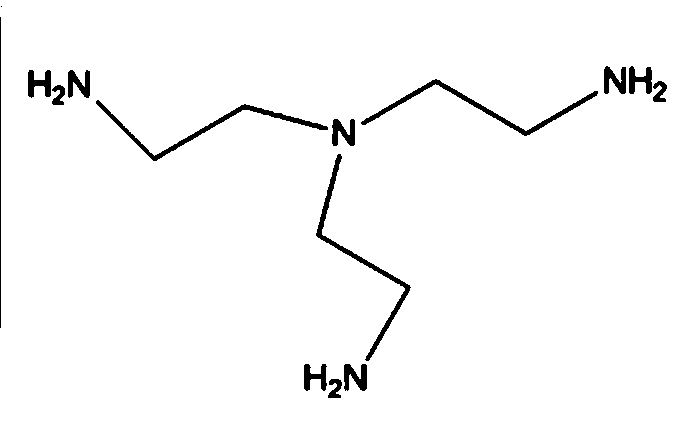
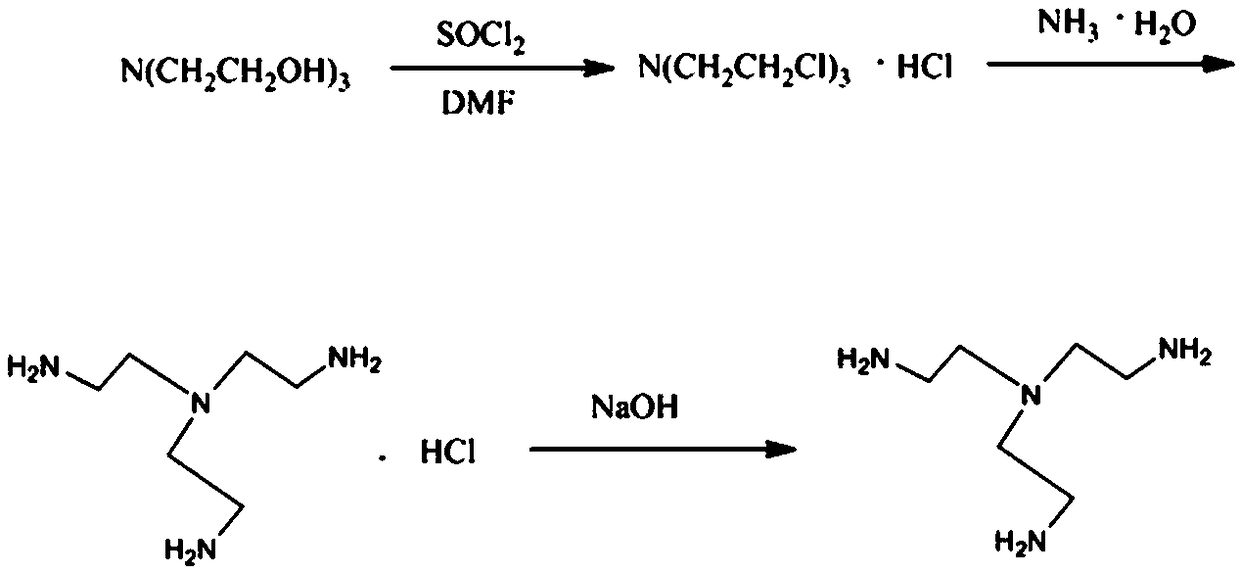
Tris(2-aminoethyl)amine synthesis process
A technology of aminoethyl and synthesis process, which is applied in the field of triamine synthesis process, can solve the problems of harm to human body and environment, cumbersome operation, low yield, etc., and achieve low harm to human body and environment, short process flow and low production cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Take 14.9g of triethanolamine solution and 29.8g of DMF into a 500ml three-necked flask, stir, take 50g of thionyl chloride solution, and slowly add it dropwise to the above reagents, and the dropping speed is controlled at 1 second per drop. At first there was a white solid accompanied by gas generation, but after the dropwise addition, the white solid disappeared and the solution was clear. Stirring was continued for 7 hours. Reflux reaction at 70°C for 6-8 hours to obtain the first intermediate tris(2-chloroethyl)amine hydrochloride. After the reaction, tris(2-chloroethyl)amine hydrochloride produced by rotary evaporation under reduced pressure salt, remove the remaining thionyl chloride solution to obtain tris(2-chloroethyl)amine hydrochloride concentrate.
[0026] Add tris(2-chloroethyl)amine hydrochloride concentrate and 35g ammonia water into a 1000ml three-neck flask, add 100g ethanol to dissolve it, stir, and heat in an oil bath to 70°C, keep the solution refl...
Embodiment 2
[0028] Take 14.9g of triethanolamine solution and 14.9g of DMF into a 1000ml three-necked flask, stir, take 50g of thionyl chloride solution, and slowly add it dropwise to the above reagents, and the dropping speed is controlled at 1 second per drop. At first there was a white solid accompanied by gas generation, but after the dropwise addition, the white solid disappeared and the solution was clear. Stirring was continued for 7 hours. After the reaction was completed, the generated tris(2-chloroethyl)amine hydrochloride was rotary evaporated under reduced pressure, and the remaining thionyl chloride solution was removed to obtain a concentrated solution of tris(2-chloroethyl)amine hydrochloride.
[0029] Add the above concentrate and 70g of ammonia water into a 1000ml three-neck flask, add 100g of ethanol to dissolve it, stir, and heat in an oil bath to 70°C, keep the solution at reflux, and react at reflux for 7 hours. The color of the reaction solution gradually changed fr...
Embodiment 3
[0031] Take 14.9g of triethanolamine solution and 21g of DMF into a 1000ml three-necked flask, stir, take 50g of thionyl chloride solution, and slowly add it dropwise to the above reagent, and the dropping speed is controlled at 1 second per drop. At first there was a white solid accompanied by gas generation, but after the dropwise addition, the white solid disappeared and the solution was clear. Stirring was continued for 7 hours. After the reaction was completed, the generated tris(2-chloroethyl)amine hydrochloride was rotary evaporated under reduced pressure, and the remaining thionyl chloride solution was removed to obtain a concentrated solution of tris(2-chloroethyl)amine hydrochloride.
[0032] Add the above concentrated solution and 140g ammonia water into a 1000ml three-necked flask, add 100g ethanol to dissolve it, stir, and heat in an oil bath to 70°C, keep the solution under reflux, and react under reflux for 7 hours. The color of the reaction solution gradually ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com