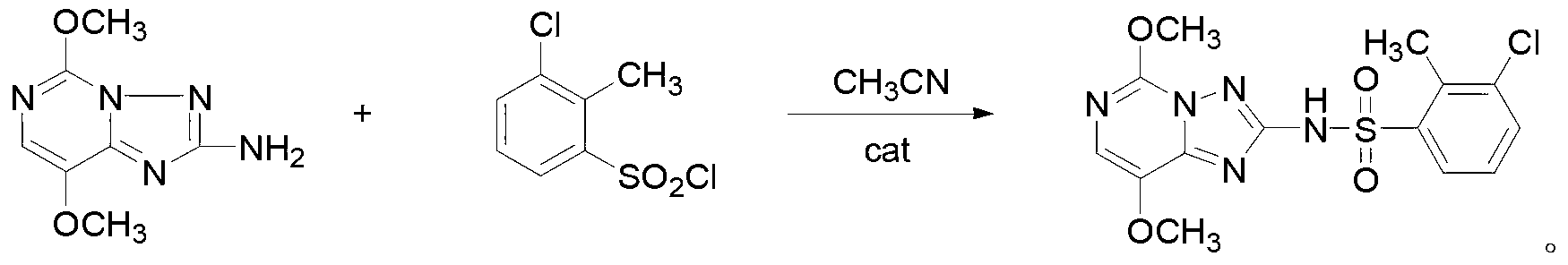Triazolopyrimidine sulfonamide compound, and synthetic method and application thereof
A technology of oxazolopyrimidine sulfonamide and synthesis method, which is applied in the field of penoxsulam analogs and synthesis, can solve the problems of high cost and difficulty in synthesizing penoxsulam, and achieves low molecular weight and simple synthesis method. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0029] Specific embodiment 1: This embodiment is a triazolopyrimidine sulfonamide compound, the chemical name is 3-chloro-2-methyl-N-(5,8-dimethoxy-[1,2,4]triazole And[1,5-c]pyrimidin-2-yl)benzenesulfonamide, the structural formula is
specific Embodiment approach 2
[0030] Specific embodiment two: This embodiment is the synthesis method of the triazolopyrimidine sulfonamide compound described in specific embodiment one, the chemical name is 3-chloro-2-methyl-N-(5,8-dimethoxy -[1,2,4]triazolo[1,5-c]pyrimidin-2-yl)benzenesulfonamide is specifically completed according to the following steps:
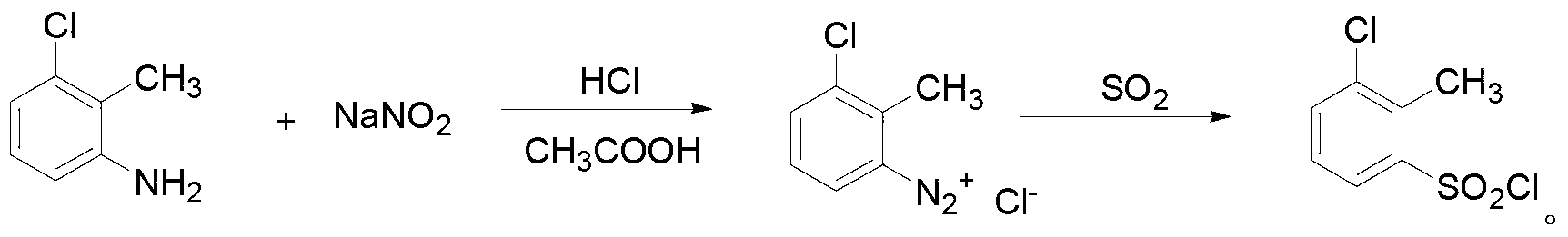
[0031] 1. Preparation of 2-methyl-3-chlorobenzenesulfonyl chloride:
[0032] ①Preparation of diazonium salt: first add glacial acetic acid and concentrated hydrochloric acid to 2-methyl-3-chloroaniline, stir at room temperature to obtain a white precipitate, and then add 2mL / min to 5mL at a temperature of -10°C to -5°C Add dropwise sodium nitrite aqueous solution with a concentration of 0.5g / mL~0.7g / mL at a rate of 1 / min to obtain an orange-yellow solution, keep it at a temperature of -10°C~-5°C for 40min~50min to obtain a diazonium salt; The volume ratio of the described glacial acetic acid to concentrated hydrochloric acid is (0.2~0.4): 1; the volu...
specific Embodiment approach 3
[0042] Specific embodiment three: This embodiment is the application of the triazolopyrimidine sulfonamide compound described in specific embodiment one, the chemical name is 3-chloro-2-methyl-N-(5,8-dimethoxy- [1,2,4]Triazolo[1,5-c]pyrimidin-2-yl)benzenesulfonamide is used as herbicide in paddy fields.
[0043] Mechanism of weeding: The triazolopyrimidine sulfonamide compound described in this embodiment has a chemical name of 3-chloro-2-methyl-N-(5,8-dimethoxy-[1,2,4]tri Azolo[1,5-c]pyrimidin-2-yl)benzenesulfonamide is an inhibitor of acetolactate synthase, which achieves herbicidal effect by inhibiting the synthesis of proteins in plants. Its mechanism of action is similar to that of sulfonylurea herbicides. similar. After plants absorb triazolopyrimidine sulfonamide herbicides, the activity of ALS in the body is severely reduced, and the synthesis of valine, leucine and isoleucine is greatly threatened, which hinders the synthesis of proteins and causes plant growth to st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com