SARS-CoV-2 vaccine and preparation method thereof
A coronavirus, sars-cov-2 technology, applied in the field of vaccines, to achieve the effect of improving immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
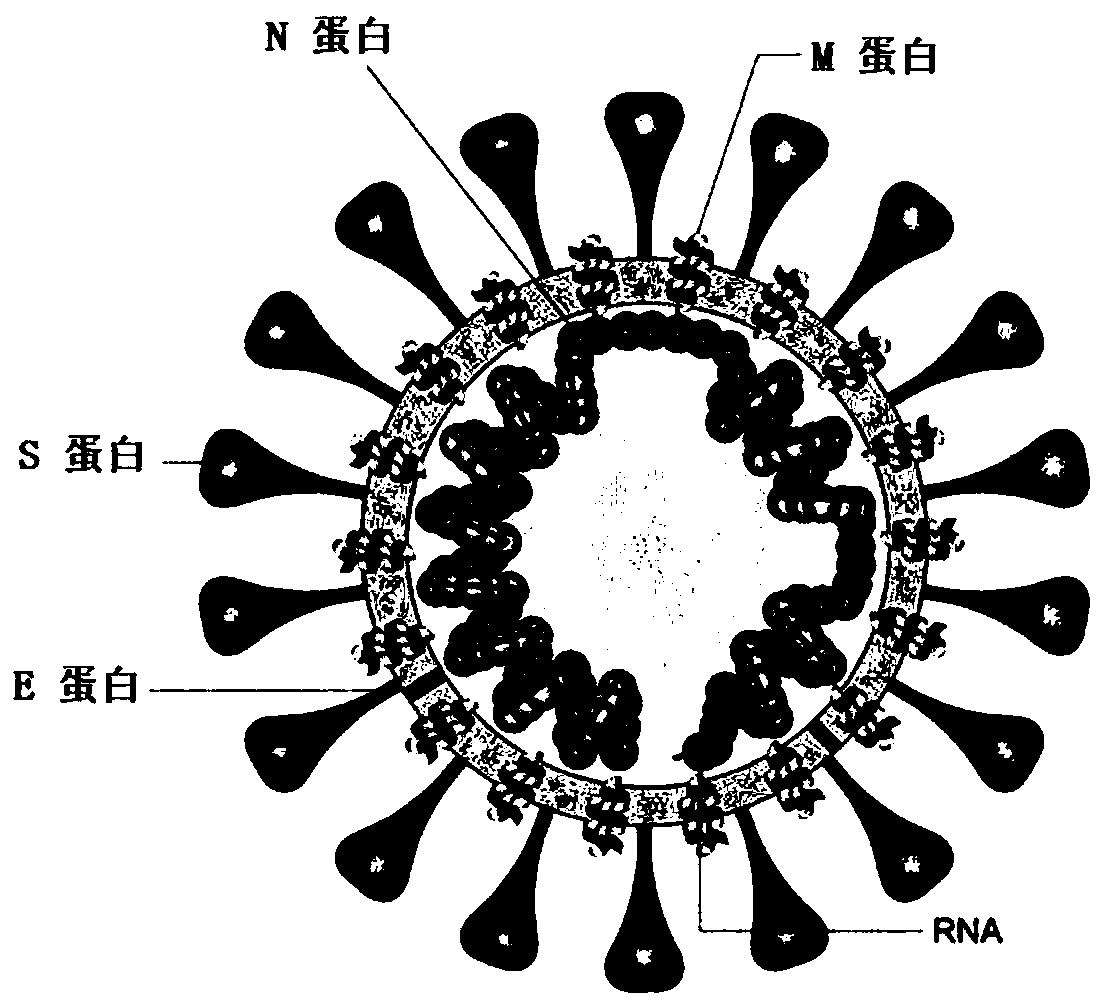
[0072] The construction of the RBD-Linker-CTB fusion gene of embodiment 1 novel coronavirus (SARS-CoV-2)
[0073] The structure of the new coronavirus (SARS-CoV-2) and the composition of the coat protein are as follows: figure 1 shown. The S protein is the most important surface protein of the coronavirus. The S protein contains two subunits: S1 and S2. The receptor binding domain (RBD) is distributed in the S1 area and is responsible for recognizing cell receptors; the S protein can pass through the receptor binding area. Genetic recombination or mutation of (RBD) achieves transmission among different hosts and leads to higher lethality. More critically, coronaviruses recognize host cell receptors through RBD. Studies have shown that RBD is the neutralizing antibody epitope of S protein.
[0074] 1-1 Preparation of RBD-Linker-CTB fusion gene. The RBD-Linker-CTB fusion gene includes codon-optimized novel coronavirus (SARS-CoV-2) RBD gene, Linker and CTB gene. Among them, ...
Embodiment 2
[0085] The construction of the RBD-Linker-CRM197 (A) fusion gene of embodiment 2 novel coronavirus SARS-CoV-2
[0086] The specific construction method refers to Example 1. The RBD-Linker-CRM197(A) fusion gene includes the codon-optimized novel coronavirus (SARS-CoV-2) RBD gene, Linker and CRM197(A) genes.
[0087] Among them, the original sequence of the novel coronavirus (SARS-CoV-2) RBD gene is from NCBI and has been codon-optimized to increase the expression of the target antigen. The specific sequence is shown in SEQ ID NO:1. Wherein, the gene sequence of the Linker is GGGGS, as shown in SEQ ID NO:2. Wherein, CRM197(A) is the A region of CRM197, and the specific sequence is shown in SEQ ID NO:9.
[0088] In the following examples, the fusion genes prepared in Example 1 and Example 2 are collectively referred to as the RBD fusion gene of the novel coronavirus (SARS-CoV-2), referred to as the RBD fusion gene for short.
Embodiment 3
[0089] The development of embodiment 3 RBD-Linker-CTB fusion protein subunit vaccine
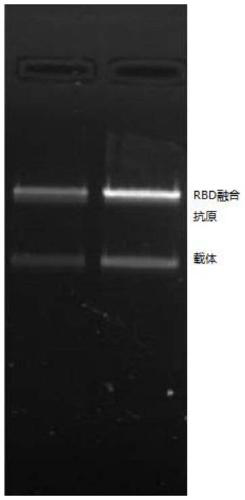
[0090] Transform Escherichia coli BL21 with the RBD-Linker-CTB fusion gene-pET9a expression vector, pick a single clone, recover in AMP-containing LB liquid medium, inoculate 100ml AMP-containing LB liquid medium for amplification, wait until OD 450 =0.5 (or in accordance with 0.4-0.6) Add 0.1 mM IPTG to induce expression. Induced expression results such as image 3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com