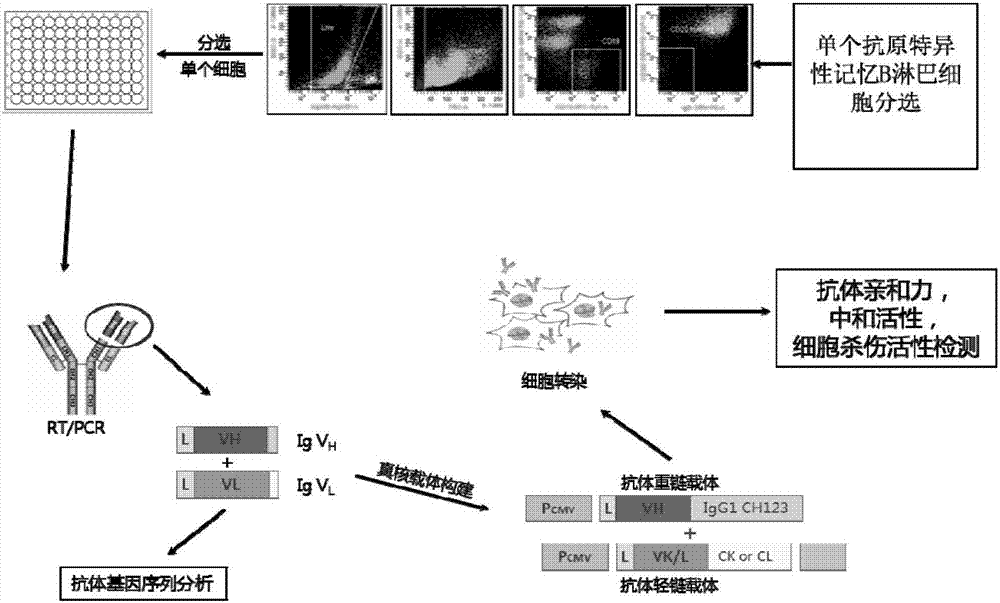
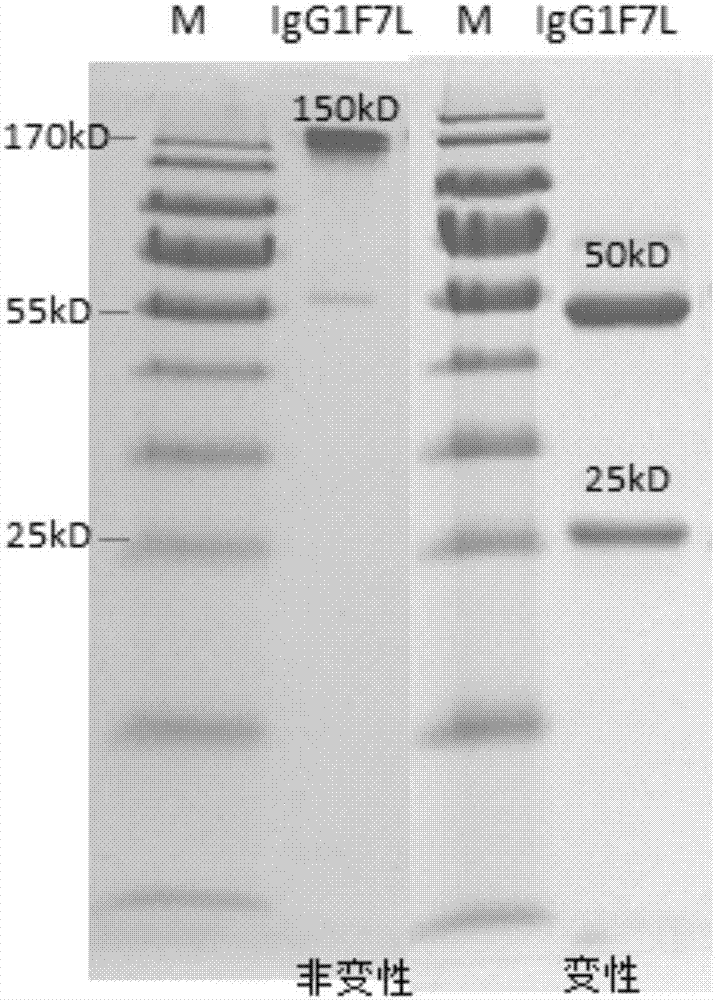
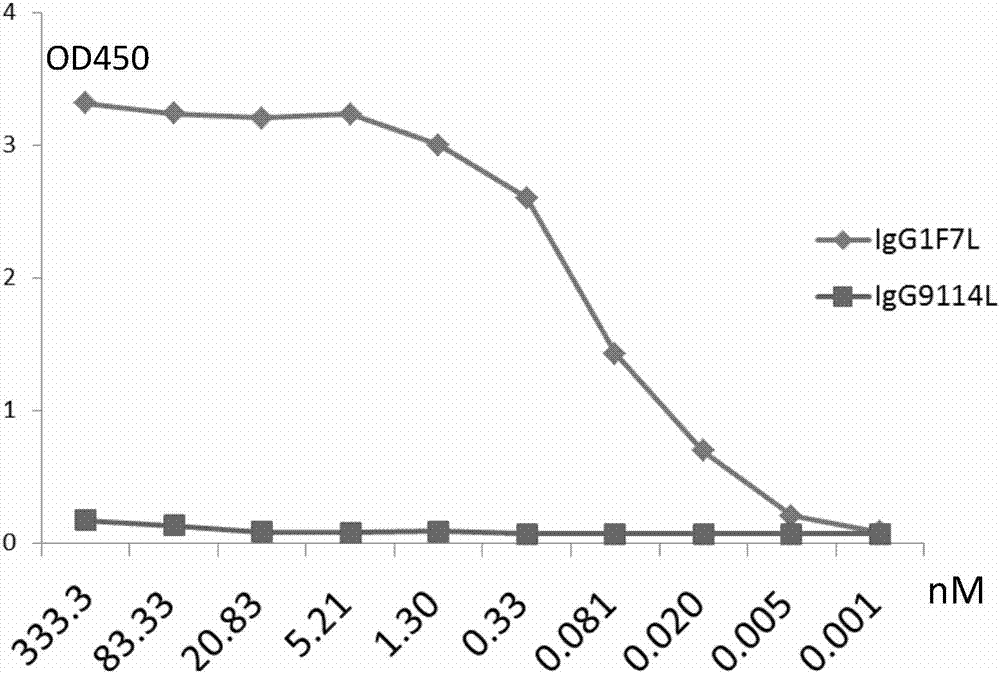
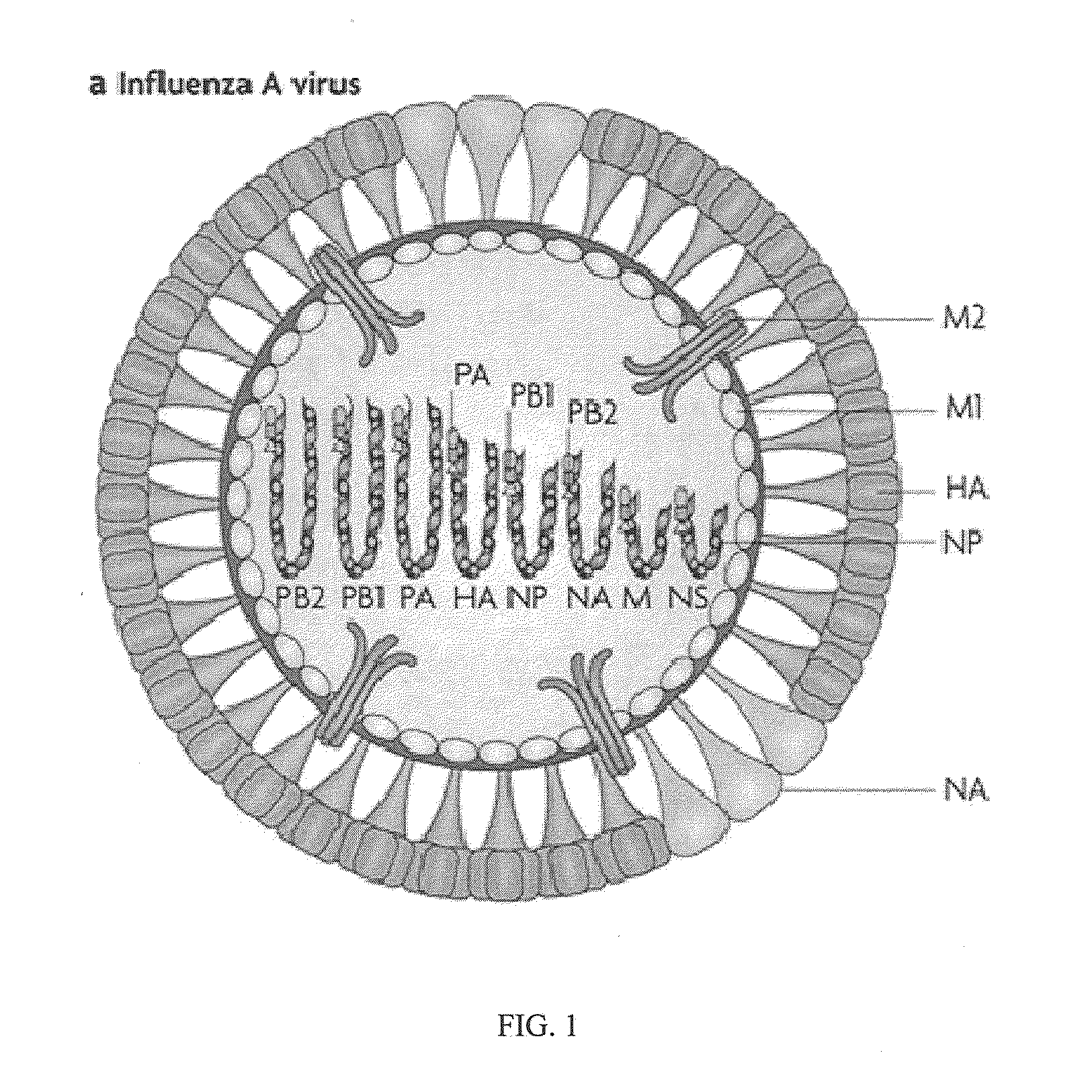
Patents
Literature
106 results about "Influenza virus Antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Vaccines and methods to treat canine influenza
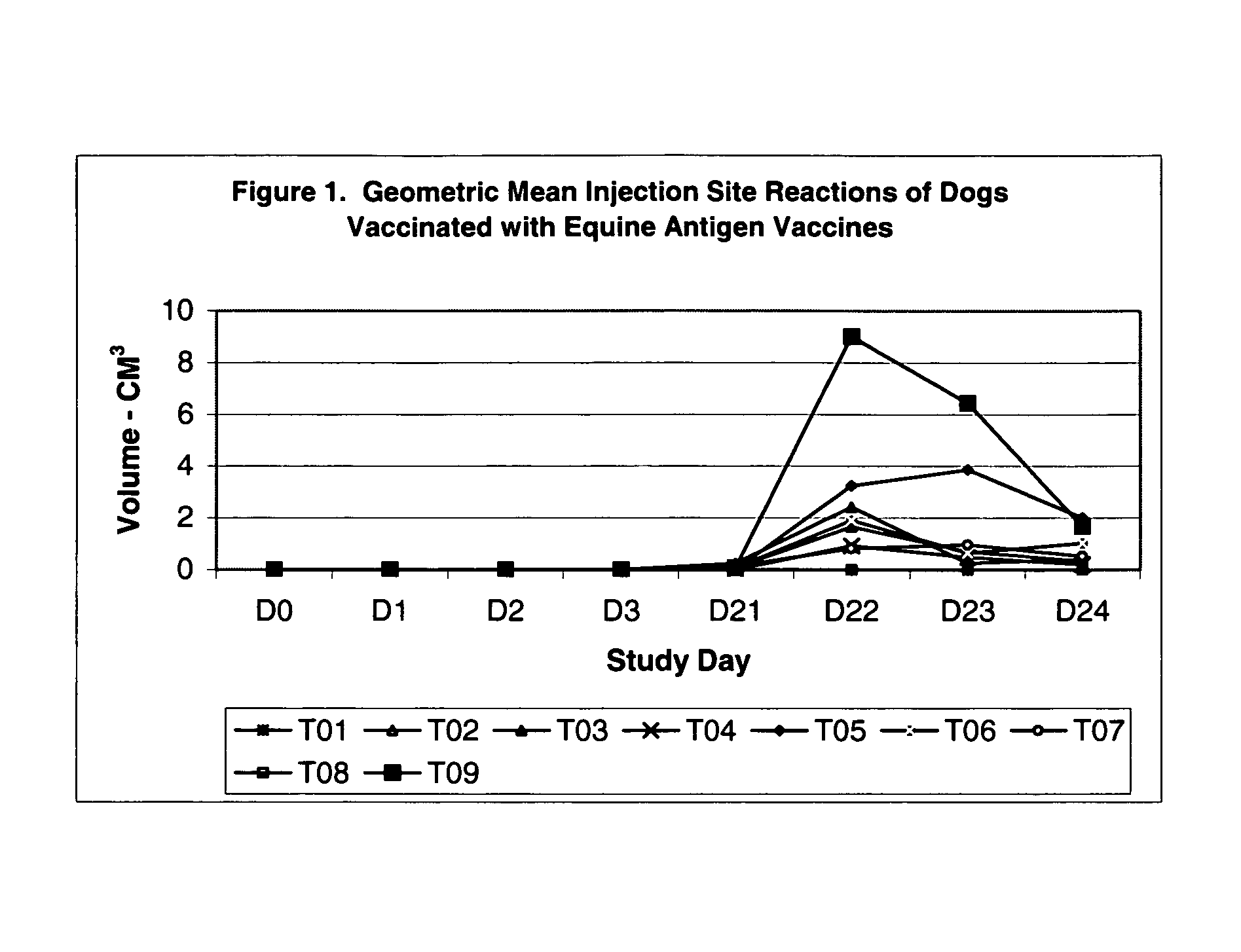
The present invention relates to providing new vaccines and treatments for the diseases related to canine influenza virus. It discloses influenza viral antigens, and methods of presenting these antigens to canines, especially dogs. It relates to attenuated and killed vaccines. The present invention relates to experimentally generated canine and equine influenza viruses. The invention also includes influenza A, including H3, N8, H3N8, H7N7 and viruses which contain at least one genome segment from an canine or equine influenza virus. The present invention also relates to the use of these viruses in therapeutic compositions to protect canines, dogs in particular, from diseases caused by influenza viruses.
Owner:ZOETIS SERVICE LLC
Oil-in-water emulsion influenza vaccine
ActiveUS20100189741A1Increase supplyLow amountSsRNA viruses negative-senseViral antigen ingredientsHuman useSterol
The present invention provides an immunogenic influenza composition in a dose volume suitable for human use, comprising an influenza virus antigen or antigenic preparation thereof and an adjuvant composition comprising an oil-in-water emulsion, wherein said oil-in-water emulsion comprises a metabolisable oil at a level of below 11 mg and an emulsifying agent at a level of below 5 mg and optionally a tocol or a sterol at a level of below 12 mg. Suitably the amount of influenza antigen per strain per dose is 15 μg HA or a low amount such as less than 15 μg HA.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Vaccine
ActiveUS20090136543A1Increase supplyLow amountSsRNA viruses negative-senseViral antigen ingredientsHuman useSterol
The present invention provides an immunogenic influenza composition in a dose volume suitable for human use, comprising an influenza virus antigen or antigenic preparation thereof and an adjuvant composition comprising an oil-in-water emulsion, wherein said oil-in-water emulsion comprises a metabolisable oil at a level of below 11 mg and an emulsifying agent at a level of below 5 mg and optionally a tocol or a sterol at a level of below 12 mg. Suitably the amount of influenza antigen per strain per dose is 15 μg HA or a low amount such as less than 15 μg HA.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Compositions of influenza viral proteins and methods of use thereof
InactiveUS20090162400A1Increase productionStimulate immune responseSsRNA viruses negative-senseSsRNA viruses positive-senseIntegral membrane proteinPathogen-Associated Molecular Pattern Molecules
Compositions, fusion proteins and polypeptides comprise at least one pathogen-associated molecular pattern and at least a portion of at least one integral membrane protein of an influenza viral antigen. The compositions, fusion proteins and polypeptides are used to stimulate an immune response in a subject.
Owner:VAXINNATE
Changing th1/th2 balance in split influenza vaccines with adjuvants
InactiveUS20090047353A1Eliminate needAvoid the needSsRNA viruses negative-sensePowder deliveryEgg proteinTh2 response
The invention seeks to avoid components in split vaccines that could cause an excessive Th2 response. Thus the invention provides an immunogenic composition comprising a split influenza virus antigen and a Th1 adjuvant, wherein the antigen is preferably prepared from a virus grown in cell culture (e.g., it is free from egg proteins).
Owner:SEQIRUS UK LTD
Influenza vaccines including combinations of particulate adjuvants and immunopotentiators
InactiveUS20090304739A1Eliminate needAvoid the needSsRNA viruses negative-senseViral antigen ingredientsParticulatesTh2 response
Influenza vaccines containing insoluble particulate adjuvants have been found to elicit an IgG response that is primarily a TH2 response (IgG1). This response can be shifted towards a TH1 response (IgG2a) by including immunopotentiators in the compositions. Thus the invention provides an immunogenic composition comprising: (i) an influenza virus antigen; (ii) an insoluble particulate adjuvant; and (iii) a immunopotentiator.
Owner:CHIRON CORP
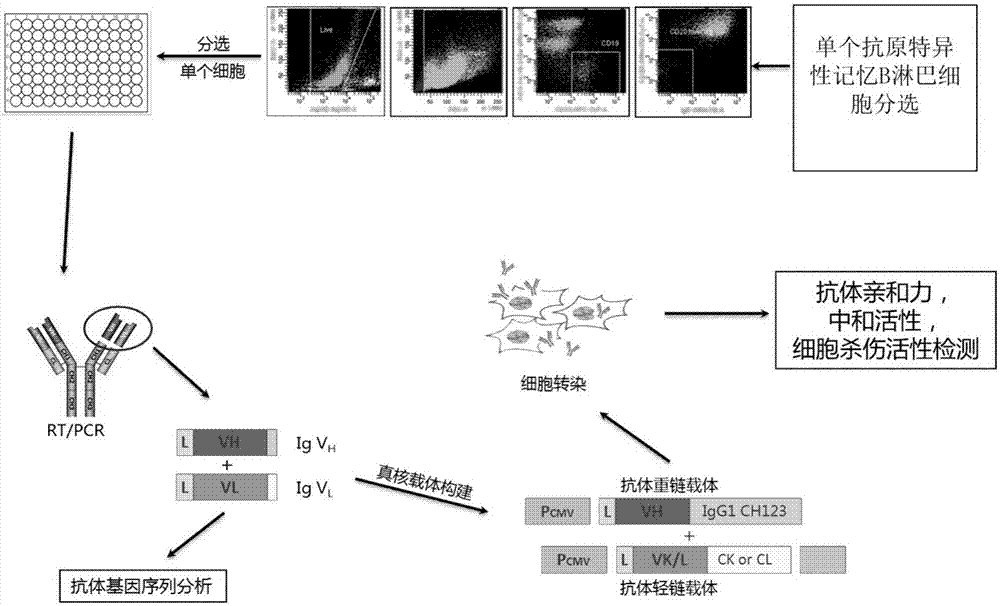
Human anti-H7N9 avian influenza virus neutralizing antibody 1F7L and its use
InactiveCN107226861AEffective neutralizationAvoid infectionImmunoglobulins against virusesAntiviralsNatural Killer Cell Inhibitory ReceptorsNeutralizing antibody
The invention discloses a human anti-H7N9 avian influenza virus neutralizing antibody 1F7L screened by a single cell sorting technology. The amino acid sequences in the light and heavy chain variable regions are shown in the formulas of SEQ ID No. 2 and SEQ ID No. 5. The antibody has the ability to neutralize the H7N9 influenza viruses in vitro and mediates the killing (ADCC) of the H7N9 influenza virus-infected cells with effector cells mainly comprising NK cells. The antibody can be used as a treatment drug for highly pathogenic avian influenza infection and can also be used for the development of H7N9 influenza virus antigen detection reagents.
Owner:THE THIRD PEOPLES HOSPITAL OF SHENZHEN
Influenza vaccines with reduced amount of emulsion adjuvant
InactiveUS20090304742A1Eliminate needAvoid the needSsRNA viruses negative-senseViral antigen ingredientsHemagglutininInfluenza vaccine
Influenza vaccines with oil-in-water emulsion adjuvants are known. The amount of emulsion adjuvant required for an influenza vaccine can be reduced, thereby allowing more vaccines to be made from a given amount of emulsion, and / or minimizing the amount of emulsion that has to be produced for a given number of vaccine doses. These vaccines can conveniently be made by mixing (i) an oil-in-water emulsion and (ii) an aqueous preparation of an influenza virus antigen. In one aspect, substantially equal volumes of components (i) and (ii) are used; in another aspect, an excess volume of component (ii) is used. When using substantially equal volumes, component (ii) has a hemagglutinin concentration of more than 60 μg influenza virus strain per ml. Components (i) and (ii) can be presented in kit form.
Owner:NOVARTIS AG
Adjuvanted influenza vaccines including cytokine-inducing agents
InactiveUS20110180430A1Quality improvementGood curative effectAntibacterial agentsSsRNA viruses negative-senseInfluenza vaccineΓ interferon
While oil-in-water emulsions are excellent adjuvants for influenza vaccines, their efficacy can be improved by additionally including other immunostimulating agent(s) to improve cytokine responses, such as γ-interferon response. Thus, a vaccine comprises (i) an influenza virus antigen; (ii) an oil-in-water emulsion adjuvant; and (iii) a cytokine-inducing agent.
Owner:NOVARTIS AG
Influenza A,B virus antigen colloidal gold combined detection test paper
InactiveCN1904615ASave the procedure of secondary inspectionThe result is clear and easy to distinguishMaterial analysis by observing effect on chemical indicatorTest sampleColloid
The invention supplies a rapid testing tape that could simultaneously test A, B virus antigen. It covers FluA-McAb, FluB-McAb and IgG on NC film, compounds FluA-McAb, FluB-McAb marked by colloidal gold, simultaneously tests A, B virus antigen in tested sample by using film chromatography double antibody cream filling method. The invention could decrease cockamamie process, and could gain result in 10-15 minutes. It is suitable to hospital and the research for large scale epidemiology to handle sporadic affair.
Owner:BEIJING ZHUANGDI HAOHE BIOMEDICINE SCI & TECH
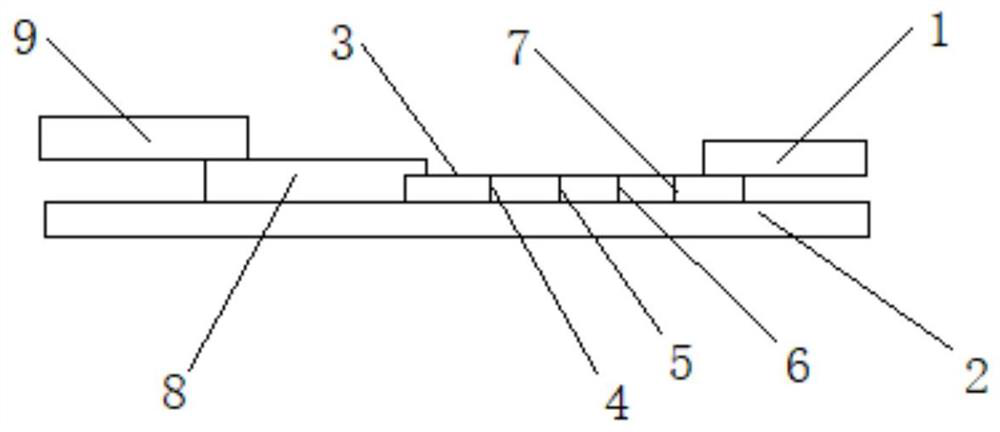
Novel coronavirus antigen and influenza virus antigen combined detection reagent strip and preparation method thereof
PendingCN112198312AReduce false positive interferenceHigh sensitivityBiological testingImmunoassaysReagent stripCoronavirus antibody
The invention discloses a novel coronavirus antigen and influenza virus antigen combined detection reagent strip which comprises a bottom plate. A sample pad, a gold-labeled pad, a nitrocellulose membrane and absorbent paper are sequentially pasted on the bottom plate, and the surface of the nitrocellulose membrane is sequentially coated with an anti-novel coronavirus antibody, an anti-influenza Avirus antibody and an anti-influenza B virus antibody. A rabbit anti-mouse IgG antibody coats the end close to the absorbent paper. A colloidal gold labeled anti-novel coronavirus antibody, an anti-influenza A virus antibody and an anti-influenza B virus antibody are sprayed on the gold-labeled pad. The binding protein A / G is marked on the surface of the colloidal gold, the protein A / G is specifically bound with the Fc end of the antibody, so that the ideal conformation of Fab end abduction is constructed, and meanwhile, the binding of the protein A / G with the Fc can reduce the false positiveinterference of rheumatoid factors on a detection structure. The reagent strip can simultaneously realize qualitative detection of the novel coronavirus antigen and the influenza antigen in one test,and is convenient to use, good in sensitivity, high in specificity and short in detection time.
Owner:南京佰抗生物科技有限公司
Influenza vaccines extemporaneously adsorbed to aluminium adjuvants
InactiveUS7959931B2SsRNA viruses negative-senseViral antigen ingredientsFlu immunizationInfluenza vaccine
Antigen and adjuvant components of an adjuvanted influenza vaccine are not mixed during manufacture, but are provided as separate components for extemporaneous mixing at the time of use, for example as a kit comprising (i) an antigen component, comprising an influenza virus antigen; and (ii) an adjuvant component, comprising an aluminium salt.
Owner:NOVARTIS AG
Neutralizing monoclonal antibodies for resisting H7N9 influenza virus
InactiveCN108794624AHigh neutralizing activityEasy to express and purifyImmunoglobulins against virusesAntiviralsMonoclonal antibodyVirus antigen
The invention discloses neutralizing monoclonal antibodies for resisting the H7N9 influenza virus and particularly discloses two monoclonal antibodies aiming at the H7N9 influenza virus. The antibodies has evident combining activity to H7N9 influenza virus antigens.
Owner:INST PASTEUR OF SHANGHAI CHINESE ACADEMY OF SCI
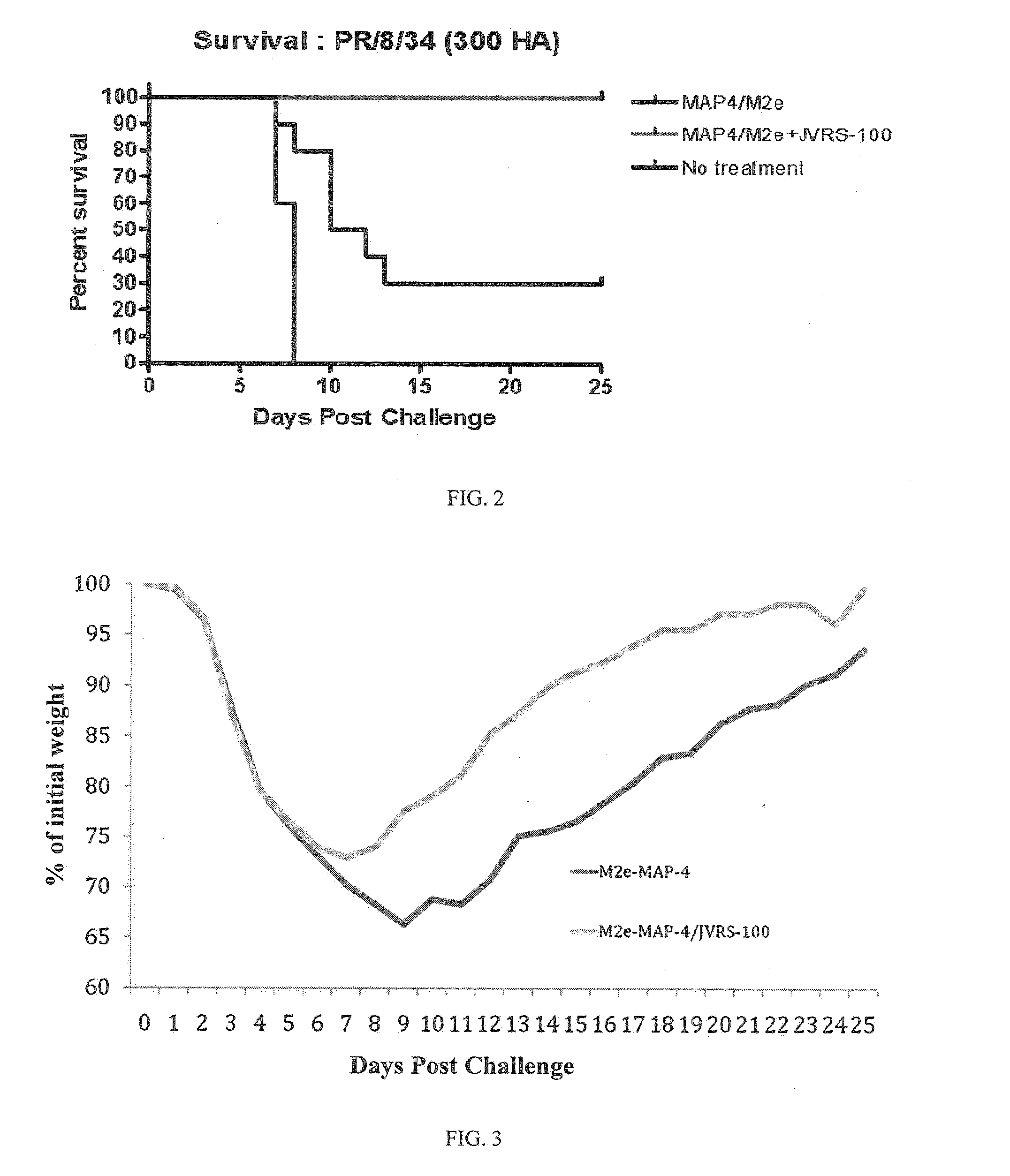
VACCINE COMPOSITIONS OF M2e, HA0 AND BM2 MULTIPLE ANTIGENIC PEPTIDES
InactiveUS20100086584A1SsRNA viruses negative-senseAntibody mimetics/scaffoldsDelivery vehicleInfluenza a
The present disclosure generally relates to a composition comprising one or more peptides selected from influenza virus antigenic peptides M2e, HA0, BM2 and a M2e-BM2 fusion peptide in a composition with a cationic liposome delivery vehicle, and the use of these compositions as a universal vaccine against influenza A and / or B viral strains.
Owner:JUVARIS BIOTHERAPEUTICS
Influenza vaccine
ActiveUS9452209B2Increase supplyLow amountSsRNA viruses negative-senseViral antigen ingredientsSterolFlu immunization
The present invention provides an immunogenic influenza composition in a dose volume suitable for human use, comprising an influenza virus antigen or antigenic preparation thereof and an adjuvant composition comprising an oil-in-water emulsion, wherein said oil-in-water emulsion comprises a metabolisable oil at a level of below 11 mg and an emulsifying agent at a level of below 5 mg and optionally a tocol or a sterol at a level of below 12 mg. Suitably the amount of influenza antigen per strain per dose is 15 μg HA or a low amount such as less than 15 μg HA.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Influenza virus splitting vaccine and preparation method thereof
InactiveCN102068692AGood immune effectLess antigenAntiviralsAntibody medical ingredientsHemagglutininMedicine
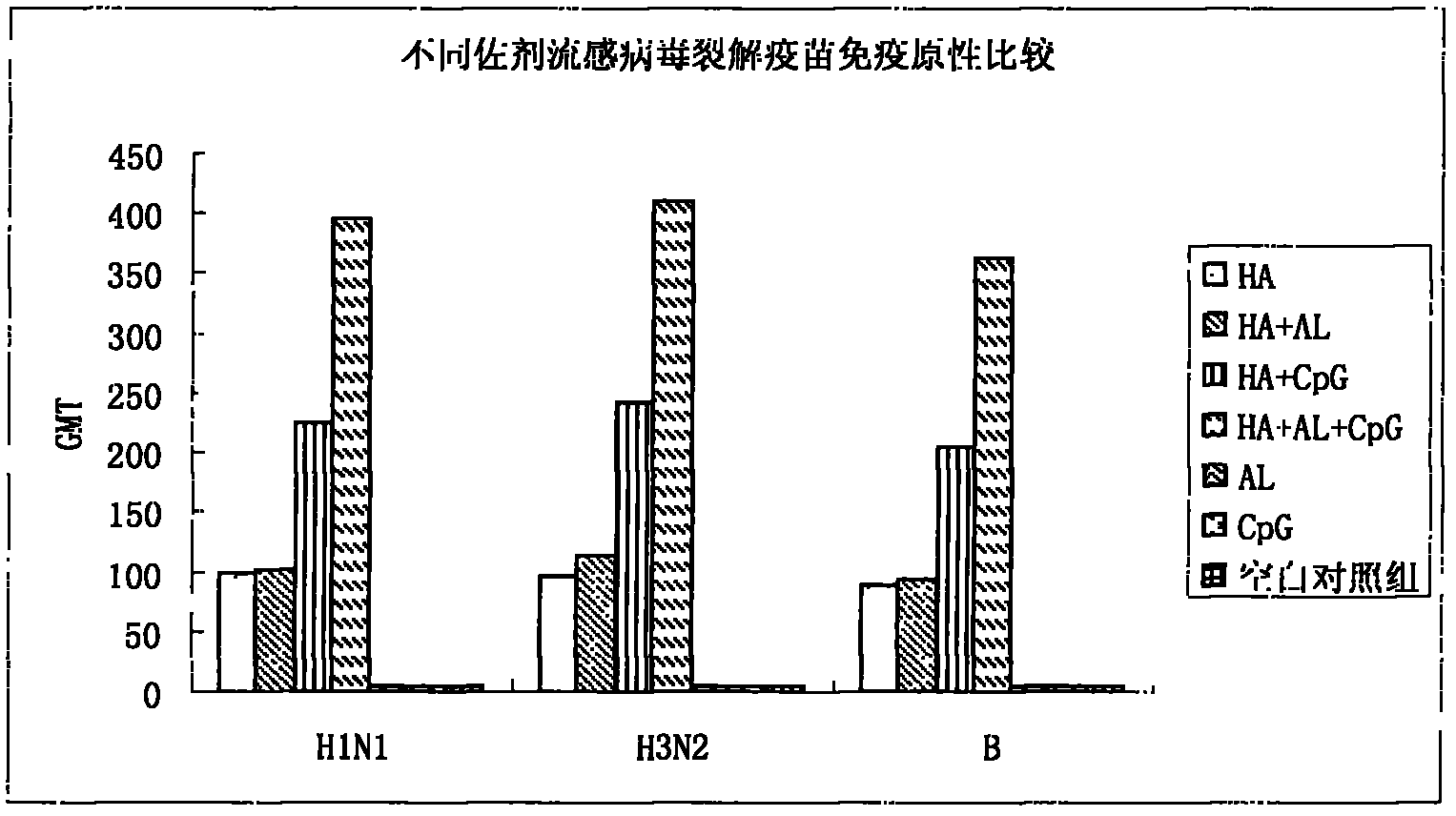
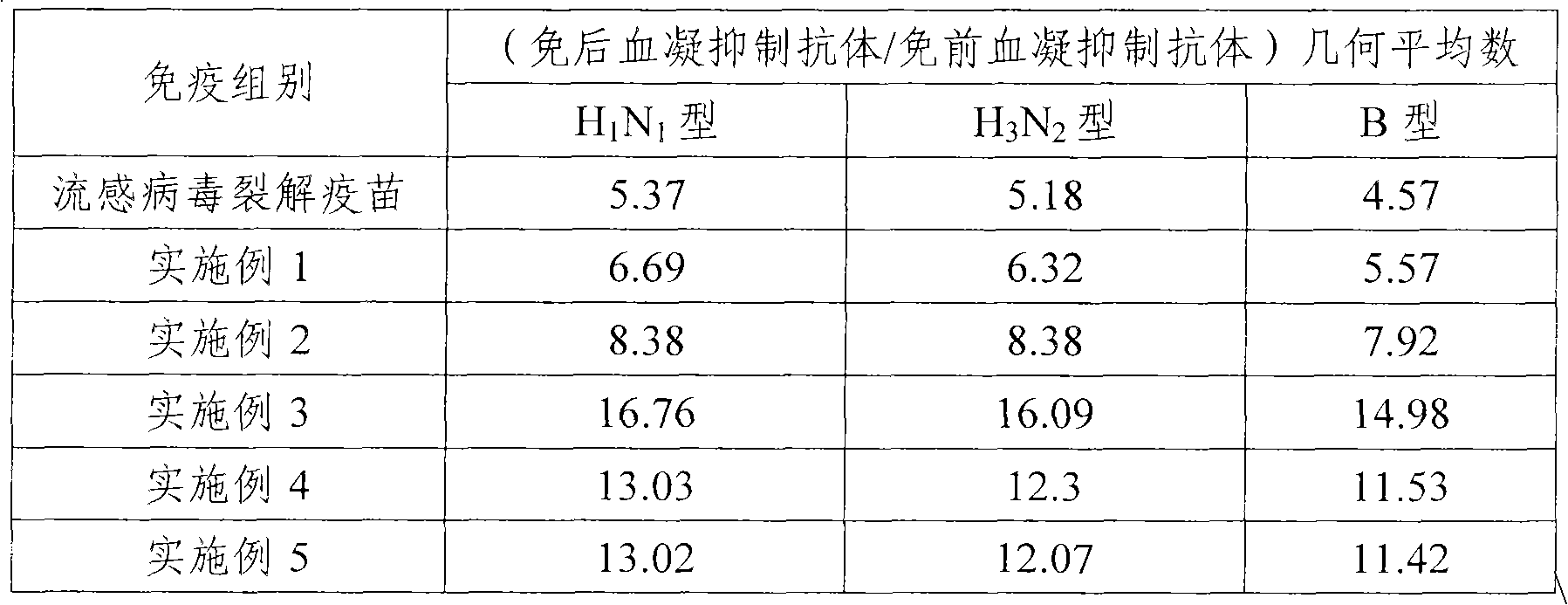
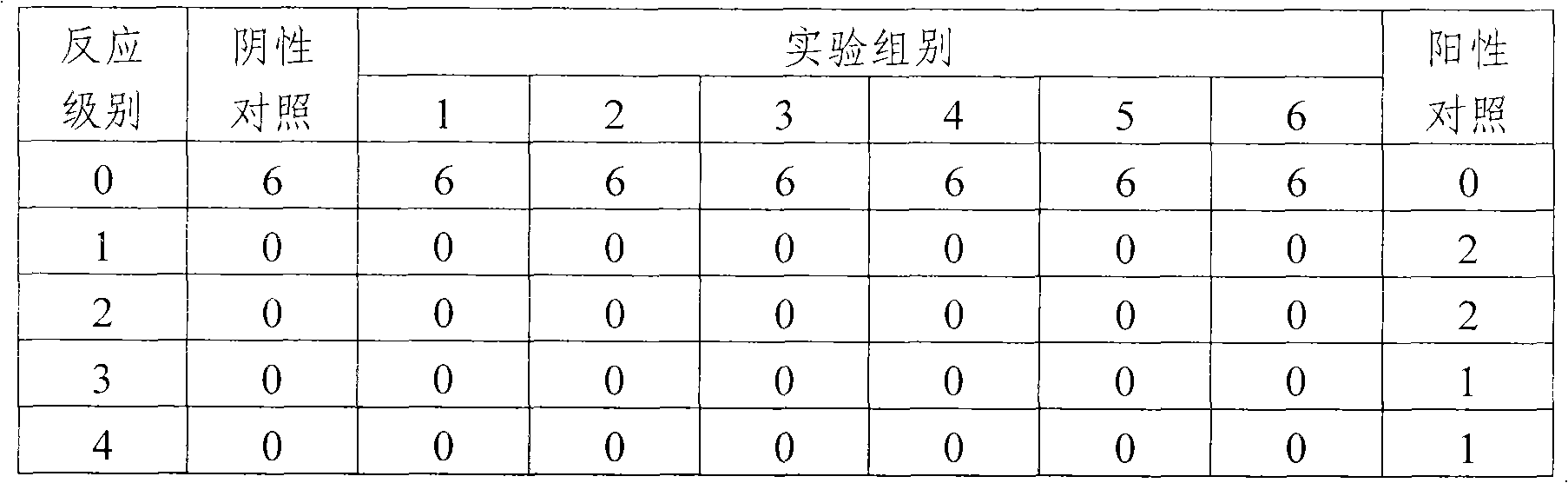
The invention provides an influenza virus splitting vaccine and a preparation method thereof. Each dose of influenza virus splitting vaccine contains three influenza hemagglutinins, namely H1N1, H3N2 and type B, a CpG ODN adjuvant and an aluminium adjuvant. The invention also provides a preparation method for the influenza vaccine, which comprises the following steps of: multiplying influenza viruses in chick embryo; performing ultrafitration and concentration; centrifuging and purifying; splitting; performing secondary purification; inactivating; and diluting and packaging. The influenza virus splitting vaccine can reduce the dosage of the influenza virus antigen and production cost of the influenza vaccine, and is suitable for quickly improving influenza vaccine supplying capacity under the threat of global influenza pandemic.
Owner:BEIJING MINHAI BIOTECH
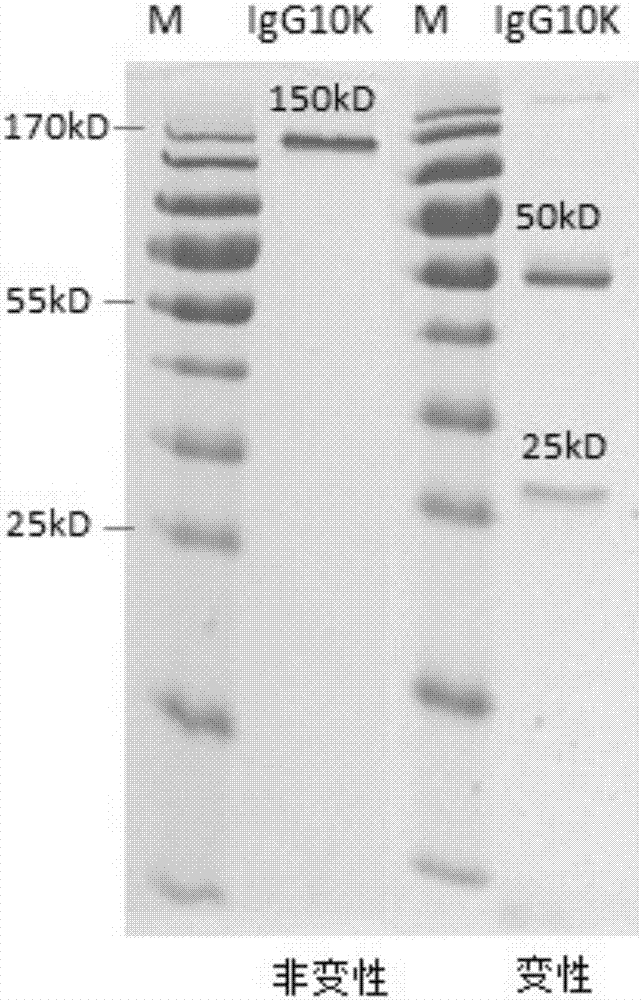
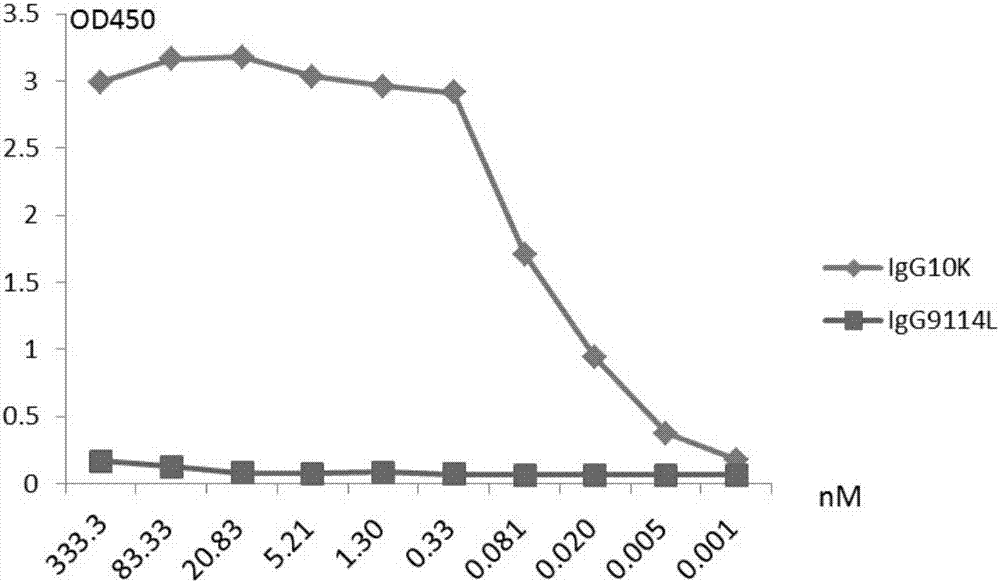
Humanized anti-H7N9 avian influenza virus high-affinity antibody 10K and application thereof
InactiveCN107056938AEfficient combinationImmunoglobulins against virusesAntiviralsHeavy chainEffector cell
The invention discloses a humanized anti-H7N9 avian influenza virus high-affinity antibody 10K filtered and obtained based on a single cell separation technology, the amino acid sequences of light chain and heavy chain variable regions of the antibody are shown as SEQ ID No. 2 and SEQ ID No. 5 respectively. The high-affinity specificity of the antibody is combined with H7N9 avian influenza virus 7 type hemagglutinin protein, and can mediate the kill and wound (ADCC) of effector cells using NK cells as main parts for H7N9 influenza virus infected cells. The antibody 10K can be used for therapeutic development of highly pathogenic avian influenza infection, and also can be used for development of H7N9 influenza virus antigen dectection reagents.
Owner:深圳普兰达科技有限公司
Chicken egg-yolk antibody capable of resisting influenza virus and preparation method thereof
ActiveCN105968195AEfficient killingLarge range of protectionEgg immunoglobulinsImmunoglobulins against virusesInfluenza A virus subtype H3N2Virology
The invention discloses a chicken egg-yolk antibody capable of resisting influenza virus. The chicken egg-yolk antibody is used for specifically resisting tetravalence influenza virus. The influenza virus includes influenza A virus subtype H1N1, influenza A virus subtype H3N2, influenza B virus subtype Victoria series and influenza A virus subtype Yamagata series. The preparation method of the chicken egg-yolk antibody comprises the following steps of 1, preparing a tetravalence influenza virus antigen; 2, performing immunity on hens; 3, collecting immunized eggs laid by the immunized hens; 4, preparing a mixed antibody stock solution from the immunized eggs; 5, detecting titer of the antibody stock solution, and a stabilizer is added cooperatively, so that the chicken egg-yolk antibody capable of resisting tetravalence influenza virus is obtained. The chicken egg-yolk antibody can be combined with the tetravalence influenza virus specificity pathogen for a short period of time, the tetravalence influenza virus can be effectively killed for a long period of time, the safety performance is good, no toxic or side effect is generated, and the chicken egg-yolk antibody can be effectively used for preventing pathogen corresponding to human pharyngeal and nasopharynx mucosas.
Owner:CHENGDU ANTIK BIOTECH CO LTD
Compositions that include hemagglutinin, methods of making and methods of use thereof
InactiveCN101394864ALess groomingDecreased physical activityBacterial antigen ingredientsAntiinfectivesHemagglutininArginine
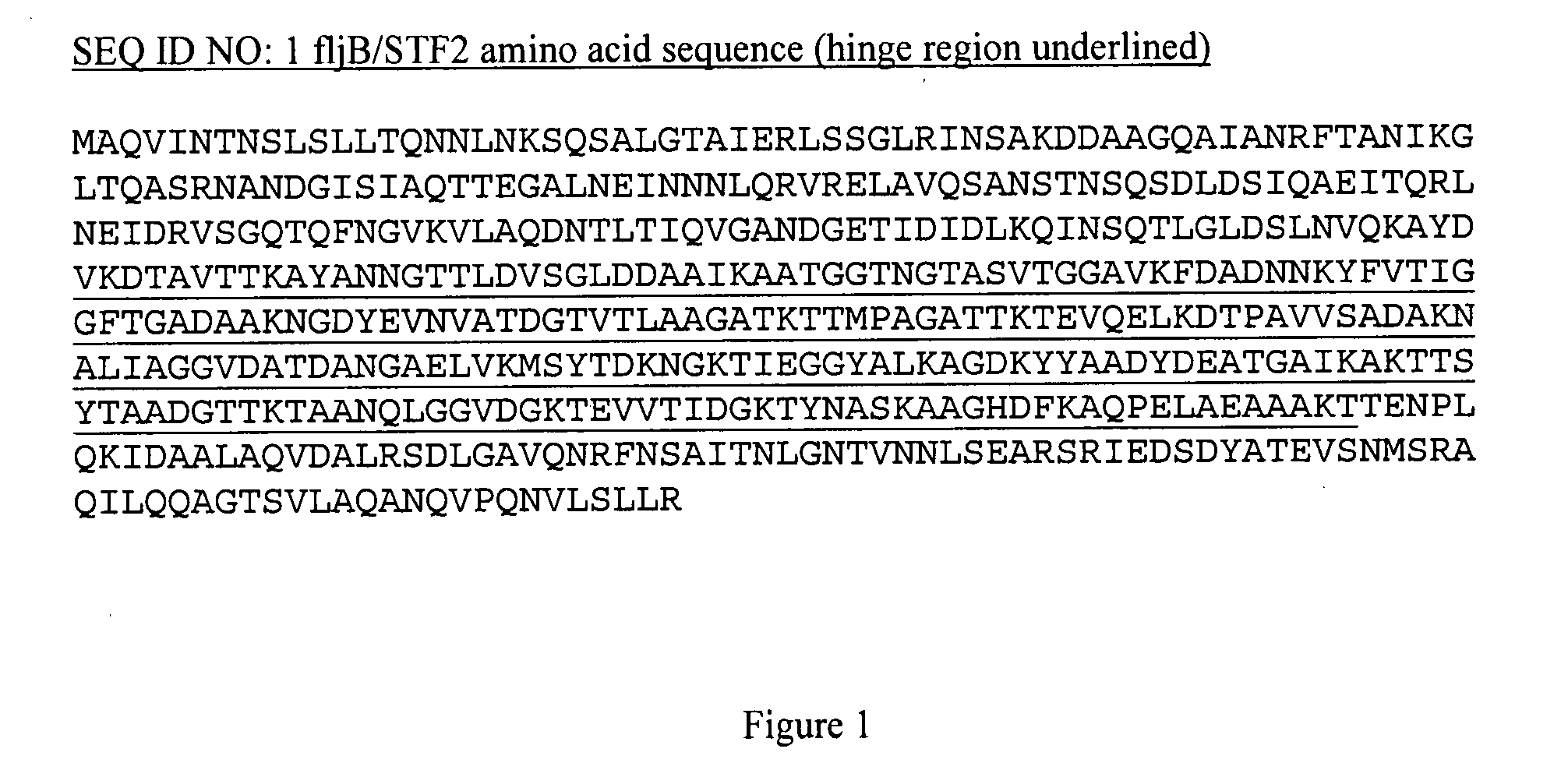
Methods of making compositions that stimulate a protective immune response in a subject include a portion of a protein from a naturally occurring viral hemagglutinin, wherein the portion includes at least a portion of a globular head, includes at least a portion of at least one secondary structure that causes the globular head to essentially retain its tertiary structure, and lacks a membrane fusion domain, a transmembrane domain and a cytoplasmic domain. Compositions comprise a flagellin component or a Toll-like Receptor agonist component that is at least a portion of a flagellin or a Toll-like Receptor agonist, wherein the flagellin component or Toll-like Receptor agonist component includes at least one cysteine residue and whereby the flagellin component or Toll-like Receptor agonist component activates a Toll-like Receptor 5 or Toll-like Receptor. Compositions comprise a flagellin component that is at least a portion of a flagellin, wherein at least one lysine of the flagellin component has been substituted with at least one arginine, serine and histidine, whereby the flagellin component activates Toll-like Receptor 5. Compositions can further include an antigen, such as an influenza antigen, a flavivirus antigen, a pathogen-related antigen, a bacterial capsular antigen and a carrier protein. The compositions are used to stimulate an immune response and a protective immune response in a subject.
Owner:VAXINNATE
Method for preparing nasal-spray type influenza virus vaccine containing CpG ODN and poly I:G adjuvant
InactiveCN101745109AImmunity-boosting propertiesLess antigenAerosol deliveryAntiviralsInfluenza virus vaccineLysis
The invention provides a method for preparing nasal-spray type influenza virus vaccine containing CpG ODN and poly I:G adjuvant, which belongs to the technical field of biology. The method comprises the following steps: adopting chicken embryo to culture proliferative influenza virus; obtaining purified influenza virus through ultrafiltration concentration and column chromatography; obtaining influenza vaccine stock solution through lysis; and proportioning the influenza vaccine stock solution of a required type to CpG ODN adjuvant or I:G adjuvant. The invention provides the method for preparing novel nasal-spray type influenza virus vaccine, which can reduce the amount of influenza virus antigen, provides convenience for immunization and vaccination, and is suitable for rapidly improving the capacity of supplying influenza vaccine under threat of global pandemic influenza.
Owner:云南沃森生物技术股份有限公司
Device for detecting avian influenza virus antibody and detection method
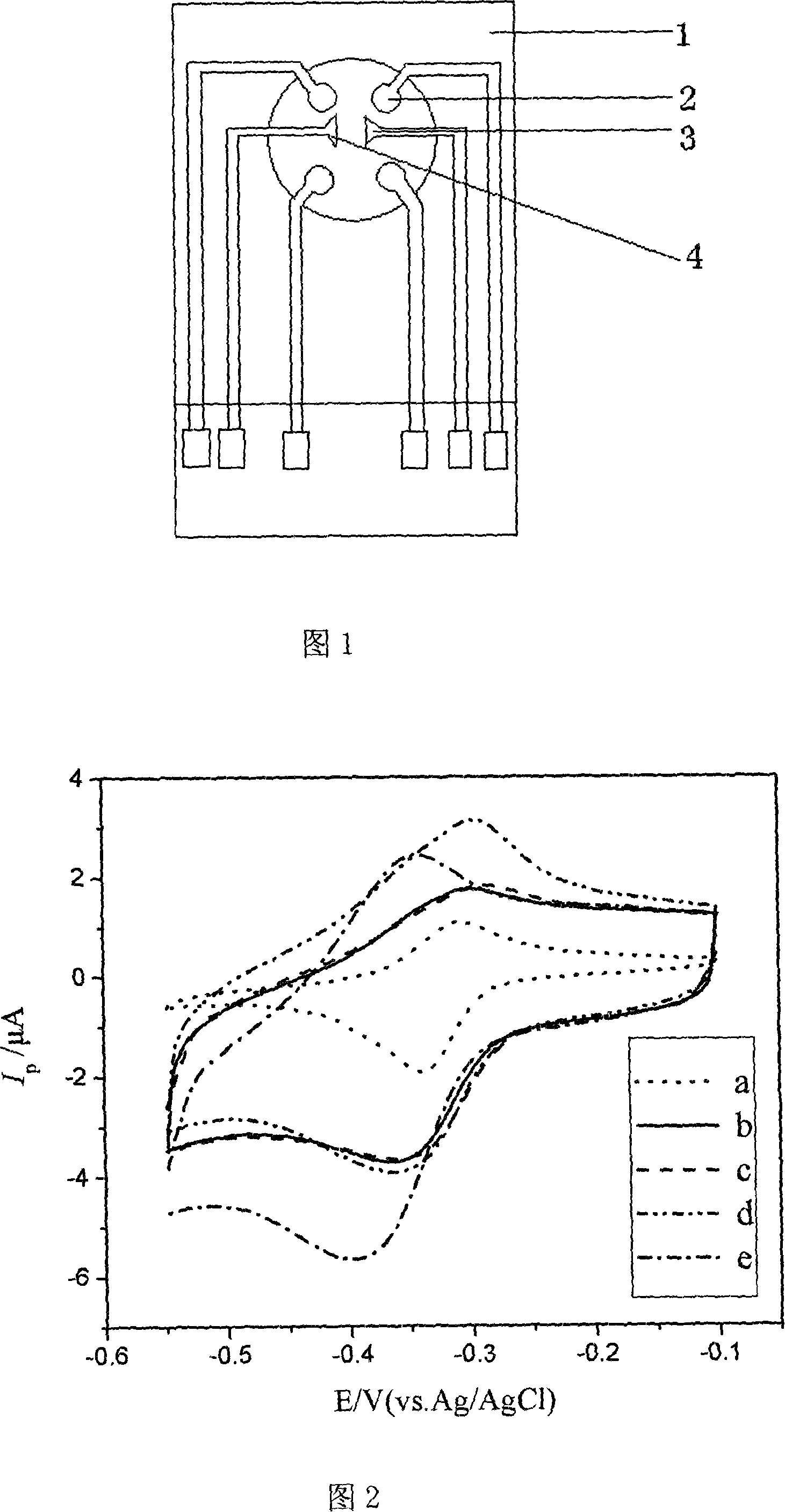
InactiveCN101149383AStrong specificityLow priceMaterial analysis by electric/magnetic meansProcess systemsSoftware system
This invention disclose a kind of equipment used to detect the fowl influenza virus antibody correctly, fast, sensitively, with high specificity and economically. The equipment includes immunity sensor, enzyme labeled double antibody reaction system, electrolytic cell and data collection and process system; the immunity sensor includes the electrode system which is composed with the work electrode, the reference electrode and the assistant electrode which are printed in the insulated base plate; the electrolytic cell includes the detection base liquid, the beater and the temperature controller; the data collection and process system includes the electrochemistry workstation, the computer and the software system; the immunity sensor connects the electrochemistry workstation by lead; the work electrode of the immunity sensor is carbon electrode, which has coated the gold size induction film embed fowl influenza virus antibody. This invention also discloses a kind of method to use the equipment to detect the fowl influenza virus antibody.
Owner:ZHEJIANG GONGSHANG UNIVERSITY +1
Vaccine adjuvant comprising lipopeptide-inserted liposome as effective ingredient and use thereof
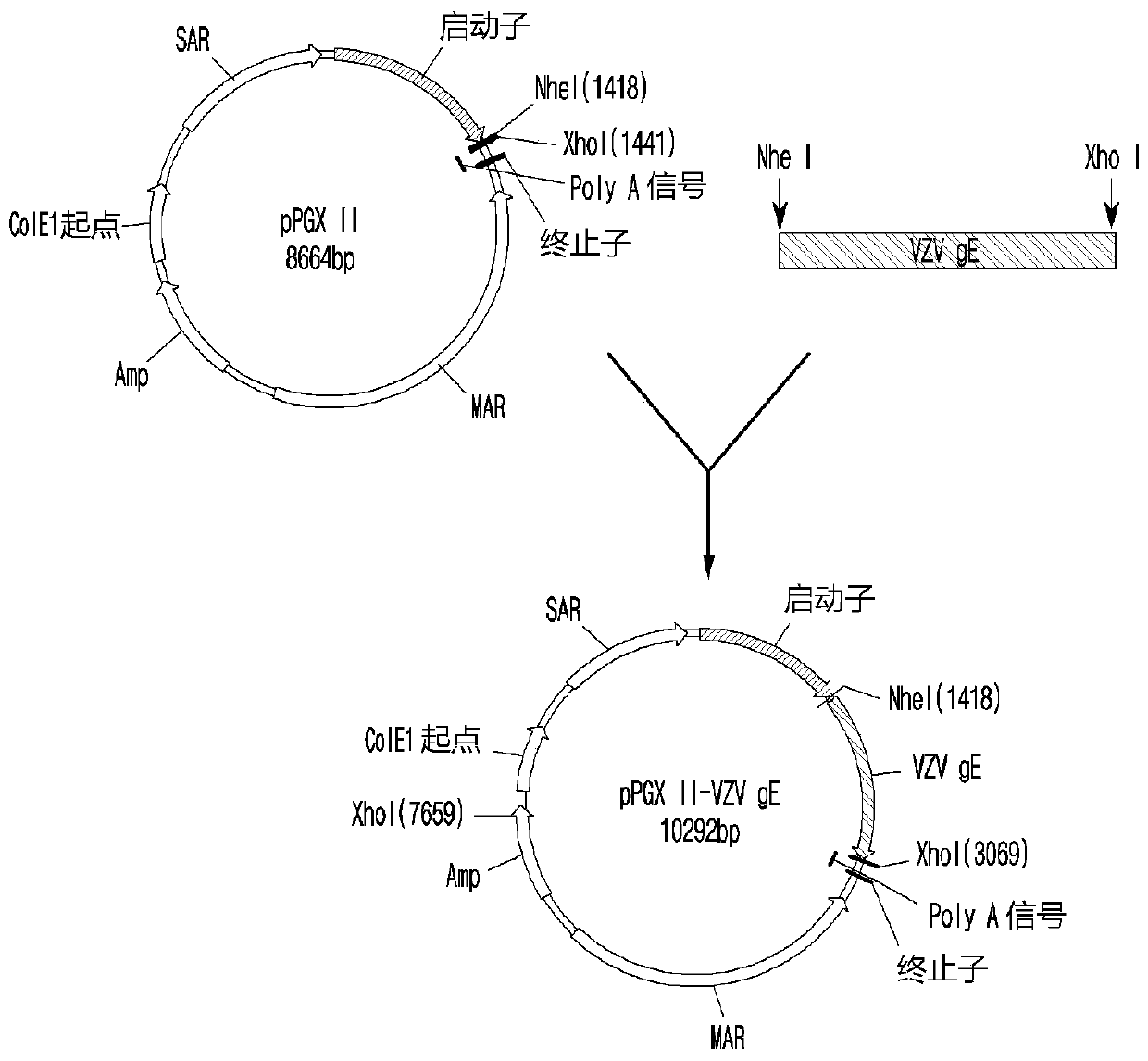
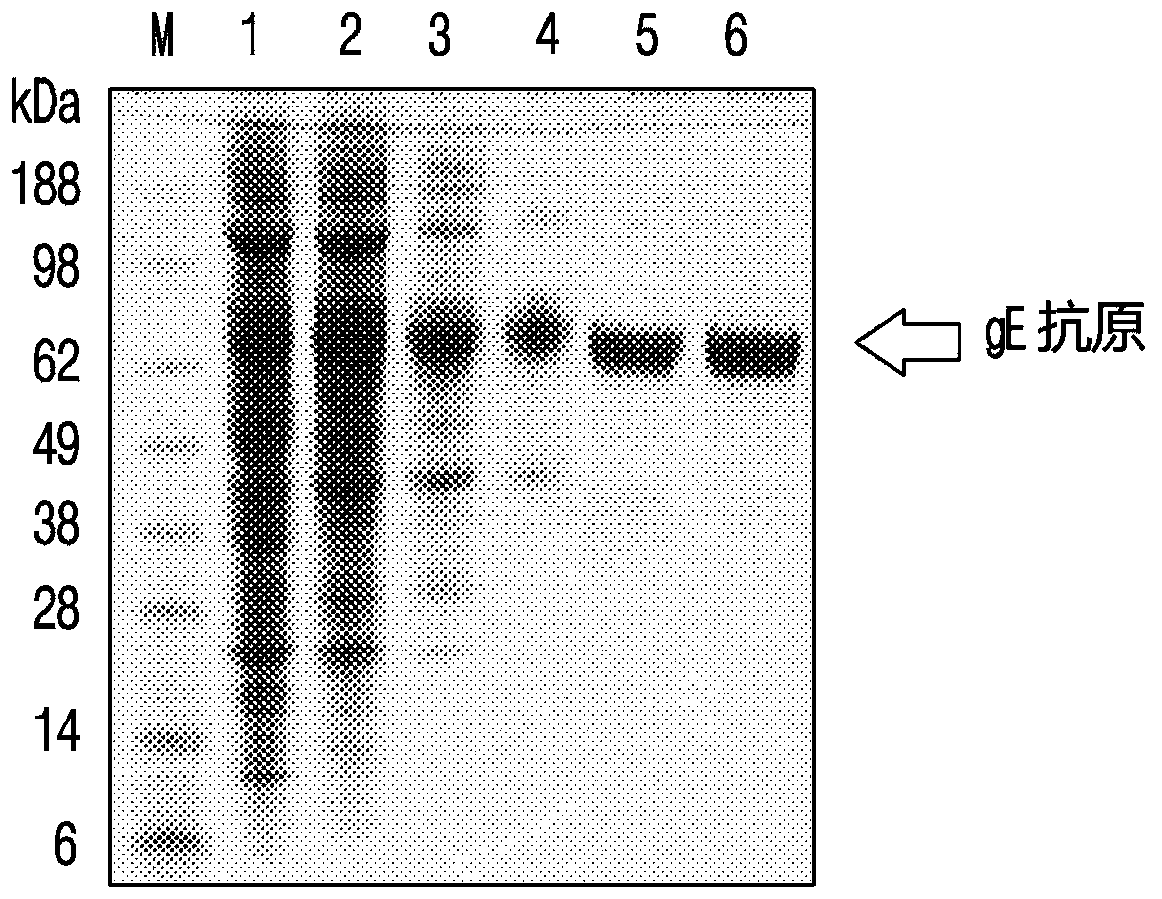
PendingCN110996999ASsRNA viruses negative-senseSsRNA viruses positive-senseChickenpoxHerpes zoster virus
The present invention relates to a recombinant herpes zoster vaccine comprising liposome and lipopeptide and a method for preparing the same. More particularly, a vaccine composition according to thepresent invention, prepared using Lipo-Pam, which is a composite adjuvant comprising a liposome and various kinds of lipopeptides, and a varicella-zoster virus gE antigen, a Japanese encephalitis virus gE antigen, or a seasonal inactivated influenza virus antigen, highly induces a cell-mediated immune response as well as a humoral immune response so that the composition of the present invention can be commercially useful.
Owner:株式会社车疫苗研究所
Influenza vaccines extemporaneously adsorbed to aluminium adjuvants
InactiveUS20090202590A1SsRNA viruses negative-senseViral antigen ingredientsTime of useInfluenza vaccine
Antigen and adjuvant components of an adjuvanted influenza vaccine are not mixed during manufacture, but are provided as separate components for extemporaneous mixing at the time of use, for example as a kit comprising (i) an antigen component, comprising an influenza virus antigen; and (ii) an adjuvant component, comprising an aluminium salt.
Owner:NOVARTIS AG
Novel epitope and mechanism of antigen-antibody interaction in an influenza virus
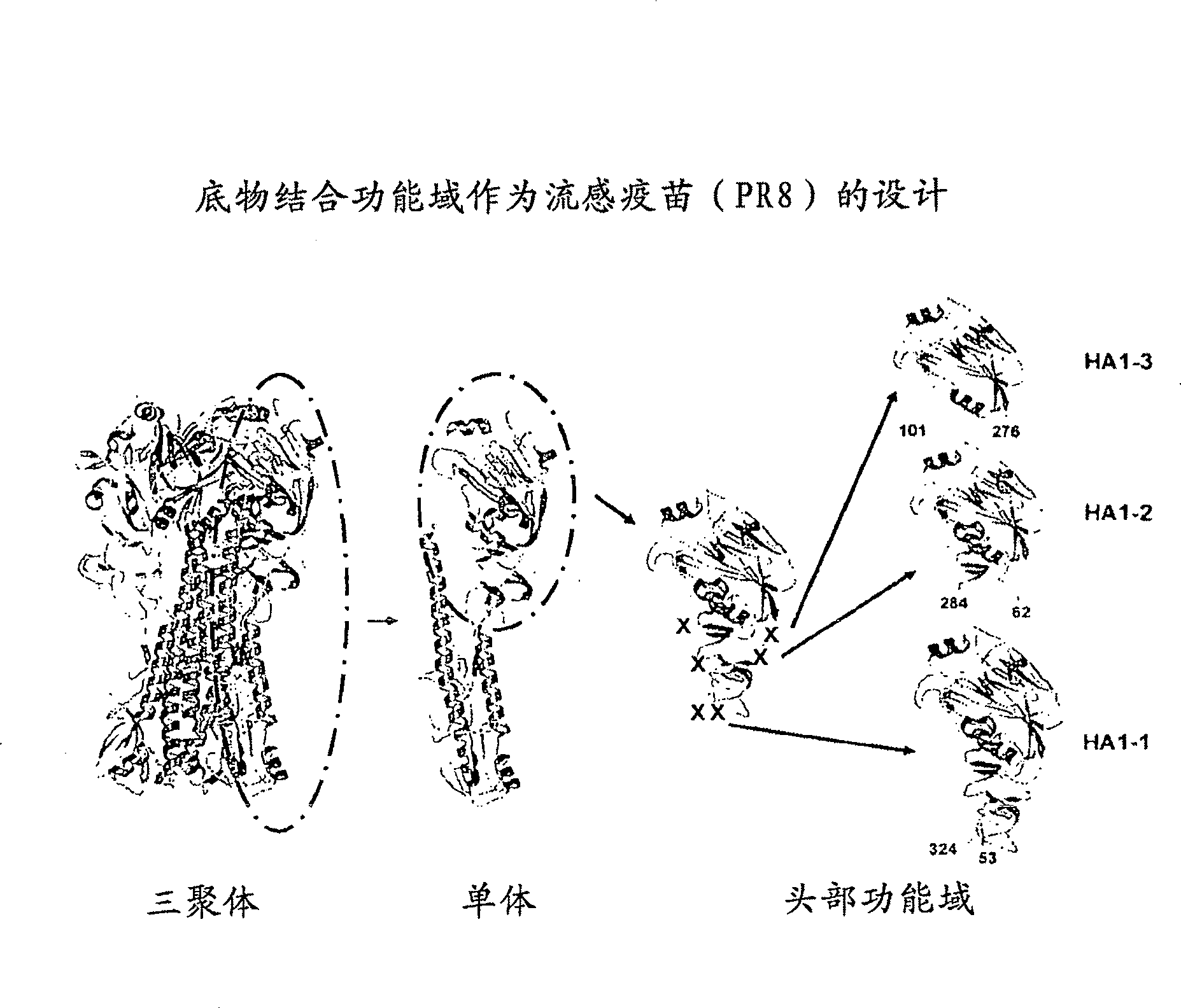
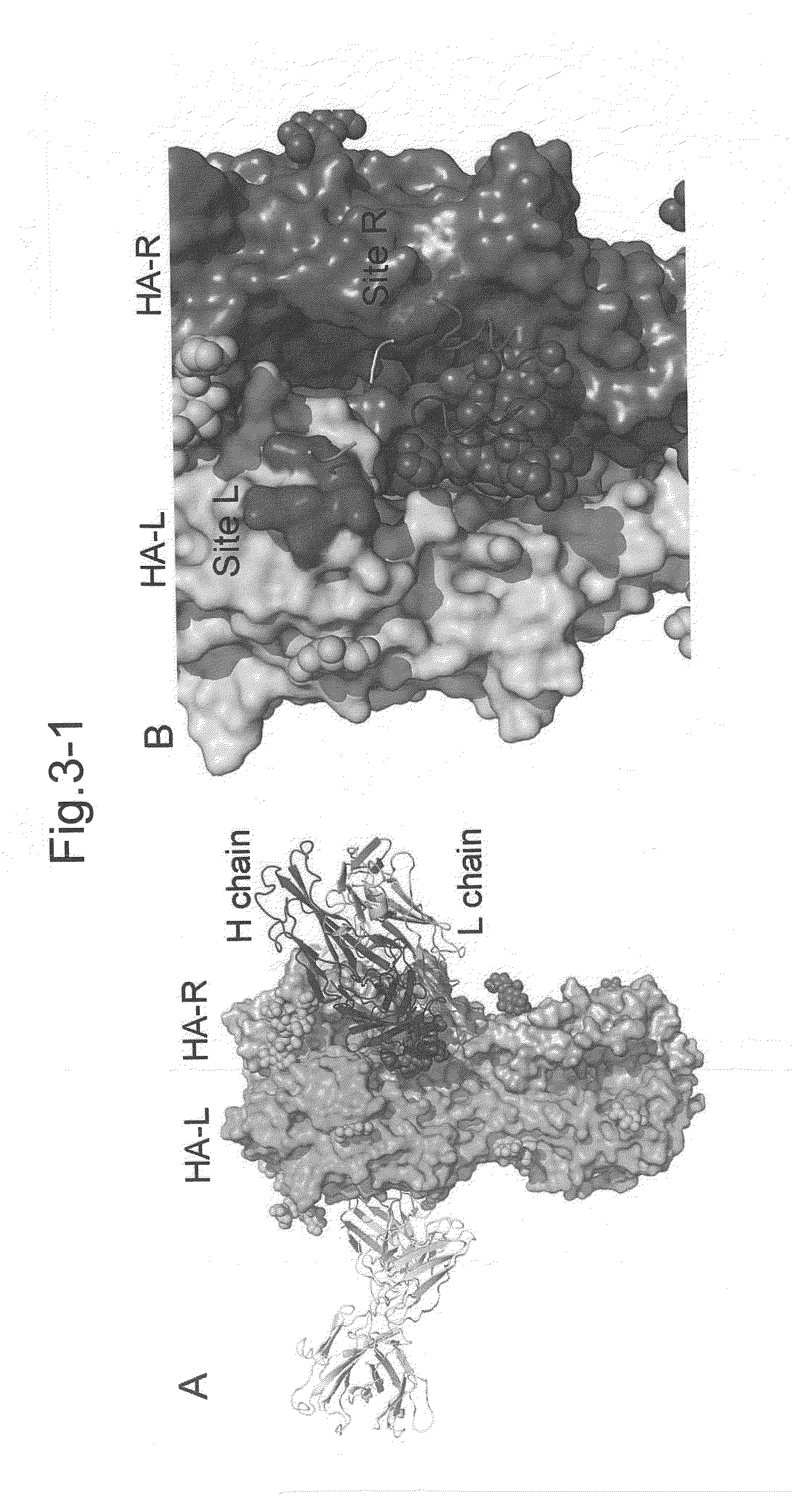
InactiveUS20140086927A1Molecular designMicrobiological testing/measurementHemagglutininNeutralizing antibody
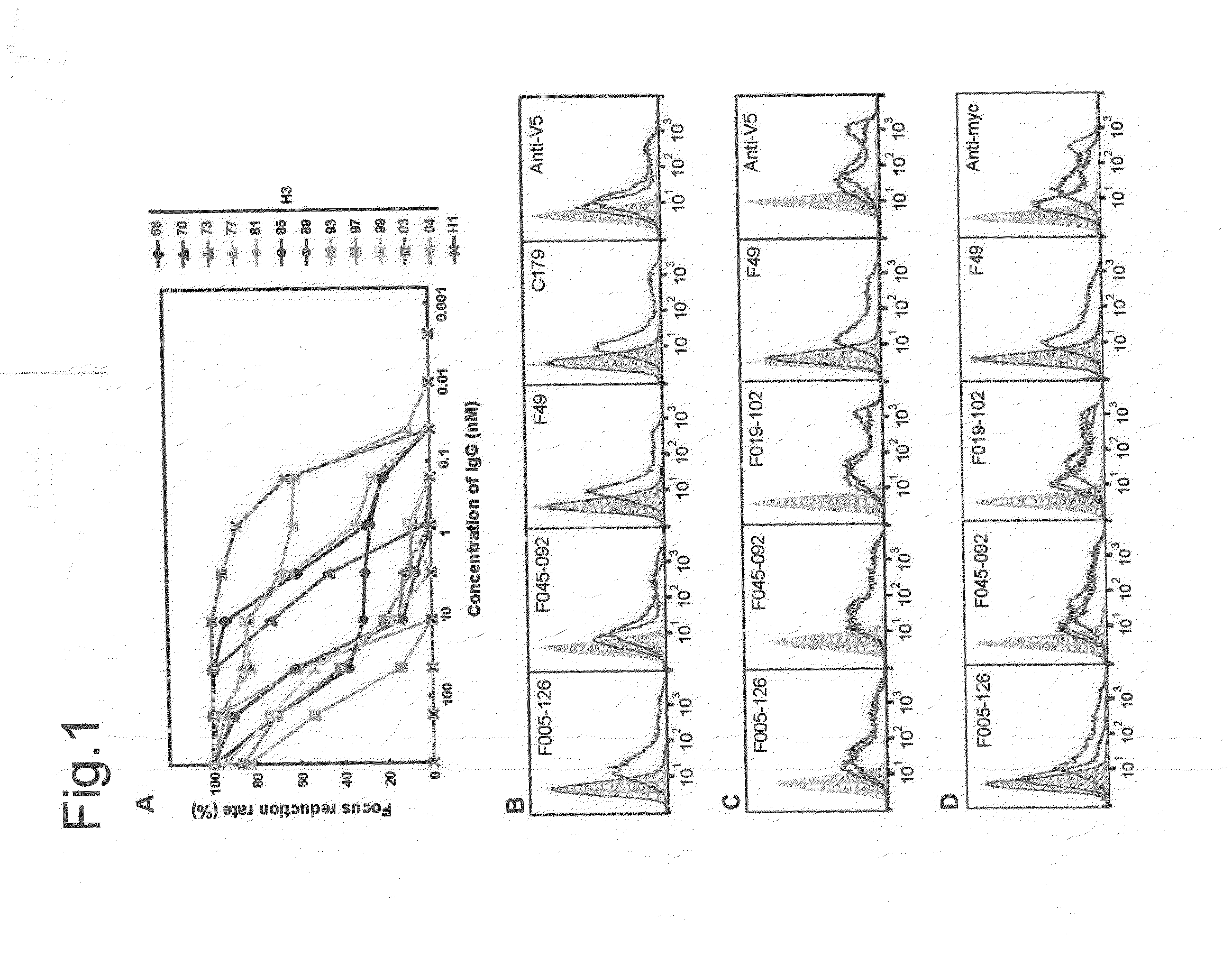
Antibodies (Abs) play roles in protection against influenza. Neutralizing Abs either inhibit the binding of hemagglutinin (HA) to cellular receptors or prevent the conformational change of HA induced by low pH. The former Ab binds to the regions near the sialic acid-binding pocket on the globular head formed by HA1 and generally shows narrow strain specificity. The latter Ab binds to the stem region formed mainly by HA2 and shows broad strain specificity. We isolated a broadly neutralizing Ab against H3N2 viruses. X-ray analysis of the HA / Ab complex indicated that the Ab binds to the valley formed by two neighboring HA monomers at the side of the globular head. The Ab shows neutralizing activity by preventing the conformational change of HA induced at low pH.
Owner:FUJITA HEALTH UNIVERSITY
Method for predicting change of influenza virus antigen
ActiveCN109448781AImprove accuracyImprove robustnessEpidemiological alert systemsSystems biologyVirus influenzaBio informatics
The invention belongs to the field of bioinformatics, and discloses a method for predicting changes of influenza virus antigens. The method comprises the following steps of: firstly, coding an influenza virus sequence pair aiming at the analysis characteristics of the influenza virus and the influenza virus antigen change; secondly, automatically extracting main characteristics of antigenic changeon the influenza virus pair by using a depth neural network, the influenza virus pair is then predicted for antigen change based on the extracted characteristics.
Owner:YUNNAN UNIV
Influenza vaccines with reduced amount of emulsion adjuvant
InactiveUS20150174234A1Eliminate needSsRNA viruses negative-senseViral antigen ingredientsHemagglutininInfluenza vaccine
Influenza vaccines with oil-in-water emulsion adjuvants are known. The amount of emulsion adjuvant required for an influenza vaccine can be reduced, thereby allowing more vaccines to be made from a given amount of emulsion, and / or minimizing the amount of emulsion that has to be produced for a given number of vaccine doses. These vaccines can conveniently be made by mixing (i) an oil-in-water emulsion and (ii) an aqueous preparation of an influenza virus antigen. In one aspect, substantially equal volumes of components (i) and (ii) are used; in another aspect, an excess volume of component (ii) is used. When using substantially equal volumes, component (ii) has a hemagglutinin concentration of more than 60 μg per influenza virus strain per ml. Components (i) and (ii) can be presented in kit form.
Owner:SEQIRUS UK LTD
Recombinant modified vaccinia virus ankara influenza vaccine
ActiveUS20130183335A1Efficient inductionSafety has been establishedSsRNA viruses negative-senseAnimal cellsEpitopeVaccinia
The invention concerns a recombinant modified vaccinia virus Ankara (MVA virus) expressing at least two external influenza virus antigens and / or an epitope of one or more of the at least two antigens and at least two internal influenza virus antigens and / or an epitope of the at least two antigens. The invention, thus, concerns a recombinant MVA virus encoding multiple external and / or internal influenza virus antigens, preferably from multiple influenza virus strains. The invention further concerns the use of said recombinant MVA in preparing a medicament and vaccine for influenza virus. Further encompassed by the present invention are methods, composition and kits.
Owner:BAVARIAN NORDIC AS
Recombinant Rhinovirus Vectors
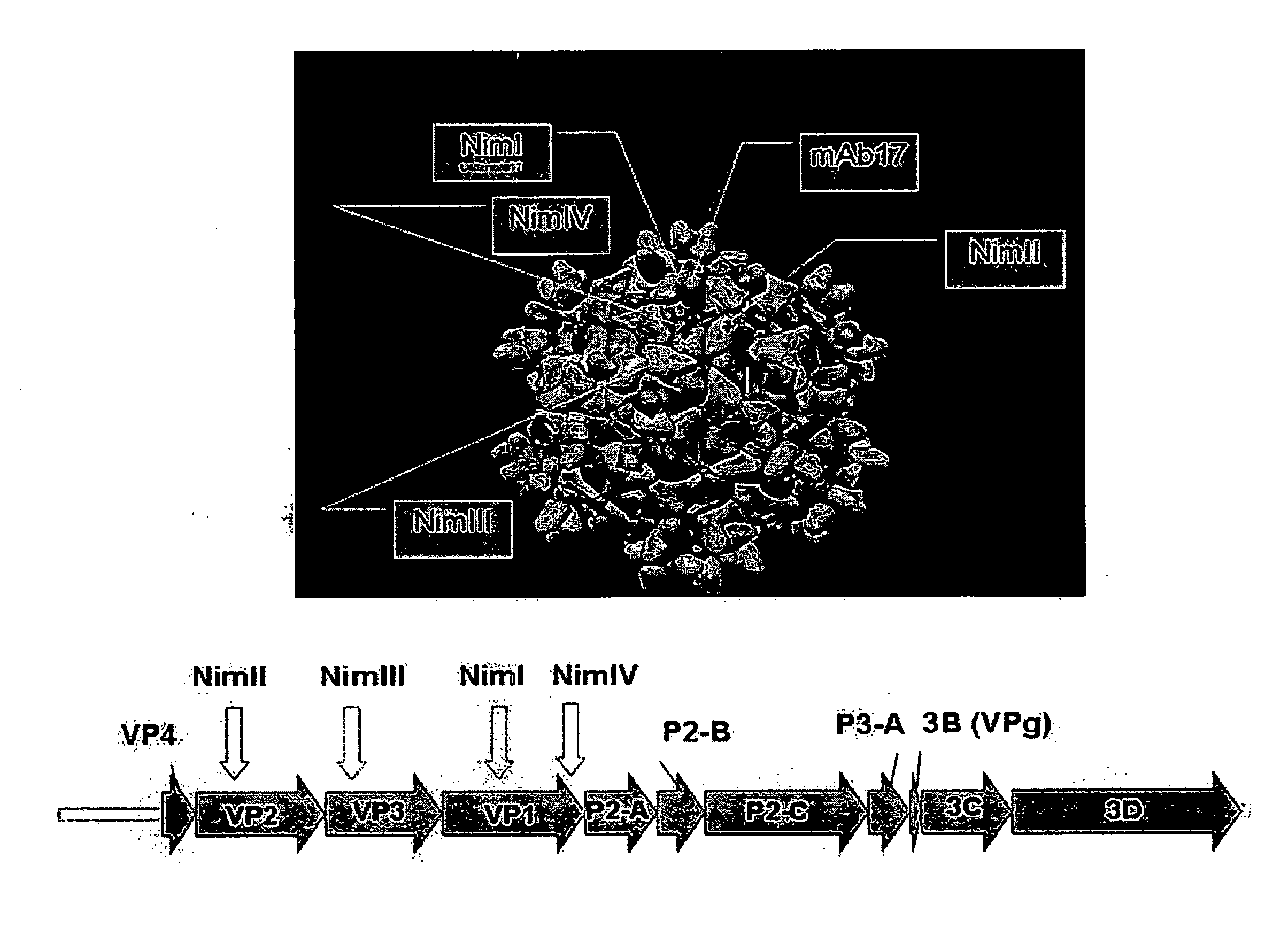
InactiveUS20100239605A1Improve good performanceLow costSsRNA viruses negative-senseBiocideViral vectorInfluenza virus Antigen
The invention provides recombinant rhinovirus vectors including, for example, influenza virus antigens. Also provided by the invention are corresponding pharmaceutical compositions and methods.
Owner:SANOFI PASTEUR BIOLOGICS CO
Vaccine for inducing an improved immune reaction
ActiveUS20130273101A1Efficient preparationGood effectAntibacterial agentsSsRNA viruses negative-senseHepatitis B virusNeisseria meningitidis
The present invention relates to a pharmaceutical vaccine composition comprising: (a) a pathogen-derived antigen selected from the group consisting of Mycobacterium tuberculosis antigen, Bacillus anthracis antigen, HAV (hepatitis A virus) antigen, HBV (hepatitis B virus) antigen, HCV (hepatitis C virus) antigen, HIV (human immunodeficiency virus) antigen, influenza virus antigen, HSV (herpes simplex virus) antigen, Hib (Haemophilus influenzae type b) antigen, Neisseria meningitidis antigen, Corynebacterium diphtheriae antigen, Bordetella pertussis antigen, Clostridium tetani antigen and Varicella virus antigen; (b) a deacylated non-toxic LOS (lipooligosaccharide); and (c) a pharmaceutically acceptable carrier.
Owner:EYEGENE INC
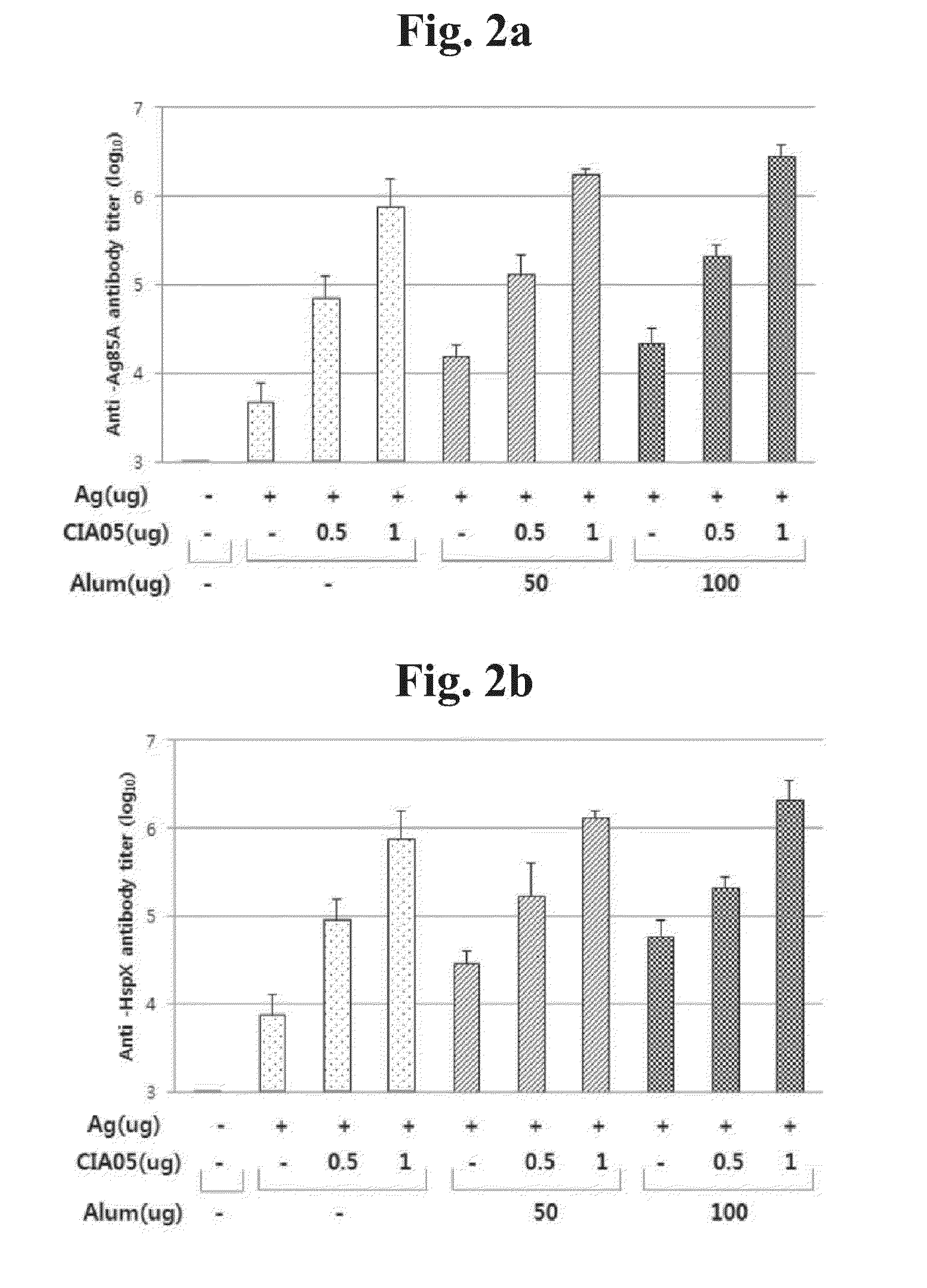
Liposome influenza virus antigen vaccine and preparing method thereof
InactiveCN104983682AReduce usageStrong immune responseAntiviralsAntibody medical ingredientsLinear epitopeLiposome
The invention relates to a liposome influenza virus antigen vaccine and a preparing method thereof. The liposome influenza virus antigen vaccine is prepared with a film evaporation method. The preparing method includes the steps of preparing an influenza virus antigen vaccine stock solution, preparing empty liposome, synthesizing liposome and the like. Compared with a linear epitope vaccine, the liposome influenza virus antigen vaccine prepared with the preparing method of the liposome influenza virus antigen vaccine has the advantages that multiple or multiple kinds of antigens can be carried at the same time, various cellular immune responses can be induced, and high immune responses can be caused; in addition, the liposome influenza virus antigen vaccine is stable in performance and long in storage life, and meanwhile the use immunogen dosage can be reduced.
Owner:SHEN ZHEN ISTEM REGENERATIVE MEDICINE SCI TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com