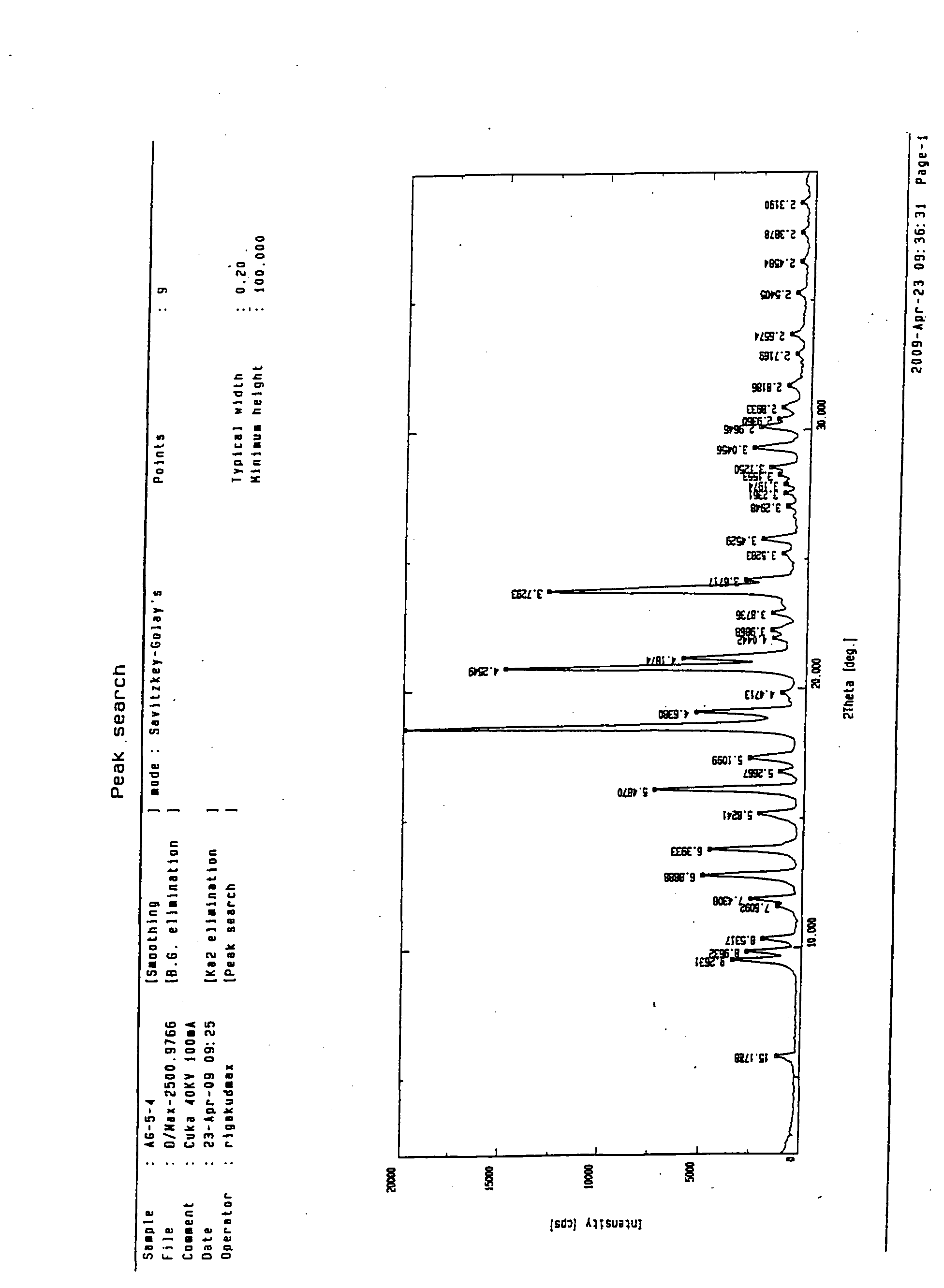
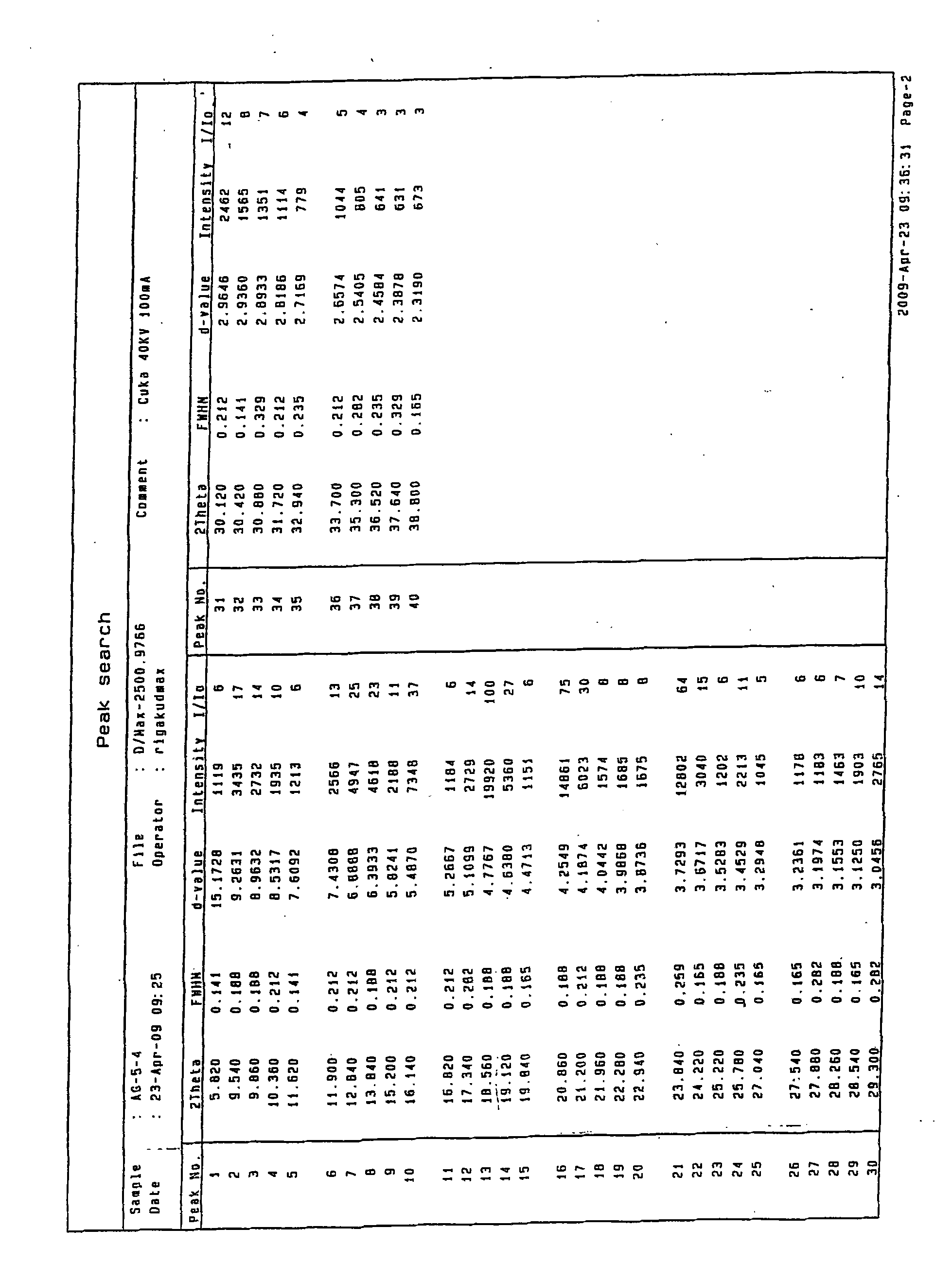
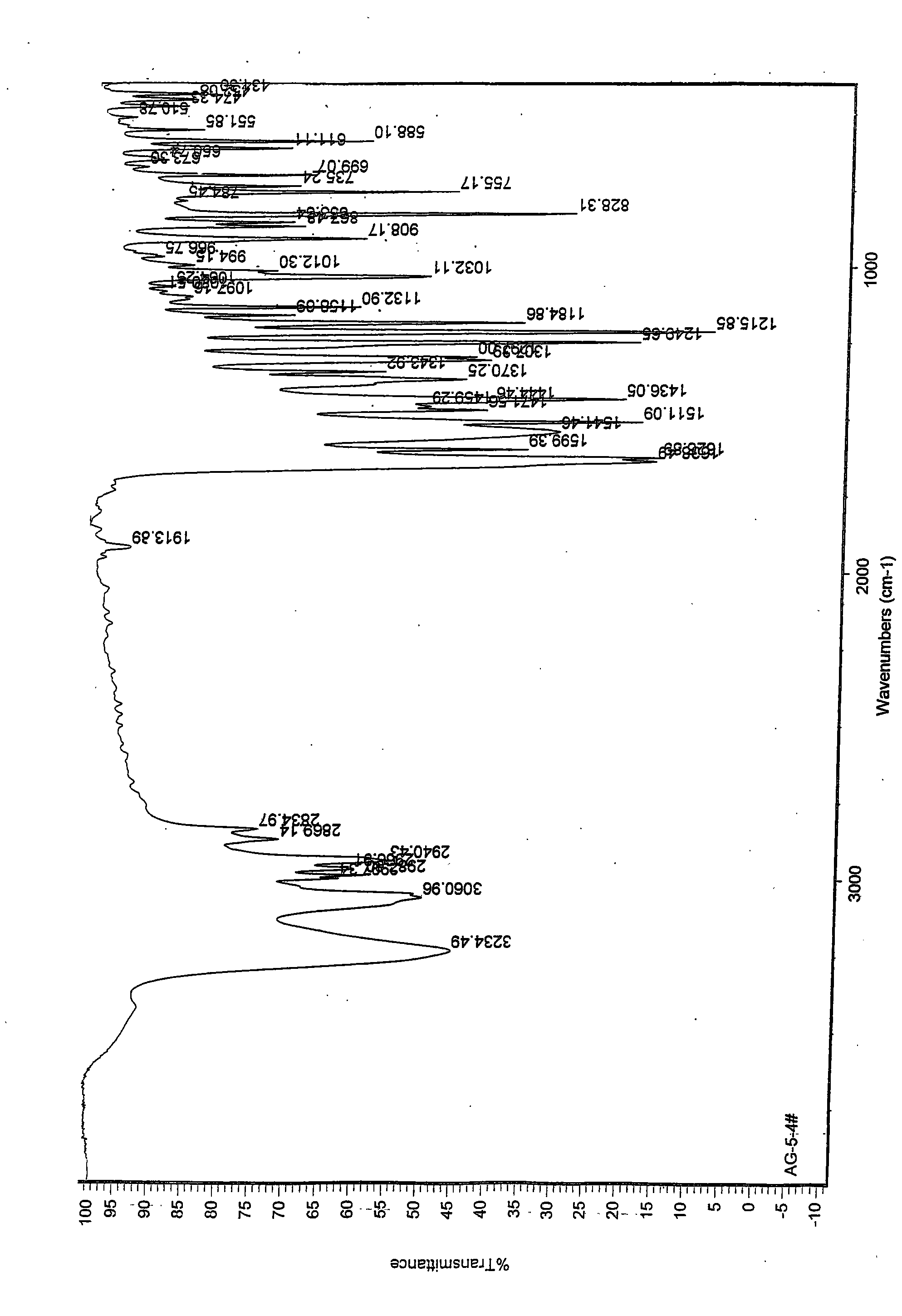
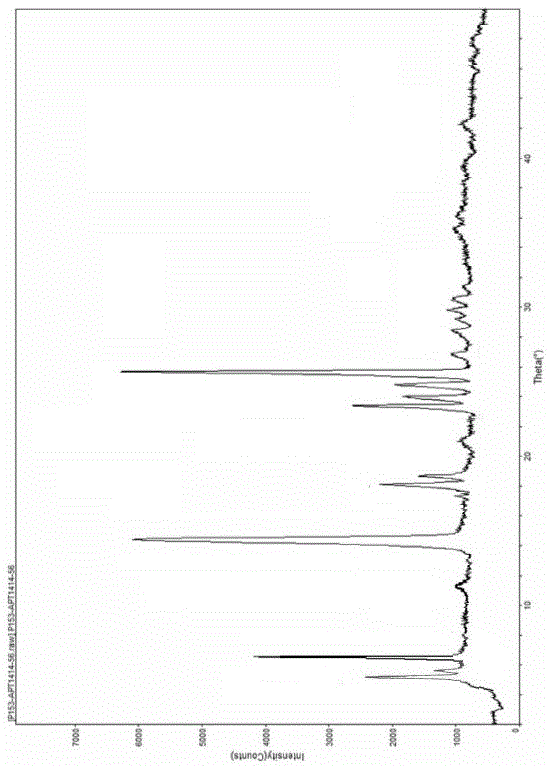
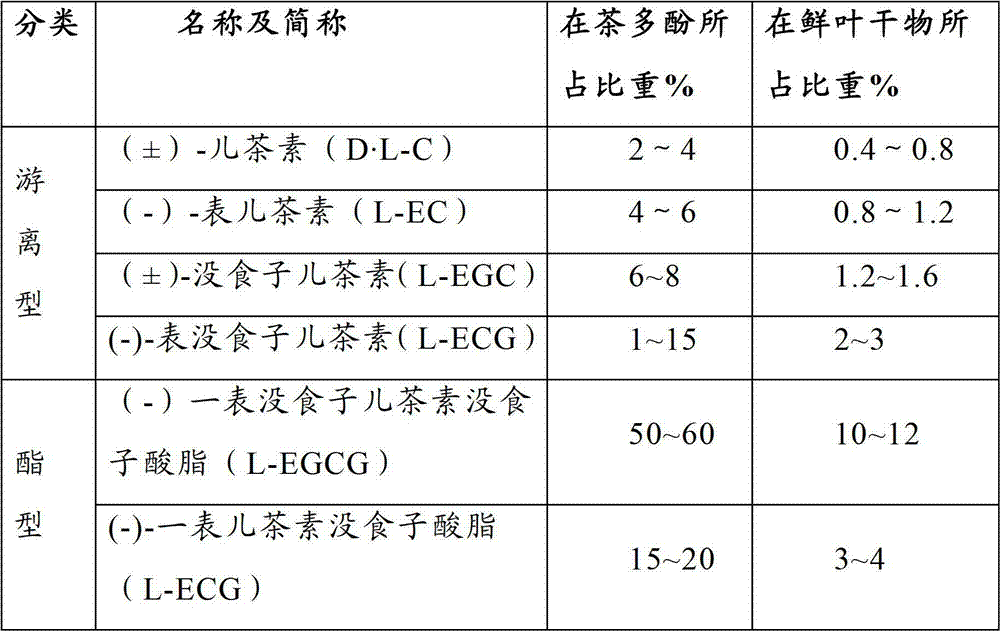
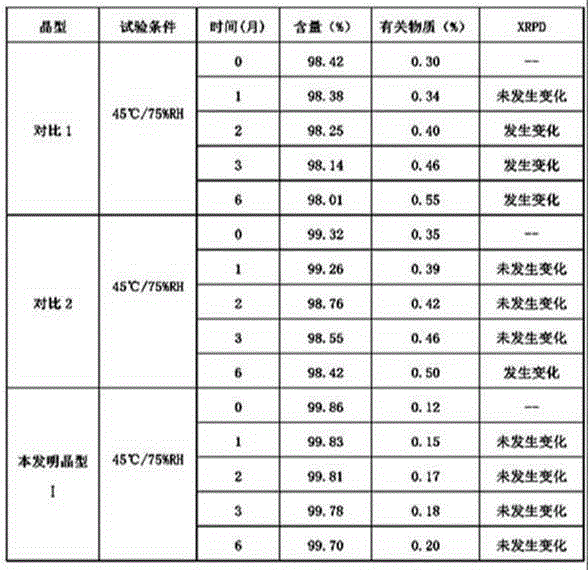
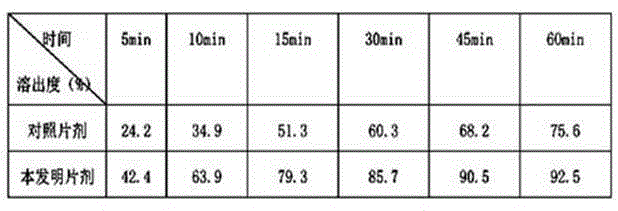
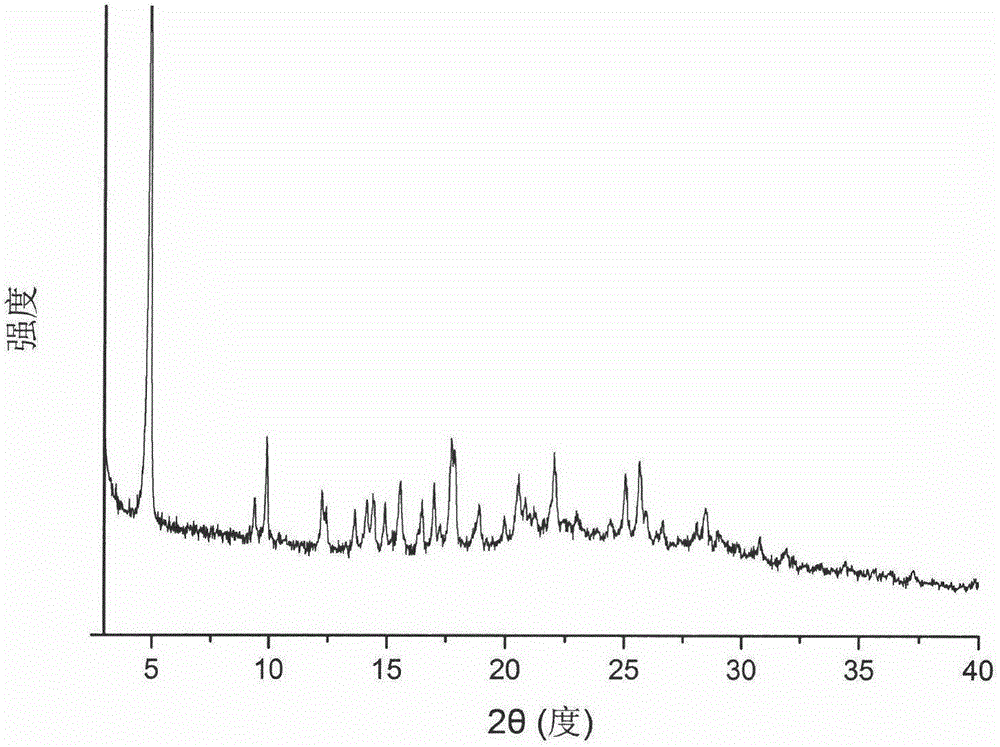
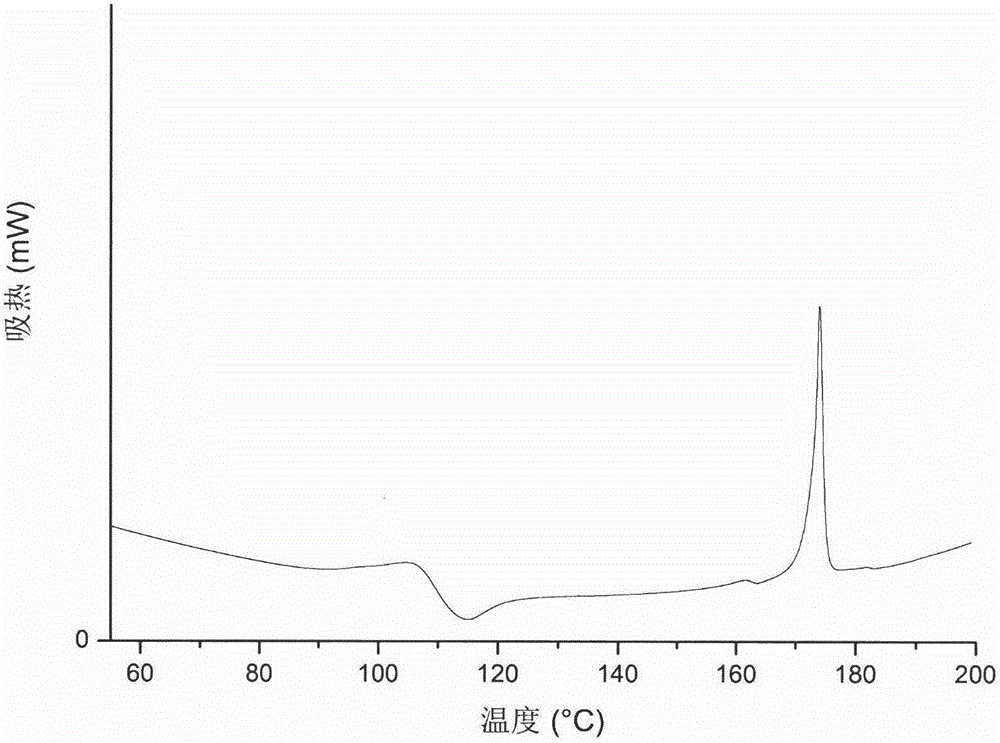
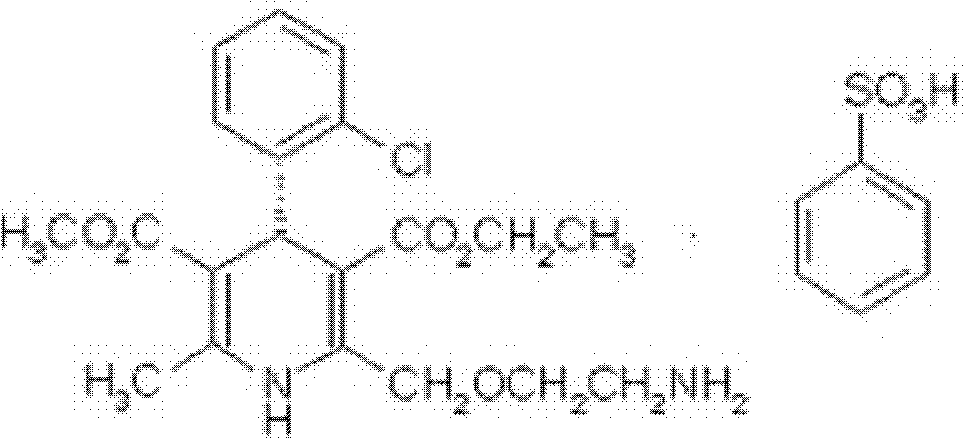Patents
Literature
183 results about "K-alpha" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In X-ray spectroscopy, K-alpha emission lines result when an electron transitions to the innermost "K" shell from a 2p orbital of the second or "L" shell. The line is actually a doublet, with slightly different energies depending on spin-orbit interaction energy between the electron spin and the orbital momentum of the 2p orbital. K-alpha is typically by far the strongest X-ray spectral line for an element bombarded with energy sufficient to cause maximally intense X-ray emission. The analogous K-alpha spectra line in hydrogen is known as Lyman alpha; however because of hydrogen's small nuclear charge, this line is in the ultraviolet, not the X-ray range. See Siegbahn notation for the newer IUPAC-recommended spectral notation system. An example of K-alpha lines are those seen for iron as iron atoms radiating X-rays spiralling into a black hole at the center of a galaxy. For such purposes, the energy of the line is adequately calculated to 2-digit accuracy by the use of Moseley's law:, where Z is the atomic number. For example, K-alpha for iron is calculated in this fashion as 10.2 eV ² = 6.38 keV energy.
Agomelatine and medicine composition thereof
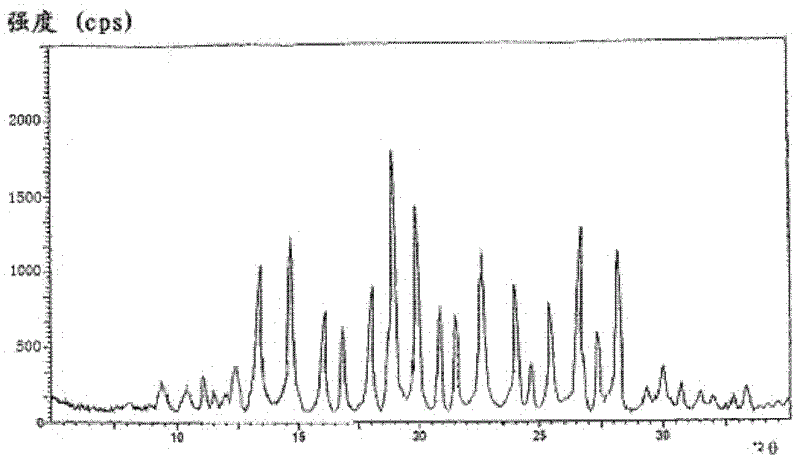
ActiveCN101781226AQuality improvementImprove solubilityOrganic active ingredientsNervous disorderK-alphaPowder diffraction
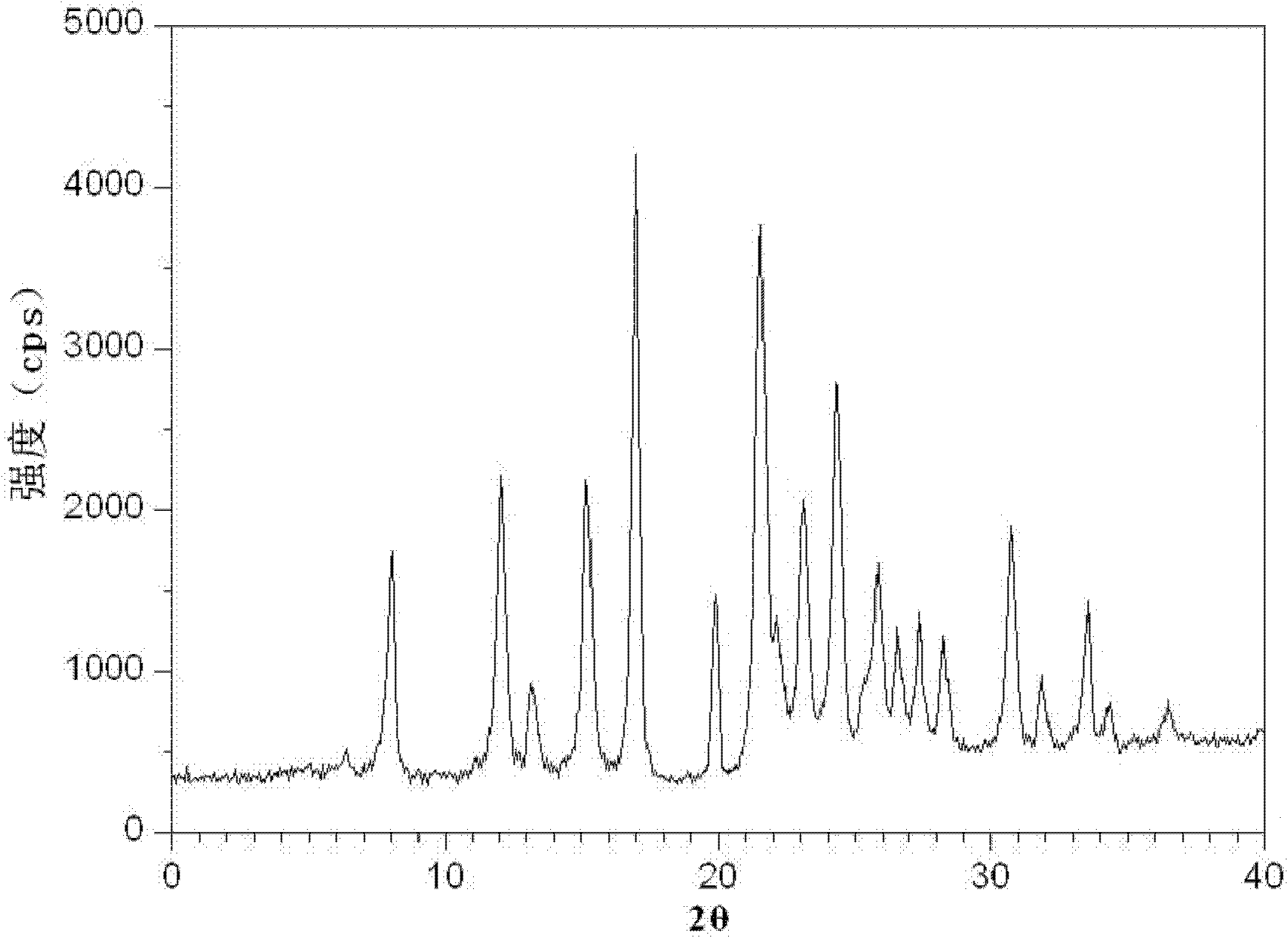
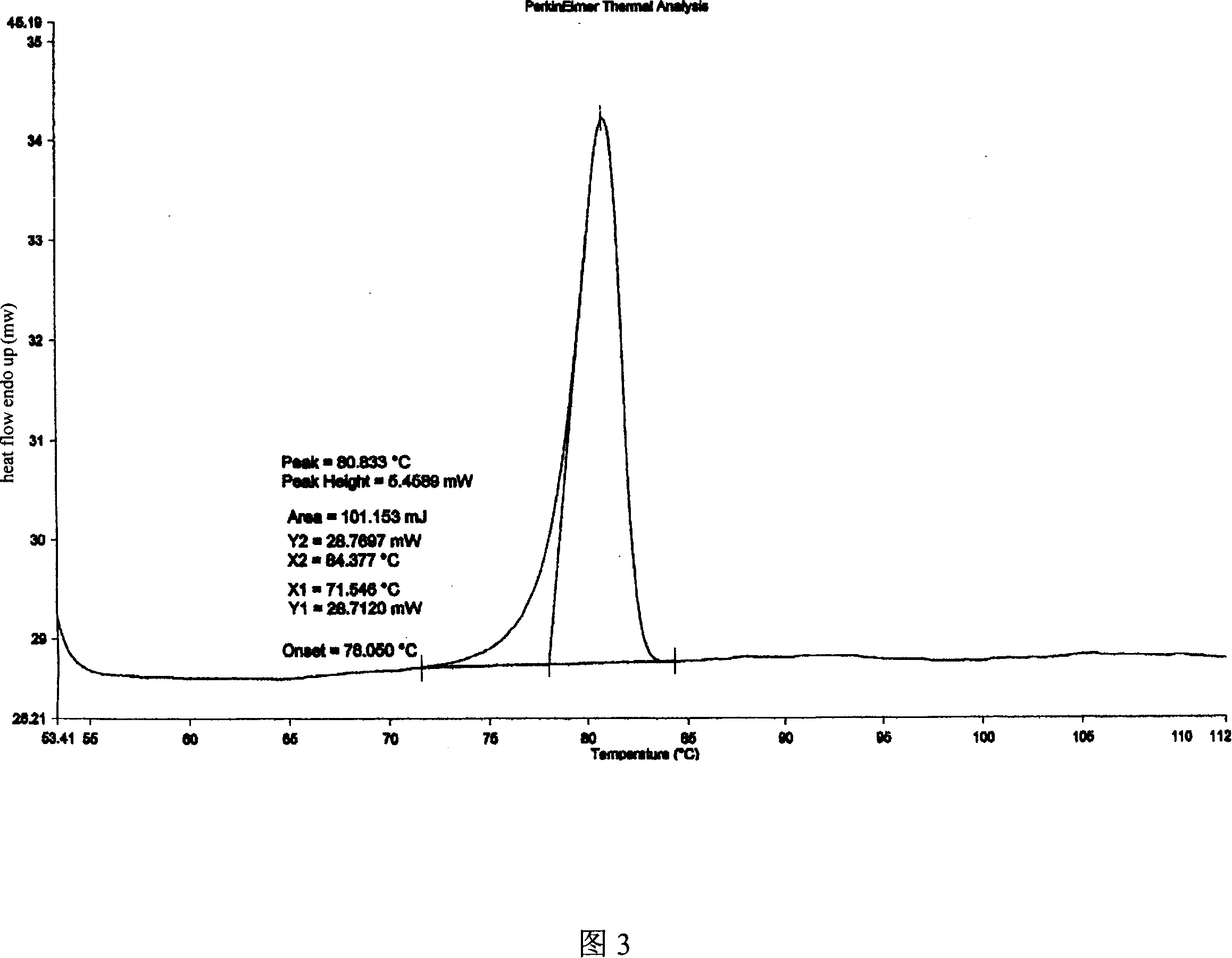
The invention discloses a medicine of Agomelatine for treating depression, in particular an Agomelatine crystal and a medicine composition thereof. The Agomelatine crystal is irradiated by using Cu-K alpha; and an X-ray powder diffraction spectrum expressed by the degree of 2theta of the Agomelatine crystal has characteristic diffraction peaks at positions of 12.84, 13.84, 16.14, 14.18, 18.56, 19.12, 20.86, 21.20 and 23.84, and an infrared absorption spectrum of the Agomelatine crystal has characteristic diffraction peaks approximately at position of 3234, 3060, 2940, 1638, 1511, 1436, 1249, 1215, 1184, 1032, 908, 828, 755 and 588cm-1. The DSP heat absorption transition is 97.6 DEG C. The invention further discloses application of the medicine composition containing the Agomelatine crystal as an active effective component to the preparation of medicines for treating the depression.
Owner:TIPR PHARM CO LTD
Crystal form B and preparation method
InactiveCN105646484AImprove stabilityStable storageOrganic active ingredientsOrganic chemistry methodsK-alphaRadiation
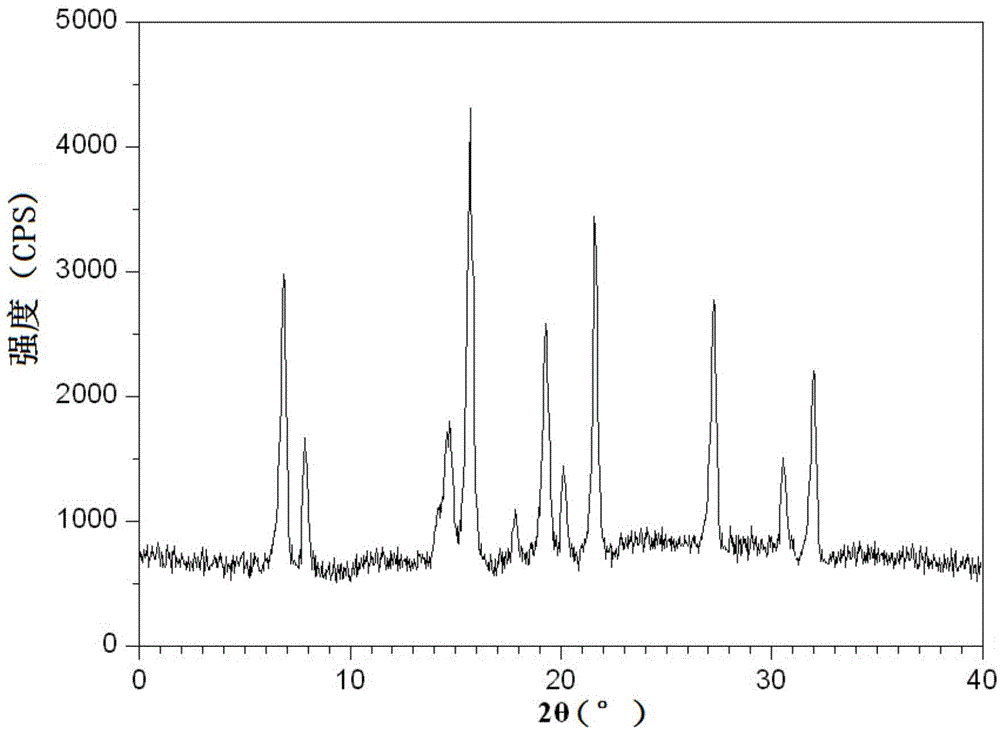
The invention discloses a crystal form B. The crystal form B is characterized in that Cu-K alpha radiation is used, X-RPD (X-ray powder diffraction) indicated by the 2 theta angle has diffraction peaks at 6.4+ / -0.2 degrees, 7.9+ / -0.2 degrees, 8.7+ / -0.2 degrees, 9.8+ / -0.2 degrees, 10.0+ / -0.2 degrees and 17.0+ / -0.2 degrees. The crystal form B can be prepared on a large scale safely and purified, can be stored stably, does not have moisture absorption, is very suitable for preparation of an inhalable preparation and has good compatibility with excipients such as lactose, starch and the like.
Owner:孙霖
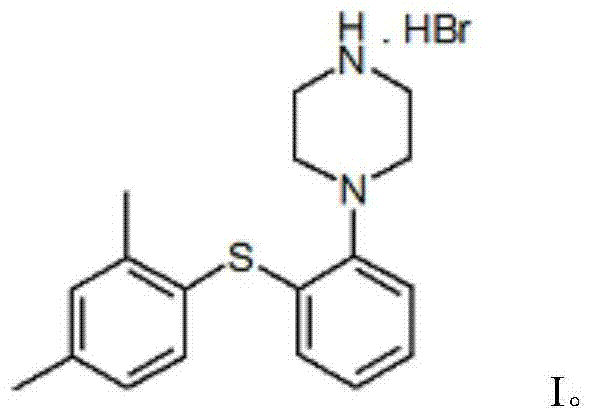
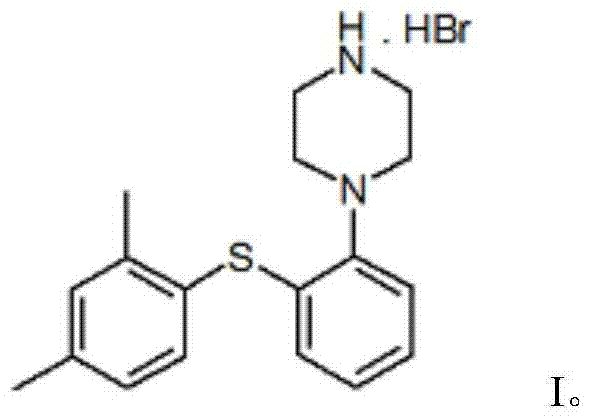
Vortioxetine hydrobromide
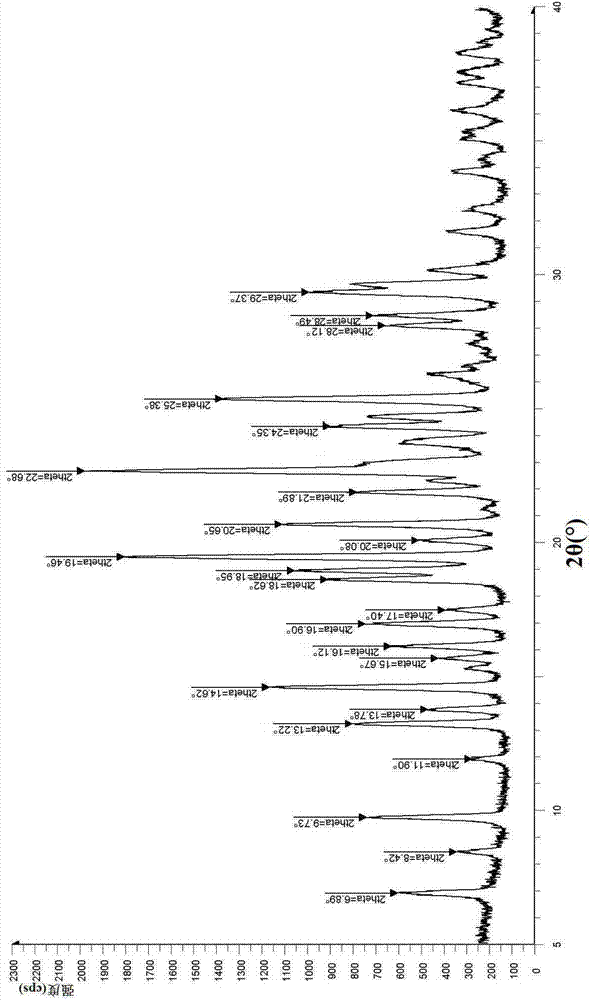
The invention relates to vortioxetine hydrobromide and in particular relates to a crystal of 1-[2-(2,4-dimethylphenylthioxo)phenyl] piperazine hydrobromide. The crystal uses Cu-K alpha radiation. In a powder X-ray diffraction pattern shown by a 2theta angle, diffraction peaks exist at about 6.89 degrees, 9.73 degrees, 13.78 degrees and 14.62 degrees. The invention also relates to a preparation method of the crystal and a drug composition containing the compound. The compound shows the serotonin reabsorption inhibitory activity, has activities towards a serotonin receptor 1A (5-HT1A) and a serotonin receptor 3 (5-HT3), can be used for treating CNS (central nervous system)-related diseases, in particular can be used for treating depressive disorder, especially major depressive disorder of adults, and can be also used for treating other CNS-related diseases.
Owner:BEIJING LABWORLD BIO MEDICINE TECH
Thermographic recording material with improved image tone and/or stability upon thermal development
InactiveUS6096486AImprove wear resistanceAvoid local deformationX-ray/infra-red processesOrganic compound preparationOrganic solventDiffractometer
A recording material comprising a support and a thermosensitive element containing silver behenate, an organic reducing agent therefor in thermal working relationship therewith and a binder, wherein the silver behenate is not associated with mercury and / or lead ions and when the recording material is irradiated with a copper K alpha 1 X-ray source the ratio, normalized to a quantity of silver in the recording material of 1 g per m2 thereof, of the sum of the peak heights of the X-ray diffraction lines attributable to silver behenate at Bragg angles, 2 THETA , of 6.01 DEG , 7.56 DEG , 9.12 DEG , 10.66 DEG , 12.12 DEG and 13.62 DEG to the sum of the peak heights of the X-ray diffraction lines at Bragg angles, 2 THETA , of 25.60 DEG , 35.16 DEG and 43.40 DEG of NIST standard 1976, rhombohedral Al2O3, determined with the same X-ray diffractometer in the same state of adjustment, is greater than 0.85; production processes for particles of substantially light-insensitive organic silver salt comprising silver behenate with these X-ray characteristics in the presence and substantial absence of organic solvent.
Owner:AGFA HEALTHCARE NV
Thermographic recording material with improved image tone and/or stability upon thermal development
InactiveUS6159667AImproved archivabilityImprove stabilityX-ray/infra-red processesOrganic compound preparationOrganic solventDiffractometer
A recording material comprising a support and a thermosensitive element containing silver behenate, an organic reducing agent therefor in thermal working relationship therewith and a binder, wherein the silver behenate is not associated with mercury and / or lead ions and when the recording material is irradiated with a copper K alpha 1 X-ray source the ratio, normalized to a quantity of silver in the recording material of 1 g per m2 thereof, of the sum of the peak heights of the X-ray diffraction lines attributable to silver behenate at Bragg angles, 2 THETA , of 6.01 DEG , 7.56 DEG , 9.12 DEG , 10.66 DEG , 12.12 DEG and 13.62 DEG to the sum of the peak heights of the X-ray diffraction lines at Bragg angles, 2 THETA , of 25.60 DEG , 35.16 DEG and 43.40 DEG of NIST standard 1976, rhombohedral Al2O3, determined with the same X-ray diffractometer in the same state of adjustment, is greater than 0.85; production processes for particles of substantially light-insensitive organic silver salt comprising silver behenate with these X-ray characteristics in the presence and substantial absence of organic solvent.
Owner:AGFA HEALTHCARE NV
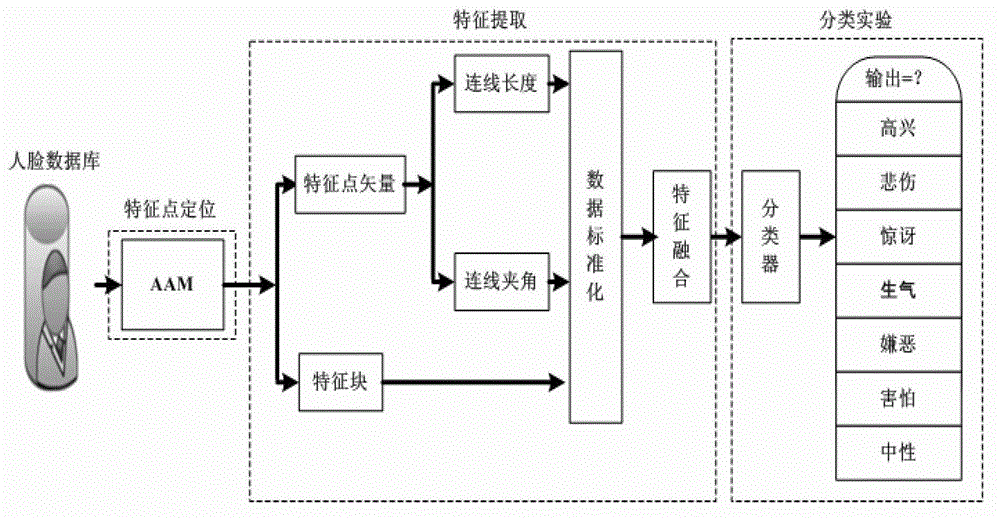
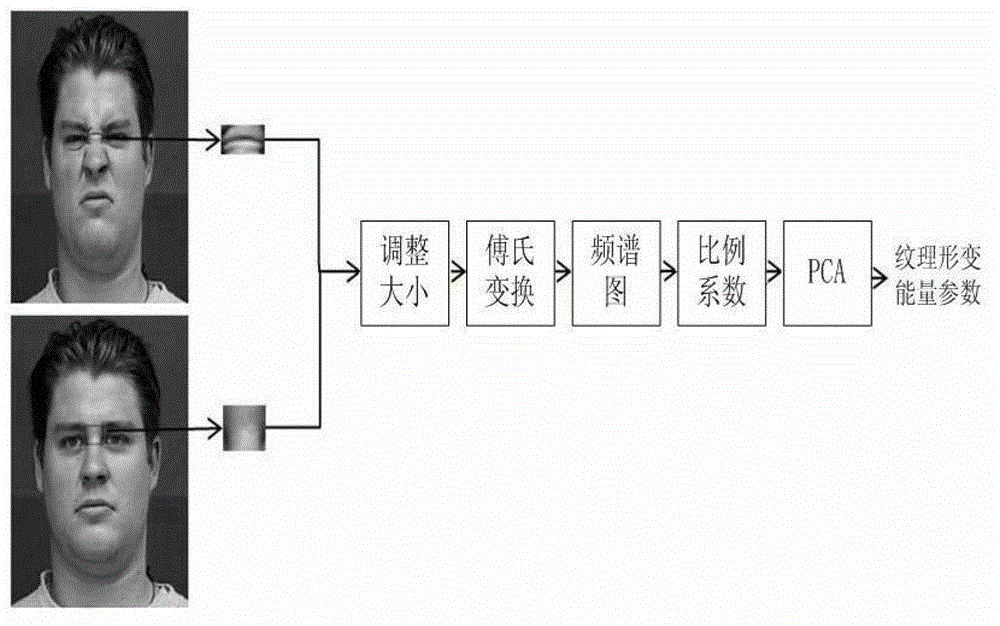
Facial expression recognition method based on feature point vectors and texture deformation energy parameter
InactiveCN102945361ASolve the impact of facial expression recognitionCharacter and pattern recognitionNerve networkPrincipal component analysis
The invention provides a facial expression recognition method based on feature point vectors and a texture deformation energy parameter. The method comprises the following steps: 1, respectively carrying out feature point positioning on neutral expressions and extreme expressions at a beginning end of a facial expression sequence by an AAM (Automatic Acoustic Management) tool of an OPENCV (Open Source Computer Vision Library); 2, forming feature point vectors with the selected 26 feature points to suit facial expression recognition, dividing the feature point vectors into a Euclidean distance d (representing a size) between the feature points and an included angle alpha (representing a direction) of connecting lines, calculating a distance coefficient ratio kd of the feature points according to d and alpha, and subtracting a redundant part kl to obtain kd - final (k alpha-final can be obtained in the same way); 3, establishing a feature block according to the feature points, calculating a texture deformation energy coefficient matrix, and finally obtaining the texture deformation energy parameter ks - final by PCA (Principal Component Analysis); and 4, inputting final features, namely using k final = kd - final + k alpha - final + ks - final as the training data of an RBF (Radial Basis Function) nerve network, and finally realizing facial expression recognition.
Owner:BEIHANG UNIV
A kind of levamlodipine besylate crystal, its preparation method and a new pharmaceutical composition containing the crystal
ActiveCN102276516AImprove solubilityGood curative effectOrganic active ingredientsOrganic chemistrySolubilityMedicine
The invention relates to levamlodipine besylate crystals, a preparation method thereof and a brand-new medicinal composition containing the crystals. Characteristic peaks in an X-ray powder diffraction pattern obtained by measuring the levamlodipine besylate crystals by using Cu-K alpha rays are displayed as 8.0, 12.1, 15.4, 17.0, 19.8, 21.6, 23.0, 24.3, 25.7, 27.4, 30.7 and 33.5 degrees at 2 theta. The medicinal composition comprises the levamlodipine besylate crystals and pharmaceutically acceptable excipients, wherein the pharmaceutically acceptable excipients are microcrystalline cellulose, sodium starch glycolate and magnesium stearate. The crystals improve the dissolubility of levamlodipine besylate; and tablets prepared from the crystals have improved bioavailability.
Owner:HAINAN JINRUI PHARMA
Nanometer iron trioxide as well as preparation method and purpose of nanometer iron trioxide
ActiveCN102897847AImprove processing efficiencyGood renewabilityWater/sewage treatment by irradiationFerric oxidesDiiron TrioxideSewage treatment
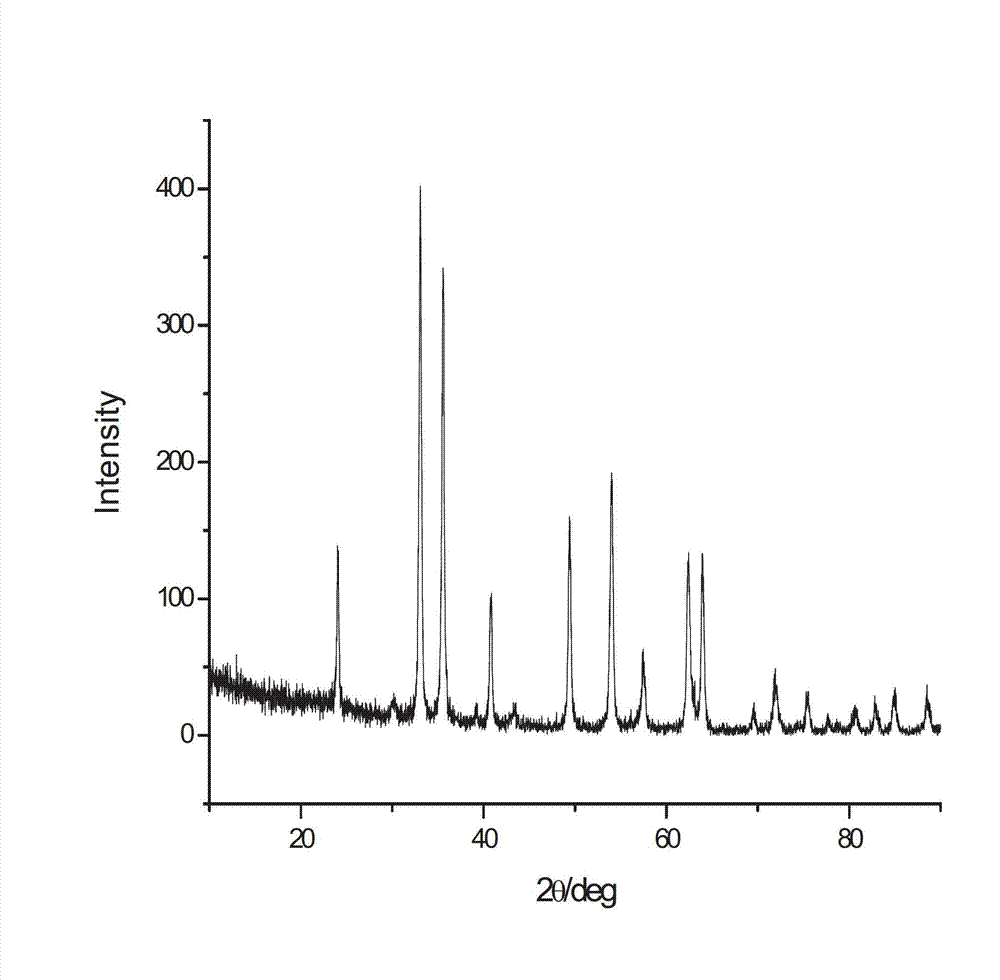
The invention provides nanometer iron trioxide as well as a preparation method and a purpose of the nanometer iron trioxide. The nanometer iron trioxide has the following structural formula of alpha-Fe2O3, and X-ray powder diffractions obtained through measurement by using a Cu-K alpha ray via the nanometer iron trioxide have characteristic peaks through display when 2 theta is 24.1 degrees, 34.4 degrees, 36.0 degrees, 41.2 degrees, 50.2 degrees, 53.3 degrees, 57.6 degrees, 62.2 degrees and 63.7 degrees. When the nanometer iron trioxide provided by the invention is adopted for carrying out sewage treatment, the advantage of high sewage treatment efficiency is realized, in addition, the nanometer iron trioxide is solid, the reproducible performance is good, and the repeated utilization can be realized, so the sewage treatment cost can be reduced, and in addition, the advantage of simplicity in operation is also realized.
Owner:W STARTECH BEIJING ENVIRONMENTAL TECH
Nickel series rechargeable battery and its producing method
InactiveCN1339844AIncrease profitIncrease energy densityMaterial nanotechnologyFinal product manufactureParticulatesCrystallography
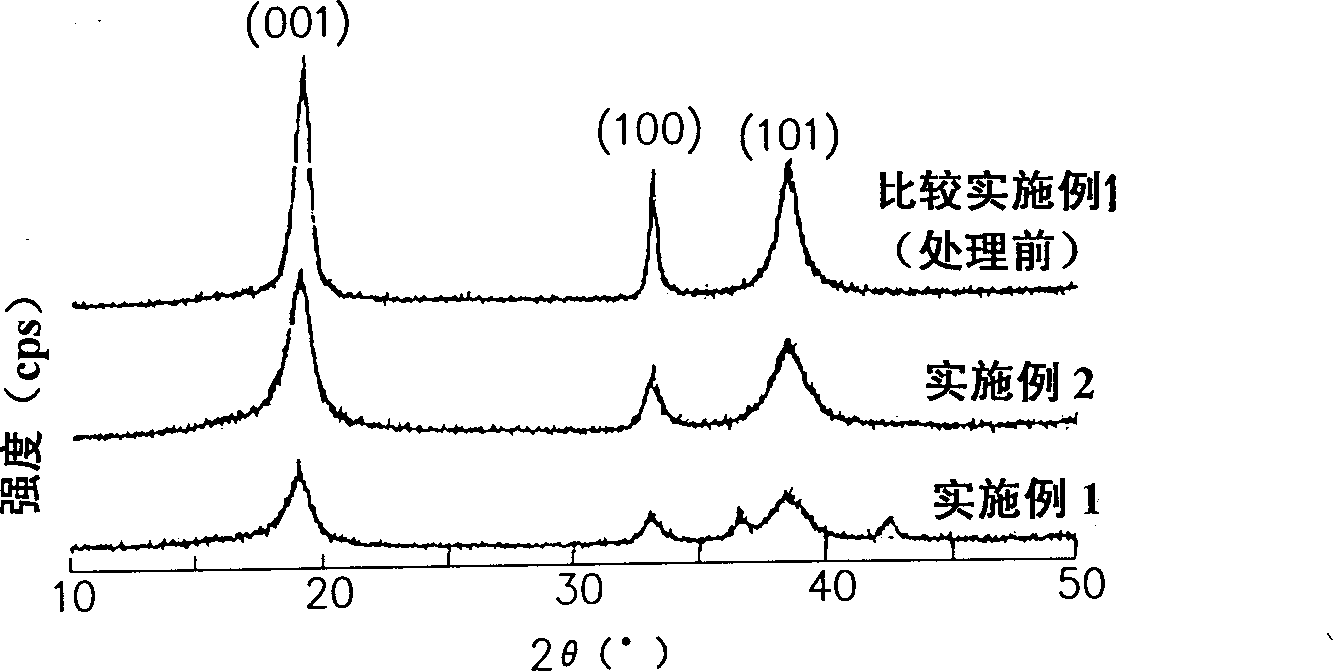
A nickel-series rechargeable battery whose cathode active material comprising a material containing an amorphous phase-bearing nickel hydroxide particulate which in X-ray diffraction using K alpha -rays of Cu as a radiation source, has a diffraction peak of a (001) face appeared near a diffraction angle 2 theta = 19 DEG having a half-value width of more than 1.2 DEG and has a diffraction peak of a (101) face appeared near a diffraction angle 2 theta = 38 DEG having a half-value width of more than 1.5 DEG . A process for the production of said rechargeable battery is also provided.
Owner:CANON KK
Novel omeprazole sodium compound and medicinal composition thereof
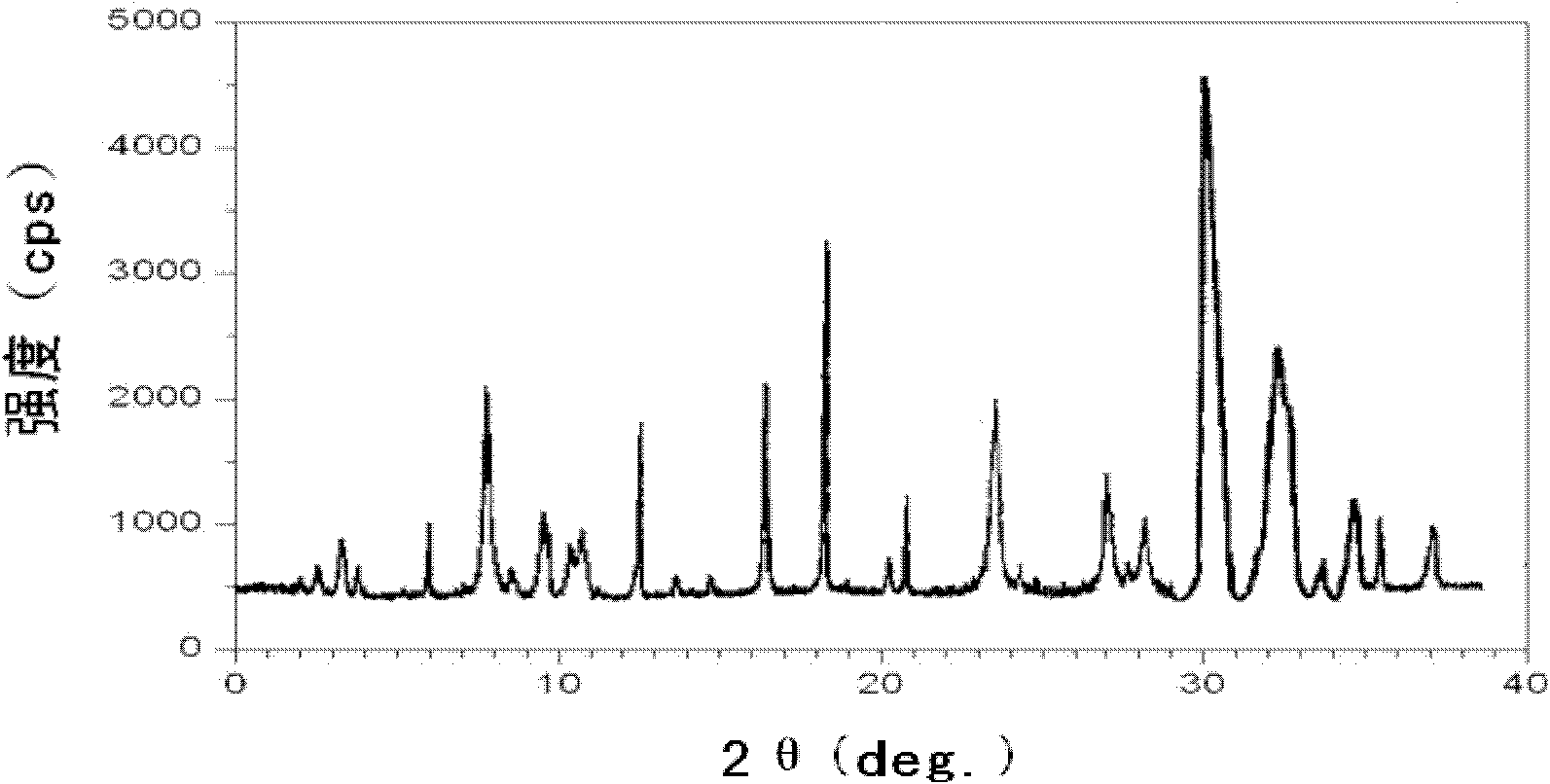
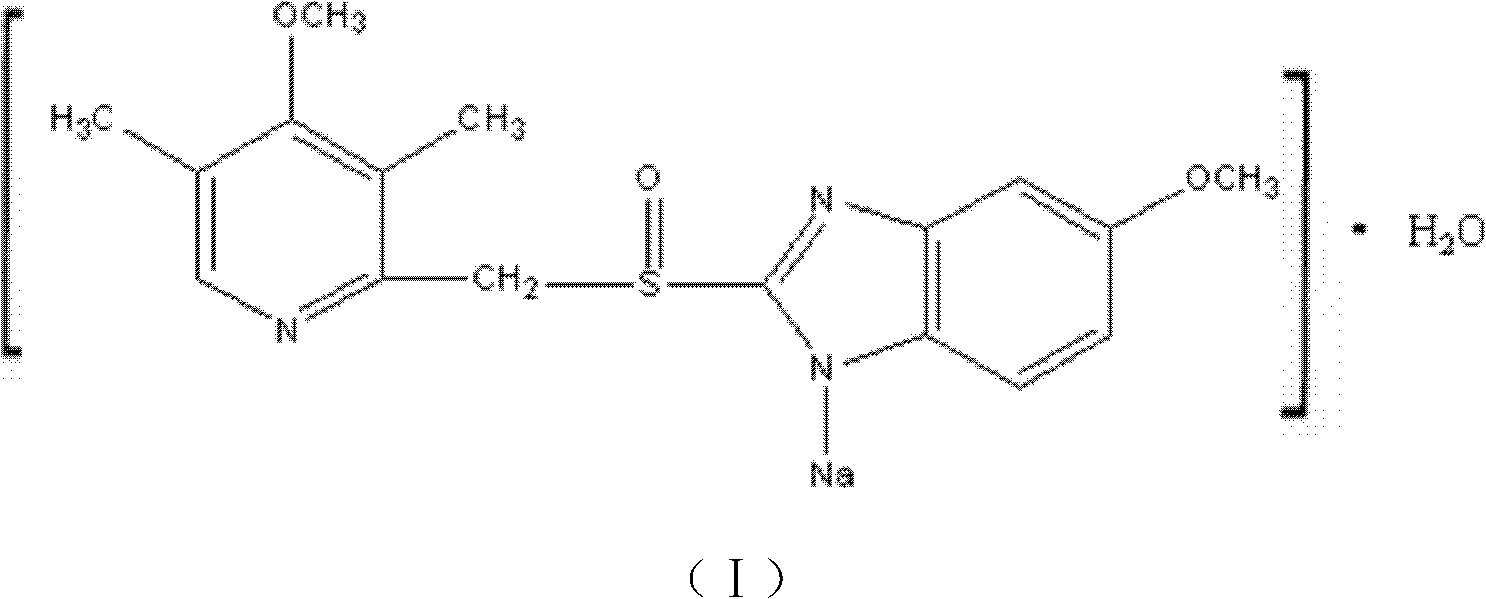
ActiveCN102351846AHigh thermodynamic stabilityHigh yieldOrganic active ingredientsPowder deliveryOmeprazole SodiumFreeze-drying
The invention discloses a novel omeprazole sodium compound. Characteristic peaks of the omeprazole sodium compound in an X-ray powder diffraction pattern obtained through Cu-K alpha-ray measurement are displayed when 2 theta is 3.2, 5.9, 7.6, 9.2, 10.5, 10.6, 12.3, 16.2, 18.2, 20.8, 23.4, 27.1, 30.4, 32.3 and 34.6. The omeprazole sodium compound has a new crystal form different from the prior art, has the thermodynamic stability higher than that with known crystal form, and has the advantage of not absorbing moisture basically. The invention also discloses a medicinal composition, which comprises the novel omeprazole sodium compound. Omeprazole sodium freeze-dried powder injection can be directly prepared under the condition of not adding lyoprotectants, and the omeprazole sodium freeze-dried powder injection has the advantages of high stability and high re-dissolubility.
Owner:周晓东
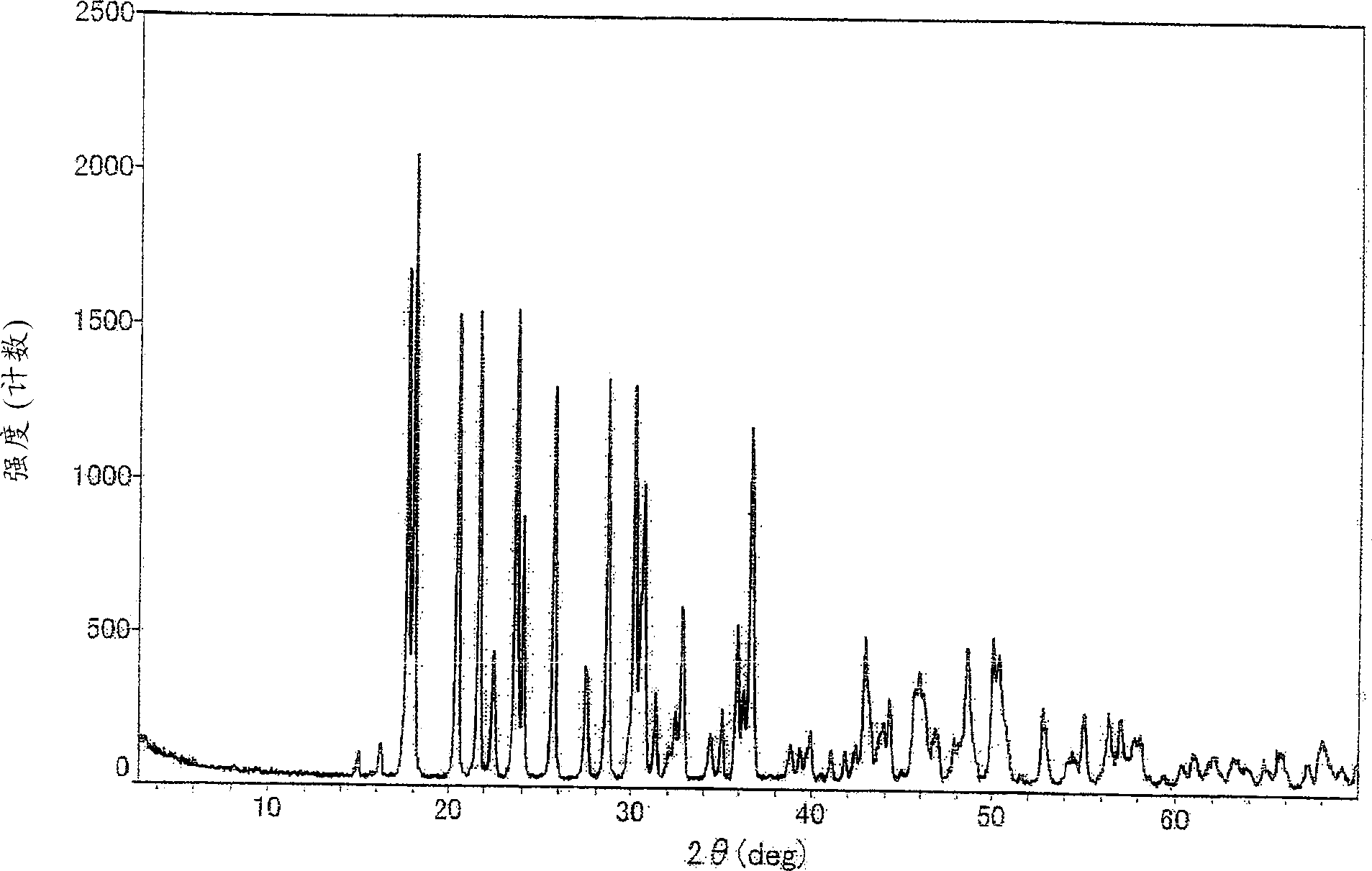
Cefixime compound and pharmaceutical composition thereof
InactiveCN103193798AUniform particle size distributionWell mixedAntibacterial agentsOrganic active ingredientsDrug compoundPharmaceutical Adjuvants
The invention relates to a drug compound, and particularly relates to a cefixime compound. An X-ray powder diffraction pattern of the cefixime compound measured through a Cu-K alpha ray is shown in a figure I. The invention also relates to a pharmaceutical composition containing the cefixime compound. The pharmaceutical composition comprises the cefixime compound and a pharmaceutical adjuvant; the pharmaceutical composition is an oral preparation including an oral normal release preparation and a controlled release preparation; and the oral normal release preparation is selected from a tablet, an enteric-coated tablet, a capsule, a dispersible tablet, dry suspension, a chewable tablet or granules. The cefixime compound disclosed by the invention is high in purity, high in bioavailability and suitable for clinical application.
Owner:四川省惠达药业有限公司
Zinc borate, and production and use thereof
InactiveCN1364142AGood lookingOvercome expensiveBiocideCosmetic preparationsChemical compositionZinc borate
A zinc borate has a particular chemical composition, has a crystallite size of not smaller than 40 nm as found from diffraction peaks of indexes of planes of (020), (101) and (200) in the X-ray diffraction image (Cu-k alpha ) and contains sodium components in amounts of not larger than 100 ppm as measured by the atomic absorptiometric method.
Owner:MIZUSAWA INDAL CHEM LTD
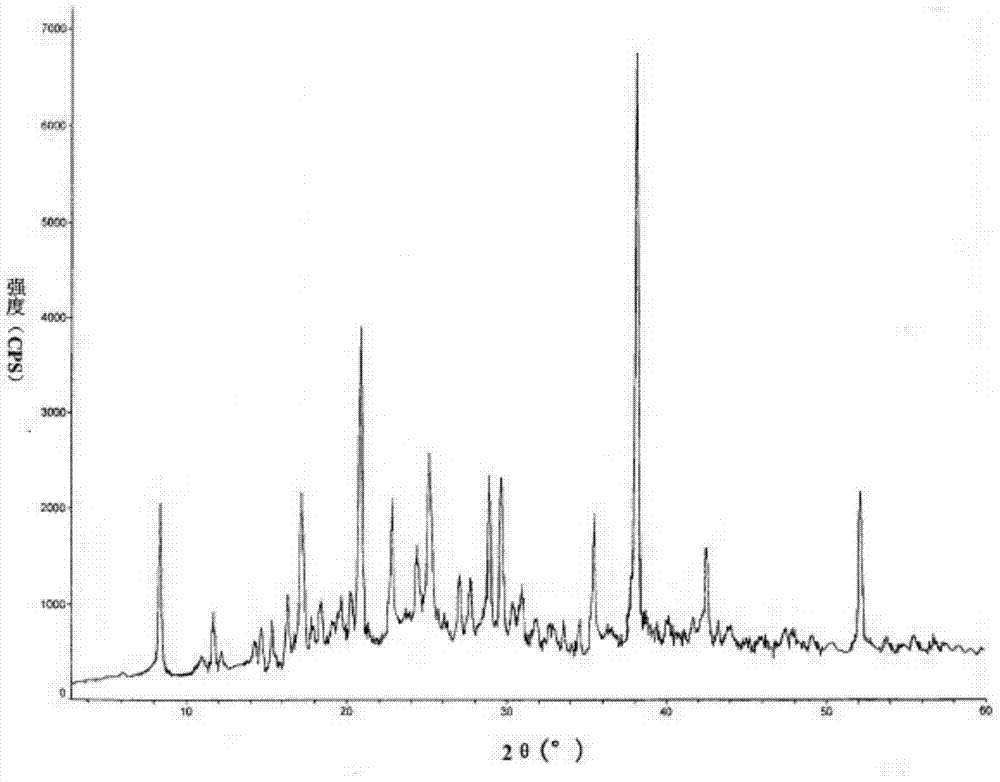
Microcrystal catechin and preparation method thereof
ActiveCN102875513AImprove quality stabilityHigh purityAntibacterial agentsAntimycoticsDiseaseSolvent
The invention relates to the field of medicine and discloses a microcrystal catechin and a preparation method thereof. The microcrystal catechin is characterized by spectral signatures of x-ray powder diffraction which uses Cu-K alpha radiation and is expressed at the angle 2 theta. The preparation method comprises the five procedures of steaming, membrane technology, organic solvent extraction, solvent recovery, and concentrating and drying. The microcrystal catechin has stable quality, long shelf life, good effects in treating hyperlipaemia and cardiovascular and cerebrovascular diseases, excellent oxidation resisting property and the excellent functions of diminishing inflammations, killing bacteria, clearing radicals, resisting tumors, resisting mutations, lowering blood fat and preventing atherosis. The microcrystal catechin is a valuable nature drug and has a wide clinic application prospect.
Owner:王孝仓
Gastrodin compound and preparation thereof
ActiveCN103788148AImprove stabilityNot easy to absorb moistureOrganic active ingredientsNervous disorderBioavailabilityK-alpha
The invention relates to the technical field of medicines, and particularly relates to a gastrodin compound, a preparation method and a preparation thereof. An X-ray powder diffraction spectrum of the gastrodin compound, measured by using a Cu-K alpha ray, is shown as a figure 1. The preparation is of a tablet, a capsule, an injection and a powder injection. The gastrodin compound provided by the invention is good in stability, difficult in absorbing moisture, and better in comprehensive physical and chemical properties; and the gastrodin preparation, especially gastrodin injection, is good in storage stability, difficult in absorbing moisture, and high in bioavailability; and the medication safety of a patient is greatly improved.
Owner:ANHUI YOUCARE KAIYUE PHARMA
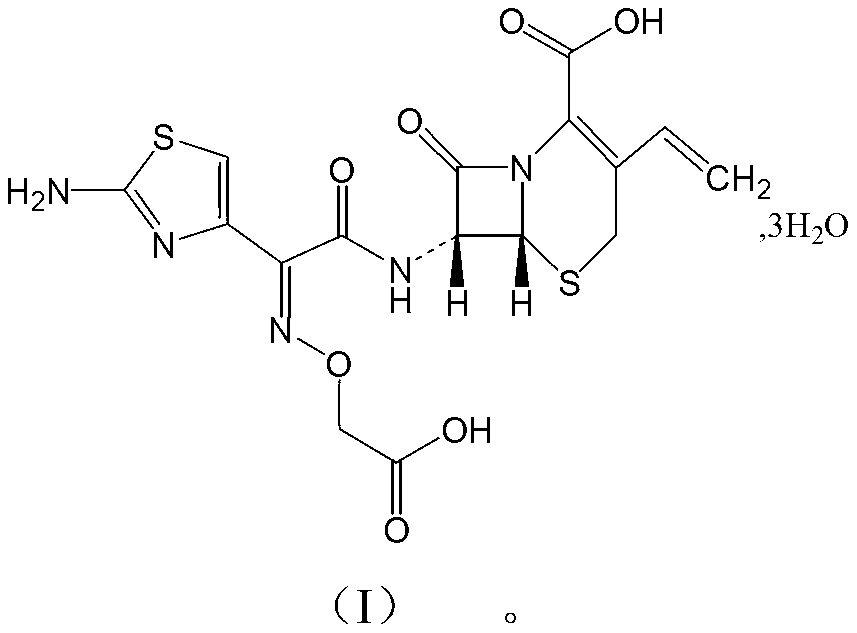
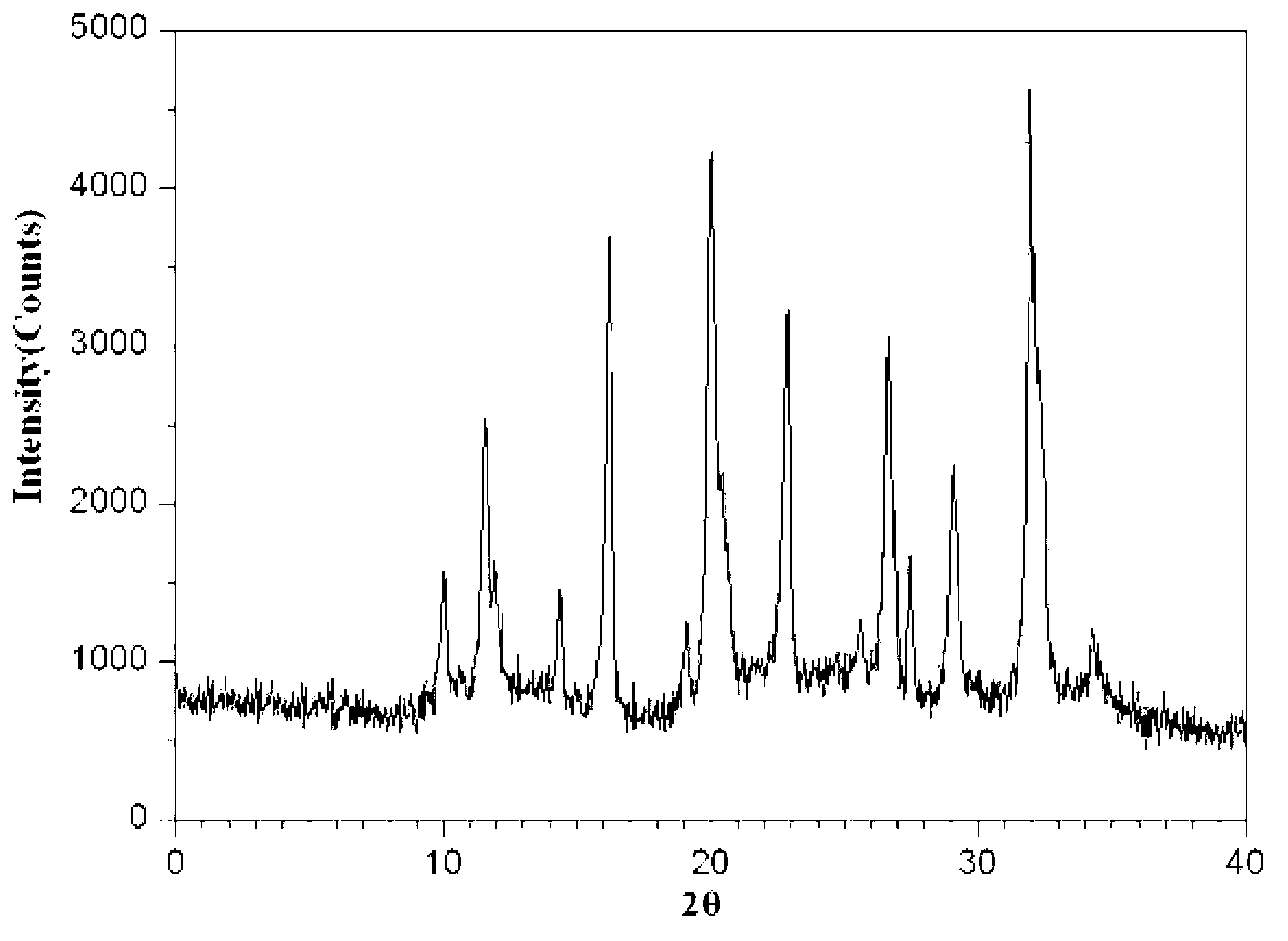
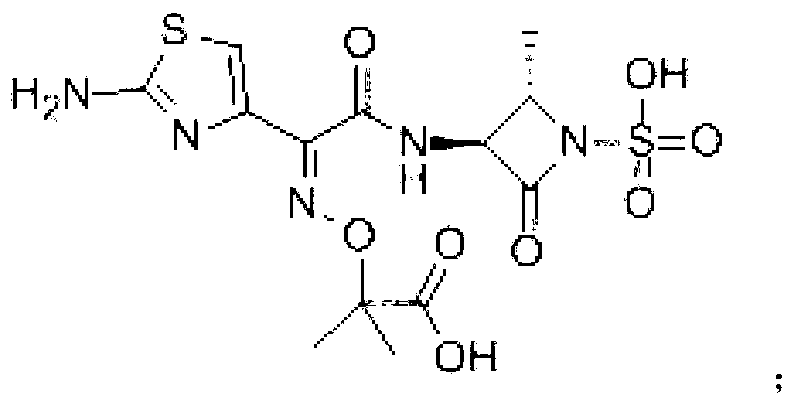
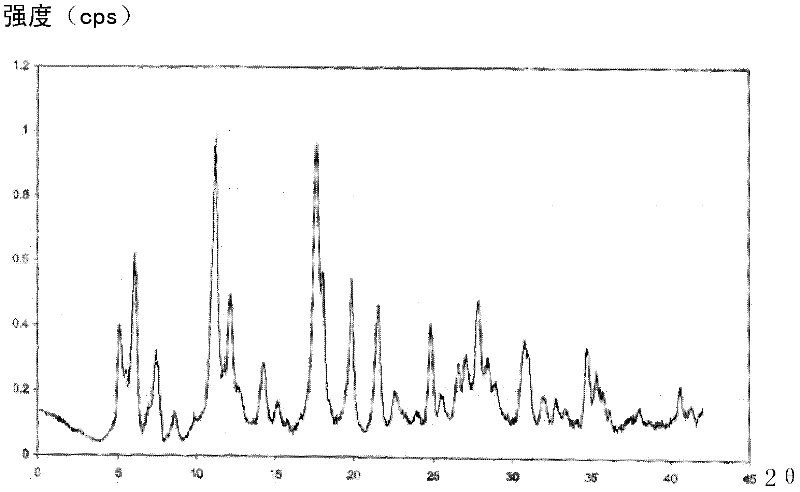
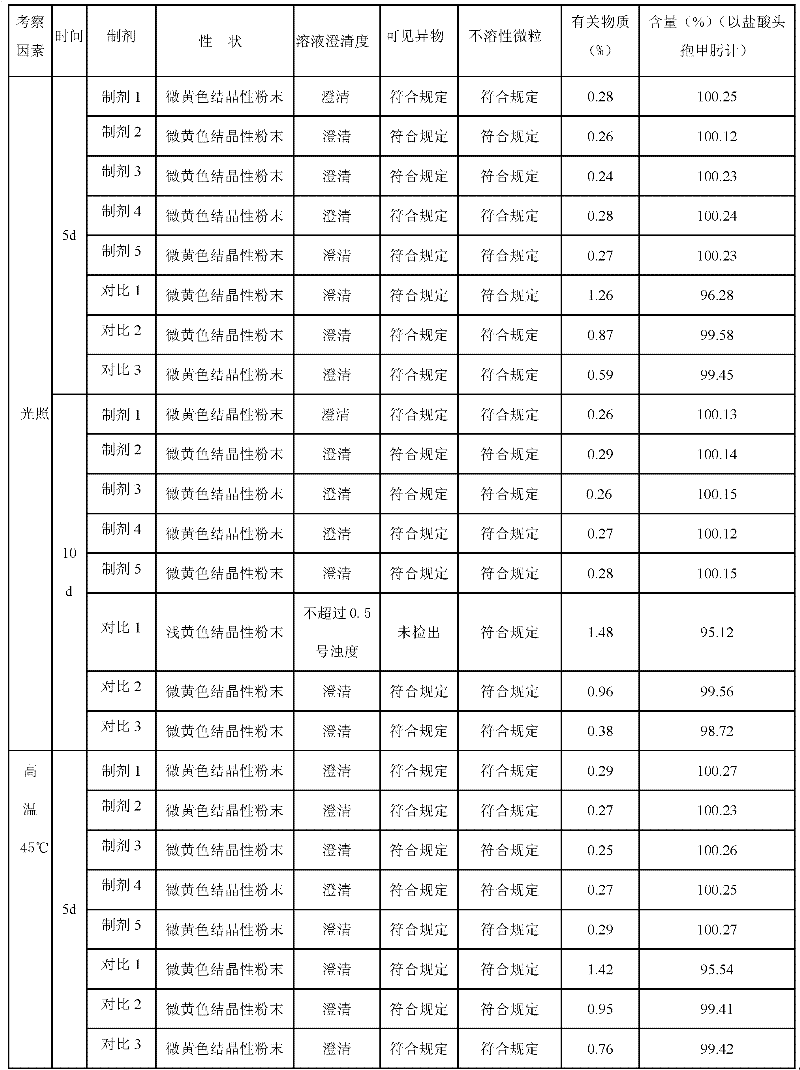
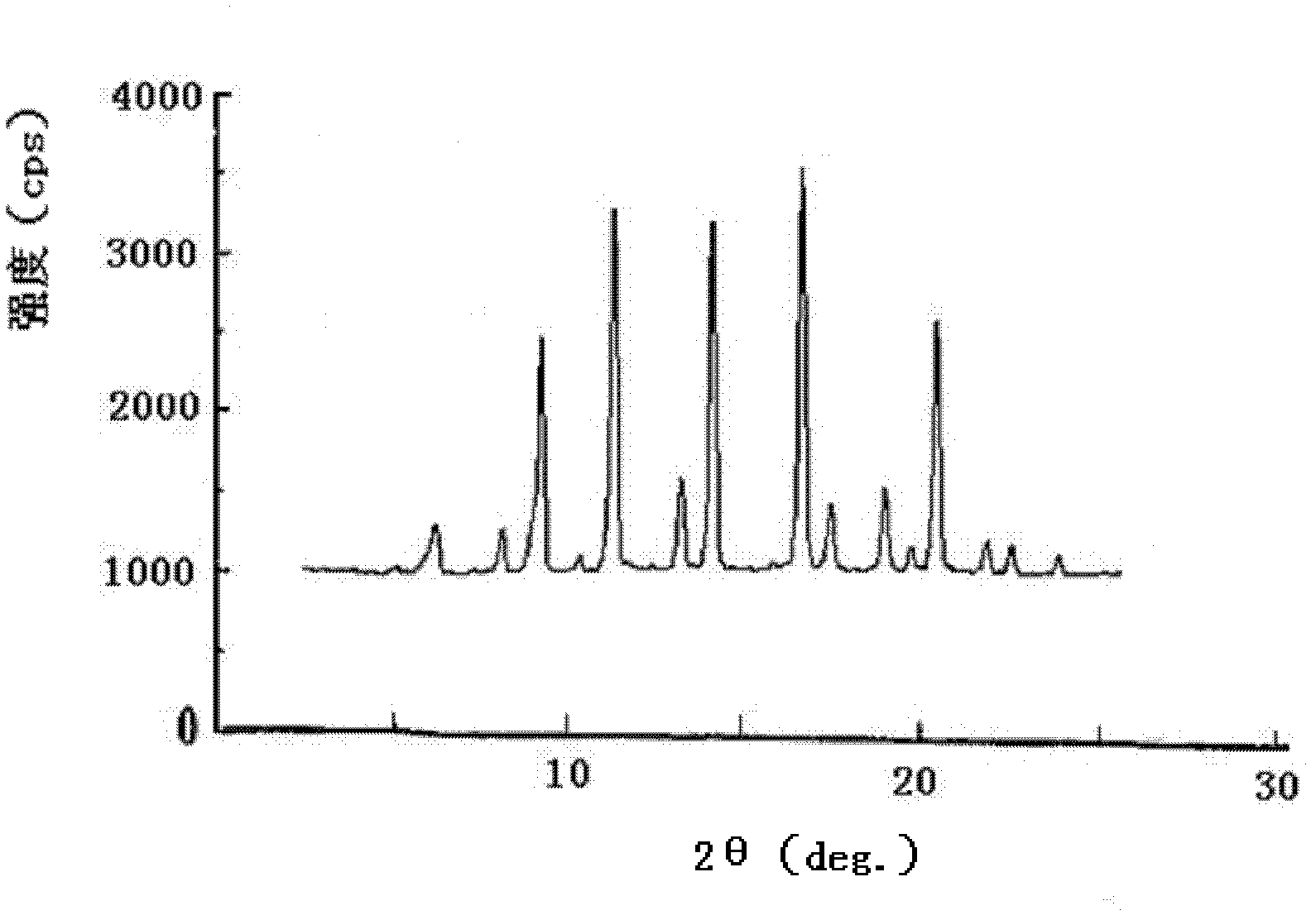
Aztreonam compound, as well as preparation method and pharmaceutical composition thereof
InactiveCN103232449AGood storage stabilityImprove liquidityOrganic active ingredientsOrganic chemistryFreeze-dryingSterile water
The invention provides an aztreonam compound. The structural formula of the aztreonam compound is as shown in the specification, and an X-ray powder diffraction spectrum of the aztreonam compound, which is obtained by using Cu-K alpha rays to measure, is as shown in Figure 1. The invention further provides a preparation method of the aztreonam compound and a pharmaceutical composition containing the aztreonam compound. The formulations of an aztreonam medicament are of sterile powder for injection and freeze-dried powder for injection. Compared with the prior art, the aztreonam compound and the pharmaceutical composition thereof, provided by the invention, have the advantages of better stability in storage and flowability, and can greatly improve the medication safety of patients.
Owner:四川省惠达药业有限公司
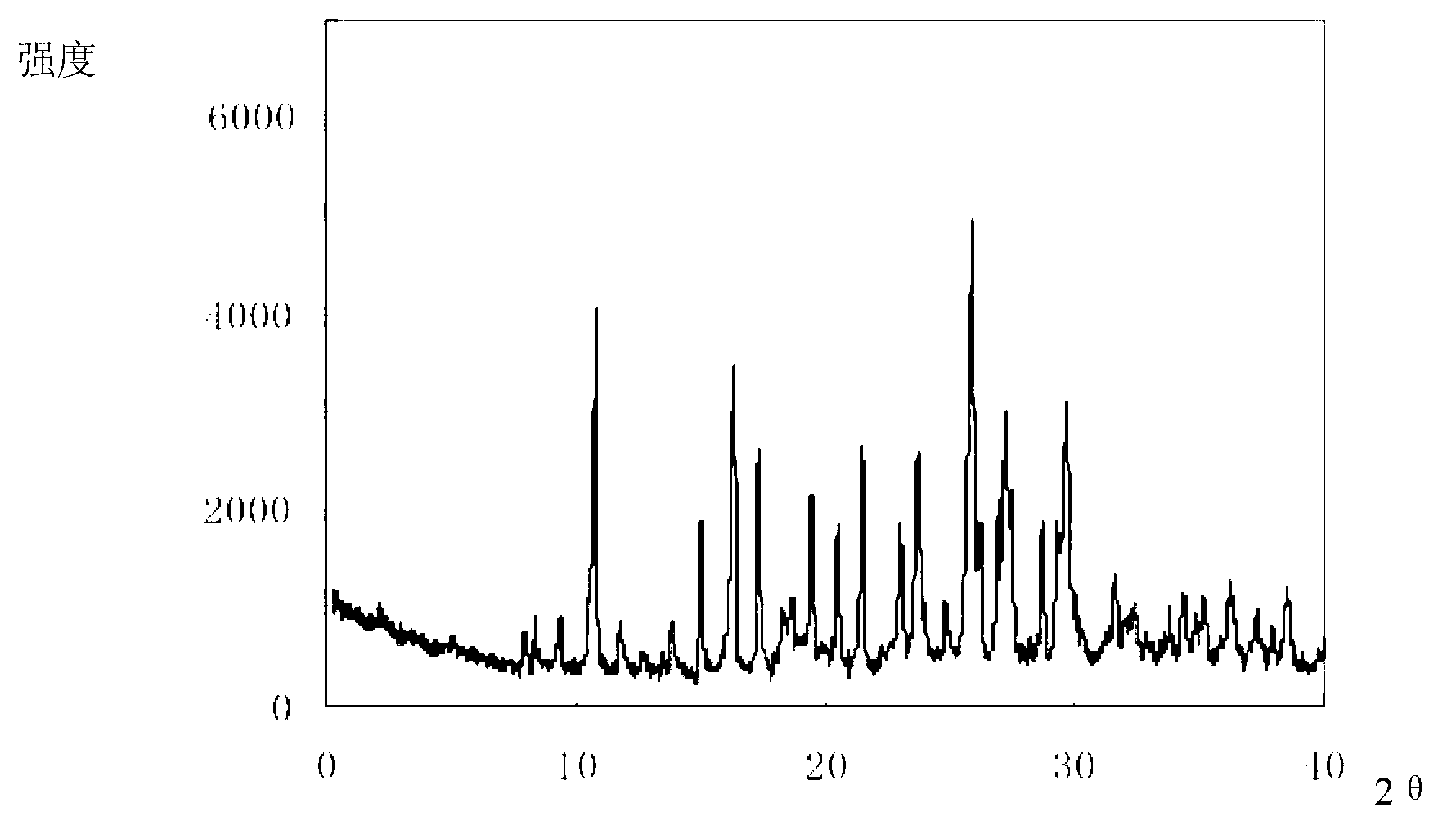
Cefmenoxime hydrochloride composition for injection and preparation thereof
ActiveCN102499922AParticle stabilityPromote generationAntibacterial agentsOrganic active ingredientsCrystal size distributionK-alpha
The invention relates to a cefmenoxime hydrochloride composition for injection which consists of 10 parts by weight of cefmenoxime hydrochloride and 1.5 to 2.5 parts by weight of anhydrous sodium carbonate, preferably 10 parts by weight of cefmenoxime hydrochloride and 1.75 to 1.8 parts by weight of anhydrous sodium carbonate. The cefmenoxime hydrochloride is crystal, having characteristic peaks at the diffraction angles 2 theta of 6.0 degrees, 7.4 degrees, 11.0 degrees, 12.2 degrees, 17.5 degrees, 19.8 degrees, 21.6 degrees, 24.8 degrees and 27.7 degrees in the X-ray powder diffraction measured using a Cu K-alpha ray. The main crystal size of cefmenoxime hydrochloride is 30 to 45 microns and the crystal size distribution width is 25 to 75 microns. The cefmenoxime hydrochloride composition of the invention is in the dosage form of injection which is dissolved quickly, suitable for clinic application, good in stability and reliable in safety.
Owner:桂林澳林制药有限责任公司
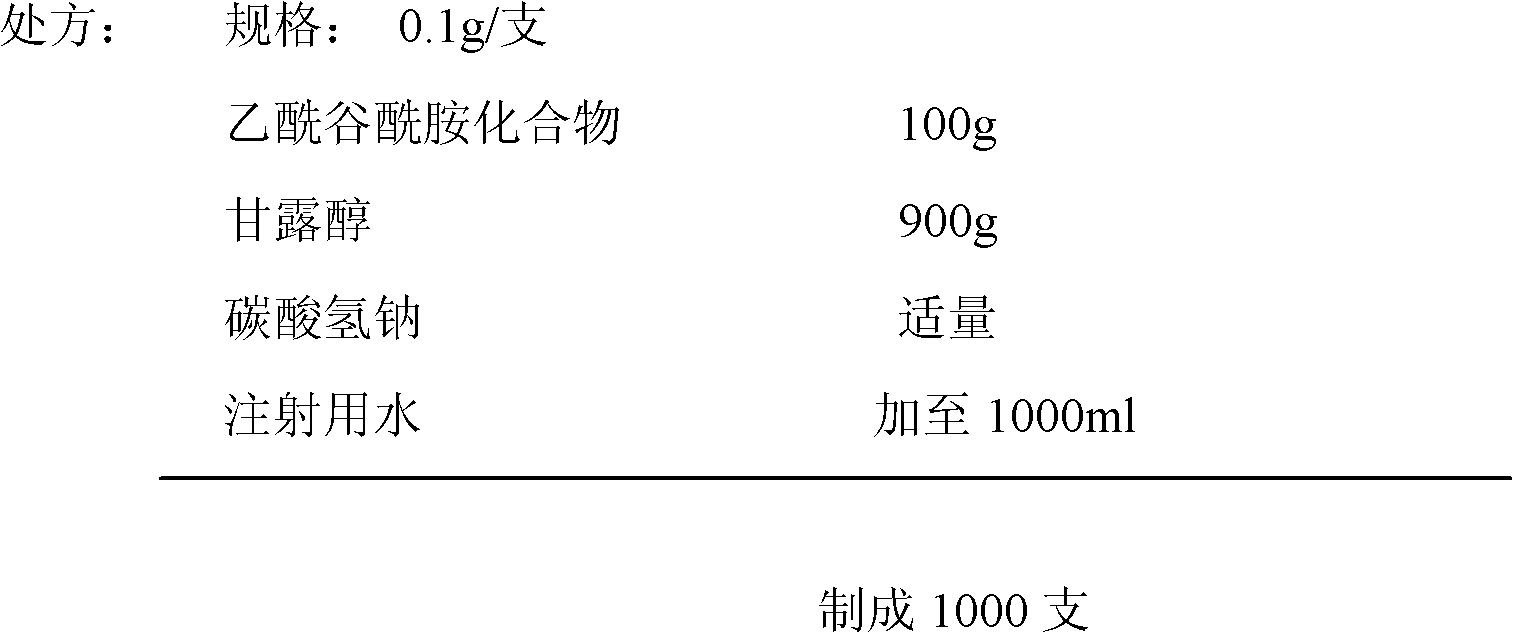
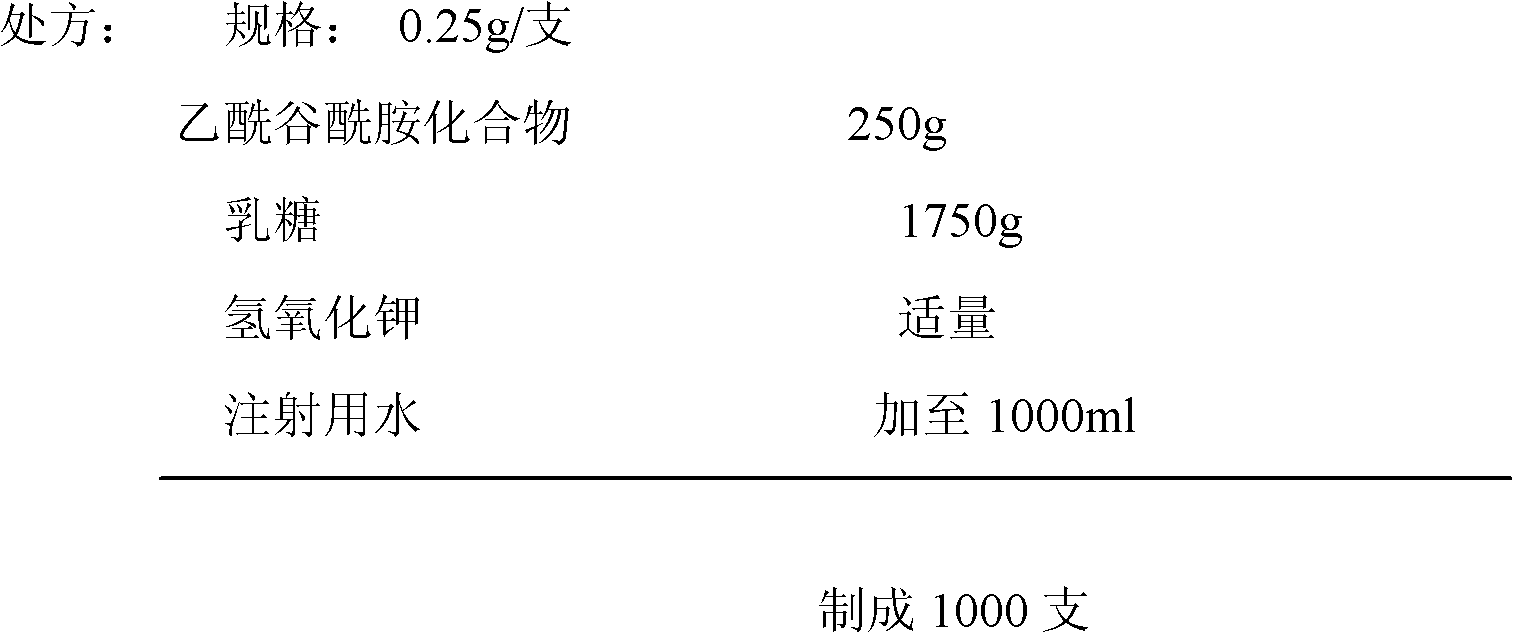
Acetylglutamine compound and pharmaceutical composition thereof
ActiveCN102276496AUniform particle sizeStable in natureNervous disorderCarboxylic acid amide separation/purificationFreeze-dryingAceglutamide
The invention discloses an aceglutamide compound. The aceglutamide compound is crystals; and characteristic peaks in an X-ray powder diffraction pattern measured by using Cu-K alpha rays are displayed as 9.3, 11.2, 14.6, 17.0 and 20.9 at 2 theta. The aceglutamide compound has the advantages of uniform particle size and steady characteristic, and cannot be hydrolyzed and oxidized easily, so that the aceglutamide compound can be used for preparing a medicinal composition. The invention also discloses the medicinal composition. The medicinal composition contains the aceglutamide compound and a pharmaceutically acceptable carrier, an excipient or a diluent; and the optimal formulation is freeze-dried powder injection or injection. The medicinal composition has the advantages of stable qualityand high yield, and the preparation process is simple, so that the period of validity of the medicine is prolonged, and the quality of the product is guaranteed.
Owner:周晓东
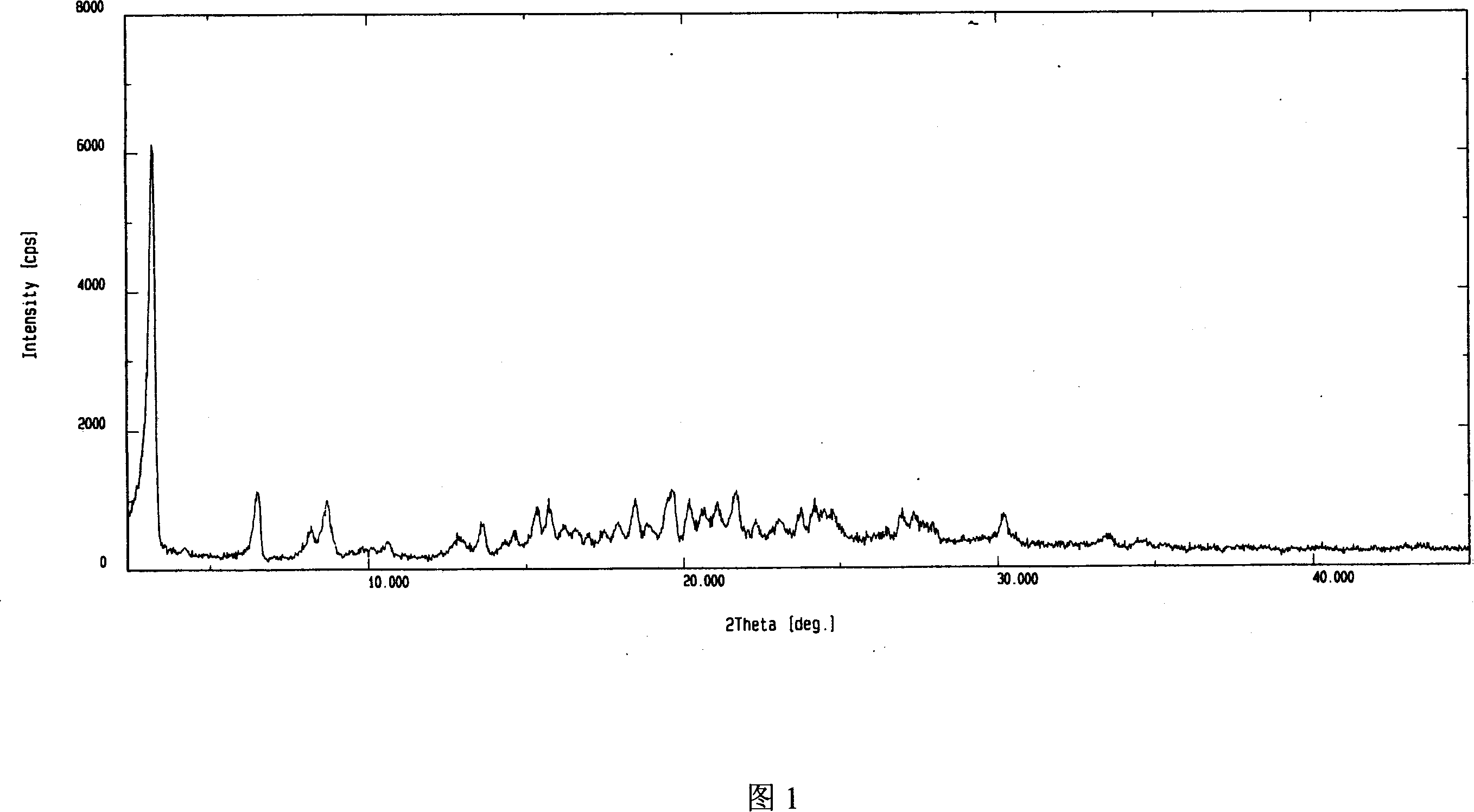
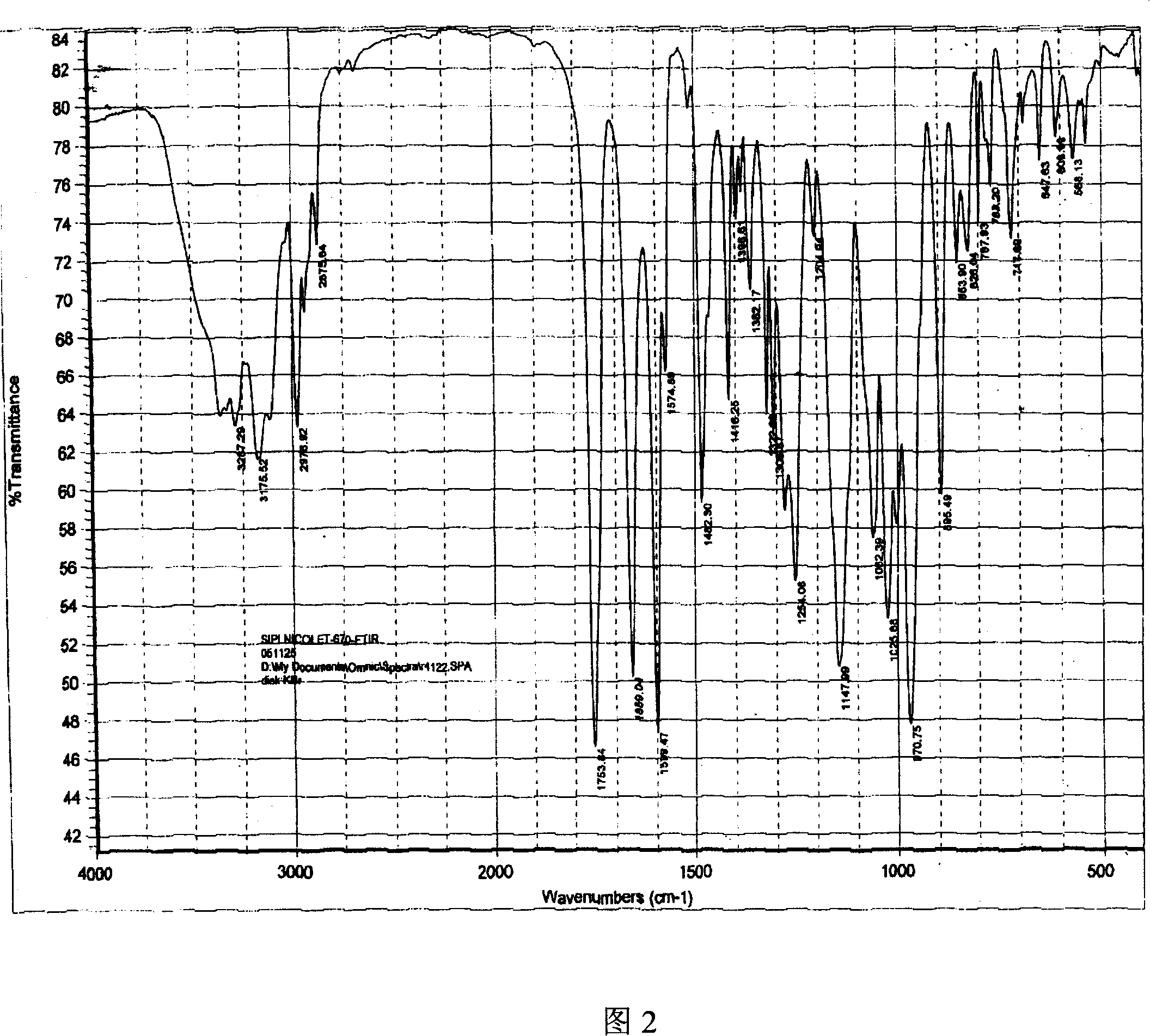
Adefovir dipivoxil anhydrous crystal, preparation method and medicine composition thereof
ActiveCN101054393AImprove liquidityEasy to prepareOrganic active ingredientsGroup 5/15 element organic compoundsDissolutionK-alpha
The invention relates to adefovir dipivoxil anhydrous crystallisate. It is characterized in that : XRD: radiating with Cu-k alpha , x ray powder diffraction spectrum expressed in 2 theta having characteristic absorption peak at 3.2,6.5,8.6, 15.7,19.6,21.6, DSC: DSC converting due to absorbing heat at 80 degree; infrared absorption spectrum : KBr pellet , characteristic absorption peak being at 3287cm-1,3175cm-1,1753cm-1,1254cm-1,1147 cm-1,1025cm-1,970cm-1. The present invention also discloses adefovir dipivoxil anhydrous crystallisate preparation method and its pharmaceutical composition. The inventive adefovir dipivoxil anhydrous crystallisate dissolves quicker than 102 degree crystal form, is high in dissolution, and is stable relative to 94 degree crystal form and not easy to be hygroscopic.
Owner:SHANGHAI SHYNDEC PHARMA CO LTD +2
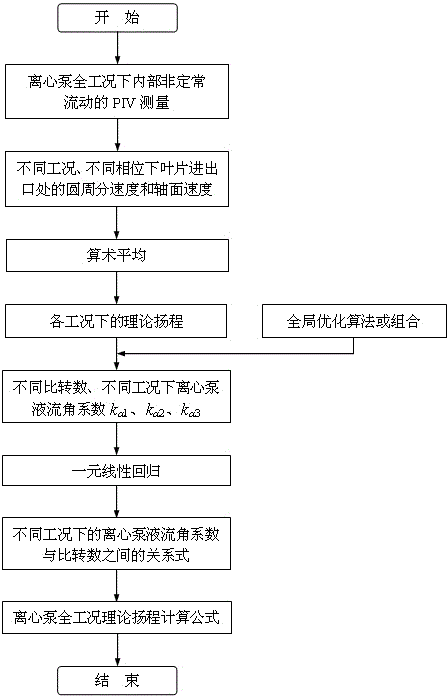
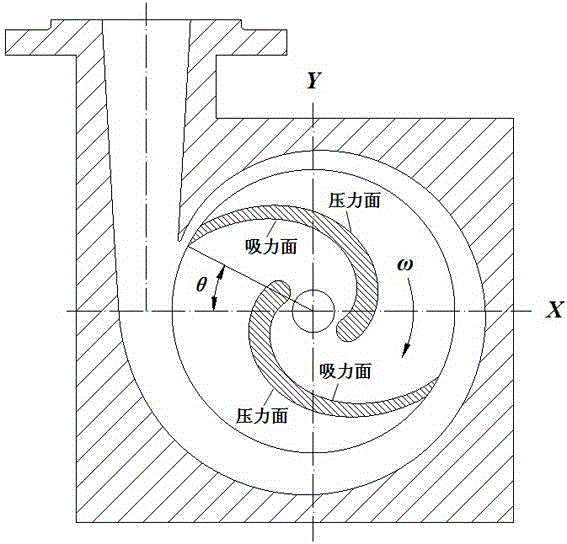
Method for determining centrifugal pump full-condition theoretical lifts based on internal flow measurement
The invention discloses a method for determining centrifugal pump full-condition theoretical lifts based on internal flow measurement. The process of the method includes using internal unsteady flow dynamic rules under different working conditions of a performance intercross valuation (PIV) experiment measurement centrifugal pump; calculating theoretical lifts of the centrifugal pump under different working conditions; using an overall optimization algorithm or combination to solve flow angle coefficients under the different working conditions, performing PIV measurement on internal flows of centrifugal pumps at different specific speeds and obtaining different flow angle coefficients k alpha 1, k alpha 2 and k alpha 3 at the different specific speeds and under the different working conditions, and utilizing a unitary linear regression method to build a relation between the flow angle coefficients and the specific speeds under the different working conditions and further build a centrifugal pump full-condition theoretical lift formula. According to the method, the centrifugal pump full condition theoretical lifts under the different working conditions can be calculated accurately, and a centrifugal pump full condition energy performance calculation model is built on the basis to optimally design a prior pump.
Owner:JIANGSU UNIV
Novel polymorph of safinamide and preparation method therefor
ActiveCN105017060AImprove stabilityHigh dissolution rateOrganic active ingredientsNervous disorderSafinamideK-alpha
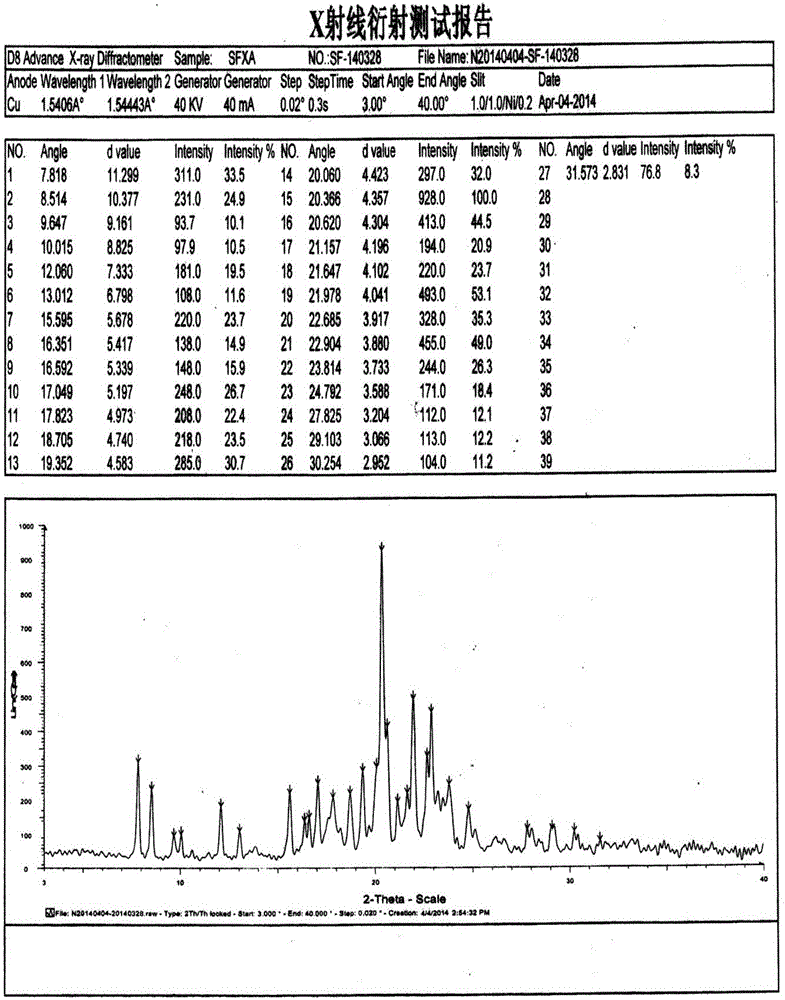
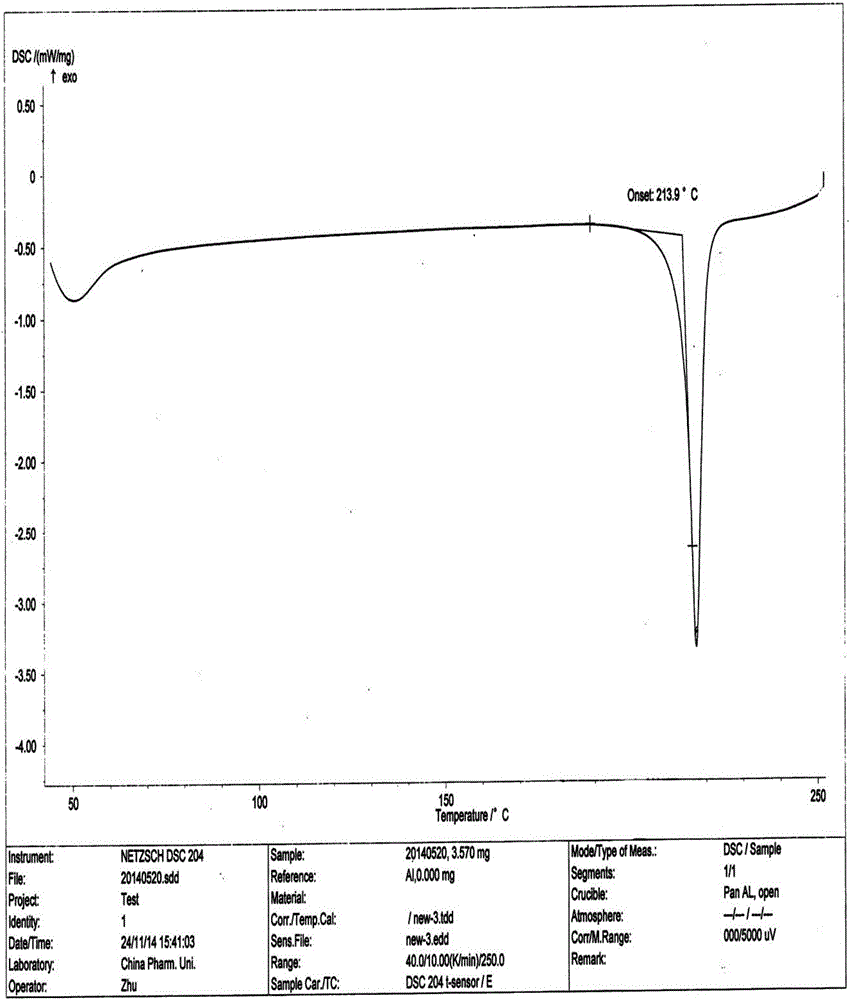
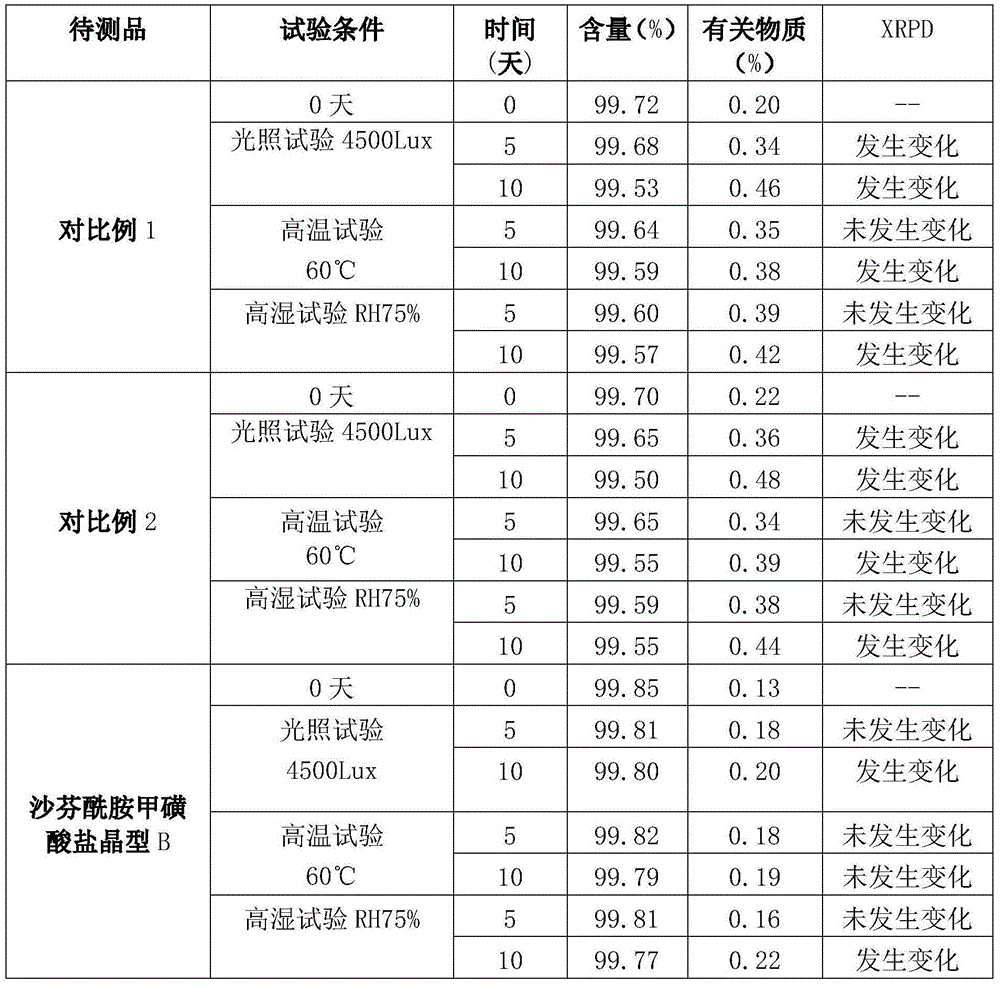
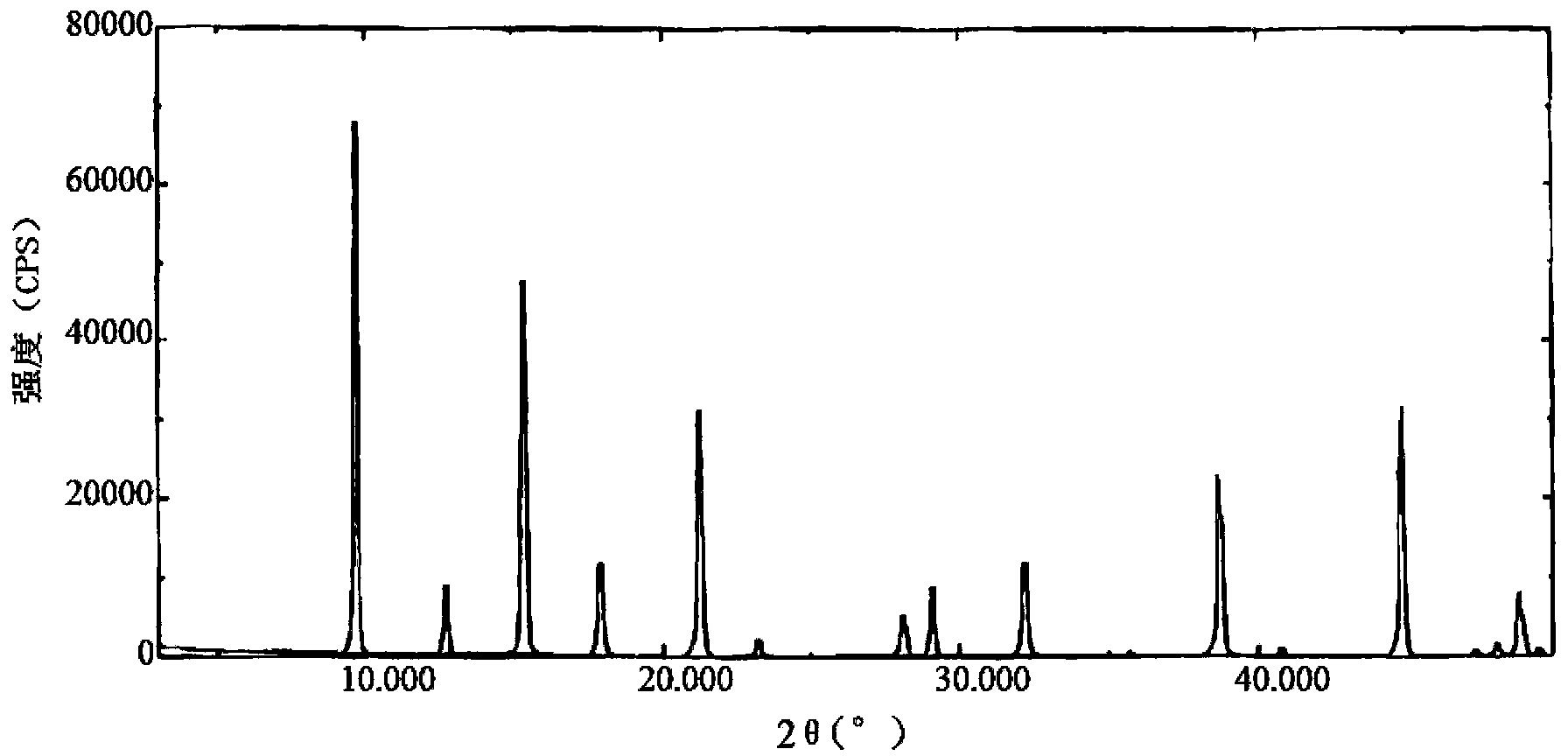
The present invention relates to the field of medicinal chemistry, and particularly relates to a novel polymorph of safinamide mesylate salt, that is a novel polymorph B, and a preparation method therefor, and also relates to a pharmaceutical composition containing the novel polymorph and applications thereof in treating Parkinson's disease. When the polymorph B of safinamide mesylate salt is under radiation of Cu-K alpha, diffraction of X-ray powder represented by 2 theta has diffraction peaks at 7.82, 8.51, 13.01, 17.82, 18.71, 20.37, 21.98, and 22.90.
Owner:NANJING CHIA TAI TIANQING PHARMA
Tropisetron hydrochloride compound, its preparation method, and pharmaceutical composition containing the same
ActiveCN103360386AImprove stabilityQuality improvementOrganic chemistryDigestive systemStructural formulaCharacteristic X-ray
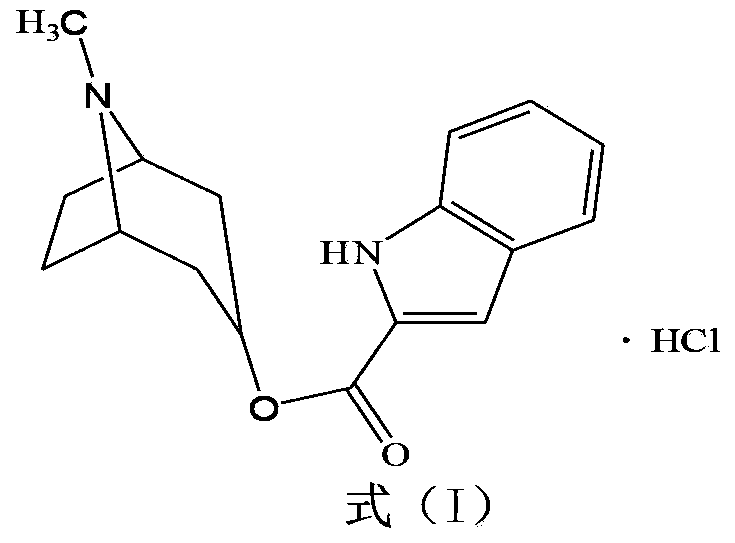
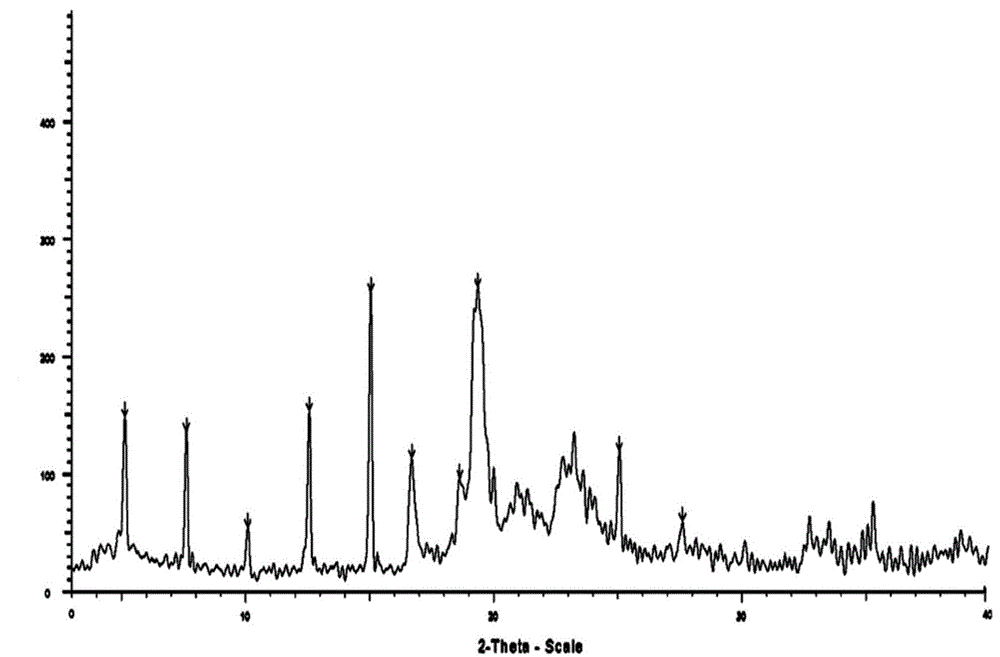
Belonging to the technical field of medicines, the invention particularly relates to a Tropisetron hydrochloride compound, its preparation method, and a pharmaceutical composition containing the same. The Tropisetron hydrochloride compound has a structural formula shown as formula (I), wherein, the Tropisetron hydrochloride compound adopts Cu-K alpha ray for characteristic X ray powder determination, the X ray powder atlas is as shown in figure 1. Compared with the prior art, the Tropisetron hydrochloride compound provided in the invention has a different crystal form, which has good stability and improved liquidity at the same time, thus improving the preparation quality.
Owner:金鸿药业股份有限公司
Catalyst for acrolein and/or acrylic acid production and process for producing acrolein and/or acrylic acid using the catalyst
ActiveCN102076411AOrganic compound preparationHeterogenous catalyst chemical elementsCatalytic oxidationAcrolein
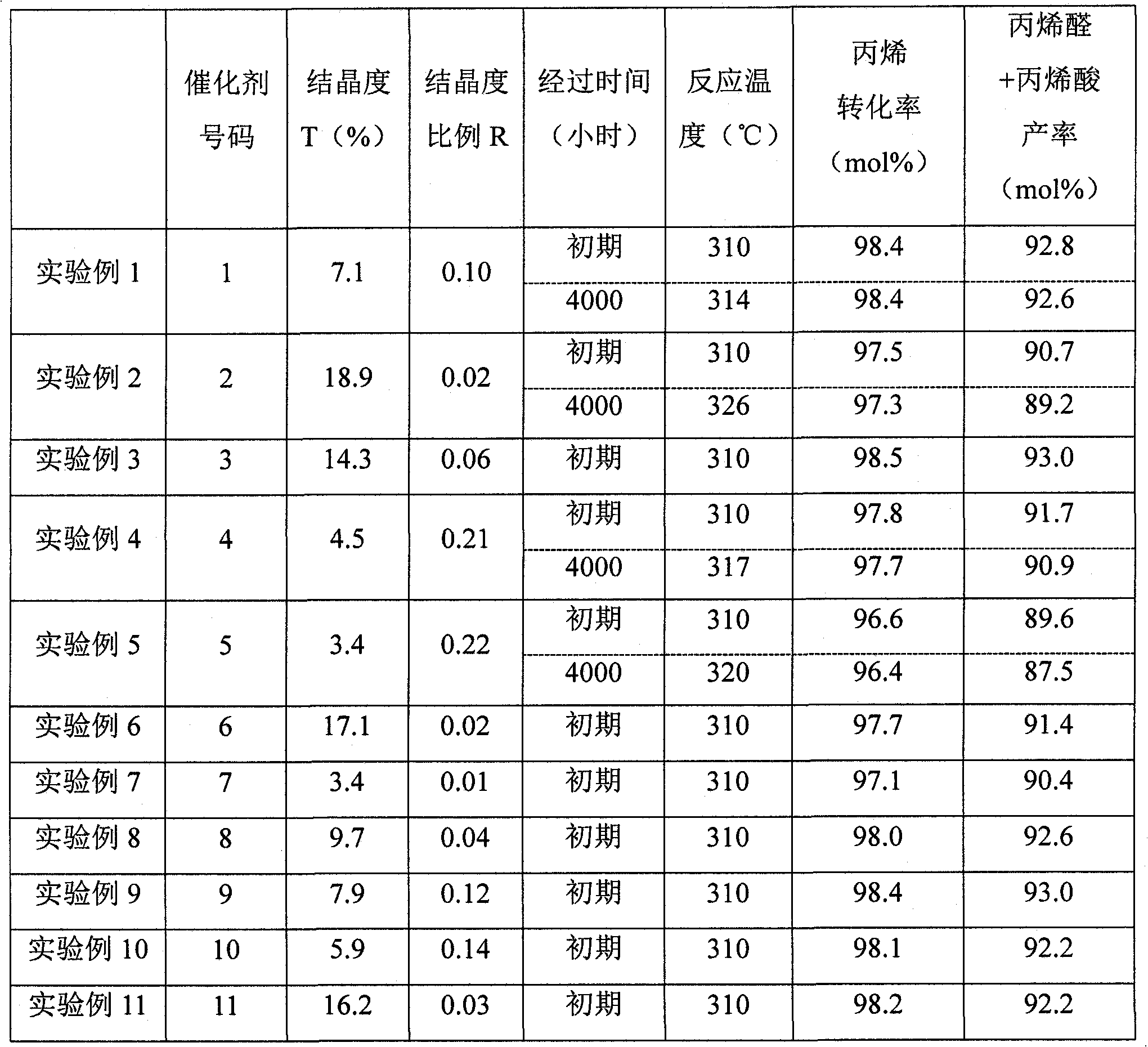
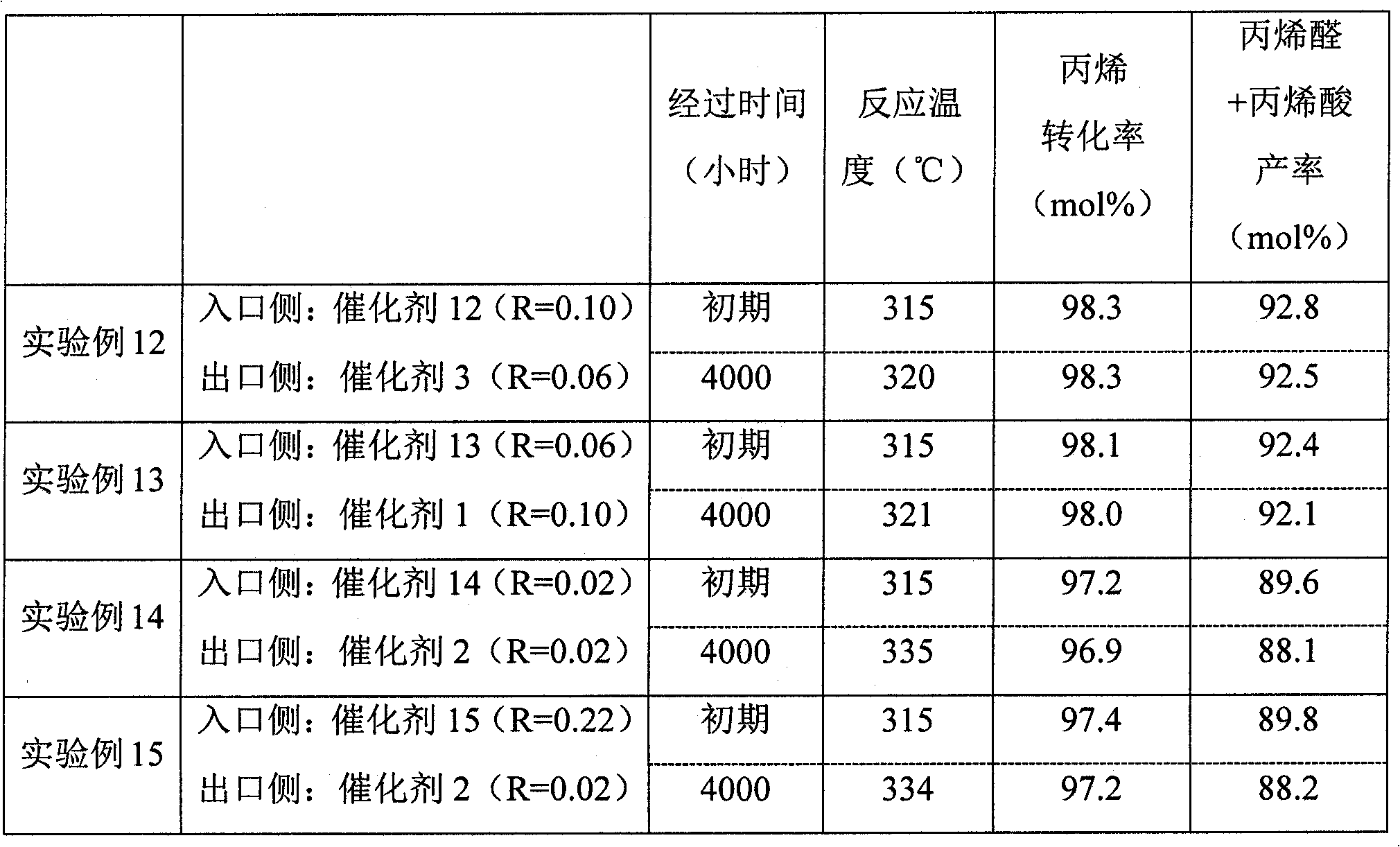
Disclosed is a catalyst for producing acrolein and / or acrylic acid by catalytically oxidizing propylene in a vapor phase with a gas comprising molecular oxygen. The catalyst is characterized by containing an active catalyst ingredient comprising molybdenum, bismuth, and cobalt as essential components, the active catalyst ingredient, in X-ray diffraction analysis with a Cu-K-Alpha line, having crystallinity (T) of 4-18% in the 2theta range of 5 to 90 DEG. Also provided is a process for producing acrolein and / or acrylic acid which comprises catalytically oxidizing propylene in a vapor phase with a gas comprising molecular oxygen. The process is characterized by including a step in which the vapor-phase catalytic oxidation is conducted in the presence of the catalyst. With the catalyst and the process for acrolein and / or acrylic acid production, acrolein and / or acrylic acid can be stably produced in a high yield over long.
Owner:NIPPON SHOKUBAI CO LTD
New crystal form of R(+)-thioctic acid-L-lysinate and preparation method thereof
ActiveCN105001195AImprove stabilitySimple processOrganic active ingredientsNervous disorderK-alphaPeripheral neuropathy
The invention relates to the field of pharmaceutical chemistry, specifically to a new crystal form of R(+)-thioctic acid-L-lysinate, namely its crystal form I and its preparation method. The invention also relates to a pharmaceutical composition containing the new crystal form and an application of the pharmaceutical composition in the preparation of drugs for treating paresthesia caused by diabetic peripheral neuropathy. By the use of Cu-K alpha radiation, the X-ray power diffraction of R(+)-thioctic acid-L-lysinate crystal form I has diffraction peaks at 5.012, 7.589, 10.072, 12.549, 15.036, 16.688, 18.627, 19.356, 25.064 and 27.611 when expressed with 2 theta degrees, wherein the error range of 2 theta value is + / - 0.2.
Owner:河北迈科生物科技有限公司
C-type crystal form of ceritinib, preparation method therefor and application thereof
InactiveCN105061397AHigh crystallinityGood water solubilityOrganic active ingredientsOrganic chemistry methodsSolubilityCrystallinity
The present invention provides a C-type crystal form of ceritinib. In an X-ray powder diffraction pattern obtained by use of Cu-K alpha ray measurement, the C-type crystal form of the ceritinib has characteristic peaks at an 2Theta angle of 4.9 degrees, 9.4 degrees, 9.9 degrees, 12.2 degrees, 13.7 degrees, 14.2 degrees, 14.4 degrees, 14.9 degrees, 15.6 degrees, 16.5 degrees, 17.0 degrees, 17.7 degrees, 18.9 degrees, 20.6 degrees, 22.0 degrees, 25.0 degrees, 26.6 degrees, 25.9 degrees and 28.5 degrees. The present invention also provides a preparation method for the C-type crystal form of the ceritinib, and an application. The C-type crystal form of the ceritinib provided by the present invention is high in crystallinity and high in water solubility. The preparation method for the C-type crystal form of the ceritinib is simple, liable to control, liable to prepare the C-type crystal form, and good in reproducibility; and the oral bioavailability of ceritinib is remarkably improved.
Owner:WUHAN YINGPURUI PHARMA CO LTD
Methylpyrazine derivative biological arginine hydrate
ActiveCN109438371AImprove stabilityHigh purityOrganic active ingredientsOrganic compound preparationSolubilityArginine
The invention belongs to the technical field of medicine and specifically provides methylpyrazine derivative biological arginine hydrate, a preparation method thereof and application thereof in preparing blood fat reducing medicine. The prepared methylpyrazine derivative biological arginine hydrate disclosed by the invention is radiated by Cu-K alpha, and an X ray diffraction spectrogram represented by 2 theta has the characteristic peaks at the 5.7+ / -0.2 degree, 9.1+ / -0.2 degree, 16.2+ / -0.2 degree, 24.5+ / -0.2 degree, 24.8+ / -0.2 degree and 27.8+ / -0.2 degree positions. The prepared methylpyrazine derivative biological arginine hydrate disclosed by the invention has high stability; the purity basically cannot change when the methylpyrazine derivative biological arginine hydrate is placed ina dissolved state and a solid state; the solubility of the methylpyrazine derivative biological arginine hydrate in different media is 2.5 times of an existing crystal form; furthermore, the methylpyrazine derivative biological arginine hydrate has the advantages of higher bioavailability and better industrial application prospect.
Owner:LUNAN PHARMA GROUP CORPORATION
Highly crystalline lithium transition metal oxides
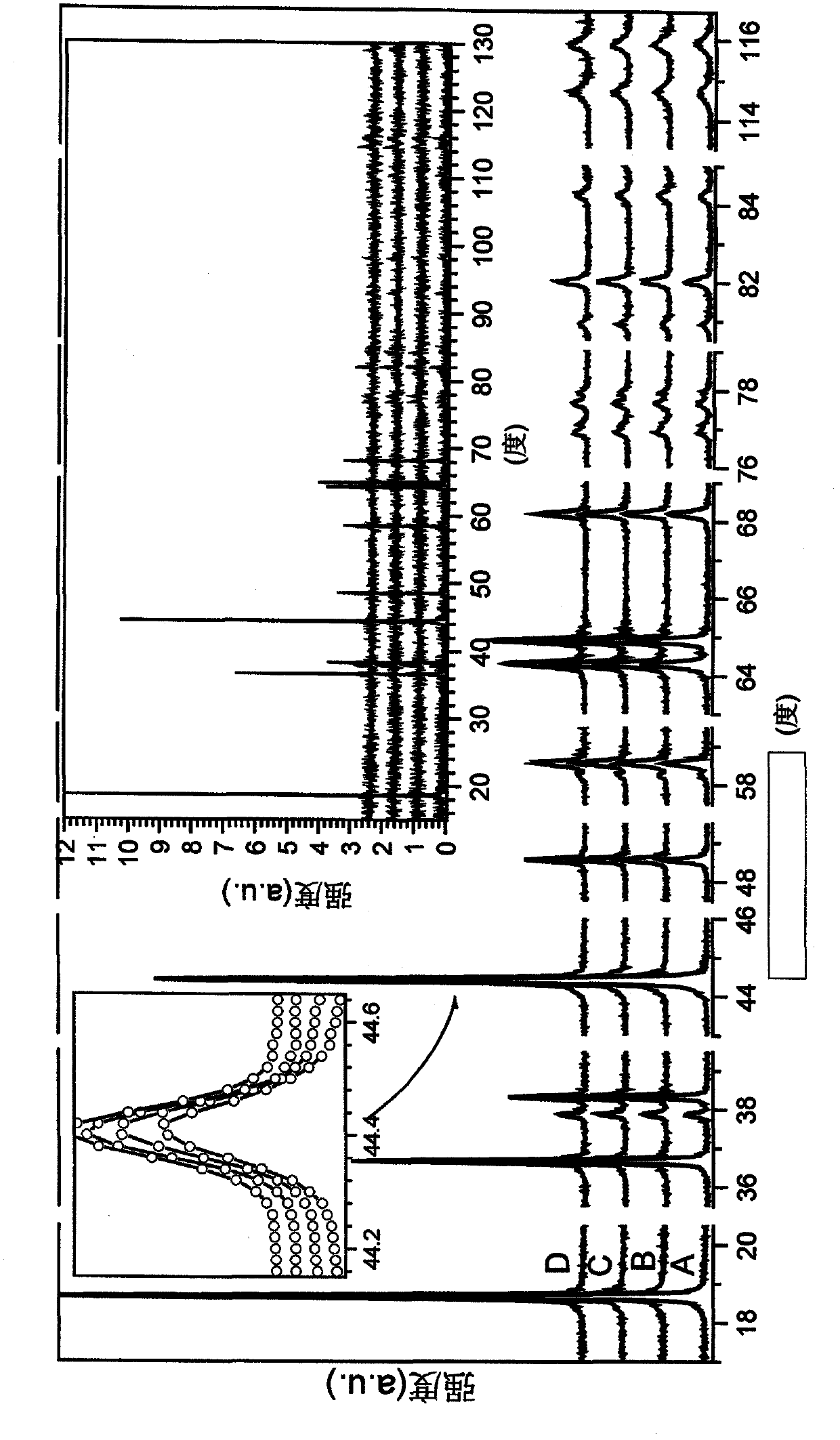
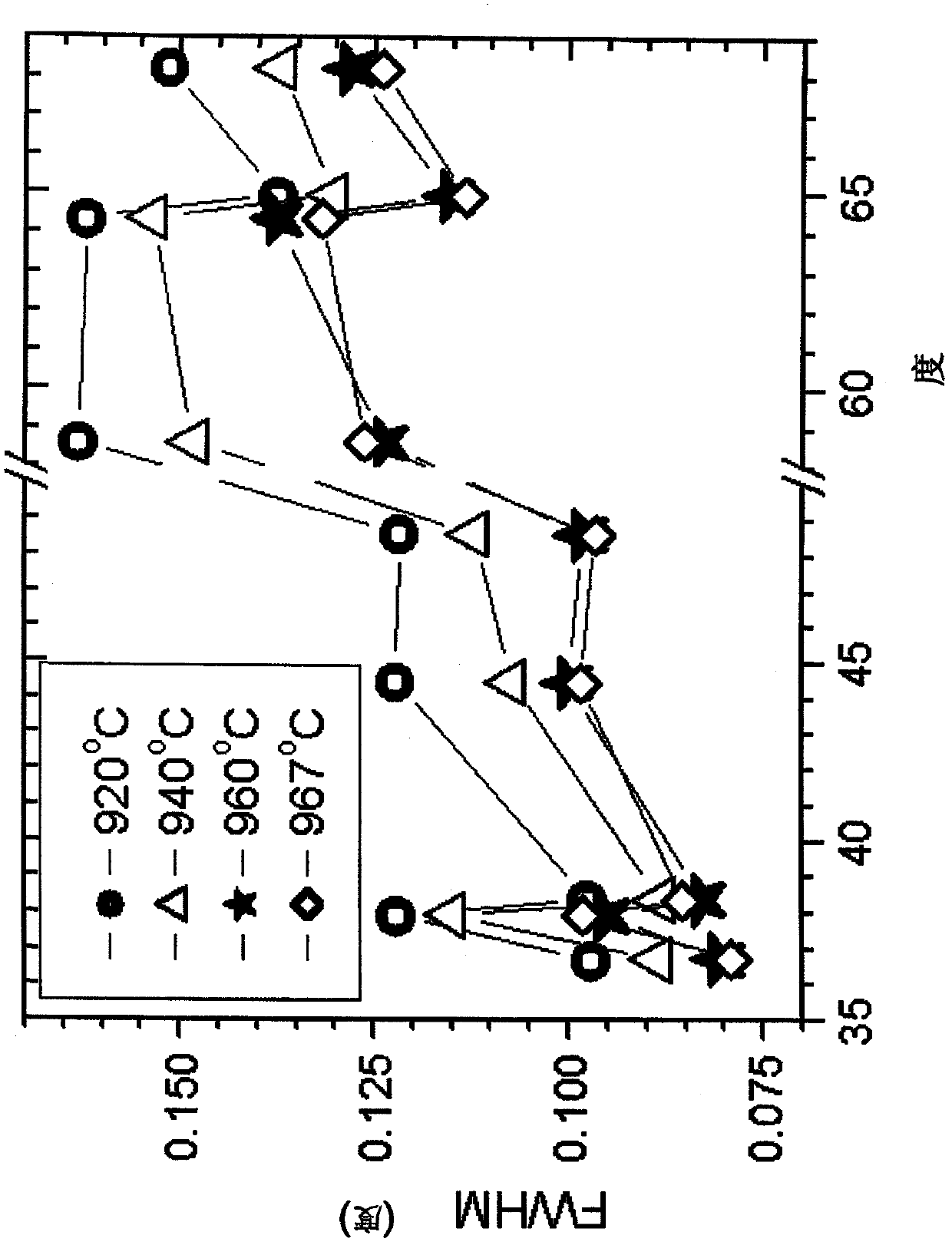
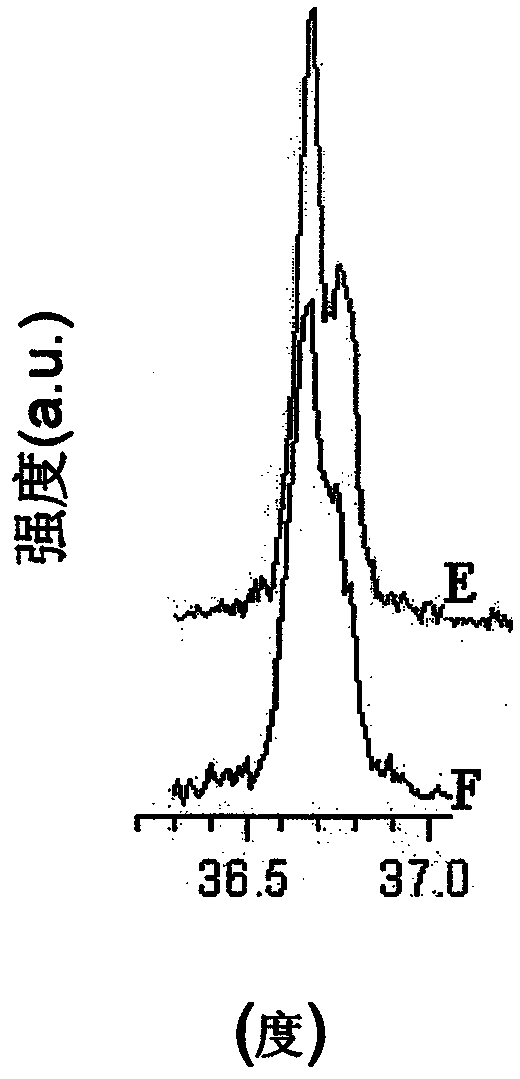
A powderous lithium transition metal oxide having a layered crystal structure Li1+aM1-aO2+-b M'k Sm with -0.03 < a < 0.06, 0 <= m <= 0.6, m being expressed in mol%, M being a transition metal compound, consisting of at least 95% of either one or more elements of the group Ni, Mn, Co and Ti; M' being present on the surface of the powderous oxide, and consisting of either one or more elements of the group Ca, Sr, Y, La, Ce and Zr, wherein: either k = 0 and M = Ni1-c-dMncCOd, with 0<c<1, and 0<d<1; or 0.015 < k < 0.15, k being expressed in wt% of said lithium transition metal oxide; characterized in that for said powderous oxide, the X-ray diffraction peak at 44.5 +- 0.3 degree, having as index 104, measured with K alpha radiation, has a FWHM value of <= 0.1 degree. By optimizing the sintering temperature of the metal oxide the FWHM value can be minimized.
Owner:UMICORE AG & CO KG
Deposit film and its producing method
InactiveCN1408896AStable barrierImprove water resistanceVacuum evaporation coatingSputtering coatingWater vapor permeabilityFluorescence
An aluminum oxide deposited film of the invention, comprising a substrate film and an aluminum oxide deposit layer of which the peel strength to the substrate film in a wet state is at least 0.3 N / 15mm, has an oxygen permeability of not more than 40 ml / m<2>.day.MPa and a water vapor permeability of not more than 4.0 g / m<2>.day. The deposition ratio (A / B) of a fluorescent X-ray intensity (A) kcps (aluminum K alpha -ray) of the aluminum oxide deposited film (1) to a fluorescent X-ray intensity (B) kcps (aluminum K alpha -ray) of an aluminum deposited film (2) obtained without feeding oxygen is (A / B) <= 0.85.
Owner:TOHCELLO CO LTD (JP)
Acetylcysteine compound and acetylcysteine solution being used for inhalation and containing same
ActiveCN104098491AIncrease humidityEasy to useOrganic chemistryOrganic compound preparationCysteineK-alpha
The invention belongs to the technical field of medicines and particularly relates to an acetylcysteine compound and an acetylcysteine solution being used for inhalation and containing the same. The acetylcysteine compound is a hydrate crystal, and the molecular formula of the acetylcysteine compound is C5H9NO3S*H2O. An X-ray powder diffraction spectrogram obtained through measurement of the acetylcysteine compound by using Cu-K alpha rays is shown in figure 1. According to the new-crystal-form acetylcysteine compound, the hygroscopicity of the compound can be significantly improved; the acetylcysteine solution which is used for inhalation and prepared from the new-crystal-form acetylcysteine compound is convenient to use, focus-targeted, fast in acting and good in curative effect.
Owner:湖南华纳大药厂手性药物有限公司
Tofacitinib crystal form compound and preparation method thereof
ActiveCN106967072AImprove liquidityImprove solubilityOrganic active ingredientsAntipyreticSolubilityTofacitinib
The invention belongs to the technical field of medicine and discloses a tofacitinib crystal form compound and a preparation method thereof. The X-ray powder diffraction spectrum shown by the 2 theta +-0.2 degree diffraction angle shows characteristic diffraction peaks at 2.42 degrees, 3.25 degrees, 4.23 degrees, 5.04 degrees, 6.12 degrees, 7.08 degrees, 8.02 degrees, 9.72 degrees, 10.63 degrees, 12.90 degrees, 14.35 degrees, 15.71 degrees, 17.82 degrees, 18.34 degrees, 19.24 degrees, 22.46 degrees, 24.56 degrees, 26.62 degrees, 30.75 degrees and 32.45 degrees, and the X-ray powder diffraction spectrum obtained by Cu-K alpha ray measurement and shown by the figure I is totally different from the prior art. The tofacitinib crystal form compound has better water solubility and high stability, the preparation method is simple and easy to operate, the medication safety is improved greatly after the compound is prepared into a drug composition, and the compound is very suitable for clinical application.
Owner:SHANDONG YUXIN PHARMA CO LTD
Riboflavin sodium phosphate compound
ActiveCN102617643AControl granularityPromote formationGroup 5/15 element organic compoundsSolubilityWater soluble
The invention relates to a riboflavin sodium phosphate compound, which exists in a crystal form. X-ray powder diffraction measured by Cu-K alpha rays shows that characteristic peaks exist at 2Theta of 12.4 degrees, 13.3 degrees, 14.7 degrees, 16.1 degrees, 16.9 degrees, 18.1 degrees, 18.9 degrees, 19.9 degrees, 20.9 degrees, 21.5 degrees, 22.7 degrees, 24.0 degrees, 24.7 degrees, 25.4 degrees, 26.7 degrees, 27.4 degrees and 28.2 degrees. The riboflavin sodium phosphate compound has main particle sizes of 5 to 15 mu m, preferably 6 to 12 mu m, and the distribution width of 2 to 20 mu m, preferably 3 to 18 mu m. A stability experiment shows that the riboflavin sodium phosphate crystal compound has high stability and better water solubility.
Owner:珠海晨安医药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com