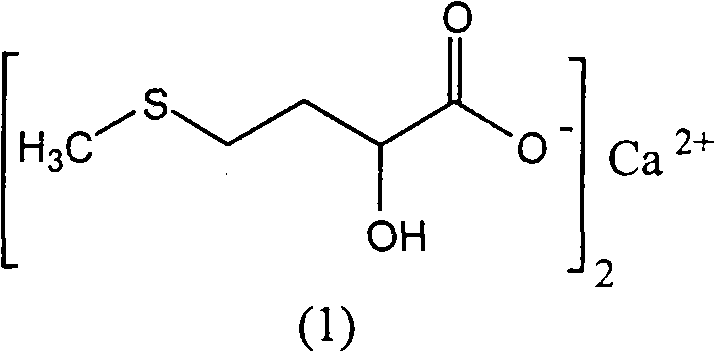
Preparation of medicinal D,L-2-hydroxy-4-methylthio calcium butyrate
A technology of calcium methylthiobutyrate and methylthiobutyric acid, which is applied in the field of medicinal D, and achieves the effects of low cost, convenient preparation, and easy control of product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
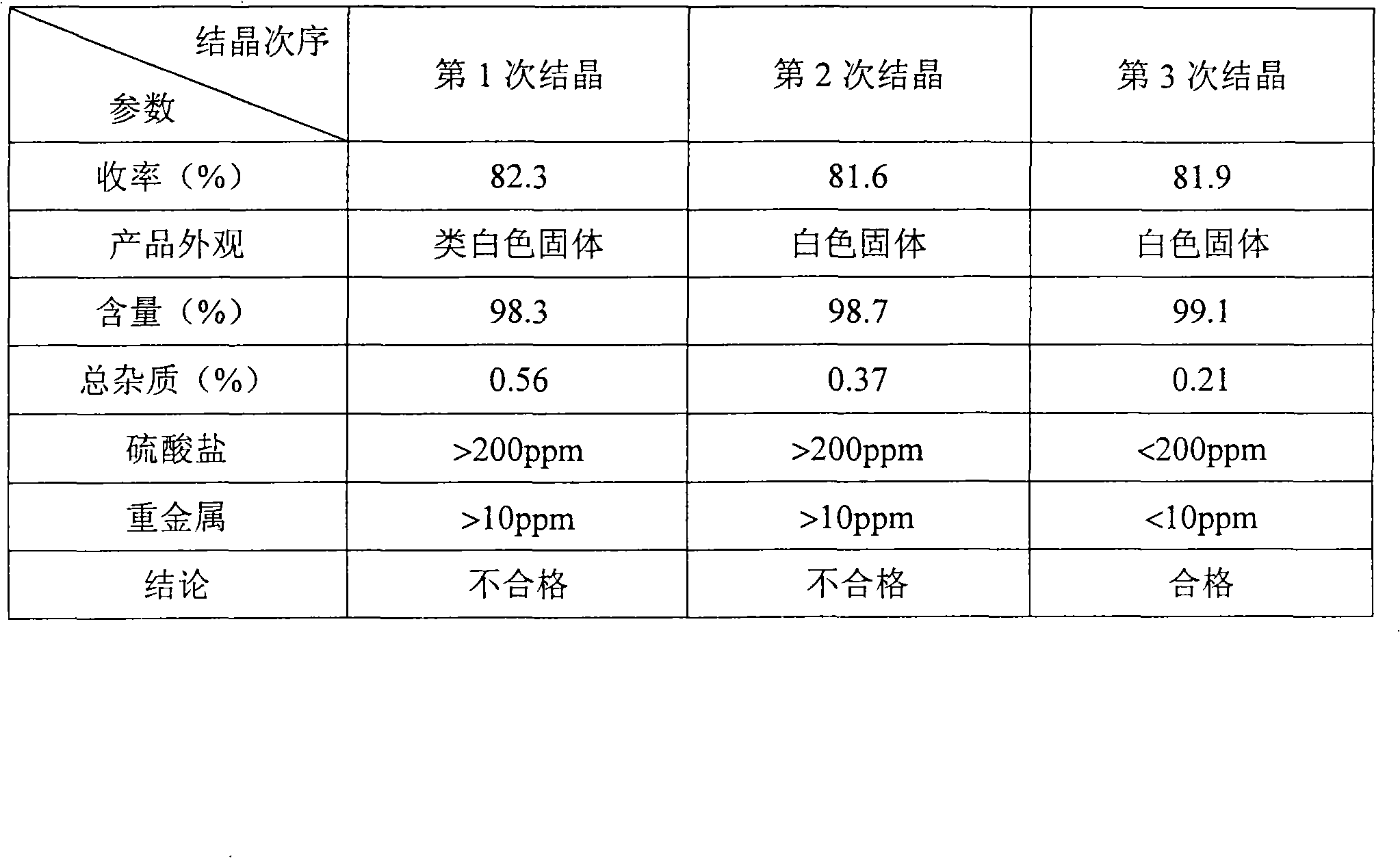
Examples
Embodiment 1
[0023] Embodiment 1: D, the preparation of L-2-hydroxyl-4-methylthiobutyric acid methyl ester
[0024] Add 60g of Alimet to a 500mL three-neck round bottom flask with mechanical stirring TM (NOVUS company), start stirring, add 120mL ethyl acetate, stir for about 5 minutes, add 120mL water; continue stirring for about 5 minutes, transfer the liquid in the flask to a separatory funnel, take the upper layer of ethyl acetate and evaporate it under reduced pressure to recover acetic acid ethyl ester to obtain 48.9 g of a dark brown concentrated solution.
[0025] In another 500mL three-neck round bottom flask with magnetic stirring and reflux condenser, add 45.1g of the above concentrated solution and 121.4mL of methanol, start stirring, add 15g of sulfuric acid (concentration: 98%), heat to reflux, and the reaction ends after 4h; Cool, evaporate under reduced pressure to recover methanol, add 90mL ethyl acetate to the concentrated solution to dilute, wash the diluted solution wit...
Embodiment 2
[0026] Embodiment 2: D, the preparation of L-2-hydroxyl-4-methylthiobutyric acid methyl ester
[0027] Add 60g of Alimet to a 500mL three-neck round bottom flask with mechanical stirring TM (NOVUS company), start stirring, add 120mL ethyl acetate, stir for about 5 minutes, add 120mL water; continue stirring for about 5 minutes, transfer the liquid in the flask to a separatory funnel, take the upper layer of ethyl acetate and evaporate it under reduced pressure to recover acetic acid ethyl ester to obtain 47.6 g of a dark brown concentrated solution.
[0028] In another 500mL three-necked round bottom flask with magnetic stirring and reflux condenser, add 45.1g of the above concentrated solution and 242.7mL of methanol, start stirring, add 12g of sulfuric acid (concentration: 98%), heat to reflux, and the reaction ends after 4h; Cool, evaporate under reduced pressure to recover methanol, add 90mL ethyl acetate to the concentrated solution to dilute, wash the diluted solution w...
Embodiment 3
[0029]Embodiment 3: D, the preparation of L-2-hydroxyl-4-methylthiobutyric acid methyl ester
[0030] Add 60g of Alimet to a 500mL three-neck round bottom flask with mechanical stirring TM (NOVUS company), start stirring, add 120mL ethyl acetate, stir for about 5 minutes, add 120mL water; continue stirring for about 5 minutes, transfer the liquid in the flask to a separatory funnel, take the upper layer of ethyl acetate and evaporate it under reduced pressure to recover acetic acid ethyl ester to obtain 48.3 g of a dark brown concentrated solution.
[0031] In another 500mL three-necked round bottom flask with magnetic stirring and reflux condenser, add 45.1g of the above concentrate and 364mL of methanol, start stirring, add 9g of sulfuric acid (concentration is 98%), heat to reflux, and the reaction ends after 5h; cooling , methanol was recovered by evaporation under reduced pressure, the concentrated solution was diluted with 90mL ethyl acetate, and the diluted solution wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com