Nanometer iron trioxide as well as preparation method and purpose of nanometer iron trioxide
A nano-iron trioxide technology, which is applied in the directions of iron oxide, chemical instruments and methods, iron oxide/iron hydroxide, etc., can solve the problems of complicated treatment process, limited popularization and application of sewage treatment process, and high cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
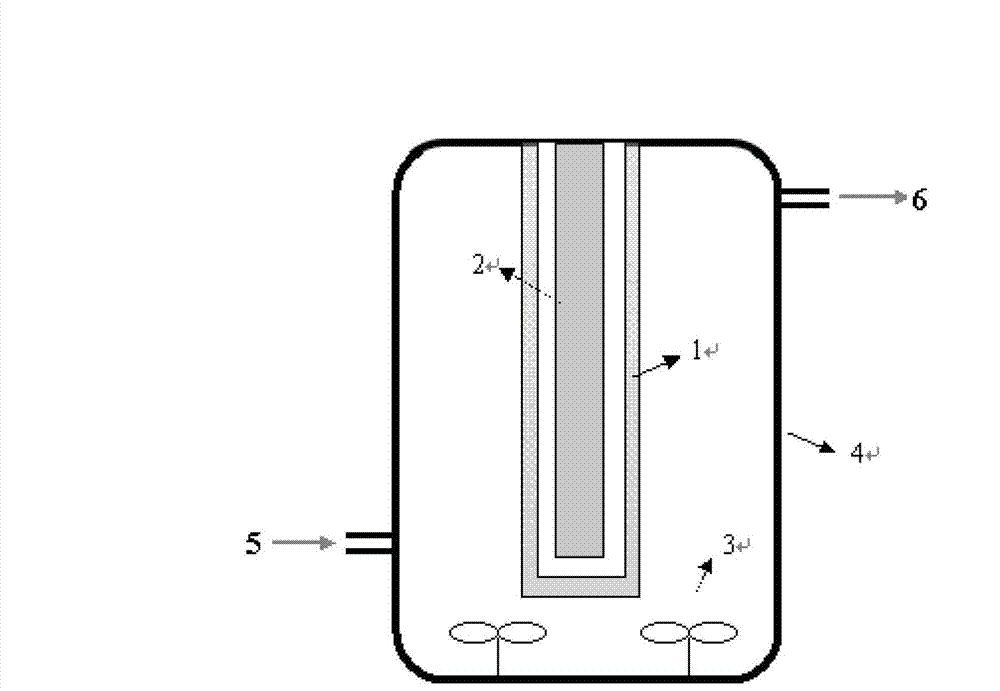
[0041] S1, 0.8gFeCl 3 ·6H 2 0, 1.8g urea and 4.8g tetrabutylammonium bromide are added in the 120ml ethylene glycol respectively, are stirred to all dissolving of solid, obtain the first mixed solution;
[0042] S2, under stirring, heat the first mixed solution obtained in S1 to 195°C, continue stirring and reflux reaction, after 12 minutes, the reaction solution appears yellow precipitate, after 8 minutes, the color of the solution turns green completely, and then reflux for 0.5 hours to react End;
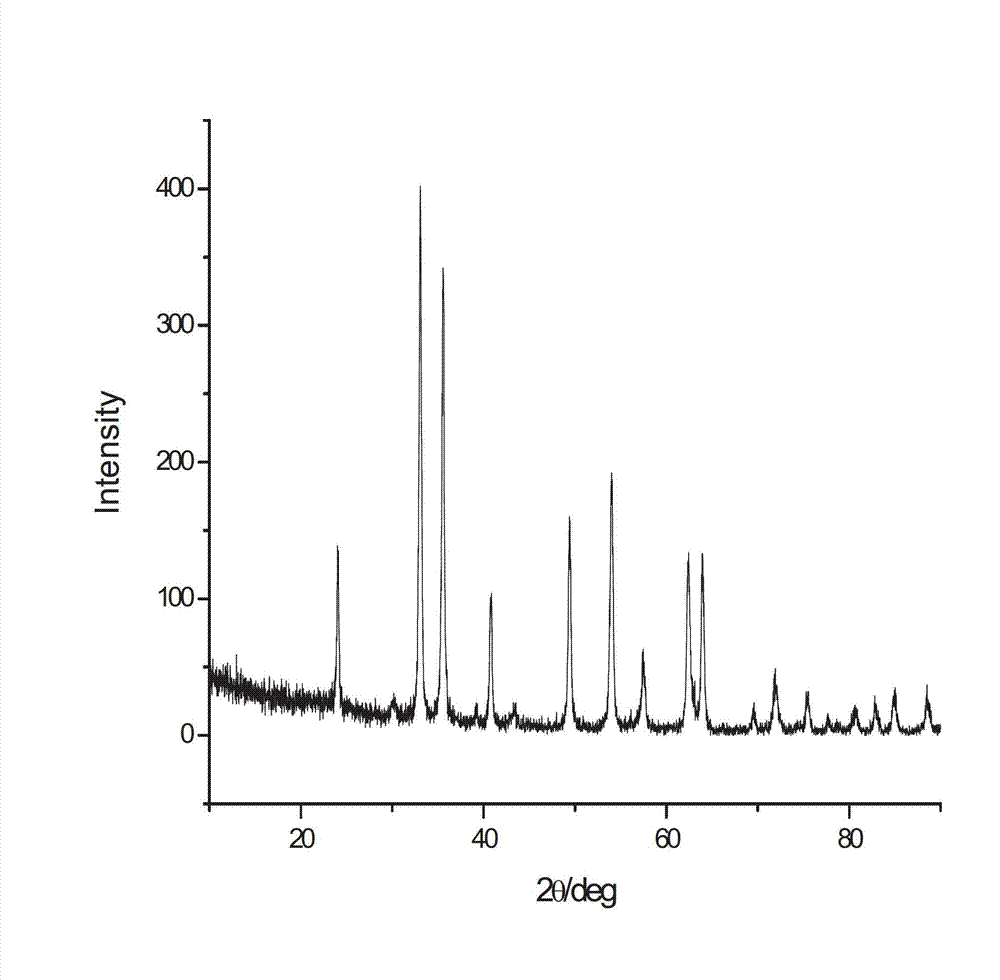
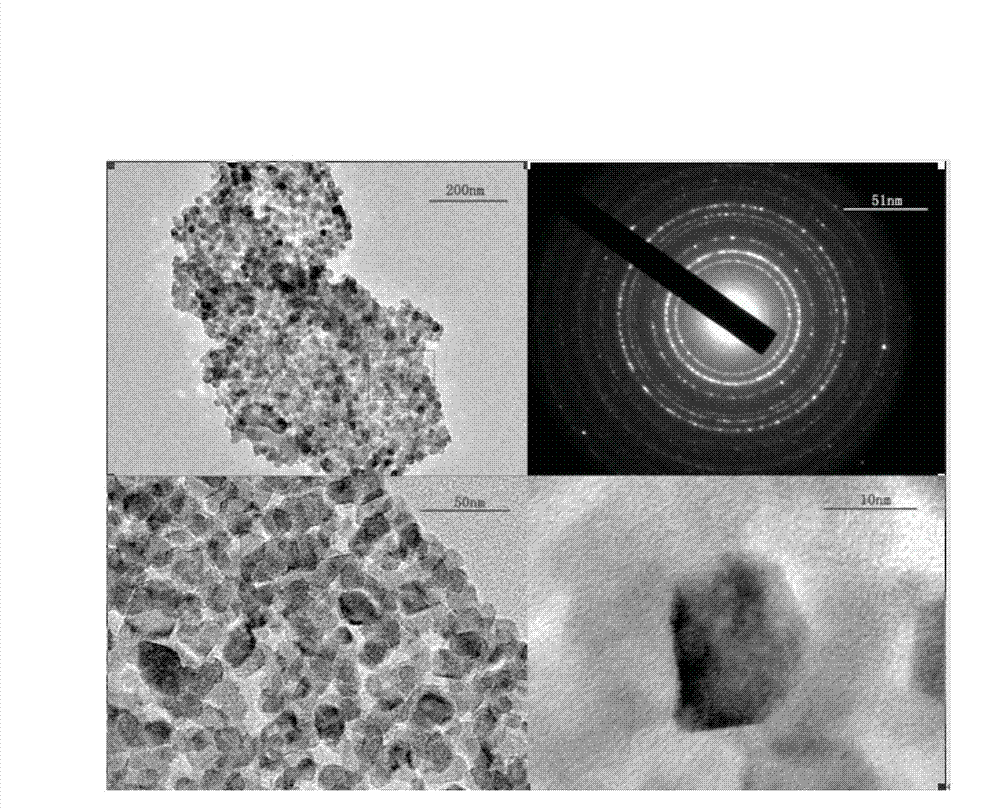
[0043] S3, cooling the reaction solution obtained in S2, cooling to 25°C, centrifuging to separate the solid, then washing the solid with ethanol 4 times, drying the washed solid in an oven at 80°C for 5h, and then transferring the solid to 450°C Calcined in the muffle furnace for 4 hours to obtain nano-iron sesquioxide; wherein, the nano-ferric oxide is black when taken out of the muffle furnace, and turns brick red after cooling in the air.
[0044] The prepared nanometer fe...
Embodiment 2
[0046] S1, 0.8gFeCl 3 ·6H 2 0, 1.6g urea and 4.2g tetrabutylammonium bromide are added in the 120ml ethylene glycol respectively, are stirred to all dissolving of solid, obtain the first mixed solution;
[0047] S2, under stirring, heat the first mixed solution obtained in S1 to 180°C, continue stirring and reflux reaction, after 14 minutes, the reaction solution appears yellow precipitate, after 7 minutes, the color of the solution turns green completely, and then reflux for 0.3 hours to react End;
[0048] S3, cooling the reaction liquid obtained in S2, cooling to 29°C, centrifuging to separate the solid, then washing the solid with ethanol for 5 times, drying the washed solid in a 100°C oven for 4h, and then transferring the solid to a 400°C Calcined in a muffle furnace for 3 hours to obtain nano-iron sesquioxide; wherein, the nano-iron sesquioxide is black when taken out of the muffle furnace, and turns brick red after cooling in the air.
[0049] The obtained nano-ferr...
Embodiment 3
[0051] S1, 0.8gFeCl 3 ·6H 2 0, 2.0g urea and 5.6g Tetrabutylammonium bromide are added in the 120ml ethylene glycol respectively, are stirred to all dissolving of solid, obtain the first mixed solution;
[0052] S2, under stirring, heat the first mixed solution obtained in S1 to 210°C, continue to stir and reflux the reaction, after 15 minutes, a yellow precipitate appears in the reaction solution, after 7 minutes, the color of the solution turns green completely, and then reflux for 1.0 hour to react End;
[0053] S3, cooling the reaction solution obtained in S2, cooling to 27°C, centrifuging to separate the solid, then washing the solid with ethanol for 3 times, drying the washed solid in a 60°C oven for 6h, and then transferring the solid to 500°C Calcined in the muffle furnace for 3.2 hours to obtain nanometer iron sesquioxide; wherein, the nanometer iron oxide is black when taken out of the muffle furnace, and turns brick red after cooling in the air.
[0054] The obtain...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com