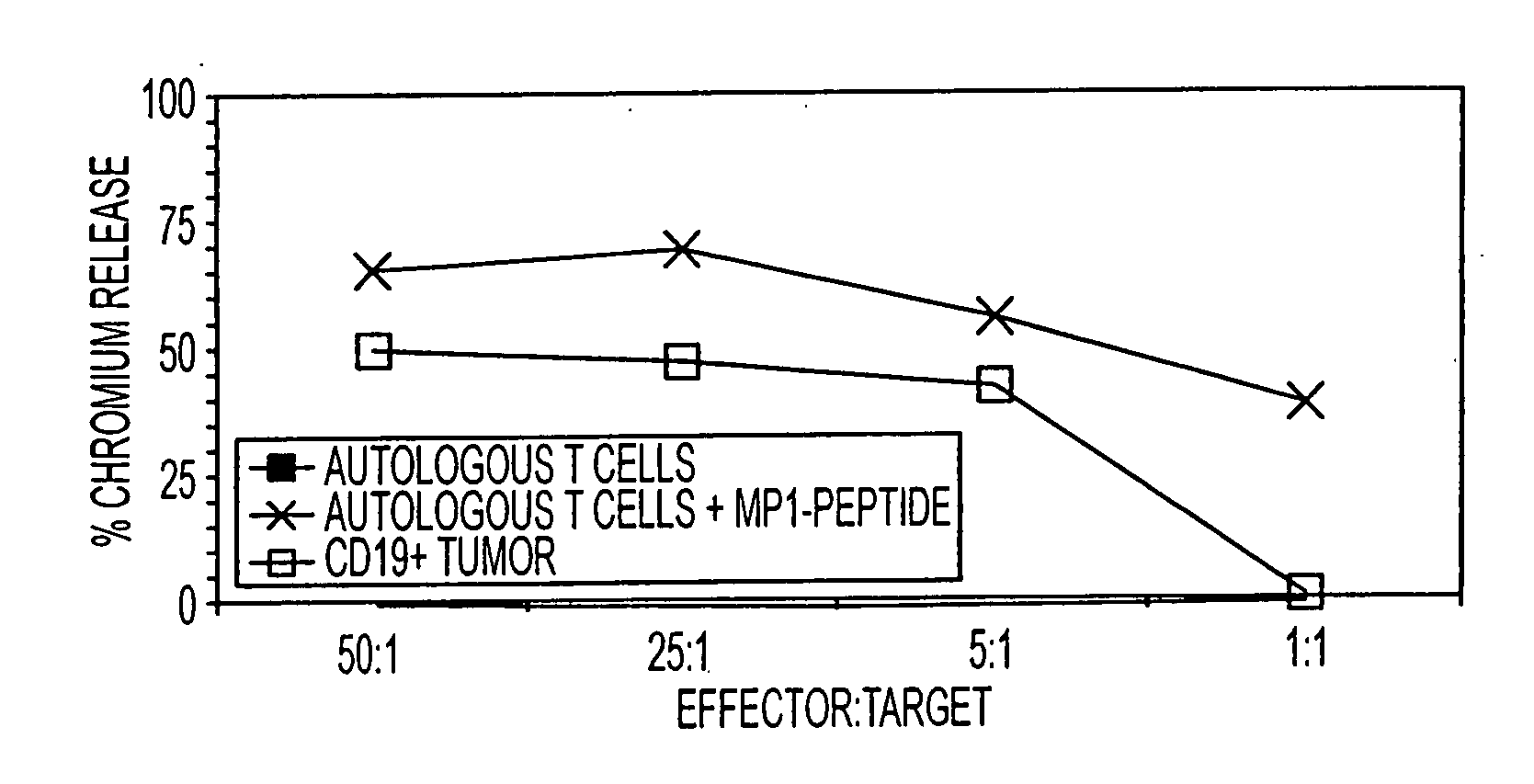
Patents
Literature
151 results about "Viral antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A viral antigen is a protein encoded by the viral genome.A viral protein is an antigen specified by the viral genome that can be detected by a specific immunological response.
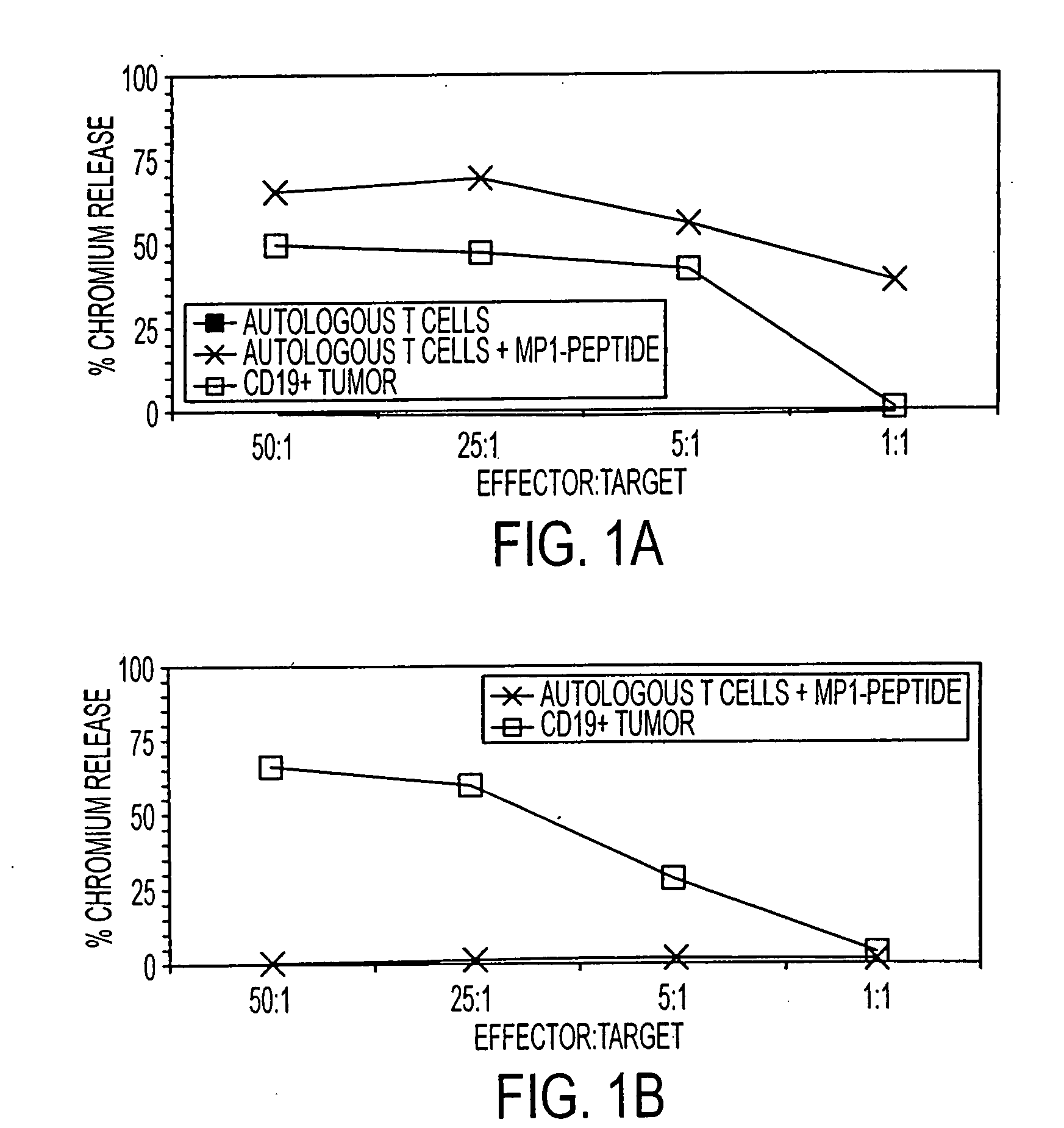
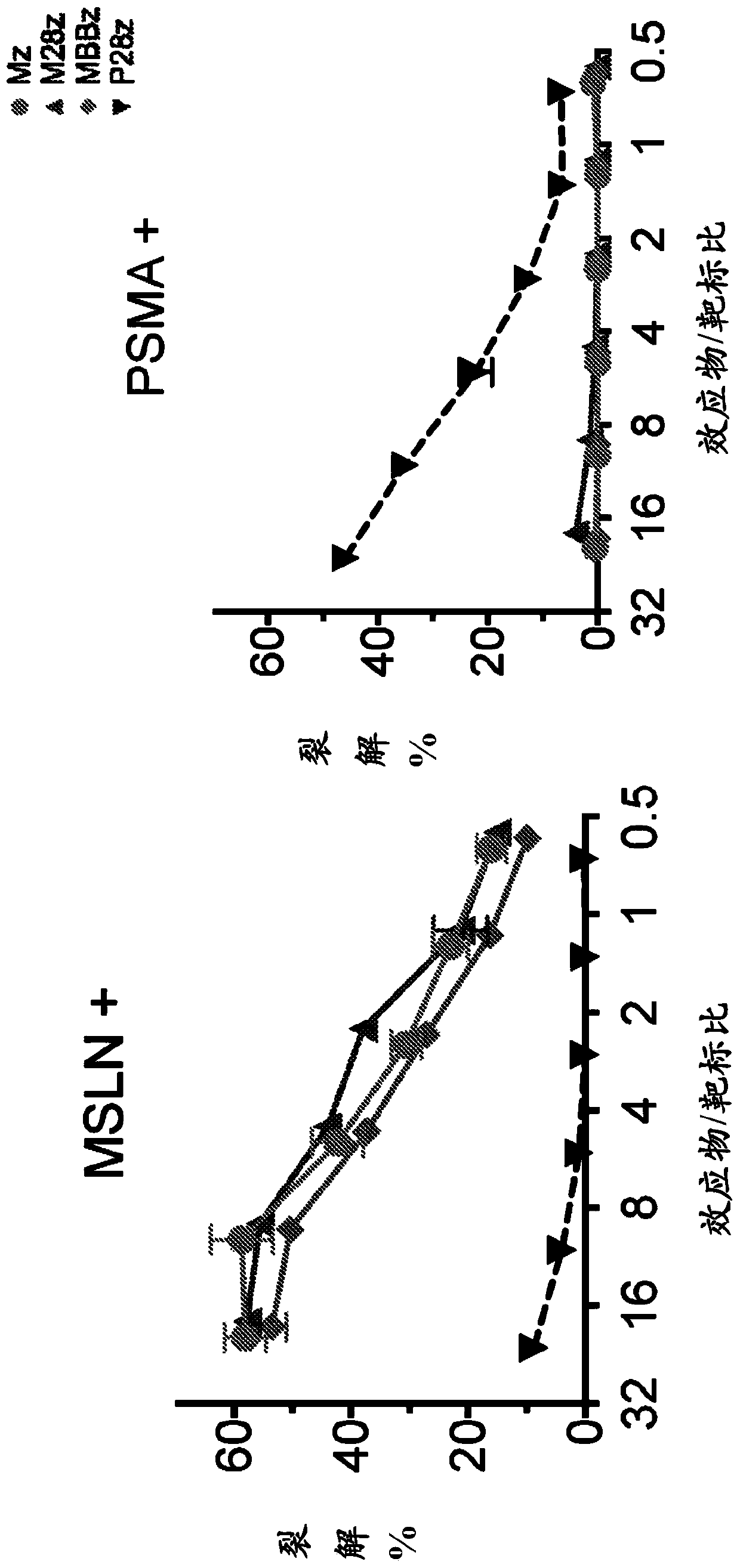
Mammalian antigen-presenting T cells and bi-specific T cells
InactiveUS20050129671A1Guaranteed functionImproving in vivo survivalBiocideGenetic material ingredientsAbnormal tissue growthAutoimmune condition
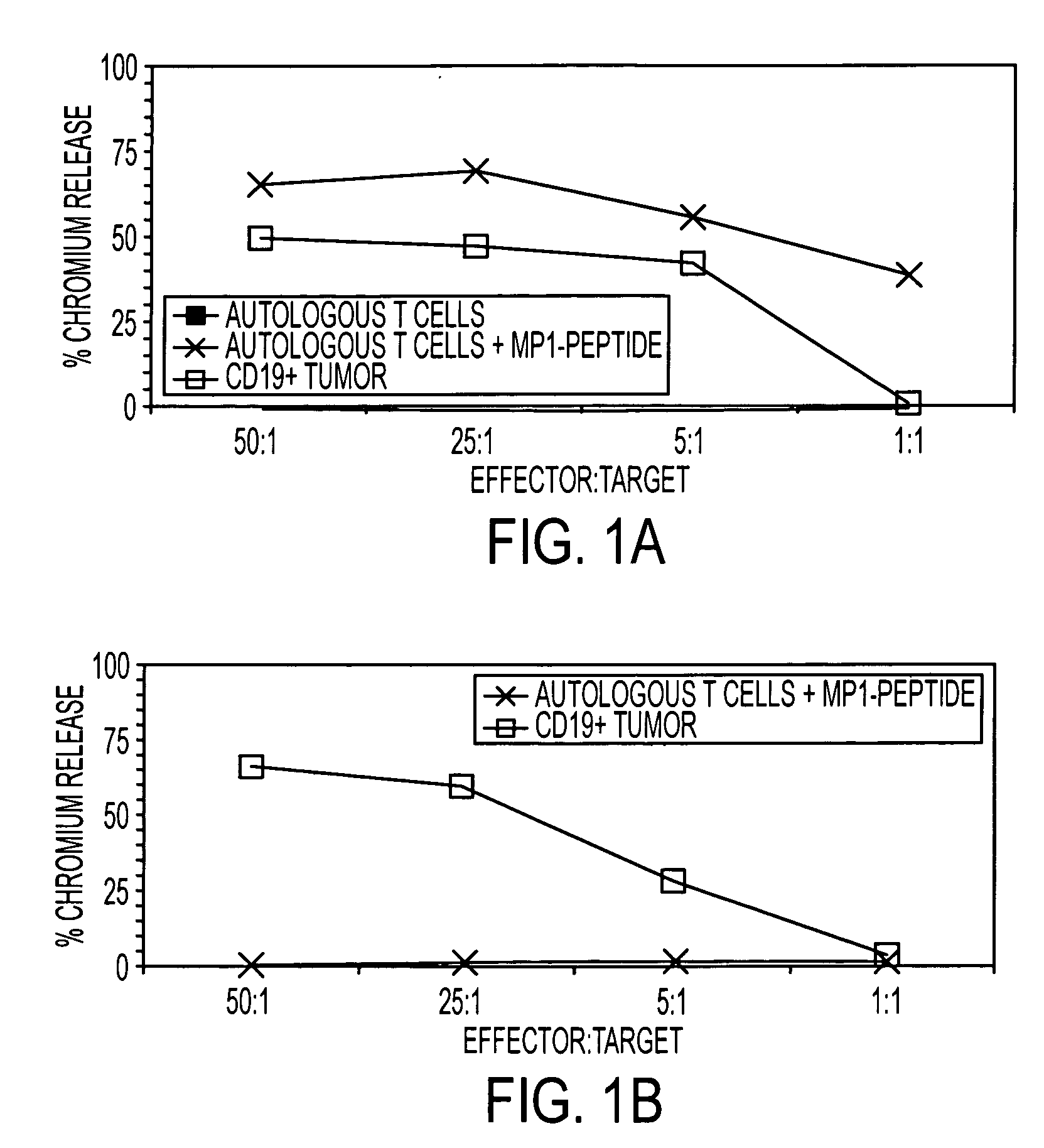
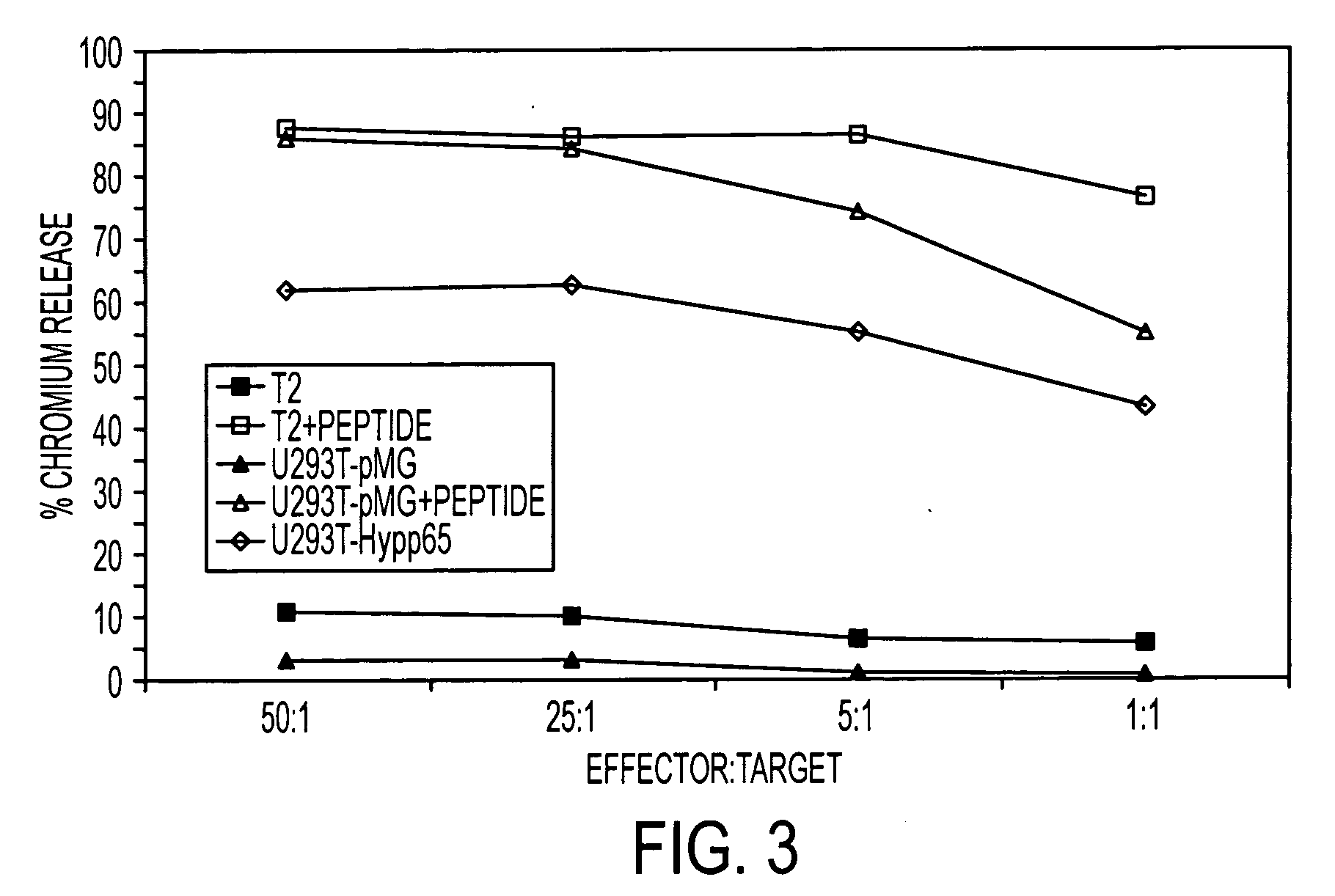
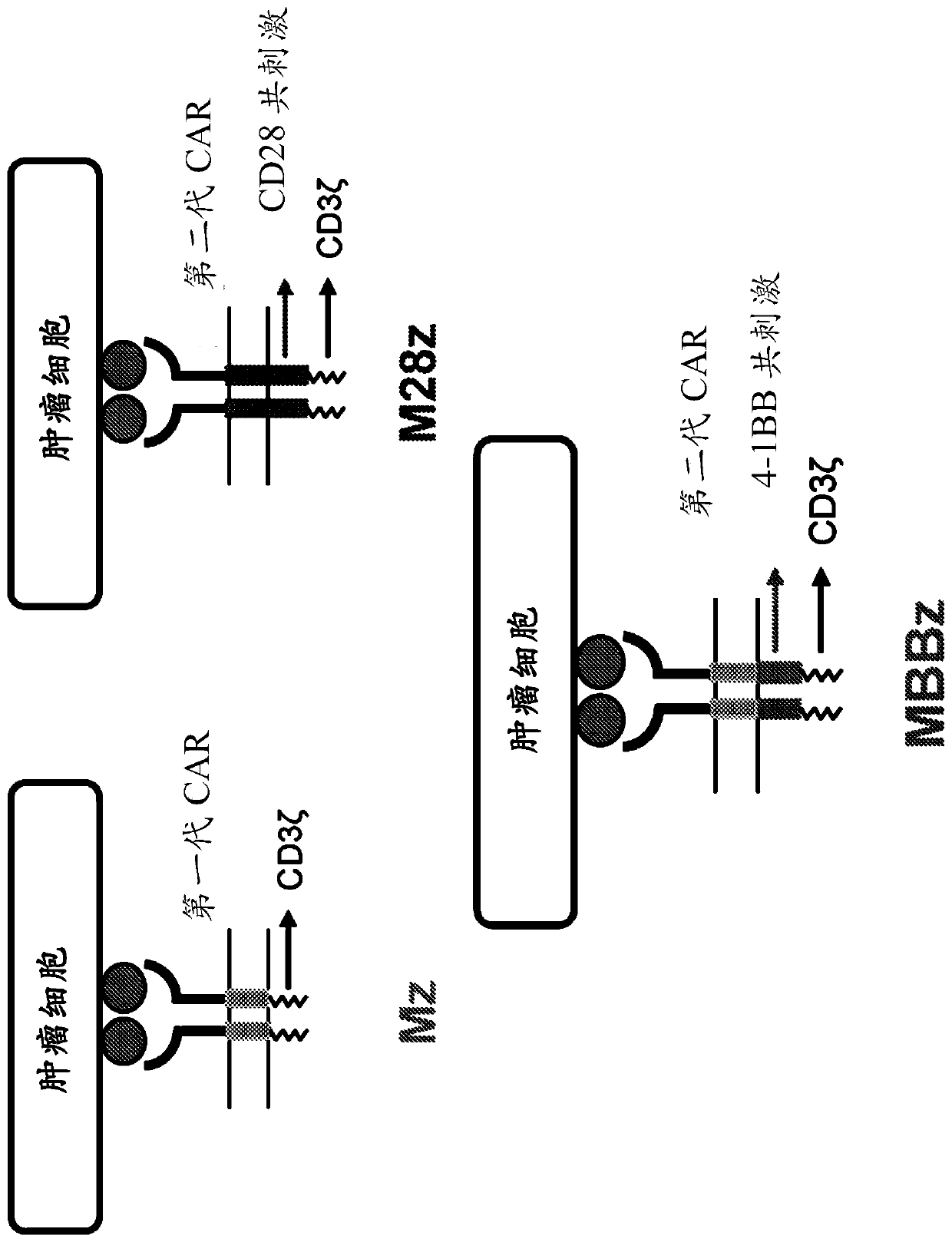
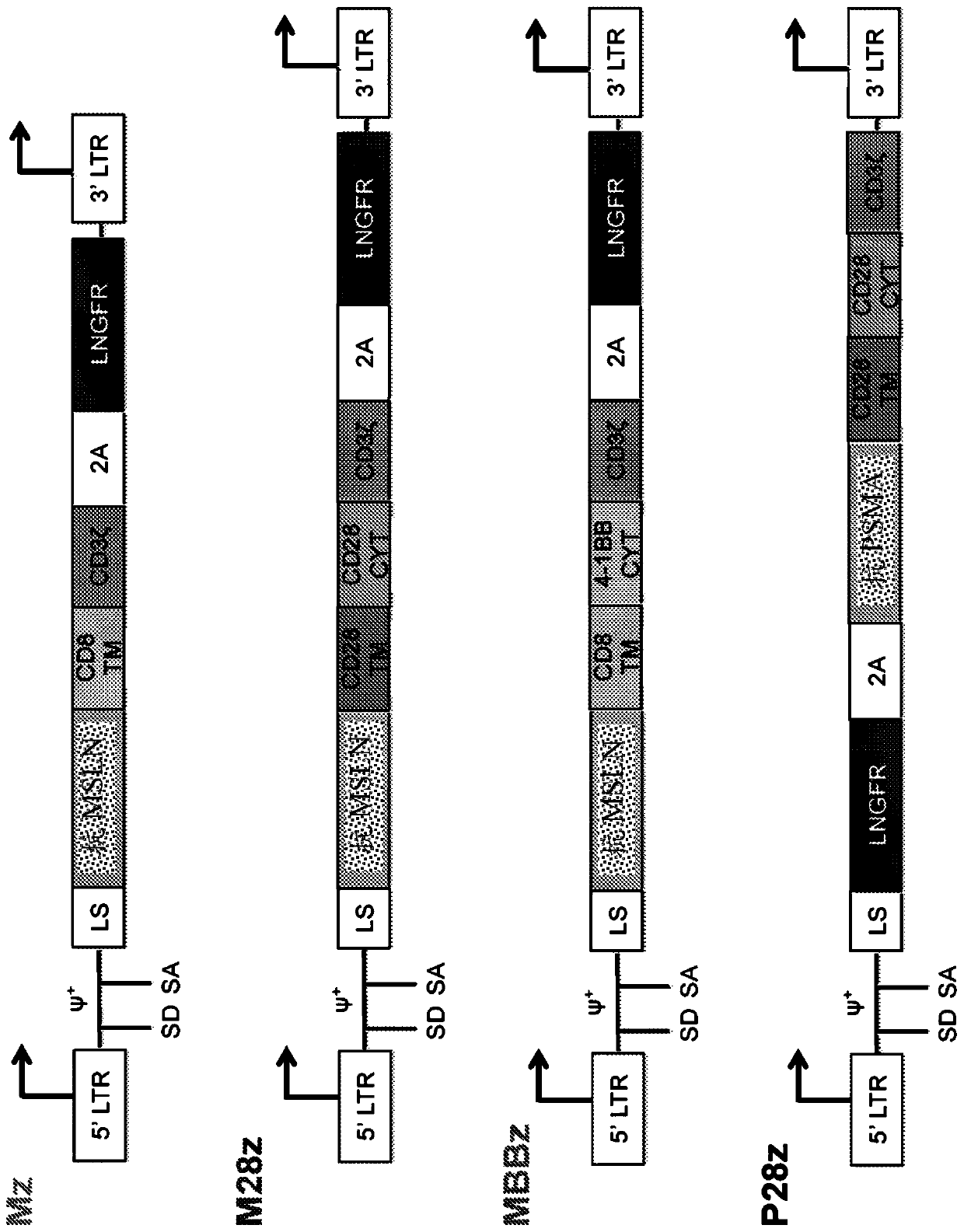
The present invention is directed to mammalian bi-specific T cells and methods for using these bi-specific T cells. More specifically, the invention relates to viral specific T cells that express chimeric anti-tumor receptors. These bi-specific T cell clones are a source of effector cells that persist in vivo in response to stimulation with viral antigen, leading to long-term function after their transfer to patients with cancer and autoimmune diseases.
Owner:CITY OF HOPE
mRNA and vaccine for coding a SARS-CoV-2 viral antigen and preparation method of vaccine
ActiveCN111218458AImproving immunogenicityRapid R&DSsRNA viruses positive-senseViral antigen ingredientsTGE VACCINECoding region
The invention provides mRNA and a vaccine for coding a SARS-CoV-2 viral antigen and a preparation method of the vaccine, and relates to the technical field of vaccines. The mRNA for coding the SARS-CoV-2 viral antigen at least contains at least one of S protein and N protein for coding SARS-CoV-2 virus and / or a coding region of a fragment of the at least one protein, and the mRNA is delivered intoa body to enable the body to generate an immune reaction.
Owner:LIVERNA THERAPEUTICS INC
Methods and reagents to detect and characterize norwalk and related viruses
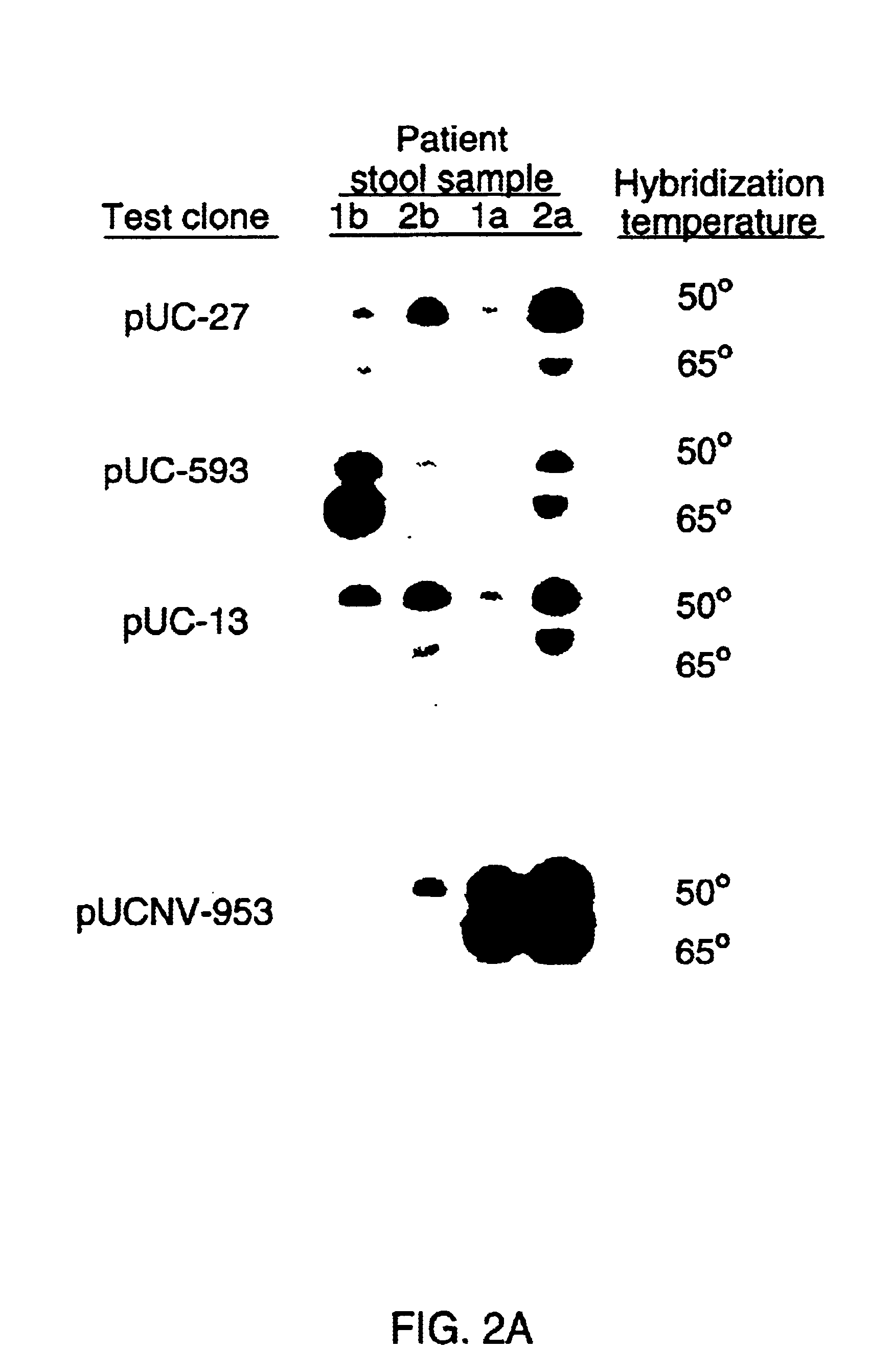
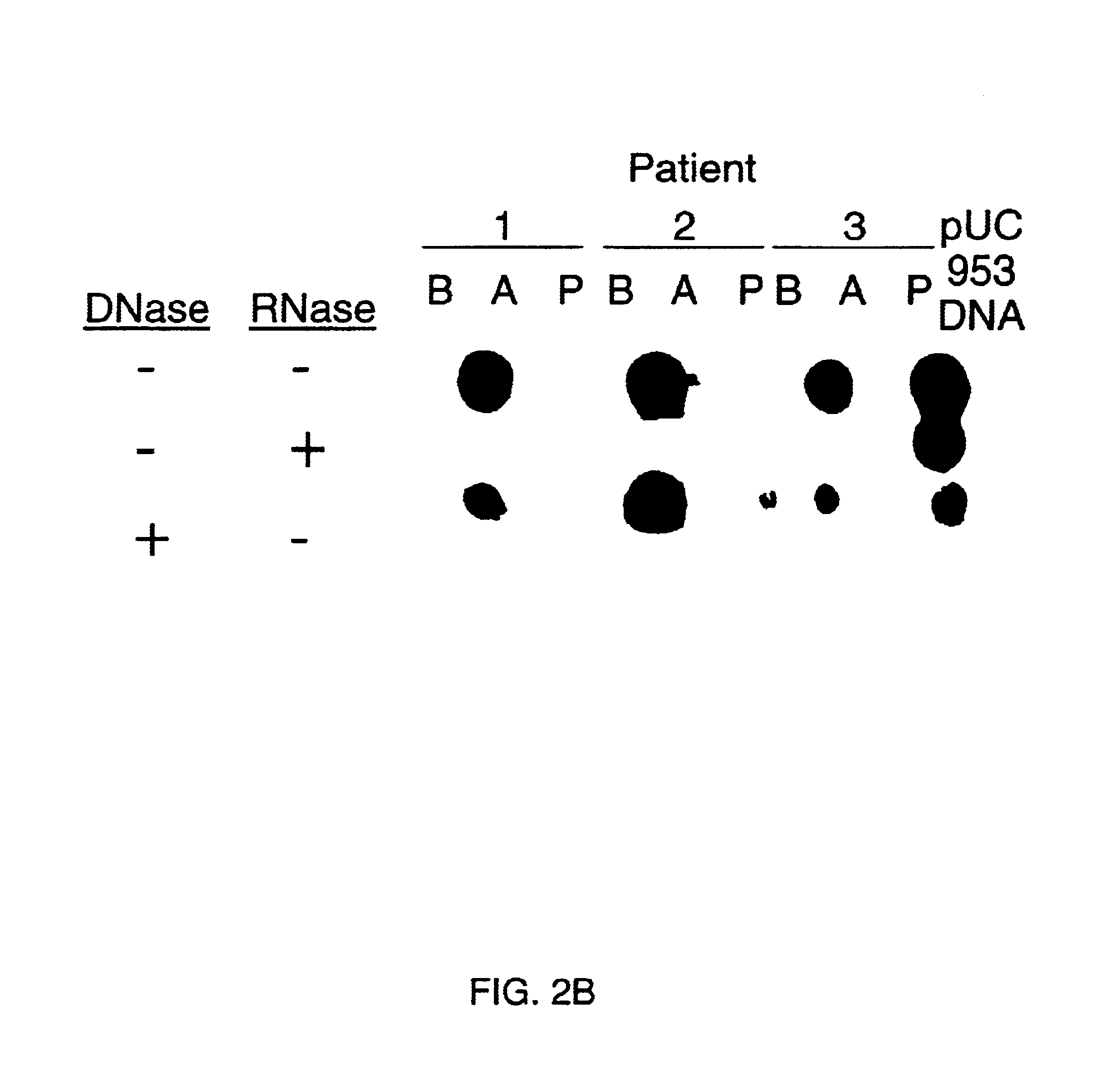
Double-stranded cDNA was synthesized from nucleic acid extracted from Norwalk virus purified from stool specimens of volunteers. One clone was isolated from a cDNA library constructed in a pUC-13 vector after amplification of the cDNA. The specificity of this cDNA (pUCNV-953) was shown by hybridization assays. The cDNA reacted with post (but not pre-) infection stool samples from Norwalk volunteers and with highly purified Norwalk virus, but not with other common enteric viruses such as hepatitis A virus and rotavirus. Finally, the probe detected virus in the same fractions of CsCl gradients in which viral antigen was detected using a specific Norwalk virus radioimmunoassay, and particles were detected by immune electron microscopy. Single-stranded RNA probes derived from the DNA clone after subcloning into an in vitro transcription vector were also used to show that the Norwalk virus contains a ssRNA genome of about 8 kb in size. The original clone was also used to detect additional cDNAs which represent at least 7 kb of nucleic acid of the Norwalk genome. The availability of a Norwalk-specific cDNA and the first partial genome sequence information allow rapid cloning of the entire genome and of establishment of sensitive diagnostic assays. Such assays can be based on detection of Norwalk virus nucleic acid or Norwalk viral antigen using polyclonal or monoclonal antibodies to proteins expressed from the cDNA or to synthetic peptides made based on the knowledge of the genome sequence. Assays using proteins deduced from the Norwlk virus genome and produced in expression systmes can measure antibody responses. Vaccines made by recombinant DNA technology are now feasible.
Owner:BAYLOR COLLEGE OF MEDICINE
Immunogenic compositions derived from poxviruses and methods of using same
Immunogenic compostions composed of poxvirus immunogens and related methods are disclosed. Specifically, immunogenic compostions useful in eliciting immune responses in animals are disclosed. In one embodiment the immunogenic compostions include viral antigens derived from vaccinia and / or variola that elicit cross-reactive immune responses. The immunogens can be made synthetically, by using recombinant DNA technology or derived from purified virus. Moreover, methods of using the immunogenic compostions are also disclosed.
Owner:MANNKIND CORP
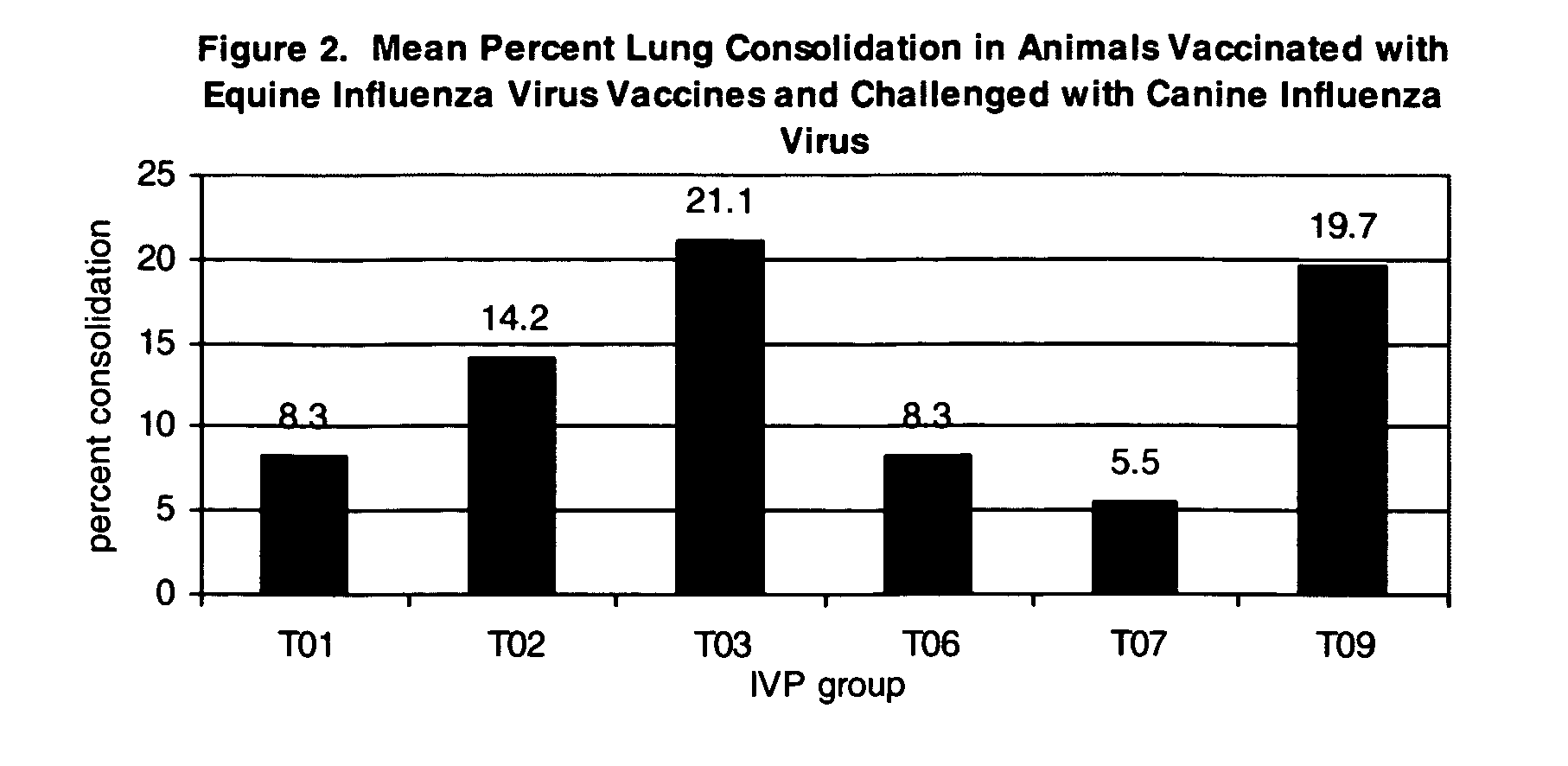
Vaccines and methods to treat canine influenza
The present invention relates to providing new vaccines and treatments for the diseases related to canine influenza virus. It discloses influenza viral antigens, and methods of presenting these antigens to canines, especially dogs. It relates to attenuated and killed vaccines. The present invention relates to experimentally generated canine and equine influenza viruses. The invention also includes influenza A, including H3, N8, H3N8, H7N7 and viruses which contain at least one genome segment from an canine or equine influenza virus. The present invention also relates to the use of these viruses in therapeutic compositions to protect canines, dogs in particular, from diseases caused by influenza viruses.
Owner:ZOETIS SERVICE LLC
Superior molecular vaccine based on self-replicating RNA, suicidal DNA or naked DNA vector, that links antigen with polypeptide that promotes antigen presentation
InactiveUS7557200B2Efficiently presentedImprove effectivenessSsRNA viruses positive-senseAntibody mimetics/scaffoldsDiseaseMHC class I
Improved molecular vaccines comprise nucleic acid vectors that encode a fusion polypeptide that includes polypeptide or peptide physically linked to an antigen. The linked polypeptide is one that (a) promotes processing of the expressed fusion polypeptide via the MHC class I pathway and / or (b) promotes development or activity of antigen presenting cells, primarily dendritic cells. These vaccines employ one of several types of nucleic acid vectors, each with its own relative advantages: naked DNA plasmids, self-replicating RNA replicons and suicidal DNA-based on viral RNA replicons. Administration of such a vaccine results in enhance immune responses, primarily those mediated by CD8+ cytotoxic T lymphocytes, directed against the immunizing antigen part of the fusion polypeptide. Such vaccines are useful against tumor antigens, viral antigens and antigens of other pathogenic microorganisms and can be used in the prevention or treatment of diseases that include cancer and infections.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Oral smallpox vaccine production and methods to evaluate safety, efficacy, and potency of orally delivered vaccine
InactiveUS20040175398A1Efficient responseSafety and efficacyViral antigen ingredientsMicrobiological testing/measurementHuman useDiagnostic test
This invention relates to methods and systems for generating a safe and effective oral smallpox vaccine for humans using a genetically defective strain of vaccinia virus to confer immunity following oral delivery of the vaccine. This invention is one that expands on current use of vaccinia virus propagation developed for gene therapy applications, and pharmaceuticals and nutraceuticals packaging and formualtion technologies. The vaccine invention can be delivered as a live virus with the ability to express viral proteins but unable to achieve complete, lytic virus replication, or it may be derived from such a virus, contain additional immunogens, or be delivered as viral antigens. Furthermore, the invention establishes innovative methods for formulation and packaging and for preclinical testing of the vaccine invention for safety, efficacy and potency with the use of human intestinal and other test cells and diagnostic test systems and kits.
Owner:INCELLS
Use of polymeric nanoparticles for vaccine delivery
InactiveUS20080044484A1Enhance antigen presentationImprove efficiencyBiocidePowder deliveryCancer cellT lymphocyte
The invention relates generally to the treatment and prevention of human cancer and viral diseases. More specifically, this invention relates to development of a new generation of vaccines that rely on eliciting cellular immune responses, specifically induction of cytotoxic T lymphocytes (CTL), against cancer cells and virus-infected cells via administration of a polymeric nanoparticle containing a vaccine comprising a fusion peptide or a modified peptide. Such a fusion peptide is composed of an insertion signal sequence and a peptide derived from a tumor antigen or a viral antigen, which improves antigen presentation and induces CTL with higher efficiency against cancer cells and virus-infected cells. An exemplary peptide utilized in the invention is Mart-1:27-35 peptide.
Owner:RGT UNIV OF CALIFORNIA
Immunotherapy of cancer through combination of local and systemic immune stimulation
Provided herein are compositions and methods for immunotherapy of cancers, said methods combining systemic vaccination with a recombinant expression vector encoding a viral antigen and / or a cancer specific tumor antigen(s) to induce activated CD8 T-cells, with a local (e.g., intratumoral) immune stimulation using standard or modified application of a TLR agonist, to induce local inflammatory and innate immune responses which recruit T-cells to the tumor.
Owner:IMMUNE DESIGN CORP
Compositions of influenza viral proteins and methods of use thereof
InactiveUS20090162400A1Increase productionStimulate immune responseSsRNA viruses negative-senseSsRNA viruses positive-senseIntegral membrane proteinPathogen-Associated Molecular Pattern Molecules
Compositions, fusion proteins and polypeptides comprise at least one pathogen-associated molecular pattern and at least a portion of at least one integral membrane protein of an influenza viral antigen. The compositions, fusion proteins and polypeptides are used to stimulate an immune response in a subject.
Owner:VAXINNATE
Liquid phase chip reagent kit detecting various anti EB viral antigen antibody
ActiveCN101063683AHigh throughputSmall amount of sampleMaterial analysisViral antigensNasopharyngeal carcinoma
This invention discloses one liquid phase chip test agent case and its process method for multiple kinds of anti-EB virus antibody, which comprises the following parts: coupling affinity element multi-color microball covering on polypeptide; at least one kind of coupling hydroxyl group multi-color microball covering on natural or gene antigen, wherein the antigen is EB virus crack object or shell antigen VCA; gene antigen is of one amino acid in list of No. 33-No. 37; the said integration polypeptide is one from No. 1-No. 32.
Owner:SUN YAT SEN UNIV CANCER CENT
Neutralizing molecules to viral antigens
The present invention concerns methods and means for identifying, producing, and engineering neutralizing molecules against influenza A viruses, and to the neutralizing molecules produced. In particular, the invention concerns neutralizing molecules against various influenza A virus subtypes, including neutralizing antibodies against H5 and / or H3 and / or H1, such as, for example all of H1, H3, and H5 subtypes, and methods and means for making such molecules.
Owner:BIOASSETS LLC
Methods and composition for oral vaccination
InactiveUS20060171960A1Provide protectionBacterial antigen ingredientsDispersion deliveryFlavorVaccination
The present invention encompasses methods and compositions both for providing protection against disease in an animal and for inducing increased intake of an orally administered vaccine by an animal. The methods of the invention are directed to admixing a bacterial or viral antigen with a water soluble palatable flavorant, further admixing the antigen and flavorant mixture with a water soluble vehicle for oral administration of the vaccine to an animal in order to provide protection against disease associated with infection by the admixed antigen and to induce the increased intake of the vaccine with the flavorant. The present invention thus encompasses methods and compositions for the oral vaccination of healthy animals through drinking water or syrups as an aid in the prevention of disease. The admixing of the palatable flavorant provides for a vaccine formulation with a desirable taste in order to promote self-administration of the vaccine formulation and / or to prevent rejection of the formulation when administered by an animal handler.
Owner:WYETH LLC
Recombinant MVA virus expressing dengue virus antigens, and the use thereof in vaccines
InactiveUS6869793B2Efficient and exceptionally safeSsRNA viruses positive-senseSugar derivativesVaccinationDengue virus Antigen
Recombinant MVA viruses containing and / or capable of expressing dengue virus antigens and the use of such recombinant MVA for vaccination.
Owner:GSF FORSCHUNGSZENT FUR UMWELT & GESUNDHEIT +2
Mammalian antigen-presenting T cells and bi-specific T cells
InactiveUS20070166327A1Guaranteed functionImproving in vivo survivalViral antigen ingredientsGenetic material ingredientsAutoimmune conditionReceptor
The present invention is directed to mammalian bi-specific T cells and methods for using these bi-specific T cells. More specifically, the invention relates to viral specific T cells that express chimeric anti-tumor receptors. These bi-specific T cell clones are a source of effector cells that persist in vivo in response to stimulation with viral antigen, leading to long-term function after their transfer to patients with cancer and autoimmune diseases.
Owner:CITY OF HOPE
Mycoplasma hyopneumoniae avirulent adjuvanted live vaccine
InactiveCN101883581AAntibacterial agentsBacterial antigen ingredientsDiseaseVirulent characteristics
Provided are immunogenic and vaccine compositions and methods for their preparation and use, which compositions are effective in protecting against, minimizing the severity of, preventing, and / or ameliorating M. hyopneumoniae infection. Administration to an animal of one or two doses of an adjuvanted live avirulent M. hyopneumoniae composition disclosed herein is effective in providing immunity to the animal and protection from infection with a virulent strain of M. hyopneumoniae thereby reducing the severity of and / or preventing disease caused by one or more virulent strain of M. hyopneumoniae. Also provided are compositions, which further comprise one or more antigen such as, for example, one or more live bacteria, bacterin, toxoid, and / or virus and / or viral antigen. Exemplified are immunogenic compositions, comprising an adjuvanted live avirulent M. hyopneumoniae and compositions, comprising Porcine Circovirus Type 1-Type 2 chimera modified live vaccine (cPCV1-2) in further combination with an adjuvanted live avirulent M. hyopneumoniae.
Owner:ZOETIS W LLC
Method for Producing Viral Vaccines
ActiveUS20090060950A1Reduce in quantityReduced responseSsRNA viruses negative-senseViral antigen ingredientsInfectious virusViral Vaccine
The present invention provides a method for the manufacture of a preparation comprising virus antigens comprising a) inoculation of cells with infectious virus in a fluid, b) propagation of said virus in said cells, c) collecting said propagated virus, d) inactivating said collected virus, and e) treating said inactivated virus with a detergent, resulting in a preparation comprising viral antigens.
Owner:RESILIENCE GOVERNMENT SERVICES INC
Composition Useful as a Vaccine
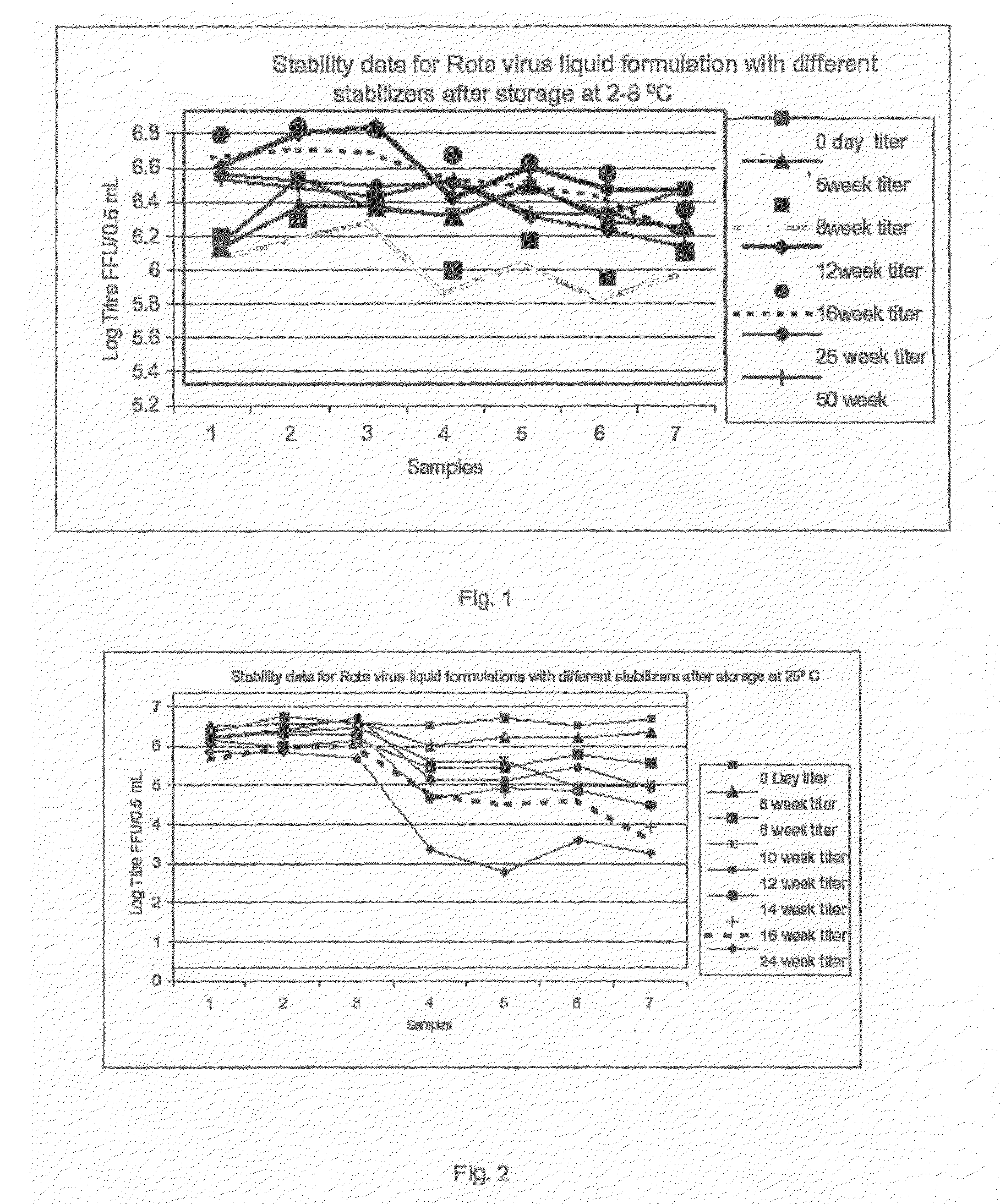
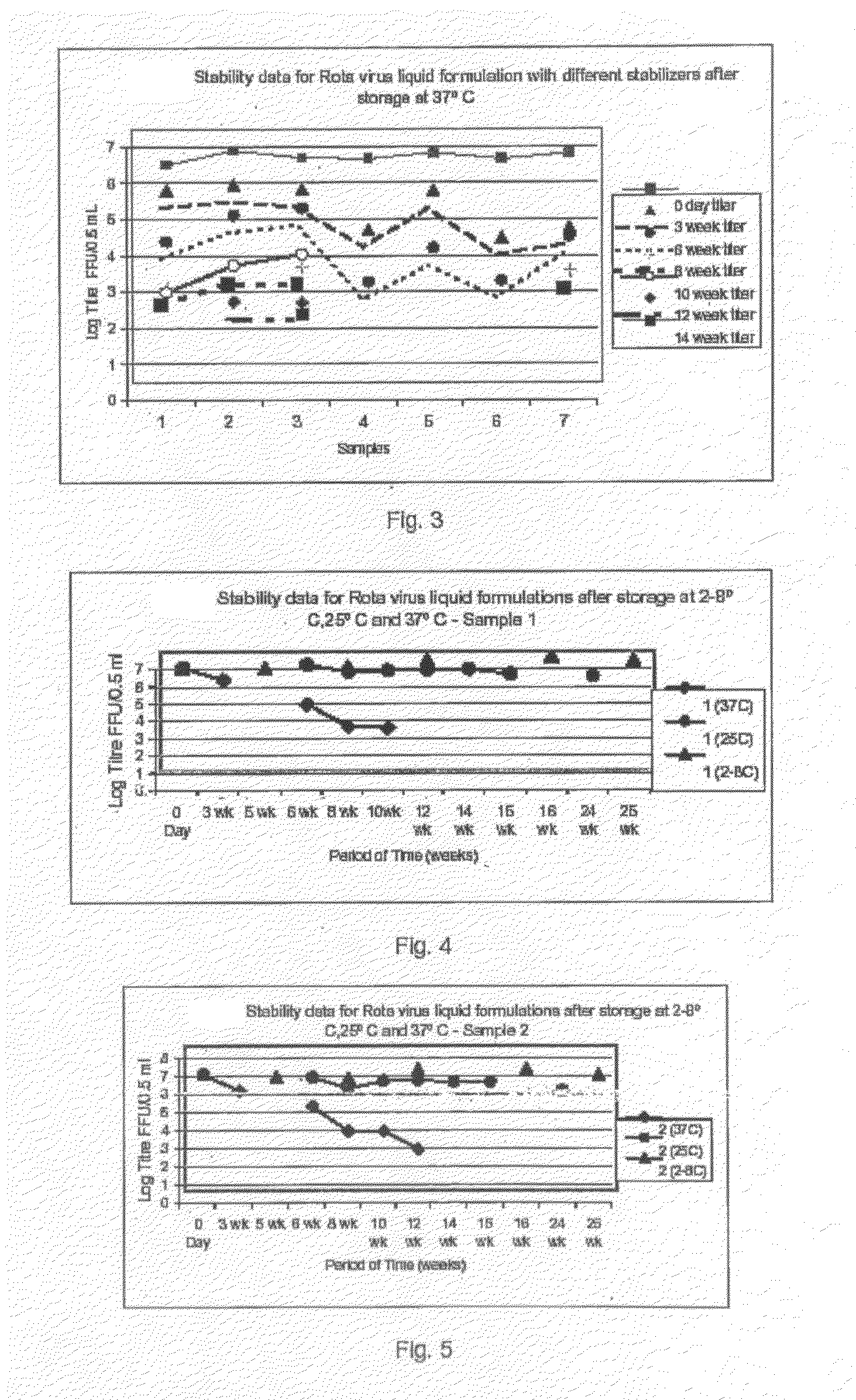
The present invention relates to a composition comprising a viral antigen, a first protein and a second protein. Optionally, the composition also comprises three different disaccharaides, or, optionally, the composition comprises a primary sugar and at least one, preferably two secondary sugars. The present invention also relates to the use of a viral antigen, a first protein and a second protein for the manufacture of a composition, preferably a vaccine. The present invention furthermore relates to a method of treatment or prevention of virus associates diseases in humans. Moreover, the present invention relates to a method of adapting a virus to a suitable cell-line. The invention is also useful for the production of virus suspensions suitable for making stable, live / inactivated, monovalent and / or polyvalent, liquid / lyophilized rotavirus vaccine compositions for oral and / or nasal or any other suitable route of administration in human.
Owner:BHARAT BIOTECH INTERNATIONAL
Large scale productions of virus antigen
InactiveUS7524676B2SsRNA viruses negative-senseArtificial cell constructsMethods of productionMicrocarrier
The present invention provides improved methods of production of viral antigen on a culture of adherent cells bound to a microcarrier, wherein the methods provide for increased viral antigen yield per culture medium volume. The invention is also directed to a cell culture biomass of adherent cells having increased cell density and microcarrier concentration compared to the respective confluent cell culture.
Owner:RESILIENCE GOVERNMENT SERVICES INC
Methods of delivery of exogenous proteins to the cytosol and uses thereof
InactiveUS20050220807A1Effective immune responseEvaluated effectively and efficientlyAntibacterial agentsVirusesProtective antigenAbnormal tissue growth
The present invention is directed to a method for delivering exogenous proteins to the cytosol, by binding a target antigen (such as a protein) to a transport factor that contains a fragment of a bipartite protein exotoxin, but not the corresponding protective antigen. Preferably, the target antigen is fused to the transport factor. Preferred transport factors include the protective antigen binding domain of lethal factor (LFn) from B. anthracis, consisting of amino acids 1-255, preferably a fragment of at least 80 amino acids that shows at least 80% homology to LFn, and a fragment of about 105 amino acids from the carboxy portion that does not bind PA. The target antigen can include any molecule for which it would be desirable to elicit a CMI response, including viral antigens and tumor antigens.
Owner:THE GENERAL HOSPITAL CORP +1
Novel mucosal vaccination approach for herpes simplex virus type-2
InactiveUS20120027841A1High potencyPrevent relapseOrganic active ingredientsViral antigen ingredientsHeterologousLiposome
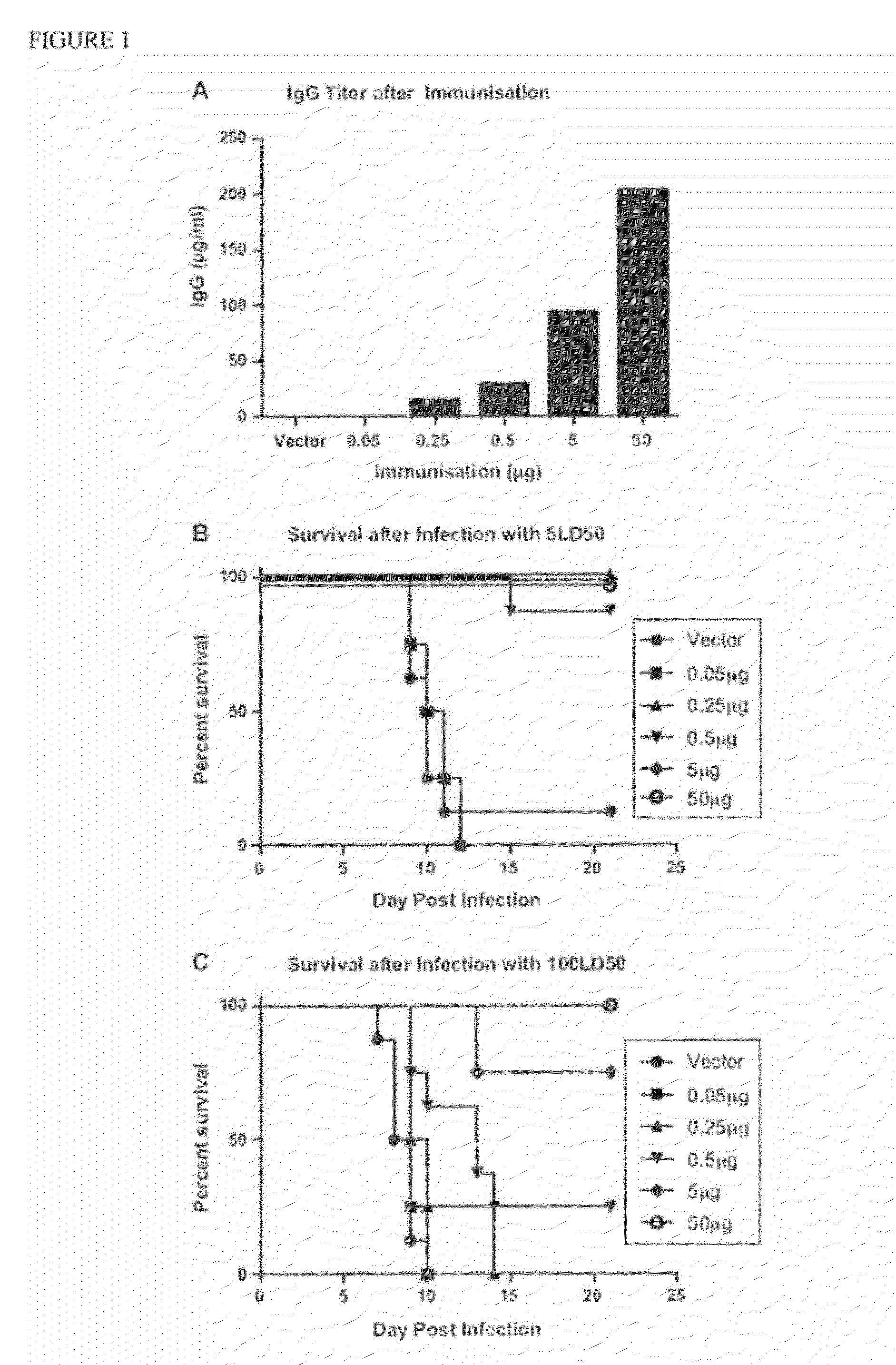
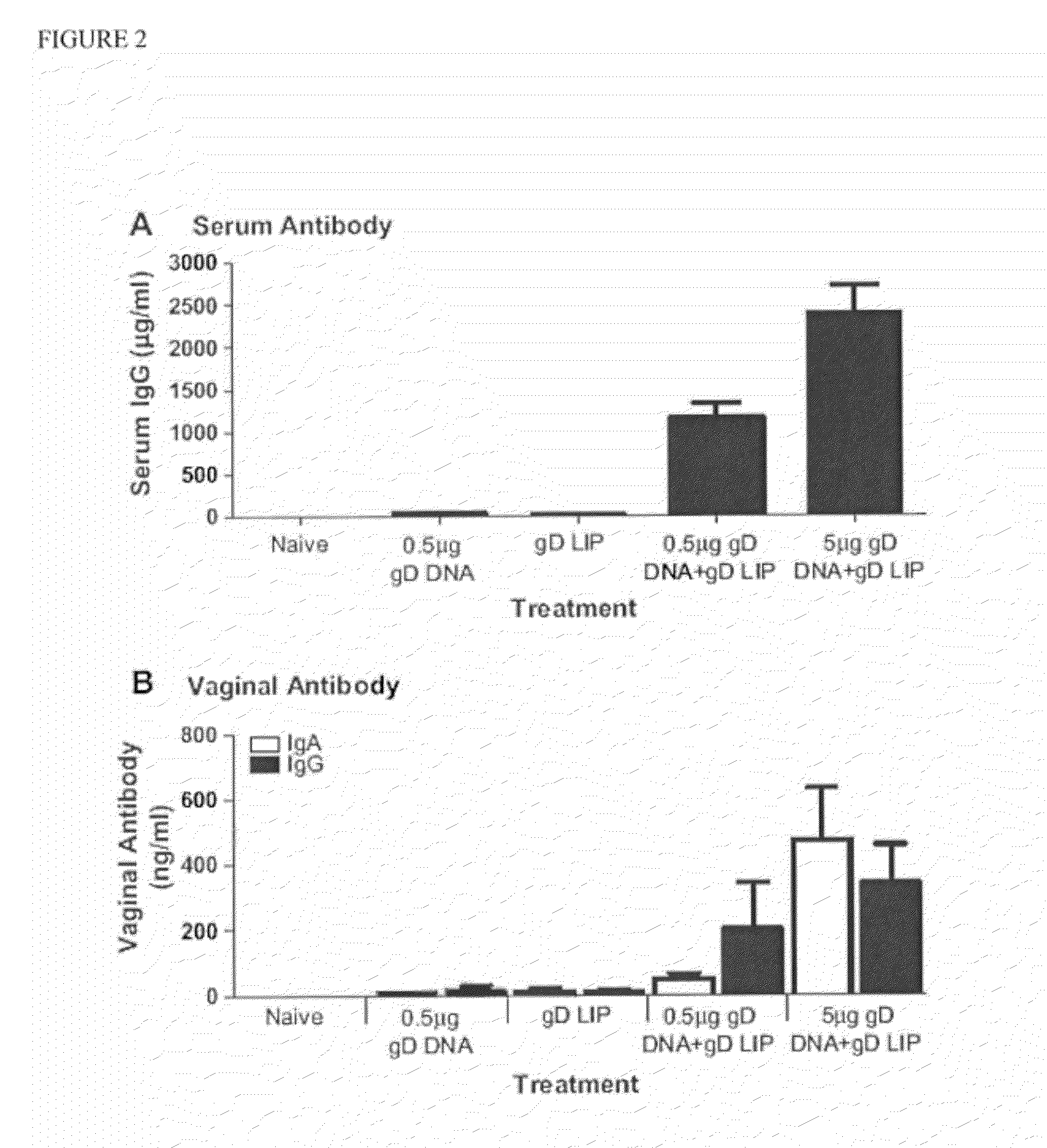
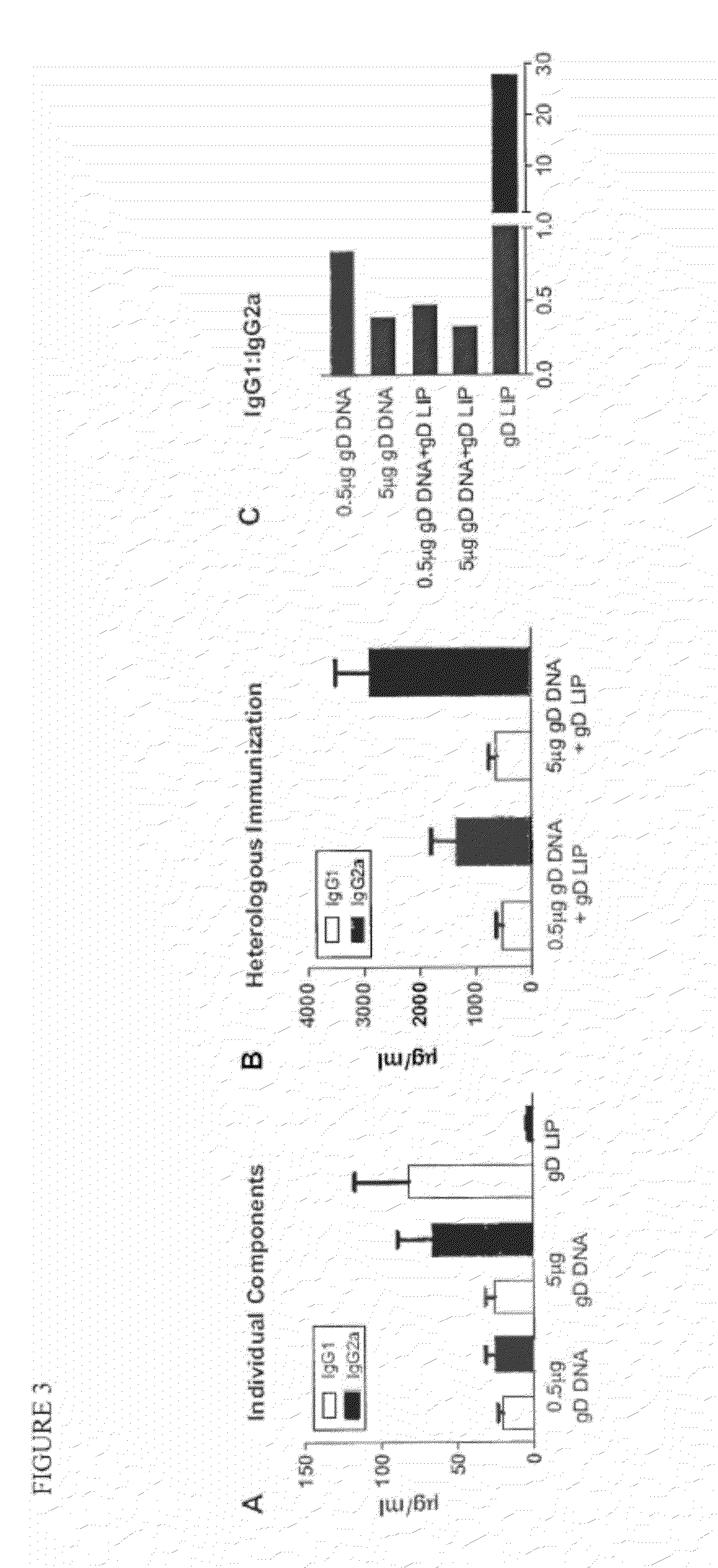
The invention provides methods and kits for immunizing animals (e.g. mammals) against viral antigens, including herpes-simplex virus type 2. The protective immune response elicited by the methods and kits of the invention is characterized by robust humoral, cellular, and mucosal immunity. In particular, the invention provides a heterologous immunization method comprising a priming DNA vaccine encoding an antigen and a boosting protein vaccine, in which the protein form of the antigen is encapsulated in liposomes. Methods of preventing primary acute, latent and recurrent viral infections, such as that caused by HSV-2 virus, and methods of providing passive protective immunity against a viral pathogen such as HSV-2 virus to a mammal are also disclosed.
Owner:MUCOSAL VACCINE TECH LLC
Highly pathogenic porcine reproductive and respiratory syndrome JXAl-R strain- porcine parvovirus disease bigeminal live vaccine and preparation method and application thereof
InactiveCN102727881AAvoid mutual interferenceImproving immunogenicityViral antigen ingredientsMicrobiological testing/measurementDiseaseHighly pathogenic
The invention particularly relates to a highly pathogenic porcine reproductive and respiratory syndrome JXAl-R strain- porcine parvovirus disease bigeminal live vaccine and the preparation method and the application of the highly pathogenic porcine reproductive and respiratory syndrome JXAl-R strain-porcine parvovirus disease bigeminal live vaccine. The highly pathogenic porcine reproductive and respiratory syndrome JXAl-R strain- porcine parvovirus disease bigeminal live vaccine is the freeze-dried vaccine of mixed viral antigen liquid containing highly pathogenic porcine reproductive and respiratory syndrome virus and porcine parvovirus, the content of the highly pathogenic porcine reproductive and respiratory syndrome virus in per dose of the freeze-dried vaccine is 105-7 TCID50 / ml, the content of the porcine parvovirus virus is 105-7 TCID50 / ml, and the highly pathogenic porcine reproductive and respiratory syndrome JXAl-R strain- porcine parvovirus disease bigeminal live vaccine is prepared via the following steps: preparing highly pathogenic porcine reproductive and respiratory syndrome virus liquid, preparing porcine parvovirus liquid, matching the vaccines and freeze-drying the vaccine. The bigeminal live vaccine provided by the invention has excellent immunogenicity and safety, can prevent two epidemic diseases including the highly pathogenic porcine reproductive and respiratory syndrome and the porcine parvovirus disease simultaneously, thereby preventing two diseases by one injection.
Owner:GUANGDONG DAHUANONG ANIMAL HEALTH PRODS +1
Compositions and methods for enhancing immune responses mediated by antigen-presenting cells
InactiveUS7063847B1High incidenceFacilitating passagePeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAdjuvantProteinase activity
Molecular adjuvants are disclosed comprising an antigen presenting cell-targeting ligand linked to an immunogen, e.g. tumor associated antigens, bacterial or viral antigens. The ligand and the immunogen are linked via a cleavable linker such as a protease-sensitive oligopeptide, to facilitate processing of the adjuvant by the antigen presenting cell. Methods are disclosed for delivery of these molecular adjuvants to patients, resulting in the transduction of activating signals to the targeted antigen presenting cell, thereby enhancing the immune response to the co-delivered immunogen.
Owner:BOARD OF RGT UNIV OF NEBRASKA
Preparation of antigen-loaded polymer lipid nanosphere and application as vaccine adjuvant
ActiveCN106177974AIncrease intakeProcessing and submission is goodPowder deliveryPharmaceutical non-active ingredientsZeta potentialBiological activation
The invention relates to a polymer lipid nanoparticle, a preparation method and application thereof as a vaccine adjuvant system. The polymer nanoparticle is a solid sphere composed of a macromoleclar polymer, positively charged cationic lipid is inlaid on the surface, the nanoparticle surface can adsorb protein antigen, polypeptide antigen, viral antigen and other active drugs; the polymer nanoparticle has an average particle size of less than 300nm, a dispersion coefficient, i.e. a PDI (polydispersity) value of less than 0.2, and a Zeta potential average value of greater than 20mV. The polymer nanoparticle provided by the invention has the characteristics of uniform size, high antigen carrying rate, and good maintenance of antigen activity, when the polymer nanoparticle carries an antigen and serves as a vaccine adjuvant, the intake of antigen presenting cells (APCs) to the antigen can be increased, the activation level of antigen presenting cells is improved, and the follow-up immune response level is strengthened.
Owner:王连艳
Compositions and methods for enhancing immune responses mediated by antigen-presenting cells
InactiveUS20080286228A1High incidenceFacilitating passageVirusesPeptide/protein ingredientsAdjuvantProteinase activity
Molecular adjuvants are disclosed comprising an antigen presenting cell-targeting ligand linked to an immunogen, e.g. tumor associated antigens, bacterial or viral antigens. The ligand and the immunogen are linked via a cleavable linker such as a protease-sensitive oligopeptide, to facilitate processing of the adjuvant by the antigen presenting cell. Methods are disclosed for delivery of these molecular adjuvants to patients, resulting in the transduction of activating signals to the targeted antigen presenting cell, thereby enhancing the immune response to the co-delivered immunogen.
Owner:BOARD OF RGT UNIV OF NEBRASKA
Direct fluorescene immunoassay for viral antigens
The present invention describes a liquid direct fluorescence antibody assay that is rapid and sensitive to detect respiratory virus in infected cells. The assay includes centrifugation of the specimen, incubation of sample and reagents in solution, and detection of the absence or presence of respiratory virus. Sapogenin is used as a detergent to permeabilize the cells for entry of the monoclonal antibodies to react with intracellular antigens. The cells are stained with fluorescently labeled monoclonal antibodies against the viral antigens along with a background stain and a fluorescent nuclear stain. This counter staining decreases background and allows co-localization of antigen and nuclear structures for enhanced detection.
Owner:DIAGNOSTIC HYBRIDS
Epitopes in viral envelope proteins and specific antibodies directed against these epitopes: use for detection of HCV viral antigen in host tissue
Antibodies to two new epitopes on the HCV envelope proteins were identified which allow routine detection of native HCV envelope antigens, in tissue or cells derived from the host. The new epitopes are: the E1 region aa 307-326 and the N-terminal hyper variable region of E2 aa 395-415. Surprisingly, we characterised an antibody that reacts with various sequences of the hypervariable domain of E2. Specific monoclonal antibodies directed against these epitopes and allowing routine detection of viral antigen are described.
Owner:INNOGENETICS NV (NL)
Immune cell compositions and methods of use for treating viral and other infections
PendingCN109863242APolypeptide with localisation/targeting motifImmunoglobulin superfamilyRegulatory T cellMedicine
Disclosed herein are cells that are immune cells, which cells recombinantly express a dominant negative form of an inhibitor of a cell-mediated immune response of the immune cell, and optionally recombinantly express a chimeric antigen receptor (CAR), wherein the CAR binds to a viral antigen. In certain embodiments, the immune cell is an immunostimulatory cell, such as a T cell. In certain embodiments, the immune cell is an immunoinhibitory cell, such as a regulatory T cell. Also disclosed herein are immune cells that recognize and are sensitized to a viral antigen, which immune cells recombinantly express a dominant negative form of an inhibitor of a cell-mediated immune response of the immune cell. The cells can be sensitized to an antigen that is a viral antigen. Additionally provided are methods of using such cells to treat a viral infection in a subject in need thereof.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
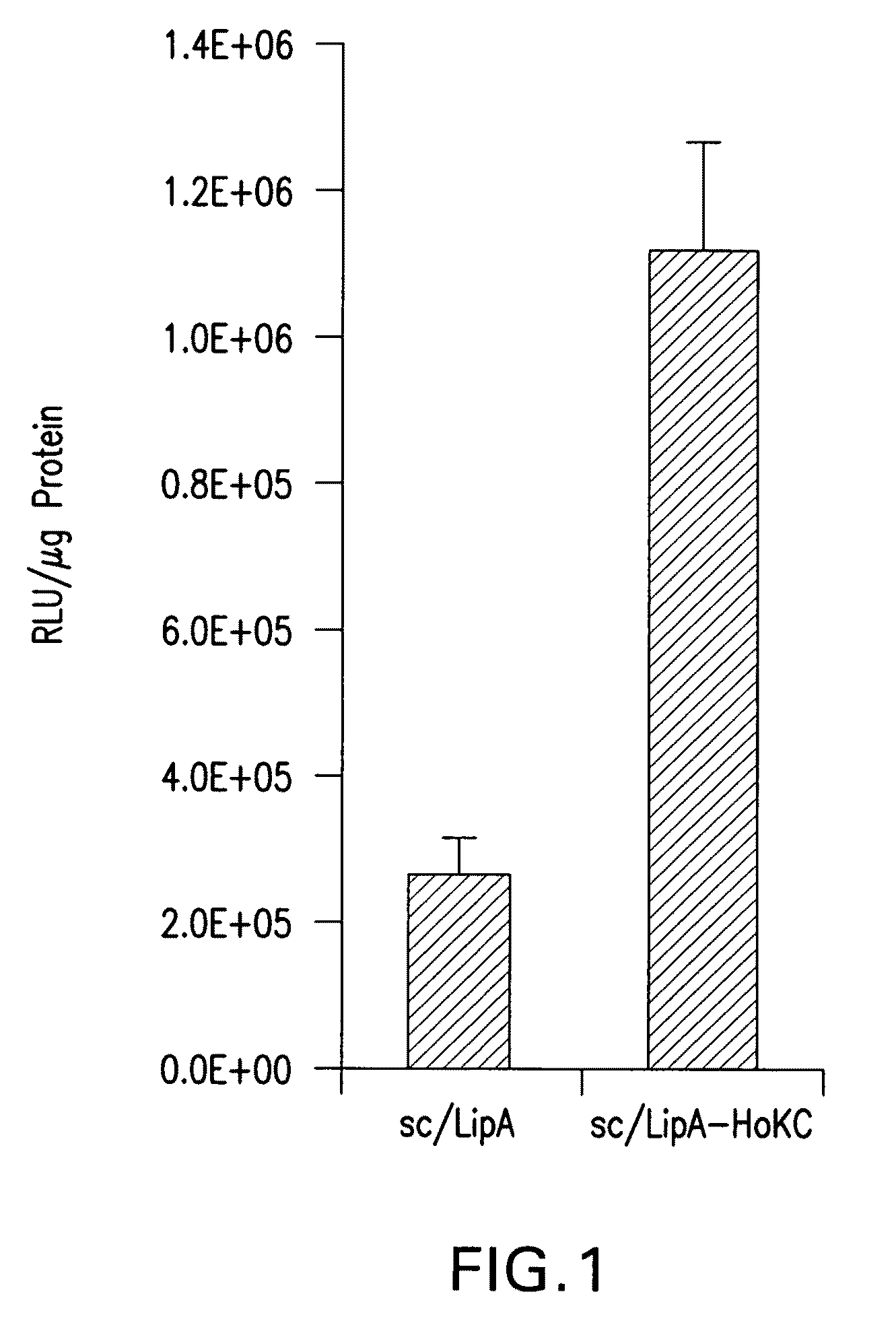
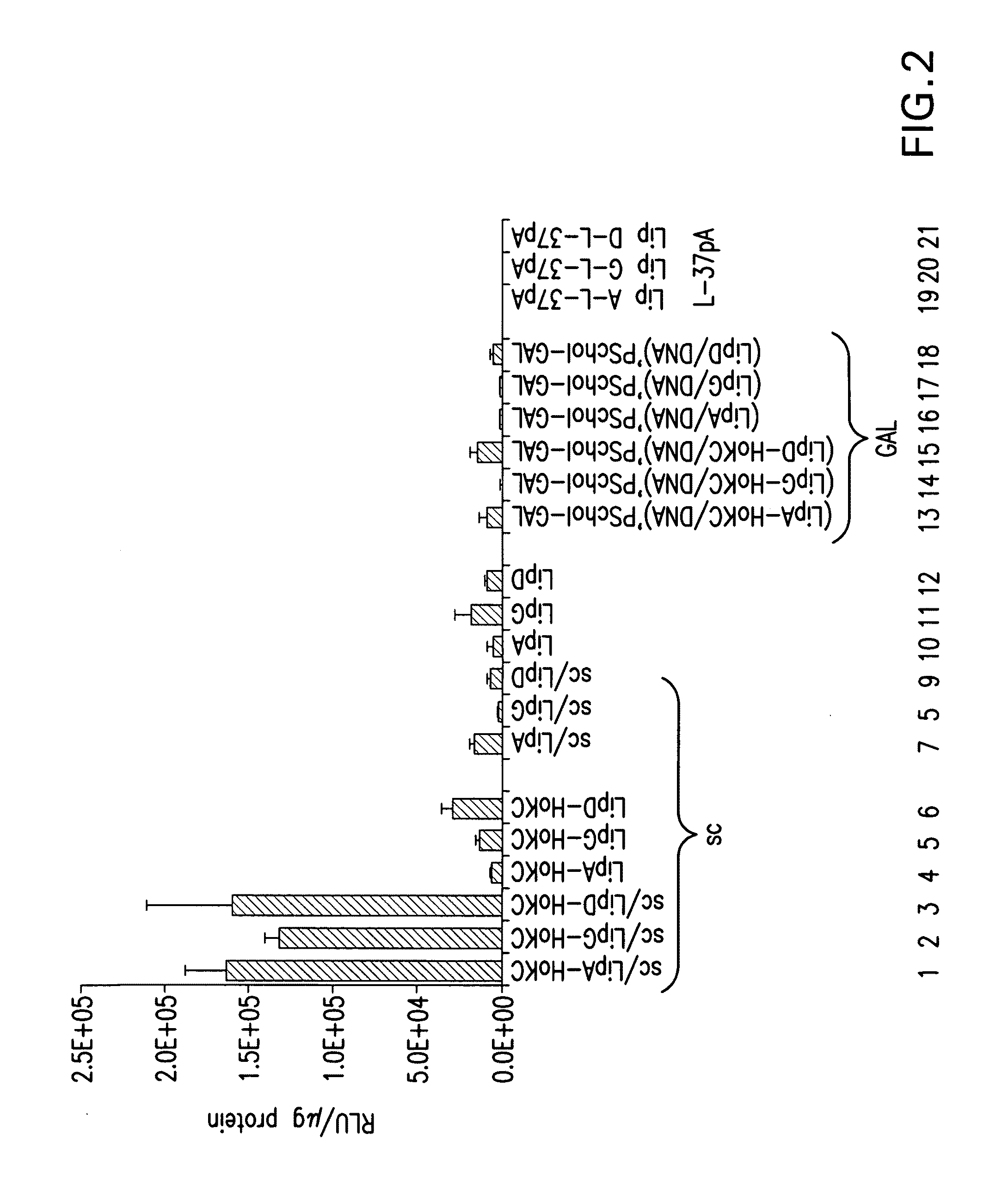
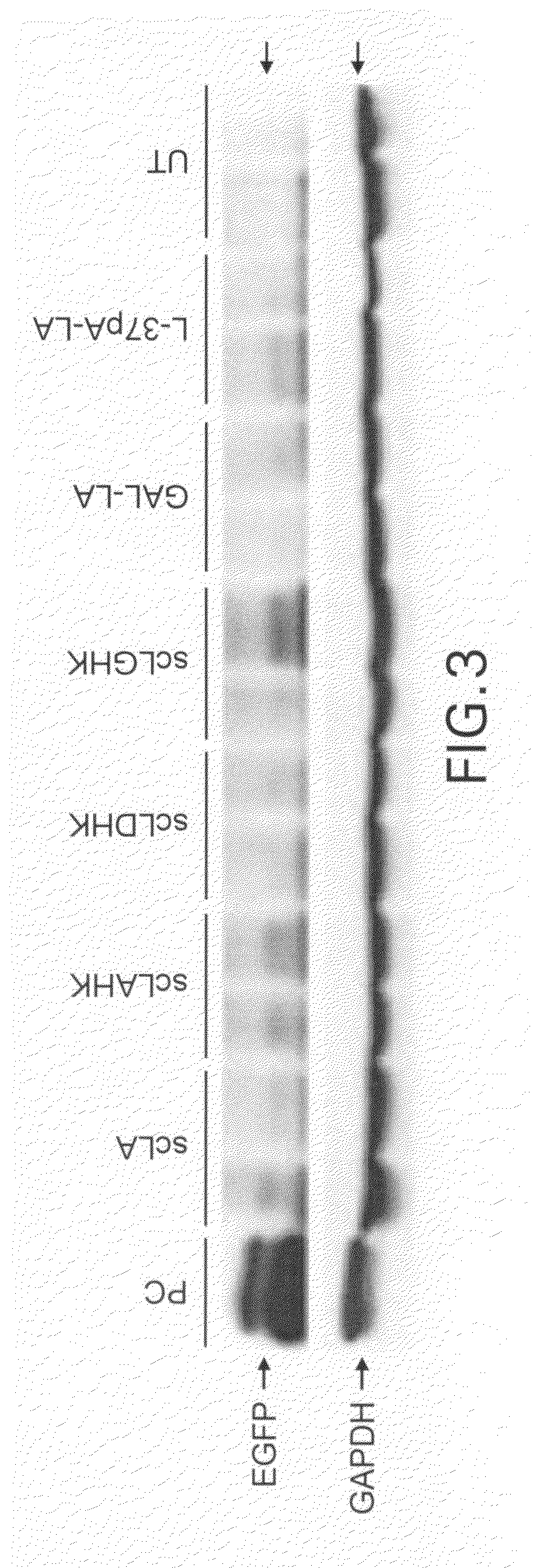
Methods for generating immune response using cationic-liposome-mediated nucleic acid delivery
InactiveUS20090053299A1SsRNA viruses negative-senseOrganic active ingredientsAntibody fragmentsViral disease
The present invention is in the fields of drug delivery, and specifically, cationic liposome-based vaccines. In embodiments, this invention provides methods of making ligand-targeted (e.g., antibody- or antibody fragment-targeted) liposomes useful for the delivery of molecules to induce immune responses against viral antigens or to treat or prevent viral diseases. The specificity of the delivery system is derived from the targeting ligands.
Owner:GEORGETOWN UNIV
A pluripotent vaccine against enveloped viruses
The present invention is directed to compositions and methods for the induction of immune responses in mammals against enveloped animal viruses. More particularly, the invention provides vaccine compositions containing multiple MHC allotypes. By generating an immune response against these MHC molecules, virus or virus-infected cells expressing foreign MHC molecules can be attacked prior to infection of cells in the immunized host. In some embodiments, the vaccine compositions contain viral antigens and adjuvants as well. The vaccine compositions may comprise intact cells, cell-derived membrane preparations or recombinantly or chemically produced MHC molecules or fragments thereof.
Owner:JOHN WAYNE CANCER INST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com