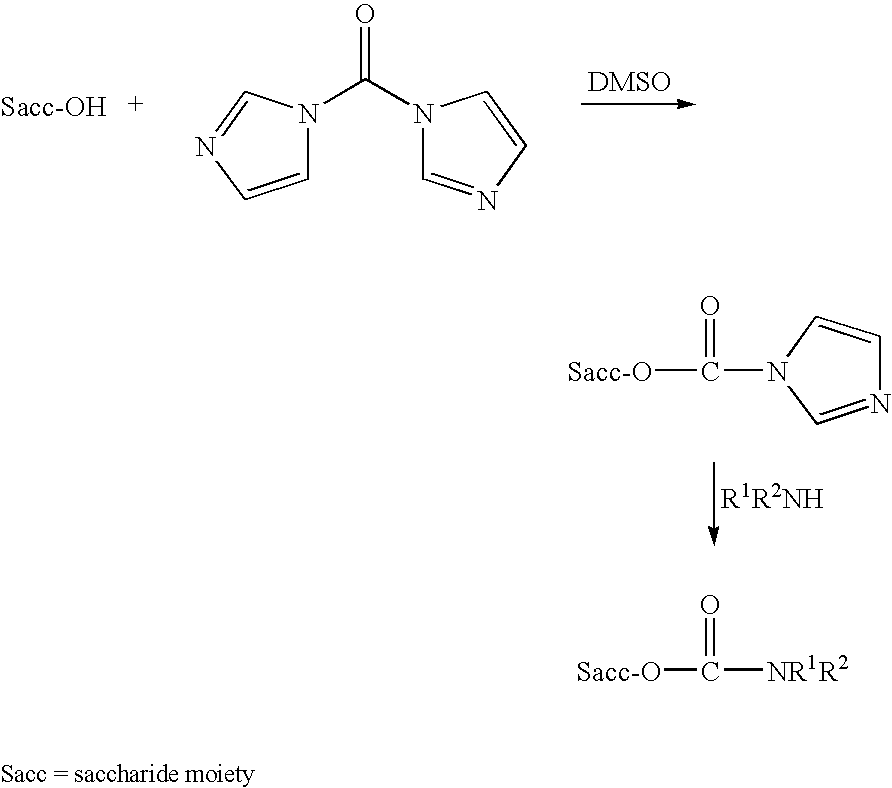
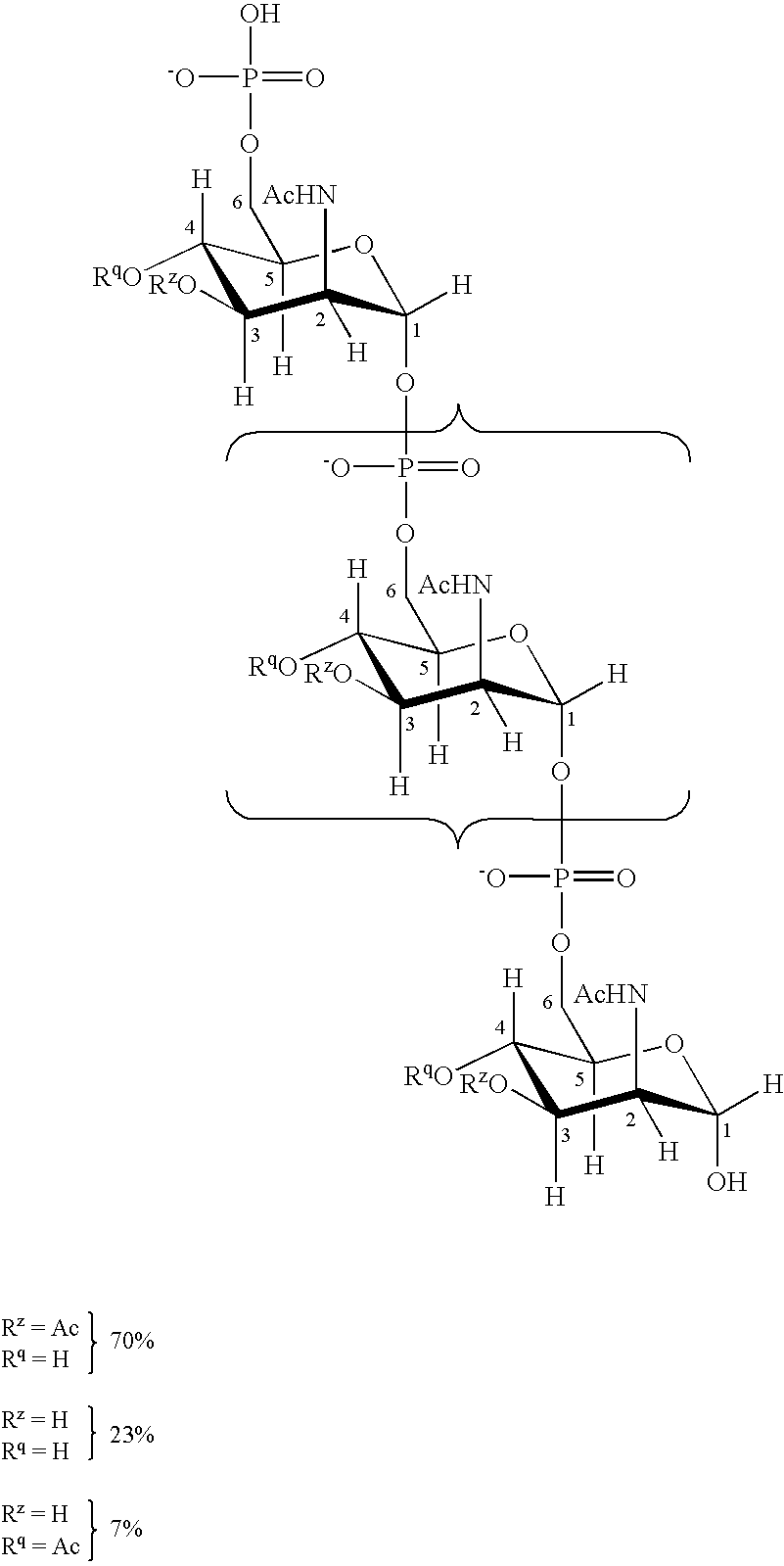
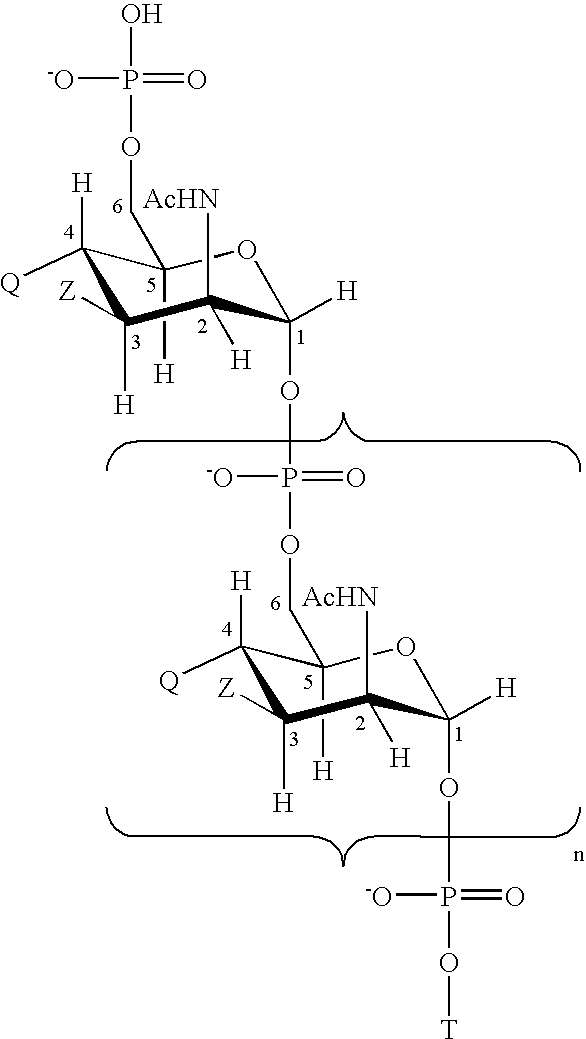
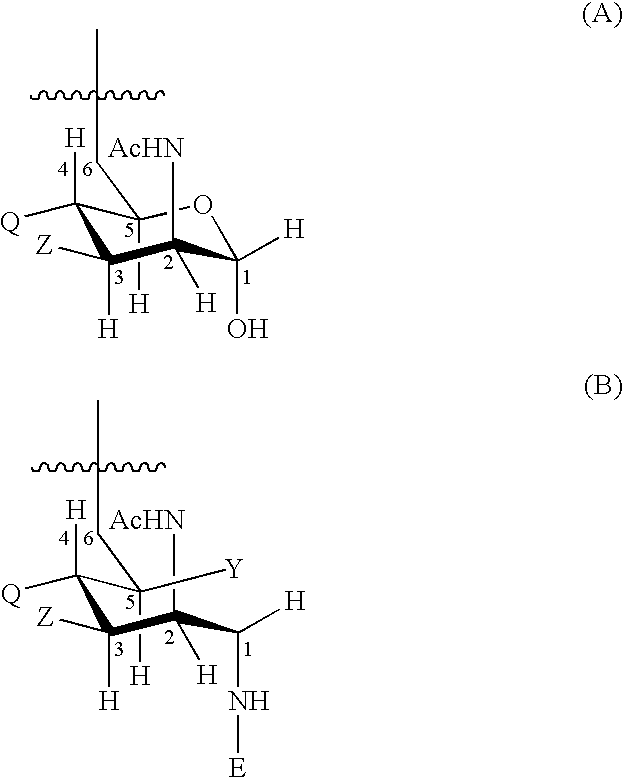
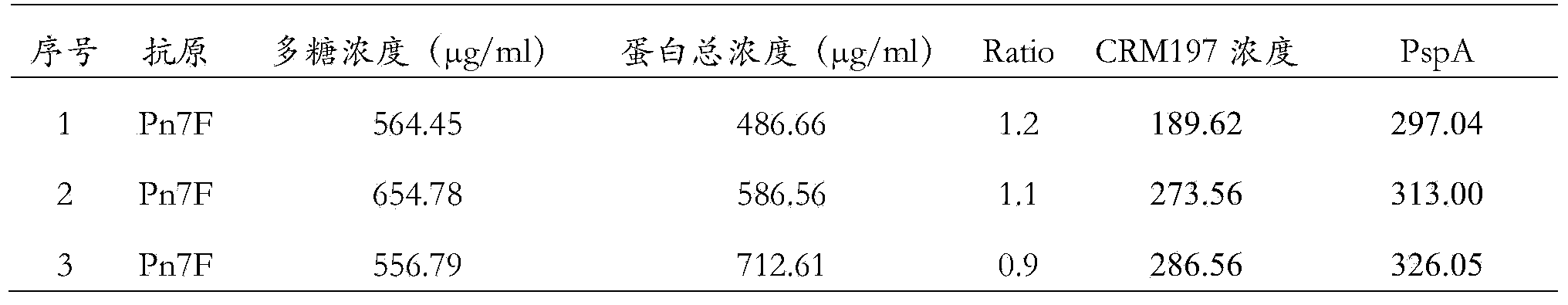
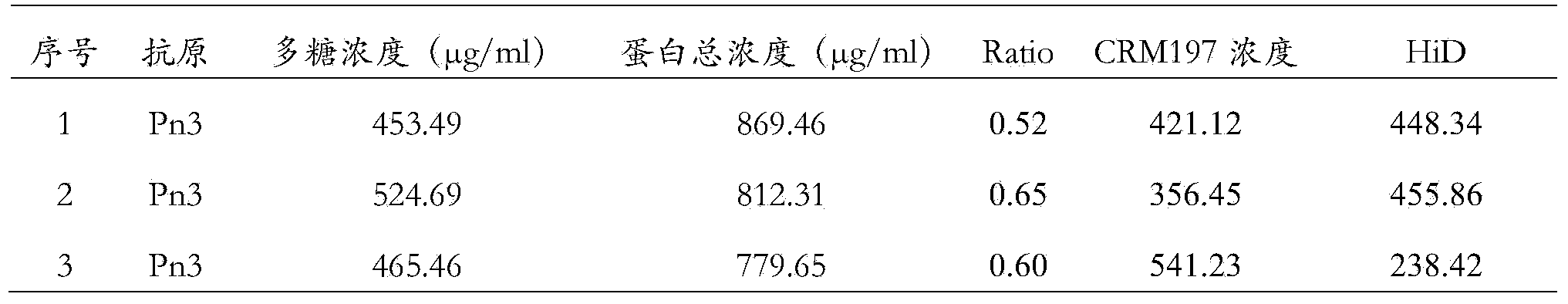
Patents
Literature
570 results about "Protein antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An exogenous protein antigen is a protein produced outside the body by another organism. An example might be a protein made by a virus which someone ingests. When the immune system sees this protein, it recognizes it as foreign, and stimulates the production of antibodies which can latch onto and attack the antigen.
Antigen delivery system and method of production
The present invention concerns polymer particle vaccine delivery systems in which a water insoluble protein antigen, e.g. a lipidated HpaA protein, is incorporated with particles comprising a polymer matrix. The present invention also concerns a method for incorporating such a water insoluble protein antigen with a polymer matrix in order to produce a polymer particle vaccine delivery system. In addition, the invention also provides a vaccine composition comprising the polymer particle delivery system. The vaccine can be used to treat and / or reduce the risk of for example Helicobacter infection.
Owner:ASTRAZENECA AB
Polypeptide-vaccines for broad protetion against hypervirulent meningococcal lineages
ActiveUS20060171957A1Improve hydrolysis resistanceAntibacterial agentsBacterial antigen ingredientsSalmonella serotype typhiMeningococcal carriage
A small number of defined antigens can provide broad protection against meningococcal infection, and the invention provides a composition which, after administration to a subject, is able to induce an antibody response in that subject, wherein the antibody response is bactericidal against two or three of hypervirulent lineages A4, ET 5 and lineage 3 of N. meningitidis serogroup B. Rather than consisting of a single antigen, the composition comprises a mixture of 10 or fewer purified antigens, and should not include complex or undefined mixtures of antigens such as outer membrane vesicles. Five protein antigens are used in particular: (1) a ‘NadA’ protein; (2) a ‘741’ protein; (3) a ‘936’ protein; (4) a ‘953’ protein; and (5) a ‘287’ protein.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
siRNA targeting inner centromere protein antigens (INCENP)
InactiveUS7582747B2Improve efficiencyGood curative effectSugar derivativesMicrobiological testing/measurementNucleotideGene silencing
Efficient sequence specific gene silencing is possible through the use of siRNA technology. By selecting particular siRNAs by rational design, one can maximize the generation of an effective gene silencing reagent, as well as methods for silencing genes. Methods, compositions, and kits generated through rational design of siRNAs are disclosed including those directed to nucleotide sequences for INCENP.
Owner:THERMO FISHER SCIENTIFIC INC
Liquid Vaccines For Multiple Meningococcal Serogroups
Conjugated capsular saccharides from meningococcal serogroups C, W135 and Y are safe and immunogenic in humans when combined in a single dose. This effect is retained when a conjugated capsular saccharide from serogroup A is added. These conjugated antigens can be stably combined in a single aqueous dose without the need for lyophilisation. Broad protection against serogroup B infection can be achieved by using a small number of defined polypeptide antigens. These polypeptide antigens can be combined with the saccharide antigens without loss of protective efficacy for any of the five serogroups. Efficacy if retained even if a Hib conjugate is added. The efficacy of a serogroup W135 conjugate is enhanced by addition of protein antigens derived from a serogroup B strain. Addition of a Hib conjugate to meningococcal conjugates enhances the overall activity against meningococcus serogroup W135.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
SARS-CoV-2 neutralizing antibody detection kit
PendingCN111562369AGood repeatabilityStrong specificityImmunoassaysImmunodiagnosticsProtein s antigen
The invention relates to an SARS-CoV-2 neutralizing antibody detection kit. The SARS-CoV-2 neutralizing antibody detection kit comprises a solid phase carrier, an S protein antigen of SARS-CoV-2 and acompetitive substance. The competitive substance is marked with a signal substance and can be specifically combined with the new coronavirus S protein antigen. Whether a tested person is infected bythe new coronavirus or not and whether infection risks exist or not are judged by detecting a neutralizing antibody through an immunodiagnosis technology, and the method is reliable in theory, practical and feasible and can be completed only in a secondary biosafety laboratory.
Owner:威海威高生物科技有限公司
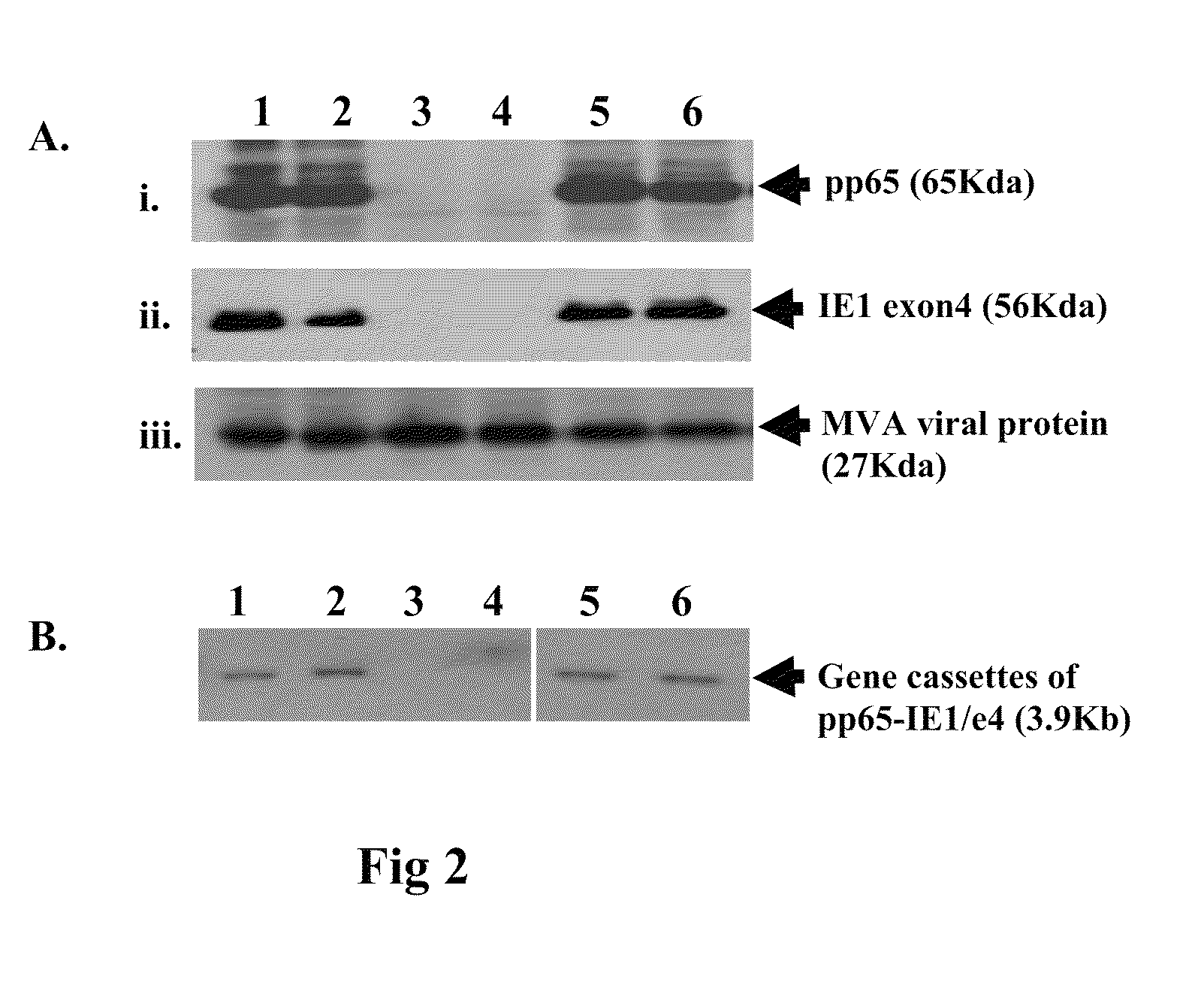
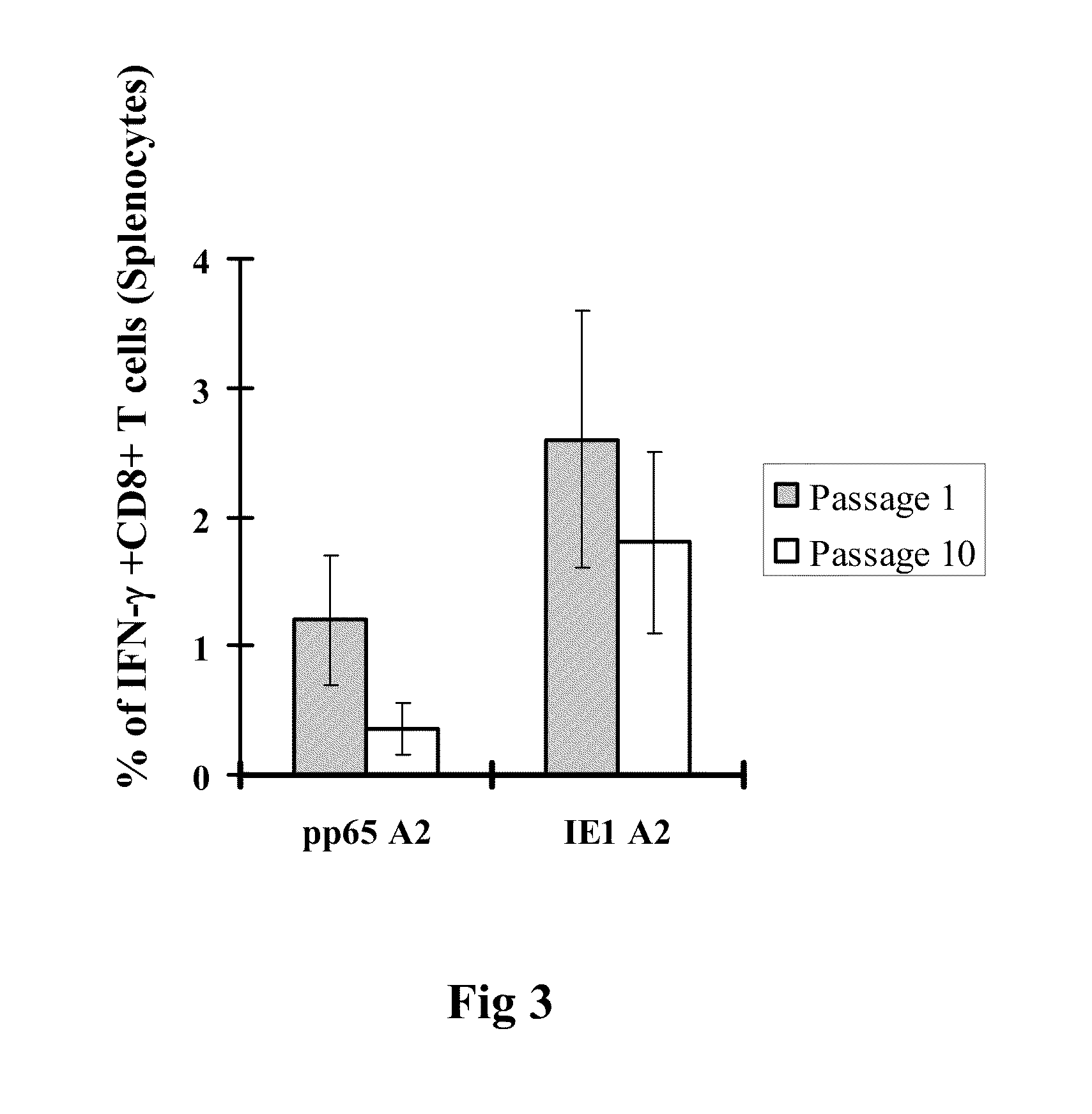
Genetically stable recombinant modified vaccinia ankara (RMVA) vaccines and methods of preparation thereof
ActiveUS20100316667A1Elicit immune responseVectorsSugar derivativesHeterologousModified vaccinia Ankara
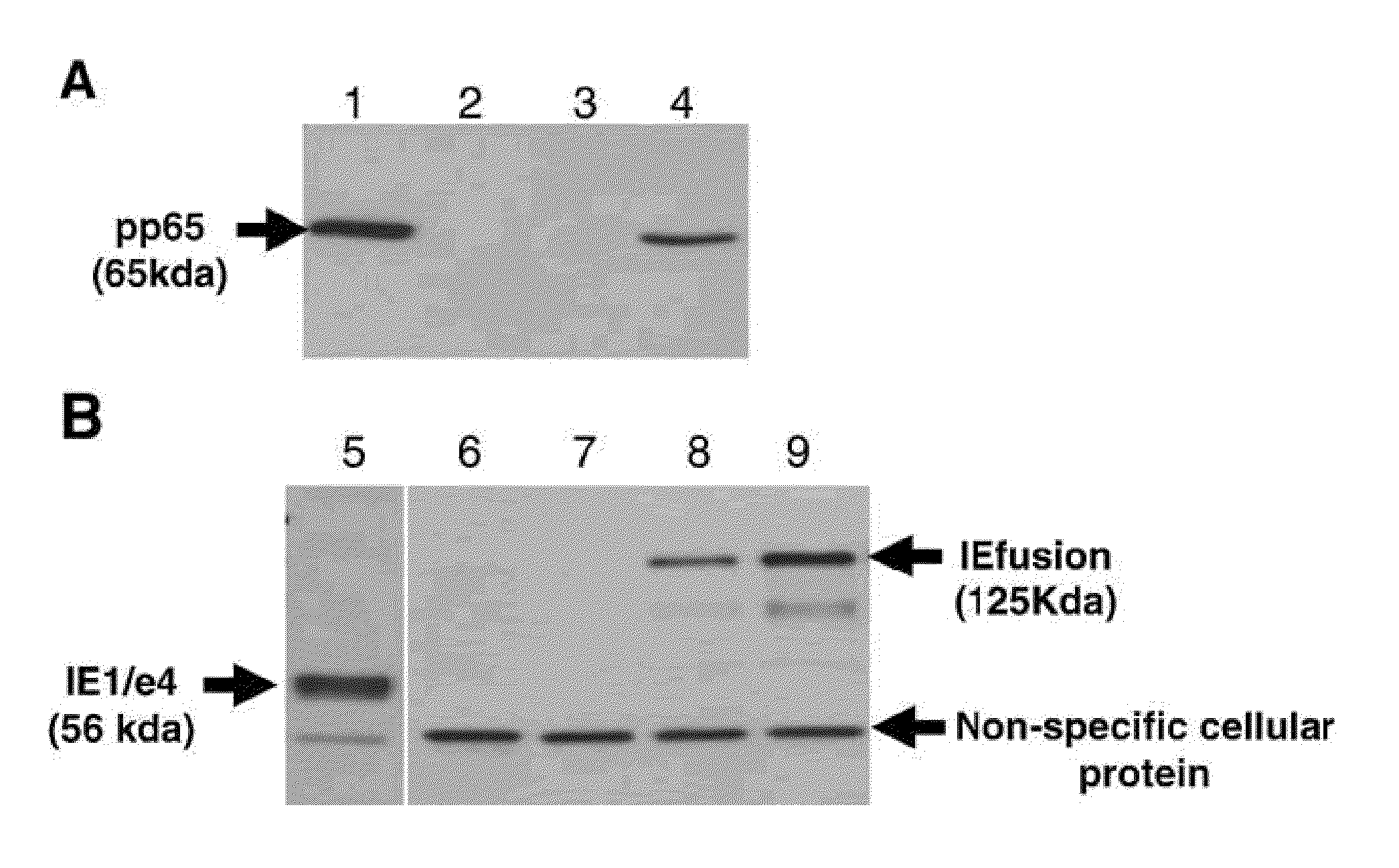
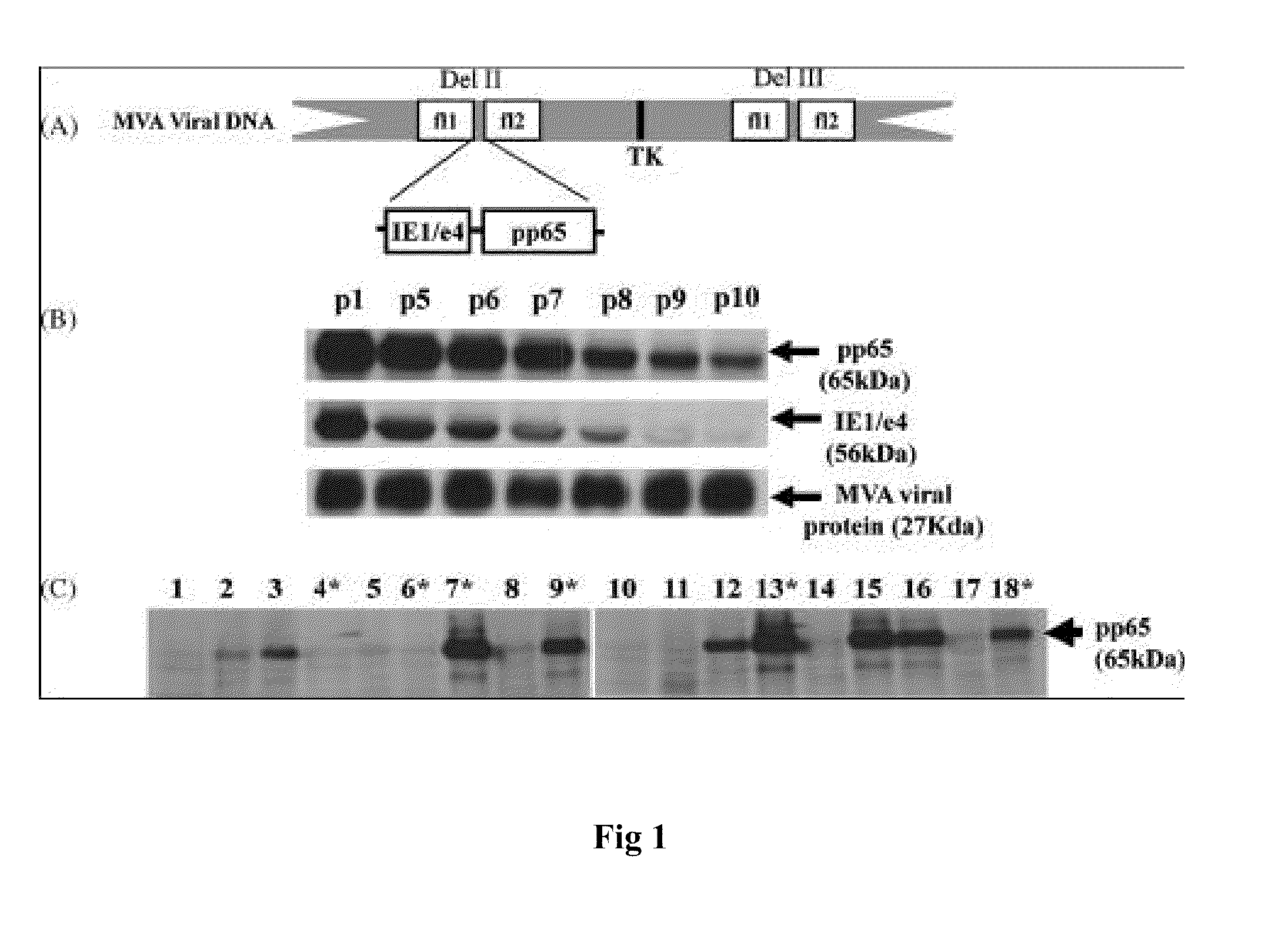
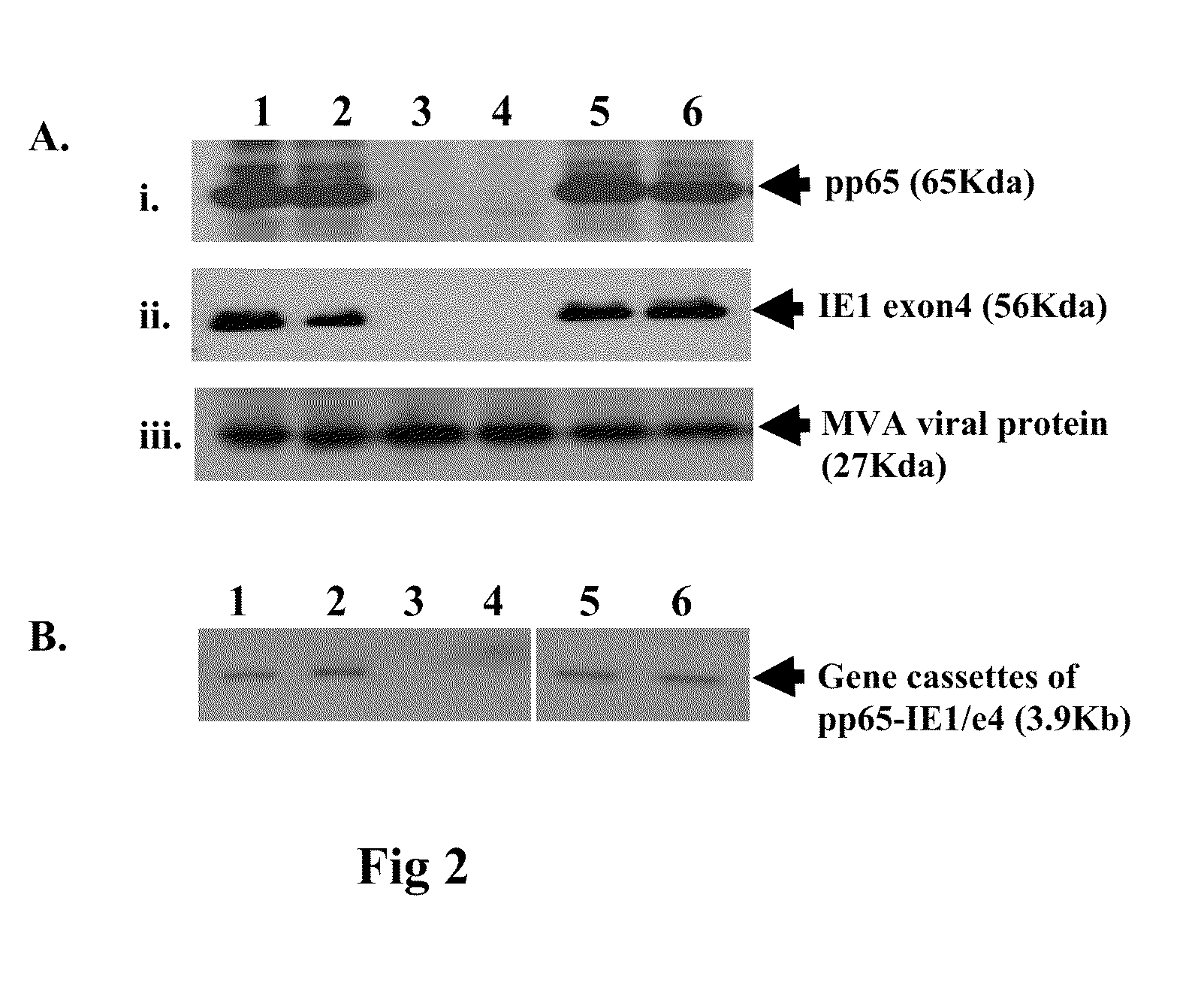
A vaccine comprising an immunologically effective amount of recombinant modified vaccinia Ankara (rMVA) virus which is genetically stable after serial passage and produced by a) constructing a transfer plasmid vector comprising a modified H5 (mH5) promoter operably linked to a DNA sequence encoding a heterologous foreign protein antigen, wherein the expression of said DNA sequence is under the control of the mH5 promoter; b) generating rMVA virus by transfecting one or more plasmid vectors obtained from step a) into wild type MVA virus; c) identifying rMVA virus expressing one or more heterologous foreign protein antigens using one or more selection methods for serial passage; d) conducting serial passage; e) expanding an rMVA virus strain identified by step d); and f) purifying the rMVA viruses from step e) to form the vaccine. One embodiment is directed to a fusion cytomegalovirus (CMV) protein antigen comprising a nucleotide sequence encoding two or more antigenic portions of Immediate-Early Gene-1 or Immediate-Early Gene-2 (IEfusion), wherein the antigenic portions elicit an immune response when expressed by a vaccine.
Owner:CITY OF HOPE
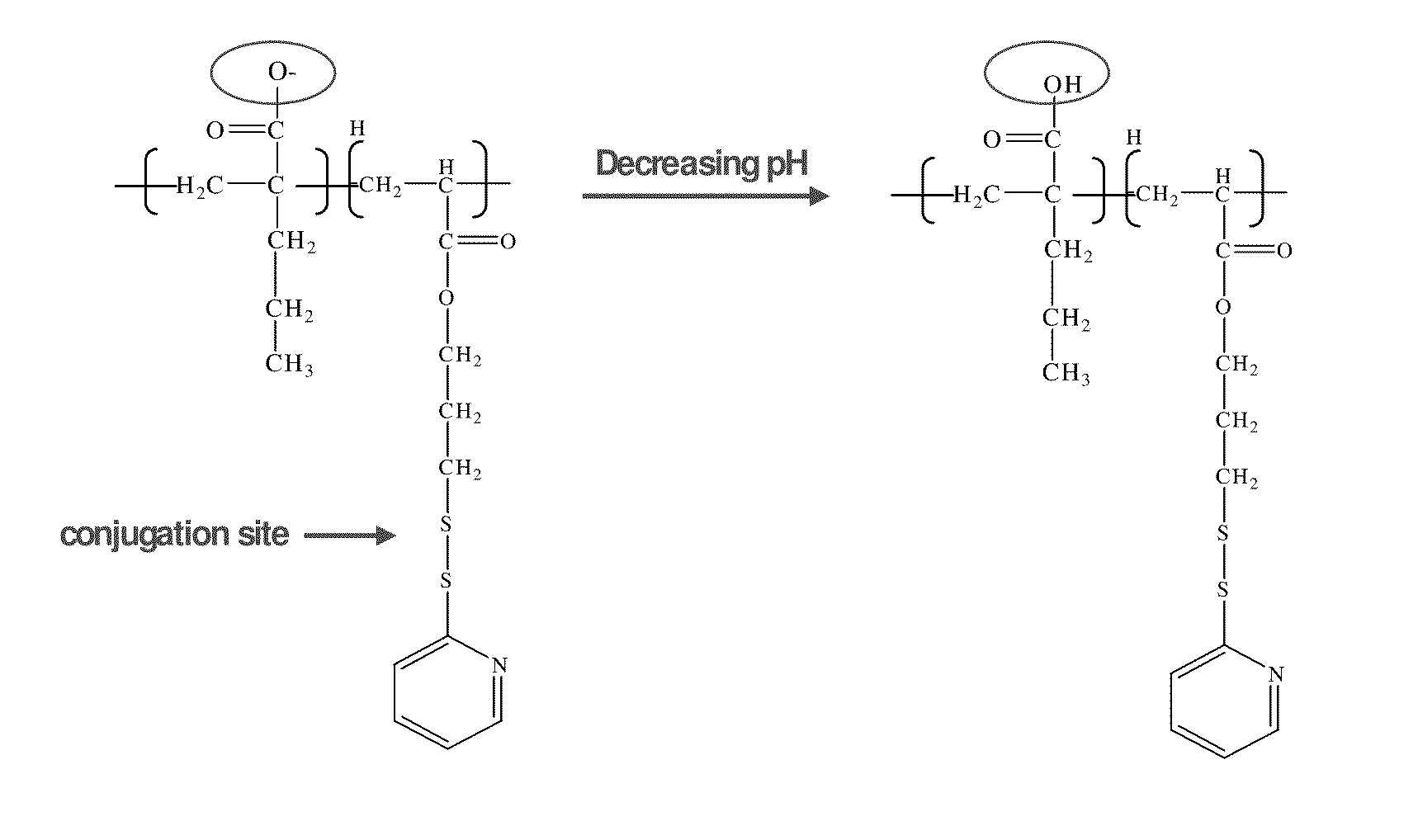
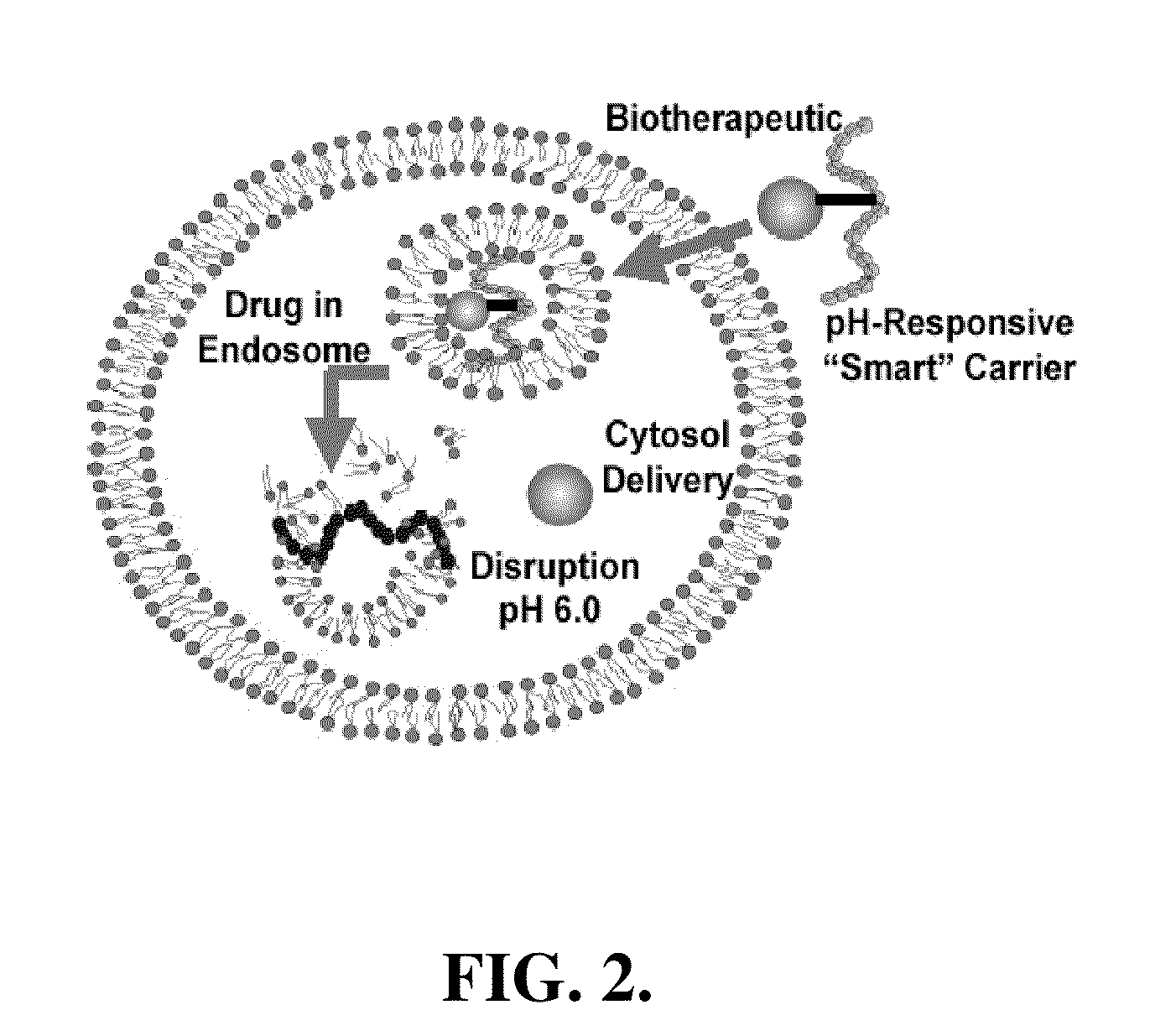
pH-RESPONSIVE POLYMER CARRIER COMPOSITIONS FOR CYTOSOLIC PROTEIN DELIVERY
InactiveUS20100150952A1Amount of remainedIncrease volumeCancer antigen ingredientsCarrier-bound antigen/hapten ingredientsAntigen deliveryLymphocyte
pH-Responsive polymer-based protein delivery carriers and compositions, methods for making the carriers and compositions, and methods for using the carriers and compositions for intracellular protein antigen delivery, inducing a cytotoxic T-lymphocyte response, introducing a tumor-specific protein antigen to an antigen presenting cell to induce an immune response, and providing tumor protection to a subject.
Owner:UNIV OF WASHINGTON
Prophylactic and therapeutic influenza vaccines, antigens, compositions and methods
The present invention relates to the intersection of the fields of immunology and protein engineering, and particularly to antigens and vaccines useful in prevention of infection by influenza virus. Provided are recombinant protein antigens, compositions, and methods for the production and use of such antigens and subunit vaccine compositions. In some embodiments, influenza antigens include hemagglutinin polypeptides neuraminidase polypeptides, and / or combinations thereof.
Owner:FRAUNHOFER USA
Novel streptococcus antigens
Streptococcus proteins and polynucleotides encoding them are disclosed. Said proteins are antigenic and therefore useful vaccine components for the prophylaxis or therapy of streptococcus infection in animals. Also disclosed are recombinant methods of producing the protein antigens as well as diagnostic assays for detecting streptococcus bacterial infection.
Owner:ID BIOMEDICAL CORP LAVAL
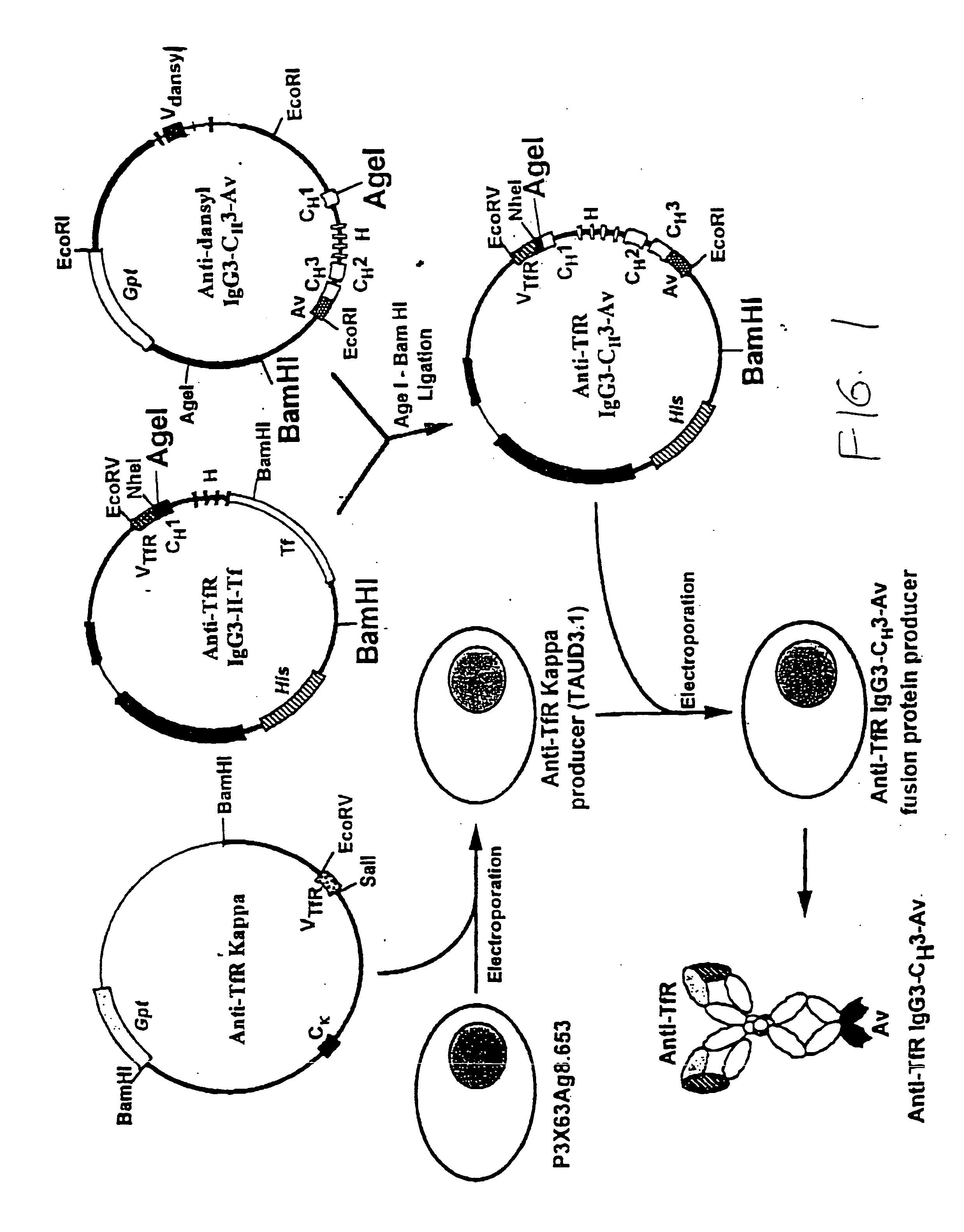
Anti-growth factor receptor avidin fusion proteins as universal vectors for drug delivery
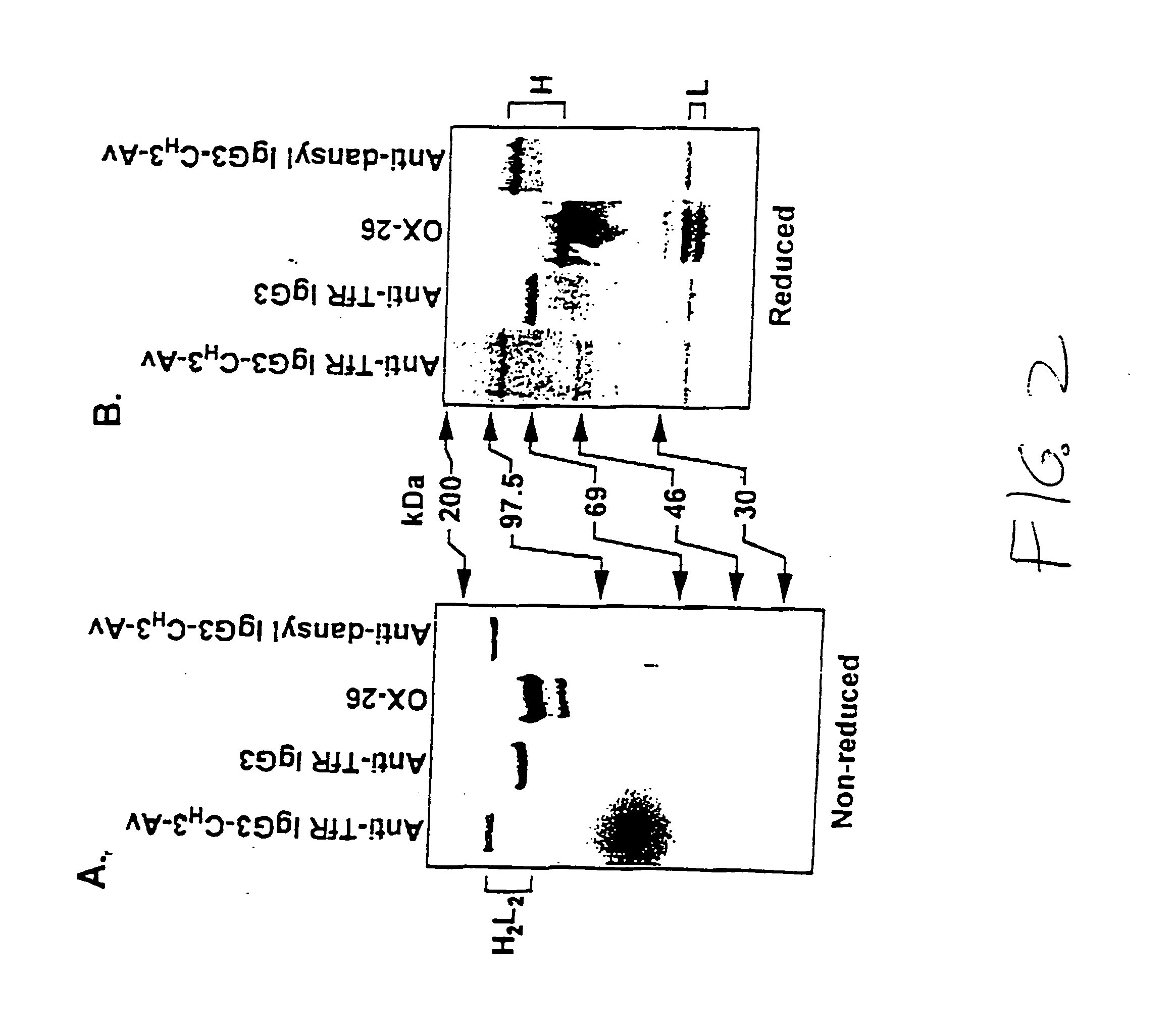
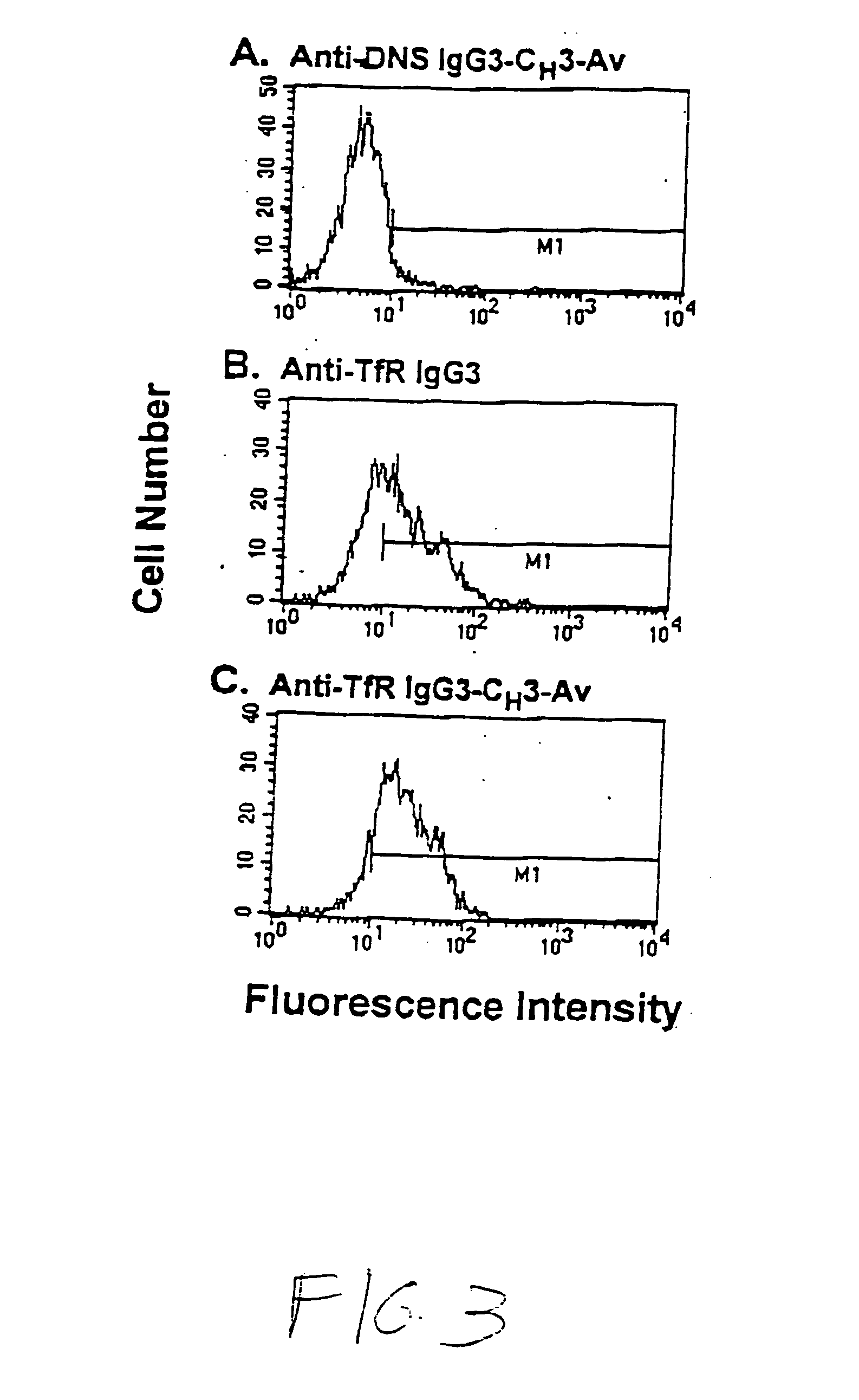
A fusion protein for delivery of a wide variety of agents to a cell via antibody-receptor-mediated endocytosis comprises a first segment and a second segment: the first segment comprising a variable region of an antibody that recognizes an antigen on the surface of a cell that after binding to the variable region of the antibody undergoes antibody-receptor-mediated endocytosis, and, optionally, further comprises at least one domain of a constant region of an antibody; and the second segment comprising a protein domain selected from the group consisting of avidin, an avidin mutein, a chemically modified avidin derivative, streptavidin, a streptavidin mutein, and a chemically modified streptavidin derivative. Typically, the antigen is a protein. Typically, the protein antigen on the surface of the cell is a receptor such as a transferrin receptor-or an insulin receptor. The invention also includes an antibody construct incorporating the fusion protein that is either a heavy chain or a light chain together with a complementary light chain or heavy chain to form an intact antibody molecule. The invention further includes targeting methods and screening methods.
Owner:RGT UNIV OF CALIFORNIA
Simultaneous detection apparatus of raman and light scattering
ActiveUS20100020312A1Radiation pyrometrySpectrum investigationDynamic light scatteringProtein antigen
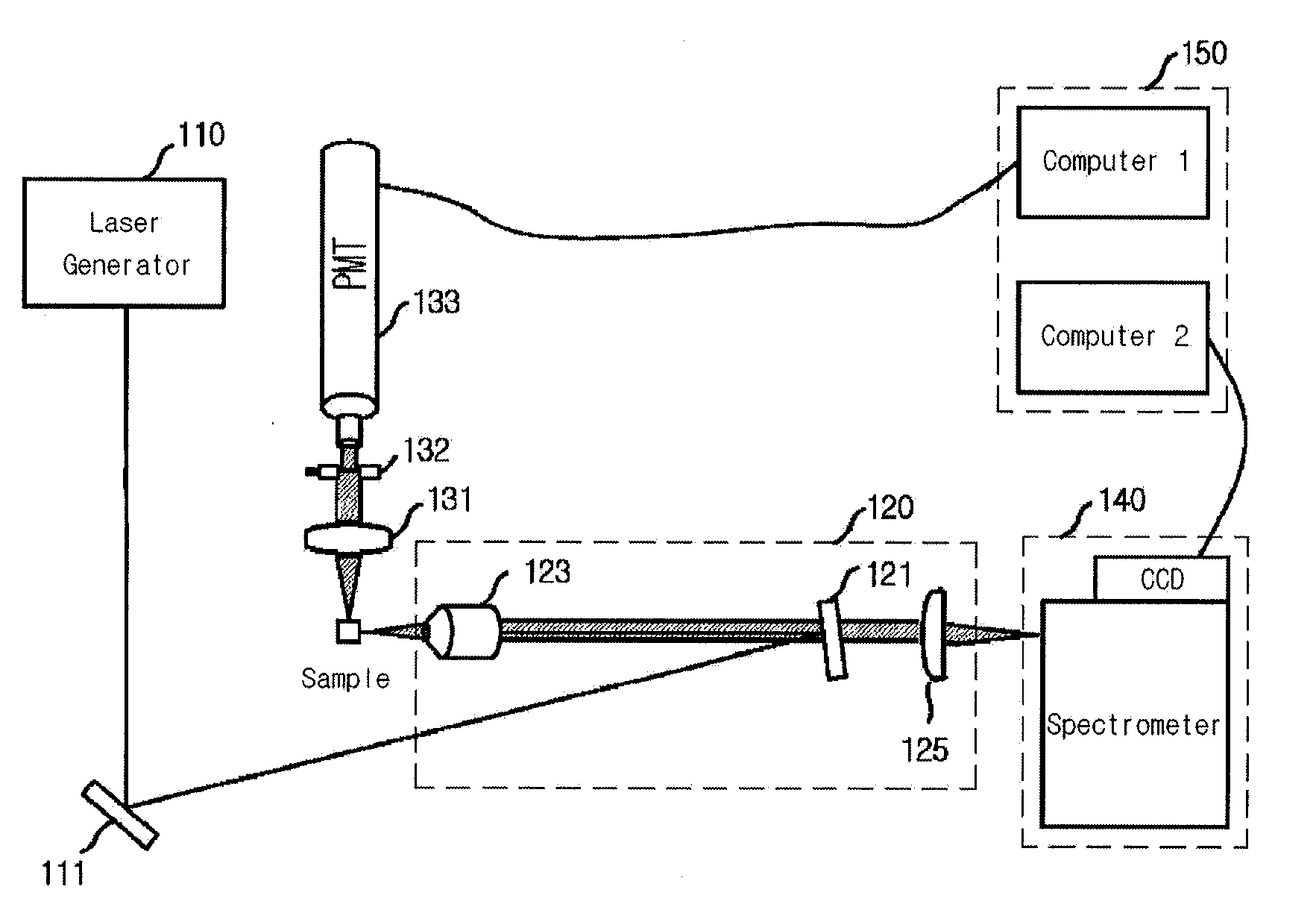
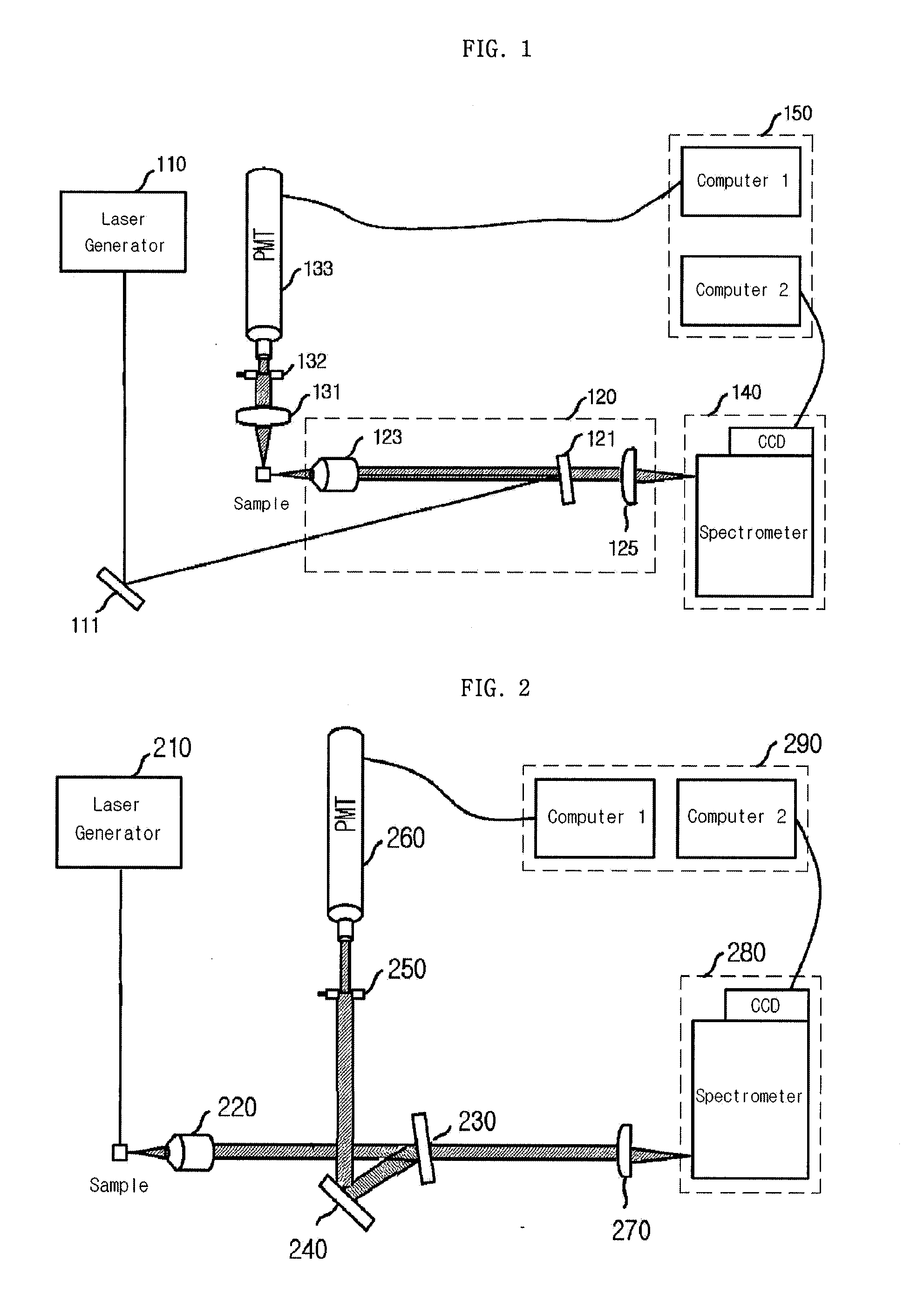
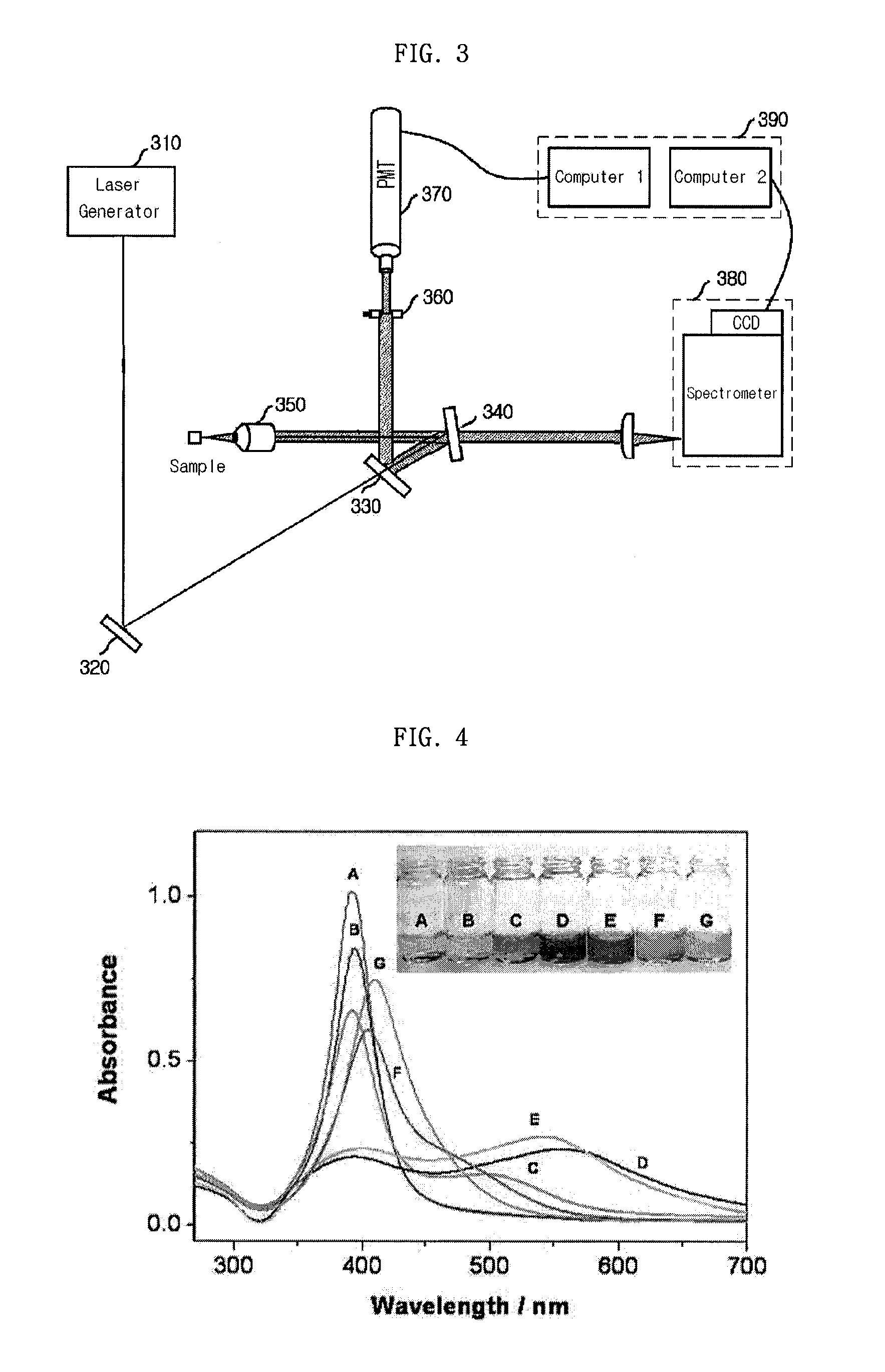
Provided is a detection apparatus of Raman scattering and light scattering, and more particularly, a simultaneous detection apparatus of Raman scattering and dynamic light scattering and a detection method using the same. The simultaneous detection apparatus of Raman scattering and light scattering includes: a detection unit for applying incident light to a sample, and detecting Raman scattering in 90° or 180° geometry and light scattering in 90° or 180° geometry in order to simultaneously collect Raman scattering and light scattering; and a computer connected to the detection unit to obtain at least one of the size and distribution of particles from the detected light scattering, and to obtain information of the molecular structure from the detected Raman scattering. This apparatus may simultaneously observe the size of nano-sized or larger material and molecular information thereof, and phenomena accompanying changes in molecular environment according to material variation and changes of the material in size and distribution, and thus is very useful for studying nano materials and protein antigens and antibodies.
Owner:SEOUL NAT UNIV R&DB FOUND
Polypeptide-vaccines for broad protection against hypervirulent meningococcal lineages
ActiveUS8663656B2Antibacterial agentsBacterial antigen ingredientsSalmonella serotype typhiMeningococcal carriage
A small number of defined antigens can provide broad protection against meningococcal infection, and the invention provides a composition which, after administration to a subject, is able to induce an antibody response in that subject, wherein the antibody response is bactericidal against two or three of hypervirulent lineages A4, ET 5 and lineage 3 of N. meningitidis serogroup B. Rather than consisting of a single antigen, the composition comprises a mixture of 10 or fewer purified antigens, and should not include complex or undefined mixtures of antigens such as outer membrane vesicles. Five protein antigens are used in particular: (1) a ‘NadA’ protein; (2) a ‘741’ protein; (3) a ‘936’ protein; (4) a ‘953’ protein; and (5) a ‘287’ protein.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Antineoplastic dibasic polypeptide and application and preparation method thereof
ActiveCN101139613ASpecific targetingWon't attackPeptide/protein ingredientsHybrid peptidesNucleotideDouble chain
The invention provides a gene, recombinant plasmid and polypeptide for an anti-tumor binary polypeptide. The gene of the recombinant anti-tumor binary polypeptide is obtained by connecting in an operable way the gene of a coding antibody simulator with a recombinant bacillus anthraci protein antigen gene. The recombinant plasmid of the invention is formed by inserting the gene of the coding antibody simulator by double-chain oligomeric nucleotide directed mutagenesis method into the recombinant bacillus anthraci protein antigen gene. The obtained recombinant plasmid is infected into engineering bacillus coli BL-21 to get engineering bacillus coli cell of anti-tumor binary polypeptide; the anti-tumor binary polypeptide can be obtained by expanding the bacillus coli, settling in centrifugal way the bacillus coli body, crushing in altrasonic way, settling and crushing bacillus coli body by hi-speed centrifuging and treating the upper clean solution. The anti-tumor binary polypeptide is of special targeting characteristic, higher efficiency in killing special physical tumor than prior anti-tumor medicine, and will not attack normal cells, and has much lower toxicity and poor-reaction than prior anti-tumor medicine.
Owner:姜荣锡
Peptide-based diagnostic reagents for SARS
InactiveUS20050100883A1Improve quality controlBiocideSsRNA viruses positive-senseSARS coronavirusAssay
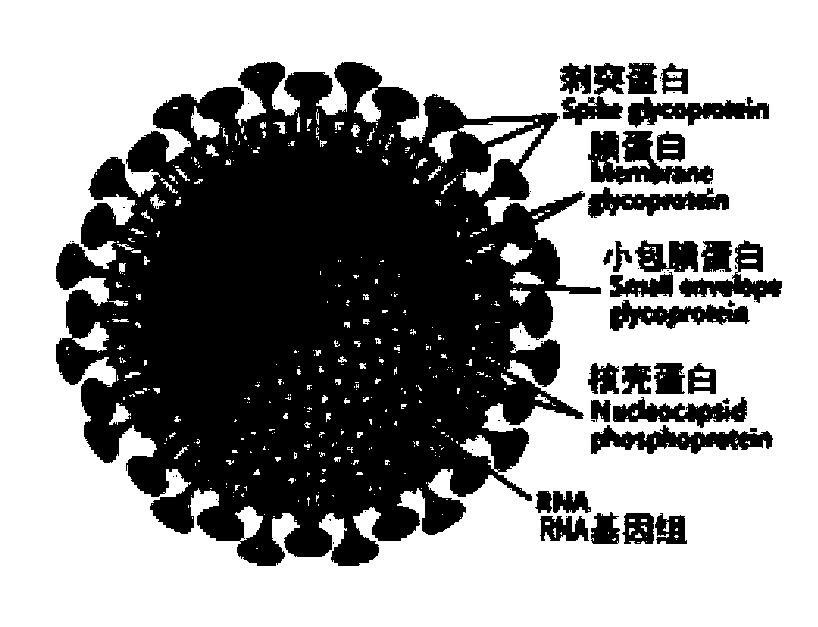
The present invention is directed to antigenic peptides and peptide compositions selected from the Membrane glycoprotein (M), the Spike glycoprotein (S), and the Nucleocapsid (N) protein antigens of the SARS coronavirus (SCoV). The present invention is also directed to methods of use of the peptides of the invention, e.g., for the detection of SARS-associated antibodies. Detection methods include enzyme-linked immunosorbent assay (ELISA) or other immunoassay procedures.
Owner:UNITED BIOMEDICAL INC
Pneumococcal polysaccharide and protein conjugated vaccine and preparation method thereof
ActiveCN103893751AEnhance immune responseImproving immunogenicityAntibacterial agentsBacterial antigen ingredientsConjugate vaccineStreptococcus pneumoniae capsular polysaccharide
The invention provides a pneumococcal polysaccharide and protein conjugated vaccine and a preparation method thereof. The pneumococcus polysaccharide protein conjugated vaccine comprises one or more streptococcus pneumoniae capsular polysaccharide and protein coupled immune conjugates, and at least one of the immune conjugates is formed by coupling a single streptococcus pneumoniae capsular polysaccharide with two or more proteins. Compared with the existing pneumococcal conjugated vaccine, the pneumococcal polysaccharide and protein conjugated vaccine has stronger immunogenicity, and can cause immune response in a wider crowd; meanwhile, the two carrier proteins are covalently coupled through the polysaccharide, the protective protein antigen epitope induces higher immune response compared with the mixed injection of the two proteins, and through mutual synergy, the immunogenicity of the carrier protein is further improved and the immune response of the body to the polysaccharide is improved; the preparation method is simple, and meets the requirement of large-scale industrial production.
Owner:CANSINO BIOLOGICS INC
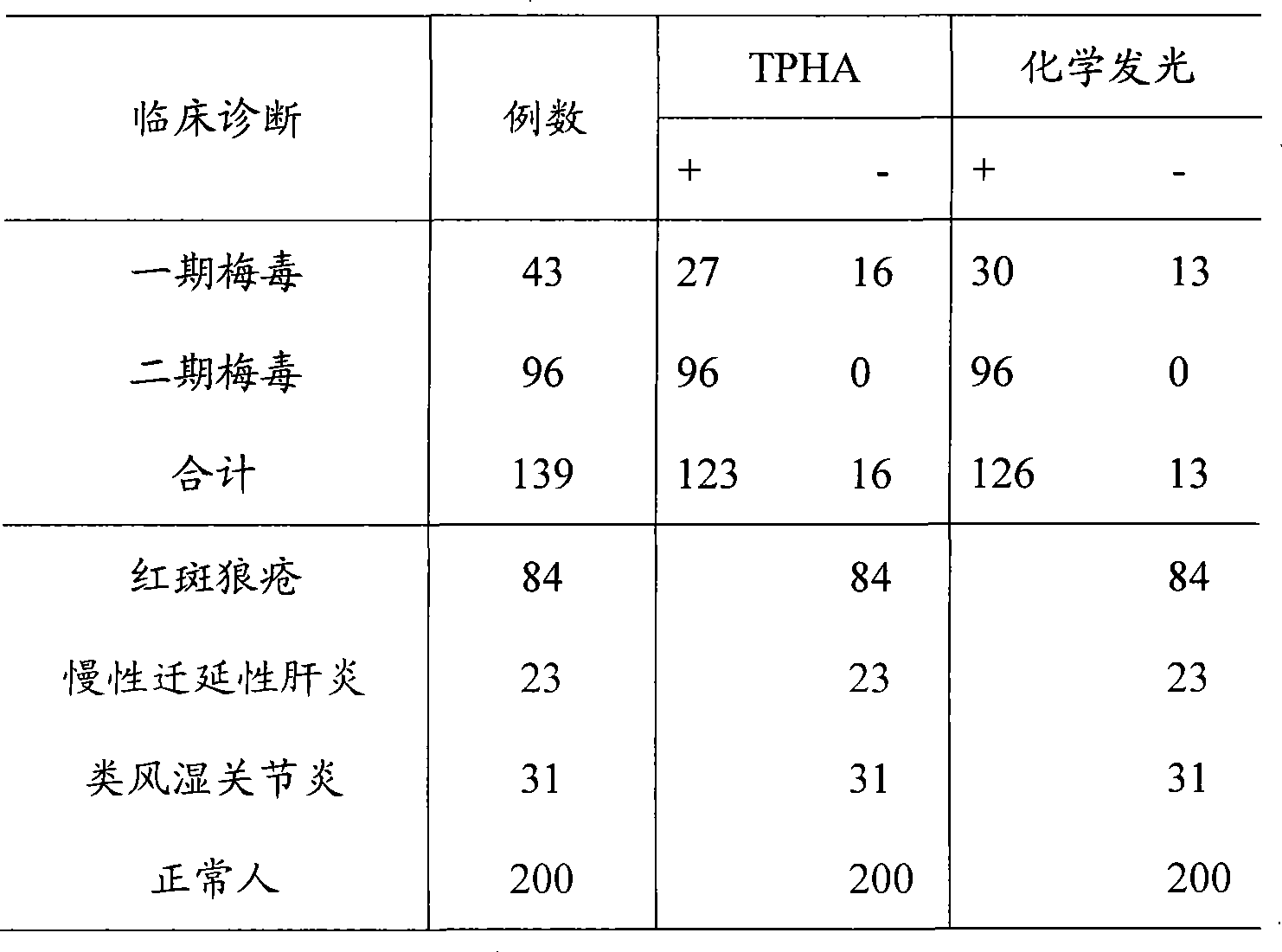
Syphilis helicoid antibody chemiluminescence immune assay determination kit and method for preparing same
InactiveCN101363860AEfficient use ofGuaranteed SensitivityChemiluminescene/bioluminescencePositive controlNon toxicity
The invention discloses a kit of chemiluminescent immunological analysis measurement of a Treponema pallidum antibody, and preparation method thereof, which belongs to the technical field of immunological analysis medical diagnosis. The kit comprises (1) a carrier coated with a treponema pallidum specific recombination protein antigen; (2) treponema pallidum antibody negative and positive reference substances; (3) an enzyme labeled treponema pallidum specific recombination protein antigen; and (4) an enzyme acted chemiluminescent primer. Furthermore, the preparation method of the kit based on the invention comprises the following steps of (1) preparing TP antibody negative and positive reference substances; (2) labeling the TP specific recombination protein antigen with an enzyme; (3) coating with the carrier; (4) sub-packaging; and (5) assembling. The kit of the detection of the Treponema pallidum antibody has the advantages of easy and simple operation, easy popularization, high sensitivity, strong specificity, good repeatability, safety, non toxicity, and no pollution.
Owner:北京科美东雅生物技术有限公司
Biological product for preventing novel coronavirus
InactiveCN111228475ASsRNA viruses positive-senseViral antigen ingredientsGenetic vaccineProtein antigen
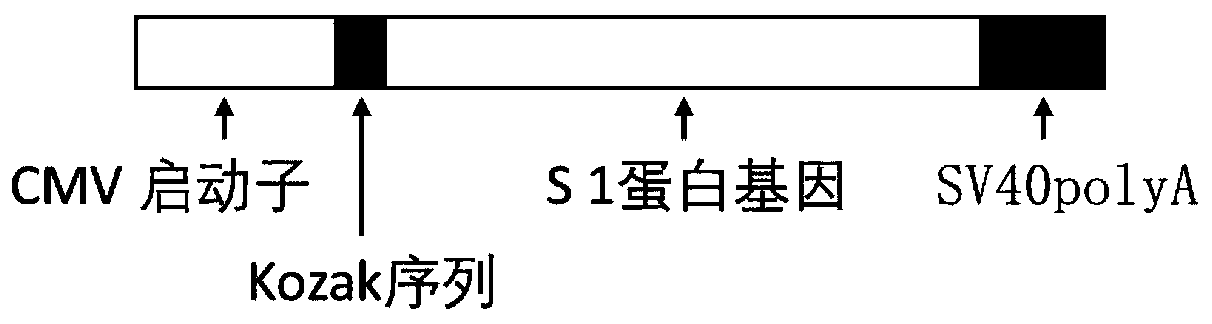
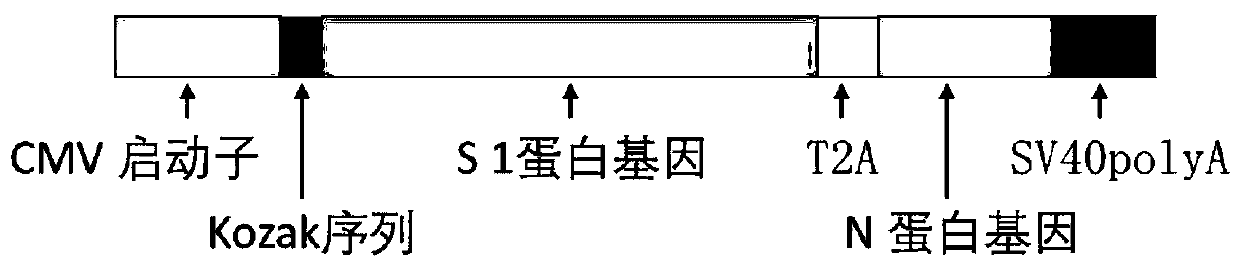
The invention discloses a biological product for preventing novel coronavirus (COVID-19). The biological product can be a gene vaccine or gene medicine, wherein the gene vaccine adopts a human type-5adenovirus with deletion of E1 and E3 genes as a vector for carrying an S1 protein antigen for expressing a Spike S1 subunit of the novel coronaviruses or simultaneously carrying an S1 expression protein antigen and N protein antigen. Through in-vivo expression of antigen proteins, the immune reaction of a body upon the novel coronavirus can be stimulated, and the effect of preventing infection and propagation of the novel coronavirus.
Owner:SYNO SHENZHEN BIOMEDICAL RES CO LTD
Lateral flow nucleic acid detector
InactiveUS20090305290A1Bioreactor/fermenter combinationsBiological substance pretreatmentsRibonucleotide synthesisNucleic acid sequencing
Point-of-care binding assays include at least one target nucleic acid binding in a multiplex structure with at least one sequence in a partner nucleic acid associated with a label, due to complementary base pairings between at least one sequence in the target nucleic acid and at least one sequence in the partner nucleic acid. The assays overcome the inherent deficiencies of antibody-protein antigen assays. In a preferred embodiment, color tagged nucleic acid sequences are used to bind a complementary target nucleic acid. The tagged nucleic acid sequences are preferably made from deoxyribonucleotides, ribonucleotides, or peptide nucleotides.
Owner:RAPID PATHOGEN SCREENING INC
Method for designing vaccines against constantly mutating pathogens
InactiveUS20090162383A1Organic active ingredientsPeptide/protein ingredientsTherapeutic antibodyImmunodominant Epitopes
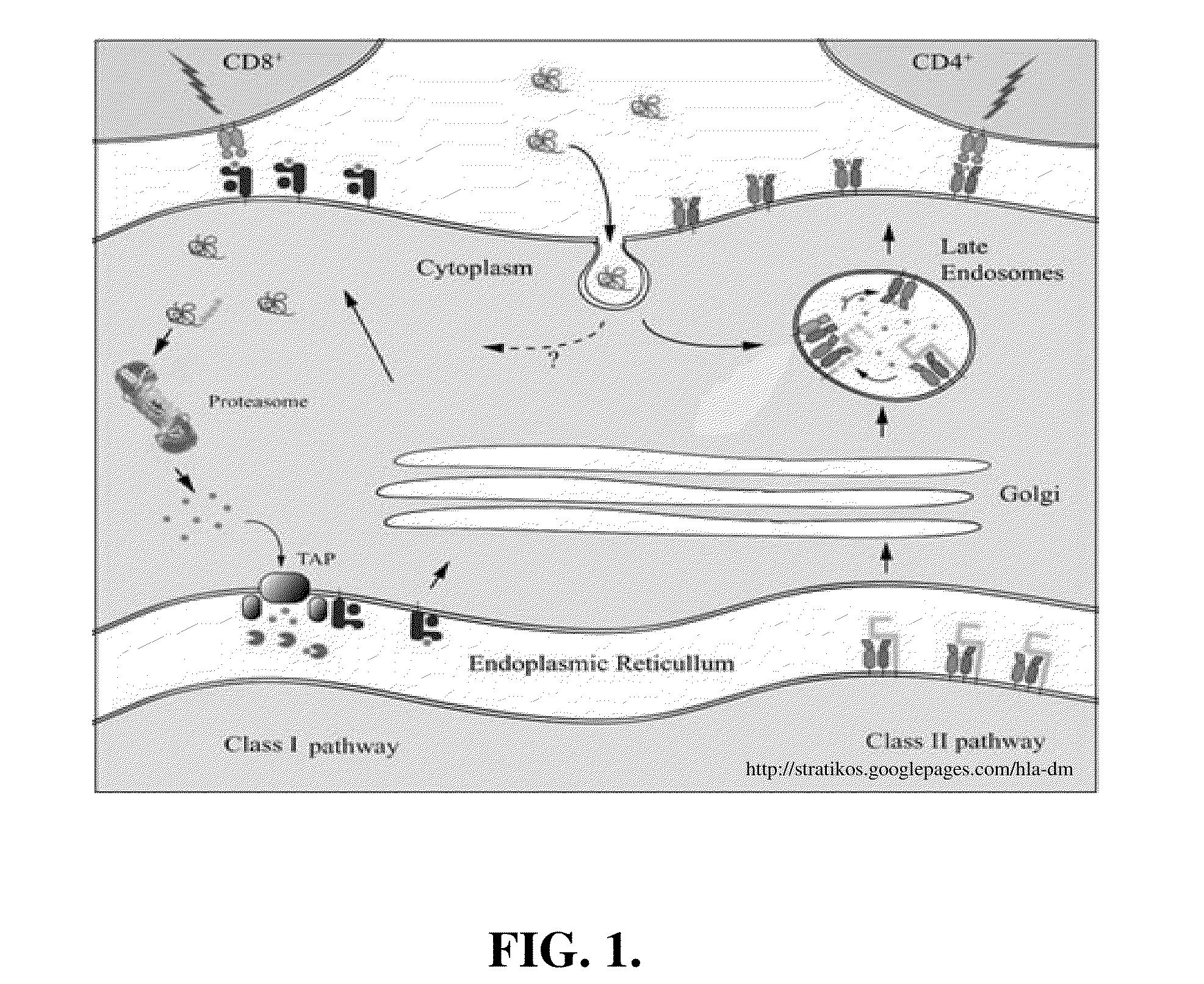
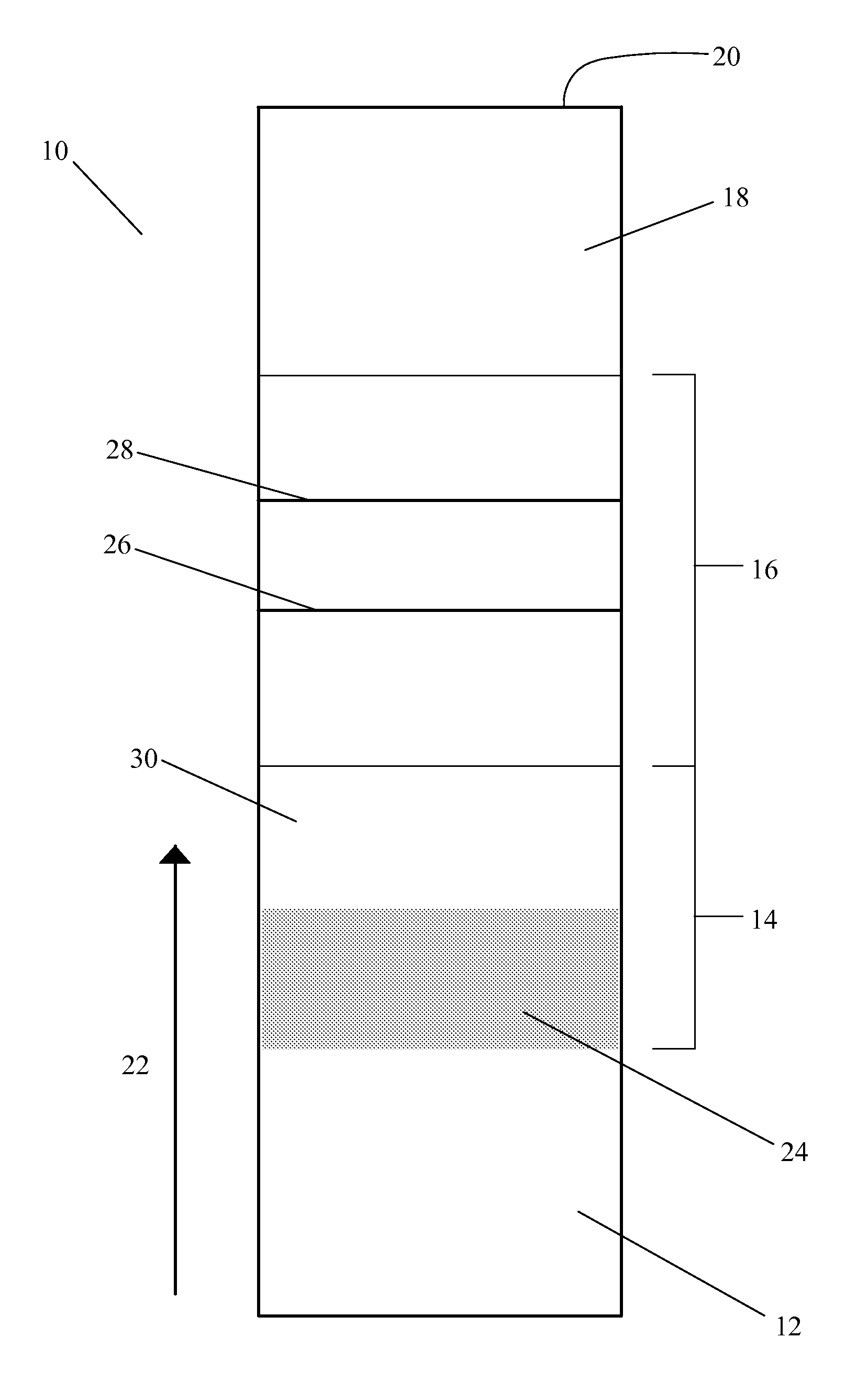
A unique method is disclosed for identifying and replacing surface amino acid residues of a protein antigen that reduces the antigenicity of the putative immunodominant epitopes of the antigen and makes all the accessible regions of the molecule essentially antigenically equivalent, so that the antibody response will be directed against more parts of the molecule and not mainly against the erstwhile immunodominant epitopes. The method will simultaneously change the antigenicity of the molecule and preserve its structure. The method is useful in the design of molecules useful for immunization, for example, as vaccines, or for the generation of therapeutic antibodies, against constantly mutating pathogens. It is also useful in the design of molecules useful for immunization against pathogens that had been intentionally mutated so as to render those pathogens able to infect erstwhile immune individuals.
Owner:PADLAN EDUARDO A
Influenza antibodies, compositions, and related methods
InactiveUS20080124272A1Inhibitory activityImmunoglobulins against virusesAntiviralsProtein engineeringProtein antigen
The present invention relates to the intersection of the fields of immunology and protein engineering, and particularly to antigens and vaccines useful in prevention of infection by influenza virus. Provided are recombinant protein antigens, compositions, and methods for the production and use of such antigens and vaccine compositions.
Owner:FRAUNHOFER USA
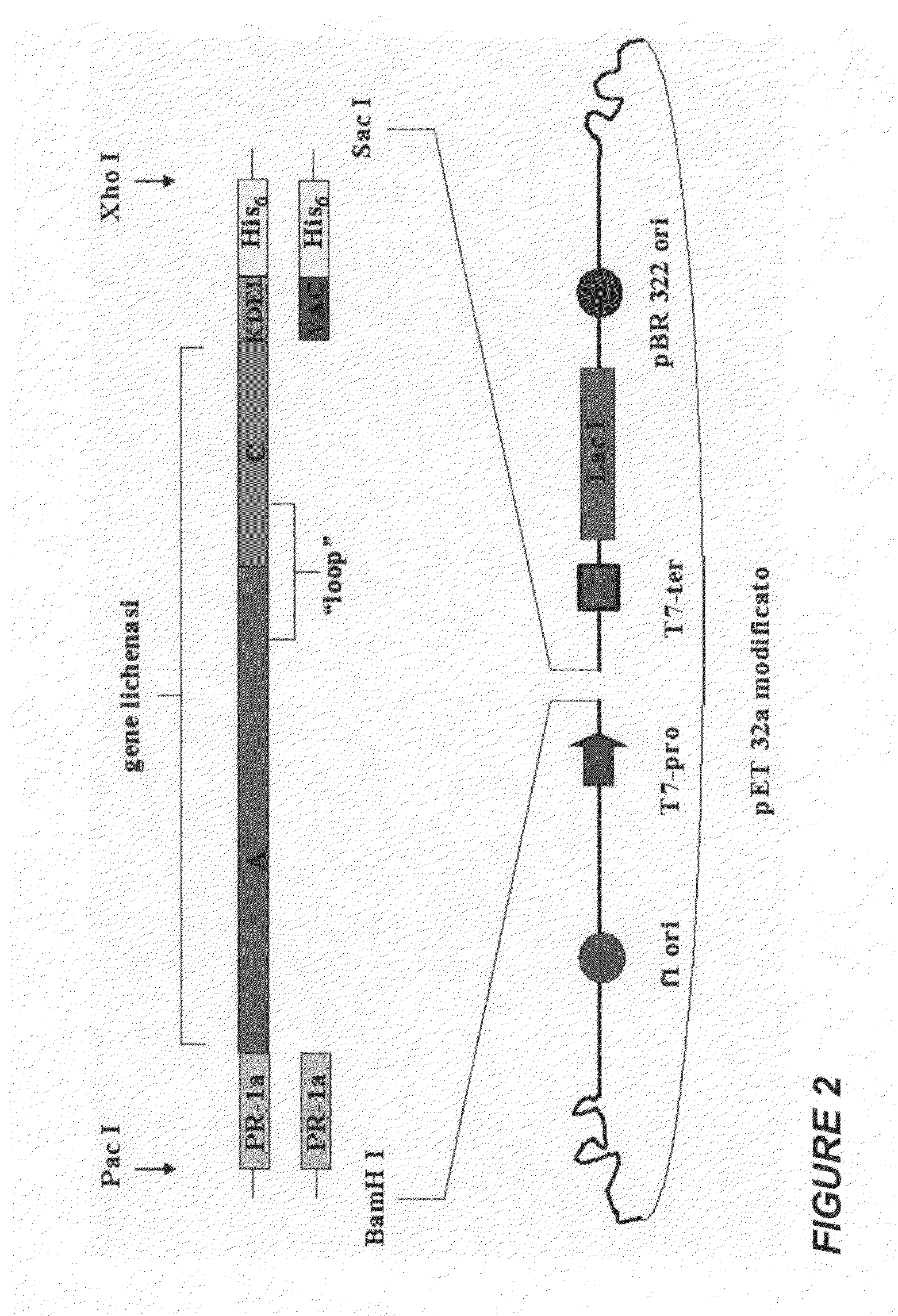
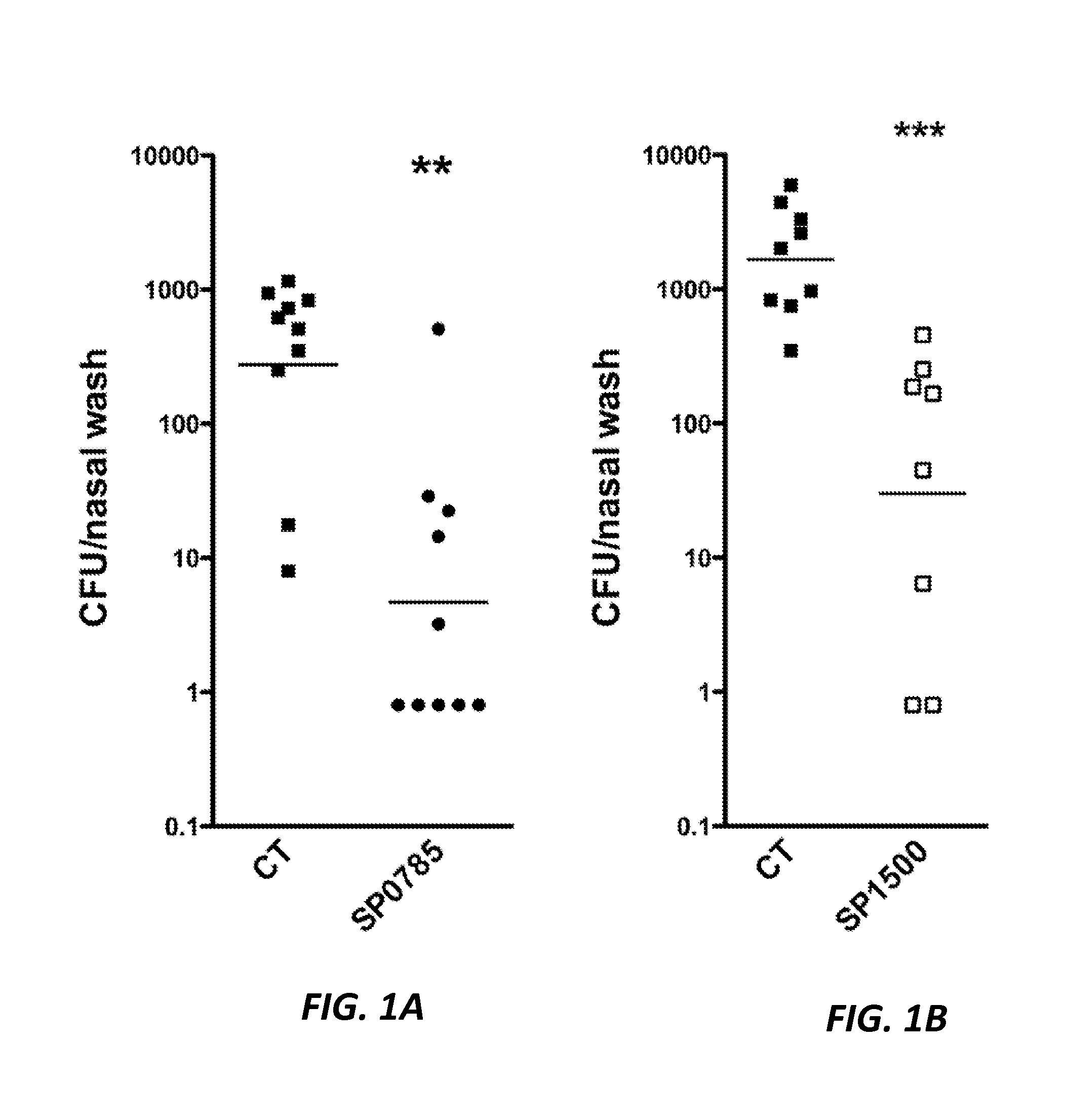
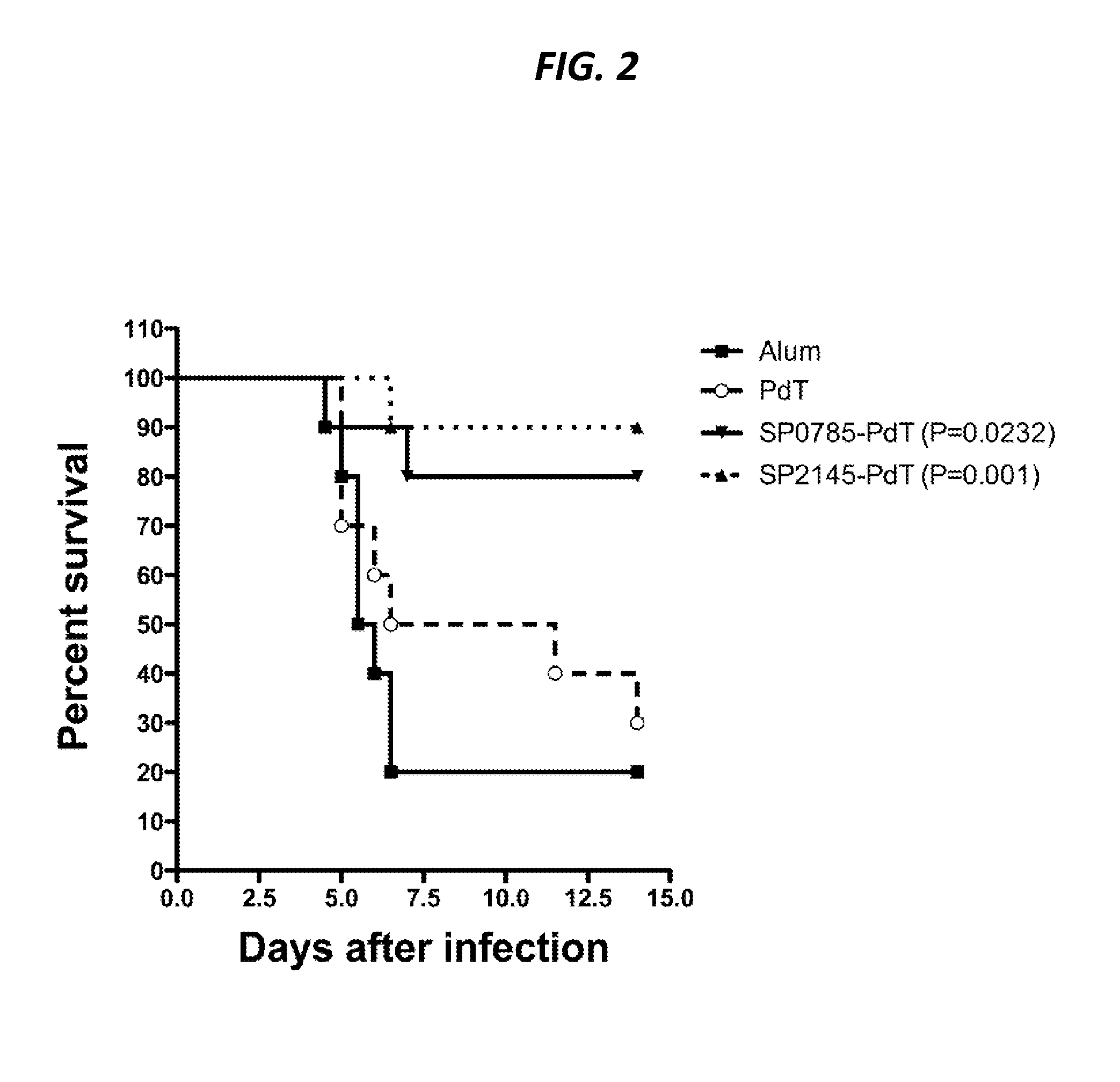
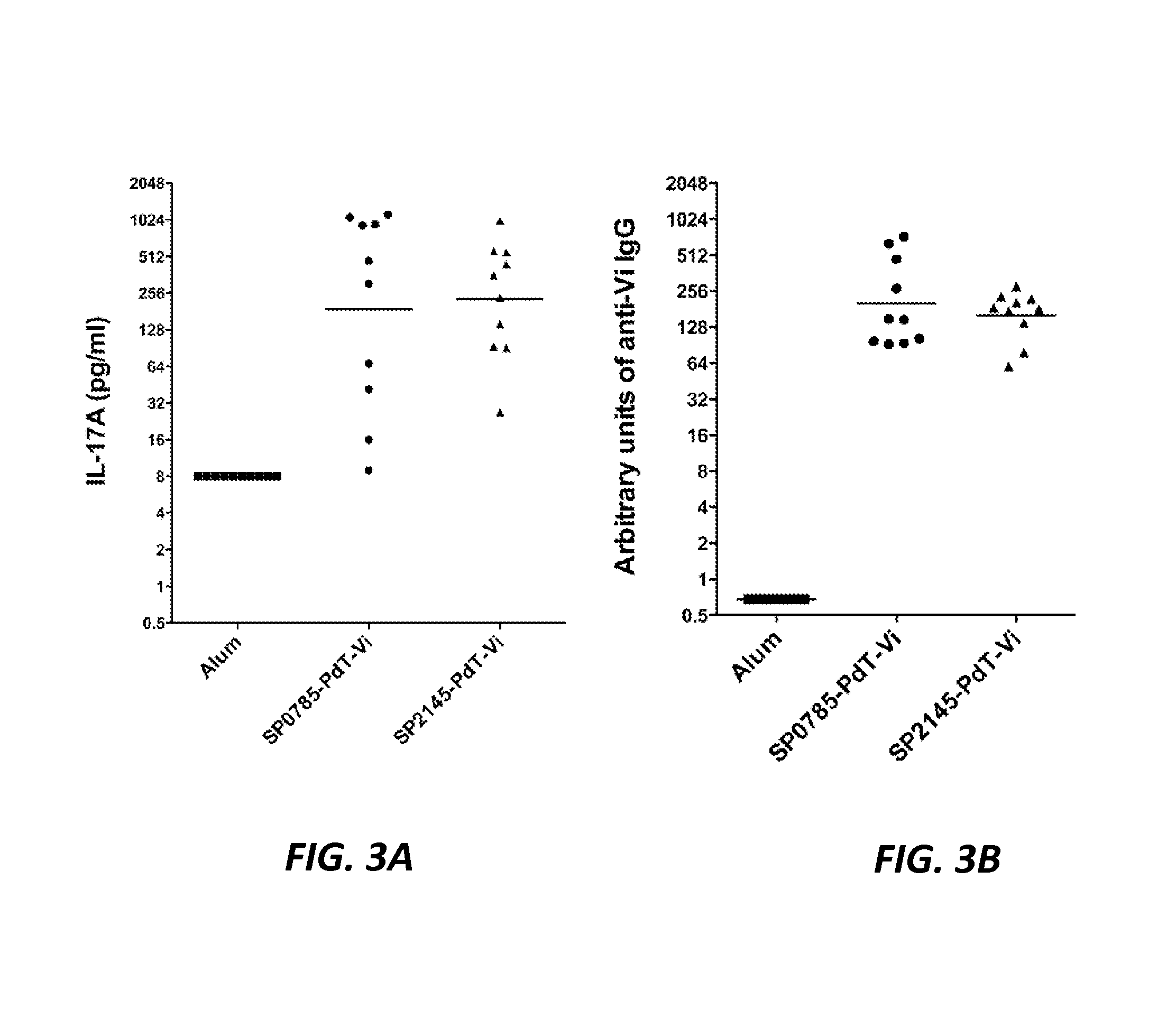
Protein antigens that provide protection against pneumococcal colonization and/or disease
ActiveUS20150374811A1Reduce colonizationReduce the risk of infectionAntibacterial agentsBacterial antigen ingredientsDiseaseT cell
The present application is generally directed to novel pneumococcal polypeptide antigens and nucleic acids encoding such antigens, and immunogenic compositions comprising such antigens for treating and preventing pneumococcal infection. The present invention further provides method of using the antigens to elicits an immune response (e.g., IL-17A response, a T cell-mediated and / or B-cell-mediated immune responses). The present invention also provides methods of prophylaxis and / or treatment of pneumococcal-mediated diseases, such as sepsis, comprising administering an immunogenic composition including one or more of a combination of pneumococcal antigens or functional fragments thereof as disclosed herein. In some embodiments, one or more pneumococcal antigens can be present in a polysaccharide conjugate. The compositions induce an anti-pneumoccocus immune response when administered to a mammal. The compositions can be used prophylactically to vaccinate an individual and / or therapeutically to induce a thereapeutic immune response to an infected individual.
Owner:CHILDRENS MEDICAL CENT CORP
Protein-chaperoned t-cell vaccines
InactiveUS20170252417A1Increase lymph node uptakeEnhance immune responseViral antigen ingredientsPharmaceutical delivery mechanismPeptide antigenTolerability
Protein antigens are provided. The protein antigens typically include a peptide antigen conjugated or fused to a chaperone protein to form a “chaperone-antigen” that increases lymph node uptake; improves an immune response; or a combination thereof relative to the peptide antigen alone. The immune response can be, for example, increased antigen-specific proliferation, enhanced cytokine production, stimulation of differentiation and / or effector functions, promotion of survival, rescue from exhaustion and / or anergy of T cells, or a combination thereof. Chaperon-antigens can also be used to induce tolerance and increase immune suppressive responses. In the most preferred embodiments, the peptide antigen is fused to the chaperone protein to form a fusion protein. The “chaperone-antigen” can be combined with an adjuvant to form a vaccine and administered to a subject to modulate an immune response to the antigen. Methods of increasing immune responses, treating cancer and infectious and inducing tolerance are also provided.
Owner:MASSACHUSETTS INST OF TECH
Genetically stable recombinant modified vaccinia ankara (rMVA) vaccines and methods of preparation thereof
A vaccine comprising an immunologically effective amount of recombinant modified vaccinia Ankara (rMVA) virus which is genetically stable after serial passage and produced by a) constructing a transfer plasmid vector comprising a modified H5 (mH5) promoter operably linked to a DNA sequence encoding a heterologous foreign protein antigen, wherein the expression of said DNA sequence is under the control of the mH5 promoter; b) generating rMVA virus by transfecting one or more plasmid vectors obtained from step a) into wild type MVA virus; c) identifying rMVA virus expressing one or more heterologous foreign protein antigens using one or more selection methods for serial passage; d) conducting serial passage; e) expanding an rMVA virus strain identified by step d); and f) purifying the rMVA viruses from step e) to form the vaccine. One embodiment is directed to a fusion cytomegalovirus (CMV) protein antigen comprising a nucleotide sequence encoding two or more antigenic portions of Immediate-Early Gene-1 or Immediate-Early Gene-2 (IEfusion), wherein the antigenic portions elicit an immune response when expressed by a vaccine.
Owner:CITY OF HOPE
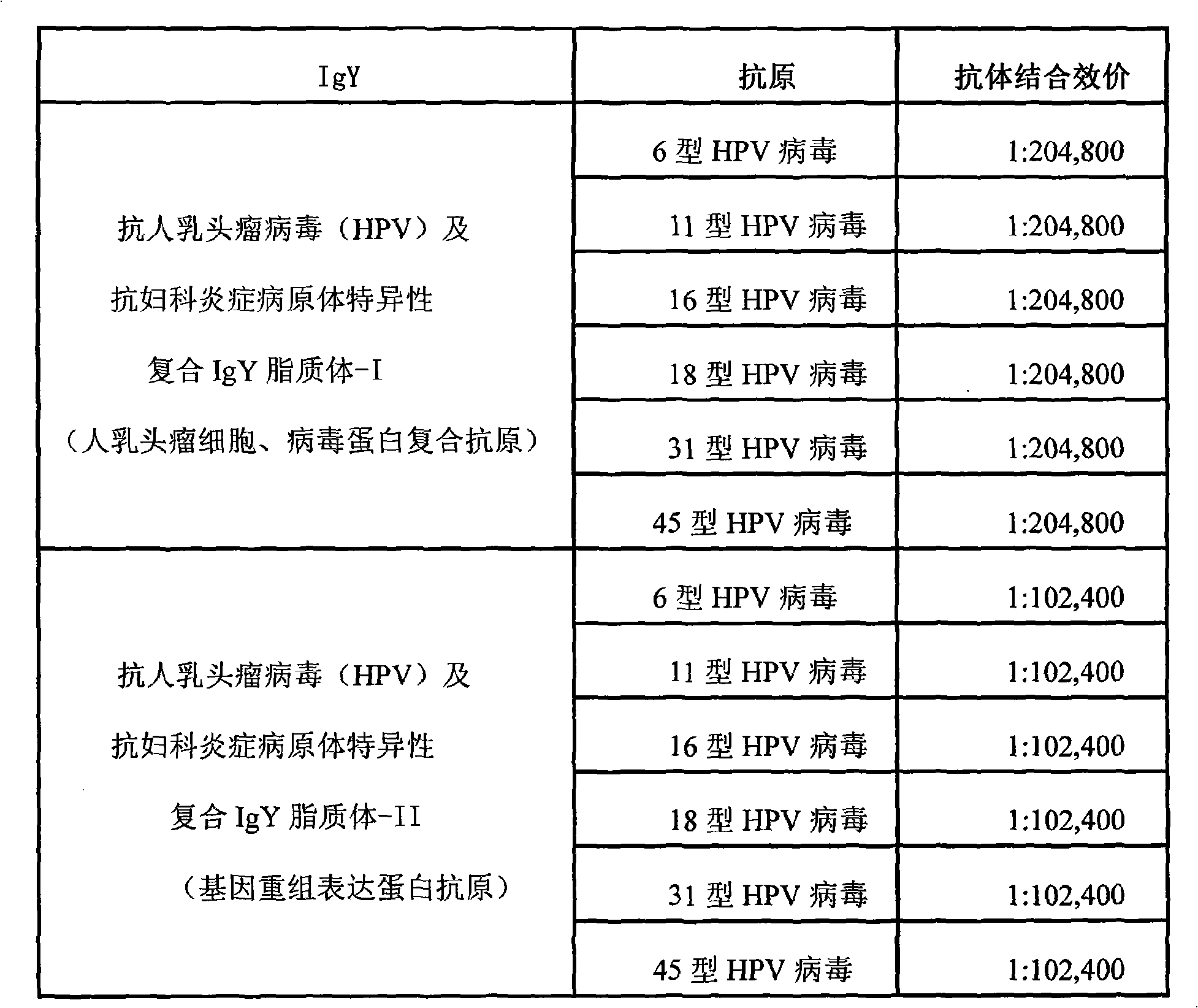
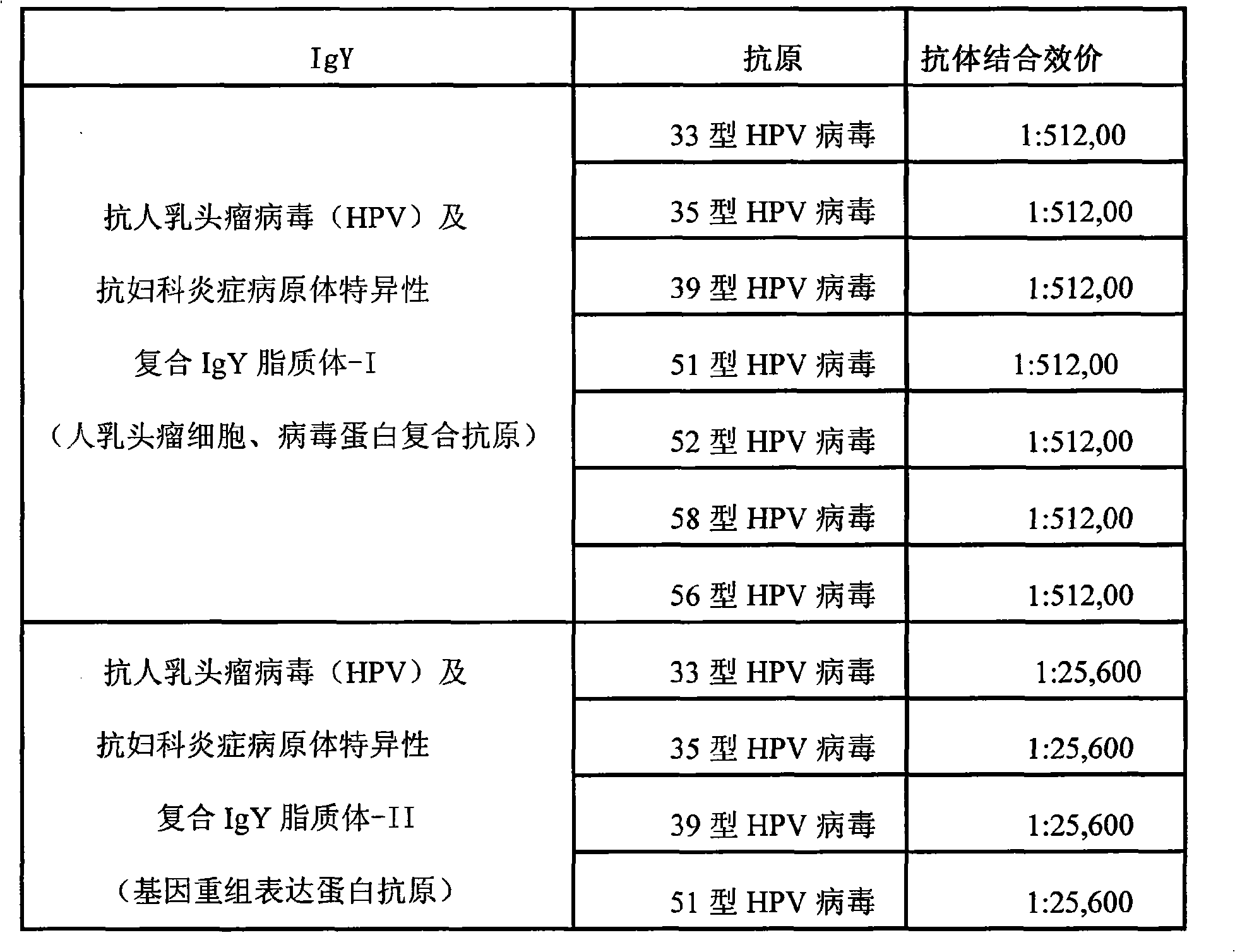
Nano liposome anti-HPV, gynecological inflammation pathogen specific compound IgY and combined preparation thereof
ActiveCN101328219AStrong targetingImprove permeabilityAntibacterial agentsEgg immunoglobulinsBacterial virusHuman papillomavirus
The invention relates to nanoliposome human papillomavirus (HPV) resistant and gynecological inflammation pathogen resistant specific complex IgY and a combined preparation for the same. Laying poultry is preferred to respectively prepare laying poultry for immunity of tumor somatic cells, viral protein complex antigens, gene recombination protein antigens and bacterial virus complex antigens; and human papillomavirus resistant special IgY and gynecological inflammation pathogen resistant specific IgY undergo affinity chromatography and purification and are converted into nanoliposomes, and then the nanoliposome special complex IgY is prepared. The nanoliposome human papillomavirus (HPV) resistant and gynecological inflammation pathogen resistant specific complex IgY and the combined preparation for the same have the advantages that: the nanoliposome technology is applied to increase the infiltration capacity and the prolongation effect and improve the curative effect. The nanoliposome human papillomavirus (HPV) resistant and gynecological inflammation pathogen resistant specific complex IgY and the combined preparation for the same can be prepared into various vaginal spraying agents, other spraying agents, lotion, gel, capsules, suppository, vaginal membranes, tablets, effervescent tablets, ointment, cream and so on. The nanoliposome complex IgY can infiltrate into a vagina mucosa to effectively remove HPV viruses and gynecological inflammation pathogens which are absorbed on the vaginal surface layer and in the vagina mucosa, is absorbed through the vagina mucosa and enters into the body to give play to the functions of prevention and treatment, and also can effectively prevent recrudescence.
Owner:SHENZHEN JASON INTELLIGENT BIOTECH CO LIMLTED PRC
Methods for detecting nucleic acids in a sample
InactiveUS20100167294A1Simple working processFinish quicklyBioreactor/fermenter combinationsBiological substance pretreatmentsSingle strandProtein antigen
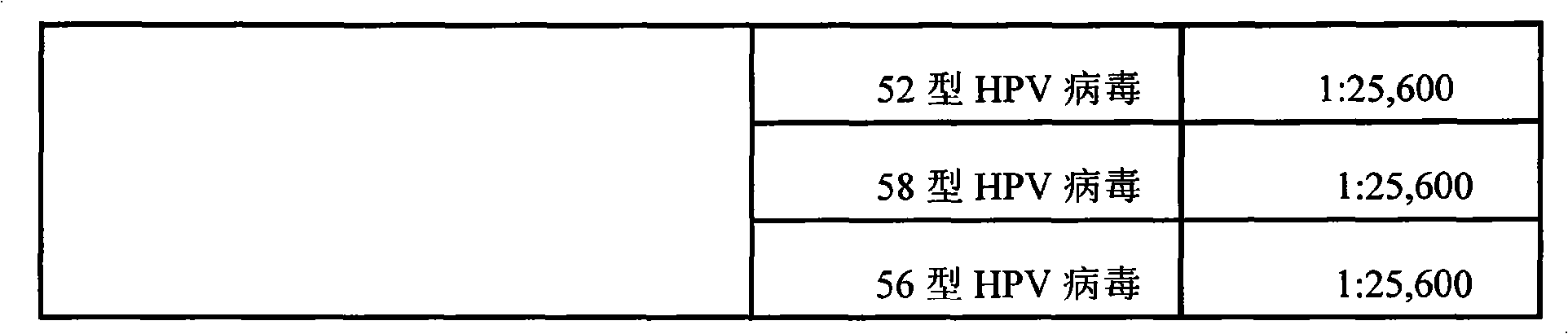
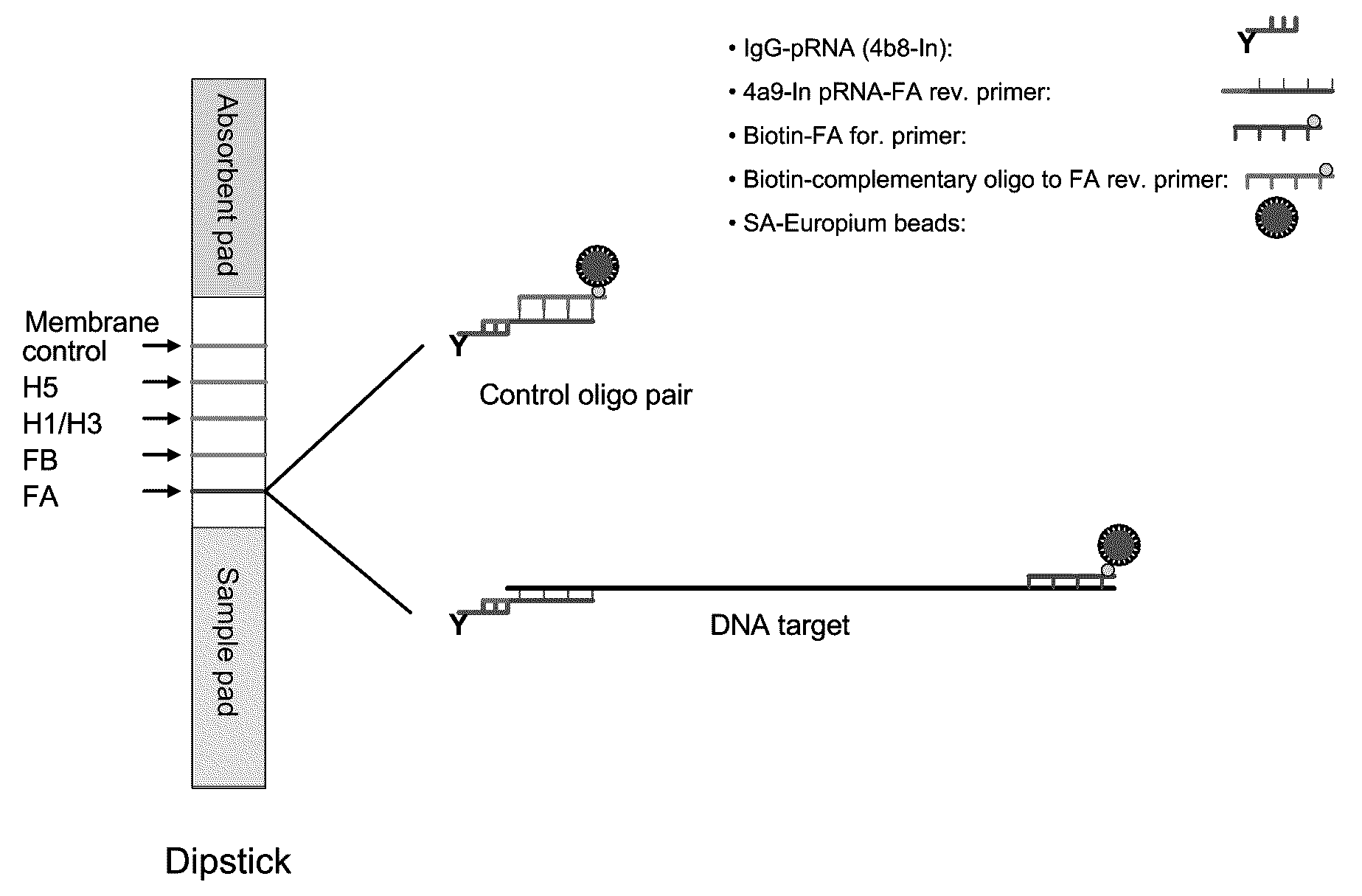
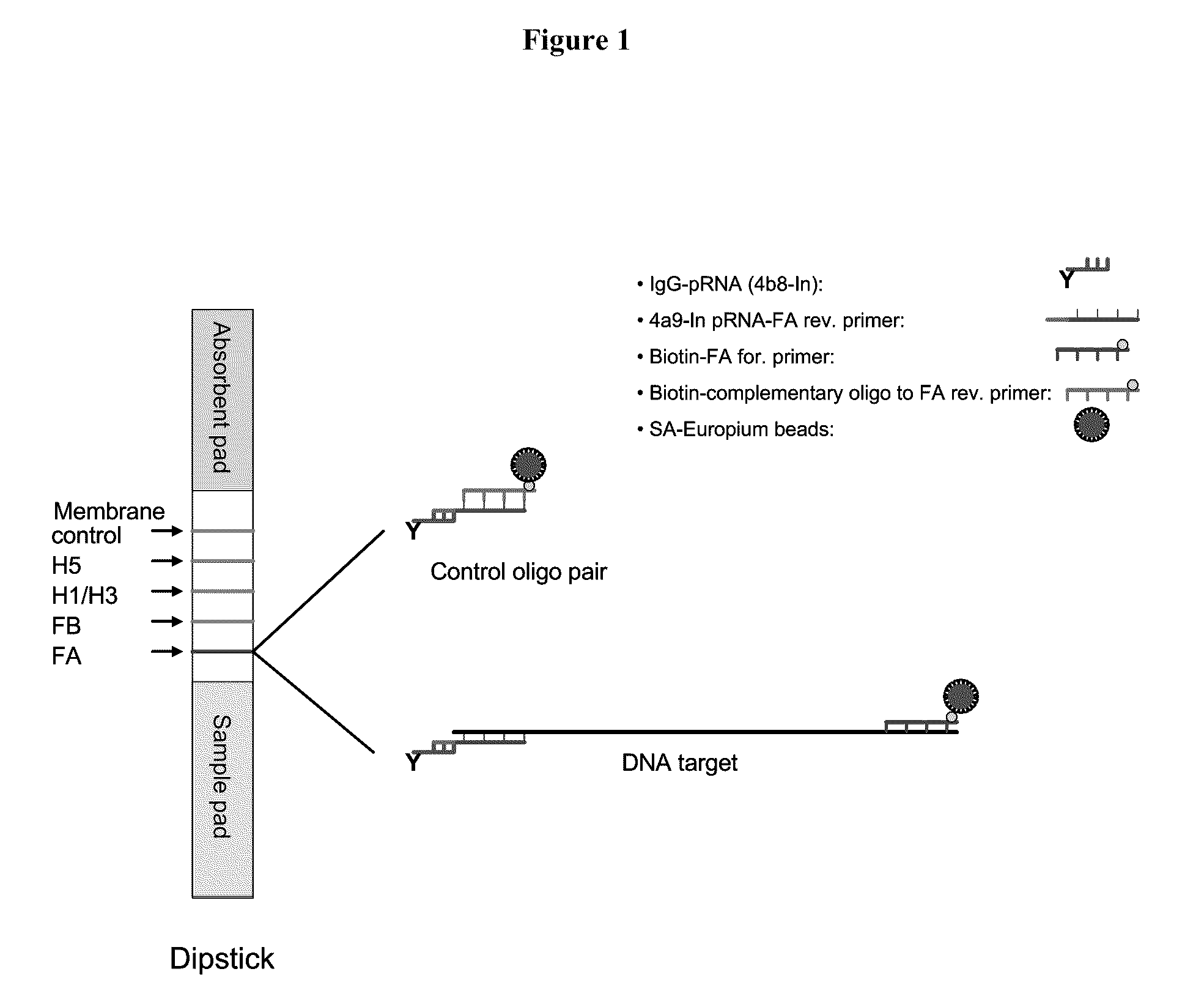
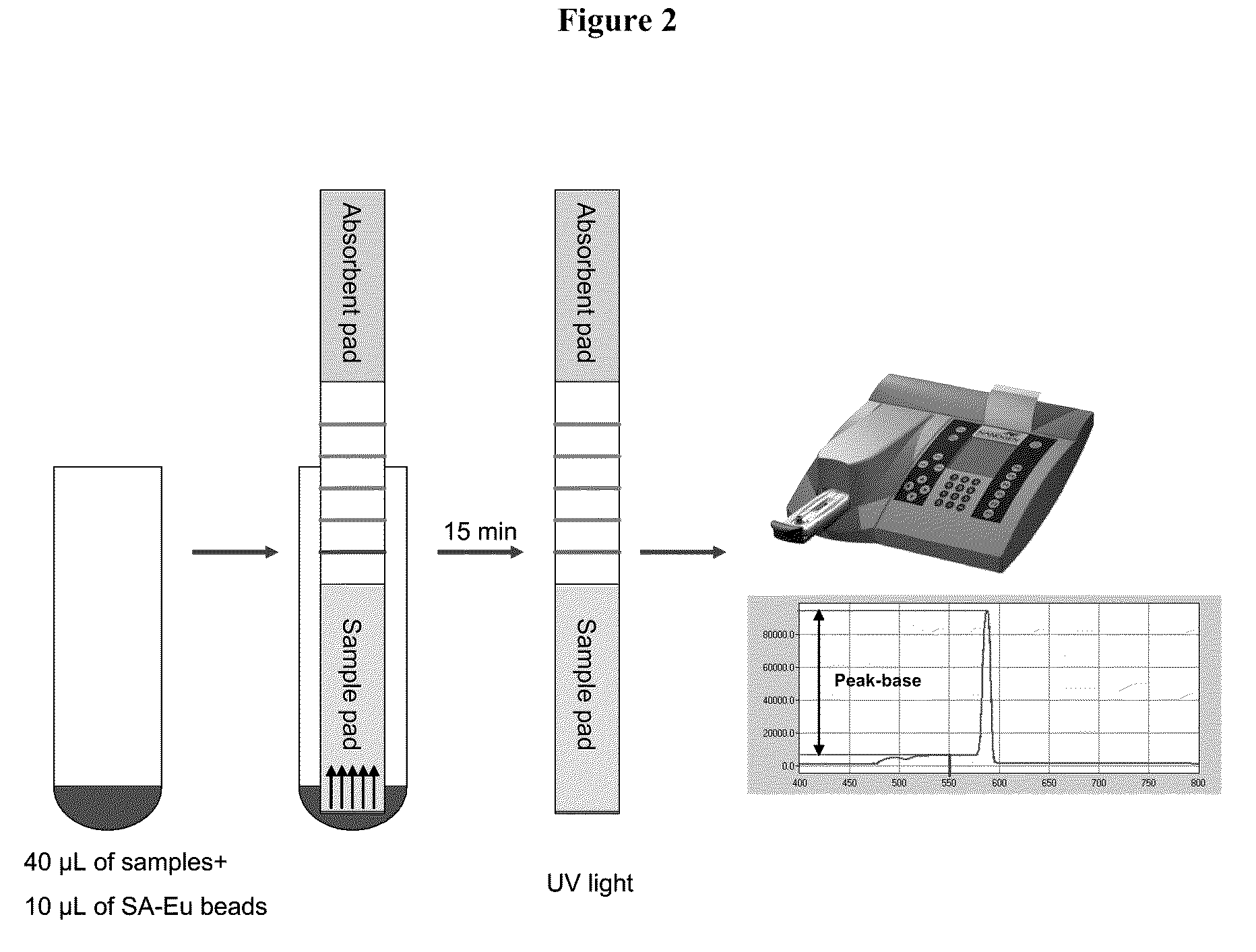
Systems and methods are provided for immobilizing nucleic acid amplicons and protein antigens on a test device. Amplicons comprising a synthetic binding unit and a detectable label are generated and immobilized at predetermined locations on a test device by specific binding interactions between the synthetic binding unit and a synthetic capture unit located at the predetermined locations. The synthetic binding unit may include a unique design such that during amplification, a region of the synthetic binding unit is not subject to the amplification reaction, and thus the amplicon remains single stranded and available for binding to the synthetic capture unit during the capture process. In certain embodiments, the synthetic binding unit and a synthetic capture unit include synthetic nucleic acid analogs that do not interact with native nucleic acids or enzymes that act thereon. In one embodiment the synthetic binding unit and synthetic capture unit comprises puranosyl RNA (pRNA).
Owner:NEXUS DX
HPV antigens, vaccine compositions, and related methods
InactiveUS20080279877A1Reduces and eliminates riskLess considerationVirus peptidesAntiviralsHPV AntigenHuman papilloma virus infection
The present invention relates to the intersection of the fields of immunology and protein engineering, and particularly to antigens and vaccines useful in prevention of infection by human papilloma virus. Provided are recombinant protein antigens, compositions, and methods for the production and use of such antigens and vaccine compositions.
Owner:FRAUNHOFER USA
Liquid vaccines for multiple meningococcal serogroups
ActiveUS8765135B2Antibacterial agentsBacterial antigen ingredientsMeningococcal carriageProtein antigen
Conjugated capsular saccharides from meningococcal serogroups C, W135 and Y are safe and immunogenic in humans when combined in a single dose. This effect is retained when a conjugated capsular saccharide from serogroup A is added. These conjugated antigens can be stably combined in a single aqueous dose without the need for lyophilisation. Broad protection against serogroup B infection can be achieved by using a small number of defined polypeptide antigens. These polypeptide antigens can be combined with the saccharide antigens without loss of protective efficacy for any of the five serogroups. Efficacy if retained even if a Hib conjugate is added. The efficacy of a serogroup W135 conjugate is enhanced by addition of protein antigens derived from a serogroup B strain. Addition of a Hib conjugate to meningococcal conjugates enhances the overall activity against meningococcus serogroup W135.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Human cytomegalovirus immunotherapy
Owner:COUNCIL OF THE QUEENSLAND INST OF MEDICAL RES
Preparation method of competitive photoelectrochemical sensor of manganese-doped cadmium selenide enhanced bismuth tungstate-cadmium sulfide beta amyloid protein
ActiveCN109060905ALow costLarge specific surface areaMaterial electrochemical variablesCadmium selenideTungstate
The invention relates to a preparation method of a competitive photoelectrochemical sensor of manganese-doped cadmium selenide enhanced bismuth tungstate-cadmium sulfide beta amyloid protein. According to the method, bismuth tungstate-cadmium sulfide is used as a base material to acquire a photoelectric current, and the photoelectric conversion efficiency of flower-like bismuth tungstate sensitized by cadmium sulfide is greatly improved; and manganese-doped cadmium selenide is used as a marker to mark a beta amyloid protein antigen, the sensitivity of the sensor is improved through competitiveimmune reactions between the marked antigen and an antibody and between a non-marked antigen and the antibody, and sensitivity detection of amyloid protein is realized, wherein the detection limit is0.068 pg / mL.
Owner:UNIV OF JINAN
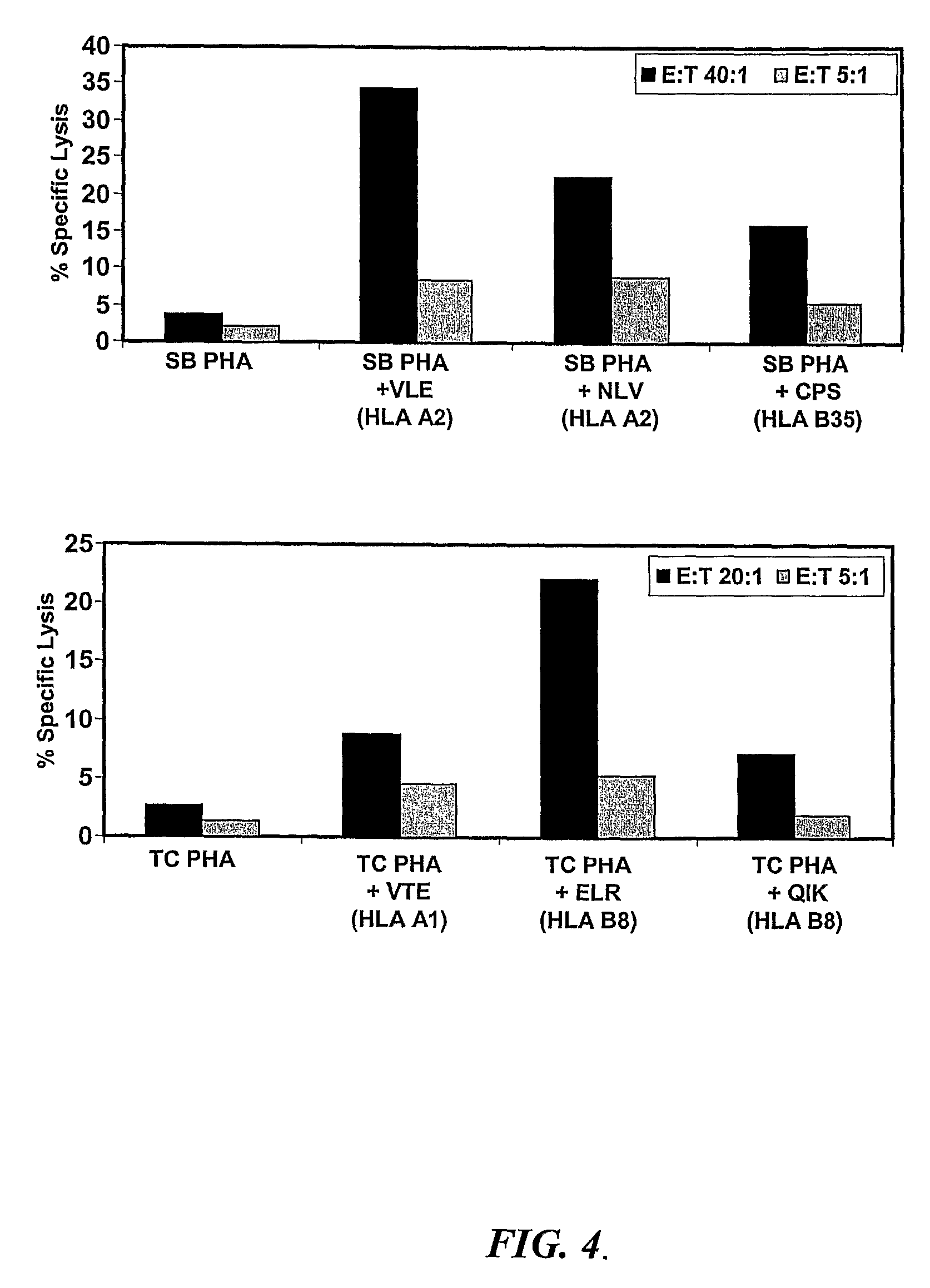
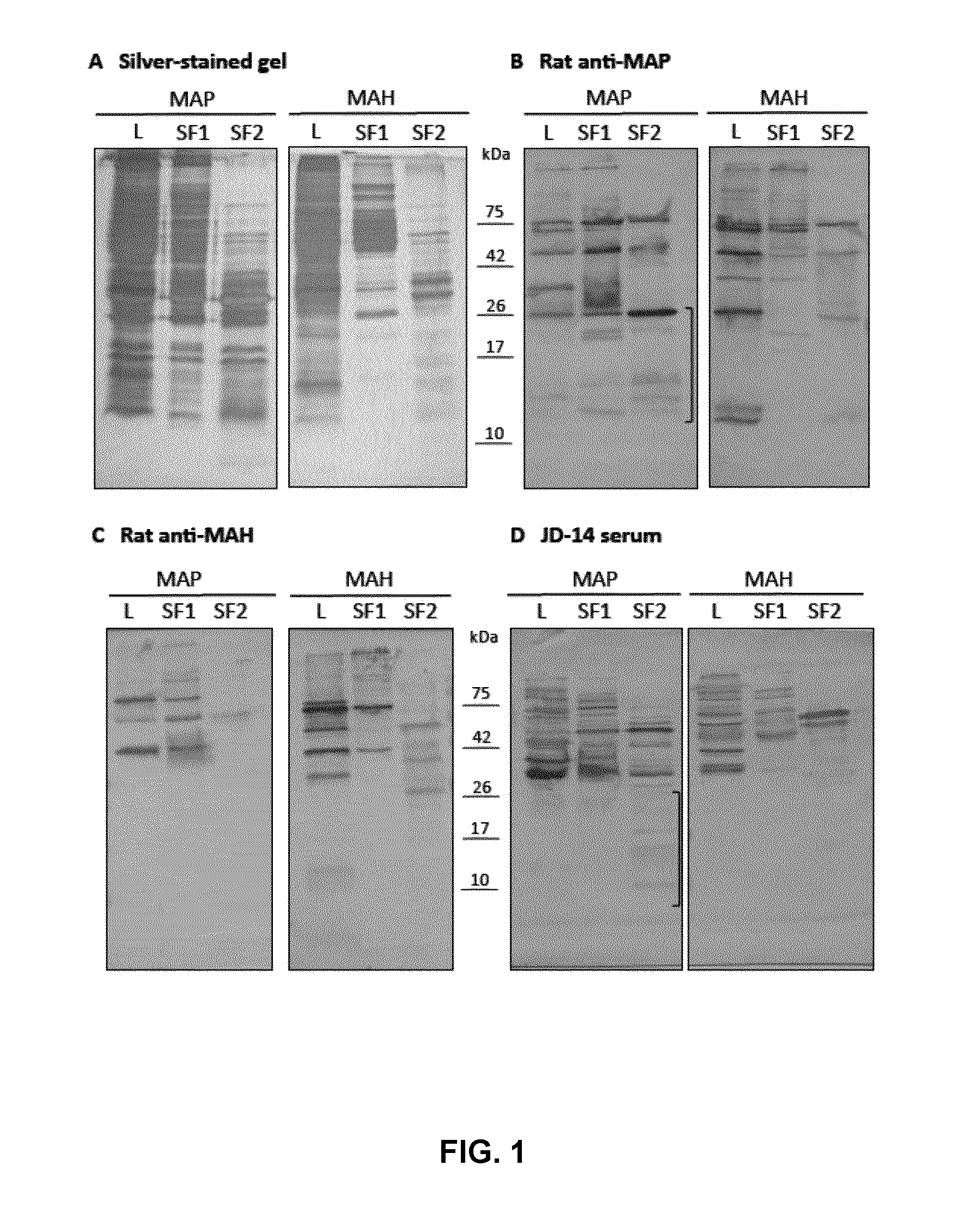
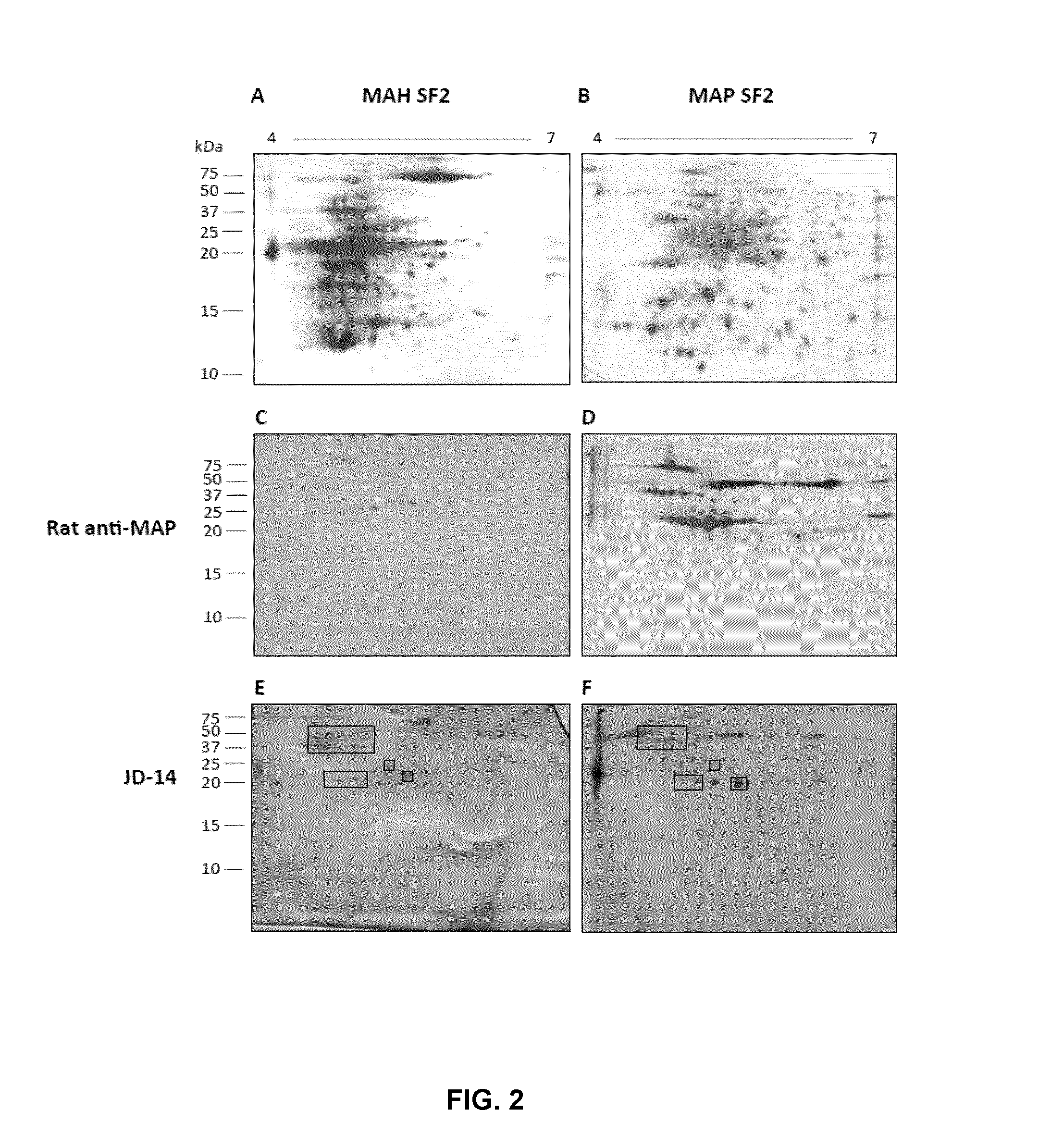
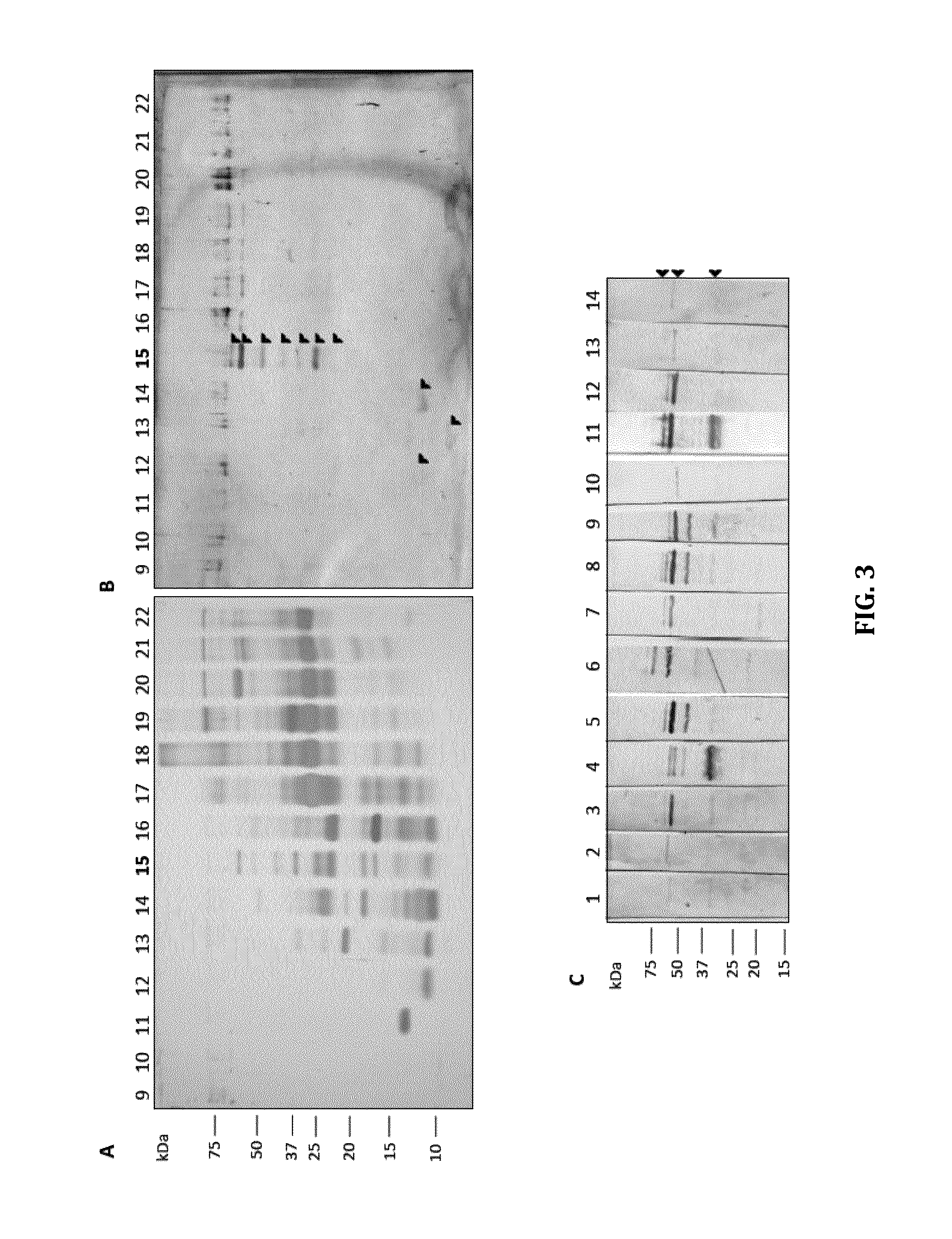
Biomarkers for mycobacterium avium paratuberculosis (MAP)
Described are methods and products useful for identifying subjects with Mycobacterium avium subspecies paratuberculosis (MAP). A number of protein antigens secreted into culture filtrate by MAP are identified and binding proteins selective for these antigens are demonstrated to be useful for detecting subjects with MAP infections including subjects with Johne's disease.
Owner:UNIVERSITY OF SASKATCHEWAN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com