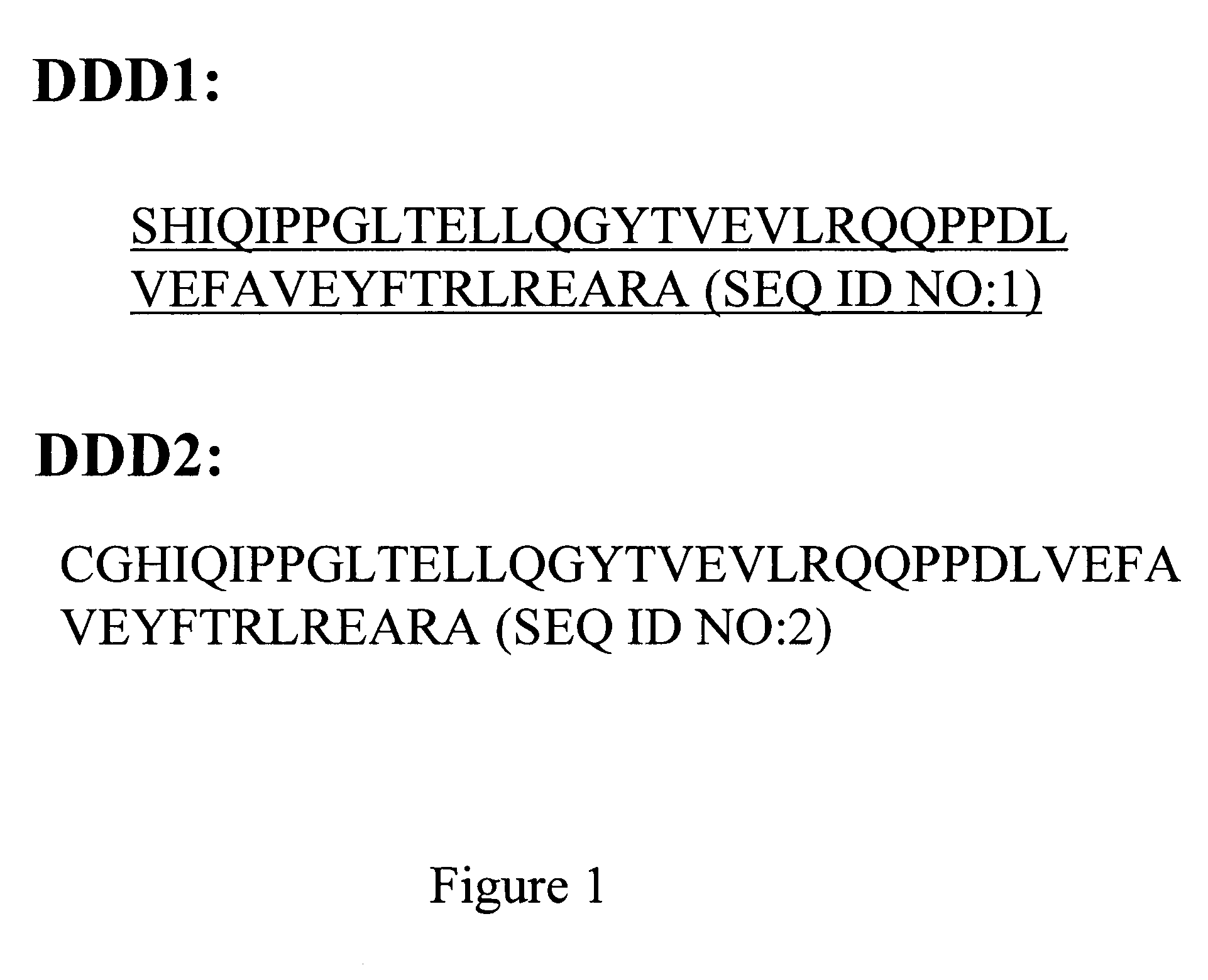
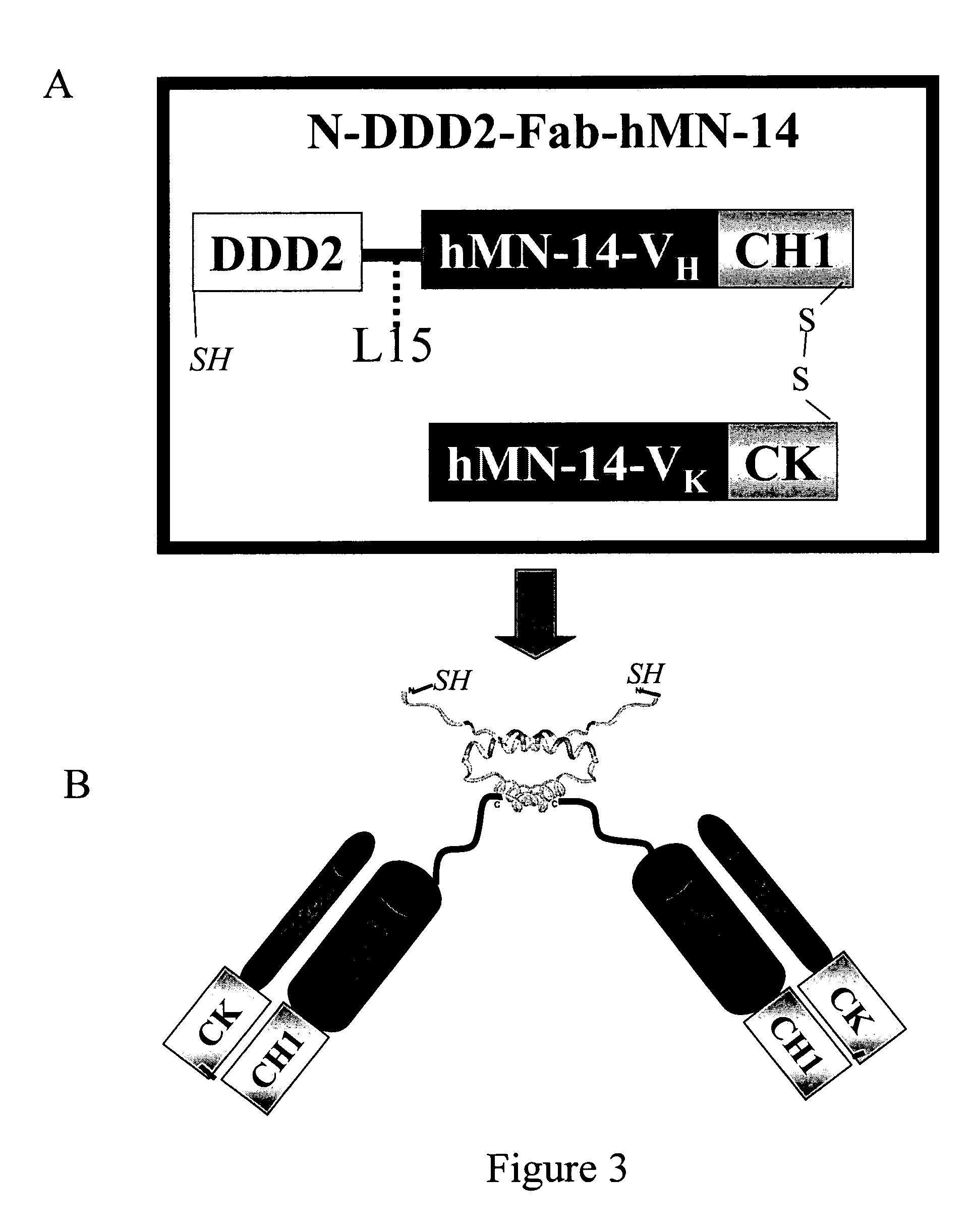
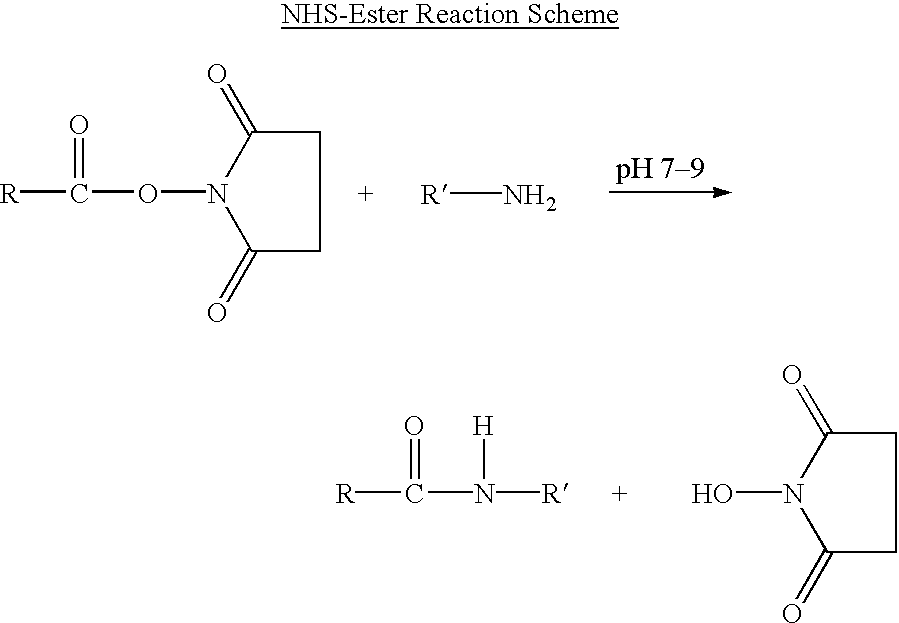
Patents
Literature
2635results about "Carrier-bound antigen/hapten ingredients" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
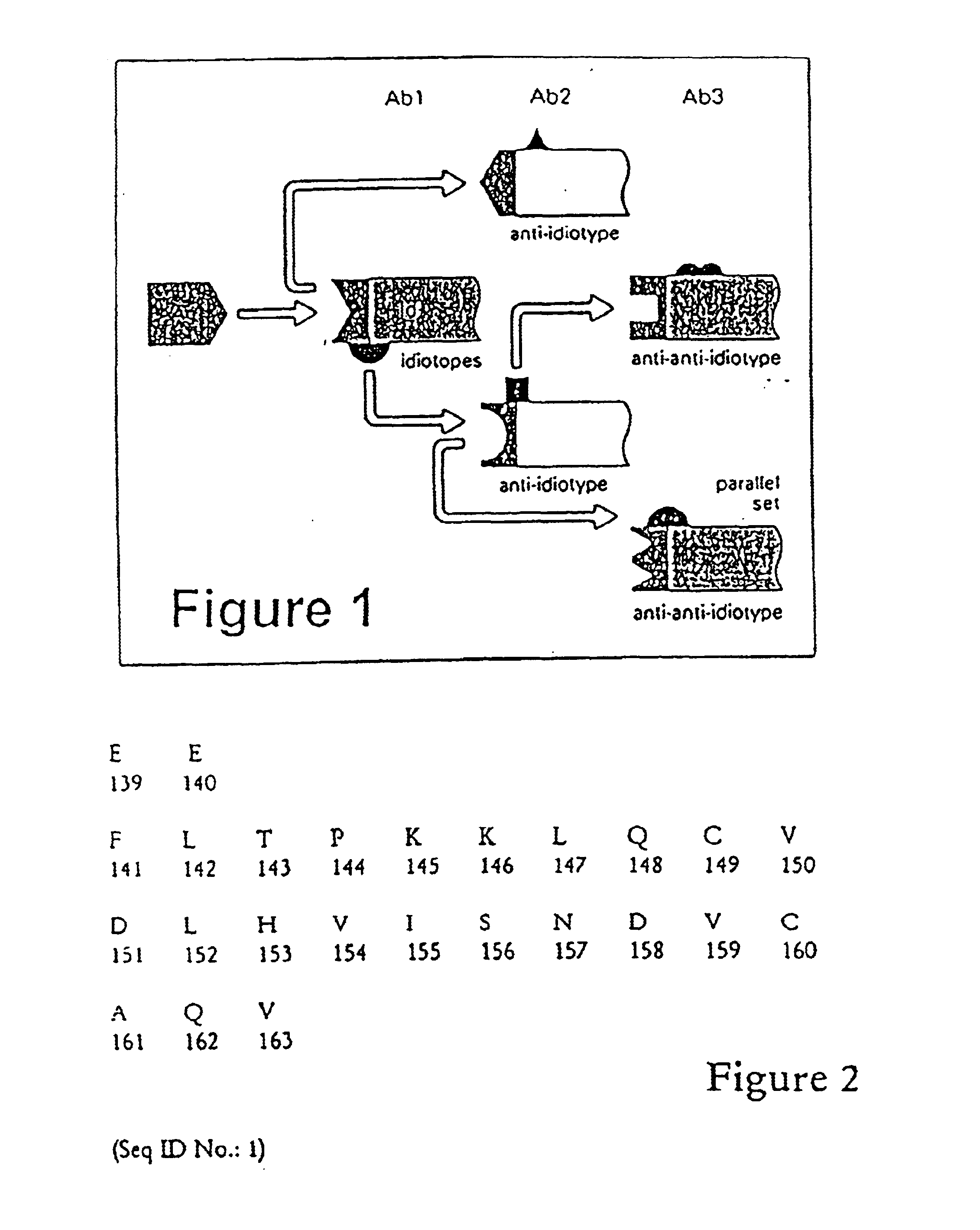
Receptor specific transepithelial transport of therapeutics
InactiveUS6030613AEffective strategyImprove abilitiesPeptide/protein ingredientsAntibody mimetics/scaffoldsAntigenTolerability
The present invention relates in general to methods and products for initiating an immune response against an antigen, and in particular relates to transepithelial delivery of antigens to provoke tolerance and immunity. The present invention further relates to methods and products for the transepithelial delivery of therapeutics. In particular, the invention relates to methods and compositions for the delivery of therapeutics conjugated to a FcRn binding partner to intestinal epithelium, mucosal epithelium and epithelium of the lung. The present invention further relates to the synthesis, preparation and use of the FcRn binding partner conjugates as, or in, pharmaceutical compositions for oral systemic delivery of drugs and vaccines.
Owner:BRANDEIS UNIV +1
Potentiation of immune responses with liposomal adjuvants
InactiveUS6090406AGood water solubilityPractical and convenientBacterial antigen ingredientsViral antigen ingredientsLipid formationOrganic acid
A high integrity liposome comprising at least one stabile lipid and at least one peptide-like therapeutic agent associated with said liposome, adapted for parenteral administration to an animal, including a human, and method according to manufacture and use. Immunizing dosage forms comprising a liposome and an immunogen, wherein said liposome and immunogen are present in an immunization dose. Additionally, a dosage form, including such form particularly adapted to producing an immune response, comprising a salt according to an organic acid derivative of a sterol and an immunogen wherein said organic acid derivative of a sterol and immunogen are present in an immunization dose, and method according to use is disclosed. Further, a dosage form, including such form particularly adapted to producing an immune response, comprising dimyristoylphosphatidylcholine (DMPC) / cholesterol liposomes, optionally in an aluminum hydroxide gel, and an immunogen wherein said DMPC / cholesterol and immunogen are present in an immunization dose, and method according to use.
Owner:TRANSAVE
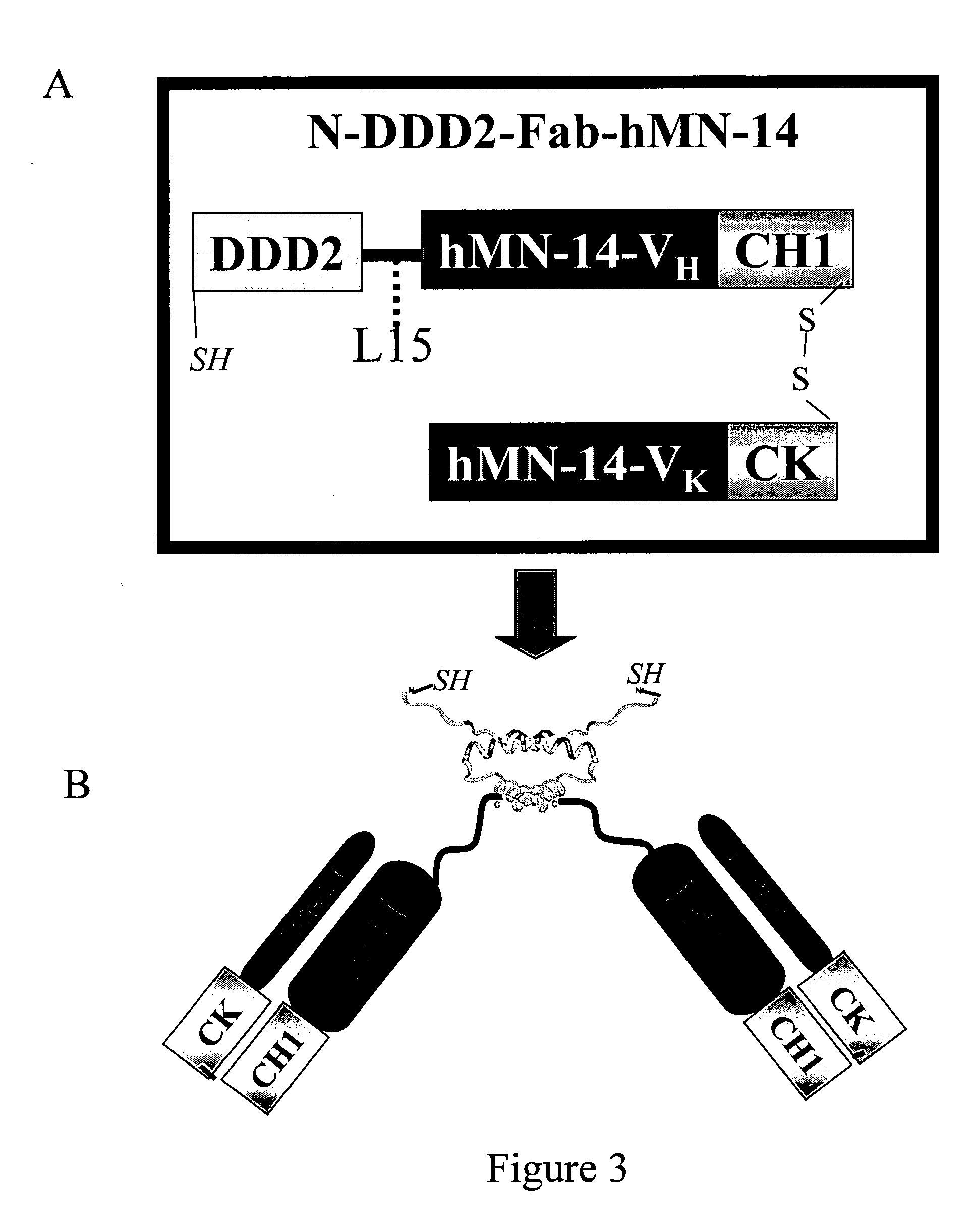
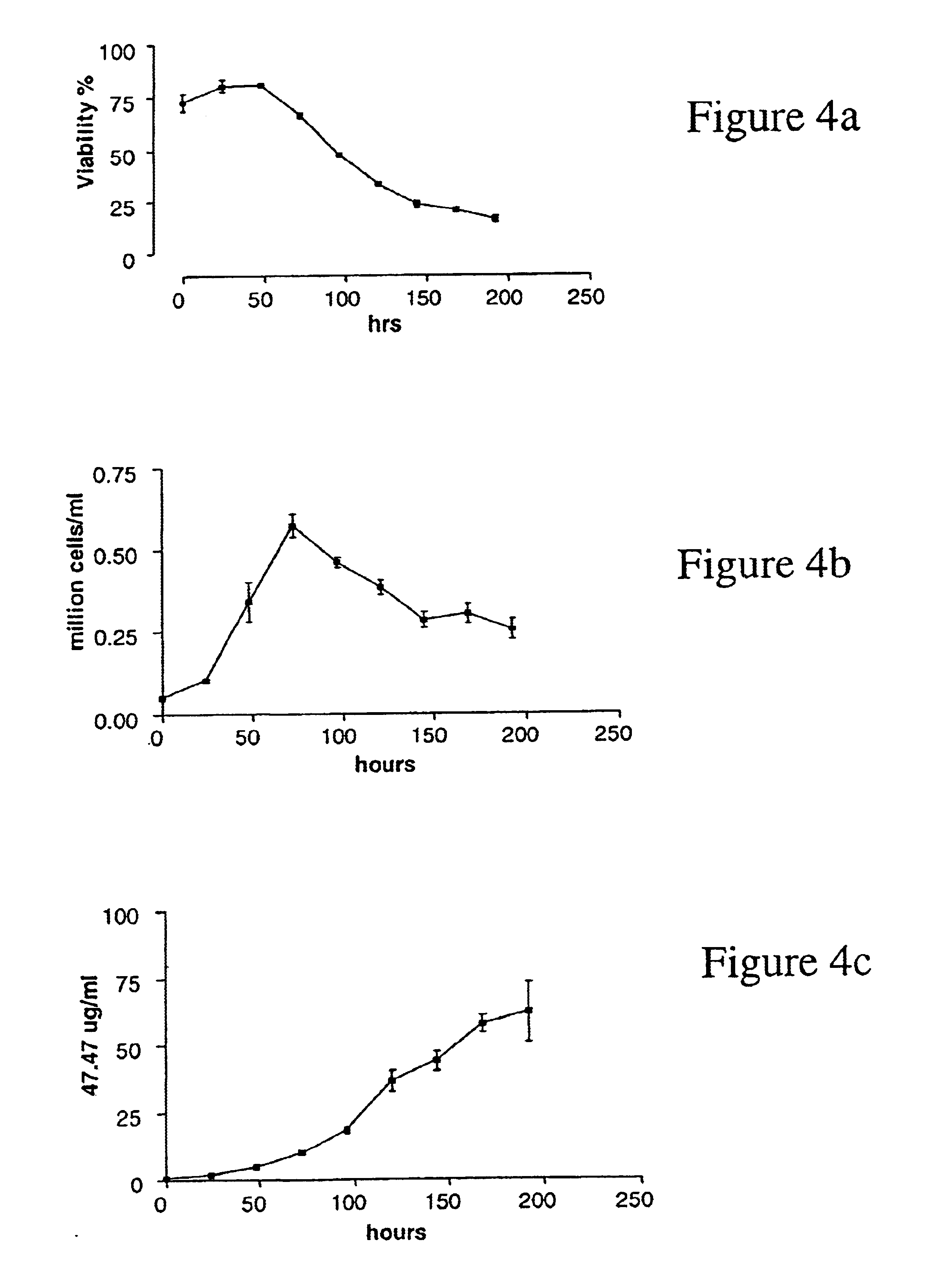
Multivalent immunoglobulin-based bioactive assemblies
ActiveUS7527787B2Efficacious for arrestingInhibition formationPeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseDiagnostic agent
Owner:IBC PHARMACEUTICALS INC
Receptor specific transepithelial transport of therapeutics
InactiveUS6485726B1Effective strategyImprove abilitiesBacterial antigen ingredientsPeptide/protein ingredientsAntigenTolerability
The present invention relates in general to methods and products for initiating an immune response against an antigen, and in particular relates to transepithelial delivery of antigens to provoke tolerance and immunity. The present invention further relates to methods and products for the transepithelial delivery of therapeutics. In particular, the invention relates to methods and compositions for the delivery of therapeutics conjugated to a FcRn binding partner to intestinal epithelium, mucosal epithelium and epithelium of the lung. The present invention further relates to the synthesis, preparation and use of the FcRn binding partner conjugates as, or in, pharmaceutical compositions for oral systemic delivery of drugs and vaccines.
Owner:BRANDEIS UNIV +1
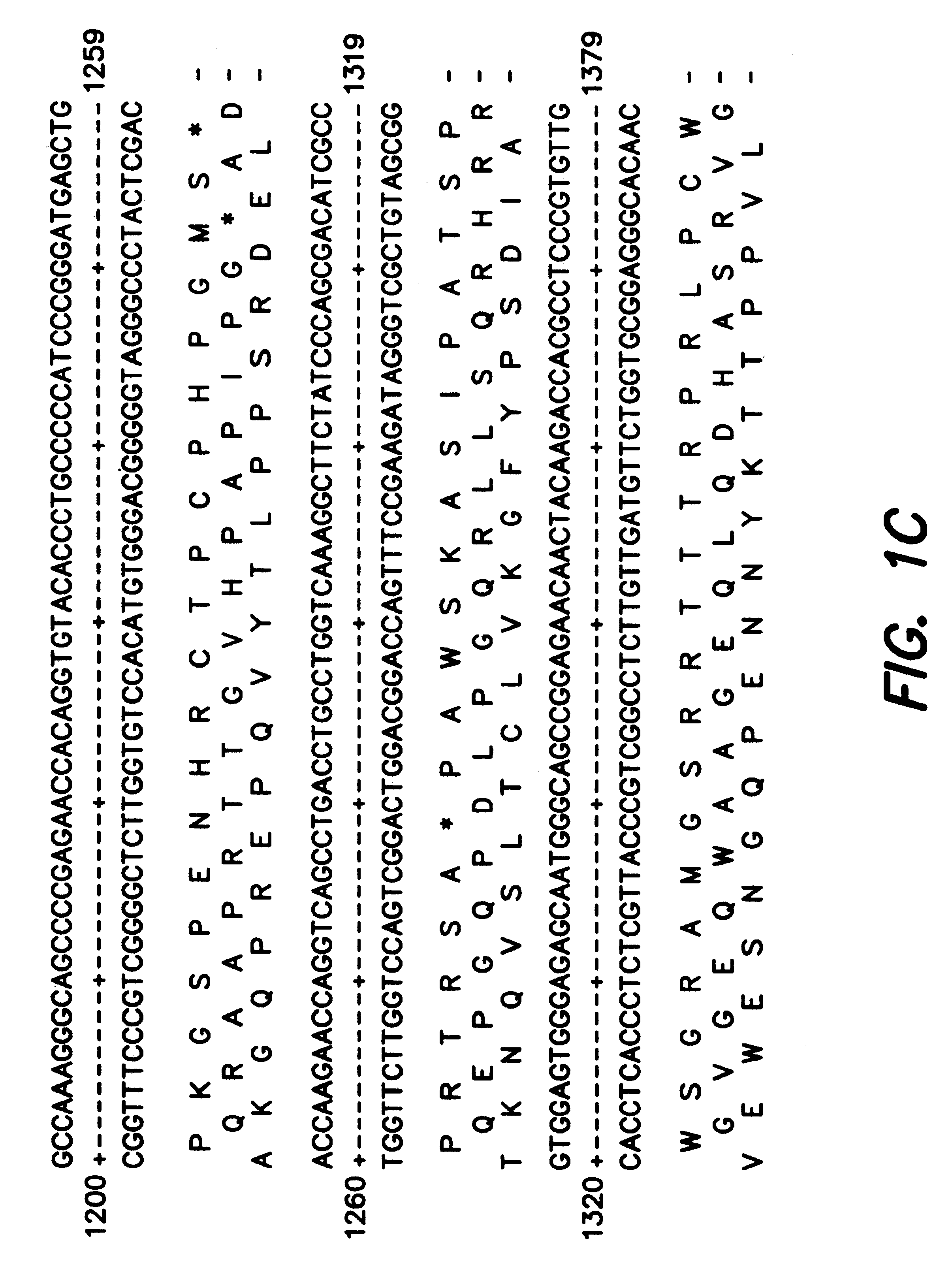
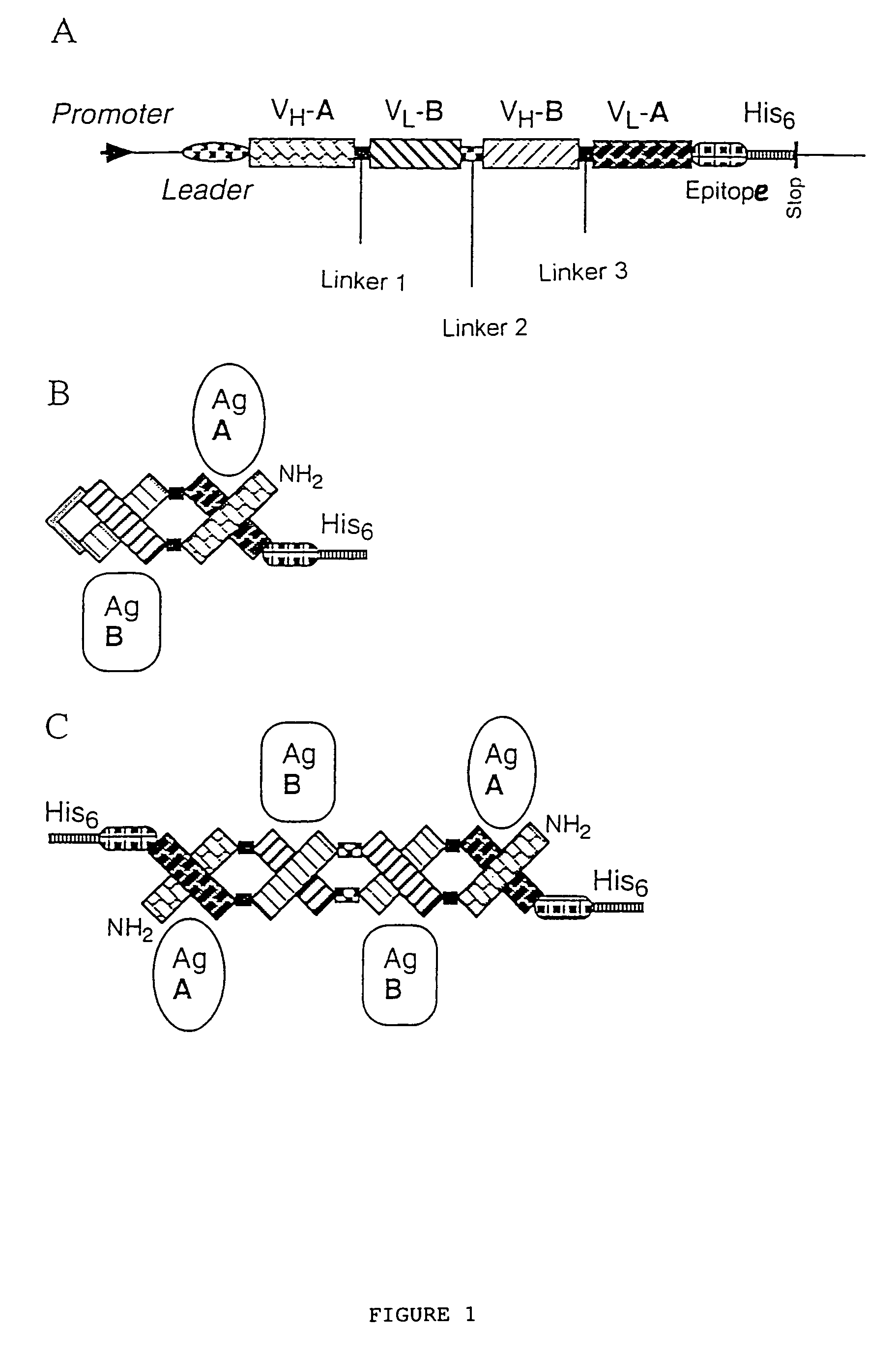
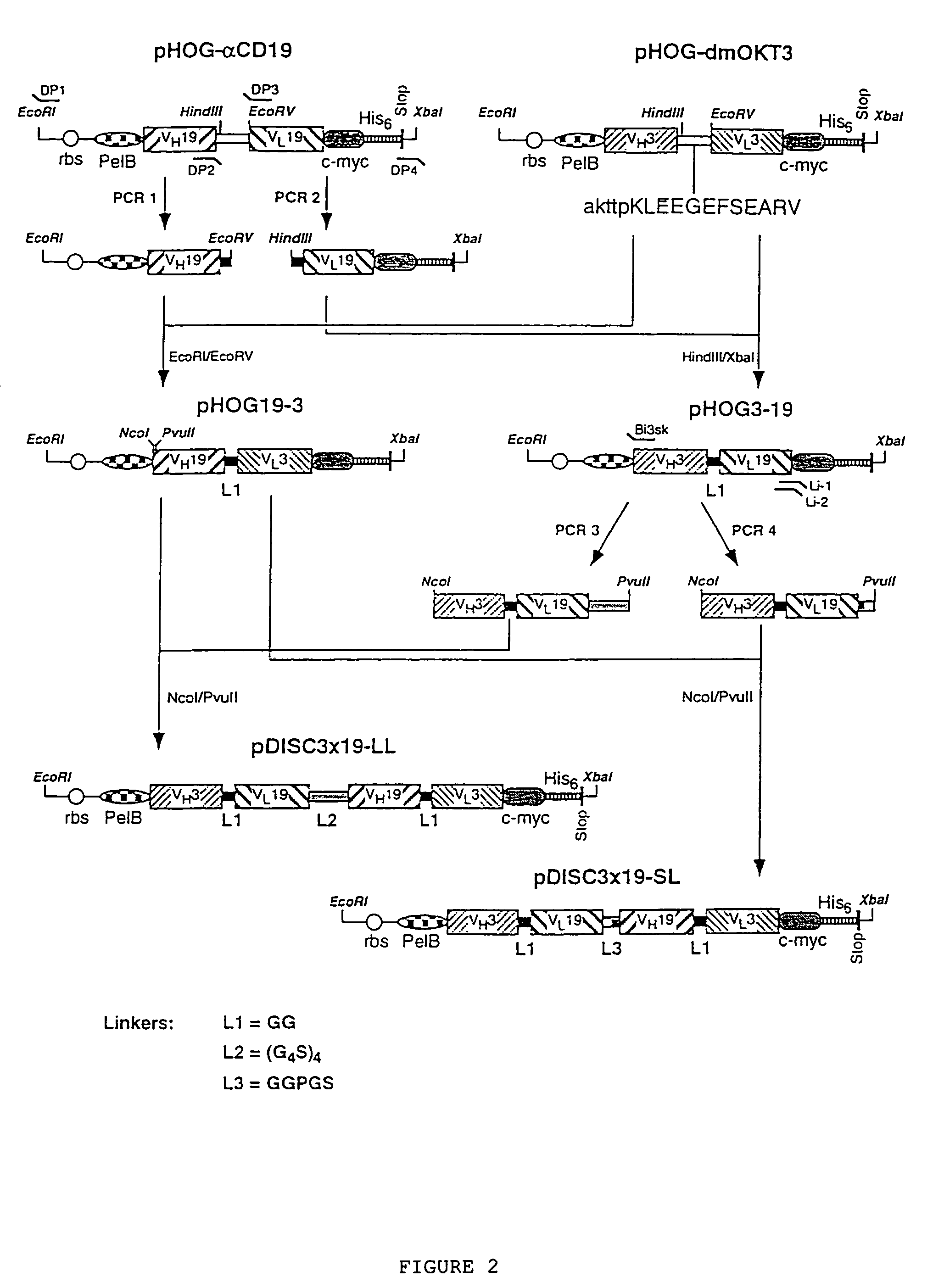
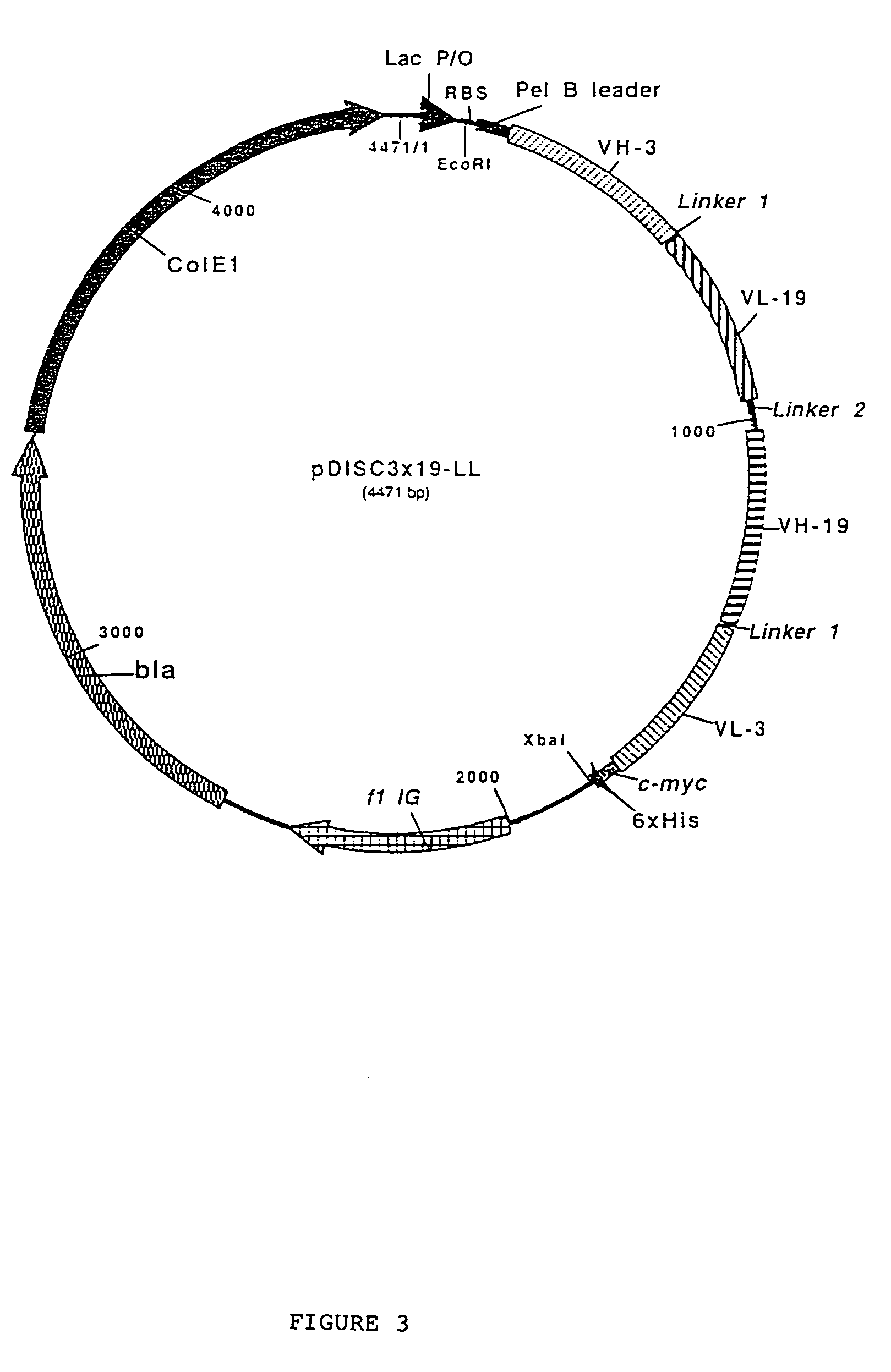
Multivalent antibody constructs
InactiveUS7129330B1Antibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsVariable domainPlasmid
The present invention relates to multivalent Fvantibody construct having at least four variable domains which are linked with each over via the peptide linkers 1, 2 and 3. The invention also concerns expression plasmids which code for such an Fvantibody construct and a method of producing the Fvantibody constructs as well as their use.
Owner:DEUTES KREBSFORSCHUNGSZENT STIFTUNG DES OFFENTLICHEN RECHTS
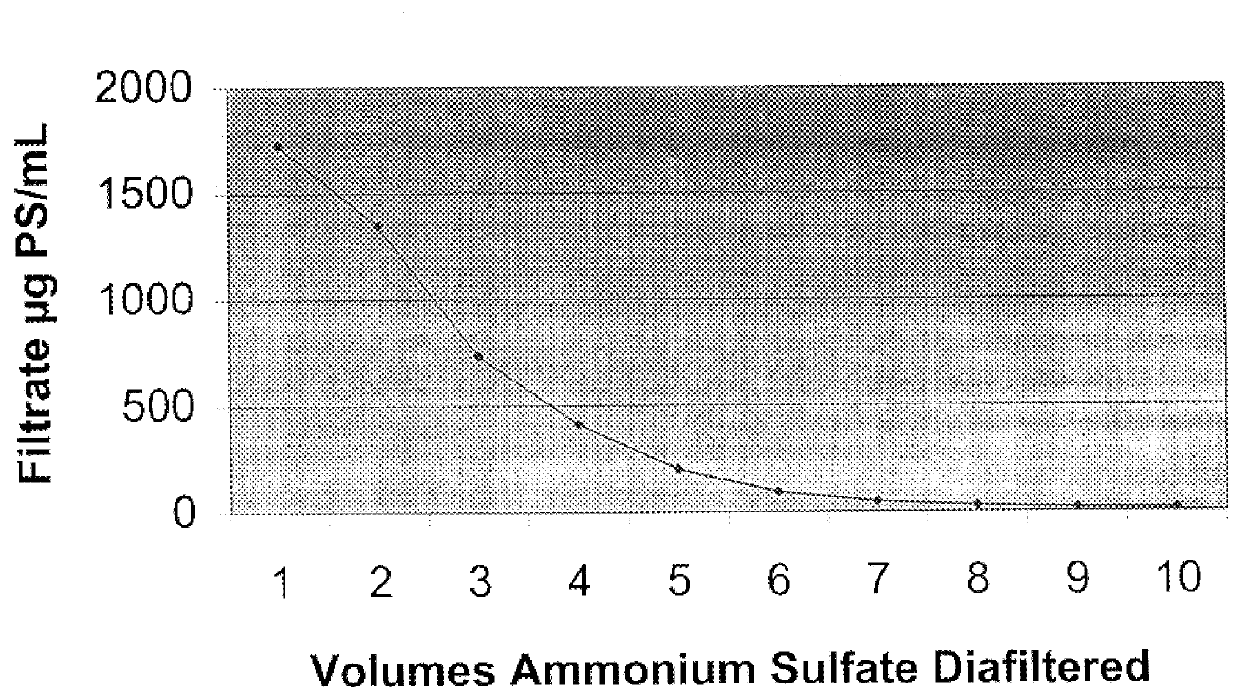
Purification of polysaccharide-protein conjugate vaccines by ultrafiltration with ammonium sulfate solutions
InactiveUS6146902AImprove scalabilityLevel of purityAntibacterial agentsSugar derivativesConjugate vaccineUltrafiltration
Disclosed and claimed are a method for the purification of polysaccharide-protein conjugate vaccines by ultrafiltration in a saturated solution of ammonium sulfate. The ultrafiltration method of the present invention provides an efficient, readily scalable method for removal of unbound polysaccharides from polysaccharide-protein vaccines, thereby improving the purity and consistency of the polysaccharide-protein vaccines.
Owner:AVENTIS PASTUER LTD
Non-invasive localization of a light-emitting conjugate in a mammal
InactiveUS6217847B1Accurate measurementEvenly distributedUltrasonic/sonic/infrasonic diagnosticsBacteriaMammalNon invasive
Methods and compositions for detecting and localizing light originating from a mammal are disclosed. Also disclosed are methods for tracking light emission to selected regions, as well as for tracking entities within the mammal. In addition, animal models for disease states are disclosed, as are methods for localizing and tracking the progression of disease or a pathogen within the animal, and for screening putative therapeutic compounds effective to inhibit the disease or pathogen.
Owner:LELAND STANFORD JUNIOR UNIV OF THE BOARD OF TRUSTEES THE
Multivalent immunoglobulin-based bioactive assemblies
ActiveUS20070140966A1Prevent further clot formationHigh affinityPeptide/protein ingredientsAntibody mimetics/scaffoldsDiagnostic agentAutoimmune condition
The present invention concerns methods and compositions for stably tethered structures of defined compositions, which may have multiple functionalities and / or binding specificities. Preferred embodiments concern hexameric stably tethered structures comprising one or more IgG antibody fragments and which may be monospecific or bispecific. The disclosed methods and compositions provide a facile and general way to obtain stably tethered structures of virtually any functionality and / or binding specificity. The stably tethered structures may be administered to subjects for diagnostic and / or therapeutic use, for example for treatment of cancer or autoimmune disease. The stably tethered structures may bind to and / or be conjugated to a variety of known effectors, such as drugs, enzymes, radionuclides, therapeutic agents and / or diagnostic agents.
Owner:IBC PHARMACEUTICALS INC
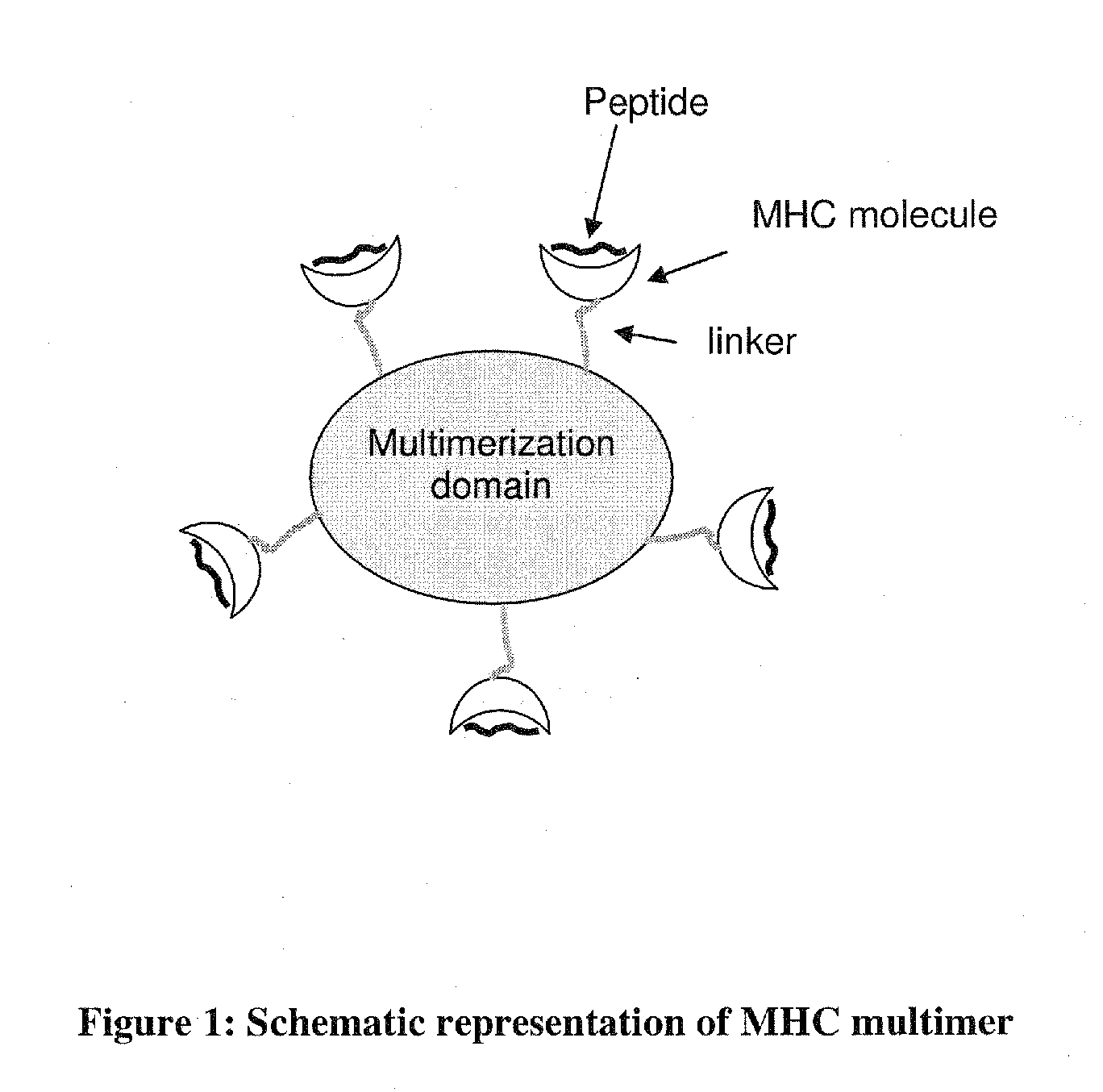
MHC Multimers in Cancer Vaccines and Immune Monitoring
InactiveUS20110318380A1Reduces infectious titerImprove efficacyPeptide/protein ingredientsImmunoglobulinsAntigenDisease
The present invention relates to MHC-peptide complexes and uses thereof in the diagnosis of, treatment of or vaccination against a disease in an individual. More specifically the invention discloses MHC complexes comprising cancer antigenic peptides and uses there of.
Owner:AGILENT TECH INC
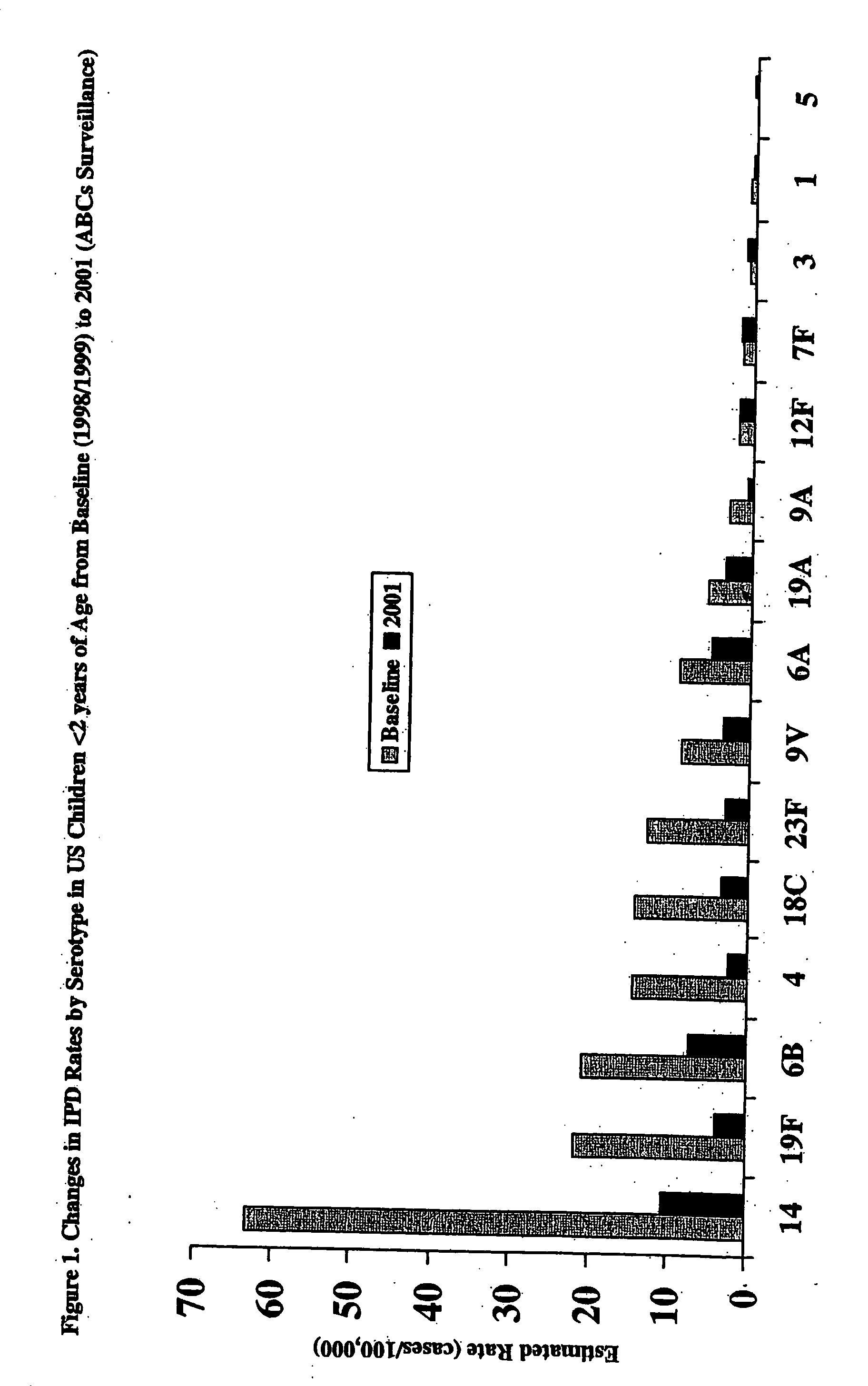
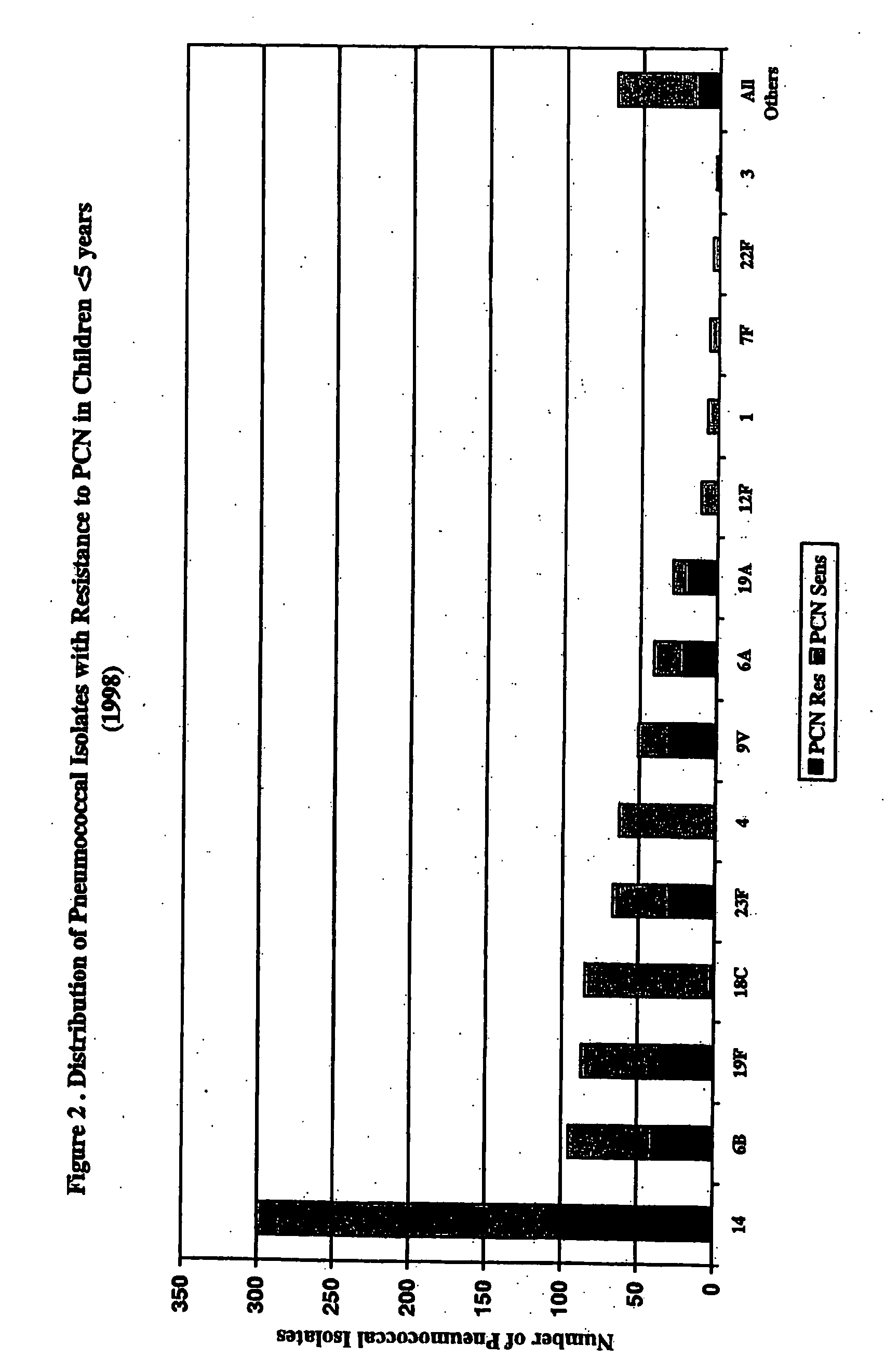
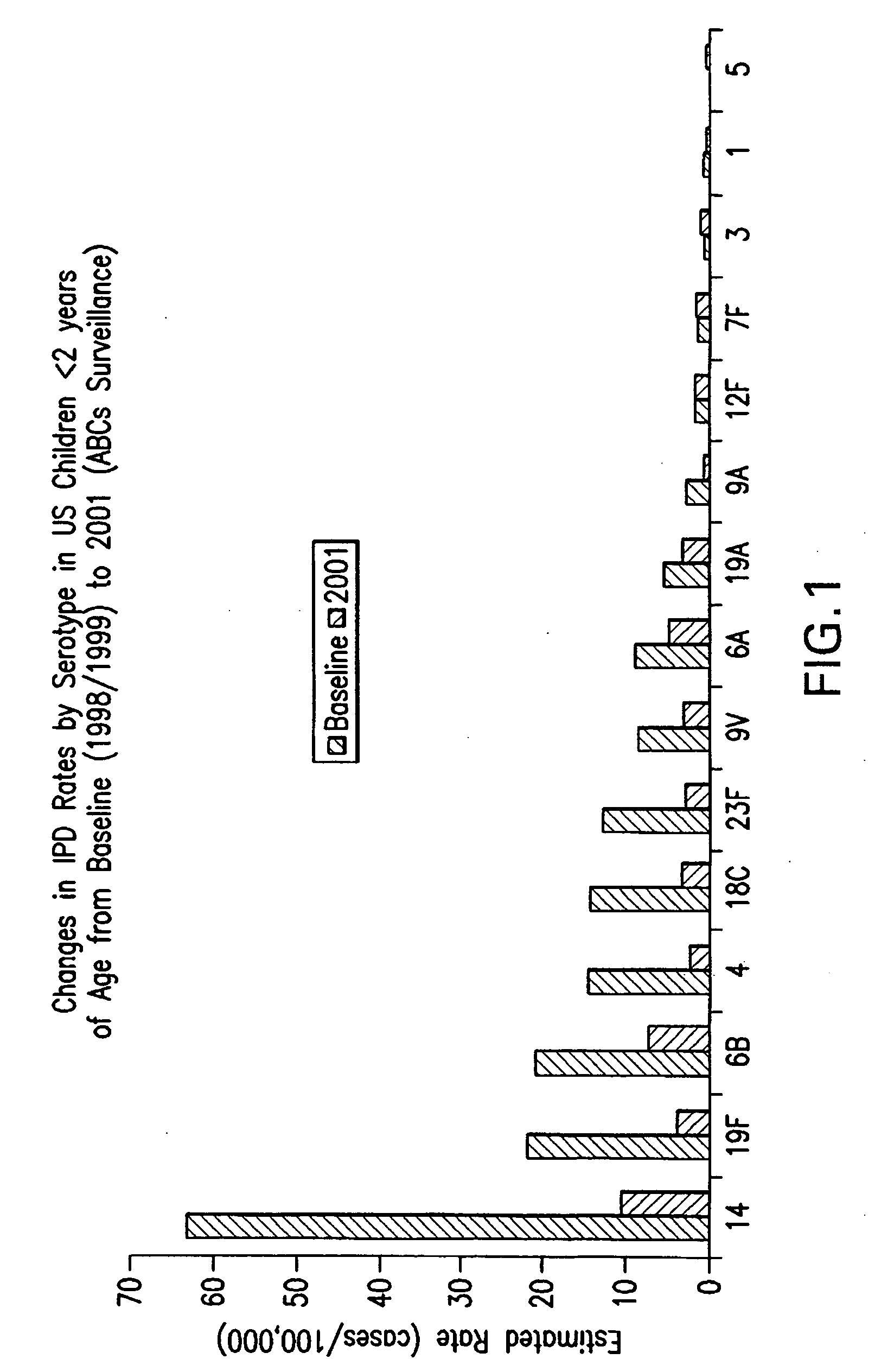
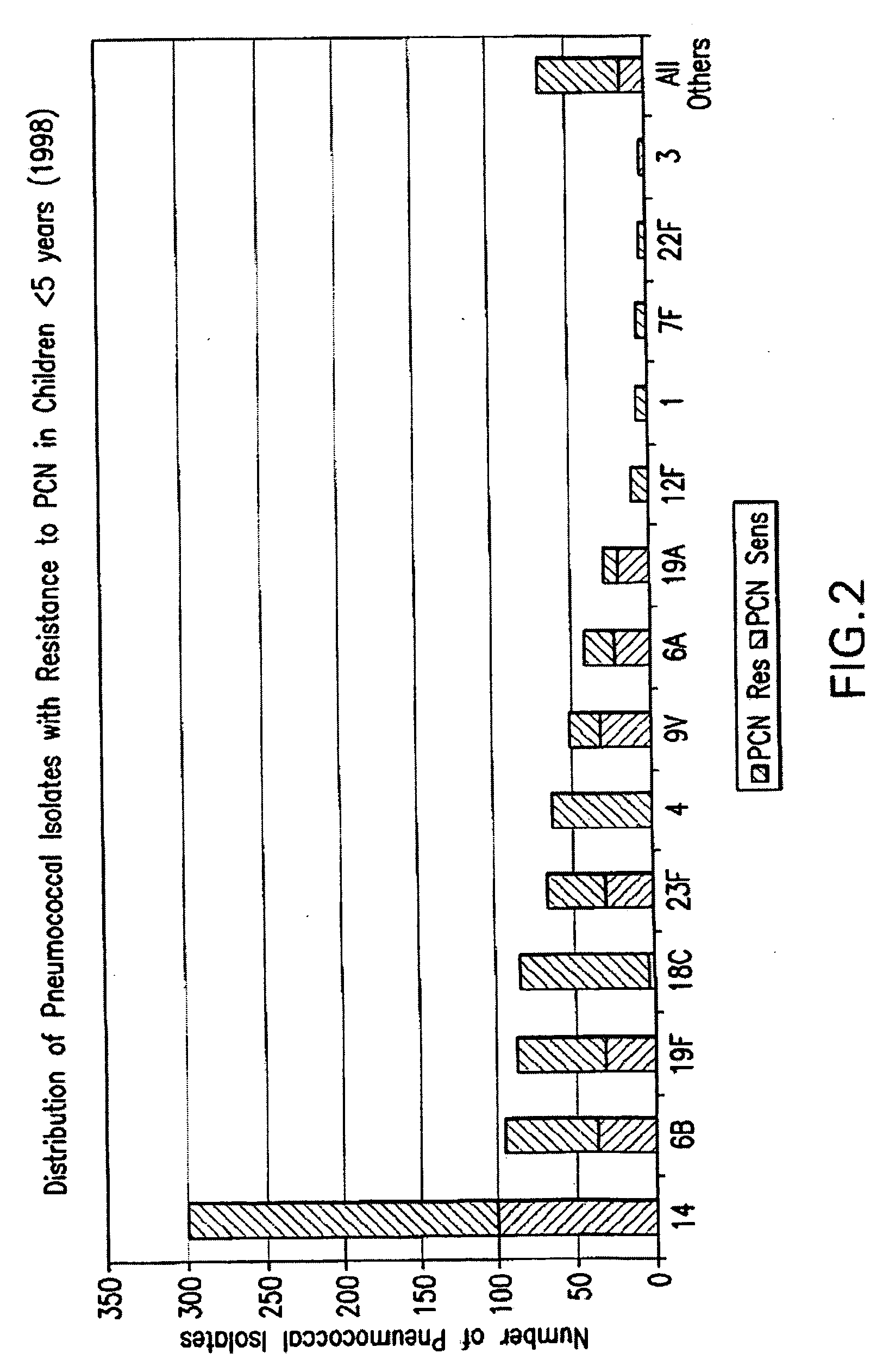
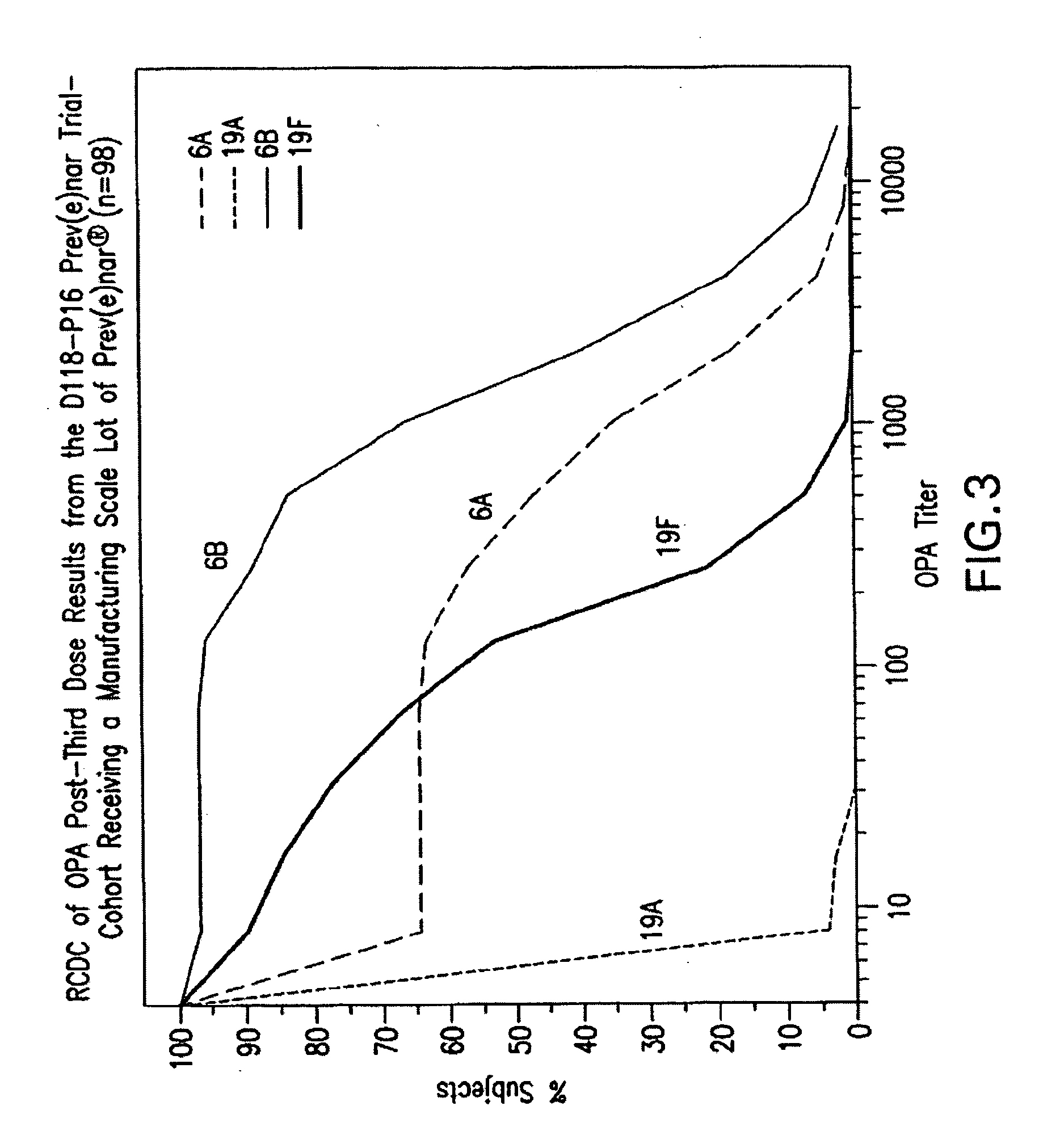
Multivalent pneumococcal polysaccharide-protein conjugate composition
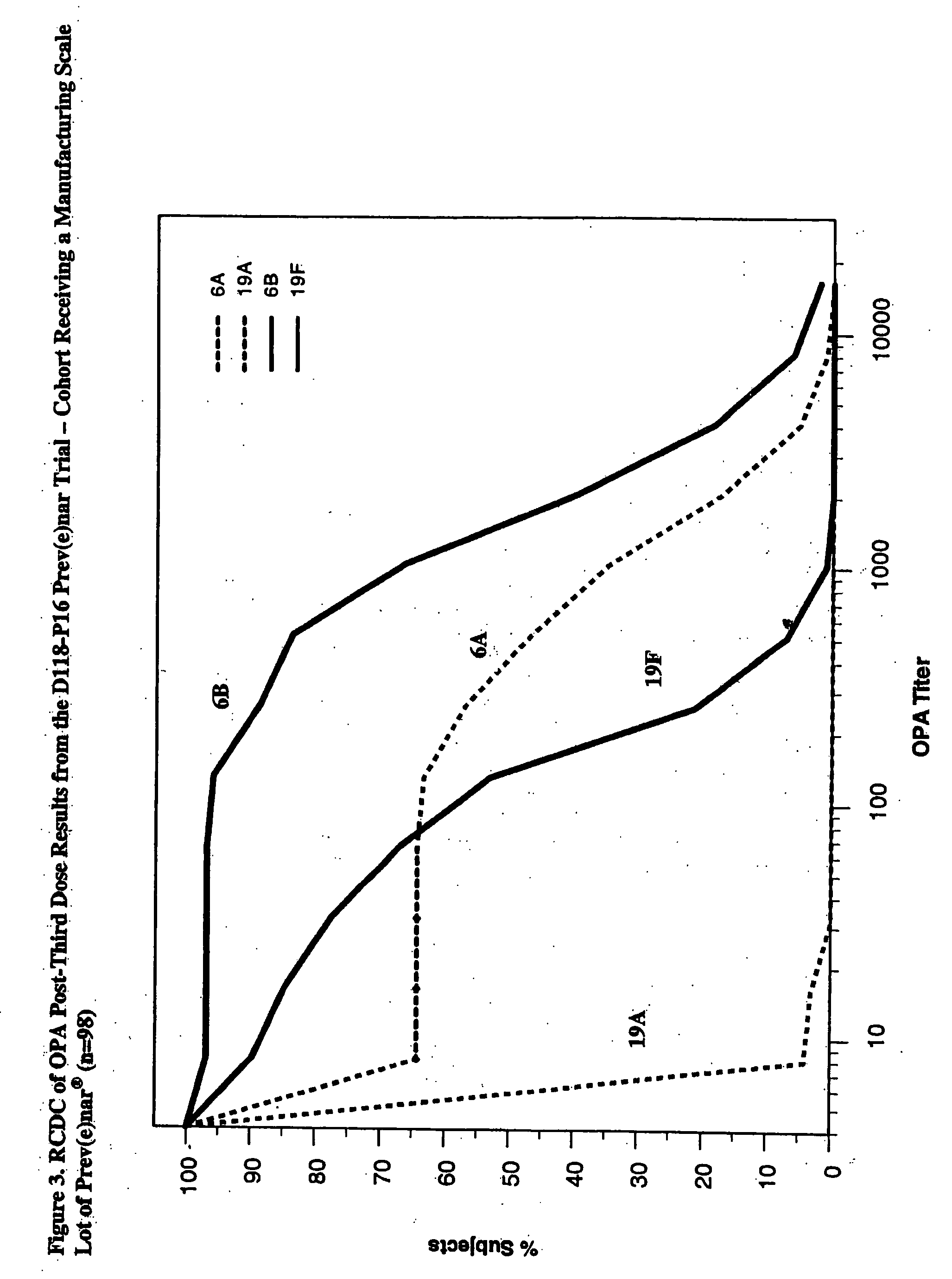
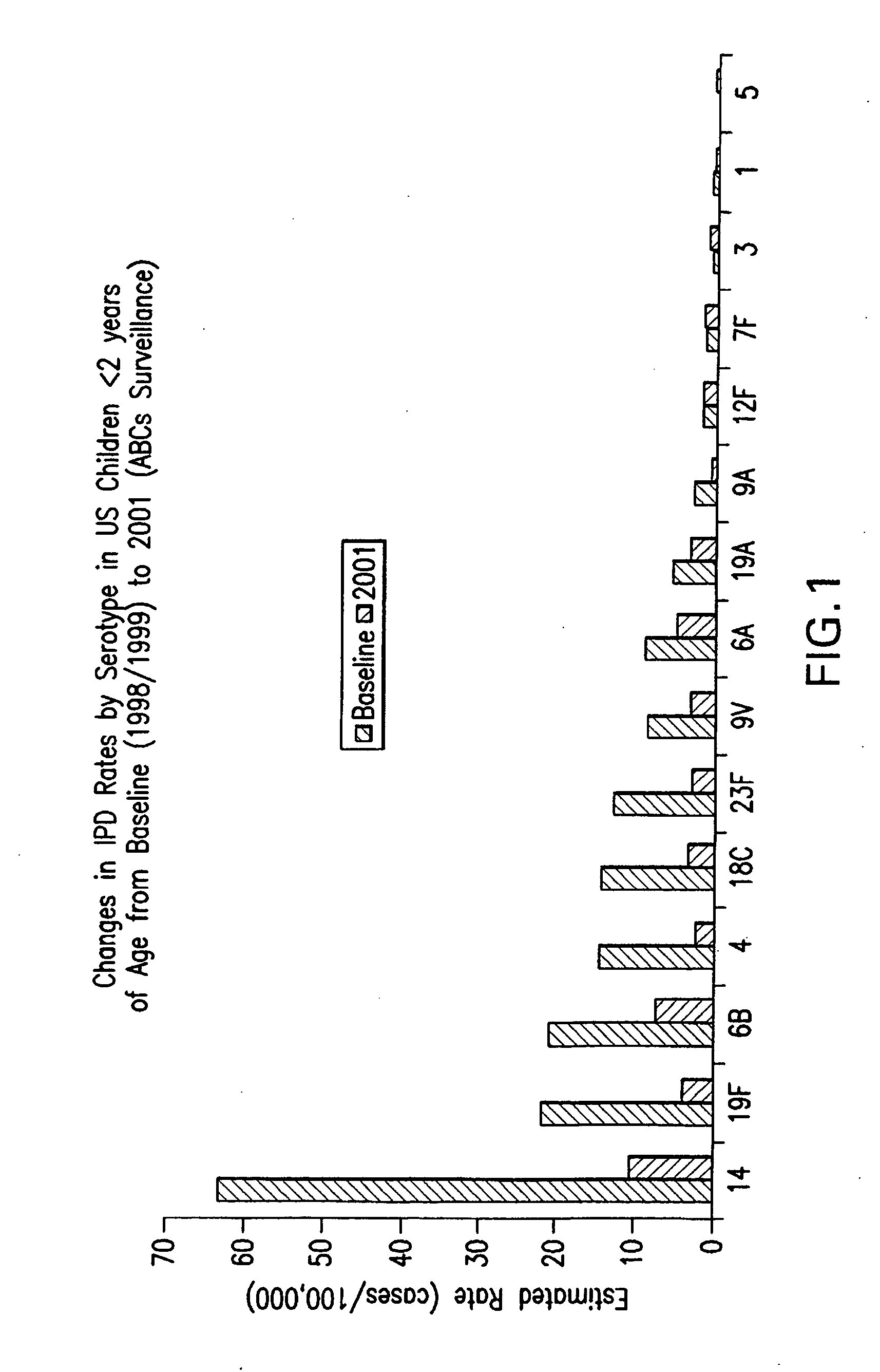
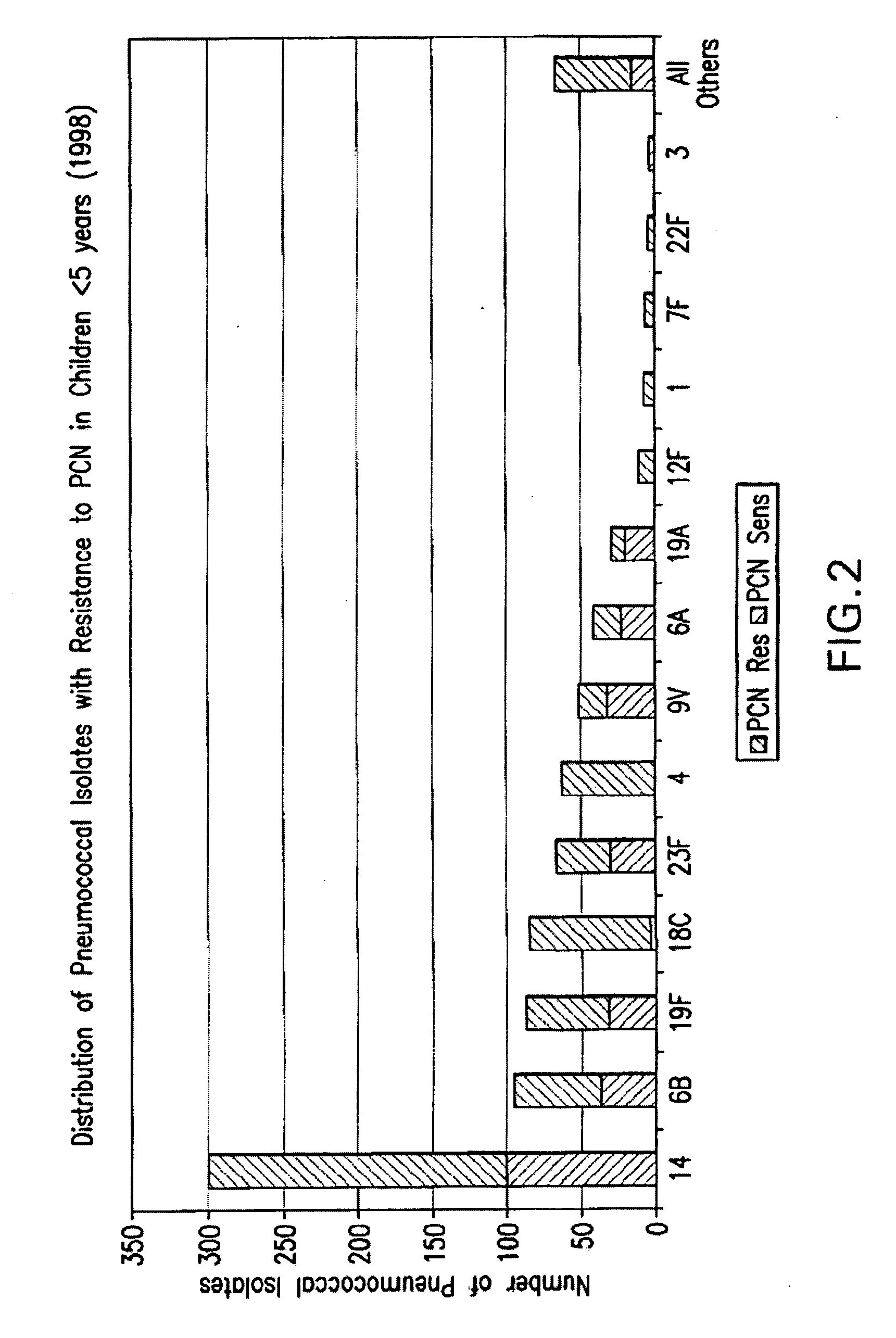
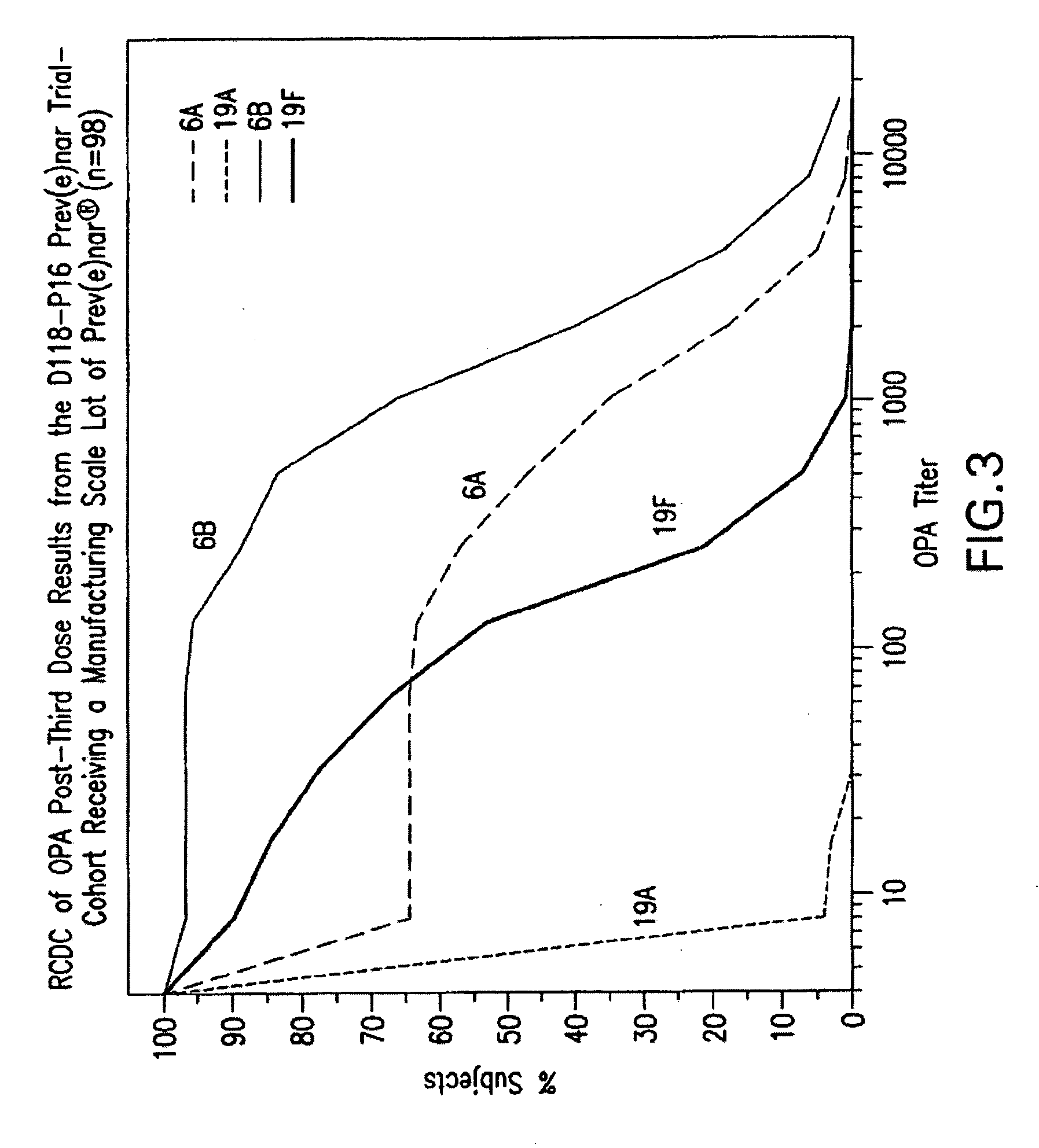
An immunogenic composition having 13 distinct polysaccharide-protein conjugates and optionally, an aluminum-based adjuvant, is described. Each conjugate contains a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F) conjugated to a carrier protein. The immunogenic composition, formulated as a vaccine, increases coverage against pneumococcal disease in infants and young children globally, and provides coverage for serotypes 6A and 19A that is not dependent on the limitations of serogroup cross-protection.
Owner:WYETH LLC
Methods and topical formulations comprising colloidal metal for treating or preventing skin conditions
In preferred embodiments, the present invention relates to compositions comprising colloidal metals and / or metals for the treatment and prevention of skin conditions and / or diseases. More specifically, the disclosed metal containing compositions are useful as antioxidants, anti-aging agents, anti-wrinkle agents, anti-peroxidation agents, antimicrobial agents, anti-inflammatory agents, pain-relieving agents, wound recovery agents, sun-screens, sunblocks, and integument and skin-supporting agents when applied to the skin / integument, or administered generally to an animal or human body.
Owner:MARGULIES JOEL +1
Compositions and methods for immunomodulation in an organism using IL-15 and soluble IL-15Ra
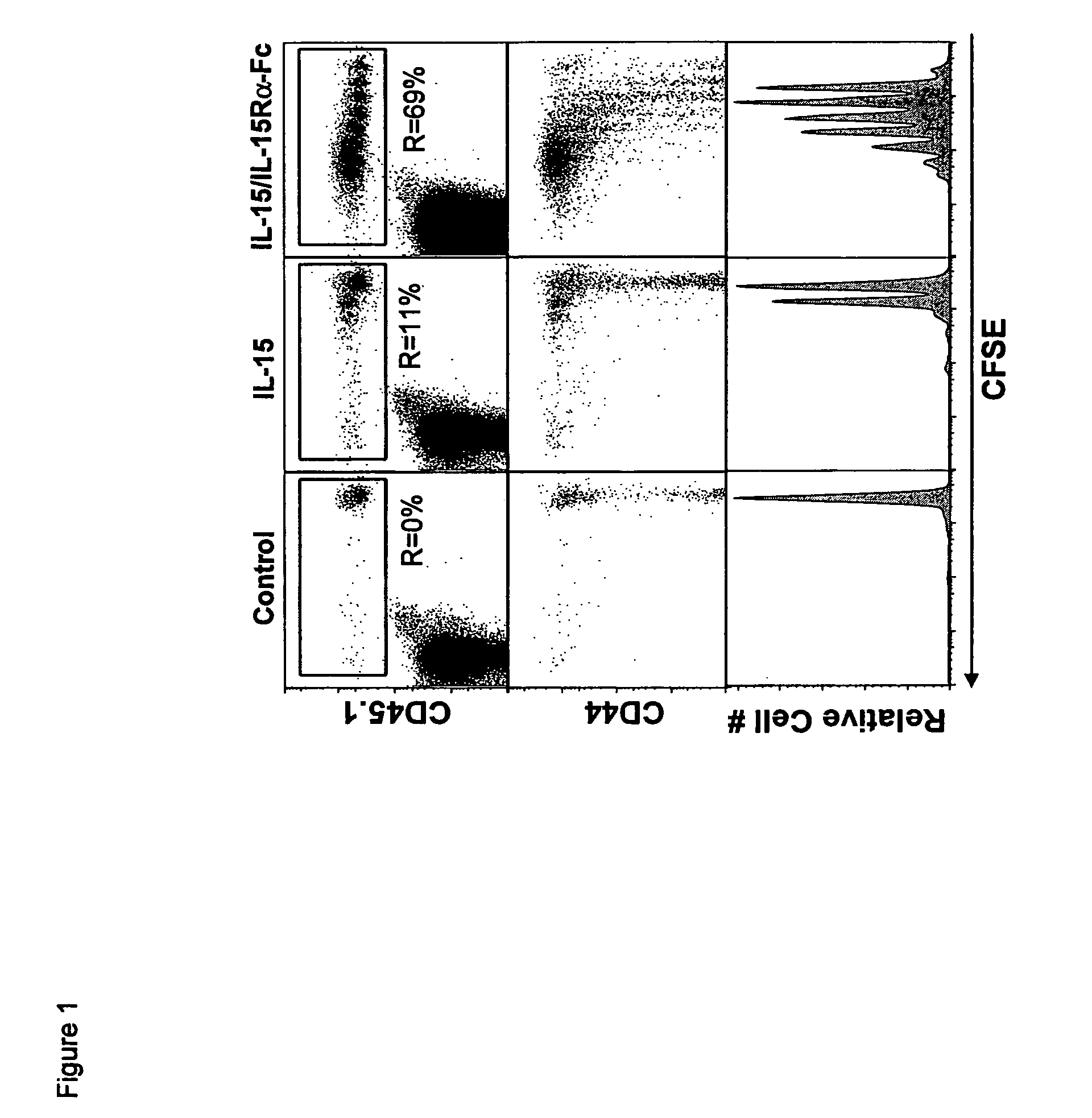
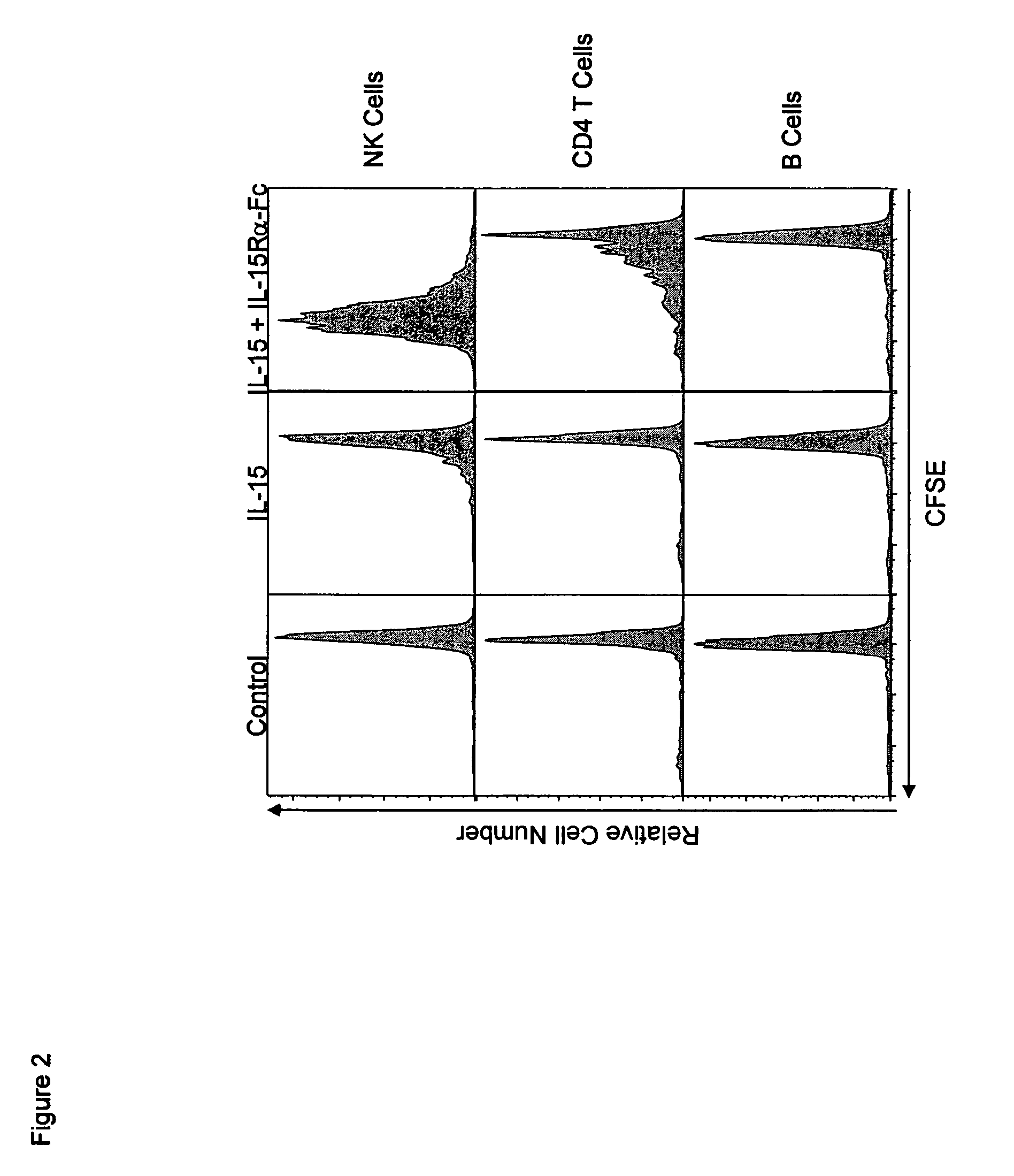
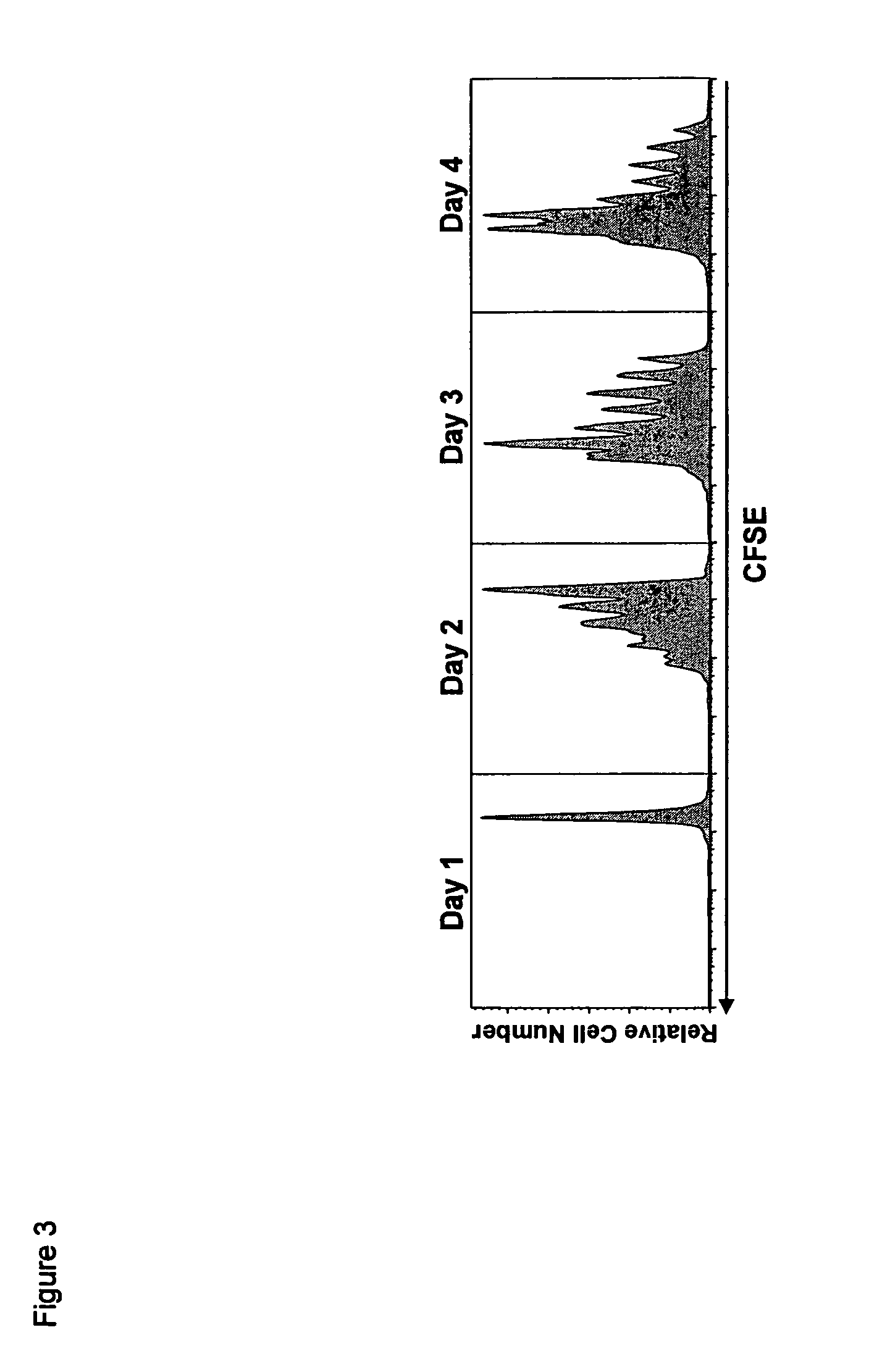
ActiveUS8124084B2Extended half-lifeImprove bioavailabilityPeptide/protein ingredientsAntibody mimetics/scaffoldsBiological bodyVaccination
The present invention relates to a therapeutic polypeptide and methods for its creation and use for modulating an immune response in a host organism in need thereof. In particular, the invention relates to the administration to an organism in need thereof, of an effective amount of a pre-coupled polypeptide complex comprising a lymphokine polypeptide portion, for example IL-15 (SEQ ID NO: 5, 6), IL-2 (SEQ ID NO: 10, 12) or combinations of both, and an interleukin receptor polypeptide portion, for example IL-15Ra (SEQ ID NO: 7, 8), IL-2Ra (SEQ ID NO: 9, 11) or combinations of both, for augmenting the immune system in, for example, cancer, SCID, AIDS, or vaccination; or inhibiting the immune system in, for example, rheumatoid arthritis, or Lupus. The therapeutic complex of the invention surprisingly demonstrates increased half-life, and efficacy in vivo.
Owner:UNIV OF CONNECTICUT
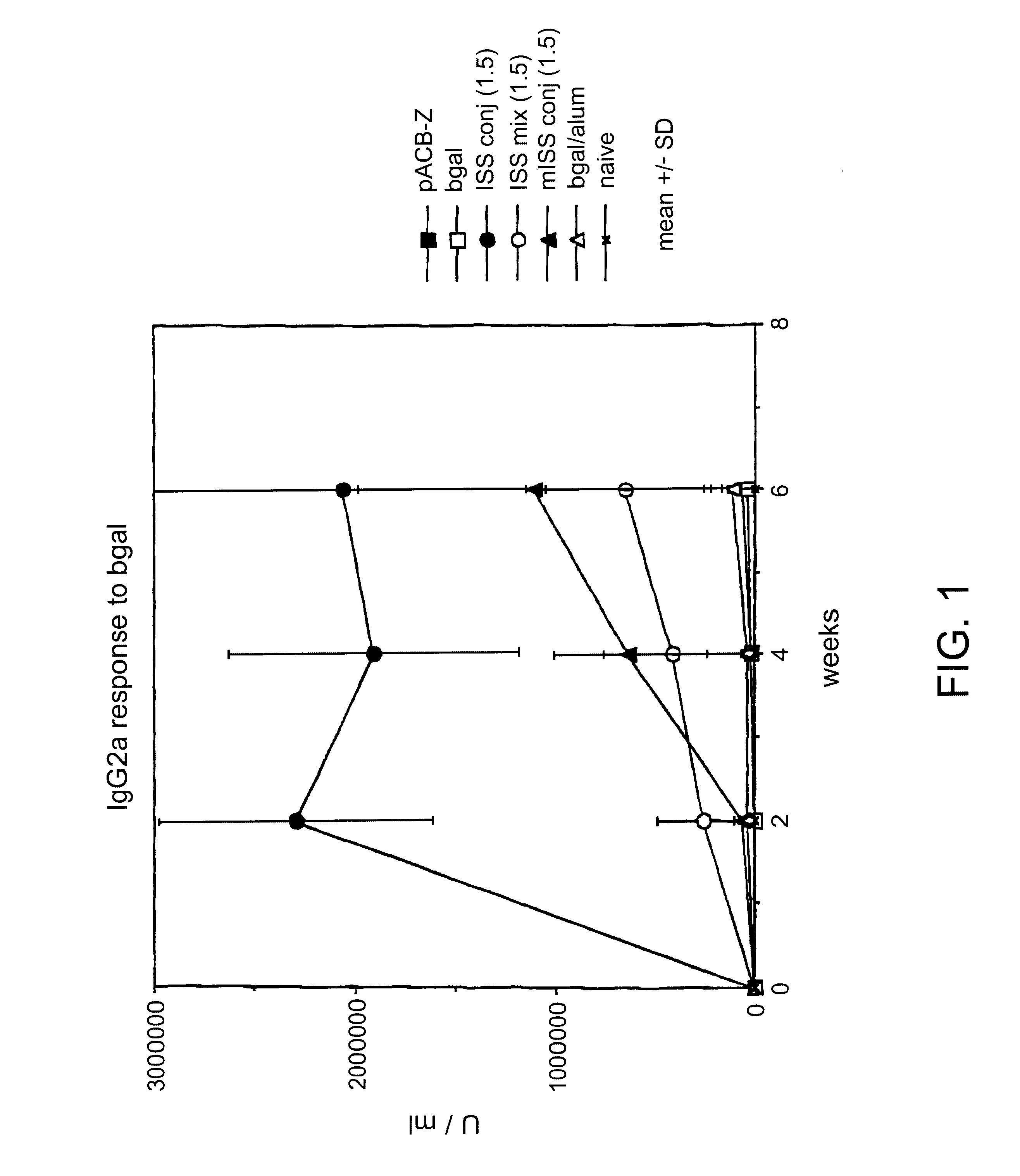
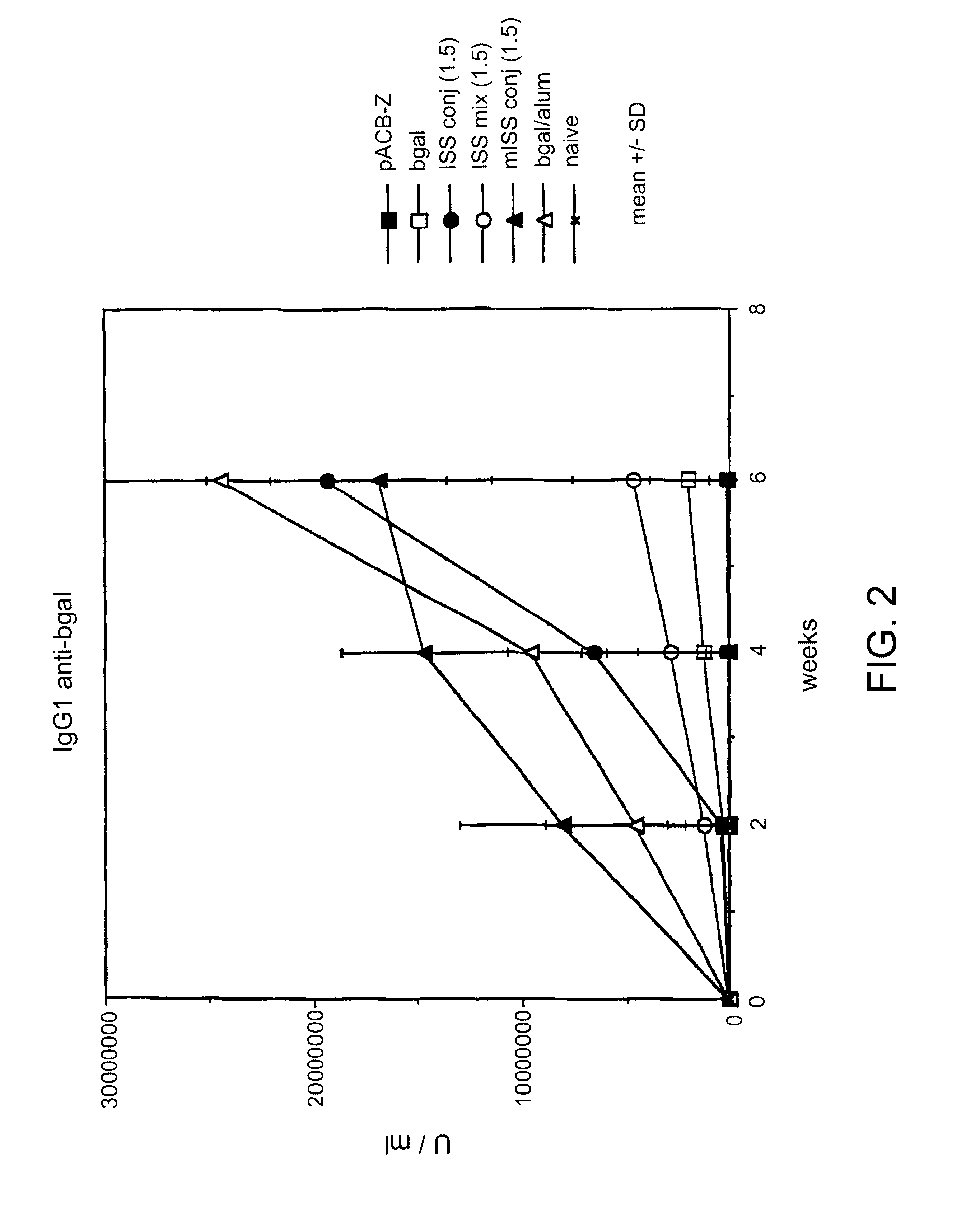
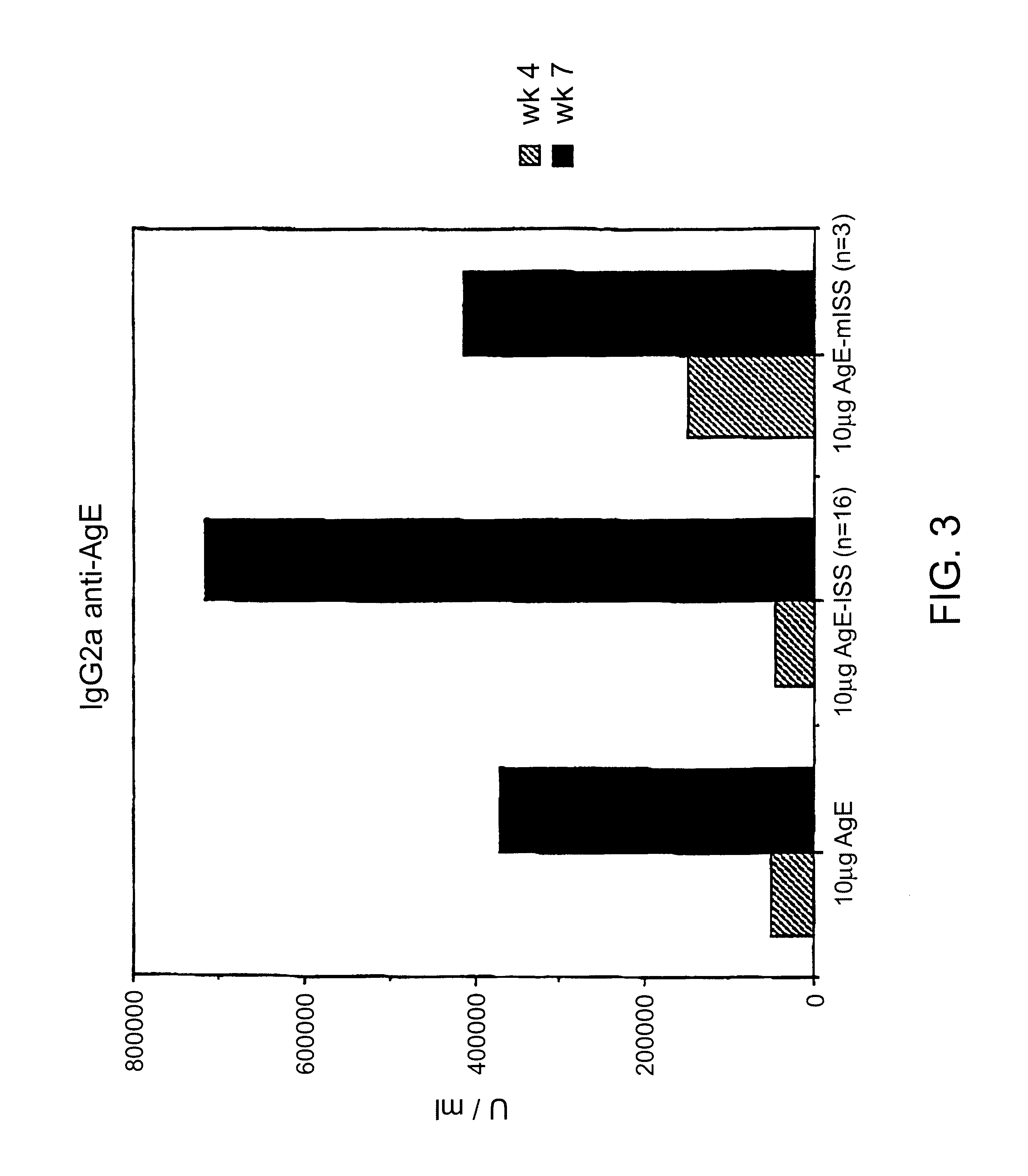
Immunostimulatory polynucleotide/immunomodulatory molecule conjugates
InactiveUS6610661B1Boost magnitudeBoost both humoral (antibody)Peptide/protein ingredientsGenetic material ingredientsAntigenAdjuvant
Immunostimulatory polynucleotide-immunomodulatory molecule conjugate compositions are disclosed. These compositions include a polynucleotide that is linked to an immunomodulatory molecule, which molecule comprises an antigen and may further comprise immunomodulators such as cytokines and adjuvants. The polynucleotide portion of the conjugate includes at least one immunostimulatory oligonucleotide nucleotide sequence (ISS). Methods of modulating an immune response upon administration of the polynucleotide-immunomodulatory conjugate preparation to a vertebrate host are also disclosed.
Owner:RGT UNIV OF CALIFORNIA
Multivalent pneumococcal polysaccharide-protein conjugate composition
An immunogenic composition having 13 distinct polysaccharide-protein conjugates and optionally, an aluminum-based adjuvant, is described. Each conjugate contains a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F) conjugated to a carrier protein. The immunogenic composition, formulated as a vaccine, increases coverage against pneumococcal disease in infants and young children globally, and provides coverage for serotypes 6A and 19A that is not dependent on the limitations of serogroup cross-protection. Methods for making an immunogenic conjugate comprising Streptococcus pneumoniae serotype 19A polysaccharide are also provided in which the serotype 19A polysaccharide is co-lyophilized with a carrier protein and conjugation is carried out in dimethyl sulfoxide (DMSO) via a reductive amination mechanism.
Owner:WYETH LLC
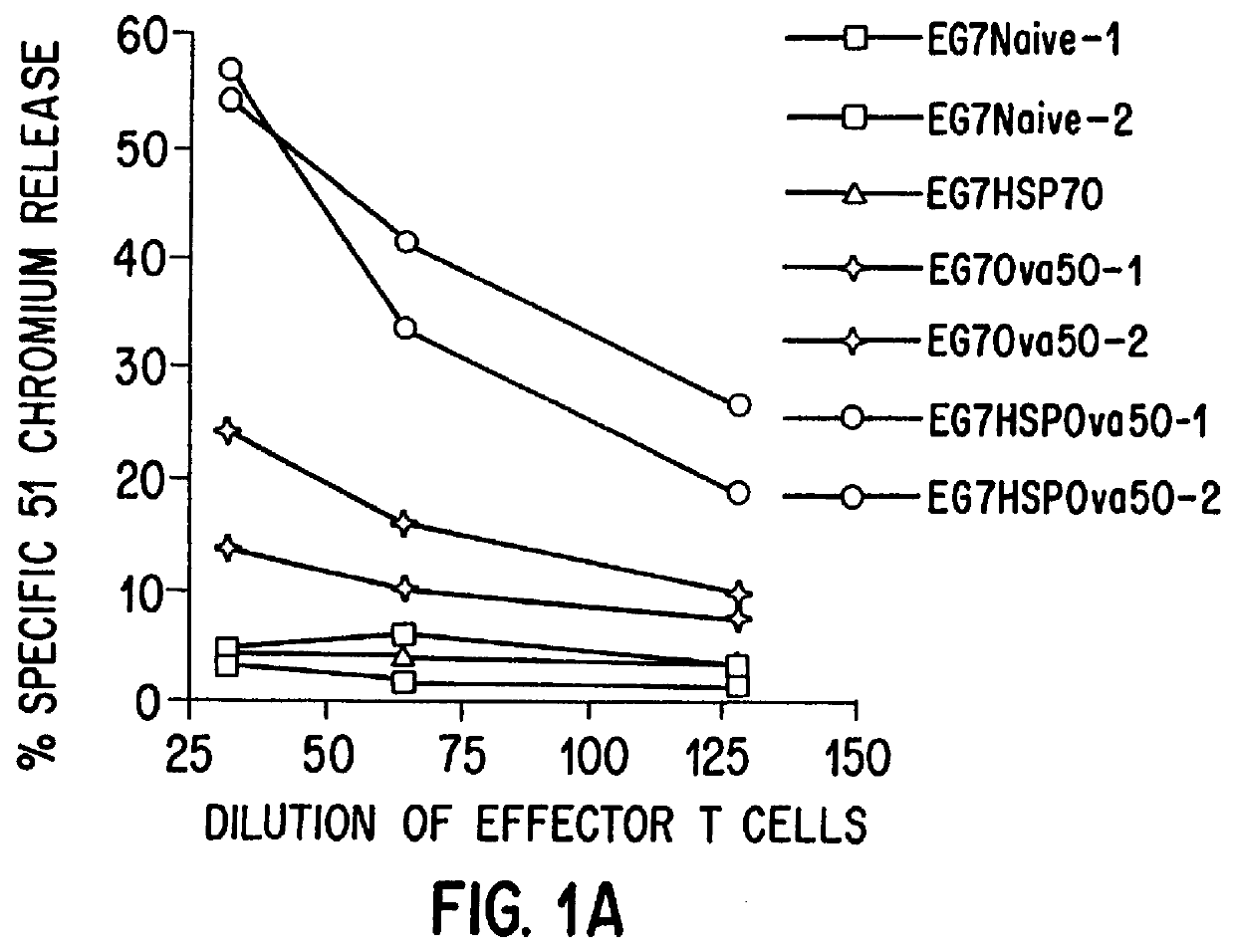
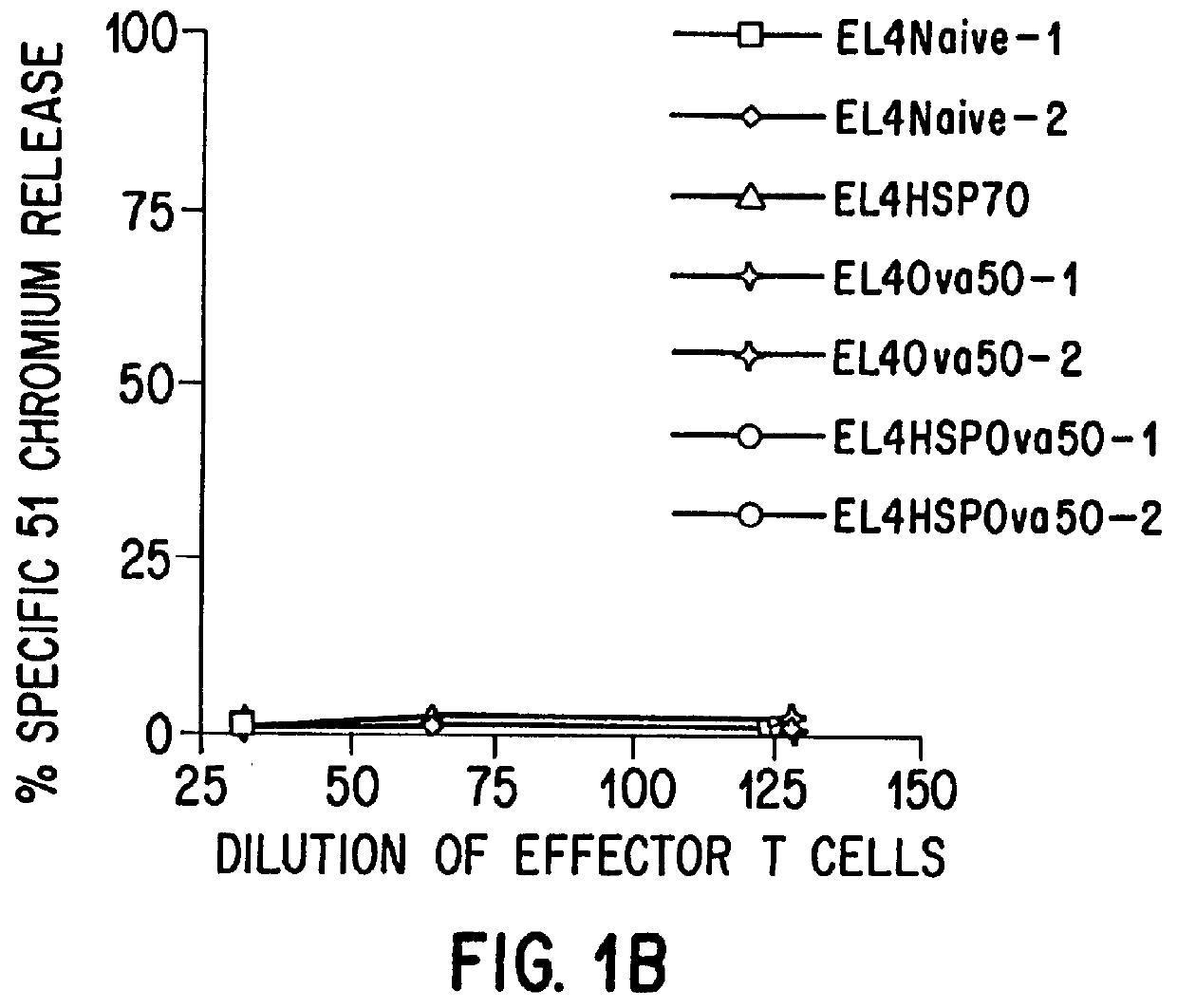
Therapeutic and prophylactic methods using heat shock proteins
The present invention relates to immunogenic complexes of heat shock proteins (hsp) noncovalently bound to exogenous antigenic molecules which when administered to an individual elicit specific immunological responses in the host. Methods of prevention and treatment of cancer and infectious disease are provided.
Owner:FORDHAM UNIVERSITY
Multivalent pneumococcal polysaccharide-protein conjugate composition
An immunogenic composition having 13 distinct polysaccharide-protein conjugates and optionally, an aluminum-based adjuvant, is described. Each conjugate contains a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F) conjugated to a carrier protein. The immunogenic composition, formulated as a vaccine, increases coverage against pneumococcal disease in infants and young children globally, and provides coverage for serotypes 6A and 19A that is not dependent on the limitations of serogroup cross-protection. Also described is a method for making an immunogenic conjugate comprising Streptococcus pneumoniae serotype 1 polysaccharide covalently linked to a carrier protein, the method including partial de-O-acetylation of the polysaccharide by mild hydrolysis in an alkaline pH buffer.
Owner:WYETH LLC
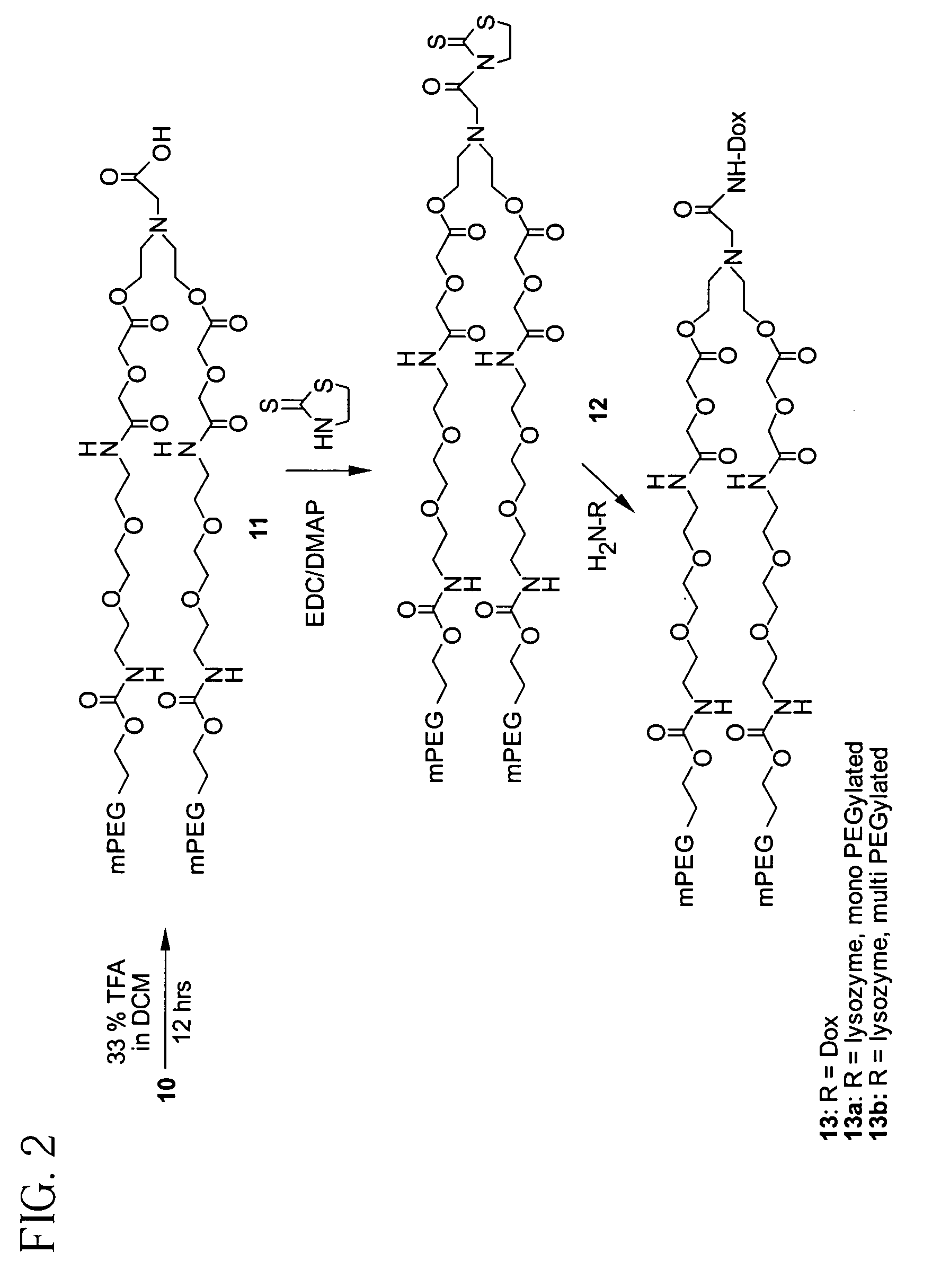
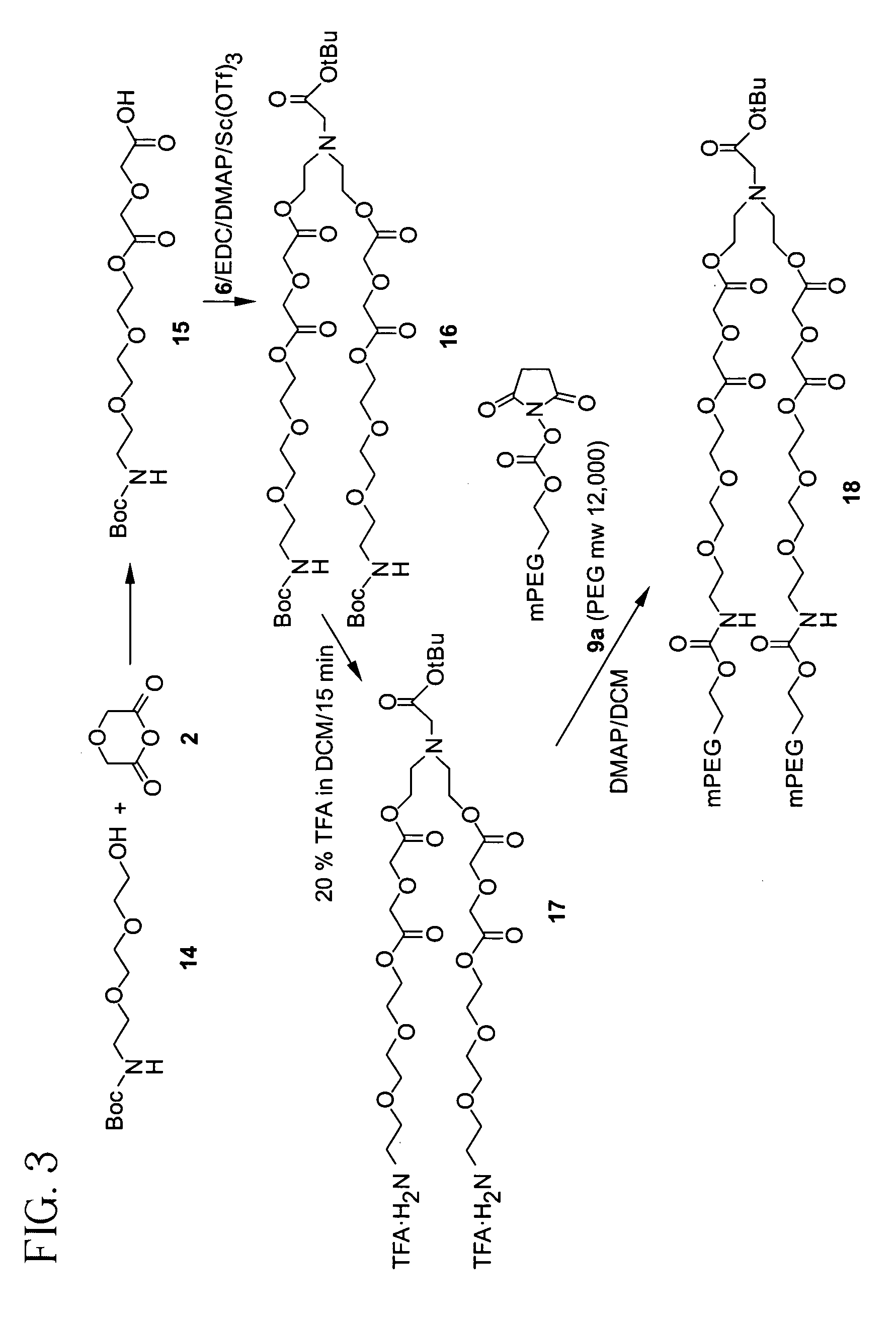
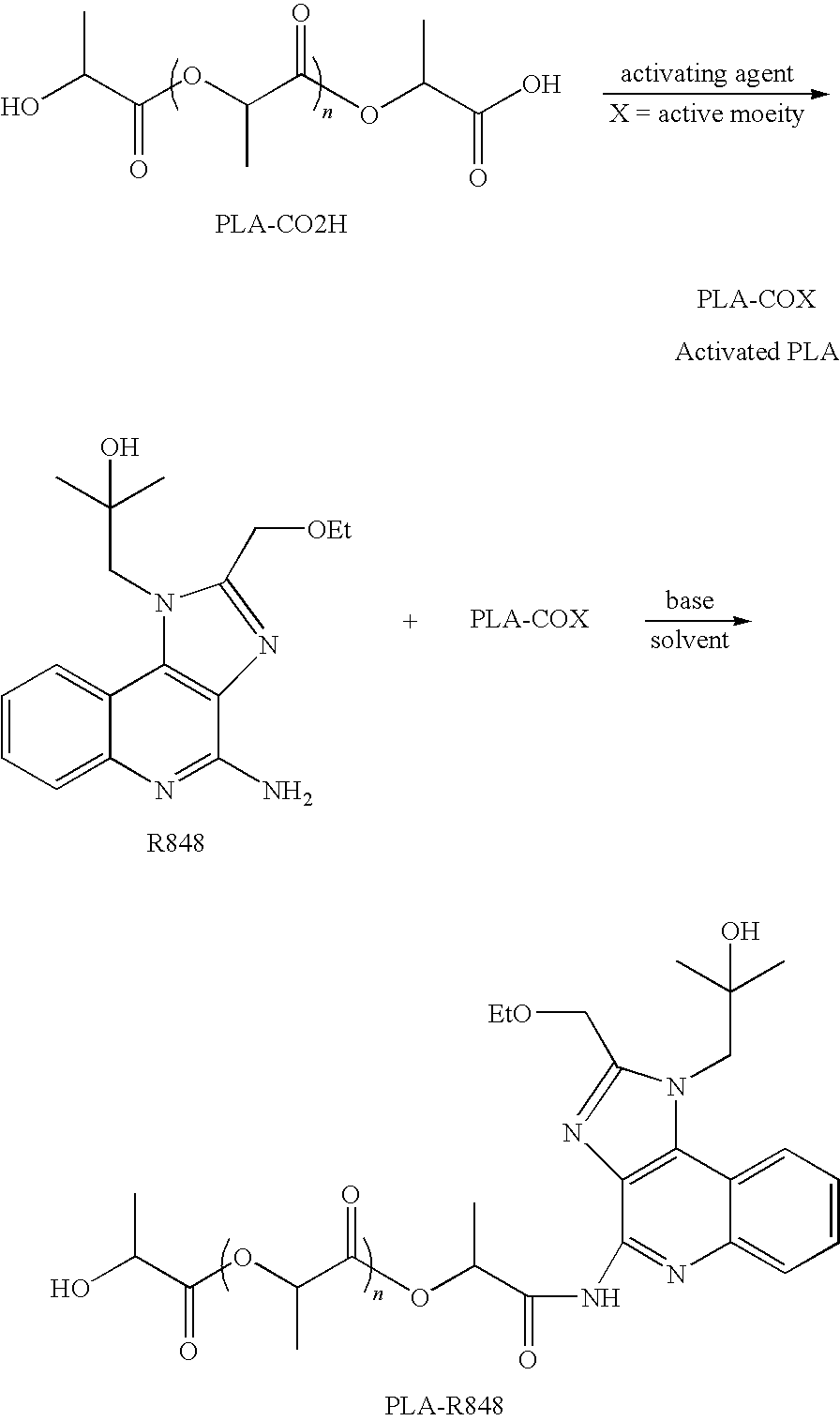
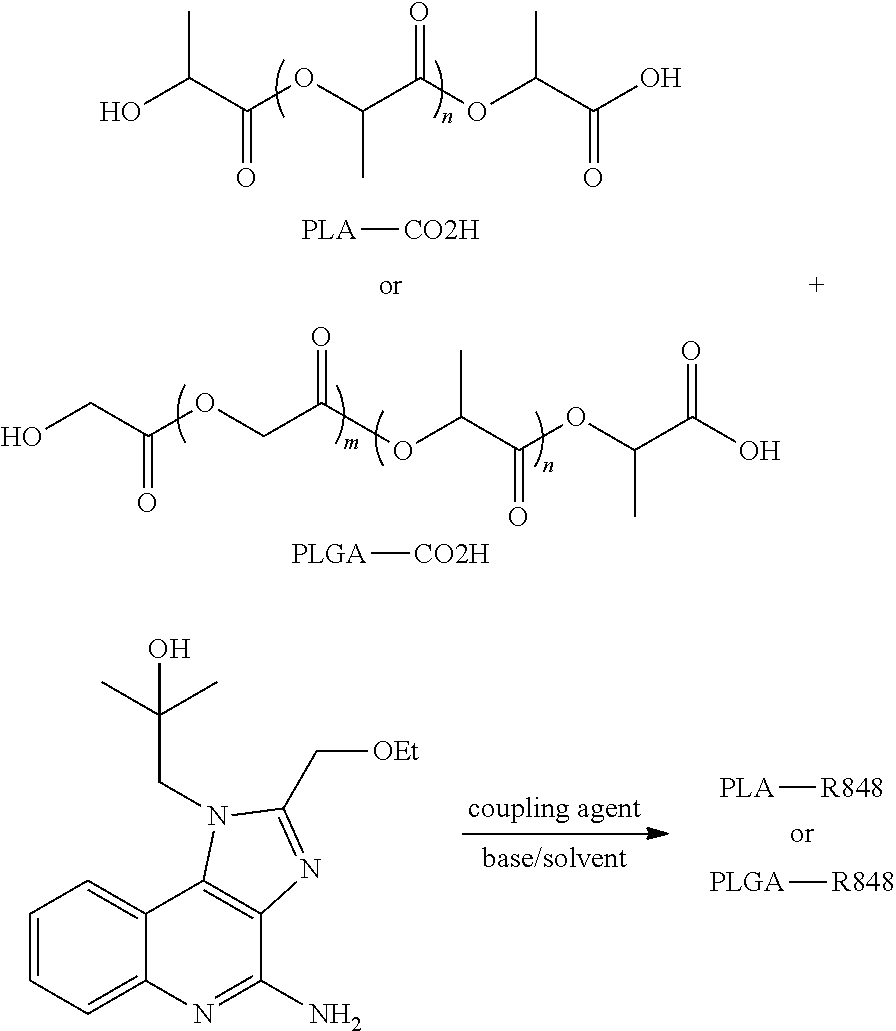
Releasable polymeric conjugates based on aliphatic biodegradable linkers
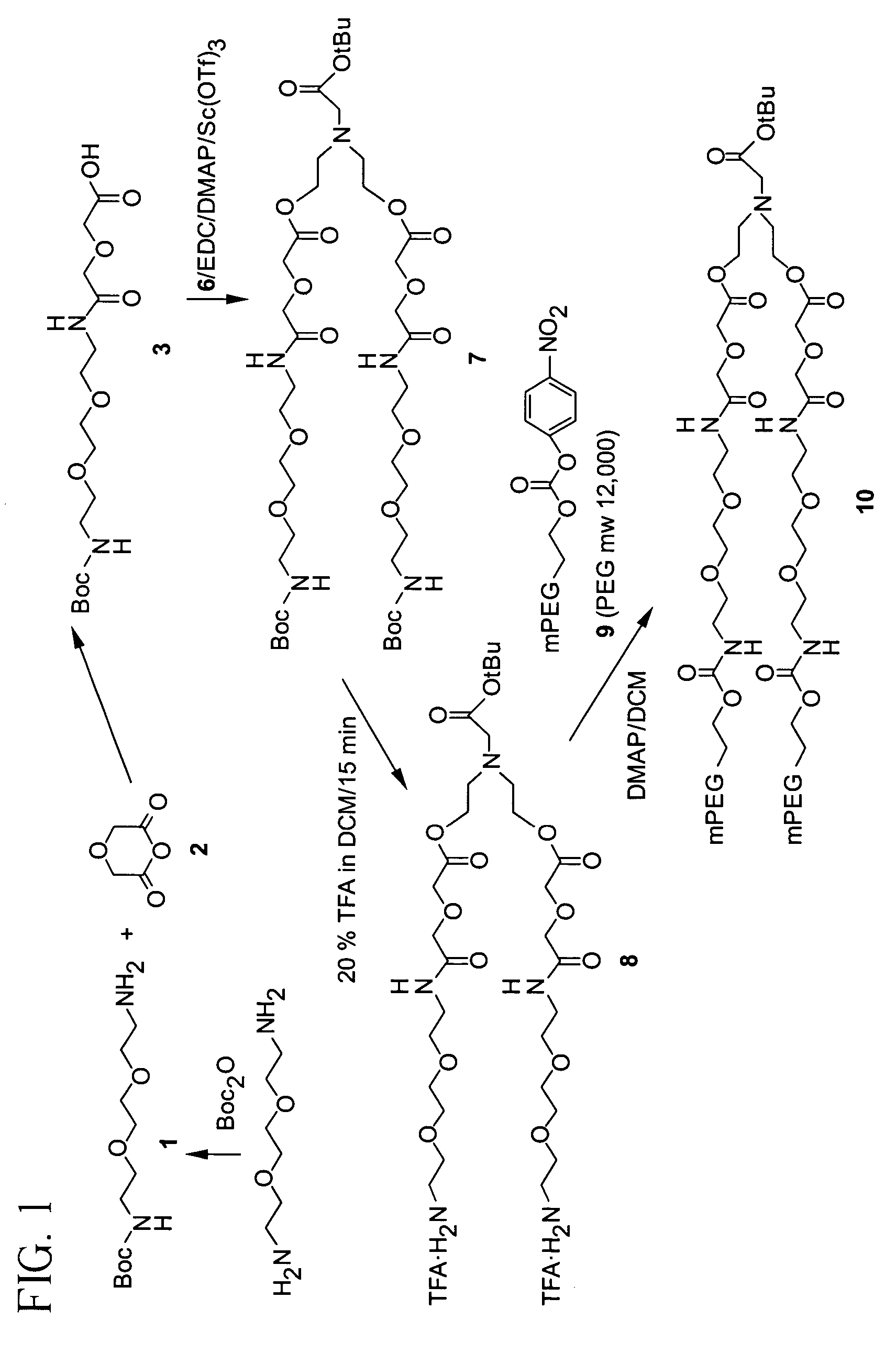
InactiveUS7087229B2Modulate the rate of hydrolysis of the prodrug and/or cellular uptakeGood water solubilityBiocideEther/acetal active ingredientsPolymerStereochemistry
Owner:BELROSE PHARMA
Receptor specific transepithelial transport of therapeutics
The present invention relates in general to methods and products for initiating an immune response against an antigen, and in particular relates to transepithelial delivery of antigens to provoke tolerance and immunity. The present invention further relates to methods and products for the transepithelial delivery of therapeutics. In particular, the invention relates to methods and compositions for the delivery of therapeutics conjugated to a FcRn binding partner to intestinal epithelium, mucosal epithelium and epithelium of the lung. The present invention further relates to the synthesis, preparation and use of the FcRn binding partner conjugates as, or in, pharmaceutical compositions for oral systemic delivery of drugs and vaccines.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC +1
Long lasting synthetic glucagon-like peptide {GLP-1}
Modified insulinotropic peptides are disclosed. The modified insulinotropic peptides are capable of forming a peptidase stabilized insulinotropic peptide. The modified insulinotropic peptides are capable of forming covalent bonds with one or more blood components to form a conjugate. The conjugates may be formed in vivo or ex vivo. The modified peptides are administered to treat humans with diabetes and other related diseases.
Owner:CONJUCHEM
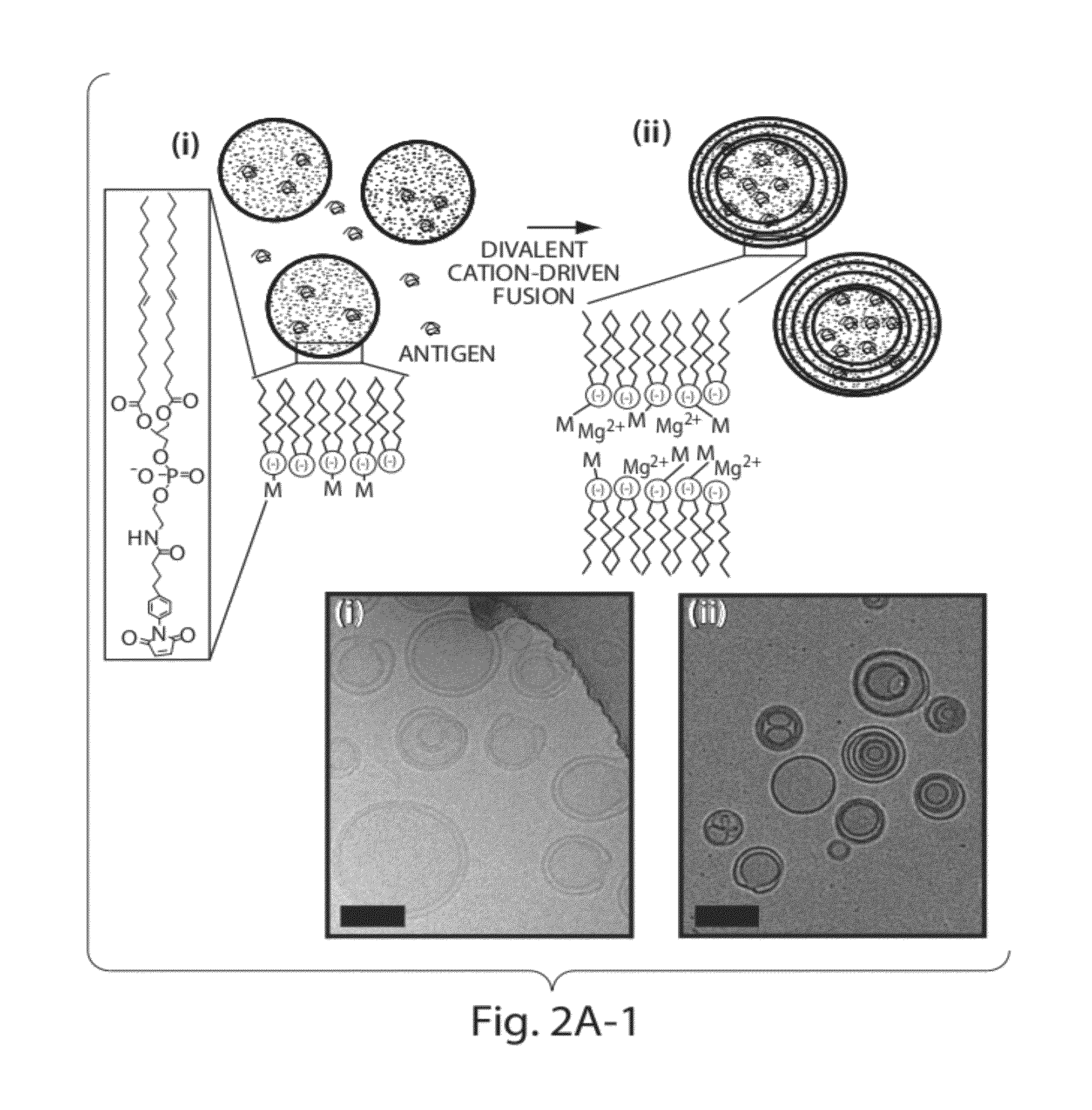
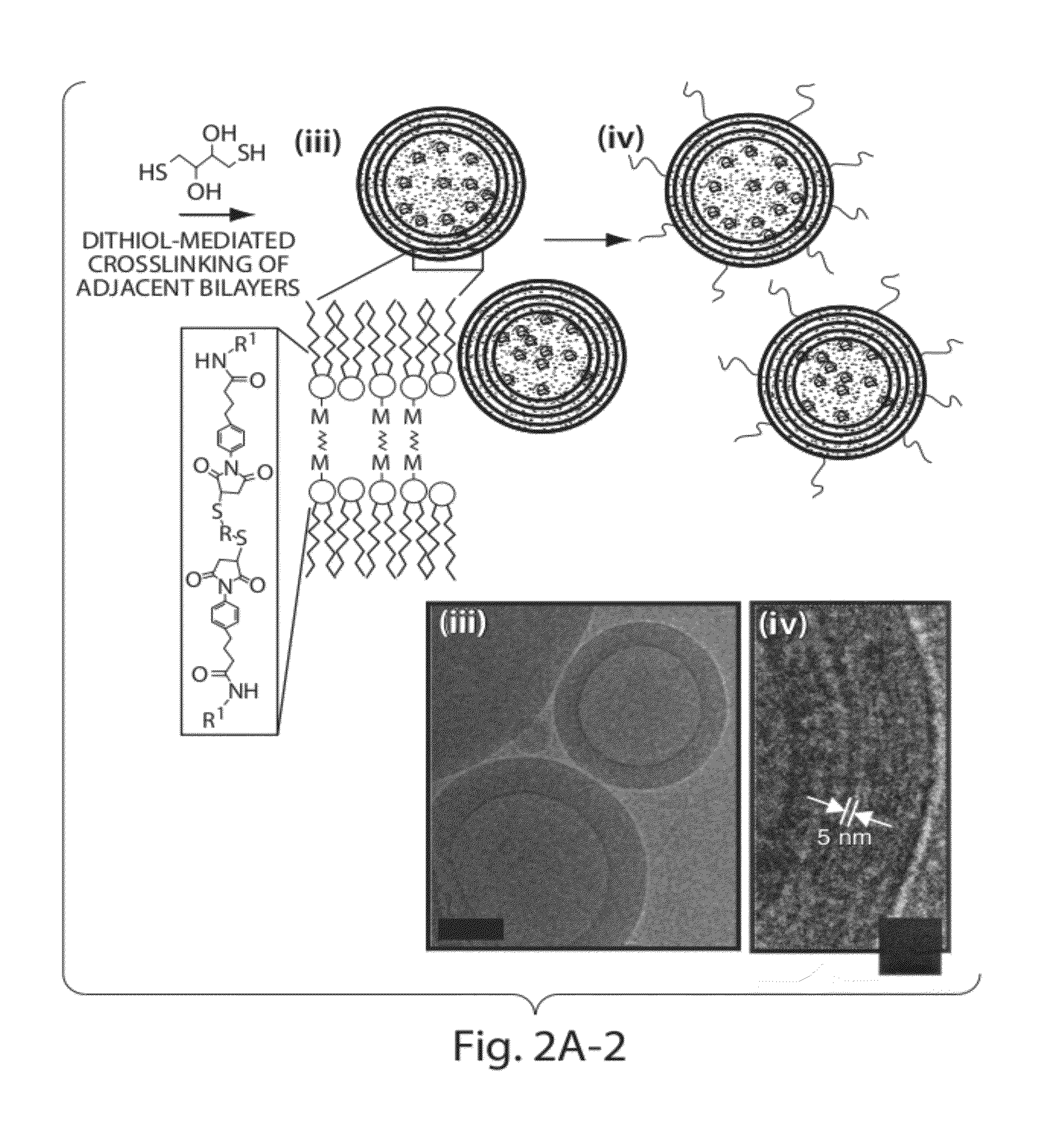
Lipid vesicle compositions and methods of use
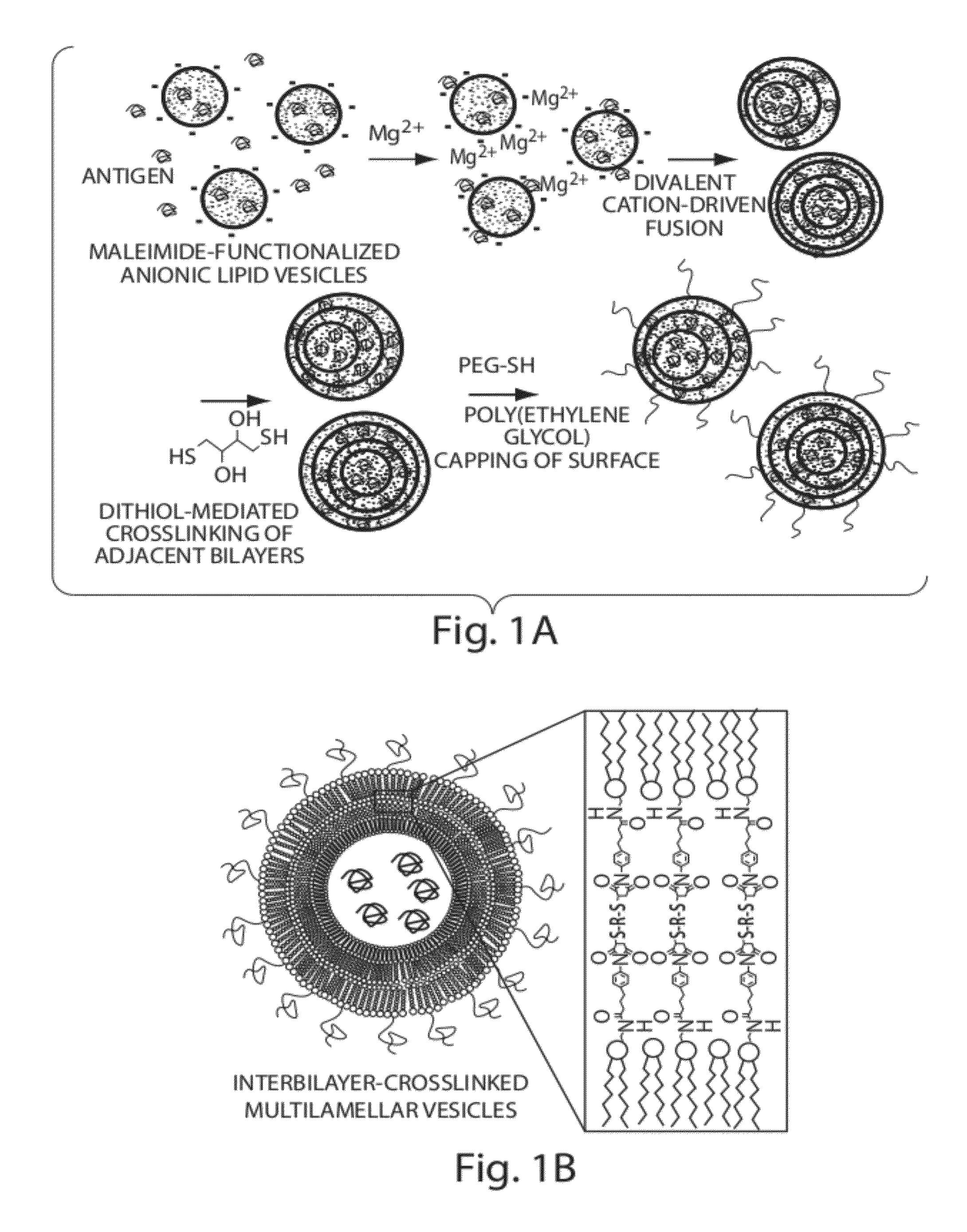
ActiveUS20120177724A1Increase load capacityImprove the level ofPeptide/protein ingredientsMicroencapsulation basedAntigenVesicle/vacuole
The invention provides delivery systems comprised of stabilized multilamellar vesicles, as well as compositions, methods of synthesis, and methods of use thereof. The stabilized multilamellar vesicles comprise terminal-cysteine-bearing antigens or cysteine-modified antigens, at their surface and / or internally.
Owner:MASSACHUSETTS INST OF TECH +1
Methods and compositions for the treatment of pancreatitis
InactiveUS6843998B1Prevents and reduces extent of fusionInhibition formationBacterial antigen ingredientsAntibody mimetics/scaffoldsProtein translocationDigestive enzyme
Methods and compositions for the treatment of acute pancreatitis in a mammal. Particular compositions comprise a binding element, a translocation element, and a therapeutic element able to prevent accumulation of digestive enzymes within the pancreas.
Owner:ALLERGAN INC
Fc fusion proteins for enhancing the immunogenicity of protein and peptide antigens
InactiveUS20050261229A1Stimulate productionEnhanceVirusesPeptide/protein ingredientsFc receptorAdjuvant
Disclosed herein are methods and compositions for enhancing the immunogenicity of a preselected protein or peptide antigen in a mammal. Immunogenicity is enhanced by fusing the preselected antigen to an immunoglobulin heavy chain constant region to produce an Fc-antigen fusion protein. The Fc-antigen fusion proteins bind Fc receptors on the surface of antigen presenting cells, thereby targeting the antigen to the antigen presenting cells in the mammal. In addition, disclosed is a family of adjuvants, for example, an Fc-adjuvant fusion protein, for use in combination with the Fc-antigen fusion proteins to enhance or modulate a particular immune response against the preselected antigen.
Owner:MERCK PATENT GMBH
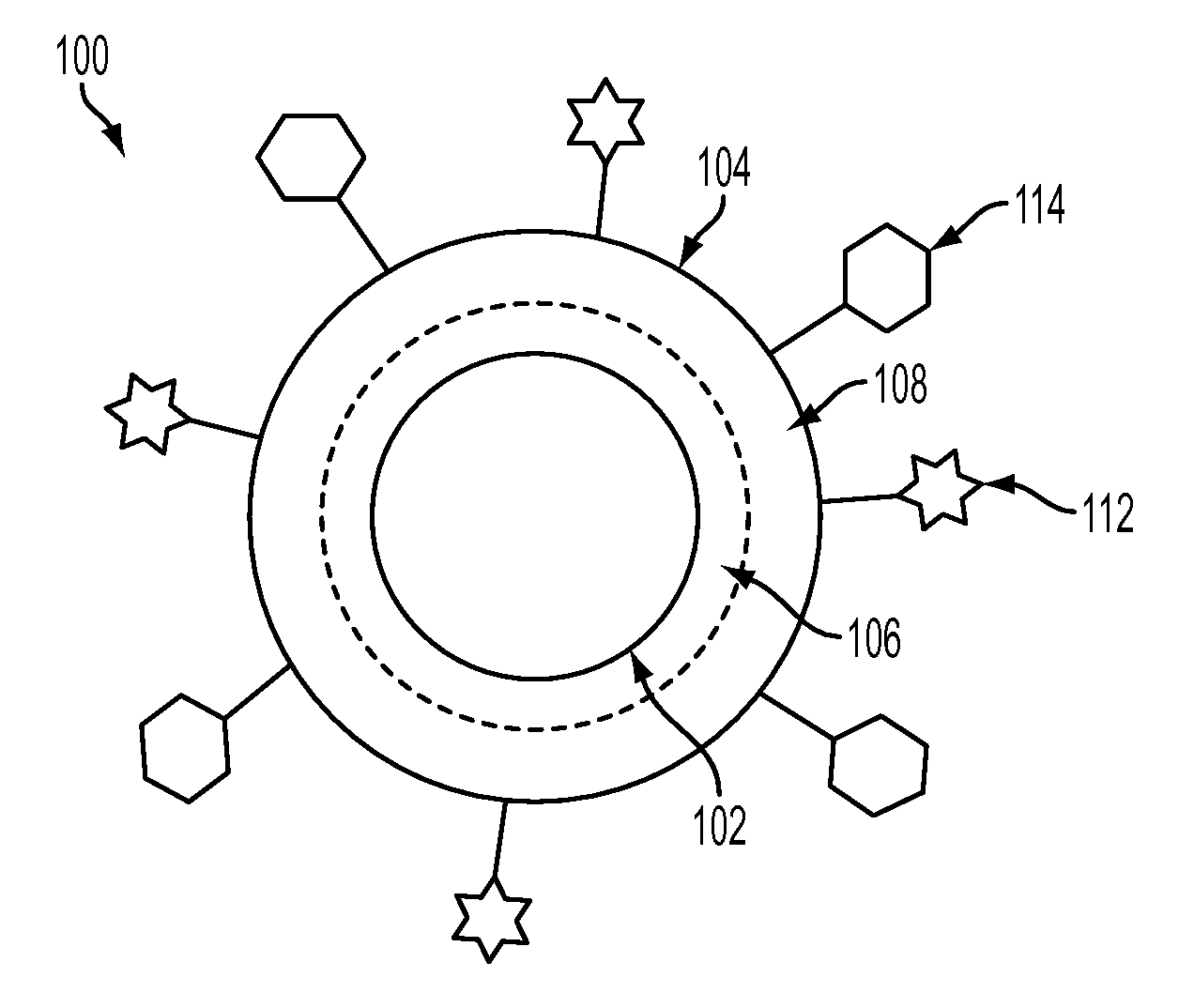
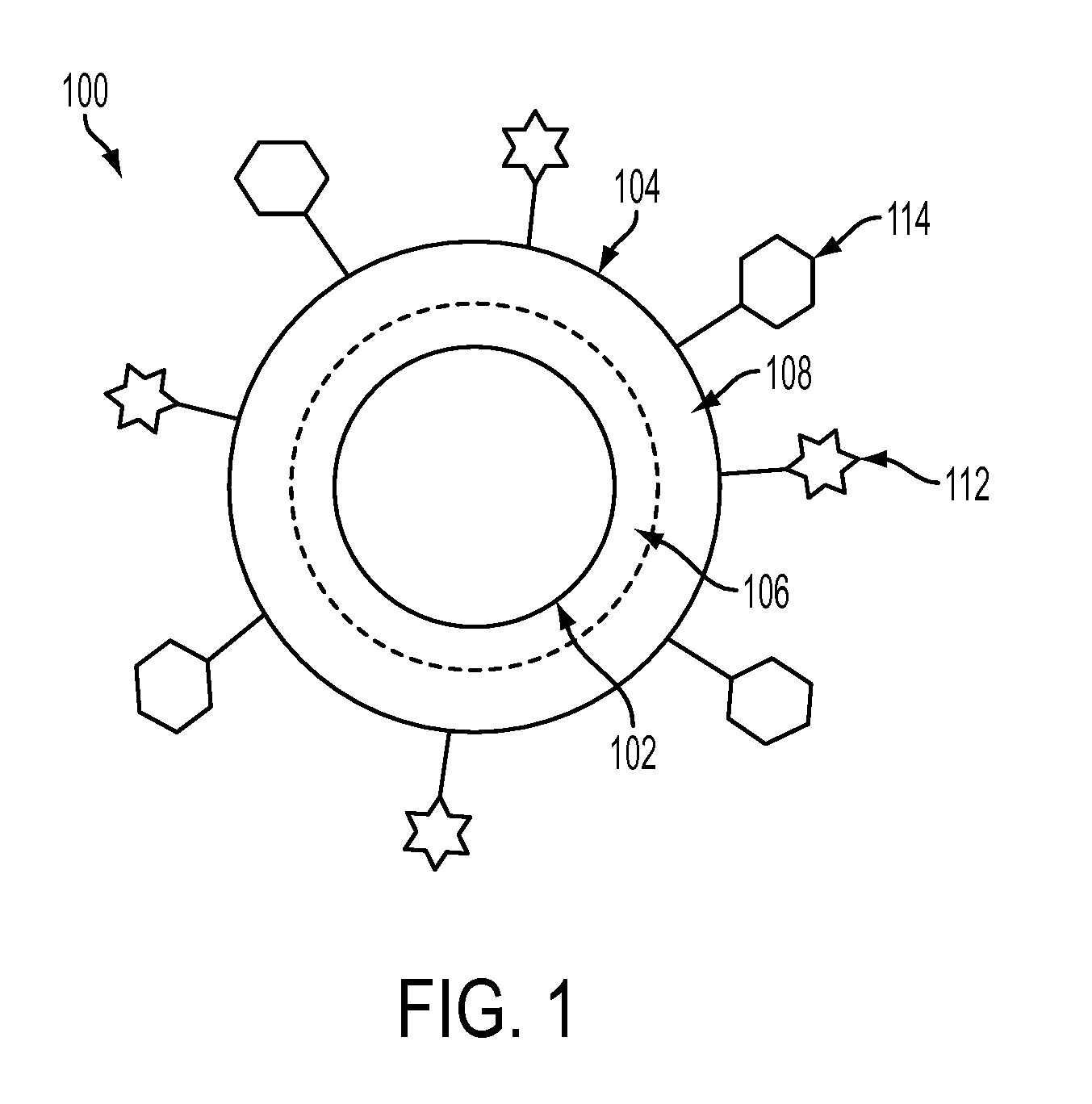
Nanoparticles for immunotherapy
ActiveUS20080031899A1High expressionPowder deliveryCarrier-bound antigen/hapten ingredientsNanoparticleImmunotherapy
Nanoparticles that activate complement in the absence of biological molecules are described. The nanoparticles are shown to specifically target antigen presenting cells in specifically in lymph nodes, without the use of a biological molecule for targeting. These particles are useful vehicles for delivering immunotherapeutics.
Owner:ECOLE POLYTECHNIQUE FEDERALE DE LAUSANNE (EPFL)
Nanoparticle Based Immunological Stimulation
A nanoparticle-based delivery system and methods for its use are disclosed. In one aspect, a nanoparticle-based delivery system comprising at least one molecule such as proteins, DNA / RNA or fragments thereof, carbohydrates, enzymes, chemicals, virus cells, bacteria, parts of a virus, parts of a bacteria, parts of a cell, part of a tissue, or a combination of one or more of these, which shall be referred to as immunogens, are chemically or physically combined with water soluble nanoparticles which, when administered to a living system, is capable of eliciting a desired immunological response. More particularly, the invention relates to nanoparticle-based delivery systems that are specifically engineered to enhance humoral or cellular immune response without the use of adjuvants.
Owner:UNIV OF HAWAII +1
Nanocarriers possessing components with different rates of release
InactiveUS20100303850A1Induce and enhance immune responseStrong and long-term humoral immune responsePowder deliveryNervous disorderAntigenNanocarriers
This invention relates to compositions, and related methods, of synthetic nanocarriers that comprise immunomodulatory agents and antigens that are differentially released from the synthetic nanocarriers.
Owner:SELECTA BIOSCI
Molecular antigen array
InactiveUS20060222652A1Improve efficiencyPeptide/protein ingredientsAntibody mimetics/scaffoldsAntigenAllergy
The invention provides compositions and processes for the production of ordered and repetitive antigen or antigenic determinant arrays. The compositions of the invention are useful for the production of vaccines for the prevention of infectious diseases, the treatment of allergies and the treatment of cancers. Various embodiments of the invention provide for a core particle that is coated with any desired antigen in a highly ordered and repetitive fashion as the result of specific interactions.
Owner:CYTOS BIOTECHNOLOGY AG
Targeting of Antigen Presenting Cells with Immunonanotherapeutics
ActiveUS20100129392A1Improve responsePowder deliverySnake antigen ingredientsNanocarriersImmunotherapeutic agent
The present invention provides compositions and systems for delivery of nanocarriers to cells of the immune system. The invention provides nanocarriers capable of stimulating an immune response in T cells and / or in B cells. The invention provides nanocarriers that comprise an immunofeature surface. The nanocarriers are capable of targeting antigen presenting cells when administered to a subject. The invention provides pharmaceutical compositions comprising inventive nanocarriers. The present invention provides methods of designing, manufacturing, and using inventive nanocarriers and pharmaceutical compositions thereof.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE +2
Targeted synthetic nanocarriers with ph sensitive release of immunomodulatory agents
This invention relates to compositions, and related methods, of synthetic nanocarriers that target sites of action in cells, such as antigen presenting cells (APCs), and comprise immunomodulatory agents that dissociate from the synthetic nanocarriers in a pH sensitive manner. Also disclosed are compositions and methods relating to synthetic nanocarriers that encapsulate labile immunomodulatory agents that dissociate from the synthetic nanocarriers in a pH sensitive manner.
Owner:SELECTA BIOSCI
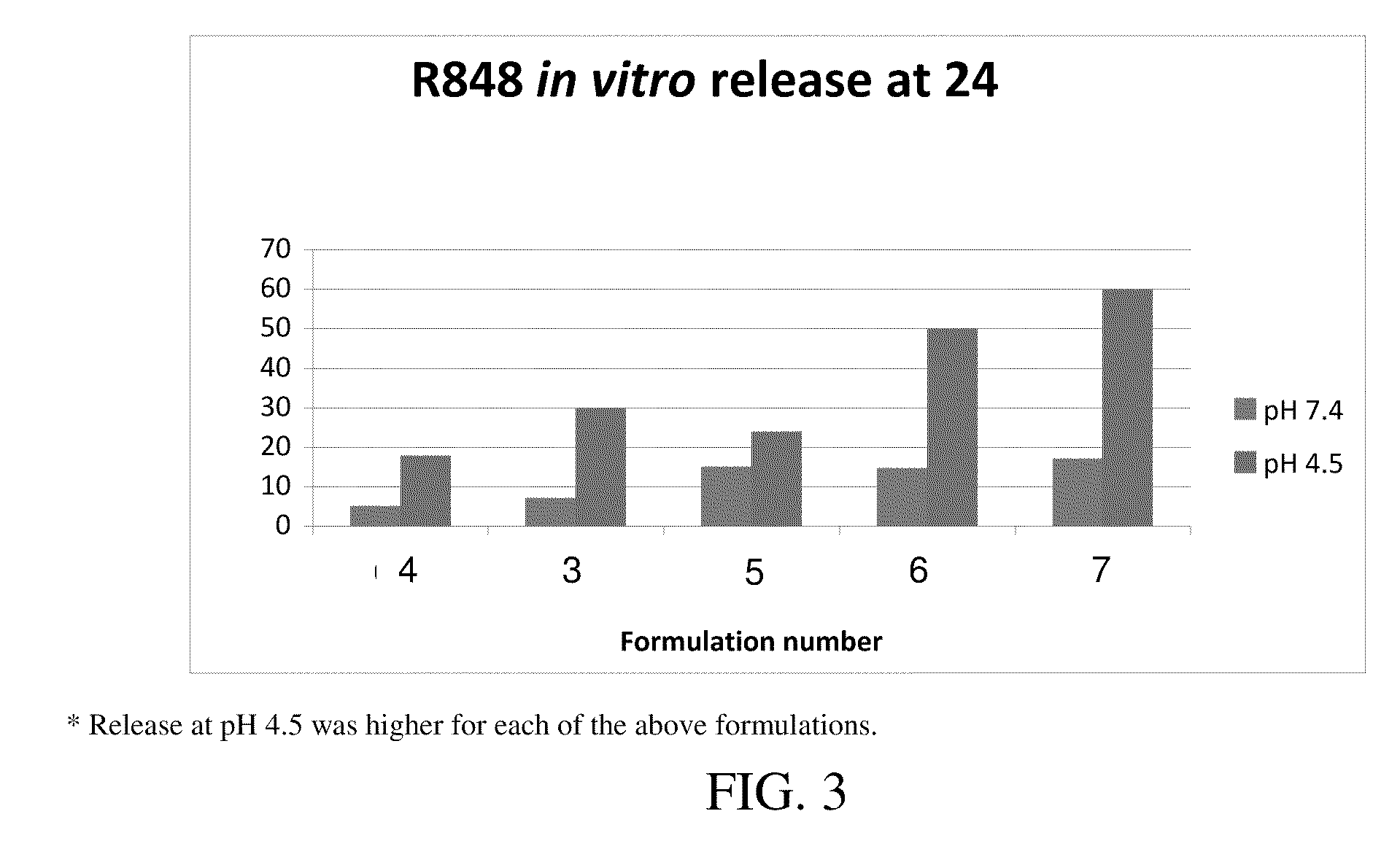
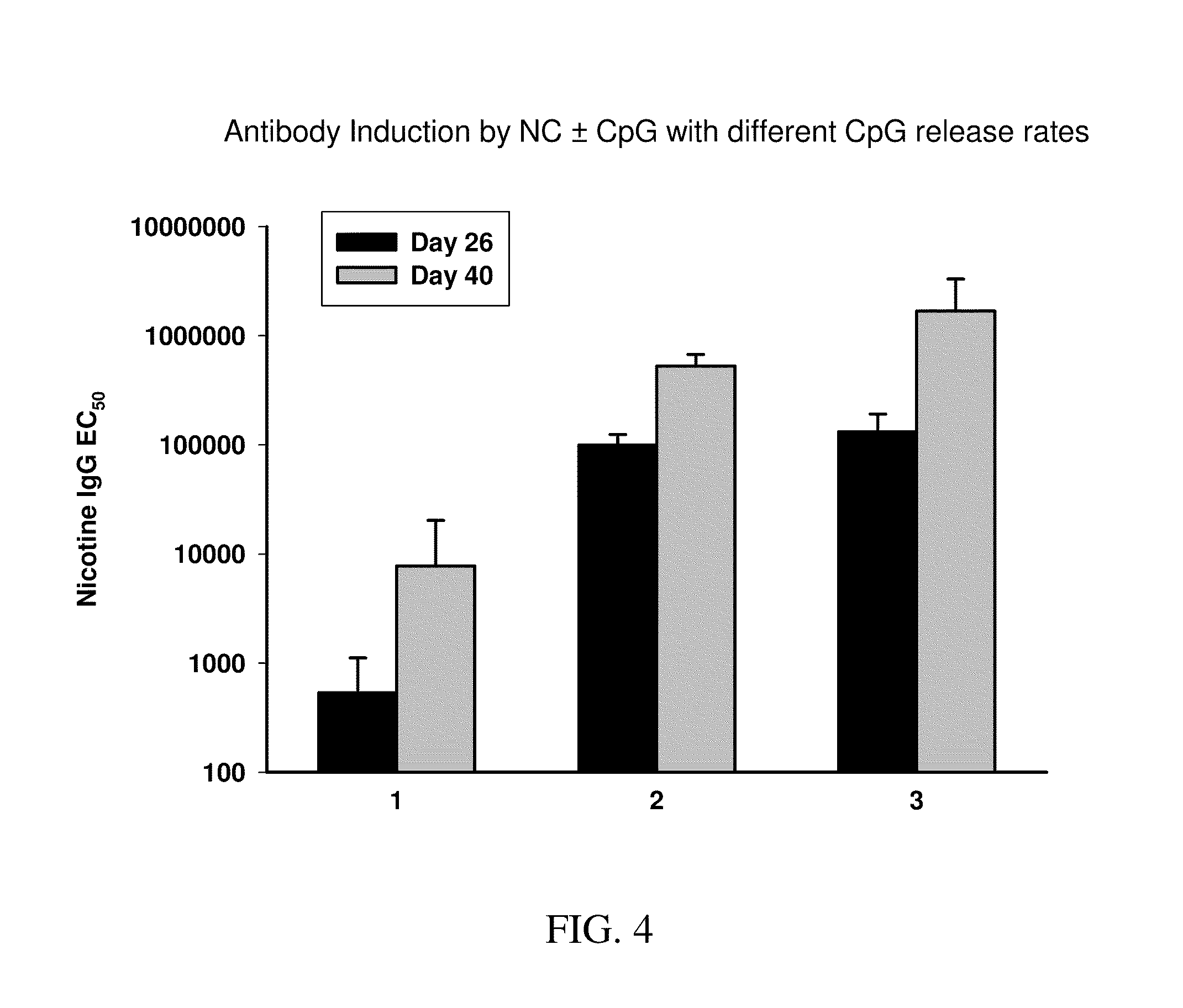
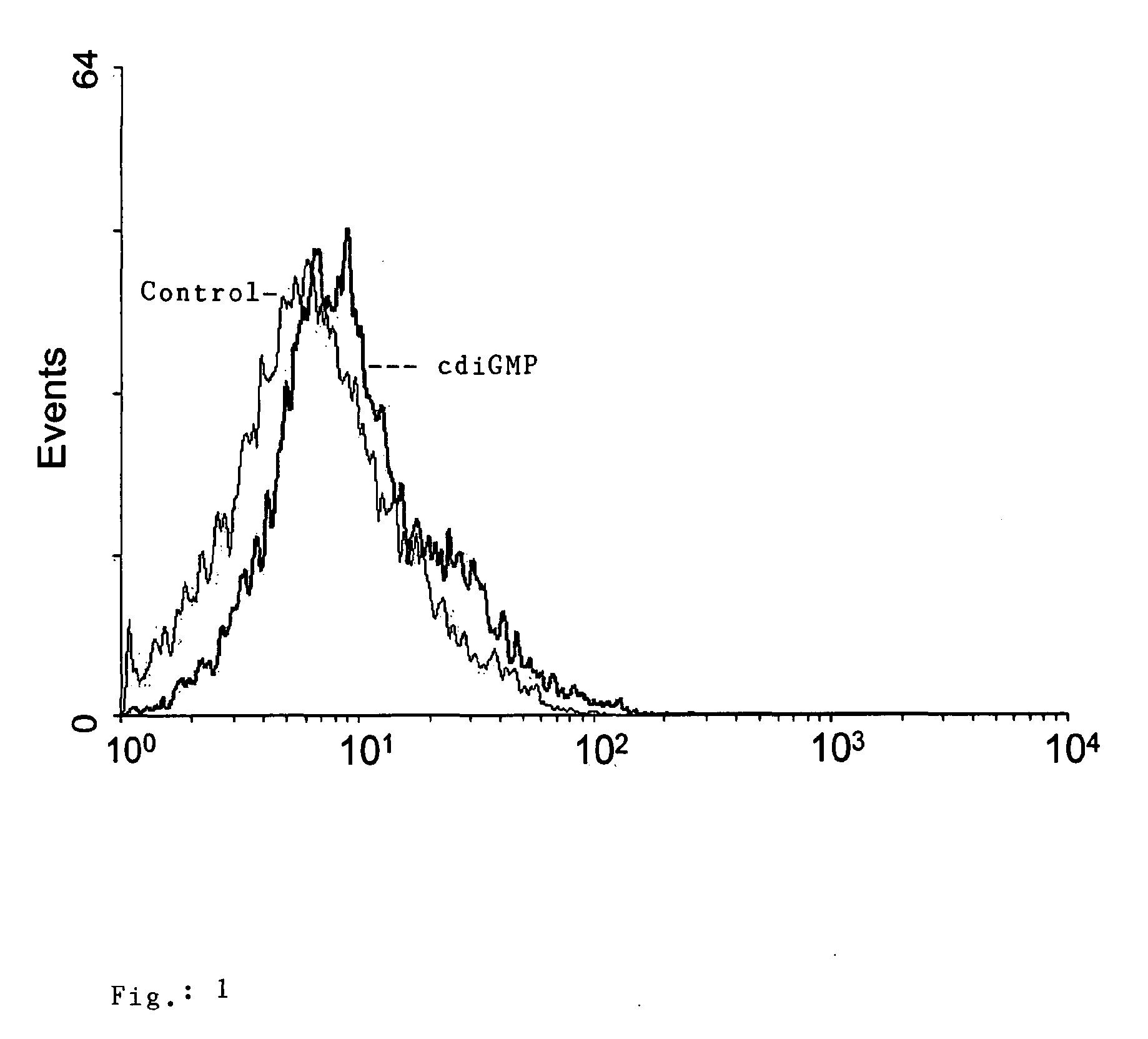
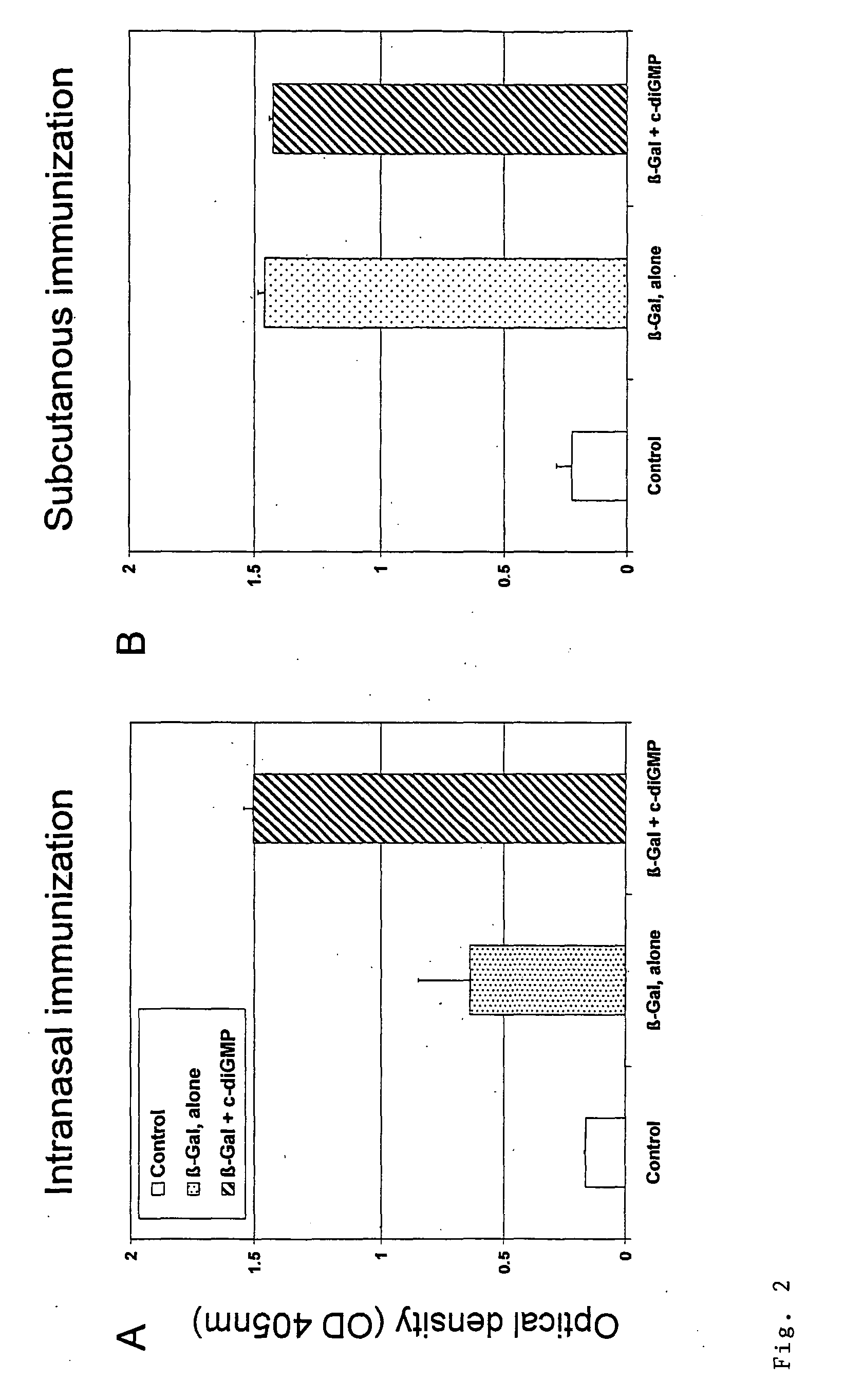
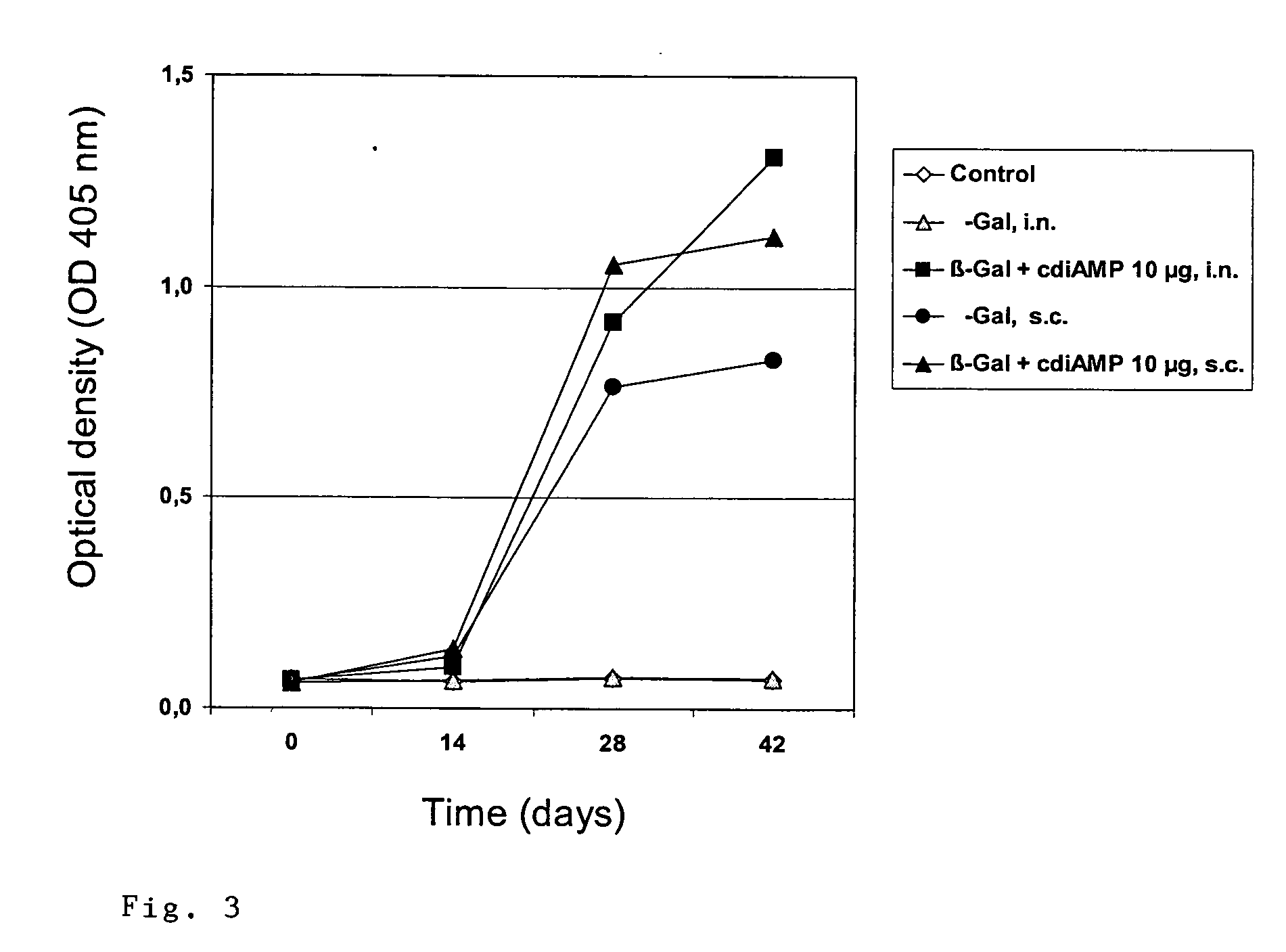
Cyclic-Dinucleotides and Its Conjugates as Adjuvants and Their Uses in Pharmaceutical Compositions
ActiveUS20080286296A1Severe formLess immunomodulatory effectOrganic active ingredientsAntipyreticDiseaseAutoimmune responses
The present invention relates to new adjuvants and the uses in pharmaceutical compositions, like in vaccines. In particular, the present invention provides new compounds useful as adjuvants and / or immunomodulators for prophylactic and / or therapeutic vaccination in the treatment of infectious diseases, inflammatory diseases, autoimmune diseases, tumors, allergies as well as for the control of fertility in human or animal populations. The compounds are particularly useful not only as systemic, but preferably as mucosal adjuvants. In addition, the invention relates to its uses as active ingredients in pharmaceutical compositions.
Owner:GESELLSCHAFT FUR BIOTECHNOLOGISCHE FORSCHUNG MBH GBF
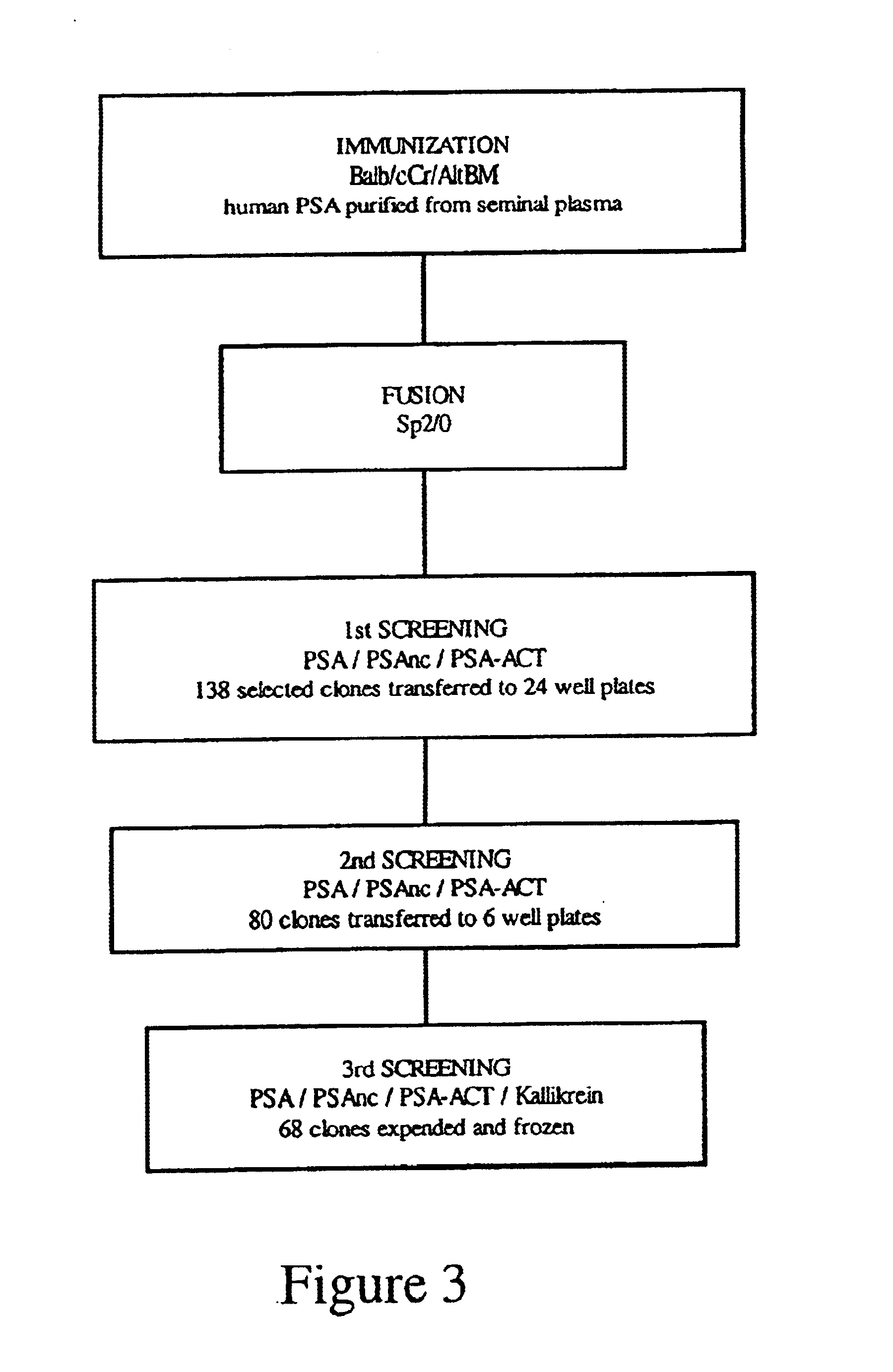
Reagents and methods for inducing an immune response to prostate specific antigen
InactiveUS6881405B2Sensitive to proteolytic degradationIncrease productionSnake antigen ingredientsAntibody ingredientsProstate-specific antigenAntibody
Owner:ONCOQUEST INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com