Highly active nano iron catalyst for the absorption of hydrogen sulfide
A technology of adsorbent and iron hydroxide, applied in physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, iron oxide/iron hydroxide, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
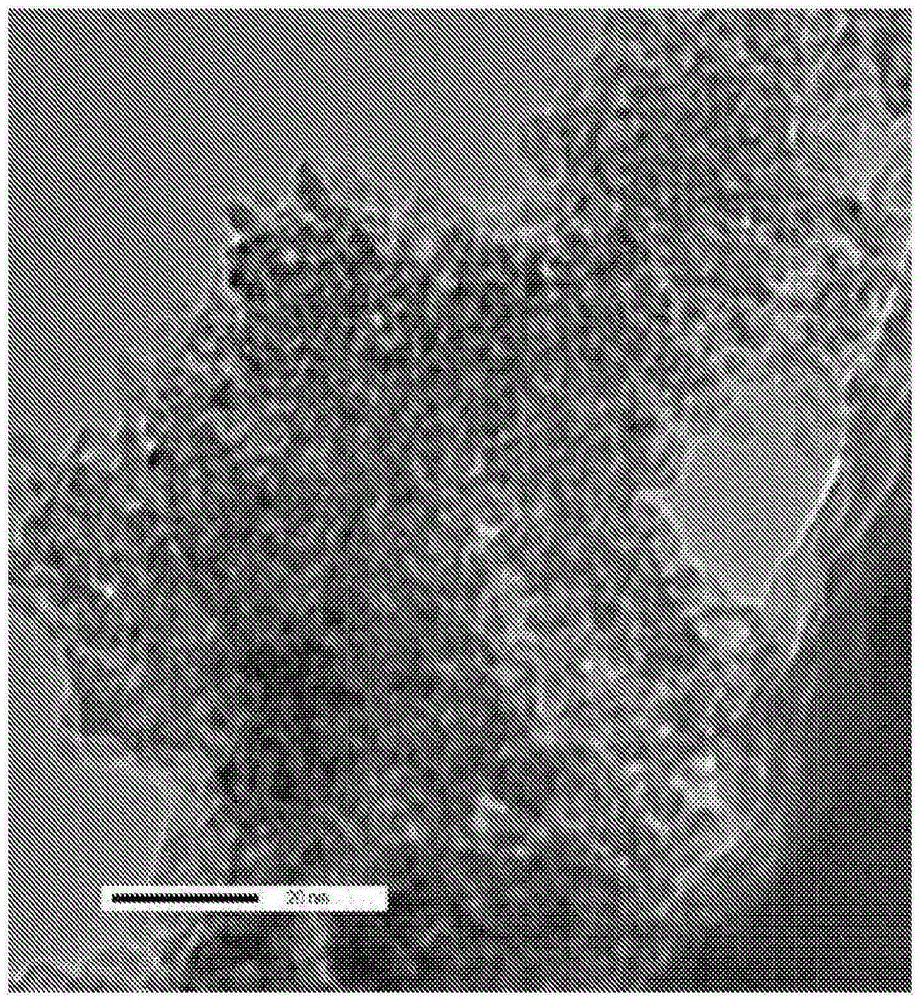
[0044] 1g siderite (mainly FeCO 3 ) was suspended in 60ml of 1M KOH solution by stirring with a magnetic stirrer and heated to 40-50°C until the color of the solid particles changed from light brown to black. This takes about 10-20 minutes. Once stirring was stopped, black particles settled to the bottom of the flask, showing a clear liquid phase at the top. They also agglomerate around the magnetic stir bar when the magnetic stir bar is not stirring due to its magnetic properties. The resulting suspension was analyzed using transmission electron microscopy, as figure 2 shown in . This demonstrates that the particles are generally in the range of 5-10 nm. This demonstrates that large particles not only react to stable iron(II) oxides and / or hydroxides, but they also deagglomerate to form iron nanoparticles.
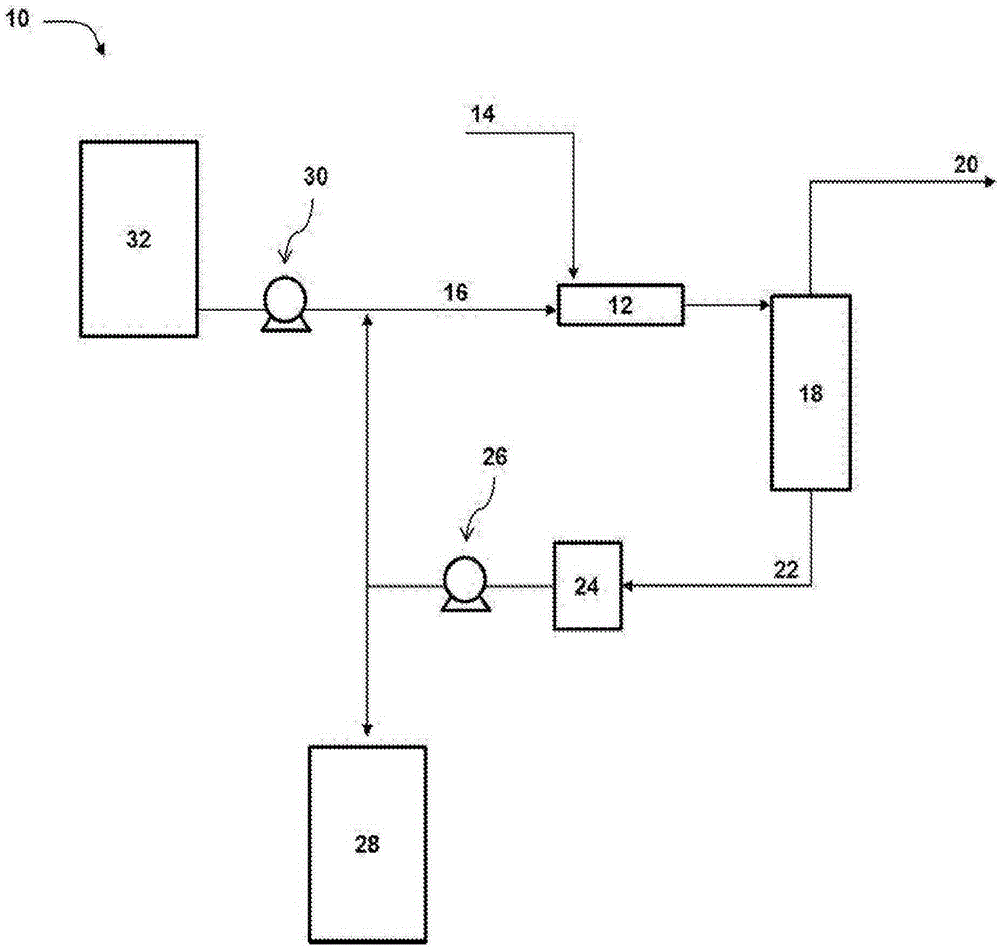
[0045] 10 ml of the resulting suspension was introduced into a glass bubbler containing the spent blank caustic solution from Example 1, and 6000 ppm H 2 S / N 2 Th...
Embodiment 3
[0053] After reaching the breakout in Example 2, 10 ml of the adsorbent prepared in the same way as given in Example 2 were added again to the spent caustic solution in the bubbler. 6000ppm H 2 S / N 2 The suspension was passed again according to the procedure stated in Reference Example 1, and the system started to absorb H again 2 S. It is important to note that in this case the lifetime of the adsorbent is 10 h before the jump, which is shorter than the first time. Therefore, it is believed that OH - and Fe +2 The molar ratio between should preferably be at least 4-6:1. Without being bound by theory, it is currently believed that this ratio allows sufficient caustic to be able to convert the ferrous carbonate in siderite to ferrous oxide / hydroxide and to stabilize it so that it can subsequently absorb H 2 S. If the ratio is lower, the caustic is able to react the siderite, but it may not exhibit absorption of H 2 S long life. Furthermore, the fact that the alkaline ...
Embodiment 4
[0055] As discussed above, when the stabilized iron (II) oxide and / or hydroxide is exposed to water, the particle color changes spontaneously from black to brown. According to the Schikorr reaction, Fe 3 o 4 Formed when Fe(II) hydroxide is exposed to water. To probe the adsorption capacity of these iron species, 60 ml of water and 5 ml of stabilized iron(II) oxide and / or hydroxide in the 1M basic solution produced according to Example 2 were added to the bubbler. Then, 20sccm of 6000ppmH 2 S / N 2 feed into the bubbler, and at H 2 Samples lasting approximately 6 hours prior to the S jump were detected by GC in the exit gas. The pH of the sample was 12.6 before absorption and 9.6 after absorption. In this case the used sample was green in color and also showed a black precipitate at the bottom. In this case, again assuming a linear relationship between the initial concentration of KOH and the hours of absorption, 5 ml of 1M KOH would be expected to last 6 h before the jump...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com