Preparation method of porous iron oxide nanorod array
A nanorod array and nanoarray technology are applied in the field of precursor iron oxyhydroxide, which can solve the problems of inconspicuous nanorod array structure and increased preparation process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
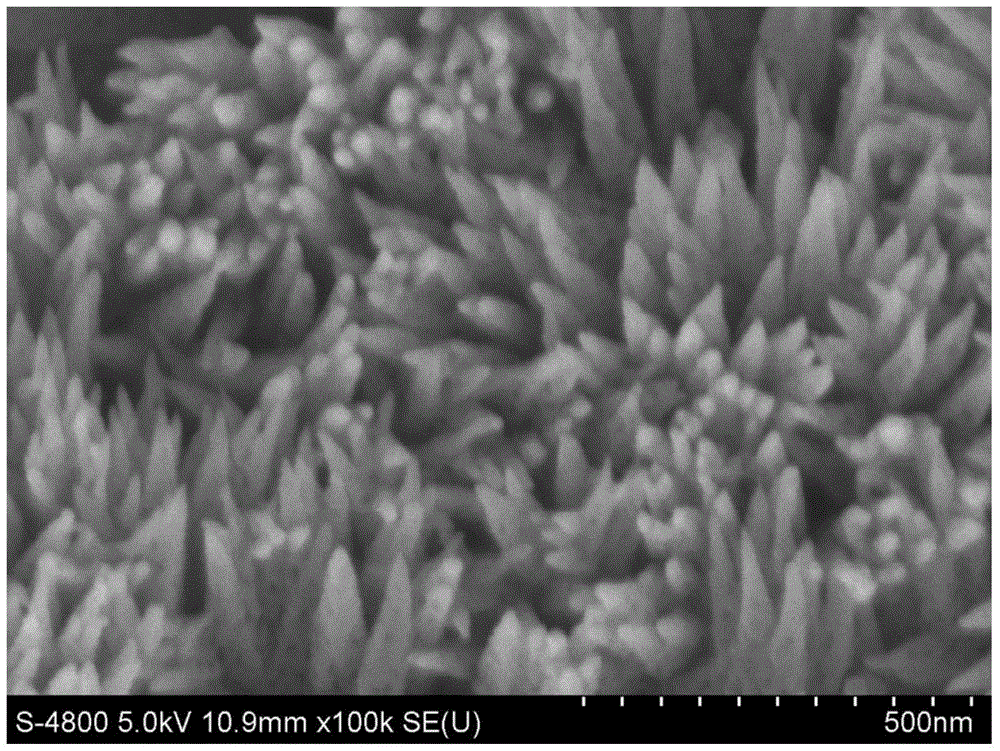
[0026] Soak the Ti plate in acetone, ethanol and deionized water for 30min, then wash it with deionized water, dry it with pure nitrogen, place it in the liner of a hydrothermal kettle, and add 1M NaNO to it. 3 and 0.15M FeCl 3 Mixed solution of water and ethanol, the volume ratio is 7:3; use 0.5M hydrochloric acid solution to adjust the pH value of the solution in the liner to 1, and mix well; seal the hydrothermal kettle and heat it in an oven at 120°C 4h; after the completion of the reaction, the hydrothermal kettle was cooled to room temperature to obtain β-FeOOH; then the conductive glass film sample was calcined at a heating rate of 10°C / min, heated for 1h and then cooled to room temperature to obtain α-FeOOH 2 o 3 nanoarray. figure 1 Shown is the α-Fe prepared in this example 2 o 3 Nanoarray FESEM top view. α-Fe can be seen 2 o 3 It is a porous tapered structure, oriented vertically upwards from the base.
Embodiment 2
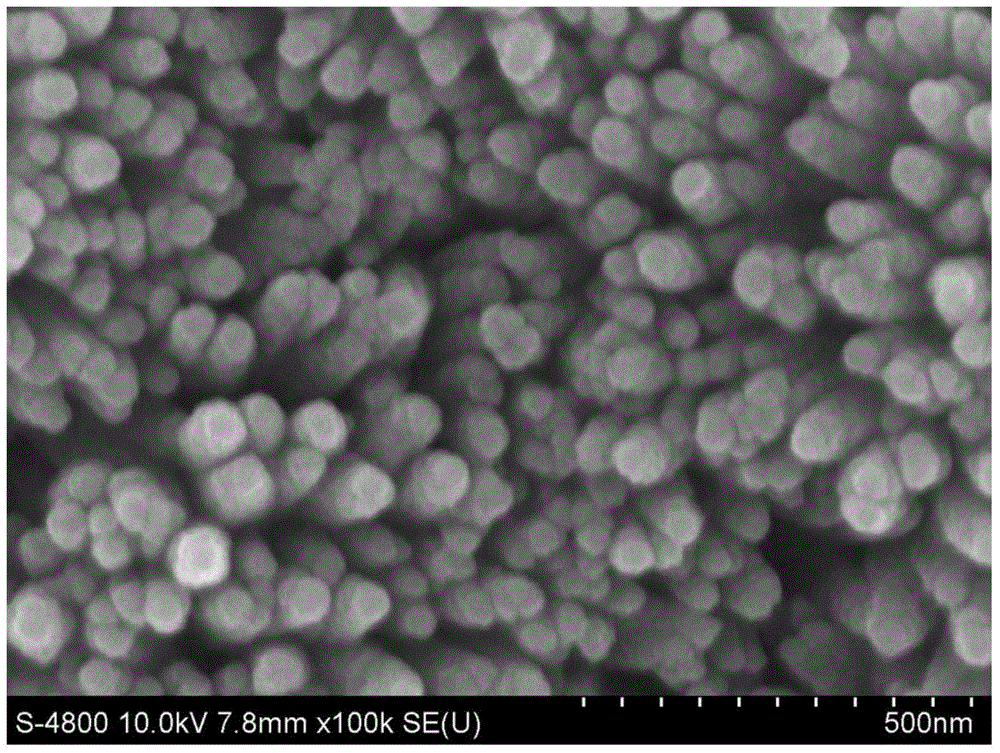
[0028] Put the W plate into acetone, ethanol and deionized water for 30min, soak in ultrasonic for 30min, wash with deionized water, blow dry with pure nitrogen, put it in the liner of a hydrothermal kettle, add 1.5M NaNO 3 and 0.2M FeCl 3 The volume ratio of the mixed solution of water and acetonitrile is 7:5; use 0.5M hydrochloric acid solution to adjust the pH value of the solution in the liner to 1, and mix well; seal the hydrothermal kettle and heat it in an oven at 100°C for 3 hours; After the reaction was completed, the hydrothermal kettle was cooled to room temperature to obtain β-FeOOH; then the conductive glass film sample was calcined at a heating rate of 2°C / min, heated for 2 hours and then cooled to room temperature to obtain α-FeOOH 2 o 3 nanoarray. figure 2 Shown is the porous conical α-Fe prepared in this example 2 o 3 Nanoarray FESEM image.
Embodiment 3
[0030] Soak the Ti plate in acetone, ethanol and deionized water for 30 minutes, then wash it with deionized water, dry it with pure nitrogen, put it in the inner tank of a hydrothermal kettle, and add 0.75M NaNO 3 and 0.075M FeCl 3 The volume ratio of the mixed solution of water and acetonitrile is 7:5; use 0.5M hydrochloric acid solution to adjust the pH value of the solution in the liner to 1, and mix well; seal the hydrothermal kettle and heat it in an oven at 100°C for 4 hours; After the reaction is completed, the hydrothermal kettle is cooled to room temperature to obtain β-FeOOH; then the conductive glass film sample is calcined at a heating rate of 10°C / min, heated for 40 minutes and then cooled to room temperature to obtain porous conical α-FeOOH 2 o 3 nanoarray.
[0031] The resulting porous conical α-Fe 2 o 3 Nanoarray sputtering of Pt metal, it can be seen that the ordered catalyst is evenly distributed on the array. image 3 Shown is the tapered α-Fe prepared...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com