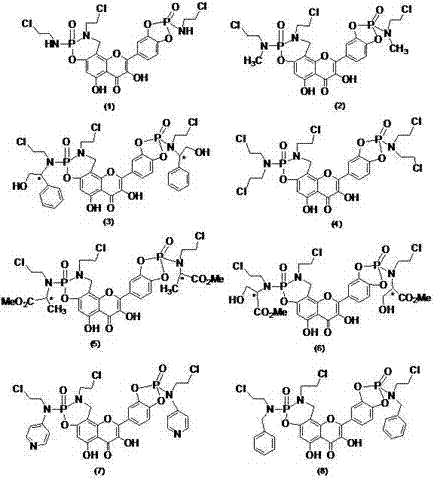
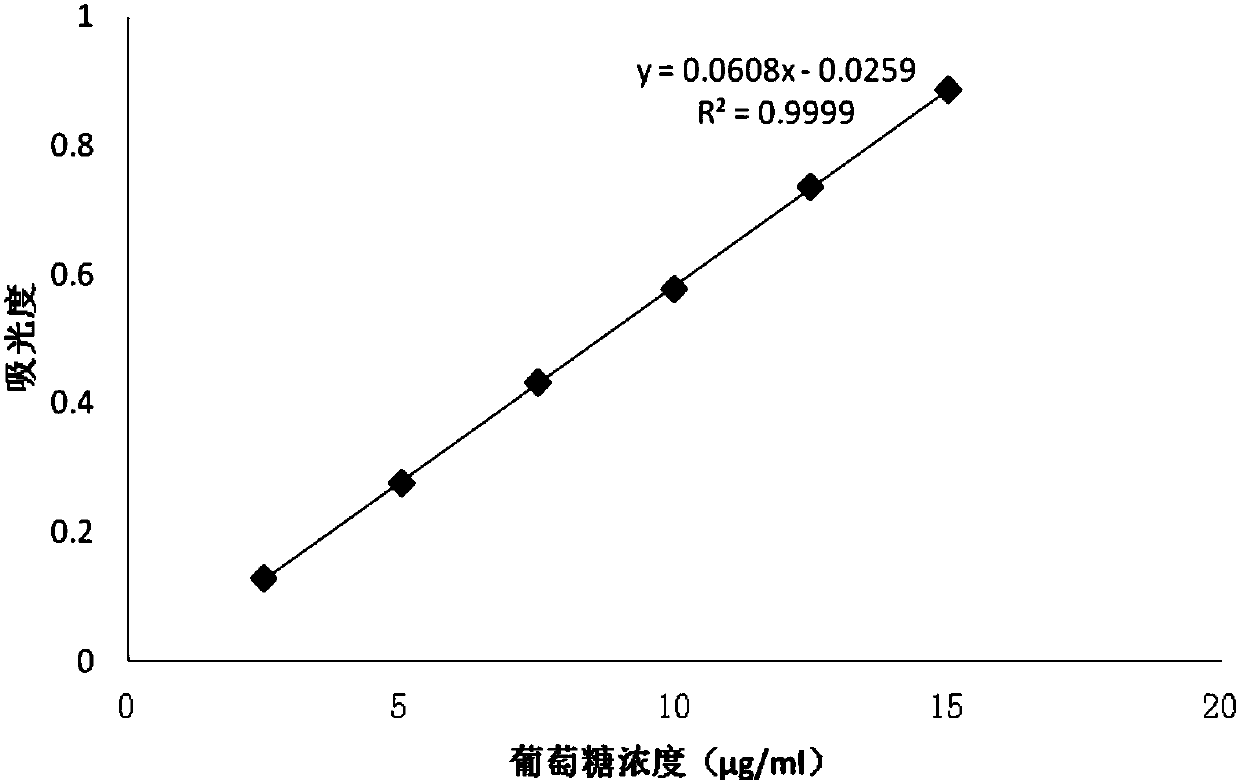
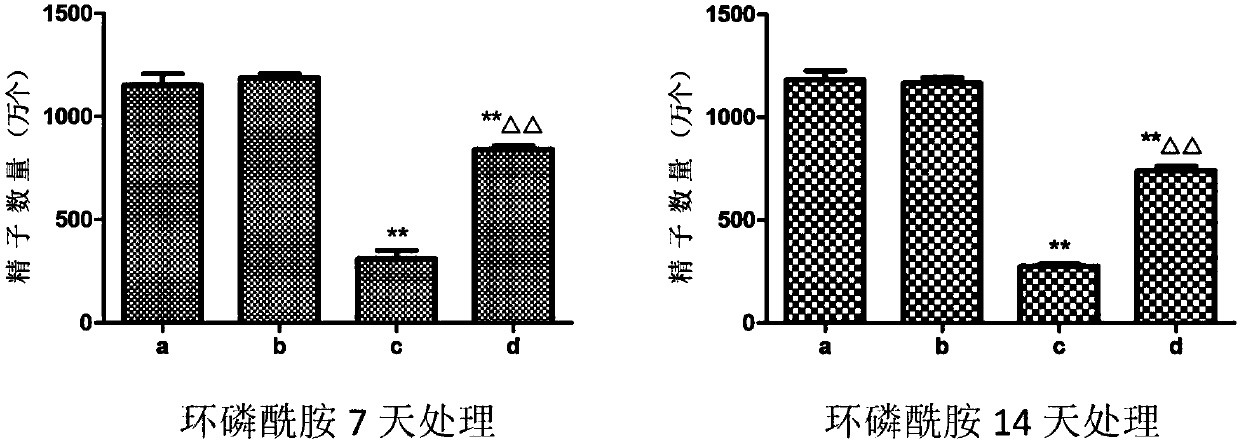
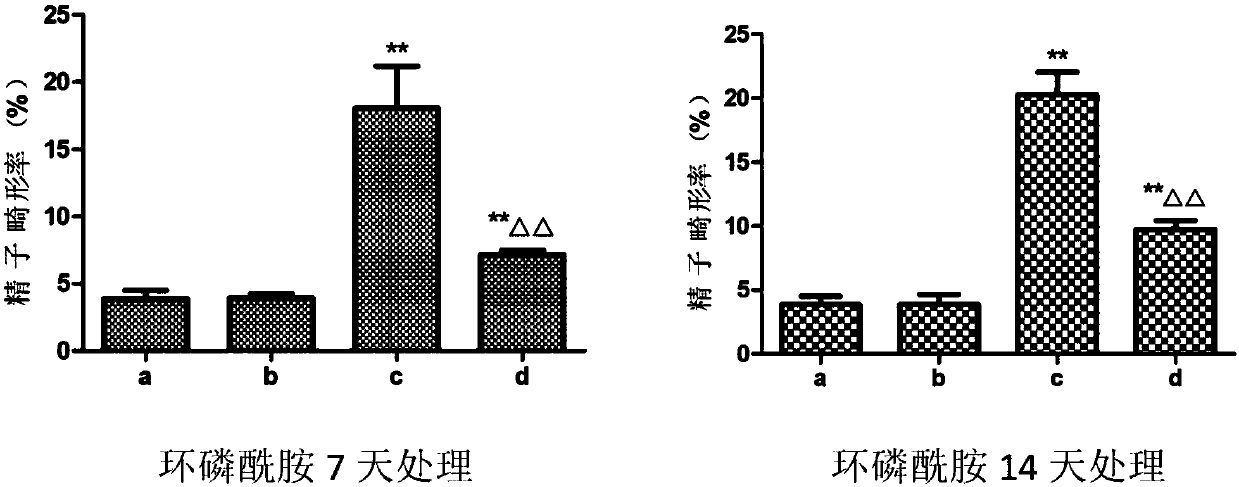
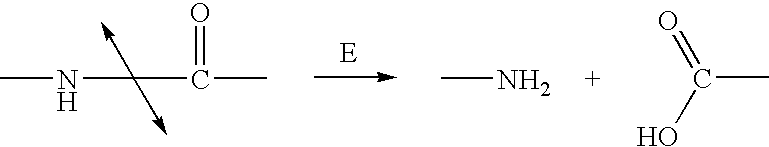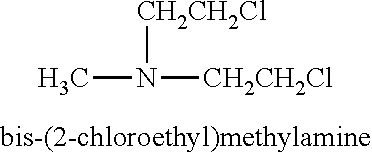Patents
Literature
89 results about "Nitrogen mustard" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Nitrogen mustards are cytotoxic chemotherapy agents derived from mustard gas. Although their common use is medicinal, in principle these compounds can also be deployed as chemical warfare agents. Nitrogen mustards are nonspecific DNA alkylating agents. Nitrogen mustard gas was stockpiled by several nations during the Second World War, but it was never used in combat. As with all types of mustard gas, nitrogen mustards are powerful and persistent blister agents and the main examples (HN1, HN2, HN3, see below) are therefore classified as Schedule 1 substances within the Chemical Weapons Convention. Production and use is therefore strongly restricted.
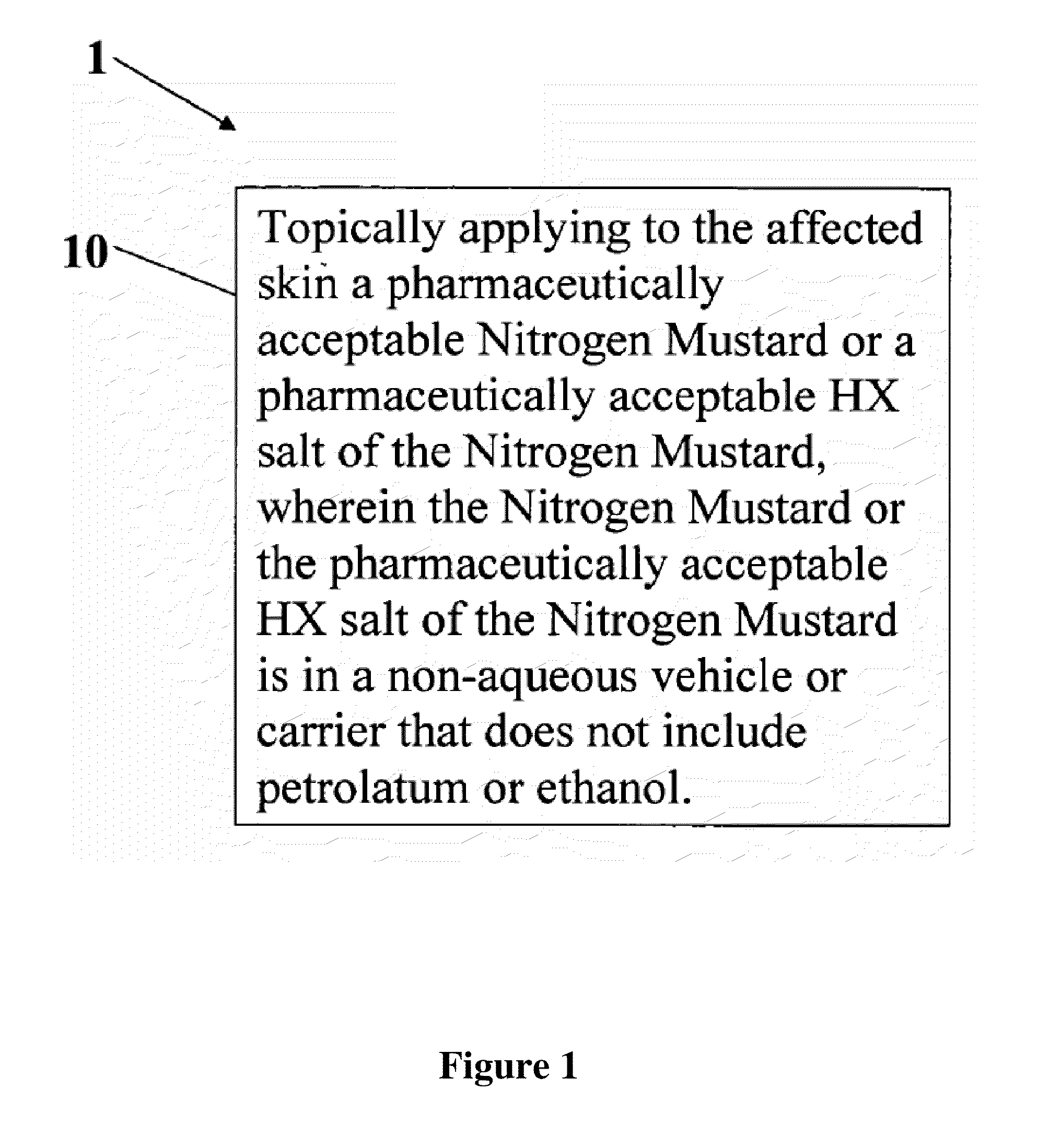
Stabilized compositions of volatile alkylating agents and methods of using thereof
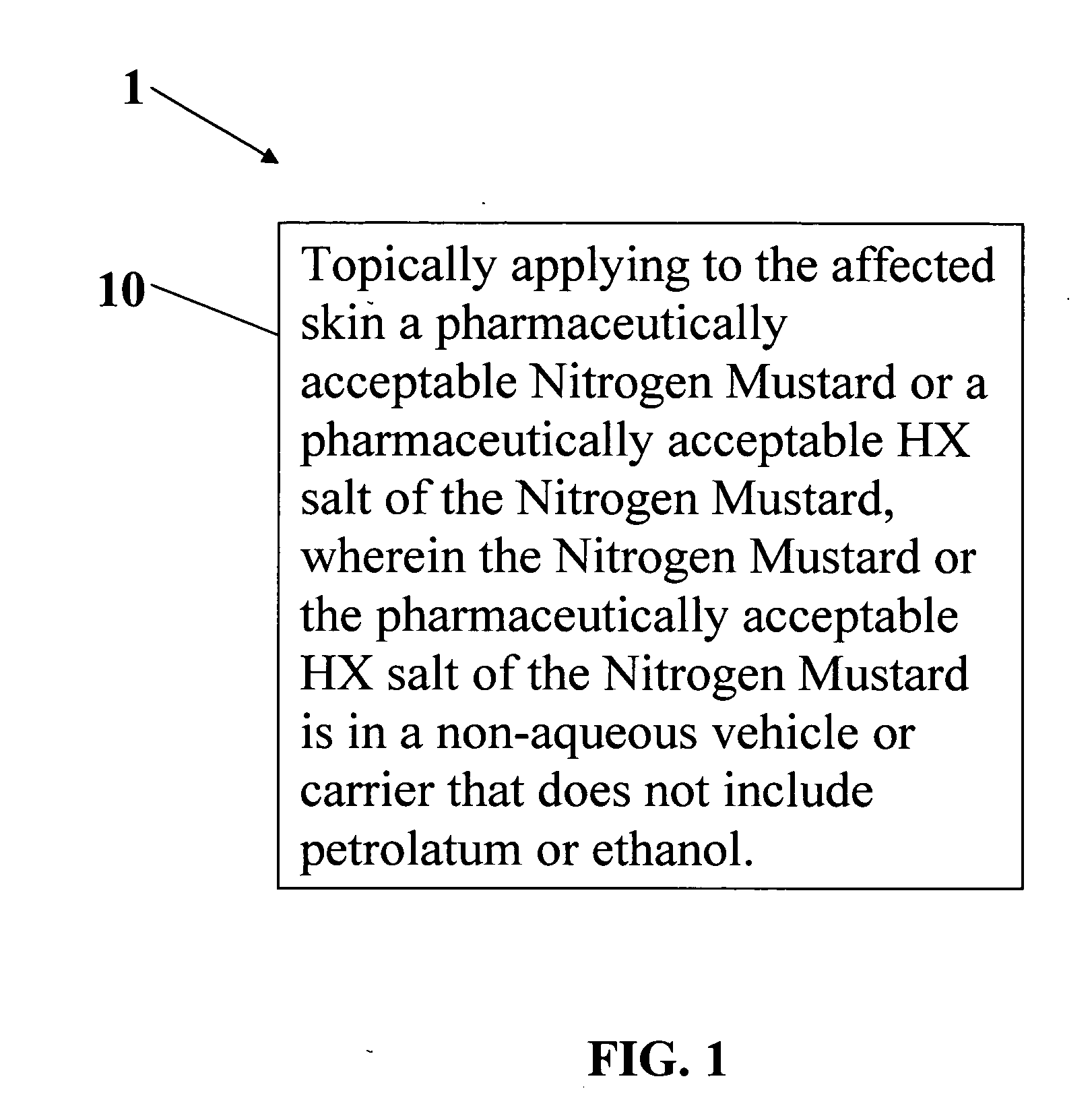
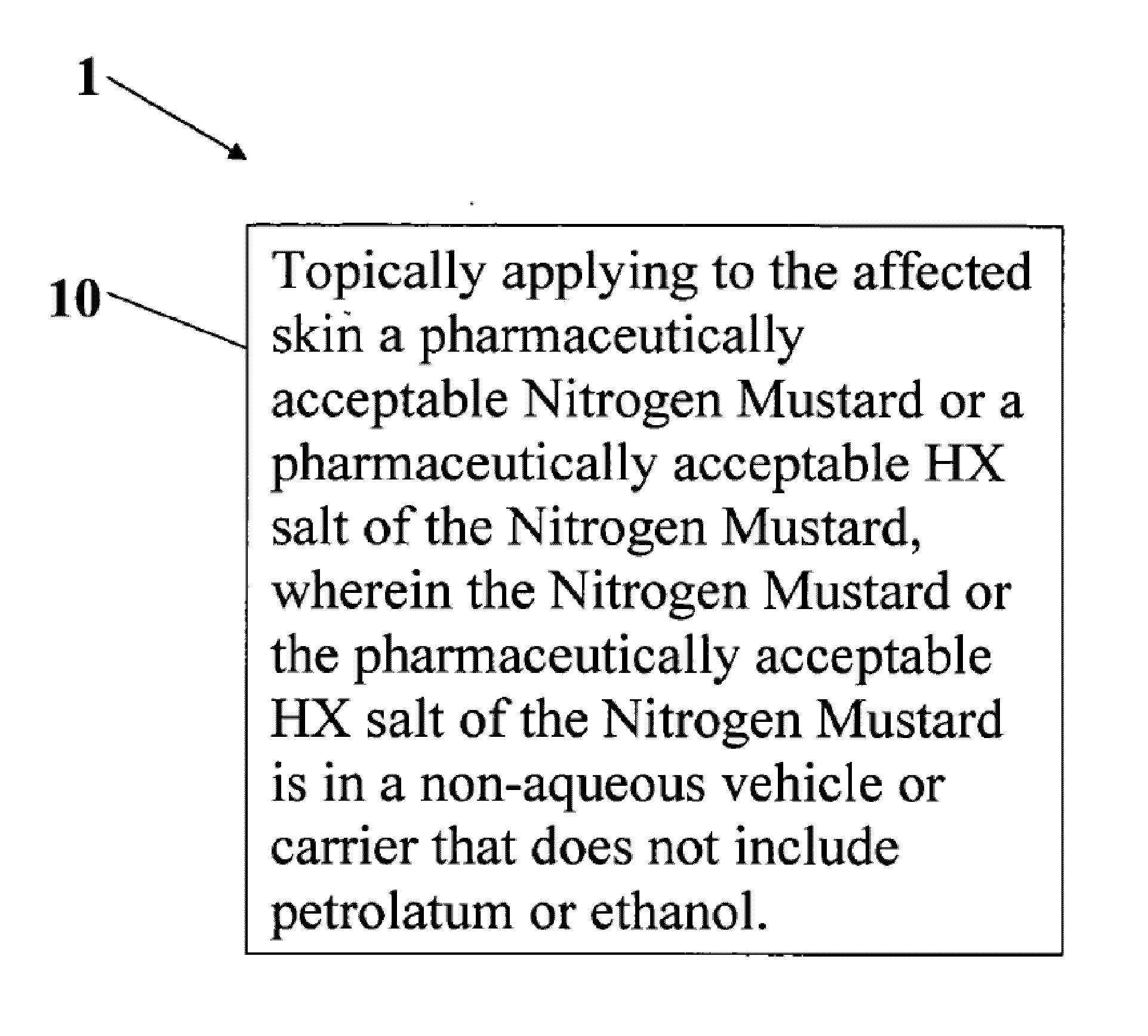
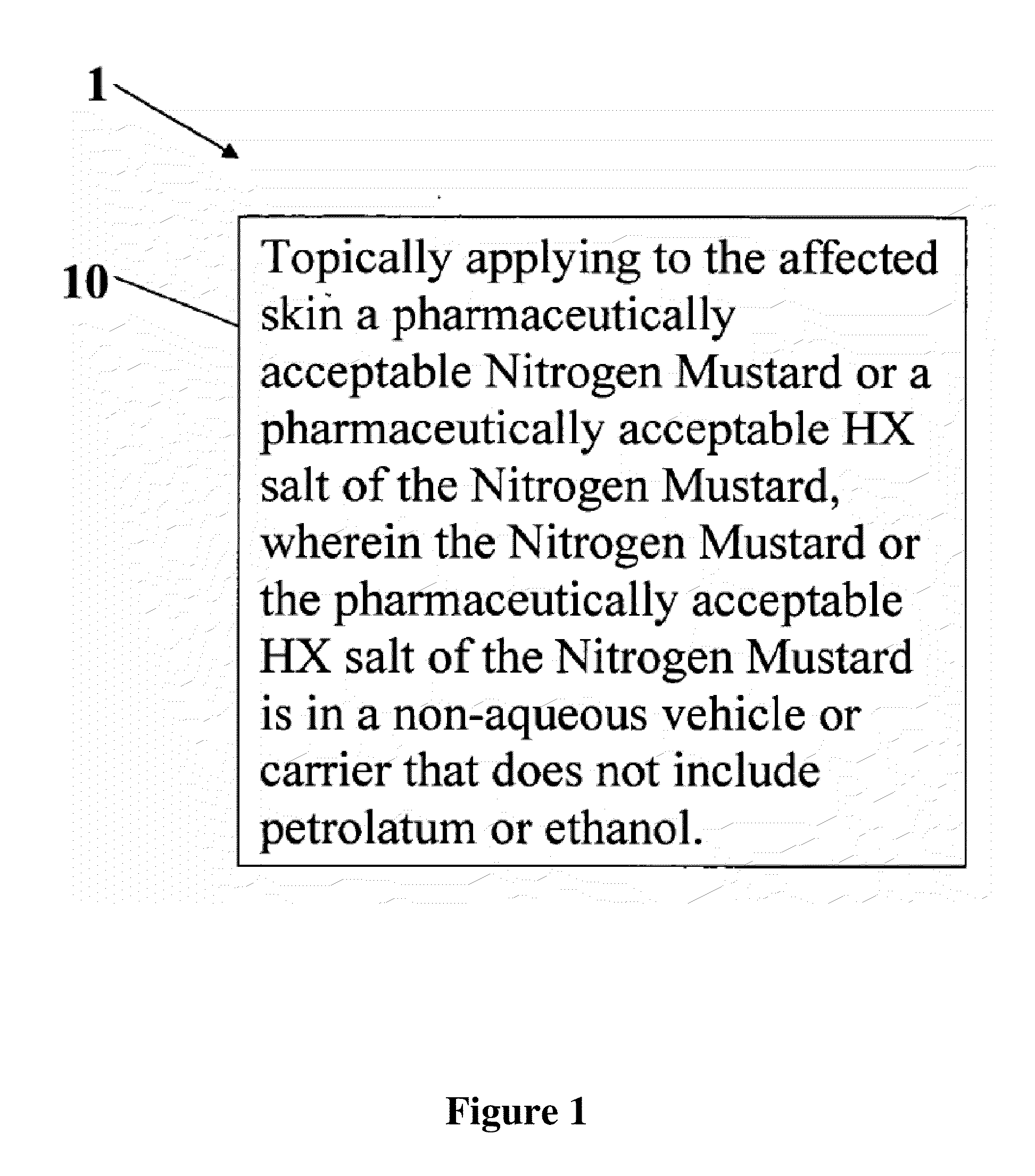
A composition and method for treatment of cancer. The composition for treating a skin disorder, comprising: a Nitrogen Mustard or an HX salt of the Nitrogen Mustard, wherein the Nitrogen Mustard or the HX salt of the Nitrogen Mustard is in a non-aqueous vehicle or carrier that does not include petrolatum or ethanol, wherein the non-aqueous vehicle or carrier that does not include petrolatum or ethanol does not include petrolatum or ethanol. The method comprises topically applying the composition of a Nitrogen Mustard or a HX salt of the Nitrogen Mustard to the affected skin, wherein the Nitrogen Mustard or the HX salt of the Nitrogen Mustard is in a non-aqueous vehicle or carrier that does not include petrolatum or ethanol, wherein the non-aqueous vehicle or carrier does not include petrolatum or ethanol.
Owner:HELSINN BIREX PHARMA
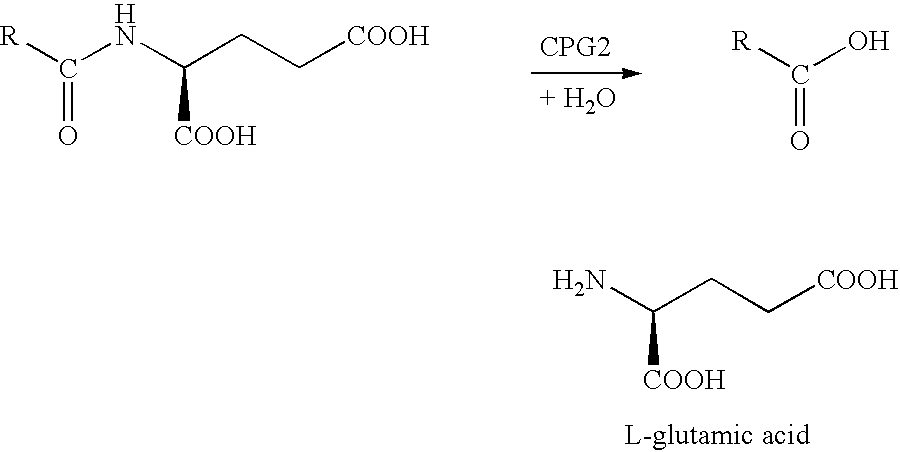
Methods of chemical systhesis of phenolic nitrogen mustard prodrugs
InactiveUS6916949B2Group 4/14 element organic compoundsCarbamic acid derivatives preparationNitrogen mustardChemical synthesis
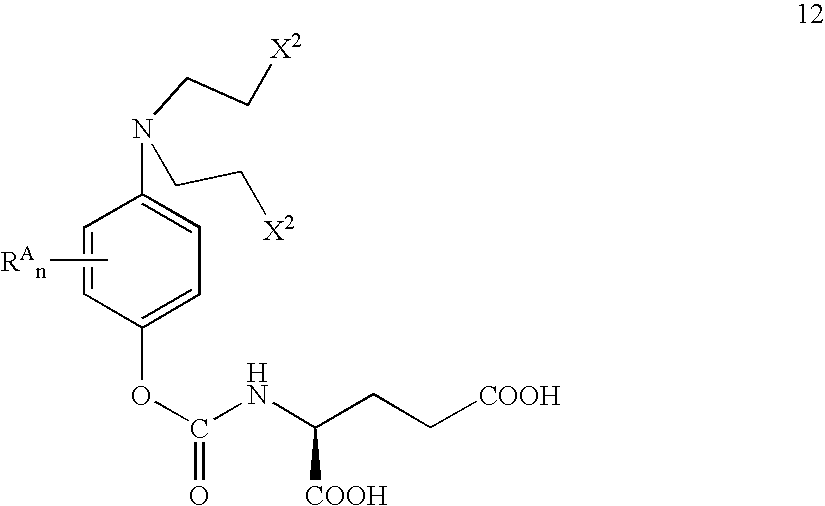
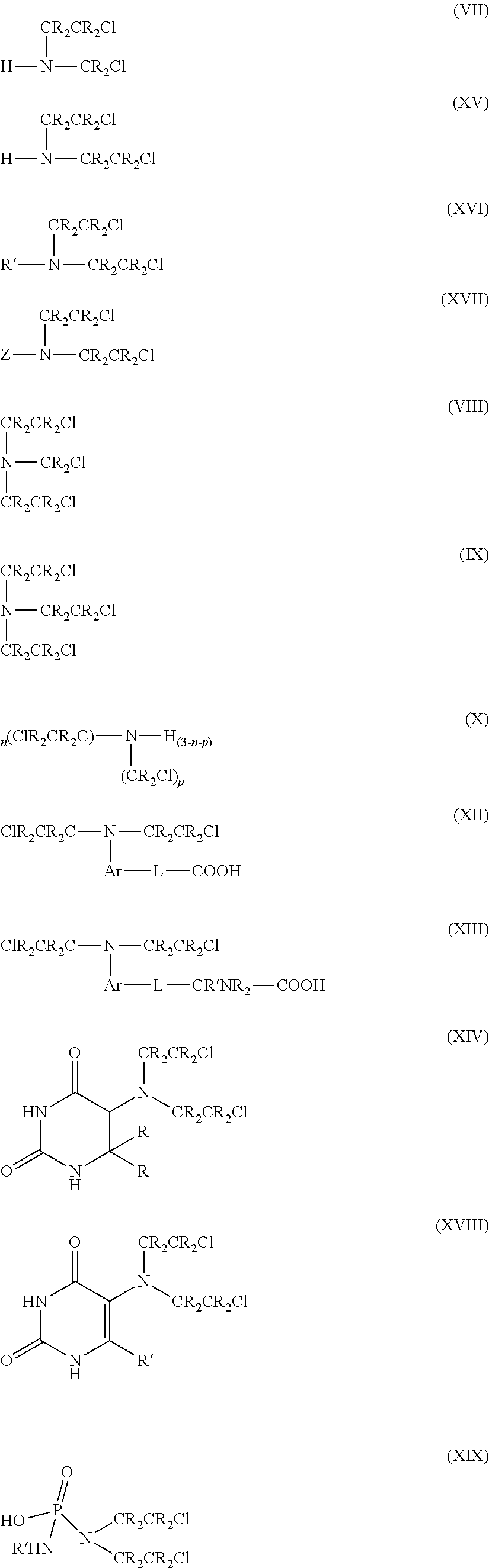
This invention pertains to novel methods for the synthesis of certain nitrogen mustard prodrugs, such as N-{4-[N,N-bis(2-haloethylamino)-phenoxycarbonyl}-L-glutamic acid: wherein: X2 is a halo group, and is —F, —Cl, —Br, or —I; n is an integer from 0 to 4; and, each RA is an aryl substituent. The methods comprise, at least, the steps of: glutamate conjugation (GC); silyloxy deprotection (SD); and, sulfonic esterification (SU). Certain preferred methods comprise the steps of: amine substitution (AS); silyloxy protection (SP); phenolic deprotection (PD); activation (AC); glutamate conjugation (GC); silyloxy deprotection (SD); sulfonic estenfication (SU); halogenation (HL); glutamate deprotection (GD); and glutamic acid protection (GP).
Owner:THE INST OF CANCER RES ROYAL CANCER HOSPITAL
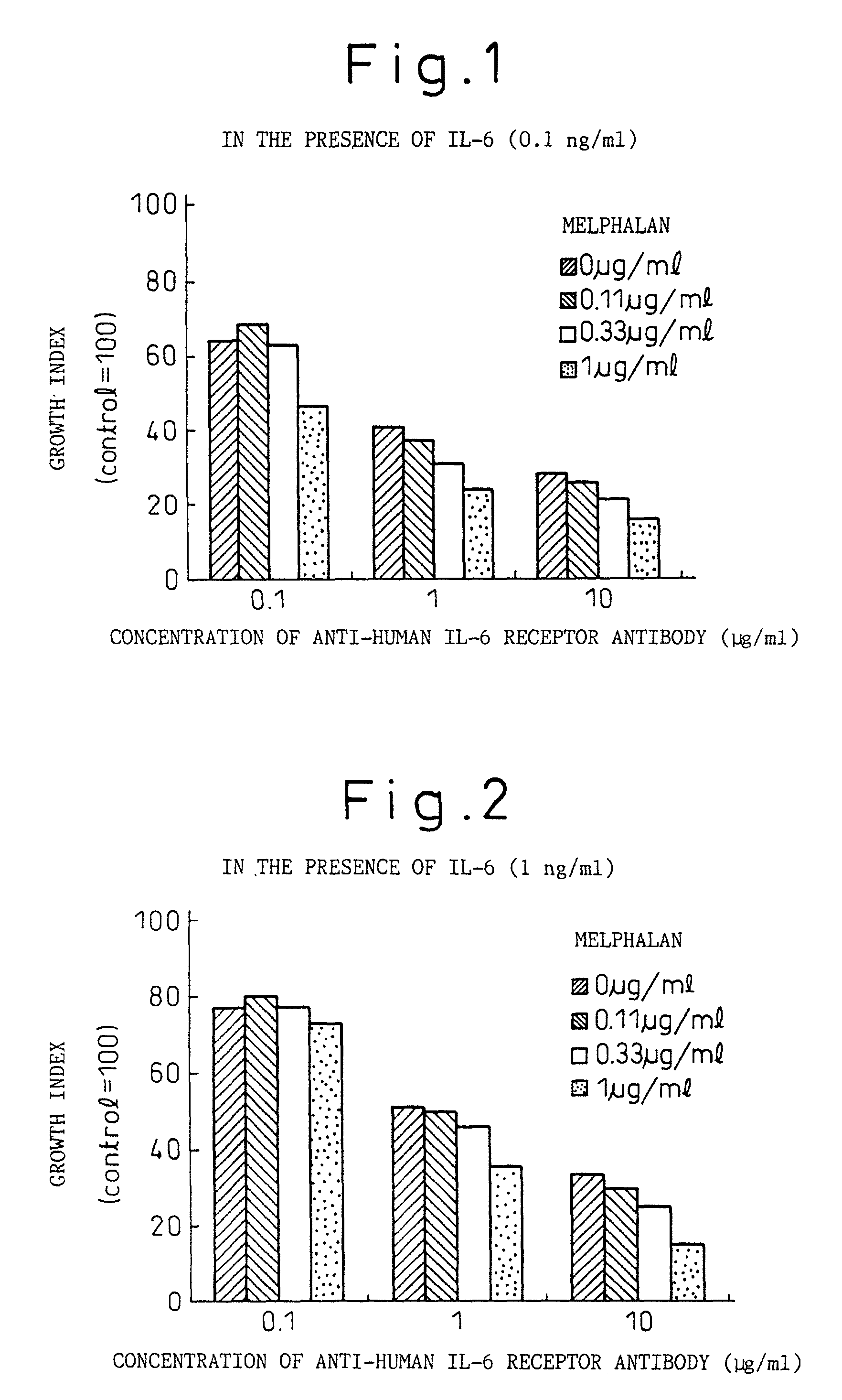
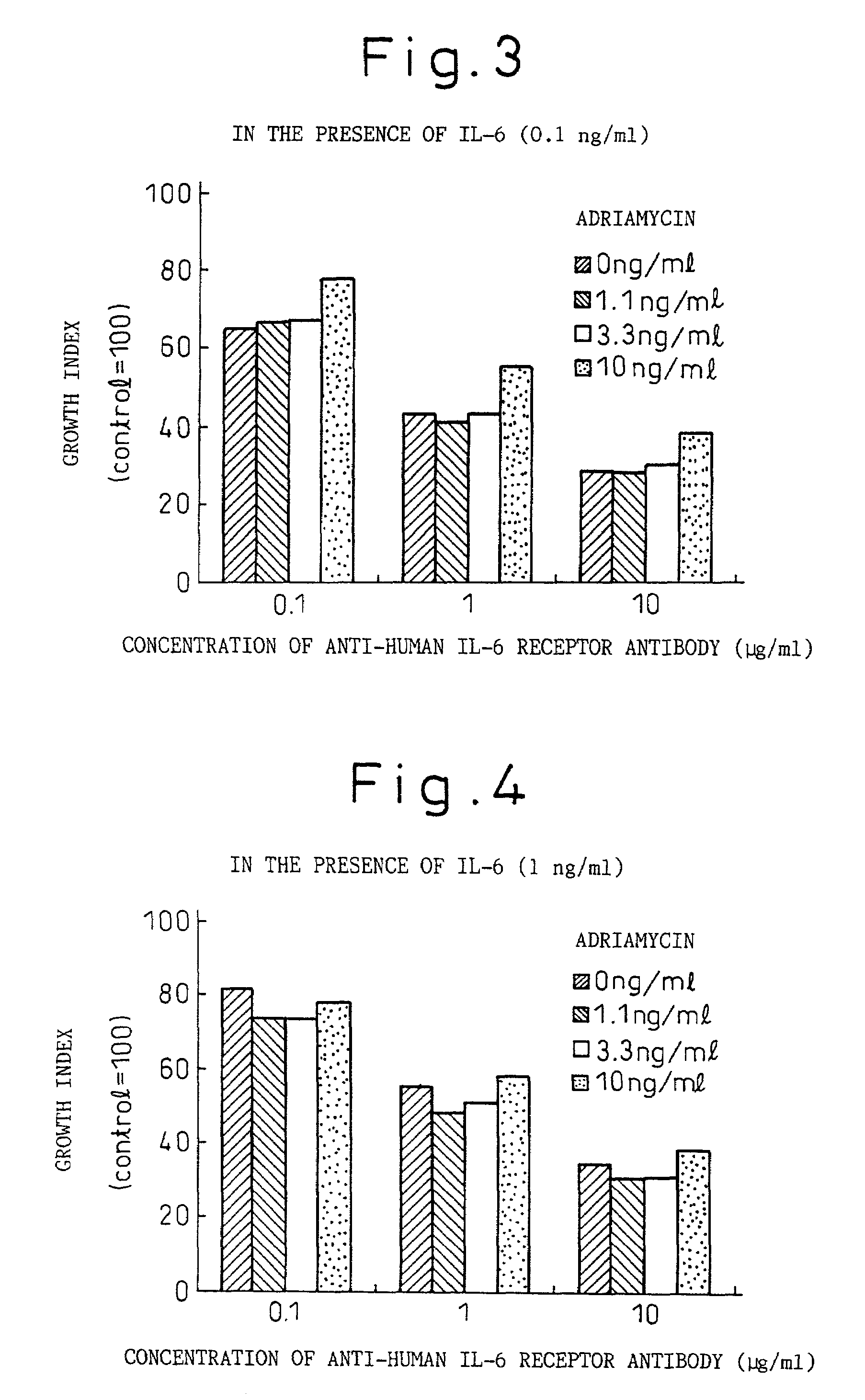
Methods for treating melanomas
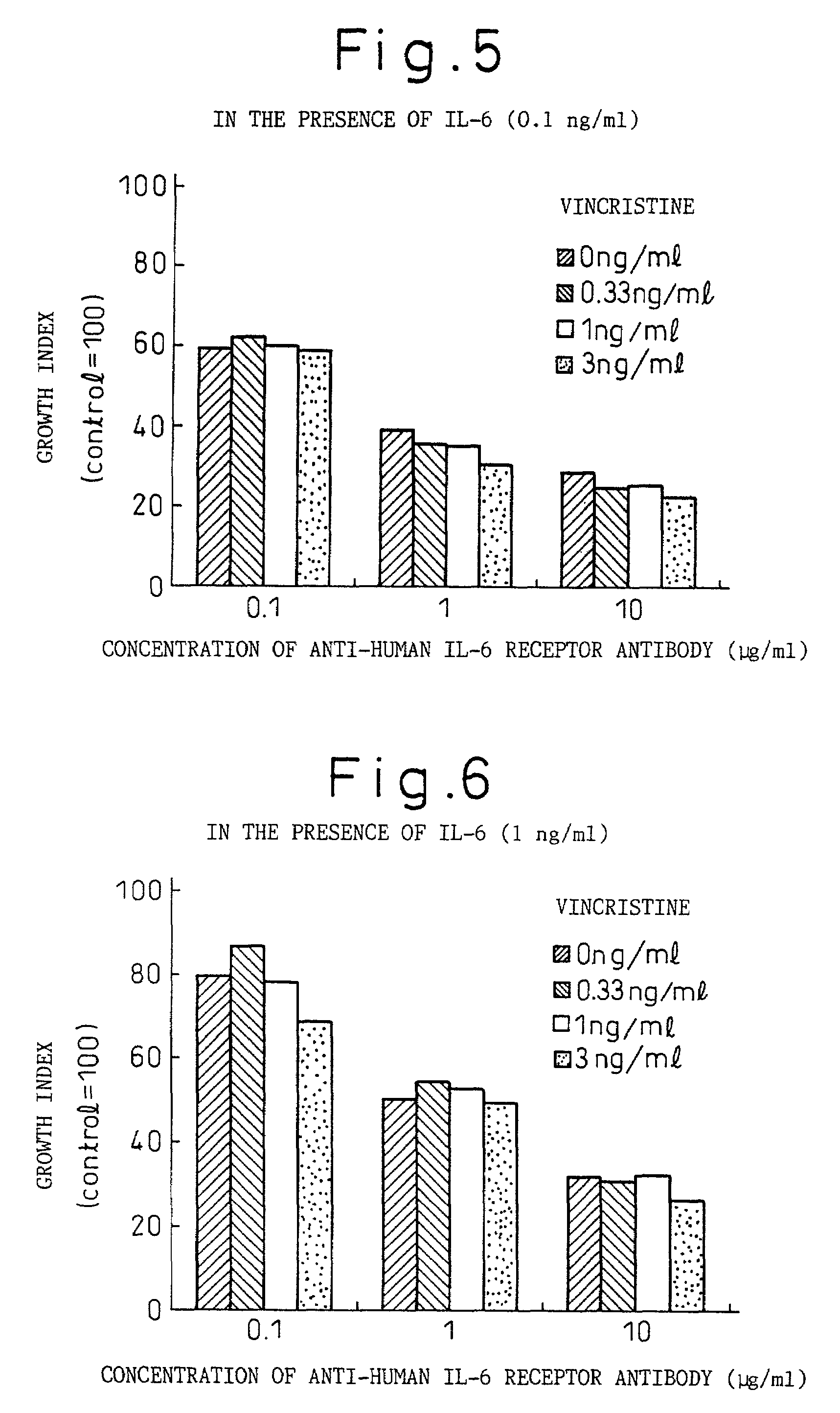
A therapeutic agent for myeloma comprising a combined use of a nitrogen mustard anticancer agent and anti-IL-6 receptor antibody. Thus, a therapeutic agent for myeloma comprising anti-IL-6 receptor antibody for use in combination with a nitrogen mustard anticancer agent; a therapeutic agent for myeloma comprising a nitrogen mustard anticancer agent for use in combination with anti-IL-6 receptor antibody; and a therapeutic agent for myeloma comprising a nitrogen mustard anticancer agent and anti-IL-6 receptor antibody.
Owner:CHUGAI PHARMA CO LTD
Method for the preparation of reactive compositions containing hydrogen peroxide
The subject invention provides a potentially economically viable method for the preparation of hydrogen peroxide (H2O2) in deep eutectic solvents (DES). H2O2 is then used for the destruction of small to large quantities of sulfur and nitrogen mustards and lewisite, their homologous / analogues, and similar chemical warfare agents at ambient conditions in DES without producing any toxic by-products. Furthermore, H2O2 has been used for the destruction of small to large quantities of halogenated hydrocarbons, their homologous / analogues, and similar hazardous chemicals at ambient conditions. H2O2 can be formed by either the electrochemical reduction of oxygen in DES in the presence of water or by dissolving Group 1 (alkali metals) or Group 2 (alkaline earth metals) superoxides, e.g. potassium superoxide, in DES in the presence of water, with / without chemicals used for the enhancement of the solubility of the metal superoxide in the DES, e.g. crown ethers.
Owner:KING SAUD UNIVERSITY
Method for the preparation of reactive hydrogen peroxide in deep eutectic solvents
The subject invention provides a potentially economically viable method for the preparation of hydrogen peroxide (H2O2) in deep eutectic solvents (DES). H2O2 is then used for the destruction of small to large quantities of sulfur and nitrogen mustards and lewisite, their homologous / analogues, and similar chemical warfare agents at ambient conditions in DES without producing any toxic by-products. Furthermore, H2O2 has been used for the destruction of small to large quantities of halogenated hydrocarbons, their homologous / analogues, and similar hazardous chemicals at ambient conditions. H2O2 can be formed by either the electrochemical reduction of oxygen in DES in the presence of water or by dissolving Group 1 (alkali metals) or Group 2 (alkaline earth metals) superoxides, e.g. potassium superoxide, in DES in the presence of water, with / without chemicals used for the enhancement of the solubility of the metal superoxide in the DES, e.g. crown ethers.
Owner:KING SAUD UNIVERSITY
Stabilized Compositions of Volatile Alkylating Agents and Methods of Using Thereof
A composition and method for treatment of cancer. The composition for treating a skin disorder, comprising: a Nitrogen Mustard or an HX salt of the Nitrogen Mustard, wherein the Nitrogen Mustard or the HX salt of the Nitrogen Mustard is in a non-aqueous vehicle or carrier that does not include petrolatum or ethanol, wherein the non-aqueous vehicle or carrier that does not include petrolatum or ethanol does not include petrolatum or ethanol. The method comprises topically applying the composition of a Nitrogen Mustard or a HX salt of the Nitrogen Mustard to the affected skin, wherein the Nitrogen Mustard or the HX salt of the Nitrogen Mustard is in a non-aqueous vehicle or carrier that does not include petrolatum or ethanol, wherein the non-aqueous vehicle or carrier does not include petrolatum or ethanol.
Owner:HELSINN BIREX PHARMA
Methods for treating skin disorders with topical nitrogen mustard compositions
Owner:ACTELION PHARM LTD
Compositions of alkylating agents and methods of treating skin disorders therewith
InactiveUS20130184243A1BiocidePharmaceutical delivery mechanismAlkylating antineoplastic agentNitrogen mustard
Owner:ACTELION PHARM LTD
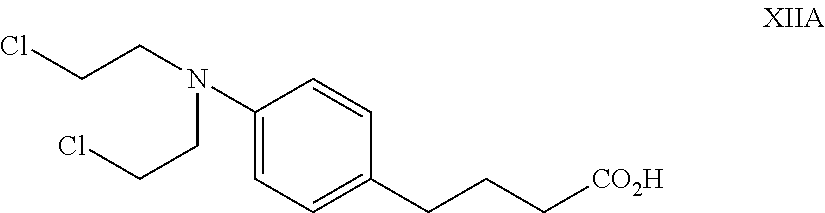
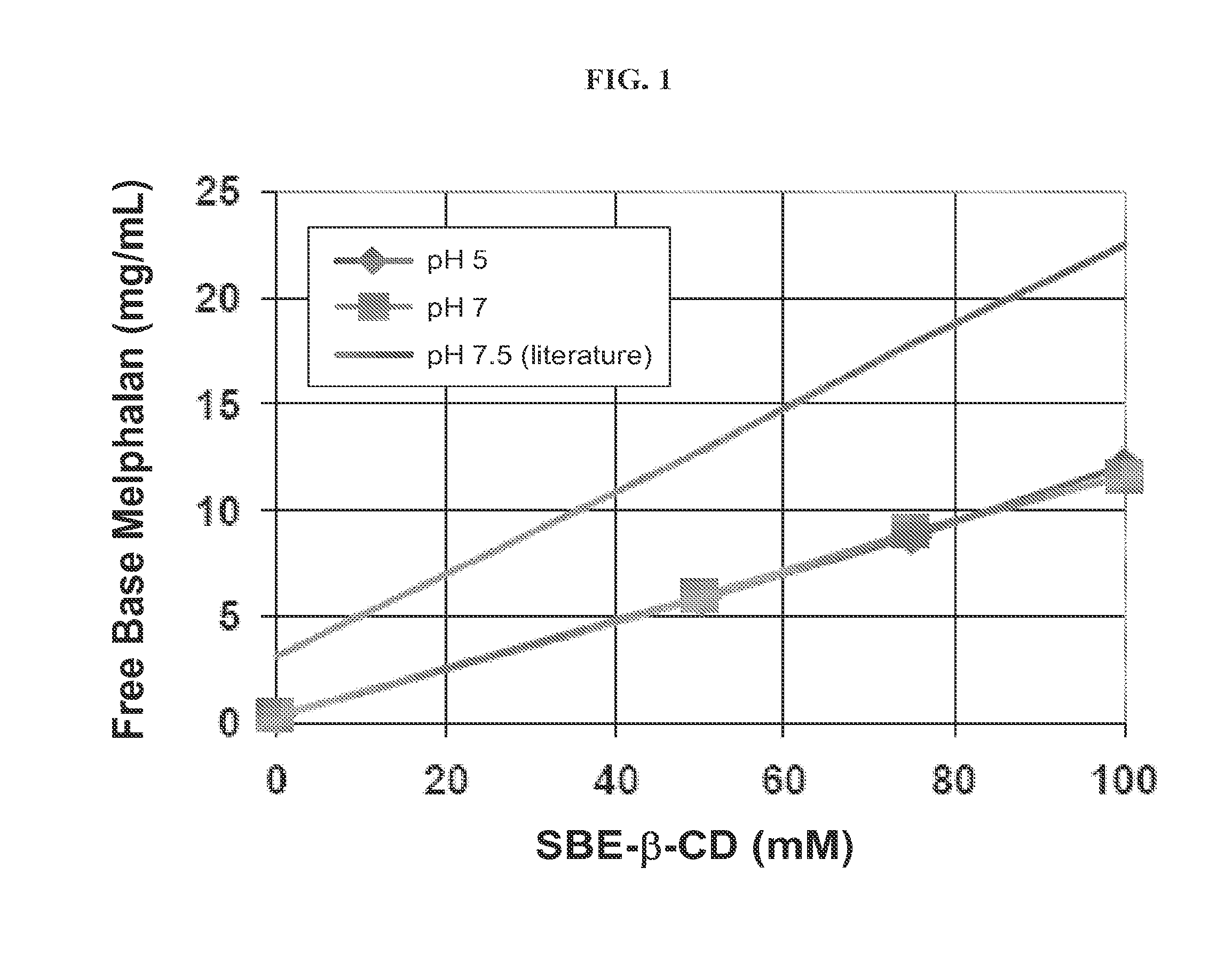
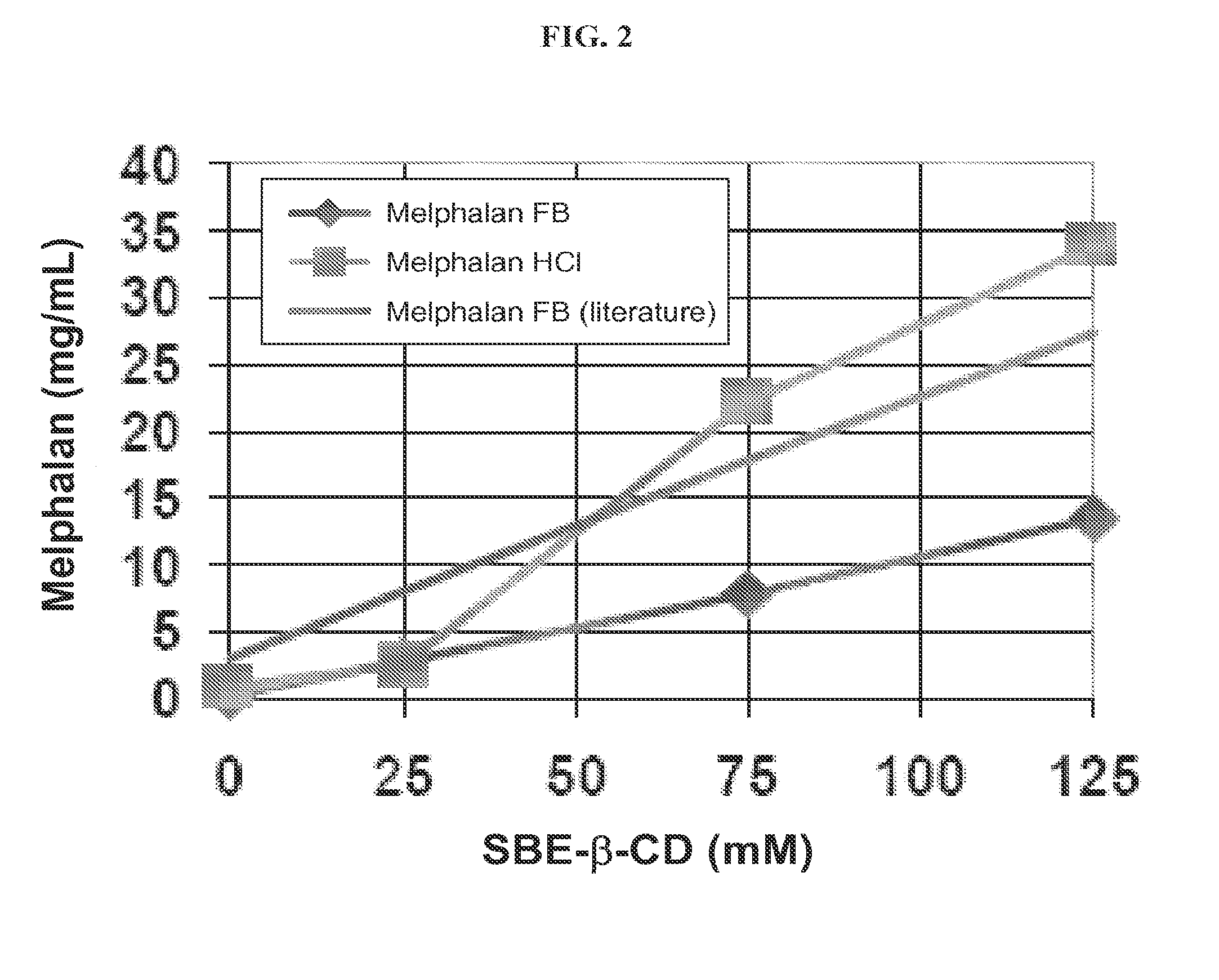
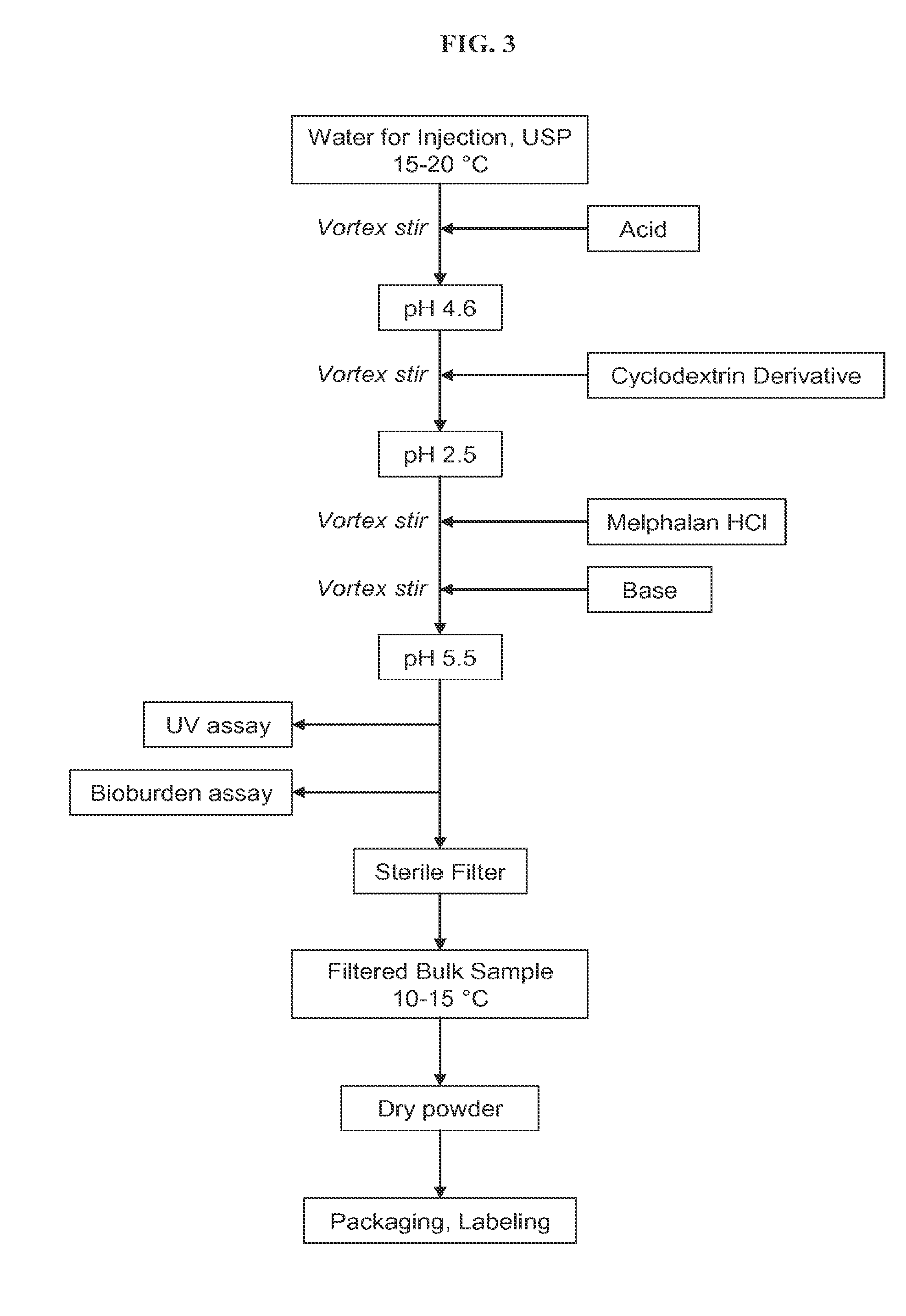
Injectable Nitrogen Mustard Compositions Comprising a Cyclodextrin Derivative and Methods of Making and Using the Same
ActiveUS20140213650A1Minimize toxicologyMinimize side-effect profileBiocideOrganic active ingredientsNitrogen mustardNitrogen
The present disclosure is directed to pharmaceutical compositions comprising a nitrogen mustard and a cyclodextrin derivative, and methods of making and using the same.
Owner:CYDEX PHARMACEUTICALS INC +1
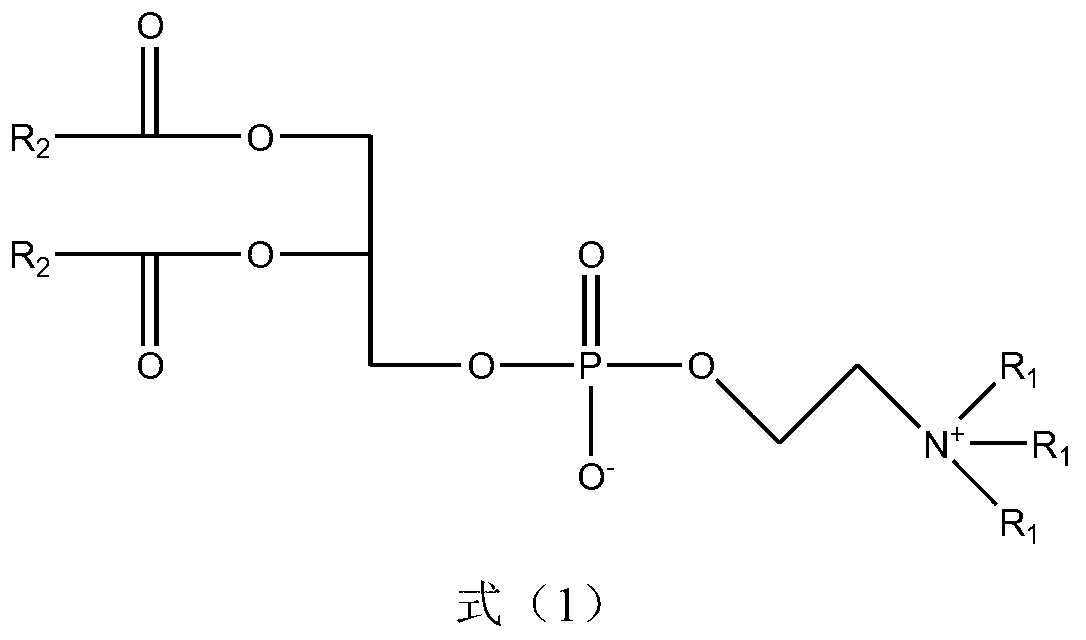
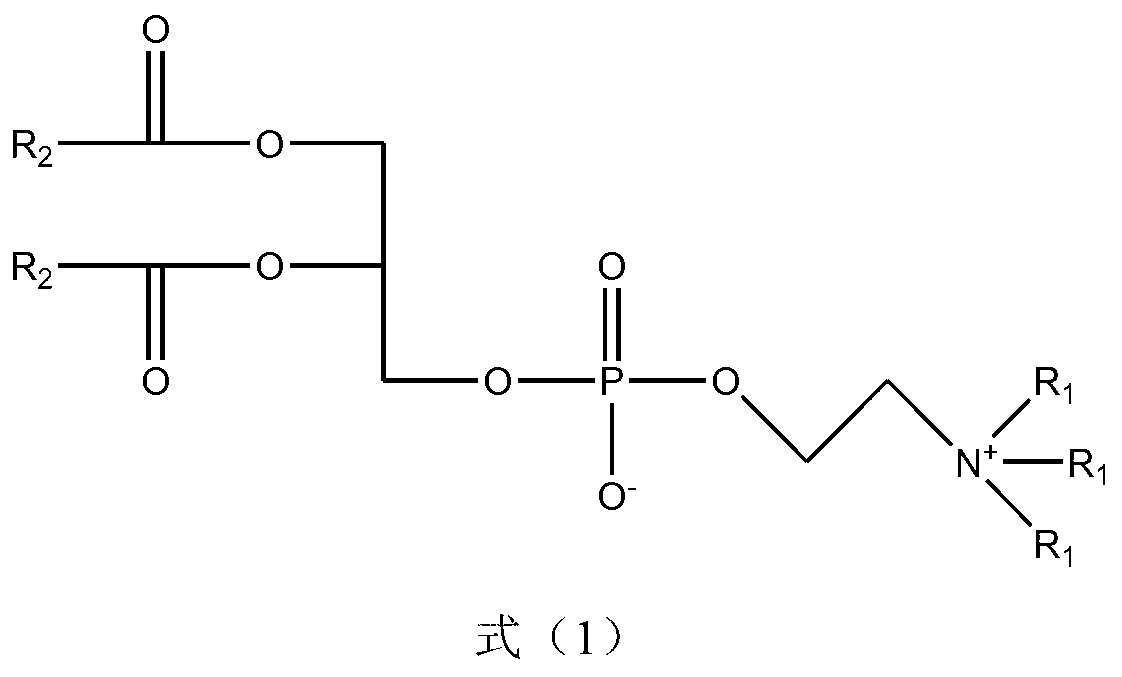
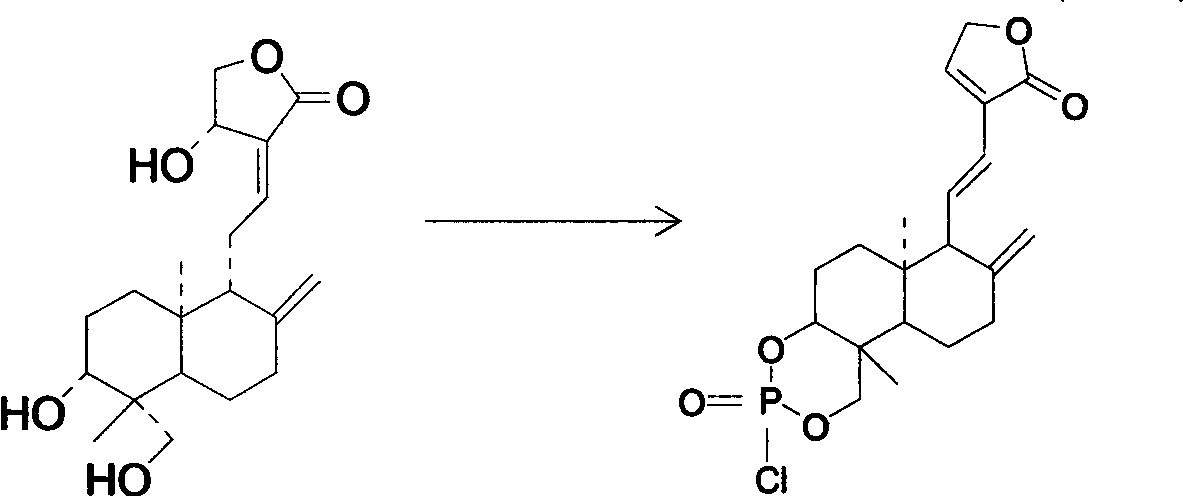
Nitrogen mustard phospholipid compound and preparation method thereof
InactiveCN103193820AHas anti-tumor effectExtended release half-lifeOrganic active ingredientsPhosphorus organic compoundsChemical synthesisPhosphoric acid
The invention discloses a nitrogen mustard phospholipid compound and a preparation method thereof. The compound expressed as the following formula in the specification and salts of pharmaceutically acceptable acids formed by the compounds are obtained through a chemical synthesis method, wherein R1 represents either -H or -CH3, R2 represents (ClCH2CH2)2NR3, R3 represents one or more of alkylene, aryl, aromatic alkylene and the like, and R1 and R2 can be identical or different; and the acids include inorganic acids such as hydrochloric acid, sulfuric acid, hydrobromic acid, phosphoric acid, carbonic acid and the like, as well as organic acids such as formic acid, acetic acid, citric acid, lactic acid, fumaric acid, tartaric acid, gluconic acid and the like. The compounds can be used as a liquid preparation, a solid preparation, a semisolid preparation, a sterilizing preparation and a sterile preparation.
Owner:SOUTHEAST UNIV
Combination Therapy with an Antitumor Alkaloid
InactiveUS20130266666A1Improve anti-tumor activitySuccessful useHeavy metal active ingredientsBiocideAntineoplastic alkaloidDepressant
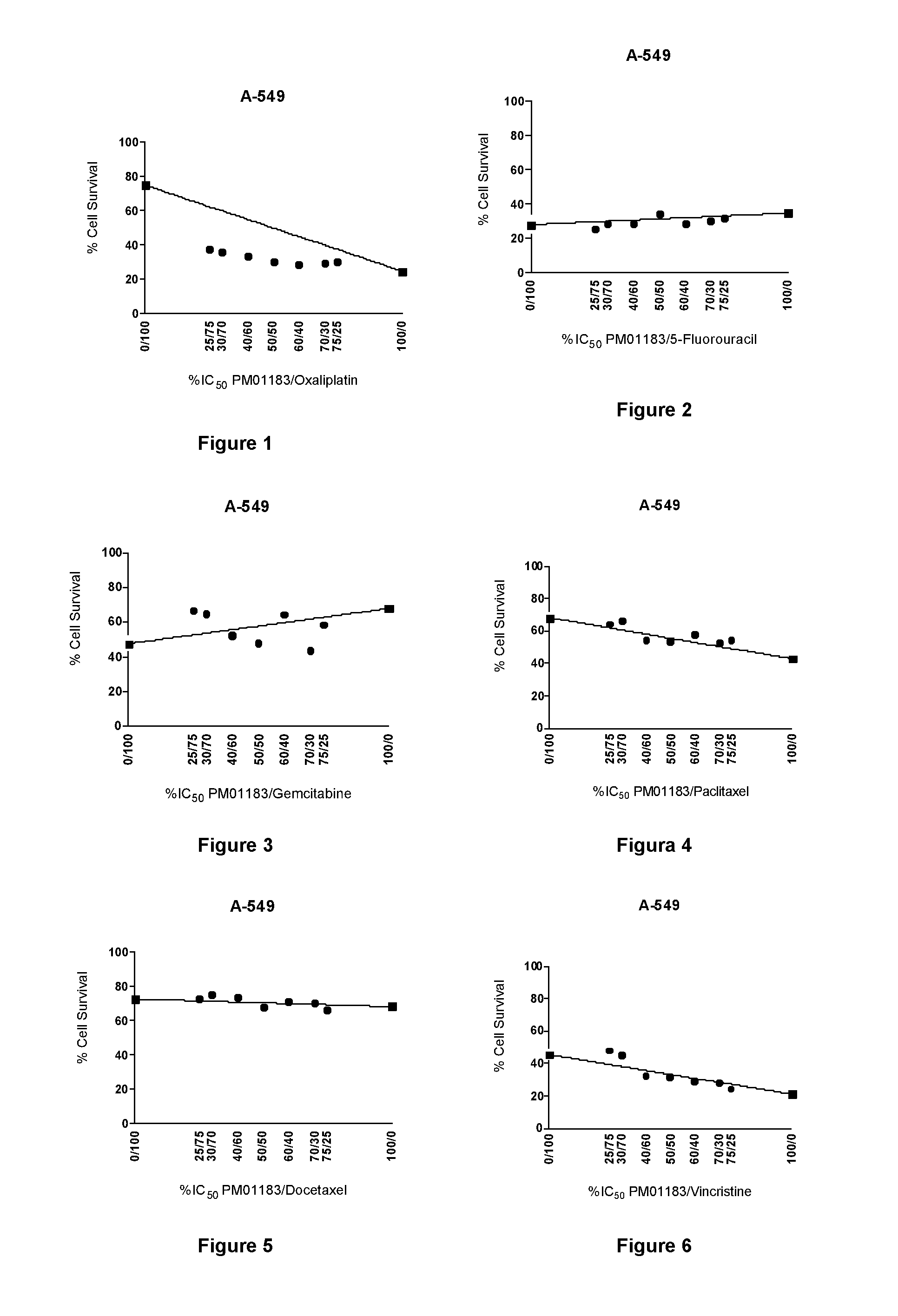
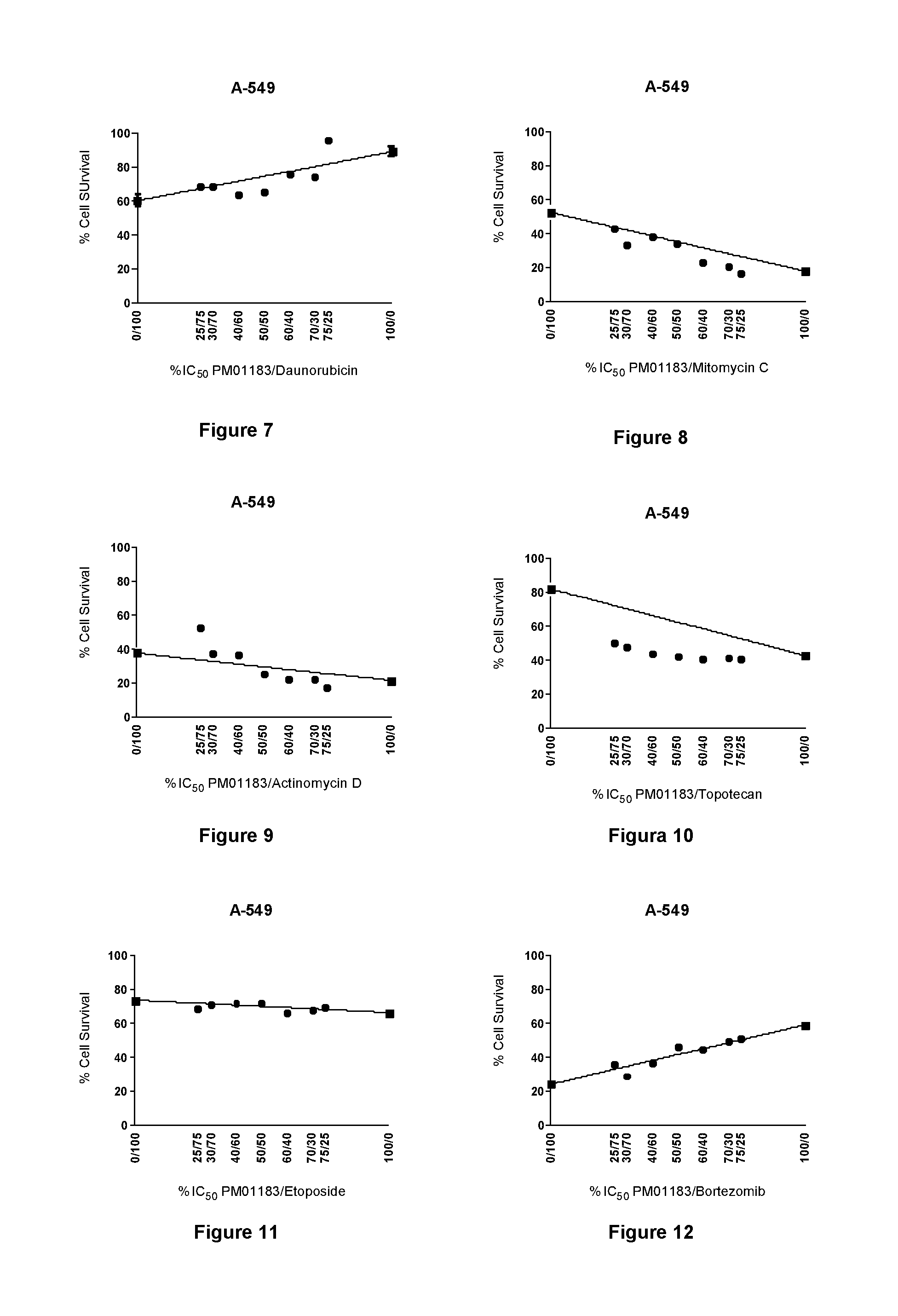
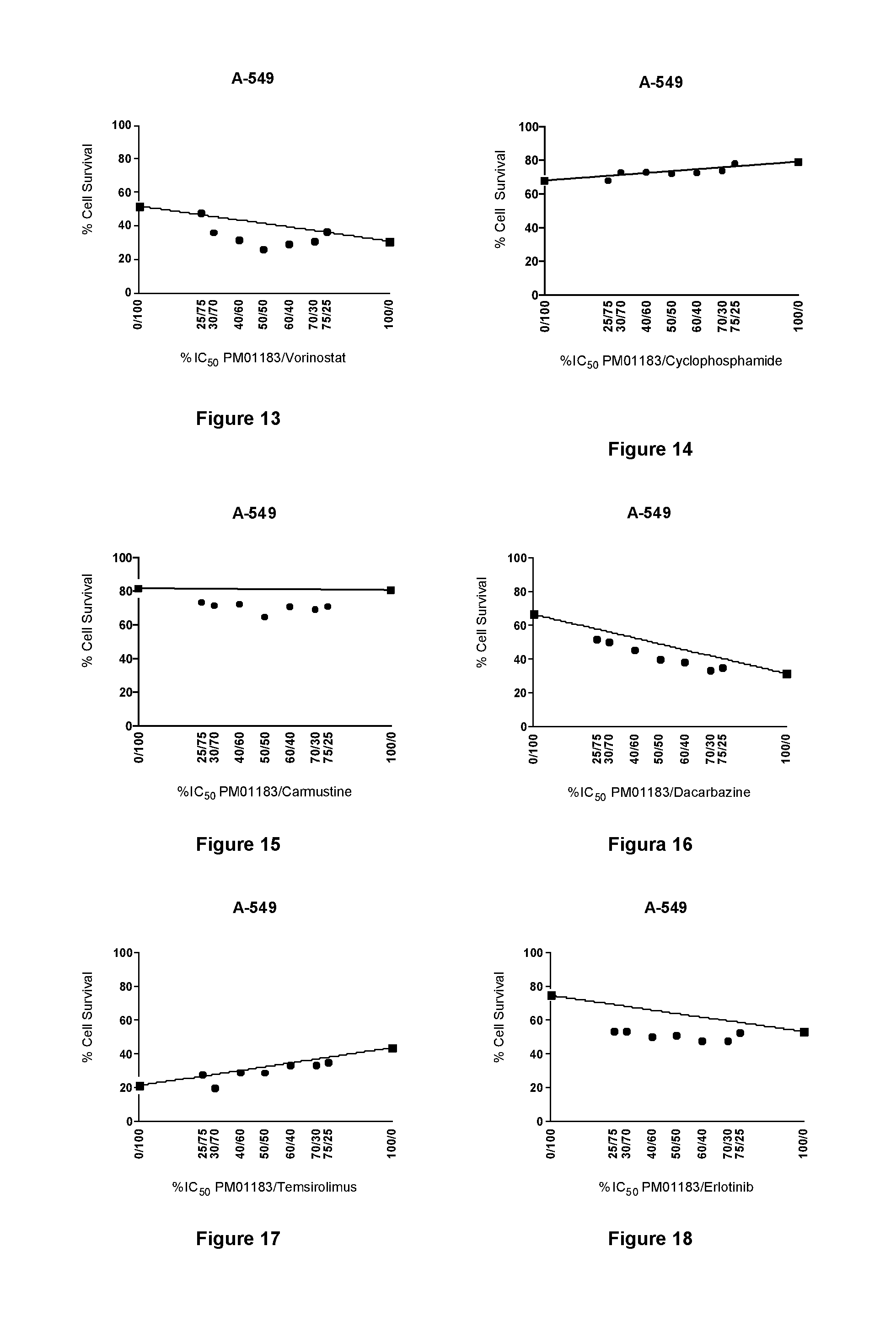
The present invention relates to the combination of PM01183 with several anticancer drugs, in particular other anticancer drugs selected from antitumor platinum coordination complexes, antimetabolites, mitotic inhibitors, anticancer antibiotics, topoisomerase I and / or II inhibitors, proteasome inhibitors, histone deacetylase inhibitors, nitrogen mustard alkylating agents, nitrosourea alkylating agents, nonclassical alkylating agents, estrogen antagonists, androgen antagonists, mTOR inhibitors, tyrosine kinase inhibitors, and other agents selected from aplidine, ET-743, PM02734 and PM00104, and the use of these combinations in the treatment of cancer.
Owner:PHARMA MAR U
Aromatic chlorethazine piperazine quaternary ammonium salt derivatives, and preparation and use thereof
InactiveCN101440073AGood water solubilityGood treatment effectOrganic active ingredientsOrganic chemistryNitrogen mustardHydrogen
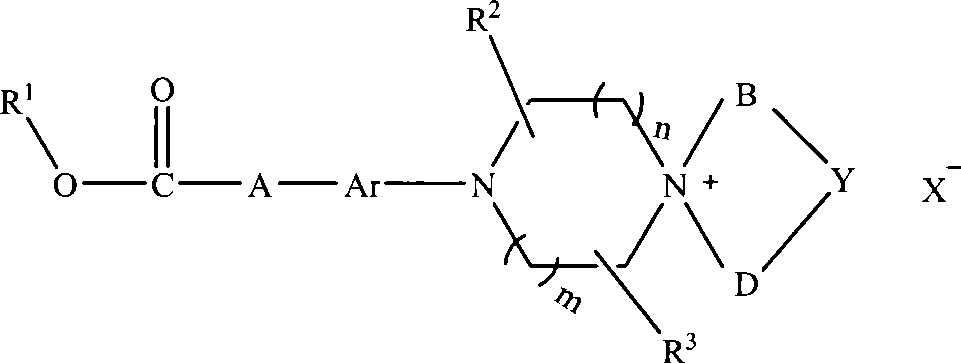
The invention discloses an aryl nitrogen mustard piperazinium derivative with a general formula I or pharmaceutically acceptable salt thereof, wherein R<1> is H, a saturated or unsaturated straight-chain or branched-chain alkyl group, and aryl or substituted aryl, and the alkyl group can be freely substituted by substitutional groups selected from halogens, amino groups, substituted amino groups, hydroxyl groups, cyano groups, nitryl, aryl and substituted aryl; A is saturated or unsaturated straight-chain or branched-chain alkylene, and the alkylene can be substituted by amido; Ar is arylene; R<2> and R<3> is independently hydrogen or methyl; n and m are integers within the range of between 0 and 2, and the n and the m are not zero synchronously; B and D independently represent straight-chain or branched-chain alkyl groups of C1-C3, and straight-chain or branched-chain alkylenes of the C1-C3; Y is -CHR4-, -O-, -S-, -S(O)-, -SO2-, -NR<4>-, and substituted or unsubstituted phenylene, wherein R<4> is H, saturated or unsaturated alkyl groups with 1 to 6 carbon atoms, optionally substituted or unsubstituted aryl, or aromatic heterocyclic-substituted methyl or ethyl; or when the B and the D are alkyl groups, the Y does not exist, that is, the derivative is ring-opened and does not form a spiro ring; and X<-> is pharmaceutically acceptable inorganic or organic anions.
Owner:PEKING UNIV
Analytic application and method of thiol nucleophilic substitution derivatization reagent
ActiveCN104198603AAchieve separationEasy to detectComponent separationDerivatizationNucleophilic substitution
The invention belongs to the field of analytic chemistry, and relates to an application of a thiol derivatization reagent to detection of mustard gas and related compounds of the mustard gas. The invention also relates to a method for detecting the mustard gas and / or related compounds of the mustard gas. Specifically, the detection method comprises the following steps: using the thiol derivatization reagent or adding the thiol derivatization reagent to a treated or untreated sample to be tested. The application and the method are suitable for the detection of the prototypes of the high-reaction activity mustard gas and the related compounds of the mustard gas, have the advantage of overcoming the abuses that the prototypes cannot be detected and the content detection is incorrect as the mustard gas and nitrogen mustard in the complicated samples undergo rapid prototype or alkylation conversion, have simplicity in operation and good reproducibility and stability, and can be applied to the liquid chromatogram-mass spectrum combined detection of the mustard gas with reaction activity and the related compounds in the environment samples and biological samples and applied to the related virus pharmacological researches.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Detoxification of chemical agents
InactiveUS20090112044A1Effect on environmentMethod securitySolid waste disposalLavatory sanitoryNerve agentChemical agent
This invention provides a process for the detoxification of chemical agents including chemical warfare agents such as sulfur mustards, nitrogen mustards, nerve agents of G and V type, lewisite and adamsite by reacting the chemical agents with hydroxyl radicals at a pH greater than 7.0 to detoxify the agents and to render them suitable for disposal. The process can be used on-site and can be easily scaled to fairly large sizes.
Owner:JAIN RAVI +1
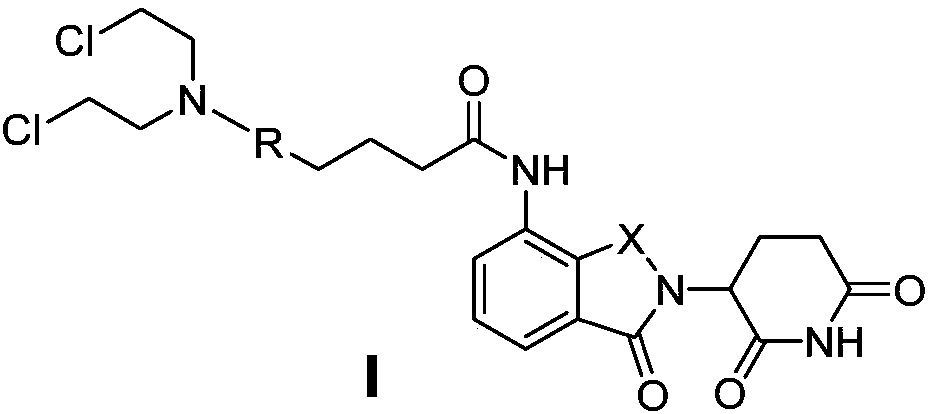
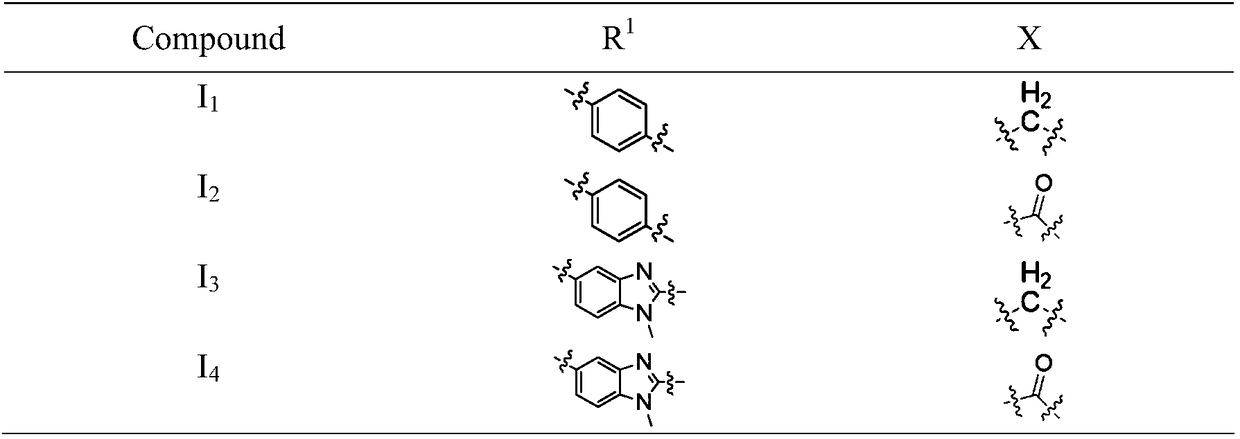
Aryl nitrogen mustard-domide conjugate and preparation method and pharmaceutical application thereof
InactiveCN108409718AImprove stabilityPromotes treatment toleranceOrganic active ingredientsOrganic chemistryDrug conjugationHATU
The invention discloses an aryl nitrogen mustard-domide conjugate and a preparation method and pharmaceutical application thereof. Compound (1) is subjected to reaction with compound (2) in the presence of polypeptide condensing agents EDCI and DMAP, or HATU and DIPEA to obtain compound I that is used to prepare antitumor drugs. The two drugs of different target types are combined herein to form aprodrug; multi-action synergic antitumor effect is achieved; antitumor activity and tolerance are improved; drug resistance of tumors is decreased. The domide drugs are linked via amido bonds, so that chances for amino groups on domide benzene rings to be degraded by in-vivo enzymes can be slimmed, and in-vivo bioavailability of the drugs is improved.
Owner:NANTONG UNIVERSITY
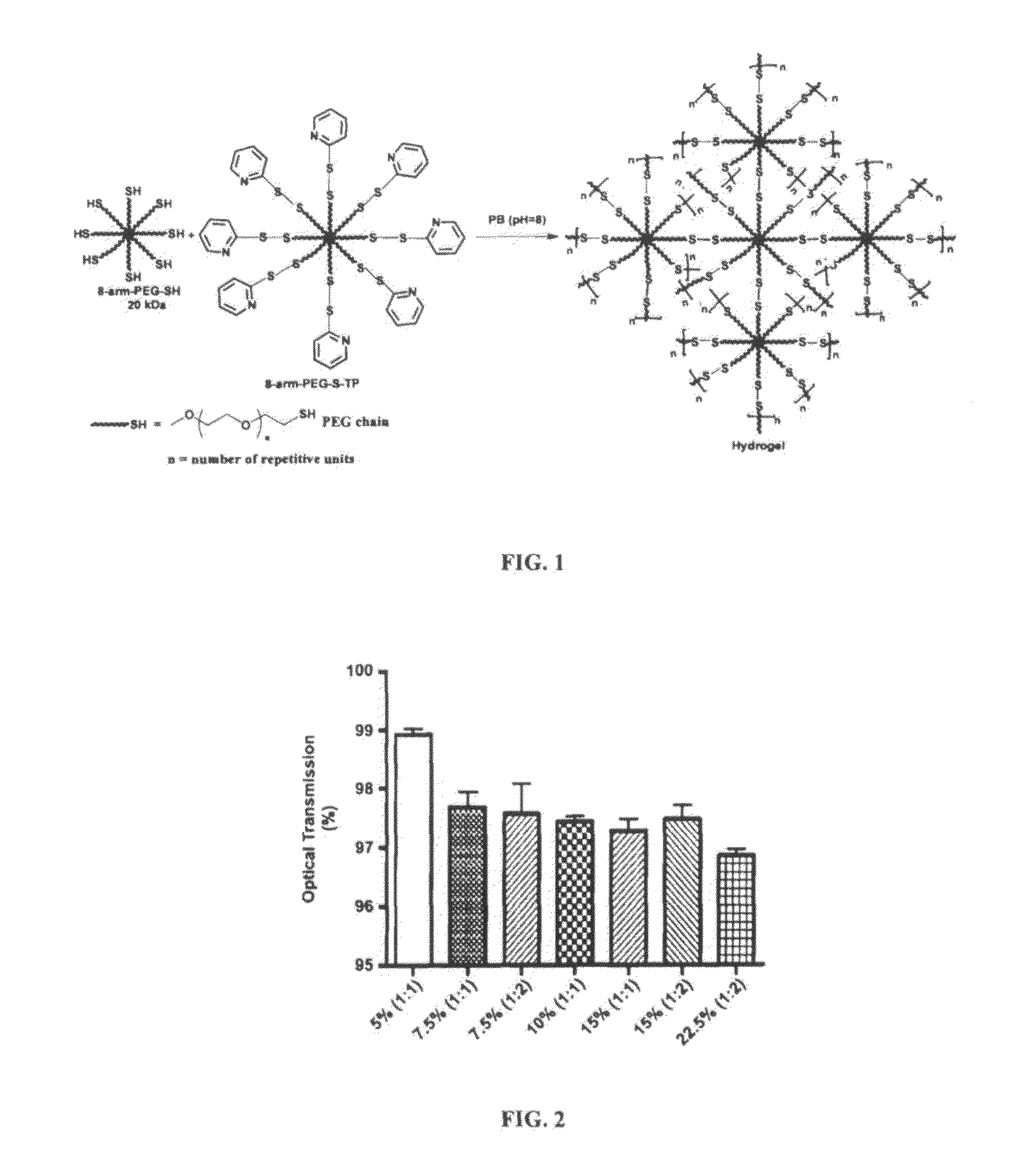
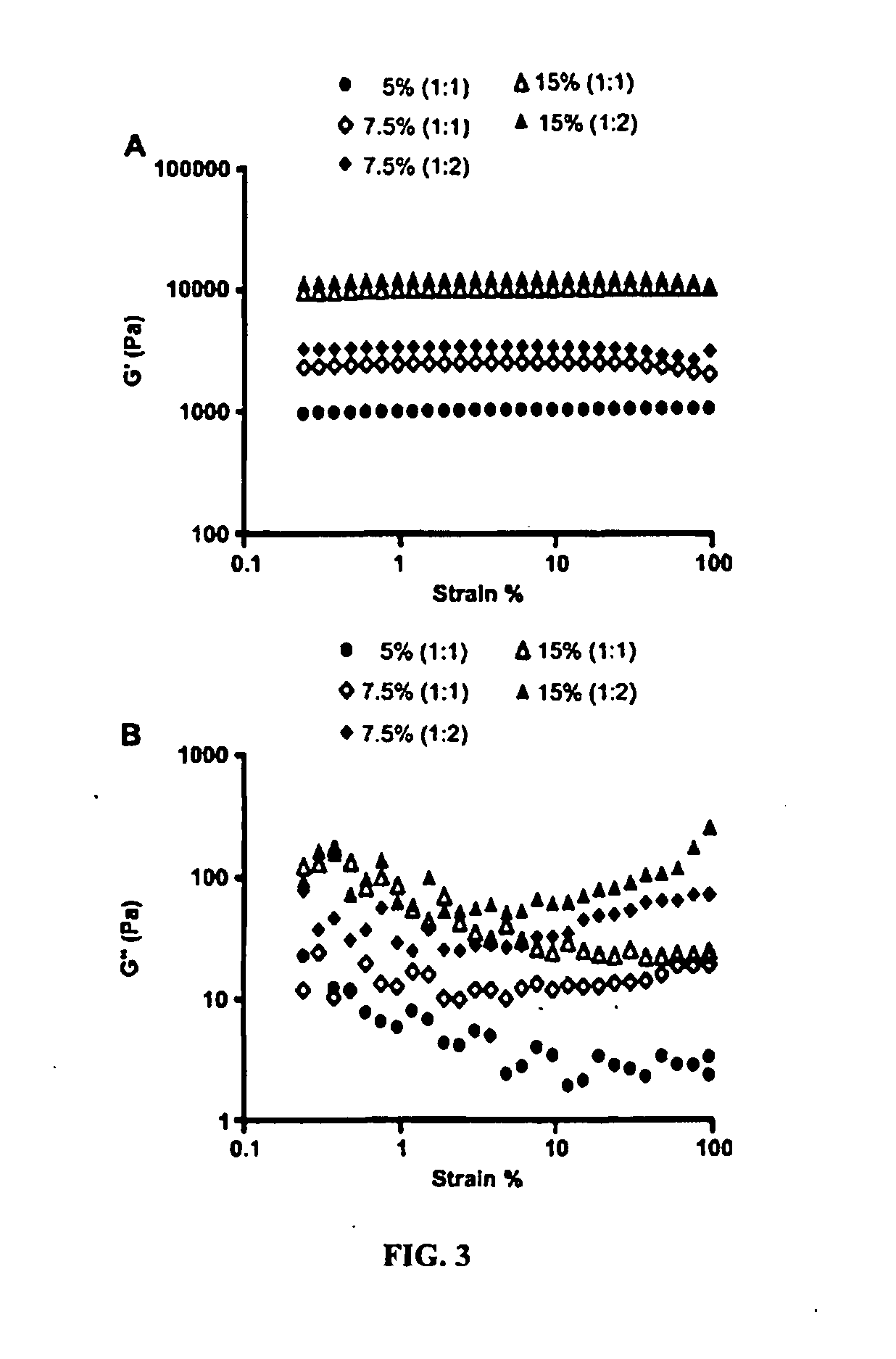
Hydrogel formulation for dermal and ocular delivery
Formulations of cross-linkable polymers, capable of forming non-toxic and biocompatible hydrogels in situ, containing at least one of doxycycline or minocycline. Methods of using the hydrogels for treating the skin or ocular tissues of mammals exposed to vesicant compounds such as sulfur mustard (SM), nitrogen mustard (NM) or half mustard (2-chloroethyl ethyl sulfide (CEES)) are also disclosed.
Owner:RUTGERS THE STATE UNIV
Targeted indoleamine-2,3-dioxygenase 1 nitrogen mustard inhibitor as well as preparation method and application thereof
InactiveCN107987031AStrong inhibitory activityBroad-spectrum antitumor activity in vitroOrganic active ingredientsOrganic chemistryAbnormal tissue growthDisease
The invention discloses a targeted indoleamine-2,3-dioxygenase 1 nitrogen mustard inhibitor as well as a preparation method and application thereof. A structure of the inhibitor is shown as a generalformula I, wherein definition of R is as shown in the claims and specification. Pharmacological experiments prove that the compound disclosed by the invention has excellent IDO1 inhibitory activity and has broad-spectrum in-vitro antitumor activity. In-vivo experiments prove that the compound disclosed by the invention can achieve effects of down-regulating the in-vivo IDO1 activity and obviouslydelaying tumor growth, and can be applied to preparing IDO1-mediated medicines for tumor diseases with pathological features in a tryptophan metabolism pathway. The structural formula is as shown in the specification.
Owner:EAST CHINA UNIV OF SCI & TECH
Preparation method and application of tumor-targeted diagnosis and treatment combined single-molecule lead compound
InactiveCN109836432AGood curative effectOrganic chemistryEnergy modified materialsChemical treatmentChemical structure
The invention discloses a preparation method and application of a tumor-targeted diagnosis and treatment combined single-molecule lead compound, and the lead compound is prepared from a terpene-mothernucleus, aryl nitrogen mustardand N, N-diethyl tertiary nitrogen, wherein the structure is shown in (I) as shown in the specification, and mitochondria targeting, fluorescence imaging diagnosis, photodynamic therapy and chemotherapy are integrated in the same molecular structure. The lead compound has the prominent characteristics of being simple in structure and small in molecular weight, determined in chemical structure, easy to prepare, purify and further modify, low in the toxicity to mice, and the like, and the basic requirements of clinical medication are met. An in-vitro experiment proves that tertiary amine electropositivity and amitochondrial electronegativity found by near infrared fluorescence imaging of the lead compound structure remarkably target tumor cells and tumor tissues, photodynamic therapy effects can be generated under near-infrared radiation, and the tertiary amine electropositivity and amitochondrial electronegativity and the nitrogen mustard in the lead compound structure can generate chemical treatment and synergistic treatment, so that the growth of tumors can be remarkably inhibited.
Owner:ZUNYI MEDICAL UNIVERSITY
Methods for treating skin disorders with topical nitrogen mustard compositions
Owner:ACTELION PHARM LTD
Combination therapy with an anti - CD19 antibody and a nitrogen mustard
ActiveCN103732252AOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsNitrogen mustardChronic lymphocytic leukemia
The present disclosure describes a pharmaceutical combination of an anti-CD19 antibody and a nitrogen mustard for the treatment of non-Hodgkin's lymphoma, chronic lymphocytic leukemia and / or acute lymphoblastic leukemia.
Owner:MORFOZIS AG
Application of nitrogen mustard based piperlongumine compound in medicine
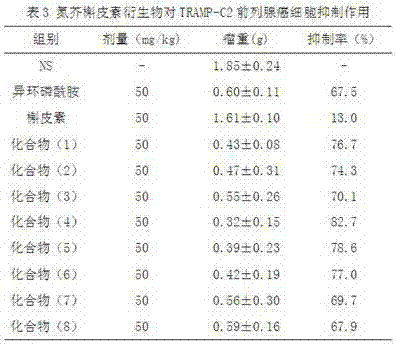
The invention provides application of a nitrogen mustard based piperlongumine compound in medicine. On the basis of the research on the nitrogen mustard based piperlongumine compound for inhibiting the activity of malignant cells, it proves that the nitrogen mustard based piperlongumine compound has the good anti-tumor activity, and a new choice is provided for preparing anti-tumor medicine.
Owner:广东永纯医药集团有限责任公司
Benzoic acid nitrogen mustard fragment-containing compound and preparation method and use thereof
ActiveCN109134487AGood antitumor activityOrganic active ingredientsOrganic chemistryBenzoic acidNitrogen mustard
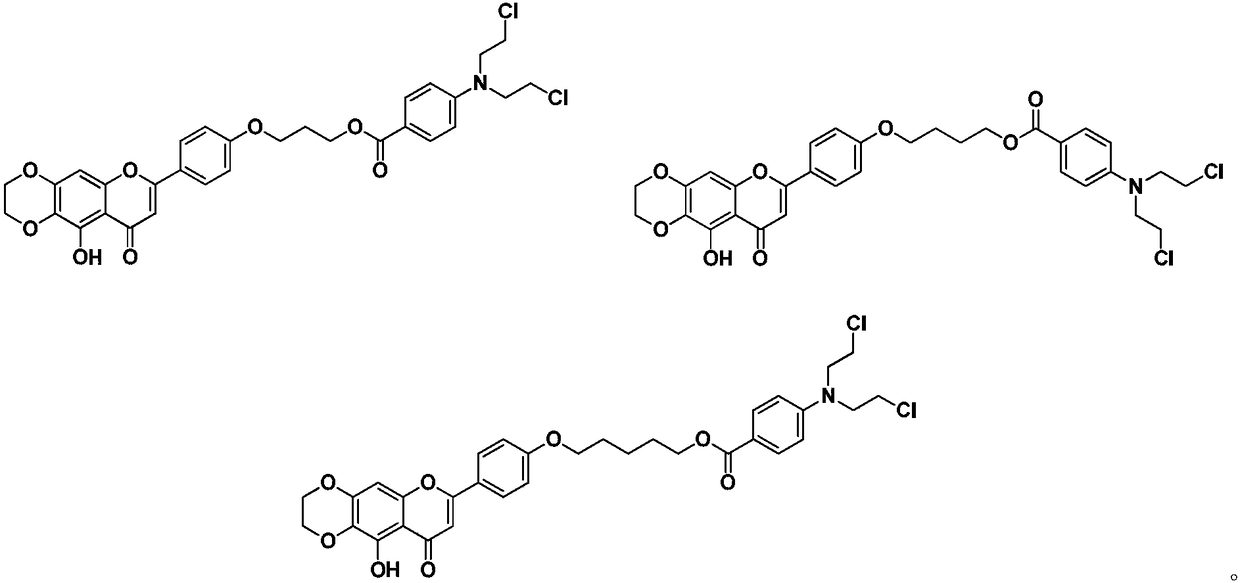
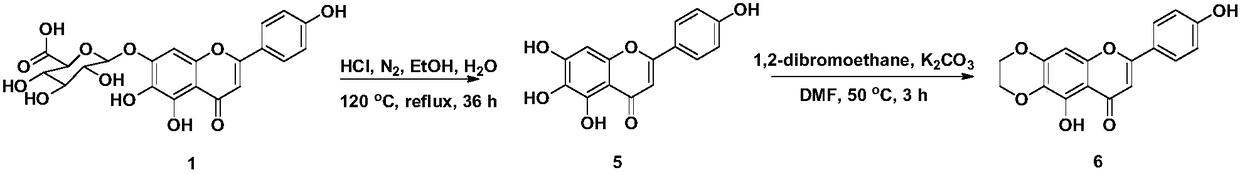
The invention relates to the field of natural medicine and medicine chemistry, and particularly relates to a compound containing benzoic acid nitrogen mustard fragment and a preparation process and use, in particular to a compound which introduces a benzoic acid nitrogen mustard fragment at a 4'-OH through a linking group after derivatization of the 6, 7-position of scutellarin, and a pharmaceutically acceptable salt thereof. The method also relates to the preparation process and antitumor activity of these compounds. The method of the benzoic acid nitrogen mustard fragment-containing compoundis shown in the general formula I, wherein m, n are as described in the claims and the specification. The formula is shown in the description.
Owner:SHENYANG PHARMA UNIVERSITY
Nitrogen mustard quercetin derivative, and preparation method and application thereof
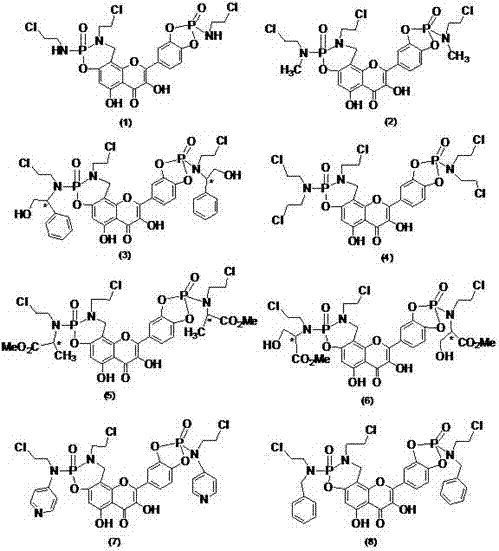
ActiveCN106854223AHas anticancer activityGroup 5/15 element organic compoundsAntineoplastic agentsNitrogen mustardActive component
The invention relates to a nitrogen mustard quercetin derivative comprising a stereisomer or a tautomer, a preparation method of the nitrogen mustard quercetin derivative, a medicinal composition taking the derivative as an active component, and application of the medicine to cancer treatment. The preparation method of the derivative comprises the following steps: preparing Mannich alkali of quercetin from the quercetin, formaldehyde and chlorethamin, and performing reaction on the Mannich alkali and substituted phosphoramidic dichloride to prepare the nitrogen mustard quercetin derivative. The nitrogen mustard quercetin derivative has an anti-tumor effect.
Owner:西安天一生物技术股份有限公司
Application of dendrobium polysaccharide in preparation of drugs to prevent or restore reproductive injury after chemotherapy
InactiveCN109985060AReduce and prevent reproductive damageWide variety of sourcesOrganic active ingredientsSexual disorderGynecologySide effect
The invention discloses an application of dendrobium polysaccharide in preparation of drugs to prevent or restore reproductive injury after chemotherapy. The dendrobium polysaccharide applied to prevent and treat male reproductive injury caused by radiotherapy and chemotherapy has obvious therapeutic effect and small side effects, and can effectively reduce the damage caused by nitrogen mustard anticancer drugs to the reproductive system, which not only makes the sperm quantity of mice rise significantly, but also improves the sperm quality, and effectively improves the antioxidant activity ofmouse testicular tissue, and in mouse testicular tissue, the level of SOD, GPx, GSH and CAT is obviously increased while the level of MDA is obviously decreased. The dendrobium polysaccharide with wide source of raw materials is simple in extraction and purification method, high in sugar content and suitable for large-scale production and popularization and has a good prospect in the preparationand / or treatment of reproductive injury after chemotherapy.
Owner:JINAN UNIVERSITY
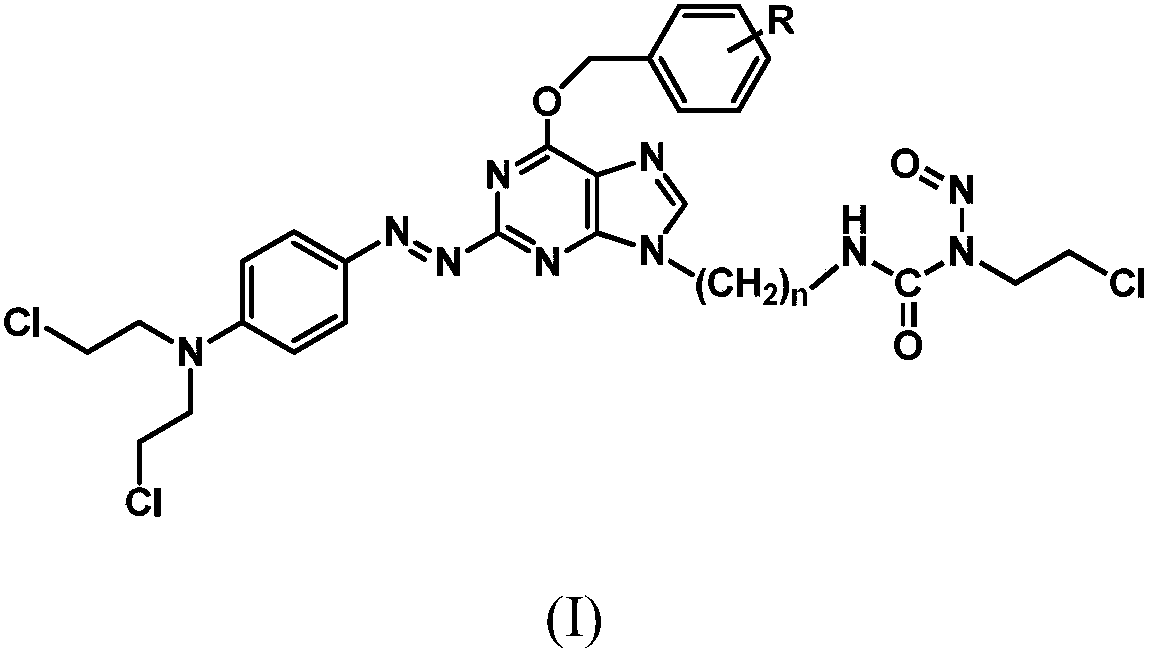
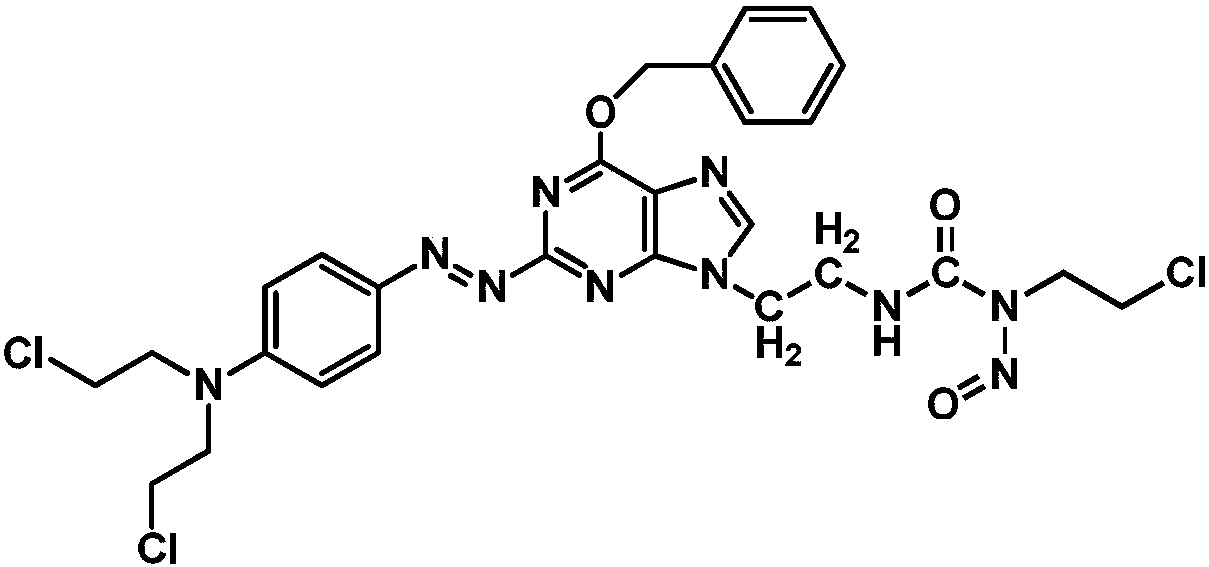
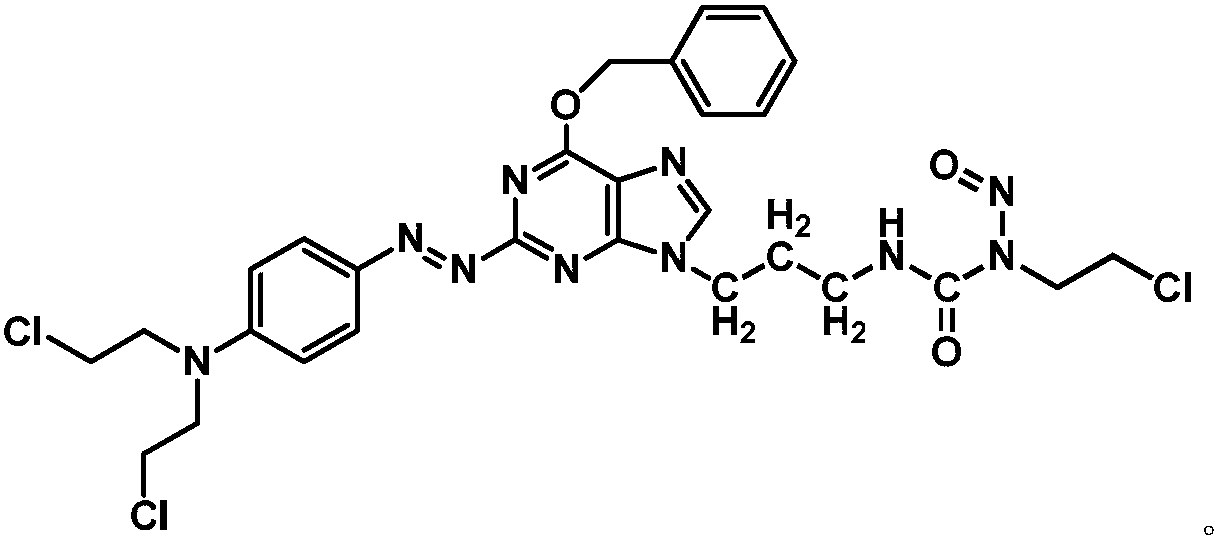
Azo aryl nitrogen mustard-chloroethylnitrosourea coupled compound, and preparation method and application thereof
ActiveCN107903267AImprove targetingGrowth inhibitionOrganic chemistryAntineoplastic agentsAlkylating antineoplastic agentNitroso
The invention relates to a compound has a structure represented by formula (I), or a pharmaceutically acceptable salt thereof. In the compound, the azo group is a low oxygen-activated pharmacophore, anitrogen-nitrogen double bond in the azo group cleaves and releases aromatic nitrogen mustard and an O6-BG analogue under tumor hypoxic conditions, and the aromatic nitrogen mustard and an O6-BG analogue act as an alkylating agent and an AGT inhibitor in a hypoxic area in a targeting manner to make tumor cells sensitive to the alkylating agent; and the CENUs pharmacophores in the compound can bedecompose to generate chloroethyl carbocations in order to cause cross-linking between DNA strands, so the growth of tumor cells is inhibited.
Owner:BEIJING UNIV OF TECH
Process for the destruction of sulfur and nitrogen mustards, lewisite, and their homologous/analogues in deep eutectic solvents
The subject invention provides a potentially economically viable process for the destruction of small to large quantities of sulfur and nitrogen mustards and lewisite, their homologous / analogues, and similar chemical warfare agents at ambient conditions without producing any toxic by-products. The process uses the superoxide ion that is either electrochemically generated by the reduction of oxygen in deep eutectic solvents or chemically by dissolving Group 1 (alkali metals) or Group 2 (alkaline earth metals) superoxides, e.g. potassium superoxide, in deep eutectic solvents.
Owner:KING SAUD UNIVERSITY
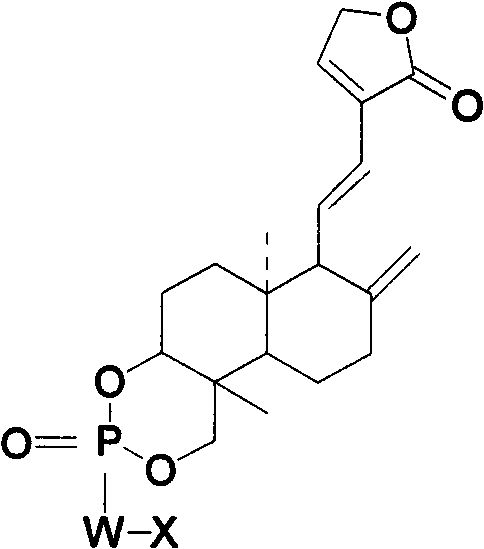
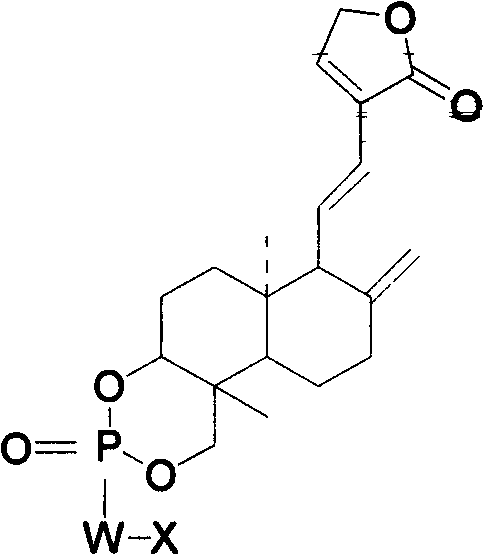
Andrographolide phosphoric acid derivatives and preparation method thereof
InactiveCN102399245AGood against viral infectionGood anti-inflammatory and antibacterialGroup 5/15 element organic compoundsNitrogen mustardPhosphate
The invention provides andrographolide phosphoric acid derivatives and a preparation method thereof. The andrographolide phosphoric acid derivatives has a chemical structural formula shown in the specification, wherein W is N or O, X is C1-C8 linear-chain group, heterocyclic radical, nitrogen mustard group, phenyl, benzyl or furfuryl. According to the preparation method, andrographolide phosphorus oxychloride, andrographolide phosphoric acid, andrographolide phosphorus diethylamide and andrographolide benzyl phosphate are respectively prepared from andrographolide serving as a raw material. The derivatives prepared by the method have excellent effects of resisting virus infection, diminishing inflammation and resisting bacteria, resisting cancers and cardiovascular diseases, stimulating immunity.
Owner:JIANGXI HERBFINE HI TECH
Hydrogel formulation for dermal and ocular delivery
ActiveUS20160271151A1Tetracycline active ingredientsPeroxide active ingredientsNitrogen mustardFoaming agent
Formulations of cross-linkable polymers, capable of forming non-toxic and biocompatible hydrogels in situ, containing at least one of doxycycline or minocycline. Methods of using the hydrogels for treating the skin or ocular tissues of mammals exposed to vesicant compounds such as sulfur mustard (SM), nitrogen mustard (NM) or half mustard (2-chloroethyl ethyl sulfide (CEES)) are also disclosed.
Owner:RUTGERS THE STATE UNIV
D-galactose/benzaldehyde nitrogen mustard/N-(2-hydroxypropyl) methacrylamide copolymer as well as preparation and application thereof
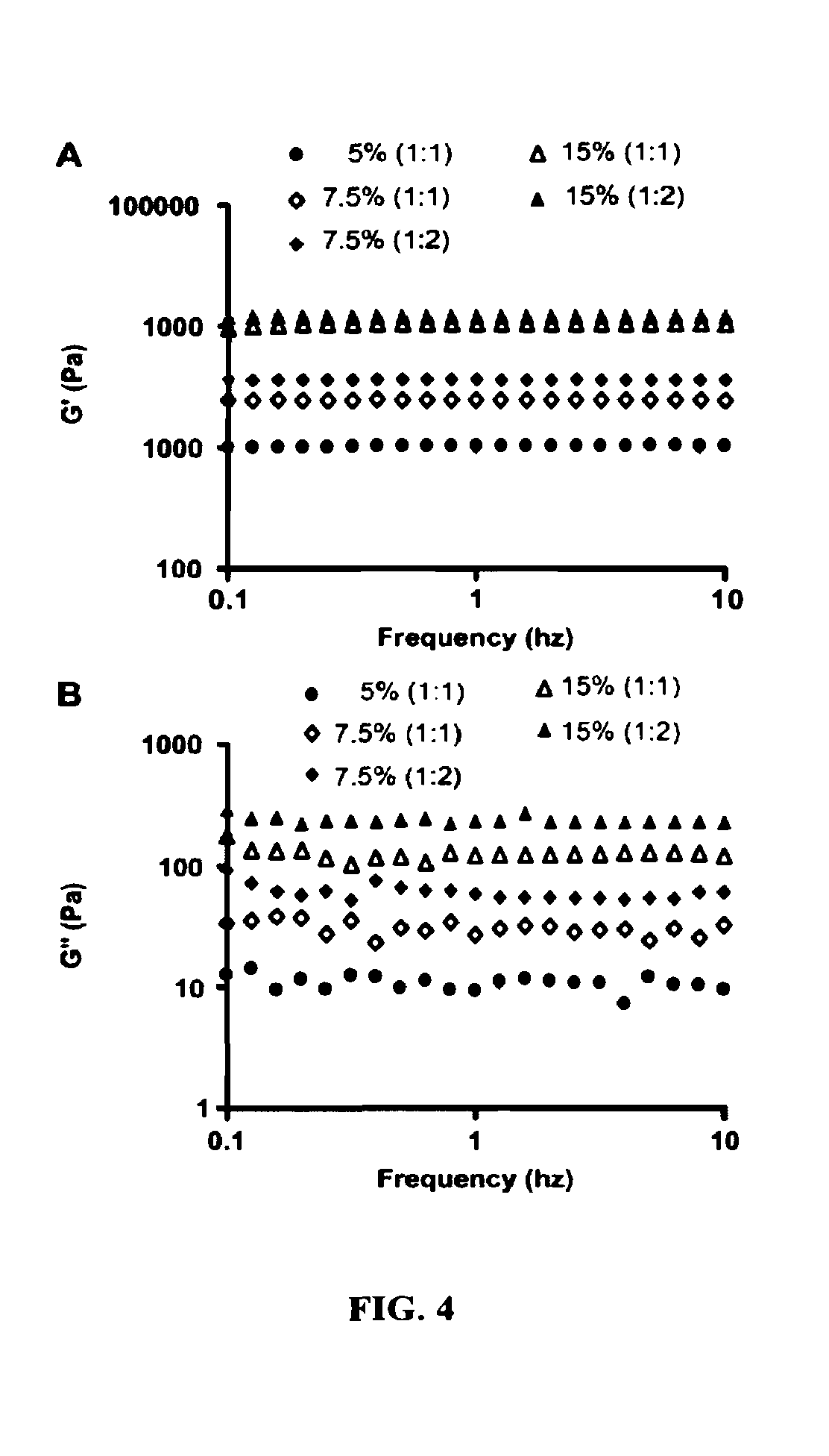
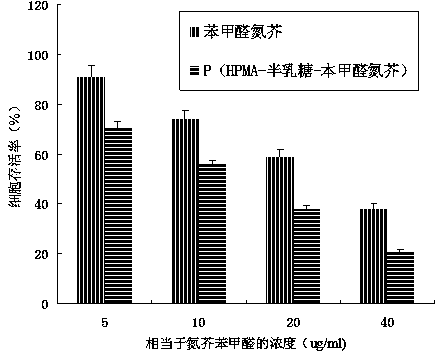
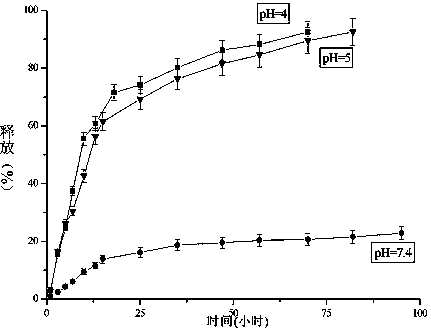
InactiveCN103965398AEnhanced inhibitory effectExtended stayOrganic active ingredientsPharmaceutical non-active ingredientsBenzaldehydeBiocompatibility Testing
The invention provides a D-galactose / benzaldehyde nitrogen mustard / N-(2-hydroxypropyl) methacrylamide copolymer, and belongs to the field of high polymer chemistry and application. The D-galactose / benzaldehyde nitrogen mustard / N-(2-hydroxypropyl) methacrylamide copolymer is a macromolecular copolymer which has good biocompatibility and is formed in the way that D-galactose and benzaldehyde nitrogen mustard are linked to N-(2-hydroxypropyl) methacrylamide (HPMA) through covalent bonds. An experiment proves that the D-galactose / benzaldehyde nitrogen mustard / N-(2-hydroxypropyl) methacrylamide copolymer has the function of intelligently releasing drugs in a targeting manner, and has excellent inhibiting effect on a liver cancer HepG2 tumor cell; besides the macromolecular copolymer is an effective drug delivery system; therefore, the copolymer has excellent prospect as being used as an inhibitor for the HepG2 tumor cell and the like to be applied to the preparation of anti-tumor drugs.
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com