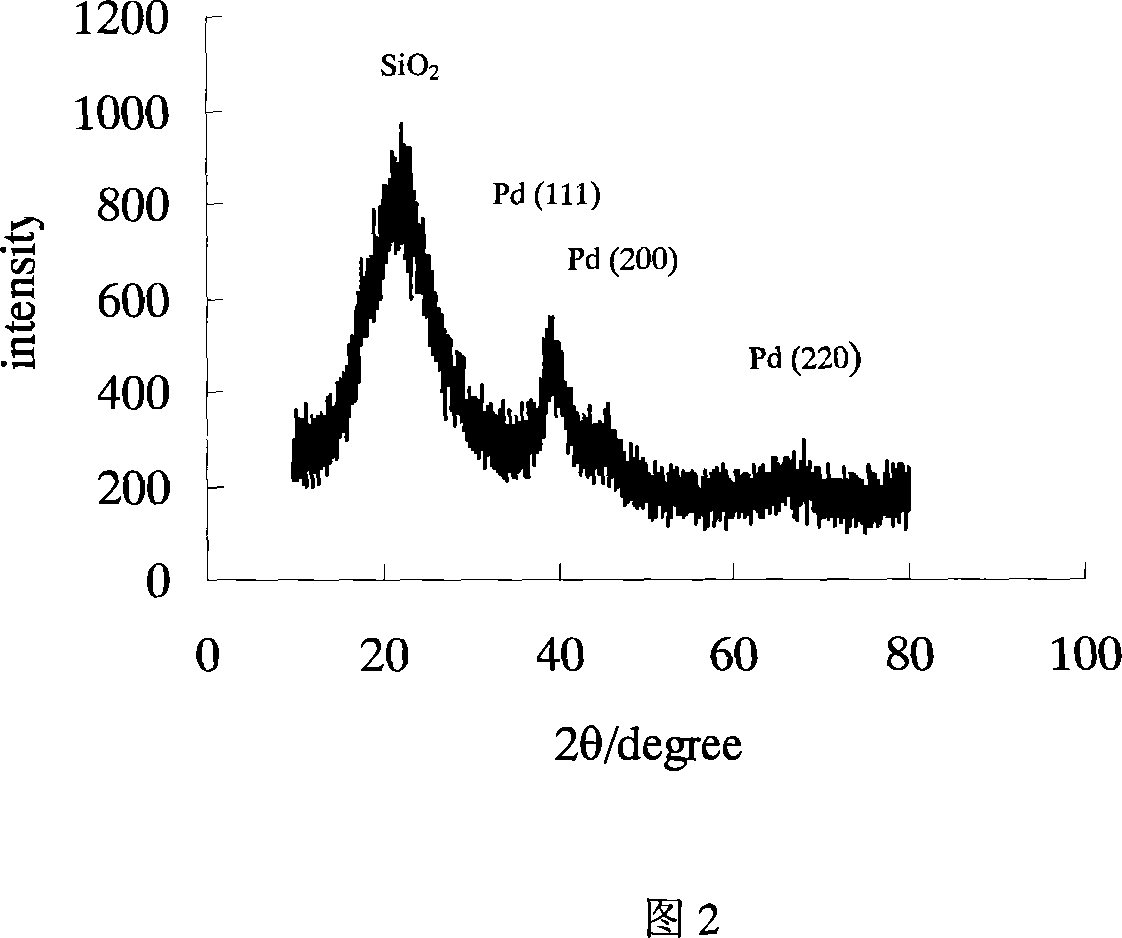
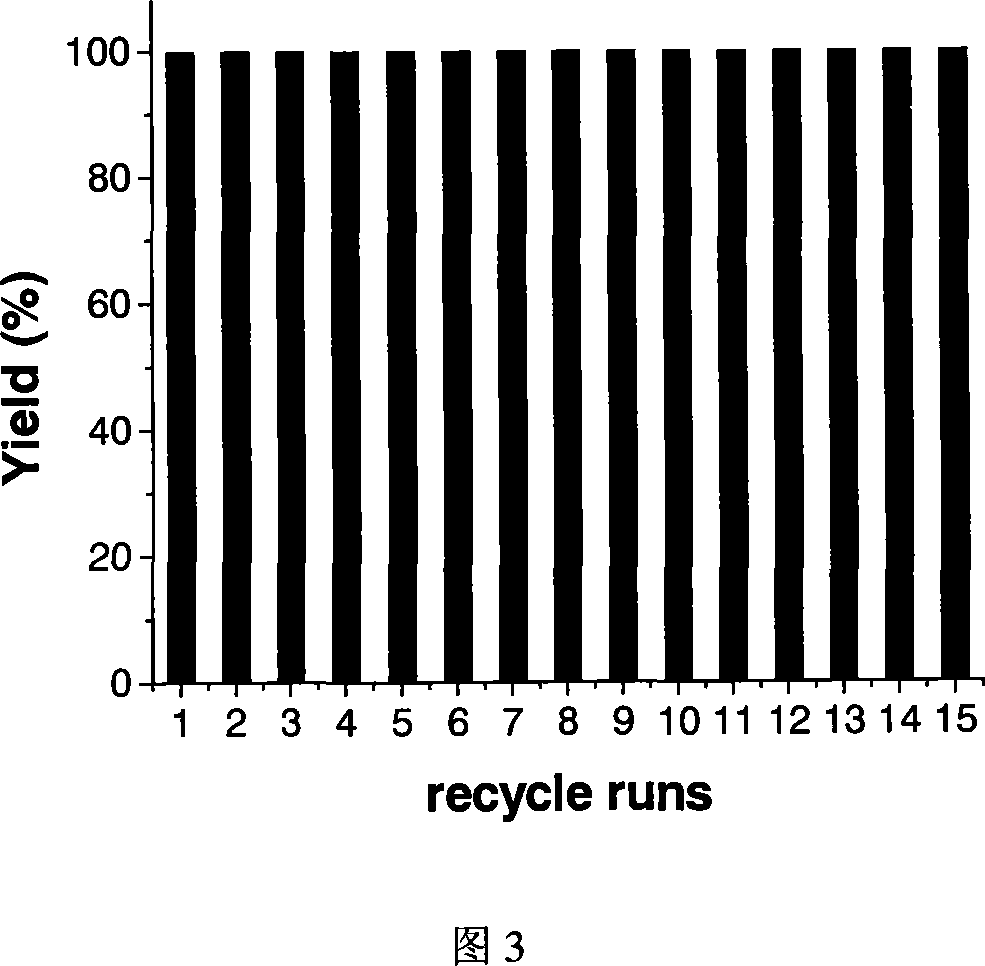
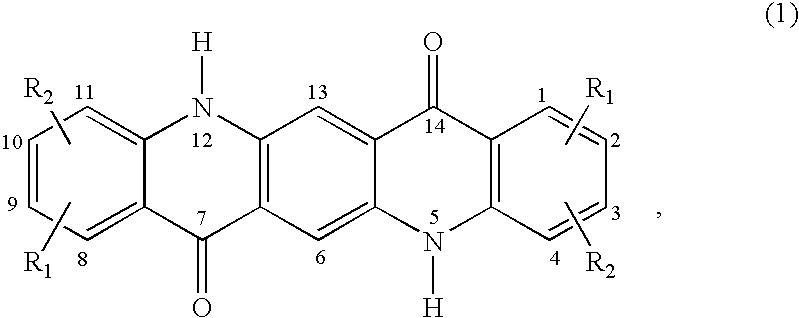
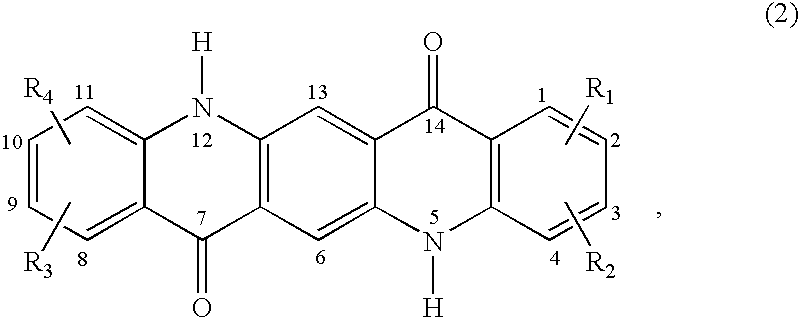
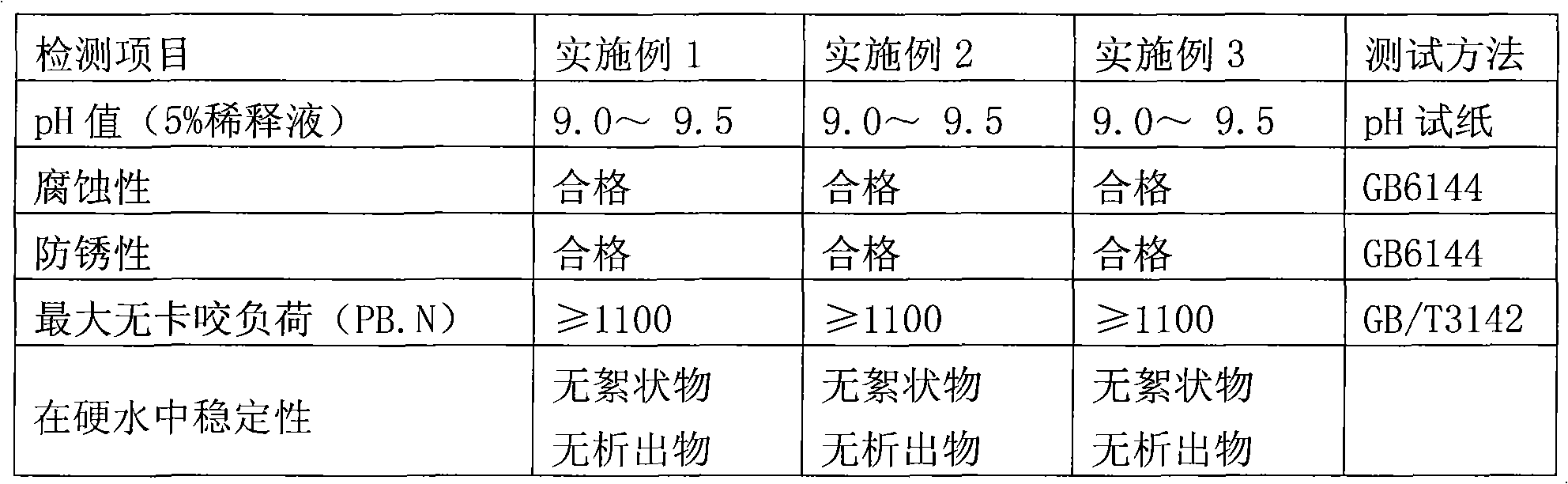
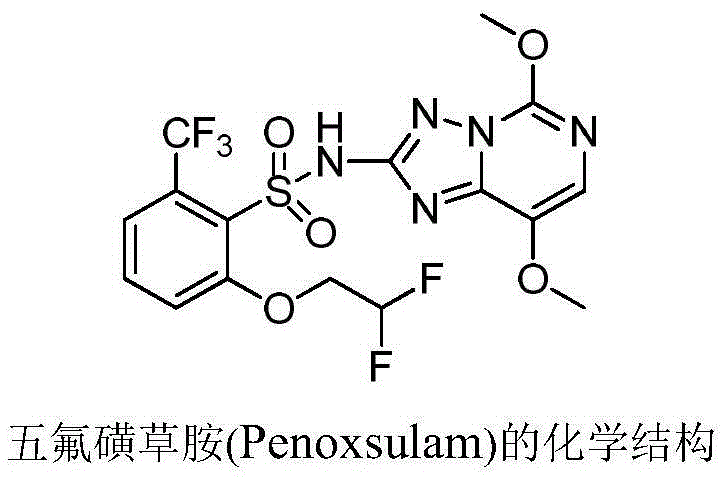
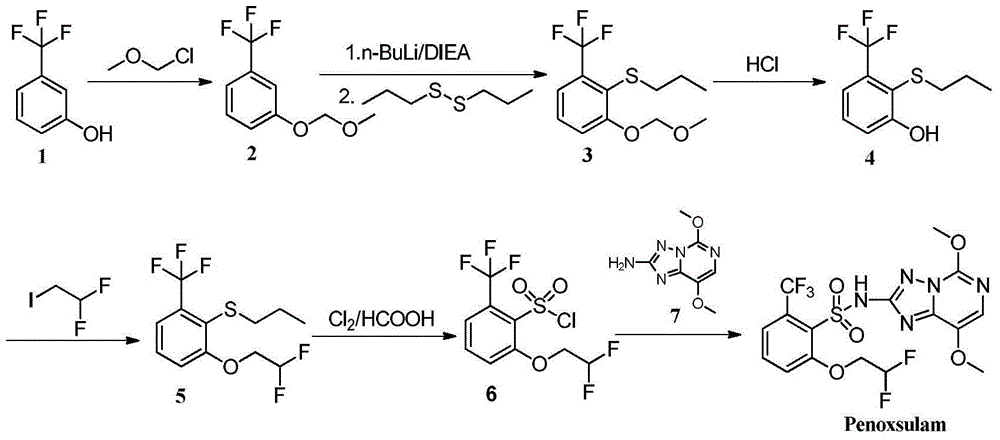
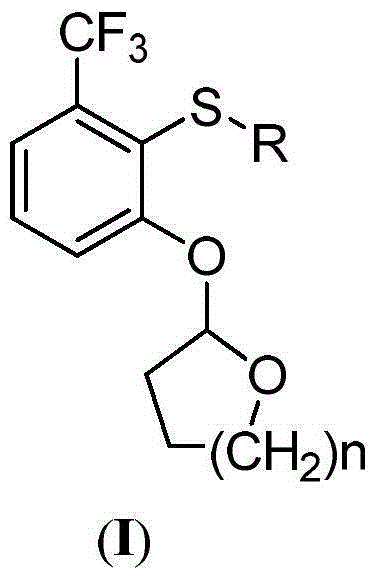
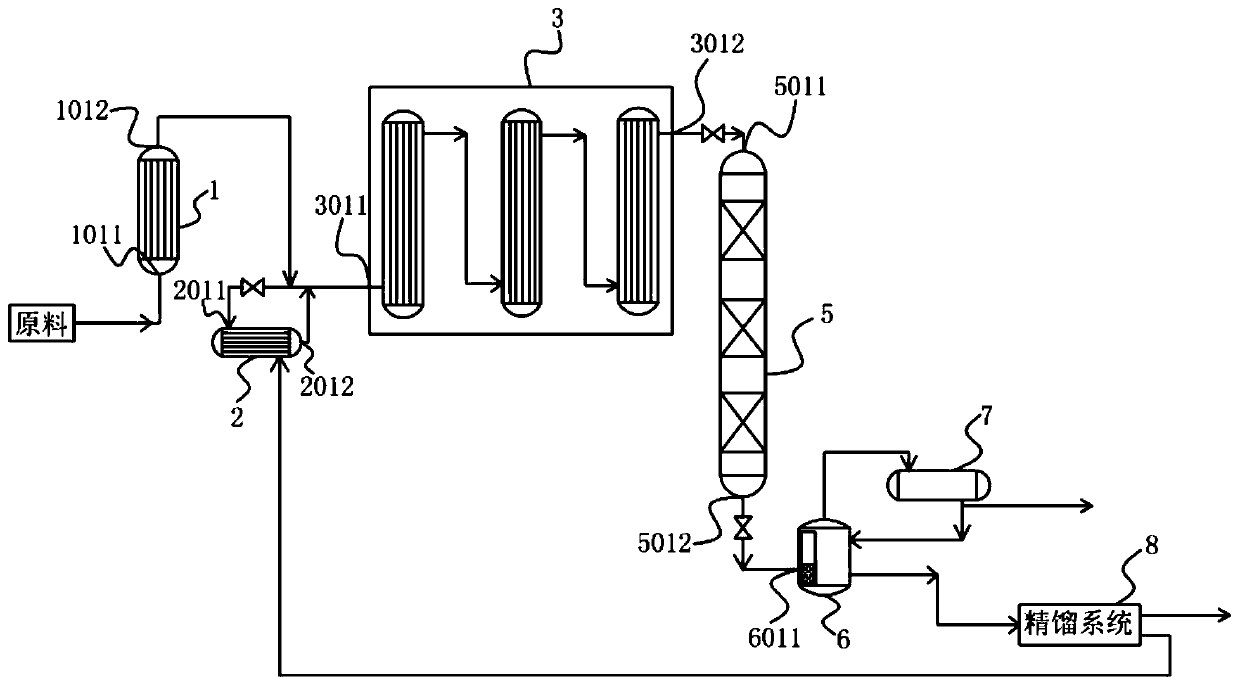
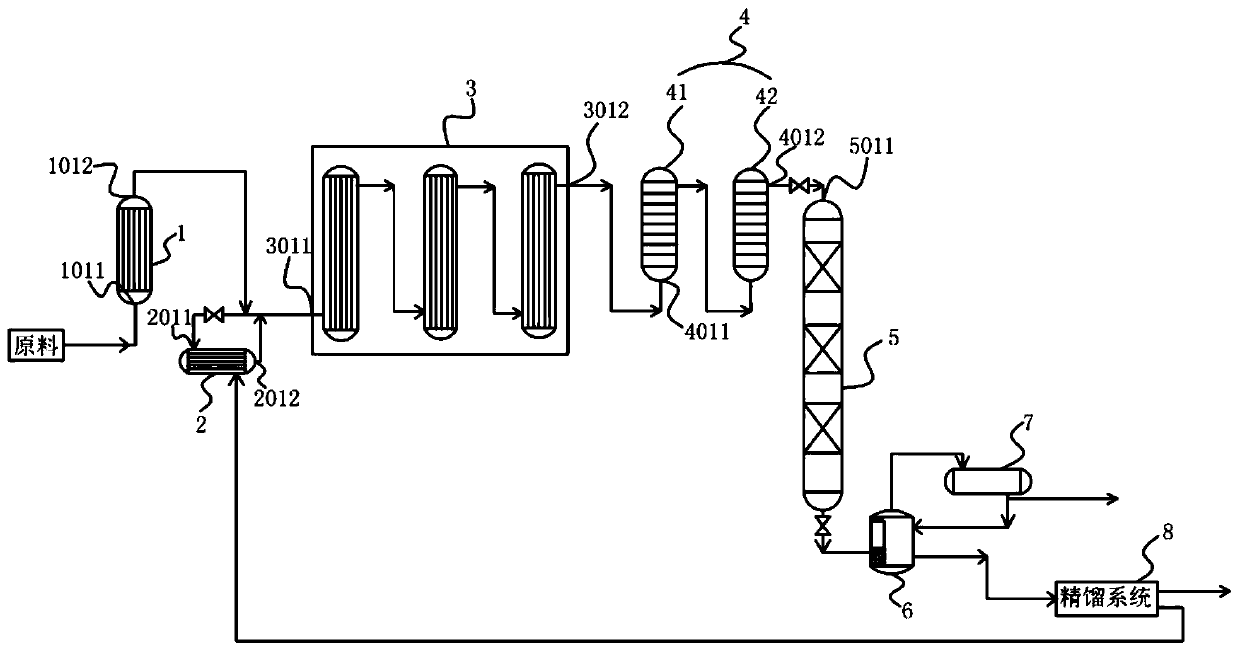
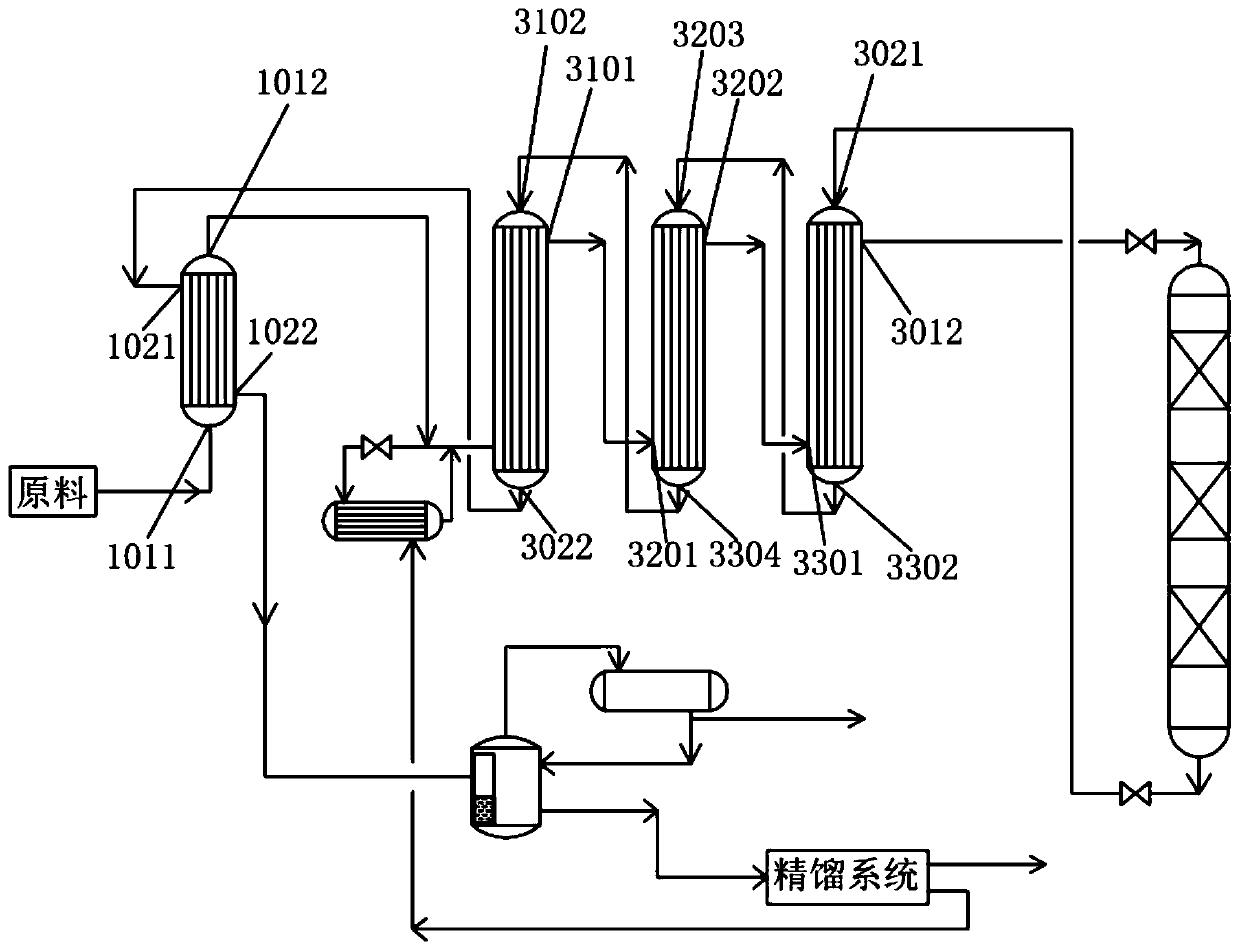
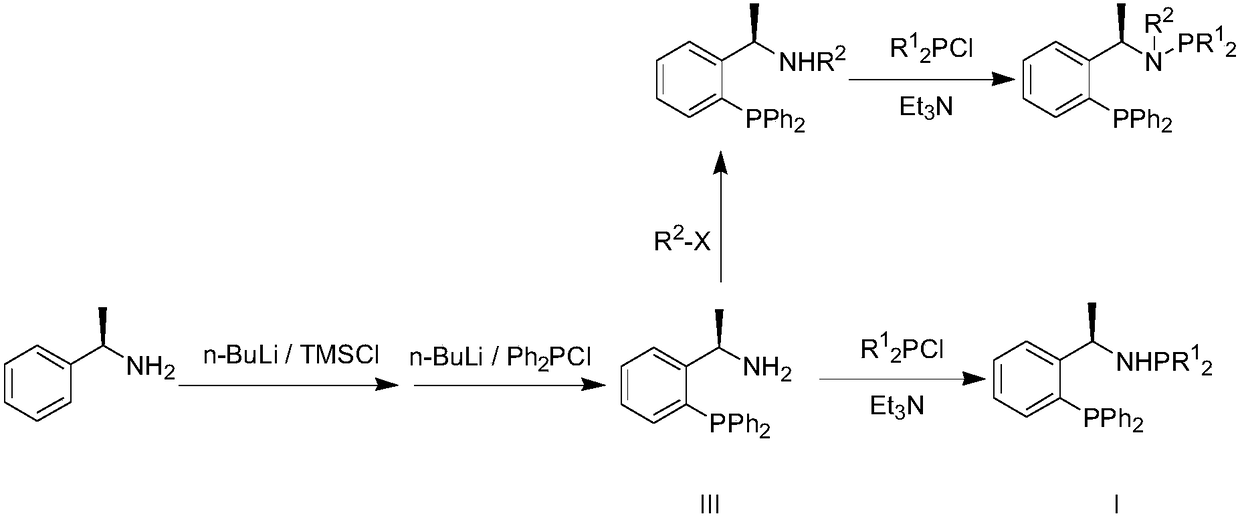
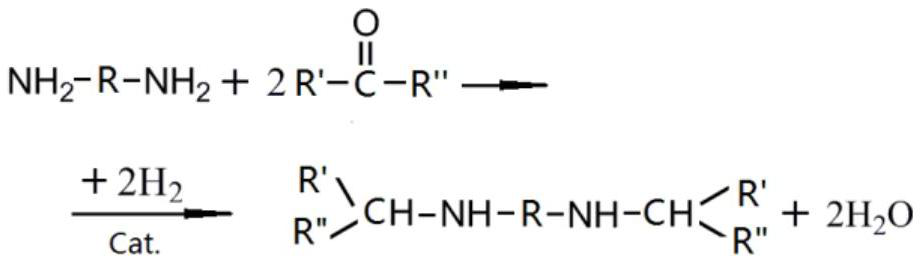
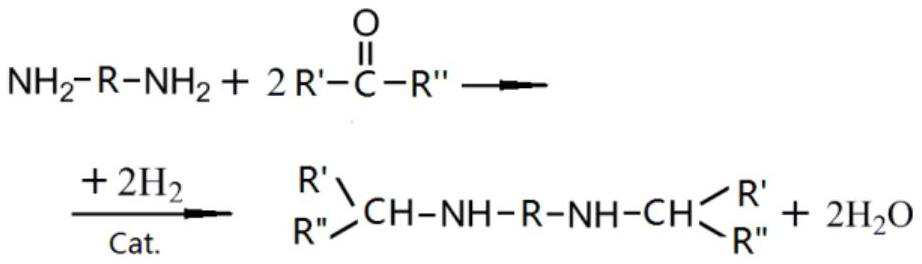
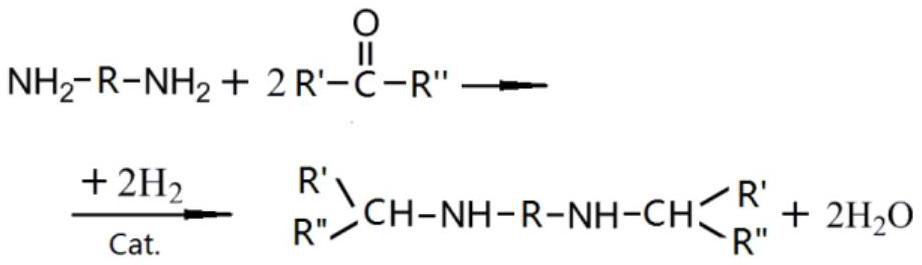
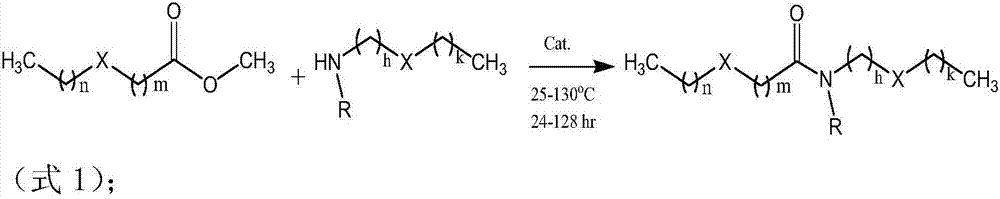
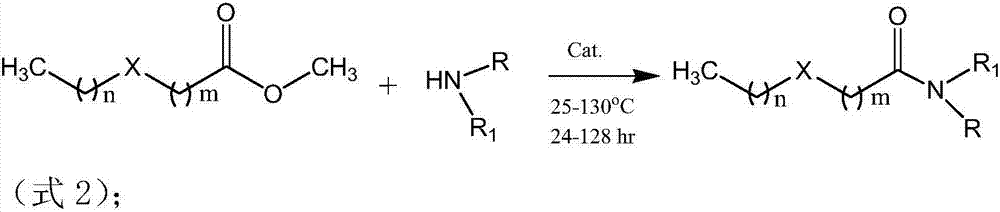
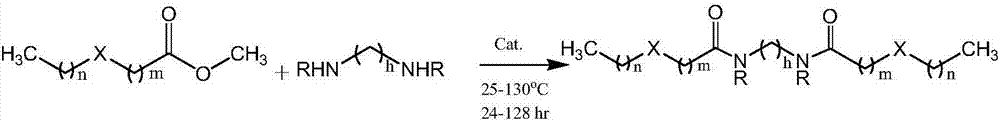
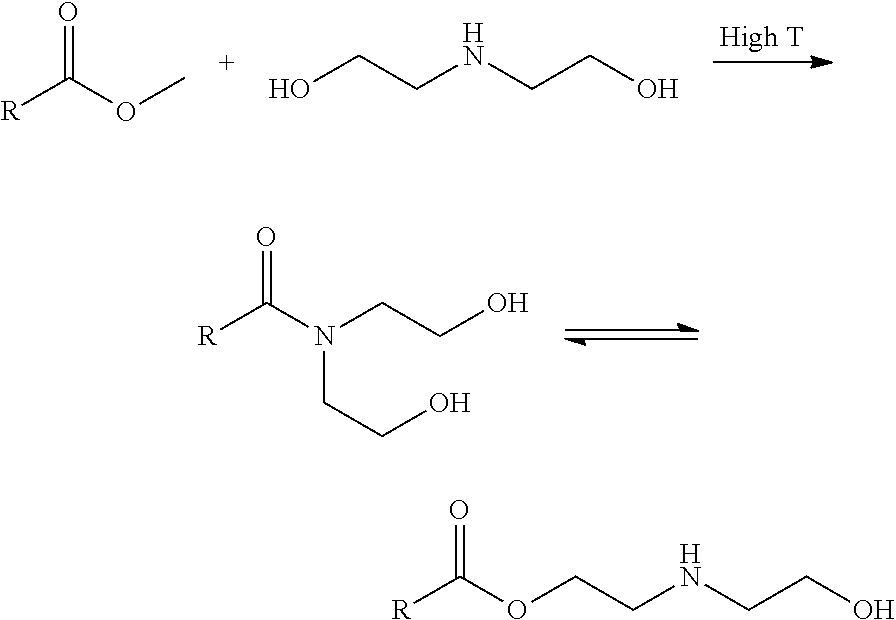
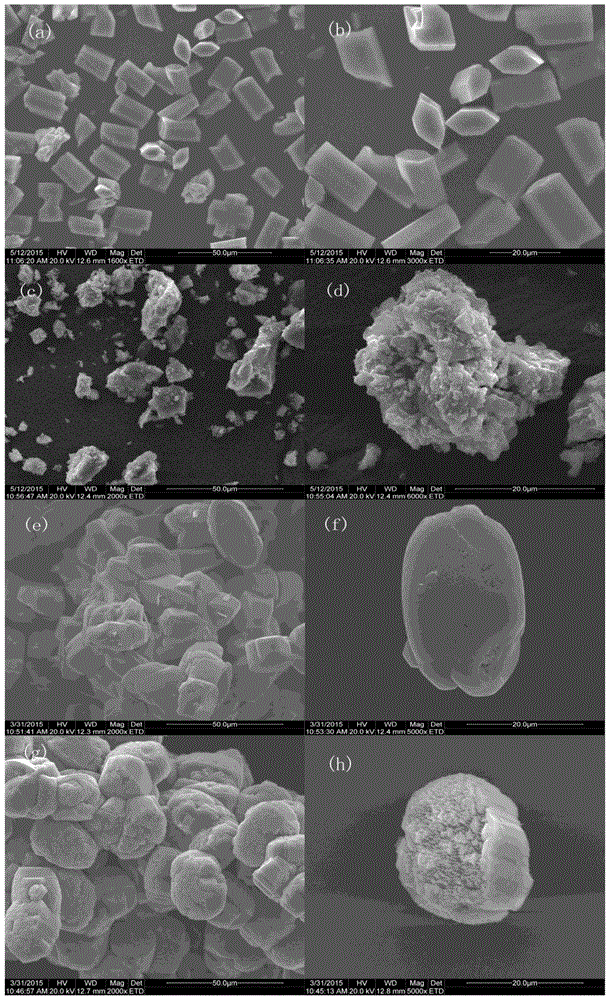
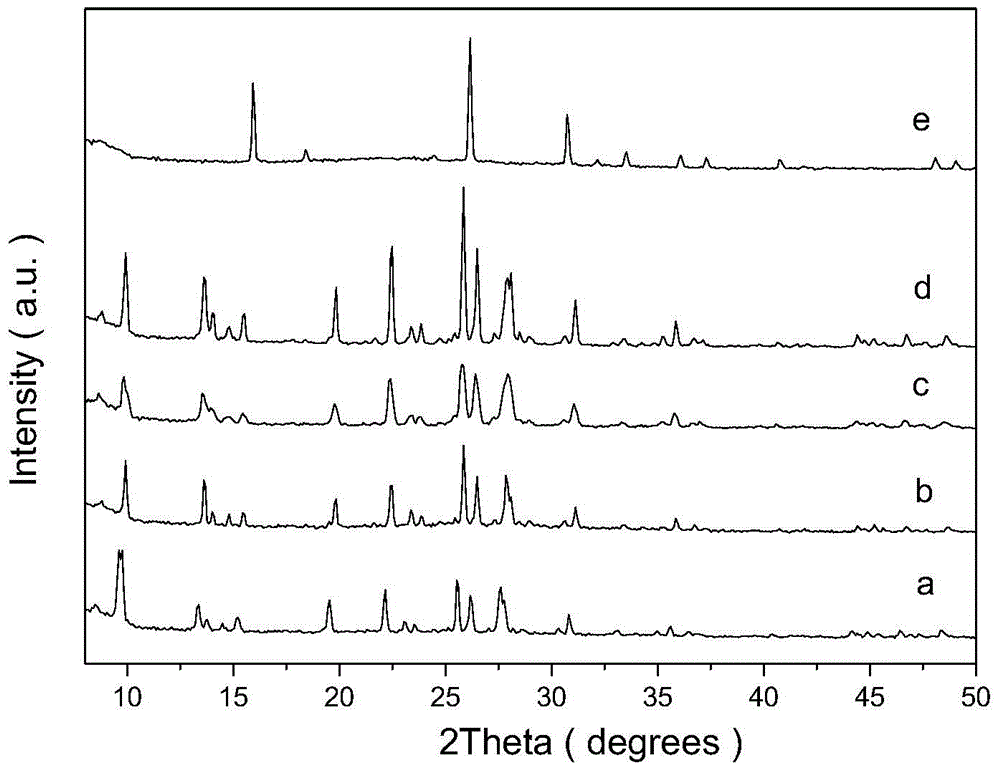
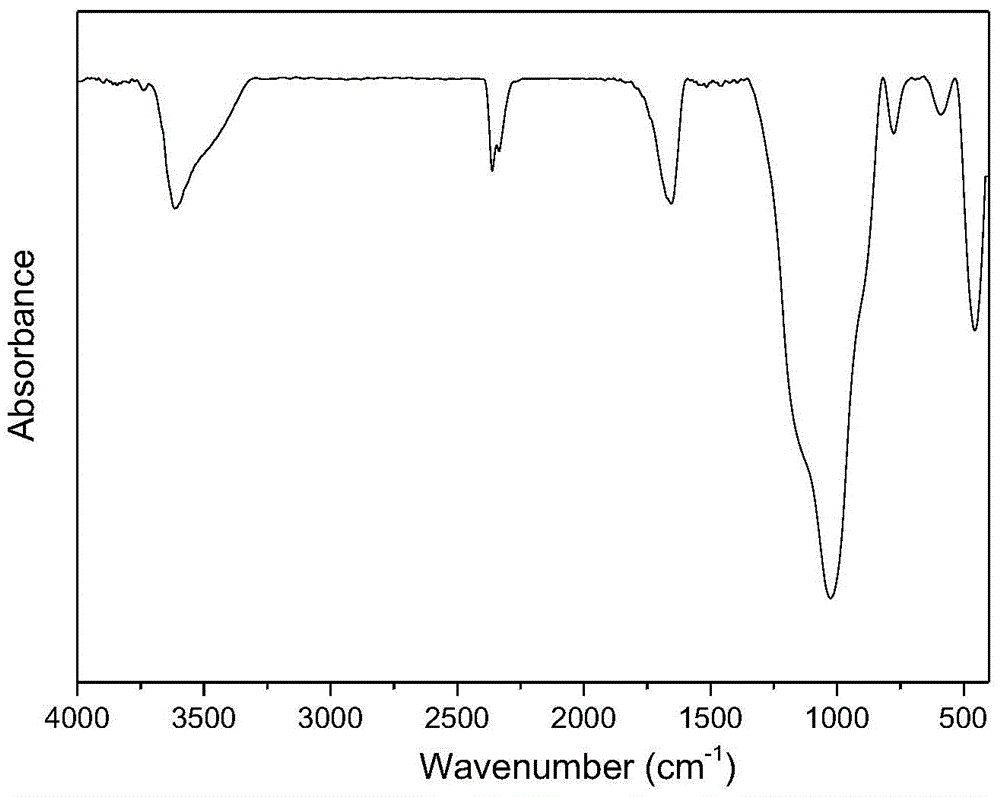
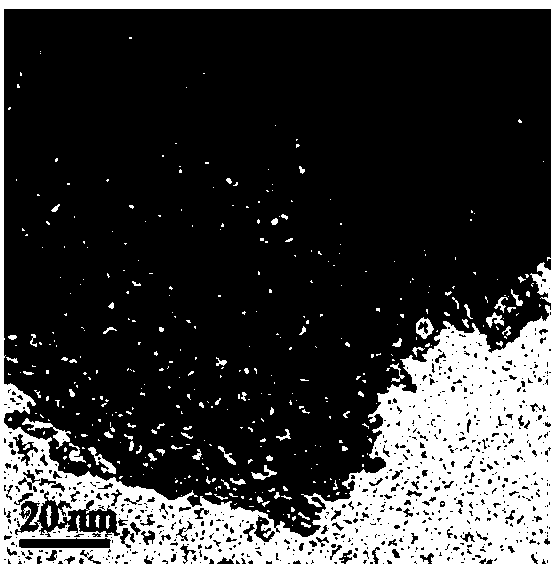
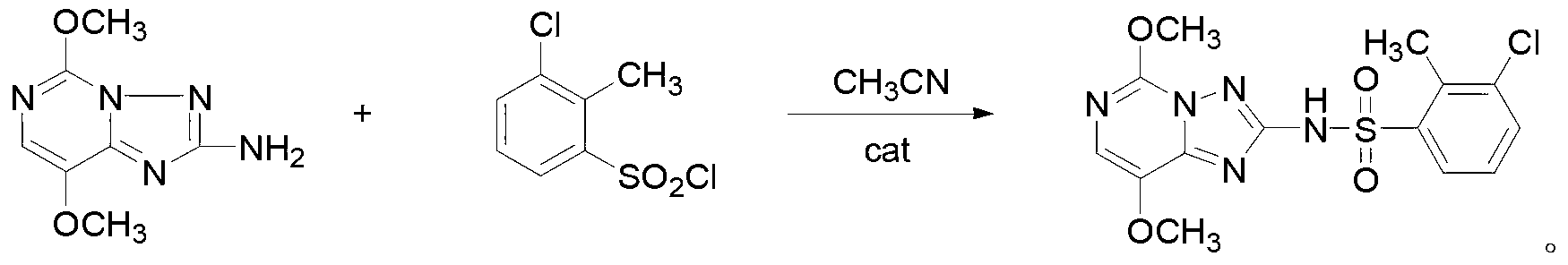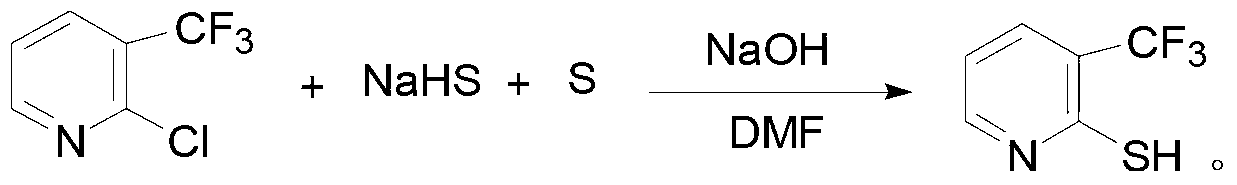Patents
Literature
140 results about "Amine synthesis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Quinacridone pigment compositions comprising unsymmetrically substituted components
The present invention relates to a novel quinacridone pigment compositions, a process using a mixed amine synthesis for the ultimate production of the compositions and to their use as colorants for pigmenting high molecular weight organic materials.
Owner:CIBA SPECIALTY CHEM CORP
Solid carried ion liquid-nanometer metal particle catalyst, and its preparing method, and application in synthesis of arylamine
InactiveCN101045213AAvoid churnNot easy to eluteOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsNitro compoundMetal particle
A catalyst carrying ionic liquid and metallic nanoparticles for preparing high-quality arylamine by hydrocatalyzing aromatic nitro compound is prepared through preparing immobilized ionic liquid by bonding the ionic liquid onto carrier, binding it with the active component containing transition metal or precursor, and reductive exchange. Its advantages are low reaction temp, long service life and no waste generation.
Owner:SHAANXI NORMAL UNIV
Quinacridone pigment compositions comprising unsymmetrically substituted components
The present invention relates to a novel quinacridone pigment compositions, a process using a mixed amine synthesis for the ultimate production of the compositions and to their use as colorants for pigmenting high molecular weight organic materials.
Owner:CIBA SPECIALTY CHEM CORP
Electronic circuit surface assembling water-soluble soldering flux and preparing method thereof
InactiveCN104191106AHigh activityImprove adaptabilityWelding/cutting media/materialsSoldering mediaAntioxidantAcrylic resin
The invention discloses electronic circuit surface assembling water-soluble soldering flux and a preparing method thereof. The electronic circuit surface assembling water-soluble soldering flux comprises the following raw materials, by weight part, of 5-8 parts of hydroxyl organic acid, 2-3 parts of alcohol amine, 0.5-1 part of surface active agent, 65-70 parts of mixed solvent, 5-8 parts of cosolvent, 17-21 parts of water-soluble acrylic resin, 0.8 part-1.2 parts of corrosion inhibitor and 0.1-0.15 part of antioxidant. The water-soluble hydroxyl organic acid with high activity, little residue and low corrosion is compounded with alcohol amine to form active material compound, a method that the surface active agent, the corrosion inhibitor, the acrylic resin, the antioxidant and the cosolvent are matched with each other is supplemented, and the active material compound is dissolved into the mixed solvent. The soldering flux is low in corrosion, a little in corrosion, easy to rinse, free of lead and halogen and used for welding electronic circuit surface assembling electronic components.
Owner:YANTAI HENGDIKE ENERGY TECH
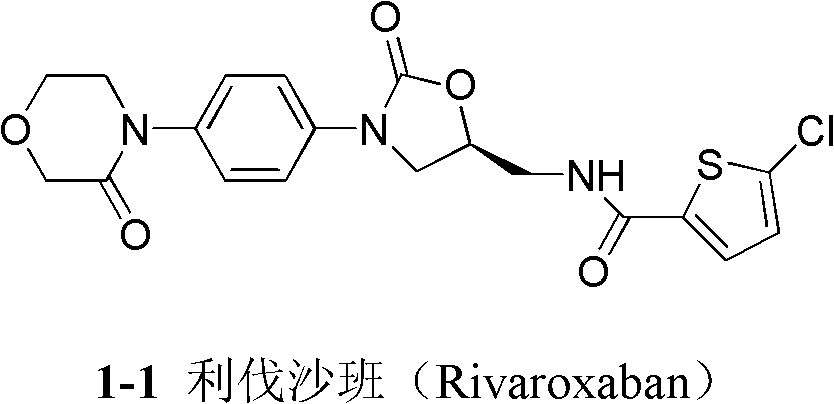
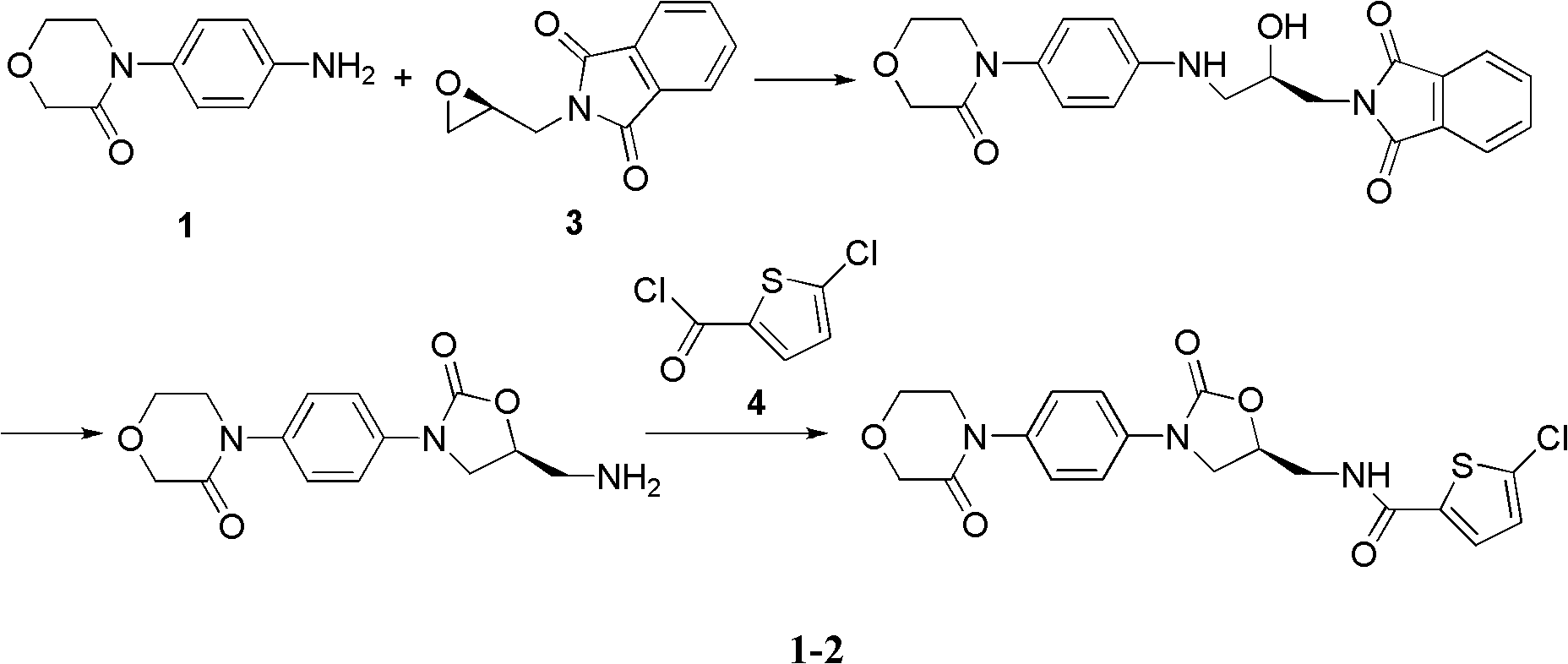
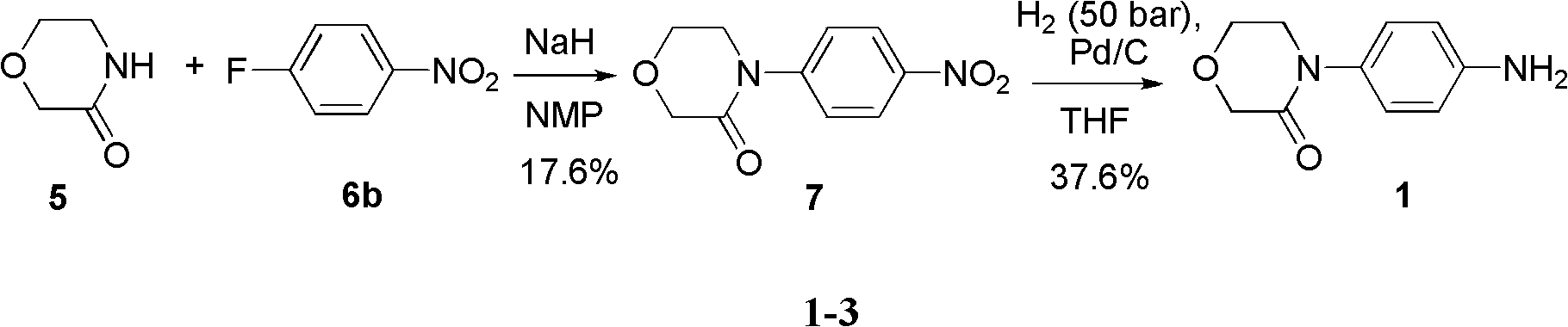
4-(4-amion phenyl)-3-morpholone intermediate amide and synthesis method and application thereof
InactiveCN102320988ARaw materials are cheap and easy to getMild reaction conditionsOrganic compound preparationCarboxylic acid amides preparationSynthesis methodsPhotochemistry
The invention relates to a 4-(4-amion phenyl)-3-morpholone intermediate amide and a synthesis method and application. The method has the advantages of cheap and readily available raw materials, mild reaction condition and high yield; and intermediate amide can be used for synthesizing 4-(4-amion phenyl)-3-morpholone and further synthesizing a rivaroxaban medicament.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Iron and nitrogen-doped carbon nanosphere preparation method and oxygen reduction application
InactiveCN108039500AGood anti-methanol effectLarge specific surface areaCell electrodesMicrosphereHigh pressure
The invention provides an iron and nitrogen-doped carbon nanosphere preparation method and application of the nanosphere in oxygen reduction. The dopamine hydrochloride is used for synthesizing a nitrogen source supply object polydopamine under alkaline condition, and then the surface of ferriferrous oxide nanosphere is coated by the polydopamine; the ferriferrous oxide nanosphere coated by the polydopamine is subjected to a reaction with glucose in a high pressure reaction vessel, and under nitrogen condition, high temperature calcining is carried out to obtain the iron and nitrogen-doped carbon nanosphere. The synthesis of the iron and nitrogen-doped carbon nanosphere takes glucose as a raw material, and the ferriferrous oxide nanosphere coated by the polydopamine is taken as a corrosiontemplate and the nitrogen source. The nitrogen-doped carbon nanosphere used in a electrocatalysis oxygen reduction reaction (ORR) has good effect and good endurance, an initial potential relative standard hydrogen electrode of reduction oxygen is 0.949 V, the efficiency after endurance test can still reach more than 85%, after addition of methanol, the usage efficiency can still reach 94%, and the nitrogen-doped carbon nanosphere has good anti-methanol effect.
Owner:UNIV OF JINAN
Water-soluble cutting fluid and preparation method thereof
ActiveCN101948711AExtended service lifeExcellent extreme pressure lubricityLubricant compositionPolyethylene glycolMonoisopropanolamine
The invention discloses water-soluble cutting fluid. The cutting fluid consists of the following components in percentage by weight: 15 to 25 percent of oleate-2-ethylhexyl ester, 2 to 8 percent of 3,5,5-trimethyl caproic acid, 3 to 10 percent of sebacic acid, 5 to 10 percent of mono ethanolamine, 15 to 20 percent of triethanolamine, 5 to 10 percent of diglycol, 3 to 10 percent of monoisopropanolamine, 15 to 20 percent of polyethylene glycol 600 and 20 to 30 percent of water. The invention also discloses a preparation method of the water-soluble cutting fluid. The method comprises the following steps of: reacting by mixing the oleate-2-ethylhexyl ester, the mono ethanolamine, the 3,5,5-trimethyl caproic acid and a part of water to obtain alkylol amine synthesis ester mixture A; reacting by mixing the sebacic acid, the triethanolamine, the monoisopropanolamine and the rest amount of water to obtain alkylol amine synthesis ester mixture B; and reacting by mixing the diglycol, the polyethylene glycol 600, the alkylol amine synthesis ester mixture A and the alkylol amine synthesis ester mixture B to obtain a finished product.
Owner:GUANGZHOU LANDNOK CHEM TECH
2-phenoxyl tetrahydrofuran (tetrahydropyrane) derivatives and application thereof in synthesis of penoxsulam
The invention discloses 2-phenoxyl tetrahydrofuran (tetrahydropyrane) derivatives and the application thereof in synthesis of penoxsulam, and belongs to the field of pesticide synthesis. A new intermediate of a penoxsulam pesticide is synthesized by the derivatives of the design of the invention. A high-toxicity raw material is not required to be used in the preparation of the intermediate; a product is convenient to purify and high in yield; a synthesizing process is environment-friendly; the synthesis cost is low. The penoxsulam pesticide can be prepared conveniently by utilizing the intermediate; the intermediate has a good application prospect. The series of derivatives are easy and convenient to synthesize; compared with the reported penoxsulam intermediate, a low-boiling-point raw material with high toxicity, namely, chloromethyl methyl ether, is not required to be used in the synthesizing process; the safety of the synthesizing process is improved; the derivatives are favorable to industrialized production and application.
Owner:CHANGZHOU UNIV
Anti-aging garbage can and preparation process thereof
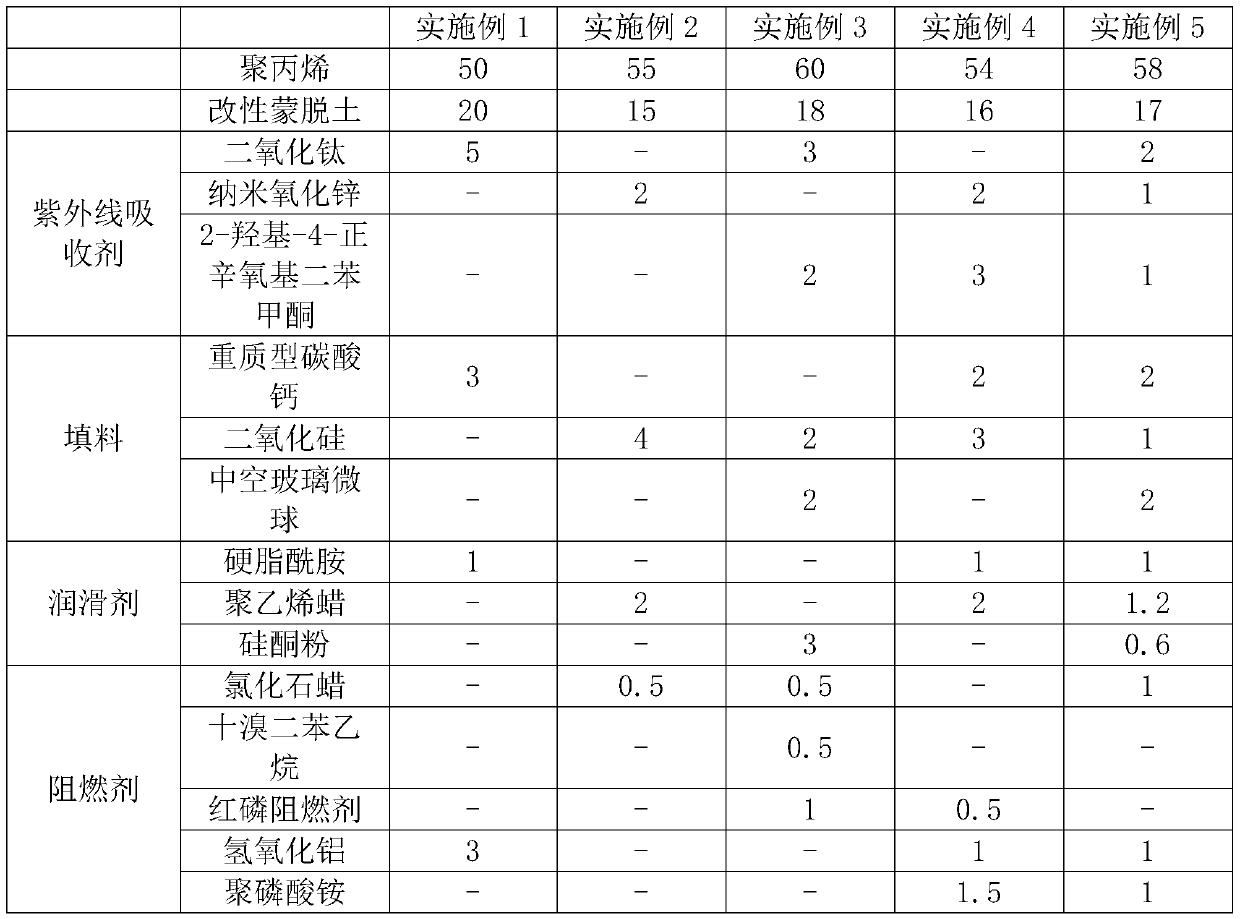
Relating to the technical field of garbage cans, the invention relates to an anti-aging garbage can and a preparation process thereof. The anti-aging garbage can comprises the following components bymass: 50-60 parts of polypropylene, 15-20 parts of modified montmorillonite, 2-5 parts of an ultraviolet light absorber, 3-5 parts of filler, 1-3 parts of a lubricant and 0.5-3 parts of a flame retardant. According to the invention, cyanuric chloride, sodium sulfanilate and tetramethylpiperidine amine are employed to synthesize a hindered amine light stabilizer, and through ion exchange, the hindered amine light stabilizer is intercalated to the interlayer of montmorillonite, the prepared modified montmorillonite has anti-aging effect, and the modified montmorillonite is uniformly dispersed inpolypropylene, thus guaranteeing a consistent anti-aging effects at all parts. The anti-aging garbage prepared according to the invention has the effects of excellent anti-aging performance and prolonged service life.
Owner:江苏林辉塑料制品有限公司
Triazolopyrimidine sulfonamide compound, and synthetic method and application thereof
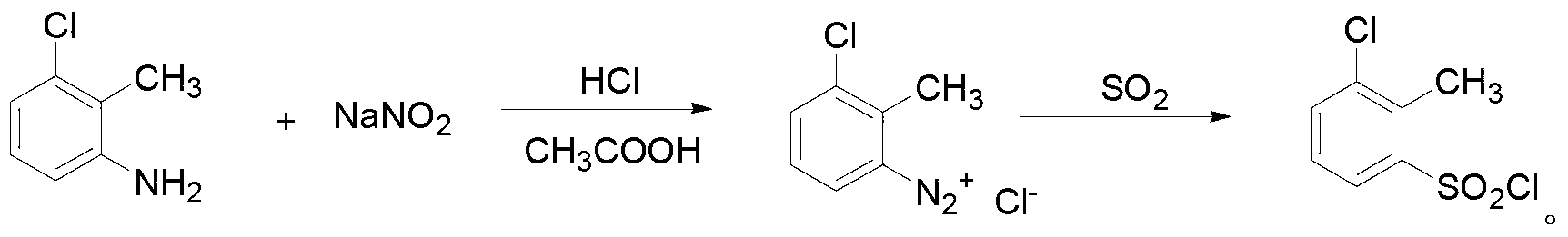
ActiveCN103319489ASmall molecular weightThe synthesis method is simpleBiocideOrganic chemistrySulfonyl chlorideChemical compound
The invention provides a triazolopyrimidine sulfonamide compound and synthetic methods and application thereof, relating to a penoxsulam analog and a synthetic method and application thereof. The objective of the invention is to overcome the problems of great synthesis difficulty and high cost of conventional penoxsulam. The triazolopyrimidine sulfonamide compound has a structural formula described in the specification. The method 1 comprises the following steps: 1, preparing 2-methyl-3-chlorobenzenesulfonyl chloride; and 2 preparing a finished product so as to obtain 3-chloro-2-methyl-N-(5,8-dimethoxy-[1,2,4] triazolo[1,5-c]pyrimidine-2-yl) benzsulfamide. The method 2 comprises the following steps: 1, preparing 2-mercapto-3-trifluoromethylpyridine; and 2, preparing 3-trifluoromethylpyridine-2-sulfonyl chloride; and 3, preparing a finished product so as to obtain 3-trifluoro-methyl-N-(5,8-dimethoxy-[1,2,4] triazolo[1,5-c]pyrimidine-2-yl) pyridine sulfamide. The triazolopyrimidine sulfonamide compound is used as a herbicide in a rice field.
Owner:HEILONGJIANG UNIV
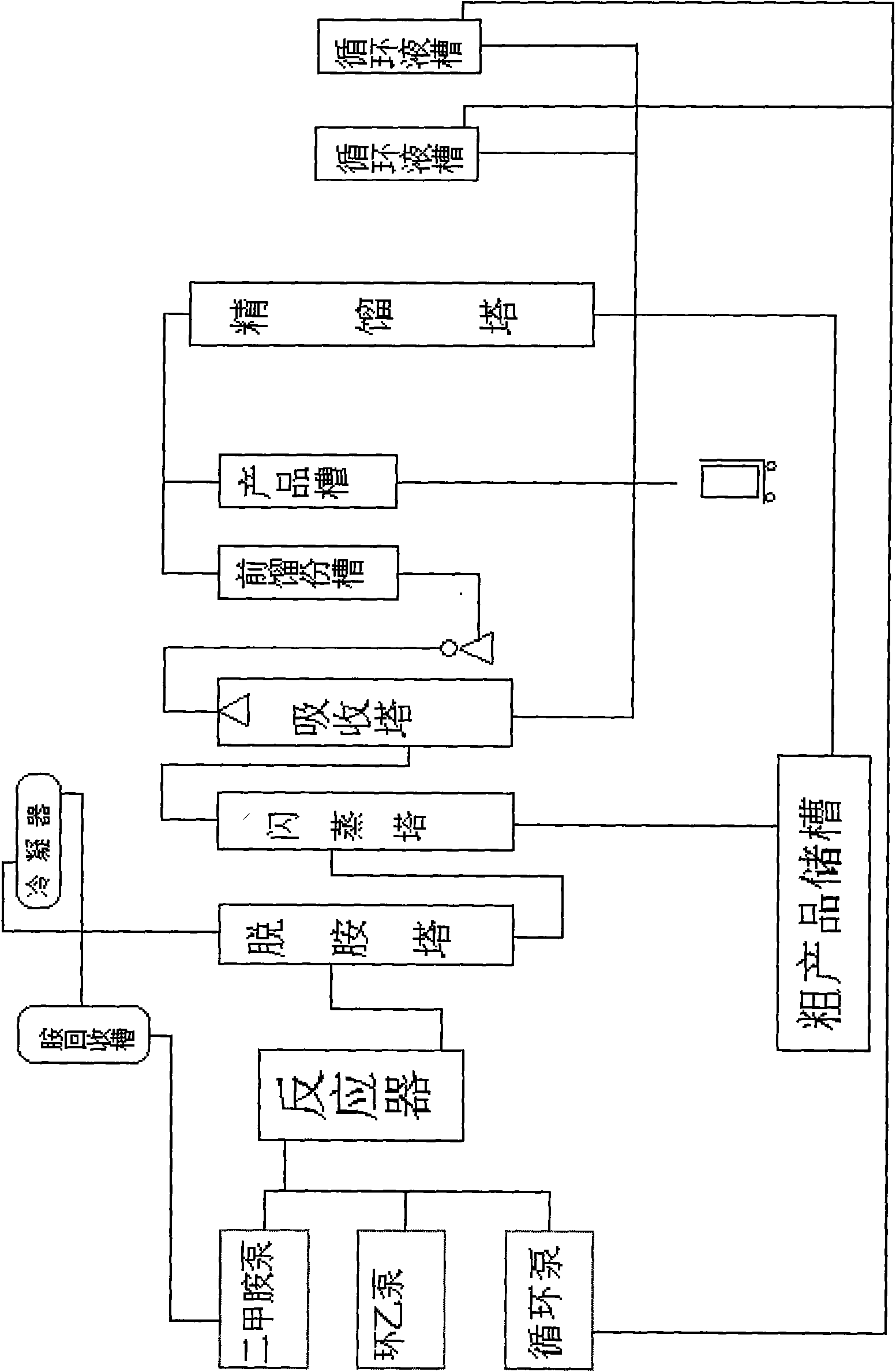
Temperature regulating system in methylamine synthesis
PendingCN110918022AEasy to recycleReduce lossesChemical/physical/physico-chemical processesThermodynamicsIntermediate heat exchanger
The invention provides a temperature regulating system in methylamine synthesis, wherein the temperature regulating system comprises a low-temperature heat exchanger; a first low-temperature outlet ofthe low-temperature heat exchanger communicates with a first medium-temperature inlet of an intermediate heat exchanger, and a first medium-temperature inlet of the intermediate heat exchanger communicates with a first high-temperature inlet of a high-temperature heat exchanger; a first high-temperature outlet of the high-temperature heat exchanger communicates with an inlet of the top of a methylamine synthesis tower, an outlet of the bottom of the methylamine synthesis tower communicates with an inlet of a crude product gas-liquid separation tank, the top end of the crude product gas-liquidseparation tank communicates with a condensation tank, and the bottom end communicates with a rectification system; an outlet of the rectification system communicates with a second medium-temperatureinlet of the intermediate heat exchanger. According to the system, the temperature of a material in the reaction process is recovered for heating a raw material at the inlet of a methylamine reactiontower, so that the raw material can reach the required initial temperature, and no extra energy and cost are consumed; in addition, the system can control the temperature in the reaction tower in time, the phenomenon that the temperature rises drastically is prevented, and the safety problem is effectively avoided.
Owner:安阳九天精细化工有限责任公司
Modified waterborne polyurethane resin and synthesis method
The invention discloses modified waterborne polyurethane resin which consists of the following raw materials in percentage by weight: 10-20 percent of isophorone diisocyanate, 10-20 percent of polyester polyol, 5-10 percent of dimethylolpropionic acid, 5-10 percent of triethylamine, 5-10 percent of diethylene glycol, 40-60 percent of deionized water and 1-3 percent of diethylenetriamine. A synthesis method comprises the steps of (1) putting the isophorone diisocyanate into a stirrer, dropping the polyester polyol and the dimethylolpropionic acid, and generating reaction at temperature of about 60-80 DEG C for 3-5 hours; (2) adding the diethylene glycol to generate chain extension reaction for 1-5 hours, and cooling to room temperature; (3) emulsifying the deionized water with triethylamine on a high-speed dispersion machine, adding the diethylenetriamine to implement modification to obtain a light-yellow and semi-transparent waterborne polyurethane dispersoid. According to the modified waterborne polyurethane resin and the synthesis method, small-molecular polyhydric alcohols are modified through gradual polymerization reaction, so that the performance of waterborne polyurethane is improved, and the waterborne polyurethane can be widely applied; furthermore, the cost can be reduced.
Owner:山东聚东新材料有限责任公司
Preparation method of N, N-dimethylethanolamine
InactiveCN101648880AIncrease usageReduce unit consumptionOrganic compound preparationAmino-hyroxy compound preparationEpoxyEthylene oxide
The invention relates to organic amine synthesis, in particular to a preparation method of N, N-dimethylethanolamine, which comprises the following steps: (a) raw material mixing; (b) synthetic reaction; (c) deamination treatment; (d) flash vaporization treatment; (e) absorption treatment; and (f) rectification treatment and separation of N, N-dimethylethanolamine products. The invention solves the problem that in the prior preparation method of N, N-dimethylethanolamine, the circulating liquid brings impurities. In the invention, the previous distillate of the N,N-dimethylethanolamine is used for replacing the circulating liquid adopted in the original process, thus the DMEA content in a crude product is increased to 95%-99% from 70%-80%, the moisture content in the crude product is reduced to 1%-5% from 20%-30%, the impurity content in the crude product is reduced to 0.3% from 1.5%-2%, the unit consumption of epoxy ethane is reduced to 0.51-0.54 from 0.56-0.59, and the unit consumption of dimethylamine is reduced to 0.53-0.56 from 0.58-0.60, thereby reducing the consumption of raw materials, reducing the consumption of energy sources and avoiding the environment pollution.
Owner:王宇平
Method for synthesizing dibenzophenazine by utilizing 2-naphthylamine under the effect of catalyst
ActiveCN102408383ANo pollution in the processLower synthesis costOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsHemicucurbiturilPtru catalyst
The invention relates to a method for synthesizing dibenzo[a,j]phenazine by utilizing 2-naphthylamine (or called naphthylamine, b-naphthylamine) under the effect of catalyst, which comprises the following steps of: taking hemicucurbituril (Hemicucurbit[n]uril, n=6, 12) as catalyst and 0.1M of hydrochloric acid solution as solvent, and catalyzing 2-naphthylamine hydrochloride to synthesize the dibenzophenazine, wherein the molar ratio of the 2-naphthylamine hydrochloride to the hemicucurbituril is 1 : 0.1, the reaction temperature is 80 degrees centigrade and the reaction time is 30min in the 0.1M of hydrochloric acid solution. The method has the advantages of simple and convenient operation, low cost, high yield, cleanness and environment-friendliness, and complies with the requirements of the green synthesis initiated in the current society.
Owner:山东格新精工有限公司
Preparation method of important intermediate of Silodosin
InactiveCN103848772AEasy to separateSimple and fast operationOrganic chemistryImideBiochemical engineering
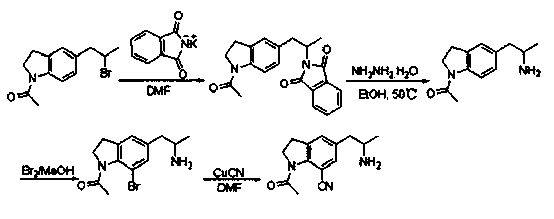
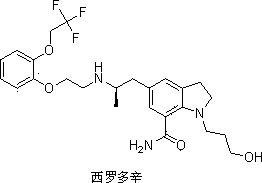
The invention discloses a preparation method of an important intermediate of Silodosin. The method comprises the following steps: carrying out a Gabriel primary amine synthesis reaction of raw materials comprising 1-acetyl-5-(2-bromopropyl)indoline and phthalimide potassium to obtain an intermediate 1-acetyl-5-(2-aminopropyl)indoline, brominating, and cyanating to obtain a product 1-acetyl-5-(2-bromopropyl)-7-cyanindoline, that is the important intermediate of Silodosin. The preparation method has the advantages of simple operation, high reaction yield, easy product separation and the like, and is suitable for industrialized production.
Owner:STONE LAKE PHARMA TECH
Method for synthesizing chiral amine compound through catalyzing asymmetric hydrogenated imine by using iridium
InactiveCN109422603AHigh selectivityHigh activityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsIridiumChloroacetyl chloride
The invention relates to a method for synthesizing a chiral amine compound through catalyzing high-activity high-selectivity asymmetric hydrogenated imine by using iridium. An adopted catalyst is generated in situ by an iridium-cyclooctadiene complex and a chiral phosphine-amino phosphine ligand. (S)-N-(1-methoxy-2-propyl)-2-methyl-6-ethylaniline [(S)-NAA] can be obtained by catalyzing a hydrogenation reaction of 2-methyl-6-ethyl-N-methylene aniline (EMA-imine) by using the catalyst, wherein the enantiomeric excess (ee) can reach 90%, and the molar ratio of the EMA-imine to the catalyst can beup to 1000000. Refined metolachlor with an S-configuration effective content of 94% can be obtained by carrying out an acylation reaction on the (S)-NAA with chloroacetyl chloride. Therefore, the ligand provided by the invention can be used for synthesizing the chiral herbicide metolachlor.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
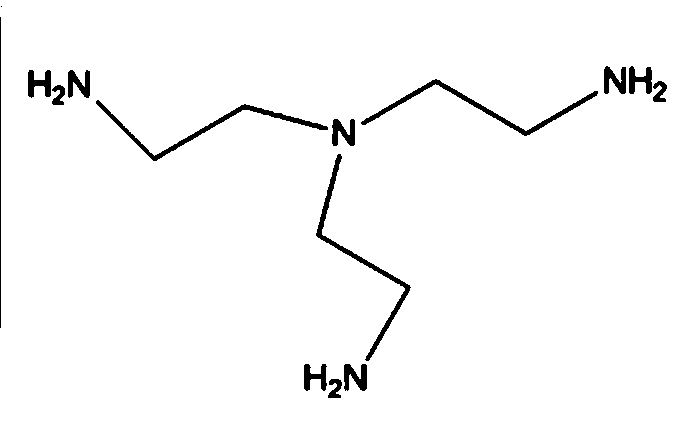
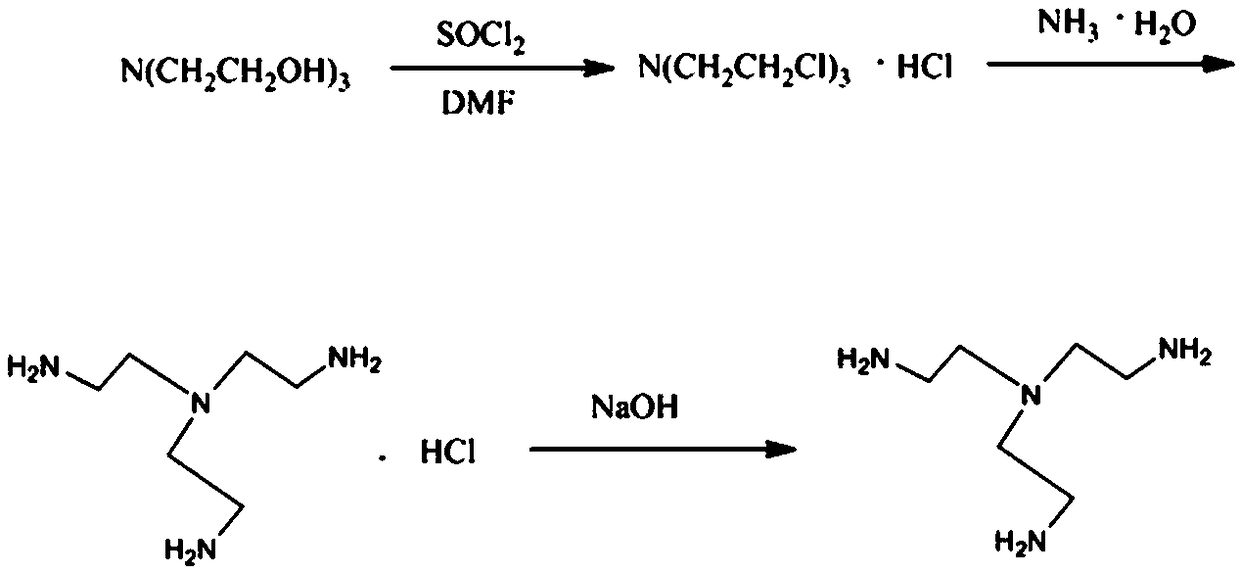
Tris(2-aminoethyl)amine synthesis process
ActiveCN109438252AHigh yieldHarm reductionOrganic compound preparationAmino compound preparationOrganic solventTris(2-aminoethyl)amine
The invention discloses a tris(2-aminoethyl)amine synthesis process, and belongs to the technical field of compound preparation. The tris(2-aminoethyl)amine synthesis process includes the steps of taking triethanolamine as a starting material, putting the triethanolamine, thionyl chloride and a catalyst DMF in a reactor, heating the three to generate tris(2-chloroethyl)amine hydrochloride, dissolving separated tris(2-chloroethyl)amine hydrochloride concentrate and ammonia water in an organic solvent in a reactor, putting the solution in the reactor, carrying out a reaction while heating to obtain tris(2-aminoethyl)amine hydrochloride, and reacting the tris(2-aminoethyl)amine hydrochloride with sodium hydroxide to obtain the tris(2-aminoethyl)amine. The tris(2-aminoethyl)amine synthesis process has the advantages of short reaction route and high controllability.
Owner:安徽工业大学科技园有限公司
Polyesteramide, preparation method thereof and fiber
ActiveCN111825838ALow yellow indexImprove thermal stabilityMonocomponent polyamides artificial filamentPolymer sciencePtru catalyst
The invention provides polyesteramide, a preparation method thereof and a fiber. The preparation method of the polyesteramide comprises the following steps: in an inert atmosphere, carrying out esterification reaction on terephthalic acid and / or derivatives thereof and dihydric alcohol in an esterification reaction kettle in the presence of a catalyst; after the esterification reaction is finished, adding a dibasic acid amine salt aqueous solution into the esterification reaction kettle for an amidation reaction, wherein the mass concentration of the dibasic acid amine salt aqueous solution is20-80%; and after the amidation reaction is finished, transferring the amidation product into a polycondensation reaction kettle to carry out polycondensation reaction, finishing the polycondensationreaction when the intrinsic viscosity of the polycondensation product reaches 0.3-1.8 dL / g, and collecting the polycondensation product to obtain the polyesteramide. According to the preparation method provided by the invention, the problem that yellowing is easy to occur in a polyesteramide synthesis process at the present stage can be solved, so that the obtained polyesteramide has a relativelylow yellow index.
Owner:CATHAY R&D CENT CO LTD +1
Method of synthesizing amide with methyl aromatics and amine in water phase
InactiveCN104761420AOrganic compound preparationCarboxylic acid amides preparationPtru catalystAcyl group
The invention provides a high-efficient method of synthesizing amide with methyl aromatics and amine. In the method, with the methyl aromatics and the amine as the raw materials in the water phase and TBHP and TBAI respectively as an oxidizing agent and a catalyst, a sp3 C-H bond and a sp3 N-H bond are broken and a C-N bond is formed. Compared with a conventional method of synthesizing the amide from oxidized amide, in which an activated acyl group or amine is required, the method is carried out with water as the solvent so that the method is not only economical but also environmental-protective. The method has a very good application prospect in the field of synthesizing polypeptide, protein and drugs in future.
Owner:JIANGXI NORMAL UNIV
Preparation method of alicyclic binary secondary amine
PendingCN111995529ALong operating timeEasy to usePreparation by reductive alkylationPolymer sciencePtru catalyst
The invention relates to a preparation method of alicyclic binary secondary amine, which is characterized by comprising the following steps: by using alicyclic binary primary amine and ketone as raw materials, carrying out catalytic hydrogenation in the presence of a catalyst, and carrying out monoalkylation substitution on the primary amino group to obtain the alicyclic binary secondary amine, wherein R is an aliphatic alkylene group containing a cycloalkyl structure, and R'and R''each independently represent an alkyl group having 1-6 carbon atoms. According to the present invention, the low-viscosity target product containing the large substituted alkyl secondary amino group is prepared, and the alicyclic diamine can be used for polyurethane / polyurea, an epoxy resin chain extender or a curing agent.
Owner:江苏湘园化工有限公司
Solvent-free amide synthesis method and its use in synthesis of polymer antioxidant stabilizer
InactiveCN106916043AHigh temperature and yieldIncrease temperatureOrganic compound preparationCarboxylic acid amides preparationPolymer sciencePhenol
The invention discloses a solvent-free amide synthesis method and its use in synthesis of a polymer antioxidant stabilizer. Through eight reaction expressions, different structural amide-linked thioether high-temperature long-term antioxidant stabilizers, sterically hindered phenol thioether hybrid antioxidants and sterically hindered phenol amide-type antioxidants as desired products are synthesized. Compared with the most carboxylate-bonded antioxidants on the market, the different types of multi-functional hybrid structure antioxidants stably bonding to amide have the advantages of structure stability, acid resistance, base resistance, hydrolysis resistance, environmental degeneration resistance and good synergistic antioxidation activity and are novel antioxidant stabilizer products having performances satisfying novel material development requirements. The novel polymer antioxidant stabilizers solve the problem that the same type of the ester-linked antioxidant stabilizer on the market has an ester bond easily decomposed under acid and base conditions and can be easily hydrolyzed and the existing product DSTDP / DLTDP on the market produces a smell in use and processing and the degraded material releases VOCs. The novel polymer antioxidant stabilizers provide a strong support and more choices for development of novel polymers.
Owner:SHAOXING RUIKANG BIOTECHNOLOGES CO INC
System for synthesizing N-methyl-2-pyrrolidone
The invention relates to a system for synthesizing N-methyl-2-pyrrolidone. The system is characterized by comprising a 1,4-butanediol dehydrogenation device, a mixed amine synthesis device and a N-methyl-2-pyrrolidone preparation device. The system provided by the invention has the advantages as follows: the system is simple in process, and low in energy consumption and input; in addition, multiple circulating exchange among devices is adopted, so that gamma-butyrolactone is completely converted into N-methyl-2-pyrrolidone, separation in a later period is easier, high-purity N-methyl-2-pyrrolidone can be synthesized and the purity can reach 99.9 wt % or above.
Owner:江苏恒祥化学股份有限公司
Ternary microemulsified diesel oil and method for preparing same
InactiveCN1962826AWide variety of sourcesReduce manufacturing costRefining by water treatmentMixed fatty acidAlcohol
The invention discloses a ternary micro-emulsifying diesel and preparing method of regenerated grease and water in the diesel, which comprises the following parts: 5-20% fatty acid ammonium soap, 5-20% water and 60-90% diesel, wherein the fatty acid ammonium soap is synthesized by 75-95% fatty acid and 5-25% polyatomic alcohol amine; the content of saturated fatty acid is less than or equals 25% in the composite fatty acid.
Owner:LIAONING WANCHENG CATALYSIS
Nanometer molecular sieve, synthetic method and applications thereof
ActiveCN111115651ALow costSmall particle sizeGas treatmentMolecular sieve catalystsMolecular sieveAdsorption separation
The invention provides a high-specific-surface-area nano zeolite molecular sieve capable of being directly synthesized without using organic amine or a seed crystal or a guiding agent, and a synthesismethod thereof. A purpose of the invention is to mainly solve the problems of small specific surface area, high cost and unfriendliness to the environment due to the fact that most of nano zeolite molecular sieves are synthesized by using organic amines in the prior art. According to the invention, inorganic ammonium or inorganic alkali is used for replacing organic amine, and the parameters in the synthesis process of the molecular sieve are optimized, so that the nano molecular sieve with the grain size of less than 200 nm is obtained, the specific surface area is 300-800 m<2> / g, and the synthesis method is good in repeatability, low in synthesis cost, simple to operate and environment-friendly, and can be applied to the industrial fields of catalysis, adsorption separation, drying purification and the like.
Owner:CHINA PETROLEUM & CHEM CORP +1
Synthesis method for novel chiral asymmetric secondary amine belonging to N-substituted amino acid esters
InactiveCN101891572ASolve the problem of poor chemoselectivityAvoid the problems of difficult post-processing and low yieldAsymmetric synthesesSynthesis methodsReaction step
The invention relates to a synthesis method for novel chiral asymmetric secondary amine belonging to N-substituted amino acid esters. The method of the invention comprises two major steps: (1) amino acid ester firstly reacts with aldehyde to form an imine intermediate; and (2) in situ reduction is carried out on the imine intermediate to form asymmetric secondary amine, wherein the reduction utilizes a Pd / C catalytic hydride process. The reaction product is separated and purified to obtain the novel chiral asymmetric secondary amine belonging to the N-substituted amino acid esters. The synthesis method solves the problem that the direct primary amine alkylate method most commonly used in asymmetric secondary amine synthesis has poor chemical selectivity, and avoids the problems that alkylation reaction is generated to produce a great amount of byproducts of tertiary ammonium and quaternary ammonium, a great amount of unreacted raw material primary amine exists, the aftertreatment is difficult and the yield is low. Compared with the amide reduction method for preparing the asymmetric secondary amine, the method of the invention has the advantages of less reaction steps, simple operation, no by-products, high yield and the like.
Owner:UNIV OF JINAN
4th phenylalaninamide hydroxylation modified endomorphin analogs and synthesis method and applications thereof
ActiveCN107987126AReduce biological activityChanges in biological activityNervous disorderTetrapeptide ingredientsTert-Butyloxycarbonyl protecting groupSynthesis methods
The invention discloses 4th phenylalaninamide hydroxylation modified endomorphin analogs and a synthesis method and applications thereof, relates to endomorphin analogs and a synthesis method and applications thereof, and provides 4th phenylalaninamide hydroxylation modified endomorphin analogs and a synthesis method and applications thereof. The endomorphin analogs are tyrosine-proline-tryptophan-phenylanine(N-hydroxyl)amide and tyrosine-proline-phenylalanine-phenylanine(N-hydroxyl)amide. The synthesis method comprises the following steps: step one, synthesizing N-t-butyloxycarboryl-phenylanine(N-hydroxyl)amide; step two, synthesizing phenylanine(N-hydroxyl)amide; step three, synthesizing carbobenzoxy-tryptophan-phenylanine(N-hydroxyl)amide; step four, synthesizing carbobenzoxy-tyrosine-proline-tryptophan-phenylanine(N-hydroxyl)amide; and step six, synthesizing tyrosine-proline-tryptophan-phenylanine(N-hydroxyl)amide. The analogs can be used to prepare polypeptide pain relieving drugs.
Owner:黑龙江省工研院资产经营管理有限公司
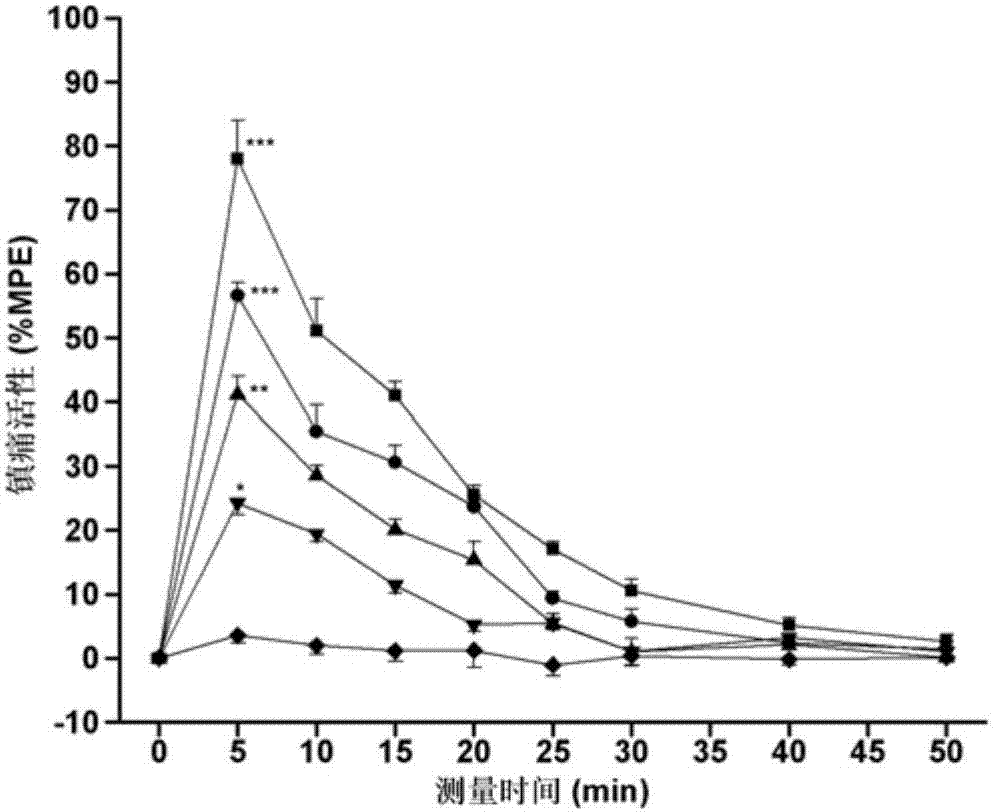
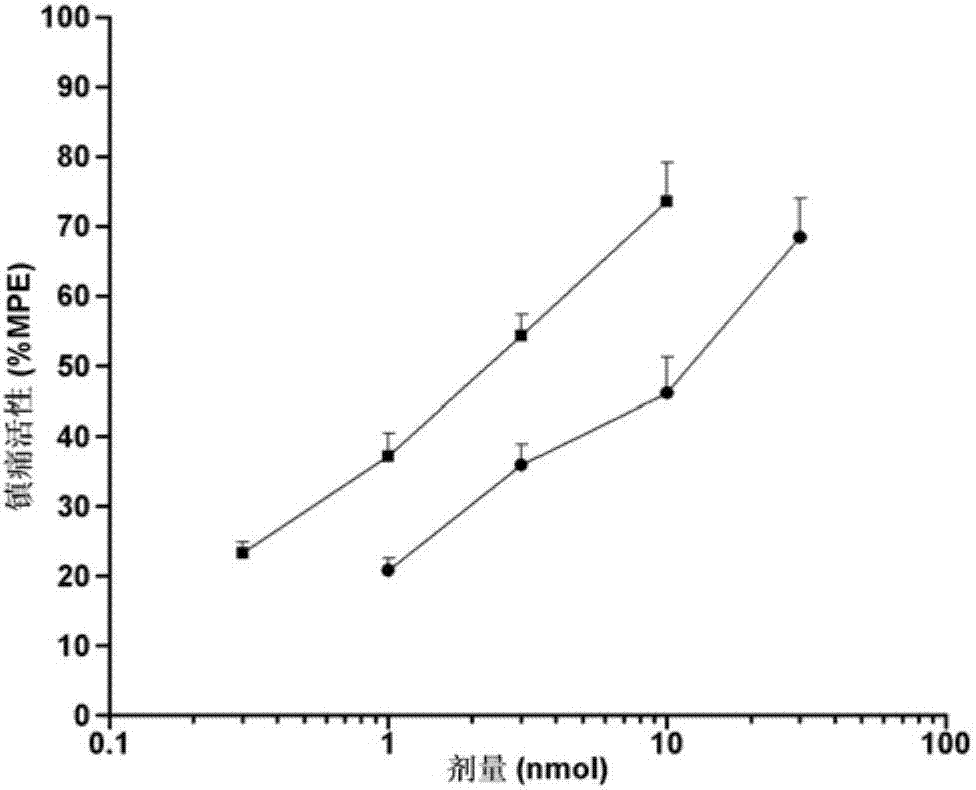
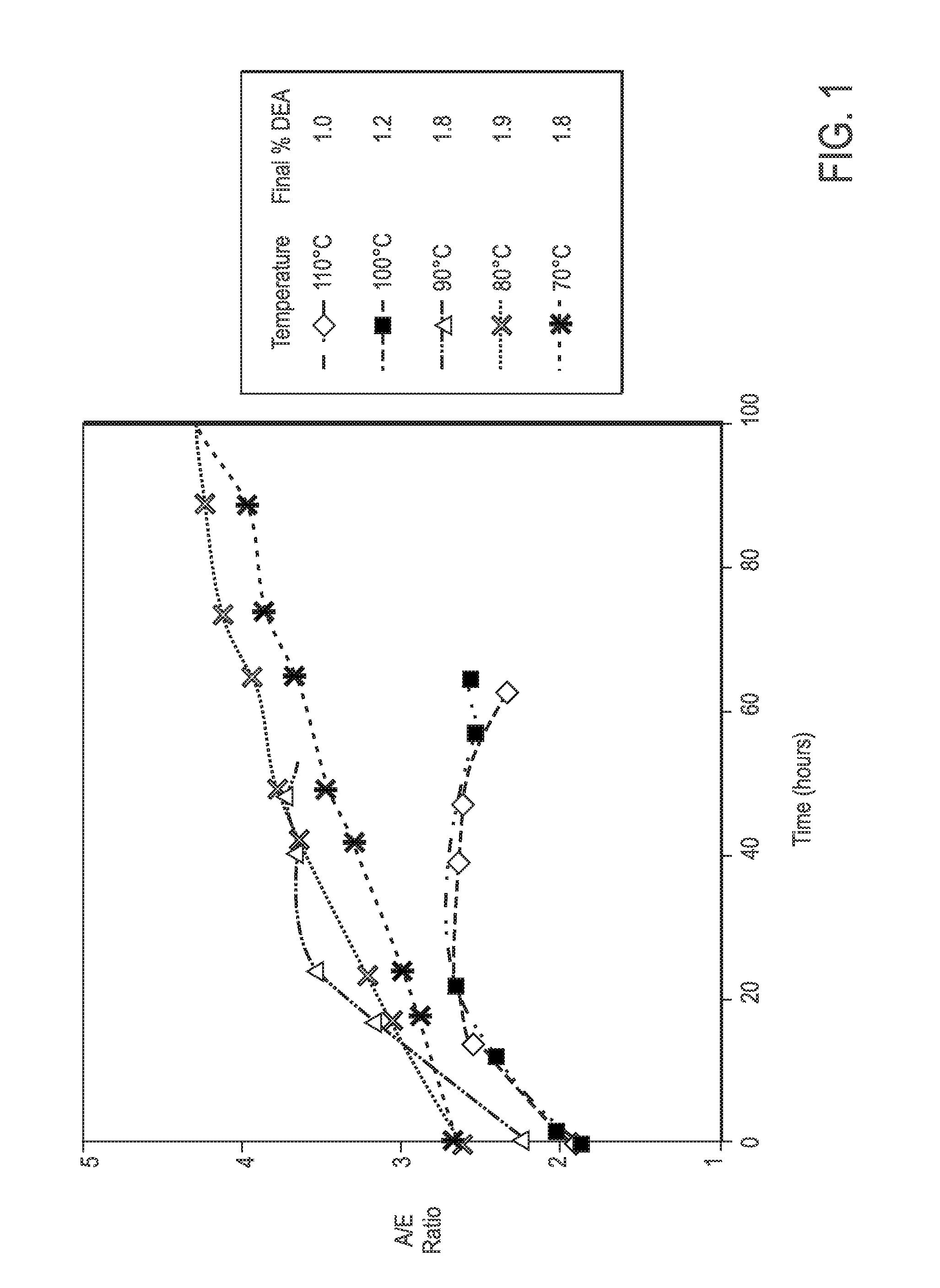
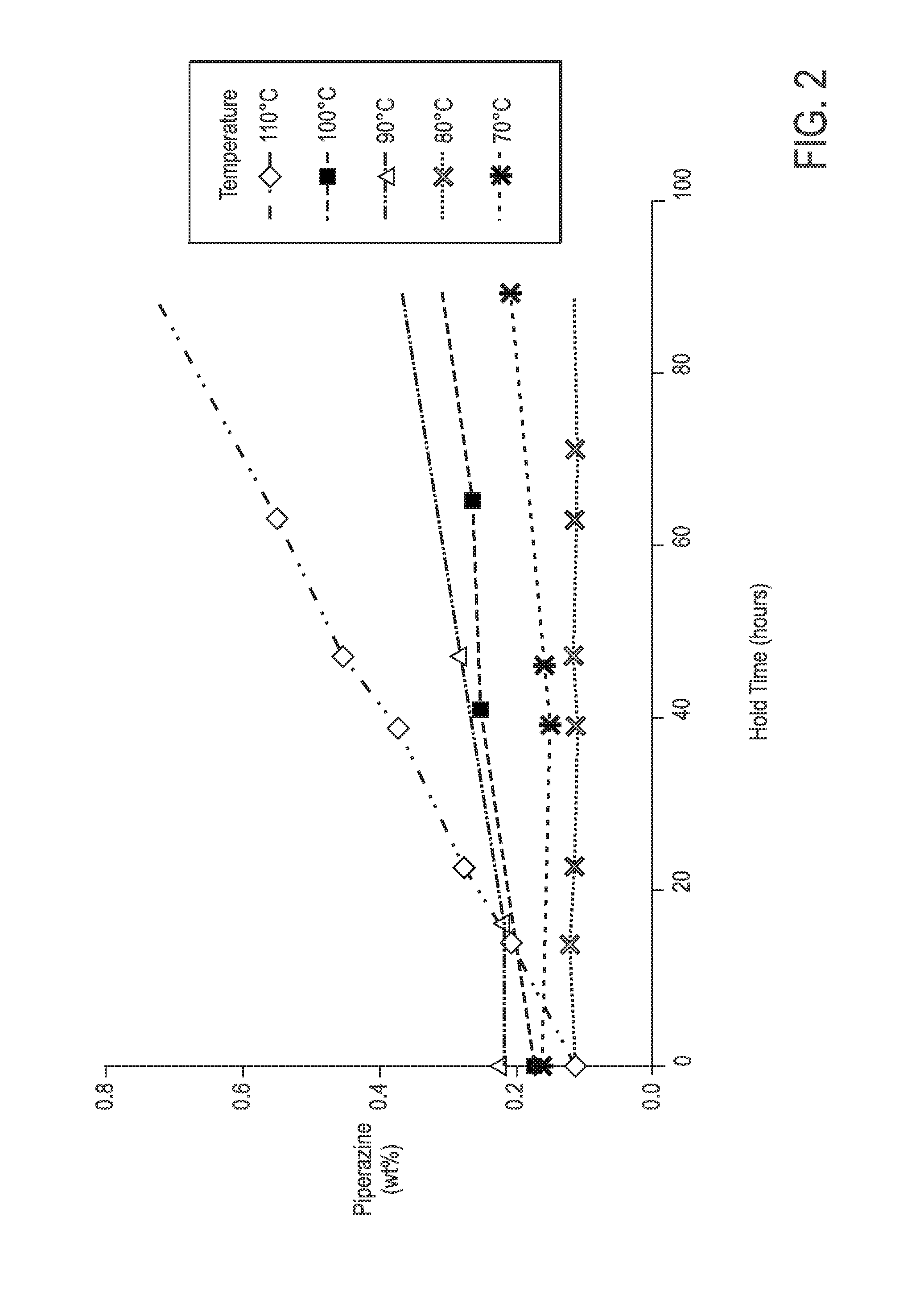
Process For Alaknolamide Synthesis
ActiveUS20160060560A1Raise the ratioOrganic compound preparationCarboxylic acid amide separation/purificationDiethanolamideAmide
The present invention is directed to a process of making alkanolamides wherein the “aging” time is reduced and the diethanol amide to ester ratio in the finished product is increased. Further provided is an additive composition comprising an alkanolamide which contains a reduced amount of DEA and BHEP.
Owner:CHEVRON ORONITE CO LLC
Synthesis and application of alcohol amine with lengthened main carbon chain
ActiveCN112920063AExtended carbon chain structureImprove grinding effectOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPolymer sciencePtru catalyst
The invention discloses synthesis and application of alcohol amine with lengthened main carbon chain, and belongs to the field of chemical building materials. Under the action of a catalyst, trihydric alcohol amine is subjected to two-step substitution reaction, hydrolysis reaction and reduction reaction to obtain the novel alcohol amine (NAA). The novel alcohol amine obtained by the invention is added into cement as a cement grinding aid, has a better grinding aid effect than that of triethanolamine, and has a wide application prospect.
Owner:BEIJING UNIV OF TECH
A high-efficiency synthesis method of dual-silicon source and amine-free large-size mordenite with controllable morphology
InactiveCN105174284BCreate pollutionEasy to operateMordenite aluminosilicate zeoliteMolecular sieveSilicic acid
The invention relates to an efficient two-silicon-source amine-free synthesis method for morphology-controllable large-size mordenite. The method comprises specific steps as follows: two kinds of different silicon sources are added sequentially, and large-size mordenite with different morphology is obtained through hydro-thermal synthesis. According to the synthesis method, tetraethoxysilane and pretreated sodium silicate are used as the silicon sources, no organic amine, inorganic ammonia or toxic fluoride is required in the synthesis method, the synthesis process is environment-friendly, the operation process is convenient, the synthesis time is short, and synthesized molecular sieve crystalline grains have the uniform size.
Owner:SHANDONG UNIV +1
Mesoporous nickel catalyst and application of same in polyether amine synthesis
ActiveCN108786816AUniform poresReduce usageMetal/metal-oxides/metal-hydroxide catalystsNickel catalystPolyol
The invention discloses a mesoporous nickel catalyst and application of the same in polyether amine synthesis. According to the catalyst, nickel nitrate serves as a nickel source, a mesoporous structure is formed under the action of surfactants after weak base prehydrolysis, and a mesoporous nickel structure is formed with the presence of reducing agents such as sodium borohydride. The mesoporousnickel catalyst can efficiently catalyze polyether polyol to synthesize polyether amine through hydrogen ammoniation, and the primary amine yield of the polyether polyol with the number-average molecular weight of 200-5000 reaches more than 98%. Compared with conventional skeletal catalysts, the mesoporous nickel catalyst has the advantages of pore uniformity, low consumption, high stability and long service life.
Owner:中科榆林能源技术运营有限责任公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com