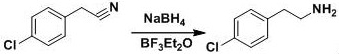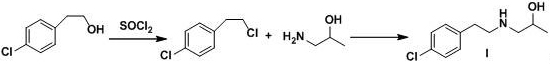Patents
Literature
43 results about "Lorcaserin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lorcaserin is used with a doctor-approved exercise, behavior change, and reduced-calorie diet program to help you lose weight. It is used by certain overweight people, such as those who are obese or have weight-related medical problems.
Compositions and methods for treating obesity and related disorders
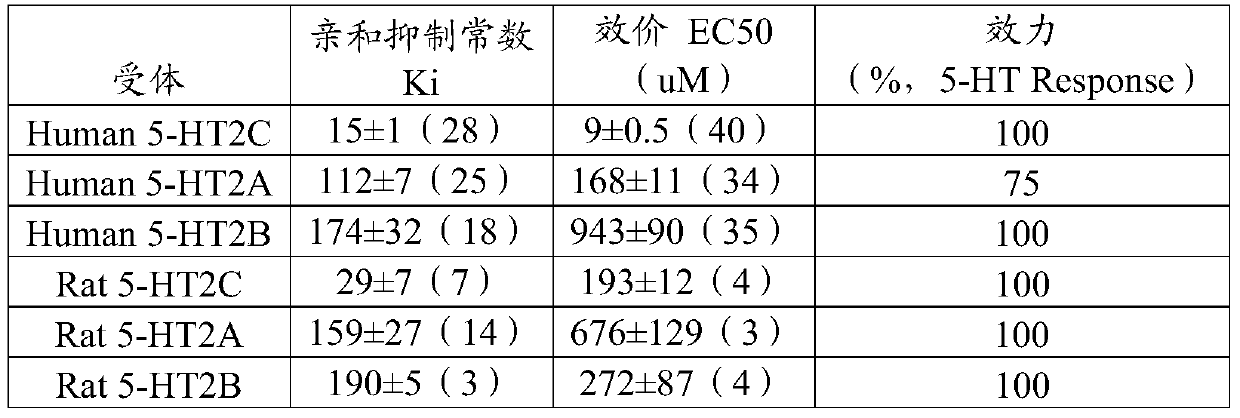
InactiveUS20080255093A1Reduces unwanted side effectGood for weight lossBiocideNervous disorderSYMPATHOMIMETIC AGENTSSerotonin receptor agonist
The present invention is drawn to combinations of pharmaceutical agents having similar chemical and / or pharmacological properties, wherein the combinations maximize the therapeutic effect of the drug while minimizing their adverse effects. The methods and compositions of the invention are particularly useful in the treatment of obesity and related conditions which involves treating a subject with a sympathomimetic agent (e.g., phentermine or a phentermine-like drug) or bupropion in combination with an anti-epileptic agent (e.g., topiramate, zonisamide), CB1 antagonists (e.g., rimonabant), or a 5HT2C-selective serotonin receptor agonist, (e.g., lorcaserin) for the treatment of obesity and related conditions. The invention also features kits for use in the practice of these novel therapies.
Owner:VIVUS
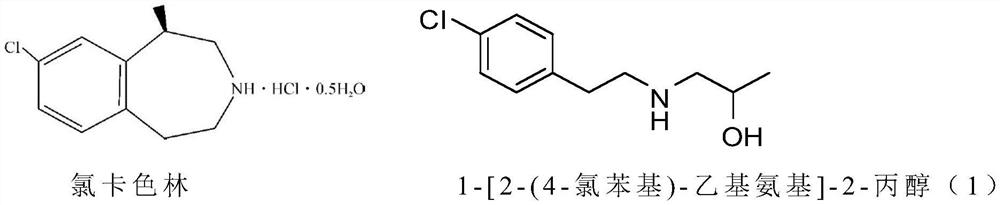
Preparation method of lorcaserin hydrochloride
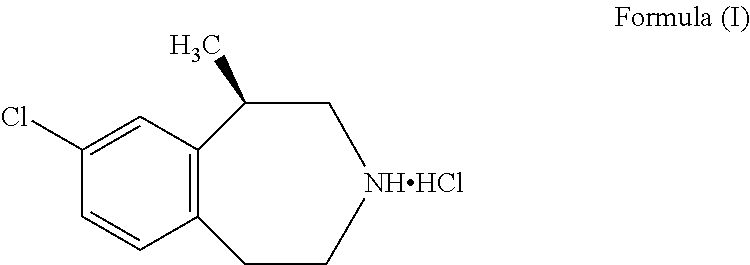
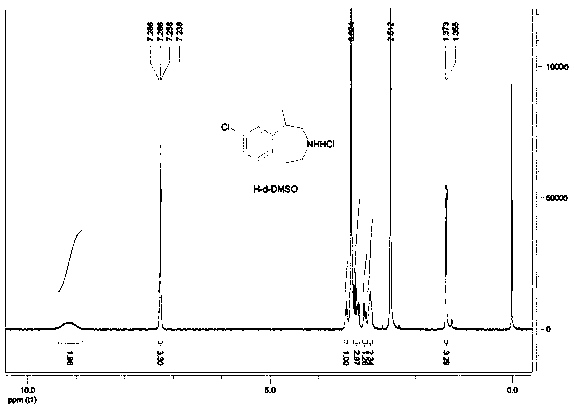
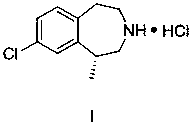
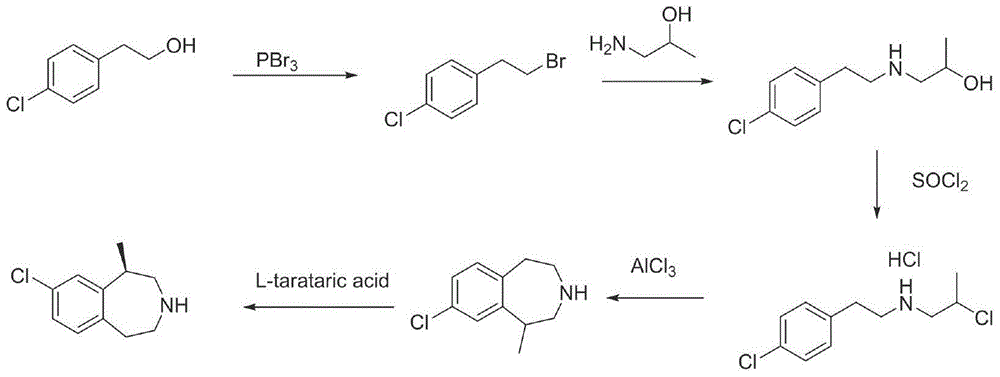
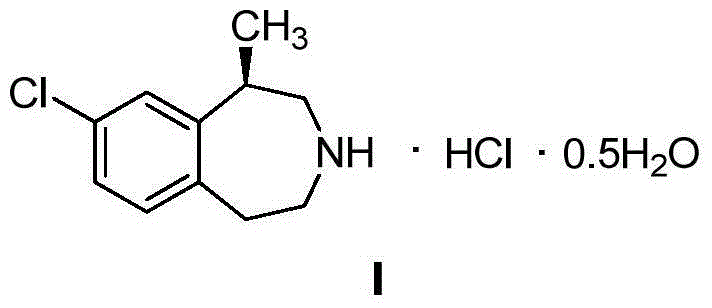
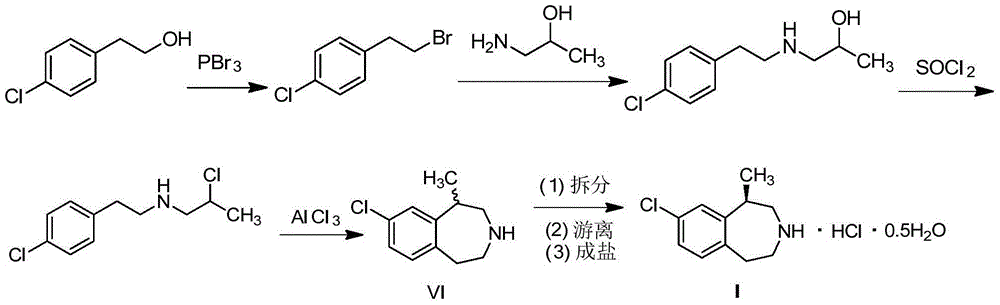
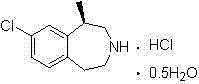
The invention provides a preparation method of lorcaserin hydrochloride, namely (R)-8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride. The method comprises the following steps: taking p-chlorophenyl acetate as raw material, performing aminolysis with isopropanol amine, then performing chlorination, reduction and Friedel-Crafts alkylation, further splitting by L-tartaric acid, and performing salt formation to get the lorcaserin hydrochloride. The invention provides the preparation method of the lorcaserin hydrochloride. The method has the advantages of low cost, simplicity in operation and higher product purity; according to the process disclosed by the invention, the continuous operation can be performed, and the production cycle is greatly reduced; meanwhile a reagent is low in price and easy to obtain, the operation is simple, and the post-treatment is simple and convenient, so that the preparation method is a brand new and economic synthesis method which can realize industrial production.
Owner:SUZHOU HUIHE PHARMA
Synthesis methods of lorcaserin derivative and salt thereof
The invention discloses a synthesis method of a lorcaserin derivative. The synthesis method comprises the following steps: resolving a compound shown as a formula (II) under the action of an optical resolving agent; performing post-treatment to obtain the lorcaserin derivative. In the formula (II), R is H, alkyl with 1-4 carbon atoms, halogen, methoxyl or nitryl; R2 is H, alkyl with 1-4 carbon atoms, methoxyl, carboxybenzyl, t-butyloxycarboryl, methylsulfonyl, tosyl and substituted or unsubstituted benzyl; the optical resolving agent is acyl-substituted tartaric acid. The revolving efficiency is increased by selecting the optical resolving agent having a specific structure, and an optical pure product of which the ee value is over 99 percent can be obtained by means of simple recrystallization; meanwhile, resolving operation is easy, a single solvent is used for resolving, materials are fed into one pot, and the method is suitable for industrial production. The invention further discloses a synthesis method of a salt of the lorcaserin derivative. The obtained salt of the lorcaserin derivative can be applied to preparation of novel weight-reducing medicaments.
Owner:CHINA JILIANG UNIV +1
Preparation method of lorcaserin intermediate
ActiveCN106631823AEasy to operateHigh yieldOrganic compound preparationOrganic chemistry methodsPropanolCyanide
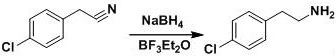
The invention discloses a preparation method of a lorcaserin intermediate (I). According to the preparation method, p-chlorobenzyl cyanide is taken as the primary raw material, and the lorcaserin intermediate (I) is obtained after reduction reactions and condensation reactions. The primary raw materials (p-chlorobenzyl cyanide and 1-chloro-2-propanol) are cheap and easily available; raw materials, which can easily get polluted and are explosive, such as sulfoxide chloride, hydrobromic acid, borane, and the like are not used; the preparation method will not produce a large amount of wastewater and is beneficial for the environment protection; moreover, the requirements on the protection of workers are lowered, and safe production is guaranteed. The route design is novel, the raw materials are easily available, the operation is simple and feasible, and the preparation method is environment-friendly and can be applied to massive industrial production.
Owner:天津泰普制药有限公司
A preparing method of (R,S)-8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazepine, a lorcaserin intermediate
ActiveCN105693610AReduce usageLow costOrganic compound preparationAmino-hyroxy compound preparationMethyl groupRaw material
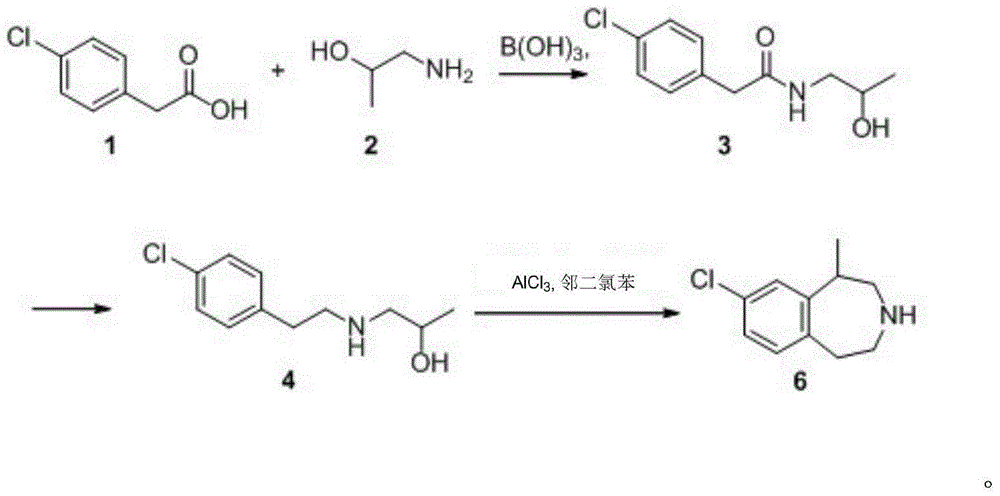
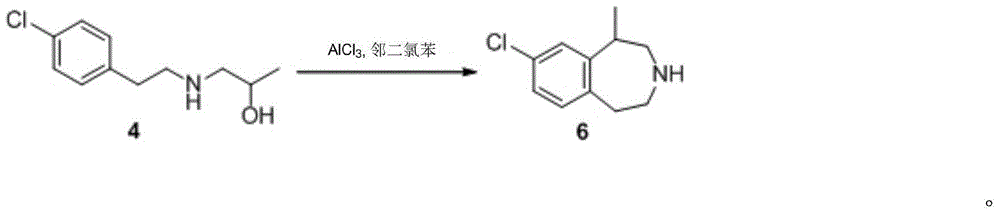
A preparing method of (R,S)-8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazepine that is a lorcaserin intermediate is disclosed. The method includes (1) dehydrating a compound 1 and a compound 2 under catalysis by boric acid to generate an amide 3, (2) reducing the intermediate 3 with a borane dimethylsulfide complex to obtain a compound 4, and (3) subjecting the compound 4 to direct ring closing with the existence of aluminum chloride to generate the lorcaserin intermediate that is the (R,S)-8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazepine that is the compound 6. The method avoids use of 3,4,5-trimethoxyphenylboronic acid that is an expensive reagent, thus reducing the cost. The method avoids use of thionyl chloride so that the method is environmental friendly. A step of hydroxy chlorination is reduced so that the method is simple in process. The conversion ratio of raw materials and the total yield of reactions are increased. The yield of the compound 6 is increased from 60% in a patent to 82%. The method is suitable for industrial production. A reaction equation is shown in the description.
Owner:SICHUAN YIBIN WULIANGYE GROUP YIBIN PHARMA +1
Application of Lorcaserin hydrochloride in preparation of medicine for inhibiting opioid addiction and withdrawal syndrome
InactiveCN103877096AInhibitory reactivityNervous disorderHeterocyclic compound active ingredientsWithdrawal syndromeStimulant
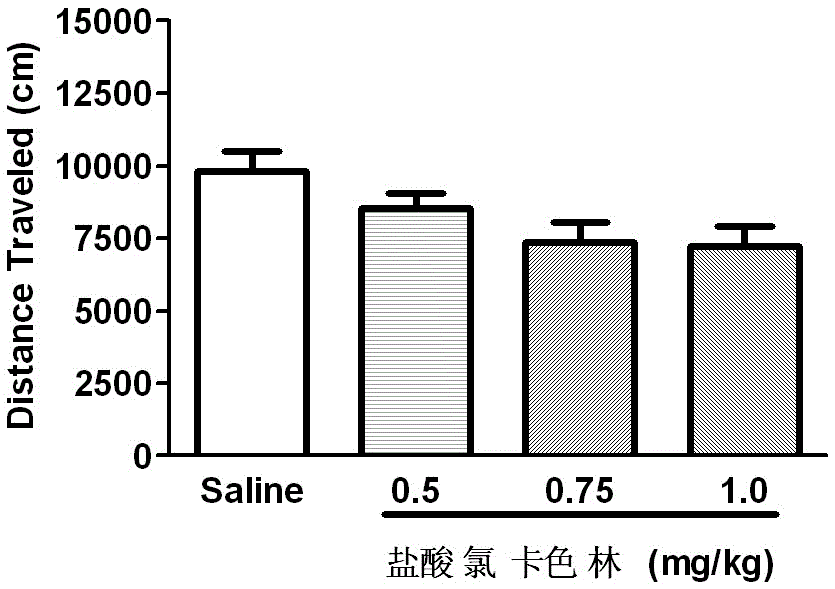
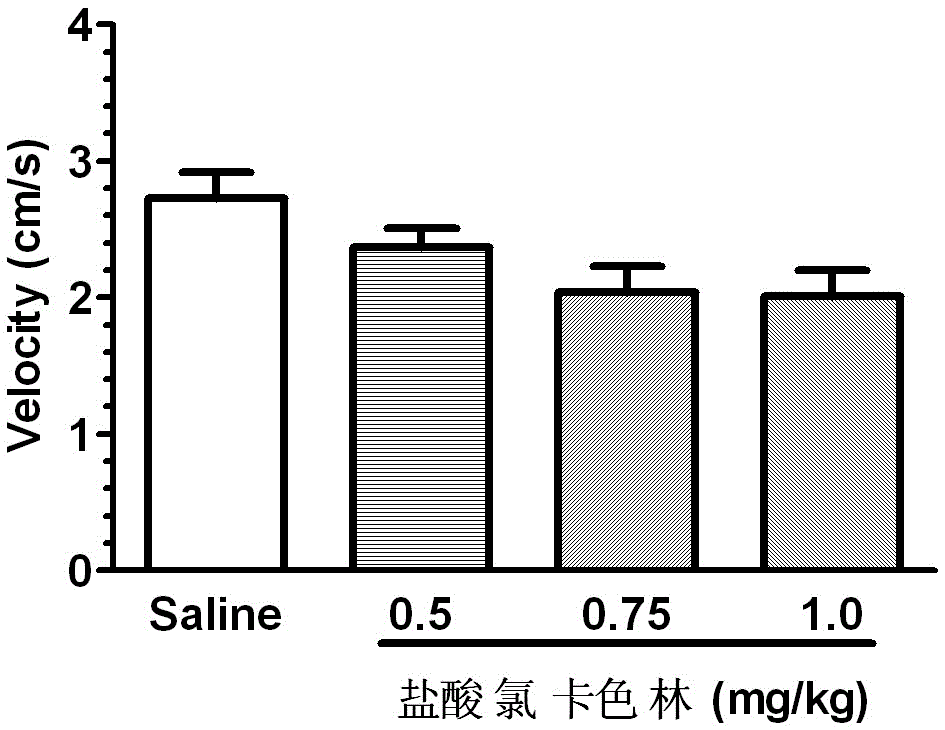
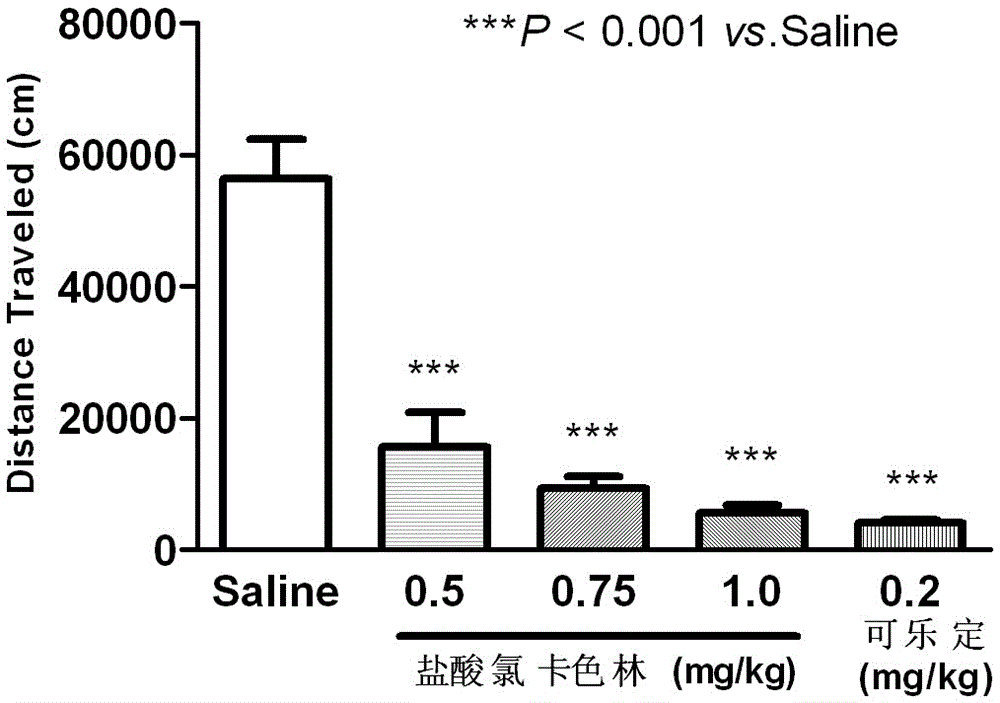
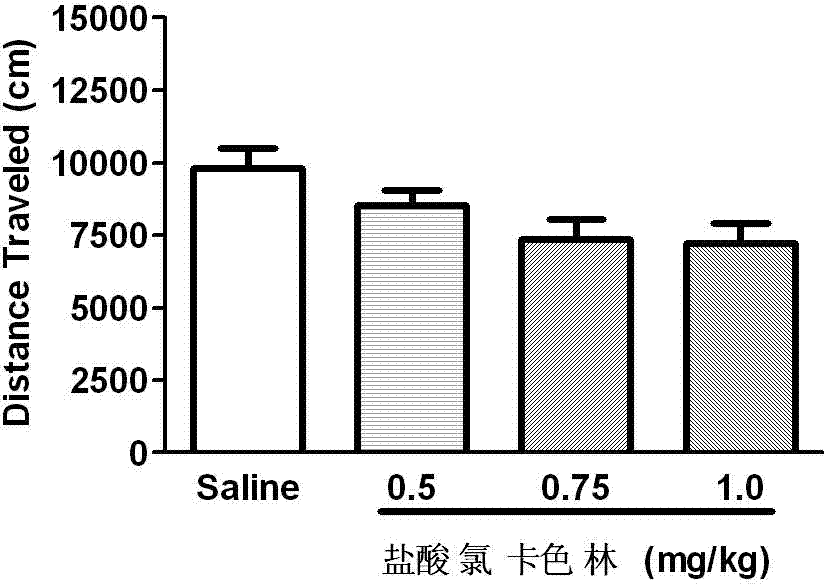
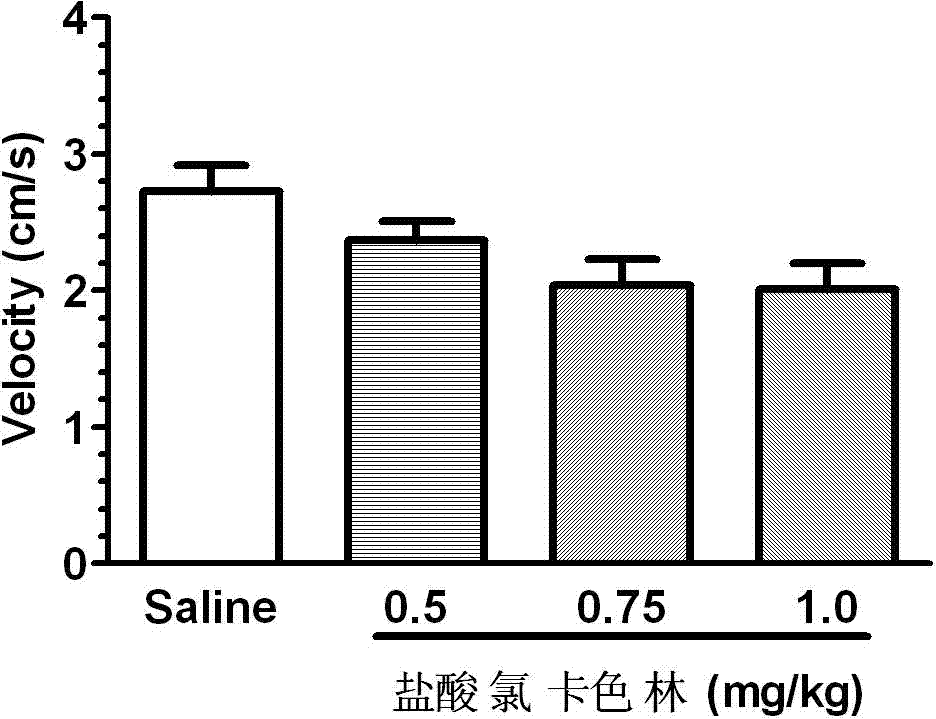
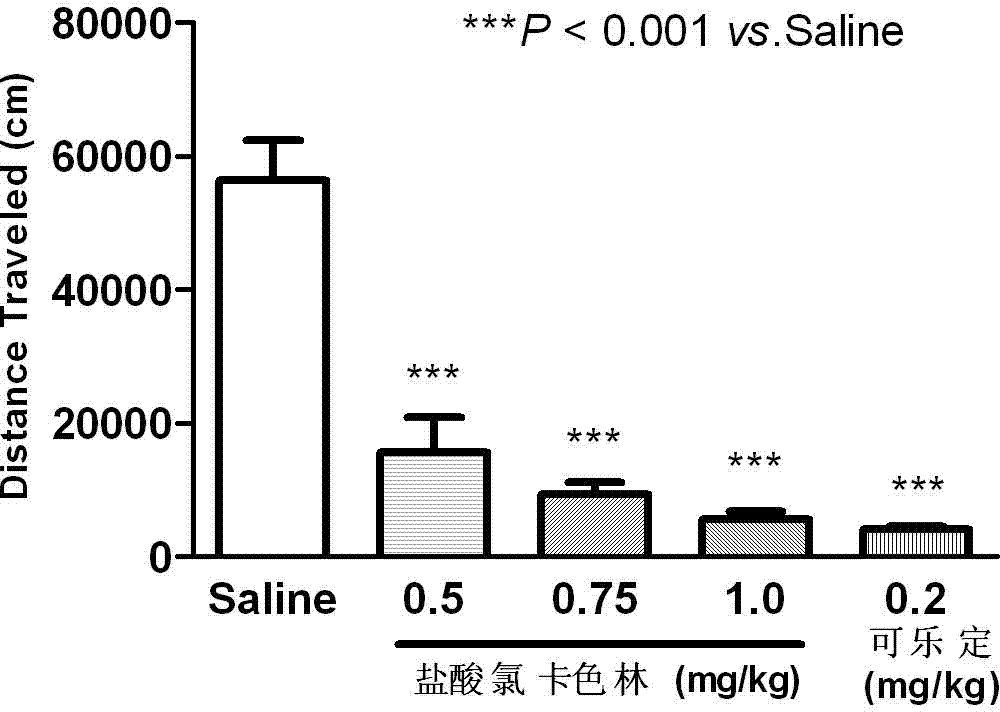
The invention relates to an application of Lorcaserin hydrochloride in preparation of a medicine for inhibiting opioid addiction and withdrawal syndrome. The Lorcaserin hydrochloride is approved to be a weight-reducing aid by the America Food and Drug Administration (FDA) in 2012. The Lorcaserin hydrochloride is a 5-hydroxytryptamine 5-HT2C receptor stimulant with high selectivity. The latest research of the invention finds that the Lorcaserin hydrochloride (0.5-1.0mg.kg<-1>) greatly reduces the naloxone-urged horizontal motion distance and speed of a morphine dependent mice and the withdrawal syndrome of the mice. It is found in the invention for the first time that the Lorcaserin hydrochloride can be applied clinically for rescuing and treating addiction and withdrawal syndrome generated by morphine and other opiates.
Owner:ANHUI MEDICAL UNIV
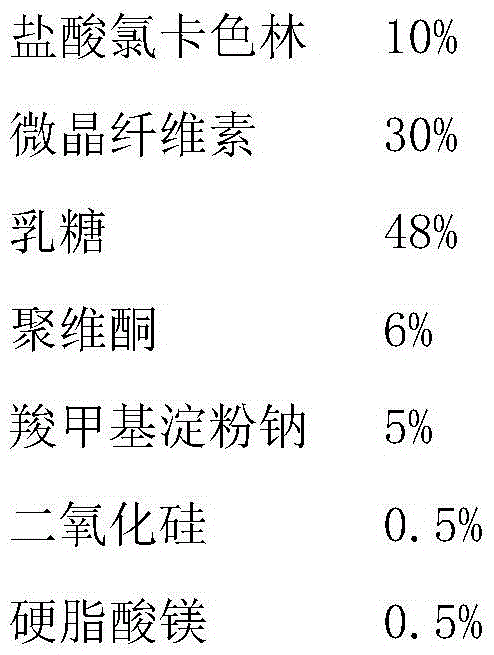
Hydrochloric lorcaserin tablets and preparing method thereof
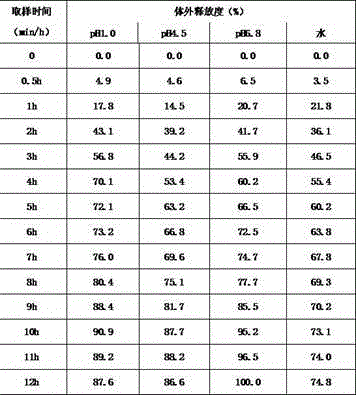
The invention belongs to the technical field of medicines and particularly relates to hydrochloric lorcaserin tablets and a preparing method thereof. The hydrochloric lorcaserin tablets are prepared from hydrochloric lorcaserin, a thinning agent, a dry binding agent, a disintegrating agent and a lubricating agent. The preparing process of the hydrochloric lorcaserin tablets comprises the step that powder is directly compressed into tablets or the powder is granulated and then compressed into tablets in a dry method, and the production process is simple, controllable, high in operability, short in production cycle, low in production cost and low in industrialization difficulty. The prepared hydrochloric lorcaserin tablets are good in appearance, fast in effect taking and stable in curative effect, the content and the dissolution rate of the hydrochloric lorcaserin tablets are both qualified, and taking compliance of a patient is not affected.
Owner:BEIJING VENTUREPHARM BIOTECH
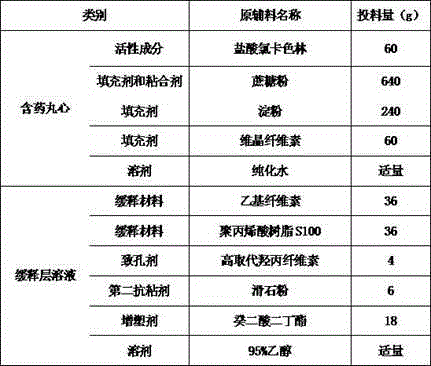
Lorcaserin hydrochloride sustained-release capsules and preparation method thereof
ActiveCN104688716AImprove complianceStable in natureMetabolism disorderPharmaceutical delivery mechanismPatient needSustained release pellets
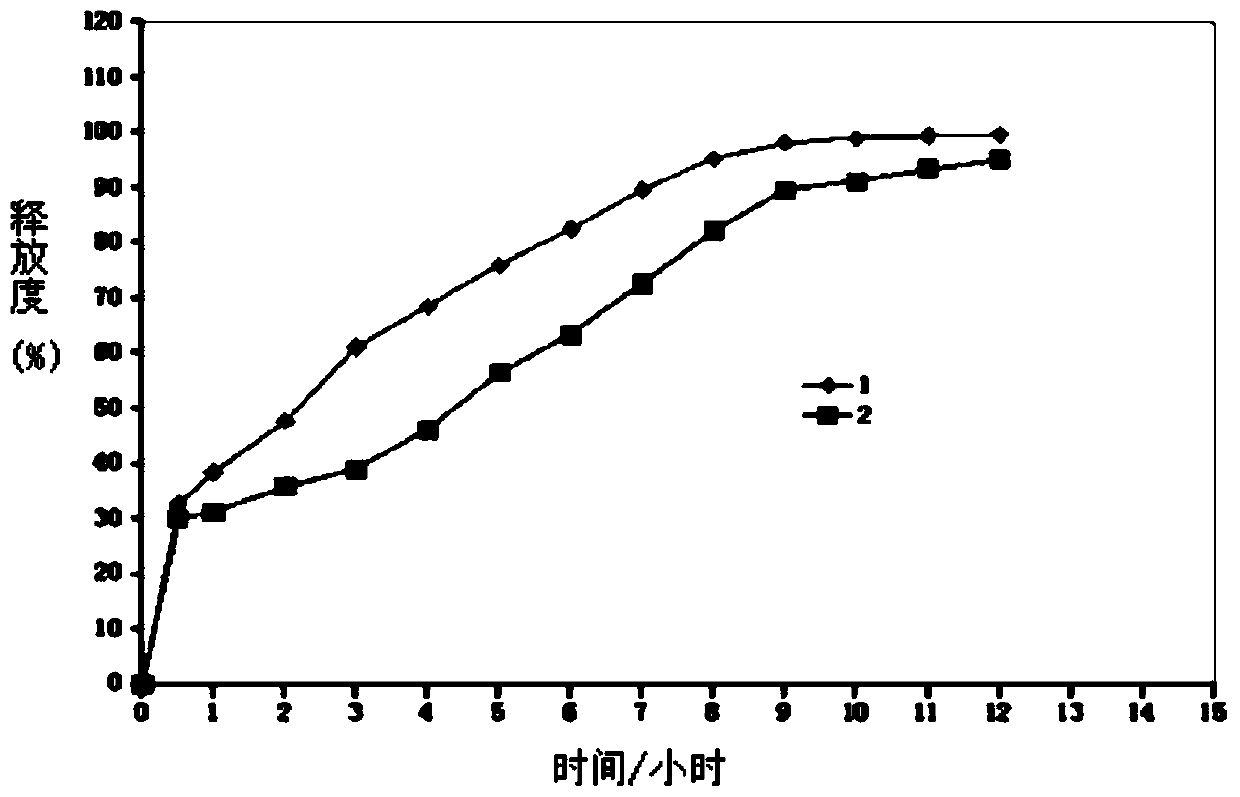
The invention belongs to the field of medicines and relates to a lorcaserin hydrochloride sustained-release capsule and a preparation method thereof. The sustained-release capsule comprises lorcaserin hydrochloride sustained-release pellets and a capsule shell, wherein each lorcaserin hydrochloride sustained-release pellet comprises a pill core and a sustained-release layer wrapping the pill core; the pill cores comprise 6wt% of lorcaserin hydrochloride or lorcaserin hydrochloride hemihydrates, 60-64wt% of sucrose powder, 20-24wt% of starch and 6-14wt% of microcrystalline cellulose; the sustained-release layers comprise 30-36wt% of polyacrylic resin S100, 30-36wt% of ethyecellulose, 4-8wt% of high-substituted hydroxyproxyl cellulose, 18-22wt% of dibutyl sebacate and 6-10wt% of talcum powder. The lorcaserin hydrochloride sustained-release capsule is stable in property and reliable in quality. A patient needs to take one common lorcaserin hydrochloride tablet twice every day, but only needs to take the hydrochloride sustained-release capsule prepared by the invention once every day, so that the compliance of the patient is improved.
Owner:WUHAN RUNXIN TECH
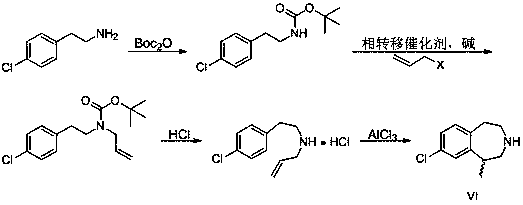
Preparation method of weigh reducing drug lorcaserin hydrochloride and intermediate thereof
ActiveCN105367497AEasy to operateConvenient and safe post-processingOrganic chemistryAlkyl transferSynthesis methods
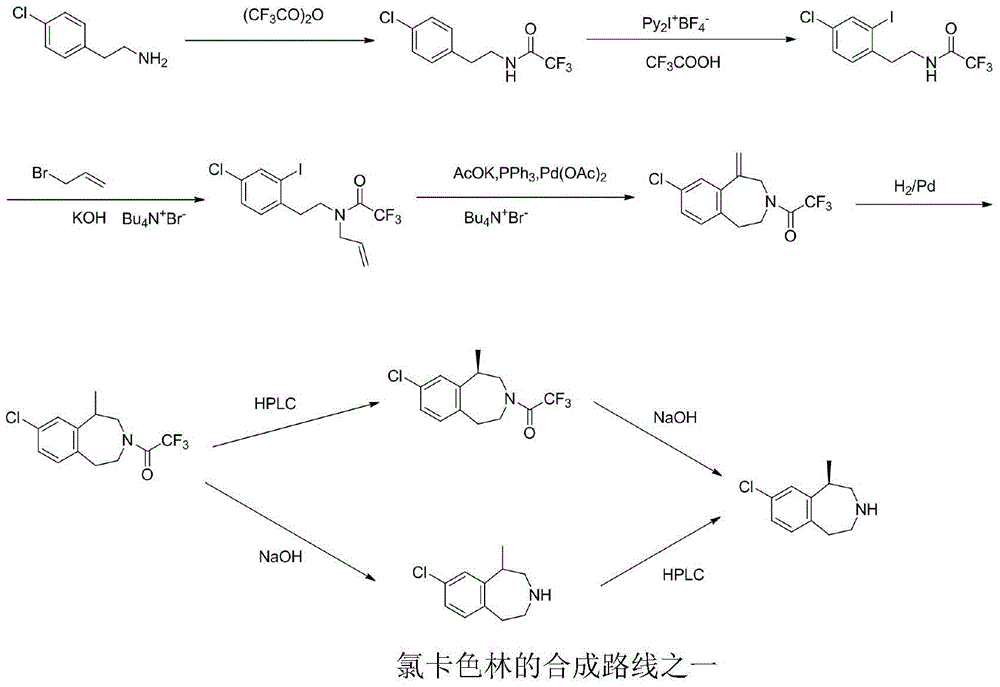
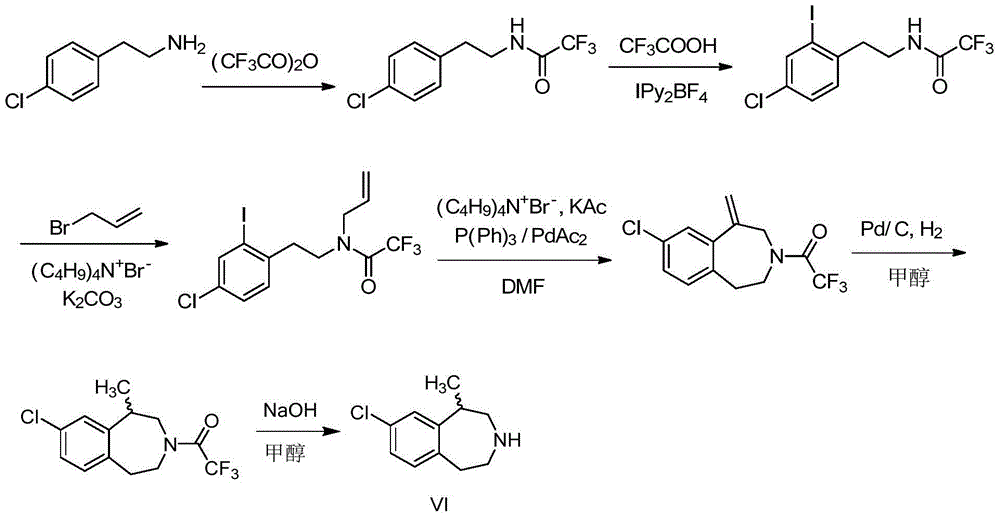
The present invention relates to the field of pharmaceutical chemistry, specifically to a preparation method of a weigh reducing drug lorcaserin hydrochloride and an intermediate thereof. The preparation method comprises: adopting p-chlorophenethylamine as a raw material, and sequentially carrying out acylation amino protection, allyl substitution, deprotection, Friedel-Crafts alkylation, splitting and salt forming to obtain the lorcaserin hydrochloride. According to the present invention, the preparation method has characteristics of low cost, simple operation and easy post-treatment, and is the economical and industrialized synthesis method.
Owner:CHINA PHARM UNIV +2
Processes for the preparation of 5-HT2C receptor agonists
InactiveUS8952197B2Yield maximizationOrganic compound preparationAmino preparation by functional substitutionSerotoninMethyl group
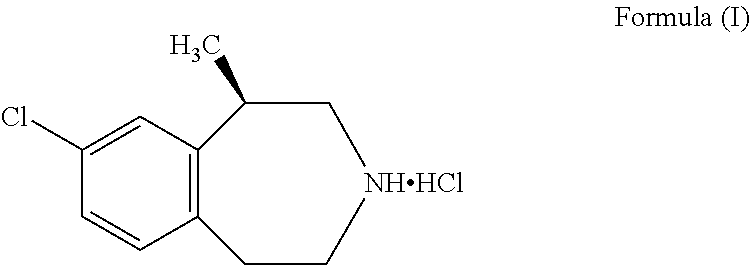
The present invention relates to processes and intermediates useful in the preparation of (R)-8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazepine (lorcaserin), a serotonin (5-HT) receptor modulator that is useful in the treatment of for example, central nervous system disorders, such as obesity.
Owner:ARENA PHARMA
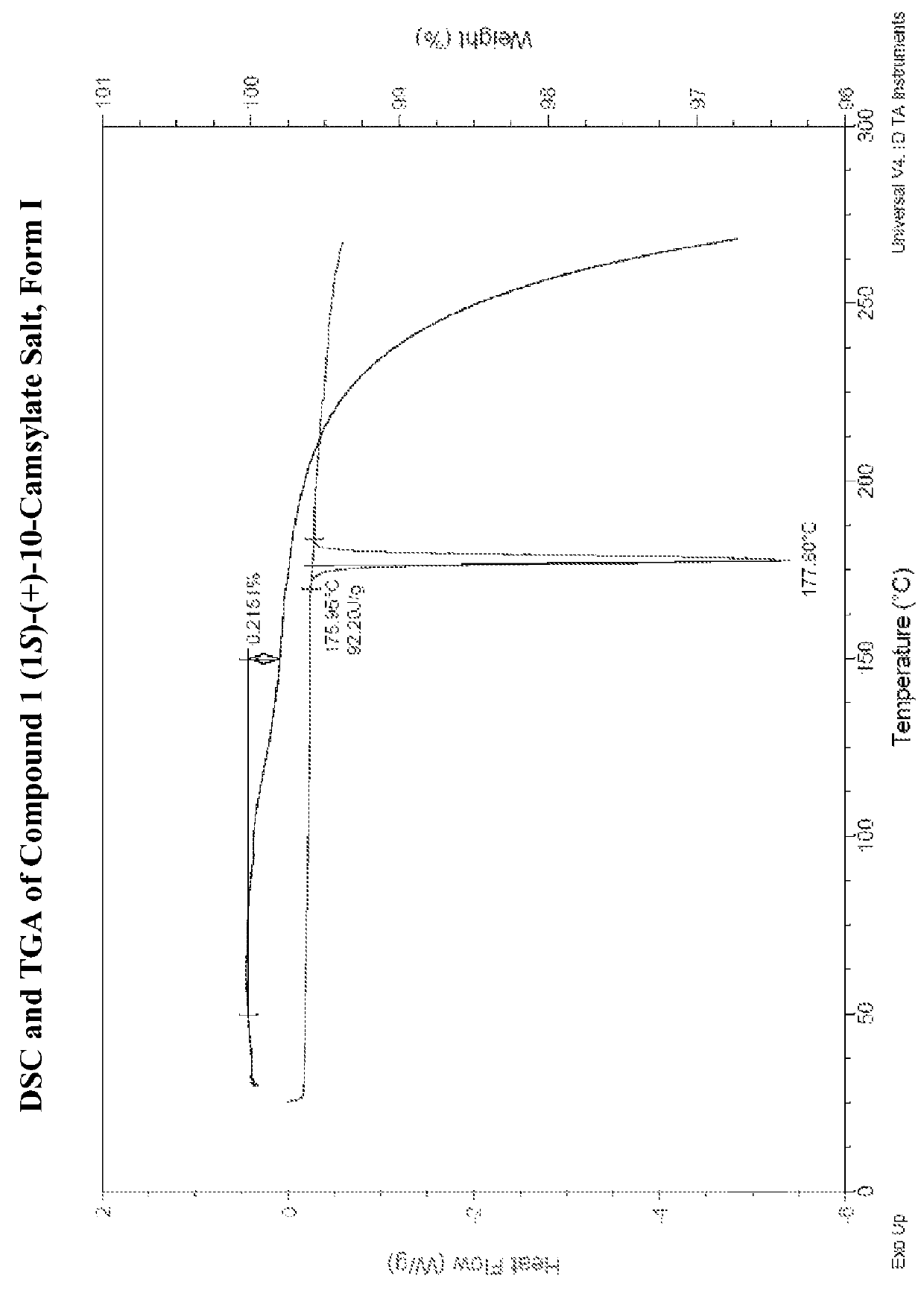
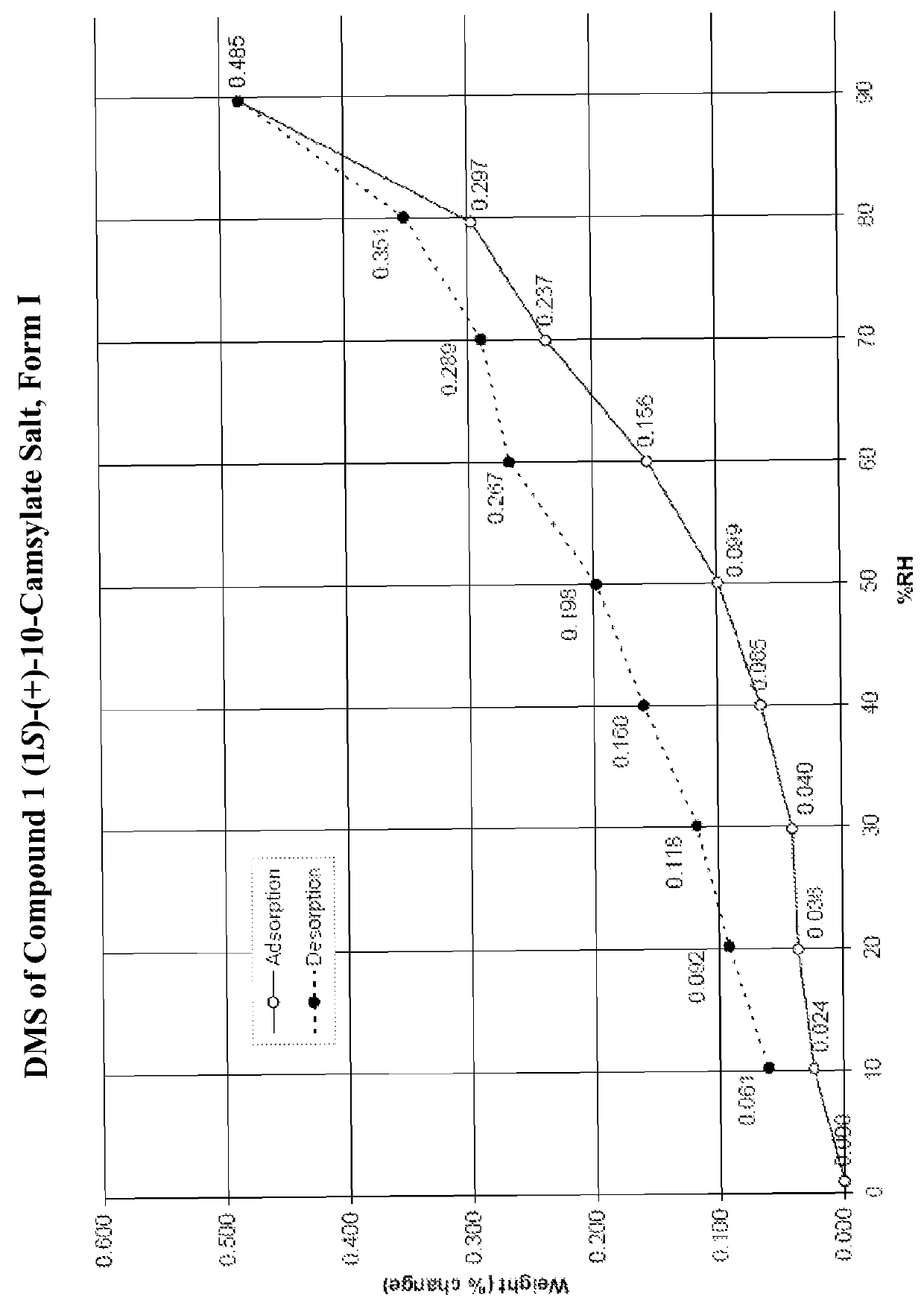
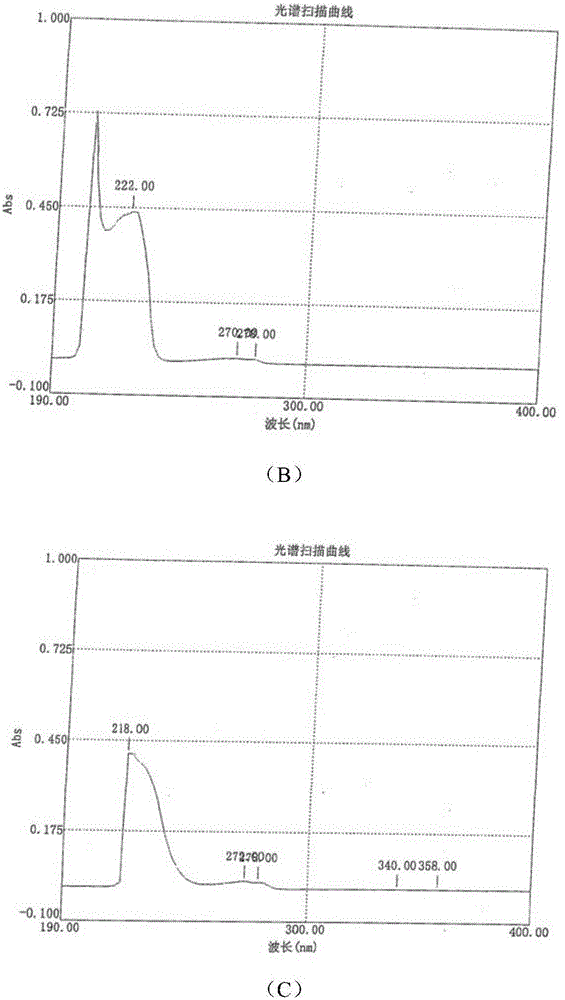
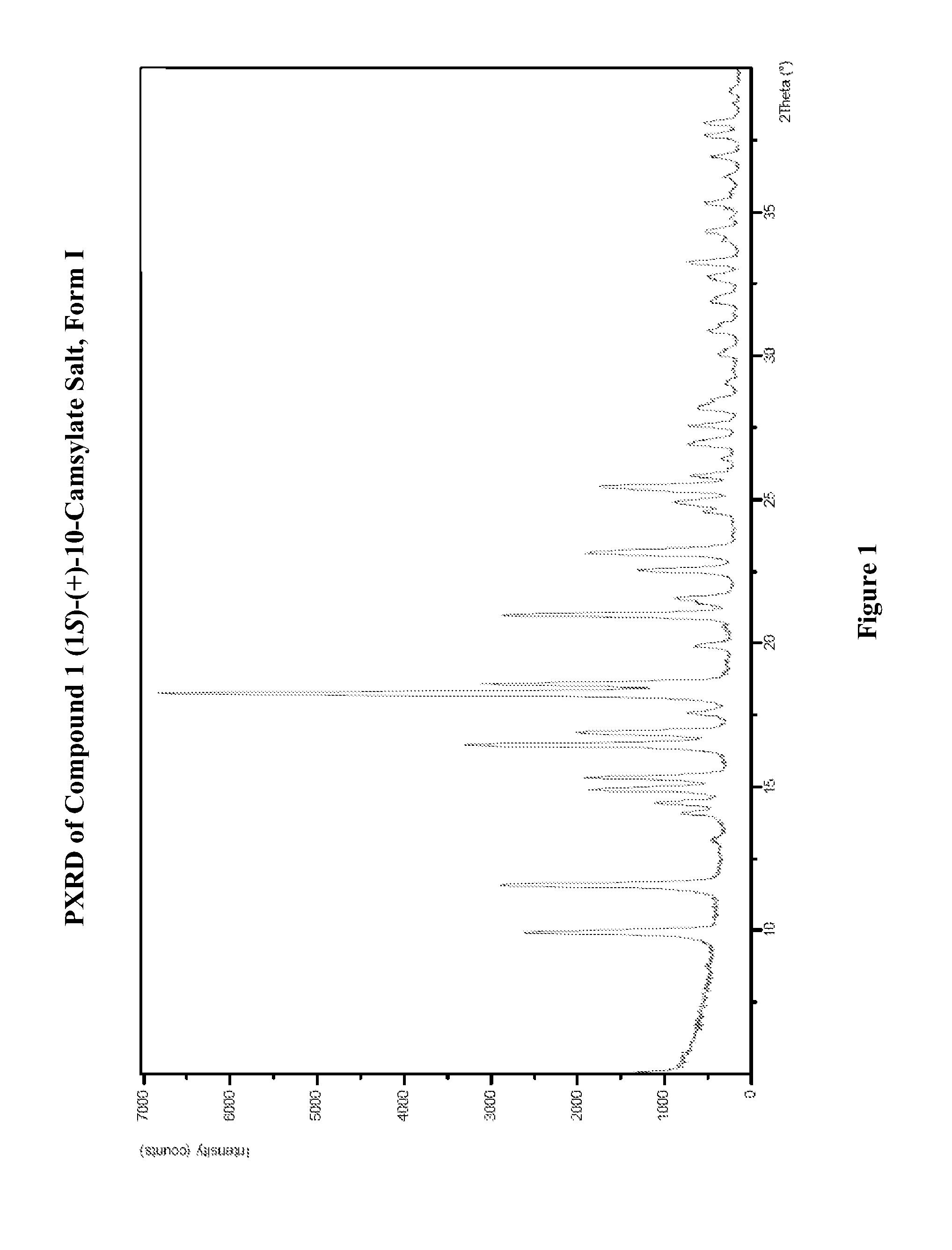
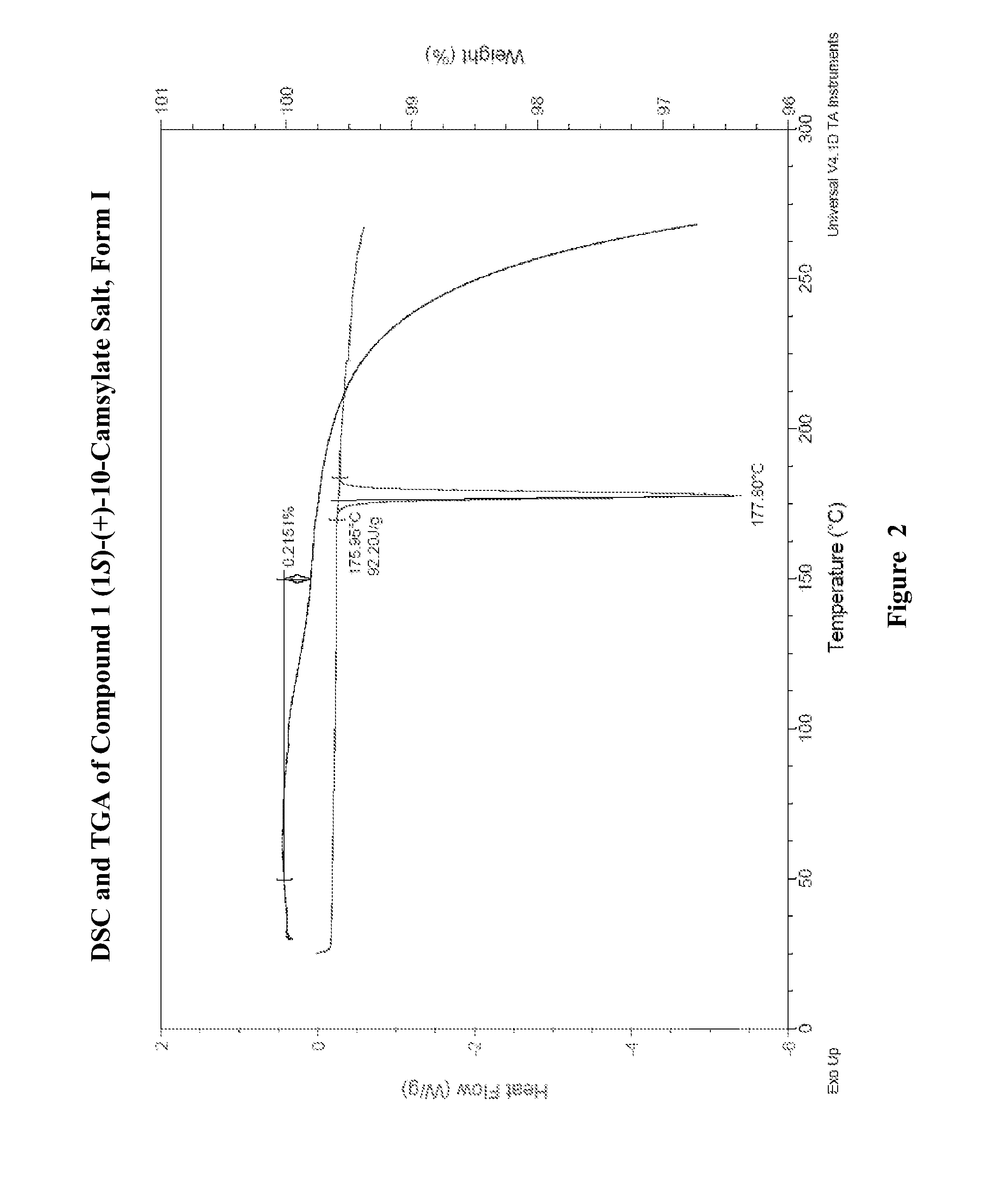
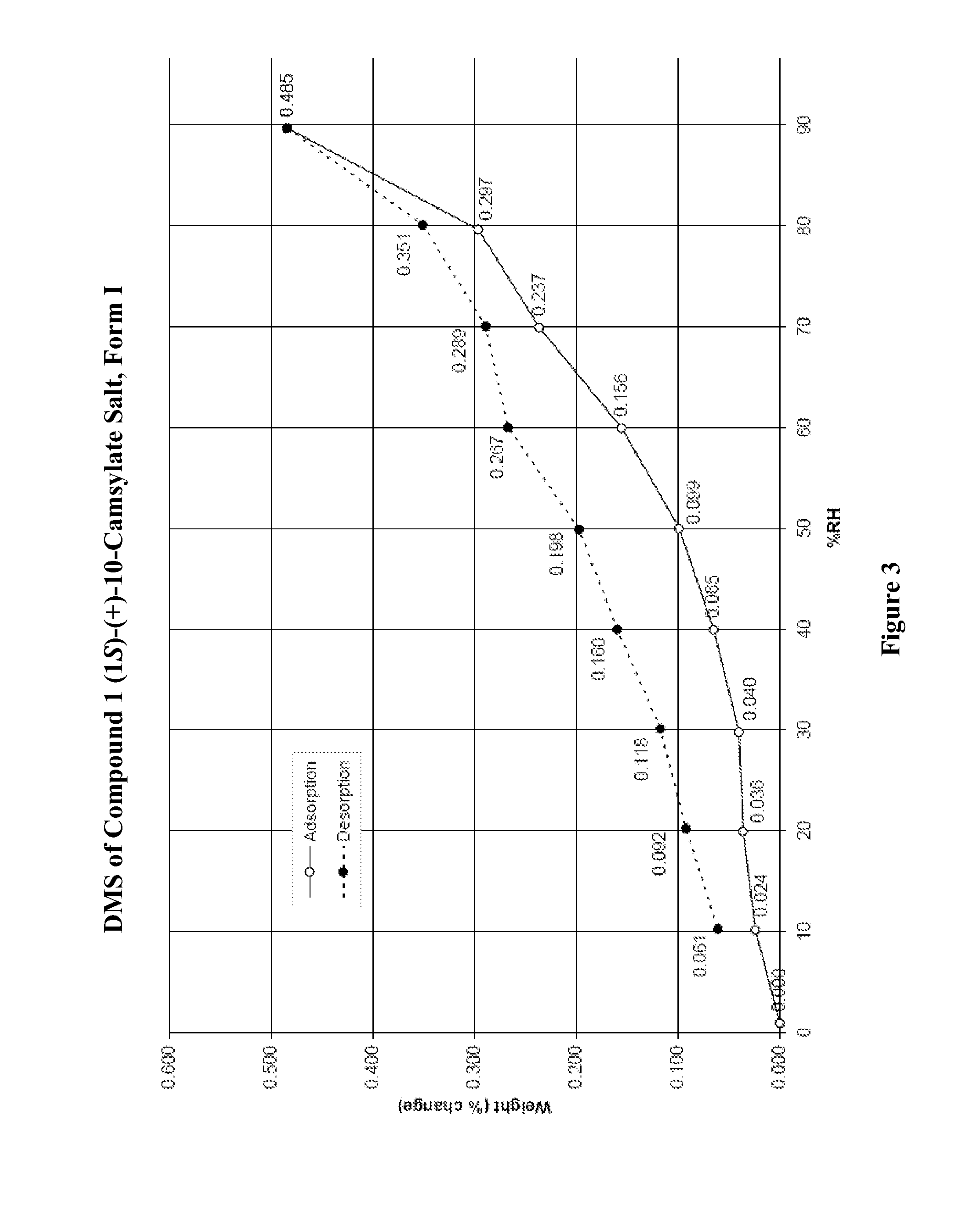
Salts of lorcaserin with optically active acids
Salts of 8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazepine with optically active acids, and pharmaceutical compositions comprising them that are useful for, inter alia, weight management.
Owner:ARENA PHARMA
Preparation method for hemihydrate lorcaserin hydrochloride
The invention discloses a preparation method for hemihydrate lorcaserin hydrochloride. The preparation method comprises the following steps: (1) making a compound shown as a formula III react with ammonia to obtain a compound shown as a formula II; (2) under the protection of nitrogen gas, dissolving the compound shown as the formula II in an organic solvent, adding a hydrogen chloride solution of which the solvent is the organic solvent to salify, and adding water and cyclohexane to form a hemihydrate in order to obtain the compound shown as a formula I, wherein the organic solvent is isopropanol or 1,4-dioxane. In the preparation method disclosed by the invention, ammonium hydroxide substitutes for potassium carbonate in the prior art, so that unqualified ignition residues of a finial product caused by potassium chloride generated after salt removal can be avoided; an isopropoxide hydrochloride solution substitutes for the conventional hydrogen chloride gas, so that other impurities can be prevented from being introduced in a preparation process under the improper control of dosage and rate of the gas.
Owner:科兴生物制药股份有限公司
Salts of lorcaserin with optically active acids
Salts of 8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazepine with optically active acids, and pharmaceutical compositions comprising them that are useful for, inter alia, weight management.
Owner:ARENA PHARMA
Lorcaserin hydrochloride jelly and preparation method thereof
A lorcaserin hydrochloride jelly and a preparation method thereof are disclosed. Jelly is a leisure food, which is welcome by the young, but the young is prone to obesity. Jelly is delicious, normally the intake amount is large, but jelly contains very few calories. The jelly is a food for reducing fat when jelly does not affect the normal functions of digestion system. Lorcaserin chloride is a 5-hydroxy tryptamine (5-HT) acceptor stimulant, and can be used as an auxiliary drug for treating chronic weight gain by reducing the diet calories and increasing the body movement. Lorcarserin hydrochloride is made into jelly so that a user can enjoy the delicious taste of jelly and is capable of reducing the weight at the same time, and the treatment and delicious taste are perfectly combined.
Owner:BEIJING SUNHO PHARMA
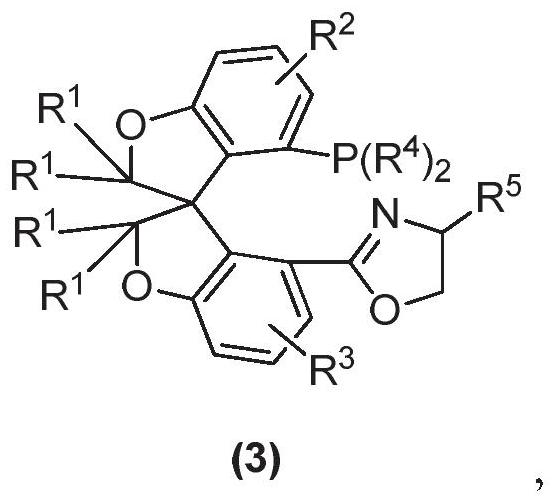
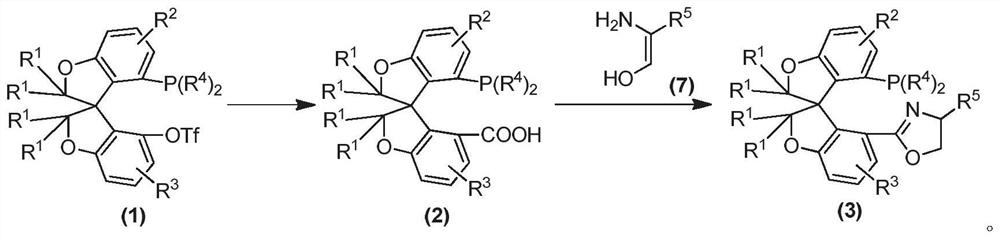
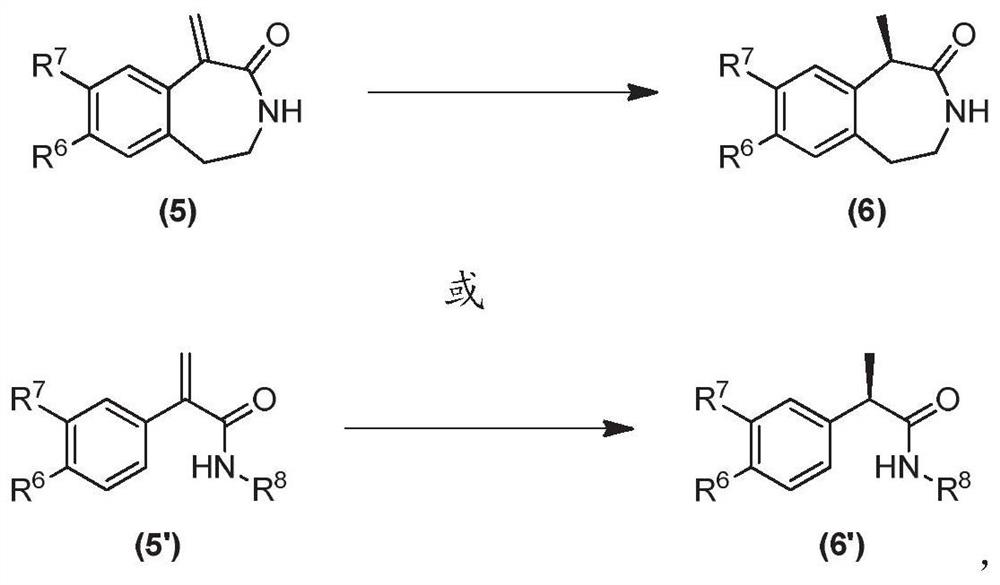
Oxa-spiro phosphine-oxazoline ligand as well as preparation method and application thereof
ActiveCN111961080AHigh catalytic activityGreat potentialOrganic compound preparationGroup 5/15 element organic compoundsPtru catalystCombinatorial chemistry
Owner:SOUTH UNIVERSITY OF SCIENCE AND TECHNOLOGY OF CHINA
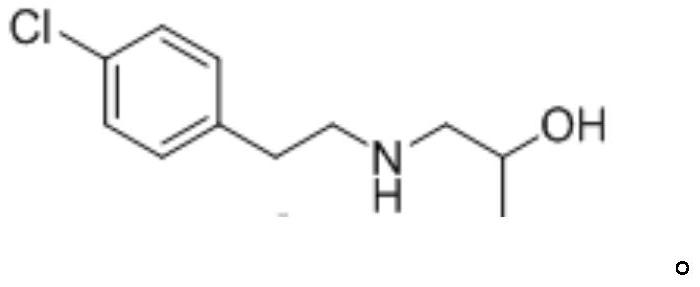
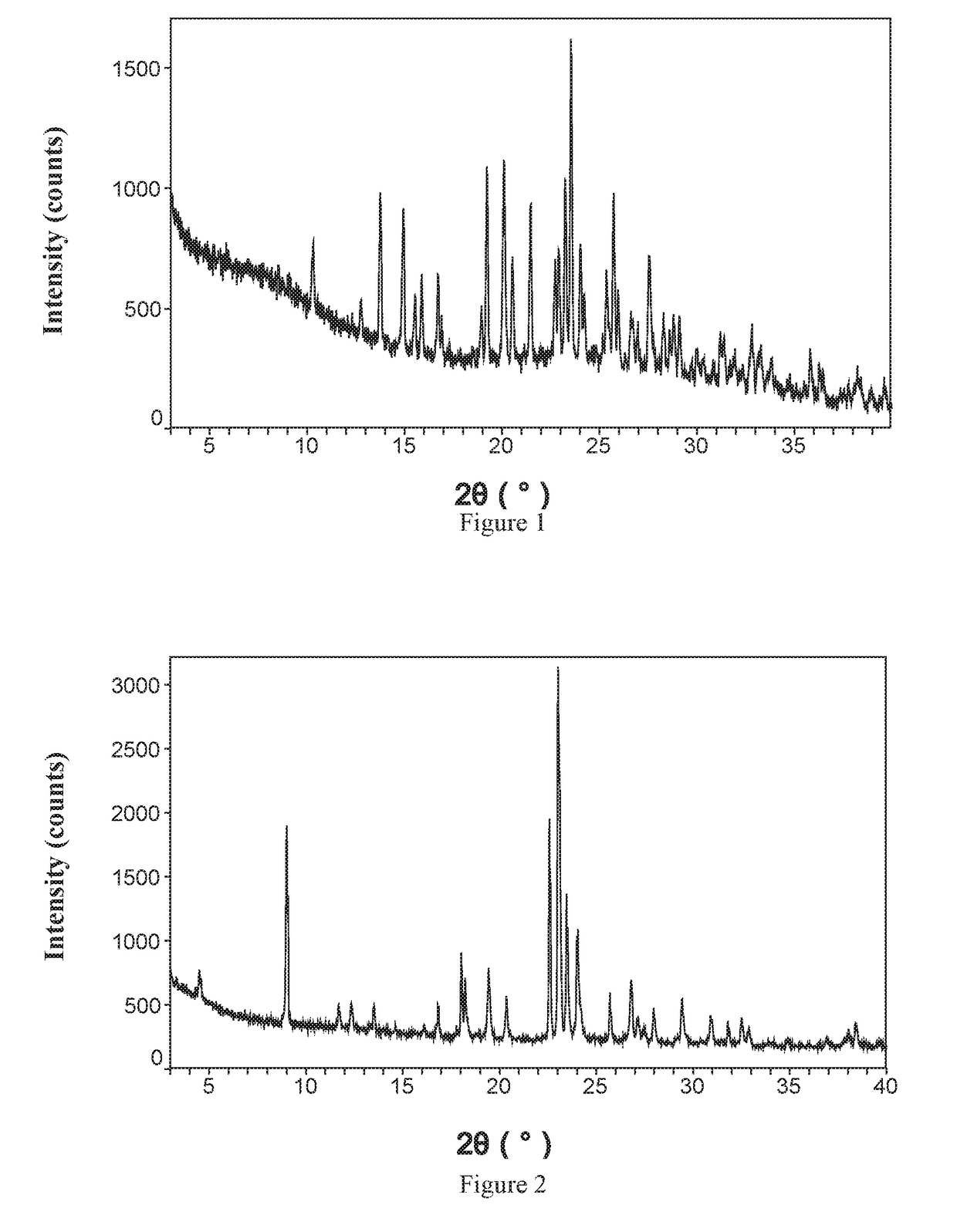
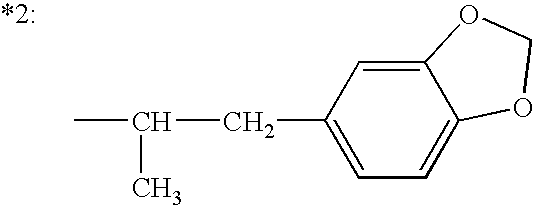
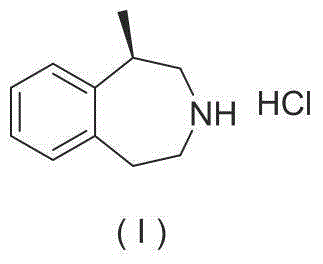
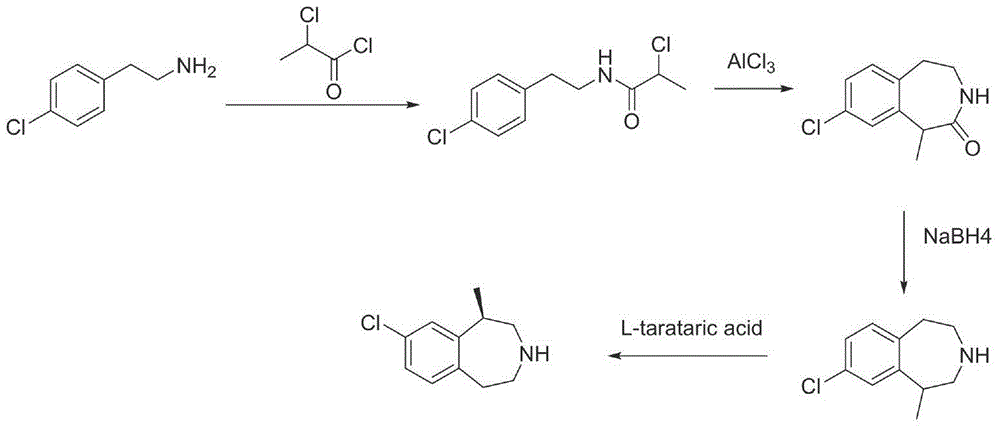
8-chloro-1-methyl-4,5-dihydro-1h-benzo[d]azepine-2(3H)-one preparation method
InactiveCN104876868AReduce dosageLow reaction temperatureOrganic chemistryAlkyl transferReaction temperature
The present invention relates to the pharmaceutical synthesis field, particularly to a preparation method of an lorcaserin intermediate 8-chloro-1-methyl-4,5-dihydro-1H-benzo[d]azepine-2(3H)-one. The preparation method is characterized in that in an intramolecular Friedel-Crafts alkylation reaction, aluminum trichloride is adopted as a catalyst and anhydrous dichlorobenzene is adopted as a solvent so as to increase the reaction at the step to 73%, such that the method has advantages of low catalyst consumption, low reaction temperature, convenient post-treatment, easy scale-up, and the like.
Owner:CHINA PHARM UNIV +1
Preparation method of lorcaserin intermediate
PendingCN112358406AHigh yieldThree wastes lessOrganic compound preparationHalogenated hydrocarbon preparationChlorobenzenePtru catalyst
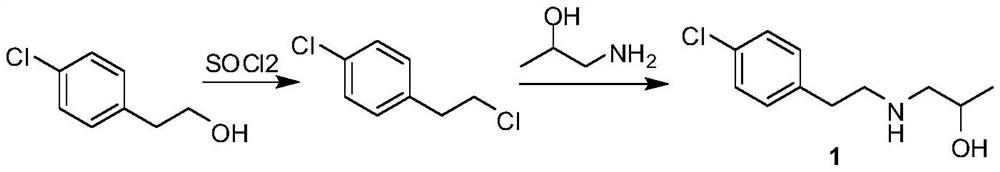
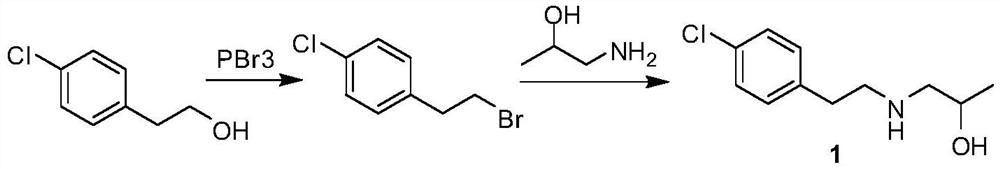
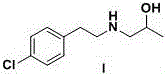
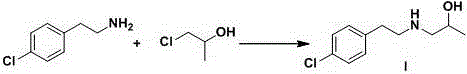
The invention relates to a preparation method of a lorcaserin intermediate 1-[2-(4-chlorphenyl)-ethylamino]-2-propanol, which specifically comprises the following steps: carrying out bromination reaction on p-chlorophenethyl alcohol serving as a starting material to obtain 4-chlorphenyl ethyl bromide, and condensing with isopropanolamine to obtain a target product. Hydrobromic acid is used as a bromination reagent, potassium carbonate is used as a condensation reaction acid-binding agent, potassium iodide is used as a condensation reaction catalyst, the yield of the technological process is high, three wastes are few, the cost is low, the operation is simple, the safety is good, and industrial production requirements are met.
Owner:SHANDONG ACADEMY OF PHARMACEUTICAL SCIENCES
Method for preparing lorcaserin
ActiveCN105924396ARealize industrial applicationQuality assuranceOrganic chemistryChlorobenzeneSolvent free
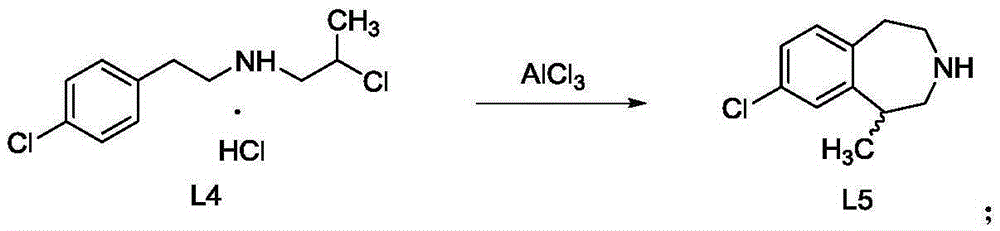
The present invention provides a method for preparing lorcaserin. The method is as below: A) synthesizing a lorcaserin racemate by using 1-[[2-(4-chlorphenyl) ethyl] amino]-2-chloropropane hydrochloride as a raw material by a solvent-free method; B) cooling the reaction system to 70-80 DEG C, adding an organic solvent, stirring well and cooling to 0 to 5 DEG C, wherein the organic solvent is tetrachloroethylene, chlorobenzene, toluene or cyclohexane; C) adding hydrochloric acid solution in the reaction system, dispensing the liquid, and removing an organic phase to obtain a water phase; and D) adding NaOH solution to the water phase, then adding cyclohexane or heptanes, and extracting and concentrating the organic phase to obtain a lorcaserin racemic pure product. After the solvent-free alkylation reaction, the organic solvent of tetrachloroethylene, chlorobenzene, toluene or cyclohexane is added, so as to solve the safety problem caused by rapid heat release due to direct addition of water; and the posttreatment process is simple and safe. The invention realizes industrial application of solvent-free synthesis of lorcaserin, at the same time ensures the quality of prepared lorcaserin.
Owner:LIANGJIANG MEDICINE CO LTD
A kind of lorcaserin hydrochloride pellets, its preparation method and its preparation
ActiveCN106031712BQuality improvementStable in natureMetabolism disorderPill deliveryCelluloseLACTOSE MONOHYDRATE
A lorcaserin hydrochloride mini-pill is provided. The mini-pill comprises a blank pill core, a medicine loading layer, a slow-release layer and a quick-release layer in order from inside to outside. The medicine loading layer comprises lorcaserin hydrochloride, lactose monohydrate, highly substituted hydroxypropylcellulose and talcum powder. The slow-release layer comprises ethyl cellulose, highly substituted hydroxypropylcellulose, talcum powder and triethyl citrate. The quick-release layer comprises lorcaserin hydrochloride, lactose monohydrate, highly substituted hydroxypropylcellulose and talcum powder. The mass ratio of the sum of the weight of the blank pill core and the weight of the medicine loading layer to the mass of the slow-release layer is 1:(0.25-0.35). The mini-pill comprises the slow-release layer and the quick-release layer, and therefore a medicine can rapidly work and functions of the medicine in a body are prolonged. A preparing method of the lorcaserin hydrochloride mini-pill and a preparation of the lorcaserin hydrochloride mini-pill are also provided.
Owner:珠海万钜生物医药有限公司
Process for the preparation of Lorcaserin hydrochloride
Owner:AUROBINDO PHARMA LTD
Method for preparing lorcaserin
ActiveCN111499575AHigh purityRaw materials are cheap and easy to getOrganic chemistryChlorobenzeneEthyl group
The invention discloses a method for preparing lorcaserin. Specifically, the method comprises the steps: taking p-chlorophenylacetonitrile as an initial raw material, preparing p-chlorophenylethylamine through reduction; carrying out a reaction with p-toluenesulfonyl chloride to form an amino occupying intermediate; enabling the intermediate to carry out a reaction with monochloroacetone under analkaline condition to form N-(2-(4-chlorphenyl)ethyl)-4-methyl-N-(2-propionyl)benzenesulfonamide, and then carrying out reduction, chlorination, p-toluenesulfonyl removal and intramolecular Friedel-Crafts alkylation to synthesize 8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzoazepine, carrying out L-(+)-tartaric acid resolution and alkalization on azepine to remove tartaric acid, and acting with hydrogen chloride diethyl ether to salify to prepare lorcaserin. The method has the characteristics of simple synthesis method, good reaction selectivity, high product purity, environmental protectionand low preparation cost.
Owner:HEBEI NORMAL UNIV
Lorcaserin hydrochloride osmotic pump controlled release preparation and preparation method thereof
ActiveCN112704670AReduce releaseRelease stabilityMetabolism disorderInorganic non-active ingredientsControlled Release TabletOsmotic pump
The invention relates to a lorcaserin hydrochloride osmotic pump controlled release preparation and a preparation method thereof. The lorcaserin hydrochloride osmotic pump controlled release preparation is a lorcaserin hydrochloride osmotic pump controlled release tablet, which comprises a tablet core and a controlled release semipermeable coating film wrapping the periphery of the tablet core. The lorcaserin hydrochloride osmotic pump controlled release preparation can be slowly and stably released in the gastrointestinal tract environment, and is simple to take and obvious in medicine effect.
Owner:ANHUI UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Cocrystal of lorcaserin, preparation methods, pharmaceutical compositions and uses thereof
ActiveUS9981912B2Improve propertiesImprove stabilityNervous disorderMetabolism disorderBenzoic acidSolubility
Owner:NI YUN
A process for the preparation of Lorcaserin Hydrochloride
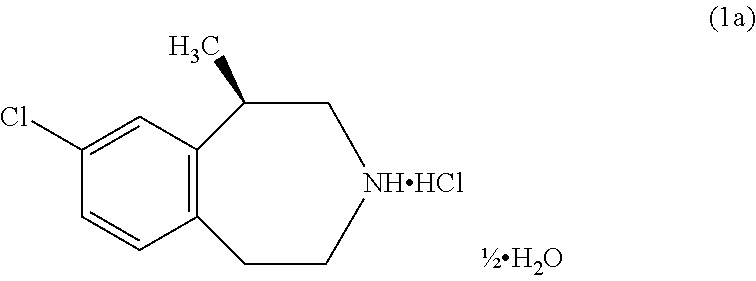
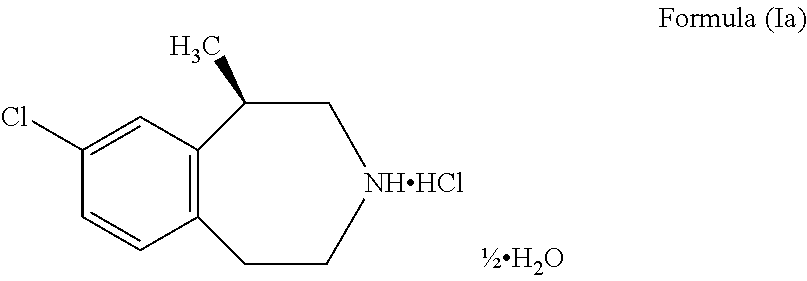
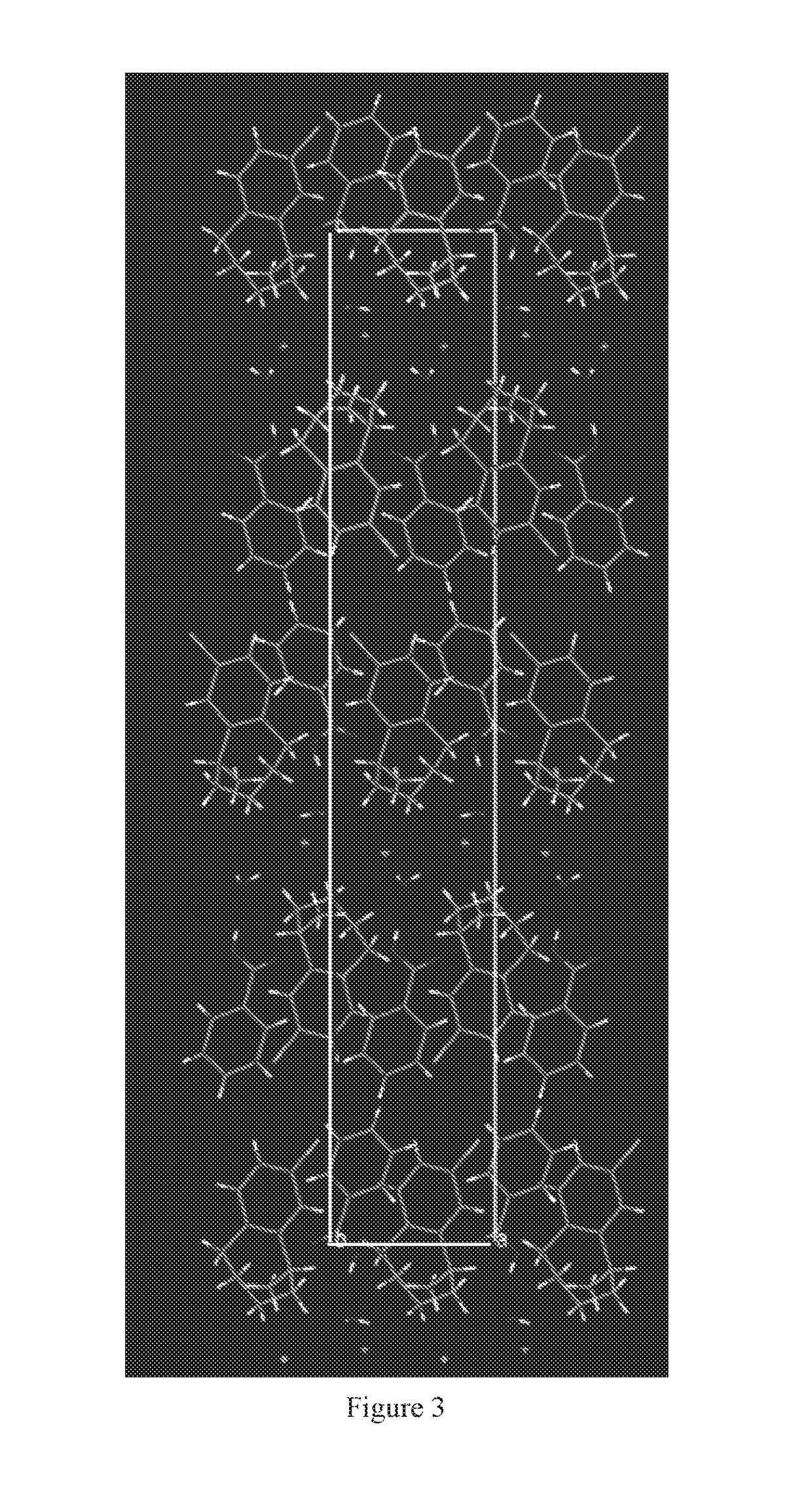
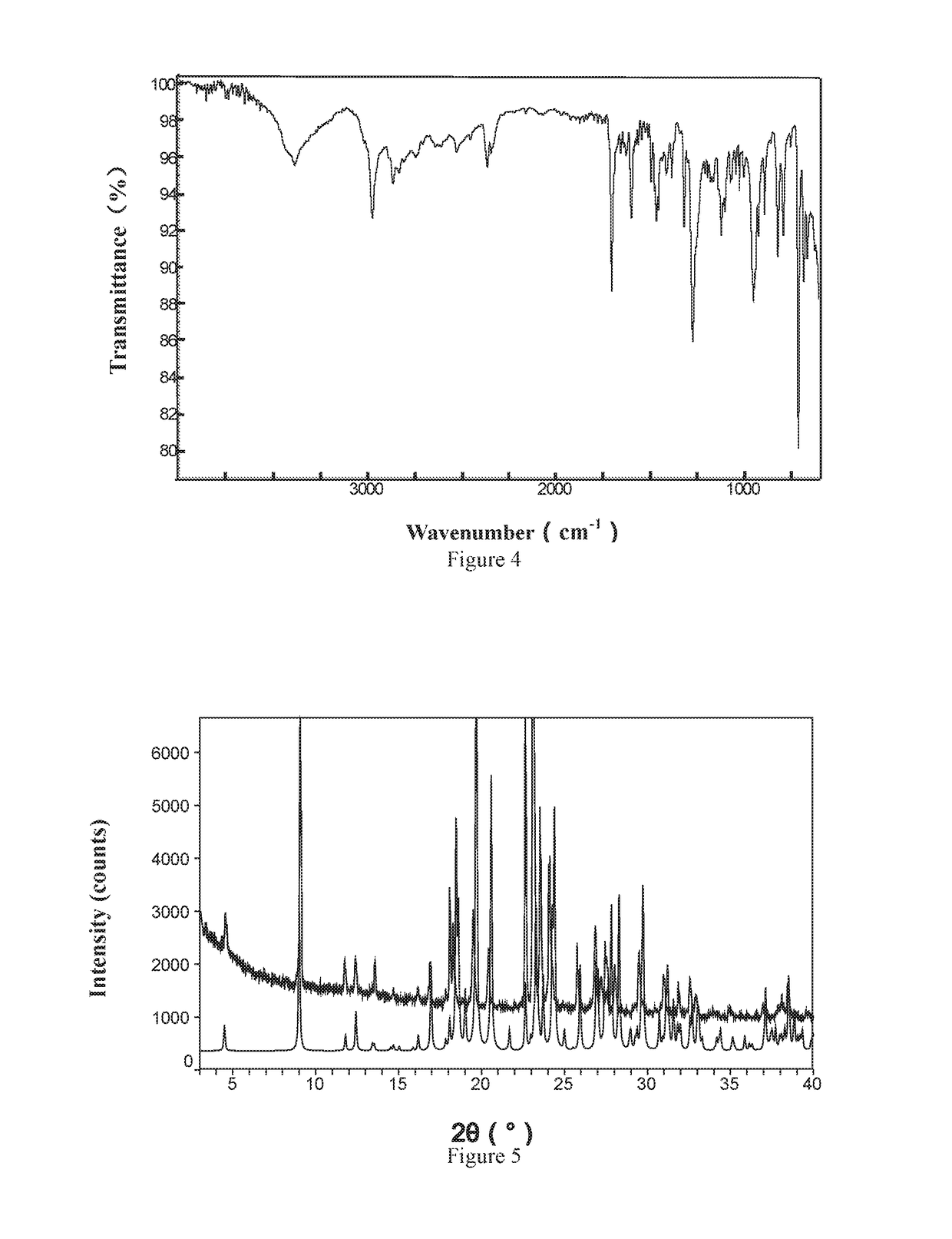
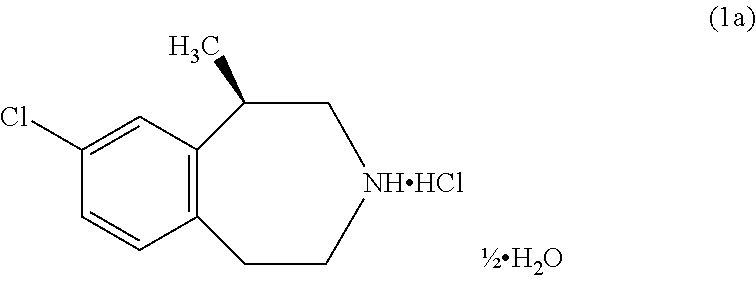
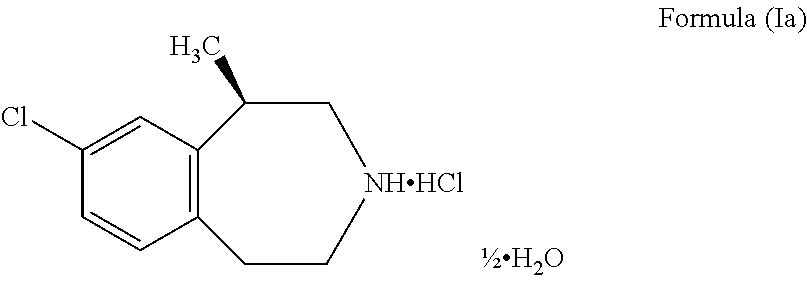
The present invention provides a process for the preparation of crystalline Lorcaserin hydrochloride hemihydrate of Formula (1a), which comprises, Formula (1a) (i) providing a solution of Lorcaserin base in a solvent; (ii) if water is present, removing water from the reaction mixture; (iii) adding hydrogen chloride to the reaction mixture; (iv) combining the reaction mixture with a suitable anti-solvent; and (v) isolating the crystalline Lorcaserin hydrochloride hemihydrate of Formula (1a).
Owner:AUROBINDO PHARMA LTD
A kind of preparation method of lorcaserin intermediate
ActiveCN106631823BEasy to operateHigh yieldOrganic compound preparationOrganic chemistry methodsPropanolChlorobenzene
The invention discloses a preparation method of lorcaserin intermediate I, which uses p-chlorophenylacetonitrile as a starting material to prepare lorcaserin intermediate I through reduction and condensation steps. The starting materials of the present invention are p-chlorophenylacetonitrile and 1-chloro-2-propanol, which are cheap and easy to obtain, avoid the use of thionyl chloride, hydrobromic acid, borane and other easily polluting and explosive raw materials, and avoid producing a large amount of industrial waste water , is more conducive to environmental protection, reduces the labor protection requirements of workers, and ensures safe production. The route is novel in design, easy to obtain raw materials, simple and feasible in process operation, and environment-friendly, and provides a simple and feasible process method for large-scale industrial production.
Owner:天津泰普制药有限公司
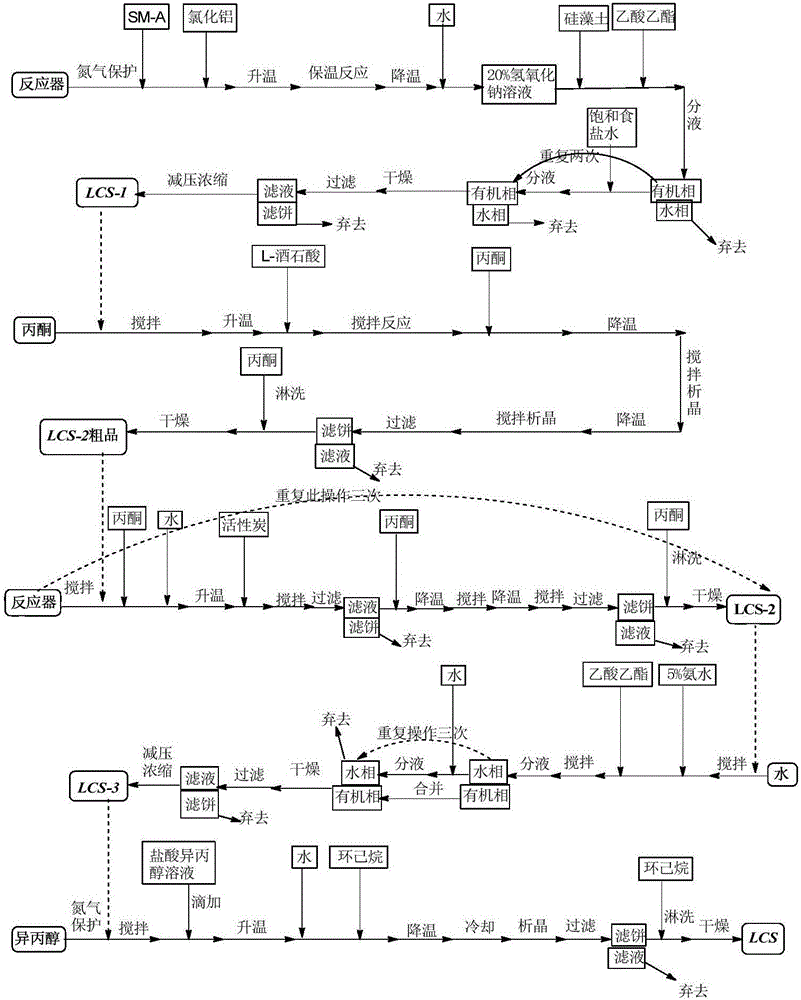
A kind of preparation method of green caserin hydrochloride hemihydrate crystal form
The present invention belongs to the preparation method of green caserin hydrochloride hemihydrate crystal form in the field of compound crystal form preparation. The method comprises the following steps: (1) completely dissolving green caserin hydrochloride in the first organic After being put into the solvent, add water, stir, concentrate to remove the solvent to obtain an oily substance; (2) add a second organic solvent to the oily substance, stir to obtain the crystal form of green caserin hydrochloride hemihydrate. The method does not need to control the feeding amount of hydrogen chloride gas and realize the crystal transformation process without heating, and has the advantages of easy control of reaction conditions, mildness, high yield and good quality of the obtained product.
Owner:NORTHEAST PHARMA GRP
Application of lorcaserin hydrochloride in preparation of medicine for suppressing opioid addiction and withdrawal reaction
InactiveCN103877096BInhibitory reactivityNervous disorderHeterocyclic compound active ingredientsWithdrawal syndromeStimulant
The invention relates to an application of Lorcaserin hydrochloride in preparation of a medicine for inhibiting opioid addiction and withdrawal syndrome. The Lorcaserin hydrochloride is approved to be a weight-reducing aid by the America Food and Drug Administration (FDA) in 2012. The Lorcaserin hydrochloride is a 5-hydroxytryptamine 5-HT2C receptor stimulant with high selectivity. The latest research of the invention finds that the Lorcaserin hydrochloride (0.5-1.0mg.kg<-1>) greatly reduces the naloxone-urged horizontal motion distance and speed of a morphine dependent mice and the withdrawal syndrome of the mice. It is found in the invention for the first time that the Lorcaserin hydrochloride can be applied clinically for rescuing and treating addiction and withdrawal syndrome generated by morphine and other opiates.
Owner:ANHUI MEDICAL UNIV
A kind of preparation method of greencaserin key intermediate I
ActiveCN108358796BAvoid harsh reaction conditionsThe reaction steps are simpleOrganic compound preparationAmino-hyroxy compound preparationChlorobenzeneOrganic solvent
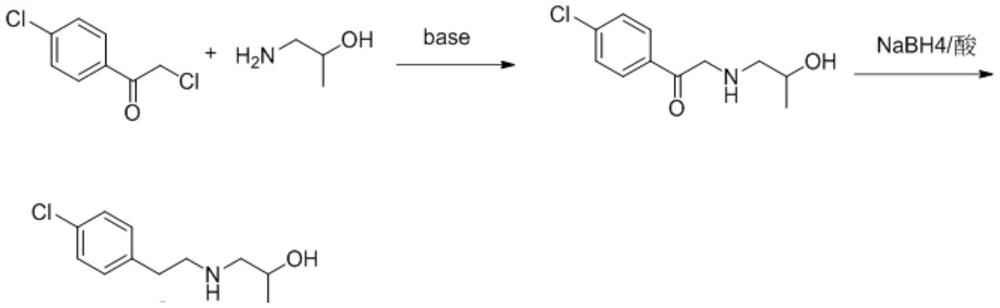
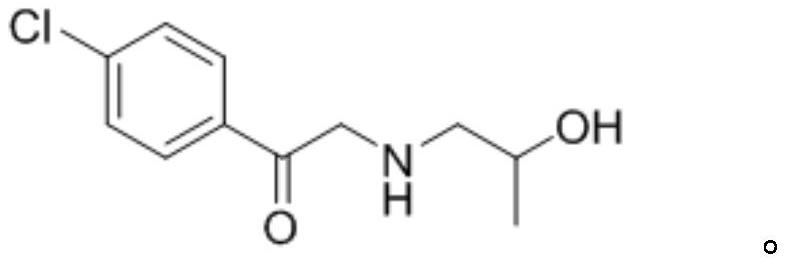
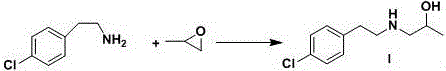
The invention discloses a preparation method of a key intermediate I of lorcaserin. The preparation method comprises the steps as follows: (1) in an organic solvent A, 2,4'-dichloroacetophenone is subjected to a condensation reaction with isopropanolamine under catalysis of alkali, and 1-(4-chlorophenyl)-2-((2-hydroxypropyl)amino)ethane-1-one is obtained; (2) the 1-(4-chlorophenyl)-2-((2-hydroxypropyl)amino)ethane-1-one obtained in the step (1) is subjected to catalytic reduction with sodium borohydride and acid in a solvent B, and the key intermediate I of lorcaserin is obtained. The preparation method can synthesize the key intermediate I of lorcaserin from cheap and easily available raw materials under mild reaction conditions, has the advantages of being simple to operate, low in costand high in yield, and is energy-saving, environmentally friendly and suitable for mass production.
Owner:GUANGZHOU TROJAN PHARMATEC LTD
Cocrystal of lorcaserin, preparation methods, pharmaceutical compositions and use thereof (as amended)
ActiveUS20170327467A1Improve propertiesImprove stabilityNervous disorderMetabolism disorderBenzoic acidDisease
The present invention relates to a new type of eutectic crystal of lorcaserin hydrochloride and benzoic acid. Compared with the prior art, the eutectic crystal has the improved properties of good stability, low solubility, and being suitable for the application of controlled-release preparation. The present invention also relates to a method for preparing the eutectic crystal, a pharmaceutical composition thereof and the use thereof in the manufacture of drugs for treating and / or preventing diseases associated with 5HT2C.
Owner:NI YUN
Synthesis process of weight-reducing drug lorcaserin hydrochloride intermediate
PendingCN110950802AReduce manufacturing costHigh yieldOrganic chemistryMetabolism disorderAcetic anhydrideMedicinal chemistry
The invention discloses a synthesis process of a weight-reducing drug lorcaserin hydrochloride intermediate (compound V), which is characterized in that p-chlorophenylethylamine is used as a raw material, acetic anhydride is subjected to acylation to protect amino, and allyl substitution, Friedel-Crafts alkylation and hydrochloric acid deprotection are performed to obtain a lorcaserin hydrochloride racemate. The synthesis method is simple in synthesis and preparation and high in yield, and is a lorcaserin hydrochloride intermediate synthesis process suitable for industrial large-scale production.
Owner:江西博雅欣和制药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
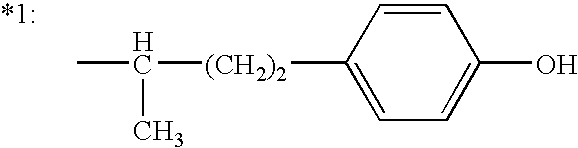





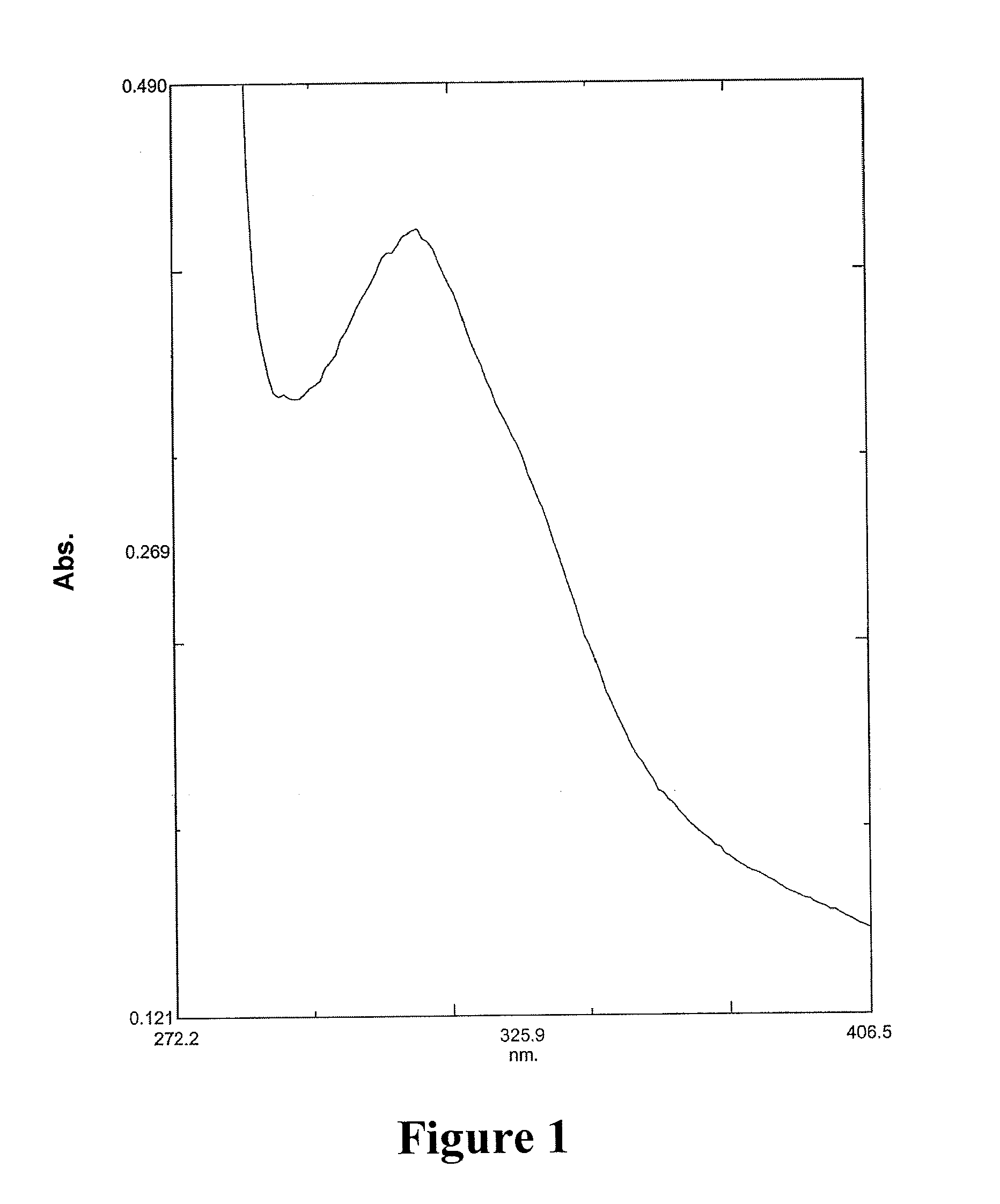
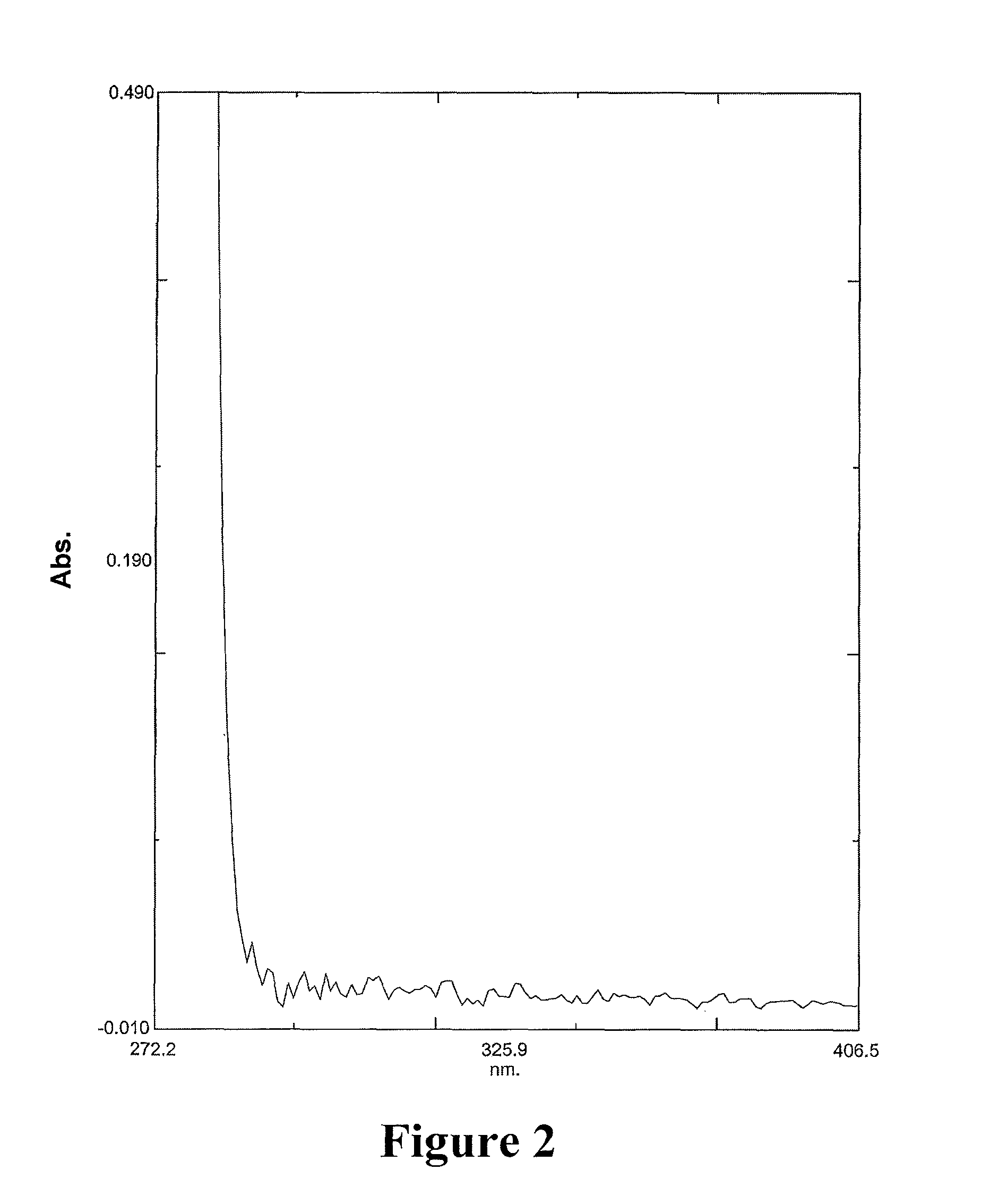
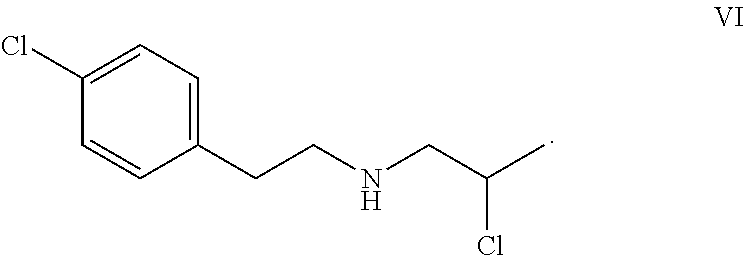
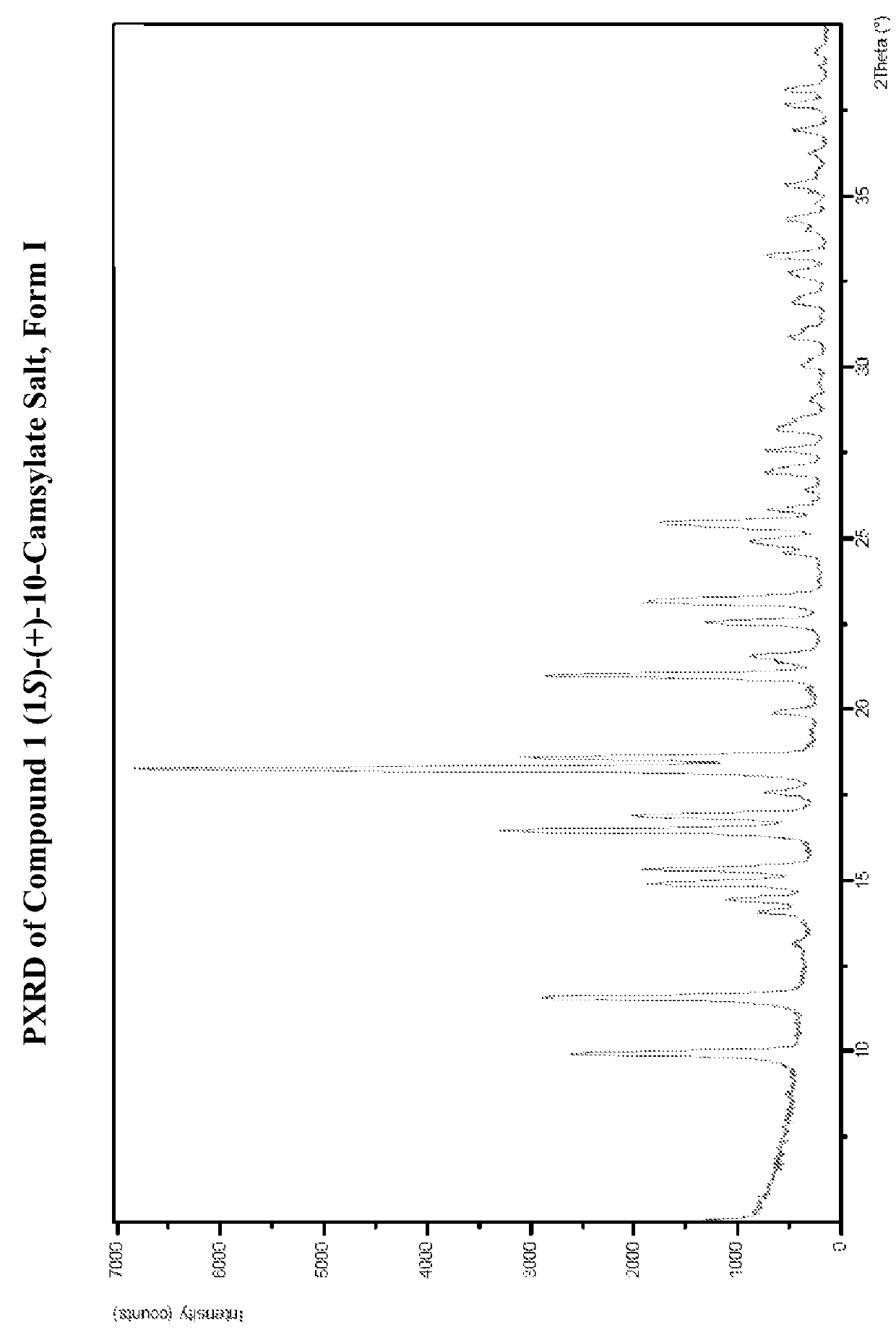
![8-chloro-1-methyl-4,5-dihydro-1h-benzo[d]azepine-2(3H)-one preparation method 8-chloro-1-methyl-4,5-dihydro-1h-benzo[d]azepine-2(3H)-one preparation method](https://images-eureka.patsnap.com/patent_img/13424c5a-7628-42c7-837f-d34a7acc865b/BSA0000101463960000011.PNG)
![8-chloro-1-methyl-4,5-dihydro-1h-benzo[d]azepine-2(3H)-one preparation method 8-chloro-1-methyl-4,5-dihydro-1h-benzo[d]azepine-2(3H)-one preparation method](https://images-eureka.patsnap.com/patent_img/13424c5a-7628-42c7-837f-d34a7acc865b/BSA0000101463960000012.PNG)
![8-chloro-1-methyl-4,5-dihydro-1h-benzo[d]azepine-2(3H)-one preparation method 8-chloro-1-methyl-4,5-dihydro-1h-benzo[d]azepine-2(3H)-one preparation method](https://images-eureka.patsnap.com/patent_img/13424c5a-7628-42c7-837f-d34a7acc865b/BSA0000101463960000013.PNG)