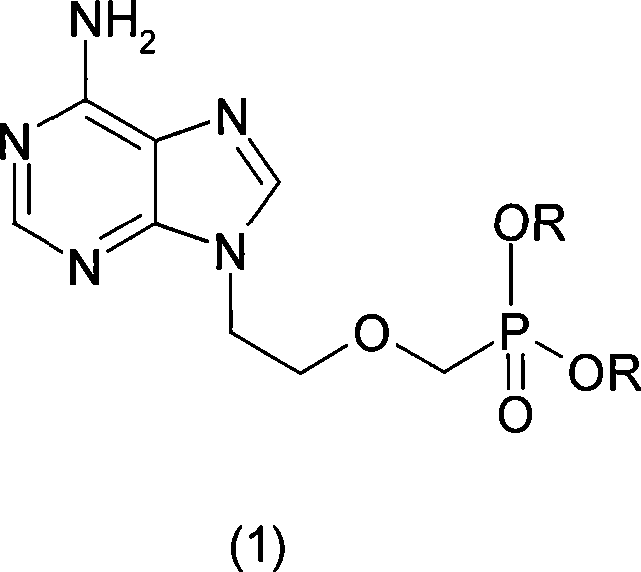
Method of Synthesizing adefovir dipivoxil
A raw material, dioxane technology, applied in chemical instruments and methods, compounds of elements of group 5/15 of the periodic table, organic chemistry, etc., can solve the problem of low total yield, reduce by-products, and reduce reaction conditions Mild, easy-to-prepare effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
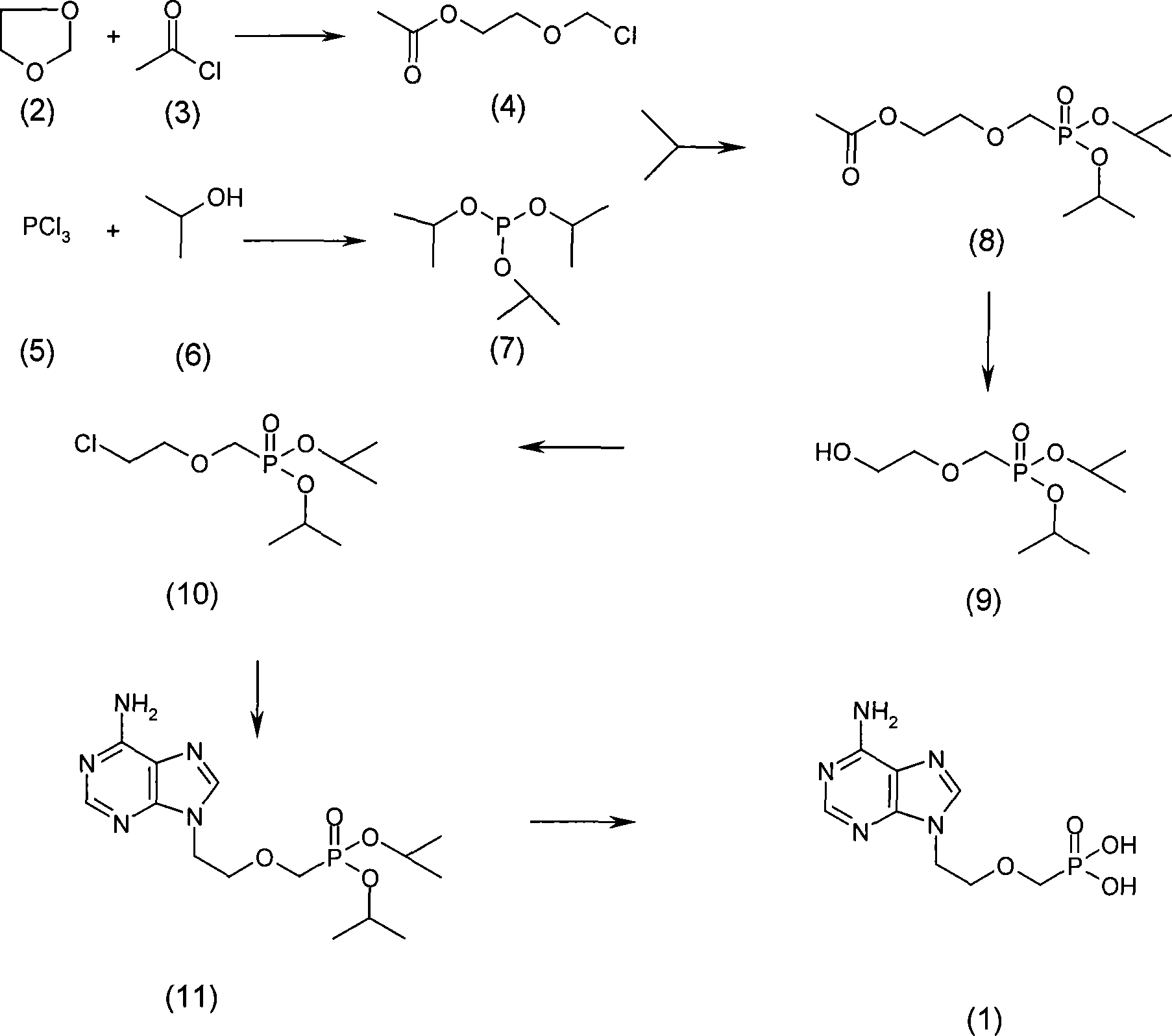
[0027] Embodiment 1: the preparation of ethyl 2-chloromethoxyacetate (4)
[0028] Add 1,3-dioxopentacycline (2) (78 g, 0.99 mol) and acetyl chloride (3) (111 g, 1.49 mol) after anhydrous treatment into a 500 ml one-necked flask, and stir evenly. Heating in an oil bath kept the reaction under reflux for 2h. After the reflux, change the distillation device to distill under reduced pressure (533Pa), and collect fractions at 65-70°C. A total of 107 g of ethyl 2-chloromethoxyacetate (2) was obtained. The yield is 82%, and the boiling point is 68°C / 4mmHg (533Pa). MS (EI): m / z = 117.1.
Embodiment 2
[0029] Embodiment 2: preparation of triisopropyl phosphite (7)
[0030] In a 150ml three-necked flask, add isopropanol (18g, 0.3mol), pyridine (25.5g, 0.3mol) and petroleum ether (100ml). Stir mechanically, cool in an ice-water bath, add dropwise a mixed solution of phosphorus trichloride (5) (13.75 g, 0.1 mol) and petroleum ether (40 ml) into the flask, and stir vigorously. With the dropwise addition of phosphorus trichloride petroleum ether solution, the reaction solution turned into white foam. After 30 minutes, the dropwise addition was completed, and changed to a 50°C water bath to heat the reaction for 1 hour, filtered, the filter cake was washed with petroleum ether, the filtrate was combined, and the 72-75°C / 30mmHg (4000Pa) fraction was collected by vacuum distillation to obtain 13.5g of a colorless liquid. Yield 65%. MS (EI+1.64e4): m / z=208.0. IR (KBr pellet, cm -1 ): 3800cm -1 (w, v CH ), 2960cm -1 (s, v CH3 ), 1185cm -1 (w, v C-O ), 980cm -1 (s, v P-O )....
Embodiment 3
[0031] Embodiment 3: the preparation of diisopropyl acetyl ethoxymethyl phosphonate (8)
[0032] Add ethyl 2-chloromethoxyacetate (4) (10.7 g, 70 mmol) into a 50 ml three-necked flask. With magnetic stirring, the oil bath was heated to 90°C, and triisopropyl phosphite (7) (18.6 g, 90 mmol) was slowly added dropwise, and the dropwise addition was completed in about 1 hour. Raise the temperature to 125°C and keep the reaction temperature for 4h. TLC followed the reaction. After heating, replace the vacuum distillation device, vacuum distillation, collect 168-172 ℃ / 1mmHg (133Pa) fraction, obtain colorless liquid 16g, yield 71%. MS (EI+6.47e4): m / z=282.1. 1 H-NMR (ppm, CDCl 3 ): δ4.73(m, OCH 2 ), δ4.21(s, OCH 2 P), δ3.80(m, CH 2 CH 2 O), δ3.64(t, CHCH 3 ), δ2.01(s, COCH 3 ), δ1.32(m, CH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com