Preparation method and application of quinonoid compound modified nylon membrane biological carrier
A technology of biological carrier and nylon membrane, which is applied in the fields of chemical engineering, environmental engineering, and material engineering. It can solve the problems of microbial system poisoning, poor mechanical strength, and easy loss, so as to reduce secondary pollution, have good biocompatibility, Effect of increasing quinone group content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Put 20 pieces of nylon membranes with a radius of 1 cm into 3 mol / L hydrochloric acid solution for hydrolysis. After hydrolysis at 35°C for 12 hours, wash with distilled water and dry for later use. Place a 250ml four-neck flask on a magnetic stirrer, add 5 pieces of hydrolyzed nylon membranes, 50ml of 2mol / L NaOH solution to the flask; dissolve 0.1g of anthraquinone-2-sulfonyl chloride into 30ml of dimethyl In the base sulfoxide, drop it into the flask with a constant pressure dropping funnel at 30°C, and continue the reaction under stirring for 6 hours to obtain a nylon membrane grafted with quinone groups, in which the quinone group content is 0.4 mmol / g. Take out the nylon membrane grafted with quinone groups, wash it with dimethyl sulfoxide, wash off the anthraquinone-2-sulfonyl chloride attached to the surface, wash it with distilled water, and dry it to obtain the nylon membrane modified by quinone compounds. Nylon membrane with quinone compounds.
Embodiment 2
[0029] Put 20 pieces of nylon membranes with a radius of 1 cm into 2mol / L hydrochloric acid solution for hydrolysis. After hydrolysis at 40°C for 10 hours, wash with distilled water and dry for later use. Place a 250ml four-neck flask on a magnetic stirrer, add 5 pieces of hydrolyzed nylon membranes, 50ml of 1mol / L NaOH solution to the flask; dissolve 0.1g of anthraquinone-2-sulfonyl chloride into 30ml of dimethyl In the base sulfoxide, drop it into the flask with a constant pressure dropping funnel at 35°C, and continue to react for 5 hours under stirring conditions to obtain a nylon membrane grafted with quinone groups, in which the content of quinones is 0.3 mmol / g. The nylon membrane grafted with quinone groups is taken out, washed with dimethyl sulfoxide to remove anthraquinone-2-sulfonyl chloride attached to the surface, washed with distilled water, and dried to obtain a nylon membrane modified with quinone compounds.
Embodiment 3
[0031]Put 20 pieces of nylon membranes with a radius of 1 cm into 4 mol / L hydrochloric acid solution for hydrolysis. After hydrolysis at 30°C for 15 hours, wash with distilled water and dry for later use. Place a 250ml four-neck flask on a magnetic stirrer, add 5 hydrolyzed nylon membranes and 50ml of 3mol / L NaOH solution to the flask; dissolve 0.1g of anthraquinone-2-sulfonyl chloride into 30ml of dimethyl In the base sulfoxide, drop it into the flask with a constant pressure dropping funnel at 30°C, and continue to react for 4 hours under stirring conditions to obtain a nylon membrane grafted with quinone groups, in which the content of quinones is 0.2 mmol / g. The nylon membrane grafted with quinone groups is taken out, washed with dimethyl sulfoxide to remove anthraquinone-2-sulfonyl chloride attached to the surface, washed with distilled water, and dried to obtain a nylon membrane modified with quinone compounds.
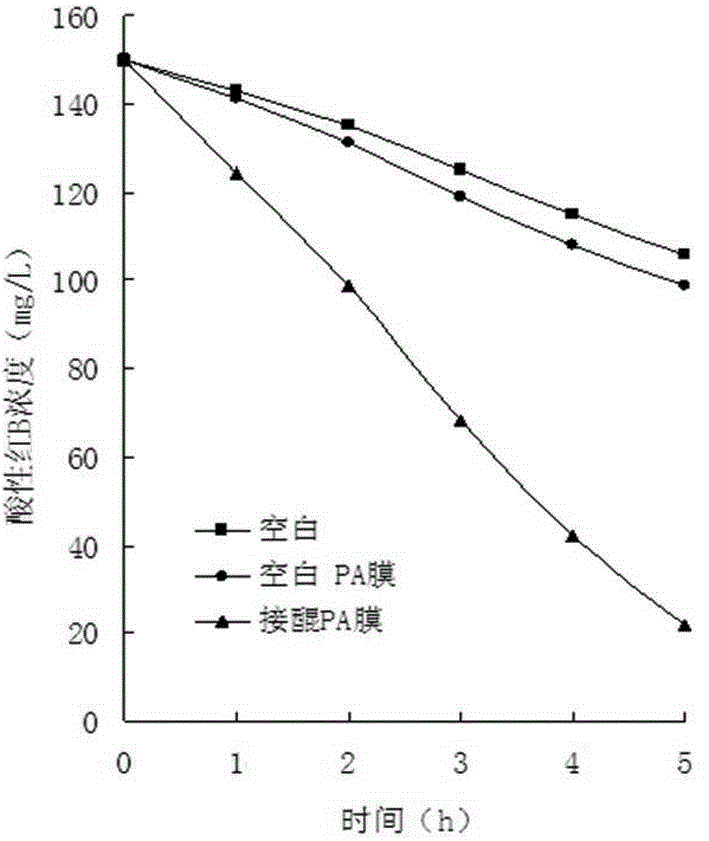
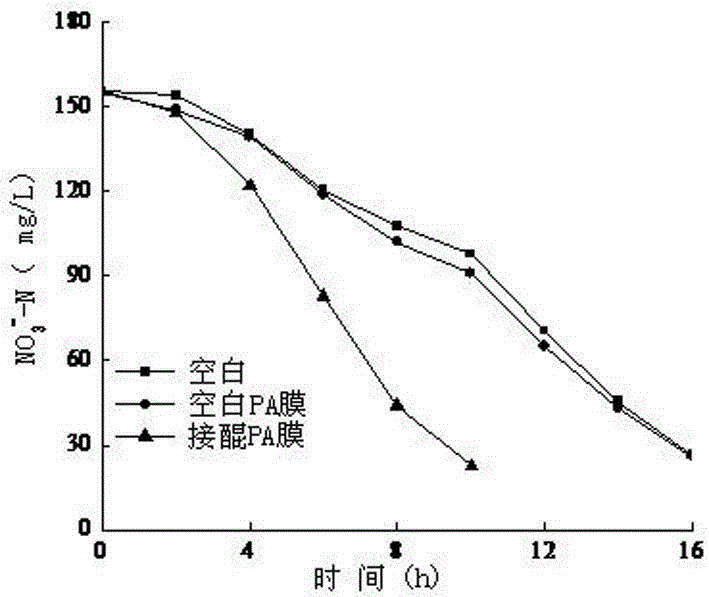
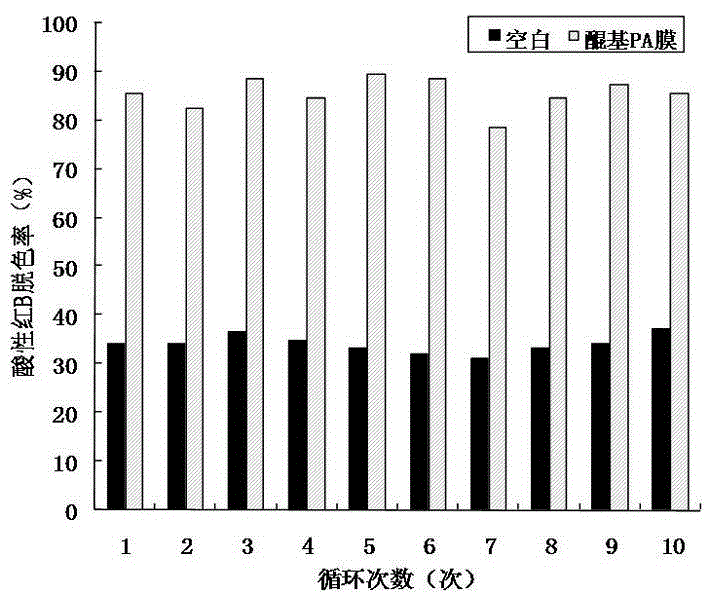
[0032] The application of the nylon membrane biocarrier mo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com