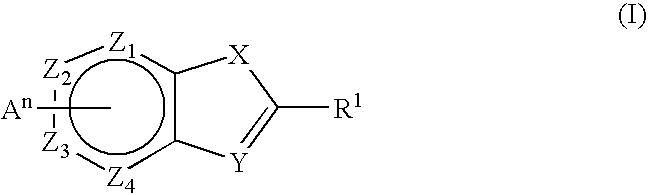Patents
Literature
6677 results about "Glutamic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
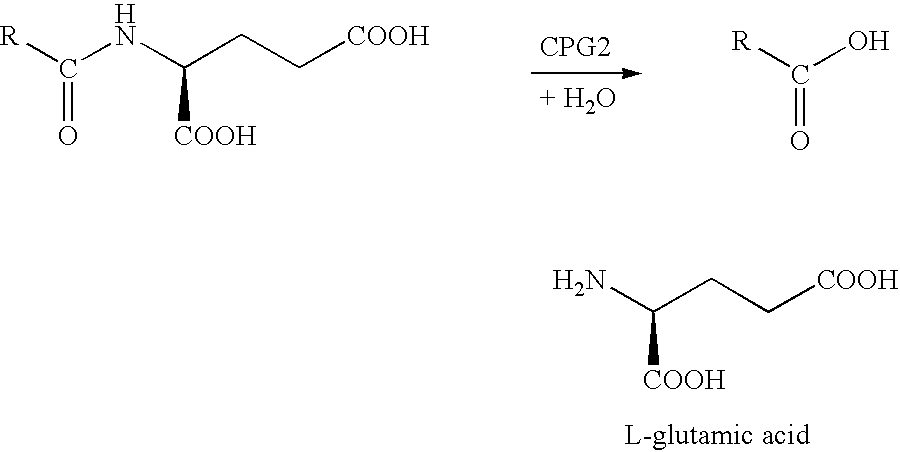
Glutamic acid (symbol Glu or E) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is non-essential in humans, meaning the body can synthesize it. It is also an excitatory neurotransmitter, in fact the most abundant one, in the vertebrate nervous system. It serves as the precursor for the synthesis of the inhibitory gamma-aminobutyric acid (GABA) in GABA-ergic neurons.
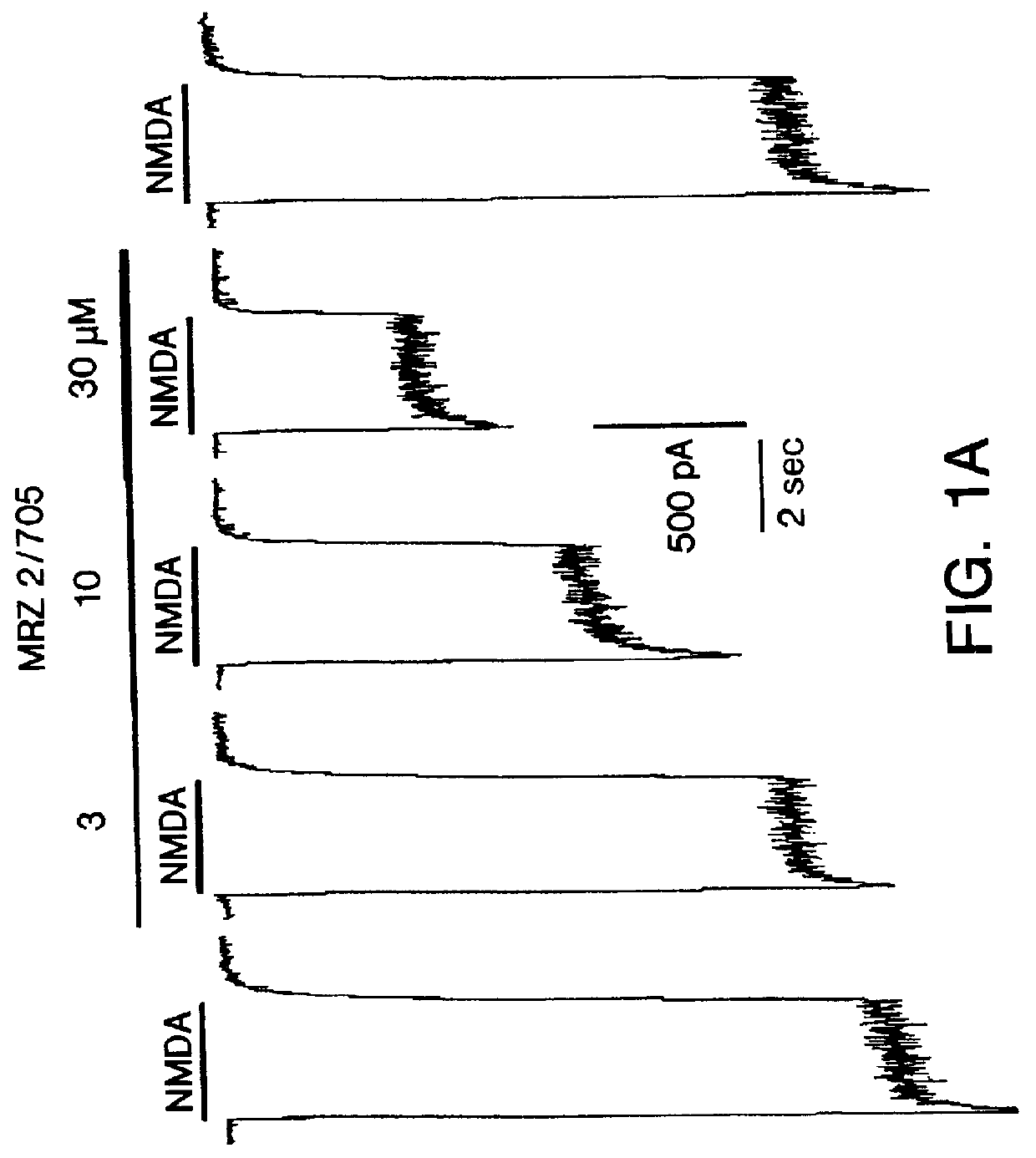
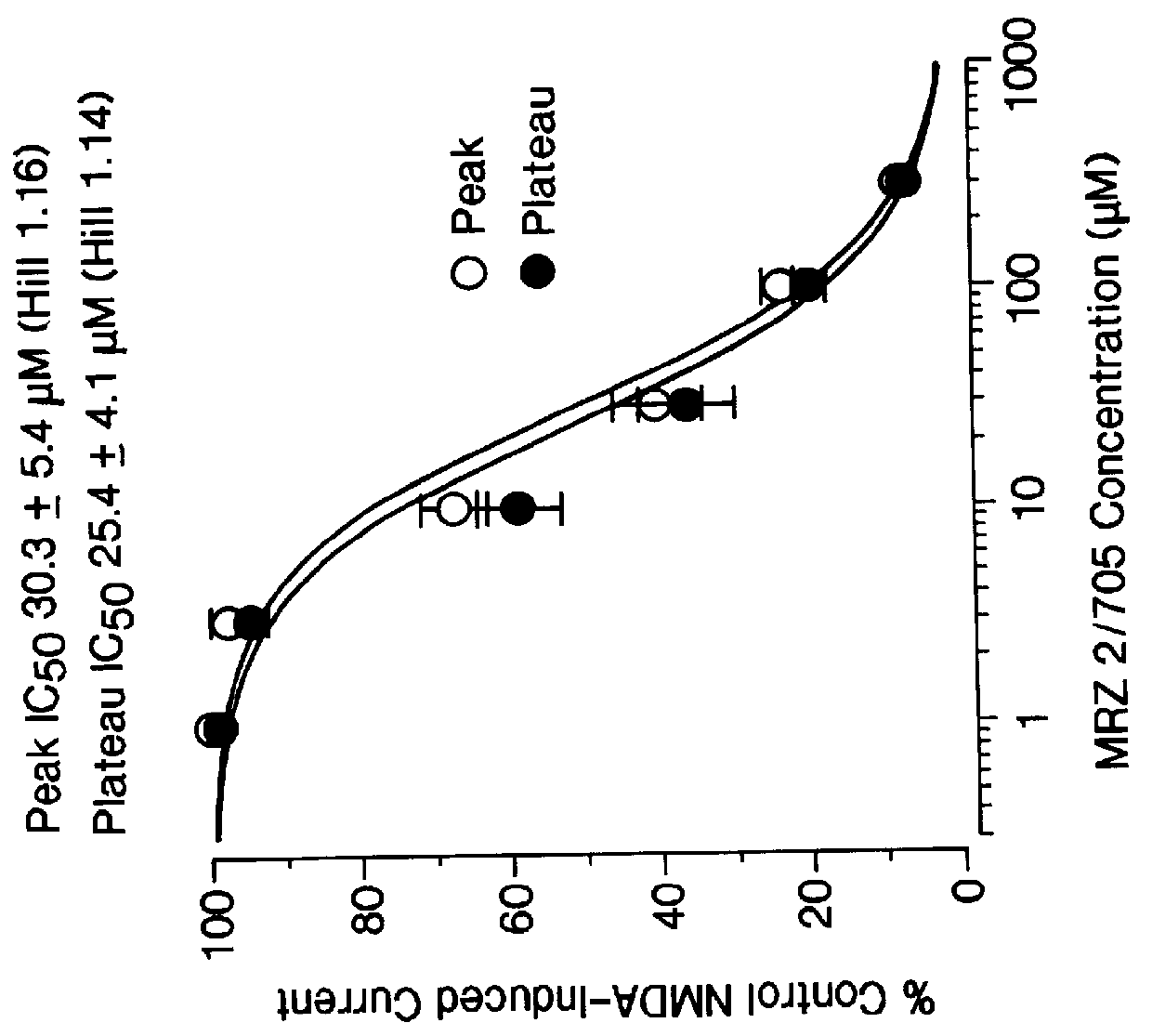
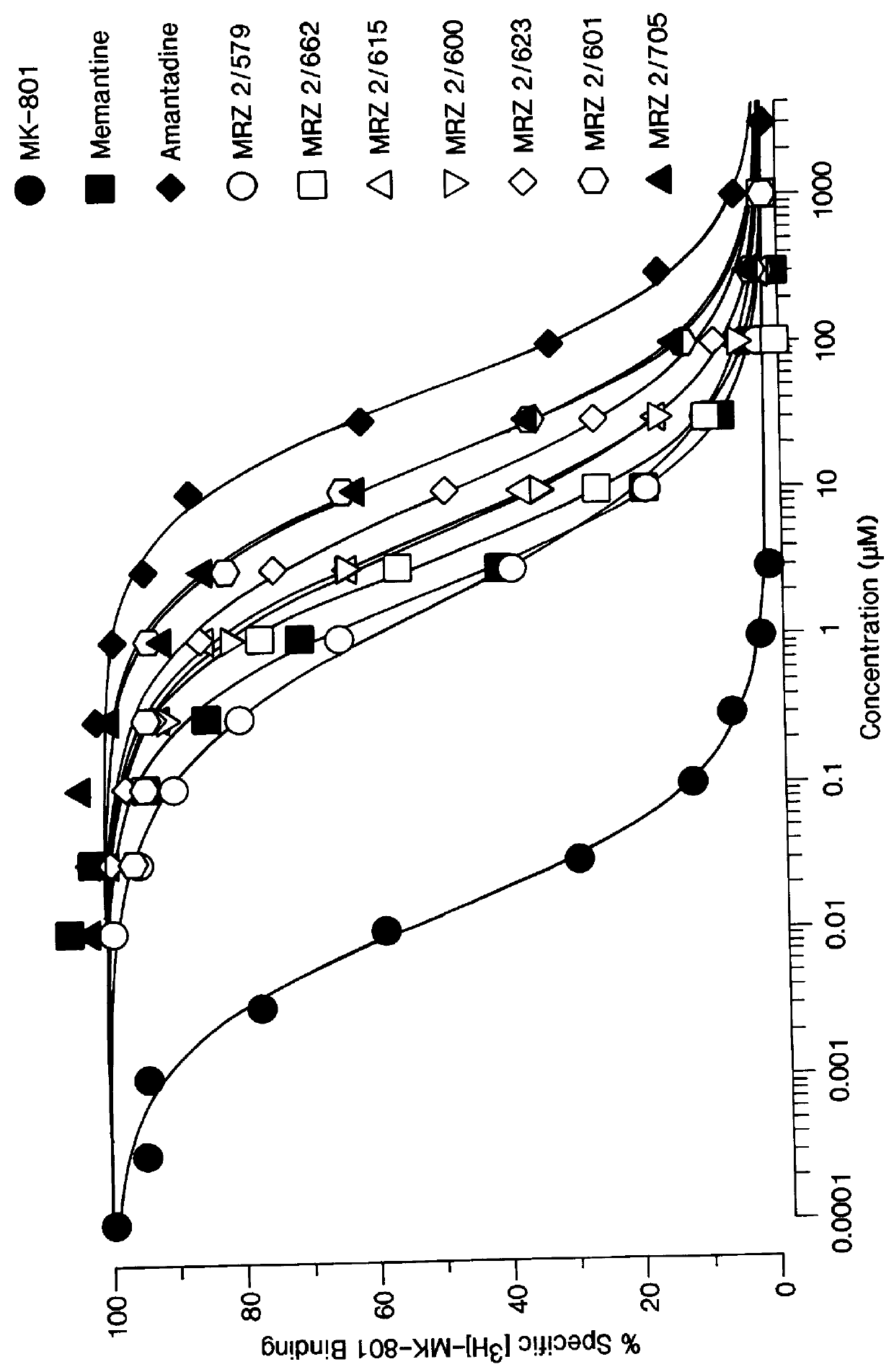
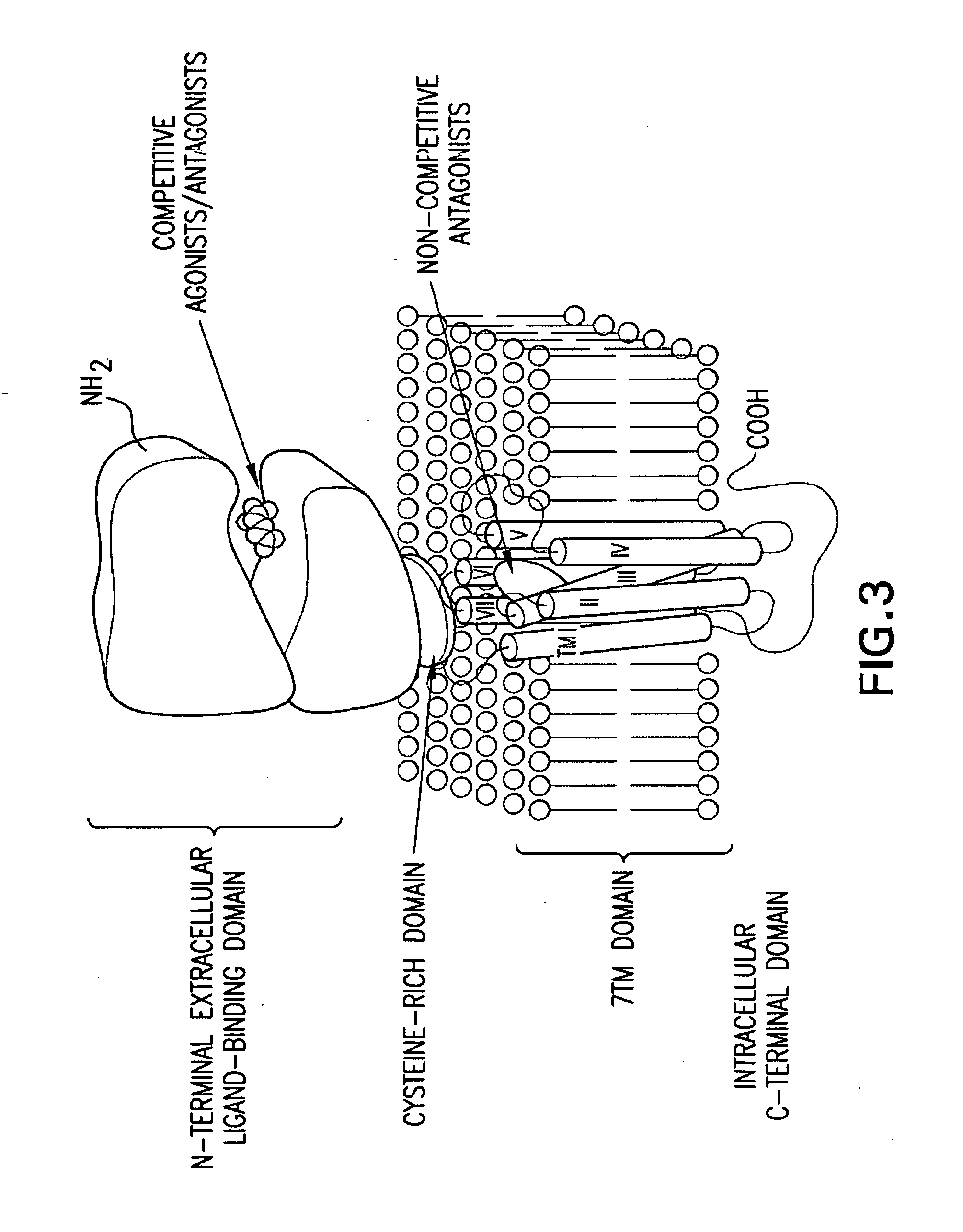
1-Amino-alkylcyclohexane NMDA receptor antagonists
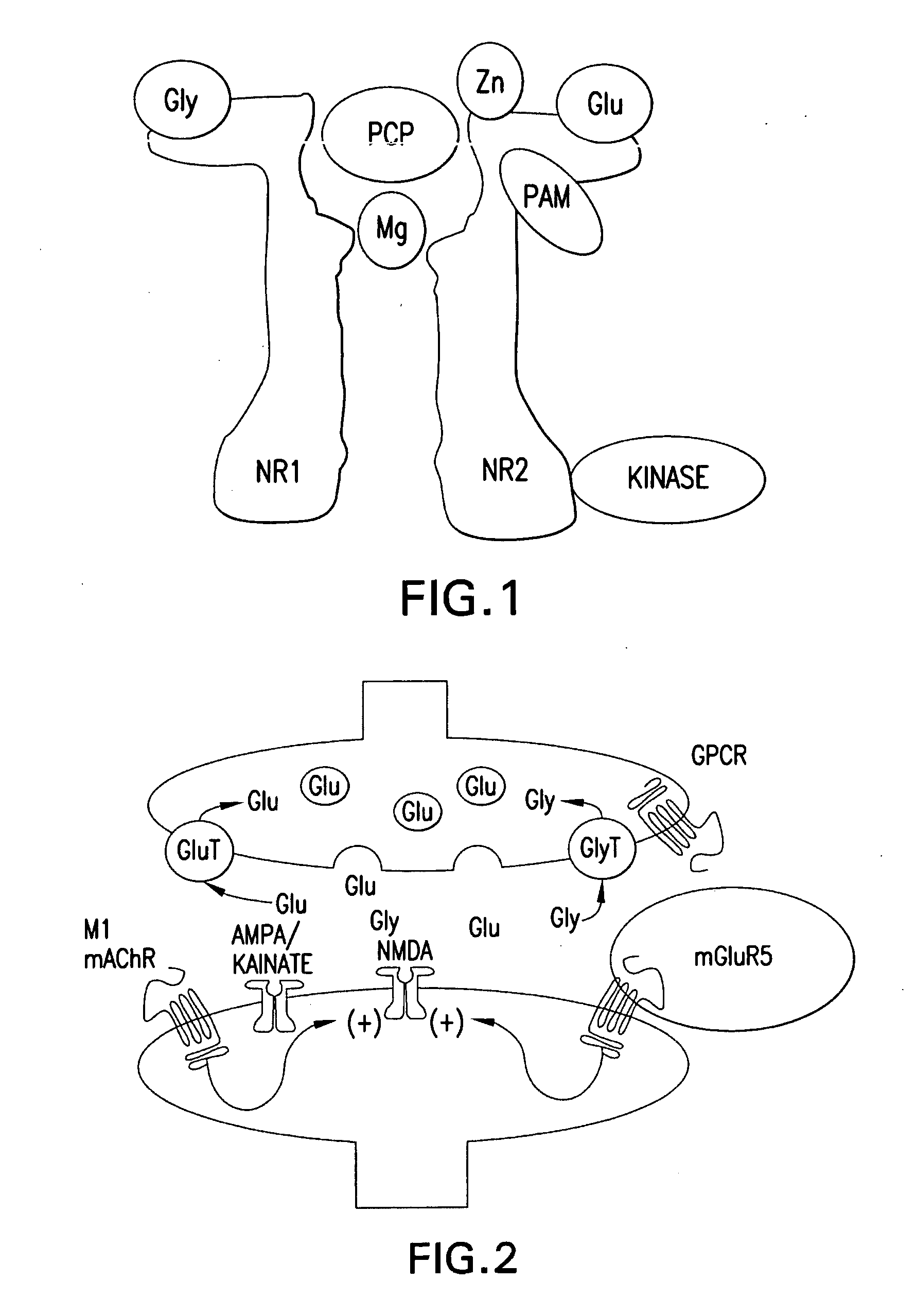
Certain 1-aminoalkylcyclohexanes are systemically-active uncompetitive NMDA receptor antagonists having rapid blocking / unblocking kinetics and strong voltage-dependency and are therefore useful in the alleviation of conditions resulting from disturbances of glutamatergic transmission giving them a wide range of utility in the treatment of CNS disorders involving the same, as well as in non-NMDA indications, due to their immunomodulatory, antimalarial, anti-Borna virus, and anti-Hepatitis C activities and utilities. Pharmaceutical compositions thereof and a method-of-treating conditions which are alleviated by the employment of an NMDA receptor antagonist, as well as the aforementioned non-NMDA indications, and a method for the preparation of the active 1-aminoalkylcyclohexane compounds involved.
Owner:MERZ PHARMA GMBH & CO KGAA
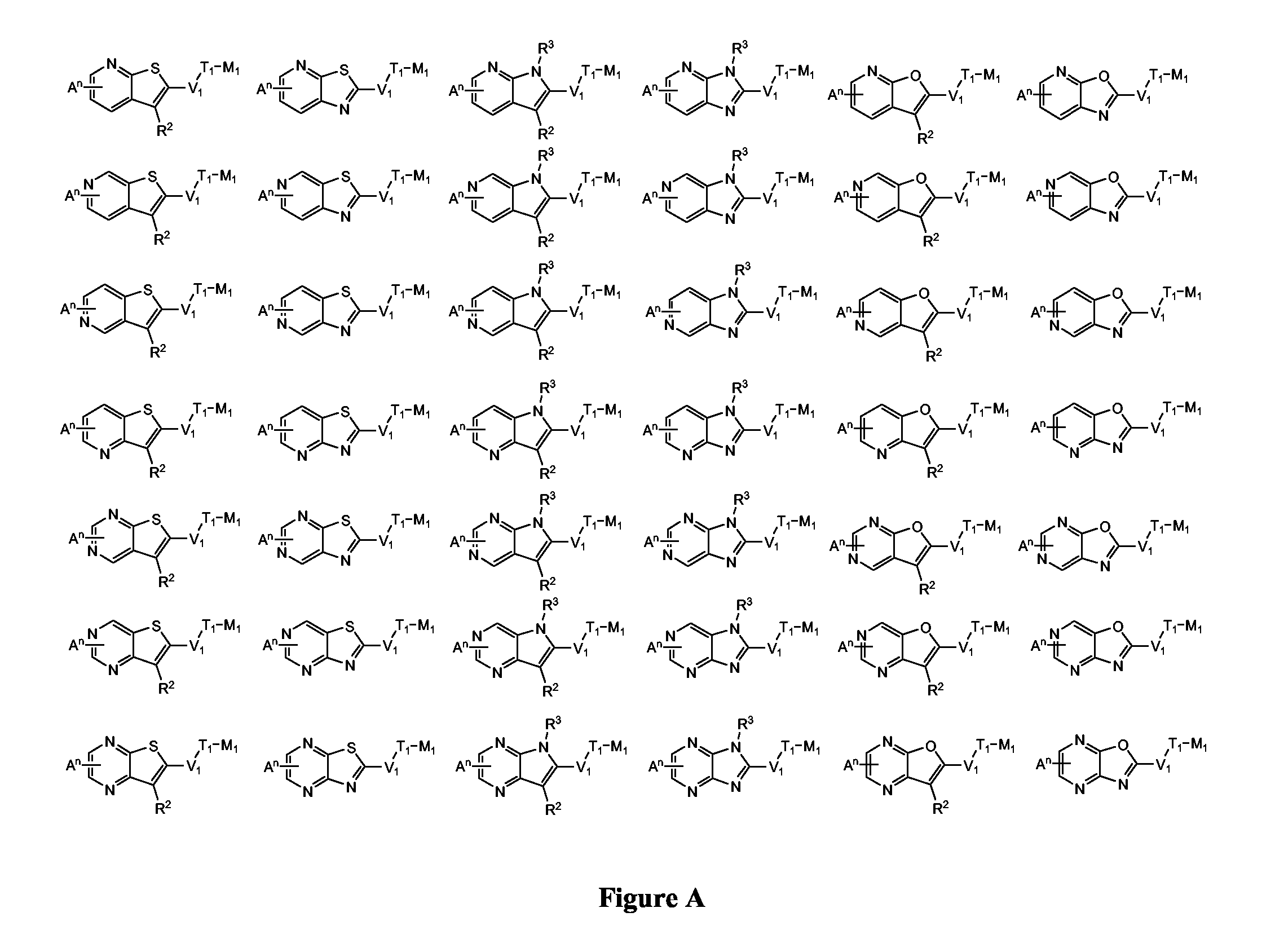
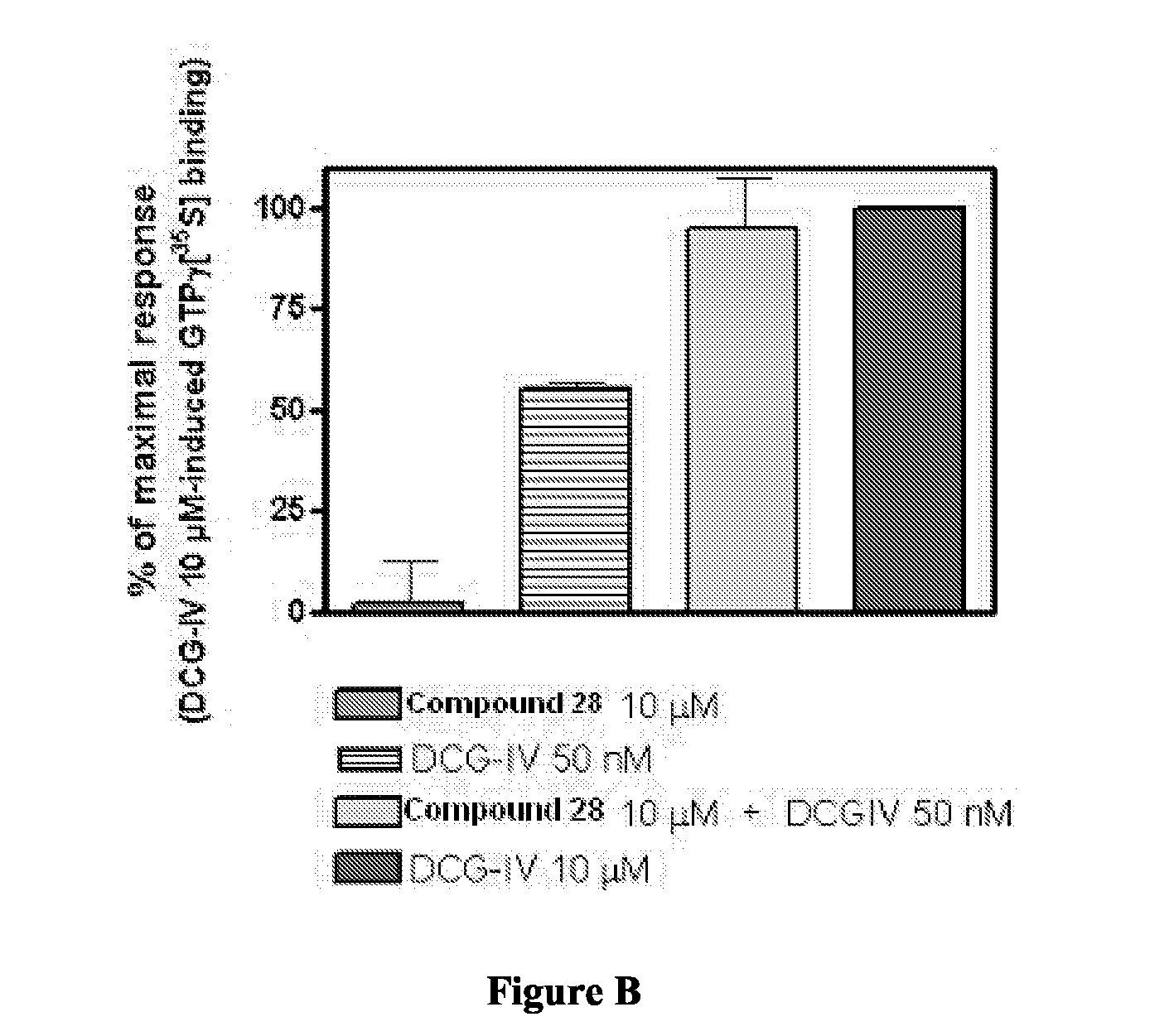
Novel Thieno-Pyridine and Thieno-Pyrimidine Derivatives and Their Use as Positive Allosteric Modulators of Mglur2-Receptors
The present invention relates to novel compounds, in particular novel thieno-pyridine and thieno-pyrimidine derivatives according to Formula (I), wherein all radicals are defined in the application. The compounds according to the invention are positive allosteric modulators of metabotropic receptors-subtype 2 ("mGluR2") which are useful for the treatment or prevention of neurological and psychiatric disorders associated with glutamate dysfunction and diseases in which the mGluR2 subtype of metabotropic receptors is involved. In particular, such diseases are central nervous system disorders selected from the group of anxiety, schizophrenia, migraine, depression, and epilepsy. The invention is also directed to pharmaceutical compositions and processes to prepare such compounds and compositions, as well as to the use of such compounds for the prevention and treatment of such diseases in which mGluR2 is involved.
Owner:ORTHO MCNEIL JANSSEN PHARMA
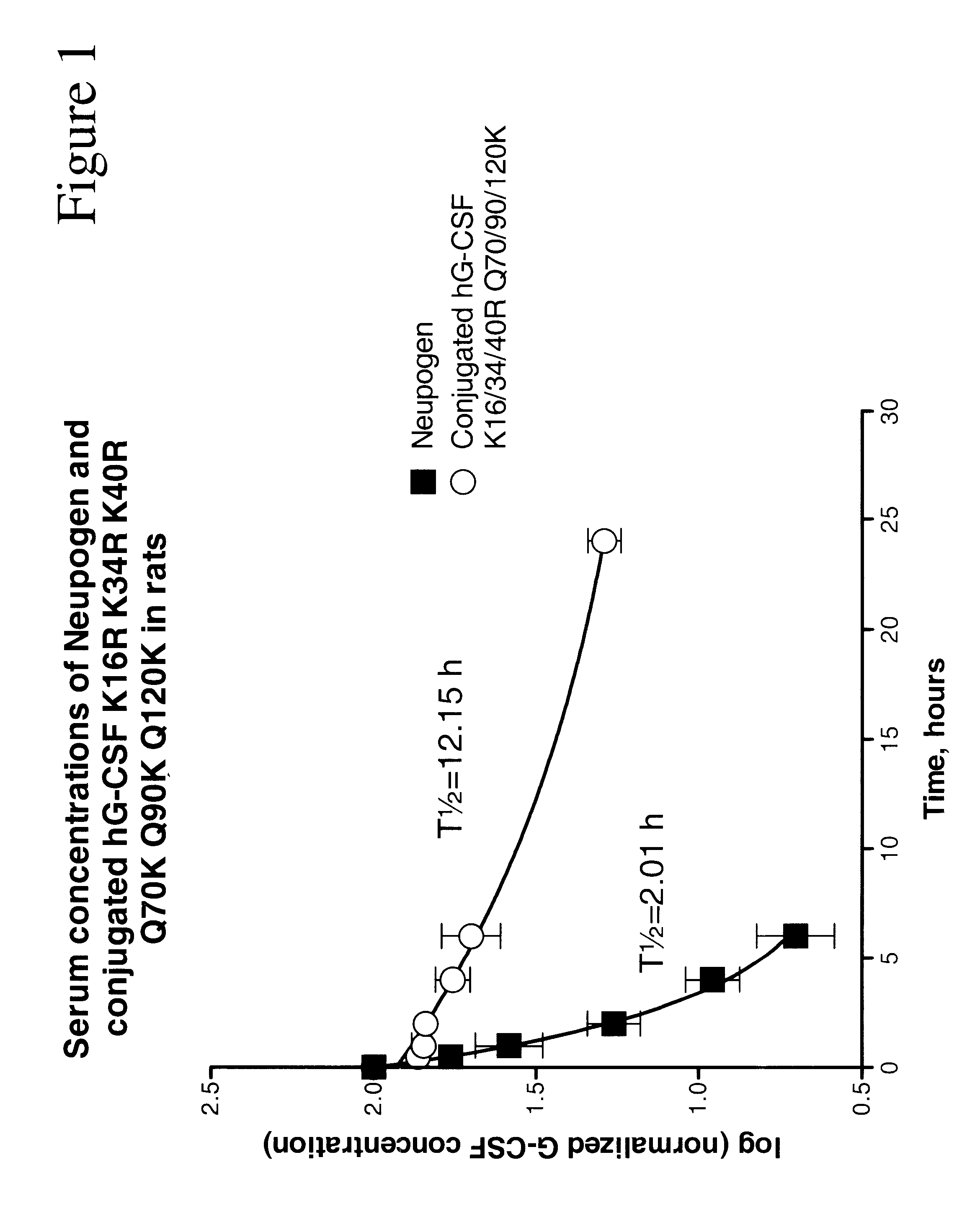
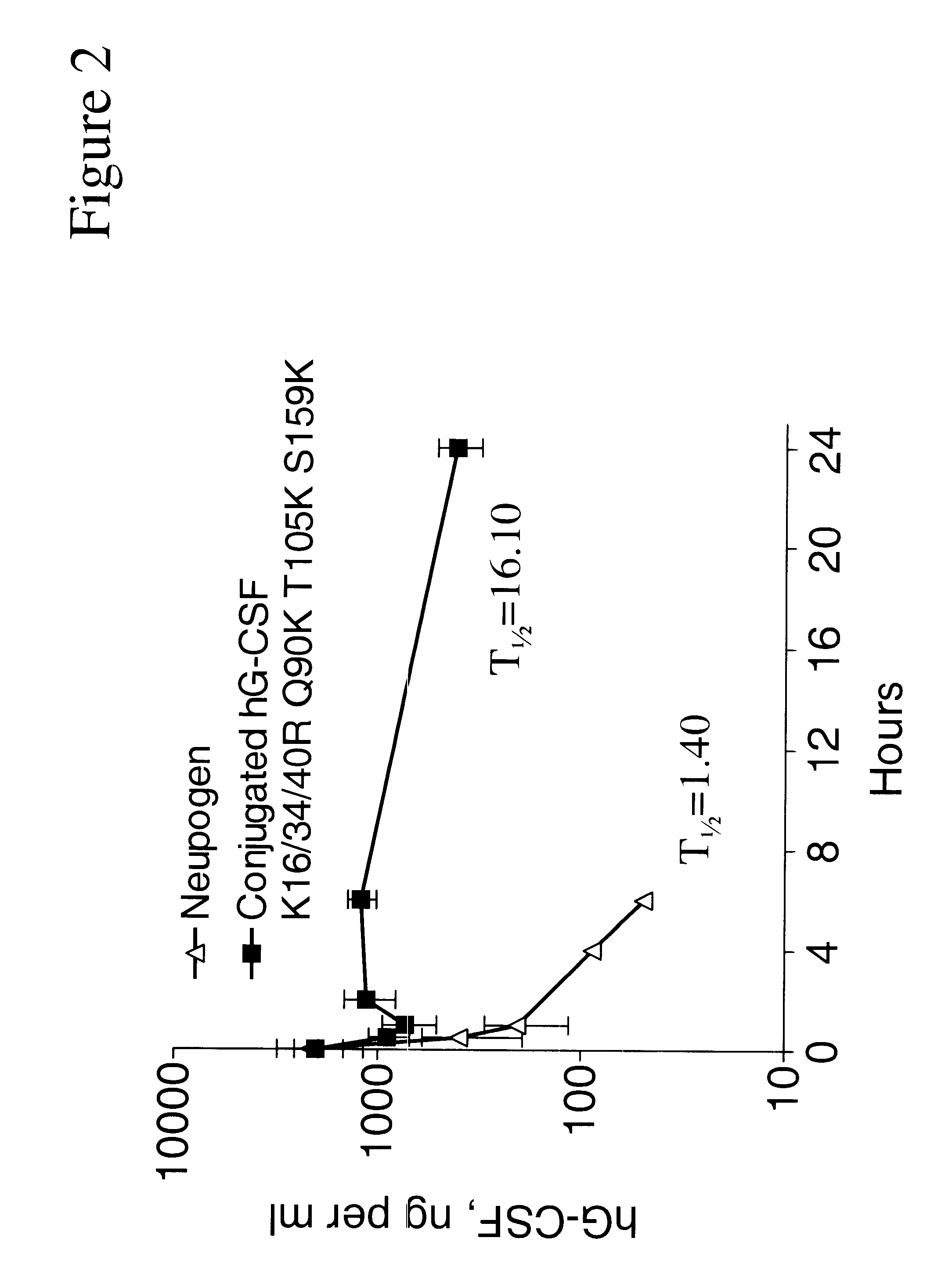
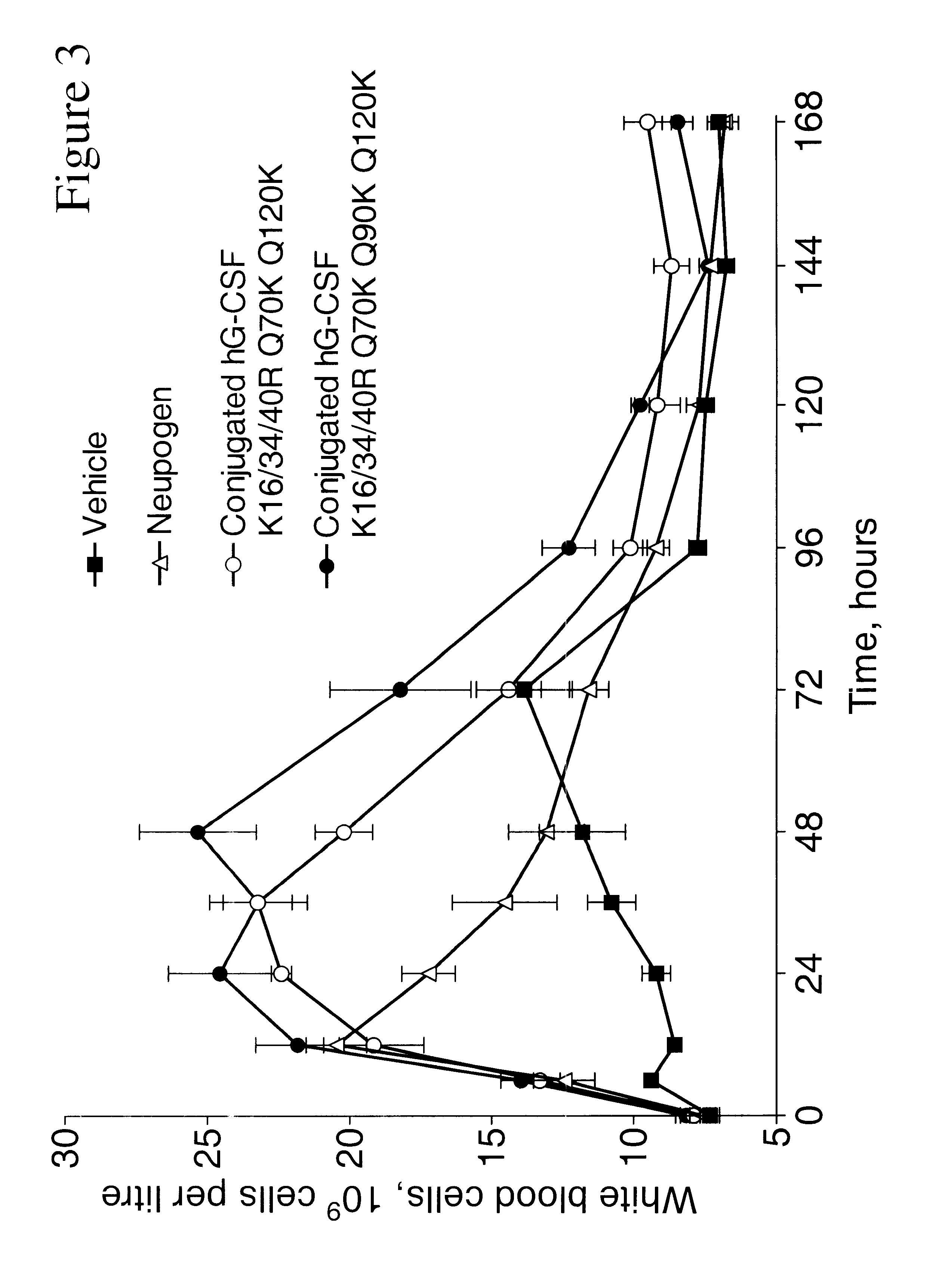
G-CSF conjugates
InactiveUS6555660B2Improved propertyReduced in vitroBiocidePeptide/protein ingredientsHalf-lifePolyethylene glycol
The invention relates to polypeptide conjugates comprising a polypeptide exhibiting G-CSF activity and having an amino acid sequence that differs from the amino acid sequence of human G-CSF in at least one specified introduced and / or removed amino acid residue comprising an attachment group for a non-polypeptide moiety, and having at least one non-polypeptide moiety attached to an attachment group of the polypeptide. The attachment group may e.g. be a lysine, cysteine, aspartic acid or glutamic acid residue or a glycosylation site, and the non-polypeptide moiety may e.g. be a polymer such as polyethylene glycol or an oligosaccharide. The conjugate, which has a reduced in vitro bioactivity compared to hG-CSF, has one or more improved properties such as increased biological half-life and increased stimulation of neutrophils.
Owner:MAXYGEN
Method of coating an intravascular stent with an endothelial cell adhesive five amino acid peptide
InactiveUS6140127APromoting cell attachmentRestore patencyBiocidePeptide/protein ingredientsCell specificArginine
Endothelial cell attachment to an intravascular stent is promoted by coating the stent with an endothelial cell specific adhesion peptide. Coating is preferably carried out by activating the intravascular stent using plasma glow discharge, applying on the stent a layer or plurality of layers of a polymer such as poly(2-hydroxyethylmethacrylate), applying a tresylation solution containing pyridine and tresyl chloride, and applying a five amino acid peptide having the sequence glycine-arginine-glutamic acid-aspartic acid-valine.
Owner:CORDIS CORP
Thermal treatment process for tobacco materials
ActiveUS20100300463A1Alter natureAlter characterTobacco preparationTobacco treatmentArgininePhenylalanine
A method of thermally processing a tobacco material is provided, the method including the steps of (i) mixing a tobacco material, water, and an additive selected from the group consisting of lysine, glycine, histidine, alanine, methionine, glutamic acid, aspartic acid, proline, phenylalanine, valine, arginine, di- and trivalent cations, asparaginase, saccharides, phenolic compounds, reducing agents, compounds having a free thiol group, oxidizing agents, oxidation catalysts, plant extracts, and combinations thereof, to form a moist tobacco mixture; (ii) heating the moist tobacco mixture at a temperature of at least about 60° C. to form a heat-treated tobacco mixture; and (iii) incorporating the heat-treated tobacco mixture into a tobacco product. Heat-treated tobacco composition prepared according to the method are also provided, such as heat-treated smokeless tobacco composition comprising a tobacco material, water, flavorant, binder, and filler, the heat-treated smokeless tobacco composition having an acrylamide content of less than about 2000 ppb.
Owner:R J REYNOLDS TOBACCO COMPANY
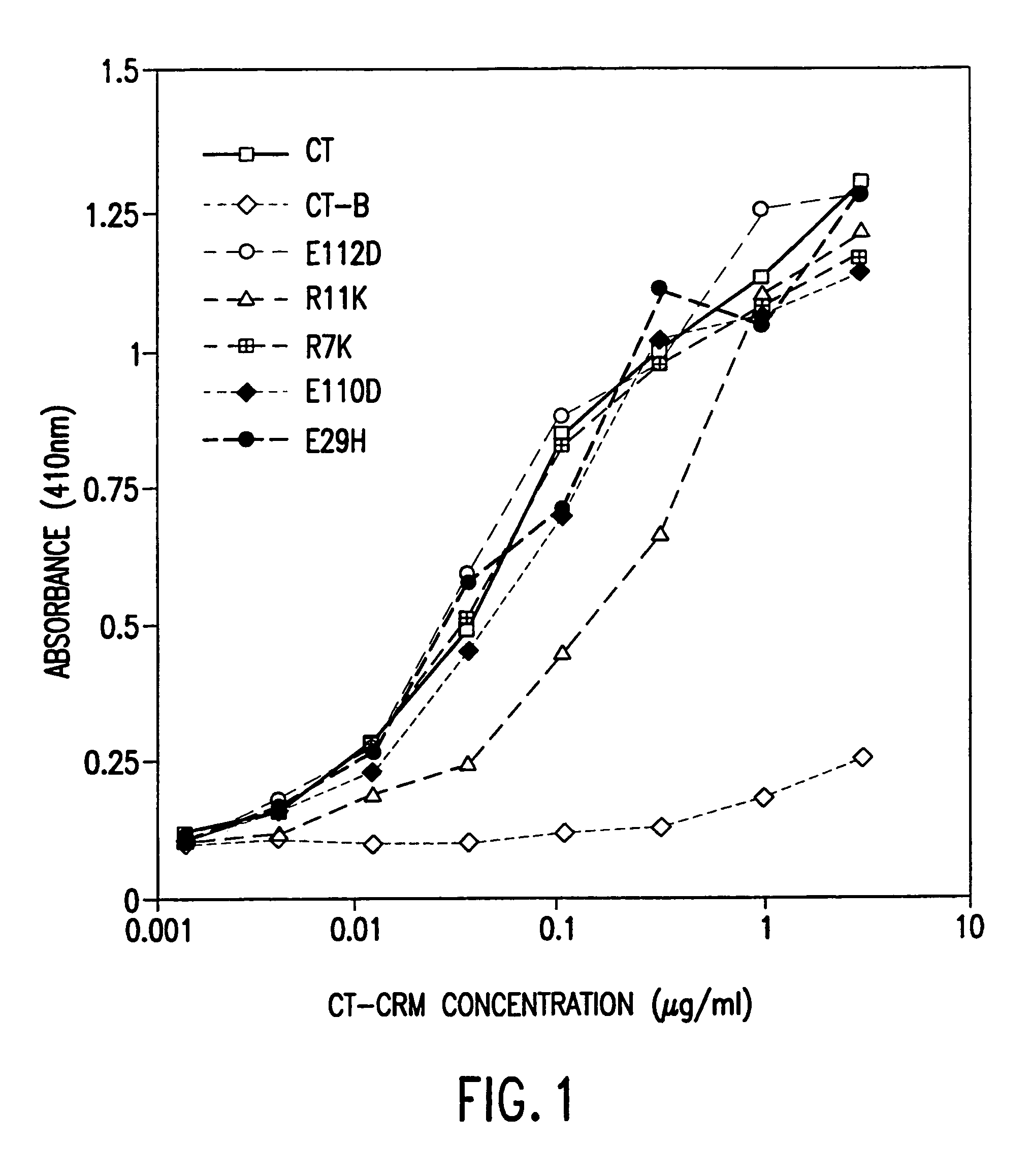
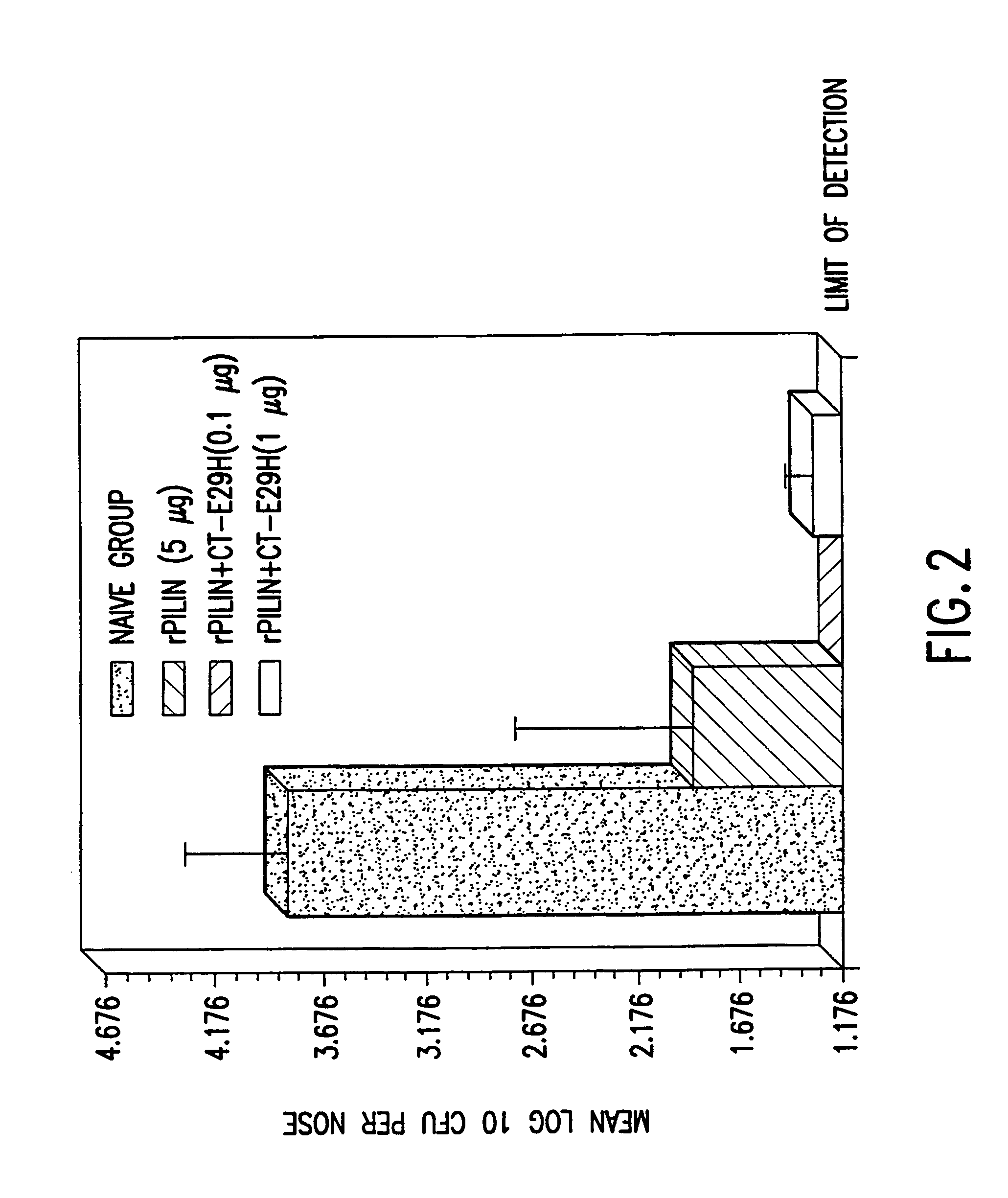
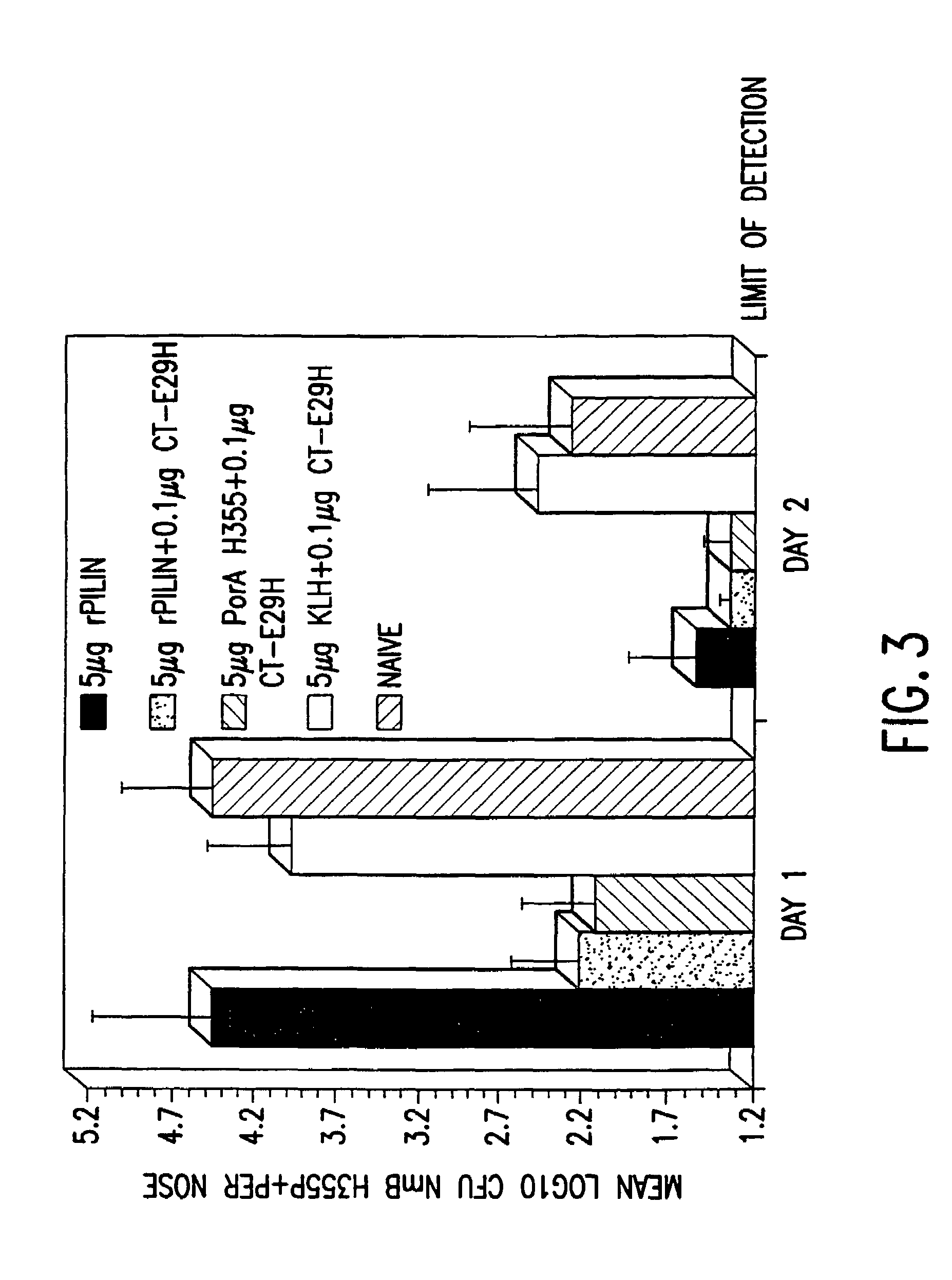
Mutant cholera holotoxin as an adjuvant
InactiveUS7384640B1Low toxicityEnhance immune responseSsRNA viruses negative-senseBacteriaAntigenAdjuvant
A mutant cholera holotoxin featuring a point mutation at amino acid 29 of the A subunit, wherein the glutamic acid residue is replaced by an amino acid other than aspartic acid, is useful as an adjuvant in an antigenic composition to enhance the immune response in a vertebrate host to a selected antigen from a pathogenic bacterium, virus, fungus or parasite. In a particular embodiment, the amino acid 29 is histidine. The mutant cholera holotoxin may contain at least one additional mutation in the A subunit at a position other than amino acid 29. The antigenic composition may include a second adjuvant in addition to the mutant cholera holotoxin.
Owner:UNIFORMED SERVICES UNIV OF HEALTH SCI UNITED STATES OF AMERICA AS REPRESENTED BY THE +1
Method for producing L-amino acids using bacteria of the Enterobacteriaceae family
ActiveUS20060088919A1Improve productivityD-xylose permease is enhancedBacteriaDepsipeptidesL-threonineArginine
There is disclosed a method for producing L-amino acid, for example L-threonine, L-lysine, L-histidine, L-phenylalanine, L-arginine or L-glutamic acid, using a bacterium of the Enterobacteriaceae family, wherein the bacterium has been modified to enhance an activity of D-xylose permease.
Owner:AJINOMOTO CO INC
Use of phosphoketolase for producing useful metabolites
Owner:AJINOMOTO CO INC
Thermal treatment process for tobacco materials
ActiveUS8434496B2Alter natureAlter characterTobacco preparationTobacco treatmentArgininePhenylalanine
A method of thermally processing a tobacco material is provided, the method including the steps of (i) mixing a tobacco material, water, and an additive selected from the group consisting of lysine, glycine, histidine, alanine, methionine, glutamic acid, aspartic acid, proline, phenylalanine, valine, arginine, di- and trivalent cations, asparaginase, saccharides, phenolic compounds, reducing agents, compounds having a free thiol group, oxidizing agents, oxidation catalysts, plant extracts, and combinations thereof, to form a moist tobacco mixture; (ii) heating the moist tobacco mixture at a temperature of at least about 60° C. to form a heat-treated tobacco mixture; and (iii) incorporating the heat-treated tobacco mixture into a tobacco product. Heat-treated tobacco composition prepared according to the method are also provided, such as heat-treated smokeless tobacco composition comprising a tobacco material, water, flavorant, binder, and filler, the heat-treated smokeless tobacco composition having an acrylamide content of less than about 2000 ppb.
Owner:R J REYNOLDS TOBACCO COMPANY
Nanoparticles for protein drug delivery
InactiveUS20090004266A1Easy to transportReduce resistancePowder deliverySaccharide peptide ingredientsActive agentNanoparticle
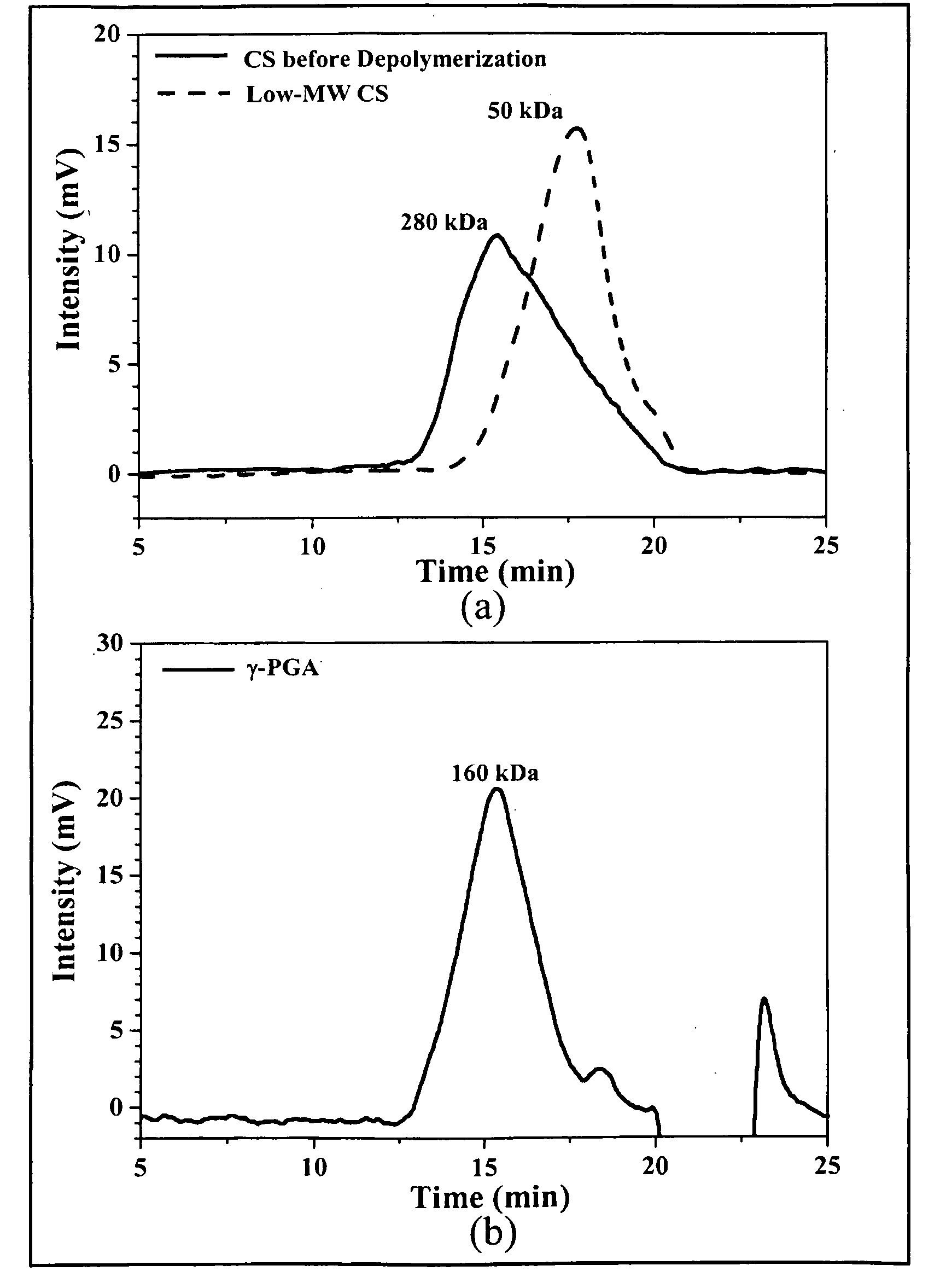
The invention discloses a chewable composition for oral delivery of bioactive agents having nanoparticles that are composed of chitosan, poly-glutamic acid, and at least one protein drug or bioactive agent characterized with a positive surface charge and their enhanced permeability for paracellular protein drug and bioactive agent delivery.
Owner:NANOMEGA MEDICAL CORP
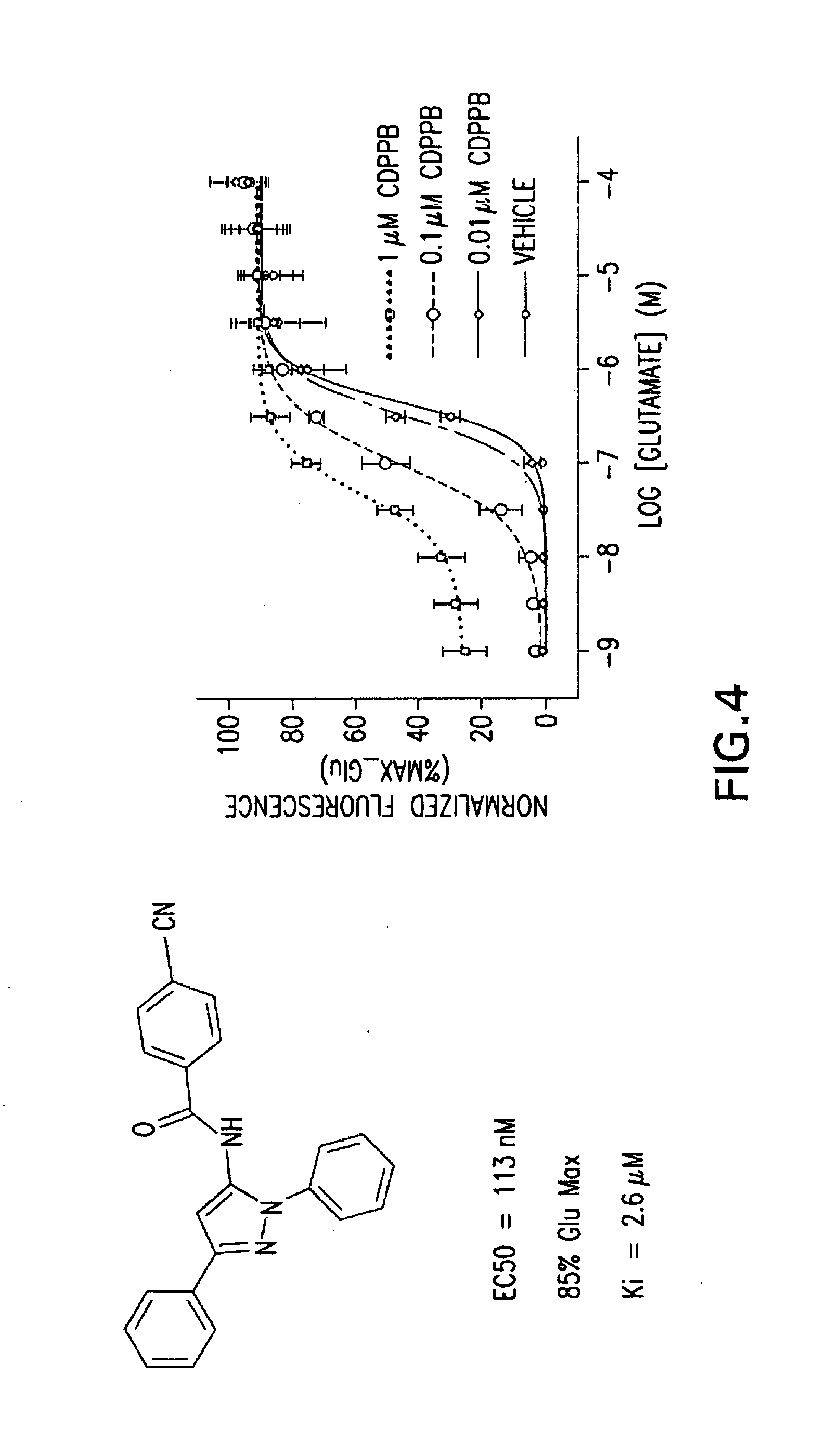
BENZAMIDE mGluR5 POSITIVE ALLOSTERIC MODULATORS AND METHODS OF MAKING AND USING SAME
InactiveUS20090042855A1Increase awarenessPotentiate metabotropic glutamate receptor activityBiocideNervous disorderAllosteric modulatorMetabotropic glutamate receptor
In one aspect, the invention relates to compounds, including phenylethynylbenzamide derivatives, cycloalkylethynylbenzamide derivatives, styrylbenzamide derivatives, 4-(3-phenyl-1,2,4-oxadiazol-5-yl)benzamide derivatives, 4-(pyridinylethynyl)benzamide derivatives, and N1-phenylterephthalamide derivatives, which are useful as positive allosteric modulators of the metabotropic glutamate receptor subtype 5 (mGluR5); synthetic methods for making the compounds; pharmaceutical compositions comprising the compounds; and methods of treating neurological and psychiatric disorders associated with glutamate dysfunction using the compounds and compositions. This abstract is intended as a scanning tool for purposes of searching in the particular art and is not intended to be limiting of the present invention.
Owner:VANDERBILT UNIV
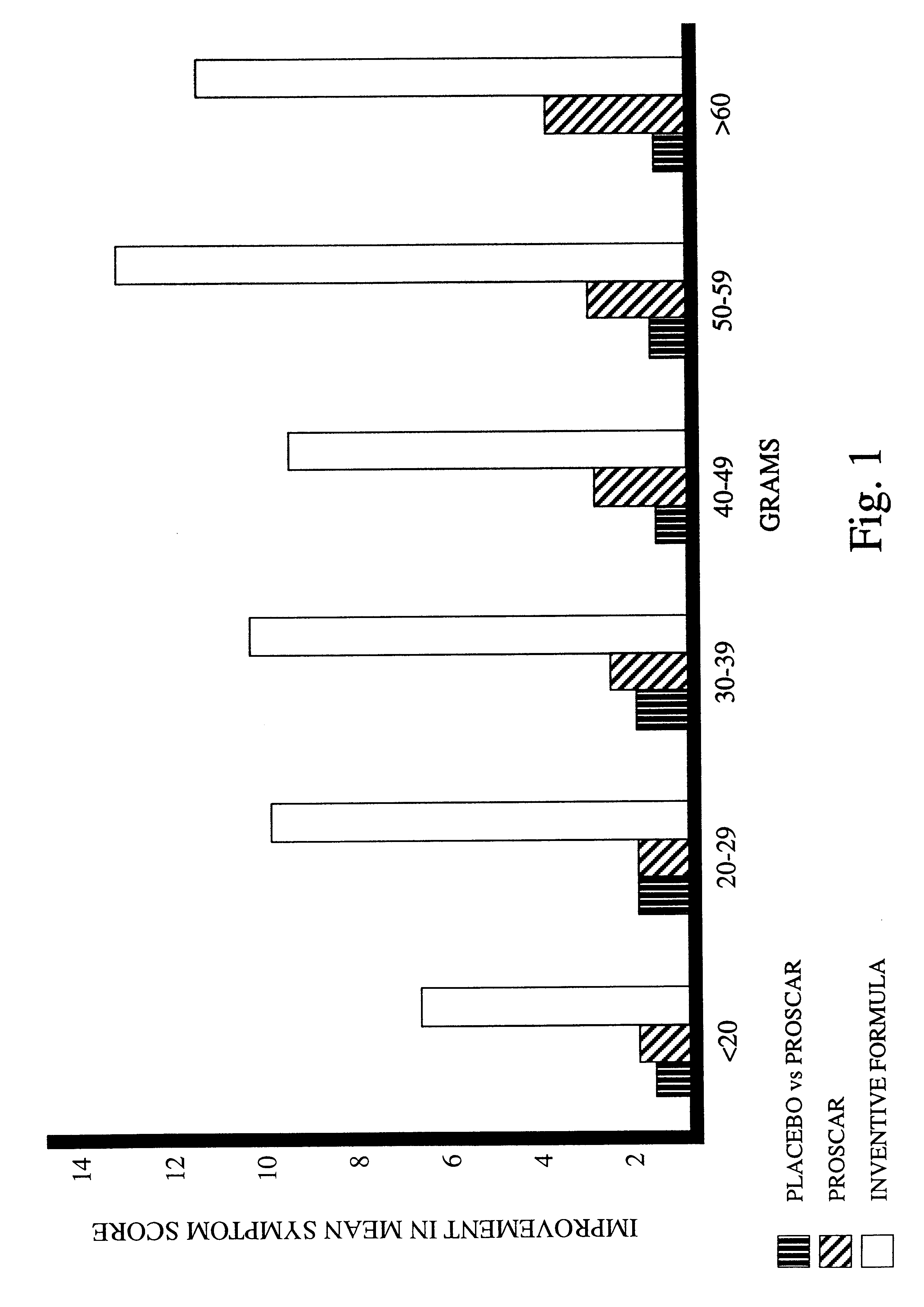
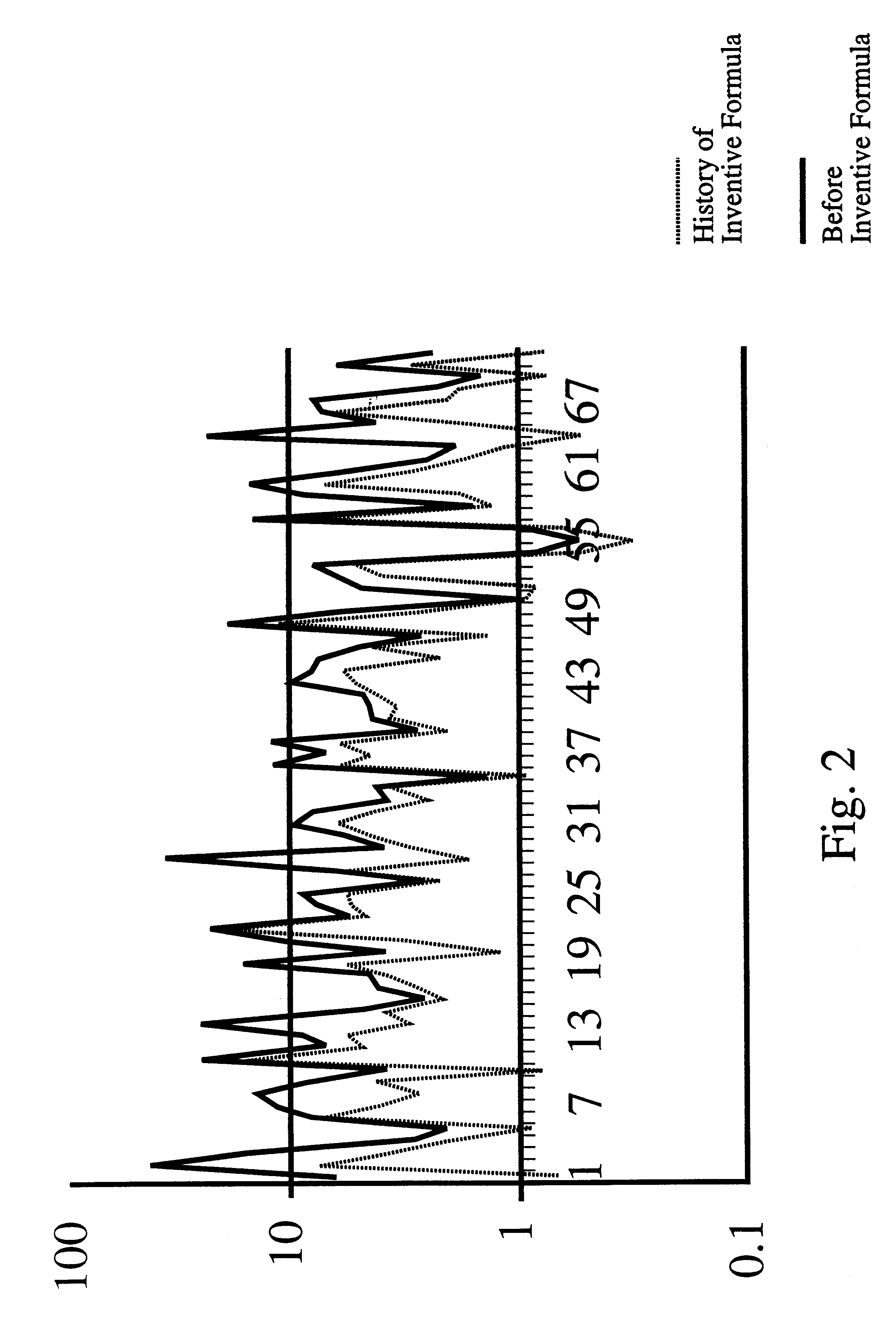
Prostate formula
InactiveUS6197309B1Good effectAlleviates and eliminates symptomHeavy metal active ingredientsOrganic active ingredientsDiseaseVitamin C
A composition providing an all-natural, non-surgical preventative of or improvement to disorders of the prostate gland is described. The invention relates to a composition for the prevention of or improvement of prostatitis, and for relieving symptoms and improving objective signs of prostatitis. The formula of the composition preferably includes the following ingredients each in a therapeutically effective amount: Vitamin C, Vitamin B6, Vitamin E, zinc, glycine, L-alanine, Glutamic acid, Saw palmetto, Pygeum extract, Pumpkin seed, Stinging nettle, Echinacea, garlic, Ginkgo leaves, and selenium.
Owner:WHEELER RONALD E
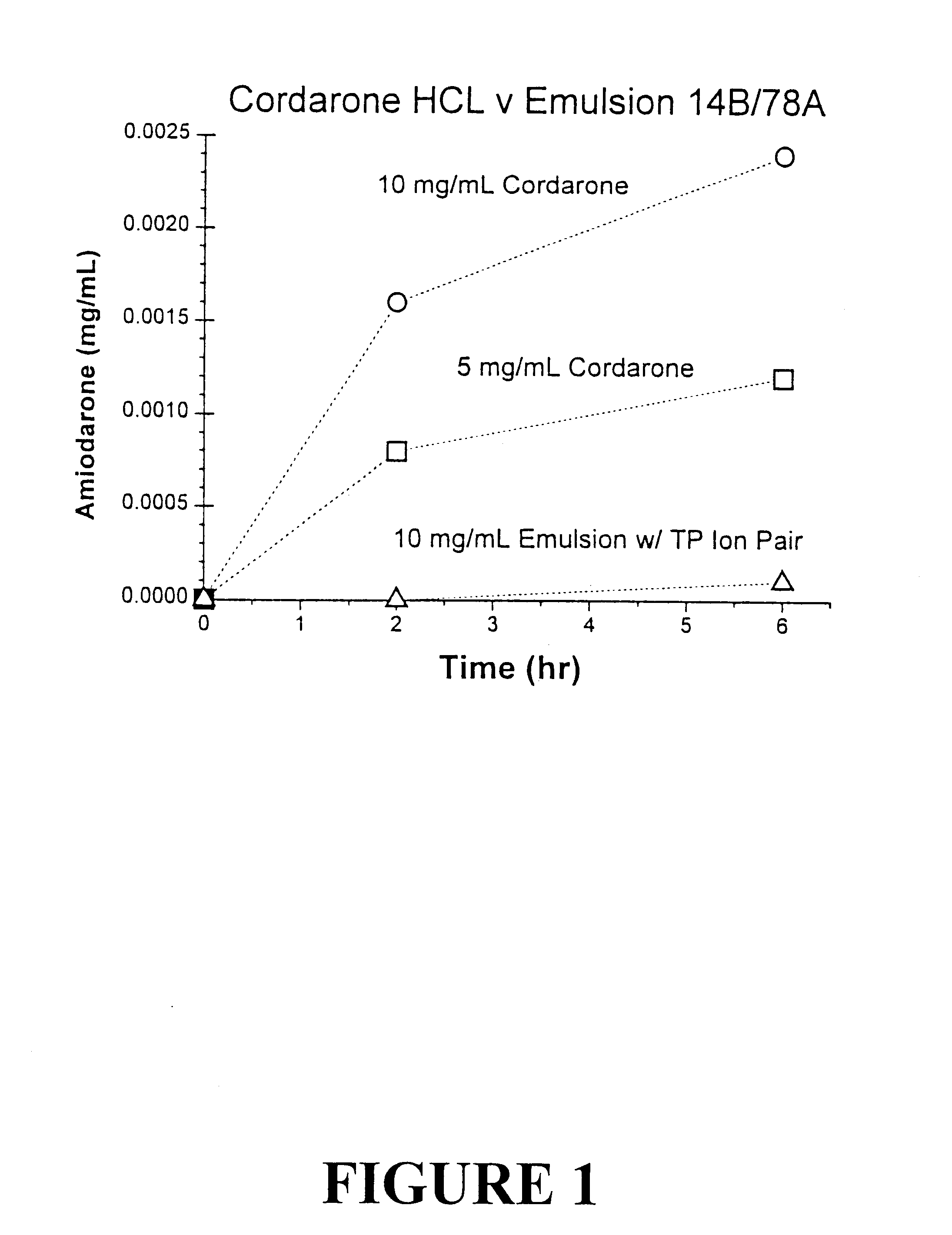
Compositions of tocol-soluble therapeutics
Tocol-based compositions of charged amphiphilic and water soluble pharmaceutically active compounds or their charged precursors are prepared by forming a tocol-soluble ion pair with an oppositely charged ion-pair forming compound capable of forming a tocol-soluble ion-pair with the active compound.Also disclosed are novel compounds tocopherolsuccinate-aspartate and tocopherolsuccinate-glutamate, which are useful as ion-pair forming compounds.
Owner:SONUS PHARM INC
Thermal treatment process for tobacco materials
ActiveUS8991403B2Alter natureAlter characterTobacco preparationTobacco treatmentArginineTobacco product
A method of thermally processing a tobacco material is provided, the method including the steps of (i) mixing a tobacco material, water, and an additive selected from the group consisting of lysine, glycine, histidine, alanine, methionine, glutamic acid, aspartic acid, proline, phenylalanine, valine, arginine, di- and trivalent cations, asparaginase, saccharides, phenolic compounds, reducing agents, compounds having a free thiol group, oxidizing agents, oxidation catalysts, plant extracts, and combinations thereof, to form a moist tobacco mixture; (ii) heating the moist tobacco mixture at a temperature of at least about 60° C. to form a heat-treated tobacco mixture; and (iii) incorporating the heat-treated tobacco mixture into a tobacco product. Heat-treated tobacco composition prepared according to the method are also provided, such as heat-treated smokeless tobacco composition comprising a tobacco material, water, flavorant, binder, and filler, the heat-treated smokeless tobacco composition having an acrylamide content of less than about 2000 ppb.
Owner:R J REYNOLDS TOBACCO COMPANY
Compositions and methods relating to reduction of symptoms of autism
InactiveUS6899876B2Relieve symptomsMany symptomPeptide/protein ingredientsOxidoreductasesNormorphineMedicine
Methods and compositions that can reduce the symptoms of autism in a human patient comprising administering a physiologically effective amount of one or both of a purified casomorphin inhibitor selected from the group consisting of a casomorphinase and a casomorphin ligand, and a physiologically effective amount of a purified gluteomorphin inhibitor selected from the group consisting of a gluteomorphinase and a gluteomorphin ligand, to a human patient in sufficient quantities to reduce the effects of the autism. In some embodiments, the compositions and methods further comprise a physiologically effective amount of an enkephalin inhibitor, preferably an enkephalinase, and a physiologically effective amount of an endorphin inhibitor, preferably an endorphinase.
Owner:PROTHERA
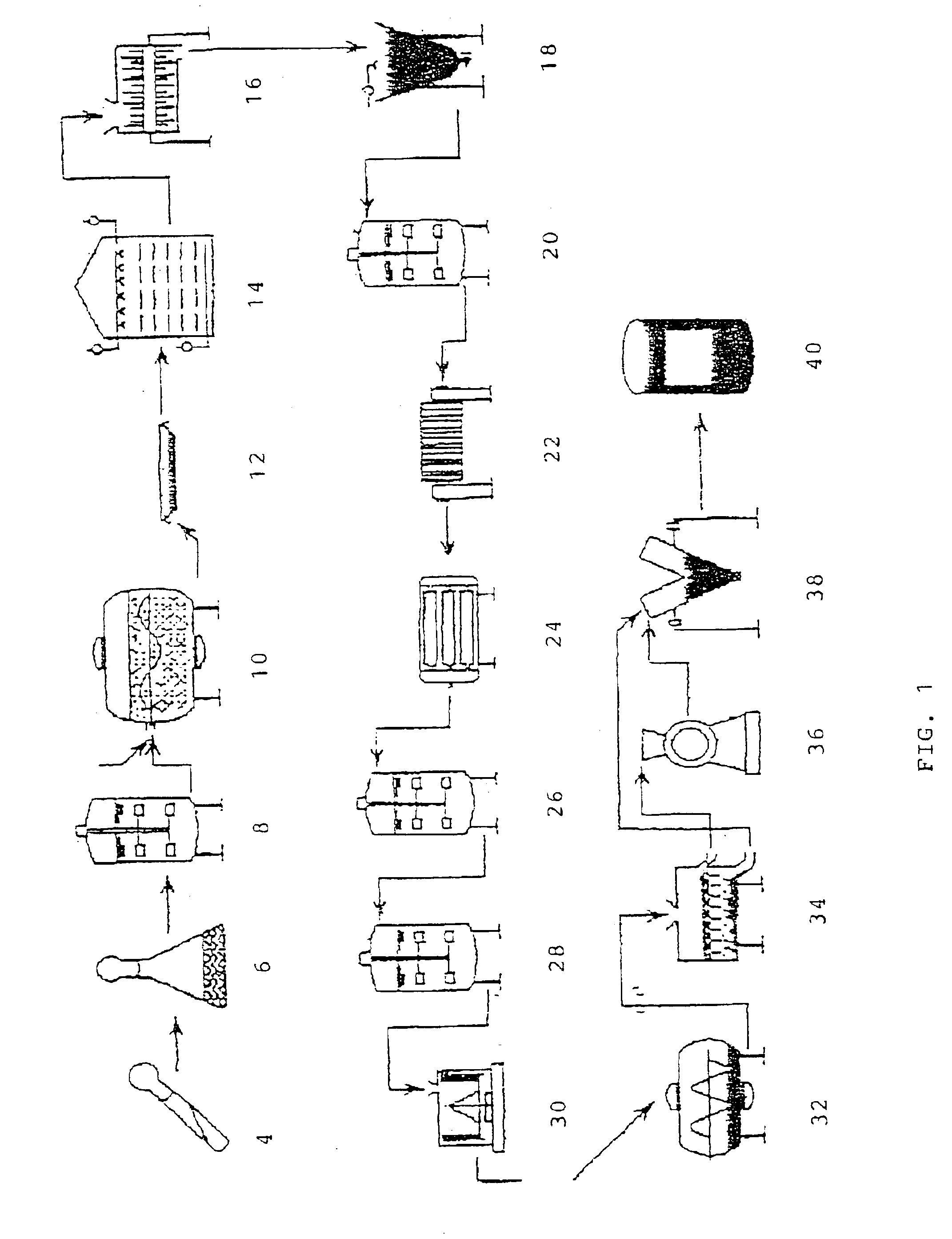
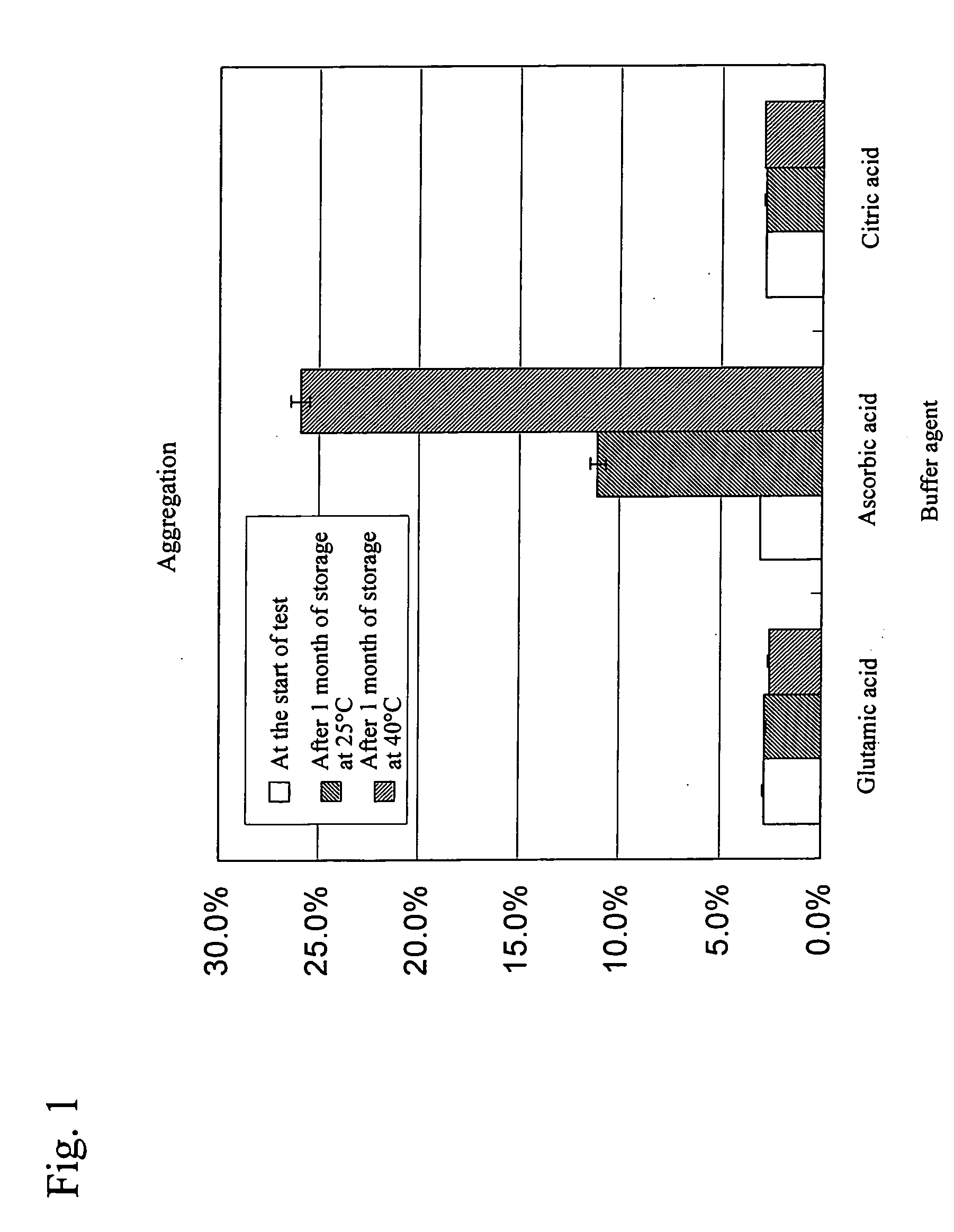
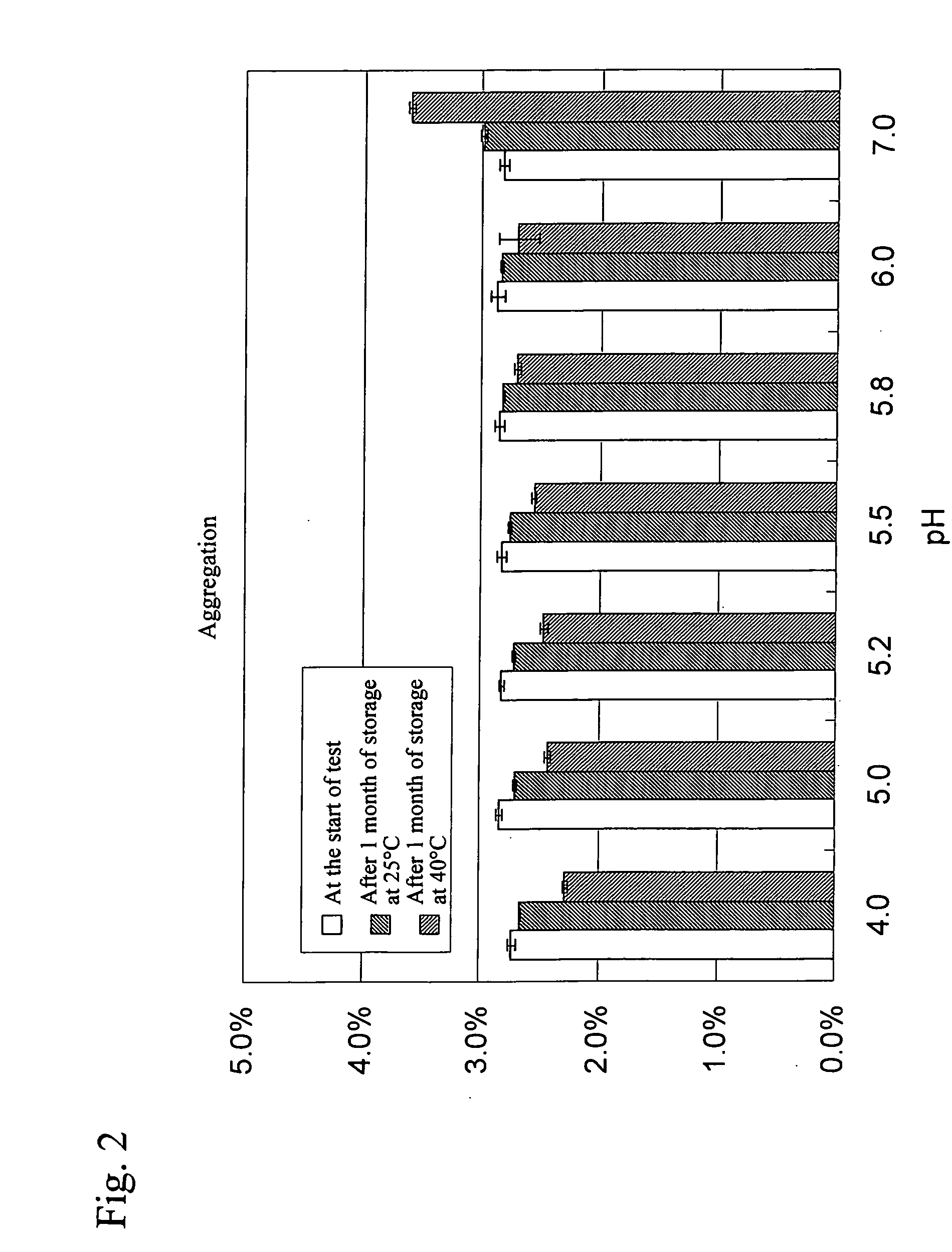
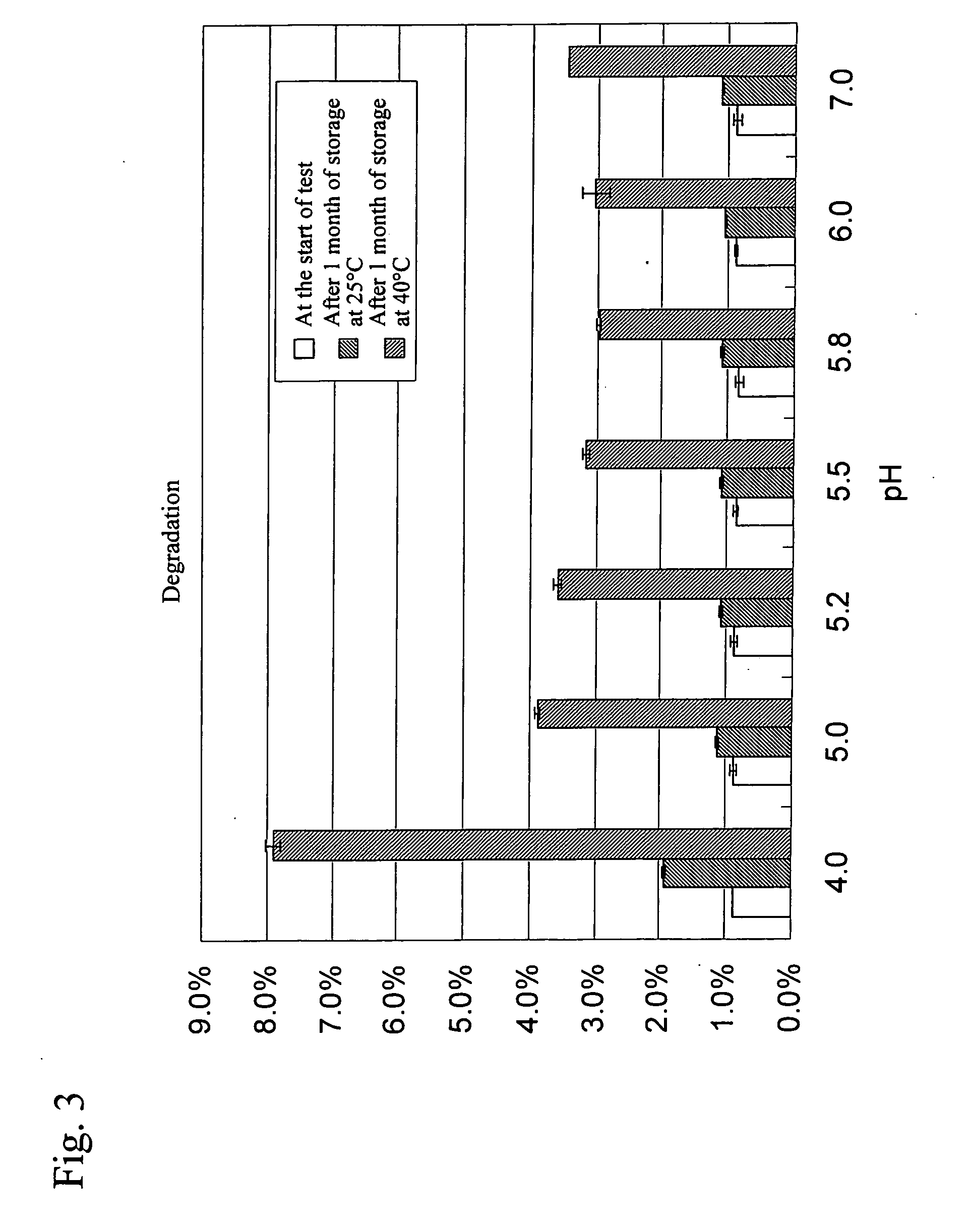
Stable water-based medicinal preparation containing antibody
InactiveUS20070184050A1Retain activityPharmaceutical delivery mechanismAntibody ingredientsWater basedPharmaceutical formulation
A stable liquid medical formulation containing an antibody is provided, which contains a therapeutically effective amount of an antibody in a glutamate buffer and / or a citrate buffer and has a pH between 4.0 and 6.0.
Owner:KYOWA HAKKO KIRIN CO LTD
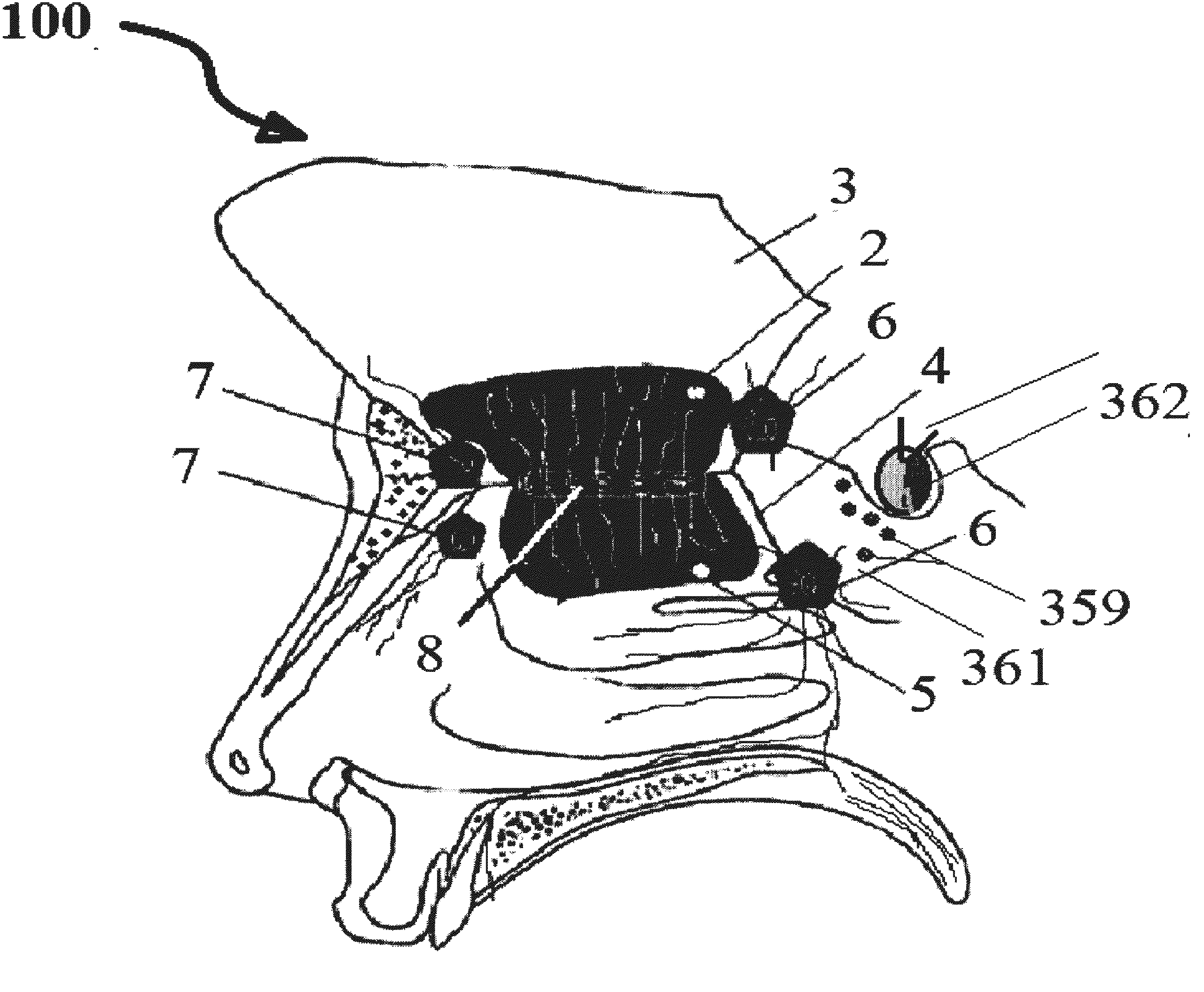
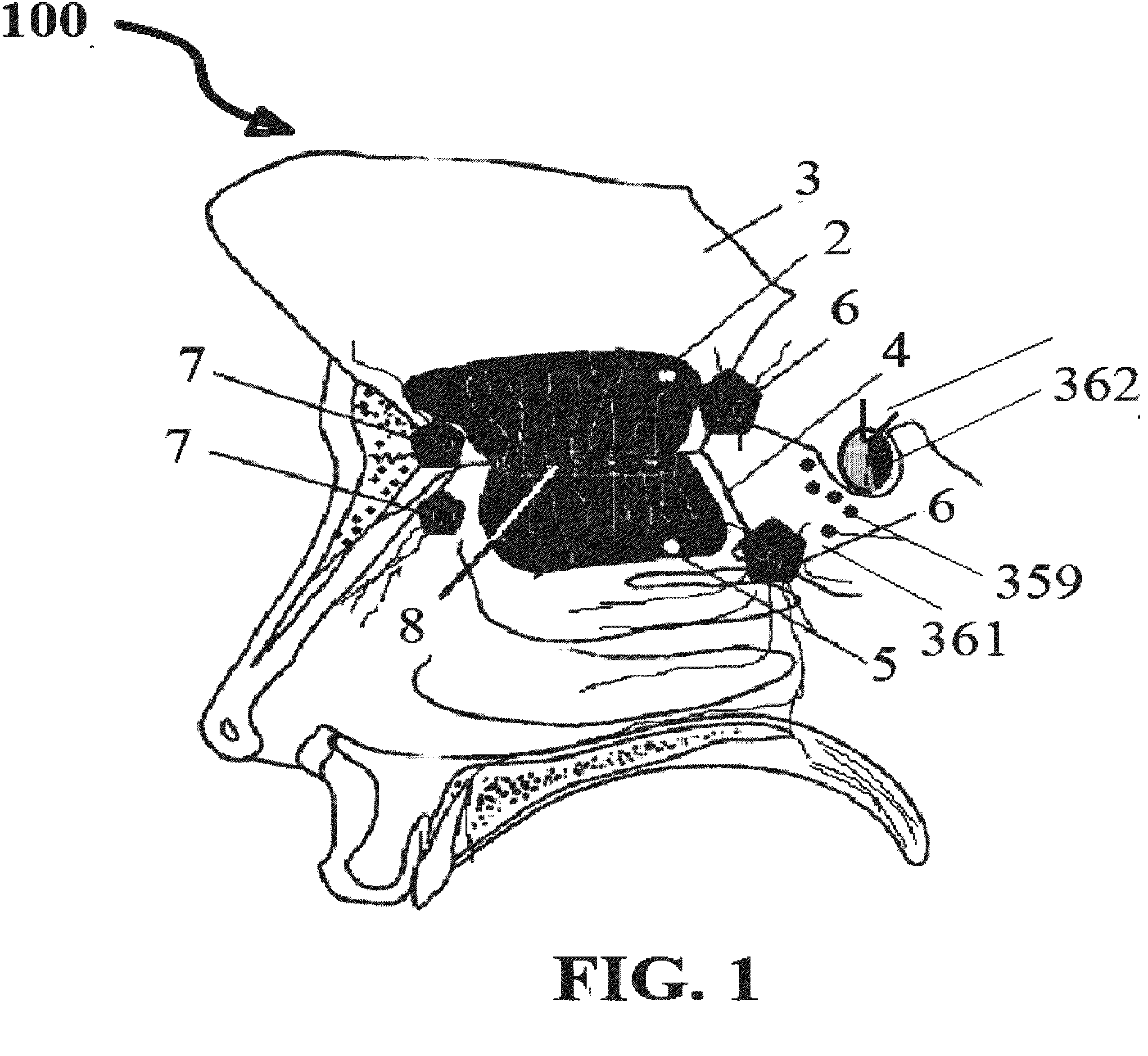
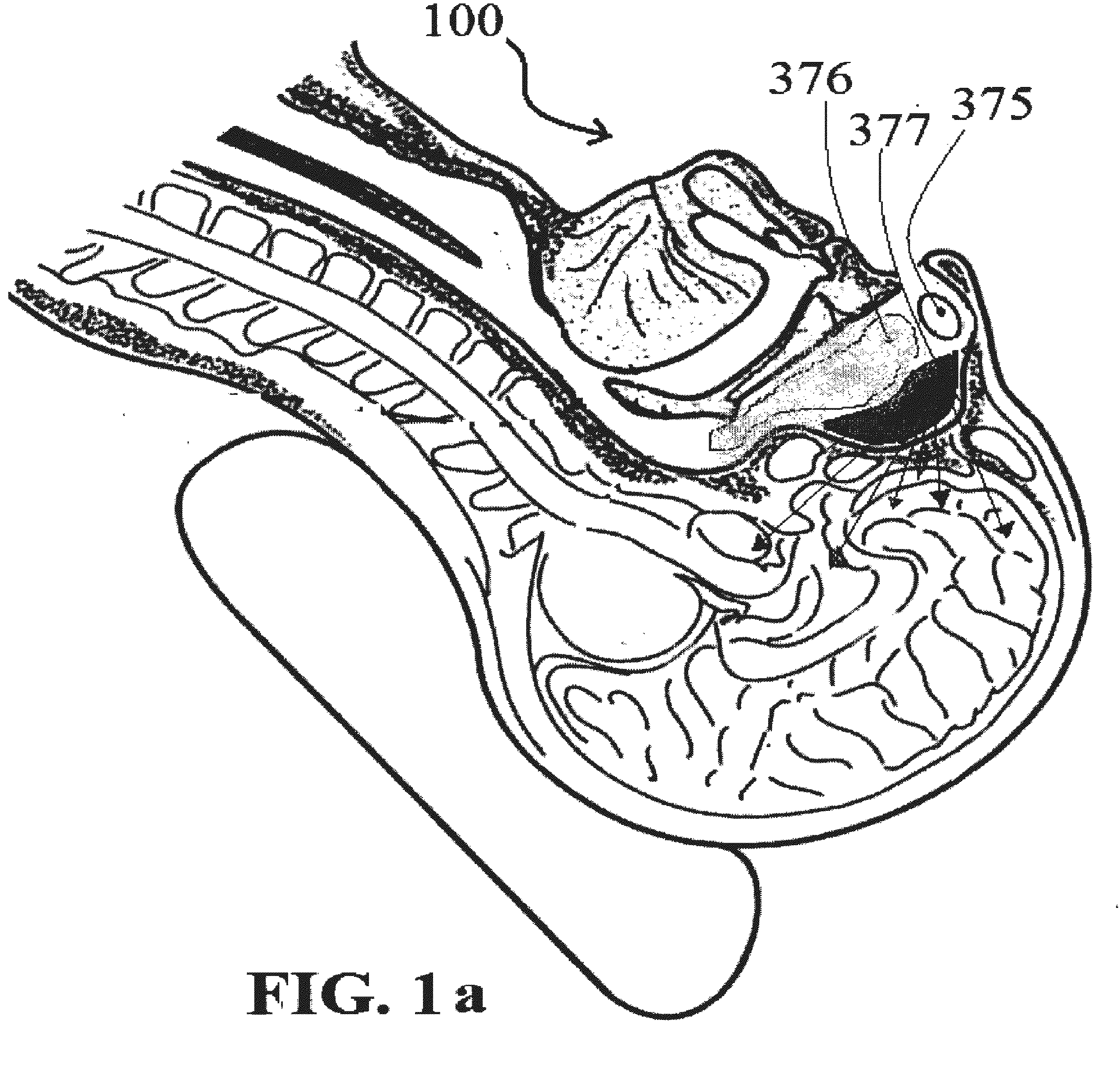
Alzheimer's disease treatment with multiple therapeutic agents delivered to the olfactory region through a special delivery catheter and iontophoresis
InactiveUS20120323214A1Reduce and preventAvoid destructionNervous disorderHead electrodesApoptosisExcitotoxicity
This invention describes the administration of multiple therapeutic agents with insulin in conjunction with bexarotene, ketamine, monoclonal antibodies Etanercept, IGF-1, and acetylcholine esterase inhibitors physostigmine, for treatment of Alzheimer's disease and other neurodegenerative diseases. Insulin, improves memory; also augments and amplifies the effects of the adjuvant therapeutic agents (paracrine and intracrine effects) and consequently reduces the β amyloid, its soluble precursors, prevents damage to the neuronal skeletal network (taupathy), and blocks glutamate excitotoxicity, reduces brain inflammation, prevents apoptosis, and increases the acetylcholine levels in the neurons and synapses; by using a combination of insulin, bexarotene, ketamine, Etanercept, IGF-1, and physostigmine therapeutic agents. The results are achieved by using the specially designed Iontophoresis incorporated olfactory mucosal delivery (ORE) catheter device located at the olfactory nerves, sphenoid sinus, and adjacent structures described here, to transport the large molecules of therapeutic agents to treat AD delivered to the CNS bypassing BBB from ORE.
Owner:WEDGE THERAPEUTICS
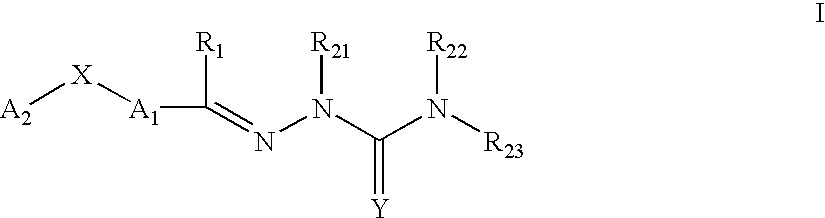
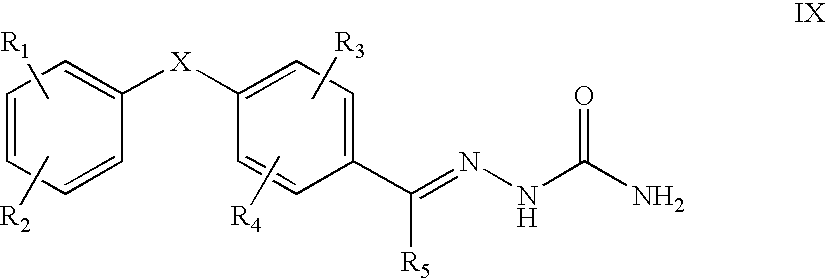
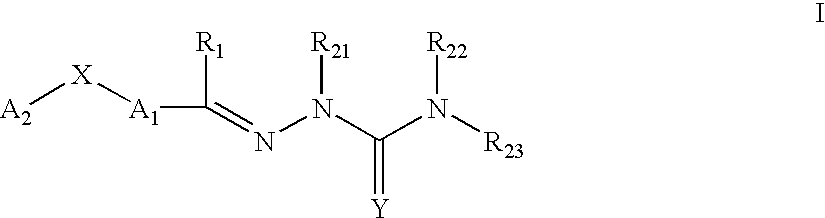
Carbocyclic and heterocyclic substituted semicarbazones and thiosemicarbazones and the use thereof
This invention is related to carbocyclic and heterocyclic substituted semicarbazones and thiosemicarbazones represented by Formula I: ##STR1## or a pharmaceutically acceptable salt or prodrug thereof, wherein: Y is oxygen or sulfur; R.sub.1, R.sub.21, R.sub.22 and R.sub.23 are independently hydrogen, alkyl, cycloalkyl, alkenyl, alkynyl, haloalkyl, aryl, aminoalkyl, hydroxyalkyl, alkoxyalkyl or carboxyalkyl; or R.sub.22 and R.sub.23, together with the N, form a heterocycle; A.sub.1 and A.sub.2 are independently aryl, heteroaryl, saturated or partially unsaturated carbocycle or saturated or partially unsaturated heterocycle, any of which is optionally substituted; X is one or O, S, NR.sub.24, CR.sub.25 R.sub.26, C(O), NR.sub.24 C(O), C(O)NR.sub.24, SO, SO.sub.2 or a covalent bond; where R.sub.24, R.sub.25 and R.sub.26 are independently hydrogen, alkyl, cycloalkyl, alkenyl, alkynyl, haloalkyl, aryl, aminoalkyl, hydroxyalkyl, alkoxyalkyl or carboxyalkyl. The invention also is directed to the use of carbocycle and heterocycle substituted semicarbazones and thiosemicarbazones for the treatment of neuronal damage following global and focal ischemia, for the treatment or prevention of neurodegenerative conditions such as amyotrophic lateral sclerosis (ALS), for the treatment and prevention of otoneurotoxicity and eye diseases involving glutamate toxicity and for the treatment, prevention or amelioration of pain, as anticonvulsants, and as antimanic depressants, as local anesthetics, as antiarrhythmics and for the treatment or prevention of diabetic neuropathy and urinary incontinence.
Owner:COCENSYS
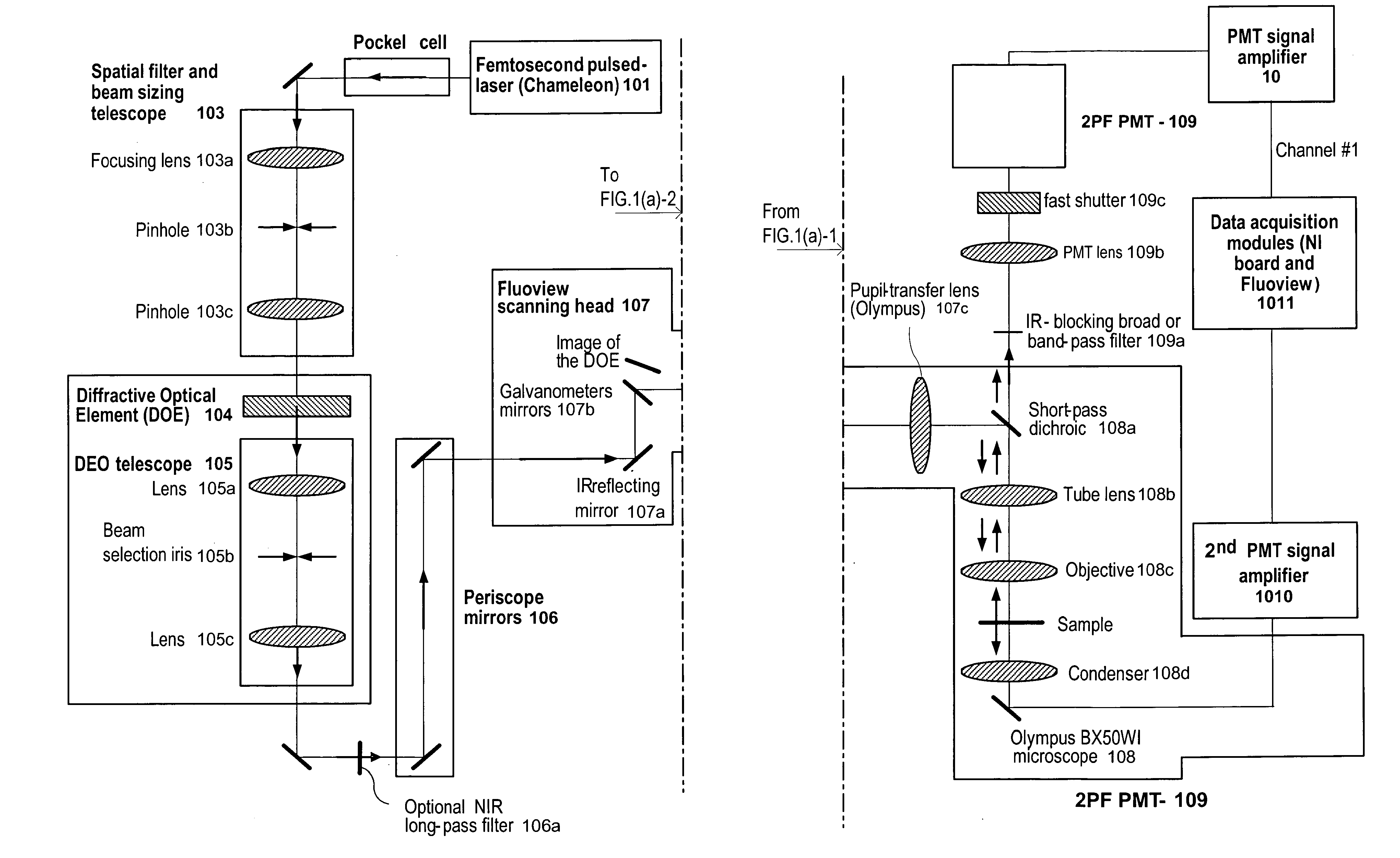
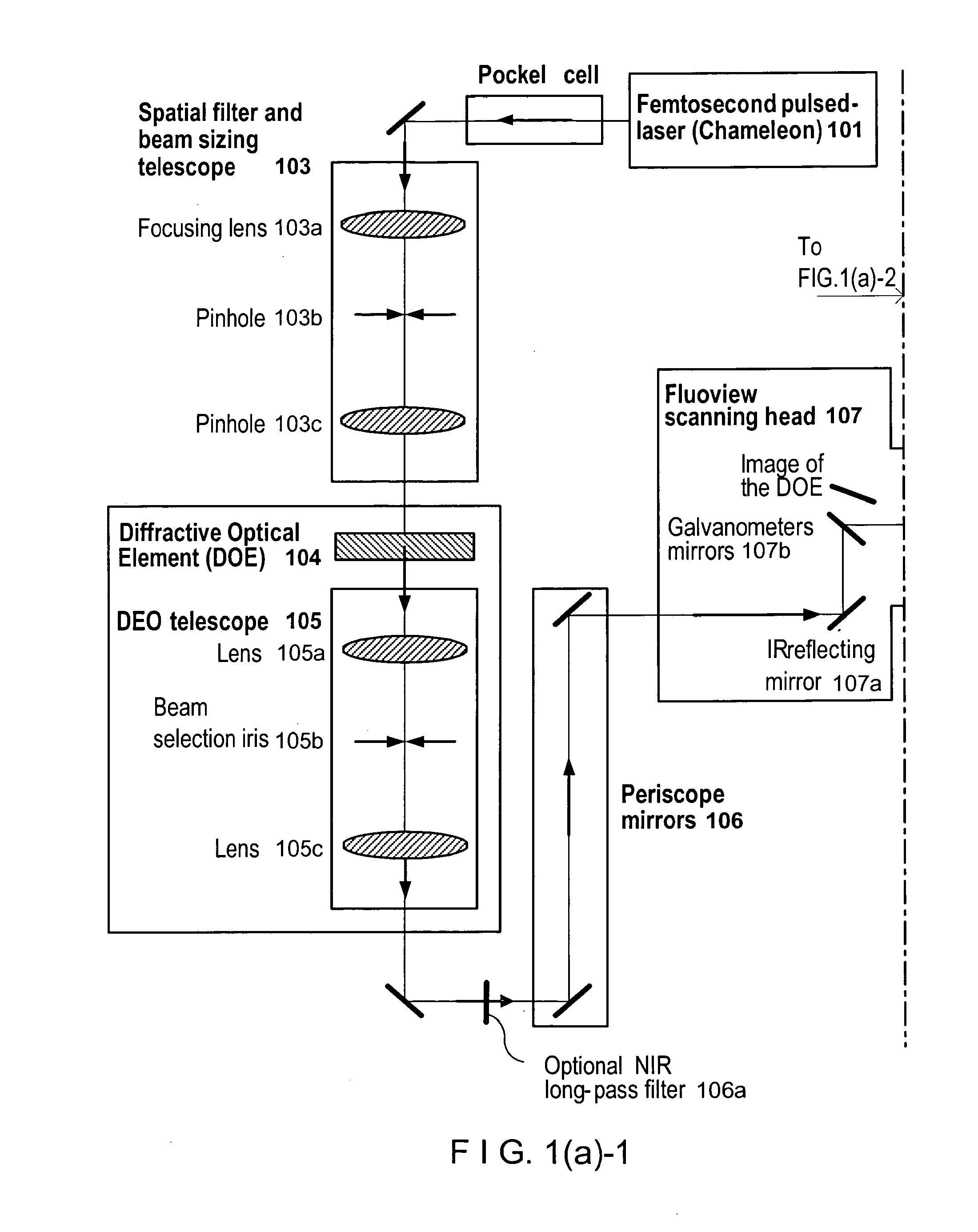
Devices, apparatus and method for providing photostimulation and imaging of structures
ActiveUS20110233046A1Reduce intensityEasy to detectMaterial analysis by optical meansHydrocarbonsNeuronSpatiotemporal pattern
According to exemplary embodiments of the present disclosure, it is possible to provide method, system, arrangement, computer-accessible medium and device to stimulate individual neurons in brain slices in any arbitrary spatio-temporal pattern, using two-photon uncaging of photo-sensitive compounds such as MNI-glutamate and / or RuBi-Glutamate with beam multiplexing. Such exemplary method and device can have single-cell and three-dimensional precision. For example, by sequentially stimulating up to a thousand potential presynaptic neurons, it is possible to generate detailed functional maps of inputs to a cell. In addition, it is possible to combine this exemplary approach with two-photon calcium imaging in an all-optical method to image and manipulate circuit activity. Further exemplary embodiments of the present disclosure can include a light-weight, compact portable device providing for uses in a wide variety of applications.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Thermal treatment process for tobacco materials
ActiveUS8944072B2Alter natureAlter characterTobacco preparationTobacco treatmentArgininePhenylalanine
A method of preparing a tobacco material for use in a smoking article is provided, including (i) mixing a tobacco material, water, and an additive selected from the group consisting of lysine, glycine, histidine, alanine, methionine, glutamic acid, aspartic acid, proline, phenylalanine, valine, arginine, di- and trivalent cations, asparaginase, saccharides, phenolic compounds, reducing agents, compounds having a free thiol group, oxidizing agents, oxidation catalysts, plant extracts, and combinations thereof; (ii) heating the mixture; and (iii) incorporating the heat-treated mixture into a smoking article as a smokable material. A smoking article in the form of a cigarette is also provided that includes a tobacco material pre-treated to inhibit reaction of asparagine to form acrylamide in mainstream smoke. Upon smoking, the smoking article is characterized by an acrylamide content of mainstream smoke that is reduced relative to an untreated control smoking article.
Owner:R J REYNOLDS TOBACCO COMPANY
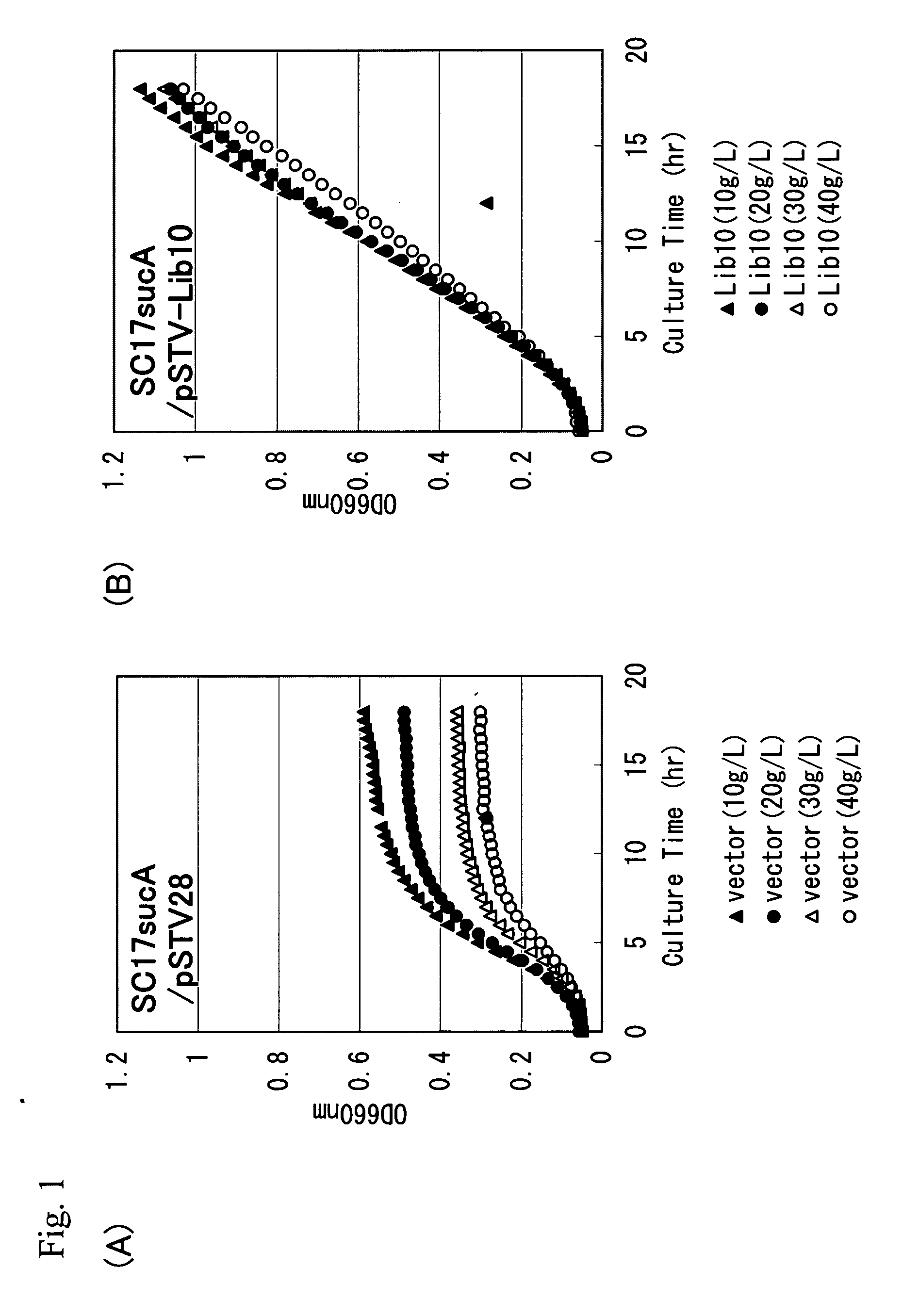
L-glutamic acid-producing microorganism and a method for producing l-glutamic acid
ActiveUS20060141588A1Improve L-glutamic acid-producing ability of coryneform bacteriumHigh expressionTelevision system detailsPulse modulation television signal transmissionMicroorganismMicrobiology
A coryneform bacterium that is modified by using a yggB gene so that L-glutamic acid-producing ability is enhanced as compared to a non-modified strains is cultured in a medium to cause accumulation of L-glutamic acid in the medium or bacterial cells, and L-glutamic acid is collected from the medium or cells.
Owner:AJINOMOTO CO INC
Nanoparticles for protein drug delivery
InactiveUS20120258176A1Enhancing intestinal paracellular transportReduce resistanceAntibacterial agentsPowder deliveryActive agentPharmaceutical Substances
The invention discloses particulate complexes composed of chitosan, poly-glutamic acid, and at least one bioactive agent, wherein equal moles of the positively charged chitosan and the negatively charged poly-glutamic acid substrate form an electrostatic network enabling improved loading the bioactive agent.
Owner:NANOMEGA MEDICAL CORP
Composition for an in vitro fertilization medium
InactiveUS6130086AImprove stabilityIncrease stimulationCulture processMedical devicesArginineTryptophan
PCT No. PCT / JP96 / 02503 Sec. 371 Date Mar. 2, 1998 Sec. 102(e) Date Mar. 2, 1998 PCT Filed Sep. 4, 1996 PCT Pub. No. WO97 / 08946 PCT Pub. Date Mar. 13, 1997The present invention aims to provide a medium composition for in vitro fertilization, in particular, a composition usable in the culture of ova or early embryos which are fertilized eggs, the preparation or culture of sperm, and the pre-treatment of ova or sperm. The composition comprises, as its essential components, L-phenylalanine, L-tryptophan, L-lysine, L-threonine, L-valine, L-methionine, L-isoleucine, L-leucine, L-proline, glycine, L-alanine, L-tyrosine, L-histidine, L-arginine, L-taurine, L-aspartic acid, L-serine, L-asparagine, L-glutamic acid, L-glutamine and L-cystine, provided that at least a part of the L-cystine may be replaced by L-cysteine.
Owner:FUSO PHARMA INDS
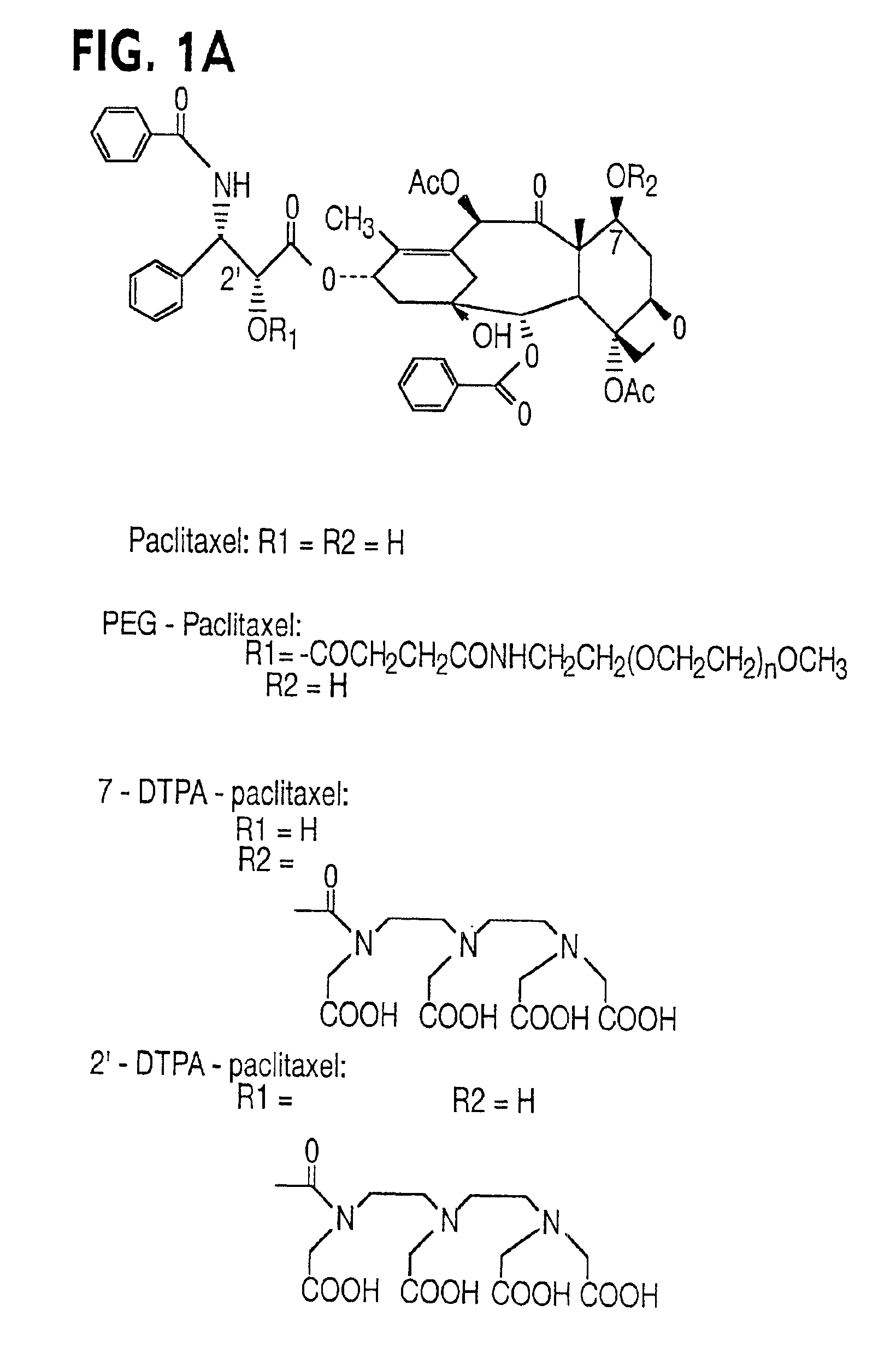
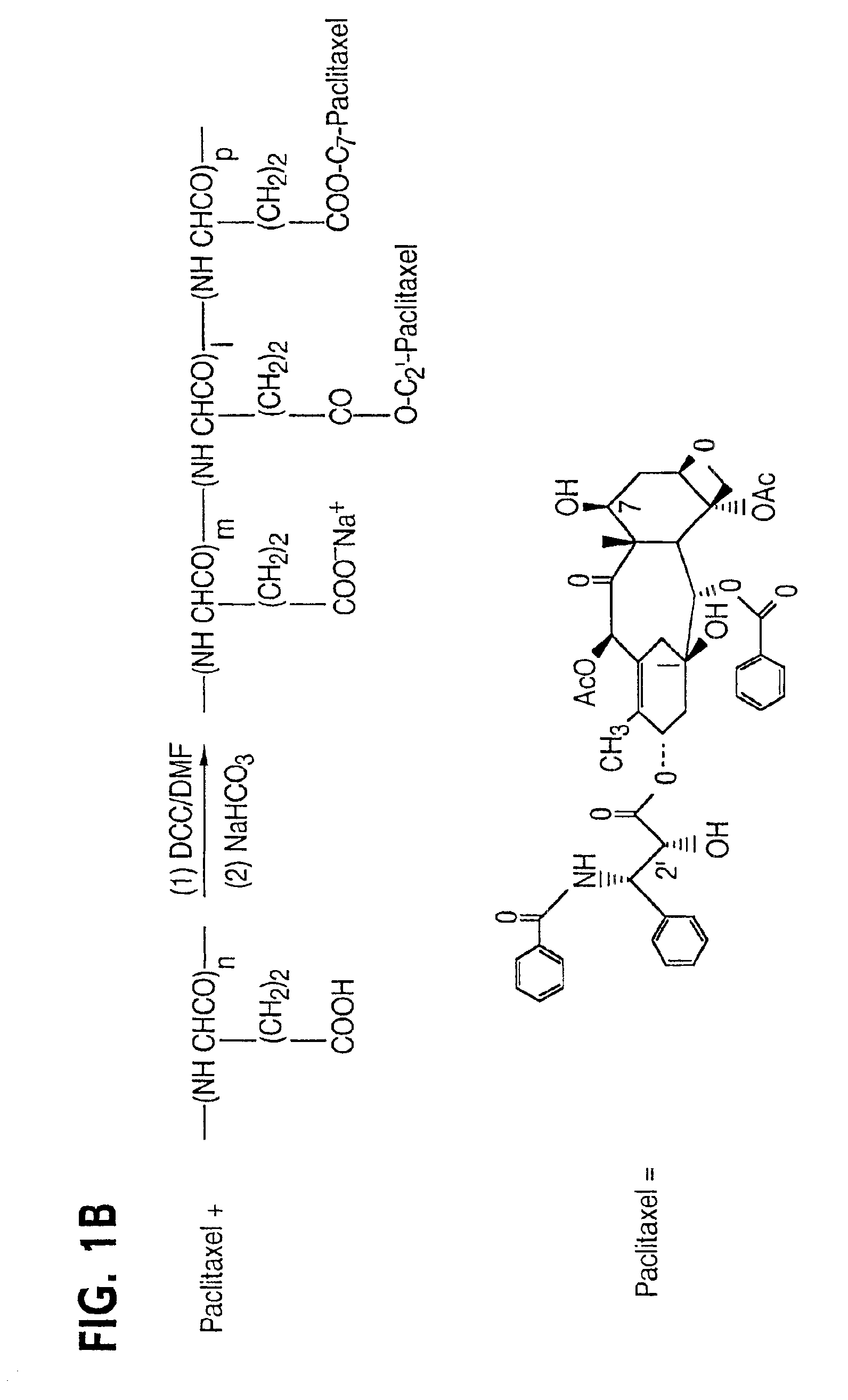
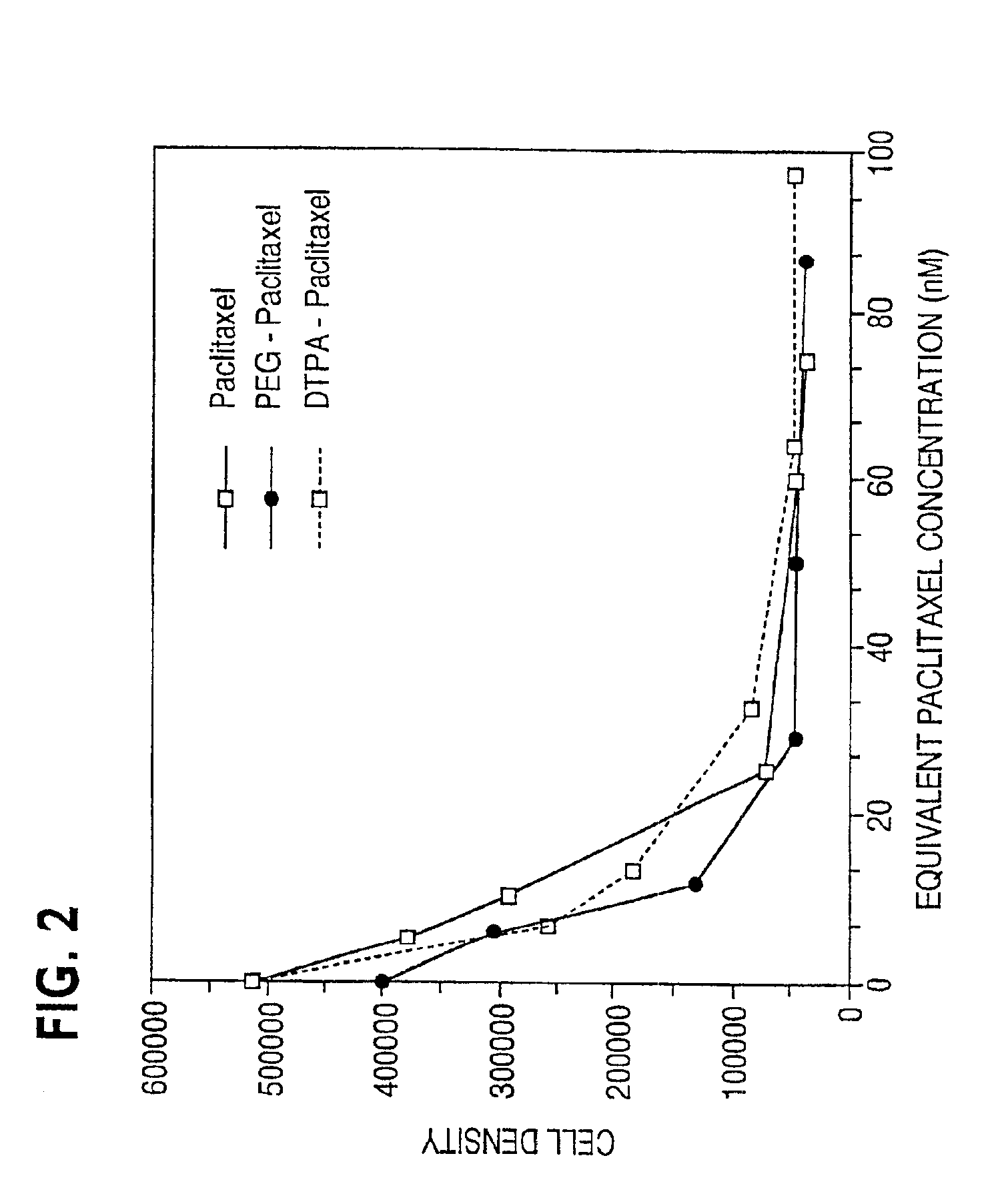
Water soluble paclitaxel derivatives
InactiveUS6884817B2Surprising antitumor activityImprove efficacyHeavy metal active ingredientsBiocideDocetaxel-PNPDocetaxel
Disclosed are water soluble compositions of paclitaxel and docetaxel formed by conjugating the paclitaxel or docetaxel to a water soluble polymer such as poly-glutamic acid, poly-aspartic acid or poly-lysine. Also disclosed are methods of using the compositions for treatment of tumors, auto-immune disorders such as rheumatoid arthritis. Other embodiments include the coating of implantable stents for prevention of restenosis.
Owner:PG TXL COMPANY
Aminobutyramide conjugate and a pharmaceutical composition for treatment of neuronal disorders
ActiveUS20060058219A1Improve efficiencyEliminate side effectsBiocidePeptide/protein ingredientsTryptophanSaccharin
A compound is provided that has the formula NH2CH2CH2CH2C(O)N—R (I) where R is a moiety capable of crossing the blood brain barrier and is as a free compound serotonin, dopamine blood brain barrier (BBB) peptide, membrane translocating protein, TAT peptides, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptidetransferrin, glucosylamnine, amino saccharin, lactylamine, leucine, tryptophan, glutamate and amino cholines.
Owner:MILLER LANDON C G
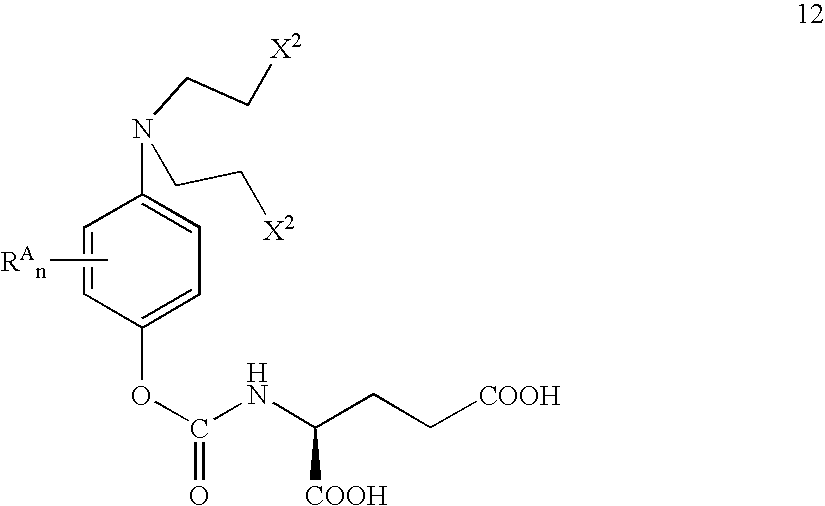
Methods of chemical systhesis of phenolic nitrogen mustard prodrugs
InactiveUS6916949B2Group 4/14 element organic compoundsCarbamic acid derivatives preparationNitrogen mustardChemical synthesis
This invention pertains to novel methods for the synthesis of certain nitrogen mustard prodrugs, such as N-{4-[N,N-bis(2-haloethylamino)-phenoxycarbonyl}-L-glutamic acid: wherein: X2 is a halo group, and is —F, —Cl, —Br, or —I; n is an integer from 0 to 4; and, each RA is an aryl substituent. The methods comprise, at least, the steps of: glutamate conjugation (GC); silyloxy deprotection (SD); and, sulfonic esterification (SU). Certain preferred methods comprise the steps of: amine substitution (AS); silyloxy protection (SP); phenolic deprotection (PD); activation (AC); glutamate conjugation (GC); silyloxy deprotection (SD); sulfonic estenfication (SU); halogenation (HL); glutamate deprotection (GD); and glutamic acid protection (GP).
Owner:THE INST OF CANCER RES ROYAL CANCER HOSPITAL
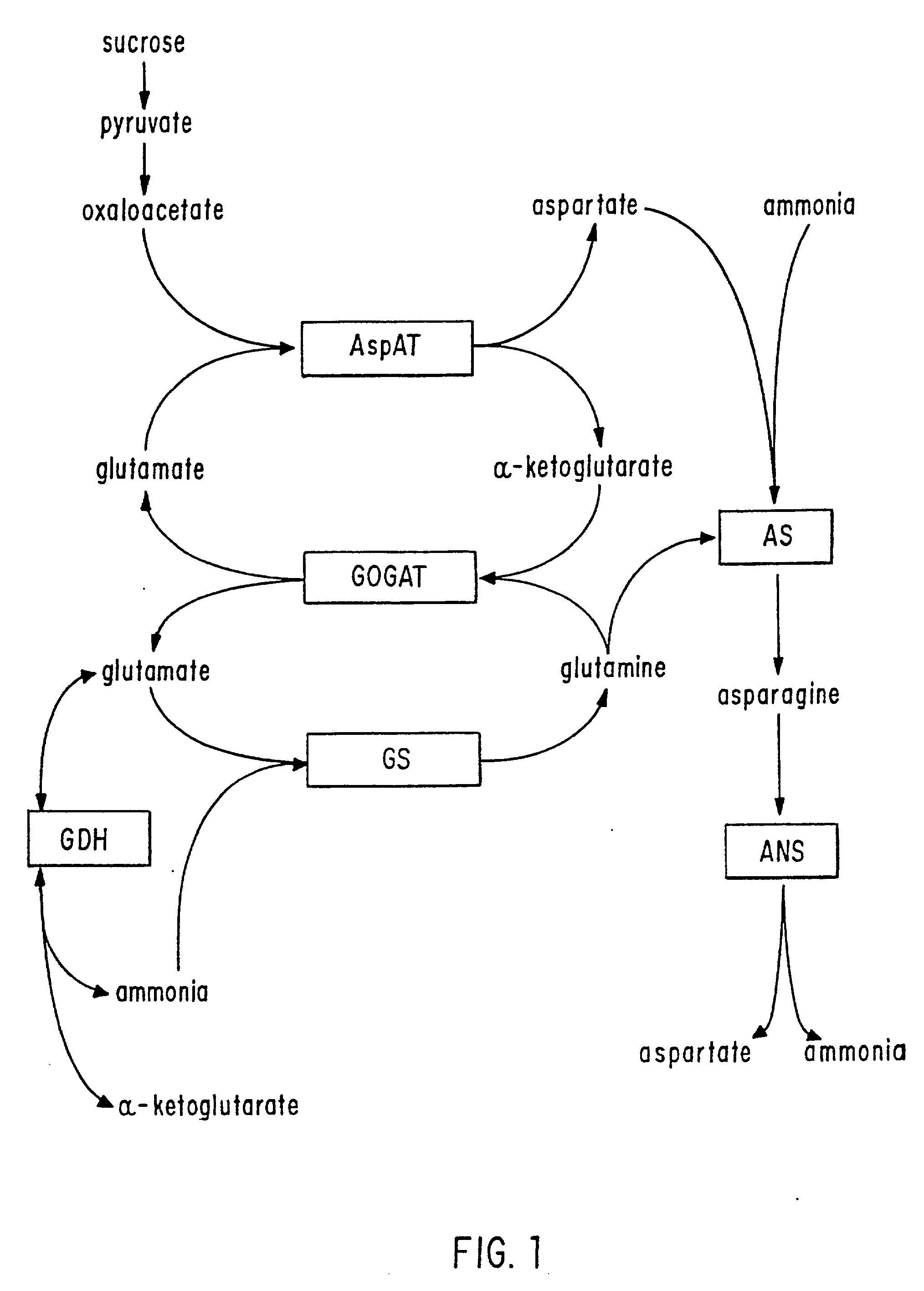
Transgenic plants that exhibit enhanced nitrogen assimilation
InactiveUS20020069430A1Improve growth characteristicsImproved vegetativeClimate change adaptationOther foreign material introduction processesPlant cellThreonine
Transgenic plants containing free amino acids, particularly at least one amino acid selected from among glutamic acid, asparagine, aspartic acid, serine, threonine, alanine and histidine accumulated in a large amount, in edible parts thereof, and a method of producing them are provided. In this method, glutamate dehydrogenase (GDH) gene is introduced into a plant together with a regulator sequence suitable for over expressing the sequence encoding GDH gene in plant cells.
Owner:AJINOMOTO CO INC
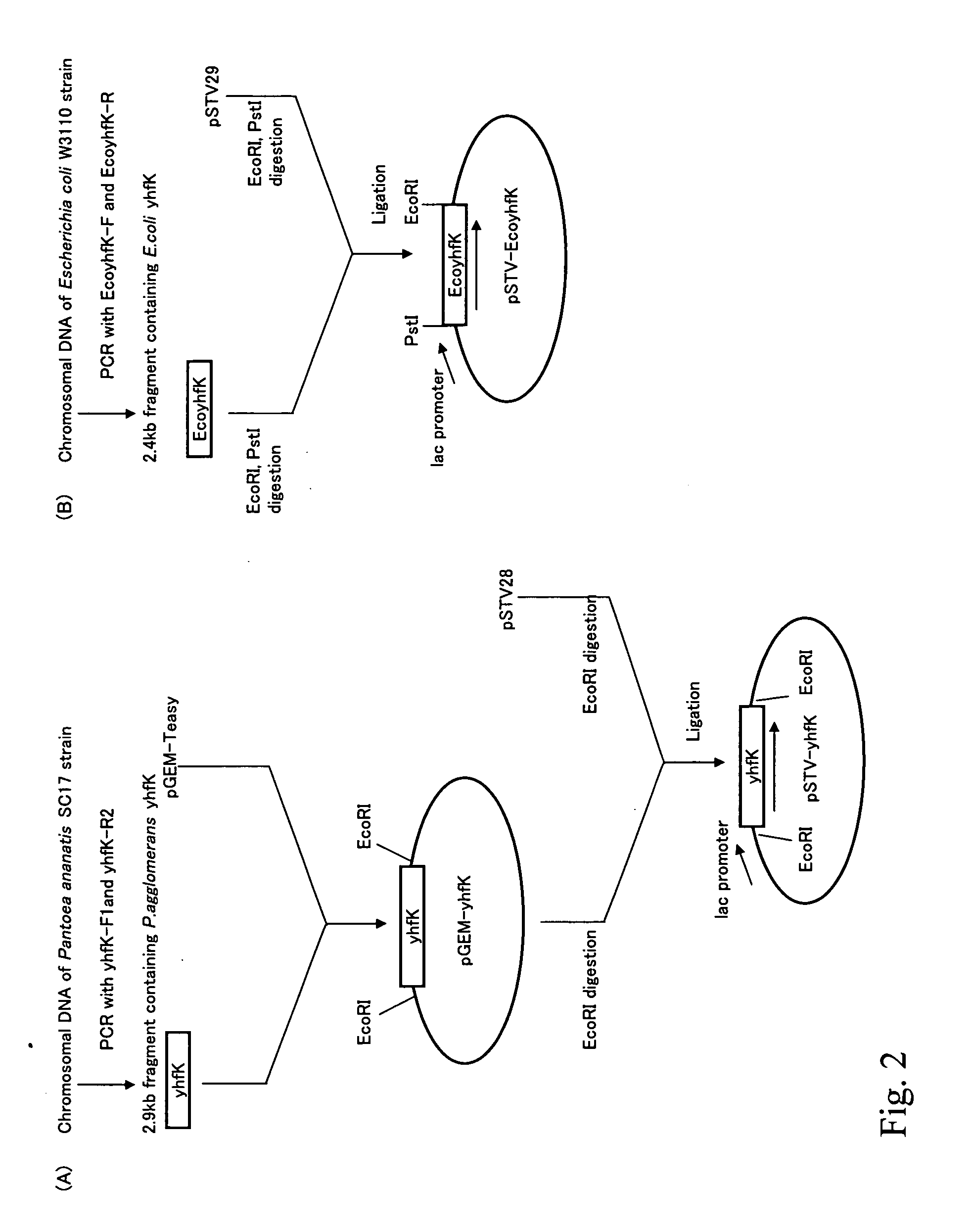
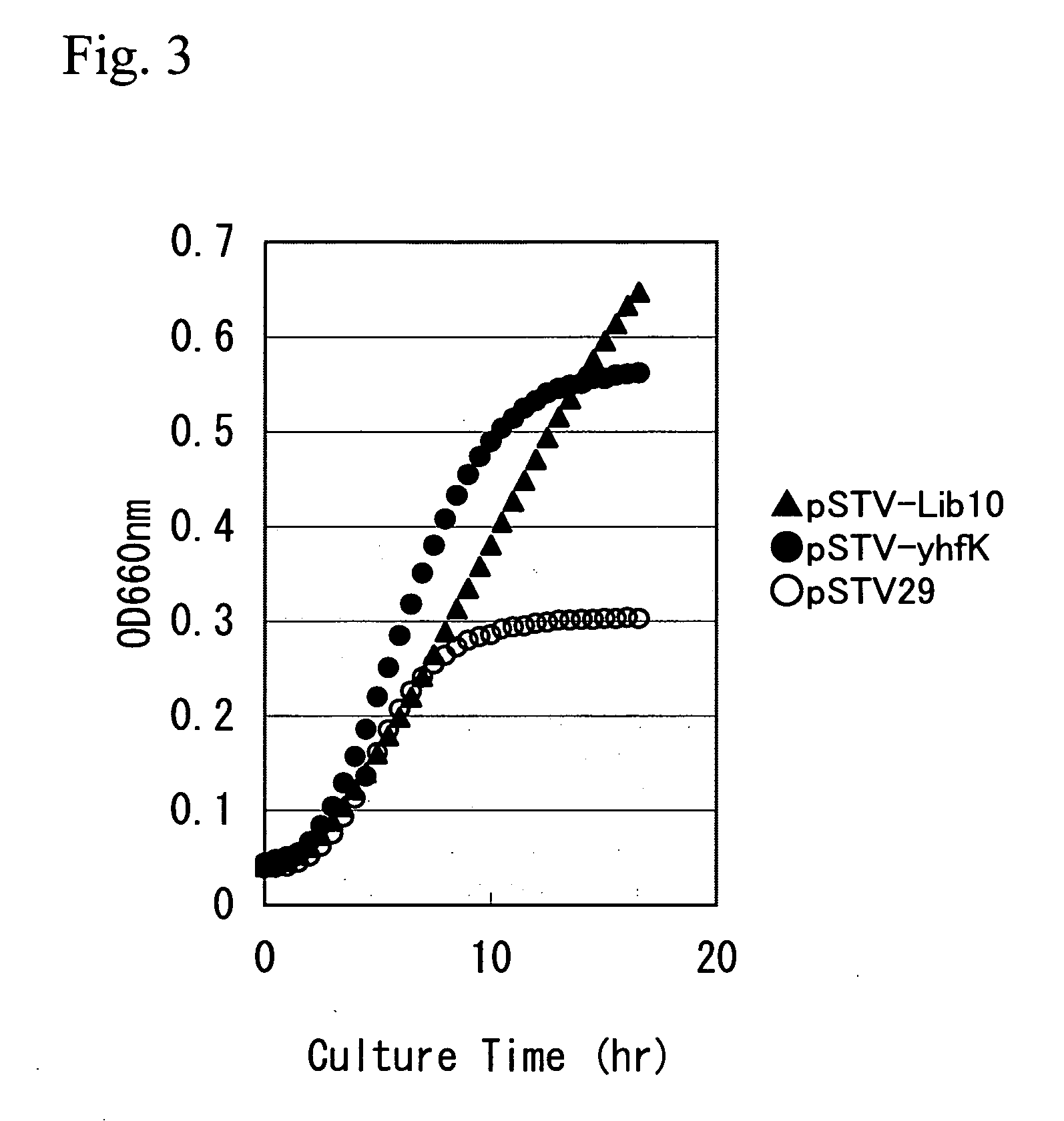
L-glutamic acid-producing microorganism and a method for producing L-glutamic acid
ActiveUS20050196846A1High expressionImprove fermentation yieldBacteriaDepsipeptidesMicroorganismMicrobiology
L-Glutamic acid is produced by culturing a microorganism in which an expression of L-glutamic acid-export gene, a yhfK gene, is enhanced or overexpressed, in a medium to produce and cause accumulation of L-glutamic acid in the medium, and collecting L-glutamic acid from the medium.
Owner:AJINOMOTO CO INC
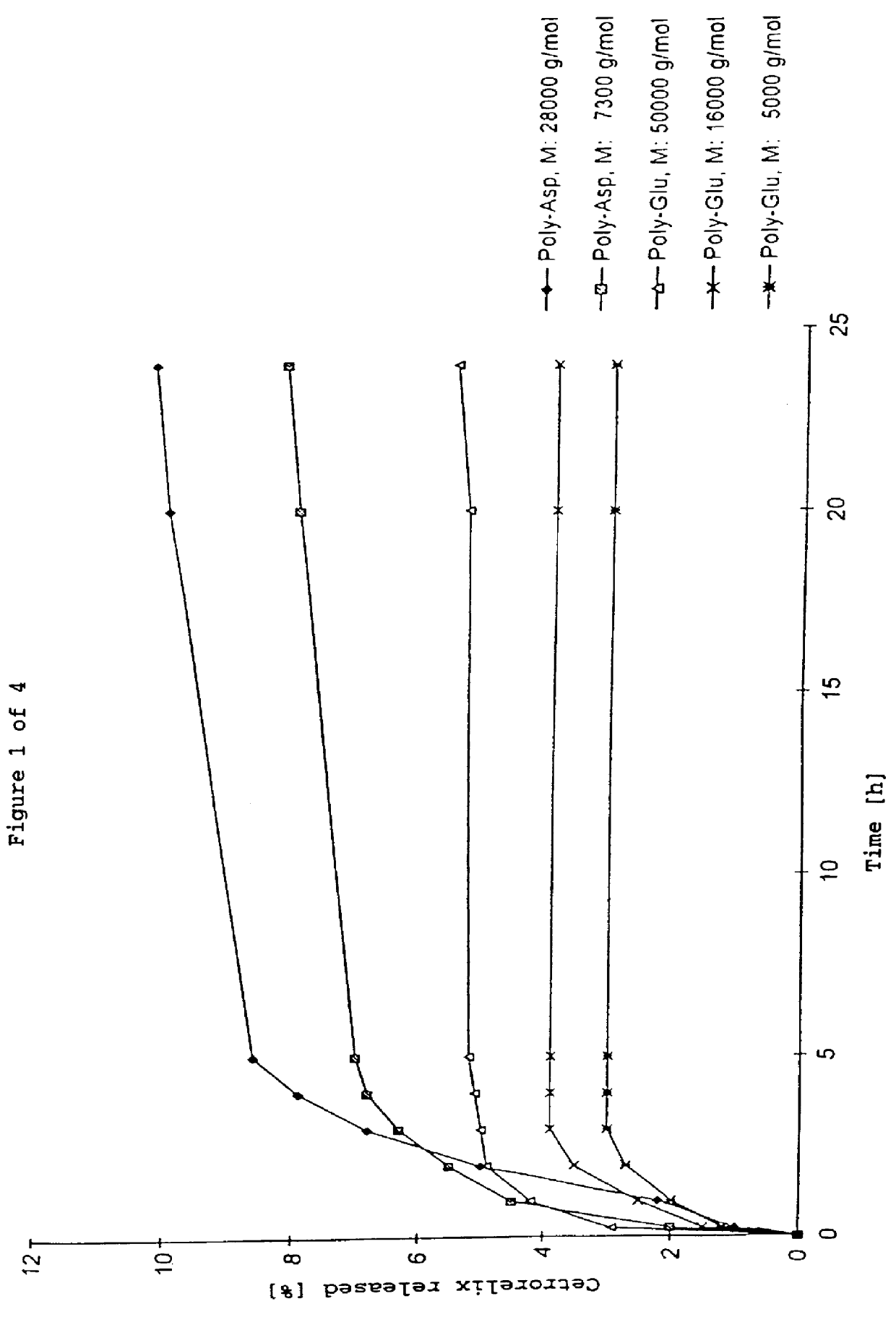
Immobilized and activity-stabilized complexes of LHRH antagonists and processes for their preparation
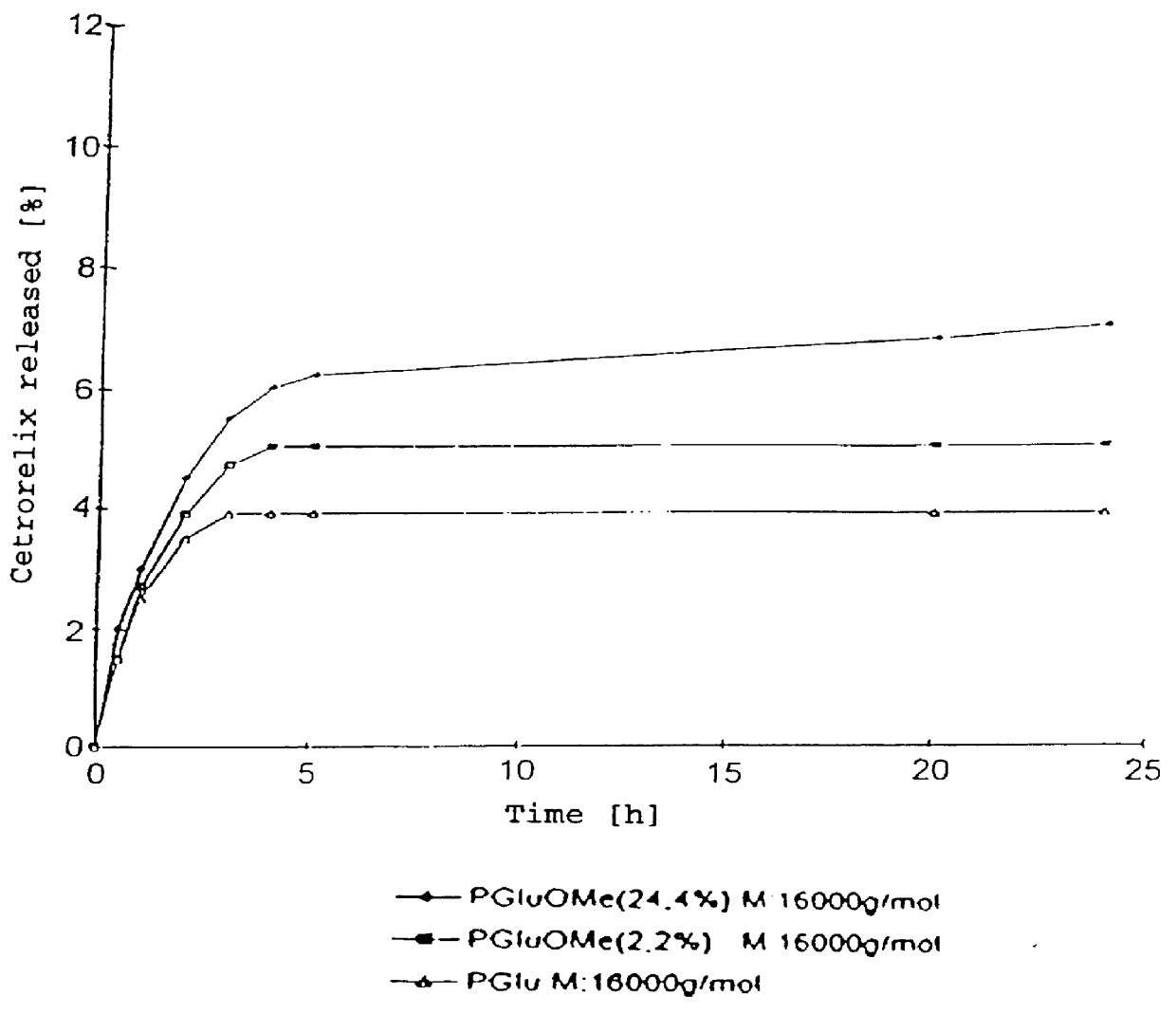
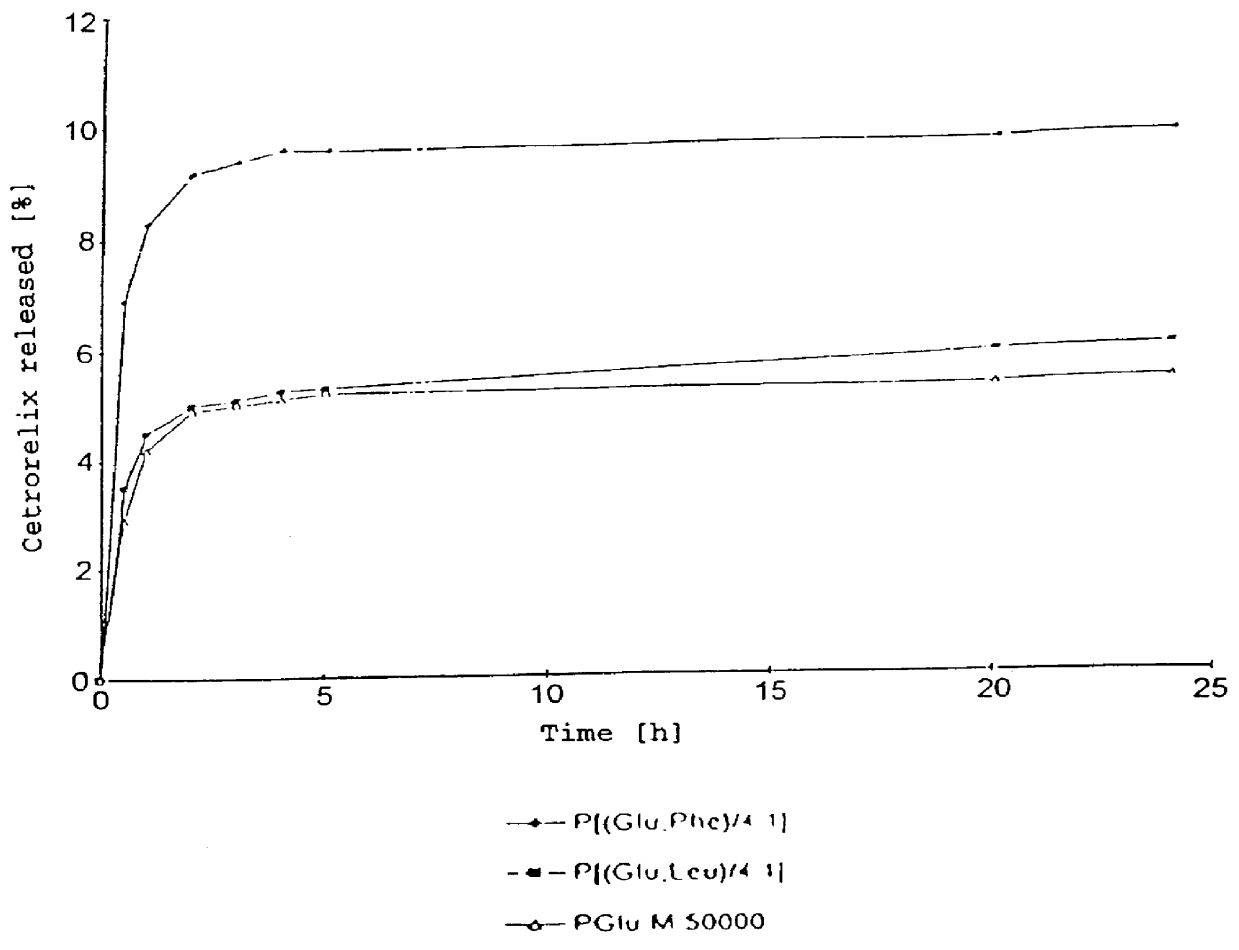
In this invention, a release-delaying system is to be developed for LHRH antagonists, in particular for cetrorelix, which allows the active compound to be released in a controlled manner over several weeks by complexation with suitable biophilic carriers. The acidic polyamino acids polyglutamic acid and polyaspartic acid were selected for complexation with cetrorelix. The cetrorelix polyamino acid complexes are prepared from aqueous solutions by combination of the solutions and precipitation of the complexes, which are subsequently centrifuged off and dried over P2O5 in vacuo. If complexes having a defined composition are to be obtained, lyophilization proves to be a suitable method. The cetrorelix-carboxylic acid complexes were also prepared from the aqueous solutions. In the random liberation system, the acidic polyamino acids poly-Glu and poly-Asp showed good release-delaying properties as a function of the hydrophobicity and the molecular mass of the polyamino acid. In animal experiments, it was possible to confirm the activity of the cetrorelix-polyamino acid complexes as a depot system in principle. It is thus possible by complexation of cetrorelix with polyamino acids to achieve testosterone suppression in male rats over 600 hours. The release of active compound here can be controlled by the nature and the molecular mass of the polymers.
Owner:ZENTARIS GMBH
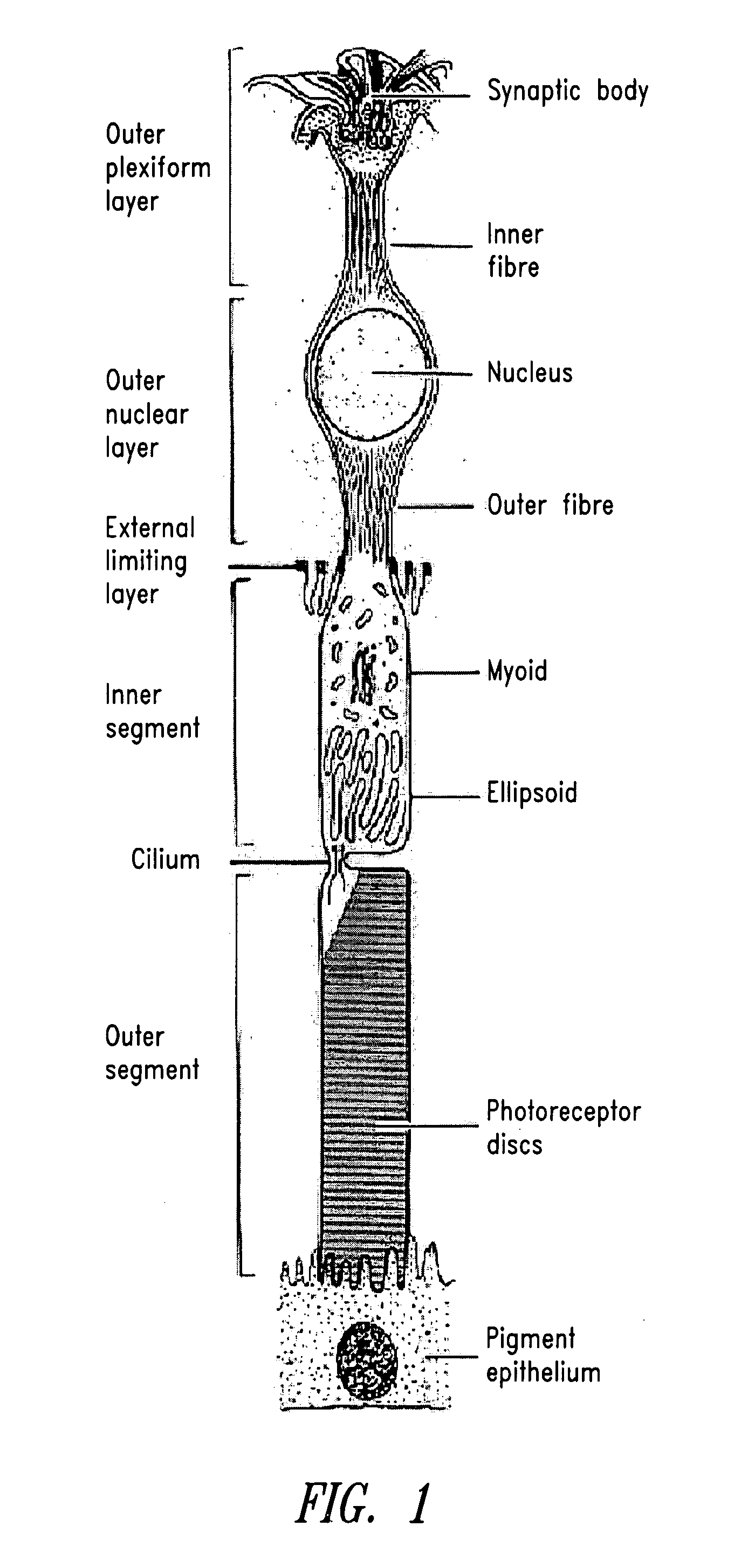
Extended primary retinal cell culture and stress models, and methods of use
A cell culture system related to extended in vitro culture of mature retinal cells and methods for preparing the cell culture system are provided. Also provided is a retinal cell culture stress model related to extended in vitro culture of mature retinal cells in the presence of a stressor and methods for using the cell culture stress model. The invention provides a cell culture system comprising a long-term culture of mature retinal cells, without requiring addition of other types of non-retinal cells such as purified glia, or cells isolated from ciliary bodies within the eye, and the addition of a stressor such as light, A2E, cigarette smoke condensate, glutamate, or hydrostatic pressure. Methods for identifying bioactive agents that alter viability, neurodegeneration, or survival of retinal cells using the retinal cell culture stress system are also provided.
Owner:ACUACELA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com