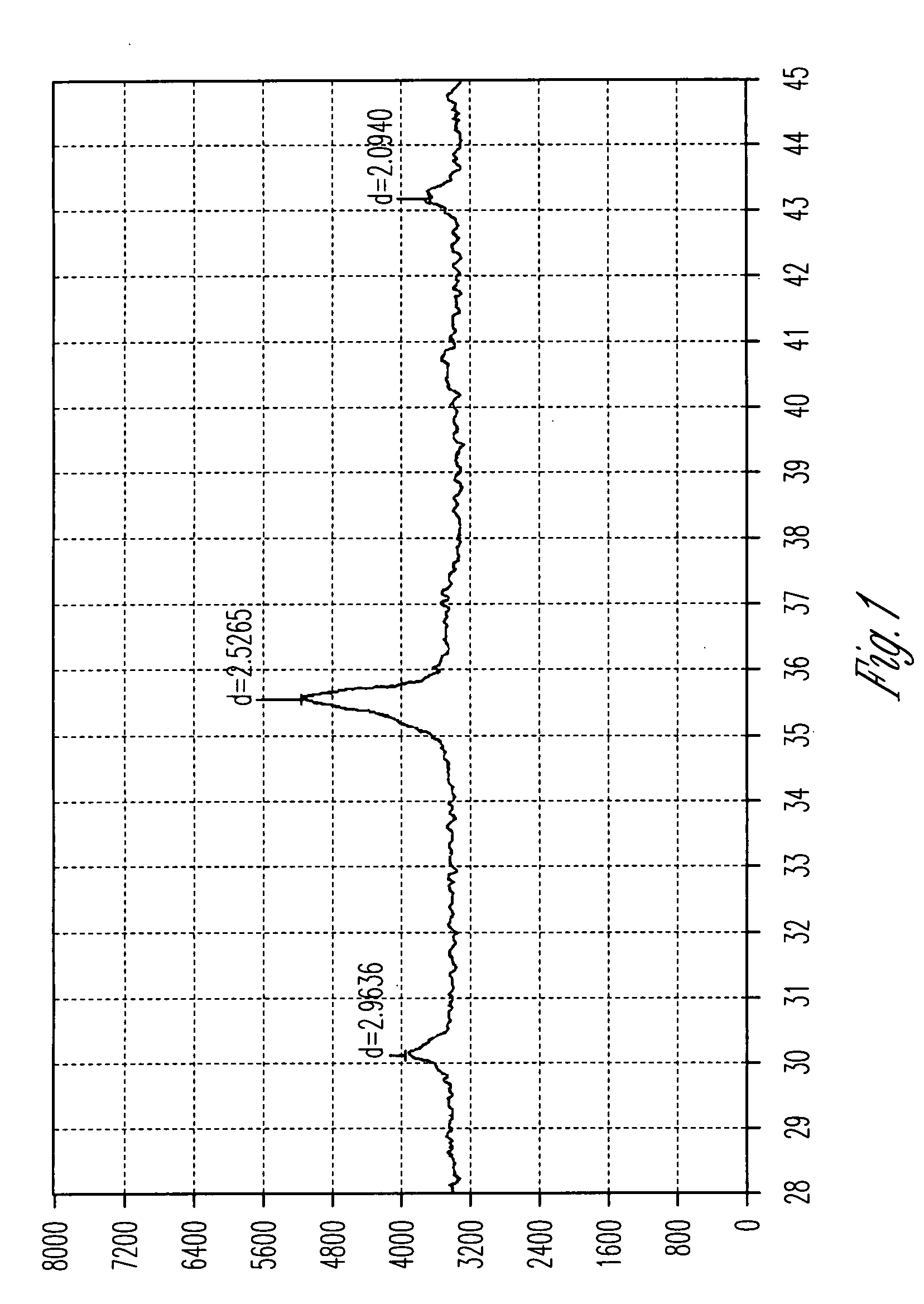
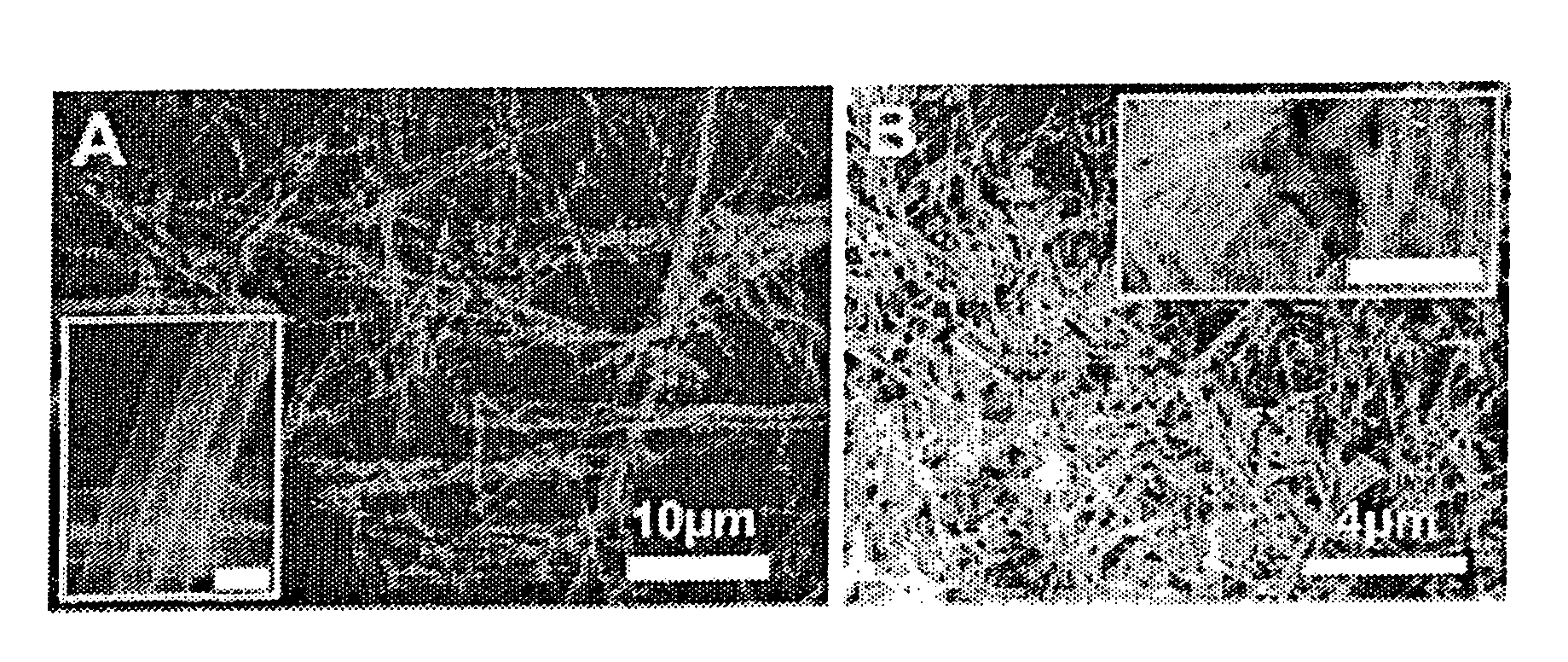
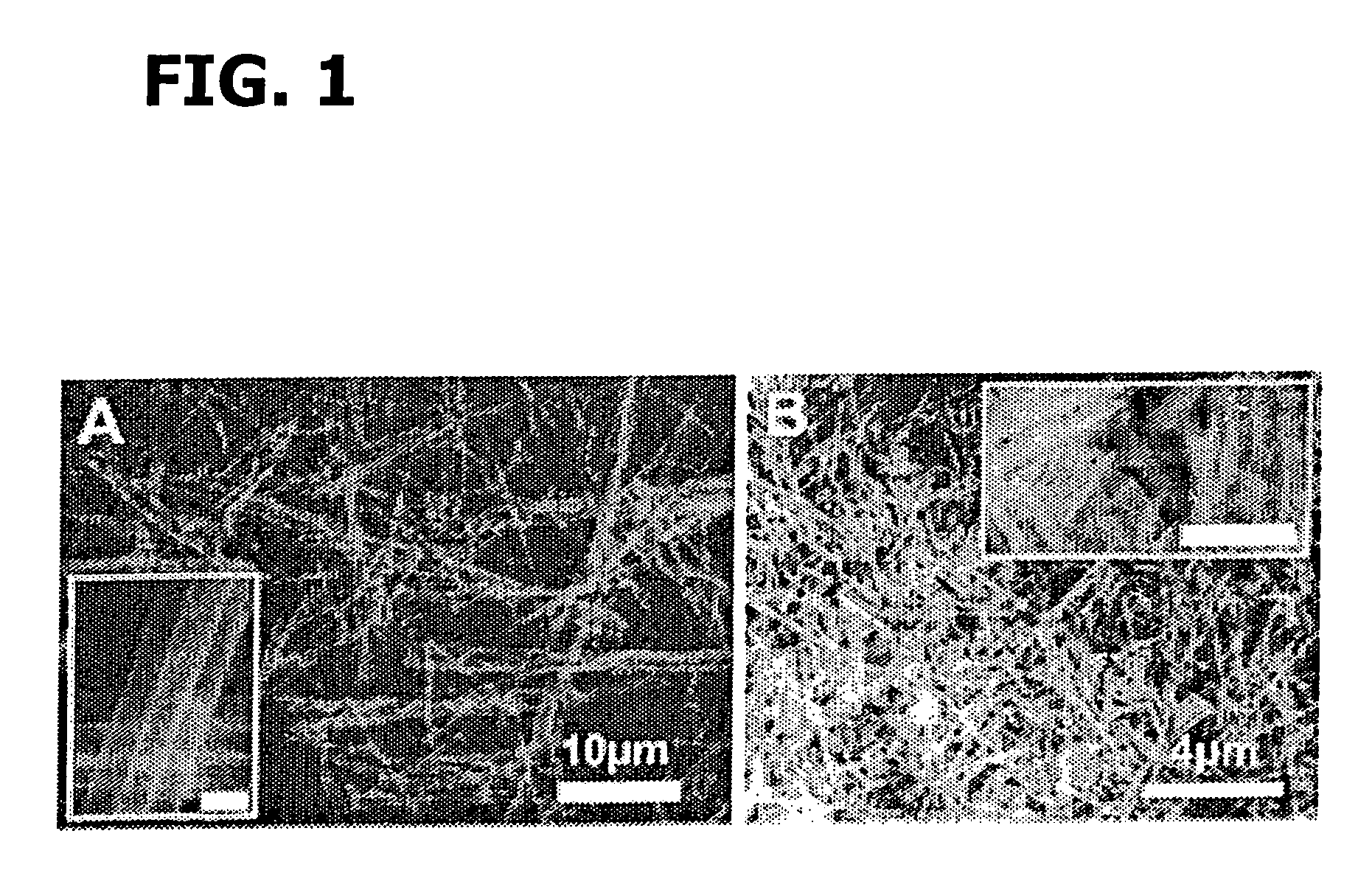
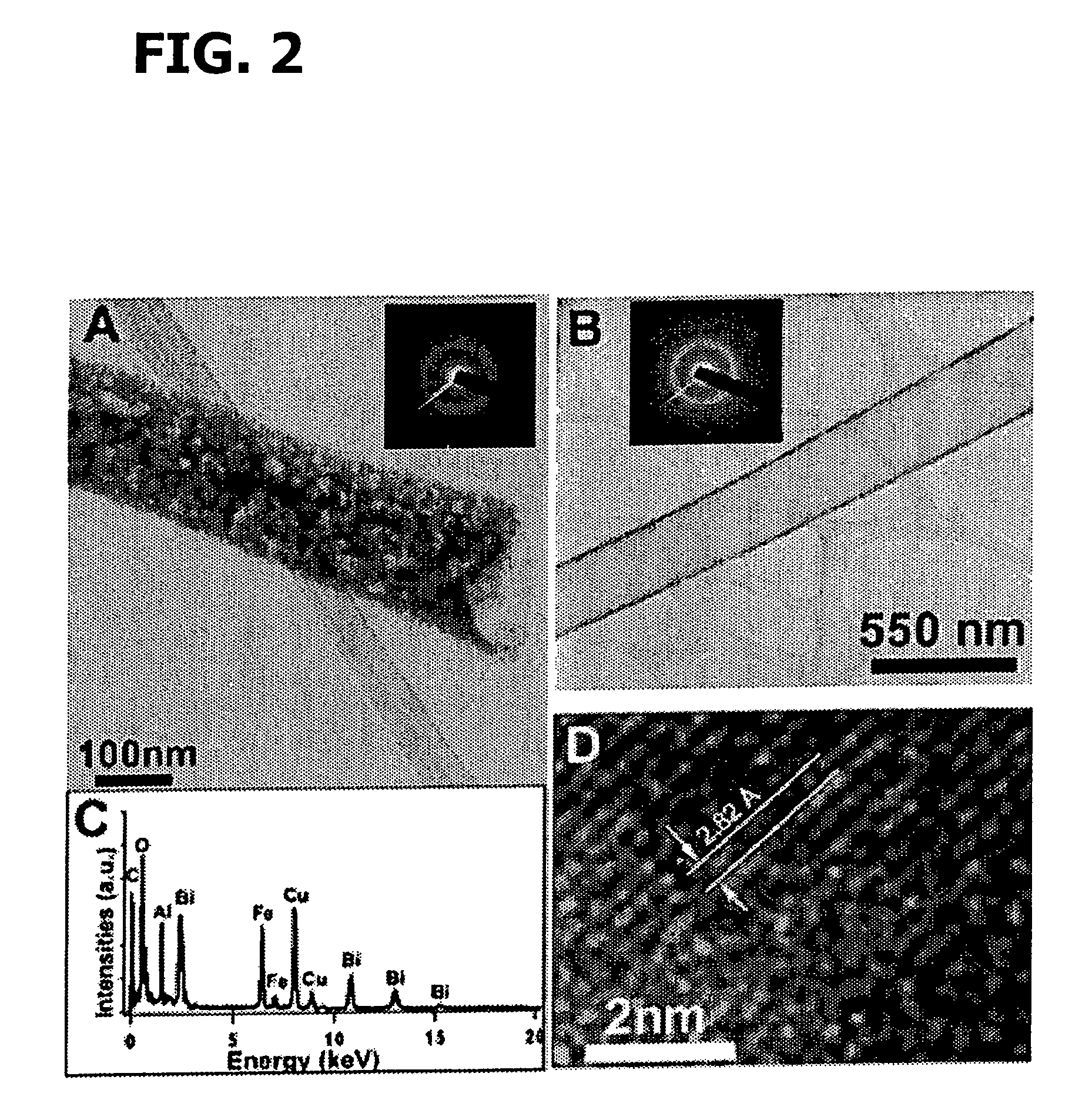
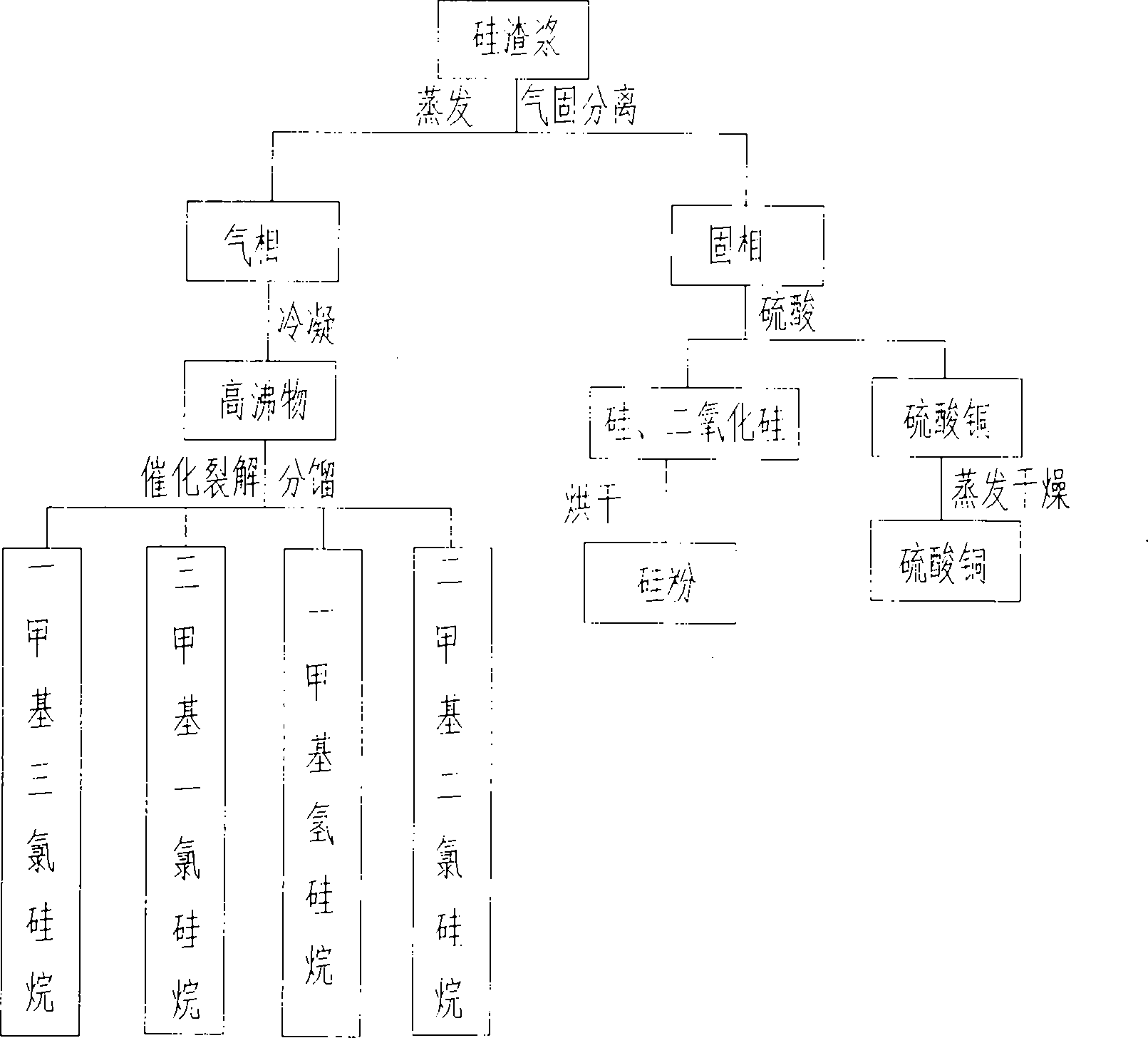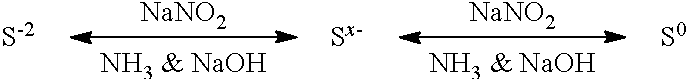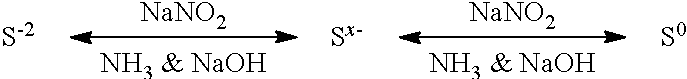Patents
Literature
68results about "Ferrous oxides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Positive electrode active materials for secondary batteries and methods of preparing same
InactiveUS6878490B2Deliver in short periodRetake in short periodAluminium compoundsAlkali titanatesPower capabilityLithium metal
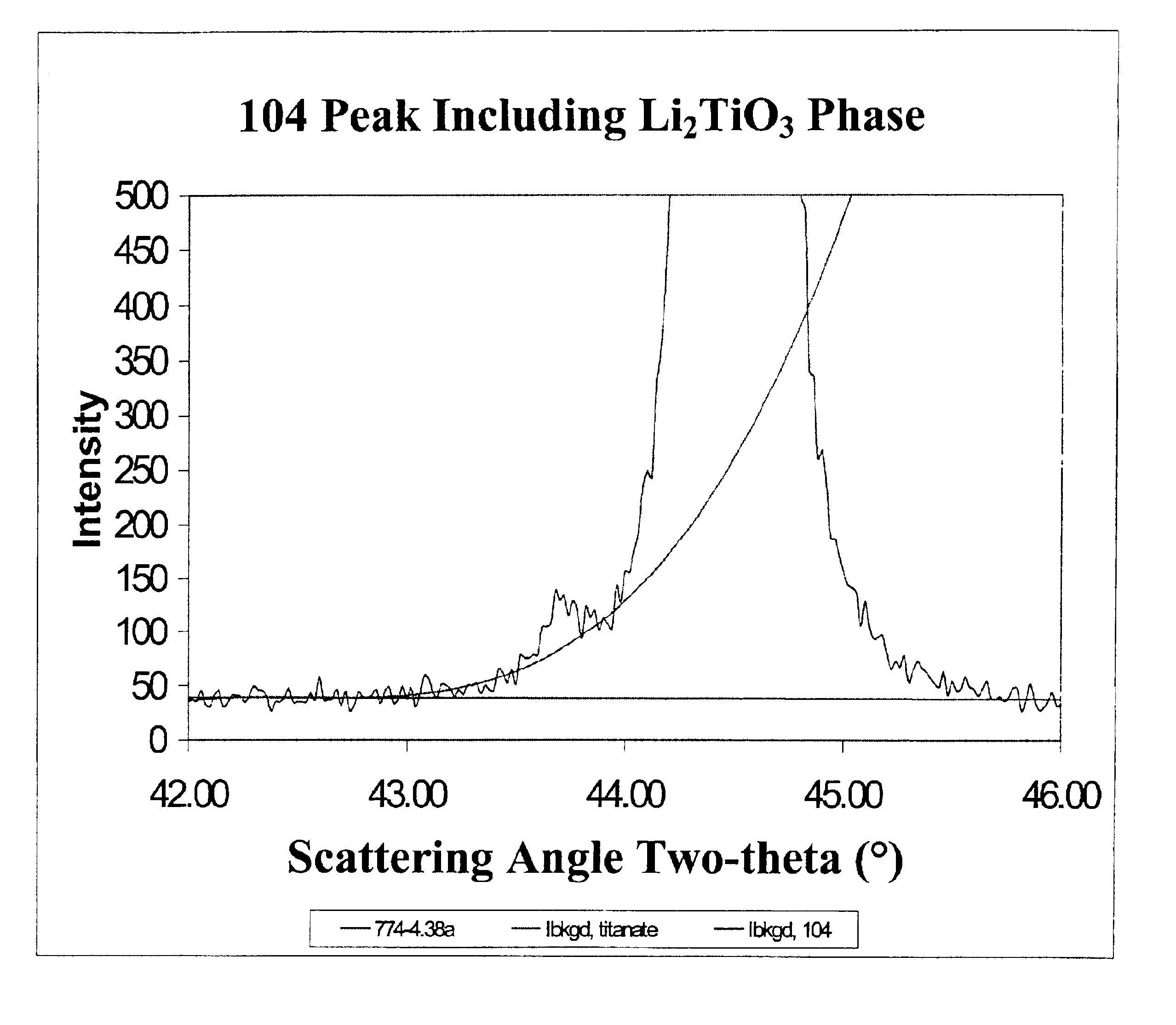
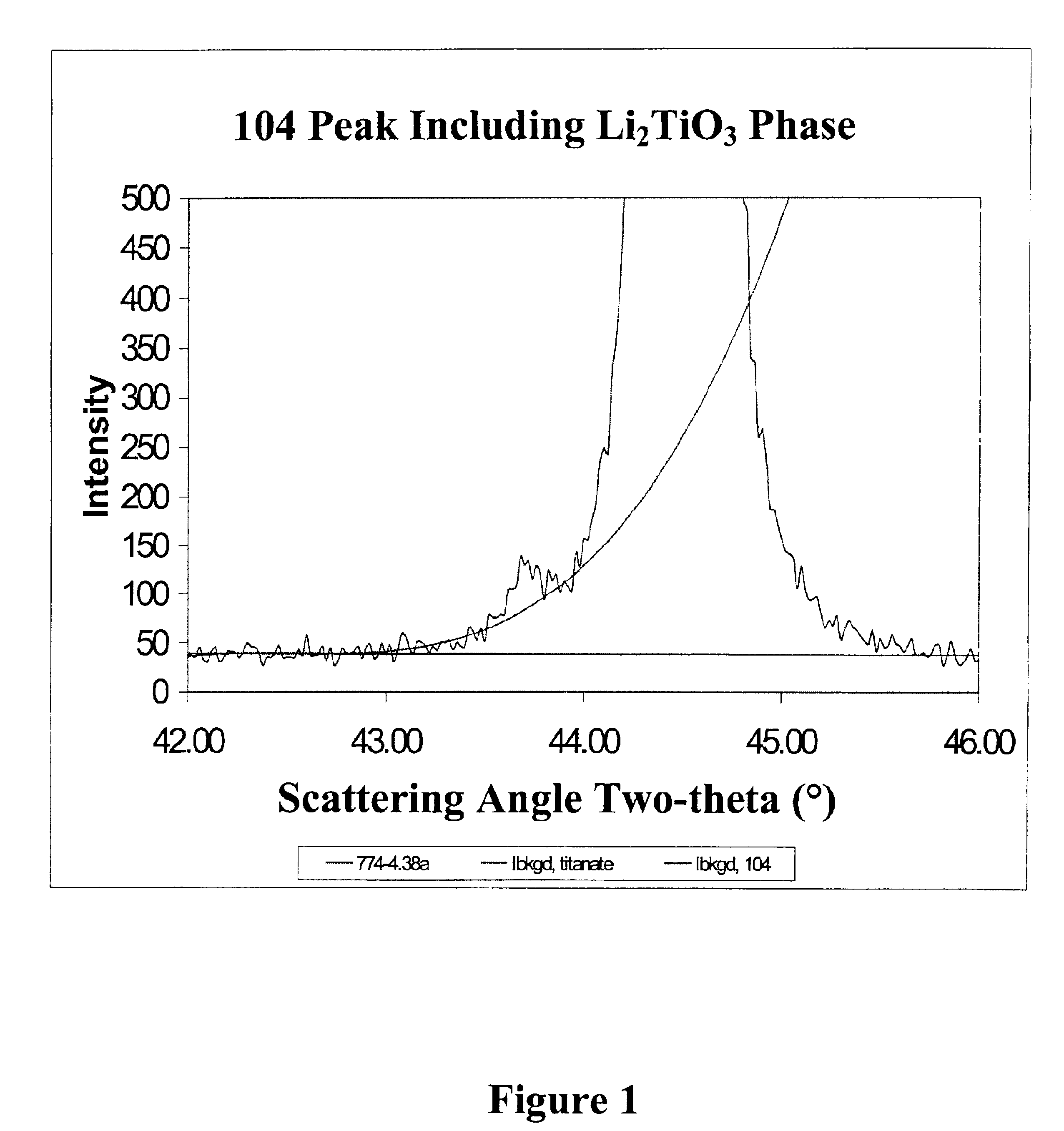
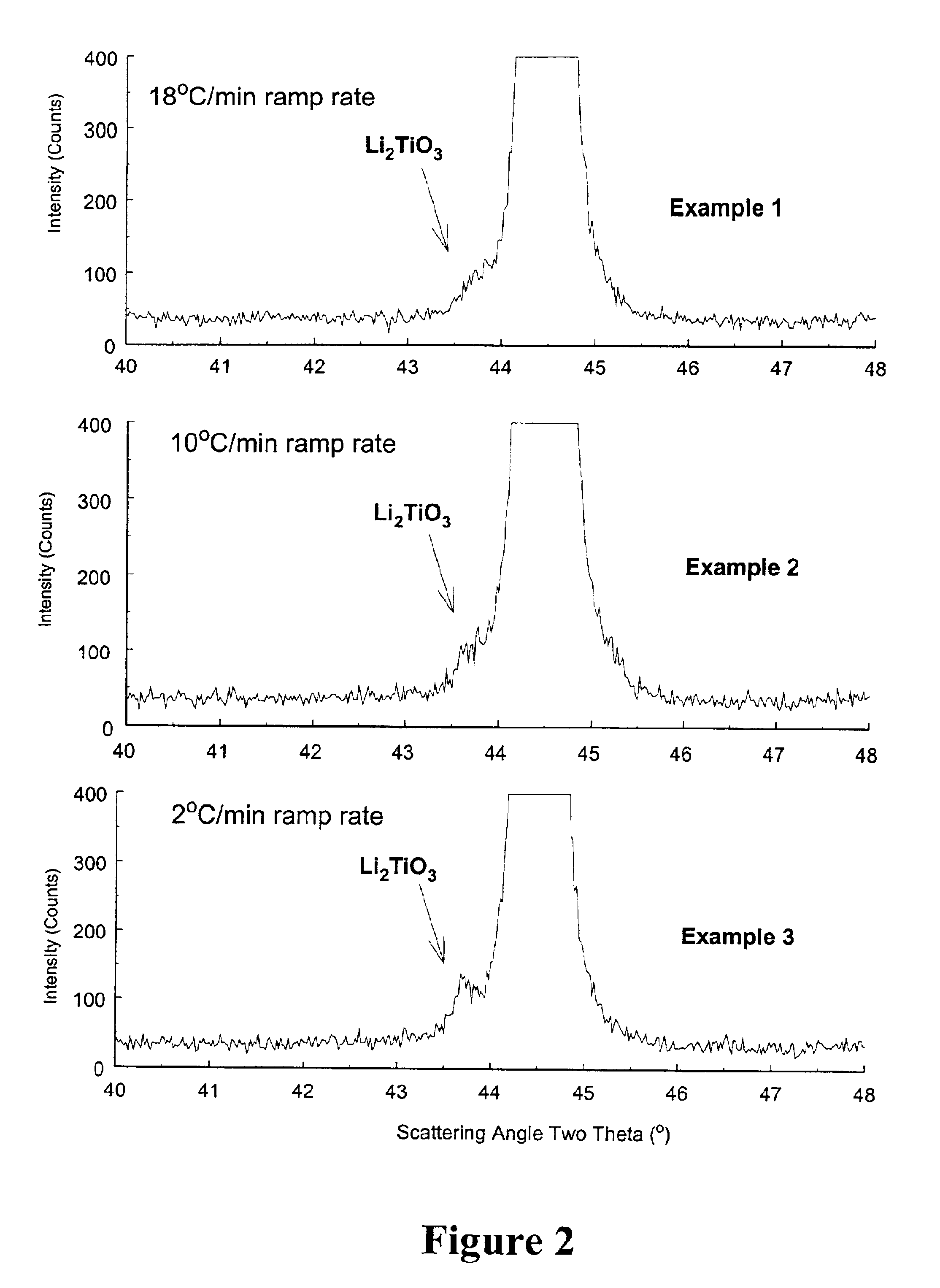
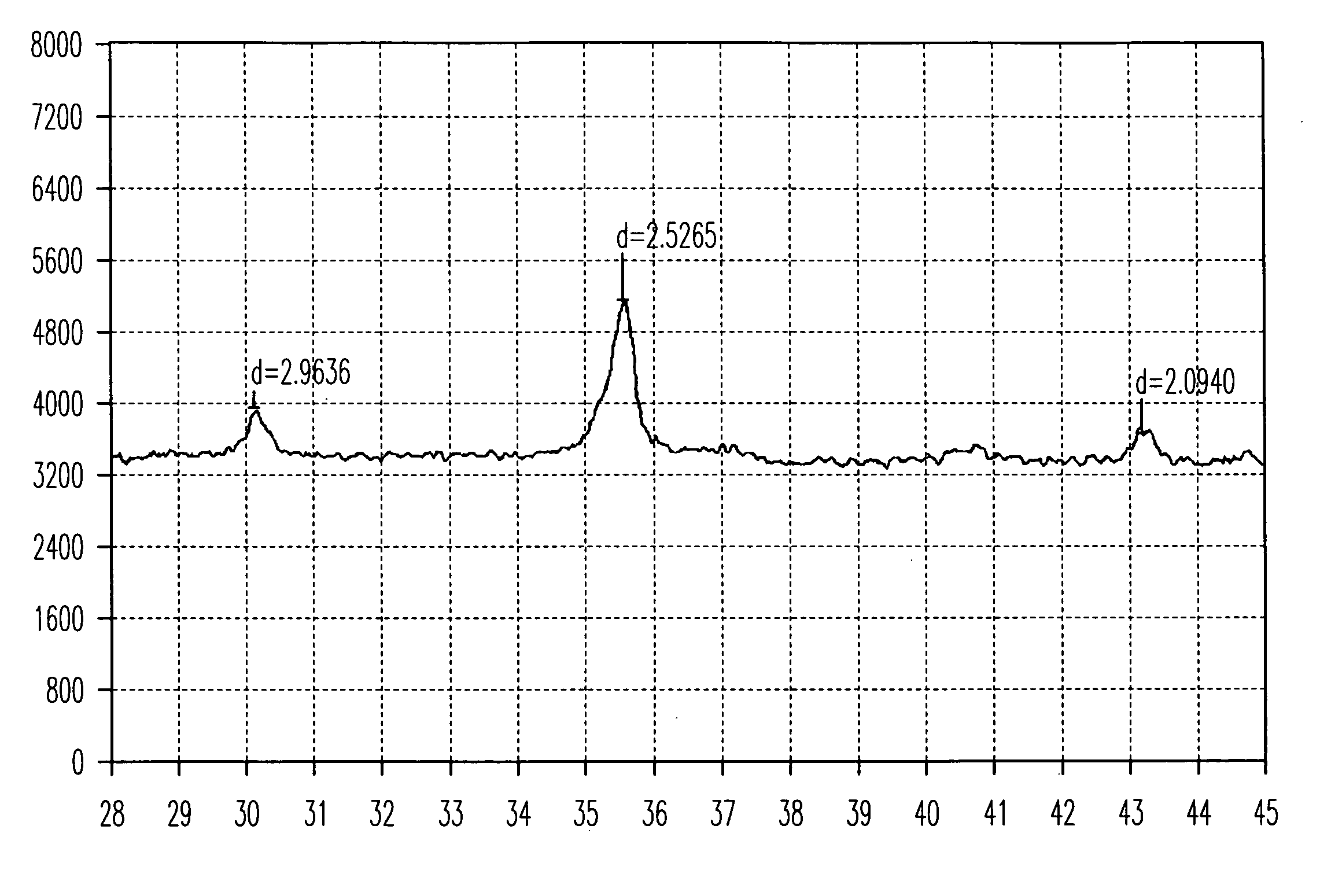
The present invention is a positive electrode active material that can be used in secondary lithium and lithium-ion batteries to provide the power capability, i.e., the ability to deliver or retake energy in short periods of time, desired for large power applications such as power tools, electric bikes and hybrid electric vehicles. The positive electrode active material of the invention includes at least one electron conducting compound of the formula LiM1x−y{A}yOz and at least one electron insulating and lithium ion conducting lithium metal oxide, wherein M1 is a transition metal, {A} is represented by the formula ΣwiBi wherein Bi is an element other than M1 used to replace the transition metal M1 and wi is the fractional amount of element Bi in the total dopant combination such that Σwi=1; Bi is a cation in LiM1x−y{A}yOz; 0.95≦x≦2.10; 0≦y≦x / 2; and 1.90≦z≦4.20. Preferably, the lithium metal oxide is LiAlO2 or Li2M2O3 wherein M2 is at least one tetravalent metal selected from the group consisting of Ti, Zr, Sn, Mn, Mo, Si, Ge, Hf, Ru and Te. The present invention also includes methods of making this positive electrode active material.
Owner:UMICORE AG & CO KG
Composition for a desulfurizer with a high sulfur capacity and the process of making the same
ActiveUS20080047395A1Quality improvementEasy to operateGas treatmentBlast furnace componentsActive componentLiquid state
The present invention discloses a composition for a desulfurizer with a high sulfur capacity and a process for making the same. The composition comprises the active components of three kinds of iron oxides and is used in the desulfurizer to remove hydrogen sulfide from the gaseous and liquid state feed stocks. The above-mentioned composition comprises cubic ferroferric oxide in the form of crystalline phase (Fe3O4), amorphous ferric oxide (Fe2O3) and amorphous ferric oxide monohydrate (Fe2O3.H2O). The composition has a sulfur capacity of at least 40%. The process for preparing the composition comprises the following steps: (1) mixing a solid ferrous compound with a solid hydroxide at a molar ratio of iron to hydroxyl being in the range from 1:2 to 1:3; (2) kneading the mixture feeds obtained in step (1) and making them react completely; (3) drying the products obtained in step (2) in the air; (4) washing and filtering the feeds obtained in the step (3); (5) naturally drying or baking the solids obtained in step (4) to form a composition for a desulfurizer with a high sulfur capacity. The process of the present invention is simple and easy to operate, consumes less energy and produces the products with a stable quality.
Owner:BEIJING HAIXIN ENERGY TECH CO LTD
Ternary oxide nanostructures and methods of making same
InactiveUS7585474B2Suitable for preparationFrom gel stateAlkaline earth titanatesSingle crystalNanostructure
A single crystalline ternary nanostructure having the formula AxByOz, wherein x ranges from 0.25 to 24, and y ranges from 1.5 to 40, and wherein A and B are independently selected from the group consisting of Ag, Al, As, Au, B, Ba, Br, Ca, Cd, Ce, Cl, Cm, Co, Cr, Cs, Cu, Dy, Er, Eu, F, Fe, Ga, Gd, Ge, Hf, Ho, I, In, Ir, K, La, Li, Lu, Mg, Mn, Mo, Na, Nb, Nd, Ni, Os, P, Pb, Pd, Pr, Pt, Rb, Re, Rh, Ru, S, Sb, Sc, Se, Si, Sm, Sn, Sr, Ta, Tb, Tc, Te, Ti, Tl, Tm, U, V, W, Y, Yb, and Zn, wherein the nanostructure is at least 95% free of defects and / or dislocations.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
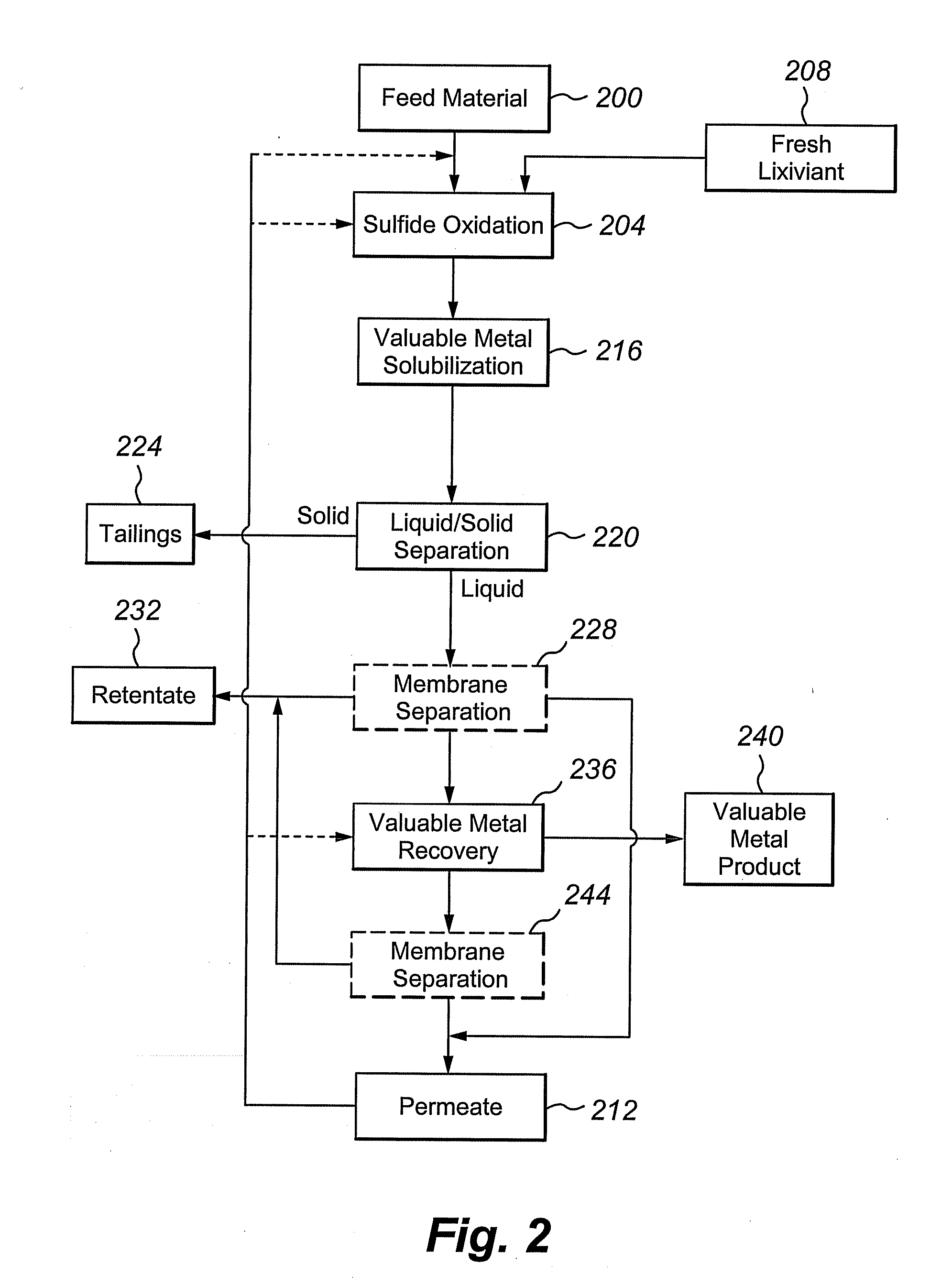
Multivalent iron ion separation in metal recovery circuits
InactiveUS20080069748A1High sulfide sulfur oxidation rateReduce electricity costsSolvent extractionFerrous oxidesPhysical chemistryFerric ion
Owner:HW PROCESS TECH
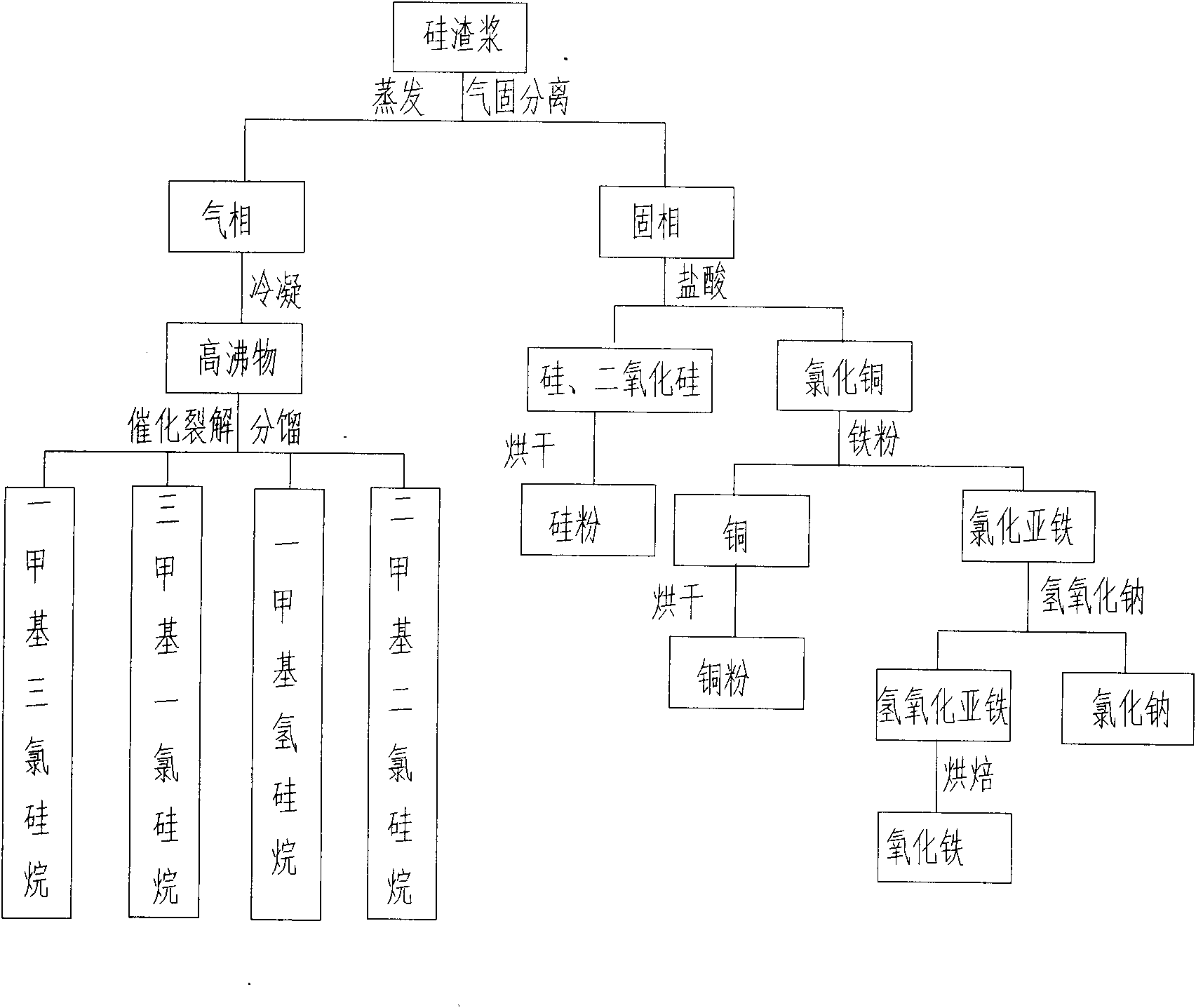
Processing and recycling process for silicon slurry
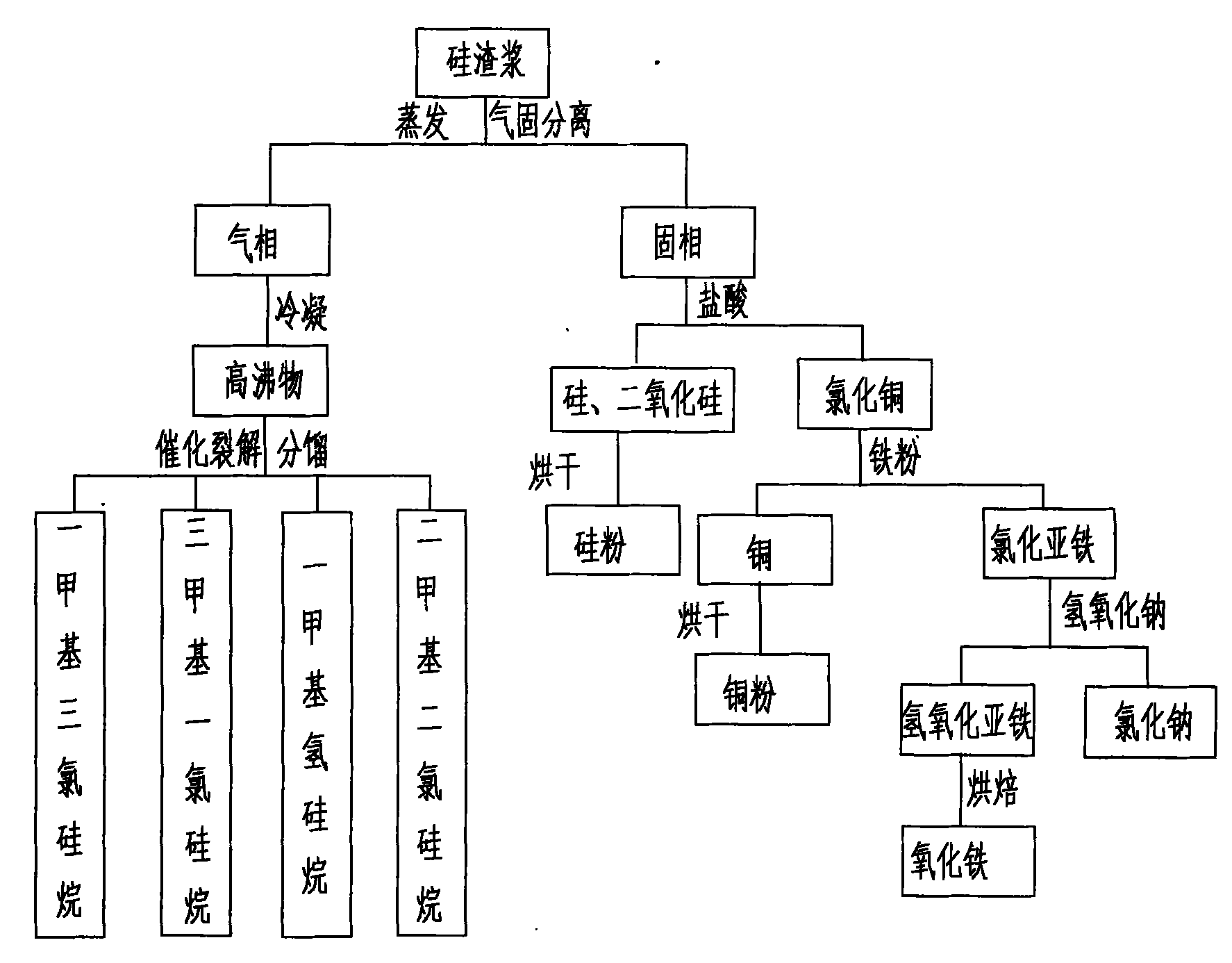
PendingCN102174674AGuaranteed to drive normallyEliminate pollutionSilicon organic compoundsFerrous oxidesGas phaseSilanes
The invention provides a processing and recycling process for silicon slurry, which comprises the steps as follows: silicon slurry is evaporated for gas and solid separation; high boiling point substances are obtained after the gas phase is condensed; the high boiling point substances react with hydrogen chloride to be cracked into mixed monomers such as methyl trichloro silicane, trimethyl chloro silicane, methyl silane, dimethyl dichloro silicane and the like; and after the fractionation, each pure monomer product can be obtained. The solid phase separated through evaporation mainly contains cuprous oxide, silicon, silicon dioxide and the like; hydrochloric acid (or sulfuric acid) is added into the solid phase to extract copper chloride (or copper sulfate, which can be sold directly) by a chemical method; and silicon and silicon dioxide are separated out. Iron powder is added into a copper chloride solution for extracting copper and ferrous chloride, and sodium hydroxide is added into the ferrous chloride solution for extracting ferrous oxide. The processing and recycling process is environment-friendly and can eliminate pollution, and meanwhile can recover various by-products from silicon slurry, thereby having well economic benefits.
Owner:上海竟茨环保科技有限公司
High purity nanoscale metal oxide powders and methods to produce such powders
InactiveUS20050063889A9High purityIncrease volumeMaterial nanotechnologyTransportation and packagingPhotonicsMaterials science
Owner:PPG IND OHIO INC
Production and producer for nanometer ferric oxide
InactiveCN1817802AImprove product qualitySmall particle sizeFerrous oxidesFerric oxidesCombustionOxygen
Production of nanometer ferric oxide and its apparatus are disclosed. The process is carried out by carrying pentacarboxylic-based iron or its steam by pressurized N2 or its inert gas, atomization ejecting it above 0.01Mpa from materials pipeline of reactive kettle, combustion reacting with oxygen or air jetted from gas pipeline of reactive kettle, dropping ferric oxide particles into liquid settling medium, separating them out by settling liquid, receiving reactant, drying and cooling to obtain final product. It has better tinting strength and dispersion and can be used for building material, coating, ink, ceramic and papermaking.
Owner:JIANGSU TIANYI ULTRA FINE METAL POWDER
Composition for a desulfurizer with a high sulfur capacity and the process of making the same
ActiveUS7717979B2Quality improvementEasy to operateGas treatmentBlast furnace componentsLiquid stateActive component
The present invention discloses a composition for a desulfurizer with a high sulfur capacity and a process for making the same. The composition comprises the active components of three kinds of iron oxides and is used in the desulfurizer to remove hydrogen sulfide from the gaseous and liquid state feed stocks. The process for preparing the composition comprises the following steps: (1) mixing a solid ferrous compound with a solid hydroxide at a molar ratio of iron to hydroxyl being in the range from 1:2 to 1:3; (2) kneading the mixture feeds obtained in step (1) and making them react completely; (3) drying the products obtained in step (2) in the air; (4) washing and filtering the feeds obtained in the step (3); (5) naturally drying or baking the solids obtained in step (4) to form a composition for a desulfurizer with a high sulfur capacity.
Owner:BEIJING HAIXIN ENERGY TECH CO LTD
Preparation method of porous metallic oxide stacked by nano-particles
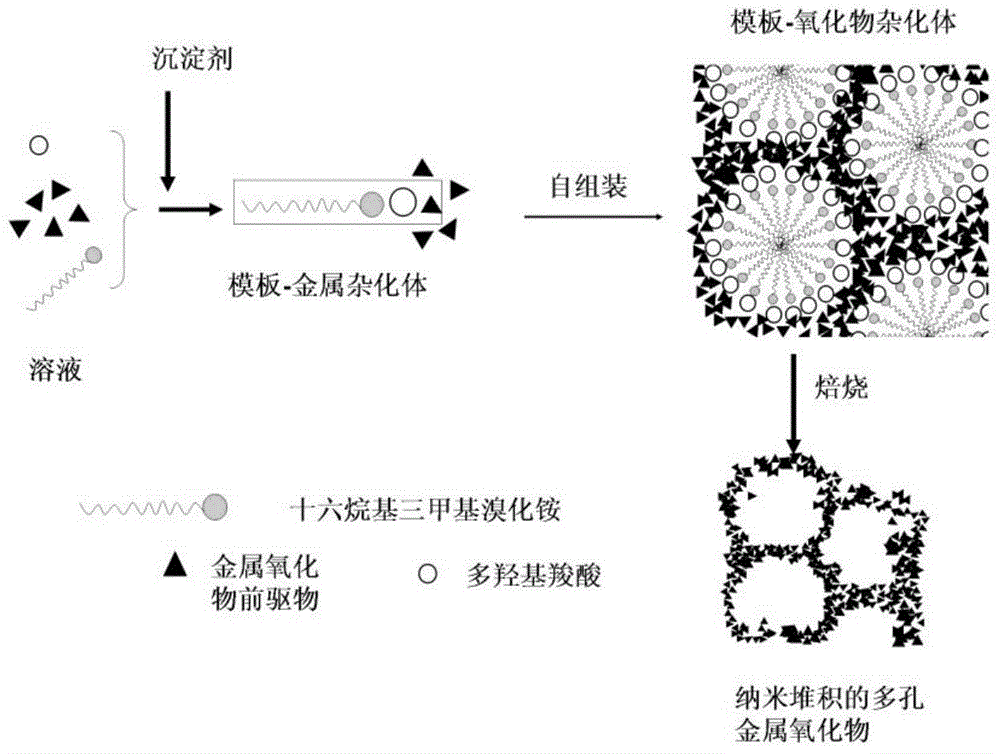
InactiveCN104445321AFast preparationSolve the problem that is difficult to filter and recycleMaterial nanotechnologyFerrous oxidesNanoparticleCarboxylic acid
The invention relates to a preparation method of porous metallic oxide stacked by nano-particles. The preparation method comprises the following steps: mixing a cationic surface active agent, a metal salt, hydroxycarboxylic acid and a precipitating agent for reaction, and recovering an intermediate product; and roasting the intermediate product to obtain the porous metallic oxide stacked by the nano-particles. By adopting the preparation method provided by the invention, the aim of quickly preparing the porous metallic oxide stacked by the nano-particles is achieved by adding hydroxycarboxylic acid into an aqueous solution of the cationic surface active agent to react with a metallic matrix. By adding the hydroxycarboxylic acid, the size of first-grade nano-particles is controlled, the assembly of nano-particles is assisted to form second-grade micron particles, and rich mesopores are constructed. By introducing the mesopores, the characteristic of a high specific surface of a nano material is kept, the problem that the nano material is difficult to filter and recover is solved, and three processes of preparation, assembly and pore forming of the nano-particles are realized in one kettle. The preparation method disclosed by the invention is simple in process, and water is adopted as a solvent, so that the preparation method is environment-friendly and the cost is greatly reduced.
Owner:QUFU NORMAL UNIV
Metal oxalate hydrate body having a certain shape, preparation method thereof, and metal oxide/carbon composite body prepared from the same
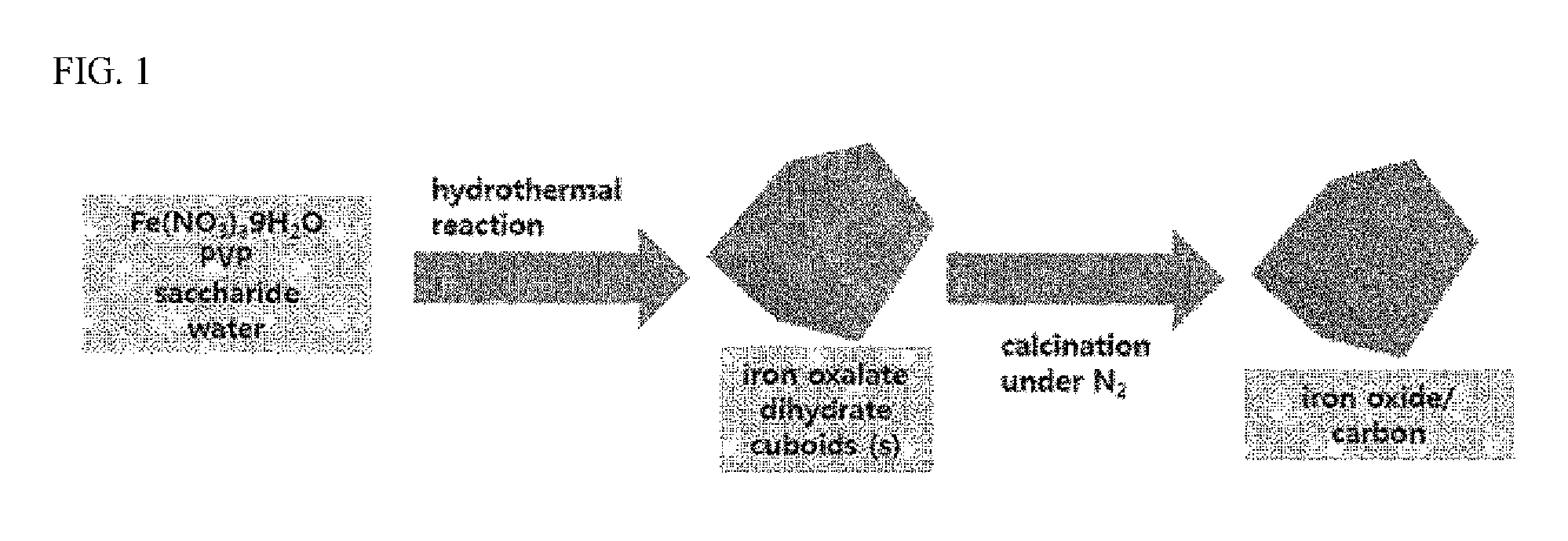
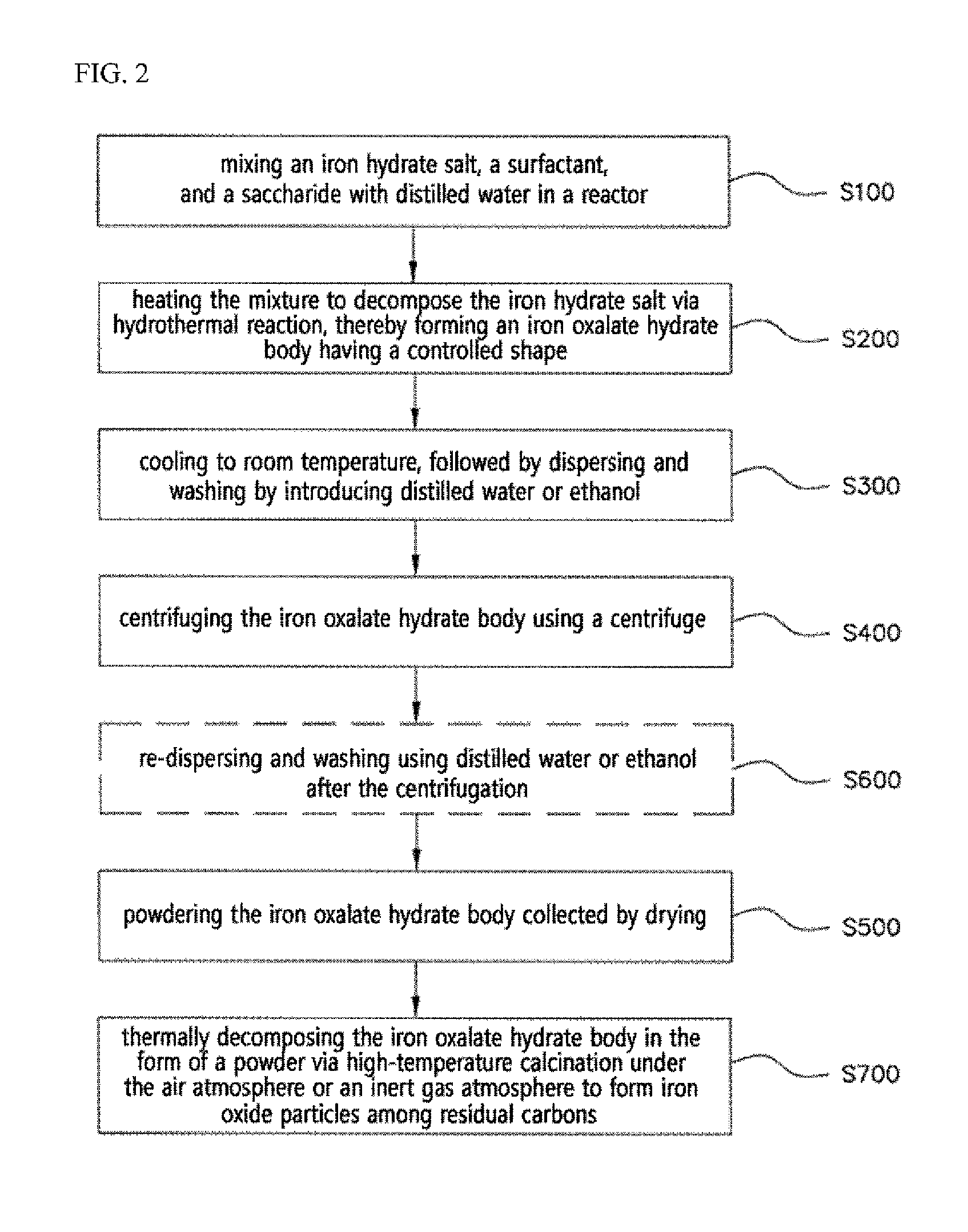
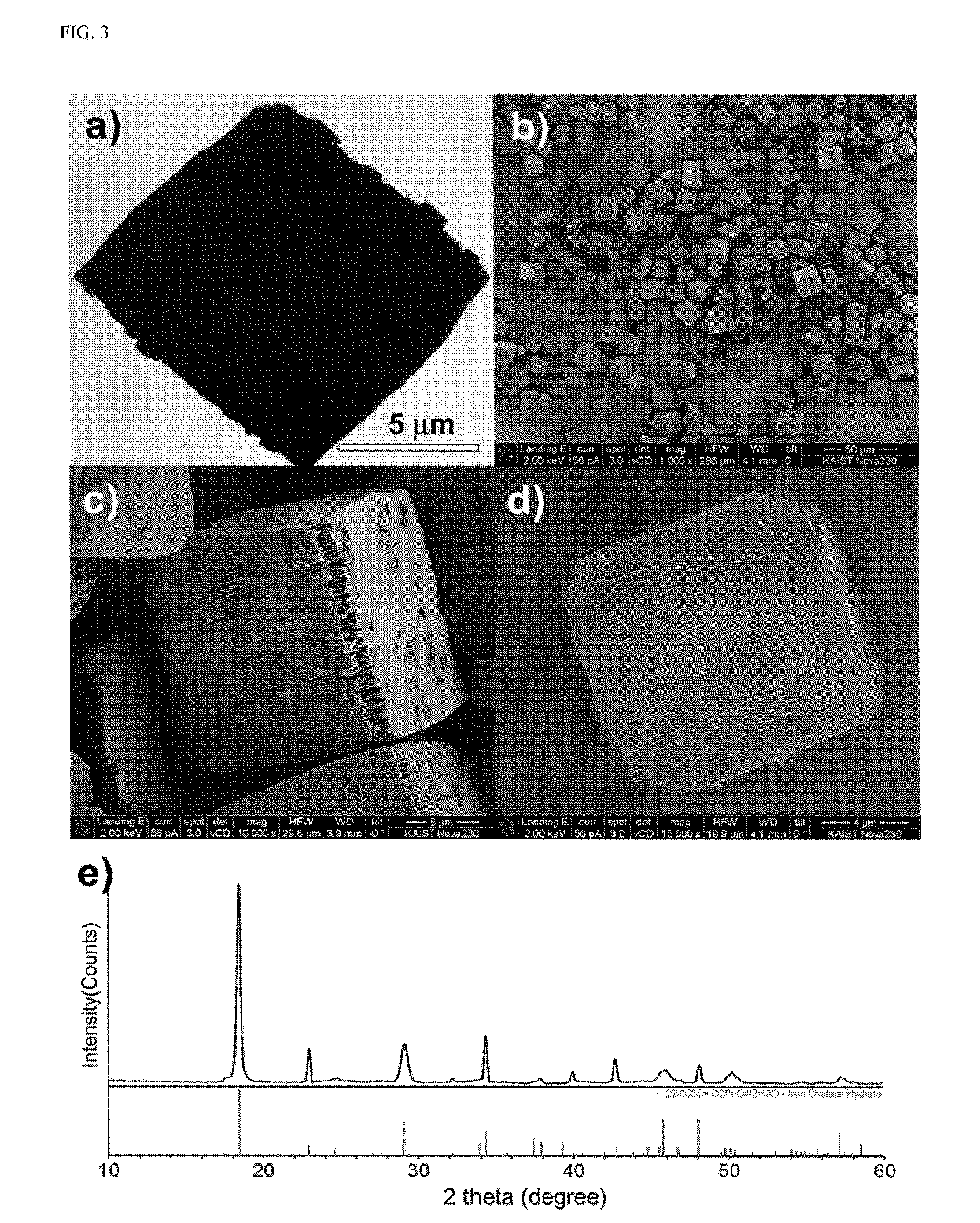
ActiveUS20160083410A1Stay in shapeOther chemical processesSynthetic resin layered productsCarbon compositesOxalate
The present invention relates to a metal oxalate hydrate body having a certain shape, a preparation method thereof, and a metal oxide / carbon composite body prepared by using the metal oxalate hydrate body. In the present invention, the metal oxalate body, whose shape is diversely controlled, and the metal oxide / carbon composite body therefrom are provided.
Owner:KOREA INST OF ENERGY RES
Novel microorganism capable of producing oxide
InactiveUS20120315437A1High yieldEasy to controlMaterial nanotechnologyBacteriaMicroorganismNanoparticle
Disclosed is a microorganism that belongs to the genus Leptothrix and is capable of producing iron oxide that has a ferrihydrite or lepidocrocite structure and has a form of aggregates of ferrihydrite nanoparticles or lepidocrocite nanoparticles; a bacterium that is capable of producing an iron oxide that has a ferrihydrite or lepidocrocite structure and has a form of aggregates of ferrihydrite nanoparticles or lepidocrocite nanoparticles; a culture medium for use in screening a bacterium that is capable of producing a metal oxide; a method for screening a bacterium that is capable of producing a metal oxide; a culture medium for culturing a bacterium that is capable of producing a metal oxide; a method for culturing a bacterium that is capable of producing a metal oxide; a method for producing a metal oxide; and iron oxide.
Owner:UNIV OKAYAMA
Mixed flora and application thereof in iron removal and whitening for kaolin
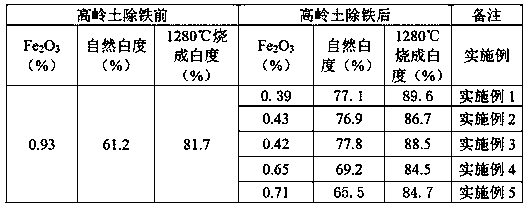
InactiveCN107603913AGood removal effectExtended service lifeBacteriaFerrous oxidesMicroorganismIron removal
The invention discloses mixed flora and application thereof in iron removal and whitening for kaolin. The mixed flora is prepared from the following components: iron-reducing bacteria RBFL, iron-reducing bacteria FeRB-FL1404, iron-reducing bacteria FL-HI, thermoanaerobacter ethanolicus, achromobacter, Geobacter bremensis Straub and Buchholz-Cleven; due to a synergistic effect of microbial strainsin the mixed flora, the iron-removing effect of the microbes for the kaolin can be improved. The bacterial liquid obtained by culturing the mixed flora is used for reducing Fe2O3 in the kaolin, and insoluble Fe2O3 is reduced into water-soluble Fe<2+> which can be removed by solid-liquid separation, so that the content of Fe2O3 in the kaolin can be reduced and the whiteness of the kaolin can be improved. The mixed flora has the advantages that the cost is low, the environmental-friendly effect is achieved, the kaolin ore with original low mining value and low grade can be mined, the service life of the mine can be prolonged, and the high quality of the low-grade kaolin can be realized.
Owner:FUJIAN NORMAL UNIV
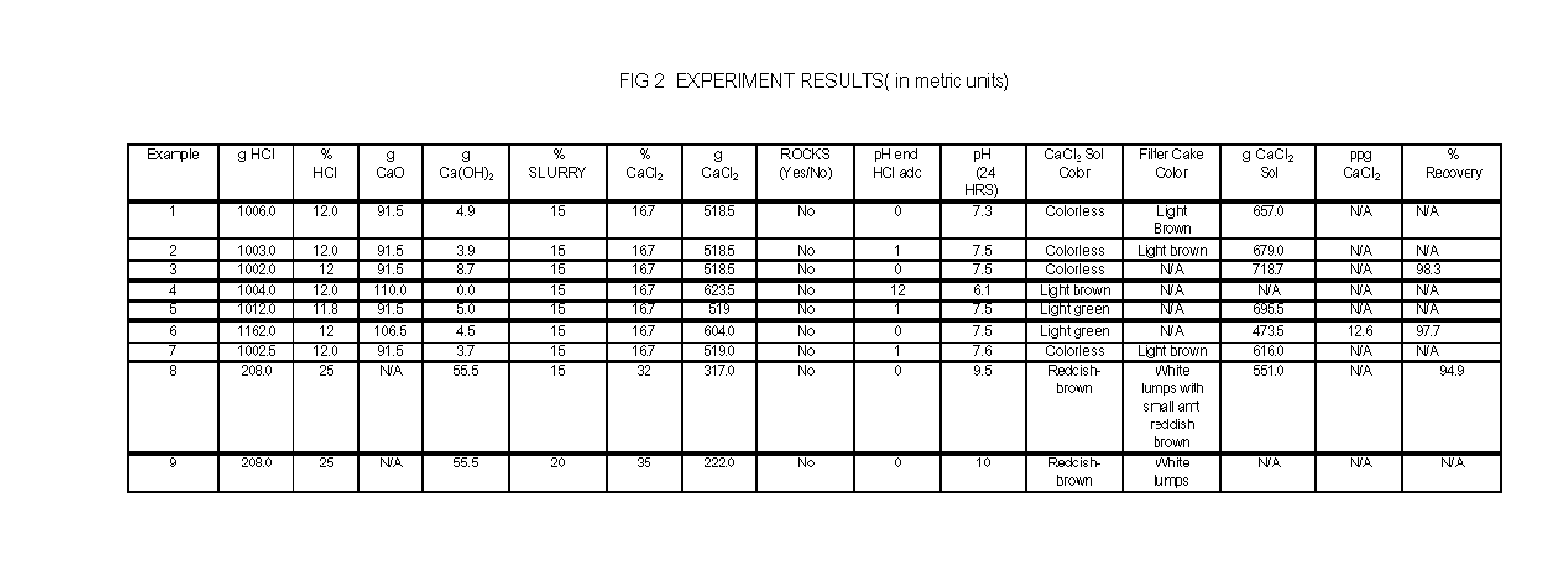
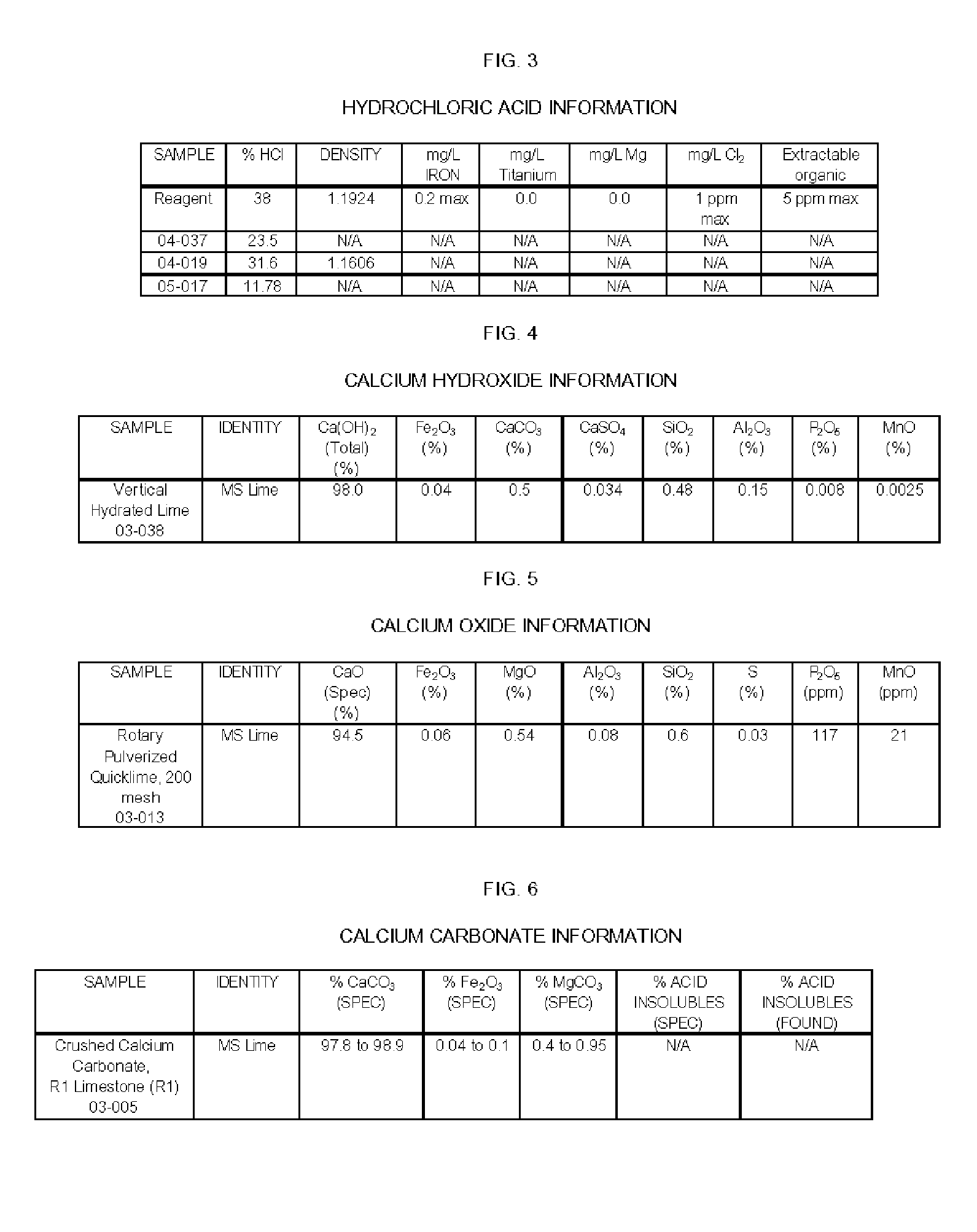
Apparatus and Methods For Producing Calcium Chloride, and Compositions and Products Made Therefrom
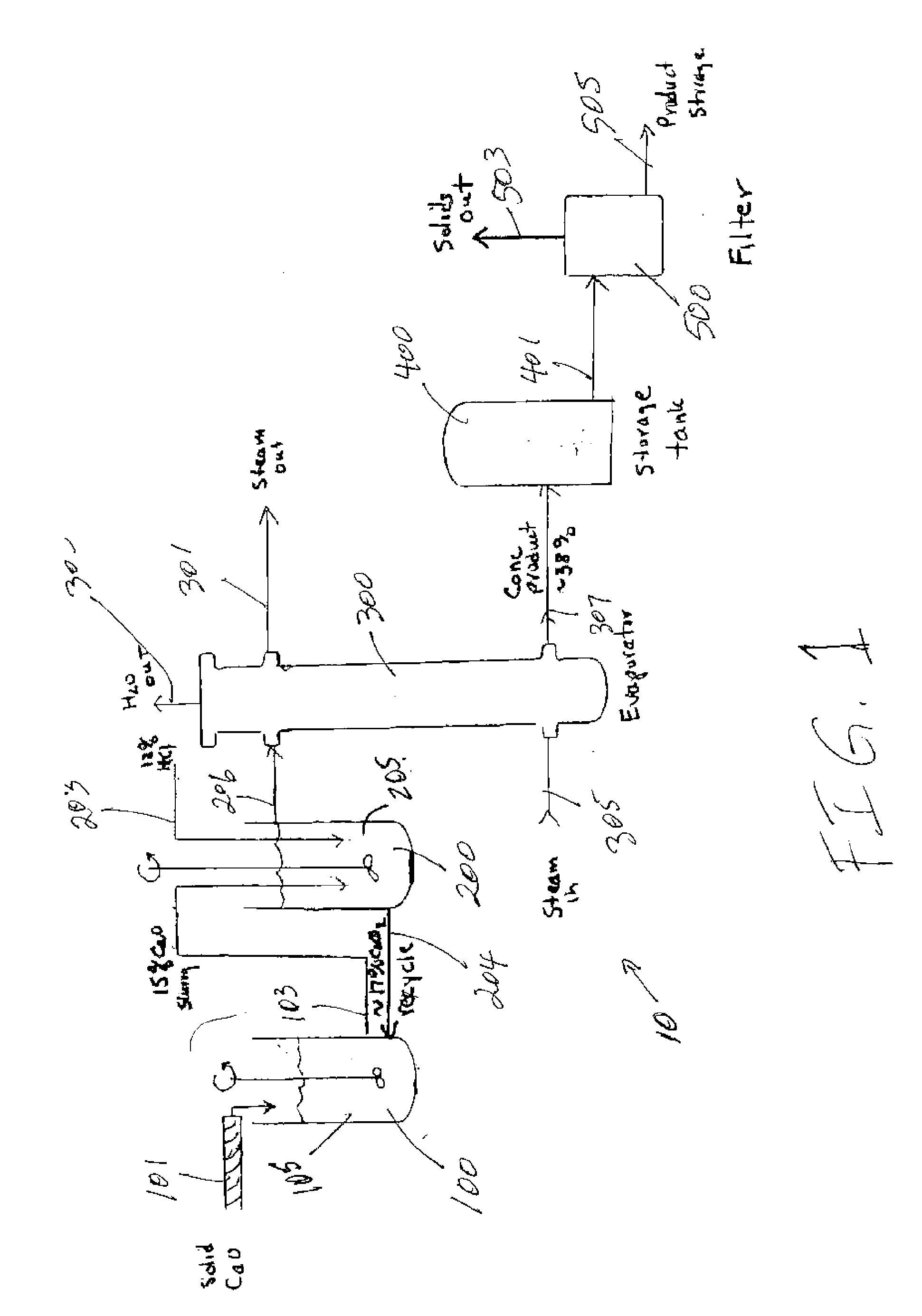
InactiveUS20070009423A1Reduce evaporative loadIncrease flow rateMagnesium halidesFerrous oxidesSlurryChloride
A method of producing calcium chloride including first forming a slurry of solid calcium oxide in an aqueous solution of calcium chloride, and then contacting the slurry with hydrochloric acid to convert at least a portion of the calcium oxide into calcium chloride.
Owner:ECO PROD SOLUTIONS LP
Method for preparing colored polyester in chain decomposing mode through waste polyester
ActiveCN110698658AQuality improvementContinuous and stable colorOrganic compound preparationCarboxylic acid esters preparationPolyesterPolymer science
The invention belongs to the technical field of waste polyester regeneration, and particularly relates to a method for preparing colored polyester in a chain decomposing mode through waste polyester.The method for preparing colored polyester in the chain decomposing mode through waste polyester includes the following steps that (1) the recovered waste polyester is subjected to pre-processing andmatching color matching; (2) a chain decomposing reaction is carried out under the action of a chain decomposing catalyst, so a chain decomposing process is that the temperature of a mother liquor is190-210 DEG C, the reaction time is 30minutes-5 hours, a chain decomposing solution is subjected to precipitation, impurity removal and multi-stage filtration treatment, the filtration fineness is sequentially improved, the fineness of the primary filtration is 50-100 mesh, and finally the filtration fineness is 500-800 mesh; and (3) a chain-decomposed matter after impurity removal is sent to a polycondensation kettle for pre-polycondensation through complementary color, and finally a finished product is formed through final polycondensation. The method can prepare continuous, stable and high-quality colored recycled polyester, and high-value recycling of the waste polyester is realized. The method can prepare continuous, stable and high-quality colored recycled polyester, and realize high-value recycling of waste polyester.
Owner:宁波大发新材料有限公司
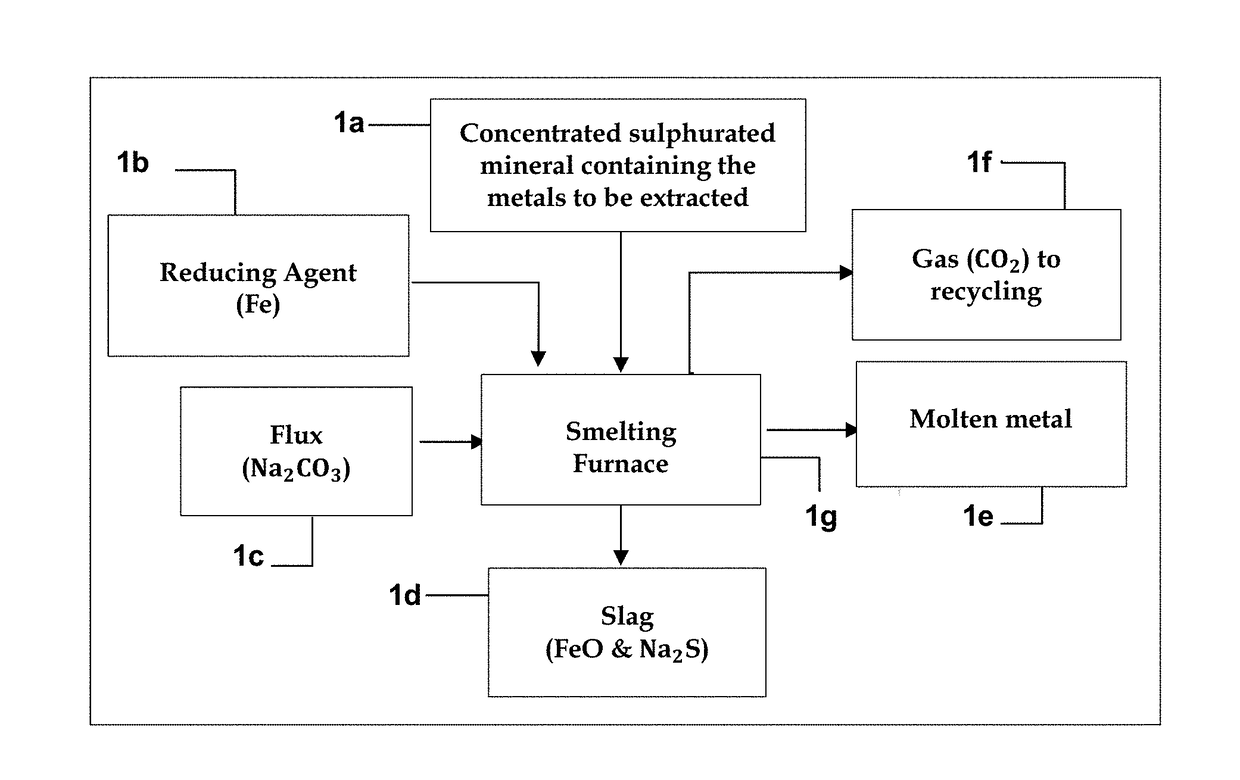
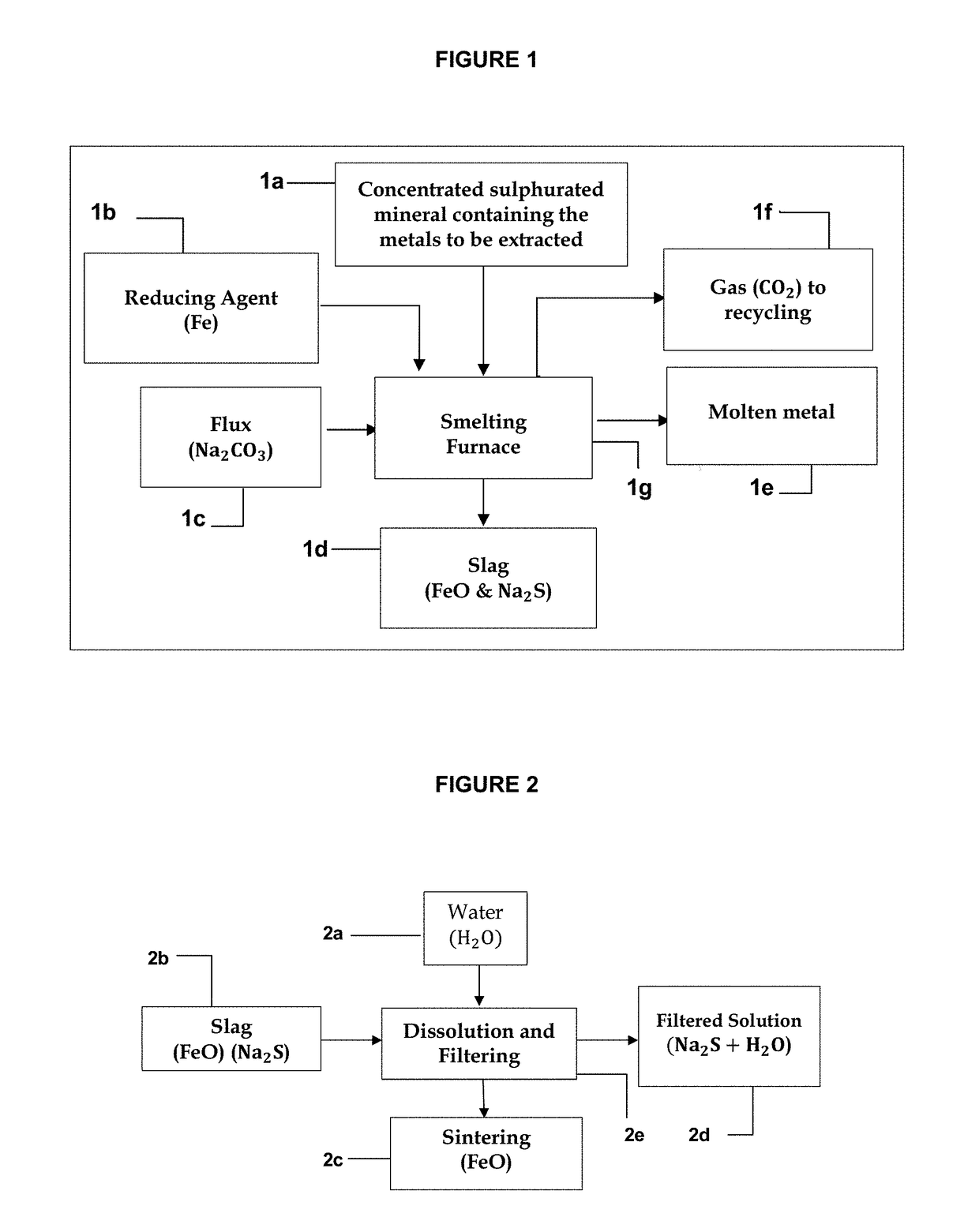
Method for extracting metals from concentrated sulphurated minerals containing metals by direct reduction with regeneration and recycling of the reducing agent, iron, and of the flux, sodium carbonate
InactiveUS20180282837A1Reduce environmental pollutionReduce processing costsFerrous oxidesSulfur preparation/purificationChemical reactionSlag
A method is disclosed for extracting metals from concentrated sulphurated minerals containing metals by direct reduction with regeneration and recycling of the reducing agent, iron, and of the flux, sodium carbonate. It is a combination of pyrometallurgical and hydrometallurgical processes which differ from the conventional processes. They do not require previous toasting of the concentrated sulphurated minerals and are technically and economically more advantageous than the presently used processes, since they directly reduce to zero the positive oxidation state of the metal, using a single reactor for extracting the metal, regenerating and recycling the metallurgical feed materials in complementary processes, the kinetics of the chemical reactions being characterised by high speed, without generating any slags or pollutant gases. The metals can be extracted at a reduced cost and in an environmentally sustainable manner
Owner:C RDENAS ARBIETO FRANCISCO JAVIER
Method of manufacturing hexagonal ferrite magnetic powder, magnetic recording medium and method of manufacturing the same
InactiveUS20100021771A1Ultra-high-density recordingImprove fill rateMaterial nanotechnologyMagnetic materials for record carriersMagnetizationMaterials science
An aspect of the present invention relates to a method of manufacturing a hexagonal ferrite magnetic powder comprising preparing a melt by melting a starting material mixture, wherein the starting material mixture comprises at least one hexagonal ferrite-forming component and glass-forming component comprising at least one B2O3 component and a content of the B2O3 component in the mixture ranges from 15 to 27 mole percent in terms of B2O3; rapidly cooling the melt to obtain a solid having a saturation magnetization level of equal to or lower than 0.6 A·m2 / kg; and heating the solid to a temperature range of 600 to 690° C. and maintaining the solid within the temperature range to precipitate a hexagonal ferrite magnetic powder having an average plate diameter ranging from 15 to 25 nm.
Owner:FUJIFILM CORP
Amine functionalized mesoporous iron oxyhydroxide and method for fabricating the same
The present invention relates to an amine functionalized mesoporous iron oxyhydroxide and a method for fabricating the same, wherein the amine functionalized mesoporous iron oxyhydroxide is provided with amino group with high reactivity with anion heavy metal on the FeOx surface with large special area, thereby effectively removing the anion heavy metal in water. The method for fabricating mesoporous FeOx structure with amino group according to one embodiment of the invention is characterized by comprising the following steps: a first step, mixing water solution of ferric chloride (FeCl12) and surfactant; a second step, mixing peroxide in the water solution of water solution of ferric chloride and surfactant; a third step, after performing centrifugal separation on the mixture solution of the second step, drying the solid matter for fabricating powder-shaped mesoporous FeOx structure; and a fourth step, after dispersing the mesoporous FeOx structure into anhydrous toluene, injecting aminosilane for causing reaction between the aminosilane and the mesoporous FeOx structure, thereby generating amino groups on the surface of the mesoporous FeOx structure.
Owner:KOREA INST OF SCI & TECH
Method for one-step synthesis of metal oxide loaded transition metal carbide
InactiveCN110683586ASmall and uniformReduce experimentTitanium carbideFerrous oxidesMaterials scienceMagnetic separation
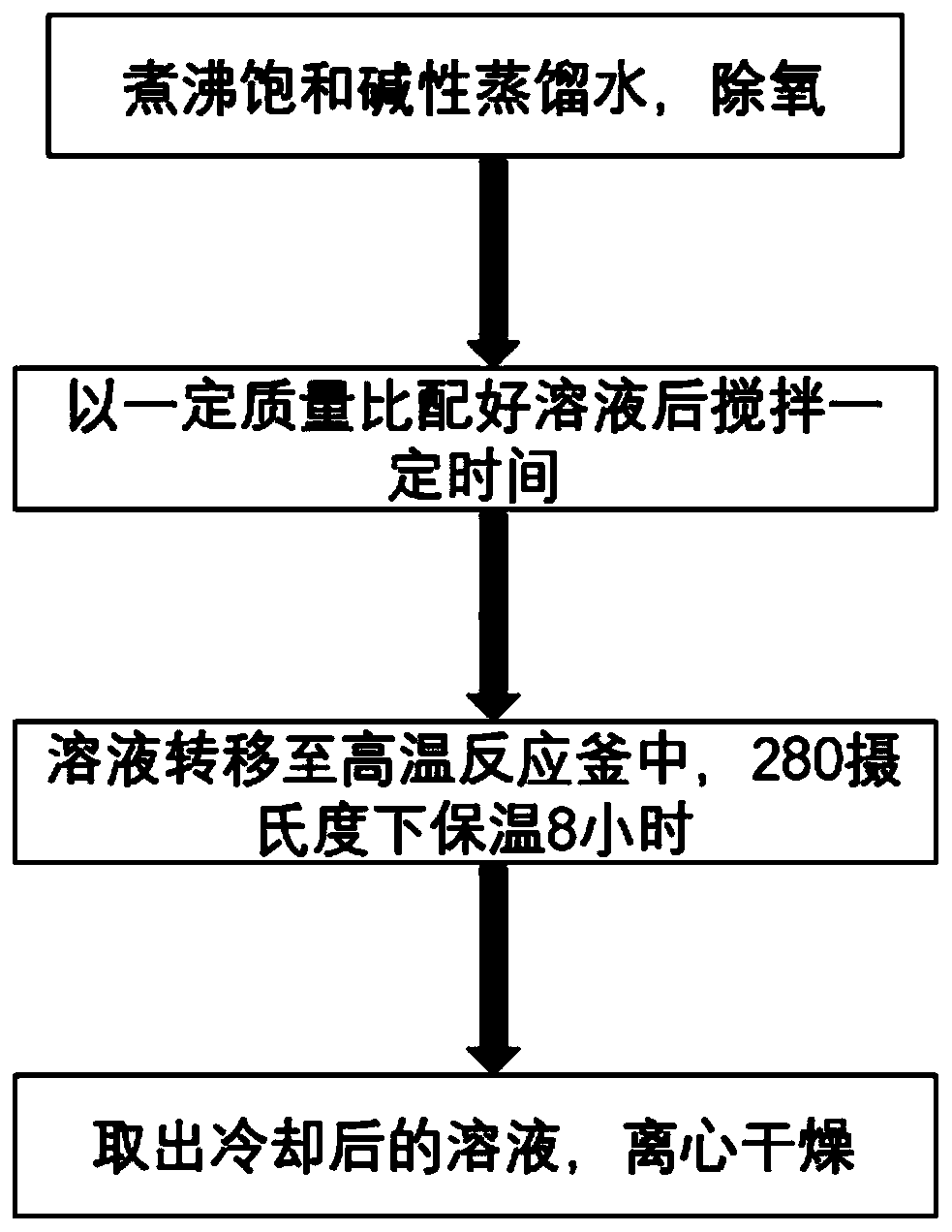
The invention provides a method for one-step synthesis of a metal oxide loaded transition metal carbide. The method comprises following steps: preparing a saturated strong alkali solution; then addinga raw material MAX phase and a metal precursor; carrying out one-step preparation of the metal oxide loaded transition metal carbide by utilizing the reactivity of the saturated strong alkali solution to the MAX phase and pyrolysis of the metal precursor at high temperature, and on the basis, selectively adding hydrazine hydrate to regulate and control the valence state of the formed transition metal oxide metal; and uniformly stirring an obtained system, heating to 280-350 DEG C, keeping the temperature for 6-10 hours, cooling, centrifugally collecting a black solid; washing, and drying to obtain a finished product. The method is easy to operate, reaction parameters are easy to control, the metal content is controllable, the method can be used for large-scale industrial production, and the obtained metal oxide / MXenes composite material has good hydrophilicity and a large specific surface area, can be subjected to magnetic separation and has outstanding technical advantages.
Owner:NANCHANG UNIV
Sulfided Iron (II) Compound and Method of Manufacture
ActiveUS20150183656A1Low costLess impuritiesSynthetic resin layered productsFerrous oxidesIron saltsSiderite
The invention involves the formation of a sulfided stable iron (II) compound from an iron (II) oxide and / or hydroxide and where the molar ratio of sulfur to iron (II) is greater than 1. Preferably these oxides and / or hydroxides are present as nanoparticles in the 5-10 nanometer range. It has been discovered that such particles can be formed at lower cost and with fewer impurities by using ferrous carbonate (FeCO3) from siderite as compared to known processes from various iron salts such as sulfates and chlorides.
Owner:NEW TECH VENTURES
Pyrophoric iron sulfide treatment using sodium nitrite
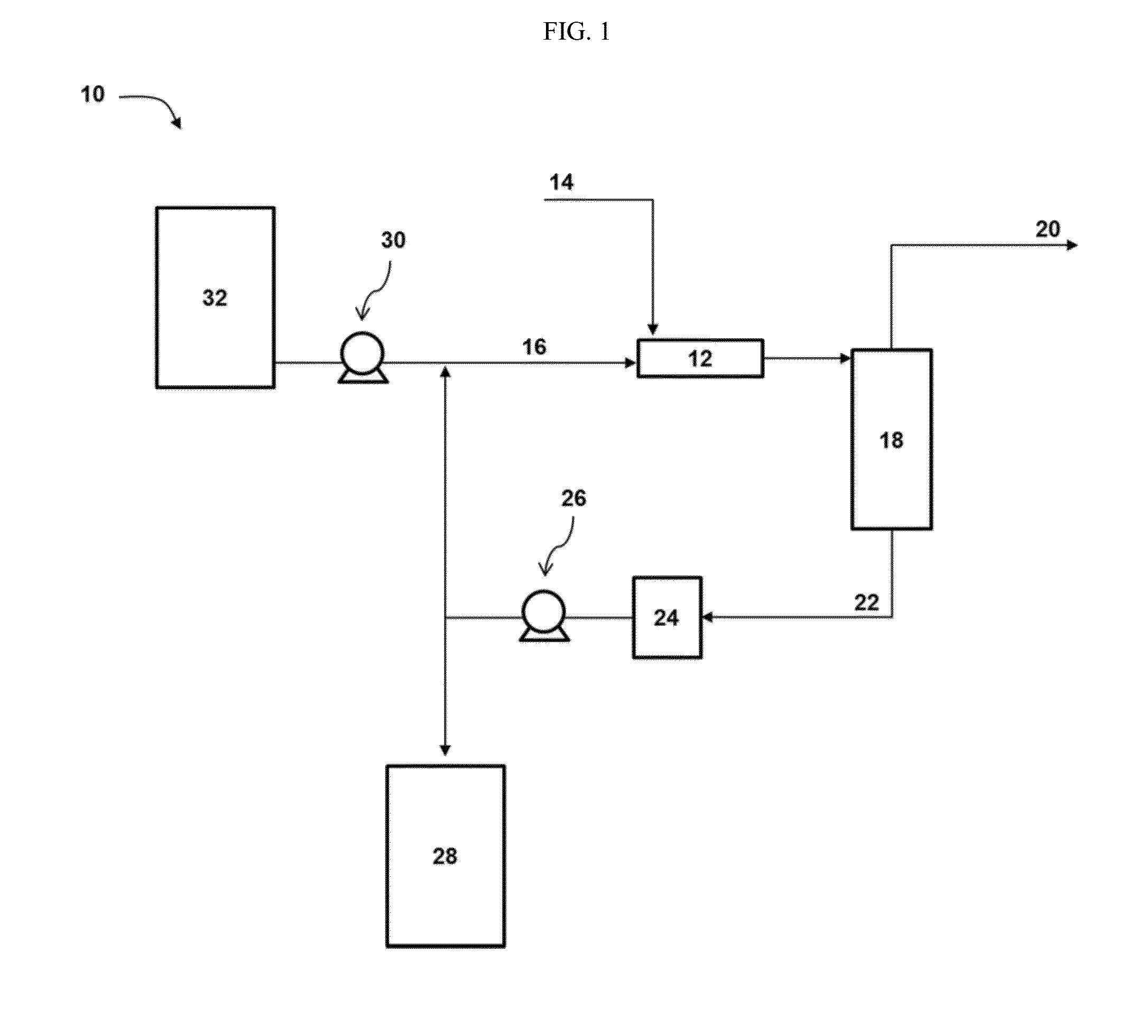
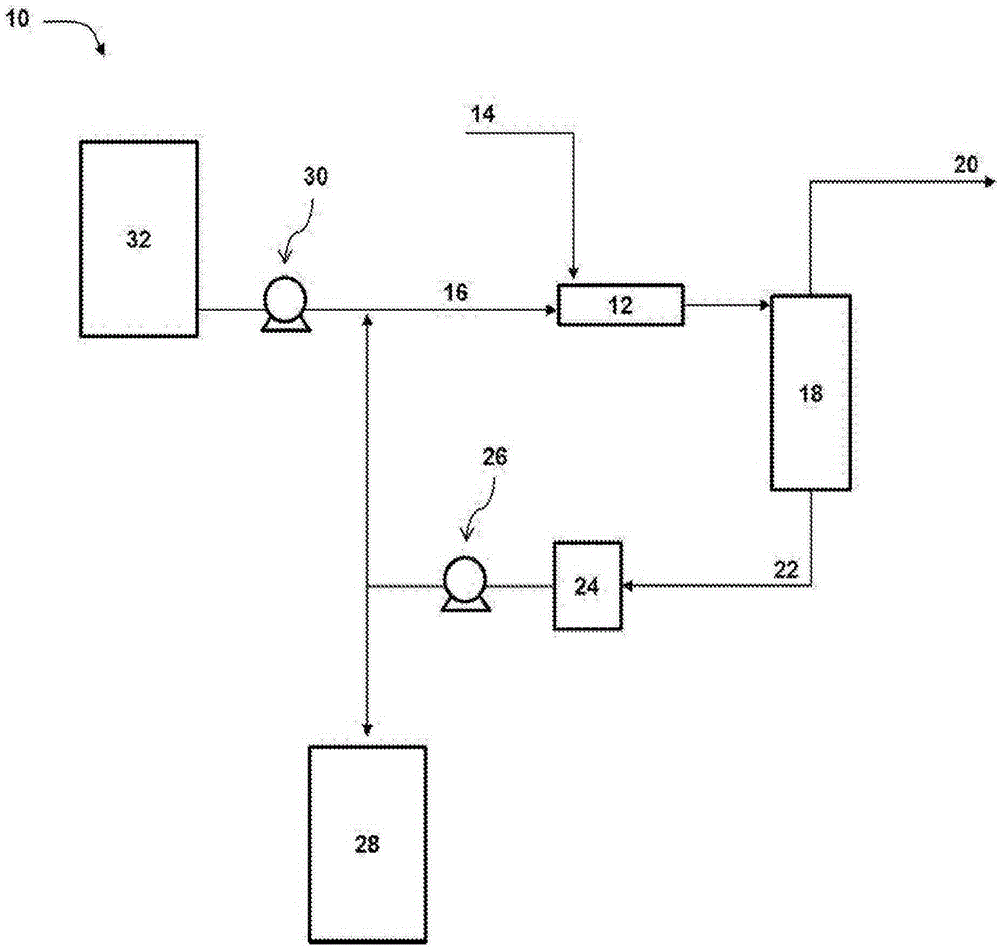
ActiveUS9452941B2Surface-active detergent compositionsNon-surface-active detergent compositionsCause injurySodium nitrite
Pyrophoric material such as iron sulfide is frequently found in refinery equipment. When the equipment is opened to the atmosphere for maintenance, an exothermic reaction can take place that may cause injury to personnel and catastrophic damage to equipment. A process used to treat pyrophoric material uses sodium nitrite injected into a gaseous carrier stream to oxidize iron sulfides to elemental sulfur and iron oxides. The sodium nitrite solution may be buffered to a pH of about 9 with disodium phosphate or monosodium phosphate. A chemical additive that provides a quantitative measure of reaction completion may be added to the treatment solution.
Owner:REFINED TECH
Highly active nano iron catalyst for the absorption of hydrogen sulfide
ActiveCN105358235ALow costLess impuritiesDispersed particle separationFerrous oxidesSideriteImpurity
The invention involves the formation of a stable iron (II) oxide and / or hydroxide. Preferably these oxides and / or hydroxides are present as nanoparticles in the 5-10 nanometer range. It has been discovered that such particles can be formed at lower cost and with fewer impurities by using ferrous carbonate (FeC03) from siderite as compared to known processes from various iron salts such as sulfates and chlorides. The novel nanoparticles are particularly adapted to removing sulfur compounds such as H2S from liquid and / or gaseous streams, such as hydrocarbon streams.
Owner:NEW TECH VENTURES
System and method for recycling iron and zinc from hot galvanizing waste acid
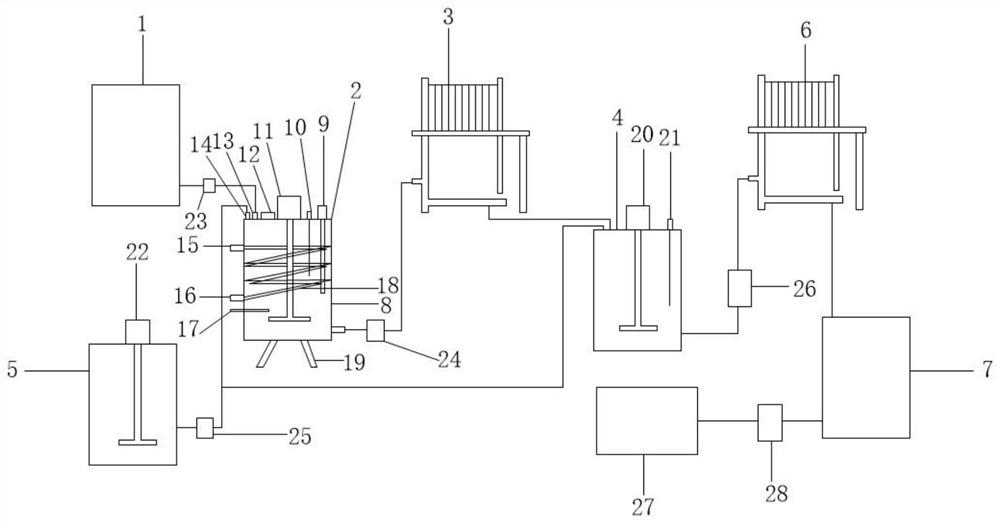
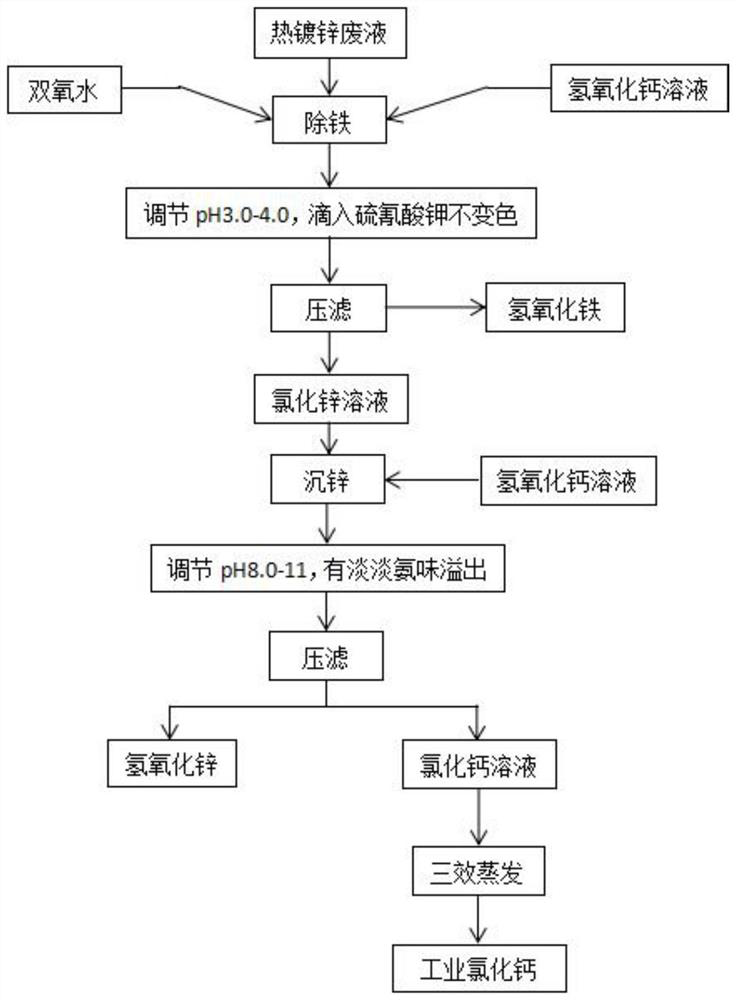
PendingCN112174184AReduce the amount addedLow costCalcium/strontium/barium chloridesZinc oxides/hydroxidesCalcium hydroxideIron removal
Owner:SHAANXI NEW WORLD SOLID WASTE COMPREHENSIVE DISPOSAL
Pyrophoric Iron Sulfide Treatment Using Sodium Nitrite
ActiveUS20150273533A1Surface-active detergent compositionsNon-surface-active detergent compositionsCause injurySodium nitrite
Pyrophoric material such as iron sulfide is frequently found in refinery equipment. When the equipment is opened to the atmosphere for maintenance, an exothermic reaction can take place that may cause injury to personnel and catastrophic damage to equipment. A process used to treat pyrophoric material uses sodium nitrite injected into a gaseous carrier stream to oxidize iron sulfides to elemental sulfur and iron oxides. The sodium nitrite solution may be buffered to a pH of about 9 with disodium phosphate or monosodium phosphate. A chemical additive that provides a quantitative measure of reaction completion may be added to the treatment solution.
Owner:REFINED TECH
Method for the processing of potassium containing materials
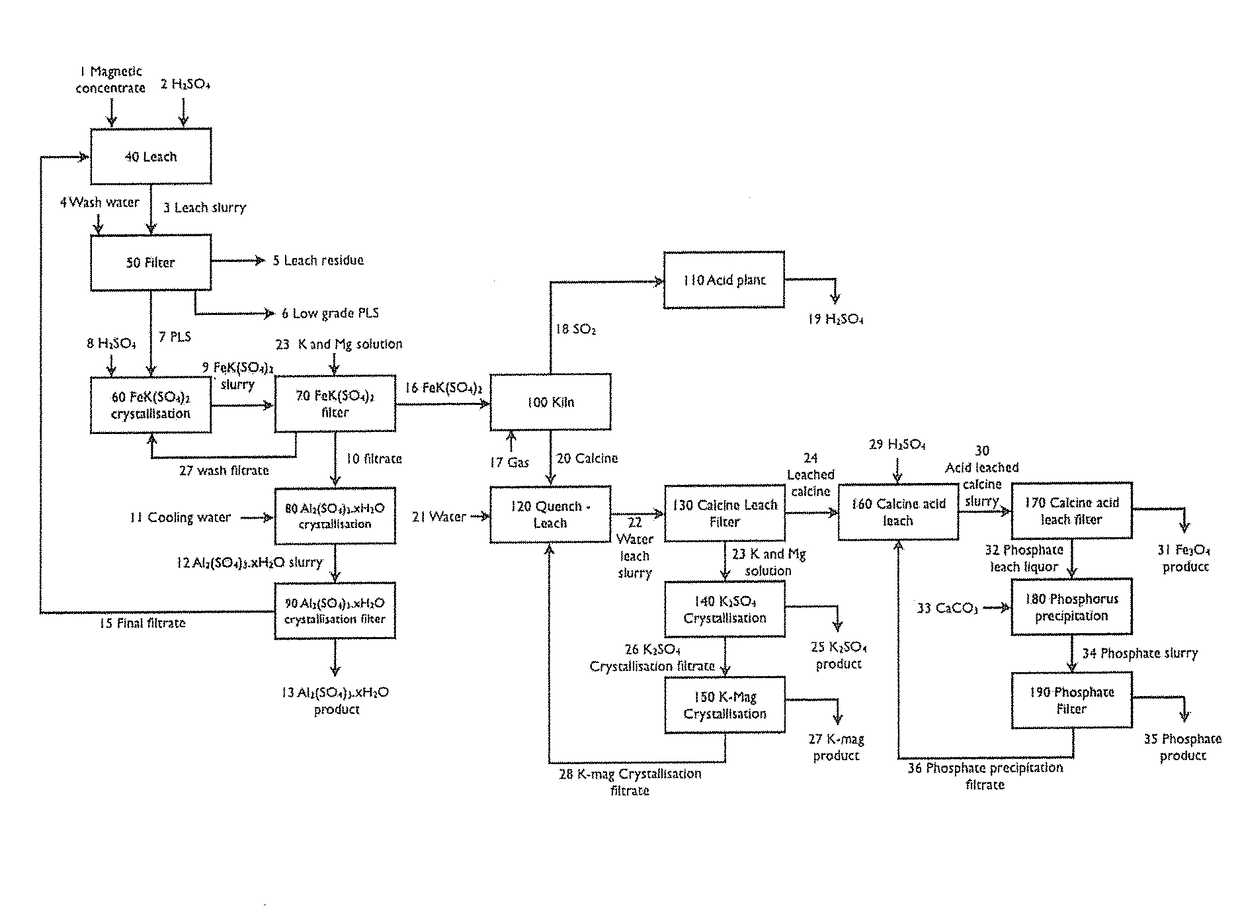
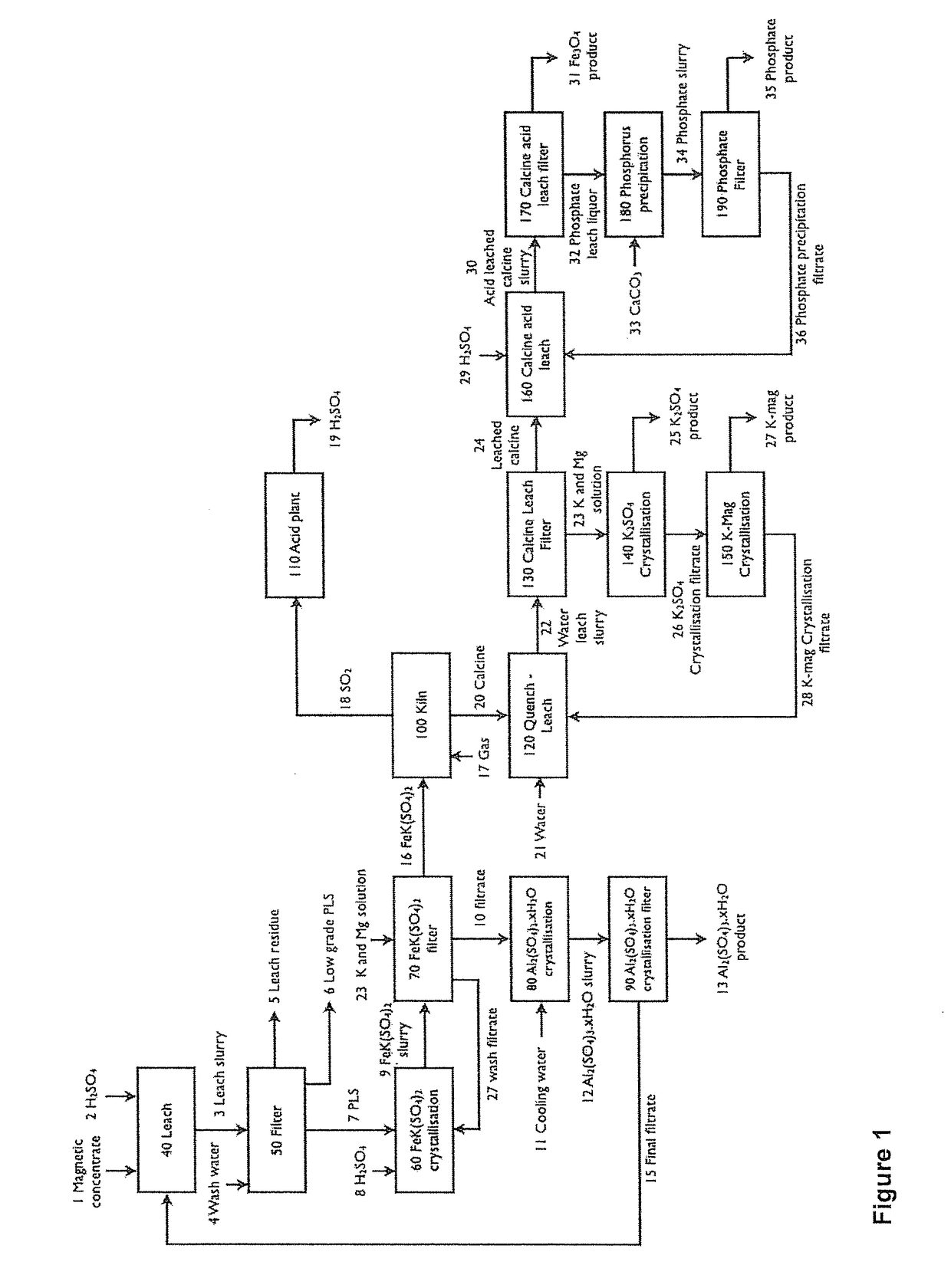
InactiveUS20170320749A1Varying degree of hydrationAvoid expensivePhosphatesSulfur compoundsPhosphatePotassium
A method for the processing of potassium containing materials comprises:(i) Separation of a potassium containing mineral from gangue minerals;(ii) Acid leaching whereby substantially all potassium, iron, aluminium and magnesium is solubilised and mixed potassium / iron double salt formed;(iii) Selectively crystallising the mixed potassium / iron double salt formed in the leach step (ii);(iv) Second separation to separate the mixed potassium / iron double salt formed in step (iii);(v) Thermal decomposition to produce an iron oxide, a potassium salt and one or more phosphates;(vi) Leaching the product of the thermal decomposition;(vii) Third separation to separate the iron oxide and phosphate from the potassium salt;(viii) Recovering the potassium salt by crystallisation;(ix) Separating the iron oxide and phosphate of step (vii) by leaching and subsequent solid liquid separation; and(x) Precipitating phosphate from liquor produced in step (ix) through the addition of a base.
Owner:K MAX PTY LTD
Method for preparing iron oxide yellow from rare earth waste acid leaching residues
PendingCN113104901ASolve pollutionWon't wasteFerrous oxidesProcess efficiency improvementPregnant leach solutionFerrous sulfate iron
The invention provides a method for preparing iron oxide yellow from rare earth waste acid leaching residues. The method comprises the following steps: (1) adding a sulfuric acid solution into the rare earth waste acid leaching residues, leaching and filtering to obtain a leachate; (2) adding a reducing agent into the leachate to obtain a ferrous sulfate solution; (3) adjusting the pH value of the ferrous sulfate solution in the step (2) to 3-4 by using a carbonate solution, removing impurities, and filtering to obtain a pure ferrous sulfate solution; and (4) introducing oxygen into the pure ferrous sulfate solution obtained in the step (3). The innovation point of the method is that sulfuric acid generated in the reaction can be consumed by scrap iron, so that sulfuric acid is not wasted, and new ferrous sulfate can be formed. The method is a low-cost, green and sustainable method.
Owner:JIANGXI UNIV OF SCI & TECH
Alpha-FE2O3 nanoparticles and method of making and use thereof in photodegradation of organic pollutants, as a photocatalyst and as an antibacterial composition
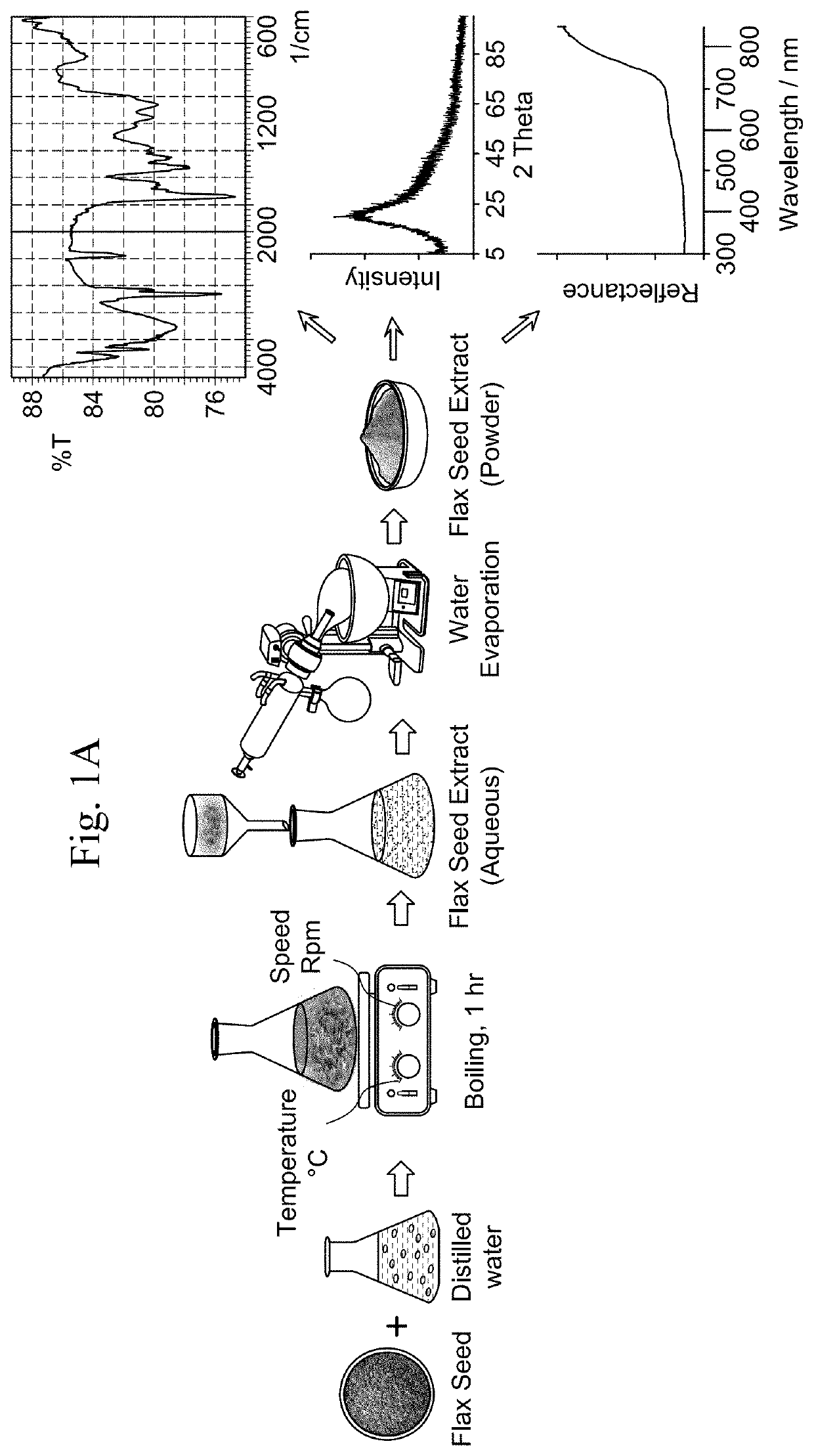
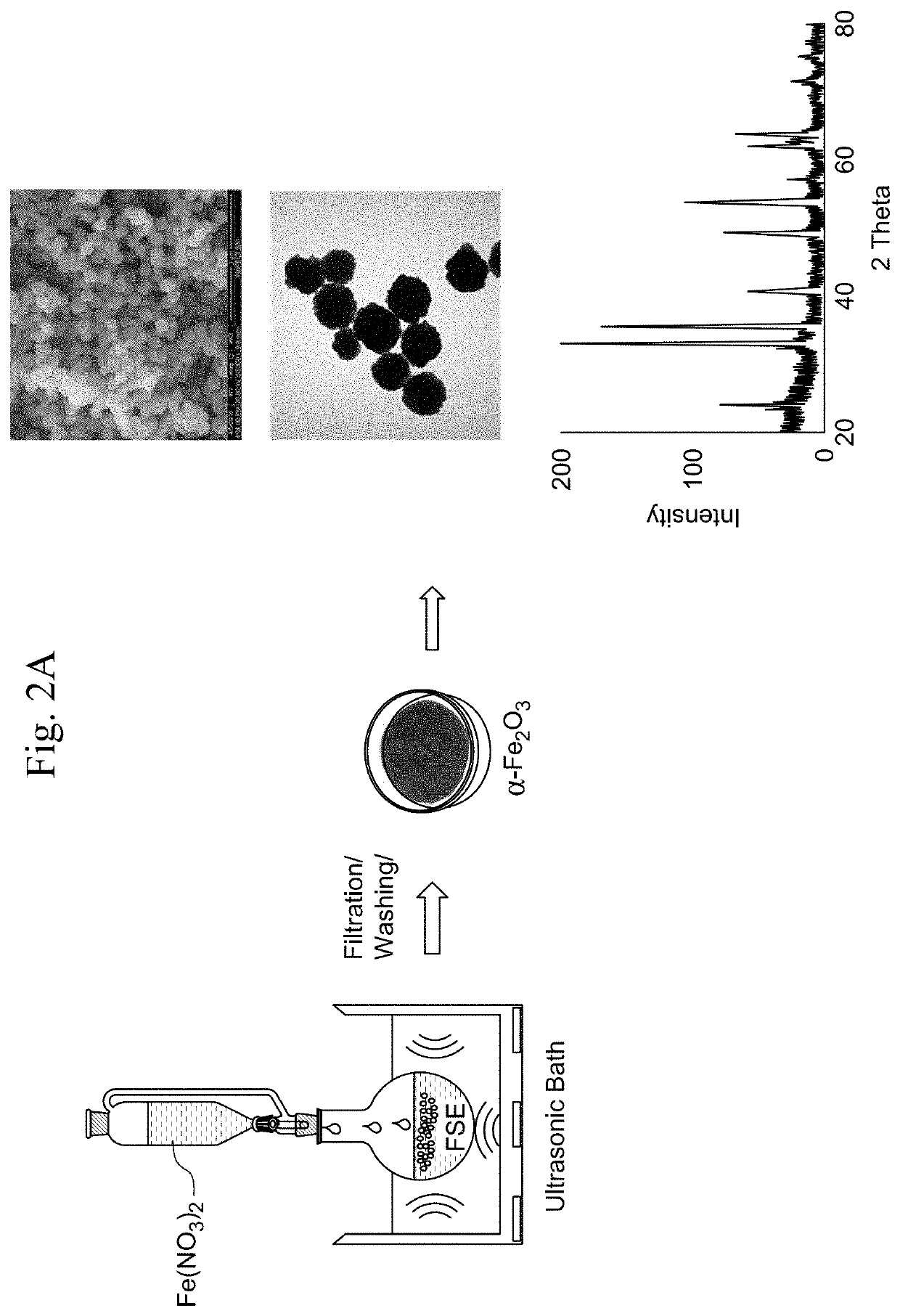
A method for producing crystalline α-Fe2O3 nanoparticles involving ultrasonic treatment of a solution of an iron (III)-containing precursor and an extract from the seeds of a plant in the family Linaceae. The method involves preparing an aqueous extract from the seeds of a plant in the family Linaceae and dropwise addition of the extract to the solution of an iron (III)-containing precursor. The method yields crystalline nanoparticles of α-Fe2O3 having a spherical morphology with a diameter of 100 nm to 300 nm, a mean surface area of 240 to 250 m2 / g, and a type-II nitrogen adsorption-desorption BET isotherm with a H3 hysteresis loop. A method for the photocatalytic decomposition of organic pollutants using the nanoparticles is disclosed. An antibacterial composition containing the crystalline α-Fe2O3 nanoparticles is also disclosed.
Owner:IMAM ABDULRAHRNAN BIN FAISAL UNIVERSITY
Hydrogen gas production method, and steel production method
A hydrogen gas production method includes a light irradiation step of applying light to a surface of a metal material immersed in water to produce gas containing hydrogen. In this hydrogen gas production method, the metal material contains iron, in the spectrum of the light, a wavelength at which the intensity is maximum is not less than 360 nm and less than 620 nm, and as the gas is produced, at least one of iron oxide and iron hydroxide is formed on the surface.
Owner:HITACHI CHEM CO LTD +1
MOFs derivative double-layer coated manganese ferrite wave-absorbing material as well as preparation method and application thereof
PendingCN114477308AIncrease complex permittivityLight in massMaterial nanotechnologyMagnetic/electric field screeningBi layerImpedance matching
The invention relates to an MOFs derivative double-layer coated manganese ferrite wave-absorbing material as well as a preparation method and application thereof. The double-layer coated manganese ferrite wave-absorbing material provided by the invention takes manganese ferrite as a core, the middle is ferrous oxide or cobalt oxide, and the outer layer is carbon. The double-layer core-shell structure of the wave-absorbing material improves interface polarization and dipole polarization, increases the complex dielectric constant of the material, enhances the interface scattering of the multilevel structure, and optimizes the impedance matching of the composite material. According to the embodiment 1, the minimum RL value of the obtained double-layer coated manganese ferrite wave-absorbing material at the frequency of 11.6 GHz is-71.65 dB, and the minimum RL value of the manganese ferrite wave-absorbing material without double-layer coating at the frequency of 13.22 GHz is-37.53 dB. Therefore, the MOFs derivative double-layer coated manganese ferrite wave-absorbing material prepared by the method can effectively absorb electromagnetic waves at a low thickness, and has good chemical stability.
Owner:江西虔悦新材料有限公司
Method for preparing active iron oxide powder materials by means of vacuum aluminothermic reduction
The invention relates to a method for preparing active iron oxide powder materials by means of vacuum aluminothermic reduction. Iron sesquioxide micro-powder is used as a raw material, aluminum micro-powder is used as a reducing agent, and the iron oxide powder materials can be manufactured by the aid of a vacuum aluminothermic reduction process. The method includes preparing steps of 1, uniformly mixing the iron sesquioxide micro-powder, namely, the raw material, and the aluminum micro-powder, namely, the reducing agent, with each other to obtain mixtures for standby application; 2, placing the mixtures obtained in the step 1 in the center of a clean porcelain boat; 3, placing the porcelain boat prepared in the step 2 in the middle of a high-temperature tube furnace, sealing the tube furnace, pumping the high-temperature tube furnace by the aid of a vacuum pump to enable the high-temperature tube furnace to be in an internal negative-pressure state and keeping the vacuum degree of the high-temperature tube furnace lower than or equal to 5pa; 4, heating the high-temperature tube furnace until the temperature of the high-temperature tube furnace reaches 900-1000 DEG C, keeping the raw material and the reducing agent reacting to each other for certain reaction time, then stopping heating the high-temperature tube furnace, cooling the high-temperature tube furnace until the temperature of the high-temperature tube furnace reaches the room temperature and taking products out of the high-temperature tube furnace. The method has the advantages that technologies are simple and controllable, prepared active iron oxide (FeO / Fe<3>O<4>, FeO, Fe / FeO) powder is high in purity and low in cost, and requirements of actual production can be met.
Owner:DONGGUAN PUREMATE ENVIRONMENTAL PROTECTION TECH CO LTD
Coloring ultraviolet protective agent
InactiveUS20190144691A1High transparencyImpair appearanceCosmetic preparationsOther chemical processesUltravioletHue
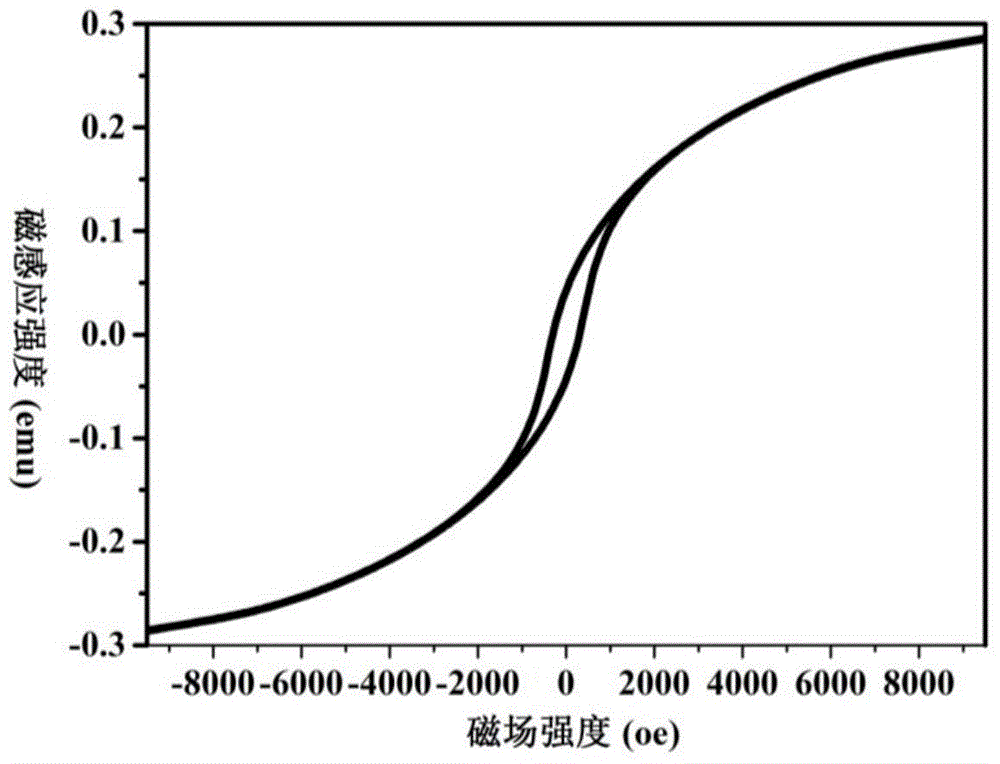
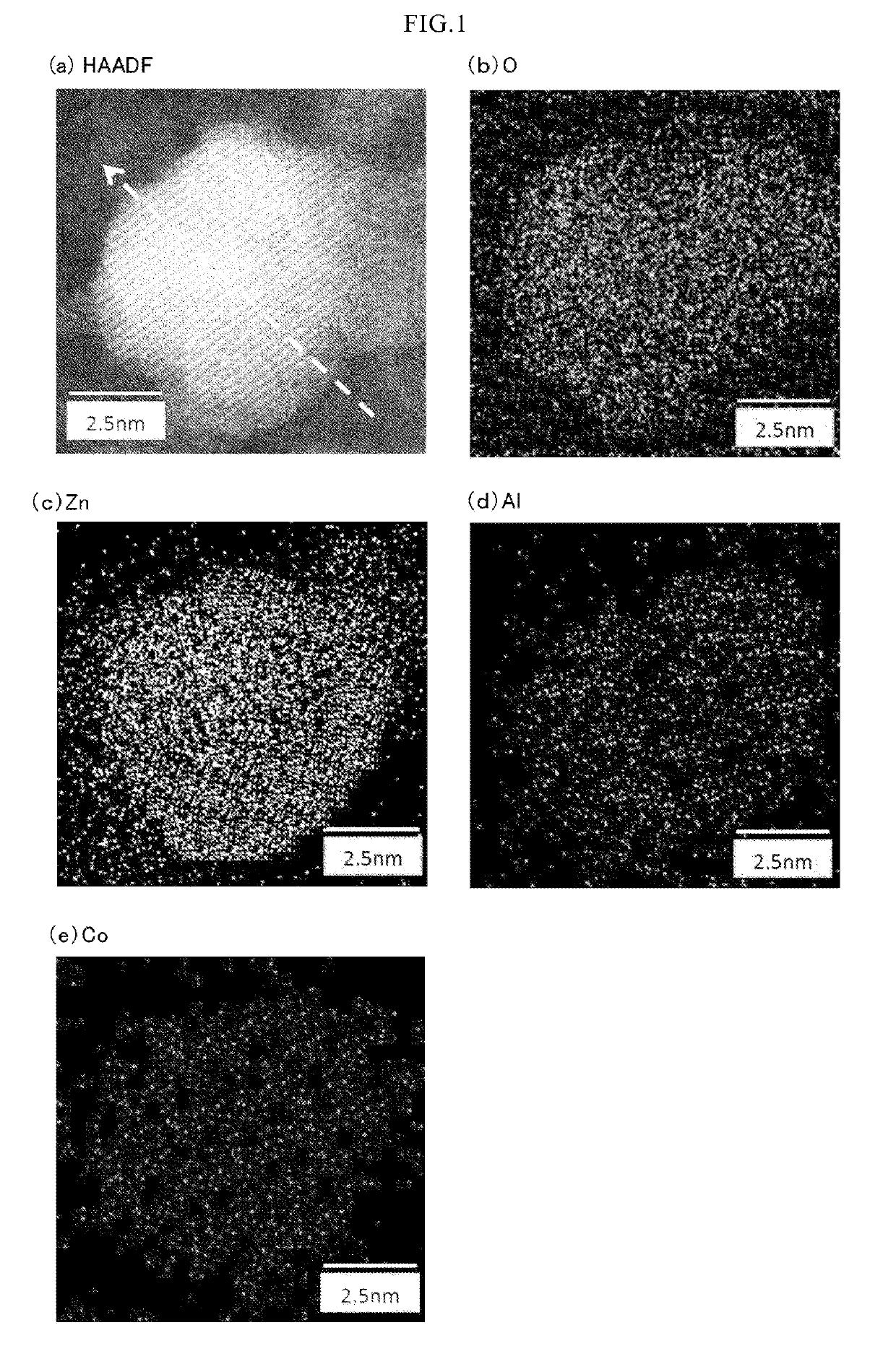
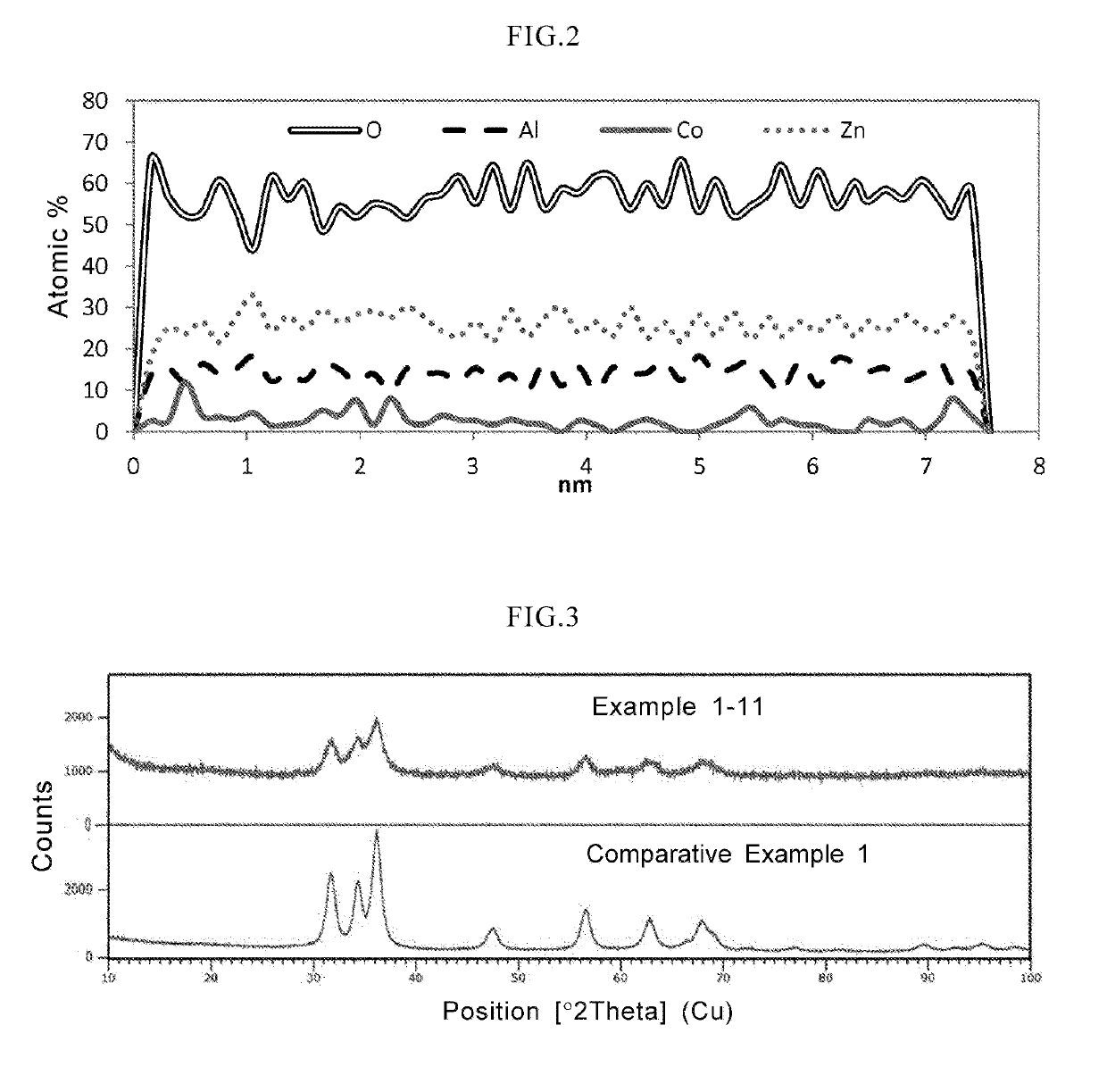
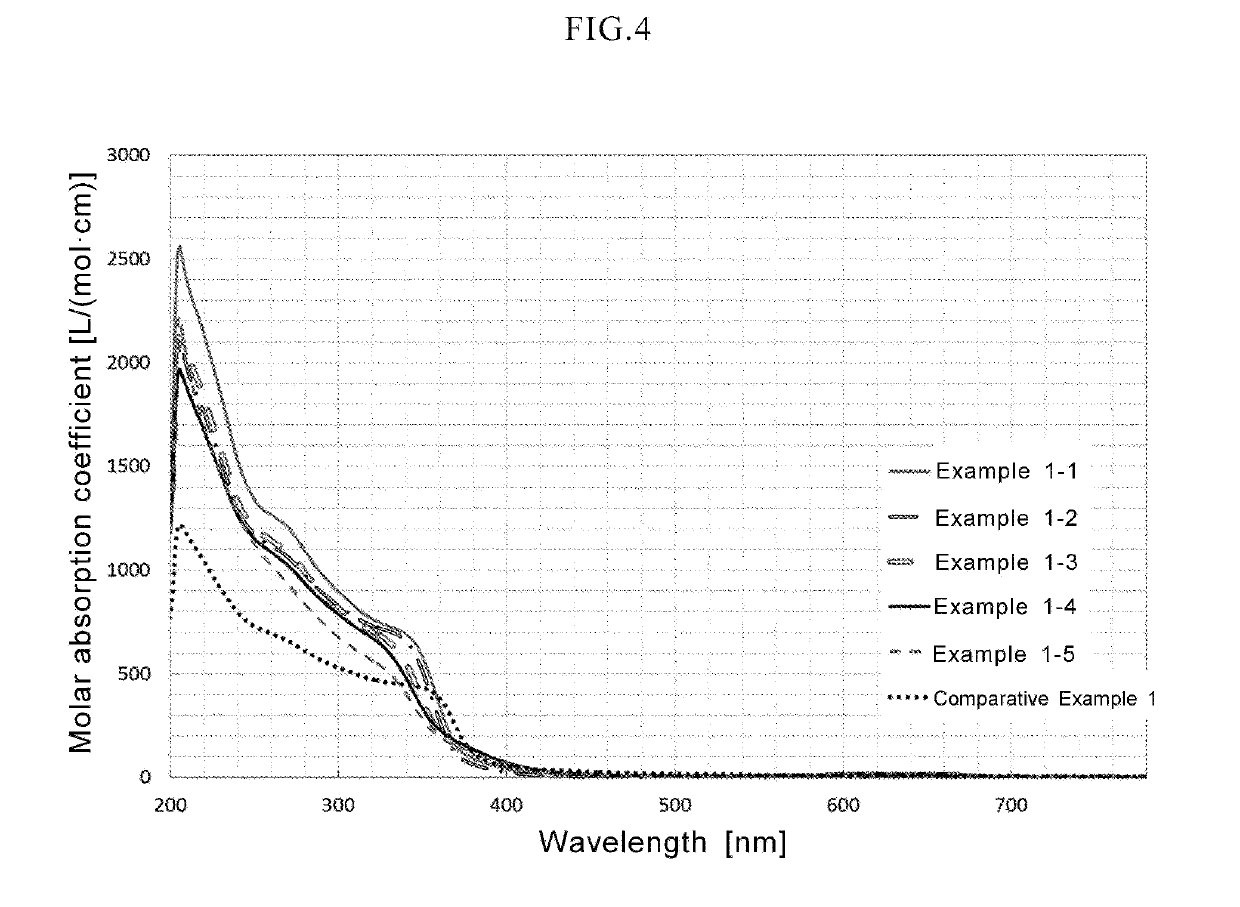
In a coloring ultraviolet protective agent, the average molar absorption coefficient in the wavelength range from 200 nm to 380 nm is increased, and the color characteristics in the visible region are controlled. The coloring ultraviolet protective agent is useful for shielding ultraviolet rays and coloring. The coloring ultraviolet protective agent comprises M2 doped oxide particles in which oxide particles (M1Ox) including at least M1 being a metal element or metalloid element, are doped with at least one M2 selected from metal elements or metalloid elements other than M1, wherein x is an arbitrary positive number, wherein an average molar absorption coefficient in the wavelength range of 200 nm to 380 nm of a dispersion in which the M2 doped oxide particles are dispersed in a dispersion medium, is improved as compared with one of a dispersion in which the oxide particles (M1Ox) are dispersed in a dispersion medium, and wherein a hue or chroma of color characteristics in the visible region of the M2 doped oxide particles is controlled.
Owner:M TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com