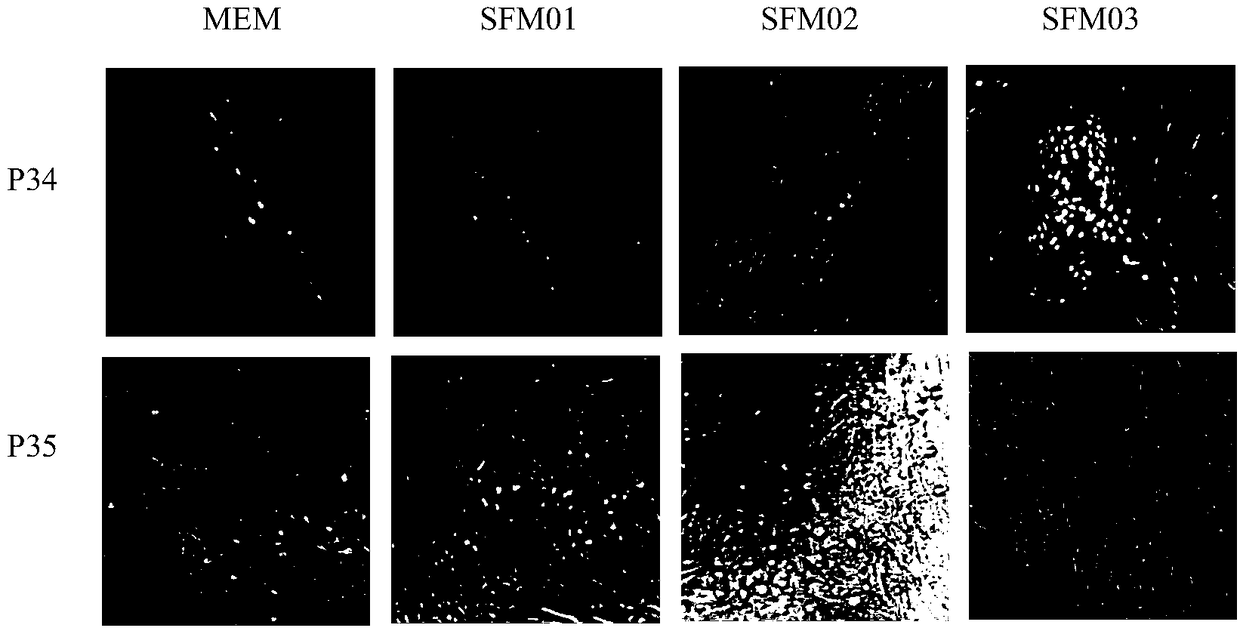
Patents
Literature
121 results about "Chickenpox" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A viral infection characterized by red blisters all over the body.
Composition for killing virus and preventing influenza and cold prevention plaster prepared by composition
InactiveCN102349991AIncrease concentrationUniform slow releaseAntibacterial agentsBiocideDiseaseRespiratory tract disease
The invention relates to a composition for killing virus and preventing influenza and a cold prevention plaster prepared by the composition. An aromatic traditional Chinese medicine volatile extract product and fragrant plant essential oil are used as effective components of the plaster; high-molecular colloid, which can be compatible with the effective components and has a stereo network structure, and an excipient are used to wrap the effective components to prepare a microcapsule; the microcapsule is used to coat on a matrix to prepare a tablet; a double faced adhesive tape is used to plaster the tablet at the position close to mouth and nose (such as collar, shoulder, chest and the like) so as to allow the effective components to slowly release at uniform speed. The plaster can be used to kill pathogen and virus in the air and enhance the immunity of upper respiratory tract, has an obvious effect of preventing influenza, and has a certain prevention effect for common cold, hand-foot-mouth disease, varicella, measles, epidemic encephalitis B, epidemic mumps, chronic respiratory tract diseases and the like. The plaster is convenient to use, has fragrant smell and is safe without side-effect.
Owner:王红芳
Pharmaceutical composition for the treatment of itch
Disclosed is a topical preparation for the treatment of topical itch in humans and animals. The said composition consists of Opuntia, Propolis, Stearic Acid, Beeswax, Vegetable Oil and β-sitosterol. Itch includes scratch reaction itch, anal itch, or irritant itch due to plants (e.g., poison ivy), insect bite, sunburn, chemical itch, eczema, pruritis dermatitis, diabetic skin itch, aging skin itch, foot-itch, chickenpox, jock itch, hives, itch of healing burns and wounds, dry winter skin itch, and stress-related scalp itch, etc
Owner:PBN PHARMA
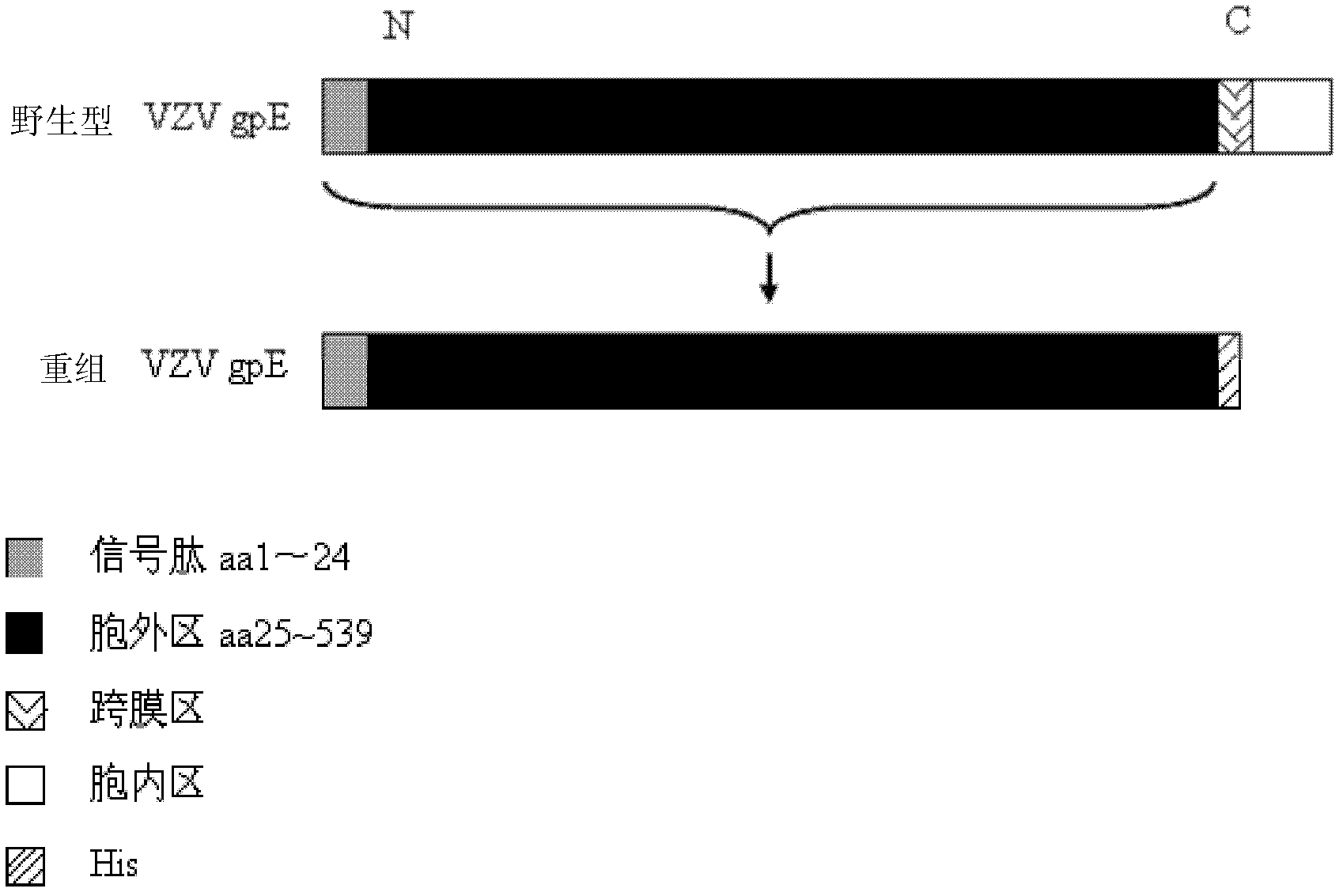
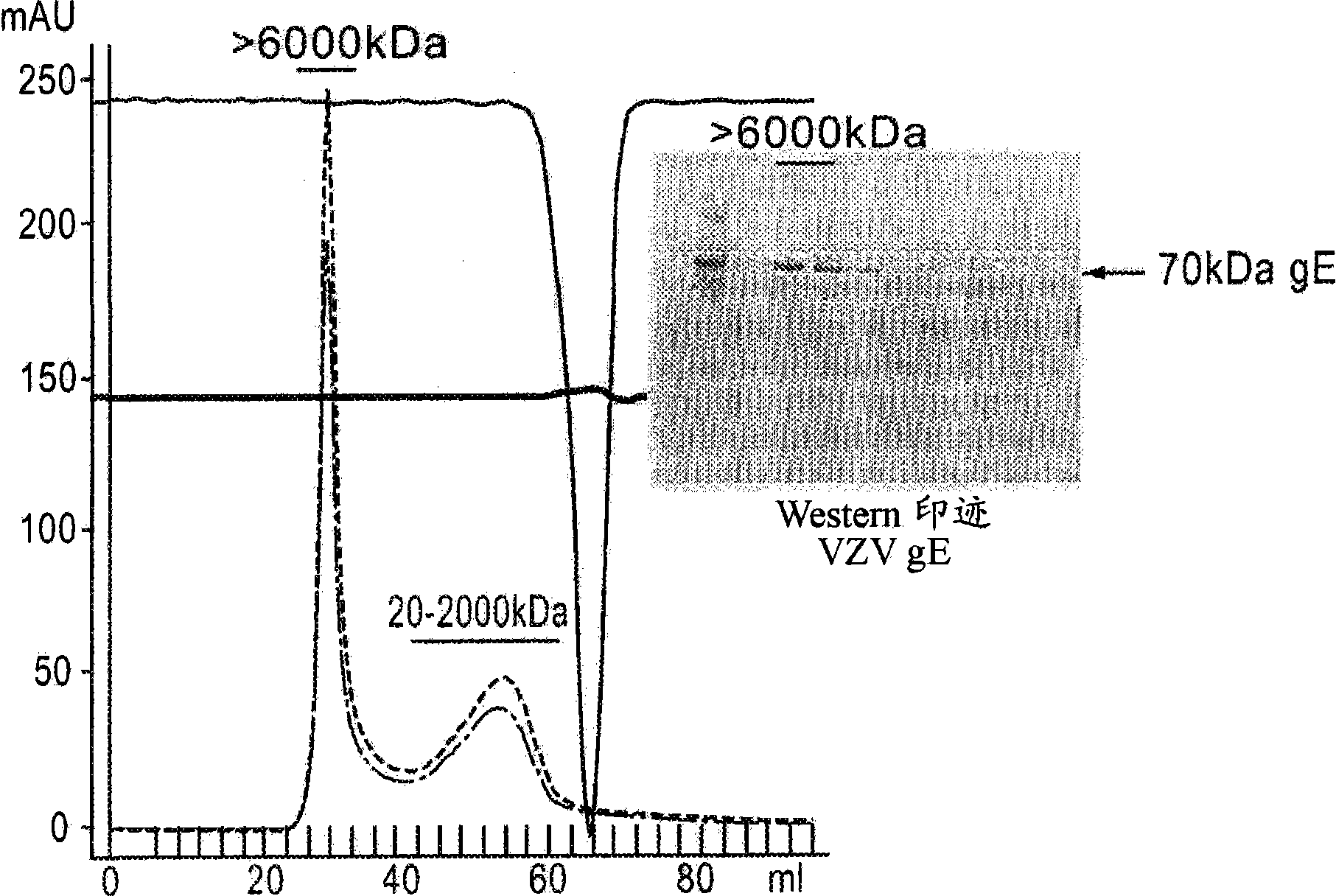
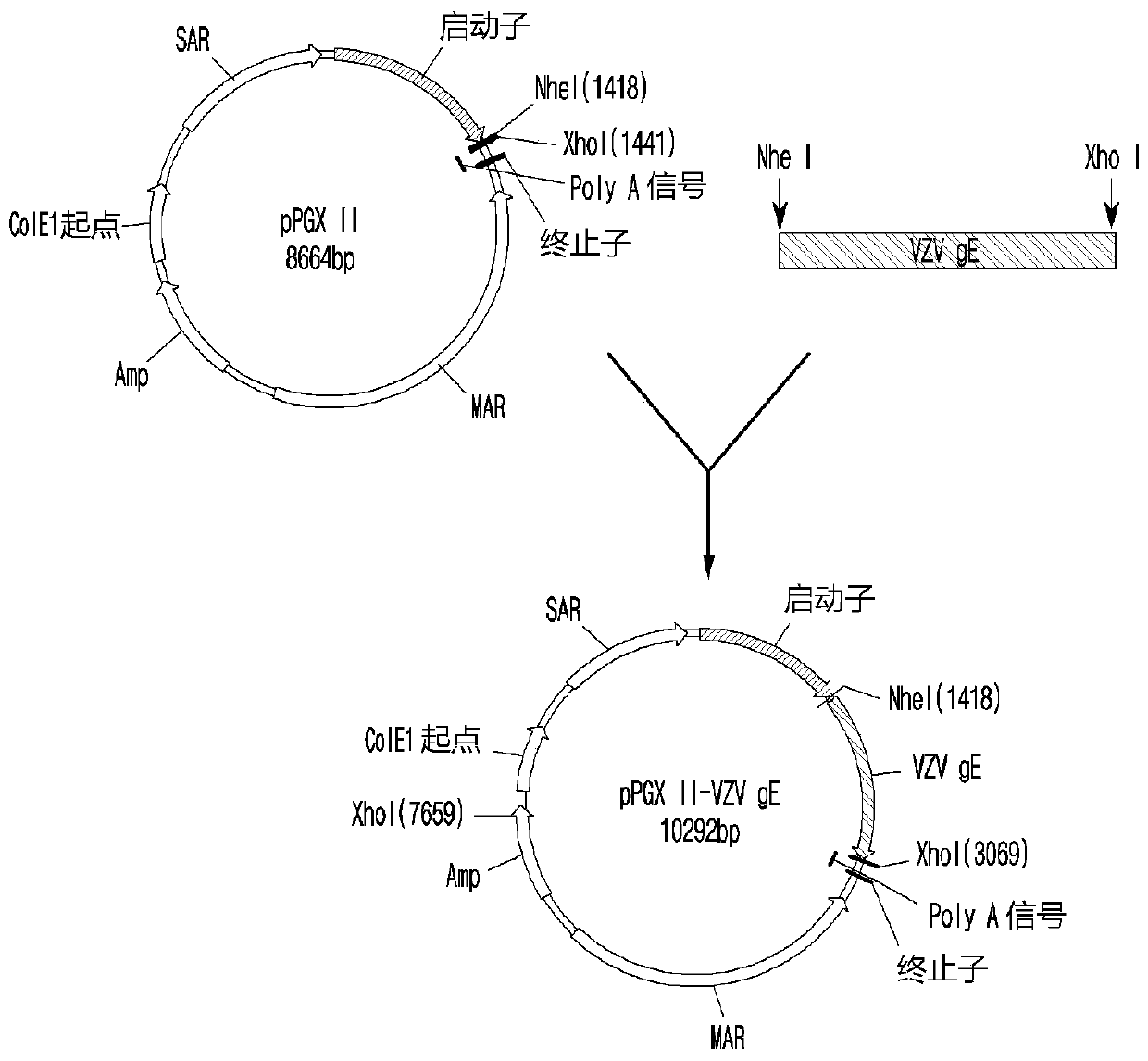
Method for recombinant expression of varicella-zoster virus truncation type glycoprotein E and application thereof
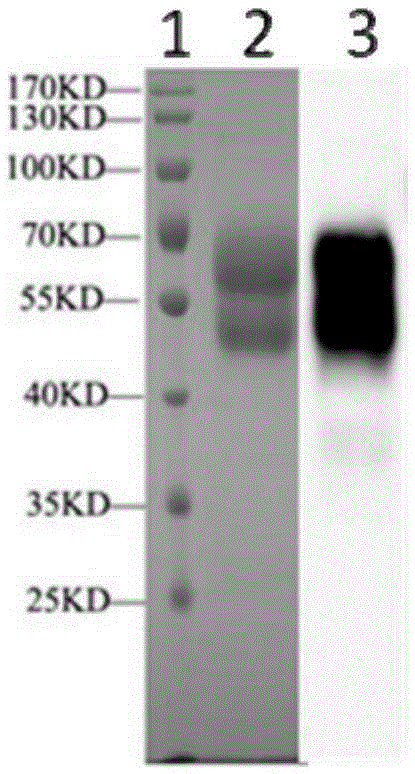
The invention discloses a method for the recombinant expression of a varicella-zoster virus truncation type glycoprotein E and application thereof. The method comprises the following steps: guiding a gene of a varicella-zoster virus (VZV) truncation type glycoprotein E (gpE) in which a transmembrane domain and an intracellular domain are removed and an His label is added into a host cell so as to obtain the recombinant varicella-zoster virus truncation type glycoprotein E by expression. The expression method is beneficial to enhancing the expression quantity of a target protein, the downstream purifying operation is simplified, and the large-scale production of the protein can be realized in an easier way; and moreover, the quality between batches is stable. The recombinant protein disclosed by the invention is used as a capturing antigen and can be used for the indirect ELISA (Enzyme-Linked Immuno Sorbent Assay) detection of specific immunoglobulin for resisting a varicella-zoster virus in a plasma specimen, the accuracy of the clinical diagnosis of VZV infection can be enhanced, and the recombinant protein is also used for other fields needing VZV specific immunoglobulin to carry out high-throughput detection.
Owner:FIELD OPERATION BLOOD TRANSFUSION INST OF PLA SCI ACAD OF MILITARY
Neutralizing epitope from varicella-zoster virus (VZV) gE protein and antibody aiming the same
ActiveCN105669838AAvoid infectionInhibition of neutralizing antibodiesVirus peptidesInactivation/attenuationDiseaseChickenpox
The invention relates to a neutralizing epitope peptide (or a variant thereof) from varicella-zoster virus virus (VZV) gE protein, a recombinant protein containing the neutralizing epitope peptide (or the variant thereof) and a carrier protein, and applications of the neutralizing epitope peptide (or a variant thereof) and the recombinant protein, and also relates to an antibody aiming to the neutralizing epitope peptide, a cell strain producing the antibody, and the applications thereof, and also relates to a vaccine containing the neutralizing epitope peptide and the recombinant protein, a medicine composition including the antibody, and the applications thereof, e.g., the application for preventing and / or treating VZV infection or one or more diseases and symptoms related to the infection.
Owner:XIAMEN UNIV +1
Synthetic peptide, inhibitor to DNA viruses
The present invention relates to the identification of the active domain of Herpoxin, a DNA virus-inhibiting-protein which was isolated from cobra venom in U.S. Pat. No. 5,648,339 and has a molecular weight of 13.5 kDa We have isolated a fragment of Herpoxin which contains the active domain and which we have named Herp. Herp mimics the activity of Herpoxin in inhibiting the replication of DNA viruses. A synthetic version of the active fragment was produced having the amino acid sequence Asn-Leu-Tyr-Gln-Phe-Lys-Asn-Met-Ile-Gln. The synthetic version of Herp consisting of ten amino acids inhibits the replication of DNA viruses such as herpes viruses types 1 and 2, cytomegalovirus and varicella zoster virus as well as Tubercle bacilli.
Owner:LIPPS BINIE V +1
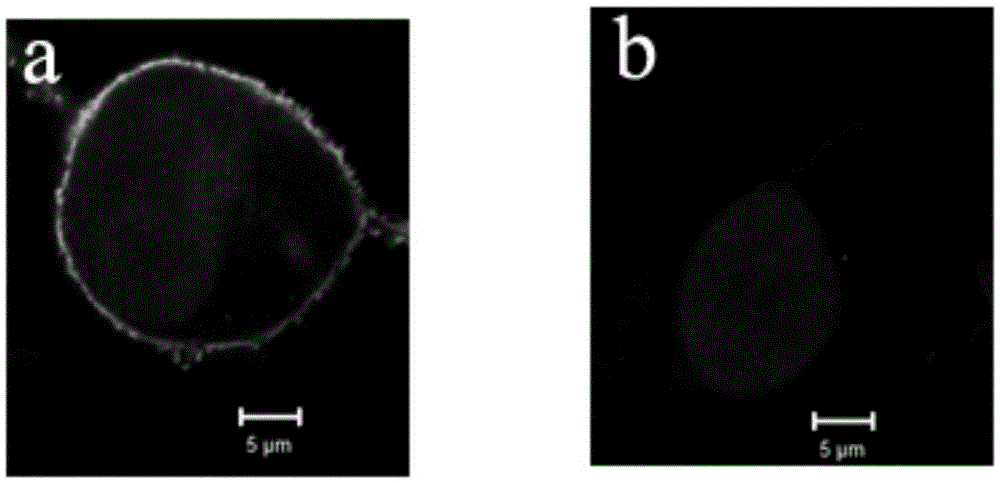
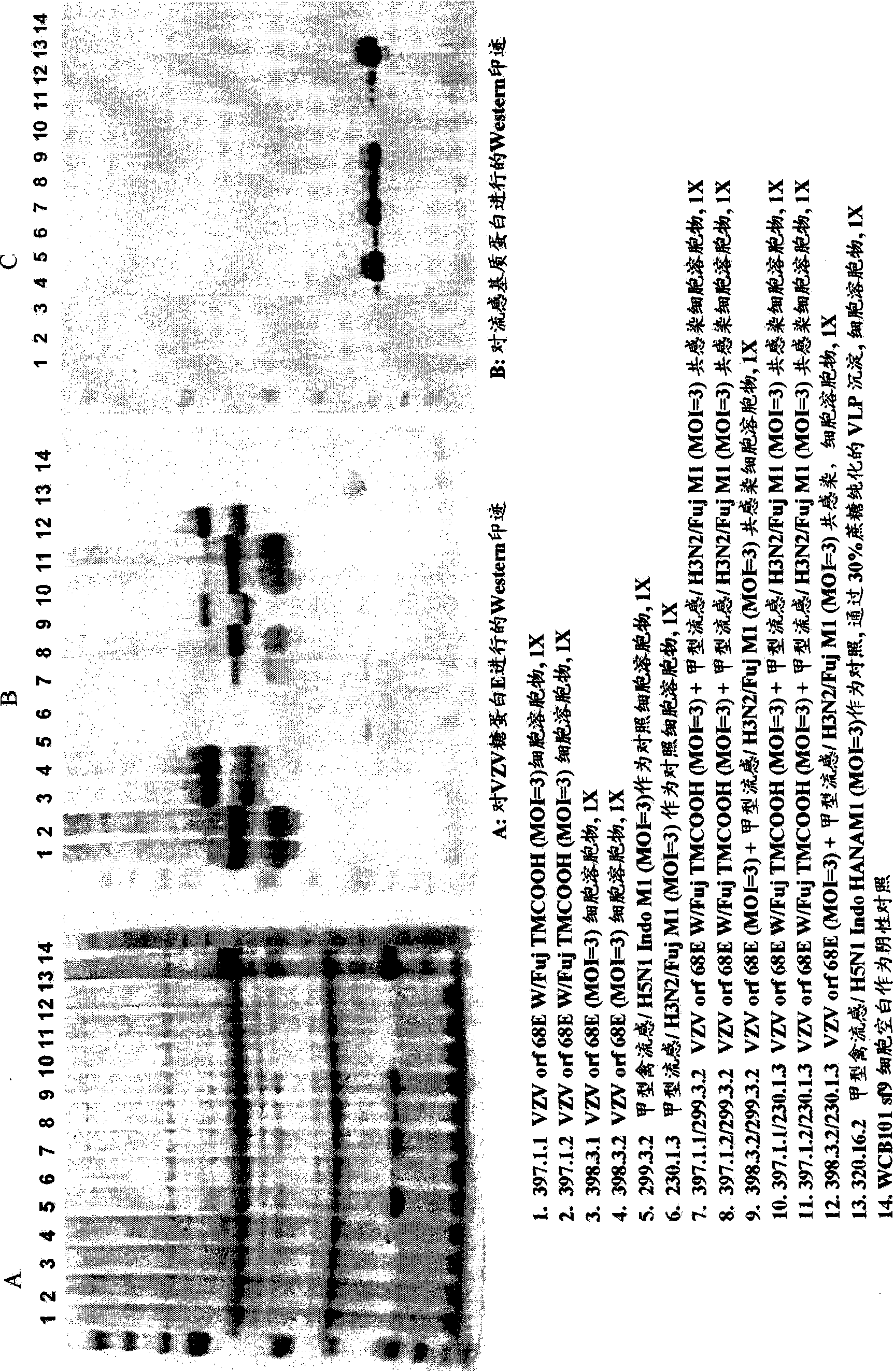
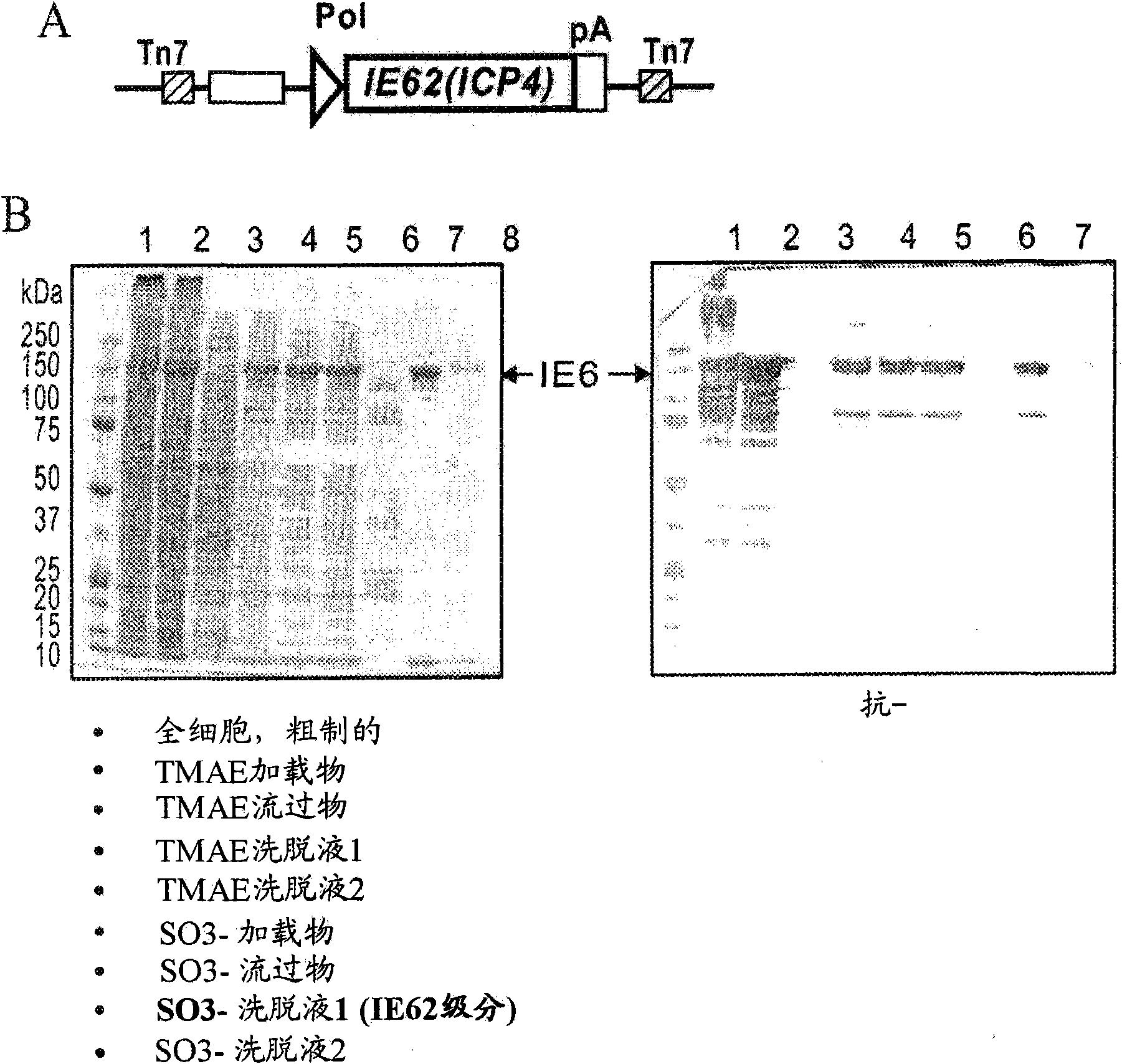
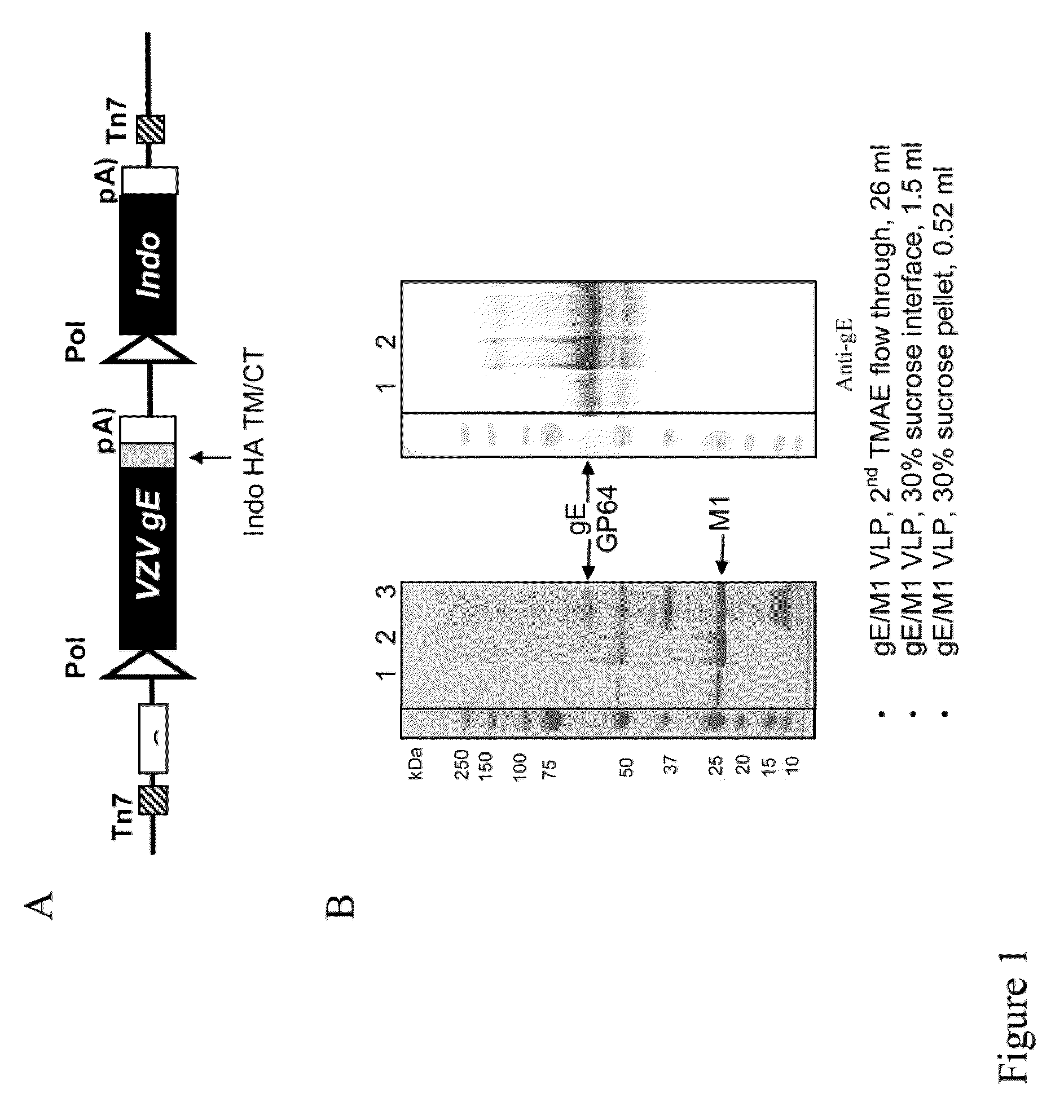
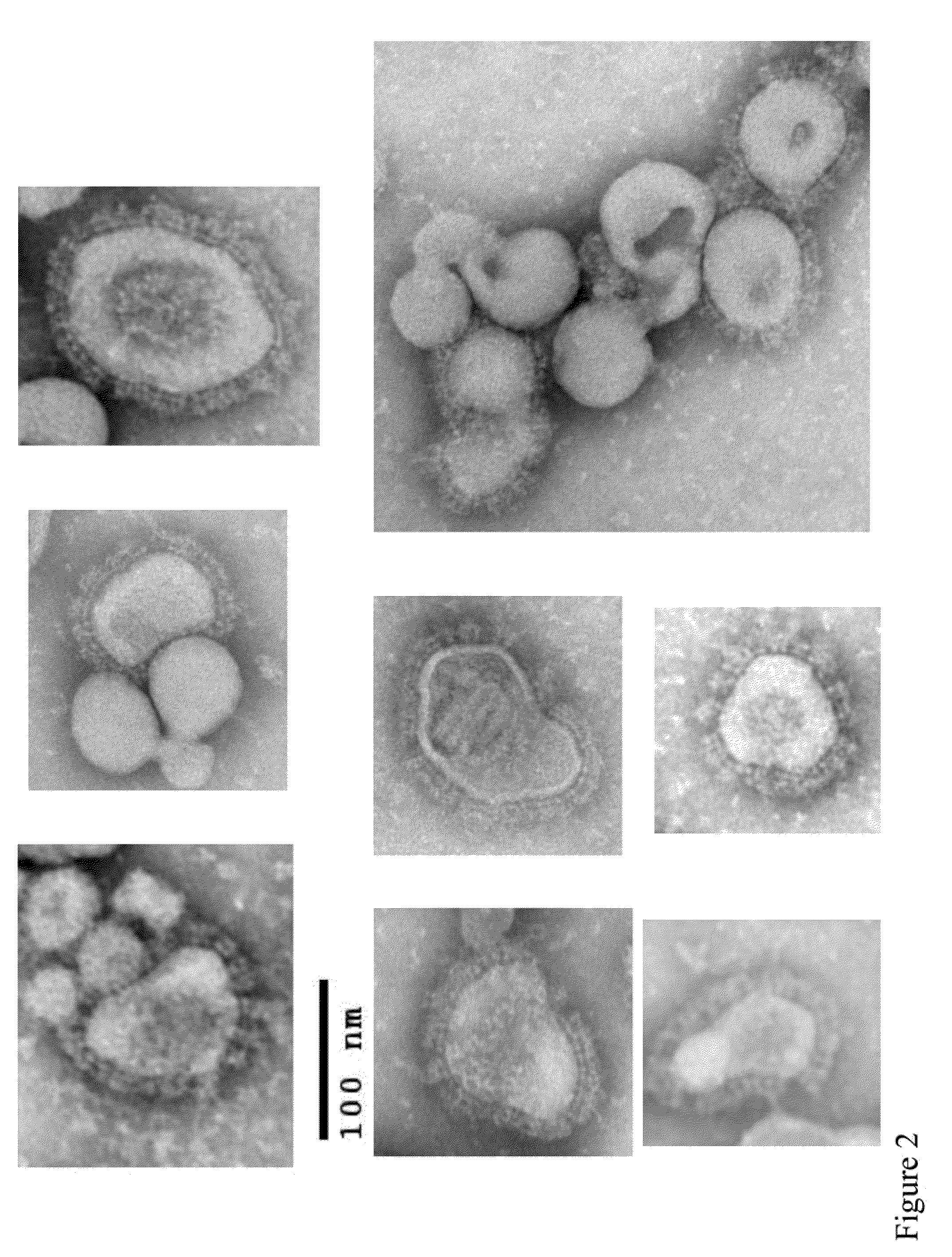
Varicella zoster virus-virus like particles (VLPS) and antigens
The present invention discloses novel Varicella Zoster Virus (VZV) virus-like particles (VLPs) comprising glycoprotein E of VZV. The invention also discloses vaccine formulations of the VZV-VLPs and methods of inducing an immune response in subjects.
Owner:NOVAVAX
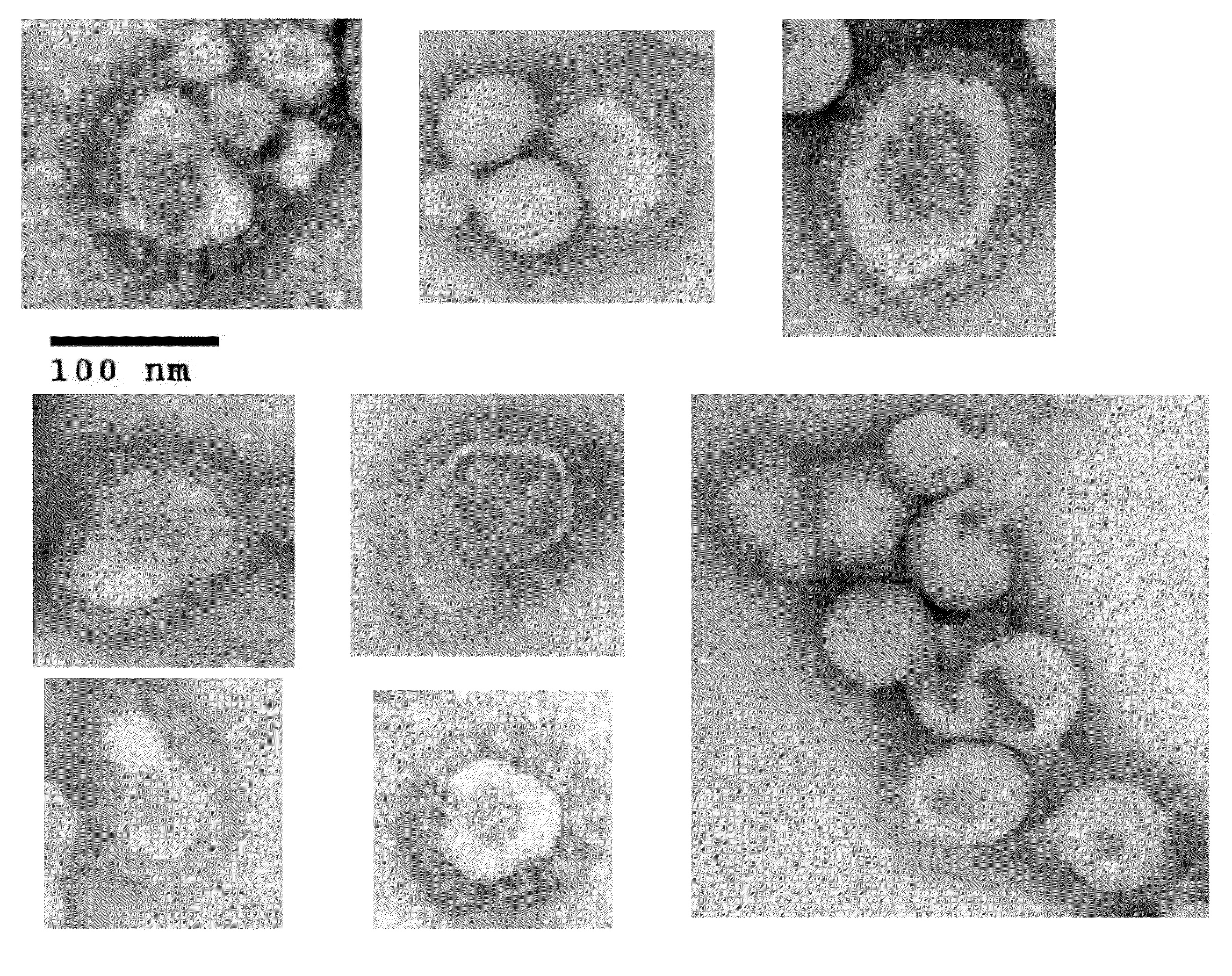
Chimeric varicella zoster virus virus-like particles
InactiveUS20110008838A1SsRNA viruses negative-senseSsRNA viruses positive-senseChickenpoxVirus-like particle
The present invention discloses novel chimeric Varicella Zoster Virus (VZV) virus-like particles (VLPs) comprising chimeric VZV glycoproteins. The invention also discloses vaccine formulations of the chimeric VZV-VLPs and methods of inducing an immune response in subjects.
Owner:NOVAVAX
Multiple real-time quantitative PCR primer, probe and detection method for identifying viral pathogens relevant to fever with eruption syndrome as infection diseases
ActiveCN102140543ADetection ExpressImprove efficiencyMicrobiological testing/measurementFluorescence/phosphorescenceChickenpoxHerpes zoster virus
The invention discloses multiple real-time quantitative PCR primer, probe and a detection method for identifying viral pathogens relevant to fevers with eruption syndromes as infection diseases, which is used for carrying out multiple real-time fluorescent quantitative PCR detection on varicella-herpes zoster viruses, human small DNA (Deoxyribonucleic Acid) viruses B19, enteroviruses (enteroviruses 71 type and coxsackie viruses A16 type), dengue viruses, rubella viruses and measles viruses. The invention can simultaneously carry out qualitative or quantitative detection on eight kinds of human viruses in various types of samples by multiple double-tubes PCR. The detection method has the advantages of simple operation, short time consumption, high sensitivity and strong specificity, is suitable for field detection, early diagnosis, epidemics detection and research and the like, and takes the actions of assistance and identification diagnosis on the fevers with eruption syndromes.
Owner:SUN YAT SEN UNIV
Recombinant varicella zoster virus vaccine
ActiveCN112870344AHigh molecular weightImproving immunogenicityPeptide/protein ingredientsAntibody mimetics/scaffoldsChickenpoxImmunogenicity
The invention discloses a recombinant varicella zoster virus vaccine, which comprises an amino acid sequence of a recombinant glycoprotein gE extracellular region of a live attenuated VZV strain (OKA strain) gene and a fusion protein formed by a human immunoglobulin Fc segment, and further comprises preparation and an application of the fusion protein, and a corresponding recombinant gene, an eukaryotic expression vector and the like. The fusion protein provided by the invention has good immunogenicity, and can induce generation of a high-level serum neutralizing antibody.
Owner:BEIJING LUZHU BIOTECH +1
Kit for detecting varicella-herpes zoster virus
ActiveCN101871013AGood reproducibilityMicrobiological testing/measurementMicroorganism based processesFluorescenceChickenpox
The invention relates to a kit for detecting varicella-herpes zoster virus, in particular to a kit for detecting varicella-herpes zoster virus in a clinical sample through the technology of fluorescence quantitative polymerase chain reaction. As the kit of the invention has higher sensitivity and specificity, the kit has significance in the confirmation of early infection and the emergency diagnosis and monitoring of virus outbreak by detecting and performing quantitative analysis to the polynucleotide of varicella-herpes zoster virus.
Owner:DAAN GENE CO LTD
Composition of starwort sulphonic acid or vitriolic acid polyoses ester total phenolic glycoside and method of preparing the same and antiviral application
The invention relates to a kind of natural medicine of broad spectrum antibiotic. At present, the broad spectrum antibiotic medicine with high effect and safety is at shortage all round the world. The invention is intended to extract laminarinsulphate or sulphonic acid sugar ester or sulphosalts from plant chickweed or other chickweed plant with two resin adsorption methods or a water extraction and alcohol precipition method. The spectrum antibiotic in the invention is distributed under 50,000 in the formula weight formed by carbon glycosidic bond and / or oxide glycosidic bond with phenol, especially the total flavones comprising apigenin. However, the invention mainly acts as total phenolic glycoside with the formula weight under 4,000. Besides, the invention can form brownish compound with the total flavones comprising apigenin and the glycosidic ingredients without sulfur element, so as to be applied as broad spectrum antibiotic drug. Therefore, the compound in the invention can be applied to cure ADIS virus, hepatitis virus, influenza virus and parainfluenza virus comprising SARS, adenovirus, verruca acuminate virus, enterovirus, mumps virus, herpes simplex virus, herpes zoster virus and varicella. No toxic effect has been found in the application. What is more, the invention can be made into 10 sorts of formulation, disinfector and health-improving products.
Owner:朱耕新
Protein chip for detecting blood and cerebro spinal fluid pathogen antibody, and its preparing method and use
InactiveCN1641355ASmall sample sizeSolve the detection speed is slowMaterial analysisAntigenChickenpox
The invention discloses a protein chip for detecting pathogen antibodies of blood and cerebrospinal fluid and its preparing method, and the protein chip includes substrate and peculiar-culture or shape protein or polypeptide antigen of array distributed pathogen and contrasted dot coating, where the substrate is a glass substrate; and the antigen and contrasted dot coating refers to 13 antigens with ten indexes including HSV-I, HSV-II, VZV, CMV, EBV, RV, JEV, MV, MT and LP, uniformly distributed on the glass substrate in a dot matrix form, and positive contrast, negative contrast and blank contrast. The protein chip of the invention can obtain multiple-index reacting result only by one reaction, judges infection of different pathogens and has the characters of quickness, high efficiency, accuracy and parallel diagnosis.
Owner:SHANDONG MEDICAL BIO TECH RES CENT
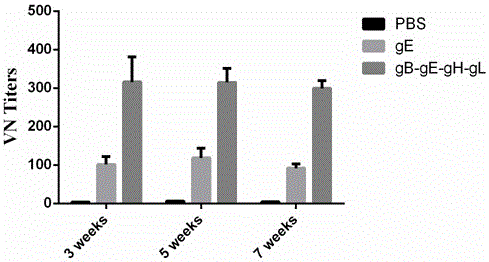
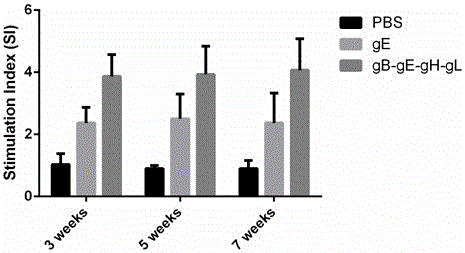
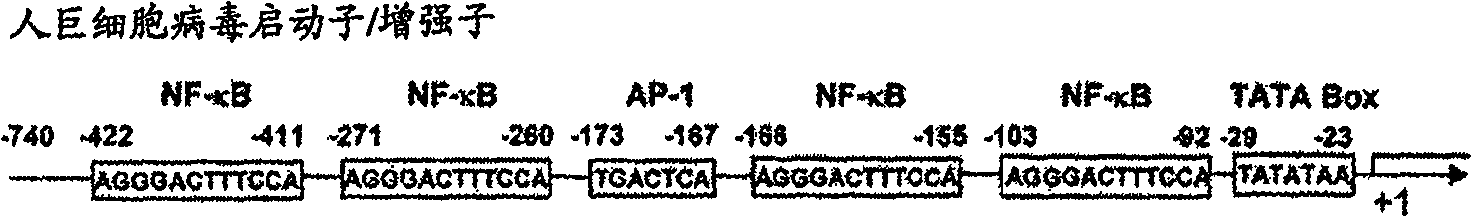
Varicella-zoster virus gB-gE-gH-gL fusion protein, genetic engineering subunit vaccine and preparation methods
ActiveCN105906721AImprove immunityStrong immune memoryAntibody mimetics/scaffoldsViral antigen ingredientsEscherichia coliAdjuvant
The invention relates to the technical field of biomedicine, and particularly provides varicella-zoster virus gB-gE-gH-gL fusion protein, a genetic engineering subunit vaccine and preparation methods. The fusion protein comprises a VZV gB extracellular region, a gE extracellular region, a gH truncated fragment and a gL truncated fragment. The amino acid sequence of encoded protein of the fusion protein is SEQ ID NO:1, and one amino acid sequence is SEQ ID NO:2. A prokaryotic expression vector is utilized for constructing escherichia coli BL21 (DE3) host bacteria capable of expressing the VZV gB-gE-gH-gL fusion protein. The fusion protein is purified and mixed with a medicinal adjuvant to be prepared into the genetic engineering subunit vaccine. Compared with a currently-used VZV attenuated live vaccine, the vaccine can induce immune mice to generate higher specific humoral immunity and cell-mediated immunity and can prevent dormant infection that VZV is spread to dorsal root ganglions and intestinal ganglions through blood flow, the safety of the VZV vaccine is effectively improved, and therefore the genetic engineering subunit vaccine is an alternative vaccine with potential clinical application value.
Owner:ANHUI MEDICAL UNIV
Recombinant multivalent vaccine
Owner:THE RES FOUND FOR MICROBIAL DISEASES OFOSAKA UNIV +1
Kit for genotyping VZV, production method of kit and application of kit
InactiveCN105132584ANo cross reactionStrong specificityMicrobiological testing/measurementMicroorganism based processesHuman DNA sequencingChemical structure
The invention provides a kit for genotyping VZV (Varicella-Zoster Viruses). The kit is characterized by comprising a nucleotide sequence shown as the Table 3 in the specification, and specific primers and specific probes corresponding to clade1-5 type VZV of chemical structures. The kit has the function of detecting various kinds of VZV DNA (Deoxyribonucleic Acid); the detection sensitivity is 10<2> copies / reaction; no cross reaction with human genome, herpes simplex viruses type I / type II, cytomegaloviruses and EB viruses exists; and the kit is applicable to the virus gene diagnosis of clinical VZV infected persons, and can also be used for the epidemiology survey of different Clade types of VZV.
Owner:CHENGDU MILITARY GENERAL HOSPITAL OF PLA
Method for producing novel freeze-dried live attenuated varicella vaccine
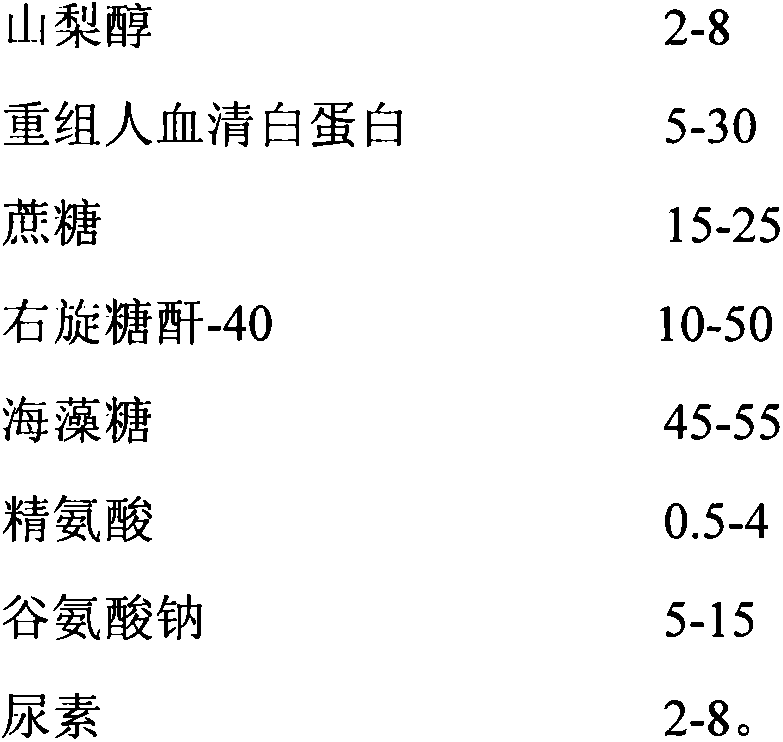
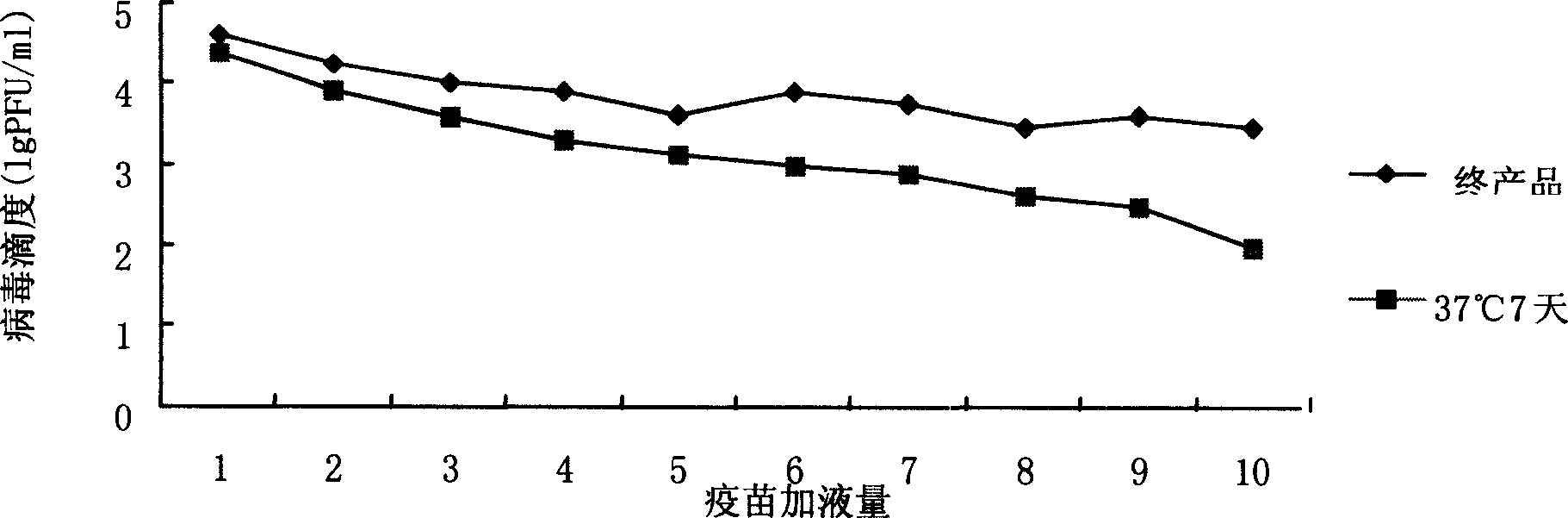
InactiveCN104188923ASolve outputSolve productivityPowder deliveryAntiviralsAdditive ingredientFreeze-drying
The invention discloses a method for producing a novel freeze-dried live attenuated varicella vaccine. The method is characterized in that a human diploid cell is used as a culture medium, a reactor culture process is applied to inoculate a varicella OKa strain, and an invented freeze-drying protective additive containing sorbitol and recombinant human serum albumin is used to produce the freeze-dried live attenuated varicella vaccine. By applying the reactor culture technology, the production cost is reduced, the defects of low yield, large production occupied area, high labor intensity and the like of a static culturing process can be overcome, the yield of vaccines can be increased, and the virus titer stability can be improved; furthermore, the protective additive containing sorbitol and recombinant human serum albumin is used and does not contain gelatin, so that animal derived ingredients can be removed, the content of toxin in the vaccine can be remarkably reduced, the stimulation and hazard of the vaccine on a human body can be greatly reduced, and the safety and stability of the vaccine can be improved.
Owner:BEIJING HEKANGYUAN BIOLOGICAL SCI & TECH
Methods and compositions for detecting CNS viruses
The present invention generally relates to a molecular test of enterovirus, herpes simplex virus-1 and -2, and / or Varicella-Zoster virus, in order to identify patients with a viral infection, in particular a viral infection of the central nervous system. Accordingly methods and compositions are disclosed to determine the presence or absence of a viral pathogen in a biological sample comprising, wherein the target nucleic acids comprise the 5′ UTR of the enterovirus genome, UL29 of herpes simplex virus and gene 36 of Varicella-Zoster virus.
Owner:QUEST DIAGNOSTICS INVESTMENTS INC
Varicella attenuation live vaccine
ActiveCN101161286AImprove securityHigh viral titerAntiviralsAntibody medical ingredientsInfected cellKanamycin
The present invention relates to herpes virus family medical microorganism. The present invention is prepared by the following method: (1) serial passage MRC-5 cell; (2) inoculating to cell and continuous infecting; (3) adding culture solution; (4) washing cell surface; (5) eluting infected cell, centrifugal separating, adding vaccine fluid containing stabilizing agent to refrigerate; (6) ultrasonic crashing and centrifugal separating to acquire virus stock solution; (7) combining and diluting, and at once processing to pack and freeze-dry, preparing attenuated pox vaccine. The stabilizing agent is prepared by dissolving NaCl, KCl, KH2PO4, Na2HPO4.12H2O, saccharose, sodium glutamate, kalamycin, erythromycin and deionized water into the water with weight ratio 3:0.1:0.2:3.3:50:0.4:0.1:0.03:1000. The present invention has good advantages of safety and immunogenicity.
Owner:SHANGHAI RONGSHENG BIOLOGICAL PHARM CO LTD
Production method of freeze dried chickenpox attenuated live vaccine and products therefrom
InactiveCN1593657AIncreased output per bottleStable titerPowder deliveryAntiviralsFreeze-dryingChickenpox
The invention provides the production method of freeze dried chickenpox attenuated live vaccine and products made thereby, wherein the method consists of using chickenpox attenuation Oka as strain, employing rotary bottle manufacturing process, and using diploid cell 2BS as culture medium mass. The invention can realize stabilized vaccine virus droplet degree and increased single bottle output compared with the static culture method.
Owner:CHANGCHUN KEYGEN BIOLOGICAL PROD
Vaccine adjuvant comprising lipopeptide-inserted liposome as effective ingredient and use thereof
PendingCN110996999ASsRNA viruses negative-senseSsRNA viruses positive-senseChickenpoxHerpes zoster virus
The present invention relates to a recombinant herpes zoster vaccine comprising liposome and lipopeptide and a method for preparing the same. More particularly, a vaccine composition according to thepresent invention, prepared using Lipo-Pam, which is a composite adjuvant comprising a liposome and various kinds of lipopeptides, and a varicella-zoster virus gE antigen, a Japanese encephalitis virus gE antigen, or a seasonal inactivated influenza virus antigen, highly induces a cell-mediated immune response as well as a humoral immune response so that the composition of the present invention can be commercially useful.
Owner:株式会社车疫苗研究所
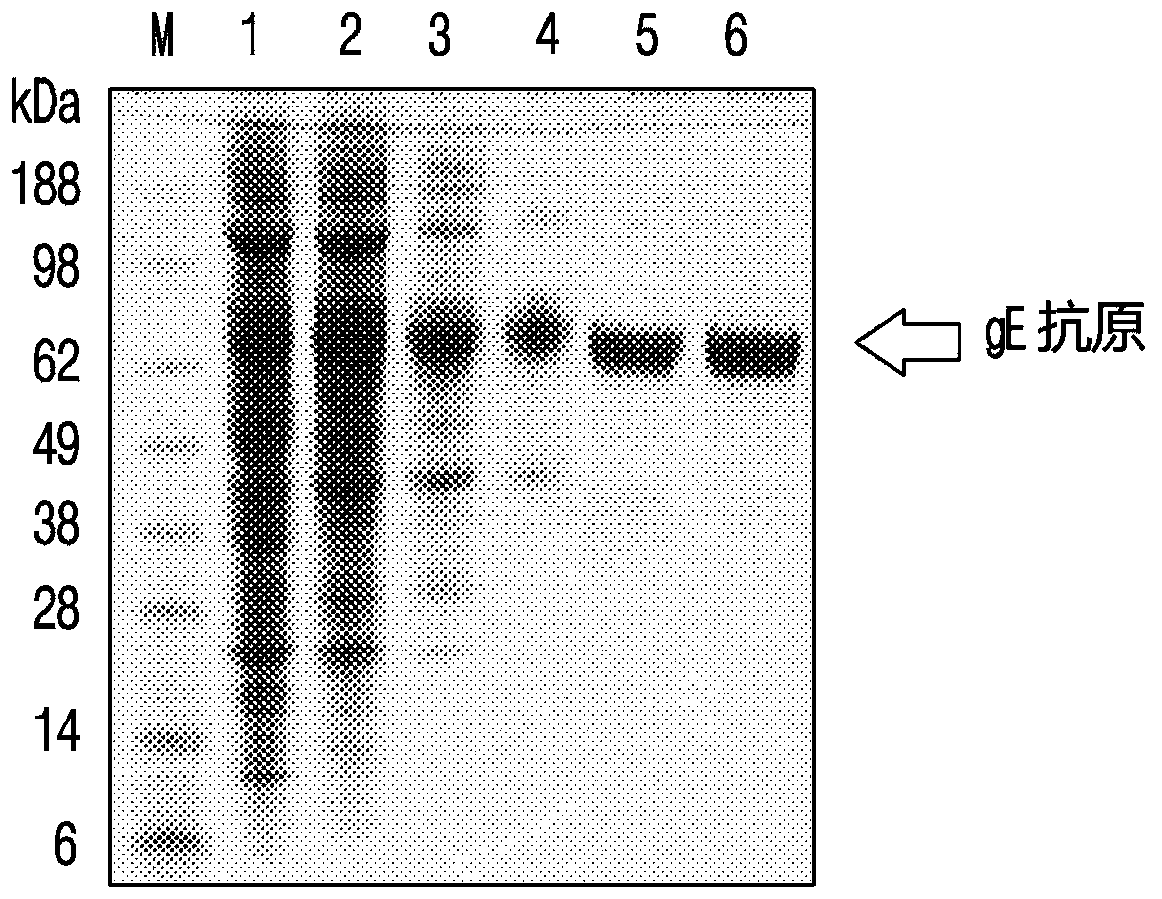
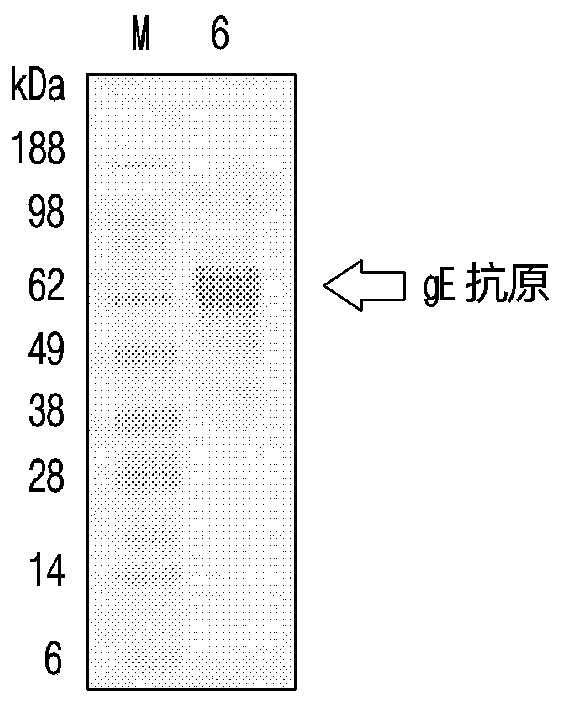
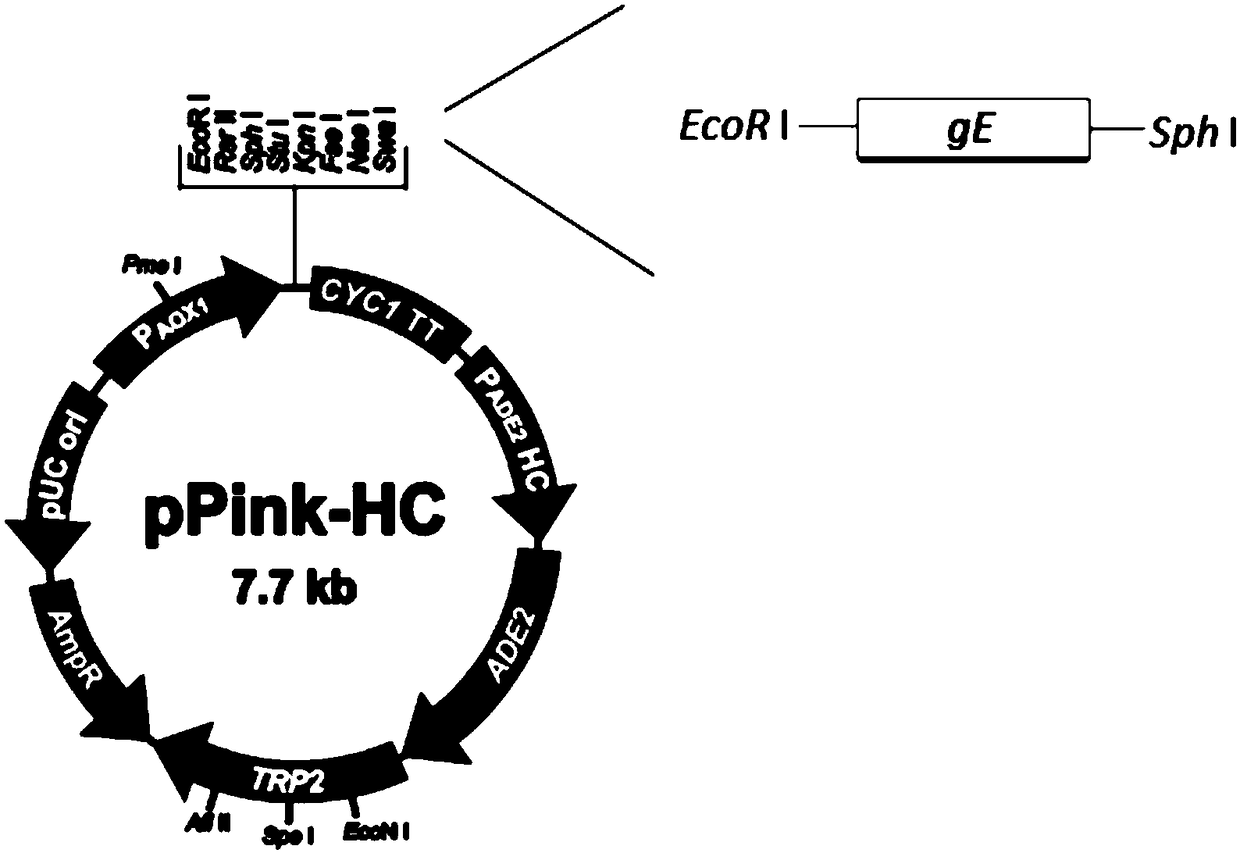
Expression method of VZV glycoprotein to pichia pastoris and application of expression method
PendingCN108383897AOptimize purification stepsHigh titerViral antigen ingredientsVirus peptidesPichia pastorisProtein target
The invention discloses an expression method of VZV glycoprotein to pichia pastoris and application of the expression method. The expression method comprises the following steps: constructing an expression vector, screening a positive transformant, linearizing a yeast expression vector, preparing a yeast electrotransformation competence, identifying a recombinant, expressing recombinant yeast andidentifying target protein. The expression method disclosed by the invention can be used for successfully expressing glycoprotein E of varicella-varicella-zoster virus and the obtained protein has good immunogenicity; the pichia pastoris can be efficiently and stably expressed.
Owner:BRAVOVAX
Compositions and methods
The invention relates to a viral vector comprising nucleic acid having a polynucleotide sequence encoding at least one epitope of the varicella-zoster virus (VZV)GlyE antigen, wherein the viral vector is an adenoviral vector. The invention also relates to uses, compositions for use in medical treatments, and methods of medical treatment.
Owner:OXFORD UNIV INNOVATION LTD
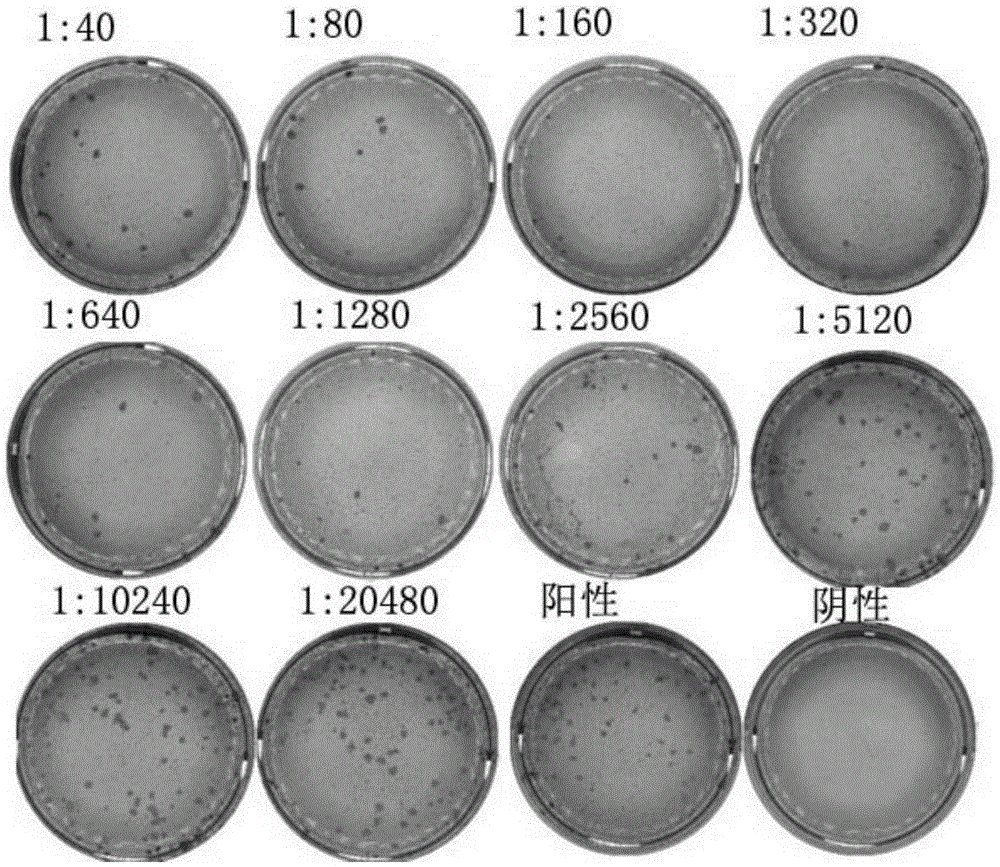
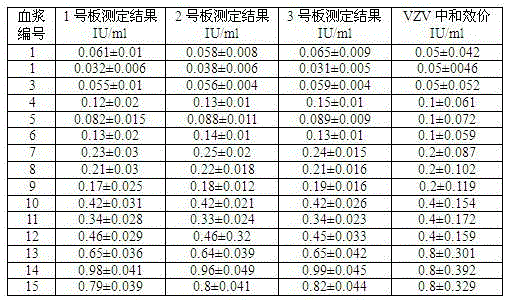
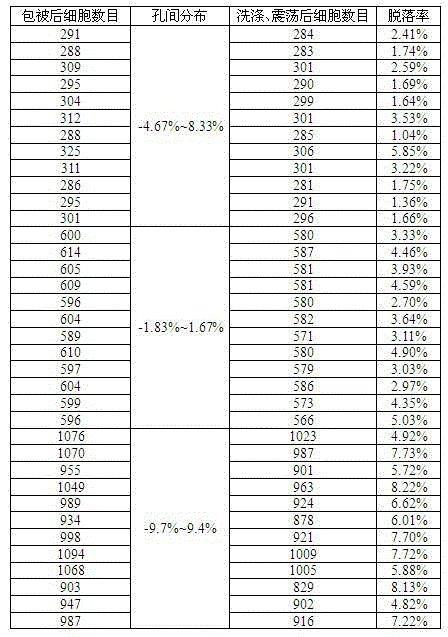
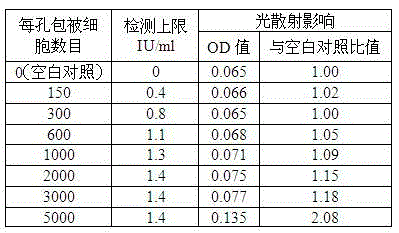
Kit capable of determining titer of neutralizing antibodies of varicella-zoster viruses and production method thereof
ActiveCN104360055AIntegrity guaranteedGuaranteed validityMaterial analysisViral glycoproteinChickenpox
The invention relates to the technical field of in-vitro diagnostic reagents and particularly relates to a kit capable of determining the titer of neutralizing antibodies of varicella-zoster viruses and a production method thereof. The kit adopts cells infected by VZV as antigens, is coated and fixed in holes of a 96-pore plate, the neutralizing antibodies in the sample to be determined are captured by using a large amount of virus glycoprotein (neutralizing glycoprotein) carried on the surfaces of the cells, simultaneously the integrity of the cells is maintained; and the other virus antigens inside are not contacted with the sample, so that the specificity of the neutralizing antibodies in determination can be guaranteed. The kit can be directly used for fast determination of the titer of the neutralizing antibodies in VZV on an automatic biochemical analyzer or an enzyme labeling instrument; the determination process is simple and fast, the flux of the determined sample is high and quantitative determination can be realized.
Owner:SHANDONG TAIBANG BIOLOGICAL PROD CO LTD
Pox-eliminating pills
InactiveCN1895587AGood effectLittle side effectsPill deliveryDermatological disorderMedicineChickenpox
A varicella pill for treating varicella is prepared from 29 Chinese-medicinal materials including black loean, red bean, honeysuckle flower, coix seed, etc through baking and treating with purified water.
Owner:王军
Varicella zoster virus vaccine
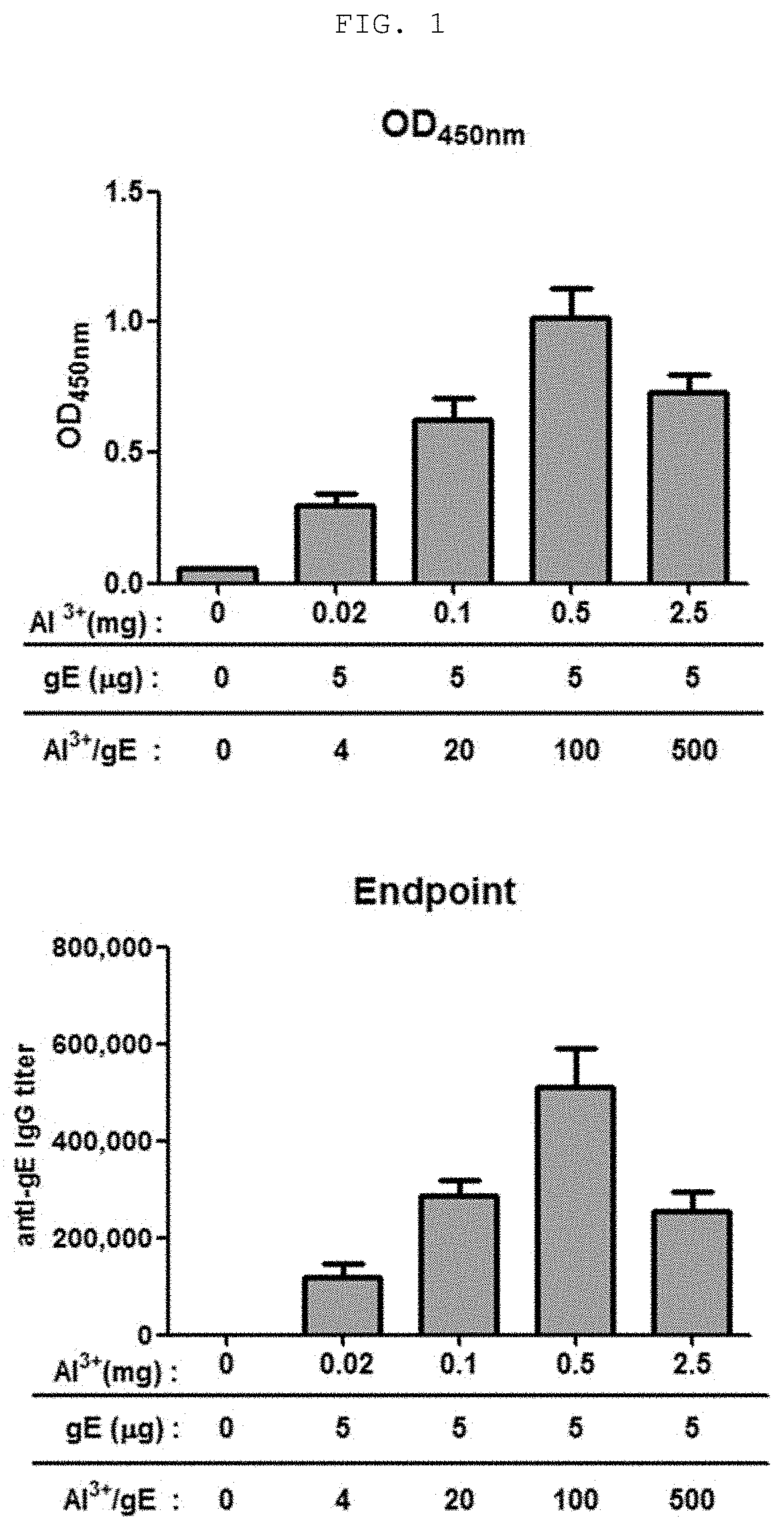
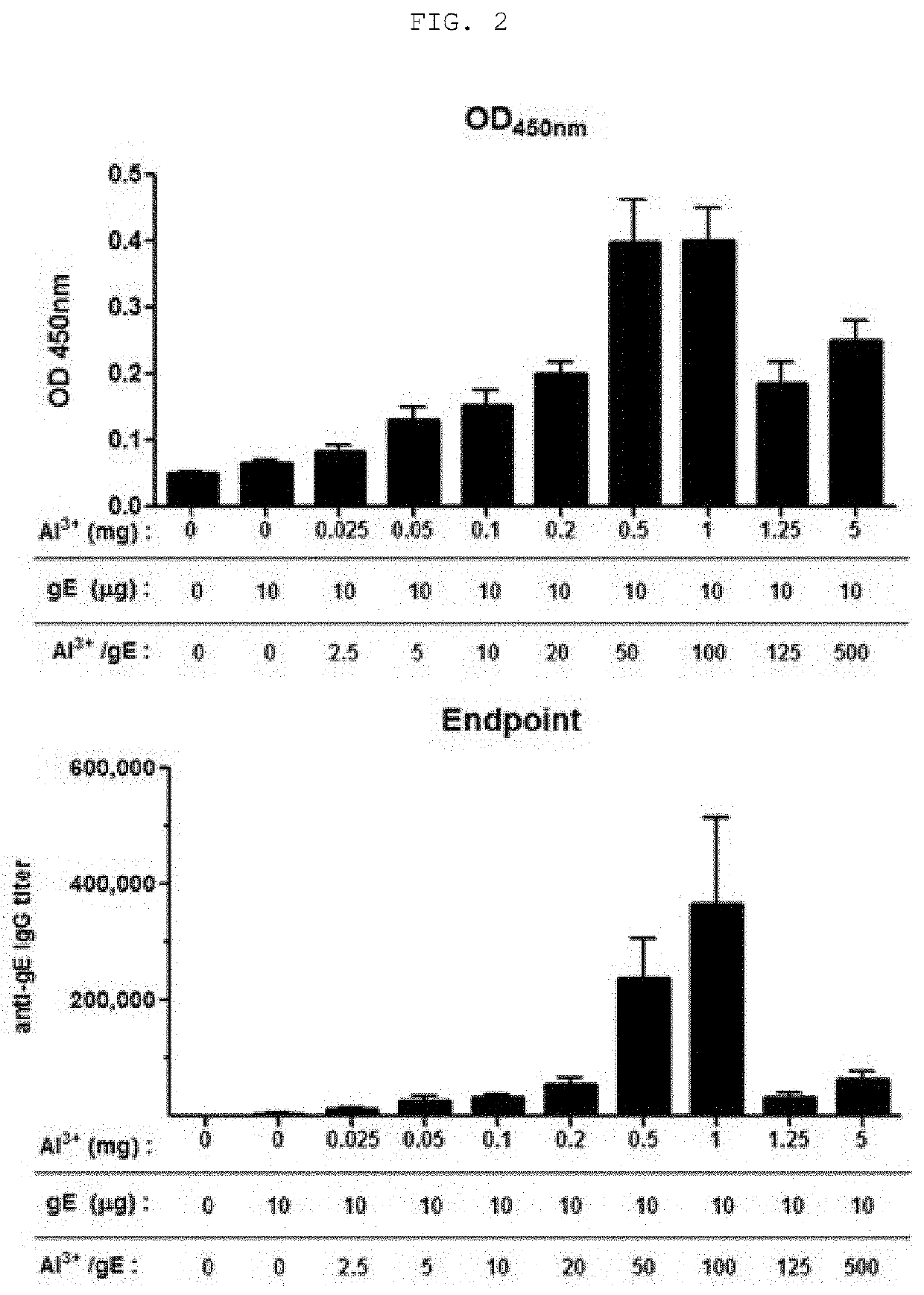
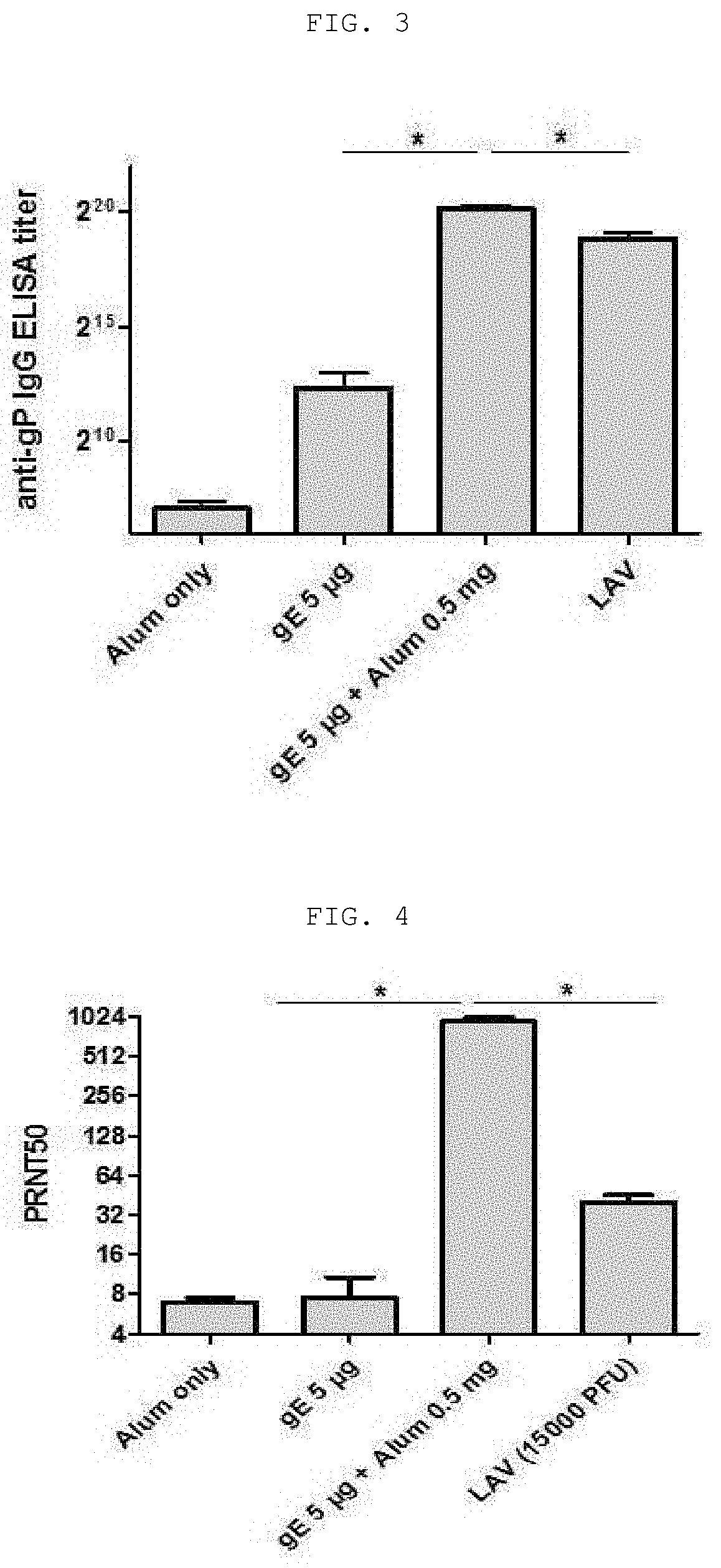
The present invention relates to a vaccine composition for prevention or treatment of chicken pox or herpes zoster, the vaccine composition comprising a surface protein (gE) of Varicella Zoster Virus and especially an aluminum salt as an adjuvant. The vaccine composition according to the present invention employs a protein antigen, thus showing greater outstanding stability than a live vaccine and has an optimized mixture ratio of adjuvants to elicit effective antibody induction, thereby being useful as a vaccine for preventing or treating Varicella Zoster Virus-caused chicken pox or herpes zoster.
Owner:MOGAM INST FOR BIOMEDICAL RES
Method for preparing varicella virus stock and application thereof
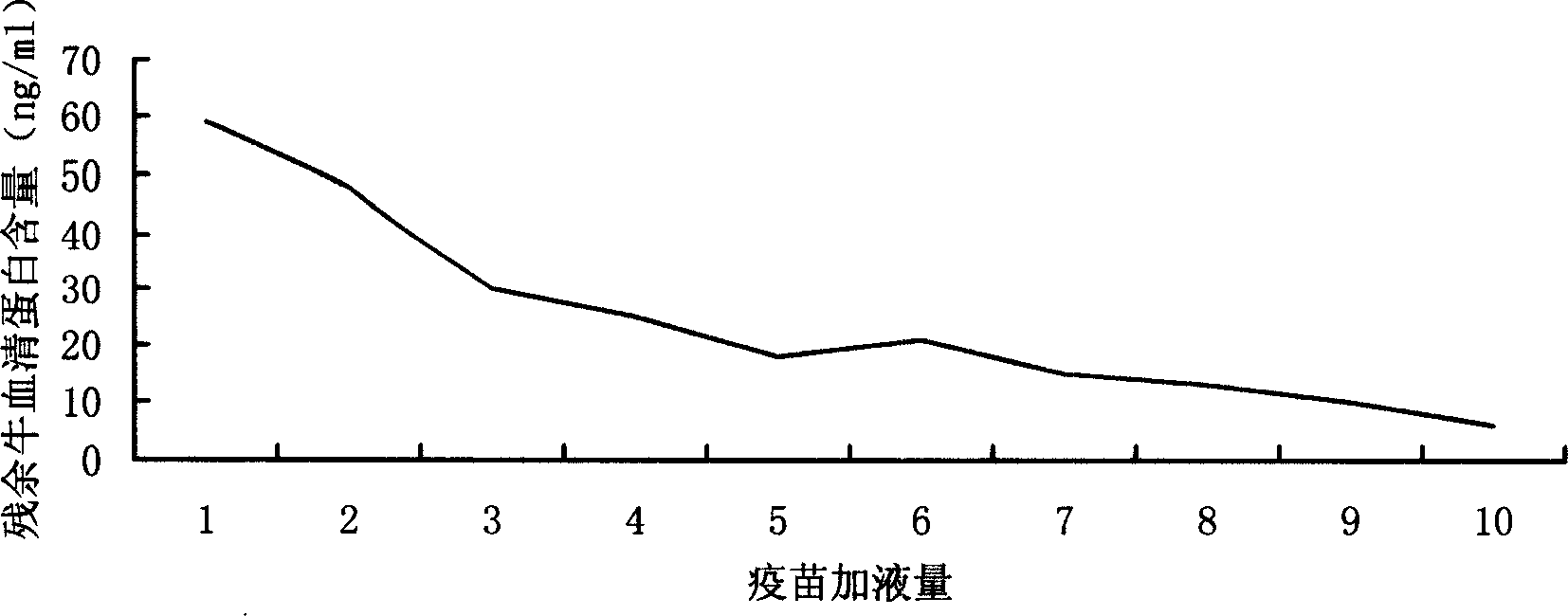
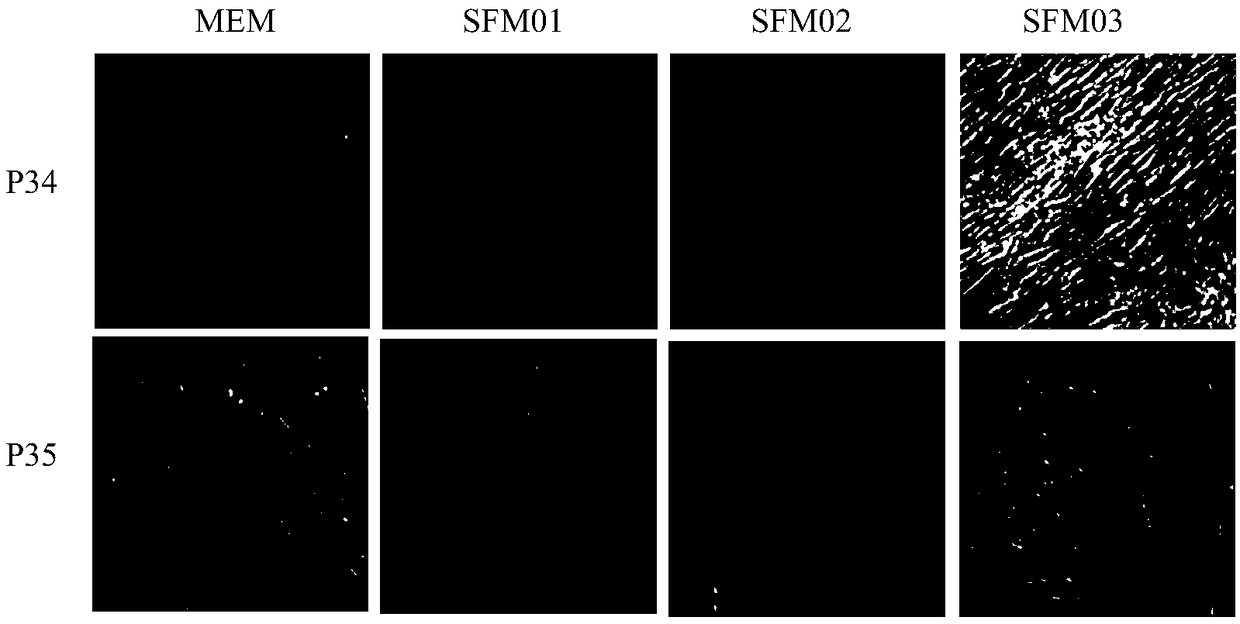
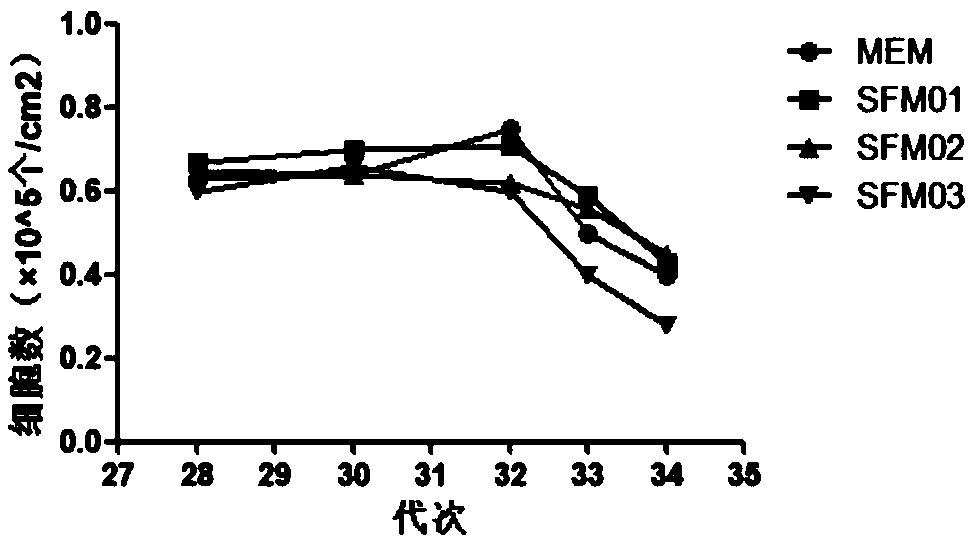
ActiveCN108753736AResidue reductionApplicable productionViral antigen ingredientsAntiviralsSerum free mediaCulture cell
The invention discloses a method for preparing varicella virus stock. The method is characterized by comprising the following steps: firstly adding 5v / v percent bovine serum into a serum-free medium at the cell culture stage of MRC-5 cell, then inoculating VZV-Oka strain, and culturing virus through the serum-free medium, so as to obtain the varicella virus stock. The method uses a suitable reduced-serum medium to culture cell, cultures VZV virus in a serum-free condition, applies the virus to the preparation of varicella attenuated live vaccine, and can greatly reduce the quantity of bovine serum albumin residue in the vaccine by 60 percent or more.
Owner:SHANGHAI RONGSHENG BIOLOGICAL PHARM CO LTD
Combined vaccine, and preparation method and application thereof
ActiveCN110743007AReduce the number of vaccinationsSsRNA viruses positive-senseViral antigen ingredientsChickenpoxEncephalitis Viruses
The invention relates to a combined vaccine, and a preparation method and application thereof. The combined vaccine comprises an inactivated japanese encephalitis virus and an inactivated varicella zoster virus; the ratio of the total protein content of the inactivated japanese encephalitis virus in the combined vaccine to the total protein content of the inactivated varicella zoster virus in thecombined vaccine is (0.25-4):1; the preparation method of the combined vaccine comprises the steps that an inactivated japanese encephalitis virus purification solution and an inactivated varicella zoster virus purification solution are mixed to obtain a mixed virus purification solution; and the inactivated japanese encephalitis virus and the inactivated varicella zoster virus are prepared by adopting the same human diploid cells as a virus culture medium to realize mixture of the inactivated japanese encephalitis virus and the inactivated varicella zoster virus to obtain the combined vaccine. The prepared combined vaccine is safe and effective, and the number of vaccinations is effectively reduced.
Owner:ANHUI ZHIFEI LONGCOM BIOPHARM CO LTD +2
Varicella-herpes zoster mRNA vaccine composition, and preparation method and application thereof
PendingCN114081943AReduce manufacturing costSave uniformityNervous disorderViral antigen ingredientsChickenpoxHerpes zoster virus
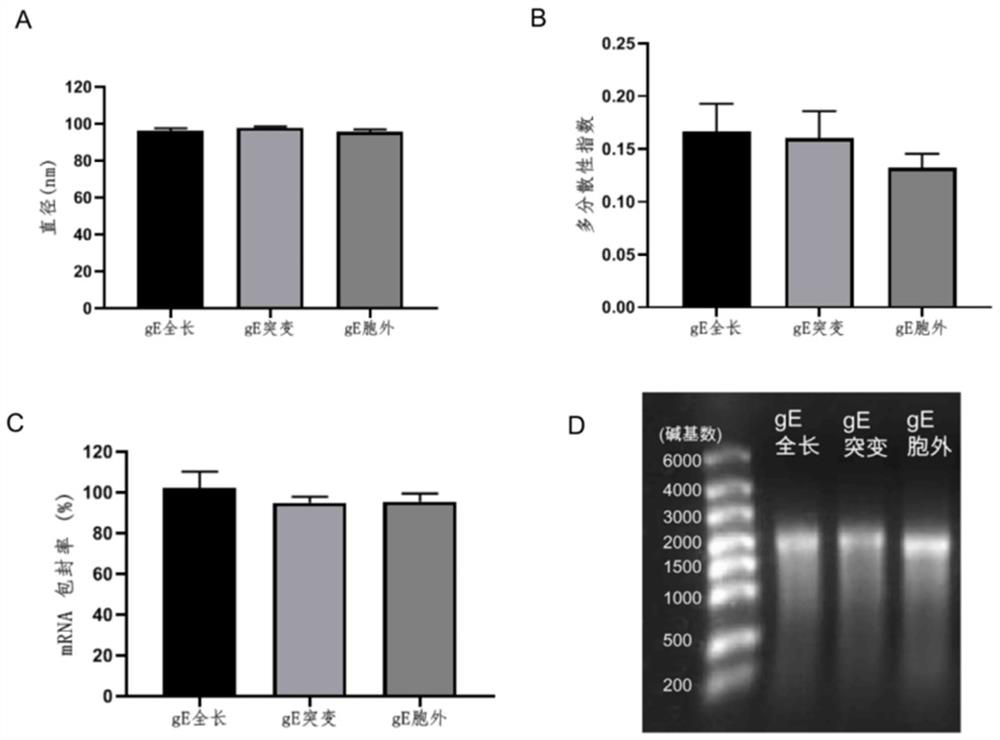
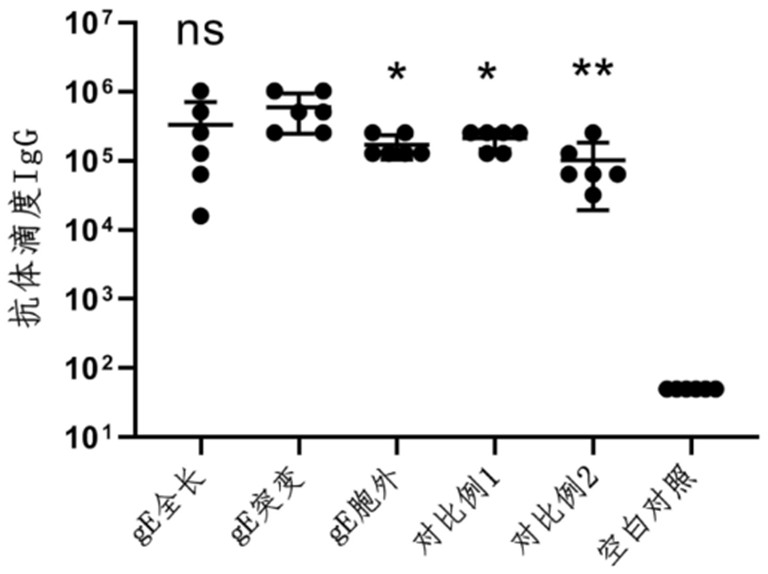
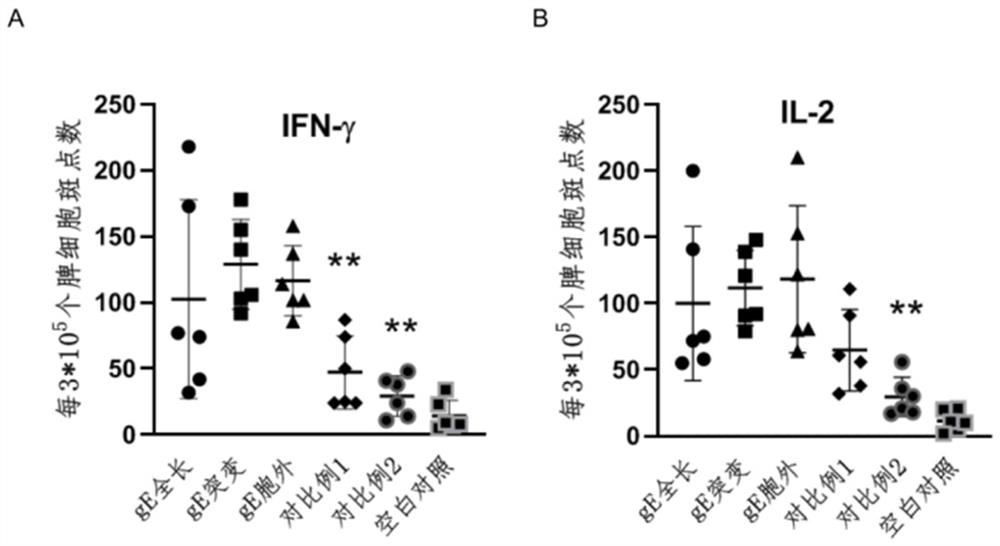
The invention provides a varicella-herpes zoster mRNA vaccine composition, and a preparation method and application thereof. The vaccine composition comprises a messenger ribonucleic acid (mRNA) sequence for coding varicella-herpes zoster virus glycoprotein E, a derivative sequence of the messenger ribonucleic acid (mRNA) sequence and lipid nanoparticles (LNP), and the messenger ribonucleic acid (mRNA) sequence is prepared into particles with the diameter of 20-400 nanometers through microfluidic equipment. The vaccine composition can specifically enhance humoral immune response and cellular immune response against varicella-herpes zoster glycoprotein E, can be used as a varicella vaccine which does not cause latent infection of vaccine strains, and can also be used as a herpes zoster vaccine with unlimited productivity. All the components in the vaccine composition can be widely obtained, so that the vaccine cost is effectively reduced, and the vaccine yield is increased.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Pien Tze Huang and new application of preparation thereof in preparing drug for treating herpes zoster
InactiveCN108042589ADefinite curative effectImprove clinical symptomsAntiviralsMammal material medical ingredientsAnti virusHerpes zoster virus
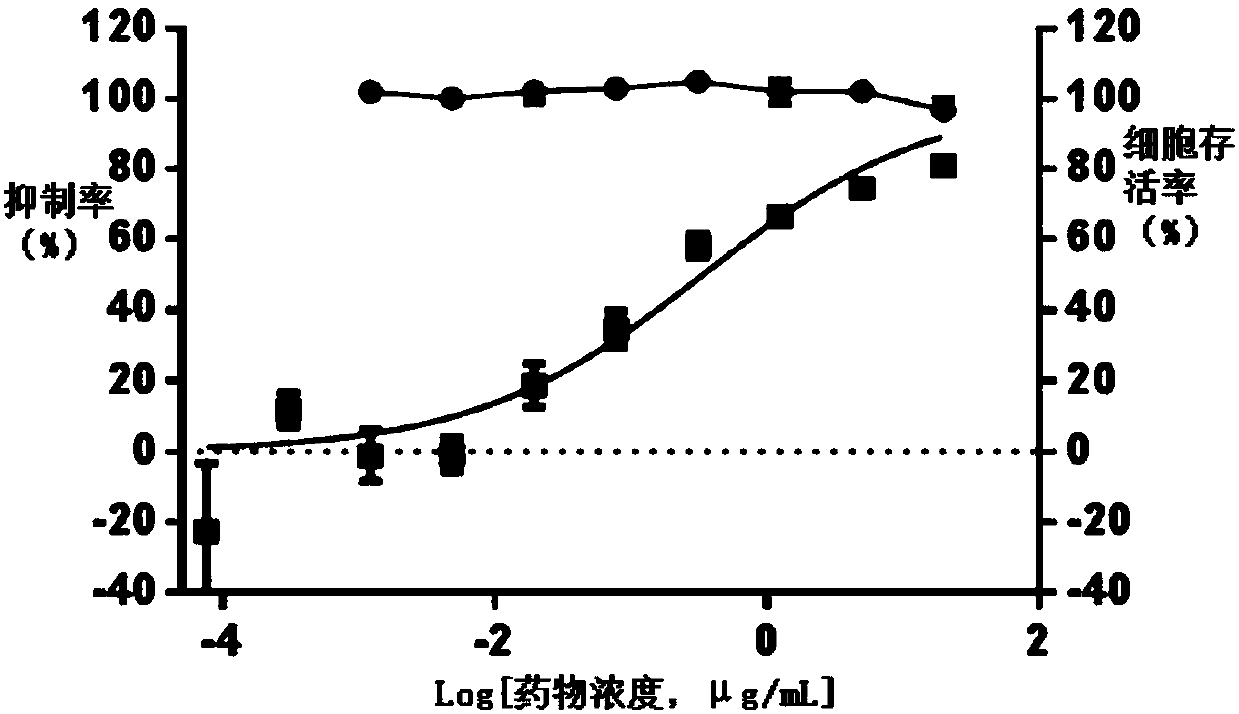
The invention belongs to the field of traditional Chinese medicine, and particularly relates to Pien Tze Huang and new application of a preparation thereof in preparing a drug for treating herpes zoster. An in-vitro experiment shows that Pien Tze Huang can dose-dependently inhibit duplication of chicken varicella-herpes zoster viruses (VZV). A clinical study finds that the combination of Pien Tzehuang and anti-virus drugs of valaciclovir and acyclovir has an obvious curative effect on alert period zoster viruses due to liver yu heat from the line, improvement of clinical symptoms of a patientcan be promoted, incrustation of herpes can be quickly promoted, new herpes can be stopped from generating, pain can be relieved, and the treatment time can also be shortened; besides, the incidenceof postherpetic neuralgia and the occurrence rate of adverse reactions occurring after treatment can also be lowered, and the clinical application has obvious advantages.
Owner:ZHANGZHOU PIEN TZE HUANG PHARM
Use of ground beetle in preparing medicine for treating virus infection disease
InactiveCN1586501AGood curative effectLow cost of treatmentAnthropod material medical ingredientsAntiviralsSide effectChickenpox
The present invention relates to the use of ground beetle, and is especially the use of ground beetle in preparing medicine for treating viral infection diseases. The present invention uses ground beetle in treating viral infection diseases, especially zoster, chickenpox, etc. and has the advantages of reliable curative effect, low cost and less side effect.
Owner:邵丹
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com