Pharmaceutical composition and application thereof
A composition and drug technology, applied in the field of chickenpox or post-herpetic neuralgia, prevention of herpes zoster, can solve problems such as huge differences in effects, and achieve the effects of excellent effects, superior immune stimulation, and high antibody levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1 Preparation method of recombinant herpes zoster vaccine composition
[0056] 1.1 Preparation of herpes gE protein: the amino acid sequence is shown in SEQ ID NO:1.
[0057] References Thomsson E, Persson L et al. report in "Journal of Virological Methods" 2011, volume 175, phase 1, pages 53-59, the specific steps are as follows:
[0058] Optimize the nucleic acid sequence according to the target protein sequence so that its codons conform to the mammalian expression system, and synthesize the target gene; connect the synthesized target gene to the pcDNA3.1(+) plasmid by enzyme digestion and ligation, and transform Top 10 competent, pick positive single clones, and perform sequencing verification on positive single clones; a large number of single clones are amplified, and a large number of plasmids that meet cell transfection are extracted with an endotoxin-free plasmid extraction kit; Transfection method: Transfect CHO suspension cells with the plasmid. Wh...
Embodiment 2
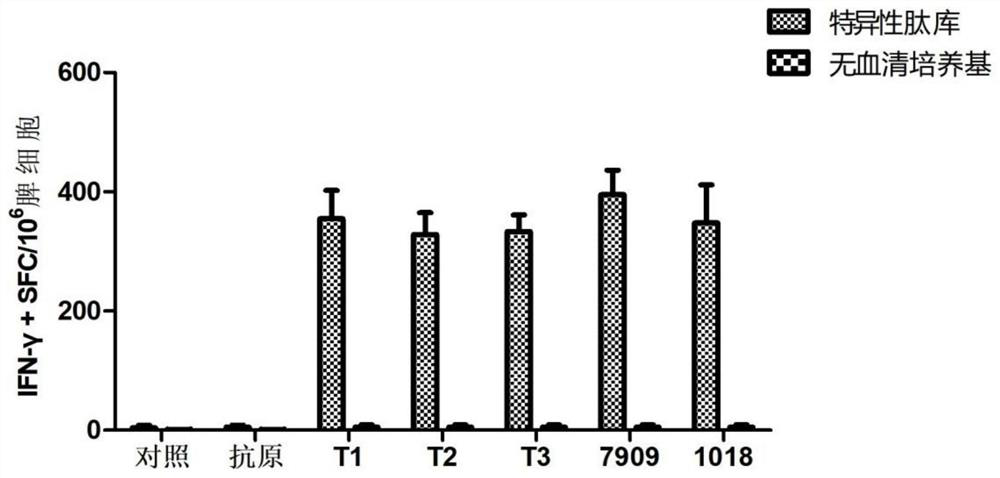
[0064] Example 2 Screening experiment of CPG oligodeoxynucleotides
[0065] 2.1 Experimental animals:
[0066] C57BL / 6(N) mice, 42 females, 5 weeks old, were purchased from Shanghai Slack Experimental Animal Co., Ltd.
[0067] 2.2 Reagent materials:
[0068] The herpes gE stock solution obtained in Example 1 was diluted to 50 μg / mL with PBS solution (purchased from Hyclone), and the CpG obtained in Example 1 was diluted to 100 μg / mL with PBS solution.
[0069] 2.3 Experimental grouping:
[0070] See Table 2, the injection volume of each mouse was 100 μL / mouse, and the control group was injected with PBS solution 100 μL / mouse.
[0071] Table 2 Grouping of experimental animals and the amount injected in each group
[0072]
[0073] 2.4 Animal immunity:
[0074] All groups were injected intramuscularly every 2 weeks, and the inoculation site was the right rear thigh, and the mice were administered twice in a row, that is, at week 0 and week 2, respectively, and all mice...
Embodiment 3
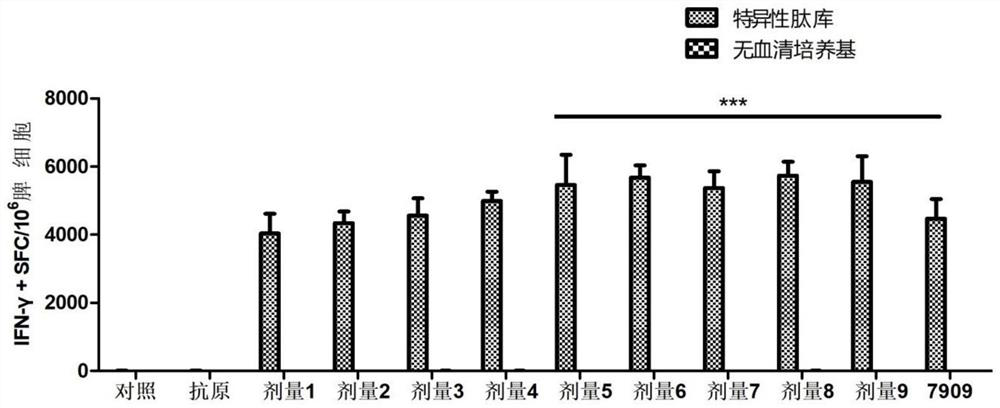
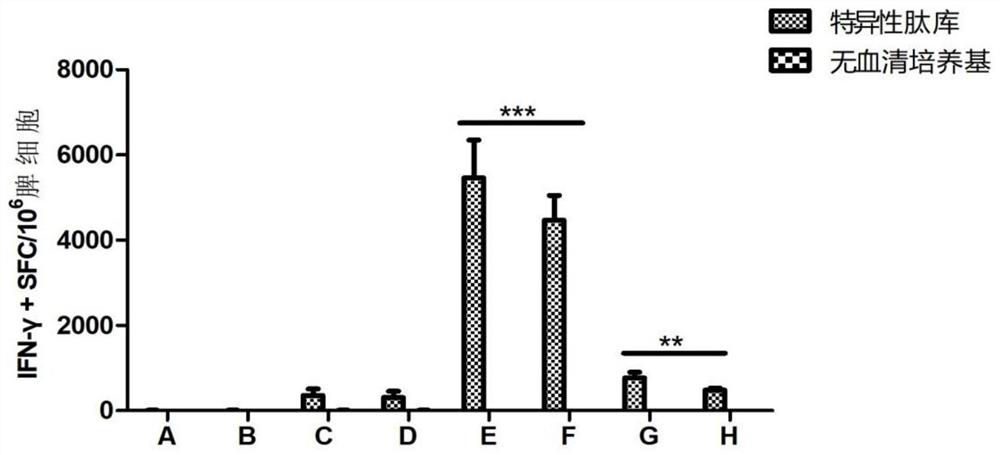
[0084] Example 3 Effects of different doses of immunostimulatory compositions on the efficacy of recombinant herpes zoster vaccine compositions
[0085] 3.1 Experimental animals and model establishment:
[0086] C57BL / 6(N) mice, 72 female, 5 weeks old, were purchased from Shanghai Slack Experimental Animal Co., Ltd.
[0087] 3.2 Reagent materials:
[0088] 1) Herpes gE protein, CpG T1, and CpG 7909 were all obtained from Example 1;
[0089] 2) QS-21 (CAS.NO.A010-023, purchased from BRENNTAG);
[0090] 3) Use PBS solution (purchased from Hyclone) to dilute herpes gE stock solution to 50 μg / mL, use PBS solution to dilute QS-21 to 5 μg / mL, 50 μg / mL, and 100 μg / mL, and use PBS solution to dissolve CpG T1 And dilute to 50μg / mL, 100μg / mL, 2mg / mL respectively, use PBS solution to dissolve CPG7909 and dilute to 100μg / mL for the next step.
[0091] 3.3 Experimental grouping:
[0092] See Table 4, the volume of each injection is 100 μL / monkey. The control group was injected with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com