Solvent-free amide synthesis method and its use in synthesis of polymer antioxidant stabilizer
A synthesis method and technology of solvent amides, which are applied in the field of synthesis and preparation of polymer antioxidant stabilizers, can solve the problems of high cost, achieve the effects of strong resistance to environmental degradation, good synergistic antioxidant performance, and overcoming unstable performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
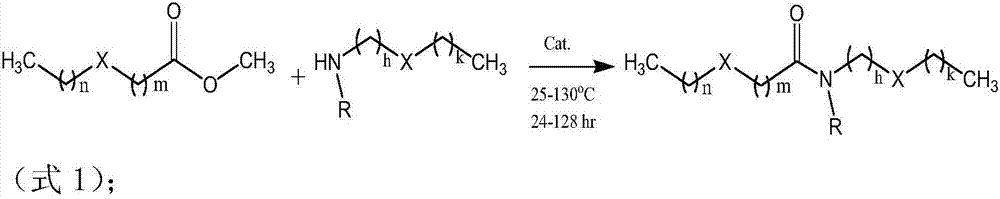
[0074] The reaction equation of monoamine and diamine with methyl ester:
[0075]
[0076] n: natural number;
[0077] R: H, Me, Et, n-Pr, i-Pr, n-Bu, i-Bu, t-Bu, long chain alkyl
[0078] RCO 2 Na:HCO 2 Na,CH 3 CO 2 Na et al. In this embodiment, heating control is an important factor. The reaction will turn yellow under high temperature for a long time, and the product quality will decline. Please see the following table for the corresponding reaction conditions: Beta-sulfide methyl ester and monobasic and dibasic organic amines Condensation reaction catalyzed by carboxylate
[0079]
[0080] Note: n.a. means not applicable to this (not applicable)
[0081] Synthetic example methyl acrylate as raw material:
[0082] The first step: Methyl acrylate and mercaptan undergo Michael 1,4-addition reaction under solvent-free carboxylate catalyzed conditions to obtain beta-sulfide methyl ester. The reaction needs to control the temperature, and it is better to stir for a...
Embodiment 2
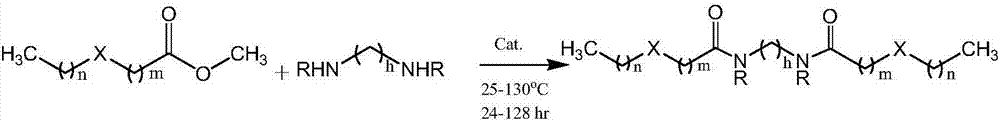
[0087] Reaction equation of methyl 3,3'-thioether dipropionate with monohydric and dihydric organic amines
[0088]
[0089]
[0090] The preparation method of disulfide adipamide:
[0091] Add Beta-12 alkyl (or 8 alkyl) methyl sulfide propionate (1 equivalent) into the reaction flask, then add sodium formate or sodium acetate catalyst, add hexamethylenediamine (0.45-0.49 equivalent) under nitrogen protection , and then heating up, TLC or GC-MS tracking reaction process, until the reaction is complete. Add ethanol aqueous solution or methanol aqueous solution, recrystallize, and cool and filter to obtain a white solid with a yield of 85-99%.
[0092] A. Product di-12 alkyl-beta-disulfide adipamide m.p.: 133-137℃ Product di-12 alkyl beta-sulfide adipamide 1 The HNMR test was completed in NMR instrument Bruker, 400MHz, 1 H NMR in CD 3 OD(δ,ppm):0.87-0.93(m,6H),1.28-1.35(m,40H),1.35-1.46(m,8H),2.47(t,4),2.55(t,4H),2.78( t,4H),3.23(t,4H),3.33(bm,CH 3 OH in CD 3 OD), 4...
Embodiment 3
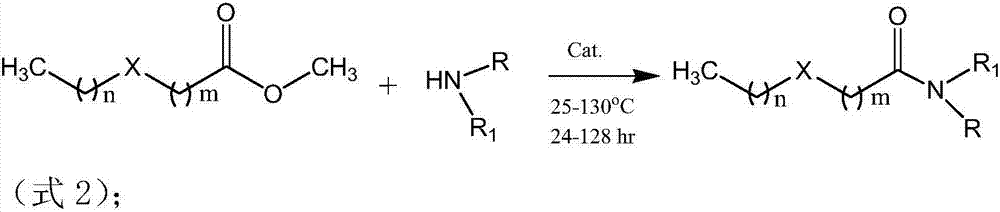
[0097] The reaction equation between dimethyl dicarboxylate and organic monoamine or thioether monoamine:
[0098]
[0099] The synthetic conditions of this embodiment:
[0100]
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com