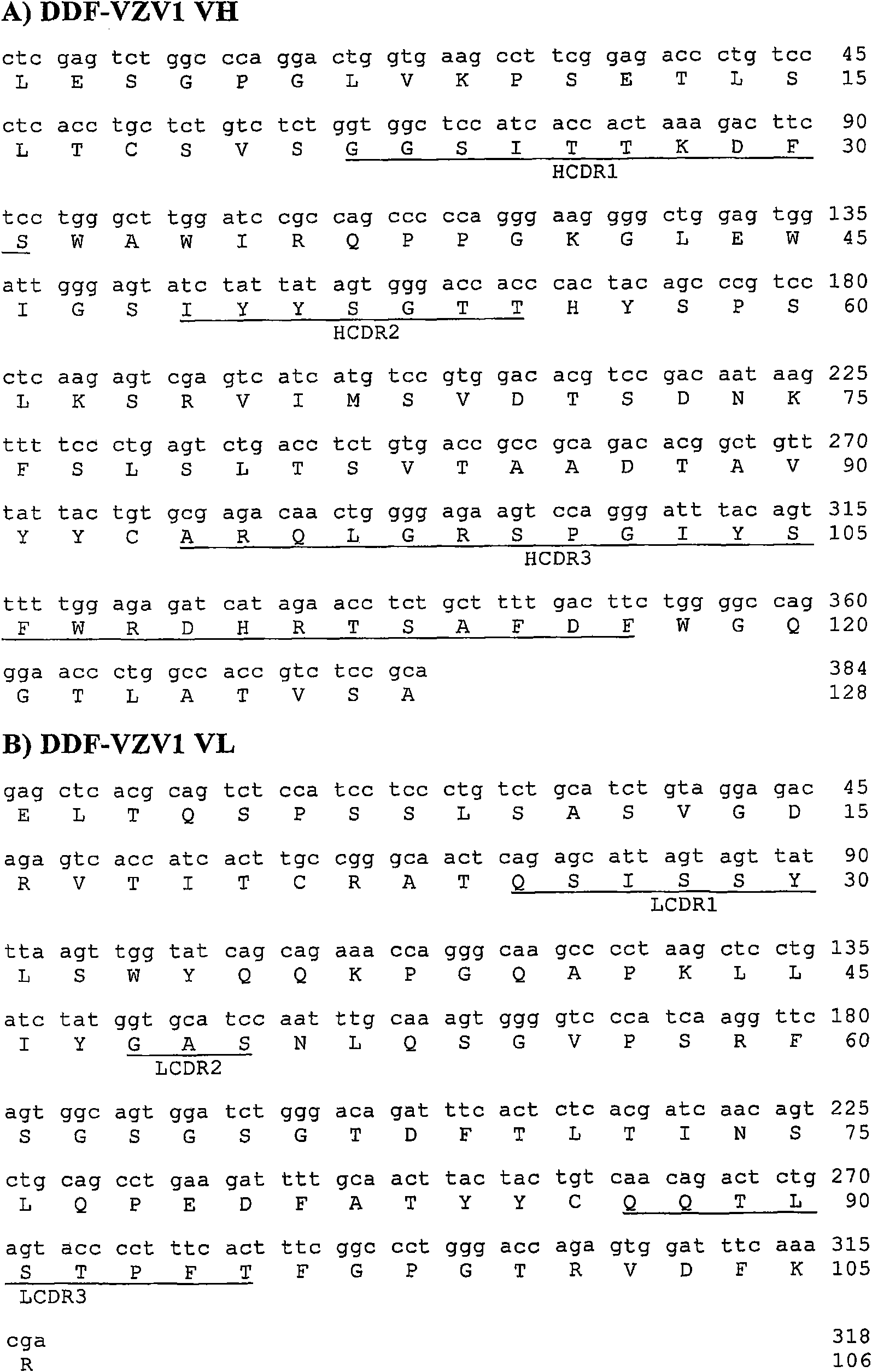Patents
Literature
98 results about "Varicella zoster virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
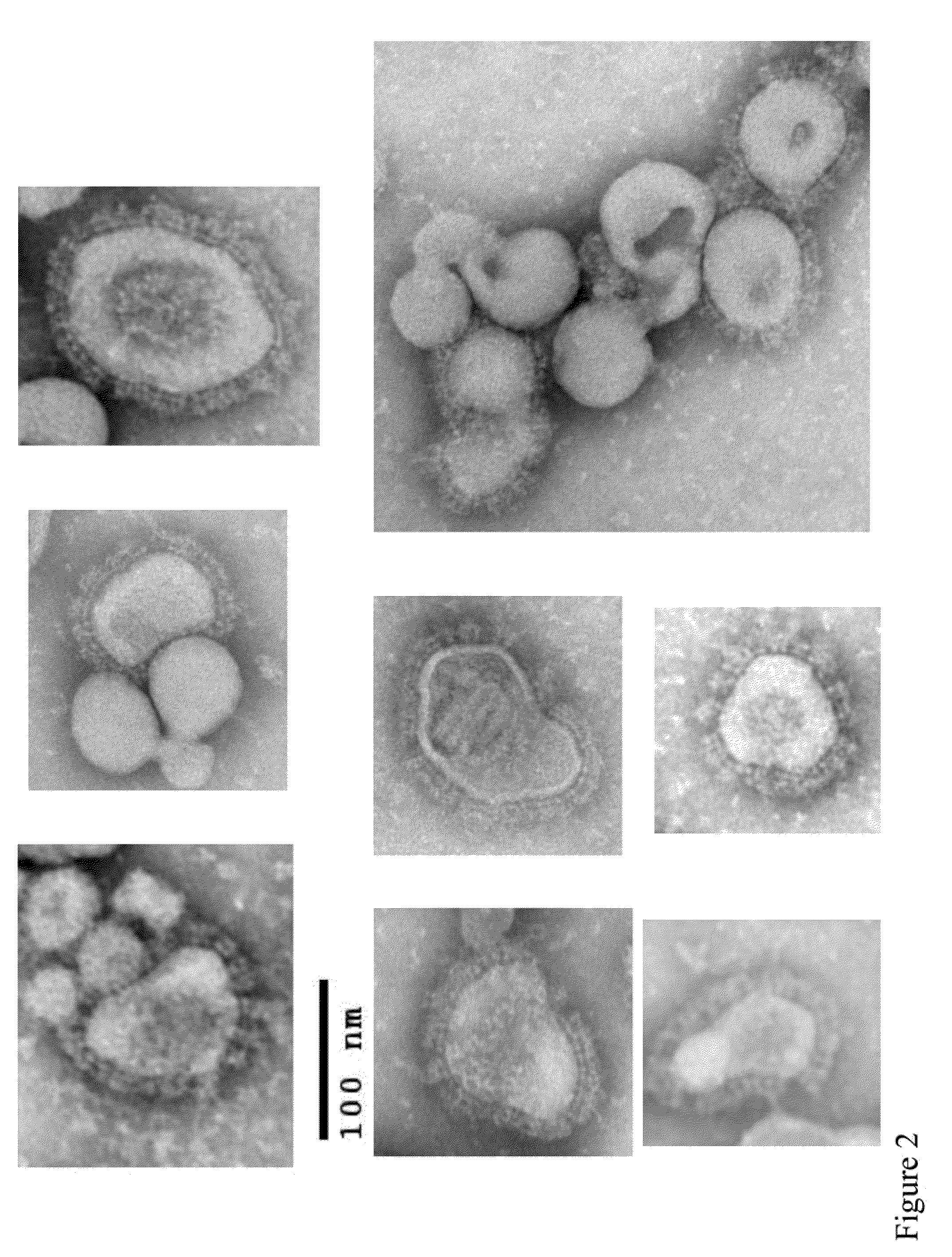
Human alphaherpesvirus 3 (HHV-3), usually referred to as the varicella-zoster virus (VZV), is one of eight herpesviruses known to infect humans. It causes chickenpox (varicella), a disease most commonly affecting children, teens, and young adults, and shingles (herpes zoster) in adults; shingles is rare in children. VZV is a worldwide pathogen known by many names: chickenpox virus, varicella virus, and zoster virus. VZV infections are species-specific to humans, but can survive in external environments for a few hours, maybe a day or two.
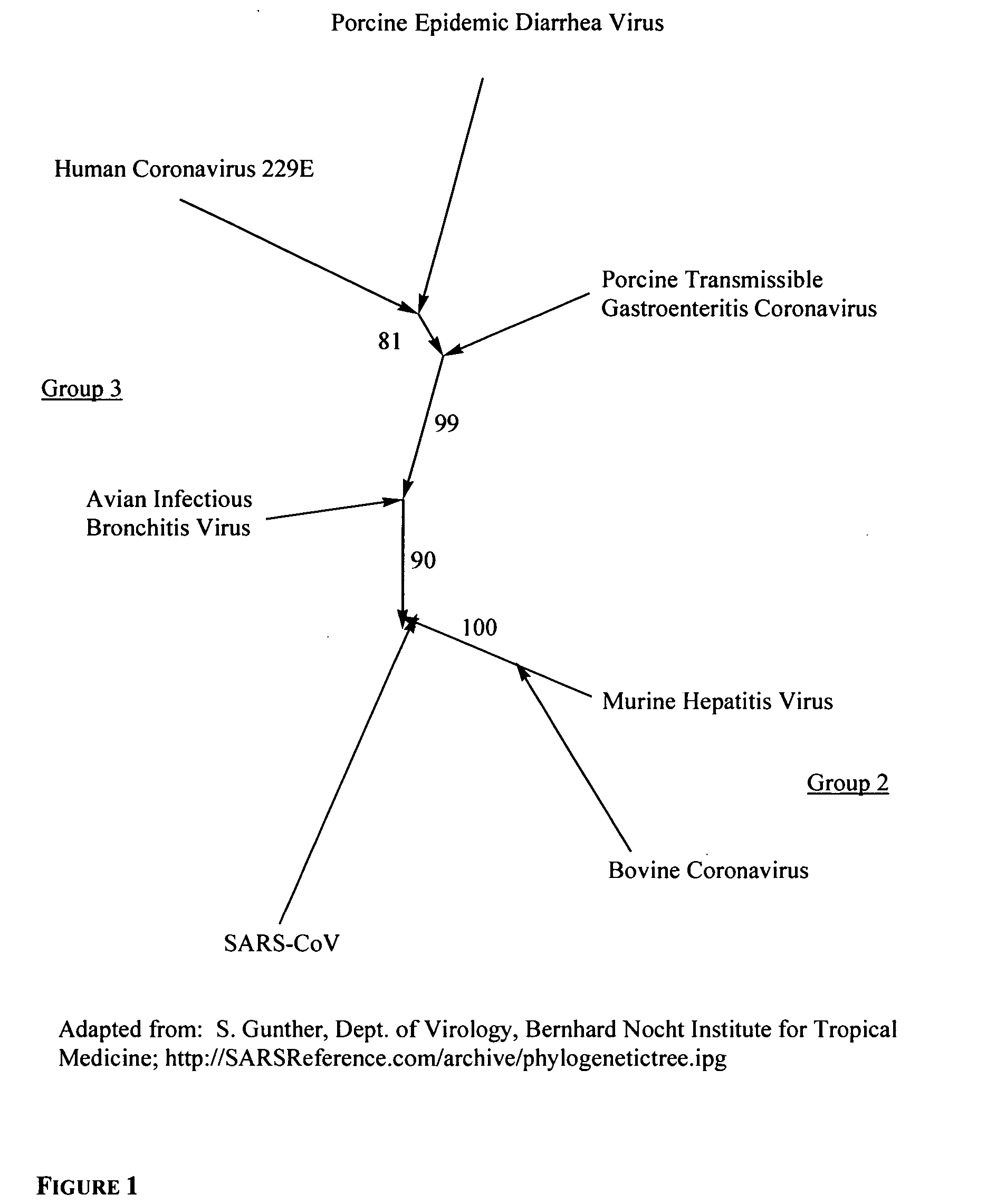
Phospholipids for the treatment of infection by togaviruses, herpes viruses and coronaviruses
InactiveUS20050187192A1BiocidePhosphorous compound active ingredientsHerpes simplex virus DNACompound (substance)
Provided are compounds, methods and pharmaceutical compositions for treating a host, especially a human, infected with a togavirus, herpes virus and / or coronavirus, and in particular SARS-CoV, cytomegalovirus or varicella-zoster virus. The method in one embodiment comprises administering to that host an effective amount of an anti-togavirus, anti-herpes virus and / or anti-coronavirus phospholipid or a pharmaceutically acceptable salt or prodrug thereof. The phospholipid compound is, e.g., a 3-alkylamido-2-alkoxypropylphosphocholine compound or salt thereof. The compound may be administered alone or in combination and / or alternation with one or more other anti-viral agents.
Owner:KUCERA PHARMA
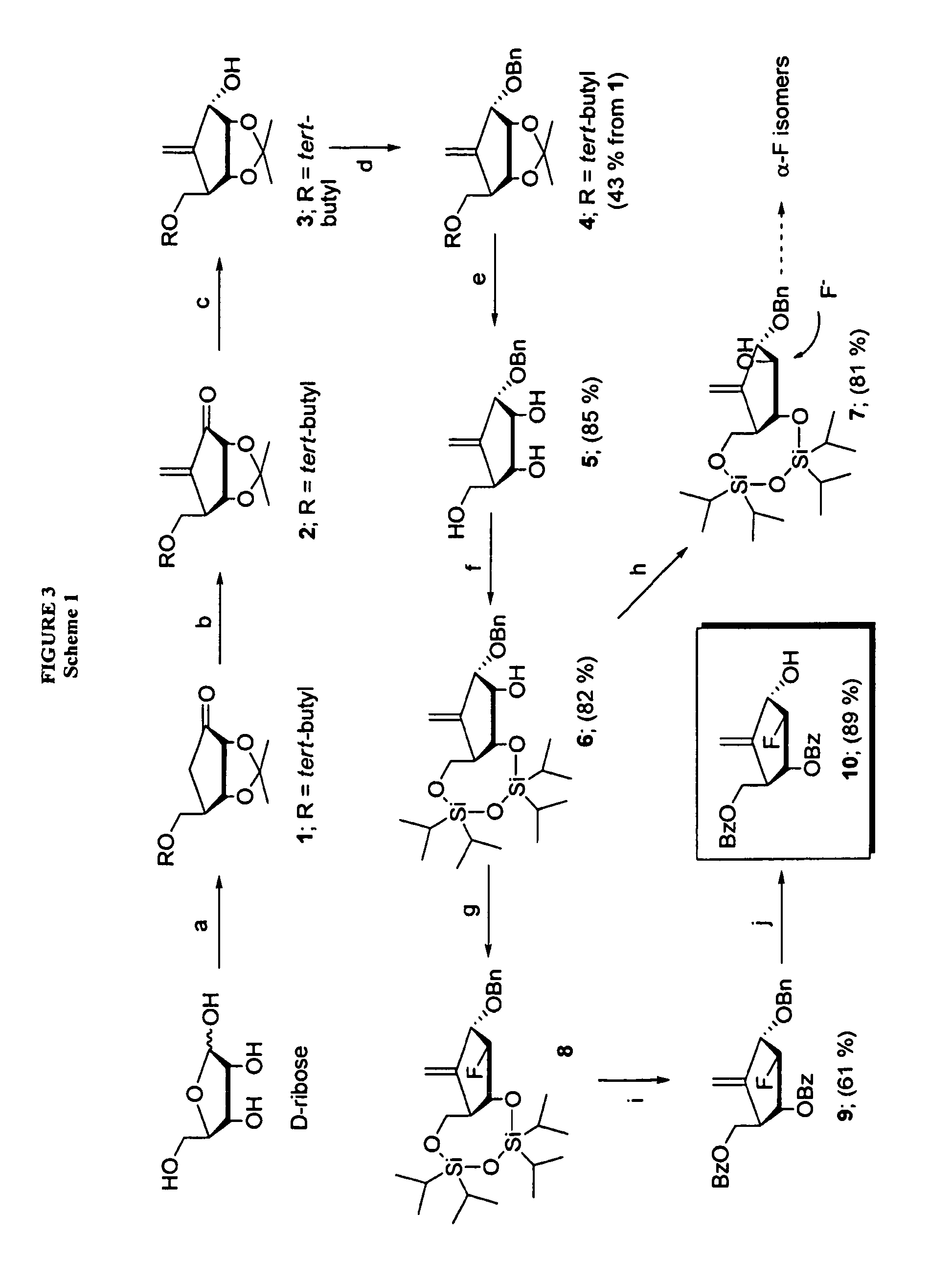
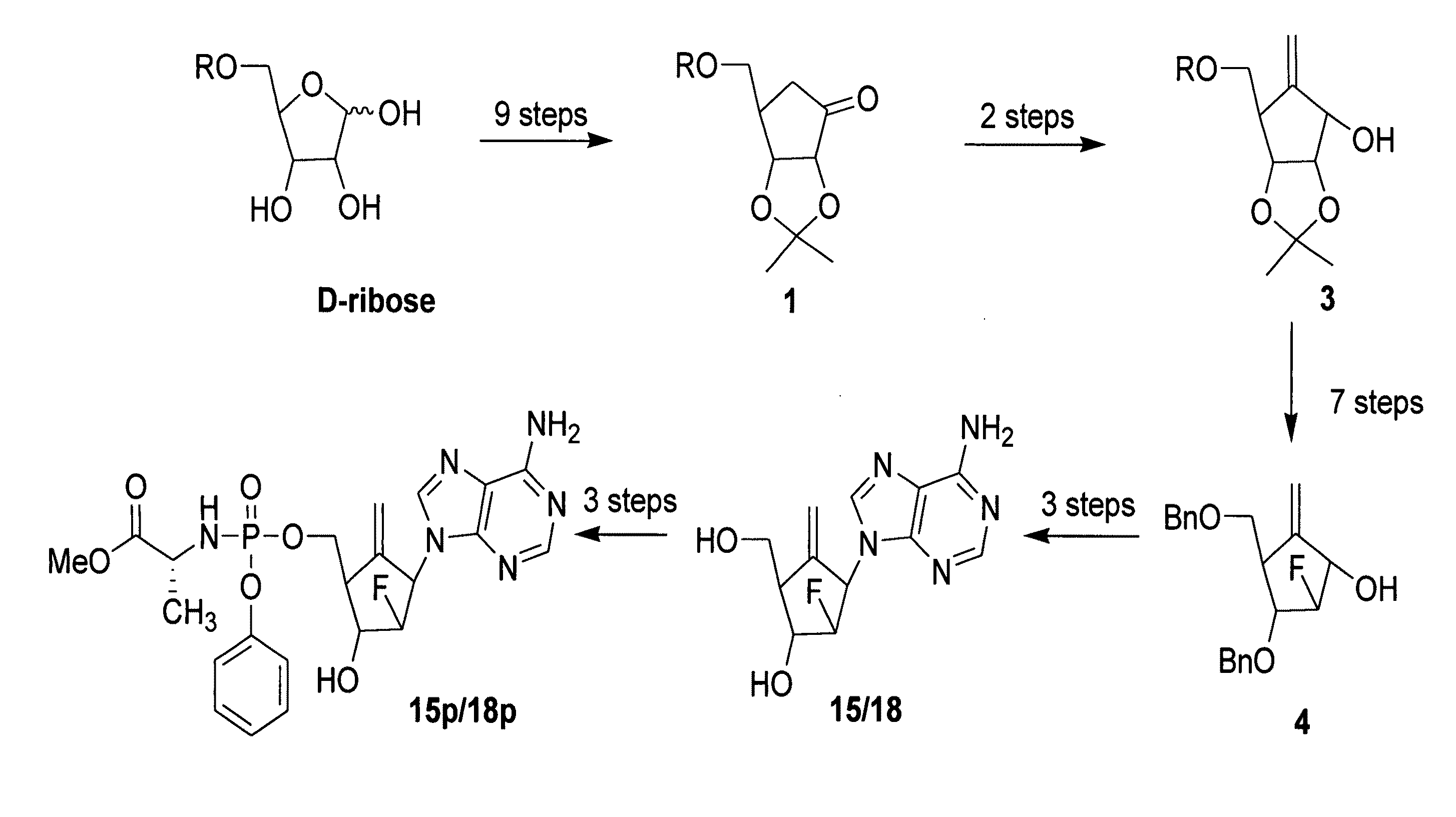
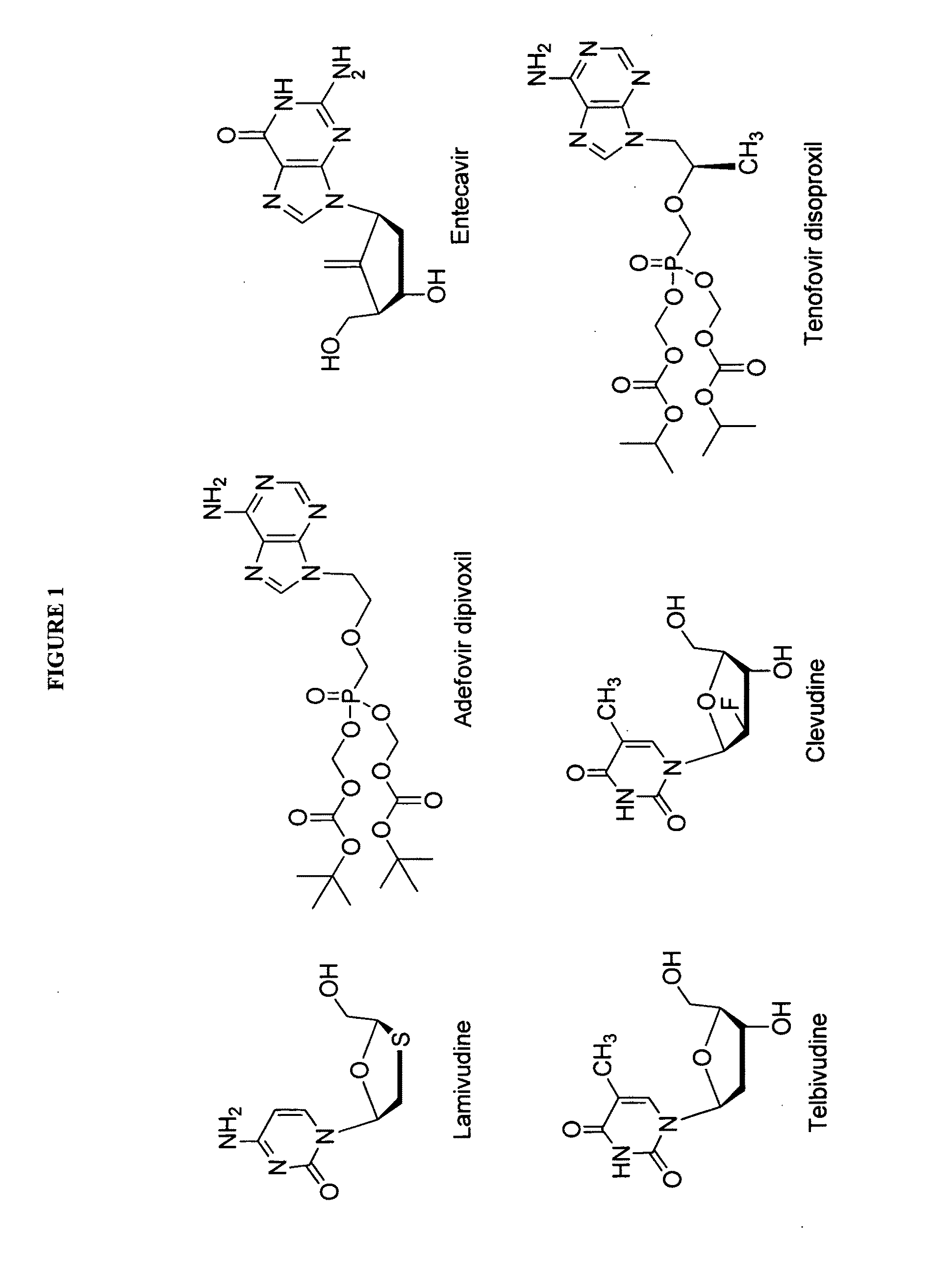
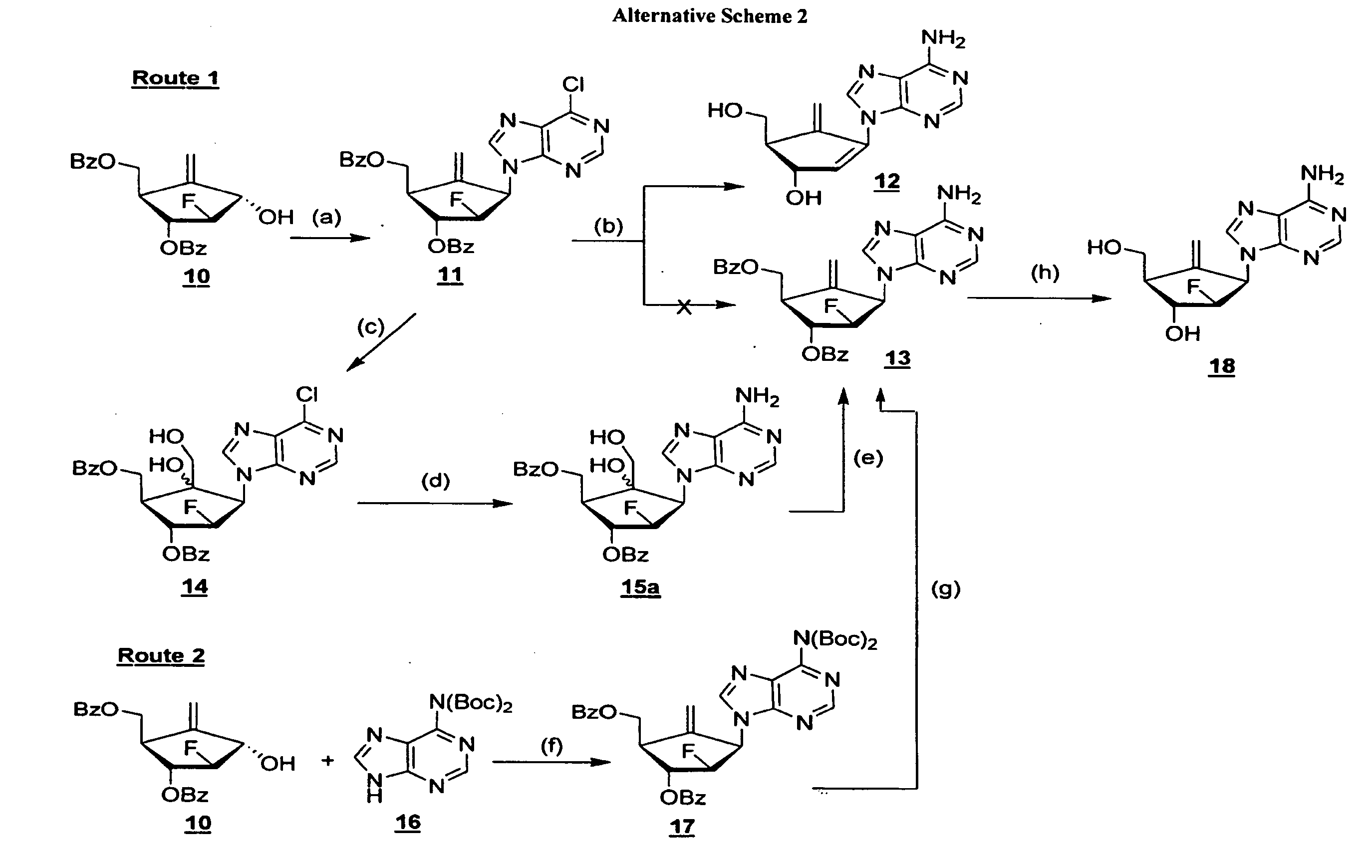
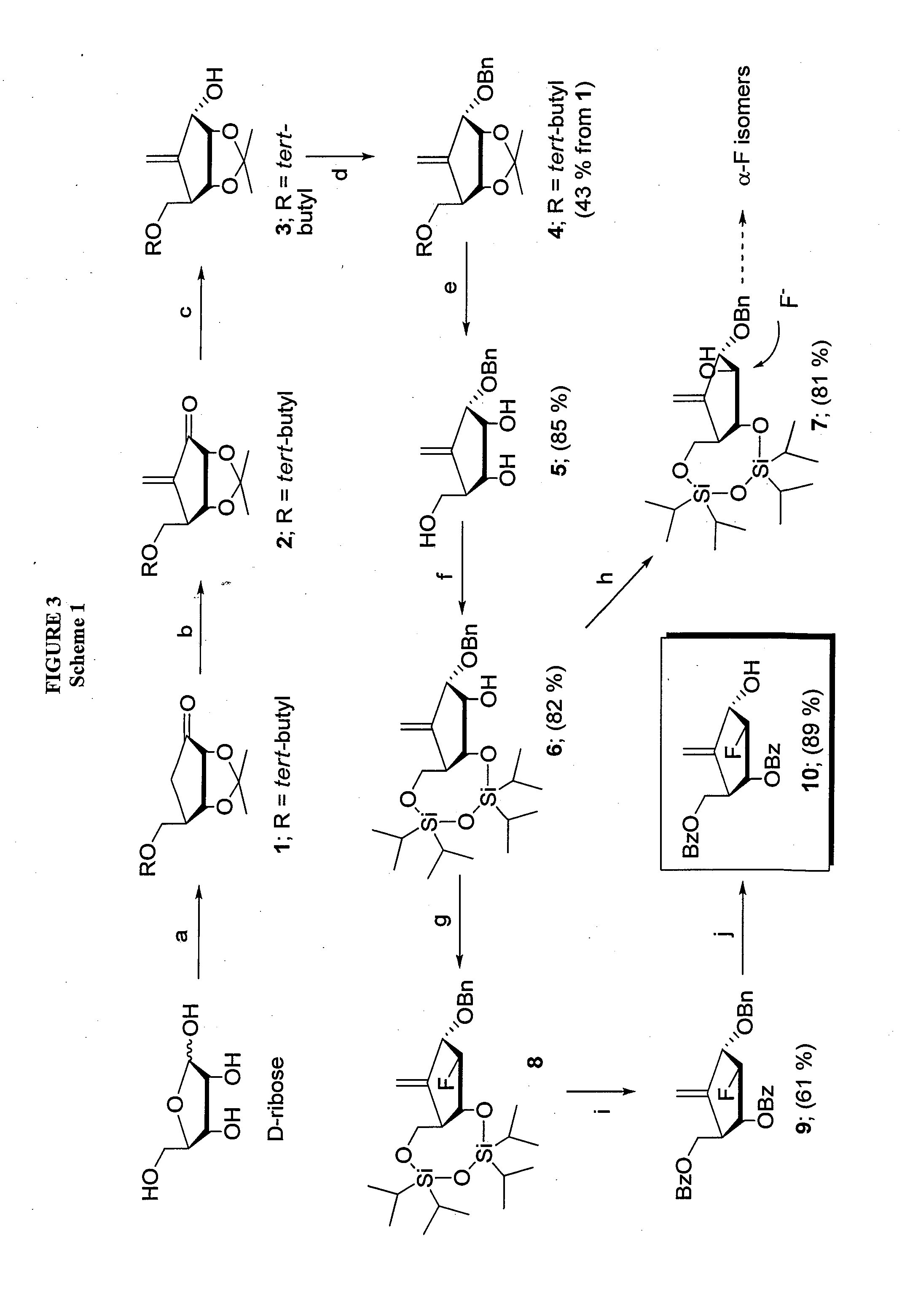
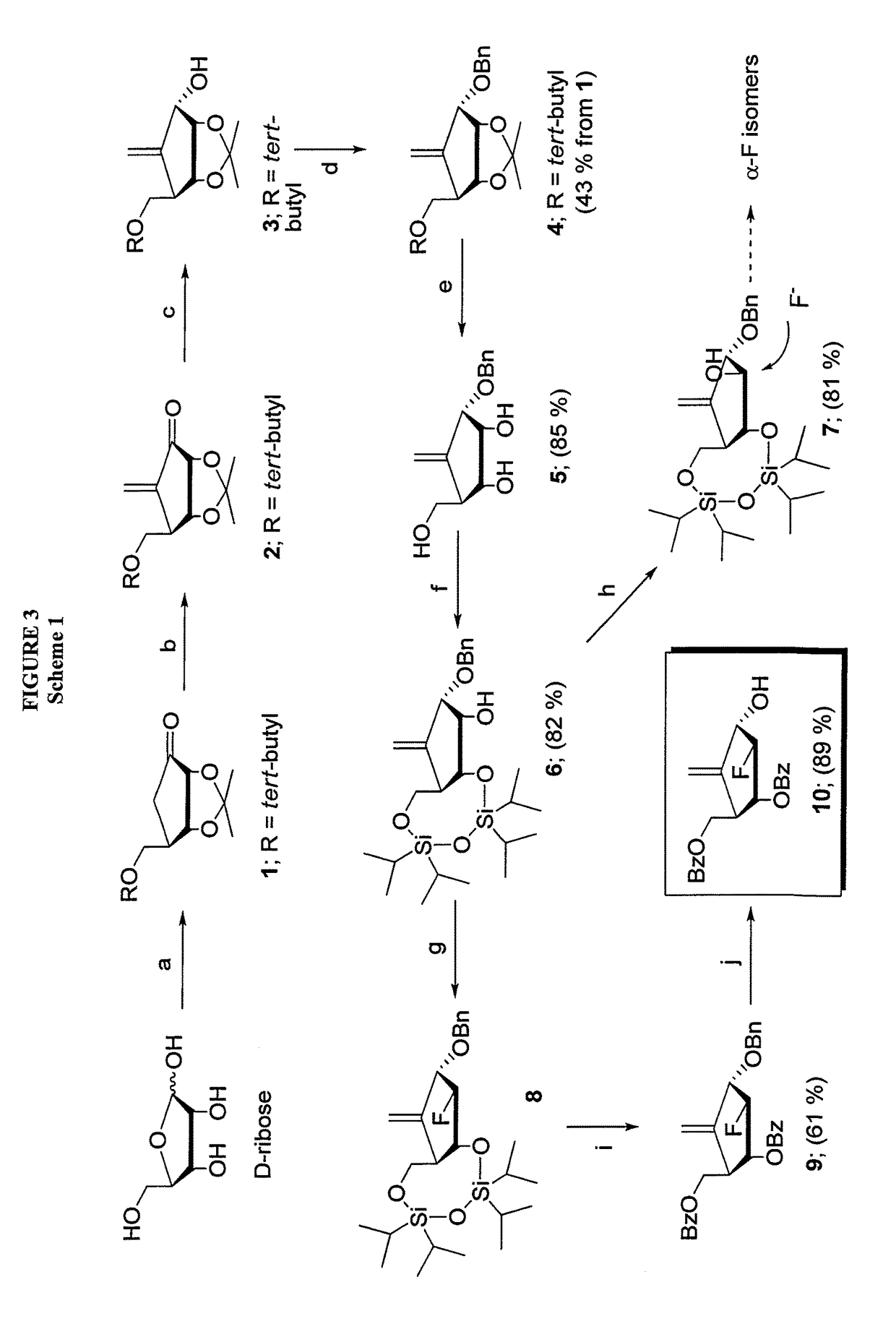
2′-fluoro-6′-methylene carbocyclic nucleosides and methods of treating viral infections
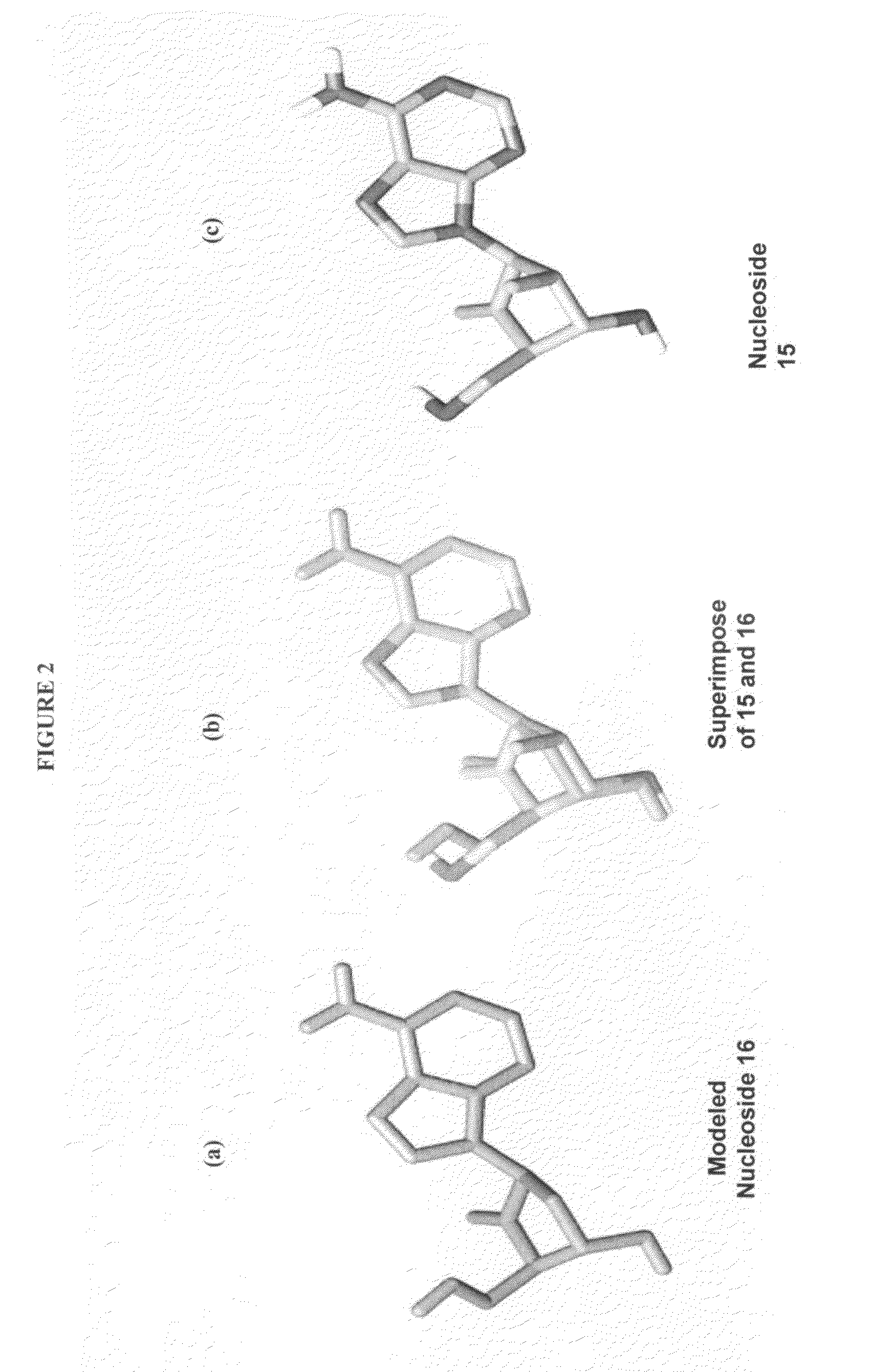
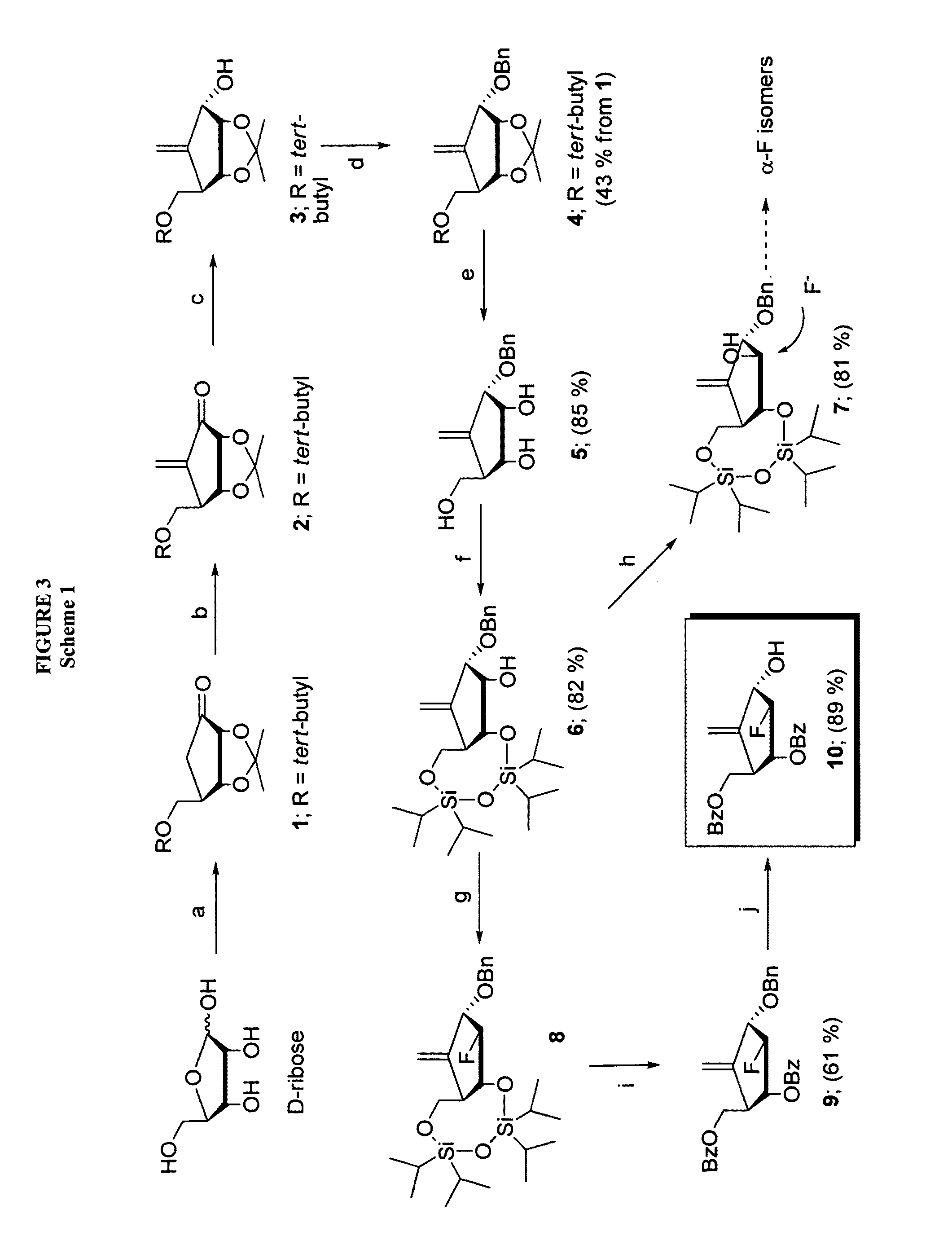
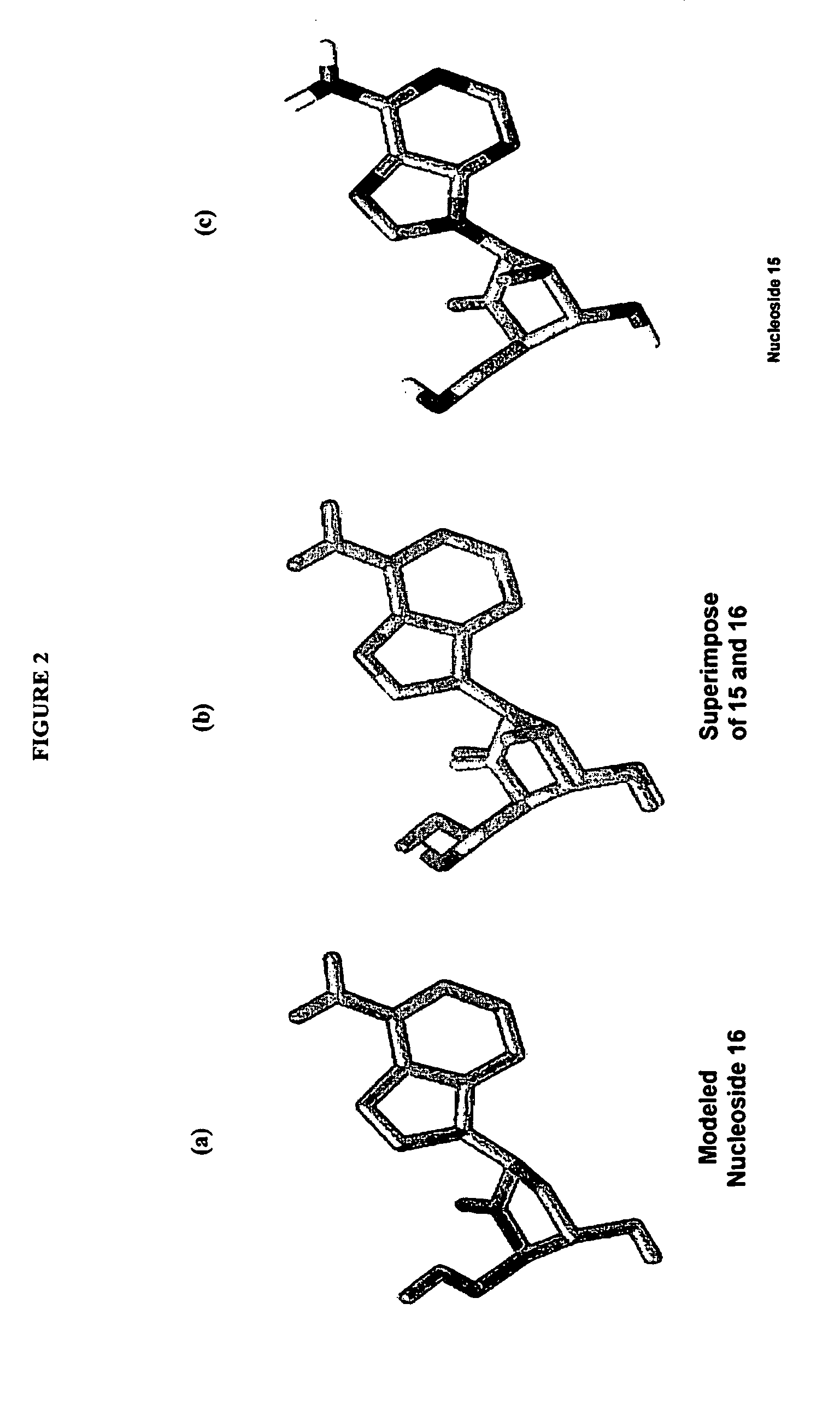
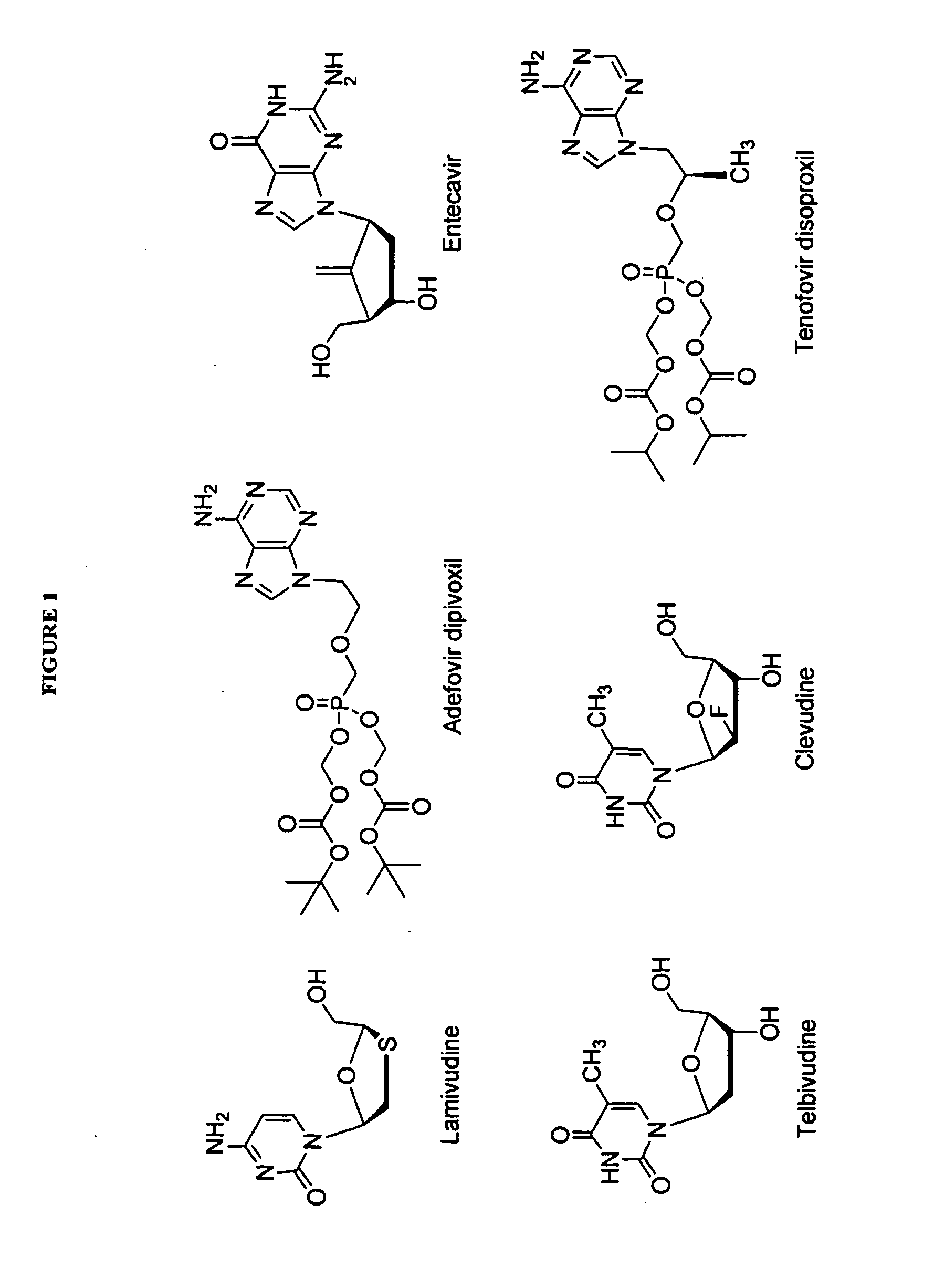
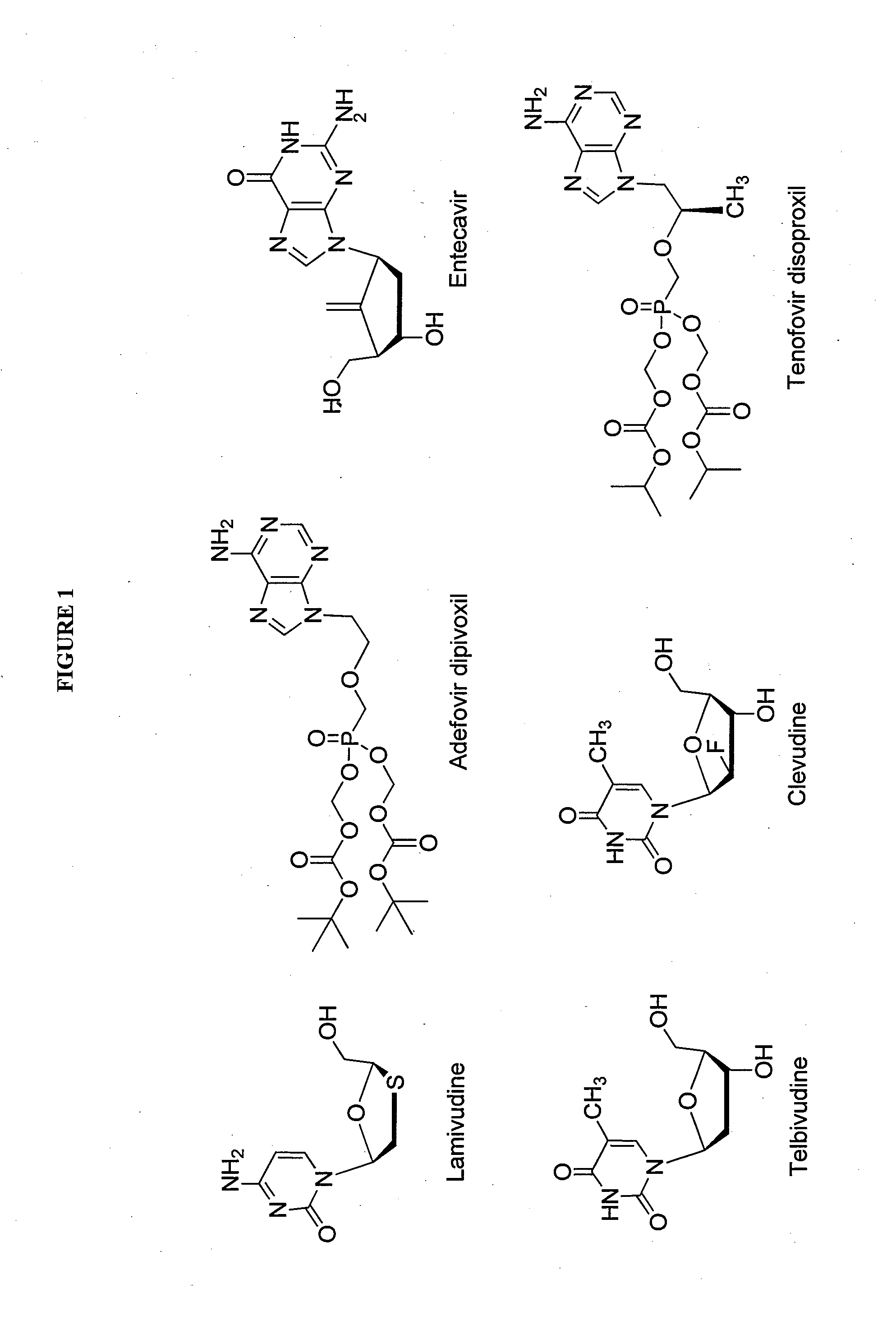
The present invention relates to 2′-Fluoro-6′-methylene carbocyclic nucleosides, pharmaceutical compositions containing these nucleosides and their use in the treatment or prophylaxis of a number of viral infections and secondary disease states and conditions thereof, especially including Hepatitis B virus (HBV) and secondary disease states and conditions thereof (cirrhosis and liver cancer), Heptatitis C virus (HCV), Herpes Simplex virus I and II (HSV-1 and HSV-2), cytomegalovirus (CMV), Varicella-Zoster Virus (VZV) and Epstein Barr virus (EBV) and secondary cancers which occur thereof (lymphoma, nasopharyngeal cancer, including drug resistant (especially including lamivudine and / or adefovir resistant) and other mutant forms of these viruses.
Owner:UNIV OF GEORGIA RES FOUND INC
2′-fluoro-6′methylene carbocyclic nucleosides and methods of treating viral infections
ActiveUS8946244B2Reduce the possibilityBiocideSugar derivativesHerpes zoster virusNasopharyngeal cancer
The present invention relates to 2′-Fluoro-6′-methylene carbocyclic nucleosides, pharmaceutical compositions containing these nucleosides and their use in the treatment or prophylaxis of a number of viral infections and secondary disease states and conditions thereof, especially including Hepatitis B virus (HBV) and secondary disease states and conditions thereof (cirrhosis and liver cancer), Hepatitis C virus (HCV), Herpes Simplex virus I and II (HSV-1 and HSV-2), cytomegalovirus (CMV), Varicella-Zoster Virus (VZV) and Epstein Barr virus (EBV) and secondary cancers which occur thereof (lymphoma, nasopharyngeal cancer, including drug resistant (especially including lamivudine and / or adefovir resistant) and other mutant forms of these viruses.
Owner:UNIV OF GEORGIA RES FOUND INC
2'-Fluoro-6'-Methylene Carbocyclic Nucleosides and Methods of Treating Viral Infections
The present invention relates to 2′-Fluoro-6′-methylene carbocyclic nucleosides, pharmaceutical compositions containing these nucleosides and their use in the treatment or prophylaxis of a number of viral infections and secondary disease states and conditions thereof, especially including Hepatitis B virus (HBV) and secondary disease states and conditions thereof (cirrhosis and liver cancer), Heptatitis C virus (HCV), Herpes Simplex virus I and II (HSV-1 and HSV-2), cytomegalovirus (CMV), Varicella-Zoster Virus (VZV) and Epstein Barr virus (EBV) and secondary cancers which occur thereof (lymphoma, nasopharyngeal cancer, including drug resistant (especially including lamivudine and / or adefovir resistant) and other mutant forms of these viruses.
Owner:UNIV OF GEORGIA RES FOUND INC
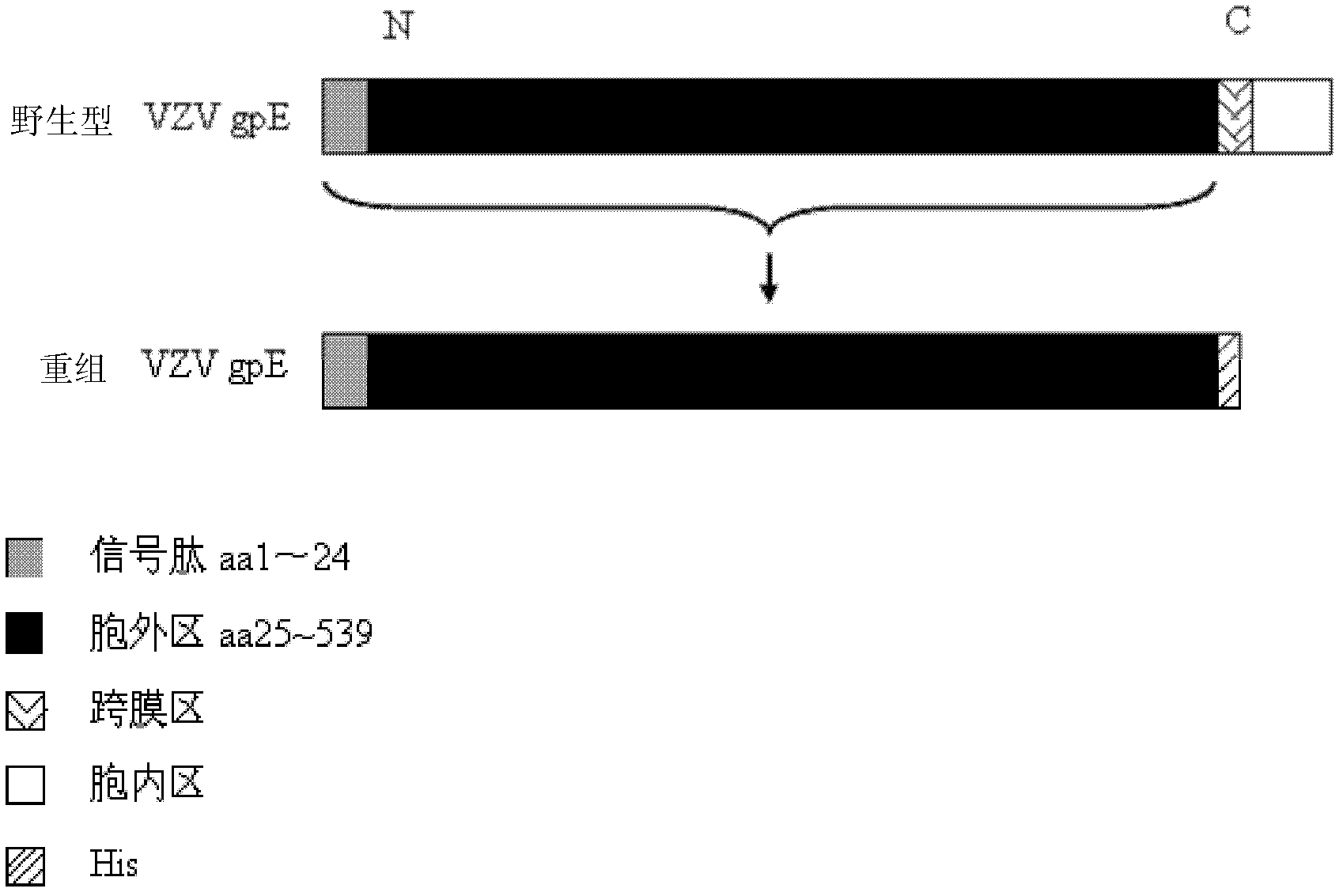
Method for recombinant expression of varicella-zoster virus truncation type glycoprotein E and application thereof
The invention discloses a method for the recombinant expression of a varicella-zoster virus truncation type glycoprotein E and application thereof. The method comprises the following steps: guiding a gene of a varicella-zoster virus (VZV) truncation type glycoprotein E (gpE) in which a transmembrane domain and an intracellular domain are removed and an His label is added into a host cell so as to obtain the recombinant varicella-zoster virus truncation type glycoprotein E by expression. The expression method is beneficial to enhancing the expression quantity of a target protein, the downstream purifying operation is simplified, and the large-scale production of the protein can be realized in an easier way; and moreover, the quality between batches is stable. The recombinant protein disclosed by the invention is used as a capturing antigen and can be used for the indirect ELISA (Enzyme-Linked Immuno Sorbent Assay) detection of specific immunoglobulin for resisting a varicella-zoster virus in a plasma specimen, the accuracy of the clinical diagnosis of VZV infection can be enhanced, and the recombinant protein is also used for other fields needing VZV specific immunoglobulin to carry out high-throughput detection.
Owner:FIELD OPERATION BLOOD TRANSFUSION INST OF PLA SCI ACAD OF MILITARY
Synthetic peptide, inhibitor to DNA viruses
The present invention relates to the identification of the active domain of Herpoxin, a DNA virus-inhibiting-protein which was isolated from cobra venom in U.S. Pat. No. 5,648,339 and has a molecular weight of 13.5 kDa We have isolated a fragment of Herpoxin which contains the active domain and which we have named Herp. Herp mimics the activity of Herpoxin in inhibiting the replication of DNA viruses. A synthetic version of the active fragment was produced having the amino acid sequence Asn-Leu-Tyr-Gln-Phe-Lys-Asn-Met-Ile-Gln. The synthetic version of Herp consisting of ten amino acids inhibits the replication of DNA viruses such as herpes viruses types 1 and 2, cytomegalovirus and varicella zoster virus as well as Tubercle bacilli.
Owner:LIPPS BINIE V +1
2'-fluoro-6'-methylene carbocyclic nucleosides and methods of treating viral infections
ActiveUS20130005677A1Reduce infectious virus titerReduce cell viabilityBiocideSugar derivativesHerpes simplex diseaseCirrhosis
The present invention relates to 2′-Fluoro-6′-methylene carbocyclic nucleosides, pharmaceutical compositions containing these nucleosides and their use in the treatment or prophylaxis of a number of viral infections and secondary disease states and conditions thereof, especially including Hepatitis B virus (HBV) and secondary disease states and conditions thereof (cirrhosis and liver cancer), Heptatitis C virus (HCV), Herpes Simplex virus I and II (HSV-1 and HSV-2), cytomegalovirus (CMV), Varicella-Zoster Virus (VZV) and Epstein Barr virus (EBV) and secondary cancers which occur thereof (lymphoma, nasopharyngeal cancer, including drug resistant (especially including lamivudine and / or adefovir resistant) and other mutant forms of these viruses.
Owner:UNIV OF GEORGIA RES FOUND INC
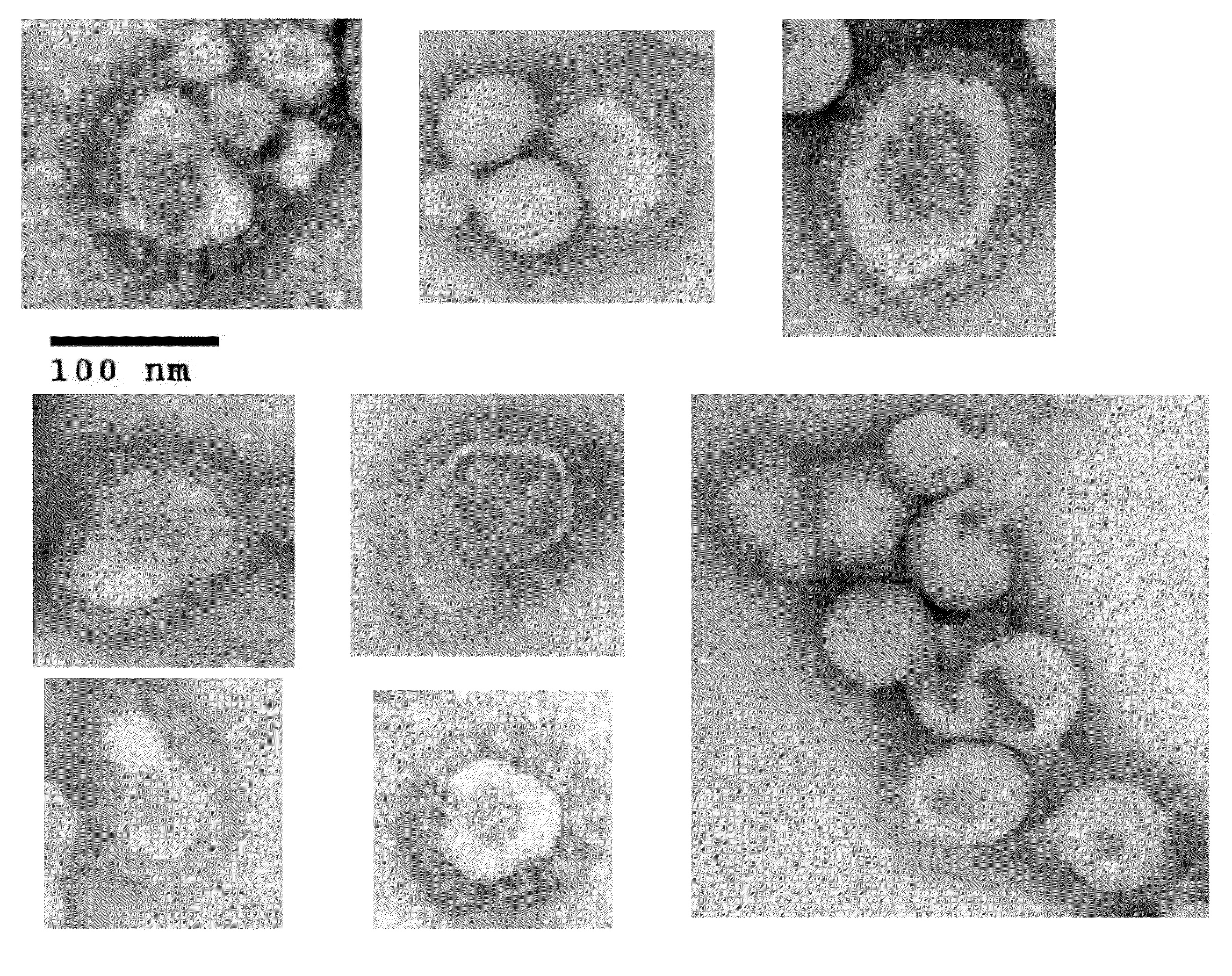
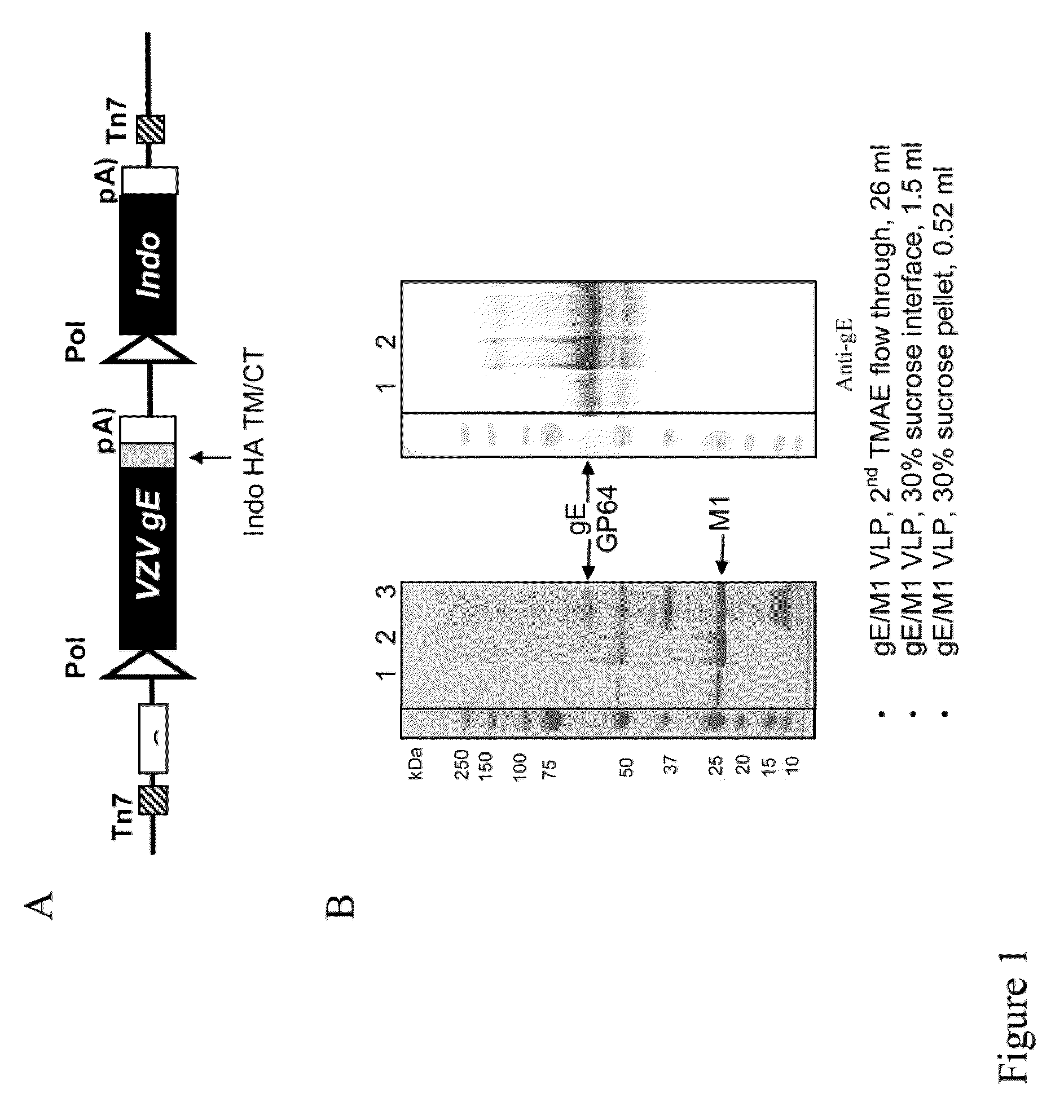
Chimeric varicella zoster virus virus-like particles
InactiveUS20110008838A1SsRNA viruses negative-senseSsRNA viruses positive-senseChickenpoxVirus-like particle
The present invention discloses novel chimeric Varicella Zoster Virus (VZV) virus-like particles (VLPs) comprising chimeric VZV glycoproteins. The invention also discloses vaccine formulations of the chimeric VZV-VLPs and methods of inducing an immune response in subjects.
Owner:NOVAVAX
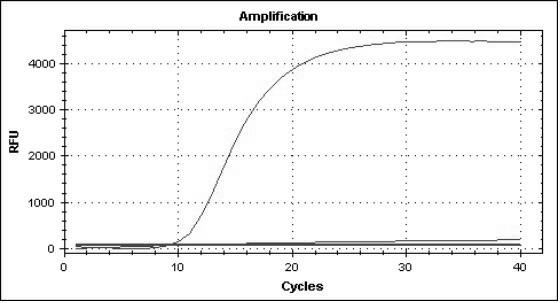
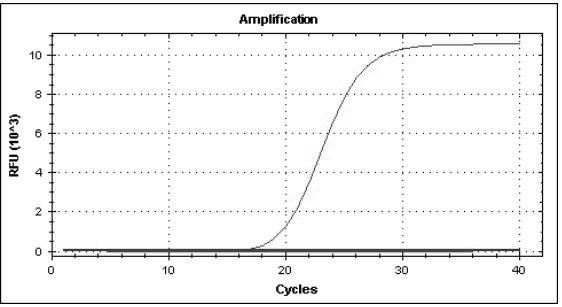
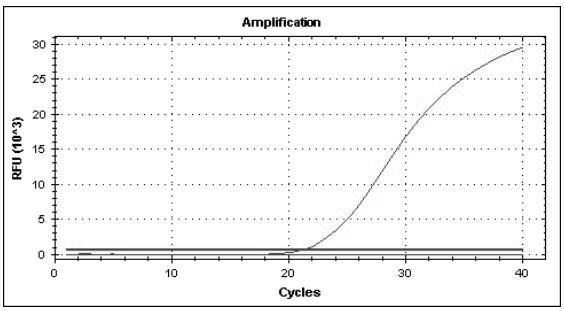
Multiple real-time quantitative PCR primer, probe and detection method for identifying viral pathogens relevant to fever with eruption syndrome as infection diseases
ActiveCN102140543ADetection ExpressImprove efficiencyMicrobiological testing/measurementFluorescence/phosphorescenceChickenpoxHerpes zoster virus
The invention discloses multiple real-time quantitative PCR primer, probe and a detection method for identifying viral pathogens relevant to fevers with eruption syndromes as infection diseases, which is used for carrying out multiple real-time fluorescent quantitative PCR detection on varicella-herpes zoster viruses, human small DNA (Deoxyribonucleic Acid) viruses B19, enteroviruses (enteroviruses 71 type and coxsackie viruses A16 type), dengue viruses, rubella viruses and measles viruses. The invention can simultaneously carry out qualitative or quantitative detection on eight kinds of human viruses in various types of samples by multiple double-tubes PCR. The detection method has the advantages of simple operation, short time consumption, high sensitivity and strong specificity, is suitable for field detection, early diagnosis, epidemics detection and research and the like, and takes the actions of assistance and identification diagnosis on the fevers with eruption syndromes.
Owner:SUN YAT SEN UNIV
Multiplex PCR assay
InactiveUS20070207453A1Microbiological testing/measurementFermentationPcr assayVaricella zoster virus
This invention relates to a multiplex PCR assay capable of screening or detecting the relevant microbial organism specific to Cytomegalo virus (CMV), Herpes Simplex virus (HSV) and Varicella zoster virus (VZV) present in a sample, comprising a reaction mixture of a combination of three sets of primers, one of said primer set for detection of CMV, a second of said primer sets for detection of HSV, a third primer set for the detection of VZV, said primers being compatible to each other.
Owner:GUPTA VISHALI +4
Stabilization of viral compositions
InactiveUS20060141483A1Improve stabilityIncrease productionSsRNA viruses negative-sensePowder deliverySugarVaricella zoster virus
This invention concerns stabilized virus compositions, preferably a herpesvirus which may be an attenuated or genetically modified herpes simplex virus or varicella zoster virus, and a method of stabilizing viruses and immunizing preparations by the addition of sugars, preferably glucose and amino acids, preferably lysine.
Owner:AURX
Topical formulation having effects on alleviating pain/inflammation caused by herpes virus infection
InactiveUS7132452B2Fasten skin recoveryPromote recoveryPowder deliveryBiocideComplete remissionTolmetin
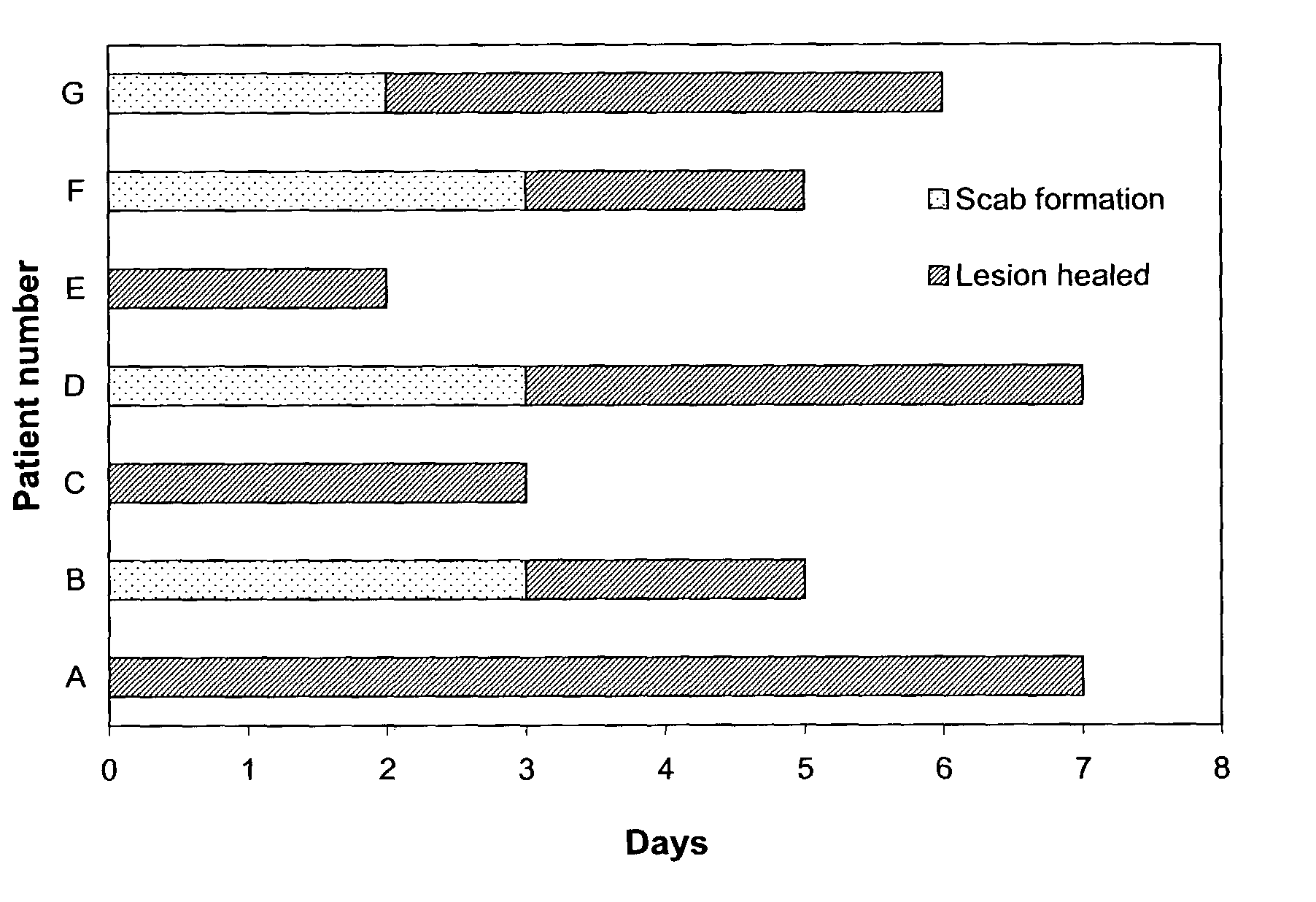
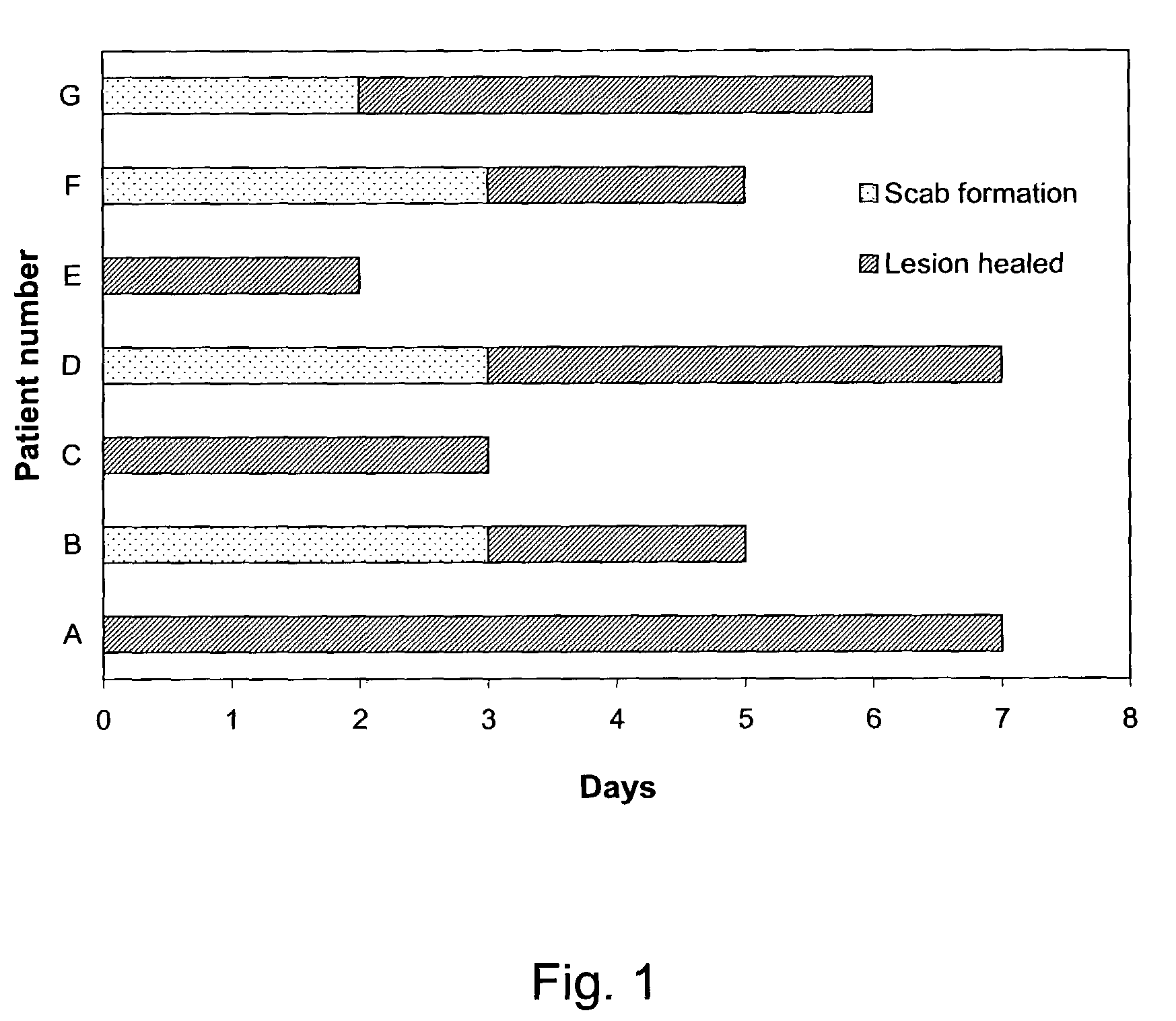
The present invention provides a topical formulation containing NSAID, particularly diclofenac. The topical formulation is particularly useful for alleviating pain / inflammation associated with infection caused by herpes virus, especially herpes simplex virus (HSV) and varicella-zoster virus (VZV). Similar relief can be achieved where diclofenac is replaced with another non-steroidal anti-inflammatory drug (NSAID), which includes, without limitation, etodolac, ketorolac, bromfenac, diflunisal, ibuprofen, fenoprofen, ketoprofen, naproxen, suprofen, meclofenamate, mefenamic acid, piroxicam, meloxicam, indomethacin, sulindac, phenylbutazone, oxyphenbutazone, and tolmetin. The topical formulation is further characterized by its fast relief on pain and / or inflammation associated with infection caused by herpes virus, i.e., a complete relief in no more than seven (7) days after the application of the topical formulation on skins of patients.
Owner:YUNG SHIN PHARMACEUTICALS INDUSTRIAL CO LTD
Glycopeptide antibiotic derivatives
InactiveUS20050250677A1Decreasing and removing antibacterial activityMaintain antiviral activityBiocideDigestive systemHerpes zoster virusGlycopeptide
Novel glycopeptide antibiotic derivatives, processes for their preparation, their use as a medicine, their use to treat or prevent viral infections and their use to manufacture a medicine to treat or prevent viral infections are provided. The present invention relates to the use of glycopeptide antibiotics and their semisynthetic derivatives to treat or prevent viral infections and their use to manufacture a medicine to treat or prevent viral infections of subjects, more in particular infections with viruses belonging to Retroviridae, Herpes viridae, Flaviviridae and the Coronaviridae, like HIV (human immunodeficiency virus), HCV (hepatitis C virus), BVDV (bovine viral diarrhoea virus), SARS (severe acute respiratory syndrome) causing virus, FCV (feline coronavirus), HSV (herpes simplex virus), VZV (varicella zoster virus) and CMV (cytomegalovirus).
Owner:BALZARINI JAN +2
Oligonucleotide therapies for modulating the effects of herpesviruses
InactiveUS6310044B1Conveniently and desirably presentedFaster replicationPeptide/protein ingredientsGenetic material ingredientsOpen reading frameHerpesvirus infection
Compositions and methods are provided for the treatment and diagnosis of herpesvirus infections. In accordance with preferred embodiments, oligonucleotides are provided which are specifically hybridizable with RNA or DNA deriving from a gene corresponding to one of the open reading frames UL5, UL8, UL9, UL13, UL29, UL30, UL39, UL40, UL42 AND UL52 of herpes simplex virus type 1. The oligonucleotide comprises nucleotide units sufficient in identity and number to effect said specific hybridization. In other preferred embodiments, the oligonucleotides are specifically hybridizable with a translation initiation site; it is also preferred that they comprise the sequence CAT. Methods of treating animals suspected of being infected with herpesvirus comprising contacting the animal with an oligonucleotide specifically hybridizable with RNA or DNA deriving from one of the foregoing genes of the herpesvirus are disclosed. Methods for treatment of infections caused by herpes simplex virus type 1, herpes simplex virus type 2, cytomegalovirus, human herpes virus 6, Epstein Barr virus or varicella zoster virus are disclosed.
Owner:IONIS PHARMA INC
Application of polycyclic polyketides in preparation of anti-HV (herpes virus) drug
The invention discloses an application of polycyclic polyketides in preparation of an anti-HV (herpes virus) drug. It is found that the polyketides can inhibit diseases caused by infection of four HVs including HSV-1 (herpes simplex virus-1), HSV-2 (herpes simplex virus-2), VZV (varicella zoster virus) and CMV (cytomegalo virus). The compounds show equivalent activity but have different acting mechanisms as compared with commercial drugs such as acyclovir and can overcome drug resistance of existing commercial drugs. Therefore, the compounds have good application prospects in treatment of related diseases caused by infection of HVs including HSV-1, HSV-2, VZV and CMV.
Owner:JINAN UNIVERSITY
Recombinant multivalent vaccine
InactiveUS20100119550A1Improve accuracySecuring and ensuring effectivenessAntibacterial agentsSsRNA viruses negative-sensePolyvalent VaccineVaricella zoster virus
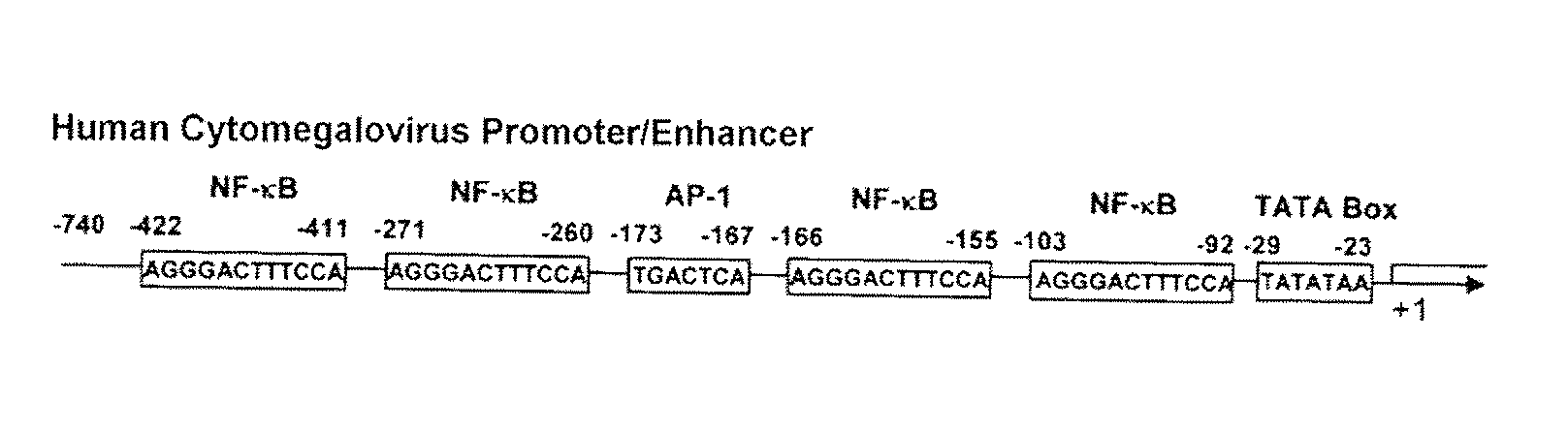
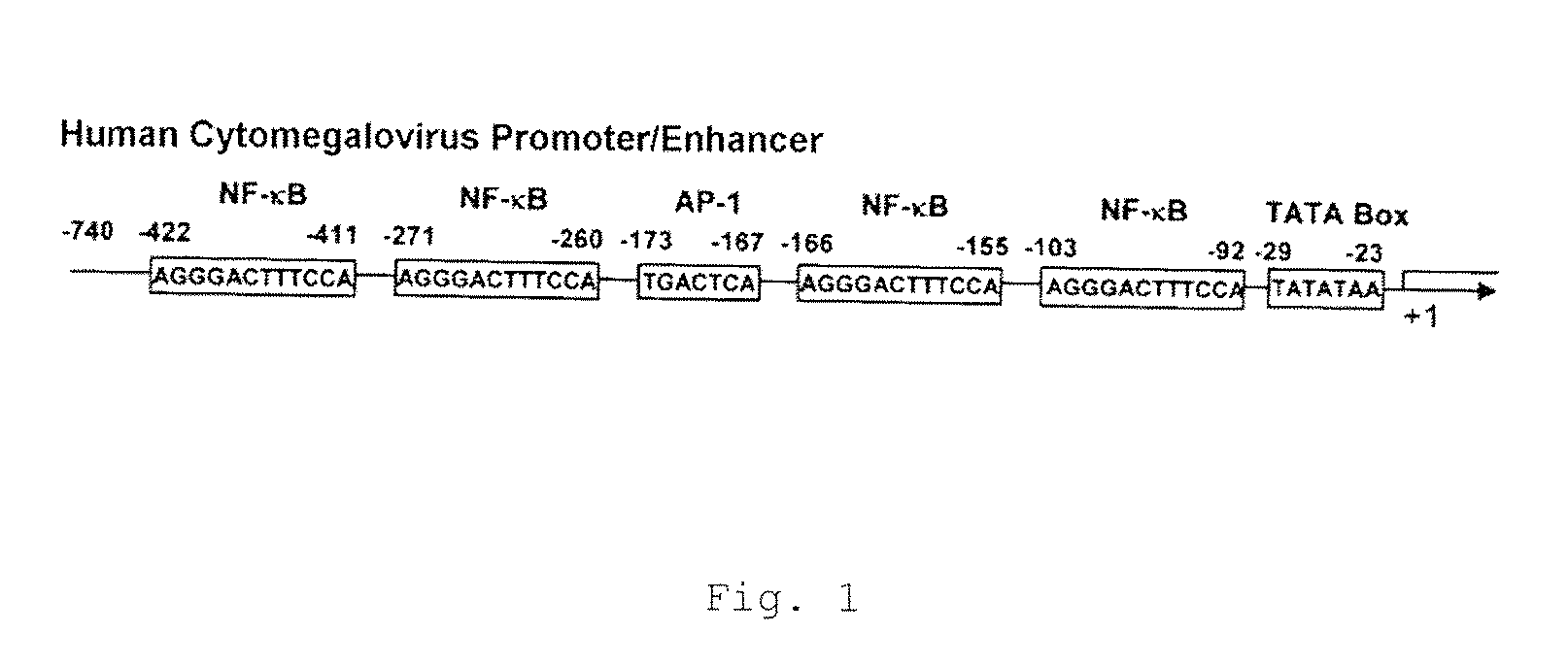
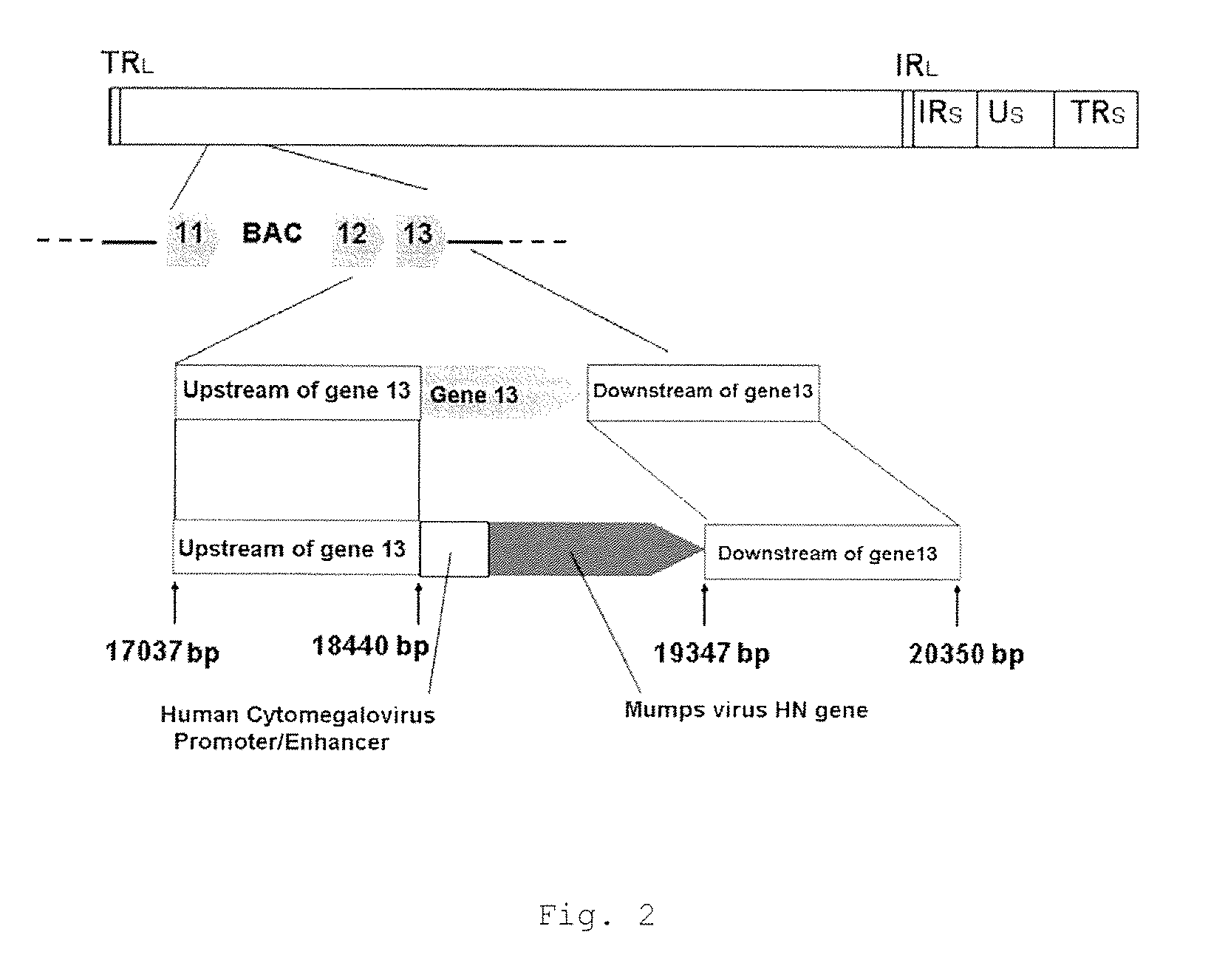
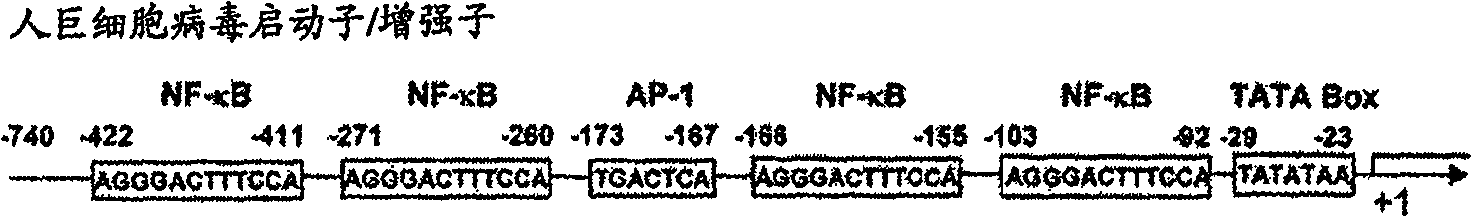
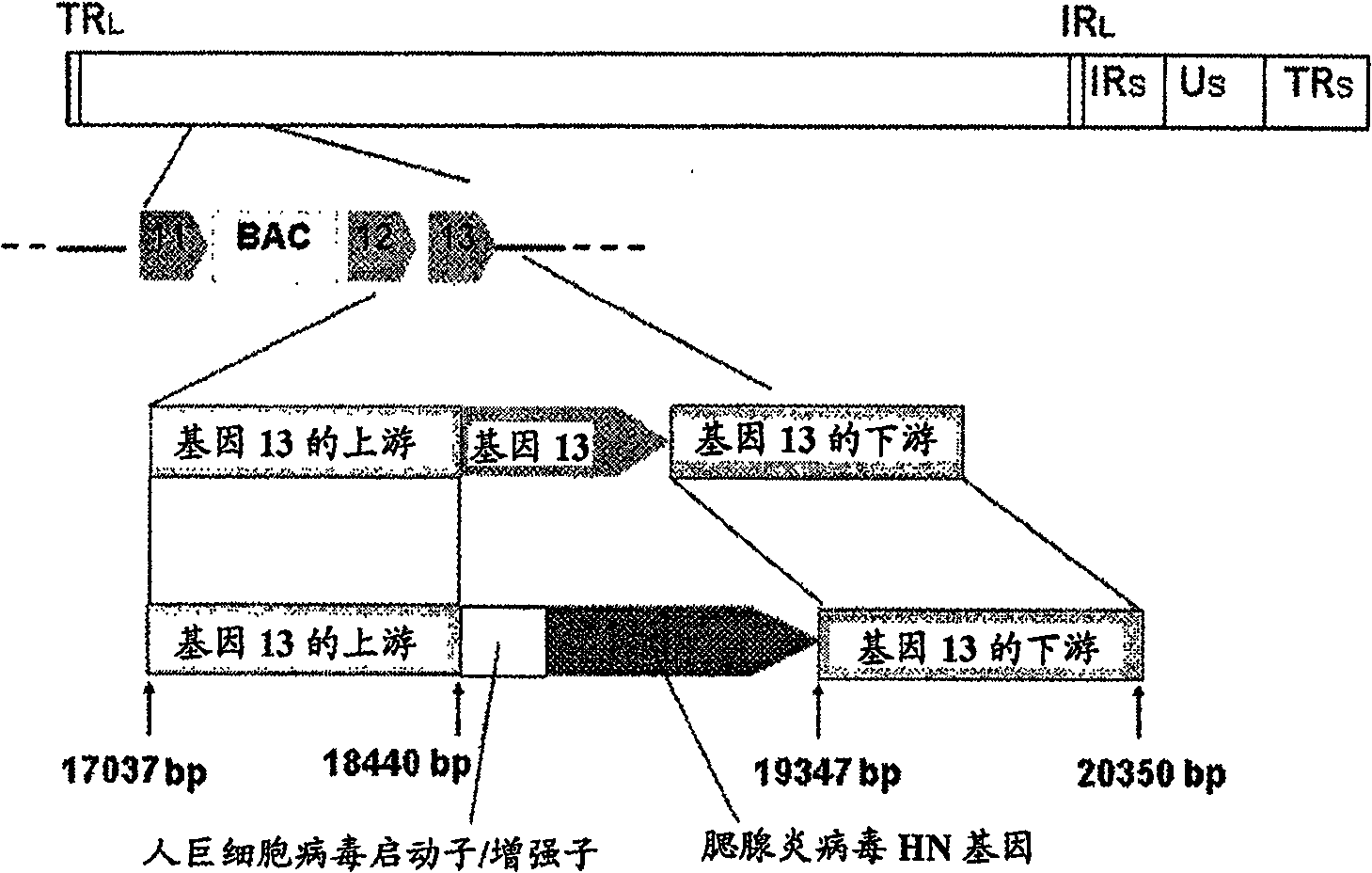
The problems to be solved by the present invention are to provide: a recombinant varicella-zoster virus; a process for producing the same; a pharmacological composition containing a recombinant varicella-zoster virus; a vector containing a BAC vector sequence in the specific gene of a genomic gene of varicella-zoster virus; cells containing such a vector; a fragment capable of homologous recombination with a genome of varicella-zoster virus; a nucleic acid cassette containing the BAC vector sequence; and a multivalent vaccine. The above problems were solved by developing a process for producing a recombinant varicella-zoster virus, wherein the BAC vector sequence is inserted into a specific virus gene.
Owner:THE RES FOUND FOR MICROBIAL DISEASES OFOSAKA UNIV +1
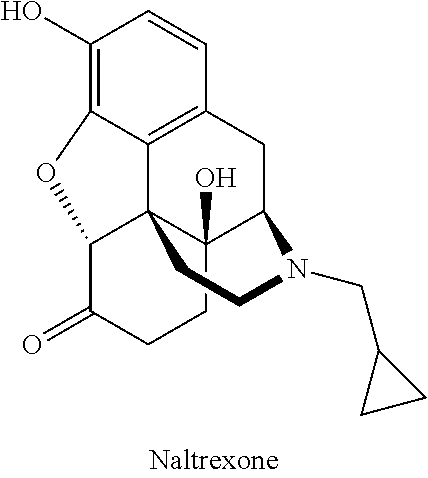
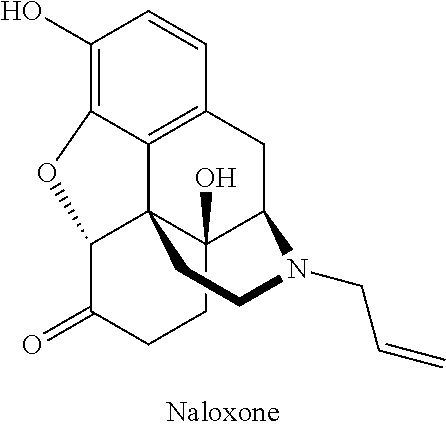
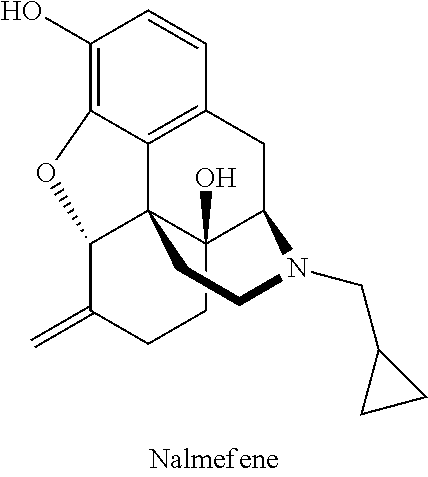
Compositions of opioid antagonists and methods for treating conditions caused by the varicella-zoster virus therewith
Provided are compositions comprising opioid antagonists, such as naltrexone naloxone, or nalmefene, or their pharmaceutically acceptable salts, and methods for treating conditions caused by the varicella-zoster virus therewith.
Owner:AK KIMYA ITHALAT IHRACAT VE SANAYII
Cosmetic treatment with nitric oxide, device for performing said treatment and manufacturing method therefor
InactiveCN101146556AIncrease supplyDiastolic increaseCosmetic preparationsToilet preparationsVirus wartsNitric oxide
Owner:NOLABS AB
Duchesnea polysaccharide, as well as preparation and use thereof
InactiveCN101486771ARich sourcesBreeding is easyOrganic active ingredientsAntiviralsMonomer compositionBiopharmaceutical
The invention relates to a preparation method of polysaccharide which is extracted from plants and usage thereof, belonging to the field of biopharmaceuticals. The preparation of polysaccharide comprises extracting total polysaccharide DIP which is extracted and separated from Duchesnea indica and has clear varicella-zoster virus activity resistance property, neutral polysaccharide neutral polysaccharide DIP1 and acidic polysaccharide DIP2 in total polysaccharide, and two major active monomer compositions polysaccharide DIP30 and polysaccharide DIP 60 in acidic polysaccharide DIP2. The raw material of Duchesnea indica has wide source range, easy regeneration, easily-operated preparation method, and high reproducibility. The polysaccharide shows significant inhibition on the varicella-zoster virus, is safe, non-toxic and stable, and is a high-quality anti-viral drug candidate.
Owner:CHINA PHARM UNIV
Recombinant multivalent vaccine
Owner:THE RES FOUND FOR MICROBIAL DISEASES OFOSAKA UNIV +1
2'-fluoro-6'-methylene carbocyclic nucleosides and methods of treating viral infections
The present invention relates to 2′-Fluoro-6′-methylene carbocyclic nucleosides, pharmaceutical compositions containing these nucleosides and their use in the treatment or prophylaxis of a number of viral infections and secondary disease states and conditions thereof, especially including Hepatitis B virus (HBV) and secondary disease states and conditions thereof (cirrhosis and liver cancer), Heptatitis C virus (HCV), Herpes Simplex virus I and II (HSV-1 and HSV-2), cytomegalovirus (CMV), Varicella-Zoster Virus (VZV) and Epstein Barr virus (EBV) and secondary cancers which occur thereof (lymphoma, nasopharyngeal cancer, including drug resistant (especially including lamivudine and / or adefovir resistant) and other mutant forms of these viruses, especially HBV.
Owner:UNIV OF GEORGIA RES FOUND INC
Kit for genotyping VZV, production method of kit and application of kit
InactiveCN105132584ANo cross reactionStrong specificityMicrobiological testing/measurementMicroorganism based processesHuman DNA sequencingChemical structure
The invention provides a kit for genotyping VZV (Varicella-Zoster Viruses). The kit is characterized by comprising a nucleotide sequence shown as the Table 3 in the specification, and specific primers and specific probes corresponding to clade1-5 type VZV of chemical structures. The kit has the function of detecting various kinds of VZV DNA (Deoxyribonucleic Acid); the detection sensitivity is 10<2> copies / reaction; no cross reaction with human genome, herpes simplex viruses type I / type II, cytomegaloviruses and EB viruses exists; and the kit is applicable to the virus gene diagnosis of clinical VZV infected persons, and can also be used for the epidemiology survey of different Clade types of VZV.
Owner:CHENGDU MILITARY GENERAL HOSPITAL OF PLA
Methods and compositions for detecting CNS viruses
The present invention generally relates to a molecular test of enterovirus, herpes simplex virus-1 and -2, and / or Varicella-Zoster virus, in order to identify patients with a viral infection, in particular a viral infection of the central nervous system. Accordingly methods and compositions are disclosed to determine the presence or absence of a viral pathogen in a biological sample comprising, wherein the target nucleic acids comprise the 5′ UTR of the enterovirus genome, UL29 of herpes simplex virus and gene 36 of Varicella-Zoster virus.
Owner:QUEST DIAGNOSTICS INVESTMENTS INC
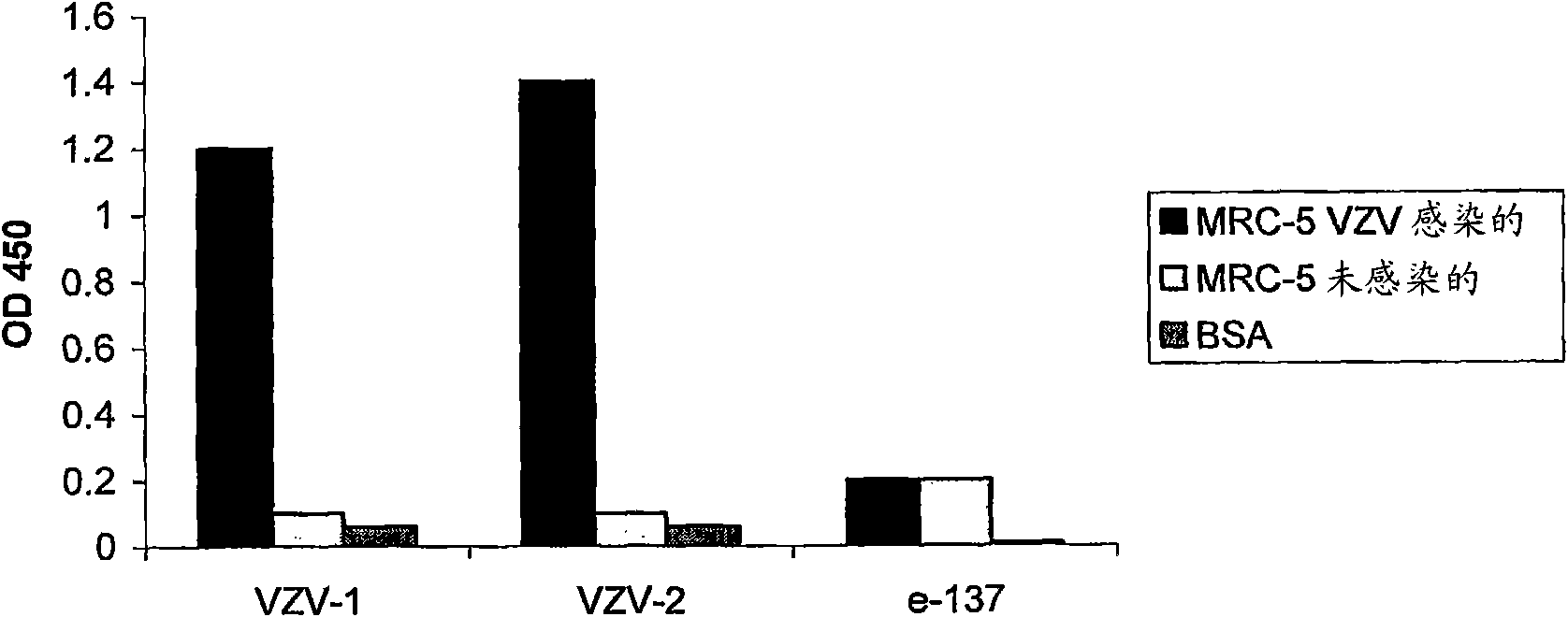
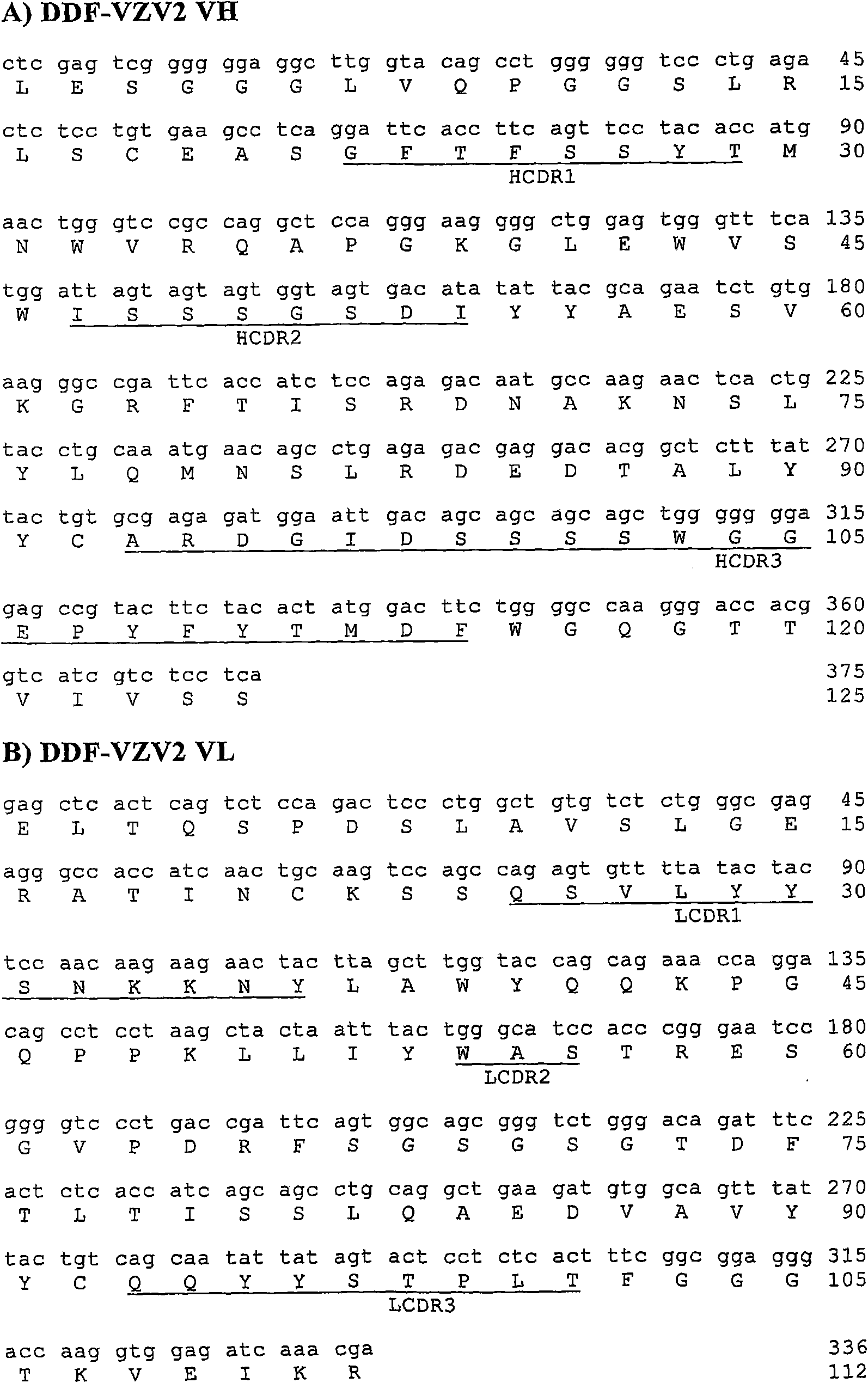
Antibodies specific for varicella zoster virus
InactiveCN101663318AVirus peptidesImmunoglobulins against virusesTherapeutic treatmentVaricella zoster virus
The present invention provides novel antibody sequences that bind Varicella Zoster Virus (VZV) and neutralize VZV infection. The novel sequences can be used for the medical management of VZV infection, in particular for detecting the virus or for preparing pharmaceutical compositions to be used in the prophylactic or therapeutic treatment of VZV infection.
Owner:RIBOVAX BIOTECHNOLOGIES SA
Glycopeptide antibiotic derivatives
Novel glycopeptide antibiotic derivatives, processes for their preparation, their use as a medicine, their use to treat or prevent viral infections and their use to manufacture a medicine to treat or prevent viral infections are provided. The present invention relates to the use of glycopeptide antibiotics and their semisynthetic derivatives to treat or prevent viral infections and their use to manufacture a medicine to treat or prevent viral infections of subjects, more in particular infections with viruses belonging to Retroviridae, Herpes viridae, Flaviviridae and the Coronaviridae, like HIV (human immunodeficiency virus), HCV (hepatitis C virus), BVDV (bovine viral diarrhoea virus), SARS (severe acute respiratory syndrome) causing virus, FCV (feline coronavirus), HSV (herpes simplex virus), VZV (varicella zoster virus) and CMV (cytomegalovirus).
Owner:K U LEUVEN RES & DEV
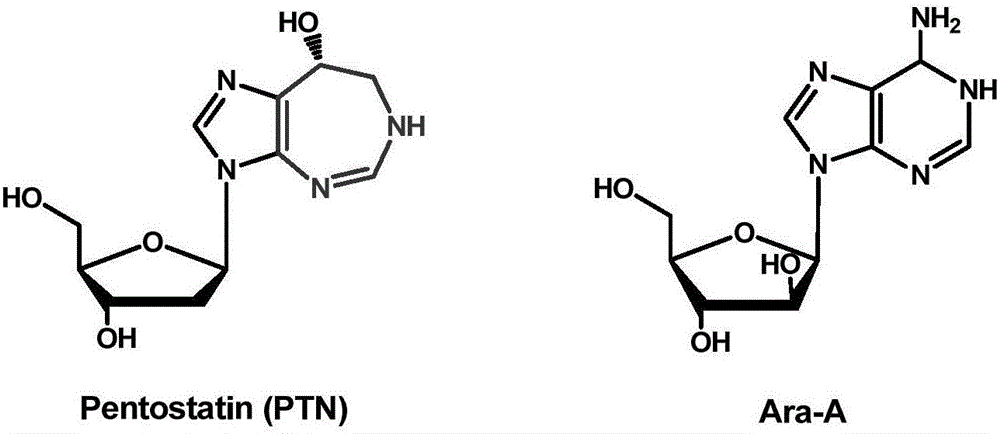
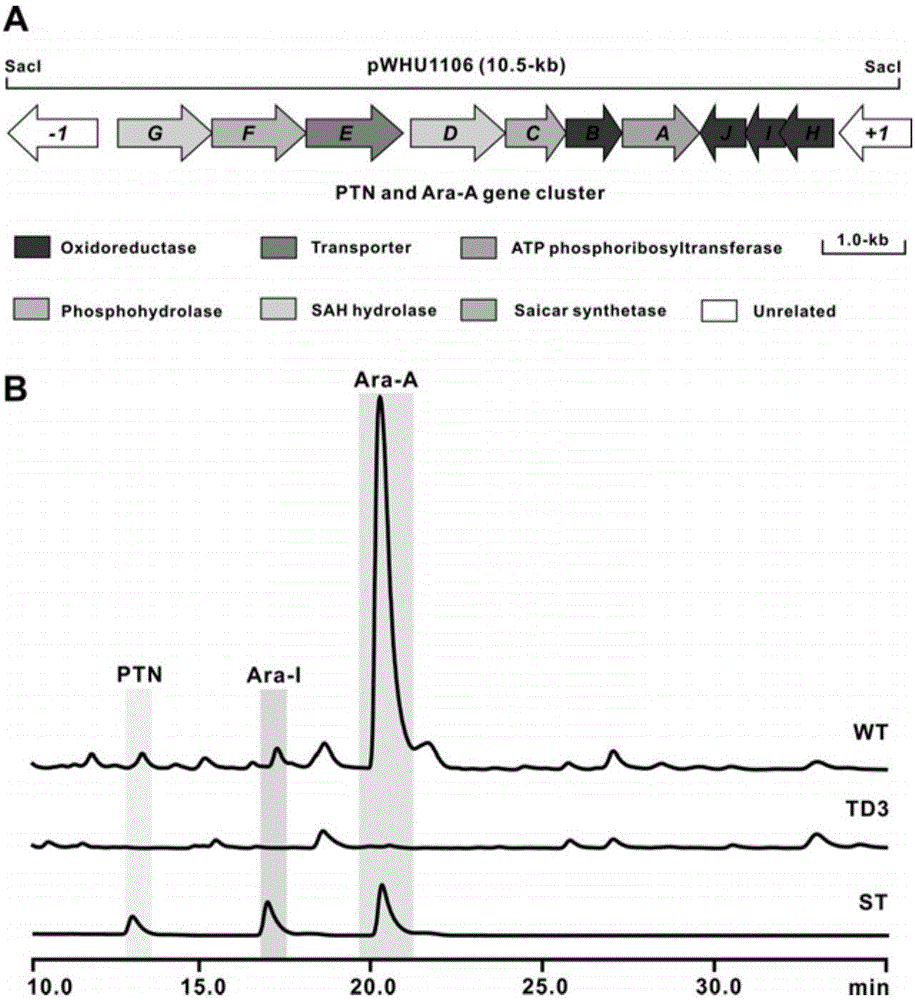
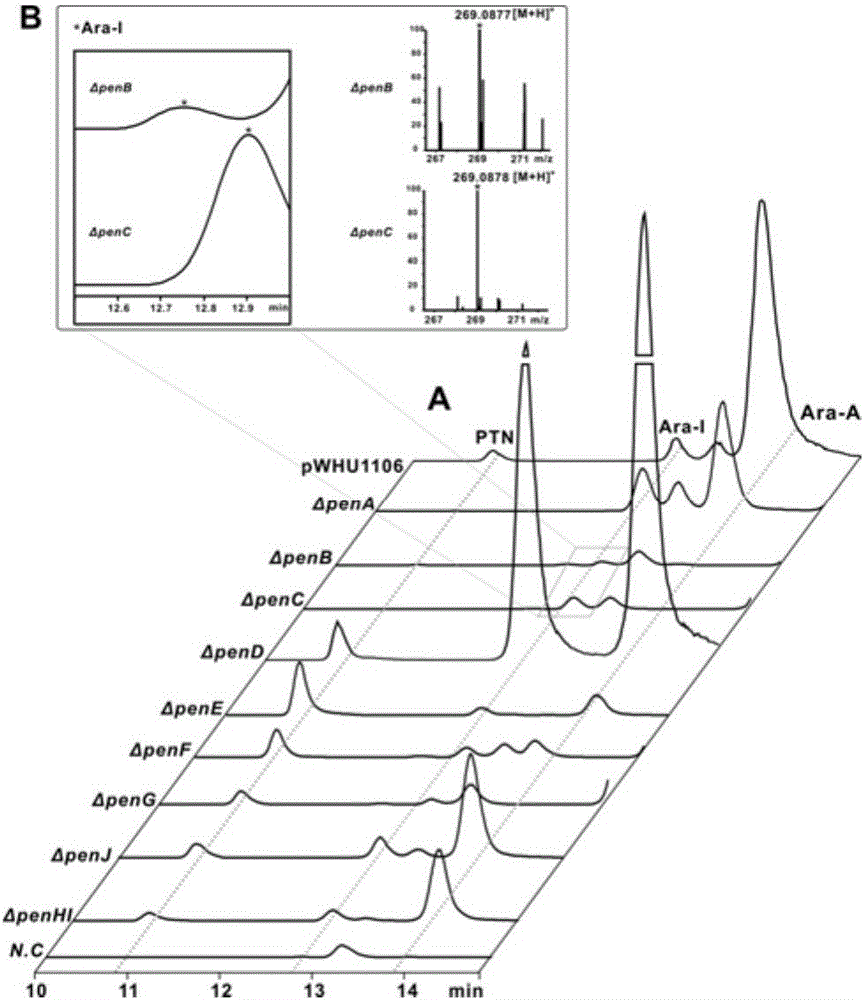
Biosynthetic gene cluster of pentostatin and arabinofuranosyladenine and application of biosynthetic gene cluster
ActiveCN106701788AImprove dynamic characteristicsIncrease productionMicroorganism based processesOxidoreductasesBiosynthetic genesStreptomyces antibioticus
The invention relates to cloning, sequencing, analysis and functional study of a biosynthetic gene cluster of a natural product pentostatin which is produced from streptomyces antibioticus and is used for treating lymphocytic leukemia and a natural product arabinofuranosyladenine for resisting Koi herpes virus and varicella-zoster virus and application of the biosynthetic gene cluster. Biosynthetic genes of the two compounds are included in the same gene cluster and are independent from each other. The whole gene cluster comprises ten genes, namely three genes related to pentostatin synthesis, five genes related to arabinofuranosyladenine synthesis and two genes related to transportation regulation of the pentostatin and arabinofuranosyladenine. According to genetic manipulations of the previous biosynthetic genes, biosynthesis of the pentostatin or arabinofuranosyladenine can be blocked or improved. The genes provided by the invention and proteins thereof can be used for genetic engineering, protein expression, enzymic catalytic reaction and the like of the compounds, and can be further used for searching and discovering compounds or genes and proteins which can be used for medicines, industry or agriculture.
Owner:WUHAN UNIV
High-efficient antiviral medicament composition in chickweed as well as preparation method and use thereof
InactiveCN101371861ASafe broad-spectrum antiviral drug actionHigh-efficiency broad-spectrum antiviral drugsAntiviralsSynthetic polymeric active ingredientsCondyloma virusHighly pathogenic
The invention discloses a highly effective antiviral medicinal composition of chickweed, a preparation method and an application thereof. Presently, highly effective, safe and universal antiviral medicine is lacking all over the world. A plant, chickweed, or other stellaria plant is extracted, by two resin adsorption methods, one water-alcohol extraction and ultra-filtration, into a dark brown composition that has a molecular distribution ranging from 2,000 to 900,000 Dolton and comprises total sulfate peptidoglycan phenolic acidic components and flavonoid components; the composition is used as a safer, more highly effective universal antiviral natural medicine. Total peptides account to 15-25 percent of the chemical structures of dark brown total sulfate peptidoglycan phenolic-acidic components, and include totaling 17 amino acids by proportion: aspartic acid, glutamic acid, serine, histidine, cystine, methionine, isoleucine; the polysaccharides essentially consist of glucose, galactose and arabinose and form sulfate polysaccharides. The composition can be used for treating virus diseases, including AIDS virus, hepatitis virus, respire virus such as influenza virus including highly pathogenic bird flu virus, para influenza virus and adenovirus, and papovavirus, enterovirus, mumps virus, herpes simplex virus, herpes zoster virus, and varicella-zoster virus, and on the like, does not show toxicity, and can be prepared into more than 10 medicinal dosage forms and healthcare products, and the production process does not cause pollution to the environment.
Owner:朱耕新
2′-fluoro-6′-methylene carbocyclic nucleosides and methods of treating viral infections
The present invention relates to 2′-Fluoro-6′-methylene carbocyclic nucleosides, pharmaceutical compositions containing these nucleosides and their use in the treatment or prophylaxis of a number of viral infections and secondary disease states and conditions thereof, especially including Hepatitis B virus (HBV) and secondary disease states and conditions thereof (cirrhosis and liver cancer), Hepatitis C virus (HCV), Herpes Simplex virus I and II (HSV-1 and HSV-2), cytomegalovirus (CMV), Varicella-Zoster Virus (VZV) and Epstein Barr virus (EBV) and secondary cancers which occur thereof (lymphoma, nasopharyngeal cancer, including drug resistant (especially including lamivudine and / or adefovir resistant) and other mutant forms of these viruses, especially HBV.
Owner:UNIV OF GEORGIA RES FOUND INC
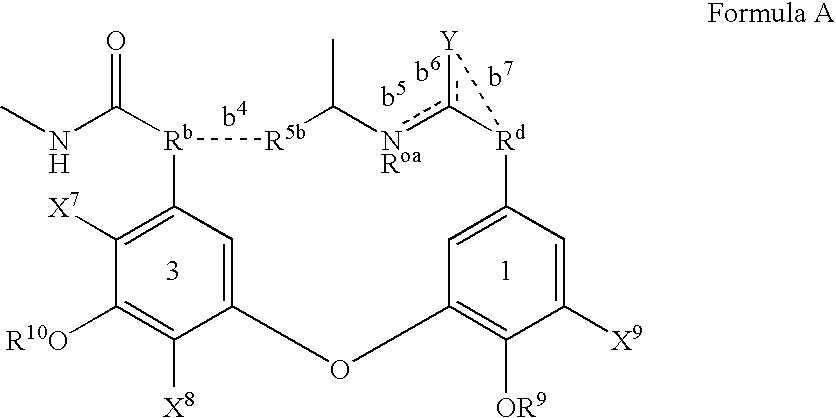
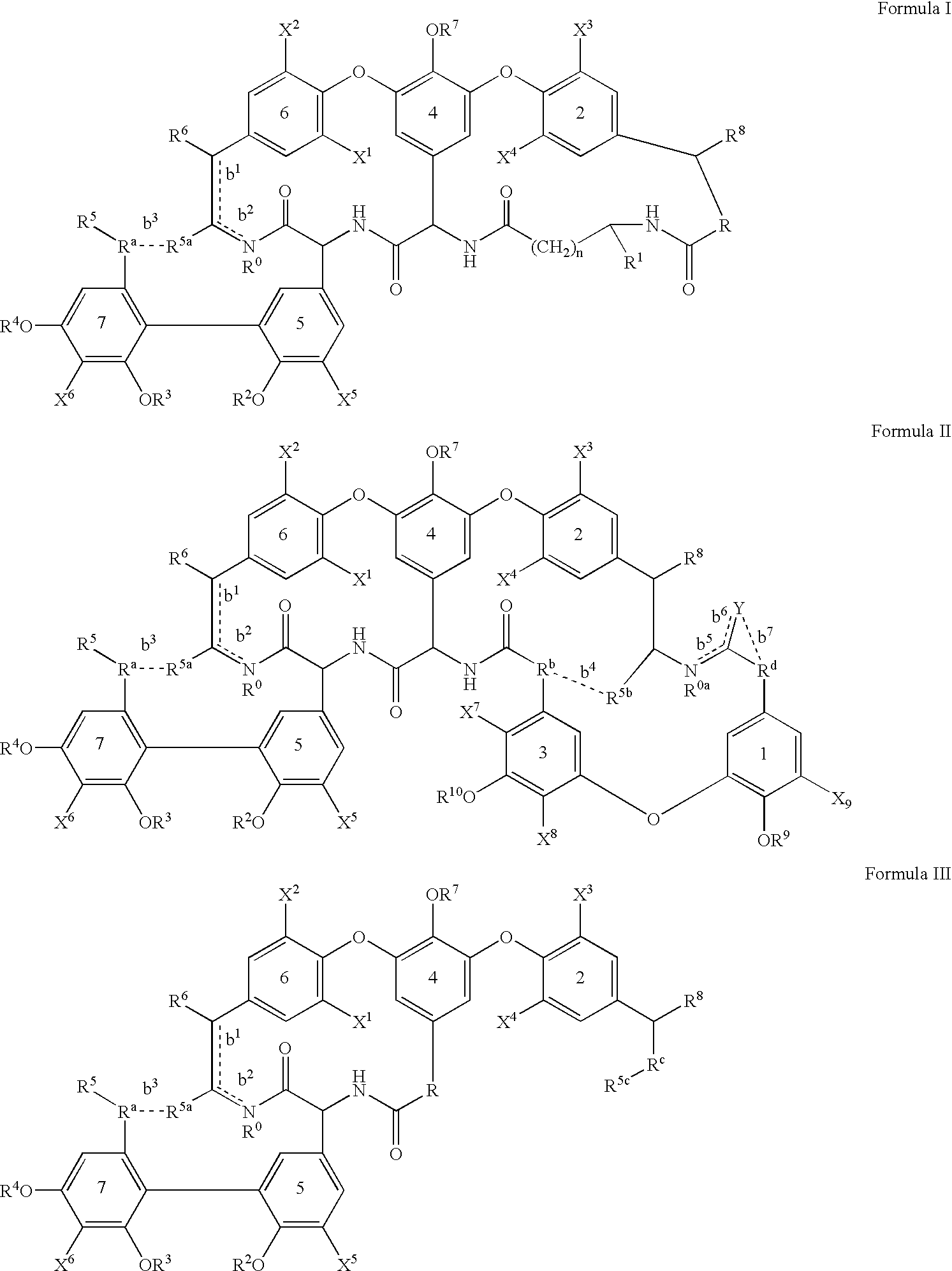
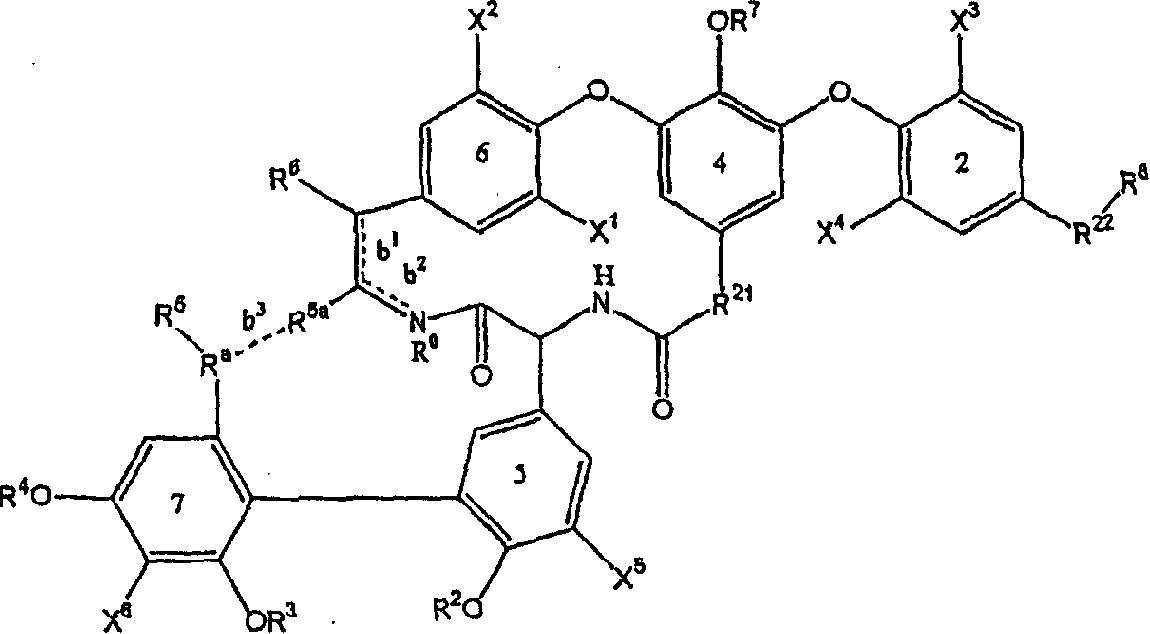
Amide derivative
There is provided a pharmaceutical drug, particularly a novel compound useful for prophylaxis or a therapeutic treatment of various diseases involving infections with viruses of the herpesvirus family, specifically various herpesvirus infections such as varicella (chicken pox) via varicella zoster virus, varicella zoster via recurrent infection with latent varicella zoster virus, herpes labialis and herpes encephalitis via HSV-1 and genital herpes via HSV-2 infection. An N-{2-[(4-substituted phenyl)amino]-2-oxoethyl}tetrahydro-2H-thiopyran-4-carboxamide derivative, of which phenyl group is substituted at position 4 with a specific 5- or 6-membered heteroaryl group, and a salt thereof have an effective anti-virus activity, and the oral administration thereof at a low dose enabled the therapeutic treatment of the above diseases.
Owner:ASTELLAS PHARMA INC
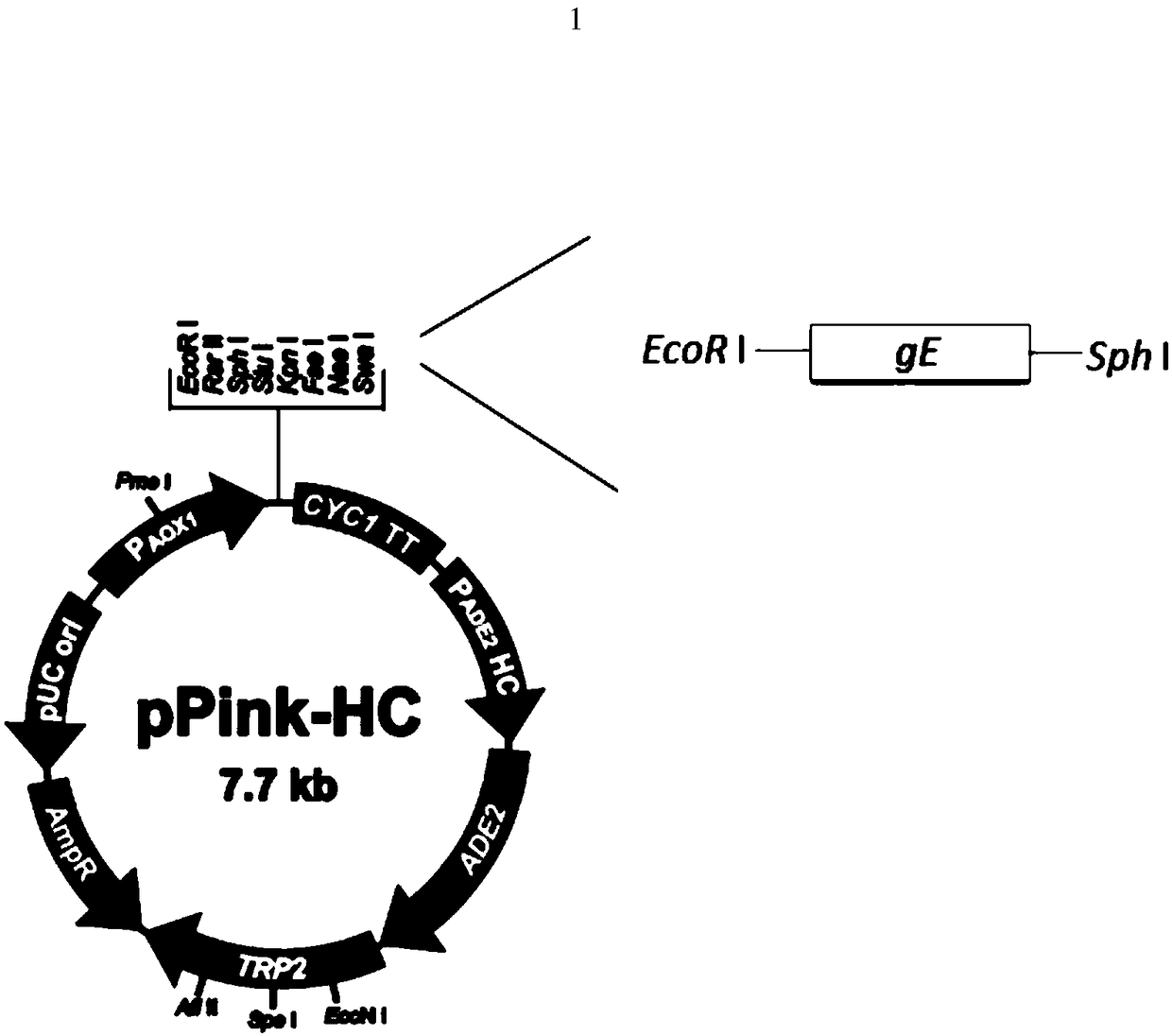
VZV glycoprotein E gene expression vector as well as recombinant yeast strain and application thereof
PendingCN108315344AOptimize purification stepsHigh titerPolypeptide with localisation/targeting motifFungiSequence signalBiotechnology
The invention discloses a VZV (varicella-zoster virus)glycoprotein E gene eukaryotic expression vector as well as a recombinant yeast strain and application thereof. The vector is a connector of alpha-gE fused gene and pPink-HC, and the alpha-gE fused gene is a gene complete sequence of VZV glycoprotein E and a fused gene alpha signal peptide. The glycoprotein E capable of successfully expressingthe VZV in an expression system of pichia pastoris discloses by the invention sets the foundation for the detection of the protein immunogenicity as well as the efficient expression of the protein inthe pichia pastoris as well as the research of the VZV vaccine.
Owner:BRAVOVAX
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com