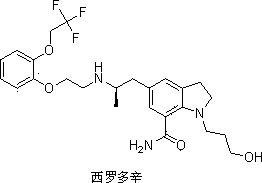
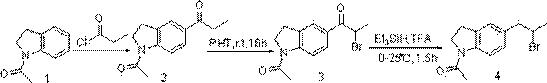Preparation method of important intermediate of Silodosin
A technology of sirodosin and intermediates, which is applied in the field of preparation of important intermediates of sirodosin and achieves the effects of high reaction yield, easy product and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
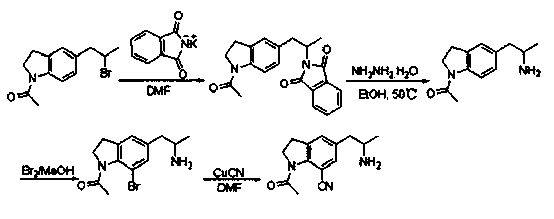
[0031] A preparation method of sirodosin important intermediate, comprising the following steps:
[0032] Step 1) In a flask, add 282g raw material 1-acetyl-5-(2-bromopropyl)indoline, 222g phthalimide potassium salt and 700mL DMF, heat at 110°C for 2h; After the completion, add an appropriate amount of water to it, wash off the excess solvent DMF and salt, and then extract and desolventize with EA to obtain 296g of crude product;
[0033] Step 2) In the flask, add 296g of the crude product obtained in step 1, dissolve it with 800mL of ethanol, add 165mL of hydrazine hydrate, and heat at 50°C until a white solid precipitates; Pressure precipitation; after dissolving with EA, wash off excess hydrazine with water, and finally remove the solvent from the organic phase to obtain 165 g of intermediate, namely 1-acetyl-5-(2-aminopropyl) indoline;
[0034] Step 3) In a three-necked flask, add 165g of the intermediate 1-acetyl-5-(2-aminopropyl)indoline, dissolve it in 600mL of met...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com