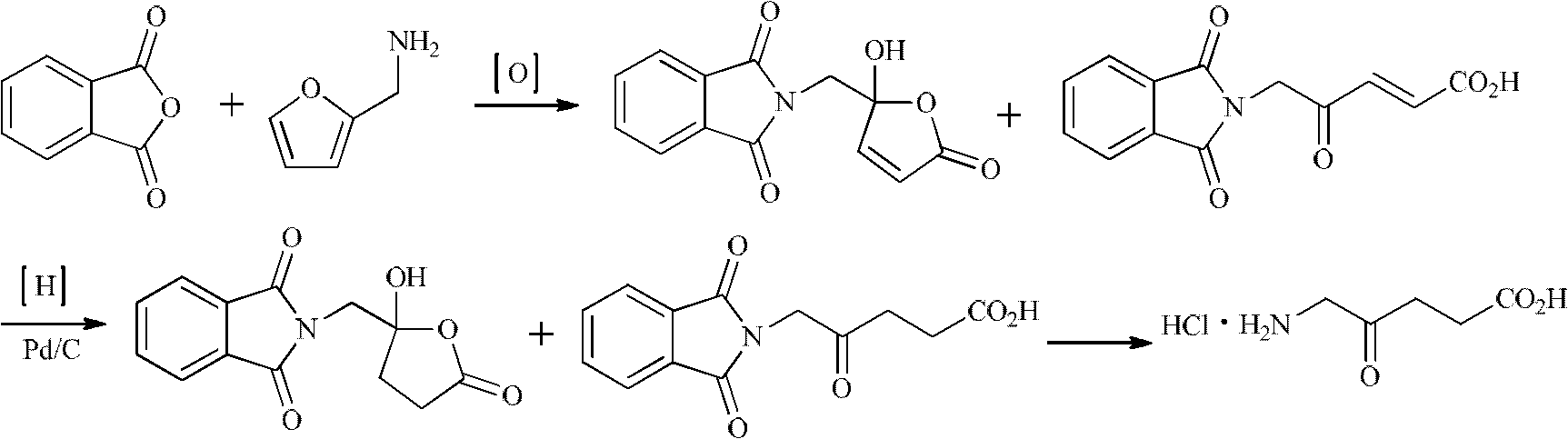
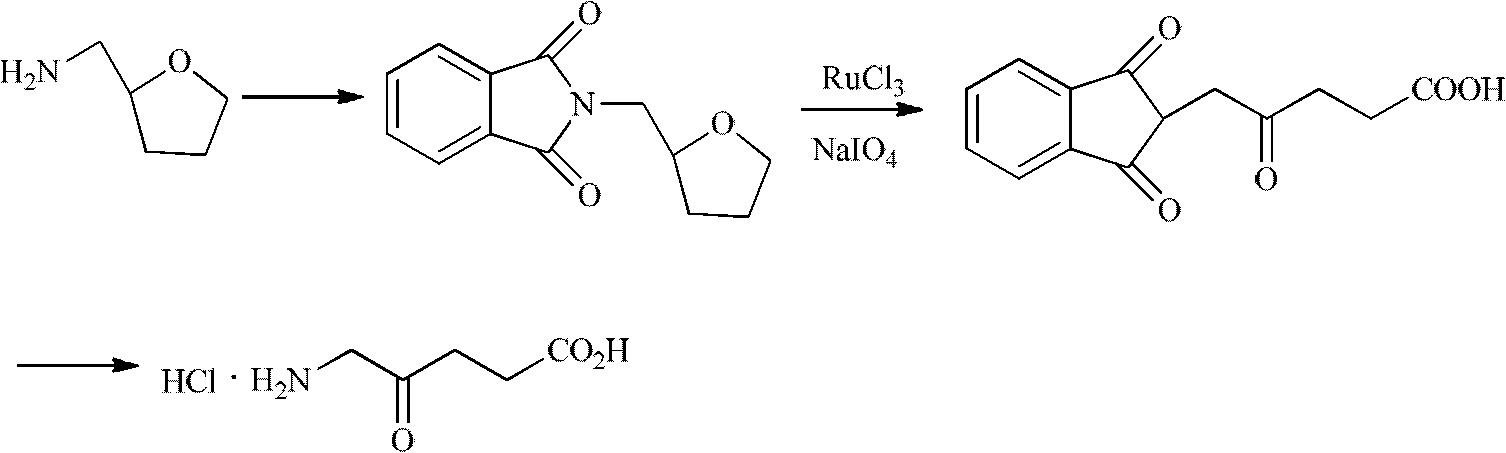
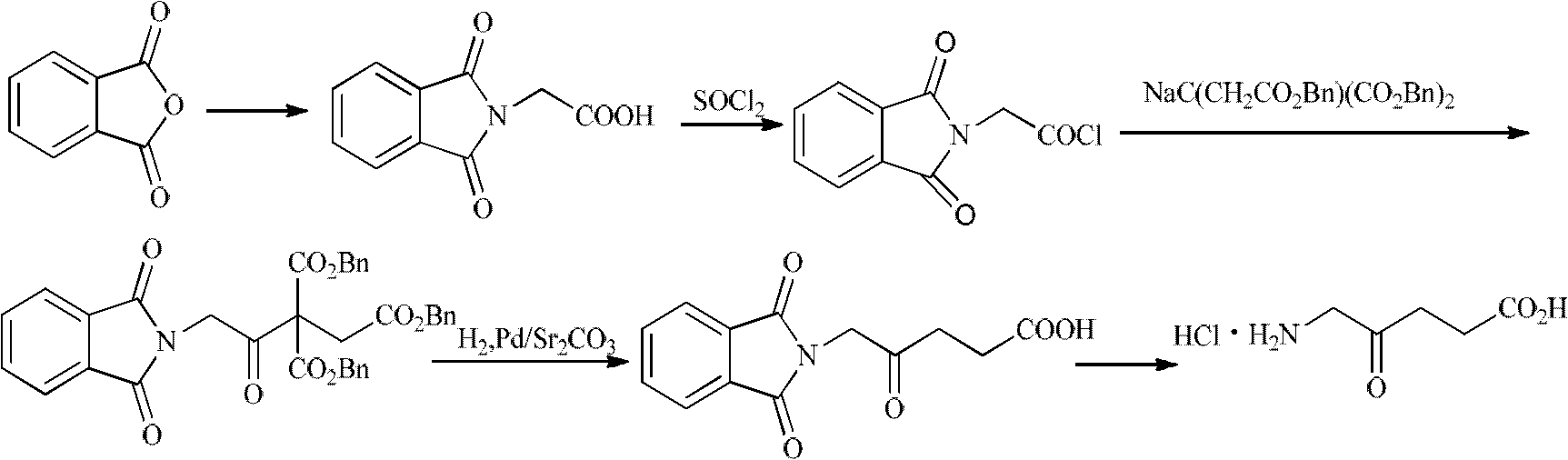
Synthesis method for 5-aminolevulinic acid hydrochloride
A kind of technology of aminolevulinic acid hydrochloride and synthesis method, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of unsuitability for industrial production, high production cost, and low total yield, etc. Achieve the effect of being conducive to large-scale industrial production, fewer steps in the synthetic route, and simple synthetic steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063]
[0064] 1) Preparation of intermediate (I) 2-(3-chloro-2-oxopropyl)isoindoline-1,3-dione:
[0065] 38.1 grams (0.3 moles) of 1,3-dichloroacetone and 25.0 grams of deionized water were added to a 150 milliliter three-necked flask, and 17.5 grams (0.1 moles) of potassium phthalimide and 11.0 grams of phthalimide potassium salt were added dropwise under mechanical stirring. gram of water, the dropwise addition was completed, and the reaction at room temperature was completed after 1 hour. The white solid intermediate (I) was obtained by filtration, washed with 20.0 g of deionized water, and dried to obtain 21.6 g of the product, with a yield of 91.1%. The filtrate can be reused, and the intermediate (I) can be directly used in the next step without purification. Melting point: 120-121°C.
[0066] 2) Intermediate (II) 2-(3-(2,2-Dimethyl-4,6-dioxo-1,3-dioxan-5-yl)-2-oxopropyl)isoind Preparation of Indoline-1,3-dione:
[0067] 7.2 g (0.05 mol) of isopropylidene malona...
Embodiment 2
[0072] 1) Preparation of intermediate (I) 2-(3-chloro-2-oxopropyl)isoindoline-1,3-dione:
[0073] 38.1 grams (0.3 moles) of 1,3-dichloroacetone and 35.0 grams of dimethyl sulfoxide were added to a 250 ml three-necked flask, and 17.5 grams (0.1 moles) of potassium phthalimide / 20.0 gram of dimethyl sulfoxide mixed solution, the dropwise addition is completed, and the reaction at room temperature is finished after 1 hour. Add 100 milliliters of water to the reaction solution, and extract 2 times with ethyl acetate. Each ethyl acetate consumption is 100 milliliters. Concentrate the solvent, and Add 27.0 ml of petroleum ether to the obtained solid, stir at 60°C for 40 minutes, then cool to room temperature, filter and dry to obtain 20.8 g of pure intermediate (I), with a yield of 87.7%. Melting point data is with embodiment 1.
[0074] 2) Intermediate (II) 2-(3-(2,2-Dimethyl-4,6-dioxo-1,3-dioxan-5-yl)-2-oxopropyl)isoind Preparation of Indoline-1,3-dione:
[0075] 7.2 g (0.05 mol...
Embodiment 3
[0078]
[0079] 1) Preparation of intermediate (I) 2-(3-chloro-2-oxopropyl)isoindoline-1,3-dione:
[0080] Add 38.1 grams (0.3 moles) of 1,3-dichloroacetone and 28.0 grams of methanol in a 150 ml three-necked flask, and add 17.5 grams (0.1 moles) of phthalimide potassium salt and 30.0 grams of methanol dropwise under mechanical stirring. Dubbed a mixed solution, the dropwise addition was completed, and the reaction at room temperature was completed after 1 hour. Evaporate the solvent, add 25.0 grams of water under stirring, filter to obtain the intermediate (I), add 27.0 milliliters of petroleum ether to the obtained solid, cool to room temperature after stirring at 60 ° C for 40 minutes, filter and dry to obtain the pure product intermediate (I ) 19.8 g, yield 83.5%. Melting point data is with embodiment 1.
[0081] 2) Preparation of intermediate (III) diethyl 2-(3-(1,3-dioxoisoindoline-2-yl)-2-oxopropyl)malonate:
[0082] 8.0 g (0.05 mol) of diethyl malonate and 2.7 g ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com