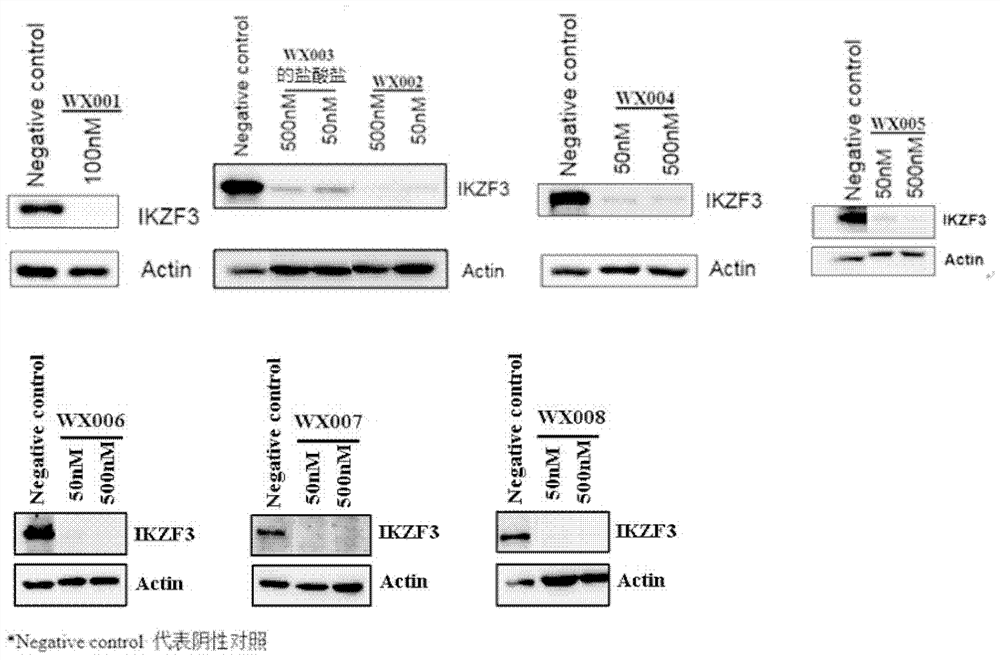
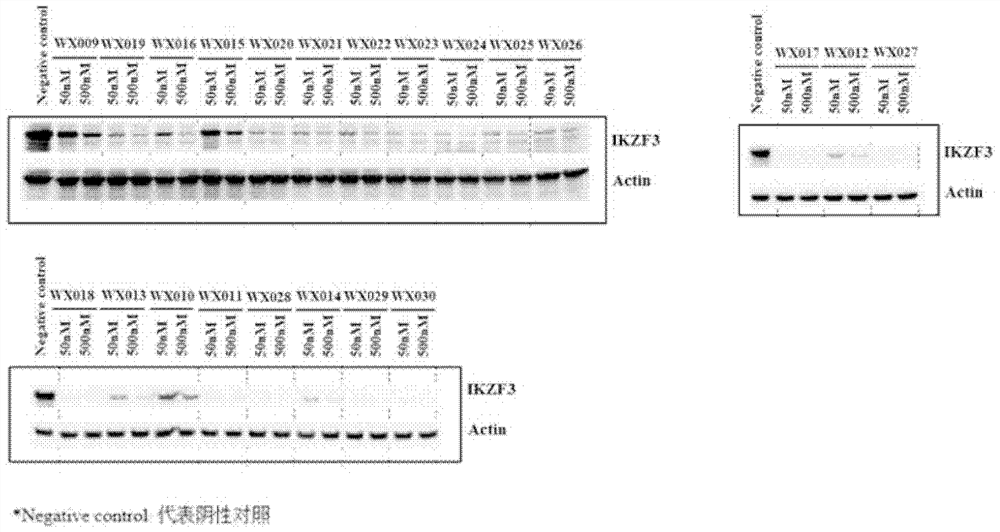
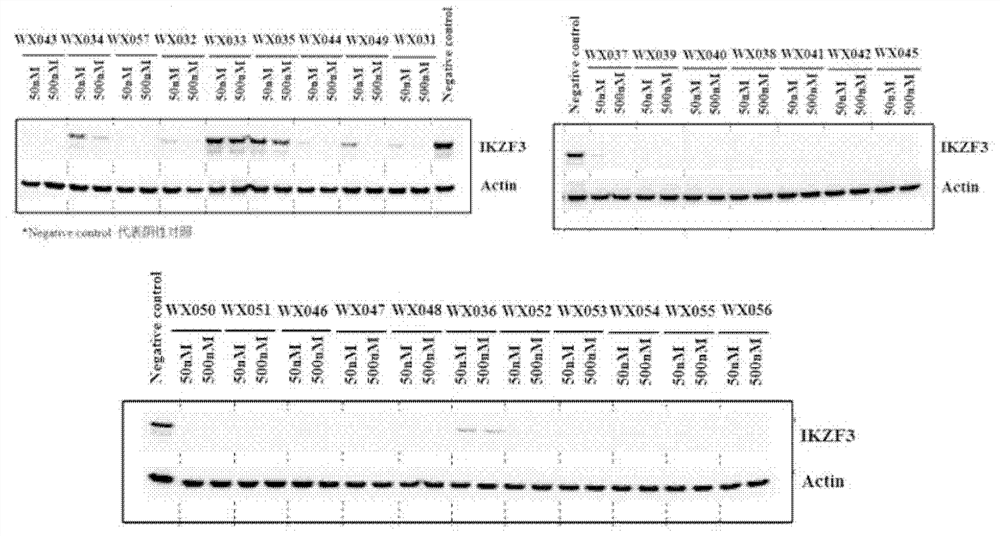
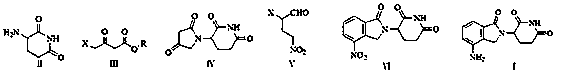
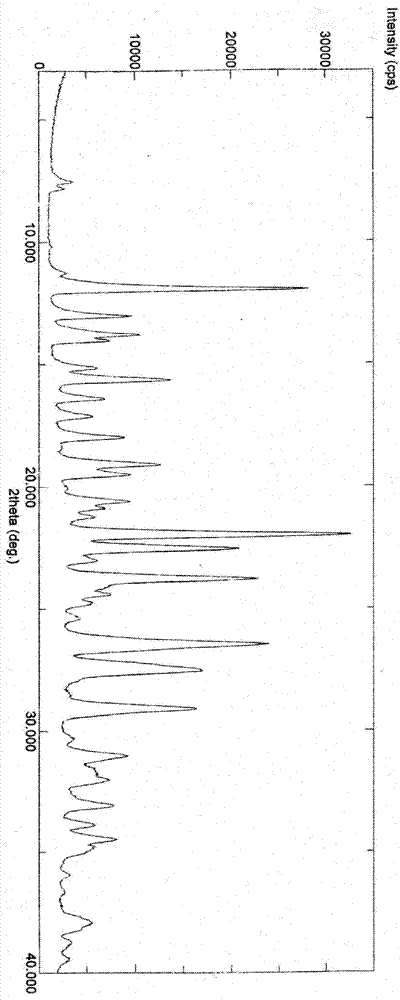
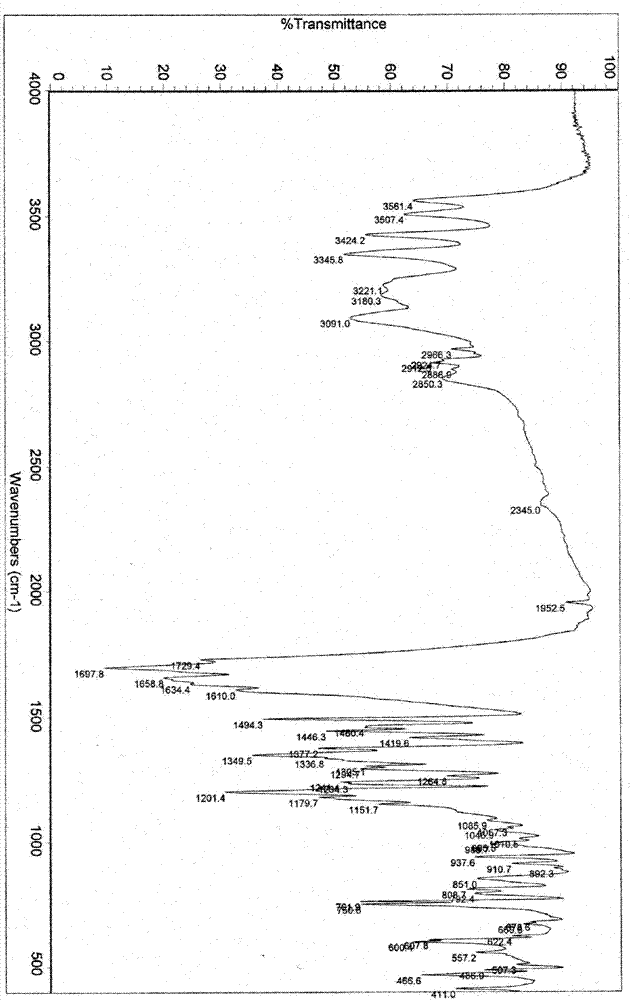
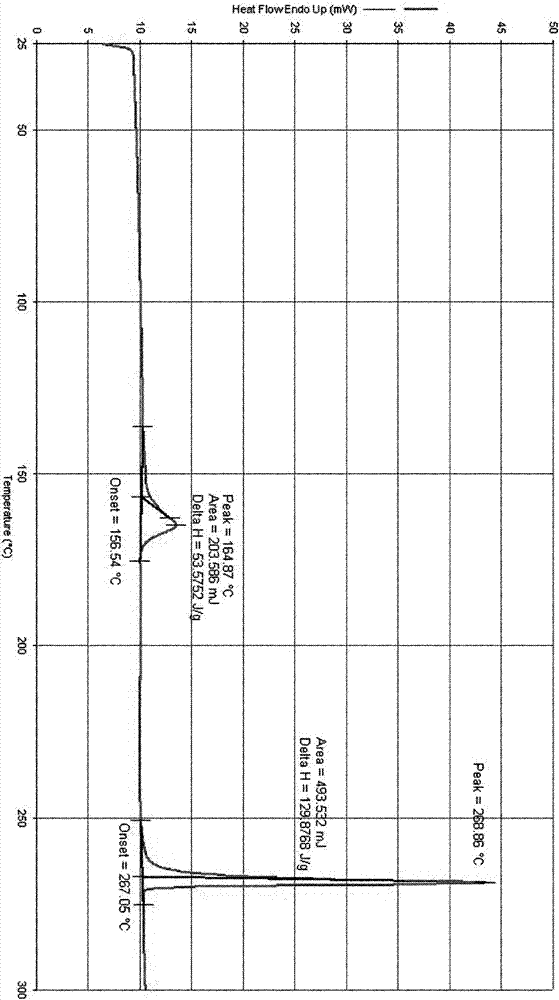
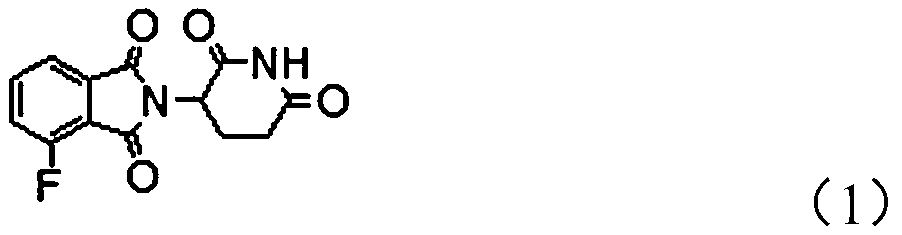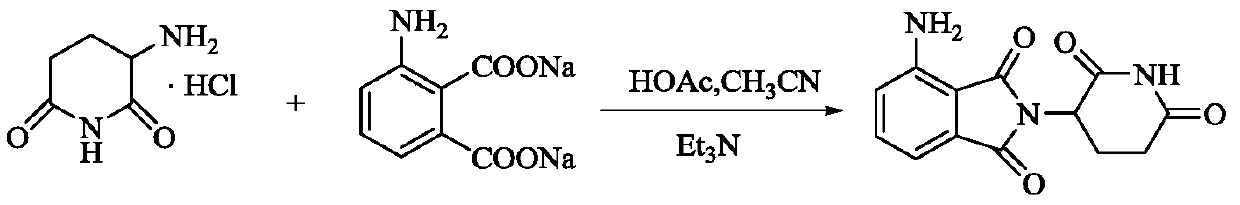Patents
Literature
52 results about "Piperidinedione" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Piperidinediones are a derivatives of piperidine with two ketone functional groups. There are six isomers, each of which has a molecular weight of 113.115 and a formula of C₅H₇NO₂. Piperidinediones form the core structure of a variety of pharmaceutical drugs.
Solid forms of 3-(5-amino-2-methyl-4-oxo-4h-quinazolin-3-yl)-piperidine-2,6-dione, and their pharmaceutical compositions and uses
Solid forms comprising 3-(5-amino-2-methyl-4-oxo-4H-quinazolin-3-yl)-piperidine-2,6-dione, compositions comprising the solid forms, methods of making the solid forms and methods of their uses are disclosed
Owner:CELGENE CORP
Process
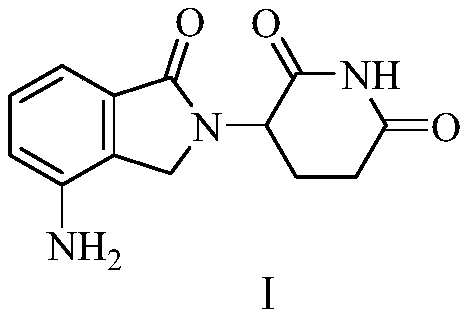
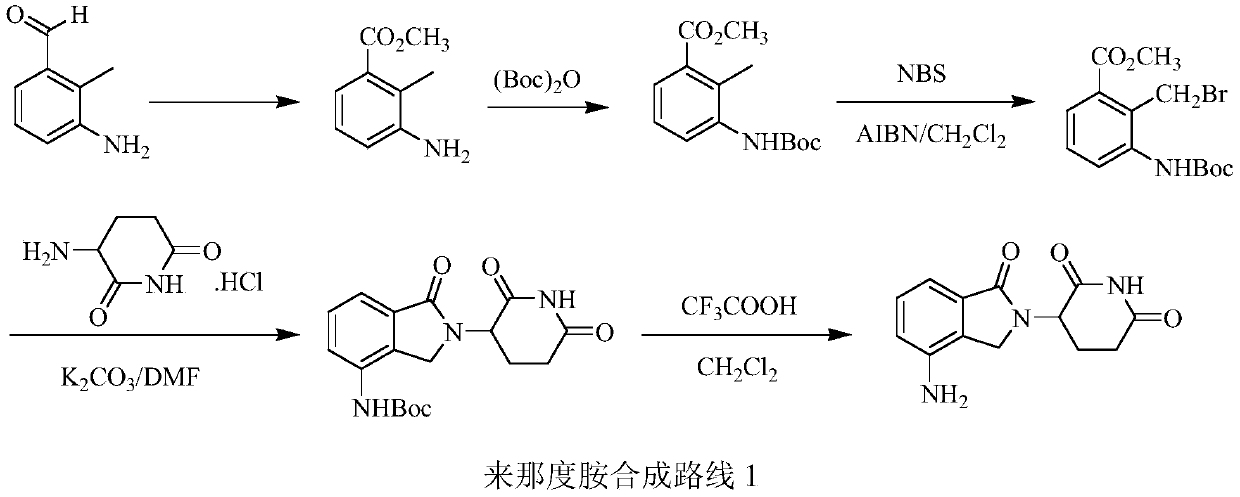
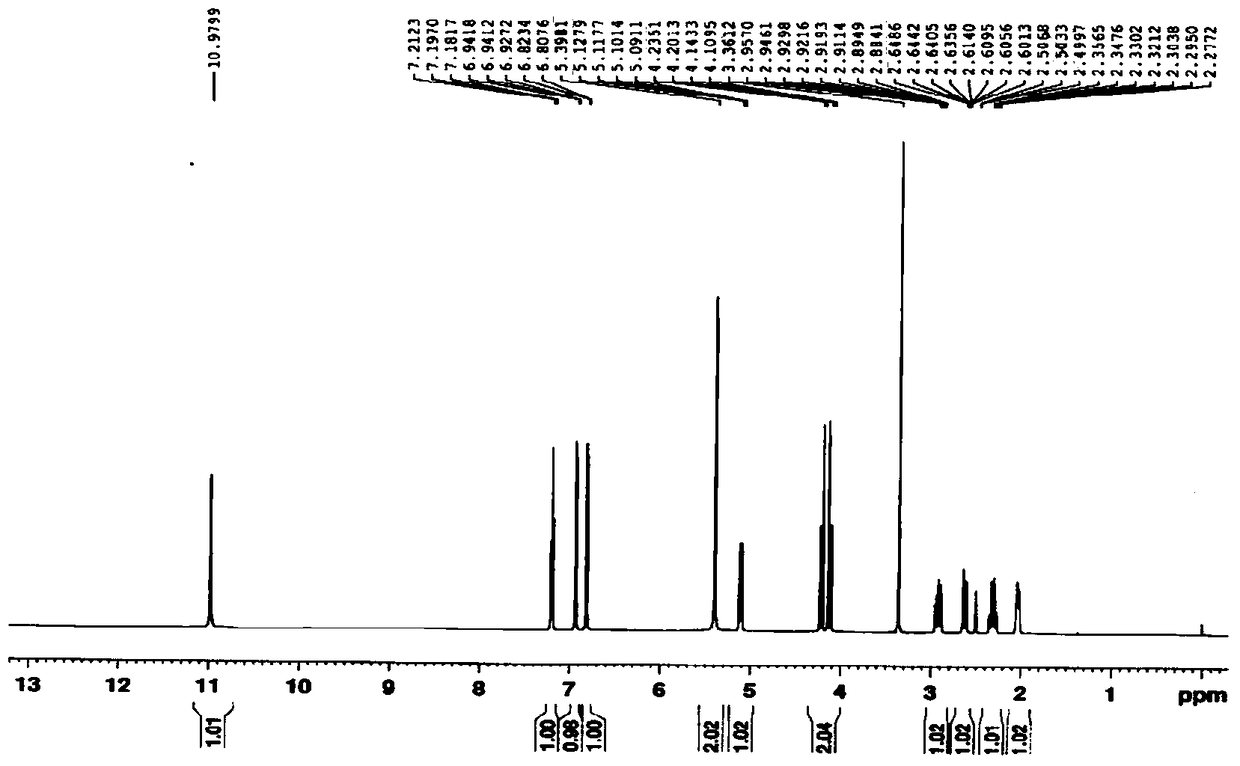
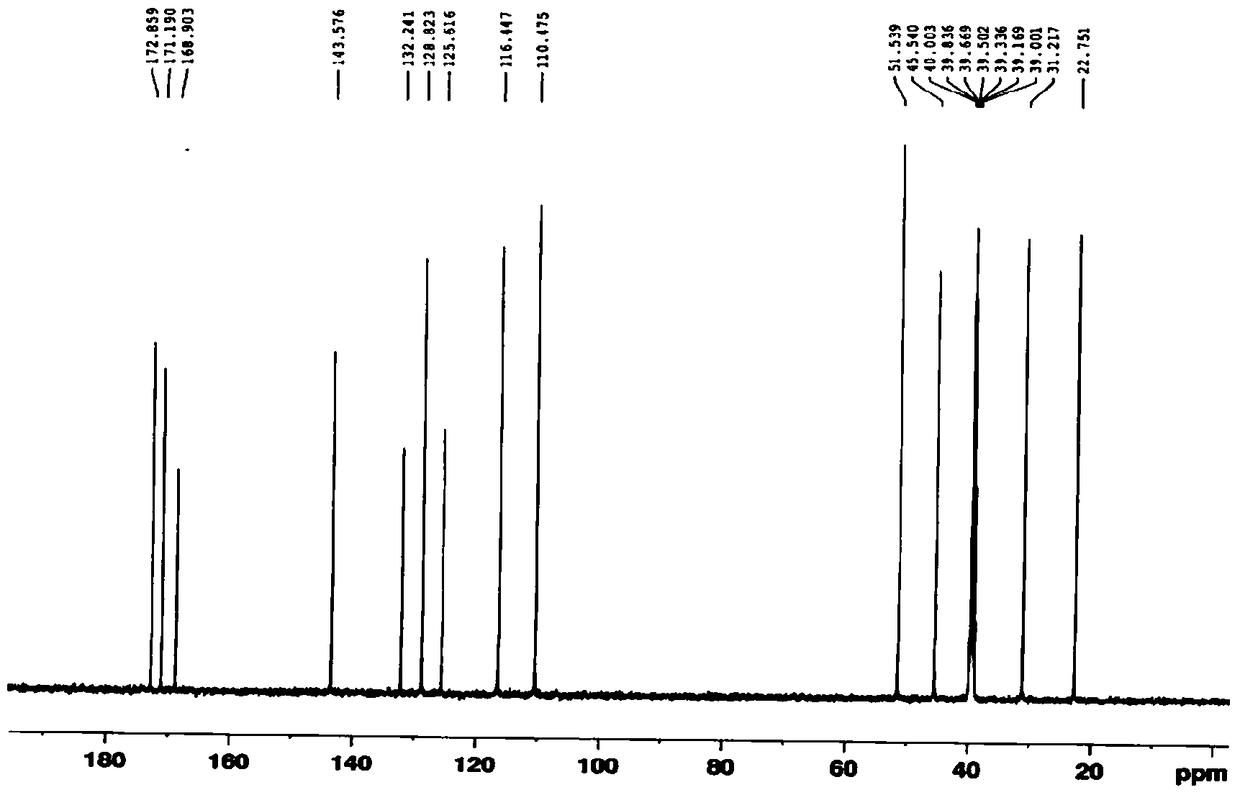
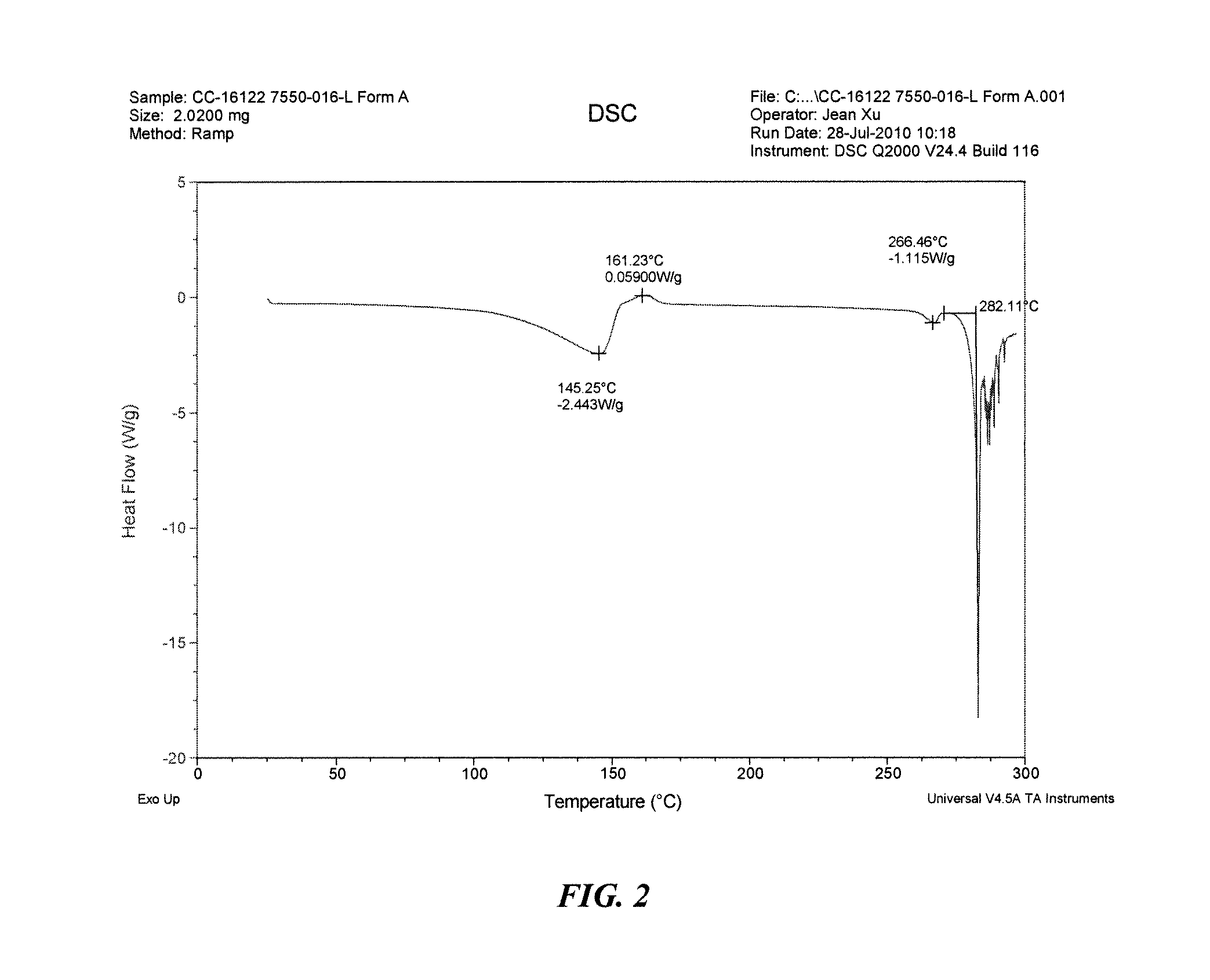
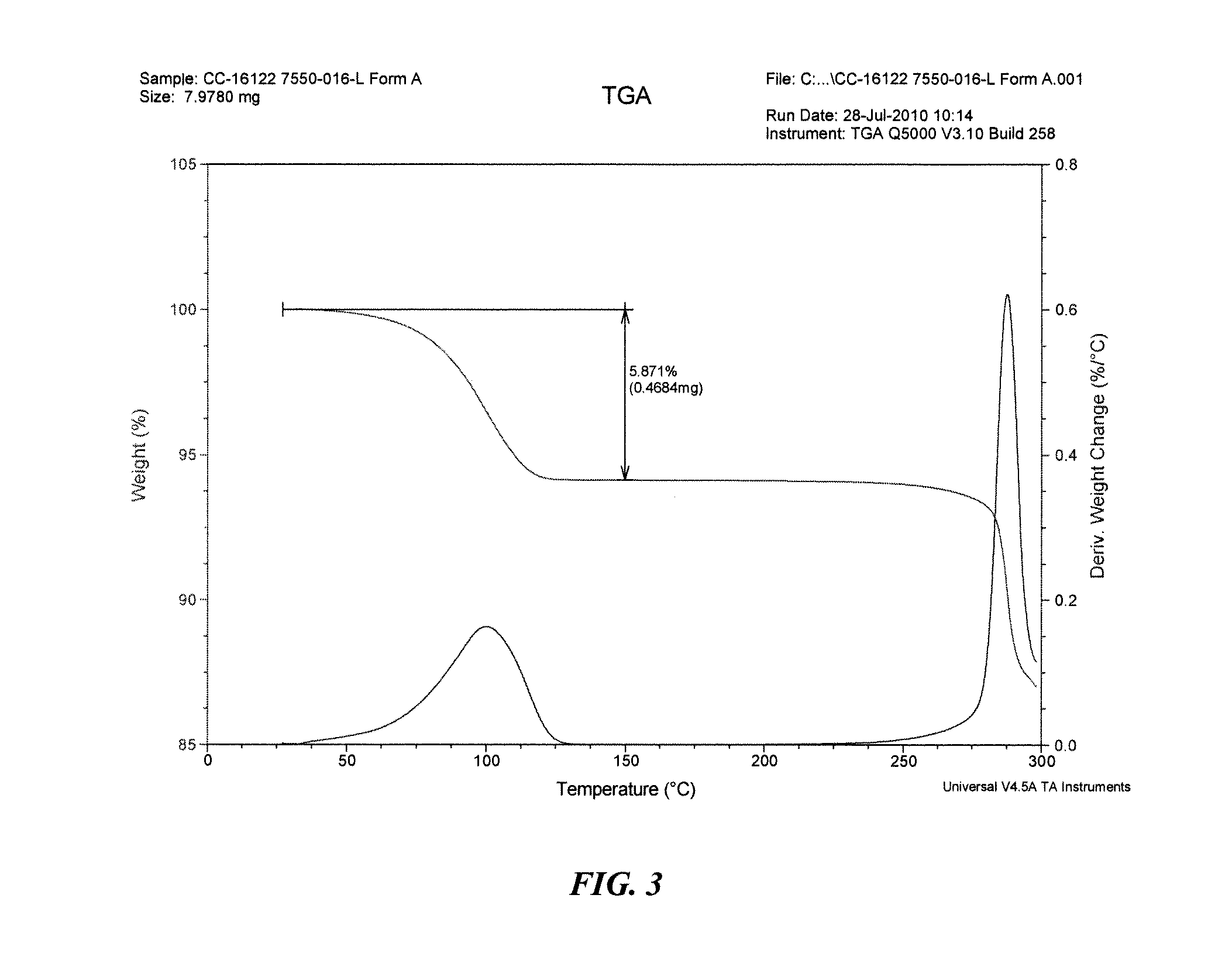
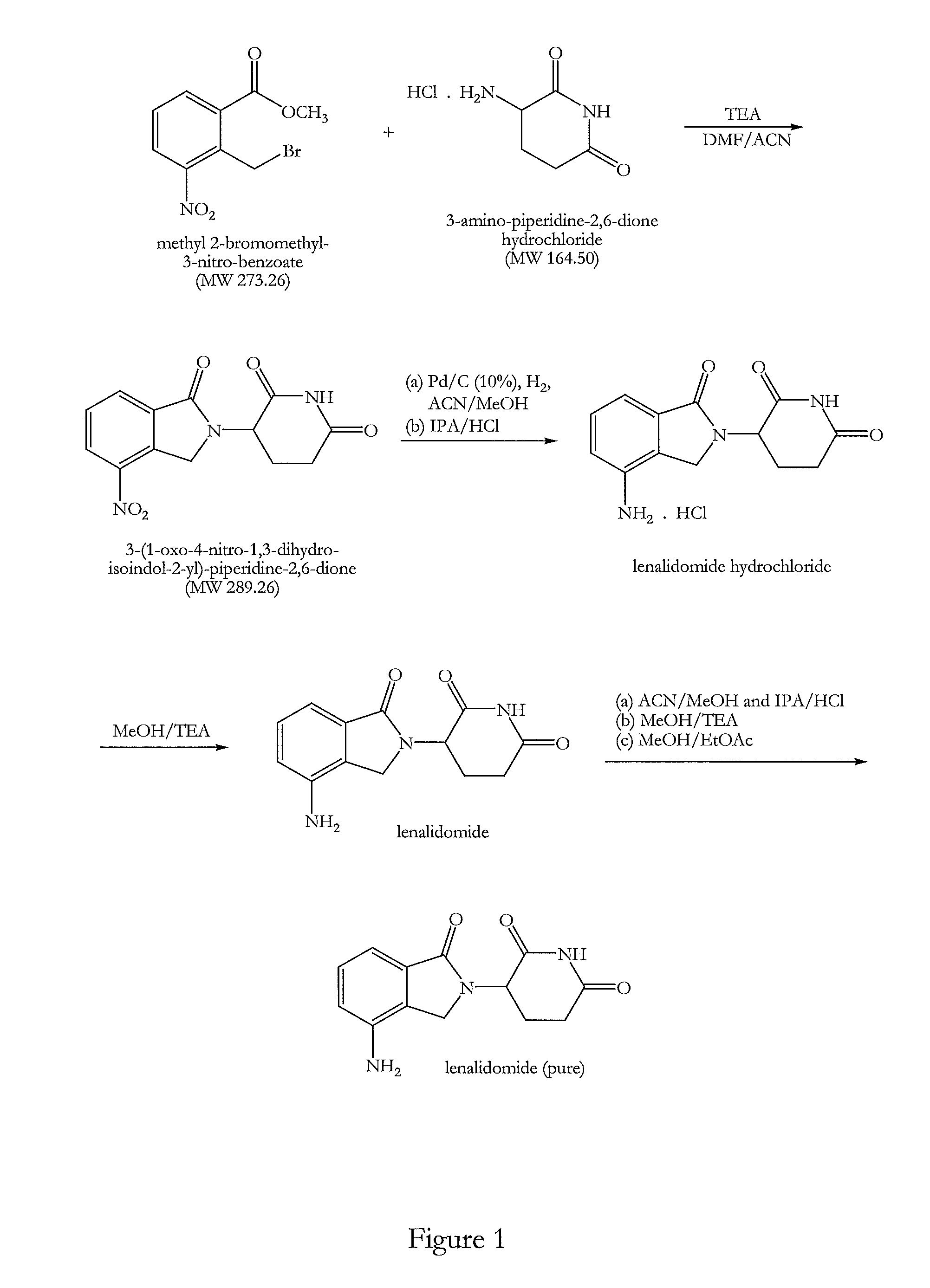
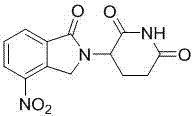
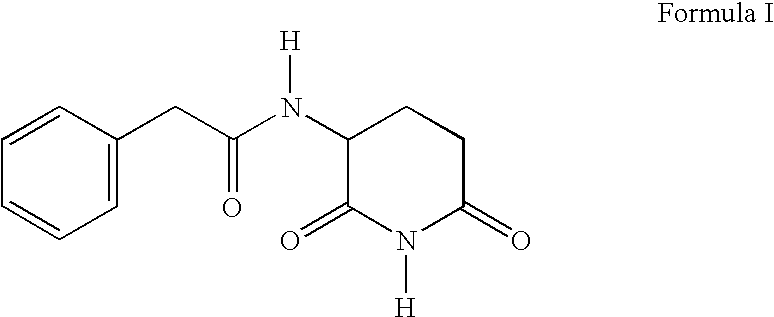
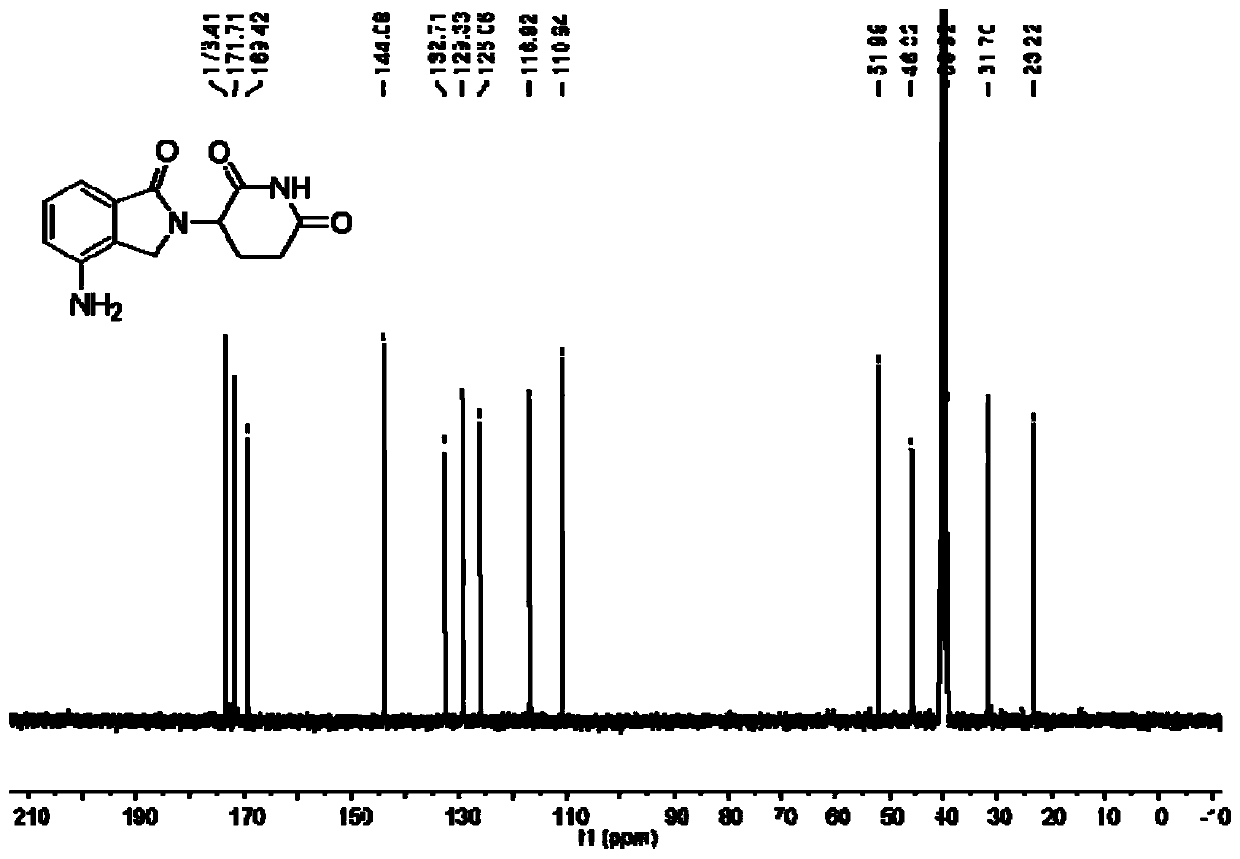
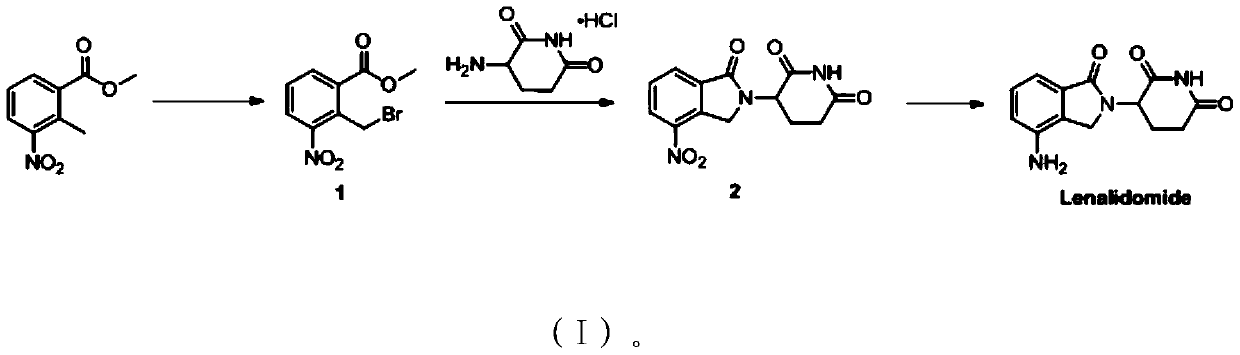
The present invention relates to improved processes for preparing 3-(4-amino-1-oxo-1,3-dihydro-isoindol-2-yl)-piperidine-2,6-dione (I) (lenalidomide) and its intermediate 3-(1-oxo-4-nitro-1,3-dihydro-isoindol-2-yl)-piperidine-2,6-dione. The present invention further relates to improved processes for preparing lenalidomide crystalline form A, use of said crystalline form A as an active pharmaceutical ingredient or as an intermediate in the preparation of further crystalline or amorphous forms of lenalidomide, compositions comprising lenalidomide crystalline form A and their use in the treatment of disease.
Owner:GENERICS UK LTD
Method for preparing lenalidomide
InactiveCN104311536ASignificant technological progressOptimize the process routeCarbamic acid derivatives preparationOrganic compound preparationDicarbonateL-Glutamin
The invention discloses a method for preparing lenalidomide. The method comprises the following steps: firstly, etherifying 2-methyl-3-nitrobenzoic acid to obtain 2-methyl-3-nitrobenzoic acid methyl ester, brominating to obtain 2-brooethyl-3-nitrobenzoic acid methyl ester, reacting L-glutamine and tert-butyl dicarbonate to obtain N-Boc glutamic acid, acquiring 3-amino-2,6-piperidine diketone protected by Boc from N-Boc-glutamic acid in the presence of a condensing agent and a catalyst, further reacting with acid to prepare 3-amino-2,6-piperidine diketone hydrochloride, reacting 3-amino-2,6-piperidine diketone with 2-brooethyl-3-nitrobenzoic acid methyl ester so as to obtain 3-(4-nitryl-1,3 dihydro-1-oxo-2 hydrogen-isobenzazole-2-yl) piperidine-2,6-diketone, and finally reducing, thereby obtaining lenalidomide. The method disclosed by the invention is high in product yield.
Owner:SHANGHAI INST OF TECH
Methods of treating cancer using 3-(5-amino-2-methyl-4-oxo-4Hquinazolin-3-yl)-piperidine-2,6-dione
Provided herein are methods of treating, preventing and / or managing cancers, which comprise administering to a patient 3-(5-amino-2-methyl-4-oxo-4H-quinazolin-3-yl)-piperidine-2,6-dione, or an enantiomer or a mixture of enantiomers thereof, or a pharmaceutically acceptable salt, solvate, hydrate, co-crystal, clathrate, or polymorph thereof.
Owner:CELGENE CORP
Preparation method of lenalidomide
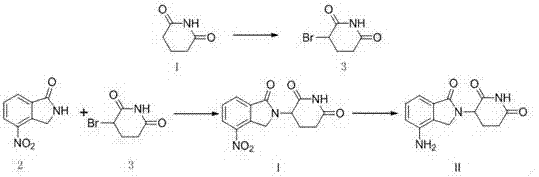
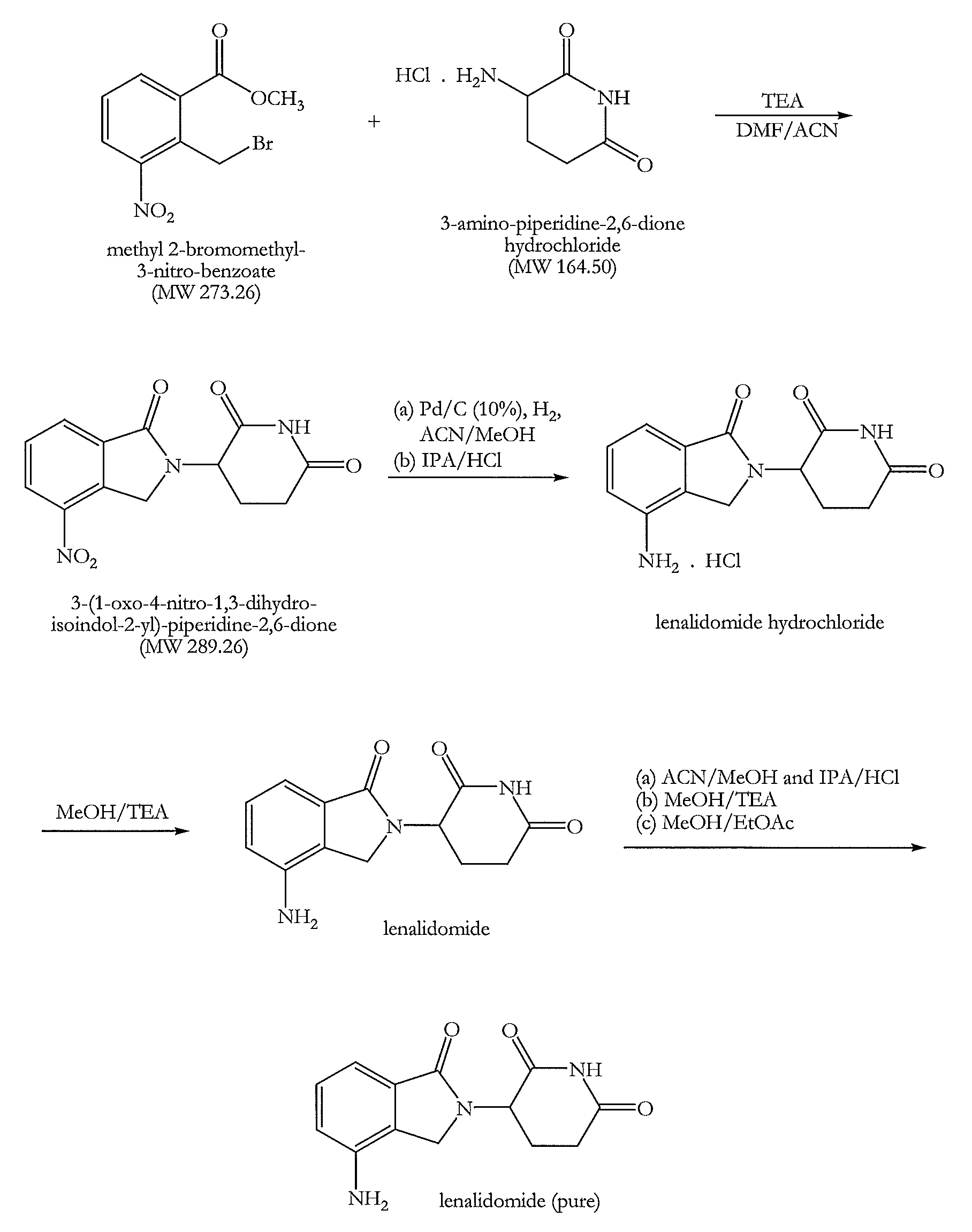
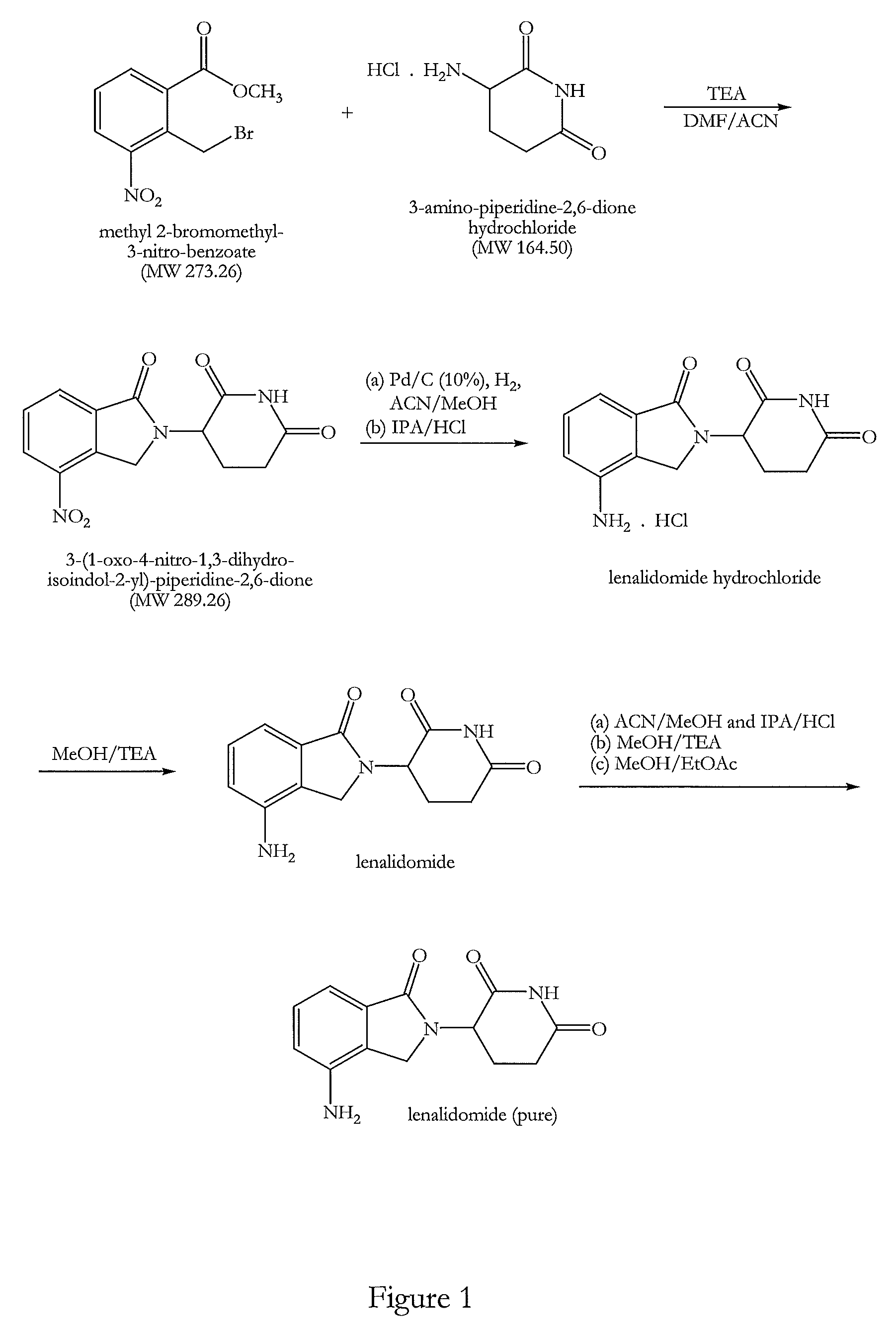
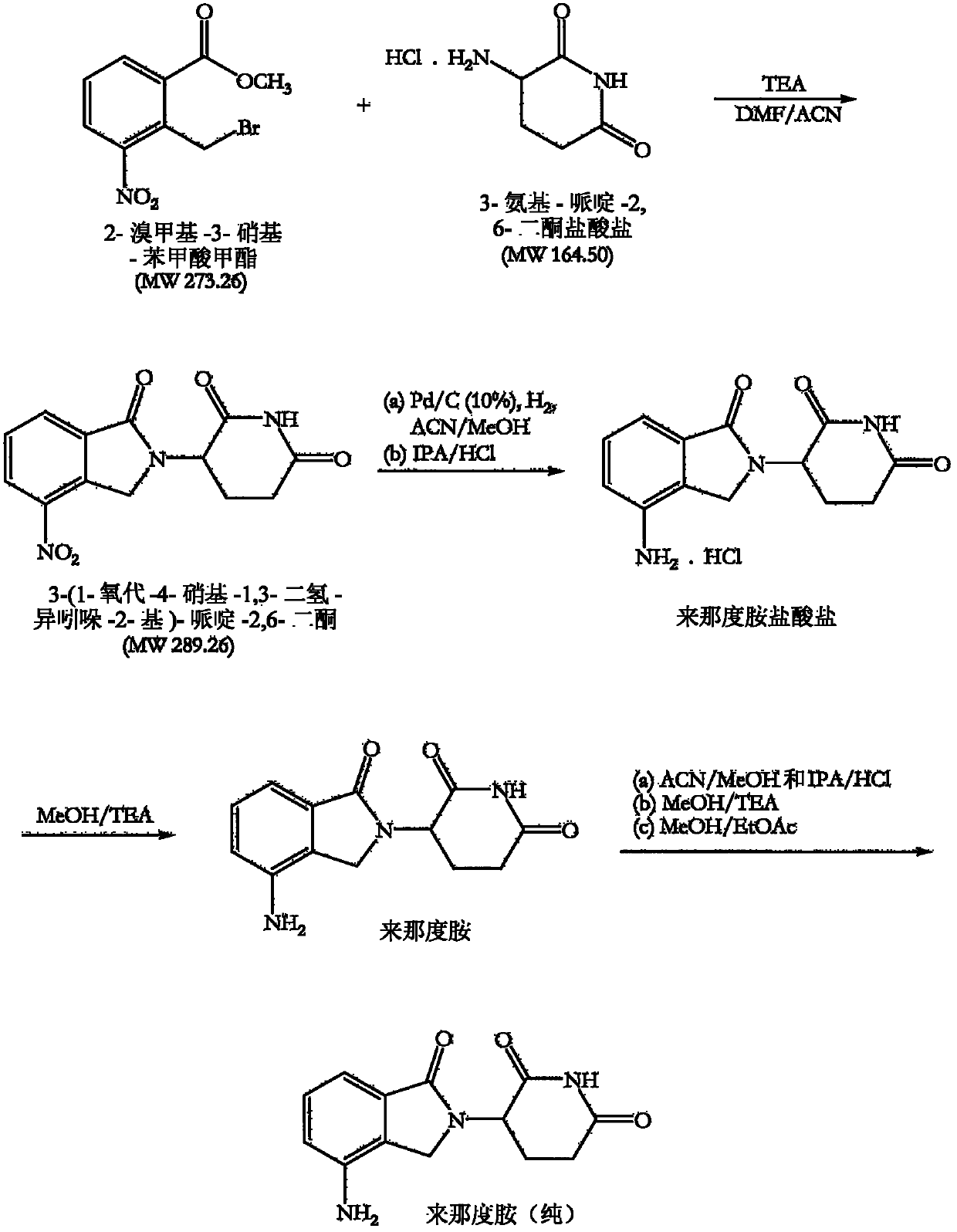
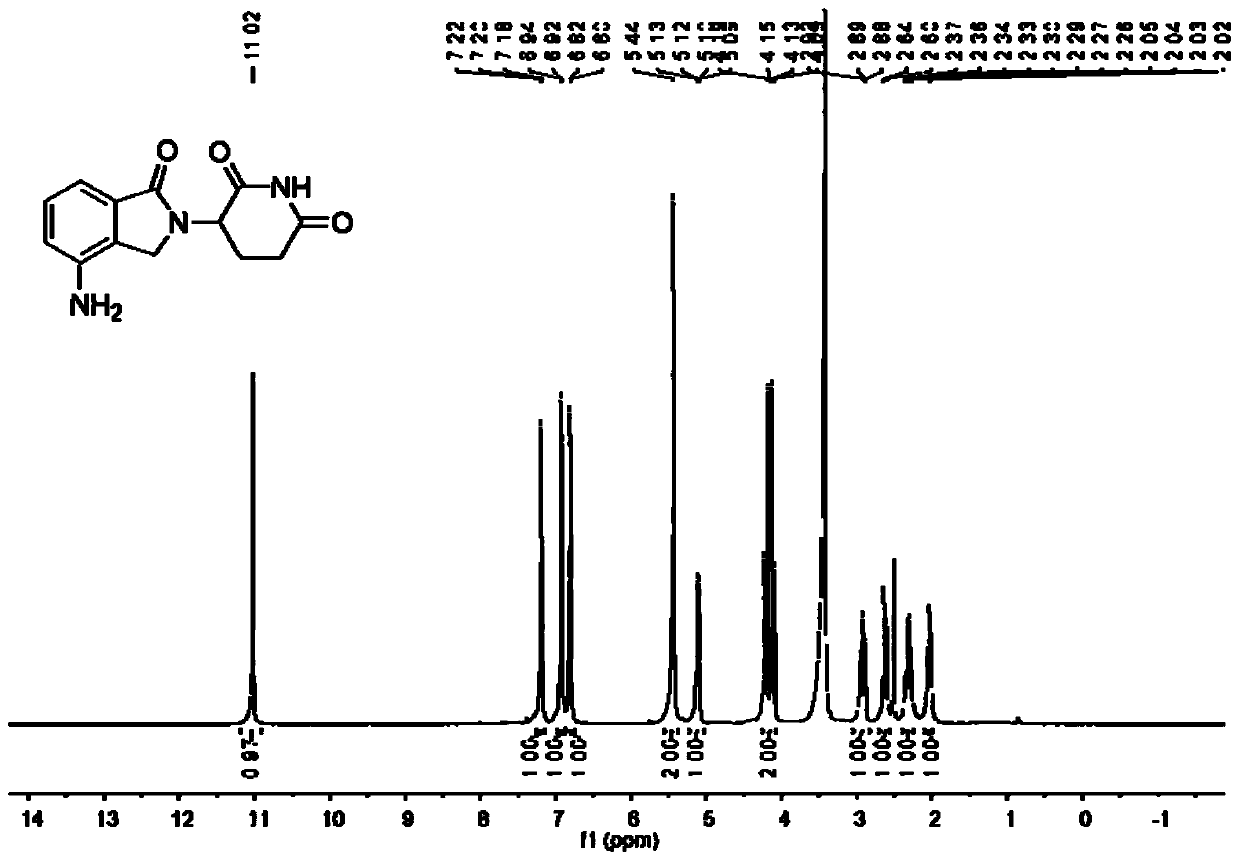
The invention relates to the field of drug synthesis, and particularly relates to a preparation method of a lenalidomide intermediate and lenalidomide. The compound is a drug for treating multiple myeloma. The method comprises the steps of adopting 2-bromomethyl-3-nitrobenzoate and 3-amino-2,6-piperidione hydrochloride as reaction substrates and an inorganic base as an acid-binding agent and obtaining a white to almost white key intermediate 3-(4-nitro-1-oxo-1,3-dihydro-2H-isoindole-2-yl) piperidine-2,6-diketone of the lenalidomide through simple post-treatment; and adopting a mixed solvent of an organic solvent and water as a reaction solvent and carrying out catalytic hydrogenation in the presence of palladium on carbon to prepare the lenalidomide (II). The process route is low in production cost, and a product is high in purity and friendly to environment, and has relatively great implement value and social and economical benefits.
Owner:CHANGZHOU PHARMA FACTORY
Process for the preparation of lenalidomide
The present invention relates to improved processes for preparing 3-(4-amino-1-oxo-1,3-dihydro-isoindol-2-yl)-piperidine-2,6-dione (I) (lenalidomide) and its intermediate 3-(1-oxo-4-nitro-1,3-dihydro-isoindol-2-yl)-piperidine-2,6-dione. The present invention further relates to improved processes for preparing lenalidomide crystalline form A, use of said crystalline form A as an active pharmaceutical ingredient or as an intermediate in the preparation of further crystalline or amorphous forms of lenalidomide, compositions comprising lenalidomide crystalline form A and their use in the treatment of disease.
Owner:GENERICS UK LTD
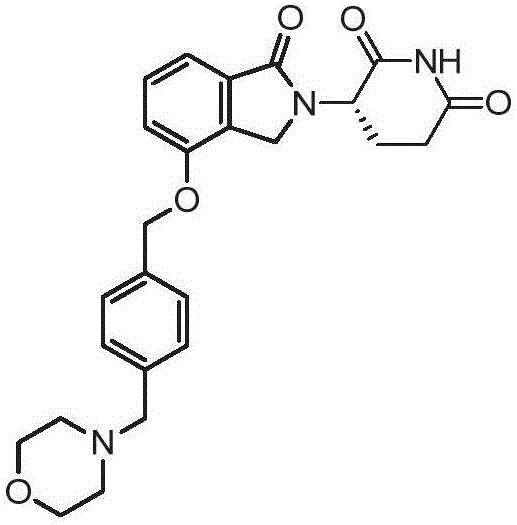
Methods of treating cancer using 3-(4-((4-(morpholinomethyl)benzyl)oxy)-1-oxoisoindolin-2-yl)piperidine-2,6-dione
Provided herein are methods of treating, preventing and / or managing cancers, which comprise administering to a patient 3-(4-((4-(morpholinomethyl)benzyl)oxy)-1-oxoisoindolin-2-yl)piperidine-2,6-dione, or an enantiomer or a mixture of enantiomers thereof, or a pharmaceutically acceptable salt, solvate, hydrate, co-crystal, clathrate, or polymorph thereof.
Owner:CELGENE CORP
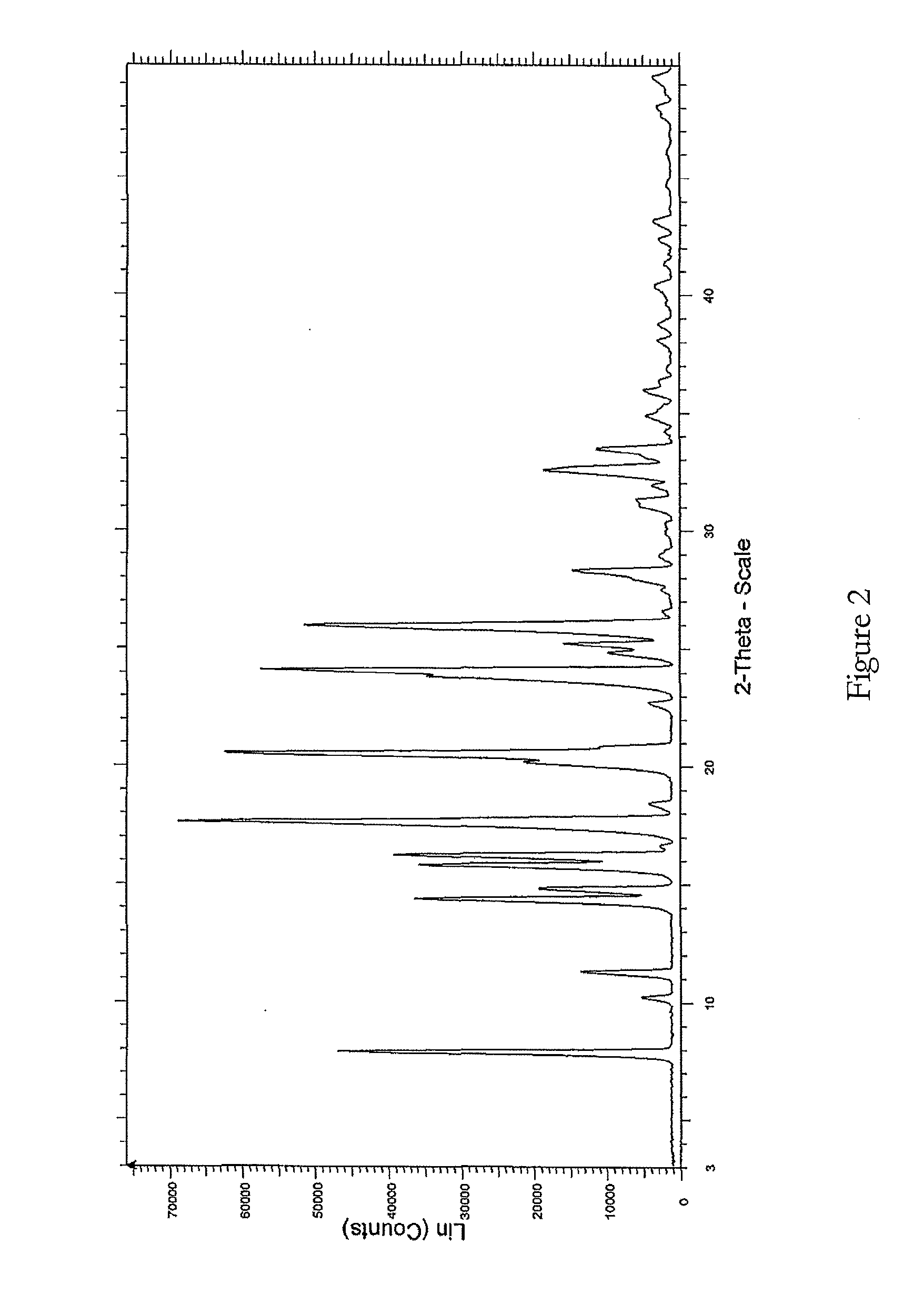
Crystal form of lenalidomide and preparation method thereof
The present invention relates to a new crystalline form of lenalidomide having the formula (I) and chemically known as 3-(4-amino-1-oxo-1,3-dihydro-isoindol-2-yl )-piperidine-2,6-dione. The invention further relates to a process for the preparation of said new crystalline form and its use in pharmaceutical formulations for the treatment of autoimmune diseases, inflammation, inflammatory diseases and the management of diseases such as cancer, especially multiple myeloma application.
Owner:GENERICS UK LTD
Toothpaste containing anticancer agents
InactiveUS20060246016A1Effective treatmentPreventive effectCosmetic preparationsToilet preparationsOral diseaseDisease
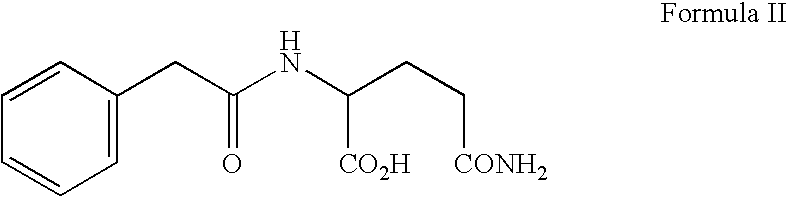
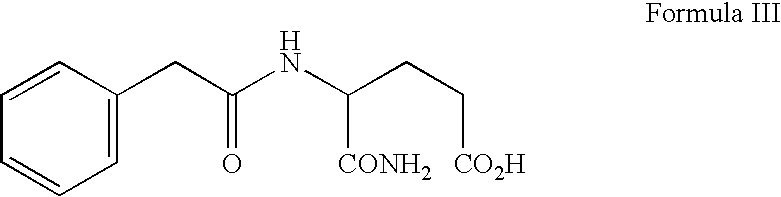
A novel dentifrice composition is provided for prevention or treatment of carcinoma of the oral cavity, caries and periodontal diseases of the oral cavity. The dentifrice composition contains a silica abrasive and medicinal agents useful in the treatment of human neoplastic disease. The medicinal agent is selected from the group consisting of 3-N-phenylacetylamino-2,6-piperidinedione, phenylacetylglutamine, phenylacetylisoglutamine, phenylbutyrate, phenylacetate, combinations thereof and pharmaceutically acceptable salts thereof. The components of the dentifrice composition act advantageously to allow the composition to remove plaque, tartar, and oral disease-causing bacteria.
Owner:BURZYNSKI STANISLAW
3-(4-amino-1, 3-dihydro-1-oxo-2 H-isoindole-2-yl)-2, 6-piperidinedione preparation method
ActiveCN104710405AThe preparation method is safeLow equipment requirementsOrganic chemistryIsoindolesPiperidinedione
The present invention relates to 3-(4-amino-1, 3-dihydro-1-oxo-2 H-isoindole-2-yl)-2, 6-piperidinedione preparation method. The method is as follows: in the presence of reducing system, a target product is prepared by reaction of formula (II) compound in an organic solvent. The method provided by the present invention has no need to use hydrogen as a reducing agent, and is safe, low in cost, high in yield, easy in purification and easy in industrialization.
Owner:JIANGSU HANSOH PHARMA CO LTD
Novel 3-(4-amino-1,3-dihydro-1-oxo-2H-isoindole-2-yl)-2,6-piperidinedione crystal forms and preparation method thereof
InactiveCN104016966AStable storageImprove liquidityOrganic active ingredientsOrganic chemistryPiperidinedioneChemistry
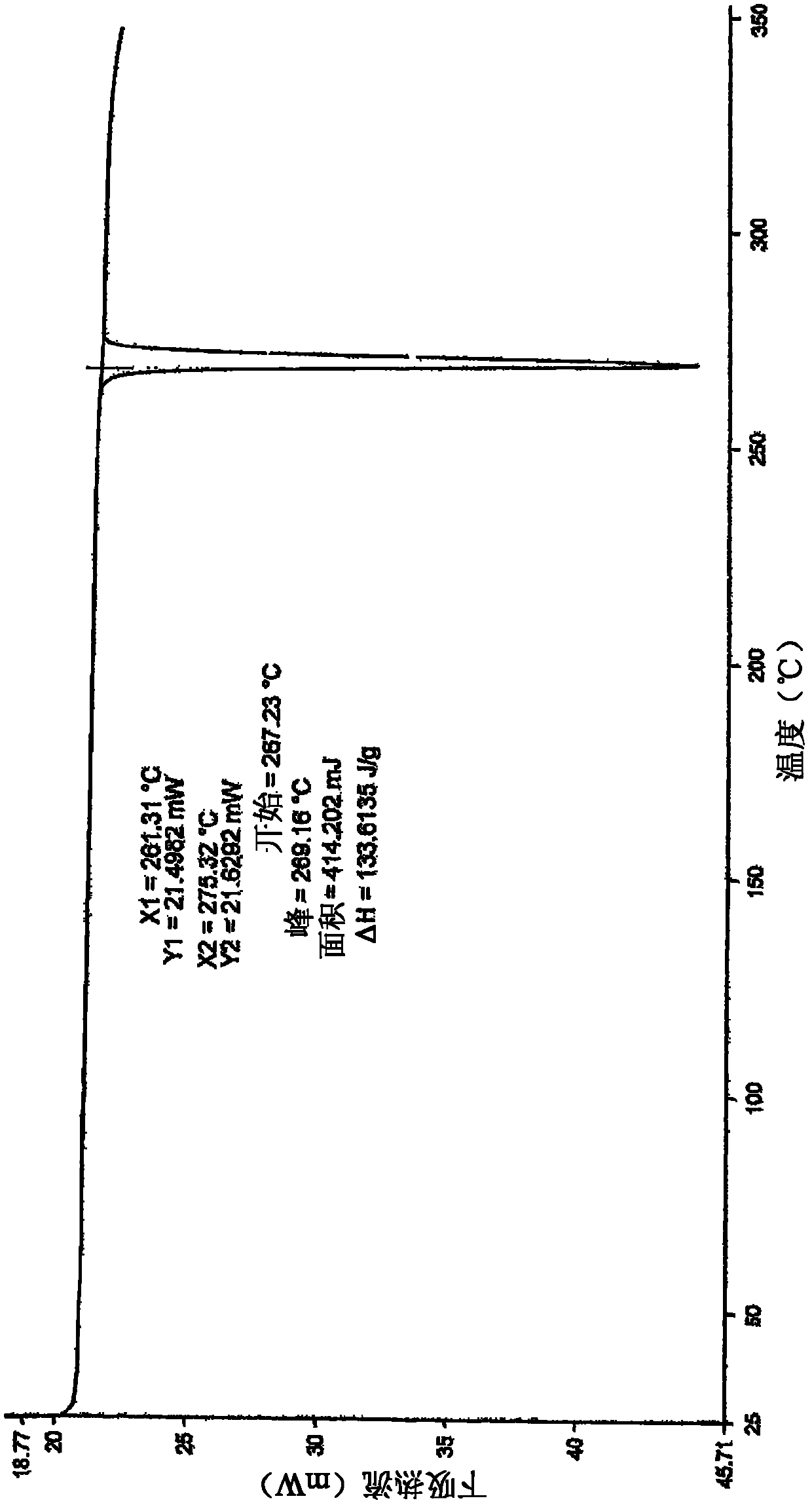
The invention relates to novel 3-(4-amino-1,3-dihydro-1-oxo-2H-isoindole-2-yl)-2,6-piperidinedione crystal form and a preparation method thereof, and particularly to four novel crystal forms, namely, crystal form X, crystal form XI, crystal form XII and crystal form XIII, and the preparation method of the novel crystal forms. The four novel crystal forms are stable to store, good in flowability, small in static electricity, more applicable to medicine preparations, simple and efficient in preparation process, capable of achieving on-scale production and environmental friendly.
Owner:SHANGHAI ACEBRIGHT PHARMA CO LTD +1
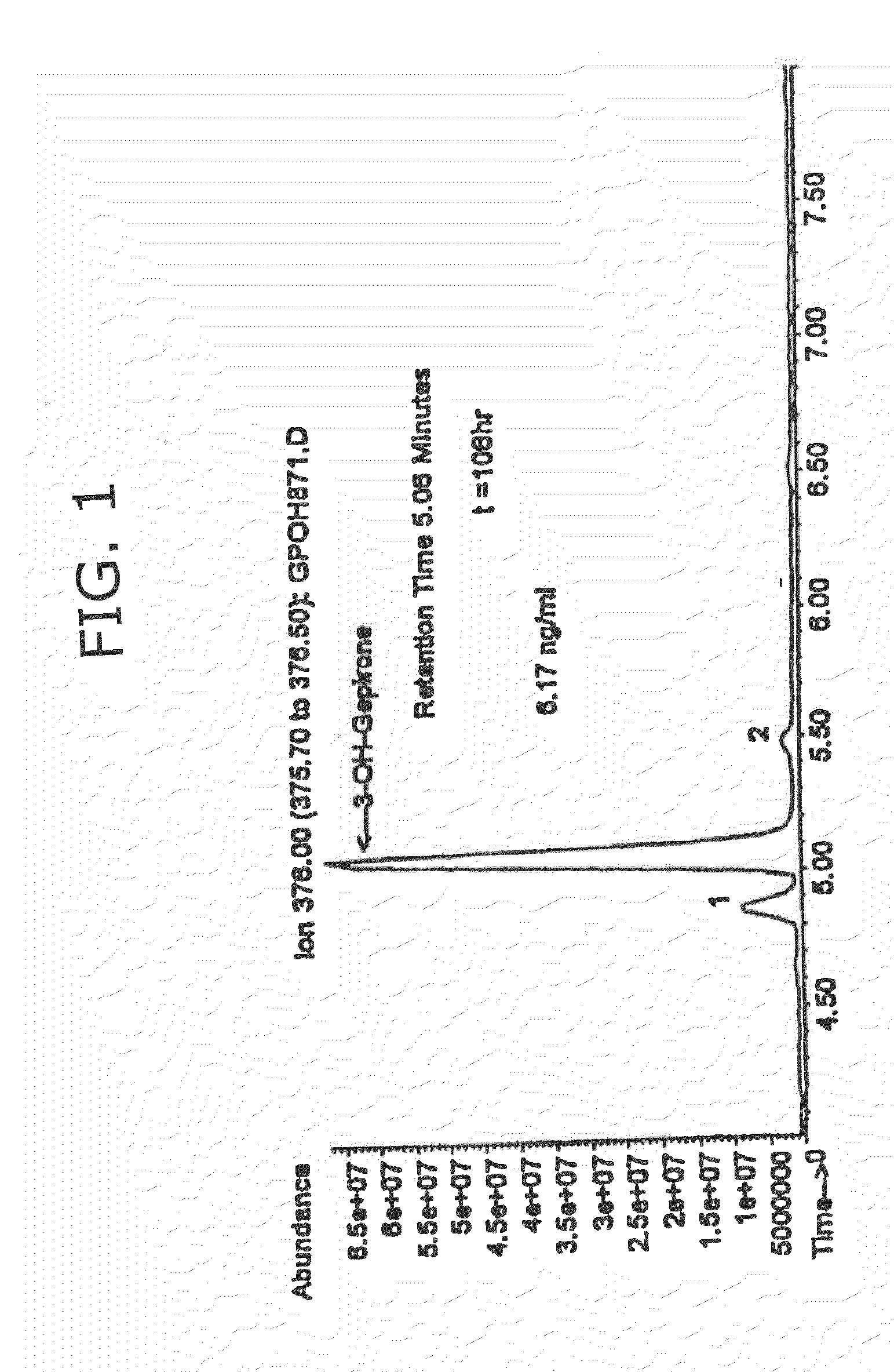
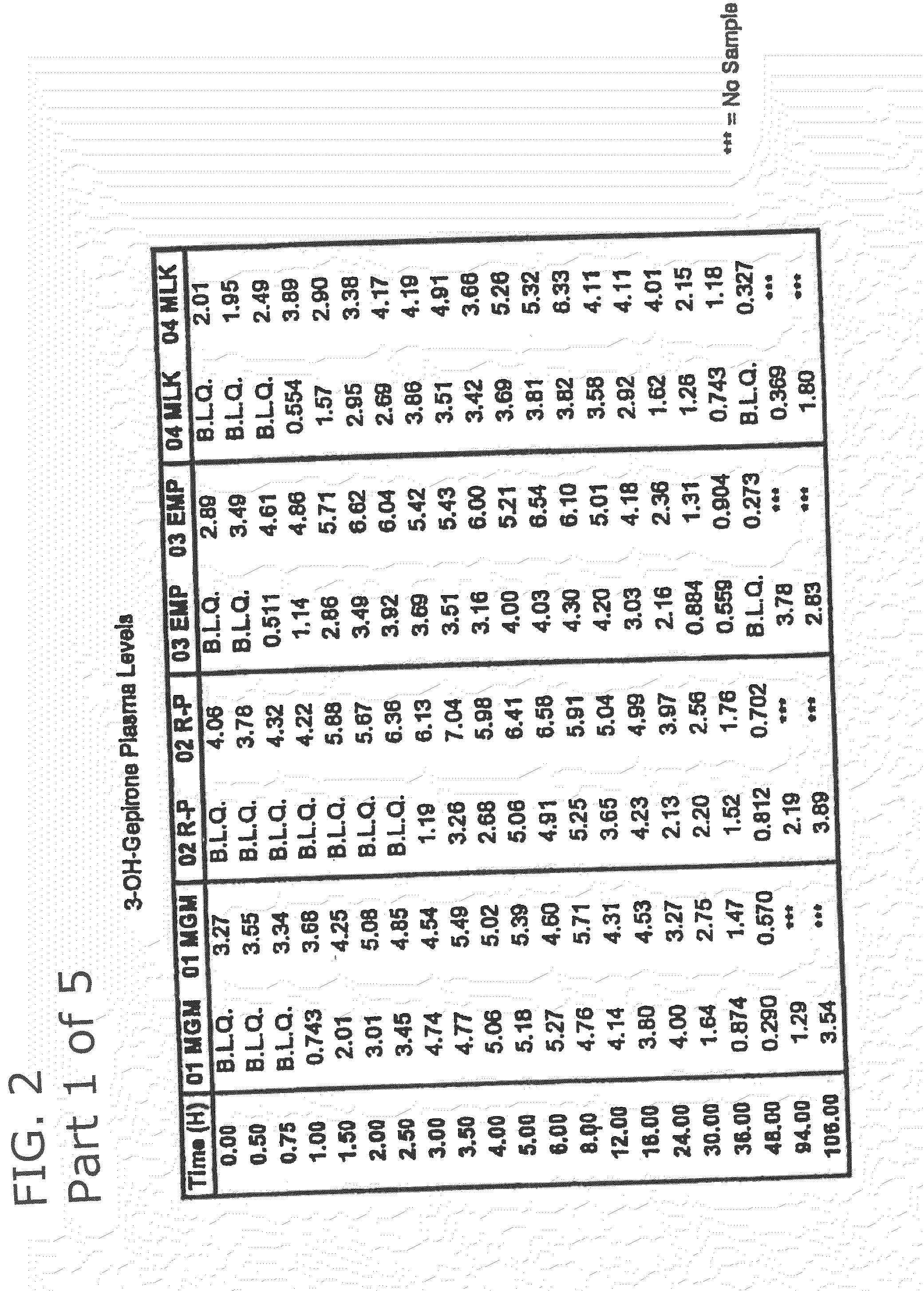
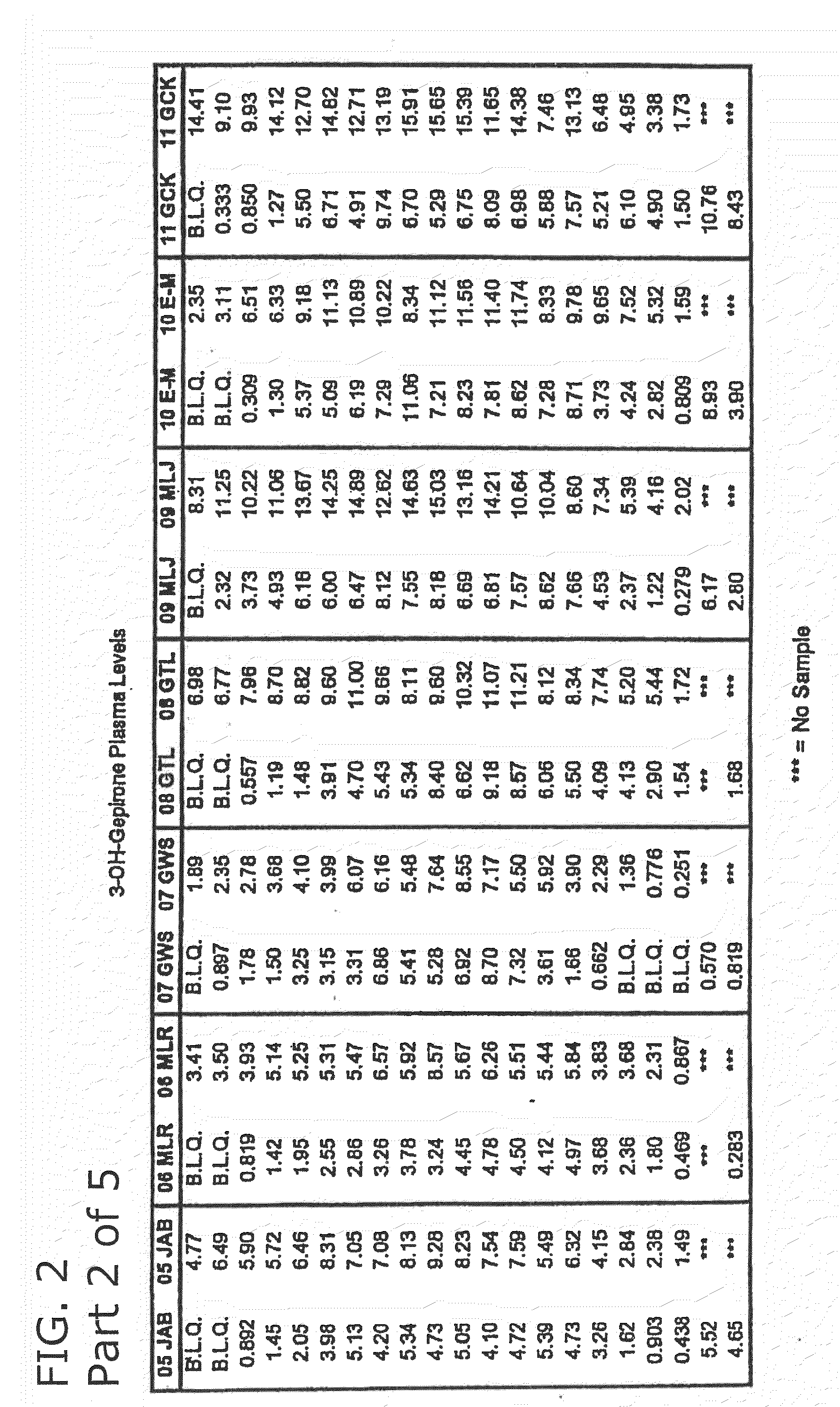
3-hydroxy gepirone for the treatment of attention deficit disorder and sexual dysfunction
The present invention relates to a method for alleviation, prevention, and treatment of attention deficit disorder, sexual dysfunction, and related conditions by administering certain bioactive metabolites of the known anti-depressant compound gepirone. In a preferred embodiment, the compound is 4,4,-dimethyl-3-hydroxy-1-[4-[4-(2-pyrimidinyl)-1-piperazinyl]butyl]-2,6-piperidinedione (3-OH gepirone).
Owner:FABRE KRAMER PHARMA INC
Synthetic process of 1-alkyl-4-p-fluorophenyl-2,6-piperadinedione-3-formic ester
InactiveCN103554010ARaw materials are cheap and easy to getHigh yieldOrganic chemistryFormic Acid EstersPtru catalyst
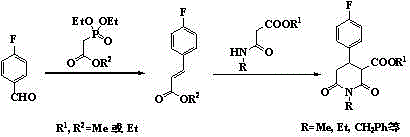
The invention discloses a synthetic process of 1-alkyl-4-p-fluorophenyl-2,6-piperadinedione-3-formic ester. The synthetic process comprises the following steps of using 1-alkyl-4-p-fluorophenyl-2,6-piperadinedione-3,5-diformic ester, sodium hydroxide water solution and the like as raw materials and lower fatty alcohol as a solvent to undergo selective hydrolysis reaction through a chemical method, cooling, diluting, filtering, recovering unreacted raw materials, acidifying the filtrate, filtering, washing and drying in vacuum, thus obtaining an intermediate 1-alkyl-4-p-fluorophenyl-2,6-piperadinedione-3-formic acid-5-formic ester; then dissolving the intermediate in an organic solvent, adding a small amount of catalyst to carry out decarboxylic reaction, cooling, filtering, adding water to the filtrate to be stirred for layering, recovering a solvent at reduced pressure with an organic phase, and carrying out cooling crystallization on the residues, thus obtaining the product 1-alkyl-4-p-fluorophenyl-2,6-piperadinedione-3-formic ester. The synthetic process is short in route, is efficient, has the characteristics of cheap and accessible raw materials, high product yield, less three wastes and the like, and is suitable for large-scale industrial production.
Owner:QUZHOU UNIV
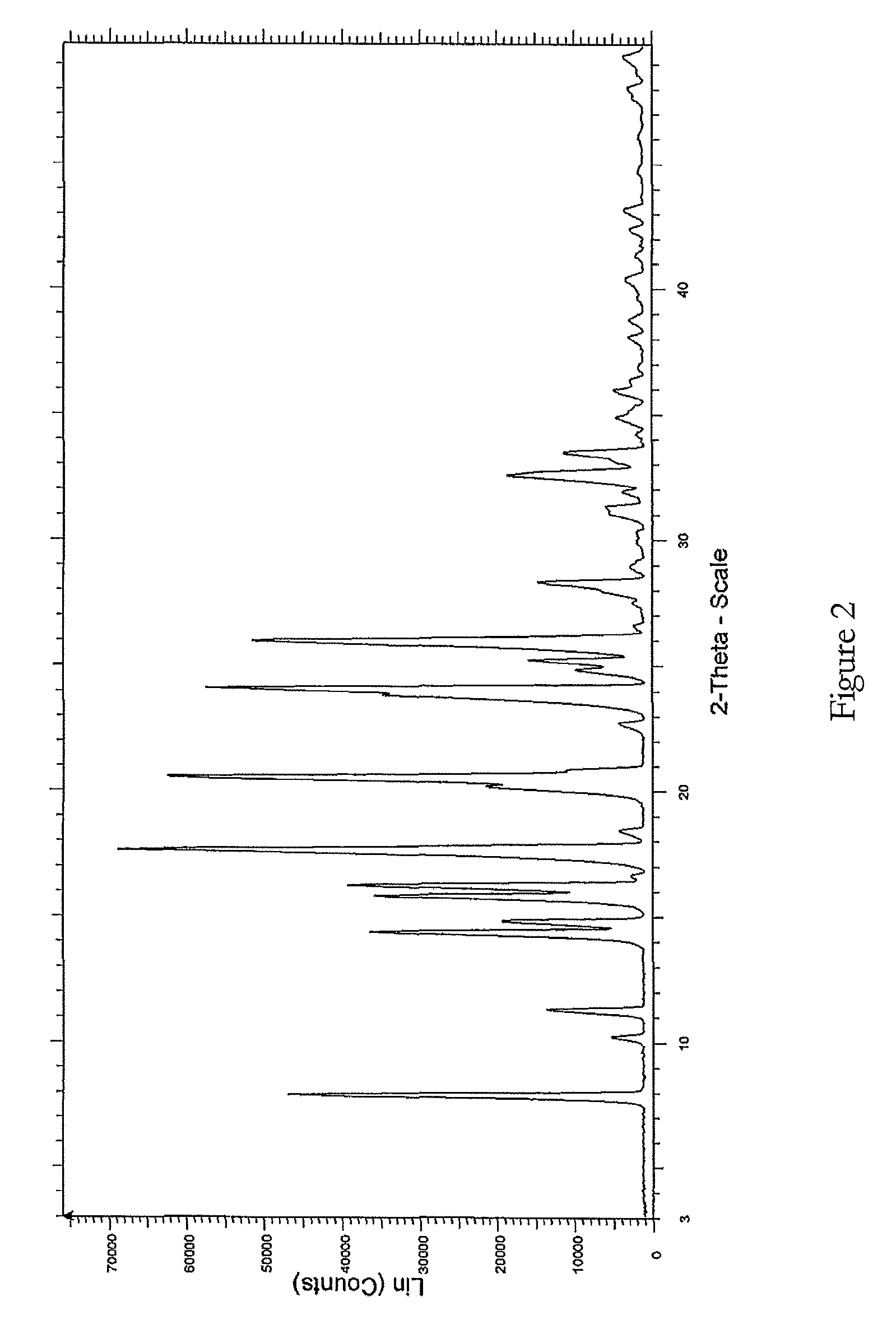
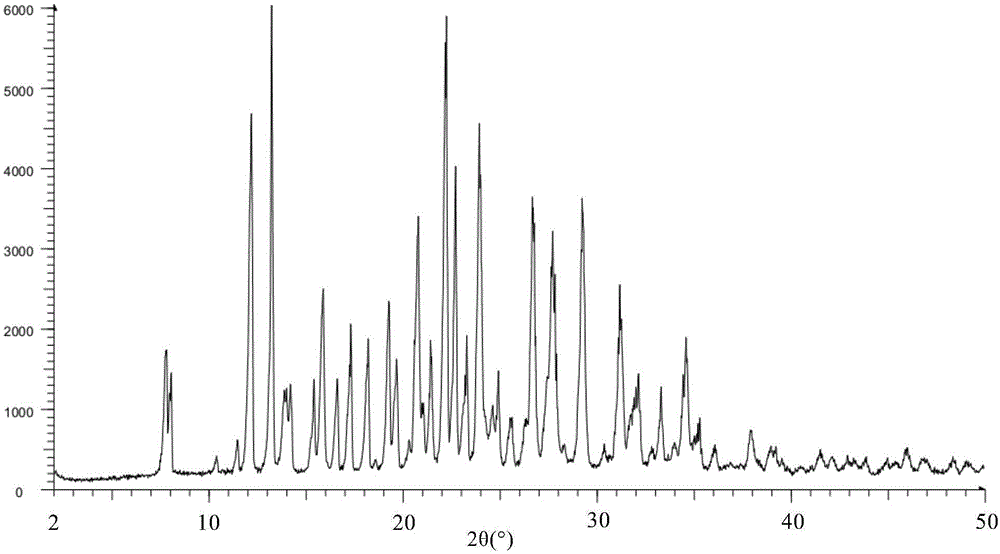
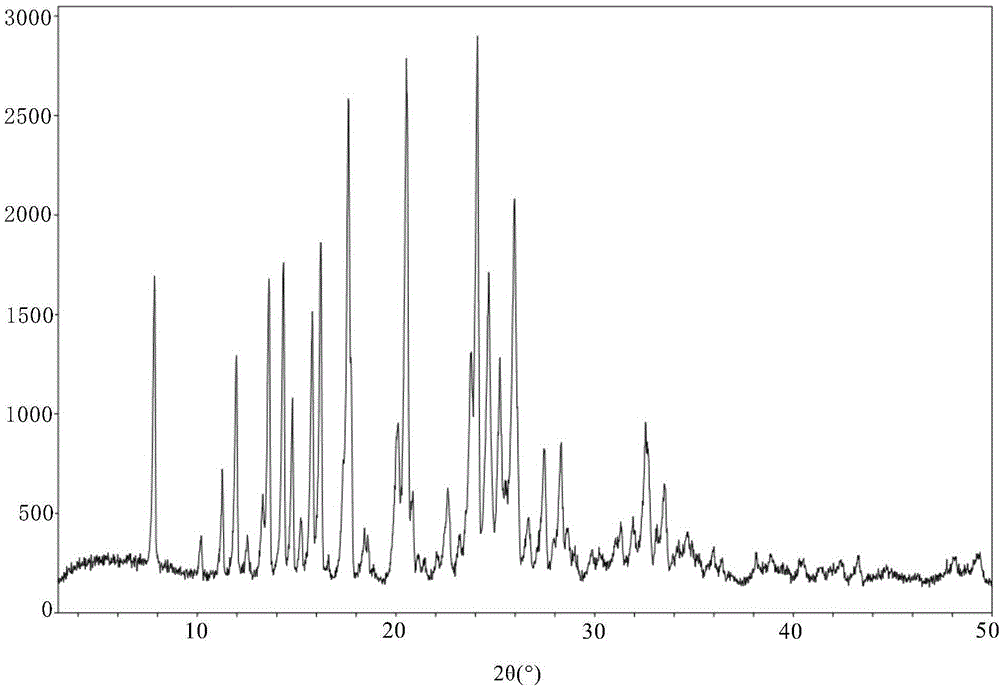
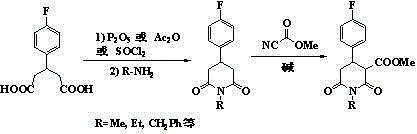
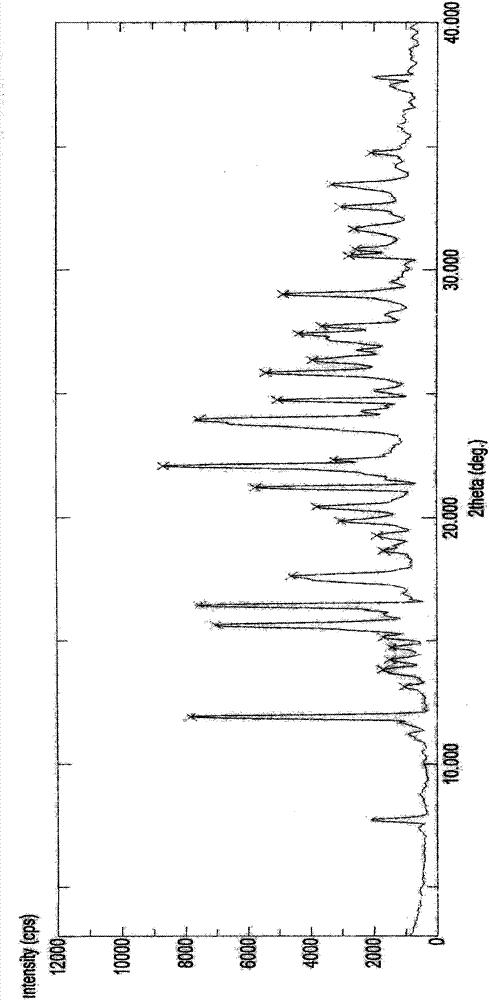
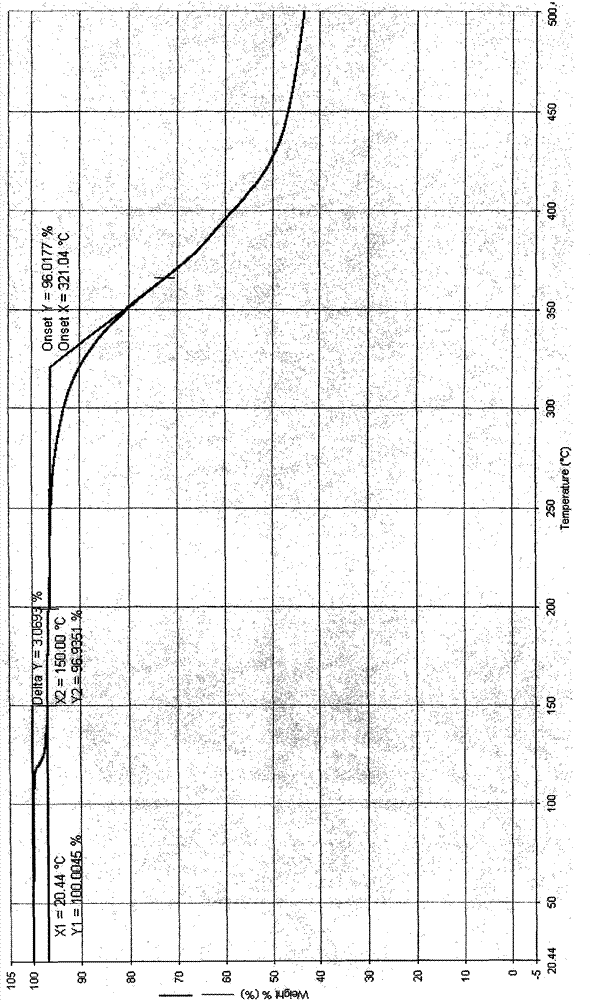
Crystal IV of 3-(substituted dihydroisoindolinone-2-yl)-2,6-piperidinediketone and medicinal composite thereof
ActiveCN101817813BNot suitable for industrial scale parallel productionImprove stabilityOrganic active ingredientsOrganic chemistryPiperidinedioneDiketone
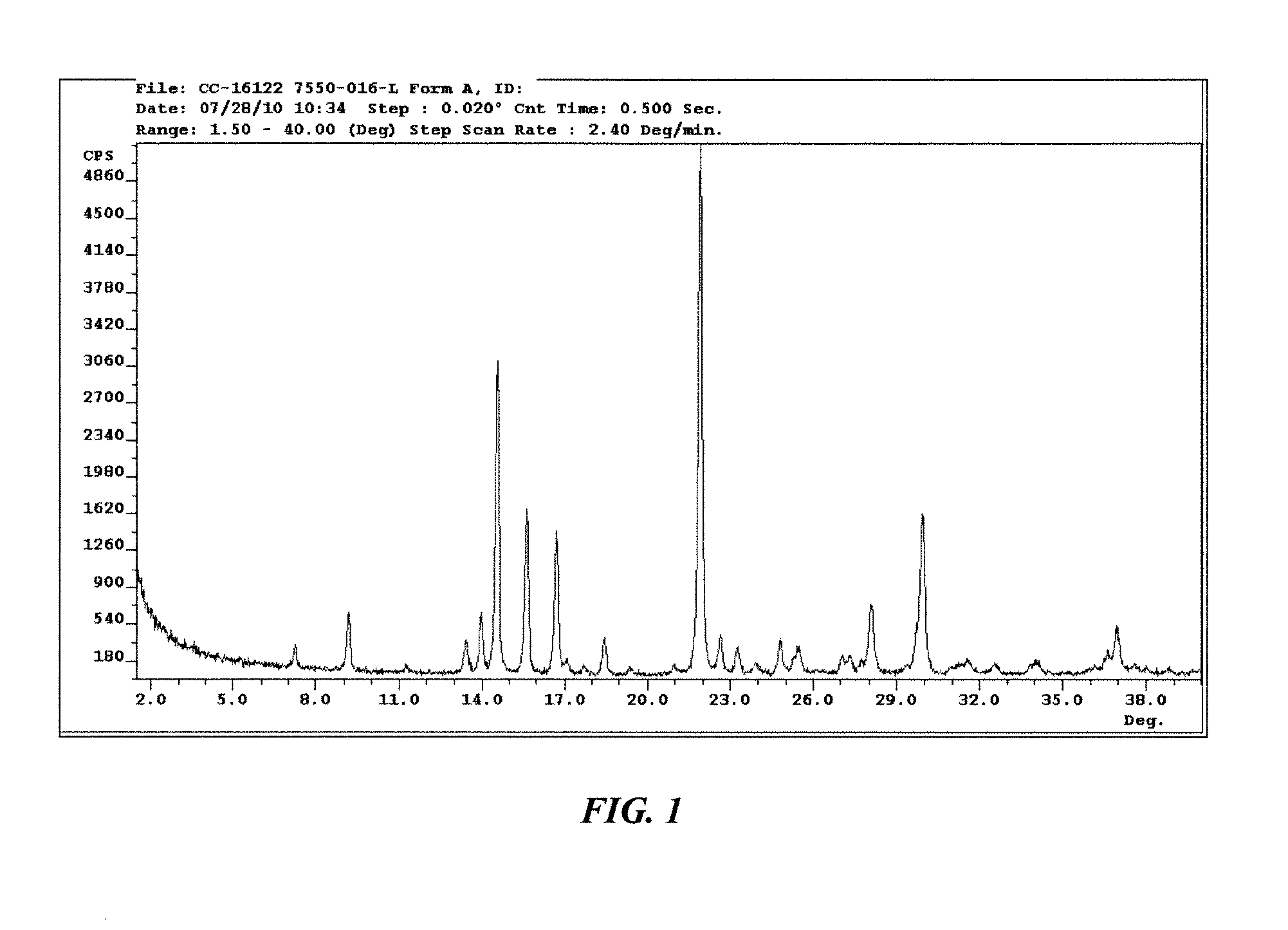
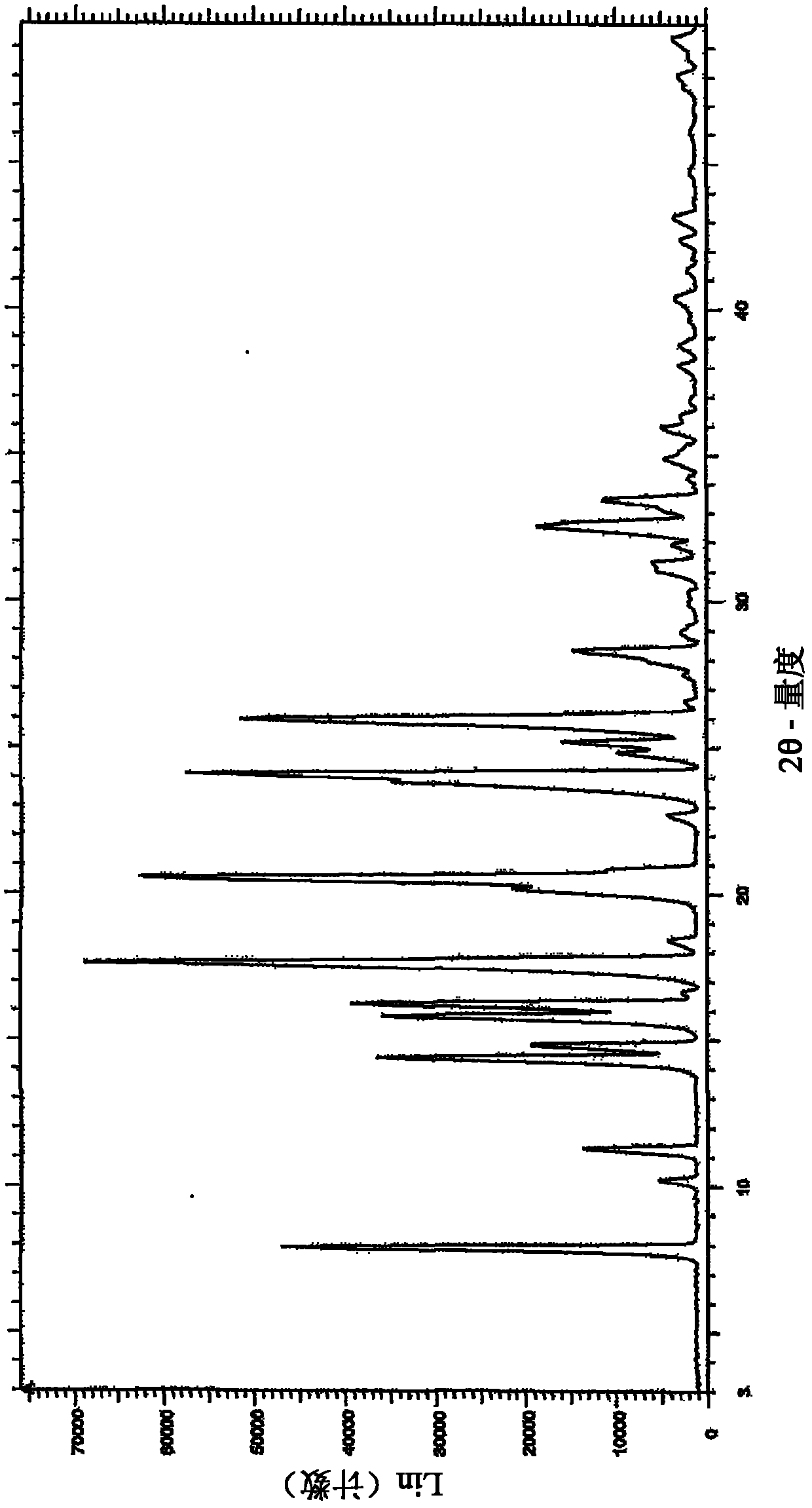
The invention discloses a crystal IV of 3-(4-amino-1-oxo-1,3-dihydro-2H-isoindole-2-yl) piperidine-2,6-diketone. 2theta expressed by degrees has diffraction peaks between 7.7+ / -0.2 and 11.9+ / -0.2 in the X-ray diffraction pattern of the crystal. Moreover, the invention also discloses a preparation method and a medicinal composite of the crystal.
Owner:NANJING CAVENDISH BIO ENG TECH +1
Antitumor drug lenalidomide intermediate preparation method
The invention discloses an antitumor drug lenalidomide intermediate preparation method. The preparation method comprises the following steps: 1) under existence of cuprous iodide and organic alkali, performing contact reaction between 4-nitro indoline and 3-bromine-2,6-piperidine to obtain 3-(4-nitro-1,3-xylylenimine-2-yl)piperidine-2,6-diketone; 2) performing oxidizing reaction on the 3-(4-nitro-1,3-xylylenimine-2-yl)piperidine-2,6-diketone obtained in the step 1) to obtain lenalidomide intermediate of 3-(4-nitro-1-oxo-1,3-xylylenimine-2-yl)piperidine-2,6-diketone. The method disclosed by the invention has obviously smaller steps, the raw materials are easy to obtain, a yield is also improved, and the method is more suitable for industrial production.
Owner:QINGDAO CHENDA BIOLOGICAL SCI & TECH
Improved process
Owner:GENERICS UK LTD
Method for synthesizing lenalidomide
InactiveCN110642834ARealize green productionProcess raw materials are easy to getOrganic chemistryChemical synthesisBenzoic acid
The invention belongs to the field of chemical synthesis, and particularly relates to a method for synthesizing lenalidomide. The method adopts three-step polymerization, and specifically comprises: (1) carrying out a bromination reaction on 2-methyl-3-nitromethyl benzoate as a starting raw material and a bromination reagent to generate a compound 1 2-bromomethyl-3-nitromethyl benzoate; (2) performing cyclization on the compound 1 and 3-aminopiperidine-2,6-dione hydrochloride under a solvent-free condition to generate a compound 2 3-(4-nitro-1-oxo-1,3-dihydroisoindole-2-yl)piperidine-2,6-dione; and (3) reducing the compound 2 with a reducing agent to obtain lenalidomide. According to the invention, the method is a novel preparation process method of lenalidomide, and has advantages of easily available process raw materials, short steps, simple and convenient operation, environmental friendliness, implementation value of industrial production, and social and economic benefits.
Owner:TIANJIN RUILING CHEM CO LTD
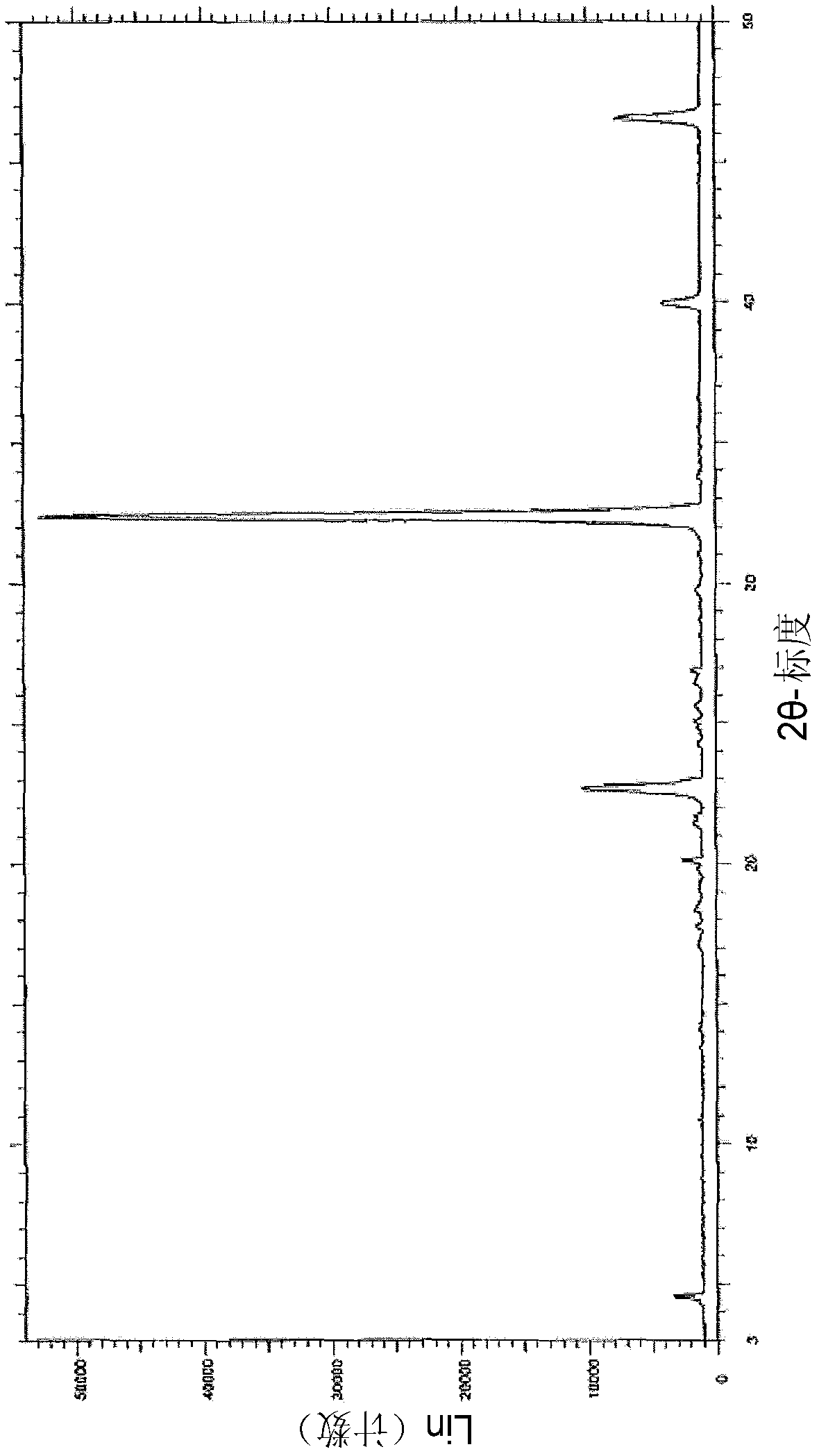
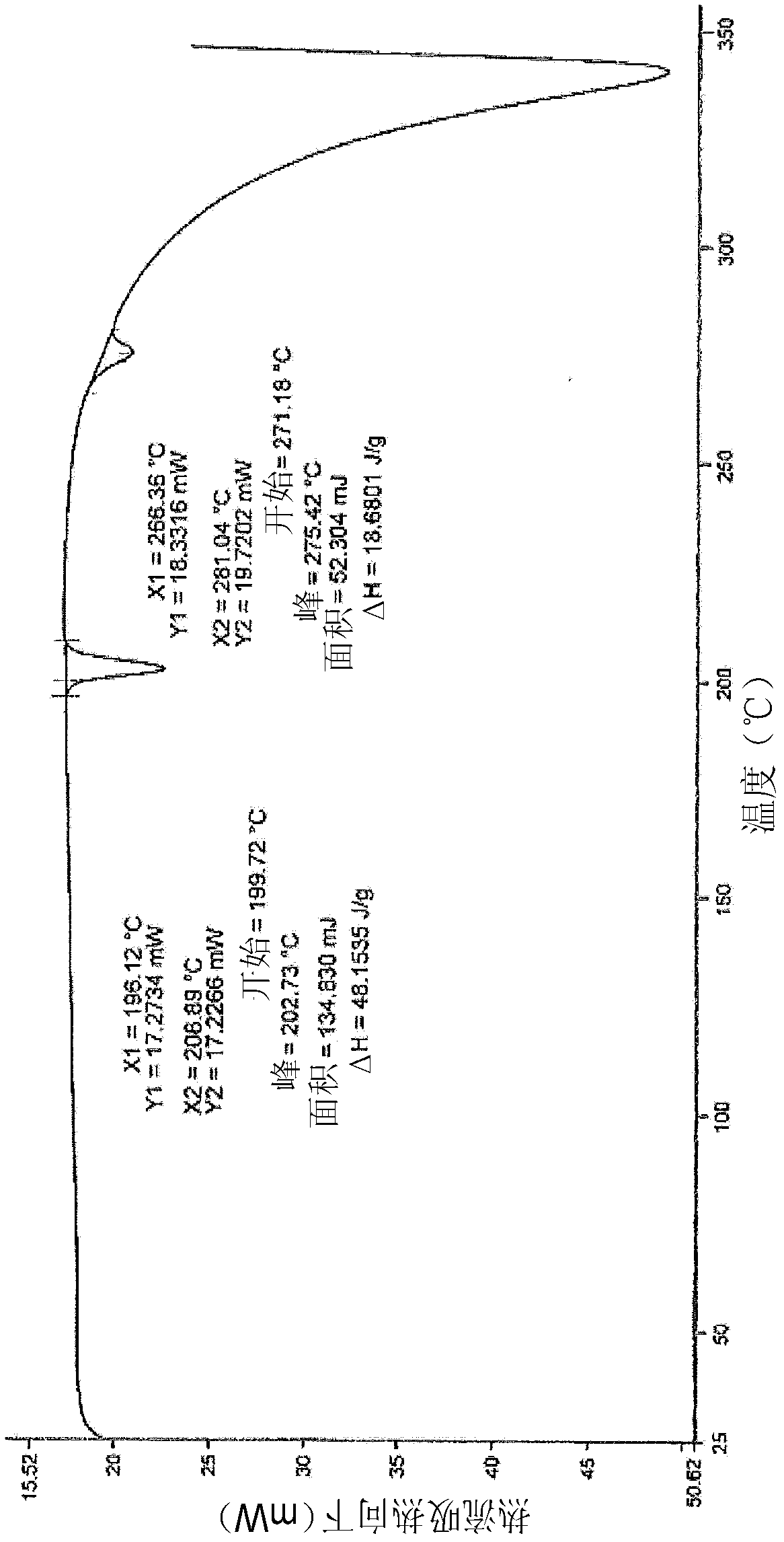
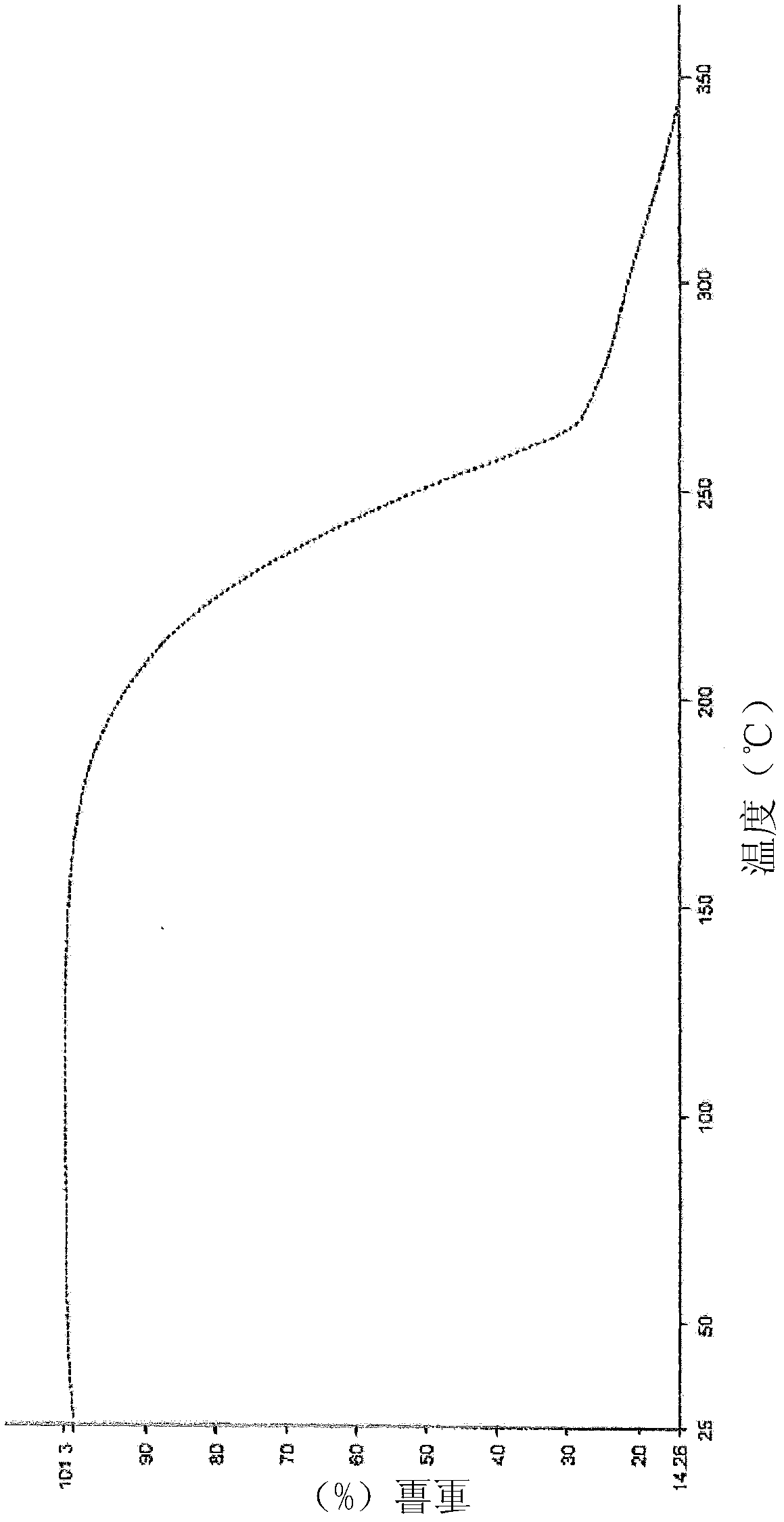
Method for preparing medical intermediate 5, 10-diarylpyrido[4, 3-b][1, 6]naphthyridine derivative
ActiveCN111393440AHigh catalytic activityImprove catalytic selectivityOrganic chemistryPiperidinedioneAryl
The invention discloses a method for preparing a medical intermediate 5, 10-diarylpyrido[4, 3-b][1, 6]naphthyridine derivative, and belongs to the technical field of biological medicine preparation. The method for preparing the medical intermediate 5, 10-diarylpyrido[4, 3-b][1, 6]naphthyridine derivative by acidic ionic liquid catalysis includes: taking aromatic aldehyde, amine and 2, 4-piperidinedione as the reaction raw materials, adopting an ethanol-dimethylformamide-water mixed solution as a reaction solvent, and performing catalysis by an acidic ionic liquid catalyst to obtain the 5, 10-diarylpyrido[4, 3-b][1, 6]naphthyridine derivative. The preparation process provided by the invention has the advantages of high product yield, recyclable catalytic system, short reaction time, simpleand convenient product purification process and the like.
Owner:马鞍山市泰博化工科技有限公司
Protein degradation targeting chimeric compound, application and preparation method thereof
PendingCN111393409AGood leaving performanceSmall molecular weightOrganic chemistryPharmaceutical non-active ingredientsProtein targetChlorobenzene
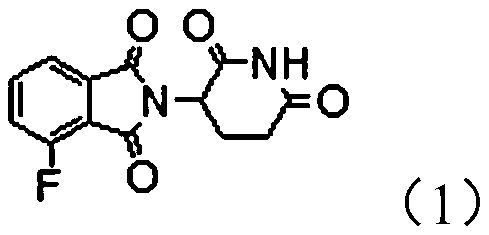
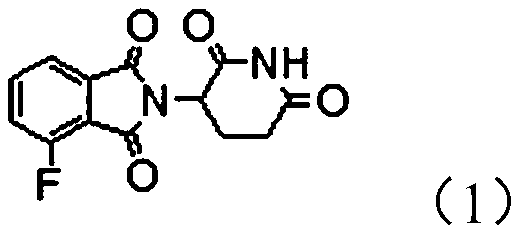
The invention provides a novel protein degradation targeting chimeric compound, application and a preparation method thereof. The protein degradation targeting chimeric compound is 2-(2, 6-dioxo-piperidine-3-yl)-4-fluoro-isoindole-1, 3-dione. The preparation method comprises the following steps: S1, carrying out a fluorination reaction on 3-chlorophthalic anhydride to generate 3-fluorophthalic anhydride; and step S2, carrying out a condensation reaction on the 3-fluorophthalic anhydride and 3-amino-2, 6-piperidinedione hydrochloride so as to obtain the 2-(2, 6-dioxo-piperidine-3-yl)-4-fluoro-isoindole-1, 3-dione. According to the protein degradation targeting chimeric body, the compound is moderate in molecular size, good in fluorine leaving performance, small in molecular weight and stable in performance, target protein and E3 ubiquitin enzyme can be effectively close to each other, and the protein degradation targeting chimeric body can be effectively used for preparing targeting drugs. And the preparation method is simple and convenient, less in three wastes and low in raw material price, so that the preparation cost is low, and the method is suitable for industrial large-scaleproduction.
Owner:SUZHOU HIGHFINE BIOTECH
Medicine for treating angina
InactiveCN106727744AIschemic typeElimination of myocardial infarction anginaPill deliveryAmide active ingredientsAnginaTherapeutic effect
The invention discloses a medicine for treating angina. The medicine for treating the angina is mainly prepared from, by weight, 8-19 parts of Phenobarbital, 17-25 parts of cannabinoid A, 5-10 parts of a ginkgo biloba extract, 2-6 parts of isosorbide-5-mononitrate, 11-18 parts of ibuprofen, 1.5-1.8 parts of heparin, 0.5-1.9 parts of crospovidone, 8-15 parts of sodium alginate and 2-4 parts of 3-ethyl-(4-aminophenyl)-piperidinedione. The prepared medicine can quickly relieve myocardial ischemia and myocardial infarction angina, the treatment effect is significant, and the angina does not relapse for a long term after being cured.
Owner:HENAN BALING ELECTRONICS TECH CO LTD
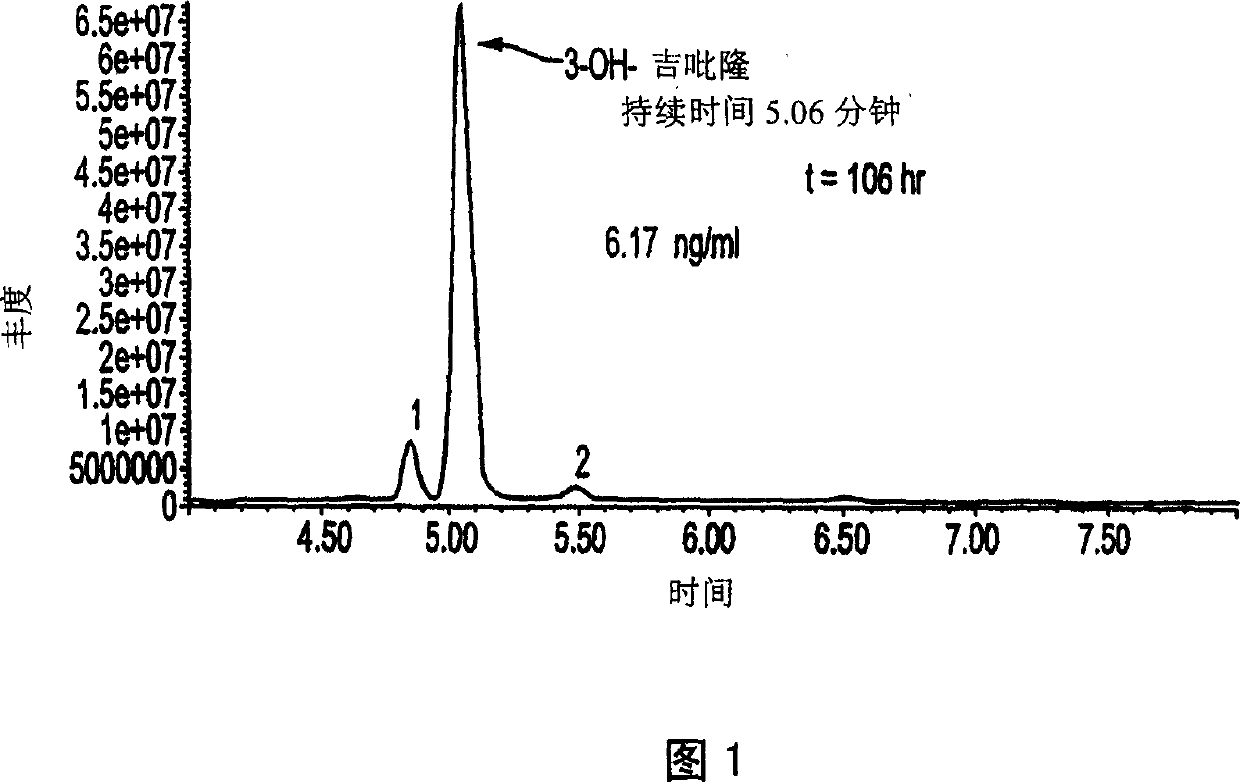
Use of bioactive metabolites of gepirone for treatment of psychological disorders
Bioactive gepirone metabolites, such as 3-OH gepirone (4,4,-dimethyl-3-hydroxy-1-[4-[4-(2-pyrimidinyl)-1-piperazinyl]butyl]-2,6-piperidinedione), and their pharmaceutically acceptable salts and hydrates, can be used to alleviate psychological disorders or the symptoms thereof. The use of these compounds provides advantages over other therapeutic azapirones as they possess superior bioavailability, faster onset of action, and more stable plasma levels when administered to a mammal.
Owner:FABRE KRAMER PHARMA INC +2
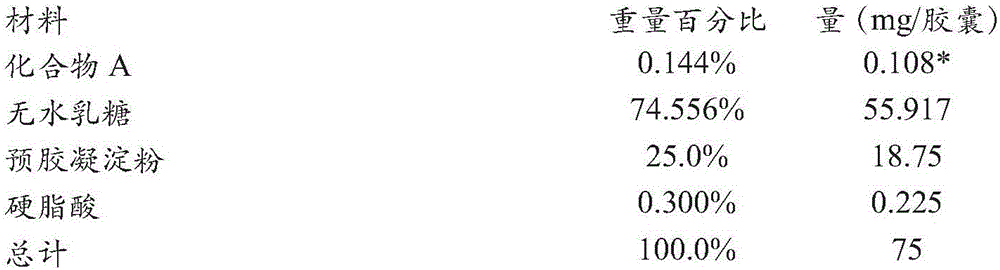
Formulations of (s)-3-(4-((4-(morpholinomethyl)benzyloxy)-1-oxoisoindolin-2-yl)piperidine-2,6-dione
Pharmaceutical compositions and single unit dosage forms of (S)-3-(4-((4-(morpholinomethyl)benzyl)oxy)-1-oxoisoindolin-2-yl)piperidine-2,6-dione, or a pharmaceutically acceptable stereoisomer, prodrug, salt, solvate, hydrate, or clathrate, are provided herein. Also provided are methods of treating, managing, or preventing various disorders, such as cancer, an inflammatory disease and / or an immune-related disorder.
Owner:CELGENE CORP
Preparation method of Pregabalin intermediate impurity
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
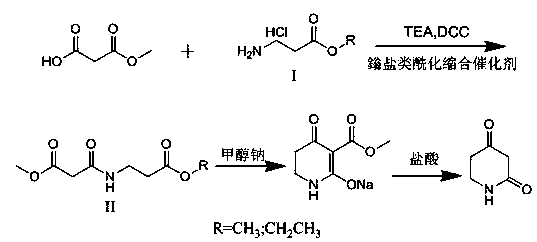
Synthetic method of 2, 4-dioxopiperidine
The invention discloses a synthetic method of 2, 4-dioxopiperidine. The method comprises the following steps: by taking monomethyl malonate, 3-amino methyl propionate hydrochloride or 3-amino ethyl propionate hydrochloride as an initial raw material, dichloromethane as a solvent, dicyclohexylcarbodiimide (DDC) as a dehydrator and triethylamine as an acid-binding agent, carrying out a reaction; acidylating a condensation product methyl-3-((3-methoxyl-3-carbonyl propyl) amino)-3-carbonyl propionate, wherein in the acidylating condensation reaction process, onium salt is used as a catalyst which is good in selectivity, few in side reaction and higher in yield; then, circularly condensing to obtain 3-(carbomethoxy(methoxycarbonyl))-4-carbonyl-1, 4, 5, 6-tetrapyridine-2-alcoholic sodium under the effect of sodium methylate; and then, decarboxylating in a hydrochloric acid system to obtain 2, 4-dioxopiperidine. In the whole reaction, as methoxyl is easy to remove, so that the synthetic method is available in the raw materials, mild in reaction condition, safe to operate and high in conversion rate.
Owner:兰州精细化工有限责任公司
3-substituted (1-oxoisoindoline-2-yl)piperidine-2,6-dione compounds and their synthetic methods
ActiveCN107739389BGroup 3/13 element organic compoundsAntineoplastic agentsMorpholineThiazolidinedione
The invention discloses a 3-site substituted (1-iso-indoxoline-2-base)piperidine-2,6-thiazolidinedione and a synthetic method thereof, and belongs to the technical field of medicine synthesis. In a formula I, Y can be Z, R1, R2, -(CH2)n-R3, wherein Z is a boric acid ester group or a boric acid group, R1 is hydroxyl, cyanogroup or trifluoromethyl, R2 is morpholinyl, piperidyl and methyl piperazinegroup; in the formula -(CH2)n-R3, R3 is alkylene, tert-butyl acetate, phenyl group and heterocyclic aromatic group, and n is 0 or 1. The compound can be used for preparing medicines capable of treating or preventing multiple myeloma, leukemia and lymphoma. The formula is shown in the description.
Owner:EAST CHINA NORMAL UNIV
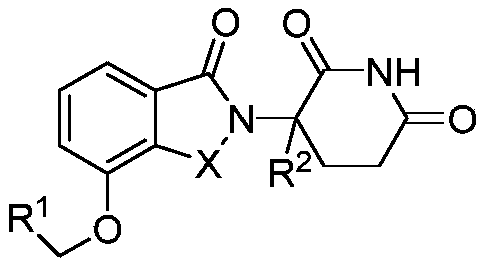
Tricyclic substituted piperidine diketones
ActiveCN112689627BGood pharmacokinetic propertiesGood inhibitory effectOrganic chemistryAntineoplastic agentsPiperidinedioneDiketone
Disclosed are a series of tricyclic substituted piperidine diketone compounds and their application in the preparation of drugs for the treatment of CRBN protein-related diseases, specifically disclosing derivative compounds represented by formula (I) or pharmaceutically acceptable salts thereof .
Owner:MEDSHINE DISCOVERY INC
A kind of production method of lenalidomide
The invention relates to a green production method of low-cost lenalidomide. According to the method, in presence of a solvent and an alkali, 3-aminopiperidine-2,6-dione and 1-halo-acetoacetate are subjected to dehydrogenation halogen acid condensation, dealcoholizing amidation, 2-halo-4-nitrobutanal dehydration and dehydrochlorination or dehydrobromination, 3-(7-nitro-3-oxo-1H-isoindol-2-yl)piperidine-2,6-dione is obtained, the process is completed with a one-pot method, nitro is reduced into amino by catalytic hydrogenation of 3-(7-nitro-3-oxo-1H-isoindol-2-yl)piperidine-2,6-dione, and lenalidomide is prepared. The method has the advantages of cheap and easily available raw materials, short technological process, simple operation and environmental protection and is a production method beneficial to industrialization.
Owner:XINFA PHARMA
Preparation method of 3-(4-amino-1,3-dihydro-1-oxo-2h-isoindol-2-yl)-2,6-piperidinedione
ActiveCN104710405BThe preparation method is safeLow equipment requirementsOrganic chemistryPiperidinedioneOrganic solvent
Owner:JIANGSU HANSOH PHARMA CO LTD
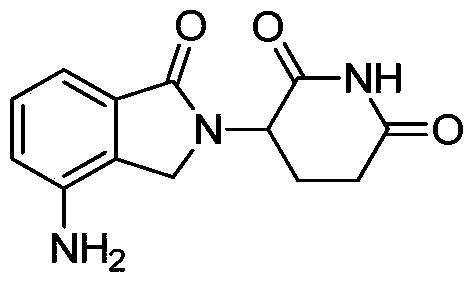
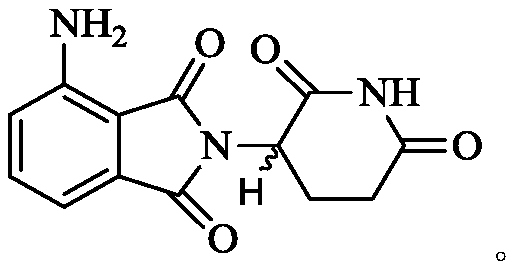
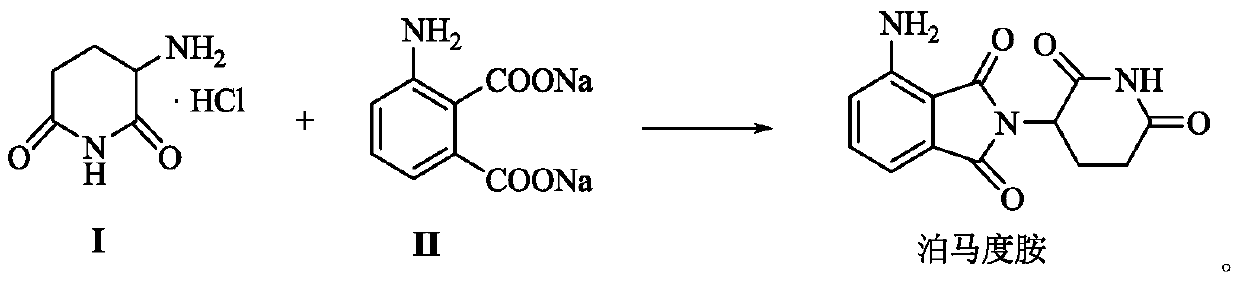
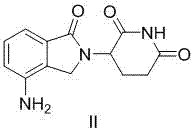
A kind of preparation method of pomalidomide
The invention relates to a preparation method of pomalidomide. The preparation method includes a process of using 3-aminopiperidine-2, 6-dione hydrochloride (which is a compound shown in the formula I) and disodium 3-amino phthalate (which is a compound shown in the formula II) as raw materials. The method is simple and short in route, simple to operate, available in raw materials, reduced in production cost, and suitable for massive industrial production.
Owner:LIANYUNGANG RUNZHONG PHARMA CO LTD
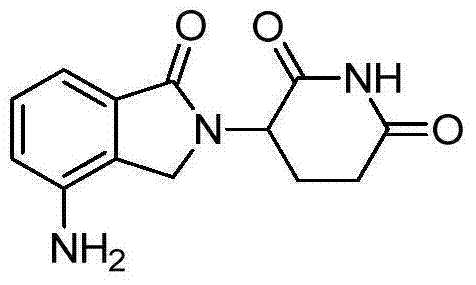
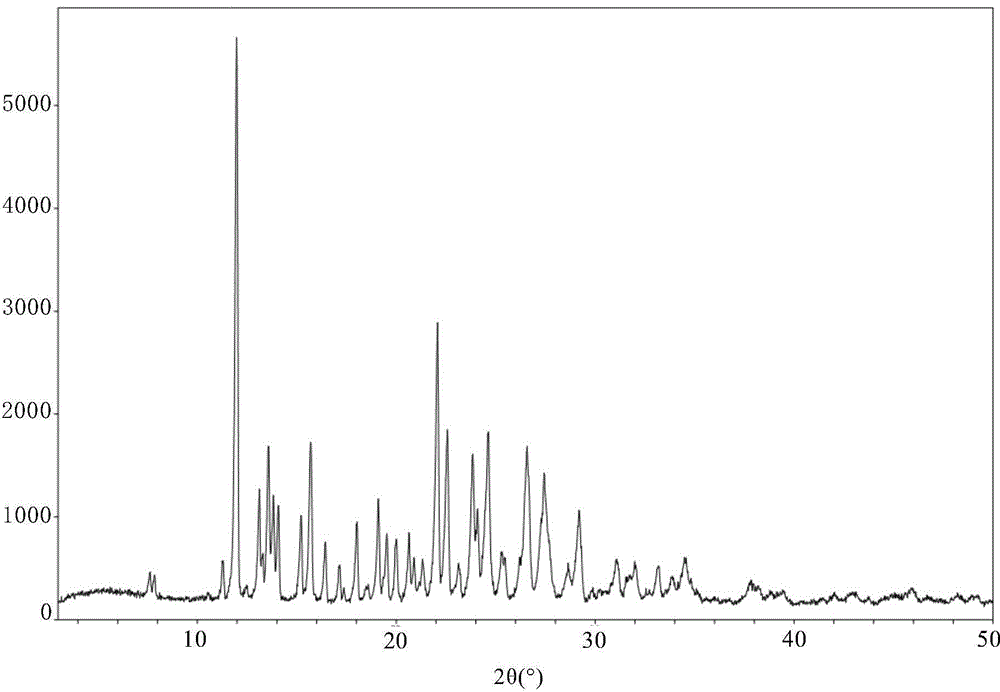
3-(substituted dihydroisoindol-2-yl)-2,6-piperidinedione polymorph and pharmaceutical composite
ActiveCN102127054BImprove stabilitySuitable for long term storageOrganic active ingredientsNervous disorderPiperidinedioneKetone
The invention discloses a 3-(4-amino-1-oxo-1,3-dihydro-2H-isoindol-2-yl)-piperidine-2,6-dione polymorph and also discloses a preparation method and pharmaceutical composite thereof.
Owner:NANJING CAVENDISH BIO ENG TECH +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
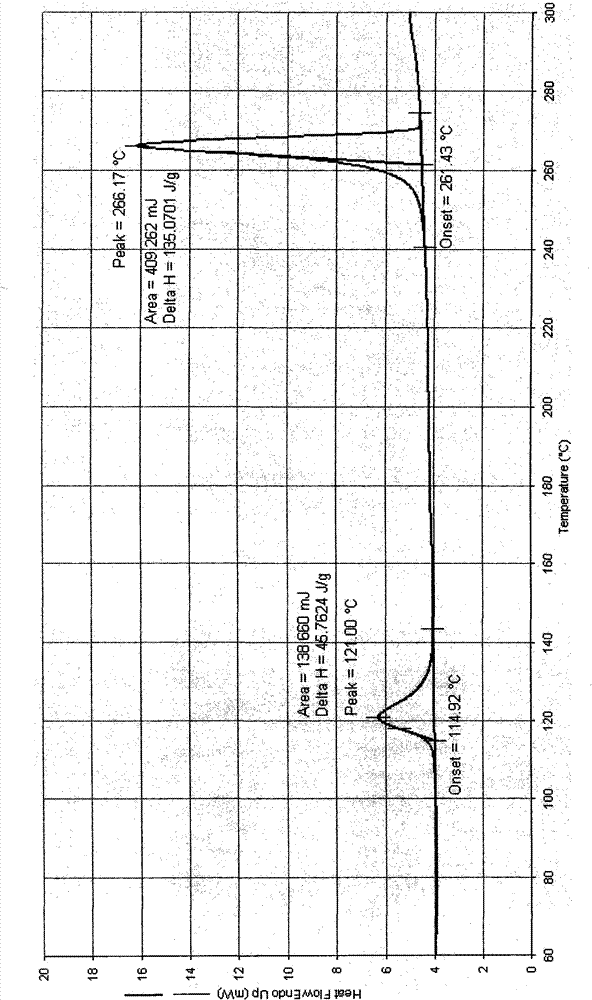
![Method for preparing medical intermediate 5, 10-diarylpyrido[4, 3-b][1, 6]naphthyridine derivative Method for preparing medical intermediate 5, 10-diarylpyrido[4, 3-b][1, 6]naphthyridine derivative](https://images-eureka.patsnap.com/patent_img/6fedb419-6872-4472-9f71-4608f9e453fe/HDA0002485208940000011.png)
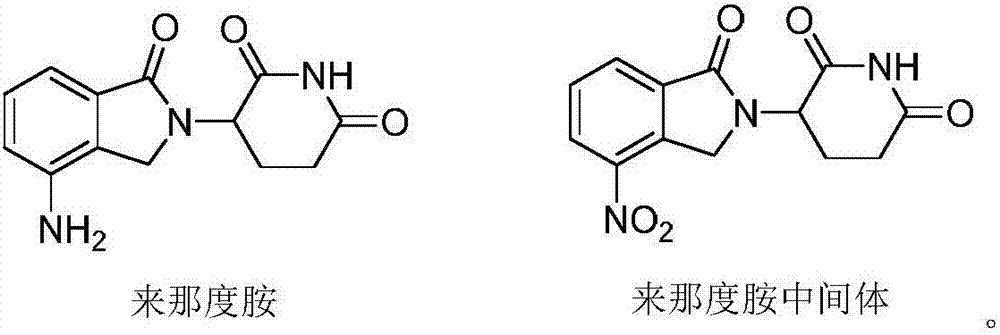
![Method for preparing medical intermediate 5, 10-diarylpyrido[4, 3-b][1, 6]naphthyridine derivative Method for preparing medical intermediate 5, 10-diarylpyrido[4, 3-b][1, 6]naphthyridine derivative](https://images-eureka.patsnap.com/patent_img/6fedb419-6872-4472-9f71-4608f9e453fe/HDA0002485208940000012.png)
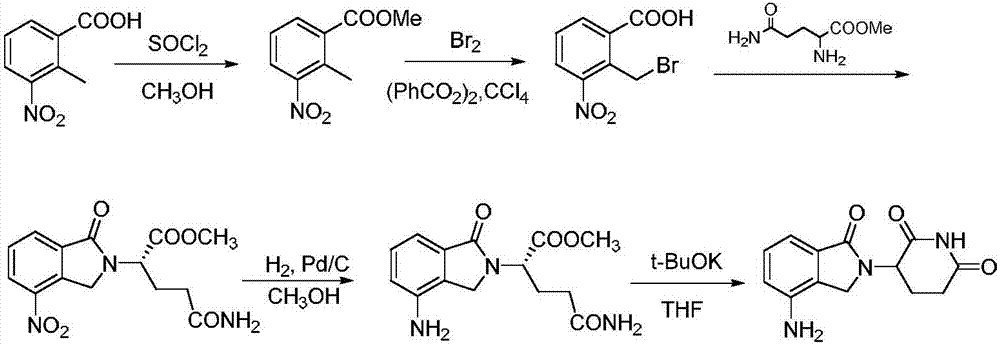
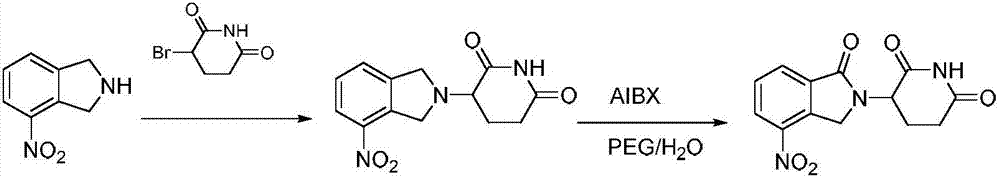
![Method for preparing medical intermediate 5, 10-diarylpyrido[4, 3-b][1, 6]naphthyridine derivative Method for preparing medical intermediate 5, 10-diarylpyrido[4, 3-b][1, 6]naphthyridine derivative](https://images-eureka.patsnap.com/patent_img/6fedb419-6872-4472-9f71-4608f9e453fe/FDA0002485208920000011.png)