Preparation of antigen-loaded polymer lipid nanosphere and application as vaccine adjuvant
A technology for polymers and polymer degradation, which is applied in the field of medicine to achieve the effects of increased intake, uniform size, and enhanced activation level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
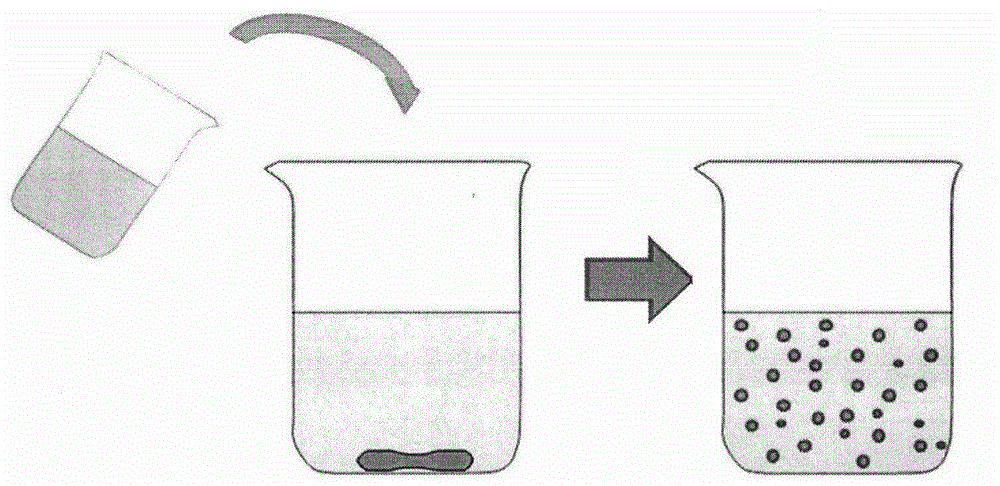
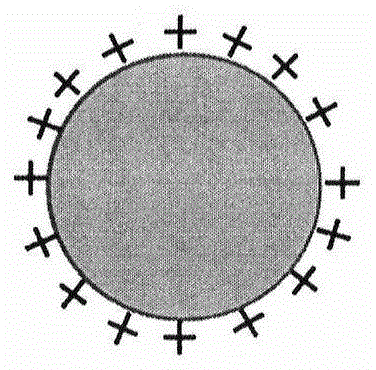
[0039] Nanoparticles were prepared by an improved self-emulsifying solvent evaporation method (the schematic diagram of the preparation process is shown in figure 1 As shown, the schematic diagram of the prepared nanoparticles is shown in figure 2 shown), 100mg PLA and 30mg didodecyldimethylammonium bromide (DDAB) were dissolved in the oil phase mixed with ethanol and acetone, the volume of the oil phase was 15mL, where V 乙醇 :V 丙酮 1:1; then use deionized water as the water phase, the volume of the water phase is 90mL; then slowly add the well-mixed oil phase into the water phase, mechanically stir at a speed of 400rpm, stir for 12 hours, and finally centrifuge, Washing, freeze drying, the obtained polymer nanoparticles. Its average particle size and particle size distribution are measured by a British Malvern nanometer (ZetasizerNano ZS90). The average particle size in water is 117nm, the particle size distribution coefficient PDI is 0.138, and the surface Zeta potential is...
Embodiment 2
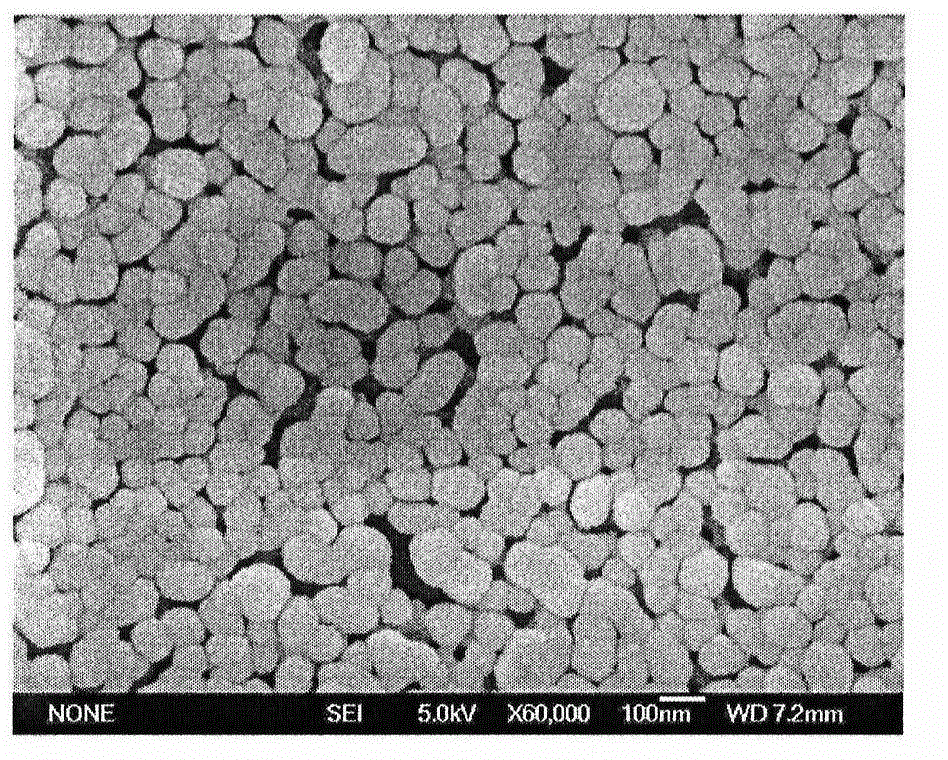
[0043] Nanoparticles were prepared by an improved self-emulsifying solvent evaporation method. 100mg PLA and 20mg didodecyldimethylammonium bromide (DDAB) were dissolved in an oil phase mixed with ethanol and acetone. The volume of the oil phase was 10mL, where V 乙醇 :V 丙酮 1:1; then use deionized water as the water phase, the volume of the water phase is 90mL; then slowly add the uniformly mixed oil phase into the water phase, mechanically stir at a speed of 400rpm, stir for 12 hours, and finally centrifuge, Wash and freeze-dry to obtain polymer nanoparticles. Its average particle size and particle size distribution are measured by a British Malvern nanometer (ZetasizerNano ZS90). The average particle size in water is 134.4nm, the particle size distribution coefficient PDI is 0.189, and the surface Zeta potential is 35.7mV. Scanning electron microscope photos as Figure 5 As shown, the results show that the prepared polymer nanoparticles have a uniform particle size.
Embodiment 3
[0045] Nanoparticles were prepared by an improved self-emulsifying solvent evaporation method. 100mg PLA and 50mg didodecyldimethylammonium bromide (DDAB) were dissolved in an oil phase mixed with ethanol and acetone. The volume of the oil phase was 15mL, where V 乙醇 :V 丙酮 1:1; then use deionized water as the water phase, the volume of the water phase is 90mL; then slowly add the uniformly mixed oil phase into the water phase, mechanically stir at a speed of 400rpm, stir for 12 hours, and finally centrifuge, Wash and freeze-dry to obtain polymer nanoparticles. Its average particle size and particle size distribution are measured by a British Malvern nanometer (ZetasizerNano ZS90). The average particle size in water is 177.7nm, the particle size distribution coefficient PDI is 0.209, and the surface Zeta potential is 35.5mV. Scanning electron microscope photos as Figure 6 As shown, the results show that the prepared polymer nanoparticles have a uniform particle size.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com