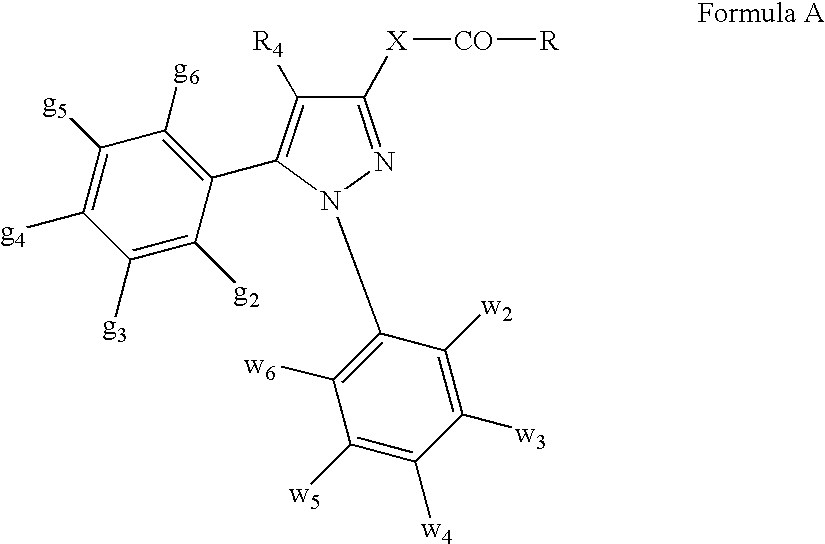
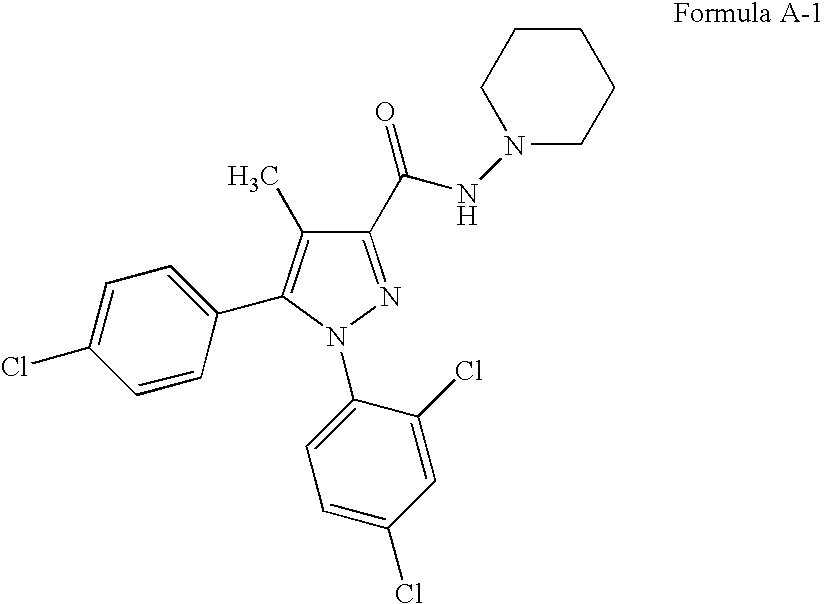
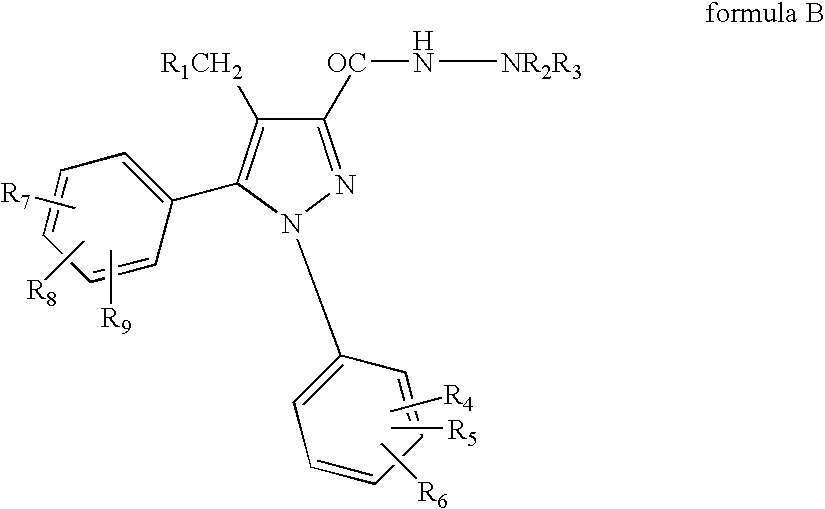
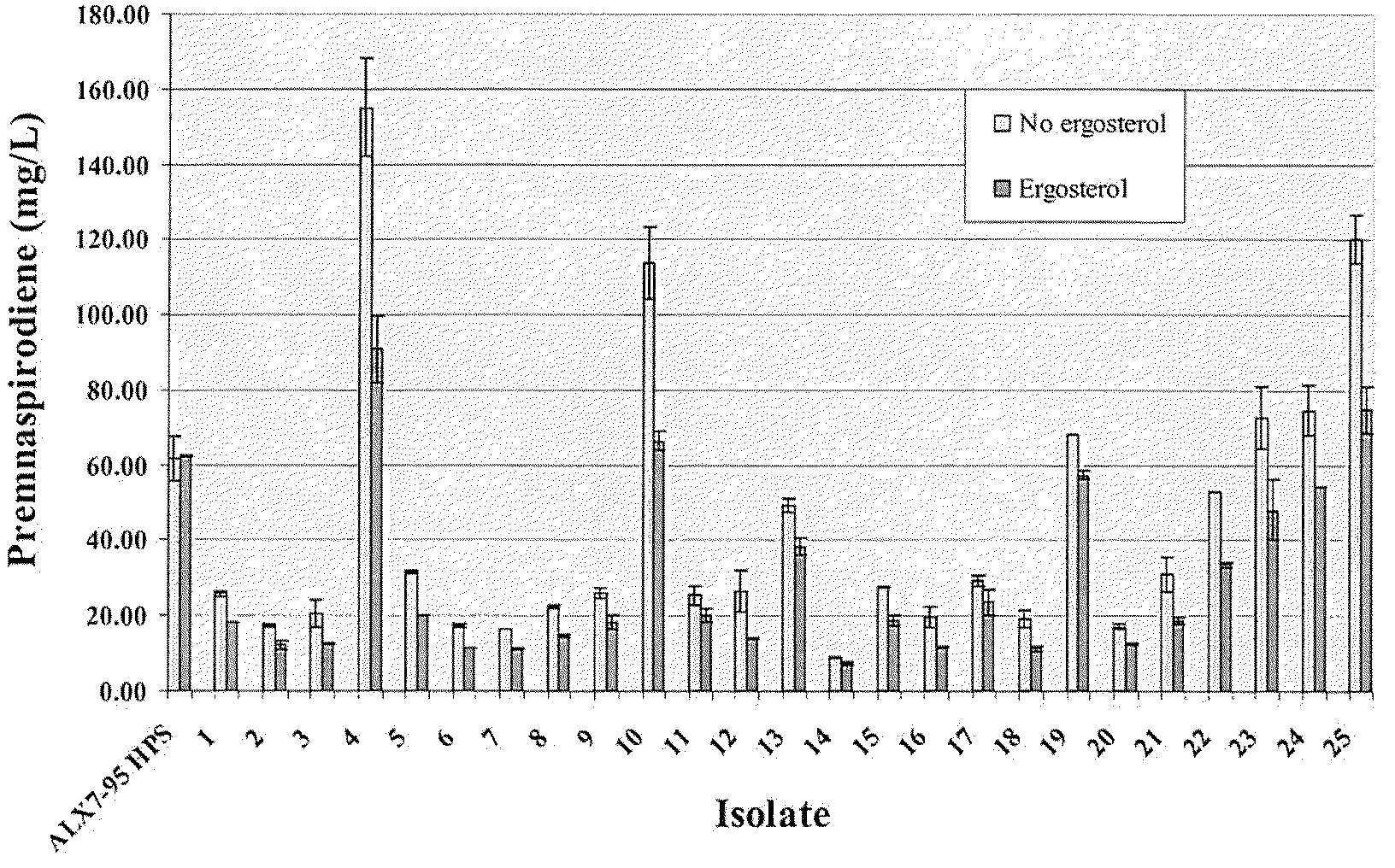
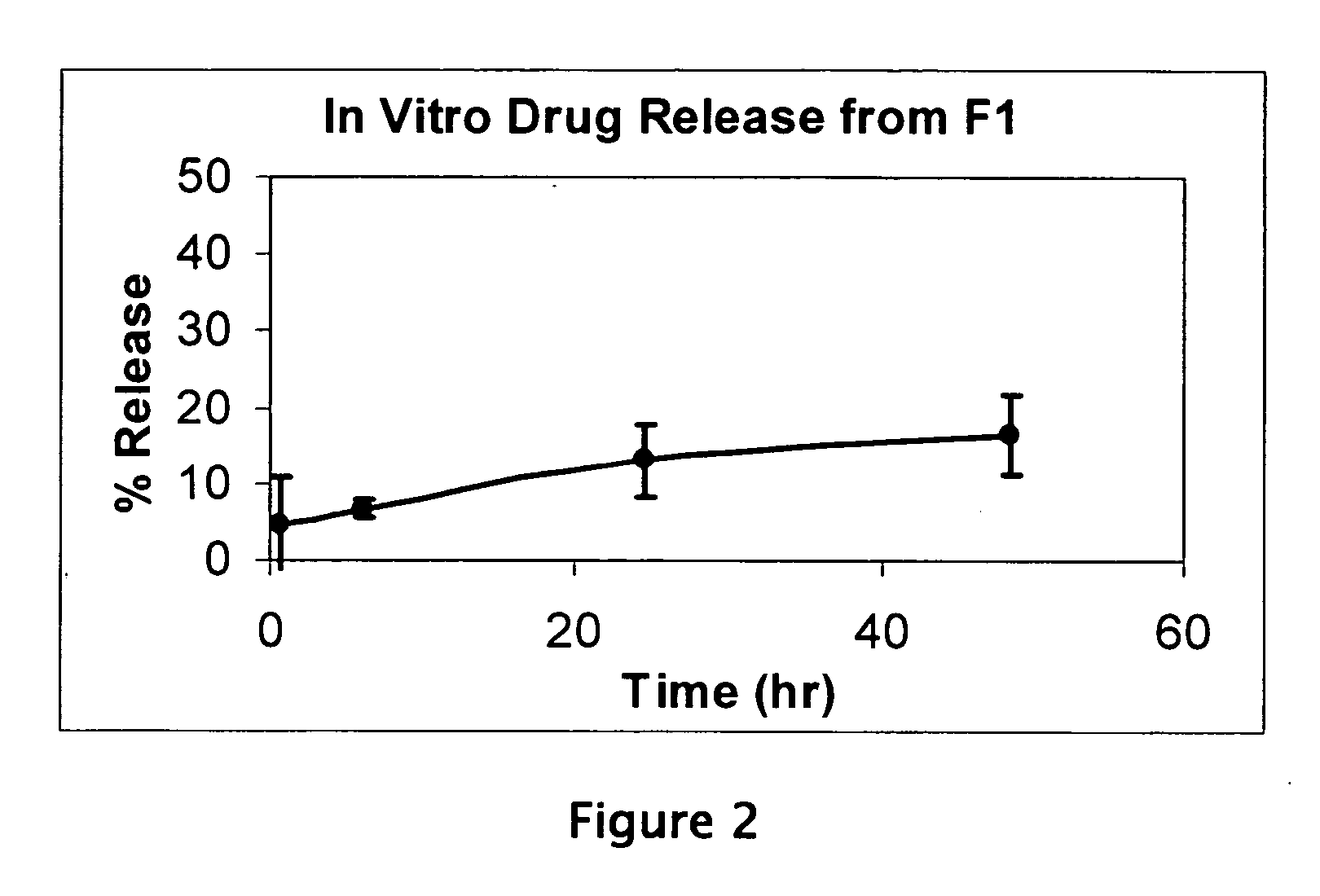
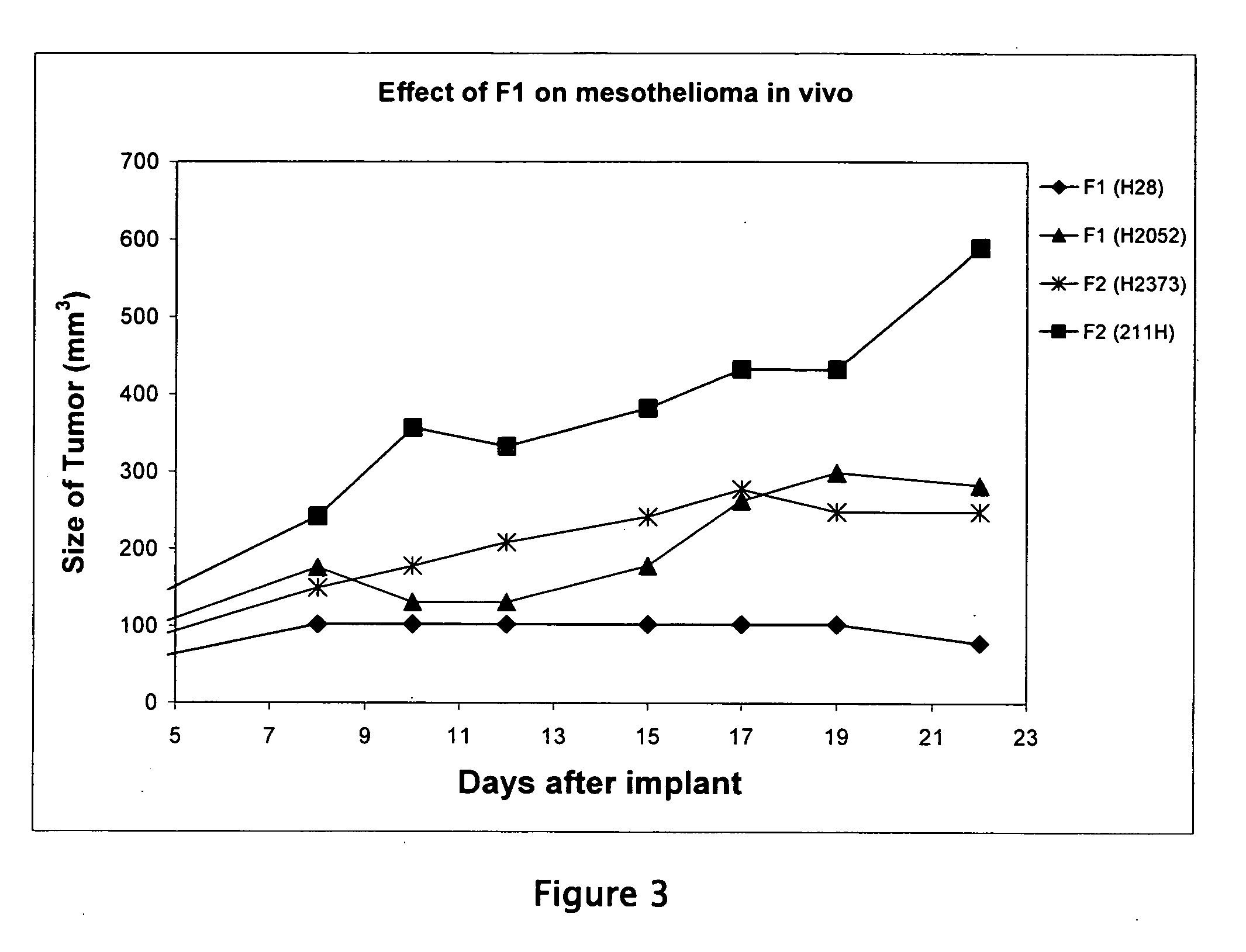
Patents
Literature
1170 results about "Sterol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
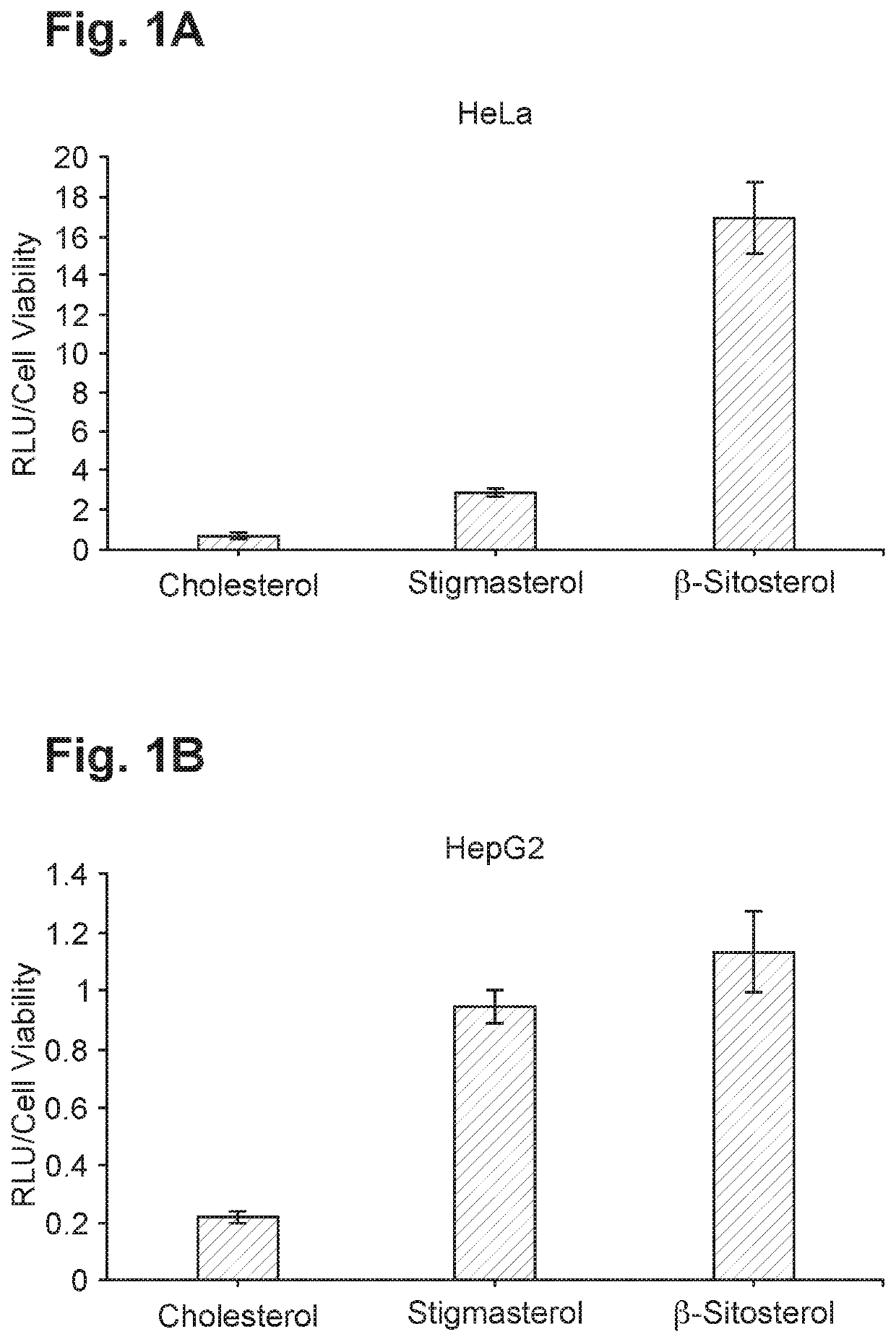
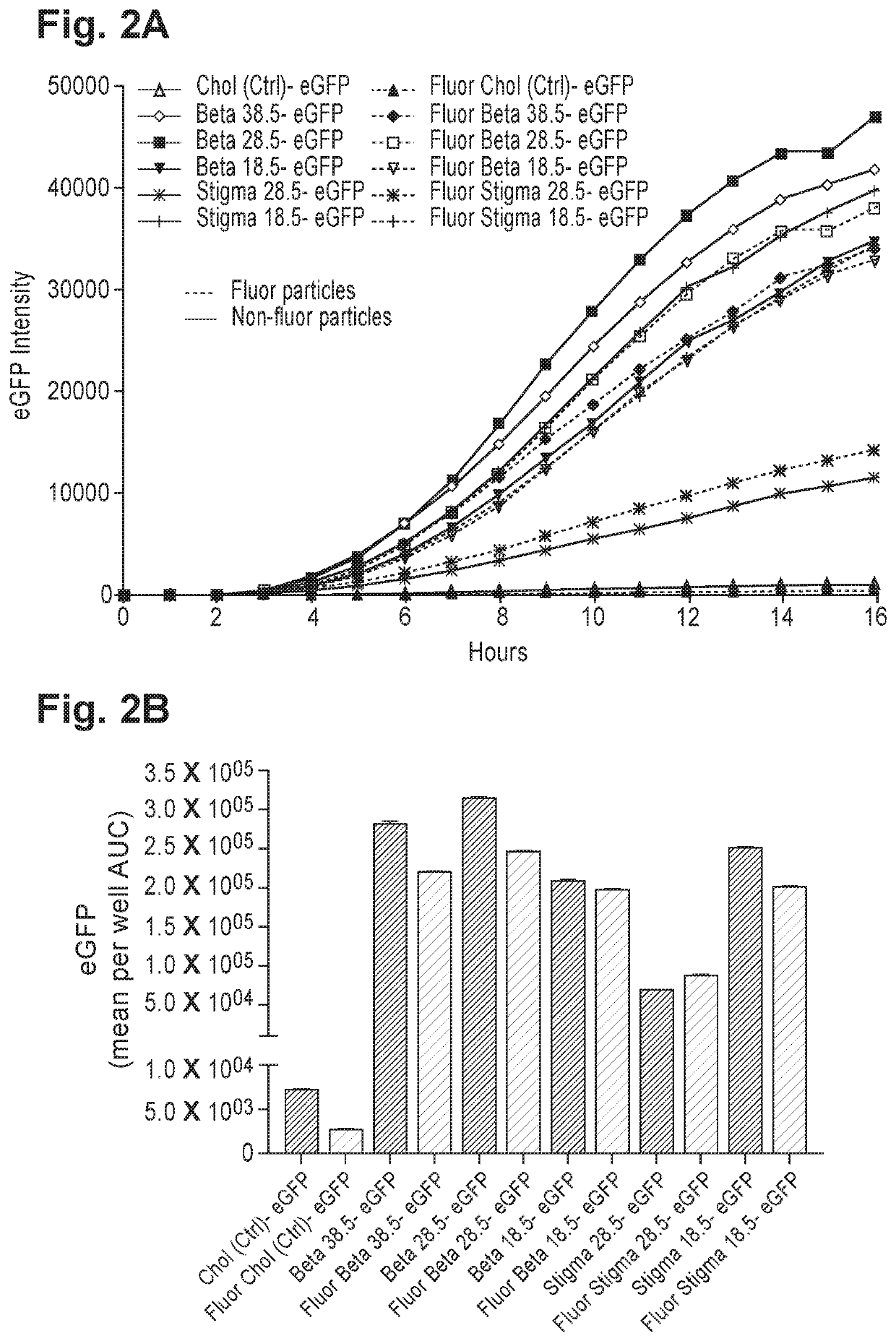
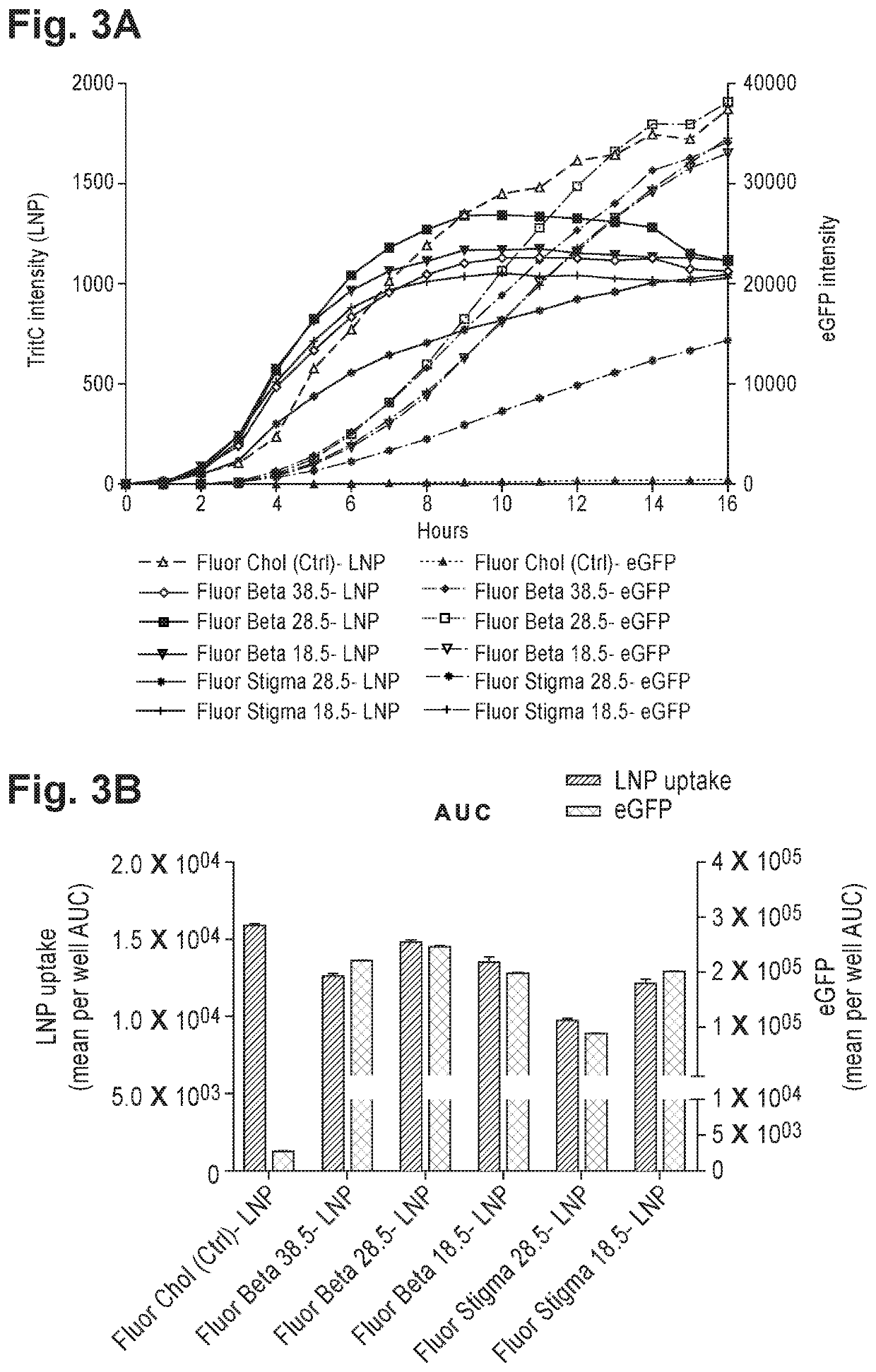
Sterols, also known as steroid alcohols, are a subgroup of the steroids and an important class of organic molecules. They are a type of lipid. They occur naturally in plants, animals, and fungi, and can be also produced by some bacteria (however likely with different functions). The most familiar type of animal sterol is cholesterol, which is vital to cell membrane structure, and functions as a precursor to fat-soluble vitamins and steroid hormones.
Potentiation of immune responses with liposomal adjuvants
InactiveUS6090406AGood water solubilityPractical and convenientBacterial antigen ingredientsViral antigen ingredientsLipid formationOrganic acid
A high integrity liposome comprising at least one stabile lipid and at least one peptide-like therapeutic agent associated with said liposome, adapted for parenteral administration to an animal, including a human, and method according to manufacture and use. Immunizing dosage forms comprising a liposome and an immunogen, wherein said liposome and immunogen are present in an immunization dose. Additionally, a dosage form, including such form particularly adapted to producing an immune response, comprising a salt according to an organic acid derivative of a sterol and an immunogen wherein said organic acid derivative of a sterol and immunogen are present in an immunization dose, and method according to use is disclosed. Further, a dosage form, including such form particularly adapted to producing an immune response, comprising dimyristoylphosphatidylcholine (DMPC) / cholesterol liposomes, optionally in an aluminum hydroxide gel, and an immunogen wherein said DMPC / cholesterol and immunogen are present in an immunization dose, and method according to use.
Owner:TRANSAVE
Lipid compositions
Disclosed herein are lipid compositions comprising a cationic lipid of formula (I), a neutral lipid, a sterol and a PEG or PEG-modified lipid, wherein formula (I) is (F). Also disclosed are methods of producing the cationic lipid of formula (I).
Owner:ARBUTUS BIOPHARMA CORPORAT ION
Topical Composition for Treating Pain
ActiveUS20080311167A1Ameliorate and eliminate painFree from painBiocideHydrocarbon active ingredientsSequelaPreventing pain
Topical compositions having as the active ingredient a lipid, fatty acid ester, natural wax, sterol, or combinations thereof referred to herein as “lipophilic vehicle” or “LV” and methods of use, have been developed for the amelioration or prevention of pain or the sequelae of pain. The composition may be in the form of an ointment, cream, gel, lotion, spray, foam, paste, patch, suspension or dispersion. In the preferred embodiment, the formulation is a gel. The LV may contain a penetration enhancer, most preferably one with membrane disruptive properties. The formulation may be applied to or impregnated into a gauze, wrap, bandage, cotton-tipped stick, adhesive bandage strip, or other support wrap or medical bandage or wound cover. For example, the compositions may be are incorporated onto or into disposables such as hemorrhoid wipes, sponge, mouth guards, dental trays; needles or catheters; adult diapers; gloves, socks or wrist bands, for ease of application. The composition is applied topically to a site at or adjacent to a painful region. The composition is reapplied as necessary. Pain relief is typically obtained within minutes and lasts for periods of variable duration ranging from minutes to several hours and even, in some cases, days. The composition is variably effective to treat visceral, somatic and neuropathic pain both acute and chronic as well as muscle pain and stiffness and joint pain and stiffness.
Owner:EPICENTRX
Transporter-enhanced corticosteroid activity and methods and compositions for treating dry eye
ActiveUS20080194468A1Extended duration of actionGood anti-inflammatory activityBiocideOrganic active ingredientsAnti-inflammatorySterol
Methods and compositions for enhancing the activity and / or duration of action of loteprednol etabonate and other soft anti-inflammatory steroids of the haloalkyl 17α-alkoxycarbonyloxy-11β-hydroxyandrost-4-cn-3-one-17β-carboxylate type and the corresponding Δ1,4-compounds are described. The enhancing agents have the formula:wherein Z1 is carbonyl, β-hydroxymethylene or methylene; R2 is H, —OH or —OCOR3 wherein R3 is C1-5 alkyl; Y is —OH, —SH or —OCOR4 wherein R4 is C1-5 alkyl, cyclopentylethyl or diethylaminoethyl; and the dotted line in ring A indicates that the 1,2-linkage is saturated or unsaturated. Ophthalmic administration in the treatment of dry eye is specifically targeted.
Owner:BODOR LAB
Lipid compositions
Disclosed herein are lipid compositions comprising a cationic lipid of formula (I), a neutral lipid, a sterol and a PEG or PEG-modified lipid, wherein formula (I) isAlso disclosed are methods of producing the cationic lipid of formula (I).
Owner:ARBUTUS BIOPHARMA CORPORAT ION
Sterol-Modified Amphiphilic Lipids
Disclosed are sterol-modified amphiphilic lipid compounds having two or more hydrophobic tails of which at least one is a sterol. Also disclosed are the processes for the synthesis of these compounds, compositions comprising such compounds, and the use of such compounds in delivery of an agent of interest, e.g., therapeutics, imaging agents, contrast materials for ultrasound applications, vaccines, biosensors, nutritional supplements and skin care products.
Owner:RGT UNIV OF CALIFORNIA
Lipid compositions
Disclosed herein are lipid compositions comprising a cationic lipid of formula (I), a neutral lipid, a sterol and a PEG or PEG-modified lipid, wherein formula (I) is (F). Also disclosed are methods of producing the cationic lipid of formula (I).
Owner:ARBUTUS BIOPHARMA CORPORAT ION
Pharmaceutical treatments and compositions
InactiveUS20070129282A1High expressionStimulate immune responseBiocidePeptide/protein ingredientsRegimenSterol
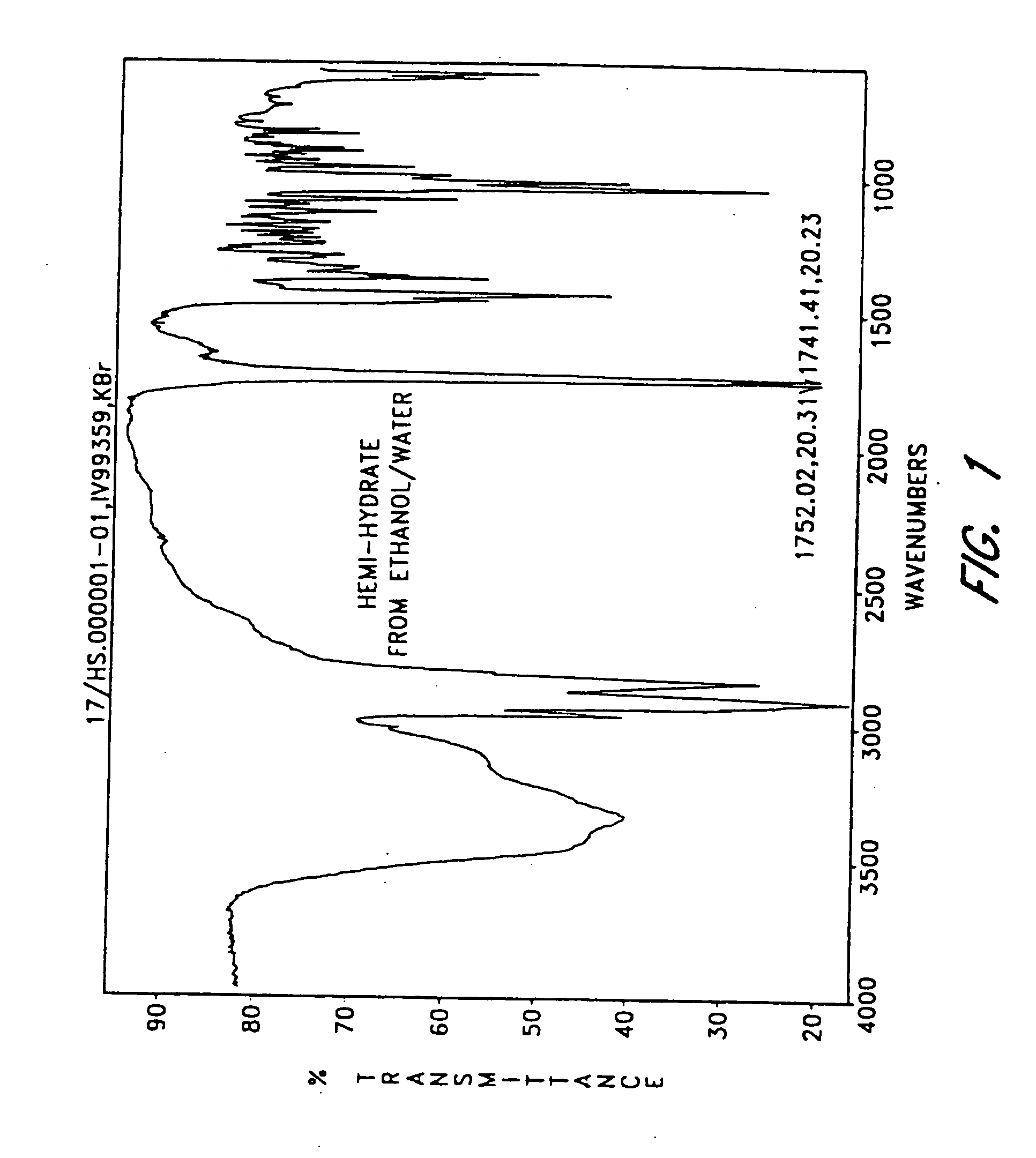
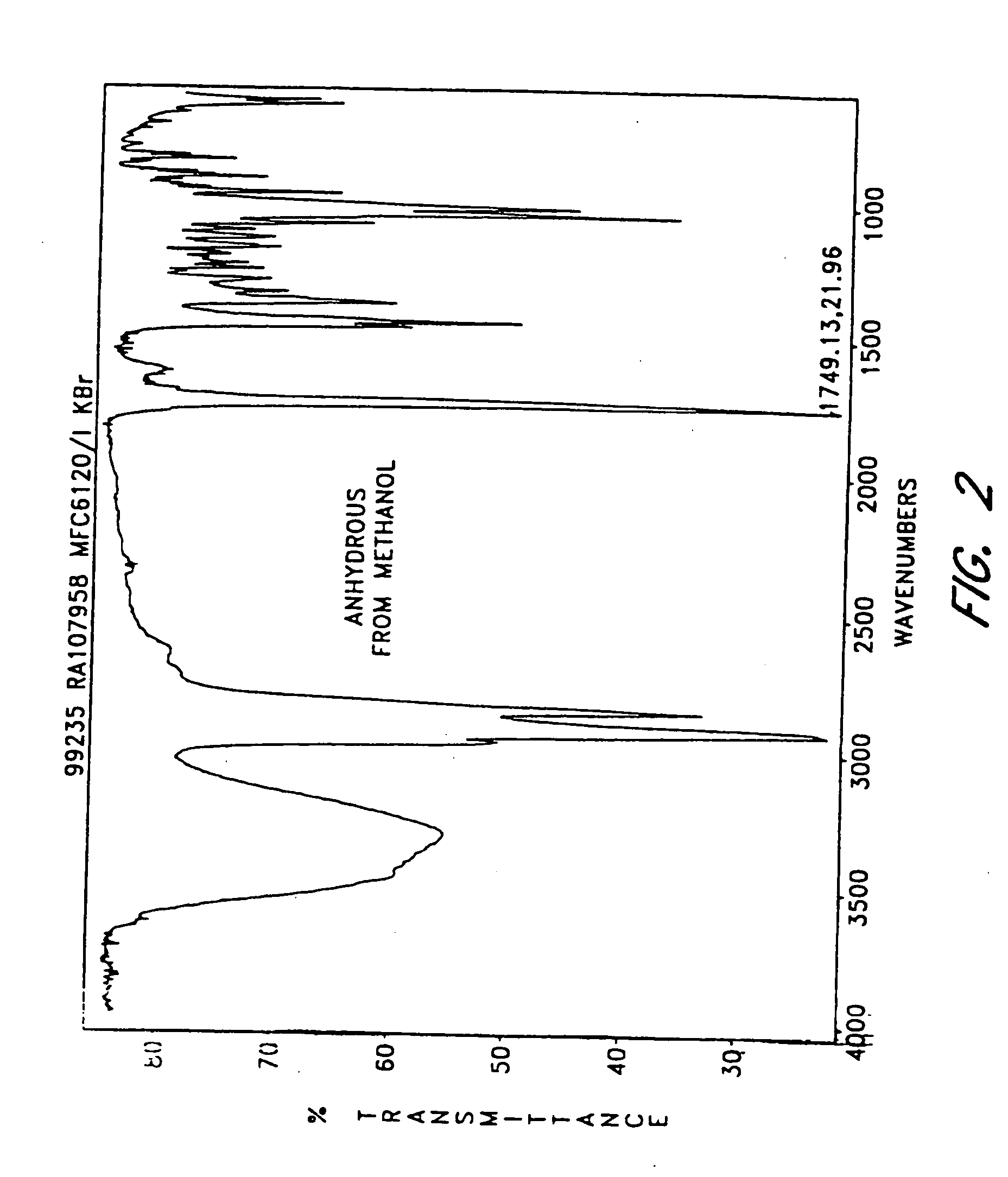
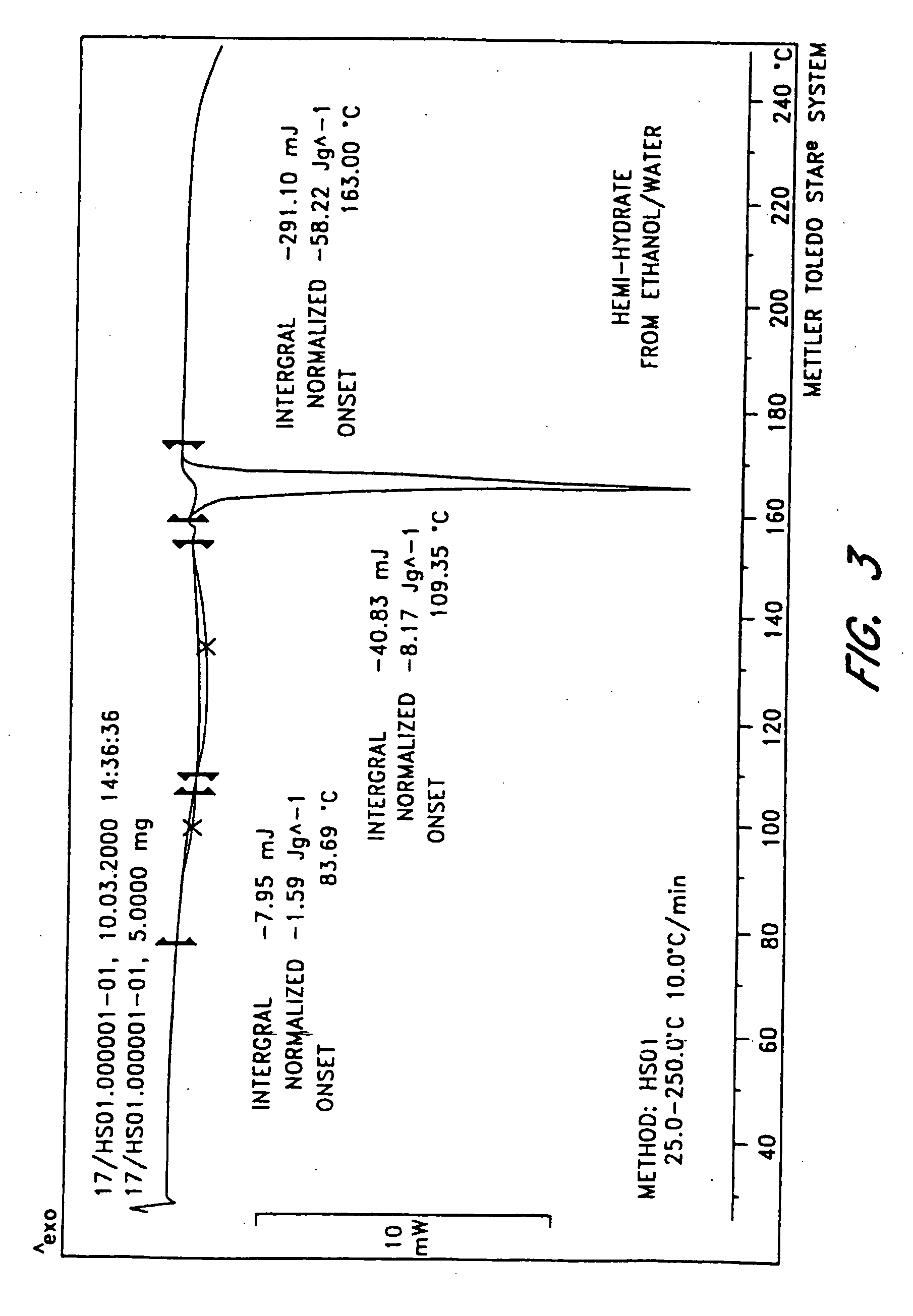
The invention provides compositions comprising formula 1 steroids, e.g., 16α-bromo-3β-hydroxy-5α-androstan-17-one hemihydrate and one or more excipients, including compositions that comprise a liquid formulation comprising less than about 3% v / v water. The compositions are useful to make improved pharmaceutical formulations. The invention also provides methods of intermittent dosing of steroid compounds such as analogs of 16α-bromo-3β-hydroxy-5α-androstan-17-one and compositions useful in such dosing regimens. The invention further provides compositions and methods to inhibit pathogen replication, ameliorate symptoms associated with immune dysregulation and to modulate immune responses in a subject using the compounds. The invention also provides methods to make and use these immunomodulatory compositions and formulations.
Owner:BIOVIE INC
Nasal delivery of cyclodextrin complexes of anti-inflammatory steroids
Aqueous, anti-inflammatory steroid compositions in solution form suitable for nasal administration and having a reduced stinging sensation are provided as well as a method for treating inflammation of the nasal mucosa by intranasal administration of anti-inflammatory steroid compositions. These solution compositions may result in enhanced nasal bio-availability. The anti-inflammatory steroid composition suitable for intranasal administration includes an anti-inflammatory steroid in an amount of from about 0.0001% to about 2.0% (w / v); a cyclodextrin in an amount of from about 0.1% to about 20% (w / v); an alcohol co-solvent in an amount of from about 0.2% to about 35% (w / v); a crystallization inhibitor where required, an effective amount of an antimicrobial preservative; an effective amount of an antioxidant; an effective amount of a chelating agent; water; and a pH adjusting agent sufficient to adjust the pH of the composition to from about 4 to about 7.
Owner:QPHARMA
Multiple-layered liposome and preparation method thereof
InactiveUS20070082042A1Good skin permeabilityImprove stabilityDermatological disorderLiposomal deliverySterolIntercellular space
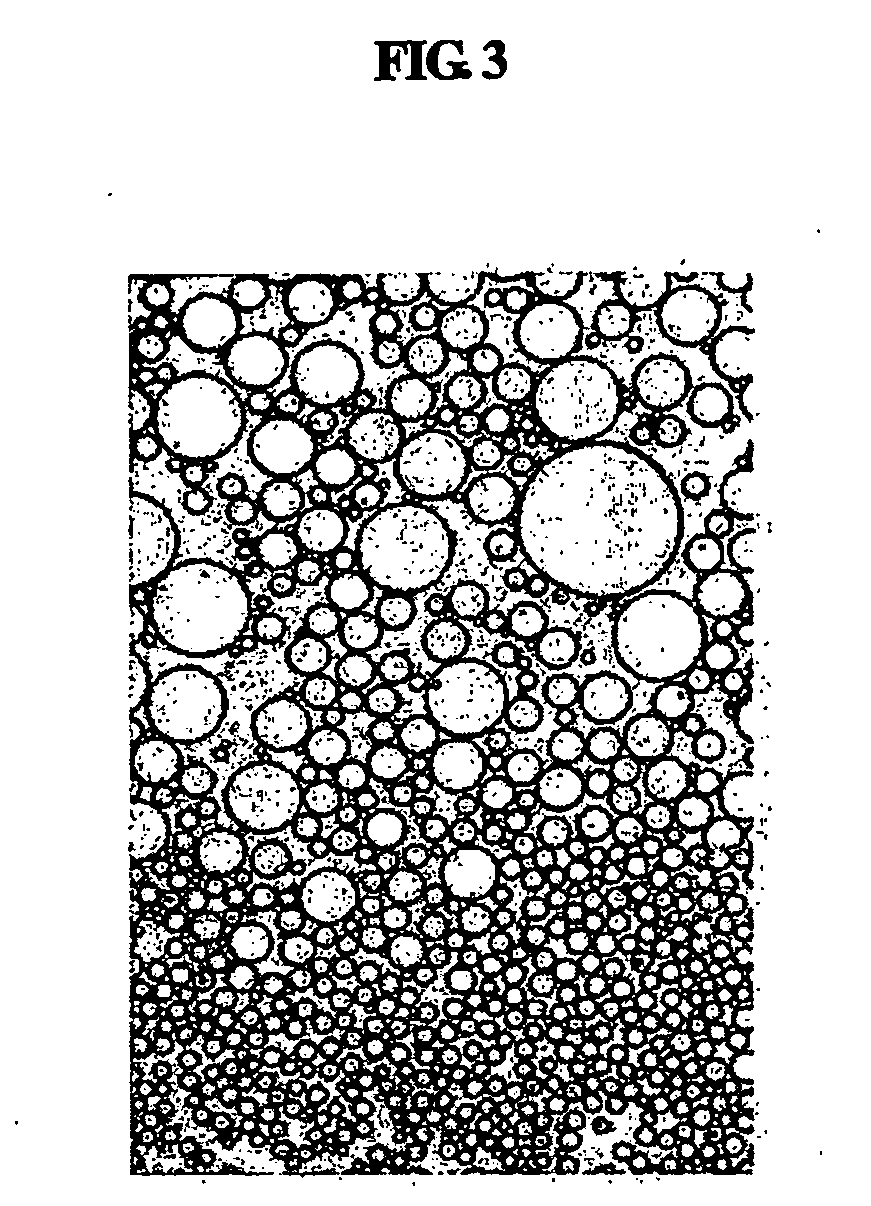
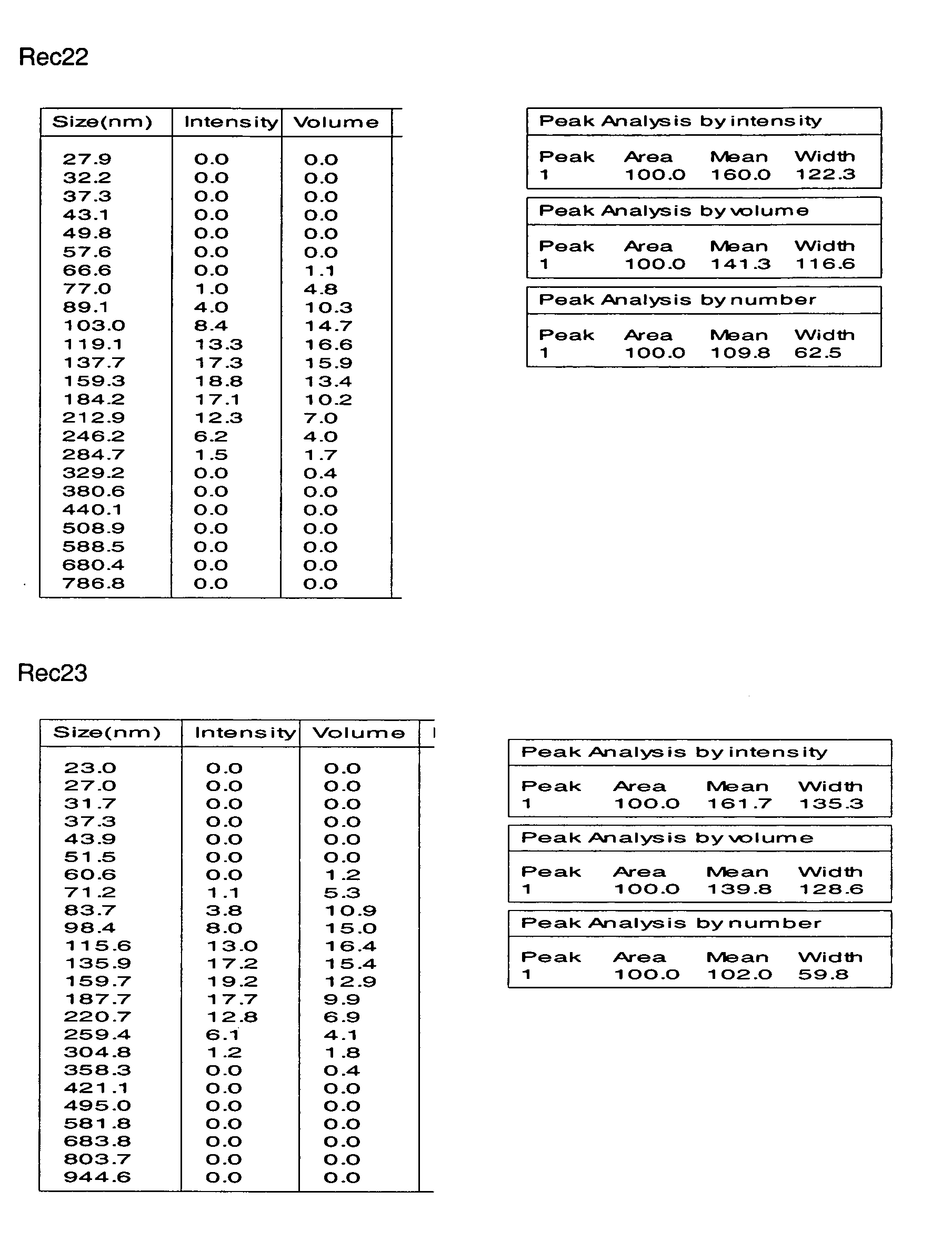
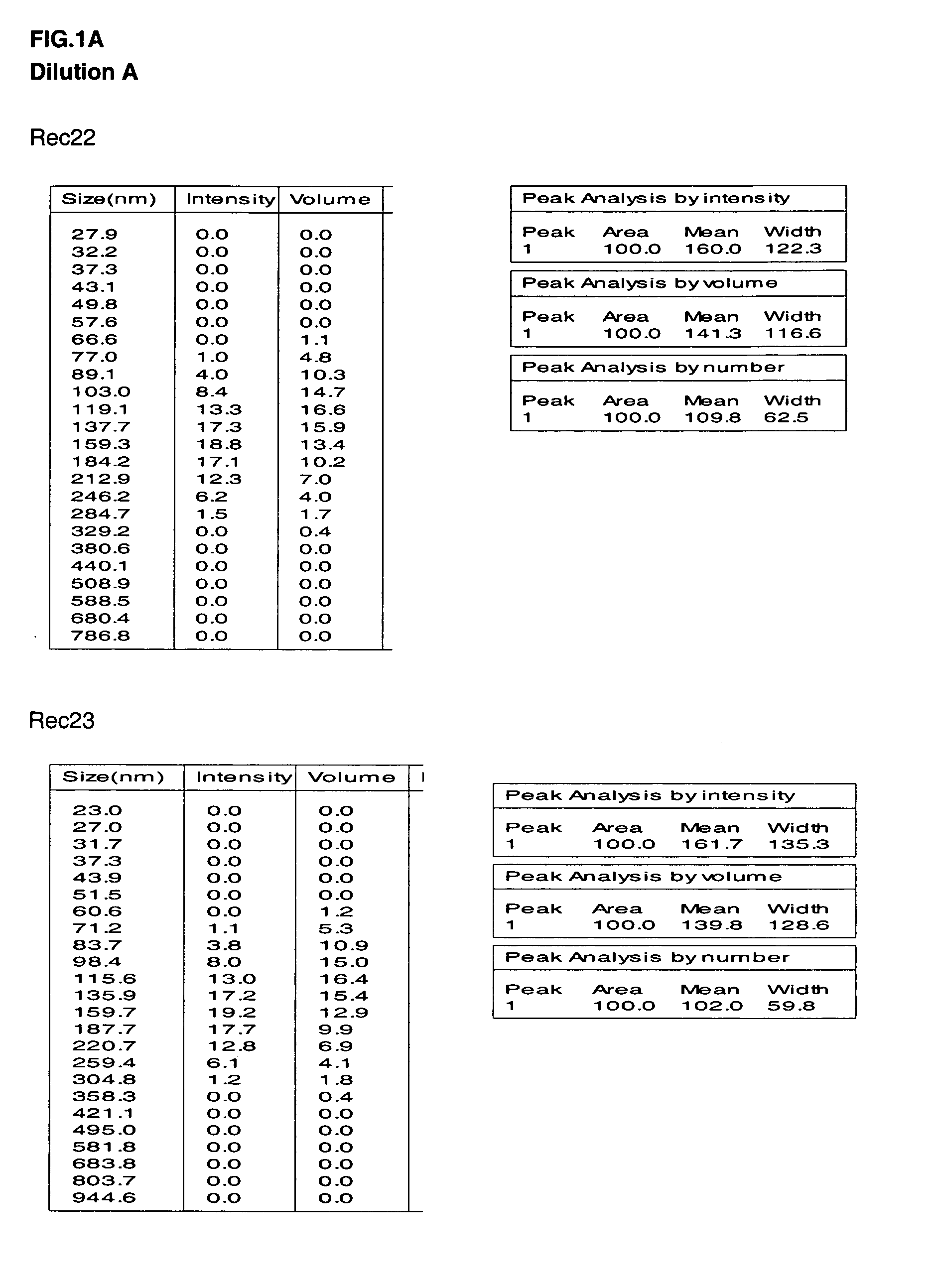
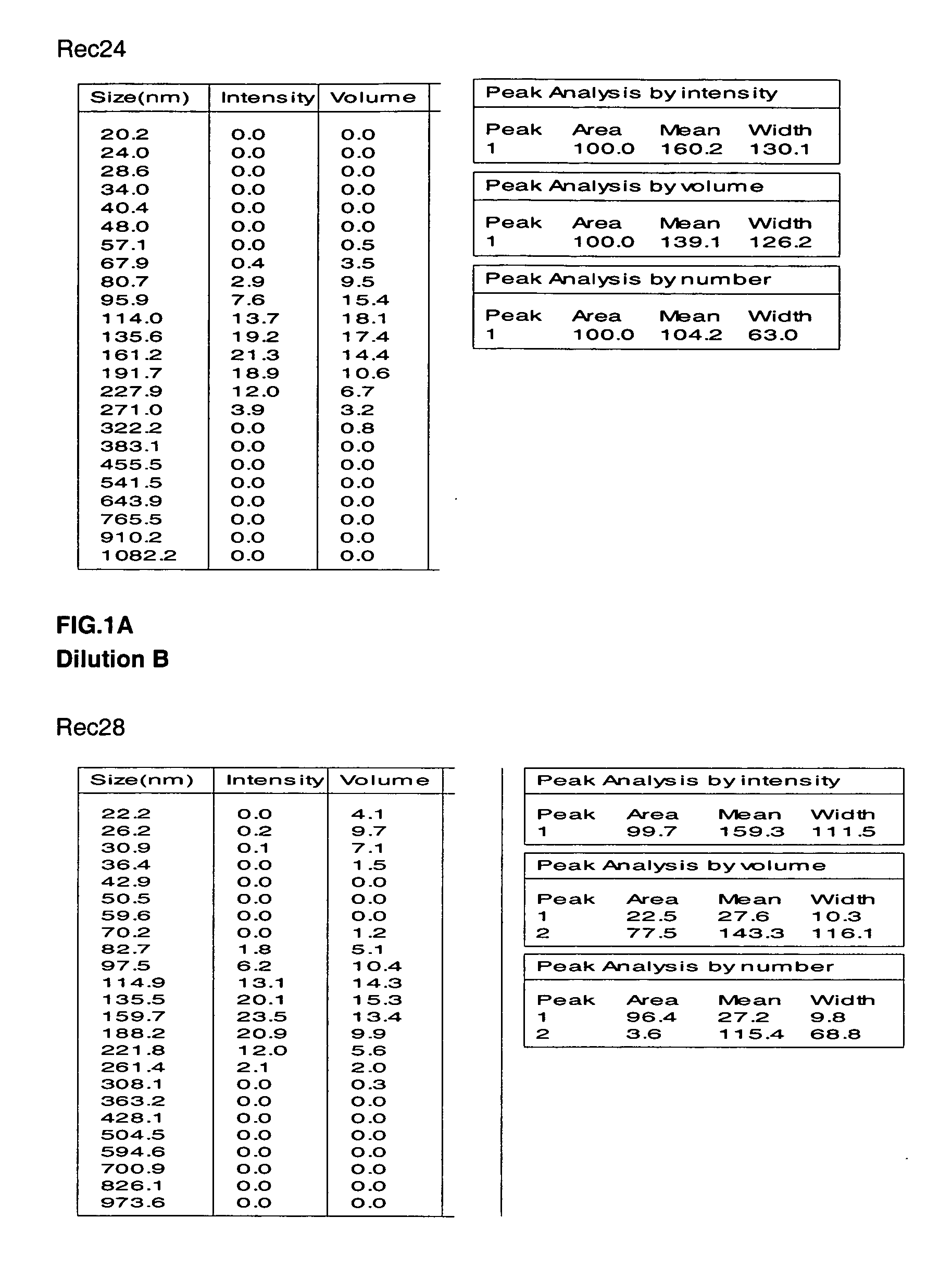
Disclosed are multilayered liposomes for transdermal absorption and a method of preparing the liposomes. The multilayered liposomes are prepared using a mixture of oil-phase components comprising squalane, sterols, ceramides, neutral lipids or oils, fatty acids and lecithins, is 200 to 5000 nm in particle size, and is capable of entrapping a physiologically active substance. The multilayered liposomes entrap a larger amount of a physiologically active substance and are structurally stable when encapsulating the physiologically active substance, compared to unilamellar liposomes. Also, they are prepared by a simple and cost-effective process not using a high-pressure homogenizer but using a general homo mixer. Further, since the multilayered liposomes are prepared in a larger size than the intercellular spaces in the stratum corneum, they overcome the tension of surrounding cells when passing through the intercellular spaces and are thus able to penetrate into the dermal layer, compared to nano-sized unilamellar liposomes. Thus, the multilayered liposomes are useful for enhancing the transdermal absorption of physiologically active substances.
Owner:BIOSPECTRUM
Adjuvant and Vaccine Compositions
InactiveUS20100226932A1Small sizeSnake antigen ingredientsInorganic non-active ingredientsSterolEmulsion
Abstract Compositions comprising an emulsion and aluminum salt nano- / micro-particles surface stabilized with at least one surfactant are useful as immunological adjuvants. The emulsion of these compositions comprises at least one oil; at least one surfactant; a plurality of surfactant vesicles; optionally at least one sterol; and an aqueous phase. The present invention also provides vaccines comprising one or more antigens combined with the emulsion and surface stabilized aluminum salt particles of the present invention, or one or more antigens combined with non-ionic surfactant vesicles.
Owner:NOVAVAX
Influenza vaccine
The present invention relates to monovalent influenza vaccine formulations and vaccination regimes for immunising against influenza disease, their use in medicine, in particular their use in augmenting immune responses to various antigens, and to methods of preparation. In particular, the invention relates to monovalent influenza immunogenic compositions comprising an influenza antigen or antigenic preparation thereof from an influenza virus strain being associated with a pandemic outbreak or having the potential to be associated with a pandemic outbreak, in combination with an oil-in-water emulsion adjuvant comprising a metabolisable oil, a sterol or a tocopherol such as alpha tocopherol, and an emulsifying agent.
Owner:SMITHKLINE BEECHAM PHARMA GMBH +1
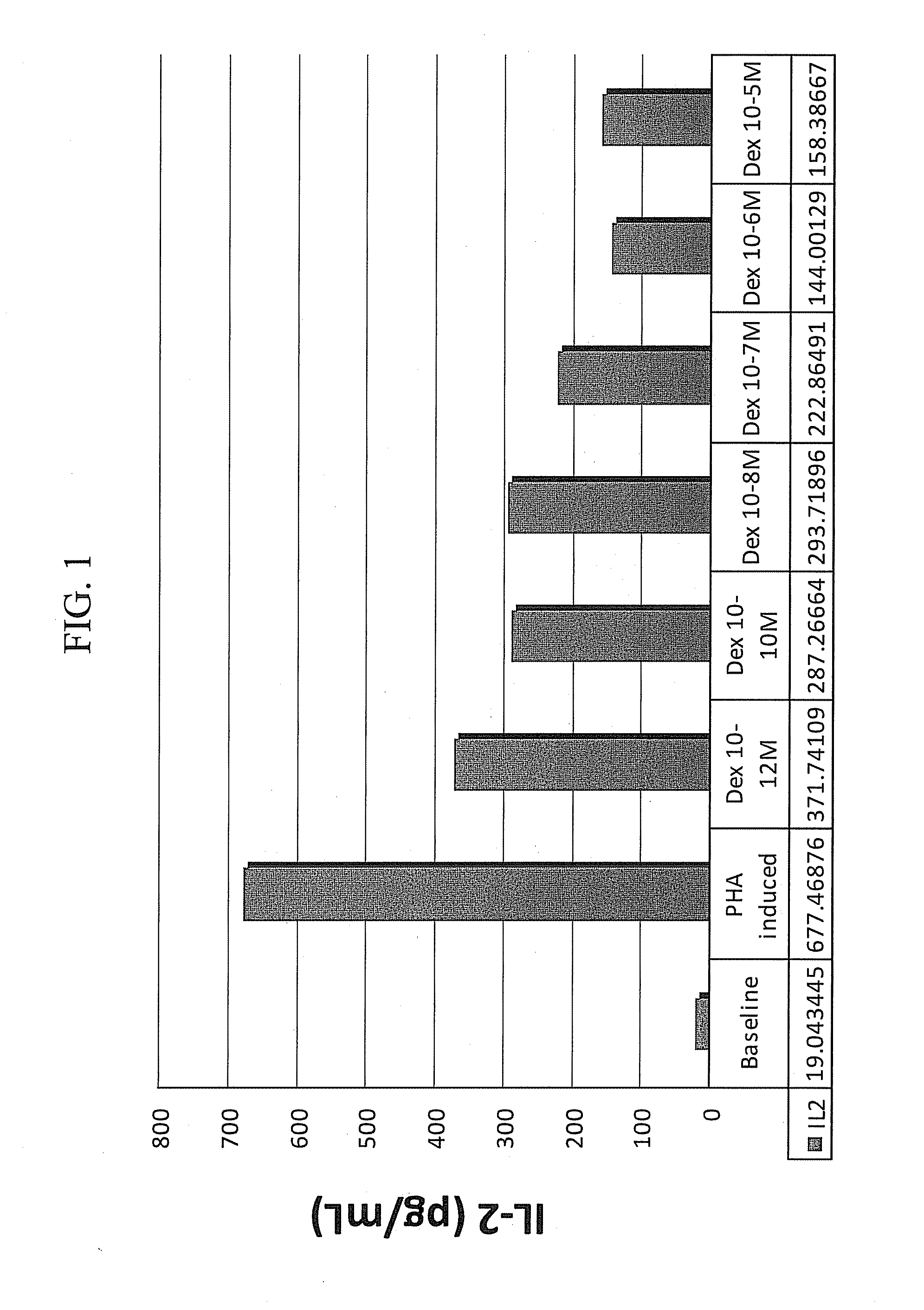
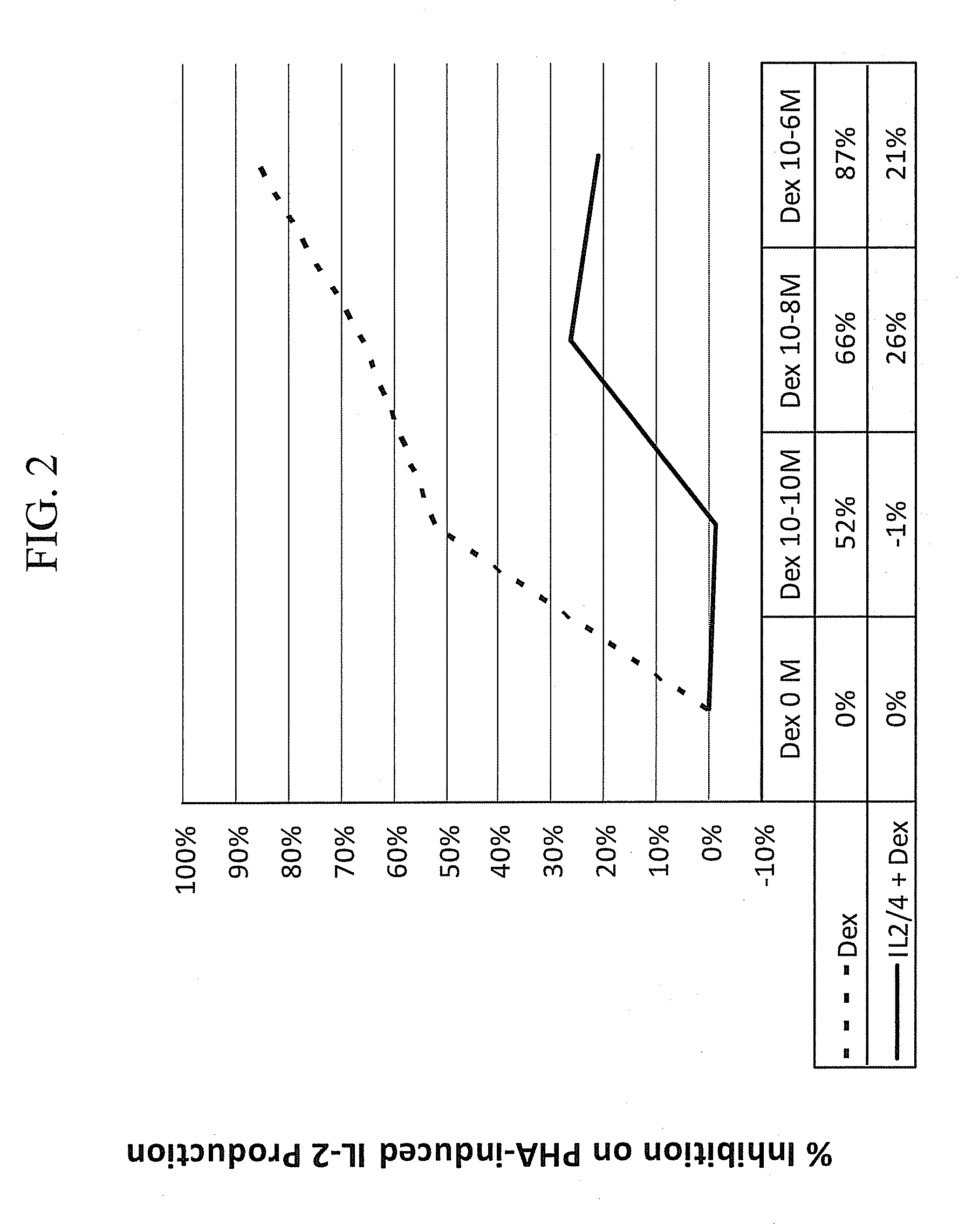
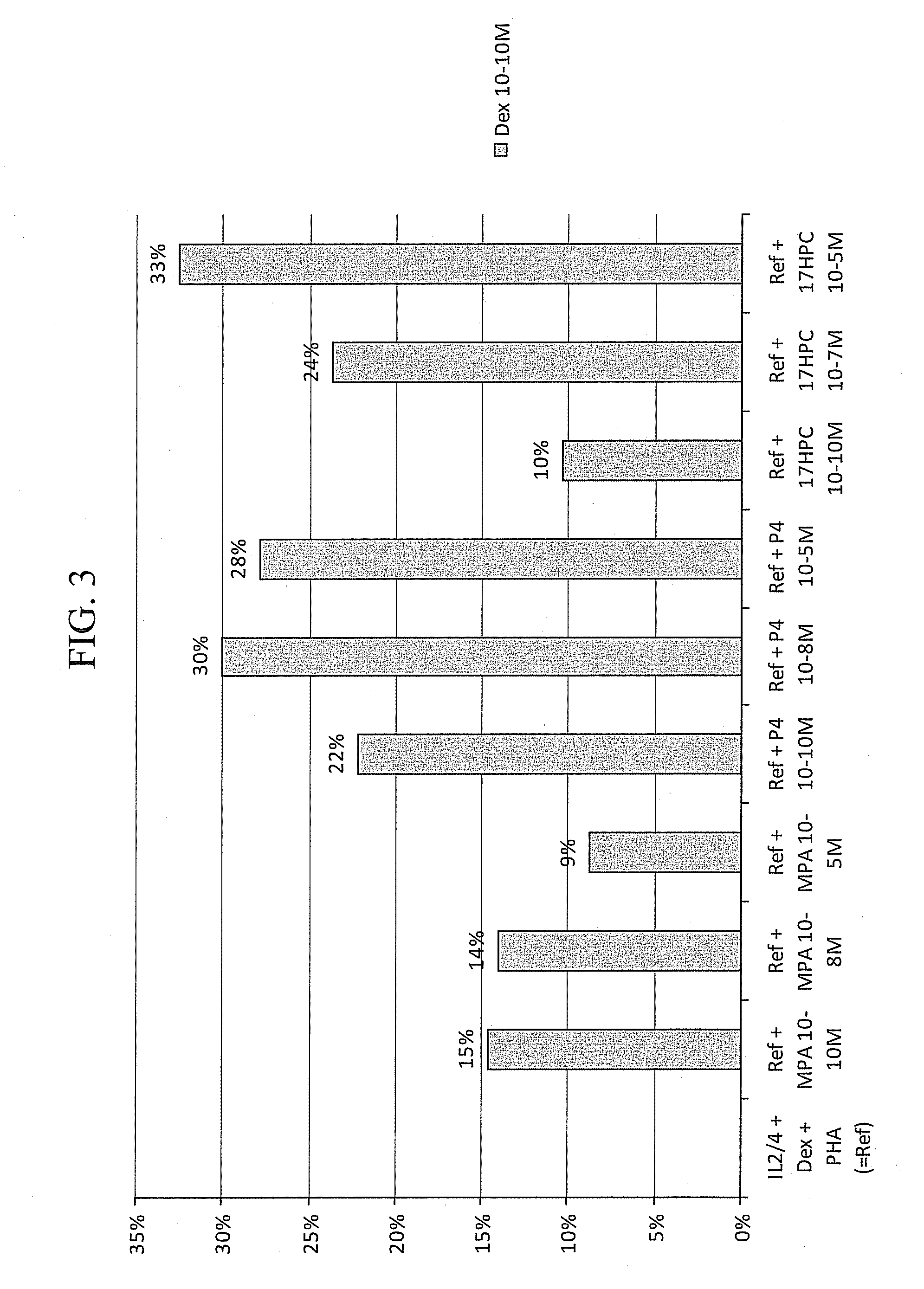
Methods for the use of progestogen as a glucocorticoid sensitizer
ActiveUS20110195031A1Improve responsivenessImprove toleranceBiocideSenses disorderSterolGlucocorticoid Sensitivity
Provided are methods and kits for administering progestogen as a glucocorticoid sensitizer to restore corticosteroid sensitivity or reverse the glucocorticoid insensitivity or enhance glucocorticoid sensitivity, in order to treat one or more glucocorticoid insensitivity related diseases or conditions. For example, these include methods for reversing the glucocorticoid insensitivity in a subject having no history of menstrual cycle-related exacerbation or allergy to self-hormones, particularly progesterone, such as premenstrual or perimenstrual deterioration in the symptoms, e.g., premenstrual worsening of atopic dermatitis or premenstrual exacerbations of asthma, and exhibiting relatively or totally refractory responses to glucocorticoid therapy, e.g., glucocorticoid resistance. The methods and kits provide for the administration of a sex hormone to the subject who is corticosteroid dependent or corticoid resistant or unresponsive or intolerant to corticosteroids.
Owner:SHENZHEN EVERGREEN THERAPEUTICS CO LTD
Extract of Maka root
InactiveCN1473594AIncrease libidoHigh densityOrganic compounds purification/separation/stabilisationGlycosidesSexual functionSterol
The present invention aims at extracting, separating and purifying the active components of Maka as one natural plant to obtain extractive with several medicinal and health care values. Fresh or dried Maka root material is extracted and separated to obtain active components and extracted residue. The active components includes glucosinolate and its decomposed product isothiocynate in 10-90 wt%,Maka amide and Maka olefin 5-70 wt% and sterol 2-30 wt%. The active components may be further prepared into capsule, tablet, and delayed releasing forms, and the prepared medicine and health food have the functions of treating climacteric syndrome, strengthening male's and female's sexuality, improving sexual function, etc.
Owner:HUAZHONG UNIV OF SCI & TECH
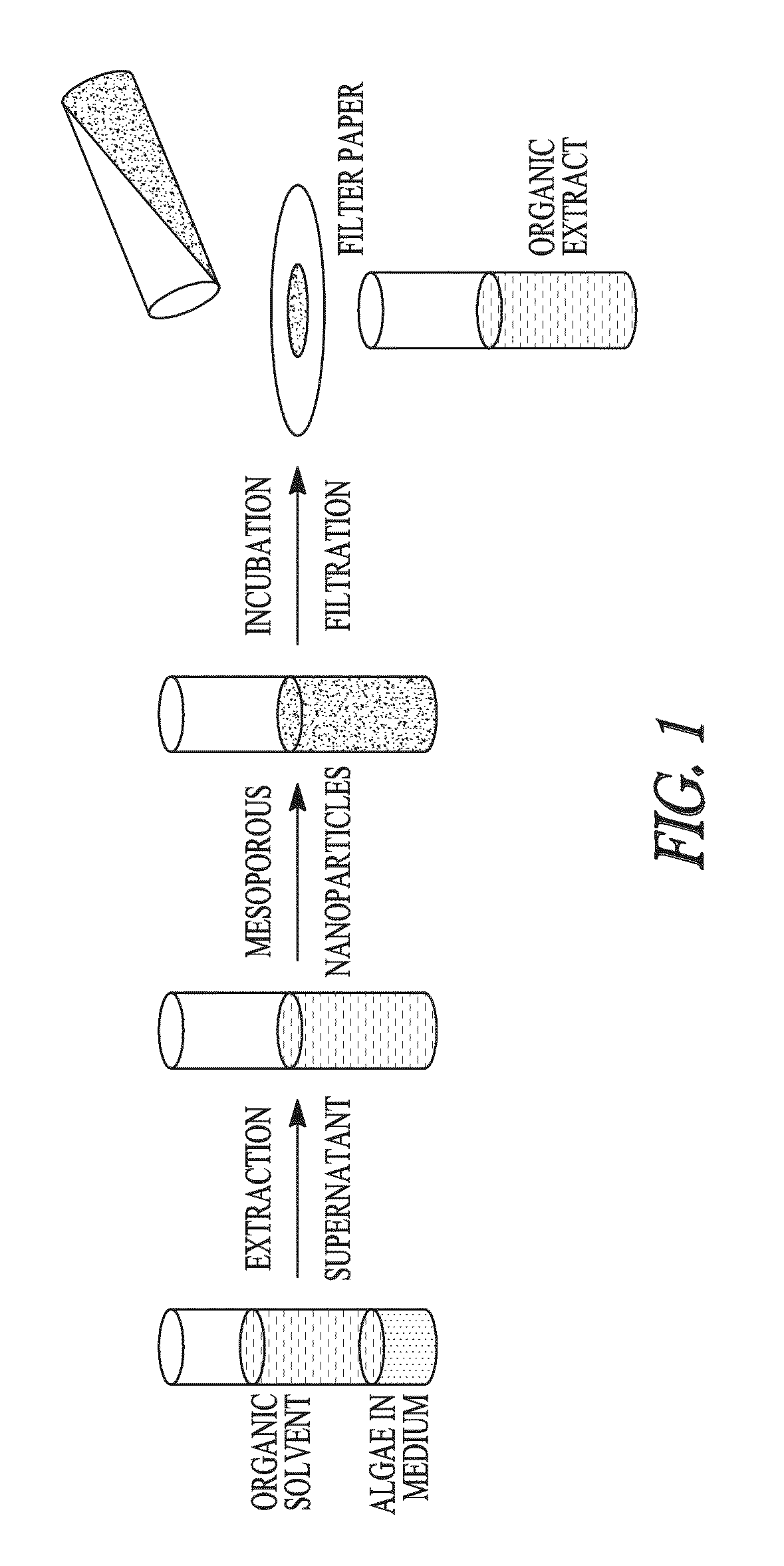
Sequestration of compounds from microorganisms
InactiveUS20100196971A1Inexpensive and clean methodEasy to cleanMaterial nanotechnologySilicaMicroorganismSterol
The invention provides novel mesoporous particles and methods for the selective sequestration of organic compounds from microorganisms, such as from various genera and species of algae that produce important organic compounds. The organic compounds can be selectively sequestered, for example, in favor of biodiesel impurities such as sterols and chlorophyll, to provide substantially pure free fatty acids. The free fatty acids can then be esterified to provide pure biodiesel.
Owner:IOWA STATE UNIV RES FOUND
Transdermal Methods and Systems for the Delivery of Corticosteroid Compounds
An integrated iontophoresis skin-worn patch and method for delivering a therapeutically effective amount of a corticosteroid drug compound in a systemically-safe and skin-safe manner for site-specific treatment of inflammation pain is disclosed.
Owner:TEIKOKU PHARMA USA INC
Combinations of substituted azetidinones and CB1 antagonists
The present invention provides compositions, therapeutic combinations and methods including: (a) at least one selective CB1 antagonist; and (b) at least one substituted azetidinone or substituted β-lactam sterol absorption inhibitor which can be useful for treating vascular conditions, diabetes, obesity, metabolic syndrome and lowering plasma levels of sterols or 5α-stanols.
Owner:SCHERING CORP
Method for production of isoprenoids
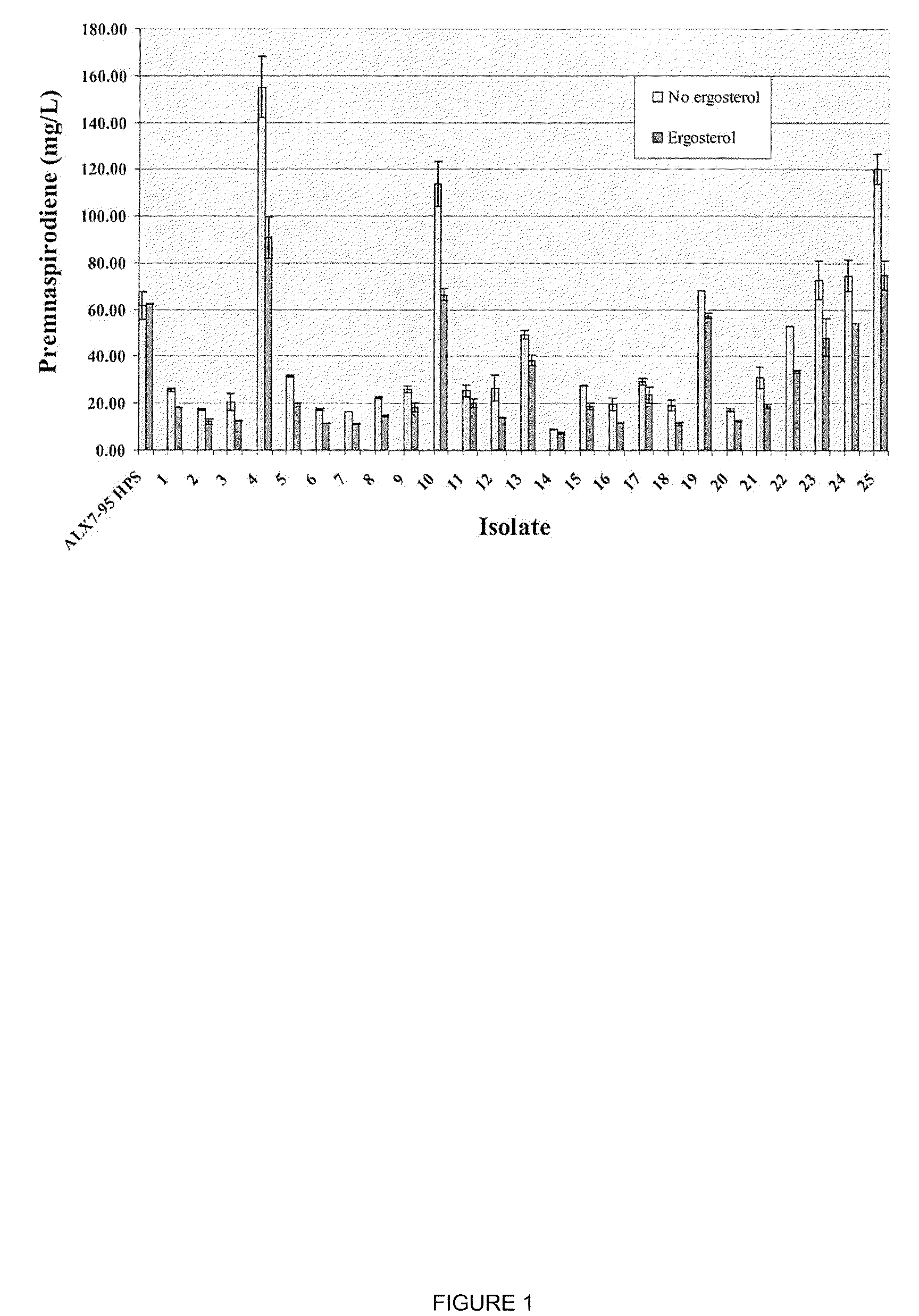
The present invention is directed to variant squalene synthase enzymes, including Saccharomyces cerevisiae squalene synthase enzymes, and to nucleic acid molecules encoding these variant enzymes. These variant enzymes produce squalene at a lower rate than the wild-type enzyme, allowing more farnesyl pyrophosphate to be utilized for production of isoprenoid compounds, while still producing sufficient squalene to allow the S. cerevisiae cells to grow without the requirement for supplementation by sterols such as ergosterol. These variant enzymes, therefore, are highly suitable for the efficient production of isoprenoids.
Owner:EVOLVA INC
Influenza vaccine
InactiveUS20090263422A1Stimulate immune responseSsRNA viruses negative-senseViral antigen ingredientsDiseaseSterol
The present invention relates to monovalent influenza vaccine formulations and vaccination regimes for immunising against influenza disease, their use in medicine, in particular their use in augmenting immune responses to various antigens, and to methods of preparation. In particular, the invention relates to monovalent influenza immunogenic compositions comprising an influenza antigen or antigenic preparation thereof from an influenza virus strain being associated with a pandemic outbreak or having the potential to be associated with a pandemic outbreak, in combination with an oil-in-water emulsion adjuvant comprising a metabolisable oil, a sterol or a tocopherol such as alphatocopherol, and an emulsifying agent.
Owner:HANON EMMANUEL JULES +1
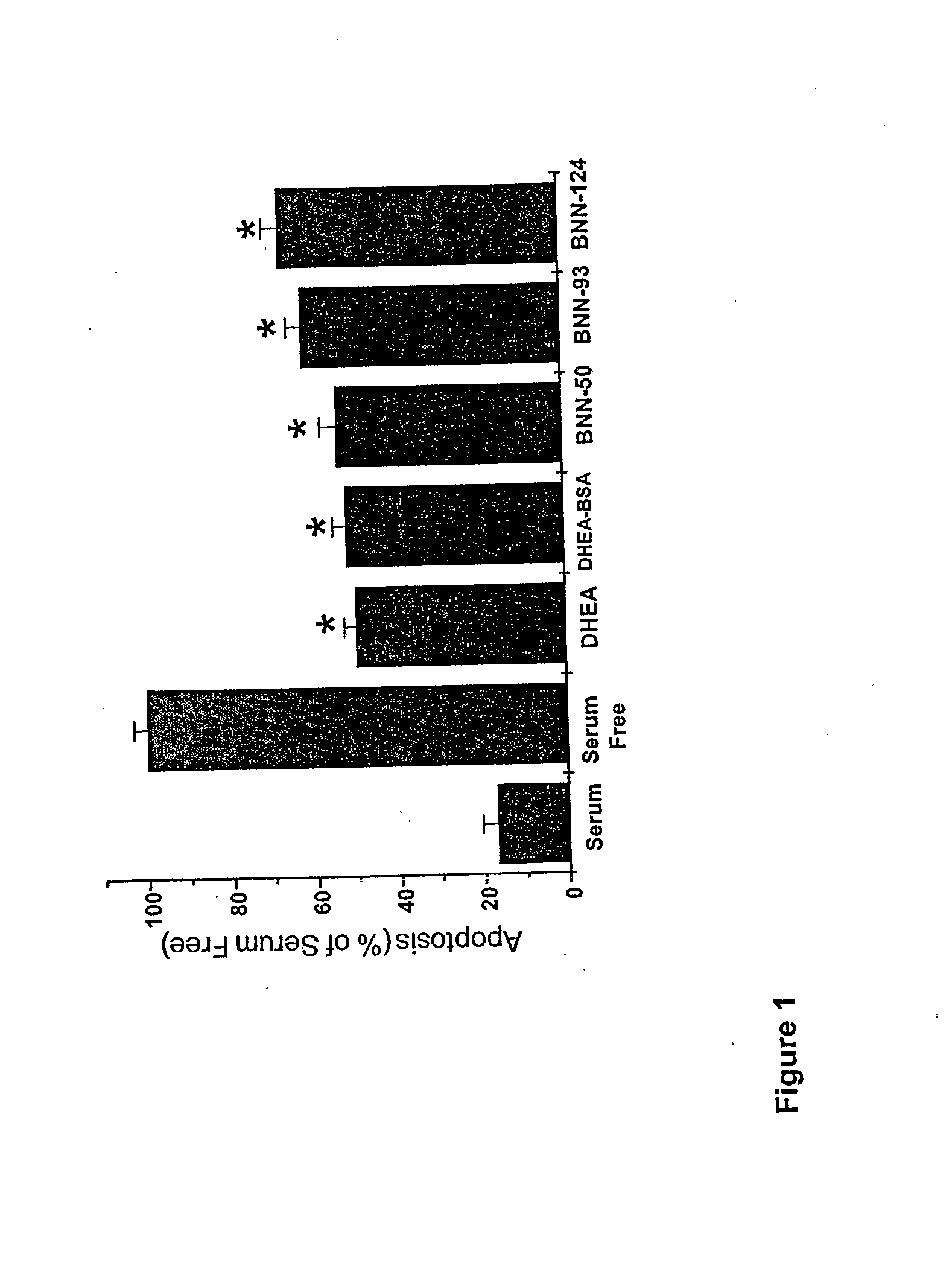
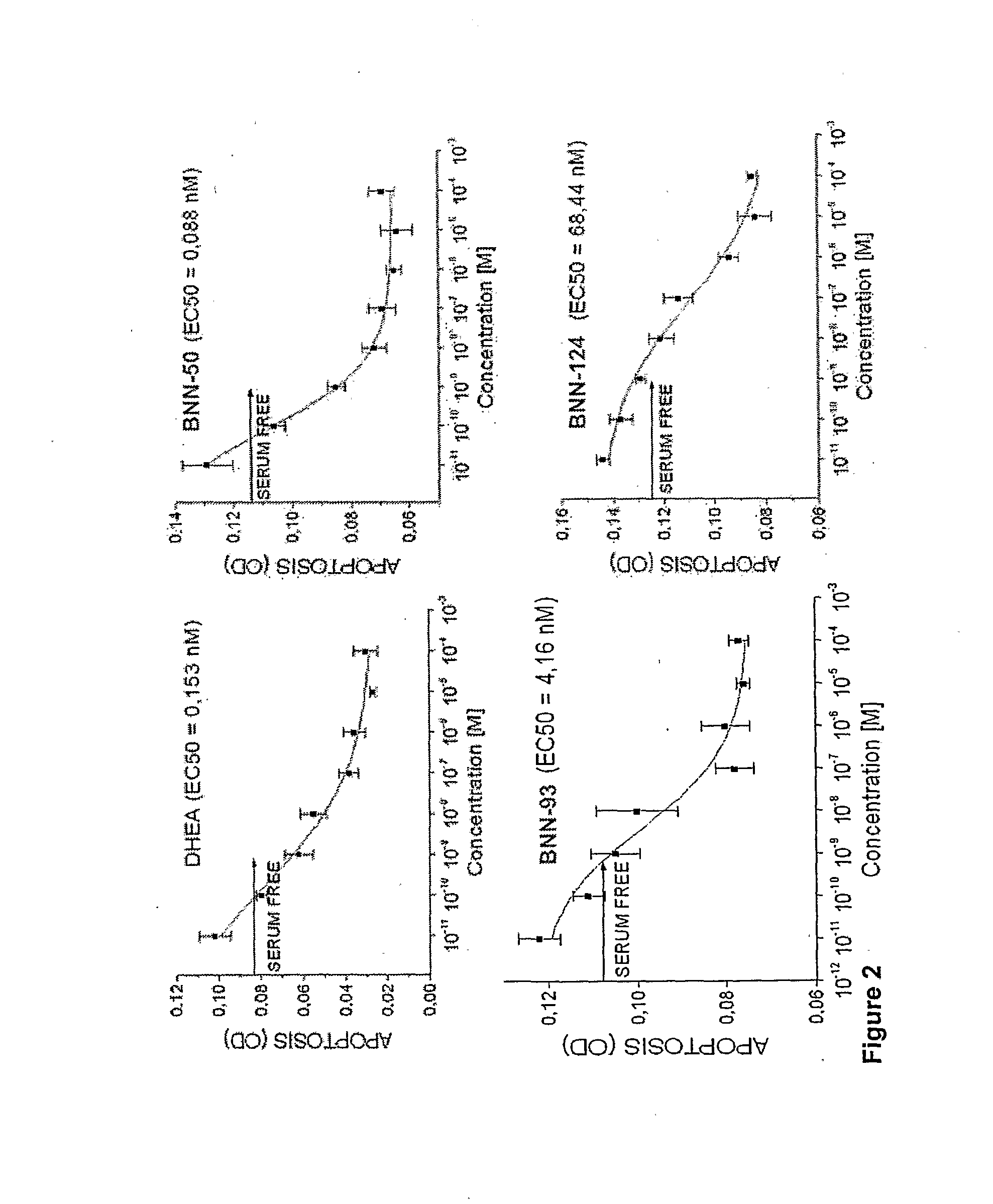
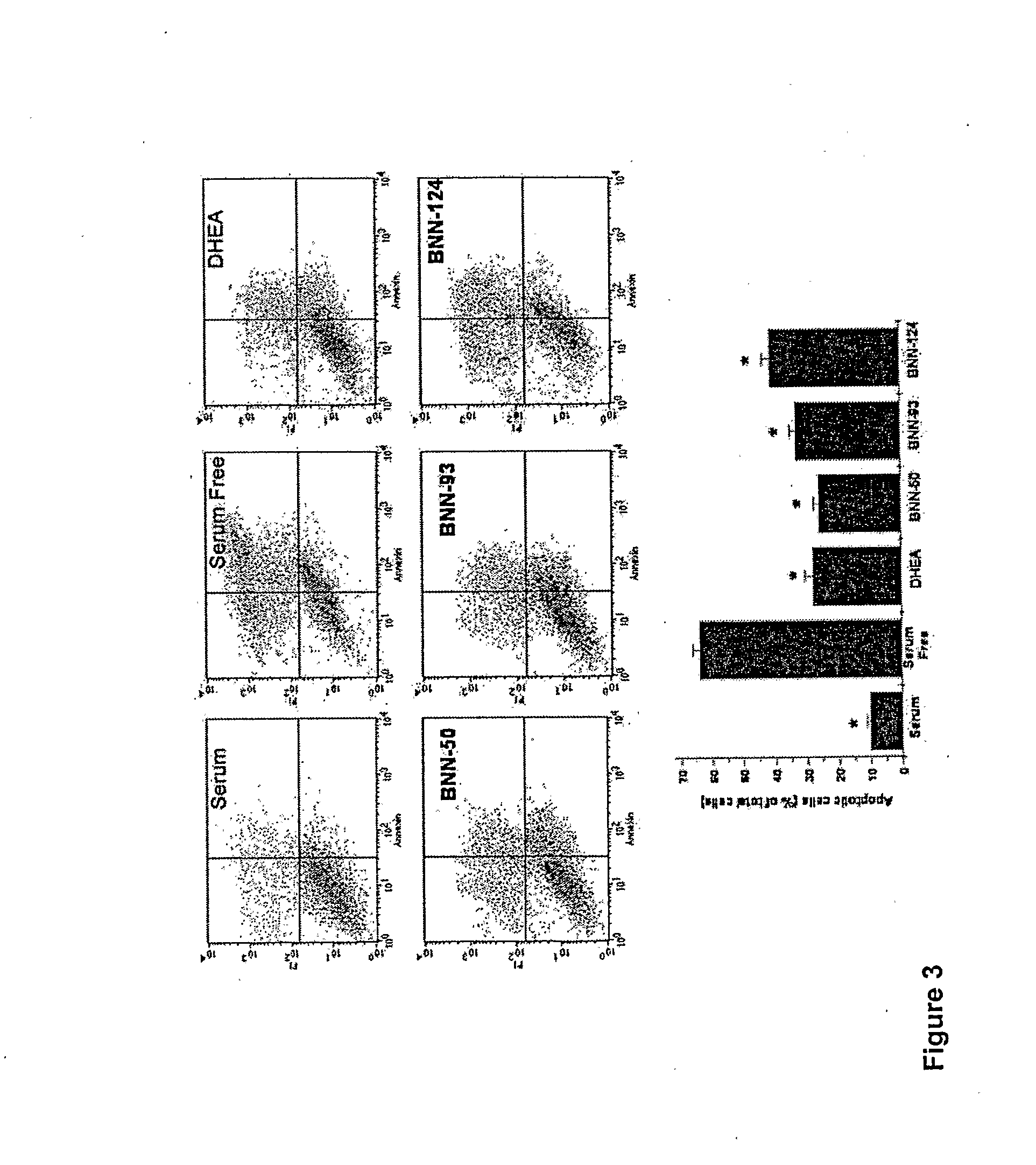
Neurosteroid compounds
The present invention relates to novel neurosteroid derivatives with anti-apoptotic, neuroprotective and neurogenic properties that act on the nervous system as well as methods for making the same and their applications in the treatment and / or prevention or amelioration of neurodegenerative diseases related to neuronal apoptosis or neuronal injury, or conditions related to or resulting from apoptosis, including but not limited to Alzheimer's disease, Parkinson's disease, Huntington's disease, multiple sclerosis and amyotrophic lateral sclerosis (ALS), retinal degeneration and detachment, peripheral neuropathy caused by genetic abnormalities, diabetes, polio, herpes, AIDS and chemotherapy, brain trauma, or ischemia and stroke. The active compounds are represented by Formula (I): wherein R1, R2, R3, R4, R5, R6, R7, A, B, X, Y and Z are defined in the description of the invention. The present invention also includes compositions which comprise one or more of the compounds of Formula (I).
Owner:BIONATURE E A LTD
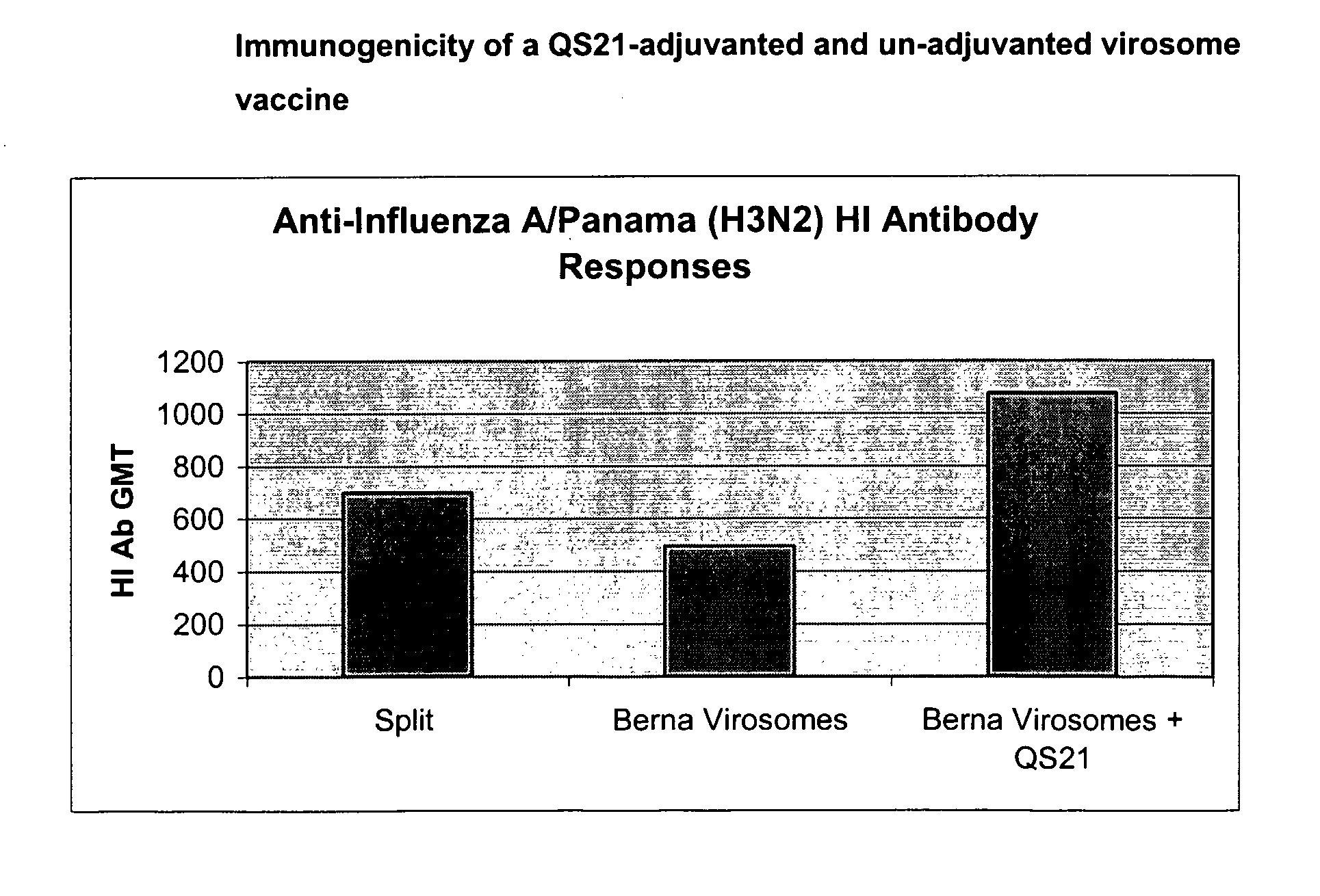
Vaccine Compositions Comprising Virosomes and a Saponin Adjuvant
InactiveUS20090263470A1Enhance immune responseSsRNA viruses negative-senseViral antigen ingredientsSterolVirosome
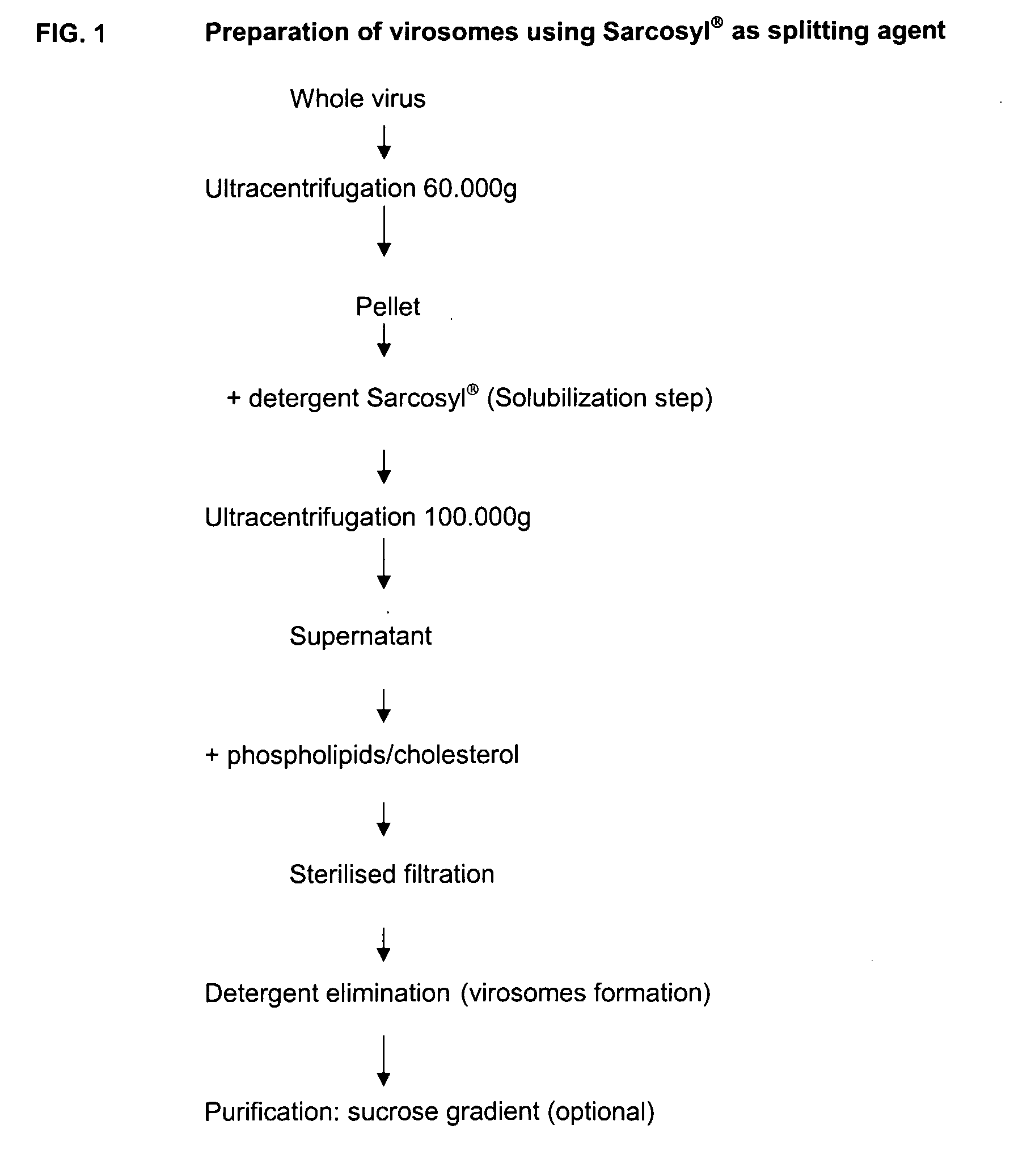
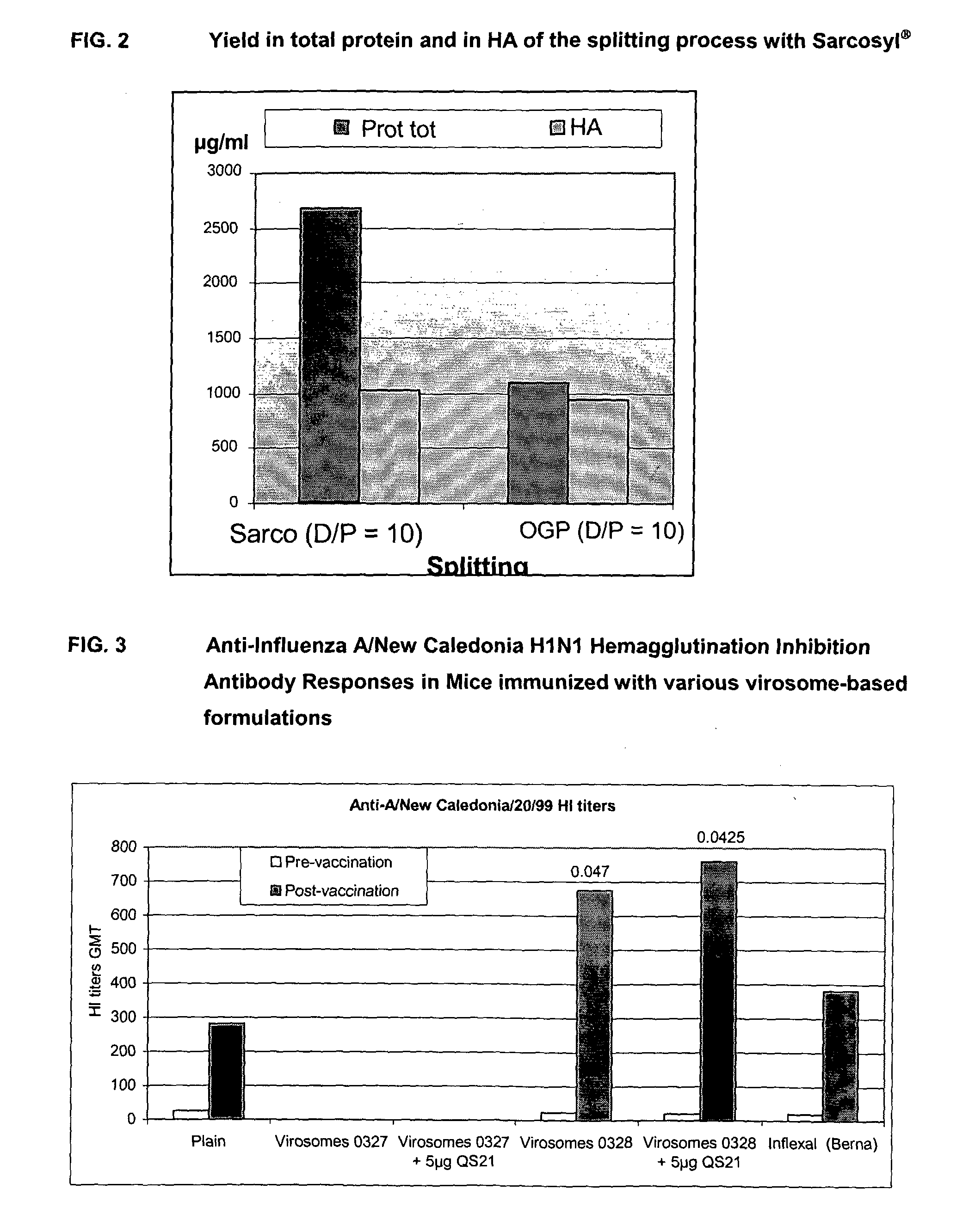
This invention provides virosome preparations from an enveloped virus, in particular from influenza virus, containing from said virus, and a saponin adjuvant. In particular the invention provides a virosome preparation from influenza virus an influenza antigen a QS21, optionally with a sterol. The invention also provides vaccine compositions containing said virosome preparations, methods of preparing said virosome preparations and vaccine containing them.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Influenza vaccine
InactiveUS20080014217A1Stimulate immune responseSsRNA viruses negative-senseViral antigen ingredientsDiseaseSterol
The present invention relates to monovalent influenza vaccine formulations and vaccination regimes for immunising against influenza disease, their use in medicine, in particular their use in augmenting immune responses to various antigens, and to methods of preparation. In particular, the invention relates to monovalent influenza immunogenic compositions comprising an influenza antigen or antigenic preparation thereof from an influenza virus strain being associated with a pandemic outbreak or having the potential to be associated with a pandemic outbreak, in combination with an oil-in-water emulsion adjuvant comprising a metabolisable oil, a sterol and / or a tocopherol such as alpha tocopherol, and an emulsifying agent.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Lipid nanoparticle formulation
The disclosure features novel lipids and compositions involving the same. Nanoparticle compositions include an ionizable lipid, a phospholipid, a first sterol or a tocopherol, and optionally a second sterol different from the first sterol. Nanoparticle compositions further including therapeutic and / or prophylactics such as RNA are useful in the delivery of therapeutic and / or prophylactics to mammalian cells or organs to, for example, regulate polypeptide, protein, or gene expression.
Owner:MODERNATX INC +1
Liposome compositions for the delivery of macromolecules
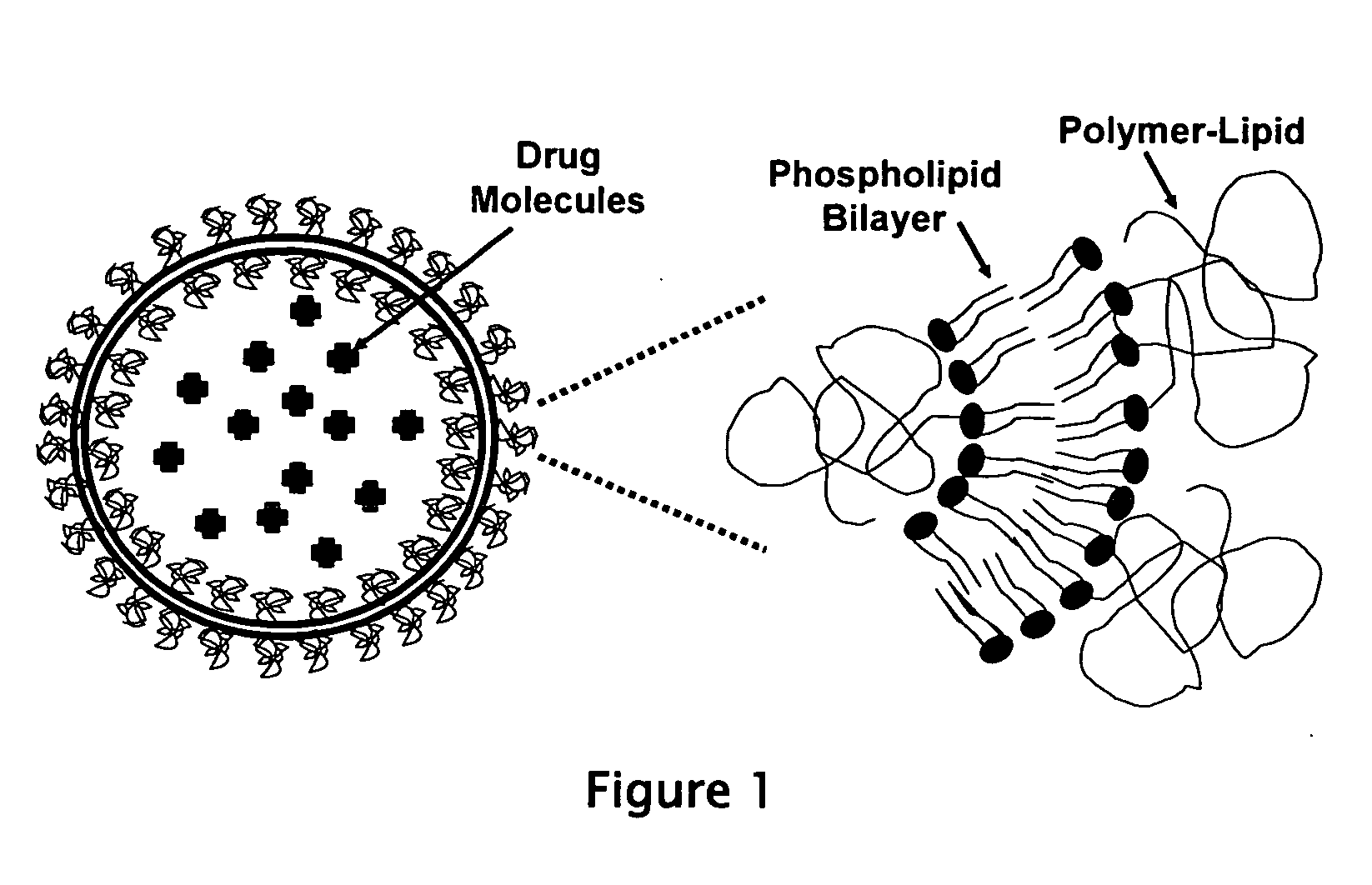
InactiveUS20050260260A1Good curative effectReduce penetrationLiposomal deliveryLipid formationSterol
This invention provides for a liposome composition which demonstrates greatly increased therapeutic efficacy when used to deliver encapsulated macromolecular drugs. The liposome composition excludes the use of sterols, sterol derivatives, and cationic lipids, contrary to conventional formulations. The invention liposome is also unique in that it utilizes low gel to fluid phase transition temperature lipids in its membrane.
Owner:KISAK EDWARD +1
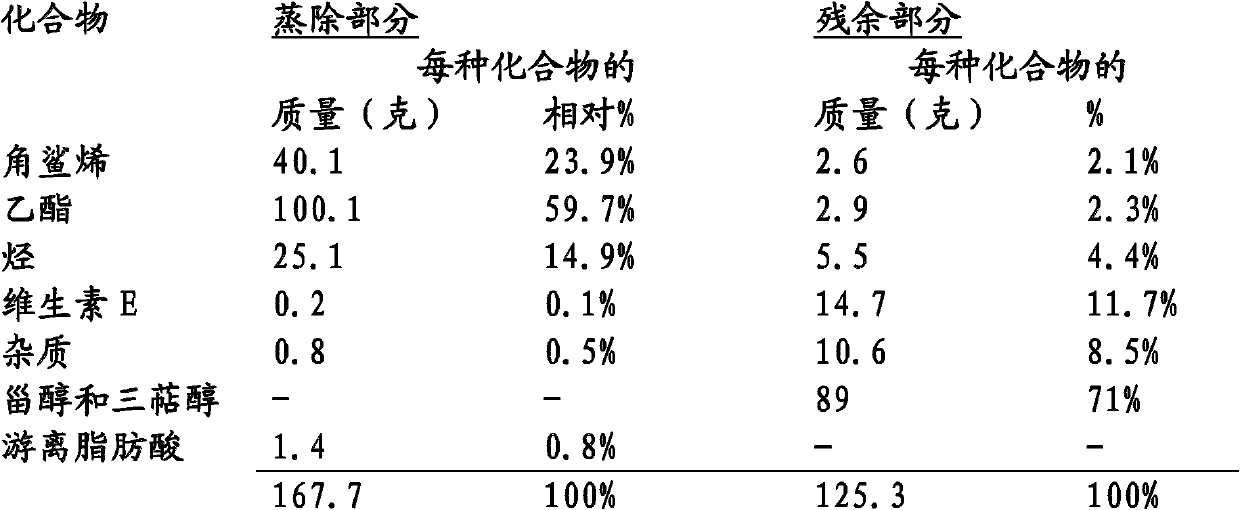
Process for the extraction of squalene, sterols and vitamin e contained in condensates of physical refining and/or in distillates of deodorization of plant oils
InactiveCN102089263AHigh priceEfficient use ofFatty acid esterificationMetabolism disorderSterolVegetable oil
The invention describes an overall process for the extraction of sterols, vitamin E, squalene and other plant-based hydrocarbons from deodorization distillates of plant oils. After an esterification of the free fatty acids, then a transesterification of the combined fatty acids (glycerides and sterides) by the same short alcohol, three successive distillations make it possible to successively recover a first fraction of hydrocarbons, the main fraction of alkyl esters, then the heaviest alkyl esters with squalene. The third distillate will be used for the production of squalene and a second fraction of hydrocarbons. The residue of the third distillation will be used for the production of sterols and vitamin E. By using bioethanol, plant-based glycerol and the plant-based hydrocarbons of the process, the process makes it possible to extract each of the four unsaponifiable substances without any solvent of oil-based origin and to claim the seals of approval for products obtained by natural physical and chemical processes.
Owner:SOPHIM
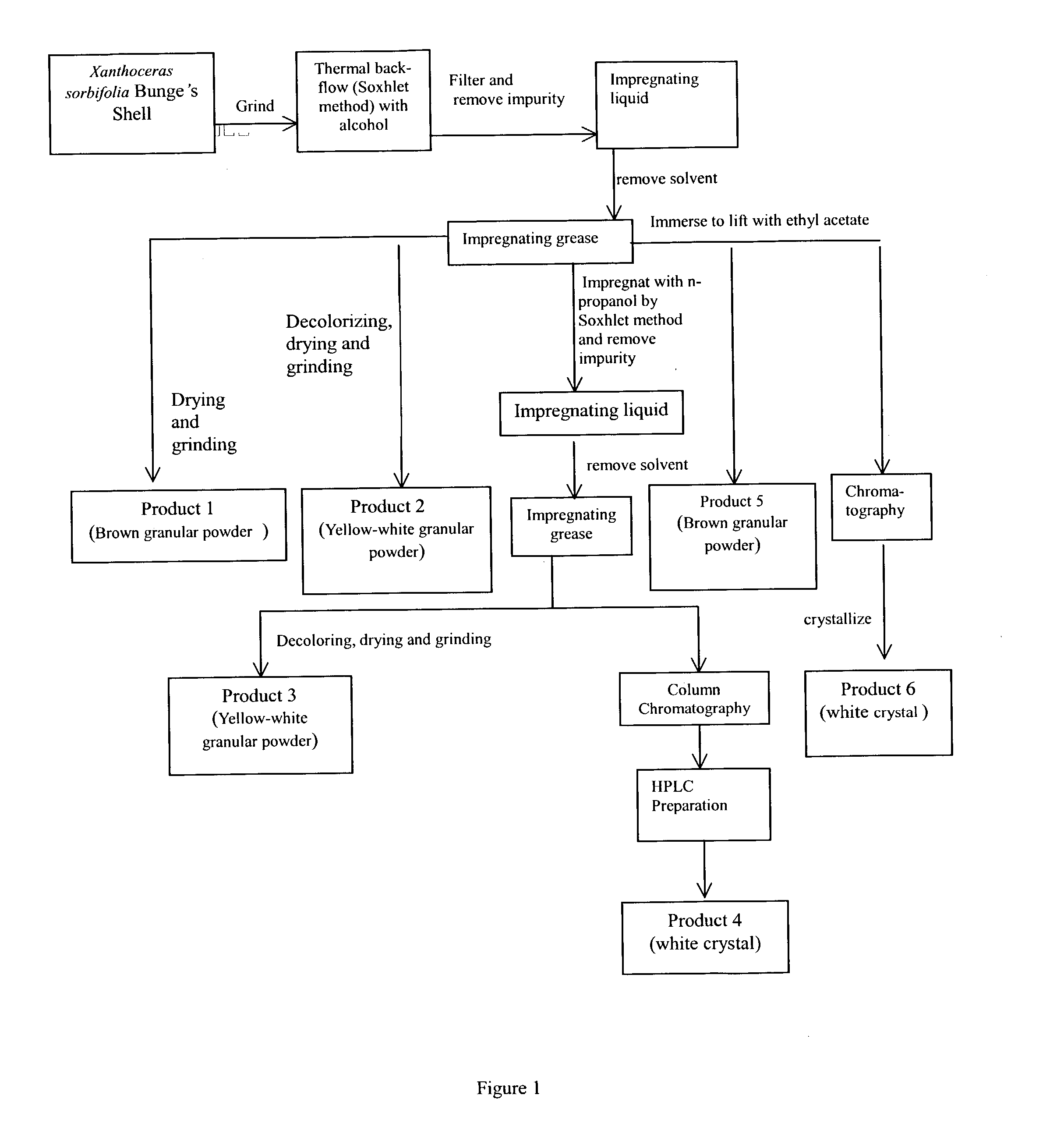
Extracting materials from the shell of Xanthoceras sorbifolia Bunge and applying the extracted materials to making drugs and functional foods
InactiveUS20030096030A1Improve brain functionImprove immunityBiocidePeptide/protein ingredientsSterolXanthoceras
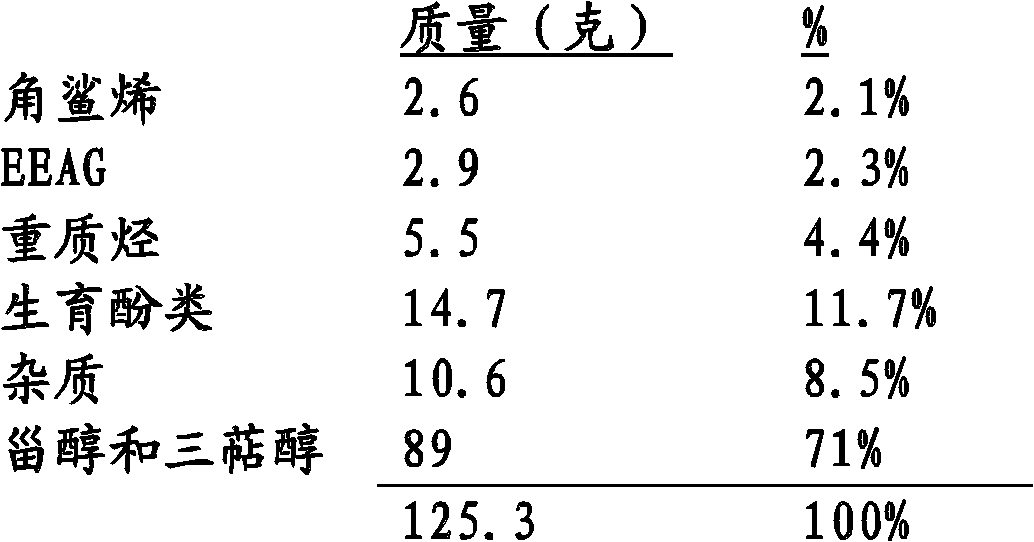
This invention proposed applications of the extracted materials from shell of Xanthoceras sorbifolia Bunge to making drugs for enhancing brain functions. The extracted materials from the shell of X. sorbifolia include 2~10 percent of crude fats, 15~20 percent of crude protein, 15~25 percent of Bunkankasaponin A. B. C. D., 20~30 percent of sugar and 7~12 percent of water as well as two kinds of sterols of (3beta,5alpha,20R,24S)-stigmasta-7, trans-22-dien-3-ol and (3beta,5alpha,20R,24R)-stigmasta-7-en-3-ol. The applications of this invention shows evidently effective for curing diseases of mental retardation and deficiency, dementia, enuresis, and increasing body's ability of resistance to activity of glycosuria. The extracted materials can also be made of functional foods for increasing brain's functions.
Owner:WANG SONGJIANG +1
Method for preparing and using water-based steroid pheromone compositions
This invention relates to methods of formulating steroid pheromones as novel stable emulsions. These emulsions can be used for administration of the steroid pheromones to living organisms such as humans or pigs in place of current organic solvent or pressurized aerosol formulations that present hazards in both shipping and application. Uses relating to pigs are in stimulation of sexual maturation, diagnosis of the onset and timing of oestrus in female pigs, and inducing boars to accept dummy sows. The water-based emulsions can include 5α-androst-16-en-3-one and 3α-hydroxy-5α-androst-16-ene for administration to pigs. The use of water-based emulsions eliminates the need for formulation of the steroid pheromones in organic solvents, delivery of these pheromones as pressurized aerosols, and following the precautions required to reduce hazards during shipping and handling. The water-based emulsions allow for treatment of living organisms where a liquid organic solvent formulation or aerosol of the pheromones might be harmful to the organism or a substrate.
Owner:CONTECH ENTERPRISES
Oil-in-water emulsion influenza vaccine
ActiveUS20100189741A1Increase supplyLow amountSsRNA viruses negative-senseViral antigen ingredientsHuman useSterol
The present invention provides an immunogenic influenza composition in a dose volume suitable for human use, comprising an influenza virus antigen or antigenic preparation thereof and an adjuvant composition comprising an oil-in-water emulsion, wherein said oil-in-water emulsion comprises a metabolisable oil at a level of below 11 mg and an emulsifying agent at a level of below 5 mg and optionally a tocol or a sterol at a level of below 12 mg. Suitably the amount of influenza antigen per strain per dose is 15 μg HA or a low amount such as less than 15 μg HA.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Shampoo compositions
InactiveUS20020119113A1Easily visualisedEnhancing performance and deliveryCosmetic preparationsHair removalSterolBULK ACTIVE INGREDIENT
A shampoo composition comprising an active ingredient and multilamellar vesicles, characterised in that the multilamellar vesicles consist essentially of anionic surfactant and a sterol.
Owner:UNILEVER HOME & PERSONAL CARE USA DIV OF CONOPCO IN C
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com