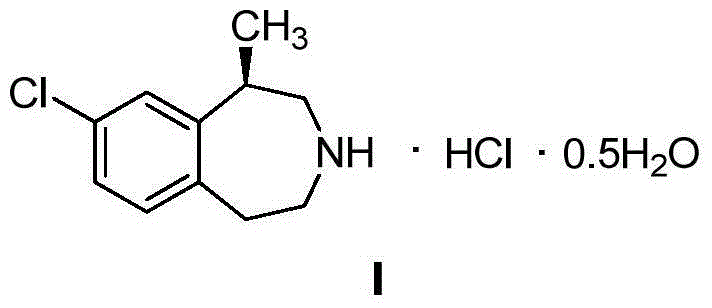
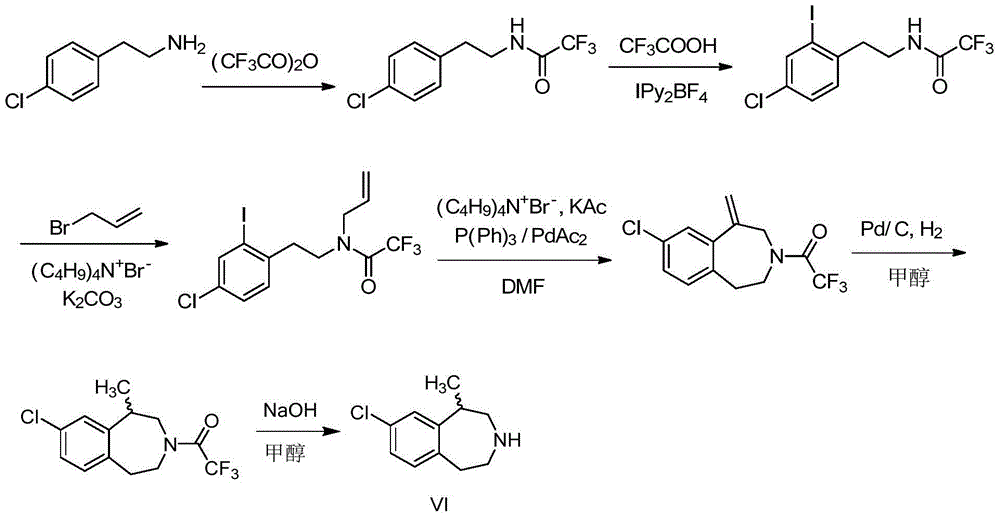
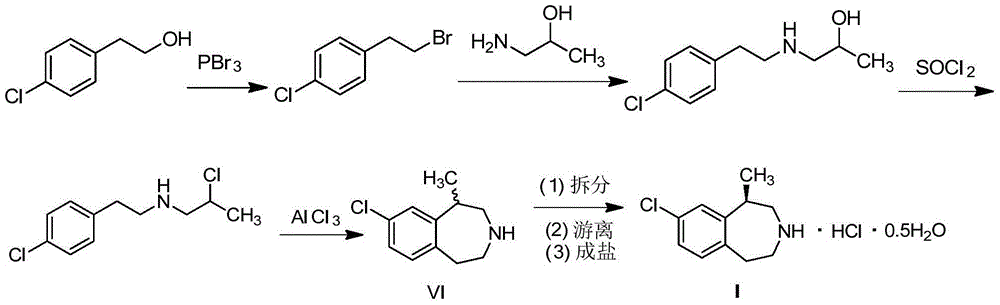
Preparation method of weigh reducing drug lorcaserin hydrochloride and intermediate thereof
A technology of catalysts and compounds, which is applied in the field of preparation of weight-loss drug greencaserin hydrochloride and its intermediates, can solve the problems of inconvenient operation, high price of borane, relatively expensive price of 2-chloropropionyl chloride, etc., and achieve simple operation, reagent Cheap and easy to obtain, convenient and safe after-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] (1) Preparation of (4-chlorophenethyl) tert-butyl carbamate (III)
[0048]Add 2-(4-chlorophenyl)ethylamine (50.0g, 321.3mmol) into a 1L eggplant-shaped flask, add 200mL of dichloromethane, stir to dissolve, and add BOC anhydride (77.1g, 353.3mmol) dropwise at 0°C ) was dissolved in 300mL of dichloromethane, and a large amount of white solid was precipitated. After the addition was completed, it was raised to room temperature, and a catalytic amount of DMAP was added, and the solution gradually became colorless and clear. After stirring for about 5 hours (monitored by TLC, the reaction was complete), pour 400mL Extract in water, combine the organic phases, wash with water and saturated sodium chloride solution successively, dry over anhydrous sodium sulfate, filter with suction, and concentrate the filtrate to dryness under reduced pressure to obtain 80.3 g of white solid with a yield of 97.7%.
[0049] 1 H-NMR (300MHz, CDCl 3 )δ7.29-7.24 (2H, m, ArH), 7.15-7.08 (2H, m...
Embodiment 2
[0066] (1) N-(4-chlorophenethyl) propyl-2-en-1-amine trifluoroacetate (V:HA=CF 3 CO 2 H) Preparation
[0067] Dissolve IV (12.0g, 40.6mml) in 25mL of dichloromethane, slowly add trifluoroacetic acid (6.0ml, 80.8mmol) dropwise, and stir the reaction at 25°C, gradually a white solid precipitates out, TLC monitors that the reaction of raw materials is complete, Cool, filter with suction, wash with a small amount of ethyl acetate to obtain a white solid, and dry to obtain intermediate V (HA=CF 3 COOH) 11.8g, yield 93.9%.
[0068] (2) 8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazepine Preparation of (VI)
[0069] Take anhydrous aluminum trichloride (8.6g, 64.5mmol), grind it into powder, put it into a 250mL four-necked bottle, add 60mL of o-dichlorobenzene, raise the temperature to 110°C under mechanical stirring, add N-(4-chloro Phenylethyl)propyl-2-en-1-amine trifluoroacetate (10.0g, 32.3mmol), after adding, stir the reaction until the raw material disappears (monitored ...
Embodiment 3
[0072] (1) Preparation of N-(4-chlorophenethyl)propyl-2-en-1-amine (VII)
[0073] IX(R=-COCF 3 , 20.0g, 68.6mmol) was dissolved in 100mL of methanol, 100mL of 15% NaOH solution was added, heated to 60°C for 3h, then concentrated under reduced pressure, the residue was extracted with dichloromethane (100mL×3), and the organic phases were combined, Wash with water and saturated sodium chloride solution, dry over anhydrous sodium sulfate, filter with suction, and concentrate the filtrate to dryness under reduced pressure to obtain 11.9 g of a colorless oil, with a yield of 88.6%.
[0074] 1 H-NMR (300MHz, CDCl 3 )δ7.25(2H,dd,J=10.7,3.8Hz,ArH),7.11(2H,dd,J=14.1,8.3Hz,ArH),5.87(1H,m,CH),5.12(2H,m, CH 2 ),3.26(2H,d,J=6.0Hz,CH 2 ),2.91-2.70(4H,m,CH 2 CH 2 ).
[0075] (2) 8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazepine Preparation of (VI)
[0076]Take anhydrous aluminum trichloride (11.5g, 86.2mmol), grind it into powder, put it into a 250mL four-necked bottle, add 60...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com