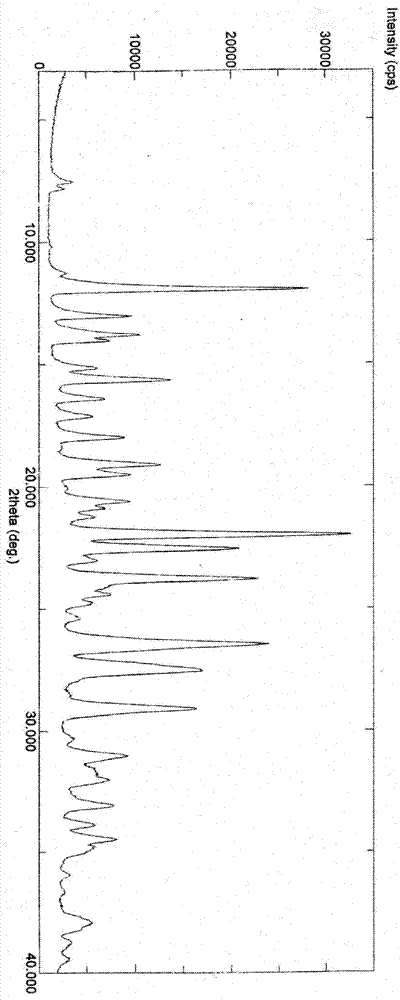
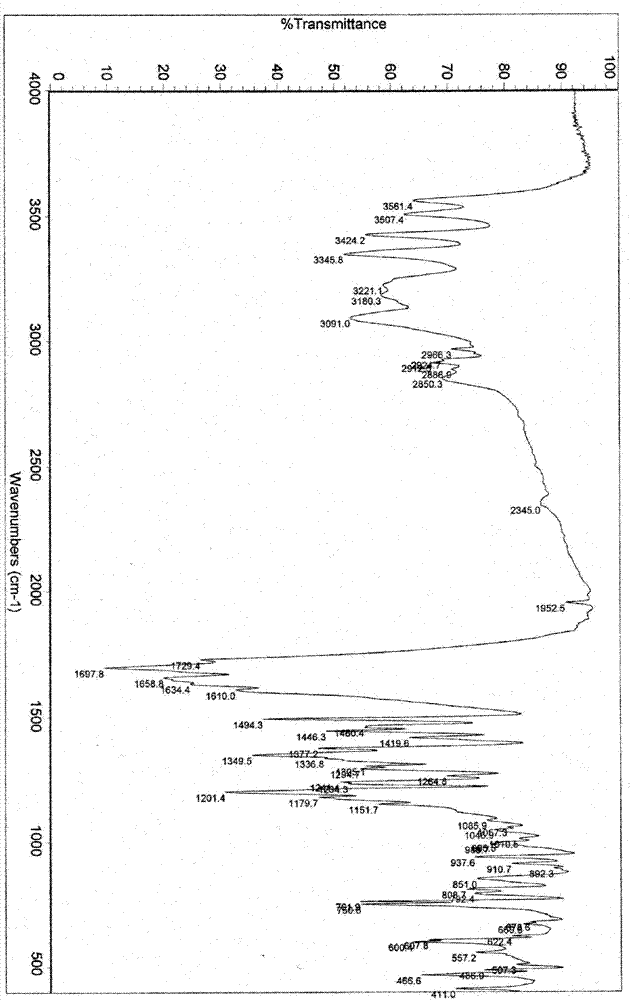
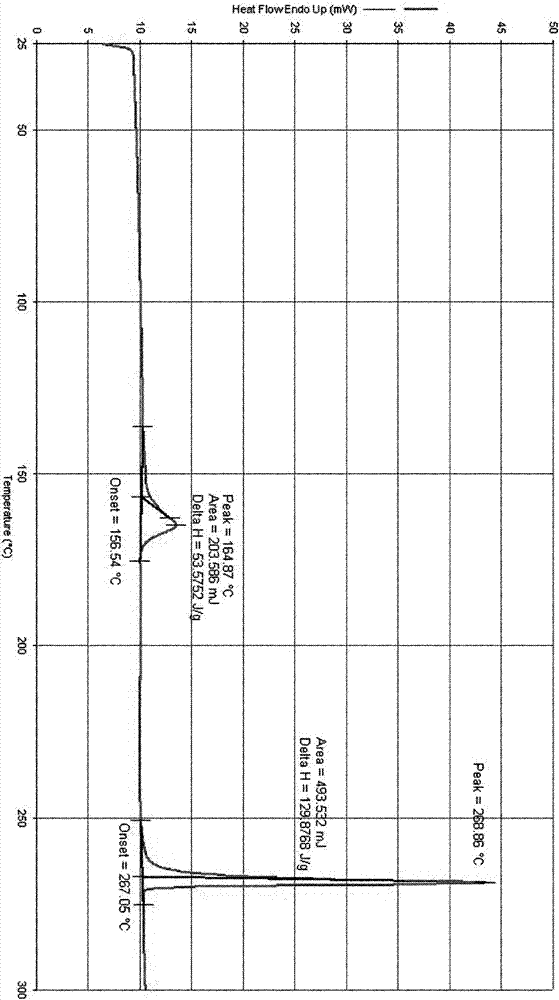
3-(substituted dihydroisoindol-2-yl)-2,6-piperidinedione polymorph and pharmaceutical composite
A polymorph, isoindole technology, applied in the field of 3-(substituted dihydroisoindol-2-yl)-2,6-piperidinedione polymorph and pharmaceutical composition, capable of Solve the problems that have not been considered and are unfavorable for industrial scale preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0202] Preparation of polymorph I:
[0203] Add 100 g of 3-(4-amino-1-oxo-1,3-dihydro-2H-isoindol-2-yl)piperidine-2,6-dione to 400 ml of DMF (or 300 ml of DMSO) In the solution, stir and heat to make it dissolve, add dropwise 1600 milliliters of pure water (or a mixed solvent system of 1000 milliliters of pure water and 600 milliliters of organic solvent (such as water and acetone, acetonitrile, ethyl acetate, dichloromethane, isopropanol, methanol , ethanol and other 3-(4-amino-1-oxo-1,3-dihydro-2H-isoindol-2-yl)piperidine-2,6-dione insoluble organic solvents or binary or Mixed system above binary system), slowly lower the temperature under stirring to precipitate the solid, recover the solid, and dry it under reduced pressure to obtain 3-(4-amino-1-oxo-1,3-dihydro-2H-isoindoline Indol-2-yl)piperidine-2,6-dione polymorph I.
[0204] DMF / water system: the product weighs 78 grams, and the yield is 78%;
[0205] DMSO / water system: the product weighed 90 grams, and the yield w...
Embodiment 2
[0208] Preparation of polymorph II:
[0209] Add 100 g of 3-(4-amino-1-oxo-1,3-dihydro-2H-isoindol-2-yl)piperidine-2,6-dione to 400 ml of anhydrous DMF, stir and heat to It dissolves; add 1800 milliliters of anhydrous ethanol (or 1600-2000 milliliters of single or mixed solvents such as methanol, acetone, ethyl acetate, acetonitrile, dichloromethane, acetonitrile) dropwise, slowly cool down under stirring to precipitate solids, and recover solids , dried in vacuo under reduced pressure to obtain 3-(4-amino-1-oxo-1,3-dihydro-2H-isoindol-2-yl)piperidine-2,6-dione polymorph II.
[0210] The product weighed 72 grams, and the yield was 72%.
[0211]
Embodiment 3
[0213] Preparation of polymorph III:
[0214] Add 100 g of 3-(4-amino-1-oxo-1,3-dihydro-2H-isoindol-2-yl)piperidine-2,6-dione into 300 ml of anhydrous DMSO, stir slowly Slowly heat to make it dissolve, add dropwise 2000 ml of anhydrous ethanol (other optional organic solvents such as: methanol, acetone, ethyl acetate, acetonitrile, dichloromethane, etc. 3-(4-amino-1-oxo- 1,3-dihydro-2H-isoindol-2-yl) piperidine-2,6-dione insoluble single or mixed solvents), slowly cool down under stirring to precipitate solids, reclaim the solids, depressurize and vacuum Drying affords 3-(4-amino-1-oxo-1,3-dihydro-2H-isoindol-2-yl)piperidine-2,6-dione polymorph III.
[0215] Product weight: 86 grams, yield 86%.
[0216]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com