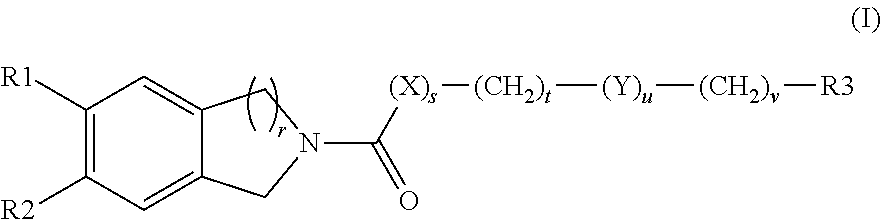Patents
Literature
300 results about "Isoindoline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Isoindoline is a heterocyclic organic compound with the molecular formula C₈H₉N. The parent compound has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing ring. The compound's structure is similar to indoline except that the nitrogen atom is in the 2 position instead of the 1 position of the five-membered ring. Isoindoline itself is not commonly encountered, but several derivatives are found in nature and some synthetic derivatives are commercially valuable drugs, e.g. pazinaclone.
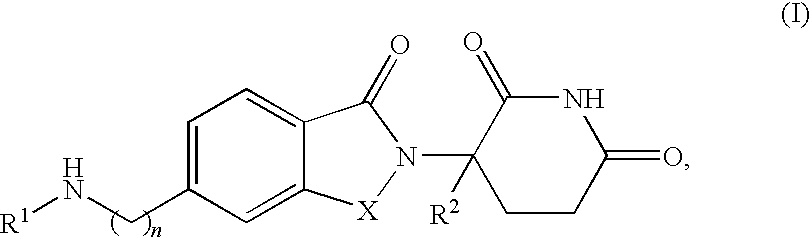
Methods and compositions for the treatment and management of hemoglobinopathy and anemia
InactiveUS20050143420A1Good effectRelieve symptomsBiocidePeptide/protein ingredientsRed blood cellThalassemia
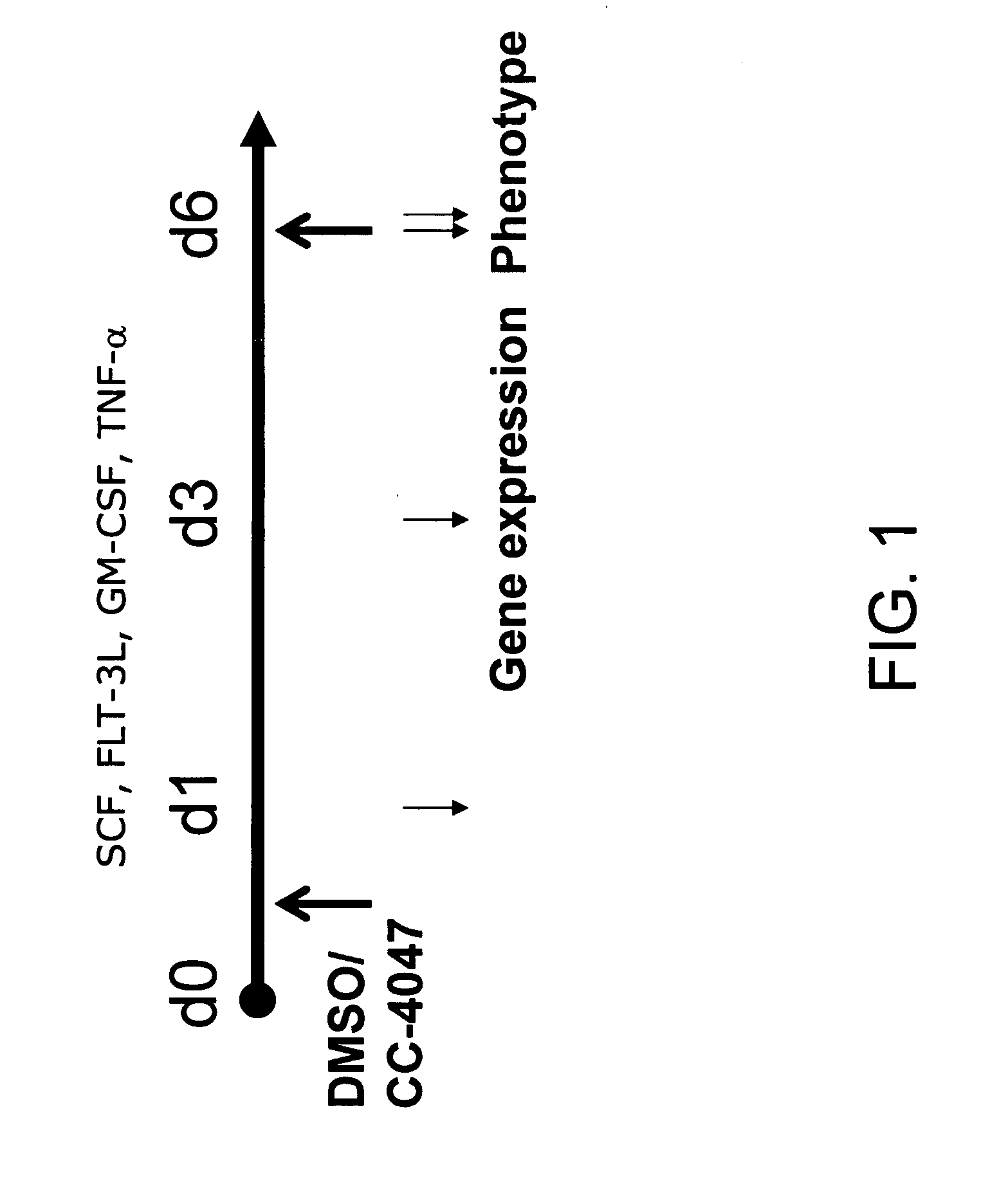
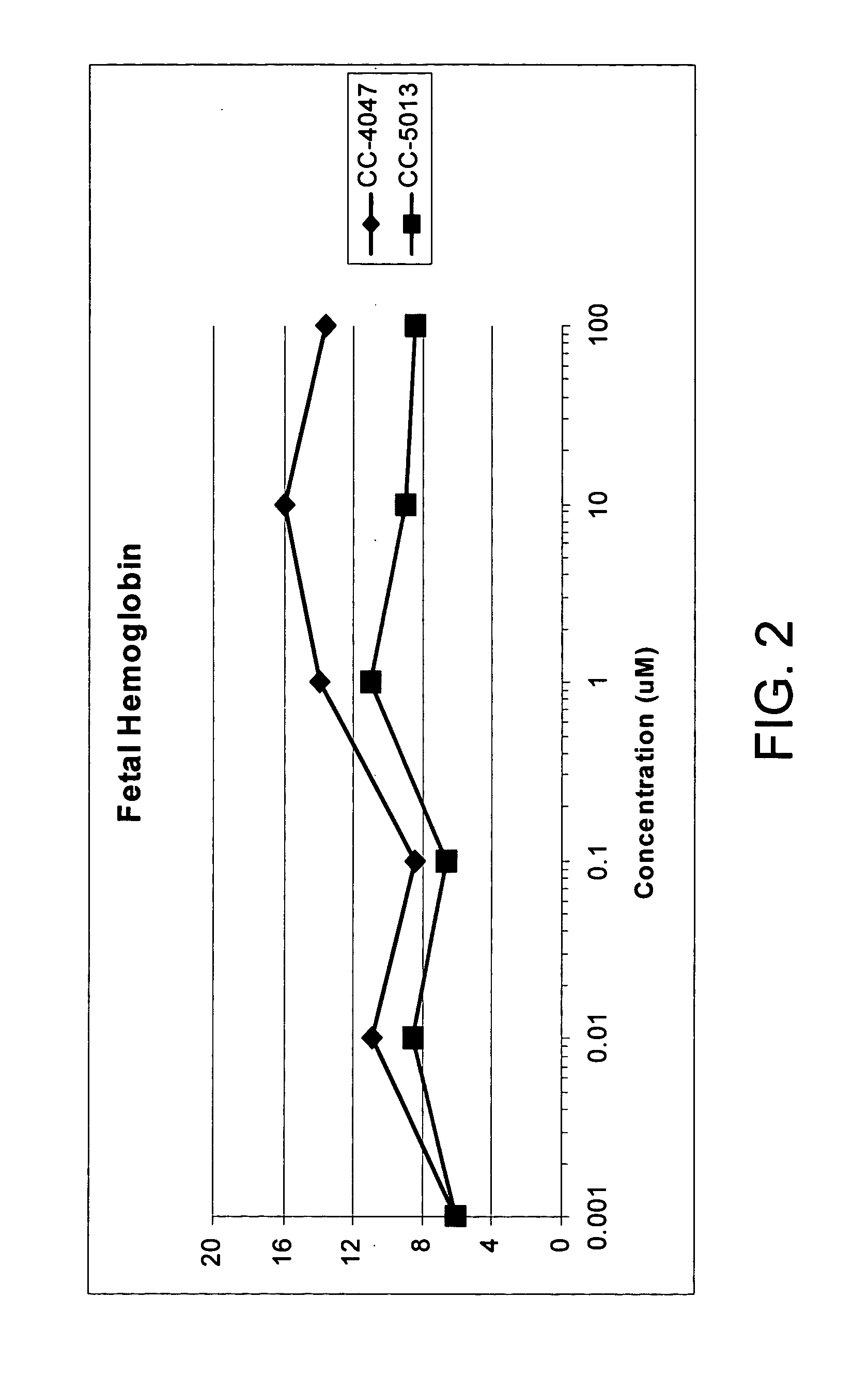
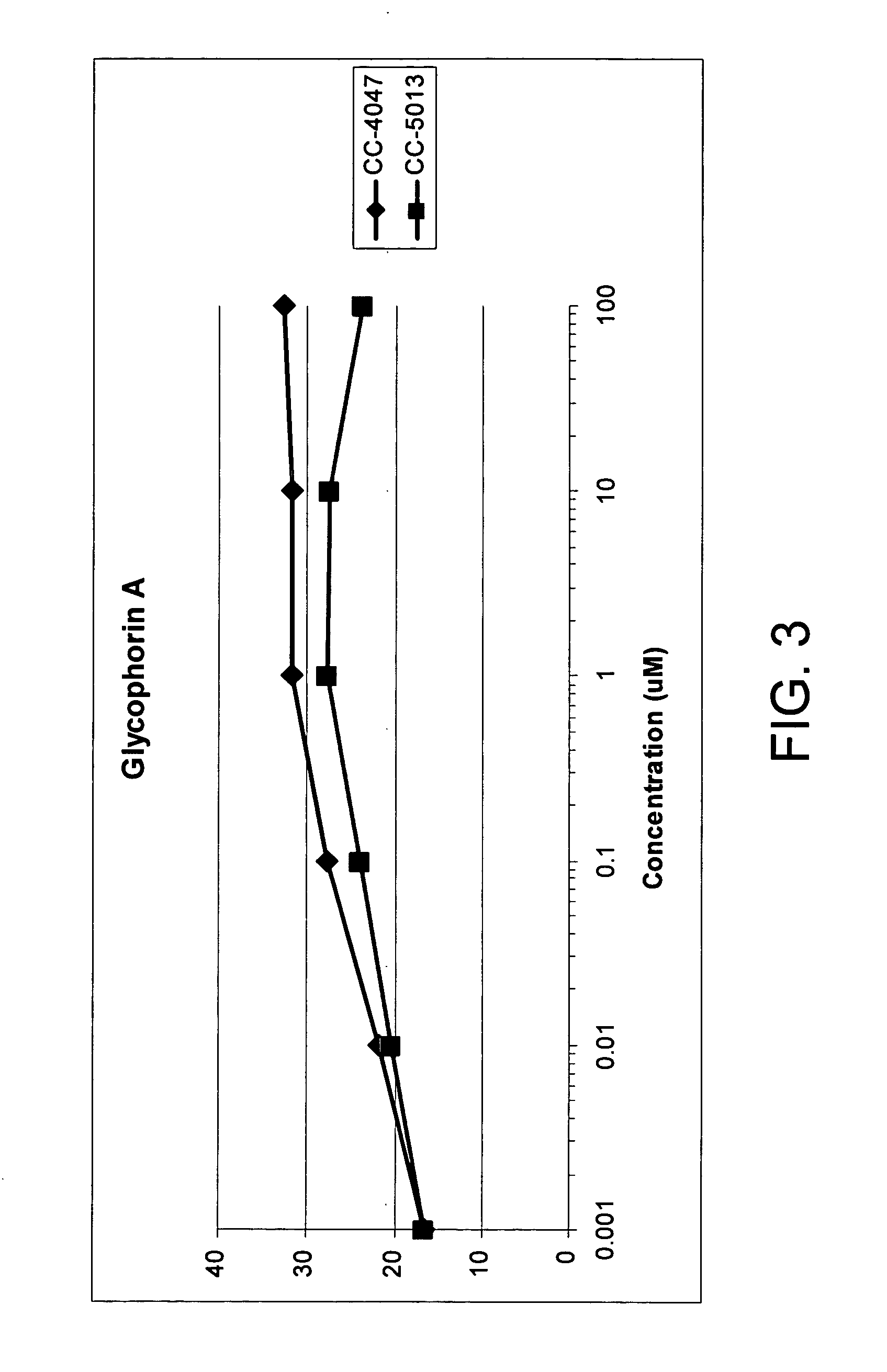
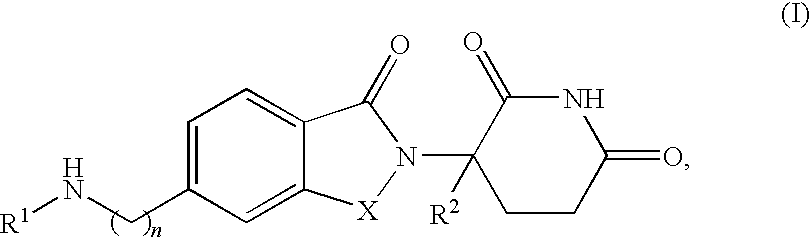
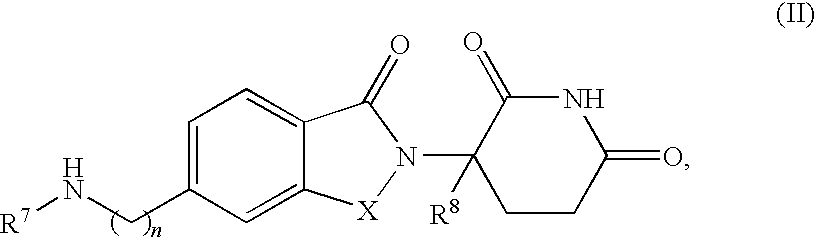
The present invention is directed to the use of immunomodulatory compounds, particularly members of the class of compounds known as IMiDs™, and more specifically the compounds 4-(Amino)-2-(2,6-dioxo(3-piperidyl))-isoindoline-1,3-dione and 3-(4-amino-1-oxo-1,3-dihydroisoindol-2-yl)-piperidine-2,6-dione, to induce the expression of fetal hemoglobin genes, genes essential for erythropoiesis, and genes encoding alpha hemoglobin stabilizing protein, within a population of CD34+ cells. These compounds are used to treat hemoglobinopathies such as sickle cell anemia or β-thalassemia, or anemias caused by disease, surgery, accident, or the introduction or ingestion of toxins, poisons or drugs.
Owner:SIGNAL PHARMA LLC
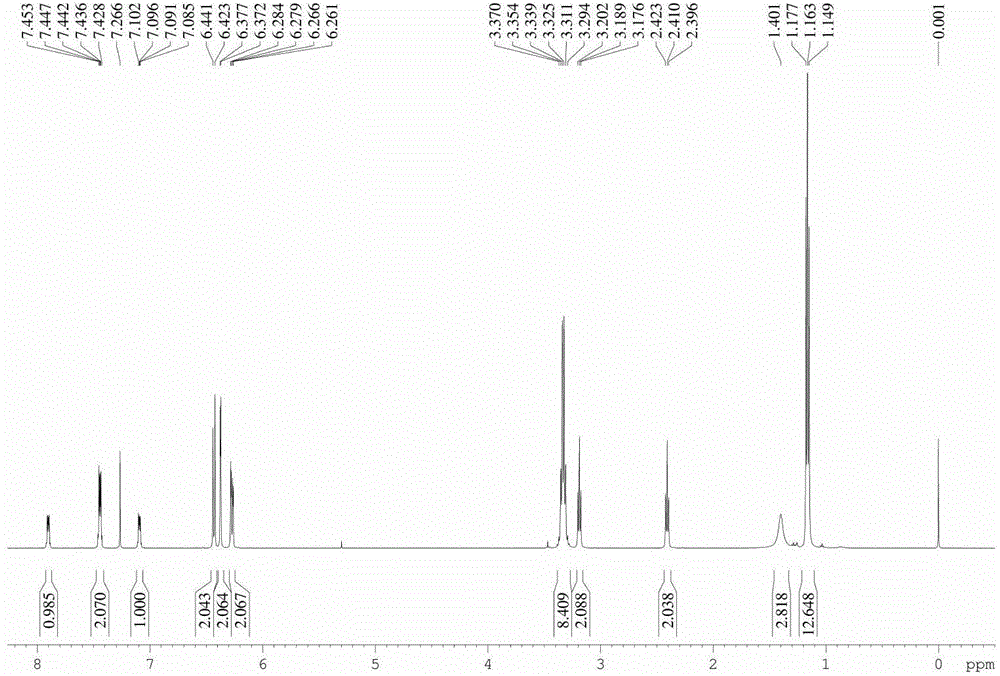
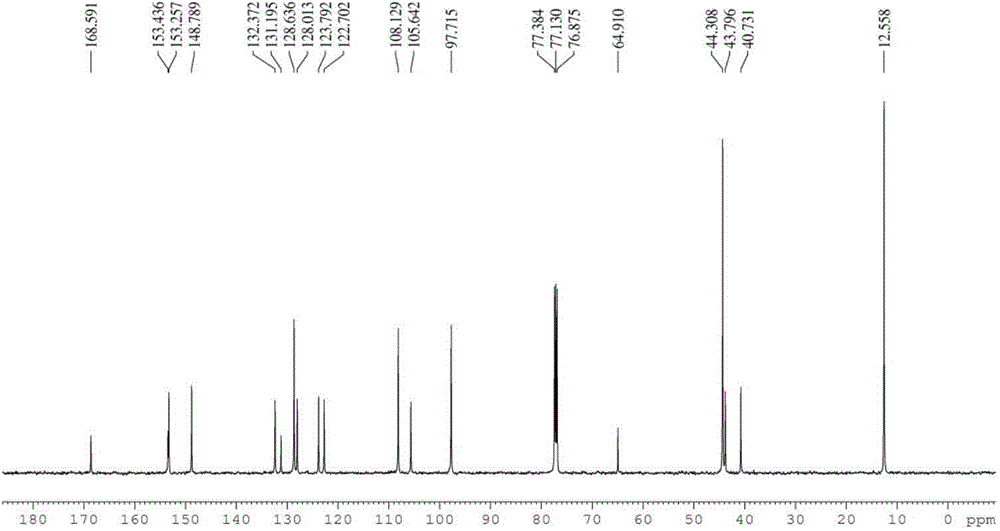
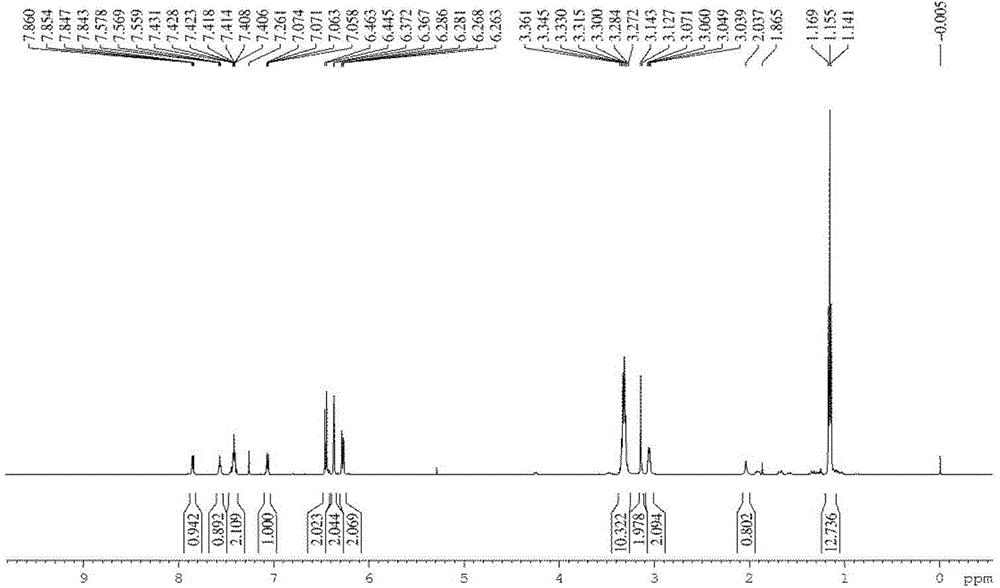
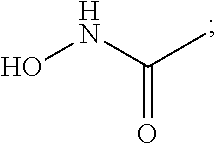
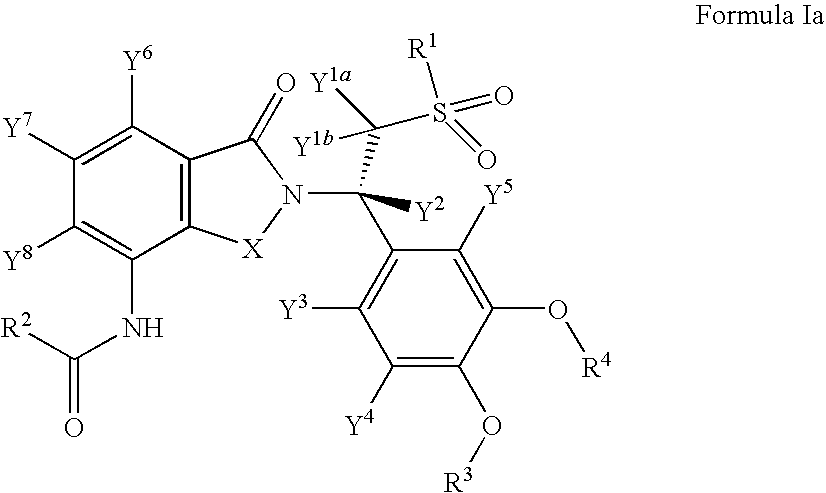
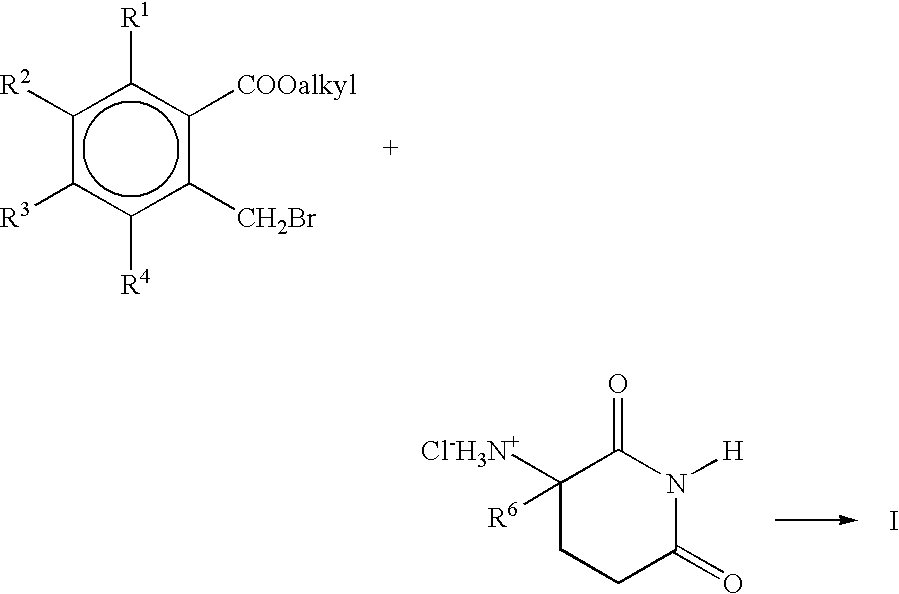
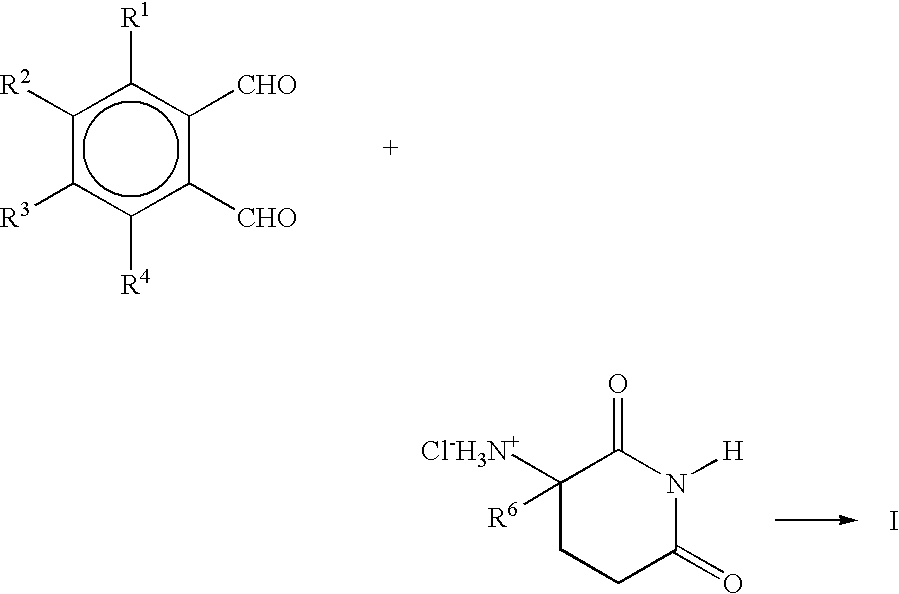
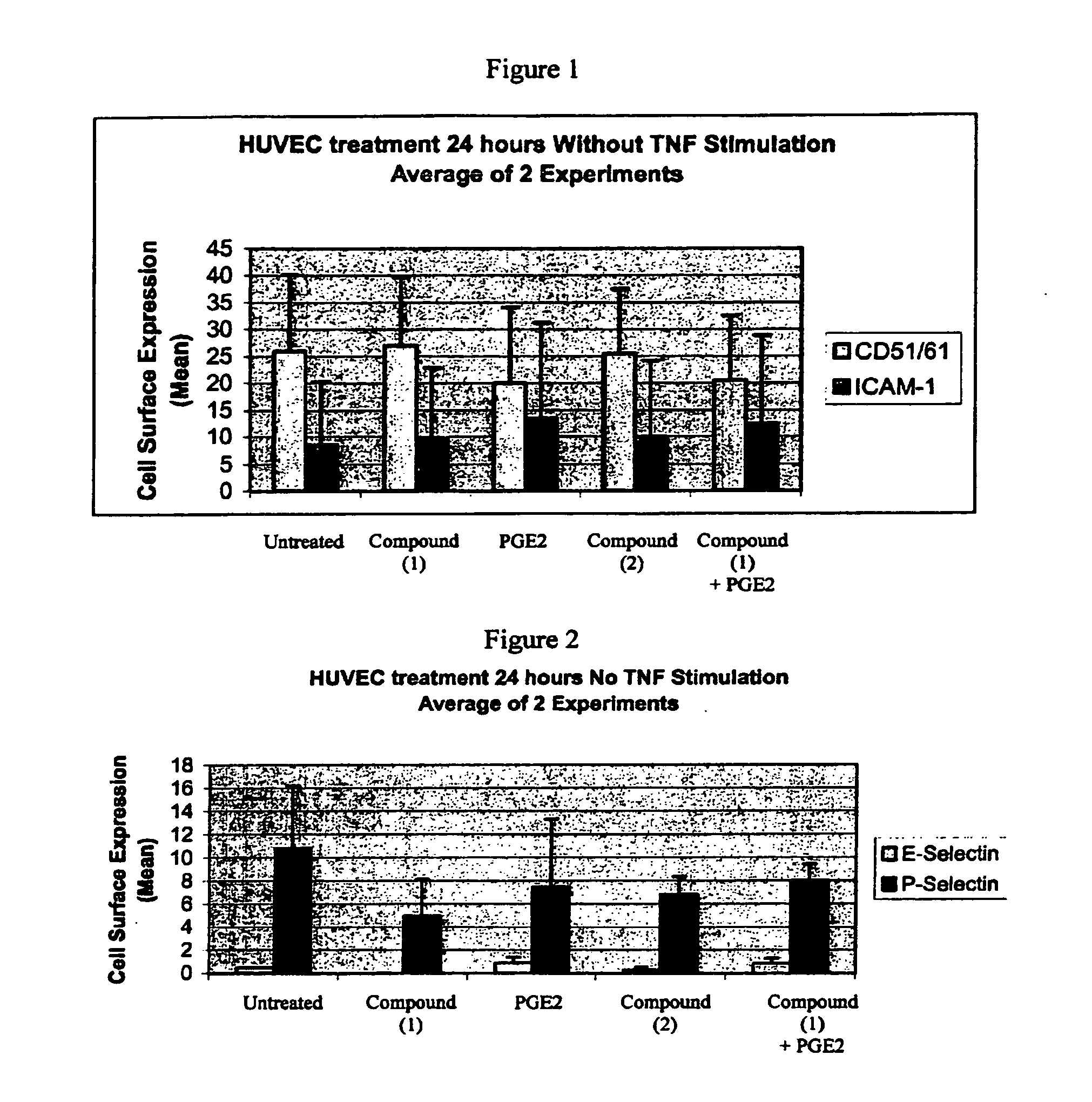
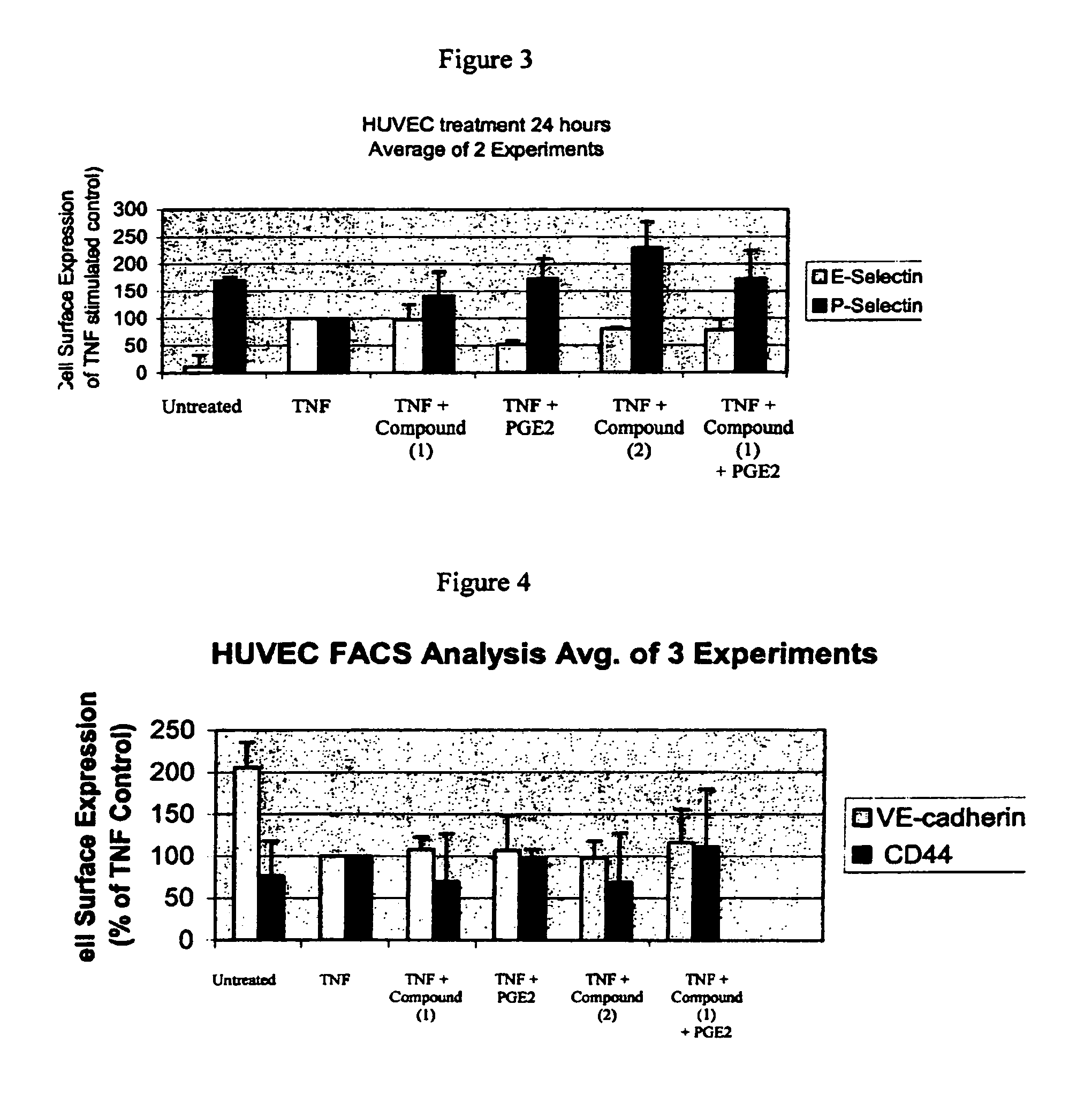
Solid forms comprising (+)-2-[1-(3-ethoxy-4-methoxyphenyl)-2- methylsulfonylethyl]-4-acetylaminoisoindoline-1,3-dione, compositions thereof, and uses thereof
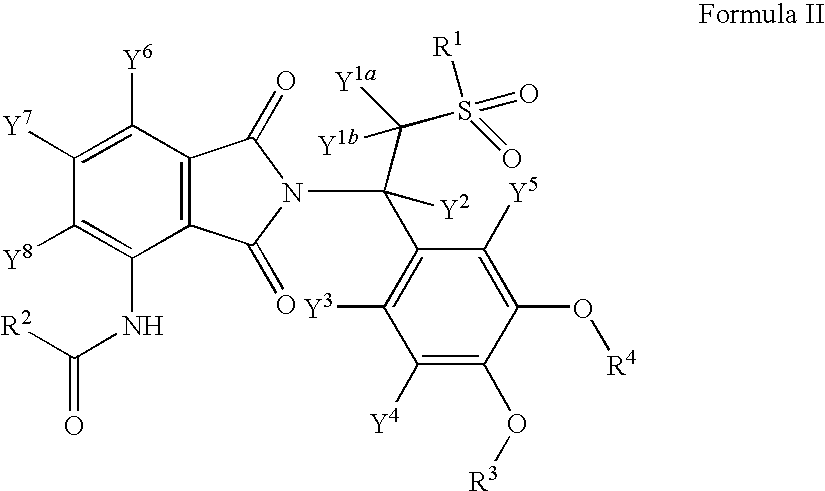
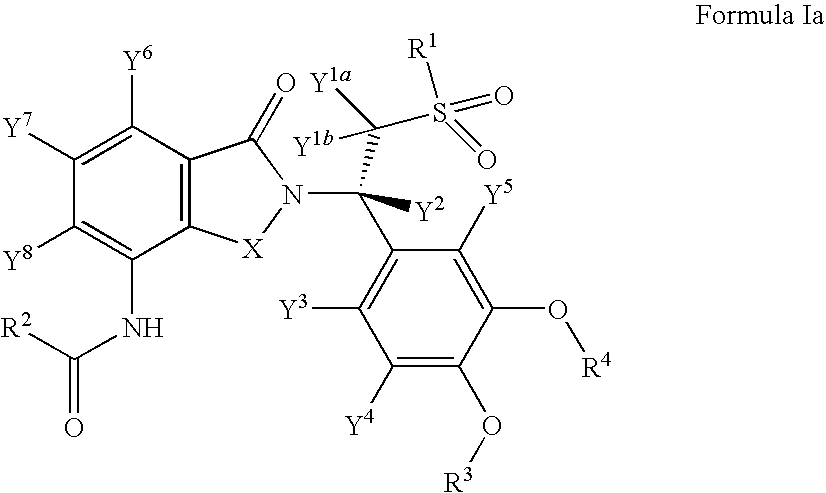
ActiveUS20080234359A1Avoidance of adverse effectReduction of adverse effectBiocideNervous disorderMedicineKetone
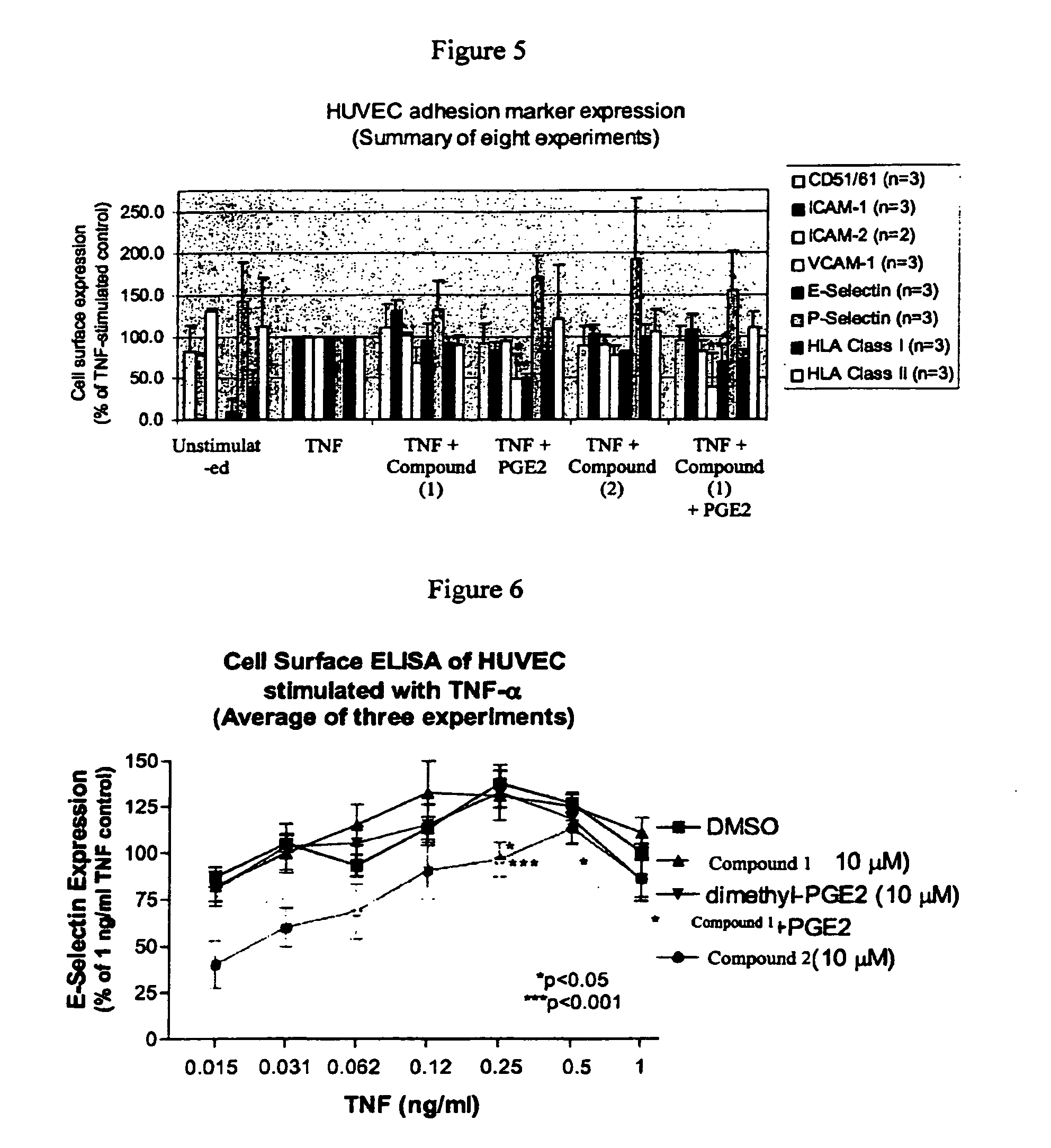
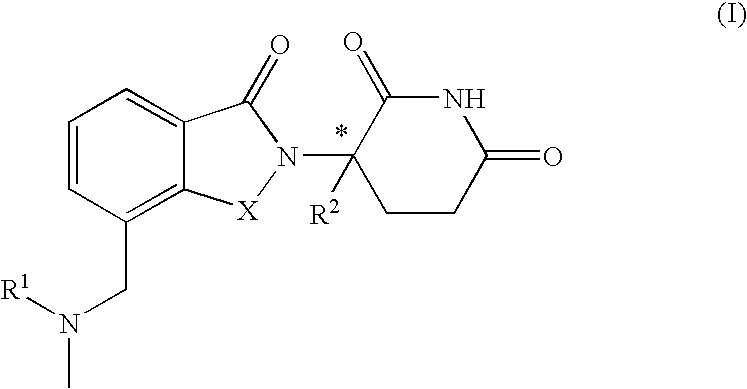
Solid forms comprising (+)-2-[1-(3-Ethoxy-4-methoxyphenyl)-2-methylsulfonylethyl]-4-acetylaminoisoindoline-1,3-dione, compositions comprising the solid forms, methods of making the solid forms and methods of their use are disclosed. The methods include methods of treating and / or preventing disorders ameliorated by the reduction of levels of TNF-α or the inhibition of PDE4.
Owner:AMGEN INC
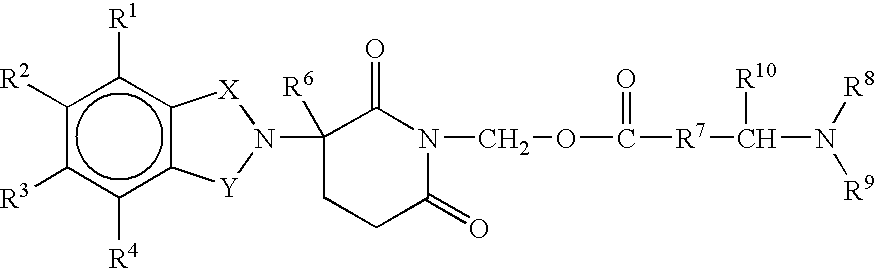
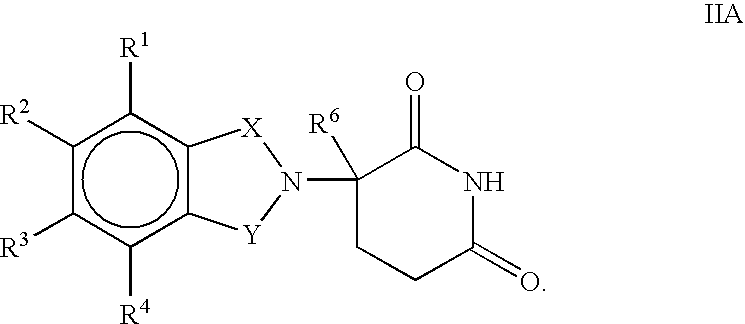
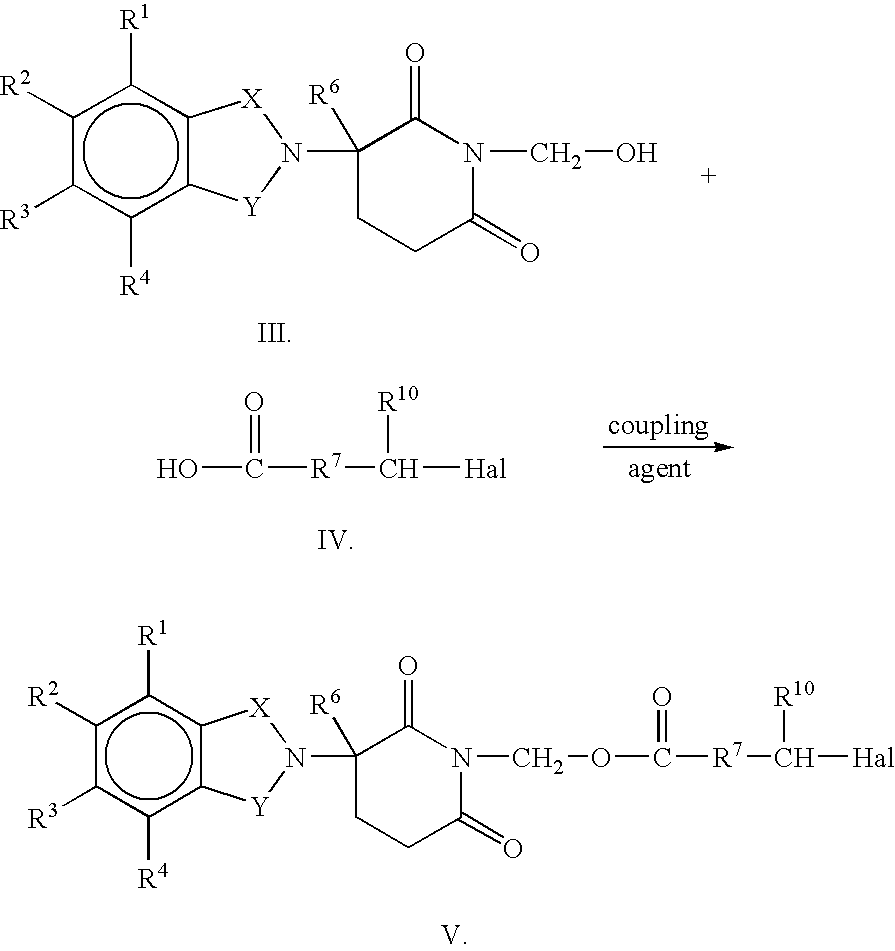
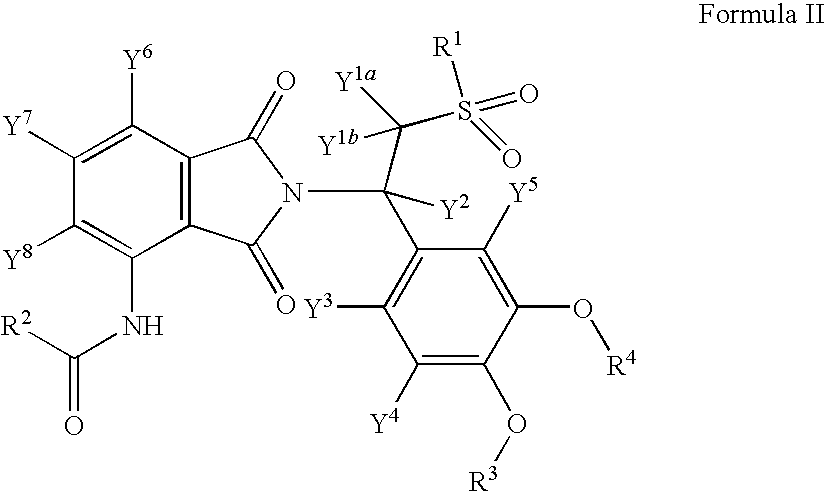
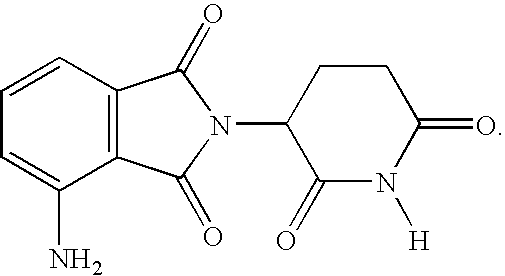
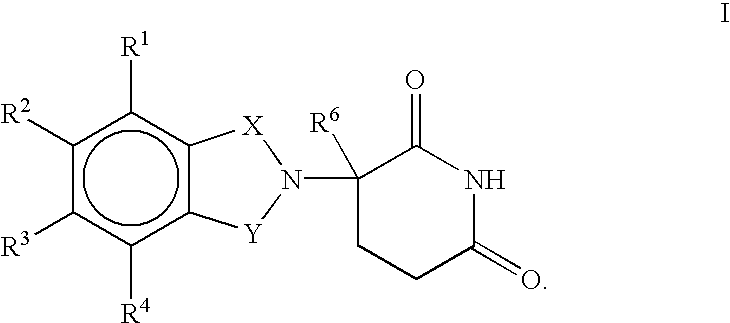
Pharmaceutical compositions of 1-oxo-2-(2,6-dioxopiperidin-3-yl)-4-aminoisoindoline
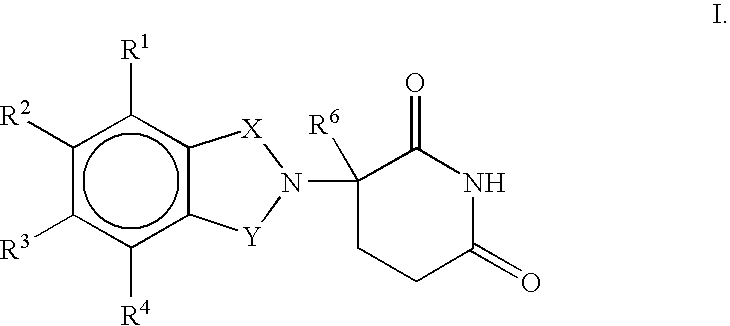
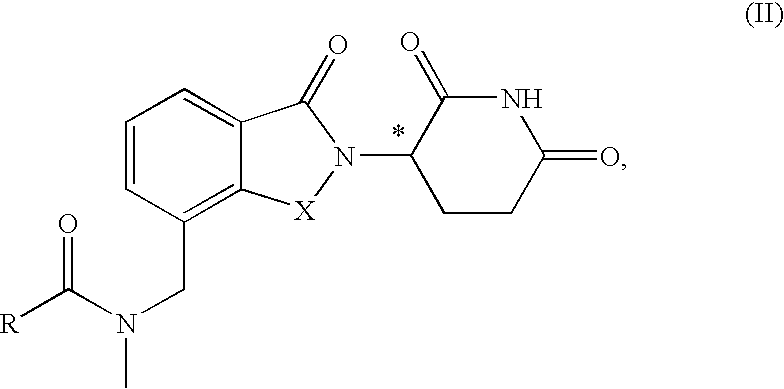
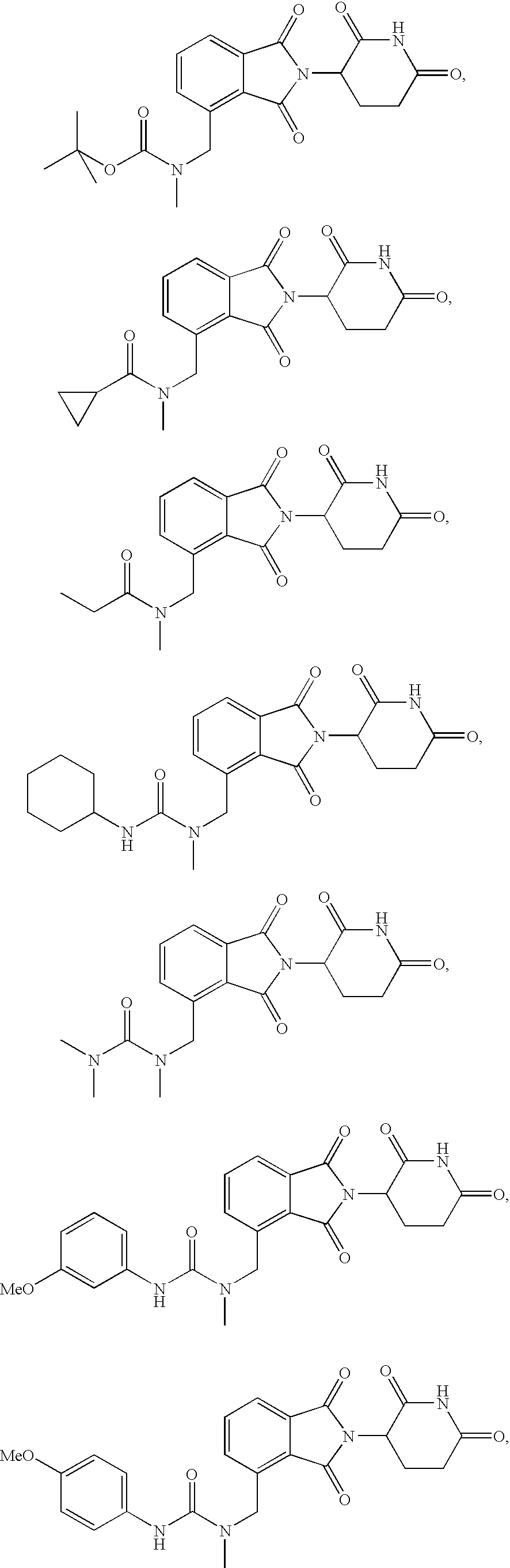
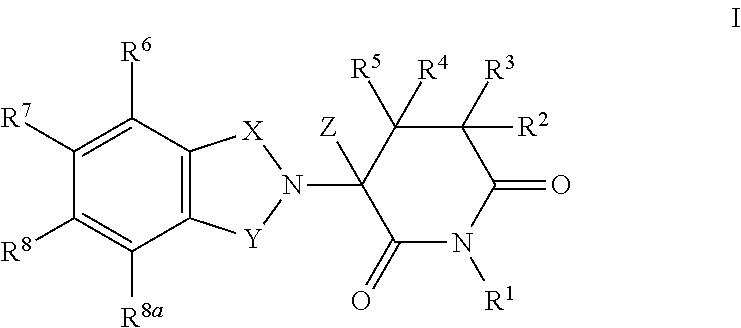
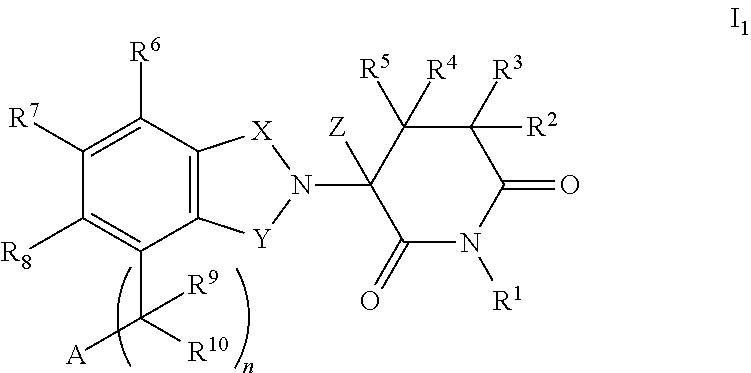
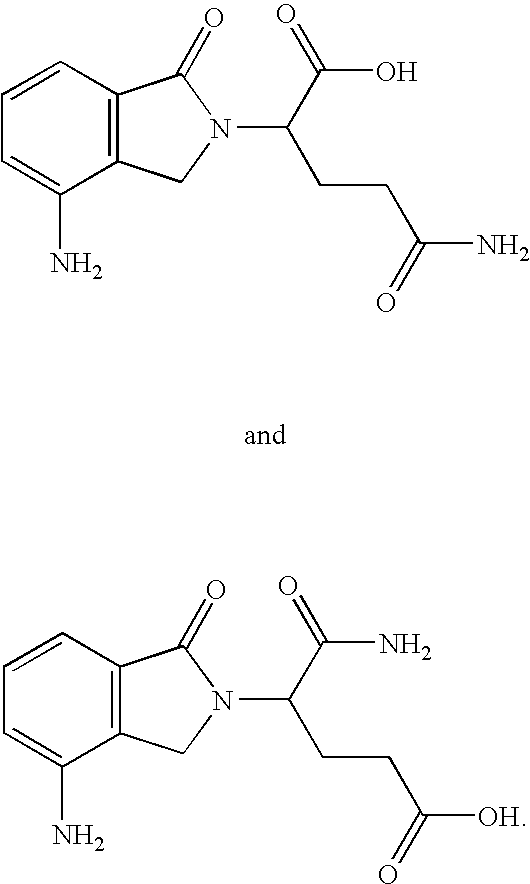
Substituted 2-(2,6-dioxopiperidin-3-yl)phthalimides and 1-oxo-2-(2,6-dioxopiperidin-3-yl)isoindolines reduce the levels of TNFα in a mammal. Typical embodiments are 1-oxo-2-(2,6-dioxo-3-methylpiperidin-3-yl)-4,5,6,7-tetrafuoroisoindoline and 1,3-dioxo-2-(2,6-dioxo-3 -methylpiperidin-3-yl)-4-aminoisoindoline.
Owner:CELGENE CORP
Methods for treating cutaneous lupus using aminoisoindoline compounds
Methods of treating cutaneous lupus in a human are disclosed. Specific methods encompass the administration of (+)-2-[1-(3-ethoxy-4-methoxyphenyl)-2-methylsulfonylethyl]-4-acetylaminoisoindoline-1,3-dione, 4-(amino)-2-(2,6-dioxo(3-piperidyl))-isoindoline-1,3-dione (ACTIMID™), 3-(4-amino-1-oxo-1,3-dihydro-isoindol-2-yl)-piperidine-2,6-dione (REVLIMID®), or cyclopropyl 2-[(1S)-1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethyl]-3-oxoisoindolin-4-yl}carboxamide, alone or alternatively, in combination with a second active agent.
Owner:CELGENE CORP
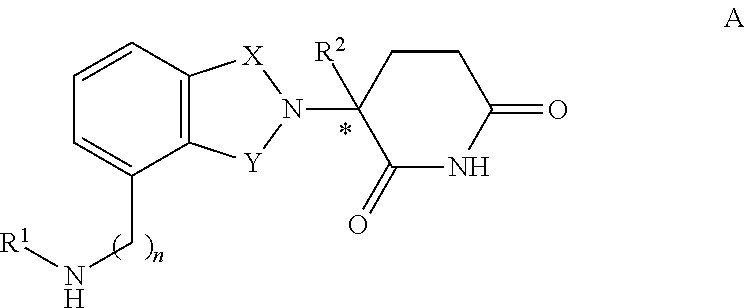
N-methylaminomethyl isoindole compounds and compositions comprising and methods of using the same
This invention relates to N-methylaminomethyl-isoindoline compounds, and pharmaceutically acceptable salts, solvates, stereoisomers, and prodrugs thereof. Methods of use, and pharmaceutical compositions of these compounds are disclosed.
Owner:CELGENE CORP
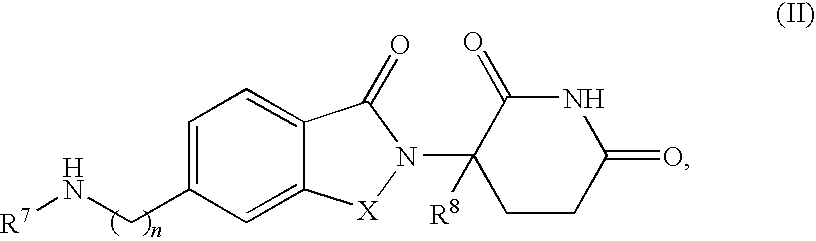
Methods for the treatment and management of myeloproliferative diseases using 4-(AMINO)-2-(2,6-dioxo(3-piperidyl)-isoindoline-1,3-dione in combination with other therapies
InactiveUS20090088410A1Reduce adverse effectsImprove toleranceBiocideAnimal repellantsJAK1 InhibitorMedicine
Methods of treating, preventing and / or managing a myeloproliferative disease are disclosed. Specific methods encompass the administration of 4-(amino)-2-(2,6-dioxo(3-piperidyl))-isoindoline-1,3-dione, or a pharmaceutically acceptable salt, solvate or stereoisomer thereof, in combination with a second active agent. Particular second active agents are is prednisone, JAK1 inhibitor, JAK2 inhibitor, FLT3 inhibitor, BCL2 inhibitor, and HDAC inhibitor.
Owner:CELGENE CORP
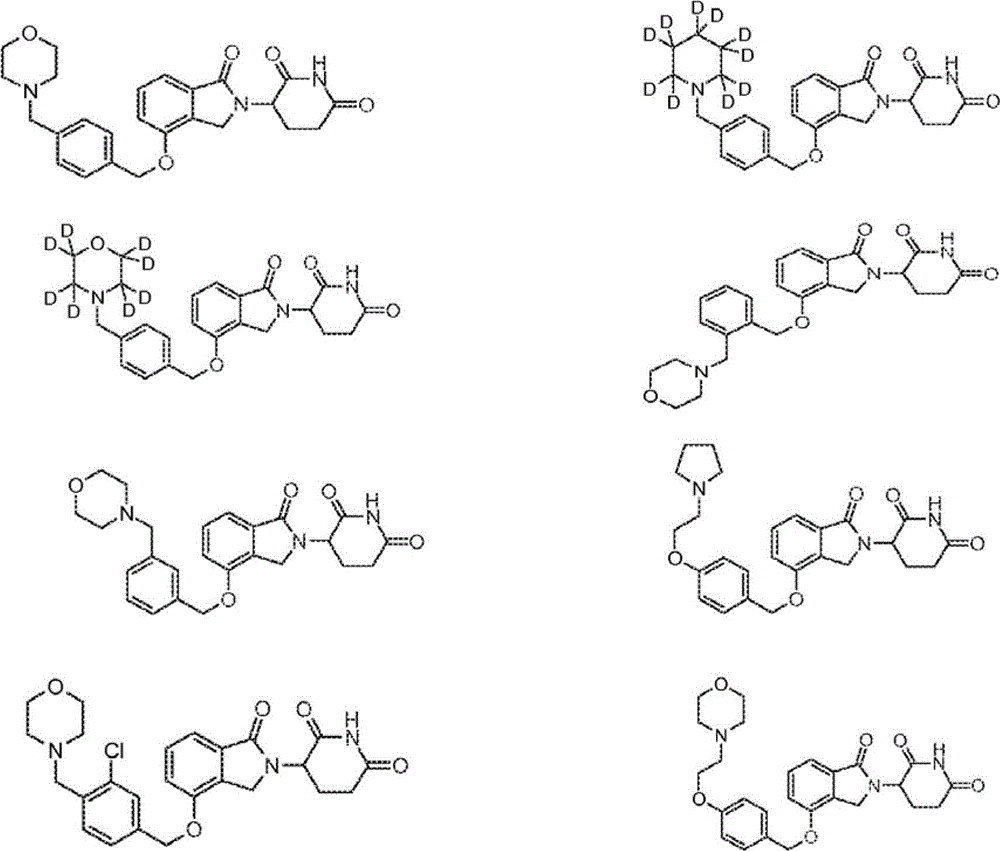
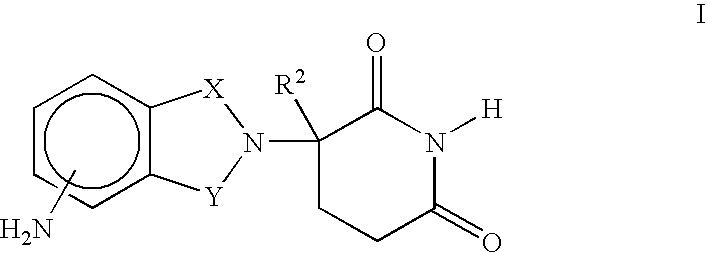
2,6-dioxo-3-deutero-piperdin-3-yl-isoindoline compounds
The present application describes 2-(2′,6′-dioxo-3′-deutero-piperidin-3′-yl)isoindoles, deuterated derivatives thereof, stereoisomers thereof, pharmaceutically acceptable salt forms thereof, and methods of treating using the same.
Owner:DEUTERX
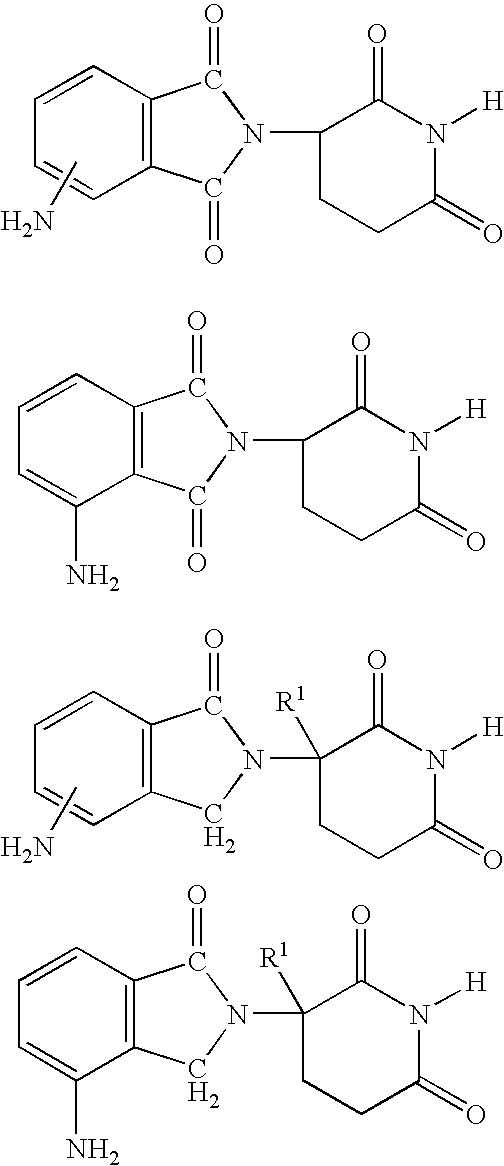
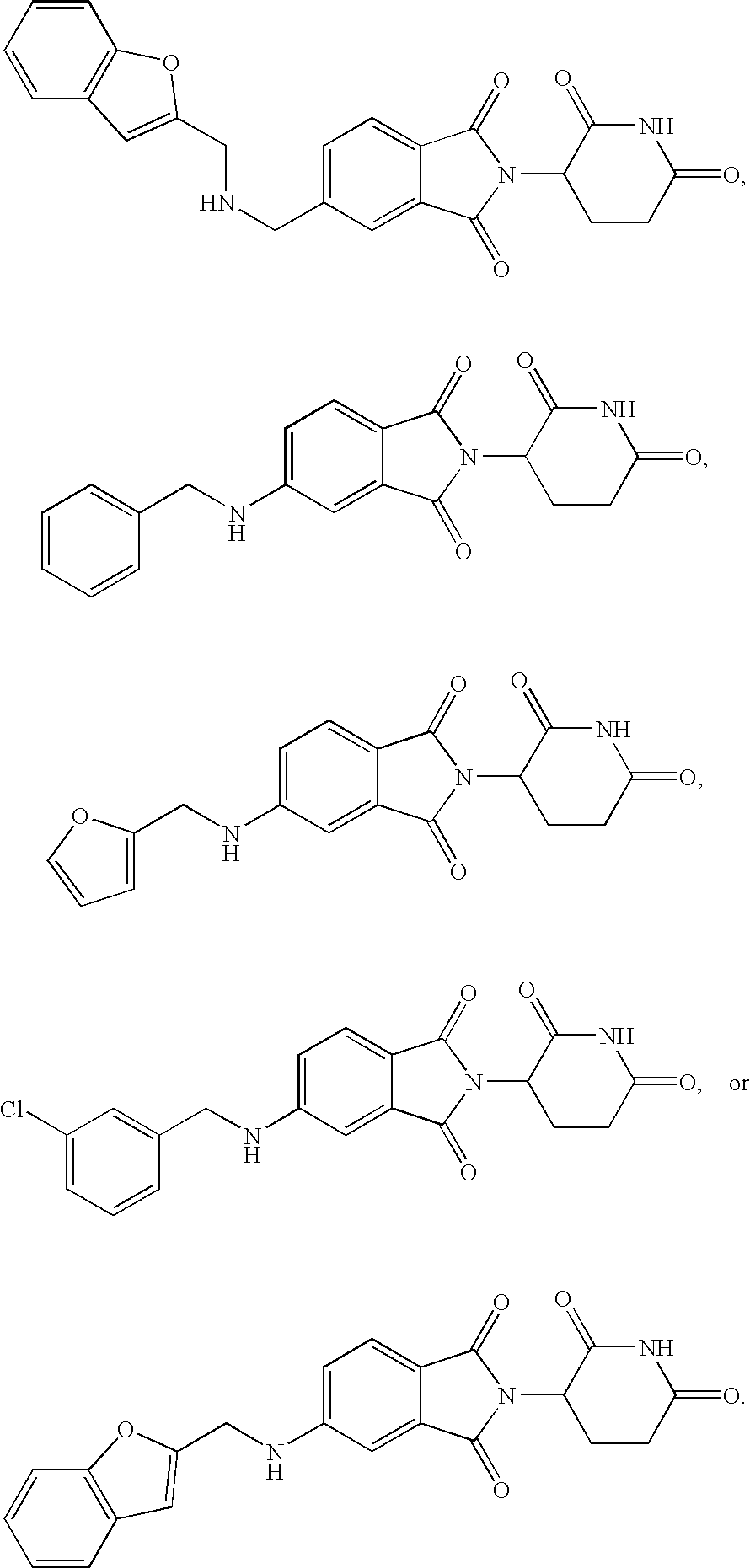
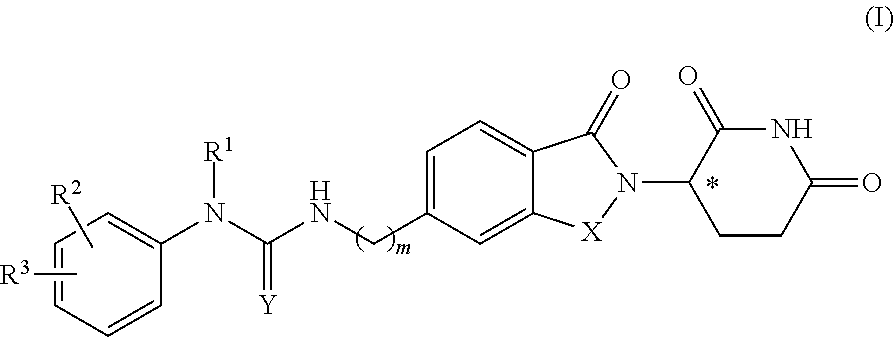
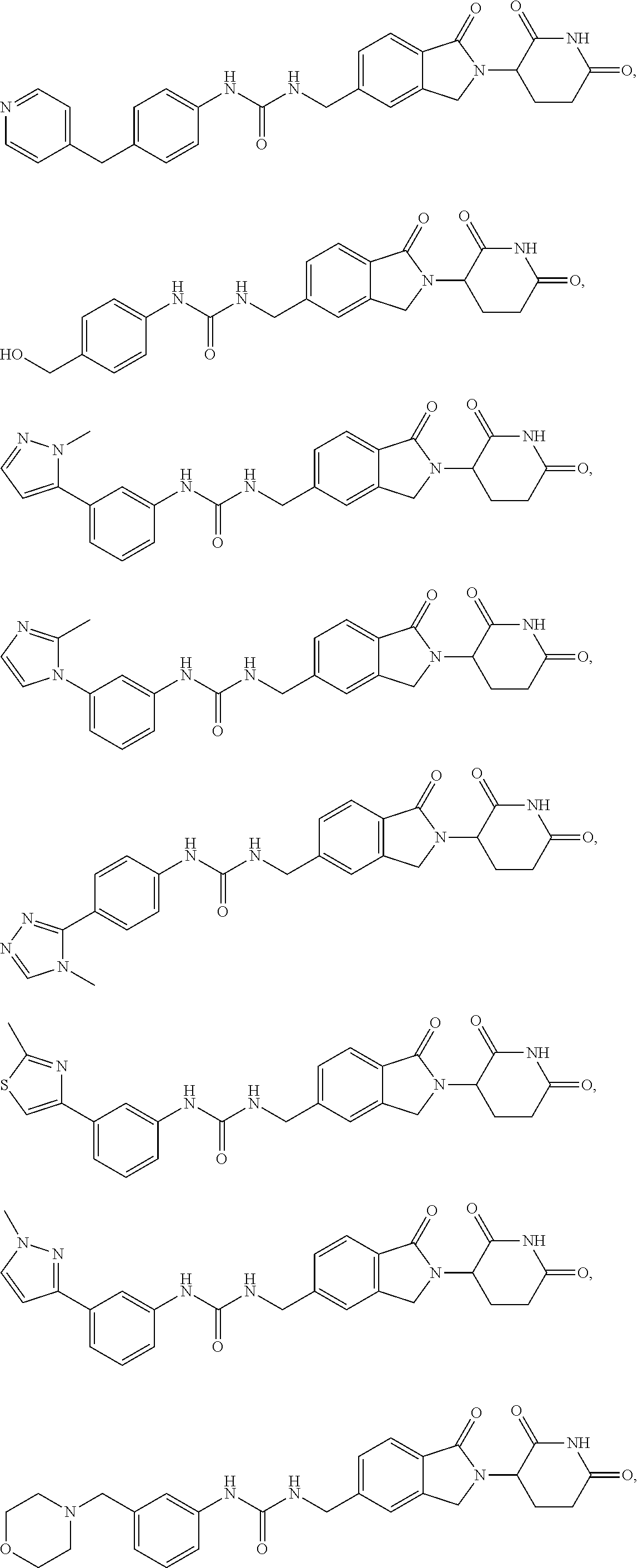
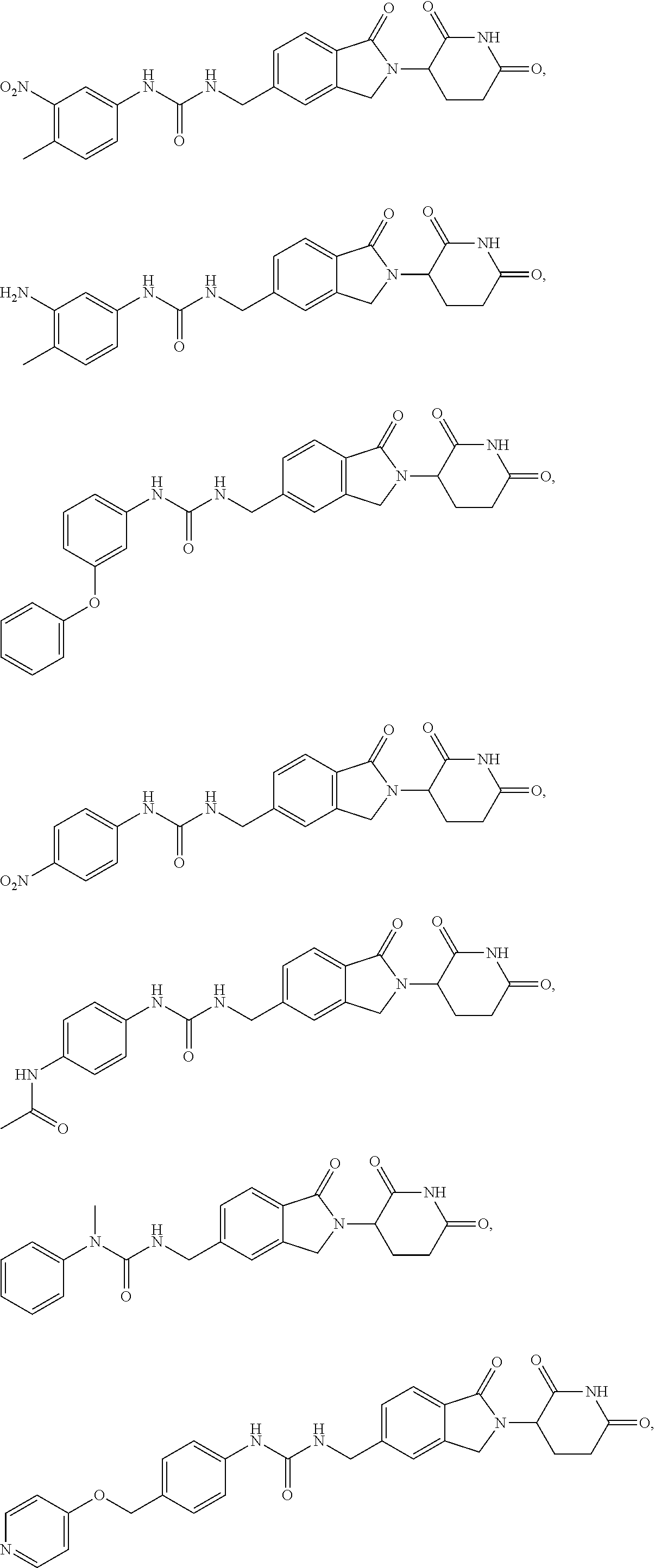
5-substituted isoindoline compounds
This invention relates to 5-substituted isoindoline compounds, and pharmaceutically acceptable salts, solvates, stereoisomers, and prodrugs thereof. Methods of use, and pharmaceutical compositions of these compounds are disclosed.
Owner:CELGENE CORP
(R) and (S) isomers of substituted 2-(2,6-dioxopiperidin-3-yl) phthalimides and 1-oxoisoindolines and methods of using the same
Substituted 2-(2,6-dioxopiperidin-3-yl) phthalimides and 1-oxo-2-(2,6-dioxopiperidin-3-yl) isoindolines reduce the levels of TNFα in a mammal. Typical embodiments are 1-oxo-2-(2,6-dioxo-3-methylpiperidin-3-yl)-4,5,6,7-tetrafluoroisoindoline and 1,3-dioxo-2-(2,6-dioxo-3-methylpiperidin-3-yl)-4-aminoisoindoline.
Owner:CELGENE CORP
Substituted isoindoline-1,3-dione derivatives
This invention relates to novel substituted isoindoline-1,3-dione derivatives and pharmaceutically acceptable salts thereof. More specifically, the invention relates to novel substituted isoindoline-1,3-dione derivatives that are analogues of apremilast. This invention also provides compositions comprising a compound of this invention and a carrier and the use of disclosed compounds and compositions in methods of treating diseases and conditions that are beneficially treated by administering apremilast.
Owner:SUN PHARMA IND INC
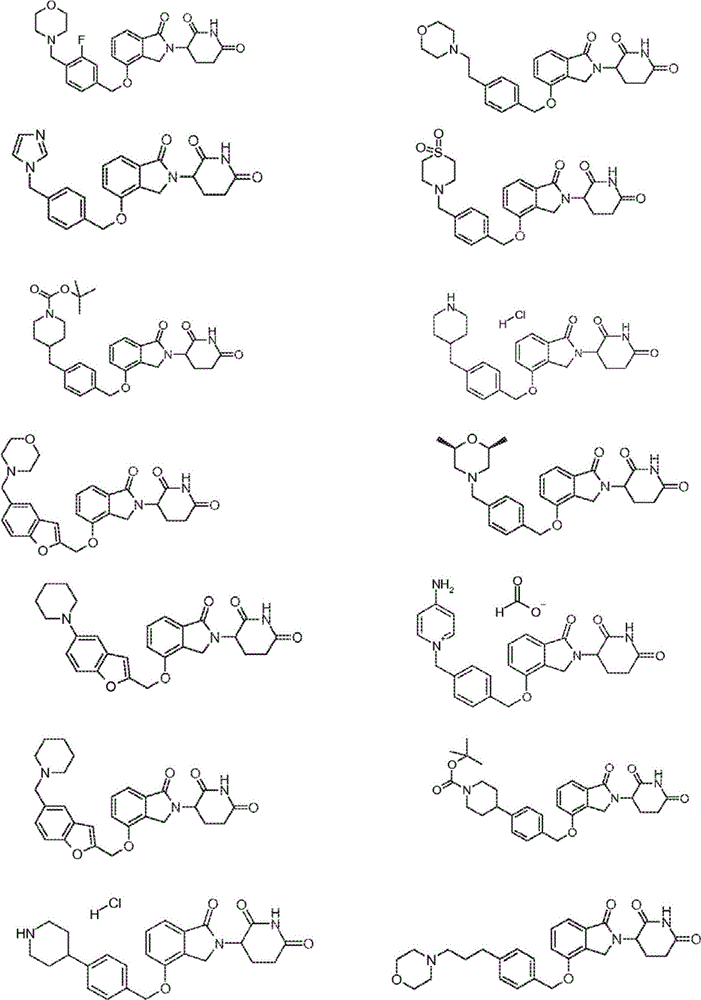
Isoindoline compounds and methods of their use
ActiveUS20060025457A1Preventing and prolonging their recurrenceLengthening time of remissionAntibacterial agentsBiocideMedicineAngiogenesis growth factor
Novel isoindoline compounds are disclosed. Methods of treating, preventing and / or managing cancer, diseases and disorders associated with, or characterized by, undesired angiogenesis, and diseases and disorders mediated by PDE 4, using the compounds are also disclosed.
Owner:AMGEN INC
Isoindoline compounds and methods of their use
ActiveUS20120122865A1Reducing and avoiding toxicityReducing and avoiding and side effectBiocideSenses disorderDiseaseStereochemistry
Provided herein are isoindoline compounds, pharmaceutical compositions comprising one or more of such compounds, and methods of their use for treating, preventing, or managing various diseases.
Owner:CELGENE CORP
Method for production of polymer-encapsulated pigments
The invention relates to a method for production of an aqueous dispersion of polymer-encapsulated pigments, characterised in that (a) an aqueous pigment dispersion, containing at least one organic pigment (P), selected from the group of azo, isoindolinone, isoindoline, anthanthrone, thioindigo, thiazinindigo, triarylcarbonium, quinophthalone, anthraquinone, dioxazine, phthalocyanine, quinacridone, quinacridonquinone, indanthrone, perylene, perinone, pyranthrone, diketopyrrolopyrrole, isoviolanthrone and azomethine pigments, at least one detergent (T), and water is prepared, (b) a monomer miniemulsion, stabilised by a hydrophobic organic compound with a water solubility at 20 DEG C of at most 5x10<-5> g / l, is prepared from a polymerisable monomer (M) and at least one detergent (T) in water, (c) a monomer pigment emulsion is prepared, whereby the aqueous dispersion from (a) and the monomer miniemulsion from (b) are mixed and homogenised and (d) the pigment-containing monomer from (c) is polymerised in the presence of a polymerisation initiator and / or by heat, whereupon an encapsulation of the pigment with the polymer thus formed occurs.
Owner:CLARIANT PROD DEUT GMBH
Methods for the treatment of cachexia
Substituted 2-(2,6-dioxopiperidin-3-yl)phthalimides and 1-oxo-2-(2,6-dioxopiperidin-3-yl)isoindolines are disclosed. The compounds are useful, for example, in reducing the levels of TNFα in a mammal.
Owner:CELGENE CORP
2,6-dioxo-3-deutero-piperdin-3-yl-isoindoline compounds
The present application describes 2-(2′,6′-dioxo-3′-deutero-piperidin-3′-yl)isoindoles, deuterated derivatives thereof, stereoisomers thereof, pharmaceutically acceptable salt forms thereof, and methods of treating using the same.
Owner:DEUTERX
Solid forms comprising (+)-2-[1-(3-ethoxy-4-methoxyphenyl)-2-methylsulfonylethyl]-4-acetylaminoisoindoline-1,3-dione, compositions thereof, and uses thereof
Solid forms comprising (+)-2-[l-(3-Ethoxy-4-methoxyphenyl)-2- methylsulfonylethyl]-4-acetylaminoisoindoline-l,3-dione, compositions comprising the solid forms, methods of making the solid forms and methods of their use are disclosed. The methods include methods of treating and / or preventing disorders ameliorated by the reduction of levels of TNF-alpha or the inhibition of PDE4.
Owner:CELGENE CORP
Application of rhodamine B based fluorescence sensor
InactiveCN103674920AHigh detection sensitivityHigh absorption coefficientFluorescence/phosphorescenceEthyl propionateEthyl ester
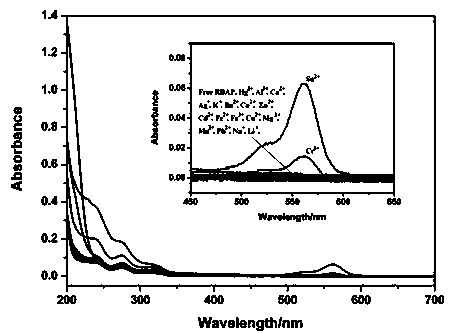
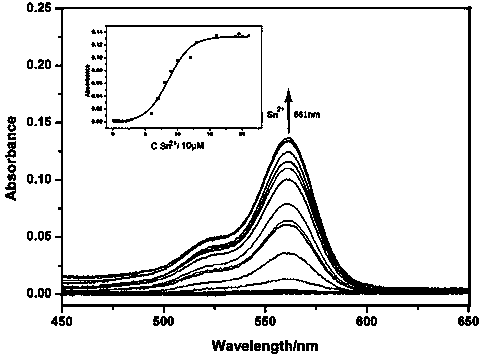
The invention discloses an application of a rhodamine B based fluorescence sensor which can specifically detect tin ions. According to the application, benzyl 3-(3', 6'-bis(diethyl amino)-3-oxo-spiro [isoindoline-1, 9'-xanthene]-2-yl) ethyl propionate is taken as a substrate, different heavy metal ions such as MgCl2*6H2O, SnCl2*H2O, CrCl3*6H2O, AgNO3, CaCl2, NaCl, PbCl2, KCl, MnCl2*4H2O, ZnCl2, CuCl2*2H2O, LiCl*H2O, Ba(NO3)2, HgCl2, CoCl2, FeCl2*4H2O, FeCl3*6H2O, CdCl2*2.5H2O, AlCl3 and the like are added, and fluorescent response is found only when SnCl2*H2O is added, so that the fluorescence sensor which can specifically detect tin ions is developed. The sensor has the advantages of high specificity and sensitivity and the like and has important application potentials in the aspects of disease diagnosis and health evaluation.
Owner:NANJING UNIV OF SCI & TECH
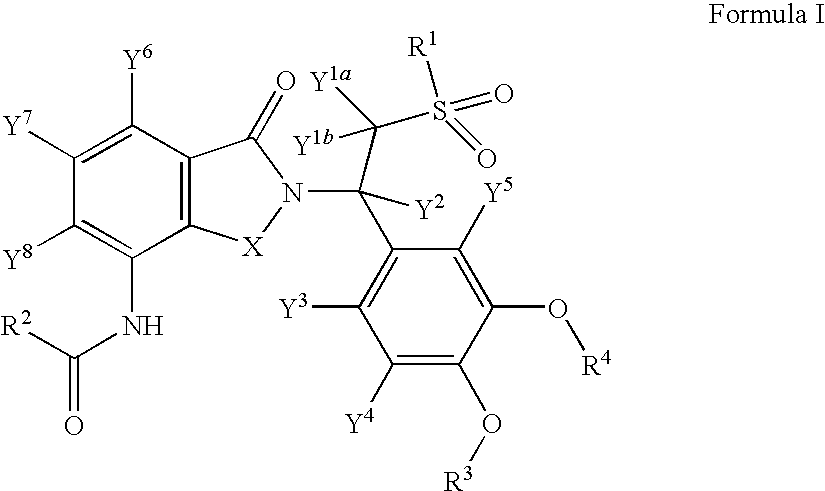
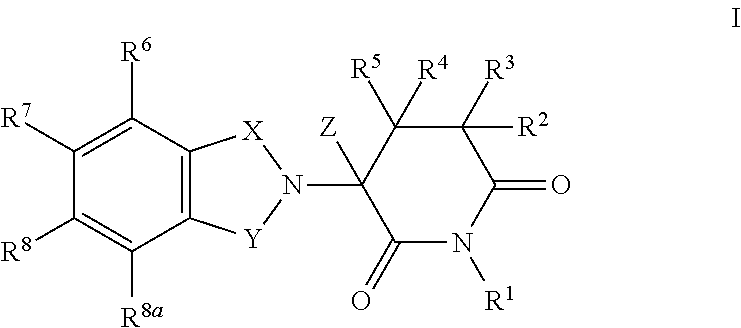
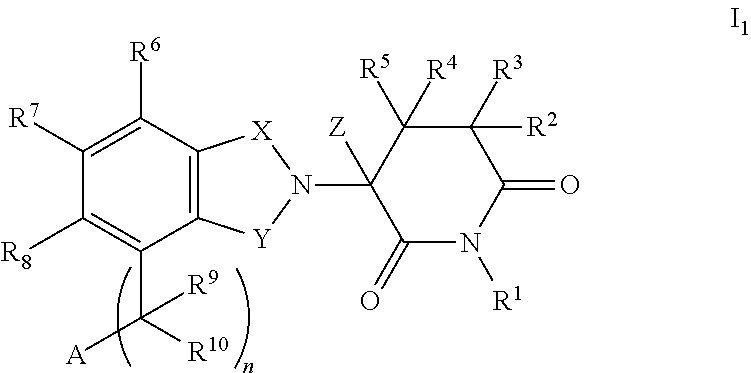
Highly selective sigma receptor ligands
Compounds having the general formula II, III, or IVwherein R1 can be a radical of an optionally substituted C-4 to C-7 N-containing heterocycle or a radical of an optionally substituted cyclic or acyclic tertiary amine or isoindoline-1,3-dione: R2,3,4,5,6 can each independently be any one or combinations of the following moieties, cyano, nitro, acyl, alkyl, amido, azido, isothiocyanate, isocyanate, optionally substituted anilino, halogens, ethers, sulfonamides, thioacyl, nitro, aromatic, heterocyclic, olefinic, acetylene, deuterium, or tritium; Y can be either CH, CH2, O, S, OCH2, N—R, N—Ar, C—R, C—Ar; Z can be either H, O, S, S—R or NR. R groups can be either H, aryls, alkyls, or cycloalkyls; “n” can be 1 to 5 carbons in length and stereoisomers, functional analogs, and pharmaceutically acceptable salts thereof and wherein the moiety bridging R1 and N can be a substituted alkylene, optionally substituted alkenylene or optionally substituted alkynylene and where the alkylene group can include an inserted C3-C5 cycloalkyl group, aromatic, and hetercocyclic group; wherein X′ is halogen, or C1-C4 haloalyl; wherein the Rx is a C1-C5 straight chain or branched chain alkyl or a C1-C4 straight chain or branched chain haloalkyl.
Owner:UNIVERSITY OF MISSISSIPPI +1
Fluorescence sensor for rhodamine B as well as preparation and application thereof
InactiveCN103913441AStrong specificityShorten the timeFluorescence/phosphorescenceLuminescent compositionsKetoneMethyl group
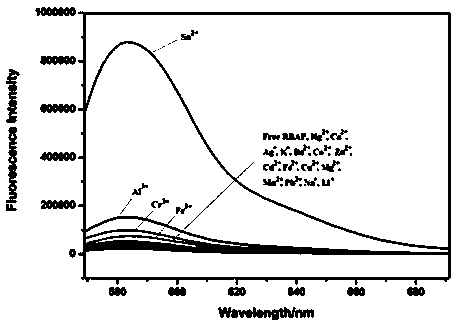
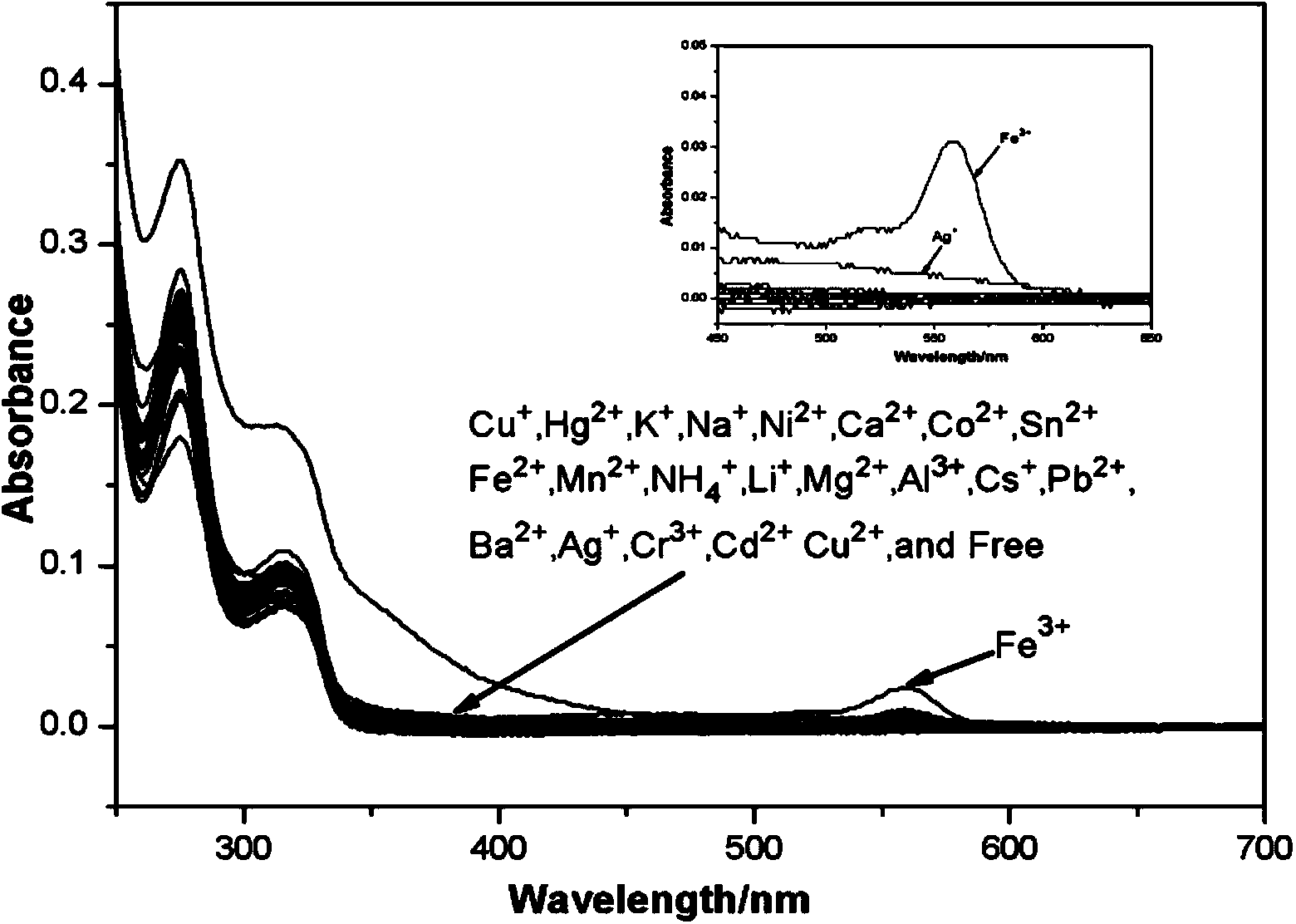
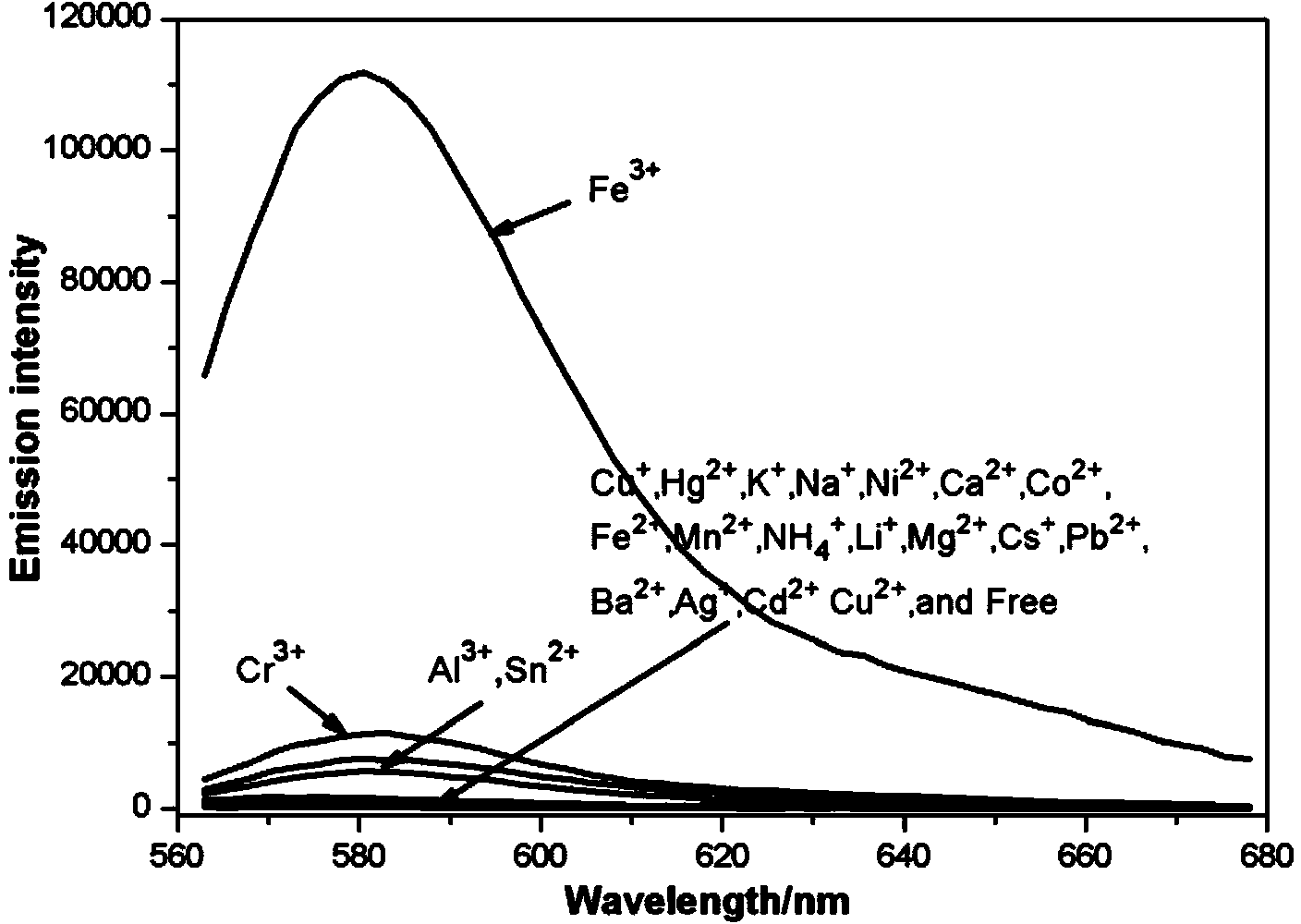
The invention discloses a fluorescence sensor for rhodamine B as well as preparation and a detection application thereof to Fe<3+>. The rhodamine B is used as a raw material to synthesize (2-(2-(((1H-pyrrol-2-yl)methyl)amino)ethyl)-3',6'-bis(dithylamino)spiro(isoindoline-1,9'-xanthen)-3-one). The structure is used as a substrate and a solution is prepared by the substrate; different metal ions are added into the solution; after ultraviolet visible light and fluorescence tests are carried out, ultraviolet and fluorescence absorption values are obviously changed after FeCl3*6H2O is added; in a certain range, the ultraviolet and fluorescence absorption values and the concentration of the Fe<3+> are in a linear relation so as to invent the fluorescence sensor for specially detecting the Fe<3+> based on the rhodamine B. The sensor has great application potential in medical and environmental sciences.
Owner:NANJING UNIV OF SCI & TECH
5-substituted isoindoline compounds
This invention relates to 5-substituted isoindoline compounds, and pharmaceutically acceptable salts, solvates, stereoisomers, and prodrugs thereof. Methods of use, and pharmaceutical compositions of these compounds are disclosed.
Owner:CELGENE CORP
Cr<3+> sensor based on rhodamine B as well as preparation and application of Cr<3+> sensor
InactiveCN104449675AGood light stabilityHigh quantum yieldOrganic chemistryFluorescence/phosphorescenceSpectrofluorometerIntensity change
The invention discloses a Cr<3+> sensor based on rhodamine B as well as a preparation and application of the Cr<3+> sensor. The intensity change of a characteristic peak of a rhodamine probe in a water phase is determined by adopting an ultraviolet-visible spectrophotometer and a fluorospectro photometer, so as to determine the presence of Cr<3+>. A target product 2,2'-sulfo-bi(N-(2-(3',6'-bi(lignocaine)-3-oxospiro[isoindoline-1,9'-xanthene]-2-yl)ethyl) acetamide) is synthesized by the rhodamine B as a precursor. The invention provides application of the target product in Cr<3+> detection; and the target product has a good detection effect on Cr<3+>. Compared with the prior art, the Cr<3+> sensor is easily available in raw materials, and simple in synthesis step, and has great application prospect in detection of the Cr<3+>; post-treatment is also convenient; and large-scale production is relatively easy to achieve.
Owner:NANJING UNIV OF SCI & TECH
Rhodamine B-based fluorescent sensor, preparation and application thereof
InactiveCN103849377AThe synthesis method is simpleMild reaction conditionsOrganic chemistryFluorescence/phosphorescenceDiseaseFluorescence spectrometry
The invention discloses a rhodamine B-based fluorescent sensor, preparation and application thereof. 2-(2-aminoethyl)-3',6'-bis(diethyl-amino)spiro[isoindoline-1,9'-xanthen]-3-one(A) and 4-(1,4,7,10-tetraoxa-13-azacycl-opentadecan-13-yl)-4-oxobutanoicacid(B) are taken as precursors to synthesize a fluorescent sensor (C). After addition of different heavy metal ions like NaCl, LiCl.H2O, KCl, NiCl, CuCl, AgNO3, SnCl2.2H2O, Ba(NO3)2, MnCl2.4H2O, ZnCl12, MgCl2.6H2O, CaCl2, CdCl2.2.5H2O, CuCl2.2H2O, FeCl2.4H2O, PbCl2, CoCl2.6H2O, HgCl2, Pd(C2H3O2)2, RhCl3.H2O, IrCl3, AlCl3, FeCl3.6H2O, and CrCl3.6H2O, analysis by ultraviolet-visible spectrum and spectrofluorimetry finds that the sensor only responses to iron ions, so that the fluorescent sensor can be used for specially detecting iron ions. The sensor has advantages of high sensitivity, simple operation and good specificity, and has important potential significance in disease diagnosis.
Owner:NANJING UNIV OF SCI & TECH
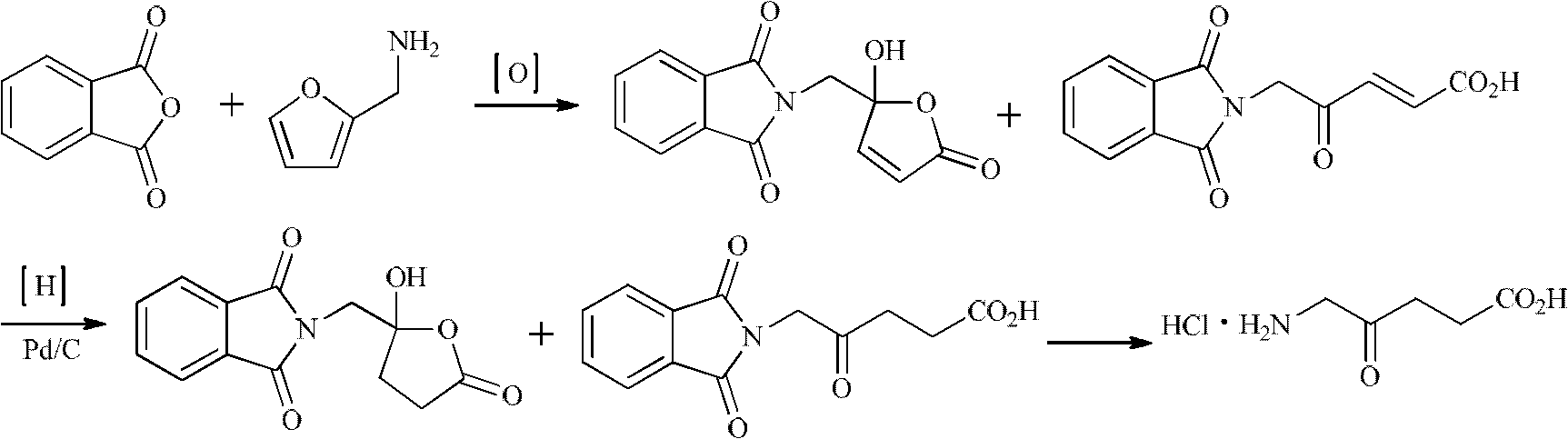
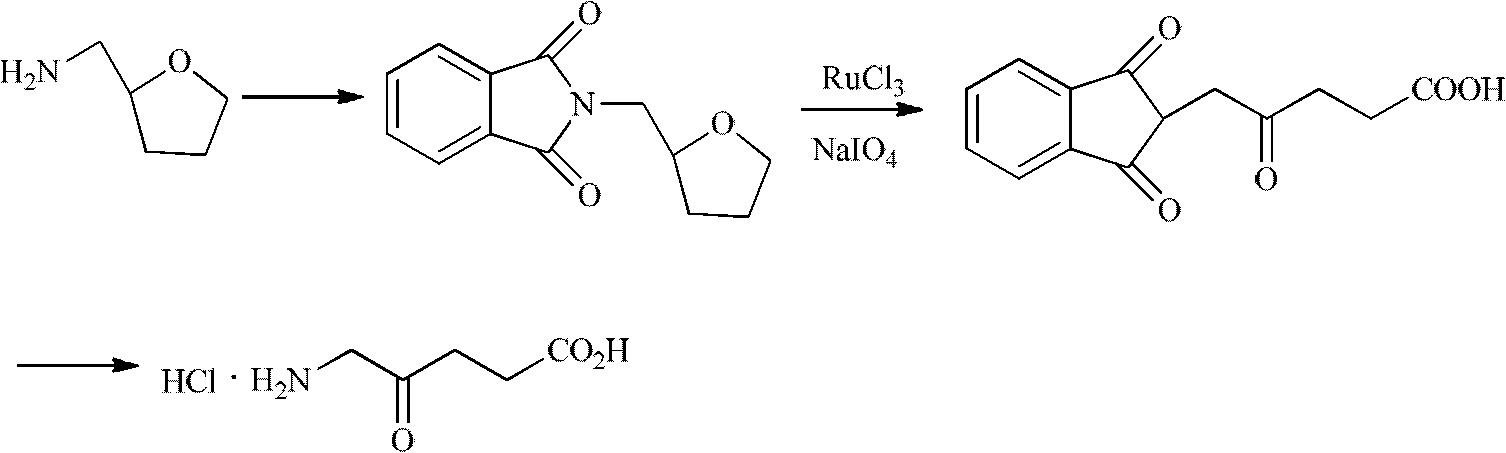
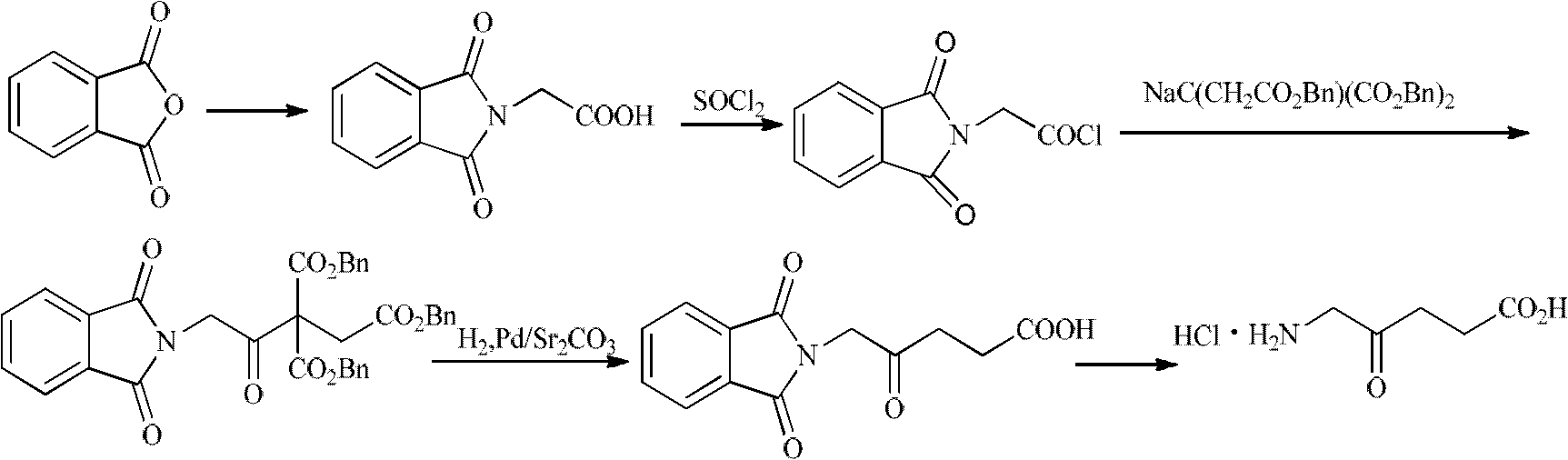
Synthesis method for 5-aminolevulinic acid hydrochloride
InactiveCN102627573AThe synthesis steps are simpleHigh selectivityOrganic compound preparationAmino-carboxyl compound preparationMalonic acidSynthesis methods
The invention relates to a synthesis method for 5-aminolevulinic acid hydrochloride. The synthesis method comprises the steps as follows: taking 1,3-dichloroacetone as a starting material, carrying out Gabriel reaction on the 1,3-dichloroacetone and phthalimide potassium in a water phase or an organic phase to obtain a high-purity intermediate (I), 2-(3-chloro-2-oxopropyl)isoindoline-1,3-diketone; reacting the intermediate (I) with malonic acid isopropyl ester or malonic acid diethyl ester in alkali condition to prepare an intermediate (II), 2-(3-(2,2-dimethyl-4,6-dioxo-1,3-dioxane-5-yl)-2-oxopropyl)isoindoline-1,3-diketone or an intermediate (III), 2-(3-(1,3-dioxoisobenzazole-2-yl)-2-oxopropyl)malonic acid diethyl ester; and hydrolyzing, decarboxylating and post-processing to obtain the high-purity 5-aminolevulinic acid hydrochloride.
Owner:SHANDONG UNIV
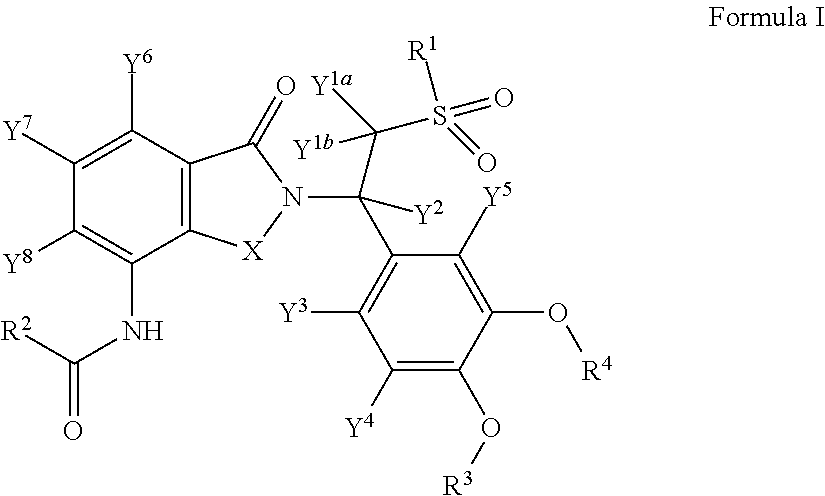
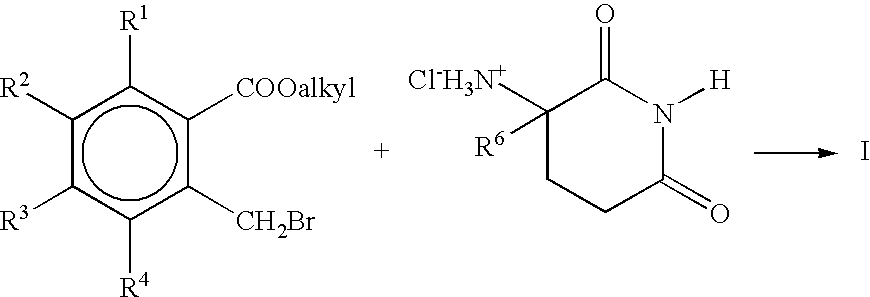
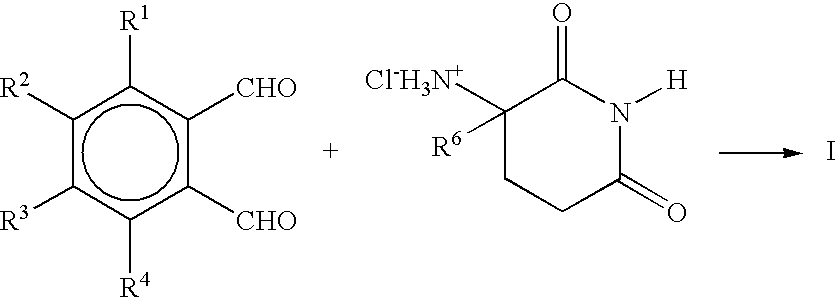
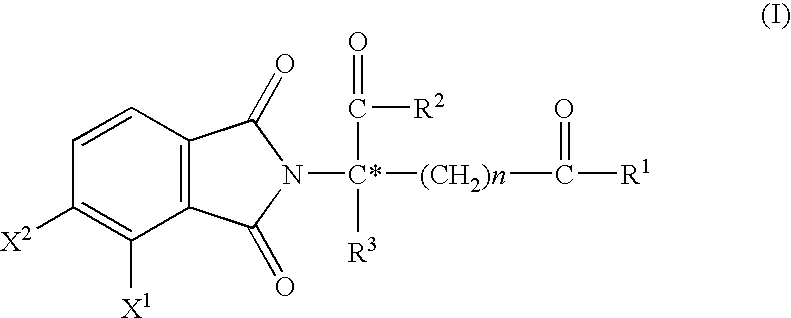
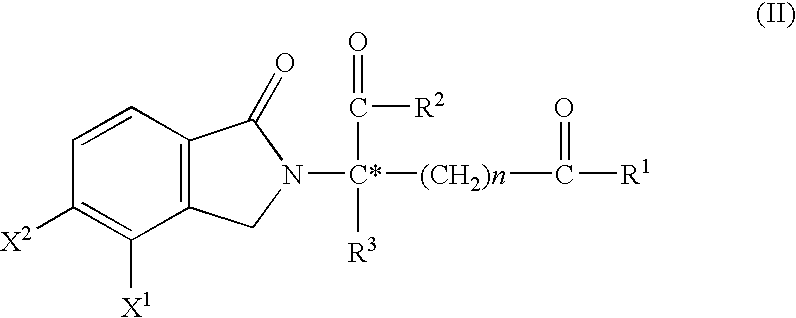
Substituted 2-(2,6-dioxopiperidin-3-yl)-phthalimides and -1-oxoisoindolines and method of reducing TNFα levels
Substituted 2-(2,6-dioxopiperidin-3-yl)-phthalimides and 1-oxo-2-(2,6-dioxopiperidin-3-yl)isoindolines reduce the levels of TNFα in a mammal. A typical embodiment is 1-oxo-2-(2,6-dioxo-3-methylpiperidin-3-yl)-4,5,6,7-tetrafluoroisoindoline.
Owner:CELGENE CORP
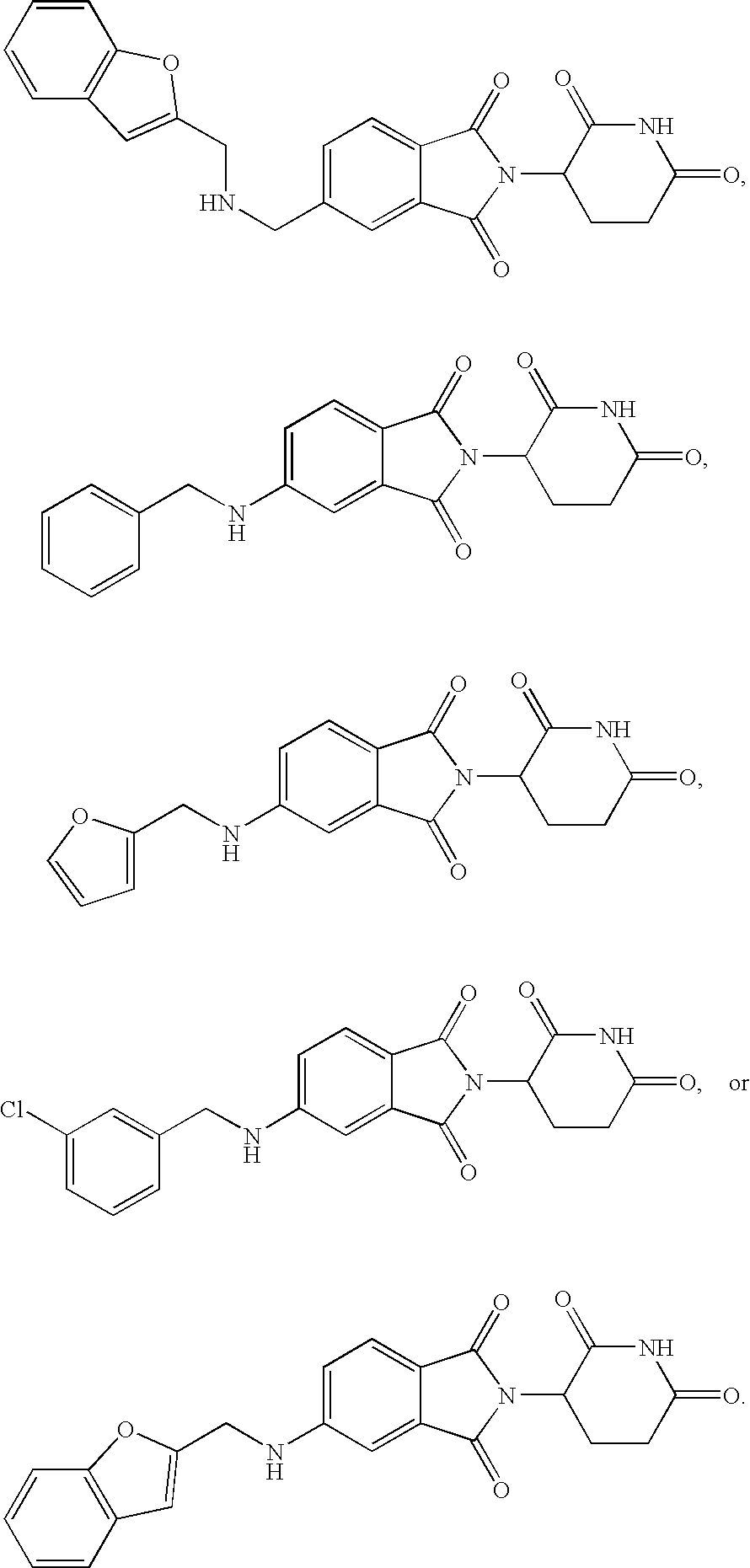
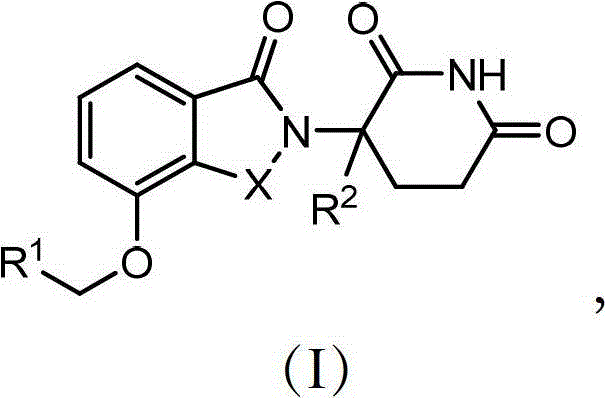
Arylmethoxy isoindoline derivatives and compositions comprising and methods of using the same
Provided are 4'-arylmethoxy isoindoline compounds, and pharmaceutically acceptable salts, solvates, clathrates, stereoisomers, and prodrugs thereof. Methods of use, and pharmaceutical compositions of these compounds are disclosed.
Owner:CELGENE CORP
Substituted isoindoline-1,3-dione derivatives
This invention relates to novel substituted isoindoline-1,3-dione derivatives and pharmaceutically acceptable salts thereof. More specifically, the invention relates to novel substituted isoindoline-1,3-dione derivatives that are analogues of apremilast. This invention also provides compositions comprising a compound of this invention and a carrier and the use of disclosed compounds and compositions in methods of treating diseases and conditions that are beneficially treated by administering apremilast.
Owner:SUN PHARMA IND INC
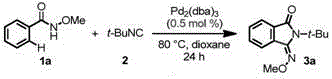
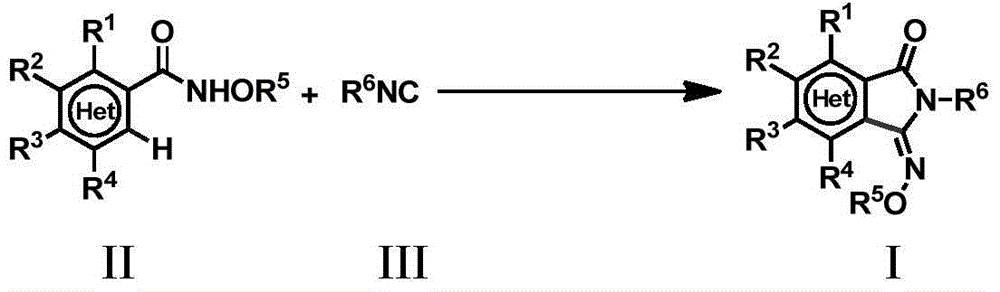
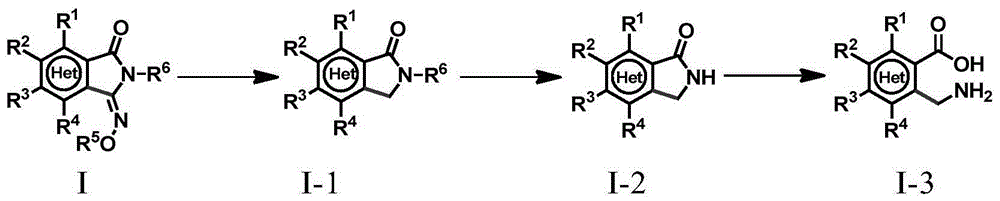
Method for preparing 3-imino isoindoline ketone compounds
ActiveCN105294536AOrganic compound preparationGroup 5/15 element organic compoundsActivation methodPalladium catalyst
The invention relates to a method for preparing 3-imino isoindoline ketone compounds and particularly discloses a method for preparing the 3-imino isoindoline ketone compounds through the activation method of palladium catalysis of the C-H bond, wherein the 3-imino isoindoline ketone compounds are as shown in the formula I (refer to the Specification). The method comprises the following steps: under the existence of an inert solvent, under the catalysis of a palladium catalyst, and under the action of an oxidizing agent, the compounds II (N-alkoxy amide compounds) and the compounds III (isonitrile compounds) react to obtain the compounds I, wherein the structures of the compounds I, compounds II and compounds III are as shown in the Specification. The method is high in efficiency, excellent in selectivity, economical, environment friendly, fewer in steps, simple and convenient to operate, wide in application range of substrates, and excellent in atom economy. The invention further provides a method for further conversion of the compounds I.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Pharmaceutical composition
InactiveUS20100273852A1Inhibits nerve cell deathPromote regenerationBiocideNervous disorder1-benzofuranSURFACTANT BLEND
There is provided a pharmaceutical composition comprising (1) (R)-5,6-dimethoxy-2-[2,2,4,6,7-pentamethyl-3-(4-methylphenyl)-2,3-dihydro-1-benzofuran-5-yl]isoindoline, (2) a lipophilic component, and (3) a surfactant, which can stably and sufficiently provide improvement in the absorbability of the drug without being affected by the amount and quality of a meal.
Owner:TAKEDA PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Solid forms comprising (+)-2-[1-(3-ethoxy-4-methoxyphenyl)-2- methylsulfonylethyl]-4-acetylaminoisoindoline-1,3-dione, compositions thereof, and uses thereof Solid forms comprising (+)-2-[1-(3-ethoxy-4-methoxyphenyl)-2- methylsulfonylethyl]-4-acetylaminoisoindoline-1,3-dione, compositions thereof, and uses thereof](https://images-eureka.patsnap.com/patent_img/3f68f0de-ad1f-4672-be1c-b0fd3de849a7/US20080234359A1-20080925-D00000.png)
![Solid forms comprising (+)-2-[1-(3-ethoxy-4-methoxyphenyl)-2- methylsulfonylethyl]-4-acetylaminoisoindoline-1,3-dione, compositions thereof, and uses thereof Solid forms comprising (+)-2-[1-(3-ethoxy-4-methoxyphenyl)-2- methylsulfonylethyl]-4-acetylaminoisoindoline-1,3-dione, compositions thereof, and uses thereof](https://images-eureka.patsnap.com/patent_img/3f68f0de-ad1f-4672-be1c-b0fd3de849a7/US20080234359A1-20080925-D00001.png)
![Solid forms comprising (+)-2-[1-(3-ethoxy-4-methoxyphenyl)-2- methylsulfonylethyl]-4-acetylaminoisoindoline-1,3-dione, compositions thereof, and uses thereof Solid forms comprising (+)-2-[1-(3-ethoxy-4-methoxyphenyl)-2- methylsulfonylethyl]-4-acetylaminoisoindoline-1,3-dione, compositions thereof, and uses thereof](https://images-eureka.patsnap.com/patent_img/3f68f0de-ad1f-4672-be1c-b0fd3de849a7/US20080234359A1-20080925-D00002.png)
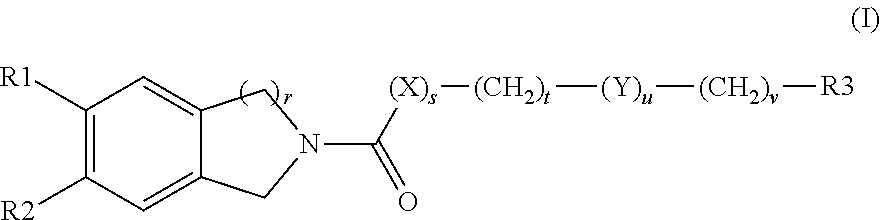
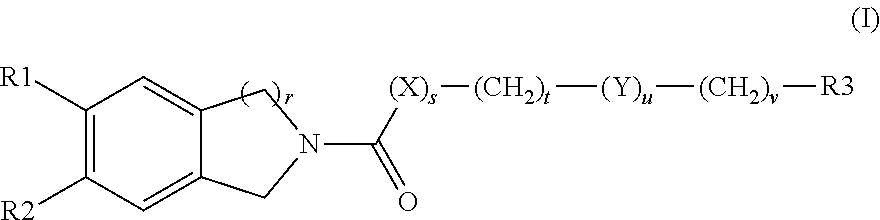
![Solid forms comprising (+)-2-[1-(3-ethoxy-4-methoxyphenyl)-2-methylsulfonylethyl]-4-acetylaminoisoindoline-1,3-dione, compositions thereof, and uses thereof Solid forms comprising (+)-2-[1-(3-ethoxy-4-methoxyphenyl)-2-methylsulfonylethyl]-4-acetylaminoisoindoline-1,3-dione, compositions thereof, and uses thereof](https://images-eureka.patsnap.com/patent_img/1ee1f265-4846-45f1-8e99-5635ea4ae65b/HPA00001259375000011.PNG)
![Solid forms comprising (+)-2-[1-(3-ethoxy-4-methoxyphenyl)-2-methylsulfonylethyl]-4-acetylaminoisoindoline-1,3-dione, compositions thereof, and uses thereof Solid forms comprising (+)-2-[1-(3-ethoxy-4-methoxyphenyl)-2-methylsulfonylethyl]-4-acetylaminoisoindoline-1,3-dione, compositions thereof, and uses thereof](https://images-eureka.patsnap.com/patent_img/1ee1f265-4846-45f1-8e99-5635ea4ae65b/HPA00001259375000021.PNG)
![Solid forms comprising (+)-2-[1-(3-ethoxy-4-methoxyphenyl)-2-methylsulfonylethyl]-4-acetylaminoisoindoline-1,3-dione, compositions thereof, and uses thereof Solid forms comprising (+)-2-[1-(3-ethoxy-4-methoxyphenyl)-2-methylsulfonylethyl]-4-acetylaminoisoindoline-1,3-dione, compositions thereof, and uses thereof](https://images-eureka.patsnap.com/patent_img/1ee1f265-4846-45f1-8e99-5635ea4ae65b/HPA00001259375000031.PNG)