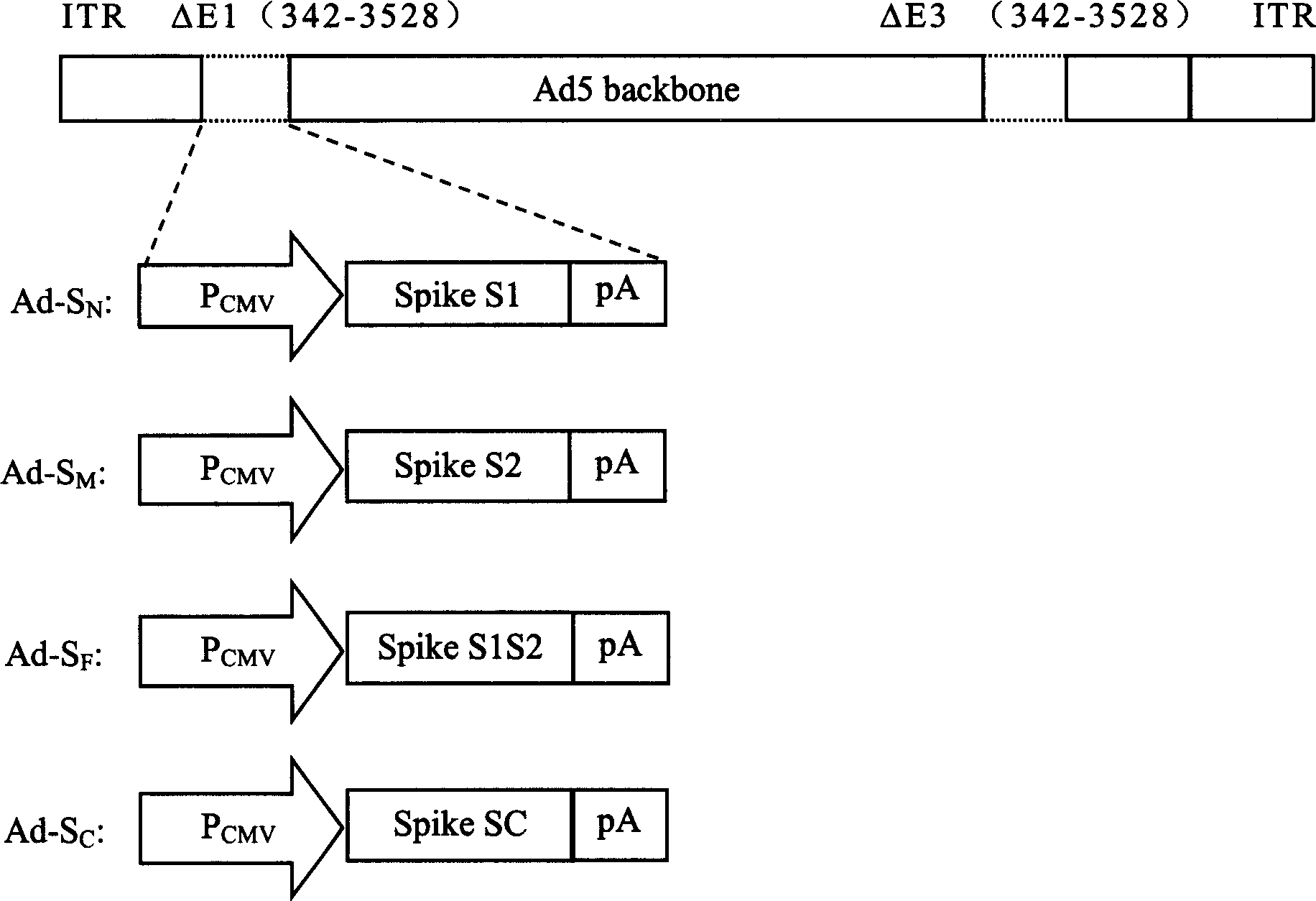
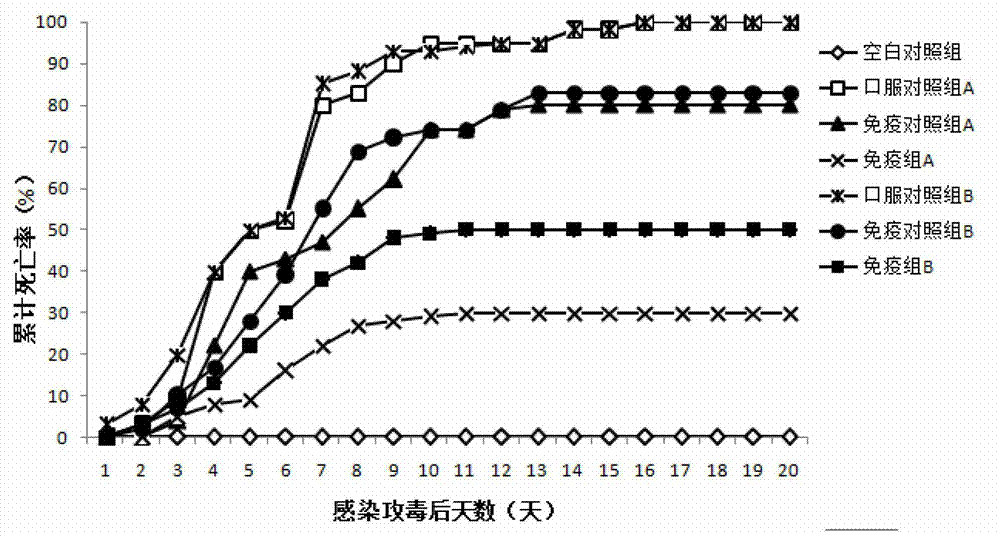
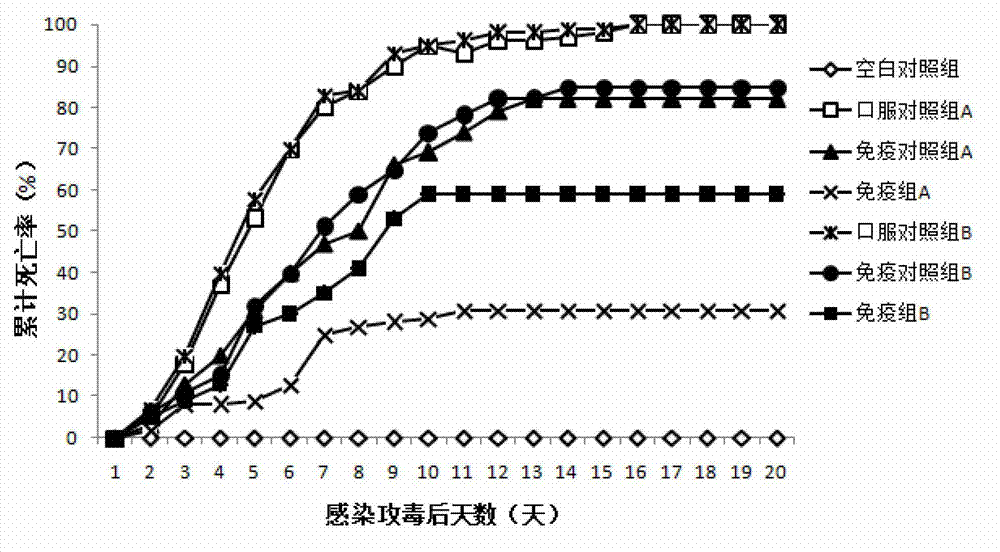
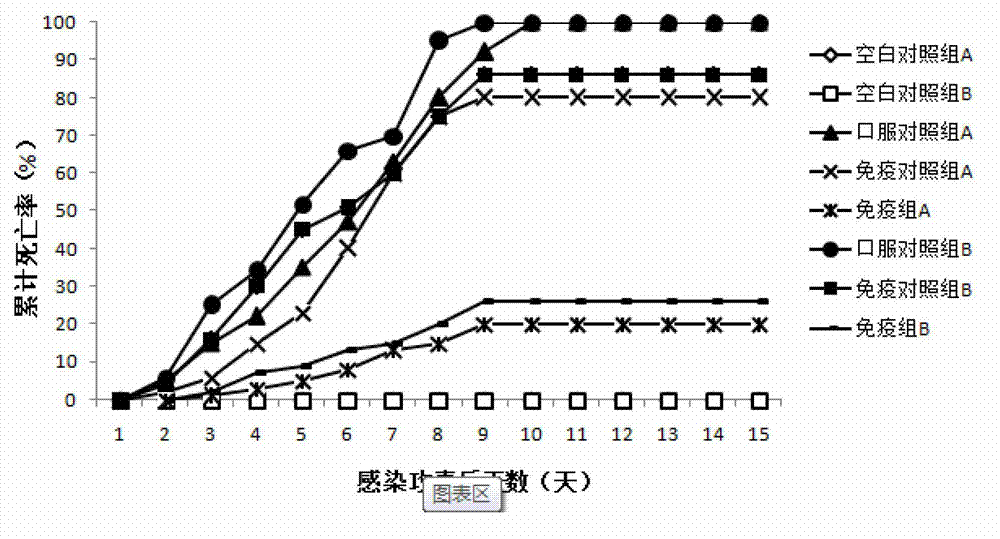
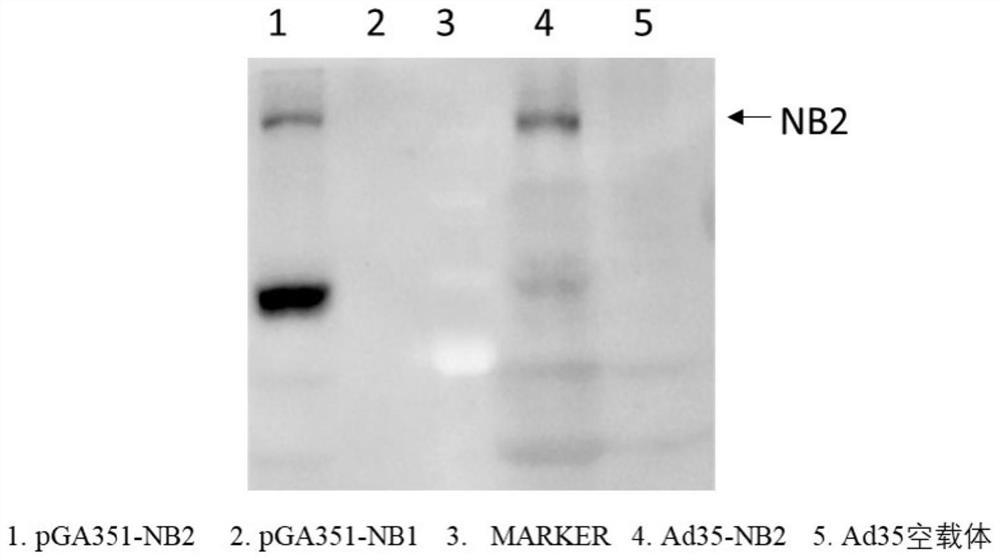
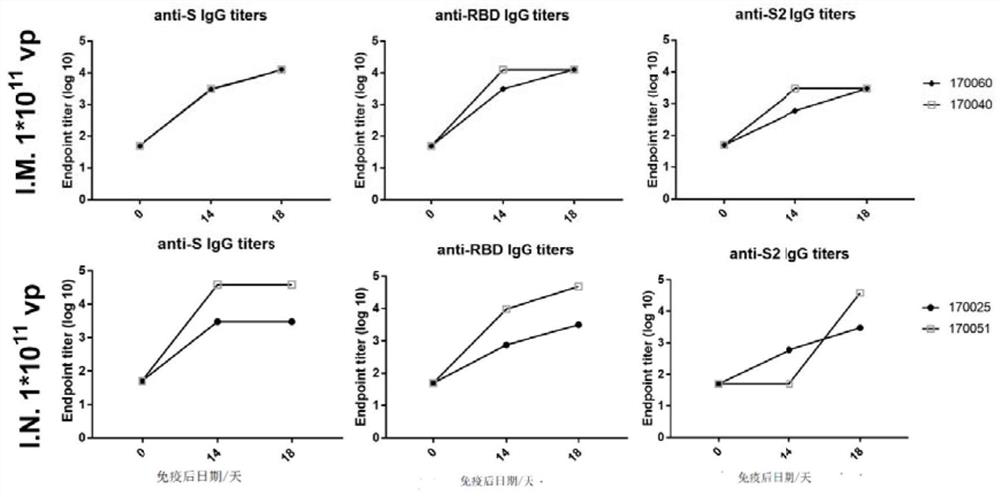
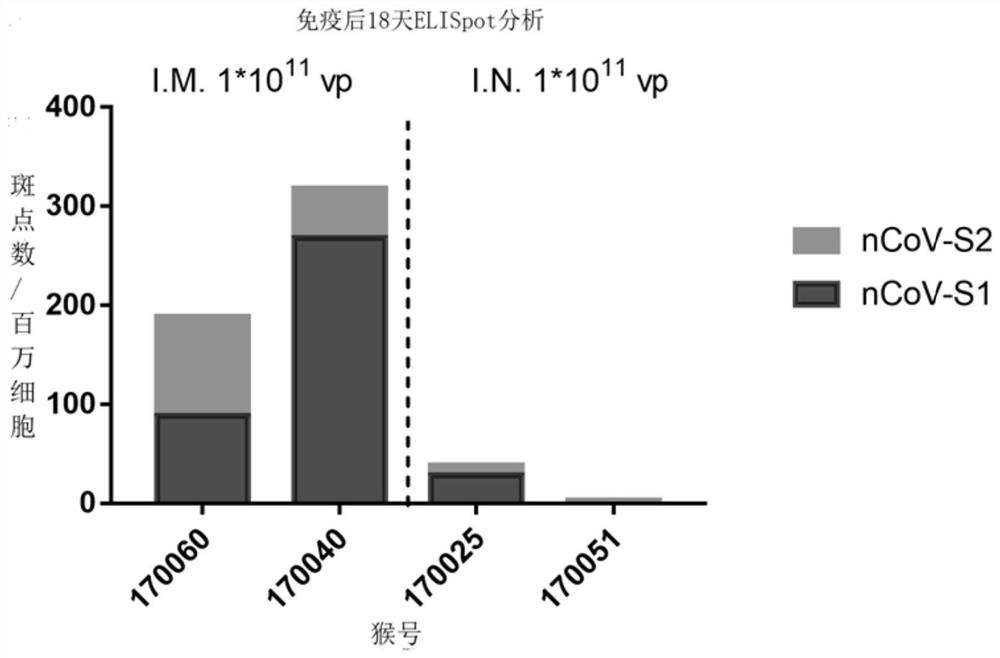
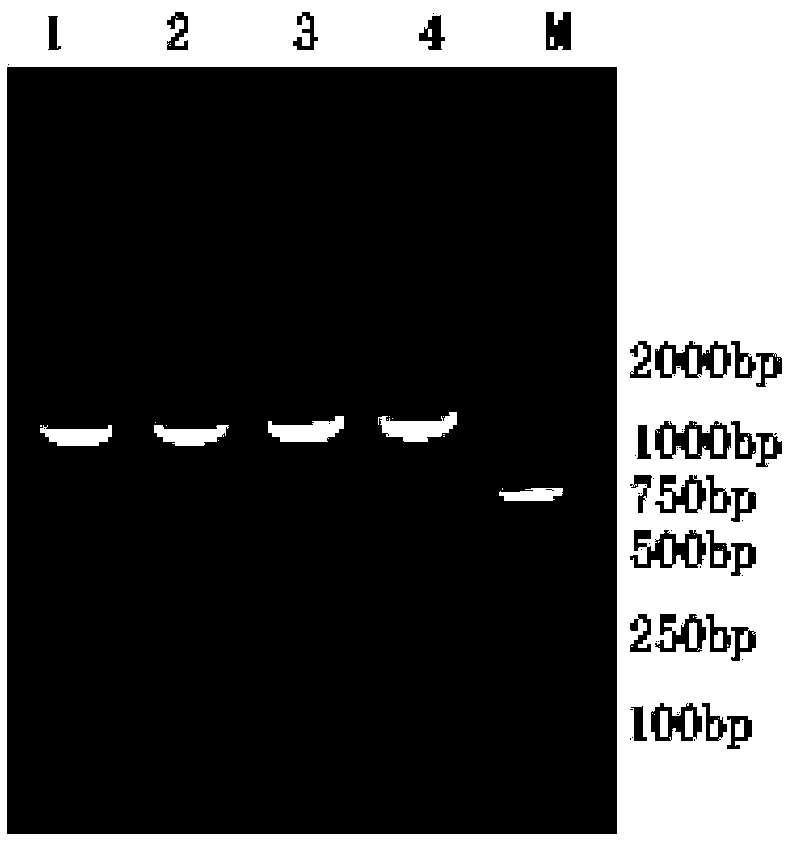
Patents
Literature
228 results about "Vector vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Vaccines developed using recombinant DNA technology.
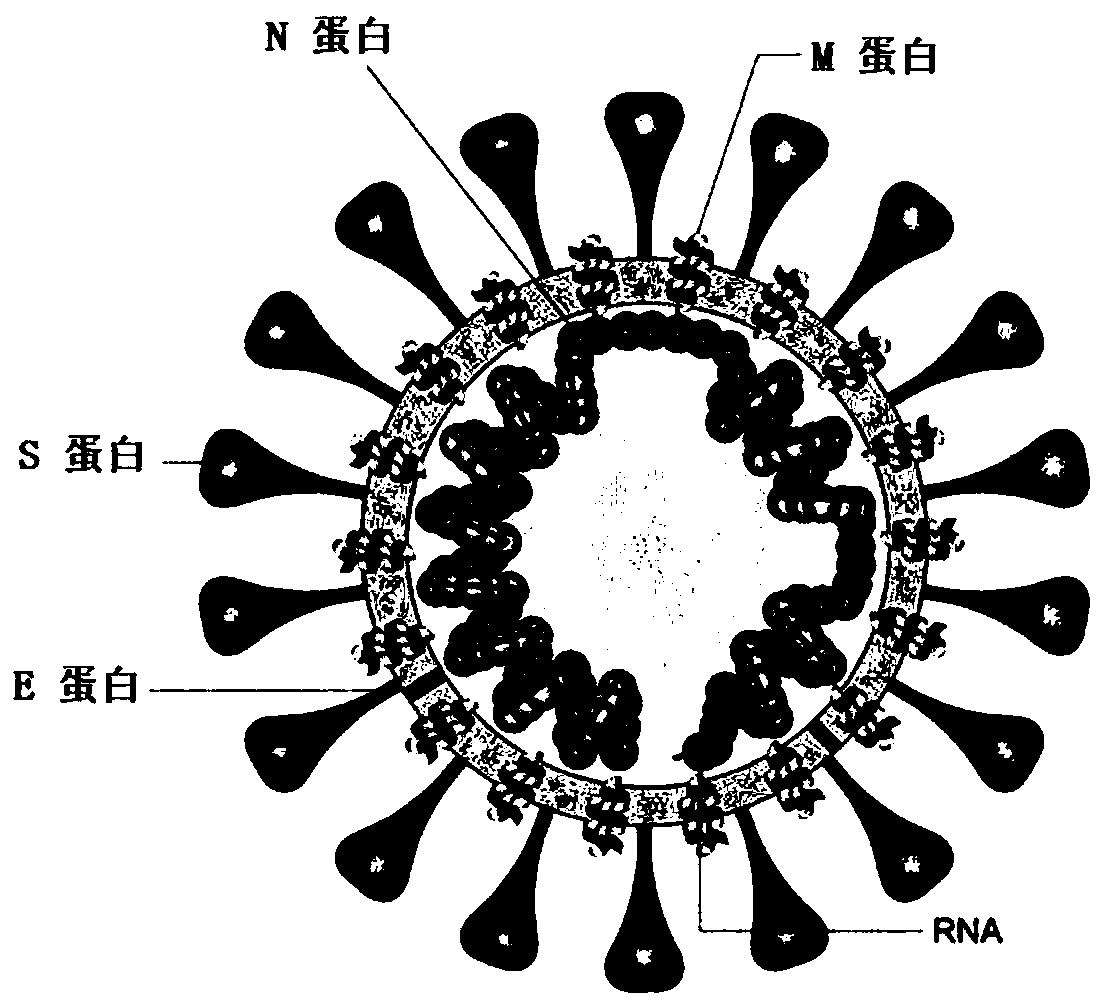
SARS-CoV-2 vaccine and preparation method thereof
ActiveCN111217917AEnter to helpImproving immunogenicityPolypeptide with localisation/targeting motifSsRNA viruses positive-senseAntigenDisease
The invention relates to a preparation method for a vaccine capable of treating and / or preventing SARS-CoV-2 infection or COVID-19 diseases. The core antigen of the vaccine comprises the RBD (receptor binding zone) fusion protein of the SARS-CoV-2, and a vaccine form comprises an RBD fusion protein subunit vaccine, an RBD fusion protein mRNA vaccine or an RBD fusion protein adenovirus vector vaccine. The above vaccine immunizes an organism, and immune reaction for treating and / or preventing the SARS-CoV-2 infection can be generated so as to be used for treating and / or preventing COVID-19. The invention also relates to an RBD fusion gene, the RBD fusion protein, a carrier, a cell, a preparation method, a treatment method or a pharmacy purpose of the SARS-CoV-2.
Owner:CANSINO BIOLOGICS INC
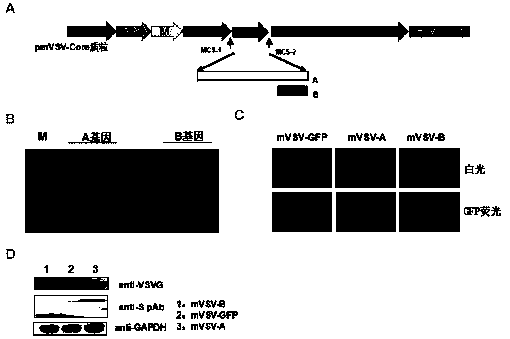
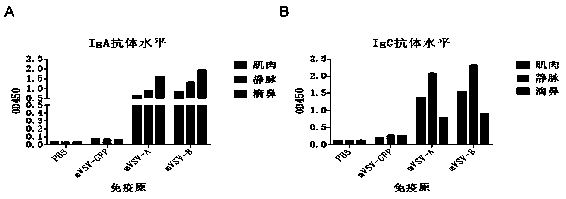
mVSV virus vector and virus vector vaccine, and COVID-19 vaccine based on mVSV mediation
ActiveCN111088283AEnhance immune responseStrong immune responseSsRNA viruses negative-senseSsRNA viruses positive-senseHeterologousReceptor
The invention provides an mVSV virus vector, i.e., attenuated mVSV obtained after multiple modification mutations occur to an M protein amino acid site of a wild Indiana strain VSV, and an optimized heterologous antigen gene is preferentially integrated to a double cloning site area of an mVSV packaging core plasmid pmVSV-Core at the same time. The mVSV virus vector vaccine comprises a heterologous antigen gene which fuses or embeds a target virus between G and L genes of an mVSV vector envelope, wherein the antigen gene comprises an enveloped and embedded antigen gene encoding the target virus, an embedded combination antigen gene or a fused antigen gene; the mVSV virus vector is embedded or fused with a dominant antigen of spike protein S of an SARS-CoV-2 pathogen; the dominant antigen is preferably selected from a receptor binding domain of spike protein S, namely RBD; and a COVID-19 vaccine based on mVSV mediation is formed. The vaccine has good prevention or treatment effect on COVID-19 infected people.
Owner:FANTASIA BIOPHARMA ZHEJIANG CO LTD
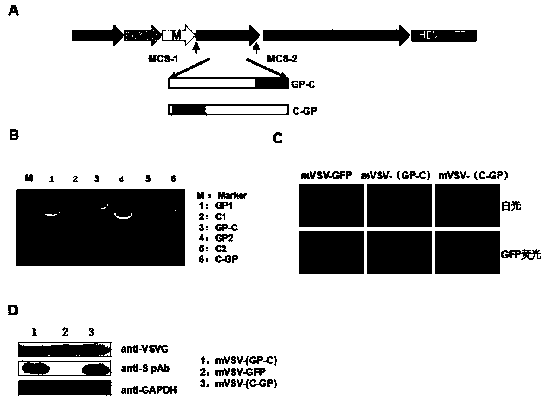
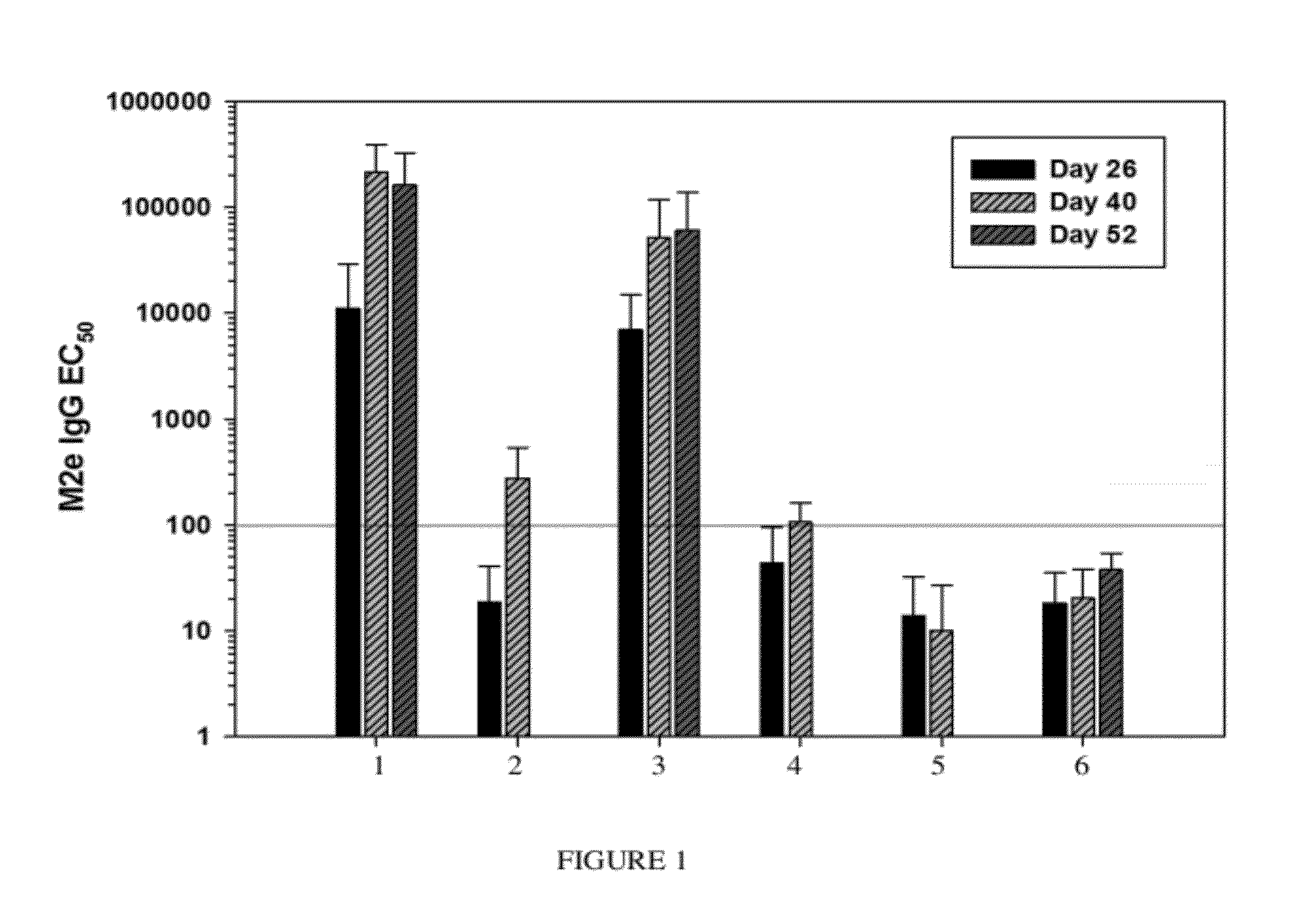
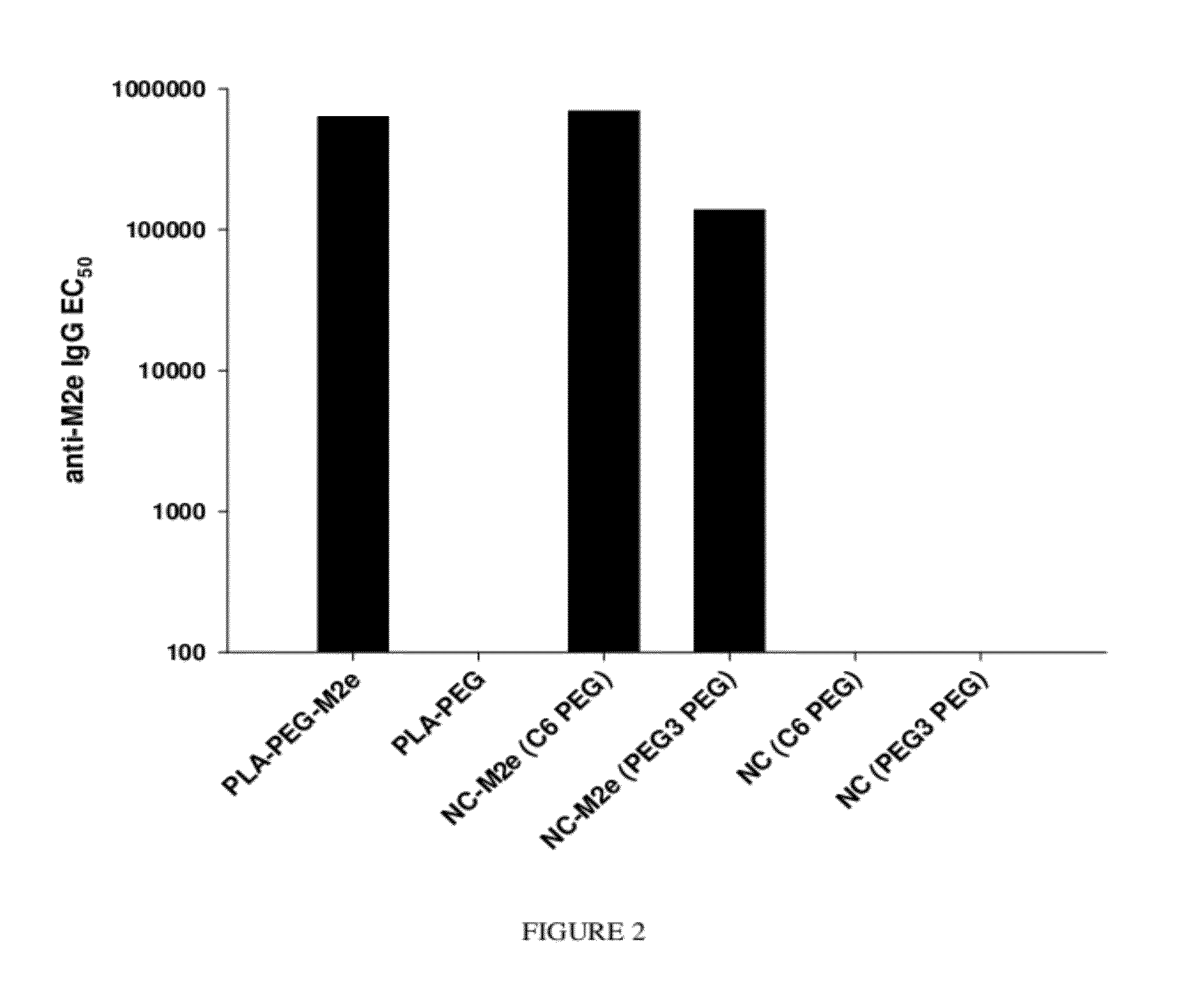
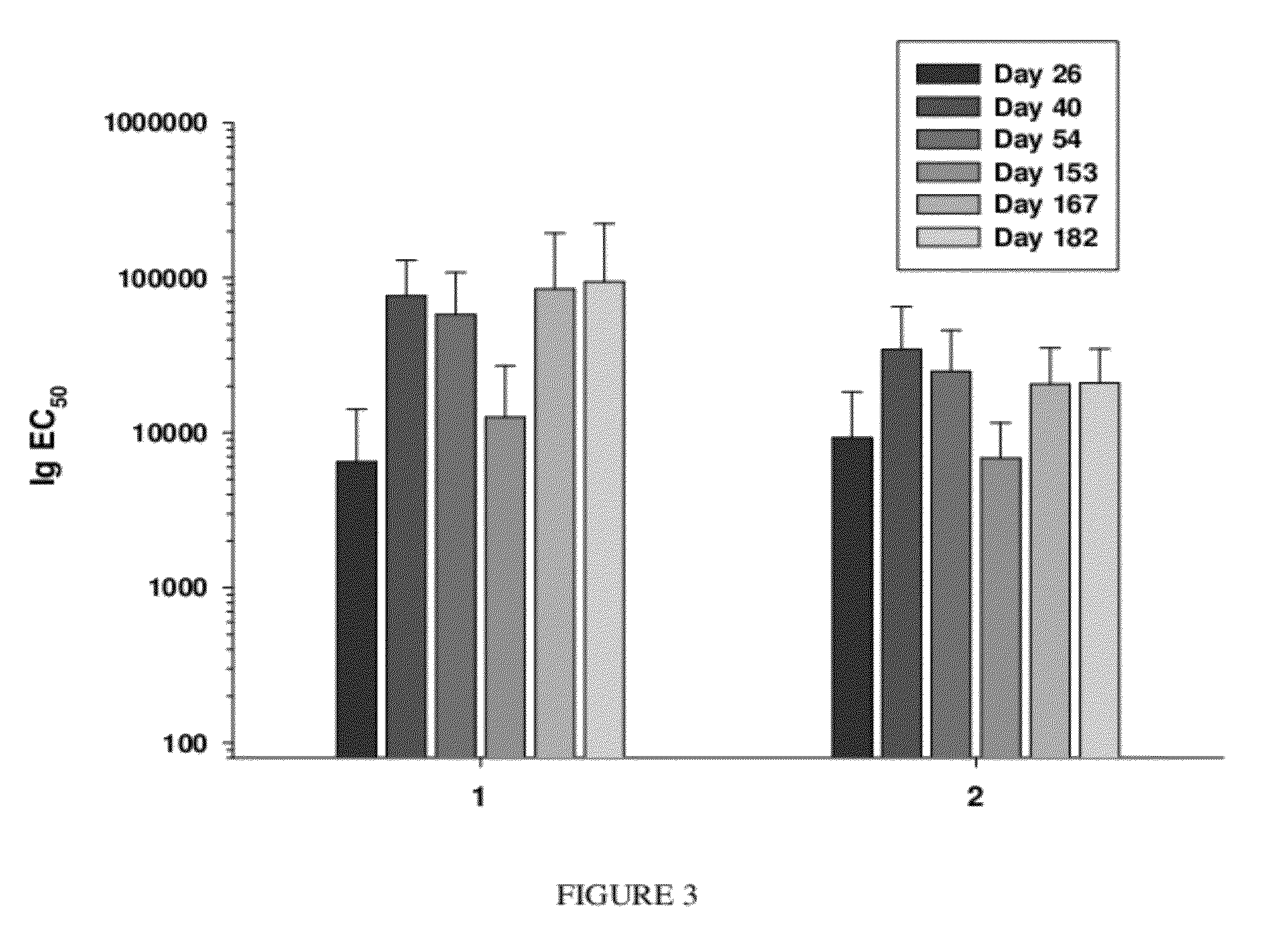
Synthetic nanocarrier vaccines comprising peptides obtained or derived from human influenza a virus m2e
InactiveUS20120058154A1Stimulate immune responseSsRNA viruses negative-sensePeptide/protein ingredientsHuman Influenza A VirusVector vaccine
This invention relates to compositions and methods that can be used immunize a subject against influenza. Generally, the compositions and methods include peptides obtained or derived from human influenza A virus M2 protein.
Owner:SELECTA BIOSCI
Adenovirus vector vaccine for preventing SARS-CoV-2 infection
ActiveCN110974950AImprove securityAvoid infectionSsRNA viruses positive-senseViral antigen ingredientsVector vaccineHuman cell
The invention discloses an adenovirus vector vaccine for preventing SARS-CoV-2 infection. The vaccine comprises a nucleic acid sequence as shown in SEQ ID NO: 1. According to a plurality of embodiments of the invention, the S protein nucleic acid sequence contained in the vaccine is easy to express in human cells, and generation of more S proteins can be induced, so the vaccine is expected to be used as a recombinant virus vaccine for preventing SARS-CoV-2 infection. According to a plurality of embodiments of the invention, the vaccine has good security.
Owner:GUANGZHOU N BIOMED LTD
Reverse genetic operation system of New castle disease LaSota vaccine strain and its applciation
The present invention is reverse genetic operation system of Newcastle disease Lasota low virulent vaccine strain and its application. The system includes one transcription plasmid including the genome cDNA sequence of the low virulent vaccine strain; one or several transcription aiding plasmids including the cDNA sequence coding the nucleoprotein of the low virulent vaccine strain, the cDNA sequence coding the phosphoprotein of the low virulent vaccine strain and the cDNA sequence coding the large polymerase protein of the low virulent vaccine strain; and the host cell of the Newcastle disease Lasota low virulent vaccine strain. Wild viral strain is obtained by means of the reverse genetic operation system. The present invention lays firm foundation for further development of Newcastle disease virus live carrier vaccine and Newcastle disease virus related research.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
HEK (human embryonic kidney) 293 cell line applicable to serum-free culture and application thereof
InactiveCN102604889AMicroorganism based processesViruses/bacteriophagesHEK 293 cellsVirulent characteristics
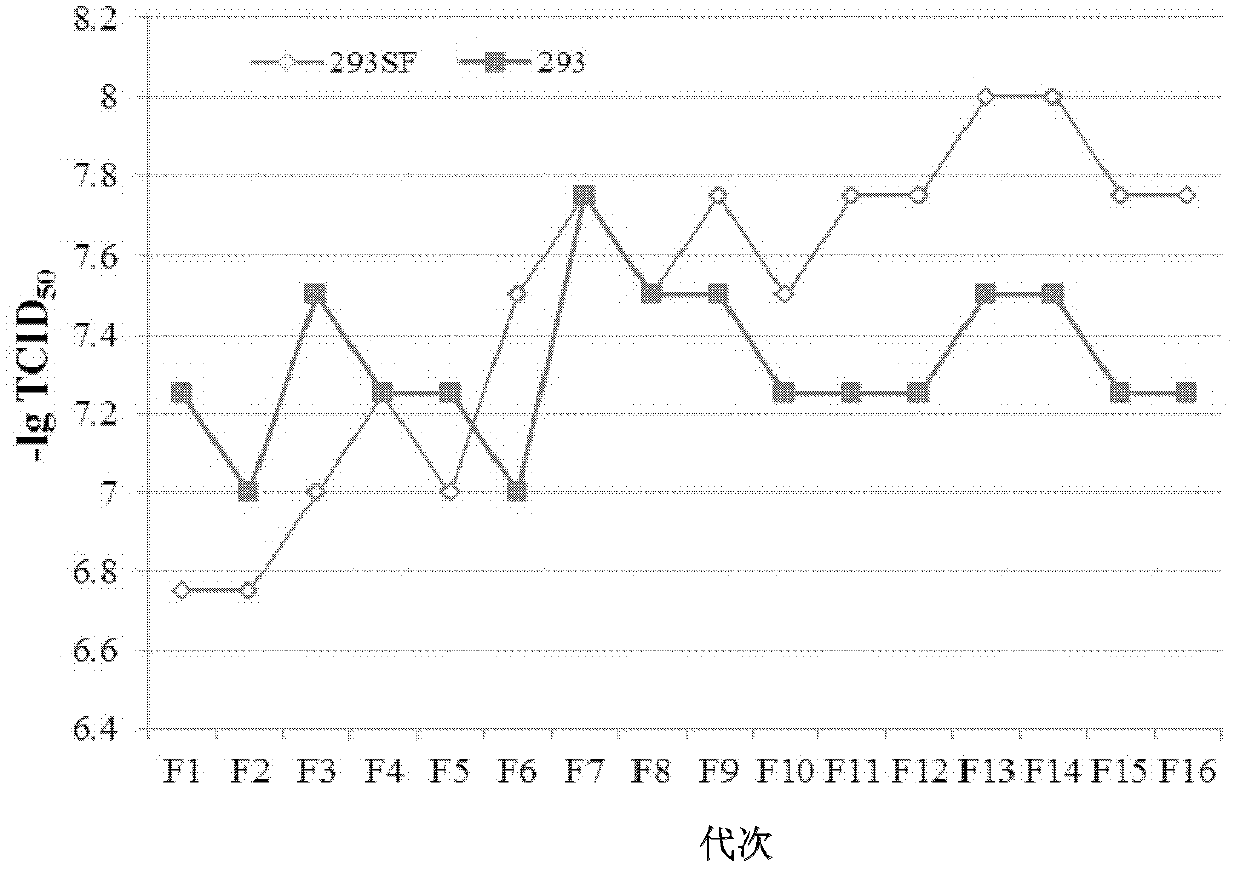
The invention discloses an HEK (human embryonic kidney) 293 cell line applicable to serum-free culture and an application thereof. According to the invention, the HEK 293 cell (293SF) applicable to serum-free culture is obtained by adopting a culture solution progressive substitution method, wherein the preservation number of the HEK 293 cell is CGMCC (China General Microbiological Culture Collection Center) No.5824. Detection shows that the 293SF cell has stable adenovirus proliferation capacity and foreign protein expressing ability; average lesion time is 97 hours and is reduced by 12.9% compared with the HEK 293 cell; virus virulence reaches up to 107.48TCID50 / mL and is improved by 43.3% compared with the HEK293 cell; and stability is good while generation number is improved, and the state and susceptibility of the 293SF cell are not changed after the 293SF cell is continuous passage is carried out for sixteen times. The cell line disclosed by the invention can be used for serum-free production of an adenovirus vector vaccine.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Disruption of programmed death 1 (pd-1) ligand to adjuvant adeno-associated virus vector vaccines
InactiveUS20100035973A1Enhance immune responseImprove efficiencyPeptide/protein ingredientsGenetic material ingredientsVector vaccineProgrammed death
The invention provides for methods of modulating an immune response against a therapeutic polypeptide or an antigenic polypeptide delivered via rAAV comprising administering a modulator of programmed death-1 (PD-1) signaling.
Owner:NATIONWIDE CHILDRENS HOSPITAL
SARS vaccine of adenovirus carrier and preparation method, application of coronavirus S gene
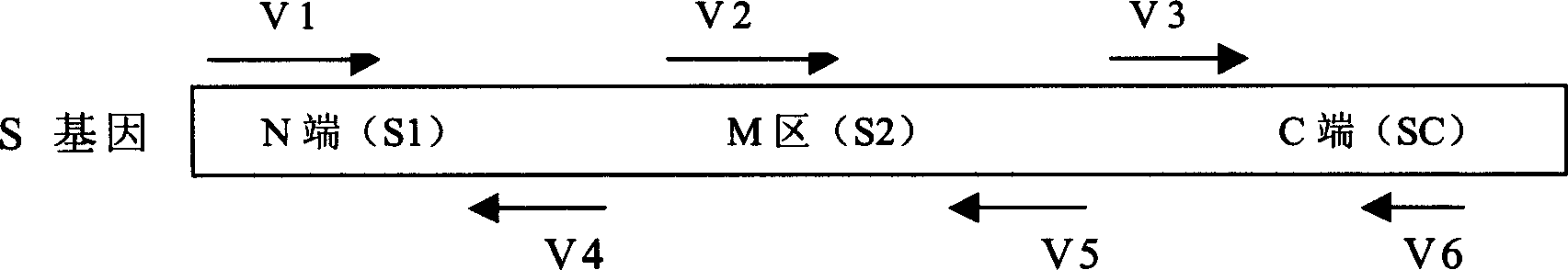
InactiveCN1562365AEffective generationEffective induction ofGenetic material ingredientsAntiviralsGenetic engineeringOrganism
The invention pertains to biological genetic engineering field, specificly relating to SARS vaccine of adenovirus carrier and preparation method, application of related coronavirus S gene in preparation of the SARS vaccine for preventing SARS. Through a bioengineering means, the related coronary virus S gene and a defective adenovirus are recombined, which makes protective immunogen protein or polypeptide expressing in it. A genic vaccine that can arouse the mucosal immunogenicity is produced through amplifying training, purification, and preparation, which induces a immunity reaction in the respiratory mucosa, makes the organism produce the corresponding antibody, and prevents virus from infection. Compared with the traditional inactivated viral particles vaccine, the invention has a high safety; it is convenient to operate; it is not limited by the specific conditions such as intramuscular injection; and it has an extensive clinical application prospect.
Owner:SUN YAT SEN UNIV CANCER CENT
Novel multivalent carrier vaccine for shrimp and application thereof
ActiveCN102895677AHigh temperature resistanceActual production application development valueAntibacterial agentsBacteriaWhite spot syndromeVector vaccine
The invention relates to a novel multivalent carrier vaccine for a shrimp. The vaccine is characterized by comprising a bacillus subtilis recombination strain obtained by a genetic engineering technique, the strain belongs to the category of bacillus subtilis HT5303 and is preserved in China Center for Type Culture Collection, and the preservation number is CCTCC NO.: M 2012395. When spores are formed by the bacillus subtilis vaccine strain, white spot syndrome virus VP281 protein can be demonstrated on the surfaces of the spores, and fusion protein of cell-penetrating peptides, white spot syndrome virus VP19 and vibrio harveyi 3-glyceraldehyde-phosphate dehydrogenase (GAPDH) can be secreted and expressed when vegetative cells are formed. Compared with other vaccines, the multivalent carrier vaccine has the advantages of cross protection and long protection period. Moreover, the vaccine can be added into feeds and has the advantages of resistance against high-temperature setting of feeds and broad prospects for commercial development.
Owner:马悦 +1
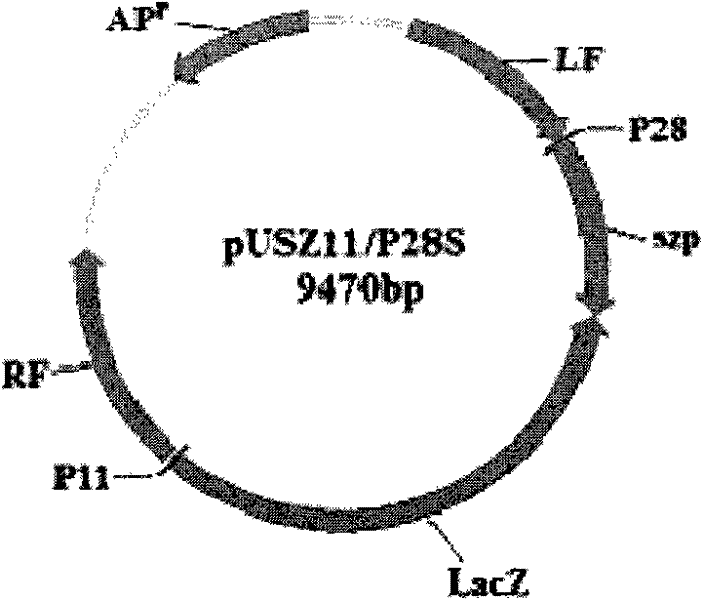
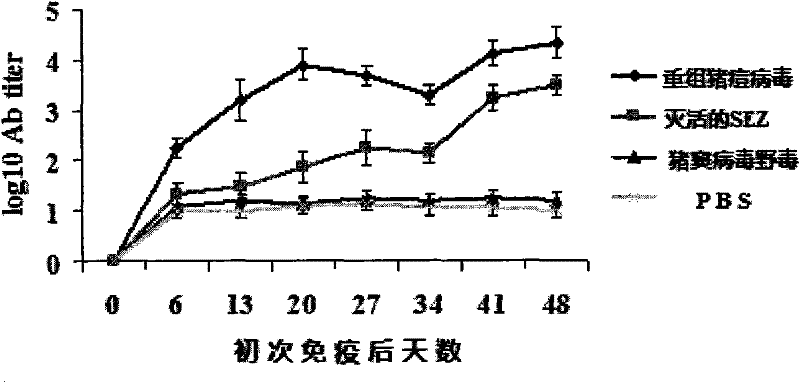
Recombinant Swine pox virus (SPV) vector vaccine for the expression of Streptococcus equi subsp zooepidemicus (SEZ) M-like protein (SzP)
InactiveCN102198268ANot pathogenicEasy Security EvaluationAntibacterial agentsBacterial antigen ingredientsProtein targetAdjuvant
The invention belongs to the field of biological pharmacy. The invention provides a recombinant vaccine, comprising a SPV and one pharmaceutically acceptable vector and / or adjuvant or a plurality of such vectors and / or adjuvants. The SPV comprises an SPV vector and the encoding genes of SzP. The recombinant SPV vaccine provided in the invention can proliferate in large quantities in immune animalbodies, express target protein SzP and induce the generation of high titer antibodies in animal bodies, and exerts a good protective effect on immune animals.
Owner:NANJING AGRICULTURAL UNIVERSITY
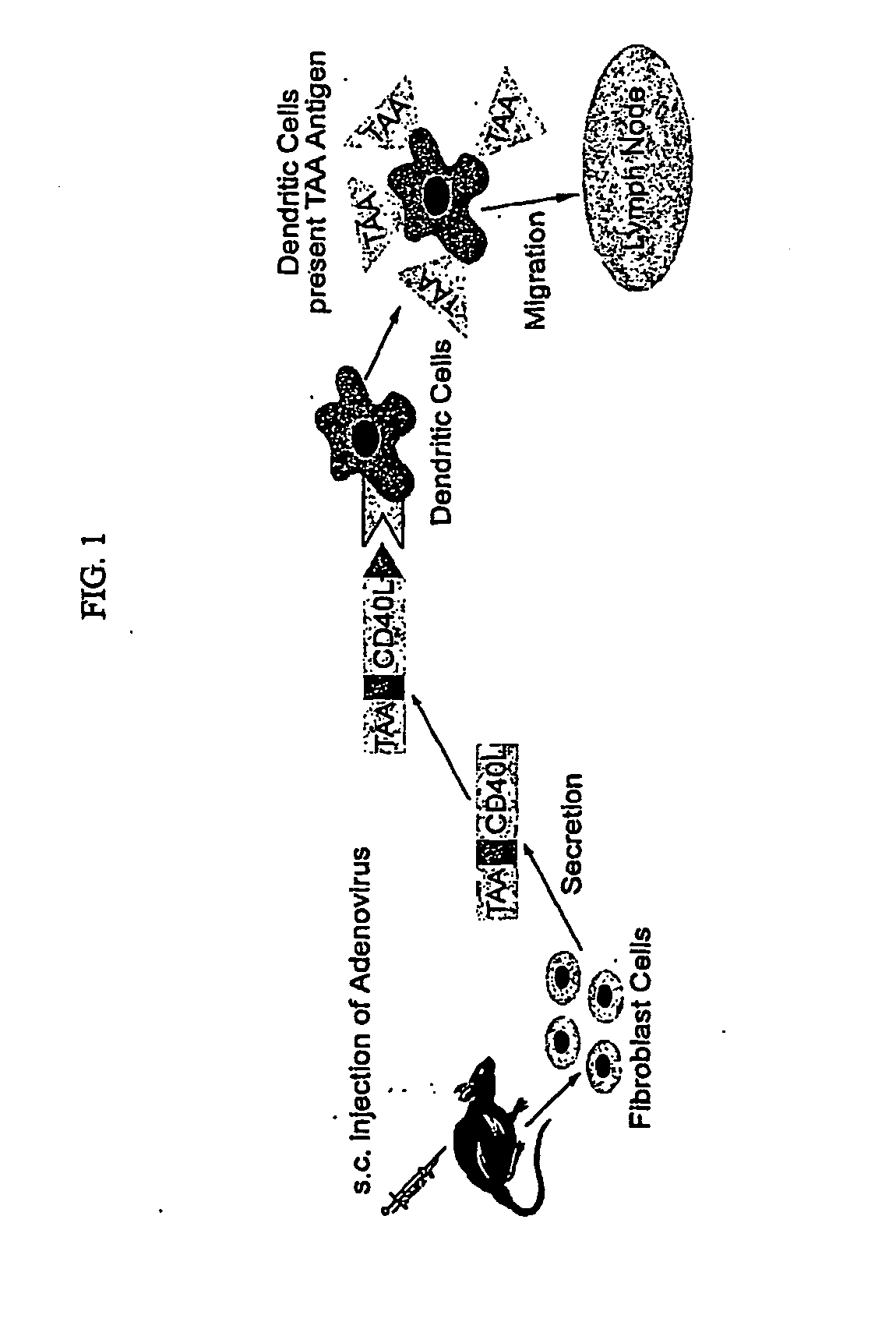
Adenoviral Vector Vaccine
ActiveUS20070269409A1Enhance immune responseLong-lasting immunityVirusesAntibody mimetics/scaffoldsHuman papilloma virus infectionVector vaccine
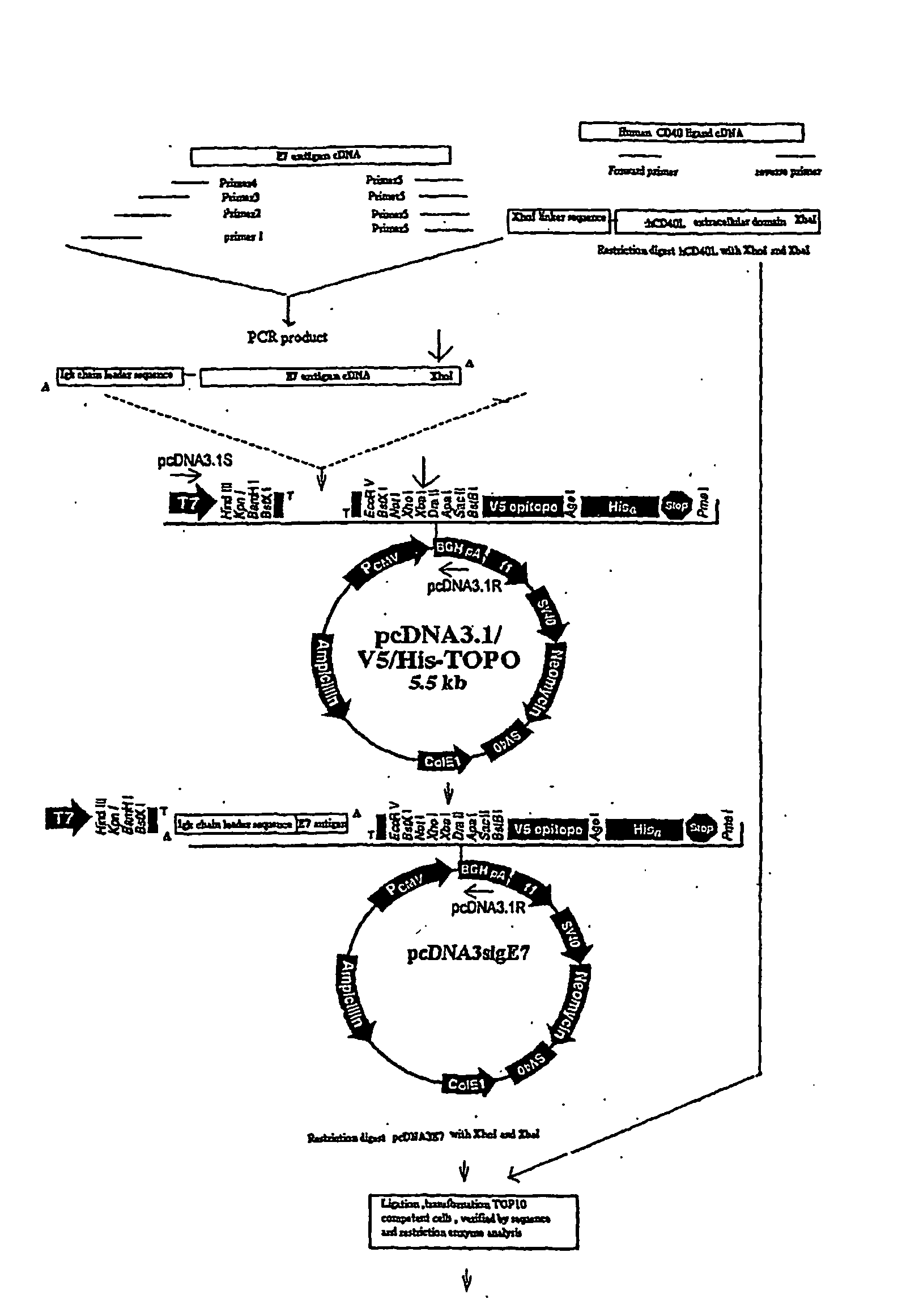
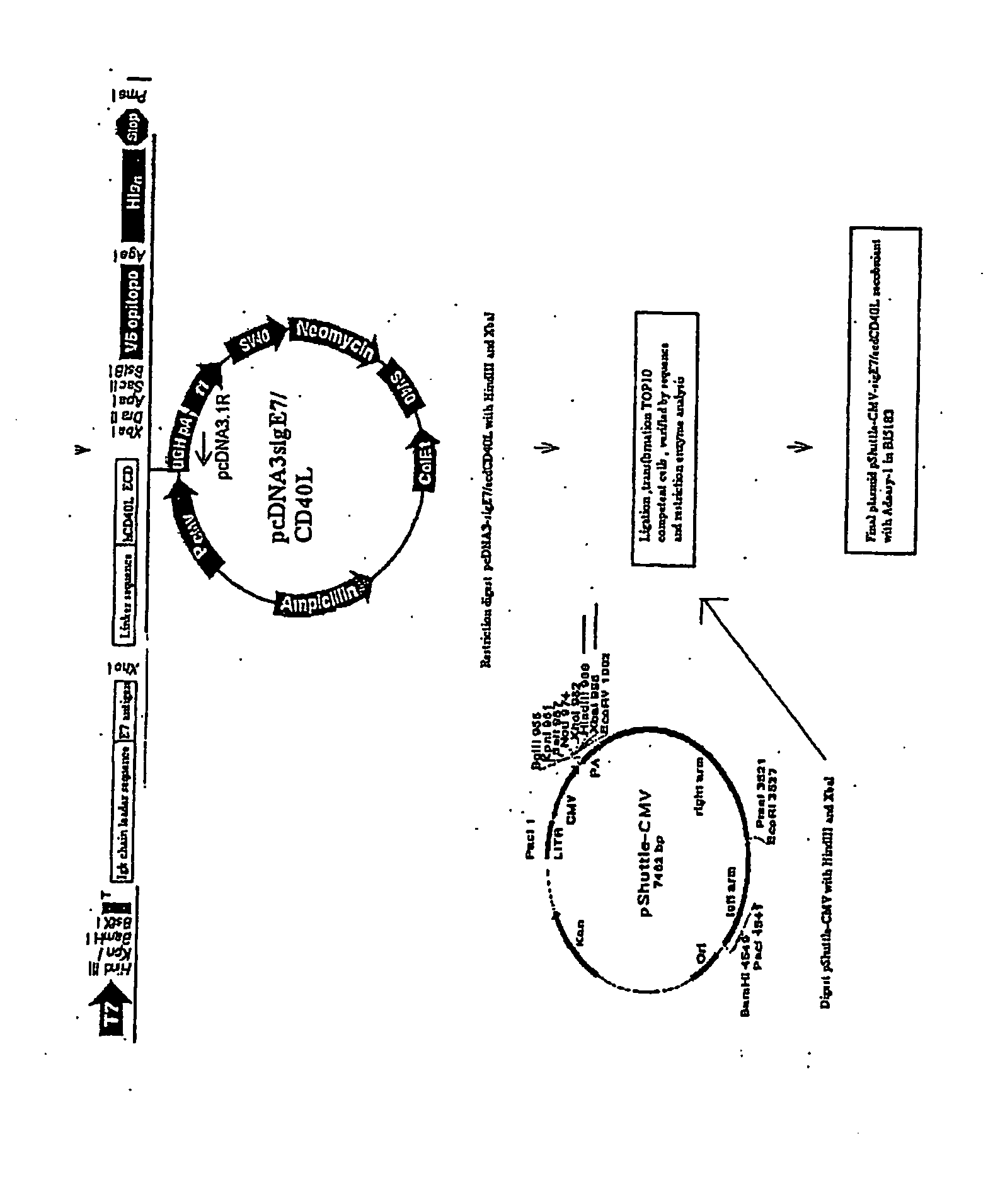
Provided are adenoviral vectors for generating an immune response to antigen. The vectors comprise a transcription unit encoding a secretable polypeptide, the polypeptide comprising a secretory signal sequence upstream of a tumor antigen upstream of CD40 ligand, which is missing all or substantially all of the transmembrane domain rendering CD40L secretable. Also provided are methods of generating an immune response against cells expressing a tumor antigen by administering an effective amount of the invention vector. Further provided are methods of generating an immune response against cancer expressing a tumor antigen in an individual by administering an effective amount of the invention vector. Still further provided are methods of generating immunity to infection by human papilloma virus (HPV) by administering an effective amount of the invention vector which enocodes the E6 or E7 protein of HPV. The immunity generated is long term.
Owner:VAXUM
Adenoviral vector-based vaccines
InactiveUS20060286121A1Increase capacityImprove stabilityAntibody mimetics/scaffoldsViral antigen ingredientsHeterologousAntigen
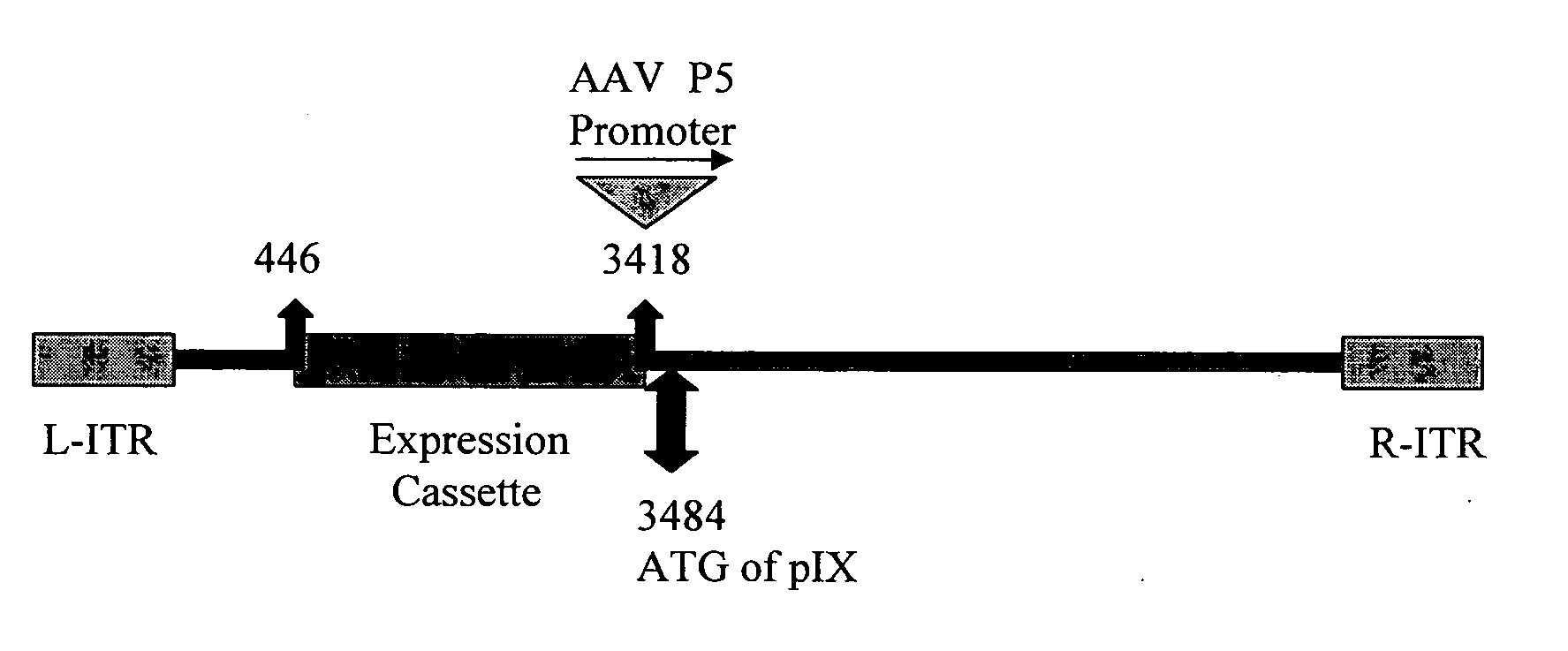
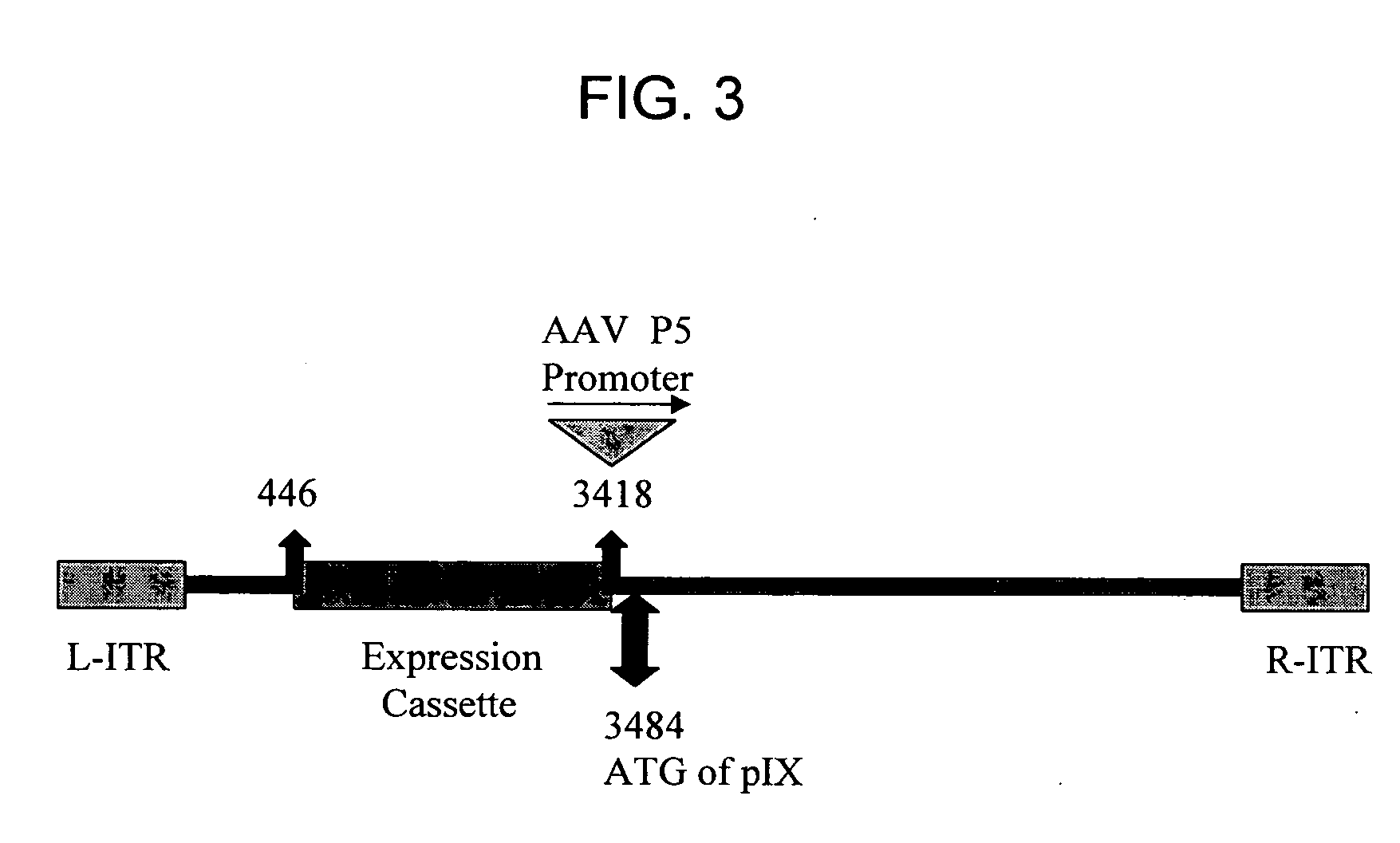
The invention provides a method of inducing an immune response in a mammal. The method comprises administering to the mammal a non-subgroup C adenoviral vector comprising an adenoviral fiber protein having an amino acid sequence comprising about 80% or more identity to an amino acid sequence encoding a subgroup C adenoviral fiber protein. The adenoviral vector further comprises a nucleic acid sequence encoding an antigen which is expressed in the mammal to induce an immune response. The invention further comprises a method of producing an adenoviral vector, and a composition comprising a serotype 41 or a serotype 35 adenoviral vector and a carrier. The invention also provides an adenoviral vector comprising a nucleic acid sequence encoding an adenoviral pIX protein operably linked to a heterologous expression control sequence, as well as a method of enhancing the stability and / or packaging capacity of an adenoviral vector.
Owner:UNITED STATES OF AMERICA +1
Baculovirus vector vaccine
InactiveUS20040071733A1Good effectAvoid damageSsRNA viruses negative-senseViral antigen ingredientsAntigenVector vaccine
It is intended to provide virus vector vaccine preparations containing as the main component a virus vector with the use of a virus showing no pathogenicity on humans and being free from a risk of the re-acquisition of pathogenicity. Namely, vaccine preparations containing as the main component a baculovirus having a gene encoding an antigen integrated thereinto are provided.
Owner:HISAMITSU PHARM CO INC
Angiotensin II receptor 1 type polypeptide-vector vaccine and application thereof
ActiveCN102247604ABlock agonistic responseLower blood pressurePharmaceutical non-active ingredientsAntibody medical ingredientsAngiotensin receptorVector vaccine
The invention discloses an angiotensin II receptor 1 type polypeptide-vector vaccine and an application thereof, belonging to the field of biotechnology drugs and biologic therapeutics. An angiotensin II receptor 1 type immunogenic peptide containing a first connection locus, a derivant thereof and one or more of carriers are preferably coupled and connected with recombined Qbeta-2aa bacteriophage viruslike particle protein to form an ordered and repeated polypeptide-carrier vaccine. The invention also discloses an application of the vaccine on the aspect of treating primary hypertension. Thevaccine can generate one section of efficient specific immunogenic peptide resisting human angiotensin II receptor 1 type and the antibody of the derivant thereof. RAS (Renin-Angiotensin System) can be effectively prevented from activating, the blood pressure of spontaneously hypertensive rats can be obviously lowered, and the vector vaccine has good function on protecting target organs.
Owner:WUHAN HUAJIYUAN BIOTECH DEV
Optimized enterotoxigenic escherichia coli-producing polyvalent antigen gene sequence and application thereof in preventing weaned piglet diarrhea
InactiveCN104593397AFull protective responseHigh expressionAntibacterial agentsBacterial antigen ingredientsEscherichia coliInclusion bodies
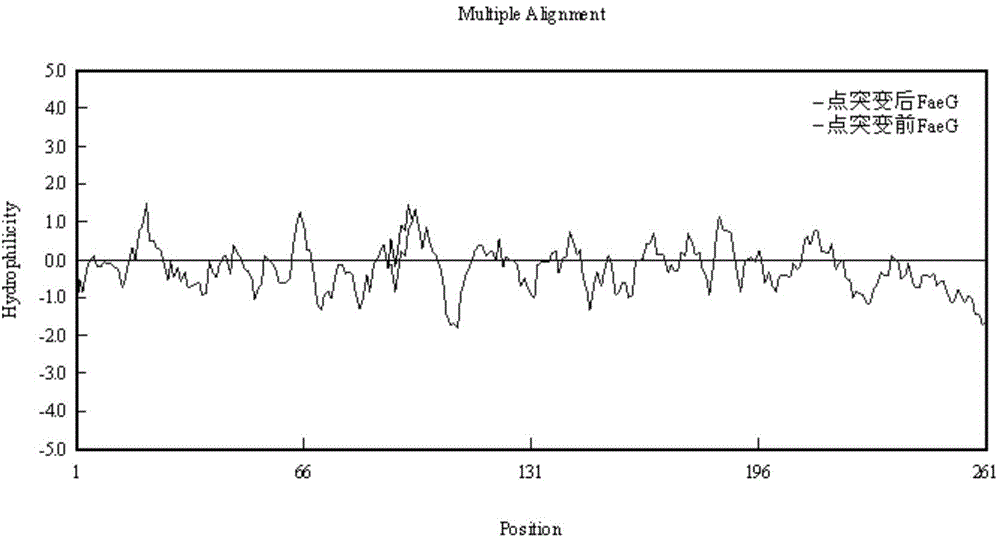
The invention discloses an optimized enterotoxigenic escherichia coli (ETEC)-producing polyvalent antigen gene sequence and the application thereof in preventing weaned piglet diarrhea, and belongs to the technical field of biology. The polyvalent antigen gene sequence is synthesized by a large fragment according to the preference of the lactococcus lactis codon, and the synthesized polyvalent antigen gene sequence contains six antigen genes, namely common ETEC dominant serotype F4<+> primary structure protein FaeG causing the weaned piglet diarrhea, the receptor binding domain RBD of F18<+>, and toxins Stx2e and STa mutant, LTB subunit and STb, and molecular peptides CO1 and YadA31 genes targeting the M cells and the intestinal cells, respectively; the genes are connected by GGGGS. The genes are applicable to constructing a lactic acid bacterium living-vector vaccine and remarkably improving the secretory expression quantity of the target protein, and have excellent immunogenicity and protection effect; the genes also are suitable for high-efficiency expression in the escherichia coli; experiments prove that the inclusion body of the genes has excellent immunocompetence and can be taken as the vaccine for preventing the weaned piglets from F4<+> and F18<+> ETEC infection.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
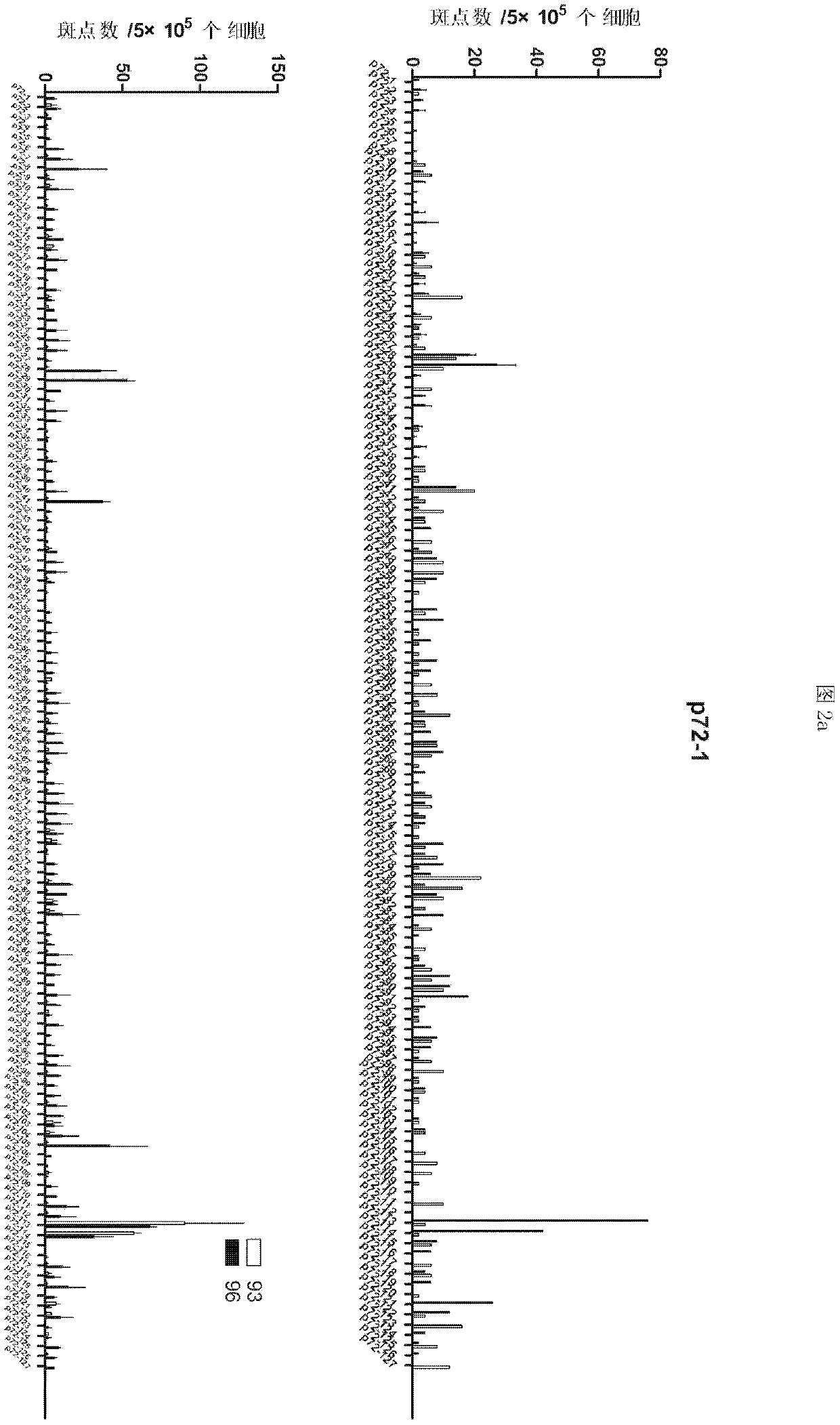
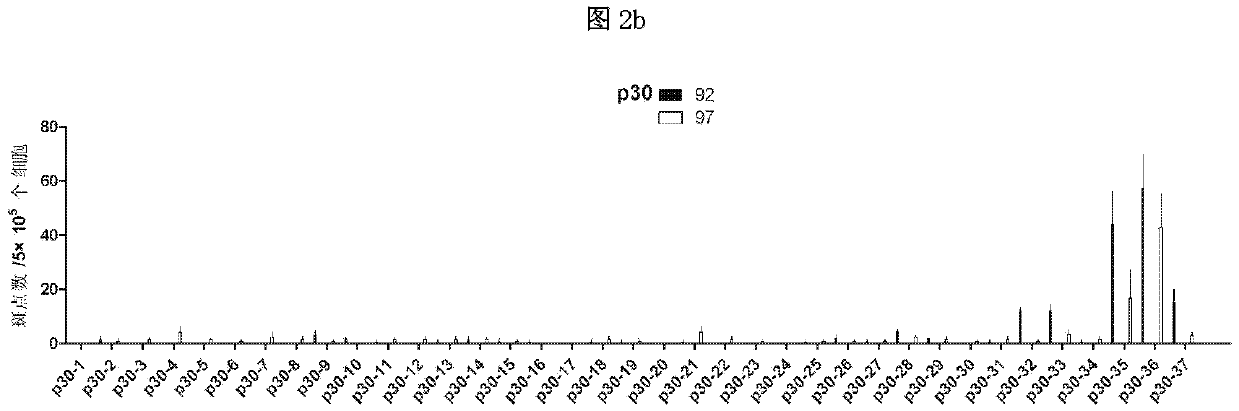
ELISPOT detection methods for screening T cell antigen polypeptides and antigen epitopes of African swine fever virus
According to the invention, the cellular immune antigen dominant epitopes of four important structural proteins p30, p54, p72 and CD2v of ASFV are screened, and the corresponding enzyme-linked immunospot test methods are established. The screened polypeptides have high amino acid sequence conservation, can effectively induce ASFV specific cellular immune response, and can be used as candidate immunogens for ASFV epitope carrier vaccines. The T cell epitope polypeptides of ASFV structural proteins obtained by the invention are screened, and the corresponding ELISPOT detection methods are established. The invention provides a powerful tool for efficacy evaluation of the ASFV candidate vaccines, and also lays a foundation for the research and development of ASFV polypeptide carrier vaccines.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Highly Attenuated Pox Virus Strains, Method for the Production Thereof and the Use Thereof as Paramunity Inducers or For Producing Vector Vaccines
The present invention relates to highly attenuated animal smallpox viral strains and to the use thereof as paramunity inducers or for producing vector vaccines. As a result of the high attenuation process, the claimed animal smallpox strains lose their virulent and immunising properties. The invention also relates to a method for producing such highly attenuated pox virus strains and the use thereof for inducing paramunity, i.e. for activating the non-specific immune system in mammals and humans or for producing vector vaccines for specific immunisation with the positive side-effect of paramunisation. The claimed highly attenuated animal smallpox viruses are thus suitable for preventing and treating diseases associated with an immune deficiency. Preferred embodiments relate to highly attenuated orthopox—(e.g. camel smallpox viruses), leporipox—(e.g. myxoma viruses), avipox-, parapox- and other orthopox viral strains, such as MVA, which have excellent paramunisation properties and in which the immunising properties have been lost.
Owner:MAYR ANTON
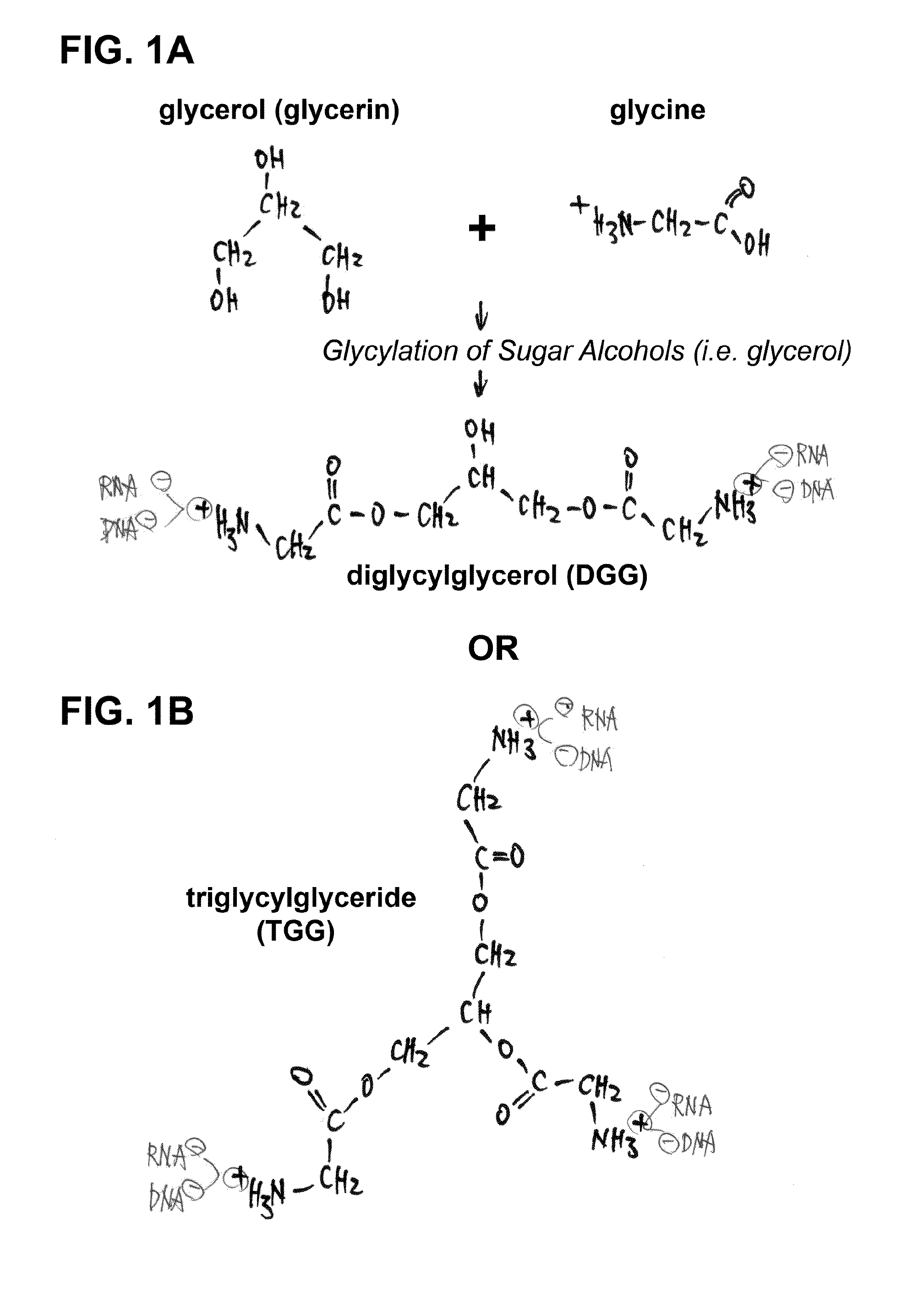
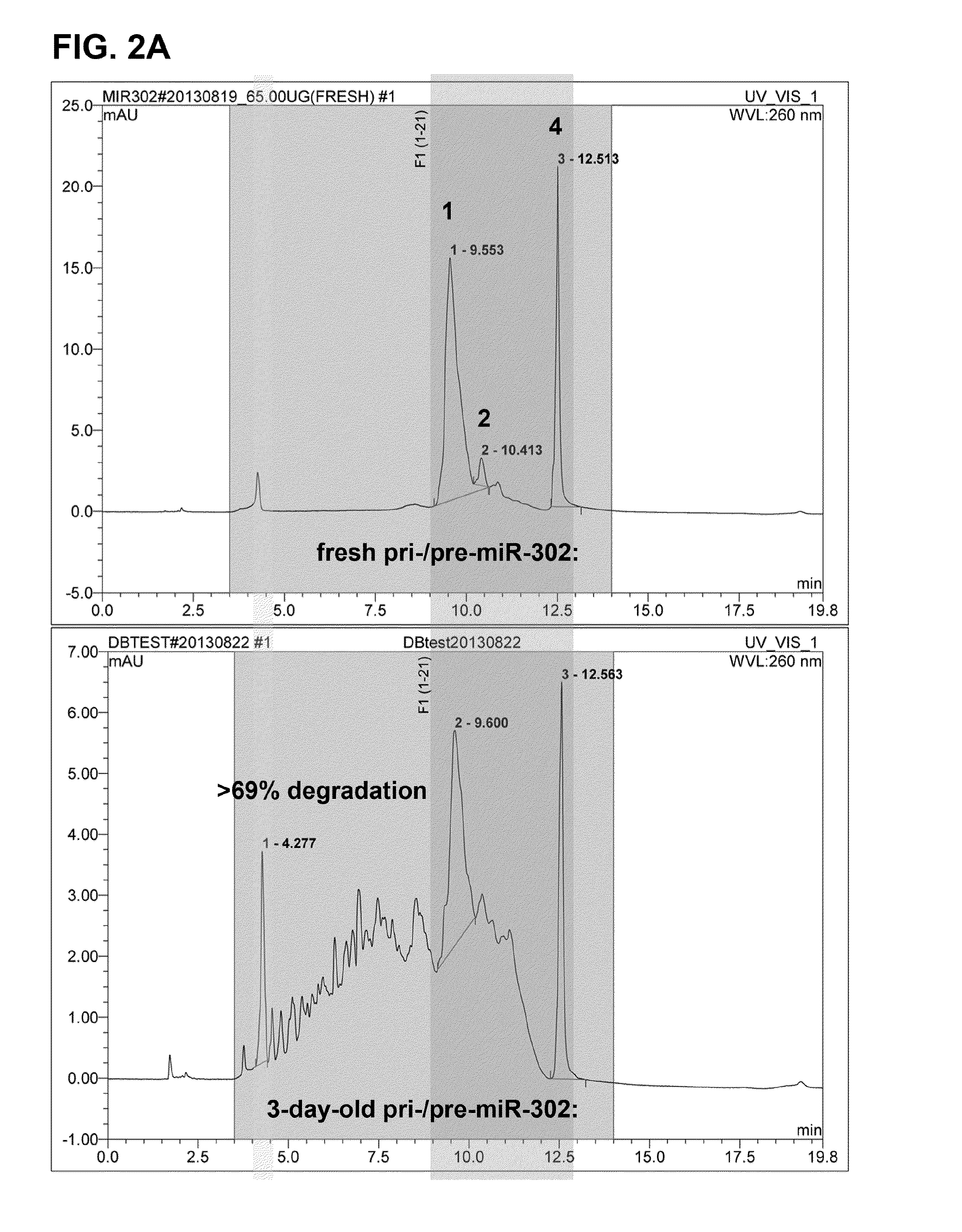
Novel sugar alcohol-based compositions for delivering nucleic acid-based drugs in vivo and in vitro
ActiveUS20140350085A1Reduce deliveryAvoid hydrolysisOrganic active ingredientsSpecial deliveryVector vaccineAlcohol sugars
This invention relates to a composition and its use for formulating nucleic acid-based drugs / vaccines with sugar alcohol compositions into complexes for both in-vitro and in-vivo delivery. Particularly, the present invention includes the ingredients and processes necessary for formulating therapeutic and pharmaceutical nucleic acid compositions, such as miRNA, microRNA precursors, shRNAs, siRNAs, ribozymes, antisense RNAs / DNAs, RNA-DNA hybrids and DNA vectors / vaccines, with glycylated sugar alcohols / sugars into delivery complexes, which can then be absorbed by cells in vivo and in vitro via active endocytosis. Also, the present invention discloses that chemical compounds containing sugar alcohol- and / or sugar-like structures can protect nucleic acids, in particular miRNAs, shRNAs, siRNAs and ribozymes, from degradation in vivo as well as in vitro. Therefore, the present invention is also a formula and method for preserving the structural integrity and functional efficacy of these nucleic acid-based drugs and / or vaccines in vivo and in vitro.
Owner:LIN SHI LUNG +1
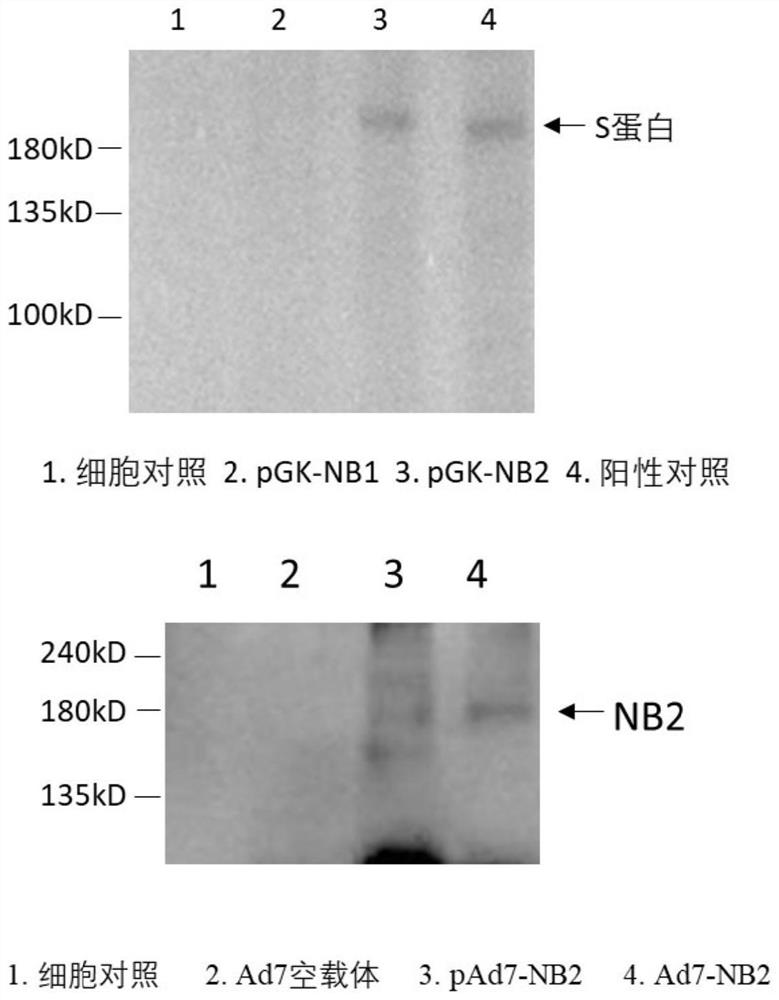
Ad7 vector vaccine for preventing SARS-CoV-2 infection
ActiveCN112206318AImprove securityEasy to useSsRNA viruses positive-senseViral antigen ingredientsVector vaccineHuman cell
The invention discloses an Ad7 vector vaccine and an adenovirus vector vaccine for preventing SARS-CoV-2 infection. The adenovirus vector vaccine comprises an Ad7 vector loaded with a nucleic acid sequence shown in SEQ ID NO: 1. In some embodiments, the adenovirus vector vaccine has better safety and are convenient to use. Experiments show that more S proteins can be generated in human cells. Theadenovirus vector vaccine is expected to be developed into the Ad7 vector vaccine for preventing SARS-CoV-2 infection. In some embodiments, the adenovirus vector vaccine can be used in combination with other vaccines, can also be used as a therapeutic vaccine for new coronal pneumonia, and can be inoculated at the initial stage of infection of a patient to quickly induce immune response in a humanbody so as to achieve a therapeutic effect.
Owner:GUANGZHOU N BIOMED LTD
Expression plasmid, cell strain for packing capacity-increased bibasic adenovirus and application of cell strain
ActiveCN110656090AReduced chance of RCAIncrease insertion volumeVirus peptidesDsDNA virusesVector vaccineCell strain
The invention discloses an expression plasmid, a cell strain for packing capacity-increased bibasic adenovirus and an application of the cell strain. The cell strain is preserved in China Center for Type Culture Collection at May 08, 2019, wherein the preservation number is CCTCC NO:C201996, and the classification designation is human embryo kidney transformant AY293-TD-37. The cell strain comprises E2a-DBP gene and E4-ORF6 gene of adenovirus, can be used for packing the E2a-DBP gene and bibasic adenovirus wit E4 gene deletion to form complete bibasic adenovirus granules having infection. Compared with the bibasic adenovirus, a first generation adenovirus has the advantages that the emergence probability of RCA is greatly reduced, foundation is established for preparation of a live load vaccine, and due to simultaneous deletion of the E2a-DBP gene and the E4 gene, the package capacity is improved once again compared with the bibasic adenovirus with E2a mutation or E4 deletion, the insertion quantity of adenovirus vector exogenous genes is further increased, and the cell strain has important significance for increment of the application level of an adenovirus vector.
Owner:JIAXING ANYU BIOTECH CO LTD
Preparation method of avian influenza virus HA gene recombinant adenovirus
InactiveCN104404005ASolve technical problems with low expression efficiencyViruses/bacteriophagesGenetic engineeringHemagglutininAvian influenza virus
The invention provides a preparation method of avian influenza virus HA gene recombinant adenovirus, which creatively comprises the following steps: carrying out a series of intermediate processes on plasmid pCAGGS, adenovirus shuttle plasmid pShuttle, adenovirus framework plasmid pAdEasy-1 and the like to obtain a gene expression plasmid and other intermediate products, and transfecting the obtained recombinant adenovirus plasmid with 293 cell; and carrying out immunohistochemical screening on the recombinant virus according to the adenovirus-infected cytopathy and specific cells. By using the CAG as the promoter to express the target gene, the method obviously enhances the expression level of the target gene. The hemagglutinin recombinant adenovirus for respectively expressing H5N1 and H9N2 subtype avian influenza viruses provides a virus model for development of the H5 / H9 subtype avian influenza virus bivalent nucleic acid vaccine, and also lays the foundation for development of the AIV (avian influenza virus) adenovirus live vector vaccine.
Owner:TIANJIN RINGPU BIO TECH
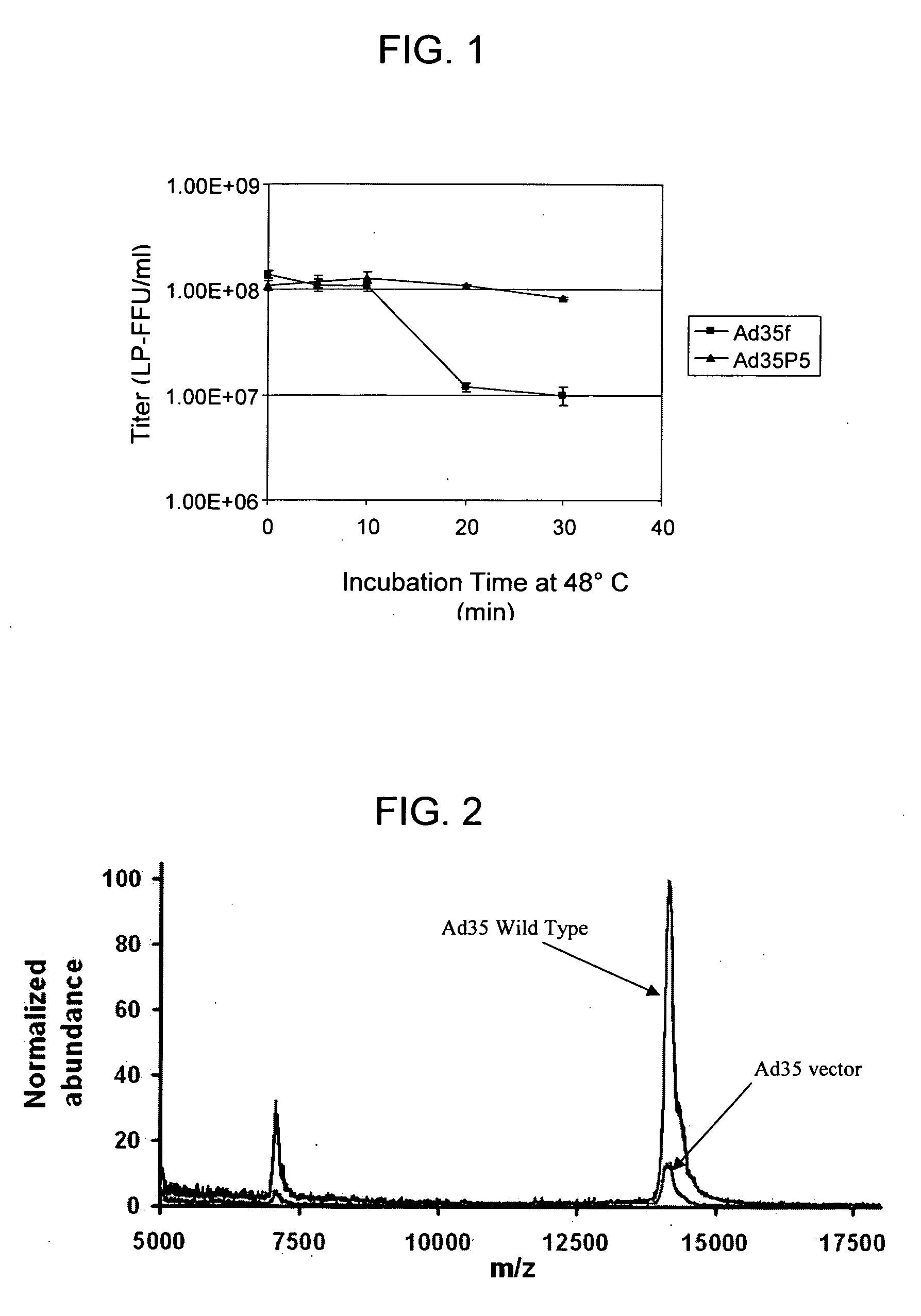
Ad35 vector vaccine for preventing SARS-CoV-2 infection
ActiveCN112220918AImprove securityEasy to useSsRNA viruses positive-senseViral antigen ingredientsVector vaccineHuman cell
The invention discloses an Ad35 vector vaccine for preventing SARS-CoV-2 infection. The Ad35 vector vaccine comprises an Ad35 vector loaded with a nucleic acid sequence as shown in SEQ ID NO: 1. Someembodiments of the invention have good safety and are convenient to use. Experiments show that the Ad35 vector vaccine can generate more S proteins in human cells. The Ad35 vector vaccine is expectedto be developed into a vaccine for preventing SARS-CoV-2 infection. Some examples of the invention can be used in combination with other vaccines, can also be used as a therapeutic vaccine for COVID-19, and can be inoculated at the initial stage of infection of a patient to quickly induce immune response in a human body so as to achieve a therapeutic effect.
Owner:GUANGZHOU N BIOMED LTD
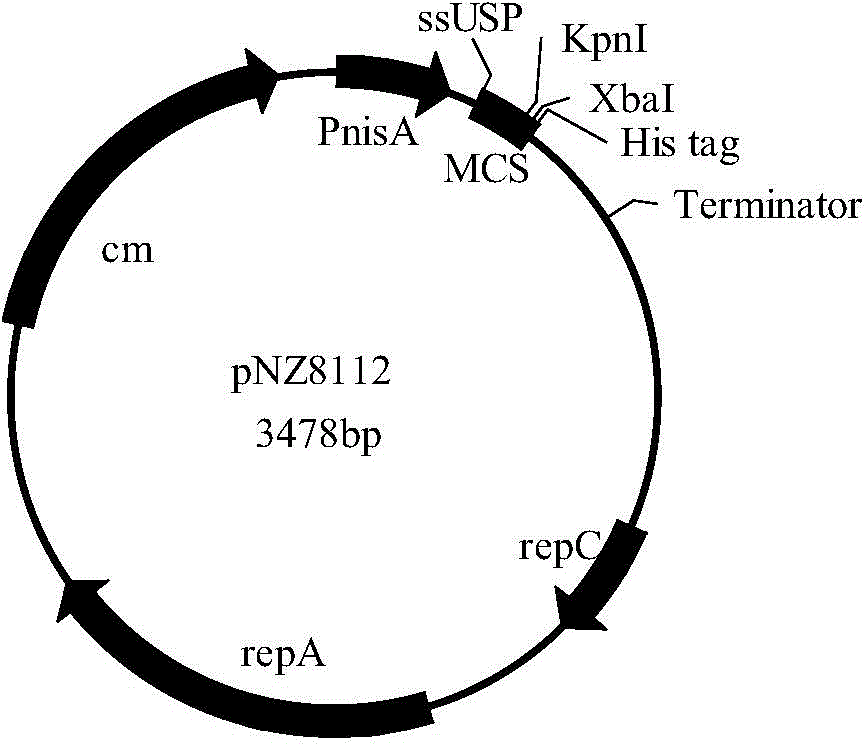
Preparation method and application of recombinant lactococcus lactis
ActiveCN107653260AImprove infection abilityAntibacterial agentsBacterial antigen ingredientsVector vaccineSerotype
The invention relates to a preparation method and application of a recombinant lactococcus lactis. The invention is characterized in that the surface immunogenic protein (Sip) of tilapia-source streptococcus agalactiae is intracellularly or secretorily expressed in lactococcus lactis cells, vectors which are used for the recombinant expression of the Sip are pNZ8124 and pNZ8148 respectively, signal peptide segments are removed from Sip gene segments inserted into the vectors, and histidine sequence tags are added to the Sip gene segments, the recombinant protein is induced to be expressed by Nisin, the optimum induction condition is 4h of induction by 100ng / mL of Nisin, the optimum oral immunization concentration is 2.24*10<10>CFU / mL, and the oral dosage is 100 mu L. The recombinant lactococcus lactis disclosed by the invention which is applied in lactobacillus living vector vaccines against tilapia streptococcus agalactiae has the advantages of wide serotype coverage, direct oral administration, high safety, easiness in operation, easy large-scale herd immunity, good immune effect and the like.
Owner:PEARL RIVER FISHERY RES INST CHINESE ACAD OF FISHERY SCI
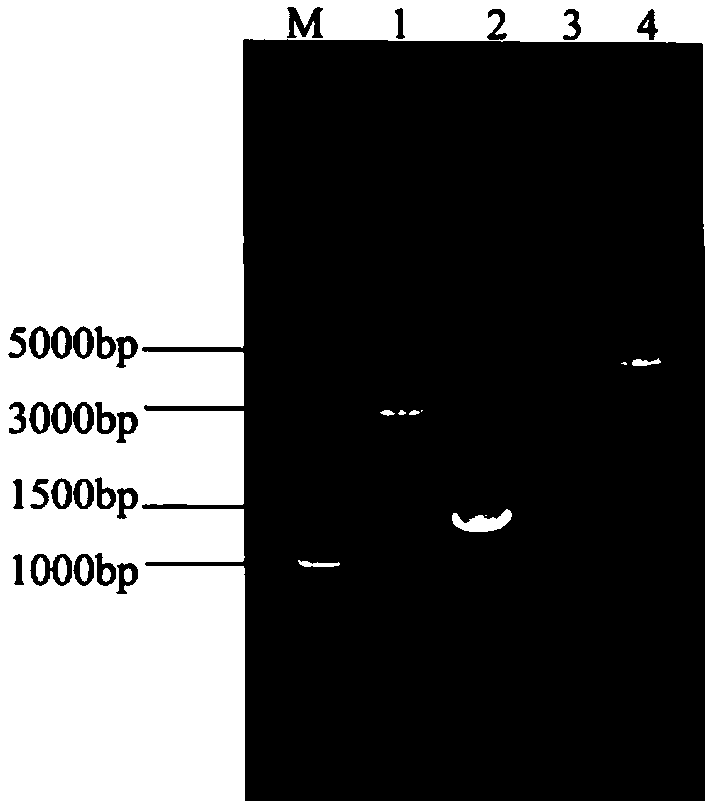
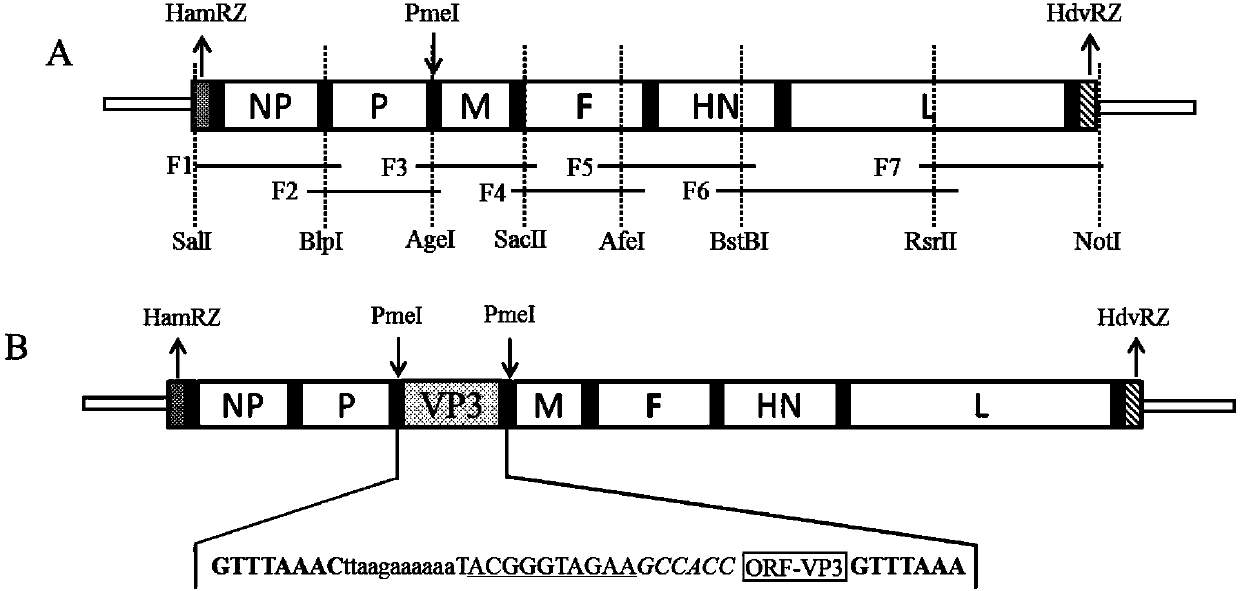
Recombinant Newcastle disease virus for expressing goose parvovirus VP3 genes and construction method thereof
ActiveCN104195116AViral antigen ingredientsMicroorganism based processesF proteinNewcastle disease virus NDV
The invention discloses a recombinant Newcastle disease virus for expressing goose parvovirus VP3 genes and a construction method thereof, belonging to the field of recombinant virus vaccines. The recombinant Newcastle disease virus is a goose isolate, the cleavage site amino acid sequence of F protein of the recombinant Newcastle disease virus is GRQGRL, and the P3 gene is positioned in a noncoding region between the P gene and M gene of Newcastle disease virus. The transcription plasmid pCI-NA-VP3(SEQ ID NO.1) and the transcription helper plasmid pcDNA-N, pcDNA-P and pcDNA-L(SEQ ID NO. 2-4) cotransfect a host cell licensed by application of the Newcastle disease virus to culture a transfected host cell, and the recombinant Newcastle disease virus can be saved from a cell suspension of the transfected host cell. The recombinant Newcastle disease virus for expressing goose parvovirus VP3 genes can be used as a bivalent living-vector vaccine for preventing Newcastle disease virus and goose parvovirus.
Owner:JILIN UNIV
AIDS vaccine based on replicative vaccinia virus vector
The present invention relates to replicative live AIDS carrier vaccine expressing HIV antigen and its use. The vaccine is constructed based on replicative vaccinia virus, such as vaccinia virus Tiantan strain. The replicative live AIDS carrier vaccine can induce high level HIV resisting body fluid and cellular immune response. The present invention provides the carrier for constructing the AIDS vaccine, and also relates to immunizing process with the AIDS vaccine.
Owner:NAT CENT FOR AIDSSTD CONTROL & PREVENTION CHINESE CENT FOR DISEASE CONTROL & PREVENTION
An adenovirus vector vaccine for the prevention of SARS-CoV-2 infection
ActiveCN110974950BAvoid infectionImprove securitySsRNA viruses positive-senseViral antigen ingredientsVector vaccineHuman cell
The invention discloses an adenovirus vector vaccine for preventing SARS-CoV-2 infection. The vaccine comprises a nucleic acid sequence as shown in SEQ ID NO: 1. According to a plurality of embodiments of the invention, the S protein nucleic acid sequence contained in the vaccine is easy to express in human cells, and generation of more S proteins can be induced, so the vaccine is expected to be used as a recombinant virus vaccine for preventing SARS-CoV-2 infection. According to a plurality of embodiments of the invention, the vaccine has good security.
Owner:GUANGZHOU N BIOMED LTD
Dual-host recombination rhabdovirus expression vector and construction method and application thereof
InactiveCN101724651ASave resourcesLow costGenetic material ingredientsGenetic engineeringAgricultural scienceShuttle vector
The invention discloses a dual-host recombination rhabdovirus expression vector and a construction method and application thereof, belonging to the technical field of gene engineering. The dual-host recombination rhabdovirus expression vector is provided with a recognizable Polyhedrin promoter of an insect cell and a recognizable CMV-IE promoter of an animal cell. Dual-host recombination rhabdovirus can be used for preparing a functional protein on the insect cell or can express a target foreign protein on the animal cell and can be used for gene therapy and nonreplication vector vaccine. The construction method of the dual-host recombination rhabdovirus expression vector comprises the following steps of: taking a transfer vector pFas in a Bac-to-Bac expression system as a base; introducing a CMV-IE promoter expression kit on the upstream of the Polyhedrin promoter; constructing the transfer vector; transforming a DH10Bac competent cell; recombining a dual-host Bacmid shuttle vector; and then transfecting insect cells in a logarithmic phase to obtain the dual-host recombination rhabdovirus expression vector.
Owner:HENAN AGRICULTURAL UNIVERSITY
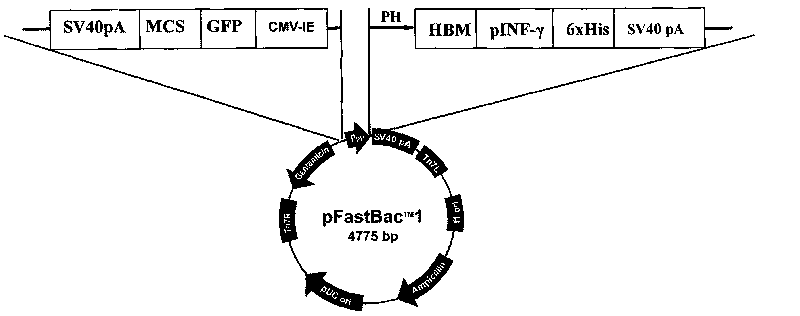
Preparation method and application of newcastle disease virus living-vector vaccine through gene recombination of canine distemper attenuated vaccine strains F and H
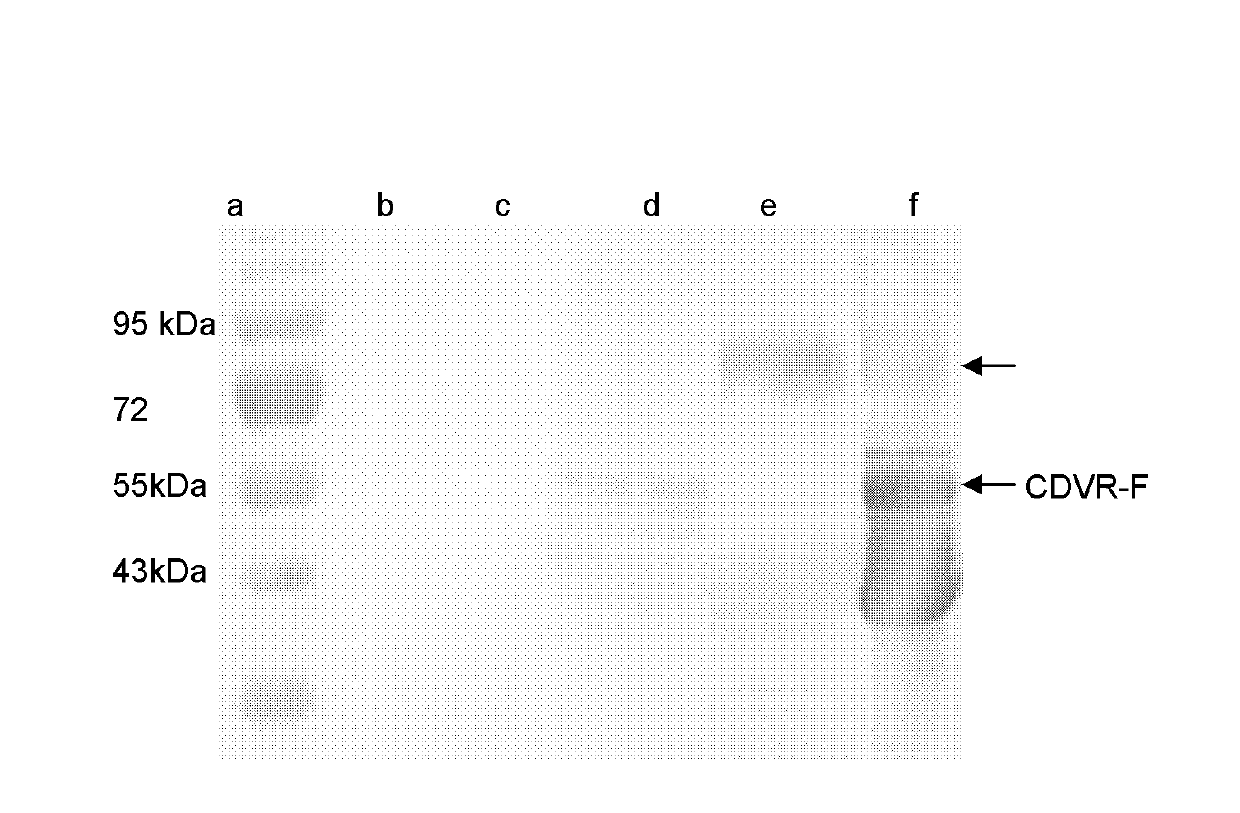
InactiveCN102816741AHigh growth titerReduce pathogenicityViral antigen ingredientsMicroorganism based processesCanine distemper virus CDVVector vaccine
The invention relates to a recombination newcastle disease LaSota attenuated vaccine for expressing canine distemper virus fusion protein (F) or canine distemper virus hemagglutinin protein (H). Particularly, the recombination newcastle disease LaSota attenuated vaccine is rLa-CDVR-F or rLa-CDVR-H. The invention further discloses a method for preparing the recombination newcastle disease LaSota attenuated vaccine and application of the recombination newcastle disease LaSota attenuated vaccine in preparation of vaccines / kits.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI +2
Respiratory syncytial virus pre-fusion F protein and application thereof
ActiveCN110054668AStable structureExpression did not decreaseSsRNA viruses negative-senseVirus peptidesF proteinWild type
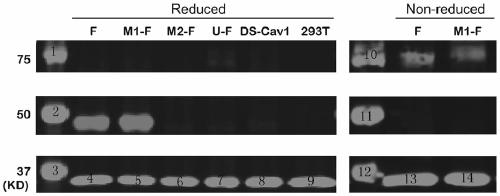
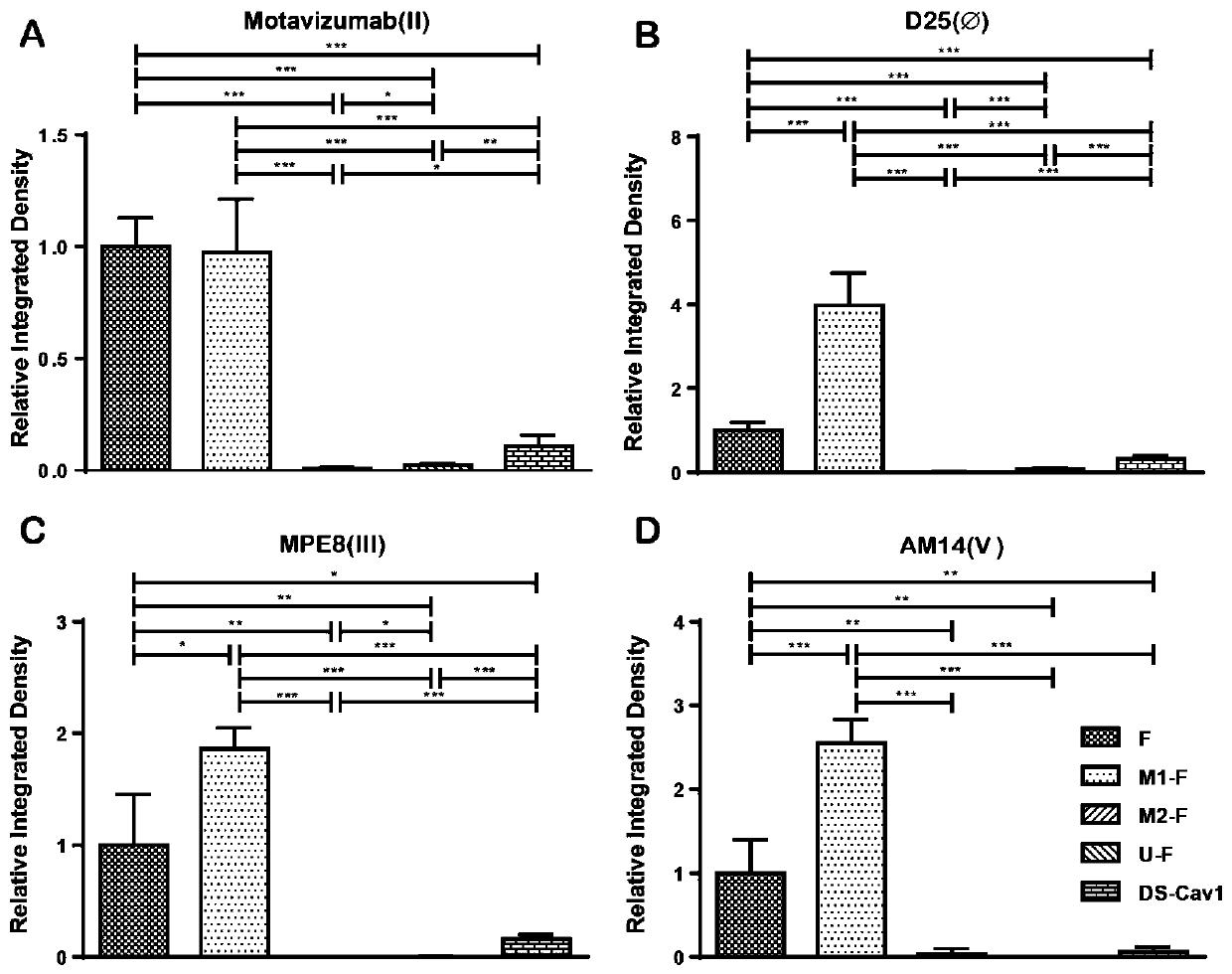
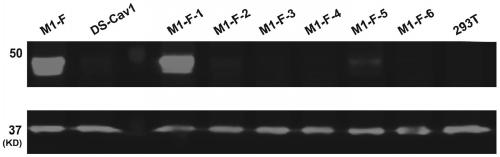
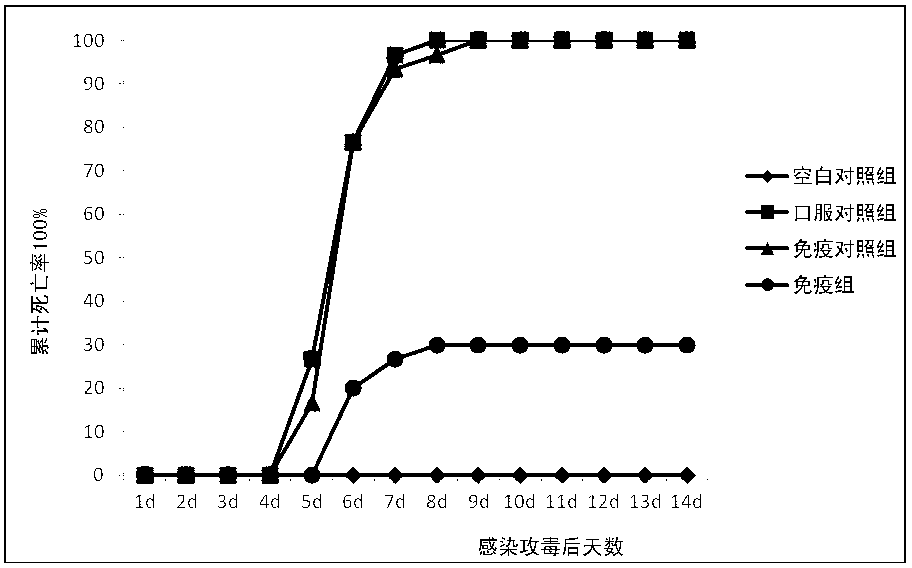
The invention provides a respiratory syncytial virus pre-fusion F protein and application thereof, and belongs to the technical field of respiratory syncytial virus vaccines. The pre-fusion F proteinis formed in the way that the 106th, 108th and 109th amino acids of a wild-type F protein are mutated from Arg to Asn, the 104th amino acid is mutated from Asn to Cys, the 155th amino acid is mutatedfrom Ser to Cys, or the 58th amino acid is mutated from Thr to Cys, and the 190th amino acid is mutated from Ser to Cys. A first furin cleavage site of the mutant wild-type F protein makes the structure of Pre-F more stable, the expression amount is not decreased, and the pre-fusion epitope of M1-F expression in 293T cells is significantly increased. The second mutation of the amino acid site of the F protein forms a disulfide bond, achieves the more stable pre-fusion epitope compared to the wild type, and is suitable for other strains of human RSV. The protein is suitable for various vaccineforms with the RSV F protein as the antigen, such as nucleic acid vaccines, protein vaccines, vector vaccines and recombinant virus particle vaccines.
Owner:BEIJING JIAOTONG UNIV
Biological control method of crucian hemorrhagic disease and application thereof
ActiveCN103272228ARealize multi-potency immune prevention and controlStimulate humoral immune responseAntibacterial agentsGenetic material ingredientsViral diseaseBacterial disease
The invention relates to a biological control method of crucian hemorrhagic disease, which is characterized in that: immunizing and inoculating a multivalent vector vaccine for cultured crucian; The multivalent vector vaccine is obtained from Bacillus subtilis recombination strain by a gene engineering method, and the classification and naming of the recombination strain is Bacillussubtilis HT5304, and the product is preserved in the China center for type culture collection, and the preservation number is CCTCCNo: M2013029. The invention also provides an application of Bacillussubtilis HT5304 in crucian culture and disease control. The biological prevention method of the present invention is used for immunizing and inoculating a multivalent vector vaccine for cultured crucian, thereby realizing multiple-effect immunization control for hemorrhagic disease with crucian viral disease cause and bacterial disease casue.
Owner:马悦 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com