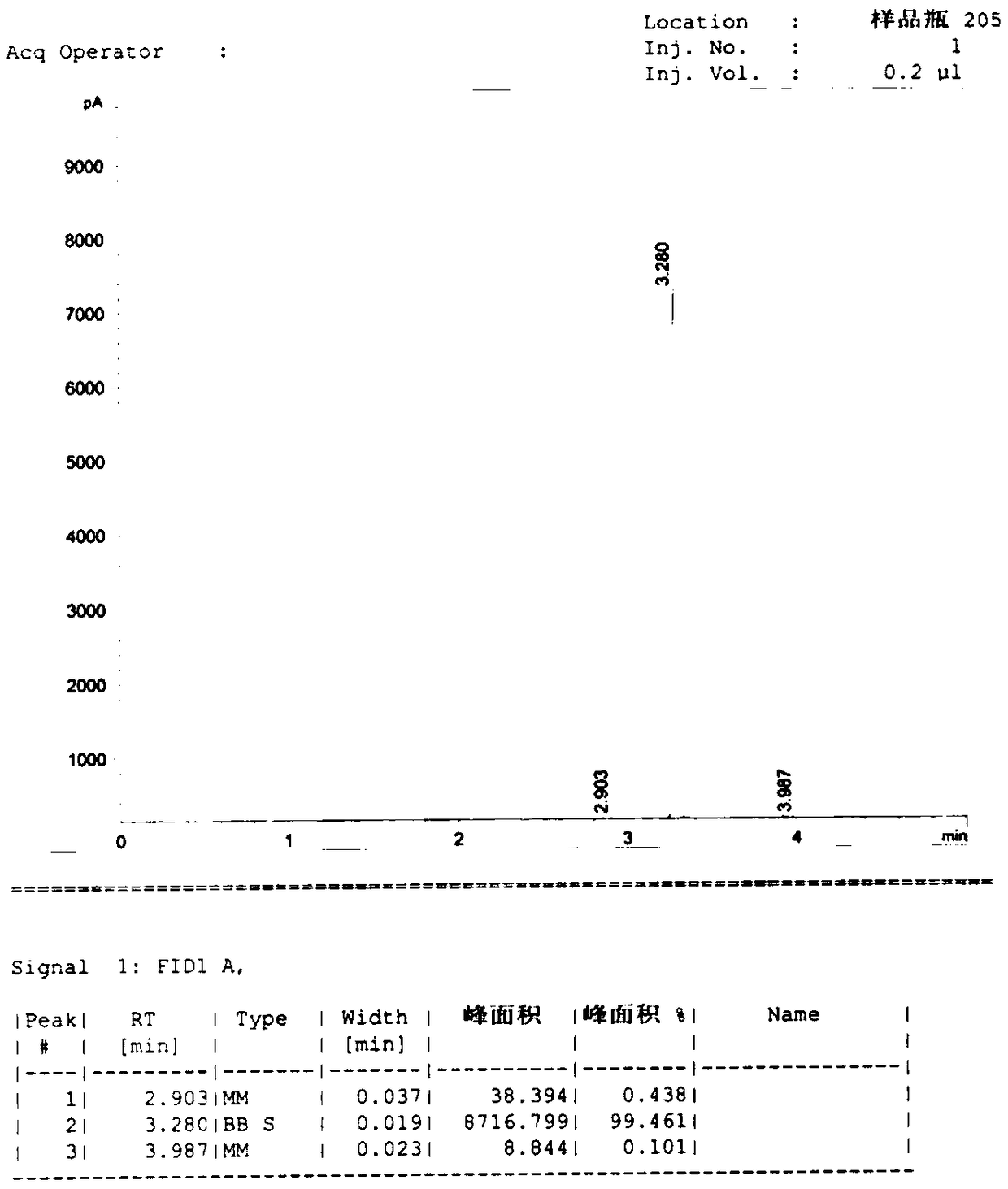
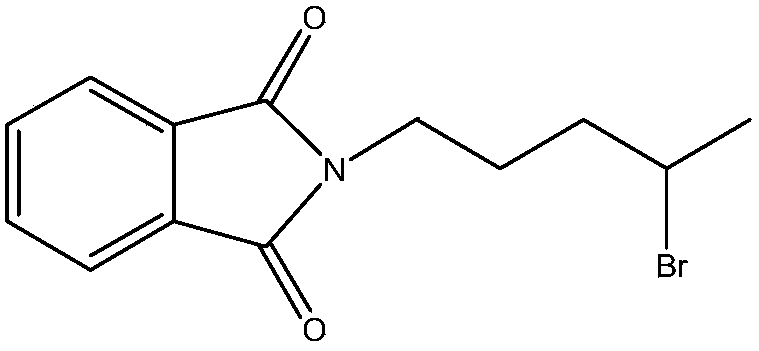
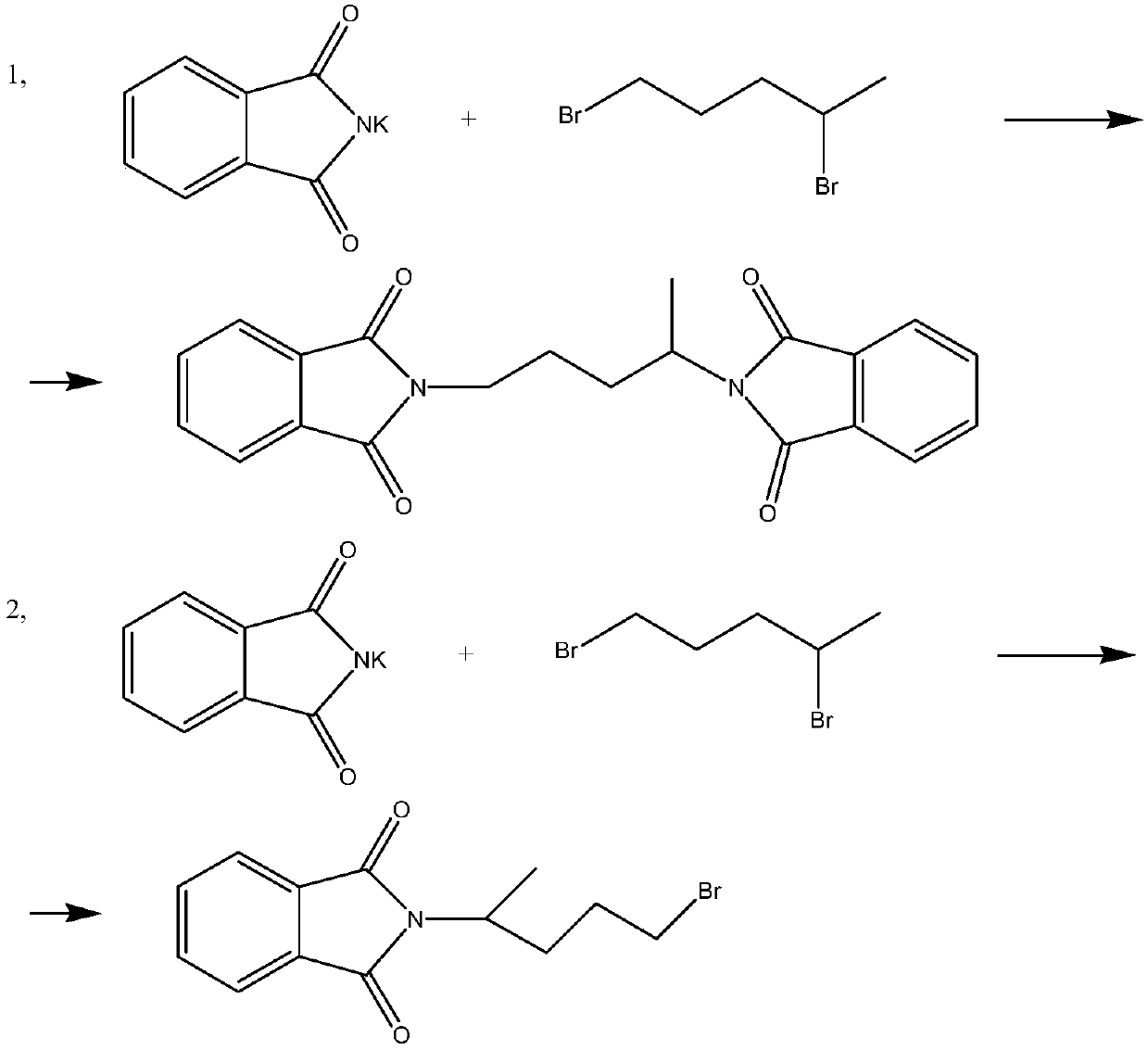
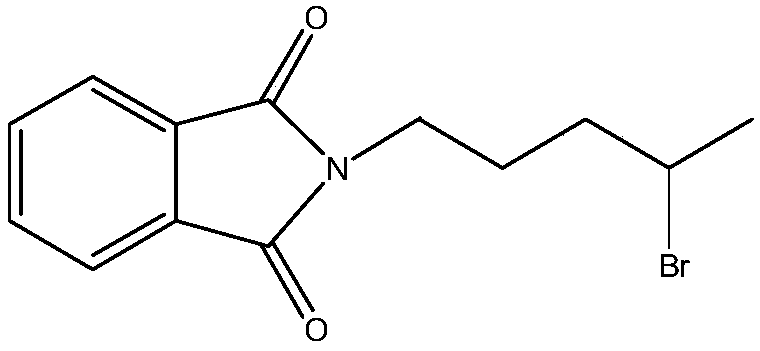
Patents
Literature
50 results about "Phthalimide potassium salt" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Phthalimide potassium salt was employed as organocatalyst for the cyanosilylation of various carbonyl compounds under extremely mild conditions. It was also employed as reagent for the transformation of allyl- and alkyl halides into protected primary amines.
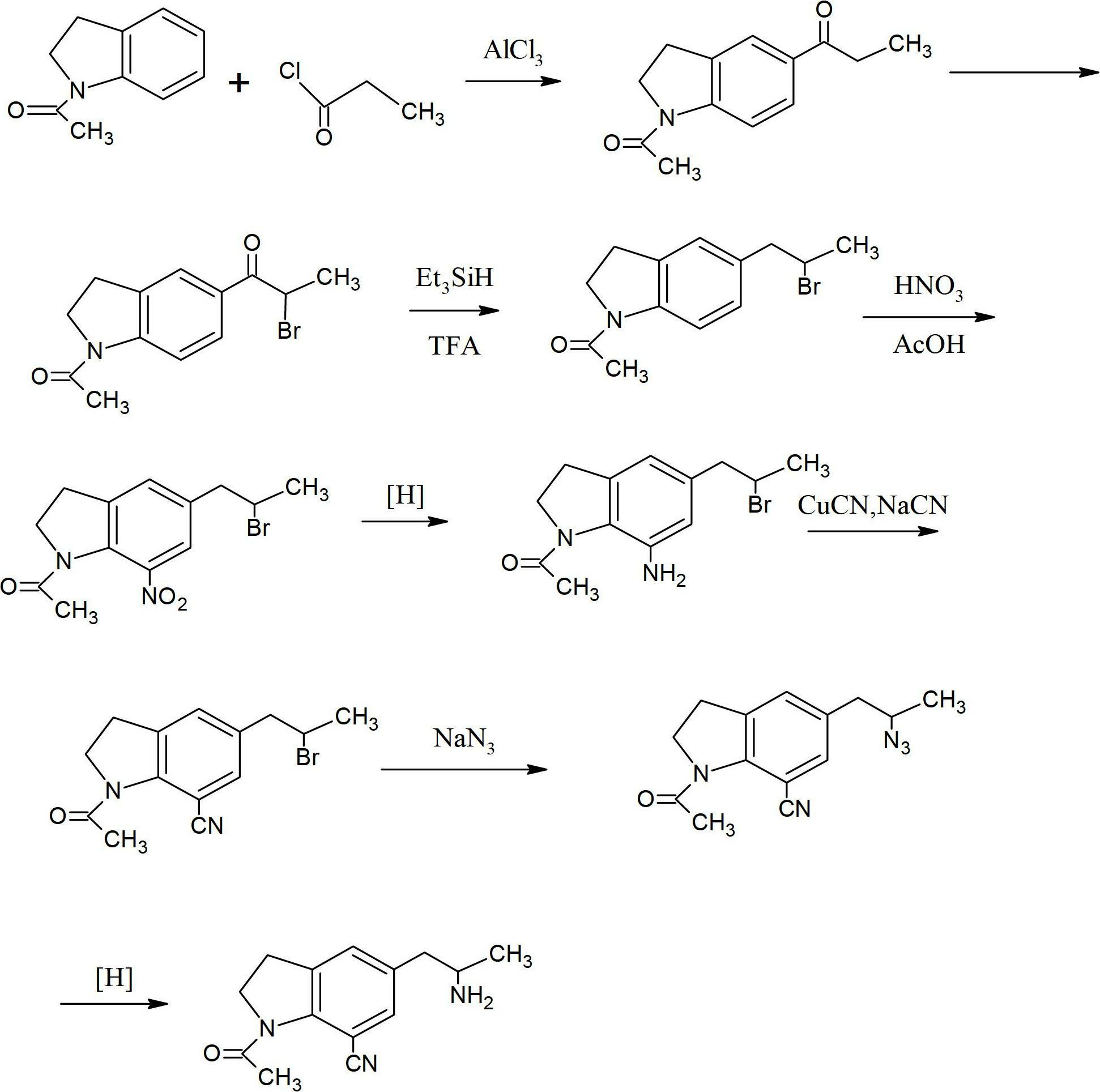
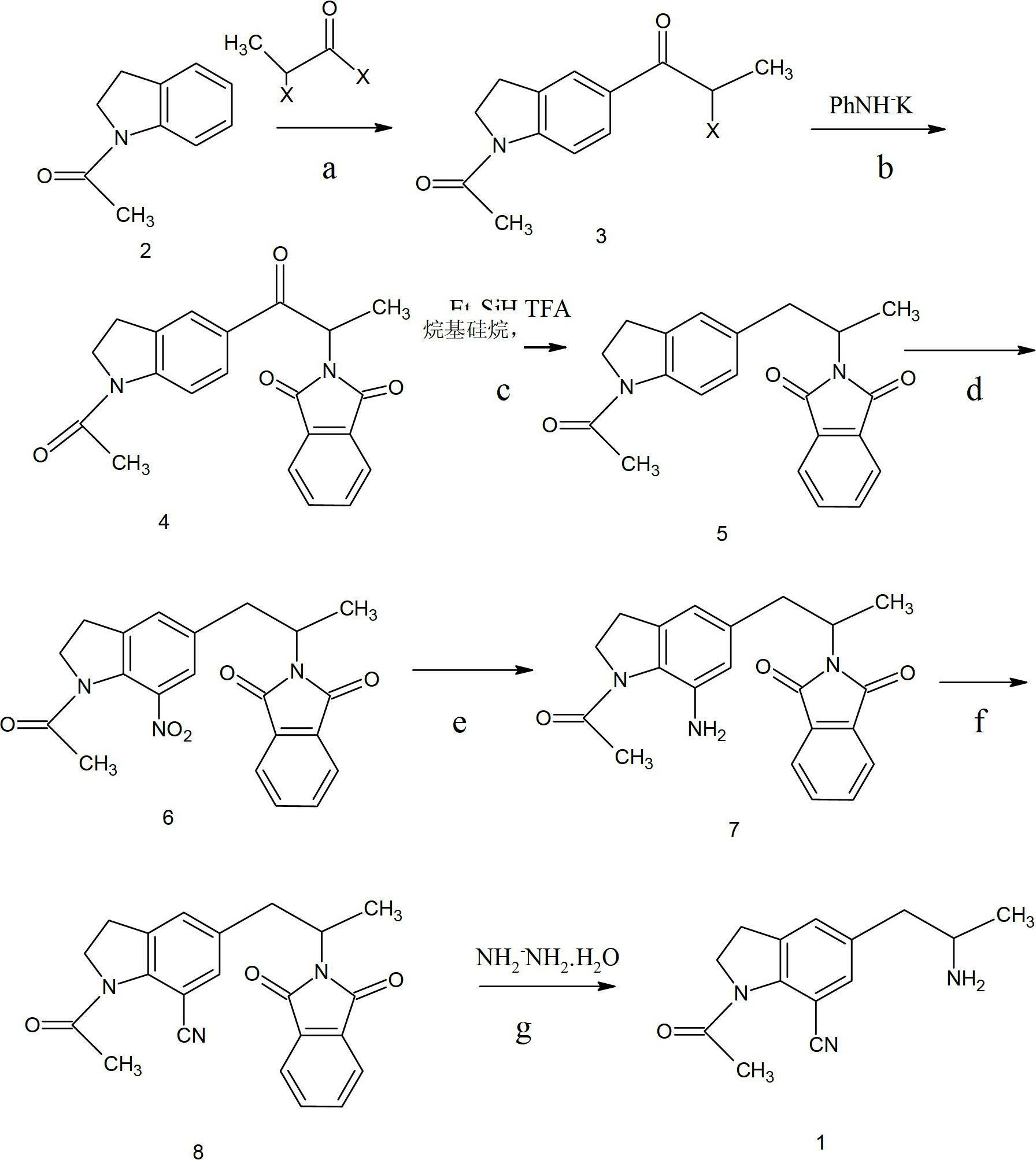
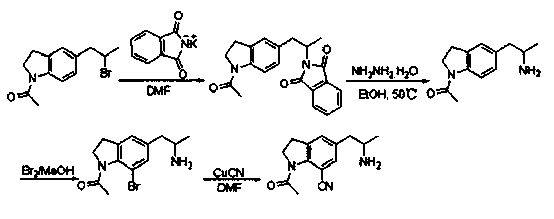
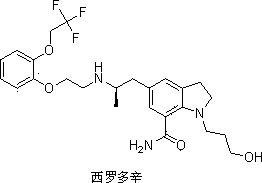
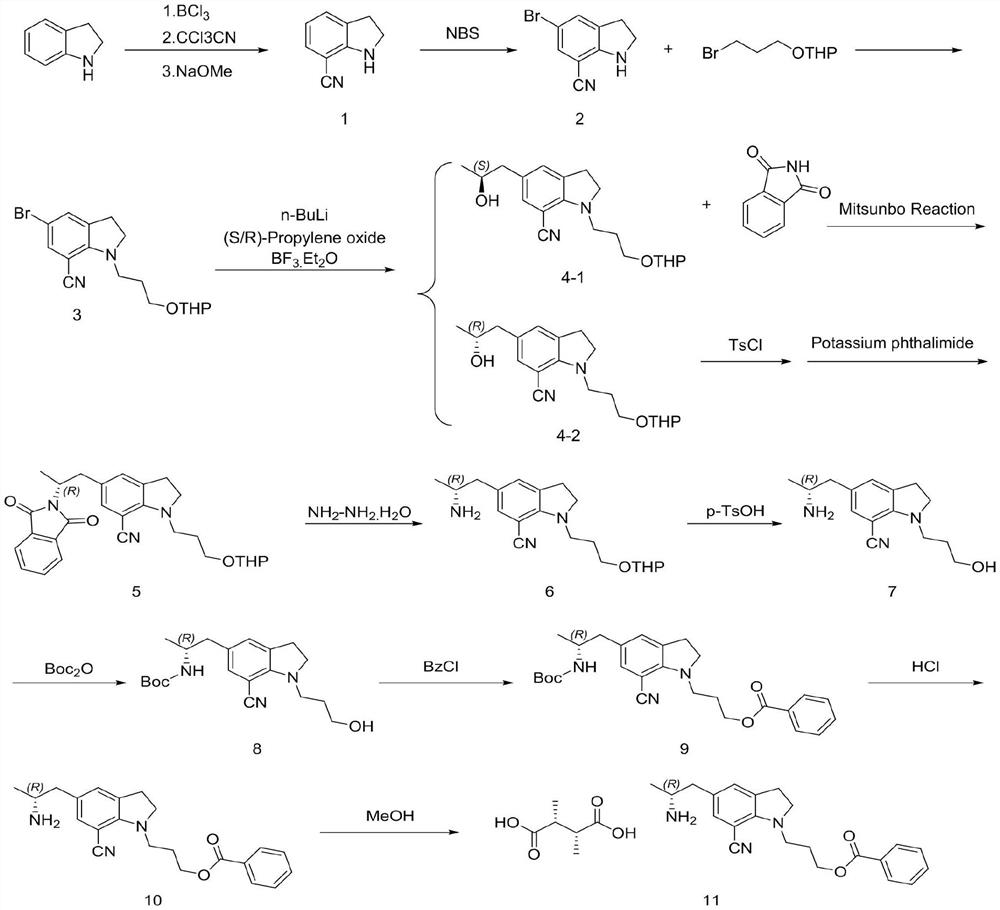
Preparation method for 1-acetyl-7-cyanopyridine-5-(2-amino propyl) indoline
The invention provides a preparation method for synthesizing 1-acetyl-7-cyanopyridine-5-(2-amino propyl) indoline which is a key intermediate of silodosin. The method comprises the following steps: 1-acetylindoline is adopted to serve as the material, and 1-acetyl-7-cyanopyridine-5-(2-amino propyl) indoline can be obtained through the such 7 steps as friedel-crafts acylation reaction, phthalimide amination, alkylsilane reduction, nitrification, reduction, heavy nitrogen cyaniding and deprotection. According to the method, the materials in the whole synthesizing line are cost-saving and easy to obtain, the operation is simple and the operation cost is low. Moreover, the yield rate of each step of reaction is higher than that in the prior art. Therefore, the method provided by the invention is very suitable for industrial production and has larger industrial application value.
Owner:LINHAI TIANYU PHARMA
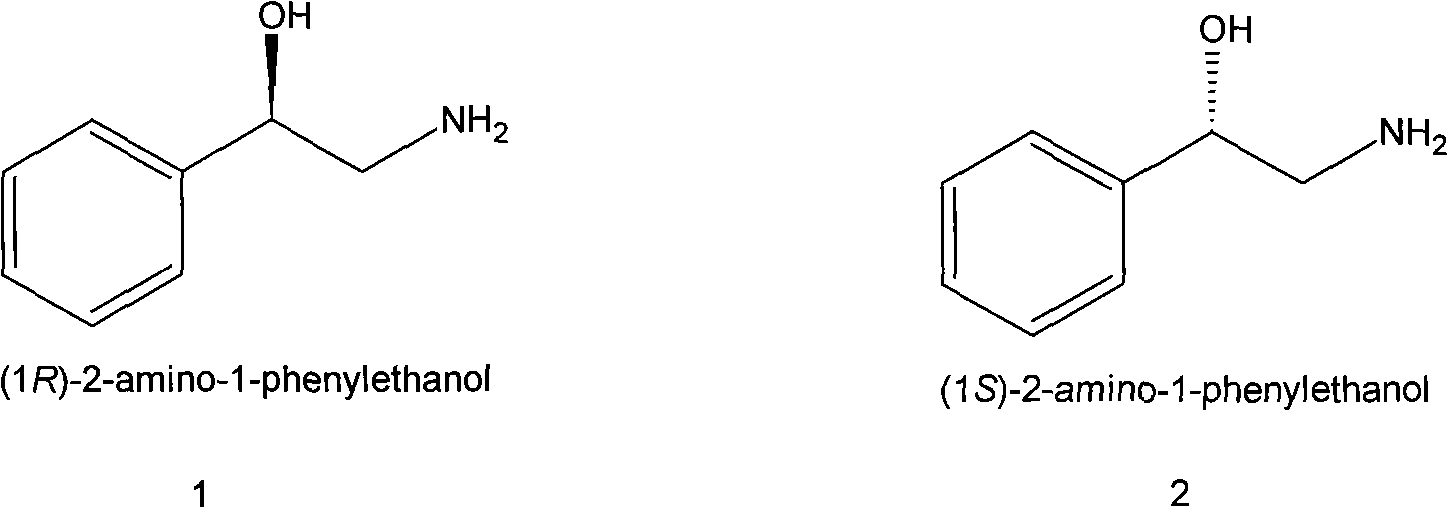
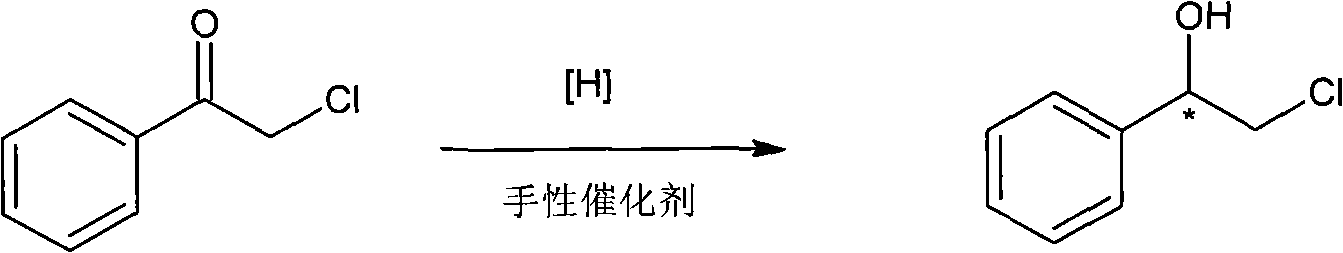
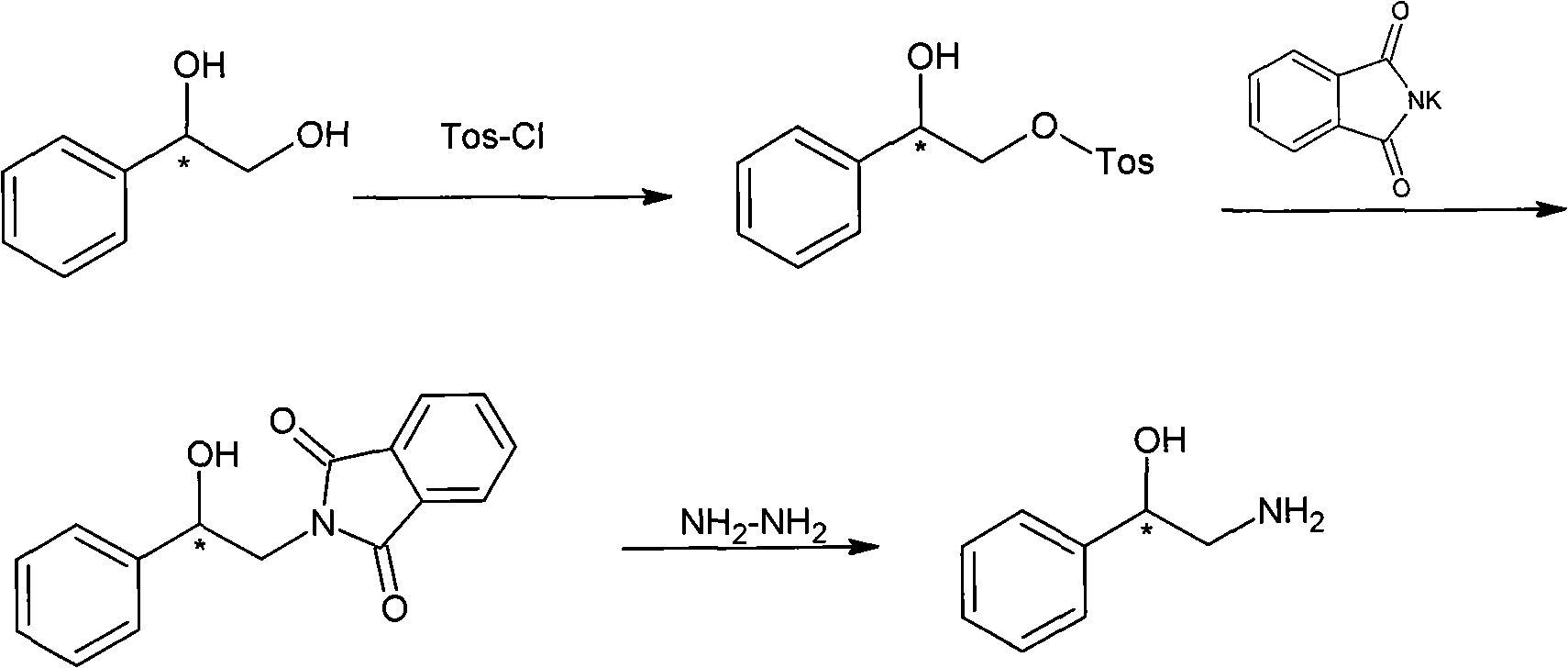
Method for preparing chiral medicinal intermediate 2-amido-1-phenylethylalcohol
ActiveCN101575298AReasonable workmanshipEasy to operateOrganic compound preparationAsymmetric synthesesPotassium phthalimideToluene
The invention relates to a method for preparing a chiral medicinal intermediate 2-amido-1-phenylethylalcohol. The method comprises the following steps that: chiral 1,2-styrolylalcohol is used as a rawmaterial to obtain chiral 2-paratoluenesulfonic-1-phenyl-1,2-glycol through a selective single para toluene sulfonylation reaction; the chiral 2-paratoluenesulfonic-1-phenyl-1,2-glycol performs a substitution reaction with potassium phthalimide to obtain chiral 2-phthalimide-1-phenylethanol; and finally, the chiral medicinal intermediate 2-amido-1-phenylethylalcohol with retained configuration isobtained through a hydrazinolysis reaction. Compared with the prior art, the method has reasonable process and simple operation, prepares the 2-amido-1-phenylethylalcohol from the low-cost chiral rawmaterial with low cost, obtains the product with high optical purity and chemical purity, is suitable for industrial large-scale production, and provides advantageous condition for industrially producing chiral 2-amido-1-phenylethylalcohol analogs.
Owner:上海予利生物科技股份有限公司
Preparation method of rivaroxaban
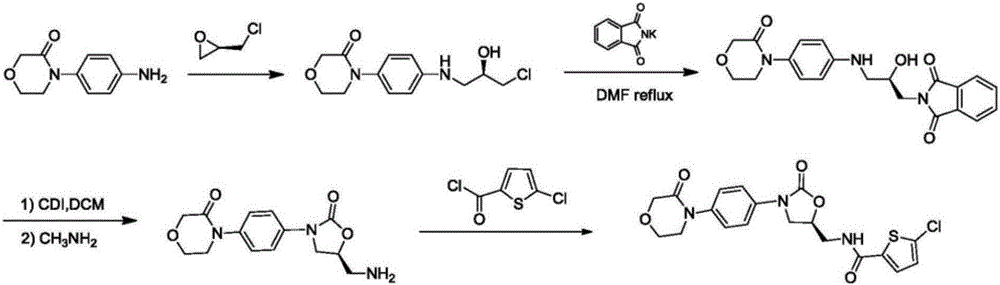
The invention discloses a preparation method of rivaroxaban. With (S)-4-chloro-3-hydroxybutyronitrile (compound 1) as a raw material, the rivaroxaban is obtained through phthalimide potassium salt substitution, cyano hydrolysis, Hofmann rearranging cyclization, Ullmann coupling, hydrazinolysis and amidation. The rivaroxaban is introduced into a chiral center instead of (S)-epoxy chloropropane which is volatile, high in toxicity and unstable; the safety is relatively high; precious catalyst, raw materials and reagent with large environmental pollution are avoided in the total process in the total synthetic route; the overall synthesis process is small in pollution and easy to treat; the yield and the purity of various steps are high; the preparation method is environmentally friendly, low in production cost and suitable for industrial production.
Owner:SHANDONG LUOXIN PHARMA GRP HENGXIN PHARMA CO LTD
Polyethylene glycol-modified phospholipid derivative taking anilino-quinazoline as targeting ligand and preparation method thereof
InactiveCN102649841APharmaceutical non-active ingredientsAntineoplastic agentsSulfonyl chlorideTumor therapy
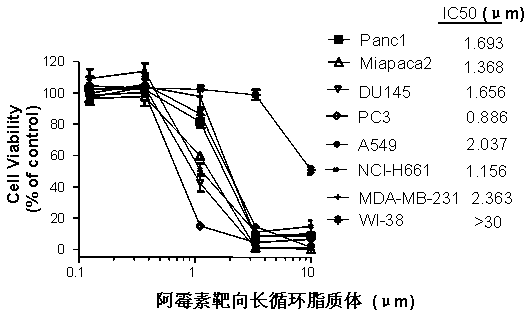
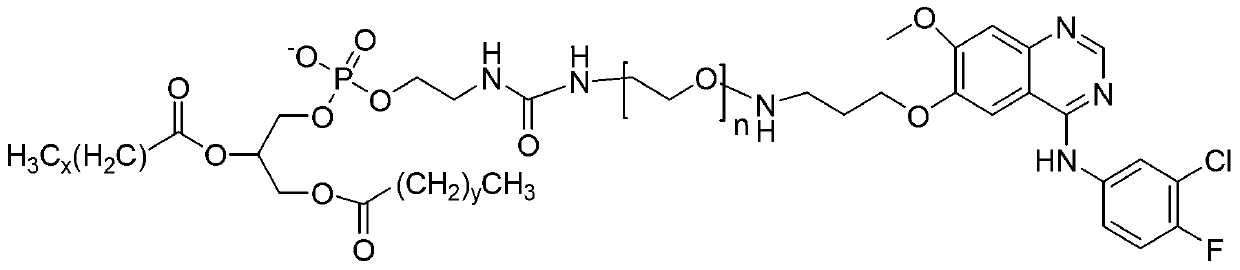
The invention provides a polyethylene glycol-modified phospholipid derivative taking a 4-substituted anilino-quinazoline group as a targeting ligand and a preparation method thereof. The method comprises the following steps of: performing condensation with para-methylbenzene sulfonyl chloride, substitution with phthalimide potassium salt and hydrolysis with a hydrazine hydrate by taking polyethylene glycol (PEG) as a starting raw material to obtain diamine PEG; and reacting with targeting ligands, i.e., N-(3-chlorin-4-fluorin)-6-(3-chloropropyl)-7-methoxylquinazoline-4-amine and distearoylphosphatidylethanolamine (DSPE) to respectively obtain 4-anilino-quinazoline group-PEG-DSPE. A liposome preparation of the 4-anilino-quinazoline group-PEG-DSPE can be used for realizing target administration of tumor cells and reducing the toxic and side effects of a tumor medicament; and the pharmacodynamic structure of gefitinib can give play to dual medicament effects together with other liposome-coated antitumor medicaments, so that the tumor treatment effect is enhanced greatly.
Owner:SOUTHEAST UNIV
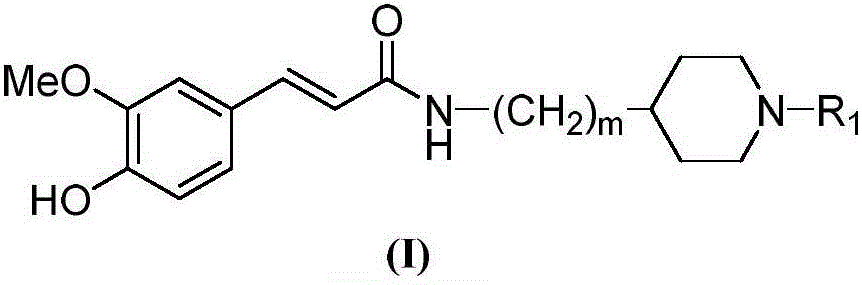
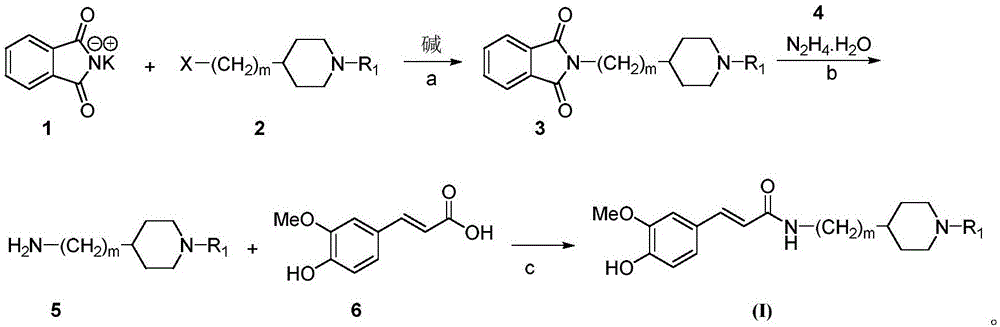
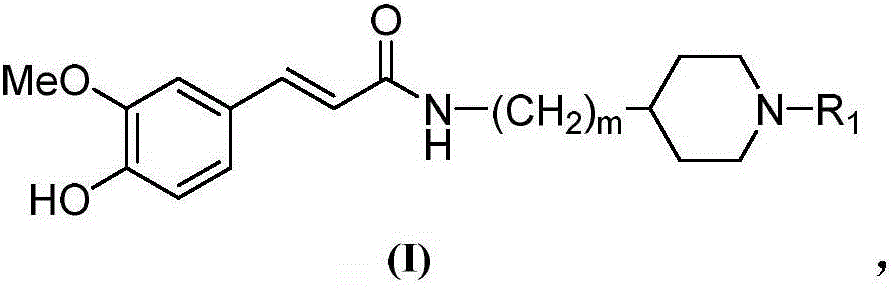
Cyclamine alkylamide ferulate compound as well as preparation method and application thereof
InactiveCN105777614AGood inhibitory effectGood selective inhibitionNervous disorderOrganic chemistryChemical structureChemical reaction
The invention belongs to the technical field of organic synthesis and particularly relates to a cyclamine alkylamide ferulate compound as well as a preparation method and application thereof. The preparation method comprises the following steps: enabling potassium phthalimide serving as a start raw material to react with 1-substituted-4-haloalkyl piperidine under the effects of a solvent and alkali to obtain a phthalimide alkylamine compound; performing hydrazinolysis of the phthalimide alkylamine compound and hydrazine hydrate to obtain a primary amine compound; and adding ferulic acid, a condensing agent and a solvent into the primary amine compound for a condensation reaction to obtain a product of cyclamine alkylamide ferulate compound. The cyclamine alkylamide ferulate compound provided by the invention is of a simple chemical structure, the chemical reactions in the preparation process are thorough, the product yield is high, the operation is convenient, the cost is low, and the product can be used for perfectly treating the neurodegenerative diseases.
Owner:NANYANG NORMAL UNIV
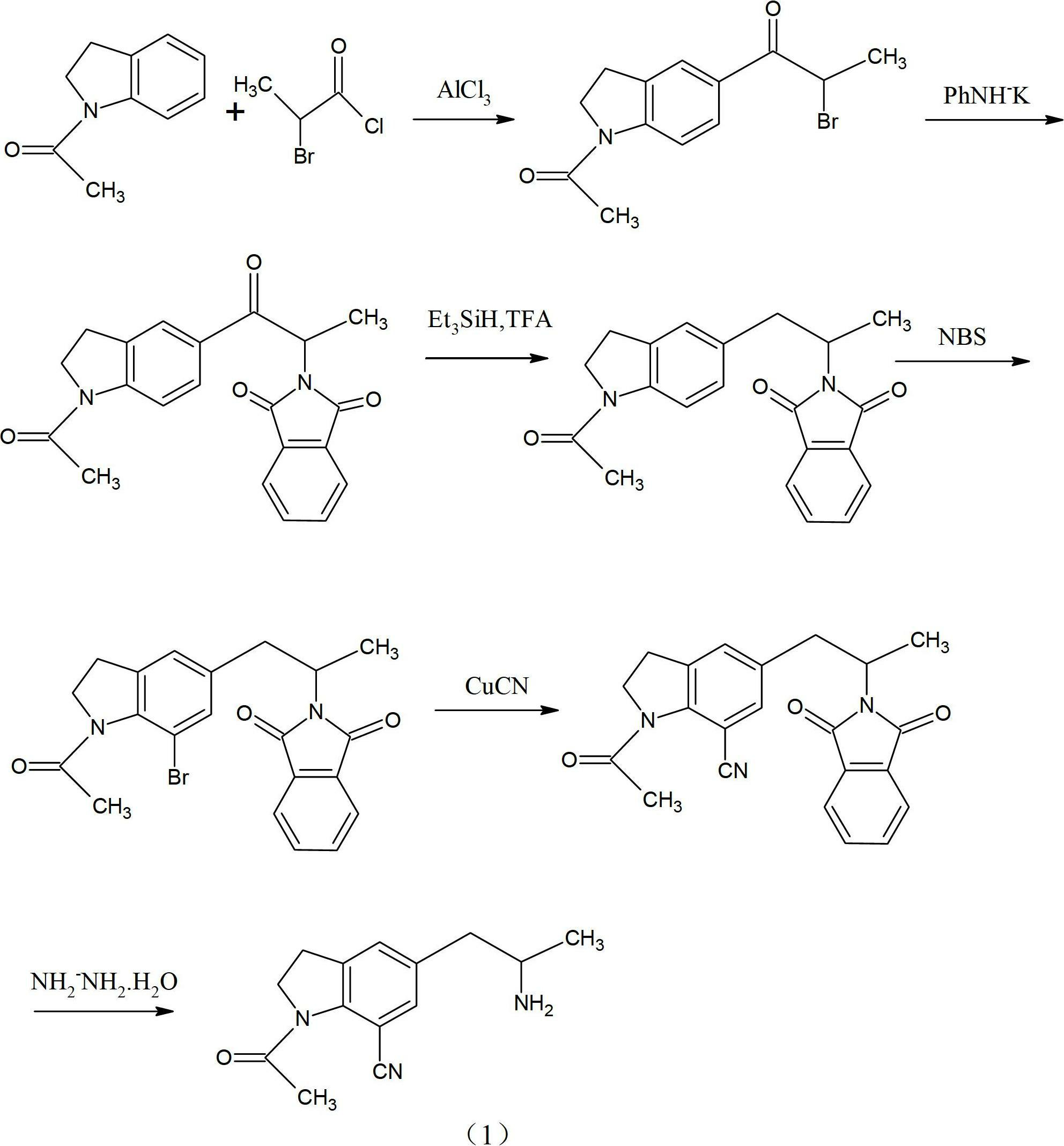
Preparation method of important intermediate of Silodosin
InactiveCN103848772AEasy to separateSimple and fast operationOrganic chemistryImideBiochemical engineering
The invention discloses a preparation method of an important intermediate of Silodosin. The method comprises the following steps: carrying out a Gabriel primary amine synthesis reaction of raw materials comprising 1-acetyl-5-(2-bromopropyl)indoline and phthalimide potassium to obtain an intermediate 1-acetyl-5-(2-aminopropyl)indoline, brominating, and cyanating to obtain a product 1-acetyl-5-(2-bromopropyl)-7-cyanindoline, that is the important intermediate of Silodosin. The preparation method has the advantages of simple operation, high reaction yield, easy product separation and the like, and is suitable for industrialized production.
Owner:STONE LAKE PHARMA TECH
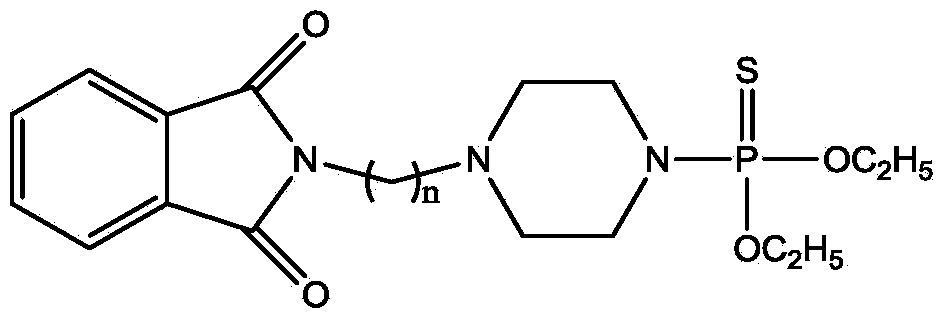
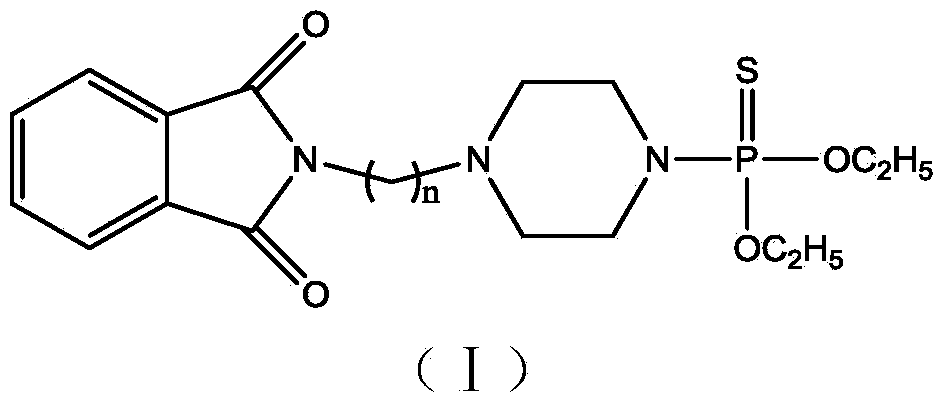
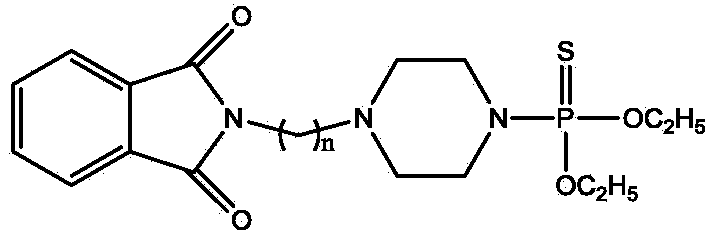
N-alkylation phthalimide piperazine derivatives as well as preparation method and application thereof
InactiveCN103923123AReduce manufacturing costGood synergyBiocideGroup 5/15 element organic compoundsAlkaneAlkyl transfer
The invention discloses N-alkylation phthalimide piperazine derivatives as well as a preparation method and an application thereof. The structural formula of the N-alkylation phthalimide piperazine derivatives is shown in descriptions, and in the structural formula, n is 1 or 2 or 3. The preparation method of the N-alkylation phthalimide piperazine derivatives comprises the following steps: dissolving phthalimide potassium salt in acetone, then adding dibromine alkane to the acetone, and reacting for 5-12 hours at the temperature of 45-65 DEG C so as to obtain a first intermediate; dissolving piperazine in dichloromethane, adding diethyl thiophosphoryl chloride to the dichloromethane at the temperature of -5 to 5 DEG C, and reacting for 0.1-1 hour at the temperature of -5 to 5 DEG so as to obtain a second intermediate; and dissolving the first intermediate in N,N-dimethylformamide, adding monovalent carbonate and the second intermediate to the N,N-dimethylformamide, and reacting for 1-5 hours at the temperature of 50-80 DEG C. The N-alkylation phthalimide piperazine derivatives provided by the invention have high effect on prevention and treatment of invertebrate insect pests. Serving as arthropod killing agents, the N-alkylation phthalimide piperazine derivatives are capable of reducing production cost and environmental load.
Owner:GUILIN JIQI BIOCHEM
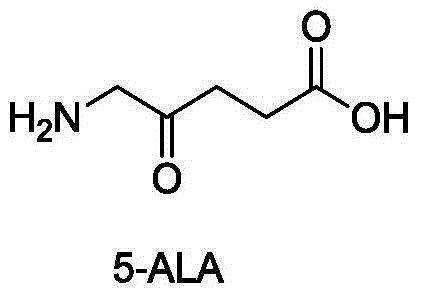
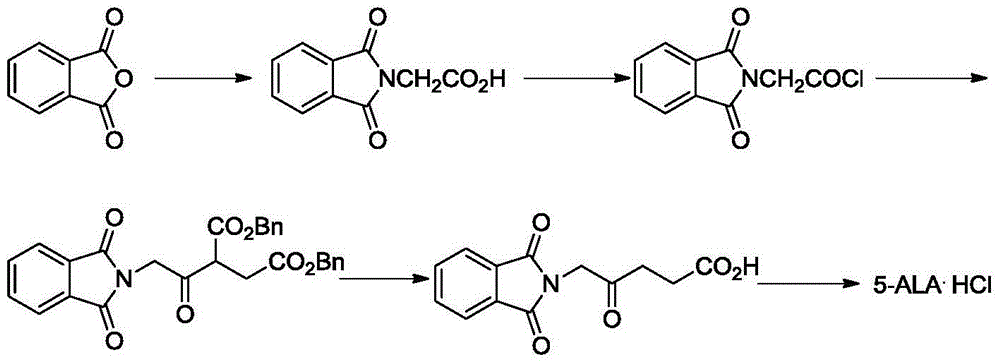
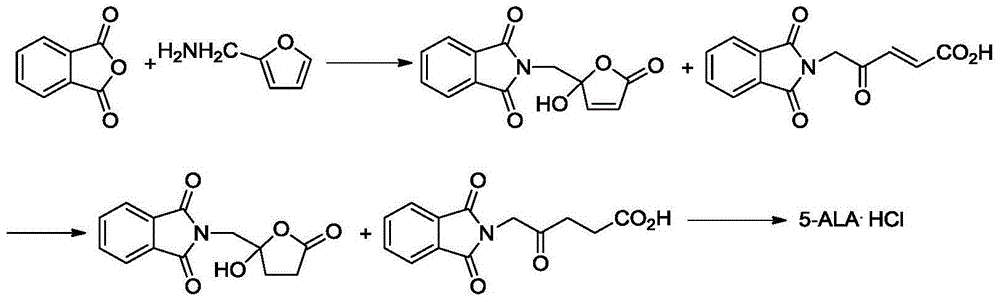
Preparation method of 5-aminolevulinic acid hydrochloride and intermediate of 5-aminolevulinic acid hydrochloride
InactiveCN104649952ASimple processEasy to operateOrganic compound preparationAmino-carboxyl compound preparationHydrogenAminolevulinic Acid Hydrochloride
The invention discloses a preparation method of a 5-aminolevulinic acid hydrochloride and an intermediate of the 5-aminolevulinic acid hydrochloride. The intermediate compound of the 5-aminolevulinic acid hydrochloride is represented by formula 3. The preparation method comprises following steps: (1) in an organic inert solvent, a compound represented by formula 1 and liquid bromine are subjected to bromination reaction; step (2), a reaction system obtained via step (1) is mixed with an alkali for reaction; and step (3), after the reaction of step (2), an obtained reaction system is directly subjected to Gabriel reaction with phthalimide potassium salt without post-processing purification so as to obtain the compound represented by formula 3, wherein R is used for representing H or C1-C4 alkyl groups. The preparation method is simple, is easy for operation, is high in yield, and is suitable for industrialized production.
Owner:SHANGHAI JIUZHOU MEDICAL TECH
Active organo montmorillonite and preparation method thereof
InactiveCN101531373AIncrease layer spacingGood dispersionSilicon compoundsDispersityHydrazine compound
The invention discloses an active organo montmorillonite and a preparation method thereof, wherein, the organo montmorillonite is the amino-containing active organo montmorillonite composed of Na-montmorillonite and an organic intercalation agent which intercalate in the Na-montmorillonite and is subjected to the modification of potassium phthalimide; the organic intercalation agent is an organic compound represented by R(CH2)<12-18N>(CH3)3R or R(CH2)<12-18>NH2 structural formulae, R in the formulae is chlorine atom or bromine atom. The preparation method thereof comprises the step of: subjecting the modified organic intercalation agent and the Na-montmorillonite to intercalation in a solvent at 80-120 DEG C and then to amination with hydrazine hydrate on condition that the solvent is present, in order to prepare the amino-containing active organo montmorillonite. The organo montmorillonite prepared by the invention has outstanding dispersity both in the organic solvent and in the water and can be better used for blending with other polymers to prepare a composite material. The invention can be used for the fields of plastic processing, the preparation of chemical building material, the preparation of nano material, biomedical material and cosmetics.
Owner:SICHUAN UNIV
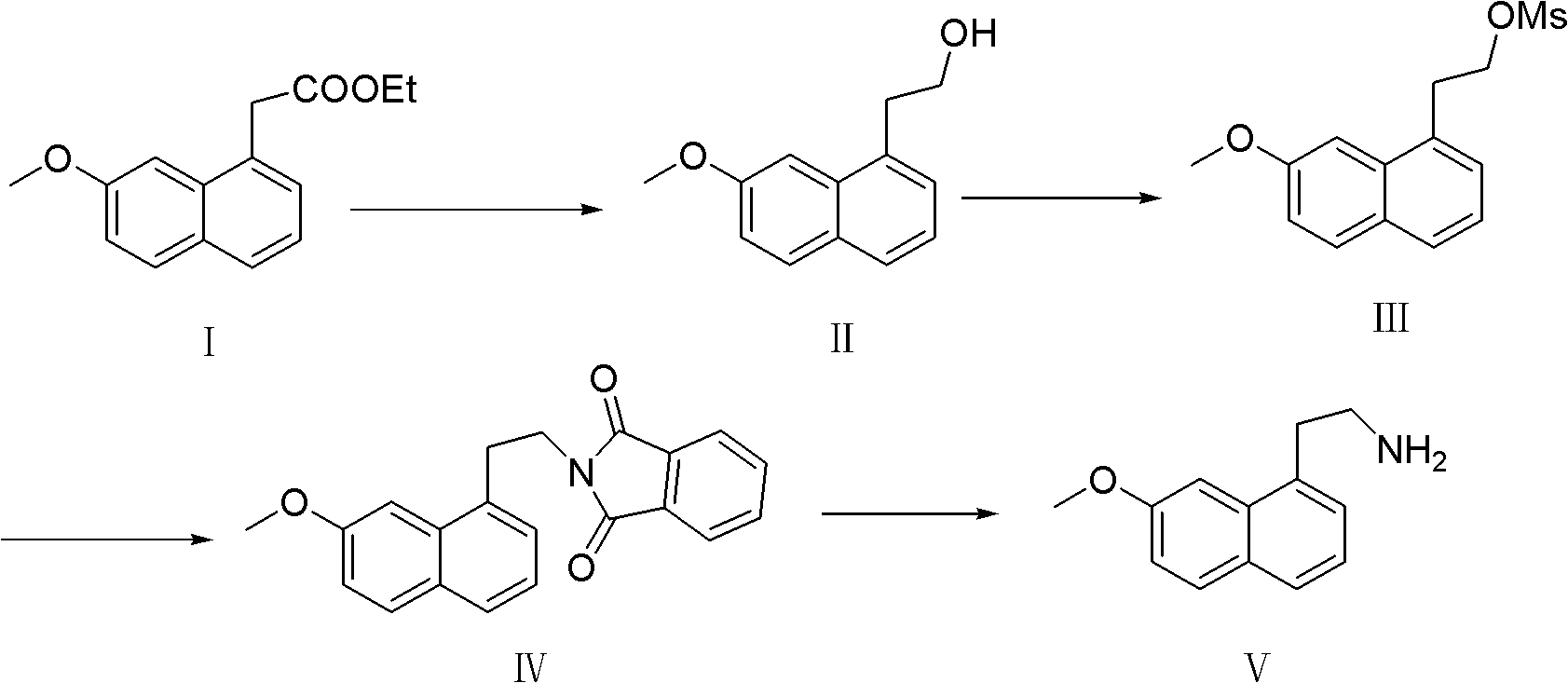
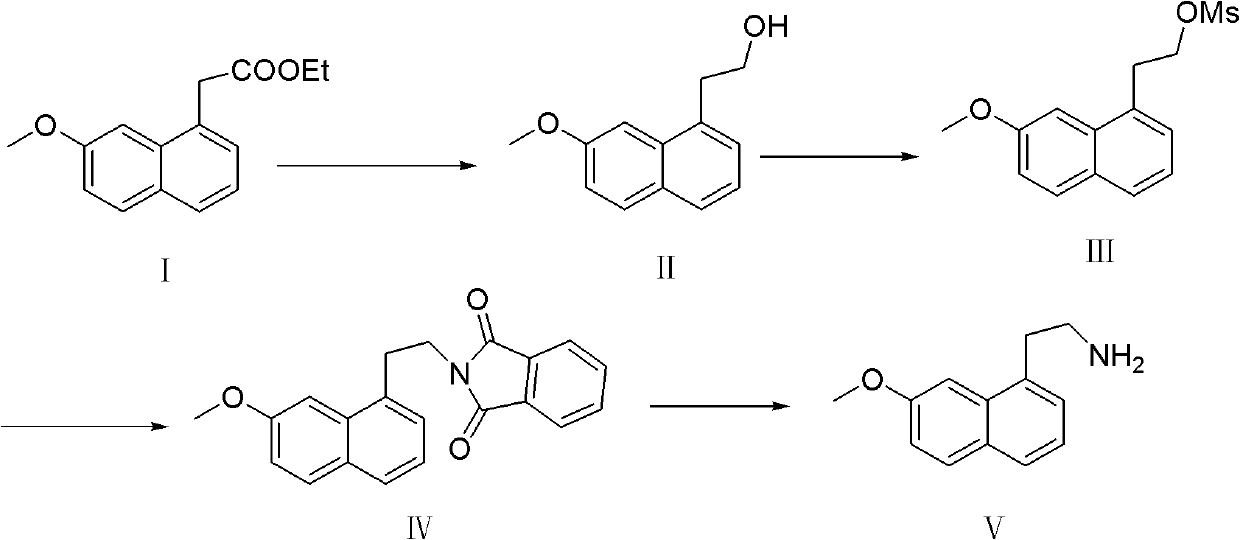
Preparation method of 7-methoxy-1-naphthylethylamine
InactiveCN102603545ALow costReduce usageOrganic compound preparationAmino-hyroxy compound preparationAcetic acidSodium borohydride
The invention belongs to the technical field of pharmaceutical intermediates, and in particular relates to a new preparation method of a key intermediate, 7-methoxy-1-naphthylethylamine of a pharmaceutical ingredient, agomelatine. The aim of the invention is to reduce the cost, optimize the process and bring convenience to the industrial production. The preparation method comprises the following steps: taking 7-methoxy-1-naphthylacetate as a raw material; reducing by sodium borohydride to obtain 7-methoxy-1-naphthaleneethanol; carrying out a methyl sulfonylation reaction to obtain 7-methoxy-1-naphthylethylmethanesulfonate; reacting 7-methoxy-1-naphthylethylmethanesulfonate with a phthalimide potassium salt to obtain 7-methoxy-1-(N-phthalimidoethyl)naphthyl; and finally hydrolyzing 7-methoxy-1-(N-phthalimidoethyl)naphthyl to obtain the product, 7-methoxy-1-naphthylethylamine. The preparation method has the advantages of being simple and convenient in operation, reasonable in reaction process, low in production cost, good in product quality, high in purity (above 98%), having no environment pollution and being suitable for industrial production.
Owner:湖北万知化工医药股份有限公司
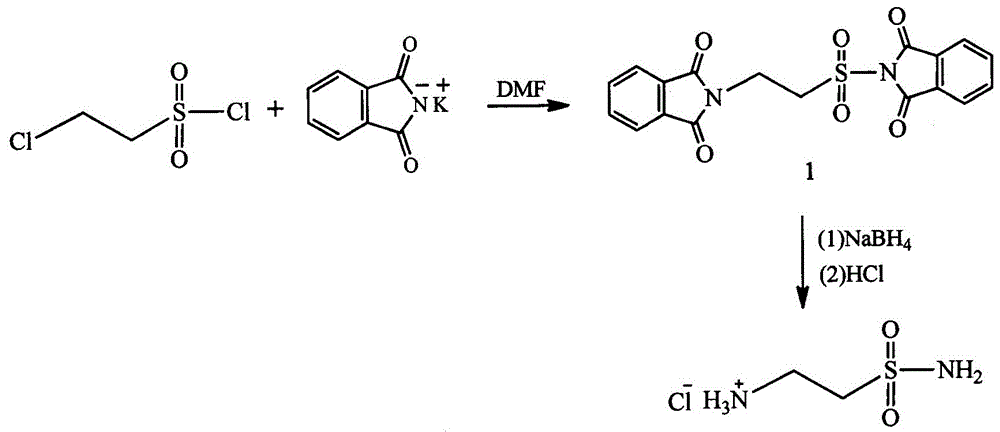
Method for preparing 2-amino ethanesulfonamide hydrochloride
ActiveCN106748905AReduced two-step reactionSimple preparation processSulfonic acid amide preparationAcetic acidChloride
The invention relates to a method for preparing 2-amino ethanesulfonamide hydrochloride. The method comprises the following steps: (1) reacting by virtue of chloroethylsulfonyl chloride with phthalimide potassium salt, so as to prepare N-(2- phthalimide ethyl sulfonyl)phthalimide (intermediate 1); and (2) reacting by virtue of the intermediate 1 with sodium borohydride, so as to prepare 2-aminoethanesulfonamide, and carrying out hydrochloric acid acidification, so as to obtain 2-amino ethanesulfonamide hydrochloride. The method is characterized in that 2-amino ethanesulfonamide hydrochloride is prepared from chloroethylsulfonyl chloride for the first time, a preparation process is simple, and waste liquids such as acetic acid, tetrahydrofuran, dioxane, hydrazine hydrate and phosphorus oxychloride are not produced.
Owner:HENAN CHEM IND RES INST
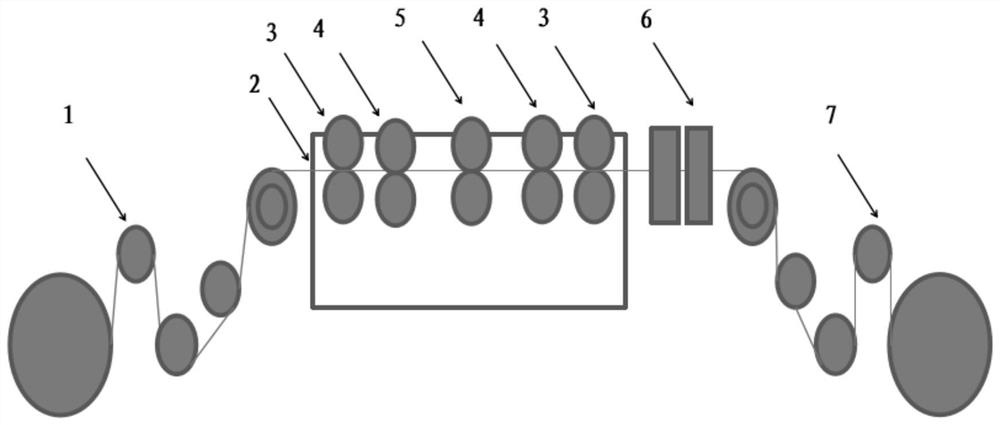
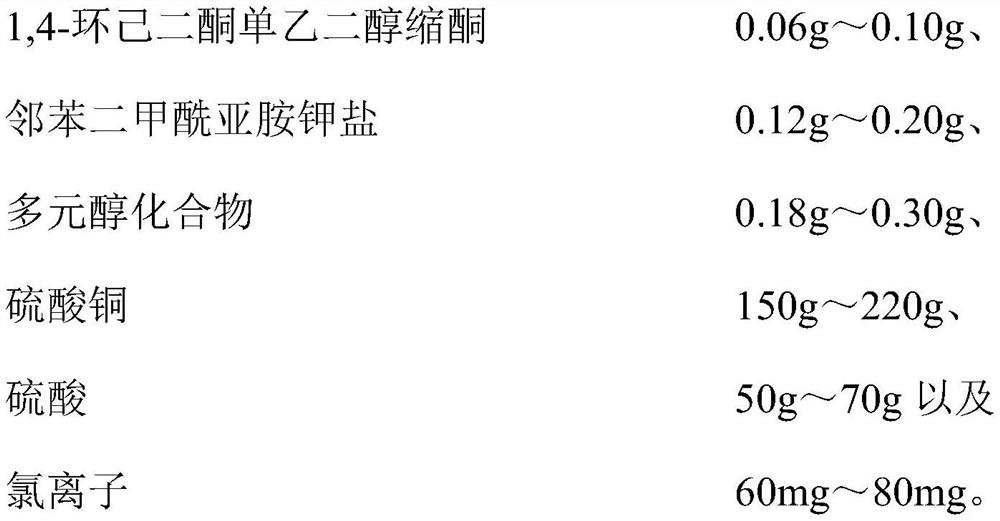
Plastic film brush copper plating process
ActiveCN114561674AImprove uniformityImprove continuityVacuum evaporation coatingSputtering coatingImidePolyol
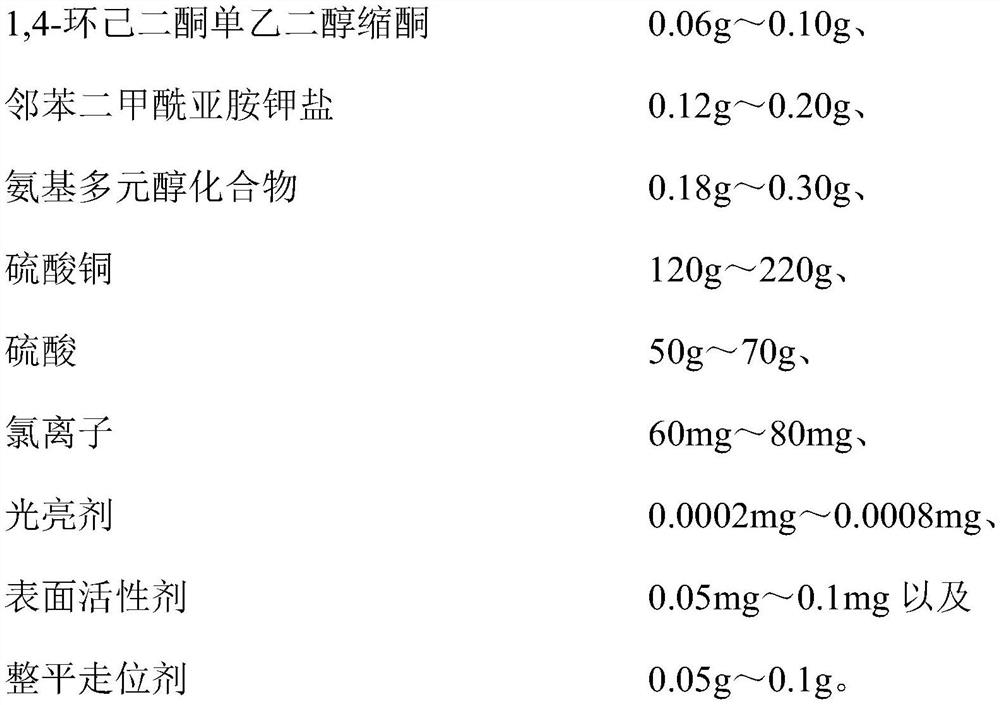
The invention relates to a plastic film brush copper plating process. The process comprises the following steps: carrying out first copper plating treatment on a plastic film by adopting a physical vapor deposition method, preparing a first copper film on one surface of the plastic film, and preparing a second copper film on the other surface of the plastic film; second copper plating treatment is conducted on the first copper film and the second copper film in a brush copper plating mode, a third copper film is prepared on the first copper film, and a fourth copper film is prepared on the second copper film; the anode of the second copper plating treatment is a brush plating roller; and a copper plating solution adopted in the second copper plating treatment comprises water, copper sulfate, sulfuric acid, chloride ions, 1, 4-cyclohexanedione monoethylene ketal, phthalimide potassium salt and a polyol compound. According to the copper plating process, the plating speed is high, the copper film layer with fine, continuous and uniform crystals can be obtained, no adverse effect is generated on the original copper plating film layer, the binding force of the plated film layer is excellent, the film surface sheet resistance can meet the finished product requirement through one-time brush plating film forming, the plastic film is not punctured, and the copper plating process is suitable for amplification application.
Owner:JIANGYIN NANOPORE INNOVATIVE MATERIALS TECH LTD
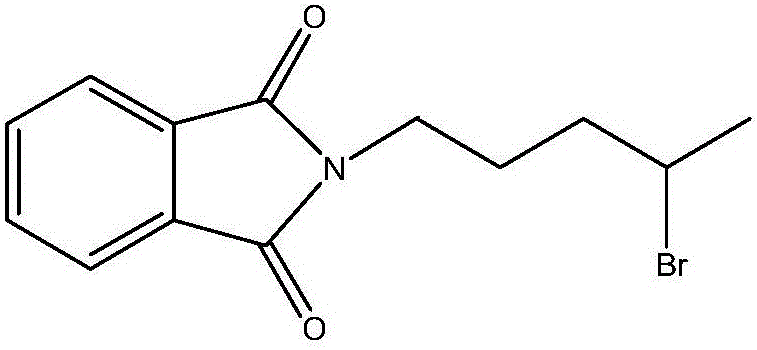
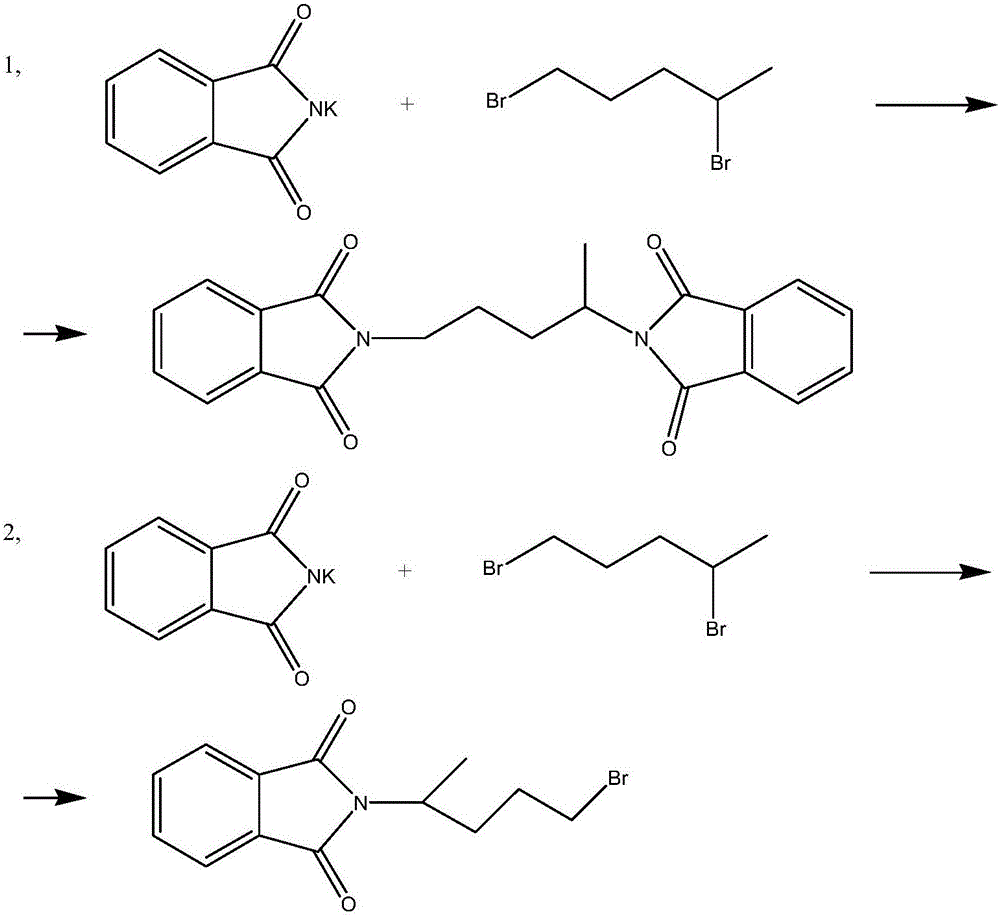
Synthesis method of antimalarial drug primaquine phosphate intermediate N-(4-bromopentyl)phthalimide
ActiveCN106008314AIncrease contentHigh selectivityOrganic chemistrySynthesis methodsHydrogen bromide
The invention provides a synthesis method of an antimalarial drug primaquine phosphate intermediate N-(4-bromopentyl)phthalimide. N-(4-bromopentyl)phthalimide is an important intermediate of an antimalarial drug primaquine phosphate. The method comprises the following steps: carrying out a condensation reaction on 5-chloro-1-pentene and phthalimide potassium salt, and carrying out an addition reaction on a condensation product 5-(o-phthalimido)-1-pentene and hydrogen bromide to obtain the final product. The method has the advantages of low cost of raw materials, high conversion rate, high purity of the final product, mild conditions of every reaction, and simple and easily controlled reaction process.
Owner:仪征市海帆化工有限公司
Preparation method and application of rivaroxaban intermediate (S)-N-epoxy propyl phthalimide
The invention provides a preparation method and application of a rivaroxaban intermediate (S)-N-epoxy propyl phthalimide. The preparation method comprises the following steps: firstly, adding absolute ethyl alcohol into a reaction container accommodating phthalimide, dropwise adding a KOH methanol solution for 1h under a stirring condition, performing continuous stirring for 3h at the room temperature, performing filtration after the reaction is finished, leaching an obtained white solid by using the absolute ethyl alcohol, and drying the white solid; secondly, adding phthalimide potassium salt into (R)-epichlorohydrin, and performing heating reaction under a condition of oil bath at the temperature of 130 DEG C to obtain the (S)-N-epoxy propyl phthalimide. The method comprises only two process routes, the steps are simple, after conditions are optimized, the reaction is wild, the cost of raw materials is low, and the yield is high.
Owner:ZHEJIANG TIANSHUN BIOTECH
Preparation method for 2-(2-methoxyphenoxy)ethylamine
InactiveCN109206328AReduce manufacturing costPromote safe productionOrganic compound preparationAmino-hyroxy compound preparationGuaiacolPotassium phthalimide
The invention provides a preparation method for 2-(2-methoxyphenoxy)ethylamine. The preparation method comprises the following steps: synthesizing 2-(2-methoxyphenoxy)ethanol with guaiacol as a starting material; then synthesizing 2-(2-methoxyphenoxy)chloroethane through chlorination; then reacting 2-(2-methoxyphenoxy)chloroethane with potassium phthalimide to obtain N-(o-methoxyphenoxyethyl)-phthalimide; and finally, performing basic hydrolysis to obtain 2-(2-methoxyphenoxy)ethylamine. The yields of the above four steps of reactions are that the yield of 2-(2-methoxyphenoxy)ethanol is 98.9%;the yield of 2-(2-methoxyphenoxy)chloroethane is 93.7%; the yield of N-(o-methoxyphenoxyethyl)-phthalimide is 86.4%; the yield of 2-(2-methoxyphenoxy)ethylamine is 91.2%; and the total yield of the four steps is 73.04%, which is higher than the yield of 43% in conventional production processes. The preparation method of the invention reduces the production cost of 2-(2-methoxyphenoxy)ethylamine and is safe in the production process.
Owner:TONGREN UNIV
Polyethylene glycol-modified phospholipid derivative taking anilino-quinazoline as targeting ligand and preparation method thereof
InactiveCN102649841BPharmaceutical non-active ingredientsAntineoplastic agentsSulfonyl chlorideSide effect
The invention provides a polyethylene glycol-modified phospholipid derivative taking a 4-substituted anilino-quinazoline group as a targeting ligand and a preparation method thereof. The method comprises the following steps of: performing condensation with para-methylbenzene sulfonyl chloride, substitution with phthalimide potassium salt and hydrolysis with a hydrazine hydrate by taking polyethylene glycol (PEG) as a starting raw material to obtain diamine PEG; and reacting with targeting ligands, i.e., N-(3-chlorin-4-fluorin)-6-(3-chloropropyl)-7-methoxylquinazoline-4-amine and distearoylphosphatidylethanolamine (DSPE) to respectively obtain 4-anilino-quinazoline group-PEG-DSPE. A liposome preparation of the 4-anilino-quinazoline group-PEG-DSPE can be used for realizing target administration of tumor cells and reducing the toxic and side effects of a tumor medicament; and the pharmacodynamic structure of gefitinib can give play to dual medicament effects together with other liposome-coated antitumor medicaments, so that the tumor treatment effect is enhanced greatly.
Owner:SOUTHEAST UNIV
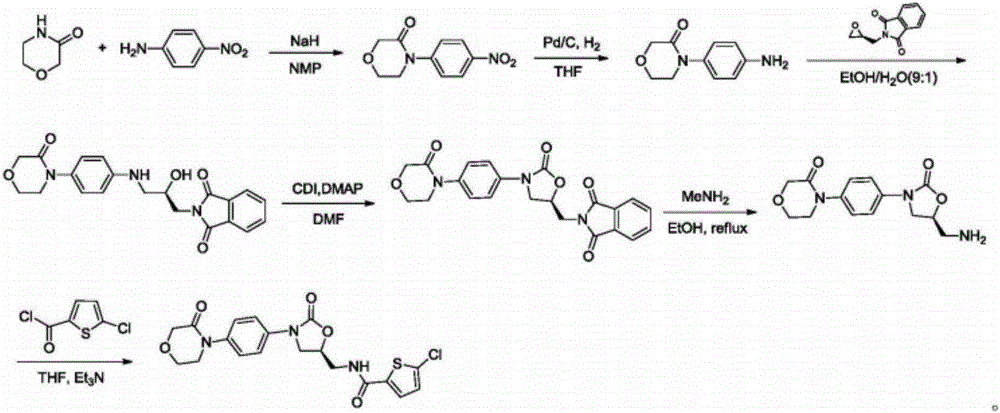
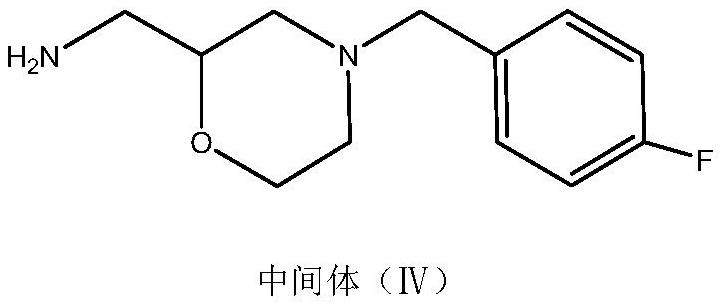
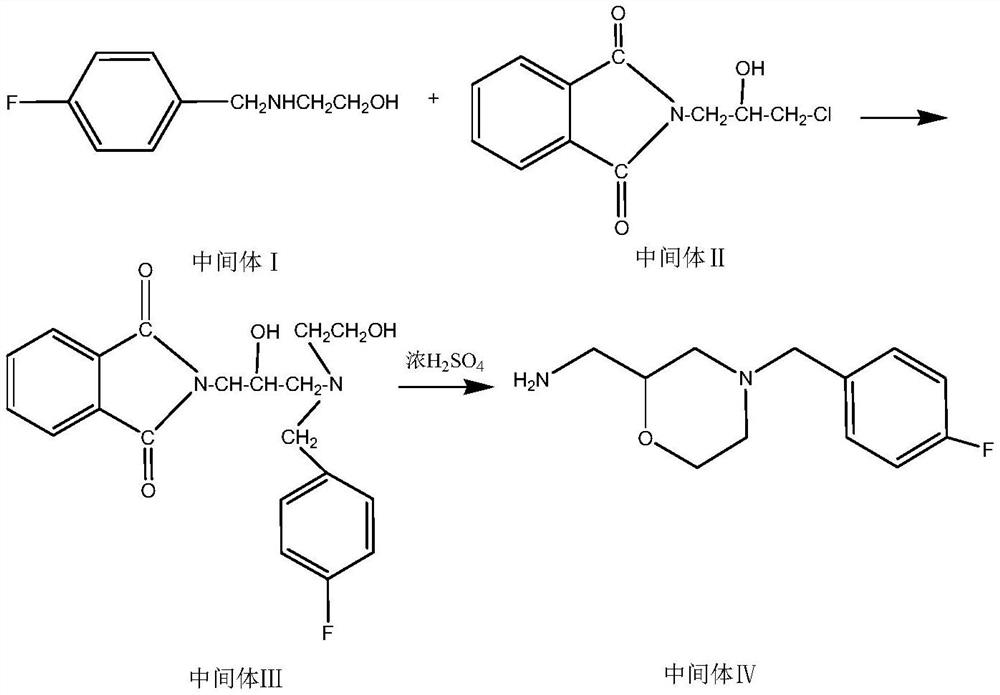
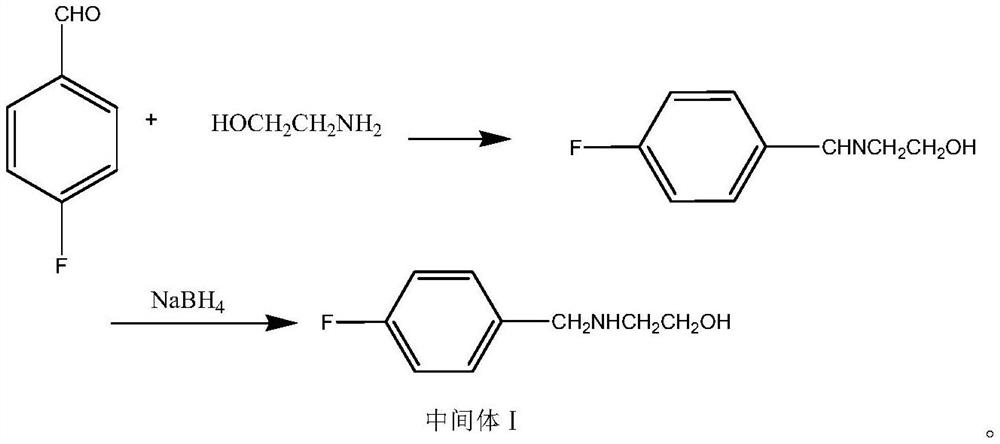
Method for preparing mosapride citrate intermediate
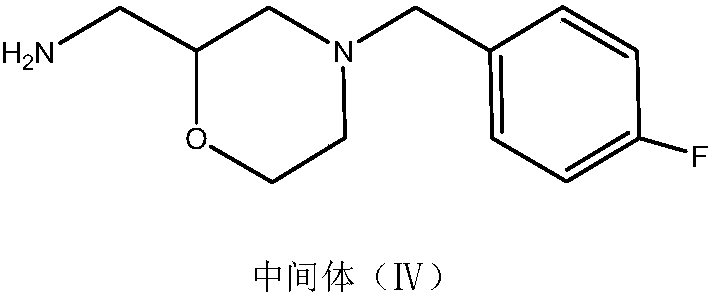
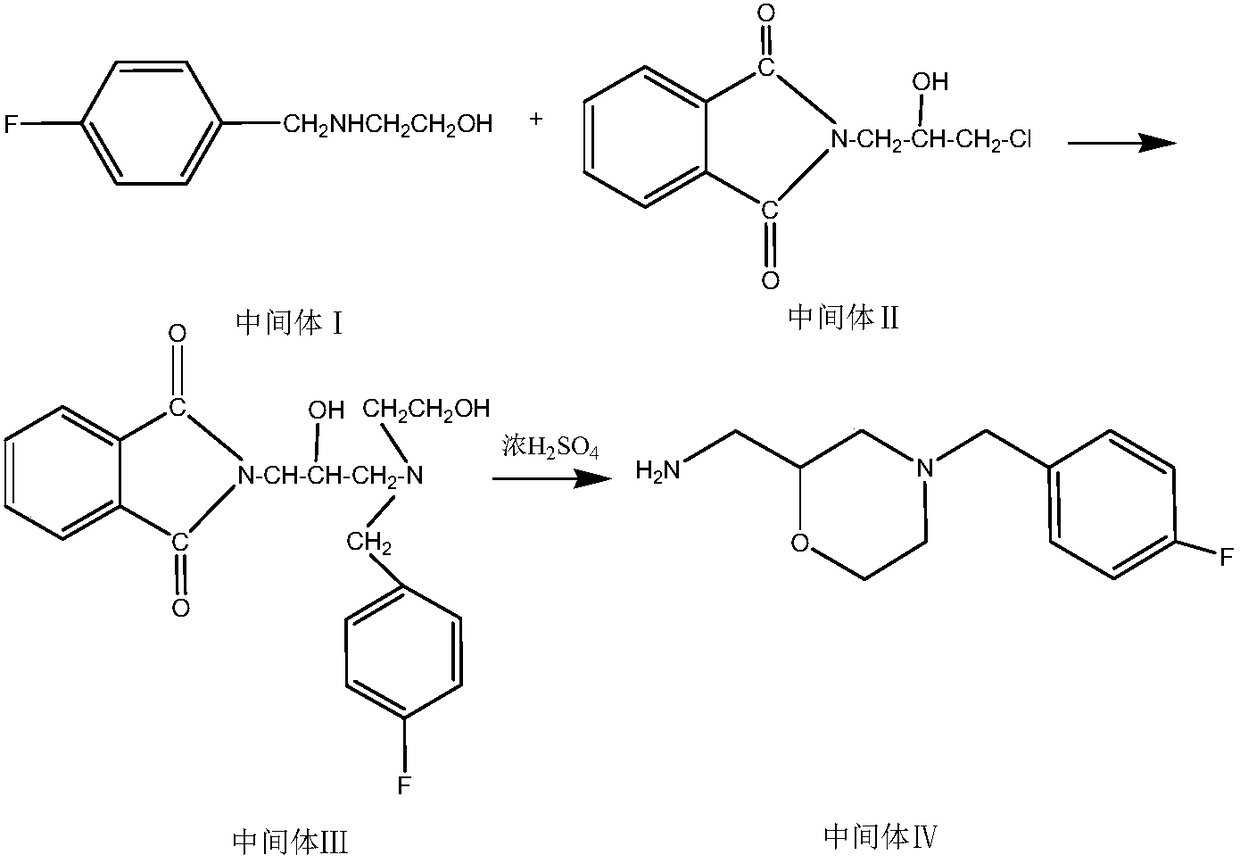
The invention belongs to the technical field of medicines and particularly relates to a method for preparing a mosapride citrate intermediate IV 4-(4-fluorophenyl)-2-aminomethyl morpholine. The methodcomprises the following steps: phthalimide potassium salt and dichloro-2-propanol react to produce an intermediate II N-(2-hydroxy-3-chloropropyl) phthalimide, then the produced intermediate II and an intermediate I 2-(4-fluorphenylamine)ethyl alcohol are condensed to prepare an intermediate III N-3-[4-fluorophenyl-2-(hydroxy-ethylamine)-2-hydroxypropyl]phthalic diamide, and the intermediate IIIis subjected to cyclization and hydrolysis to obtain the intermediate IV 4-(4-fluorophenyl)-2-aminomethyl morpholine. The method is short in route, lower in production cost and suitable for industrialproduction.
Owner:LUNAN PHARMA GROUP CORPORATION
Method for preparing pentazocine intermediate
ActiveCN112358403AWide variety of sourcesLow priceOrganic compound preparationCarboxylic acid esters preparationSulfonyl chlorideChemical synthesis
The invention belongs to the technical field of chemical synthesis, and provides a method for preparing a pentazocine intermediate, which comprises the following steps: by using low-price methyl acetoacetate as a raw material, carrying out methylation, methoxyformylation and removal of one methoxyformyl group, reducing by using a reducing agent, and reacting the reduction product with alkyl sulfonyl chloride to obtain the pentazocine intermediate; generating a compound with a dialkyl sulfonyl group; reacting a compound with dialkyl sulfonyl with phthalimide potassium salt, removing one alkyl sulfonyl, and grafting phthalimide; carrying out dehydrogenation reaction on the phthalimido product under an alkaline condition to generate a vinyl compound, and reacting the vinyl compound with hydrazine hydrate to obtain the pentazocine intermediate. Cheap compounds are used as initial raw materials, the whole route avoids high-pressure and high-temperature dangerous reactions, and industrial production is facilitated.
Owner:HEADING NANJING PHARMTECH CO LTD
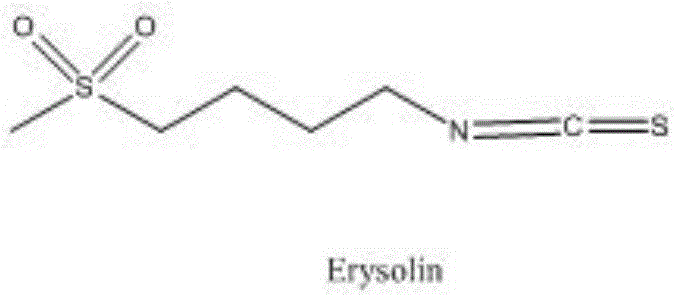
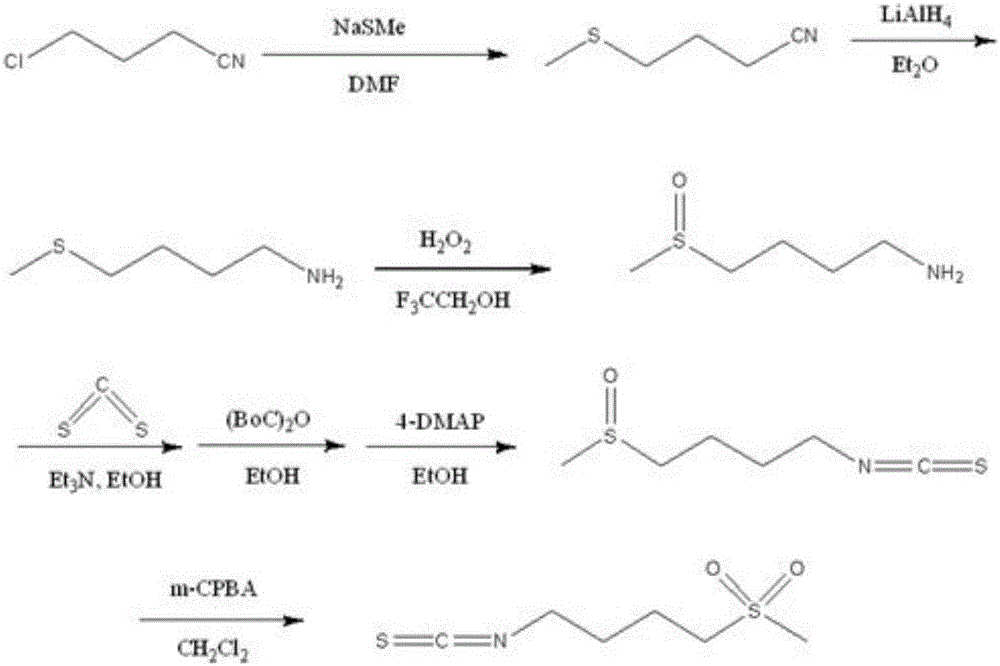
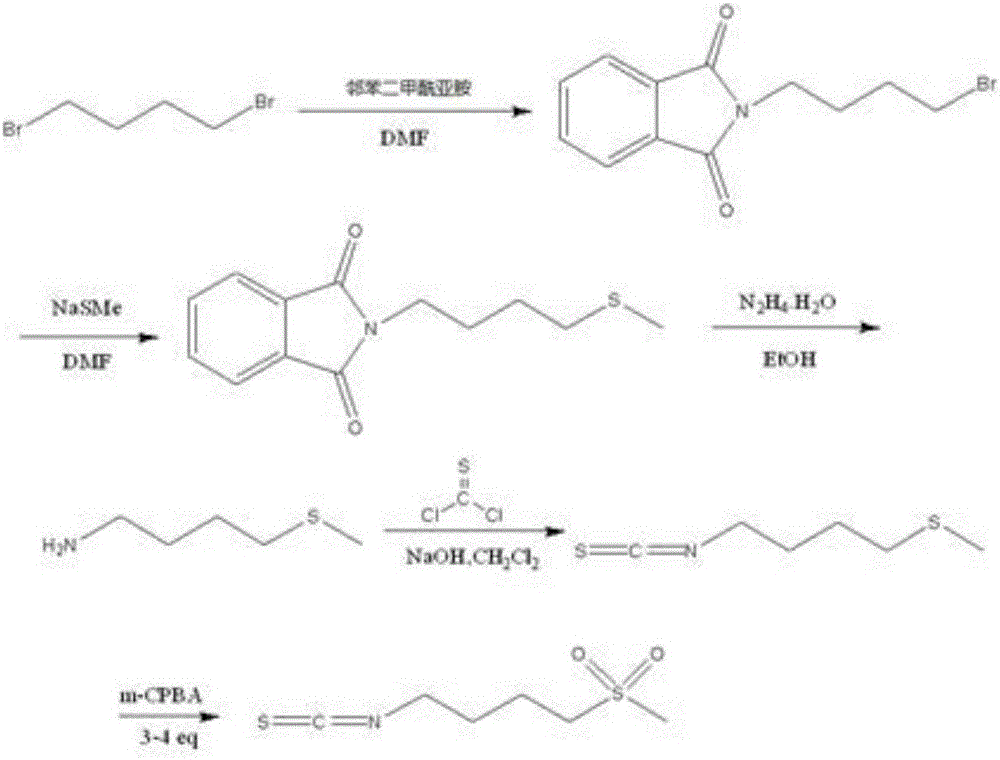
Synthesis method of 4-methanesulfonylbutyl isothiocyanate
ActiveCN106496086AThe synthesis steps are simpleReduce yieldOrganic chemistryChemical synthesisSynthesis methods
The invention relates to a chemical synthesis method of 4-methanesulfonylbutyl isothiocyanate (Erysolin). The method comprises that 1-bromo-4-chlorobutane and hydrous sodium methyl mercaptide as starting raw materials undergo a reaction to produce chlorothioether, the chlorothioether and phthalimide potassium salt undergo a reaction to produce a tertiary amine conjugate, the tertiary amine conjugate undergoes a hydrazinolysis reaction to produce thioether primary amine, the thioether primary amine forms isothiocyanate under action of thiophosgene, and the isothiocyanate is oxidized to form Erysolin. The chemical synthesis method is simple in operation, uses a small amount of dangerous reagents, gives consideration to a cost and a yield and is suitable for Erysolin industrial production.
Owner:SHENYANG PHARMA UNIVERSITY
Preparation process for rivaroxaban
InactiveCN108690010AMild reaction conditionsEasy to separateOrganic chemistry methodsDiketoneMorpholine
The invention provides a preparation process for rivaroxaban, and particularly relates to the technical field of pharmaceutical chemistry. The preparation process comprises the following steps: (S)-4-chloro-3-hydroxylbutyronitrile reacts with phthalimide potassium salt, (S)-4-(1,3-dioxoisoindol-2-yl)-3-hydroxybutyramide is obtained by nitrile group hydrolysis, (S)-2-[[2-oxo-1,3-oxazolidine-5-yl]methyl]-1H-isoindol-1,3(2H)-diketone is obtained by rearrangement under the action of iodobenzene, and carries out Ullmann coupling with 4-(4-bromophenyl)morpholine-3-ketone, so that 2-[[(S)-2-oxo-3-[4-(3-oxo-4-morpholinyl)phenyl]-1,3-oxazolidine-5-yl]methyl]-1H-isoindol-1,3(2H)-diketone is obtained, and by hydrazinolysis and amidation, rivaroxaban is obtained. The preparation process has the advantages of easiness in operation, moderate reaction conditions and higher yield.
Owner:苏州盛达药业有限公司
Quinacridone derivatives containing urea bonds and gels thereof
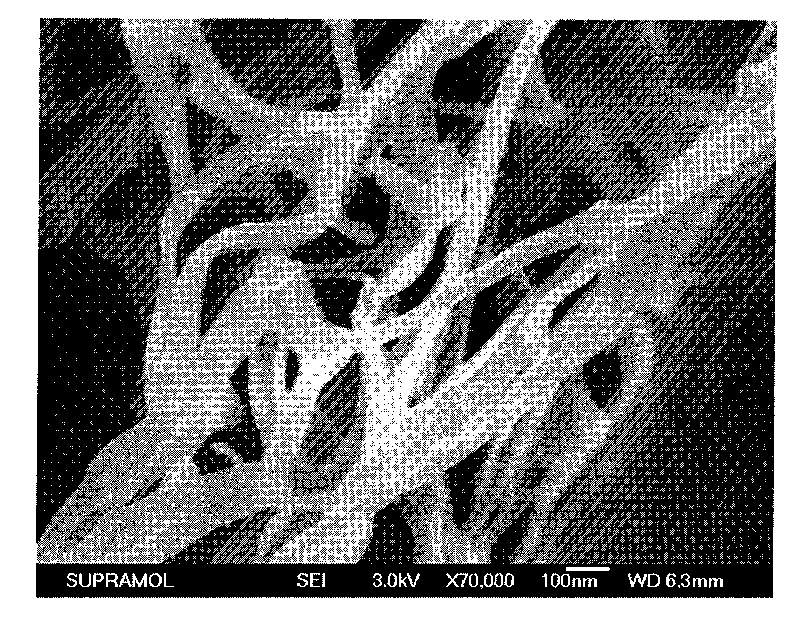
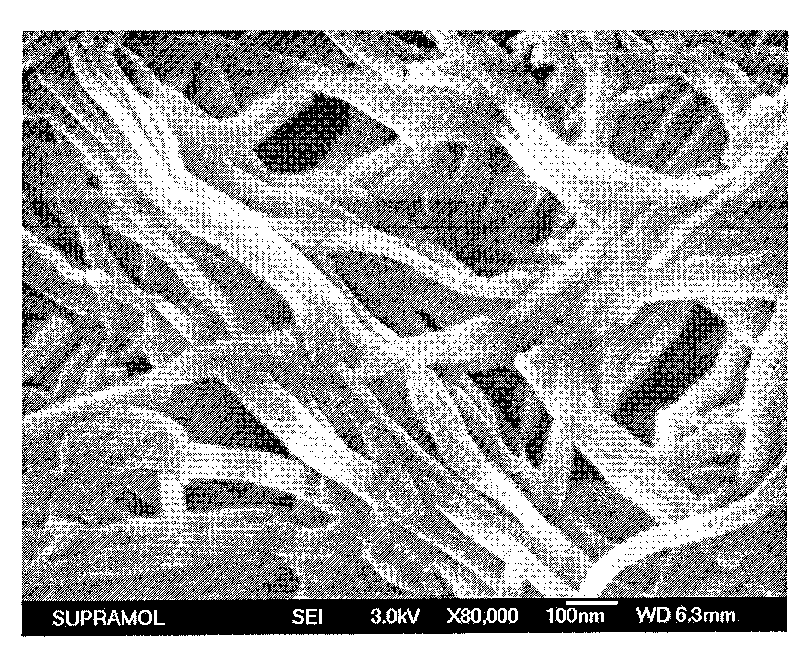
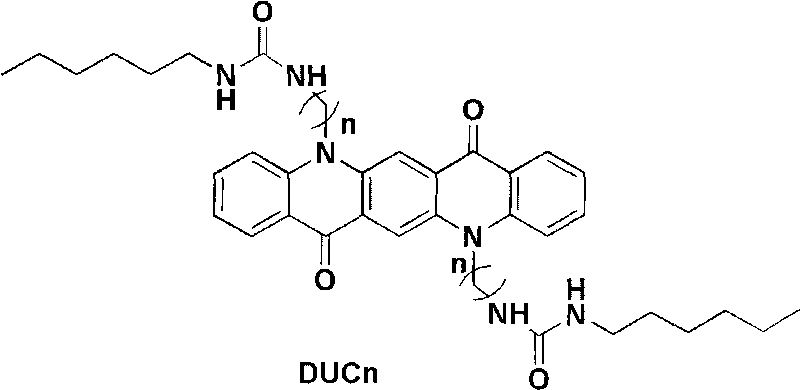
The invention belongs to the technical field of organic photoelectric material and particularly relates to four quinacridone derivative luminescent materials containing urea bond functional groups and gels thereof. The structural formula of the luminescent material is shown as follows, wherein n is 6, 8, 10 or 12, and the luminescent material is obtained through the following steps: DCBn reacts with potassium phthalimidate in DMF to obtain DUPn, a pure product is obtained through simple column chromatography, a protection group is separated in solution with hydrazine hydrate as alkaline environment to form end amino, and the product directly reacts with cyanate. The luminescent material can obtain stable gel in the organic solvent and reflects stronger yellow green fluorescence emission.
Owner:JILIN OPTICAL & ELECTRONICS MATERIALS
Preparation method of silodosin key intermediate
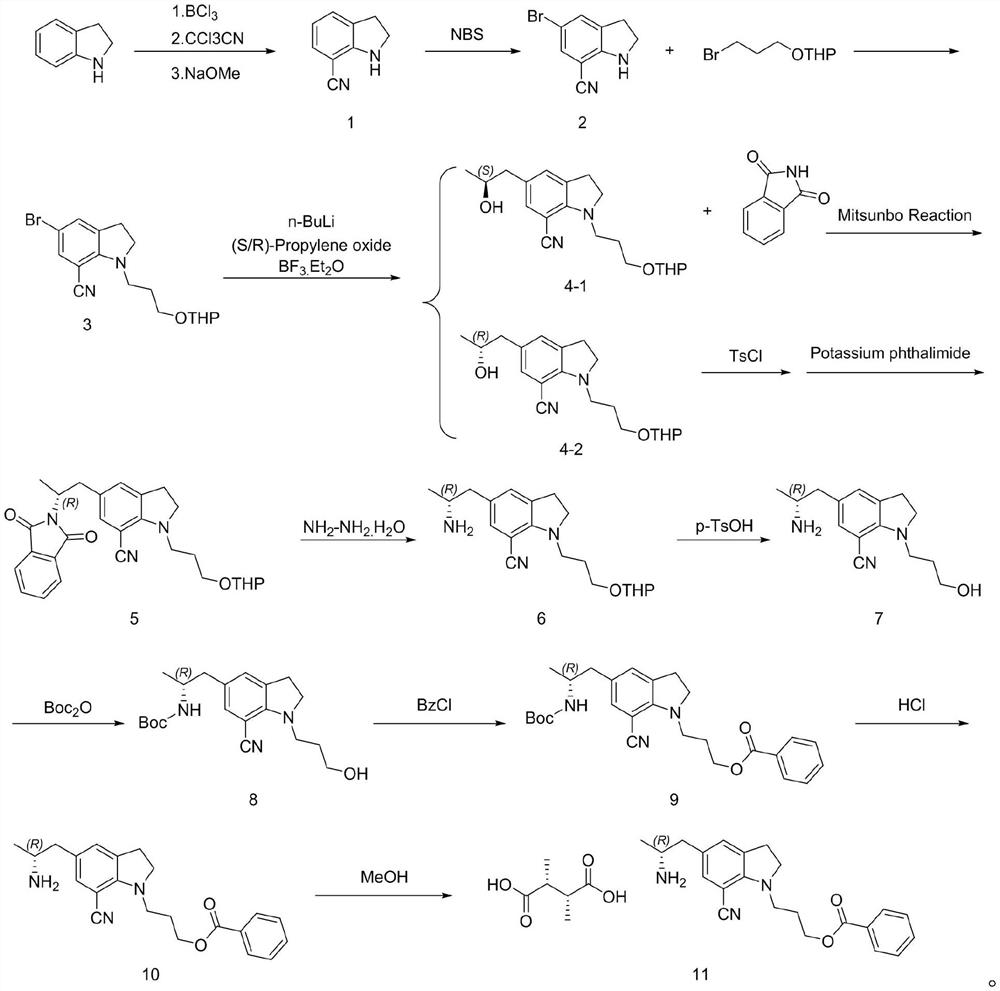
PendingCN114751852ASplit atom utilization is lowReduce utilizationOrganic compound preparationCarboxylic acid salt preparationTrichloroacetonitrilePyran
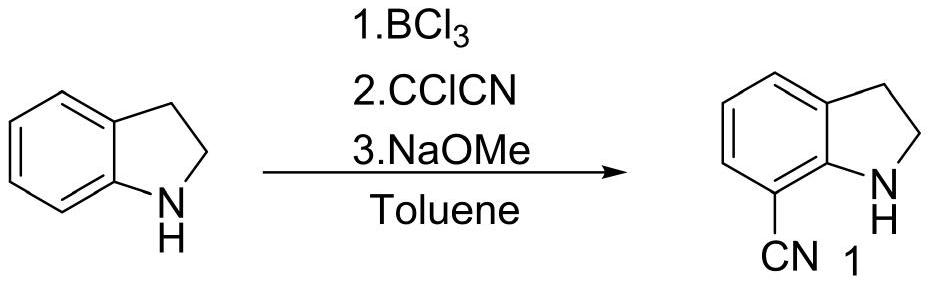
The invention discloses a preparation method of a silodosin key intermediate, and belongs to the technical field of medicine synthesis. Carrying out Friedel-Crafts reaction on indoline and trichloroacetonitrile to obtain a compound 1; brominating to obtain a compound 2; then carrying out substitution with 2-(3-bromopropoxy) tetrahydro-2H-pyran to obtain a compound 3; then carrying out nucleophilic addition with (S)-epoxypropane or (R)-epoxypropane at an ultralow temperature to obtain a compound 4; then carrying out Mitsunobu reaction with phthalimide to obtain a compound 5 (configuration inversion) or esterifying with paratoluensulfonyl chloride, and reacting with potassium phthalimide under the condition of inorganic alkali to obtain the compound 5; reducing through hydrazine hydrate to obtain a compound 6; then removing tetrahydropyran protection from p-toluenesulfonic acid to obtain a compound 7; performing amino Boc protection under an alkaline condition to obtain a compound 8; esterifying with benzoyl chloride to obtain a compound 9; removing Boc protection with hydrochloric acid to obtain a compound 10; and salifying with L-tartaric acid to obtain a compound 11. Compared with the prior art, the preparation method has the advantages that cyano groups synthesized by Vilsmeier reaction, hydroxylamine oximation and acetic anhydride dehydration at the 7 site and introduction of amino groups at the 5 site through nitro groups or reductive amination are avoided, and heavy metals such as Pd / Pt / Zn and the like do not need to be used; the continuous operation of the whole steps is increased, the production cost of the silodosin intermediate is greatly reduced, and industrial large-scale production is facilitated.
Owner:山西库邦生物医药科技有限公司
Copper plating additive, copper plating solution and application thereof
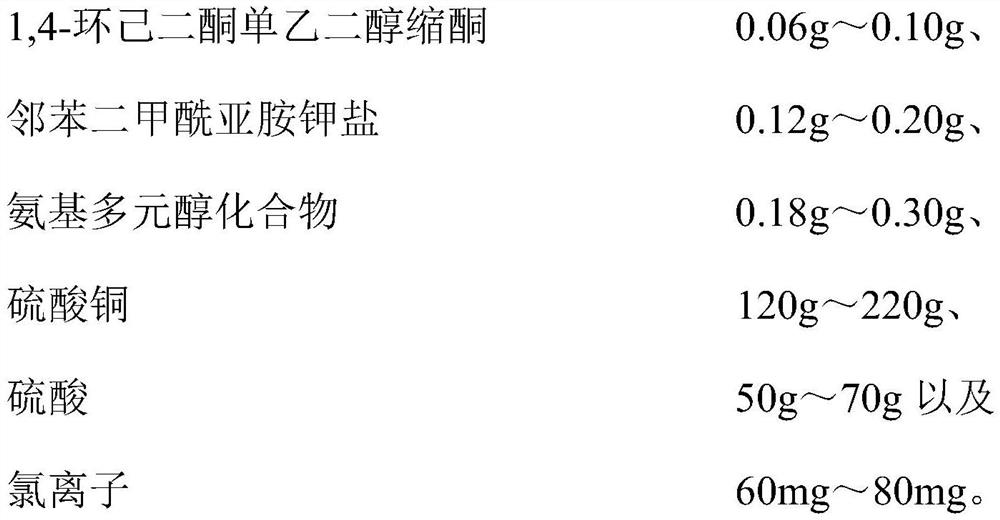
The invention relates to a copper plating additive, a copper plating solution and application thereof. The copper plating additive comprises 1, 4-cyclohexanedione monoethylene ketal, a potassium phthalimide salt and an amino polyol compound. Wherein the 1, 4-cyclohexanedione monoethylene glycol ketal can obviously improve the brightness of a brush plating copper layer, the phthalimide potassium salt can effectively improve the dissolving efficiency of the 1, 4-cyclohexanedione monoethylene glycol ketal, meanwhile, the effects of refining grains, leveling and improving the uniformity and thickness of a plating layer are achieved, and the amino polyalcohol compound can greatly change the color tone of the copper layer; the color is brighter. In addition, the copper plating additive has the advantages of being environmentally friendly, non-toxic, low in cost and the like, and is suitable for large-scale industrial production. When the copper plating additive is added into a copper plating solution, the thickness, the brightness and the plumpness of a plating layer can be greatly improved by adding a very small amount of the copper plating additive, and the stability of the original copper plating solution and the performance of the original copper plating layer are not changed.
Owner:JIANGYIN NANOPORE INNOVATIVE MATERIALS TECH LTD
Preparation method of 4-aminobutanol
ActiveCN108658792ALow costHigh yieldOrganic compound preparationAmino-hyroxy compound preparationEconomic benefitsPotassium phthalimide
The invention provides a preparation method of 4-aminobutanol. The method comprises the steps of taking potassium phthalimide and 4-chlorbutanol as raw materials, and preparing an intermediate in thepresence of a solvent and a phase transfer catalyst; and then hydrolyzing the intermediate under the effect of an alkaline solution to obtain the 4-aminobutanol. The raw materials used in the method are simple, the cost is low, the reaction conditions are gentle, potential safety hazards do not exist, and additional reactions hardly exist in a reaction process of the various used raw materials; and in a preparation process, substances which are difficult to separate are not generated, and aftertreatment is simple and is easy to operate; and therefore, the yield of the 4-aminobutanol is greatlyimproved, the utilization rate of the raw materials is increased, and the production cost is reduced. Under the condition of low cost, the yield of products is increased obviously, and the economic benefit is remarkable.
Owner:HENAN CHEM IND RES INST
Synthetic method of antimalarial drug primaquine phosphate intermediate n-(4-bromopentyl)phthalimide
ActiveCN106008314BIncrease contentHigh selectivityOrganic chemistrySynthesis methodsHydrogen bromide
The invention provides a synthesis method of an antimalarial drug primaquine phosphate intermediate N-(4-bromopentyl)phthalimide. N-(4-bromopentyl)phthalimide is an important intermediate of an antimalarial drug primaquine phosphate. The method comprises the following steps: carrying out a condensation reaction on 5-chloro-1-pentene and phthalimide potassium salt, and carrying out an addition reaction on a condensation product 5-(o-phthalimido)-1-pentene and hydrogen bromide to obtain the final product. The method has the advantages of low cost of raw materials, high conversion rate, high purity of the final product, mild conditions of every reaction, and simple and easily controlled reaction process.
Owner:仪征市海帆化工有限公司
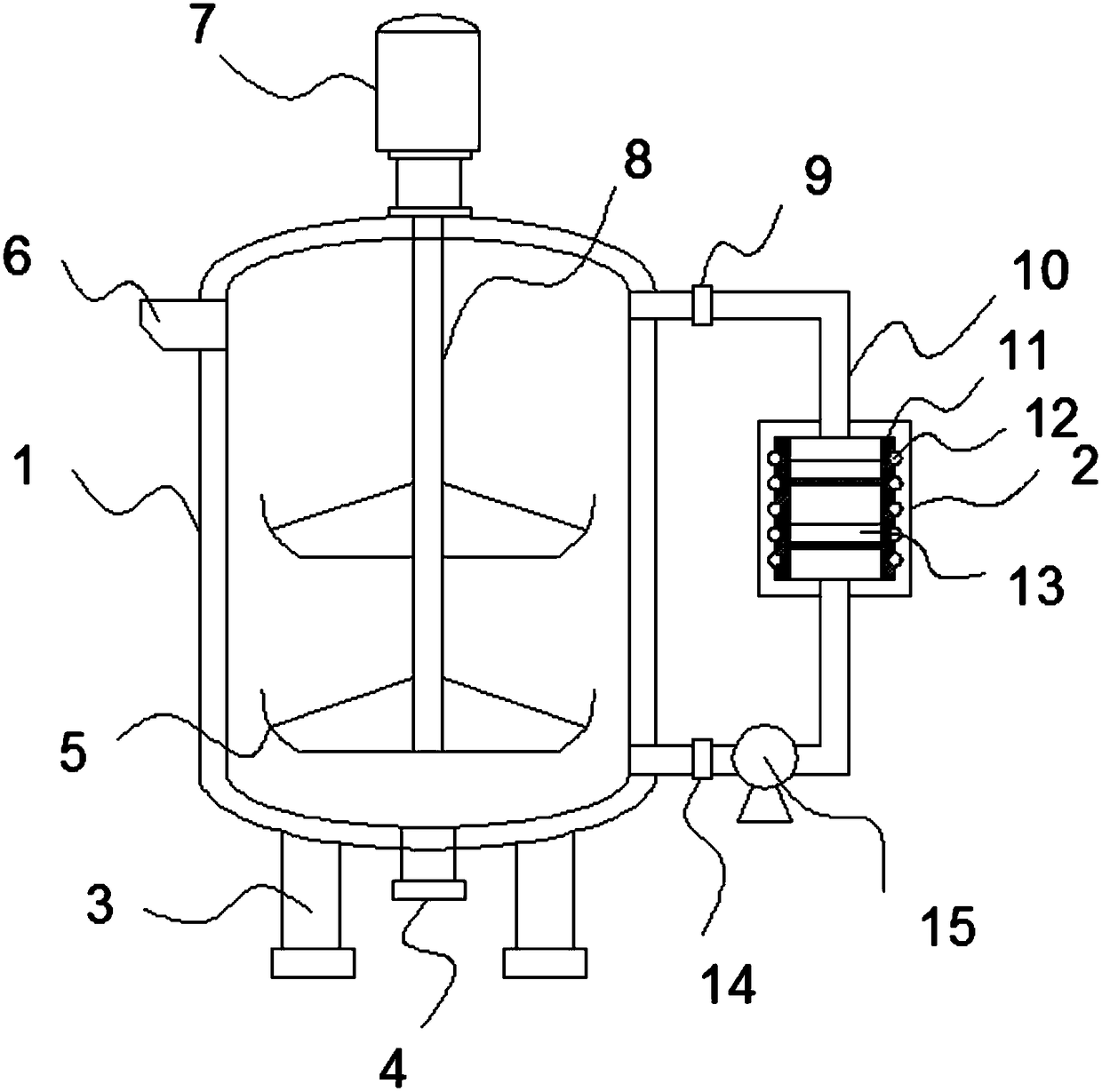
Device reducing phthalimide potassium salt to prepare amine
PendingCN108689872AIncrease reaction rateImprove conversion rateOrganic compound preparationCarboxylic acid amides preparationReaction ratePotassium
The invention relates to a device reducing phthalimide potassium salt to prepare amine. The device comprises a device body and a recycling tank and is characterized in that support legs are arranged on two sides of the bottom of the device body, the bottom surface of the device body is provided with a discharge outlet, and a motor is fixedly connected to the upper end of the device body. The device has the advantages that the recycling tank is arranged on the outer side of the device body, the upper end and the lower end of the recycling tank are communicated with the device body through slurry conveying pipes, two rows of membrane filters are arranged inside the recycling tank, and accordingly solid potassium phthalate can be recycled, reaction rate can be increased, and conversion rate can be increased; the heat conduction layers are arranged on two sides of the recycling tank, electric heating rods are embedded into the outer surfaces of the heat conduction layers, and accordingly asolution inside the device body can be heated.
Owner:NANJING CARBONDE TECH
A kind of preparation method of mosapride citrate intermediate
The invention belongs to the technical field of medicines and particularly relates to a method for preparing a mosapride citrate intermediate IV 4-(4-fluorophenyl)-2-aminomethyl morpholine. The methodcomprises the following steps: phthalimide potassium salt and dichloro-2-propanol react to produce an intermediate II N-(2-hydroxy-3-chloropropyl) phthalimide, then the produced intermediate II and an intermediate I 2-(4-fluorphenylamine)ethyl alcohol are condensed to prepare an intermediate III N-3-[4-fluorophenyl-2-(hydroxy-ethylamine)-2-hydroxypropyl]phthalic diamide, and the intermediate IIIis subjected to cyclization and hydrolysis to obtain the intermediate IV 4-(4-fluorophenyl)-2-aminomethyl morpholine. The method is short in route, lower in production cost and suitable for industrialproduction.
Owner:LUNAN PHARMA GROUP CORPORATION
Preparation method of ftibamzone
The invention discloses a preparation method of ftibamzone. The preparation method comprises the following steps: firstly, preparing 3-phthalimide butanone from phthalimide potassium salt and 3-chlorobutanone; reacting the 3-phthalimide butanone with dry hydrogen bromide gas in a dimethyl sulfoxide solution to prepare a dimethyl sulfoxide solution of 3-phthalimide-2-oxy-n-butyraldehyde; dropwise adding a thiosemicarbazide solution into the dimethyl sulfoxide solution of the 3-phthalimide-2-oxy-n-butyraldehyde, controlling the reaction temperature, carrying out heat preservation reaction to prepare a crude product, and recrystallizing the crude product to obtain the ftibamzone. The technical scheme has the advantages of few reaction steps, high yield and high purity.
Owner:山东嘉成医药科技有限公司
Preparation method of 7-methoxy-1-naphthylethylamine
InactiveCN102603545BLow costReduce usageOrganic compound preparationAmino-hyroxy compound preparationAcetic acidSodium borohydride
The invention belongs to the technical field of pharmaceutical intermediates, and in particular relates to a new preparation method of a key intermediate, 7-methoxy-1-naphthylethylamine of a pharmaceutical ingredient, agomelatine. The aim of the invention is to reduce the cost, optimize the process and bring convenience to the industrial production. The preparation method comprises the following steps: taking 7-methoxy-1-naphthylacetate as a raw material; reducing by sodium borohydride to obtain 7-methoxy-1-naphthaleneethanol; carrying out a methyl sulfonylation reaction to obtain 7-methoxy-1-naphthylethylmethanesulfonate; reacting 7-methoxy-1-naphthylethylmethanesulfonate with a phthalimide potassium salt to obtain 7-methoxy-1-(N-phthalimidoethyl)naphthyl; and finally hydrolyzing 7-methoxy-1-(N-phthalimidoethyl)naphthyl to obtain the product, 7-methoxy-1-naphthylethylamine. The preparation method has the advantages of being simple and convenient in operation, reasonable in reaction process, low in production cost, good in product quality, high in purity (above 98%), having no environment pollution and being suitable for industrial production.
Owner:湖北万知化工医药股份有限公司
A kind of preparation method of 4-aminobutanol
ActiveCN108658792BLow costHigh yieldOrganic compound preparationAmino-hyroxy compound preparationImidePtru catalyst
The invention provides a method for preparing 4-aminobutanol, which uses phthalimide potassium salt and 4-chlorobutanol as raw materials, and prepares an intermediate in the presence of a solvent and a phase transfer catalyst , and then the intermediate is hydrolyzed under the action of an alkaline solution to obtain 4-aminobutanol. The raw materials used in the method are simple, the cost is low, the reaction conditions are mild, and there is no potential safety hazard. There is almost no additional reaction in the reaction process of various raw materials used; no difficult-to-separate substances are generated during the preparation process, and the post-treatment is simple and easy to operate; Therefore greatly improved the yield of 4-aminobutanol, improved the utilization rate of raw material, reduced production cost. That is, the yield of the product is obviously improved under the condition of low cost, and has remarkable economic benefits.
Owner:HENAN CHEM IND RES INST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com