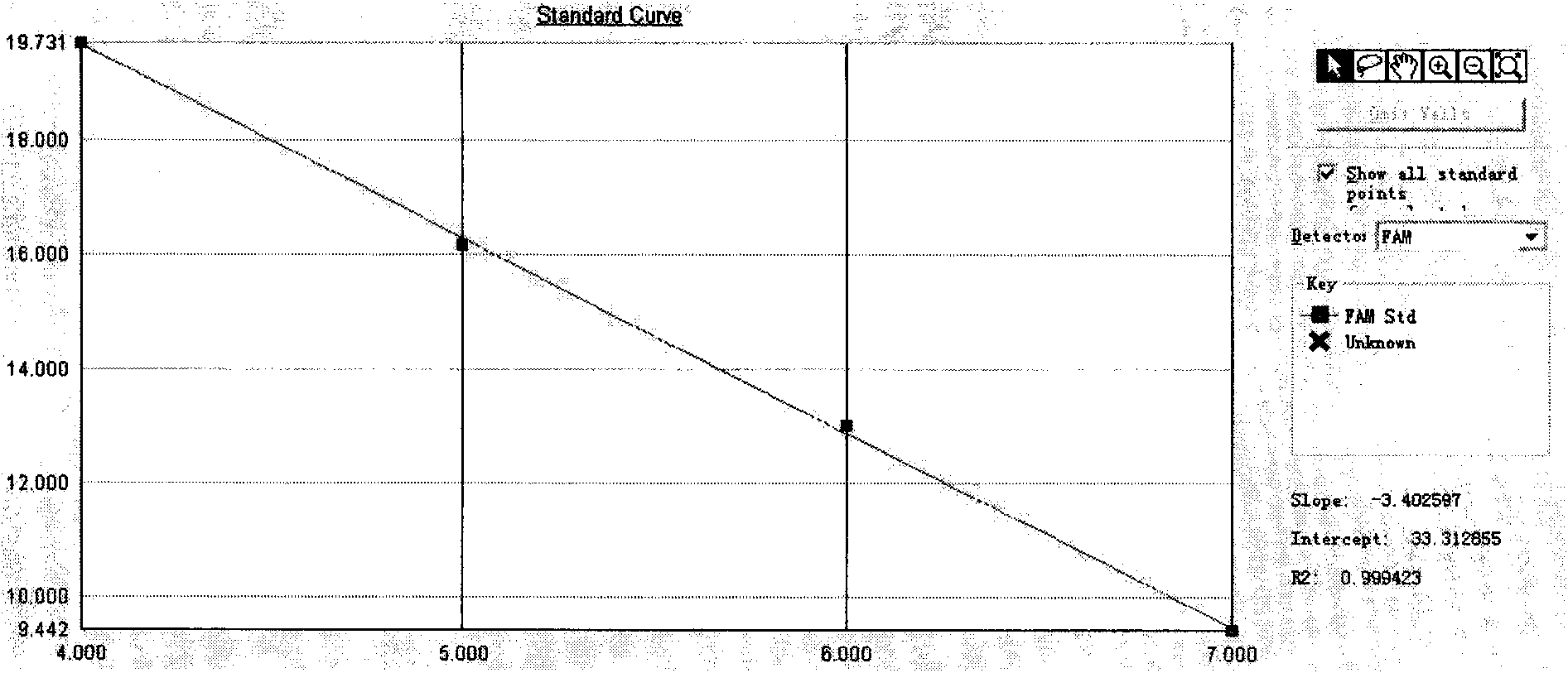
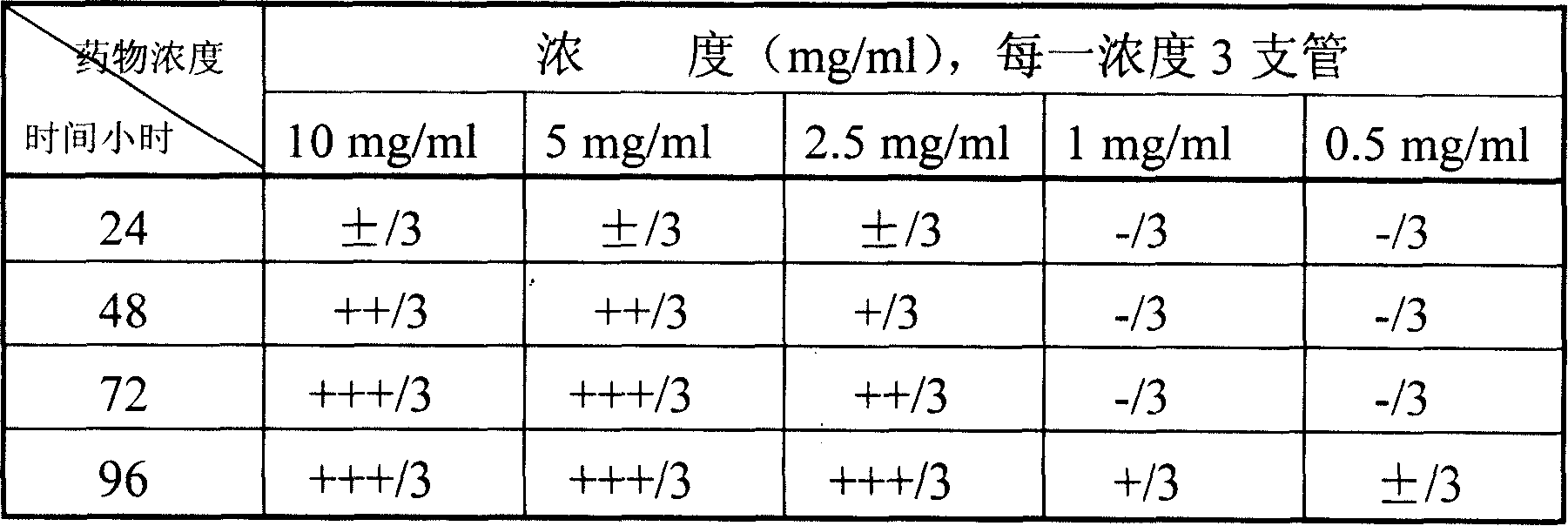
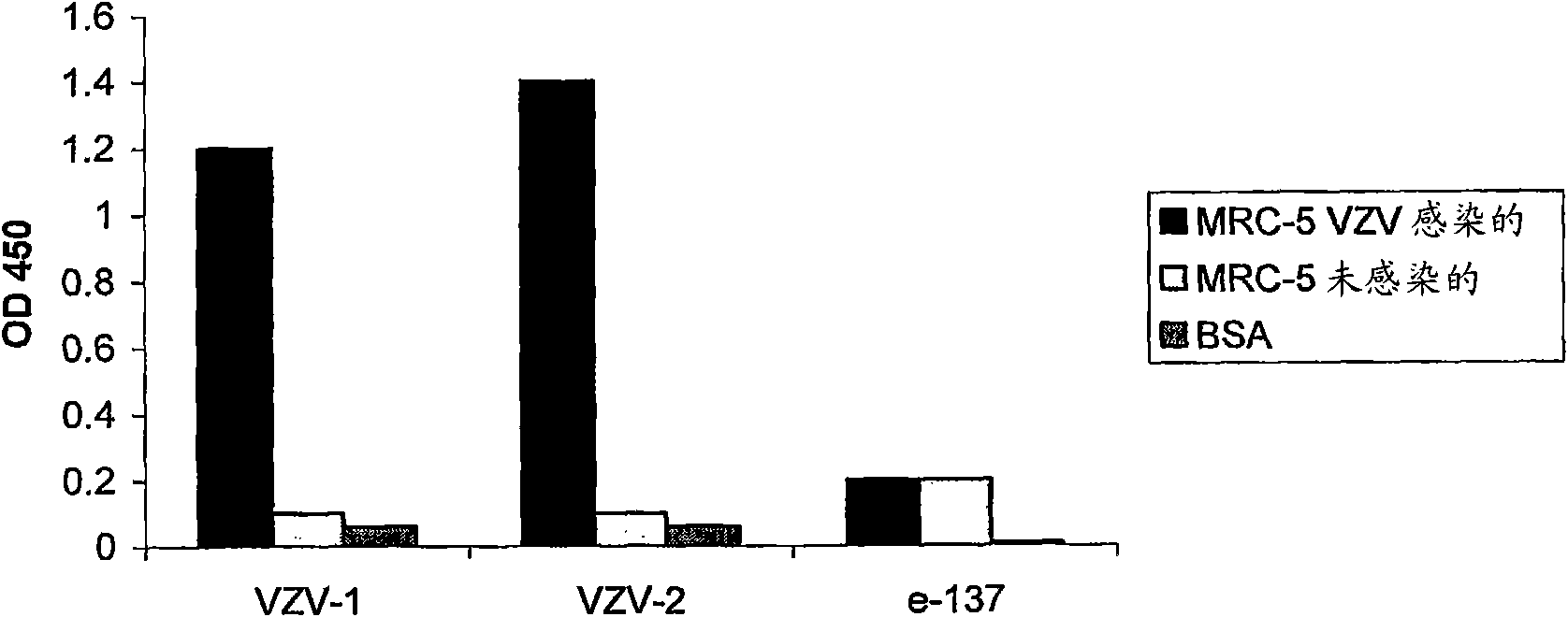
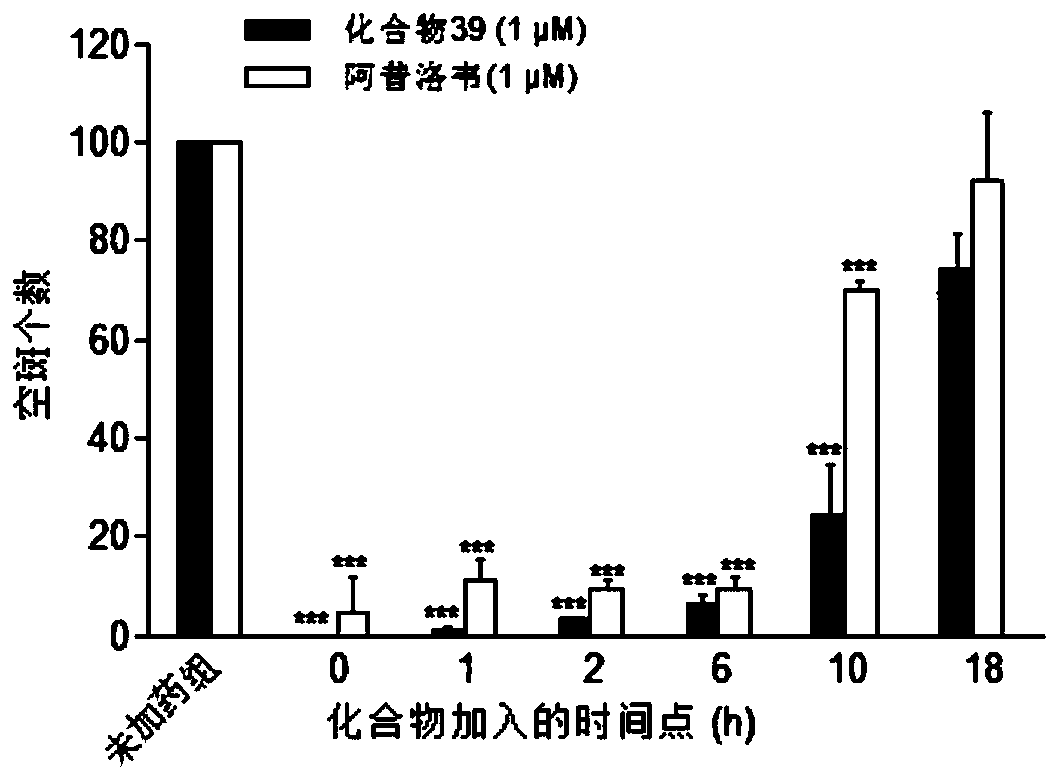
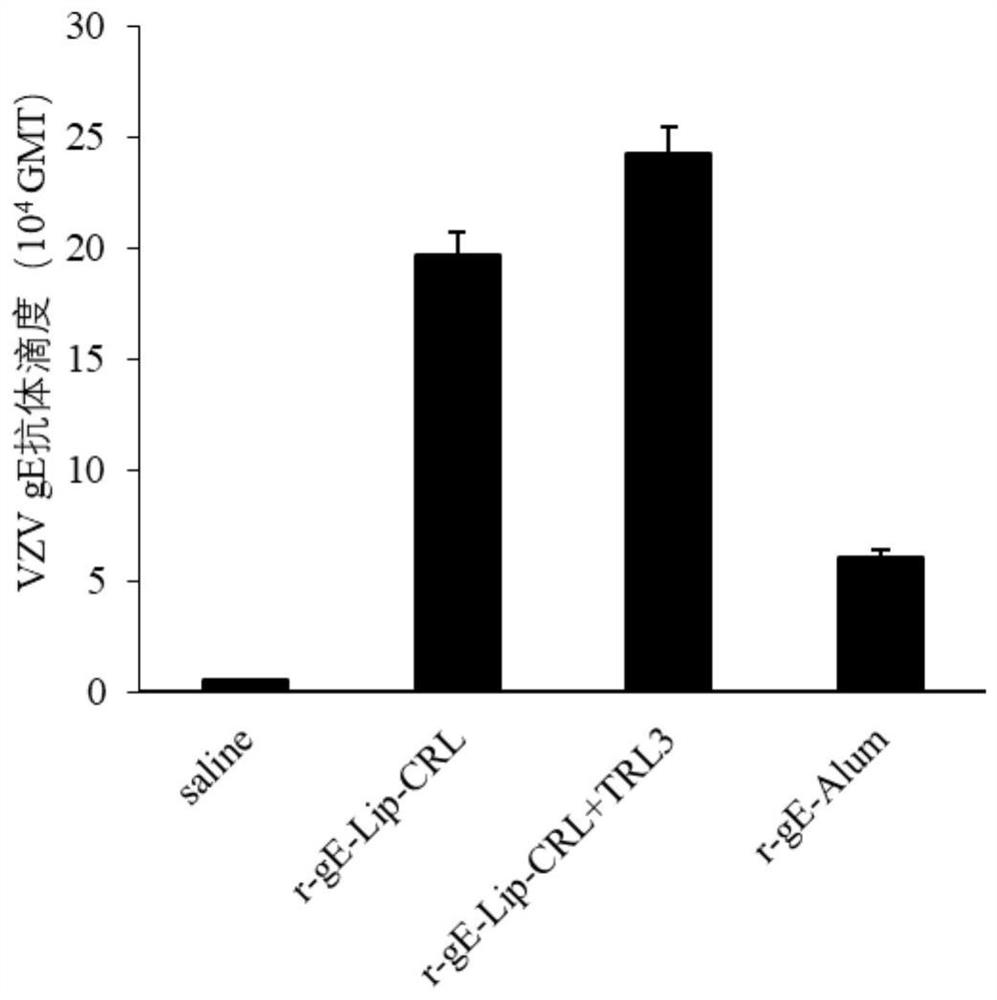
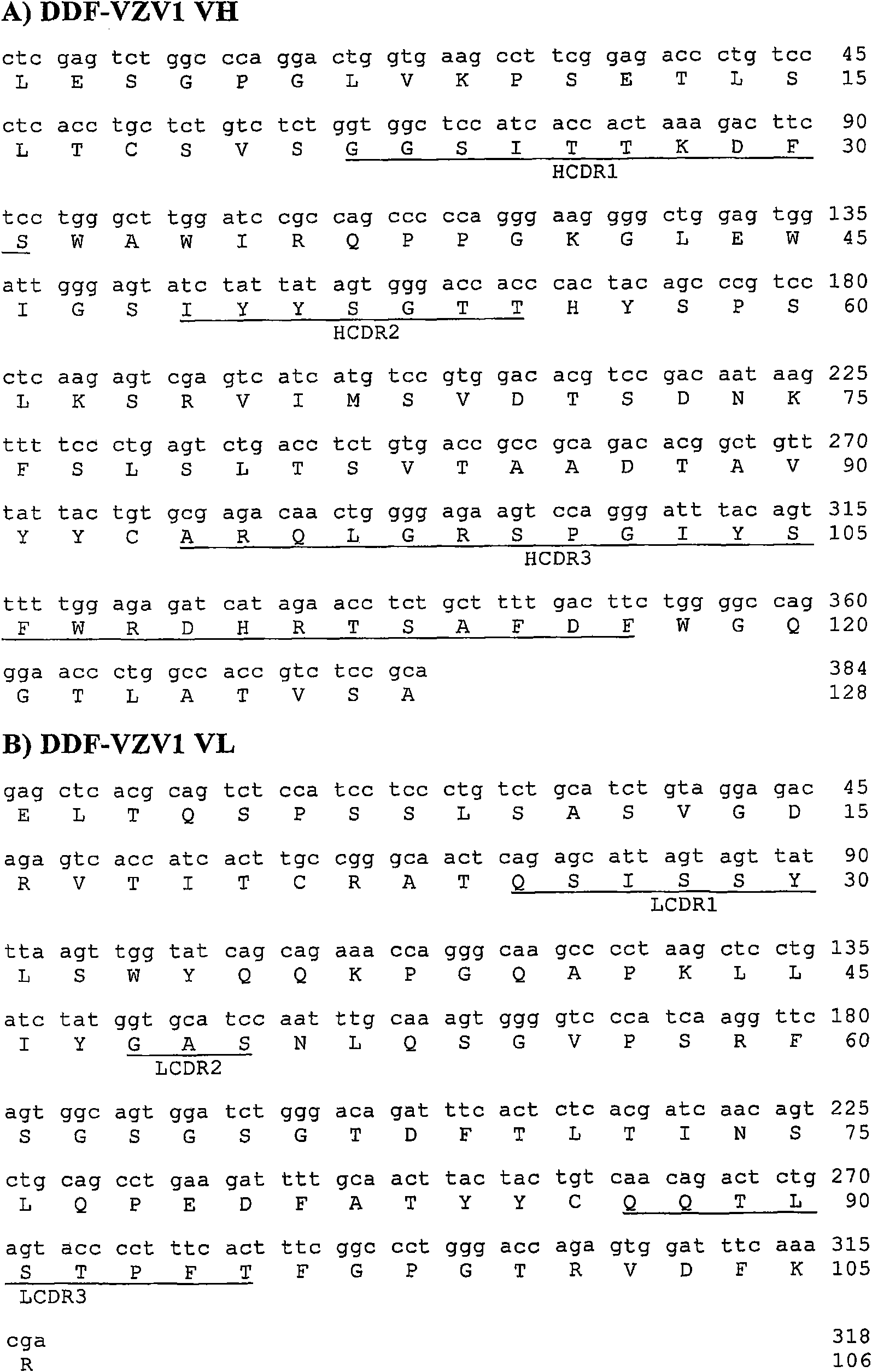Patents
Literature
98 results about "Herpes zoster virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Herpes zoster (also known as shingles) is a condition caused by a reinfection with the varicella-zoster virus. The varicella-zoster virus that causes herpes zoster is the same virus that causes chickenpox. The infection with this virus tends to occur during different decades of a person's life.
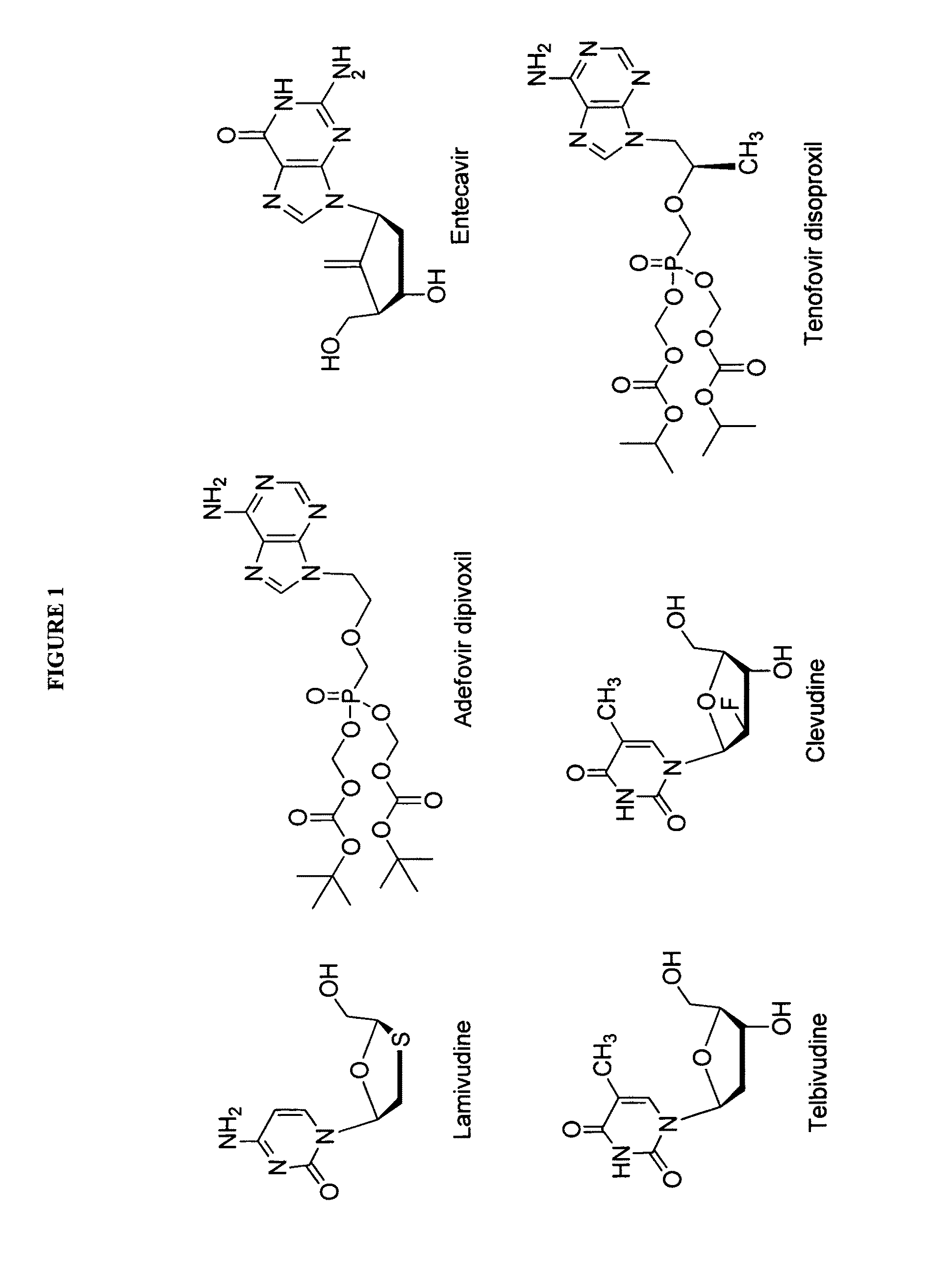
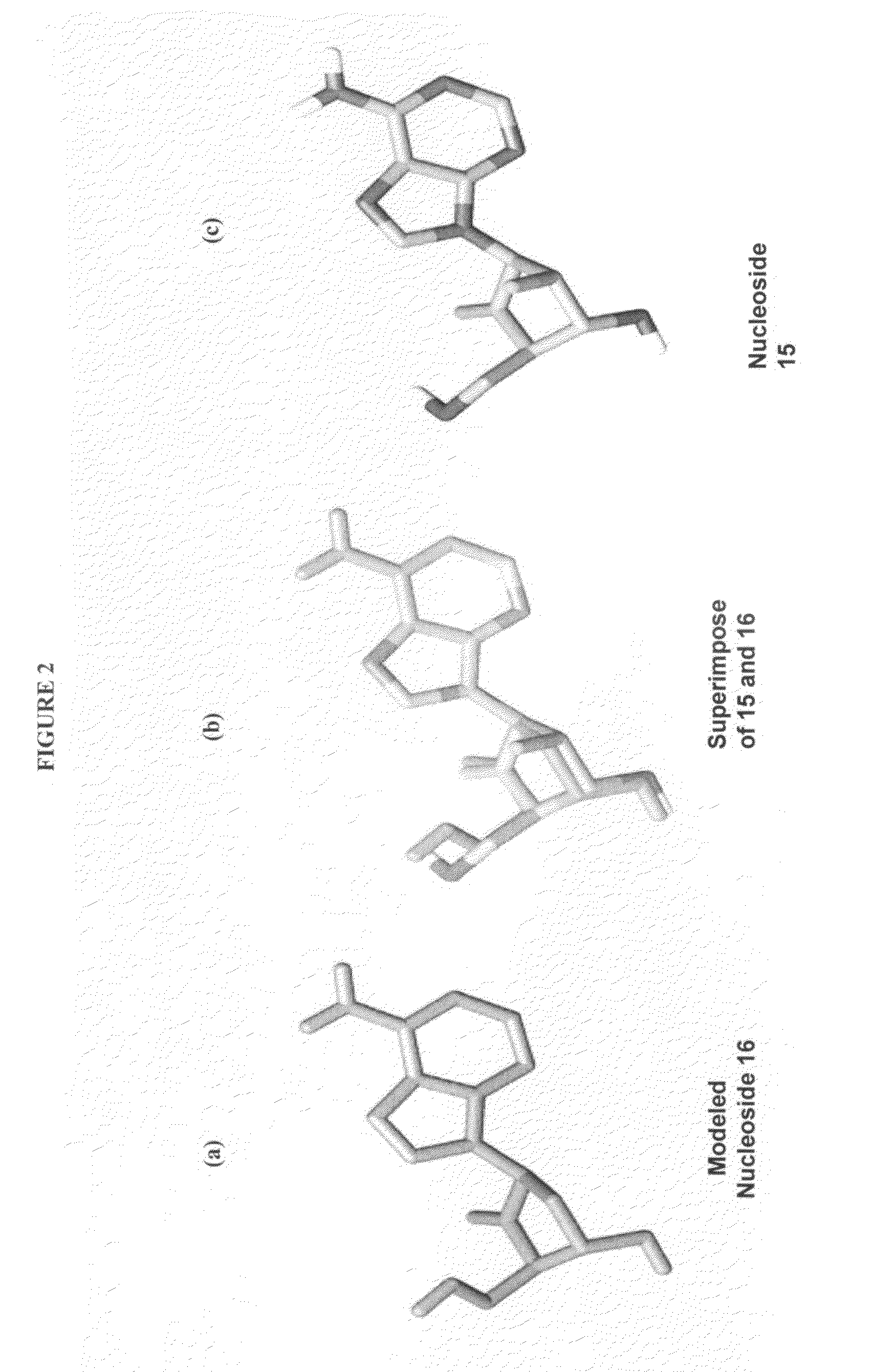
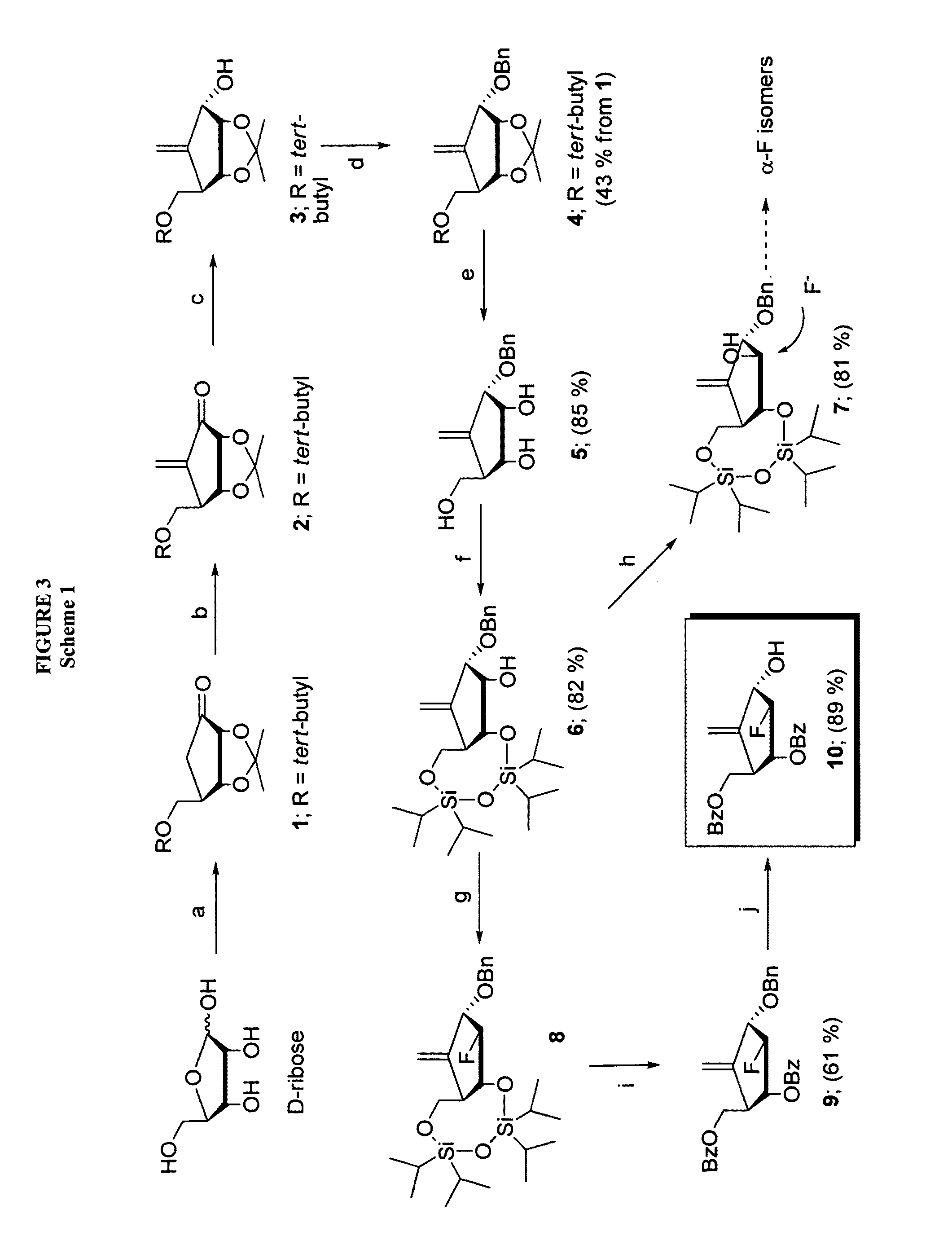
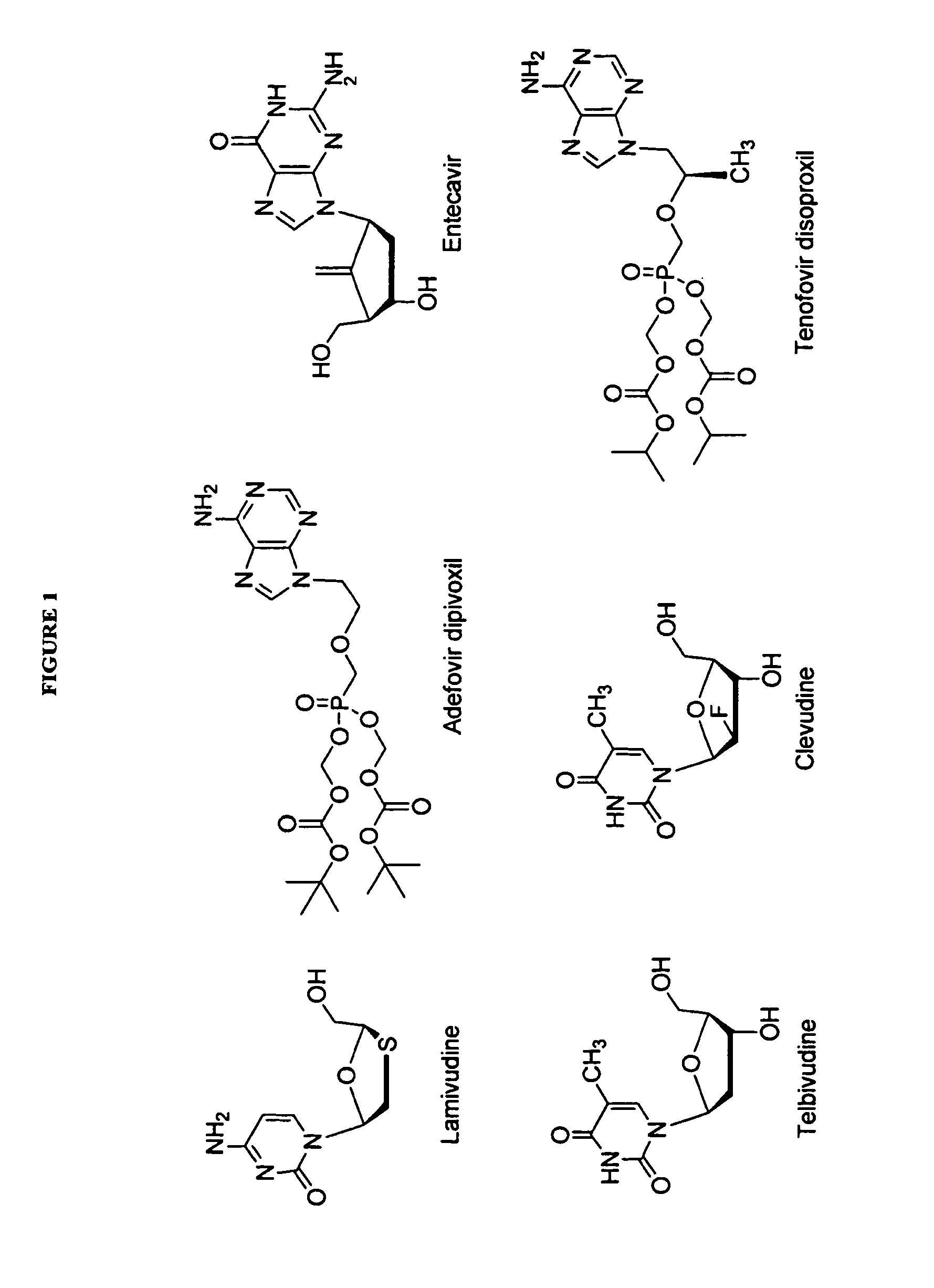
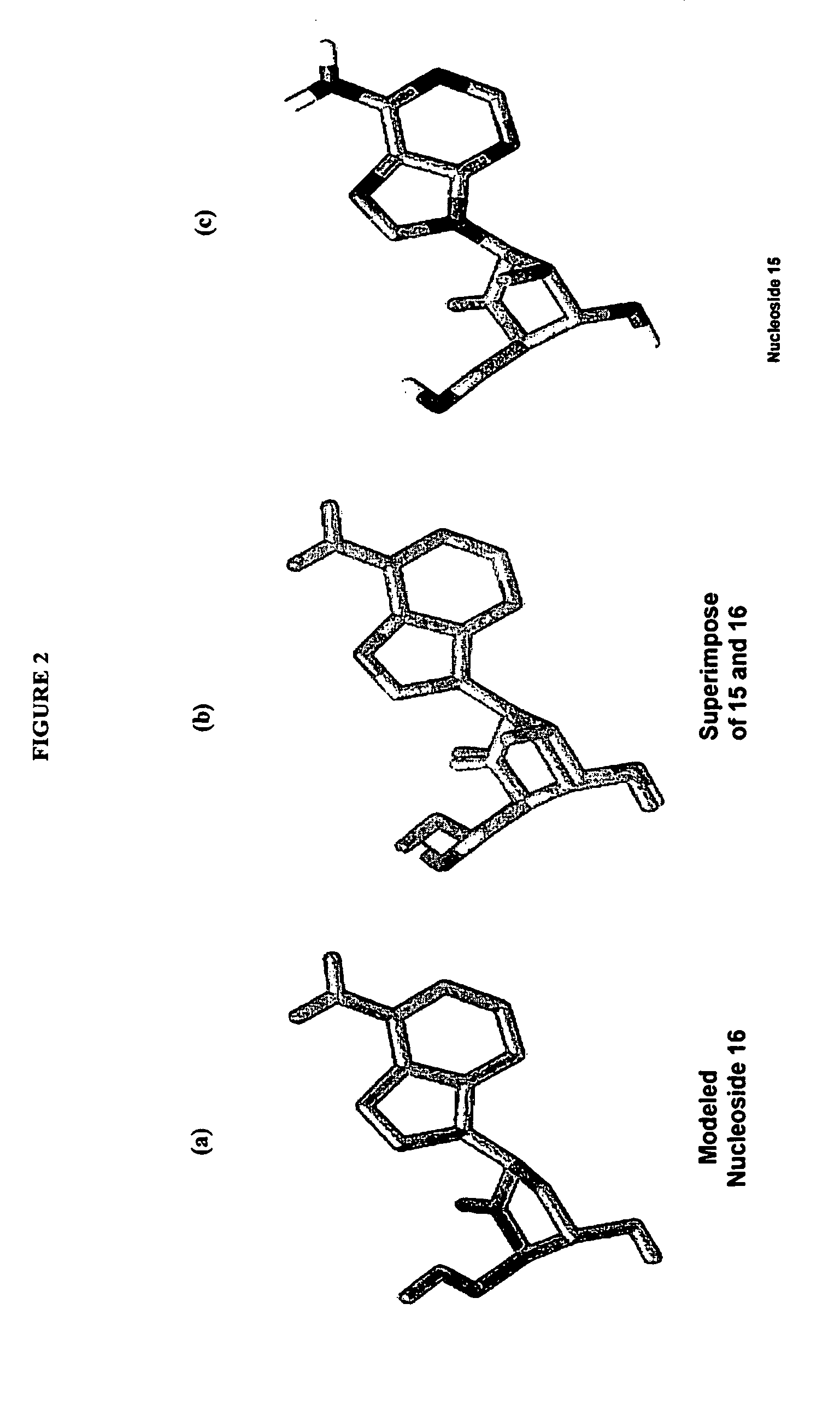
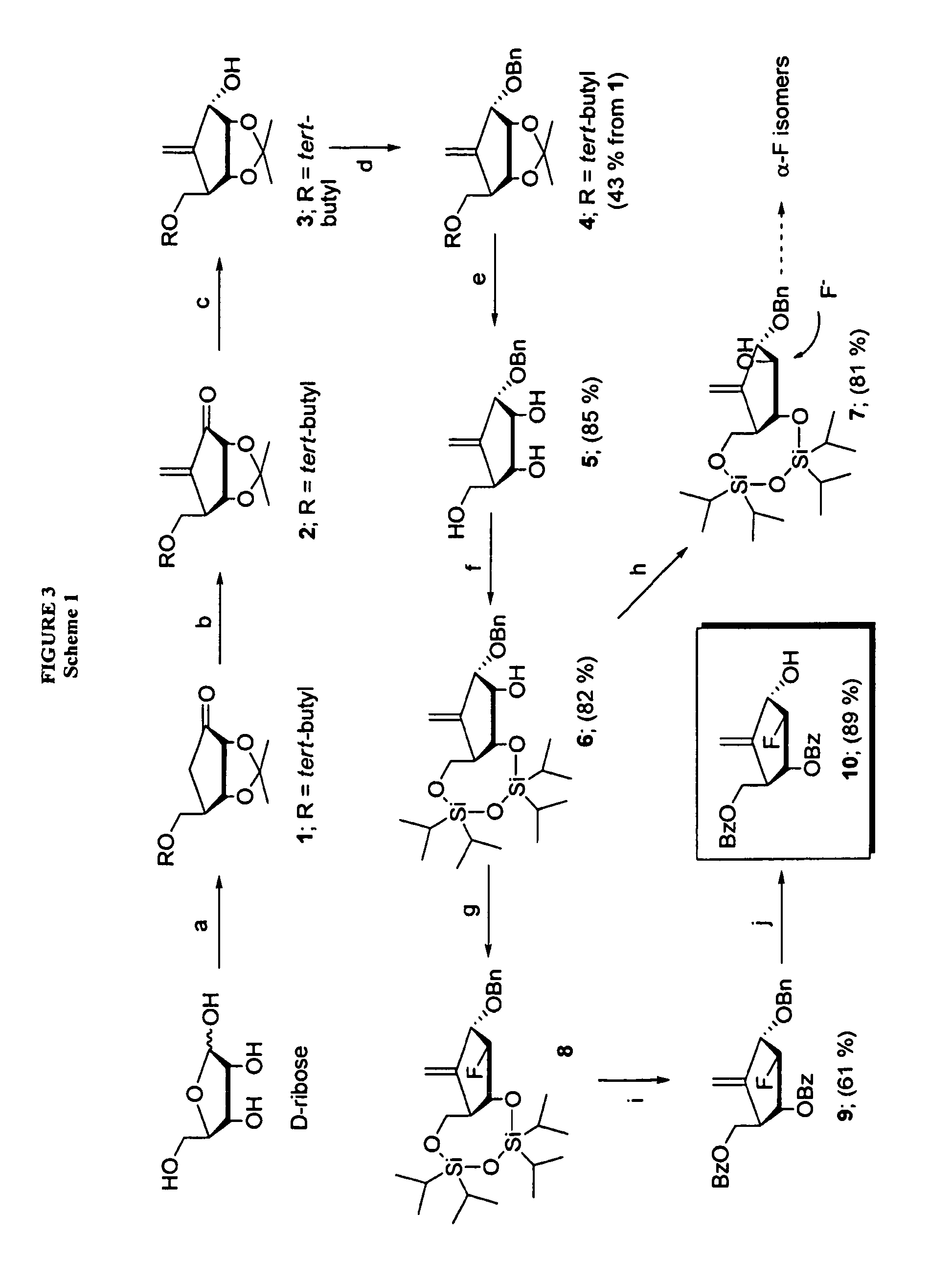
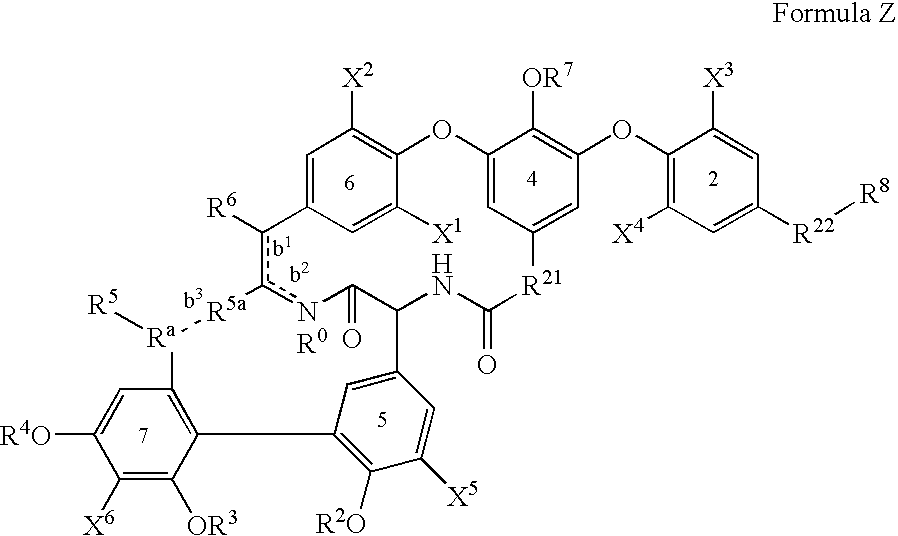
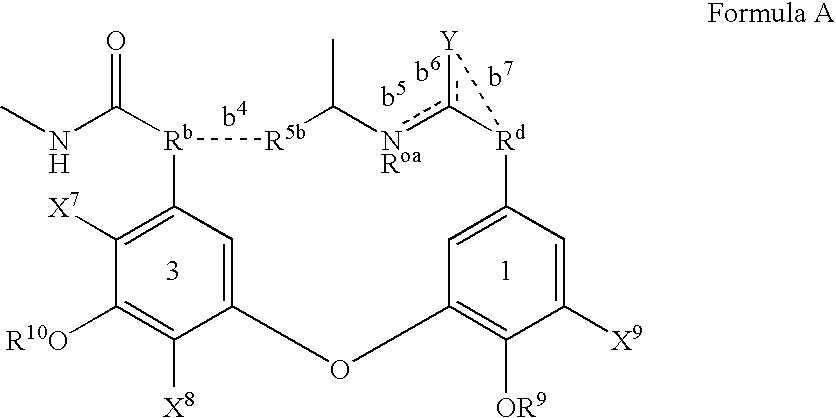
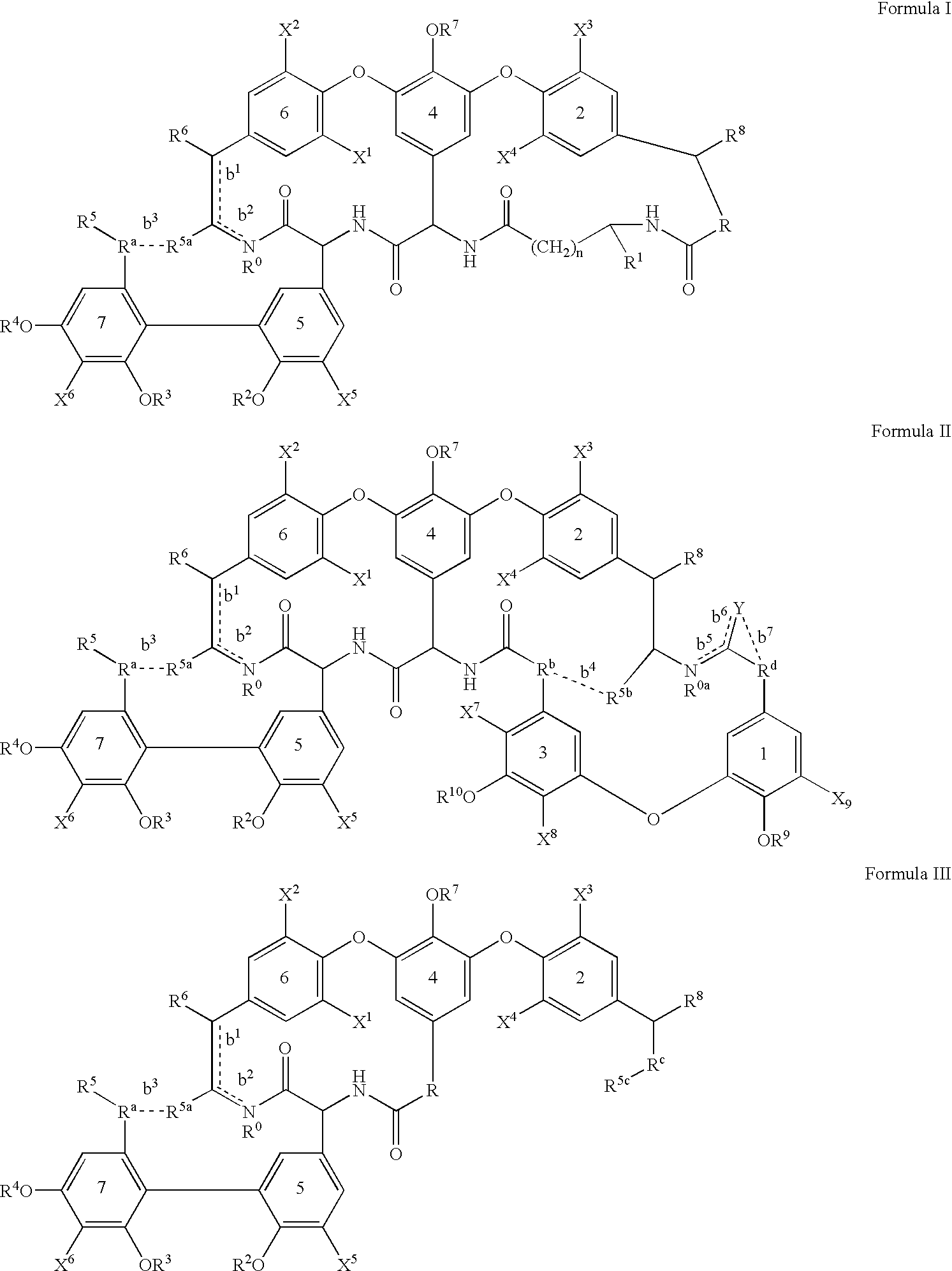
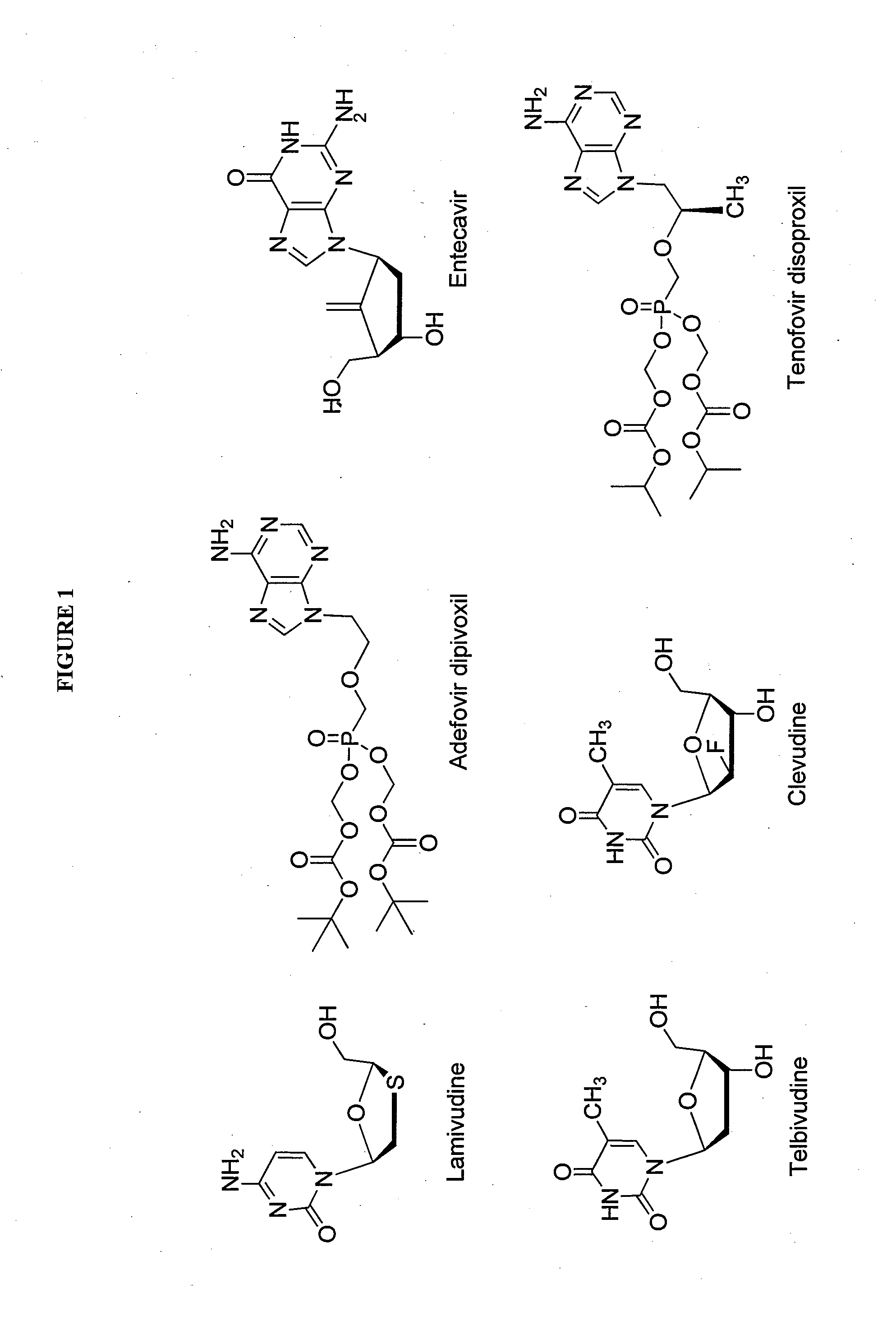
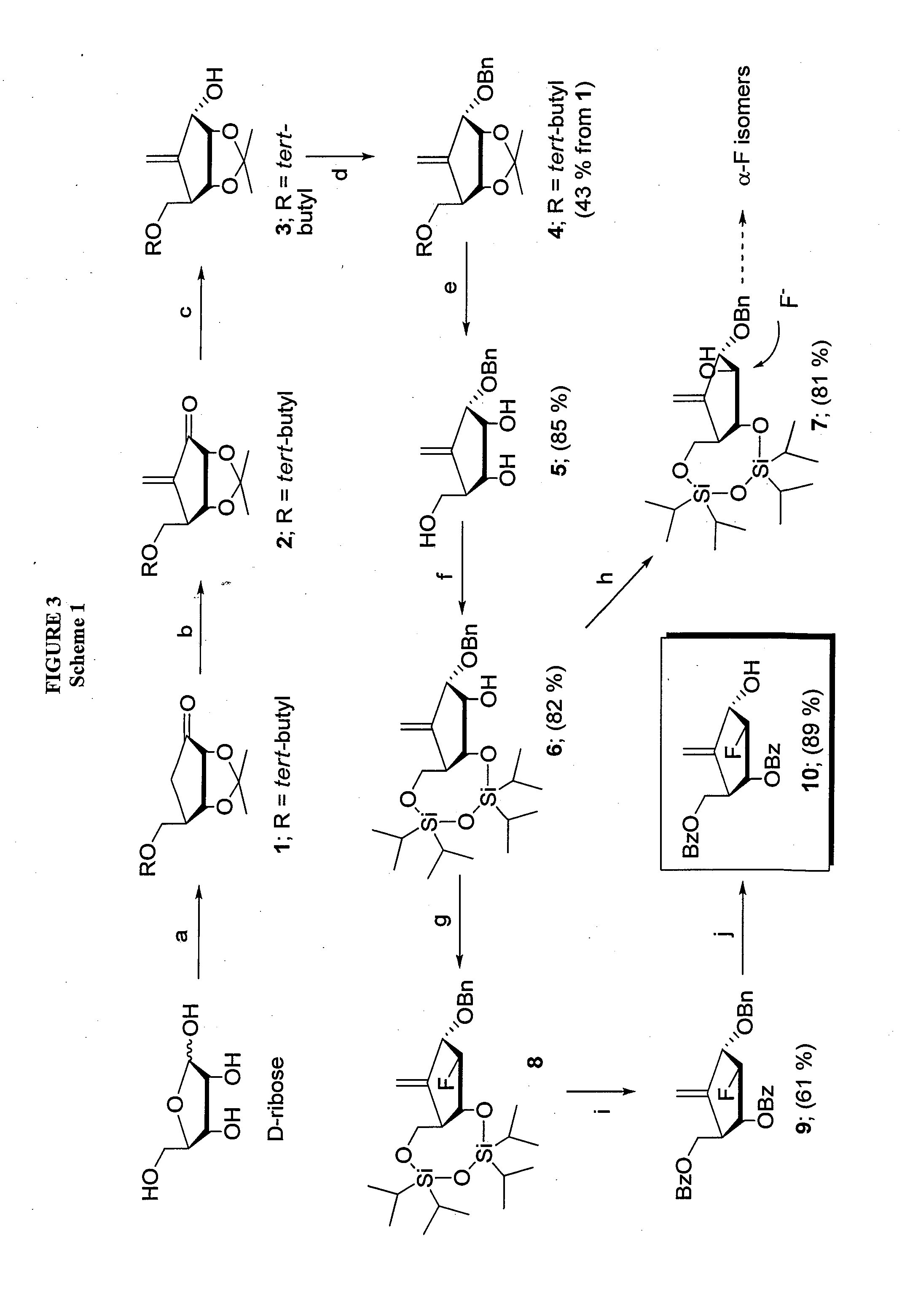
2′-fluoro-6′-methylene carbocyclic nucleosides and methods of treating viral infections
The present invention relates to 2′-Fluoro-6′-methylene carbocyclic nucleosides, pharmaceutical compositions containing these nucleosides and their use in the treatment or prophylaxis of a number of viral infections and secondary disease states and conditions thereof, especially including Hepatitis B virus (HBV) and secondary disease states and conditions thereof (cirrhosis and liver cancer), Heptatitis C virus (HCV), Herpes Simplex virus I and II (HSV-1 and HSV-2), cytomegalovirus (CMV), Varicella-Zoster Virus (VZV) and Epstein Barr virus (EBV) and secondary cancers which occur thereof (lymphoma, nasopharyngeal cancer, including drug resistant (especially including lamivudine and / or adefovir resistant) and other mutant forms of these viruses.
Owner:UNIV OF GEORGIA RES FOUND INC
2′-fluoro-6′methylene carbocyclic nucleosides and methods of treating viral infections
ActiveUS8946244B2Reduce the possibilityBiocideSugar derivativesHerpes zoster virusNasopharyngeal cancer
The present invention relates to 2′-Fluoro-6′-methylene carbocyclic nucleosides, pharmaceutical compositions containing these nucleosides and their use in the treatment or prophylaxis of a number of viral infections and secondary disease states and conditions thereof, especially including Hepatitis B virus (HBV) and secondary disease states and conditions thereof (cirrhosis and liver cancer), Hepatitis C virus (HCV), Herpes Simplex virus I and II (HSV-1 and HSV-2), cytomegalovirus (CMV), Varicella-Zoster Virus (VZV) and Epstein Barr virus (EBV) and secondary cancers which occur thereof (lymphoma, nasopharyngeal cancer, including drug resistant (especially including lamivudine and / or adefovir resistant) and other mutant forms of these viruses.
Owner:UNIV OF GEORGIA RES FOUND INC
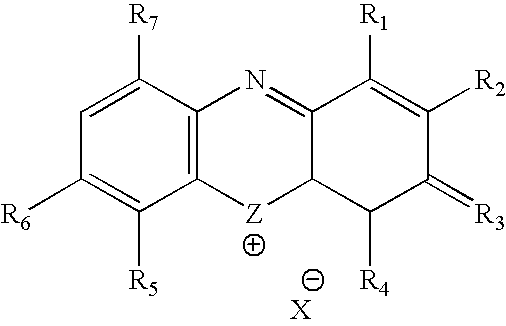
Methylene Blue Therapy of Viral Disease
InactiveUS20060264423A1Avoid infectionHeterocyclic compound active ingredientsAgainst vector-borne diseasesVirus typeHerpes zoster virus
A method for using thiazine dyes, especially methylene blue or methylene blue derivatives, in an immediate or controlled release formulation, alone or in combination with low levels of light or other drugs, to selectively inactivate or inhibit hepatitis infection, has been developed. Clinical trial results demonstrate efficacy in a human clinical trial for treatment of hepatitis C by oral administration of methylene blue immediate release formulation, in a dosage of 65 mg twice daily, over a period of at least 100 days. A method for using thiazine dyes, especially methylene blue or methylene blue derivatives, in an immediate or controlled release formulation, along or in combination with low levels of light or other drugs, to prevent or decrease reactivation of viruses, is also described. The preferred class of patient is infected with, or has been exposed to, viruses such as Herpes simplex virus type 1 & 2, Varicella zoster virus, Epstein-Barr virus, Cytomegalovirus, and Herpes virus type 6 & 7, Adenovirus, and Human polyoma viruses, e.g. JC virus and BK virus. In one embodiment the thiazine dye is administered to a patient experiencing symptoms or disease caused by reactivation of a virus. In a preferred embodiment the thiazine dye is administered to a patient at risk for or experiencing symptoms or disease caused by reactivation of a virus, prior to or during immunosuppression or chemotherapy.
Owner:BIOENVISION
Neutralizing epitope from varicella-zoster virus (VZV) gE protein and antibody aiming the same
ActiveCN105669838AAvoid infectionInhibition of neutralizing antibodiesVirus peptidesInactivation/attenuationDiseaseChickenpox
The invention relates to a neutralizing epitope peptide (or a variant thereof) from varicella-zoster virus virus (VZV) gE protein, a recombinant protein containing the neutralizing epitope peptide (or the variant thereof) and a carrier protein, and applications of the neutralizing epitope peptide (or a variant thereof) and the recombinant protein, and also relates to an antibody aiming to the neutralizing epitope peptide, a cell strain producing the antibody, and the applications thereof, and also relates to a vaccine containing the neutralizing epitope peptide and the recombinant protein, a medicine composition including the antibody, and the applications thereof, e.g., the application for preventing and / or treating VZV infection or one or more diseases and symptoms related to the infection.
Owner:XIAMEN UNIV +1
Formulations useful for the treatment of varicella zoster virus infections and methods for the use thereof
InactiveUS20050203187A1Inhibiting varicella zoster virus replicationBiocideHydroxy compound active ingredientsVaricella-zoster virus infectionActive agent
Jojoba alcohol, a mixture of long chain monounsaturated alcohols, is an oily liquid at moderate ambient temperatures. It is readily absorbed by human skin where it relieves irritation and inhibits the formation of lesions caused by viruses. The inhibitory action is applicable to enveloped viruses which express as sores at dermal surfaces in humans. When applied topically to an incipent herpes episode, it will quickly penetrate the epidermis to the subdermal vascular cells and suppress viral replication which leads to inflammation and the formation of blisters on the face, genital and other skin and mucosal areas. Fumaric acid and malonic acid at low concentrations also inhibit the replication of varicella zoster virus in human cell cultures, with no cellular toxicity. Compositions of certain low molecular weight organic acids in jojoba alcohol enhance antiviral activity. Topical treatment of shingles with a low concentration of fumaric acid in jojoba alcohol terminates the episode. This combination drug acts by a dual mechanism wherein the jojoba alcohol blocks viral fusion by a lipoidal mode, and the polycarboxylic acids inhibit viral fusion by an ionic mode. The combination drug can also be effective in treating chicken pox. Jojoba alcohol is a carrier and transdermal delivery system for these and other pharmacologically active agents for the relief of pain and treatment of other conditions which occur at or under the surface of the skin. Topically applied jojoba alcohol is non-toxic and safe for animals and humans.
Owner:VERBISCAR ANTHONY J
Multiple real-time quantitative PCR primer, probe and detection method for identifying viral pathogens relevant to fever with eruption syndrome as infection diseases
ActiveCN102140543ADetection ExpressImprove efficiencyMicrobiological testing/measurementFluorescence/phosphorescenceChickenpoxHerpes zoster virus
The invention discloses multiple real-time quantitative PCR primer, probe and a detection method for identifying viral pathogens relevant to fevers with eruption syndromes as infection diseases, which is used for carrying out multiple real-time fluorescent quantitative PCR detection on varicella-herpes zoster viruses, human small DNA (Deoxyribonucleic Acid) viruses B19, enteroviruses (enteroviruses 71 type and coxsackie viruses A16 type), dengue viruses, rubella viruses and measles viruses. The invention can simultaneously carry out qualitative or quantitative detection on eight kinds of human viruses in various types of samples by multiple double-tubes PCR. The detection method has the advantages of simple operation, short time consumption, high sensitivity and strong specificity, is suitable for field detection, early diagnosis, epidemics detection and research and the like, and takes the actions of assistance and identification diagnosis on the fevers with eruption syndromes.
Owner:SUN YAT SEN UNIV
Glycopeptide antibiotic derivatives
InactiveUS20050250677A1Decreasing and removing antibacterial activityMaintain antiviral activityBiocideDigestive systemHerpes zoster virusGlycopeptide
Novel glycopeptide antibiotic derivatives, processes for their preparation, their use as a medicine, their use to treat or prevent viral infections and their use to manufacture a medicine to treat or prevent viral infections are provided. The present invention relates to the use of glycopeptide antibiotics and their semisynthetic derivatives to treat or prevent viral infections and their use to manufacture a medicine to treat or prevent viral infections of subjects, more in particular infections with viruses belonging to Retroviridae, Herpes viridae, Flaviviridae and the Coronaviridae, like HIV (human immunodeficiency virus), HCV (hepatitis C virus), BVDV (bovine viral diarrhoea virus), SARS (severe acute respiratory syndrome) causing virus, FCV (feline coronavirus), HSV (herpes simplex virus), VZV (varicella zoster virus) and CMV (cytomegalovirus).
Owner:BALZARINI JAN +2
Kit for detecting varicella-herpes zoster virus
ActiveCN101871013AGood reproducibilityMicrobiological testing/measurementMicroorganism based processesFluorescenceChickenpox
The invention relates to a kit for detecting varicella-herpes zoster virus, in particular to a kit for detecting varicella-herpes zoster virus in a clinical sample through the technology of fluorescence quantitative polymerase chain reaction. As the kit of the invention has higher sensitivity and specificity, the kit has significance in the confirmation of early infection and the emergency diagnosis and monitoring of virus outbreak by detecting and performing quantitative analysis to the polynucleotide of varicella-herpes zoster virus.
Owner:DAAN GENE CO LTD
Composition of starwort sulphonic acid or vitriolic acid polyoses ester total phenolic glycoside and method of preparing the same and antiviral application
The invention relates to a kind of natural medicine of broad spectrum antibiotic. At present, the broad spectrum antibiotic medicine with high effect and safety is at shortage all round the world. The invention is intended to extract laminarinsulphate or sulphonic acid sugar ester or sulphosalts from plant chickweed or other chickweed plant with two resin adsorption methods or a water extraction and alcohol precipition method. The spectrum antibiotic in the invention is distributed under 50,000 in the formula weight formed by carbon glycosidic bond and / or oxide glycosidic bond with phenol, especially the total flavones comprising apigenin. However, the invention mainly acts as total phenolic glycoside with the formula weight under 4,000. Besides, the invention can form brownish compound with the total flavones comprising apigenin and the glycosidic ingredients without sulfur element, so as to be applied as broad spectrum antibiotic drug. Therefore, the compound in the invention can be applied to cure ADIS virus, hepatitis virus, influenza virus and parainfluenza virus comprising SARS, adenovirus, verruca acuminate virus, enterovirus, mumps virus, herpes simplex virus, herpes zoster virus and varicella. No toxic effect has been found in the application. What is more, the invention can be made into 10 sorts of formulation, disinfector and health-improving products.
Owner:朱耕新
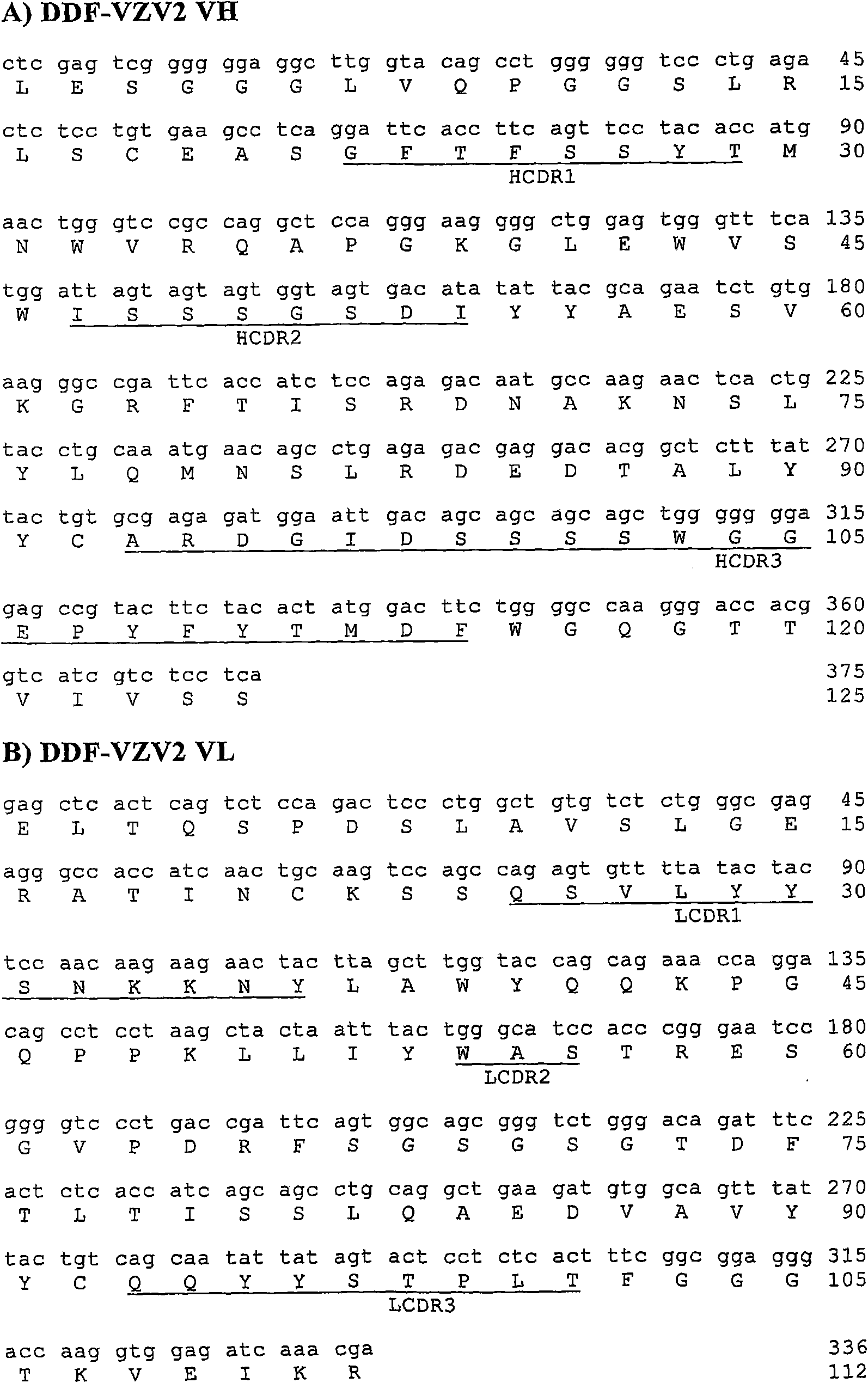
Antibodies specific for varicella zoster virus
InactiveCN101663318AVirus peptidesImmunoglobulins against virusesTherapeutic treatmentVaricella zoster virus
The present invention provides novel antibody sequences that bind Varicella Zoster Virus (VZV) and neutralize VZV infection. The novel sequences can be used for the medical management of VZV infection, in particular for detecting the virus or for preparing pharmaceutical compositions to be used in the prophylactic or therapeutic treatment of VZV infection.
Owner:RIBOVAX BIOTECHNOLOGIES SA
2'-fluoro-6'-methylene carbocyclic nucleosides and methods of treating viral infections
The present invention relates to 2′-Fluoro-6′-methylene carbocyclic nucleosides, pharmaceutical compositions containing these nucleosides and their use in the treatment or prophylaxis of a number of viral infections and secondary disease states and conditions thereof, especially including Hepatitis B virus (HBV) and secondary disease states and conditions thereof (cirrhosis and liver cancer), Heptatitis C virus (HCV), Herpes Simplex virus I and II (HSV-1 and HSV-2), cytomegalovirus (CMV), Varicella-Zoster Virus (VZV) and Epstein Barr virus (EBV) and secondary cancers which occur thereof (lymphoma, nasopharyngeal cancer, including drug resistant (especially including lamivudine and / or adefovir resistant) and other mutant forms of these viruses, especially HBV.
Owner:UNIV OF GEORGIA RES FOUND INC
Kit for genotyping VZV, production method of kit and application of kit
InactiveCN105132584ANo cross reactionStrong specificityMicrobiological testing/measurementMicroorganism based processesHuman DNA sequencingChemical structure
The invention provides a kit for genotyping VZV (Varicella-Zoster Viruses). The kit is characterized by comprising a nucleotide sequence shown as the Table 3 in the specification, and specific primers and specific probes corresponding to clade1-5 type VZV of chemical structures. The kit has the function of detecting various kinds of VZV DNA (Deoxyribonucleic Acid); the detection sensitivity is 10<2> copies / reaction; no cross reaction with human genome, herpes simplex viruses type I / type II, cytomegaloviruses and EB viruses exists; and the kit is applicable to the virus gene diagnosis of clinical VZV infected persons, and can also be used for the epidemiology survey of different Clade types of VZV.
Owner:CHENGDU MILITARY GENERAL HOSPITAL OF PLA
Process for simultaneously abstracting essential oil, chromocor and triterpenes components from agastache
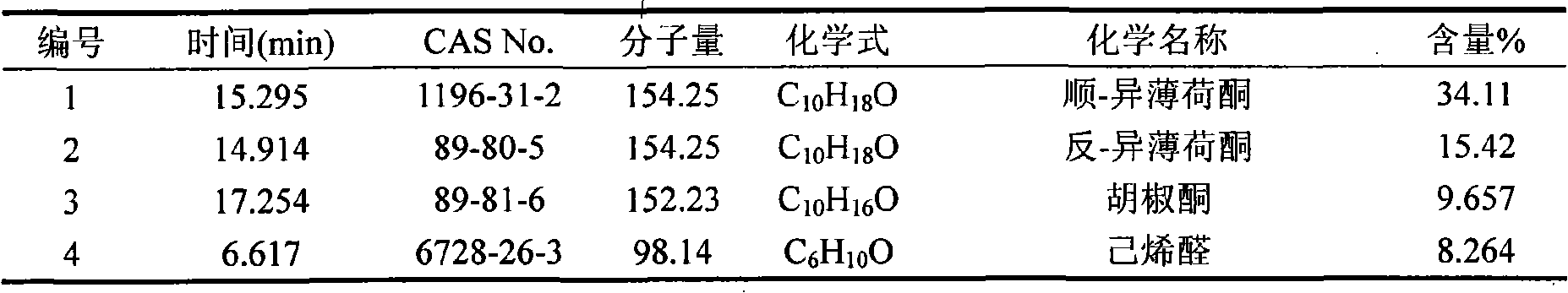
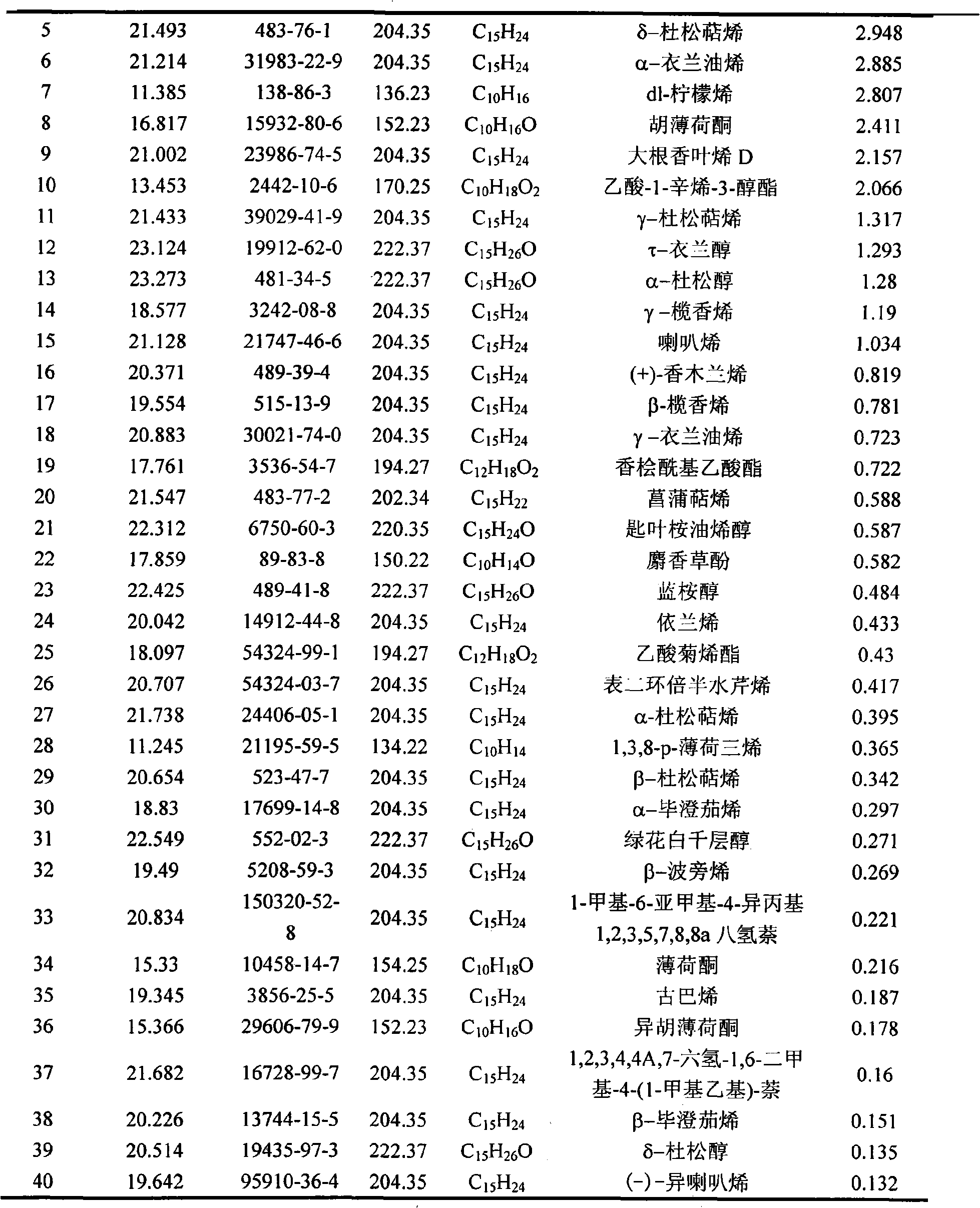
InactiveCN101285021AAchieve separationHigh extraction rateEssential-oils/perfumesSteroidsHerpes zoster virusEssence oil
The invention belongs to natural products separating and extracting technical field, particularly relating to a process for simultaneously extracting essential oil, flavone and triterpene out of wrinkled giant hyssop. The invention provides a novel separating and extracting process, which can simultaneously separate and extract essential oil, flavone and triterpene by taking wrinkled giant hyssop as raw materials; the used raw materials can be wrinkled giant hyssop as well as cablin pacholi, fresh or dried herbs as well as fresh or dried leaves, stems or other parts. When fresh wrinkled giant hyssop leaves are used as the raw materials, the essential oil thereof comprises more than 40 volatile components such as cis form - isomenthone, anti form - isomenthone piperitone, hexenoic aldehyde, delta-cadinene, cadinol, alpha-muurolene, limonene and pulegone, wherein main volatile components are the cis form - isomenthone and the anti form- isomenthone, contents of which are 34.11 percent and 15.42 percent respectively; the total flavone comprises flavonoids such as acacia substance, tilianin, linarin, agastachoside, isoagastachoside, agastachin, apigenin, apigenin-7- glucoside, 6-methoxyl group apigenin, luteolin and luteolin-7- glucoside, etc., wherein main flavone components are the agastachoside and the isoagastachoside , contents of which are 25.8 percent and 8.21 percent respectively; the triterpene materials comprises triterpene compounds such as cralaegolic acid, oleanolic acid, 3-acetyl oleanolic aldehvde, 3- acetyl oleanolic acid, alpha-amyrin, beta- amyrin, campesterol, campestanol, ursolic acid, erythrodiol, erythrodiol-3-acetic ester, wherein the main triterpene component is the cralaegolic acid , the content of which is 34.6 percent. The components have wide biological activities and a plurality of effects such as liver protection, anti-tumor, anti-HIV-1, anti-proteinase activity of HIV-1, anti-herpes zoster virus and immunity improvement, etc.
Owner:JIANGNAN UNIV
Water solution of penciclovir and its prepn. method
InactiveCN1461643AImprove solubilityExpand the scope of clinical applicationAntiviralsHeterocyclic compound active ingredientsDiseaseHerpes zoster virus
A water soluble of Penxiluowei contains the Penxiluowei or its salt, solubilizer, cosolvent and pH regulator. It can be used to prepare injection, eye drops and nose drops for treating the diseases caused by herpes simple virus, herpes zoster virus and hepatitis B virus.
Owner:XICHUAN INST OF ANTIBIOTIC IND NAT MEDICINE SUPERVISION BUREAU
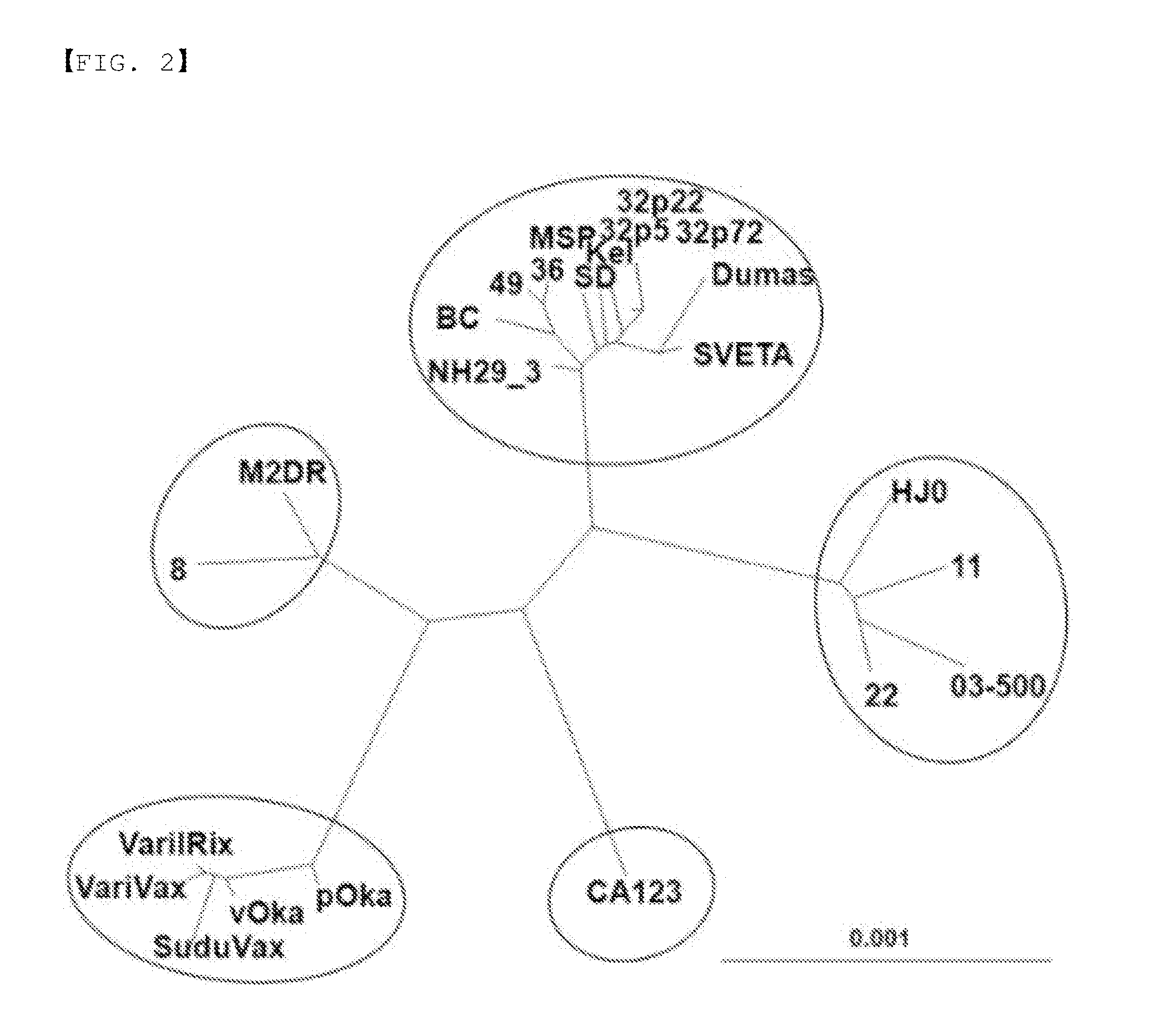
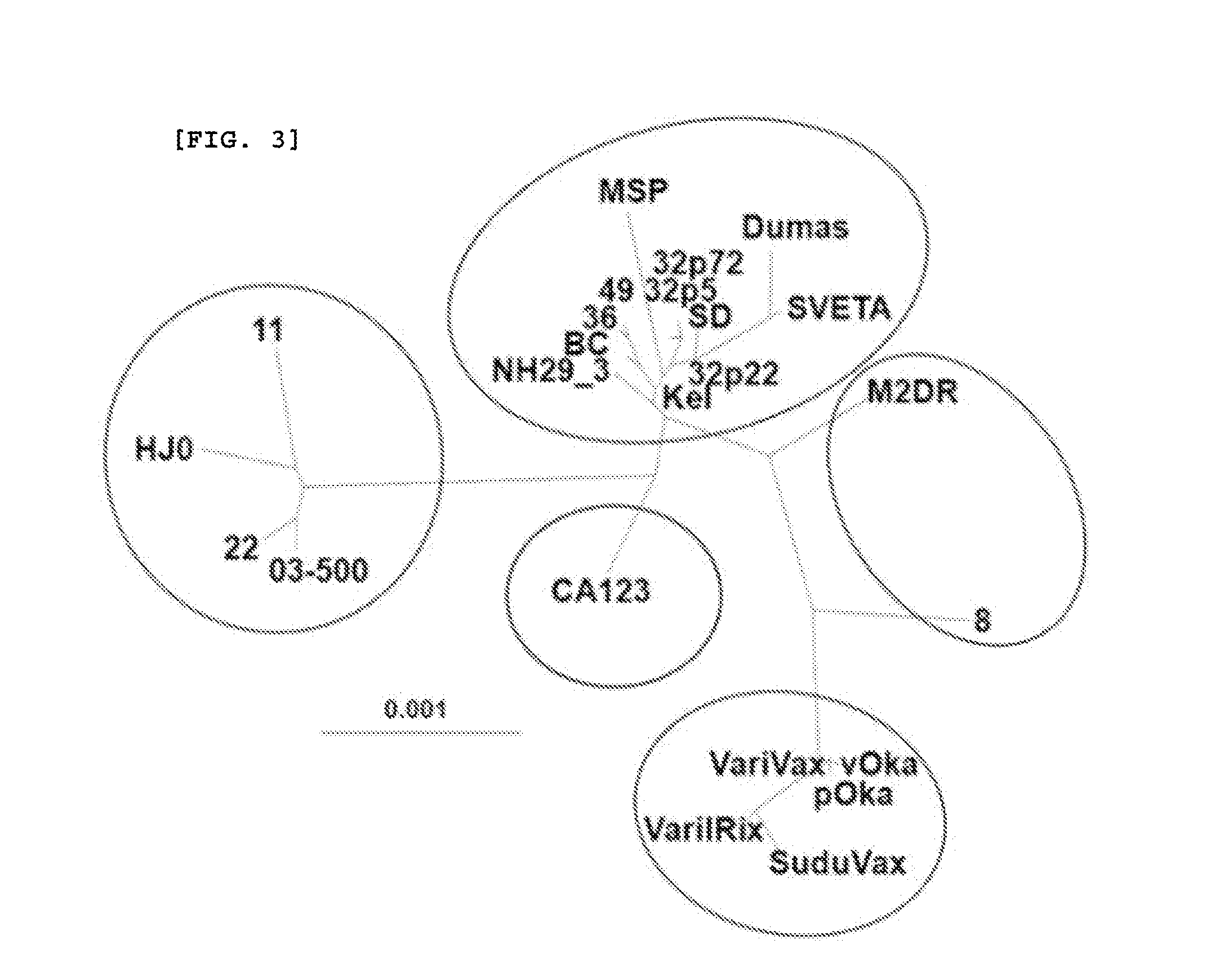
Novel varicella-zoster virus strains, and chicken pox and herpes zoster virus vaccine using same
The present invention relates to novel Varicella-zoster virus strain and to a chicken pox and herpes zoster virus vaccine using the same. More particularly, the present invention relates to genome DNA of VZV MAV / 06 strain isolated from a Korean patient, to an open reading frame (ORF) thereof, to a protein coded by the genome DNA of VZV MAV / 06 strain and the ORF thereof, and to a vaccine composition which contains the protein as an active ingredient.
Owner:MOGAM BIOTECH RES INST
High-efficient antiviral medicament composition in chickweed as well as preparation method and use thereof
InactiveCN101371861ASafe broad-spectrum antiviral drug actionHigh-efficiency broad-spectrum antiviral drugsAntiviralsSynthetic polymeric active ingredientsCondyloma virusHighly pathogenic
The invention discloses a highly effective antiviral medicinal composition of chickweed, a preparation method and an application thereof. Presently, highly effective, safe and universal antiviral medicine is lacking all over the world. A plant, chickweed, or other stellaria plant is extracted, by two resin adsorption methods, one water-alcohol extraction and ultra-filtration, into a dark brown composition that has a molecular distribution ranging from 2,000 to 900,000 Dolton and comprises total sulfate peptidoglycan phenolic acidic components and flavonoid components; the composition is used as a safer, more highly effective universal antiviral natural medicine. Total peptides account to 15-25 percent of the chemical structures of dark brown total sulfate peptidoglycan phenolic-acidic components, and include totaling 17 amino acids by proportion: aspartic acid, glutamic acid, serine, histidine, cystine, methionine, isoleucine; the polysaccharides essentially consist of glucose, galactose and arabinose and form sulfate polysaccharides. The composition can be used for treating virus diseases, including AIDS virus, hepatitis virus, respire virus such as influenza virus including highly pathogenic bird flu virus, para influenza virus and adenovirus, and papovavirus, enterovirus, mumps virus, herpes simplex virus, herpes zoster virus, and varicella-zoster virus, and on the like, does not show toxicity, and can be prepared into more than 10 medicinal dosage forms and healthcare products, and the production process does not cause pollution to the environment.
Owner:朱耕新
Vaccine adjuvant comprising lipopeptide-inserted liposome as effective ingredient and use thereof
PendingCN110996999ASsRNA viruses negative-senseSsRNA viruses positive-senseChickenpoxHerpes zoster virus
The present invention relates to a recombinant herpes zoster vaccine comprising liposome and lipopeptide and a method for preparing the same. More particularly, a vaccine composition according to thepresent invention, prepared using Lipo-Pam, which is a composite adjuvant comprising a liposome and various kinds of lipopeptides, and a varicella-zoster virus gE antigen, a Japanese encephalitis virus gE antigen, or a seasonal inactivated influenza virus antigen, highly induces a cell-mediated immune response as well as a humoral immune response so that the composition of the present invention can be commercially useful.
Owner:株式会社车疫苗研究所
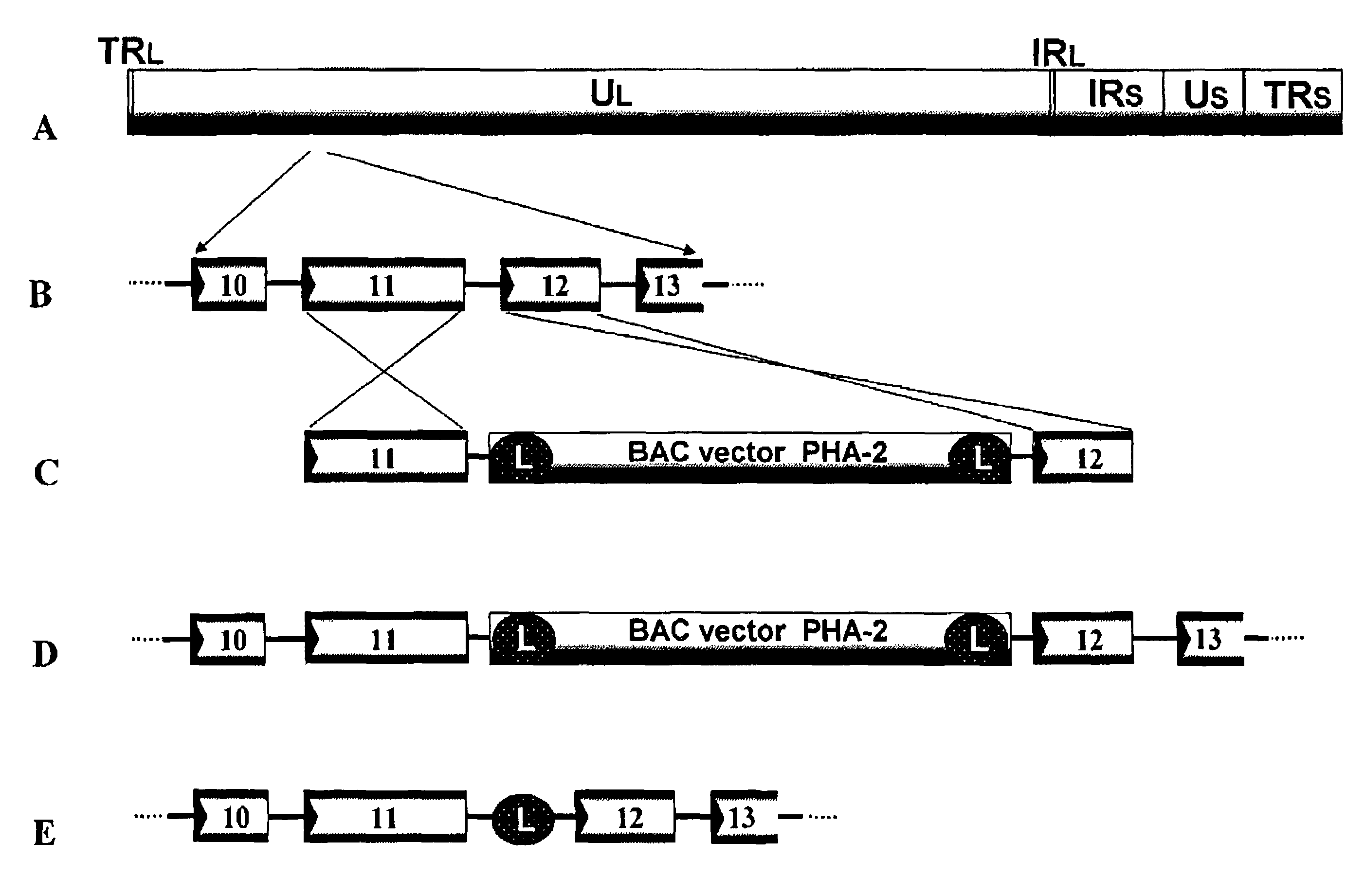
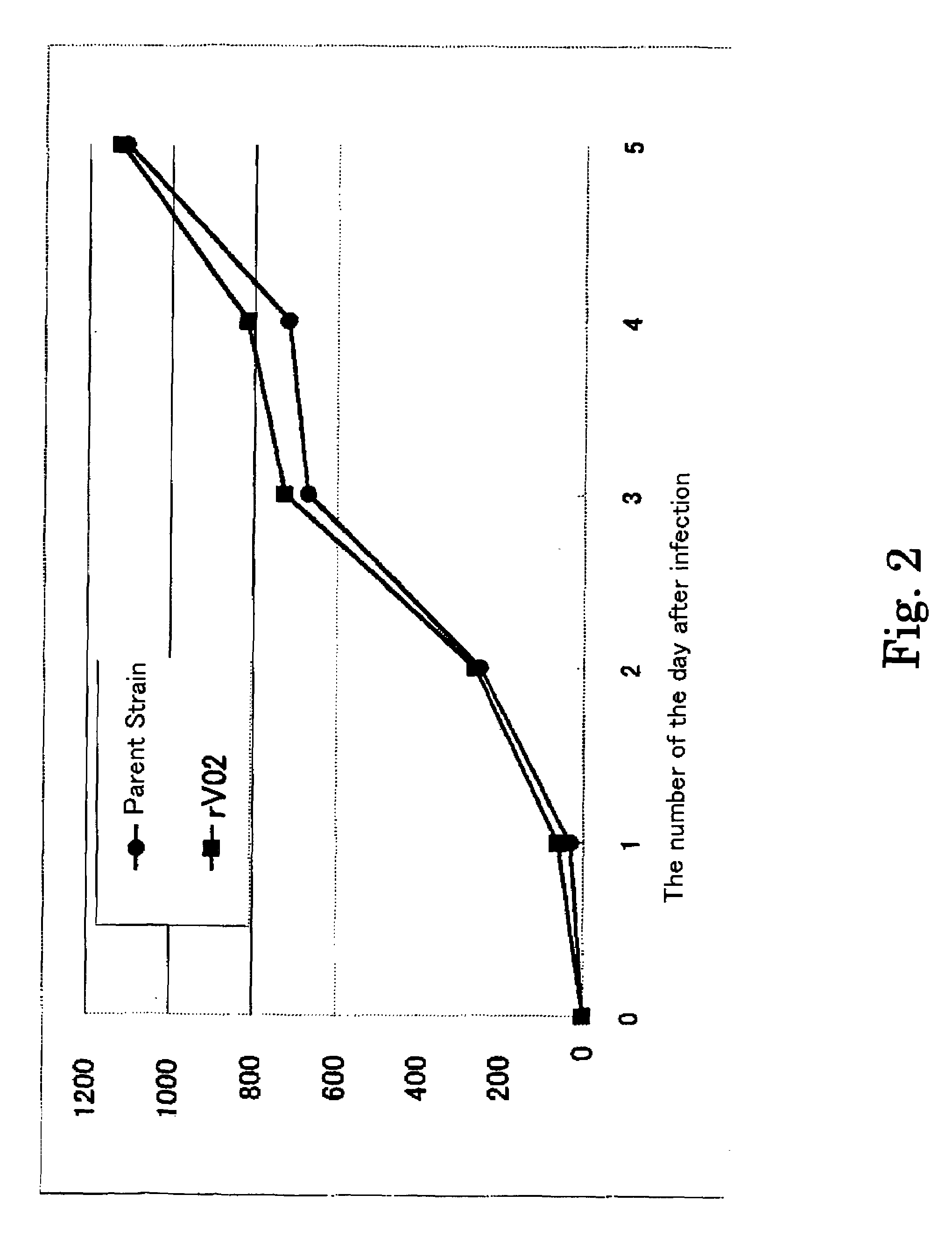
Recombinant Varicella-Zoster Virus
InactiveUS20110189233A1Improve accuracySecuring and ensuring effectivenessBacteriaVirus peptidesVaricella zoster virusGenome
A recombinant varicella-zoster virus; a process for producing the same; a pharmacological composition containing recombinant varicella-zoster virus; a vector containing a genomic gene of varicella-zoster virus and BAC vector sequence; cells containing the above vector; a fragment capable of homologous recombination with a genome of varicella-zoster virus; and a nucleic acid cassette containing the BAC vector sequence. For these, there is provided a process for producing recombinant varicella-zoster virus, comprising use of the BAC vector sequence.
Owner:THE RES FOUND FOR MICROBIAL DISEASES OFOSAKA UNIV
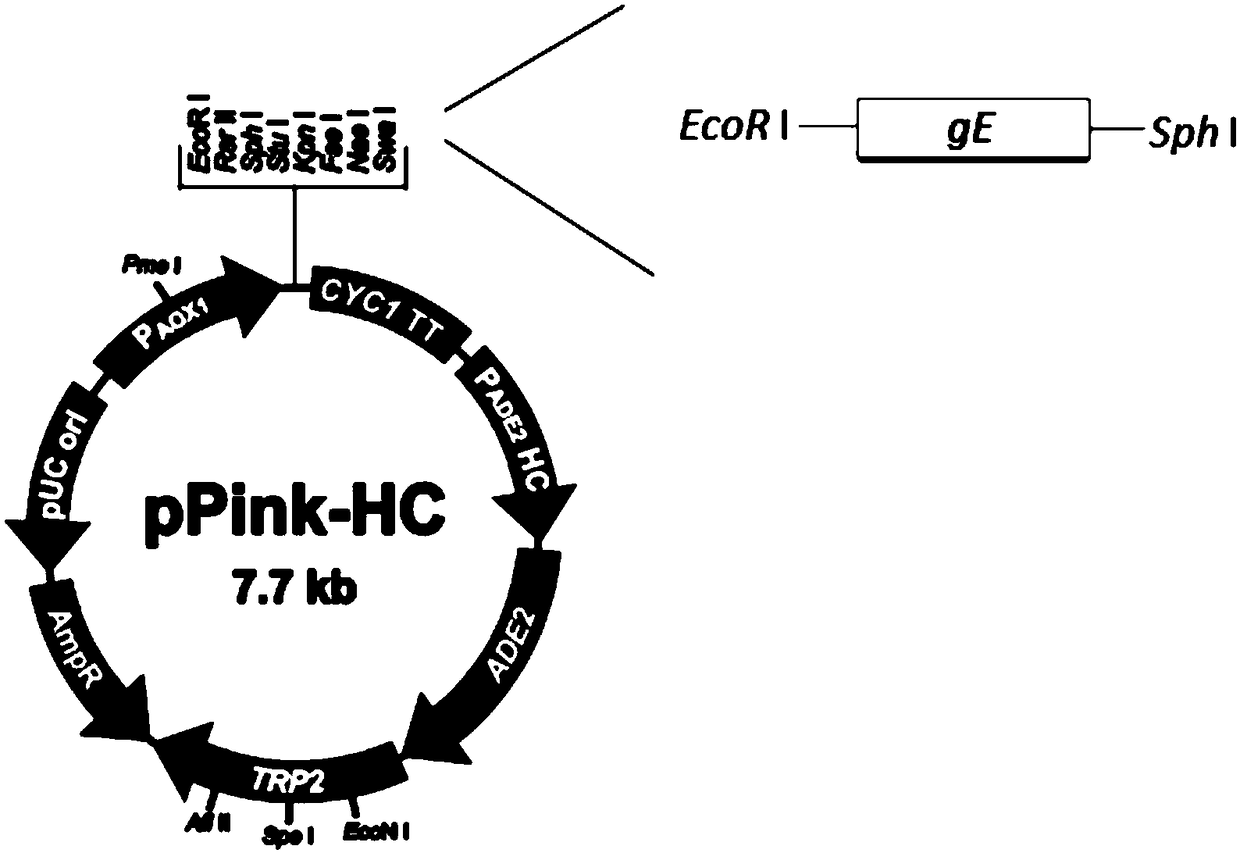
Expression method of VZV glycoprotein to pichia pastoris and application of expression method
PendingCN108383897AOptimize purification stepsHigh titerViral antigen ingredientsVirus peptidesPichia pastorisProtein target
The invention discloses an expression method of VZV glycoprotein to pichia pastoris and application of the expression method. The expression method comprises the following steps: constructing an expression vector, screening a positive transformant, linearizing a yeast expression vector, preparing a yeast electrotransformation competence, identifying a recombinant, expressing recombinant yeast andidentifying target protein. The expression method disclosed by the invention can be used for successfully expressing glycoprotein E of varicella-varicella-zoster virus and the obtained protein has good immunogenicity; the pichia pastoris can be efficiently and stably expressed.
Owner:BRAVOVAX
Application of polycyclic polyketides in preparation of anti-herpesvirus drugs
The invention discloses application of polycyclic polyketides in the preparation of anti-herpesvirus drugs. According to the invention, the polyketides are found to inhibit diseases caused by herpes simplex virus (HSV) type I, HSV-2, varicella zoster virus (VZV) and cytomegalo virus (CMV) infection. Compared with drugs such as acyclovir on the market, the compound in the invention exhibits considerable activity, but the mechanism of action is different, and the compound overcomes the drug resistance of the drugs currently on the market. Thus, these compounds have good application prospects inthe treatment of diseases caused by HSV-1, HSV-2, VZV and CMV infection.
Owner:JINAN UNIVERSITY
Varicella-zoster virus r-gE fusion protein, recombinant varicella-zoster vaccine and preparation method and application of varicella-zoster virus r-gE fusion protein and recombinant varicella-zoster vaccine
ActiveCN113683704AEffective and significant immunityEffective and significant humoral immunitySsRNA viruses negative-senseAntibody mimetics/scaffoldsDiseaseAntigen
The invention provides a varicella-zoster virus r-gE fusion protein, a recombinant varicella-zoster vaccine and a preparation method and application of the varicella-zoster virus r-gE fusion protein and the recombinant varicella-zoster vaccine. An antigen of the recombinant varicella-zoster vaccine is the varicella-zoster virus r-gE fusion protein, the recombinant varicella-zoster vaccine can be an adjuvant compatible varicella-zoster vaccine which is formed by compatibility of an r-gE fusion protein and an adjuvant, or a recombinant adenovirus vector varicella-zoster vaccine which is formed by gene recombination of the r-gE fusion protein and an adenovirus vector or an adenovirus vector containing a TLR receptor stimulant. The recombinant varicella-zoster vaccine is suitable for preventing or improving varicella-zoster and / or post-varicella-zoster neuralgia; the varicella-zoster virus r-gE fusion protein can further be used for preparing a respiratory syncytial virus and varicella-zoster combined vaccine, and the combined vaccine is formed by compatibility of the r-gE fusion protein, RSV Pre-F protein mixed liquor and the adjuvant; and the prepared combined vaccine can be used for preventing or improving respiratory syncytial virus infection diseases and varicella-zoster.
Owner:ANHUI ZHIFEI LONGCOM BIOPHARM CO LTD +2
Folium artemisiae argyi plaster for treating herpes zoster
InactiveCN104415075AEasy to prepareNo side effectsHydroxy compound active ingredientsInanimate material medical ingredientsSide effectPoultice
Owner:李霞
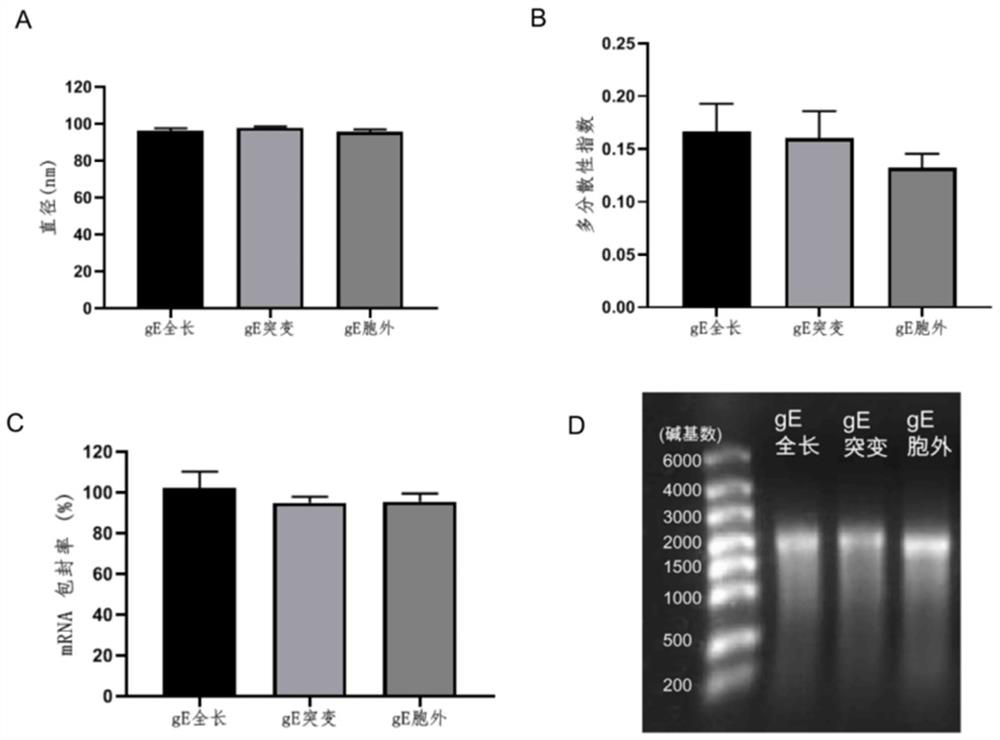
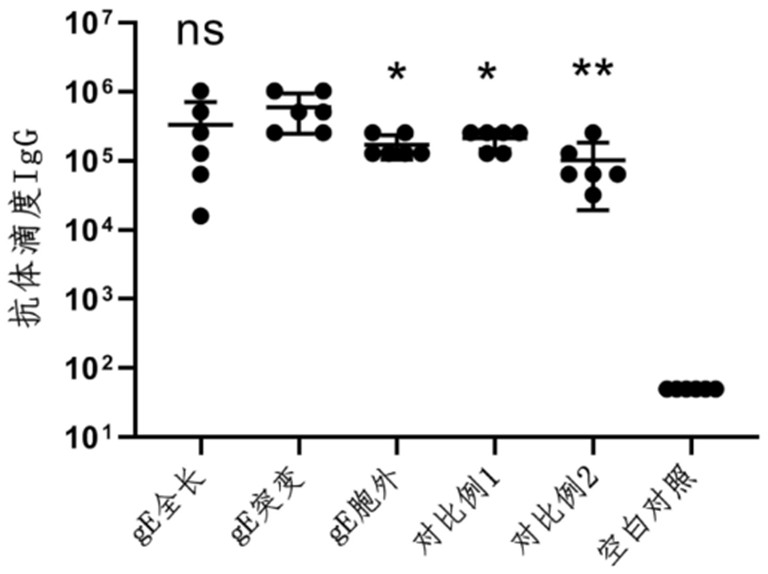
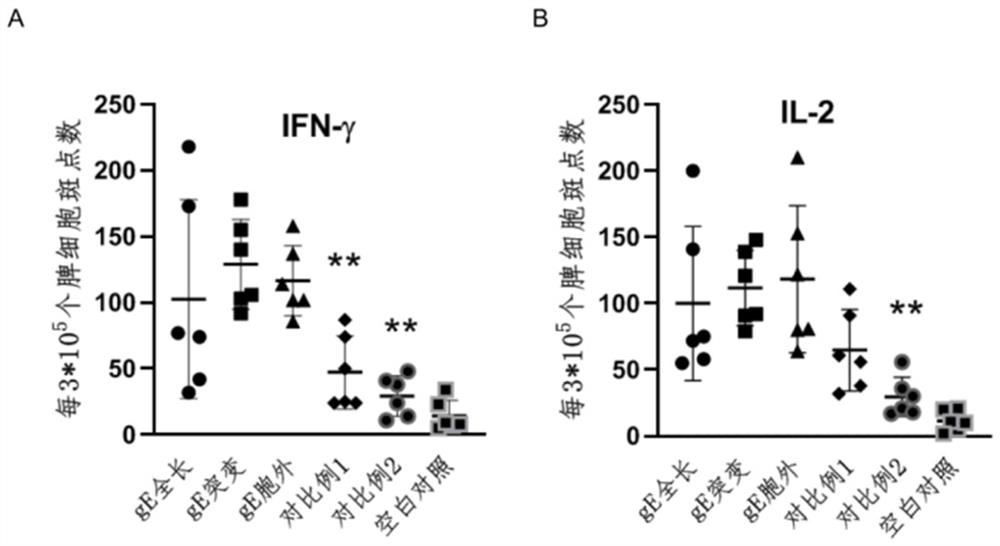
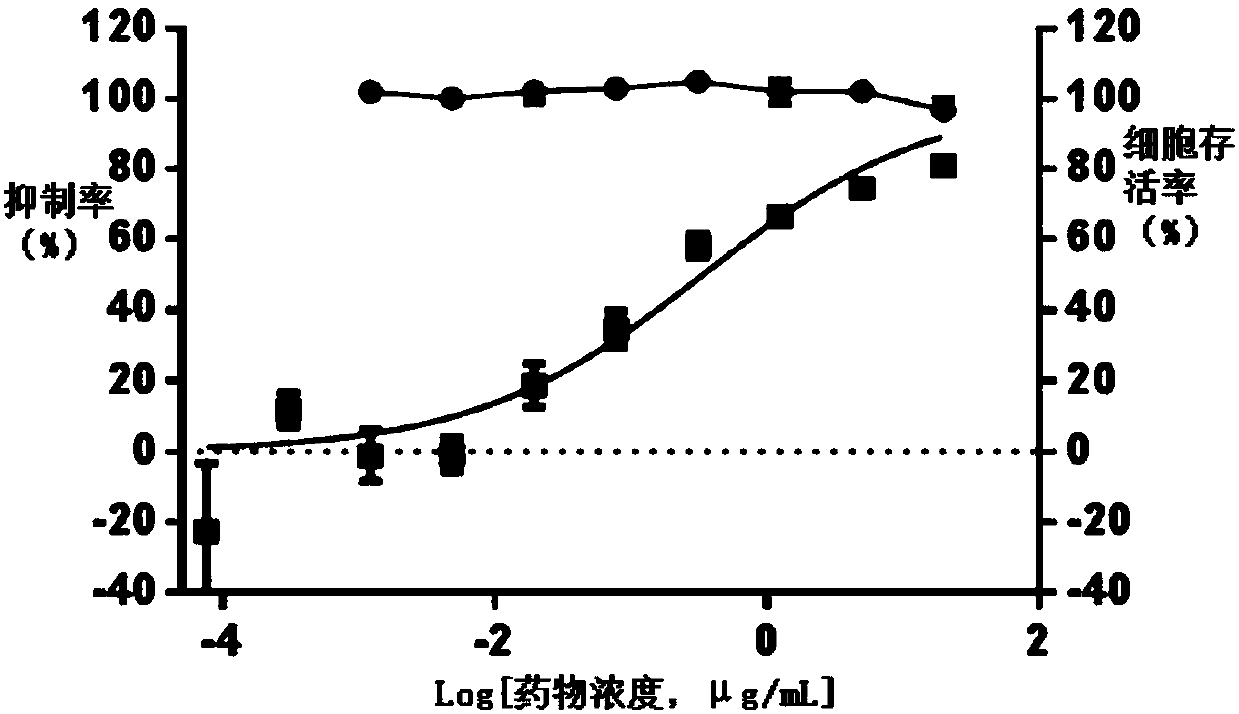
Varicella-herpes zoster mRNA vaccine composition, and preparation method and application thereof
PendingCN114081943AReduce manufacturing costSave uniformityNervous disorderViral antigen ingredientsChickenpoxHerpes zoster virus
The invention provides a varicella-herpes zoster mRNA vaccine composition, and a preparation method and application thereof. The vaccine composition comprises a messenger ribonucleic acid (mRNA) sequence for coding varicella-herpes zoster virus glycoprotein E, a derivative sequence of the messenger ribonucleic acid (mRNA) sequence and lipid nanoparticles (LNP), and the messenger ribonucleic acid (mRNA) sequence is prepared into particles with the diameter of 20-400 nanometers through microfluidic equipment. The vaccine composition can specifically enhance humoral immune response and cellular immune response against varicella-herpes zoster glycoprotein E, can be used as a varicella vaccine which does not cause latent infection of vaccine strains, and can also be used as a herpes zoster vaccine with unlimited productivity. All the components in the vaccine composition can be widely obtained, so that the vaccine cost is effectively reduced, and the vaccine yield is increased.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Pien Tze Huang and new application of preparation thereof in preparing drug for treating herpes zoster
InactiveCN108042589ADefinite curative effectImprove clinical symptomsAntiviralsMammal material medical ingredientsAnti virusHerpes zoster virus
The invention belongs to the field of traditional Chinese medicine, and particularly relates to Pien Tze Huang and new application of a preparation thereof in preparing a drug for treating herpes zoster. An in-vitro experiment shows that Pien Tze Huang can dose-dependently inhibit duplication of chicken varicella-herpes zoster viruses (VZV). A clinical study finds that the combination of Pien Tzehuang and anti-virus drugs of valaciclovir and acyclovir has an obvious curative effect on alert period zoster viruses due to liver yu heat from the line, improvement of clinical symptoms of a patientcan be promoted, incrustation of herpes can be quickly promoted, new herpes can be stopped from generating, pain can be relieved, and the treatment time can also be shortened; besides, the incidenceof postherpetic neuralgia and the occurrence rate of adverse reactions occurring after treatment can also be lowered, and the clinical application has obvious advantages.
Owner:ZHANGZHOU PIEN TZE HUANG PHARM
Preparation method of herpes zoster vaccine
PendingCN110237248AImprove immune activityHigh similarityAntibody mimetics/scaffoldsViral antigen ingredientsDisseminated herpes zosterImmunocompetence
The invention belongs to the field of virus vaccines and discloses a preparation method of a herpes zoster vaccine. Through combination use of a glycoprotein E envelope outer structural domain of the herpes zoster and a glycosylation locus area of envelope glycoprotein I, the herpes zoster vaccine is established, the envelope glycoprotein I, interacting with glycoprotein E of the herpes zoster, on a herpes zoster virus envelope, of the herpes zoster virus assists the glycoprotein E envelope outer structural domain of the herpes zoster in preparation of the herpes zoster vaccine, the glycosylation modification level of a vaccine protein complex is improved, the similarity of the vaccine protein complex and a natural virus envelope is improved, and the immunocompetence of the herpes zoster vaccine is improved.
Owner:DALIAN NATIONALITIES UNIVERSITY
Reagent device and method for detecting varicella-zoster-virus antibody
ActiveCN103185794AStrong specificityHigh sensitivityColor/spectral properties measurementsChickenpoxVaricella-Zoster Virus Antibody
The invention provides a reagent device and a method used for detecting varicella-zoster-virus antibodies. The reagent device has a long strip shape, and has a base body comprising 8 hole positions and a handle positioned on one end of the base body. From the end proximal to the handle, the 8 hole positions are sequentially a sample hole, an auxiliary agent hole, an enzyme conjugate hole, a substrate hole, a termination liquid hole, a dilution liquid hole, a reaction hole, and a dilution hole. According to the invention, based on a principle of enzyme-linked immunoassay, varicella-zoster-virus antibody is detected by using the reagent device. The method is an independent single-person analysis and detection method. The device can be used in cooperation with a corresponding specific analysis instrument. During a detection process, detection reagents or samples are injected by using a full-automatic precise dosing device. The device and the method have the advantages of automatic operation, precise dosing, high detection result accuracy, high detection result precision, and wide application prospect.
Owner:SHENZHEN YHLO BIOTECH
Pharmaceutical composition and application thereof
PendingCN112972671AStrong immunostimulatory effectEnhance immune stimulationAntibacterial agentsOrganic active ingredientsDiseaseAdjuvant
The present invention provides a pharmaceutical composition. The pharmaceutical composition comprises i) a herpes gE protein, an active fragment of the protein, a variant of the protein, or a mixture of at least two of the herpes gE protein, the active fragment of the protein and the variant of the protein; Ii) an immunostimulatory composition, wherein the immunostimulatory composition comprises a saponin and a CpG oligodeoxynucleotide, or the immunostimulatory composition comprises an adjuvant comprising the saponin, and the CpG oligodeoxynucleotide. The invention provides application of the pharmaceutical composition in preparation of a medicine for preventing and / or treating chicken pox-herpes zoster virus infection and / or chicken pox-herpes zoster virus mediated diseases. The pharmaceutical composition provided by the invention achieves an unexpected technical effect and can mediate stronger immune response.
Owner:JIANGSU THERAVAC BIO PHARMA
Chickenpox-zoster virus glycoprotein E gene expression vector, recombinant yeast strain thereof and application thereof
PendingCN109234302AAchieve secretory expressionOptimize purification stepsFungiVirus peptidesPichia pastorisChickenpox
The invention discloses a chickenpox-zoster virus glycoprotein E gene expression vector, a recombinant yeast strain thereof and the application thereof. The vector is chickenpox as shown in SEQ ID NO:1. The complete gene sequence of herpes zoster virus glycoprotein E and the linker of pGAPZ alpha A. The expression system of the Pichia pastoris disclosed by the invention can successfully express chickenpox. The glycoprotein E of herpes zoster virus lays a foundation for the following immunogenicity detection and high expression in Pichia pastoris as well as the development of varicella zostervaccine.
Owner:BRAVOVAX
Genital Herpes Vaccine
Owner:CHANGCHUN BCHT BIOTECH
Method for preparing antigen tablet for detecting varicella-zoster virus antibody
ActiveCN104371981AComplete formFully distributedViruses/bacteriophagesBiological testingVaricella antibodyChickenpox
The invention provides a method for preparing an antigen tablet for detecting the varicella-zoster virus antibody. According to the method, a varicella-zoster virus VZV84-7 strain is used as an infected virus strain to prepare the antigen tablet; compared with an Oka strain infected diploid cell, the virus has the characteristics that the virus CPE is in a dispersed manifestation and is widely distributed on the surface of the cell after infecting a sensitive cell; a Vero cell is selected as a cell matrix, and can be dispersed more sufficiently than the diploid cell, and the cellular morphology is more completed; after the infected virus is reproduced, the CPE widely occurs to the cell, so that observation is facilitated and results can be determined when the virus infected cell is used for preparing the antigen tablet. By adopting the method, the problems of low antigen tablet quality and complicated tablet producing process in varicella-zoster antibody detection by an FAMA method can be solved, the preservation time of the antigen tablet can be prolonged, and conditions are provided to massive preparation of the antigen tablet. Compared with a traditional method, the method has the advantages of simplicity and practicality, the antigen tablet can be frozen at -20-70 DEG C and stored for over one year without remarkable quality change, and the result of tablet microscopic examination is not influenced.
Owner:CHANGCHUN KEYGEN BIOLOGICAL PROD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com