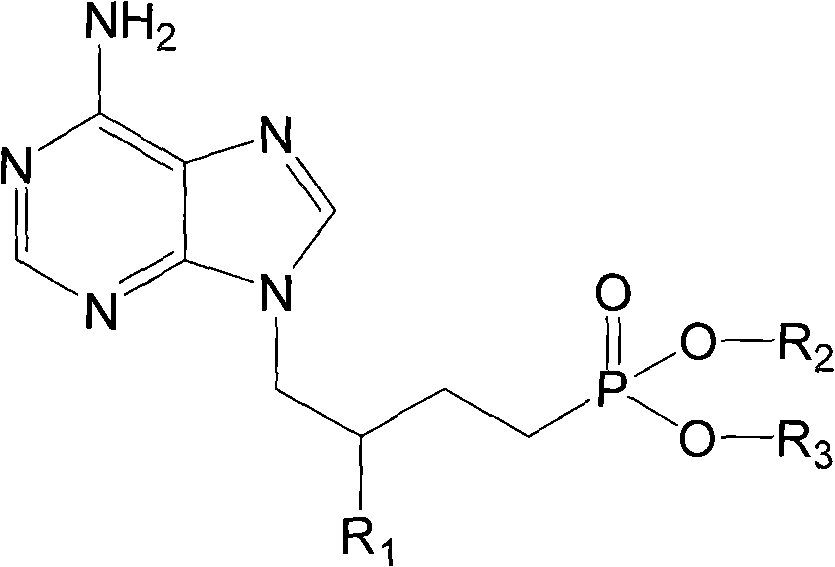
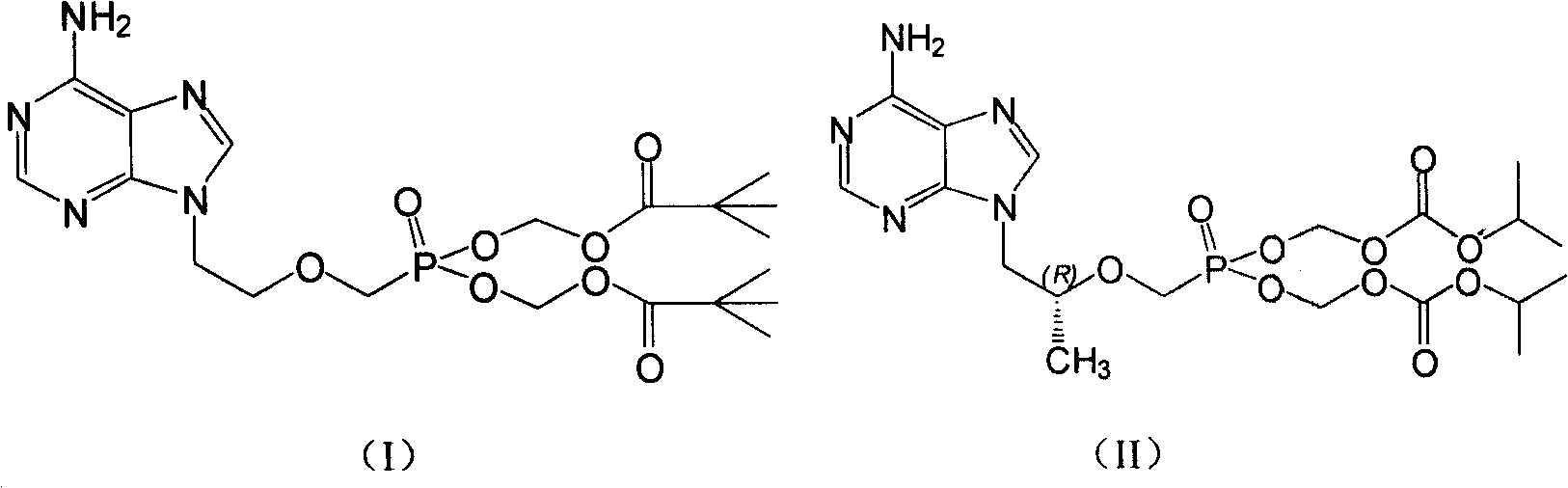
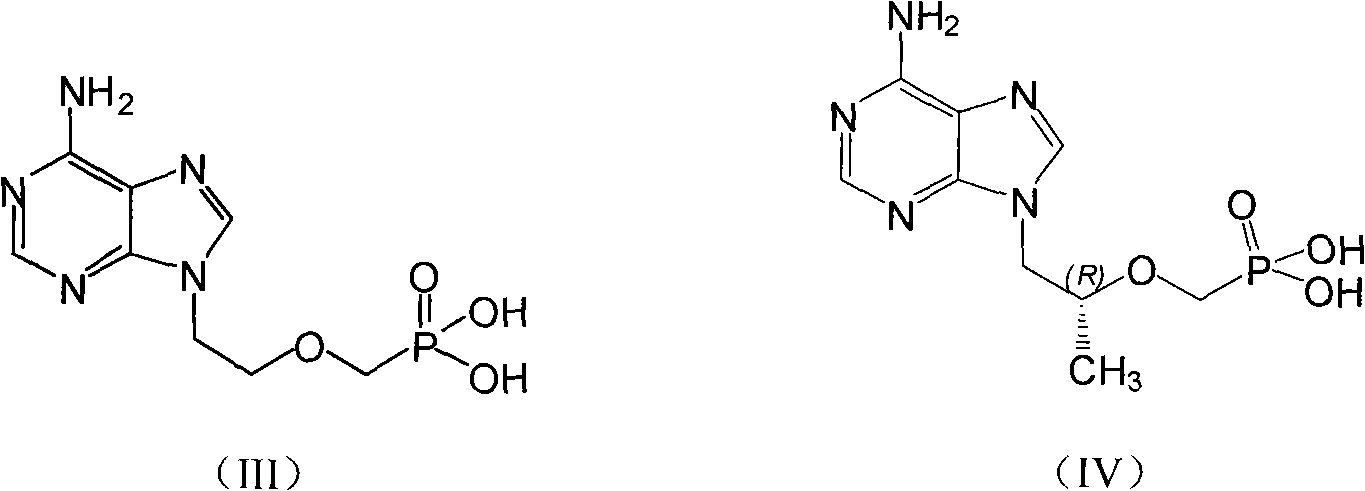
Patents
Literature
161 results about "Adefovir" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
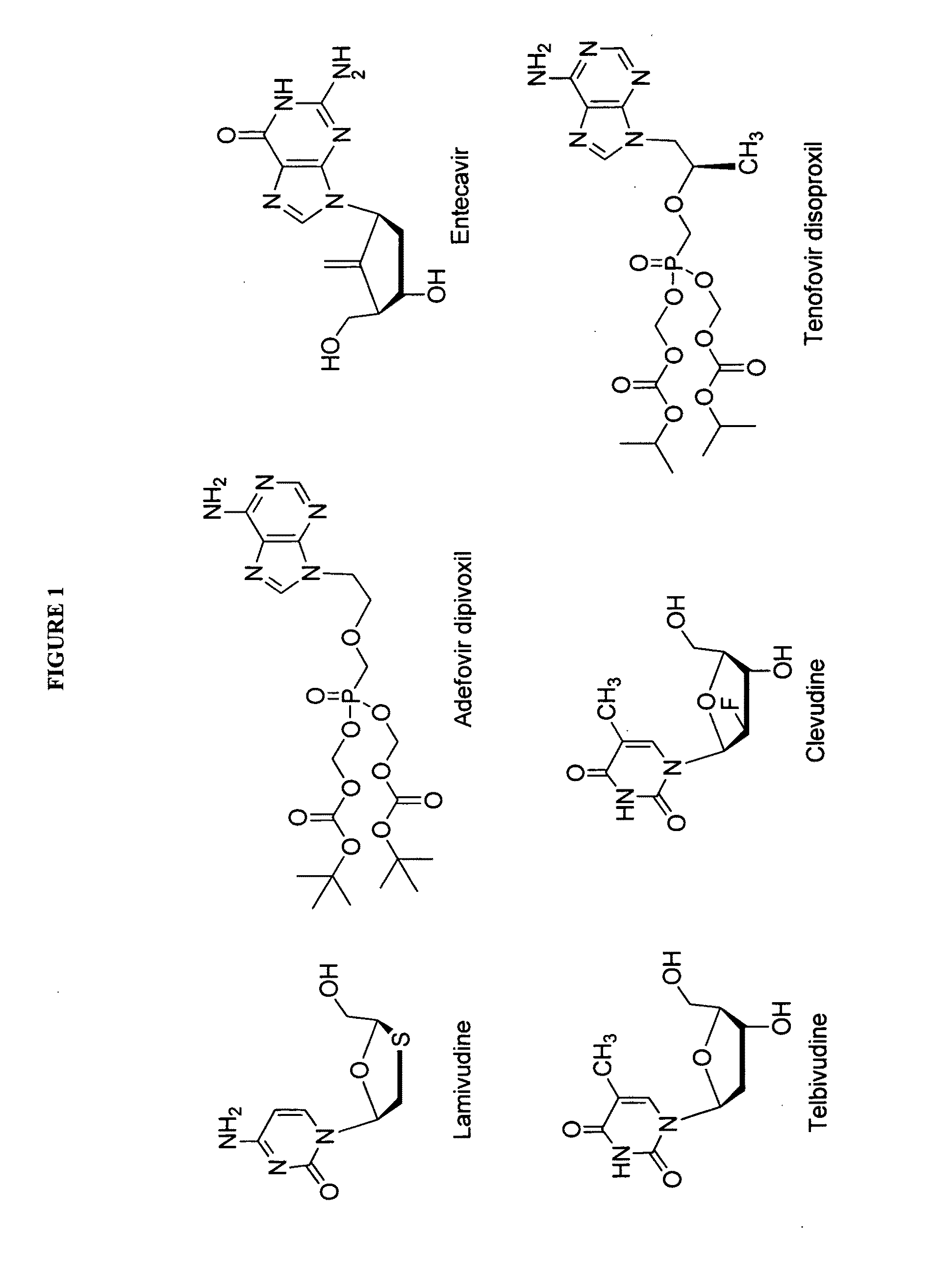
This medication is used to treat a chronic viral infection of the liver (hepatitis B) in people 12 years of age and older.
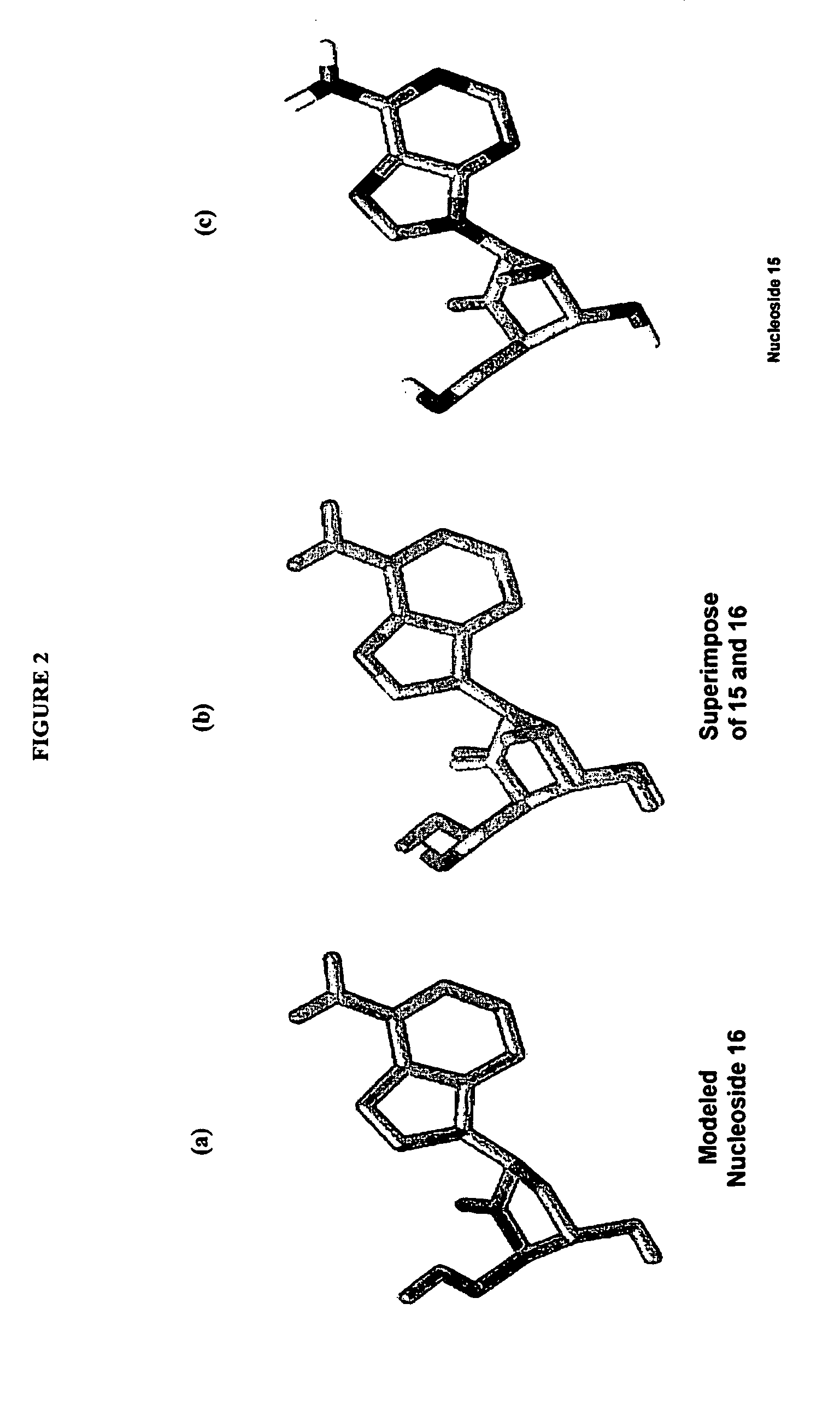
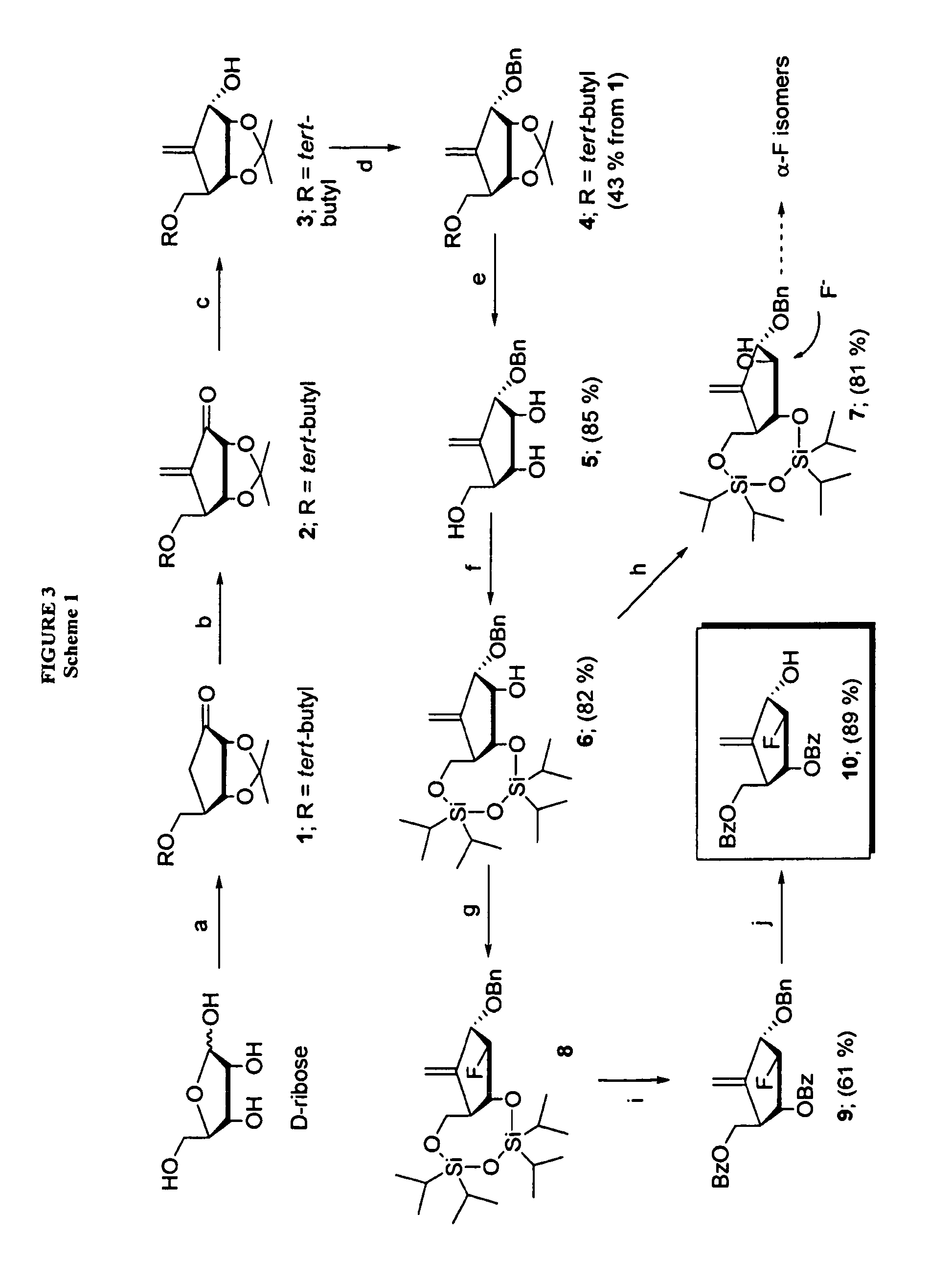
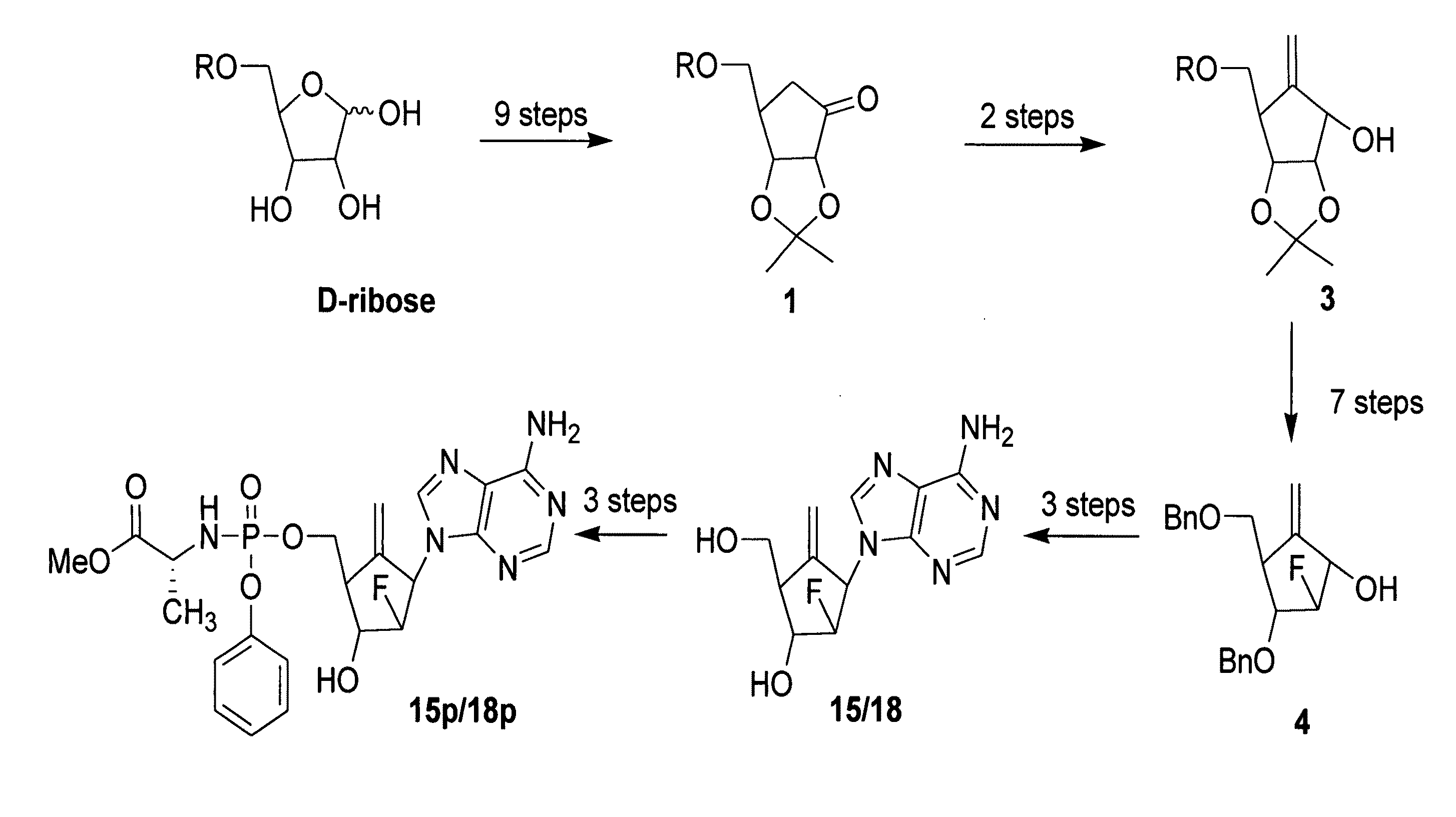
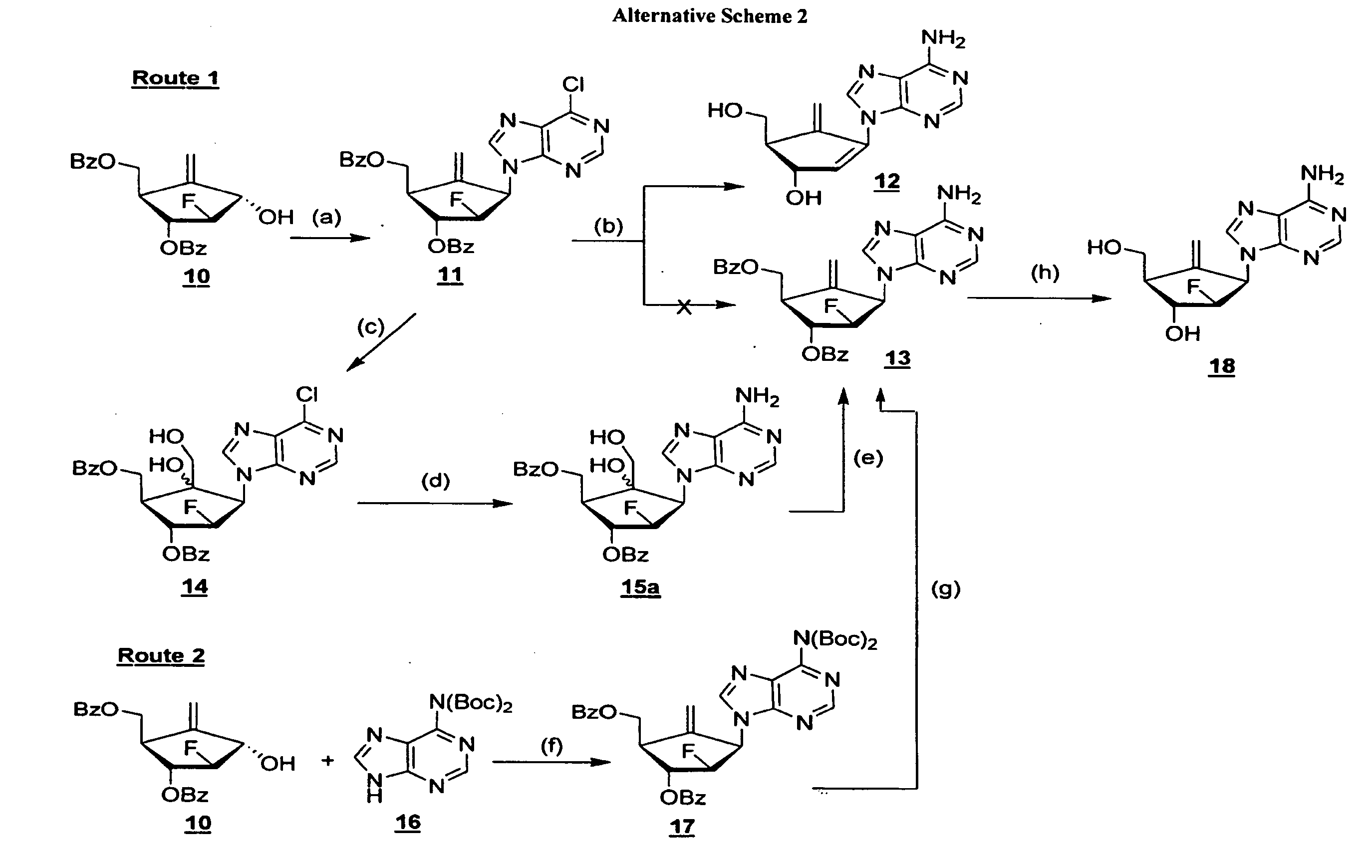
2′-fluoro-6′-methylene carbocyclic nucleosides and methods of treating viral infections
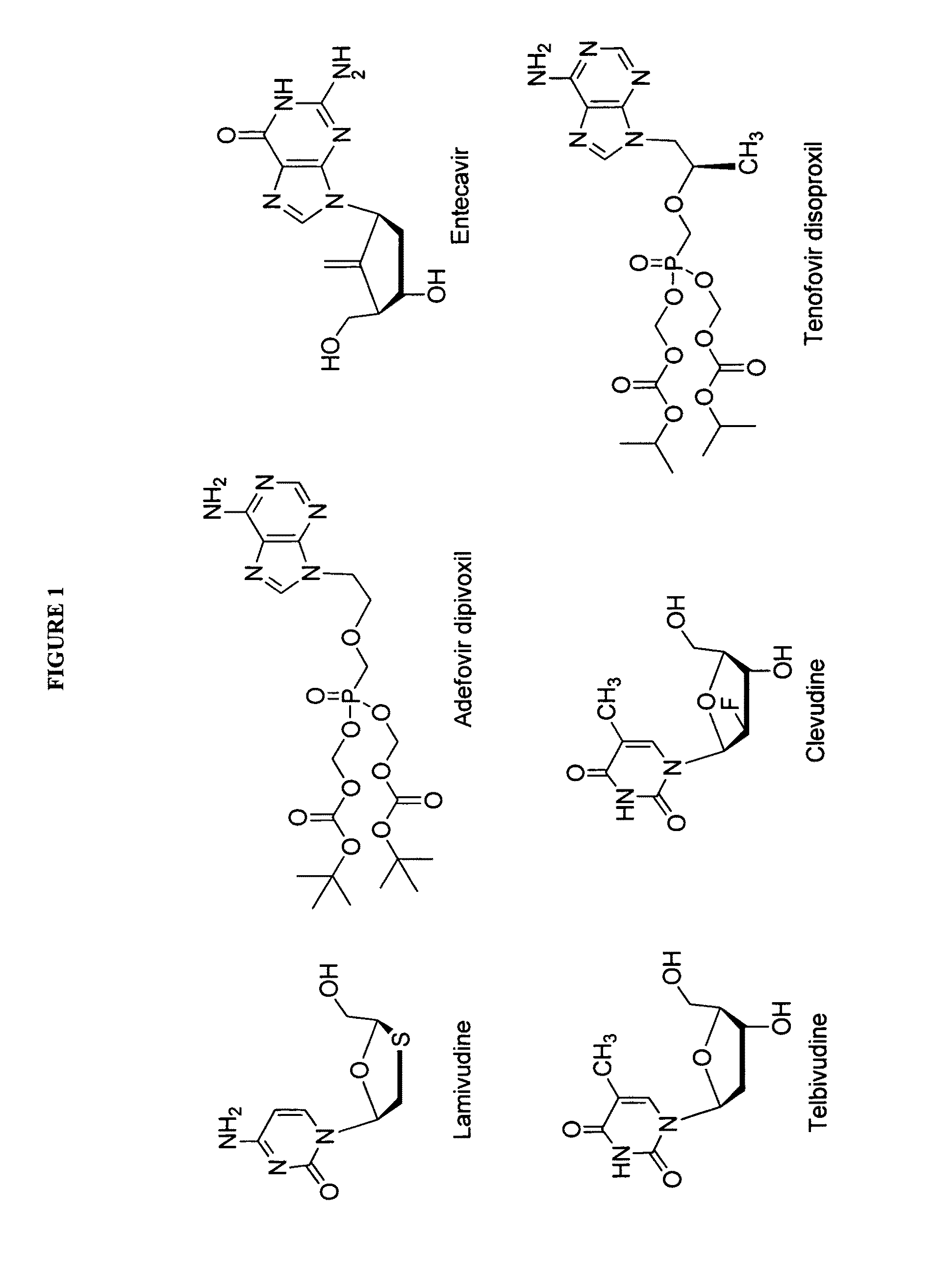
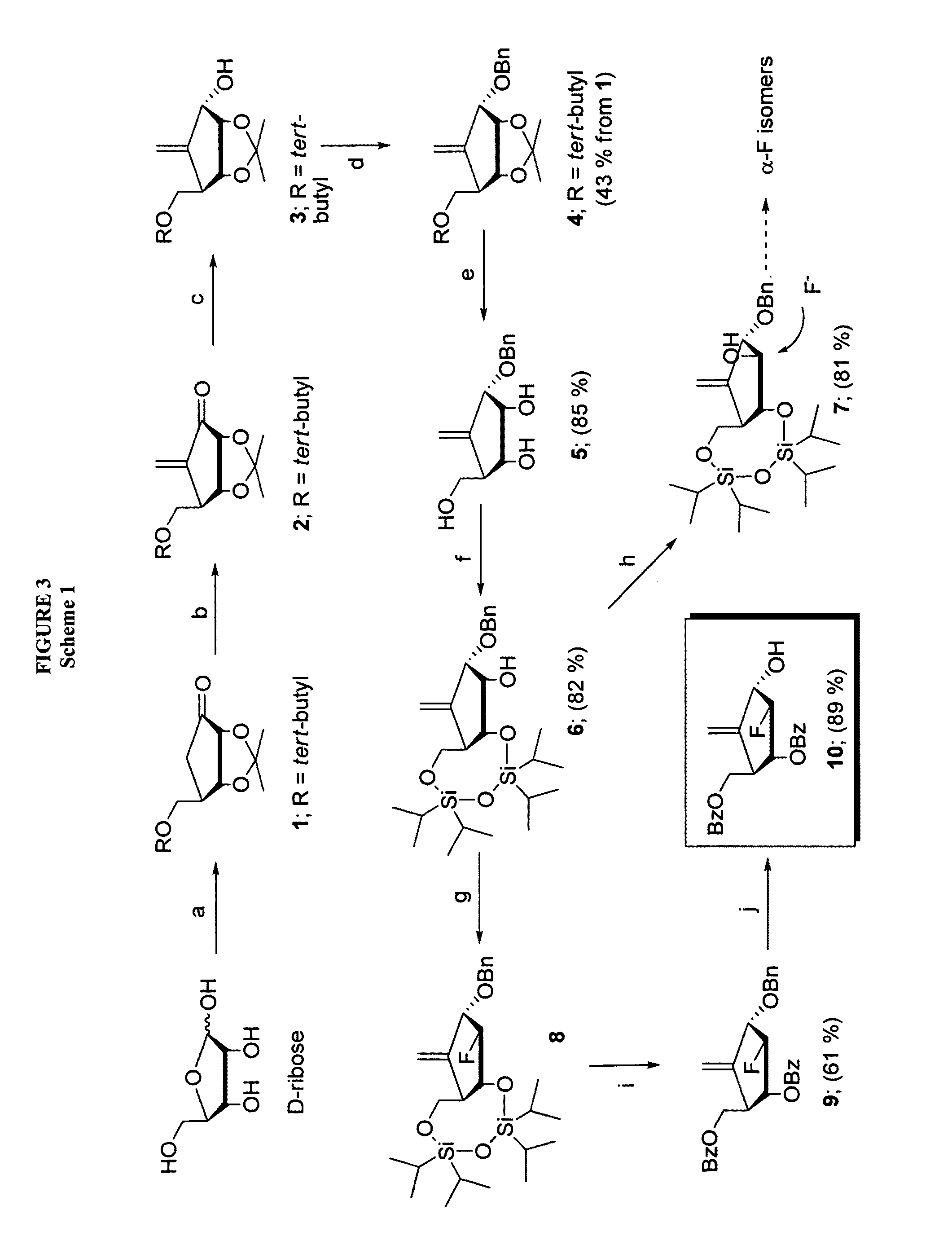
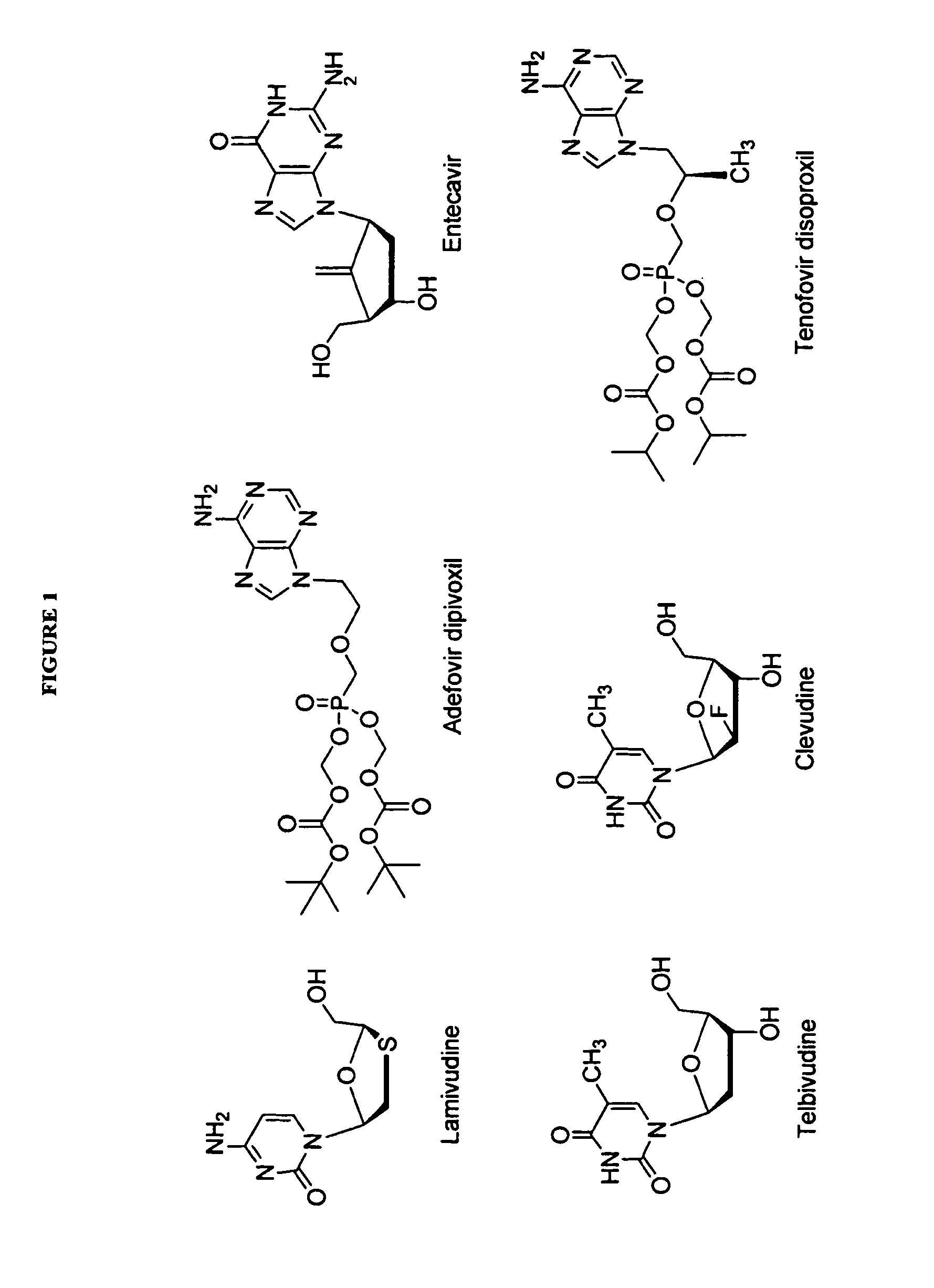
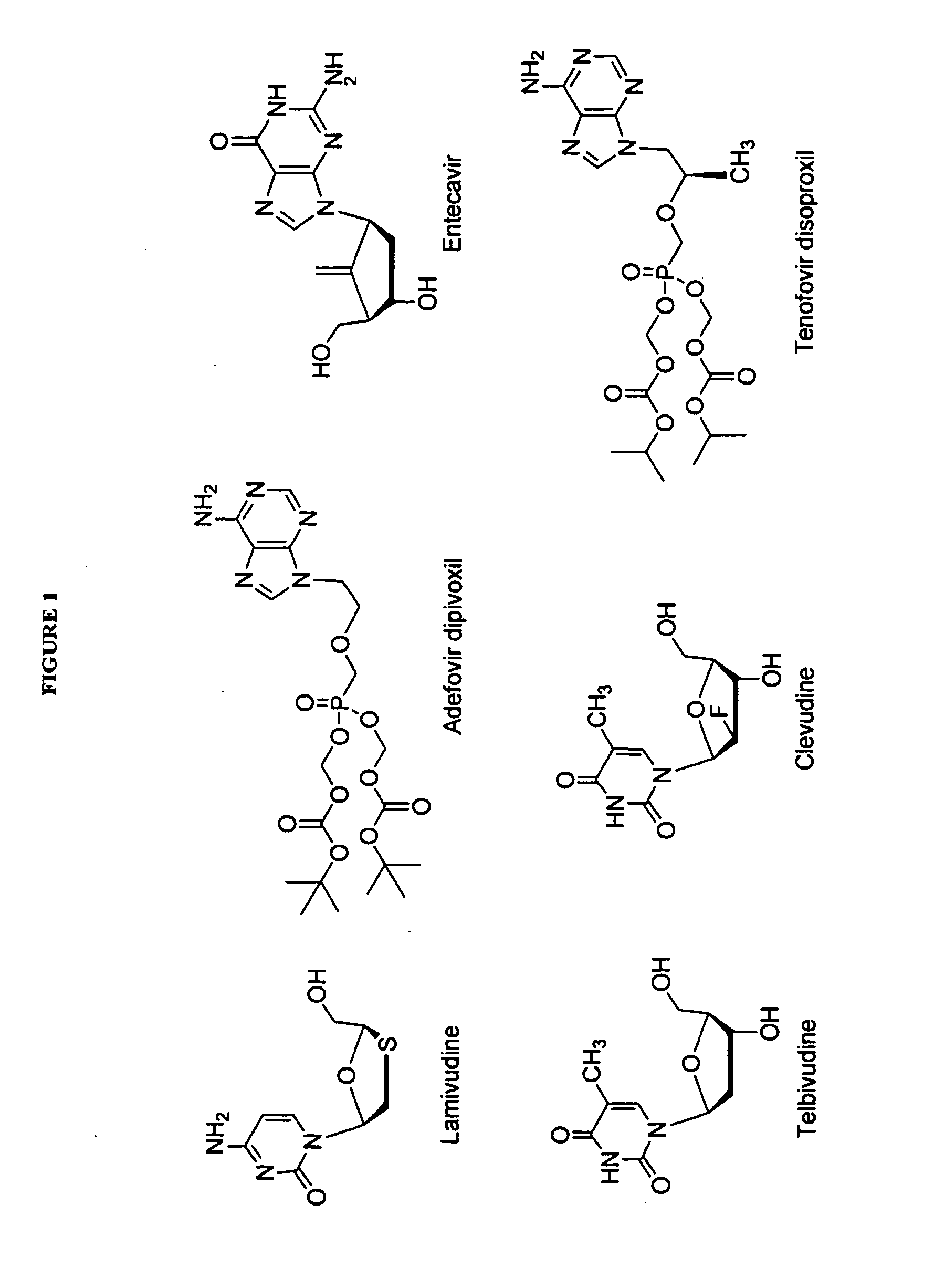
The present invention relates to 2′-Fluoro-6′-methylene carbocyclic nucleosides, pharmaceutical compositions containing these nucleosides and their use in the treatment or prophylaxis of a number of viral infections and secondary disease states and conditions thereof, especially including Hepatitis B virus (HBV) and secondary disease states and conditions thereof (cirrhosis and liver cancer), Heptatitis C virus (HCV), Herpes Simplex virus I and II (HSV-1 and HSV-2), cytomegalovirus (CMV), Varicella-Zoster Virus (VZV) and Epstein Barr virus (EBV) and secondary cancers which occur thereof (lymphoma, nasopharyngeal cancer, including drug resistant (especially including lamivudine and / or adefovir resistant) and other mutant forms of these viruses.
Owner:UNIV OF GEORGIA RES FOUND INC
2′-fluoro-6′methylene carbocyclic nucleosides and methods of treating viral infections
ActiveUS8946244B2Reduce the possibilityBiocideSugar derivativesHerpes zoster virusNasopharyngeal cancer
The present invention relates to 2′-Fluoro-6′-methylene carbocyclic nucleosides, pharmaceutical compositions containing these nucleosides and their use in the treatment or prophylaxis of a number of viral infections and secondary disease states and conditions thereof, especially including Hepatitis B virus (HBV) and secondary disease states and conditions thereof (cirrhosis and liver cancer), Hepatitis C virus (HCV), Herpes Simplex virus I and II (HSV-1 and HSV-2), cytomegalovirus (CMV), Varicella-Zoster Virus (VZV) and Epstein Barr virus (EBV) and secondary cancers which occur thereof (lymphoma, nasopharyngeal cancer, including drug resistant (especially including lamivudine and / or adefovir resistant) and other mutant forms of these viruses.
Owner:UNIV OF GEORGIA RES FOUND INC
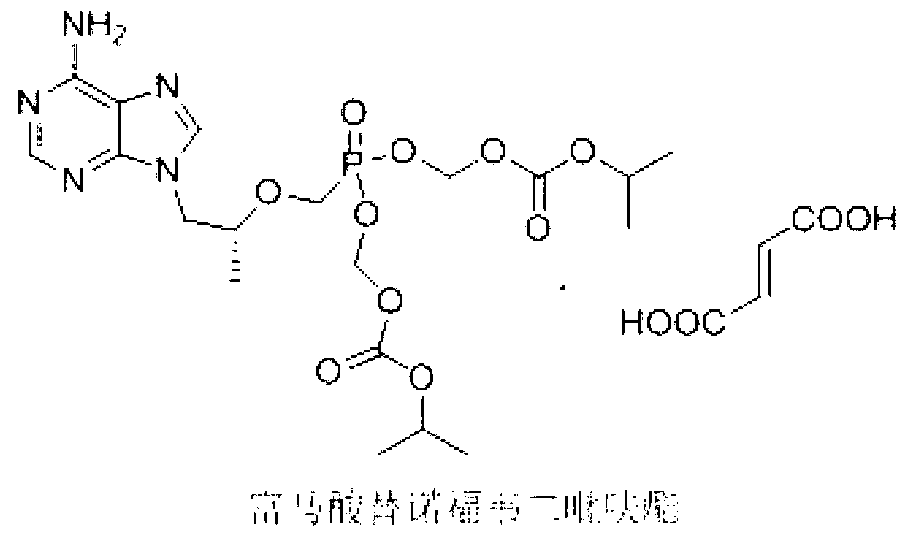
Amorphous Adefuweizhi ester amorphous solid matter and its prepn
InactiveCN1374314AEasy to filterKeep dryOrganic active ingredientsGroup 5/15 element organic compoundsSolid matterChemistry
The present invention is amorphous Adefuweichi ester solid matter and its preparation process. The amorphous Adefuweichi ester solid matter is solid powder obtained through solidifying oil amorphous Adefuweichi ester and has, X-ray test shows, at least 80% amorphous component. The amorphous Adefuweichi ester solid matter of the present invention is easy to filter and dry, has excellent flowability and bulk density performance, and the present invention makes it easy to produce medicine composite containing Adefuweichi ester and its suitable for industrial production.
Owner:上海仲夏化学有限公司
2'-Fluoro-6'-Methylene Carbocyclic Nucleosides and Methods of Treating Viral Infections
The present invention relates to 2′-Fluoro-6′-methylene carbocyclic nucleosides, pharmaceutical compositions containing these nucleosides and their use in the treatment or prophylaxis of a number of viral infections and secondary disease states and conditions thereof, especially including Hepatitis B virus (HBV) and secondary disease states and conditions thereof (cirrhosis and liver cancer), Heptatitis C virus (HCV), Herpes Simplex virus I and II (HSV-1 and HSV-2), cytomegalovirus (CMV), Varicella-Zoster Virus (VZV) and Epstein Barr virus (EBV) and secondary cancers which occur thereof (lymphoma, nasopharyngeal cancer, including drug resistant (especially including lamivudine and / or adefovir resistant) and other mutant forms of these viruses.
Owner:UNIV OF GEORGIA RES FOUND INC
Crystalline form of Adefovir dipivoxil and preparing process thereof
InactiveCN1435420AOrganic active ingredientsGroup 5/15 element organic compoundsChronic hepatitisEthyl group
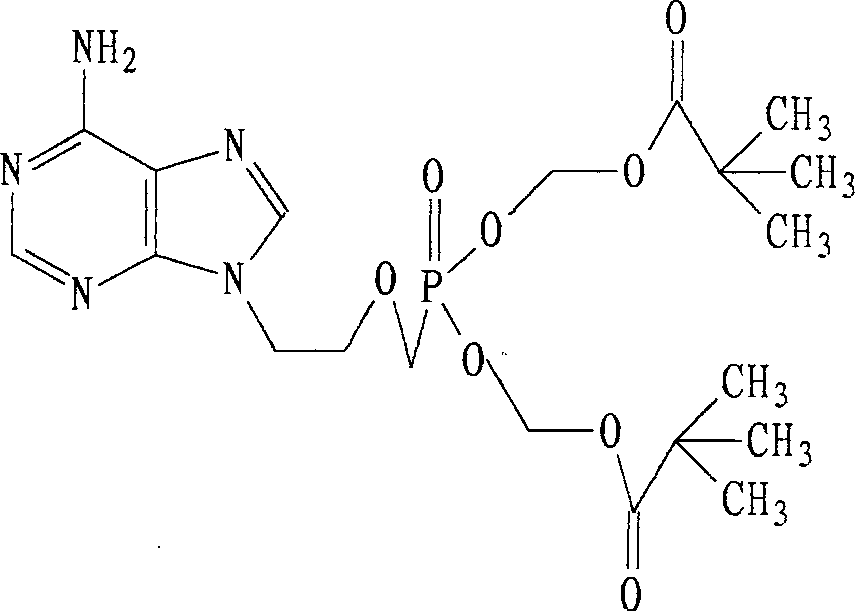
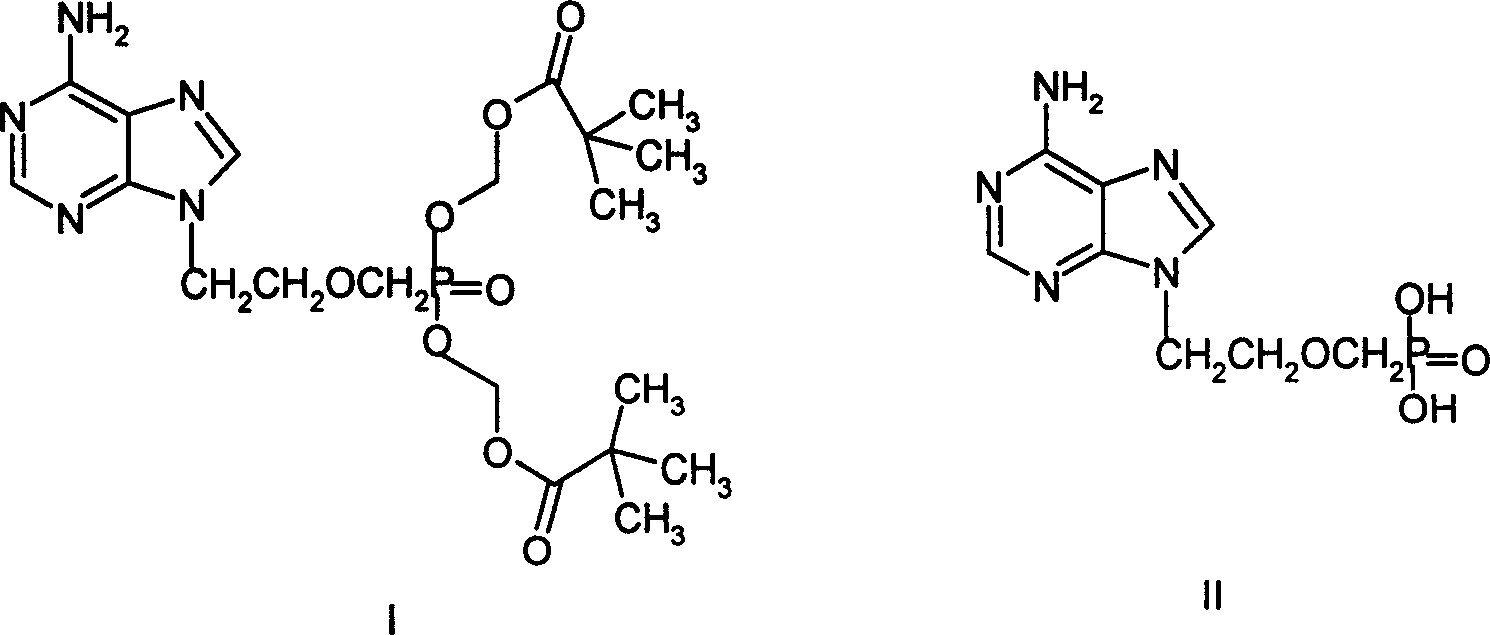
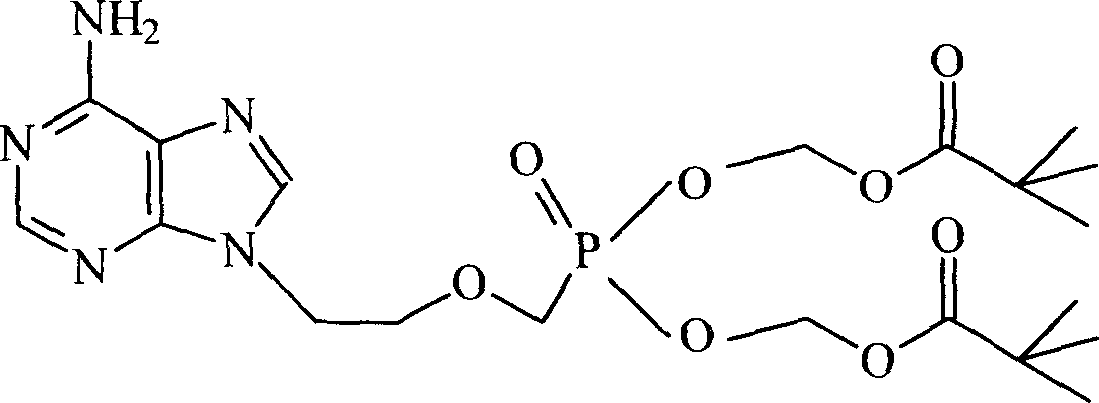
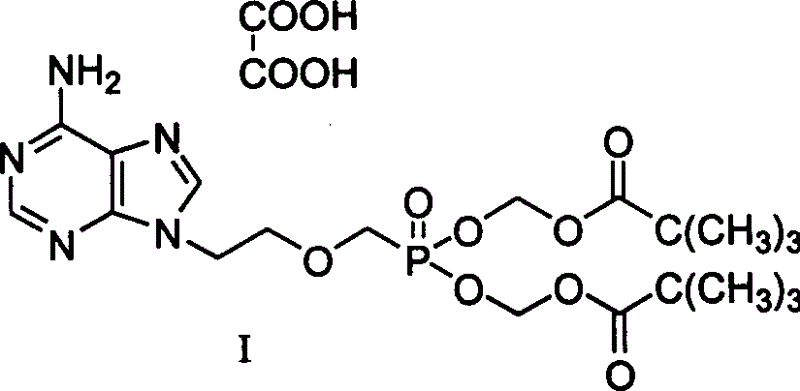
A compound Adefovir Dipivoxil (AD) in the form of anhydrous crystal for curing chronic hepatitis B is disclosed. It is just 9-[2-[bis(neopentanoyloxy methoxy)phosphorylmethoxy]ethyl] adenine. Its features are its high stability to light, moisture and heat. Its preparing process is also disclosed.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Crystal form of Pifuadefuwei
InactiveCN1396170AOrganic active ingredientsGroup 5/15 element organic compoundsMedicineViral hepatitis
A crystal form of Adefovir dipivoxil, 9-[2-[[bis (neovaleroxy) methyl] phosphoroso] methoxy]ethyl] adenine, its application to treating hepatism, such as viral hepatitis, its medical composition, and the process for preparing it are disclosed.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Adefovir dipivoxil new crystal state, new crystal state composition and its preparing method
InactiveCN1425673ARaise quality standardsGood crystal stabilityOrganic active ingredientsGroup 5/15 element organic compoundsAdefovirChemistry
The present invention describes a new crystal state of adefovir depivoxil and its preparation process as well as the composite of the new crystal state and its preparation process. The new crystal state and the composite are easy to prepare, stable and suitable for industrial production and storage.
Owner:SUZHOU ERYE PHARMA CO LTD
Adefovir dipivoil dispersion tablet and preparing method
InactiveCN1562046AGood disintegration timeGood dissolution rateOrganic active ingredientsAntiviralsCarboxymethyl starchLactose
A dispersing tablet of adefovirdipivoxil ester is prepared from adefovirdipivoxil ester, pregelatinized starch, microcrystalline fibres, lactose, carboxymethyl starch sodium, sodium laurylsulfate, and magnesium stearate through respective sieving, proportionally mixing and die pressing.
Owner:JIANGZHONG PHARMA
2'-fluoro-6'-methylene carbocyclic nucleosides and methods of treating viral infections
ActiveUS20130005677A1Reduce infectious virus titerReduce cell viabilityBiocideSugar derivativesHerpes simplex diseaseCirrhosis
The present invention relates to 2′-Fluoro-6′-methylene carbocyclic nucleosides, pharmaceutical compositions containing these nucleosides and their use in the treatment or prophylaxis of a number of viral infections and secondary disease states and conditions thereof, especially including Hepatitis B virus (HBV) and secondary disease states and conditions thereof (cirrhosis and liver cancer), Heptatitis C virus (HCV), Herpes Simplex virus I and II (HSV-1 and HSV-2), cytomegalovirus (CMV), Varicella-Zoster Virus (VZV) and Epstein Barr virus (EBV) and secondary cancers which occur thereof (lymphoma, nasopharyngeal cancer, including drug resistant (especially including lamivudine and / or adefovir resistant) and other mutant forms of these viruses.
Owner:UNIV OF GEORGIA RES FOUND INC
Variants of hepatitis B virus resistant against some nucleoside analogues, but sensitive to others, and uses thereof
ActiveUS20070042356A1Reduce sensitivityRapid and reliable detectionBiocideVirus peptidesHepatitis B immunizationReverse transcriptase
The present invention relates generally to the field of Hepatitis B variants exhibiting a reduced sensitivity to nucleoside analogues both in vivo and in vitro. More in particular, reverse transcriptase mutant rt I233V is provided. Present invention provides assays and methods for detecting such variant, which assays are useful in monitoring anti-viral therapeutic regimes and adjusting patient therapy. A diagnostic kit for detecting the presence of an HBV variant in a biological sample has also been described. Finally, the use of a farmaceutical composition to cure a subject suffering from a HBV infection, which HBV is resistant to lamuvidine and / or adefovir has been provided, which farmaceutical composition comprises the nucleoside analogue tenofovir.
Owner:UNIVERSITY OF BONN +2
Synthesis process for Adefovir ester of anti hepatitis type B virus medicine
InactiveCN1560059AReduce generationReduce pollutionGroup 5/15 element organic compoundsDigestive systemHepatitisHepatitis B virus
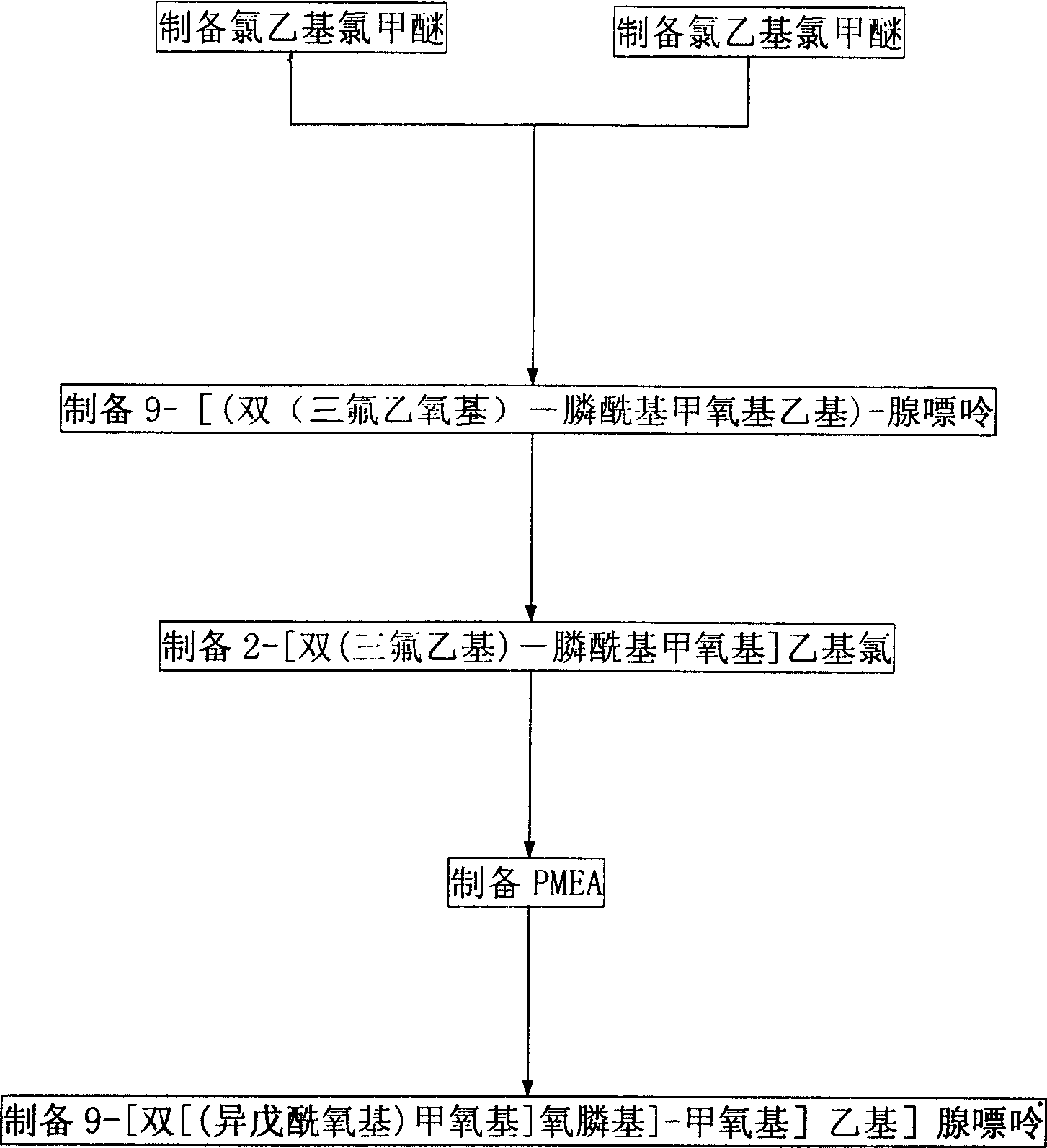
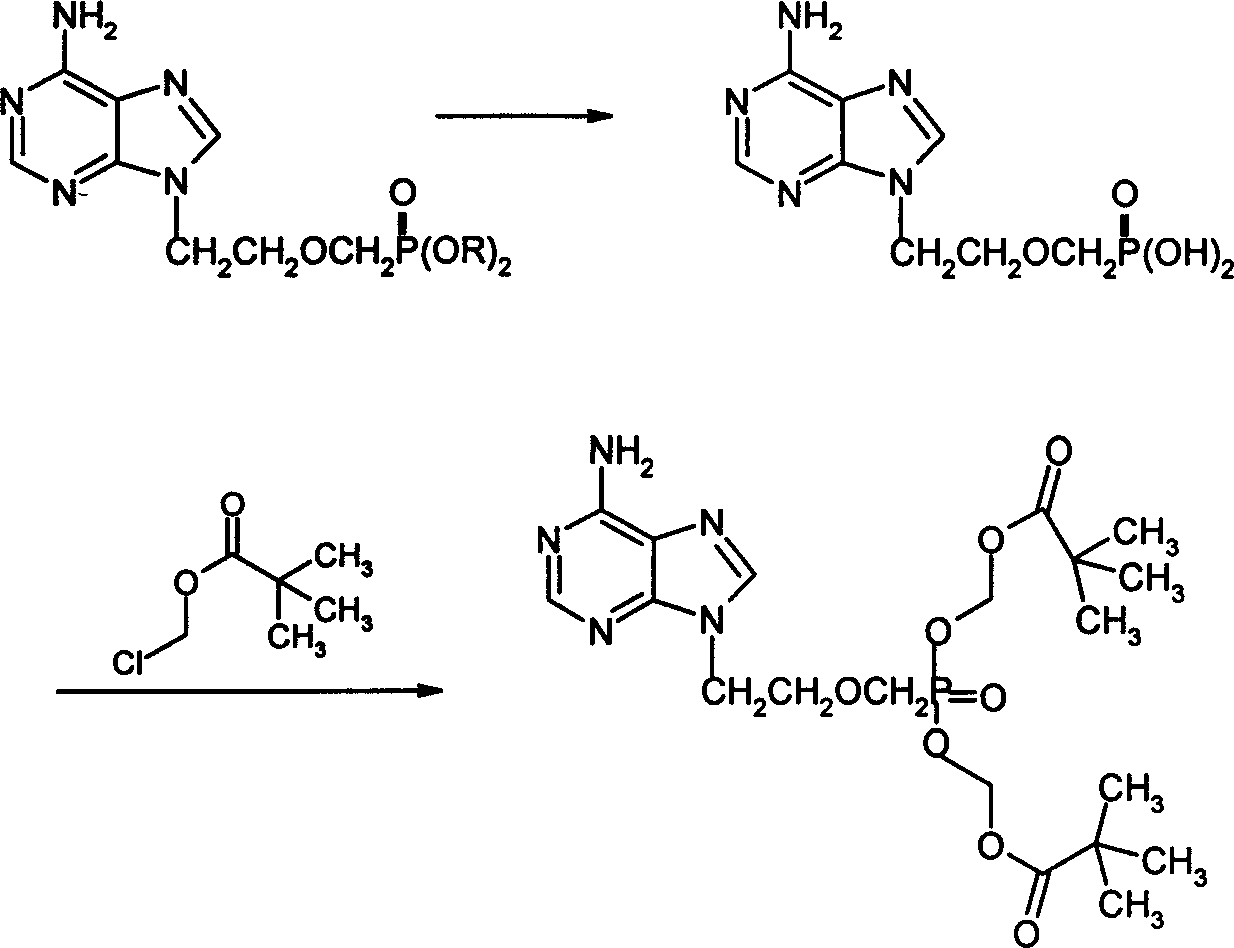
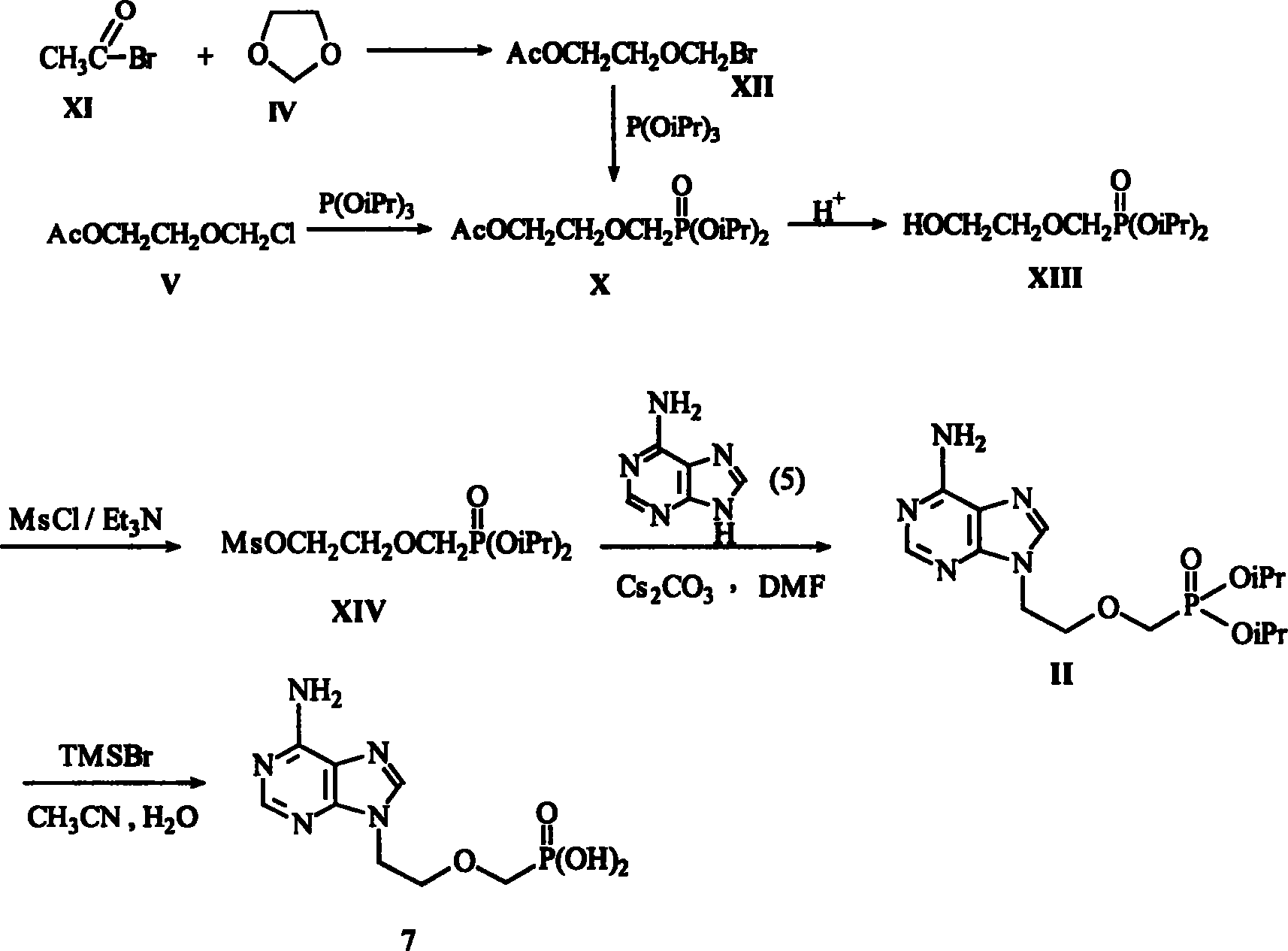
The invention provides a synthetic method of anti-hepatitis B virus medicine Adefovir dipivoxil, including the steps: (1) preparing chloroethyl chloromethylether; (2) preparing chloroethyl methylether; (3) preparing 2-[bis(trifluoroethyl)-phosphoryl methoxyl] ethyl chloride; (4) preparing 9-[bis(trifluoroethoxyl)-phosphoryl methoxyl ethyl]-adenine; (5) preparing PMEA; (6) preparing 9-[bis[(isopentanoyl)methoxyl]phosphinyl]-methoxyl]adenine; it has the characters of process easy to apply, environmental protection and higher yield.
Owner:嘉兴锐奇服饰有限公司
Anti-infective compositions, methods and systems for treating pathogen-induced disordered tissues
InactiveUS20060135464A1Stimulates rapid immunological attackExtraordinary therapeutic effectBiocidePharmaceutical delivery mechanismCidofovirMetabolite
Compositions, methods and systems for treating disordered epithelial tissues, such as is caused by pathogens and / or by toxins produced thereby. The invention relates to the use of an anti-infective and / or antimicrobial active agent in a carrier, with vigorous agitation of the disordered epithelial tissue for topical treatment thereof under such conditions sufficient to achieve clinically discernable improvement of the disordered epithelial tissue. The preferred anti-infective and / or antimicrobial active agent comprises a nucleoside, such as acyclovir, valcyclovir, penciclovir, famciclovir, ganciclovir, cidofovir, adefovir, and tenofovir, and derivatives, analogs, or metabolites thereof, or a mixture thereof, or 1-docosanol, optionally in combination with an organohalide. The inventive compositions and methods may employ the use of an applicator adapted for use in promoting the penetration of the treatment composition and / or the vigorous agitation of the disordered tissue.
Owner:QUADEX PHARMA
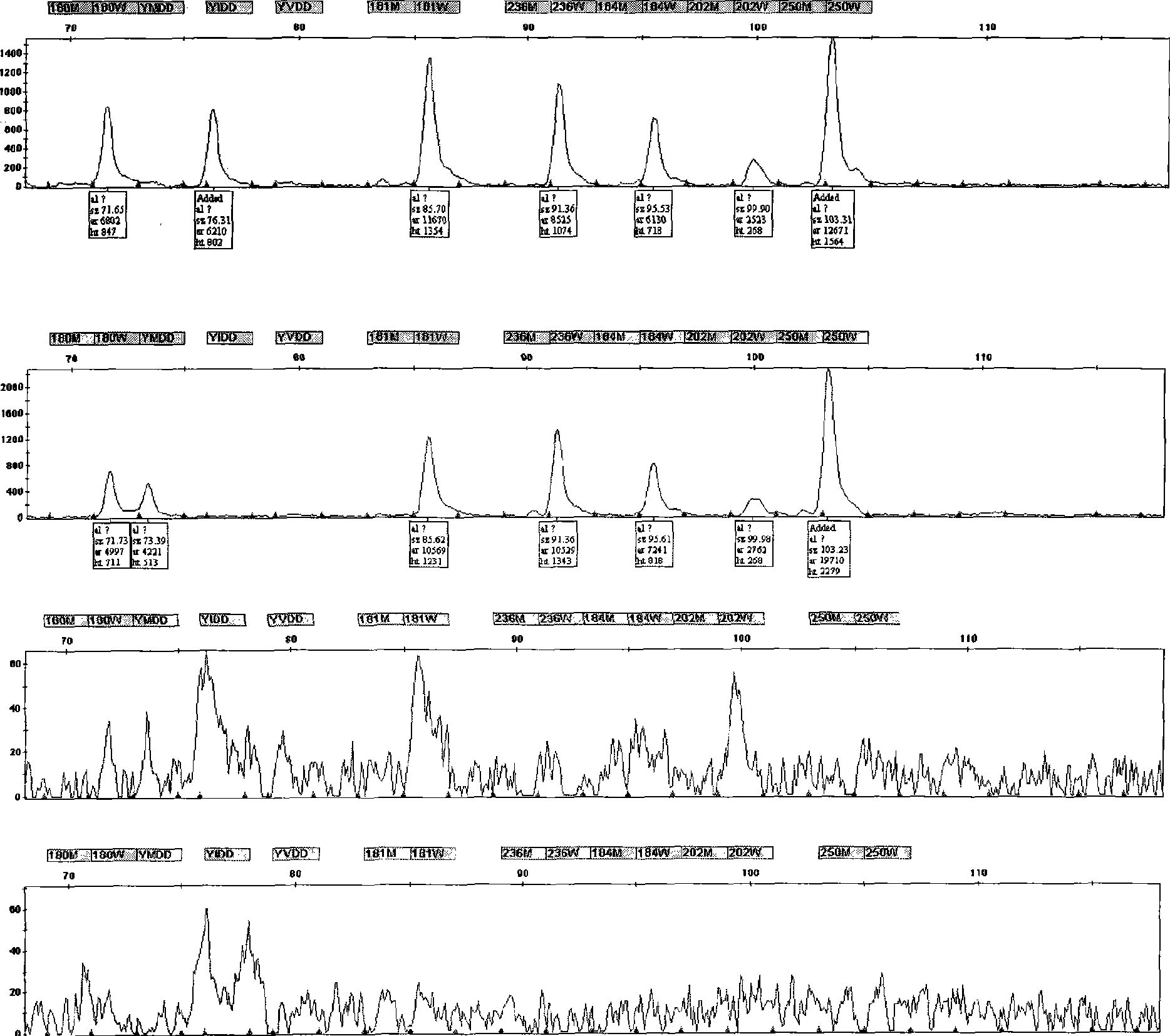
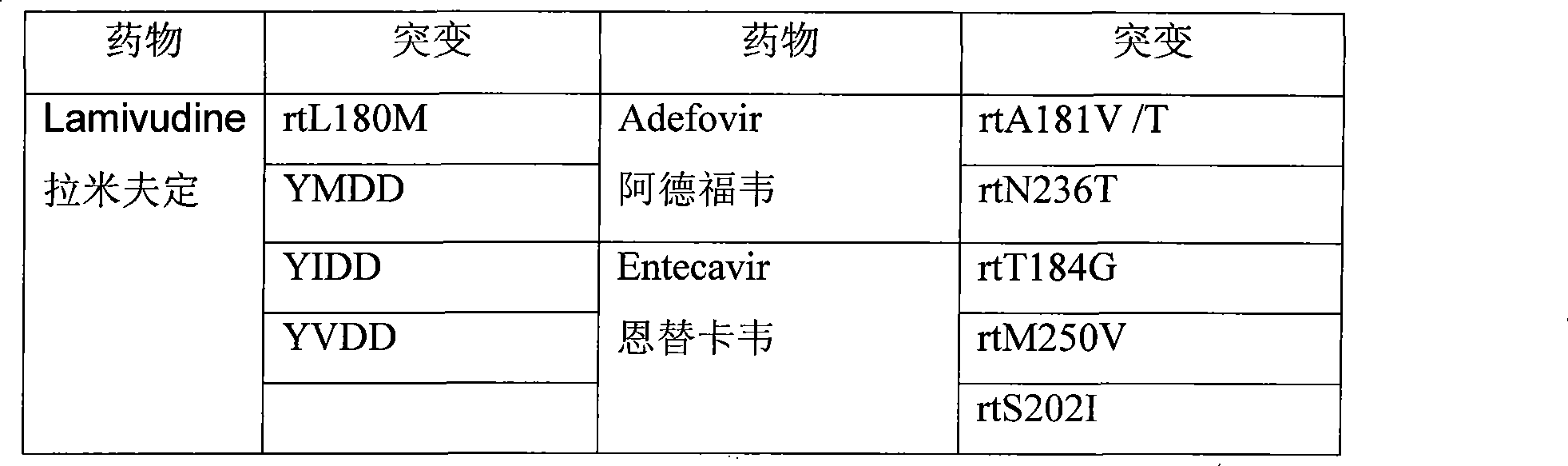
Hepatitis B virus multi-drug resistant gene locus typing detection kit
ActiveCN101545013AReduce the number of medicationsRelieve painMicrobiological testing/measurementMicroorganism based processesForward primerGenotype
The invention relates to a hepatitis B virus multi-drug resistant gene locus typing detection method and a kit thereof, wherein the detection method comprises the following steps that: DNA extraction is performed, wherein PCR amplification is applied to the abstracted DNA, the used PCR primers are two pairs of positive primers and negative primers; the PCR primers contain all correlative drug resistant locuses; amplified products of PCR are subjected to LDR amplification; the used probes are probes for correlation locus, which comprise a fluorescence labeling probe and a detection probe; a sequencing machine analyzes LDR products to judge the genotype of drug resistant locus; the multi-drug resistant gene locus is a drug resistant locus of lamivudine, ADV and ETV. The hepatitis B virus multi-drug resistant gene locus typing detection kit has reliable stable result, simple and quick operation; the kit can detect the drug resistant condition of various hepatitis B viruses, and supply clinical individuation medicine application instruction to hepatitis B virus patients. The kit can reduce the ineffectivity of drugs and administration times of the patients, thereby alleviating pains and economic burden of the patients.
Owner:上海翼和应用生物技术有限公司
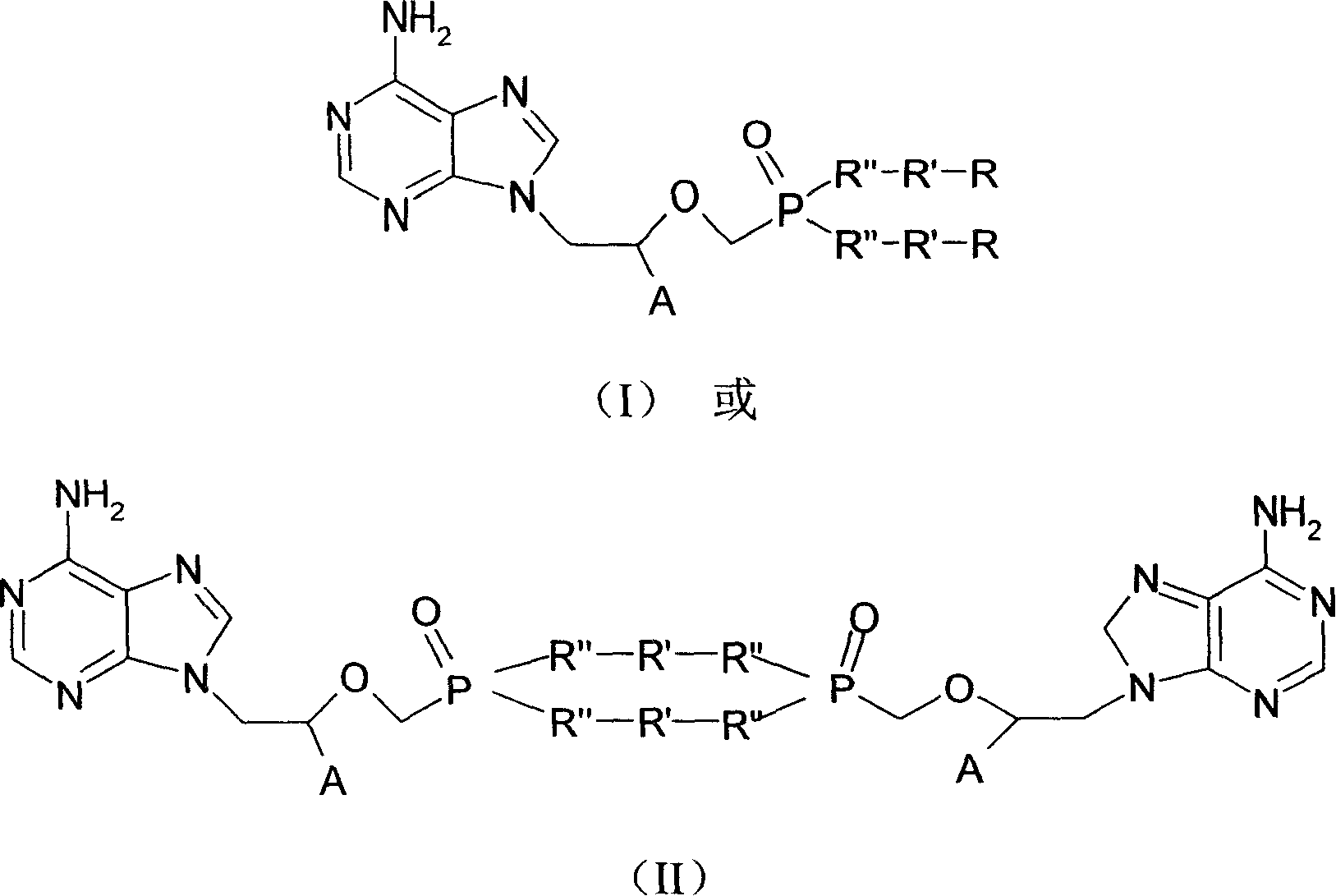
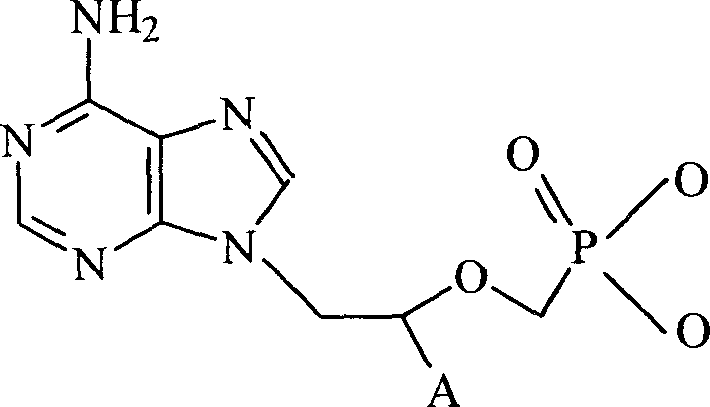
Chemical modified adefovir and tynofovir
ActiveCN1947796AReduced renal clearanceReduce nephrotoxicityPowder deliveryOrganic active ingredientsPolyethylene glycolChemistry
Owner:LIANYUNGANG RUNZHONG PHARMA CO LTD
Crystalline form of Adefovir ester and preparation method
InactiveCN1498890ASuitable for industrial mass productionHigh purityGroup 5/15 element organic compoundsNucleotideCrystallization
A new crystal form of nucleotide analog 9-[2-bis(tert-amylacyloxymethoxy) phosphomethoxy ethyl] adenine (or adefovir dipivoxil-AD), its preparing process and its application are disclosed. Its process for synthesizing, purifying and preparing AD is also disclosed.
Owner:XICHUAN INST OF ANTIBIOTIC IND NAT MEDICINE SUPERVISION BUREAU
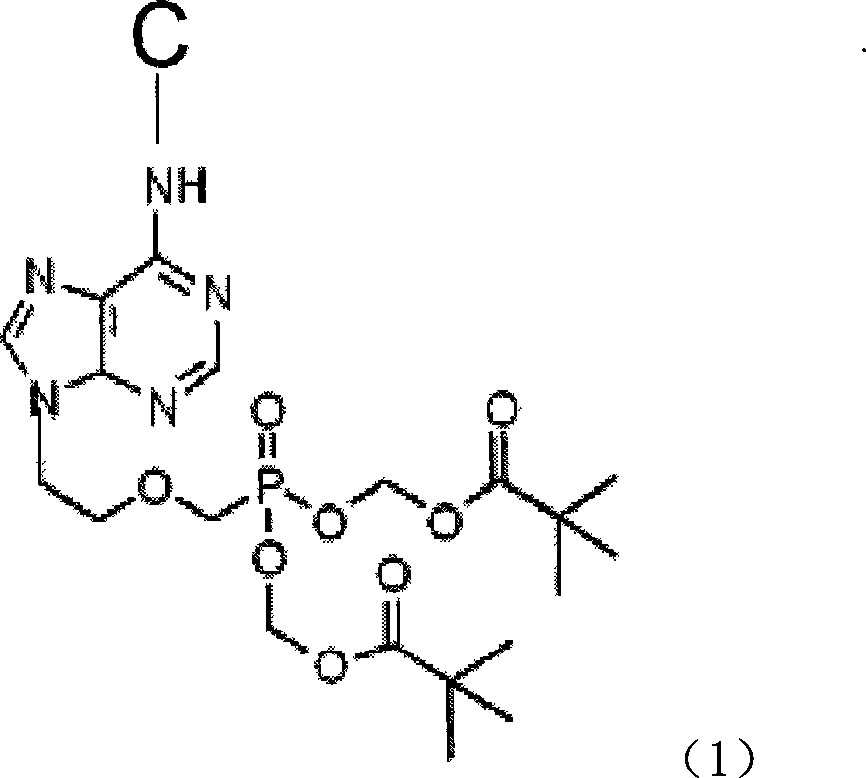
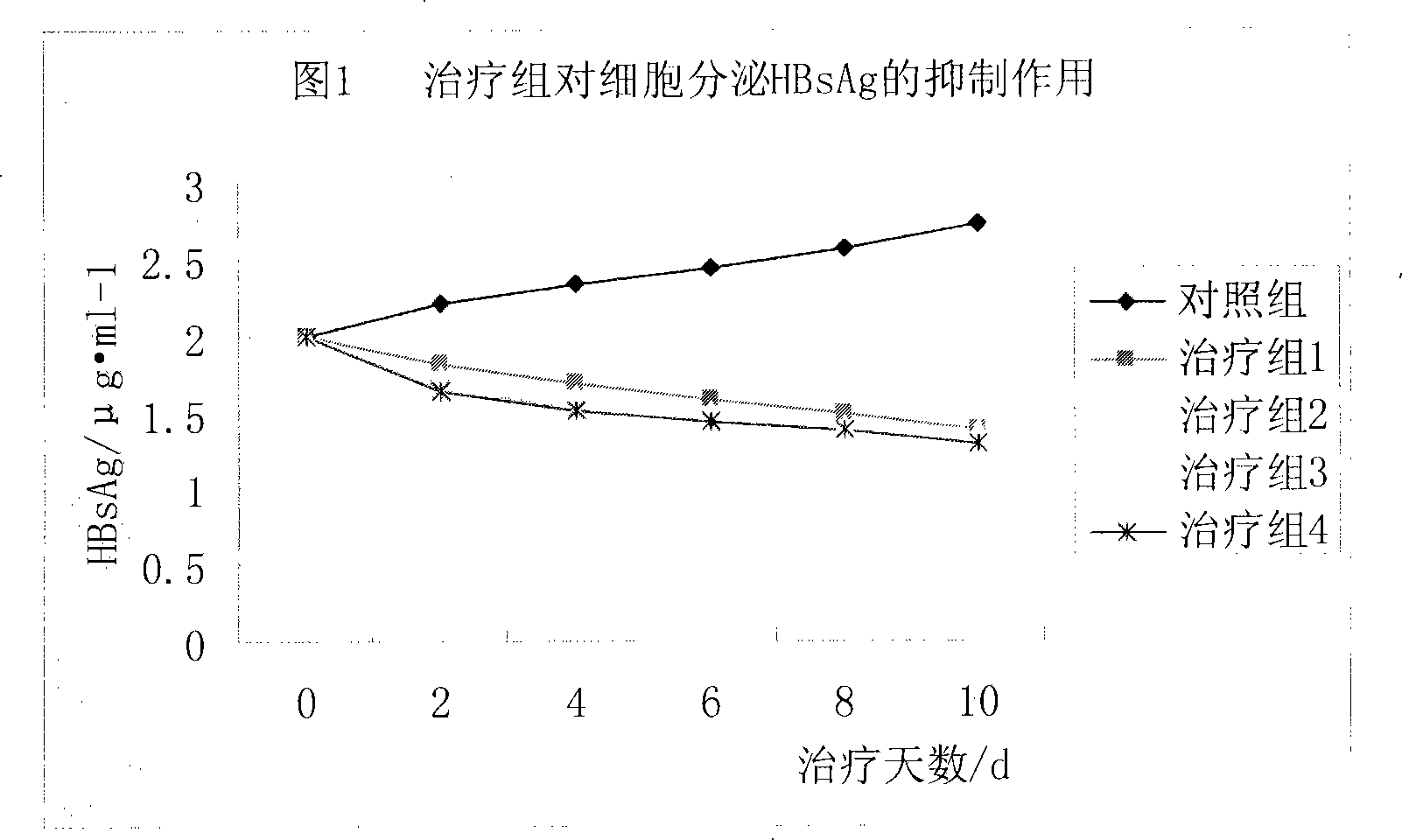
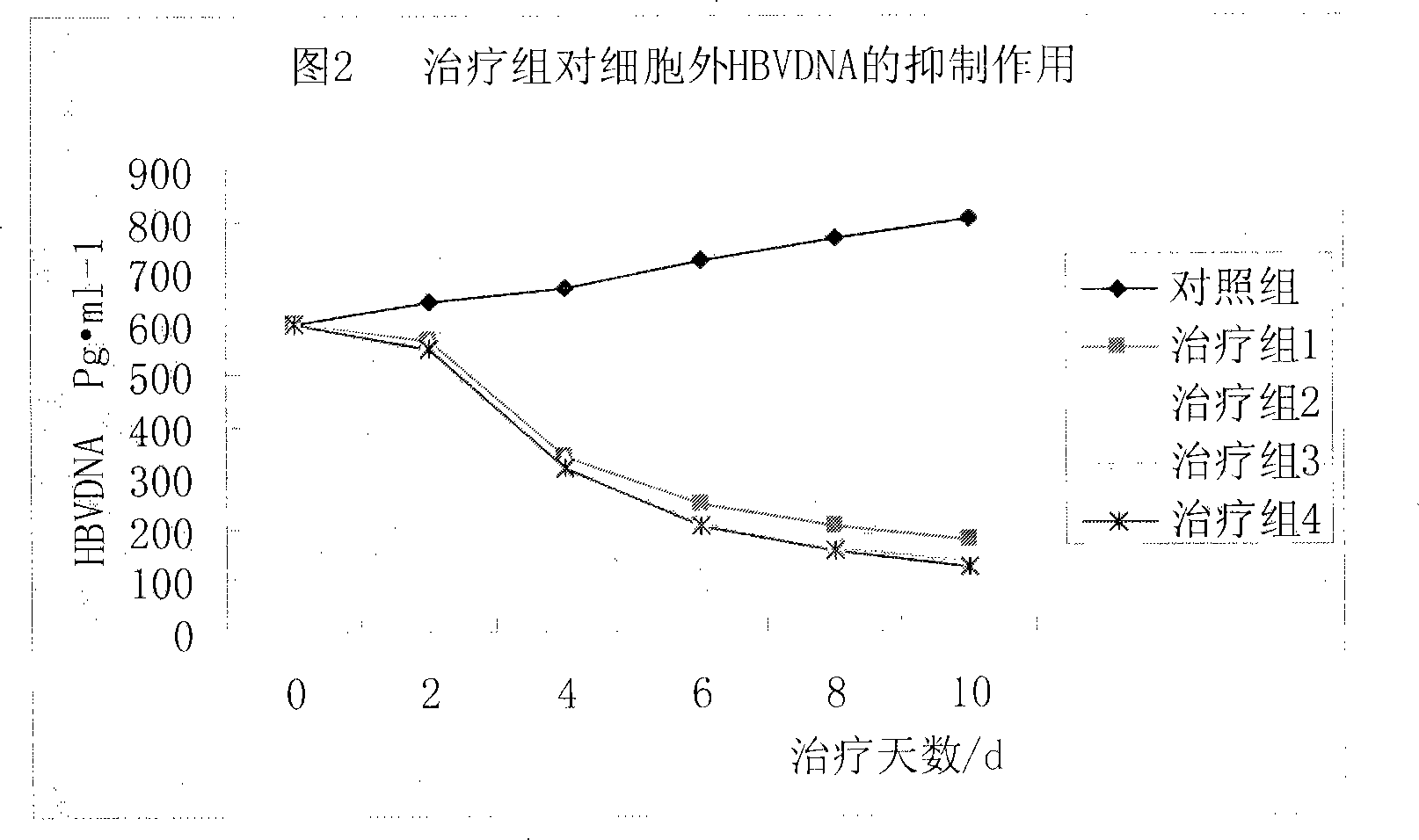
Prodrug with liver-targeted anti-HBV effect
InactiveCN101307076AReduce concentrationGood curative effectOrganic active ingredientsGroup 5/15 element organic compoundsCholic acidSide effect
The invention provides a prodrug of a hepatitis b virus resistant drug with a liver targeting action and a preparation method for the same. The prodrug is a prodrug of Adefovir or Tenofovir. Higher fatty acid, glyceride, cholic acid compounds or the base groups of derivatives thereof with the liver targeting action are connected with Adefovir or Tenofovir to prepare the prodrug. After being taken into a human body, the prodrug is concentrated in the liver and then is decomposed, thereby having the effects of reducing the dosage, improving the curative effect, reducing the drug concentration in a kidney and reducing the toxic and side effect. The invention also provides a method for synthesizing the prodrug.
Owner:江苏吴中苏药医药开发有限责任公司
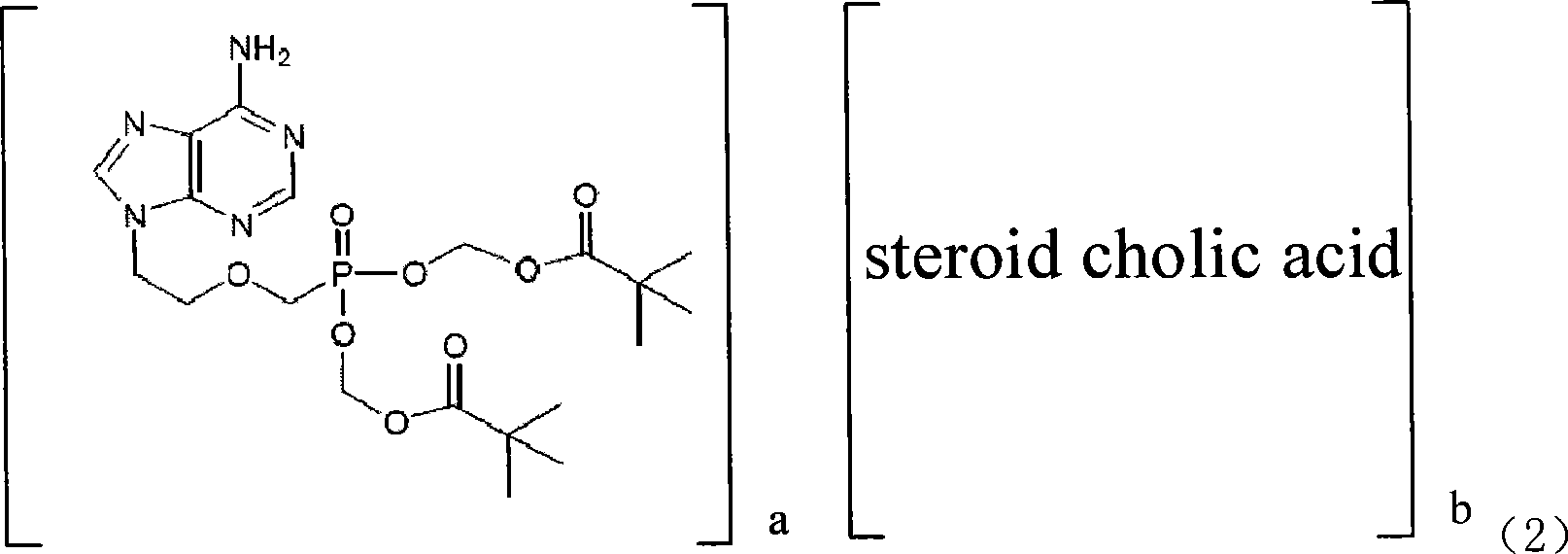
Adefovir dipivoxil ester colalin derivatives as well as preparation method and uses thereof
InactiveCN101182331AIncrease concentrationGood treatment effectOrganic active ingredientsGroup 5/15 element organic compoundsCholeic AcidStereochemistry
The present invention discloses an adefovir dipivoxil cholic acid class derivative and also relates to a preparation method and applications thereof at the medical aspect. The adefovir dipivoxil cholic acid class derivative is a hepatic targeting precursor drug of adefovir; compared with the adefovir dipivoxil, the adefovir dipivoxil cholic acid class derivative can improve the concentration of the adefovir in liver after being taken orally, and the adefovir dipivoxil cholic acid class derivative can be used as a preferential drug for the treatment of resisting virus and especially resisting hepatitis virus.
Owner:CHINA PHARM UNIV
Compound medicine compounds containing adefovir dipivoxil, preparing method and uses thereof
The invention relates to a medical composition with active components of adefovir dipivoxil and another rnucleotide (acid) anti-virus medicine and the preparation method and usage thereof. The adefovir dipivoxil and anther rnucleotide (acid) anti-virus medicine are taken as the active components, and mixed with a plurality of pharmaceutically acceptable supplements to prepare the medical composition; and the invention can be applied in treatment of viral hepatitis B. The adefovir dipivoxil and anther rnucleotide (acid) anti-virus medicine are taken as raw materials of the invention content, a plurality of supplements with special type and proportion are added, and various kinds of oral preparations such as tablet, capsule, dispersion, chewable tablet, oral disintegrating tablet, buccal tablet, dropping pill and soft capsule are prepared and developed according to the technical method described by the invention.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
Adefovir dipivoxil CHARIOTEER crystallographic form and preparation method thereof
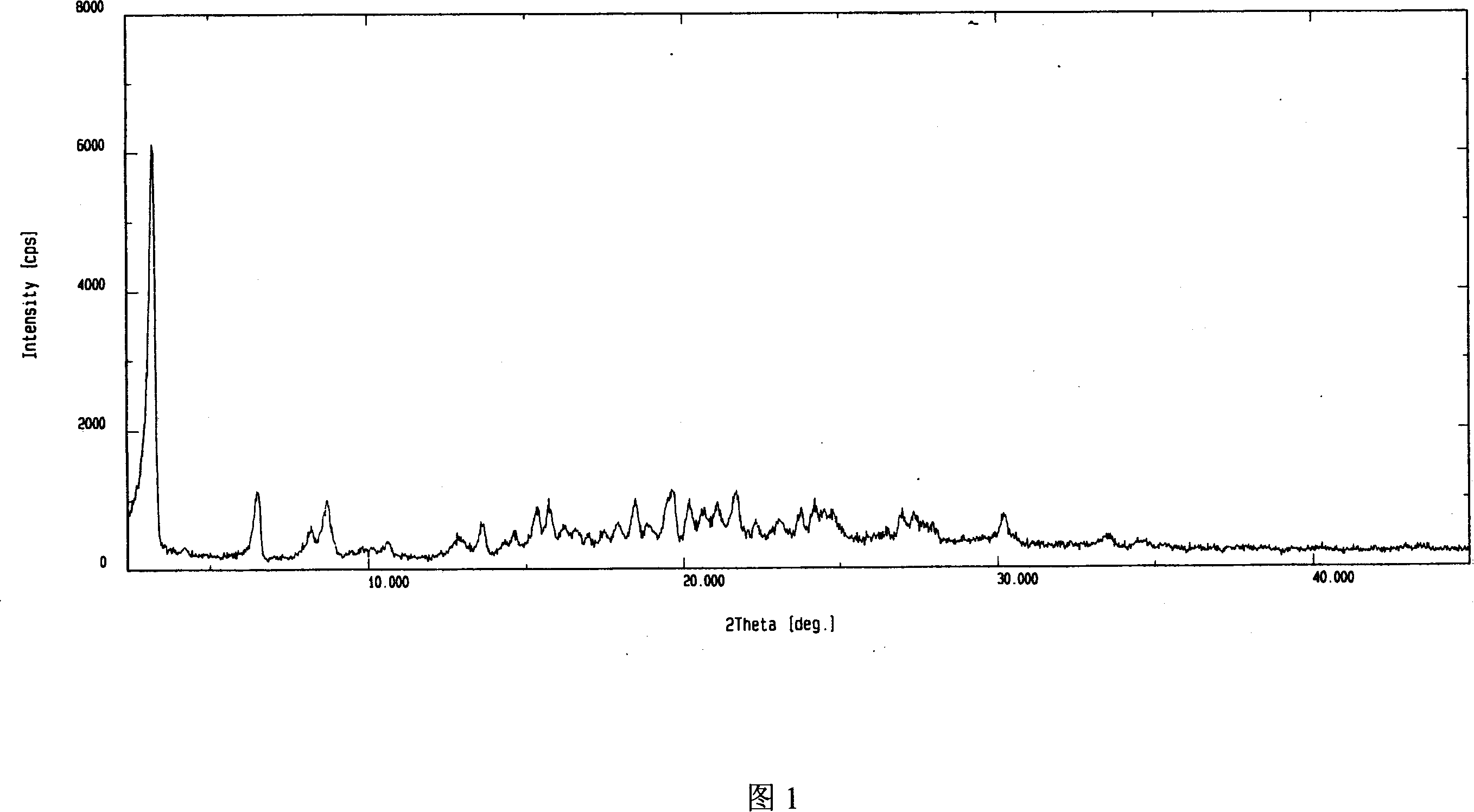
ActiveCN1995048AGood inhibitory effectSimple preparation processGroup 5/15 element organic compoundsCrystal systemIrradiation
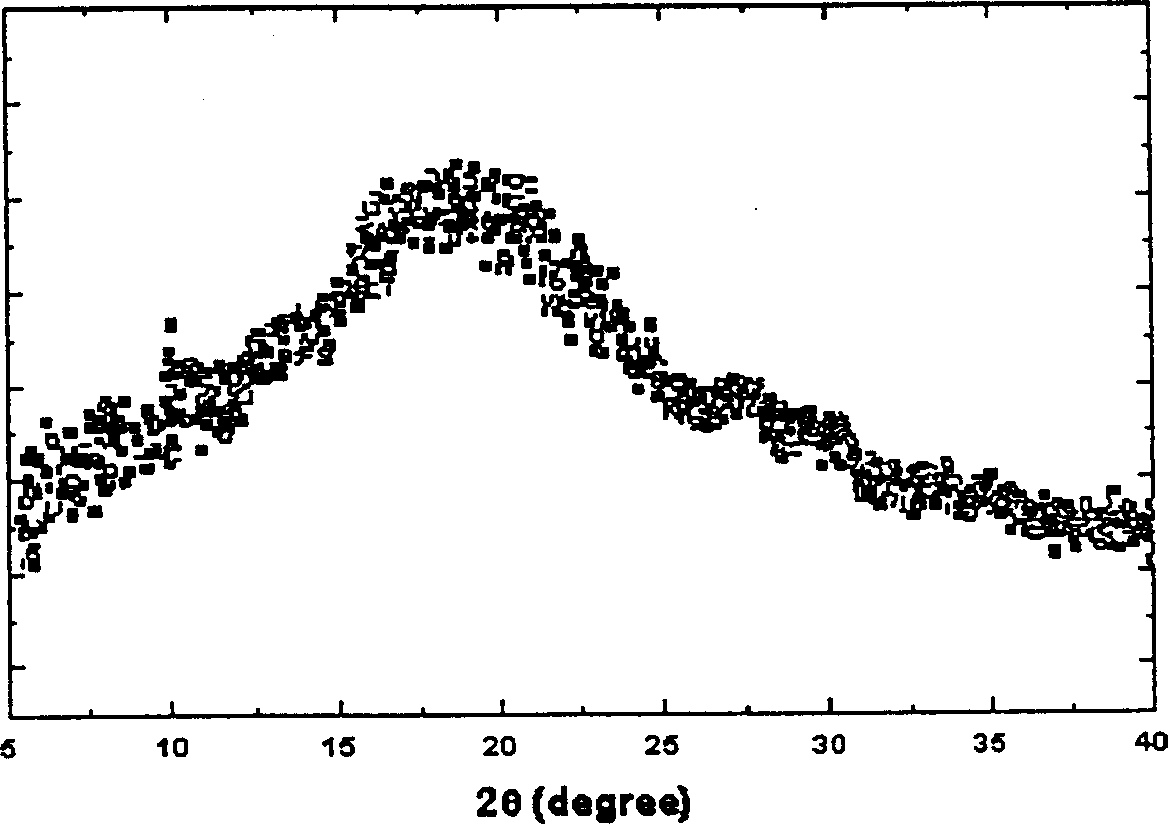
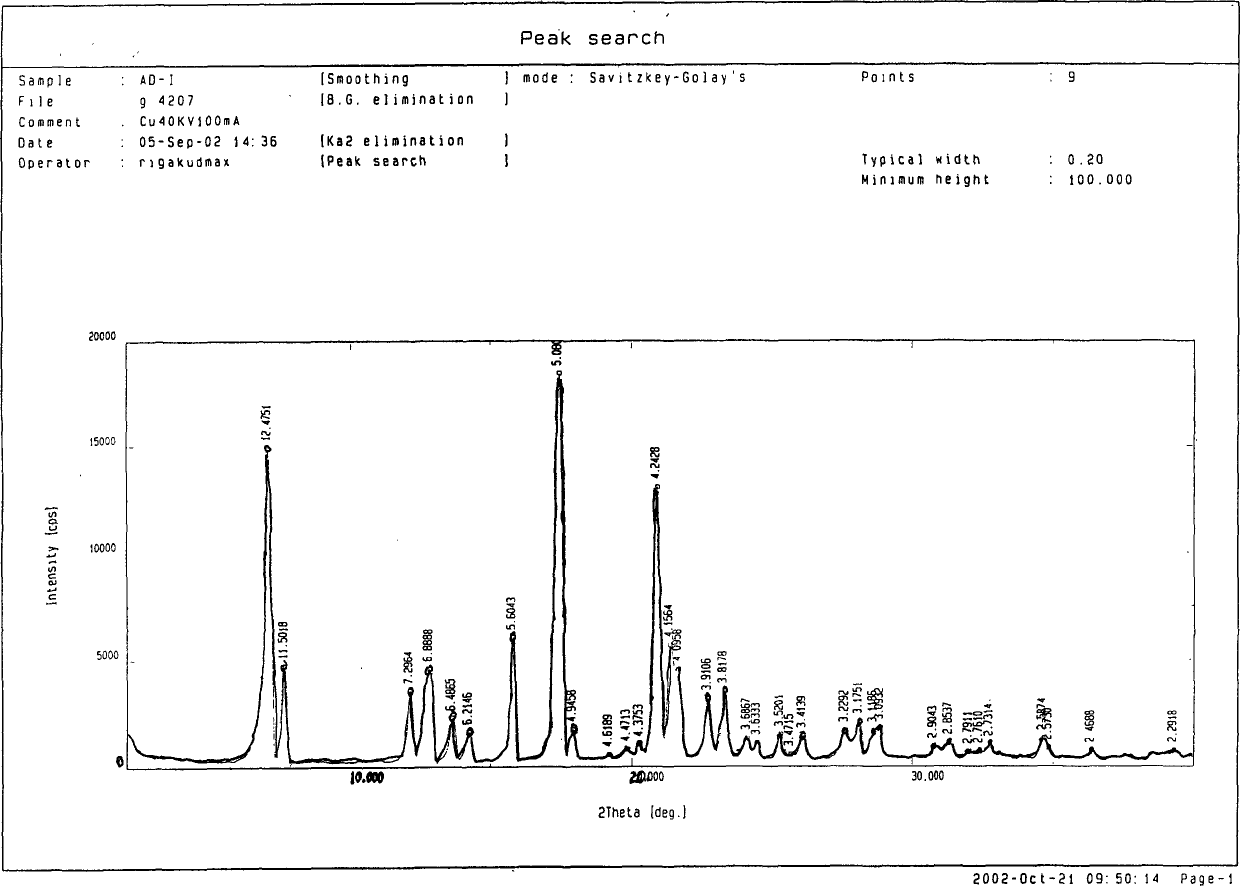
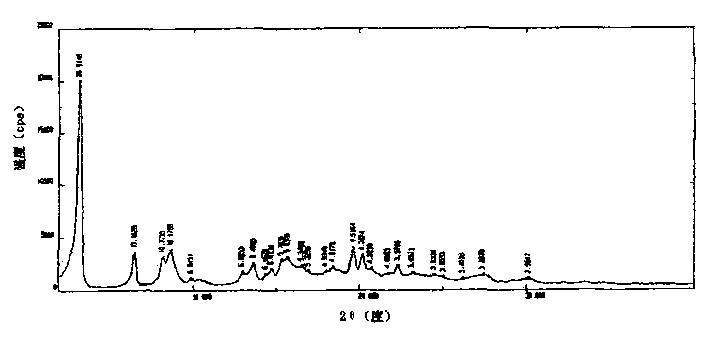
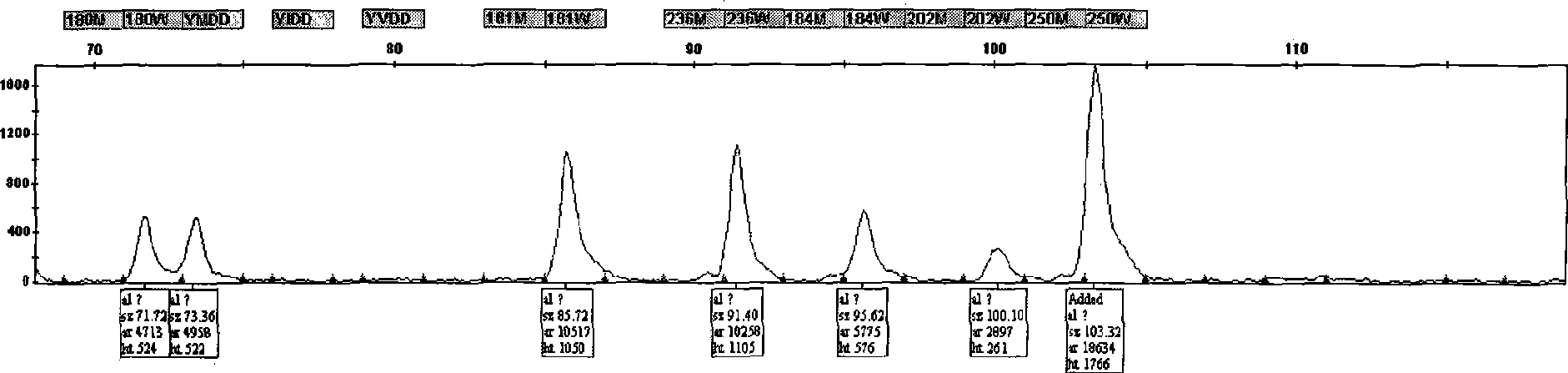
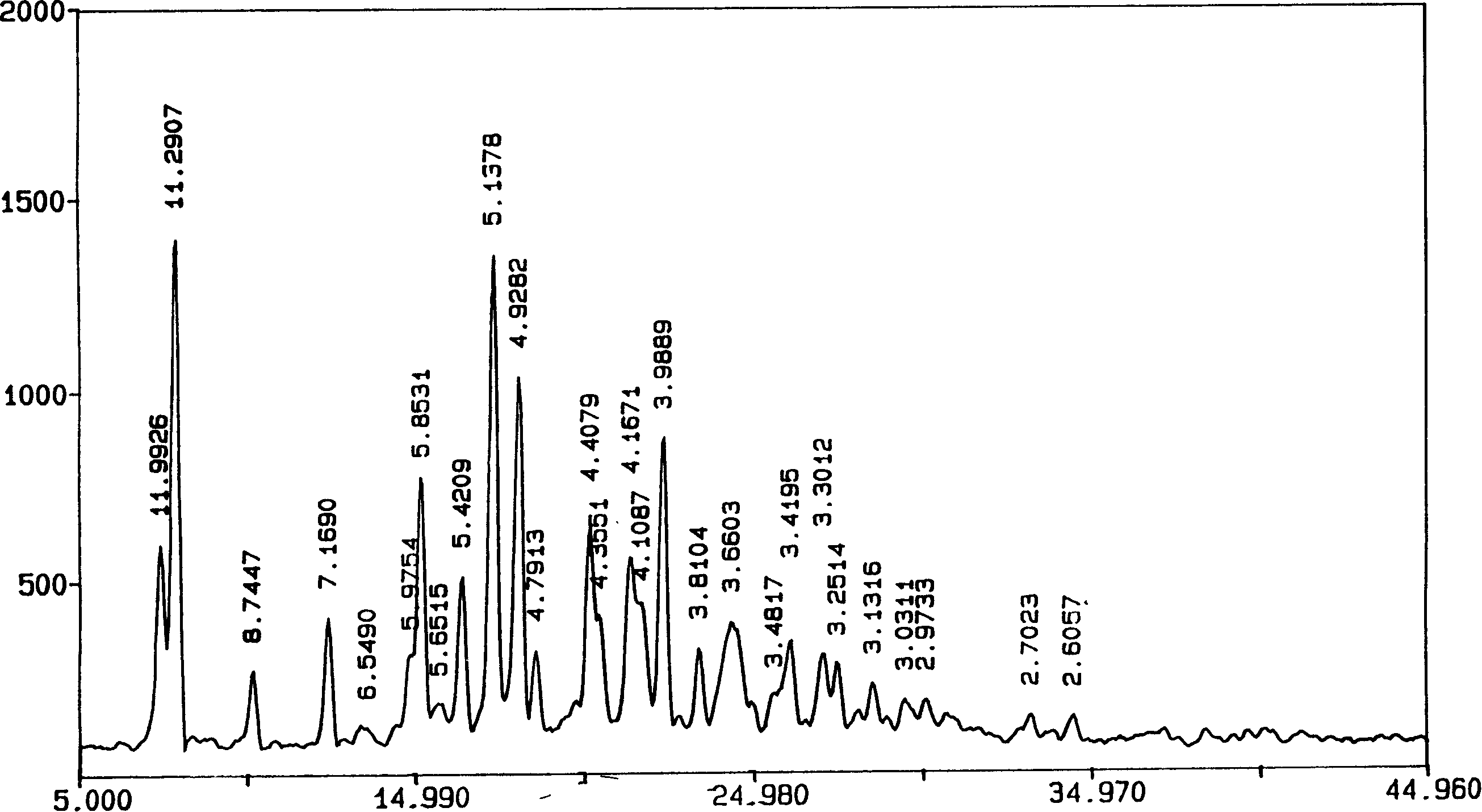
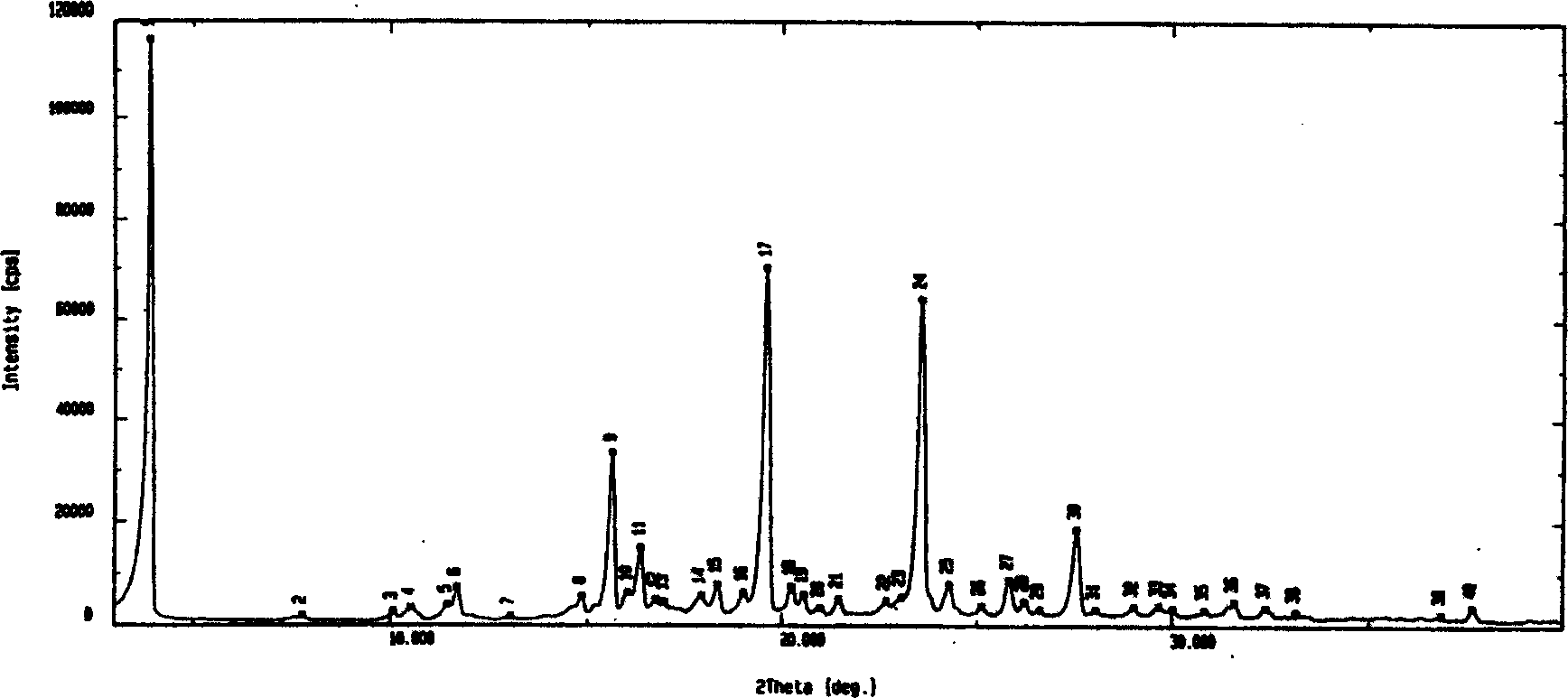
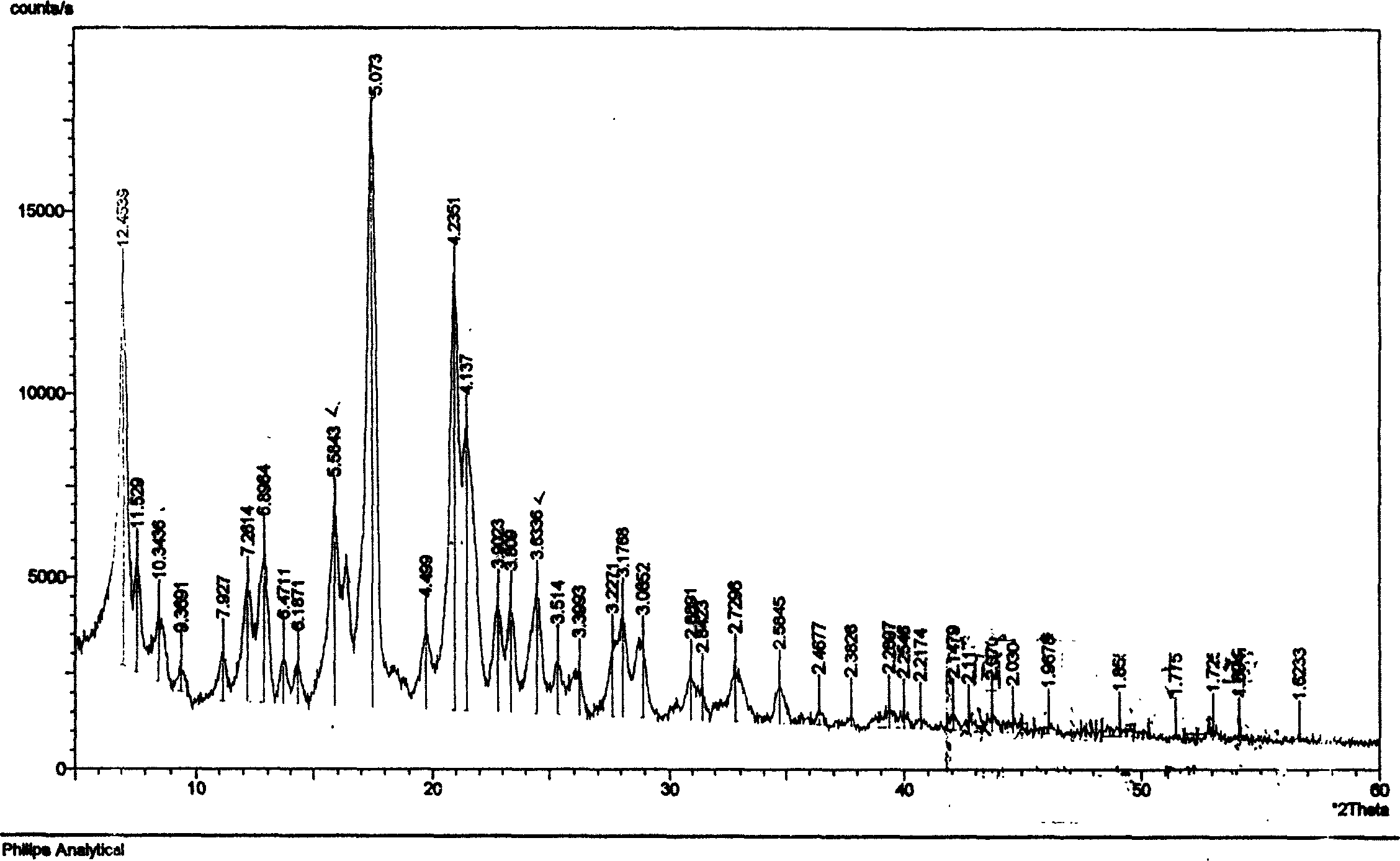
The invention discloses an adefovir dipivoxil (AD) as medical nucleoside compound and making method, whose chemical name is 9-[2-[[di (neopentyl acyloxy group) methoxy] oxyphosphino] methoxy] ethyl) adenine], wherein the CHARIOTEER crystal system uses Cu-K alpha irradiation, which displays characteristic adsorbing peak under X-ray powder diffraction with 2thita angle and crystal face distance (d) at 8. 2(10.9), 9. 8(9. 0), 14. 2(6. 3), 16. 5(5. 5), 17. 0(5. 2), 19. 8(4. 5), 21. 1(4. 2), 21. 5(4. 2), 22. 5(3. 9), 23. 8(3. 7), 28. 8(3. 1) and 33. 3(2. 6).
Owner:ZHEJIANG CHARIOTEER PHARMA
Method for synthesizing adefovir dipivoxil ester
ActiveCN101134765AThe starting material is easy to getHigh yieldGroup 5/15 element organic compoundsPhosphateHydrolysis
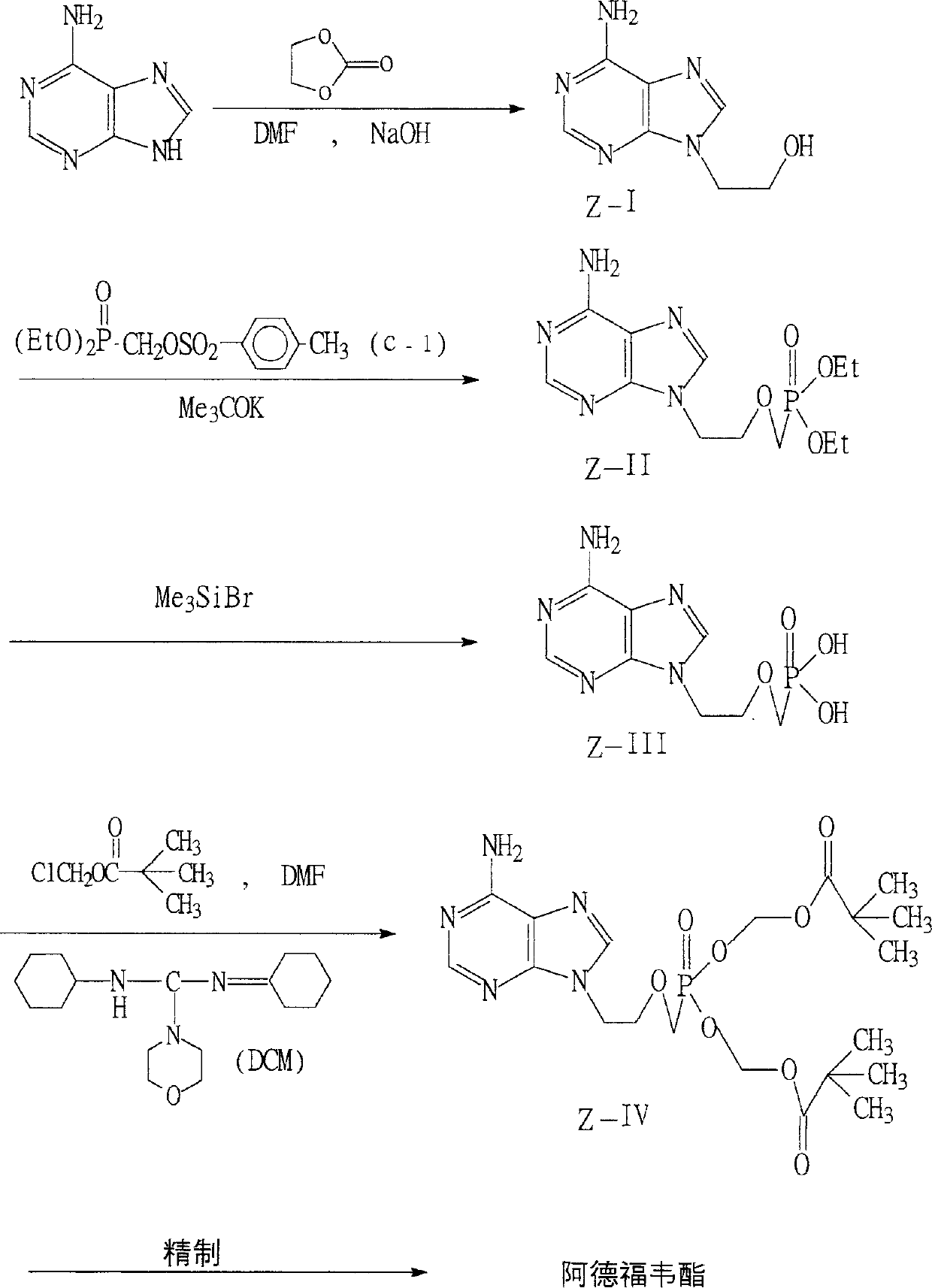
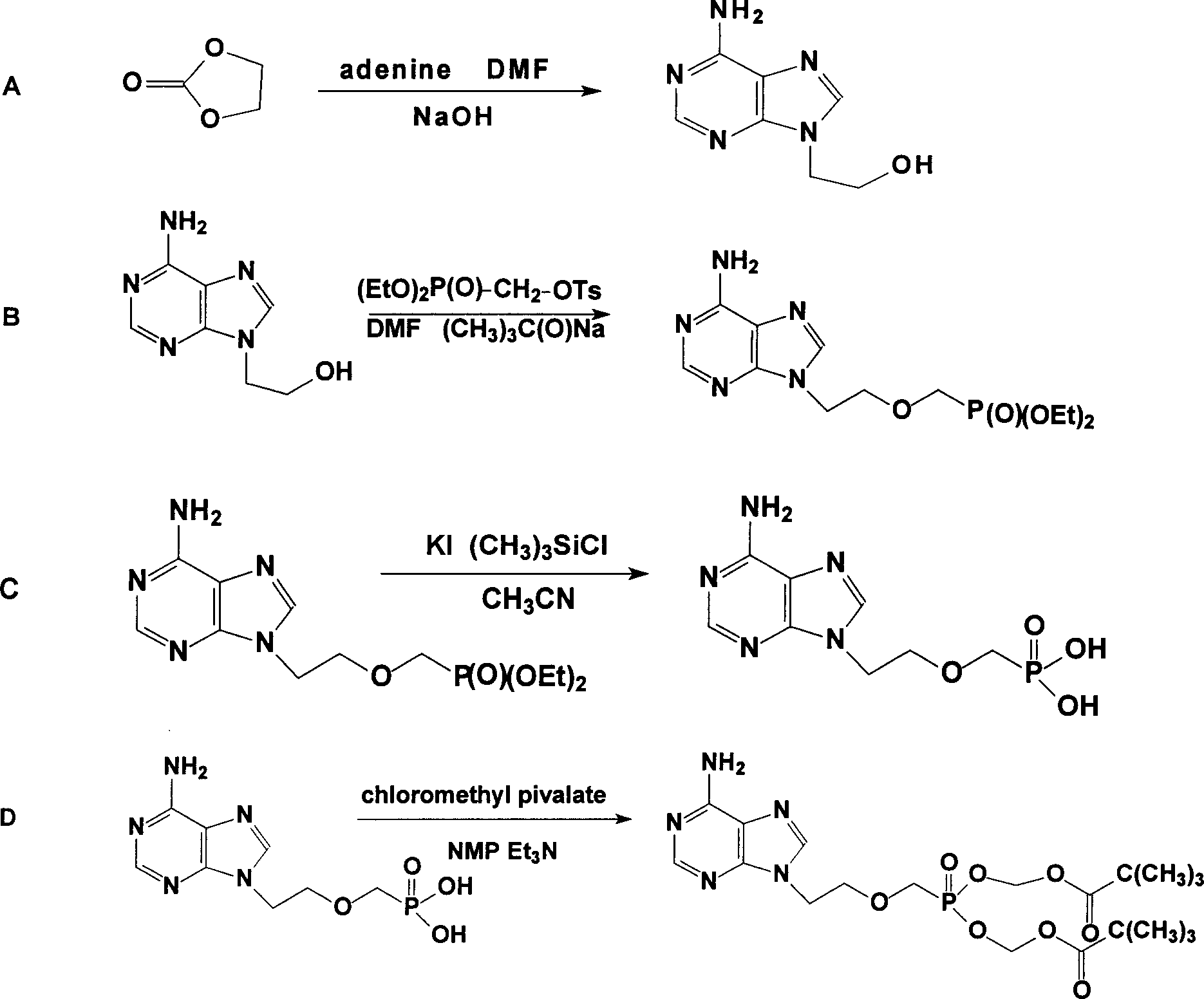
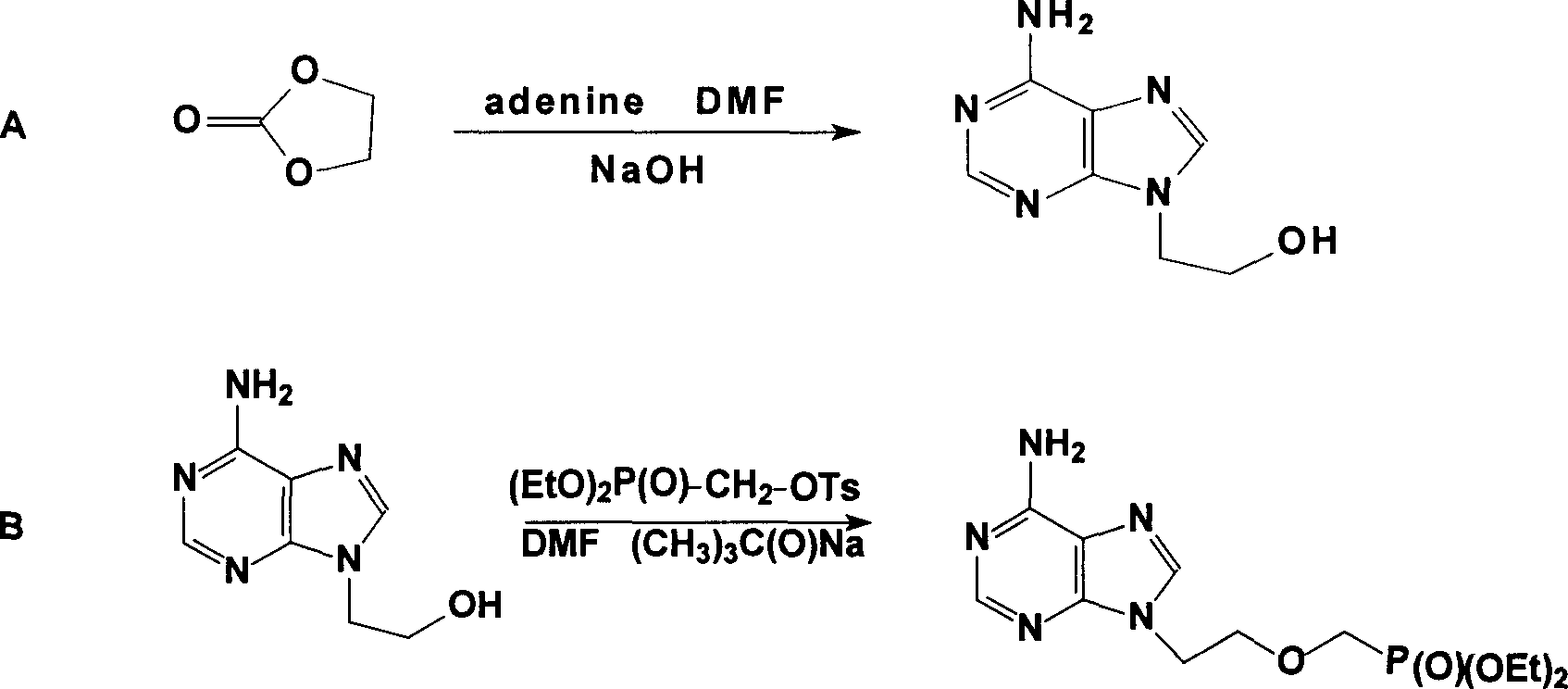
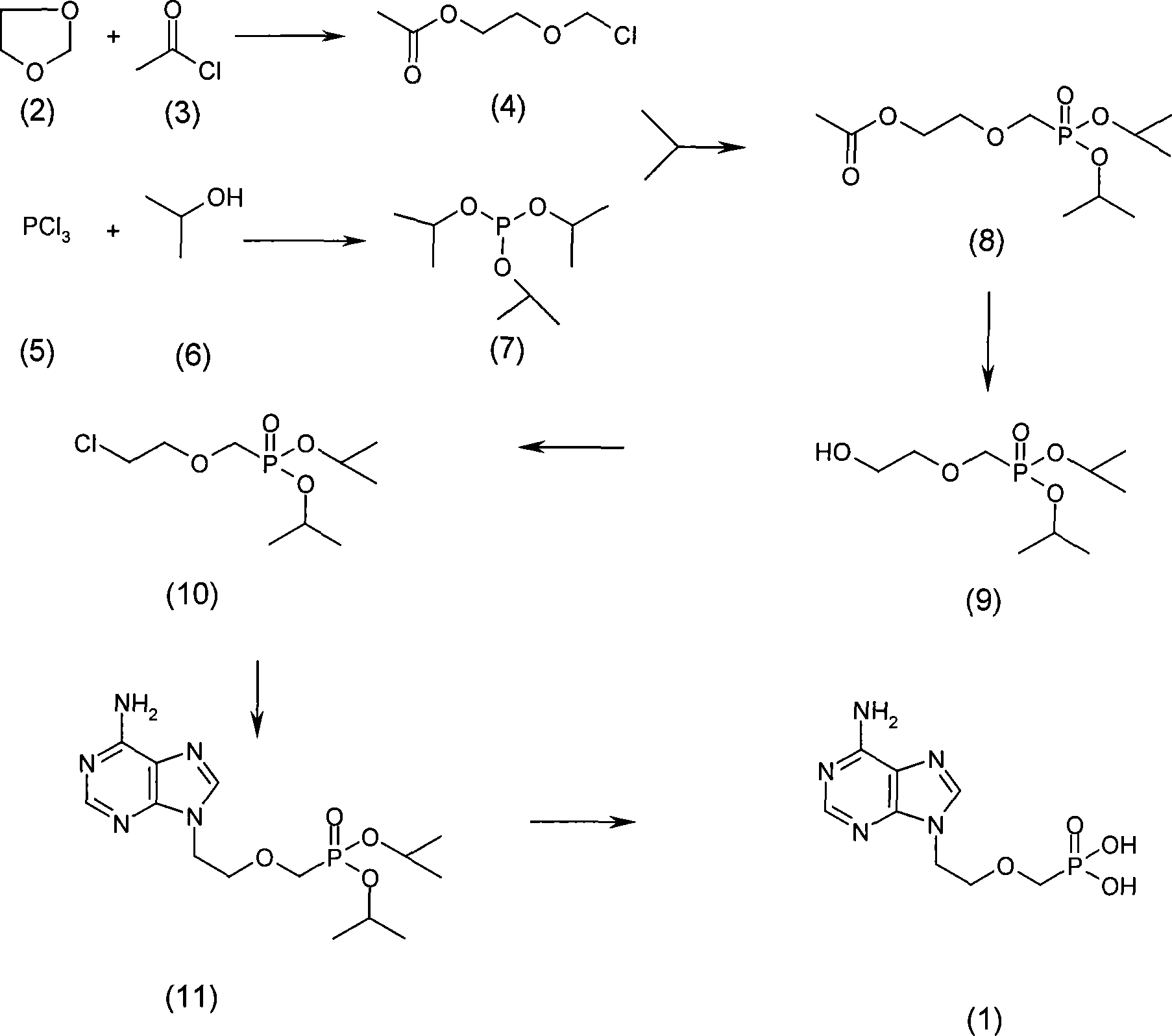
The present invention relates to medicine synthesis technology, and is especially Adefovir dipivoxil synthesizing process, which includes the following steps: 1. methylation reaction of 2-chlorethanol, paraformaldehyde and anhydrous hydrogen chloride to produce 1-chloro-2-chloromethoxyl ethane; 2. reaction of 1-chloro-2-chloromethoxyl ethane and trimorpholinyl phosphate to obtain 2-dimorpholinyl chloroethoxyl methyl phosphonate; 3. condensation of 2-dimorpholinyl chloroethoxyl methyl phosphonate and adenine in DMF in the presence of anhydrous K2CO3 catalyst to obtain 9-(2-dimorpholinylphsphonomethoxylethyl) adenine; 4. hydrochloric acid hydrolysis of 9-(2-dimorpholinylphsphonomethoxylethyl) adenine in DMF aqua to obtain 9-(2-phsphonomethoxylethyl) adenine; and 5. reaction of 9-(2-phsphonomethoxylethyl) adenine and chloromethyl pivalate in DMF under the catalysis of triethylamine to obtain Adefovir dipivoxil.
Owner:FUJIAN COSUNTER PHARMA
Prepn process of Adefovir dipivalate
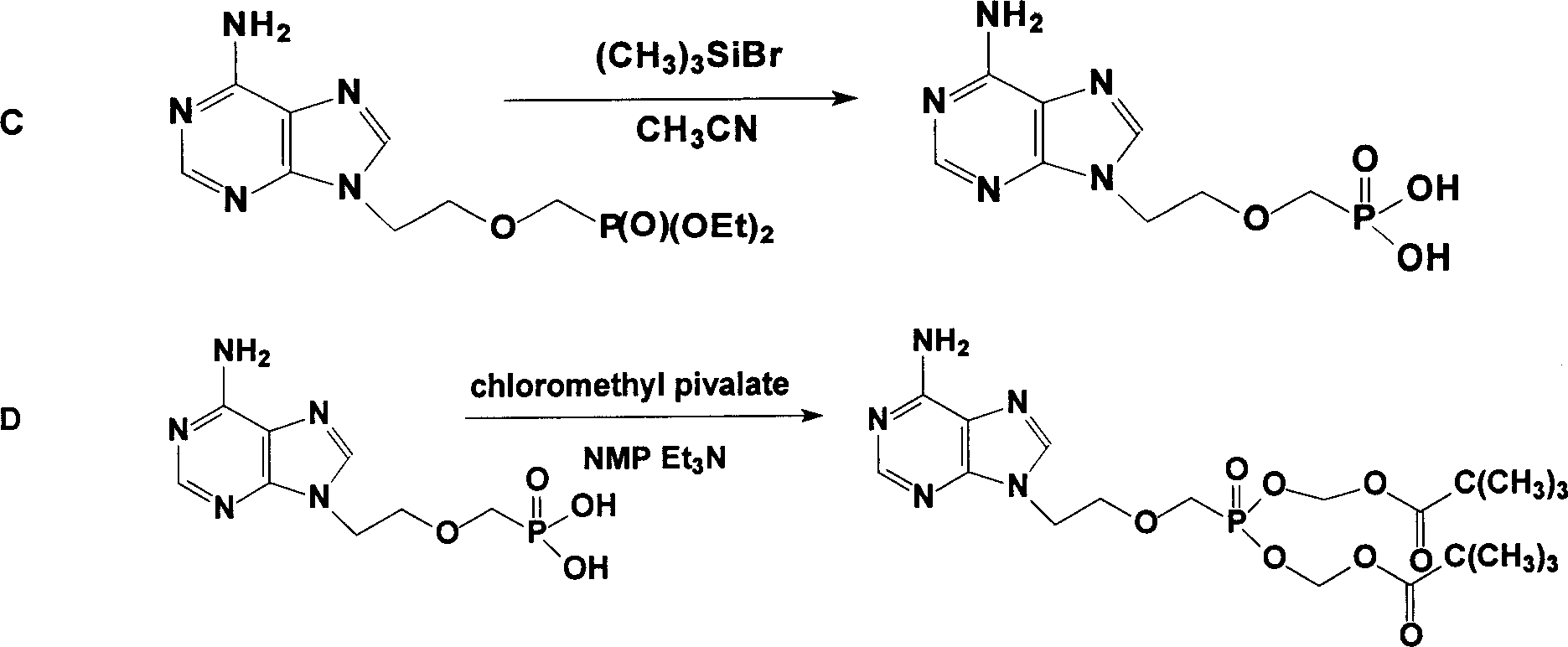
InactiveCN1506370ASuitable for industrializationReduce manufacturing costGroup 5/15 element organic compoundsAntiviralsTrimethylsilyl chloridePurine
The present invention discloses one improved preparation process of Adefovir dipivalate. In the synthesis of the mother compound 9-[2-(phosphinocarboxyl methoxy) ethyl adenine, trimethyl chlorsilane, rather than expensive trimethyl bromosilane, as material and potassium iodide as nucleophilic reagentare used to reduce cost while the product yield and quality is maintained. The production process of the present invention may be used in industrial production.
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST
Synthesis process of adefovir dipivoxil raw medicine
ActiveCN101830932AReduce medication burdenSimple processGroup 5/15 element organic compounds2-ChloroethanolReagent
The invention provides an industrialized production and synthesis process of an adefovir dipivoxil raw medicine. The synthesis process comprises the following process routes of: reacting 2-chlorohydrin subjected to cholromethylation and triisopropyl phosphite to generate an adefovir lateral chain which is condensed and hydrolyzed with adenine to generate adefovir, and then carrying out condensation on the adefovir and chloromethyl pivalate to prepare the adefovir dipivoxil product. The invention has simple process, easy operation, safety, environment protection, mild reaction condition and high product purity, uses the most elementary raw materials and common reagents, and can be used for industrialized production. The invention is used for producing the adefovir dipivoxil raw material.
Owner:湖南欧亚药业有限公司
Variants of hepatitis B virus resistant against some nucleoside analogues, but sensitive to others, and uses thereof
ActiveUS7807437B2Reduce sensitivityRapid and reliable detectionBiocideVirus peptidesReverse transcriptaseHepatitis B virus
Owner:UNIVERSITY OF BONN +2
Oxalic adefovir dipivoxil, and crystalline form and preparing method and use thereof
InactiveCN1528766AEasy to purifyImprove filtering effectOrganic active ingredientsGroup 5/15 element organic compoundsX-rayHepatitis
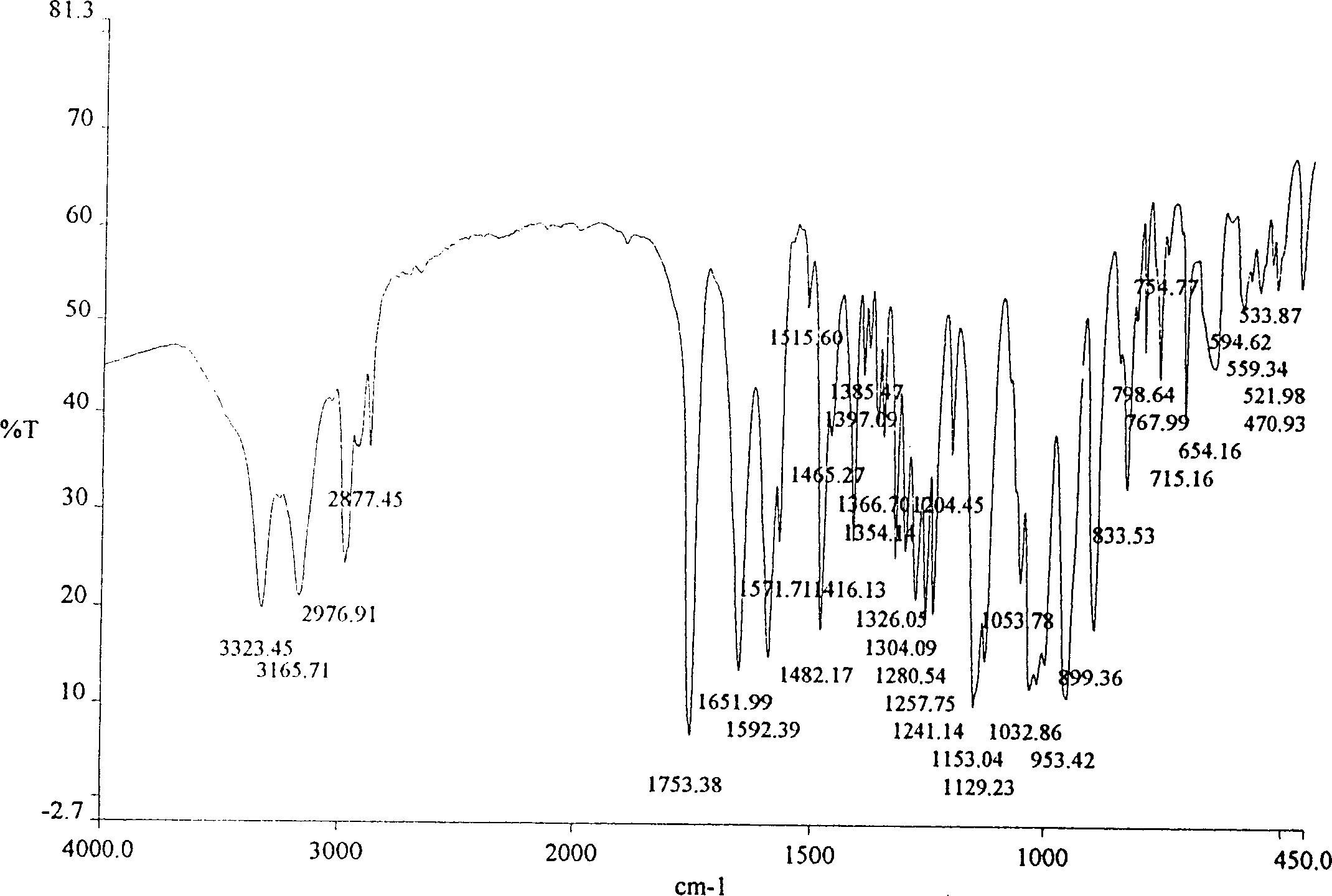
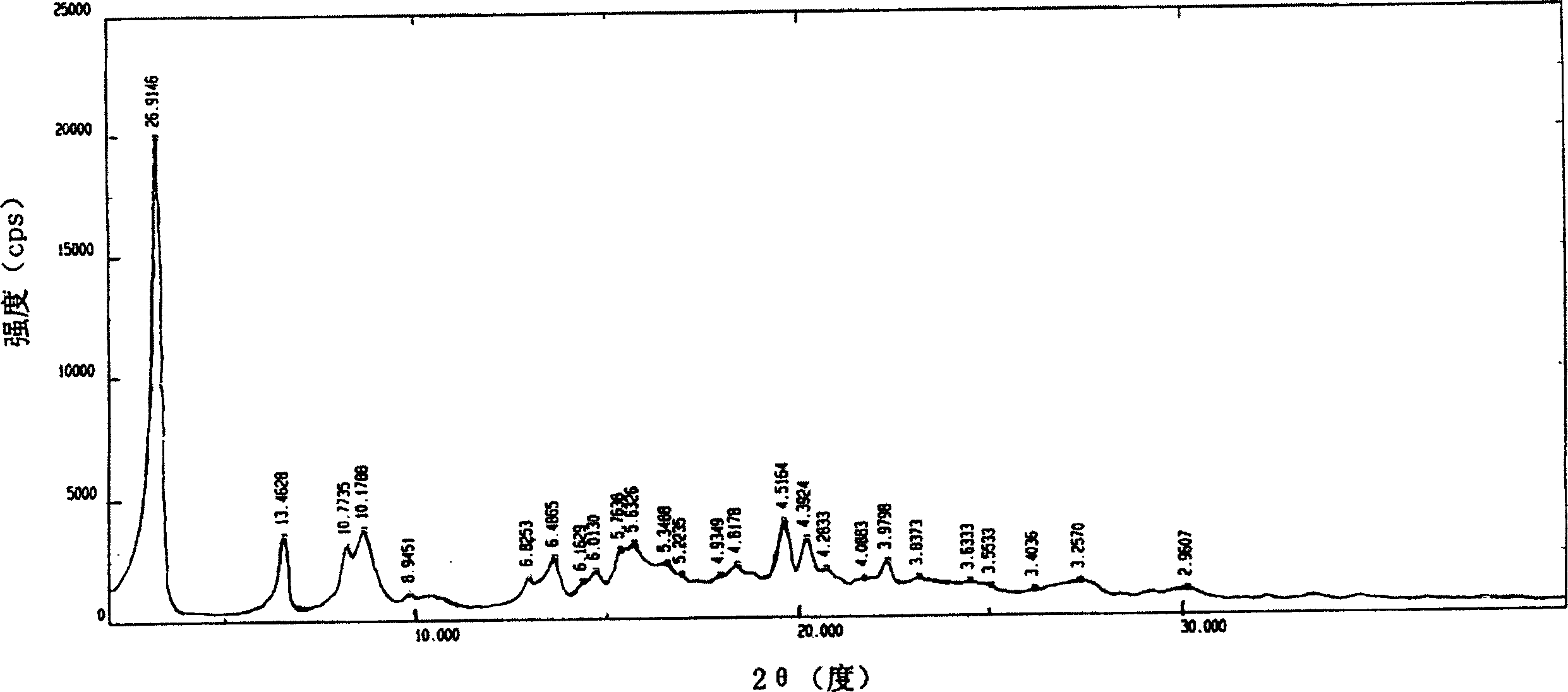
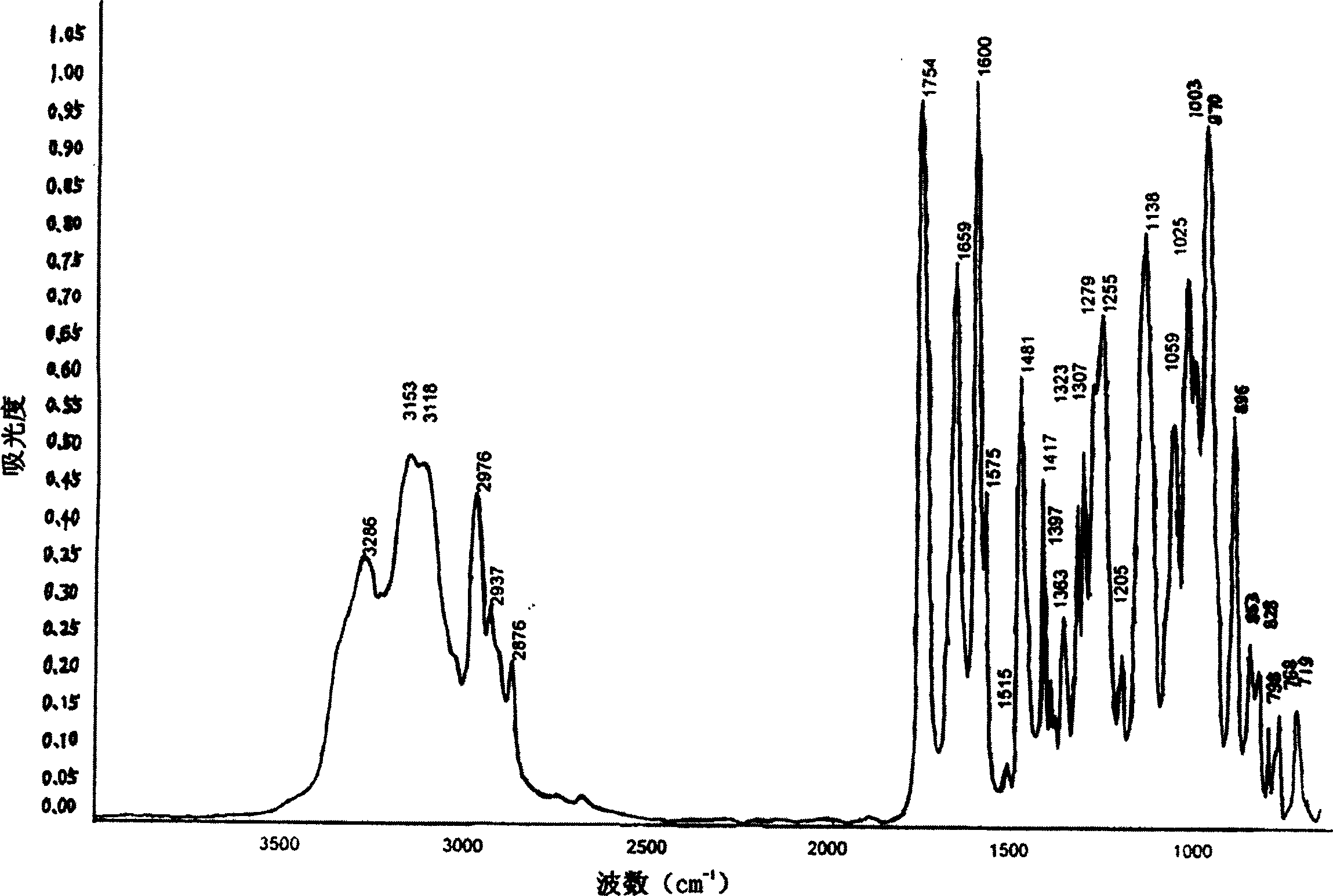
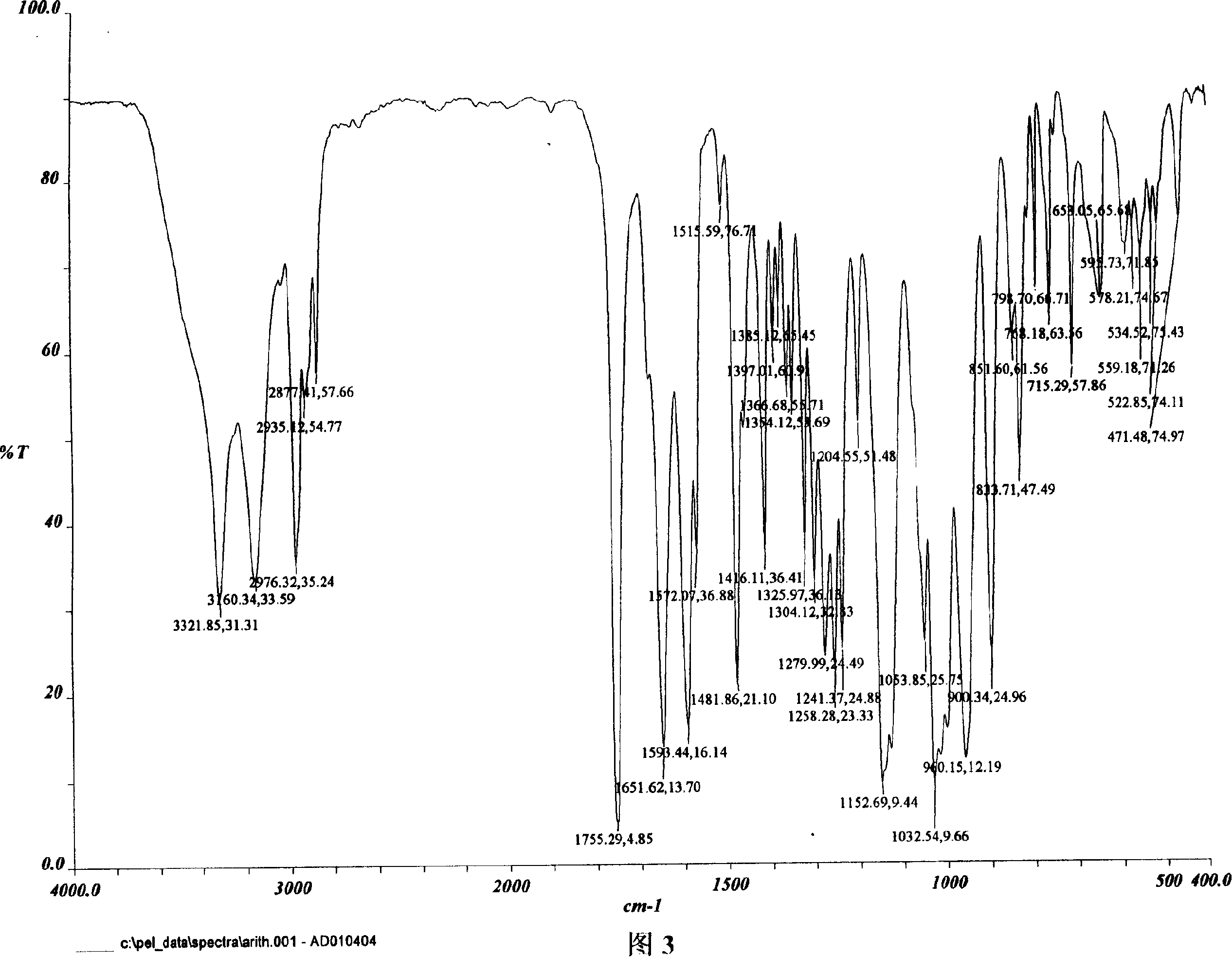
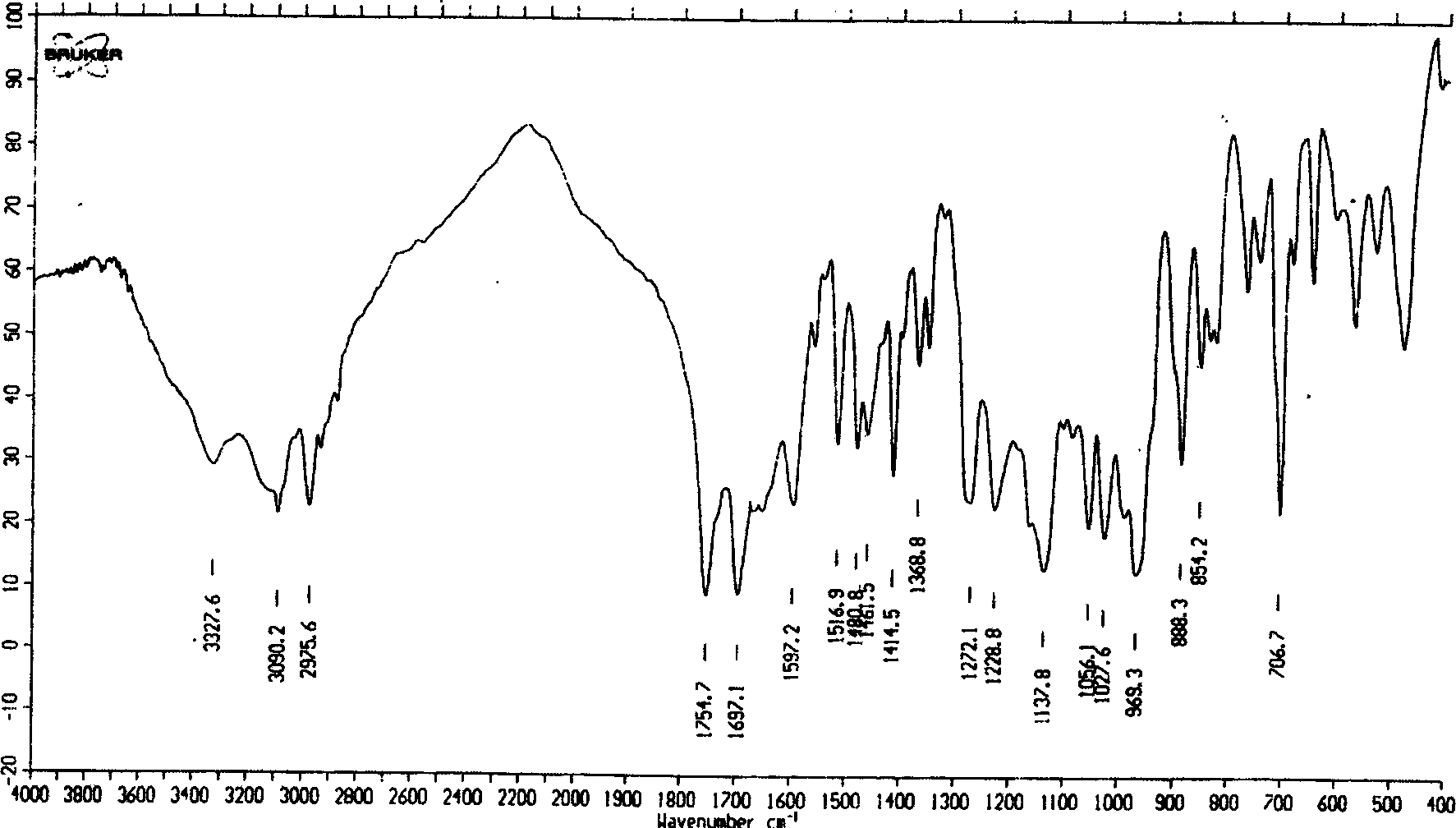
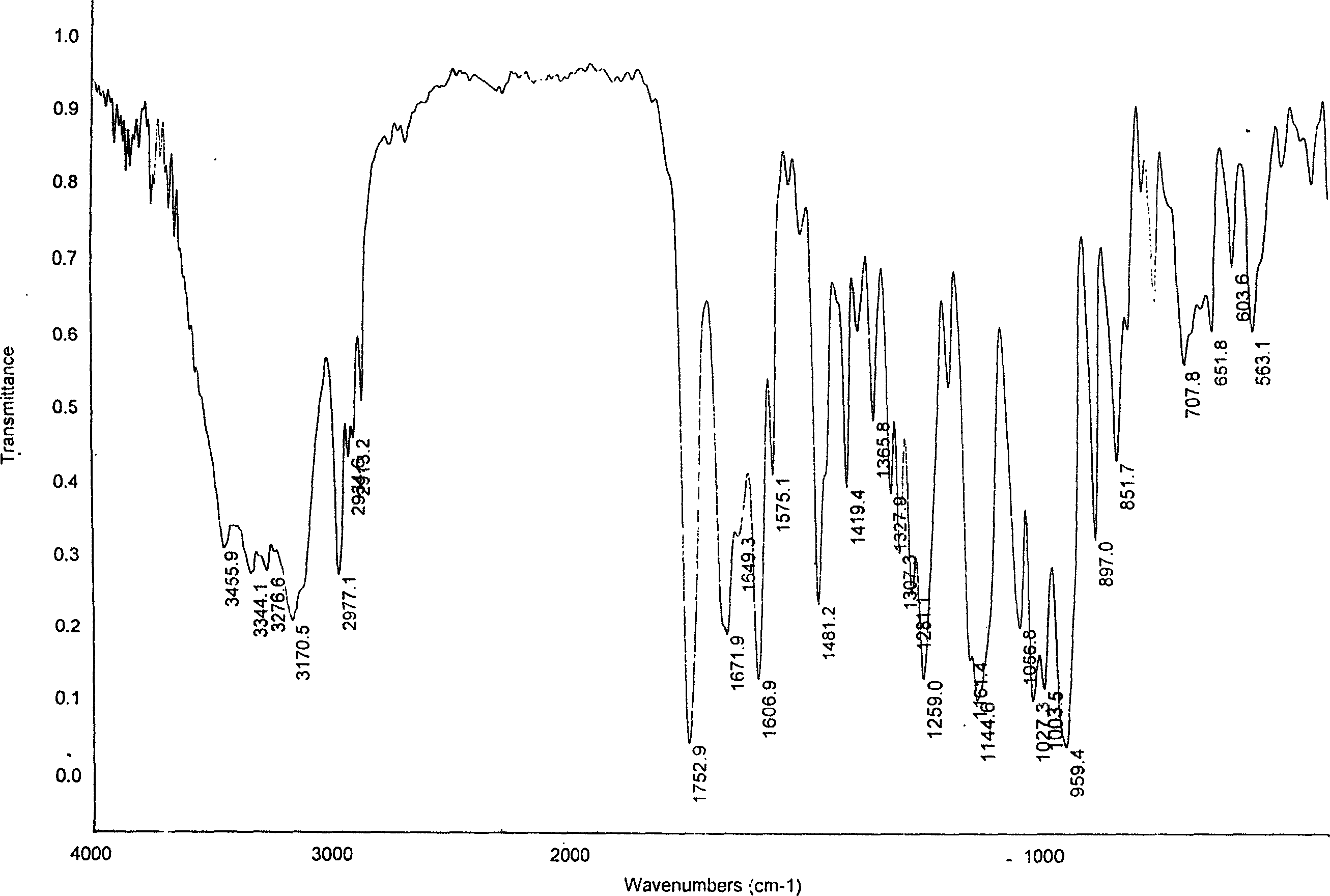
The invention is an oxalic acid adefovir dipivoxil and its crystal form as well as preparing method and usage, having stable property and good fluidity, easy to purify and convenient to operate. It is chemically called oxalic acid 9-[2-[bis(pivaloyloxymethoxy)phosphorylmethoxyl]ethyl] adenine. The infrared absorption spectrum measured by KBr sheet has absorption peaks at about 3090, 2975, 1754, 1697 and 1597 cm-1; X-ray powder diffraction spectrum radiated by Cu-Ka and expressed by 20 degrees has characteristic peaks at about 3.9, 15.6, 19.6, 23.6 and 27.5. It is applied to industrialized production and widely applied to prepare the drug of curing virus hepatitis.
Owner:HARBIN PHARMA GRP CO LTD GENERAL PHARMA FACTORY +1
Method of Synthesizing adefovir dipivoxil
InactiveCN101058588AMild reaction conditionsEasy to prepareGroup 5/15 element organic compoundsChemistryBy-product
The invention discloses a making method of adefovir dipivoxil, which comprises the following steps: adopting 1,3-pentacyclic dioxide as raw materia; opening ring; rearranging altrose; hydrolyzing; chlorinating; proceeding N-alkylating reaction; obtaining the product with mild reacting condition, little by-product and superior object product.
Owner:TOPFOND PHARMA CO LTD
Novel use of adefovir dipivoxil or medicinal salt thereof
The invention relates to novel use of adefovir dipivoxil or a medicinal salt thereof, and particularly relates to application in preparation of a drug for treating influenza A or B, and application in preparation of the drug for preventing the influenza A or B.
Owner:FUJIAN COSUNTER PHARMA
Kit and method for testing hypotype of virogene of hepatitis B and drug fast mutation
ActiveCN101092651AReduce financial burdenRich reference informationMicrobiological testing/measurementHypotypeOligonucleotide
This invention provides method and test kit for synchronously detecting sub-genotypes and drug-resistant mutation of hepatitis B virus (HBV). The test kit comprises: two specific primers for amplifying gene or transcript of HBV, three specific oligonucleotide probes for detecting sub-genotypes of HBV, and thirteen specific oligonucleotide probes. The primers and the probes can also be replaced with the complementary sequences. The test kit can synchronously differentiate three sub-genotypes (B, C and D) of HBV, and detect lamivudine- and adefovir dipivoxil-resistant mutations. The test kit can reduce the detection cost.
Owner:亚能生物技术(深圳)有限公司
Adefovir dipivoxil anhydrous crystal, preparation method and medicine composition thereof
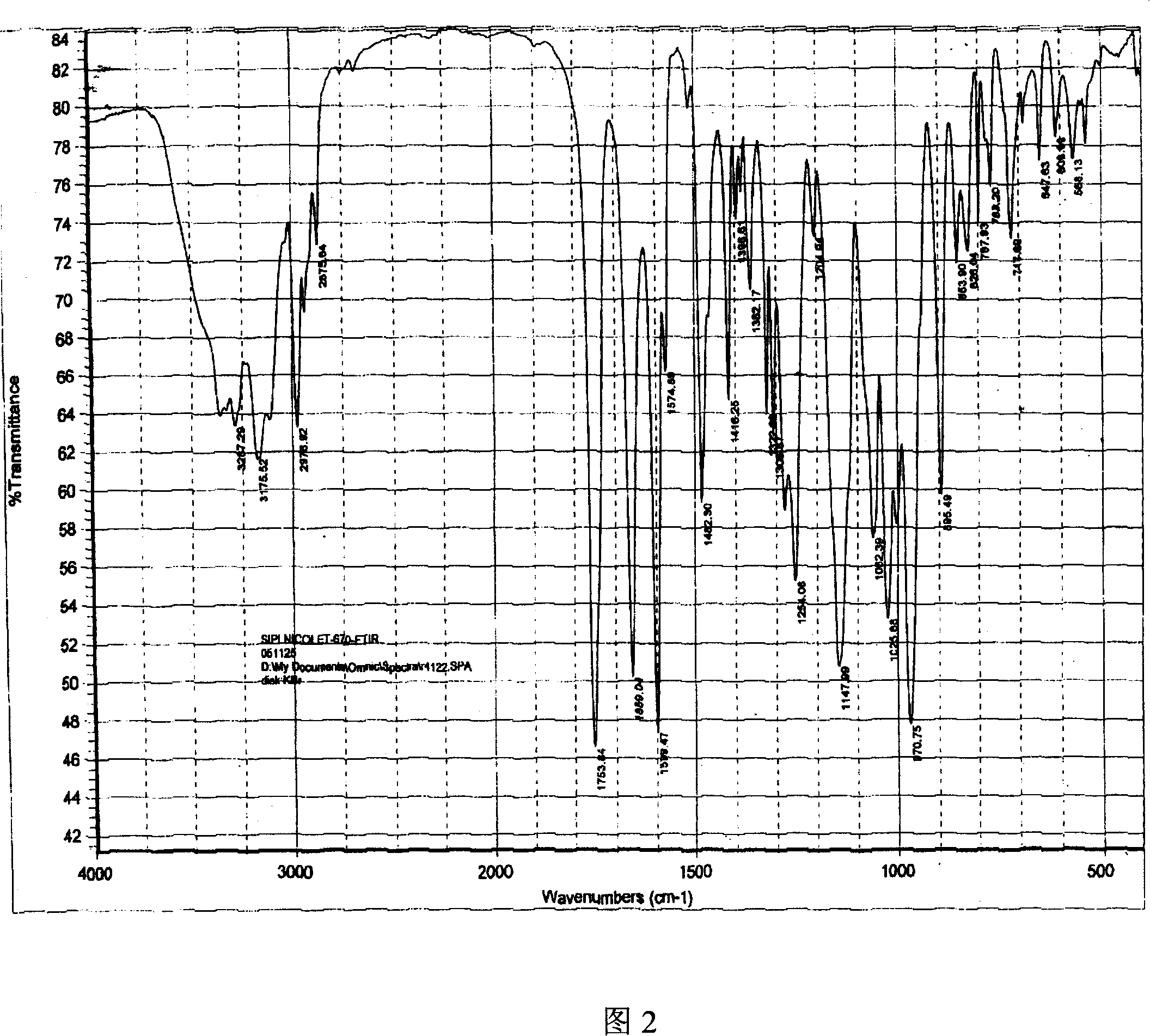
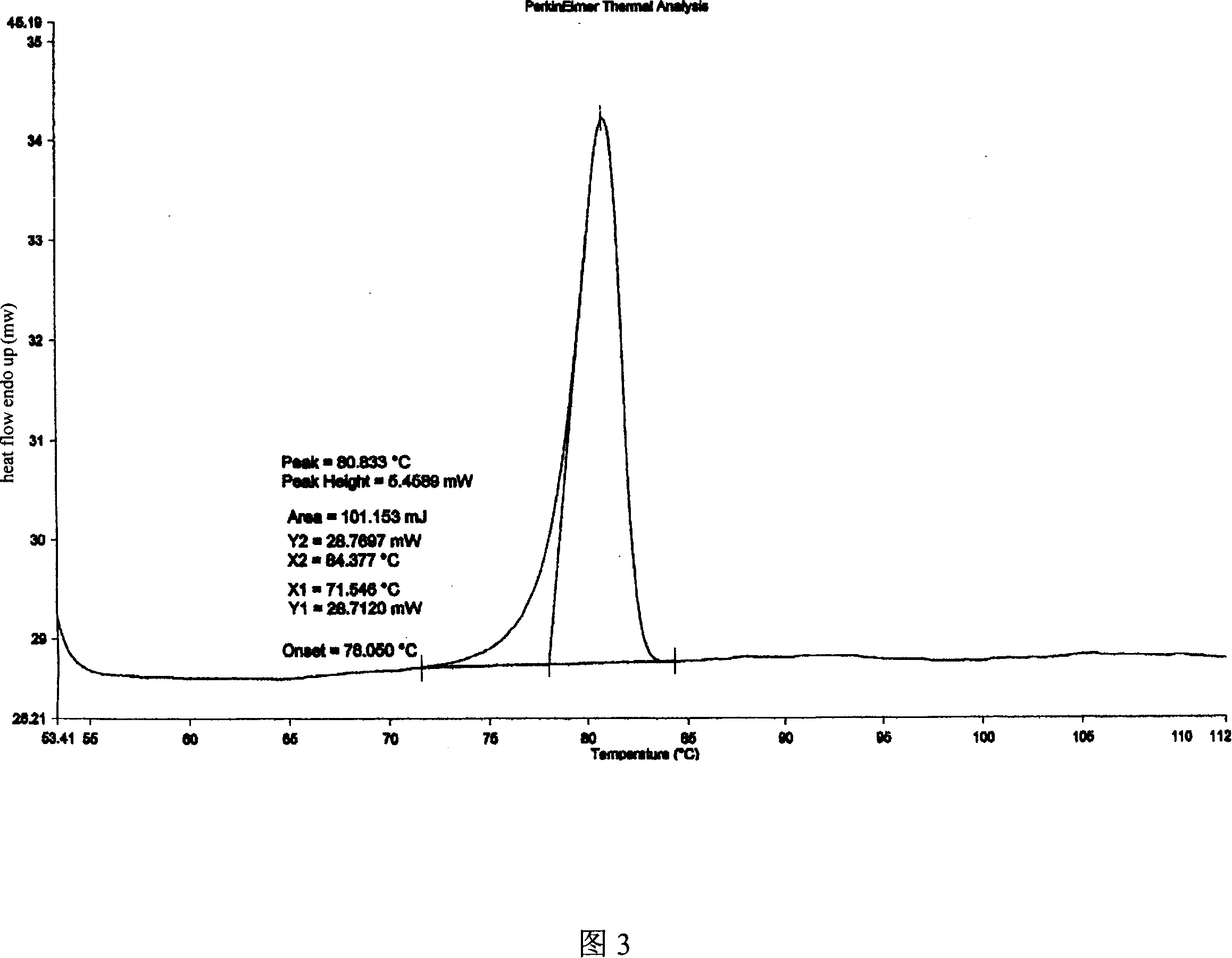
ActiveCN101054393AImprove liquidityEasy to prepareOrganic active ingredientsGroup 5/15 element organic compoundsDissolutionK-alpha
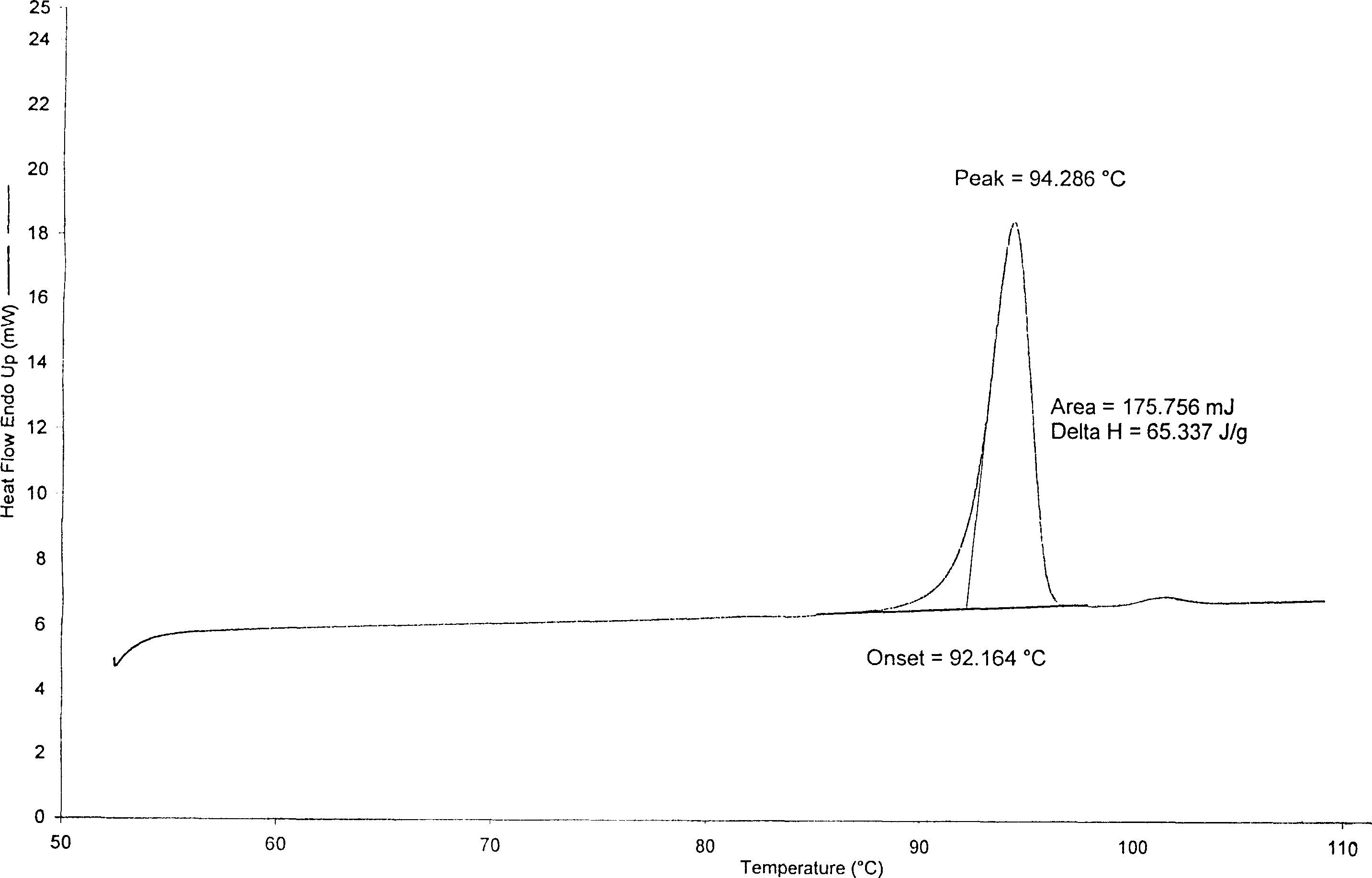
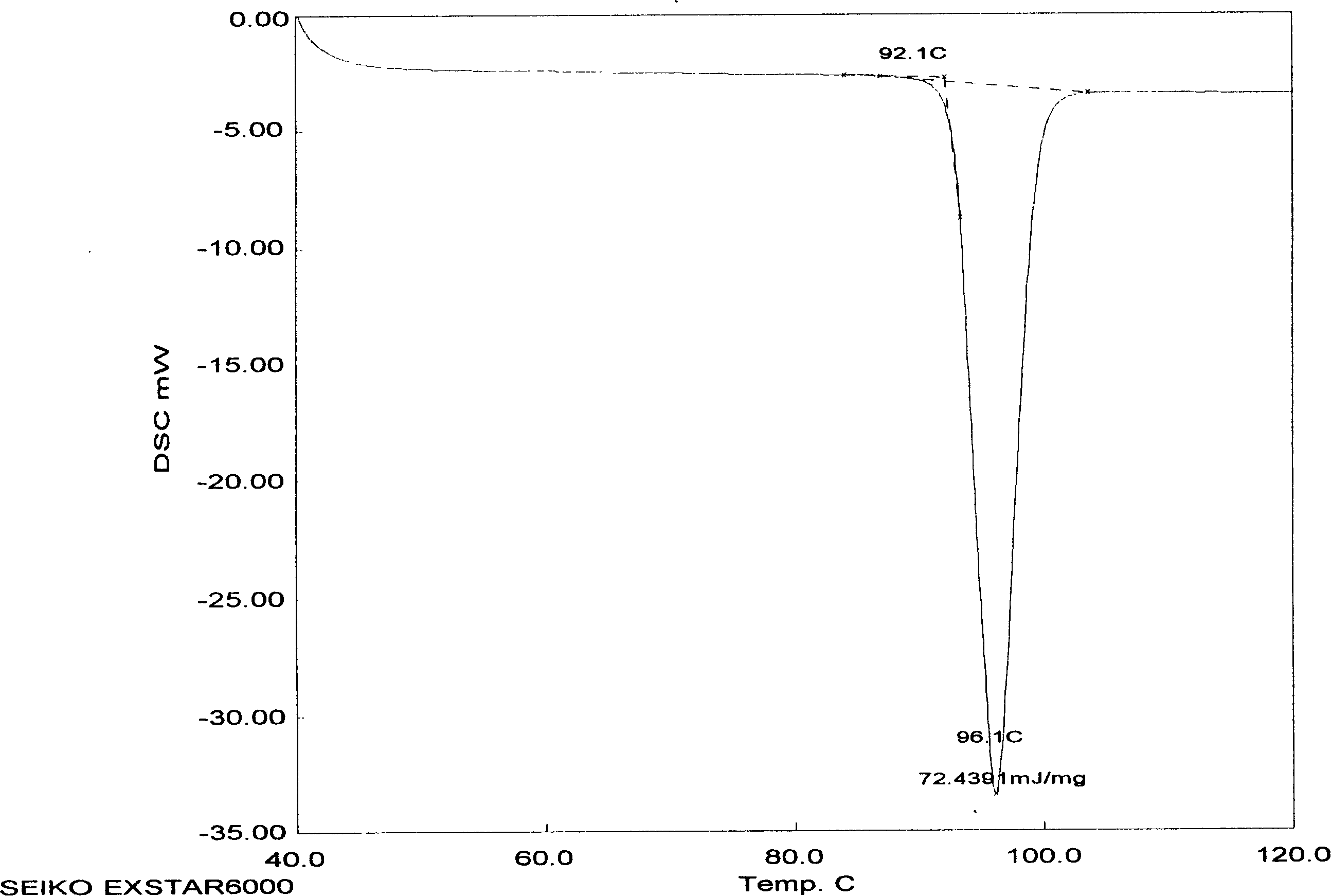
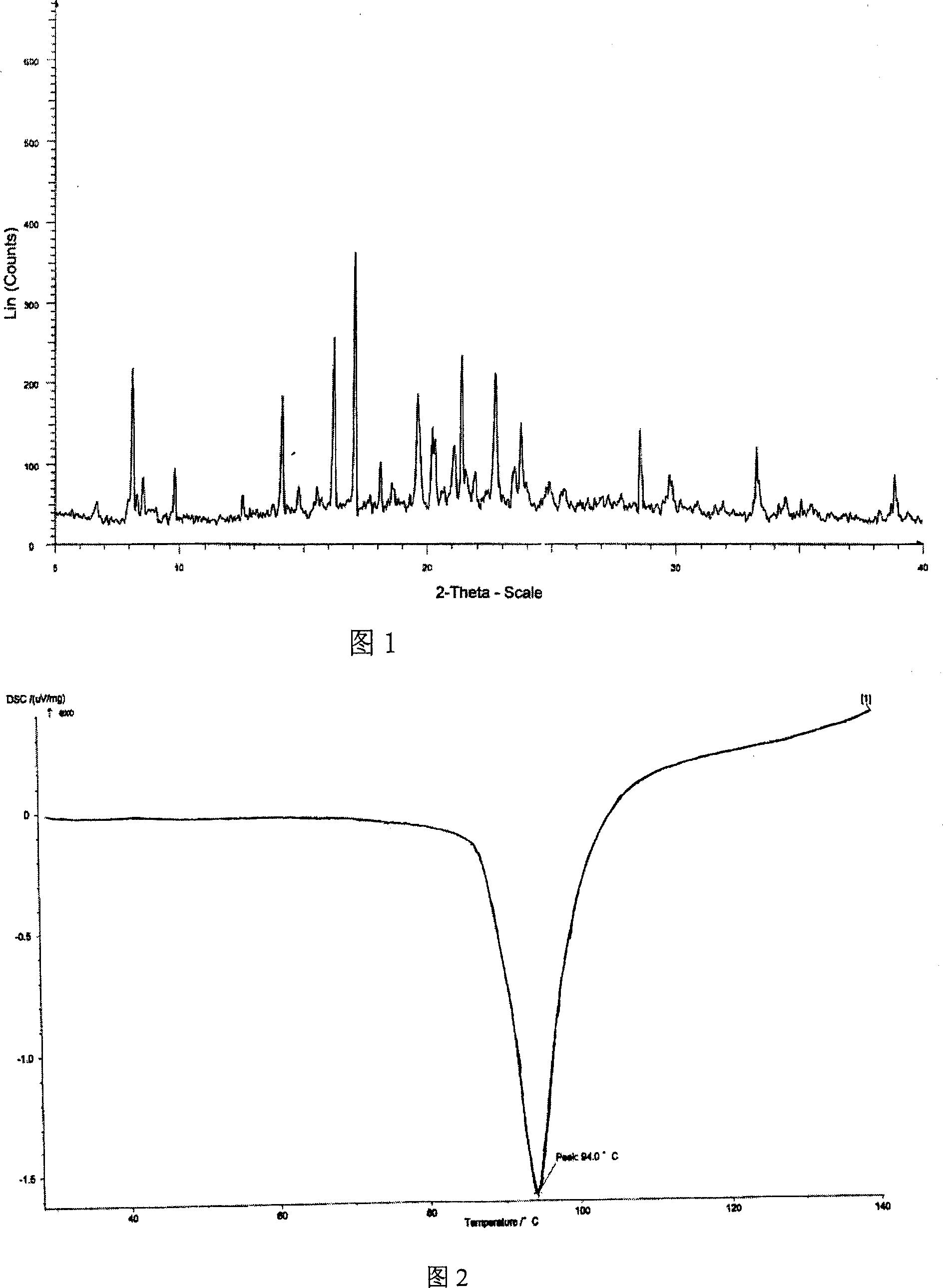
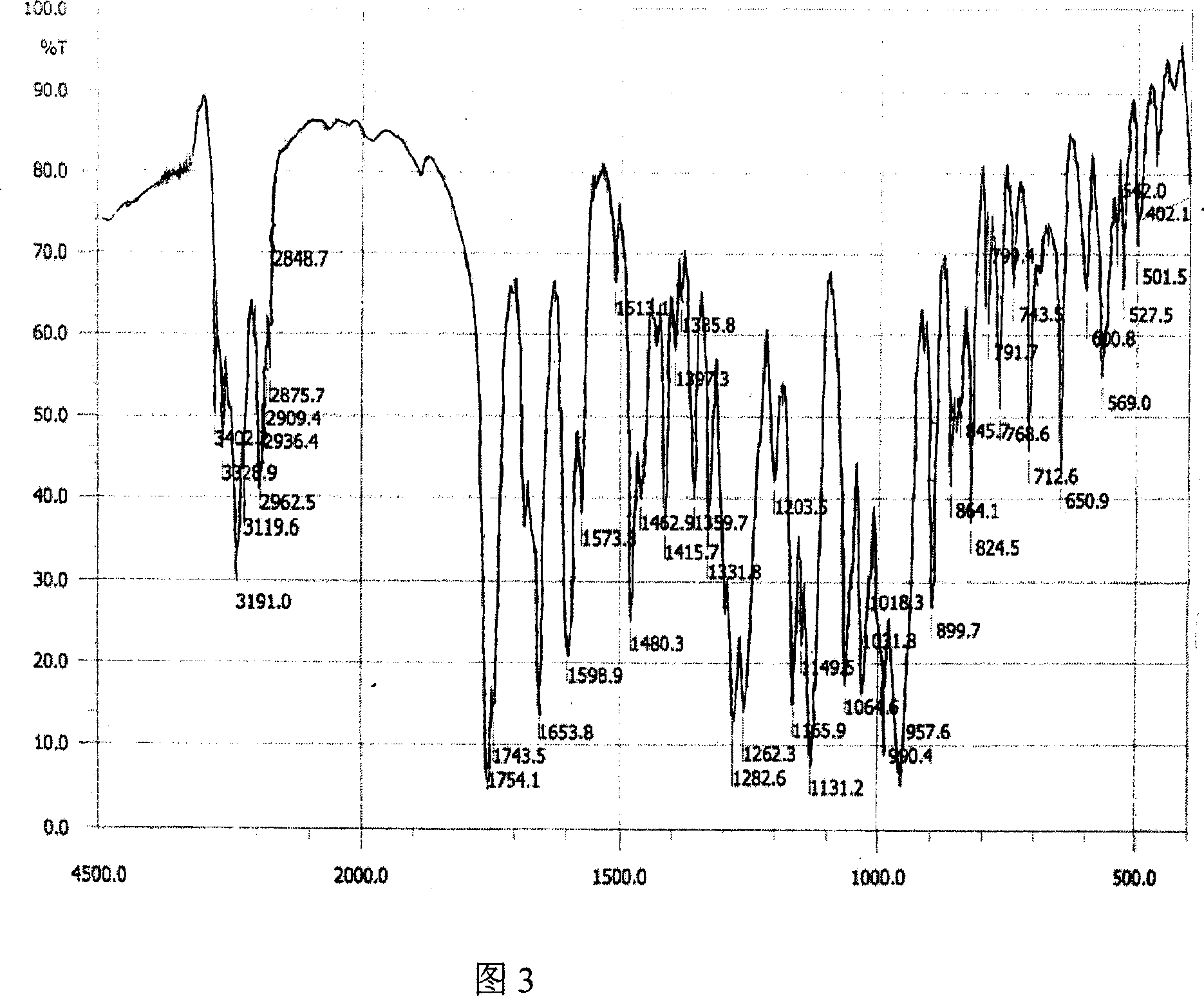
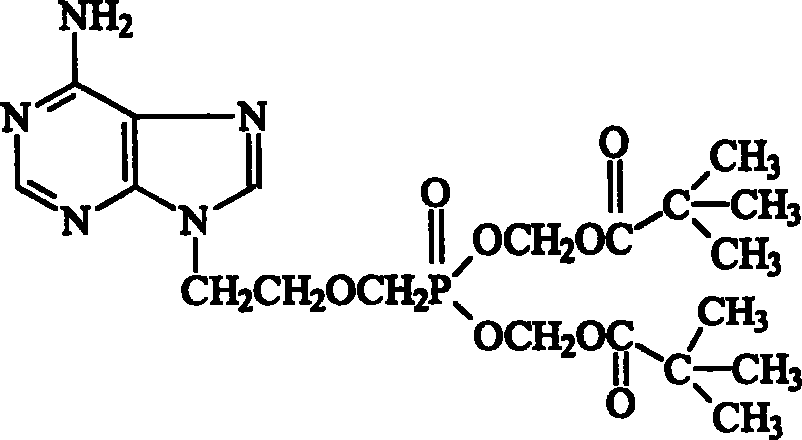
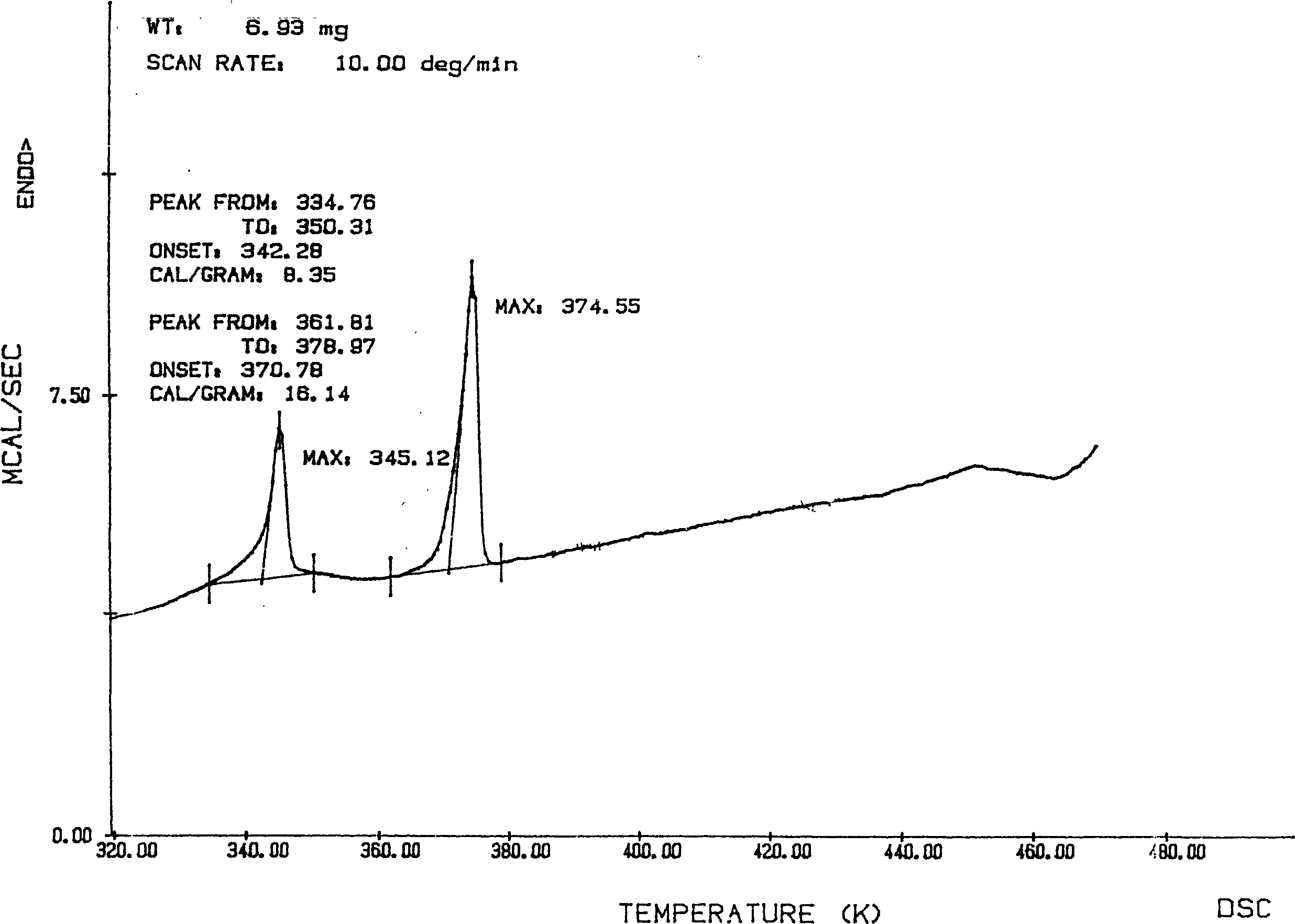
The invention relates to adefovir dipivoxil anhydrous crystallisate. It is characterized in that : XRD: radiating with Cu-k alpha , x ray powder diffraction spectrum expressed in 2 theta having characteristic absorption peak at 3.2,6.5,8.6, 15.7,19.6,21.6, DSC: DSC converting due to absorbing heat at 80 degree; infrared absorption spectrum : KBr pellet , characteristic absorption peak being at 3287cm-1,3175cm-1,1753cm-1,1254cm-1,1147 cm-1,1025cm-1,970cm-1. The present invention also discloses adefovir dipivoxil anhydrous crystallisate preparation method and its pharmaceutical composition. The inventive adefovir dipivoxil anhydrous crystallisate dissolves quicker than 102 degree crystal form, is high in dissolution, and is stable relative to 94 degree crystal form and not easy to be hygroscopic.
Owner:SHANGHAI SHYNDEC PHARMA CO LTD +2
Crystal structure for medicinal compound adefovir divoxil, and its preparing method and medicine
InactiveCN1580060AOrganic active ingredientsGroup 5/15 element organic compoundsMedicineAdditive ingredient
Owner:四川倍达尔新技术开发有限公司
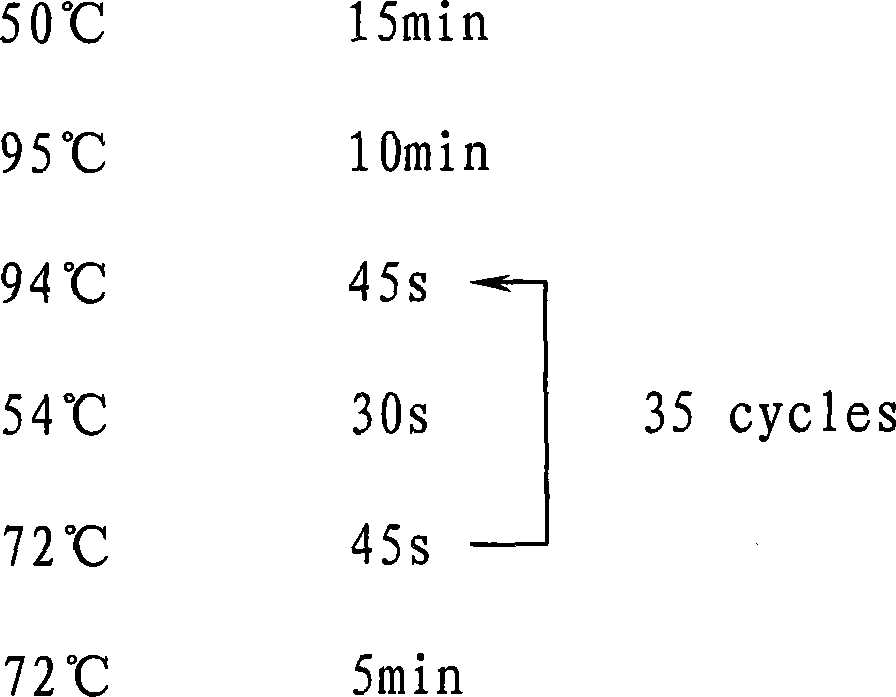
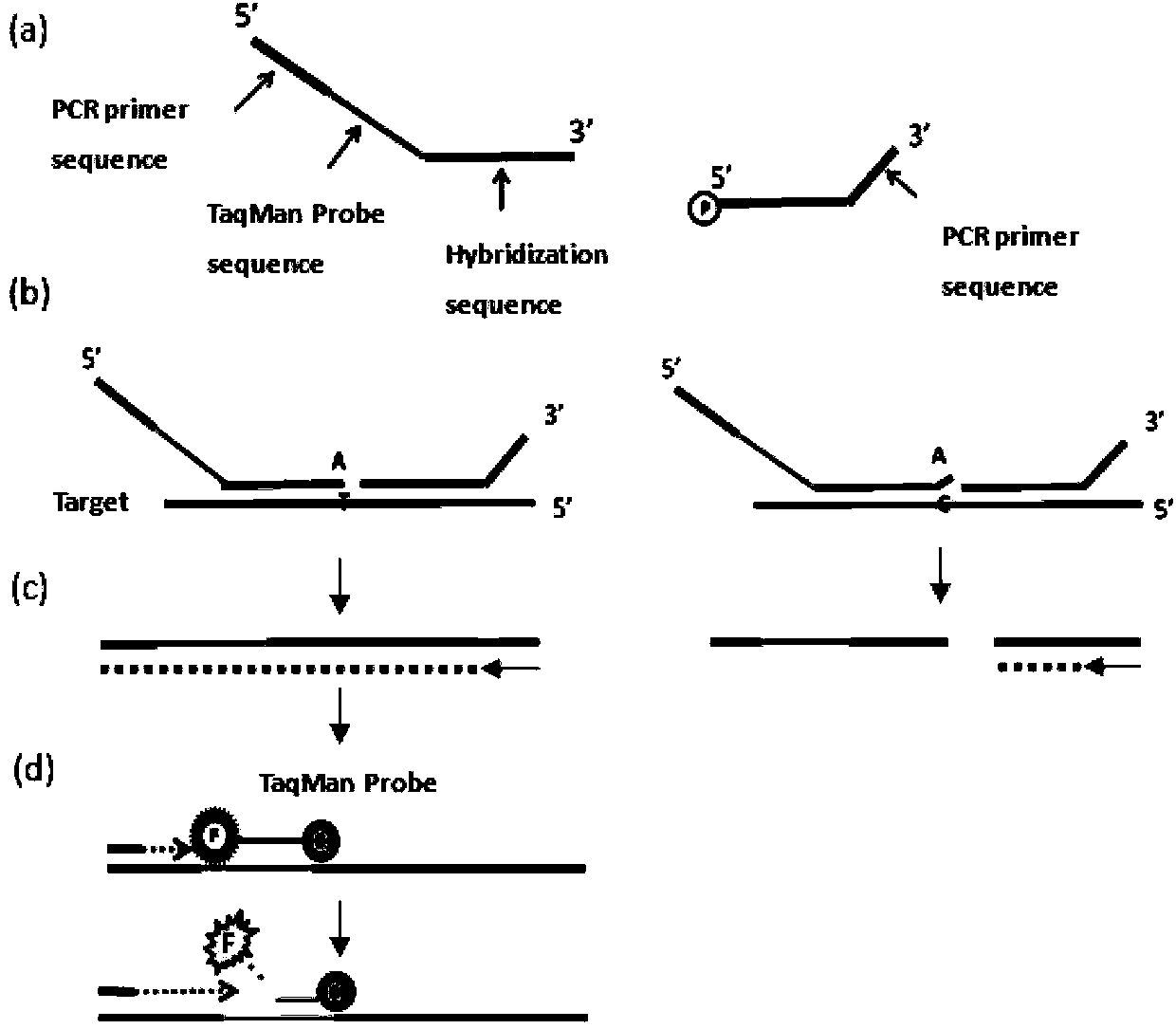
Multiplex ligation-dependent probe real-time fluorescence PCR (Polymerase Chain Reaction) kit for detecting drug resistance of HBV (Hepatitis B Virus) lamivudine and/or adefovir
InactiveCN103409551AEfficient detectionLow costMicrobiological testing/measurementDNA/RNA fragmentationMultiplex ligation-dependent probe amplificationFluorescence
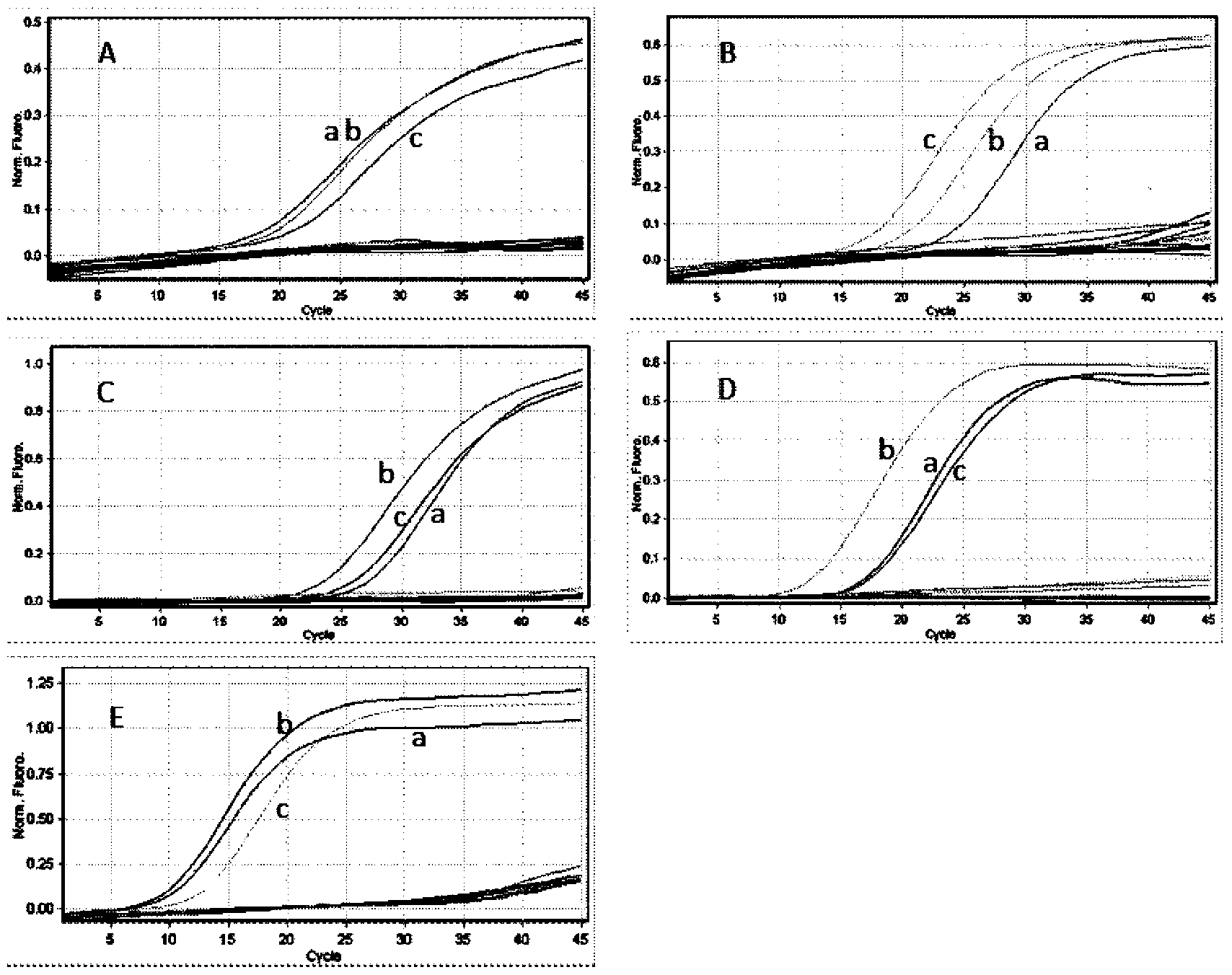
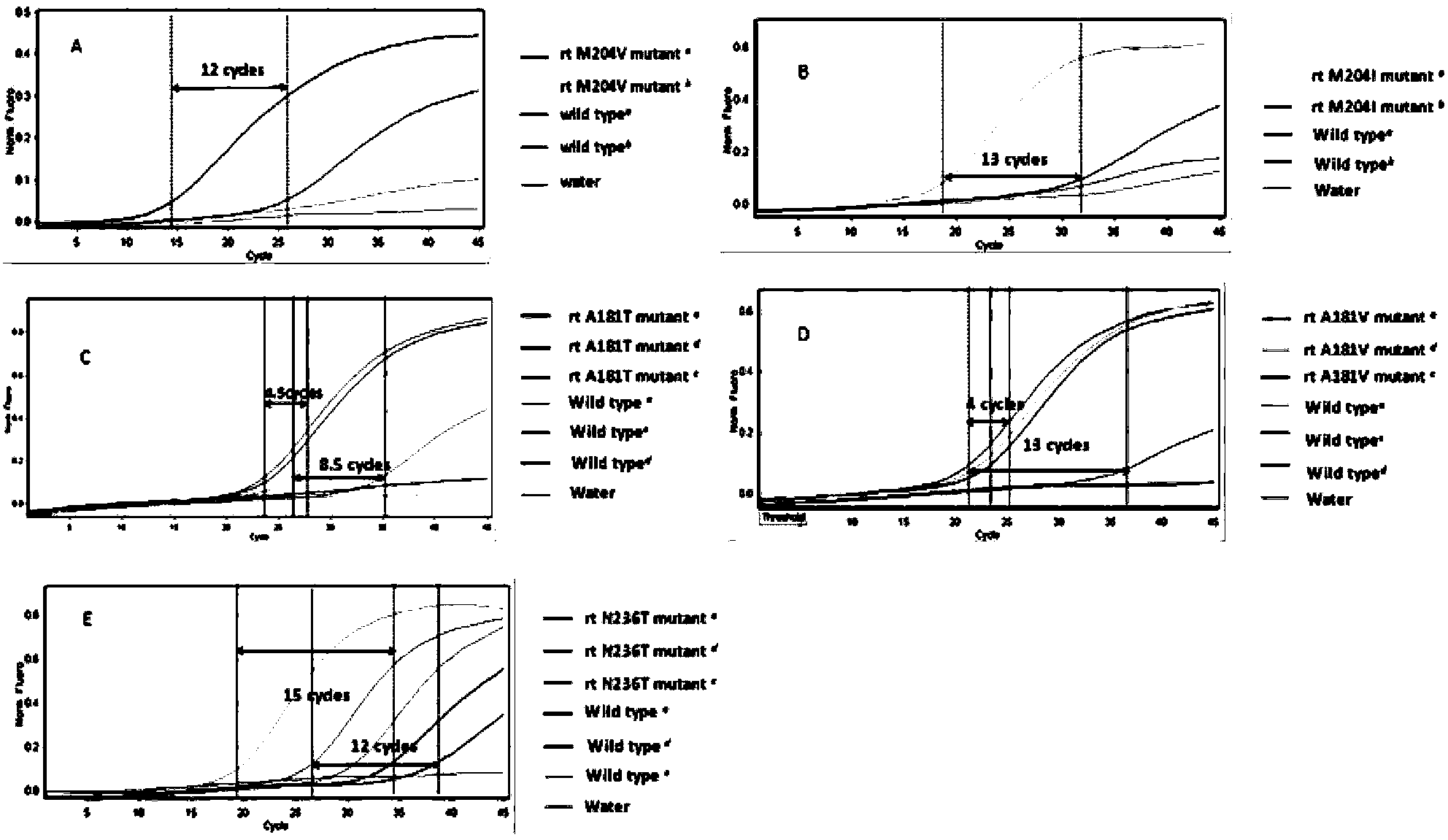
The invention belongs to the biotechnology field and particularly relates to a multiplex ligation-dependent probe real-time fluorescence PCR (Polymerase Chain Reaction) technology for detecting the drug resistance of chronic hepatitis B. A multiplex ligation-dependent probe real-time fluorescence PCR kit comprises an MLPA (Multiplex Ligation-dependent Probe Amplification) probe set, a filling sequence and a universal primer, wherein probes are respectively designed according to mutation sites rtM204V, rtM204I, rtA181T, rtA181V and rtN236T of a P gene reverse transcription region of HBV (Hepatitis B Virus); the sequences of the probes are represented by SEQ ID NO: 1-10. The invention further relates to a method for detecting the drug resistance of the HBV lamivudine and adefovir by virtue of the kit. The method combines the high throughput of an MPLA technology, and the rapidness, sensitivity and real-time detection of real-time fluorescence PCR, and has a high coincidence rate compared with a direct sequencing method, and can be used for detecting samples which cannot be detected by the sequencing method due to insufficient sensitivity; a whole detection process takes 4-5 hours; the clinic hepatitis B drug resistance detection method provided by the invention is low in cost and quick in detection.
Owner:THE THIRD AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIV OF PLA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com