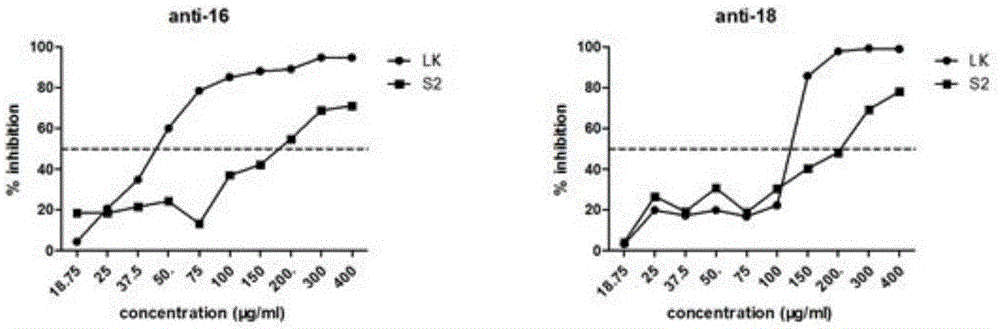
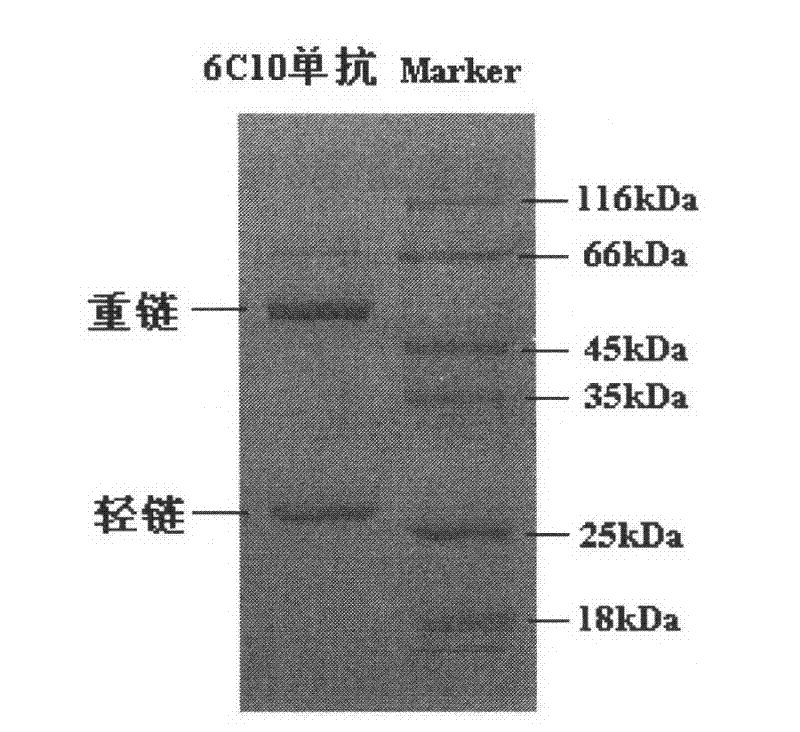
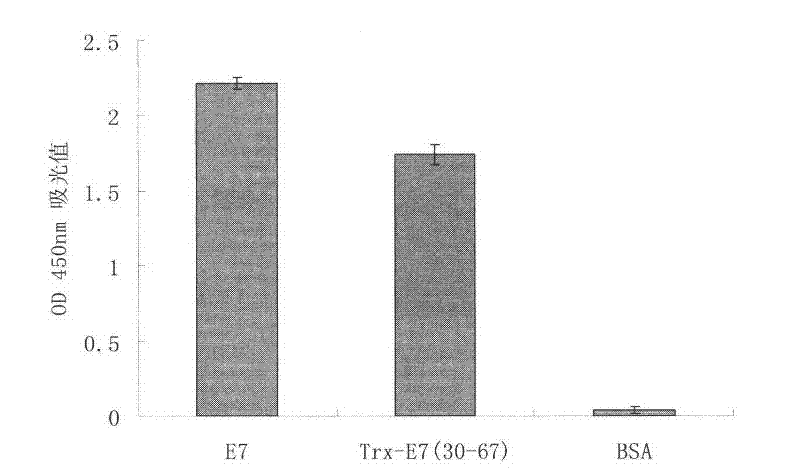
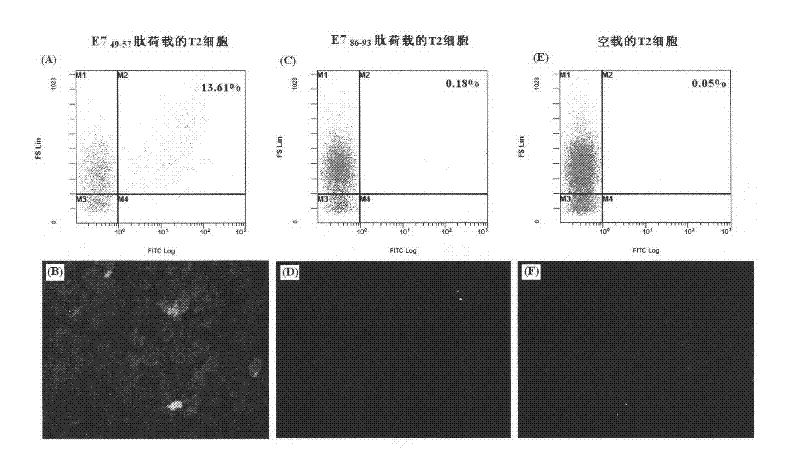
Patents
Literature
118 results about "Human papilloma virus infection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
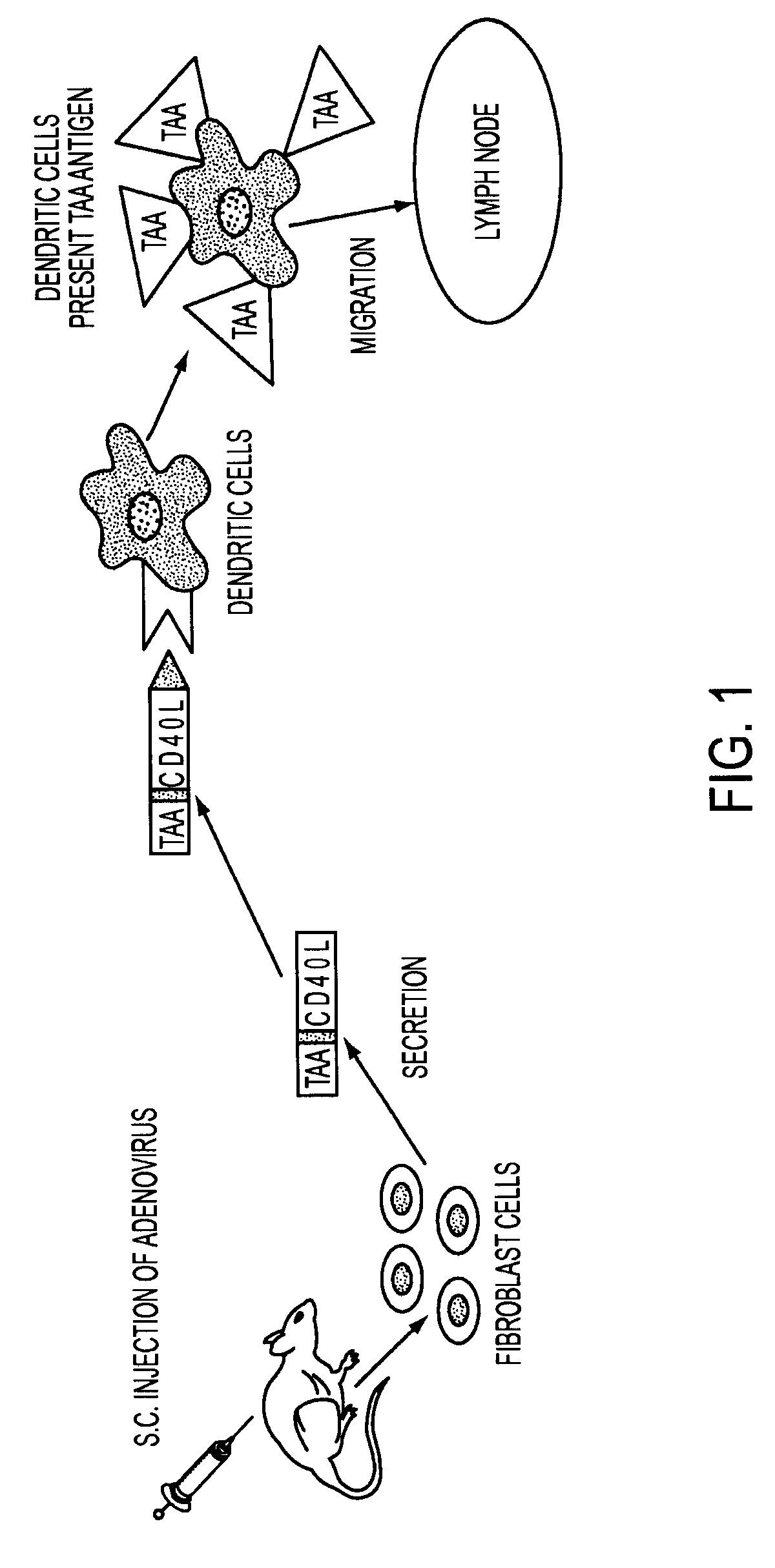
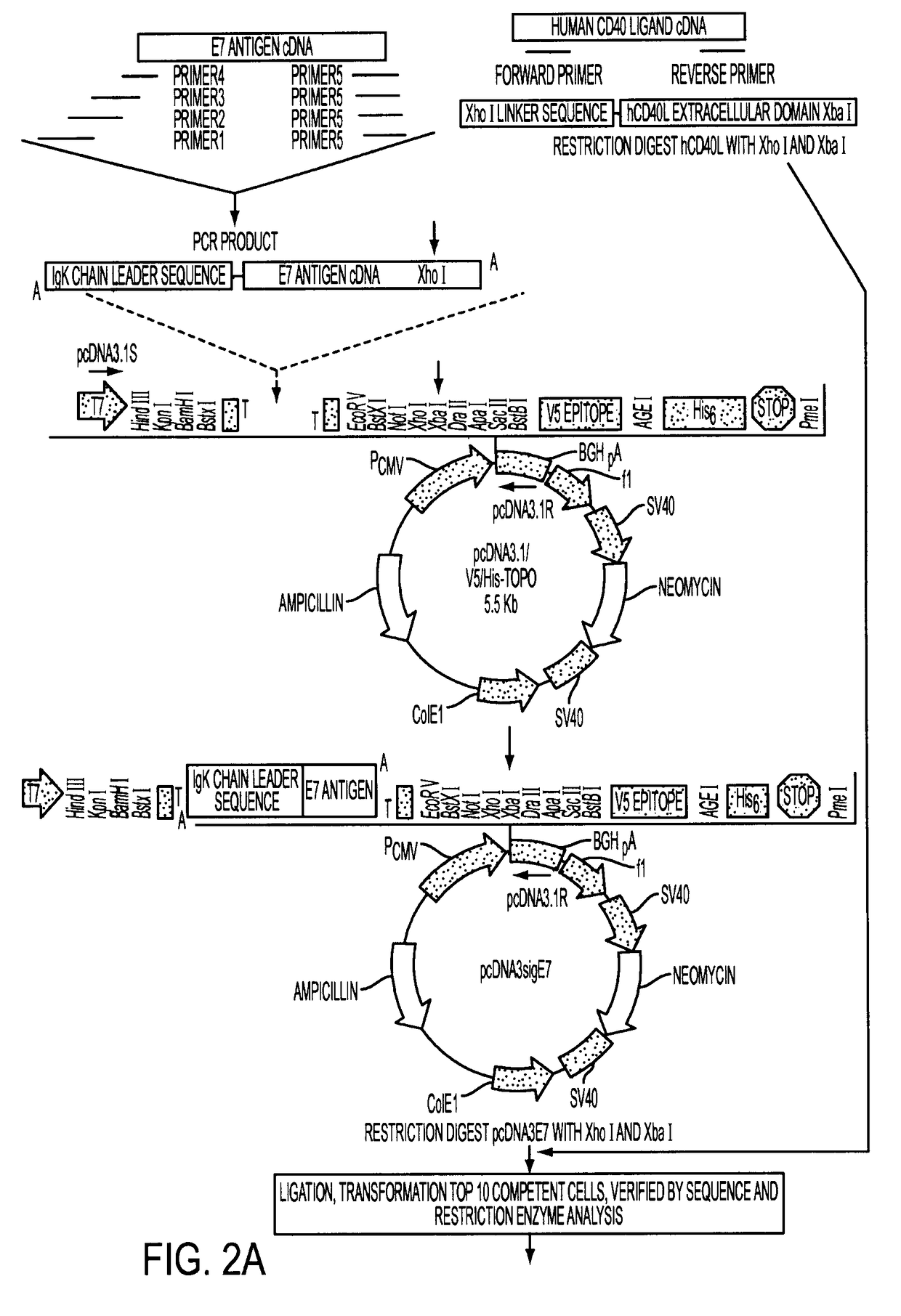
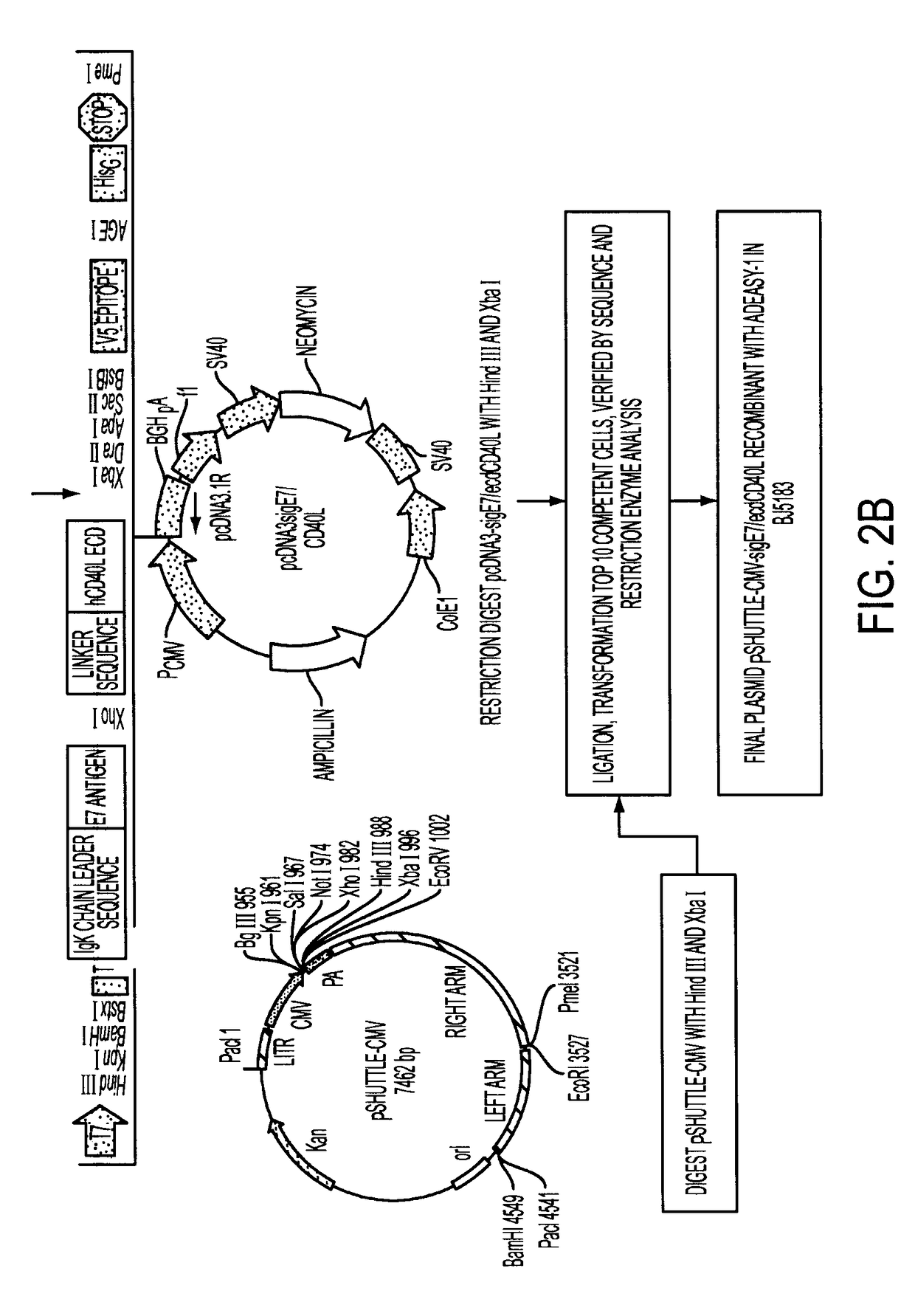
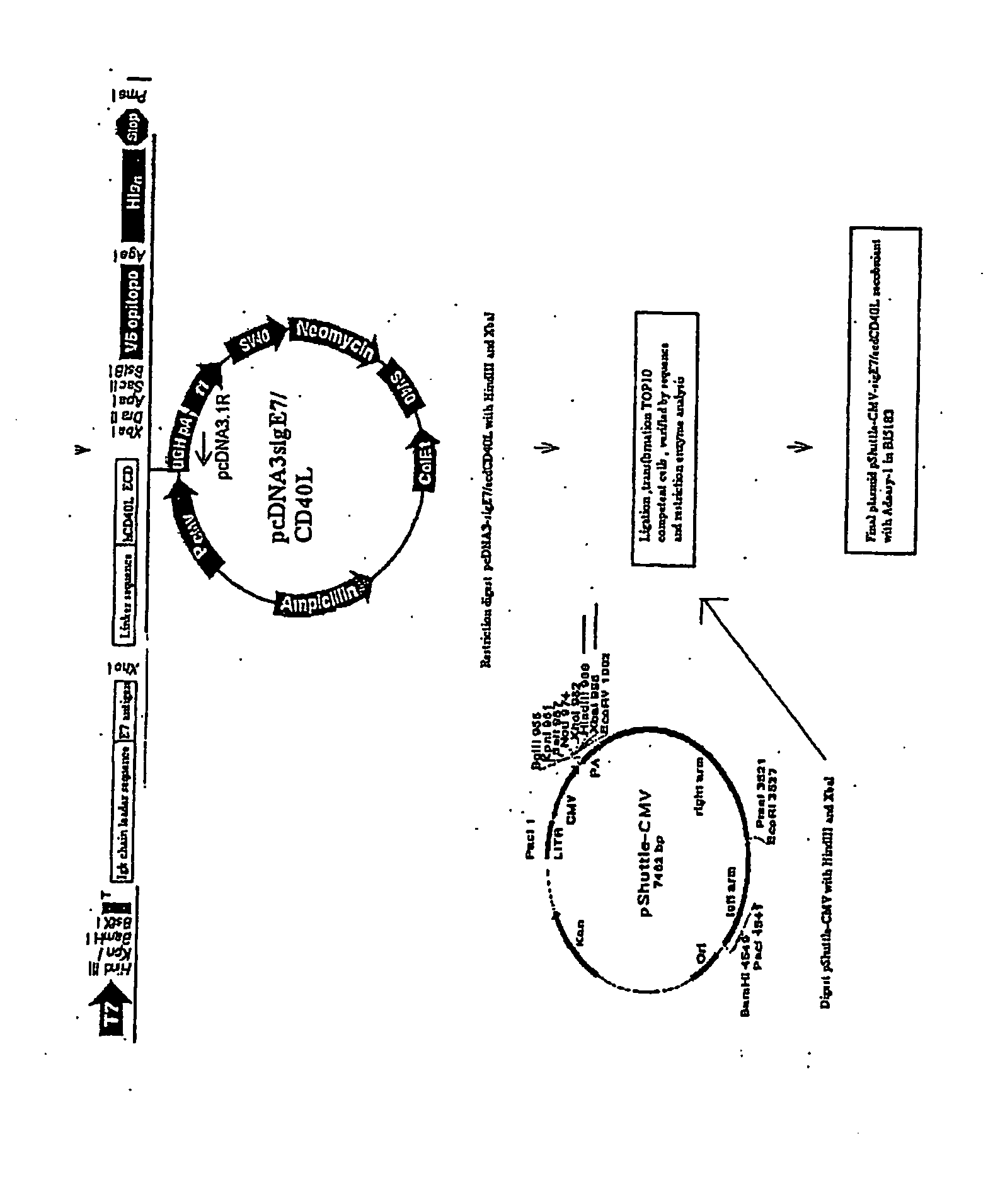
Adenoviral expression vector comprising a CD40L fusion protein adapted to elicit cellular immunity
ActiveUS8119117B2Enhance immune responseLong-lasting immunityVirusesAntibody mimetics/scaffoldsTransmembrane domainTumor antigen
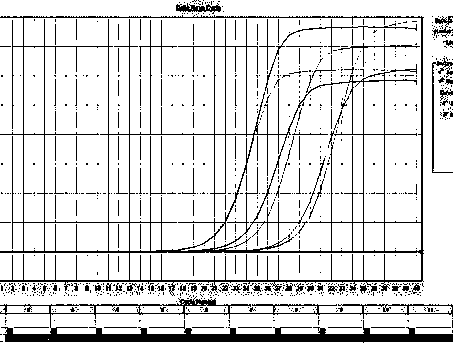
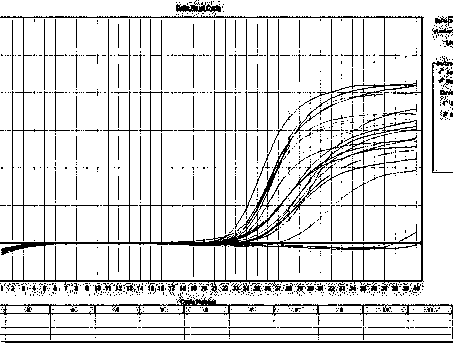
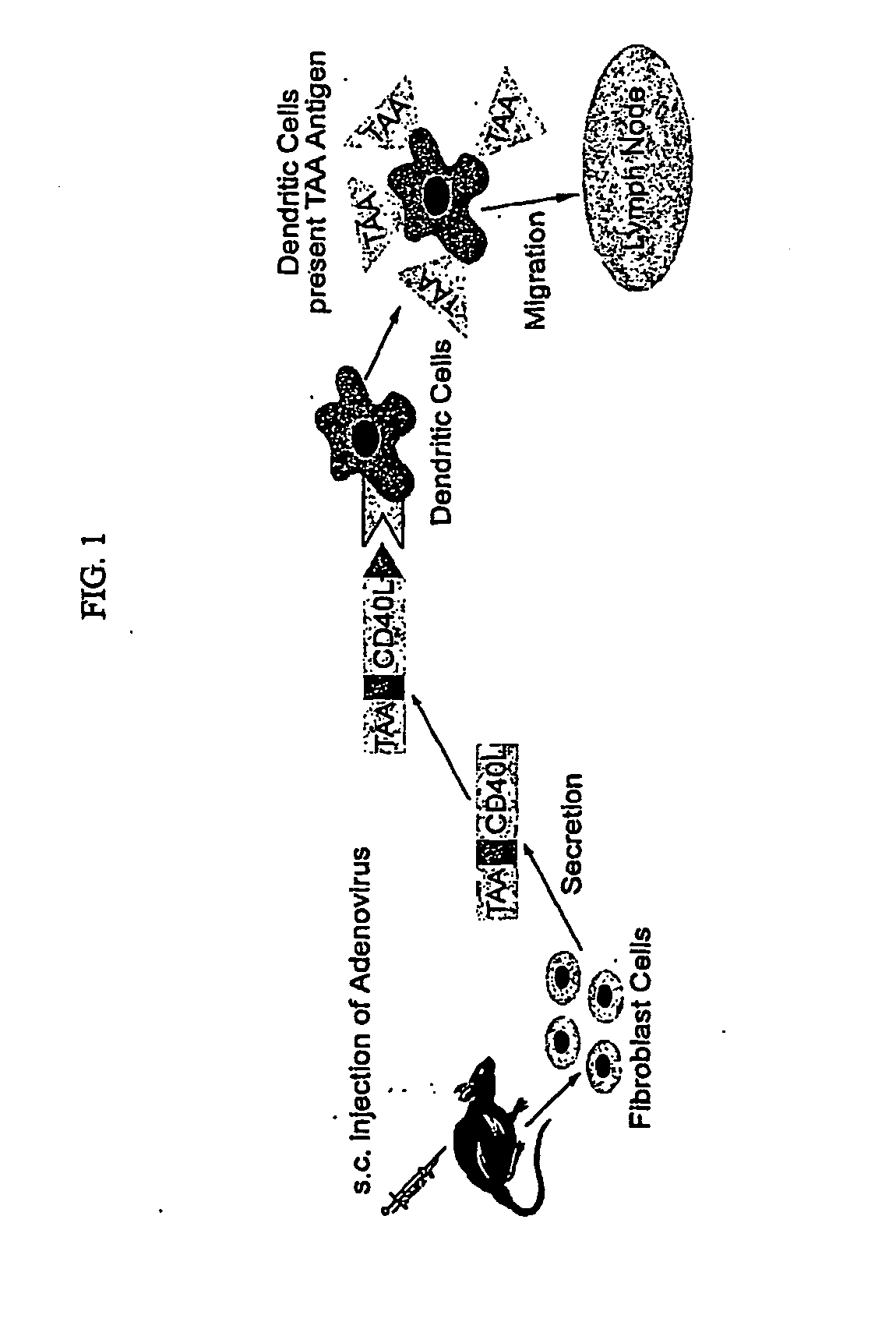
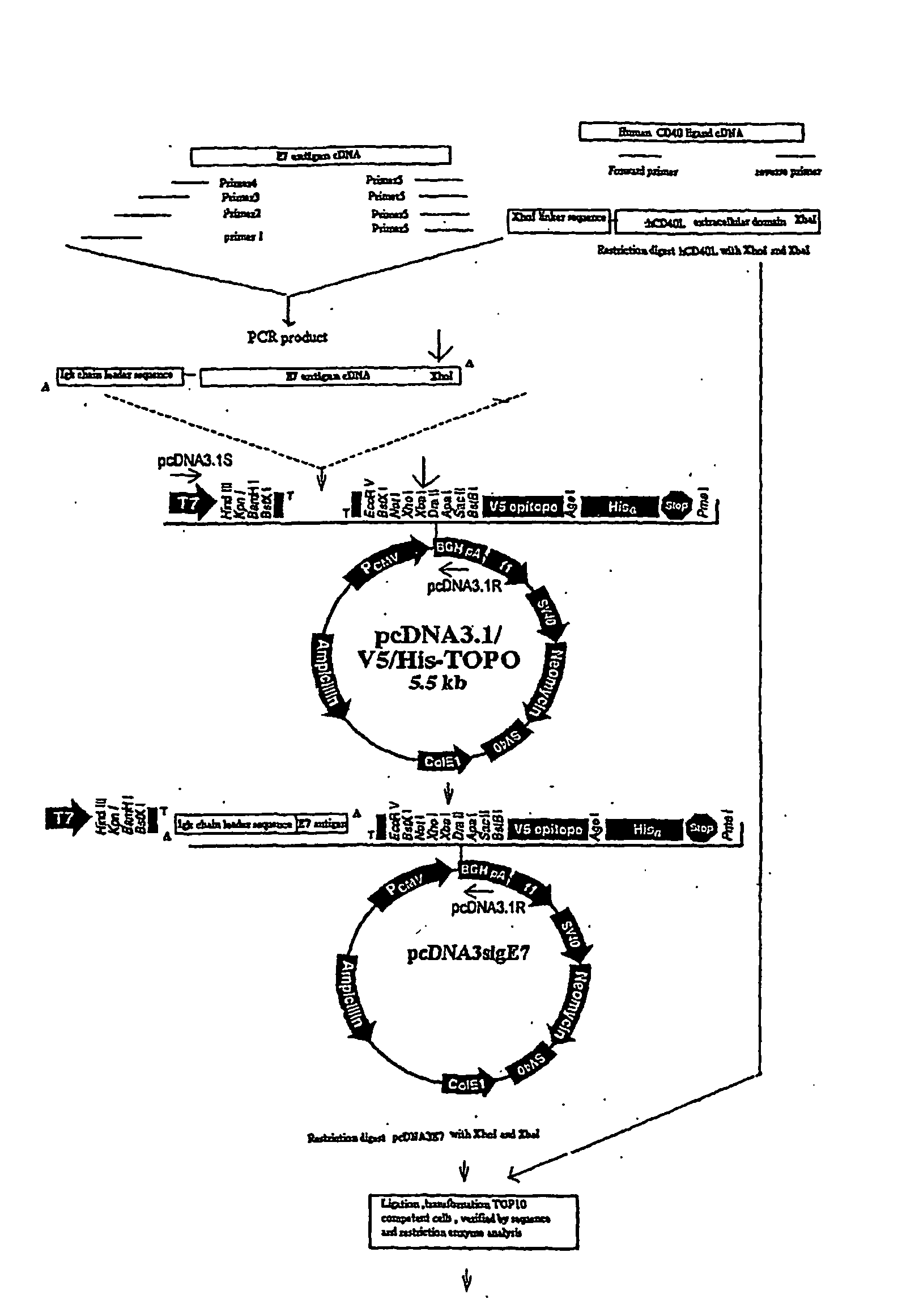
Provided are adenoviral vectors for generating an immune response to antigen. The vectors comprise a transcription unit encoding a secretable polypeptide, the polypeptide comprising a secretory signal sequence upstream of a tumor antigen upstream of CD40 ligand, which is missing all or substantially all of the transmembrane domain rendering CD40L secretable. Also provided are methods of generating an immune response against cells expressing a tumor antigen by administering an effective amount of the invention vector. Further provided are methods of generating an immune response against cancer expressing a tumor antigen in an individual by administering an effective amount of the invention vector. Still further provided are methods of generating immunity to infection by human papilloma virus (HPV) by administering an effective amount of the invention vector which enocodes the E6 or E7 protein of HPV. The immunity generated is long term.
Owner:VAXUM
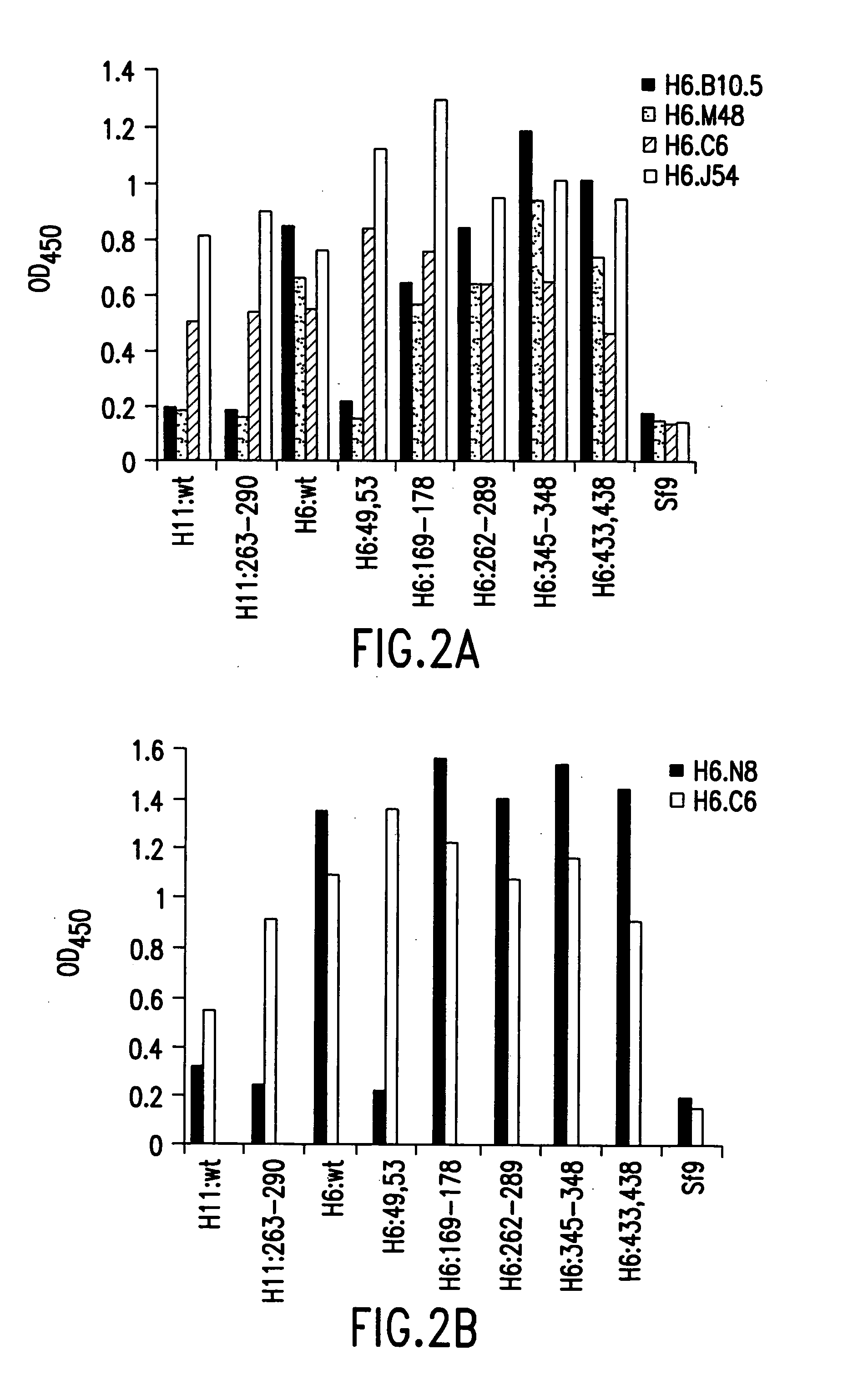
Screening for papilloma viruses
InactiveUS7135281B2Microbiological testing/measurementImmunoglobulins against virusesHuman papilloma virus infectionMalignancy
The invention relates to a method of screening for precursor lesions which can lead to cervical malignancy, methods of detecting and typing human papilloma virus infections, and reagents of use in these methods.
Owner:MEDICAL RESEARCH COUNCIL
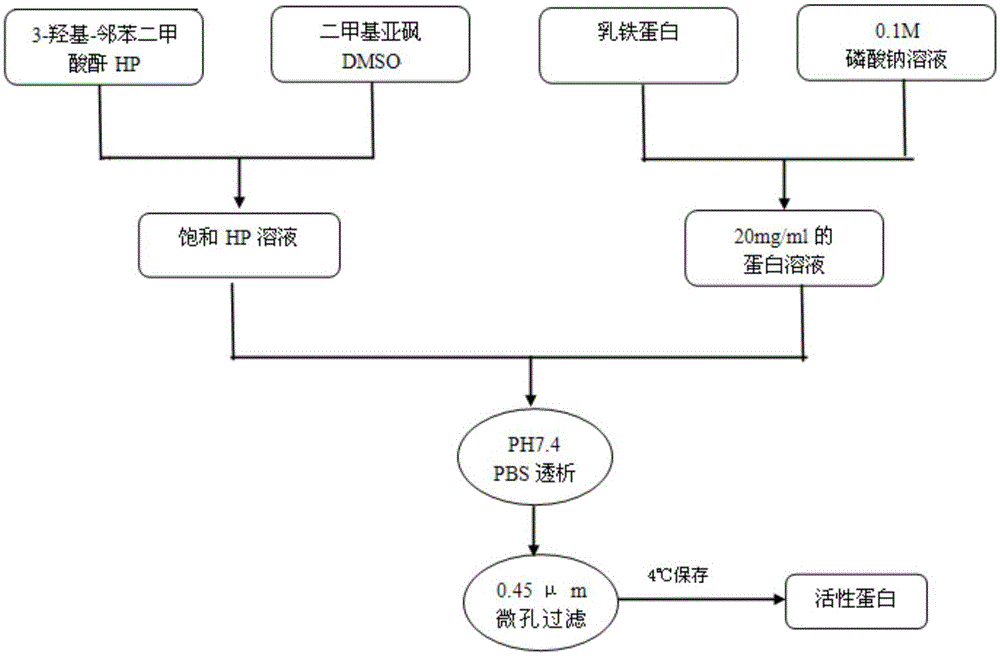
Preparation method for biological agent for preventing and controlling human papilloma virus infection
ActiveCN102631666AImprove stabilityLow costPeptide/protein ingredientsAntiviralsProtein solutionHuman papilloma virus infection
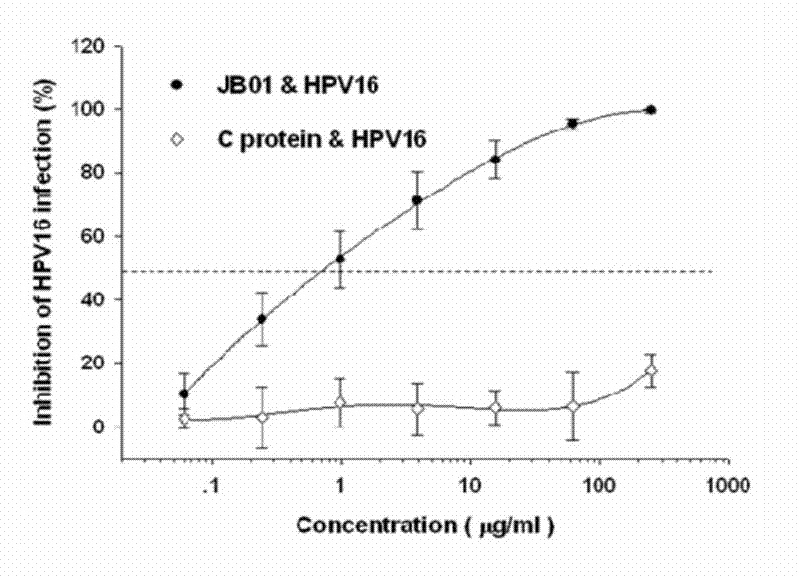
The invention discloses a preparation method for a biological agent for preventing and controlling human papilloma virus infection. The preparation method includes following steps: dissolving 3-hydroxy-phthalic anhydride HP into dimethyl sulfoxide DMSO to obtain saturated HP solution; dissolving beta-lactoglobulin beta-LG into 0.1M of sodium phosphate solution with pH (potential of hydrogen) of 8.5 to obtain protein solution with protein final concentration of 20mg / mL; and equally dividing the HP solution into five parts, adding the HP solution into the protein solution at every 12 minutes, shaking and mixing, adjusting pH of IMNaOH to be 8.5, leading the final concentration of anhydride in a reaction system to be 60mM, standing for 1 hour at the temperature of 25 DEG C, dialyzing by PBS (phosphate buffer solution) with pH of 7.4, performing filtration sterilization by a 0.45uM microfiltration membrane, and storing at the temperature of 4 DEG C so as to obtain a finished product. The biological agent has the advantages of capabilities of stopping HPV (human papilloma virus) from invading cells and stopping virus infection from spreading, safety, stability, low cost and the like.
Owner:SHANXI JINBO BIO PHARMA CO LTD
Methods of treating human papillomavirus
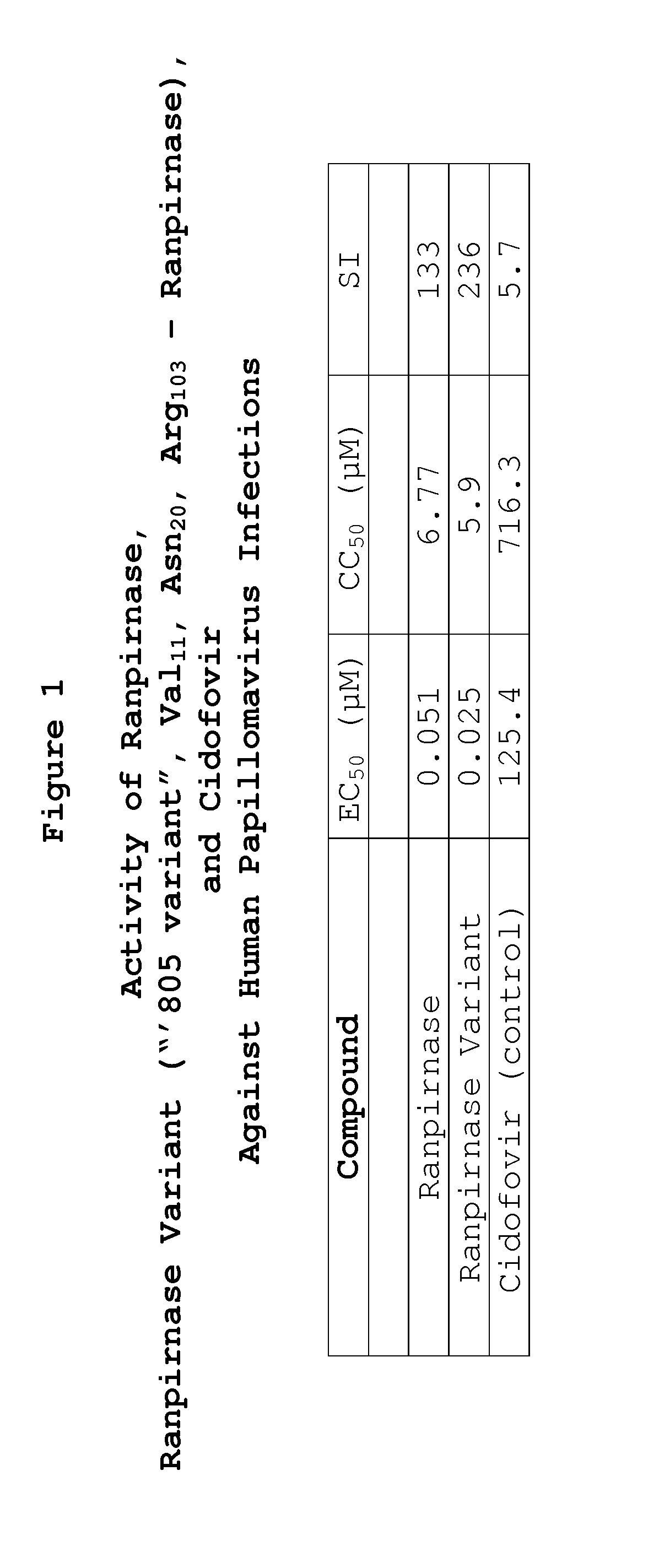
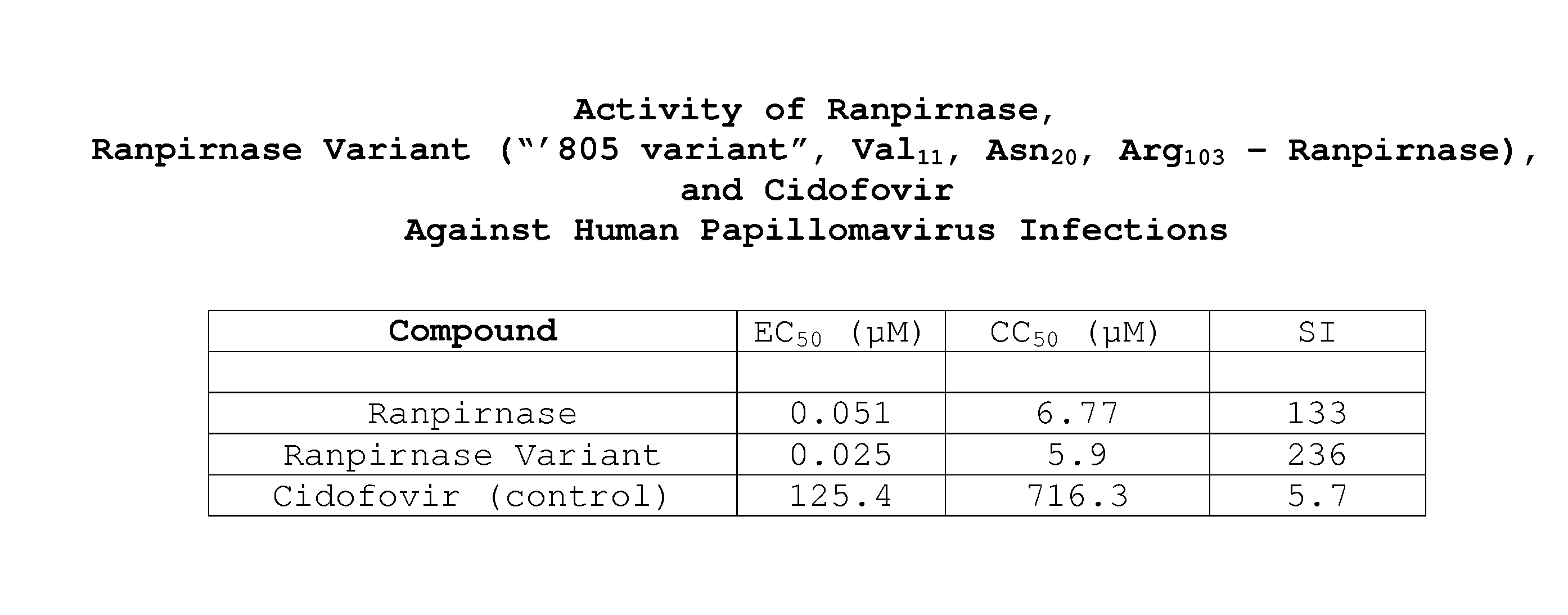
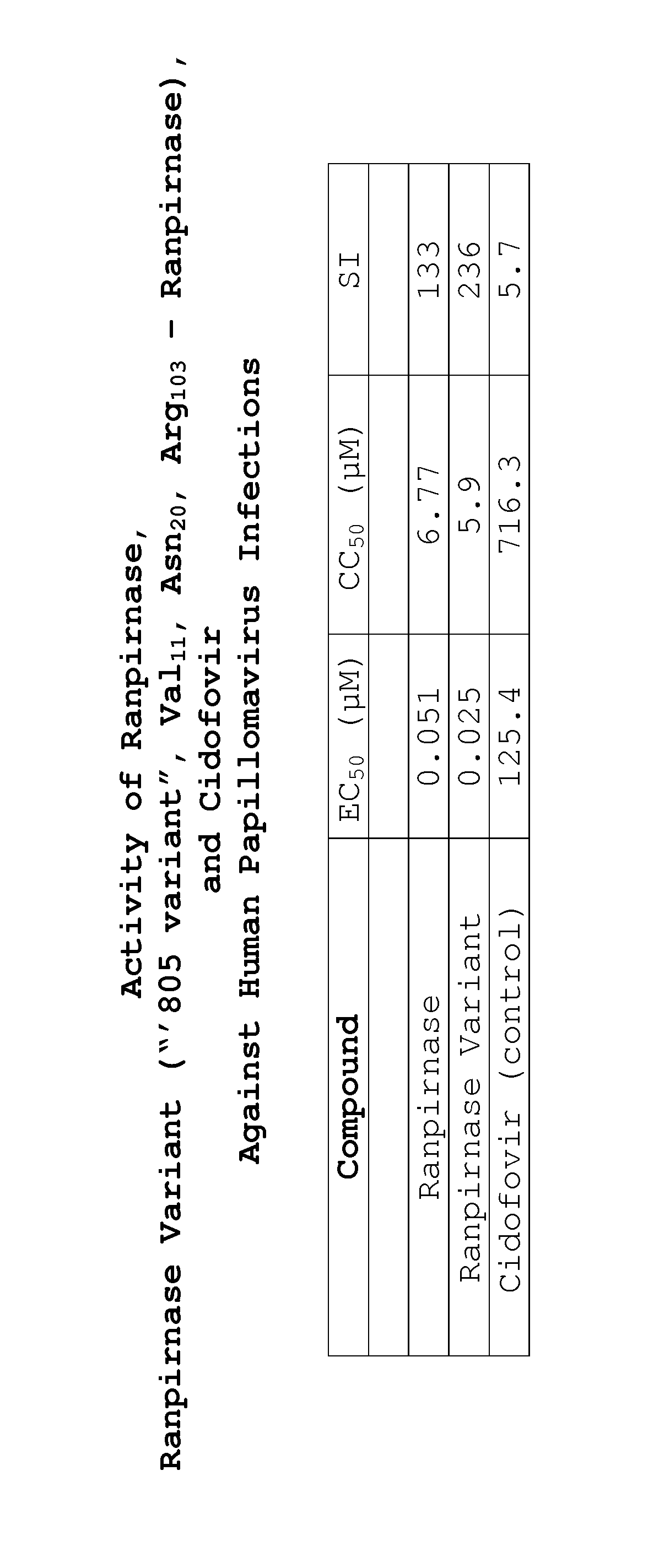
Two RNases (ranpirnase and the second embodiment disclosed in U.S. Pat. No. 5,728,805) are tested against human papillomavirus infections. QRT-PCR assays indicate that the RNases have anti-viral activity against type 11 HPV.
Owner:ORGENESIS INC
Methods of treating human papillomavirus
Owner:ORGENESIS INC
HPV antigens, vaccine compositions, and related methods
InactiveUS20080279877A1Reduces and eliminates riskLess considerationVirus peptidesAntiviralsHPV AntigenHuman papilloma virus infection
The present invention relates to the intersection of the fields of immunology and protein engineering, and particularly to antigens and vaccines useful in prevention of infection by human papilloma virus. Provided are recombinant protein antigens, compositions, and methods for the production and use of such antigens and vaccine compositions.
Owner:FRAUNHOFER USA
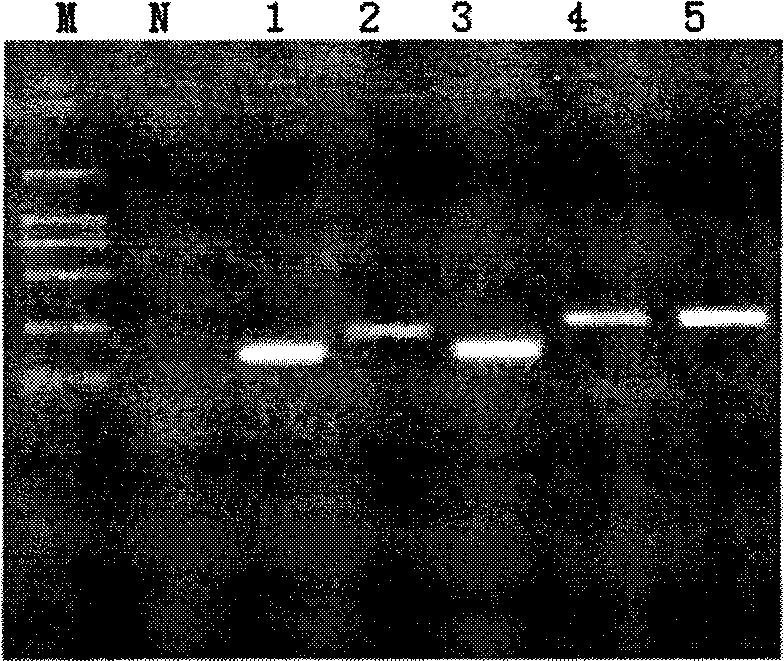
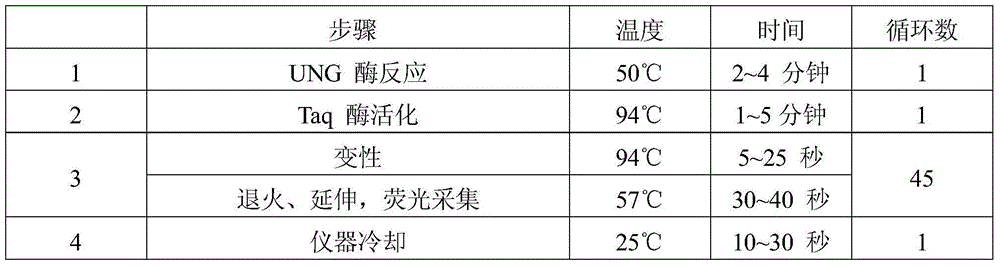
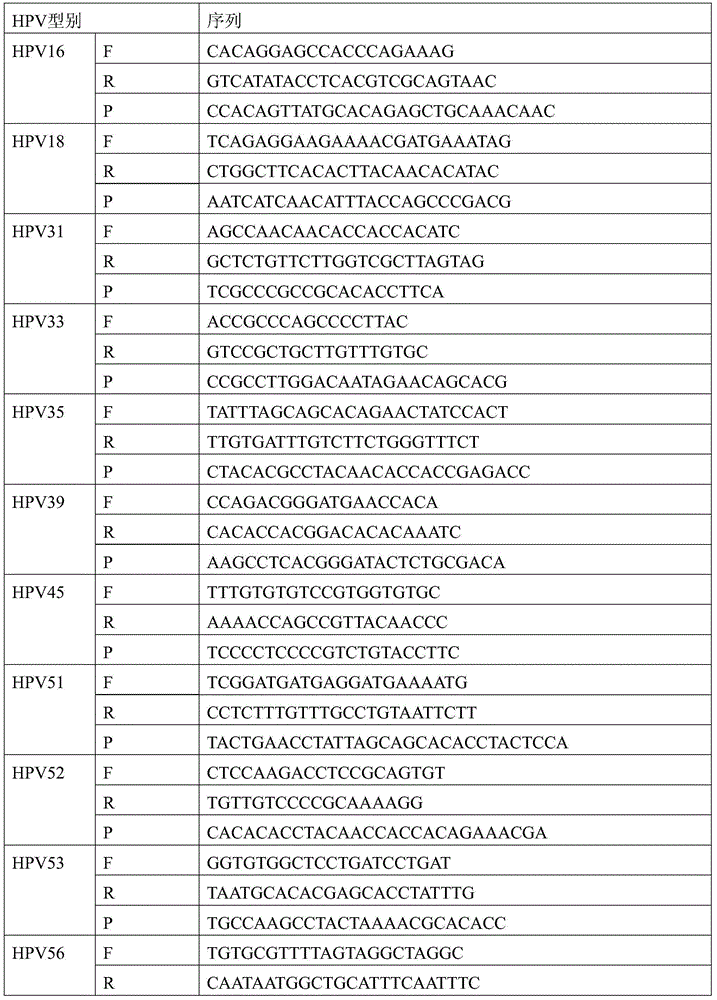
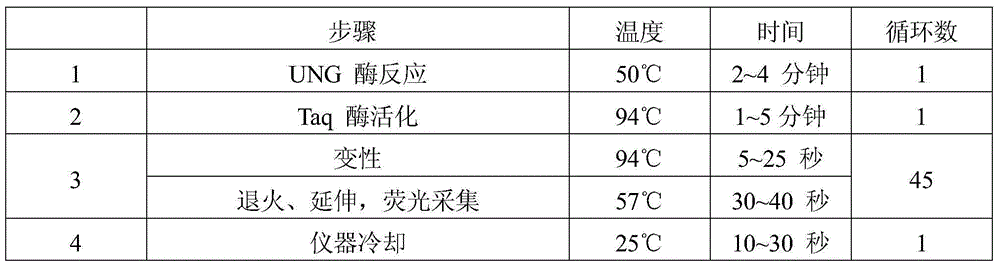
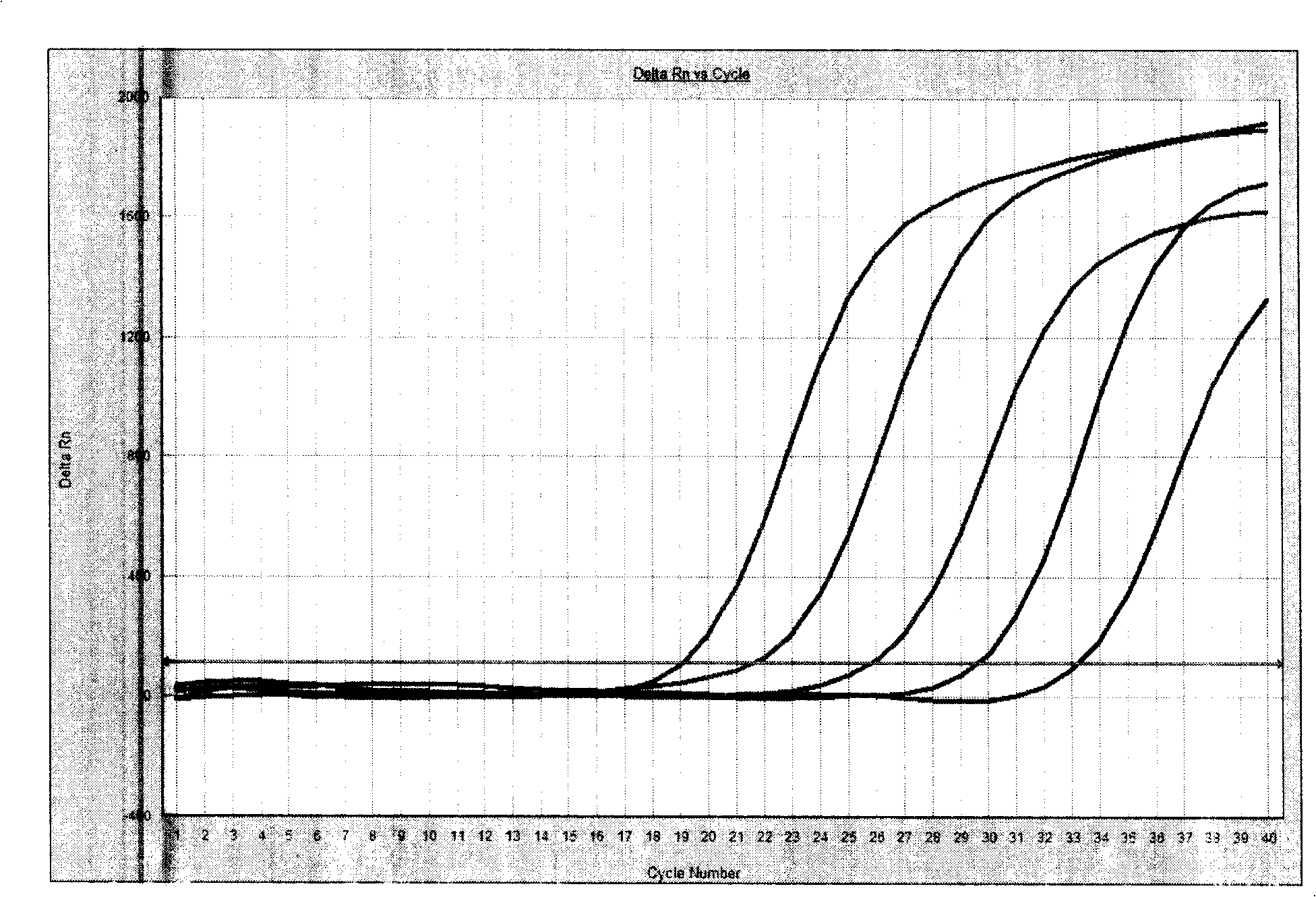
Human papilloma virus infection gene amplification fluorescent detection kit
ActiveCN101487063AEasy to acceptHigh sensitivityMicrobiological testing/measurementMicroorganism based processesHuman papilloma virus infectionFluorescence
The invention discloses a probe and a primer which have nucleotide sequence complementary with HPV DNA, wherein, the probe is selected from SEQ ID Nos: 1 to 13; the primer is selected from SEQ ID SEQ ID Nos: 14 to 39; the invention also discloses a fluorescence identification kit comprising the probe and the primer and used for indentifying nucleic acid amplification infected by high-risk human papillomavirus, which contains 13 varieties of high-risk human papillomavirus subtypes and can finish extracting and detecting 80 to 100 portions of clinical samples DNA in 3 hours. The invention has the advantages of high sensitivity and specificity and low price, thus being easy to be accepted by patients and easy for popularizing and spreading.
Owner:潮州凯普生物化学有限公司 +1
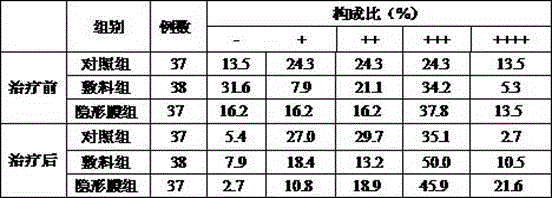
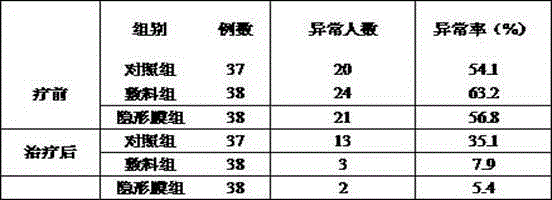
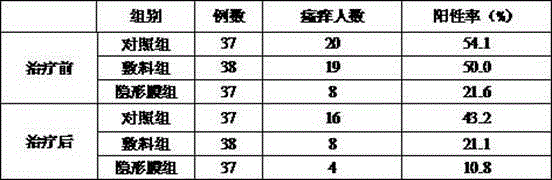
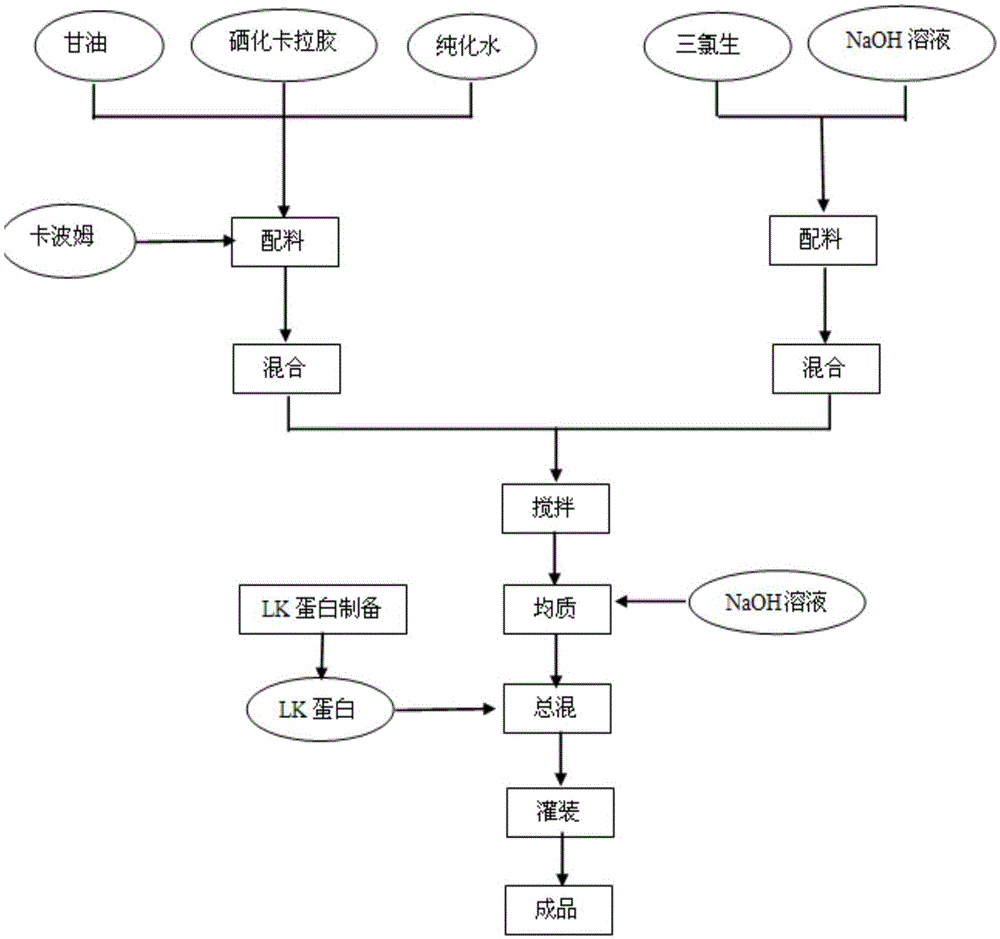
Bioprotein dressing and bioprotein invisible film for preventing and controlling human papilloma virus infection
ActiveCN102908611AExcellent pruritusReduce loadPeptide/protein ingredientsAerosol deliveryTriclosanHuman papilloma virus infection
The invention discloses a bioprotein dressing and a bioprotein invisible film for preventing and controlling human papilloma virus infection, relates to products for gynecological diseases and solves the problem of absence of medicines capable of effectively preventing and controlling HPV (human papilloma virus) infection in foreign and domestic markets. The bioprotein dressing comprises, by mass concentration, 0.05-1%o of JB protein, 1-3% of carbomer, 0.5-2% of green tea extracts, 1-5% of glycerol, 0.1-0.3% of triclosan and the balance water. The bioprotein invisible film comprises, by mass concentration, 0.05-1%o of JB protein, 0.05-1% of carbomer, 0.5-2% of green tea extracts, 1-5% of glycerol, 0.11-0.25% of enhanceparaben, 0.1-2% of phenoxyethanol and the balance water. The bioprotein dressing is anti-HPV biological agent, is good in biocompatibility, nonirritant, safe and free of side effect.
Owner:SHANXI JINBO BIO PHARMA CO LTD
Human papilloma virus (24 types) detection (fluorescent PCR method) kit and detection method
InactiveCN103725792AAvoiding the False Negative ProblemSolve the problem of small fluxMicrobiological testing/measurementFluorescence/phosphorescenceForward primerHuman papilloma virus infection
Owner:JIANGSU MOLE BIOSCI
Adenoviral Vector Vaccine
ActiveUS20070269409A1Enhance immune responseLong-lasting immunityVirusesAntibody mimetics/scaffoldsHuman papilloma virus infectionVector vaccine
Provided are adenoviral vectors for generating an immune response to antigen. The vectors comprise a transcription unit encoding a secretable polypeptide, the polypeptide comprising a secretory signal sequence upstream of a tumor antigen upstream of CD40 ligand, which is missing all or substantially all of the transmembrane domain rendering CD40L secretable. Also provided are methods of generating an immune response against cells expressing a tumor antigen by administering an effective amount of the invention vector. Further provided are methods of generating an immune response against cancer expressing a tumor antigen in an individual by administering an effective amount of the invention vector. Still further provided are methods of generating immunity to infection by human papilloma virus (HPV) by administering an effective amount of the invention vector which enocodes the E6 or E7 protein of HPV. The immunity generated is long term.
Owner:VAXUM
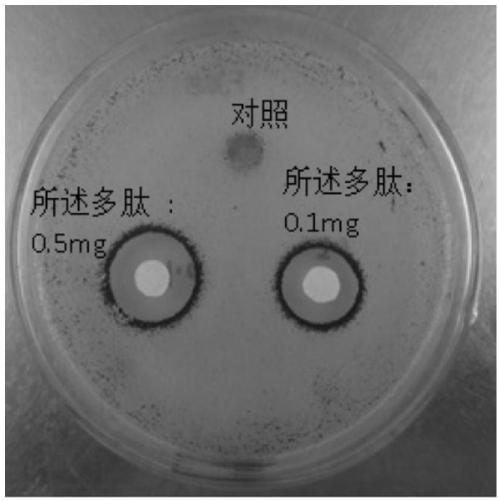
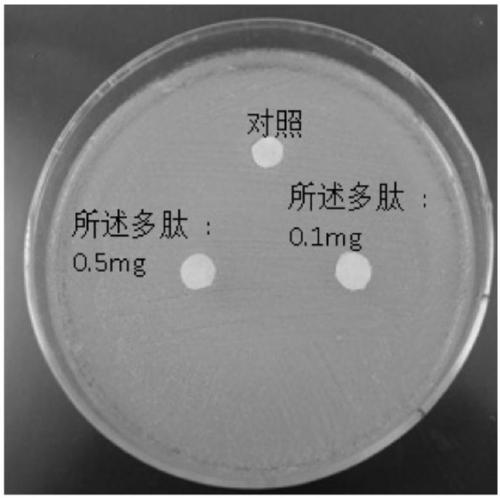
Application of polypeptide in preparing preparations for preventing and treating human papilloma virus infection
PendingCN111375051AImprove securityNo inhibitory effectPeptide/protein ingredientsSuppositories deliveryHuman papilloma virus infectionInfectious Disorder
The invention discloses an application of polypeptide in preparing preparations for preventing and treating human papilloma virus infection, and belongs to the field of infectious disease treatment. Apolypeptide preparation for preventing and treating human papilloma virus infection is also provided; and the polypeptide preparation takes the polypeptide with effective dose as active ingredients and is prepared by adding pharmaceutically acceptable subsidiary materials or auxiliary components. The polypeptide can be used for the treatment and prevention of human papilloma virus infection, so that the polypeptide is significant in effect; and the polypeptide has no inhibition effects on the normal flora of female genital tracts, so that the polypeptide is good in safety.
Owner:GENLOCI BIOTECH
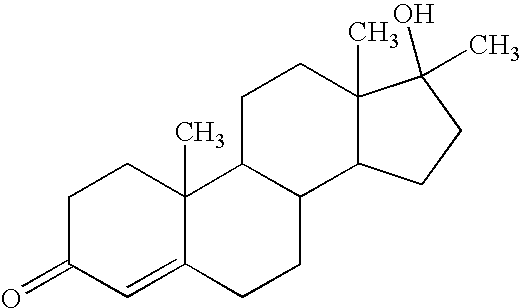
Use of methyltestosterone as a drug for treatment of human papilloma virus infections
The present invention discloses the use of methyltestosterone as a novel pharmaceutical for treatment of anti-HPV infections, especially for treatment of verruca planae, condyloma acuminatum, and the relapse of these disorders. The therapeutic efficacy of the aforementioned indications proves satisfactory.
Owner:LIANG YOUGOU +2
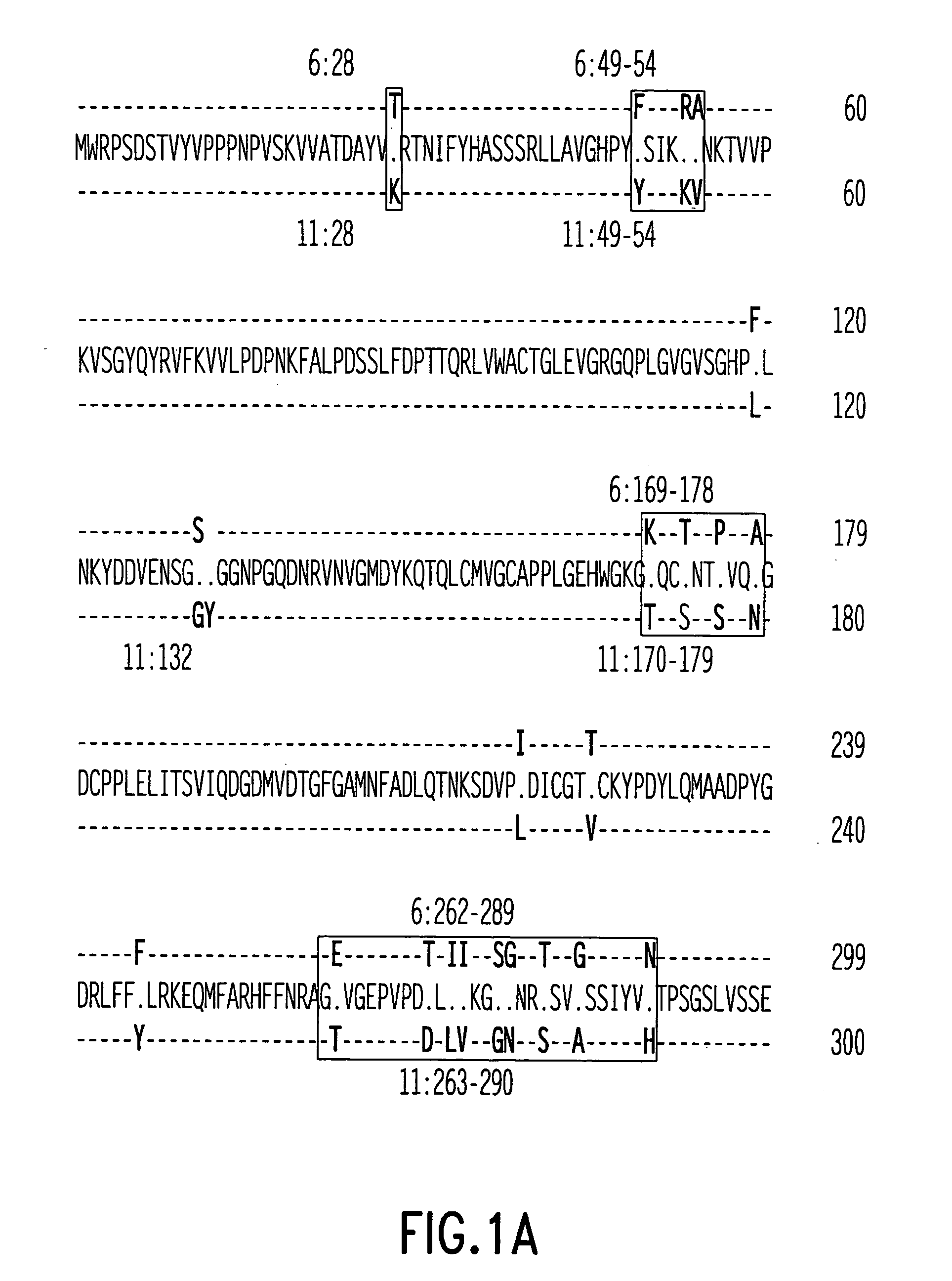
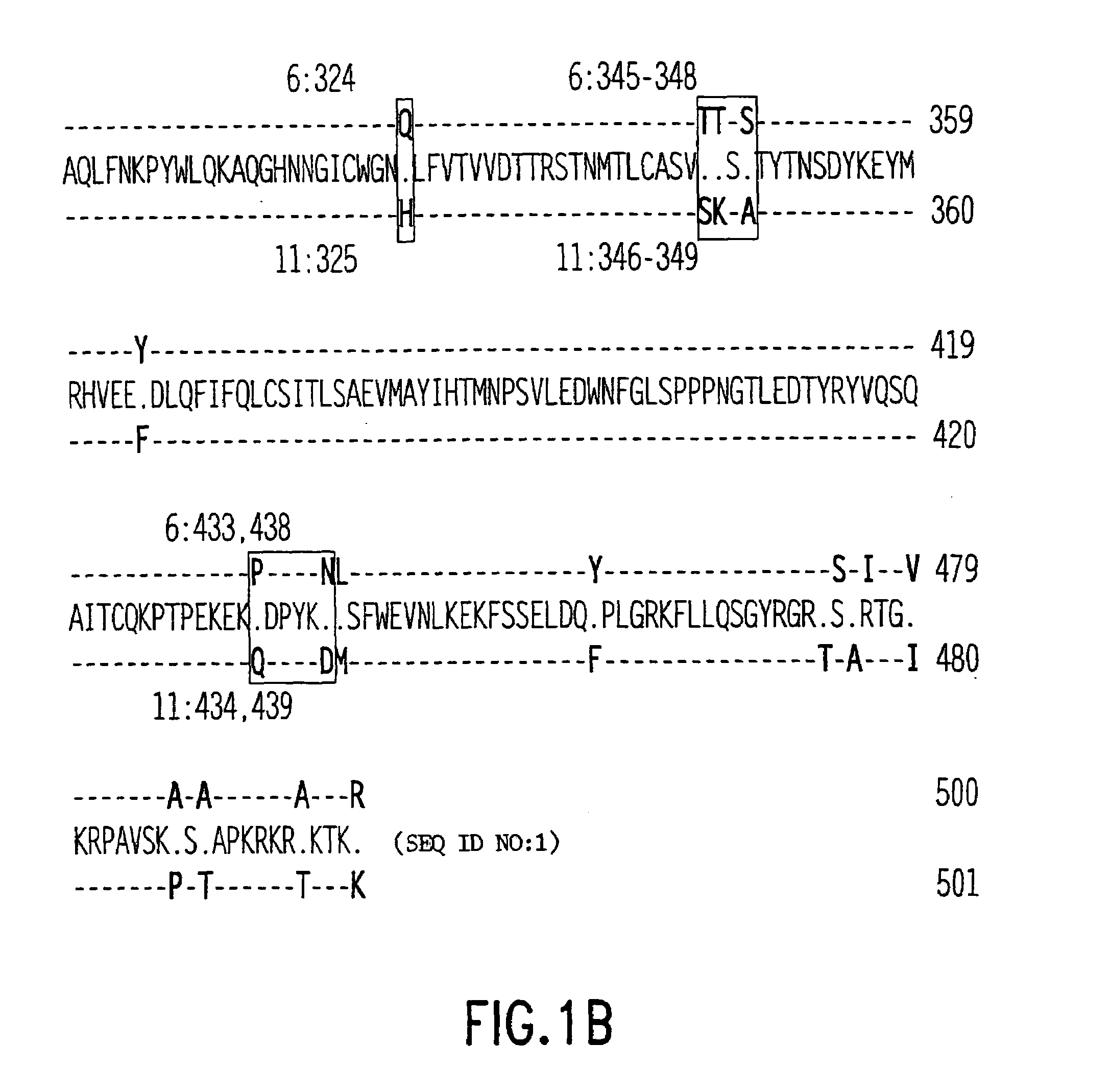
Modification sequence of recombinant human mammilla tumor virus L1 capsid protein
InactiveCN101481407AStructural stability has no appreciable effectStructural Stability EffectsViral antigen ingredientsAntiviralsAntigenHuman papillomavirus
The invention relates to the prevention and treatment field of human papillomavirus infection, in particular to an amino acid modification sequence of recombinant human papillomavirus (HPV) L1 capsid protein, a nucleotide sequence encoding the amino acid modification sequence, and a carrier and a transformant containing the nucleotide sequence; and the invention further relates to application of an HPV L1 protein polymer consisting of the amino acid modification sequence to preparing vaccines, pharmaceutical compositions and diagnostic antigen or diagnostic antibodies. In the invention, an HPV L1 protein wild-type sequence is modified to prevent forming of a disulfide bond in the HPV L1 protein, the modification has no significant effect on the structural stability of the recombinant HPV L1 protein, but significantly improves the efficiency of purification process, and directly reduces the reagent consumption, thus effectively lowering the industrial production cost and having great economical benefit.
Owner:马润林 +1
HPV DNA Vaccines and Methods of Use Thereof
InactiveUS20080260765A1Enhance antigen-specific immune responseEnhance immune responseSugar derivativesViral antigen ingredientsDiseaseTreatment effect
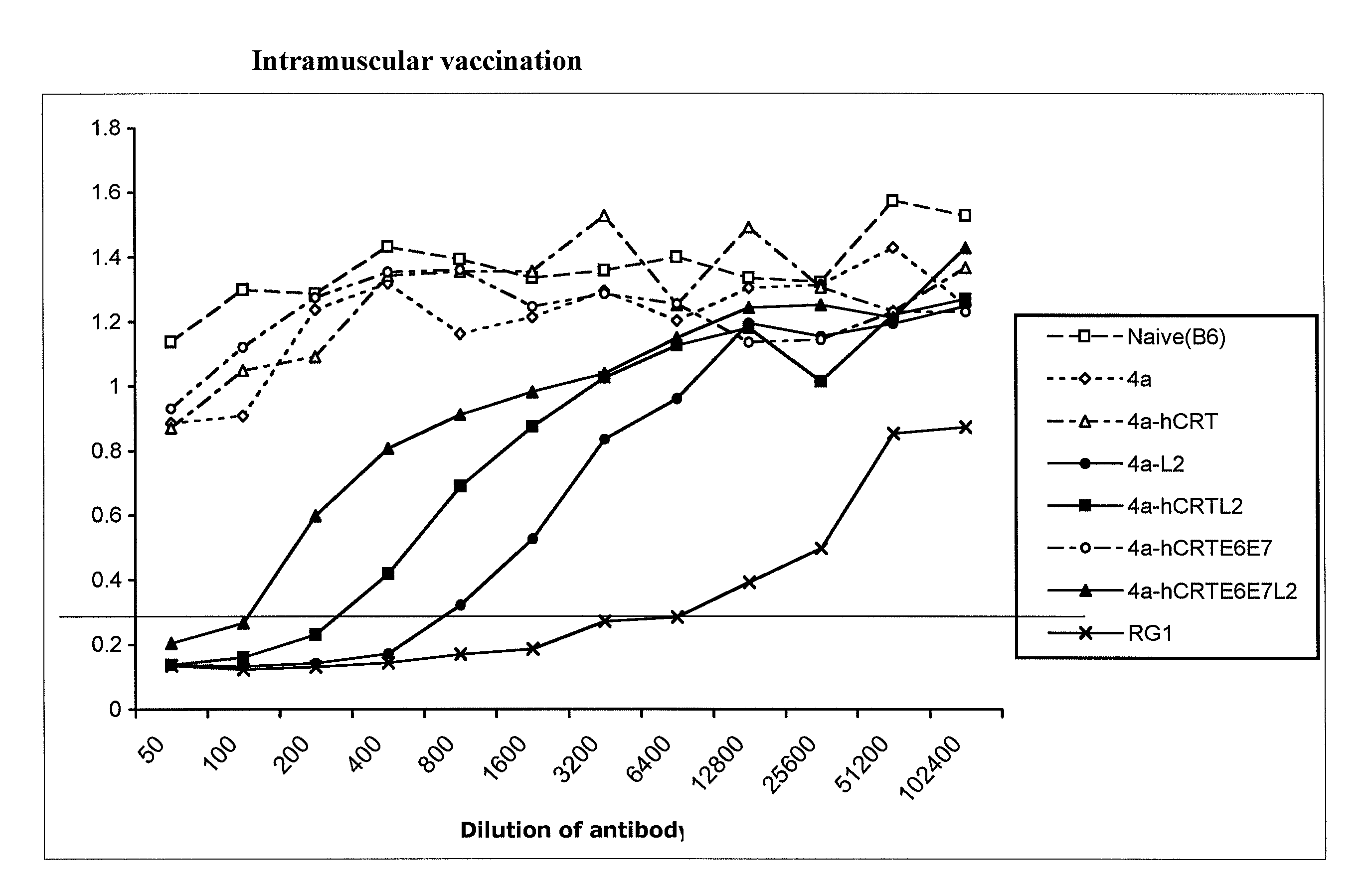
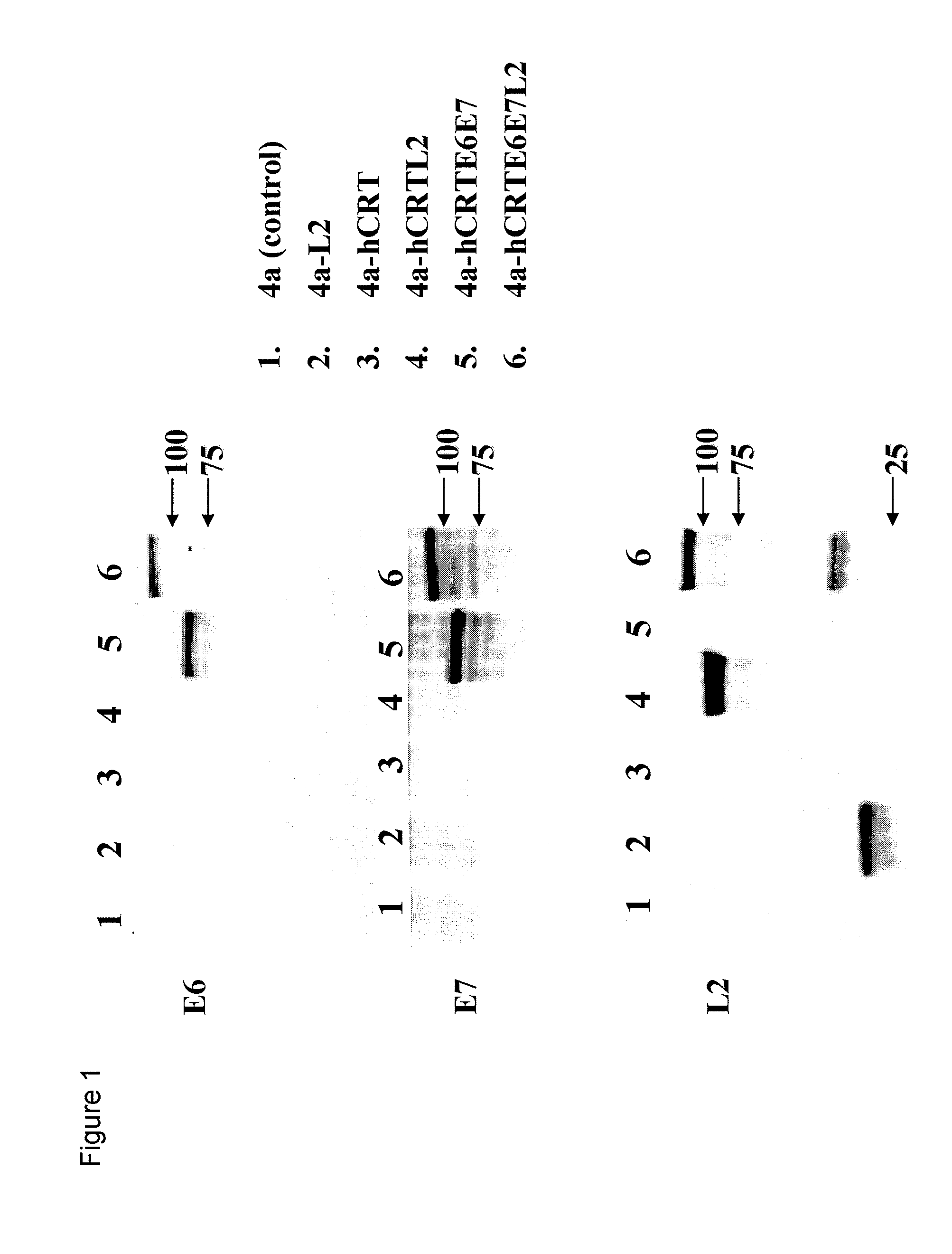
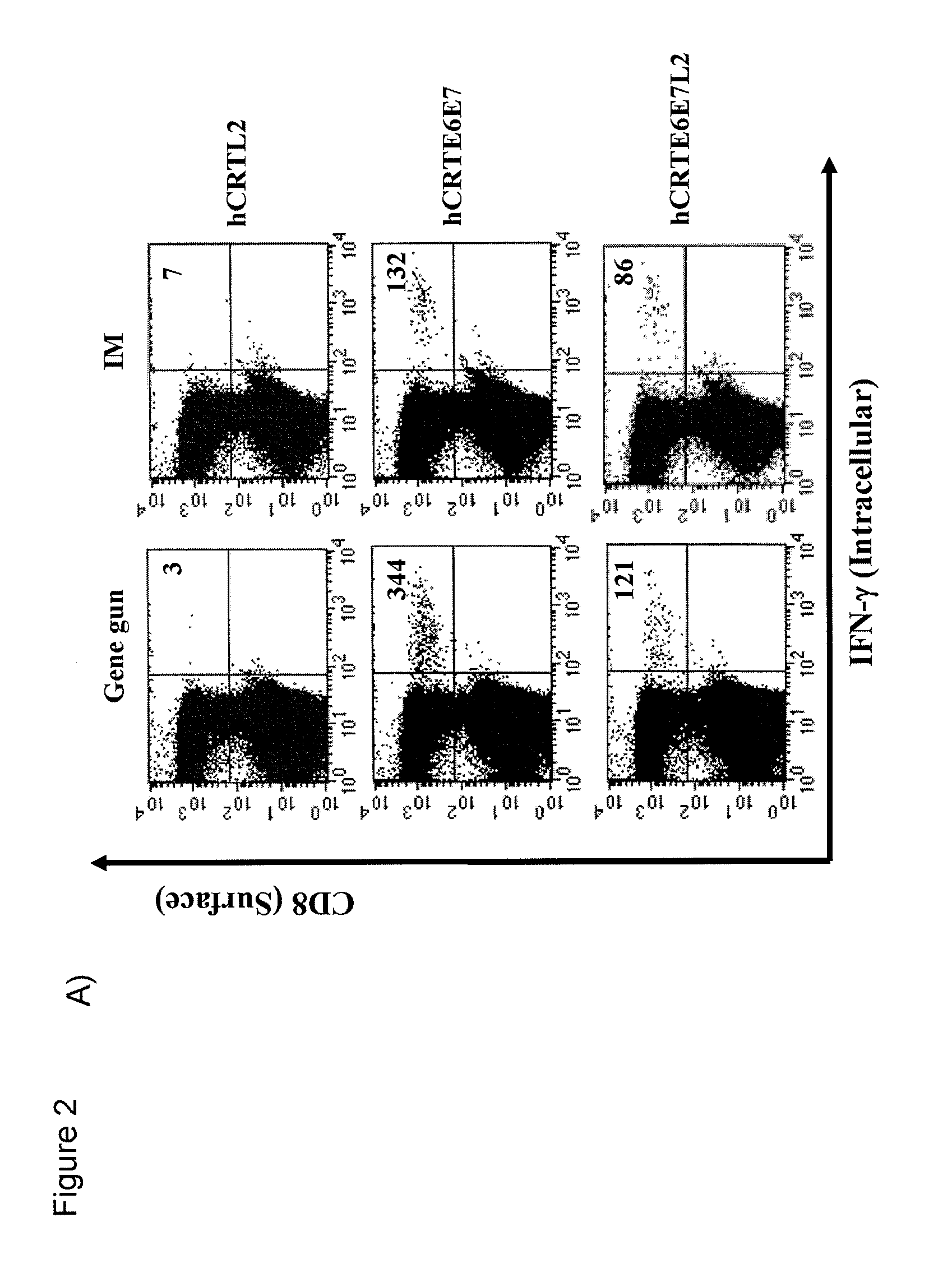
Human papillomavirus (HPV) infection is the etiological factor for cervical cancer. Provided are HPV vaccines that generate a humoral immune response to prevent new infection, as well as cell-mediated immunotherapy to eliminate established infection or HPV-related disease. HPV vaccines include nucleic acid sequences encoding HPV16 early proteins E6 and E7. Additional nucleic acid sequences in the vaccines include sequences encoding calreticulin and / or the HPV16 late protein L2. Methods using these vaccines are provided that result in therapeutic effects.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Method for treating cervical cancer
InactiveUS7582287B2Heavy metal active ingredientsBiocideHuman papilloma virus infectionWhite blood cell
Use of Interleukin-20 for treating cervical cancer or cells infected with human papilloma virus. IL-20 can be administered alone or in conjunction with radiation or chemotherapeutic agents or surgical excision of the involved cells or lesions.
Owner:ZYMOGENETICS INC
Topical treatment of skin disease and eye afflictions
A method of treating patients having viral infections including herpes simplex virus 1 and 2 infections and human papillomavirus infections by topically administering Product R, a peptide-nucleic acid preparation, by itself or in a composition comprising Product R and other pharmaceutically acceptable carriers for topical administration, is disclosed.
Owner:BBM HLDG
Compound vaccine composition for preventing and controlling human viral infection, compound vaccine vaginal mist and uses thereof
ActiveCN101181636ABroad immune spectrumFor personal usePeptide/protein ingredientsViral antigen ingredientsHuman papilloma virus infectionHuman papillomavirus
The invention relates to a compound vaccine composition for preventing and treating human papillomavirus infection, a compound vaccine vaginal spray and uses thereof, and belongs to the field of vaccine preparations with targeted treatment indications. The product of the present invention can prevent cervical cancer and treat HPV infection; it is suitable for married women of any age; it can prevent and treat chronic cervicitis and cervical epithelial dysplasia caused by sexually transmitted HPV infection, including CIN I, II, III Degree, inhibit HPV reproduction, prevent cervical cancer.
Owner:北京金迪克生物技术有限责任公司
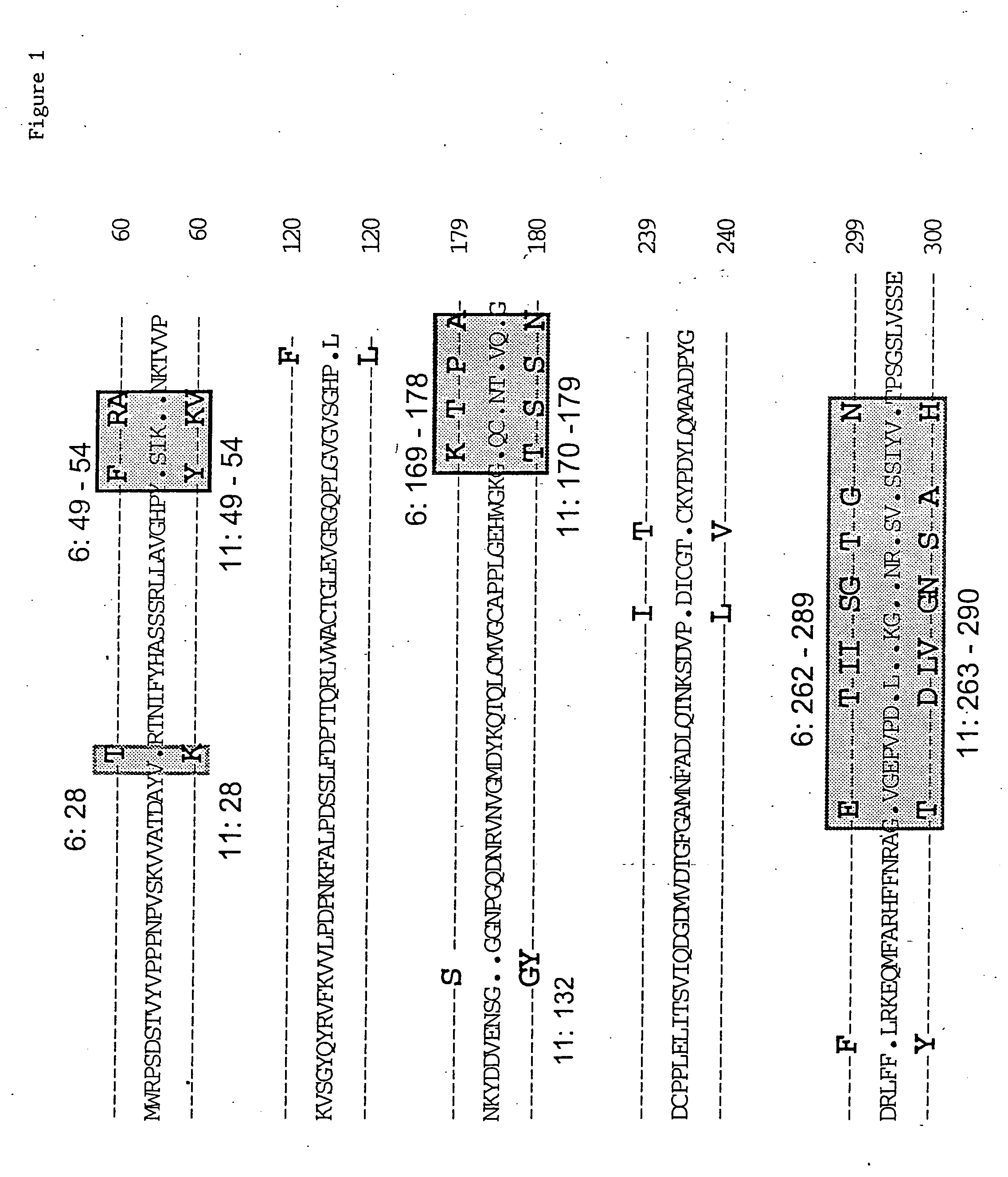
Synthetic virus-like particles with heterologous epitopes
Owner:MERCK SHARP & DOHME CORP
Charge modified lactoferrin and carrageenin combination medicine and preparation method thereof
ActiveCN105031625AIncrease conversion rateGood curative effectAntibacterial agentsOrganic active ingredientsHuman papilloma virus infectionCarrageenan
The invention discloses a charge modified lactoferrin and carrageenin combination medicine which comprises 0.01 part by weight of lactoferrin, 1.5 parts by weight of carrageenin, 5 parts by weight of carbomer, 50 parts by weight of glycerol and 0.1 part by weight of ethylparaben. The combination medicine is one of a gel, a suppository and a dressing. The combination medicine disclosed by the invention can be used for preventing and treating female human papilloma virus infection as well as preventing and treating female gynecological gram-negative bacterium infection.
Owner:NANJING LAKESEN BIOPHARM TECH CO LTD
Traditional Chinese medicine used for treating human papillomavirus infection
ActiveCN1861105AStrong specificityEfficient removalAntiviralsPlant ingredientsHuman papillomavirusHuman papilloma virus infection
A Chinese medicine for treating the human papillomavirus infection and clearing the papillomavirus from human body is prepared from safflower and scouring herb.
Owner:杭州清净和生物工程有限公司
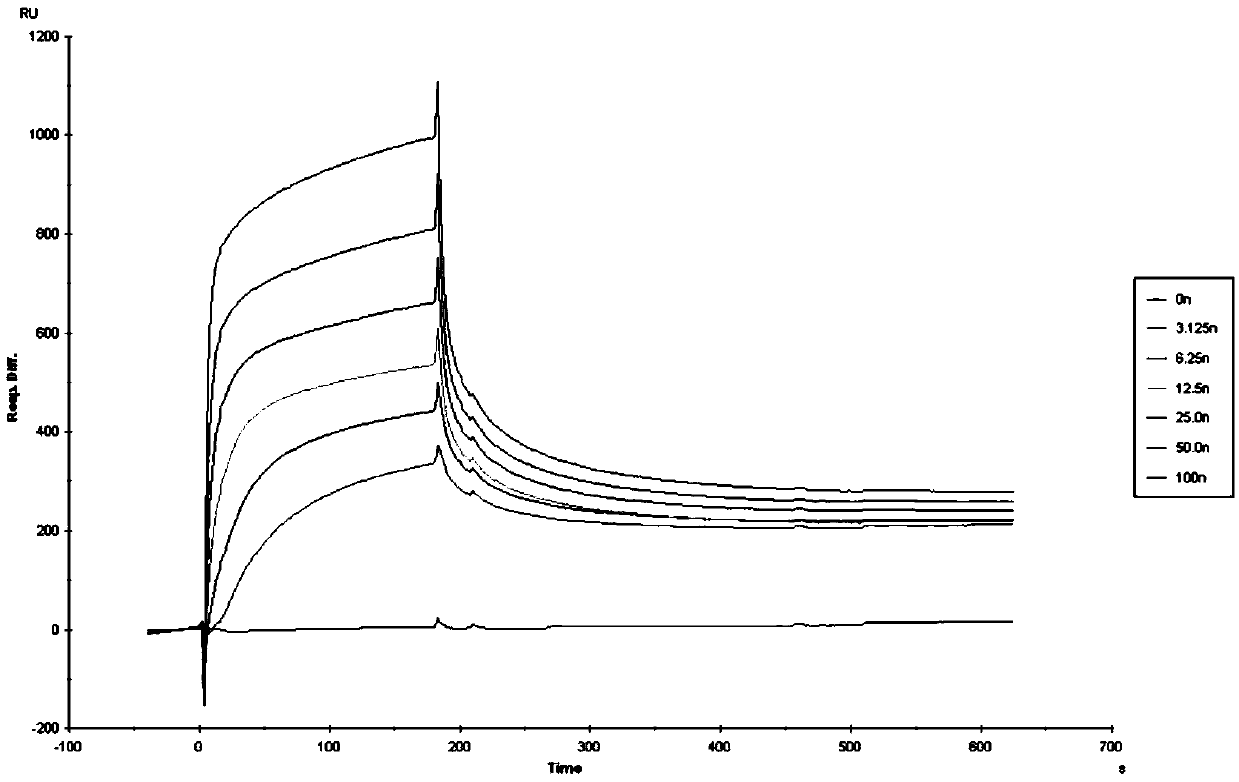
Anti-HPV (human papilloma virus) antibody and preparation method and application thereof
ActiveCN102443060AImmunoglobulins against virusesMicroorganism based processesDiseaseHuman papilloma virus infection
The invention relates to an antibody used for treatment of diseases, in particular to cervical cancer, related to human papilloma virus infection, and a preparation method and an application thereof.
Owner:SHANGHAI ZERUN BIOTECHNOLOGY CO LTD
Synthetic virus-like particles with heterologous epitopes
The invention is a series of synthetic virus-like particles comprising a heterologous conformational epitope useful in the characterization of human papillomavirus infection, and useful to vaccinate individual for protection against HPV 6 and HPV 11 infections, and assays employing the synthetic virus-like particles.
Owner:MERCK SHARP & DOHME CORP
Compositions and methods for treating skin ailments
InactiveUS20030133893A1Organic active ingredientsCosmetic preparationsDiseaseHuman papilloma virus infection
Compositions that comprise a polymer entrapping an oxidizing agent are disclosed. The disclosed compositions are used in the treatment of skin ailments such as human papilloma virus infections.
Owner:DEGANIA SILICON
Immunogenic HPV L2-Containing VLPs and Related Compositions, Constructs, and Therapeutic Methods
ActiveUS20140105924A1Highly immunogenicReduce the possibilityViral antigen ingredientsDsDNA virusesImmunogenicityImmunotherapy
The invention provides immunotherapeutic and prophylactic bacteriophage viral-like particle (VLPs) which are useful in the treatment and prevention of human papillomavirus (HPV) infections and related disorders, including cervical cancer and persistent infections associated with HPV. Related compositions (e.g. vaccines), nucleic acid constructs, and therapeutic methods are also provided. VLPs and related compositions of the invention induce high titer antibody responses against HPV L2 and protect against HPV challenge in vivo. VLPs, VLP-containing compositions, and therapeutic methods of the invention induce an immunogenic response against HPV infection, confer immunity against HPV infection, protect against HPV infection, and reduce the likelihood of infection by HPV infection.
Owner:STC UNM
Kit for fluorescent PCR (Polymerase Chain Reaction) detection of high-risk HPV (Human Papilloma Virus)
ActiveCN104017907AEasy to detectMicrobiological testing/measurementMicroorganism based processesHuman papilloma virus infectionVerruca acuminata
The invention provides a kit for fluorescent PCR (Polymerase Chain Reaction) detection of a high-risk HPV (Human Papilloma Virus). The kit comprises a nucleic acid releasing agent and a PCR reaction solution, wherein the nucleic acid releasing agent comprises 0.01-05mmol / L surfactin, 20-300mmol / L potassium chloride, 0.01-2% sodium dodecyl sulfate and 0.05-1% ethanol; the PCR reaction solution comprises high-risk HPV16-type and 18-type primer probe sequences and internal standard primer probe sequences. The invention aims at providing a kit for fluorescent quantitative PCR detection of high-risk Human Papilloma Virus, and the kit has the advantages of fastness in operation, simple method, high detection sensitivity and wide detection range. By virtue of the kit, a common fast fluorescent PCR detection is carried out on DNA nucleic acid fragments of the high-risk human papilloma virus in unknown samples, such as cast-off cells of the surface of a verruca acuminata body, women cervical epithelial cells and genital secretion and the detection results can be applied in the auxiliary diagnosis of the high-risk human papilloma virus infection and the early screening of cervical cancer.
Owner:SANSURE BIOTECH INC
HPV (human papilloma virus) high-risk typing fluorescence PCR (polymerase chain reaction) detection kit
ActiveCN104017906AMicrobiological testing/measurementMicroorganism based processesFluorescenceCervix
The invention provides an HPV (human papilloma virus) high-risk typing fluorescence PCR (polymerase chain reaction) detection kit which comprises a nucleic acid releaser and two or more PCR reaction solutions, wherein the nucleic acid releaser comprises 0.01-0.5 mmol / L surfactin, 20-300 mmol / L potassium chloride, 0.01-2% of sodium dodecylsulfate and 0.05-1% of ethanol. One of the PCR reaction solutions comprises high-risk HPV 16 type and / or 18 type primer probe sequences, and another PCR reaction solution comprises an internal reference primer probe sequence. The kit provided by can be used for carrying out quick fluorescence PCR typing detection on DNA (deoxyribonucleic acid) nucleic acid segments of high-risk HPV in wart surface shedding cells, female cervix epithelial cells, genital secretion and other unknown samples; and the detection result can be used for auxiliary diagnosis of high-risk HPV infection, early screening of cervical carcinoma, follow-up survey of cervix pathological changes and instruction of vaccine development.
Owner:SANSURE BIOTECH INC
Traditional Chinese medicine composition used for treating human papillomavirus infection as well as preparation method and application thereof
ActiveCN103100007AReasonable prescription designStrict compatibilityAntiviralsPlant ingredientsHuman papilloma virus infectionHuman papillomavirus
The invention provides a traditional Chinese medicine composition used for treating human papillomavirus infection. The traditional Chinese medicine composition is prepared from the following raw materials in parts by weight: 8-12 parts of rhizoma atractylodis stir-fried with bran, 8-12 parts of white atractylodes rhizome stir-fried with bran, 8-12 parts of golen cypress, 20-40 parts of coix seed, 8-12 parts of paris polyphylla, 8-12 parts of isatis root, 20-40 parts of oldenlandia diffusa and 8-12 parts of rhizoma smilacis glabrae; and the aim of treating human papillomavirus infection is achieved by tonifying spleen, benefiting qi, promoting diuresis, clearing heat and detoxifying. The invention also provides a preparation method and an application of the traditional Chinese medicine composition.
Owner:南京融昱医药产业有限公司
Fluorescence PCR method for diagnosis of human papilloma viral infection
PendingCN101251469ALow efficiencyReduce complexityMicrobiological testing/measurementMaterial analysis by optical meansForward primerHuman papilloma virus infection
Owner:GENETEL PHARMA SHENZHEN
Method of immunotherapy for treament of human papillomavirus infection
ActiveUS9333238B2Avoid developmentAntibacterial agentsOrganic active ingredientsLangerhan cellHuman papilloma virus infection
A method of treating human papillomavirus (HPV), by administering a therapeutically effective amount of a primary cell-derived biologic to a patient infected with HPV, and inducing an immune response to HPV. A method of overcoming HPV-induced immune suppression of Langerhans cells (LC), by administering a therapeutically effective amount of a primary cell-derived biologic to a patient infected with HPV, and activating LC. A method of increasing LC migration towards lymph nodes, by administering a therapeutically effective amount of a primary cell-derived biologic to a patient infected with HPV, activating LC, and inducing LC migration towards lymph nodes. A method of generating immunity against HPV, by administering an effective amount of a primary cell derived biologic to a patient infected with HPV, generating immunity against HPV, and preventing new lesions from developing.
Owner:BROOKLYN IMMUNOTHERAPEUTICS LLC
HPV vaccines
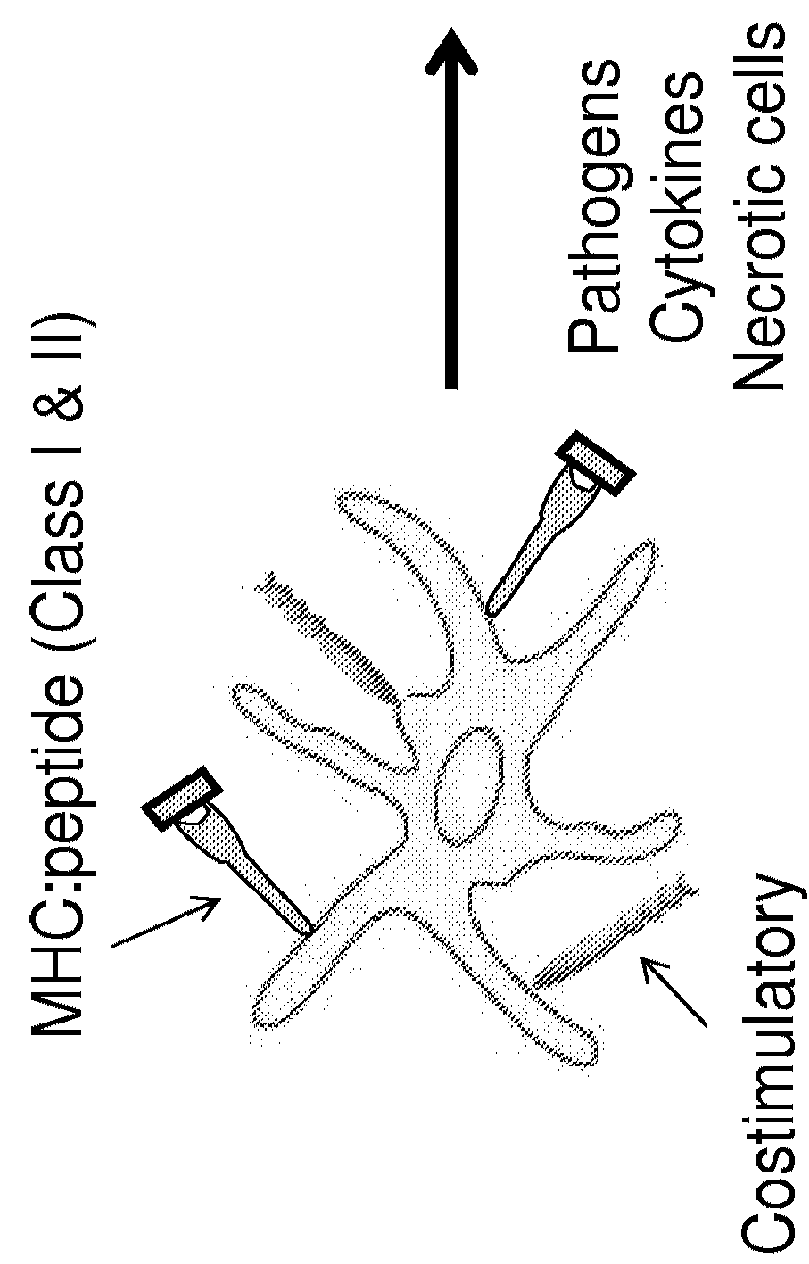
ActiveUS10669315B2Reduce severityReduce frequencySsRNA viruses negative-senseViral antigen ingredientsDiseaseHuman papilloma virus infection
Provided herein are genetically modified arenaviruses suitable as vaccines against neoplastic diseases or cancer. The invention also relates to pharmaceutical compositions and methods for the prevention or treatment of certain infections causing neoplastic diseases or cancer, such as infections with oncogenic viruses. Specifically, provided herein are pharmaceutical compositions, vaccines, and methods of preventing or treating diseases and conditions caused by and associated with infections with Human Papillomavirus (HPV), such as cervical cancer, anogenital cancer, head and neck cancer and skin cancers. Also provided herein are immunotherapies for the treatment of a neoplastic disease, such as a neoplastic disease caused by infection with oncogenic viruses.
Owner:HOOKIPA BIOTECH GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com