Adefovir dipivoxil anhydrous crystal, preparation method and medicine composition thereof
An adefovir dipivoxil and anhydrous crystallization technology, applied in the field of nucleoside drugs, can solve the problems of high preparation cost and high solvent residue, and achieve the effects of simple preparation method, good fluidity and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
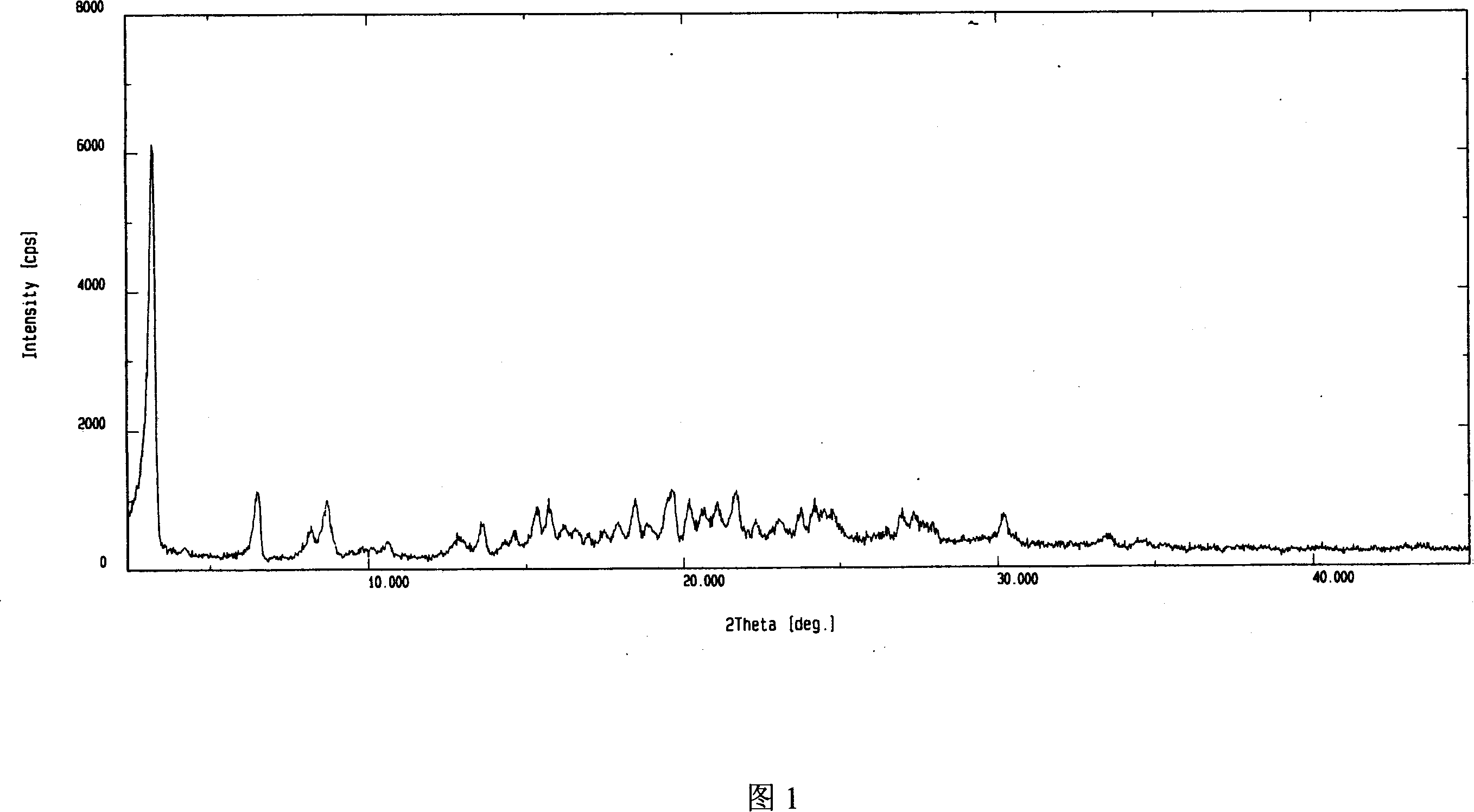
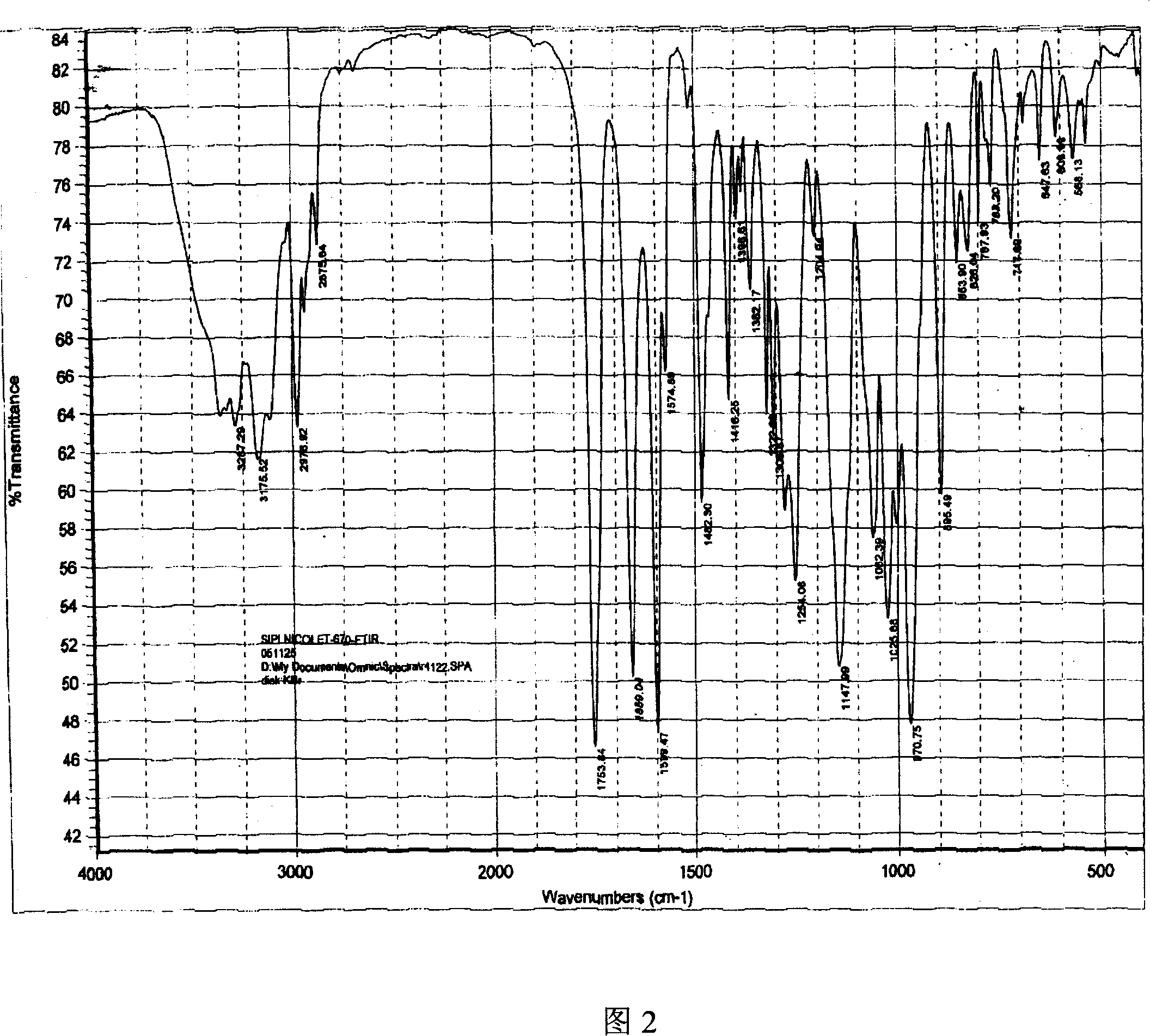
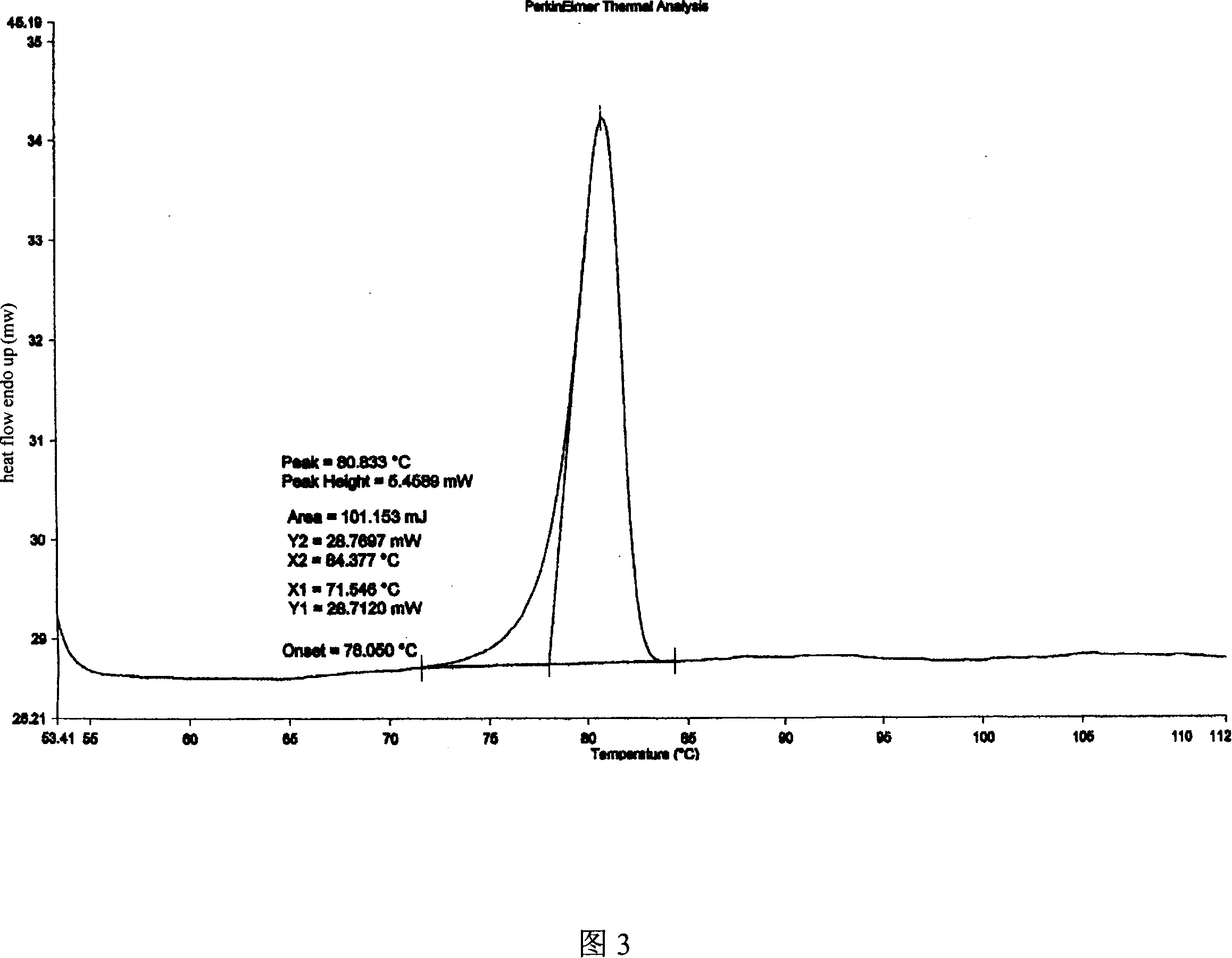
[0032] Dissolve 100g of adefovir dipivoxil crude product in 1500ml of isopropanol aqueous solution (isopropanol: water = 1:3), heat to 70°C, dissolve, add 5g of activated carbon, filter, and cool the filtrate to 0°C, stirring and crystallizing After 5 hours, filter and air-dry at about 50°C to obtain 95 g of the product. As determined by HPLC, the content is 99.5%. Its X-ray powder diffraction spectrum is shown in Figure 1, its infrared absorption spectrum is shown in Figure 2, and its DSC is shown in Figure 3.
Embodiment 2
[0034] Dissolve 100g of adefovir dipivoxil crude product in 1500ml of acetone aqueous solution, (acetone: water = 1:5), heat to 60°C, dissolve, add 5g of activated carbon, stir for 20 minutes, filter, the filtrate is cooled to 0°C, stir and crystallize After 5 hours, filter and air-dry at about 30°C to obtain 91 g of the product. As determined by HPLC, the content is 99.5%. Its X-ray powder diffraction spectrum is as in Example 1, and its infrared absorption spectrum is as in Example 1.
Embodiment 3
[0036] Dissolve 100g of adefovir dipivoxil crude product in 1500ml of acetonitrile aqueous solution (acetonitrile: water = 1: 4), heat to 70°C, dissolve, add 5g of activated carbon, stir for 20 minutes, filter, the filtrate is cooled to 0°C, stir and crystallize After 5 hours, filter and air-dry at about 60°C to obtain 92g of the product. Its X-ray powder diffraction spectrum is as in Example 1, and its infrared absorption spectrum is as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com