Patents
Literature
35 results about "Carboxybenzyl" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Carboxybenzyl is a carbamate which is often used as an amine protecting group in organic synthesis. It is commonly used in peptide synthesis where the carboxybenzyl protection group is introduced by reacting the amine functionality with benzyl chloroformate in the presence of a weak base: Alternatively, as in the Curtius rearrangement, it is made by the trapping of an isocyanate with benzyl alcohol. It is used to protect amines from electrophiles. The protected amine can be deprotected by catalytic hydrogenation or treatment with HBr, yielding a terminal carbamic acid that then readily decarboxylates to yield the free amine. The method was first used by Max Bergmann and Leonidas Zerwas in 1932 for the synthesis of peptides. The abbreviation Z is in honor of Zerwas.
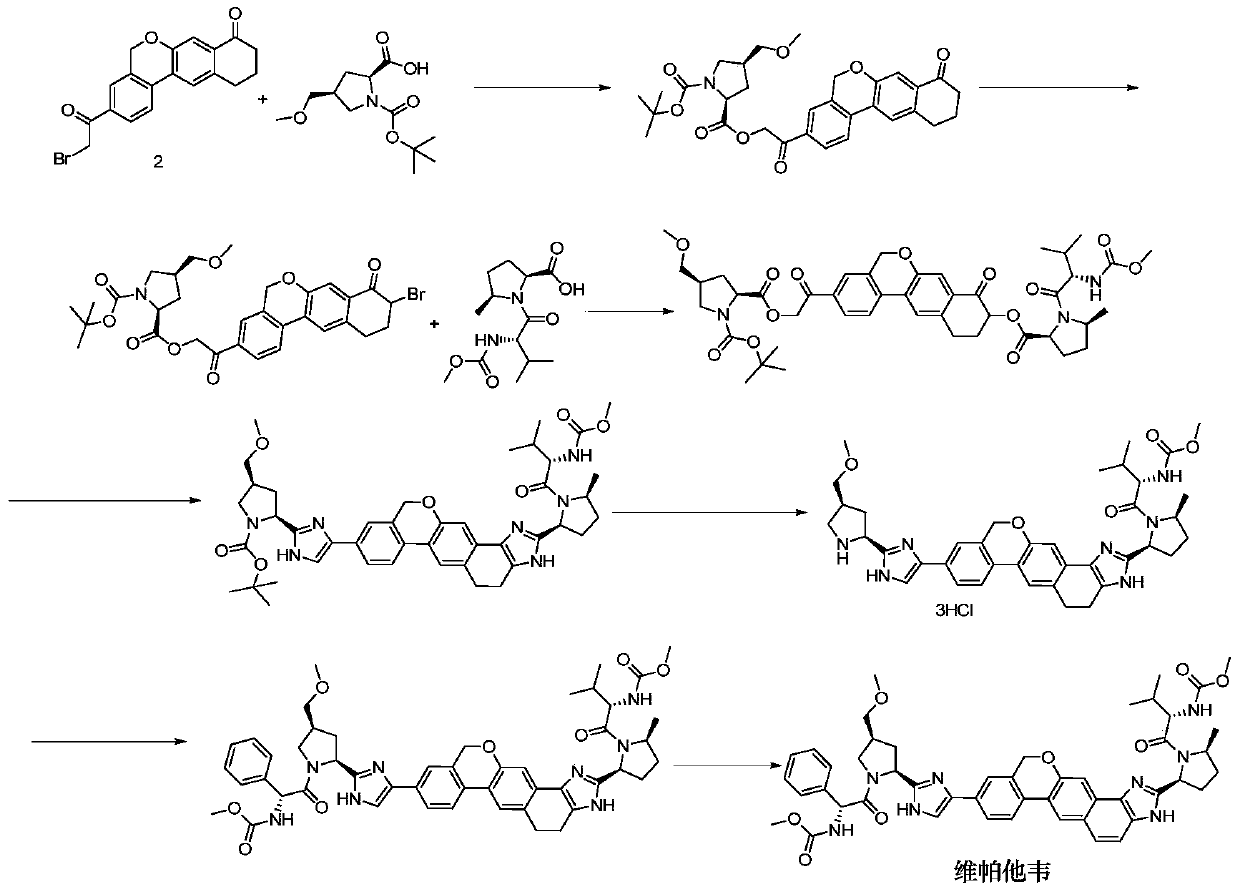
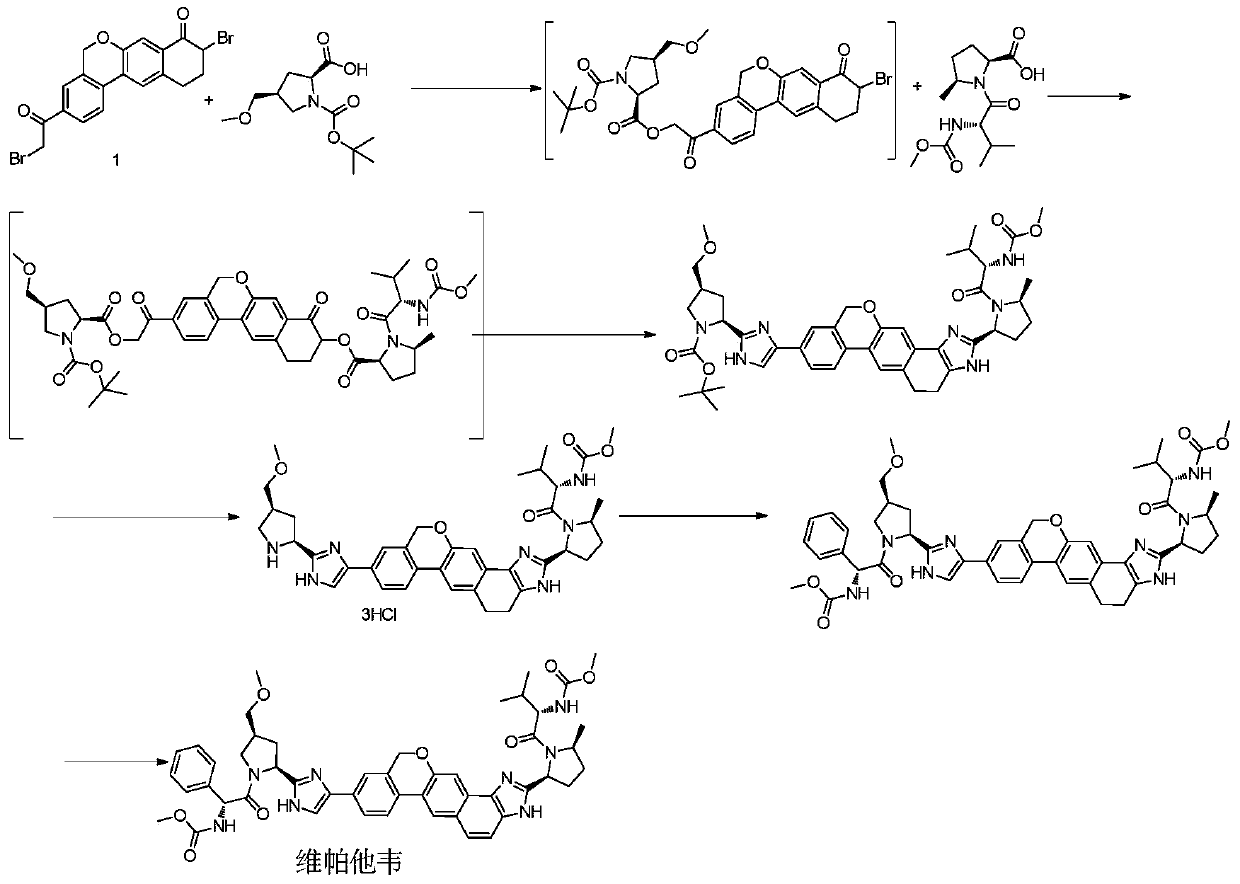
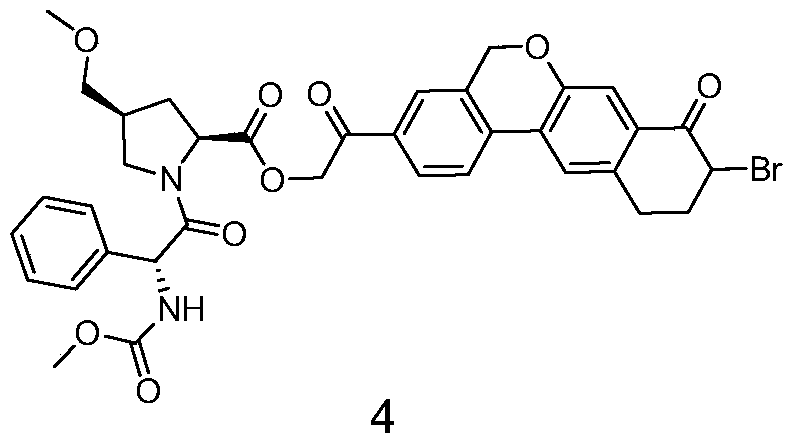
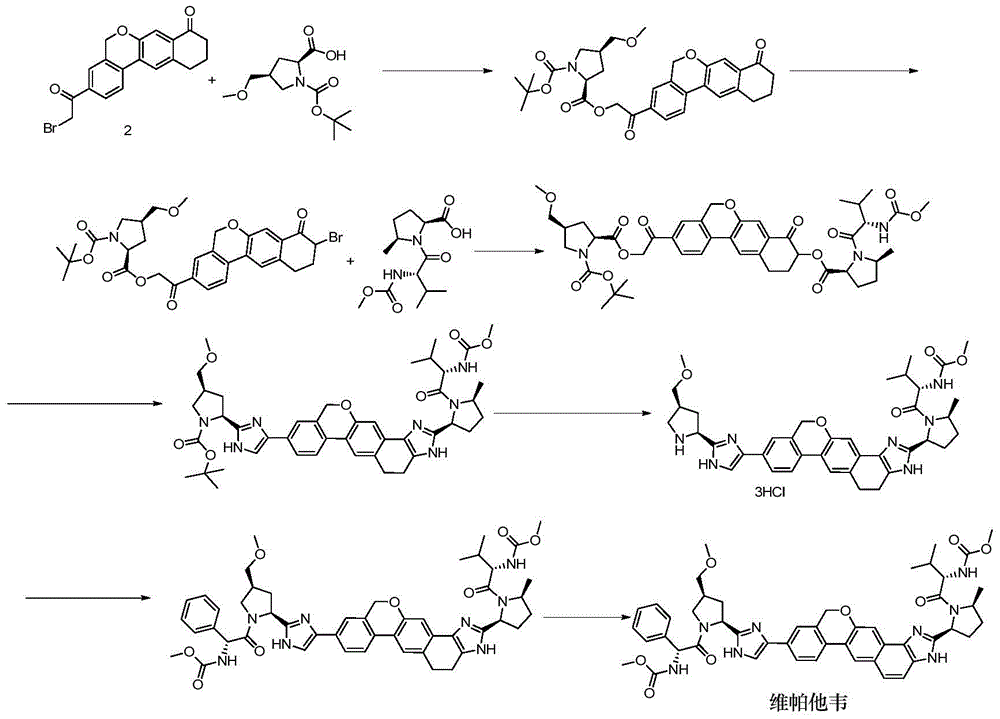
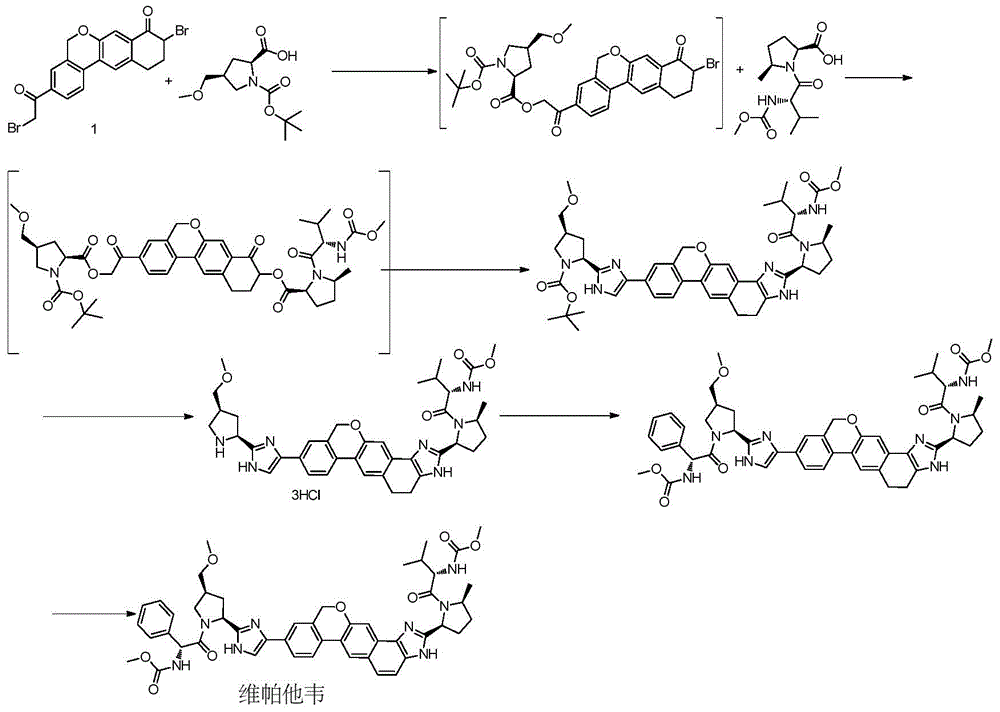
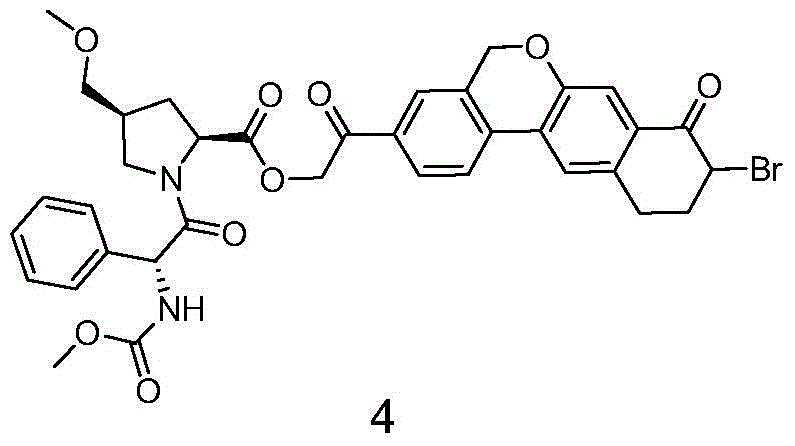
Novel synthesis method of hepatitis drug velpatasvir
ActiveCN105732765AIncrease profitEfficient synthesisPeptidesTert-Butyloxycarbonyl protecting groupSynthesis methods
The invention provides a novel synthesis method of hepatitis drug velpatasvir.Two intermediate compounds including a compound 4 and a compound III are utilized to synthesize the velpatasvir, the structures of the two compounds are as shown in the following formulas, wherein PG radical is t-butyloxycarboryl (Boc), carboxybenzyl (Cbz), acetyl, benzoyl or (S)-2-methoxyl acyl carbonyl amino-3-methyl-butyryl group (Moc-L-Valyl).
Owner:山东科巢生物制药有限公司
4-carboxybenzylamino derivatives as histone deacetylase inhibitors
ActiveUS20100324046A1Prevent proliferationArrest cell growthBiocideCarbamic acid derivatives preparationDiseaseDosing regimen
The present invention relates to a novel class of 4-carboxybenzylamino derivatives. The 4-carboxybenzylamino compounds can be used to treat cancer. The 4-carboxybenzylamino compounds can also inhibit histone deacetylase and are suitable for use in selectively inducing terminal differentiation, and arresting cell growth and / or apoptosis of neoplastic cells, thereby inhibiting proliferation of such cells. Thus, the compounds of the present invention are useful in treating a patient having a tumor characterized by proliferation of neoplastic cells. The compounds of the invention may also be useful in the prevention and treatment of TRX-mediated diseases, such as autoimmune, allergic and inflammatory diseases, and in the prevention and / or treatment of diseases of the central nervous system (CNS), such as neurodegenerative diseases. The present invention further provides pharmaceutical compositions comprising the 4-carboxybenzylamino derivatives and safe dosing regimens of these pharmaceutical compositions, which are easy to follow, and which result in a therapeutically effective amount of the 4-carboxybenzylamino derivatives in vivo.
Owner:MERCK SHARP & DOHME LLC
Synthesis methods of lorcaserin derivative and salt thereof
The invention discloses a synthesis method of a lorcaserin derivative. The synthesis method comprises the following steps: resolving a compound shown as a formula (II) under the action of an optical resolving agent; performing post-treatment to obtain the lorcaserin derivative. In the formula (II), R is H, alkyl with 1-4 carbon atoms, halogen, methoxyl or nitryl; R2 is H, alkyl with 1-4 carbon atoms, methoxyl, carboxybenzyl, t-butyloxycarboryl, methylsulfonyl, tosyl and substituted or unsubstituted benzyl; the optical resolving agent is acyl-substituted tartaric acid. The revolving efficiency is increased by selecting the optical resolving agent having a specific structure, and an optical pure product of which the ee value is over 99 percent can be obtained by means of simple recrystallization; meanwhile, resolving operation is easy, a single solvent is used for resolving, materials are fed into one pot, and the method is suitable for industrial production. The invention further discloses a synthesis method of a salt of the lorcaserin derivative. The obtained salt of the lorcaserin derivative can be applied to preparation of novel weight-reducing medicaments.
Owner:CHINA JILIANG UNIV +1
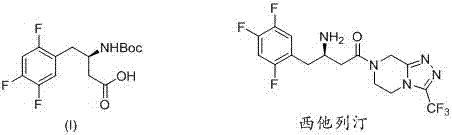
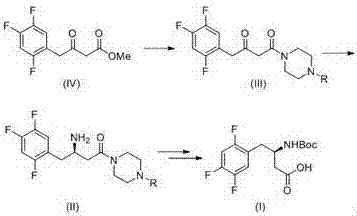
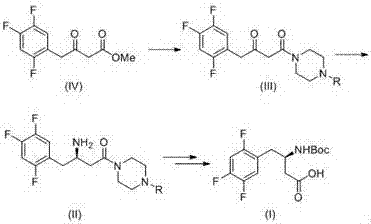
Method for synthetizing (R)-N-BOC-3-amino-4-(2,4,5-trifluorophenyl) butyric acid by adopting transaminase method
InactiveCN107365809AIngenious designThe synthetic route is simpleFermentationButyramideCarboxybenzyl
The present invention provides a method for synthesizing (R)-N-BOC-3-amino-4-(2,4,5-trifluorophenyl) butyric acid (I) by transaminase method, in which 3-oxo-4- (2,4,5-trifluorophenyl) methyl butyrate (IV) is a starting material, reacted with nitrogen-protected piperazine to obtain 3-oxo-4-(2,4,5-trifluorophenyl) butane Amide intermediate (III); (III) reacts in the presence of amino donor and transaminase, and is converted into 3-amino-4-(2,4,5-trifluorophenyl) butyramide intermediate (II); with ( The amide of II) is hydrolyzed into an acid, and the amino group is protected by BOC to obtain the target intermediate (I); wherein the protecting group of piperazine in formula (III) and formula (II) is C1-4 alkoxycarbonyl, benzyloxycarbonyl or methyl. The invention has the advantages of ingenious design, simple and efficient synthesis route, simple and easy process flow, and environmental protection, and provides a new method for industrial scale production of formula (III).
Owner:SHANGHAI PUYI CHEM CO LTD
Squarylium cyanine chemical sensor used for Fe<3+> detection and preparation method thereof
InactiveCN102795995AThe synthesis method is simpleReaction conditions are easy to controlMethine/polymethine dyesOrganic compound preparationSolubilityBenzene
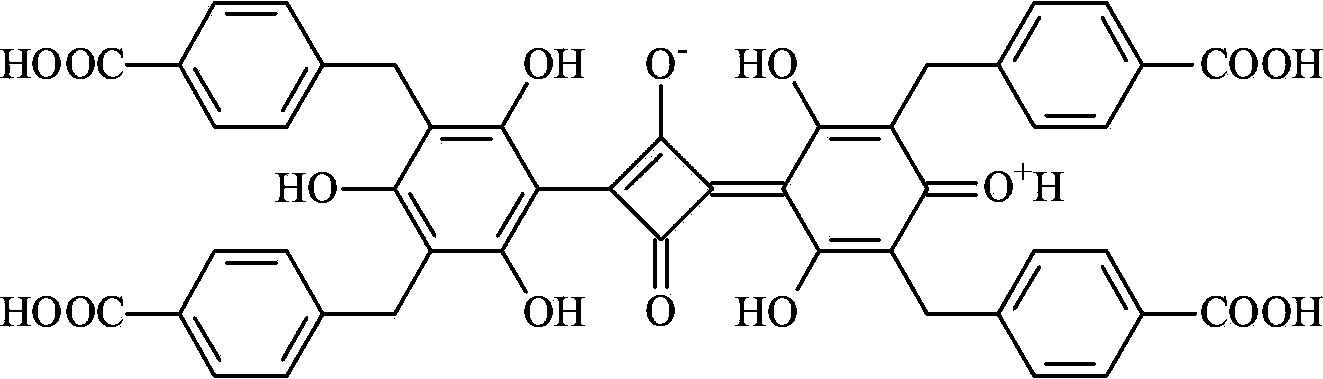
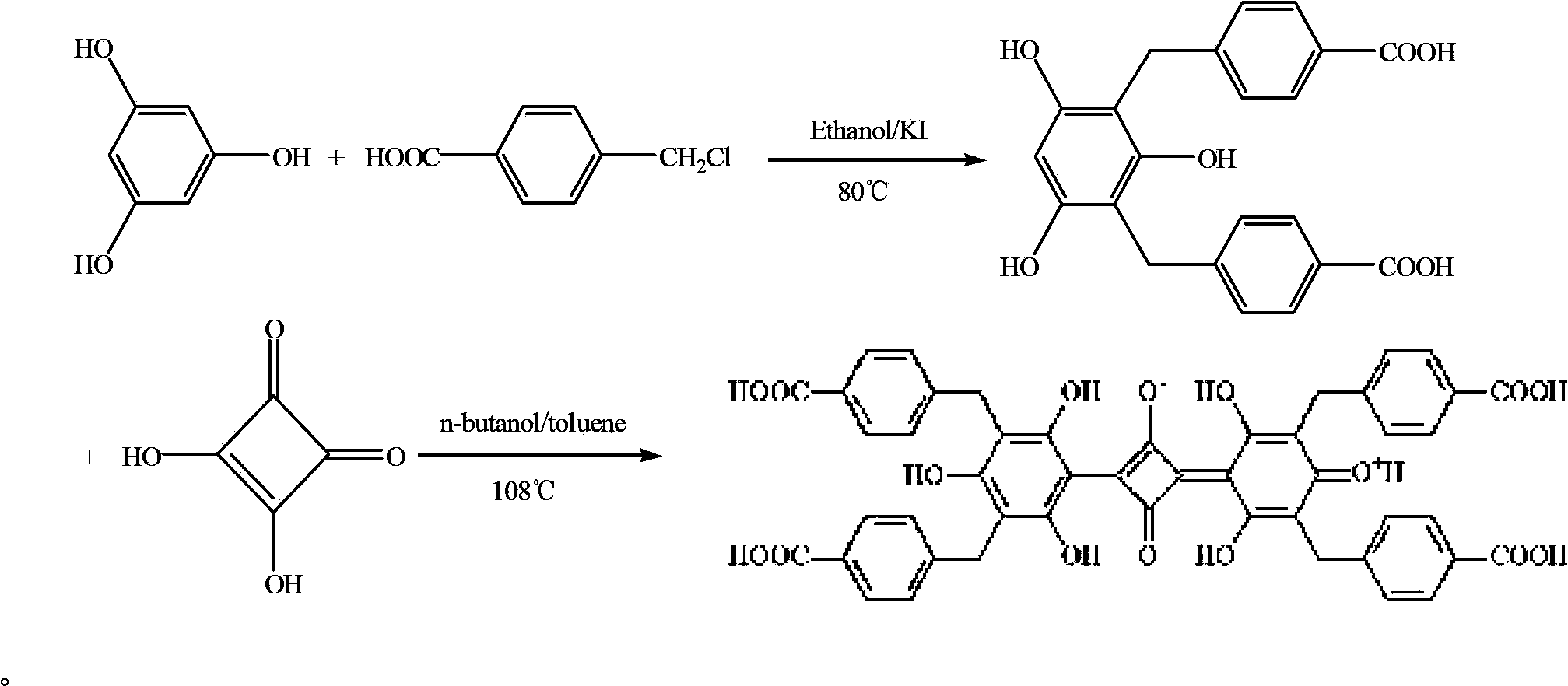
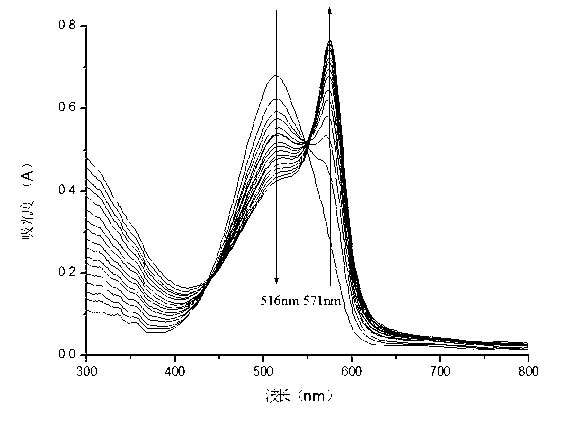
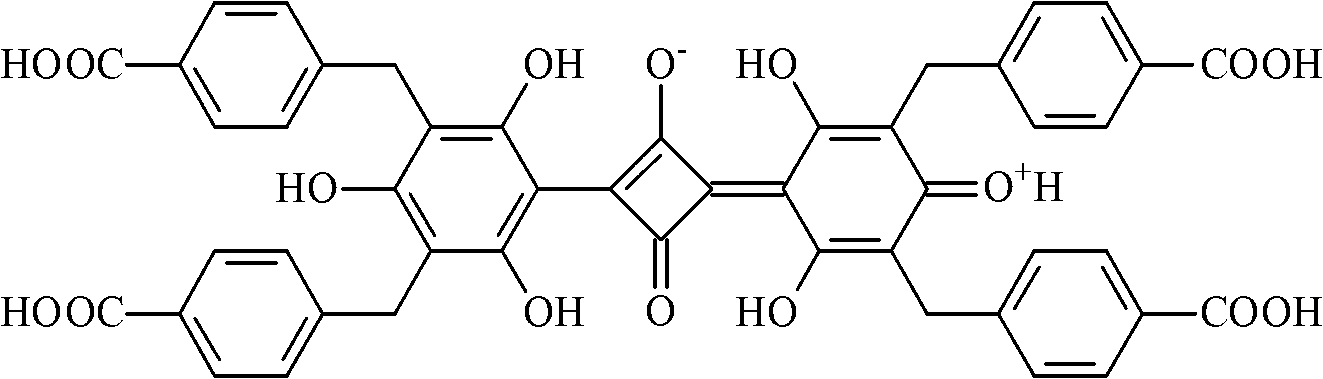
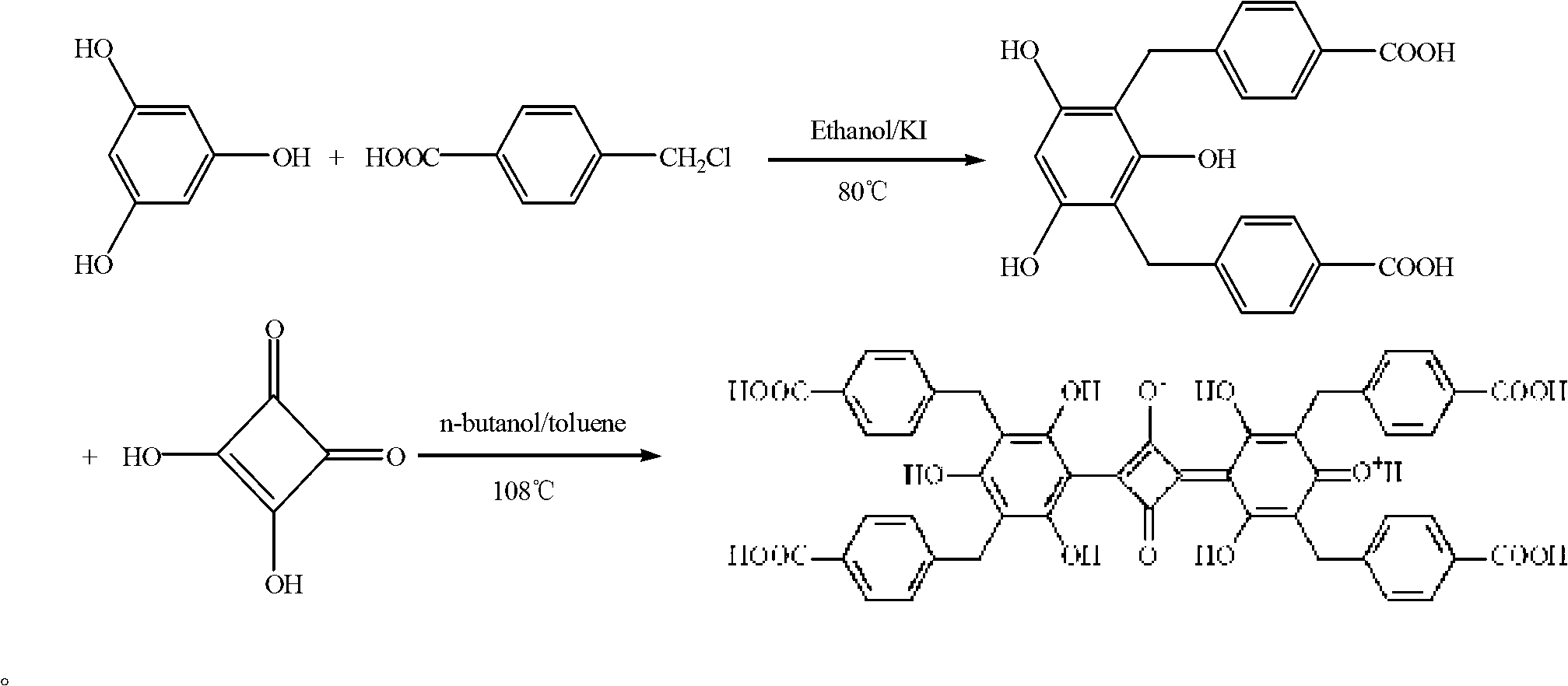
Belonging to the field of chemical analysis and test, the invention relates to a squarylium cyanine chemical sensor used for Fe<3+> detection and a preparation method thereof. The squarylium cyanine chemical sensor has a symmetrically structured 2, 4, 6-trihydroxy-3, 5-di(p-carboxybenzyl)phenyl)squarylium cyanine compound. The preparation method of the squarylium cyanine chemical sensor used for Fe<3+> detection consists of: preparation of an intermediate 1, 3, 5- trihydroxy-2, 4-di(p-carboxybenzyl)phenyl; and preparation of bis(2, 4, 6-trihydroxy-3, 5-di(p-carboxybenzyl)phenyl)squarylium cyanine. The squarylium cyanine compound provided in the invention has a simple synthesis method and easily controllable reaction conditions, and by means of a simple treatment, a pure product can be obtained. The obtained squarylium cyanine compound has excellent optical performance and optical stability. The squarylium cyanine compound provided in the invention contains carboxyl and hydroxyl groups, has certain water-solubility, and has good selectivity for iron ions.
Owner:CHANGZHOU UNIV +1
Preparation method of N-carbobenzoxy-2-amino-alkyl sulfonamide
InactiveCN102675165ARaw materials are non-toxic and easy to obtainSimple and fast operationSulfonic acid amide preparationImideCombinatorial chemistry
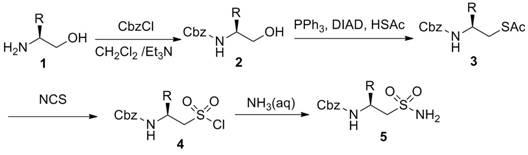
The invention provides a preparation method of N-carbobenzoxy-2-amino-alkyl sulfonamide. The preparation method of N-carbobenzoxy-2-amino-alkyl sulfonamide includes using vicinal alkamine as material, and sequentially performing carboxybenzyl protection, Mitsunobu reaction, NCS (N-chlorosuccinimide) oxidation and ammonolysis reaction to obtain the N-carbobenzoxy-2-amino-alkyl sulfonamide. The materials used in the method are nontoxic and easy to obtain, simple and convenient to operate, high in reproducibility and high in yield. The preparation method is a current most effective method of synthesizing amino-alkyl sulfonamide. The obtained compound can be used as an enzymatic inhibitor, raw materials to prepare sulfonopeptide and the like.
Owner:BEIJING UNIV OF CHEM TECH
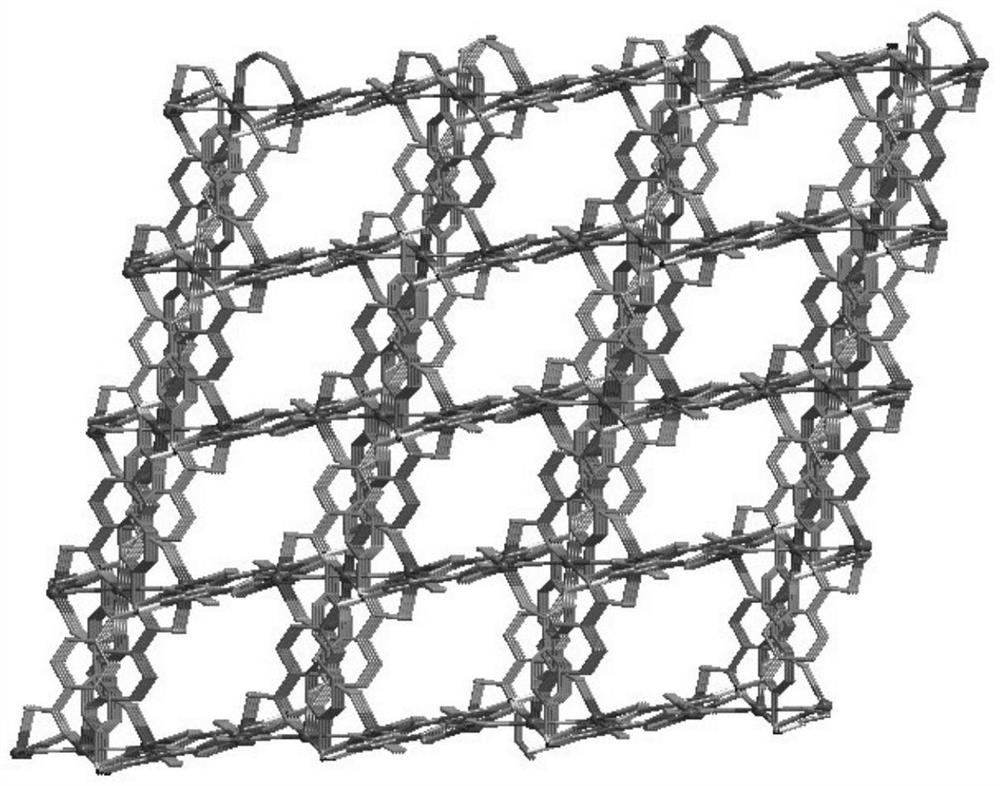
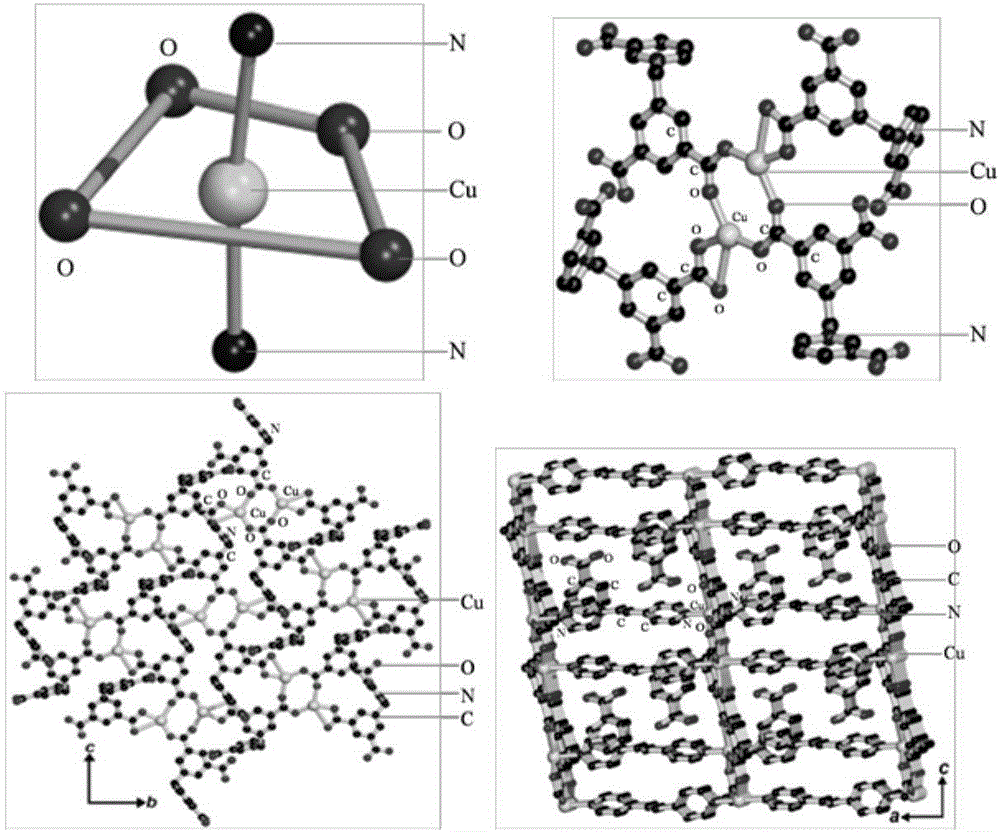
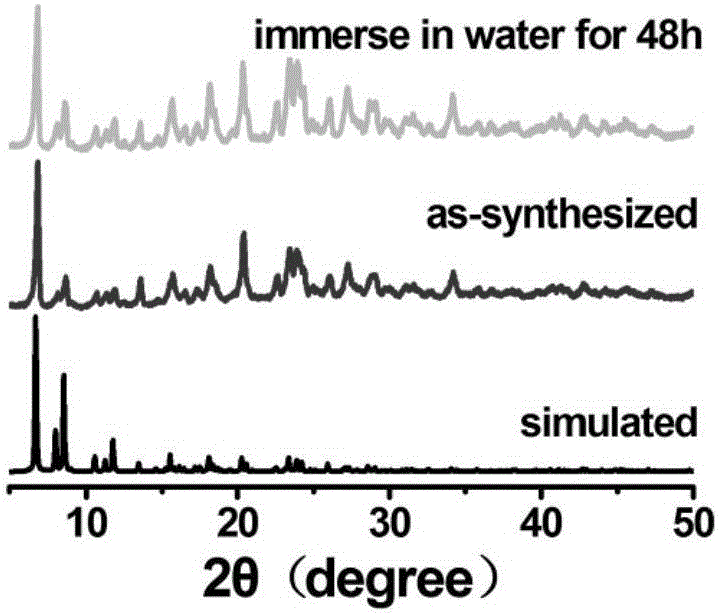
Preparation method and application of cadmium-based metal organic framework Cd-MOF material
InactiveCN112300400AImprove flexibilityLarge conjugated structureFluorescence/phosphorescenceLuminescent compositionsCarboxyl radicalMetal-organic framework
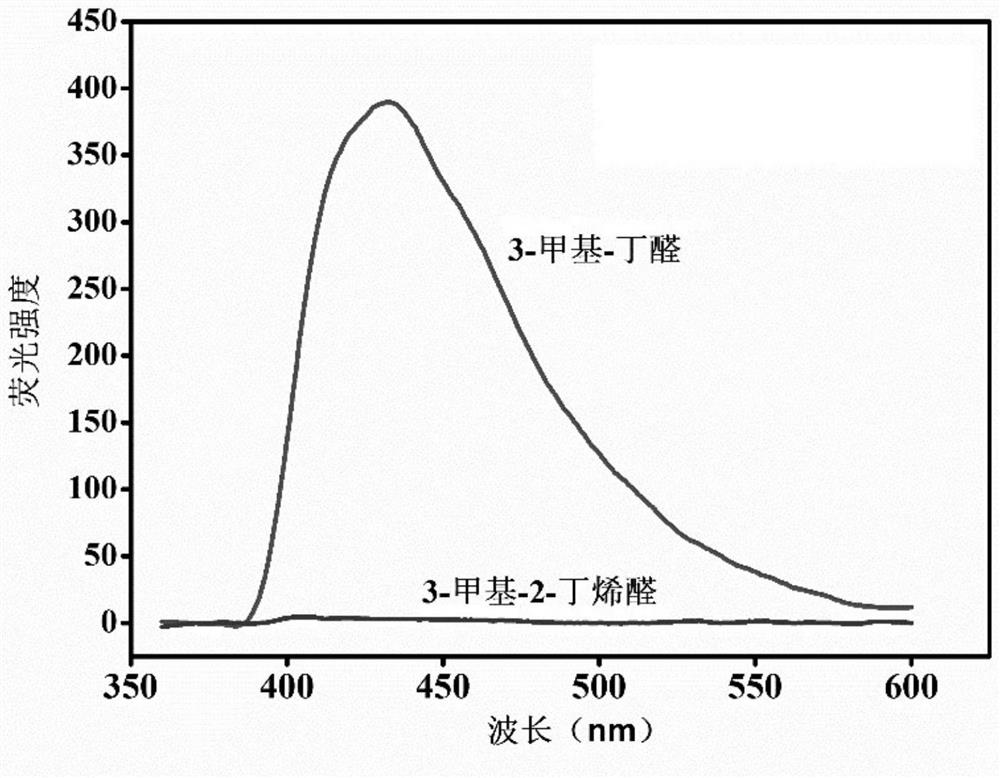
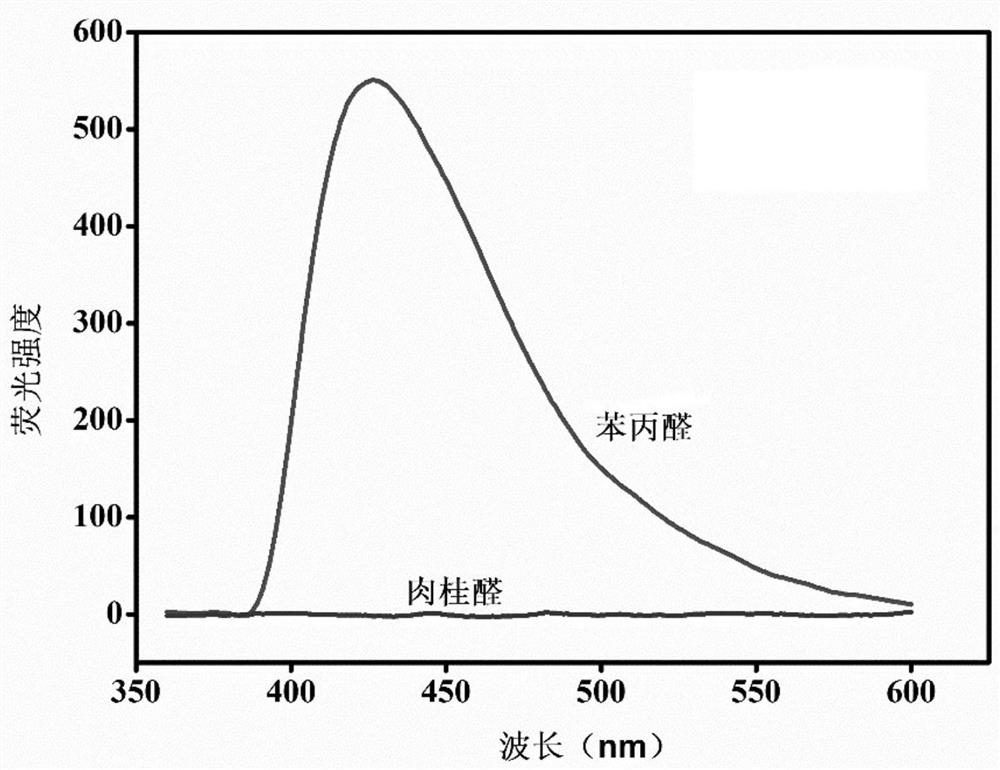
The invention provides a preparation method and application of a cadmium-based metal organic framework Cd-MOF material. The preparation method is characterized by comprising the following steps: step1, dissolving cadmium nitrate in water, dissolving a ligand 5-(bis(4-carboxylbenzyl)amino isophthalic acid in N,N-diethylformamide, and mixing the ligand 5-(bis(4-carboxylbenzyl)amino isophthalic acidand N,N-diethylformamide with ethanol; step 2, pouring the mixed solution into a polytetrafluoroethylene reaction kettle lining, and carrying out ultrasonic treatment; step 3, putting the reaction kettle into a blast drying oven for heating; and step 4, cooling the reaction kettle to room temperature, and filtering the reaction solution to obtain the yellow petal-shaped Cd-MOF crystal. The synthesis method of the Cd-MOF fluorescent material is simple, the yield and the purity are high, the accurate structure of MOF can be analyzed through single crystal diffraction, and the Cd-MOF fluorescentmaterial synthesized by the method has the characteristics of simple operation, less time consumption and low cost when alpha,beta-unsaturated aldehyde and saturated aldehyde are distinguished and detected.
Owner:XUCHANG UNIV
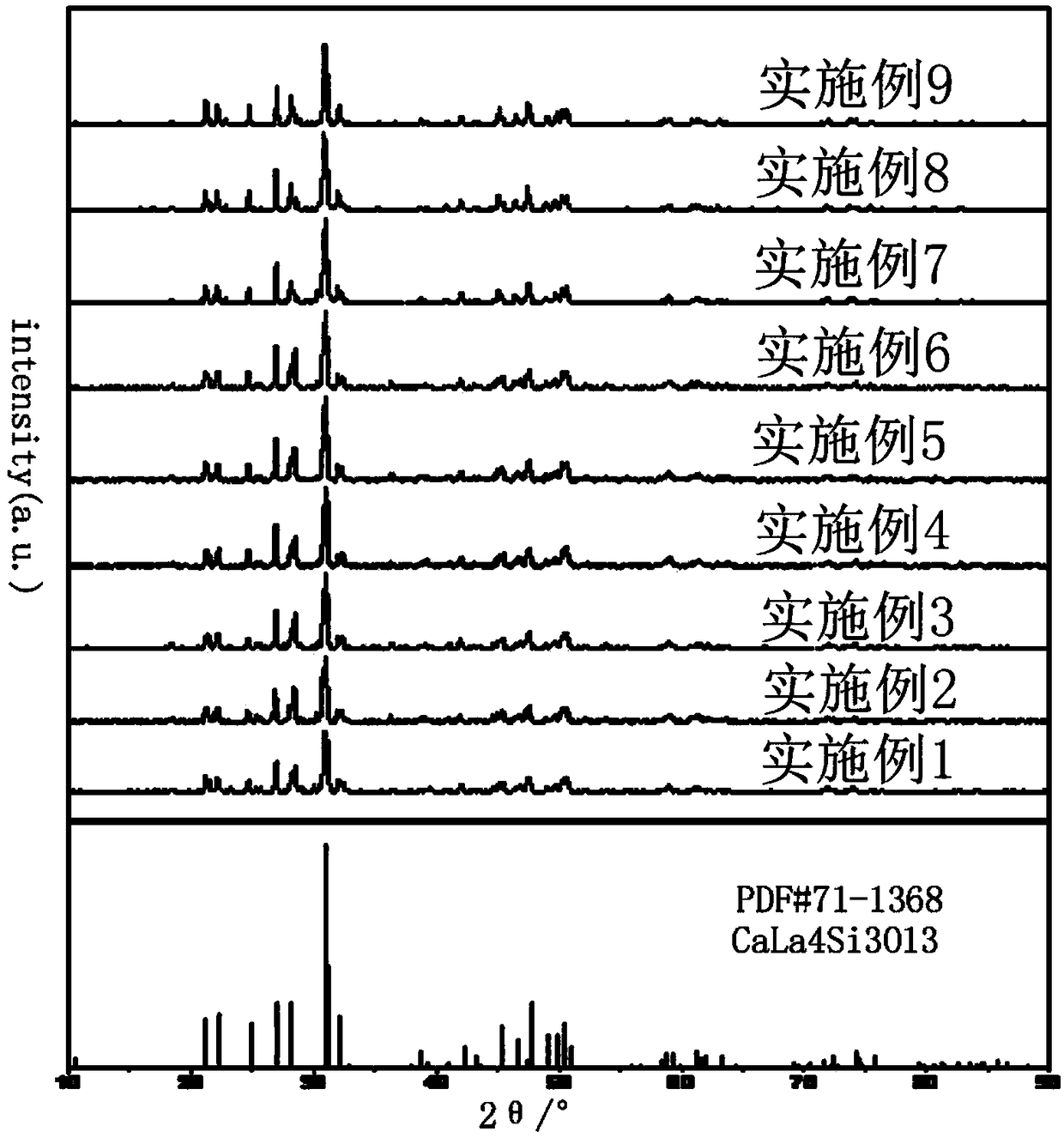
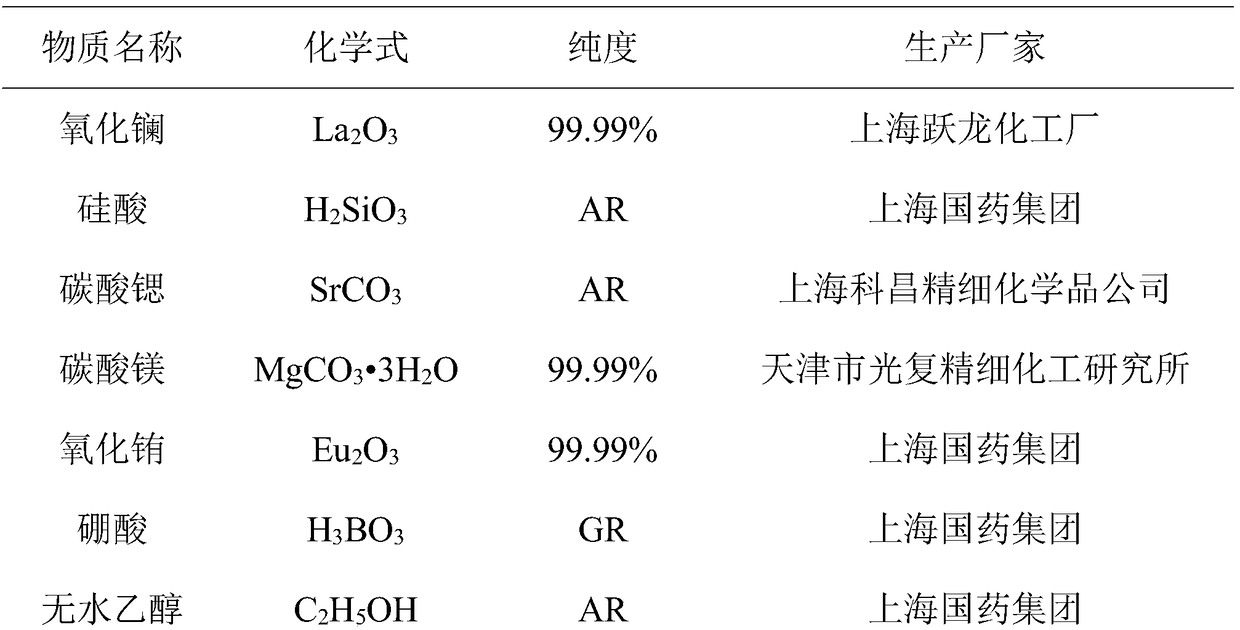
Mixed-valence europium-doped strontium magnesium lanthanum oxygen-based apatite silicate luminescent material and preparation method thereof
ActiveCN108913136AConducive to high-efficiency light emissionGood chemical stabilityEnergy efficient lightingLuminescent compositionsSilicic acidApatite
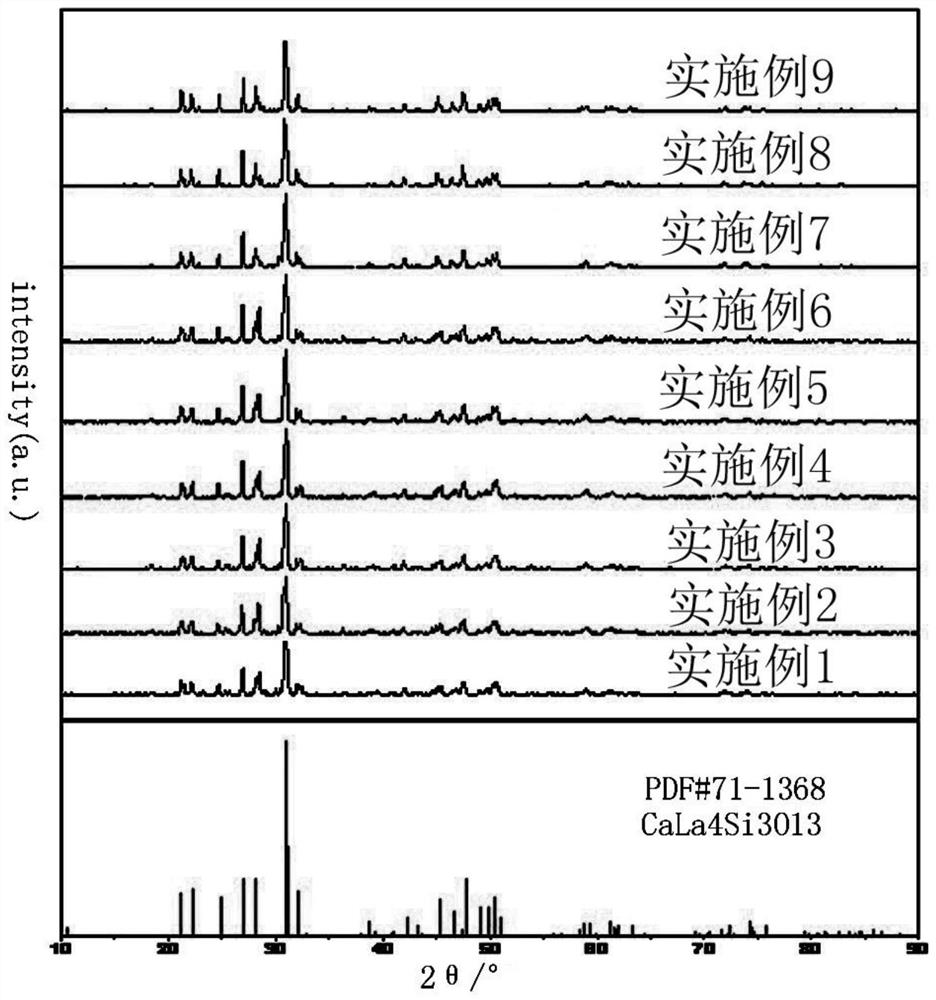
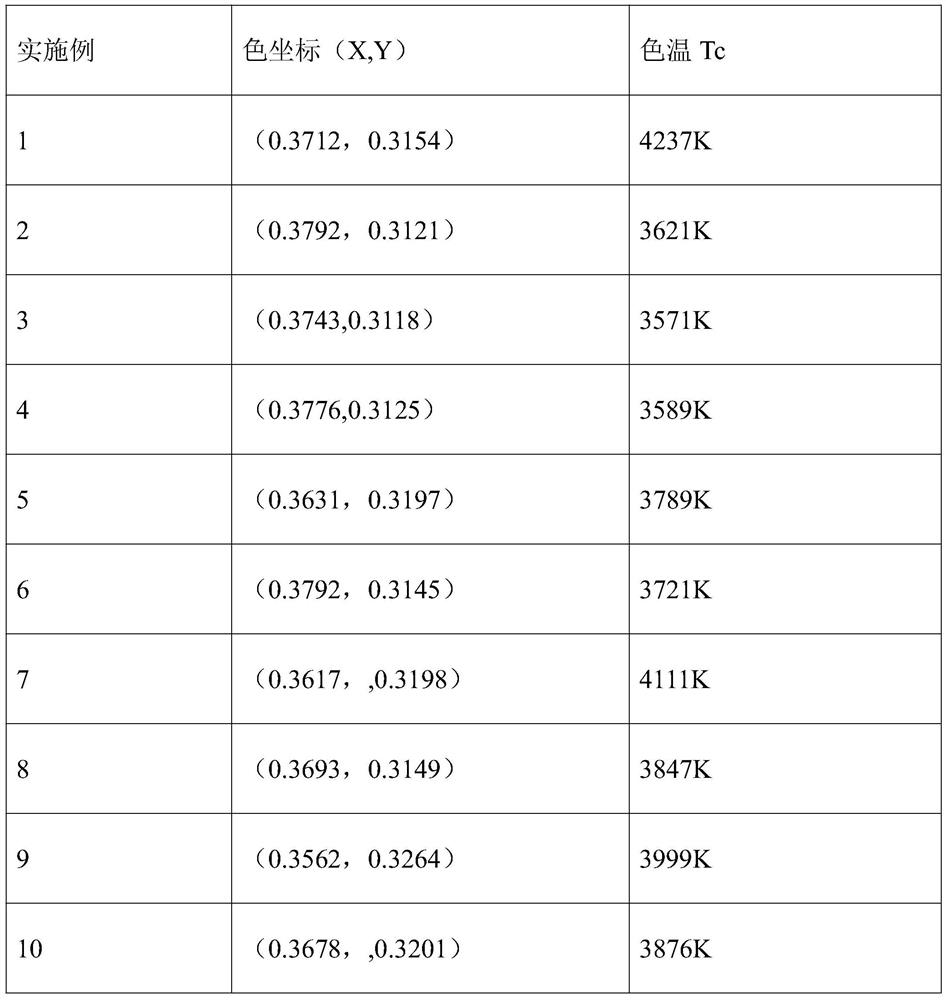
The invention discloses a mixed-valence europium-doped strontium magnesium lanthanum oxygen-based apatite silicate luminescent material. Strontium magnesium lanthanum silicates are used as a fluorescent body matrix, europium ions are used as doping ions, and the raw materials are strontium carbonate, magnesium carbonate, lanthanum oxide, silicic acid and europium oxide. At the same time, a fluxingagent boric acid and an accelerant are added, wherein the addition amount of the boric acid is 2%-2.5% of the total mass of the raw materials, and the addition amount of the accelerant is 1.5% of thetotal mass of the raw materials. The luminescent material is prepared by a high-temperature solid-phase method. The accelerator comprises the following components: titanium bis(triethanolamine)diisopropoxide, 2,5-difluorobenzylzinc bromide, (3,4-epoxycyclohexyl)ethyltriethoxysilane, (4-carboxybenzyl)methylbicarbamic acid dibenzyl ester, bis(2,2,6,6-tetramethyl-3,5-heptanedionato)calcium, and tetrakis(ethylmethylamino)zirconium. The final luminescent material gives out pure white light, the color rendering index can reach 90, the color temperature is around 4000 K, and the luminescent materialcan be used for indoor lighting.
Owner:WENZHOU UNIVERSITY
Preparation method and application of benzofuran derivate
The invention discloses a preparation method and application of a benzofuran derivate. The preparation method of the benzofuran derivate comprises the following steps: (a) carrying out an organic reaction on a compound shown in formula II with a compound shown in formula III to obtain a compound shown as formula I, wherein X represents chlorine, bromine, iodine, amino, substituted amino, hydroxyl or substituted hydroxyl; the substituted amino is -NR1R2, the substituted hydroxyl is -O-SO2R3, wherein R1 and R2 respectively represent hydrogen, tert-butoxycarbonyl, carboxybenzyl, toluenesulfonyl, trifluoroacetyl, formoxyl and triphenylmethyl; R3 represents methyl, ethyl, propyl, phenyl and p-methylphenyl; and Y represents fluorine, chlorine or bromine. The invention also discloses the application of the prepared benzofuran derivate as an intermediate in preparing Dronedarone.
Owner:ZHEJIANG ACAD OF MEDICAL SCI +1
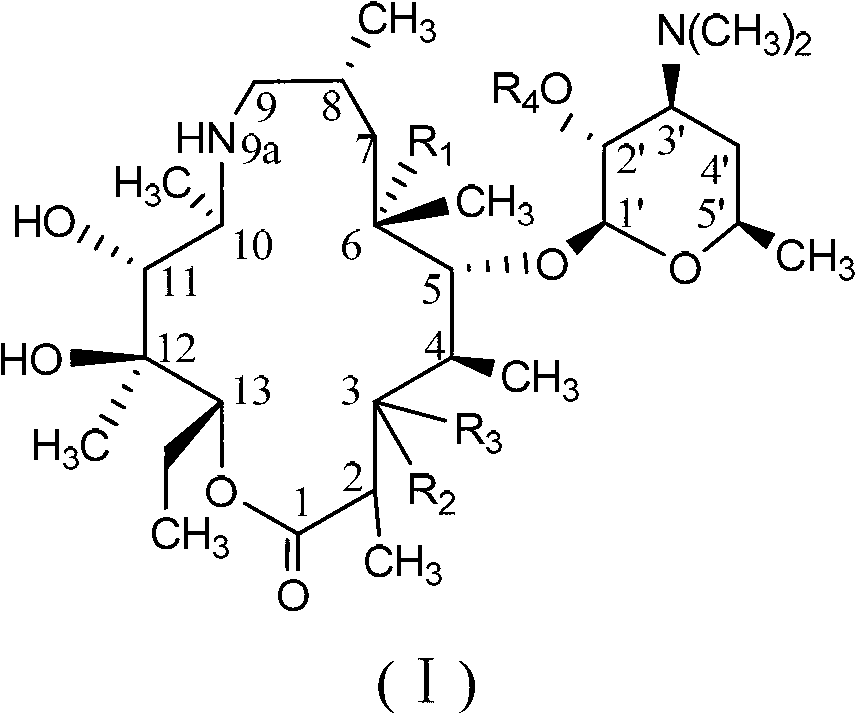
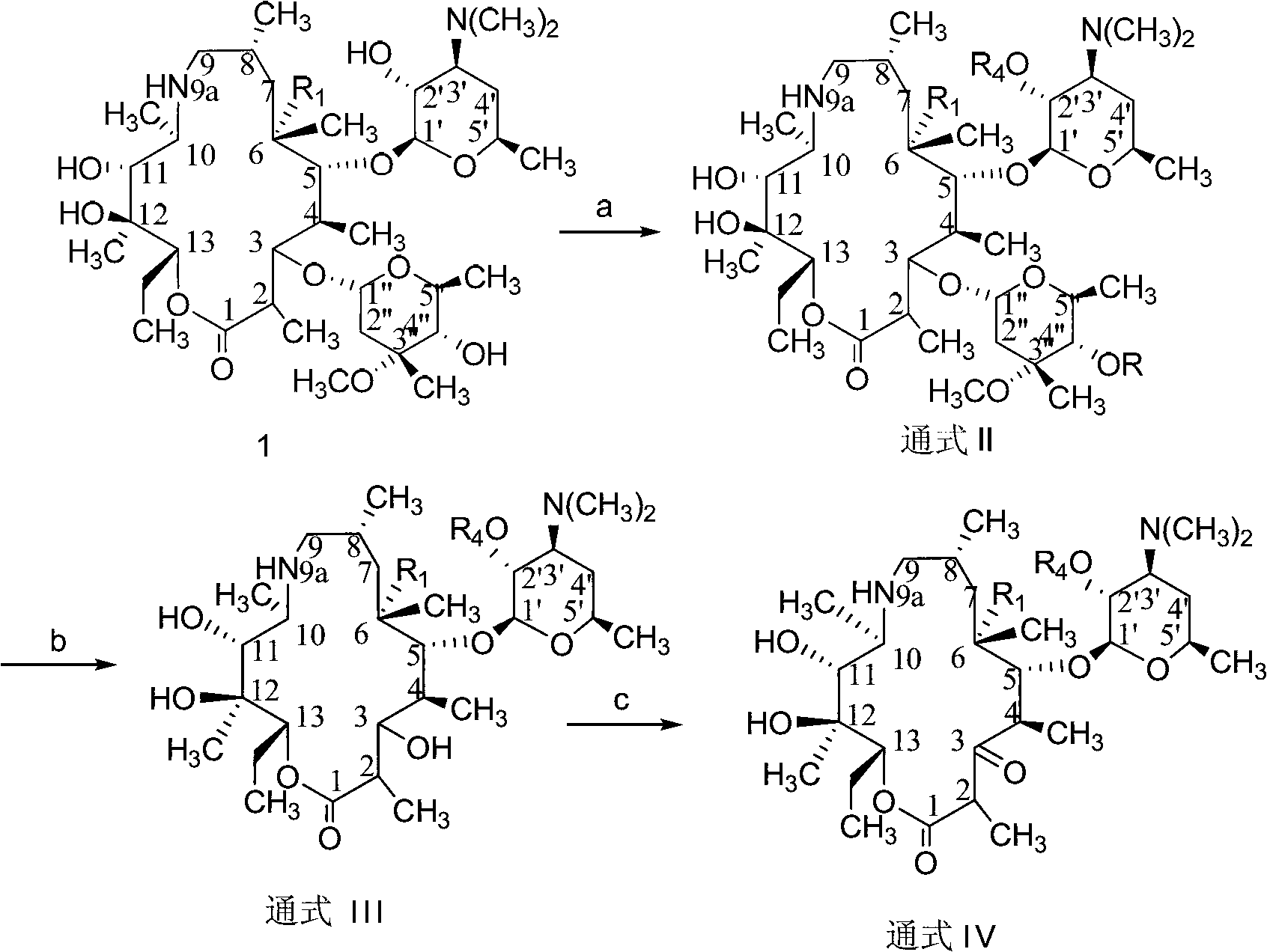
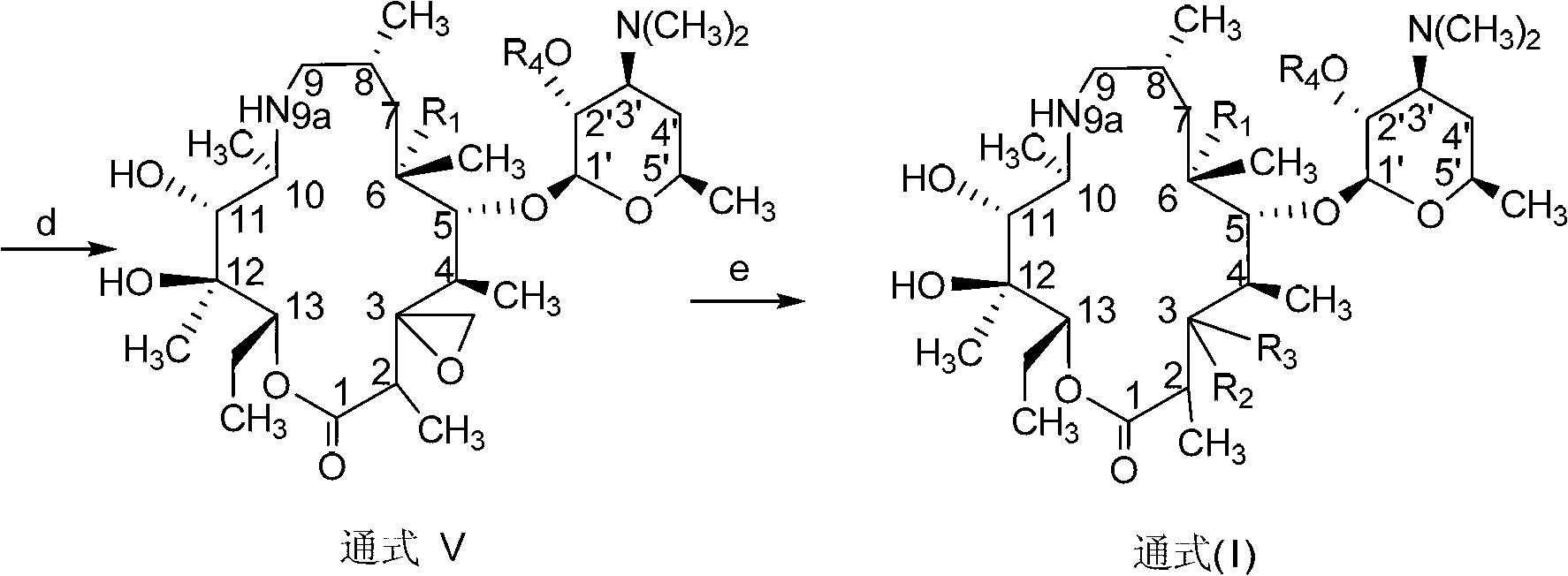
C-3 substituted-9-deoxidized-9A-aza-9A-high erythromycin A derivative
The invention provides a C-3 substituted-9-deoxidized-9A-aza-9A-high erythromycin A derivative which has a structure shown as formula (I), wherein R1 is H, hydroxyl or methoxyl; R2 is hydroxyl; R3 is an organic matter substituted or unsubstituted by a heteroatom. The heteroatom is one or more of halogen, N, O and S. R4 is H, carboxybenzyl, benzoyl or acetyl. The compound shown by formula (I) is an antibacterial agent and can be used to treat various bacterial and protozoon infections. The invention further relates to an application and a preparation method of the compound, and a composition formed by the compound.
Owner:LUOYANG HUIZHONG ANIMAL MEDICINE
High-purity and/or high-content penicillin sodium salt solid powder and preparing method
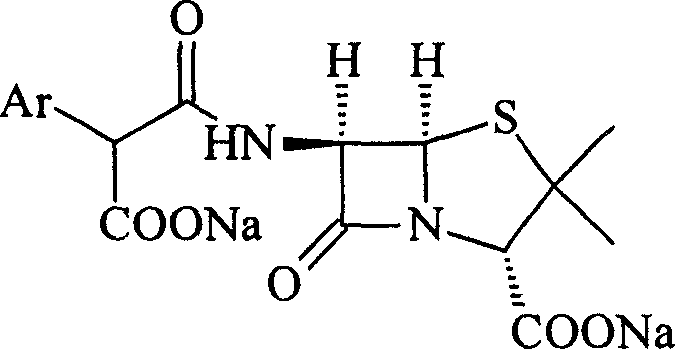
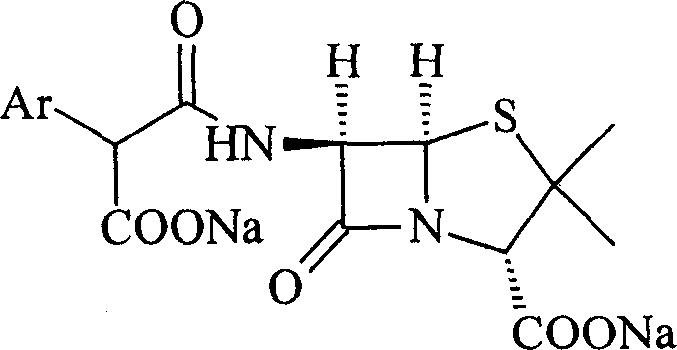
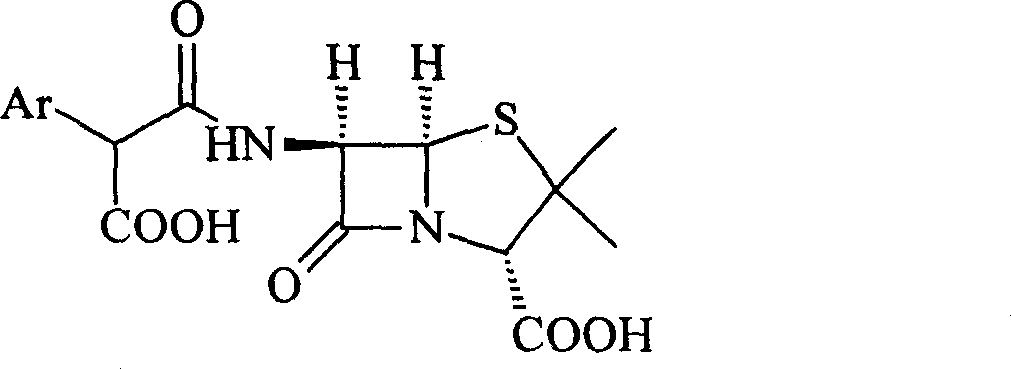
This invention relates to a high-purity and / or high-content sodium benzylpenicillin sodium solid powder and their medicine combination, as well as preparation method. Such as: sodium salt of Ticarcillin and carbenicillin carboxybenzyl penicillin.
Owner:马启明
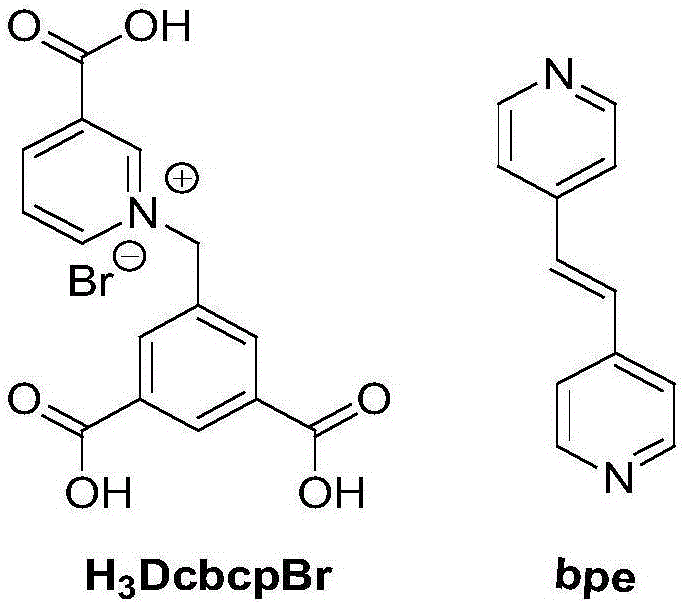
Amphoteric carboxylic acid three-dimensional metal coordination polymer, and preparation method and application thereof
The invention relates to an amphoteric carboxylic acid three-dimensional metal coordination polymer, and a preparation method and application thereof. The invention specifically discloses a water-soluble amphoteric carboxylic acid three-dimensional metal coordination polymer, wherein the main ligand of the three-dimensional copper coordination polymer is N-(3,5-dicarboxylbenzyl)-(3-carboxyl)-pyridine bromide; the auxiliary ligand of the polymer is 1,2-bis(4-pyridyl)ethylene; and central atom of the polymer is copper. According to the invention, raw materials are widely and easily available; the preparation method is simple in process, mild in reaction conditions and good in repeatability; and the amphoteric carboxylic acid three-dimensional metal copper coordination polymer has good water-solubility, can be successfully applied to detection of nucleic acid sequences of AIDS, SUDV, dengue viruses and the like, has the advantages of rapidness, convenience, high efficiency and sensitivity, and shows good application prospects in detection of related diseases.
Owner:SOUTHERN MEDICAL UNIVERSITY
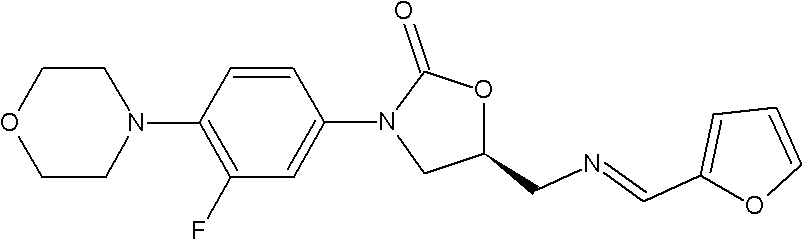
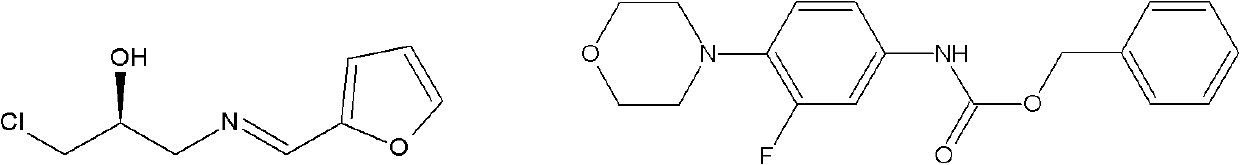
Intermediate for preparing linezolid
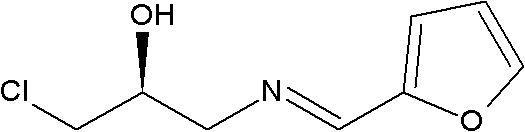
The invention discloses an intermediate for preparing linezolid, which is a compound shown in a structural formula I. The invention further discloses a method for preparing the compound shown in the structural formula I. The method comprises the steps of reacting S)-1-chlorine-3-[(furan-2-methylene)amino]propyl-2-alcohol and N-carboxybenzyl-3-fluorin-4-morpholine phenylamine and generating the compound shown in the structural formula I. According to the method disclosed by the invention, raw and auxiliary materials are low in cost and are easy to obtain; furthermore, the process route is short; the cost is low; the reaction condition is moderate and safe; the device does not have specific requirements; and the method is applied to industrial production.
Owner:ZHEJIANG YATAI PHARMA
Melt direct spinning porous superfine polyester fiber and preparation method thereof
PendingCN114108120AGuaranteed qualityEliminate reticulationSpinning head liquid feederMonocomponent polyesters artificial filamentFiberPolymer science
The invention relates to a melt direct-spinning porous superfine polyester fiber and a preparation method thereof, sodium borohydride is added in esterification reaction of terephthalic acid and ethylene glycol, after esterification reaction, the melt direct-spinning porous superfine polyester fiber is obtained through condensation polymerization and a spinning process; wherein the addition amount of the sodium borohydride is 30-50ppm of the weight of terephthalic acid, and due to the reaction characteristics and chemical selectivity of the sodium borohydride, the sodium borohydride reacts with p-carboxybenzaldehyde in the terephthalic acid before esterification in polyester synthesis to generate p-carboxybenzyl alcohol, so that a net structure formed by the p-carboxybenzaldehyde in the polyester synthesis process is eliminated; according to the melt direct-spinning porous superfine polyester fiber, the filtering performance of polyester melt is improved, the quality of the porous superfine polyester fiber is guaranteed, the replacement period of a filter and a spinning assembly is prolonged, and the prepared melt direct-spinning porous superfine polyester fiber has excellent breaking strength and breaking elongation performance.
Owner:JIANGSU HENGKE ADVANCED MATERIALS CO LTD
3-trifluoromethyl-2,5-diazabicyclo[2.2.1] heptane derivant and preparation method thereof
InactiveCN102167702AChange solubilityAlter biological metabolic stabilityOrganic chemistryBulk chemical productionSolubilitySide chain
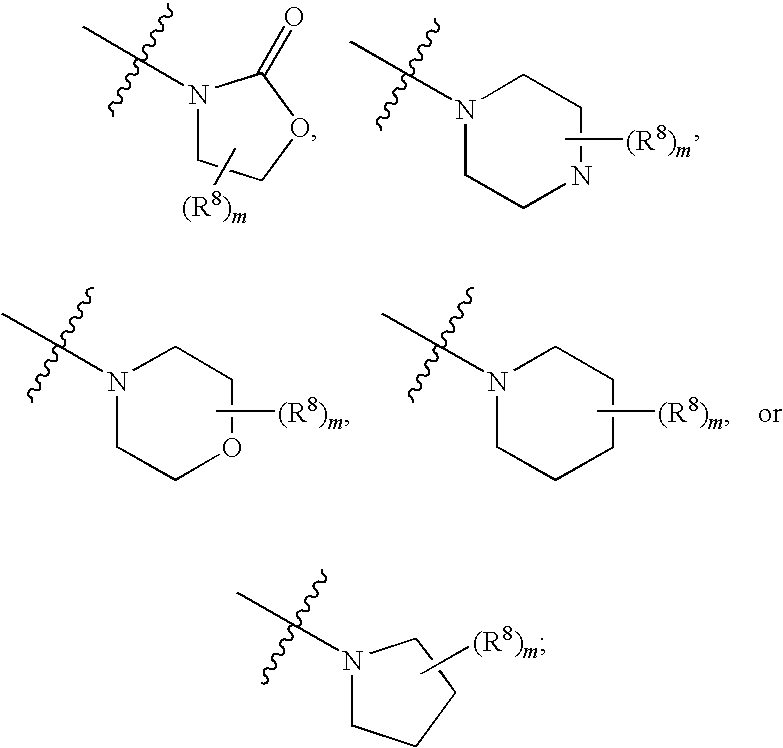
The invention discloses a 3-trifluoromethyl-2,5-diazabicyclo[2.2.1] heptane derivant and a preparation method thereof, mainly aiming to solve the technical problems that bridged compounds with structure of 2,5-diazabicyclo[2.2.1] heptane are restrained in extension of a space structure, and the compounds have poor water solubility. The chemical structural formula is as follows in the specification, wherein X is NR1 or O, R1 is a functional group or amino-substituted protective group, and is selected from one of H, C1-10 linear-chain or substituent-side-chain-containing alkyl, benzyl, 2,4-dimethoxybenzyl, 4-methoxybenzyl, tertiarybutoxy carbonyl, carboxybenzyl, alkylacyl, aroyl, alkylsulfonyl or aryl sulfonyl, and R2 is a functional group or amino-substituted protective group, and is selected from one of H, C1-10 linear-chain or substituent-side-chain-containing alkyl, benzyl, 2,4-dimethoxybenzyl, 4-methoxybenzyl, carboxybenzyl, alkylacyl, aroyl, alkylsulfonyl or aryl sulfonyl.
Owner:上海药明康德新药开发有限公司
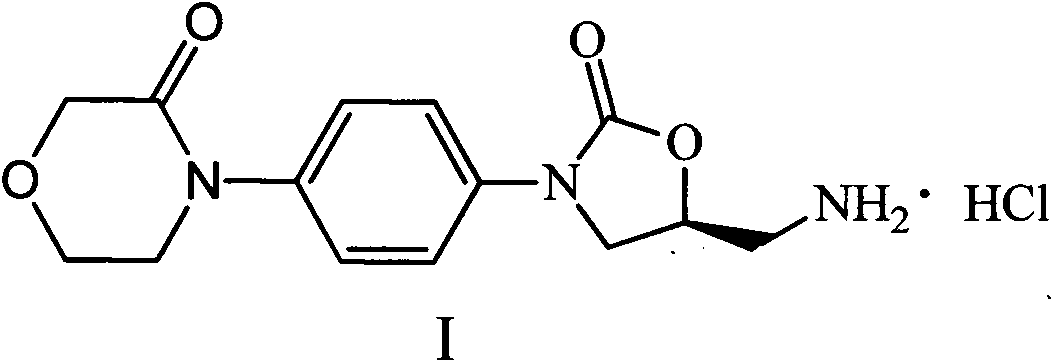
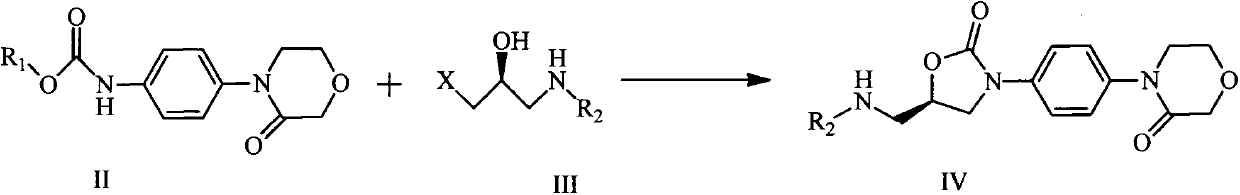
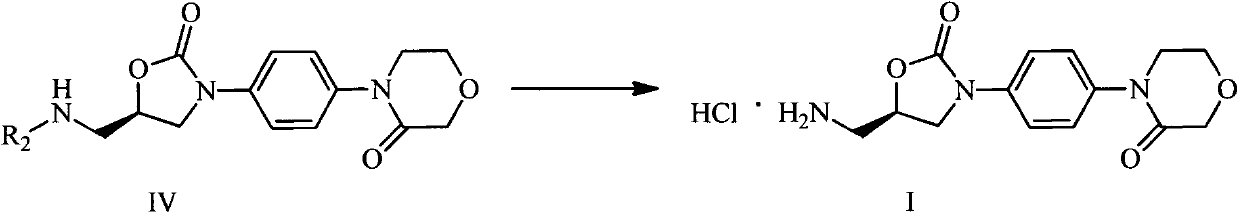
Rivaroxaban intermediate preparation method
InactiveCN103012389ARaw materials are cheap and easy to getSimple and fast operationOrganic chemistryArylOrganic solvent
The invention discloses a rivaroxaban intermediate 4-{4-[(5S0-5-(aminomethyl)-2-oxo-1,3-oxazolidine-3-radical]phenyl}morpholine-3-ketohydrochloride preparation method. The method includes the steps: step (1) reacting a compound II and a compound III in organic solvent under the action of alkaline to obtain a compound IV, wherein R1 refers to benzyl, substituent benzyl, aryl or substituent aryl, X refers to halogen and R2 refers to methoxycarbonyl, ethoxycarbonyl, tert-butoxycarbonyl, carboxybenzyl or trityl; and step (2) preparing a compound I by means of hydrolysis reaction of the compound IV in organic solvent under the action of acid. The rivaroxaban intermediate preparation method is simple and convenient in operation, mild in reaction condition, stable in quality, simple in post-processing and suitable for industrial production.
Owner:CHINA PHARM UNIV
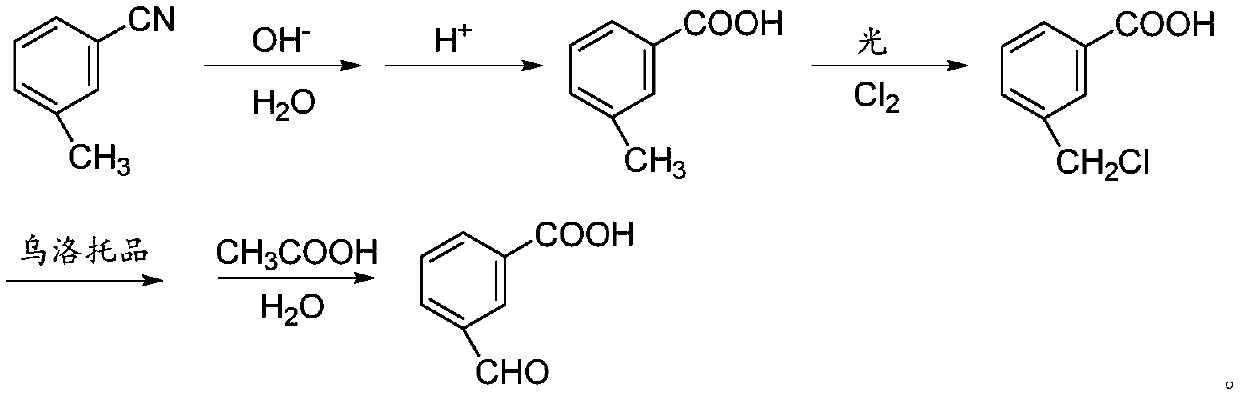
Preparation method of 3-carboxybenzaldehyde
InactiveCN110981719AHigh purityHigh yieldOrganic compound preparationPreparation from nitrilesBenzoic acidCarboxyl radical
The invention discloses a preparation method of 3-carboxybenzaldehyde. The method comprises the following steps: by using m-toluonitrile as a starting raw material, carrying out a first hydrolysis reaction, and adding an acid to carry out an acidification reaction to obtain m-toluic acid; carrying out chlorination reaction on m-toluic acid to obtain 3-carboxyl benzyl chloride; mixing the 3-carboxyl benzyl chloride and urotropin for an oxidation reaction, then adding glacial acetic acid and water for a second hydrolysis reaction to obtain the 3-carboxybenzaldehyde. According to the method disclosed by the invention, m-toluonitrile with low cost is used as a raw material, the 3-carboxybenzaldehyde is synthesized through a series of processes of hydrolysis, chlorination, oxidation and hydrolysis, and the product purity and yield are relatively high; the method has the advantages of simple and safe process operation, easily available raw materials and low cost and is suitable for industrial production.
Owner:黄石市利福达医药化工有限公司
An improved process for the preparation of boronic acid esters
ActiveUS20200031850A1Avoid tedious and long work-up procedureOrganic chemistry methodsGroup 3/13 element organic compoundsCarboxyl radicalTert-Butyloxycarbonyl protecting group
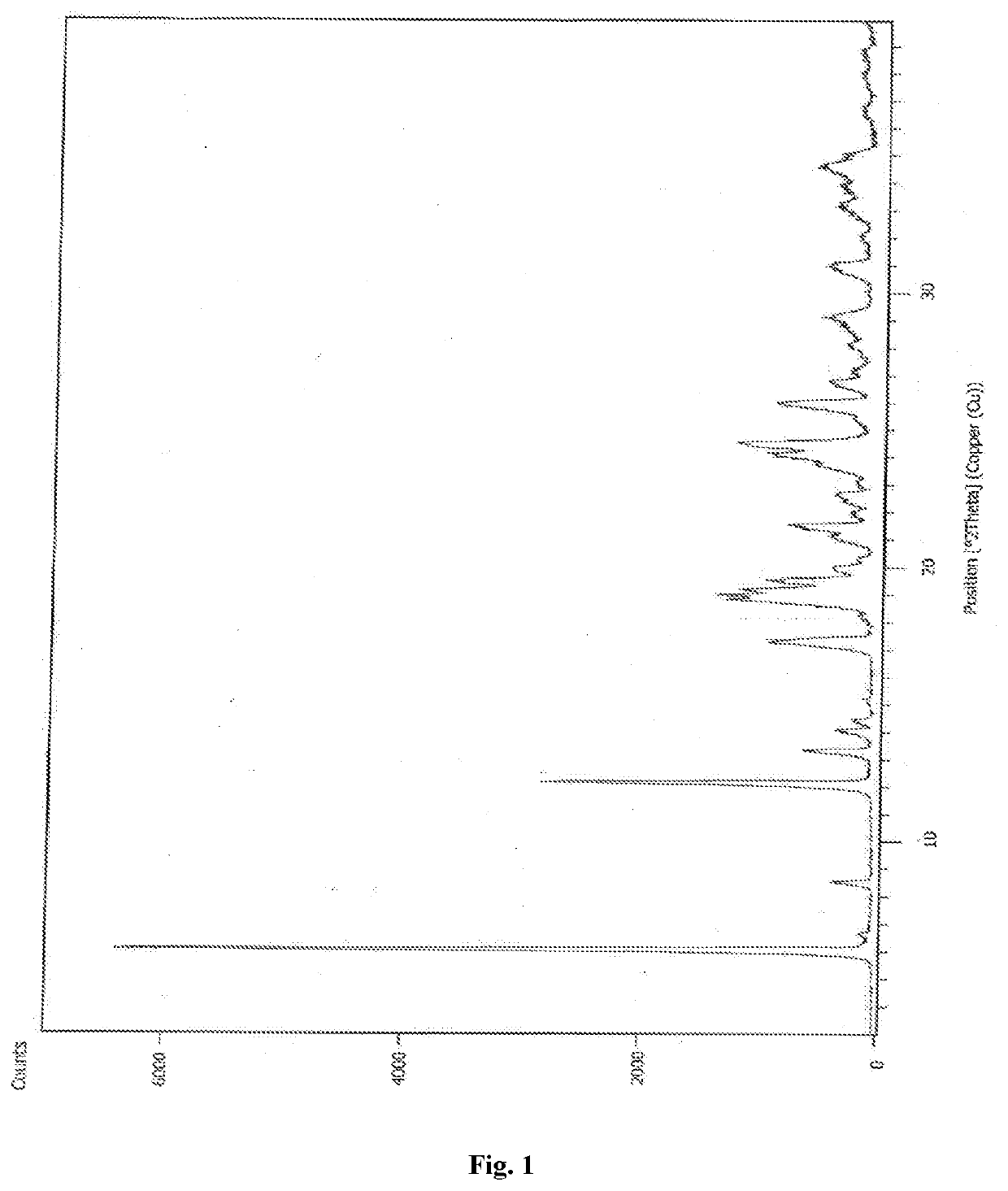
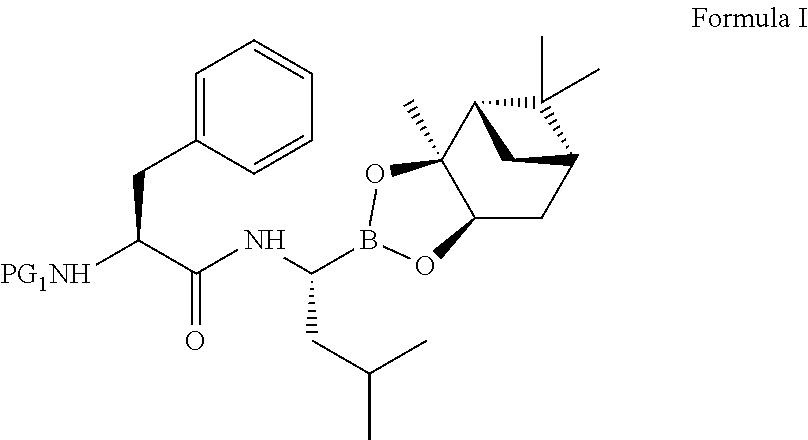
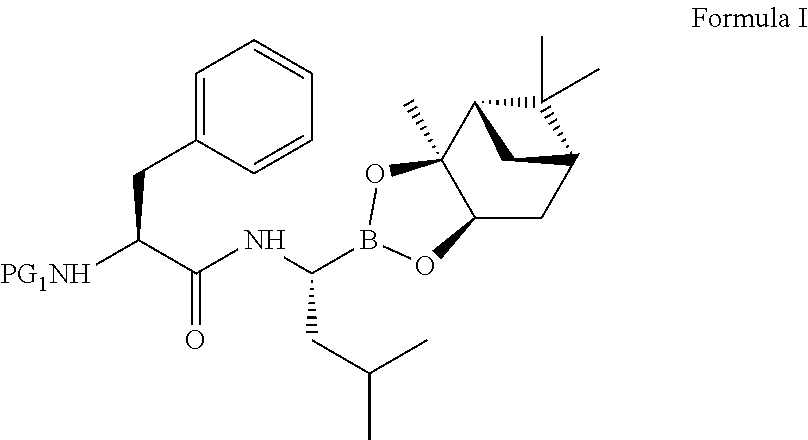
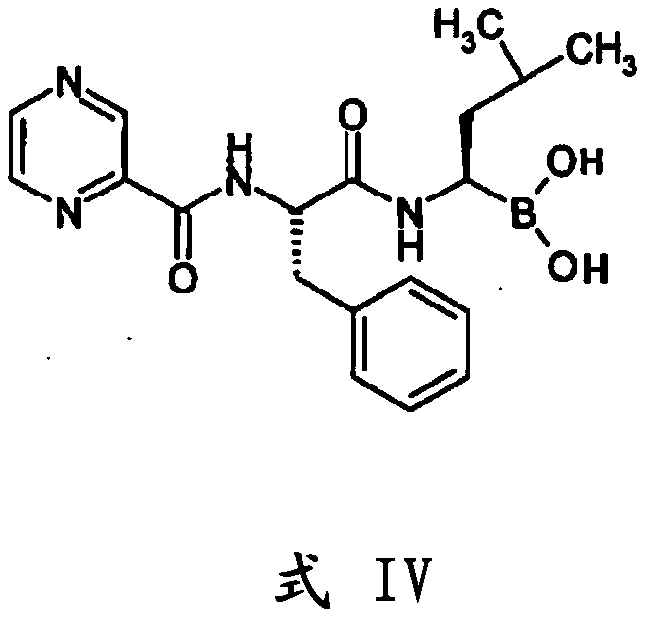
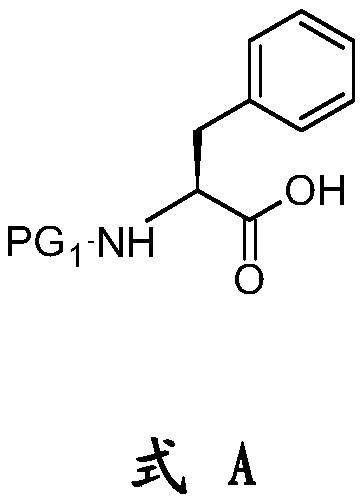
The present invention relates to an improved process for the preparation of a compound of formula (I), wherein PG1 may be independently selected from tert-butyloxycarbonyl (Boc), phthaloyl, 9-fluorenylmethyloxycarbonyl (Fmoc), triphenylmethyl (Trityl), carboxybenzyl (Cbz), trifluoroacetyl, benzyl (Bn), benzylidene, methanesulfonyl (Mesyl), toluene sulfonyl (Tosyl) or acyl; its isolation as solid and use for the preparation of the compound of formula (IV), in particular the compound of formula (IV) i.e. [(1R)-3-methyl-1[[(2S)-1-oxo-3-phenyl-2-[(pyrazinylcarbonyl) amino]propyl]amino]butyl] boronic acid with more than 99.95% chiral purity, as measured by HPLC.
Owner:FRESENIUS KABI ONCOLOGY LTD
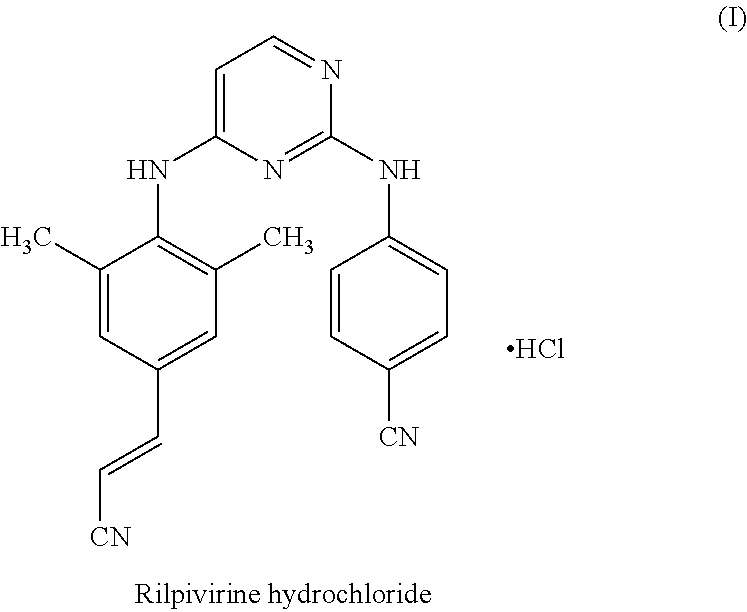
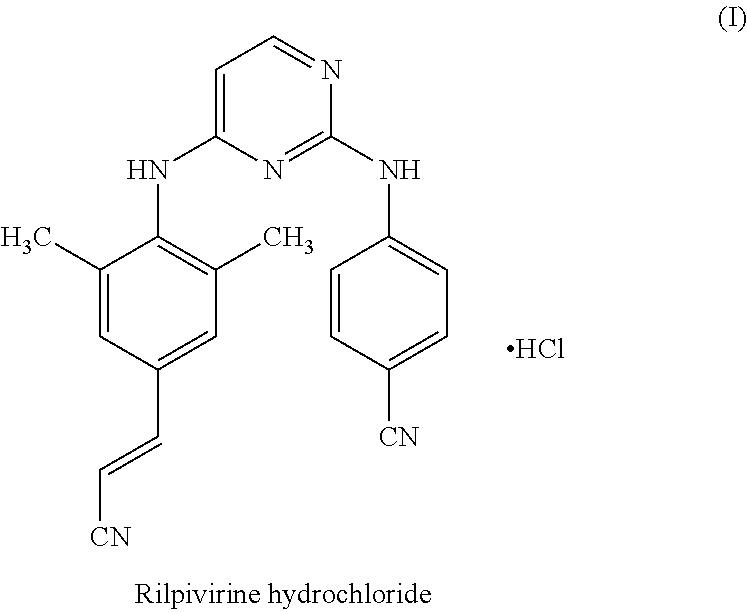
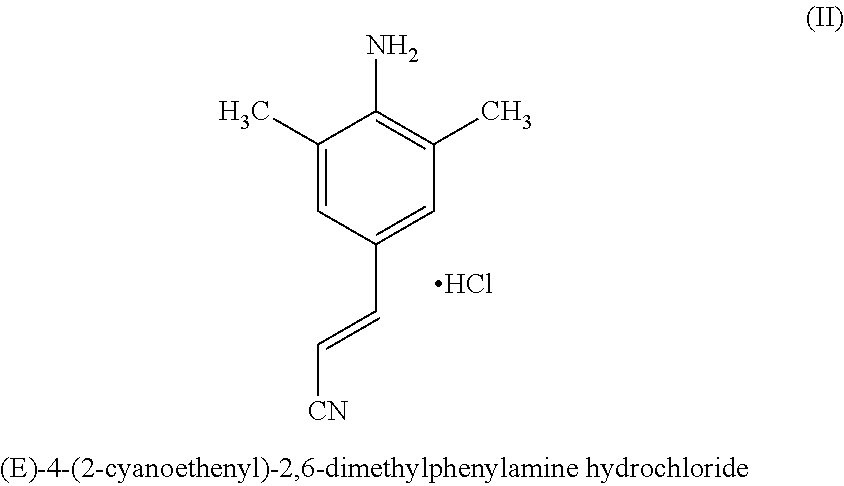
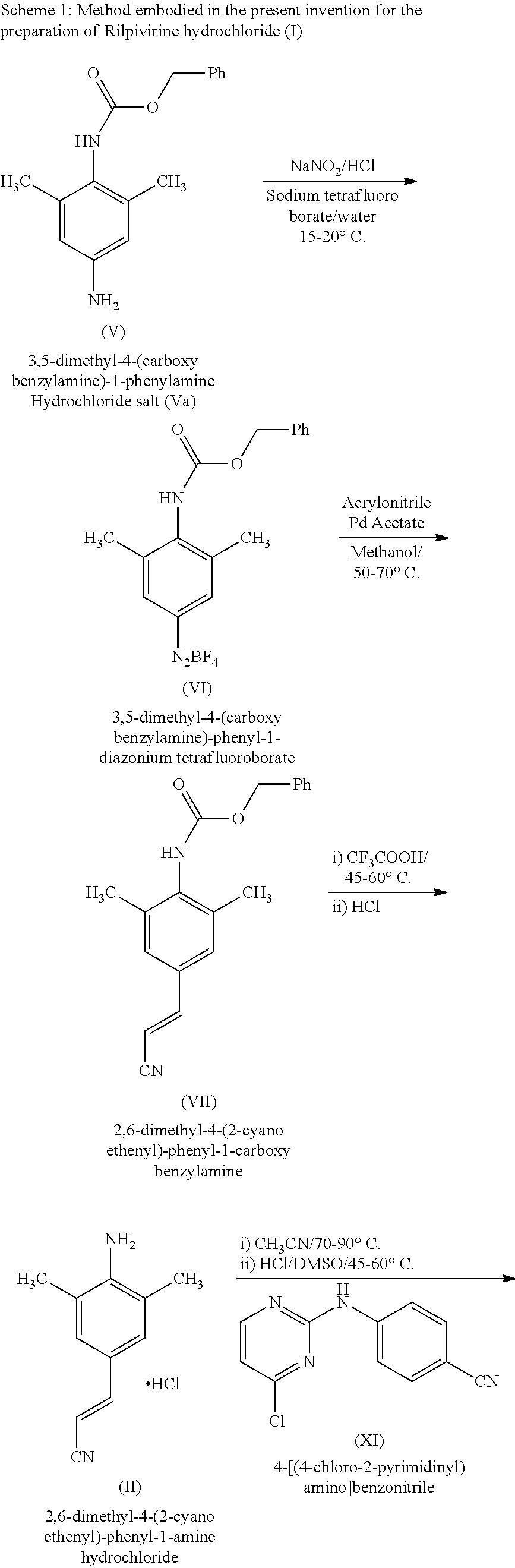
Rilpivirine process
InactiveUS20140275538A1Convenient and cost-effective and industrially viableCost-effectiveCarbamic acid derivatives preparationOrganic compound preparationDimethylaniline N-oxideTetrafluoroborate
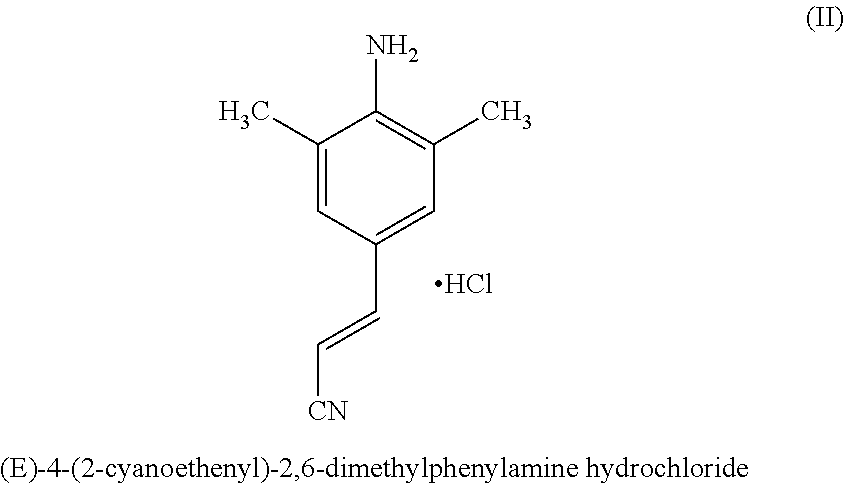
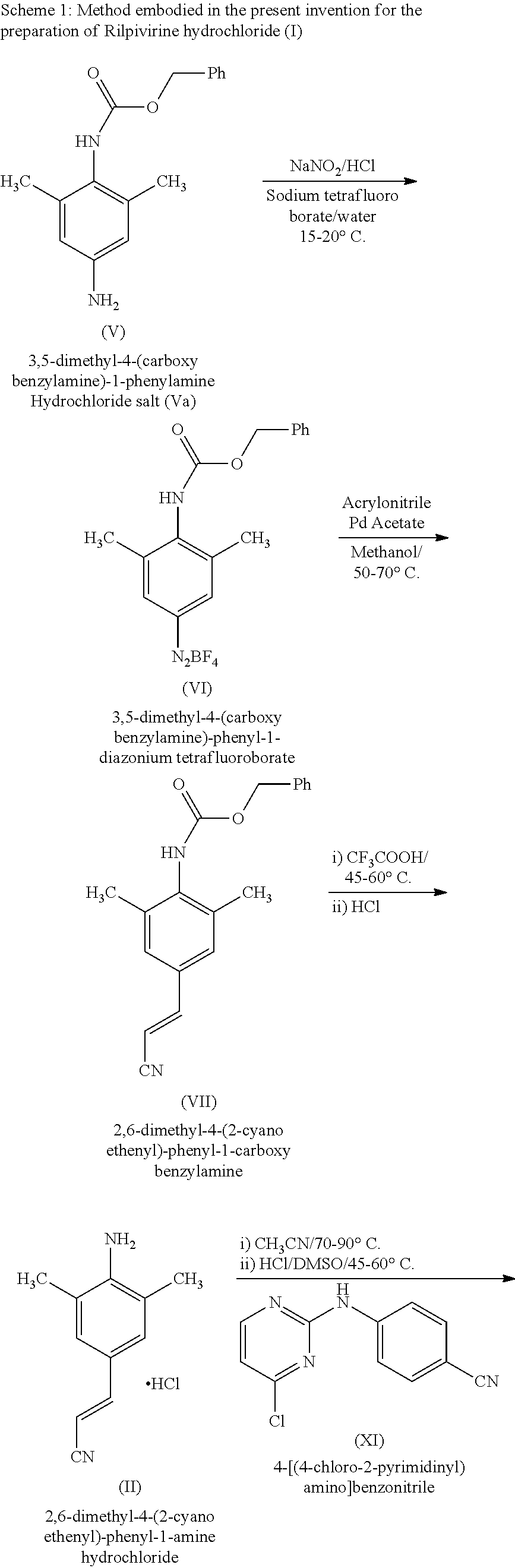
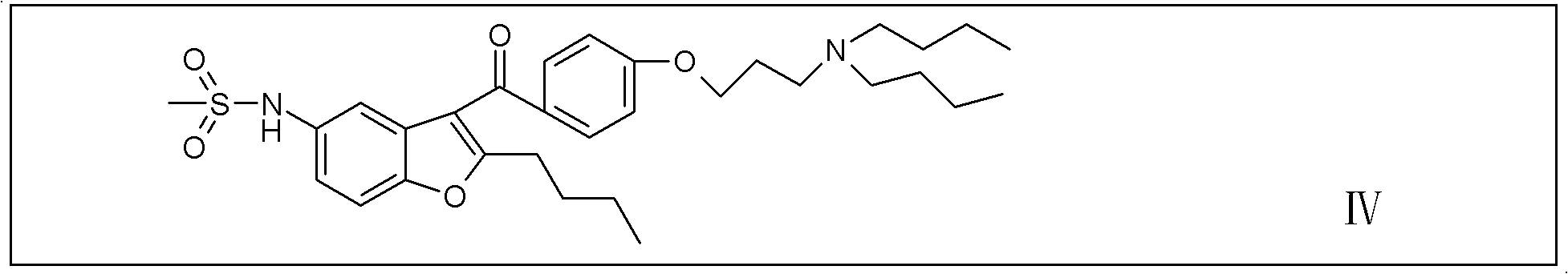
Disclosed is process for the preparation of a key Rilpivirine intermediate namely, (E)-4-(2-cyanoethenyl)-2,6-dimethylphenylamine hydrochloride (II) by a process comprising reaction of the tetrafluoroborate salt of the diazonium ion of 2,6-dimethyl-4-amino-1-carboxybenzyl phenylamine (VI) with acrylonitrile in presence of palladium acetate, followed by treatment with an acid and its subsequent conversion to the hydrochloride salt (II), wherein the undesired Z isomer is less than 0.5% and provides Rilpivirine hydrochloride having Z isomer less than 0.1%.
Owner:EMCURE PHARAMACEUTICALS LTD
Squarylium cyanine chemical sensor used for Fe<3+> detection and preparation method thereof
InactiveCN102795995BThe synthesis method is simpleReaction conditions are easy to controlMethine/polymethine dyesOrganic compound preparationSolubilityBenzene
Belonging to the field of chemical analysis and test, the invention relates to a squarylium cyanine chemical sensor used for Fe<3+> detection and a preparation method thereof. The squarylium cyanine chemical sensor has a symmetrically structured 2, 4, 6-trihydroxy-3, 5-di(p-carboxybenzyl)phenyl)squarylium cyanine compound. The preparation method of the squarylium cyanine chemical sensor used for Fe<3+> detection consists of: preparation of an intermediate 1, 3, 5- trihydroxy-2, 4-di(p-carboxybenzyl)phenyl; and preparation of bis(2, 4, 6-trihydroxy-3, 5-di(p-carboxybenzyl)phenyl)squarylium cyanine. The squarylium cyanine compound provided in the invention has a simple synthesis method and easily controllable reaction conditions, and by means of a simple treatment, a pure product can be obtained. The obtained squarylium cyanine compound has excellent optical performance and optical stability. The squarylium cyanine compound provided in the invention contains carboxyl and hydroxyl groups, has certain water-solubility, and has good selectivity for iron ions.
Owner:CHANGZHOU UNIV +1
An improved process for the preparation of boronic acid esters
InactiveCN110312727AOrganic chemistry methodsGroup 3/13 element organic compoundsCarboxyl radicalPyrazine
The present invention relates to an improved process for the preparation of a compound of formula (I), wherein PG1 may be independently selected from tert-butyloxycarbonyl (Boc), phthaloyl, 9-fluorenylmethyloxycarbonyl (Fmoc), triphenylmethyl (Trityl), carboxybenzyl (Cbz), trifluoroacetyl, benzyl (Bn), benzylidene, methanesulfonyl (Mesyl), toluene sulfonyl (Tosyl) or acyl; its isolation as solid and use for the preparation of the compound of formula (IV), in particular the compound of formula (IV) i.e. [(1R)-3-methyl-1[[(2S)-1-oxo-3-phenyl-2- [(pyrazinylcarbonyl) amino]propyl]amino]butyl] boronic acid with more than 99.95% chiral purity, as measured by HPLC.
Owner:FRESENIUS KABI ONCOLOGY LTD
A mixed valence europium co-doped strontium magnesium lanthanum oxyapatite silicate luminescent material and its preparation method
ActiveCN108913136BImprove thermal stabilityHigh color rendering indexEnergy efficient lightingLuminescent compositionsZinc bromideMeth-
The invention discloses a mixed valence europium co-doped strontium magnesium lanthanum oxyapatite silicate luminescent material. The strontium magnesium lanthanum silicate is used as a phosphor matrix and europium ions are used as doping ions. The raw material is Strontium carbonate, magnesium carbonate, lanthanum oxide, silicic acid and europium oxide, while also adding flux boric acid and accelerator, the addition of boric acid is 2%-2.5% of the total mass of raw materials, and the addition of accelerator is 1.5% of the total mass, the luminescent material was prepared by a high-temperature solid-phase method, wherein the accelerator selected bis(triethanolamine) diisopropyl titanate, 2,5-difluorobenzyl zinc bromide, (3,4 ‑Epoxycyclohexyl)ethyltriethoxysilane, (4‑carboxybenzyl)methyldibenzyldicarbamate, bis(2,2,6,6,‑tetramethyl‑3,5 ‑Heptanedionate) calcium and tetrakis (ethylmethylamino) zirconium; the final luminescent material emits pure white light, the color rendering index can reach 90, and the color temperature is around 4000K, which can be used for indoor lighting.
Owner:WENZHOU UNIV
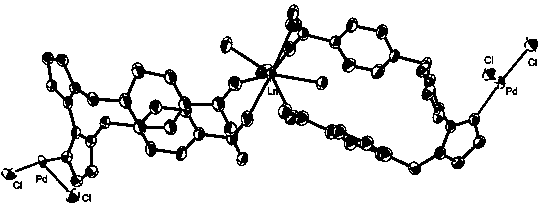
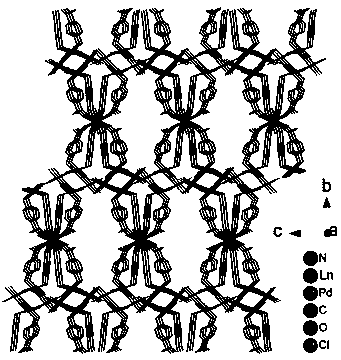
PD/ln heterometallic organic framework and its preparation method and application
ActiveCN106866986BEasy to synthesizeHigh yieldOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystChemical compound
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
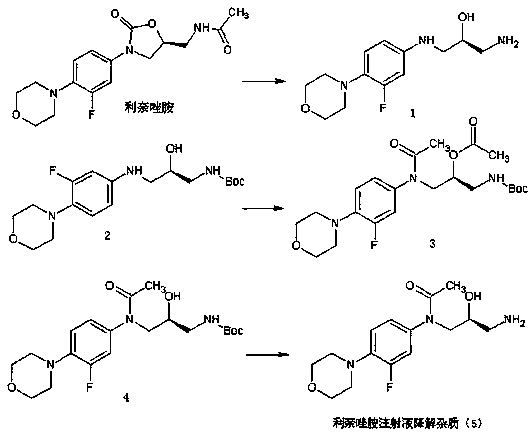
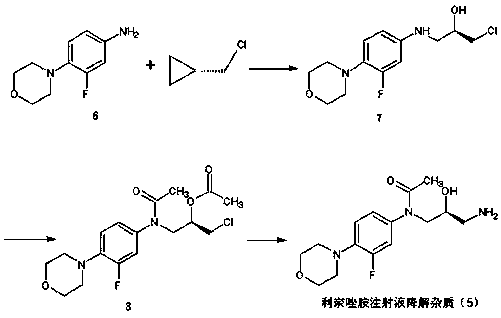
Preparation method of linezolid injection for degrading impurities
ActiveCN106316988BMild reaction conditionsEasy to operateOrganic chemistryChemical structureAminolysis
Owner:CHONGQING CHANGJIE MEDICINE CHEM
Method for preparing Fmoc-Thr(tBu)-OH
The invention discloses a method for preparing Fmoc-Thr(tBu)-OH, and relates to the field of biochemical engineering. The method comprises the following steps of (1) dissolving, wherein Z-Thr(tBu)-OMeis dissolved; (2) hydrogenolysis, wherein a hydrogen donor, pure water and a catalyst are added to be hydrogenated; (3) filtration, wherein the hydrogenated solution is filtered, and precipitation isremoved; (4) concentration, wherein a benign solvent is removed; (5) saponification, wherein acetone and the pure water are added, the pH value is adjusted to be 11-12 by using an alkaline solution,saponification is carried out to obtain Thr(tBu)-OH; (6) synthesis, wherein the Thr(tBu)-OH and Fmoc-OSu react to generate the Fmoc-Thr(tBu)-OH; and (7) drying. The method for preparing the Fmoc-Thr(tBu)-OH has the advantages that the catalyst is adopted to perform catalytic hydrogenation reaction, it is achieved that Z-Thr(tBu)-OH reacts with 1,3-cyclopentane diene at the normal temperature and pressure, the dehydrogenation of carboxybenzyl is achieved, not only is the production safer, but also the reaction speed is effectively improved, and then the production efficiency is improved.
Owner:成都市科隆化学品有限公司
Preparation of electrically-induced resistive switching polycatenane crystalline material and application thereof in memory
ActiveCN111234247AReduce operating voltageEasy to changeElectrical apparatusPhosphoric acidResistive switching
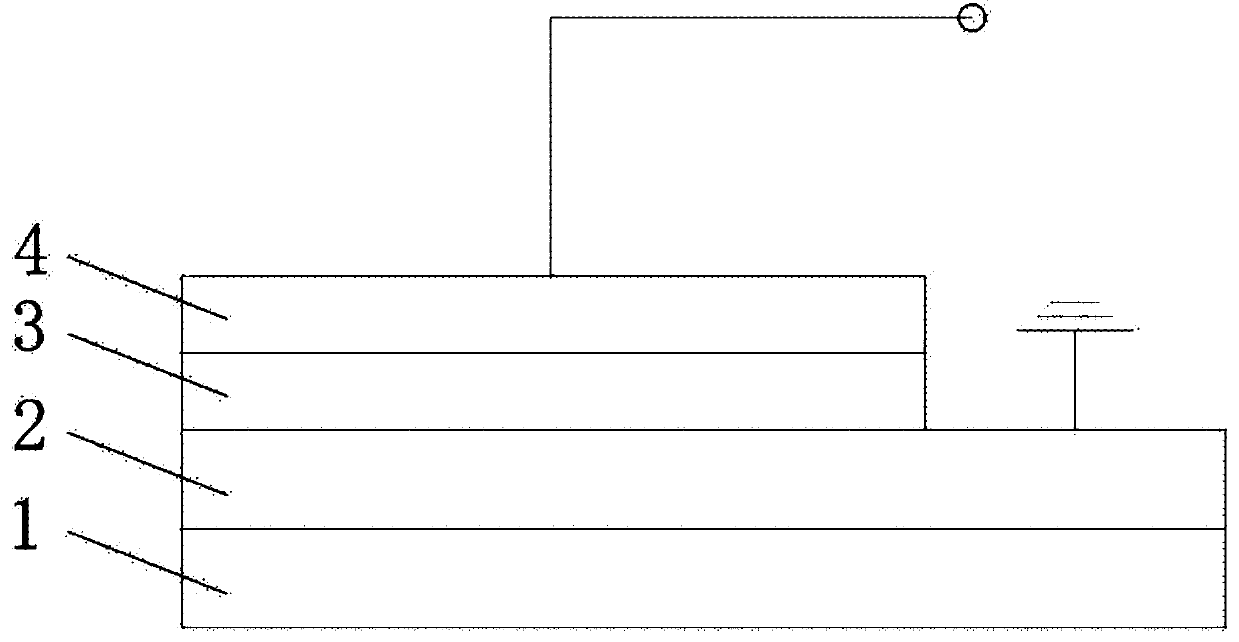
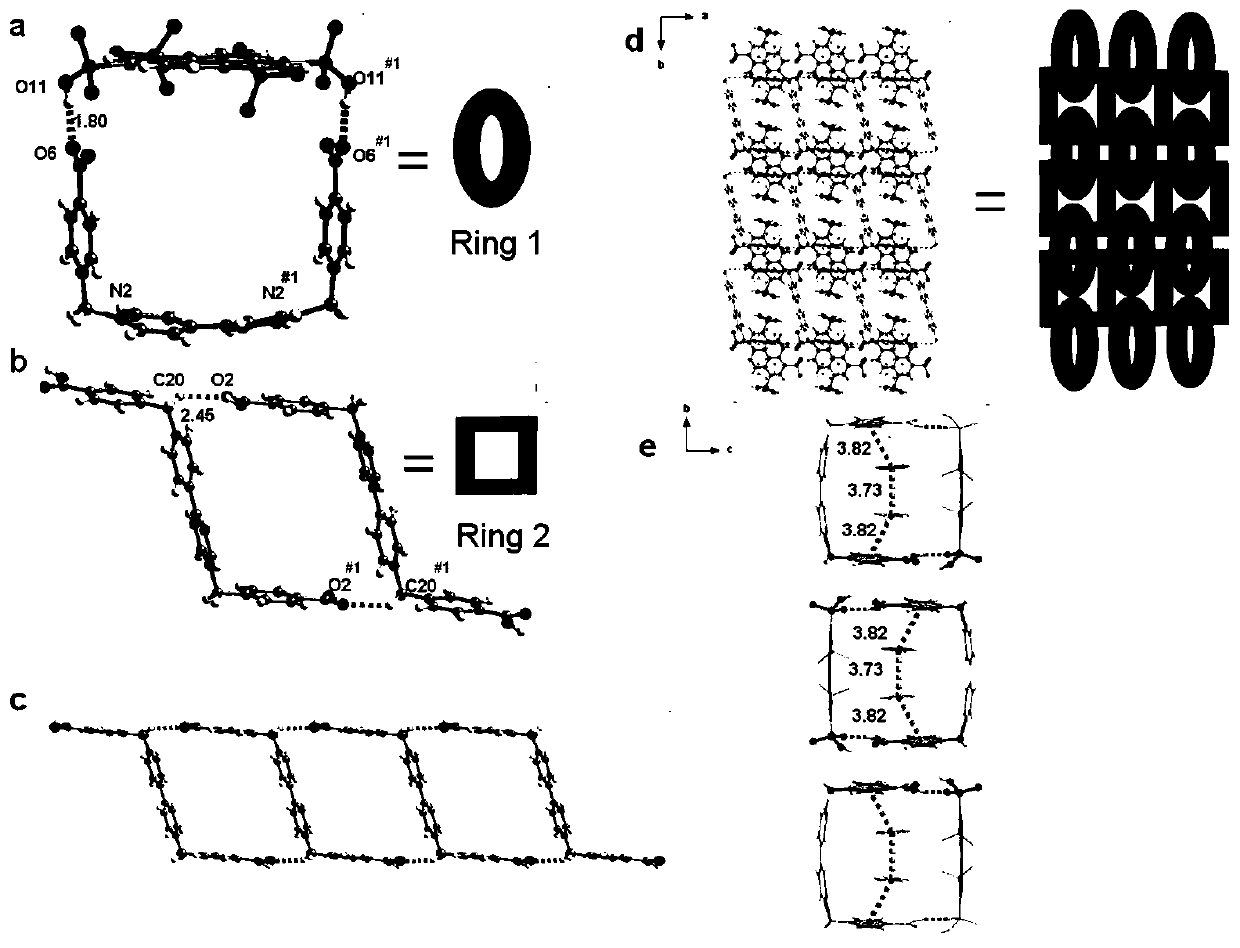
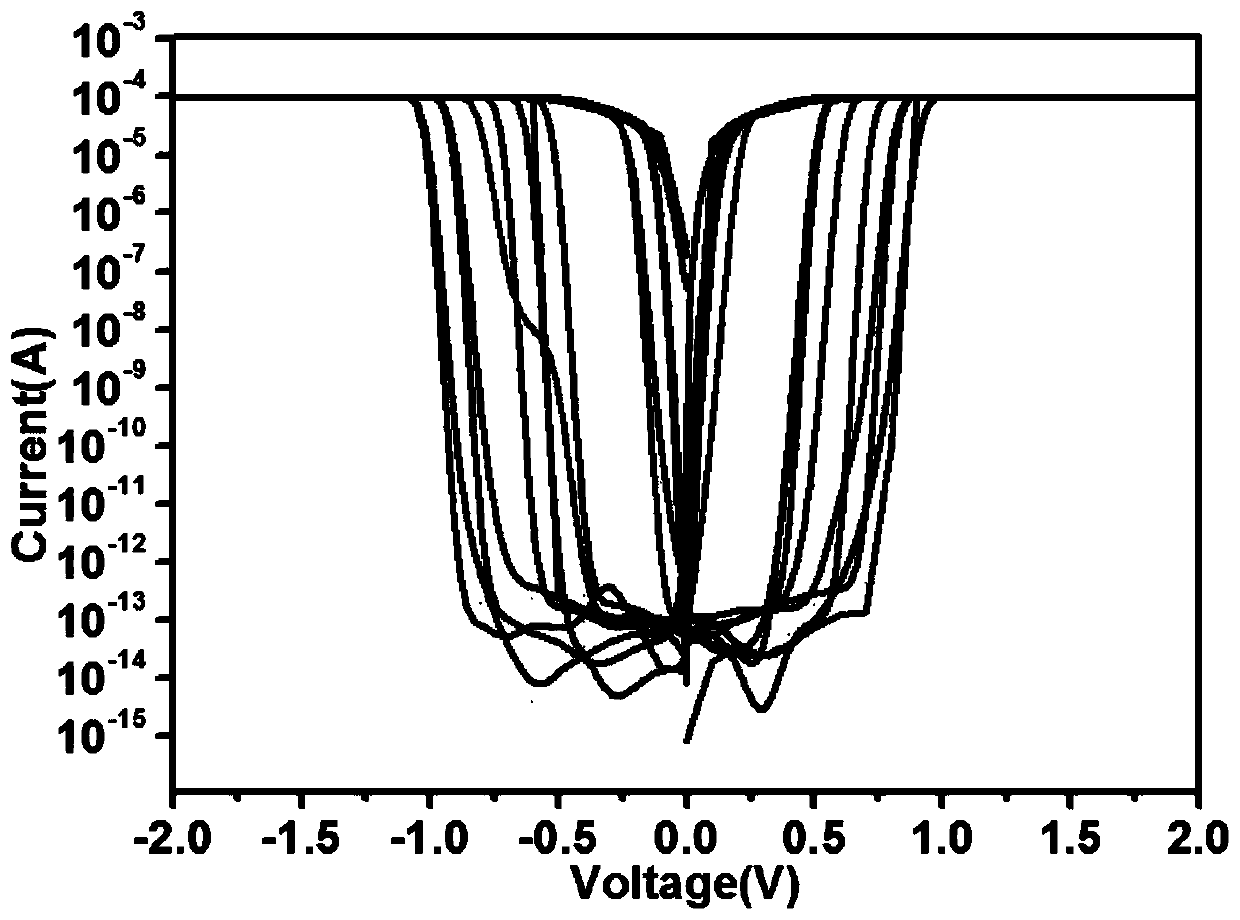
The invention provides a preparation method of an electrically-induced resistive switching polycatenane crystalline material. The preparation method comprises the following steps: S1, adding a mixed solvent of methanol and water into a glass container, then adding 1,3,6,8-pyrene tetraphosphoric acid, 1,1'-bis(4-carboxyl benzyl)-4,4'-dipyridyl dichloride and a cobalt salt into the mixed solution ofmethanol and water, and carrying out ultrasonic treatment for 5-15 minutes; S2, sealing the glass container, then placing the glass container in an oven, heating the glass container from room temperature to 60-100 DEG C, carrying out heat preservation for 24-36 hours, and cooling the glass container to room temperature; and S3, filtering the solution to obtain solid, washing the solid with distilled water, and naturally drying the solid at room temperature to obtain blocky orange crystals, namely the electrically-induced resistive switching polycatenane crystalline material. An electrically-induced resistive switching organic medium memory prepared from the material is simple in manufacturing process, high in switch ratio, low in operation voltage and stable in performance.
Owner:FUJIAN NORMAL UNIV
New synthesis method of hepatitis C drug velpatasvir
ActiveCN105732765BIncrease profitEfficient synthesisPeptidesTert-Butyloxycarbonyl protecting groupSynthesis methods
The invention provides a novel synthesis method of hepatitis drug velpatasvir.Two intermediate compounds including a compound 4 and a compound III are utilized to synthesize the velpatasvir, the structures of the two compounds are as shown in the following formulas, wherein PG radical is t-butyloxycarboryl (Boc), carboxybenzyl (Cbz), acetyl, benzoyl or (S)-2-methoxyl acyl carbonyl amino-3-methyl-butyryl group (Moc-L-Valyl).
Owner:山东科巢生物制药有限公司
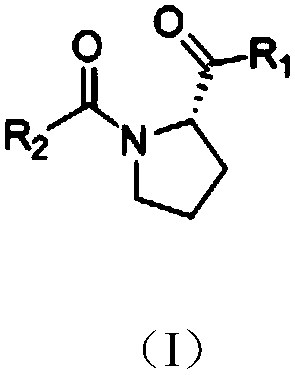
Proline derivatives having beta-lactamase inhibitory effect
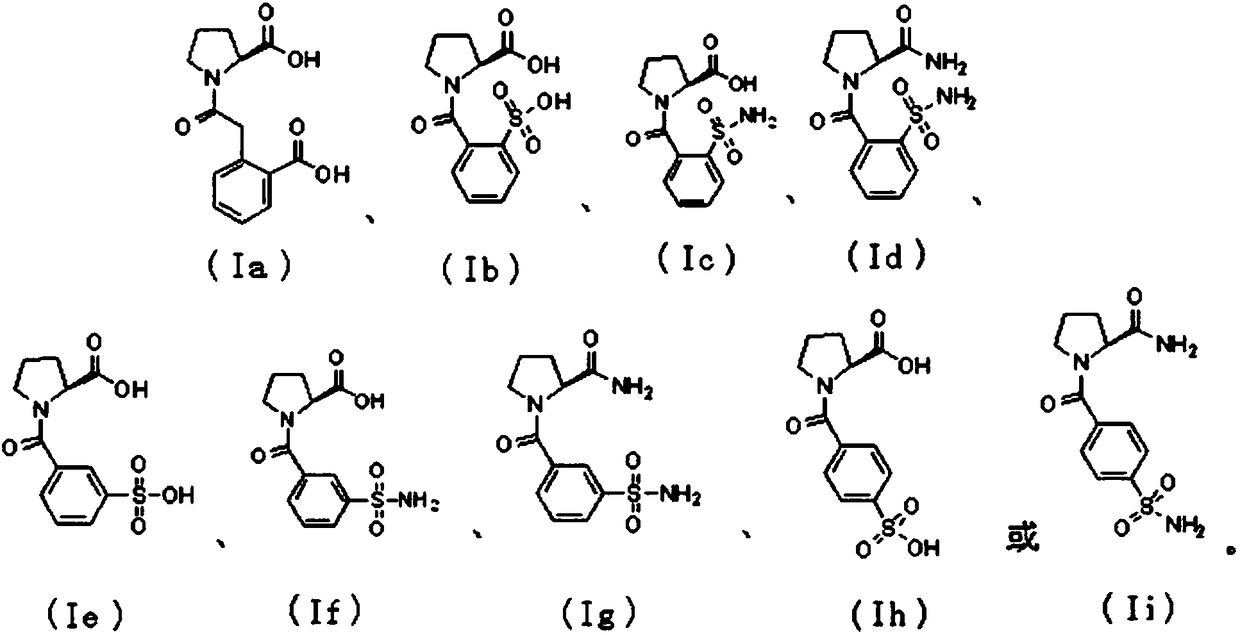
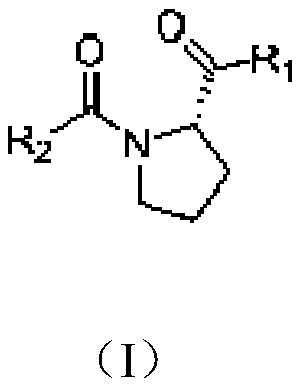
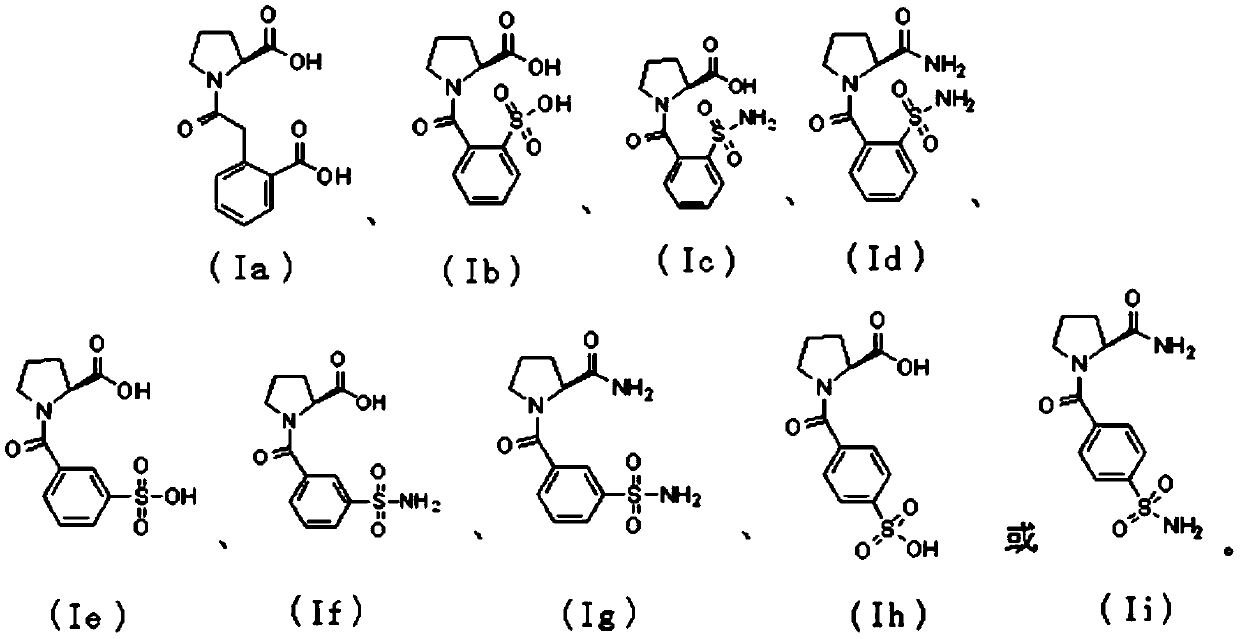
InactiveCN108084075ADelay drug resistanceReduce or even eliminate hydrolysisAntibacterial agentsOrganic active ingredientsBeta lactam antibioticAntibiotic Y
The present invention discloses a proline derivative having a beta-lactamase inhibitory effect. The proline derivative is represented by formula (I), wherein the amide bond in the formula (I) comprises a naturally occurring cis or trans rotamer; R1 is OH or NH2; R2 is o-sulfophenyl, o-sulphonylaminophenyl, m-sulfophenyl, m-sulfonamidophenyl, p-sulfophenyl, p-sulfonamidophenyl, or o-carboxybenzyl.Activity tests show that the proline derivative represented by the formula (I) has a good inhibitory effect on beta-lactamase, especially on NDM-1. By inhibiting of the activity of the beta-lactamase,especially the NDM-1, hydrolysis of beta-lactam antibiotics by the NDM-1 can be reduced or even eliminated, drug resistance caused by the NDM-1 can be alleviated, the curative effect of the antibiotics on bacteria can be restored, and drug-resistant bacterial infections caused by the NDM-1 can be treated.
Owner:TIANJIN UNIV
Use of proline derivatives in the preparation of β-lactamase inhibitors
InactiveCN108078982BDelay drug resistanceReduce hydrolysisAntibacterial agentsOrganic active ingredientsCarboxyl radicalAmidase activity
Owner:TIANJIN UNIV
Rilpivirine process
InactiveUS8952155B2Cost-effectiveAmenable for synthesisCarbamic acid derivatives preparationOrganic compound preparationDimethylaniline N-oxideTetrafluoroborate
Disclosed is process for the preparation of a key Rilpivirine intermediate namely, (E)-4-(2-cyanoethenyl)-2,6-dimethylphenylamine hydrochloride (II) by a process comprising reaction of the tetrafluoroborate salt of the diazonium ion of 2,6-dimethyl-4-amino-1-carboxybenzyl phenylamine (VI) with acrylonitrile in presence of palladium acetate, followed by treatment with an acid and its subsequent conversion to the hydrochloride salt (II), wherein the undesired Z isomer is less than 0.5% and provides Rilpivirine hydrochloride having Z isomer less than 0.1%.
Owner:EMCURE PHARAMACEUTICALS LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com



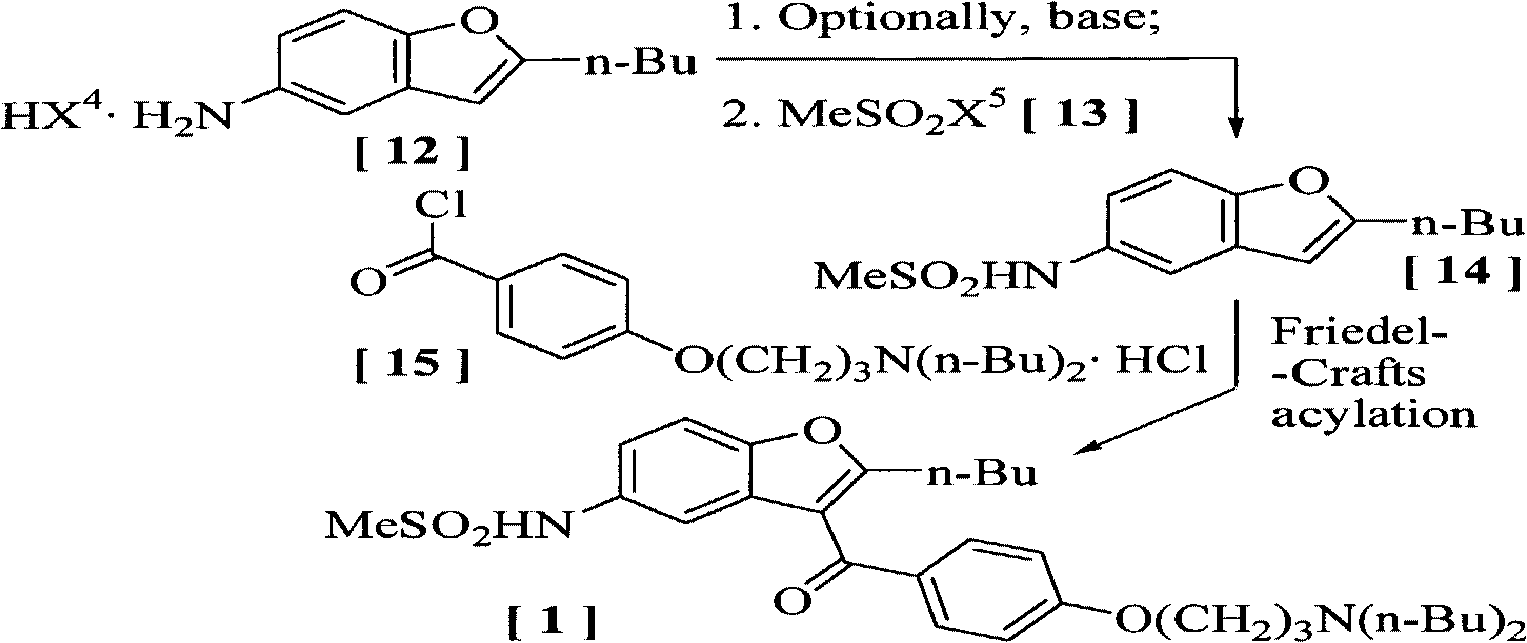
![3-trifluoromethyl-2,5-diazabicyclo[2.2.1] heptane derivant and preparation method thereof 3-trifluoromethyl-2,5-diazabicyclo[2.2.1] heptane derivant and preparation method thereof](https://images-eureka.patsnap.com/patent_img/2547f914-1758-41be-bd07-a8060bdb397e/FSA00000037763800011.png)
![3-trifluoromethyl-2,5-diazabicyclo[2.2.1] heptane derivant and preparation method thereof 3-trifluoromethyl-2,5-diazabicyclo[2.2.1] heptane derivant and preparation method thereof](https://images-eureka.patsnap.com/patent_img/2547f914-1758-41be-bd07-a8060bdb397e/FSA00000037763800012.png)
![3-trifluoromethyl-2,5-diazabicyclo[2.2.1] heptane derivant and preparation method thereof 3-trifluoromethyl-2,5-diazabicyclo[2.2.1] heptane derivant and preparation method thereof](https://images-eureka.patsnap.com/patent_img/2547f914-1758-41be-bd07-a8060bdb397e/FSA00000037763800021.png)