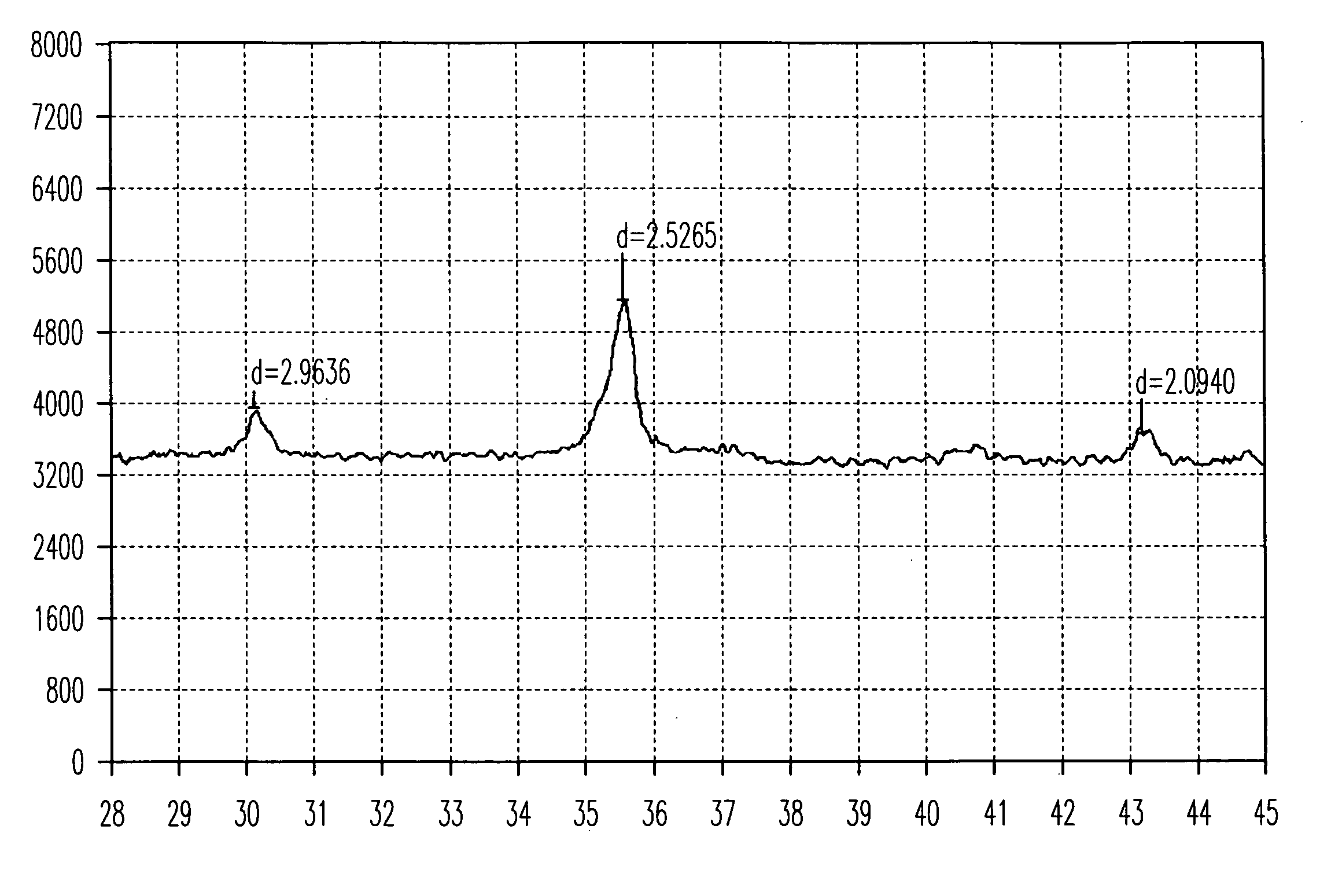
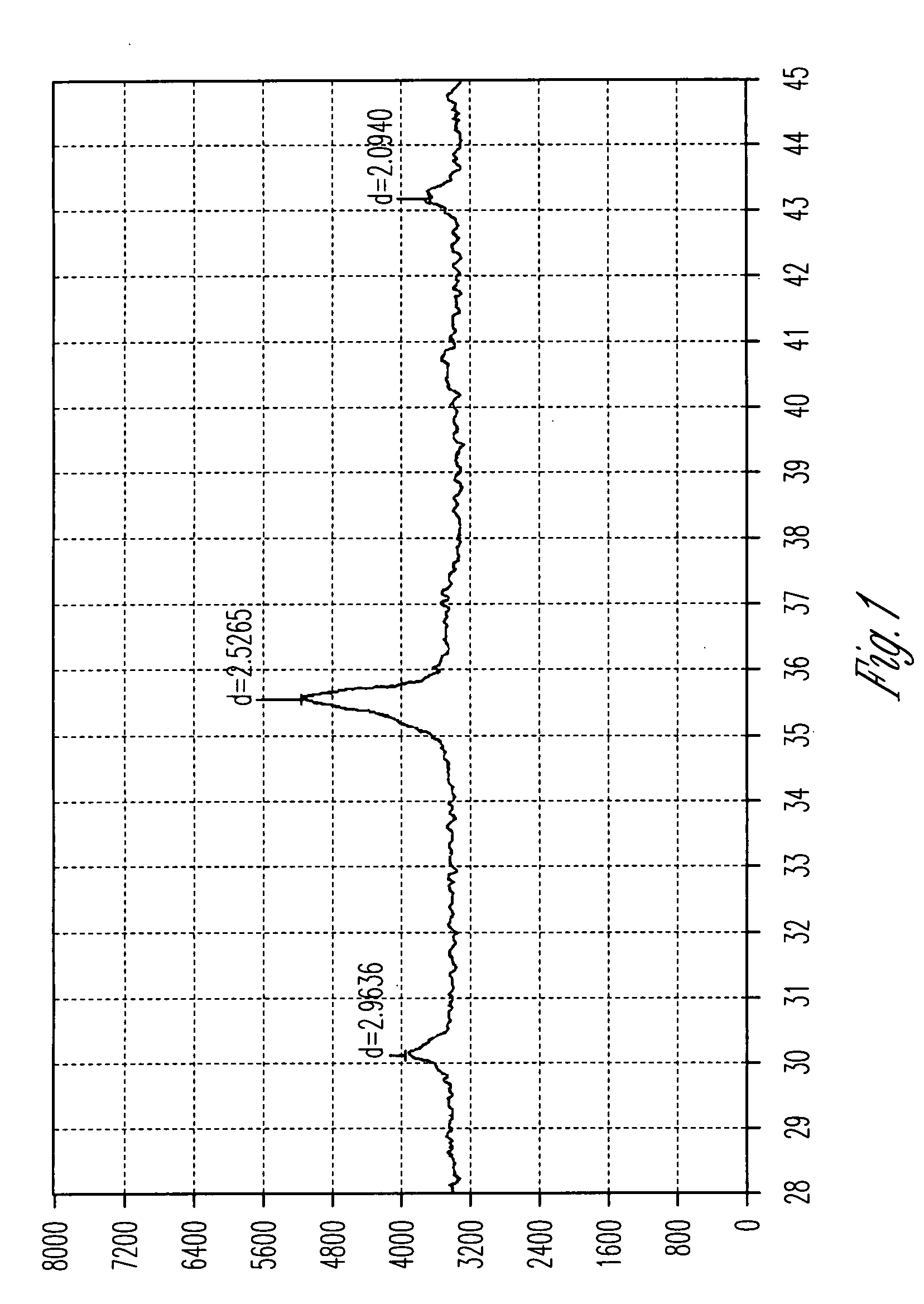
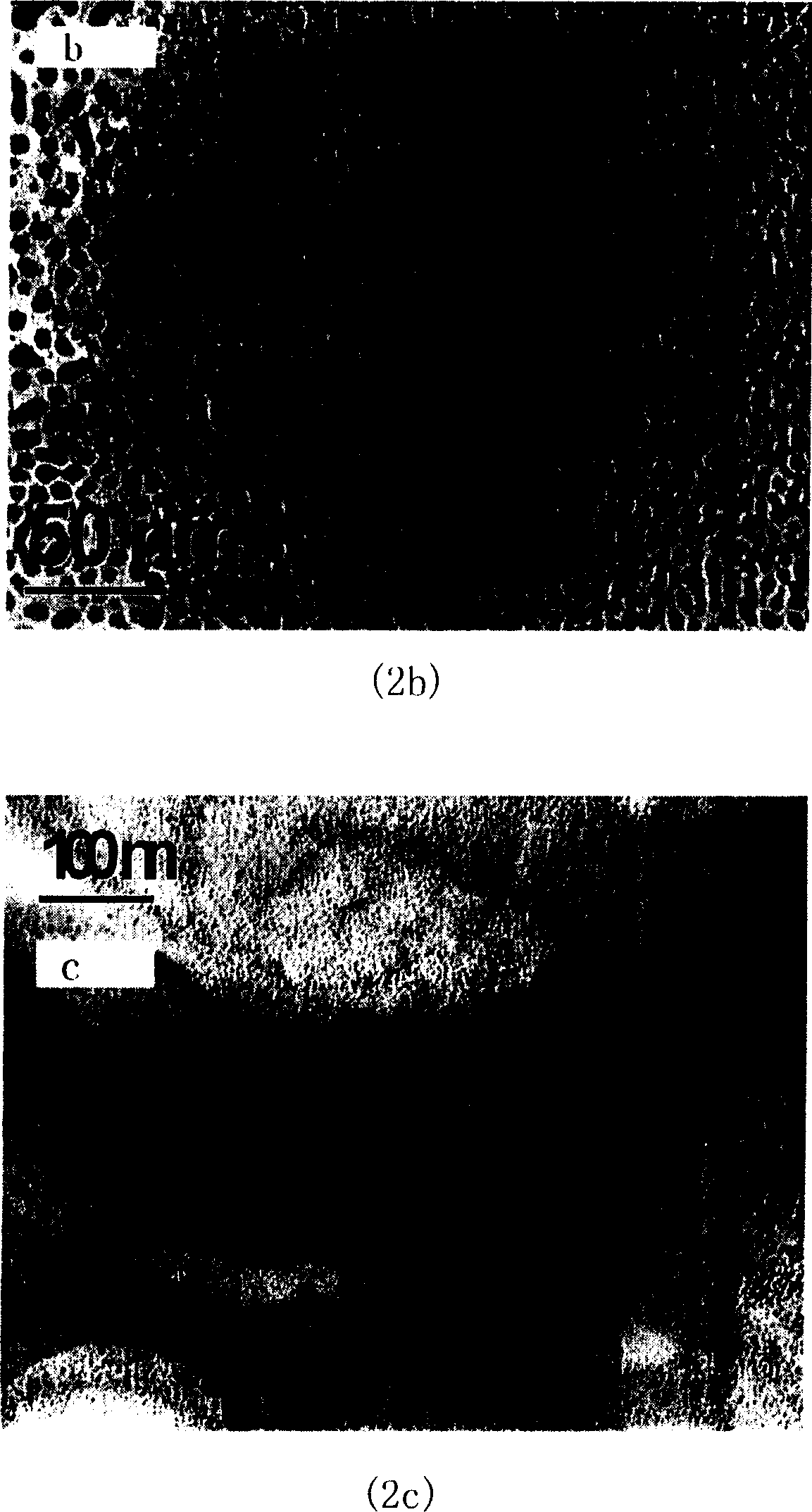
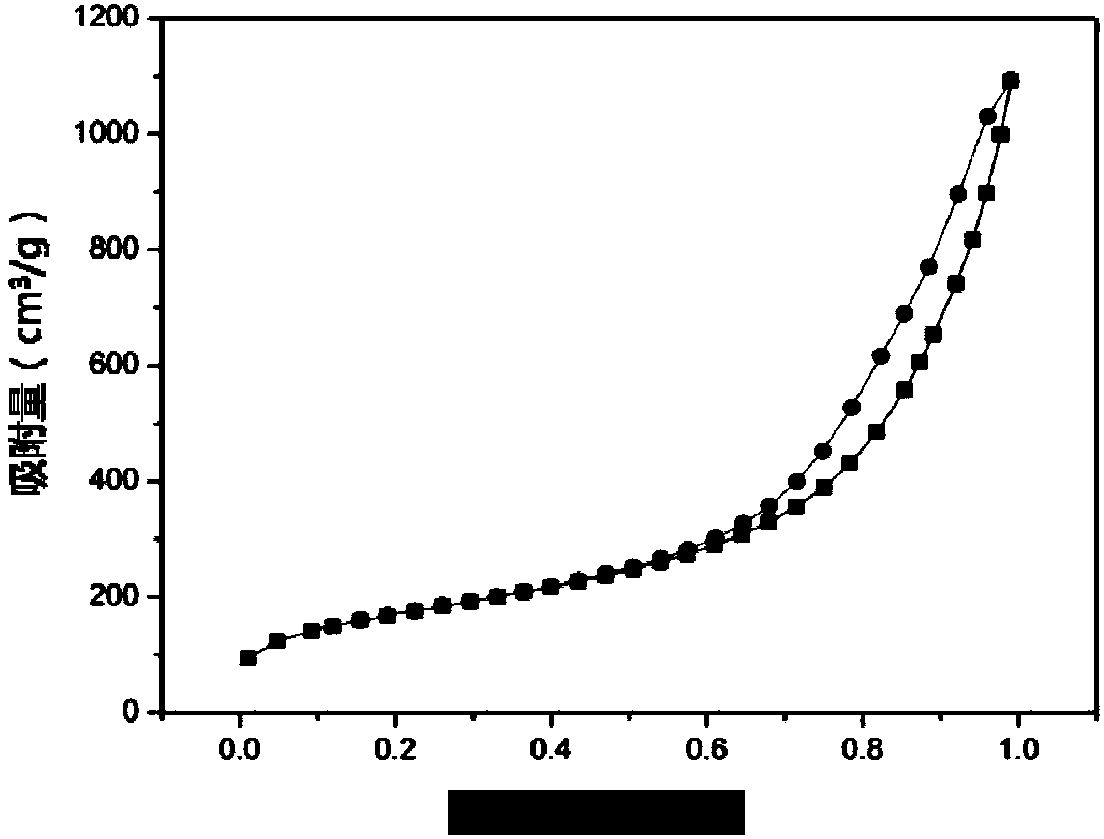
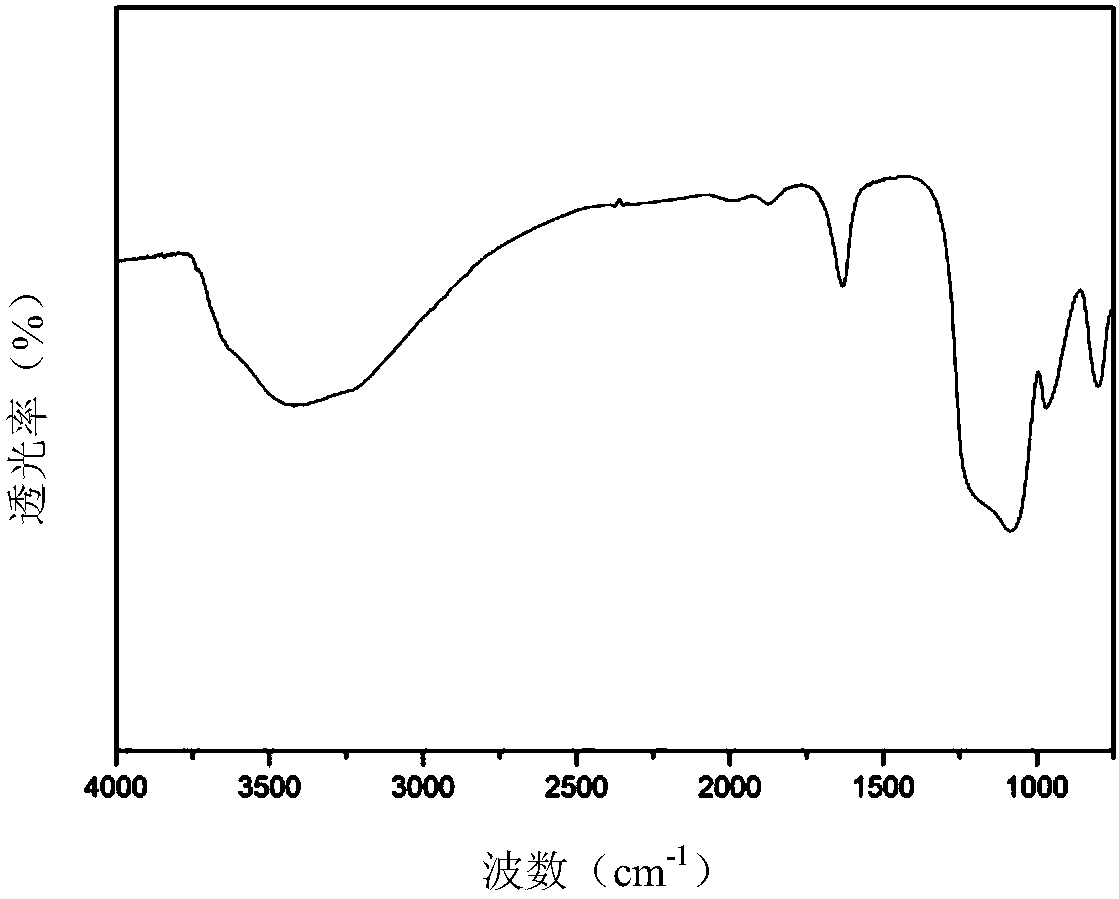
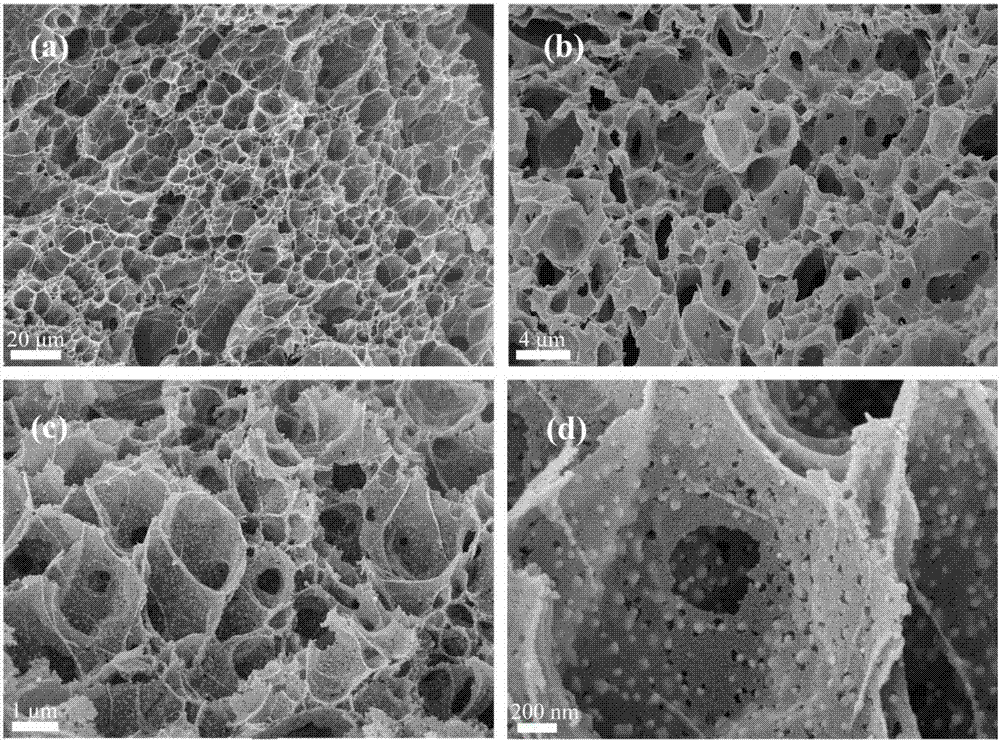
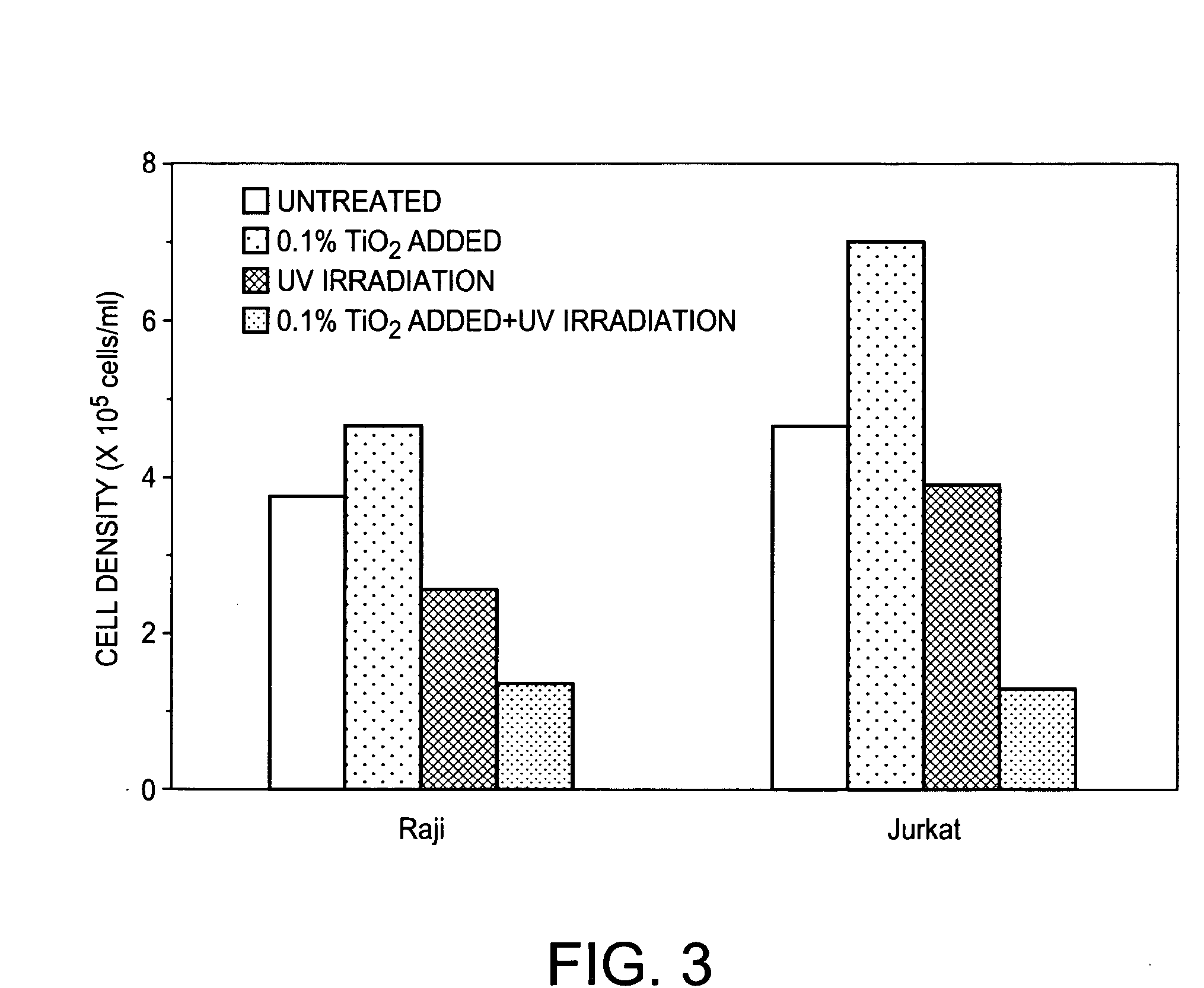
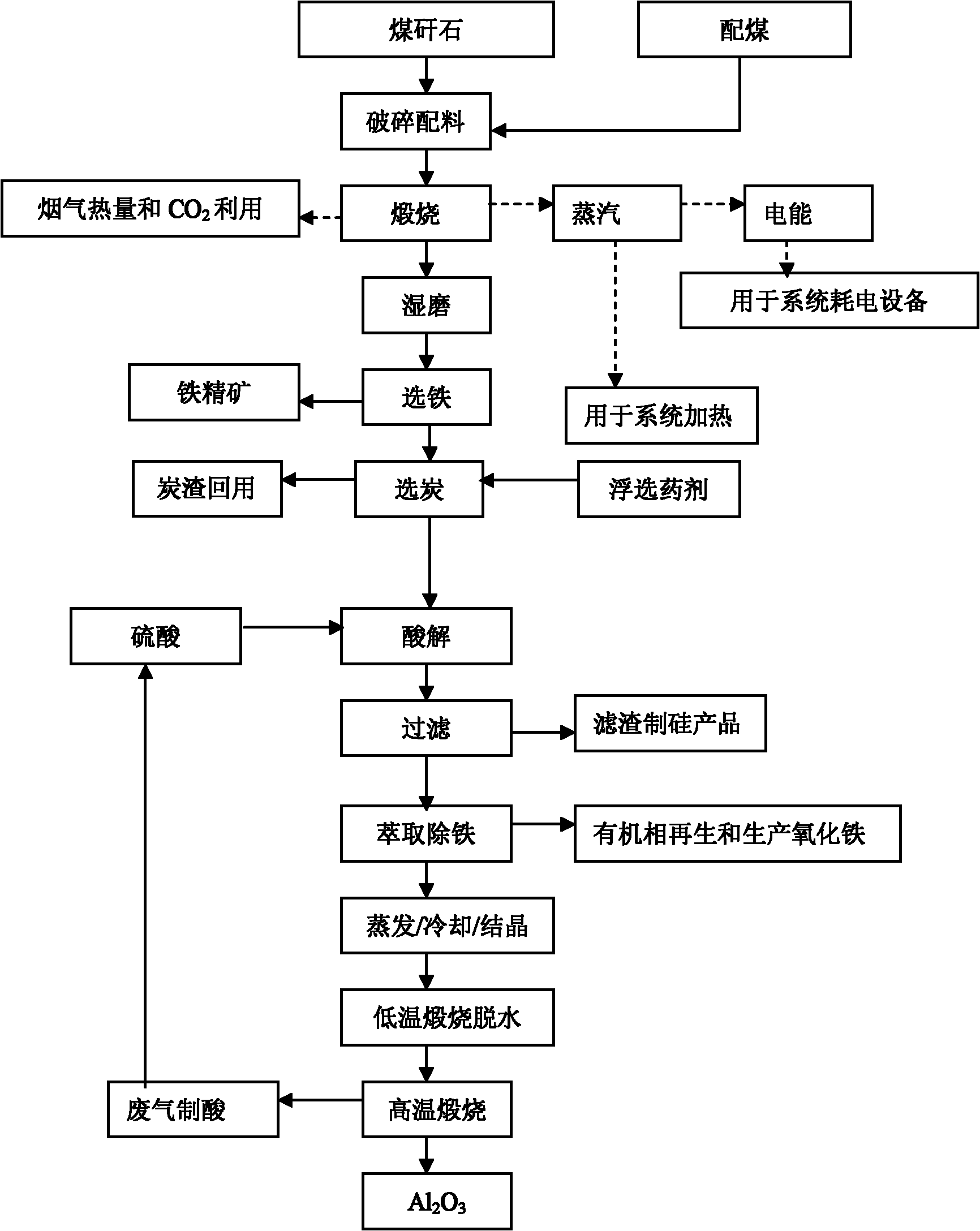
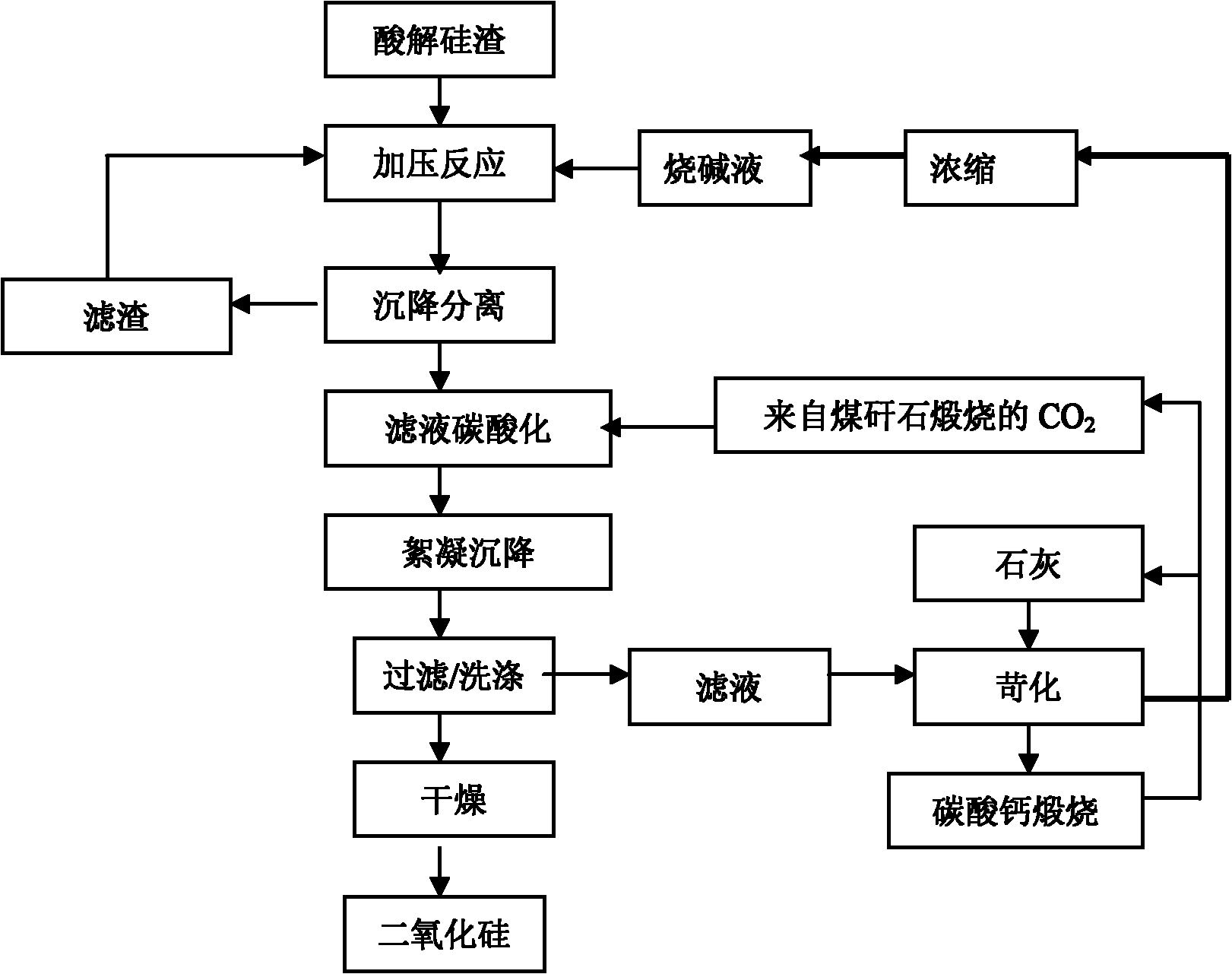
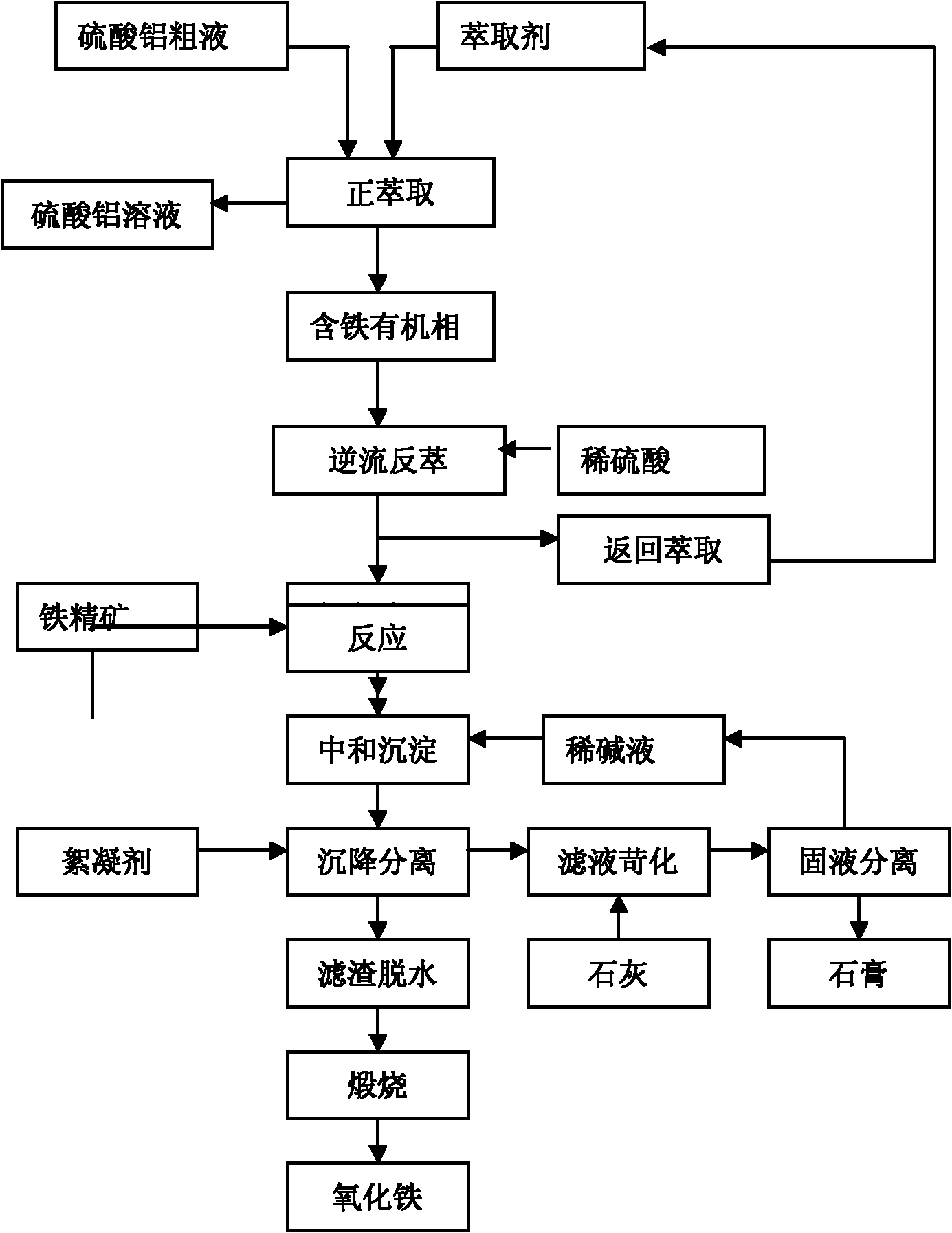
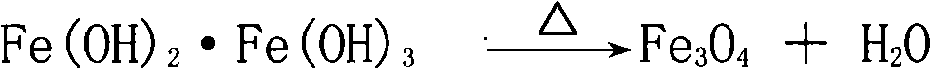Patents
Literature
2133results about "Ferric oxides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
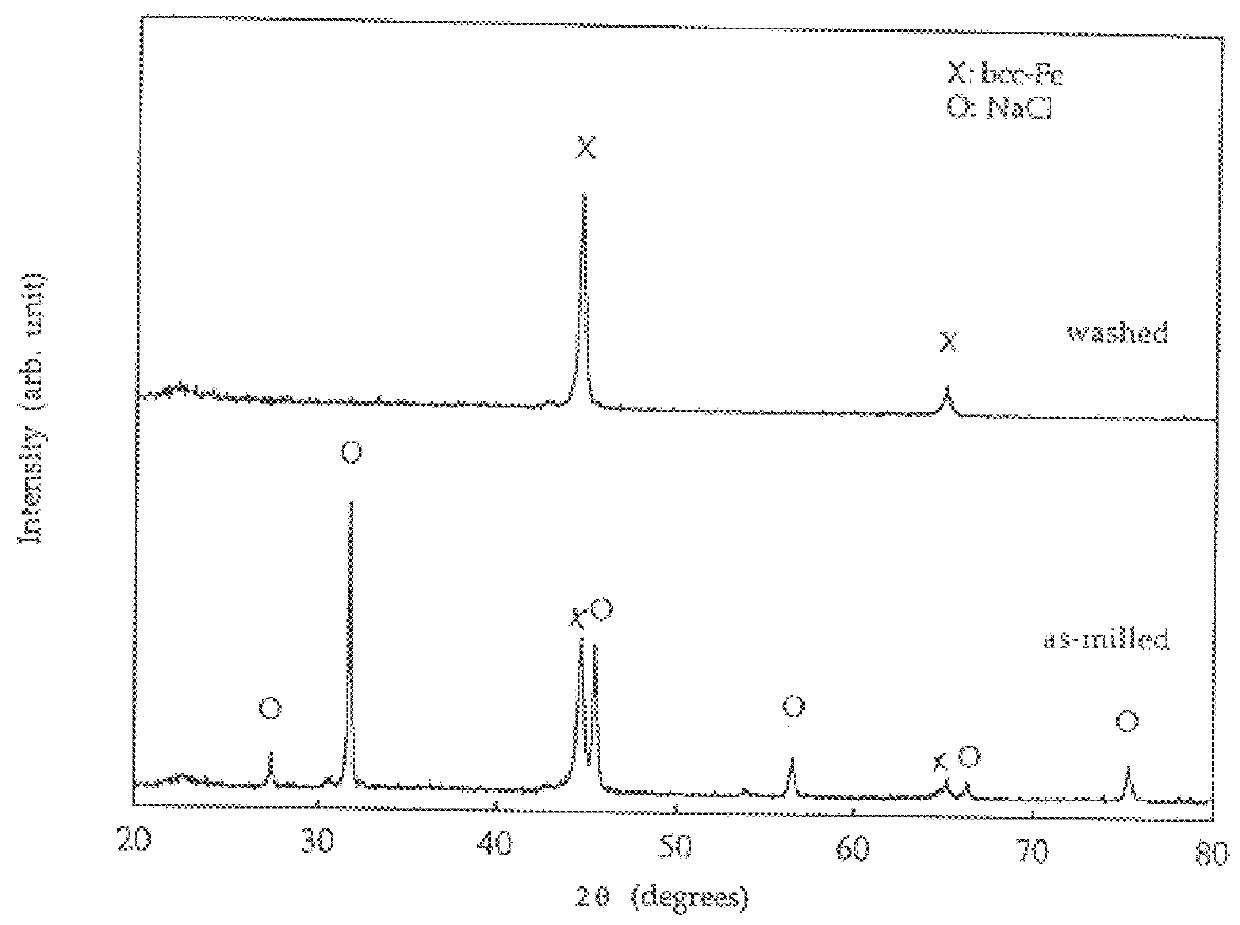
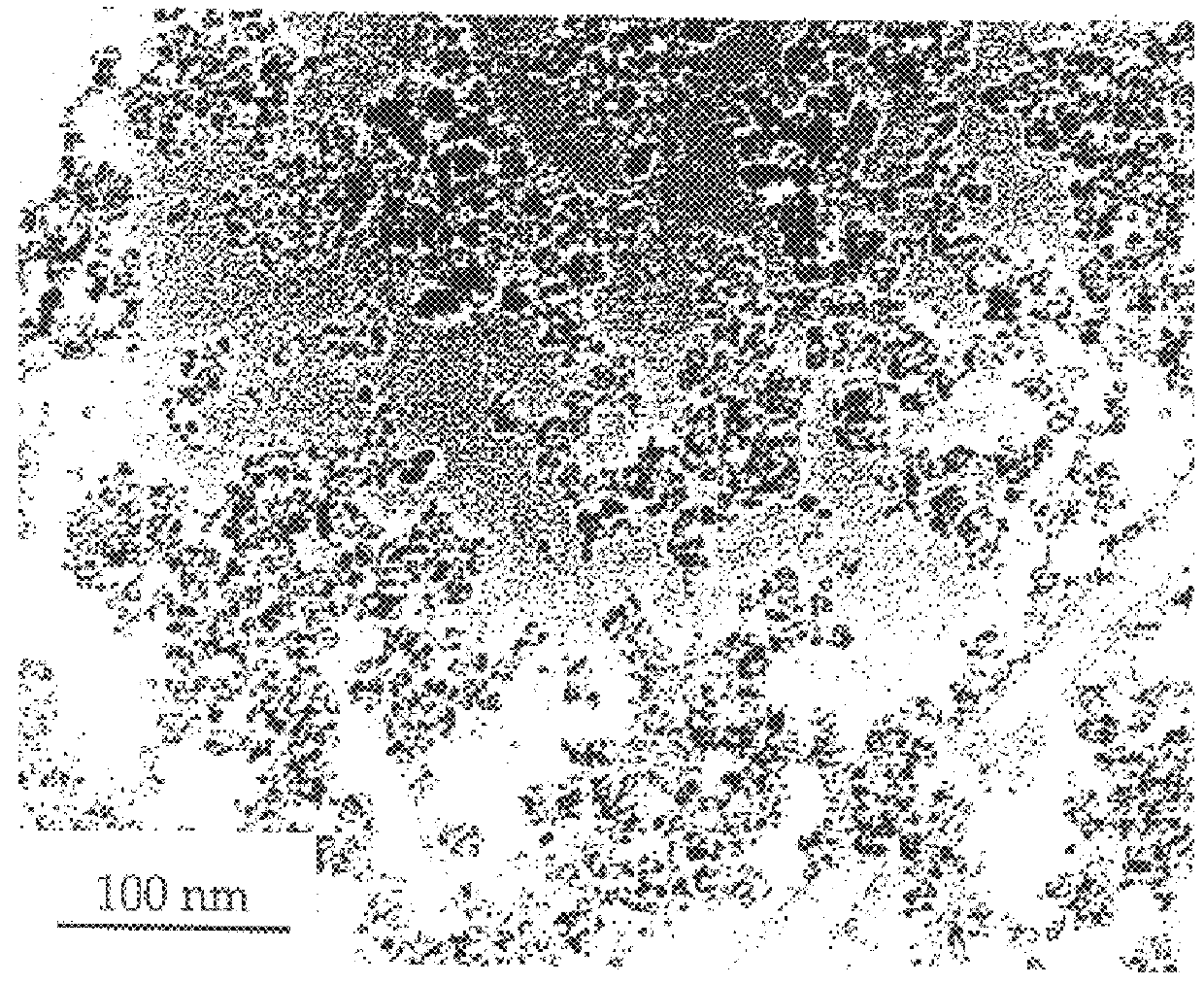
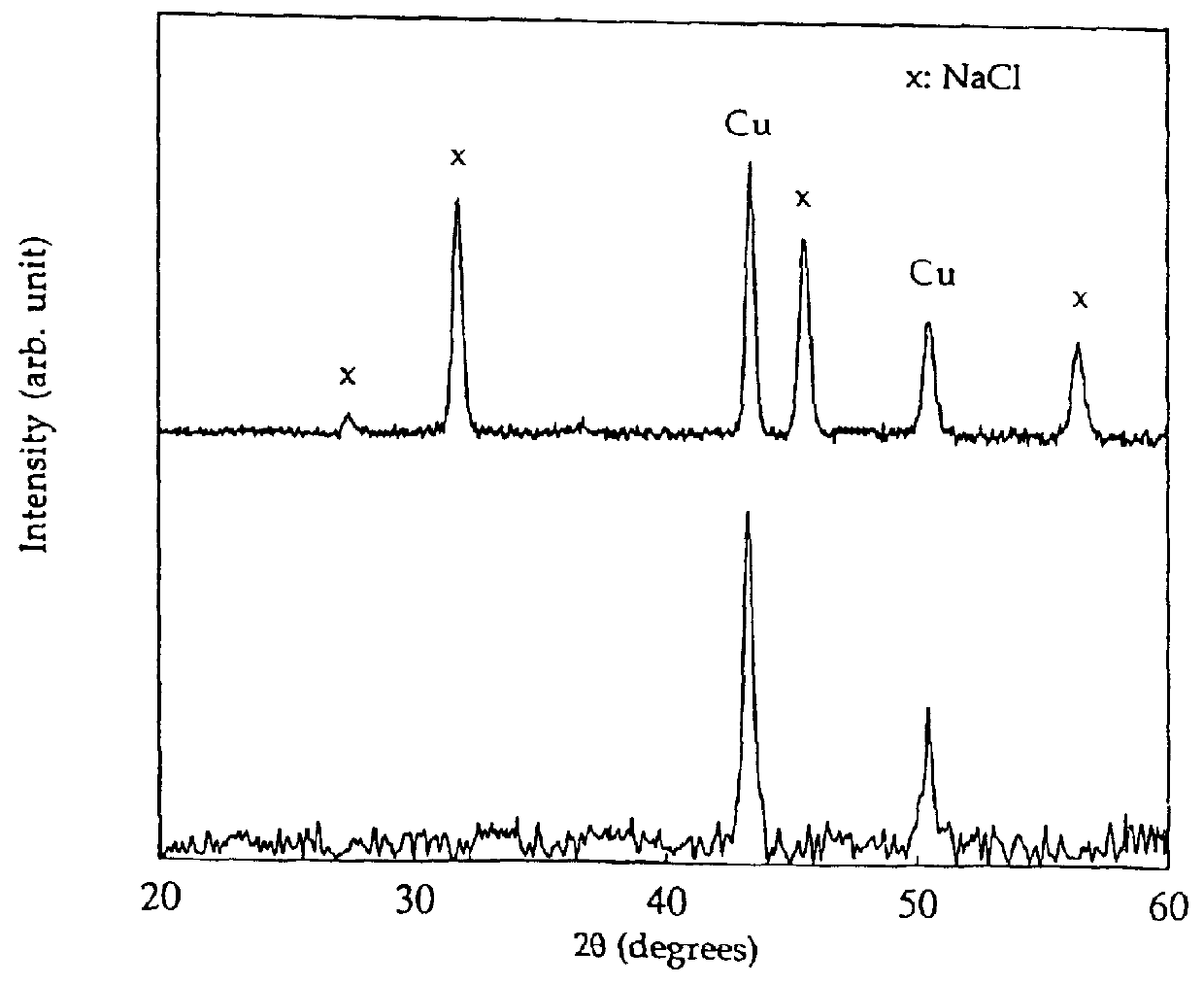
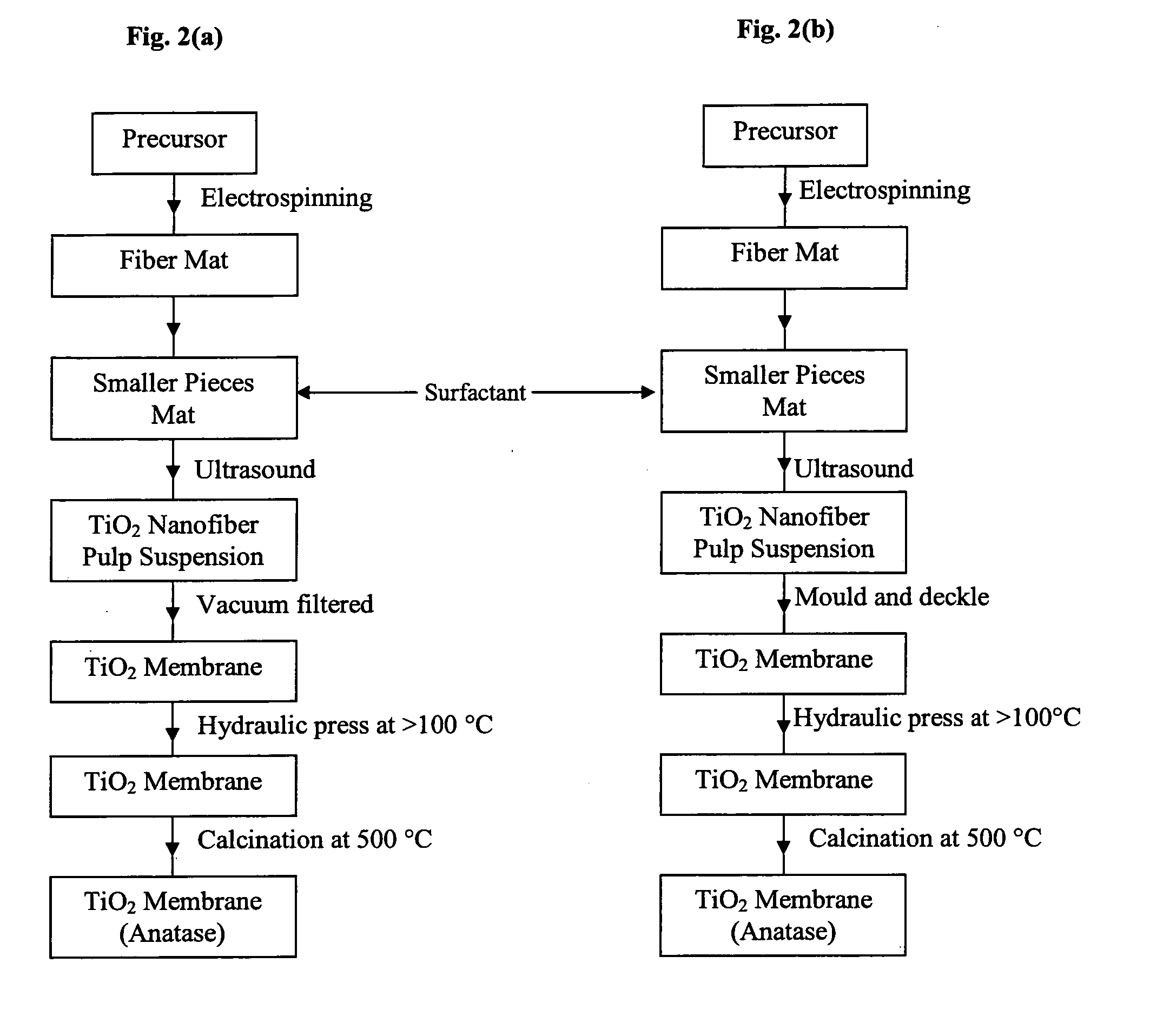
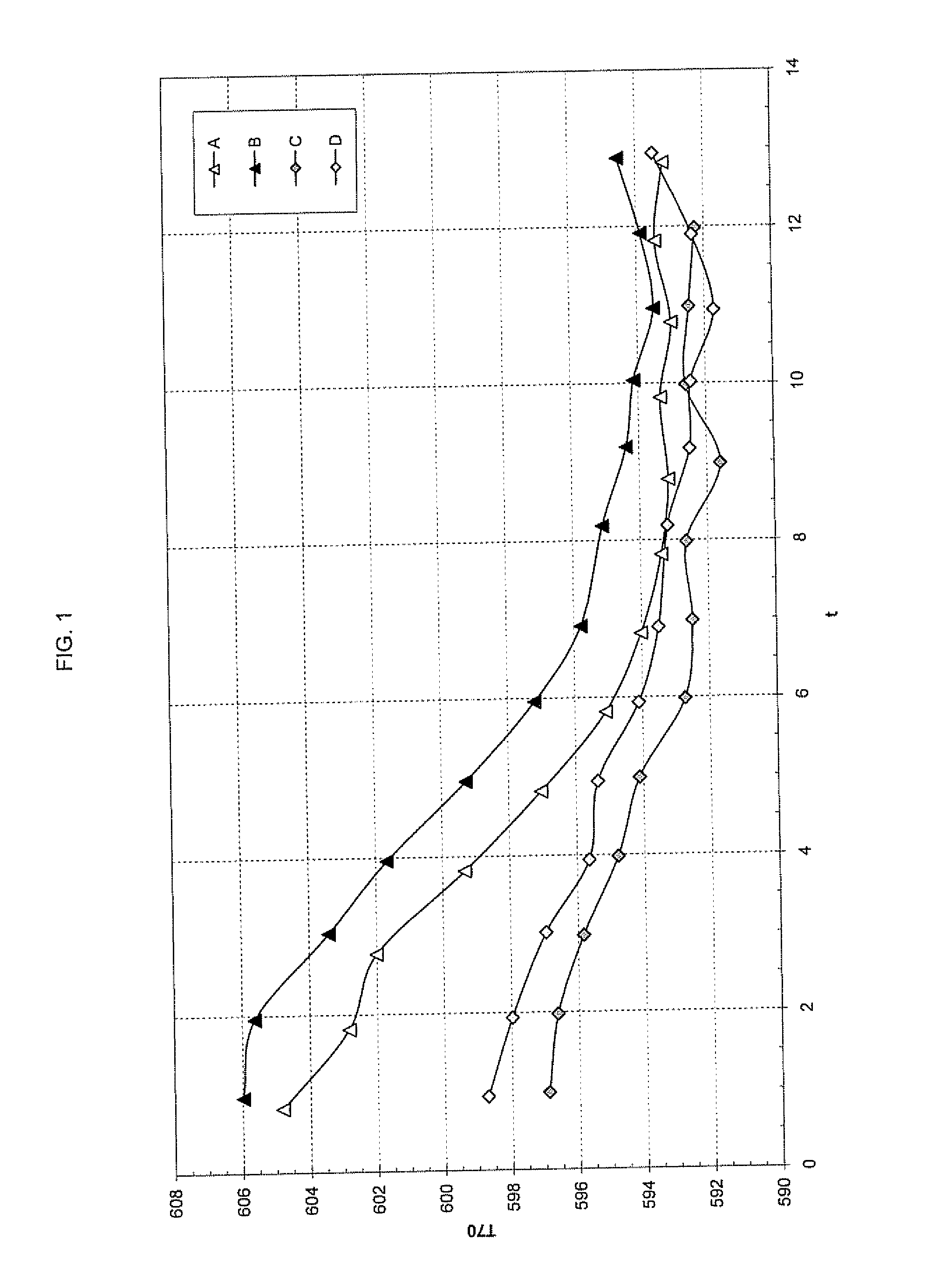
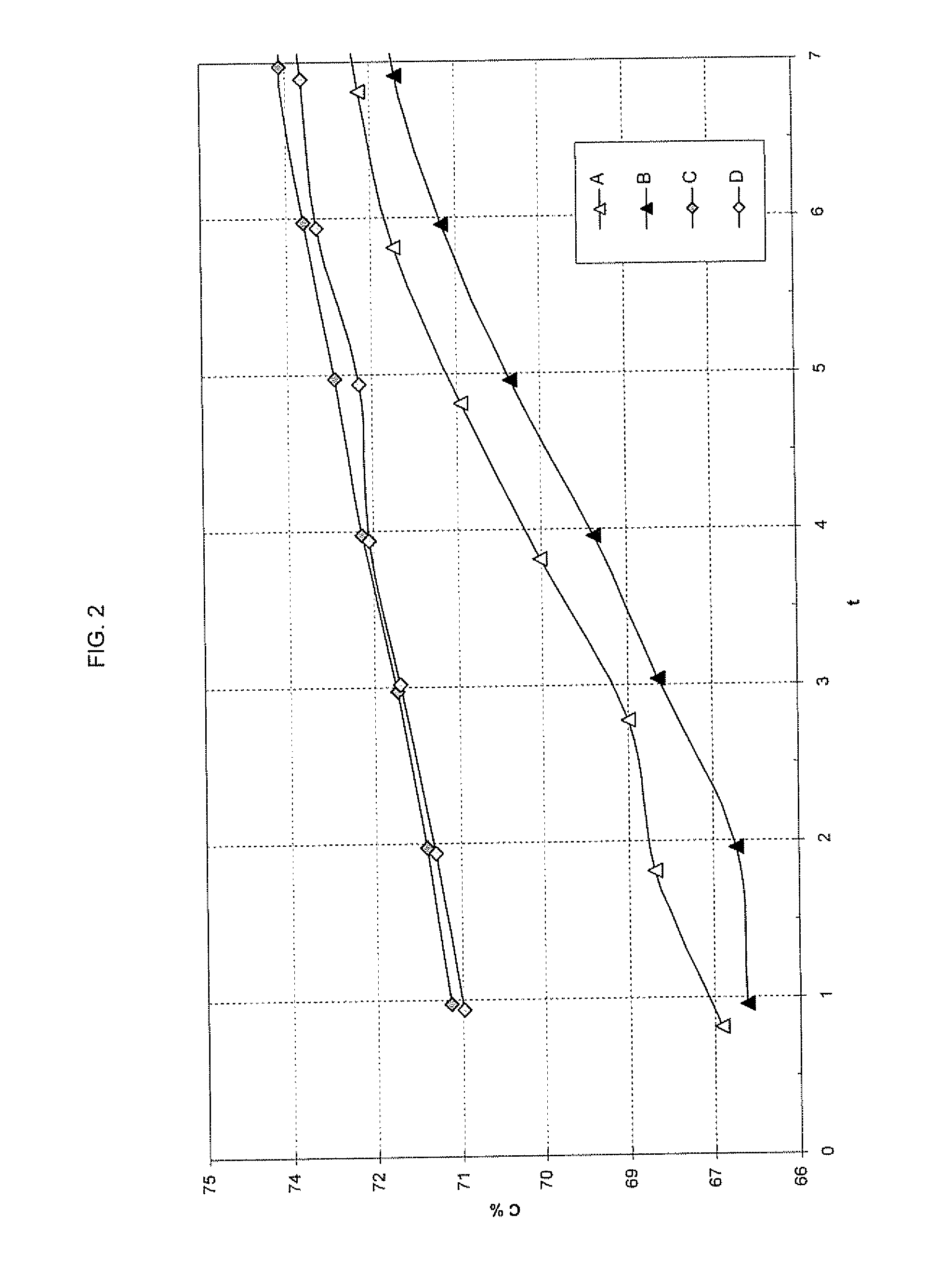
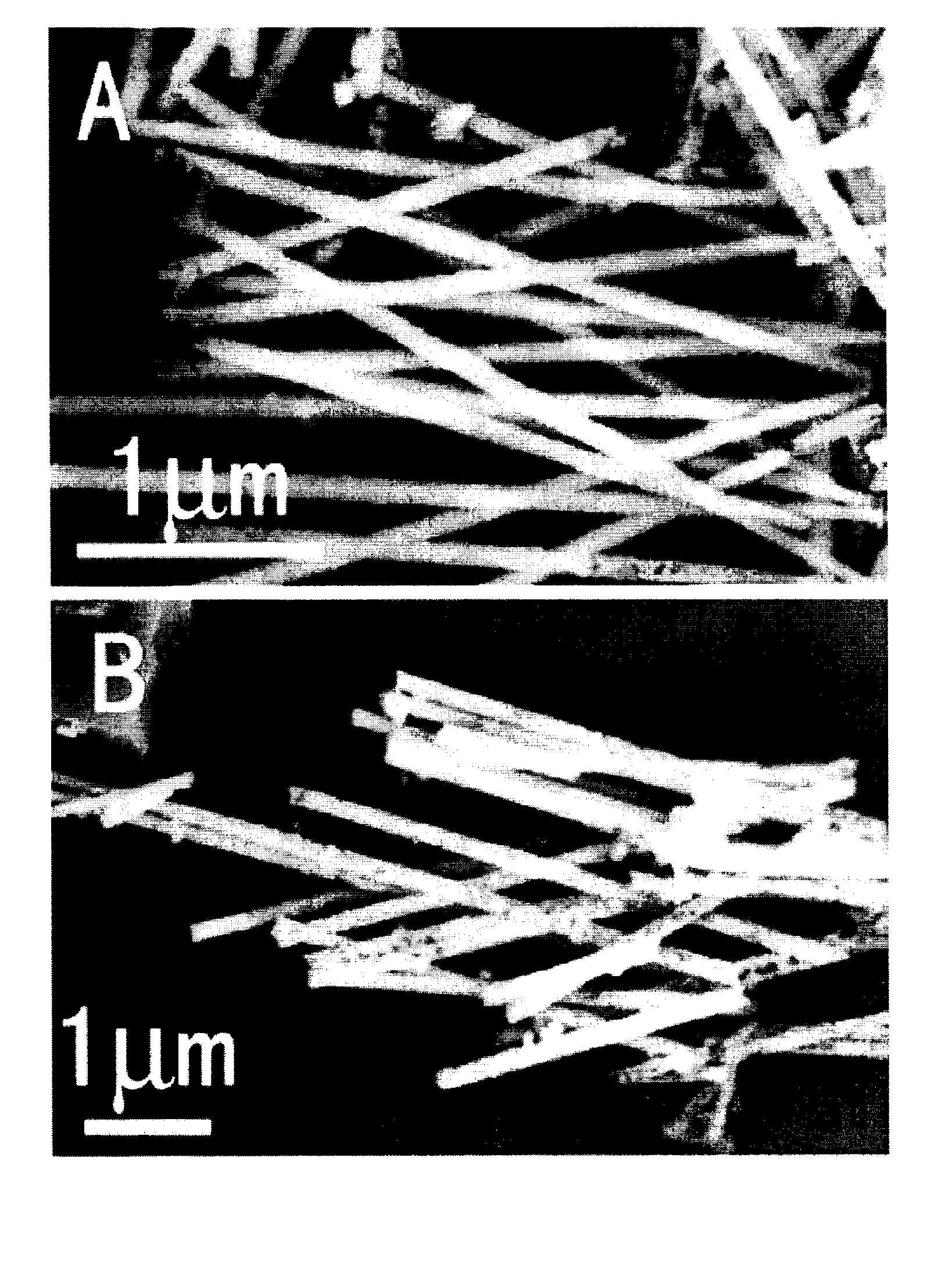
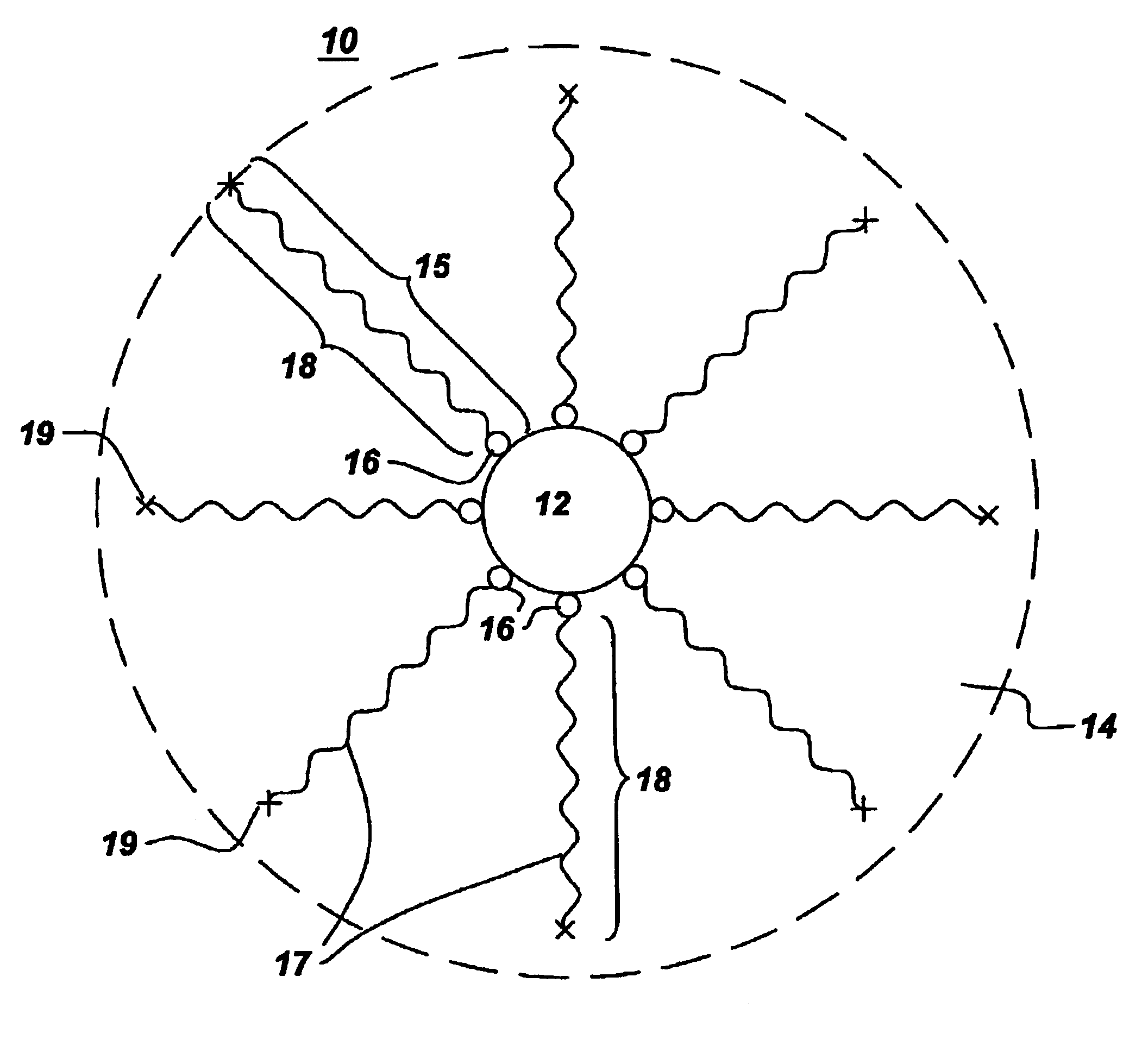
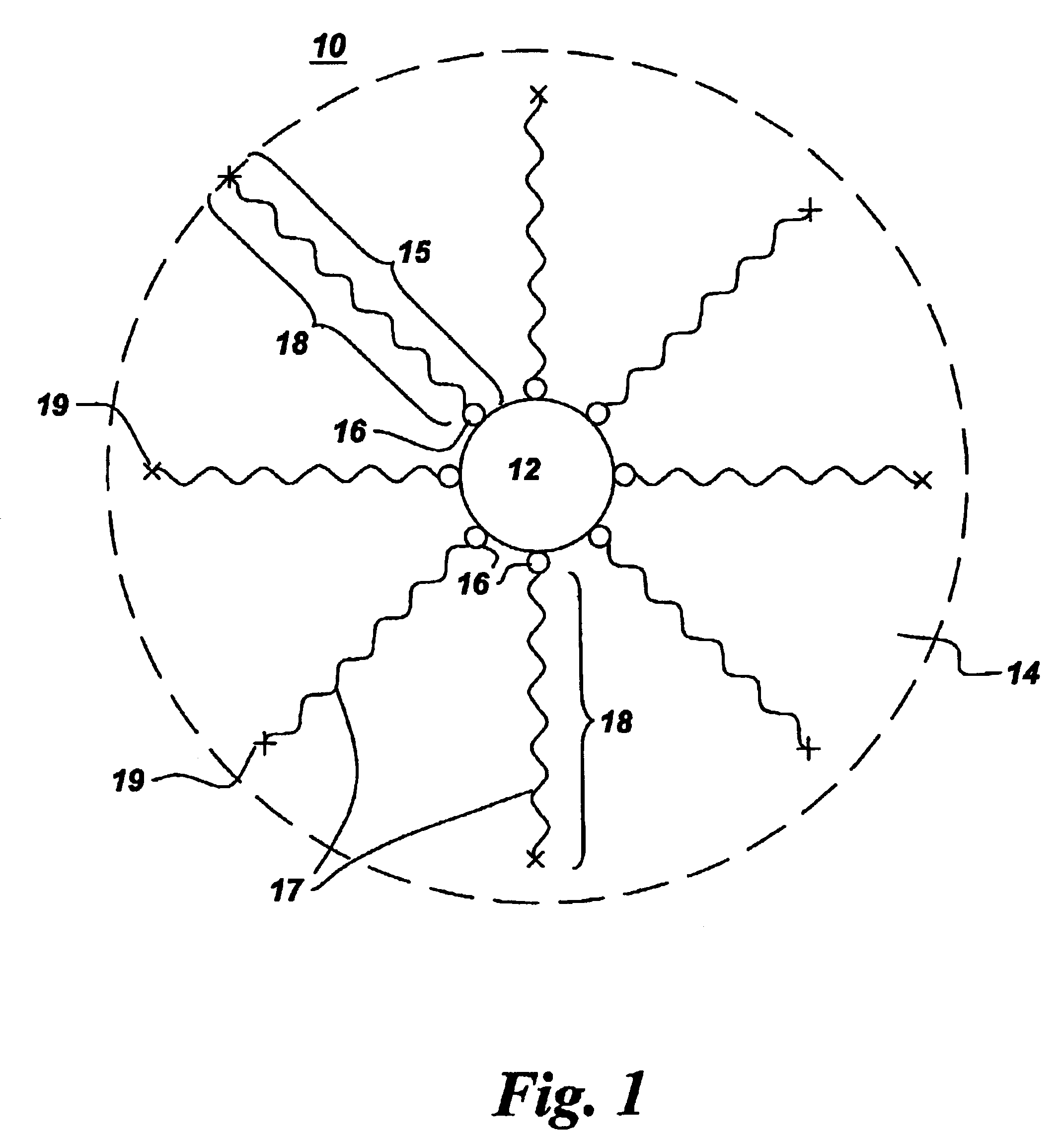
Process for the production of ultrafine particles
A new, cost effective process for the production of ultrafine particles which is based on mechanically activated chemical reaction of a metal compound with a suitable reagent. The process involves subjecting a mixture of a metal compound and a suitable reagent to mechanical activation to increase the chemical reactivity of the reactants and / or reaction kinetics such that a chemical reaction can occur which produces a solid nano-phase substance. Concomitantly, a by-product phase is also formed. This by-product phase is removed so that the solid nano-phase substance is left behind in the form of ultrafine particles. During mechanical activation a composite structure is formed which consists of an intimate mixture of nano-sized grains of the nano-phase substance and the reaction by-product phase. The step of removing the by-product phase, following mechanical activation, may involve subjecting the composite structure to a suitable solvent which dissolves the by-product phase, while not reacting with the solid nano-phase substance. The process according to the invention may be used to form ultrafine metal powders as well as ultrafine ceramic powders. Advantages of the process include a significant degree of control over the size and size distribution of the ultrafine particles, and over the nature of interfaces created between the solid nano-phase substance and the reaction by-product phase.
Owner:WESTERN AUSTRALIA UNIV OF THE
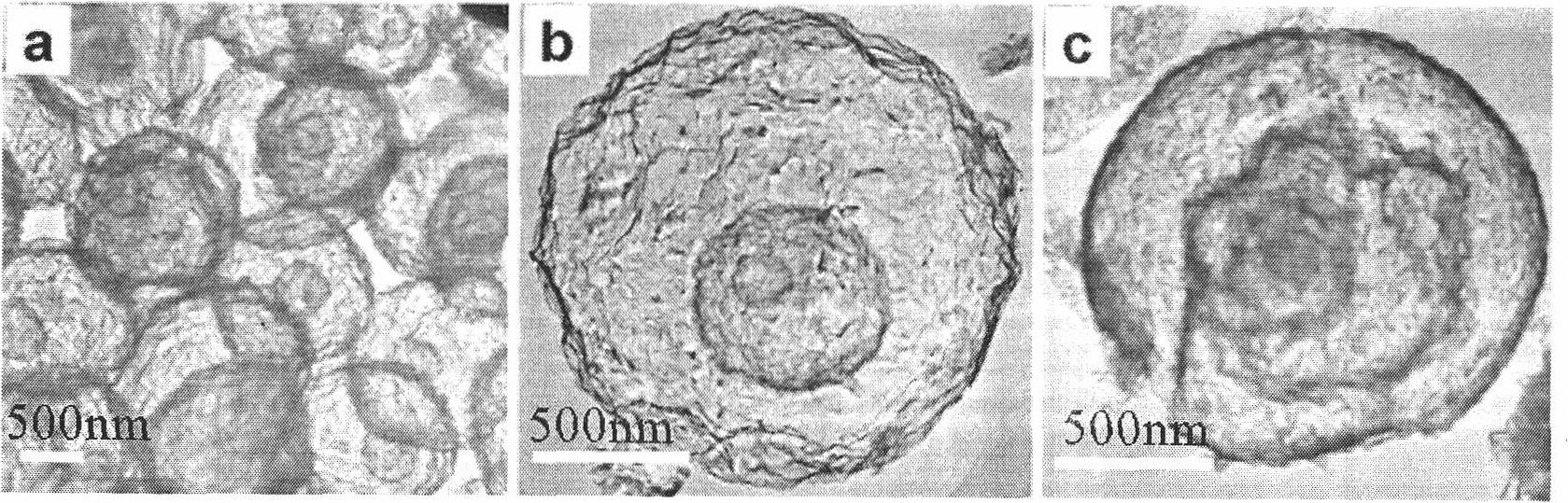
Multi-shell-layer metal oxide hollow ball and preparation method thereof
ActiveCN102464304AHigh specific surface areaSimple processOxide/hydroxide preparationZinc oxides/hydroxidesControllabilityNanoscopic scale
The invention provides a multi-shell-layer metal oxide hollow ball and a preparation method thereof. A hydrothermal method is used for preparing a carbon ball template; metal salts are dissolved in carbon ball suspension liquid, and the gradient distribution, the depth and the number of metal salts entering carbon balls are controlled through regulating adsorption conditions such as metal salt concentration, solution pH value, soaking temperature and time and the like; and the heat treatment is carried out on the carbon balls adsorbing metal ions, and the multi-shell-layer metal oxide hollow ball can be obtained. The shell-layer of the hollow ball prepared by the method is formed by accumulating nanometer crystal particles of metal oxides, the shell layer number can be regulated and changed from two to four, and both the size of the hollow ball and the thickness of the shell layers are controllable. The method provided by the invention is simple and is easy to implement, the controllability is high, the pollution is little, the cost is low, and in addition, the general applicability is realized. The prepared product has a hollow structure and the shell layers with the thickness inthe nanometer level, simultaneously, the internal space can be effectively utilized through the multilayer structure, and the multi-shell-layer metal oxide hollow ball is applied to gas sensitivity and photocatalysis and has the more excellent performance through being compared with the traditional nanometer material and a single-layer hollow ball.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
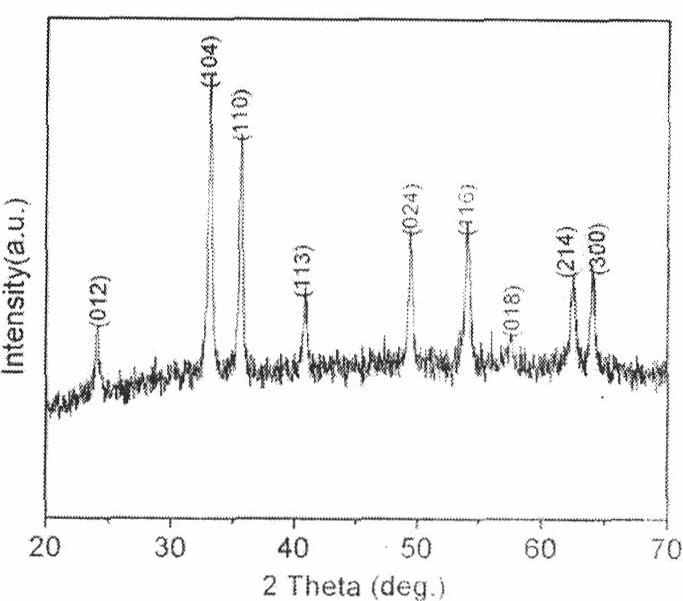
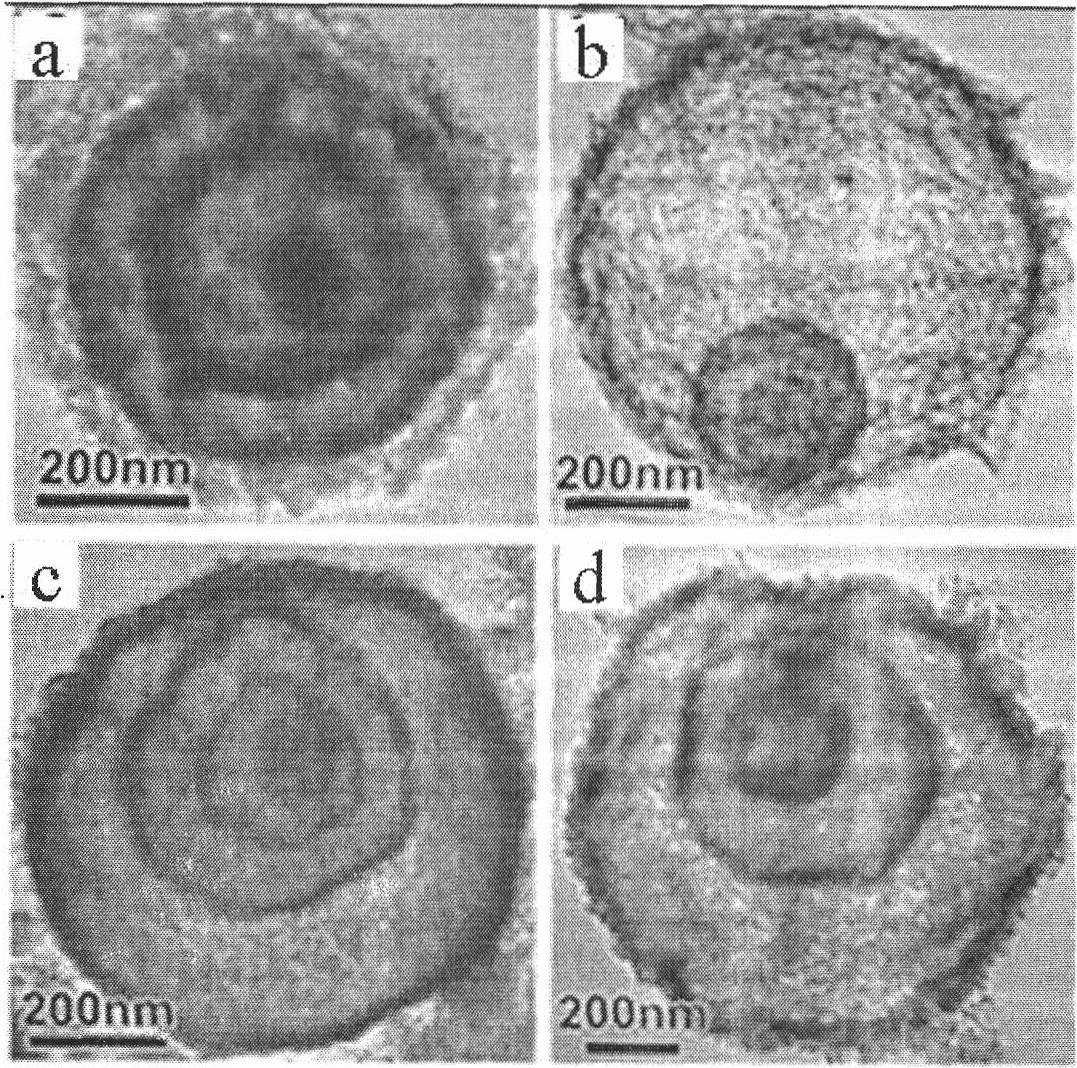
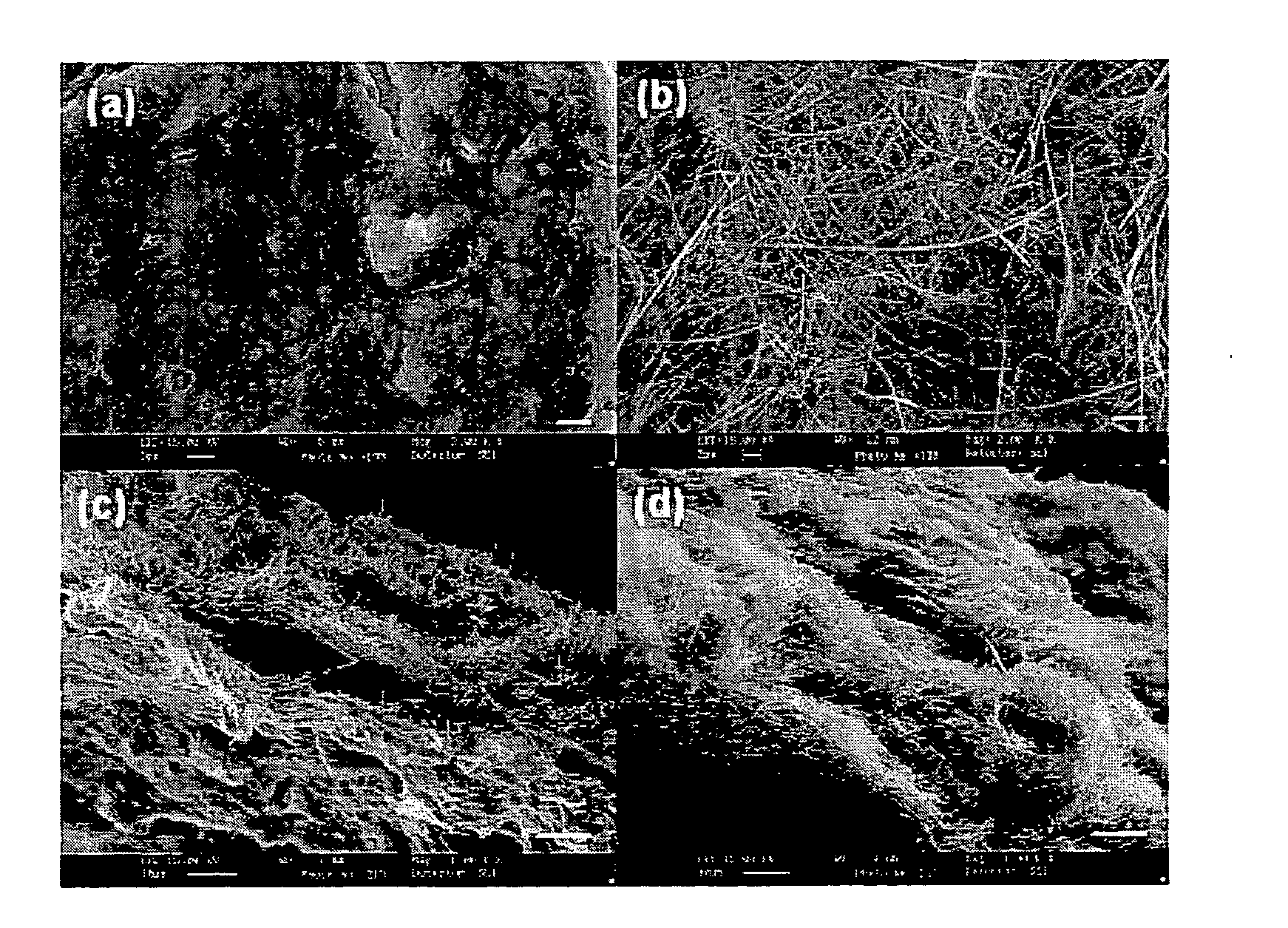
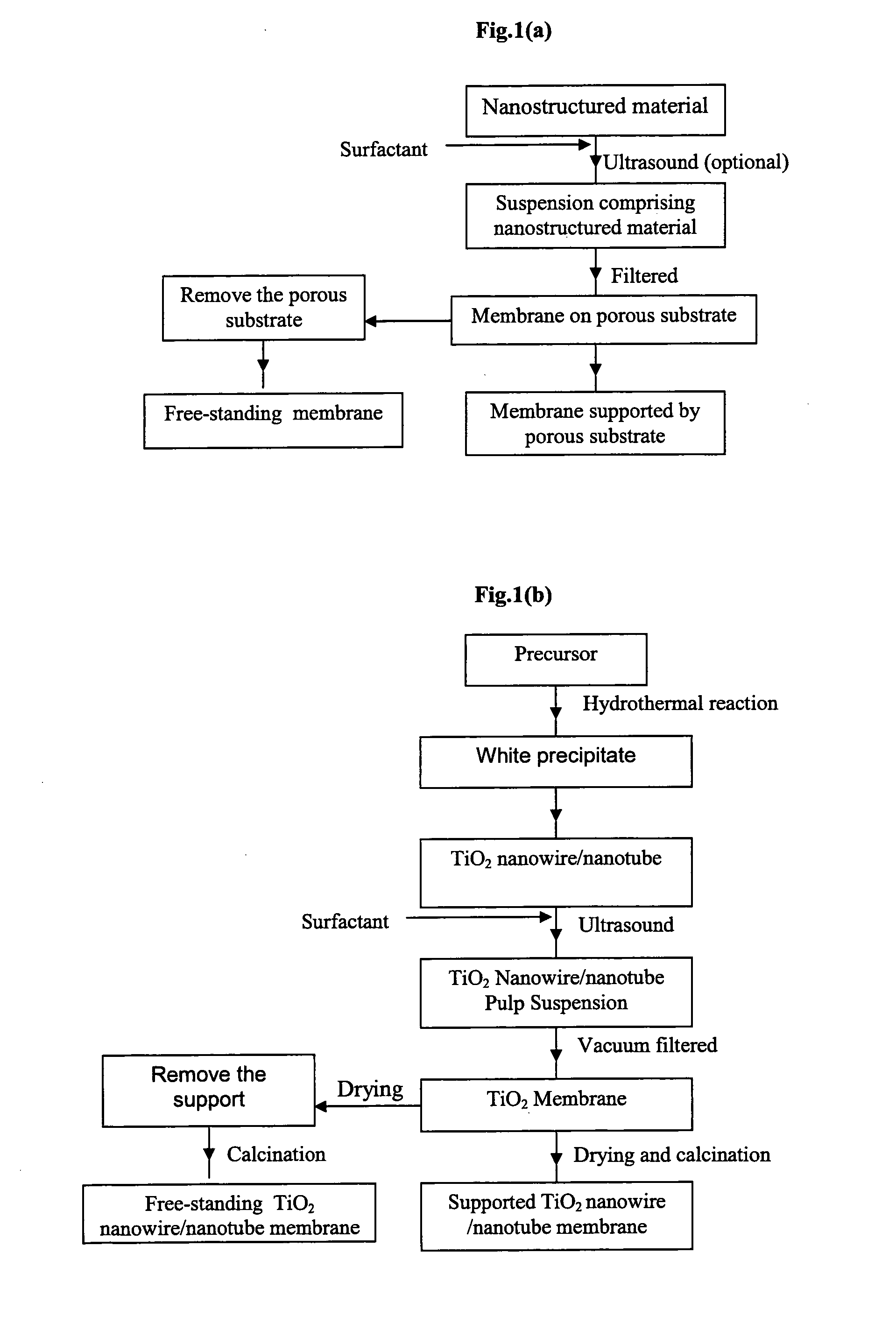
Membrane made of a nanostructured material
Owner:NANYANG TECH UNIV +1
Catalyst, its preparation and use
A process for preparing a catalyst which process comprises preparing a mixture comprising iron oxide and at least one Column 1 metal or compound thereof, wherein the iron oxide is obtained by heating a mixture comprising an iron halide and at least 0.05 millimoles of a Column 6 metal per mole of iron; a catalyst made by the above described process; an iron oxide composition; a process for the dehydrogenation of an alkylaromatic compound which process comprises contacting the alkylaromatic compound with the catalyst; and a method of using an alkenylaromatic compound for making polymers or copolymers, in which the alkenylaromatic compound has been produced by the dehydrogenation process.
Owner:SHELL OIL CO
Methods of Making Binary Metal Oxide Nanostructures and Methods of Controlling Morphology of Same
ActiveUS20100278720A1Reduce crystallinityControl dimensionalityCopper oxides/halidesManganese oxides/hydroxidesPorous membraneNanostructure
The present invention includes a method of producing a crystalline metal oxide nanostructure. The method comprises providing a metal salt solution and providing a basic solution; placing a porous membrane between the metal salt solution and the basic solution, wherein metal cations of the metal salt solution and hydroxide ions of the basic solution react, thereby producing a crystalline metal oxide nanostructure.
Owner:WONG STANISLAUS S +1
Method for making a lithium mixed metal compound
A method for making a lithium mixed metal compound includes: preparing a reactant mixture that contains a metal compound, a lithium compound, and optionally, a phosphate-containing compound; and exposing the reactant mixture to an atmosphere in the presence of suspended carbon particles, and conducting a reduction to reduce oxidation state of at least one metal ion of the reactant mixture at a temperature sufficient to form a reaction product containing lithium and the reduced metal ion.
Owner:AQUIRE ENERGY CO LTD
Method of forming iron oxide core metal shell nanoparticles
InactiveUS7829140B1Narrow size distributionMaterial nanotechnologyPigmenting treatmentWater dispersibleComposite nanoparticles
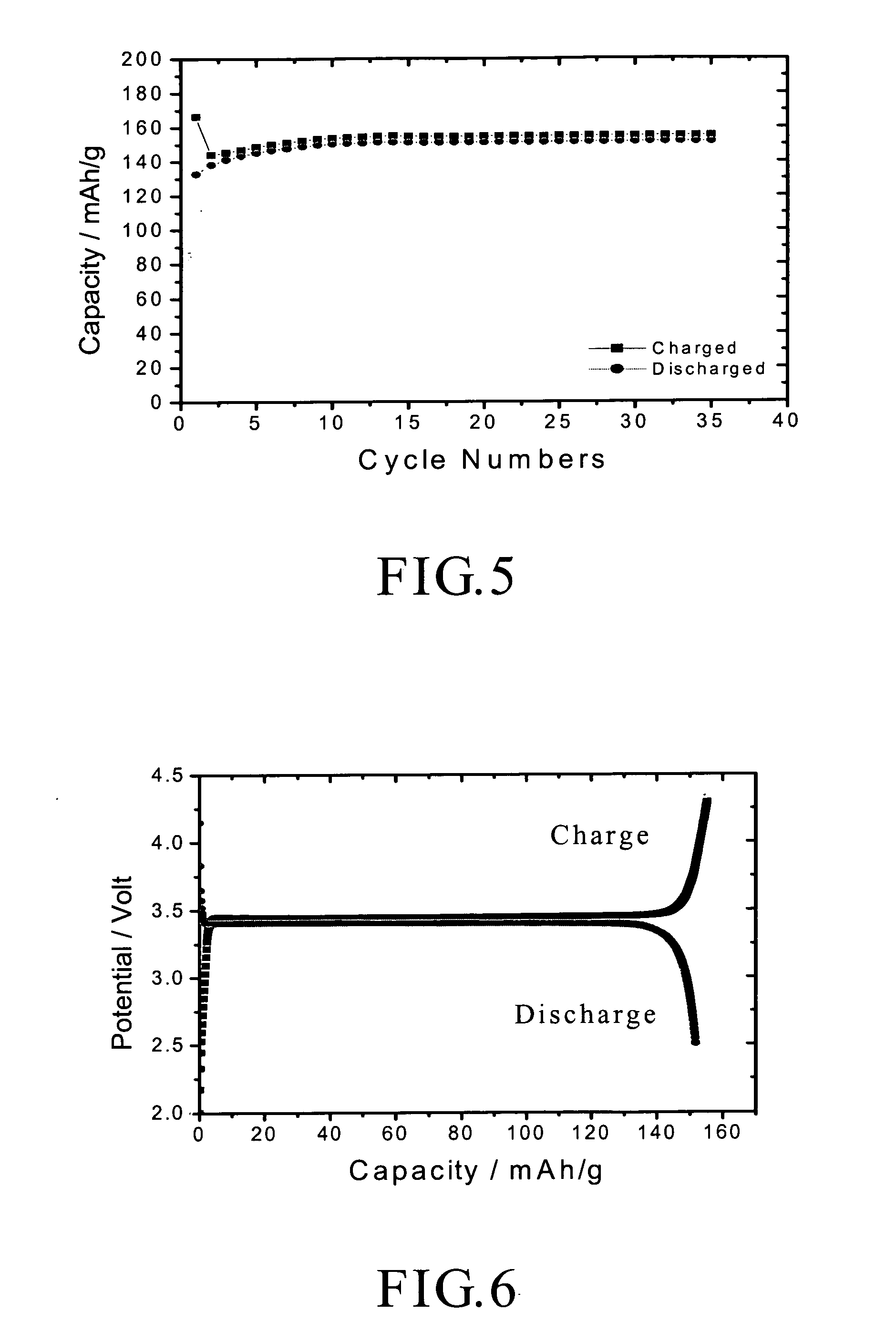
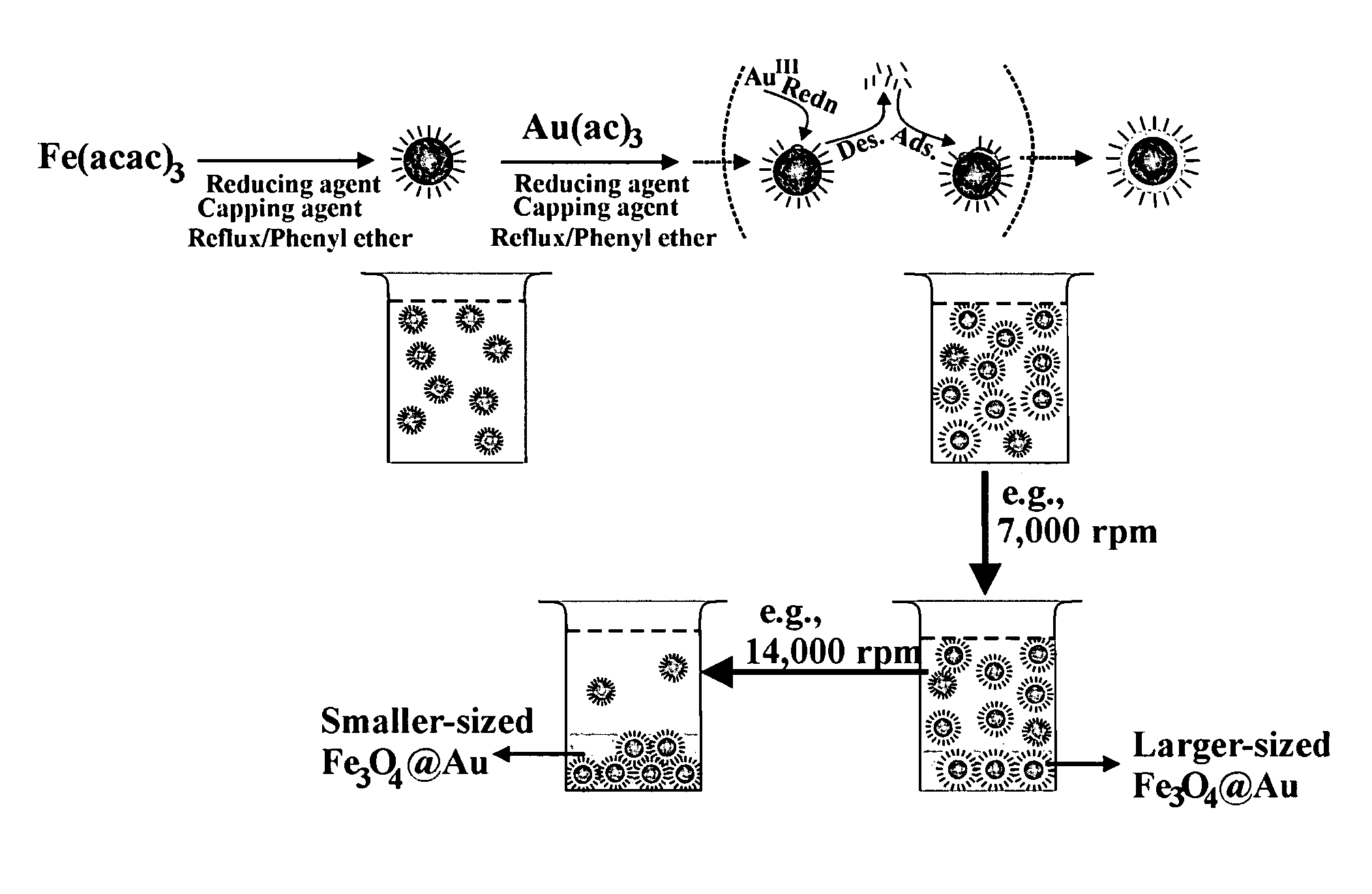
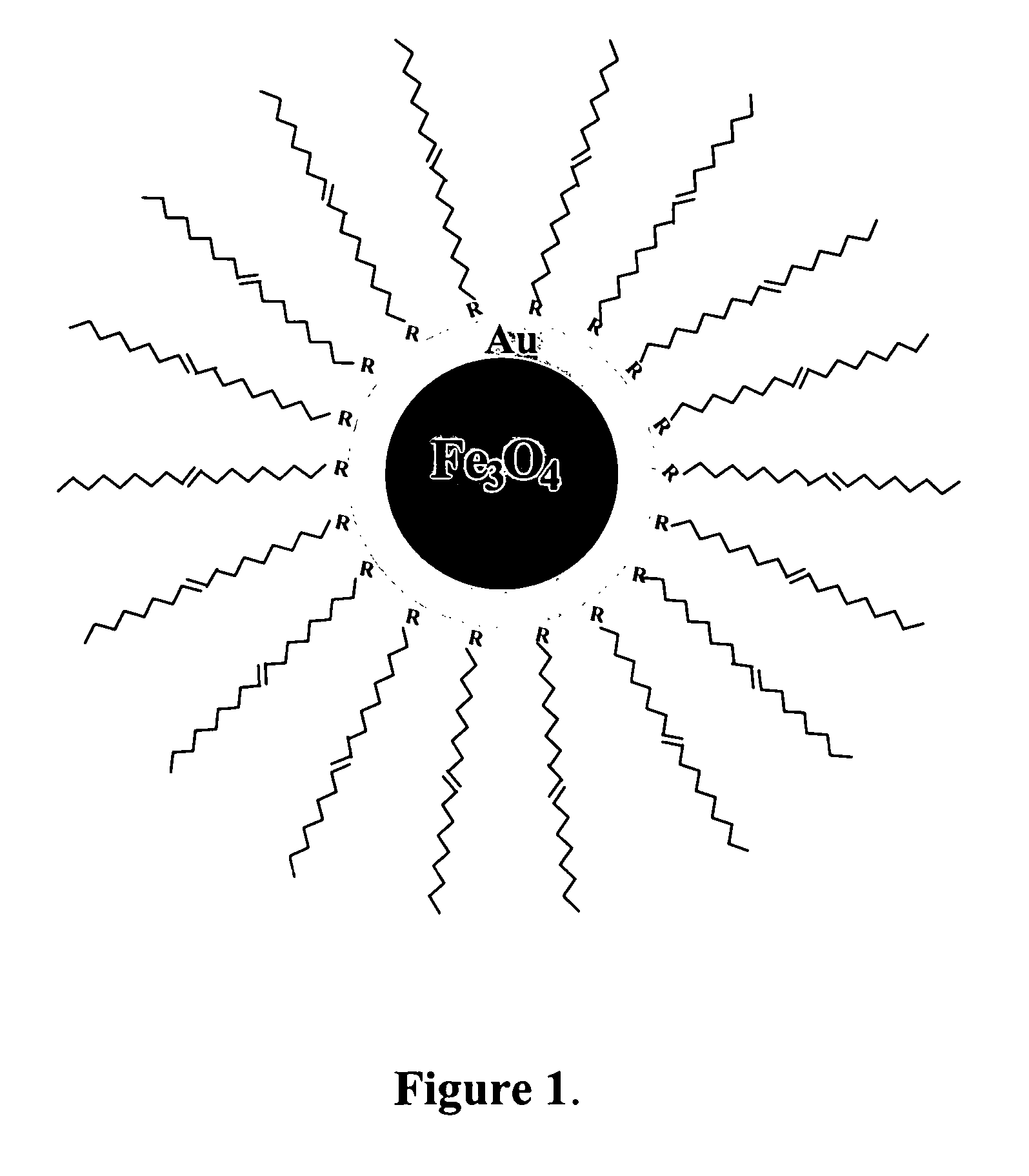
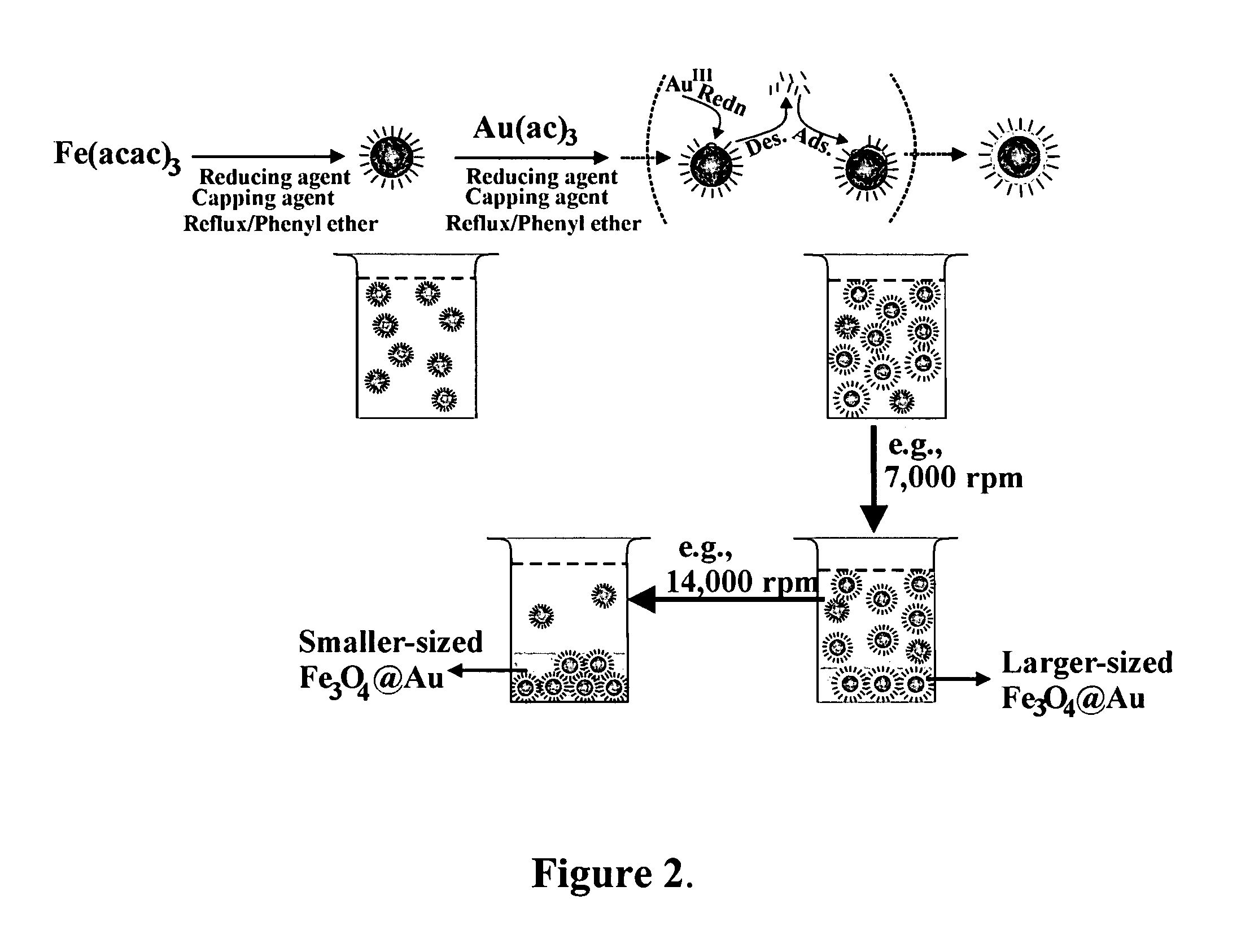
A method of forming mono-disperse iron-oxide core metal shell nanoparticles is disclosed. Particle size of the oxide core seeds is controlled and capped seeds are formed. The capping layer is desorbed by a thermally activated process and metal such as gold is chemically deposited on the core seeds in situ. This process can be repeated to produce multi-metal or different metal shells. A second capping layer is applied on the core / shell composite nanoparticles. In another step, the particles are sized by centrifuging to obtain a tightly controlled and narrow particle size distribution. The water-dispersibility of the particles is achieved by a thiol exchange reaction on the gold shell of the core / shell nanoparticles or by deposition of gold on ferritin-derived iron oxide cores in aqueous solution. Mono and multilayer thin films are assembled on different substrates using the core / shell particles and linking molecules.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Composition for a desulfurizer with a high sulfur capacity and the process of making the same
ActiveUS20080047395A1Quality improvementEasy to operateGas treatmentBlast furnace componentsActive componentLiquid state
The present invention discloses a composition for a desulfurizer with a high sulfur capacity and a process for making the same. The composition comprises the active components of three kinds of iron oxides and is used in the desulfurizer to remove hydrogen sulfide from the gaseous and liquid state feed stocks. The above-mentioned composition comprises cubic ferroferric oxide in the form of crystalline phase (Fe3O4), amorphous ferric oxide (Fe2O3) and amorphous ferric oxide monohydrate (Fe2O3.H2O). The composition has a sulfur capacity of at least 40%. The process for preparing the composition comprises the following steps: (1) mixing a solid ferrous compound with a solid hydroxide at a molar ratio of iron to hydroxyl being in the range from 1:2 to 1:3; (2) kneading the mixture feeds obtained in step (1) and making them react completely; (3) drying the products obtained in step (2) in the air; (4) washing and filtering the feeds obtained in the step (3); (5) naturally drying or baking the solids obtained in step (4) to form a composition for a desulfurizer with a high sulfur capacity. The process of the present invention is simple and easy to operate, consumes less energy and produces the products with a stable quality.
Owner:BEIJING HAIXIN ENERGY TECH CO LTD
Super paramagnetic ferric oxide composite nanometre particle preparation method
InactiveCN1736881AReduce nucleationReduced growth rateNanostructure manufactureFerric oxidesSal ammoniacNanoparticle
The invention discloses a preparation method for composite nano particcle of superparamagnetic ferric oxide. Wherein, adding ammonia and sodium citrate synchronously and speed controlled to make ions of Fe3+ and Fe2+ coprecipitate and form Fe3O4 nano particle with water-phase dispersion surface adsorbed by citric acid radical that can be substituted by multi chemical functional group on basic condition as crystal seed for sodium citrate-goden chloric acid reduction reaction and form SPION nano particle.
Owner:HUAZHONG UNIV OF SCI & TECH
Aerogel micro powder and preparation method thereof
InactiveCN108689412AImprove drying efficiencyShorten freeze-drying timeChemical industryFerric oxidesPorosityOrganic solvent
The invention discloses aerogel micro powder and a preparation method thereof. The method comprises the following steps: a hydrogel is provided, and fragmentation is carried out on the hydrogel and dispersing the hydrogel to obtain gel fluid or gel dispersion liquid; an atomizing device is employed for performing atomization on the gel fluid or the gel dispersion liquid, gel droplets are formed, the gel droplets are collected by a low-temperature container, and the gel freezing particles are obtained; the freeze drying is carried out, and the aerogel micro powder is obtained. The aerogel micropowder can realize integration of granulation and refrigeration, pre-refrigeration is not required, rapid and continuous production of the aerogel micro powder can be realized, organic solvent displacement is not required, the preparation method has the advantages of simple process, short production period, low cost, energy saving, and environmental protection, the preparation process can be amplified to industrial production, and the obtained aerogel micro powder has the excellent physical properties of controllable morphology, uniform particle size, large specific surface area, high porosity amount, and low thermal conductivity.
Owner:SUZHOU INST OF NANO TECH & NANO BIONICS CHINESE ACEDEMY OF SCI
Carbon aerogel/metal oxide composite material and preparation method and application thereof
ActiveCN107134373AEvenly dispersedIncrease contentHybrid capacitor electrodesFerric oxidesCross-linkPorous carbon
The invention discloses carbon aerogel / metal oxide composite material and a preparation method and an application thereof. A cross-linking agent and soluble metal salt are added in a high-molecular aqueous solution so that the inorganic nanoparticles are enabled to be precipitated and dispersed in the organic hydrogel in an in-situ way and the organic and inorganic composite hydrogel can be obtained, a unidirectional porous structure is formed by the hydrogel through multistage freezing and drying and then carbonized and thus the nitrogen-containing multistage porous carbon aerogel / metal oxide composite material is obtained; or the hydrogel is put in the atmosphere of ammonia to be mineralized and then dried under the normal temperature and finally carbonized under the nitrogen protection so that the nitrogen-containing multistage porous carbon aerogel / metal oxide composite material is obtained. The two originally relatively independent stages of carbon aerogel material predation and subsequent metal oxide generation are effectively combined so that the uniform composite characteristic of the inorganic and organic nanoscale in the in-situ precipitation method can be taken, the multistage freezing and drying pore forming function is also developed and the preparation steps can be greatly simplified.
Owner:WUHAN UNIV
Method for processing fly ash used for circulating fluidized bed
ActiveCN104445212ASolve environmental pollutionHarm reductionChlorine/hydrogen-chlorideSilicaAluminium hydroxideHydrolysis
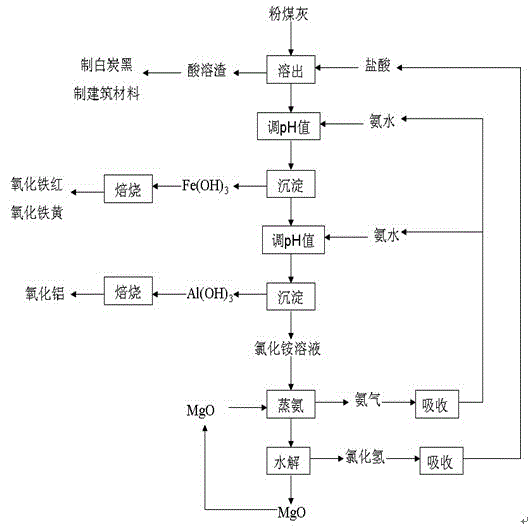
The invention relates to a method for processing fly ash used for a circulating fluidized bed, which comprises the following steps: dissolving fly ash from the circulating fluidized bed out by hydrochloric acid, filtering to obtain filter residue and a leaching liquid; adjusting pH value of the leaching liquid to 1.5-3.5 by ammoniacal liquor, deposing, filtering to obtain the filter residue and a filtrate, washing filter residue, drying to obtain a compound of iron; adjusting the pH value of the filtrate after extracting iron to 3.8-5.2 by the ammoniacal liquor, deposing, filtering to obtain the filter residue and the filtrate, washing filter residue, drying to obtain aluminium hydroxide, further calcining to obtain metallurgy alumina; adding magnesium oxide in an ammonium chloride solution after extracting aluminium and performing ammonia distilling and hydrolysis reactions to obtain hydrogen chloride gas, ammonia gas and magnesium oxide. According to the invention, stepwise extraction of useful elements such as aluminium, silicon and iron in fly ash can be realized, cycle utilization of the materials can be realized, process is simple, cost is low, and the method opens new approach for high value utilization of fly ash from the circulating fluidized bed.
Owner:GUIYANG AL-MG DESIGN & RES INST
Surface-modified titanium dioxide fine particles and dispersion comprising the same, and method for producing the same
InactiveUS20060264520A1Good dispersionGood dispersibilityAntibacterial agentsHeavy metal active ingredientsWater solubleMicroparticle
Surface-modified titanium dioxide particles which have a surface chemically modified with a hydrophilic polymer, wherein a carboxyl group of the hydrophilic polymer and titanium dioxide are bound through an ester bonding; and a method for producing the surface-modified titanium dioxide fine particles, which comprises mixing a dispersion comprising titanium dioxide fine particles having a particle size of 2 to 200 nm and a solution of a water-soluble polymer, heating the resultant mixture to a temperature of 80 to 220° C., to thereby bind both the components through an ester bonding, and removing an unbound water-soluble polymer, to purify the resuultant particles. The surface-modified titanium dioxide fine particles exhibit excellent dispersibility and stability in an aqueous solvent over a wide pH region including a neutral range.
Owner:STARBOARD TECH +1
Method for extracting aluminum oxide, monox and ferric oxide from gangue combustion ashes
InactiveCN101913632AEfficient use ofMeet steamSilicaCalcium/strontium/barium sulfatesBiological activationCoal blending
The invention relates to a method for extracting aluminum oxide, monox and ferric oxide from gangue combustion ashes. Coal blending is carried out according to heating value conditions after the gangue is pretreated, gangue activation is achieved while calcination generation is carried out, and the produced electricity and steam are supplied for a system to use; the aluminum oxide is extracted from the ashes by an acid method, the monox is extracted by an alkaline method, and the ferric oxide is extracted by comprehensive utilization of by-products; and acid, alkaline, lime, an extractant andCO2 which are required by each craft link are recycled inside the system. The invention has the beneficial effects that gangue reclamation is implemented by using the method; the energy and the chemical components in the gangue are fully utilized; the discharge amounts of greenhouse gases and waste ashes are greatly reduced, and the economic benefit of the system is improved; and the method is a novel technology of gangue greening and high added-value utilization, and has obvious competitive advantage.
Owner:KUNMING UNIV OF SCI & TECH +1
Textile composite with iron oxide film
A colored textile composite is produced by forming an iron (III) oxide film on a textile surface. This is accomplished by contacting the textile with an aqueous solution having an iron (II) or iron (III) species present. The iron (II) ion resulting from the dissociated iron (II) salt, if an iron (II) salt is utilized, is first hydrolyzed within the aqueous solution and then oxidized under controlled conditions to form iron (III) oxide (hydroxide). The iron (III) ion resulting from the dissociated iron (III) salt, if an iron (III) salt is utilized, is only hydrolyzed under controlled condition to form iron (III) oxide (hydroxide). The iron (III) oxide is then nucleated and forms a smooth and coherent iron (III) oxide film or coating on the surface of the textile without forming an insoluble iron (III) hydroxide precipitate in the solution. This reaction occurs because the reaction conditions are controlled in such a manner as to form sub-colloidal sized iron oxide particles which, in turn, permits a faster rate of adsorption of the iron (III) oxides onto the substrate surface than the rate of formation of the same particles. The iron (III) oxide formed may be goethite, hematite, or magnetite or any mixture thereof. Varying the type of oxide formed allows control over the color shade and other properties of the treated textile composite.
Owner:MILLIKEN & CO
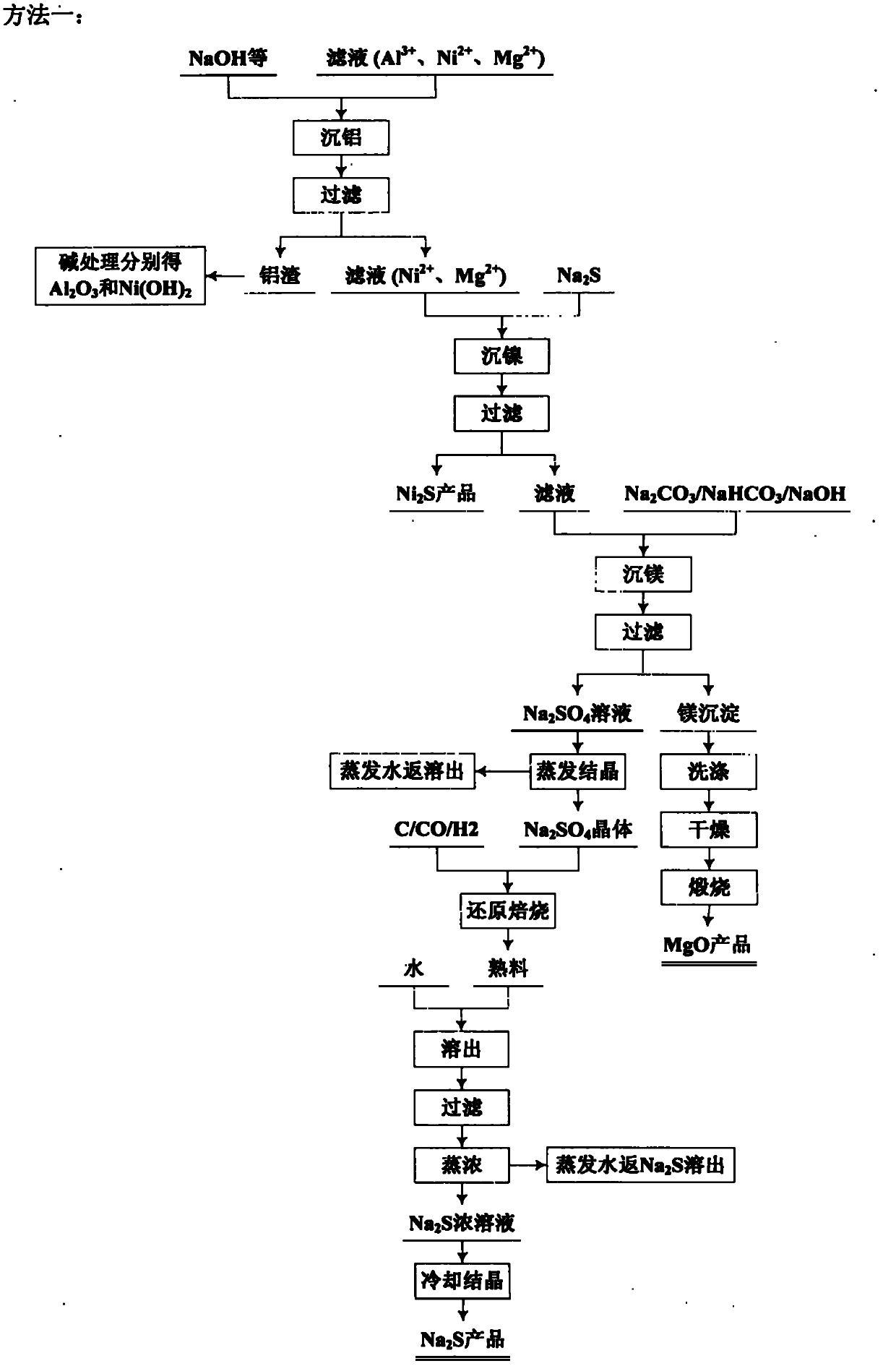
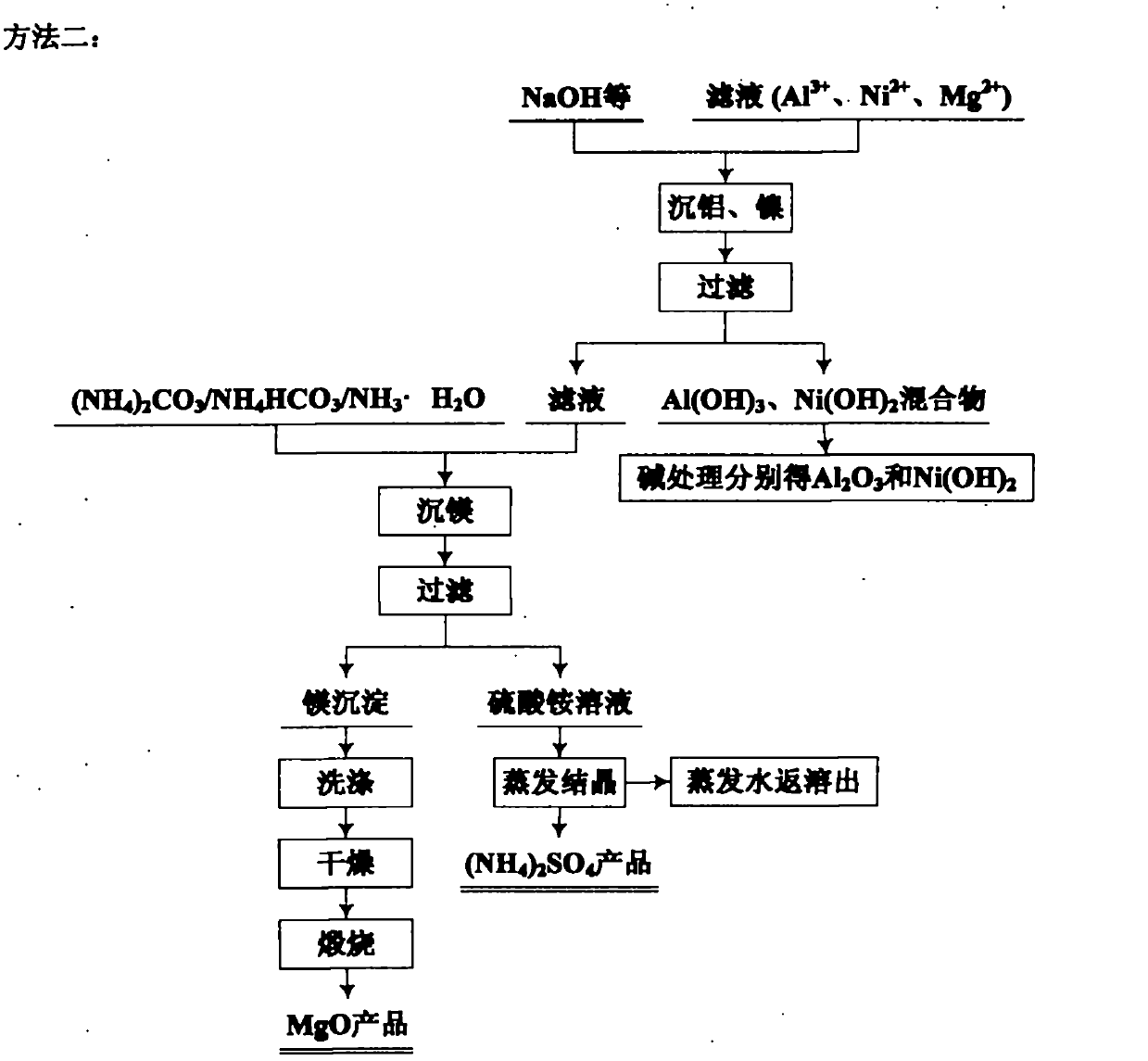
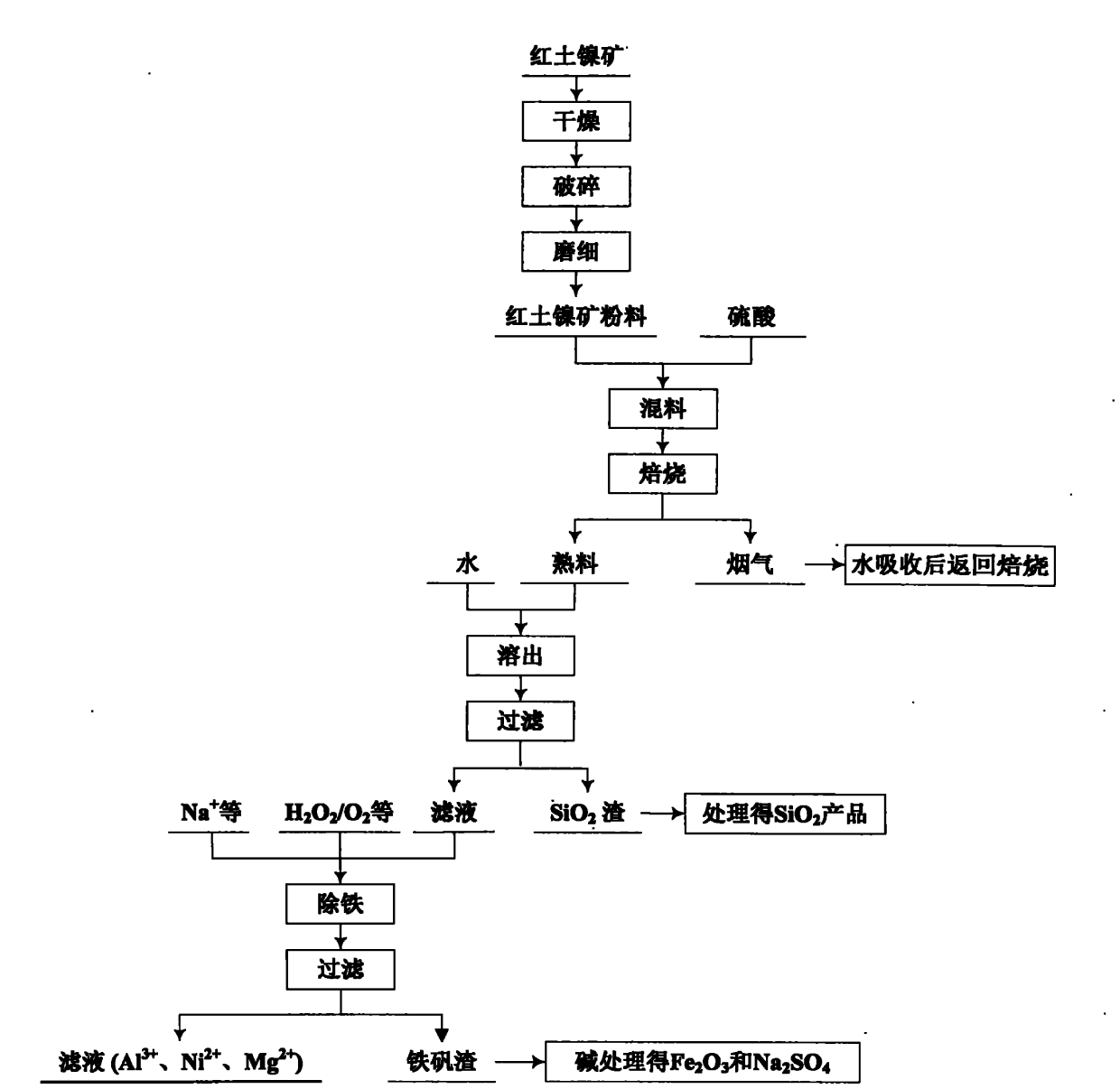
Comprehensive utilization method for laterite-nickel ore
The invention relates to an environmental-friendly comprehensive utilization method for a laterite-nickel ore, which comprises the following steps of: (1) grinding the laterite-nickel ore, mixing with sulfuric acid, roasting, dissolving out roasted clinker and filtering to obtain silicon dioxide and dissolution liquid; (2) deironing the dissolution liquid to obtain liquid No.2 and filter residue (iron compounds), wherein the liquid No.2 comprises aluminum, nickel and magnesium and can be treated by the step (3) or (4); (3) precipitating the aluminum in the liquid No.2 by using alkali, filtering, precipitating the nickel in filtrate by using sodium sulfide, filtering, precipitating the magnesium by using the alkali, and treating filter residue to obtain aluminum oxide, nickel hydroxide, nickel sulfide and magnesium oxide respectively; and (4) precipitating the aluminum and the nickel in the liquid No.2 by using the alkali, treating mixed slag containing the aluminum and the nickel by using the alkali to obtain aluminum hydroxide and nickel hydroxide products, and precipitating the magnesium in filtrate subjected to aluminum and nickel precipitation by using ammonia or ammonium saltto obtain a magnesium oxide product. The method is suitable for treating various laterite-nickel ores, three wastes (waste gas, waste water and waste residue) are not generated, and valuable components magnesium, nickel, iron, aluminum and silicon in the laterite-nickel ore are separated and extracted.
Owner:NORTHEASTERN UNIV
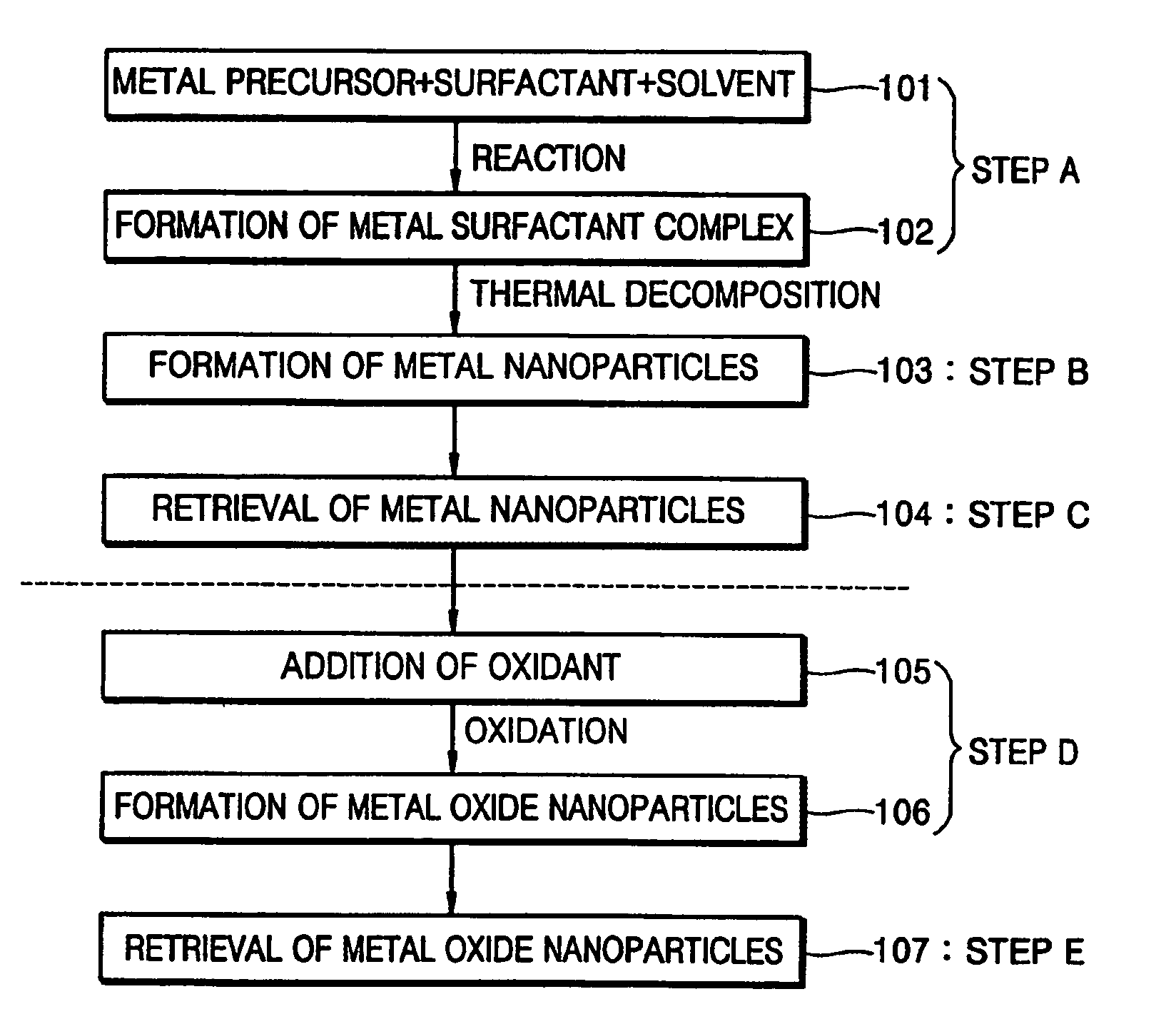
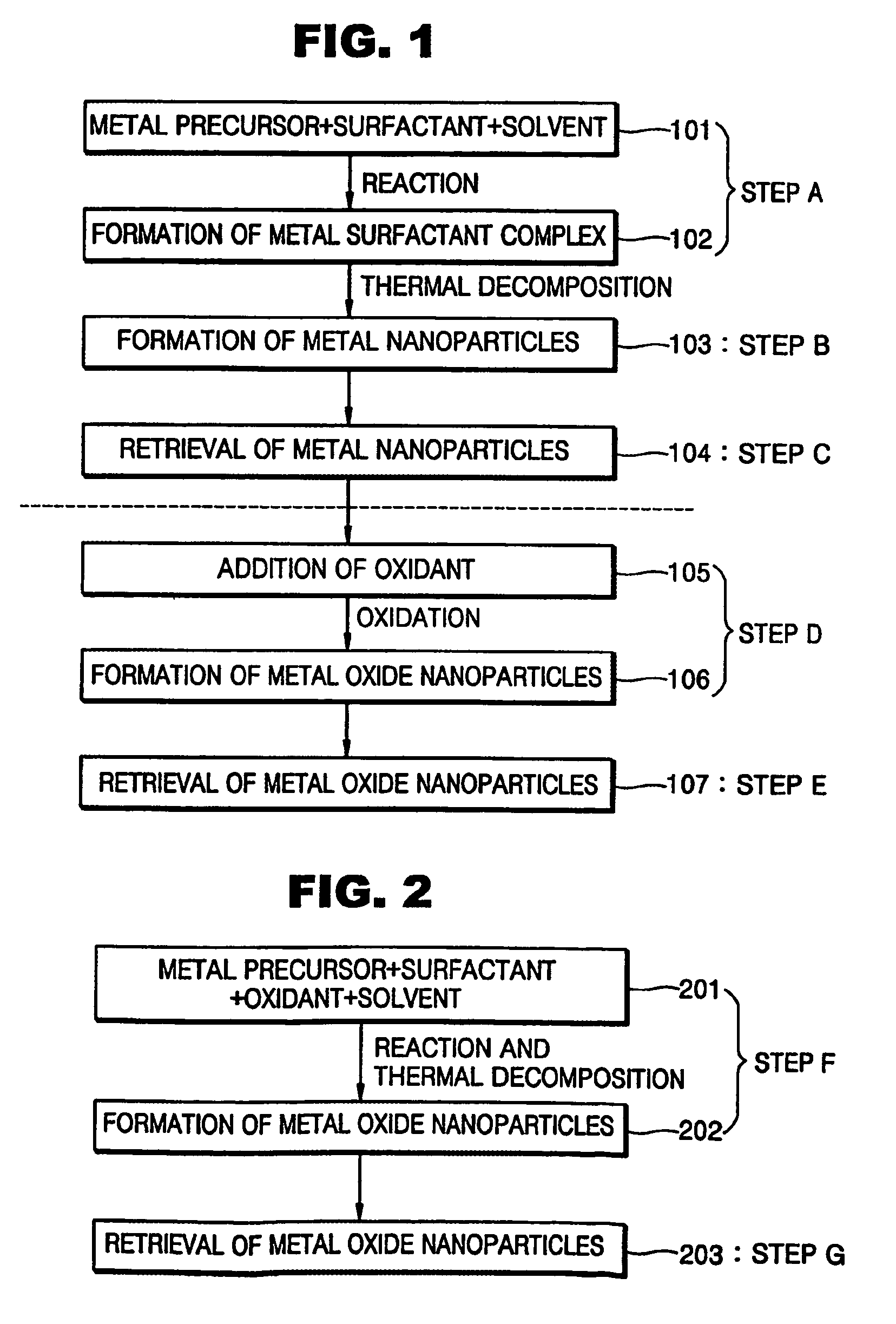
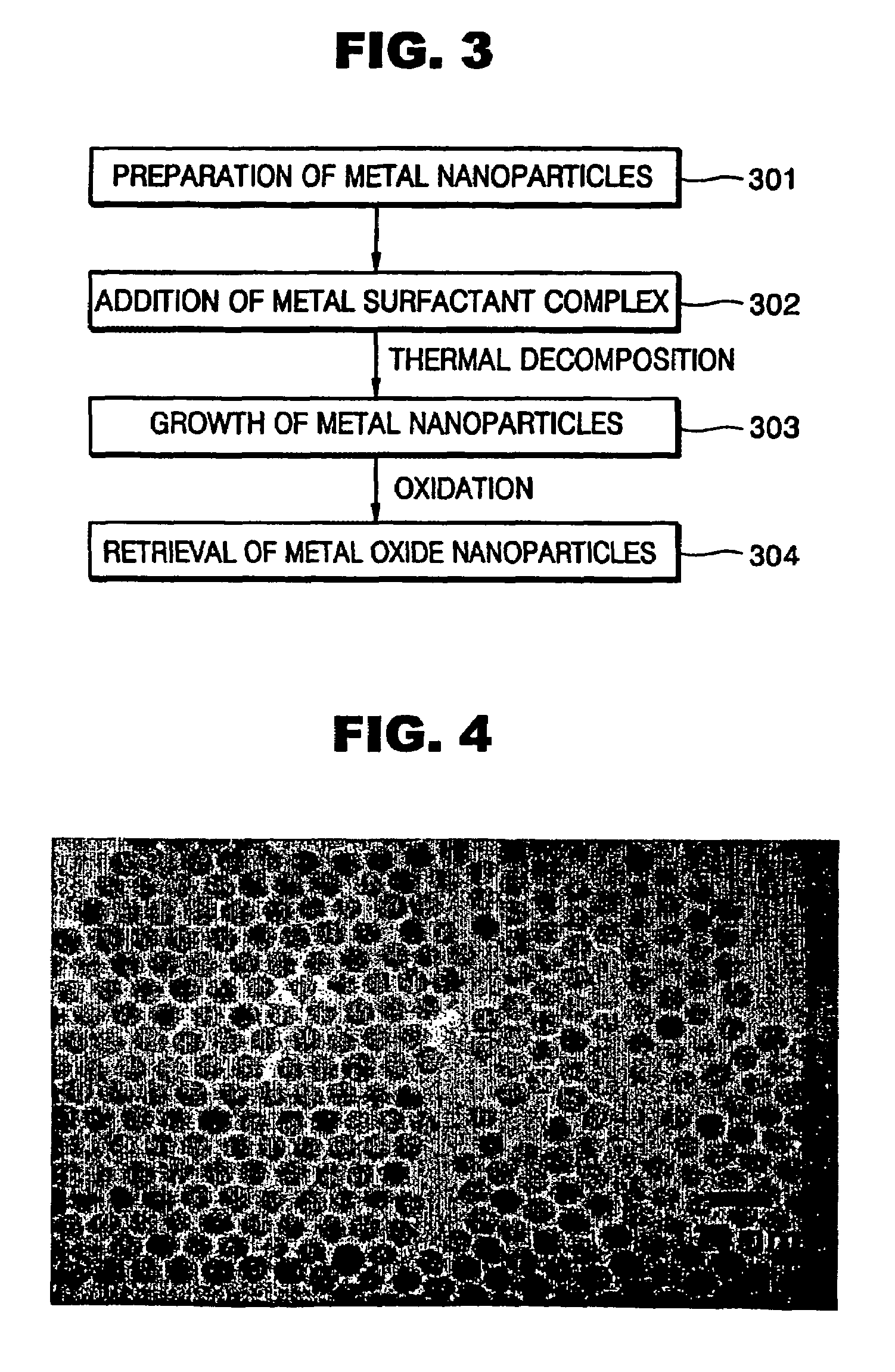
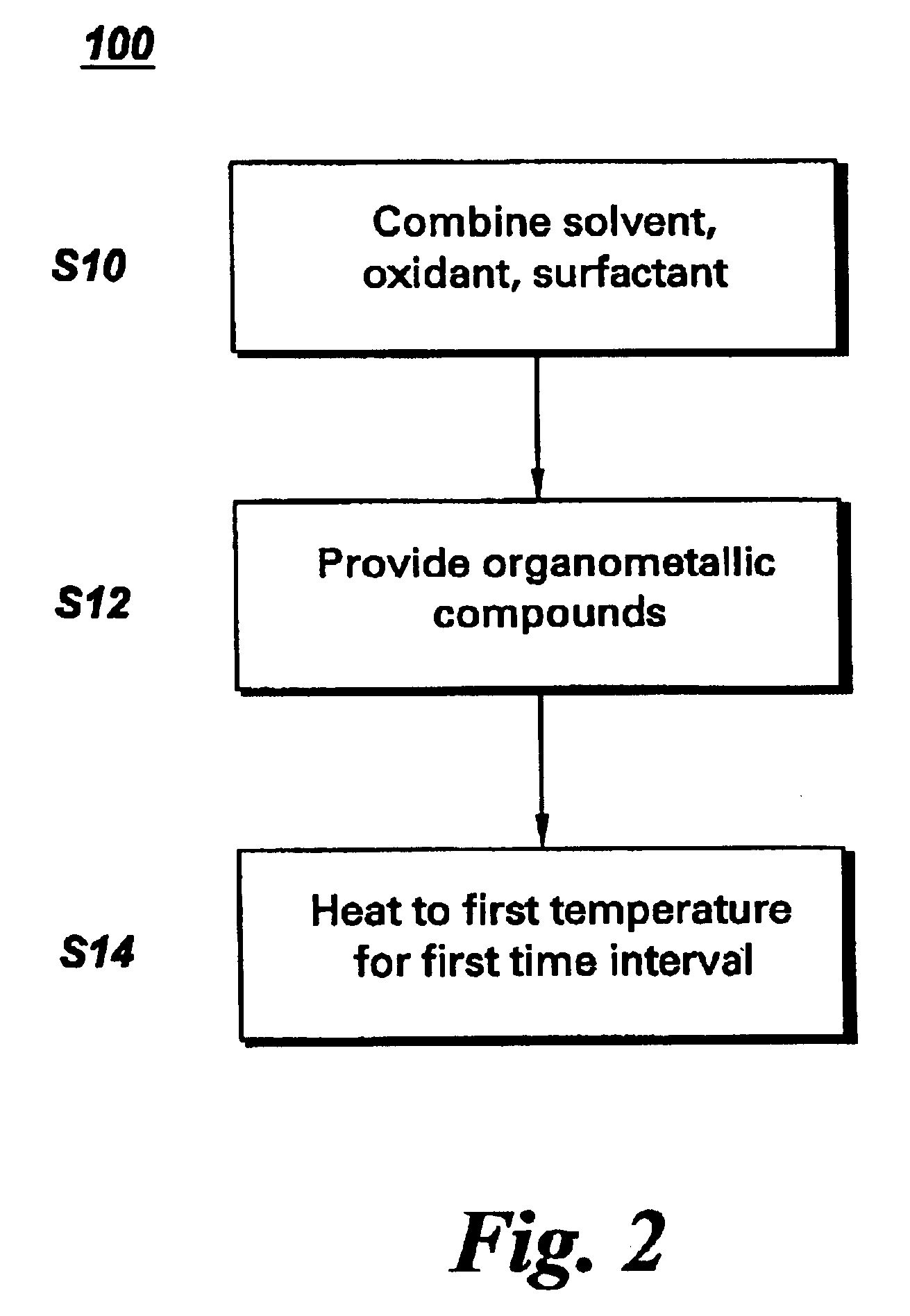
Synthesis of mono-disperse and highly crystalline nano-particles of metals, alloys, metal-oxides, and multi-metallic oxides without a size-selection process
InactiveUS7407527B2Sufficient reaction timeInduce precipitationMaterial nanotechnologyOxygen/ozone/oxide/hydroxideMetal oxide nanoparticlesSynthesis methods
A synthetic method of fabricating highly crystalline and monodisperse nanoparticles of metals, multi-metallic alloys, monometallic oxides and multi-metallic oxides without a size selection process are disclosed. A typical synthetic method comprises the steps of, synthesis of a metal surfactant complex from the reaction of a metal precursor and a surfactant, high temperature thermal decomposition of the metal surfactant complex to produce monodisperse metal nanoparticles, and completing the formation of synthesized metal, metal alloy or metal oxide nanoparticles by adding a poor solvent followed by centrifuging. For obtaining highly crystalline monodisperse nanoparticles, additional steps are necessary as described in the invention. The resulting nanoparticles have excellent magnetic property for many applications.
Owner:SEOUL NAT UNIV R&DB FOUND
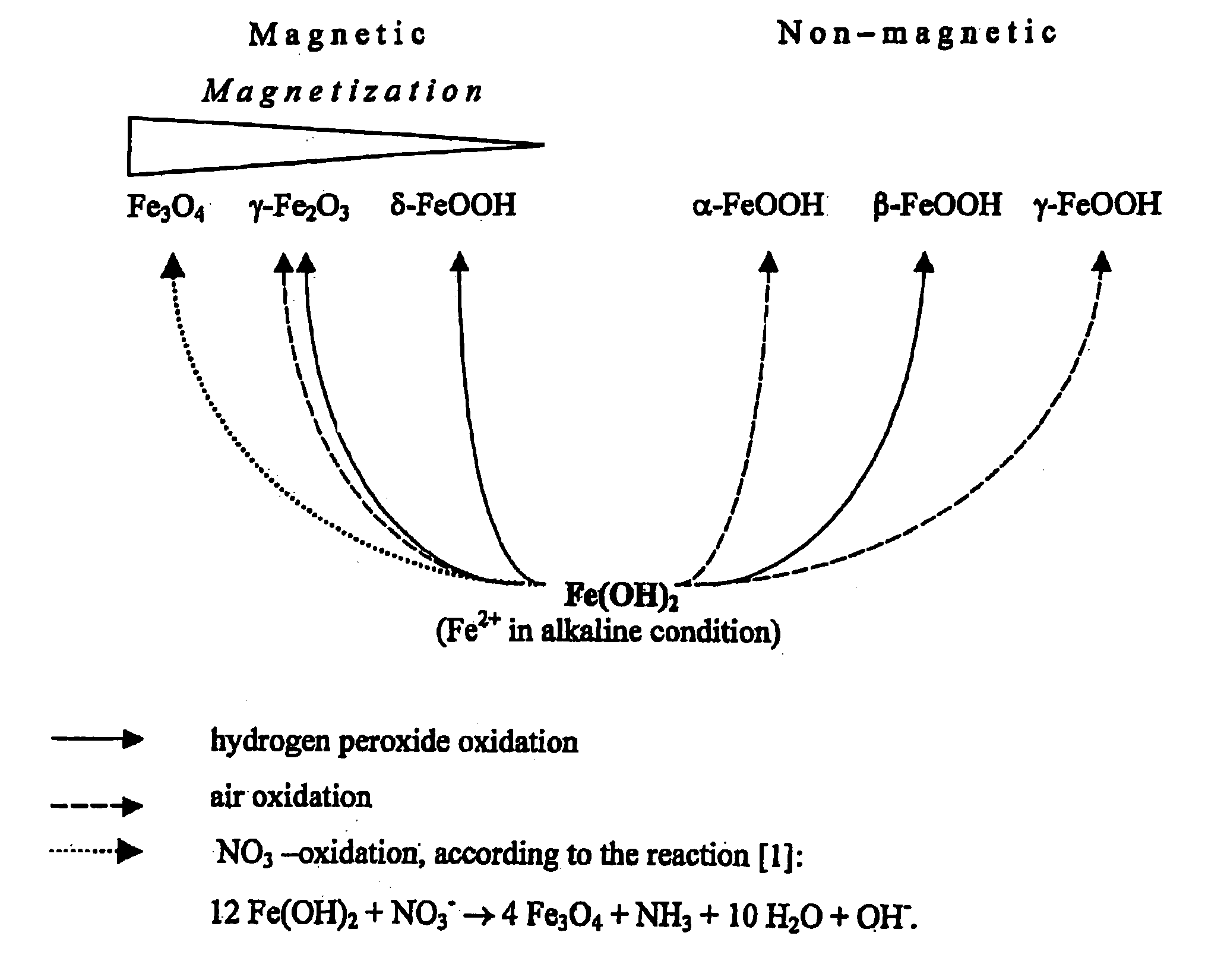
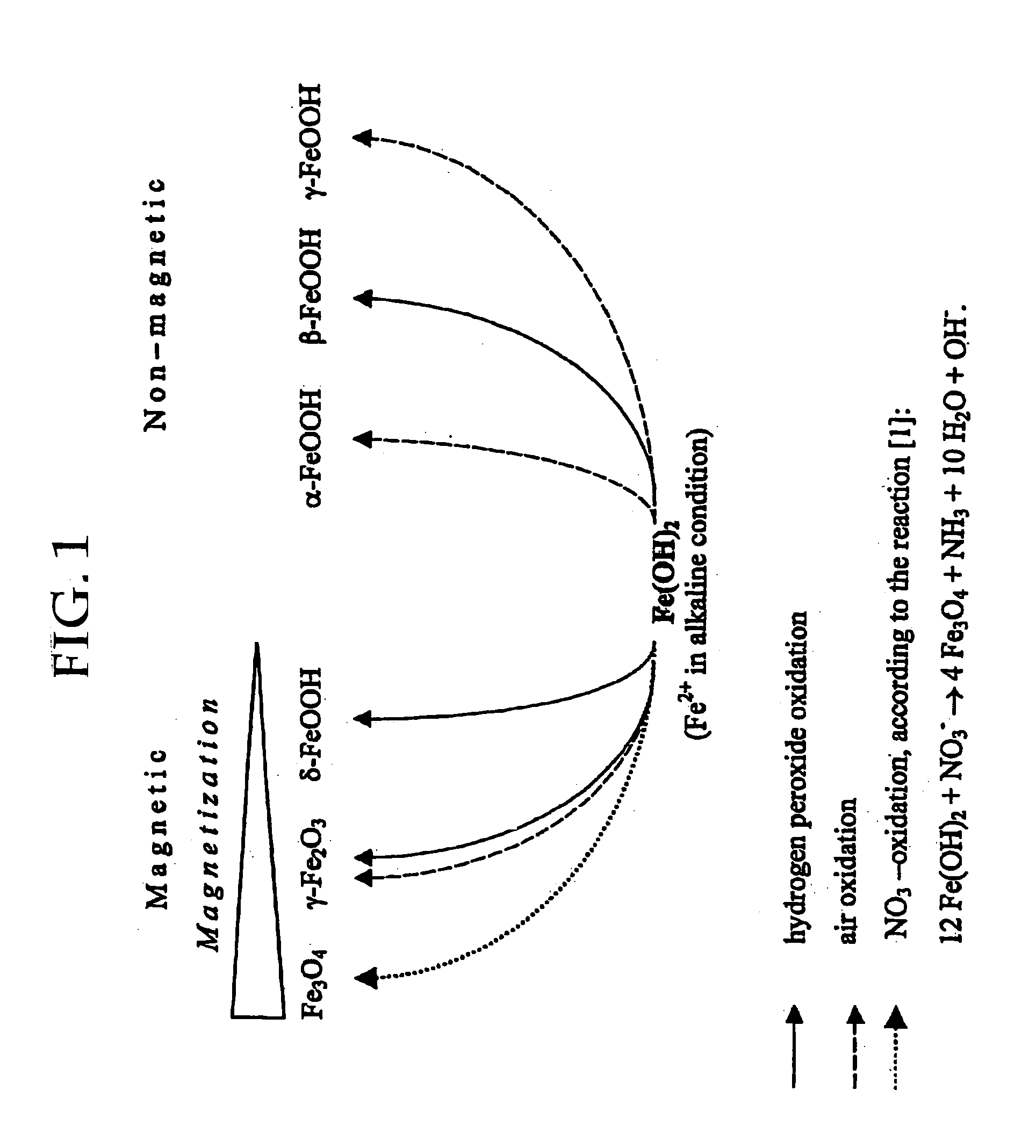
Formation of superparamagnetic particles
InactiveUS20050019755A1Efficient removalImprove conductivityNanomagnetismMicrobiological testing/measurementCross-linkNitrate
The present invention features a method for preparing superparamagnetic iron particles by the in situ formation of these particles in a cross-linked starch matrix or by the formation of a superparamagnetic chitosan material. The superparamagnetic materials are formed by mild oxidation of ferrous ion, either entrapped into a cross-linked starch matrix or as a chitosan-Fe(II) complex, with the mild oxidizing agent, nitrate, under alkaline conditions. The present invention further features superparamagnetic iron compositions prepared by the method of the invention. The compositions of the invention are useful for the separation, isolation, identification, or purification of biological materials.
Owner:MARCHESSAULT ROBERT H +5
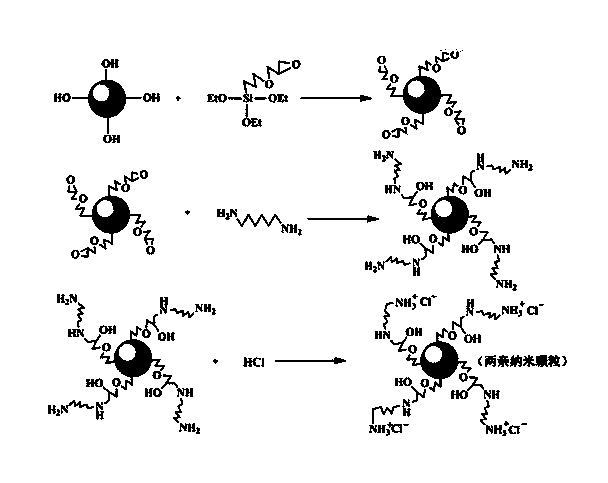
Preparation method for amphiphilic nanometer particle and application of amphiphilic nanometer particle to prepare Pickering emulsion
ActiveCN104016361ASimple preparation processImprove stabilityMaterial nanotechnologySilicaEmulsion polymerizationPickering emulsion
The invention relates to a preparation method for an amphiphilic nanometer particle and application of amphiphilic nanometer particle to prepare Pickering emulsion. The novel amphiphilic nanometer particle is formed according to the following preparation method: firstly performing epoxy-group modification on the surface of a nanometer particle by employing a silane coupling agent, then utilizing hexylenediamine to react with epoxy groups, so as to further introduce amino groups, and finally adding proper amount of hydrochloric acid for neutralization. The particle is capable of replacing conventional surfactants for preparing highly-stable Pickering emulsions of silicone oil / water and or toluene / water. The amphiphilic nanometer particle is simple in preparation technology, good in stability and uneasy for being influenced by temperature, pH value, salinity and other factors, and therefore the amphiphilic nanometer particle possesses great application prospect and industrialization capability. The preparation method of the emulsion can be further expanded for preparing different oil / water emulsions, and even has potential application prospect in emulsion polymerization field.
Owner:SKSHU PAINT
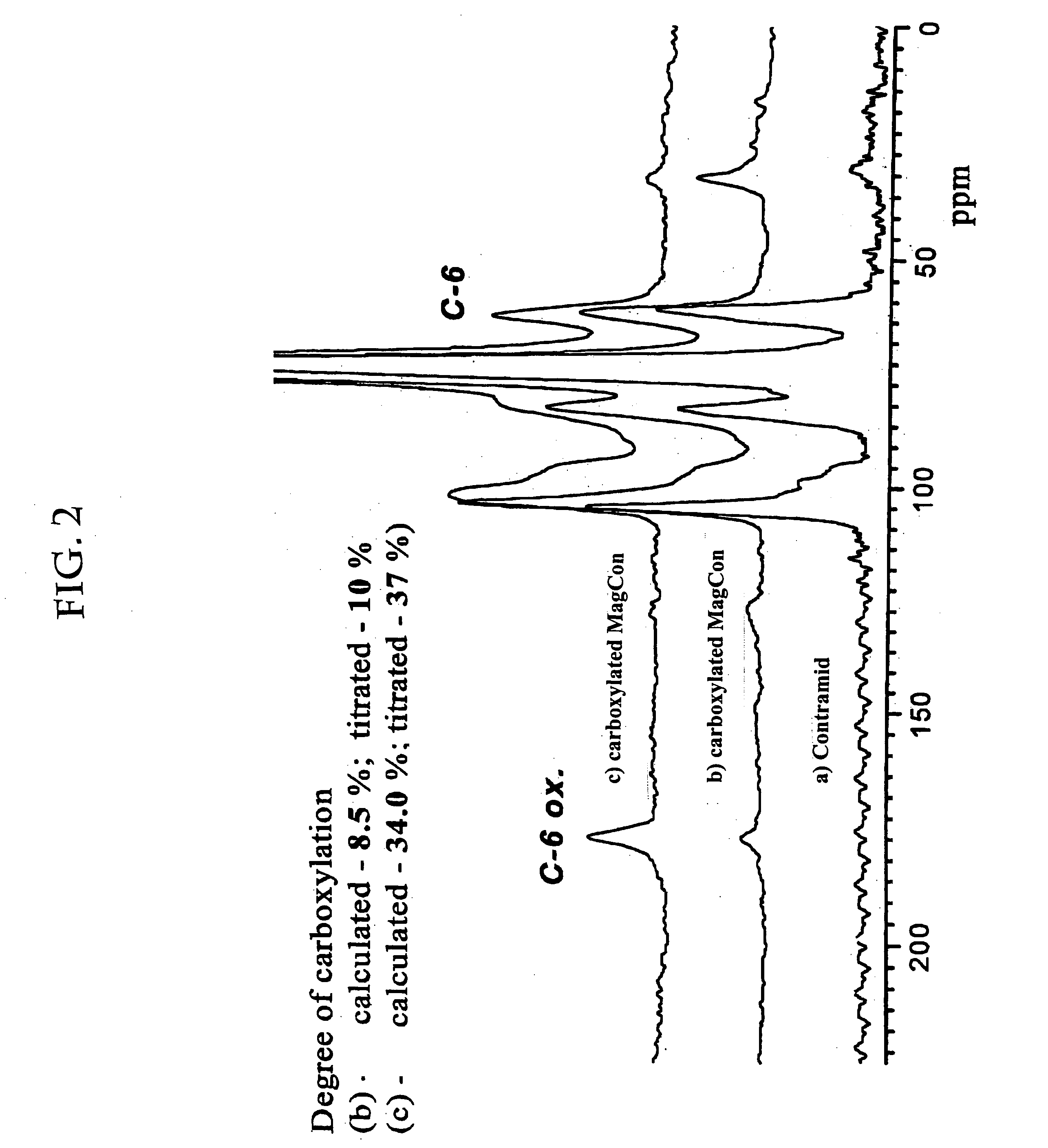
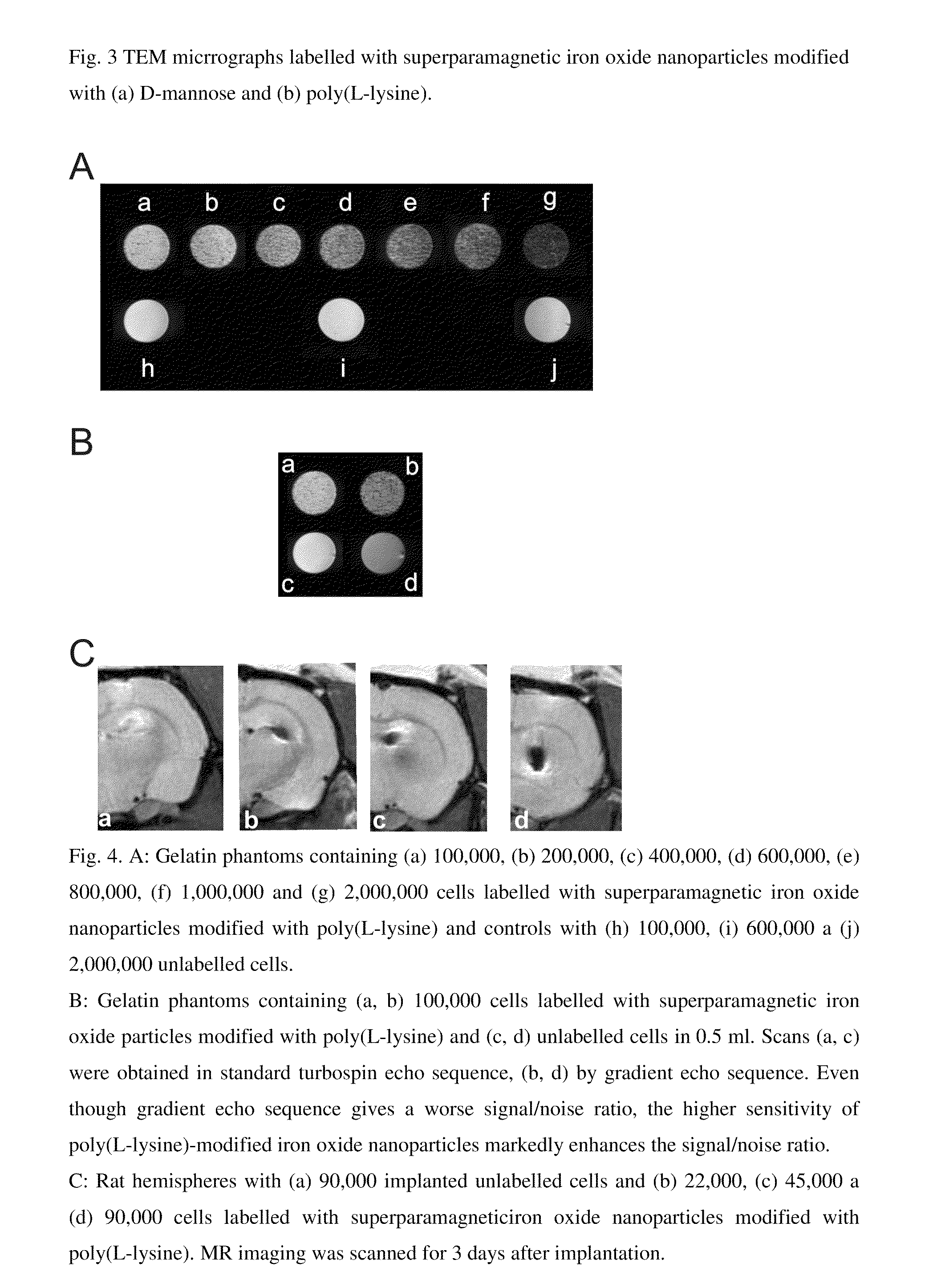
Superparamagnetic Nanoparticles Based on Iron Oxides with Modified Surface, Method of Their Preparation and Application
InactiveUS20090309597A1Less loadImprove abilitiesPigmenting treatmentMaterial nanotechnologyArginineDextran
The subject of the invention is superparamagnetic nanoparticle probes based on iron oxides, to advantage magnetite or maghemite, with modified surface, coated with mono-, di- or polysaccharides from the group including D-arabinose, D-glucose, D-galactose, D-mannose, lactose, maltose, dextrans and dextrins, or with amino acids or poly(amino acid)s from the group including alanine, glycine, glutamine, asparagine, histidine, arginine, L-lysine, aspartic and glutamic acid or with synthetic polymers based on (meth)acrylic acid and their derivatives selected from the group containing poly(N,N-dimethylacrylamide), poly(N,N-dimethylmethacrylamide), poly(N,N-diethylacrylamide), poly(N,N-diethylmethacrylamide), poly(N-isopropylacrylamide), poly(N-isopropylmethacrylamide), which form a colloid consisting of particles with narrow distribution with polydispersity index smaller than 1.3, the average size of which amounts to 0.5-30 nm, to advantage 1-10 nm, the iron content is 70-99.9 wt. %, to advantage 90 wt. %, the modification agent content 0.1-30 wt. %, to advantage 10 wt. %.The particles of size smaller than 2 nm with polydispersity index smaller than 1.1 can be obtained by a modified method of preparation.Superparamagnetic nanoparticle probes according to the invention are prepared by pre-precipitation of colloidal Fe(OH)3 by the treatment of aqueous 0.1-0.2M solution of Fe(III) salt, to advantage FeCl3, with less than an equimolar amount of NH4OH, at 21° C., under sonication, to which a solution of a Fe(II) salt, to advantage FeCl2, is added in the mole ratio Fe(III) / Fe(II)=2 under sonication and the mixture is poured into five- to tenfold, to advantage eightfold, molar excess of 0.5M NH4OH. The mixture is left aging for 0-30 min, to advantage 15 min, and then the precipitate is repeatedly, to advantage 7-10 times, magnetically separated and washed with deionized water. Then 1-3 fold amount, to advantage 1.5 fold amount, relative to the amount of magnetite, of 0.1 M aqueous solution of sodium citrate is added and then, dropwise, 1-3 fold amount, to advantage 1.5 fold amount, relative to the amount of magnetite, of 0.7 M aqueous solution of sodium hypochlorite. The precipitate is repeatedly, to advantage 7-10 times, washed with deionized water under the formation of colloidal maghemite to which, after dilution, is added dropwise, to advantage under 5-min sonication, an aqueous solution of a modification agent, in the weight ratio modification agent / iron oxide=0.1-10, to advantage 0.2 for amino acids and poly(amino acid)s and 5 for saccharides.The particles smaller than 2 nm with polydispersity index smaller than 1.1 are prepared by mixing at 21° C. 1 volume part of 10-60 wt. %, to advantage 50 wt. %, of an aqueous solution of a saccharide, disaccharide or polysaccharide, such as D-arabinose, D-glucose, D-galactose, D-mannose, lactose, maltose, dextran and dextrins, and 1 volume part of aqueous solution of a Fe(II) and Fe(III) salt, to advantage FeCl2 and FeCl3, where the molar ratio Fe(III) / Fe(II)=2. A 5-15%, to advantage 7.5%, solution of NH4OH is added until pH 12 is attained and the mixture is heated at 60° C. for 15 min. The mixture is then sonicated at 350 W for 5 min and then washed for 24 h by dialysis in water using a membrane with molecular weight cut-off 14,000 until pH 7 is reached. The volume of solution is reduced by evaporation so that the final dry matter content is 50-100 mg / ml, to advantage 80 mg per 1 ml.Superparamagnetic nanoparticle probes according to the invention can be used for labelling cells used in magnetic resonance imaging for monitoring their movement, localization, survival and differentiation especially in detection of pathologies with cell dysfunction and of tissue regeneration and also for labelling and monitoring cells administered for cell therapy purposes, in particular embryonal stem cells, fetal stem cells, stem cells of an adult human including bone marrow stem cells, olfactory glial cells, fat tissue cells, in the recipient organism by magnetic resonance.The preparation of labelled cells proceeds by adding to the complete culture medium 5-20 μl, to advantage 10 μl, of a colloid containing 0.05-45 mg iron oxide per ml, to advantage 1-5 mg iron oxide per ml of the medium, and culturing the cells for a period of 1-7 days, to advantage for 1-3 days, at 37° C. and 5% of CO2.
Owner:INST OF MACROMOLECULAR CHEM ASCR V V I +1
Nanoparticle having an inorganic core
A nanoparticle comprising an inorganic core and a polymerizable outer coating. The inorganic core comprises a substantially crystalline inorganic material such as a superparamagnetic material. In one embodiment, the inorganic core comprises a single crystal mixed spinel ferrite comprising iron in a first oxidation state and at least one metal in a second oxidation state, wherein the second oxidation state is different from the first oxidation state.
Owner:GENERAL ELECTRIC CO
E-iron oxide type ferromagnetic powder and magnetic recording medium
ActiveUS20180147626A1Improve featuresExcellent electromagnetic conversion characteristicMaterials with ironTransportation and packagingIron oxideMaterials science
Provided is an ε-iron oxide type ferromagnetic powder with a ratio Hc173K / Hc296K between a coercive force Hc173K measured at a temperature of 173 K and a coercive force Hc296K measured at a temperature of 296 K is higher than 1.00 and less than 2.00, and a magnetic recording medium containing the ε-iron oxide type ferromagnetic powder in a magnetic layer.
Owner:FUJIFILM CORP
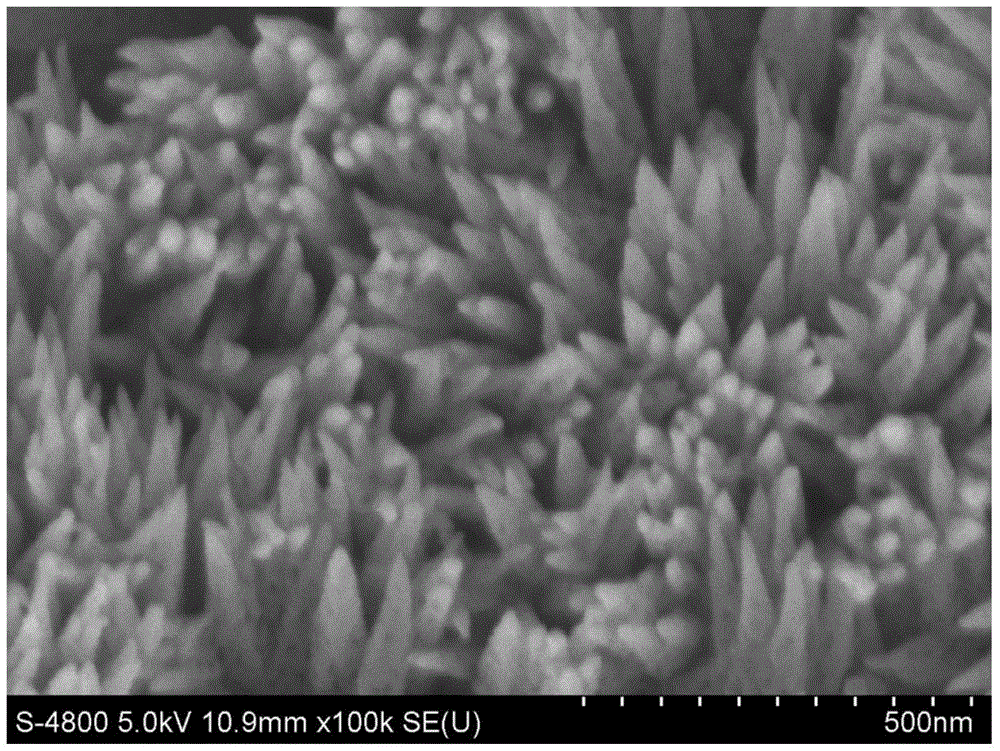
Preparation method of porous iron oxide nanorod array
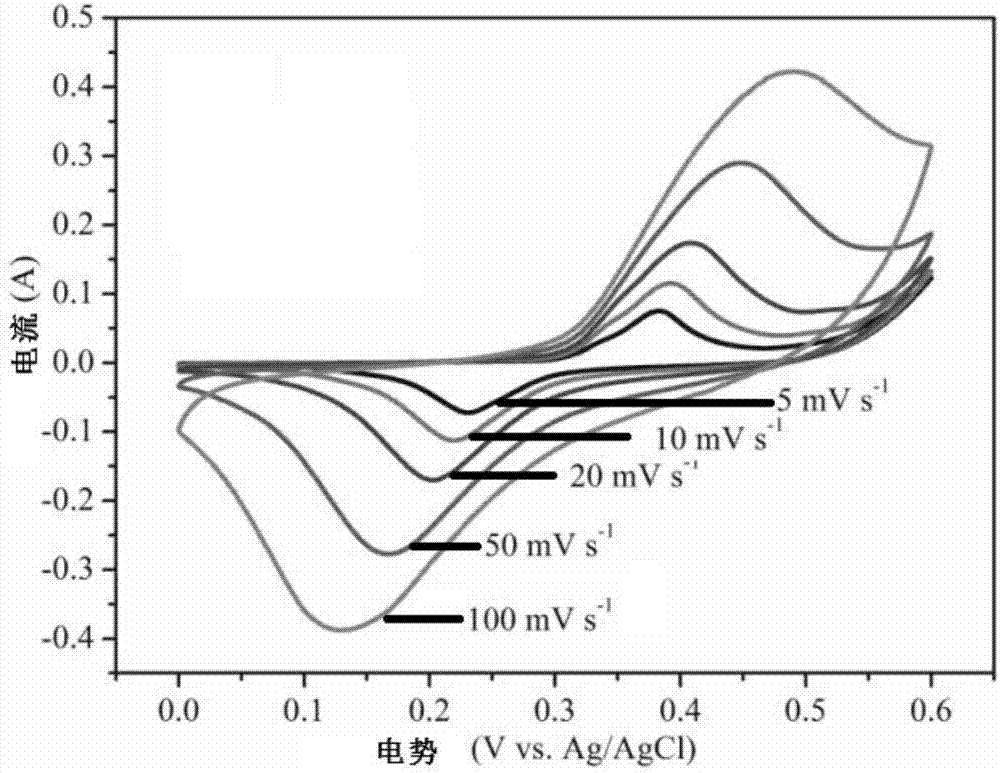
The invention relates to a preparation method of porous alpha-Fe2O3. The porous alpha-Fe2O3 has a regular and orderly nanorod array structure. The method comprises the following steps: preparing a precursor FeOOH through a hydrothermal synthesis process, and roasting to obtain the porous alpha-Fe2O3 tapered nanorod array structure. The alpha-Fe2O3 prepared through the method is a porous nanorod structure, and nanorods are vertical to a substrate in order to form the regular and orderly array structure. The above material has important application potential in fuel cells and other energy transfer devices as a catalyst carrier, and can also be used in the fields of photoelectrocatalysis and electrochemical catalysis.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for preparing superfine/nano iron oxide/iron powder
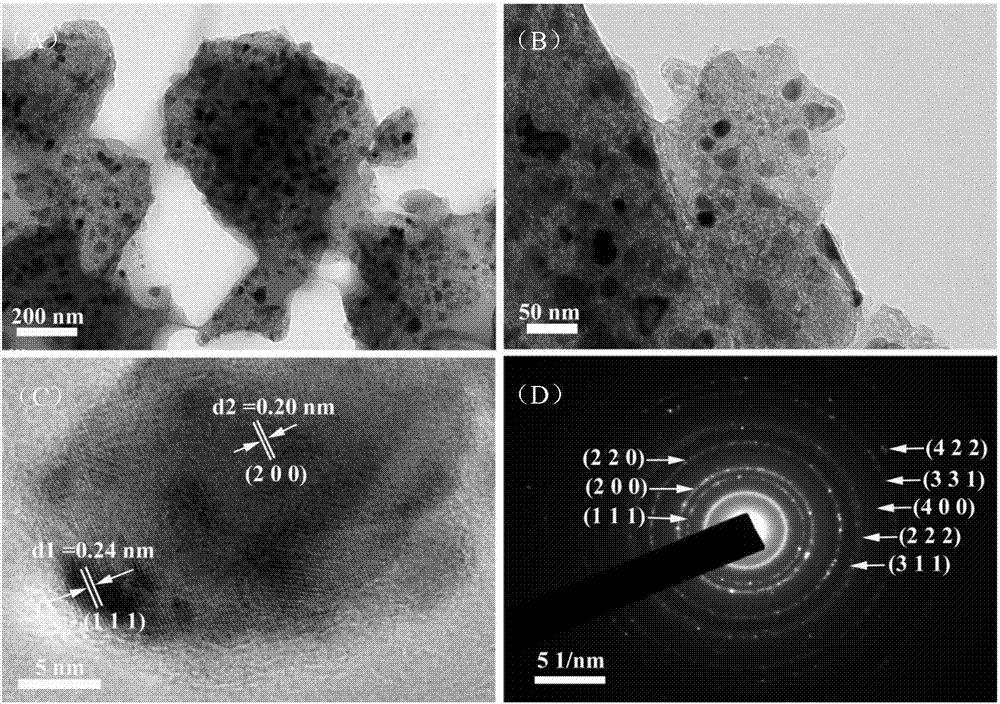
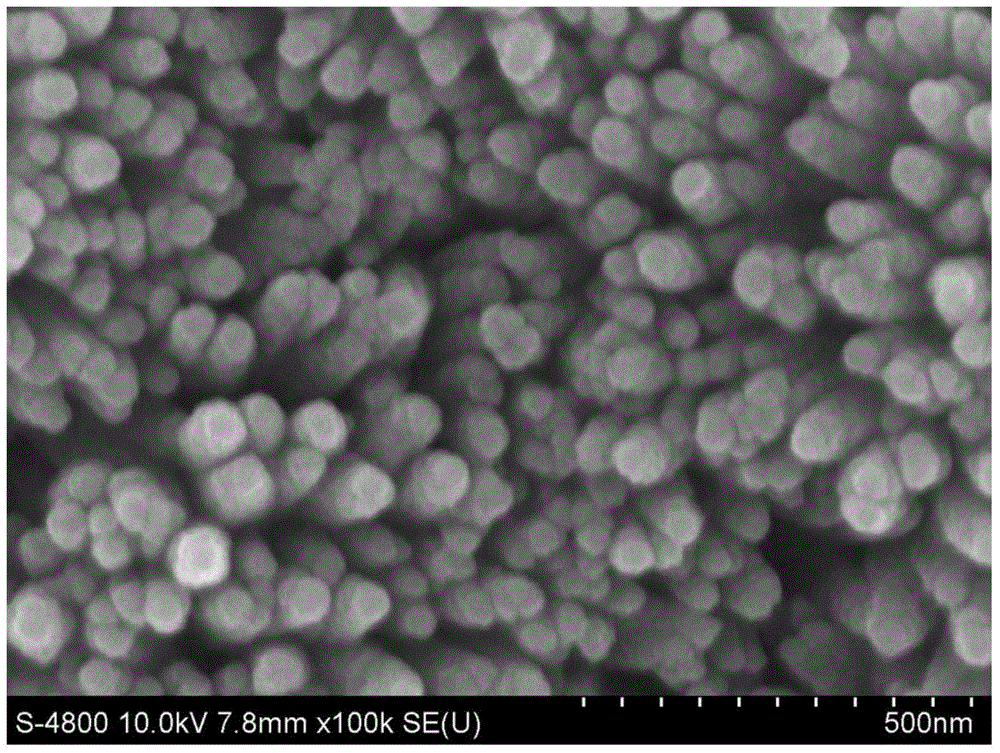
The invention relates to the powder metallurgy domain, specially used in the preparation method of nano / superfine ferric oxide. Tts characteristic lies in: Uses Fe(NO)3 the crystal (Fe (NO3) 3 - 9H2O), the ferrous sulfate crystal (FecSO4 - 7H2O), the ferric chloride crystal (FeCl3 - 6H2O) as the raw material, which matches the density - 30wt% molysite solution; Joins the ammonia water and adjusts pH to 1.5-3; Then joins 0.1-1.0% surface active agent and 0.01-0.1% crystal grain inhibitor, vibrates 10 - 60min after the ultrasonic waving, obtains the transparent colloid; Then dry, then gets the superfine mix powder forerunner body; Under 350 - 700 íµ temperatures for calcination, then obtains the nanometer / superfine ferric oxide powder. This invention preparation powder granularity is thin, which is smaller than 100nm, high purity which can reaches 99% - 99.5%, which is serviceable and makes the high performance magnetic powder and the absorbing material; The craft is simple, the process is easy to control, the powder output is high.
Owner:CENT SOUTH UNIV
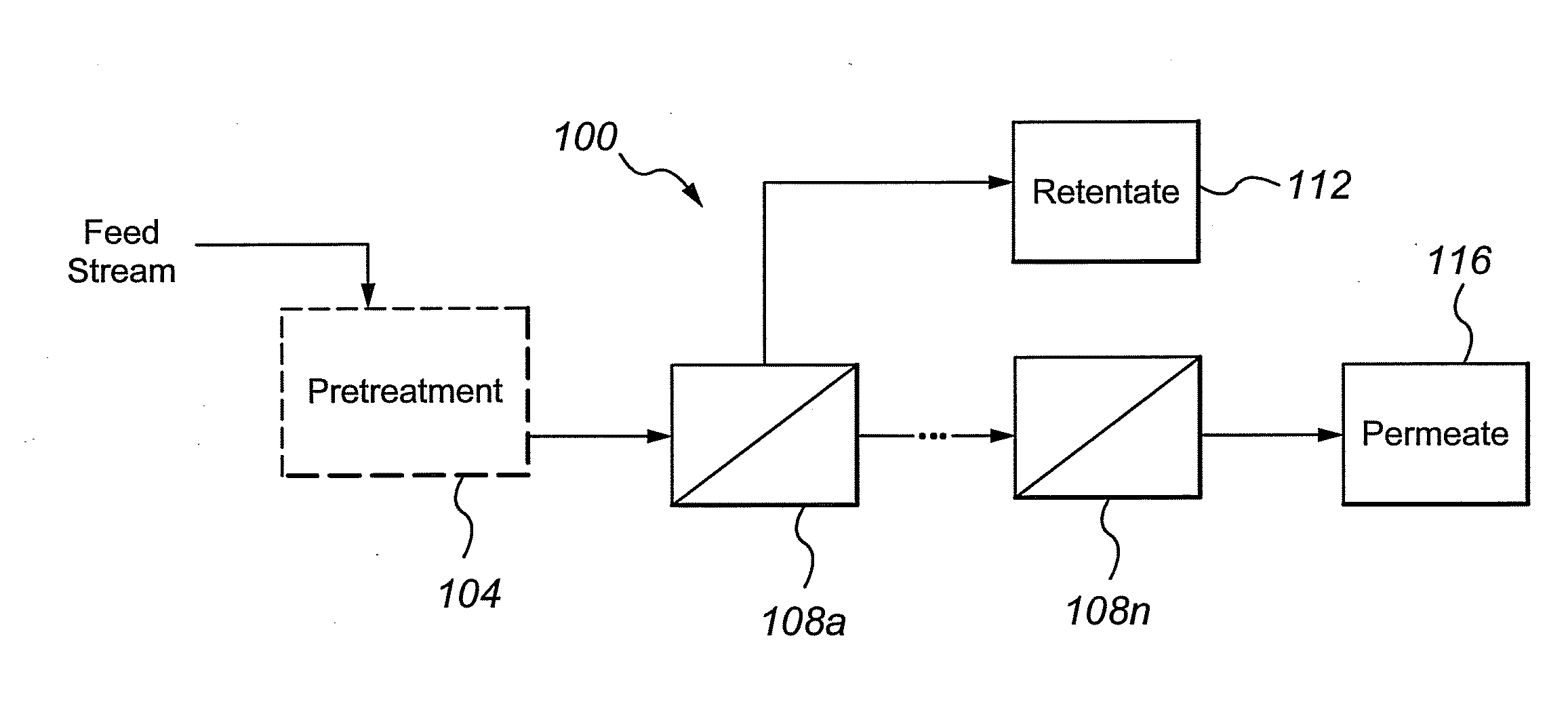
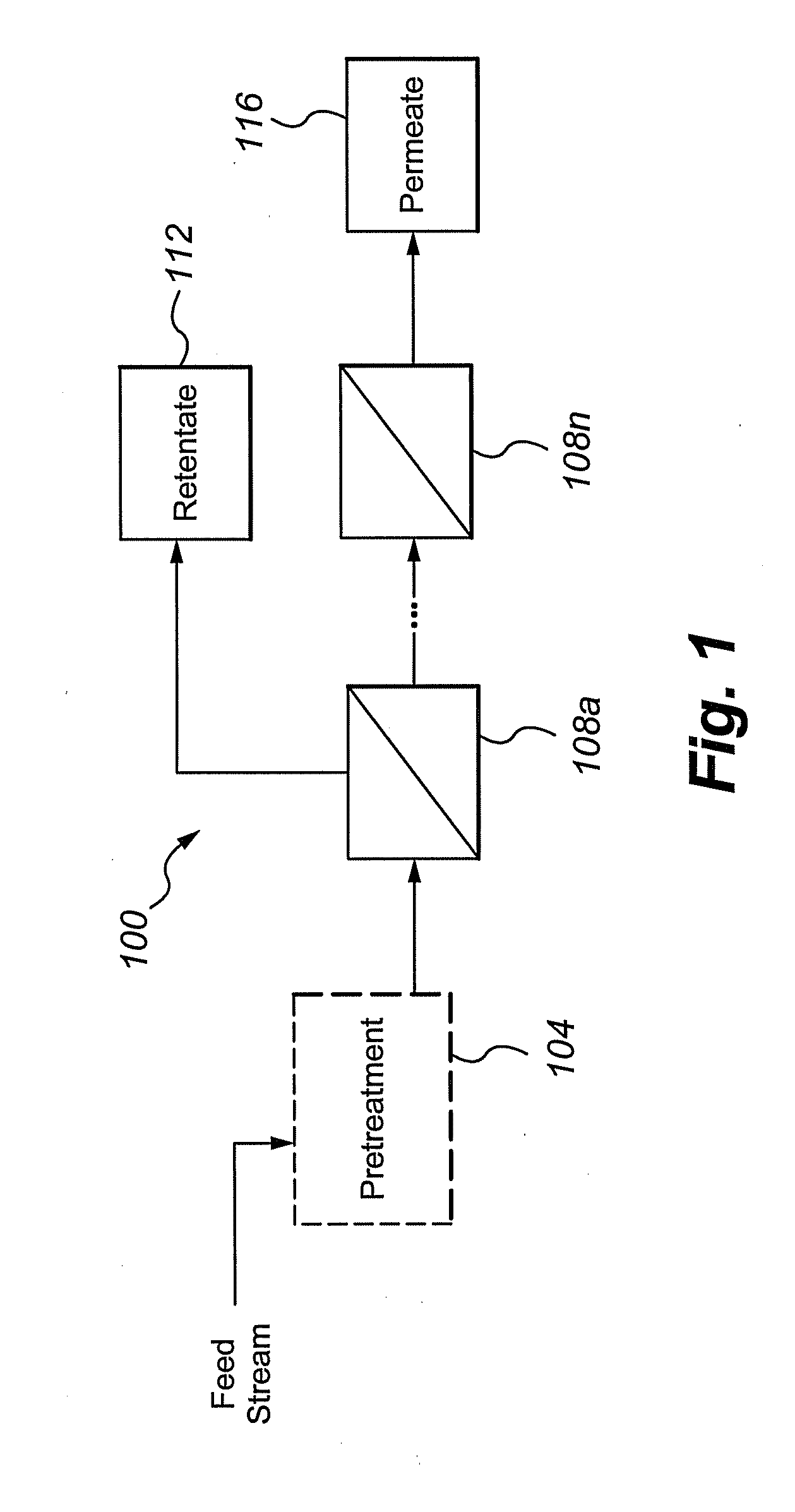
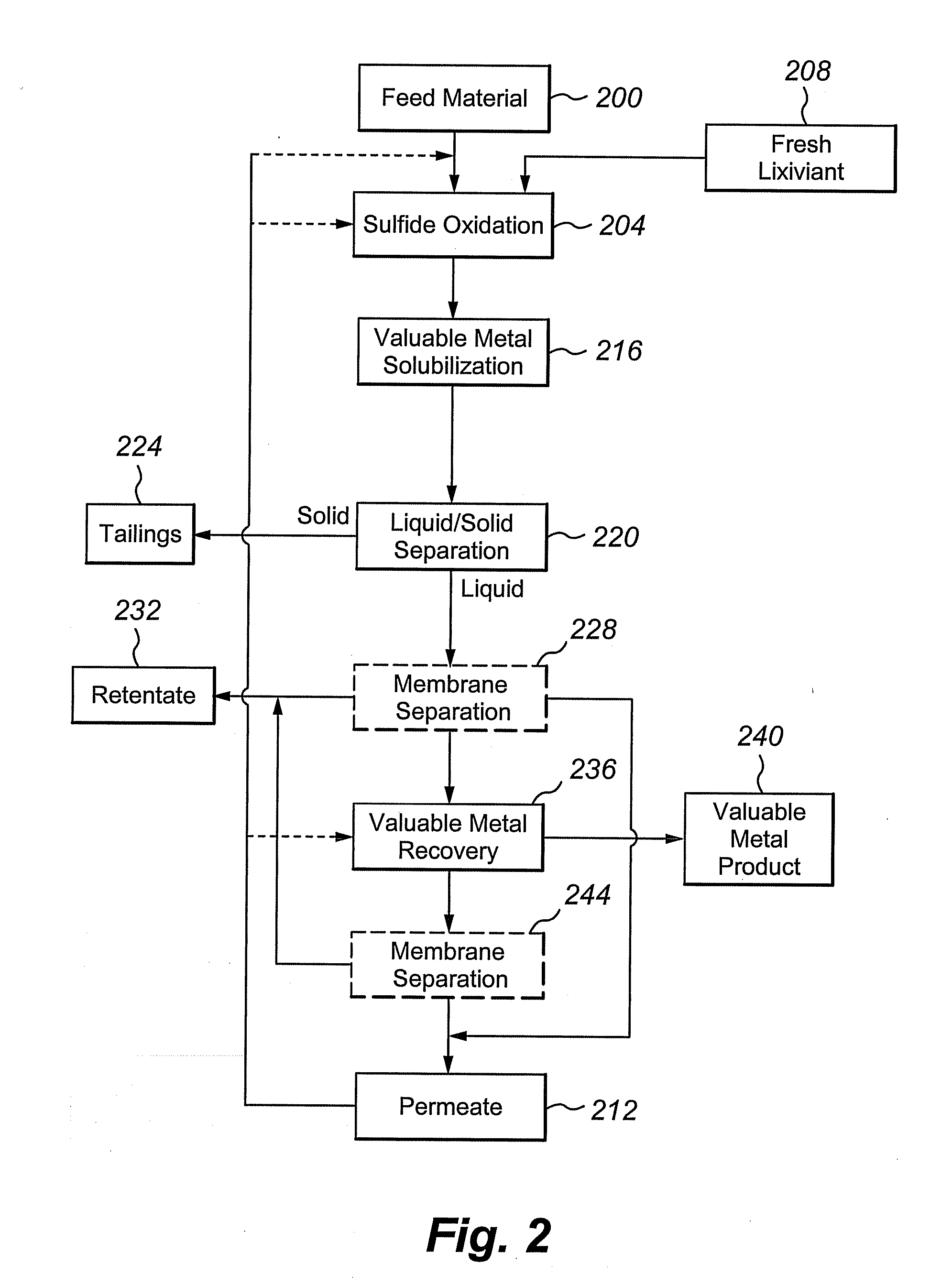
Multivalent iron ion separation in metal recovery circuits
InactiveUS20080069748A1High sulfide sulfur oxidation rateReduce electricity costsSolvent extractionFerrous oxidesPhysical chemistryFerric ion
Owner:HW PROCESS TECH
Fire resistant black iron oxide pigment and preparation method thereof
ActiveCN101314678AImprove acid resistanceGood alkali resistanceInorganic pigment treatmentFerric oxidesEngineering plasticManganese oxide
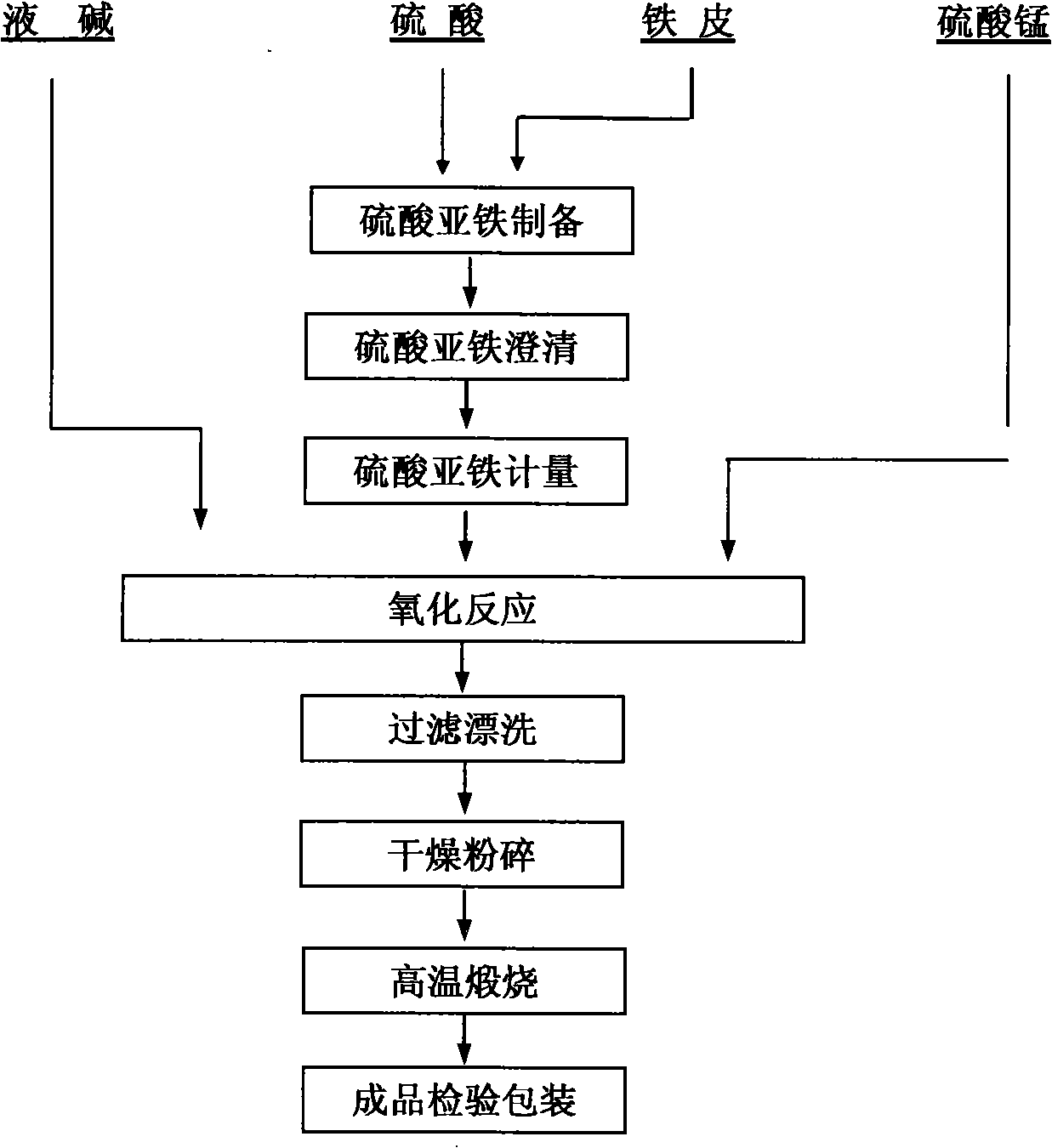
The invention relates to an inorganic iron oxide black pigment, in particular to a heat-resistant iron oxide black pigment (manganese ferrite black) and the preparation method thereof. The heat-resistant iron oxide black pigment comprises manganese ferrite black prepared from ferroferric oxide and manganese oxide by high-temperature lattice reaction, wherein the molar ratio of iron element to manganese pigment in the pigment is 1.5:1 to 5:1. The heat-resistant iron oxide black pigment has high heat resistance up to 700 DEG C or above, and can be widely used for ceramic, engineering plastic, color sand, etc., for products manufactured at high temperature, and for coloring agent for products. Meanwhile, the inorganic iron oxide black pigment has the advantages of high acid resistance, high alkali resistance and high light absorbability.
Owner:ZHEJIANG HUAYUAN PIGMENT CO LTD
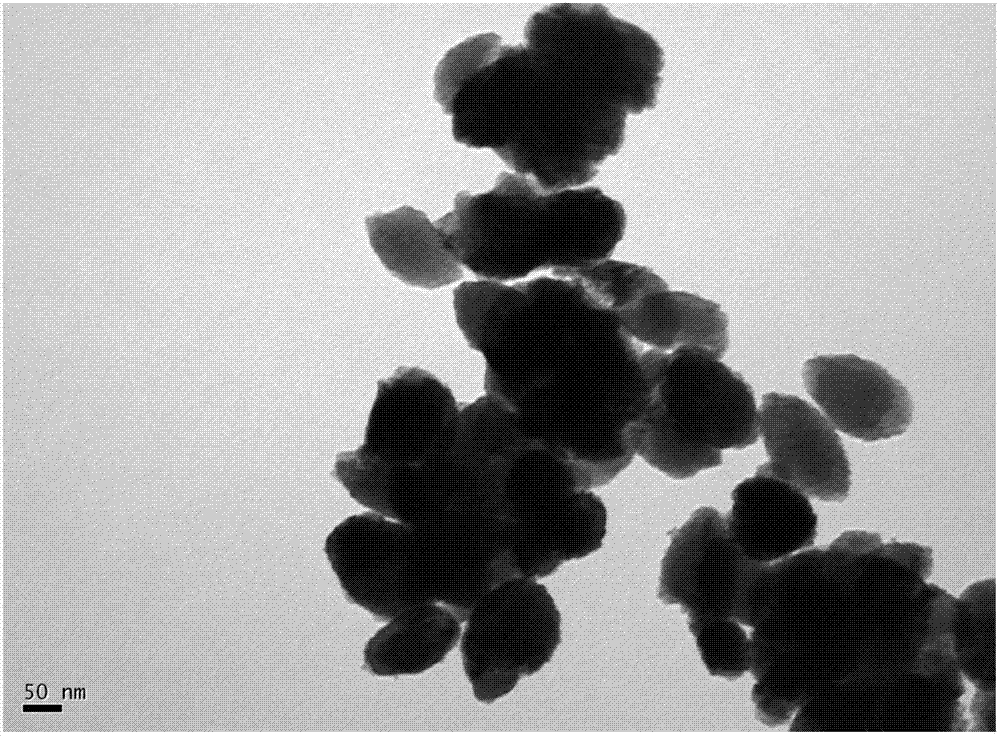
Preparation method of ellipsoidal particle size-controllable alpha-Fe2O3 nano particle
ActiveCN103043726ALarge controllable rangeLow costMaterial nanotechnologyFerric oxidesHydrogenEllipsoidal particle
The invention discloses a preparation method of an ellipsoidal particle size-controllable alpha-Fe2O3 nano particle. By adopting the raw material combination of trivalent iron salts and hydroxyl or / and carbonyl surfactant, the hydroxyl or the carbonyl and -OH of Fe(OH)3 for hydrogen bonds to be absorbed on the surfaces of crystals; the irregular motion of the surfactant molecules containing the hydroxyl or the carbonyl is intensified so as to form an ellipsoidal alpha-Fe2O3 seed crystal through induction; an obtained product is uniform in particle size; in the preparation process, the overall participation of the surfactant effectively suppresses hard agglomeration, the controllable range of the particle size is wide, raw materials are easily available and the cost is low, and the preparation process is simple and convenient; the particle size distribution of the prepared ellipsoidal alpha-Fe2O3 nano particle is 90-800nm + / -10 nanometers, the particle size can be regulated and controlled within the range of 90-800nm, the capacity is increased, and industrialization is realized.
Owner:CHONGQING YUNTIANHUA HIGH END NEW MATERIALS DEV CO LTD +1
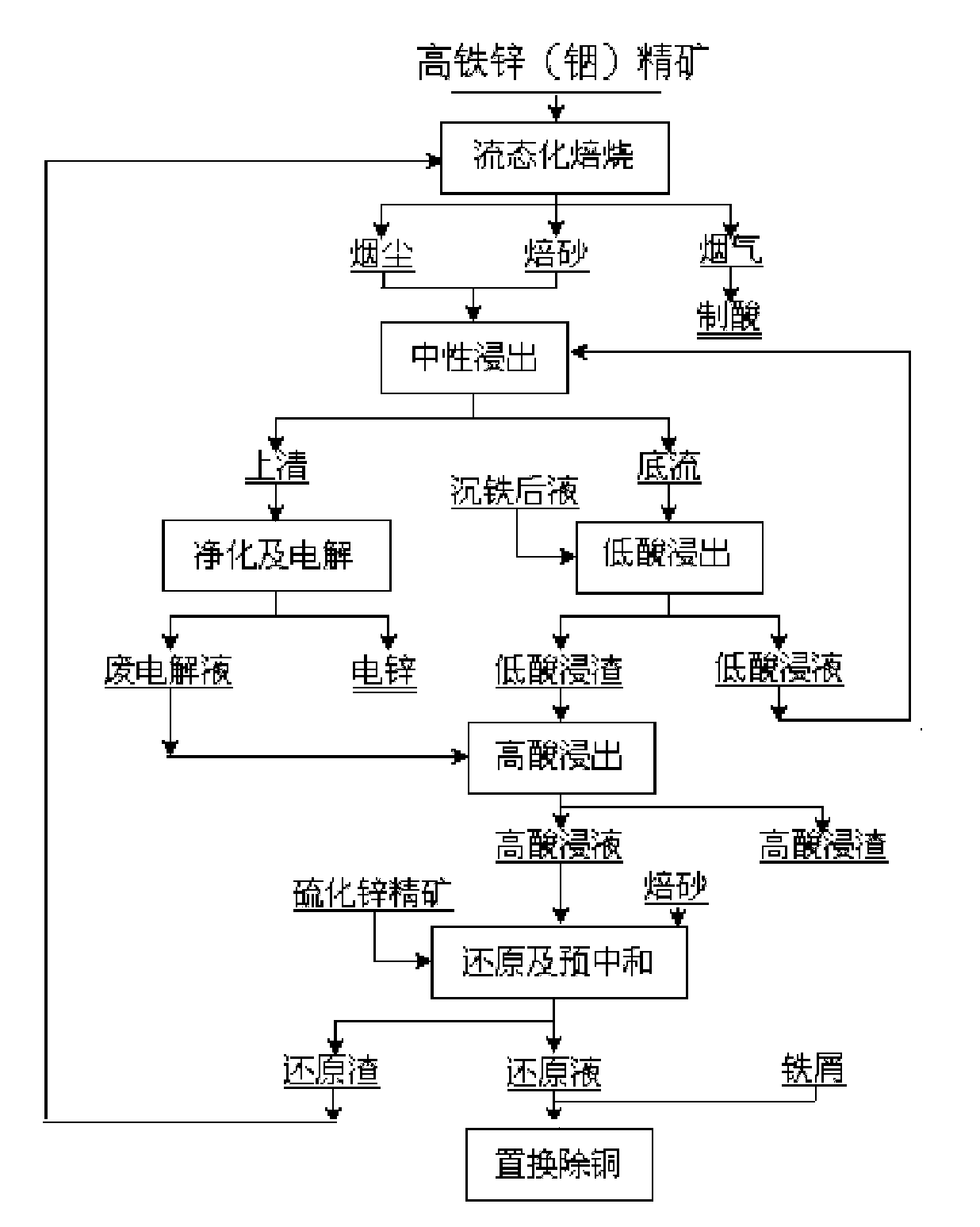
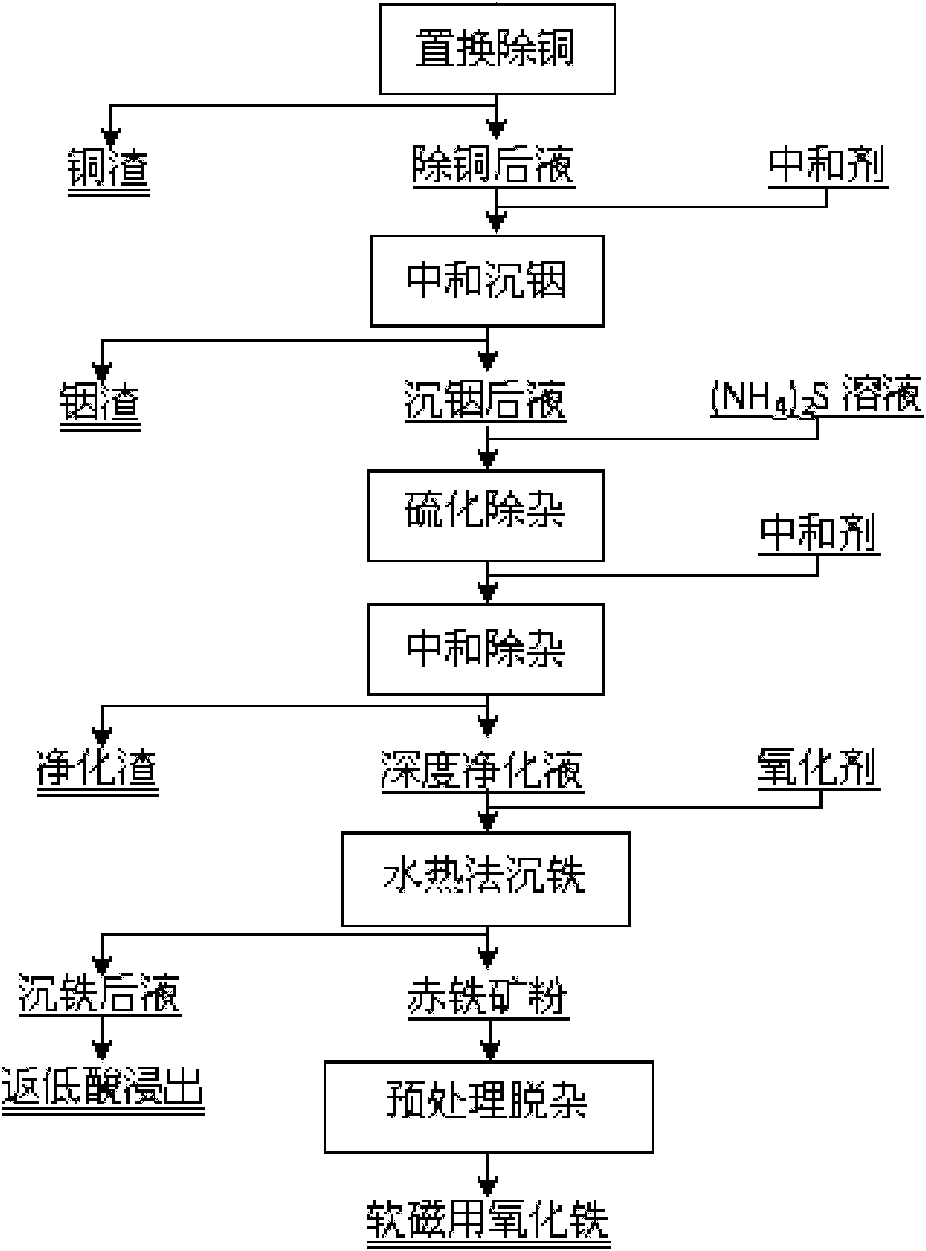
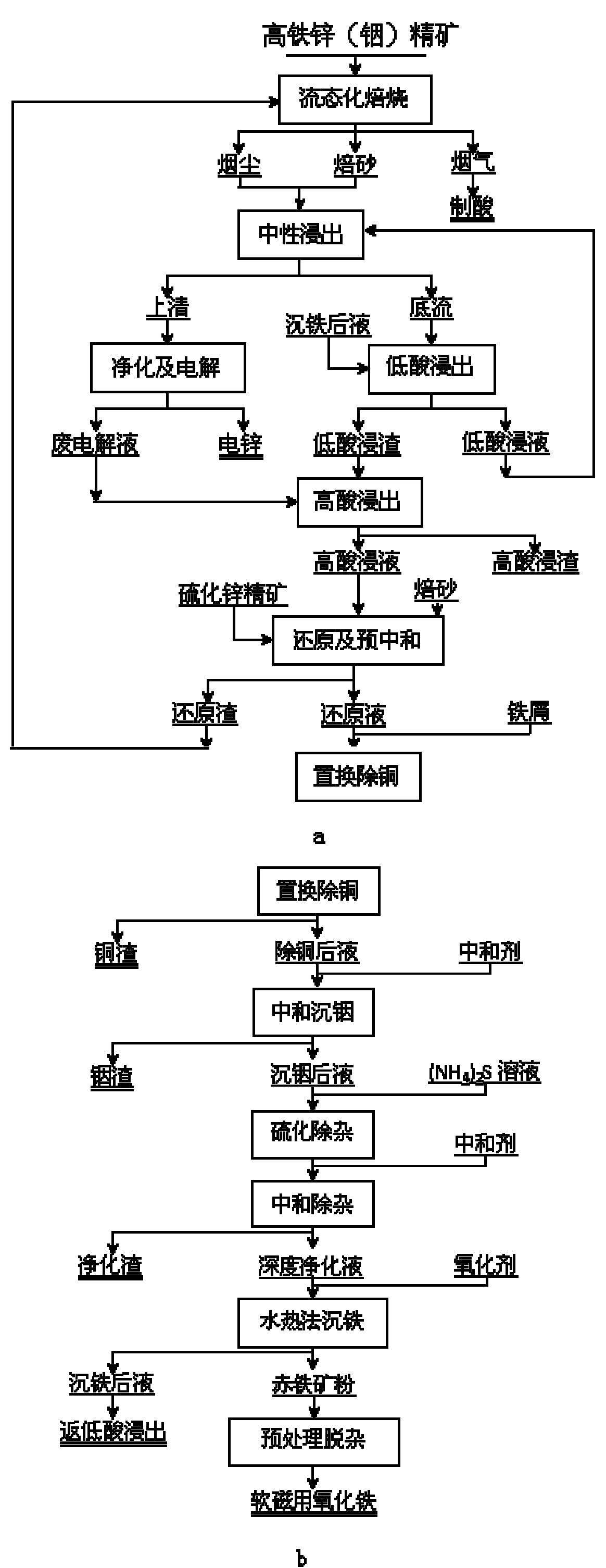
Method for extracting indium and preparing iron oxide by slag-free zinc hydrometallurgy of zinc concentrate
InactiveCN101886272AHigh recovery rateOvercome the problem of efficient usePhotography auxillary processesFerric oxidesIndiumSlag
The invention relates to a method for extracting indium and preparing iron oxide by slag-free zinc hydrometallurgy of zinc concentrate, which comprises the following steps of: 1, performing fluidized bed roasting, neutral leaching, low acid leaching, purification and electrodeposition on the zinc concentrate to prepare electric zinc; 2, performing high acid leaching, reduction, preneutralization and displacement to remove copper on low acid leaching residue and waste electrolyte after the electrodeposition to prepare the electric zinc; 3, neutralizing the liquid from which the copper is removed to settle the indium; 4, vulcanizing the liquid in which the indium is settled to remove heavy metal and then adding lime milk to neutralize the liquid to obtain deeply purified liquid; 5, settling iron in the deeply purified liquid by a hydrothermal method to obtain hematite powder; and 6, removing impurities from the hematite powder to obtain soft magnetic iron oxide. In the method, the indium is settled by neutralizing and the iron is settled by the hydrothermal method, so that the indium is separated from the iron, the indium is separated from the zinc, the hematite powder is formed, and the requirement of the soft magnetic iron oxide is met through impurity removal treatment. The method has the advantages of simple process, high recovery rate of the indium and the zinc, short flow for separating the iron from the zinc, high purity of the iron, environmental friendliness, suitability for industrial application, and capability of replacing the conventional slag-free zinc hydrometallurgy process for extracting the indium and making effective use of the iron resource in the zinc concentrate in a form of the soft magnetic iron oxide.
Owner:CENT SOUTH UNIV
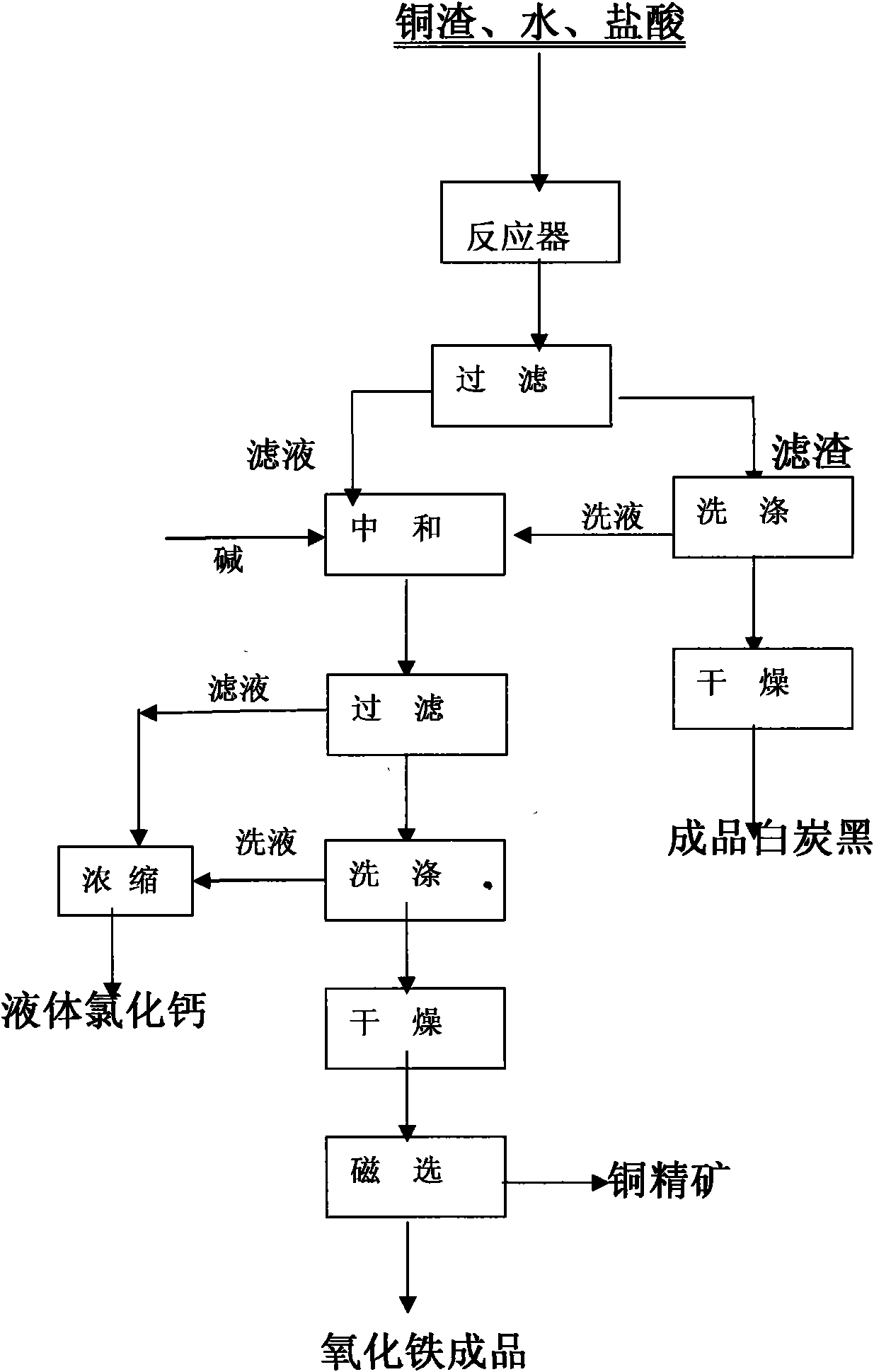
Method for comprehensively recovering Fe, Cu and Si from copper smelting slag
InactiveCN101555551AImprove resource utilizationImprove product added valueSilicaCalcium/strontium/barium chloridesSilicon dioxideMaterials science
The invention discloses a method for comprehensively recovering iron, copper and silicon dioxide from copper smelting slag. The method takes copper smelting slag as a raw material, and comprehensively recovers Fe, Cu and Si in copper slag by adopting wet chemistry metallurgical technology. A muriatic acid and an inorganic acid are mainly adopted for leaching the copper smelting slag, the leaching acid concentration, the solid to liquid ratio, the leaching temperature and the leaching time are selected according to the quality requirement of silicon dioxide products under certain conditions, and silicon dioxide is firstly separated through filtering and drying to prepare silica pigment; the leaching filtered liquid is counteracted, settled, filtered, dried and ground, and ferric oxide phase and copper-bearing phase are selectively separated by adopting a conventional mineral processing method.
Owner:KUNMING UNIV OF SCI & TECH
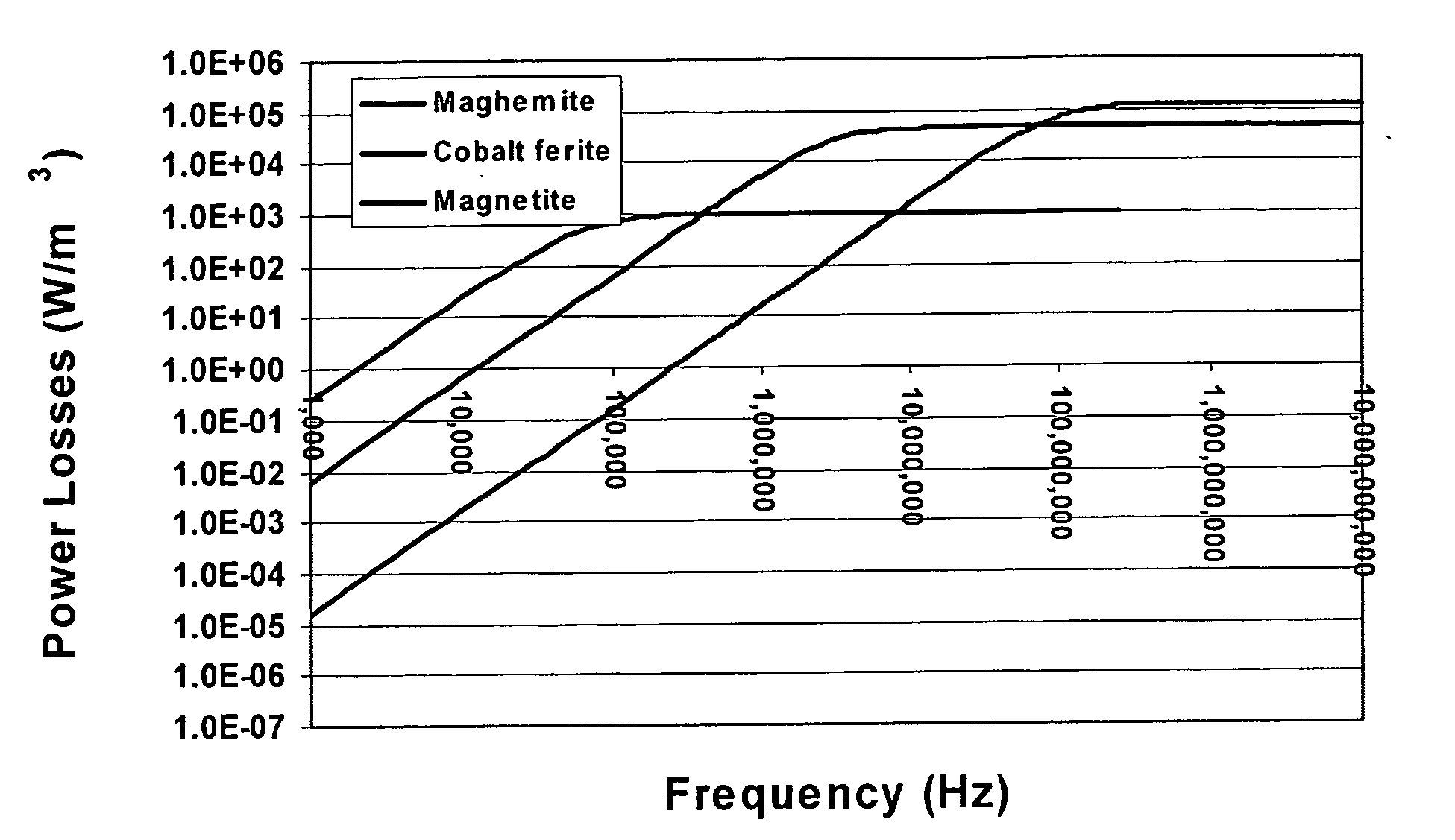
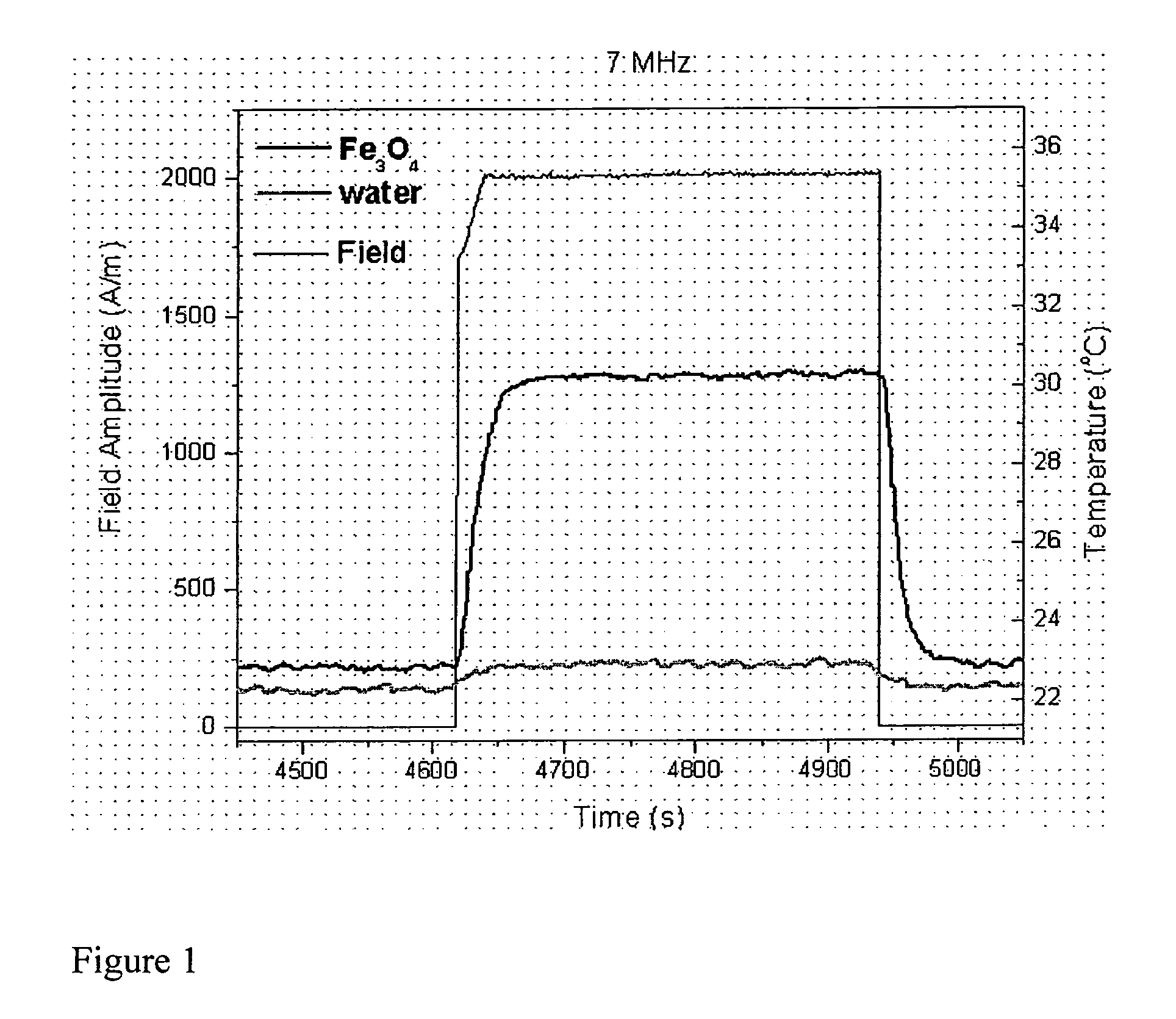
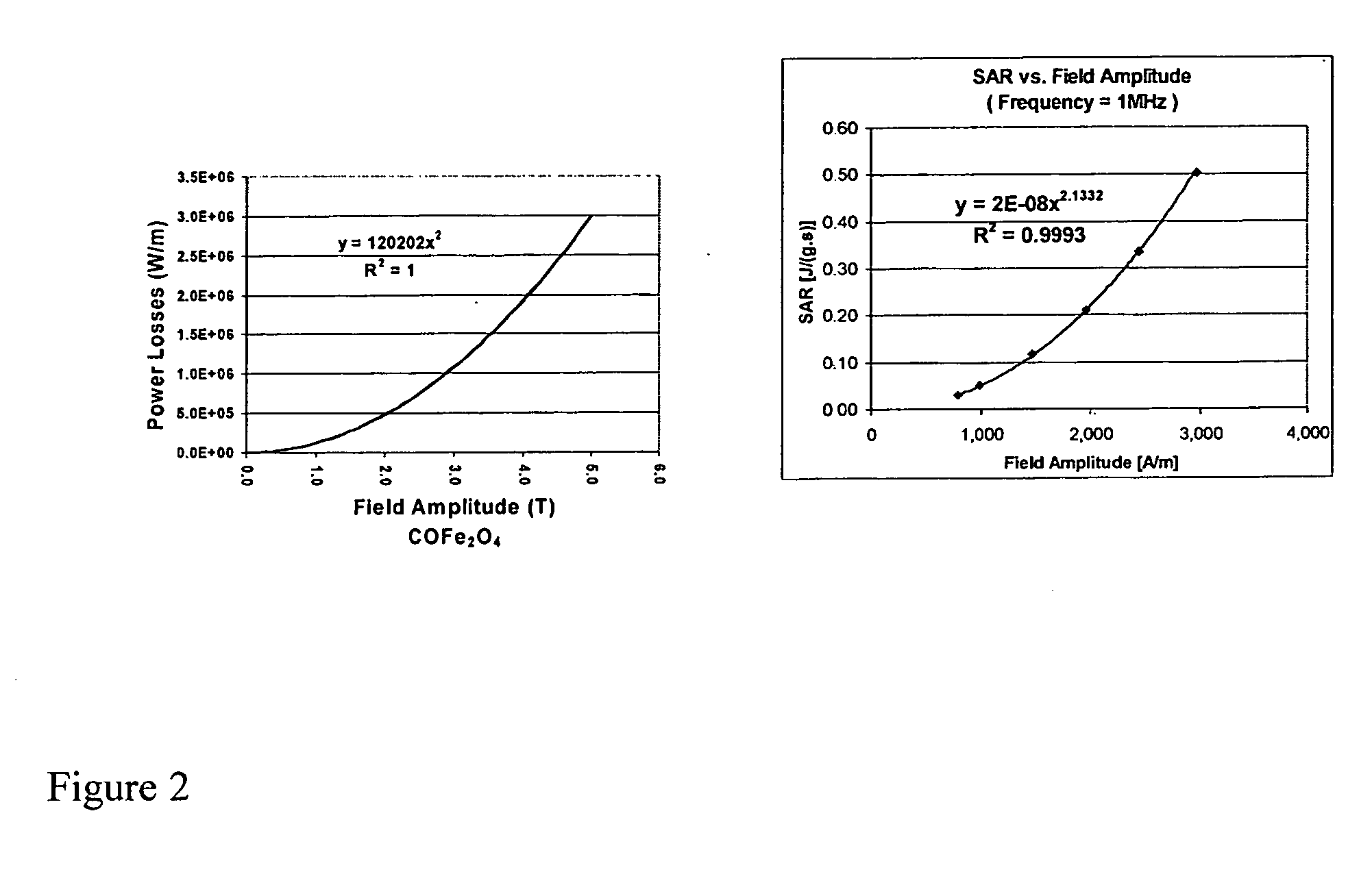
Nanoparticle heating and applications thereof
This invention provides magnetic nanoparticles, which when placed in a magnetic field are selectively heated at a certain frequency of the magnetic field, as a function of their size, composition, or both. The invention also provides for use of such nanoparticles, in applications including, inter alia, selective nanoparticle heating and applications thereof, hyperthermia induction in cells or tissue, remote alteration of protein structure and / or drug delivery.
Owner:MASSACHUSETTS INST OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com