Aryl nitrogen mustard-domide conjugate and preparation method and pharmaceutical application thereof
A kind of aromatic nitrogen mustard and conjugate technology, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
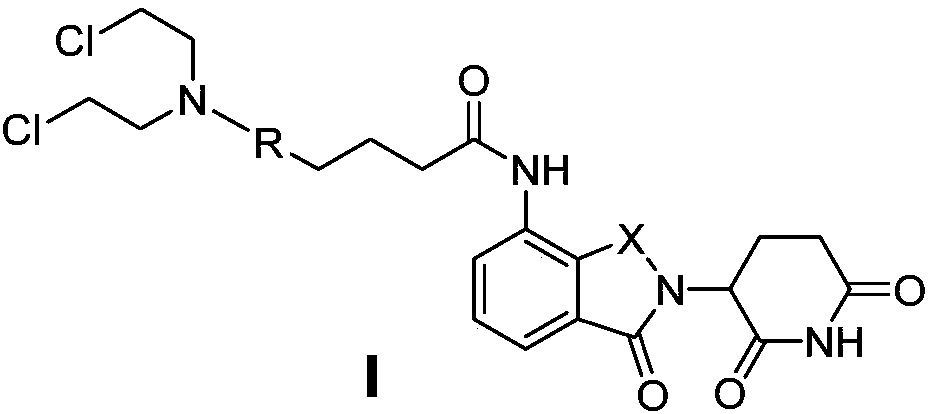
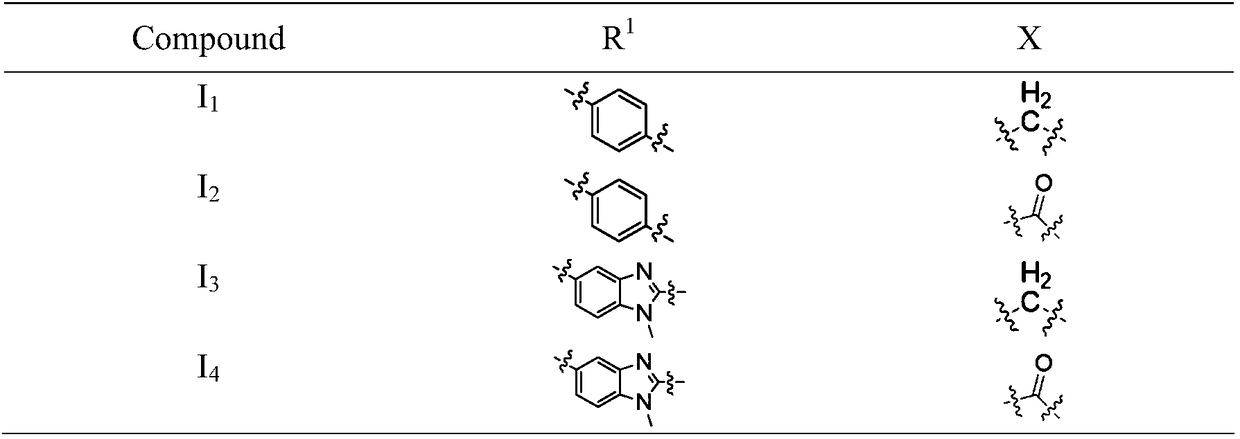
[0047] 4-(4-(bis(2-chloroethyl)amino)phenyl)-N-(2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindoline- 4-yl) butanamide (I 1 ) preparation
[0048] Lenalidomide (1a, 259mg, 1.0mmol) was dissolved in 10ml of anhydrous CH 2 Cl 2 , then sequentially added chlorambucil (2a, 333mg, 1.1mmol), EDCI (384mg, 2.0mmol) and DMAP (25.9mg, 10%), and heated to 40°C for reflux reaction after all of them were dissolved. After the reaction was detected by TLC, the solvent was distilled off under reduced pressure, and the crude product was purified by column chromatography (mobile phase ethyl acetate:petroleum ether=2:1-4:1) to obtain 338mg of white solid, yield 62%.ESI-MS (m / z):545[M+H] + . 1 H NMR (DMSO-d 6 ,400MHz): δ11.04(s,1H,NH),9.79(s,1H,NH),7.81(d,1H,J=8.0Hz,Ar-H),7.52(d,1H,J=8.0Hz ,Ar-H),7.49(m,1H,Ar-H),7.06(d,2H,J=8.0Hz,Ar-H),6.68(d,2H,J=8.0Hz,Ar-H),5.15 (m,1H,CH),4.37(m,2H,CH 2 ),3.70(m,8H,CH 2 ),2.59(m,2H,CH 2 ),2.51(m,2H,CH 2 ),2.36(m,2H,CH 2 ),2.03(m,2H,CH 2 ),1.87(m,2H,CH 2 ...
Embodiment 2
[0050] 4-(5-(bis(2-chloroethyl)amino)-1-methyl-1H-benzo[d]imidazol-2-yl)-N-(2-(2,6-dioxopiper Pyridin-3-yl)-1-oxoisoindoline-4-yl)butanamide (I 2 ) preparation
[0051] Lenalidomide (1a, 259mg, 1.0mmol) was dissolved in 10ml of anhydrous CH 2 Cl 2 , and then sequentially added bendamustine hydrochloride hydrate (2b, 454mg, 1.1mmol), HATU (2.0mmol), DMAP (130mg, 50%) and triethylamine (404mg, 4.0mmol), until it was completely dissolved After heating to 40 ° C reflux reaction. After the reaction was detected by TLC, the solvent was distilled off under reduced pressure, and the crude product was purified by column chromatography (mobile phase methanol: dichloromethane = 1:30-1:15) to obtain 305 mg of a light yellow solid with a yield of 51%. ESI-MS (m / z):599[M+H] + . 1 H NMR (DMSO-d 6 ,400MHz):δ11.03(s,1H,NH),9.87(s,1H,NH),7.81(m,1H,Ar-H),7.50(m,1H,Ar-H),7.34(d, 1H,J=8.0Hz,Ar-H),6.93(m,1H,Ar-H),6.79(m,1H,Ar-H),5.15(m,1H,CH),4.37(m,2H,CH 2 ),3.71(s,8H,CH 2 ),3.69(s,3H,CH...
Embodiment 3
[0053] 4-(4-(bis(2-chloroethyl)amino)phenyl)-N-(2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindol Indoline-4-yl)butanamide (I 3 ) preparation
[0054] Referring to compound (I) in Example 1 1 ) preparation method, pomalidomide (1b, 273mg, 1.0mmol) was substituted for lenalidomide and chlorambucil to react to obtain light yellow solid 301mg, yield 54%, ESI-MS (m / z): 559[M+H] + . 1 HNMR (DMSO-d 6 ,400MHz):δ11.15(s,1H,NH),9.69(s,1H,NH),8.45(d,1H,J=8.0Hz,Ar-H),7.82(t,1H,J=8.0Hz ,Ar-H),7.61(d,1H,J=8.0Hz,Ar-H),7.05(d,2H,J=8.0Hz,Ar-H),6.67(d,2H,J=8.0Hz,Ar -H),5.15(m,1H,CH),3.69(s,8H,CH 2 ),2.90(m,2H,CH 2 ),2.62(m,2H,CH 2 ),2.46(t,2H,J=8.0Hz,CH 2 ),2.05(m,2H,CH 2 ),1.89(m,2H,CH 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com