Amine functionalized mesoporous iron oxyhydroxide and method for fabricating the same
A structure and mesoporous technology, applied in the field of mesoporous FeOx structure and its preparation, can solve the problems of long time required, small pore volume, and uneven pore shape.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] x >
[0025] Mix 200ml of 0.20M FeCl 2 and 45ml of 0.08M sodium dodecylsulfate (SDS, sodium dodecylsulfate) and then stirred for 6 hours. Next, in FeCl 2 Add 50ml of 0.3M H 2 o 2 The solution was reacted and stirred (stirring) for about 1 hour or so. The above-mentioned sodium dodecyl sulfate (SDS, sodium dodecylsulfate) is a surfactant, and the above-mentioned H 2 o 2 solution for the oxidation of FeCl 2 of oxidants.
[0026] Then, the kneaded solution was subjected to solid-liquid separation using a centrifugal separator (3000 rpm, 15 minutes). After washing the separated solid substance three times with distilled water, centrifugation was performed again to separate the liquid substance. The finally separated solid matter was dried in an oven at 100 °C for about 4 hours to prepare FeO with a light bronze-colored mesoporous structure. x powder (mesoporous FeO x structure).
[0027] Next, inject the prepared mesoporous FeO into the flask containing anhydrou...
Embodiment 2
[0030] x Material properties>
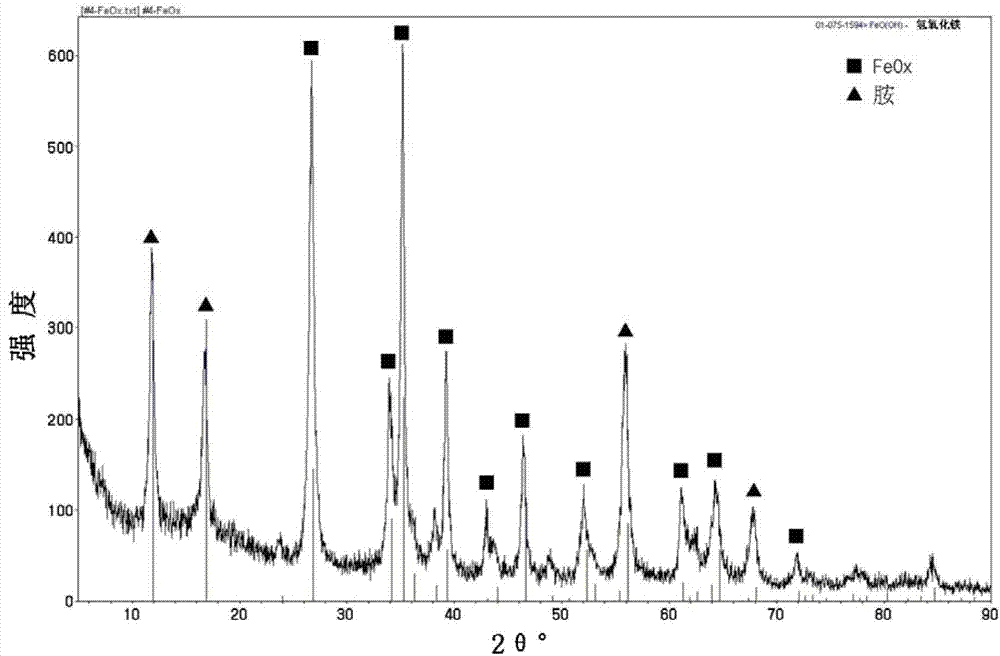
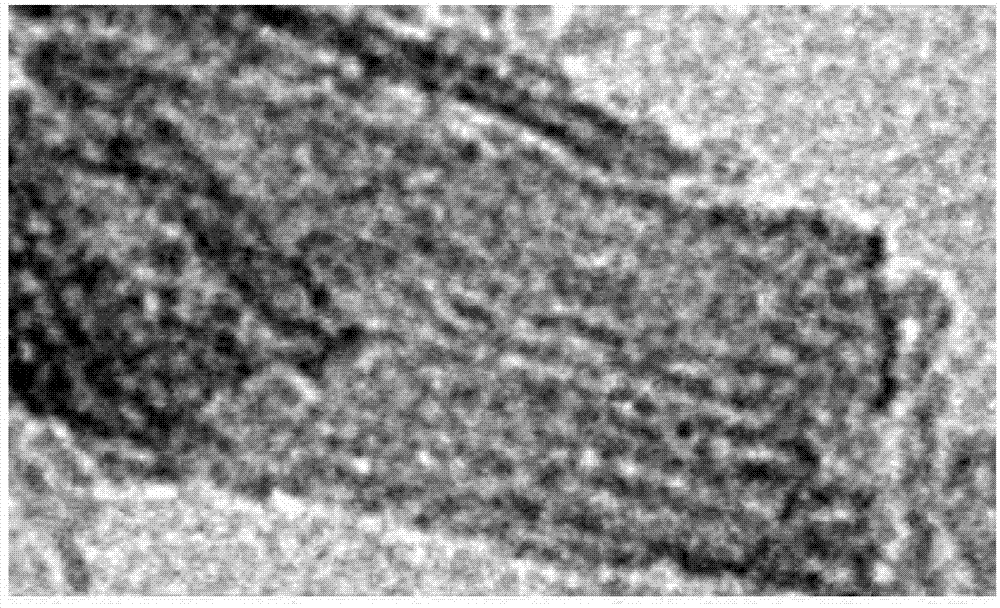
[0031] The mesoporous FeO with amine groups prepared in Example 1 was confirmed by XRD analysis and TEM analysis x The material properties of the structure. refer to figure 2 The XRD chart can confirm that FeO x Peaks and amine peaks are well defined, by image 3 The TEM photo can confirm the mesoporous structure of FeO with amine groups x .
Embodiment 3
[0032]
[0033] Implemented the mesoporous FeO with amine group prepared by Example 1 x Arsenic adsorption experiments on structures. The experimental method and experimental results are as follows.
[0034] Aqueous solutions of copper, cadmium, lead, and arsenic were prepared at a concentration of 10 mg / L in a 50 ml Teflon-made conical tube. Then, after measuring the concentration of each heavy metal aqueous solution with an ion chromatography (IC, ion chromatography), it was confirmed whether it conformed to the experimental concentration.
[0035] Next, the mesoporous FeO with amine groups prepared in Example 1 was injected into each heavy metal aqueous solution. x Structure 0.05g. The heavy metal adsorption performance was determined after stirring for 6 hours. At this time, for comparison, under the same conditions, mesoporous FeO without amine groups was also injected into the heavy metal aqueous solution. x The structure was then stirred. The mesoporous FeO with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com