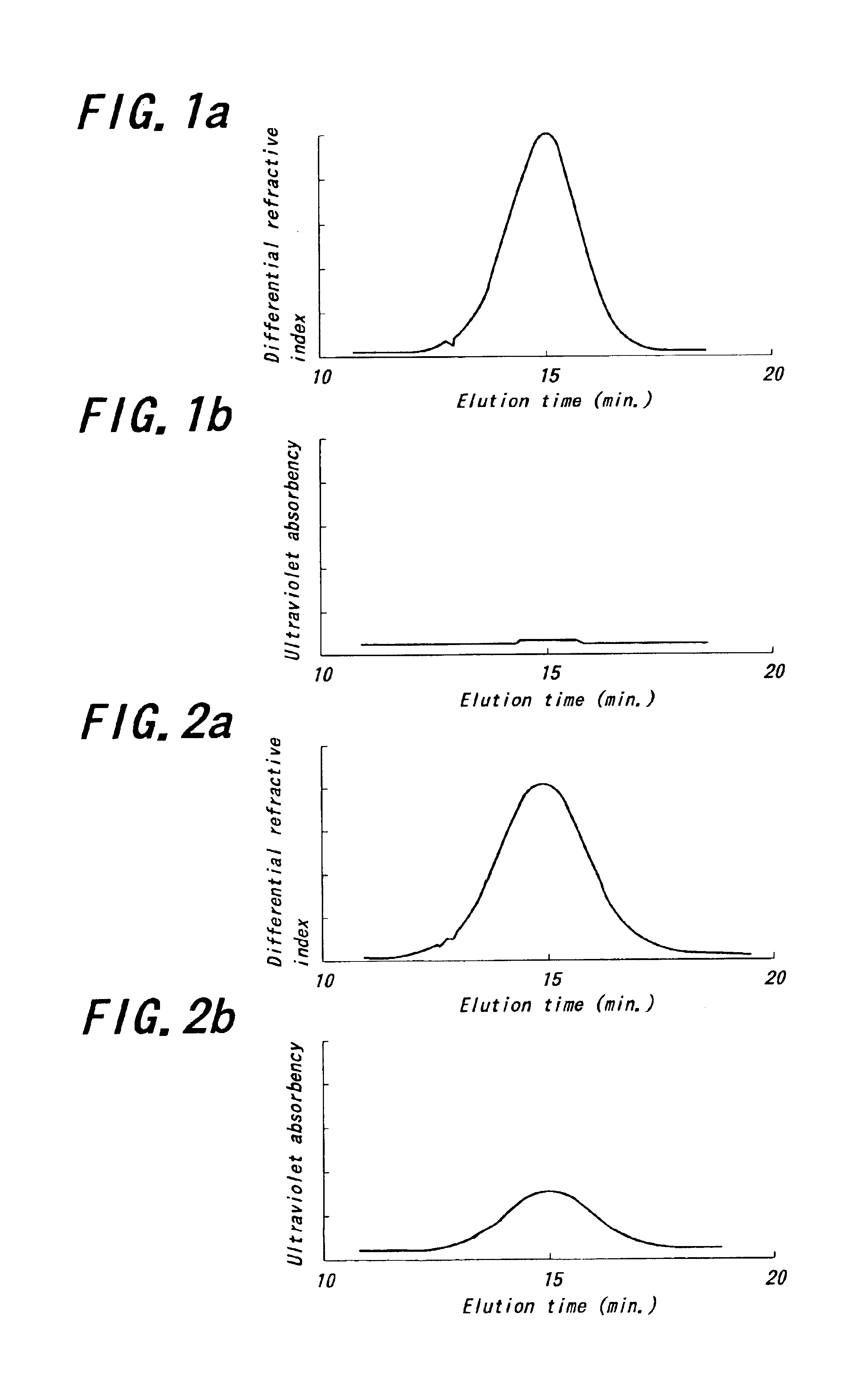
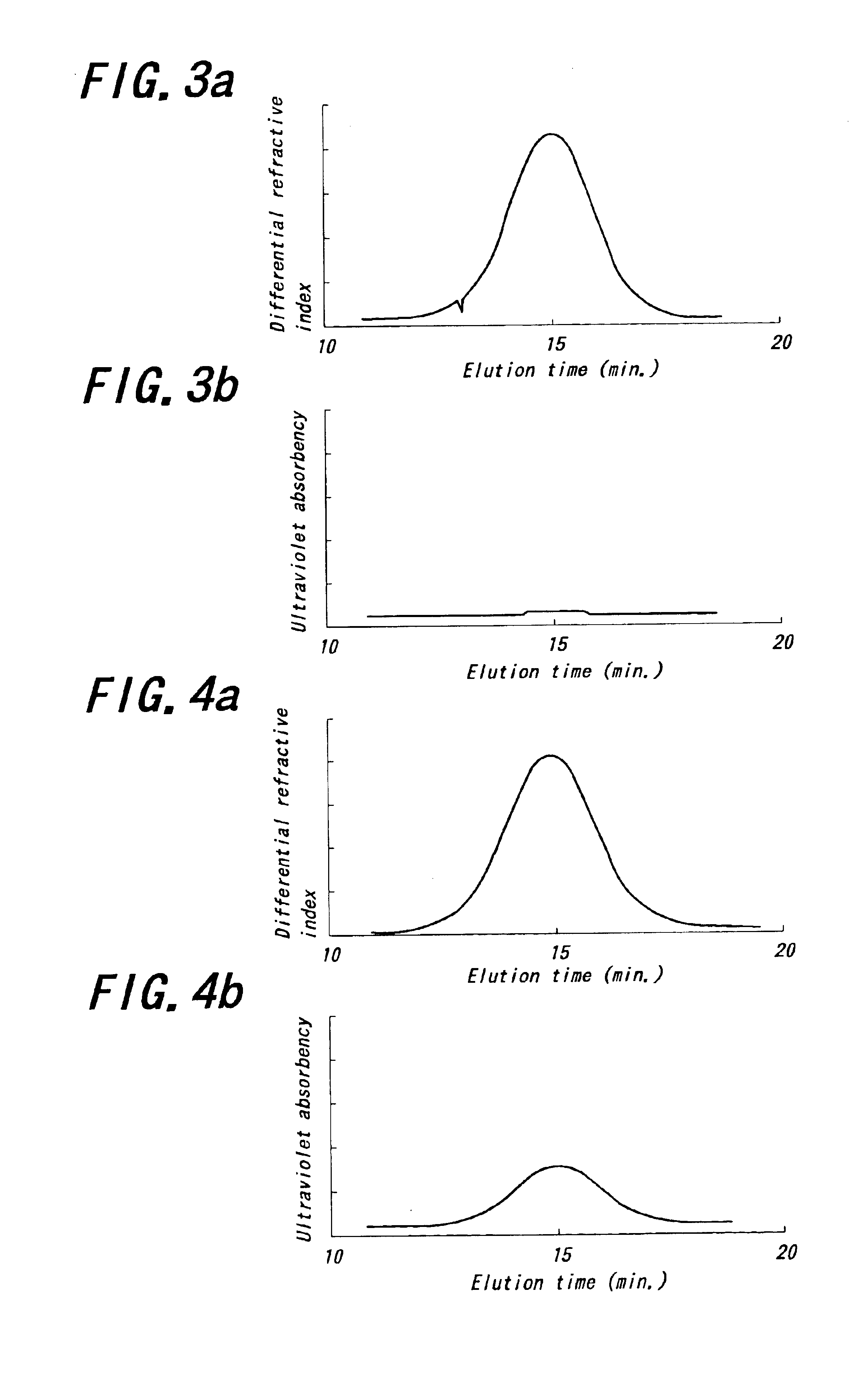
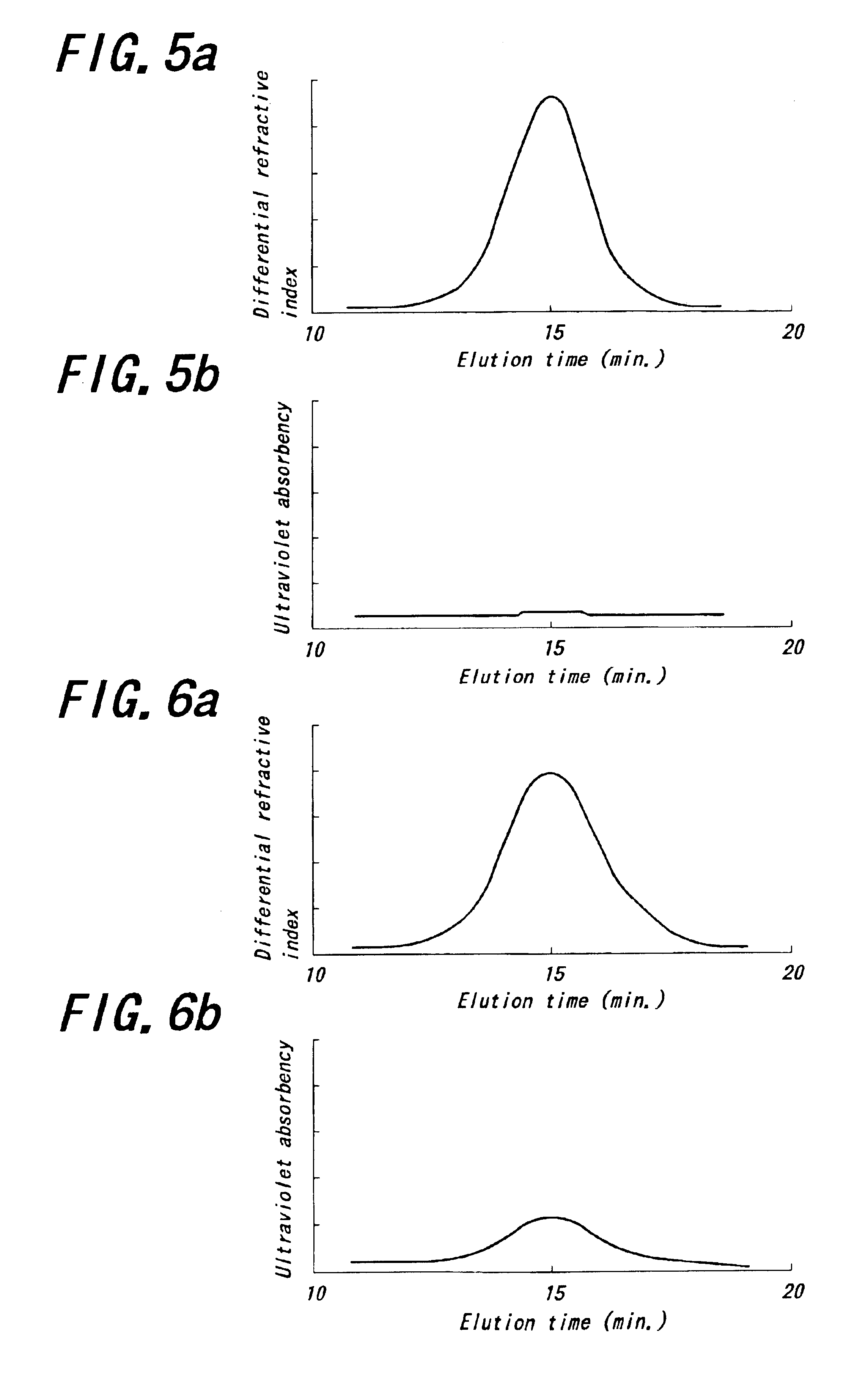
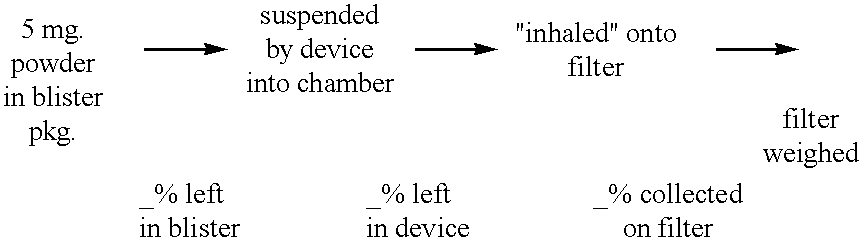
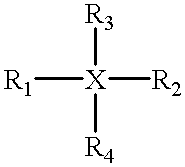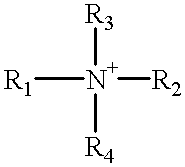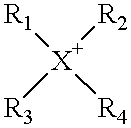Patents
Literature
812results about How to "Good dispersibility" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
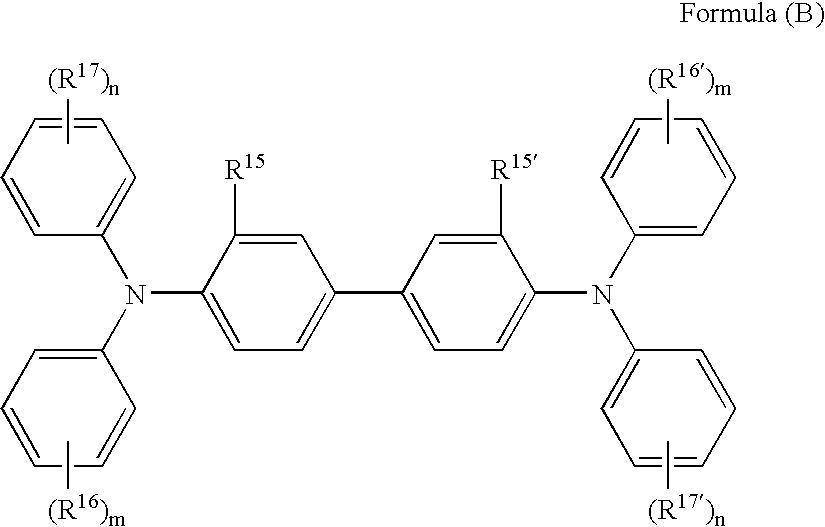
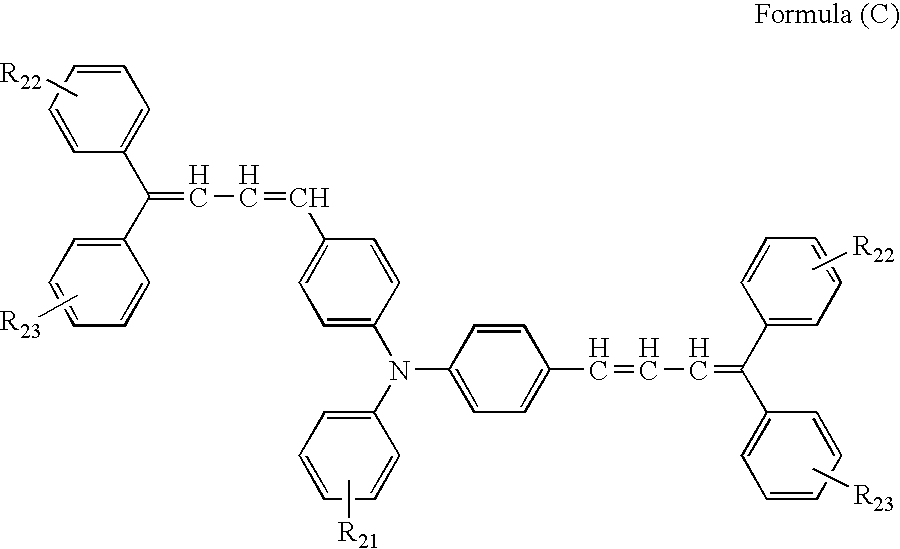
Modified conjugated diene polymer and method of producing the same and rubber composition
A novel modified conjugated diene polymer is obtained by polymerizing a conjugated diene compound with a specified catalyst consisting of components (a)-(c) and then reacting with at least one specified compound selected from the group consisting of components (d)-(l), and has a content of cis-1,4-bond of not less than 85% and a ratio of weight average molecular weight to number average molecular weight of not more than 4. And also, a rubber composition comprises the modified conjugated diene polymer a rubber ingredient.
Owner:JSR CORPORATIOON +1
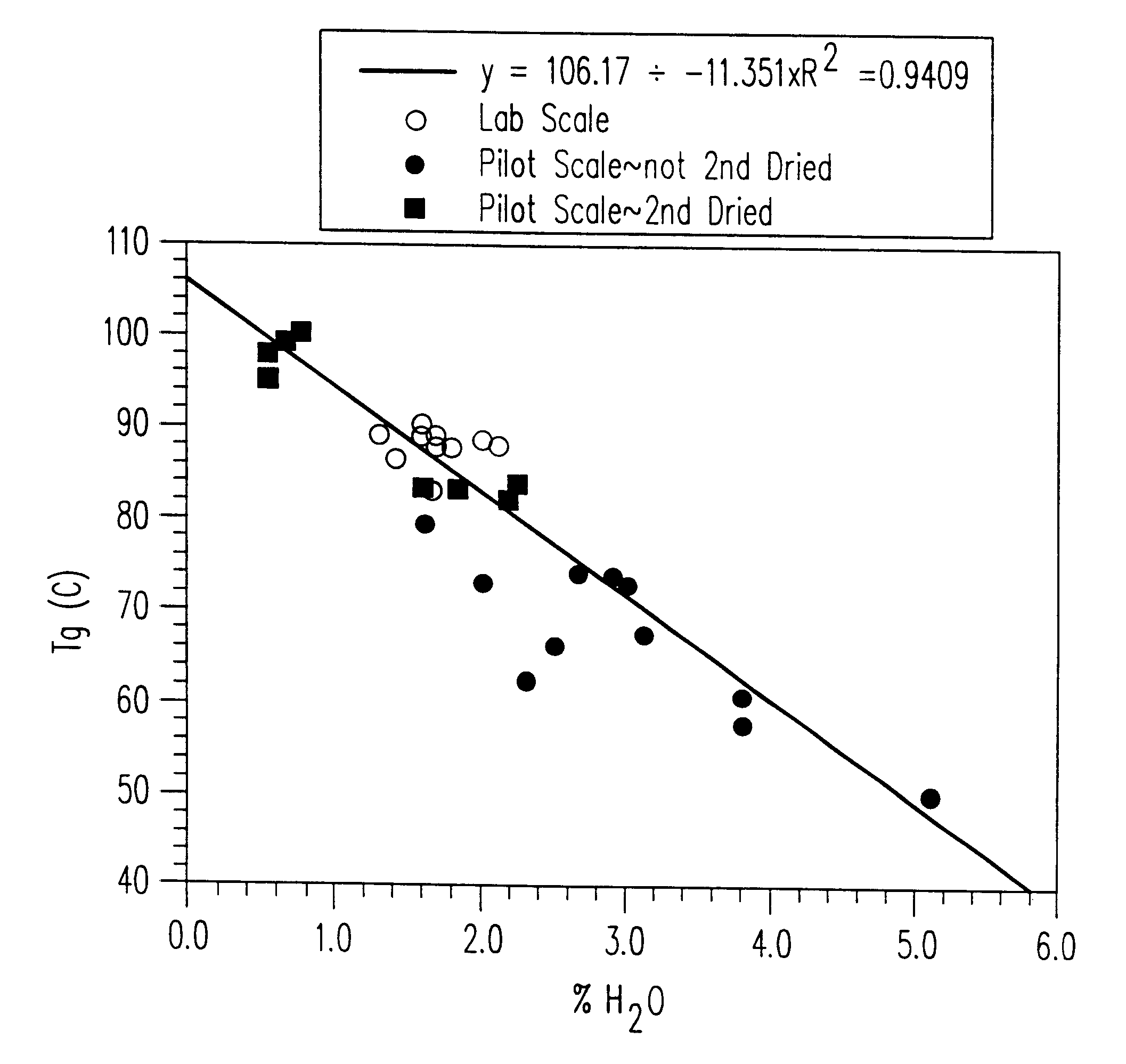
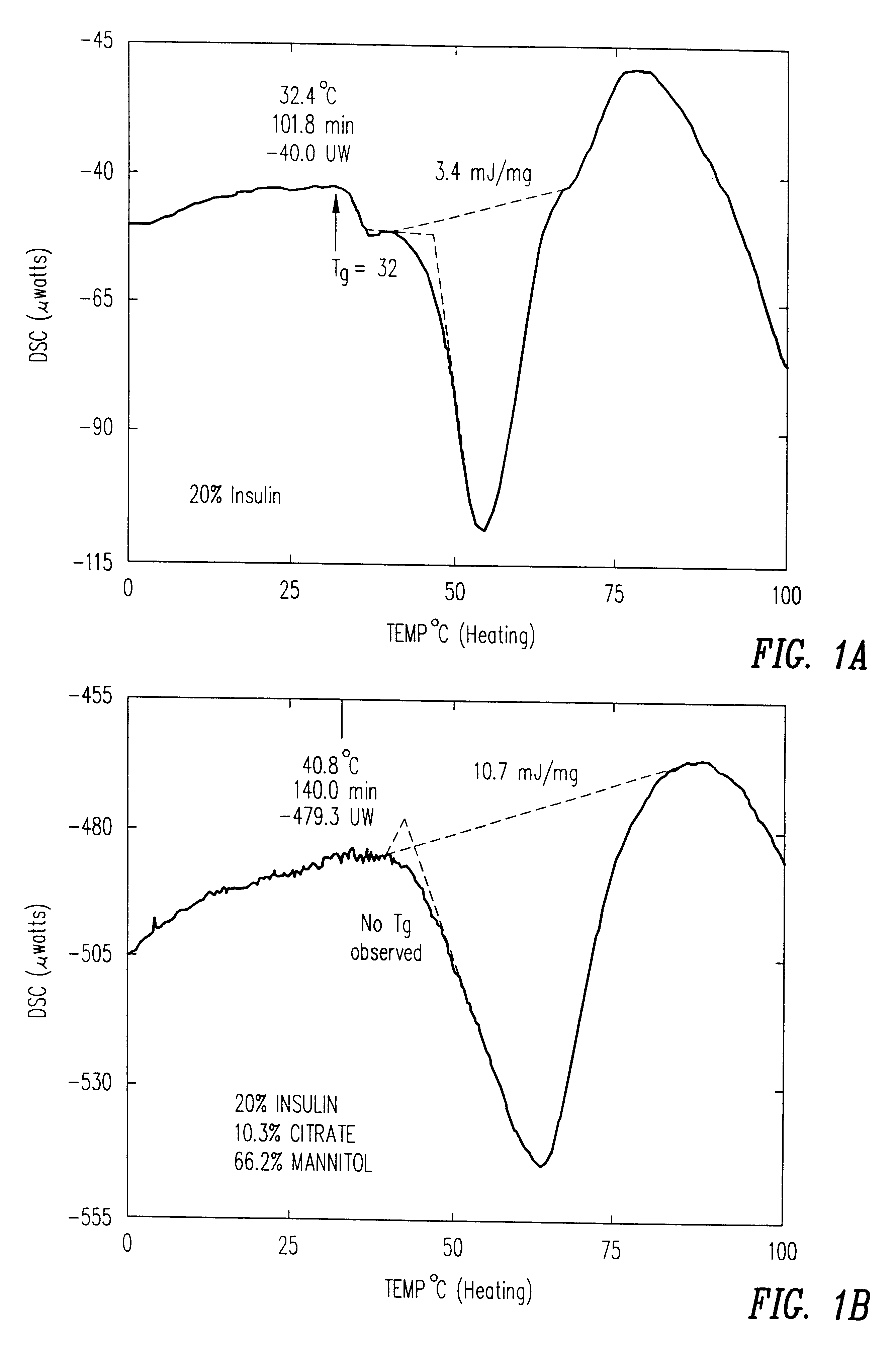
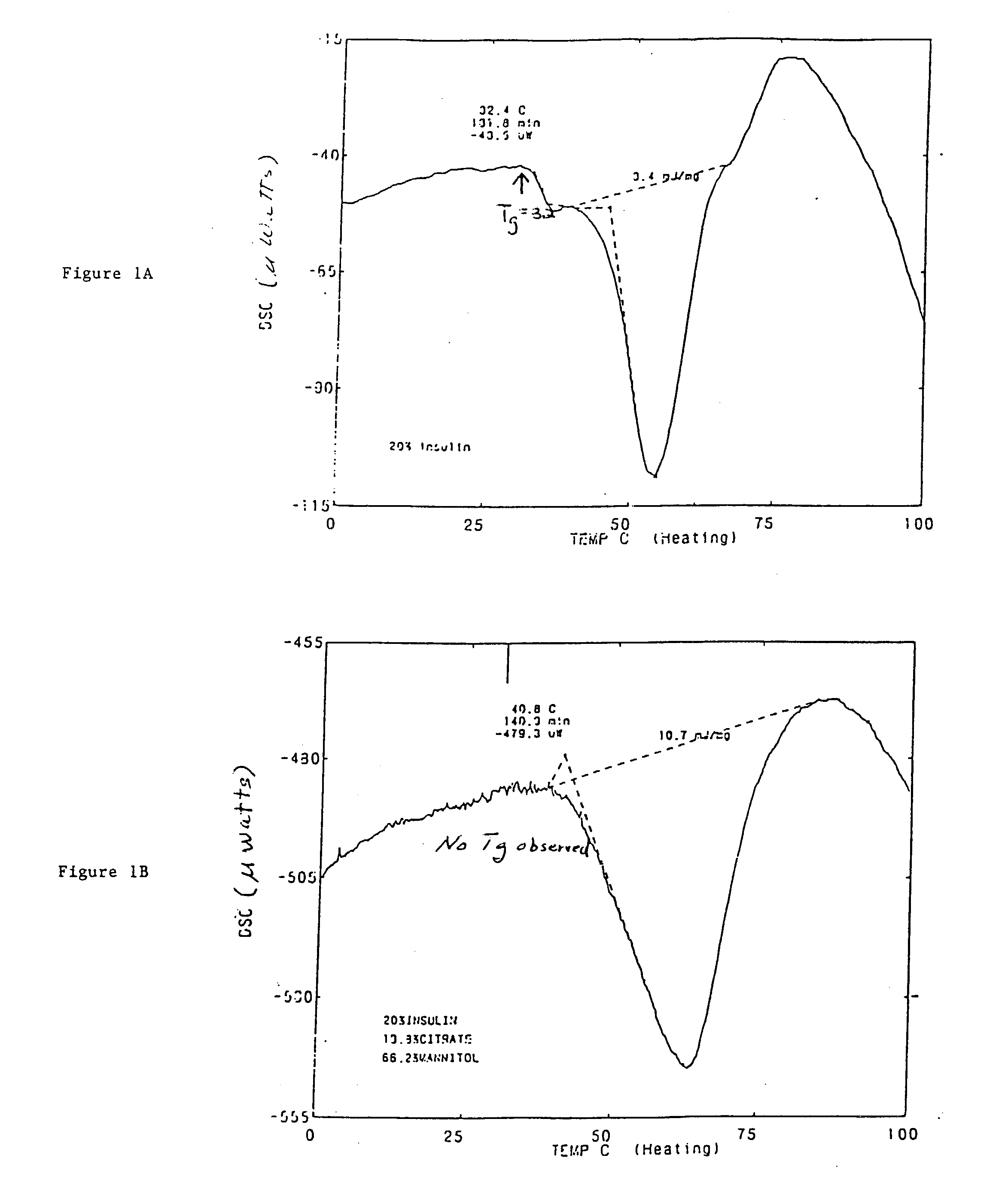
Stable glassy state powder formulations
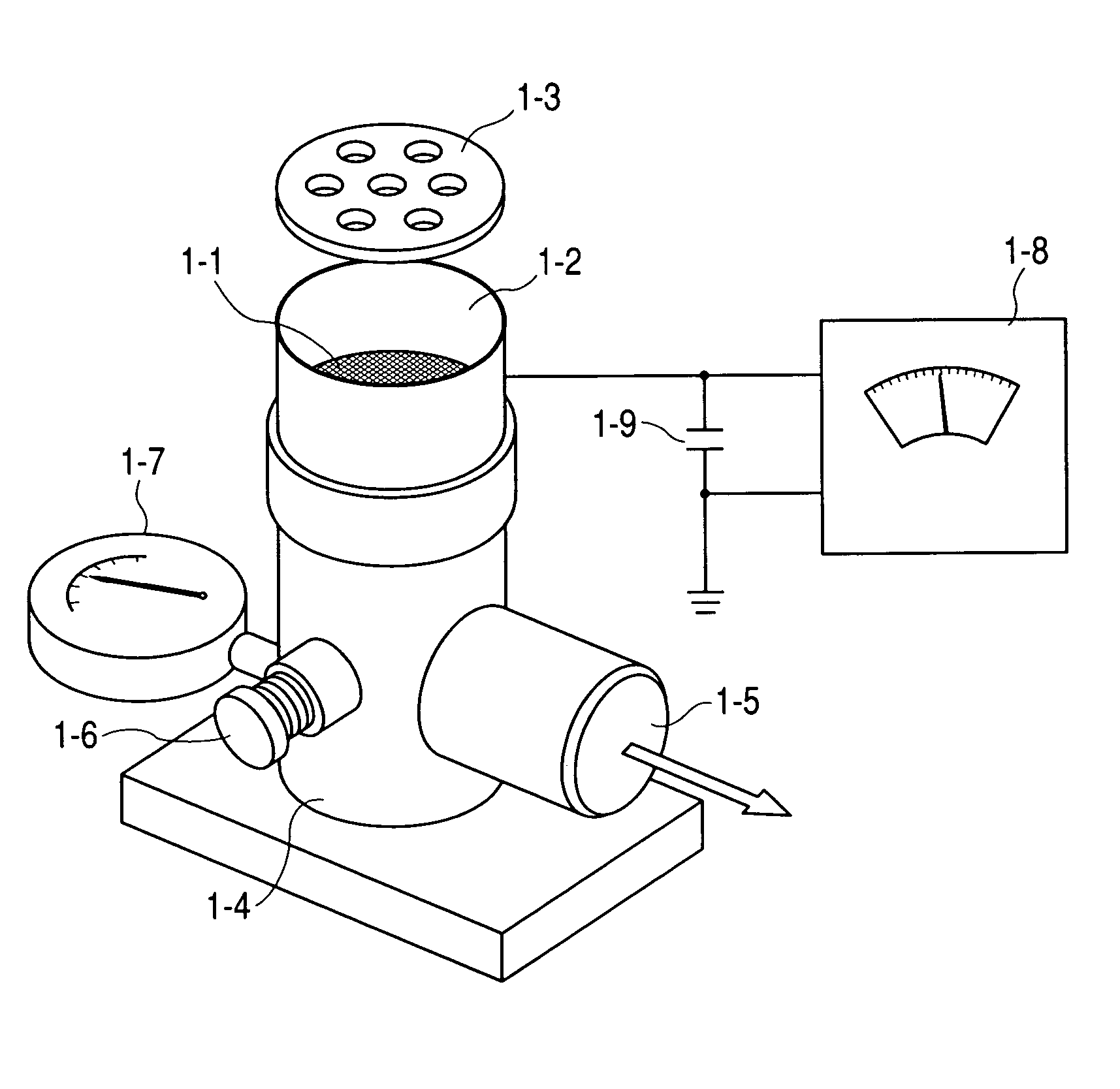
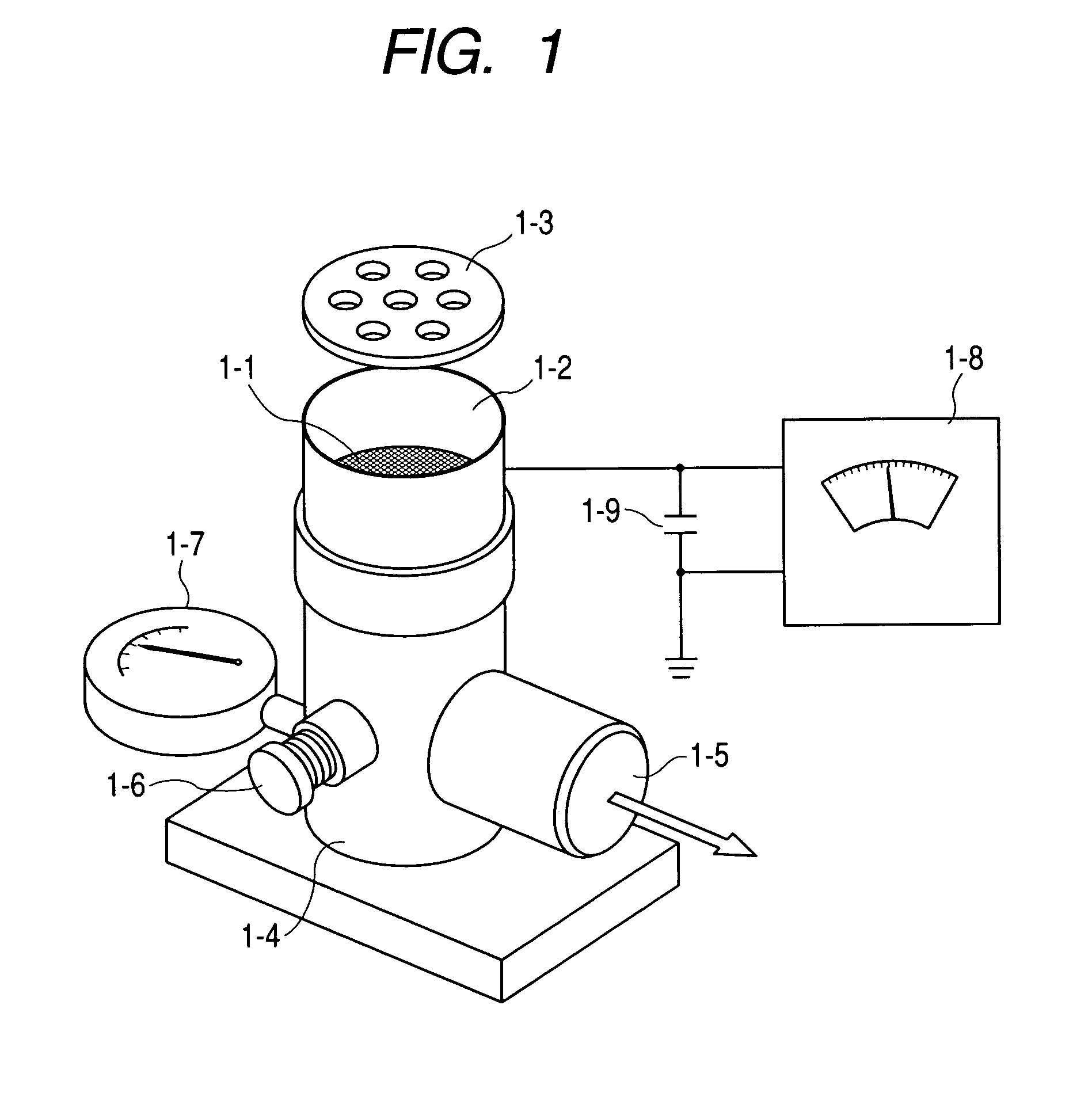
InactiveUS6309671B1Good dispersionGood dispersibilityPowder deliveryPeptide/protein ingredientsSolventChemistry
A powdered, dispersible composition having stable dispersibility over time is provided. The composition exhibits a characteristic glass transition temperature (Tg) and a recommended storage temperature (Ts), wherein the difference between Tg and Ts is at least about 10° C. (i.e. Tg-Ts is greater than 10° C.). The composition comprises a mixture of a pharmaceutically-acceptable glassy matrix and at least one pharmacologically active material within the glassy matrix. It may be further mixed with a powdered, pharmaceutically-acceptable carrier. It is particularly valuable in unit dosage form having a moisture barrier, in combination with appropriate labelling instructions. A process for producing a powdered dispersible composition is also provided, wherein the process comprises removing the solvent from a solution comprising a solvent, a glass former and a pharmacologically active material under conditions sufficient to form a glassy matrix having the pharmacologically active material within the matrix.
Owner:NOVARTIS FARMA
Method of denaturing whey protein
InactiveUS20110003975A1Improve textureGreat tastePeptide preparation methodsDepsipeptidesOrganic solventWhey protein
Provided is a method of producing a denatured whey protein which has an improved heat stability without using an additive such as an organic solvent. Also provided is a denatured whey protein produced by this method. A method of producing a denatured whey protein which comprises contacting and mixing a whey protein solution with a whey protein solution that is flowing as a thin film in the form of a rotating cylinder, and thus shearing the same at a temperature ranging form 76 to 120° C. at a shear speed of 5,000 s−1 to 25,000 s−1 for 8 minutes to 0.1 second; and a denatured whey protein obtained by this method.
Owner:MORINAGA MILK IND CO LTD
Dispersion composition, polymerizable composition, light-shielding color filter, solid-state image pick-up element, liquid crystal display device, wafer level lens, and image pick-up unit
ActiveUS20110124824A1Good shading effectGood dispersionPhotomechanical apparatusNon-linear opticsLiquid-crystal displayTitanium
A dispersion composition is provided in which the dispersibility of titanium black is high, the sedimentation of titanium black over time is suppressed, and overall dispersibility and storage stability are high. Further, a polymerizable composition is provided in which favorable coating property on a substrate and even film thickness can be obtained, generation of residue in an unexposed region when a pattern is formed can be suppressed, and favorable pattern shape having any steps after exposure / development can be obtained. The dispersion composition contains (A) titanium black, (B) a graft copolymer and (C) a solvent.
Owner:FUJIFILM CORP
Toner, developer, toner container, process cartridge, image forming apparatus and image forming method
InactiveUS20060210903A1Superior offset property and charge property and storage stabilityFavorable coloring property and OHP transparencyDevelopersImage formationAcid value
The present invention provides a toner and a developer which includes the toner. The toner is produced in an aqueous medium and includes at least a binding resin, a colorant and a dispersant which disperses the colorant. The binding resin contains 50% by mass to 100% by mass of a polyester resin, and the colorant is a pigment whose surface is given an acid treatment. The acid value of the dispersant is 1 mg KOH / g to 30 mg KOH / g, and the amine value of the dispersant is 1 mg KOH / g to 100 mg KOH / g.
Owner:RICOH KK
Electrophotographic photoreceptor, process cartridge and image forming apparatus
InactiveUS20040086794A1Improve stain resistanceViscosity control of solutionElectrographic process apparatusCorona dischargePeak areaSilicon
An electrophotographic photoreceptor comprising a conductive support and a photosensitive layer disposed on the conductive support, wherein the photosensitive layer comprises a silicon compound-containing layer containing a silicon compound, and the silicon compound-containing layer further contains a resin, and wherein the photosensitive layer has a peak area in the region of -40 to 0 ppm (S1) and a peak area in the region of -100 to -50 ppm (S2) in a <29>Si-NMR spectrum satisfying the following equation (1): S1 / (S1+S2)>=0.5 (1).
Owner:FUJIFILM BUSINESS INNOVATION CORP
Cationically polymerizable pigmented composition
InactiveUS6166100AGood dispersibilityMaintain good propertiesOrganic chemistryFilm/foil adhesivesEpoxyPolymer science
A cationically polymerizable pigmented composition comprising (A) a cationically polymerizable binder component containing at least one resin or compound selected from the group consisting of (A-1) a cationically polymerizable acrylic resin consisting of a copolymer of (a) a (meth) acrylic ester monomer having C6-31 aliphatic hydrocarbon group, (b) a polymerizable unsaturated monomer containing a polymerizable unsaturated group and at least one cationically polymerizable moiety selected from the group consisting of an epoxy group and an oxetane ring, and optionally (c) other polymerizable unsaturated monomer, and (A-2) a fatty acid-modified epoxy compound containing C6-32 aliphatic hydrocarbon group and epoxy group, (B) a cationic polymerization initiator initiating polymerization by irradiation or heating, and (C) a color pigment.
Owner:KANSAI PAINT CO LTD
Superparamagnetic colloidal nanocrystal structures
ActiveUS20100224823A1High magnetizationGood water dispersibilityMaterial nanotechnologyPigmenting treatmentMagnetitePhotonics
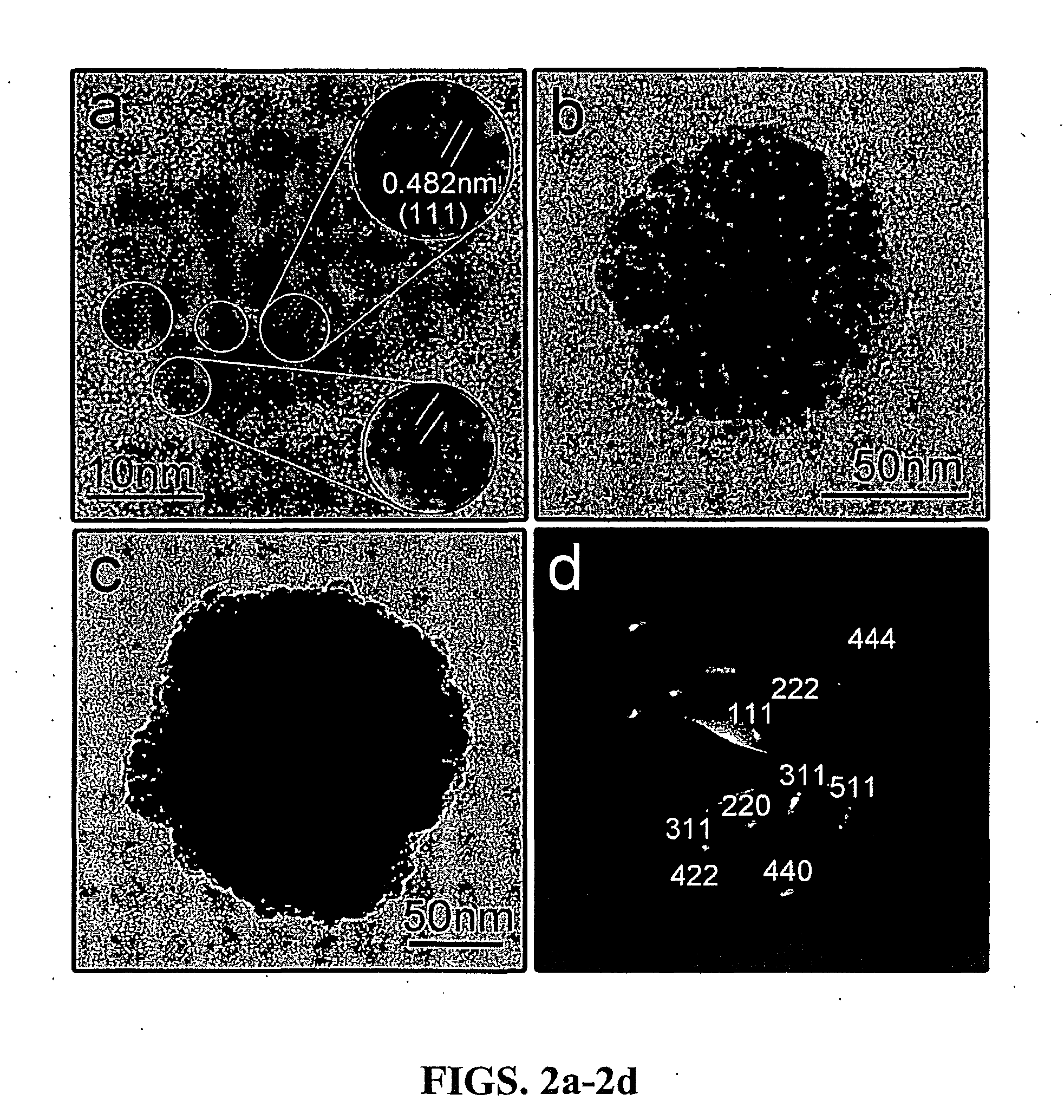
Monodisperse colloidal nanocrystal clusters of magnetite (Fe3O4) with tunable sizes from about thirty to about three hundred nanometers have been synthesized using a high-temperature hydrolysis process. The colloidal nanocrystal clusters are capped with polyelectrolytes, and highly water soluble. Each cluster is composed of many single magnetite crystallites, thus retaining the superparamagnetic behavior at room temperature. The combination of superparamagnetic property, high magnetization, and high water dispersibility makes the colloidal nanocrystal clusters ideal candidates for various important biomedical applications such as drug delivery and bioseparation. The present invention is further directed to methods for forming colloidal photonic crystals from both aqueous and nonaqueous solutions of the superparamagnetic colloidal nanocrystal clusters with an external magnetic field applied thereto. The diffraction of the photonic crystals can be tuned from near infrared to visible and further ultraviolet spectral region by varying the external magnetic field.
Owner:RGT UNIV OF CALIFORNIA
Intercalates formed with polypropylene/maleic anhydride-modified polypropylene intercalants
InactiveUS20010033924A1Good dispersionImprove distributionThin material handlingWater-setting substance layered productPolyolefinPolymer science
A nanocomposite concentrate composition comprising about 10 weight percent to about 90 weight percent of a layered silicate material and about 10 weight percent to about 90 weight percent of a matrix polymer comprising about 90-99.8% by weight of a polyolefin and about 0.2% to about 10%, preferably about 0.2% to about 3%, more preferably about 1% to 3% by weight, of a maleic anhydride-modified polyolefin, based on the total weight of polyolefins, wherein the layered silicate material is dispersed uniformly throughout the matrix polymer. Shearing of the concentrate and later (after shear) addition of an added matrix polymer avoids thermal degradation of the added matrix polymer and optimizes the dispersion of the nanomer throughout the matrix polymer; provides increased tensile strength; and reduces degradation of the polymer by melt formation of a concentrate thereby decreasing heat degradation of added matrix polymer.
Owner:AMCOL INTERNATIONAL CORPORATION
Self-emulsifying composition of omega3 fatty acid
InactiveUS20170348273A1Reduce the amount requiredImprove compatibilityOrganic active ingredientsNervous disorderAlcoholEmulsion
A self-emulsifying composition contains: 70 to 90% by weight of at least one compound selected from the group consisting of ω3 polyunsaturated fatty acids and their pharmaceutically acceptable salts and esters; 0.5 to 6% by weight of water; 1 to 29% by weight of a polyoxyethylene sorbitan fatty acid ester as an emulsifier (optionally including a polyoxyl castor oil, and not including lecithin); and lecithin in an amount of 3 to 40 parts by weight in relation to 100 parts by weight of ω3 polyunsaturated fatty acids and the like. The self-emulsifying composition is excellent in self-emulsifying property, composition dispersibility, emulsion stability, and absorbability, is free from ethanol and polyhydric alcohols or only has such an alcohol added thereto at a reduced concentration, and is useful for foods and pharmaceuticals.
Owner:MOCHIDA PHARM CO LTD
Flame retardant composition with improved fluidity, flame retardant resin composition and molded products
ActiveUS20070176154A1Improve flame retardant performanceImproved powder property and hygroscopic propertyDyeing processMelamine phosphateAdditive ingredient
The invention provides a flame retardant composition comprising 1-99 weight parts of a salt of piperazine and an inorganic compound selected from among piperazine phosphate, piperazine pyrophosphate and piperazine polyphosphate, or a mixture of two or more of these piperazine salts (ingredient (A)), 99-1 weight parts of a salt of melamine and an inorganic compound selected from among melamine phosphate, melamine pyrophosphate and melamine polyphosphate, or a mixture of two or more of these melamine salts (ingredient (B) ) (wherein, the sum of ingredient (A) and ingredient (B) is 100 weight parts), 0-50 weight parts of an arbitrary ingredient (ingredient (C)), and 0.01-20 weight parts of a silicone oil having a viscosity at 25° C. of 5000 mm2 / s (ingredient (D)) which is added thereto. This flame retardant not only has superior flame retarding properties, but also has enhanced powder properties and anti-hygroscopic properties, and when it is added to a resin, there is little change of electrical resistance.
Owner:ADEKA CORP
Amphiphilic star block copolymers
InactiveUS20070160561A1Improve solubilityGood dispersibilityCosmetic preparationsBiocidePolymer scienceAqueous solution
The present invention relates to a basically spherical hyperbranched block copolymer having an internal hydrophobic block and an external hydrophilic block. Within the spherical copolymer, the hydrophobic block constitutes a hydrophobic layer, suitable to associate or encapsulate hydrophobic bioactive agents, while the hydrophilic block provides an outer layer, which is suitable to render the copolymer soluble or dispersible in aqueous solutions. Also claimed is a method for preparing the copolymer, which is suitable to encapsulate fragrances, flavours, drugs, agrochemicals, for example.
Owner:FIRMENICH SA
Method for purification of aqueous fluoropolymer emulsions, purified emulsions, and fluorine-containing finished articles
InactiveUS20060041051A1Good dispersibilityFatty oils/acids recovery from wasteOrganic compound preparationEmulsionIon exchange
The present invention provides a method of purifying an aqueous fluoropolymer emulsion by which fluorine-containing surfactants can be removed without lowering the dispersibility of the aqueous fluoropolymer dispersions. The present invention provides a method of purifying an aqueous fluoropolymer emulsion comprising; purifying an aqueous fluoropolymer emulsion by a specific technique of concentration wherein the aqueous fluoropolymer emulsion comprises a fluoropolymer and a fluorine-containing surfactant, said specific technique of concentration comprises concentration by phase separation, electric concentration and / or ion exchange concentration, and said concentration by phase separation, electric concentration and / or ion exchange concentration is carried out for removing the fluorine-containing surfactant.
Owner:DAIKIN IND LTD
Spherical silver power and method for producing same
InactiveUS20050279970A1Good dispersibilityGood degree of sinterTransportation and packagingConductive materialSilver particlesAqueous solution
A spherical silver powder which has a good dispersibility and which is capable of obtaining a good degree of sintering even if it is used for forming a paste to be fired at a low temperature of 600° C. or less to form a conductor. An aqueous solution containing a reducing agent is added to a water reaction system containing silver ions, to deposit silver particles by reduction, to produce a spherical silver powder which has a shrinkage of 5 to 15% at 500° C., a shrinkage of 10 to 20% at 600° C., a mean particle size of not greater than 5 μm, a tap density of not less than 2 g / cm3, and a BET specific surface area of not greater than 5 m2 / g.
Owner:DOWA ELECTRONICS MATERIALS CO LTD
Electrophotographic photoconductor, electrophotography method using the same, electrophotographic apparatus, electrographic apparatus process cartridge and electrophotographic photoconductor outermost surface layer coating solution
InactiveUS7018755B2High-resolution imageDecrease in resolutionRadiation applicationsConductive materialOxygen atomSimple Organic Compounds
An electrophotographic photoconductor having at least a photosensitive layer on a conductive support, wherein the electrophotographic photoconductor comprising, in the outermost layer thereof: a filler, an organic compound having an acid value of 10–400 mgKOH / g, and at least one of compounds represented by the following general formulas 1 and 2: where R1, R2 are substituted or unsubstituted alkyl groups or aromatic hydrocarbon rings, and may be identical or different. R1, R2 may also be bonded together to form a substituted or unsubstituted heterocycle containing a nitrogen atom. R3, R4, R5 are substituted or unsubstituted alkyl or alkoxy groups, or halogen atoms. Ar is a substituted or unsubstituted aromatic hydrocarbon ring or aromatic heterocycle. n is an integer in the range 2 to 4, and k, l, m are respectively integers in the range 0 to 3. X is an oxygen atom, or a sulfur atom.
Owner:RICOH KK
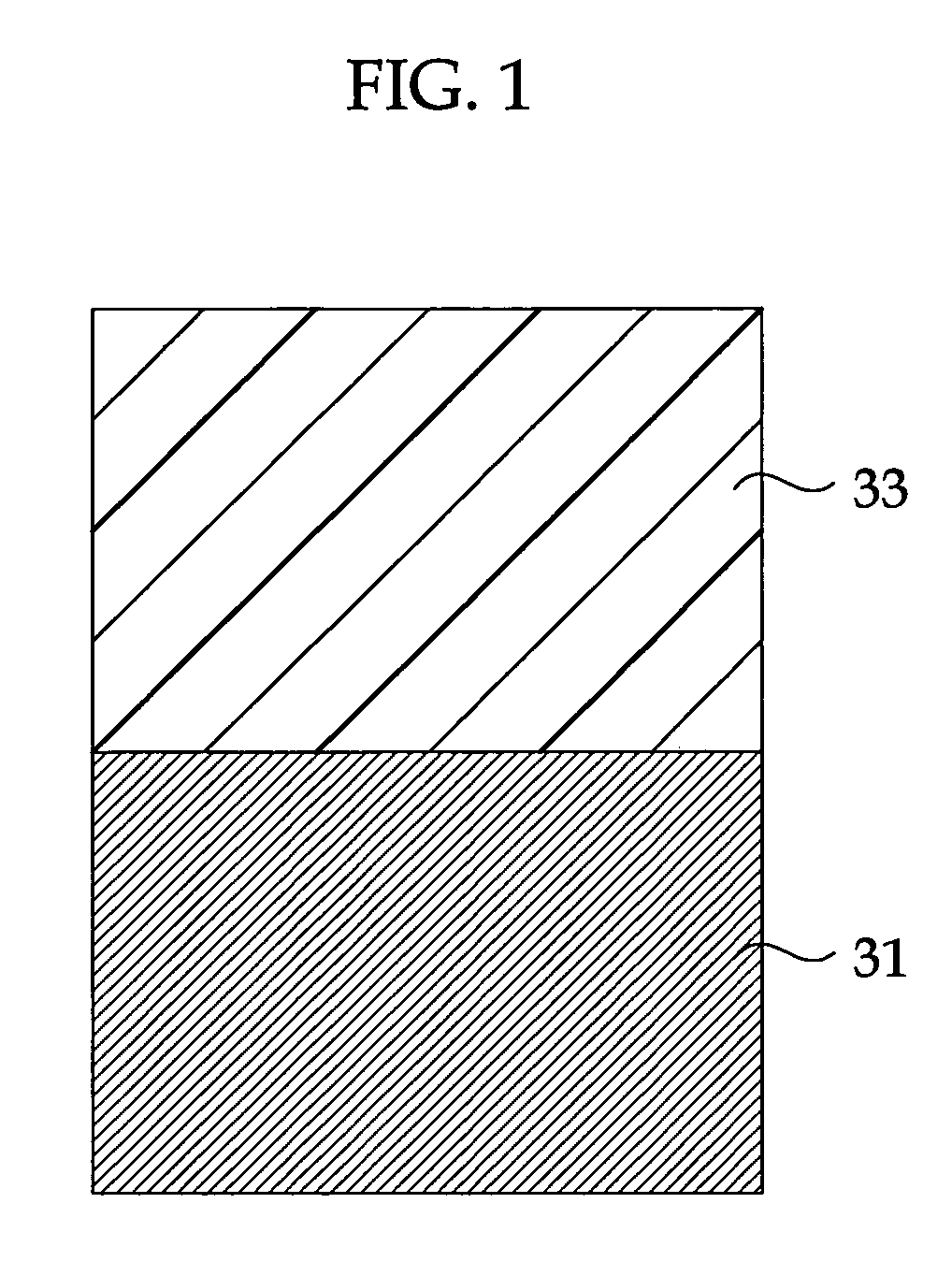
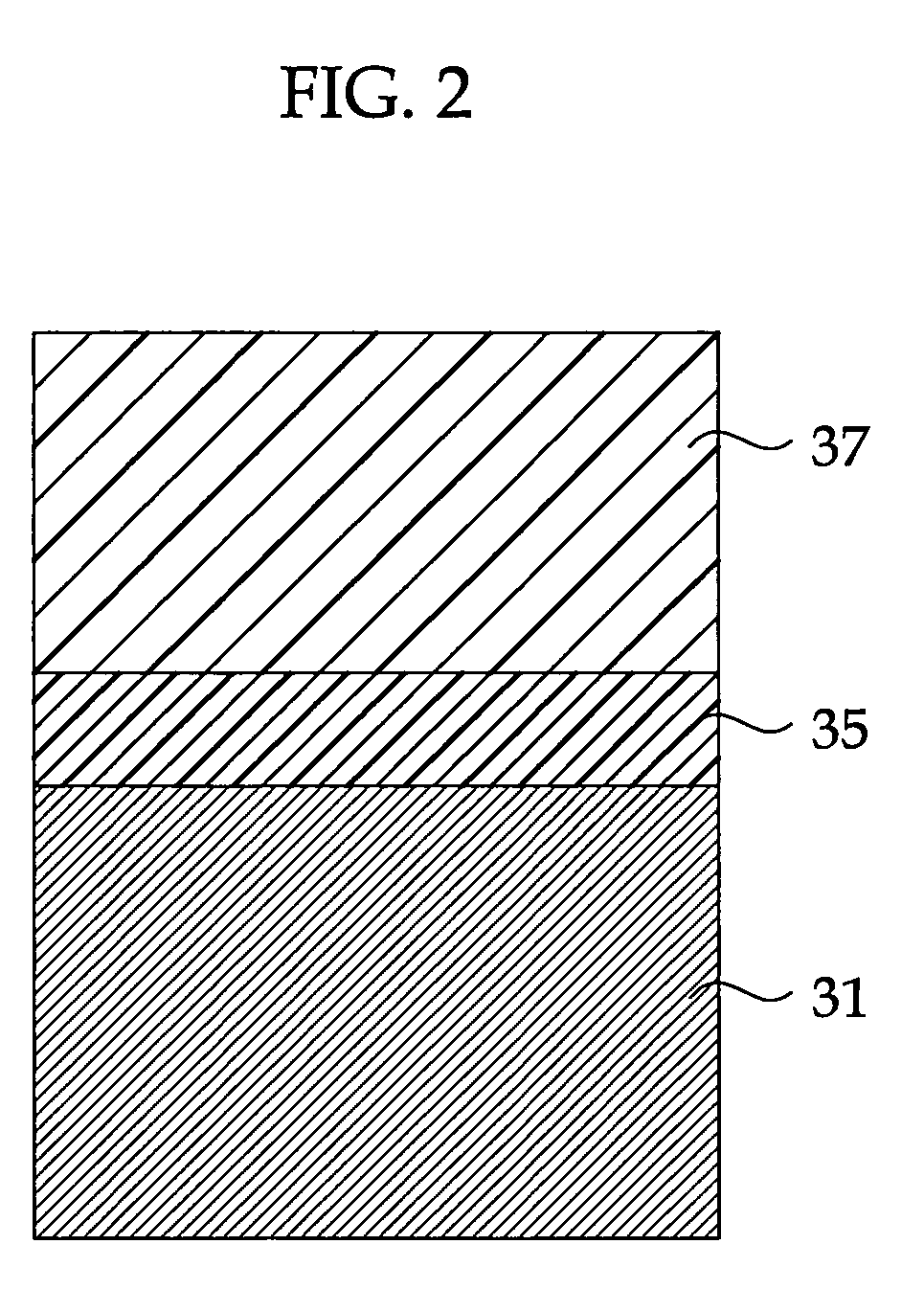
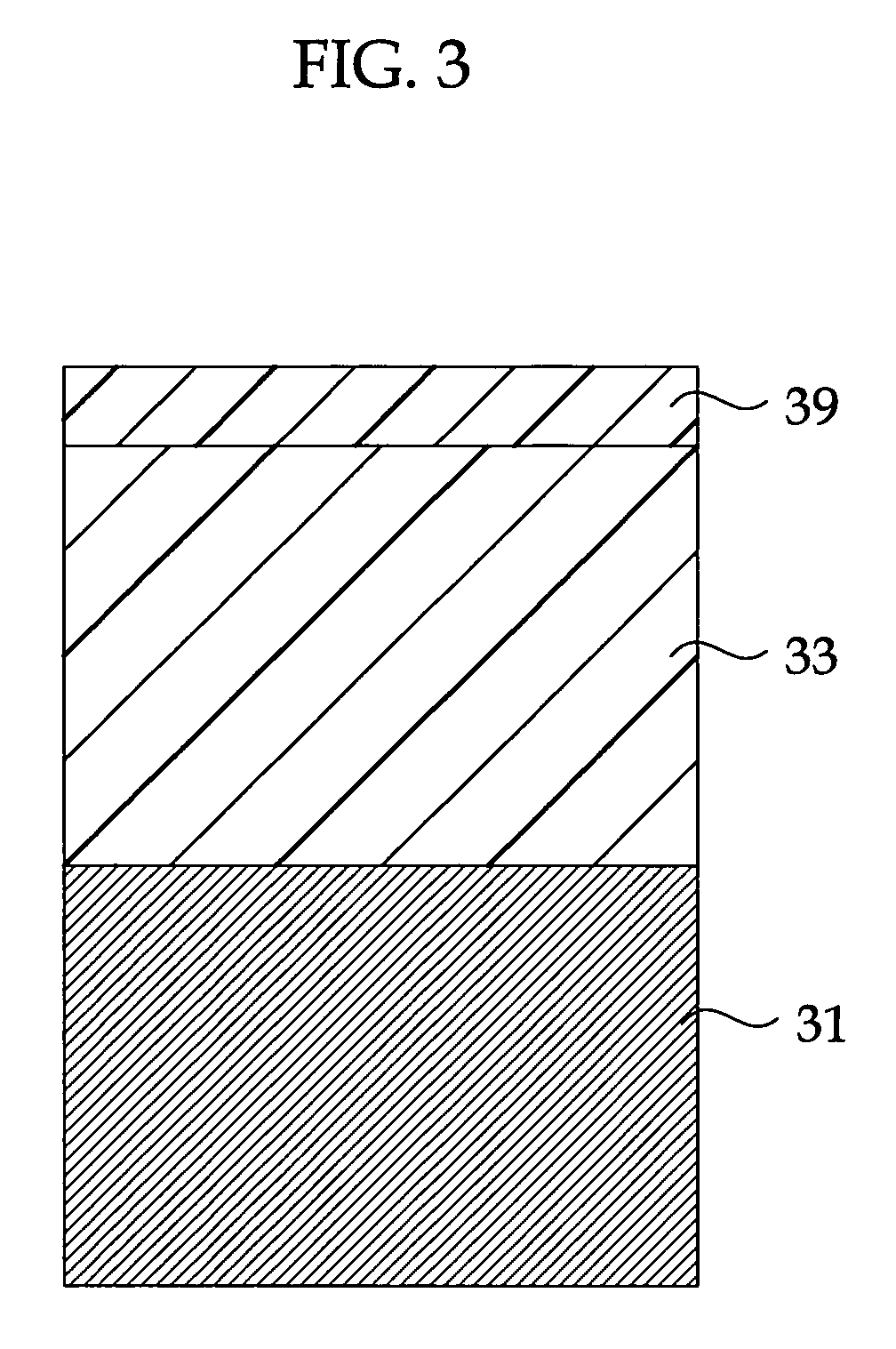
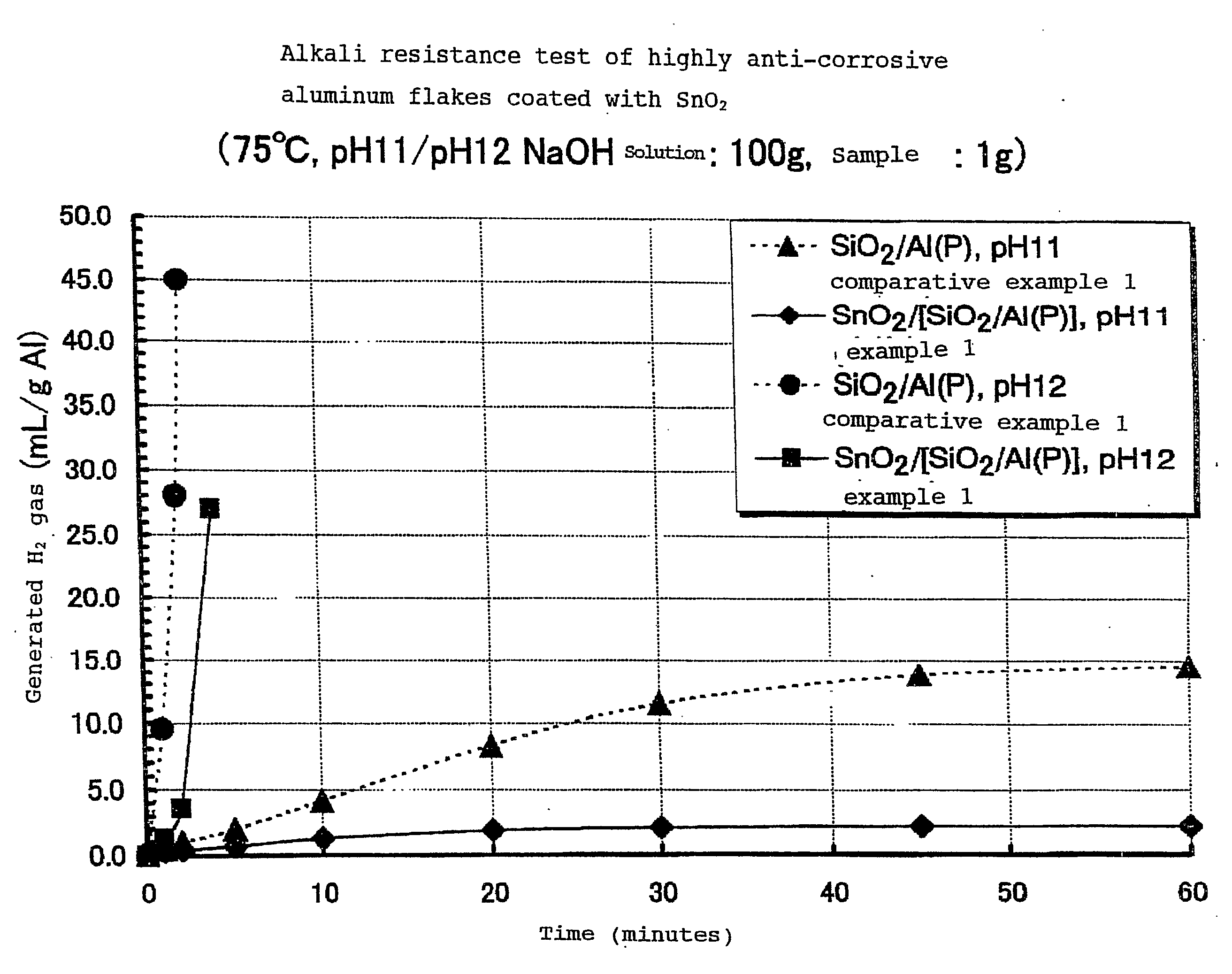
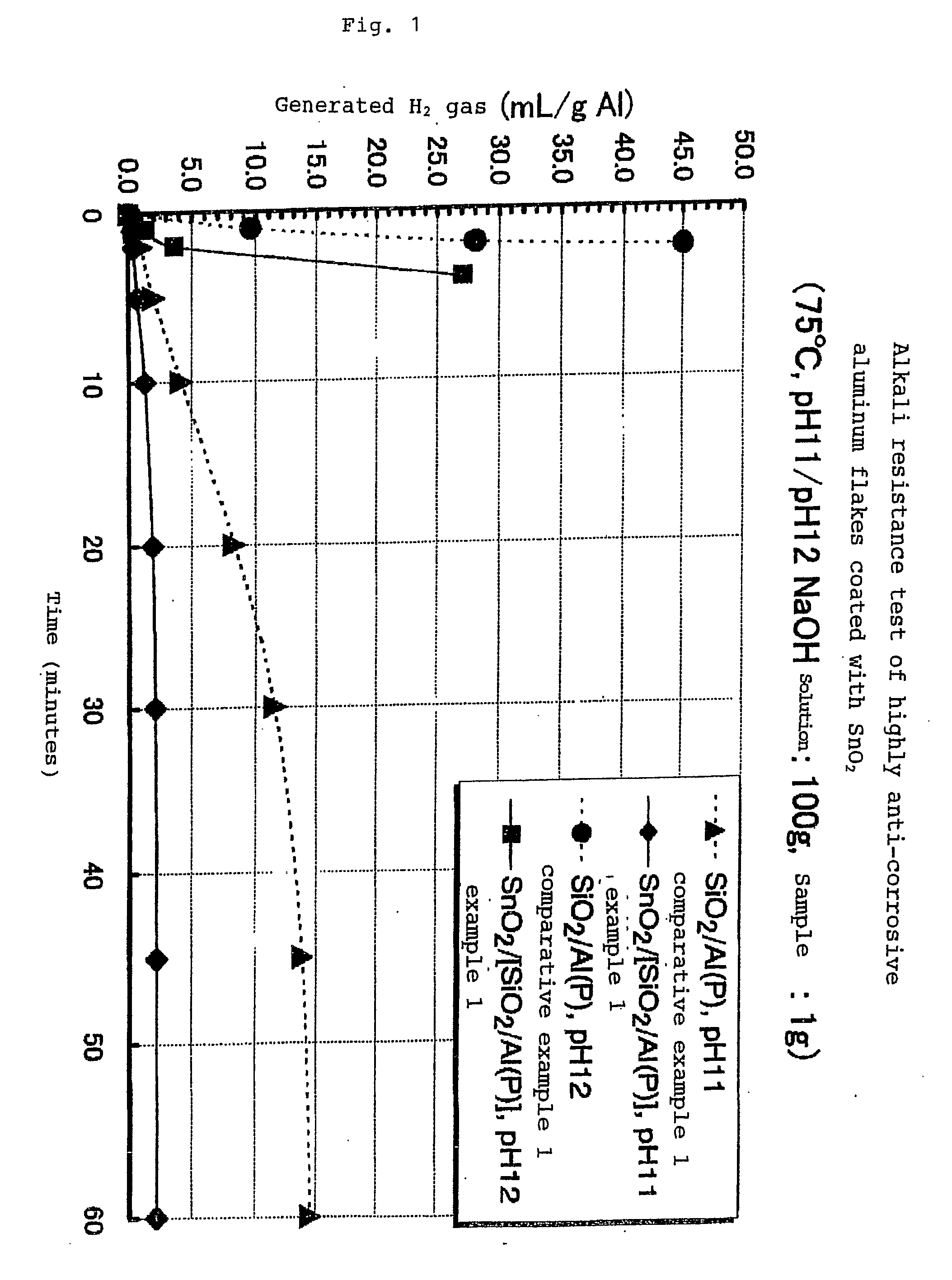
Highly Anti-Corrosive Thin Platelet-Like Metal Pigments, Preparing Method of the Same, and Colored Interference Pigments Having Metallic Luster Based on the Same
InactiveUS20080314284A1High corrosion resistanceGood dispersibilityCosmetic preparationsLiquid surface applicatorsPhosphoric acidMetal substrate
Owner:MERCK PATENT GMBH
Use of high-purity phenylsilsesquioxane liquids for the preparation of cosmetic and pharmaceutical compositions
InactiveUS20030077240A1Feel goodBroaden applicationCosmetic preparationsSilicon organic compoundsSilanolOrganic compound
Owner:CLARIANT INT LTD
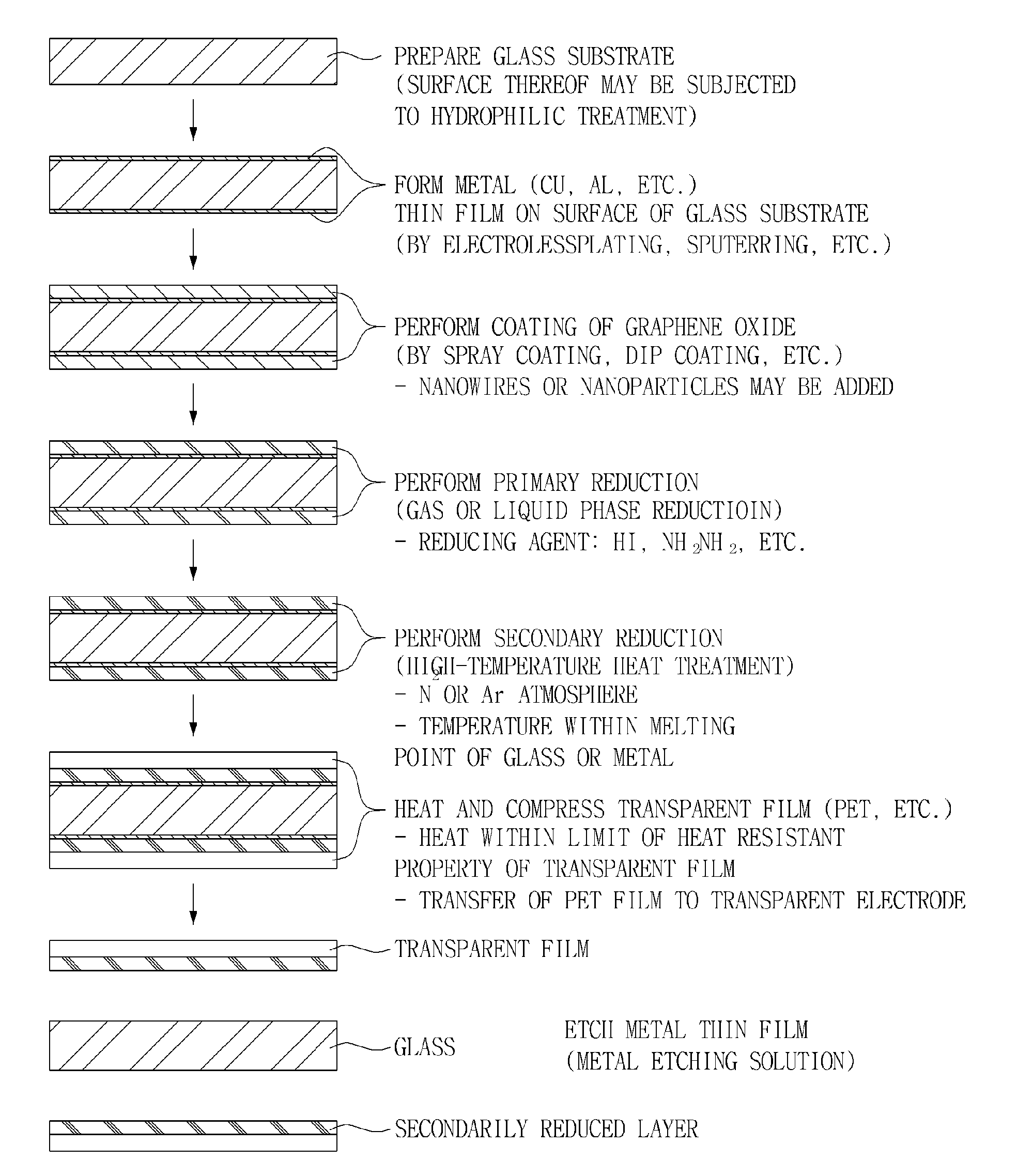
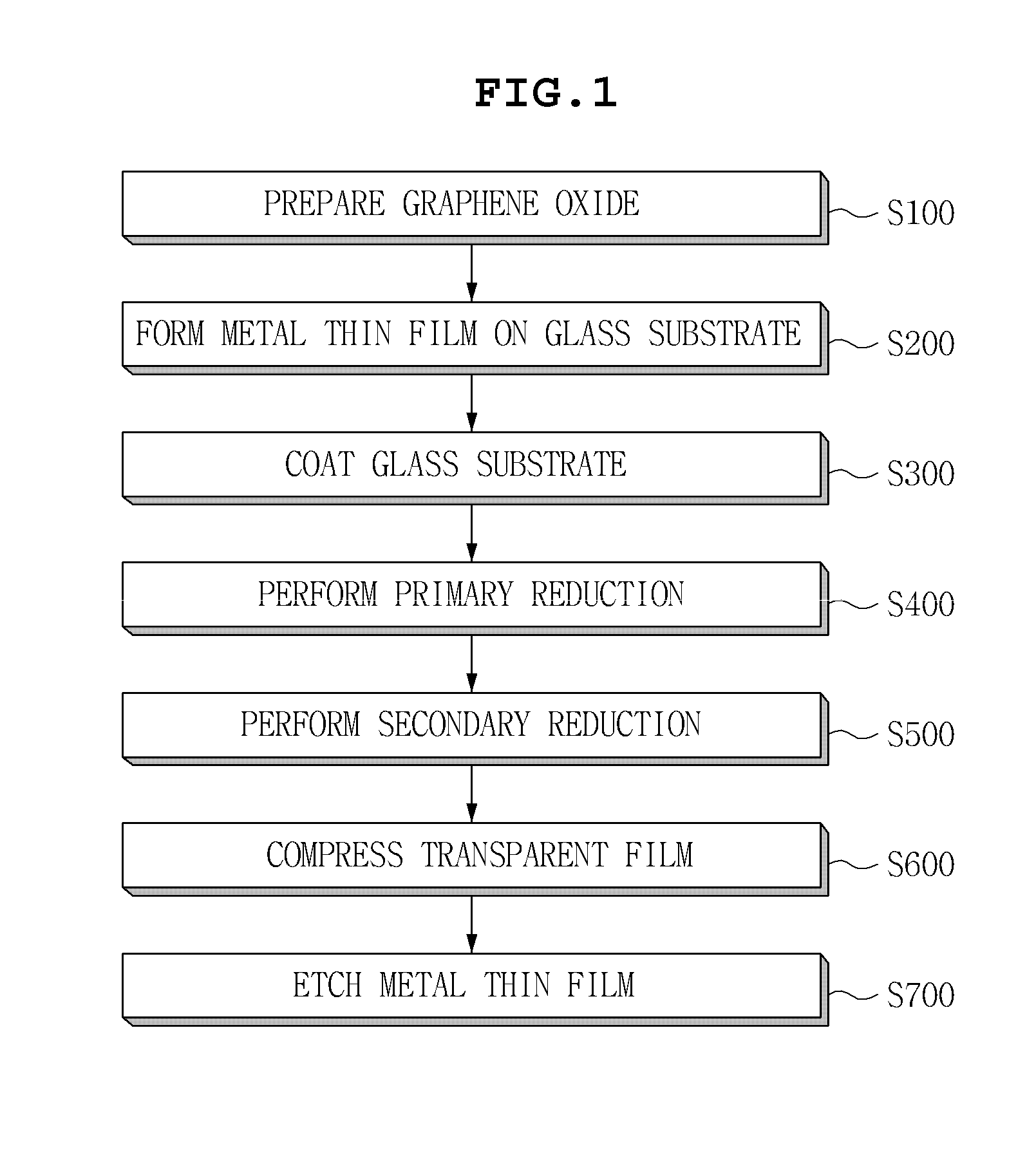
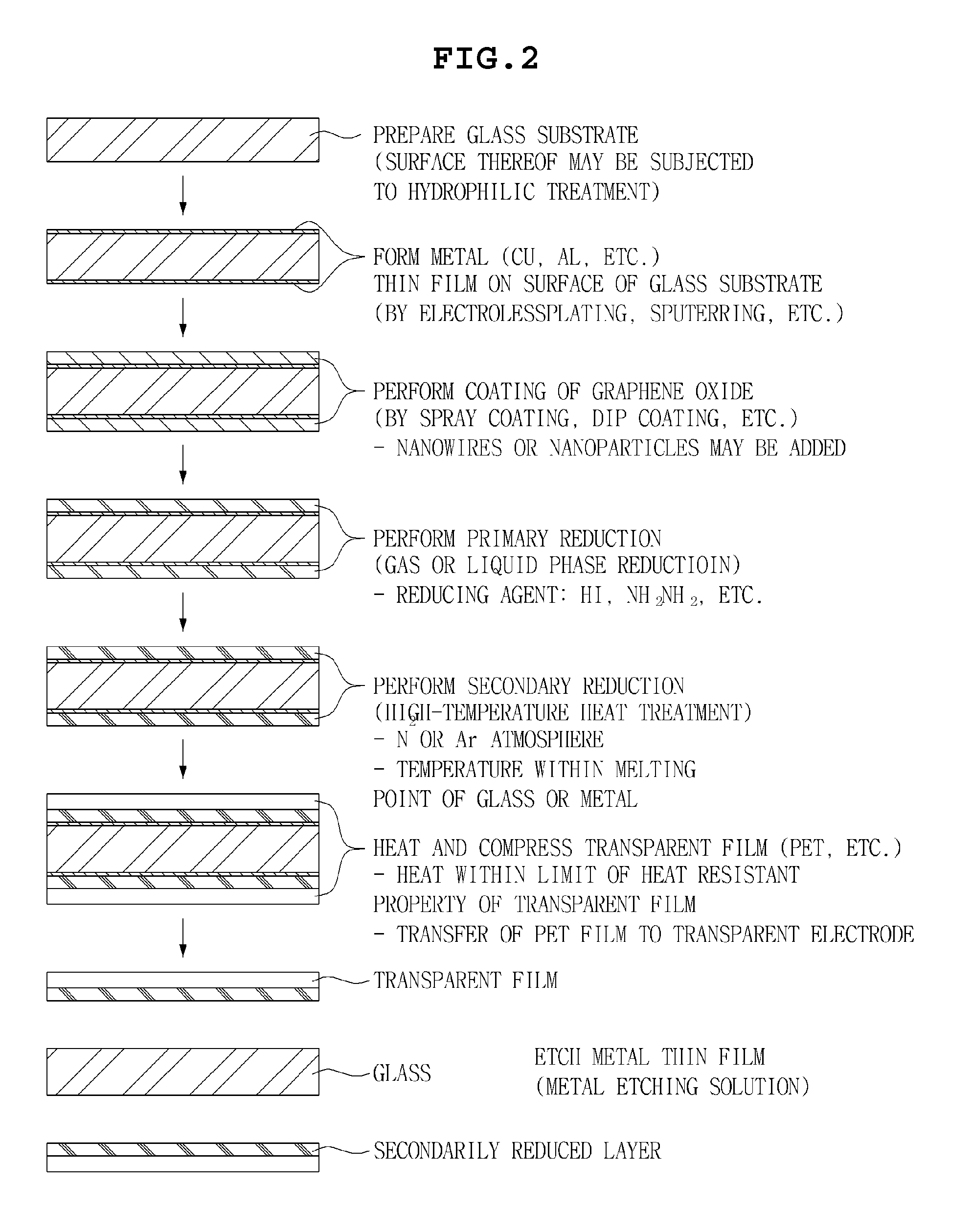
Graphene transparent electrode and method for manufacturing the same
InactiveUS20130133925A1Excellent dispersibilityGood electrical conductivityMaterial nanotechnologyConductive layers on insulating-supportsCvd grapheneGraphite oxide
Disclosed herein are a method for manufacturing a graphene transparent electrode and a graphene transparent electrode manufactured by the method. The method includes: providing a graphene oxide solution: forming a metal thin film on a glass substrate; coating the graphene oxide solution on the metal thin film, followed by drying; primarily reducing the thus obtained graphene oxide by using a reducing agent, to obtain reduced graphene oxide; secondarily reducing the reduced graphene oxide by heat treatment under the inert atmosphere, to form a reduced layer; compressing a transparent film on the reduced layer; and etching the metal film by an etching solution. The method enables a graphene transparent electrode having economical feasibility and excellent electric conductivity to be manufactured.
Owner:SAMSUNG ELECTRO MECHANICS CO LTD
Toner for electrostatic image development, electrostatic image developer and image forming method using the same
InactiveUS20070092821A1Good compatibilityGood dispersibilityDevelopersElectrographic processes using charge patternAlcoholCompound (substance)
The present invention relates to a toner for electrostatic image development, comprising a crystalline ester compound synthesized by polymerizing a carboxylic acid component with an alcohol component, a non-crystalline resin, a colorant and a releasing agent, wherein the weight-average molecular weight of the crystalline ester compound is 5000 or less, and the number of carbon atoms in at least one component selected from the carboxylic acid component and the alcohol component is 10 or more.
Owner:FUJIFILM BUSINESS INNOVATION CORP
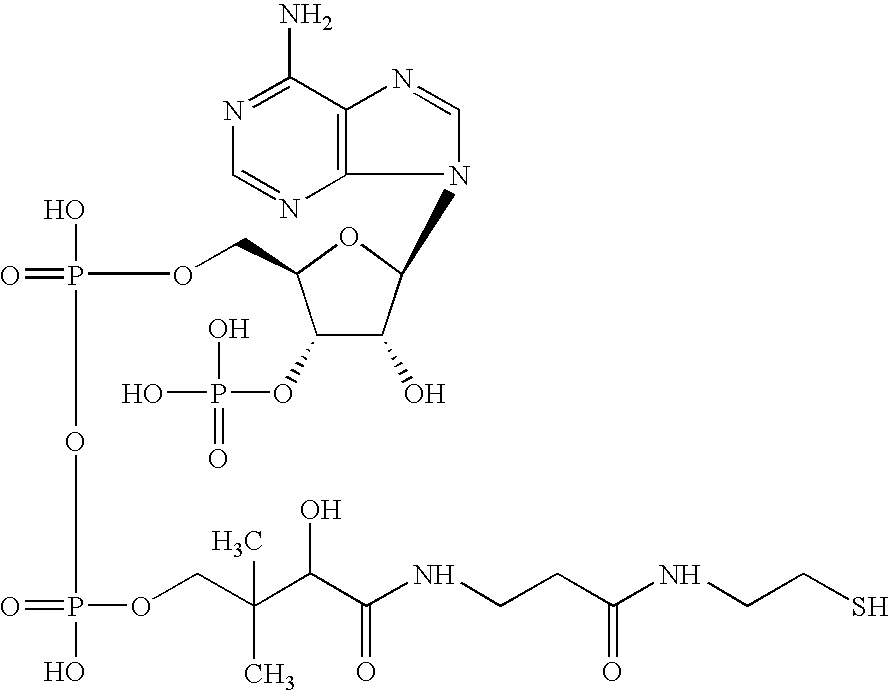
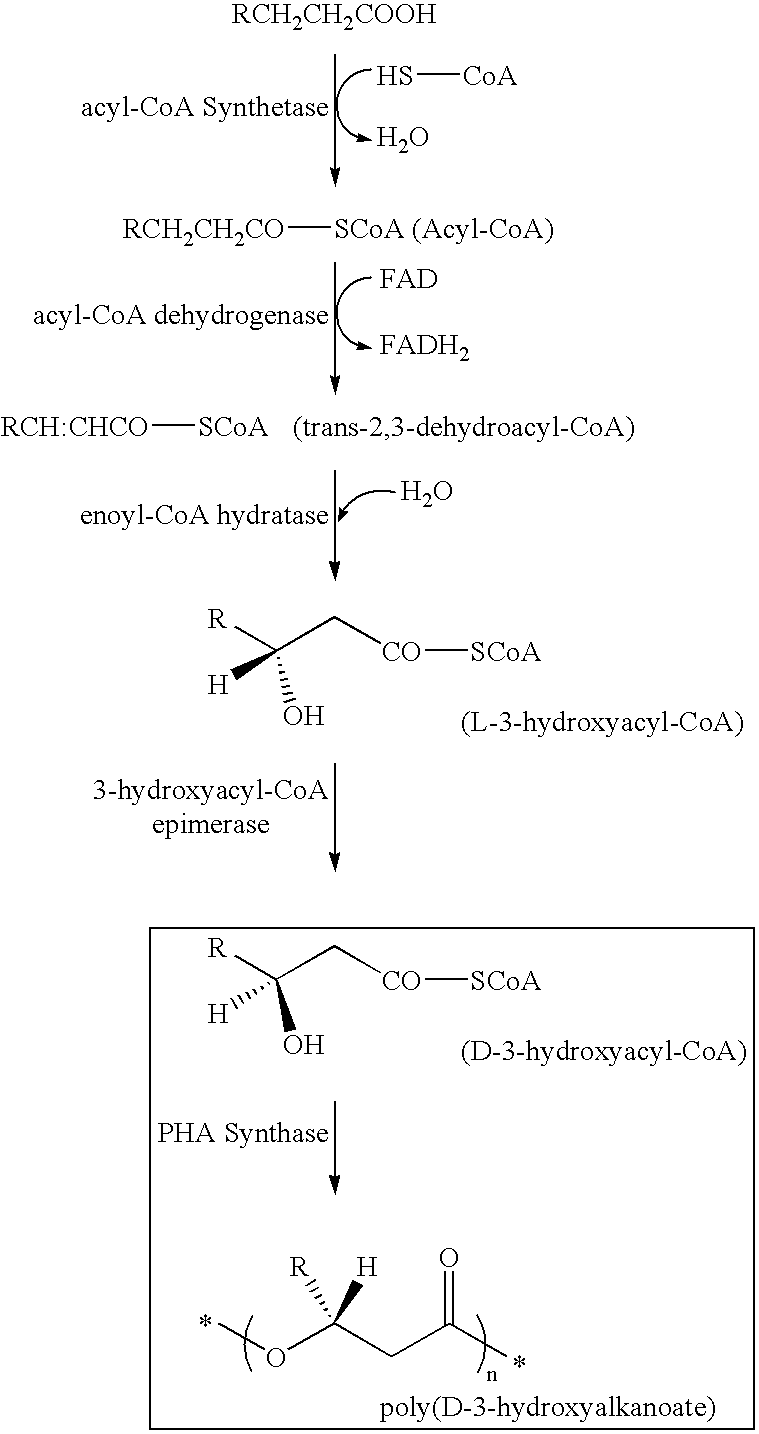
Pigment containing ink and production method thereof
InactiveUS6916861B2Contained in a fine microcapsule easilyImprove propertiesHydrolasesMicroorganism based processesDispersion stabilityPolyhydroxyalkanoates
A micro-capsulated pigment containing pigment ink being excellent in density, fineness, transparency, and coloring and color rendering properties required for ink solutions and having excellent dispersibility and dispersion stability with time due to the reduced particle size is provided. At least a color material with at least part of the surfaces of pigment particles covered with polyhydroxyalkanoate, and a medium for dispersion of the color material are used to obtain the pigment ink.
Owner:CANON KK
Process for the preparation of a pigment comprising a core material and at least one dielectric layer
InactiveUS20050013934A1Good absorbance of microwave energyImprove adhesionLiquid surface applicatorsPigment preparation by wet methodsAqueous solutionDielectric layer
The present invention relates to a process for the preparation of a pigment comprising a core material and at least one dielectric layer using microwave deposition of a metal oxide from an aqueous solution of fluorine scavenger onto a core material.
Owner:CIBA SPECIALTY CHEM CORP
Method for Producing Modified Conjugated Diene Polymer, Modified Conjugated Diene Polymer, Modified Conjugated Diene Polymer Composition, Rubber Composition and Tire
ActiveUS20140371383A1Improve balanceLow hysteresis loss propertySpecial tyresAlkaline earth metalPolymer science
A method for producing a modified conjugated diene polymer comprising a polymerization step of obtaining a conjugated diene polymer containing a nitrogen atom in a polymer chain and an active end by copolymerizing a conjugated diene compound and a nitrogen atom-containing vinyl compound, or a conjugated diene compound, an aromatic vinyl compound and a nitrogen atom-containing vinyl compound by use of an alkali metal compound and / or an alkaline earth metal compound as a polymerization initiator, and a modification step of reacting a modifier.
Owner:ASAHI KASEI CHEM CORP
Toner, method of producing the toner, developer including the toner, and image forming method and apparatus using the developer
ActiveUS7056635B2Good dispersibilityImproved pulverizabilityDevelopersElectrographic processes using charge patternWaxCrystallography
A toner including a thermoplastic resin; a colorant; a wax; and a crystalline polymer, wherein a DSC endothermic peak temperature of the wax or the crystalline polymer determined by subjecting the toner to a differential scanning calorimetric analysis is lower by not less than 2° C. than a DSC endothermic peak temperature thereof determined when only the wax or the crystalline polymer is subjected to the differential calorimetric analysis.
Owner:RICOH KK
Composition board binding material
InactiveUS6291558B1Good dispersibilityImprove stabilityInksLignin derivativesLignosulfonatesReaction step
This invention features a graft copolymer of lignosulfonates reacted with an unsaturated carbonyl compound and an aldehyde, in two separate reaction steps. The resin can be used as a substitute for a majority of pure urea formaldehyde resin in the manufacture of composite board products. The resin can also be introduced directly into the urea formaldehyde resin production process.
Owner:CELLUTECH
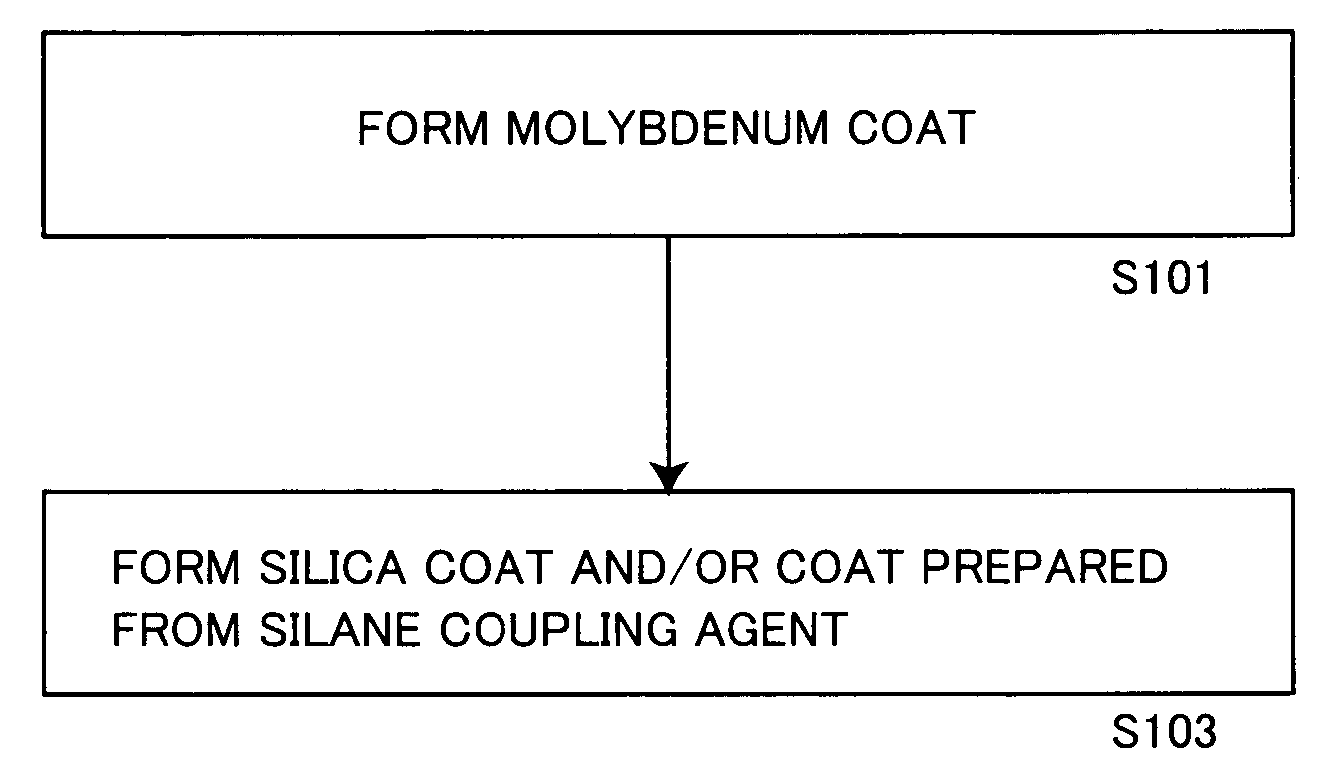
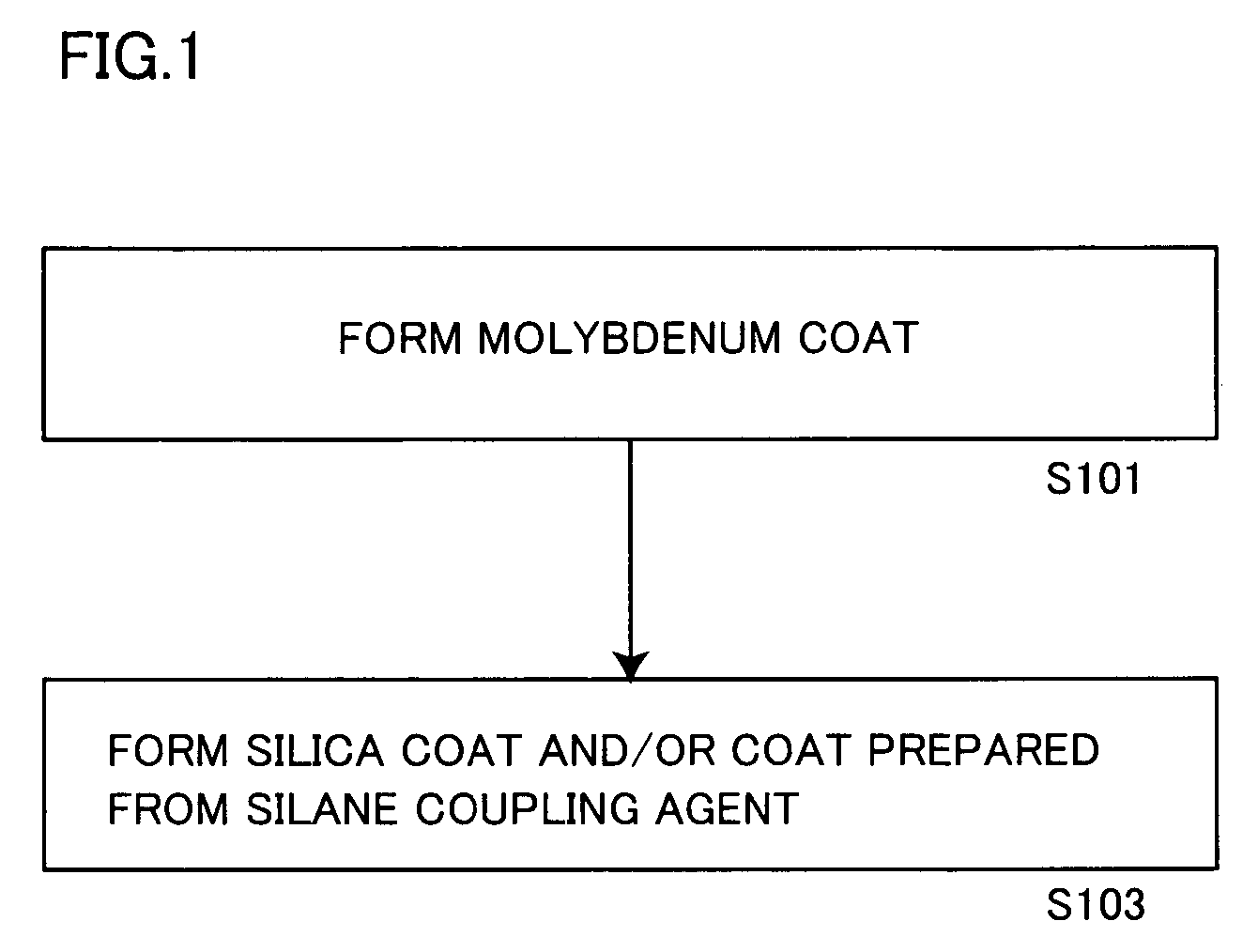
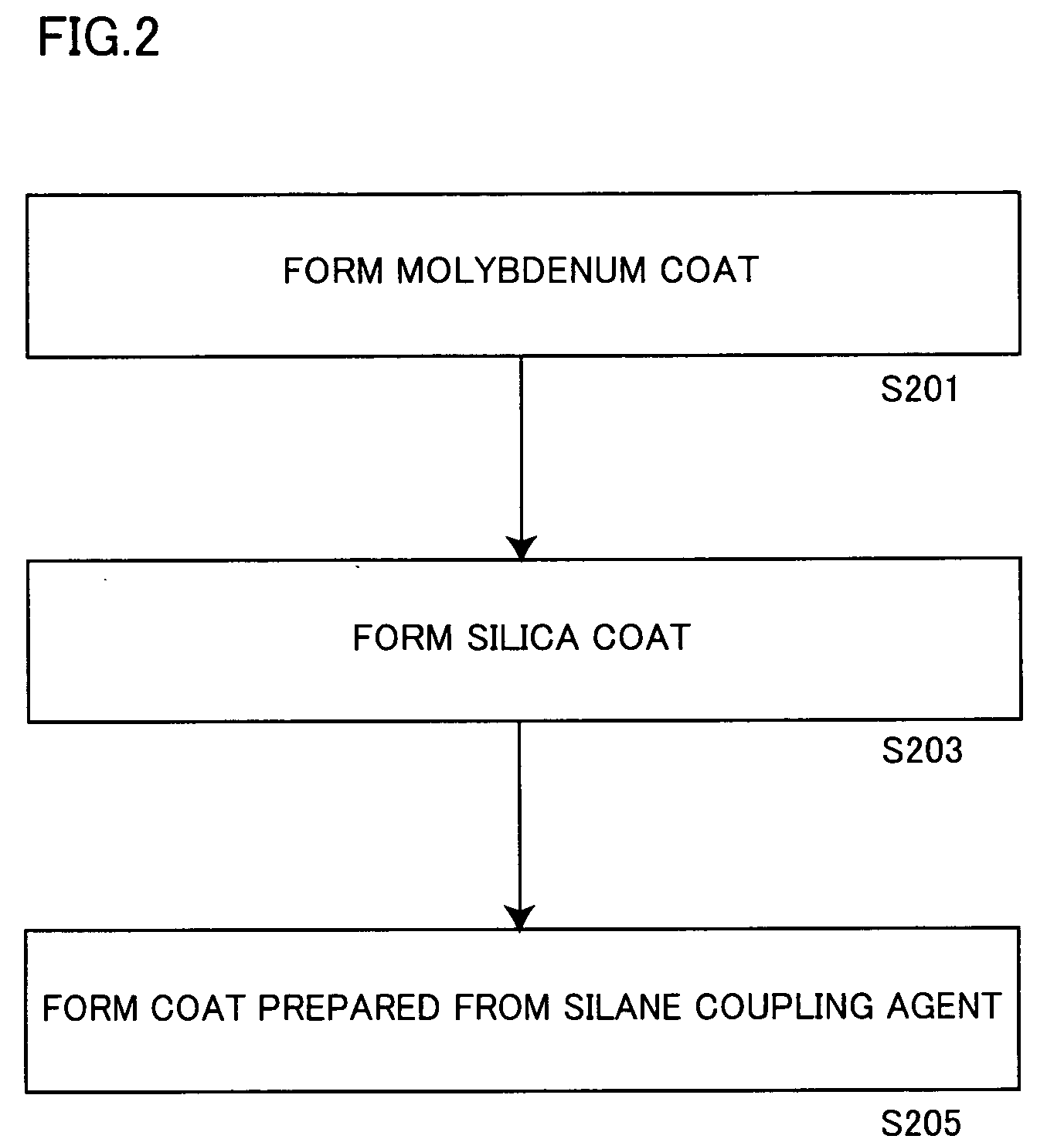
Aluminum pigment, process for production thereof and resin composition
ActiveUS20060150864A1Excellent dispersibility and stabilityExcellent designabilityInorganic pigment treatmentInksSilane couplingSilica coating
An aluminum pigment having aluminum particles, a molybdenum coat comprising a molybdenum oxide and / or a molybdenum hydrate covering the surface of each aluminum particle and a silica coat comprising amorphous silica and / or a coat prepared from a silane coupling agent further covering this molybdenum coat is provided as an aluminum pigment having excellent dispersibility and stability, neither generating hydrogen gas nor agglomerating during storage and providing excellent designability for the appearance of a film. With respect to 100 parts by mass of aluminum, the content of molybdenum is preferably in the range of 0.01 to 5 parts by mass, and the content of silicon is preferably in the range of 1 to 20 parts by mass.
Owner:TOYO ALUMINIUM KK
Filler for Porous Film and Porous Film Containing the Same
InactiveUS20080182933A1Good dispersionPoor adhesionSemi-permeable membranesGranular deliveryLiquid-crystal displayInorganic particle
A filler for porous films is provided which is easy to be mixed with a resin, has good dispersibility in a resin, and provides a porous film useful for a light reflector of, for example, a liquid crystal display and a lighting apparatus and a porous film useful for a diaphragm (separator) between electrodes of a battery.A filler comprises inorganic particles surface treated with a surfactant (A) and a compound (B) having a chelating function to an alkaline earth metal.
Owner:MARUO CALCIUM COMPANY
Metal nanoparticle dispersion and production process of the same
InactiveUS20090198009A1Superior self-assembling abilityStable dispersed stateMaterial nanotechnologyConductive materialPolymer scienceNanometre
A metal nanoparticle dispersion comprising: a dispersion of a polymer compound (X), which comprises a polyalkyleneimine chain (a), a hydrophilic segment (b) and a hydrophobic segment (c), and metal nanoparticles (Y).
Owner:DAINIPPON INK & CHEM INC
Slurry for secondary battery porous membranes, secondary battery porous membrane, secondary battery electrode, secondary battery separator, secondary battery, and method for producing secondary battery porous membrane
ActiveUS20130280584A1Excellent cycle characteristicsIncrease flexibilityActive material electrodesWaste accumulators reclaimingInorganic particlePorous membrane
To provide a secondary battery porous membrane which is produced using a slurry for secondary battery porous membranes having excellent coatability and excellent dispersibility of insulating inorganic particles and is capable of improving the cycle characteristics of a secondary battery that is obtained using the secondary battery porous membrane, said secondary battery porous membrane having high flexibility and low water content and being capable of preventing particle fall-off. [Solution] A slurry for secondary battery porous membranes of the present invention is characterized by containing: insulating inorganic particles, each of which has a surface functional group that is selected from the group consisting of an amino group, an epoxy group; a mercapto group and an isocyanate group; a binder which has a reactive group that is crosslinkable with the surface functional group; and a solvent.
Owner:ZEON CORP
Toner, and image forming method
ActiveUS7297455B2Improve performanceMaintain good propertiesDevelopersElectrographic processes using charge patternWaxPolyester
A toner is composed primarily of toner particles containing at least a binder resin, a colorant and a wax, and inorganic fine particles. The binder resin is one which has at least a polyester unit and is synthesized by using as a catalyst one or more compounds selected from titanium chelate compounds each having a specific structure and hydrates of the titanium chelate compounds. The toner has superior fixing performance and high-temperature anti-offset properties and is superior in charge stability even when used for a long time.
Owner:CANON KK
Stable glassy state powder formulations
InactiveUS20030215512A1Good dispersionGood dispersibilityPowder deliveryPeptide/protein ingredientsSolventMoisture barrier
A powdered, dispersible composition having stable dispersibility over time is provided. The composition exhibits a characteristic glass transition temperature (Tg) and a recommended storage temperature (Ts), wherein the difference between Tg and Ts is at least about 10° C. (i.e. Tg-Ts is greater than 10° C.). The composition comprises a mixture of a pharmaceutically-acceptable glassy matrix and at least one pharmacologically active material within the glassy matrix. It may be further mixed with a powdered, pharmaceutically-acceptable carrier. It is particularly valuable in unit dosage form having a moisture barrier, in combination with appropriate labelling instructions. A process for producing a powdered dispersible composition is also provided, wherein the process comprises removing the solvent from a solution comprising a solvent, a glass former and a pharmacologically active material under conditions sufficient to form a glassy matrix having the pharmacologically active material within the matrix.
Owner:NOVARTIS FARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com