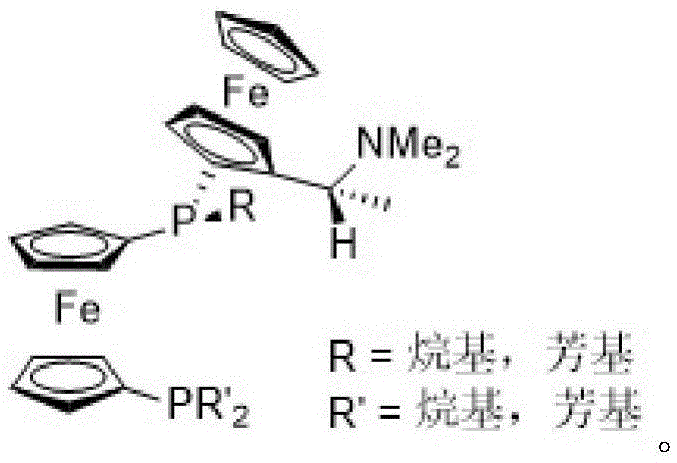
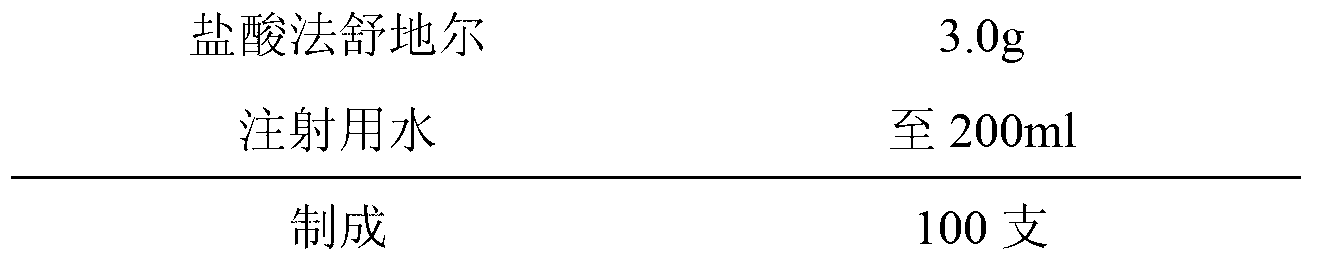Patents
Literature
237 results about "Food and drug administration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
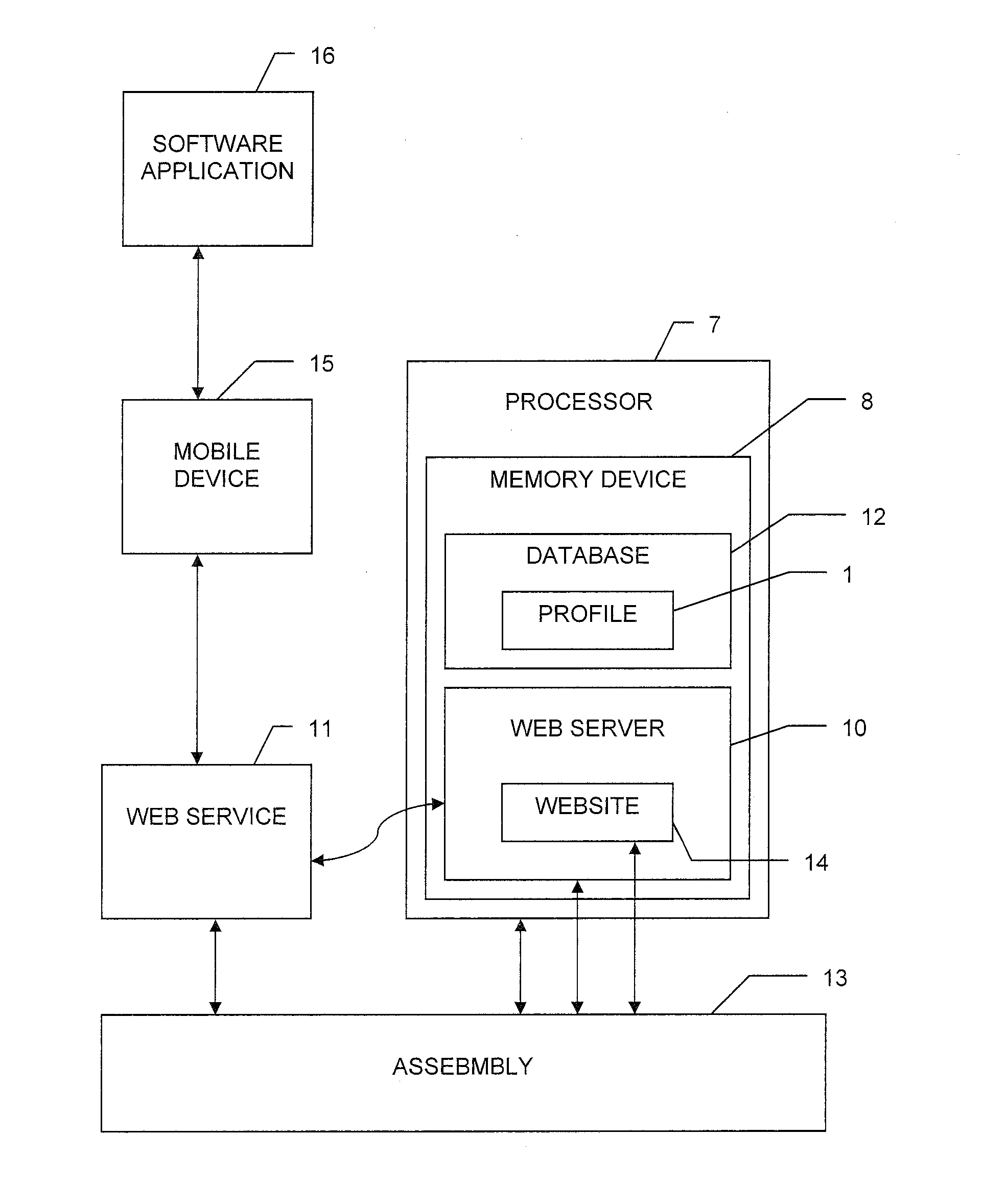
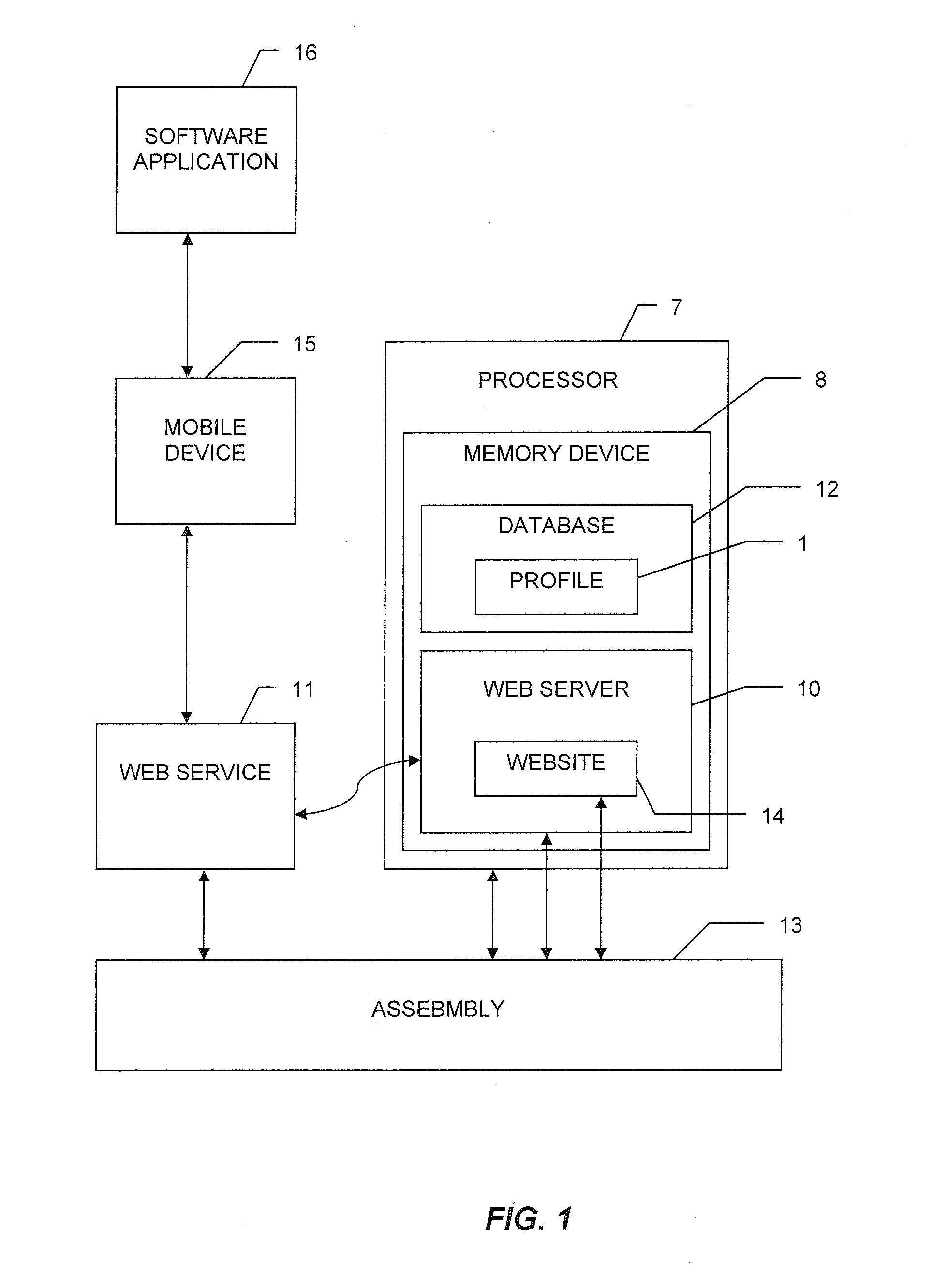
System, method, and computer-readable medium for collection of environmental data and generation of user report for compliance with FDA requirements
InactiveUS6904370B1Data processing applicationsGeneral water supply conservationParticulatesWater quality
A system, method, and computer-readable medium for the collecting and storing of environmental data, and generating a user report of the environmental data, the user report providing document compliance with U.S. Food and Drug Administration requirements. The collected and stored environmental data includes a wide variety of manufacturing facility parameter data, including but is not limited to, the presence of viable microbiological organisms, the presence of particulates and other environmental conditions within the facility, such as humidity, pressure, temperature, water quality (e.g., pH, conductivity, total organic content (TOC), endotoxin, coliform, and metals), and the respective amounts of different materials involved in the manufacture of the end product(s).
Owner:COMPLIANCE SOFTWARE SOLUTIONS
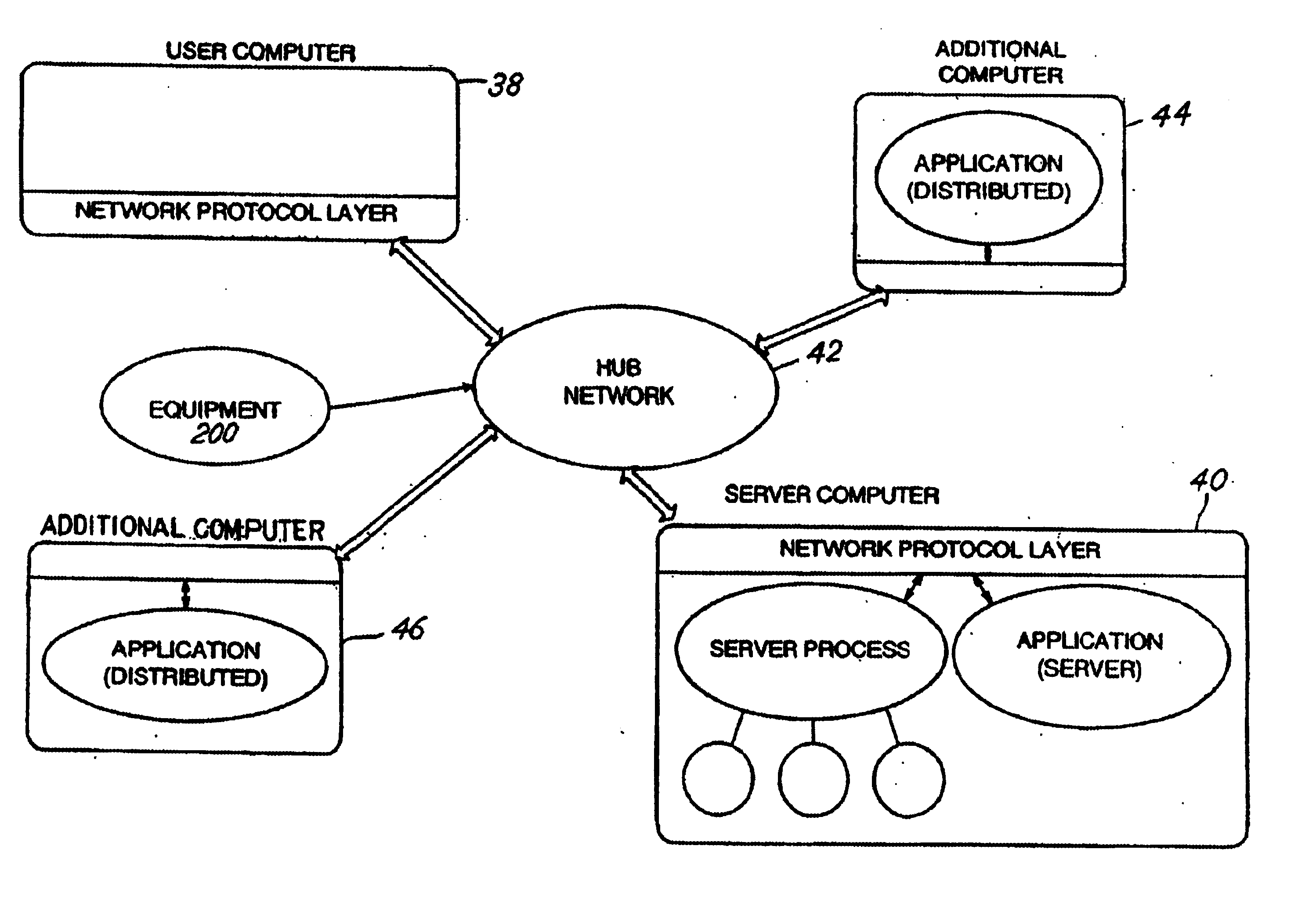
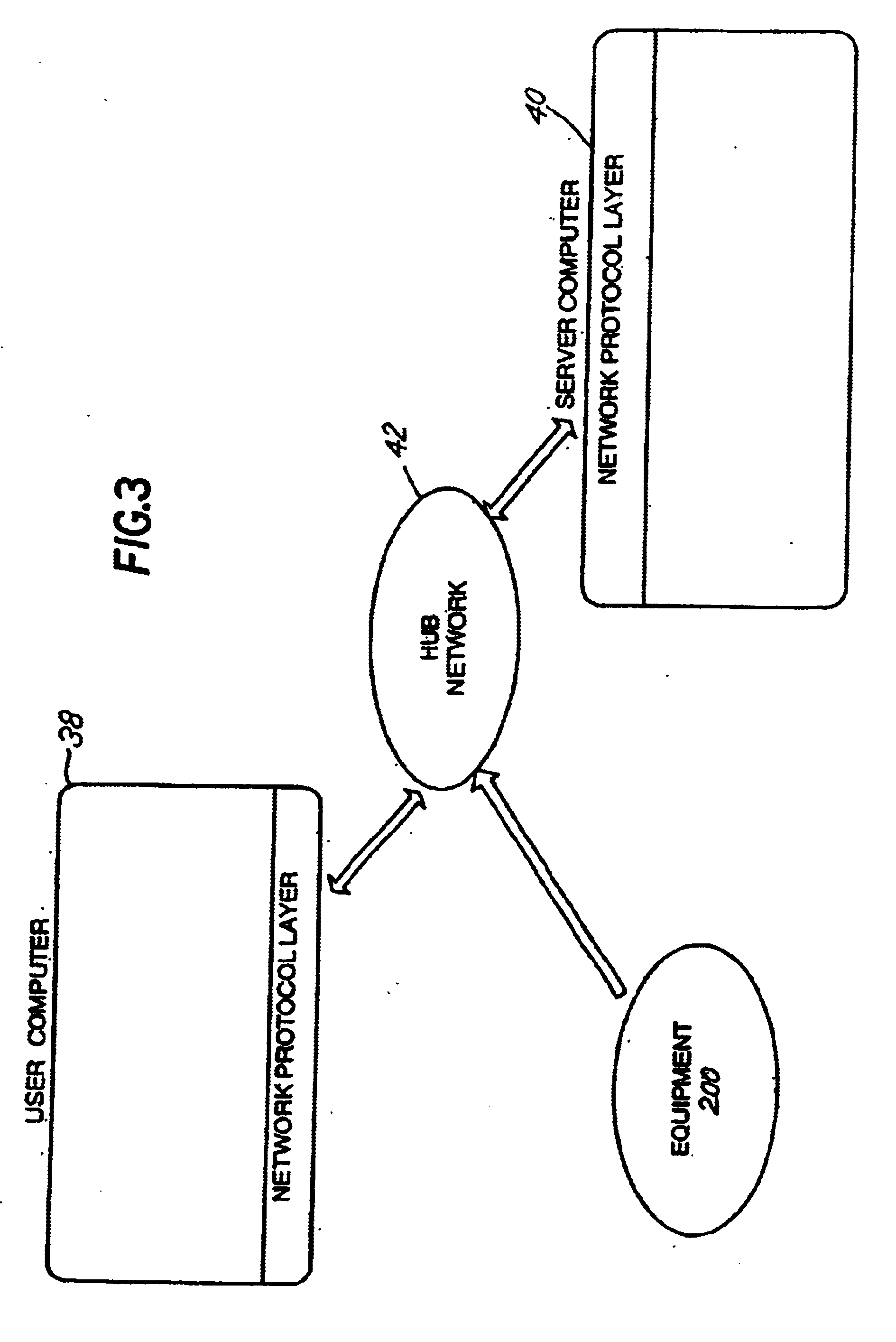
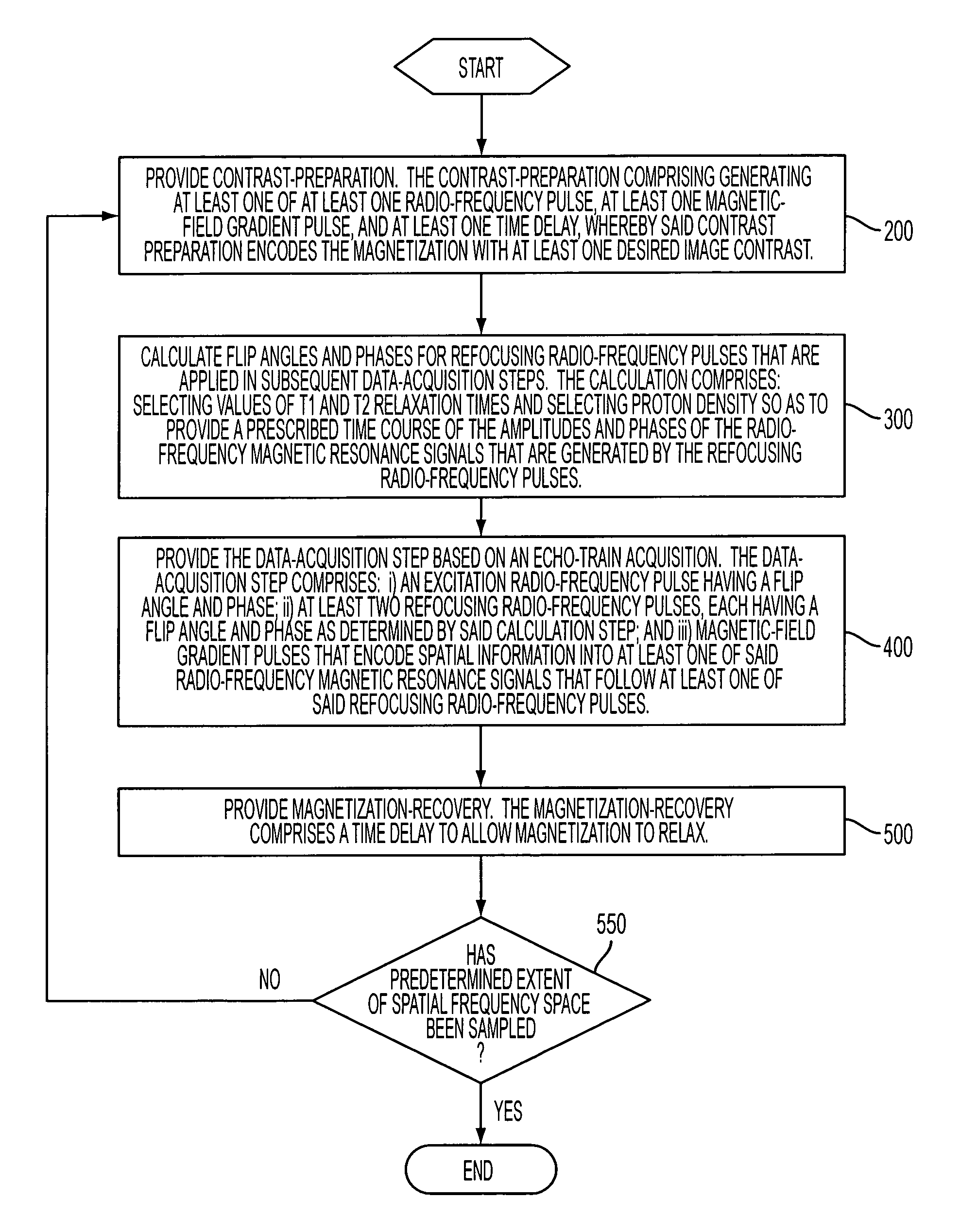
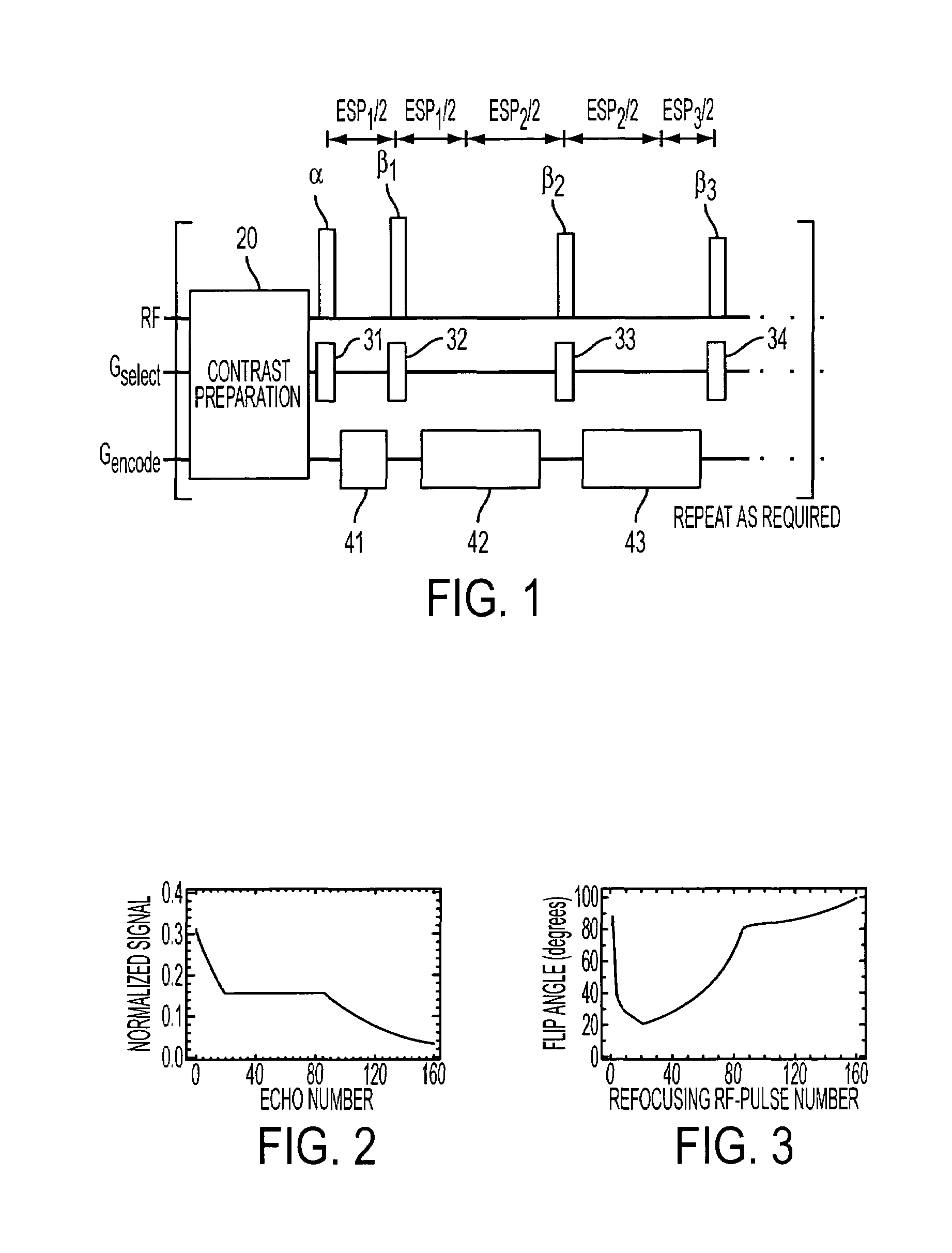
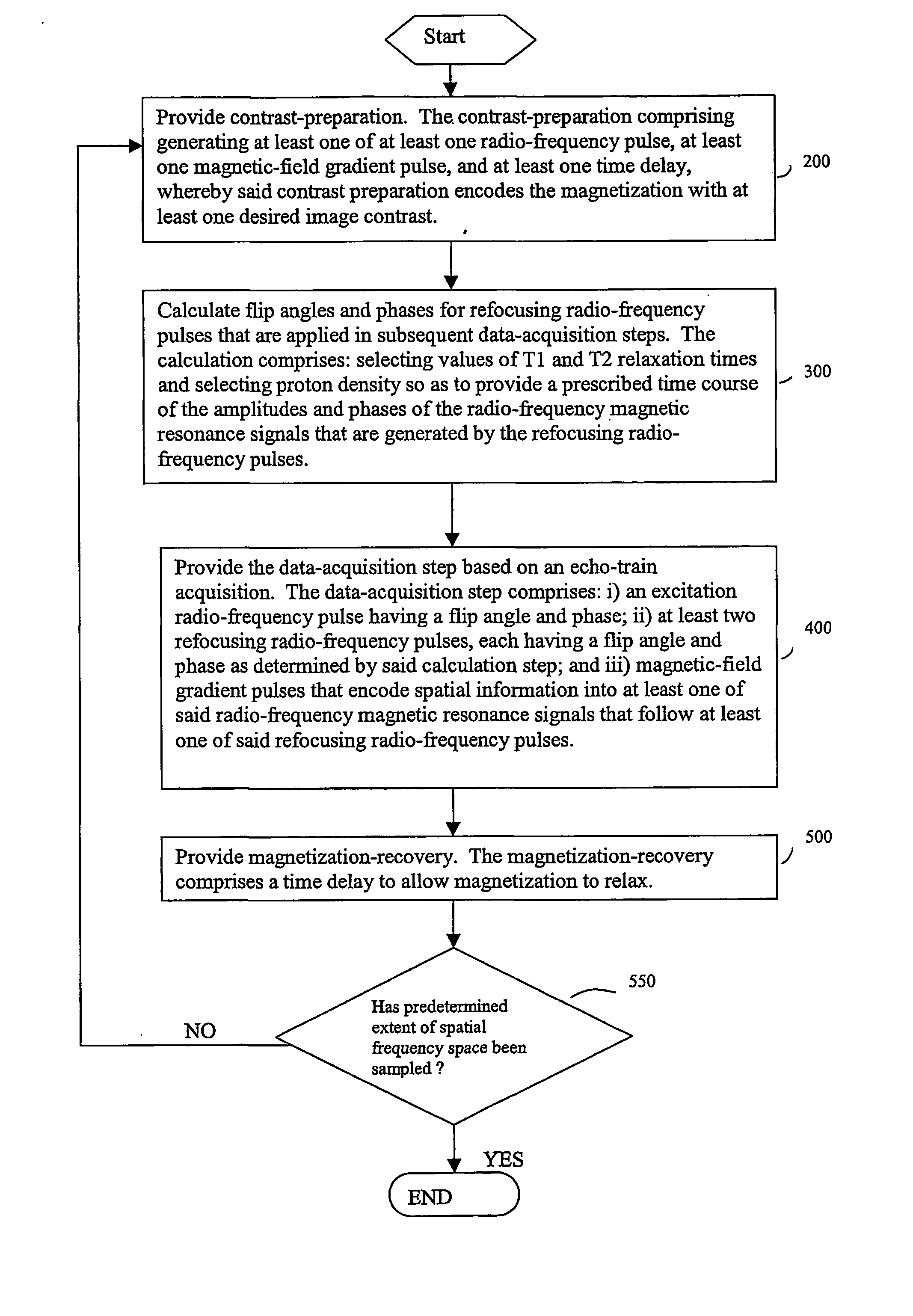
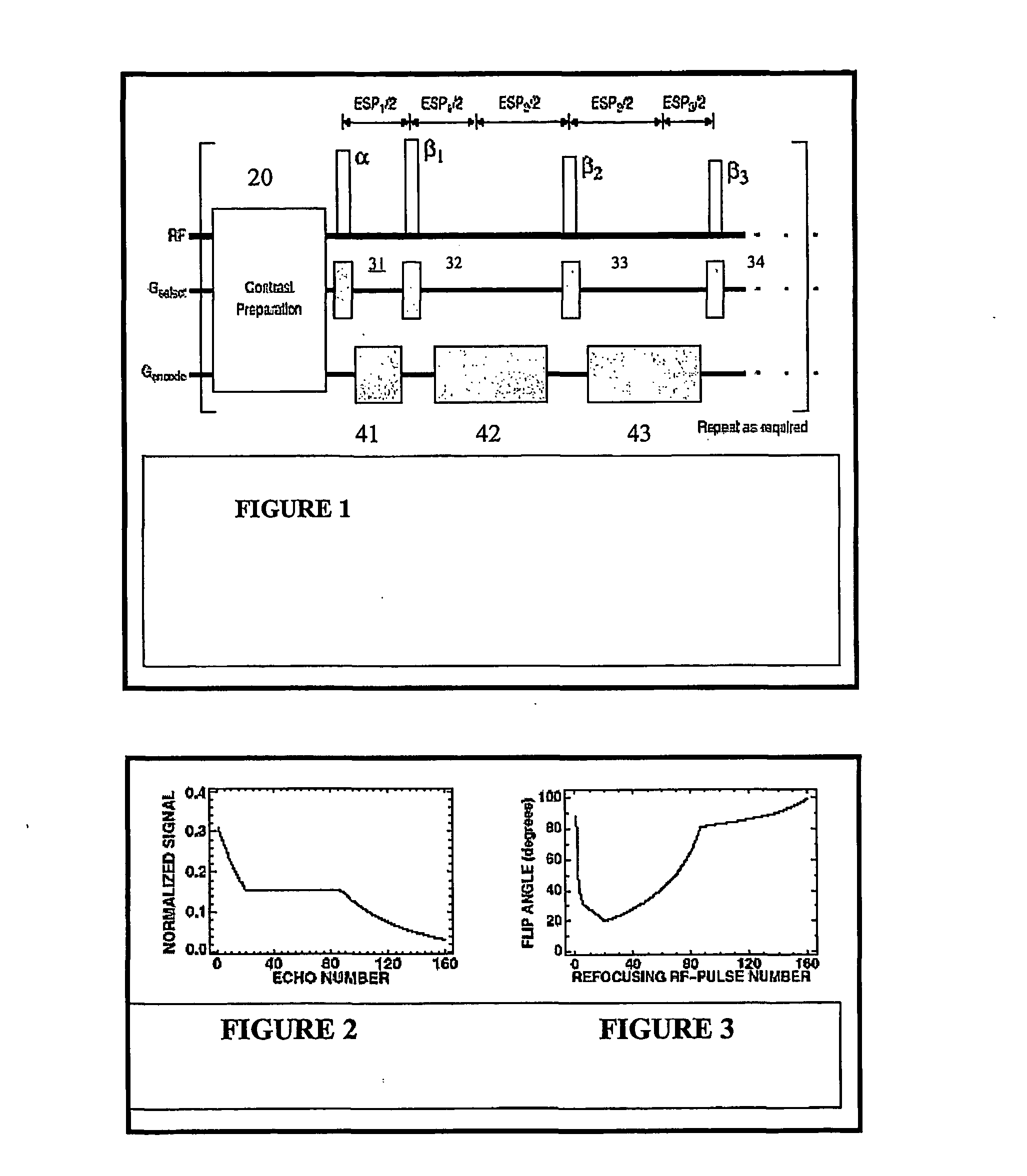
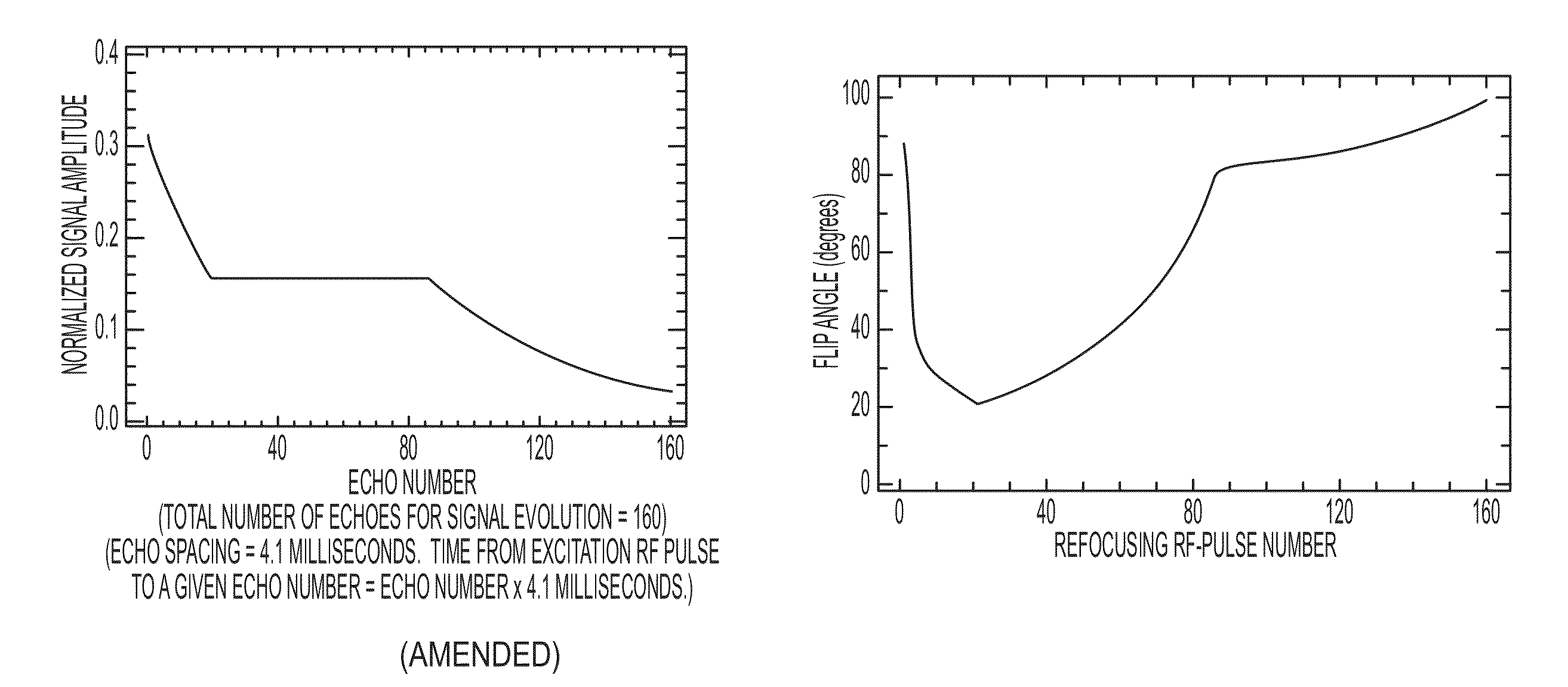
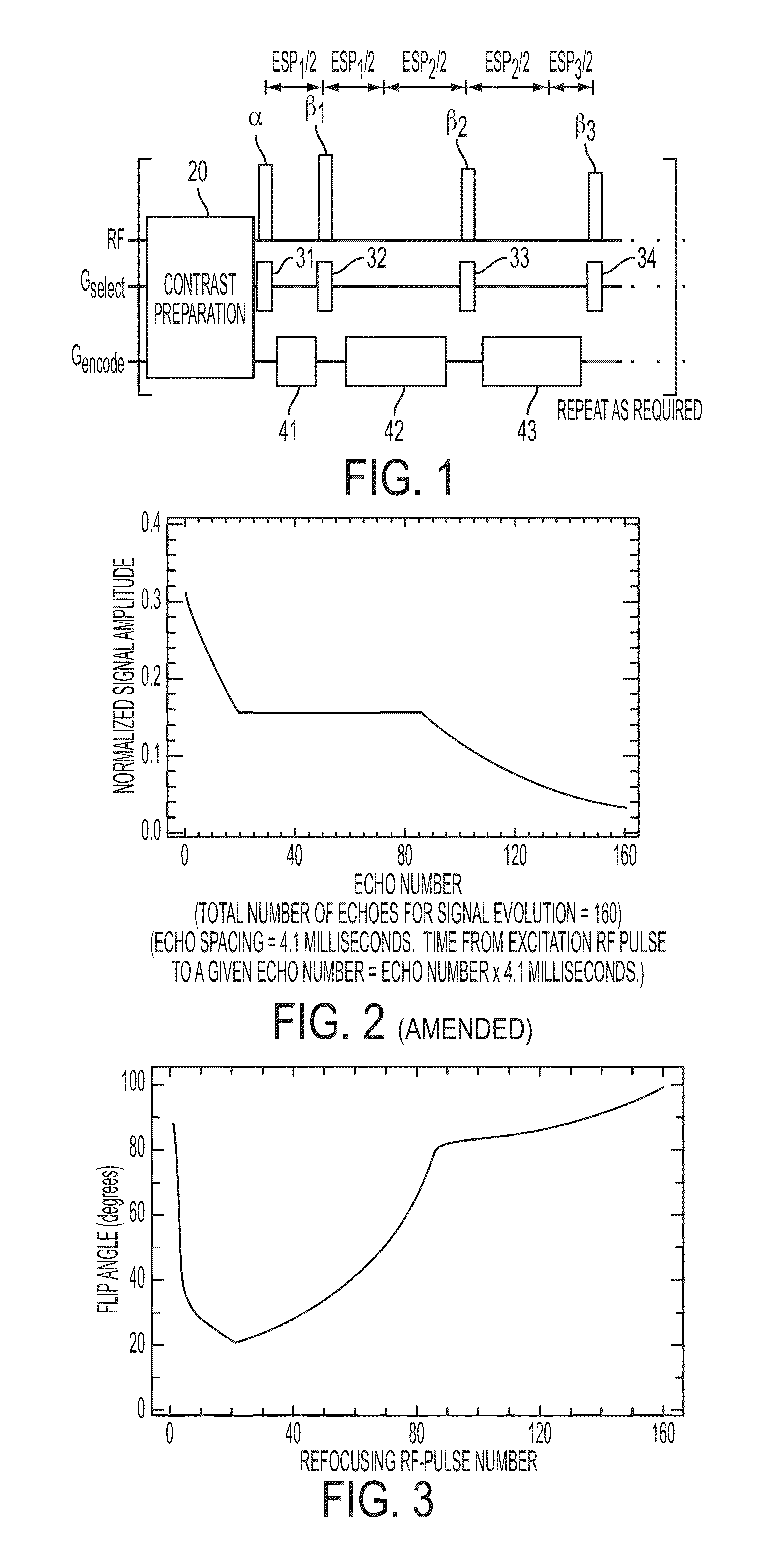
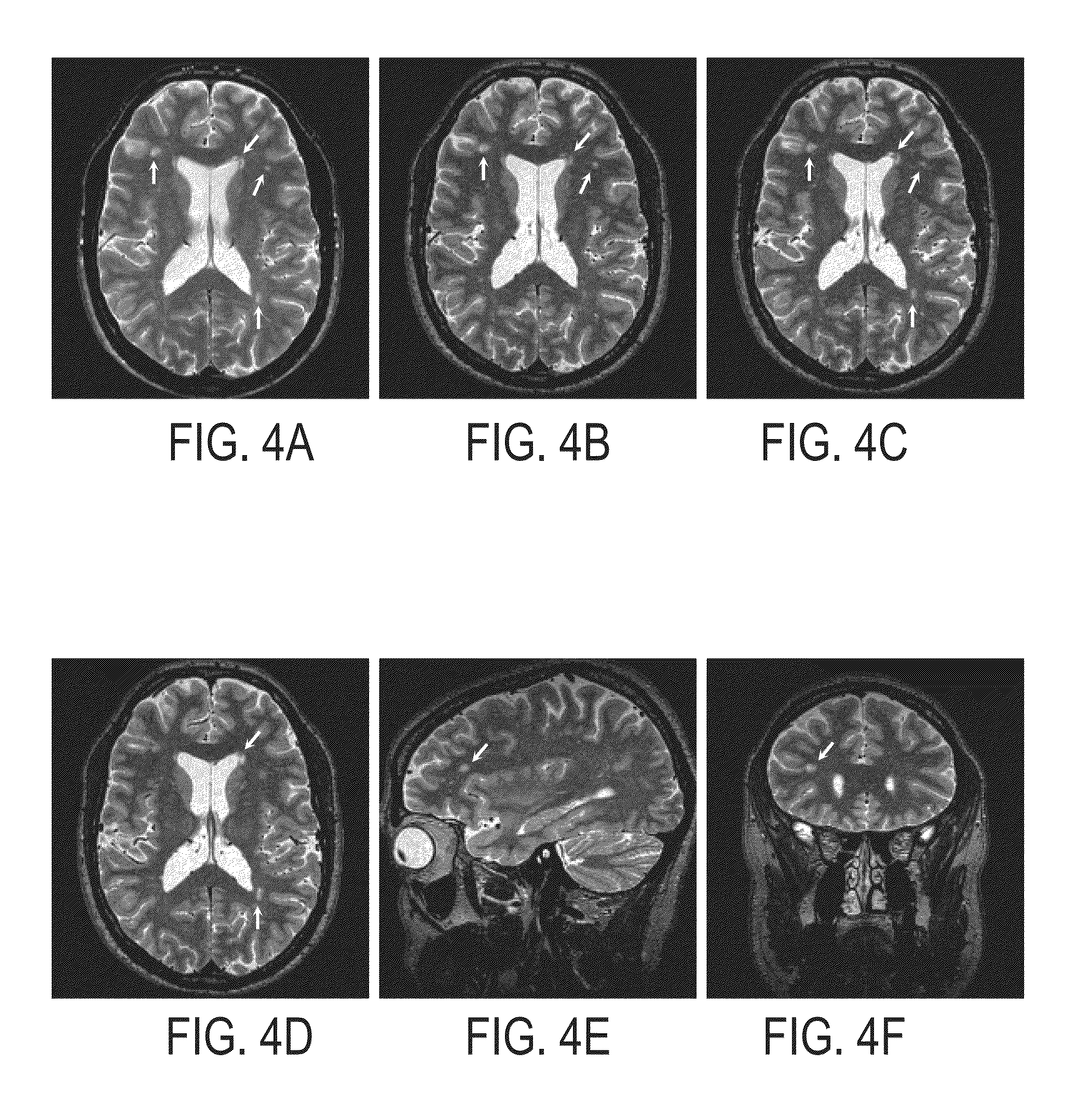
Method and apparatus for spin-echo-train MR imaging using prescribed signal evolutions
InactiveUS7164268B2Extended durationShorten Image Acquisition TimeDiagnostic recording/measuringMeasurements using NMR imaging systemsHigh fieldMagnetic resonance technique
A magnetic resonance imaging “MRI” method and apparatus for lengthening the usable echo-train duration and reducing the power deposition for imaging is provided. The method explicitly considers the t1 and t2 relaxation times for the tissues of interest, and permits the desired image contrast to be incorporated into the tissue signal evolutions corresponding to the long echo train. The method provides a means to shorten image acquisition times and / or increase spatial resolution for widely-used spin-echo train magnetic resonance techniques, and enables high-field imaging within the safety guidelines established by the Food and Drug Administration for power deposition in human MRI.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
System, method, and computer-readable medium for collection of environmental data and generation of user report for compliance with FDA requirements
InactiveUS20050222778A1Data processing applicationsGeneral water supply conservationParticulatesWater quality
A system, method, and computer-readable medium for the collecting and storing of environmental data, and generating a user report of the environmental data, the user report providing document compliance with U.S. Food and Drug Administration requirements. The collected and stored environmental data includes a wide variety of manufacturing facility parameter data, including but is not limited to, the presence of viable microbiological organisms, the presence of particulates and other environmental conditions within the facility, such as humidity, pressure, temperature, water quality (e.g., pH, conductivity, total organic content (TOC), endotoxin, coliform, and metals), and the respective amounts of different materials involved in the manufacture of the end product(s).
Owner:COMPLIANCE SOFTWARE SOLUTIONS
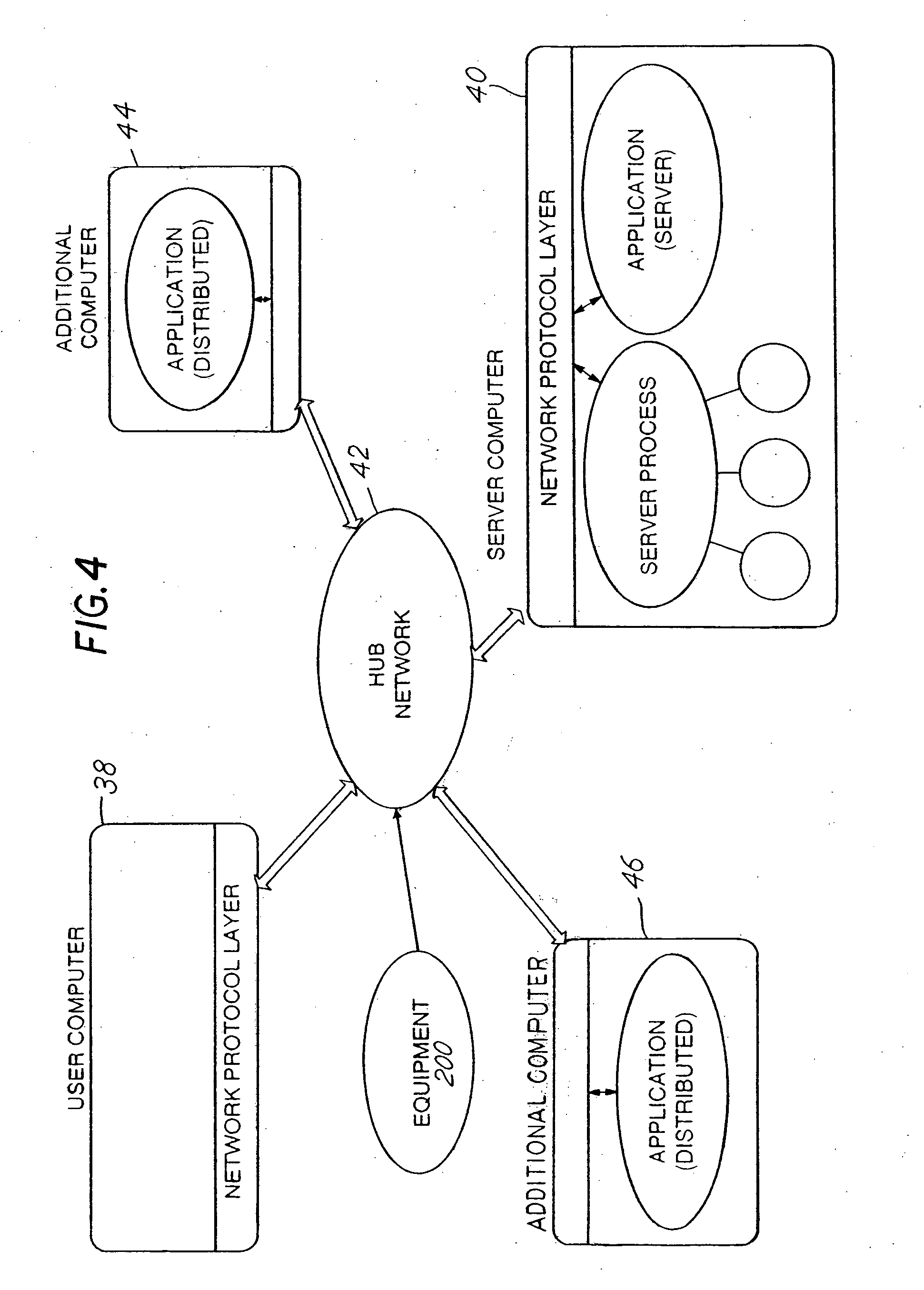
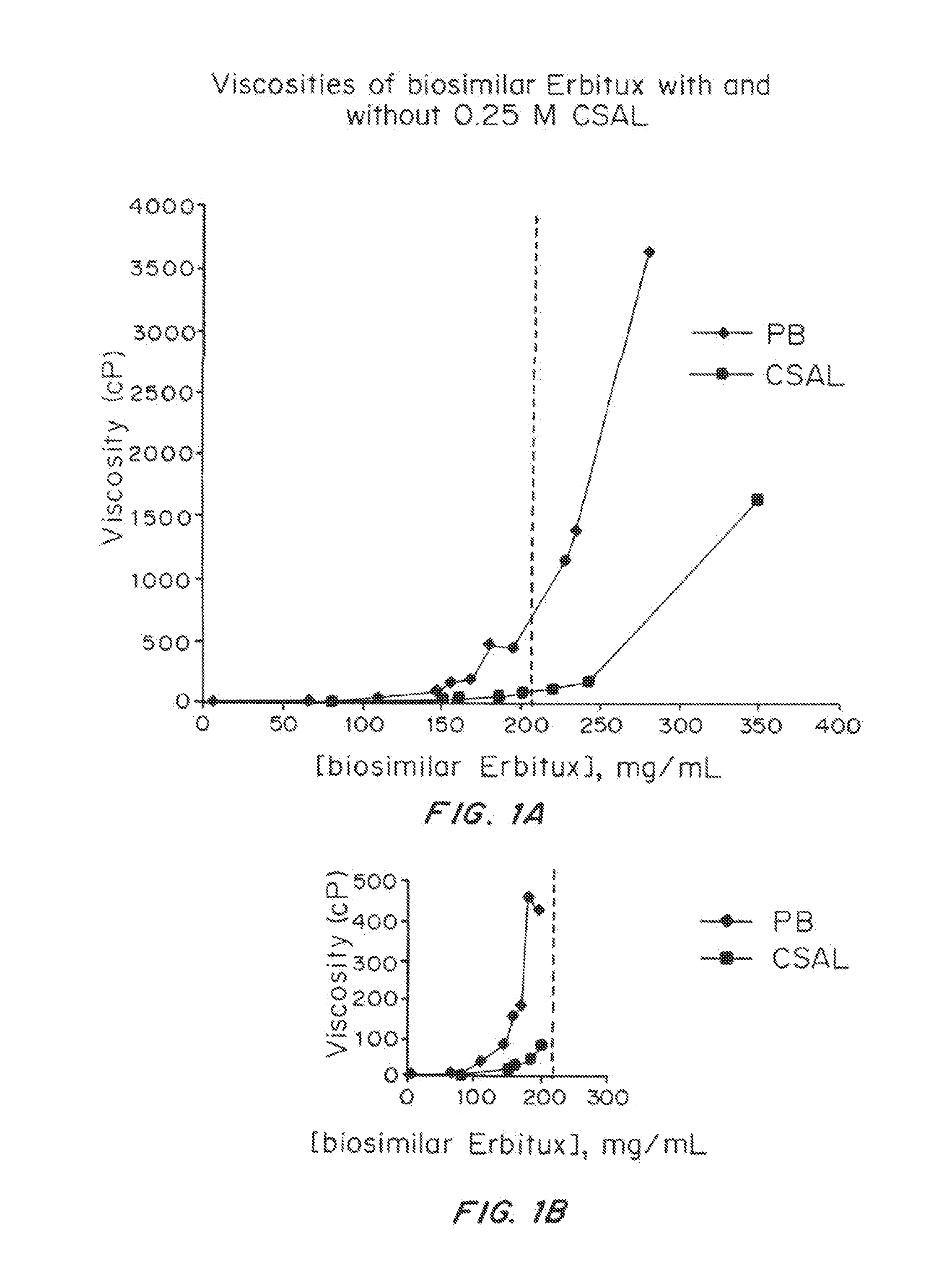
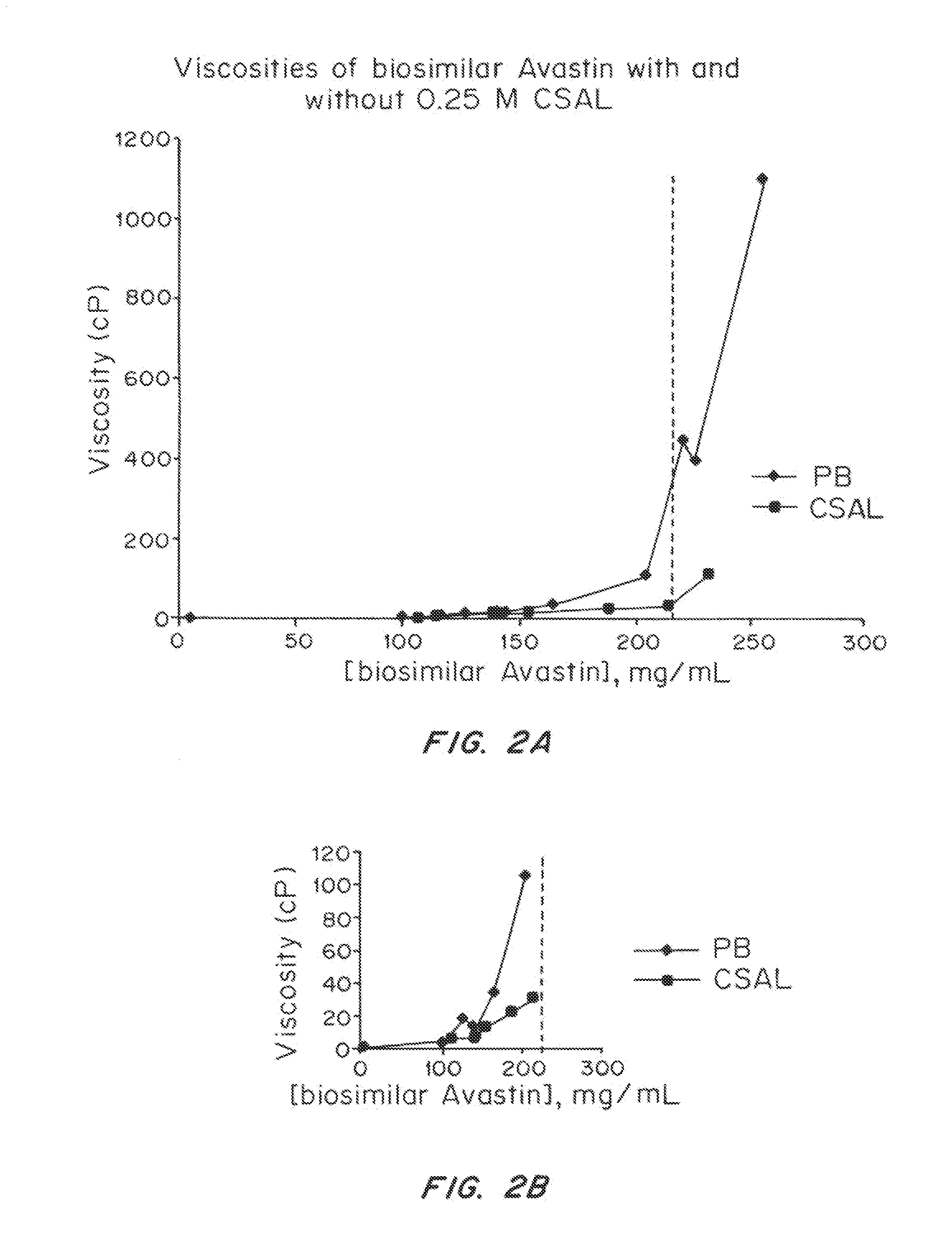
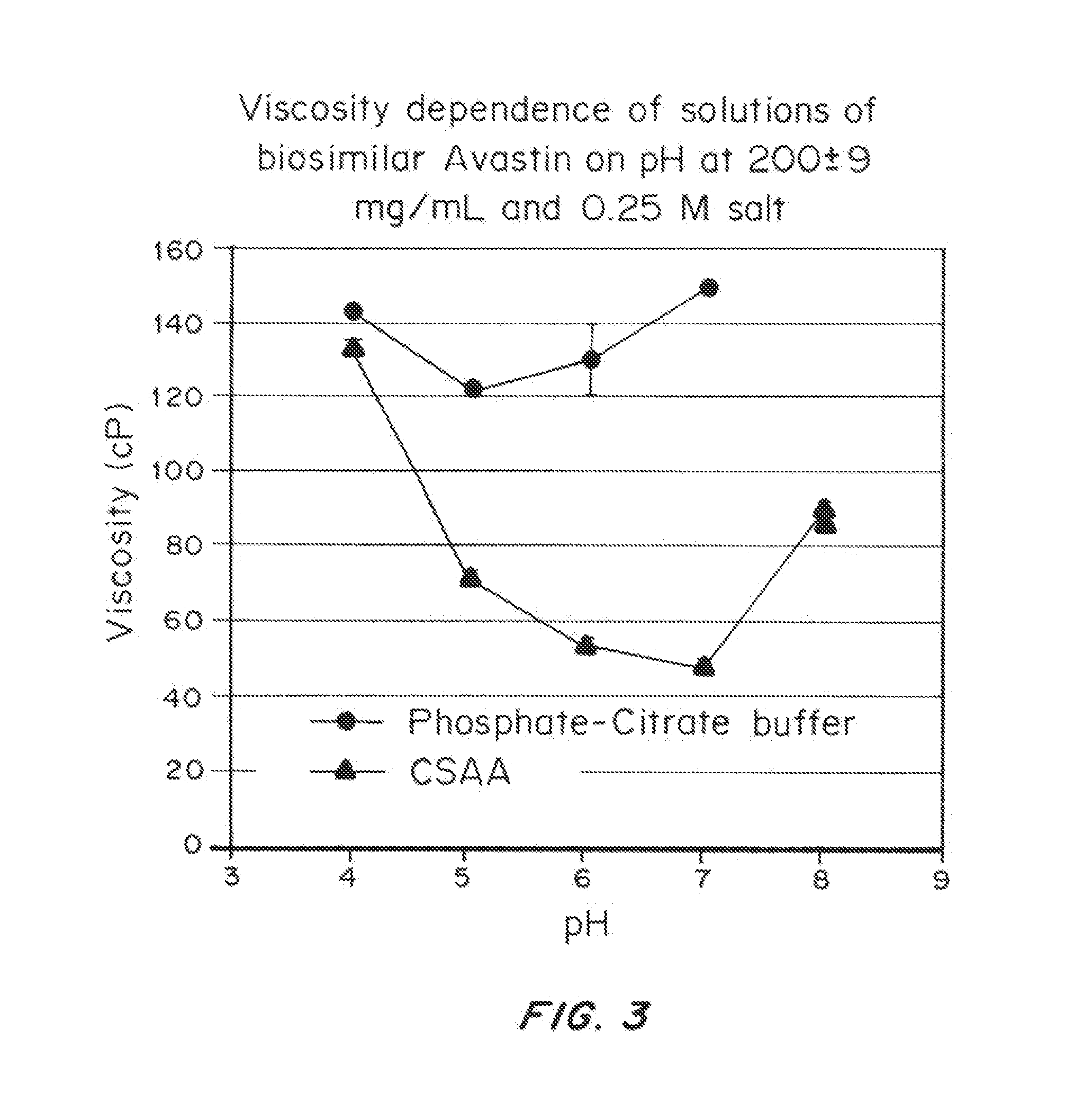
Liquid protein formulations containing viscosity-lowering agents
ActiveUS20150071925A1Facilitates and accelerates reconstitutionEasy to processNervous disorderPeptide/protein ingredientsIntramuscular injectionAdditive ingredient
Concentrated, low-viscosity, low-volume liquid pharmaceutical formulations of proteins have been developed. Such formulations can be rapidly and conveniently administered by subcutaneous or intramuscular injection, rather than by lengthy intravenous infusion. These formulations include low-molecular-weight and / or high-molecular-weight proteins, such as mAbs, and viscosity-lowering agents that are typically bulky polar organic compounds, such as many of the GRAS (US Food and Drug Administration List of compounds generally regarded as safe) and inactive injectable ingredients and FDA approved therapeutics.
Owner:EAGLE BIOLOGICS INC
Method and apparatus for spin-echo-train MR imaging using prescribed signal evolutions
InactiveUS20040051527A1Measurements using NMR imaging systemsElectric/magnetic detectionHigh fieldMagnetic resonance technique
A magnetic resonance imaging "MRI" method and apparatus for lengthening the usable echo-train duration and reducing the power deposition for imaging is provided. The method explicitly considers the t1 and t2 relaxation times for the tissues of interest, and permits the desired image contrast to be incorporated into the tissue signal evolutions corresponding to the long echo train. The method provides a means to shorten image acquisition times and / or increase spatial resolution for widely-used spin-echo train magnetic resonance techniques, and enables high-field imaging within the safety guidelines established by the Food and Drug Administration for power deposition in human MRI.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
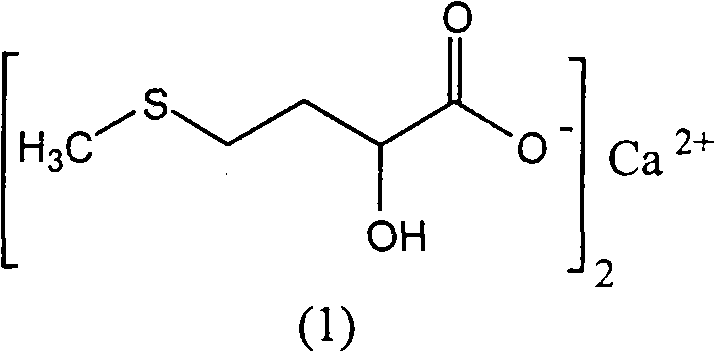
Preparation of medicinal D,L-2-hydroxy-4-methylthio calcium butyrate
The invention discloses a method for preparing a D, L-2-hydroxyl-4-methylthio butanoic calcium salt for medicinal purpose. The method comprises following steps of: a. using a D,L-2-hydroxyl-4-methylthio butanoic acid and an alcohol with a general formula of ROH as the raw materials to carry out esterification to obtain a D, L-2-hydroxyl-4-methylthio butyrate, and; b. hydrolyzing the D, L-2-hydroxyl-4-methylthio butyrate in step a and calcium oxide in a solvent to produce the D, L-2-hydroxyl-4-methylthio butanoic calcium salt. The method provided by the invention for the production of the D, L-2-hydroxyl-4-methylthio butanoic calcium salt has the advantages of short course, readily available the raw materials, low cost, easy control over the quality of the product, and more importantly, the method can prepare high-purity D, L-2-hydroxyl-4-methylthio butanoic calcium salt for medicinal purpose, thereby satisfying the requirements of State Food and Drug Administration Bureau and Good Manufacturing Practice (GMP) for drug production, and facilitating the preparation of pharmaceutical preparations.
Owner:NANJING LIFENERGY R & D +1
Cosmetics Applicator System and Method
ActiveUS20150366327A1Additive manufacturing apparatusWood working apparatusPowder mixtureAdditive ingredient
A manufacturing process for three-dimensionally printing cosmetic products, and / or custom facial mask applicators using makeup powder as a fabrication medium. The process may use the versatile nature of makeup powder ingredients to stretch the usability of a makeup powder mixture across many platforms of facial cosmetics. The process may create custom, digital files that can be manufactured with high resolution, high complexity, and with a full spectrum of colors using Food and Drug Administration (FDA) compliant cosmetic grade dye. In particular, full-face makeup mask applicators that are based on an exact three-dimensional scan of the user's face.
Owner:LAHOOD SR RICHARD JOSEPH +2
Quantitative detection kit for neuronspecific enolase (NSE) and preparation method and application thereof
InactiveCN102914650AImprove stabilityEasy to operate manuallyMaterial analysisBiotin-streptavidin complexAntigen
The invention relates to a quantitative detection kit for NSE and a preparation method and application of the quantitative detection kit. The kit comprises a calibrator, a magnetic separation reagent, an enzyme reactant, a stable reinforcing agent and a chemiluminiscent substrate, wherein the calibrator is obtained by treating NSE antigen through a reducing agent solution and diluting the NSE antigen to a buffer solution containing a nonionic surfactant; the magnetic separation reagent is obtained by immunofixation of a biotinylation antibody and streptavidin magnetic particles; the enzyme reactant comprises a NSE tracing antibody marked by alkaline phosphatase; and the stable reinforcing agent comprises a multicomponent immune compound interfered by an anti-heterophilic antibody. The invention further relates to a preparation method of the kit and a method of applying the kit to quantitatively detect a tumor marker NSE. The kit is reliable in performance, high in flexibility, and wide in linear range, and matched up with an automatic instrument for use. At present, the kit has already obtained a third registration certificate of a diagnostic reagent in SFDA (State Food and Drug Administration).
Owner:BEIJING DIACHA BIO ENG
Stem cell freezing and storing medium and preparation method and freezing and storing method thereof
The invention discloses a stem cell freezing and storing medium and a preparation method and freezing and storing method thereof. The stem cell freezing and storing medium comprises, by weight, 3-10 parts of dimethyl sulfoxide, 2-7 parts of human serum albumin, 0.5-3 parts of mycose, 0.2-2 parts of dextran 40 and 2-6 parts of hetastarch. Cells can be frozen for a long time by the stem cell freezing and storing medium, freezing damage of the cells can be remarkably reduced, the resuscitated cells have a high degree of survival rate and adherent property, and freezing and storing effect of the cells is improved; meanwhile, the compositions of the stem cell freezing and storing medium are clear and are medical compendial injection-grade excipients without containing serum, the risk of contamination and allergen introduced by the use of heterogeneous serum is effectively prevented, the content of DMSO (dimethylsulfoxide) is low, the negative effect of the DMSO on the cells is lowered, highsecurity and good stability are achieved, requirements of CFDA (China food and drug administration) and FDA (food and drug administration) are met, and the stem cell freezing and storing medium can be directly used in human infusion and is suitable for clinical research and treatment.
Owner:广州赛隽生物科技有限公司
High solid solvent type epoxy varnish anti-corrosion paint
InactiveCN101386767AHigh quality solidsReduce contentAnti-corrosive paintsEpoxy resin coatingsEpoxyLacquer
The invention discloses high-solid solvent type antiseptic epoxy resin paint, which comprises a main agent and a curing agent, wherein the ratio of the main agent to the curing agent is between 20 to 1 and 5 (weight). Compared with the prior art, the antiseptic epoxy resin paint has high mass solid content and lower content of organic volatile substances, can adapt to flow coating of the prior container, can be thickly coated to the specified film thickness at one time, has good anticorrosion property and good physical and mechanical properties such as shock resistance, wear resistance, a paint film has no toxicity and can contact foodstuff such as grain and other food materials, and the antiseptic epoxy resin paint passes the performance detection of Food and Drug Administration (USA) and achieves the corresponding authorization certificate.
Owner:中涂化工(上海)有限公司
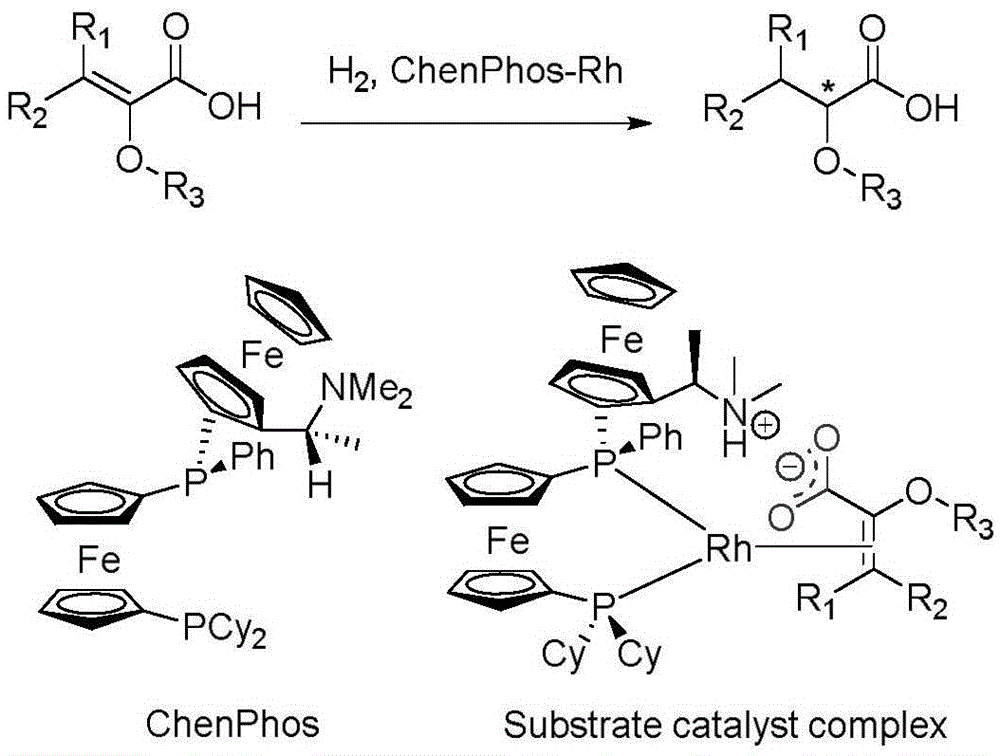
Asymmetric hydrogenation method of alpha-oxo-alpha, beta-unsaturated carboxylic acid
InactiveCN105481622AHigh catalytic activityHigh enantioselectivityOrganic reductionOrganic compound preparationEnkephalinase inhibitorAsymmetric hydrogenation
The invention relates to an asymmetric hydrogenation method of alpha-oxo-alpha, beta-unsaturated carboxylic acid. A metal complex containing ChenPhos chiral ligand is a catalyst high in conversion efficiency, and particularly, the catalyst can be used for synthesizing a core framework in enkephalinase inhibitor Sacubitril through asymmetric hydrogenation. The inhibitor is one of components of medicine LCZ 696 approved by American Food and Drug Administration. The asymmetric hydrogenation method of the alpha-oxo-alpha, beta-unsaturated carboxylic acid is efficient, and the application range of substrate is wide.
Owner:WUHAN CATALYS TECH CO LTD
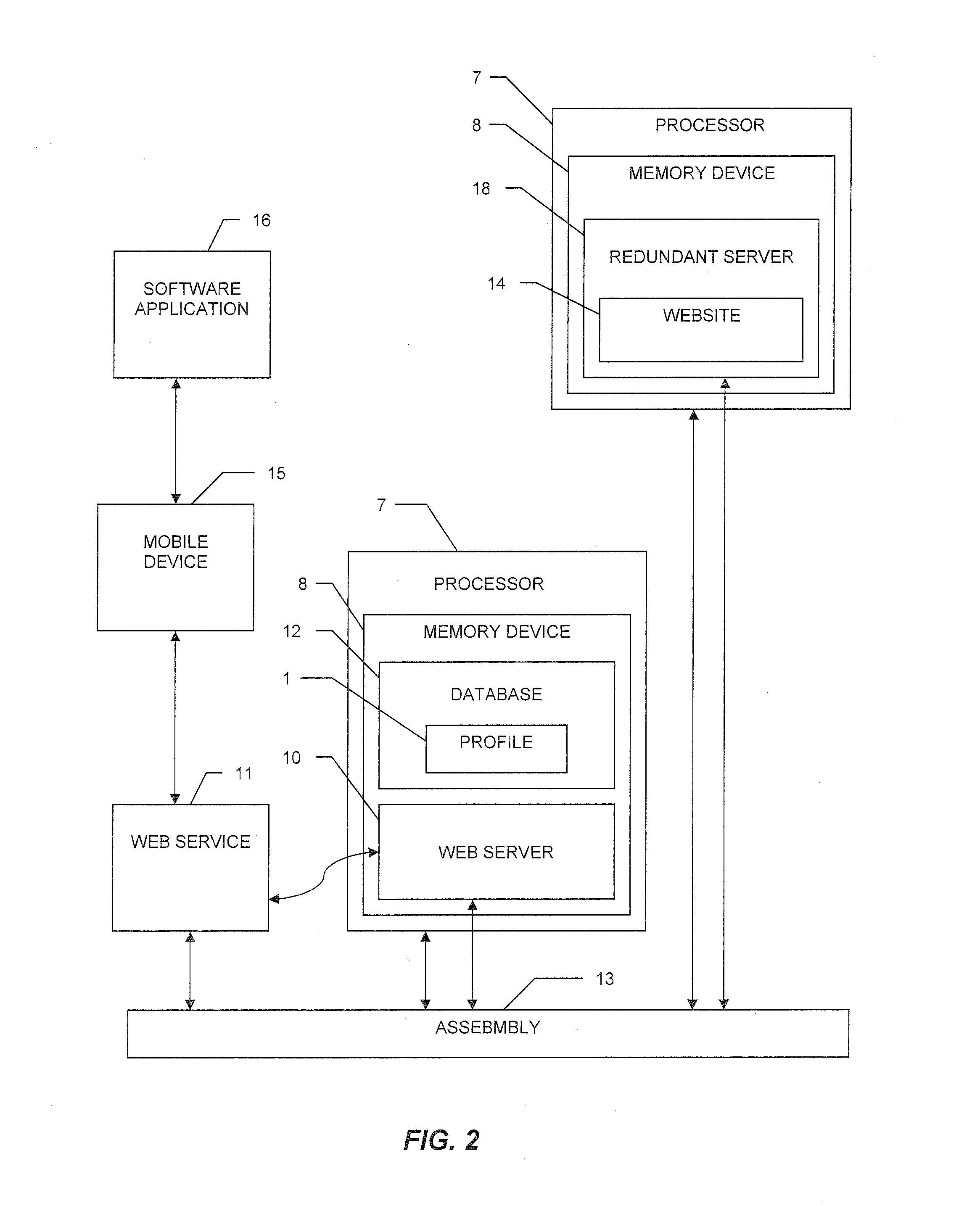
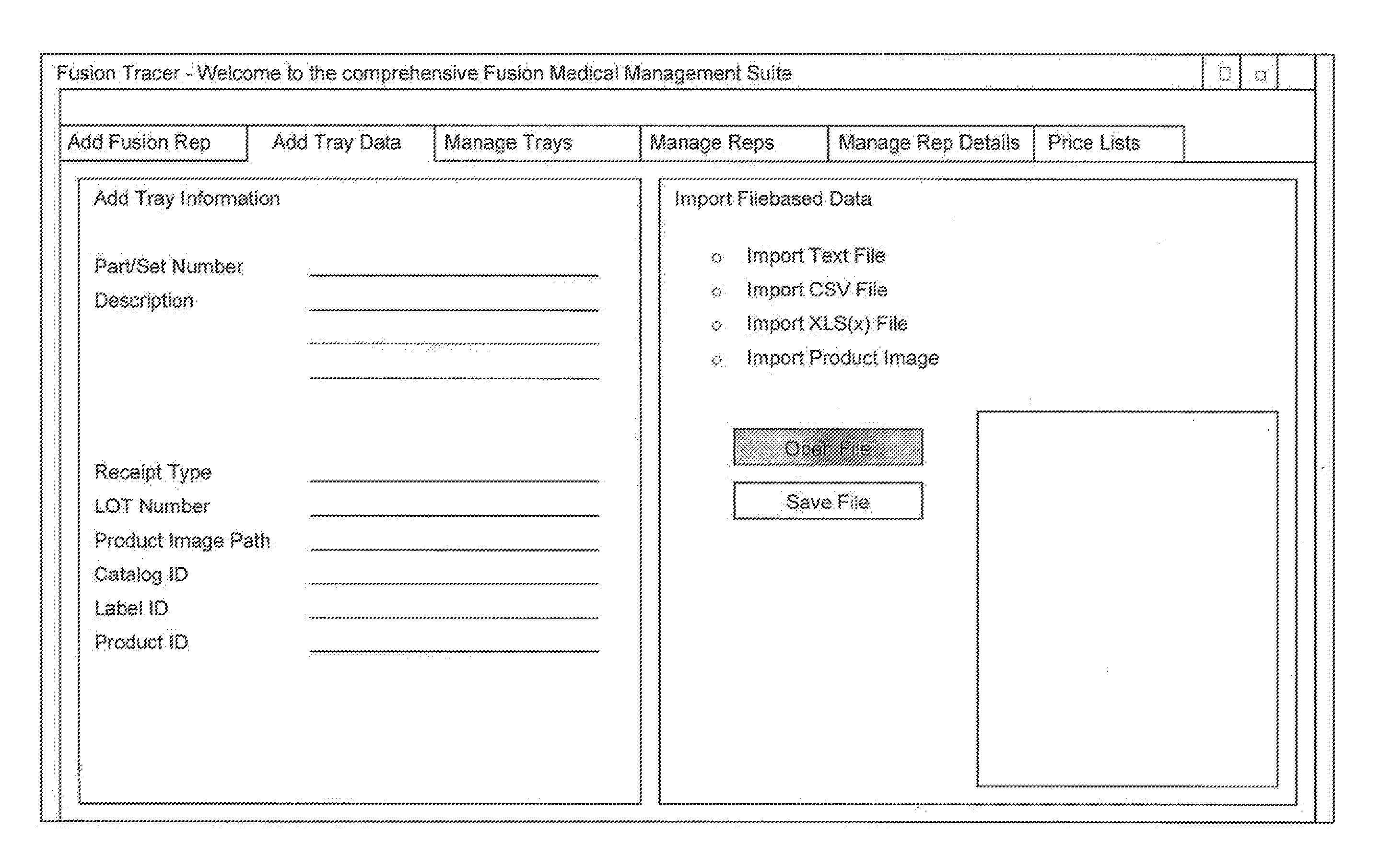
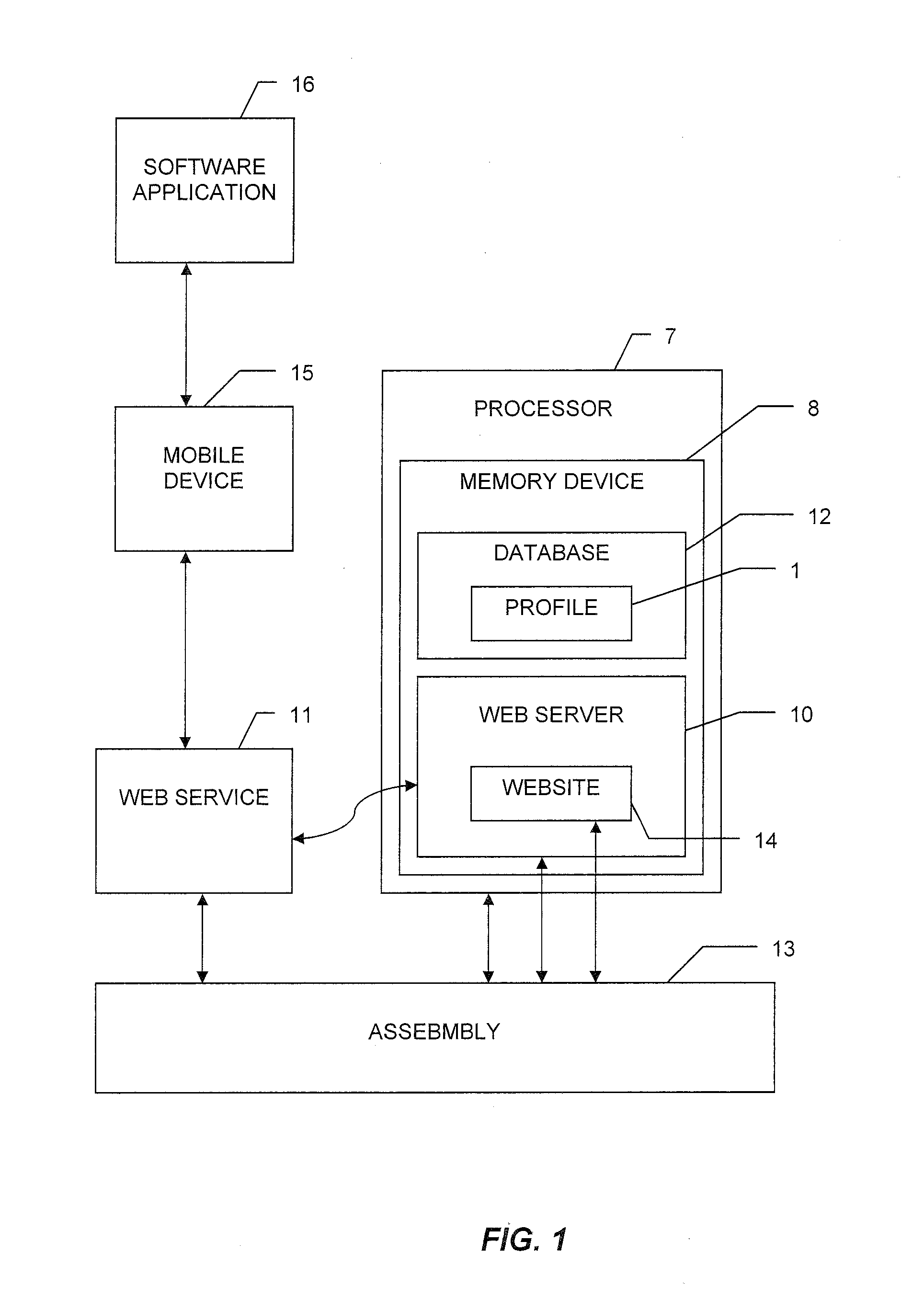
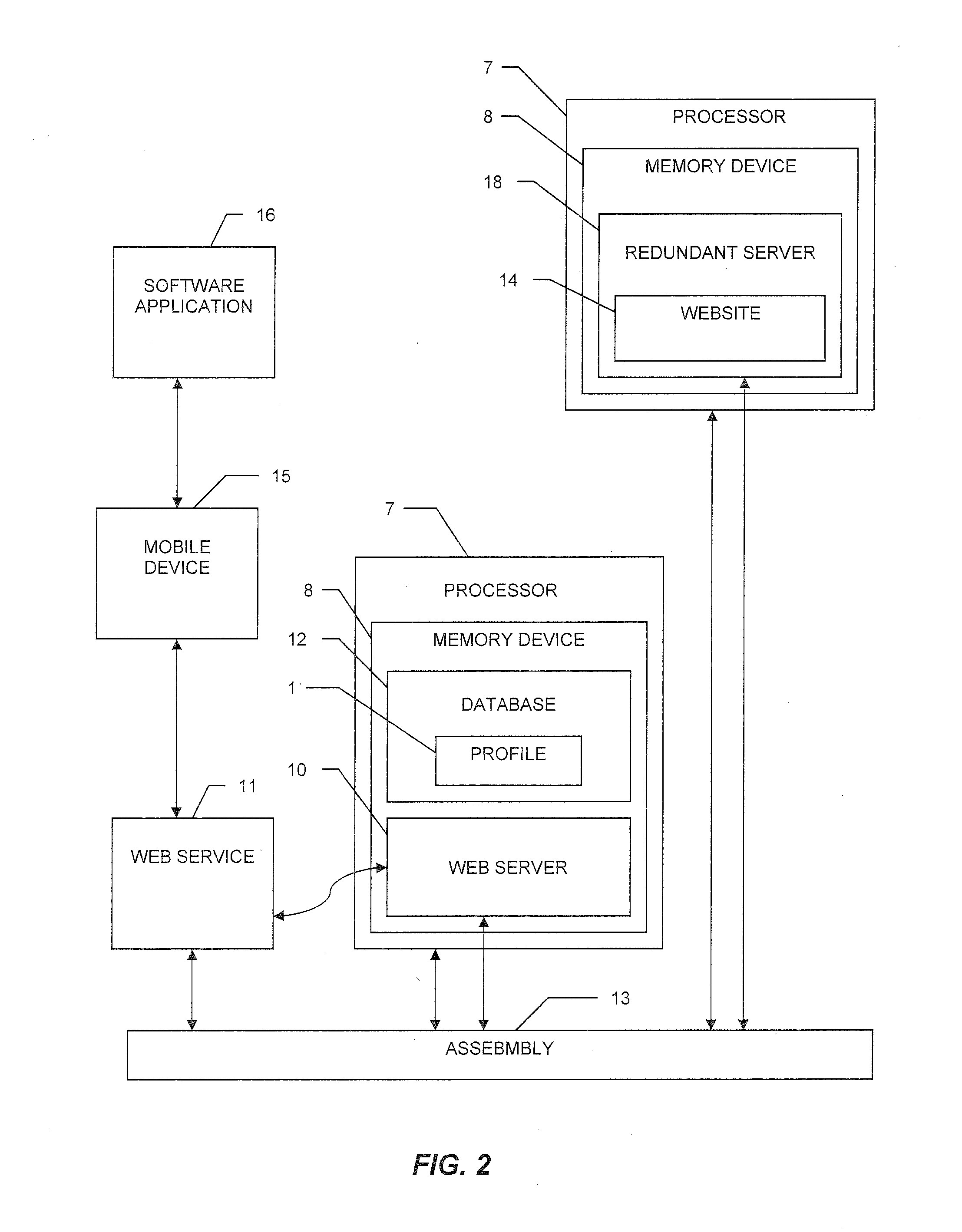
System and method for tracking and managing medical device inventory
A system and method of managing the logistics of trays and lots of medical inventory, from the manufacturer's initial release until final discharge by the end user healthcare provider. The system is driven by one or more software applications running on a central, secure server accessible by remote handheld telecommunication devices. The system comprises software modules providing functionality for the management of specific medical cases, the billing process, warehouse reconciliation of used inventory, a loaner program for borrowing otherwise unavailable inventory, and many other processes. Additional features of the system include a geographic map, an inventory list of medical devices, an imaging assembly to read barcodes or other optic identifiers, an Food and Drug Administration news assembly incorporating a live RSS news feed for releases about product defects or recalls.
Owner:MEDICAL TRACKING SOLUTIONS
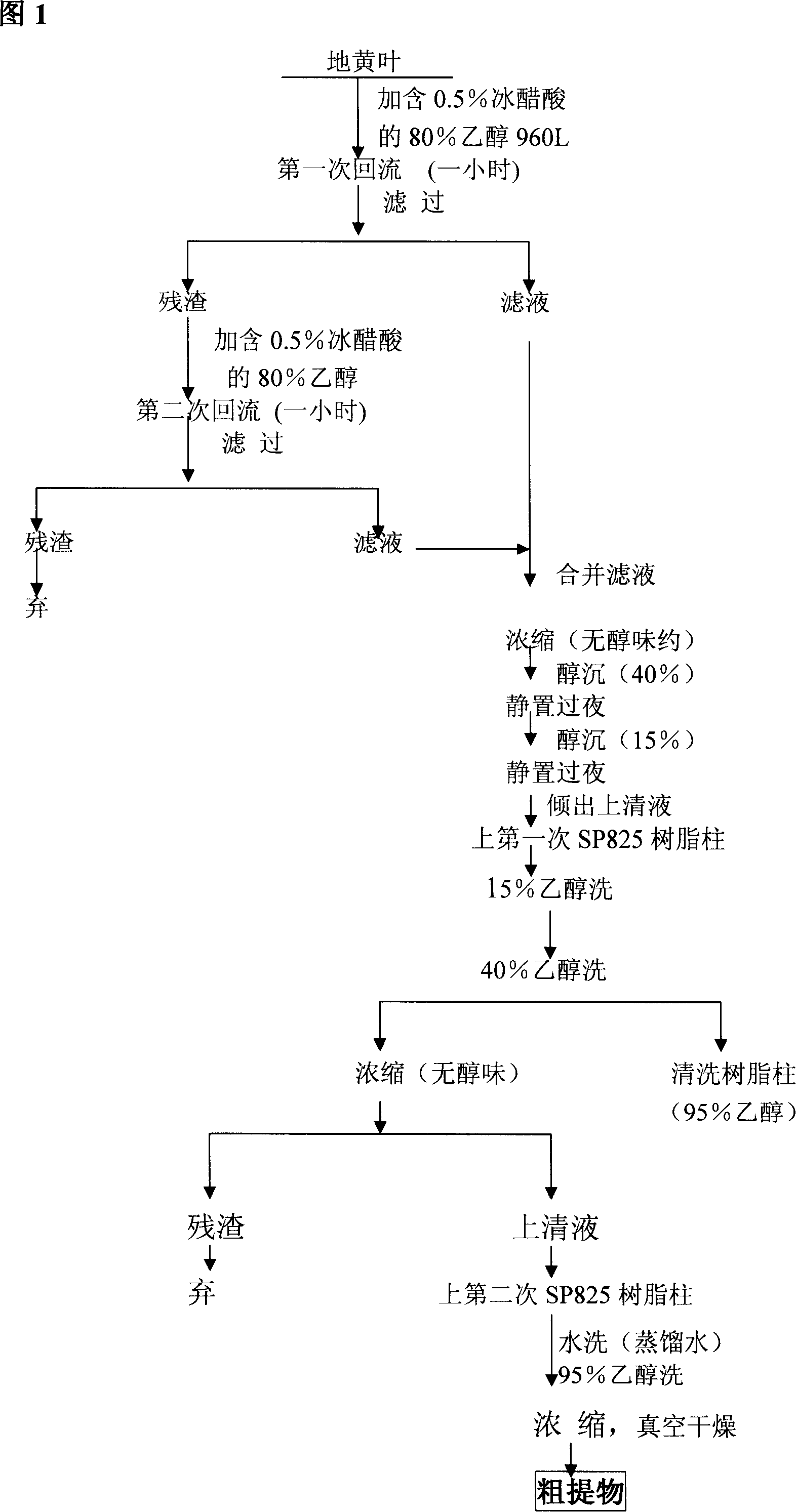
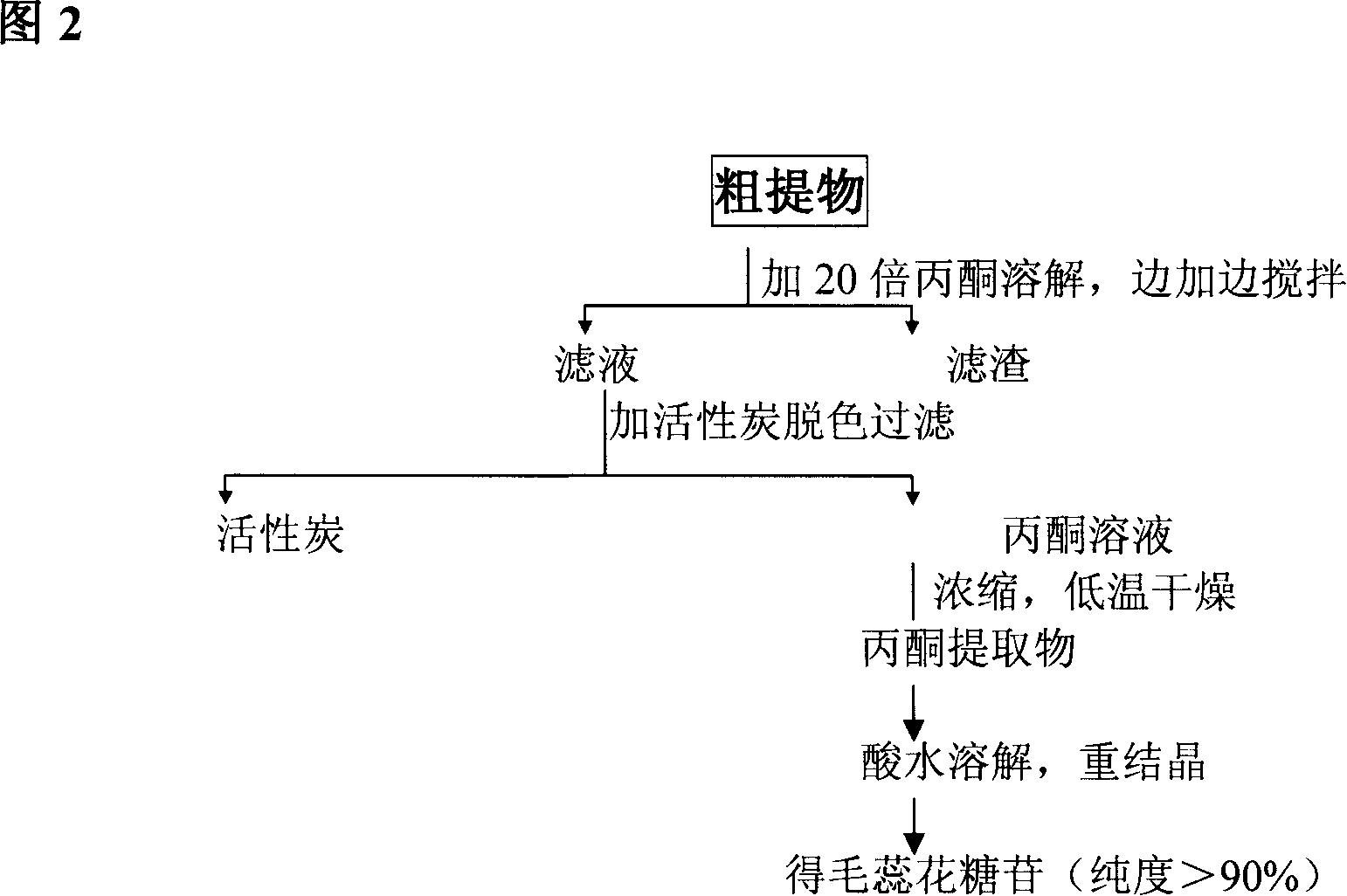
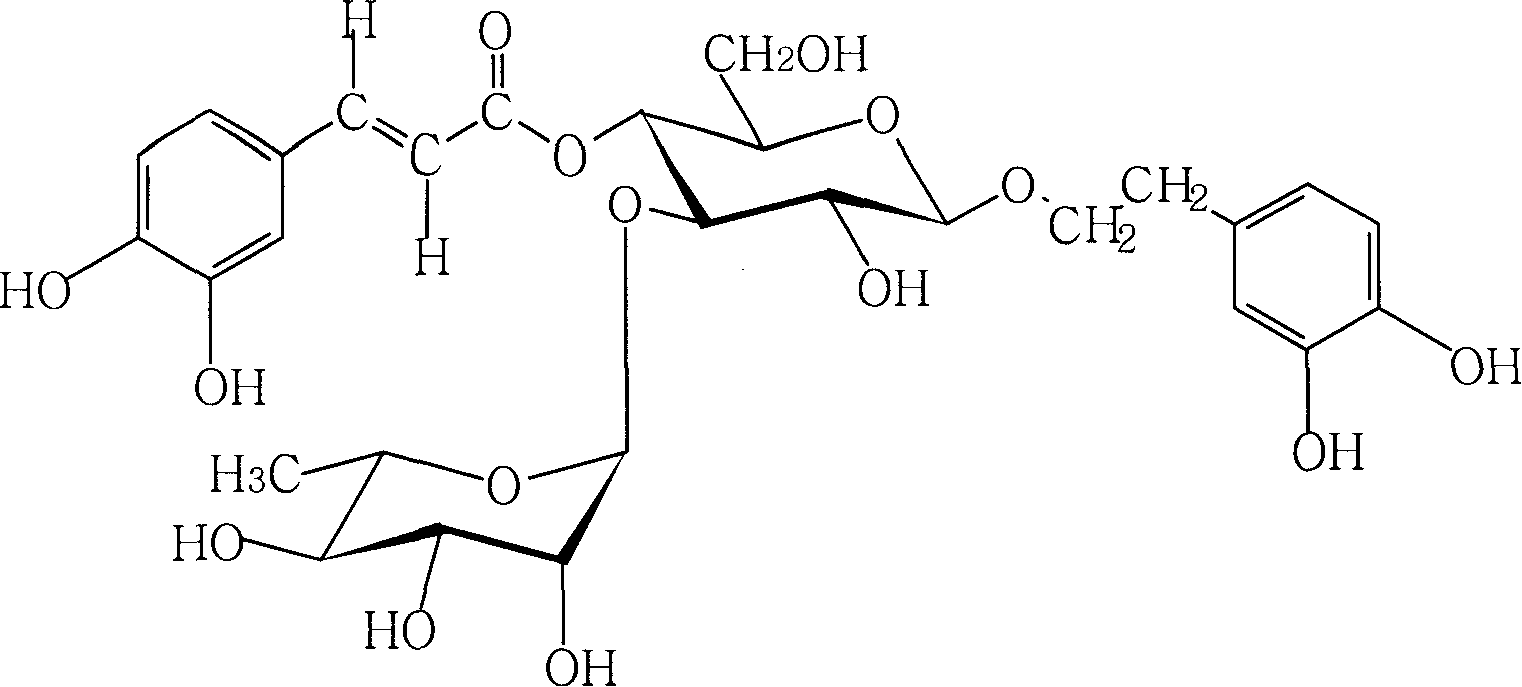
Technique for preparing verbascoside with function of curing chronic glomerulonephritis in glutinous rehmannia leaf
InactiveCN101121740ANon-toxic and safeConducive to extensive clinical applicationEsterified saccharide compoundsSugar derivativesAcute toxicity testingDigitalis
The invention belongs to the technological field of the Chinese medicine, particularly relating to an extraction and separation processes of the active ingredients with the active function of treating the chronic glomerulonephritis. The mullein indican is the active compound researched and developed for many years in our research institute; the mullein indican is extracted, separated and refined from the digitalis leaf and is an active compound with the function of treating the chronic glomerulonephritis. The invention is the research result of many years held and developed by the Chinese Medicine Research Department of the Chinese Medicine Research Institute of China and is supported by the special fund of the Central Institute of the National Science and Technology Department; now the compound has been approved by the State Food and Drug Administration Bureau as the novel drug and the mechanism research of the treatment of the chronic nephritis has been completed; the pre-clinical research work includes the main pharmacodynamic experiment, the production technology experiment (including the industrialized extraction and separation processes), the quality control standard, the experiment of the affecting factors of the quality, the experiment of the long-term stability, the acute toxicity of the rat, the experiment of the long-term toxicity, the teratogenic and mutagenic experiment, the general pharmacological experiment, the pharmacokinetics experiment, and so on. The digitalis leaf is mainly produced in the Huaiqing area of the Henan province, and is also grown wild and cultivated in the Shaanxi, Hebei and other places of China.
Owner:INST OF CHINESE MATERIA MEDICA CHINA ACAD OF CHINESE MEDICAL SCI
Multi-purpose hand disinfectant
InactiveUS20120208894A1Rule out the possibilityBiocideCosmetic preparationsCare personnelDrug product
A multi-purpose hand disinfectant and in particular, a hand disinfectant which meets the FDA (Food and Drug Administration) requirements for use both as an antiseptic handwash or health-care personnel handwash drug product and as a surgical hand scrub drug product is described herein.
Owner:BODE CHEMIE
Injectable active bone repair material
The invention relates to an injectable active bone repair material. The material has good liquidity, can reach a bone defect part through injection and quickly solidify the defect part, and can obviously promote the healing of bone trauma along with gradual release of rear bone growth factors so as to shorten the healing period. Main components of the injectable active bone repair material consist of multiple calcium salts such as phosphoric acid calcium salt, calcium sulfate, calcium carbonate and the like, stowage factor microspheres and solidifying conditioner. The injectable active bone repair material has good injectability and collapse resistance. The solidifying time is 15 to 30 minutes, the temperature rise is not over 45 DEG C during solidifying, and the material does not cause adverse effect on tissues of an implanted part. After solidifying for half an hour, the material reaches the strength of spongy bone. All the components of the material are medicinal raw materials approved by the United States Food and Drug Administration, and can meet the safety of clinical use.
Owner:SHENZHEN LANDO BIOMATERIALS
Comprehensive application method of salicornia bigelovii
The invention relates to a comprehensive application of salicornia bigelovii. The salicornia bigelovii comprises fresh vegetables and tender stems of plants; active substances are extracted after the salicornia bigelovii is pulped or dried and crushed; the active substances comprise components such as polysaccharide, ferulic acid, general flavone and the like; the residues of salicornia bigelovii after the active substances are extracted are inoculated with food-security microorganism aureobasidium pullulans for fermentation so as to produce fermented products rich in single-cell protein (SCP) and viscous pullulan which has been allowed by the FDA (food and drug administration) in Japan to be used as a food additive, the pullulan produced by self fermentation is utilized as a binding agent, infrared and microwave technologies are adopted to dewater, dry and form fermentation mud, and the fermented products are prepared into instant vegetable paper with rich nutrition, so that the variety of leisure foods is richened and the high-additional-value comprehensive utilization system of the salicornia bigelovii is constructed.
Owner:YANCHENG INST OF TECH
High-content sodium alginate nanofiber membrane and electrostatic spinning manufacturing method thereof
InactiveCN105586716AHigh porosityFine fiber diameterFilament/thread formingNon-woven fabricsPorosityFiber
The invention provides a high-content sodium alginate nanofiber membrane and an electrostatic spinning manufacturing method thereof. The material is formed by sodium alginate or sodium alginate and polyvinyl alcohol, wherein the absolute content of the sodium alginate is 42.5 wt% to 100 wt%, and average diameter of the fiber is 100 nm to 150 nm. A solvent used in a preparation process of an electrostatic spinning solution is deionized water, and a surfactant is TX-100 which is certificated by US Food and Drug Administration. The manufacturing method is simple and feasible, and the high-content sodium alginate nanofiber membrane is not added with any hazardous substance. The manufactured sodium alginate nanofiber membrane is characterized by high content of sodium alginate, thin average diameter, and high porosity. The sodium alginate nanofiber membrane can be applied in the fields of wound dressing and tissue engineering (for example, a support material), etc.
Owner:TIANJIN TEDA JINSHAN PACKING MFR
Ganoderma lucidum black tea processing technology
InactiveCN104286277AShape effectThe shape is tight and well-proportionedPre-extraction tea treatmentAdditive ingredientBlack tea
The invention belongs to the field of tea processing and mainly provides ganoderma lucidum black tea and a processing technology thereof. The problems in the prior art that ganoderma lucidum and tea activated ingredients cannot be effectively combined and absorbed by a human body, the taste and the color are poor, the soup is turbid, and the product quality is difficult to control when spore powder is used (China Food and Drug Administration stipulates that ganoderma lucidum spore powder cannot be used as a common food raw material). The raw materials comprise black tea fresh leaves and ganoderma lucidum granules. The processing technology comprises the procedures: cutting ganoderma lucidum into granules, putting on a steamer and steaming, selecting the black tea fresh leaves, withering the black tea fresh leaves, simultaneously rolling the ganoderma lucidum and the tea, fermenting, drying, extracting fragrance and examining quality. As the ganoderma lucidum black tea is prepared through the mixed fermentation of the ganoderma lucidum and the black tea, the interior quality of the tea is enriched, and the health-care function of the tea is improved. The tea can perform the functions of protecting the liver, nourishing the stomach, calming the nerves, pacifying the mind, brightening the eyes, reducing blood pressure, blood fat and blood sugar, improving body immunity, delaying aging and the like. Besides, the soup is clear, the taste is great, and the black tea variety in China is enriched.
Owner:詹聪 +1
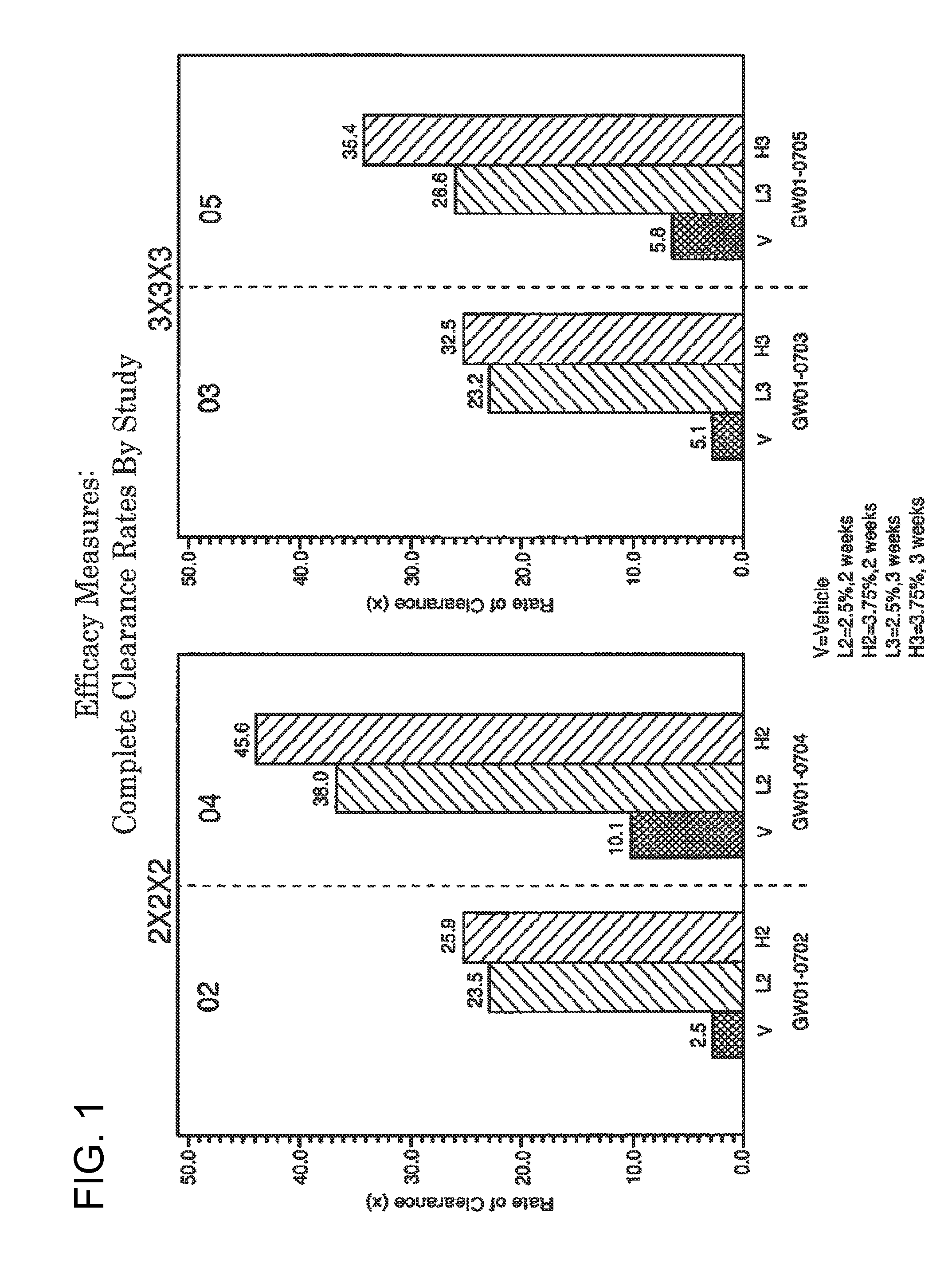
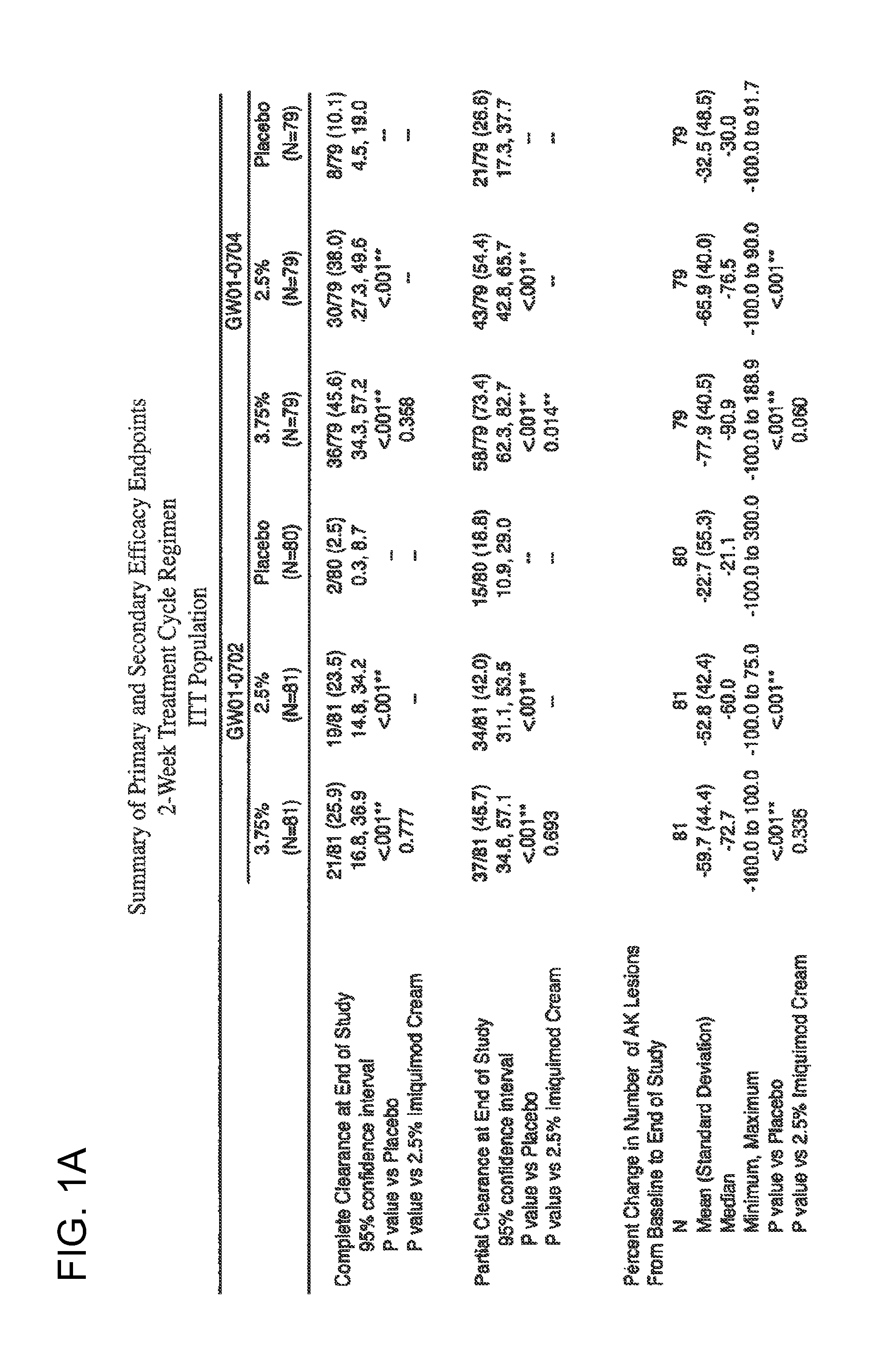
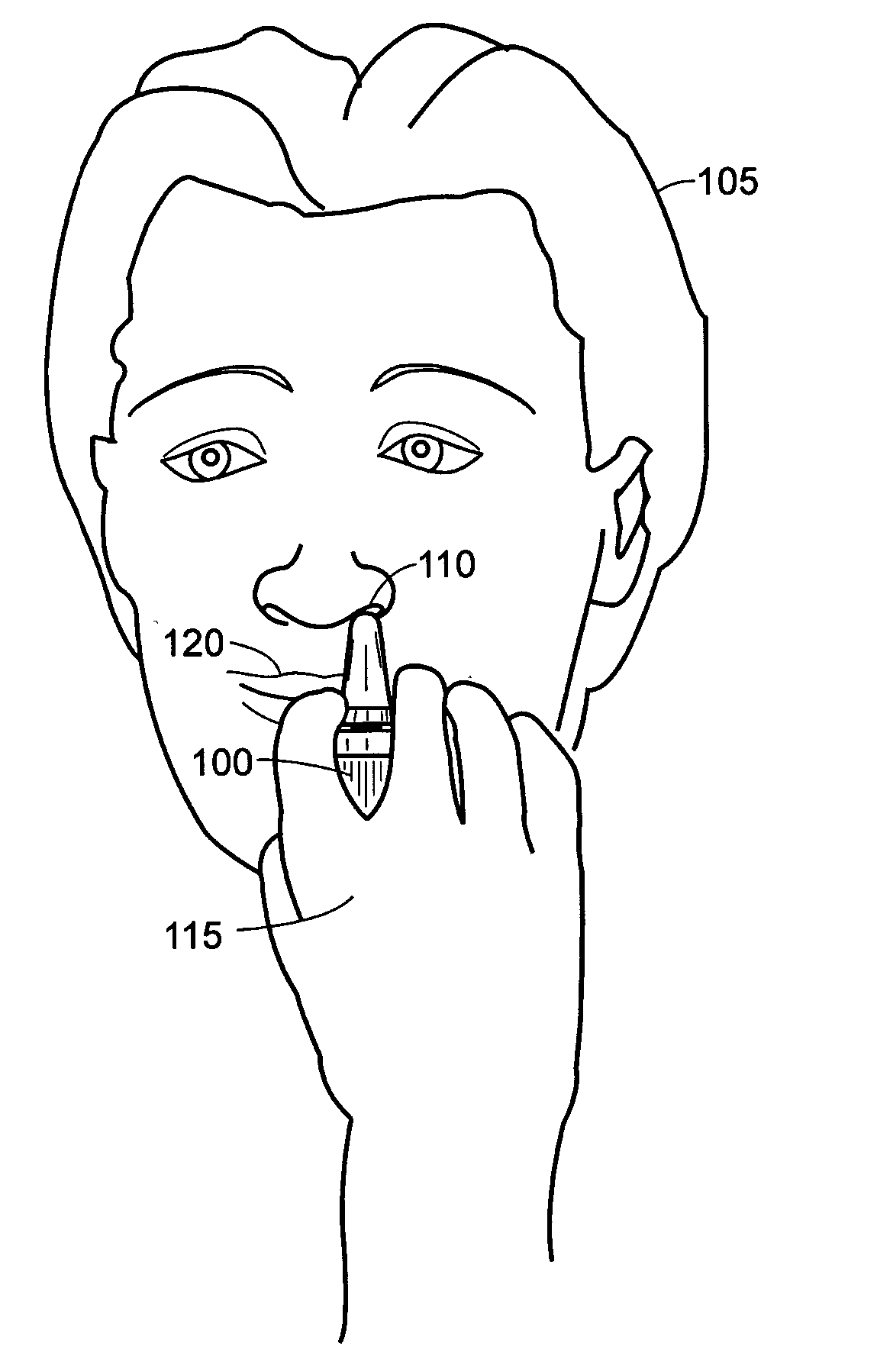
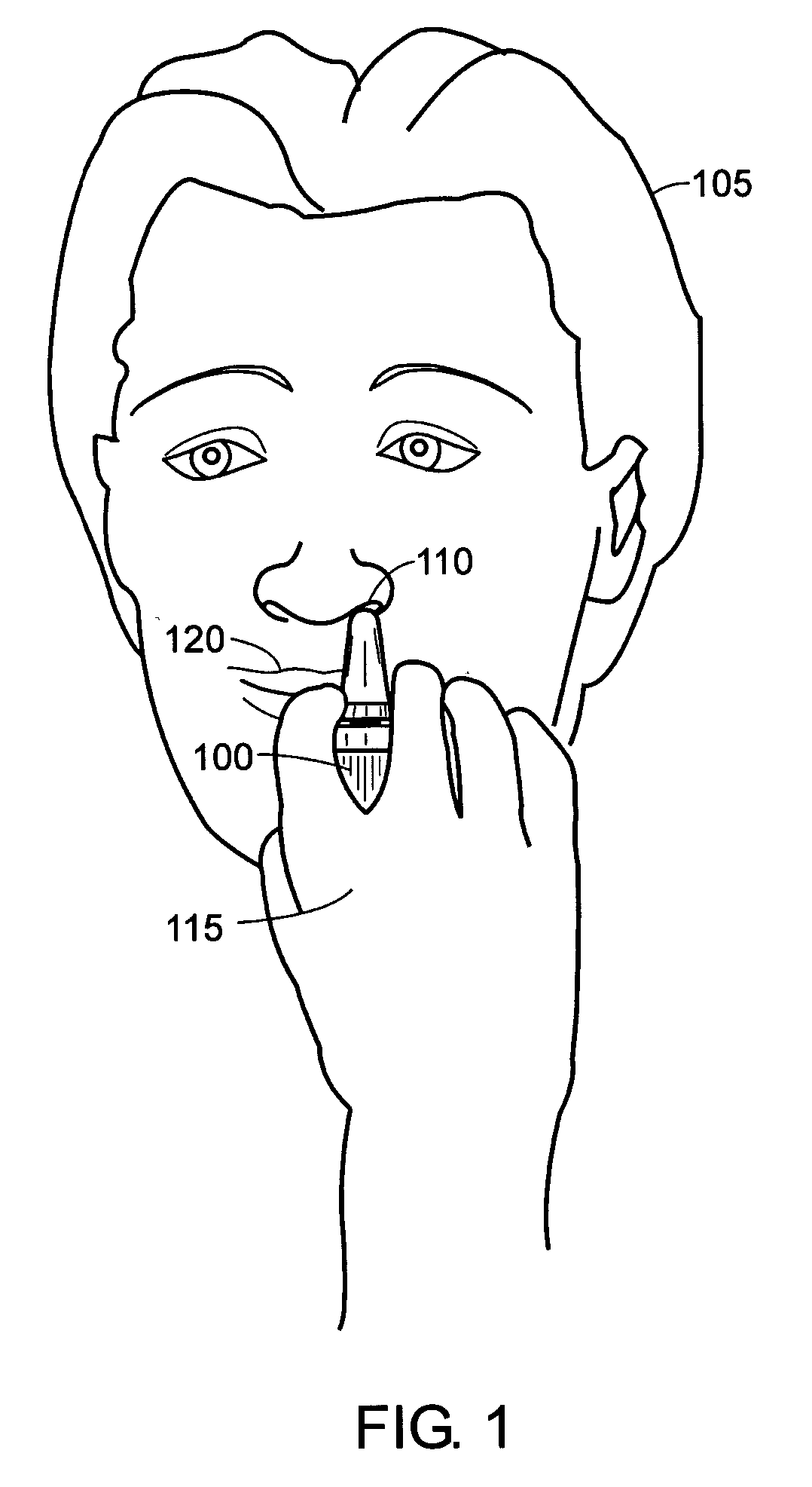
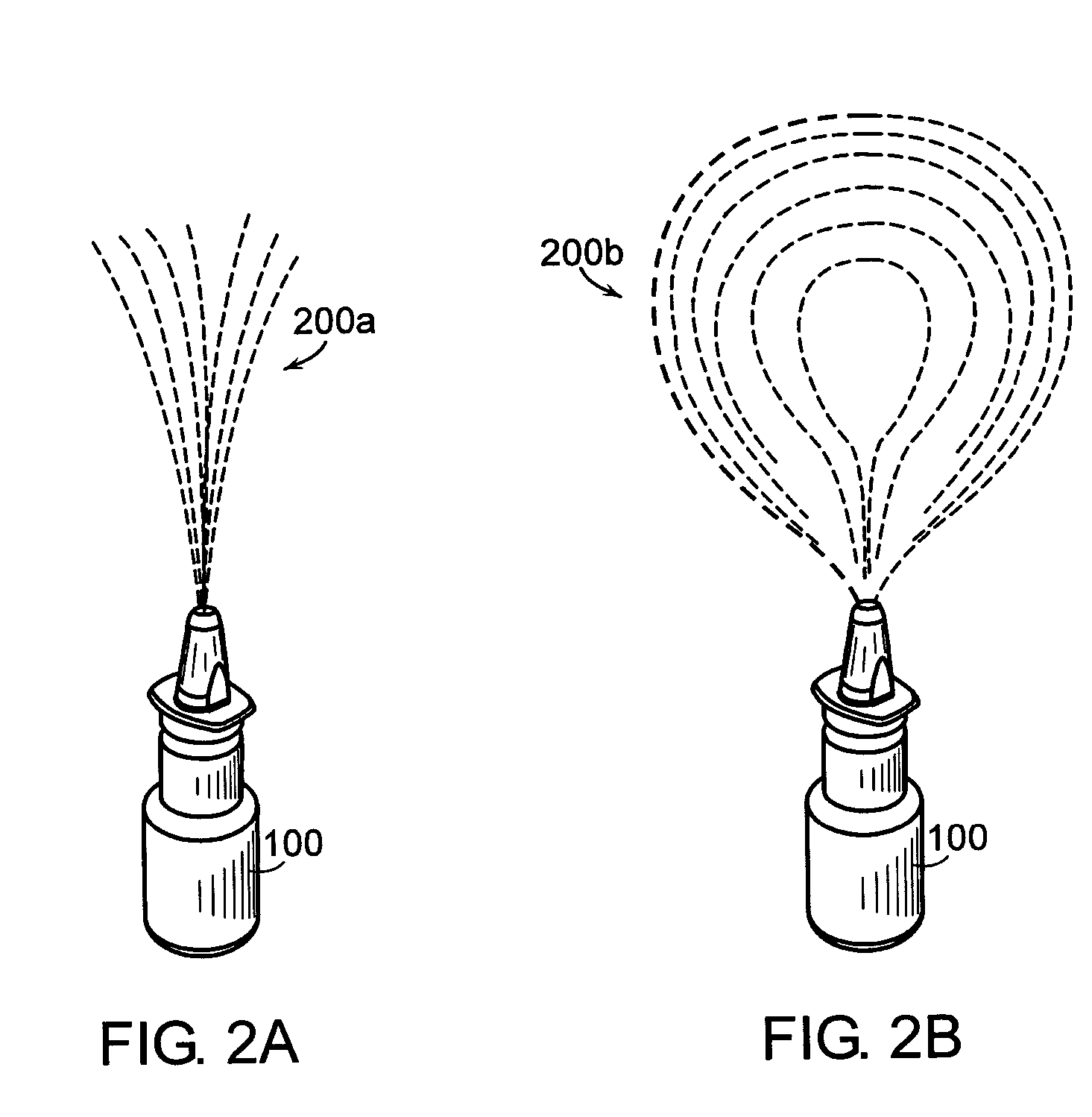
2 x 2 x 2 WEEK TREATMENT REGIMEN FOR TREATING ACTINIC KERATOSIS WITH PHARMACEUTICAL COMPOSITIONS FORMULATED WITH 2.5% IMIQUIMOD
ActiveUS20110257216A1Improved imiquimodReduced strengthBiocideOintment deliveryActinic keratosesDosing regimen
Pharmaceutical formulations and methods for the topical or transdermal delivery of 1-isobutyl-1H-imidazo[4,5-c]-quinolin-4-amine or 1-(2-methylpropyl)-1H-imidazo[4,5-c]quinolin-4-amine, i.e., imiquimod, to treat actinic keratosis with short durations of therapy, than currently prescribed for the commercially available Aldara® 5% imiquimod cream, as now approved by the U.S. Food & Drug Administration (“FDA”), are disclosed and described. More specifically, lower dosage strength imiquimod formulations to deliver an efficacious dose of imiquimod for treating actinic keratosis with an acceptable safety profile and dosing regimens that are short and more convenient for patient use than the dosing regimen currently approved by the U.S. Food & Drug Administration (“FDA”) for Aldara® 5% imiquimod cream to treat actinic keratosis are also disclosed and described.
Owner:MEDICIS PHARMA CORP
Method of servicing companies associated with a spray device operating under guidelines of a regulatory body
ActiveUS20050077369A1Low variabilityHigh sensitivityLiquid surface applicatorsWatering devicesGuidelineEngineering
A method for servicing companies associated with a spray device operating under guidelines of a regulatory body includes capturing in vitro actuation data associated with operation of the spray device and distributing the data to a company associated with an aspect of the spray device. The company may use the data for testing the spray device to ensure continued compliance with prior approval of the spray device by the regulatory body. The method may also include converting the data to parameters and distributing the parameters. Capturing the in vitro actuation data may include sorting the data based on in vitro age groups. Distributing the data may include providing the data in a format executable by an automated actuation system. The company receiving the data may be a drug development company, spray device manufacturer, or testing service. The regulatory body may be the Food and Drug Administration (FDA).
Owner:PROVERIS SCI CORP
Preparation method of medical rubber stopper
The invention relates to a preparation method of a medical rubber stopper, which comprises the steps as follows: a rubber stopper is immersed into alkaline aqueous solution; the rubber stopper treated with the alkaline aqueous solution is immersed into acid aqueous solution for neutralization; the rubber stopper neutralized with the acid aqueous solution is rinsed with purified water or water forinjection and siliconized with silicone oil; the siliconized rubber stopper is dried at 115-130 DEG C; and the medical rubber stopper meeting the requirement of compatibility of particular drugs is obtained. The preparation method of the medical rubber stopper has simple process and realizes industrialization easily. High-performance medical rubber stoppers treated by the method do not affect thesealing performance of the original halogenated butyl rubber stopper, and can meet the requirements of Standard YBB00042005 and / or Standard YBB00052005 issued by the State Food and Drug Administration and the requirement of compatibility of particular drugs.
Owner:郑州翱翔医药科技股份有限公司
Method and apparatus for spin-echo-train MR imaging using prescribed signal evolutions
InactiveUSRE45725E1Shorten Image Acquisition TimeExtended durationDiagnostic recording/measuringSensorsHigh fieldMagnetic resonance technique
A magnetic resonance imaging “MRI” method and apparatus for lengthening the usable echo-train duration and reducing the power deposition for imaging is provided. The method explicitly considers the t1 and t2 relaxation times for the tissues of interest, and permits the desired image contrast to be incorporated into the tissue signal evolutions corresponding to the long echo train. The method provides a means to shorten image acquisition times and / or increase spatial resolution for widely-used spin-echo train magnetic resonance techniques, and enables high-field imaging within the safety guidelines established by the Food and Drug Administration for power deposition in human MRI.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
System and method for tracking and managing medical device inventory
InactiveUS20160371639A1FinanceHealthcare resources and facilitiesMedical equipmentApplication software
A system and method of managing the logistics of trays and lots of medical inventory, from the manufacturer's initial release until final discharge by the end user healthcare provider. The system is driven by one or more software applications running on a central, secure server accessible by remote handheld telecommunication devices. The system comprises software modules providing functionality for the management of specific medical cases, the billing process, warehouse reconciliation of used inventory, a loaner program for borrowing otherwise unavailable inventory, and many other processes. Additional features of the system include a geographic map, an inventory list of medical devices, an imaging assembly to read barcodes or other optic identifiers, an Food and Drug Administration news assembly incorporating a live RSS news feed for releases about product defects or recalls.
Owner:MEDICAL TRACKING SOLUTIONS
Fish-source anti-freezing polypeptide and preparation method thereof
ActiveCN103483440AAntifreeze activity efficiently achievedAntifreeze activity achievedFermentationAnimals/human peptidesBiotechnologyCollagenan
The invention provides a fish-source anti-freezing polypeptide and a preparation method thereof. Fish skin collagen is used as a raw material which is subject to enzymolysis of papain and then separated and purified to obtain the purified specific anti-freezing polypeptide, and the amino acid complete sequence is Lys-Gly-Glu-Ala-Gly-Asp-Asn-Gly-Ala-Lys-Gly-Asp-Ala-Gly-Ser-Pro-Gly. The invention breaks through the extant research concepts and methods of the anti-freezing protein at home and abroad, avoids limitation on quantity of anti-freezing protein purified from natural living bodies and safety worries about applying the transgenic anti-freezing protein to food from the international FDA (Food and Drug Administration), and acquires the food-source-based efficient anti-freezing polypeptide, thereby laying a theoretical basis for developing the food-source-based anti-freezing polypeptide and exploring the wide application of the food-source-based anti-freezing polypeptide to food and medicines.
Owner:FUZHOU UNIV
Fasudil hydrochloride injection composition and its preparation method
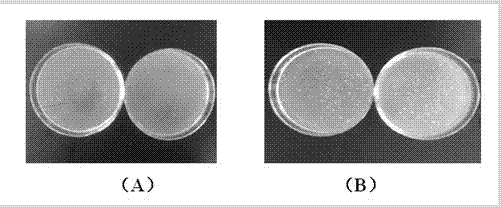
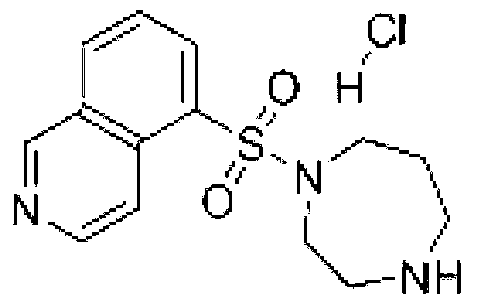
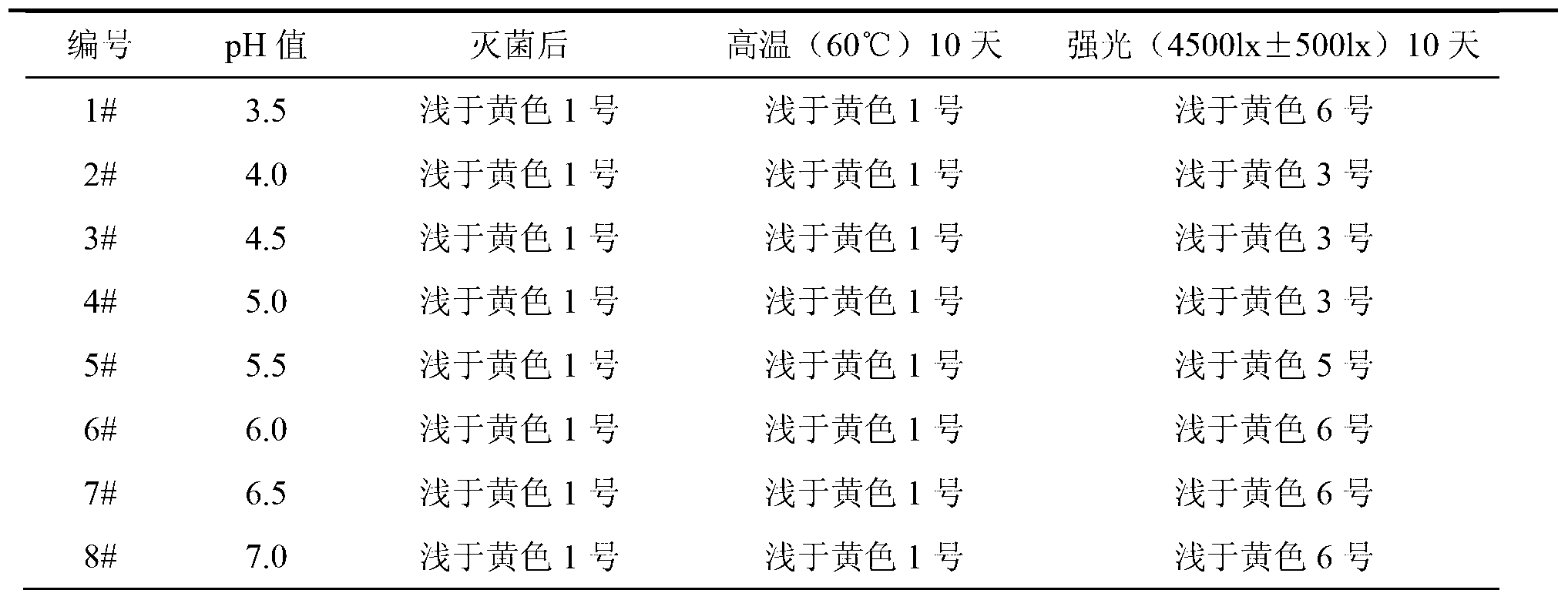
ActiveCN103222953AReduce contentSimple prescriptionOrganic active ingredientsPharmaceutical delivery mechanismSodium calcium edetateMedical prescription
The invention provides a novel Fasudil hydrochloride injection composition and its preparation method. A prescription and a preparation technology are simple. As a metal ion chelating agent, sodium calcium edetate in the prescription can be used to reduce degradation of the product under a high-light condition. In addition, the preparation technology provided by the invention is easy to operate. The quality of the prepared product is controllable, and the product has good stability and meets regulations of SFDA (State Food and Drug Administration).
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD
Antifreeze polypeptide for protecting bacteria from low temperature freeze-thawing damage and preparation method thereof
ActiveCN103467586AAntifreeze activity achievedDead animal preservationPeptide preparation methodsBiotechnologyFreeze-drying
The invention discloses an antifreeze polypeptide for protecting bacteria from low temperature freeze-thawing damage and a preparation method thereof. The preparation method is characterized by using fish-derived collagens as raw materials, adopting alkaline protease to carry out enzymolysis on the fish-derived collagens and carrying out separation and purification and freeze drying, thus obtaining the antifreeze polypeptide for protecting bacteria from low temperature freeze-thawing damage. The amino acid complete sequence of the antifreeze polypeptide is Gly-Gln-Arg-Gly-Gly-Arg-Gly-Leu-Pro-Gly-Glu-Arg-Gly-Arg-Val-Gly-Pro-Ser-Gly-Pro-Ala-Gly-Ala-Arg-Gly-Ala-Asp-Gly. By adopting the antifreeze polypeptide and the preparation method, the research ideas and methods of antifreeze proteins (polypeptides) existing at home and abroad are broken through, limitation of the quantity of the antifreeze proteins purified form natural organisms and considerations of international FDA (food and drug administration) for security of transgenic antifreeze proteins in food application are overcome, an efficient antifreeze polypeptide based on food sources is obtained, and a theoretical foundation is laid for developing the antifreeze polypeptide based on food sources and exploring extensive application of the antifreeze polypeptide to food and medicines.
Owner:FUZHOU UNIV
Vulcanizing system and medical chlorinated butyl rubber plug
ActiveCN102702574ARaw materials are easy to getSimple processPharmaceutical containersMedical packagingPolymer sciencePtru catalyst
The invention discloses a vulcanizing system, which consists of the following components in parts by weight: 0.5-5 parts of a vulcanizing agent, 0.1-2 parts of a catalyst and 0.5-6 parts of an activating agent, wherein the vulcanizing agent is a silane coupling agent of which the general formula is shown as Y(CH2)nSiX3; in the formula, Y represents alkyl, vinyl, an epoxy group, amino or sulfydryl, and X represents chloro or alkoxyl; the catalyst is thiolimidazoline or 2-thiolbenzimidazole; and the activating agent is zinc oxide or magnesium oxide. A medical chlorinated butyl rubber plug has the advantages of readily-available raw materials and simple process. The medical chlorinated butyl rubber plug prepared by adopting the vulcanizing system can meet the requirements of standards YBB00042005 and / or YBB00052005 issued by the State Food and Drug Administration, can meet the requirements of the Japanese Pharmacopoeia, can meet the requirement of compatibility of special medicaments simultaneously, and is equivalent to the quality of national and international products of the same type.
Owner:郑州翱翔医药科技股份有限公司
Preparation method and technology of alliin reference substance
ActiveCN101560174ASimple extraction and purification processLow priceOrganic chemistryOrganic compound preparationPhysical constantFood and drug administration
The invention relates to a preparation method and technology of alliin reference substance, which comprises the following steps of: first extracting alliin from fresh garlic; then preparing the alliin reference substance by using two methods: the first method is to prepare the alliin reference substance by ethanol recrystallization; and the second method is to prepare the alliin reference substance by acetone recrystallization; and finally determining the alliin physical constant, analyzing four spectrum, and determining the purity; if all the indicators thereof are eligible, the product complies with the 'alliin reference substance' required by the 'plant extract reference substance' regulated by the State Food and Drug Administration.
Owner:新疆胡蒜研究院(有限公司)
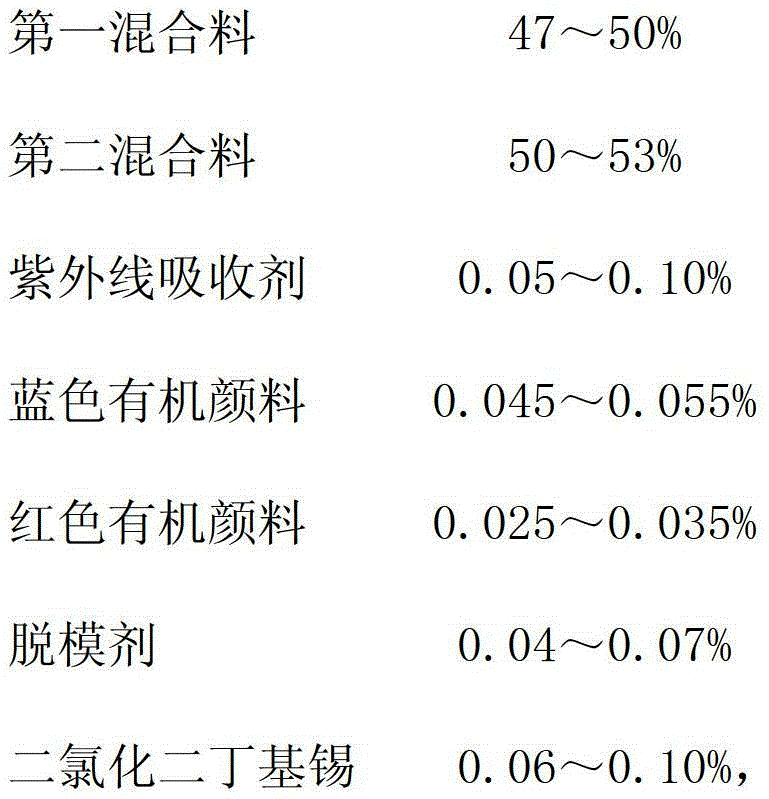
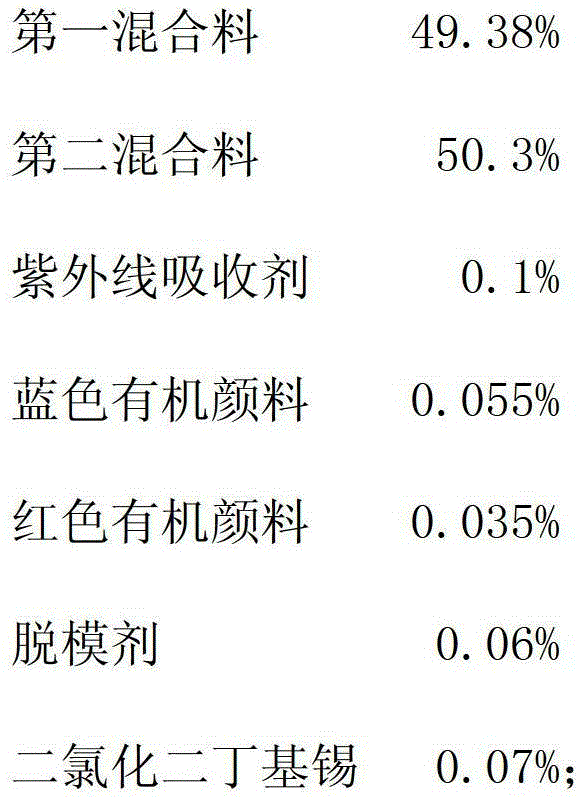
PMC (Polymer-matrix composite) radiation-proof resin lens and manufacturing process thereof
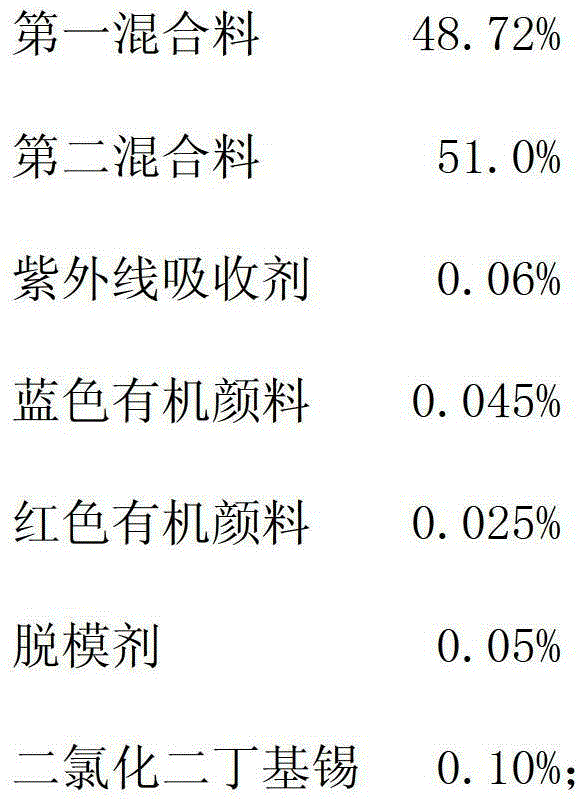
The invention relates to a PMC (polymer-matrix composite) radiation-proof resin lens, comprising the following components in percentage by weight:47-50% of a first mixture, 50-53% of a second mixture, 0.05-0.10% of an ultraviolet absorbent, 0.045-0.055% of a blue organic pigment, 0.025-0.035% of a red organic pigment, 0.04-0.07% of a mold releasing agent, and 0.06-0.10% of dibutyltin dichloride. By adopting the PMC radiation-proof resin lens, the visual field aberration caused by edge dispersion of a substrate can be greatly improved, the image sharpness is improved, and the shock resistance passes through and surpasses an FDA (Food and Drug Administration) shock resistance safety certification of America, so that the safety for wearing the resin lens is greatly improved.
Owner:JIANGSU MINGYUE PHOTOELECTRICS TECH
Compositions for drug administration
InactiveUS20120021980A1Improve absorption and bioavailabilityToxic effectsPeptide/protein ingredientsMetabolism disorderChain lengthDrug administration
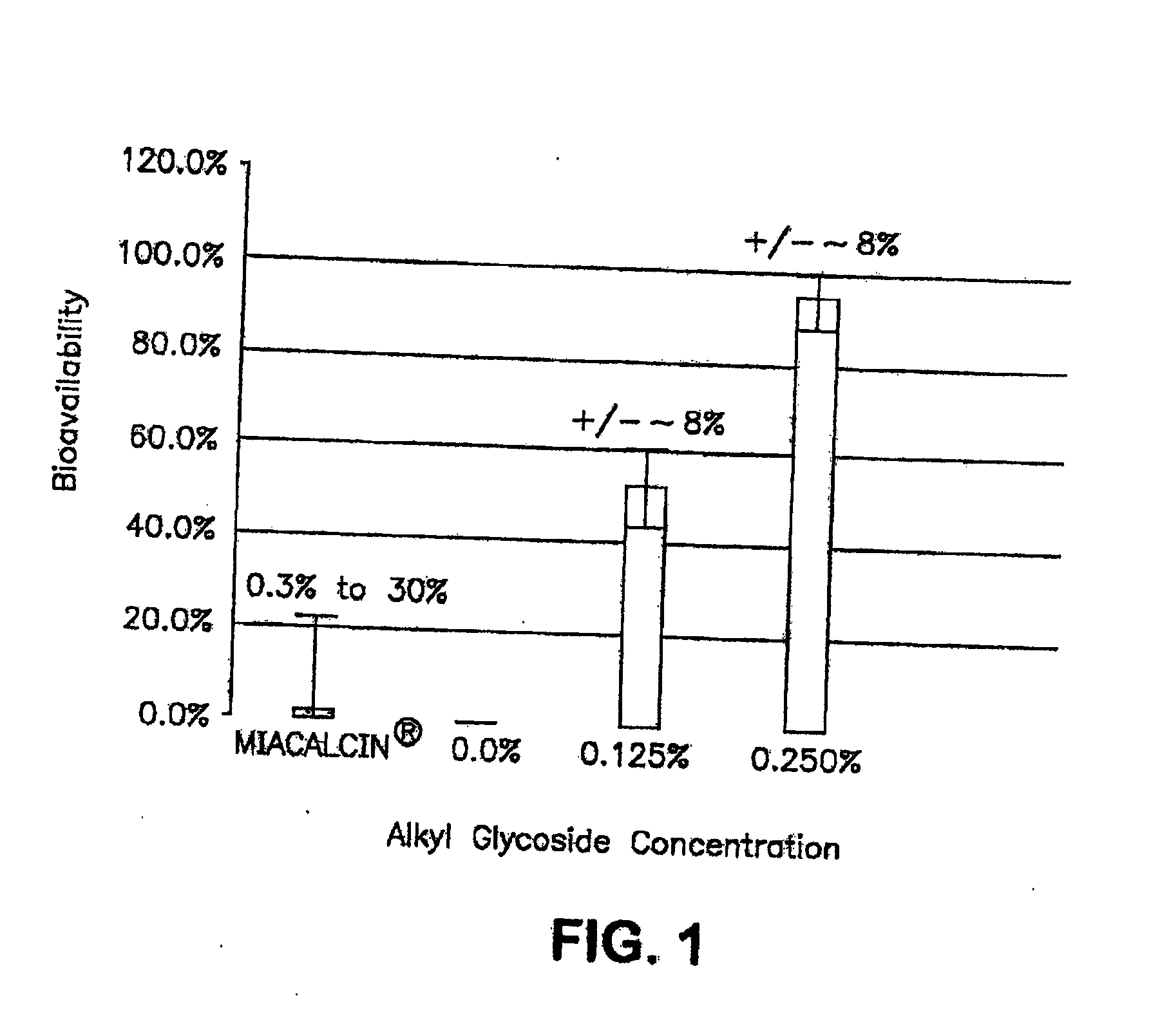
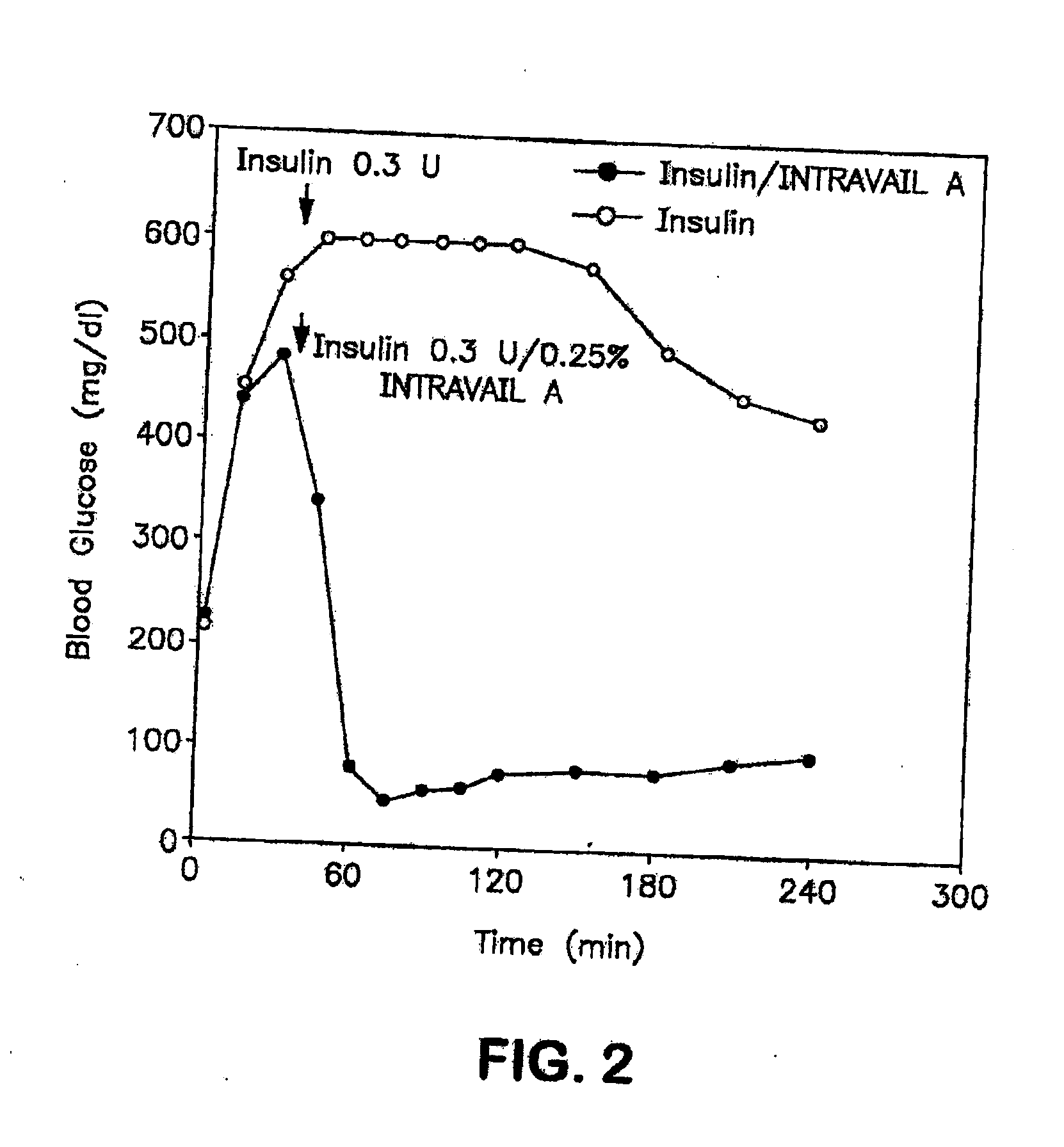
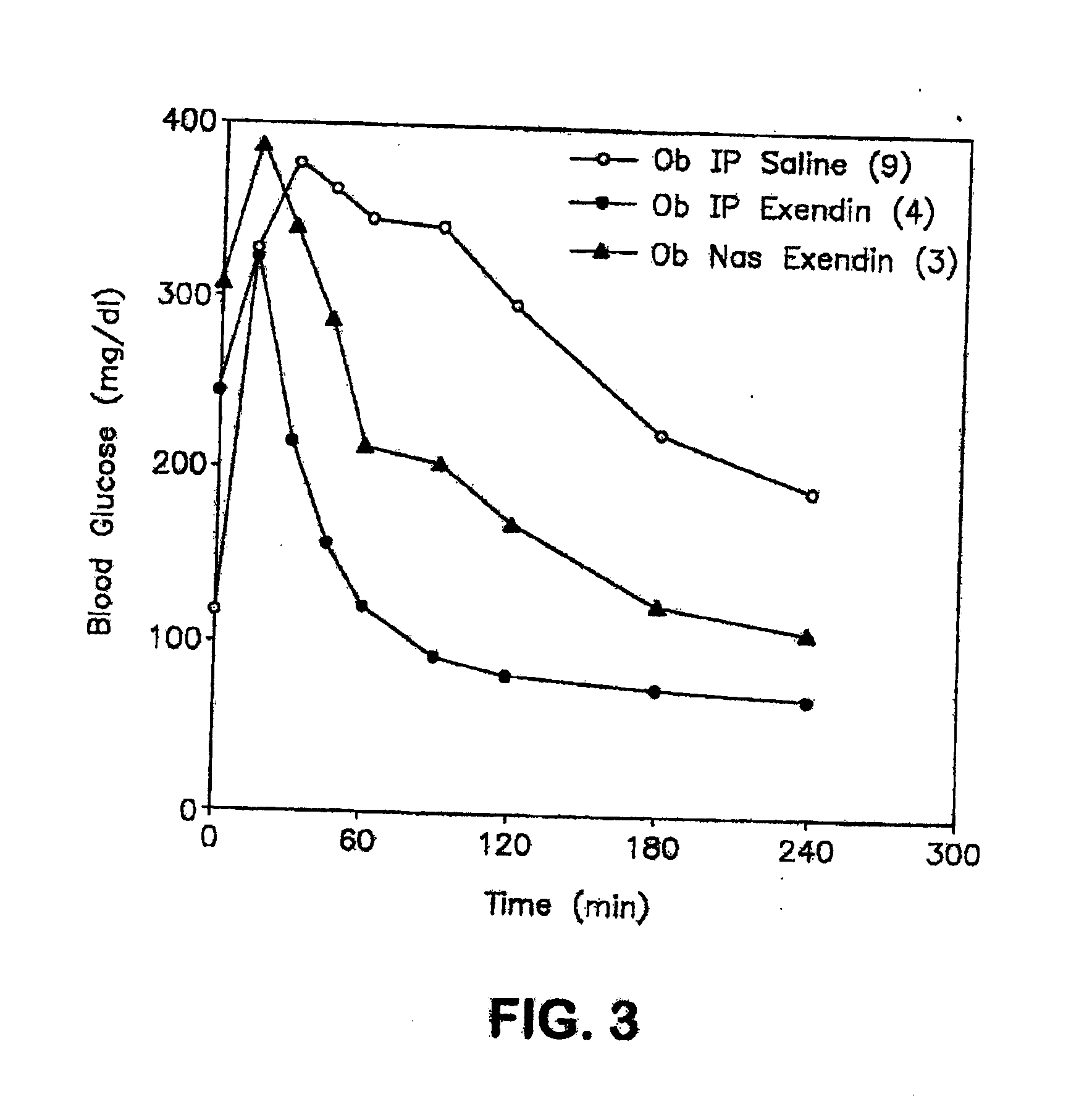
The present invention provides compositions and methods and for increasing the bioavailability of therapeutic agents in a subject. The compositions include at least one alkyl glycoside and at least one therapeutic agent, wherein the alkylglycoside has an alkyl chain length from about 10 to about 16 carbon atoms. In various aspects, the invention provides compositions and methods for oral delivery of glucagon-like peptide-1 analogs, such as exenatide, albiglutide, taspoglutide, liraglutide and lixisenatide.
Owner:AEGIS THERAPEUTICS LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com