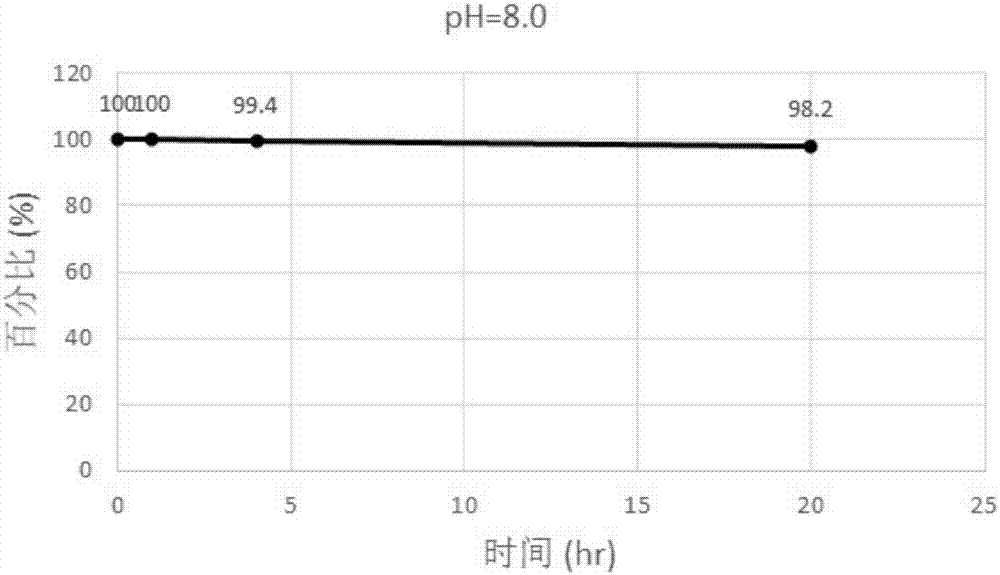
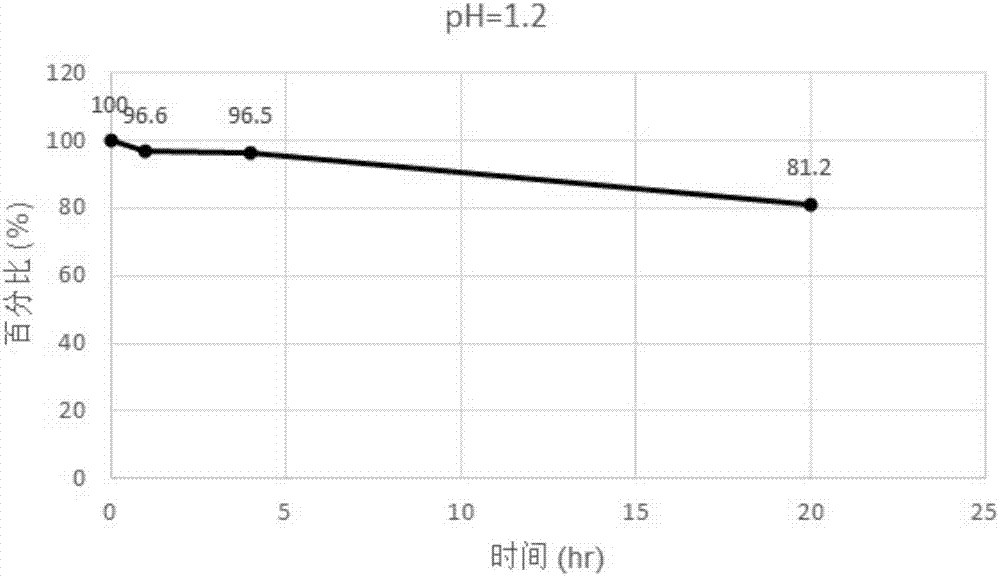
Patents
Literature
918 results about "Tert-Butyloxycarbonyl protecting group" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
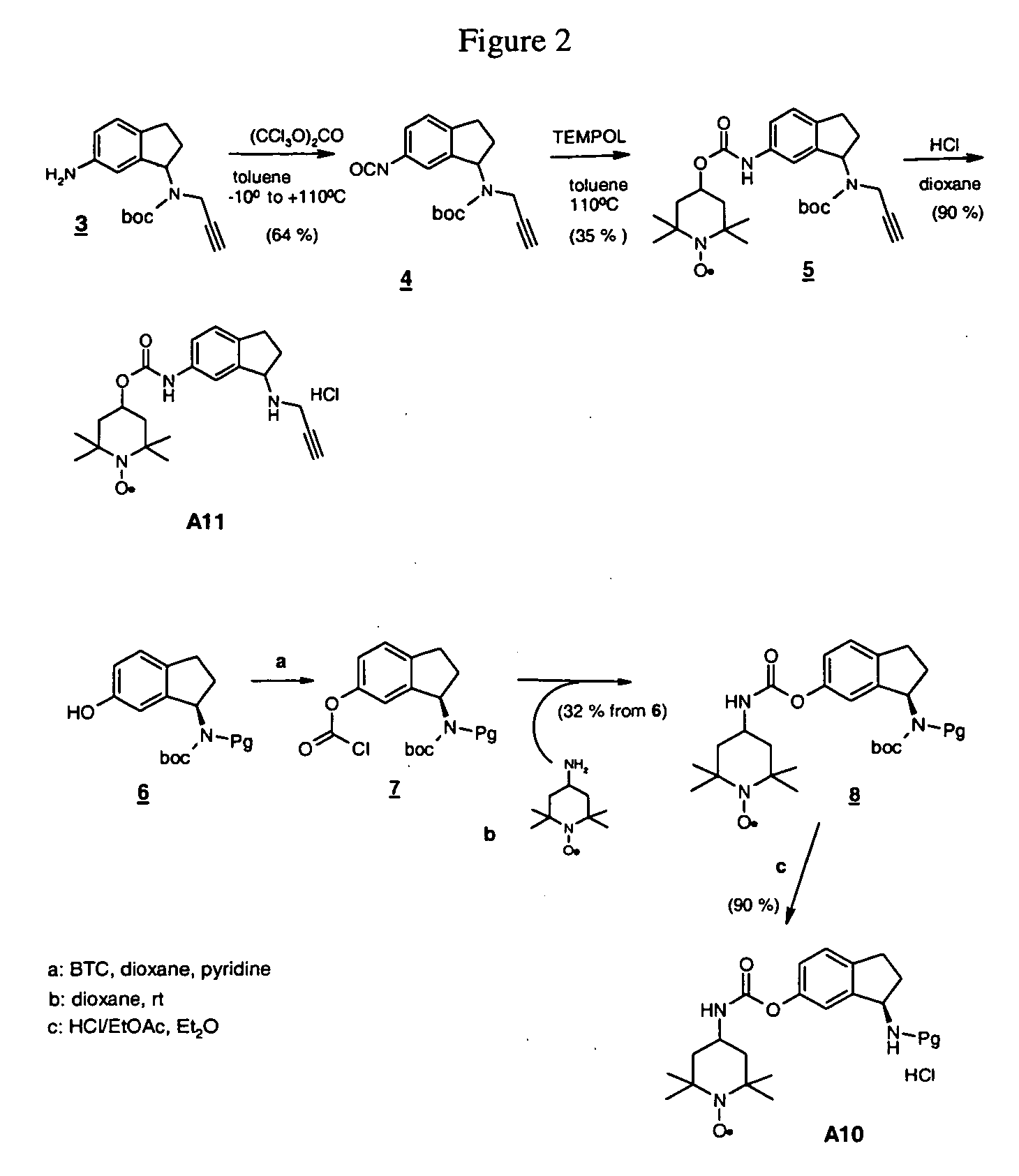
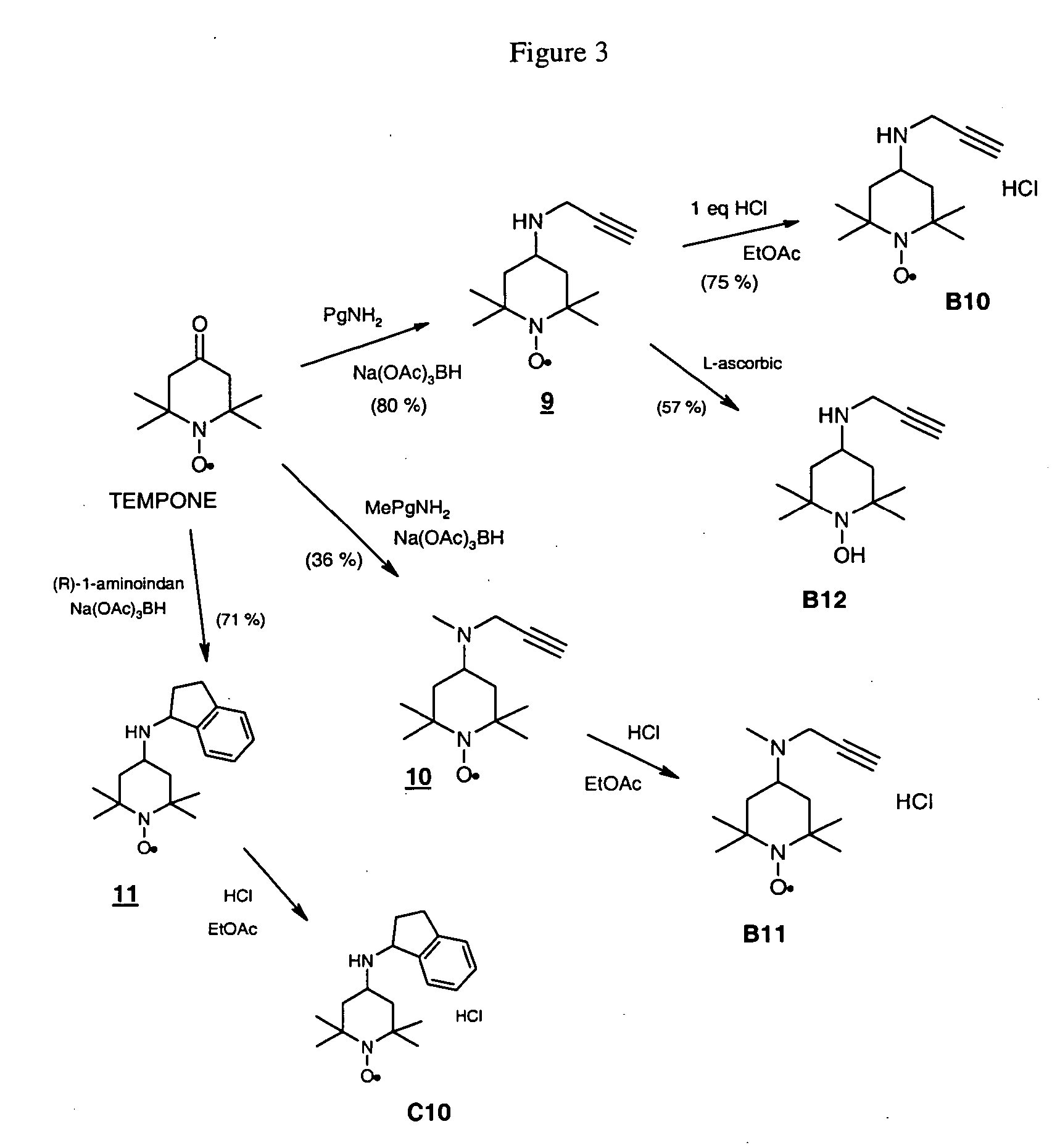
The tert-butyloxycarbonyl protecting group or tert-butoxycarbonyl protecting group (BOC group) is a protecting group used in organic synthesis.
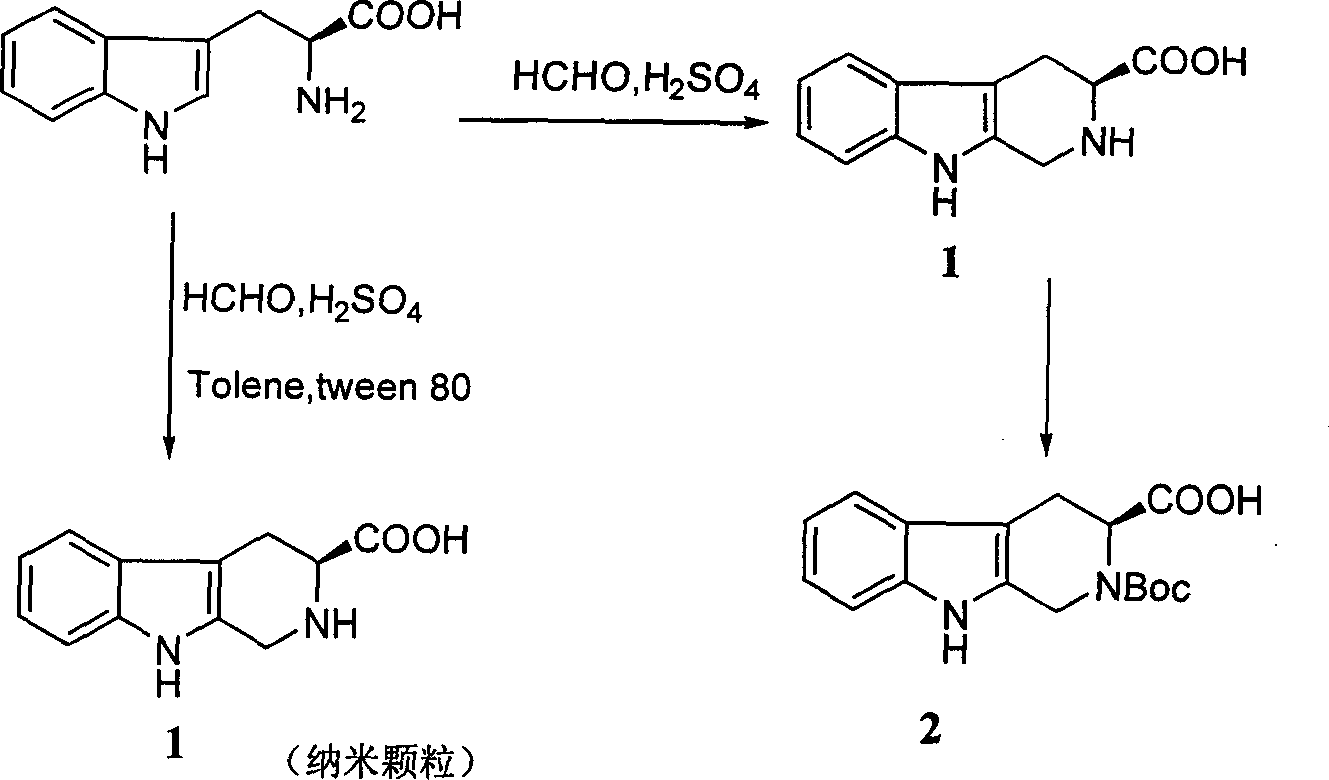
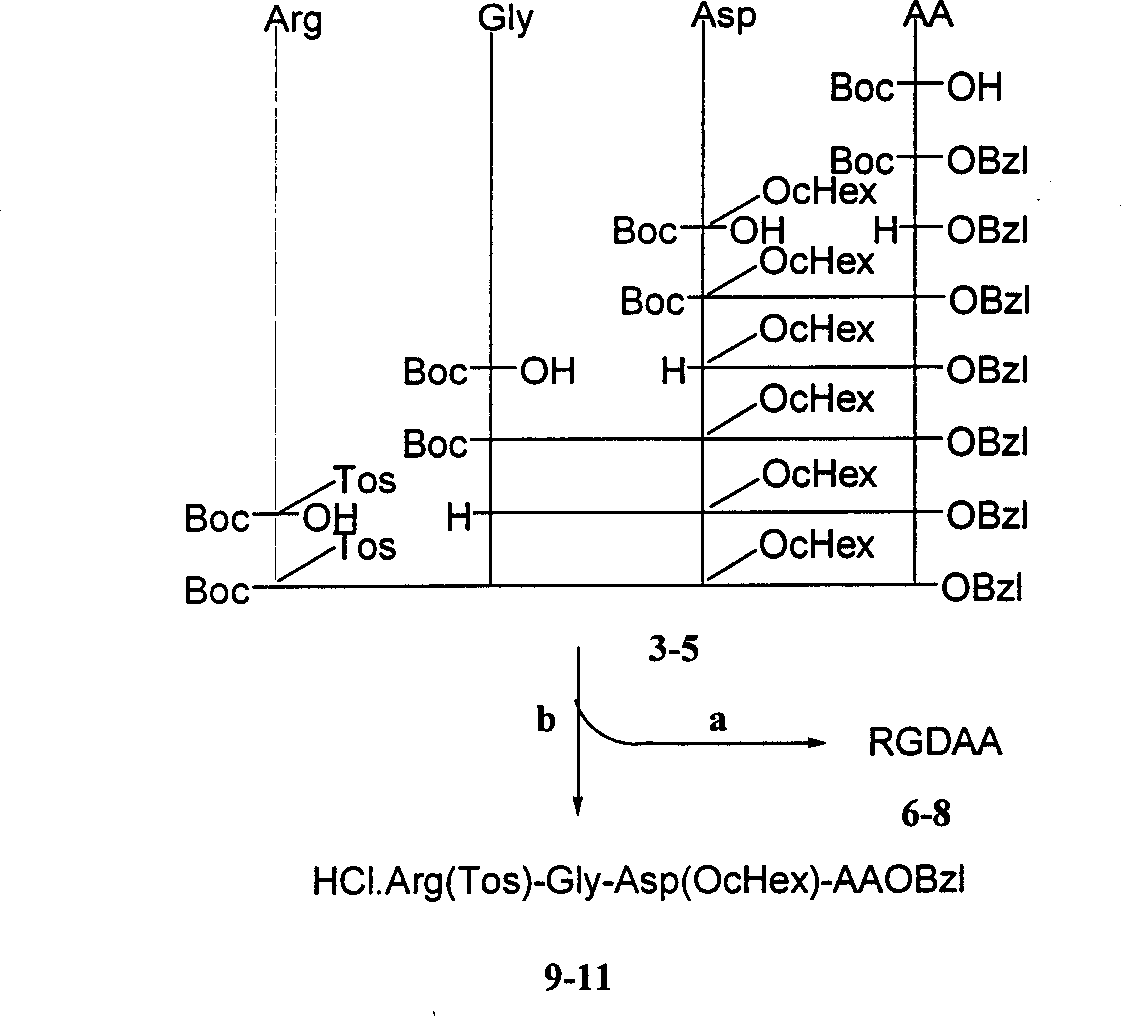
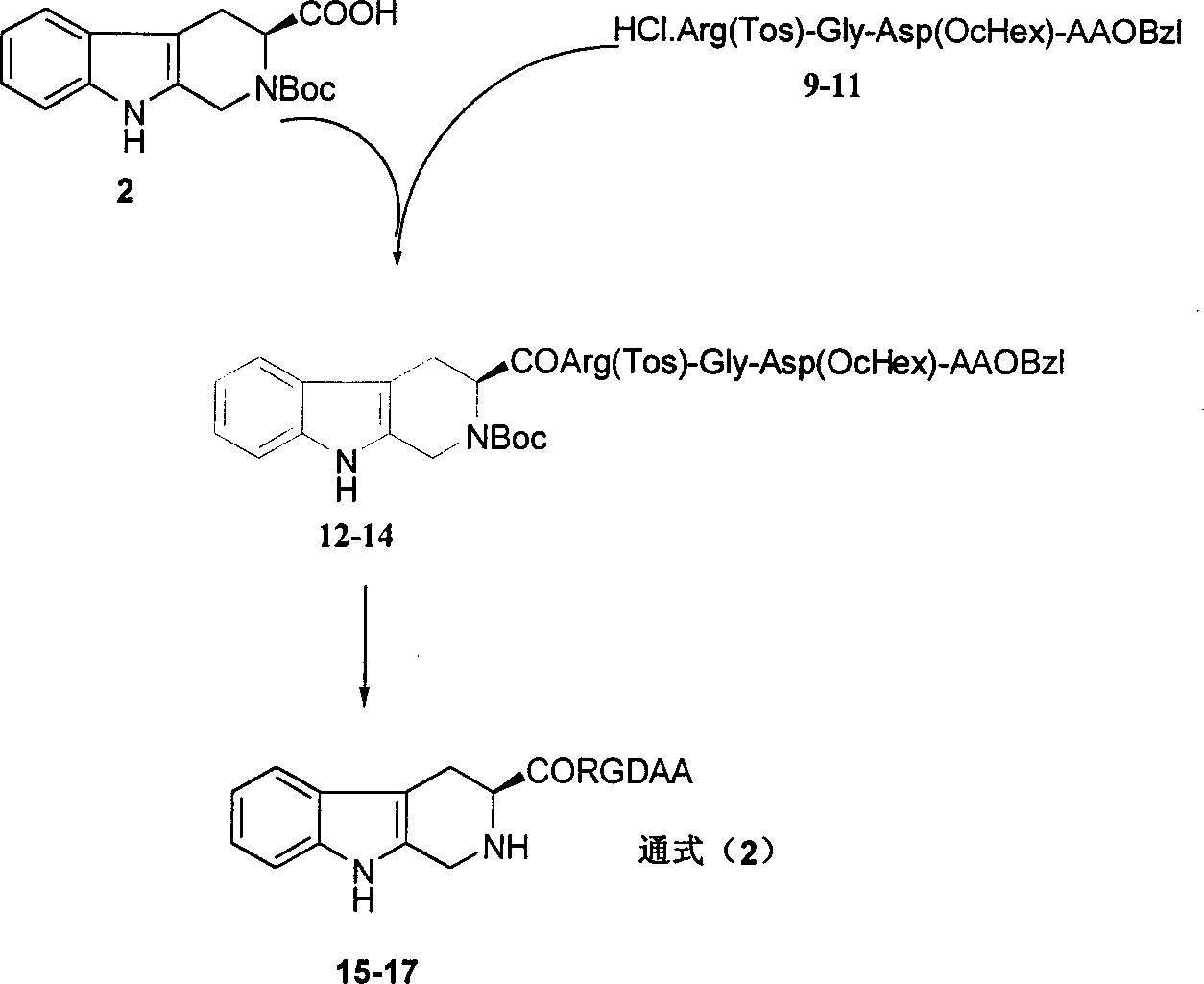
Beta-tetrahydro carboline carboxylic acid, its RGD conjugate, their synthesis and medical application
The present invention relates to the synthesis of beta-tetrahydro carboline carboxylic acid and its nanoparticle preparation; liquid phase process of gradually grafting peptide to obtain protected tetrapeptide intermediate, which, after eliminating its Boc, is condensated with N-Boc-beta-tetrahydro carboline carboxylic acid and further HF treated to eliminate its protection to obtain the said compound; the nanoparticle of tetrahydro carboline carboxylic acid and the in-vitro and in-vivo activity of said compound in-vitro and in-vivo experiments show that the compound of the present invention has excellent activity of resisting blood platelet aggregation, effect of resisting cell adhesion and effect of resisting thrombus.
Owner:ZHEJIANG MEDICINE CO LTD XINCHANG PHAMACEUTICAL FACTORY
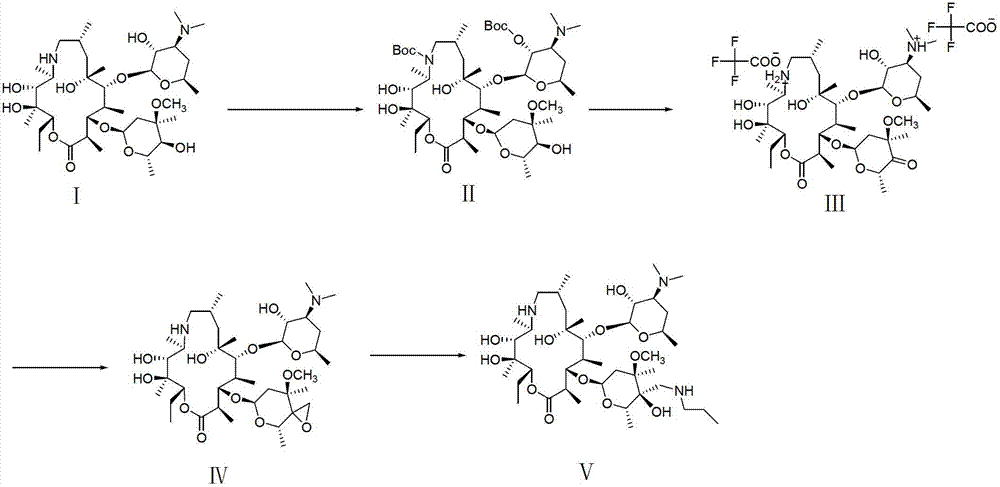
Tulathromycin intermediate and preparation method thereof, as well as preparation method of tulathromycin
ActiveCN102786569AReduce manufacturing costMild conditionsSugar derivativesSugar derivatives preparationEpoxyTert-Butyloxycarbonyl protecting group
The invention provides a tulathromycin intermediate, a preparation method of the tulathromycin intermediate, and a preparation method of the tulathromycin. The preparation method of the tulathromycin has the advantages of mild condition, convenience for operation, and low cost. The preparation method of the tulathromycin comprises the following steps of: using azithromycin A as a raw material; protecting 2'-hydroxy and 6'-amino in the azithromycin A through di-tert-butyl dicarbonate so as to obtain double-protective azithromycin A; carrying out Swern oxidation to 4''-hydroxy to the double-protective azithromycin A; salifying along with trifluoroacetic acid; and synchronously removing boc t-butyloxycarbonyl to obtain the azithromycin A bitrifluoroacetic acid salt of 4''-carbonyl; and then reacting with trimethylsulfonium bromide to obtain 4''-epoxy compound; and finally carrying out nucleophilic addition on the 4''-epoxy compound by n-propylamine so as to obtain the phosphate of tulathromycin; and further neutralizing via alkaline to obtain the target compound tulathromycin; and synchronously obtaining the tulathromycin intermediate of azithromycin A bitrifluoroacetic acid salt of 4''-carbonyl.
Owner:TIANJIN ZHONGSHENG TIAOZHAN BIOTECH
Preparation method of (R)-2-(N-tertbutyloxycarbonylamino)biphenylpropanol
InactiveCN105330569AMild reaction conditionsImprove conversion rateCarbamic acid derivatives preparationOrganic compound preparationAlcoholTert-Butyloxycarbonyl protecting group
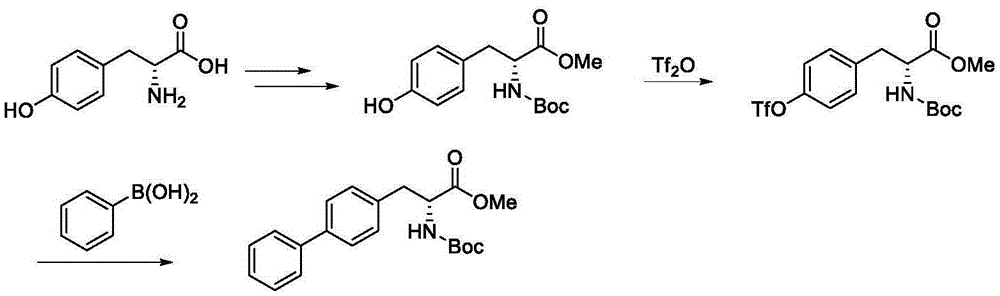
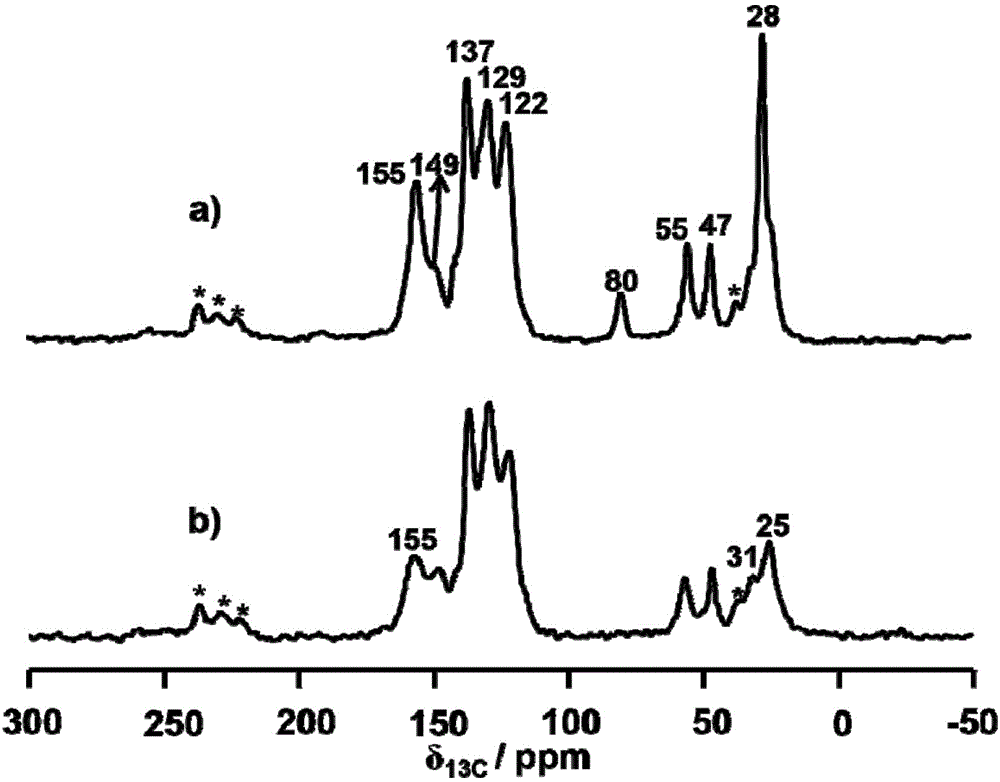
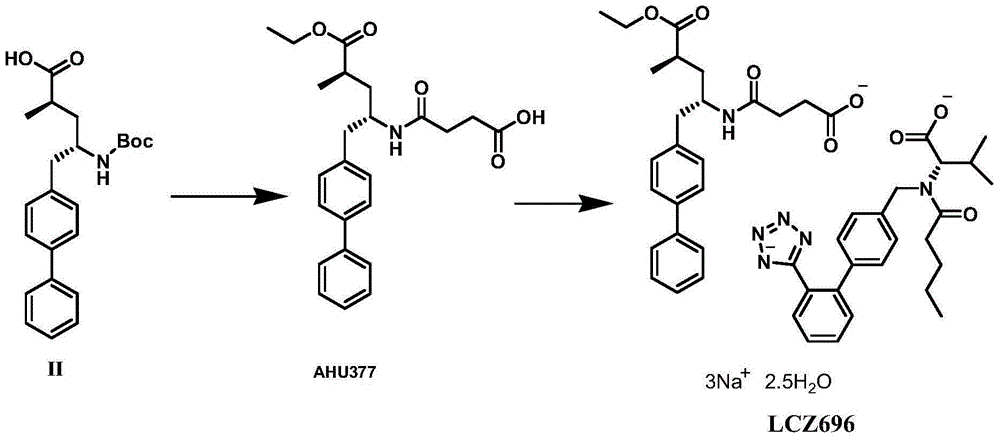
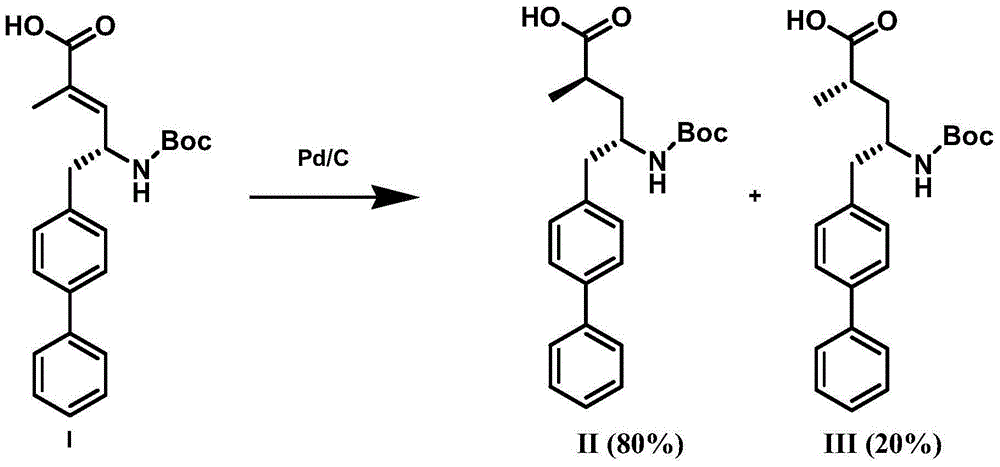
The invention discloses a preparation method of (R)-2-(N-tertbutyloxycarbonylamino)biphenylpropanol represented by formula (I). The method comprises the following steps: carrying out an Erlenmeyer-Plochl cyclization reaction on biphenylcarboxaldehyde and N-acylglycine, hydrolyzing or alcoholyzing, and carrying out asymmetric hydrogenation to obtain (R)-N-acylbiphenylalanine or an ester thereof; and carrying out acid hydrolysis, reduction and amino protection on the (R)-N-acylbiphenylalanine or the ester thereof, or carrying out reduction, acid hydrolysis and amino protection on the (R)-N-acylbiphenylalanine or the ester thereof, or directly reducing the (R)-N-acylbiphenylalanine or the ester thereof in order to obtain the product (R)-2-(N-tertbutyloxycarbonylamino)biphenylpropanol. The product is a key intermediate of Sacubitril (AHU-377) which is one of a novel blood pressure reducing medicine LCZ696. The method has the advantages of easily available raw materials, and suitableness for industrial production.
Owner:天台宜生生化科技有限公司
Oligoaniline compound
ActiveCN101679206AImprove conductivityImprove featuresOrganic chemistrySolid-state devicesPhosphoric Acid EstersCarboxyl radical
Any of the oligoaniline compounds with a triphenylamine structure represented by the formula (1) exhibits satisfactory light emitting efficiency and brightness performance when used in either an OLEDdevice or a PLED device, and is further satisfactory in the solubility in organic solvents so as to be applicable to various coating methods. Each of R<1> and R<2> independently is a hydrogen atom, anoptionally substituted monovalent hydrocarbon group, t-butoxycarbonyl, etc.; each of R<3> to R<34> independently is a hydrogen atom, hydroxyl, silanol, thiol, carboxyl, a phosphoric group, a phosphoric ester group, ester, thioester, amido, nitro, an optionally substituted monovalent hydrocarbon group, etc.; and each of m and n is an integer of 1 or greater provided that they satisfy the relationship m+n=20.
Owner:NISSAN CHEM CORP
Method for synthesizing trans-(1R,2S)-2-(3,4-difluorophenyl)cyclopropylamine
InactiveCN102249929AReduce difficulty of reactionHigh reaction yieldOrganic compound preparationAmino compound preparationBiochemical engineeringTert-Butyloxycarbonyl protecting group
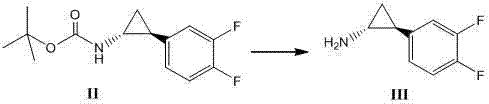
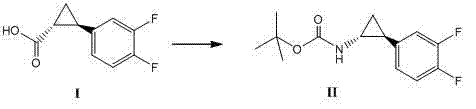
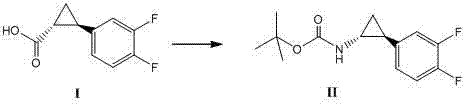
The invention relates to a method for synthesizing trans-(1R,2S)-2-(3,4-difluorophenyl)cyclopropylamine which is an intermediate for preparing an anticoagulation medicine Ticagrelor. The method provided by the invention mainly comprises the following steps of: synthesizing the trans-(1R,2S)-2-(3,4-difluorophenyl)cyclopropylamine protected by tertiarybutoxy carbonyl through carrying out a rearrangement reaction of DPPA (Diphenylphosphoryl Azide); then removing the protective group of the trans-(1R,2S)-2-(3,4-difluorophenyl)cyclopropylamine protected by the tertiarybutoxy carbonyl and then alkalifying to obtain the product. The whole reaction can be finished through a one-pot boiling synthetic method so that synthesizing steps and synthesizing time are greatly saved, the cost is effectively reduced and the yield is improved; and the method for synthesizing the trans-(1R,2S)-2-(3,4-difluorophenyl)cyclopropylamine has the very active meaning in the industrial production.
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD
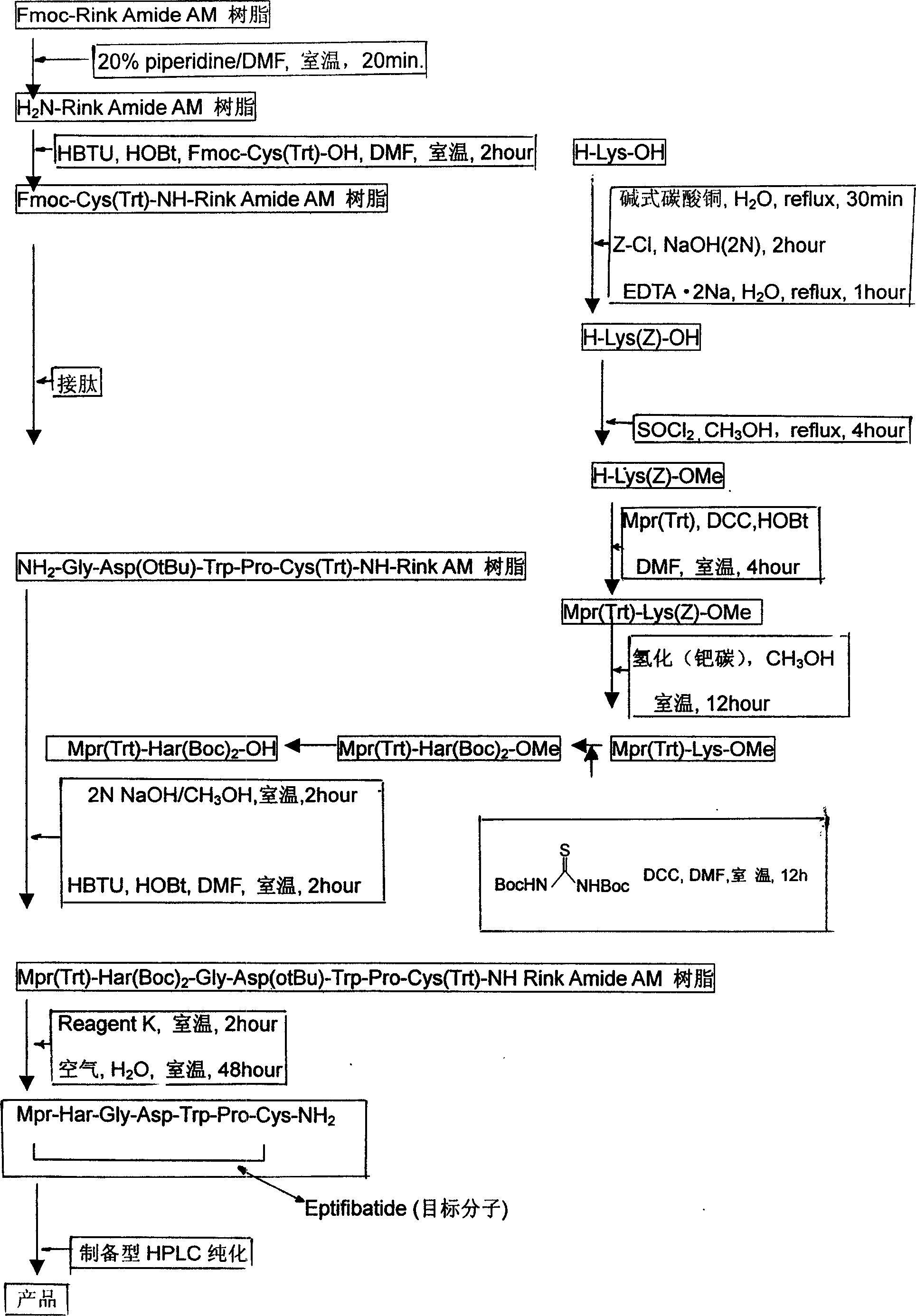
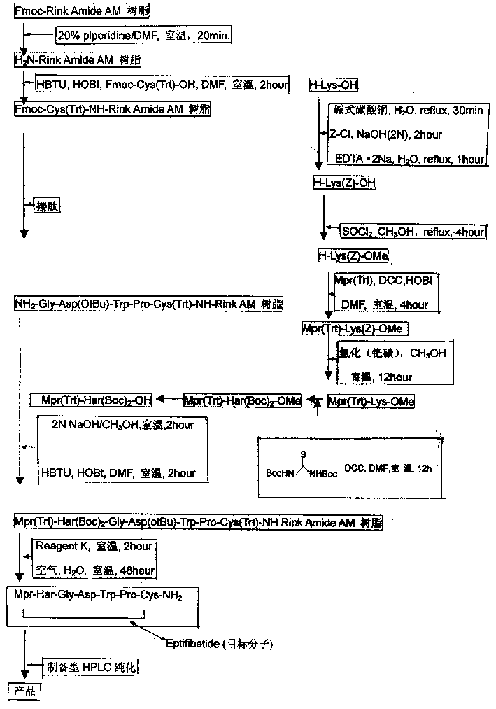
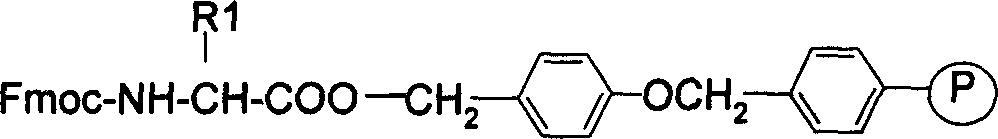
Preparing process for Eptifibatide
InactiveCN1500805AEasy to trackPromote safe productionPeptidesBlood disorderCyclic peptideTert-Butyloxycarbonyl protecting group
The present invention relates to the preparation of cyclic peptide, and is especially new Fmoc-solid phase process of preparing Eptifibatide. The new process is superior to liquid phase process, which has long synthesis period, and BOC solid phase process, which uses virulent and corrosive material. The technological scheme is that the Eptifibatide preparing process includes the following steps: eliminating Fmoc protection of Fmoc-Rink Amide AM resin to obtain H2N-Rink Amide AM resin; connecting various protective amino acids successively to obtain corresponding resin; eliminating Fmoc-protection radical and Kaiser test to detect reaction procedure; preparing S-triphenyl mercapto propionyl-N, N-ditert butyl oxycarbonyl-homoarginine with lysine; grafting S-triphenyl mercapto propionyl-N, N-ditert butyl oxycarbonyl-homoarginine; eliminating side chain protecting radical and resin to reduce into coarse product; and cyclization, oxidation, HPLC tracking purification to obtain pure product.
Owner:GL BIOCHEM SHANGHAI +1
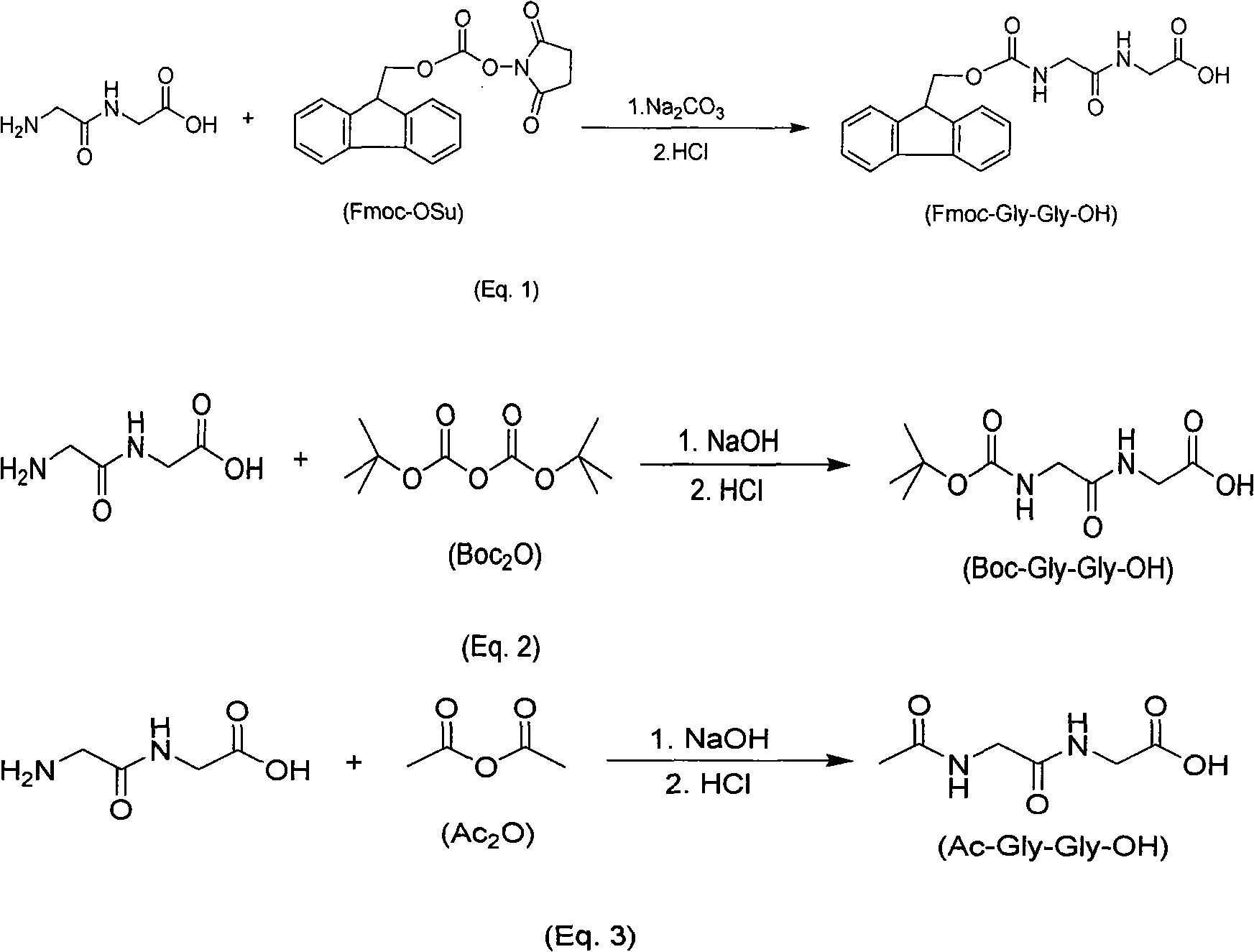
Synthesis method of amino-protecting glycine dipeptidase derivant
The invention relates to a synthesis method of amino-protecting glycine dipeptidase derivant. The amino-protecting glycine dipeptidase derivant is N-9-Fmoc glycine dipeptidase, N-Boc glycine dipeptidase and N-acetyl glycine dipeptidase. The invention has the technology that glycine dipeptidase is dissolved in 1-20% of inorganic aqueous alkali, and the organic solution of Fmoc-OSu or Boc2O or Ac2Ois dropwise added; reaction is finished within 1-12 hours, the yield of the product Fmoc-Gly-Gly-OH, Boc-Gly-Gly-OH and Ac-Gly-Gly-OH is between 80-95%, and the content of the product is above 99%. The invention has simple technology, easy industrialization and low cost. The product of the invention is the important raw material for synthesizing polypeptide compound.
Owner:上海力智生化科技有限公司
Method for preparing ceritinib
ActiveCN104356112AReduce pollutionReduce manufacturing costOrganic chemistryBulk chemical productionTert-Butyloxycarbonyl protecting groupBromine
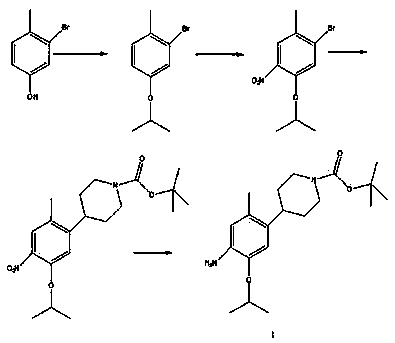
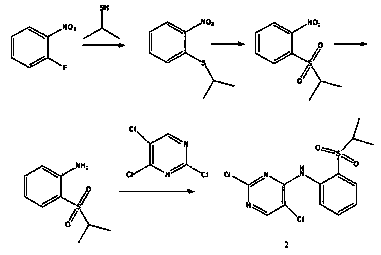
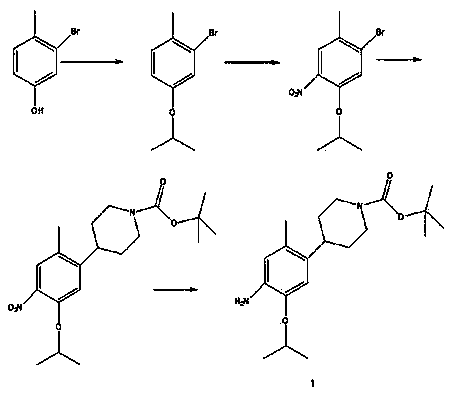
The invention discloses a method for preparing ceritinib, belonging to the field of chemical pharmacy. The method comprises the following steps: by taking 3-bromine-4-methylphenol as an initial raw material, performing phenolic hydroxyl isopropylation, nitration, coupling and reduction reaction, obtaining a midbody 1, by further taking o-nitro fluorobenzene as another initial raw material, performing isosulfhydrylation, oxidation, reduction and pyrimidine, obtaining a midbody 2, performing coupling reduction on the midbody 1 and the midbody 1, obtaining ceritinib which is protected by BOC acid anhydride, and finally removing a t-butyloxycarboryl protecting group, and obtaining ceritinib. The method is simple and feasible to operate, relatively high in yield, small in pollution and applicable to industrial production.
Owner:安庆奇创药业有限公司
Prolinol derivative induced chiral MOFs material with asymmetric catalysis
ActiveCN101830920AEasy to makeSave raw materialsGroup 4/14 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsTert-Butyloxycarbonyl protecting groupProper treatment
The invention discloses a prolinol derivative induced chiral MOFs material with asymmetric catalysis, which belongs to the technical field of a chiral catalytic material. L-BCIP or D-BCIP is used as a chiral source; 5,5'-methylene diiso-phthalic acid, 4,4'-biphenyl acid, 3,3',4,4'-biphenyltetrazole acid or 4,4'-sulfonyl terephthalic acid is used as a connecting ligand; Ln3+ is used as a node; a three-dimensional hole channel structure is constructed by a hydrothermal method; the general formula is as follows: Ln<3+>+L+L-BCIP or D BCIP->Ln-L, wherein Ln<3+> is a rare earth metal ion; L is a connecting ligand; L-BCIP is L-N-tert-butoxycarbonyl-2-imidazole-1-pyrrolidine; and D-BCIP is D-N tert-butoxycarbonyl-2-imidazole-1-pyrrolidine. The material can be used as a heterogeneous catalyst used for an asymmetric silicon cyanation reaction; therefore, the catalyst can be recycled with a yield of 100% and ee value of 99%; and the chiral MOFs material has good application prospect in the aspects of synthesis of pure enantiomer compounds, synthesis of medical intermediates and the like. The material can be recycled after proper treatment.
Owner:DALIAN UNIV OF TECH
Method for catalytically synthesizing atazanavir intermediate
ActiveCN104911224AImprove recycling ratesImprove regeneration efficiencyChemical industryFermentationTert-Butyloxycarbonyl protecting groupButanone
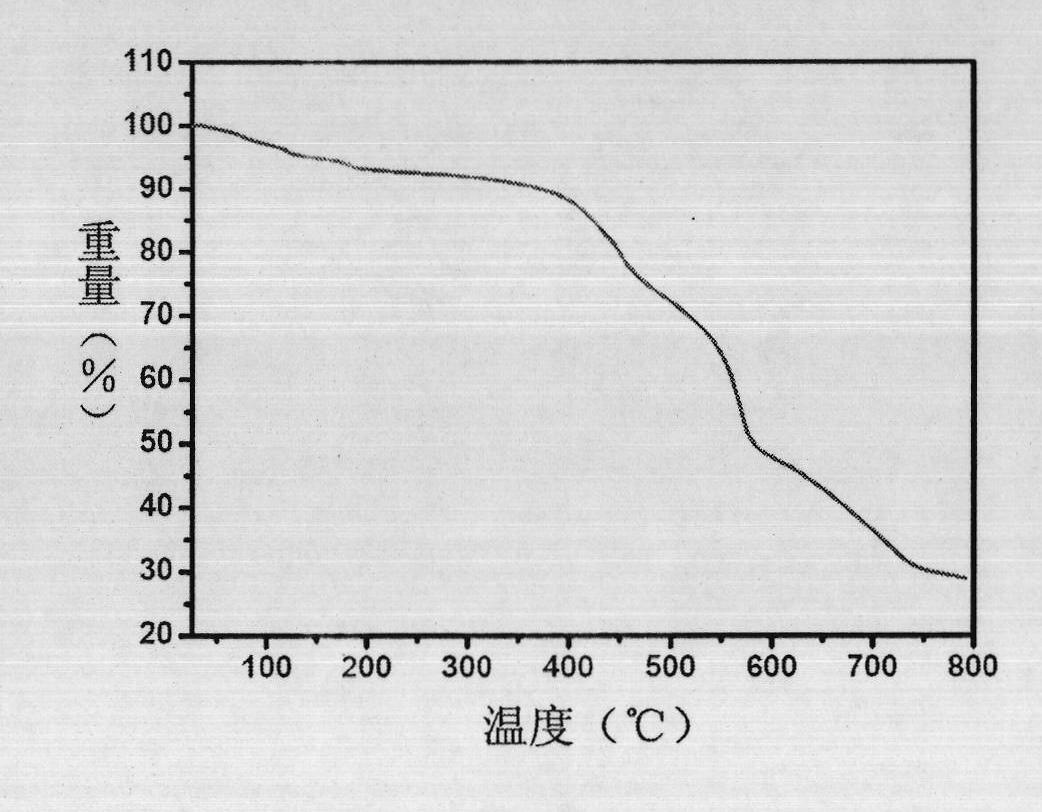
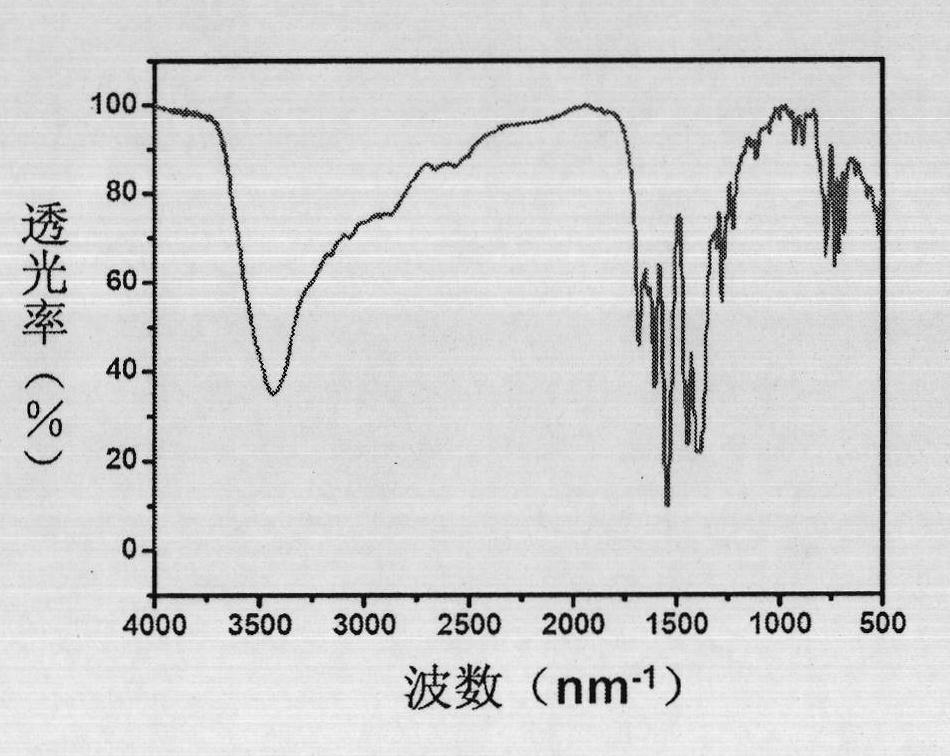
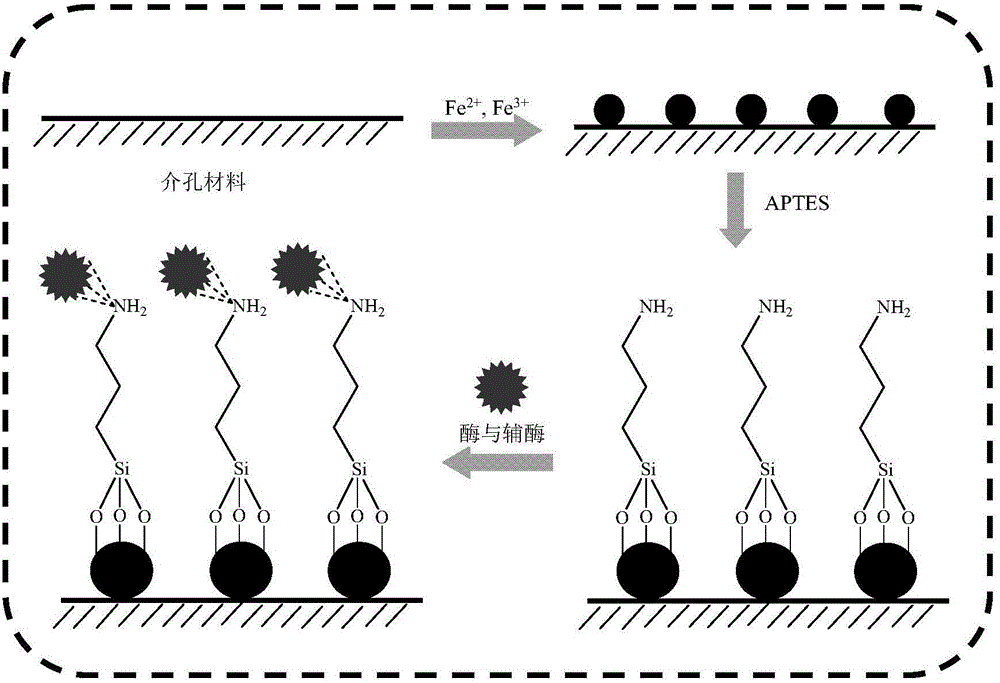
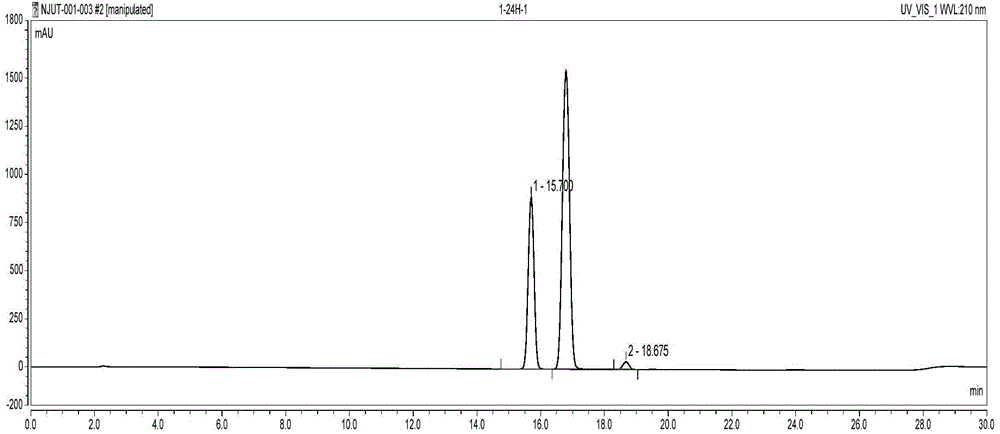
The invention discloses a method for catalytically synthesizing an atazanavir intermediate, which comprises the following steps: preparing a magnetic modified mesoporous material, co-immobilizing a carbonyl reduction enzyme and a coenzyme in the magnetic mesoporous material, and catalyzing (3S)-3-(tert-butyloxycarbonyl)amino-1-chloro-4-phenyl-(2R)-butanone by using the immobilized enzyme to generate the (3S)-3-(tert-butyloxycarbonyl)amino-1-chloro-4-phenyl-(2R)-butanol intermediate. The method has the advantages of high recovery rate of the carbonyl reduction enzyme and coenzyme, high regeneration efficiency of the coenzyme, low cost, wide market prospects and the like.
Owner:NANJING UNIV OF TECH
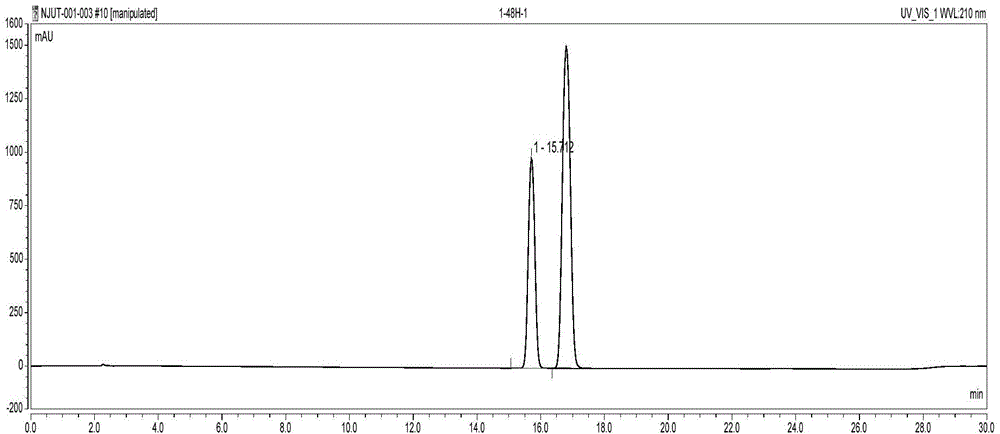
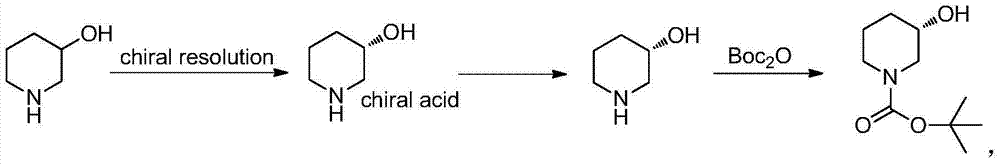
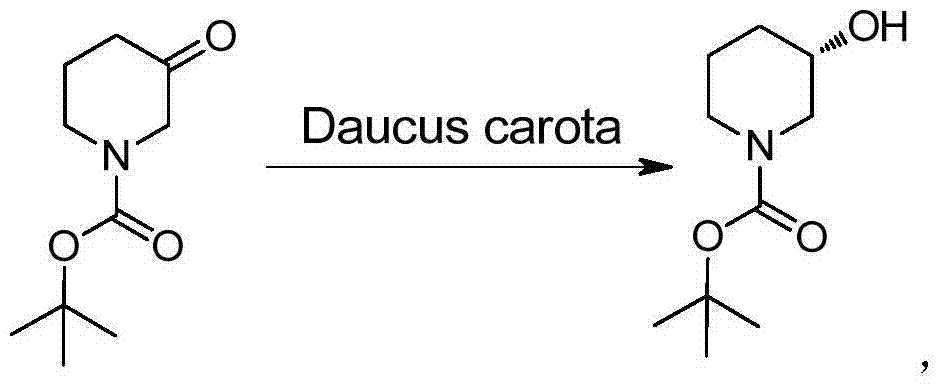
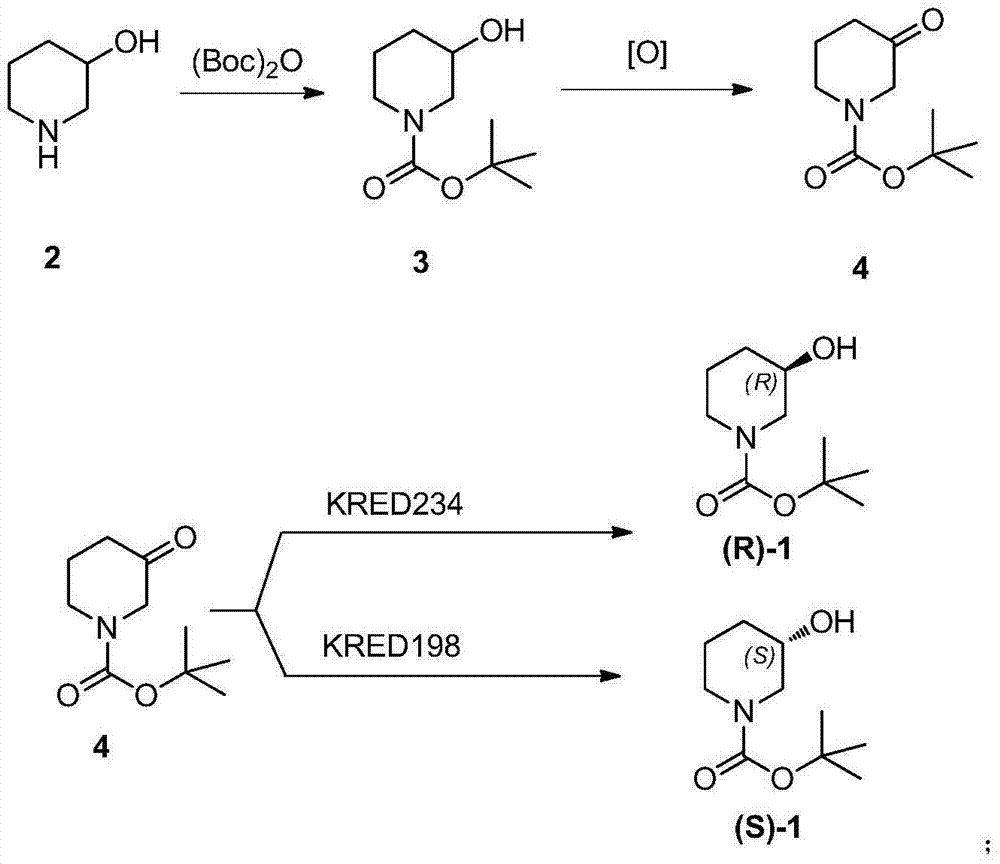
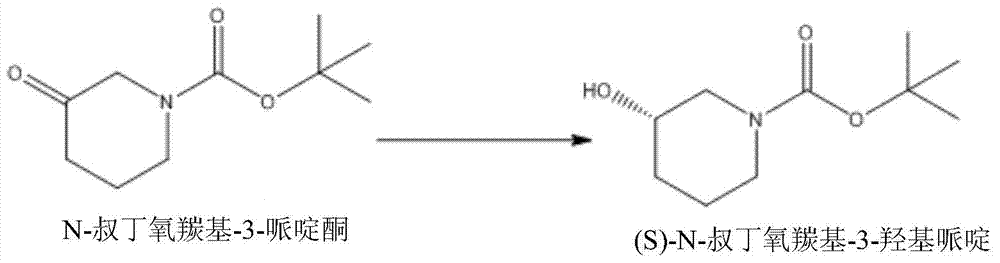
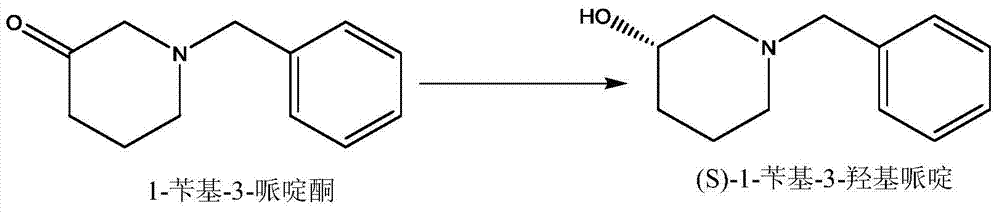
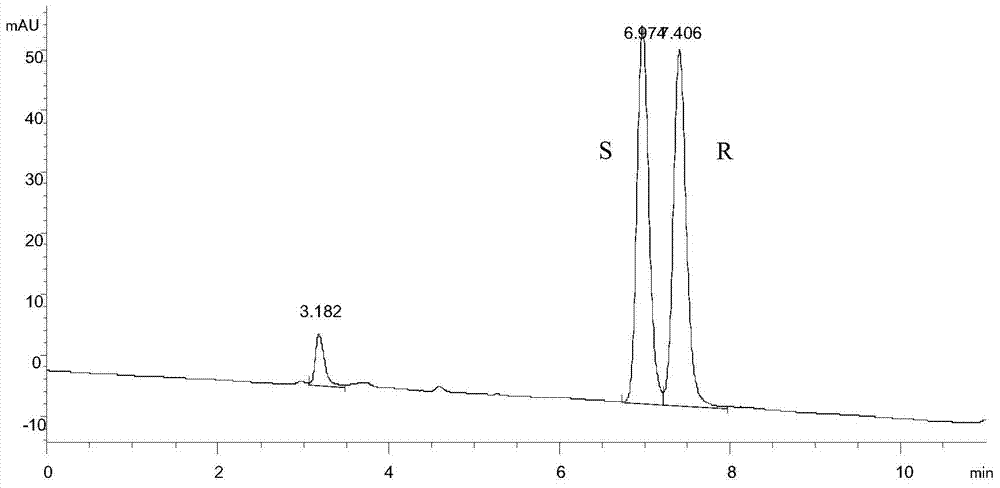
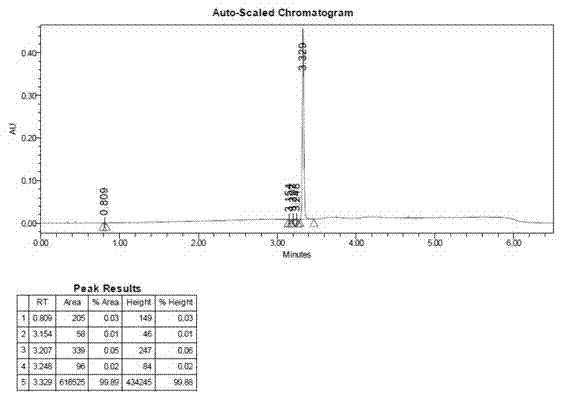
Method for preparing chiral N-tert-butyloxycarboryl-3-hydroxypiperidine
InactiveCN103571908ARaw materials are cheap and easy to getMild reaction conditionsFermentationTert-Butyloxycarbonyl protecting groupPyridine
The invention discloses a method for preparing chiral N-tert-butyloxycarboryl-3-hydroxypiperidine. The method comprises the following steps: a) enabling racemic 3-hydroxypiperidine and di-tert-butyl dicarbonate ester to react to generate racemic N-tert-butyloxycarboryl-3-hydroxypiperidine; b) oxidizing the racemic N-tert-butyloxycarboryl-3-hydroxypiperidine into racemic N-tert-butyloxycarboryl-3-piperidone under the action of an oxidizing agent; c) reducing the racemic N-tert-butyloxycarboryl-3-piperidone by using a ketoreductase into the chiral N-tert-butyloxycarboryl-3-hydroxypiperidine. Compared with the prior art, the method provided by the invention can be used to prepare the chiral N-tert-butyloxycarboryl-3-hydroxypiperidine with the optical purity of 99% by using the racemic 3-hydroxypiperidine as a raw material, in addition, the material is cheap and easy to get, the reaction condition is mild, the operation is simple and feasible, the production cost is greatly lowered, and great industrial application value is achieved.
Owner:SYNCOZYMES SHANGHAI
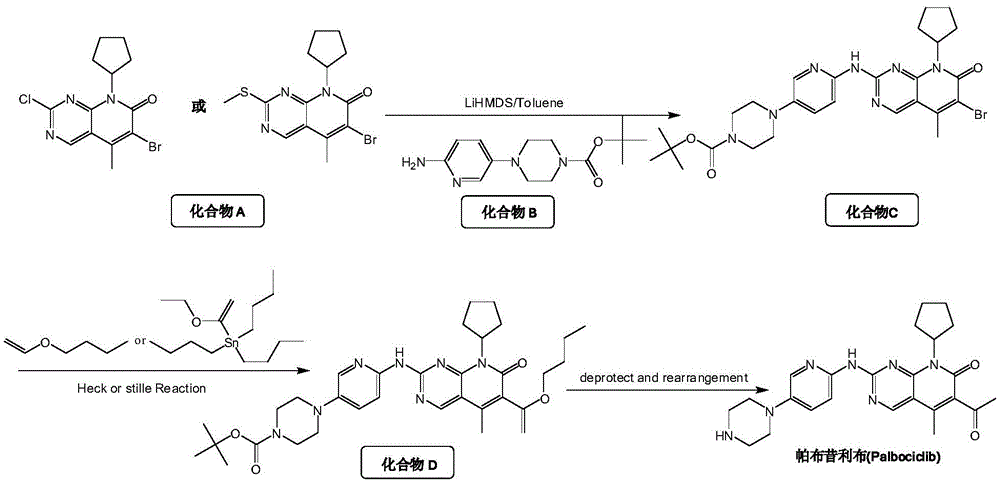
Method for preparing high-purity palbociclib and reaction intermediate of palbociclib
InactiveCN105418603AImprove solubilityReduce the difficulty of purificationOrganic chemistryBulk chemical productionTert-Butyloxycarbonyl protecting groupReaction intermediate
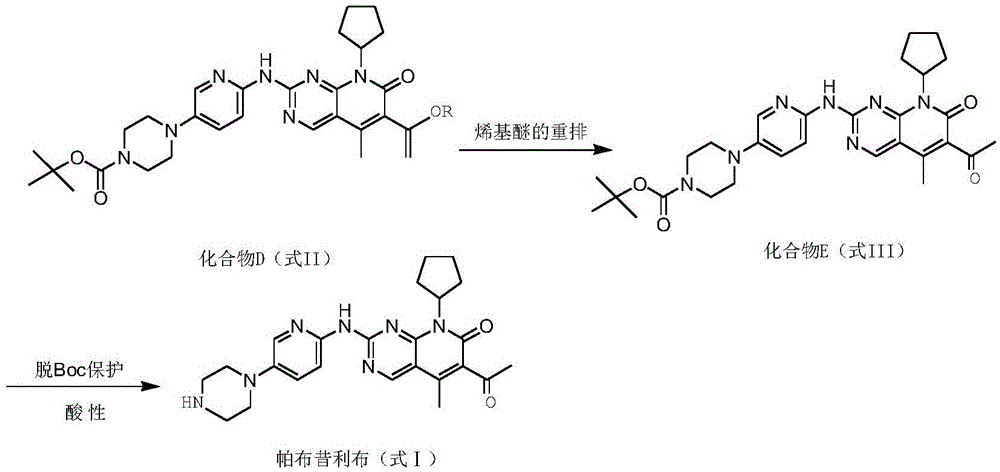
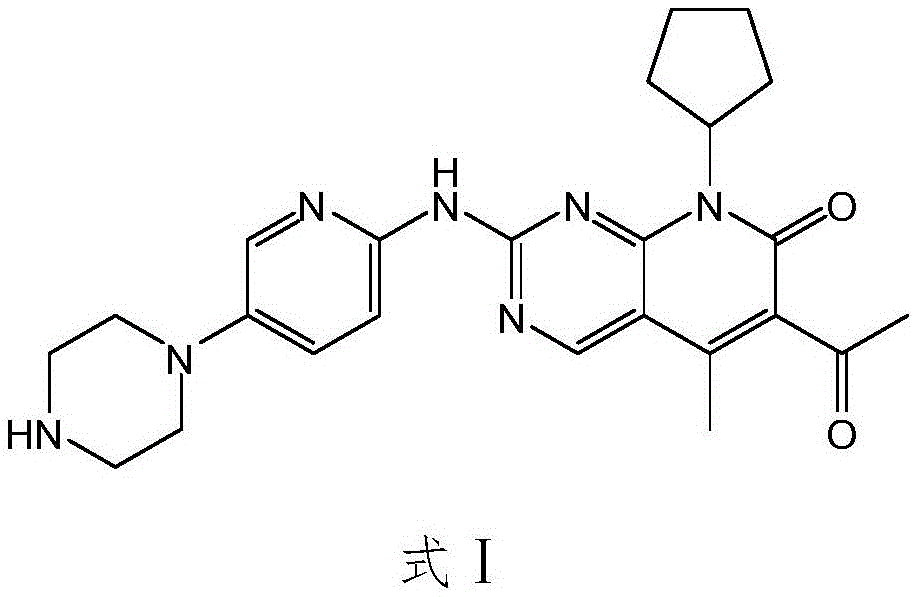
The invention relates to a method for preparing compound palbociclib (shown as the formula I). A compound D (shown as the formula II) serves as a raw material, and a compound E (shown as the formula III) is obtained through a rearrangement reaction of alkenyl ether; then the compound E is subjected to t-butyloxycarboryl protection group removal under the acidic condition, and target product palbociclib is obtained; an R group in a compound D is selected from any one of a C1-C6 alkyl group, a C1-C6 halogenate alkyl group, C1-C6 hydroxyalkyl and a C1-C6 naphthenic base. The process procedure and operation steps are easy and convenient, prepared palbociclib is high in purity, and good popularization prospects are achieved.
Owner:重庆莱美隆宇药业有限公司
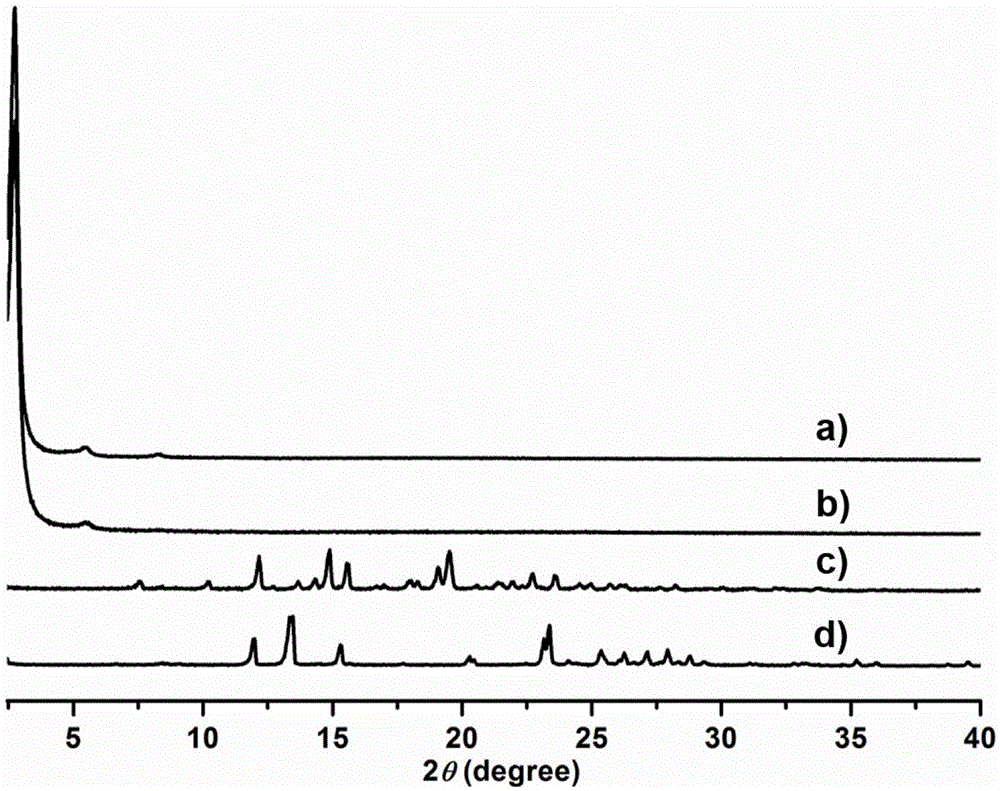
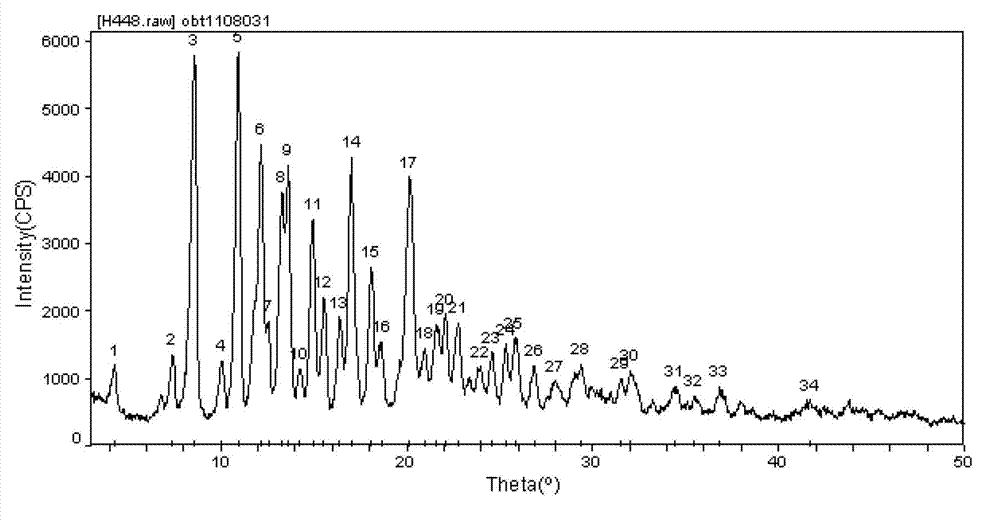
Cabazitaxel crystal and preparation method thereof
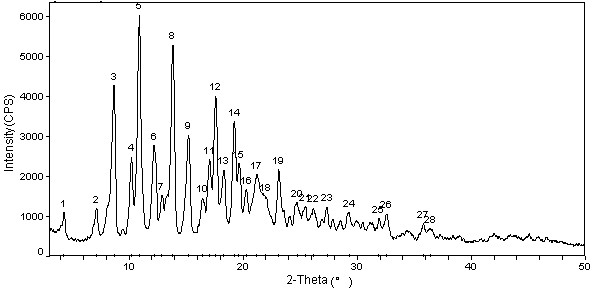
The invention relates to a cabazitaxel crystal which is solvent-free and non-crystallization water crystal form of 7, 10-dimethoxy docetaxel or (2R, 3S)-3-tert-butoxycarbonyl amino-2-hydroxyl-3- phenylpropionic acid 4-acetoxyl-2 alpha-benzoyloxy-5 beta, 20-epoxy-1-hydroxyl-7 beta, 10 beta- dimethoxy-9-oxo-11-taxadiene-13 alpha-ester; and the cabazitaxel crystal is shown to be positioned at 4.3, 7.1, 8.7, 10.2, 10.9, 12.2, 13.8, 15.2, 16.4, 17.0, 17.6, 18.3, 19.2, 19.6, 20.3, 21.2, 23.1, 24.7, 26.1, 27.3, 29.3, 31.9, 32.5 and 35.8 degrees 2 theta characteristic peak by powder X ray diffraction (PXRD). The invention also discloses a preparation method of the cabazitaxel crystal. The cabazitaxel crystal is prepared under the condition of reduced pressure at the room temperature, and is high in yield and good in purity.
Owner:SHANGHAI JINHE BIO TECH
New synthesis technology of anti-cancer drug Raltitrexed
InactiveCN102127063ALow costOptimize the synthetic routeOrganic chemistryAntineoplastic agentsBenzoic acidTert-Butyloxycarbonyl protecting group
The invention relates to a new synthesis technology of anti-cancer drug Raltitrexed. The technology comprises the following steps: 1) using L-glutamic acid as raw material to perform esterification with alcohol under the action of halogenating agent and obtain L-glutamic acid diester hydrochloride; 2) using 2-amino-5-methyl-benzoic acid as raw material to prepare 6-bromomethyl-3,4-dihydro-2-methyl-4-oxo-6-quinazoline through cyclization, amination and bromination; 3) using 2-thienyl-propanedioic acid as raw material to prepare N-[5-[N-(tert-butoxycarbonyl)-N-methylamino]-2-thenoyl]-L-glutamic acid diethyl ester through nitrification, esterification, reduction, amino protection, N-methylation and device-esterification; 4) using L-glutamic acid diester hydrochloride and N-[5-[N-(tert-butoxycarbonyl)-N-methylamino]-2-thenoyl]-L-glutamic acid diethyl ester to prepare N-[5-(N-methylamino)-2-thenoyl]-L-glutamic acid diester through dehydrant condensation and deamination protection; and 5) using N-[5-(N-methylamino)-2-thenoyl]-L-glutamic acid diester and 6-bromomethyl-3,4-dihydro-2-methyl-4-oxo-6-quinazoline to perform condensation under the catalysis of alkali, recycling preparative chromatography, purifying, and performing de-esterification to obtain Raltitrexed.
Owner:深圳市普迈达科技有限公司
Chiral preparation method for N-t-butyloxycarboryl-(4S)-(p-phenyl phenyl methyl)-4-amino-(2R)-methylbutyric acid
ActiveCN105152980AAvoid cost reductionSimple reaction conditionsCarbamic acid derivatives preparationOrganic compound preparationTert-Butyloxycarbonyl protecting groupReaction conditions
The invention relates to a chiral preparation method for N-t-butyloxycarboryl-(4S)-(p-phenyl phenyl methyl)-4-amino-(2R)-methylbutyric acid. The chiral preparation method comprises the following step: enabling a compound (1) and hydrogen to react in the presence of Pd / C and (S-phenylethylamine), thereby preparing a compound (2-a) as shown in the specification. The chiral preparation method is simple in reaction condition, easy to operate, easy in obtaining reagents, and applicable to industrial production, the cost can be lowered as an expensive chiral catalyst is not used, and both the reaction efficiency is improved and the product purity is ensured.
Owner:ZHEJIANG YONGNING PHARMA
Curcumin micellar drug carrying system and preparation method thereof
InactiveCN104758255ABig spaceGood compatibilityPowder deliveryKetone active ingredientsTert-Butyloxycarbonyl protecting groupEnd-group
The invention relates to a novel micellar drug carrying system formed by an amphiphilic block copolymer and curcumin. The amphiphilic block copolymer comprises a hydrophilic chain segment and a hydrophobic chain segment, the hydrophilic chain segment is methoxypolyethylene glycols, the hydrophobic chain segment is polycaprolactone, and the hydrophobic chain segment end group is terminated with a hydrophobic group. The hydrophobic group is a group having a t-butyloxycarboryl or benzene ring structure, compatibility of drug molecules with the hydrophobic chain segment of the block copolymer is improved, the mutual force is increased, and besides, a greater space is provided for accommodating the drug molecules. Prepared micelles can more effectively limit the drug molecules in micelle cores so as to allow the drug molecules to be not easily dissolved out, and thus the drug carrying micelles having high stability are obtained.
Owner:CHANGZHOU TARGET MEDICINE TECH CO LTD
Target mitochondrion antioxidant as well as preparation method and application of target mitochondrion antioxidant
The invention discloses a compound. The compound is a compound shown as a formula I or a stereoisomer, a geometric isomer, a tautomer, nitrogen oxide, hydrate, a solvate, a metabolite and a pharmaceutically acceptable salt of the compound shown as the formula I, wherein Cbz represents benzyloxycarbonyl; Bn represents benzyl; Boc represents tert-butoxycarbonyl. The compound has stable physiochemical properties; compared with XJB-5-131, the compound has the advantages of improved water solubility, stable physiochemical properties, good bioactivity, small medicinal dosage, improved in-vivo stability, high bioavailability, small side effect and prominent target anti-oxidization property; the compound can be used for effectively repairing oxidization injuries of mitochondria, and preventing or treating neurodegenerative diseases including age-related macular degeneration and the like. The formula I is shown in the description.
Owner:XW LAB INC
Star-shaped cationic polymer containing dendriform polylysine element and preparation method thereof
ActiveCN102604114AGood biocompatibilityLow toxicityOther foreign material introduction processesMacromolecular non-active ingredientsTert-Butyloxycarbonyl protecting groupCationic polymerization
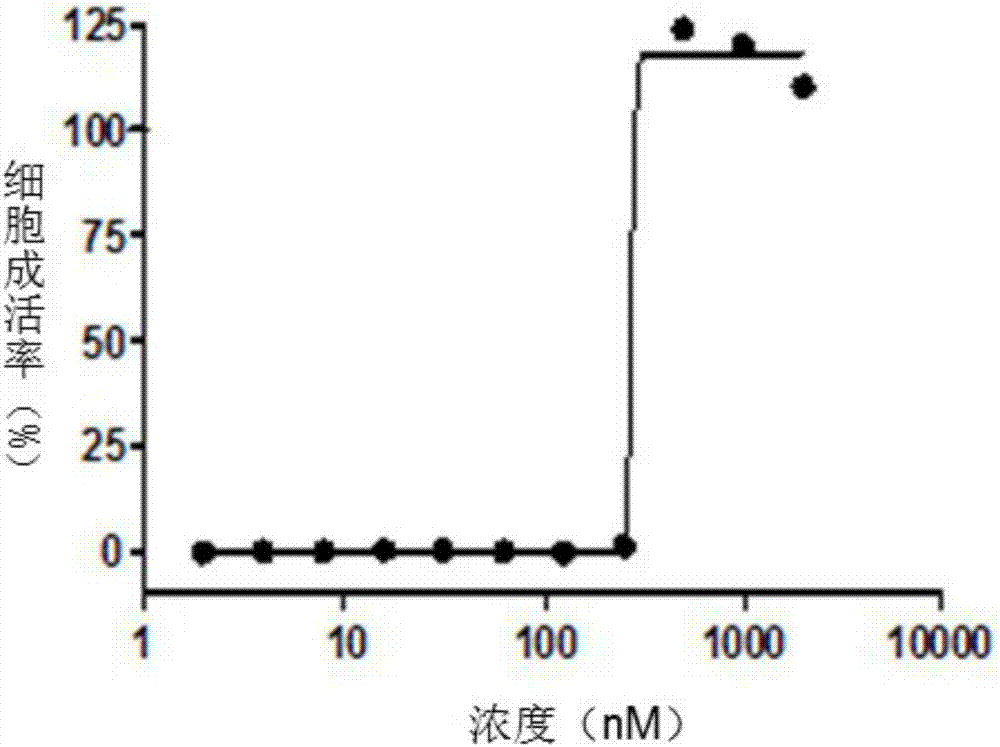
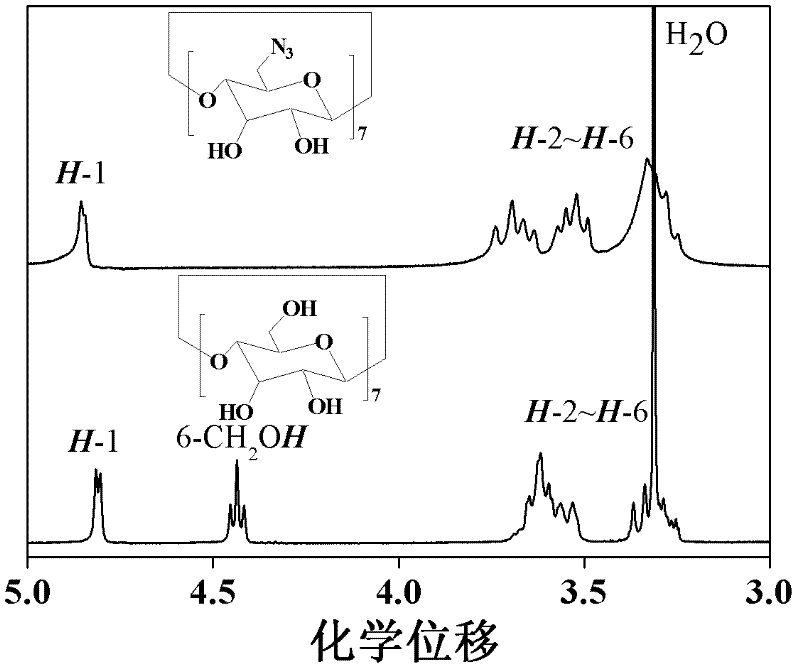
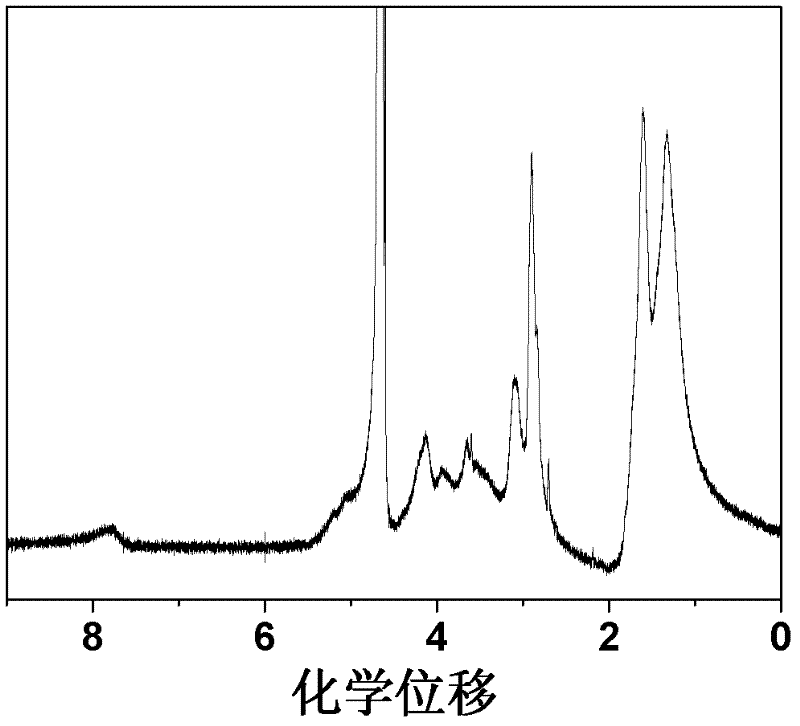
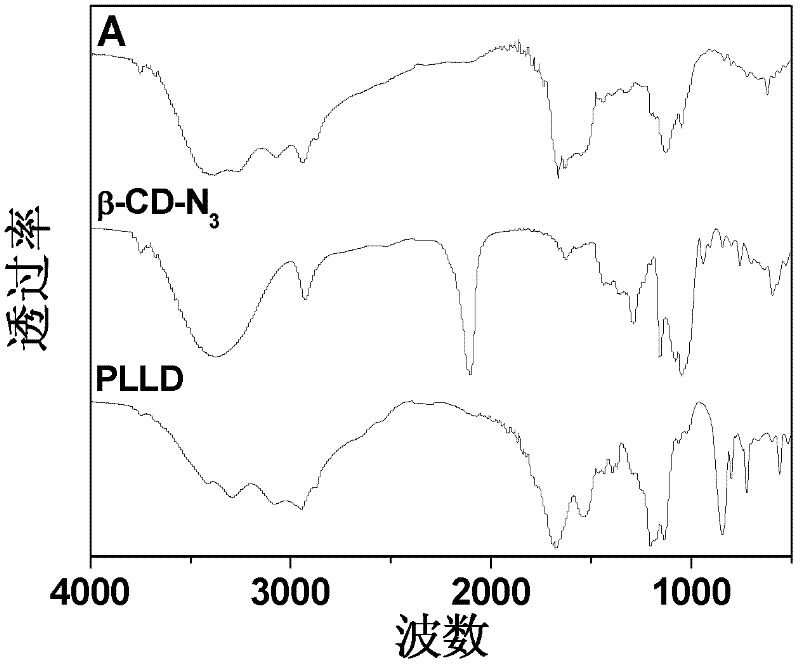
The invention discloses a star-shaped cationic polymer containing dendriform polylysine element, and a preparation method and application thereof. The method comprises the following steps: carrying out substitution reaction to introduce an azido group onto the C6 site of beta-cyclodextrin; carrying out coupling reaction on propargylamine and N,N'-di-tert-butoxycarbonyl-L-lysine, and carrying out repeated condensation and deprotection reactions to synthesize the alkynyl-containing dendriform polylysine; and coupling the two products by a click chemical process to obtain the star-shaped cationic polymer beta-CD-PLLD containing dendriform polylysine element. The polymer has the advantages of regular and easily controlled structure, single molecular weight distribution, favorable biocompatibility, biodegradability and the like, and is prospected to be widely used in the field of gene therapy.
Owner:SUN YAT SEN UNIV
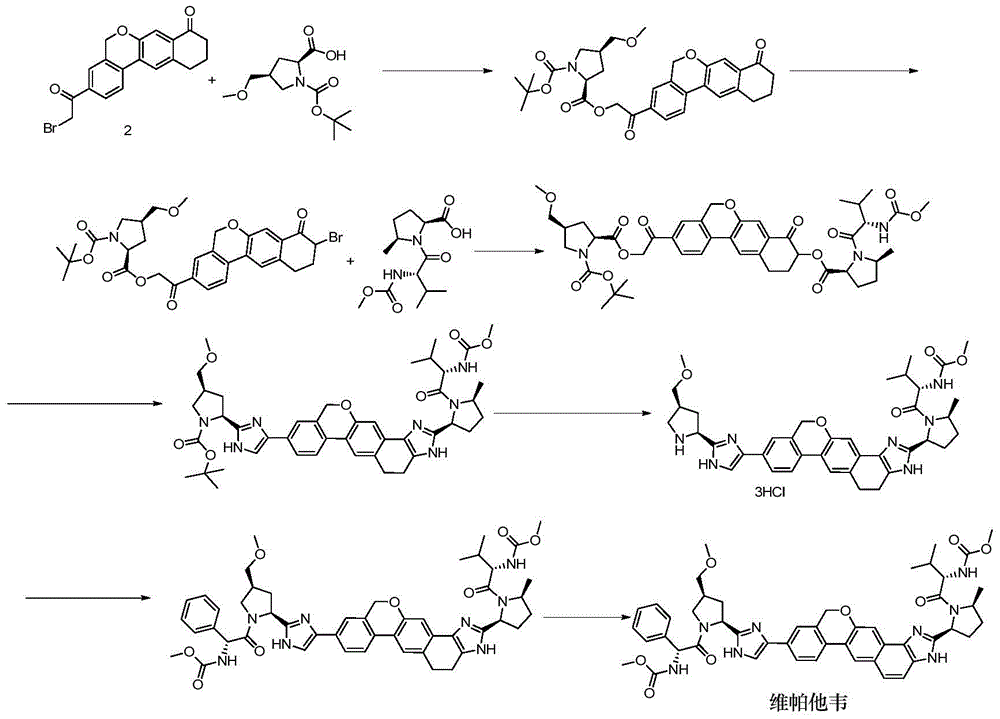
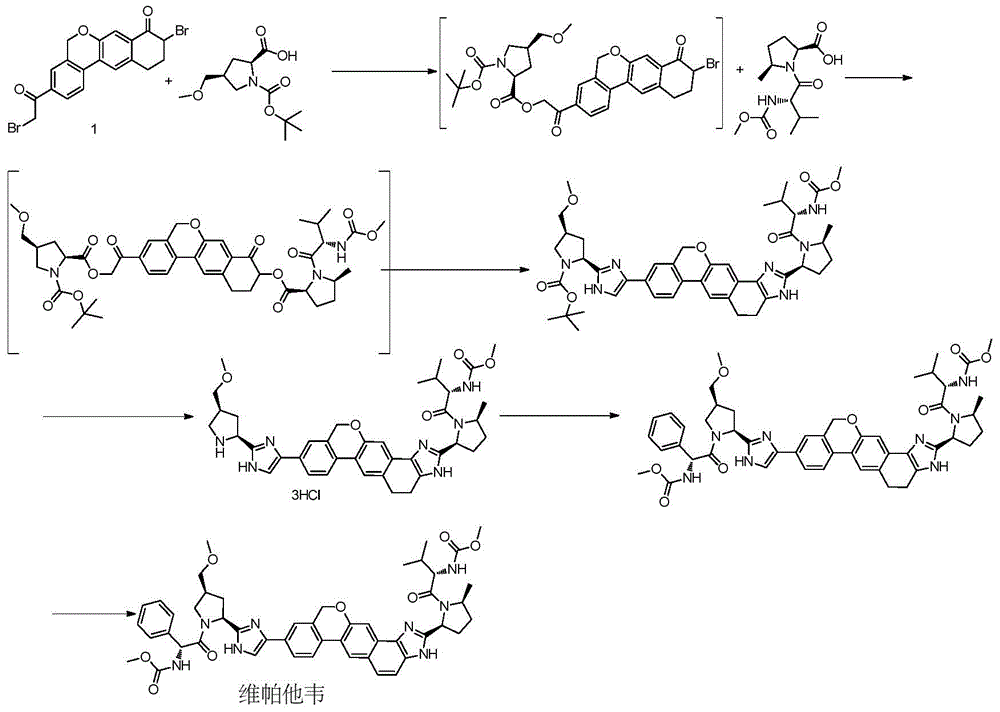
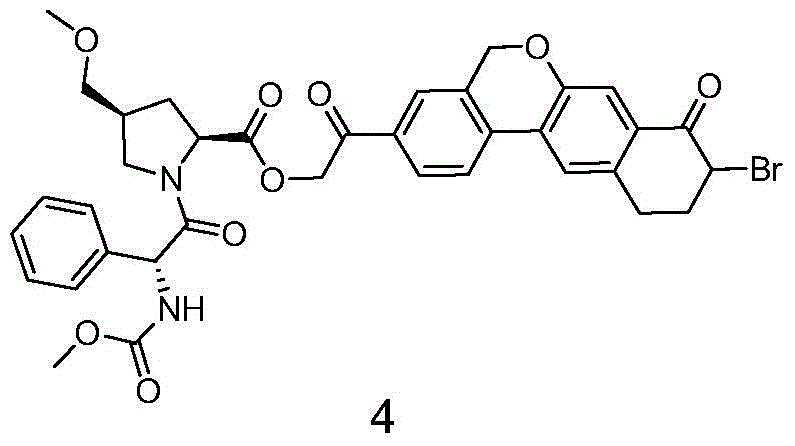
Novel synthesis method of hepatitis drug velpatasvir
ActiveCN105732765AIncrease profitEfficient synthesisPeptidesTert-Butyloxycarbonyl protecting groupSynthesis methods
The invention provides a novel synthesis method of hepatitis drug velpatasvir.Two intermediate compounds including a compound 4 and a compound III are utilized to synthesize the velpatasvir, the structures of the two compounds are as shown in the following formulas, wherein PG radical is t-butyloxycarboryl (Boc), carboxybenzyl (Cbz), acetyl, benzoyl or (S)-2-methoxyl acyl carbonyl amino-3-methyl-butyryl group (Moc-L-Valyl).
Owner:山东科巢生物制药有限公司
Chirality covalent organic framework and synthesis method and application thereof
ActiveCN105622579ALarge specific surface areaRegular pore structureOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsTert-Butyloxycarbonyl protecting groupSynthesis methods
The invention discloses a synthesis method of a chirality covalent organic framework. The method includes the following steps that firstly, a chirality precursor and benzenetricarboxaldehyde are catalyzed by acetic acid under the inert atmosphere to react and generate a solid product, wherein the structural formula of the chirality precursor can be seen in the description, and Boc is t-butyloxycarboryl; secondly, t-butyloxycarboryl is removed from the solid product obtained in the first step to obtain the chirality covalent organic framework material. The chirality covalent organic framework is successfully synthesized, has a large specific area and a regular pore structure, has good catalytic activity and circulation for the asymmetric Aldol reaction of ketone and aldehyde, and is a good multiphase chirality catalyst, and the application range of the molecular sieve material in the field of chirality catalysis is greatly expanded.
Owner:LANZHOU UNIVERSITY
Preparation method of NEP (neutral endopeptidase) inhibitor intermediate
ActiveCN105061263ACarbamic acid derivatives preparationOrganic compound preparationHydrogenTert-Butyloxycarbonyl protecting group
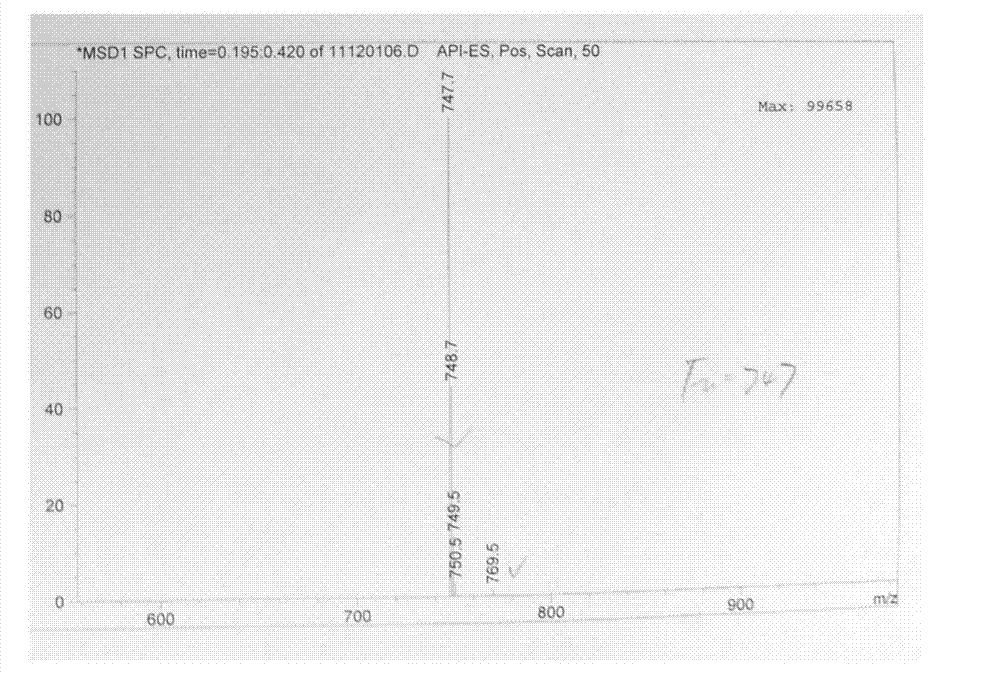
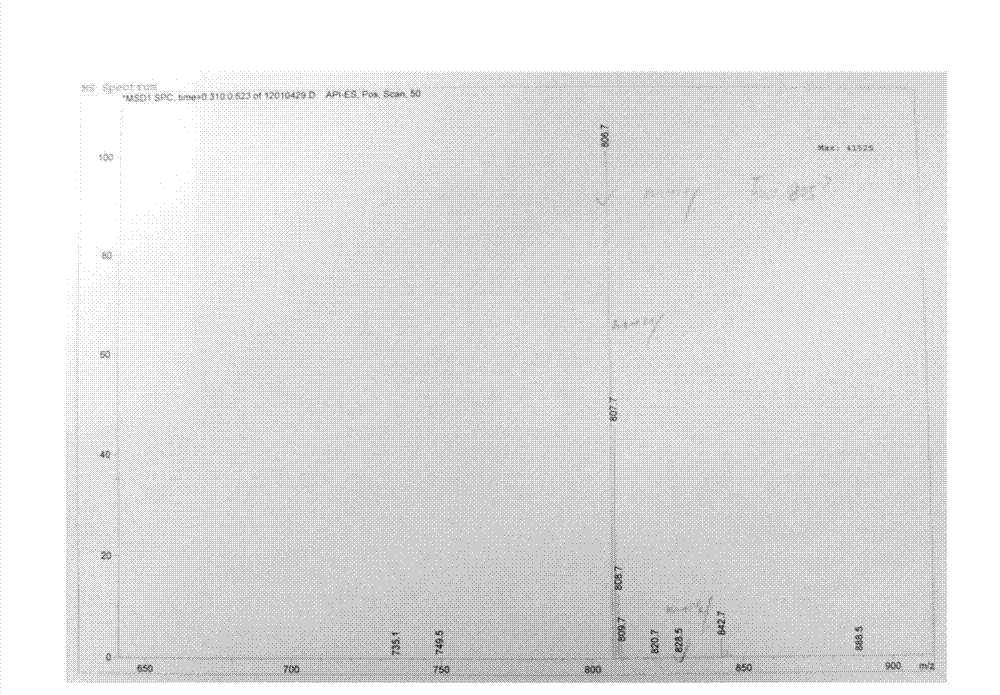
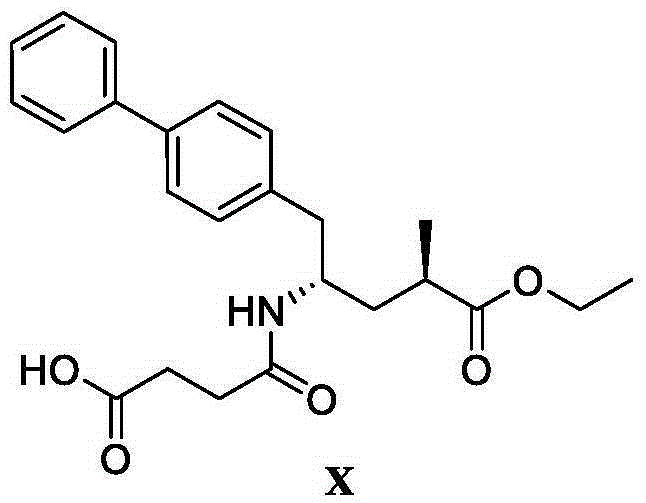
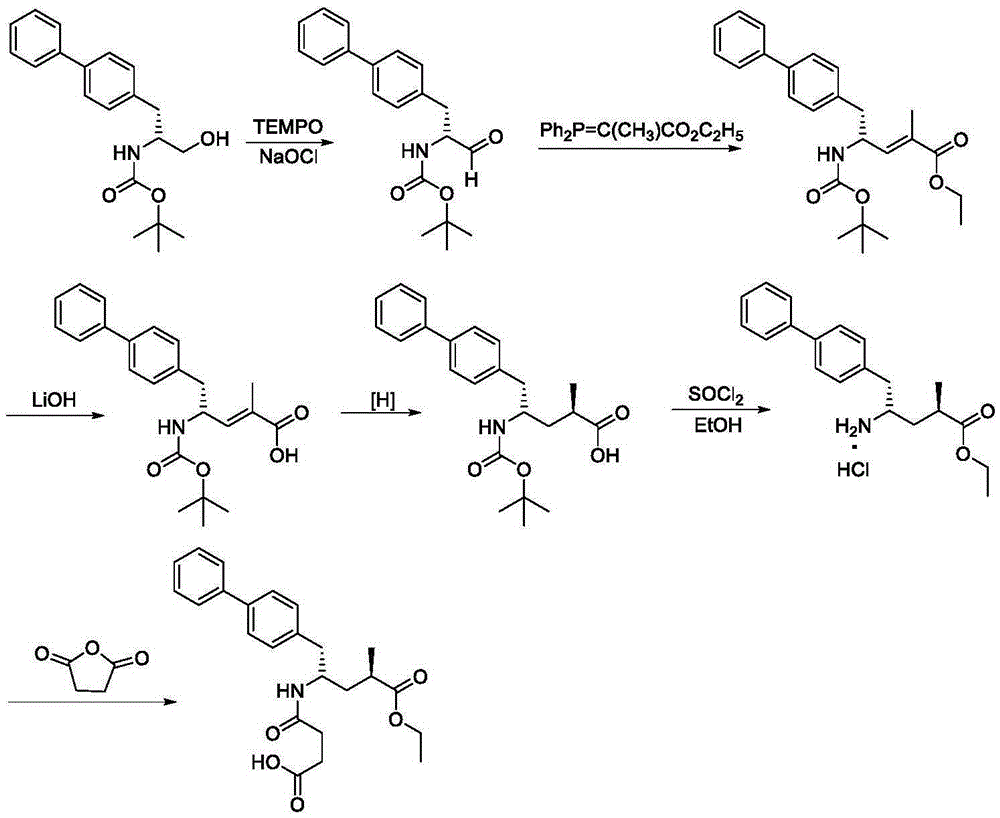
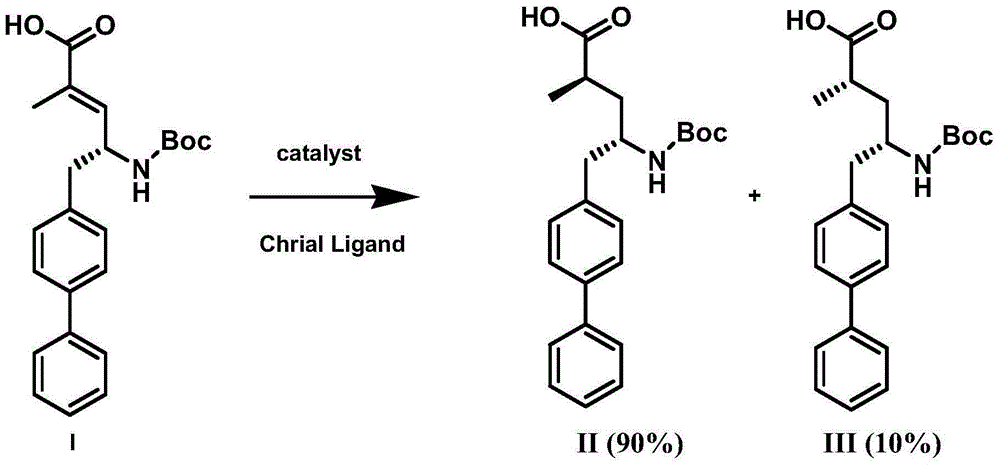
The invention discloses a preparation method of an NEP (neutral endopeptidase) inhibitor intermediate, further discloses a selective reduction preparation method of the NEP inhibitor intermediate (2R, 4S)-5-([1,1'-biphenyl]-4-yl)-4-((t-butyloxycarboryl)amino)-2-methylpentanoic acid in presence of a chiral ligand, and particularly discloses a diastereoselective hydrogenation synthetic method with hydrogen under the condition of presence of a transition metal catalyst and the chiral ligand. The metal catalyst used in the method is cheap, the chiral ligand is obtained easily, high yield is realized, a high-purity product is produced preferably, and the product with the proportion of diastereoisomers being 90:10 is produced preferably.
Owner:苏州楚凯药业有限公司
Preparation method of polyether amine
ActiveCN102604072AAchieve synthesisLess side effectsPolyethylene oxideTert-Butyloxycarbonyl protecting group
The invention relates to a preparation method of polyether amine. In the structure of polyether amine, the main chain is polypropylene oxide, polyethylene oxide, polytetrahydrofuran or a copolymer of polypropylene oxide, polyethylene oxide and polytetrahydrofuran; and one end of the main chain is hydroxyl, and the other end of the main chain is an alcohol amine compound terminated end having a H2N-R-O-H structure. Polyether amine is prepared by the following steps: (1) protecting the amino of the alcohol amine compound by using BOC (tertbutyloxycarbonyl) and maintaining hydroxyl to obtain BOC-NH-R-OH; (2) in the catalysis of strong base or double-metal cyanide, by using BOC-NH-R-OH, initiating polyethylene oxide, polypropylene oxide, tetrahydrofuran or the mixture of polyethylene oxide, polypropylene oxide and tetrahydrofuran to react for ring opening polymerization; and (3) under the acidic condition, carrying out BOC deprotection reaction to obtain polyether amine. According to the invention, polyether main chains having different hydrophilic properties can be obtained by regulating the ratio of polypropylene oxide to polyethylene oxide to polytetrahydrofuran in the polyether chain; and one end of the polyether chain contains the amino, thus the amino can provide strong hydrophilic property after acidification. Polyether amine prepared by using the method can be applied to the fields of a surfactant, a flocculant, textile dyeing and finishing auxiliary, electrophoretic paint and the like.
Owner:WUXI ACRYL TECH
Practical synthesis method for feritin inhibitor aliskiren
InactiveCN101016253AReasonable choice of reaction processFew reaction stepsOrganic compound preparationCarboxylic acid amides preparationSynthesis methodsTert-Butyloxycarbonyl protecting group
The invention discloses a synthesizing method of Alikelun of hypertension proteinogenase inhibitor, which comprises the following steps: adopting (1S, 4S, 2'S)-(4-benzyloxy methyl-2-hydroxy-1-{2-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-3-methyl-butyl}-5-methyl-hexyl)-carbonic tert-butyl as raw material; catalyzing; hydrogenating; removing benzyl; separating two R and S-typed diastereomers through column chromatography; obtaining (1S, 2S, 4S, 2'S)-(2-hydroxy-4-methylol-1-{2-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-3-methyl-butyl}-5-methyl-hexyl)-carbonic tert-butyl ester; oxidizing into (1S, 3S, 1'S, 4'S)-{1-(4-isopropyl-5-oxo-tetrahydrofuran-2-radical)-3-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-4-methyl-amyl}-carbonic tert-butyl ester; proceeding amide ester exchange to obtain (1 S, 3S, 1'S, 4'S)-(4-(2-amino formyl-2-methyl-propyl amino formyl)-2-hydroxy-1-{2-[4-methoxy-3-(3-methoxy-propoxy-benzyl]-3-methyl-butyl}-5-methyl-hexyl) carbonic tert-butyl ester; removing terbu-carbonyl to produce Aliskiren.
Owner:上海药明康德新药开发有限公司
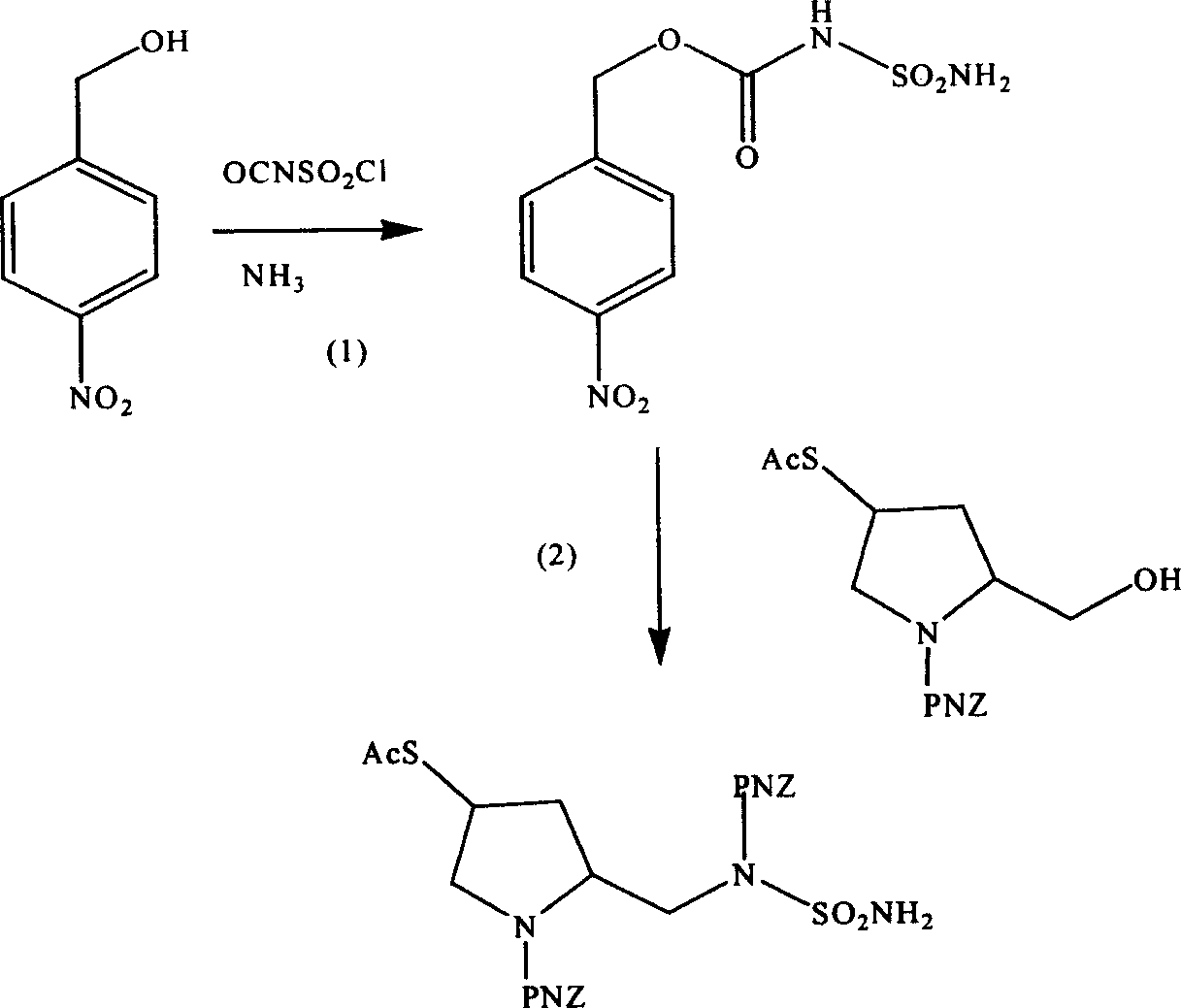
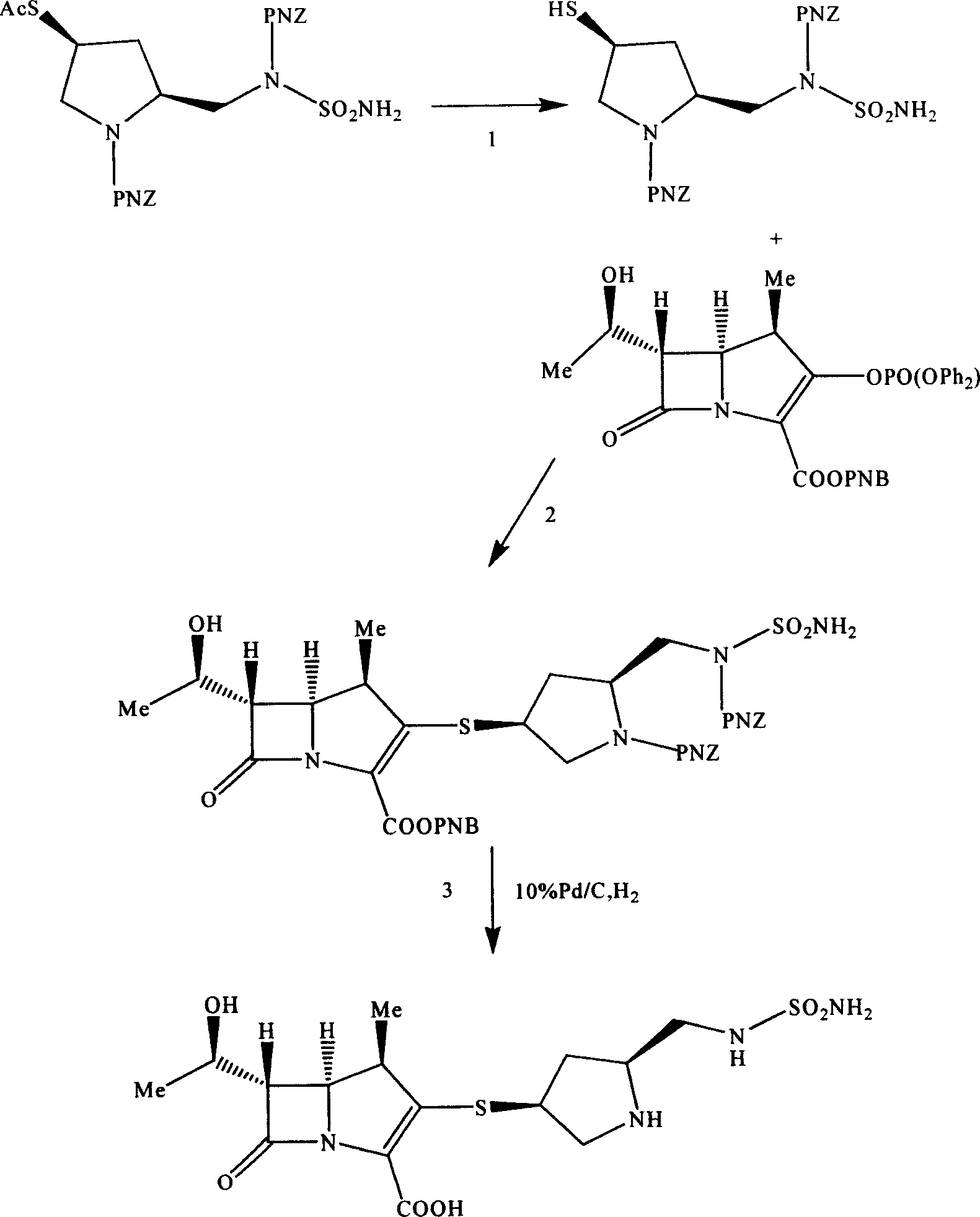
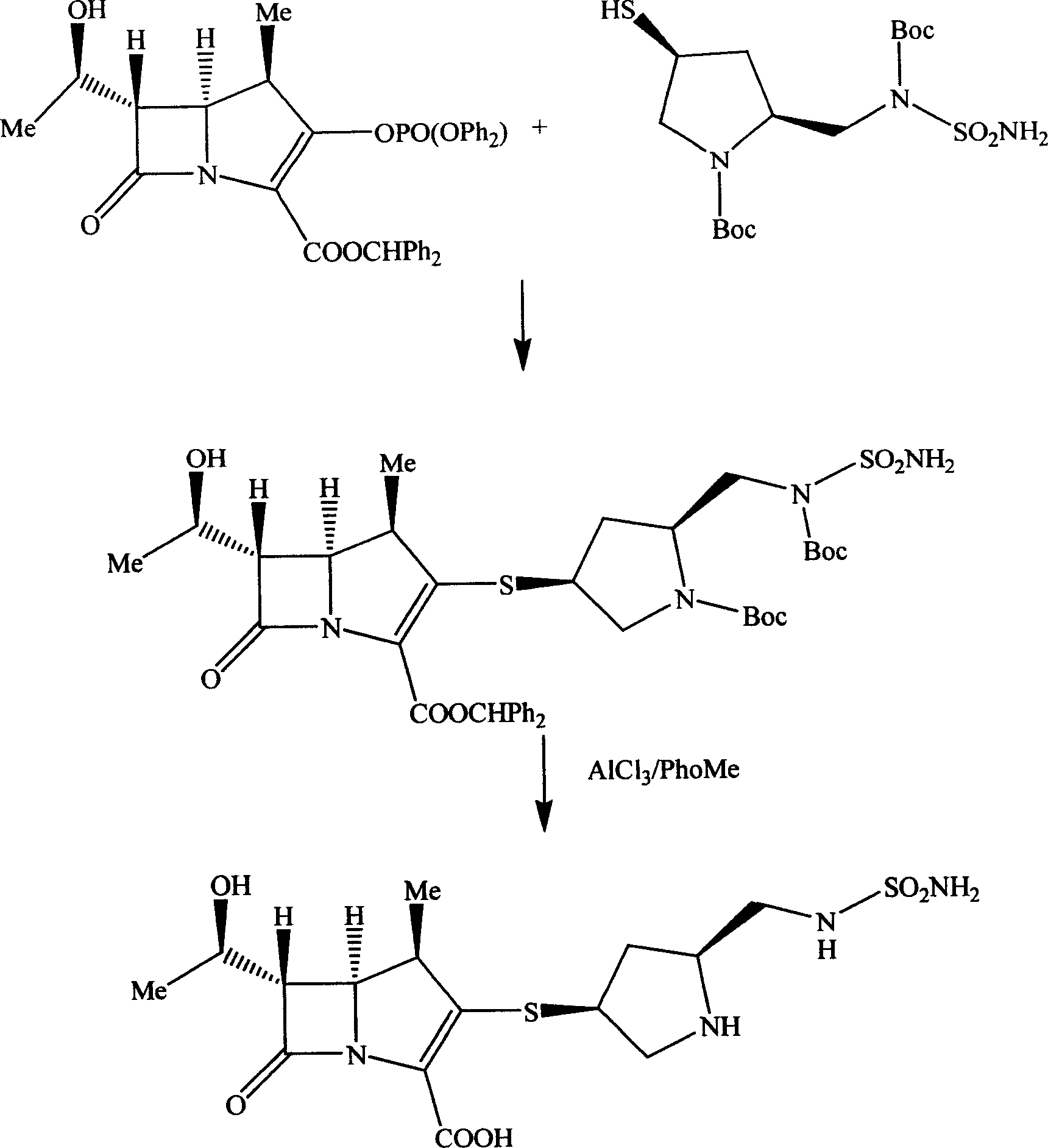
Pentazane derivative intermediate, its preparation and use
ActiveCN1896057AEasy to prepareInhibit side effectsOrganic chemistryUrinary disorderTert-Butyloxycarbonyl protecting groupPyrrolidine
A pentazane derivative intermediate, its production and use are disclosed. PNZ expresses p-nitrocarbobenzoxy, R represents Ac(acetyl) or-H. It is simple and has no side reaction. It can be used for donippenan.
Owner:CHENGDU DIAO JIUHONG PHARMA FACTORY
Production method of N-protection pipradrol
ActiveCN103789368AThe method is simple and efficientFermentationTert-Butyloxycarbonyl protecting groupIsopropyl alcohol
The invention discloses a production method of N-protection pipradrol. The structural formula of the N-protection pipradrol is shown in the specification, wherein R is tert-butyloxycarbonyl or benzyl. The production method is characterized in that the N-protection piperidone is taken as a substrate, and is obtained through a reaction in the presence of alcohol dehydrogenase, ADH-A, isopropyl alcohol and NAD<+1>; the alcohol dehydrogenase is cpsADH or cmADHmut. The method disclosed by the invention is efficient and simple and has relatively high value in industrial application.
Owner:SHANGHAI RES & DEV CENT OF INDAL BIOTECH +1
Cabazitaxel crystal and preparation method thereof
ActiveCN102898406AEfficient removalImprove solubilityOrganic chemistryTert-Butyloxycarbonyl protecting groupCabazitaxel
The invention relates to a solvate-free and crystal water-free crystal form of 4-accetoxy-2alpha-benzoyloxy-5beta,20-epoxy-1-hydroxy-7beta,10beta-dimethoxy-9-oxotax-11-en-13alpha-yl(2R,3S)-3tert-butoxycarbonylamino-2-hydroxy-3-phenylpropanoate 1-hydroxy-7beta,10beta-dimethoxy-9-oxo-5beta,20-epoxytax-11-ene-2alpha,4,13alpha-triyl4-acetate 2-benzoate13-[(2R,3S)-3-{[(tertbutoxycarbonyl]amino}-2-hydroxy-3-phenylpropanoate. A powder X-ray diffraction (PXRD) diagram shows that the crystal is positioned on the characteristic peaks of 4.3, 7.4, 8.6, 10.0, 11.0, 12.2, 12.6, 13.3, 13.6, 14.2, 15.0, 15.5, 16.4, 17.0, 18.1, 18.6, 20.2, 21.0, 21.6, 22.1, 22.8, 24.0, 24.6, 25.3, 25.8, 26.9, 28.0, 29.4, 31.5, 32.0, 34.4, 35.5, 36.8 and 41.7 DEG 2theta. The invention also discloses a preparation method for the crystal. The crystal prepared under reduced pressure at room temperature is high in yield and purity, and does not have any solvent residue.
Owner:SHANGHAI JINHE BIO TECH
Synthetic method of linagliptin
ActiveCN105936634AHigh purityPurity does not affectOrganic chemistryBulk chemical productionTert-Butyloxycarbonyl protecting groupXanthine
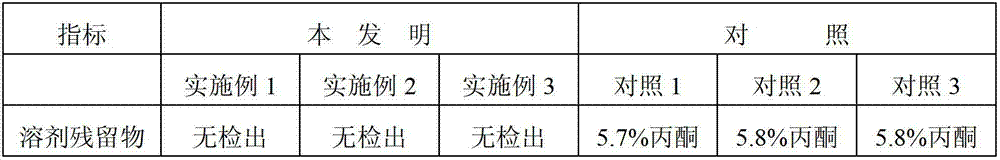
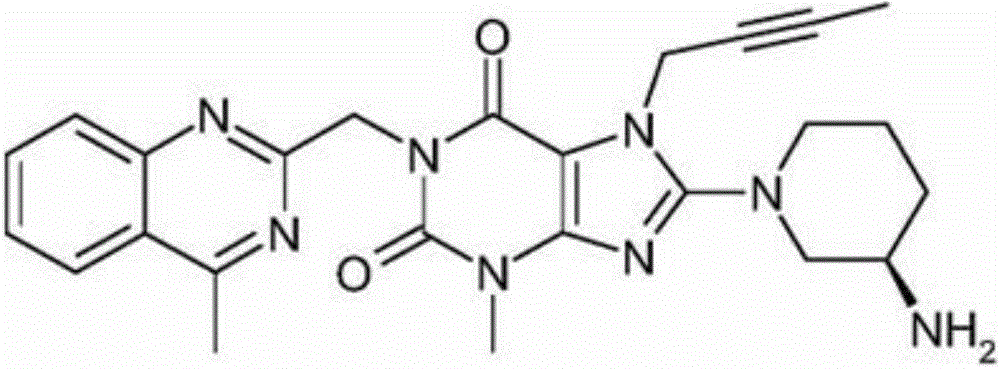
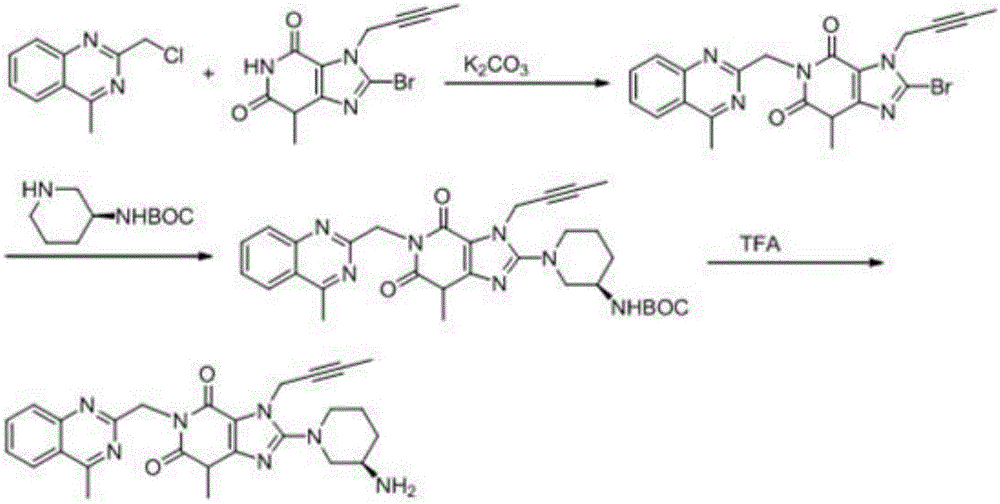
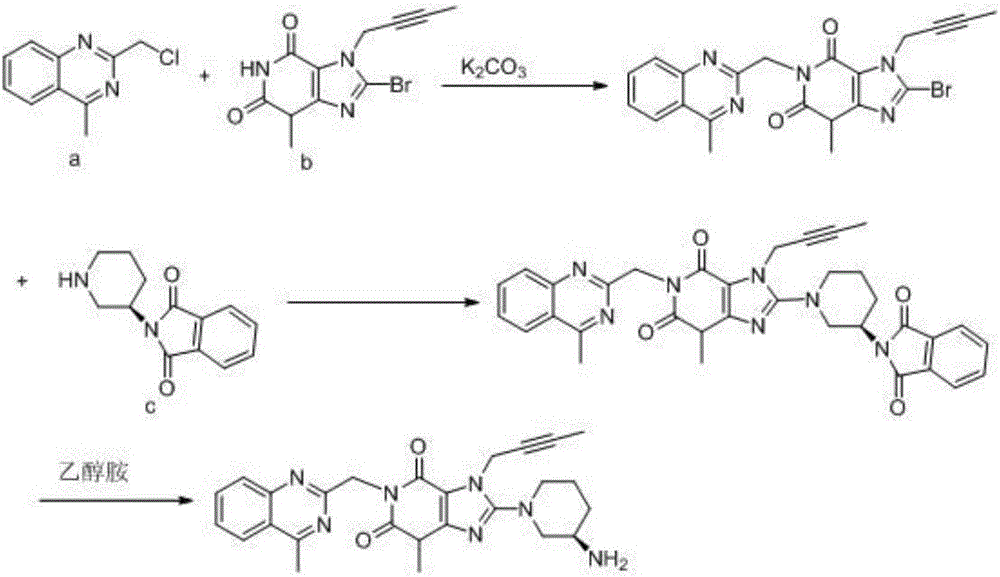
The invention discloses a synthetic method of linagliptin, wherein the method includes the following steps: (1) carrying out a reaction of 8-bromo-3-methylxanthine (a) and 1-bromo-2-butyne (b), to obtain 3-methyl-7-(2-butyne-1-yl)-8-bromo-xanthine (c); (2) carrying out a reaction of 3-methyl-7-(2-butyne-1-yl)-8-bromo-xanthine (c) and 2-chloromethyl-4-methylquinazoline (d), to obtain 1-[(4-methylquinazolin-2-yl)methyl]-3-methyl-7-(2-butyne-1-yl)-8-bromoxanthine (e); (3) adding 1-[(4-methylquinazolin-2-yl)methyl]-3-methyl-7-(2-butyne-1-yl)-8-bromoxanthine (e), (R)-3-Boc-aminopiperidine (f), potassium carbonate and acetonitrile into a reactor and mixing evenly, and carrying out a reaction under a state of heating reflux, to obtain t-butyloxycarboryl-linagliptin (g); and (4) removing a Boc protective group of t-butyloxycarboryl-linagliptin (g) in a methanol aqueous solution, to obtain linagliptin. The synthetic method has the advantages of environmental protection, no pollution, high production rate, and no impurities.
Owner:赤峰赛林泰药业有限公司
Enzyme-activity-improved ethanol dehydrogenase mutant and preparing method and application thereof
ActiveCN105861457AReduce dosageHigh activityOxidoreductasesFermentationChemical industryEthanol dehydrogenase
The invention discloses an enzyme-activity-improved ethanol dehydrogenase mutant and a preparing method and application thereof, and belongs to the field of biological medicine and the field of chemical industry. According to the enzyme-activity-improved ethanol dehydrogenase mutant and the preparing method and application thereof, the amino acid sequence nearby the active site of ethanol dehydrogenase is optimized, the ethanol dehydrogenase mutant is obtained, and the activity of the ethanol dehydrogenase mutant can be mostly improved 36%. When the enzyme-activity-improved ethanol dehydrogenase mutant is applied, the dosage of enzymes can be greatly decreased in the conversion process, and when S-N-t-butyloxycarboryl-3-hydroxypiper is prepared in a large-scale mode, production cost can be effectively reduced, and remarkable economic benefits are generated.
Owner:BIORTUS BIOSCI
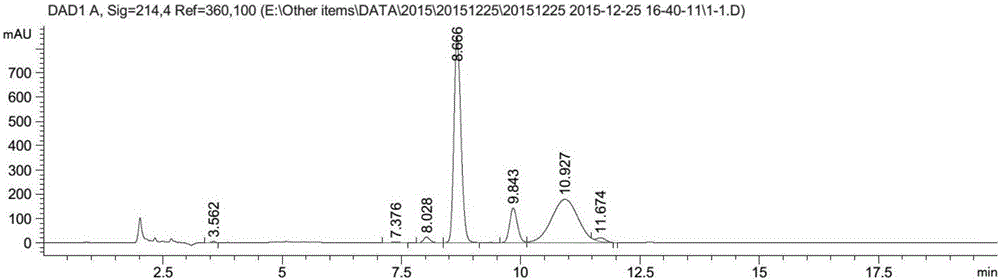
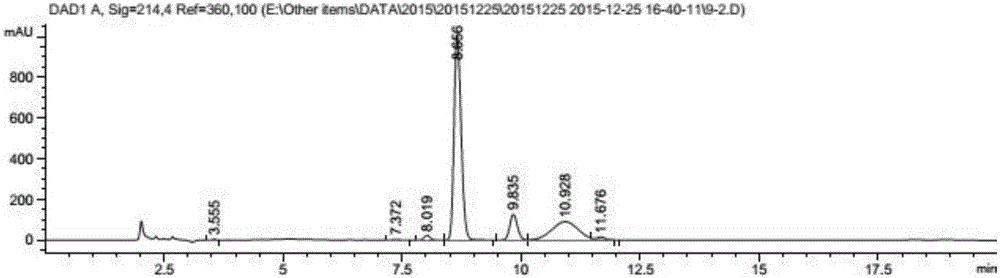
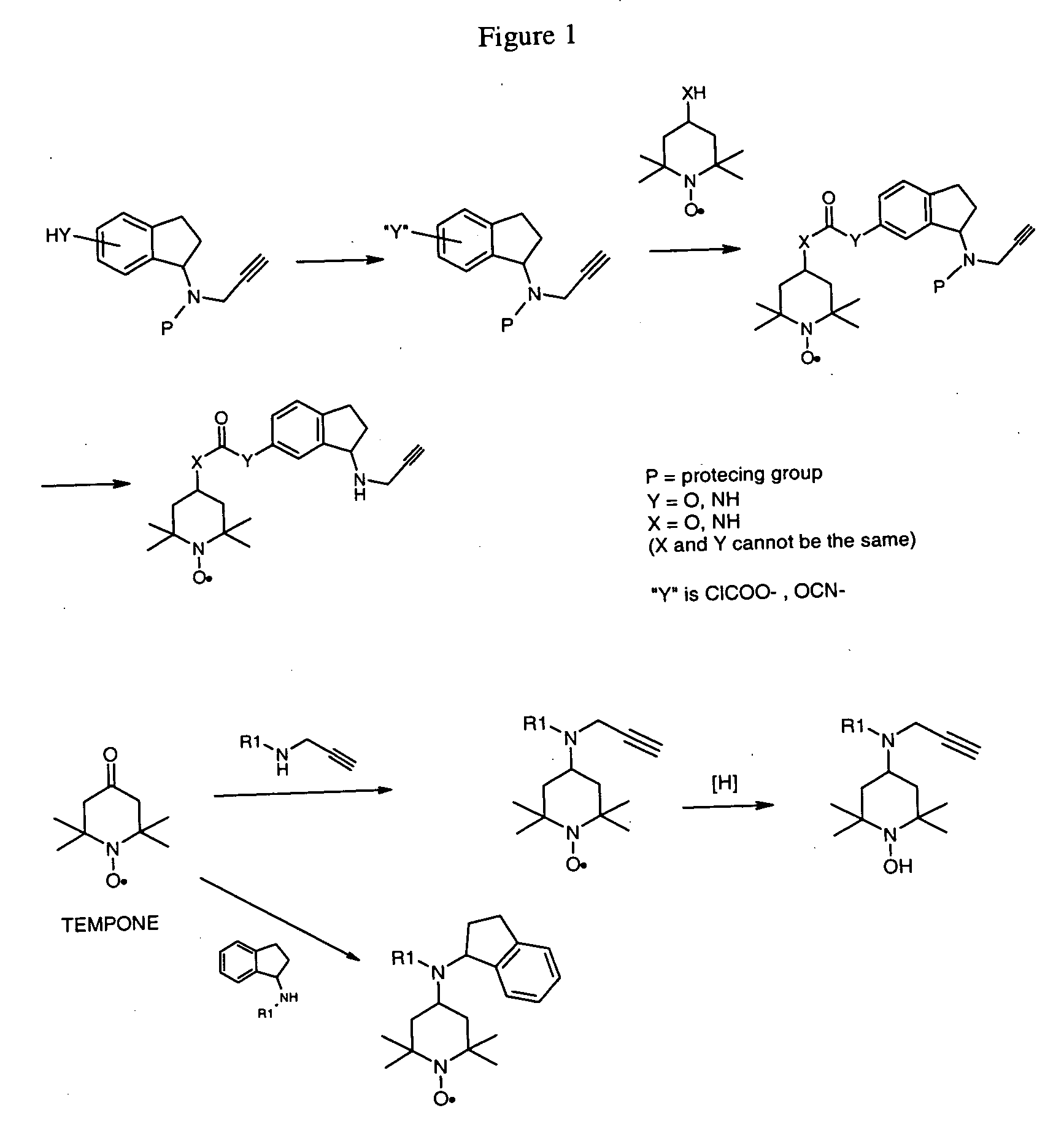
Propargyl nitroxides and indanyl nitroxides and their use for the treatment of neurologic diseases and disorders
Owner:TEVA PHARMA IND LTD
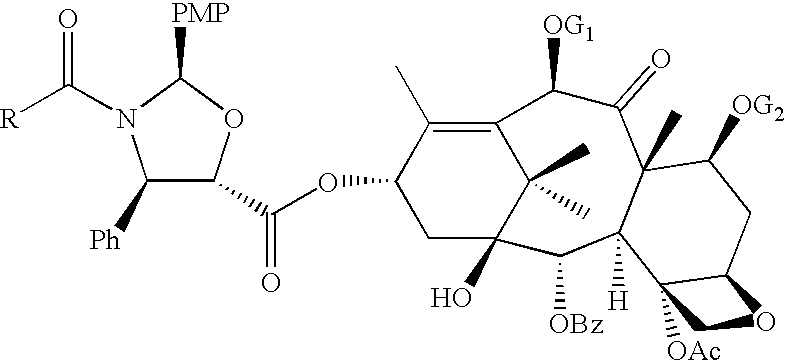
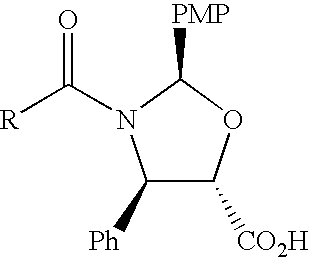
Anticancer taxanes such as paclitaxel, docetaxel and their structural analogs, and a method for the preparation thereof
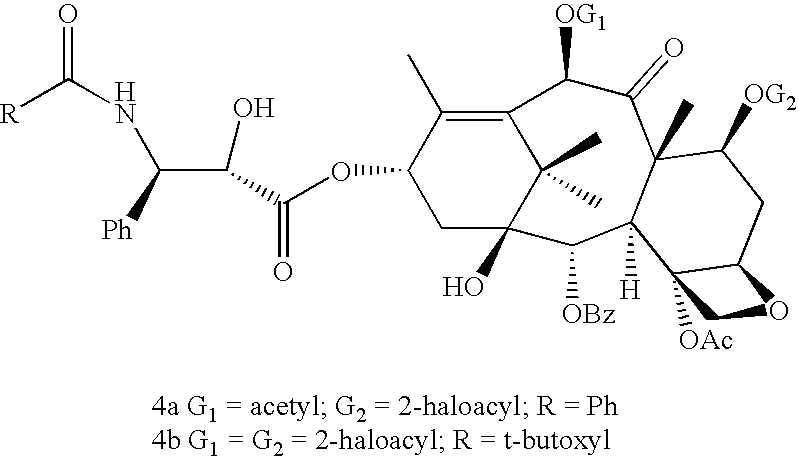
A process for the preparation of taxanes comprising wherein R is a tert. butoxycarbonyl or benzoyl group; PMP is p-methoxyphenyl group; G1 is acetyl group; G2 is haloacetyl group comprisinga) protecting the C-7 hydroxyl group of 10-deacetylbaccatin III with haloacetyl chlorides and then acetylating the C-10 hydroxyl group with acetyl chloride to obtain a protected 10-deacetylbaccatin III (1);b) subjecting the protected 10-deacetylbaccatin III (1) to coupling with an oxazolidine-5-caboxylic acid of formula 2 wherein R is tert. butoxycarbonyl or benzoyl; PMP is p-methoxyphenyl group in the presence of a condensation agent and an activating agent to obtain C-13 esters of formula 3;c) treating the coupled products 3 with weak acidic medium to open the oxazolidine ring to obtain intermediates of formula 4; wherein R is a tert. butoxycarbonyl or benzoyl group; G1 is acetyl group; G2 is haloacetyl groupd) subjecting the intermediates of compound 4 to selective deprotection of haloacyl group in the presence of acetyl group under mild alkaline condition at −20 to +40° C. for 6-24 h in the presence of ammonia or aliphatic amine or aromatic amines or their combination to obtain paclitaxel or docetaxel.
Owner:DABUR PHARM LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com